Patents
Literature
153 results about "Triazolopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
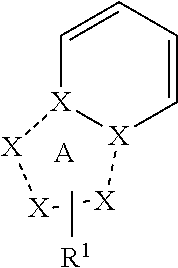
Triazolopyridine is a class of antidepressants chemically and pharmacologically unrelated to other similarly performing antidepressants; clinical effectiveness appears pharmacologically similar to other tricyclic antidepressants, although it seems to have less anticholinergic effects.
Imidazopyridines and triazolopyridines
The present invention relates to imidazopyridine and triazolopyridine derivatives, pharmaceutical compositions and methods of use thereof.
Owner:WARNER-LAMBERT CO
Triazolopyridine JAK Inhibitor Compounds and Methods
InactiveUS20100048557A1Reduce severityReduce the severity of the diseaseBiocideNervous disorderDiseaseKinase activity
A compound of Formula I, enantiomers, diasteriomers, tautomers or pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4 and R5 are defined herein, are useful as JAK kinase inhibitors. A pharmaceutical composition that includes a compound of Formula I and a pharmaceutically acceptable carrier, adjuvant or vehicle, and methods of treating or lessening the severity of a disease or condition responsive to the inhibition of JAK kinase activity in a patient are disclosed.
Owner:GENENTECH INC
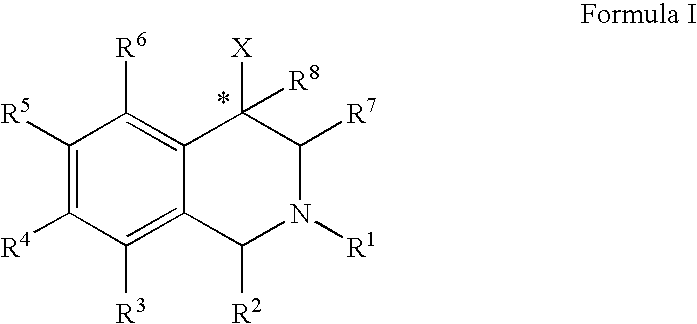
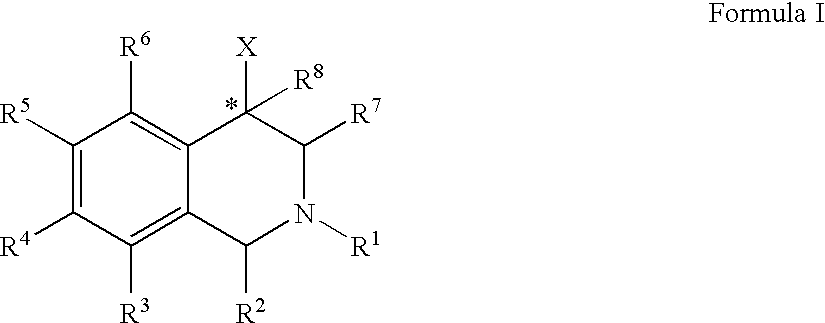
Aryl- and heteroaryl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine, and serotonin
ActiveUS20060052378A1Good curative effectQuick effectBiocideNervous disorderBenzoxazoleChemical structure
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
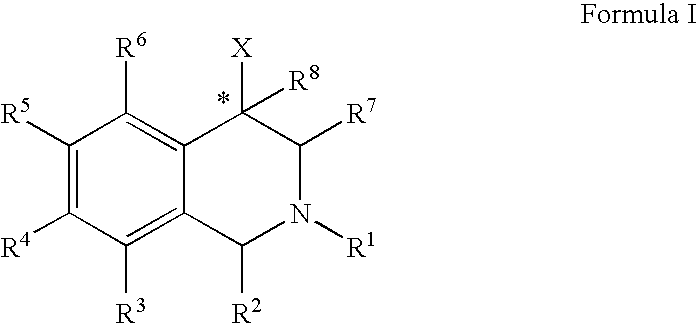
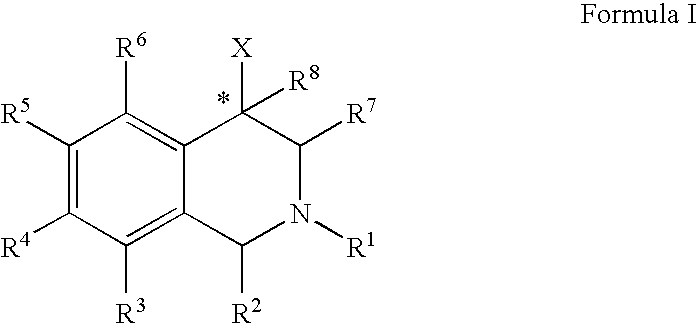
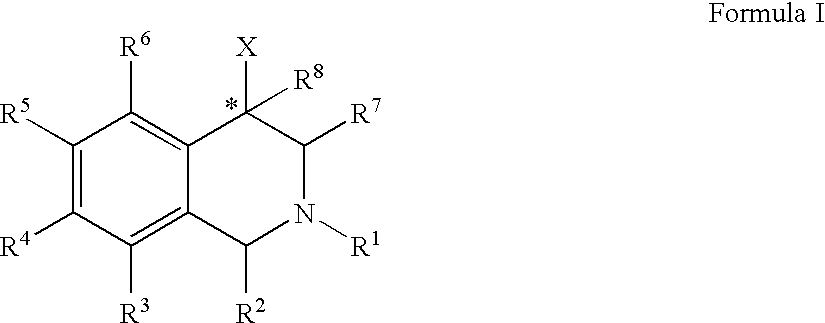
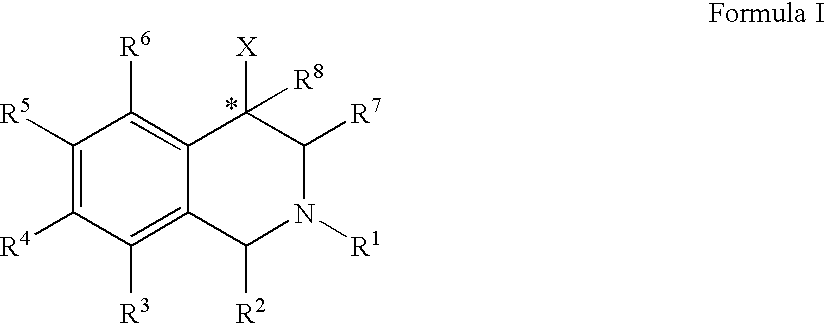
Use of aryl- and heteroaryl-substituted tetrahydroisoquinolines to block reuptake of norepinephrine, dopamine, and serotonin
InactiveUS20060063766A1Little and no activityMinimal potential for substance abuseBiocideNervous disorderBenzoxazoleBenzene
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
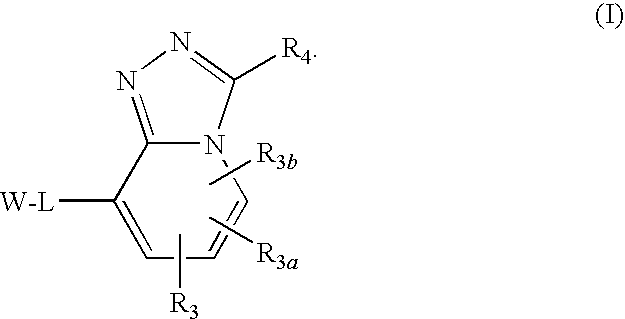
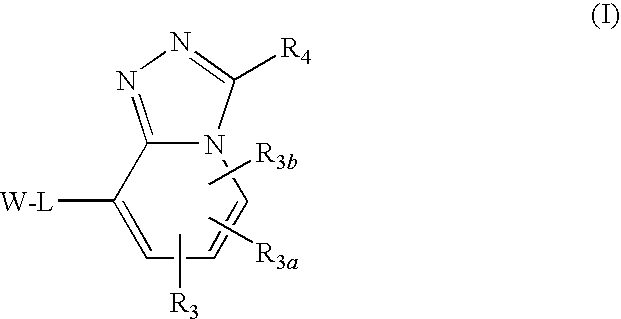
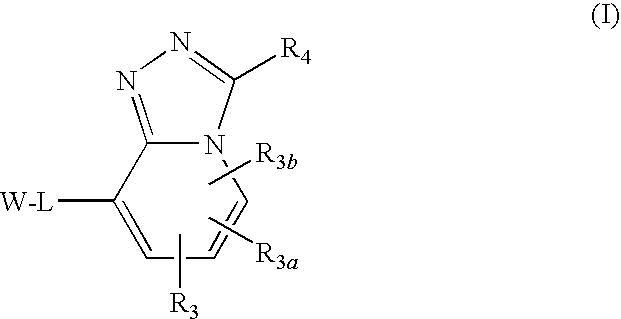
Triazolopyridine 11-beta hydroxysteroid dehydrogenase type i inhibitors
Novel compounds are provided which are 11-beta-hydroxysteroid dehydrogenase type I inhibitors. 11-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 11-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds have the structure:or stereoisomers or prodrugs or pharmaceutically acceptable salts thereof wherein W, L, R3, R3a, R3b and R4 are defined herein.
Owner:BRISTOL MYERS SQUIBB CO
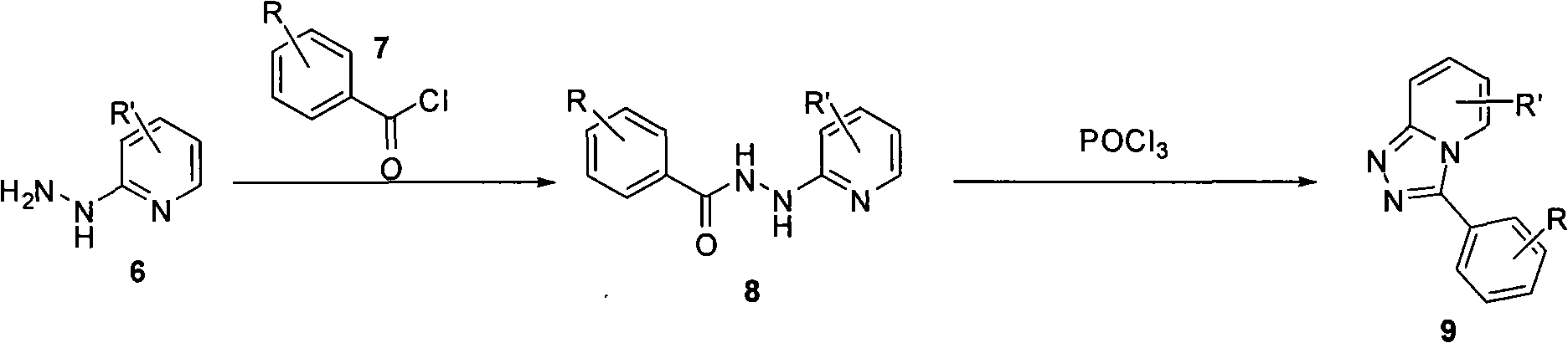
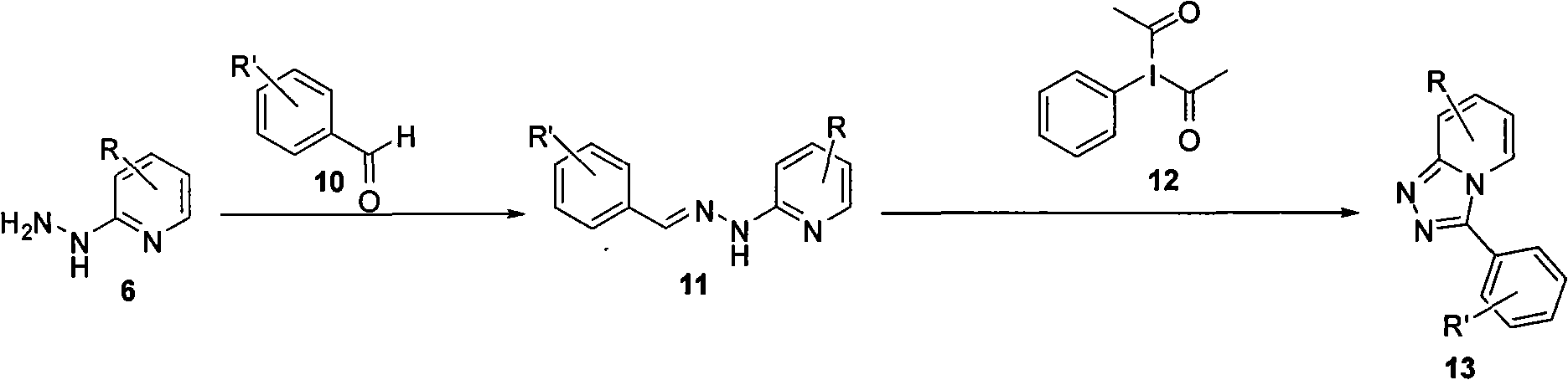
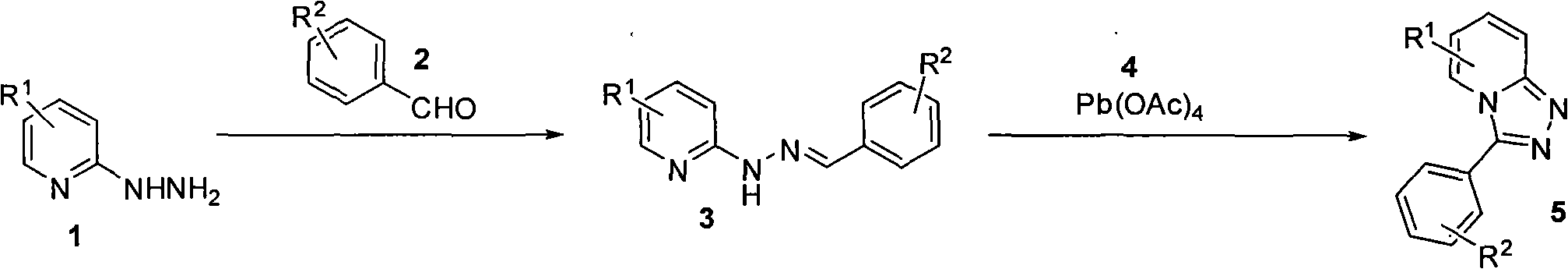
Synthesis method of triazol pyridine ring compound
InactiveCN102002040AEasy to preparePrepared successfullyOrganic chemistrySynthesis methodsBenzaldehyde
The invention relates to a synthesis method of a triazol pyridine ring compound, which mainly solves the technical problems of low yield, high synthesizing cost, poor applicability and the like existing in the traditional synthesis method. The synthesis method comprises the following steps of: (1) making substituted 2-hydrazine-based pyridine react with substituted benzaldehyde, and (2) filtering sediments generated by reaction, dissolving the sediments in a solvent and adding lead tetraacetate, and refluxing to obtain a target product. The target product can be also obtained with a one-pot method by directly carry out the step (2) without separating the sediments. The synthesis method has the advantages of easy obtainment of simple raw materials, simple synthesizing steps, mild reaction condition and higher yield.
Owner:上海药明康德新药开发有限公司 +1
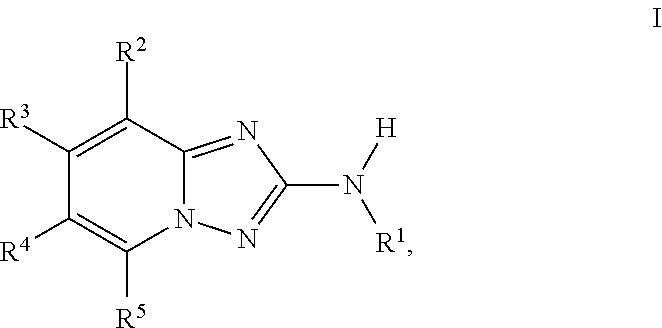
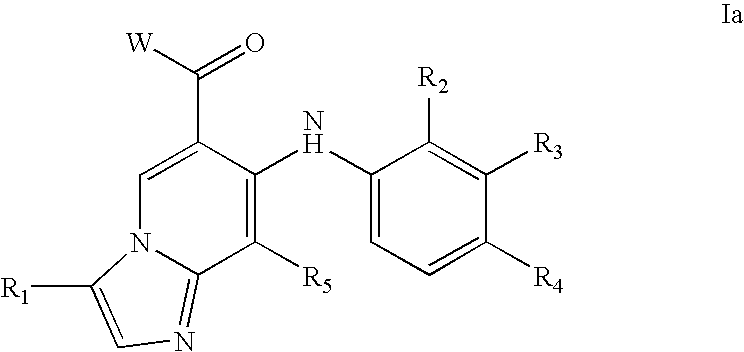
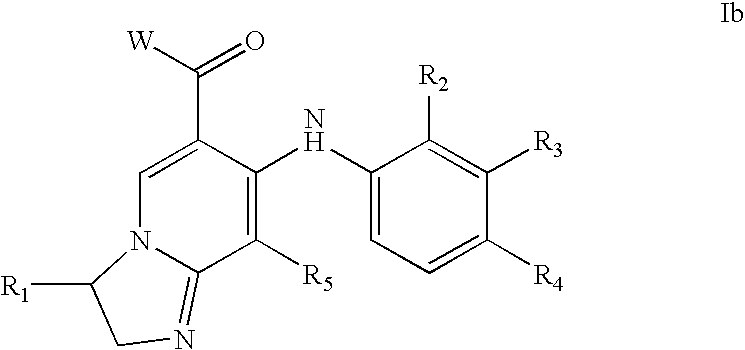
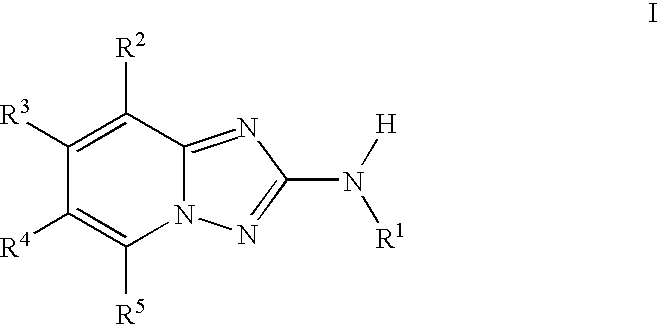
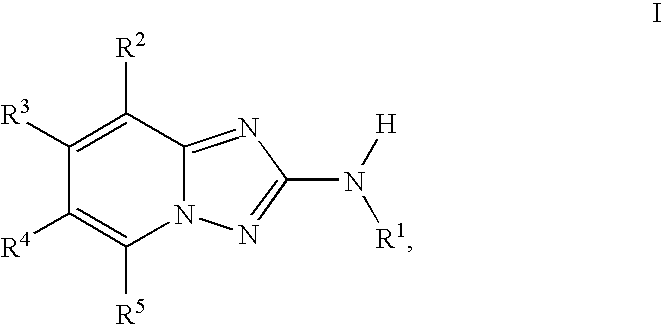
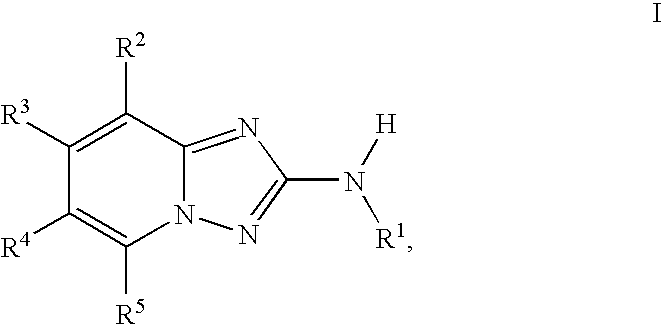
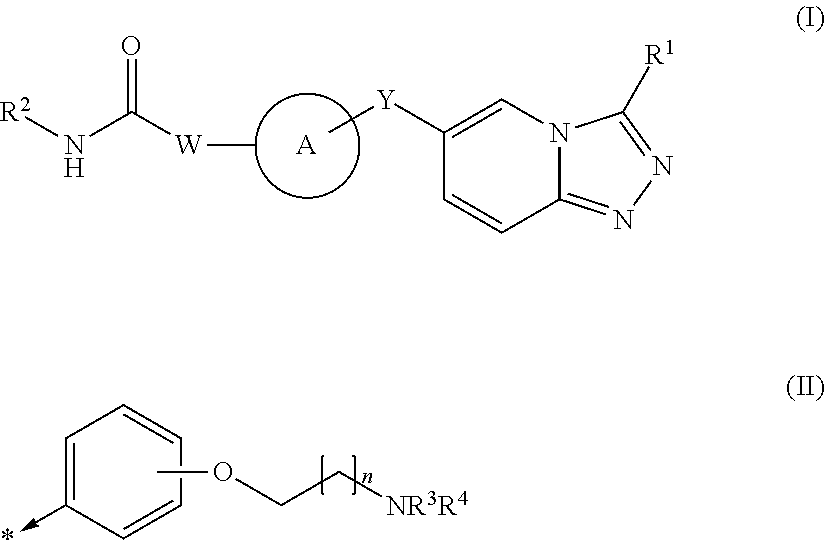
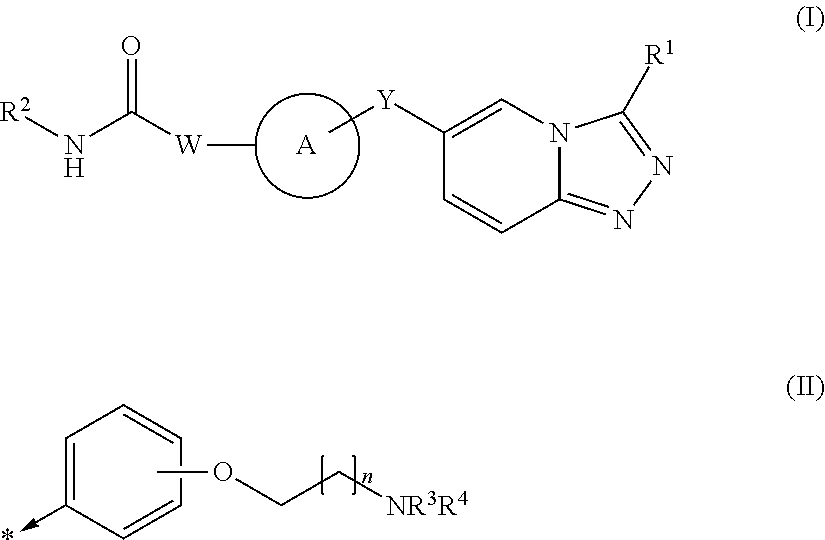
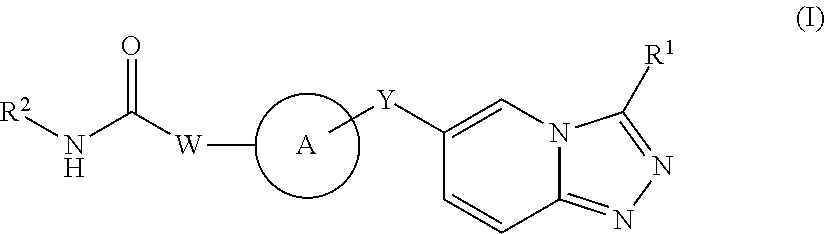
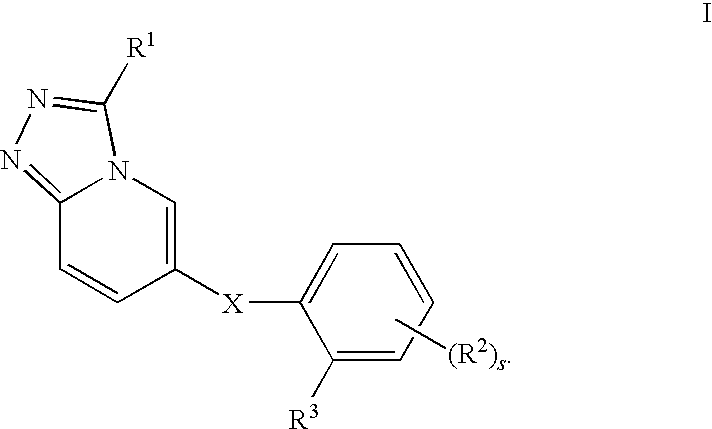
1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders
The present invention relates to novel triazolo[4,3-a]pyridine derivatives of Formula (I) wherein all radicals are as defined in the claims. The compounds according to the invention are positive allosteric modulators of the metabotropic glutamate receptor subtype 2 (“mGluR2”), which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. The invention is also directed to pharmaceutical compositions comprising such compounds, to processes to prepare such compounds and compositions, and to the use of such compounds for the prevention or treatment of neurological and psychiatric disorders and diseases in which mGluR2 is involved.
Owner:JANSSEN PHARMA INC +1
Cmp polishing solution and polishing method
ActiveUS20110318929A1Reduce generationReduce productionOther chemical processesSemiconductor/solid-state device manufacturingZeta potentialPhysical chemistry
The CMP polishing solution of the invention comprises (A) a metal corrosion inhibitor containing a compound with a 1,2,3-triazolo[4,5-b]pyridine skeleton, (B) an abrasive grain having a positive zeta potential in the CMP polishing solution, (C) a metal oxide solubilizer and (D) an oxidizing agent. The polishing method of the invention comprises a first polishing step in which the conductive substance layer of a substrate comprising an interlayer insulating filth having an elevated section and a trench at the surface, a barrier layer formed following the surface of the interlayer insulating film and the conductive substance layer formed covering the barrier layer, is polished to expose the barrier layer located on the elevated section of the interlayer insulating film, and a second polishing step in which the barrier layer exposed in the first polishing step is polished using the CMP polishing solution to expose the elevated section of the interlayer insulating film.
Owner:RESONAC CORP
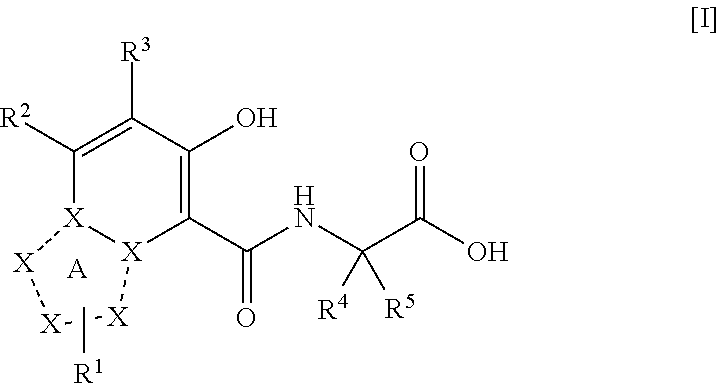
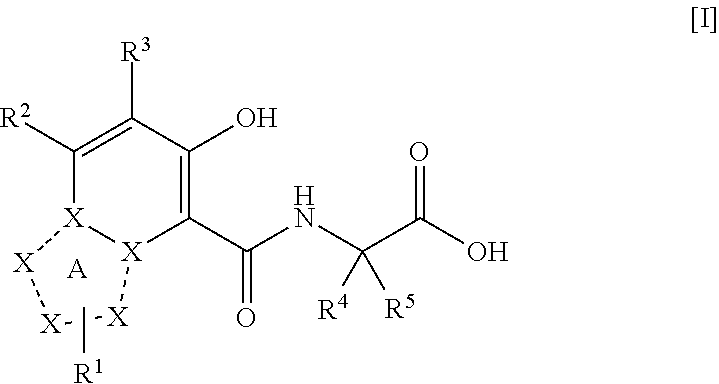
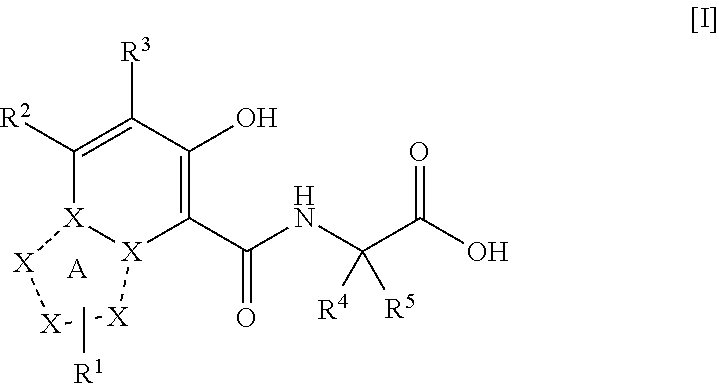
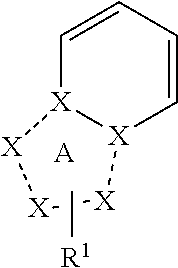
Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor or erythropoietin production-inducing agent
ActiveUS20110077267A1Easy to produceReduce productionBiocideGroup 5/15 element organic compoundsDiseaseMedicine
The present invention provides a triazolopyridine compound having a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability. The present invention relates to a compound represented by the following formula [I]:wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, as well as a prolyl hydroxylase inhibitor or erythropoietin production-inducing agent containing the compound. The compound of the present invention shows a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability and is useful as a prophylactic or therapeutic agent for various diseases and pathologies (disorders) caused by decreased production of erythropoietin.
Owner:JAPAN TOBACCO INC
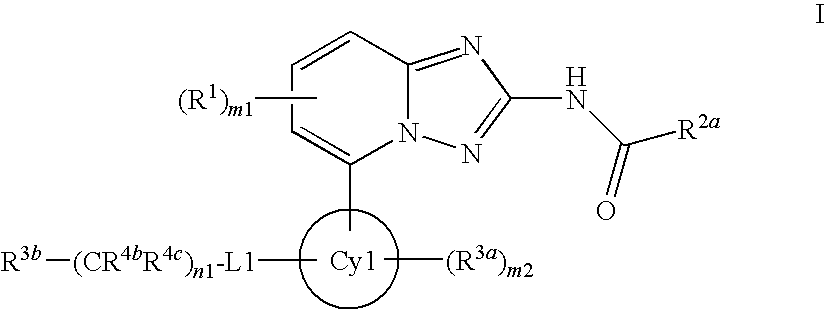
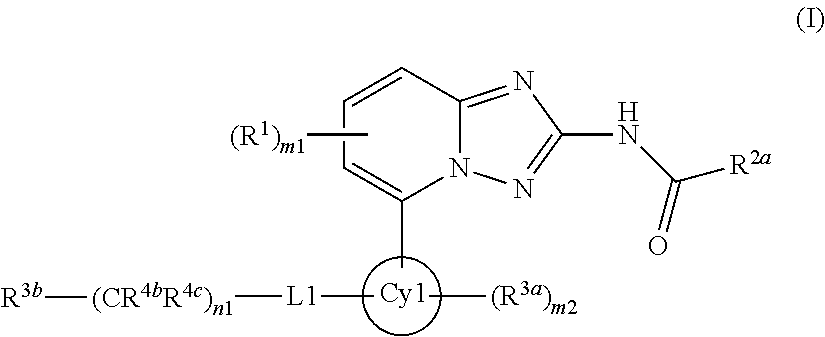
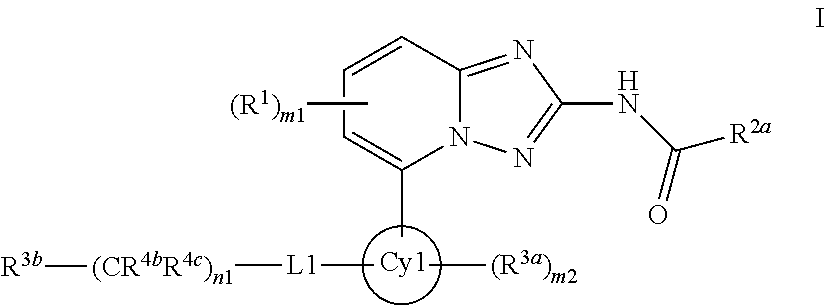
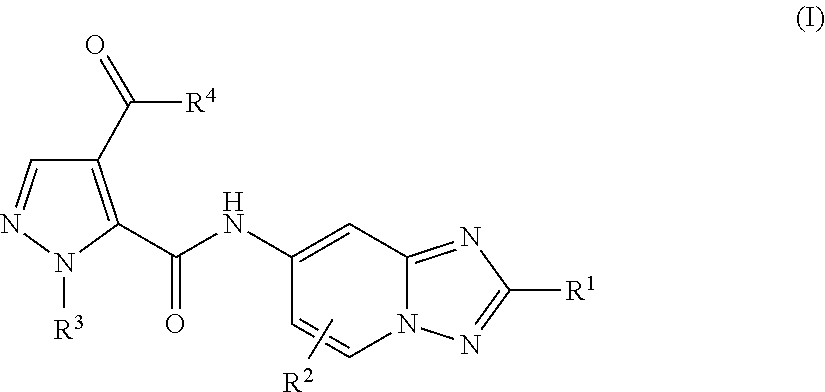
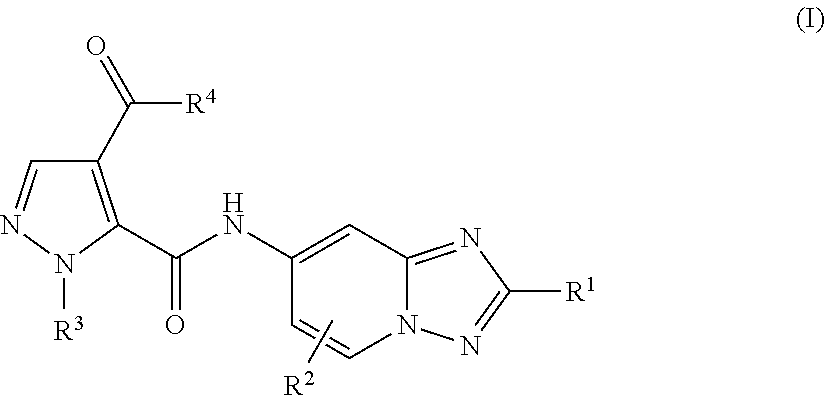
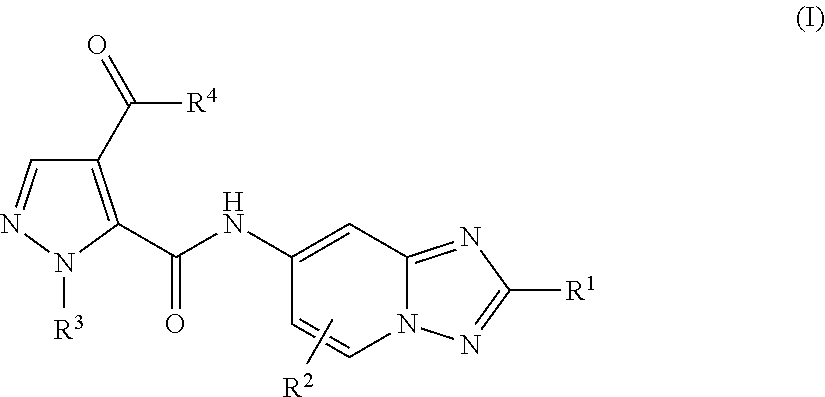
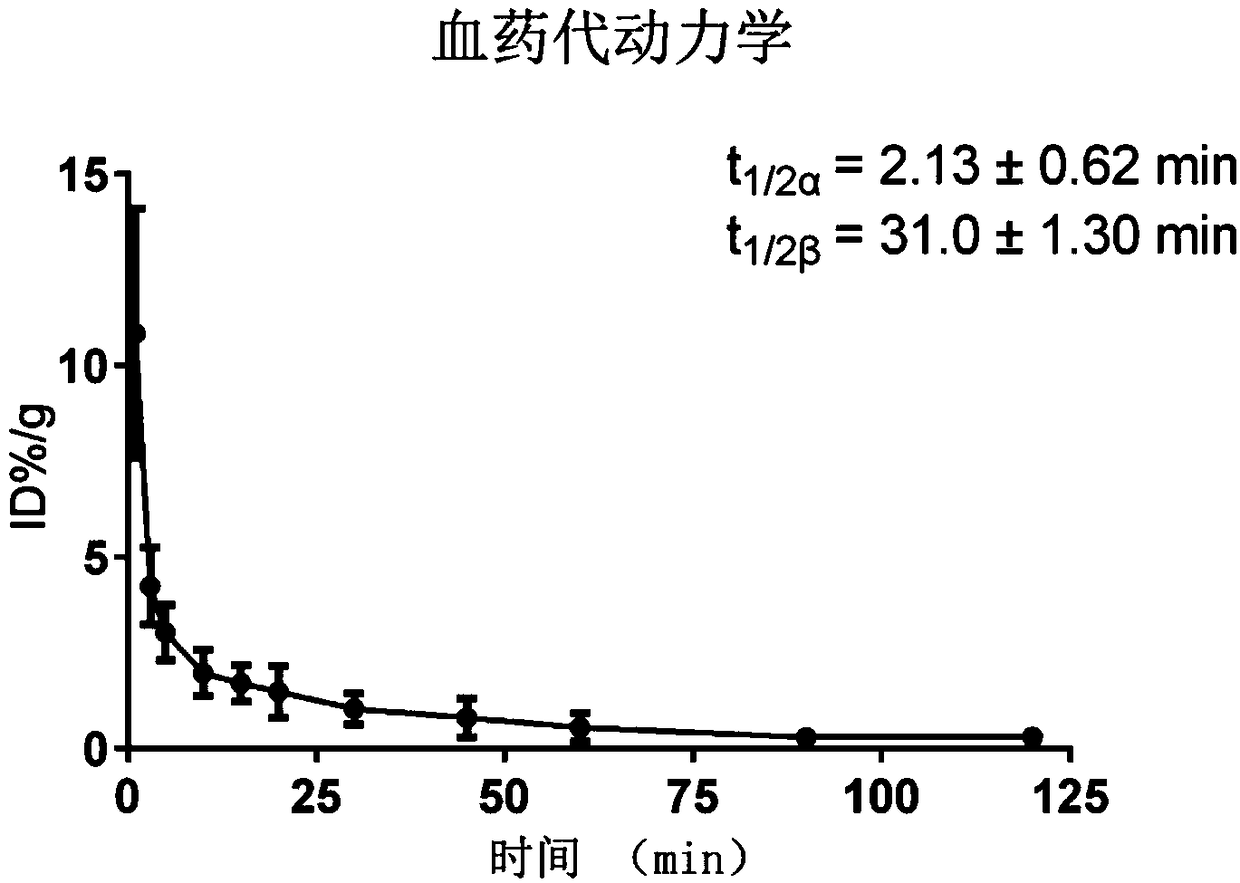
[1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof
The invention relates to a [1,2,4]triazol[1,5-a] pyridine compound as well as a preparation method and medical application thereof. Particularly, the invention relates to the compound shown in a general formula I, the preparation method of the compound, a medicinal composition containing the compound and application of the compound as a Janus kinase inhibitor. The compound and the medicinal composition containing the compound can be used for treating diseases related to the activity of Janus kinase, such as inflammation, autoimmune disease and cancer. The definitions of all substituent groupsin the general formula I are identical with those of the description. (The general formula is shown in the description).
Owner:NAT INST OF PHARMA R & D CO LTD
Novel compounds useful for the treatment of degenerative and inflammatory diseases
ActiveUS20100029709A1Prevent and treat any maladyModify activityBiocideOrganic chemistryMammalArthritis
A novel [1,2,4]triazolo[1,5-a]pyridine compound is disclosed that has a formula represented by the following:This compound may be prepared as a pharmaceutical composition, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example, diseases involving cartilage degradation, bone and / or joint degradation, for example osteoarthritis; and / or conditions involving inflammation or immune responses, such as Crohn's disease, rheumatoid arthritis, psoriasis, allergic airways disease (e.g. asthma, rhinitis), juvenile idiopathic arthritis, colitis, inflammatory bowel diseases, endotoxin-driven disease states (e.g. complications after bypass surgery or chronic endotoxin states contributing to e.g. chronic cardiac failure), diseases involving impairment of cartilage turnover (e.g. diseases involving the anabolic stimulation of chondrocytes), congenital cartilage malformations, diseases associated with hypersecretion of IL6 and transplantation rejection (e.g. organ transplant rejection) and proliferative diseases.
Owner:GALAPAGOS NV
Triazolopyridine 11-beta hydroxysteroid dehydrogenase type i inhibitors
ActiveUS20090093516A1High purityFew process stepsBiocideSenses disorderDisease11-beta-Hydroxysteroid Dehydrogenases
Novel compounds are provided which are 11 -beta-hydroxysteroid dehydrogenase type I inhibitors. 11-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 11-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds of formula I:or stereoisomers or pharmaceutically acceptable salts thereof, wherein G, Q, X, Y, R3, R3a, and R3b are defined herein.
Owner:BRISTOL MYERS SQUIBB CO
Piperazinylimidazopyridine and piperazinyltriazolopyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS20060270848A1Organic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorLutenizing hormone
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
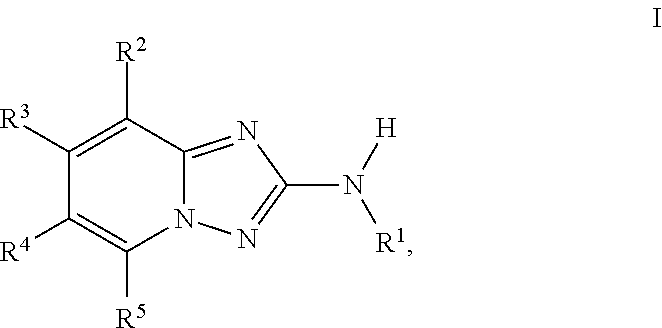
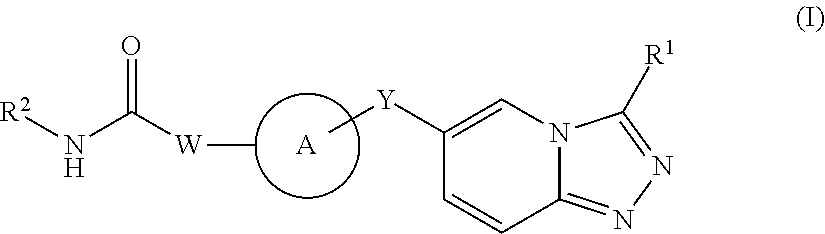
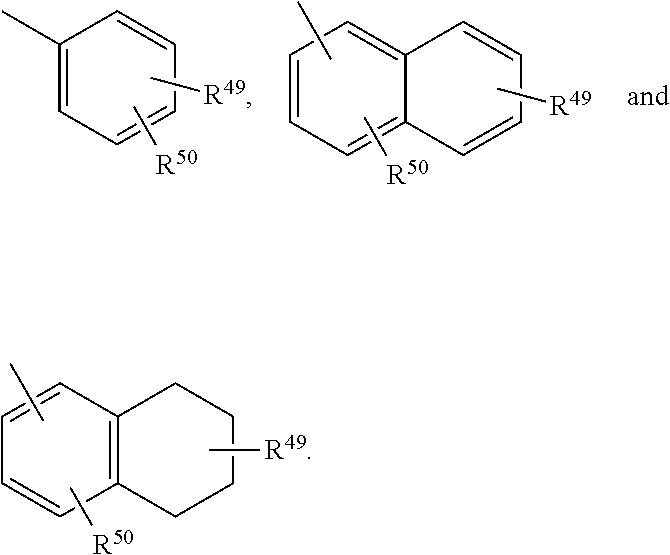
1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors
InactiveUS9012448B2Organic active ingredientsNervous disorderAllosteric modulatorMetabotropic glutamate receptor
The present invention relates to novel triazolo[4,3-a]pyridine derivatives of Formula (I)wherein all radicals are as defined in the claims. The compounds according to the invention are positive allosteric modulators of the metabotropic glutamate receptor subtype 2 (“mGluR2”), which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. The invention is also directed to pharmaceutical compositions comprising such compounds, to processes to prepare such compounds and compositions, and to the use of such compounds for the prevention or treatment of neurological and psychiatric disorders and diseases in which mGluR2 is involved.
Owner:JANSSEN PHARMA INC
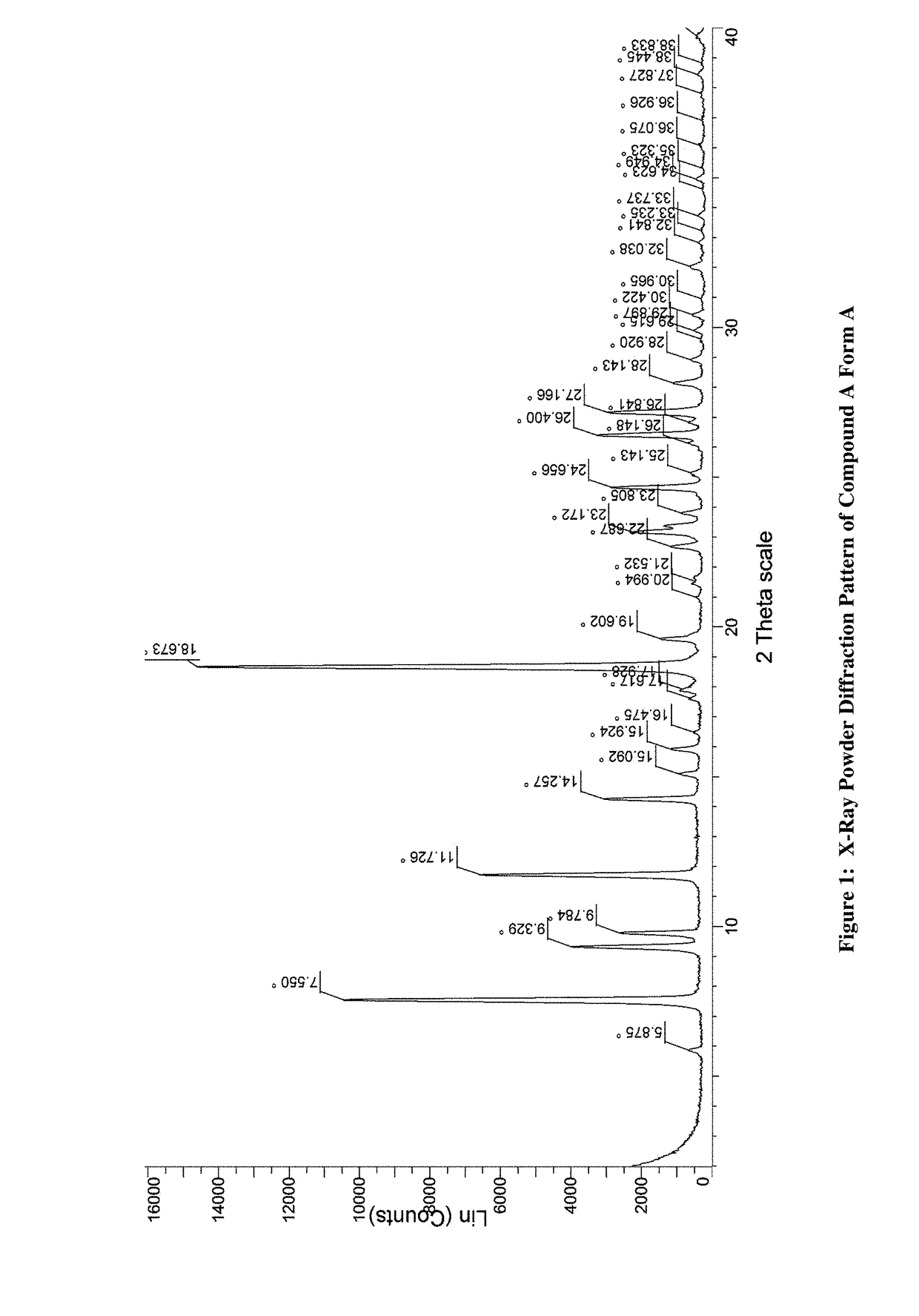
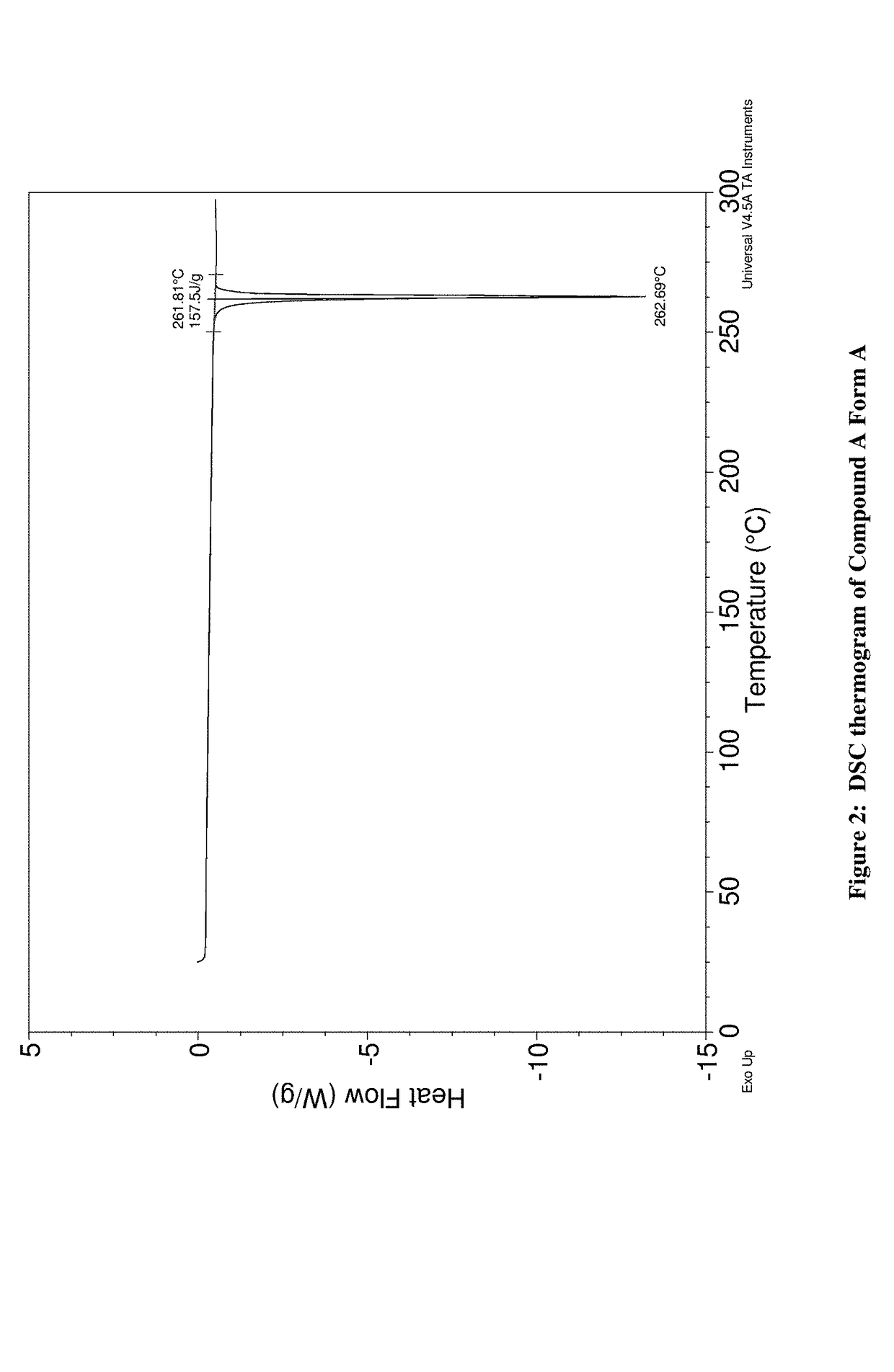
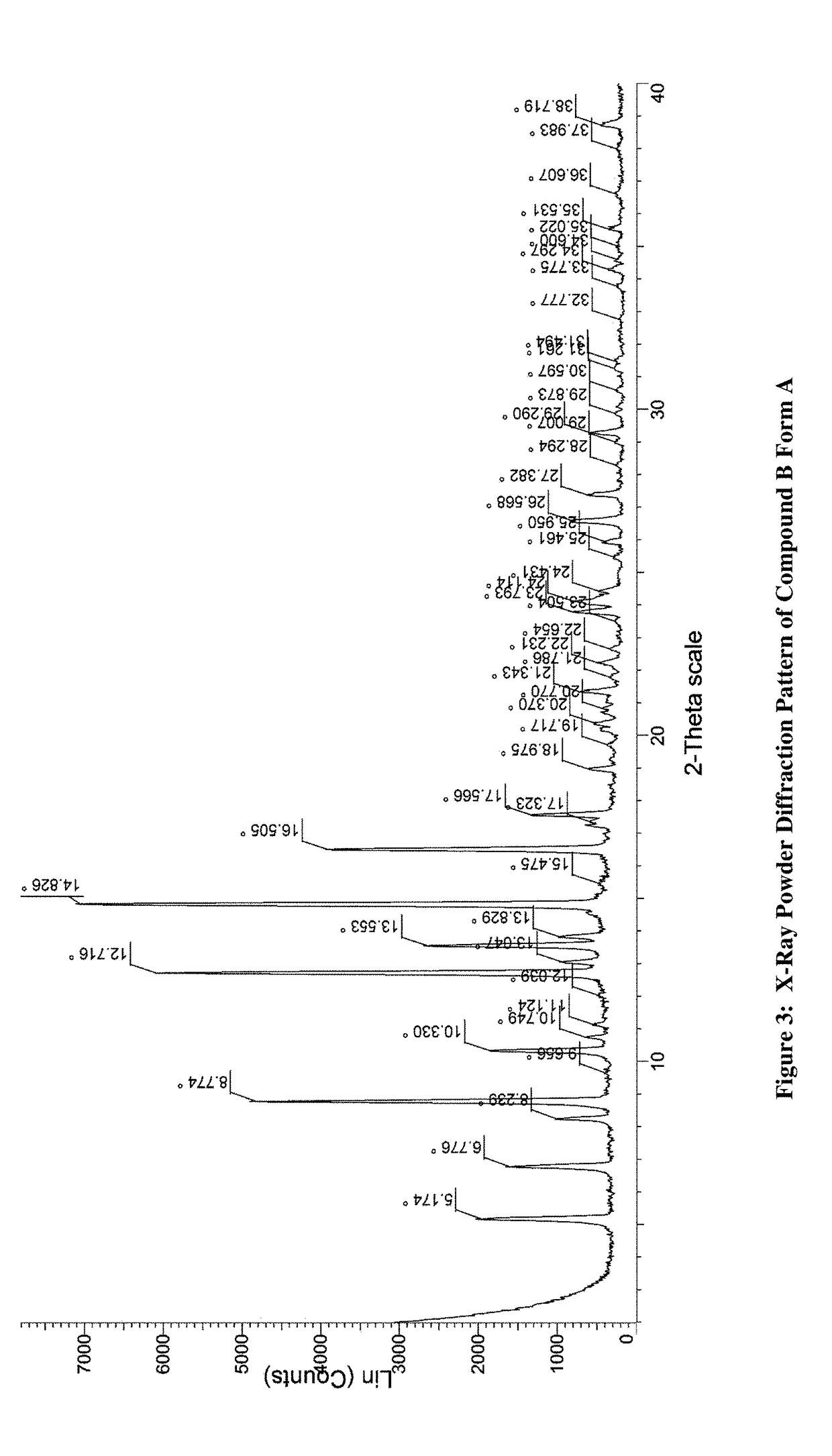
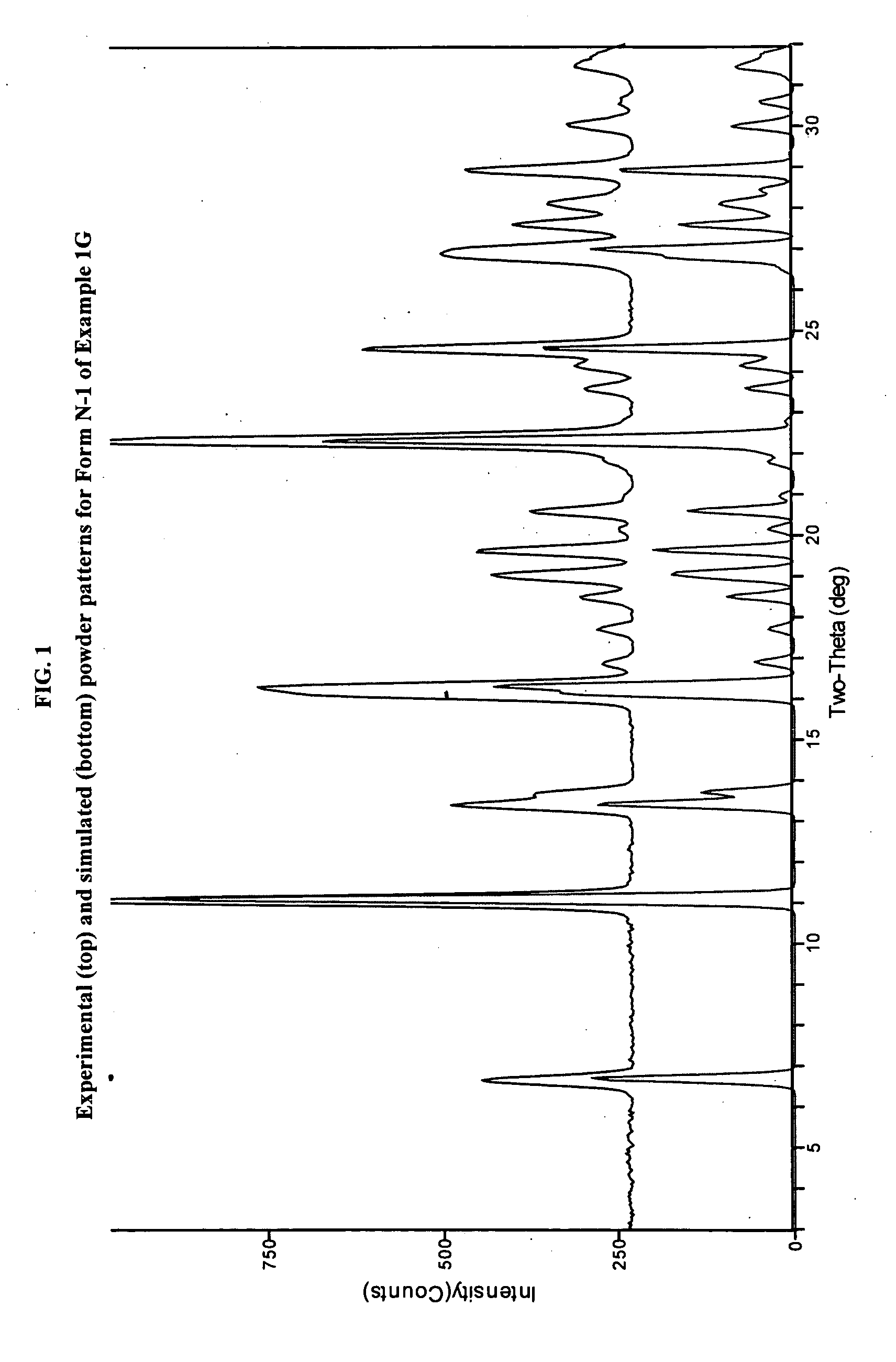
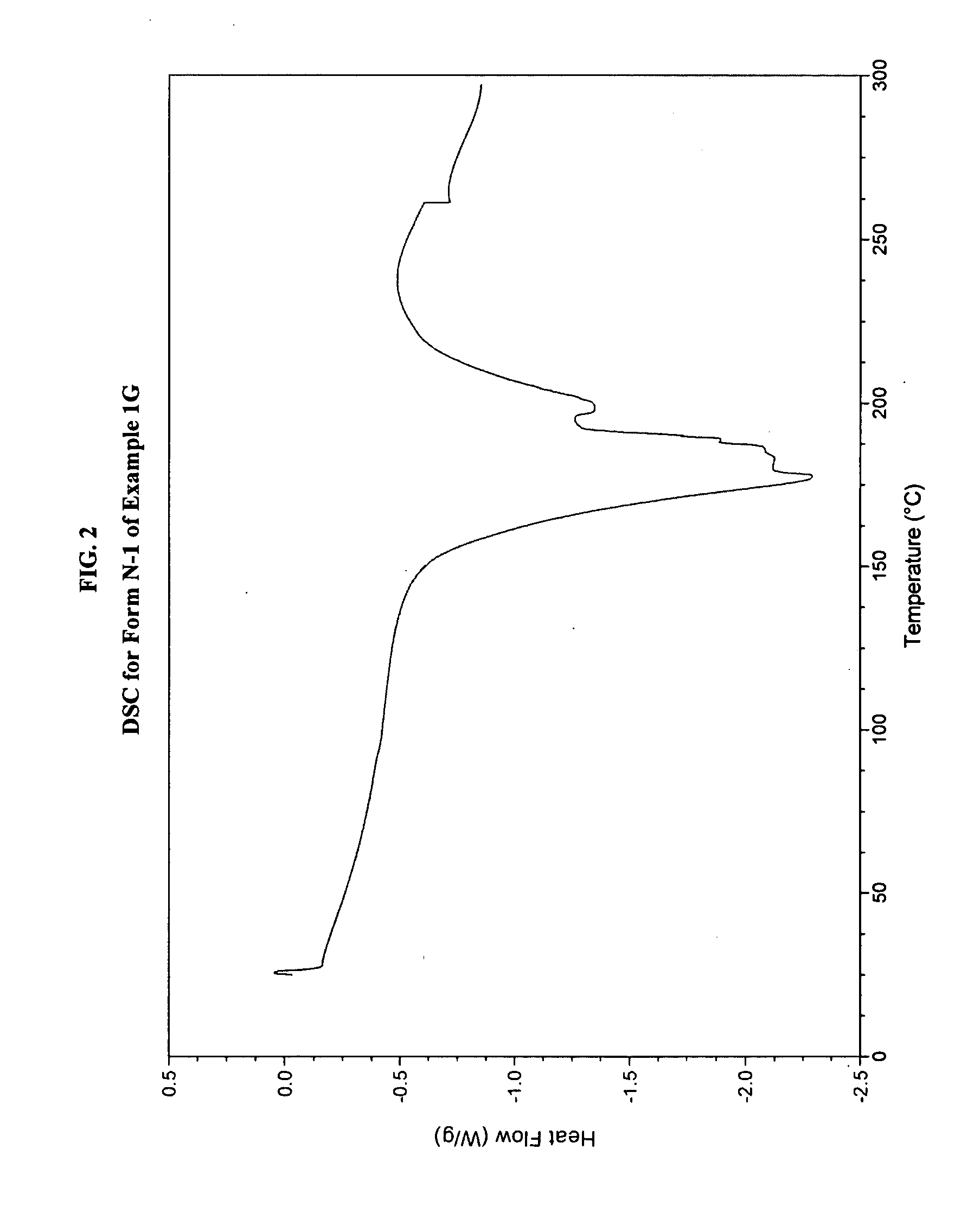
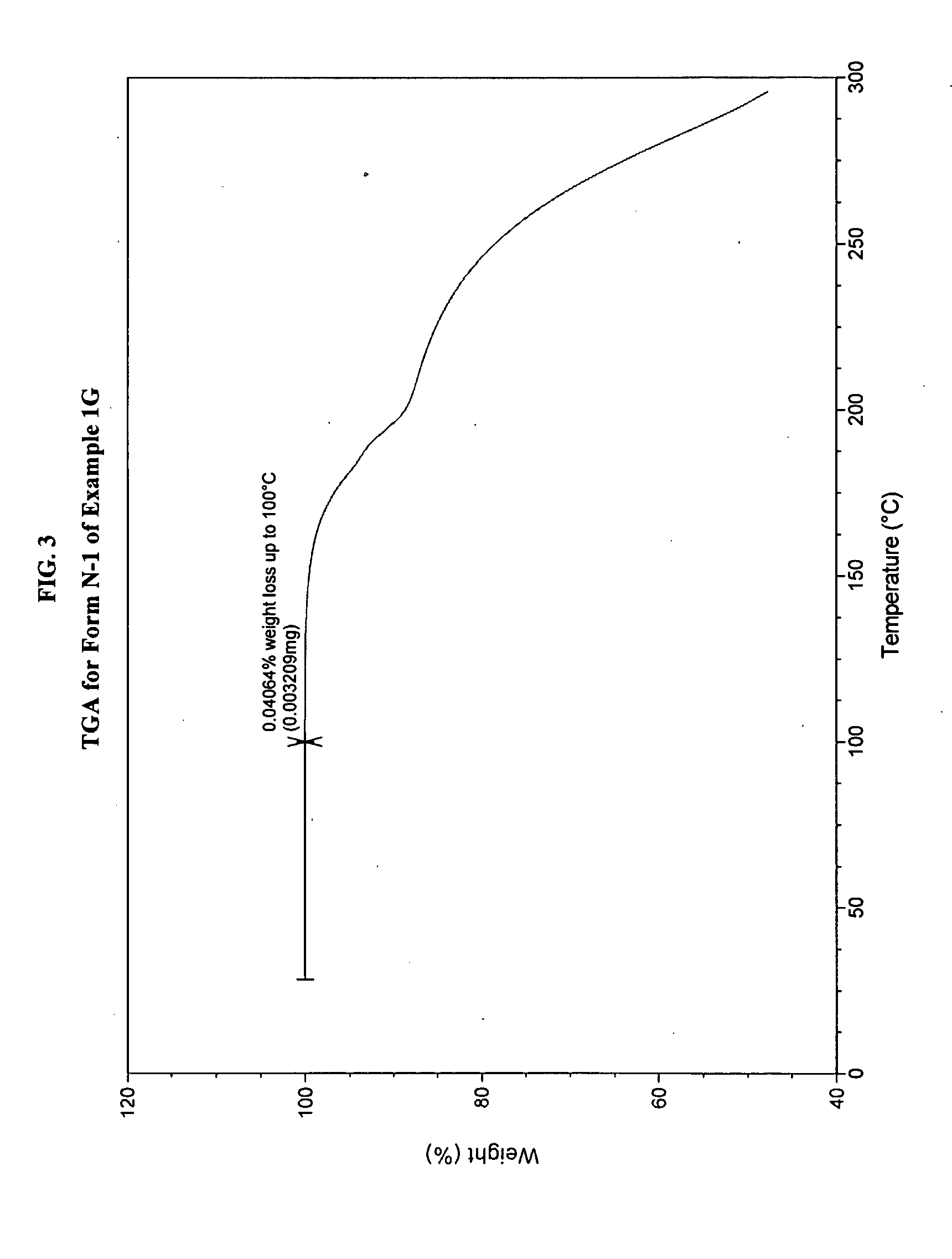
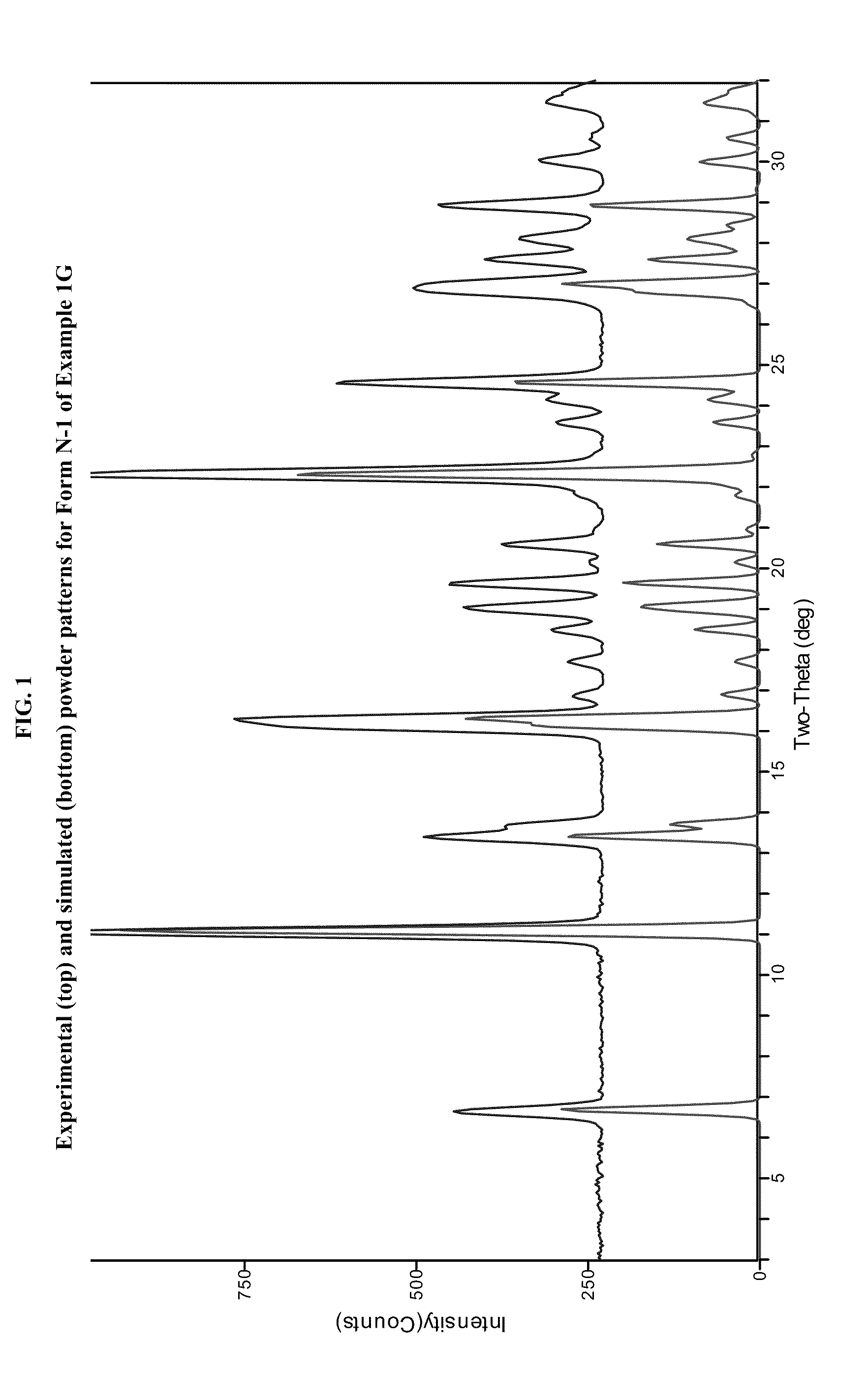
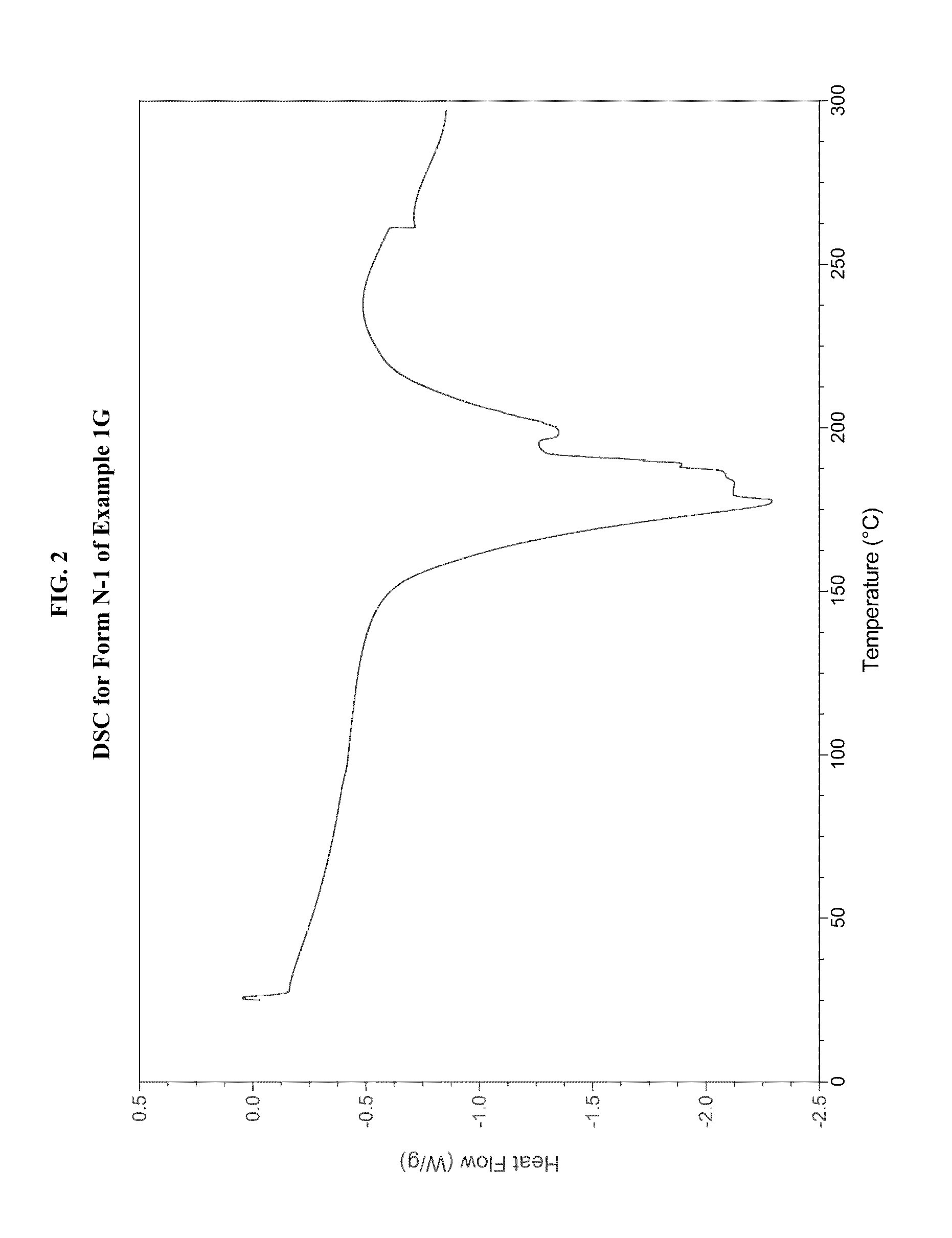
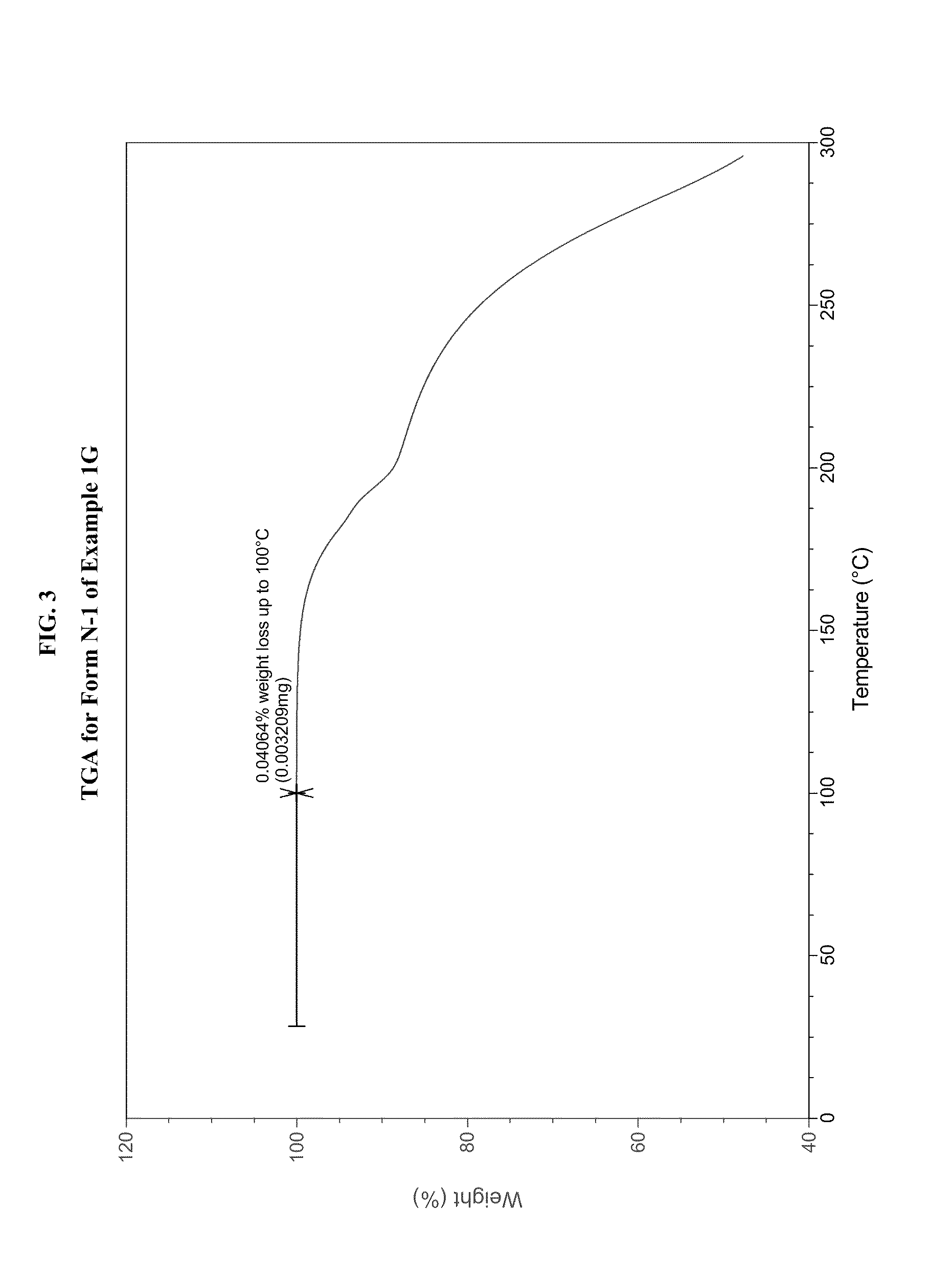
JAK inhibitor crystal forms, preparation methods and applications thereof
InactiveCN105061420AGood physical and chemical propertiesImprove stabilityOrganic active ingredientsAntipyreticPharmaceutical formulationChemical property
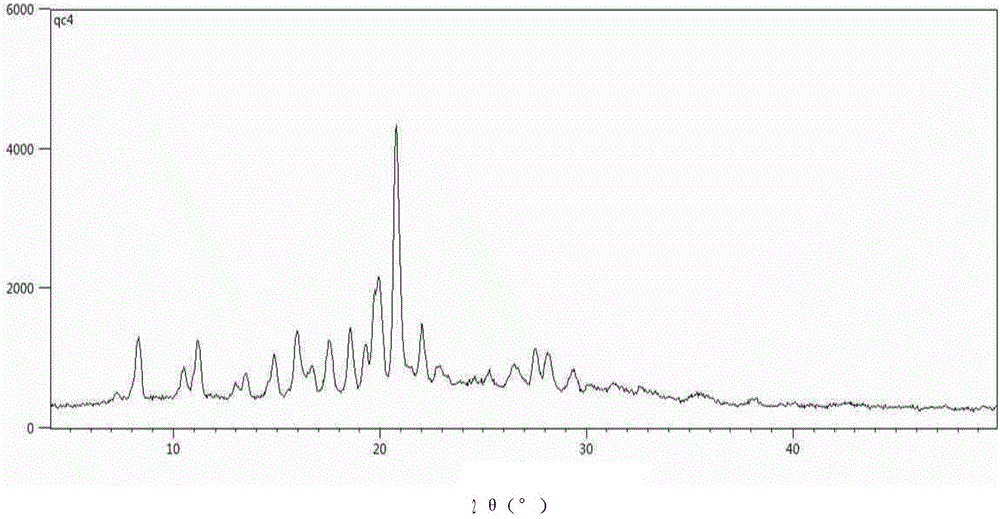
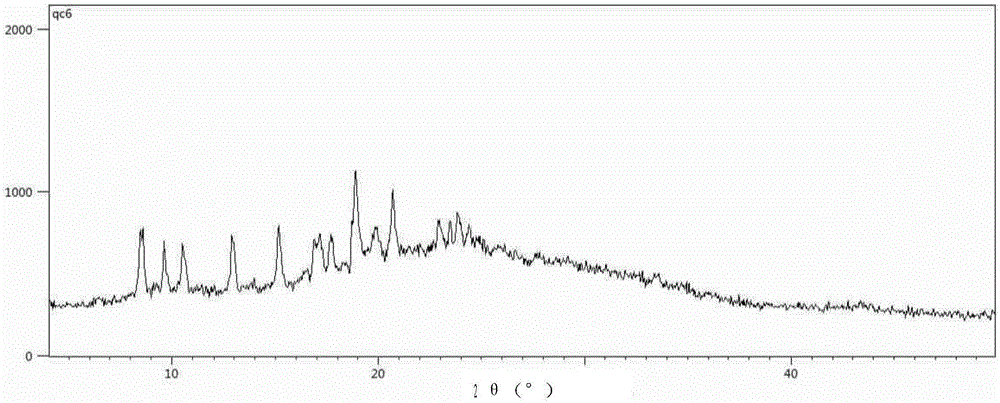
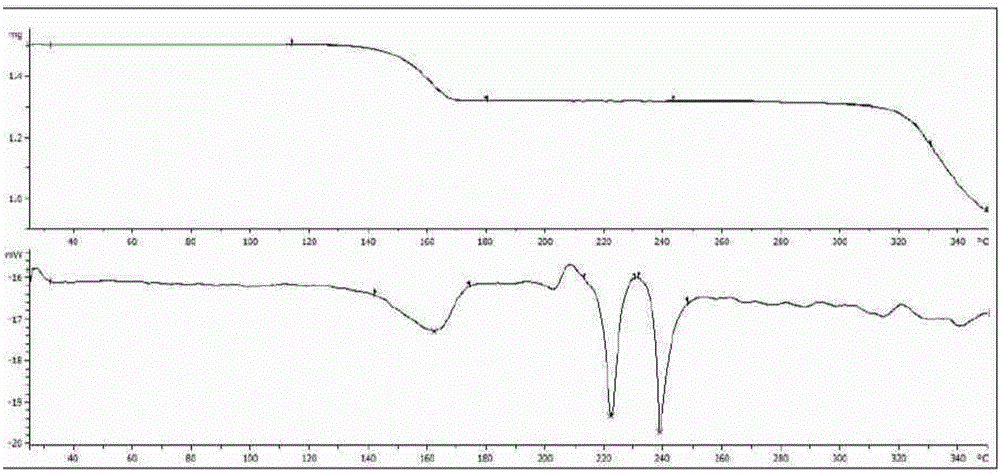
The present invention discloses four crystal forms of a JAK inhibitor N-(5-(4-(1,1-dioxothiomorpholinyl)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide, and methods for preparing the four crystal forms, wherein the four crystal forms respectively are a crystal form H1, a crystal form H2, a crystal form H3 and a crystal form H4, the crystal form H1 has the characteristic absorption peaks when the diffraction angle 2[theta] is 8.3 DEG, 11.2 DEG, 16.0 DEG, 17.5 DEG, 18.5 DEG, 19.3 DEG, 19.7 DEG, 20.0 DEG, 20.7 DEG, 22.0 DEG and the like, the crystal form H2 has the characteristic absorption peaks when the diffraction angle 2[theta] is 9.3 DEG, 12.8 DEG, 14.0 DEG, 16.4 DEG, 18.7 DEG, 20.5 DEG, 23.5 DEG, 29.4 DEG, 33.1 DEG, 33.4 DEG and the like, the crystal form H3 has the characteristic absorption peaks when the diffraction angle 2[theta] is 9.6 DEG, 9.8 DEG, 10.7 DEG, 15.1 DEG, 15.3 DEG, 16.8 DEG, 16.9 DEG, 19.8 DEG, 20.0 DEG, 24.9 DEG and the like, and the crystal form H1 has the characteristic absorption peaks when the diffraction angle 2[theta] is 8.6 DEG C, 9.6 DEG, 10.5 DEG, 12.9 DEG, 15.1 DEG, 17.2 DEG, 18.9 DEG, 19.9 DEG, 20.7 DEG, 23.8 DEG and the like. According to the present invention, the four crystal forms have advantages of excellent physical and chemical properties, good stability, simple preparation operation and the like, are suitable for pharmaceutical formulation applications.
Owner:CHARM PHARMATECH NANJING
Triazolopyridine derivatives and their therapeutic use
Compounds of formula (I) are inhibitors of p38 MAP kinase, useful as anti-inflammatory agents in the treatment of inter alia, diseases of the respiratory tract wherein; R1 is C1-C6 alkyl, C3-C6 cycloalkyl, phenyl which is optionally substituted, 5- or 6 membered monocyclic heteroaryl which is optionally substituted, or a radical of formula (II) wherein n is 1 or 2, and R3 and R4 are independently H or C1-C6 alkyl, or R3 and R4 taken together with the nitrogen to which they are attached form a 6-membered heterocyclic ring optionally containing a further heteroatom selected from N and O; Y is —O— or —S(O)p— wherein p is 0, 1 or 2; A is an optionally substituted divalent arylene radical, or a mono- or bicyclic heteroarylene radical, or a C3-C6 divalent cycloalkylene radical having 5 or 6 ring atoms, or a piperidinylene radical wherein the ring nitrogen is linked to R2NHC(═O)W—; W is a bond, —NH— or —C(RA)(RB), wherein RA and RB are independently H, methyl, ethyl, amino, hydroxyl or halo; and R2 is a radical as defined in the claims.
Owner:CHIESI FARM SPA
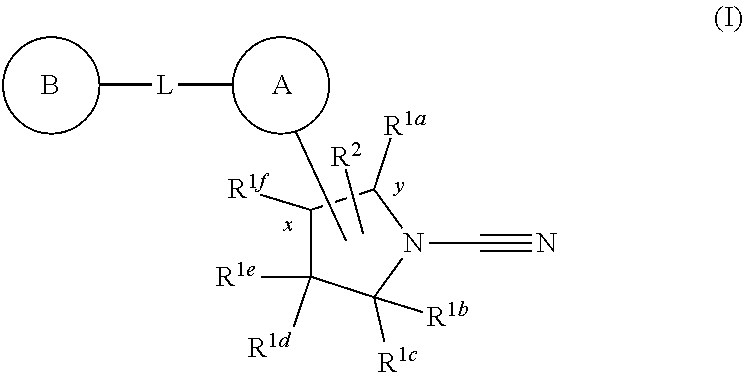
1-cyano-pyrrolidine derivatives as dub inhibitors
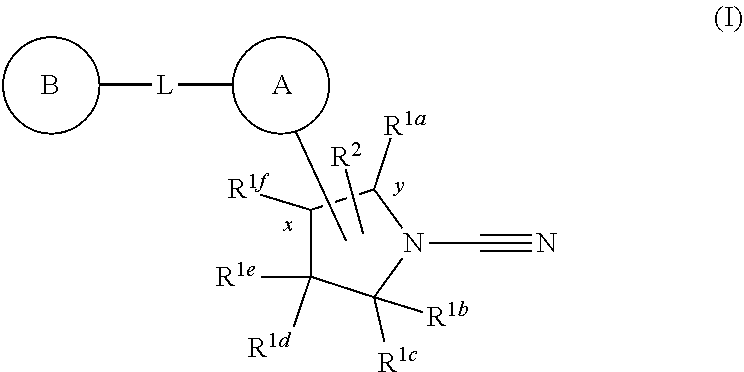
The present invention relates to novel compounds and methods for the manufacture of inhibitors of deubiquitylating enzymes (DUBs). In particular, the invention relates to the inhibition of ubiquitin C-terminal hydrolase 30 or ubiquitin specific peptidase 30 (USP30). The novel compounds have formula (I): (Formula (I)) or are pharmaceutically acceptable salts thereof, wherein: R1a, R1b, R1c, R1d, R1e and R1f each independently represent hydrogen, optionally substituted C1-C6 alkyl or optionally substituted C3-C4 cycloalkyl, or R1b and R1c together form an optionally substituted C3-C6 cycloalkyl ring, or R1d and R1e together form an optionally substituted C3-C6 cycloalkyl ring; R2 represents hydrogen or optionally substituted C1-C6 alkyl; A represents an optionally further substituted 5 to 10 membered monocyclic or bicyclic heteroaryl, heterocyclyl or aryl ring; L represents a covalent bond or linker; B represents an optionally substituted 3 to 10 membered monocyclic or bicyclic heterocyclyl, heteroaryl, cycloalkyl or aryl ring; and when -A-L-B is at position x attachment to A is via a carbon ring atom of A, and either: A cannot be triazolopyridazinyl, triazolopyridinyl, imidazotriazinyl, imidazopyrazinyl or pyrrolopyrimidinyl; or B cannot be substituted with phenoxyl; or B cannot be cyclopentyl when L is an oxygen atom.
Owner:MISSION THERAPEUTICS
Triazolopyridine 11-beta hydroxysteroid dehydrogenase type i inhibitors
Novel compounds are provided which are 11-beta-hydroxysteroid dehydrogenase type I inhibitors. 11-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 11-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds of formula I:or stereoisomers or pharmaceutically acceptable salts thereof, wherein G, Q, X, Y, R3, R3a, and R3b are defined herein.
Owner:BRISTOL MYERS SQUIBB CO
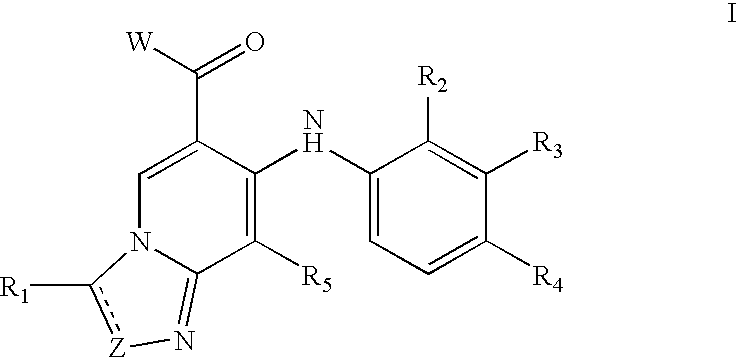
Triazolopyridine compounds
ActiveUS20120142665A1Avoid problemsAnti-inflammatory propertyBiocideNervous disorderPyridineTriazolopyridine
The invention is concerned with triazolopyridine compounds of formula (I)wherein R1, R2, R3 and R4 are as defined in the description and in the claims, as well as physiologically acceptable salts thereof. These compounds inhibit PDE10A and can be used as pharmaceuticals.
Owner:F HOFFMANN LA ROCHE INC
Triazolopyridine derivatives and their therapeutic use
Compounds of formula (I) are inhibitors of p38 MAP kinase, useful as anti-inflammatory agents in the treatment of inter alia, diseases of the respiratory tract wherein; R1 is C1-C6 alkyl, C3-C6 cycloalkyl, phenyl which is optionally substituted, 5- or 6 membered monocyclic heteroaryl which is optionally substituted, or a radical of formula (II) wherein n is 1 or 2, and R3 and R4 are independently H or C1-C6 alkyl, or R3 and R4 taken together with the nitrogen to which they are attached form a 6-membered heterocyclic ring optionally containing a further heteroatom selected from N and O; Y is —O— or —S(O)p— wherein p is 0, 1 or 2; A is an optionally substituted divalent arylene radical, or a mono- or bicyclic heteroarylene radical, or a C3-C6 divalent cycloalkylene radical having 5 or 6 ring atoms, or a piperidinylene radical wherein the ring nitrogen is linked to R2NHC(═O)W—; W is a bond, —NH— or —C(RA)(RB), wherein RA and RB are independently H, methyl, ethyl, amino, hydroxyl or halo; and R2 is a radical as defined in the claims.
Owner:CHIESI FARM SPA
Novel Triazolopyridine Compounds
InactiveUS20090209577A1Inhibiting kinase activityInhibiting pBiocideSenses disorderKinase activityCyclo oxygenase 2
This invention is directed generally to triazolopyridine compounds that generally inhibit p38 kinase, TNF, and / or cyclooxygenase activity. Such triazolopyridine include compounds generally corresponding in structure to the following formula:wherein R1, R2 and R3, are as defined in this specification. This invention also is directed to compositions of such triazolopyridines (particularly pharmaceutical compositions), intermediates for the syntheses of such triazolopyridines, methods for making such triazolopyridines, and methods for treating (including preventing) conditions (typically pathological conditions) associated with p38 kinase activity, TNF activity, and / or cyclooxygenase-2 activity.
Owner:PFIZER INC
Novel compounds useful for the treatment of degenerative and inflammatory diseases
ActiveUS20110190260A1Prevent and treat any maladyModify activityBiocideOrganic chemistryArthritisOsteocyte
[1,2,4]Triazolo[1,5-a]pyridine compounds are disclosed that have a formula represented by the formula (I). The compounds may be prepared as pharmaceutical compositions, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example, diseases involving cartilage degradation, bone and / or joint degradation, for example osteoarthritis; and / or conditions involving inflammation or immune responses, such as Crohn's disease, rheumatoid arthritis, psoriasis, allergic airways disease (e.g. asthma, rhinitis), juvenile idiopathic arthritis, colitis, inflammatory bowel diseases, endotoxin-driven disease states (e.g. complications after bypass surgery or chronic endotoxin states contributing to e.g. chronic cardiac failure), diseases involving impairment of cartilage turnover (e.g diseases involving the anabolic stimulation of chondrocytes), congenital cartilage malformations, diseases associated with hypersecretion of IL6, transplantation rejection (e.g. organ transplant rejection) and proliferative diseases.
Owner:GALAPAGOS NV
Triazolopyridine compounds
The invention is concerned with triazolopyridine compounds of formula (I)wherein R1, R2, R3 and R4 are as defined in the description and in the claims, as well as physiologically acceptable salts thereof. These compounds inhibit PDE10A and can be used as pharmaceuticals.
Owner:F HOFFMANN LA ROCHE & CO AG
P2X7 receptor imaging agent and preparation method thereof
InactiveCN109400605AImprove bindingThe synthesis process is simple and fastIsotope introduction to heterocyclic compoundsRadioactive preparation carriersMedicineImaging agent
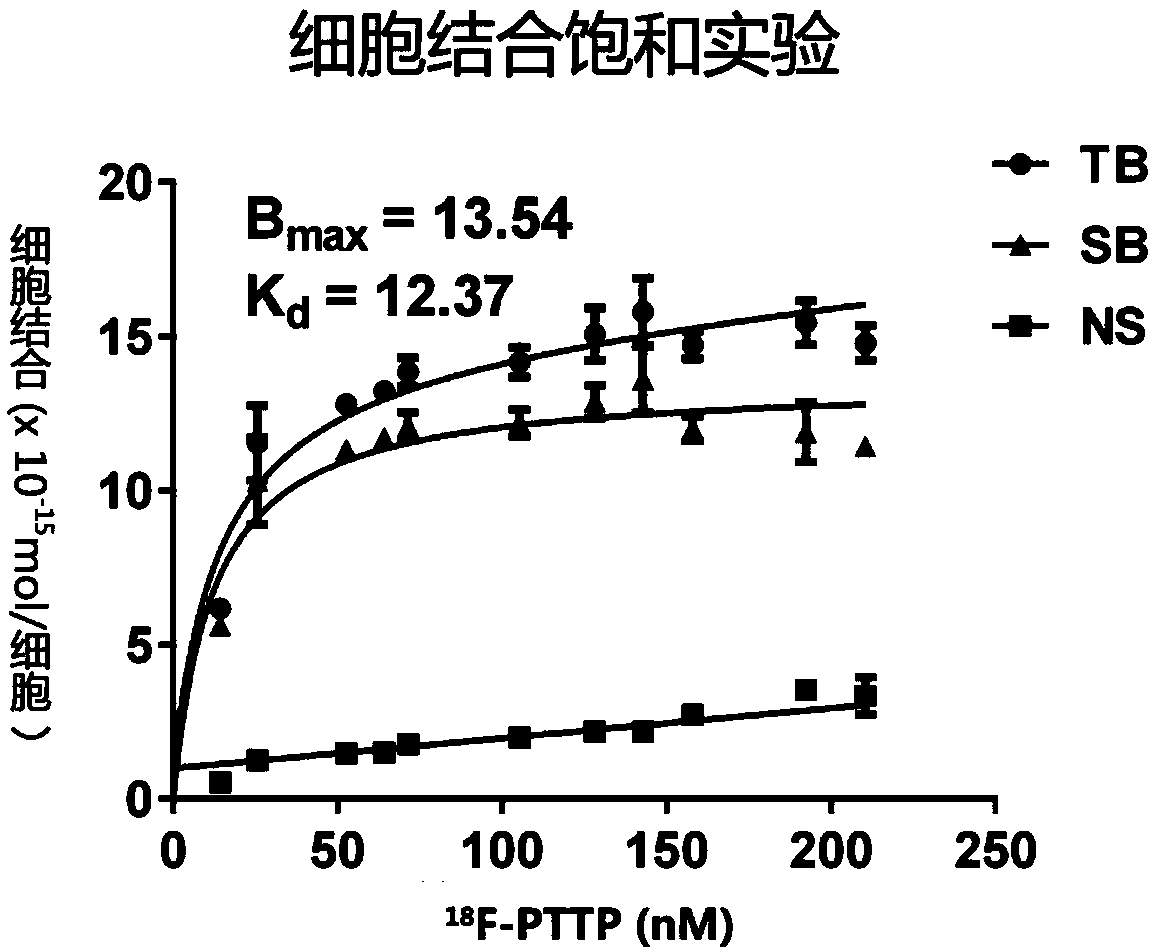
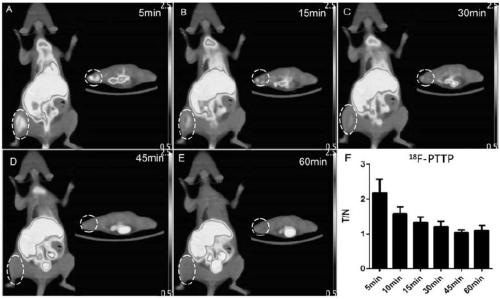
The invention discloses a F-18 tagged P2X7 receptor imaging agent which is a compound 18F-{(2-chloro-3-trifluorophenyl)(1-(pyrimidin-2-yl)-6,7-dihydro-1H-[1,2,3]triazolo[4,5-c]pyridin-5(4H)-yl)methanone shown as formula I. The P2X7 receptor imaging agent is suitable for identifying and diagnosing pulmonary inflammation and tumors.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
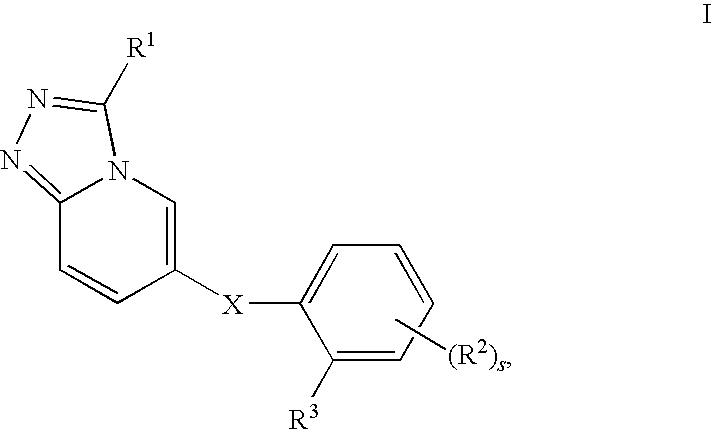
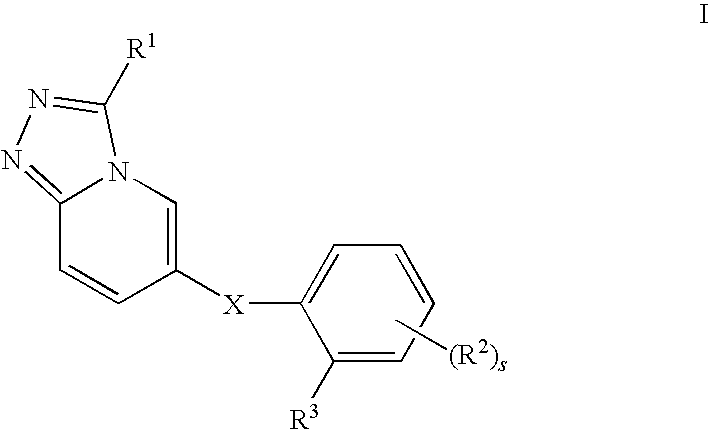
1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors
InactiveUS8946205B2Treatment and prevention and control and amelioration and reduction of risk of diseaseReduce disease riskBiocideNervous disorderAllosteric modulatorMetabotropic glutamate receptor
The present invention relates to novel triazolo[4,3-a]pyridine derivatives of Formula (I) wherein all radicals are as defined in the claims. The compounds according to the invention are positive allosteric modulators of the metabotropic glutamate receptor subtype 2 (“mGluR2”), which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. The invention is also directed to pharmaceutical compositions comprising such compounds, to processes to prepare such compounds and compositions, and to the use of such compounds for the prevention or treatment of neurological and psychiatric disorders and diseases in which mGluR2 is involved.
Owner:JANSSEN PHARMA INC +1
Organic electroluminescent material and organic electroluminescent device based on triazolopyridine
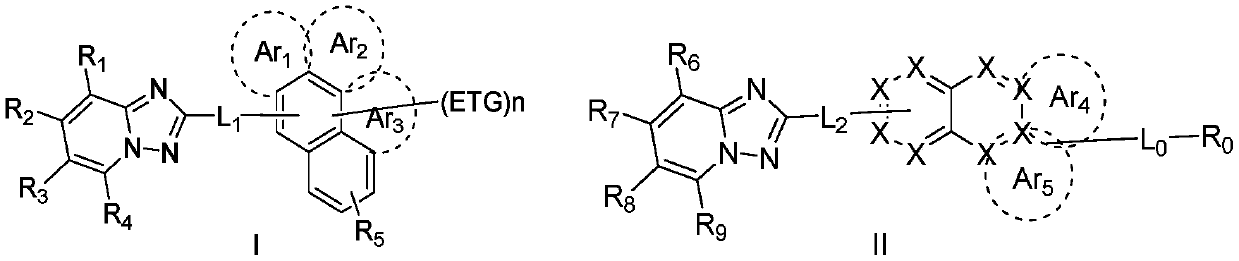
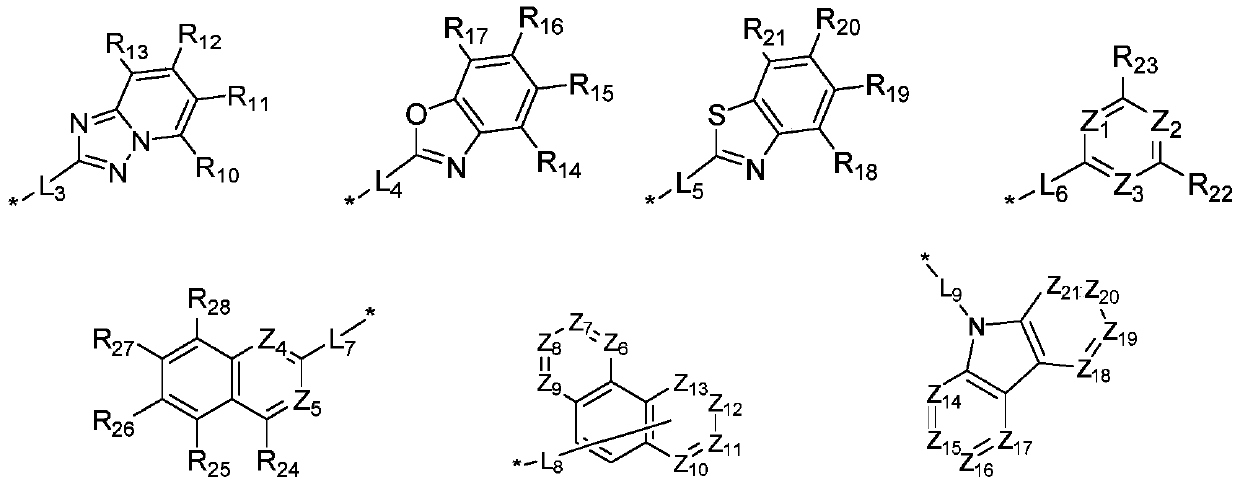
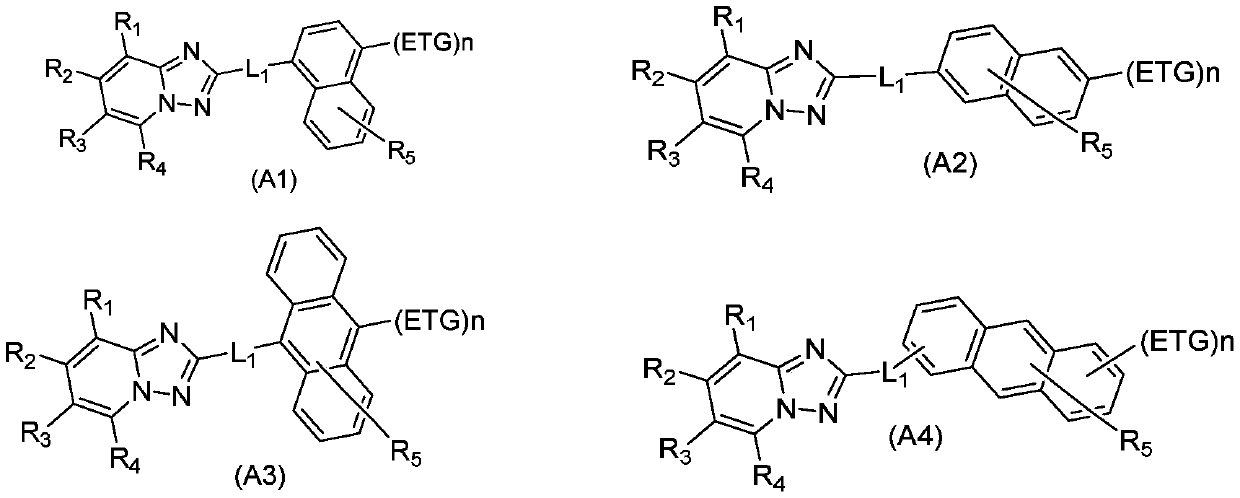
ActiveCN110452689ALower triplet energy levelAvoid efficiency roll-offOrganic chemistrySolid-state devicesHost materialGreen-light
The invention relates to the field of the photoelectric material applied technology, and discloses an organic electroluminescent material and an organic electroluminescent device based on triazolopyridine. The organic electroluminescent material finely regulates and controls triazolopyridyl groups through specific functional groups. The invention provides an electron transport material and a light-emitting host material with excellent comprehensive performance. The technical problems that an electron transport material is not matched with the hole transport rate, and the stability is poor in the prior art are solved, the technical problems of roll-off of the efficiency of a green light host material and impure light color are also solved effectively, thus, the comprehensive performance ofthe device in the aspects of driving voltage, efficiency, light color, thermal stability, service life and the like is improved, and industrialization development of optoelectronic materials is speeded up.
Owner:湖北尚赛光电材料有限公司
Triazolopyridine JAK inhibitor compounds and methods
A compound of Formula I, enantiomers, diasteriomers, tautomers or pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4 and R5 are defined herein, are useful as JAK kinase inhibitors. A pharmaceutical composition that includes a compound of Formula I and a pharmaceutically acceptable carrier, adjuvant or vehicle, and methods of treating or lessening the severity of a disease or condition responsive to the inhibition of JAK kinase activity in a patient are disclosed.
Owner:GENENTECH INC
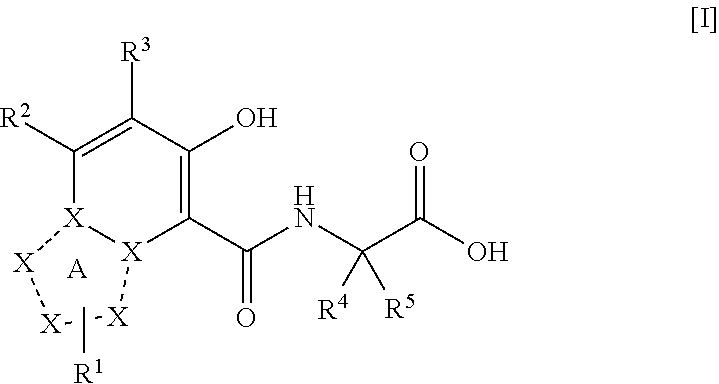
Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor or erythropoietin production-inducing agent
ActiveUS8283465B2Organic active ingredientsGroup 5/15 element organic compoundsMedicineTriazolopyridine
The present invention provides a triazolopyridine compound having a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability. The present invention relates to a compound represented by the following formula [I]:wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, as well as a prolyl hydroxylase inhibitor or erythropoietin production-inducing agent containing the compound. The compound of the present invention shows a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability and is useful as a prophylactic or therapeutic agent for various diseases and pathologies (disorders) caused by decreased production of erythropoietin.
Owner:JAPAN TOBACCO INC
Triazolopyridine jak inhibitor compounds and methods
A compound of Formula I, enantiomers, diasteriomers, tautomers or pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4 and R5 are defined herein, are useful as JAK kinase inhibitors. A pharmaceutical composition that includes a compound of Formula I and a pharmaceutically acceptable carrier, adjuvant or vehicle, and methods of treating or lessening the severity of a disease or condition responsive to the inhibition of JAK kinase activity in a patient are disclosed.
Owner:GENENTECH INC
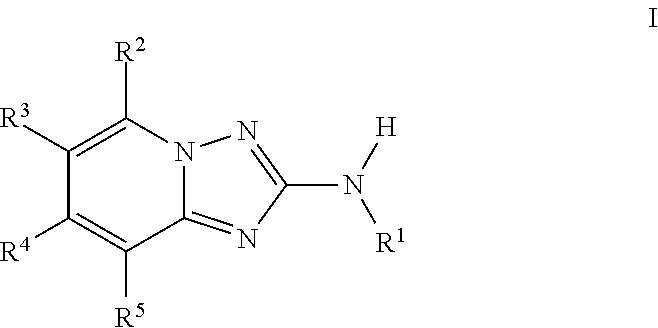
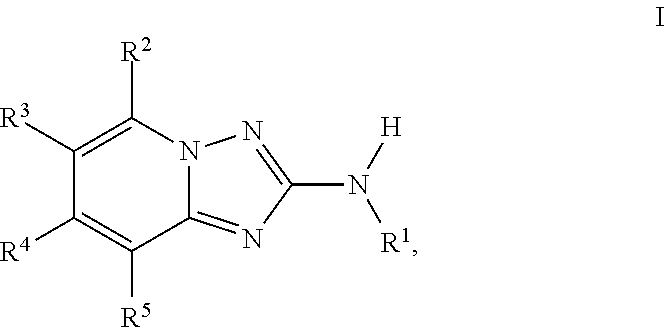
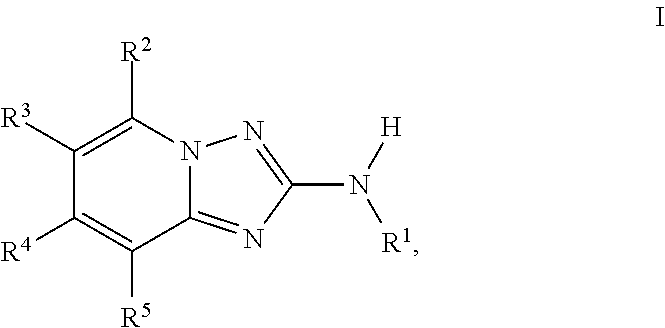
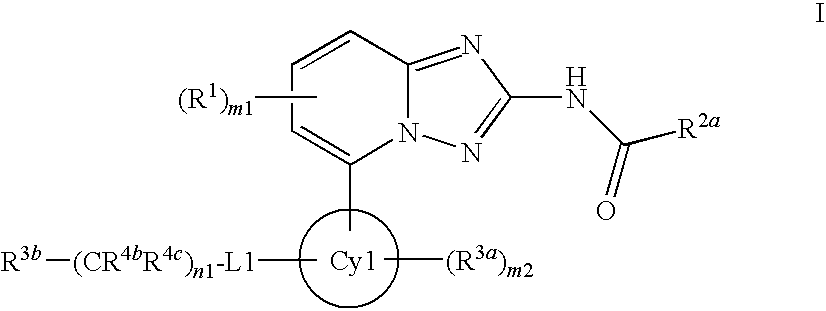
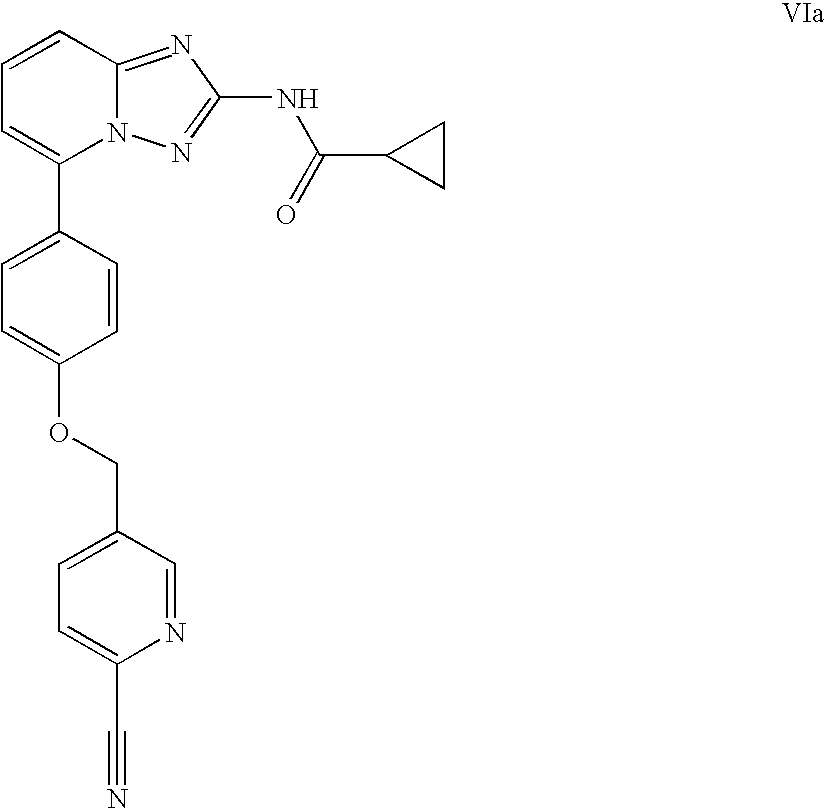
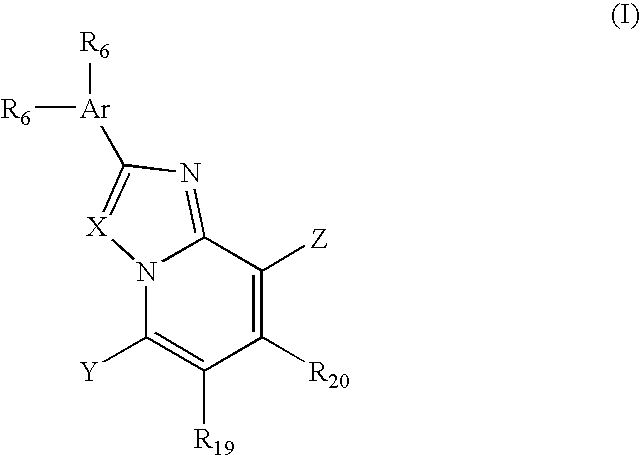
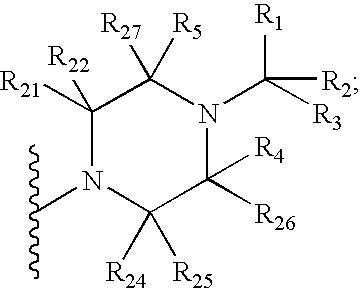
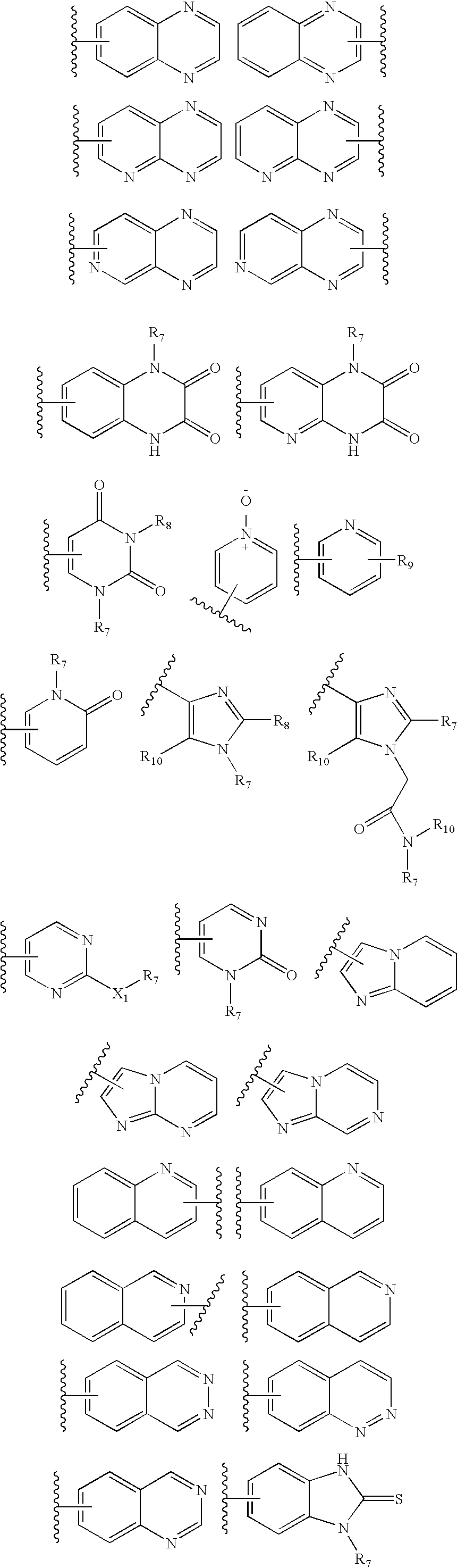
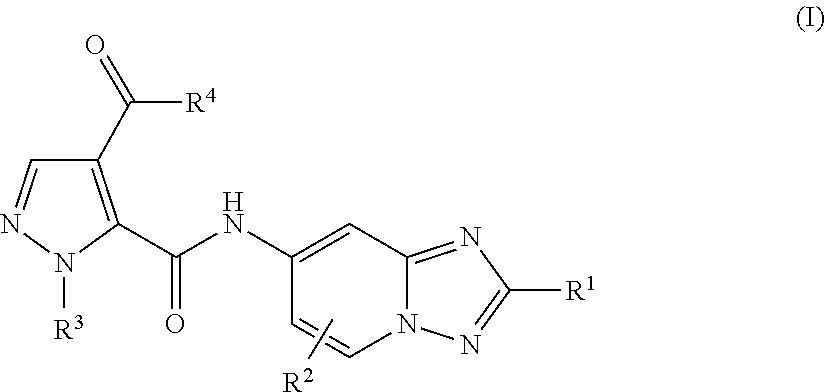
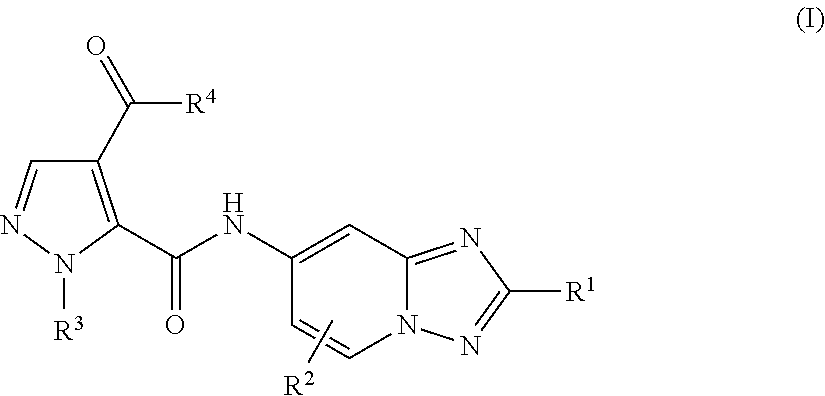
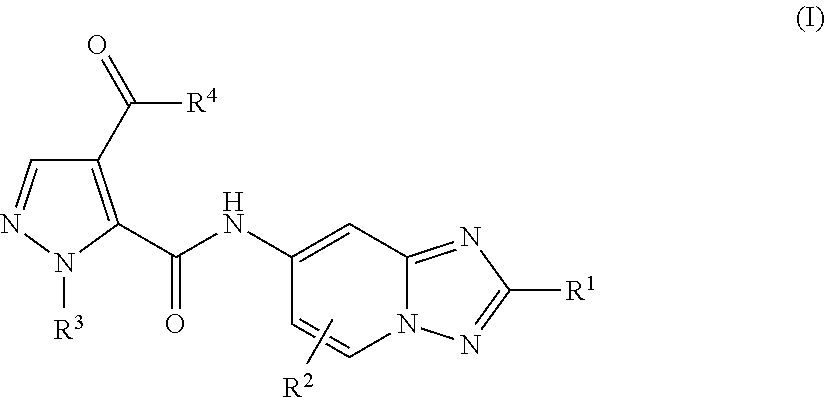
Amino-triazolopyridine Compounds and Their Use in Treating Cancer
The specification generally relates to compounds of Formula (I):and pharmaceutically acceptable salts thereof, where R1 and R2 have any of the meanings defined herein. The specification also relates to the use of such compounds and salts thereof to treat or prevent DNA-PK mediated disease, including cancer. The specification further relates to pharmaceutical compositions comprising such compounds and salts; kits comprising such compounds and salts; methods of manufacture of such compounds and salts; intermediates useful in the manufacture of such compounds and salts; and to methods of treating DNA-PK mediated disease, including cancer, using such compounds and salts.
Owner:ASTRAZENCA UK LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders 1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders](https://images-eureka.patsnap.com/patent_img/d7c7b245-5bc3-42e7-80c0-7e46aa191791/US08937060-20150120-C00001.PNG)
![1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders 1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders](https://images-eureka.patsnap.com/patent_img/d7c7b245-5bc3-42e7-80c0-7e46aa191791/US08937060-20150120-C00002.PNG)
![1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders 1,2,4-triazolo [4,3-A] pyridine derivatives and their use for the treatment of prevention of neurological and psychiatric disorders](https://images-eureka.patsnap.com/patent_img/d7c7b245-5bc3-42e7-80c0-7e46aa191791/US08937060-20150120-C00003.PNG)
![[1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof [1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof](https://images-eureka.patsnap.com/patent_img/6068898e-27c2-4f5d-bad3-cdedf7dcddf0/FDA0001381371540000011.png)
![[1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof [1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof](https://images-eureka.patsnap.com/patent_img/6068898e-27c2-4f5d-bad3-cdedf7dcddf0/FDA0001381371540000021.png)
![[1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof [1,2,4]triazol[1,5-a]pyridine compound as well as preparation method and medical application thereof](https://images-eureka.patsnap.com/patent_img/6068898e-27c2-4f5d-bad3-cdedf7dcddf0/FDA0001381371540000031.png)
![1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors](https://images-eureka.patsnap.com/patent_img/5dfc5c4d-3f20-4882-a275-337c2bc6f9c8/US09012448-20150421-C00001.PNG)
![1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors](https://images-eureka.patsnap.com/patent_img/5dfc5c4d-3f20-4882-a275-337c2bc6f9c8/US09012448-20150421-C00002.PNG)
![1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of MGLUR2 receptors](https://images-eureka.patsnap.com/patent_img/5dfc5c4d-3f20-4882-a275-337c2bc6f9c8/US09012448-20150421-C00003.PNG)
![1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors](https://images-eureka.patsnap.com/patent_img/e6472739-307f-477a-aed8-8c71d5f447e1/US08946205-20150203-C00001.PNG)
![1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors](https://images-eureka.patsnap.com/patent_img/e6472739-307f-477a-aed8-8c71d5f447e1/US08946205-20150203-C00002.PNG)
![1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors 1,2,4-triazolo[4,3-a]pyridine derivatives and their use as positive allosteric modulators of mGluR2 receptors](https://images-eureka.patsnap.com/patent_img/e6472739-307f-477a-aed8-8c71d5f447e1/US08946205-20150203-C00003.PNG)