Patents
Literature
36 results about "Lutenizing hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
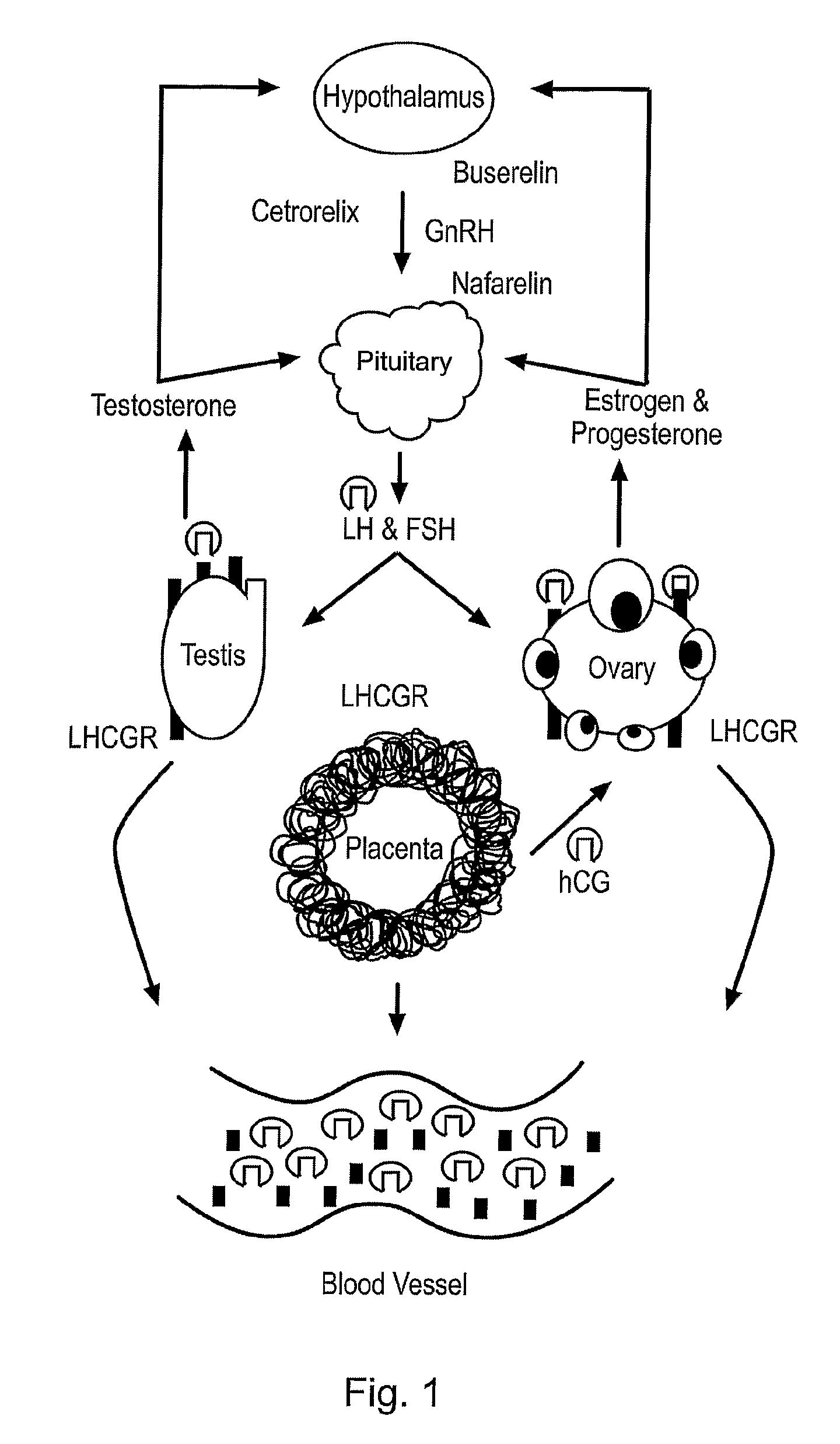
Luteinizing hormone (LH) is a hormone that prompts ovulation, the release of an egg cell from the ovary, in women and the production of the hormone testosterone in men.
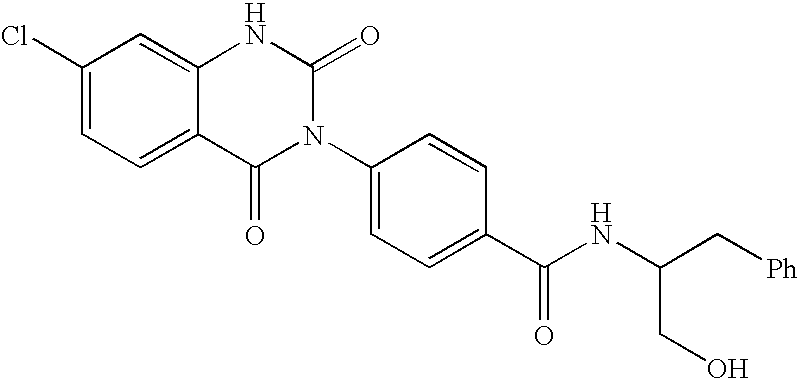
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Fused pyramidine derivative and use thereof
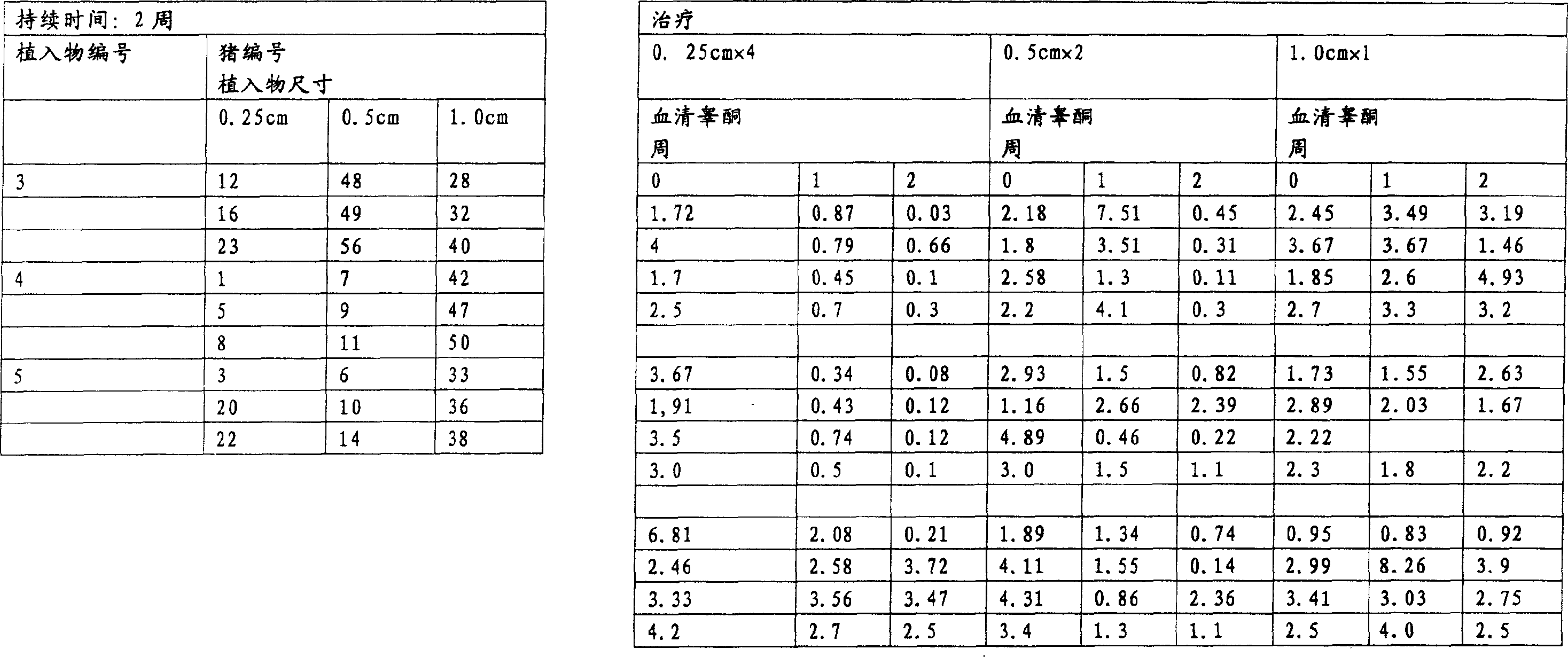
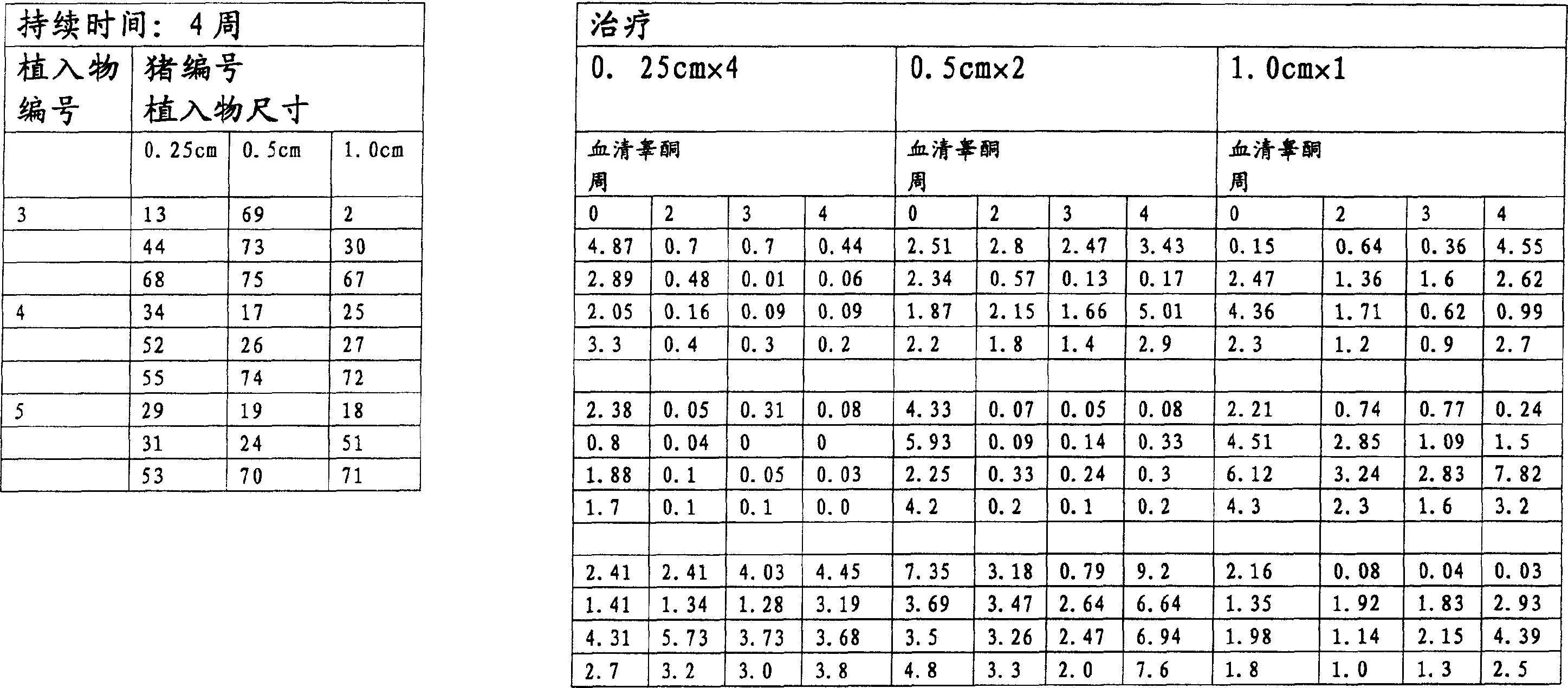
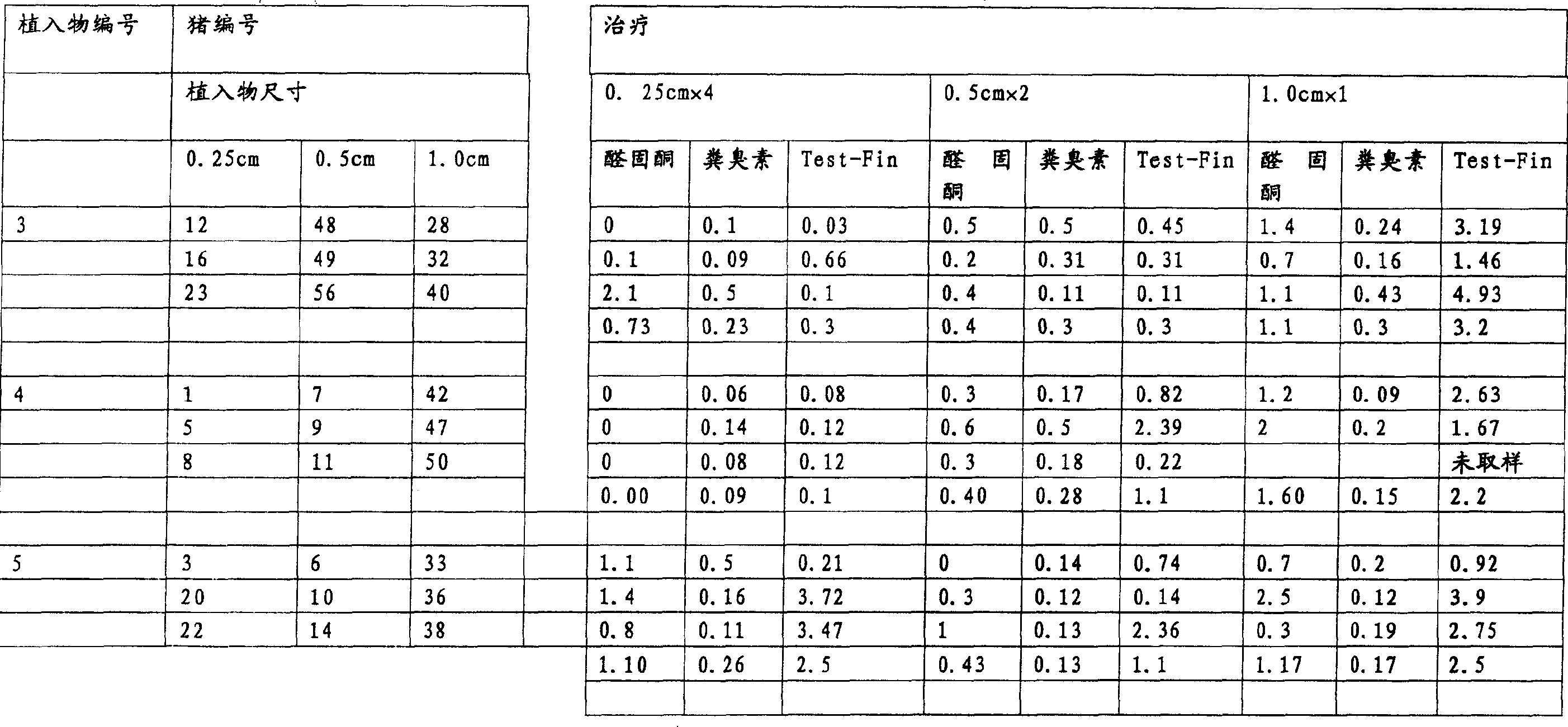
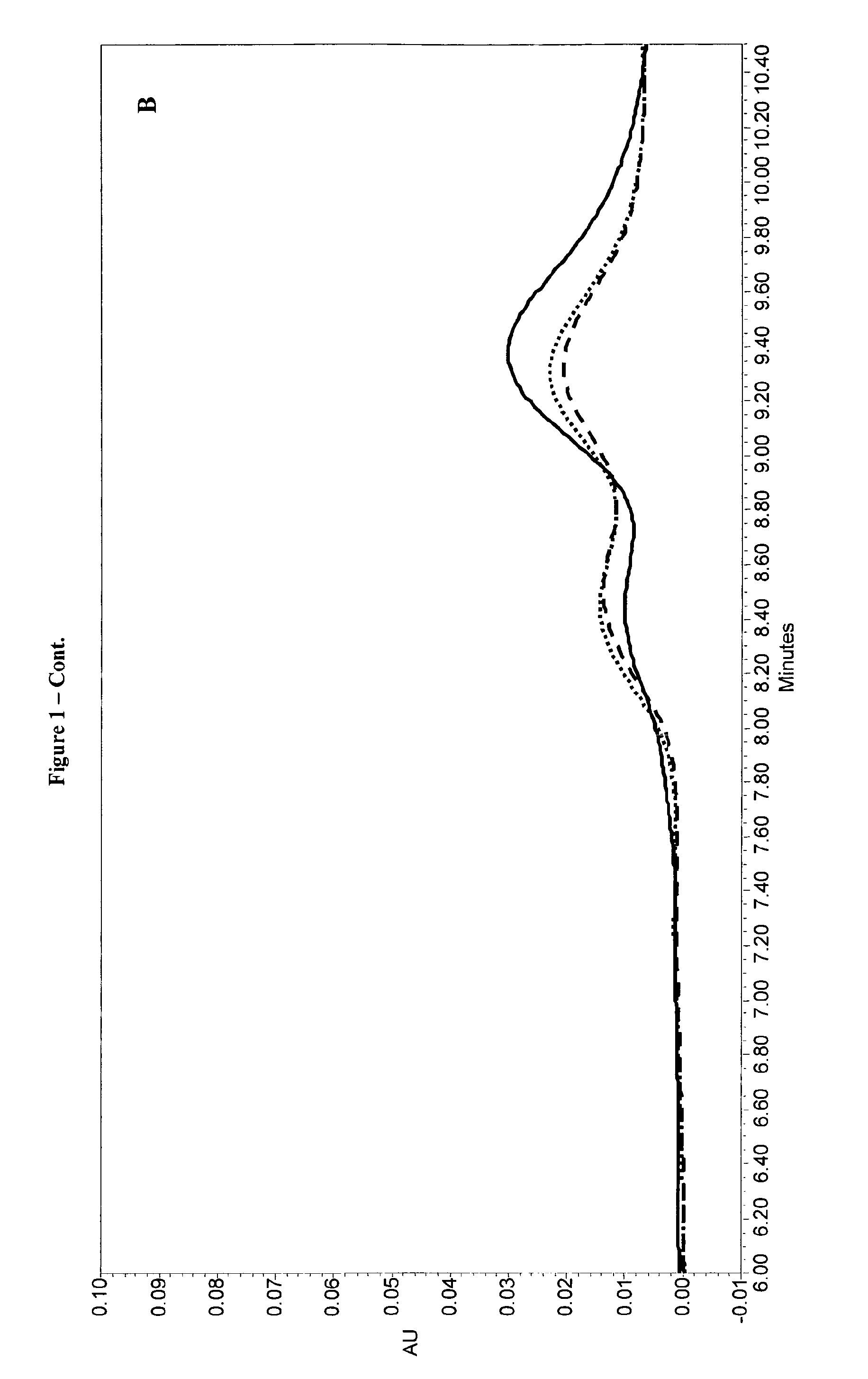
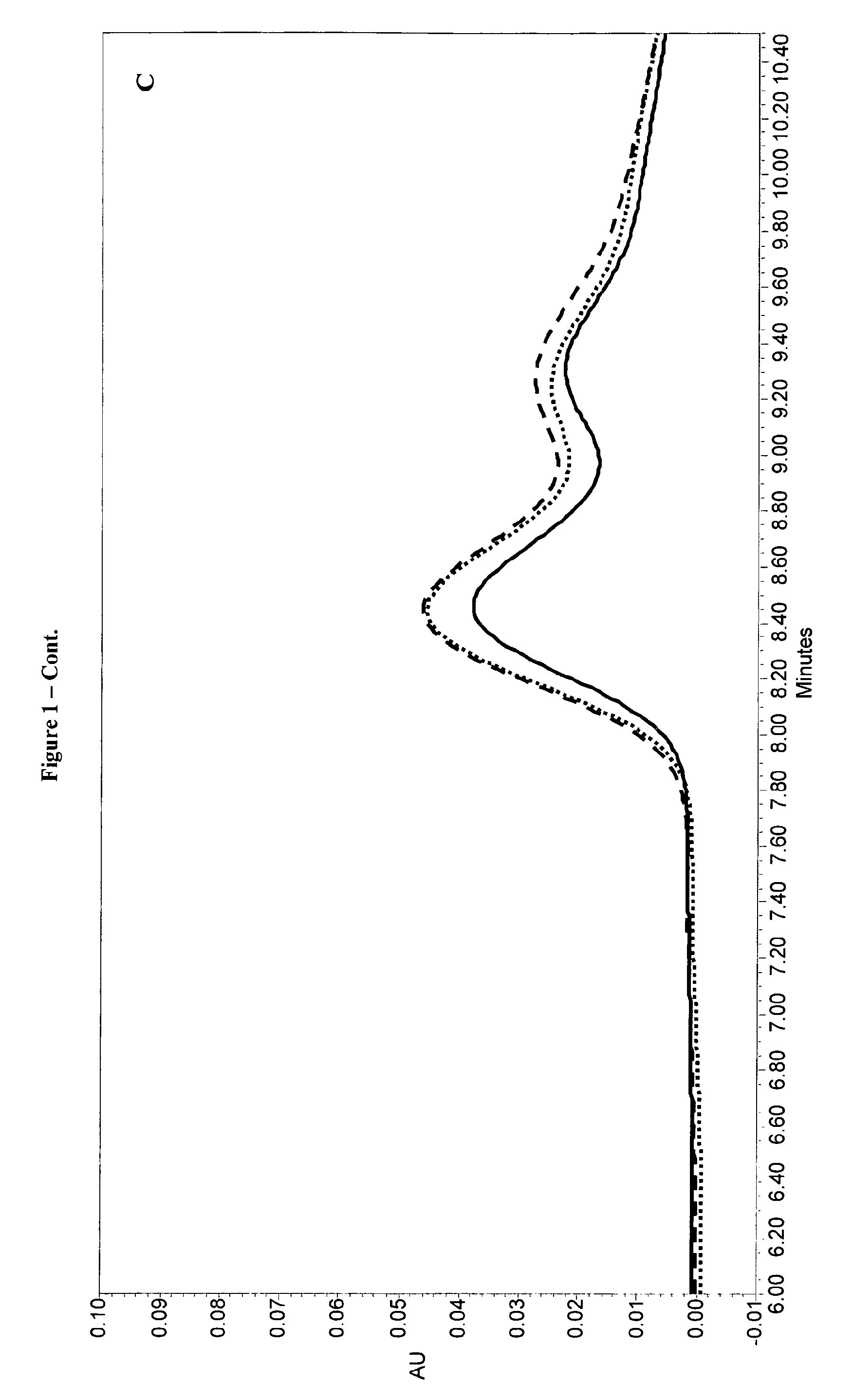
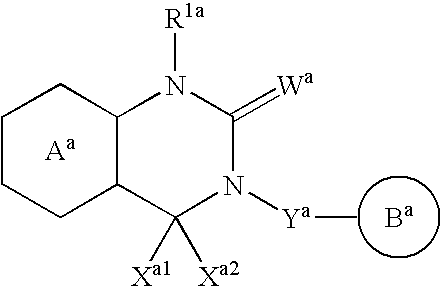
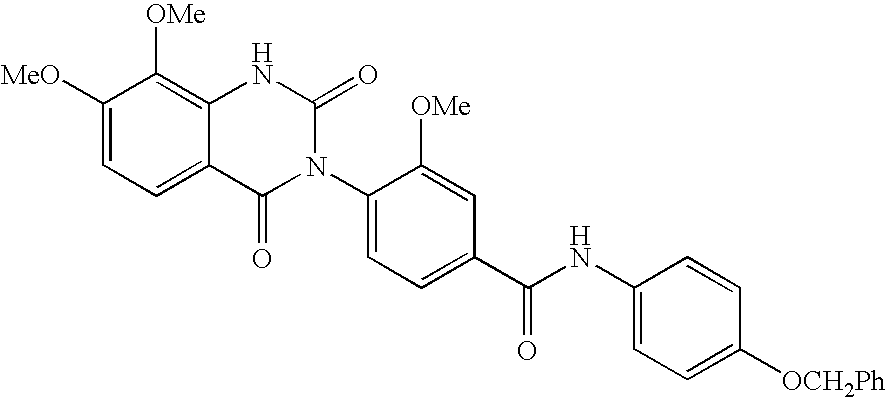
InactiveUS20070010537A1Excellent GnRH antagonizing activityHigh antagonistic activityBiocideOrganic active ingredientsLutenizing hormonePharmaceutical drug
There are provided a fused pyrimidine compound having antagonistic activity against luteinizing hormone releasing hormone, and a medicine containing the compound. A luteinizing hormone releasing hormone antagonist containing a compound represented by the formula: wherein R1a is a hydrocarbon group which may be substituted or a hydrogen atom, ring Aa is a 6-membered aromatic ring which may be further substituted, ring Ba is a homocyclic or heterocyclic ring which may be further substituted, Wa is an oxygen atom or a sulfur atom, Xa1 and Xa2, which may be identical or different, are each a hydrogen atom, a hydrocarbon group which may be substituted, or a heterocyclic group which may be substituted, or Xa1 and Xa2 together may form an oxygen atom, a sulfur atom or NR3a (wherein R3a is a hydrocarbon group which may be substituted or a hydrogen atom), and Ya is C1-6 alkylene which may be substituted or a bond, or a salt or prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
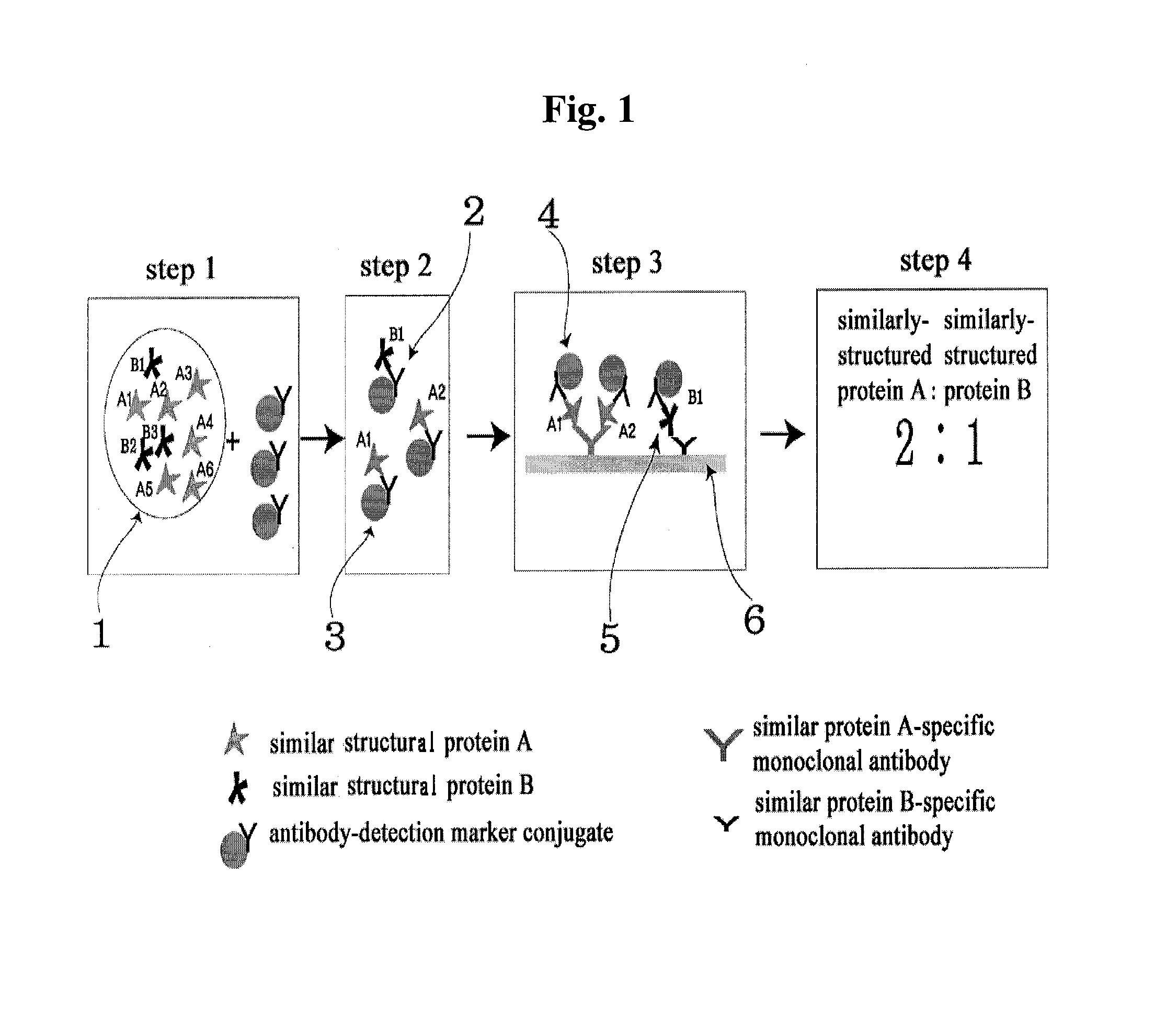
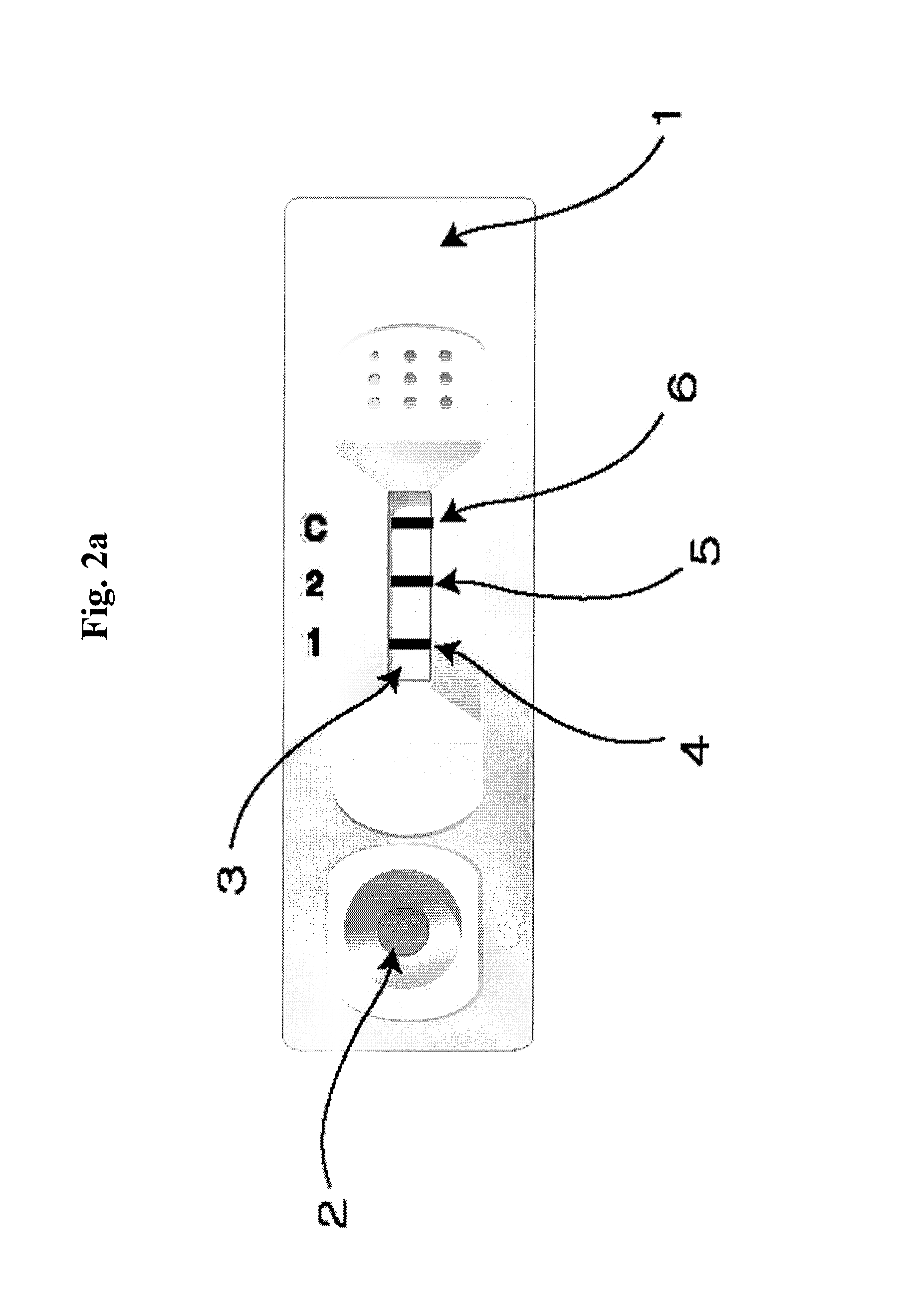
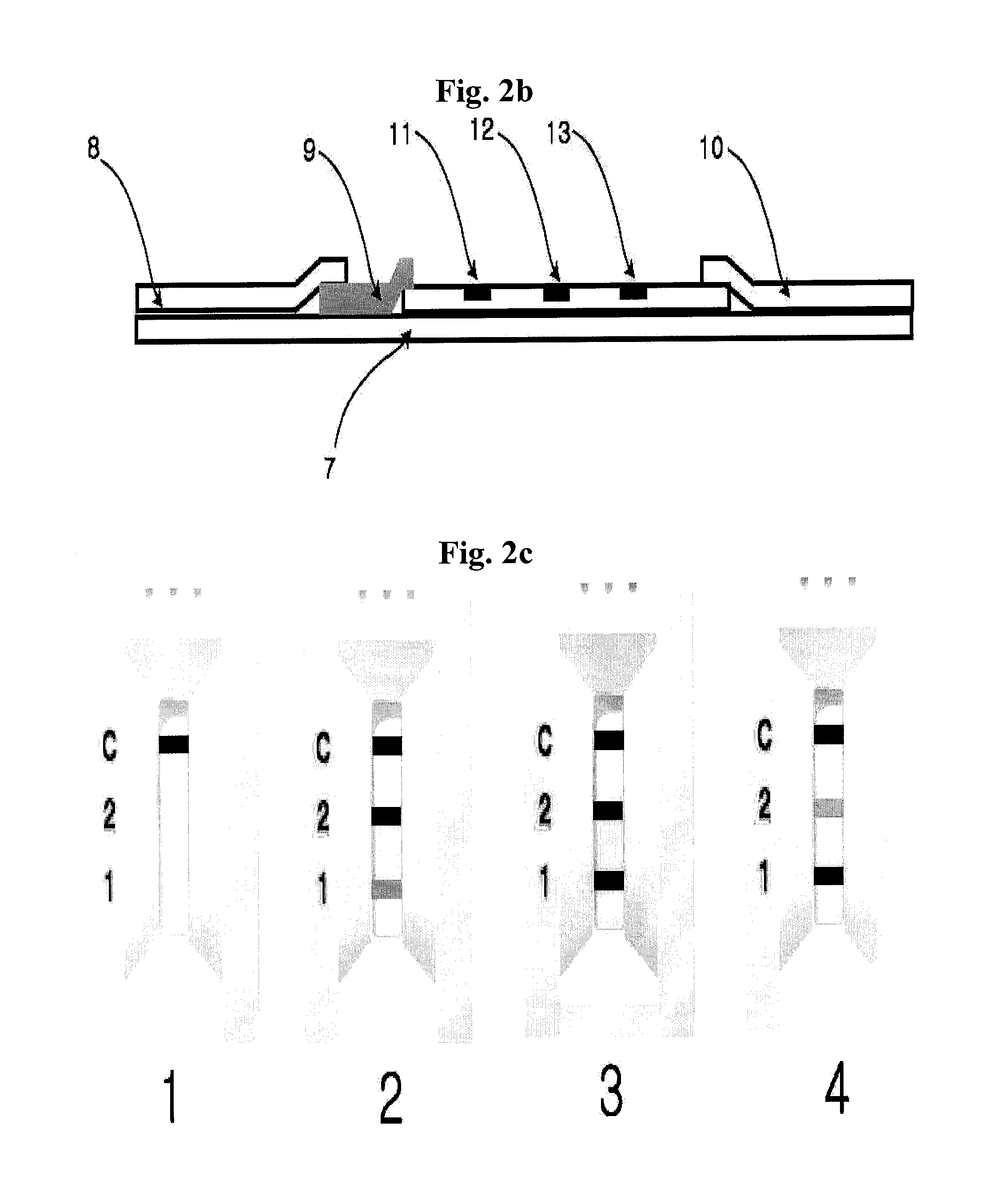
Diagnose device for measuring the ratio of proteins with similar structure
ActiveUS8101369B2Advantage of simplicityEasy to testSamplingPeptide/protein ingredientsAntiendomysial antibodiesObstetrics
The present invention relates to a diagnostic device for measuring the ratio of similar structural proteins among the proteins secreted in a liquid test sample taken from diagnosis subject. In further detail, the test device according to the present invention comprises detection marker-antibody conjugate recognizing the same site on two or more similar structural proteins and a detection zone in which antibody specifically recognizes each of said proteins via formation of sandwich type complex, wherein said antibodies form a set, and the present Invention relates to a diagnostic device for early diagnosis of polycystic ovary syndrome, abnormal pregnancy, prostatic carcinoma etc. based on determination of the ratio of follicle stimulating hormone and luteinizing hormone in case of polycystic ovary syndrome, the ratio between hCG isomers in case of abnormal pregnancy, and the ratio of prostate-specific antigens (PSA) in case of prostatic carcinoma.
Owner:HUMASIS
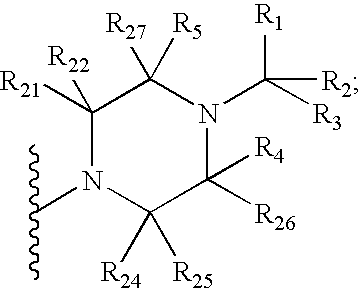
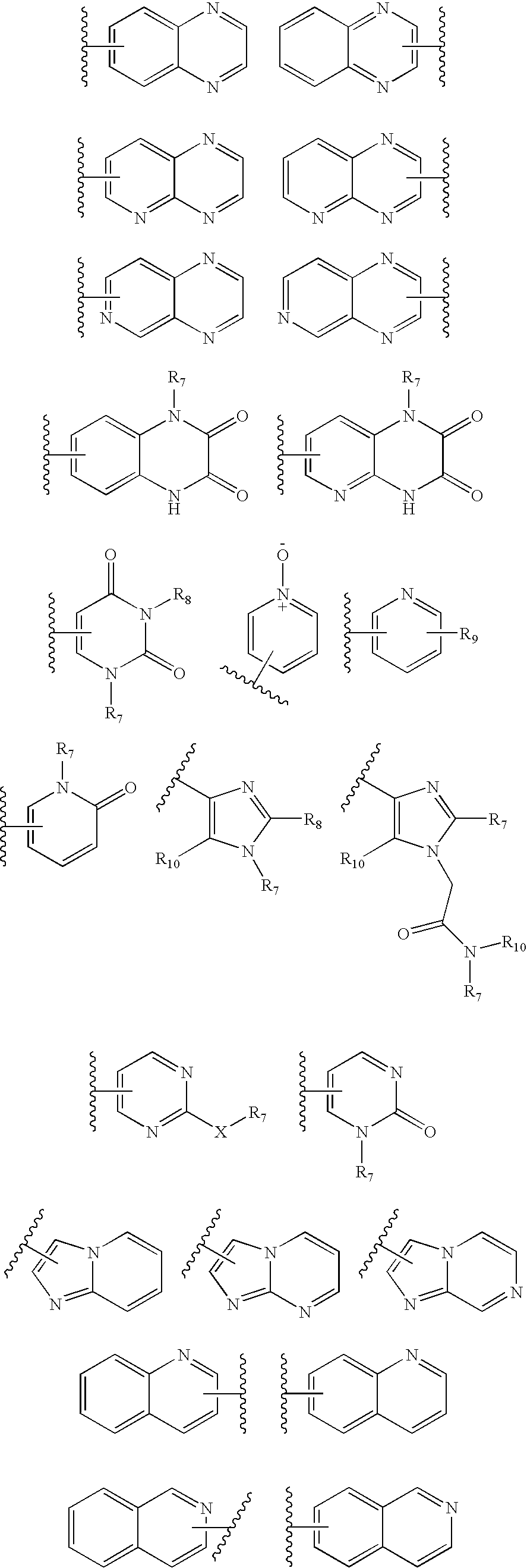
4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor
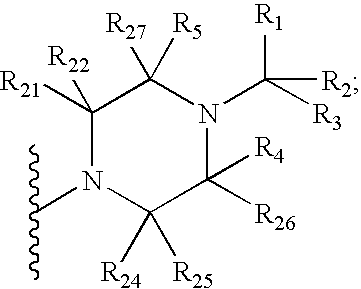
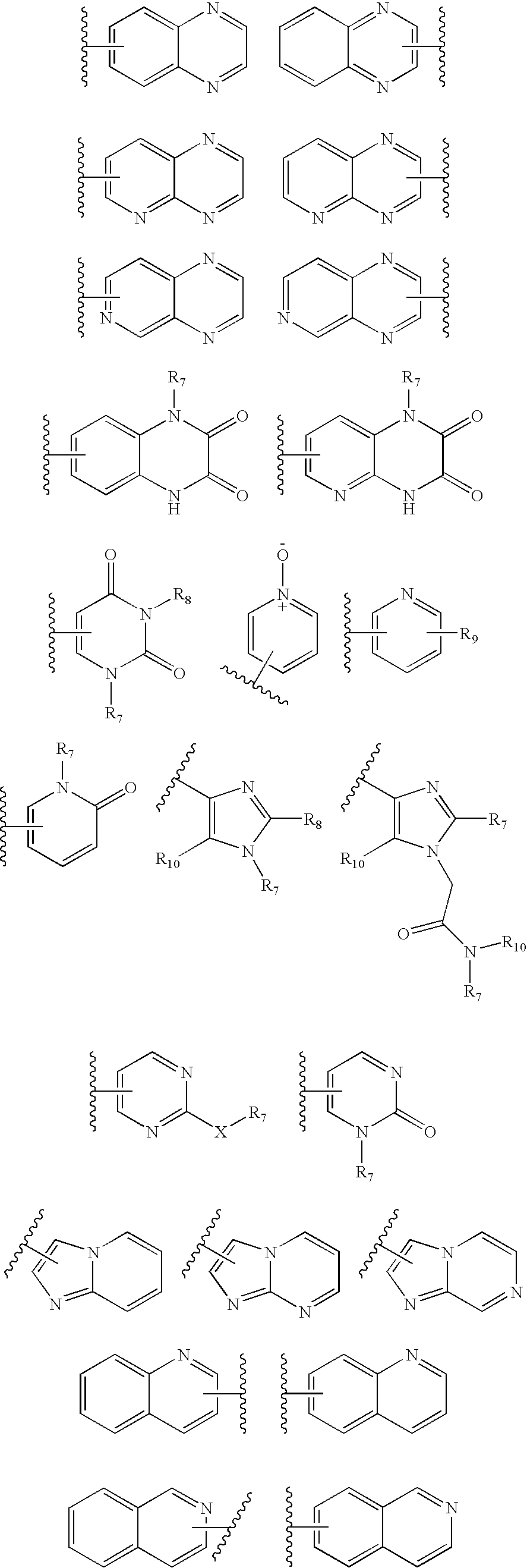
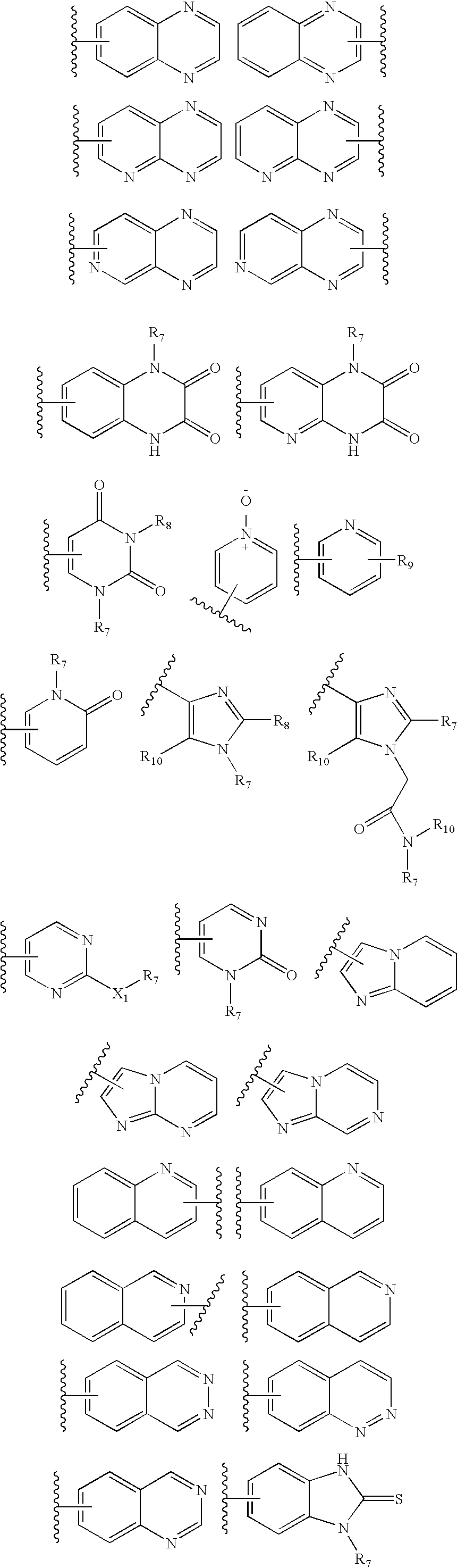
InactiveUS20060189618A1BiocideOrganic chemistryGonadotropin-releasing hormone receptorLutenizing hormone
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
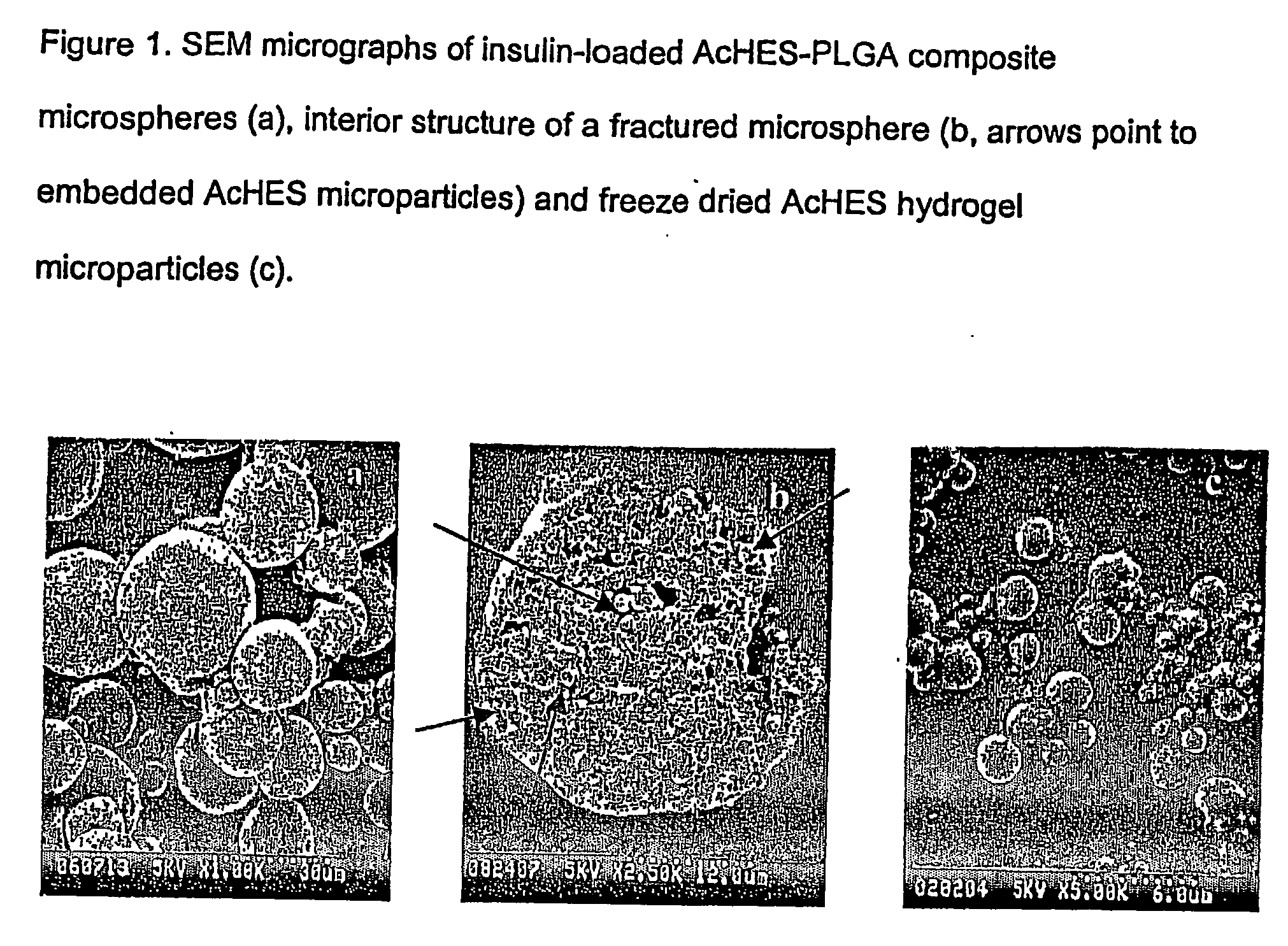
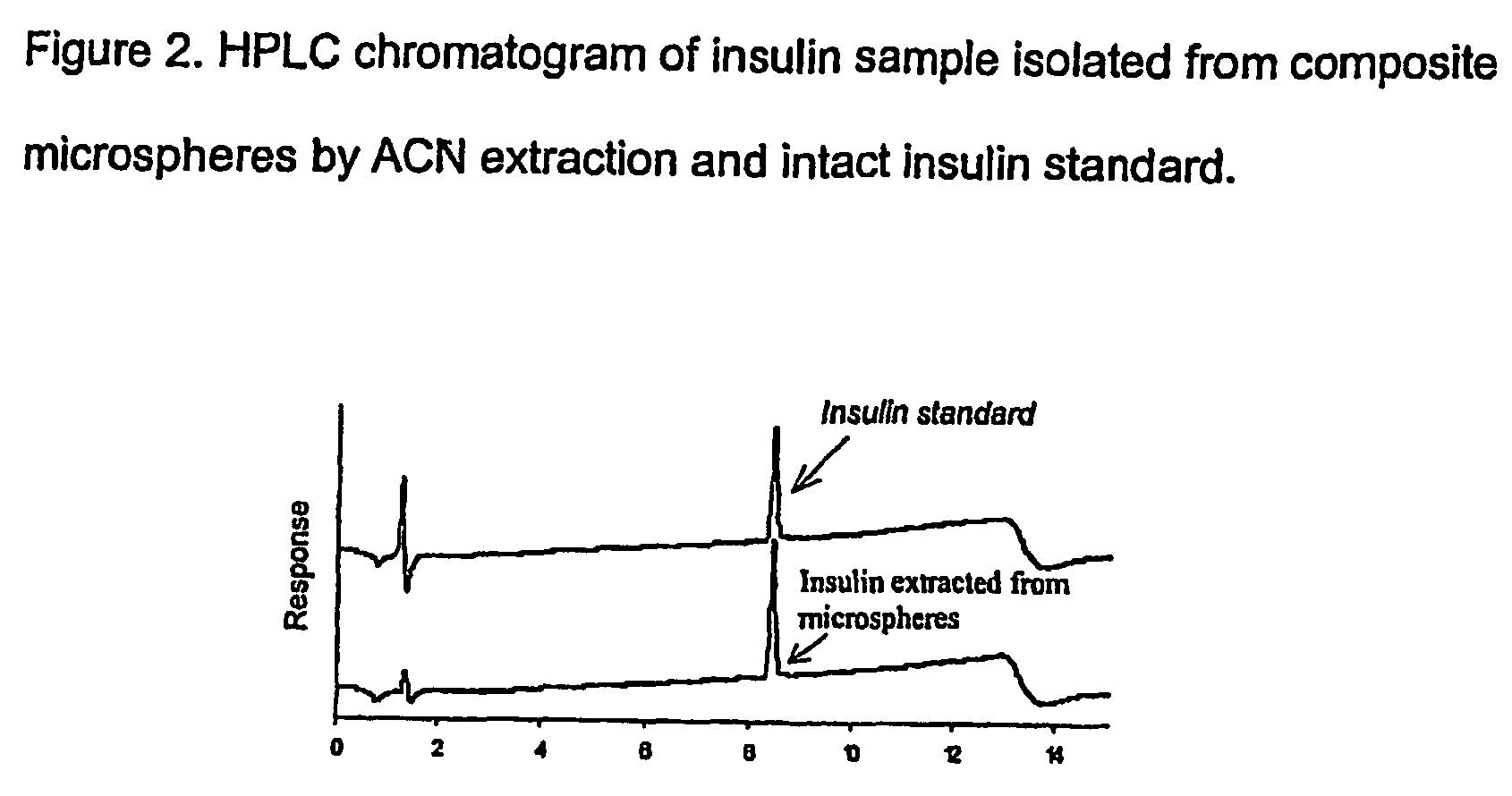
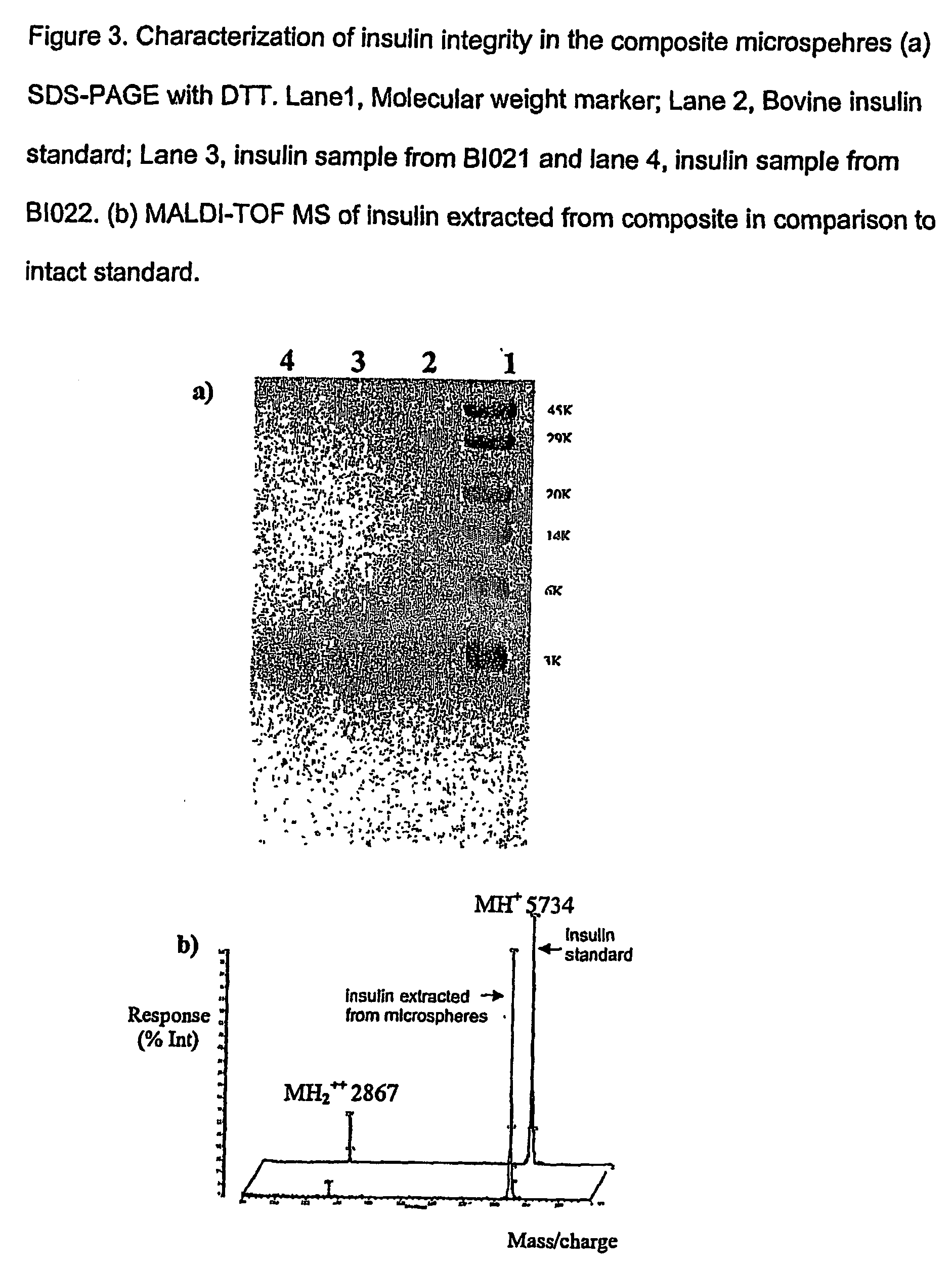
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Benzooxazole and benzothiazole antagonists of gonadotropin releasing hormone receptor
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH
Method for the treatment of fertility disorders
InactiveUS6319192B1Organic active ingredientsPeptide/protein ingredientsObstetricsHormones regulation
An improvement to the method of intrauterine insemination by the administration of luteinizing hormone-releasing hormone antagonists (LHRH antagonists).
Owner:ZENTARIS IVF
Piperazinylimidazopyridine and piperazinyltriazolopyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS20060270848A1Organic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorLutenizing hormone
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
Method of controlled ovarian hyperstimulation and pharmaceutical kit for use in such method
InactiveUS20050235374A1Easy to solveImprove developmentPeptide/protein ingredientsPeptide preparation methodsGanirelixCo administration
One aspect of the present invention is concerned with a method of controlled ovarian hyperstimulation in a mammalian female, said method comprising the co-administration to said female of a substance having follicle stimulating hormone activity (FSH substance) in an amount effective to stimulate multiple follicular development;—gonadotropin releasing hormone (GnRH) antagonist in an amount equivalent to a daily subcutaneous dose of at least 0.5 mg ganirelix to prevent a premature LH-surge; and—a LH substance in an amount effective to prevent or suppress symptoms of luteinising hormone (LH) deficiency resulting from the administration of the GnRH antagonist; followed by administering a meiosis and luteinisation inducing substance (ML substance) in an amount effective to stimulate resumption of meiosis and luteinisation, and wherein the LH substance is not obtained from the urine of human females. Another aspect of the to invention relates to a pharmaceutical kit for use in a method of controlled hyperstimulation, which kit comprises:—at least one parenteral or oral dosage unit containing one or more FSH substances in an amount equivalent to a subcutaneous dose of 50-1500 I.U. FSH;—at least one parenteral dosage unit containing one or more GnRH antagonists in an amount equivalent to a subcutaneous dose of 0.5-25 mg ganirelix;—at least one parenteral dosage unit containing one or more LH substances in an amount equivalent to a subcutaneous dose of 50-3000 I.U. recombinant LH; wherein the LH substance is not obtained from the urine of human females.
Owner:ZONE IND DE IOURIETTAZ
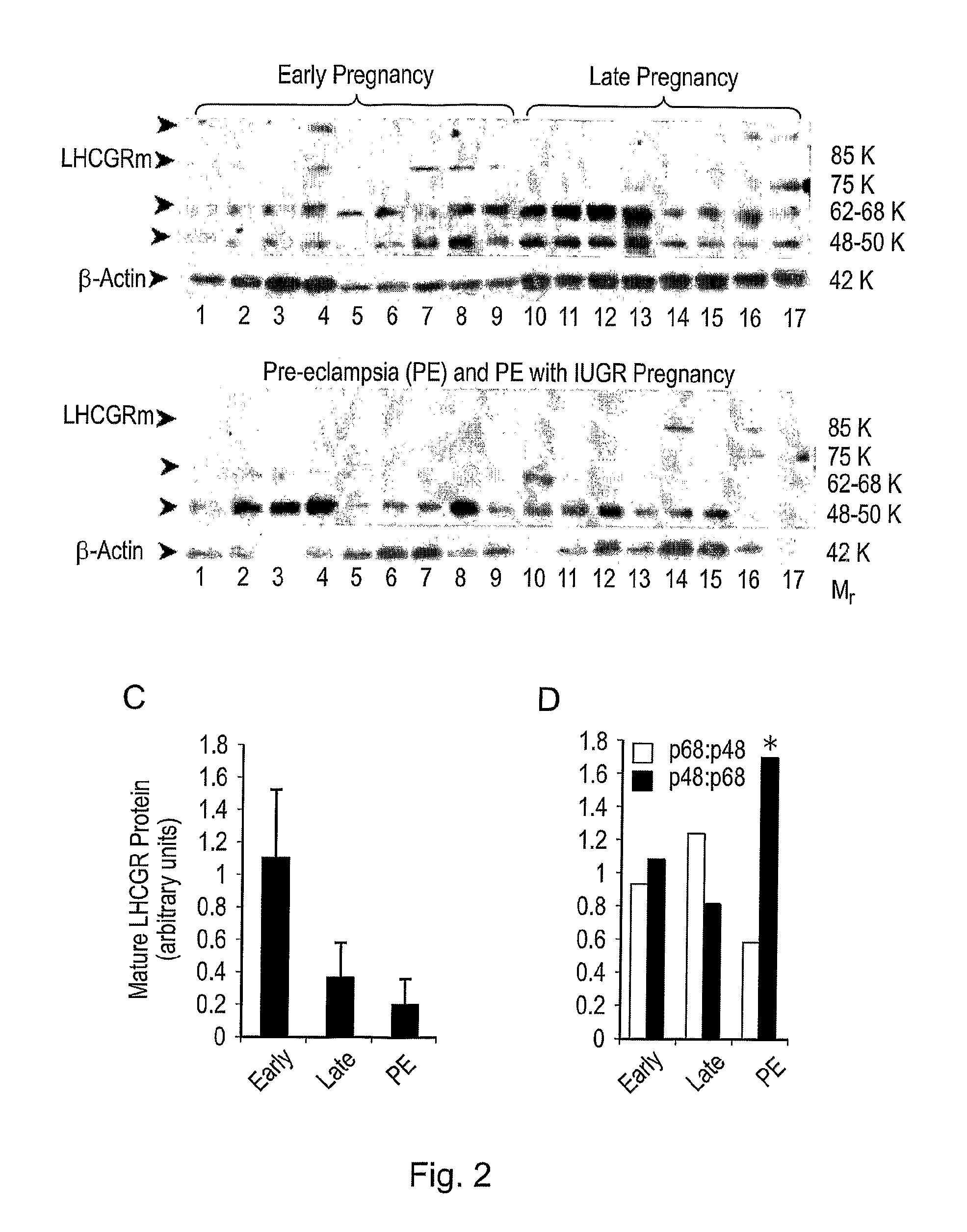
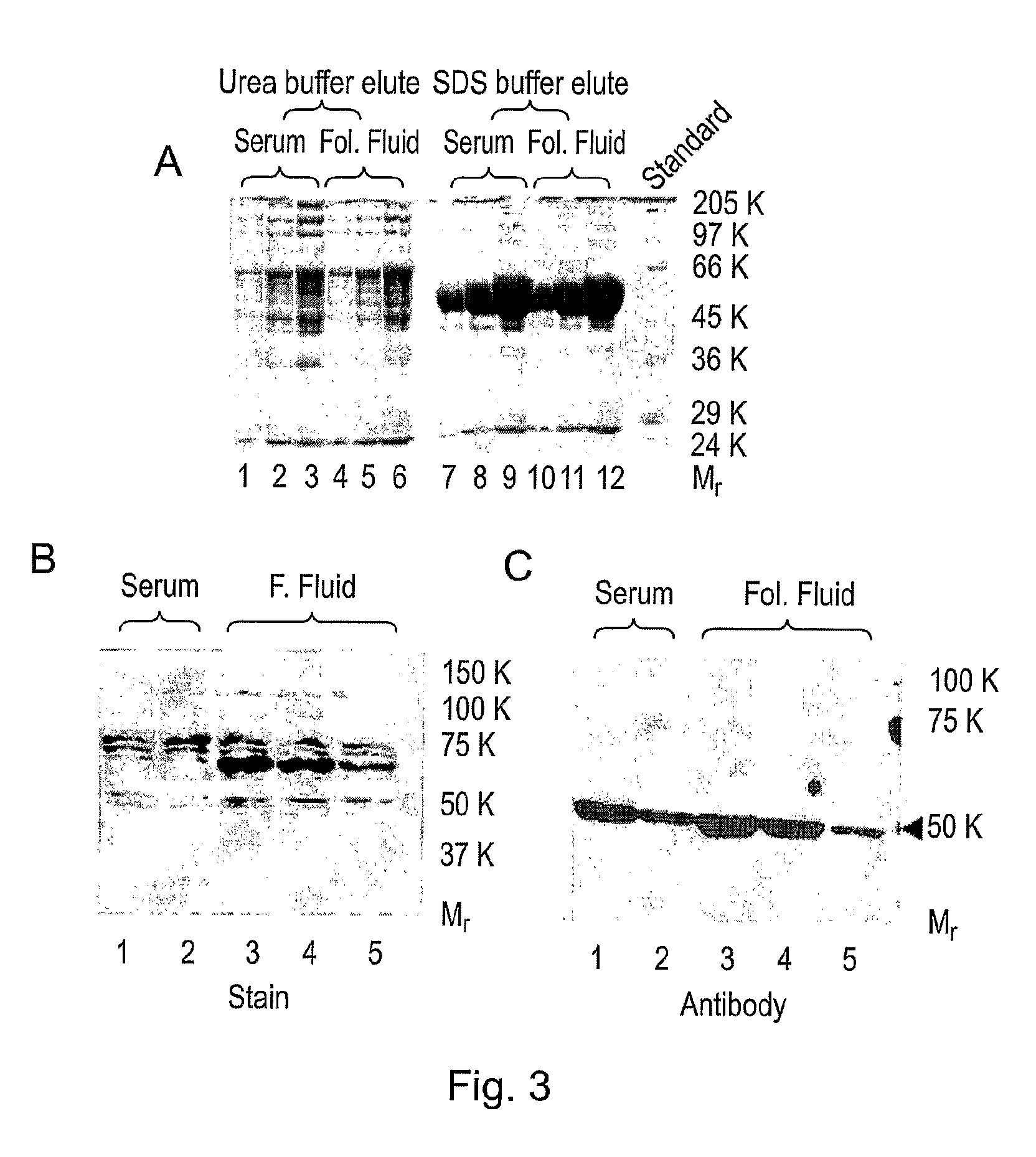
Diagnostic and therapeutic applications of soluble LHCGR protein
InactiveUS7892753B1Easy to measureImprove survival rateReceptors for hormonesBiological testingDiseasePhysiology
The invention relates to a soluble luteinising hormone / chorionic gonadotropin receptor (LHCGR) protein and its use in diagnosing, treating and preventing conditions associated with over- and under-production of the said receptor, with over- and under-production of luteinising hormone, with over- and under-production of chorionic gonadotropin, with reproductive failure, with gonadal cancer and metastases, and Alzheimer's disease.
Owner:BANERJEE SUBHASIS
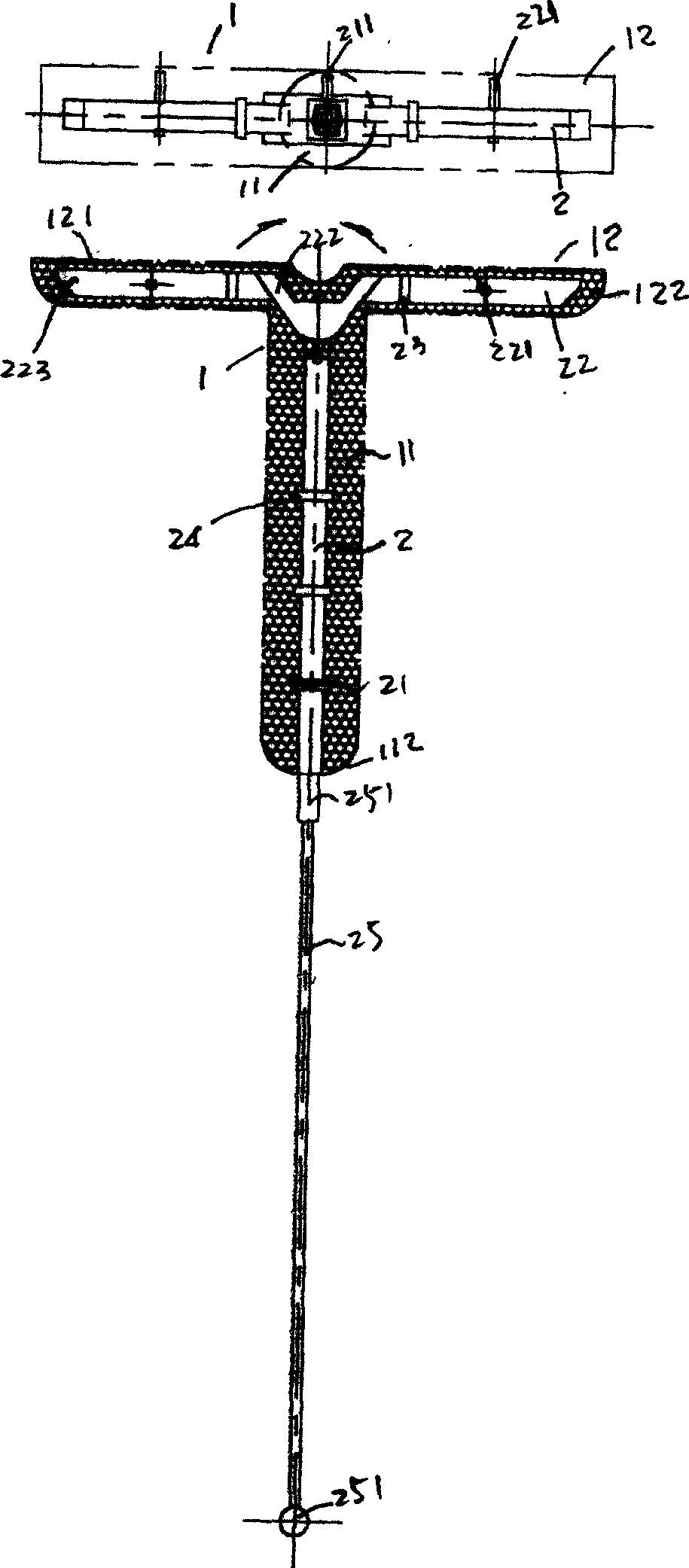
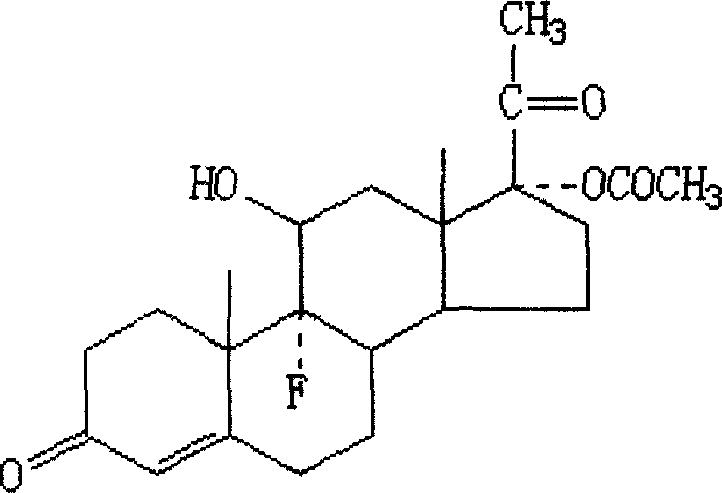
Progestational hormone composition, and its slow-release suppository device and preparation of said device
InactiveCN1891224AInhibit inflammationReduce adhesionOrganic active ingredientsSuppositories deliveryAlcoholPhysiology
The present invention relates to a progestogen composition. Said progestogen composition is formed from 27-31% of fluoropregestin, 2-2.7% of ethinyl estradiol, 8-10% of lauric acid, 37-40% of ethyl alcohol, 0.3-0.6% of carbomer and 6-10% of propylene glycol, and the rest is pure water. Besides, said invention also provides the delayed-release suppository device of said progestogen composition and its preparation method.
Owner:天津市中意畜牧科技发展有限公司
4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
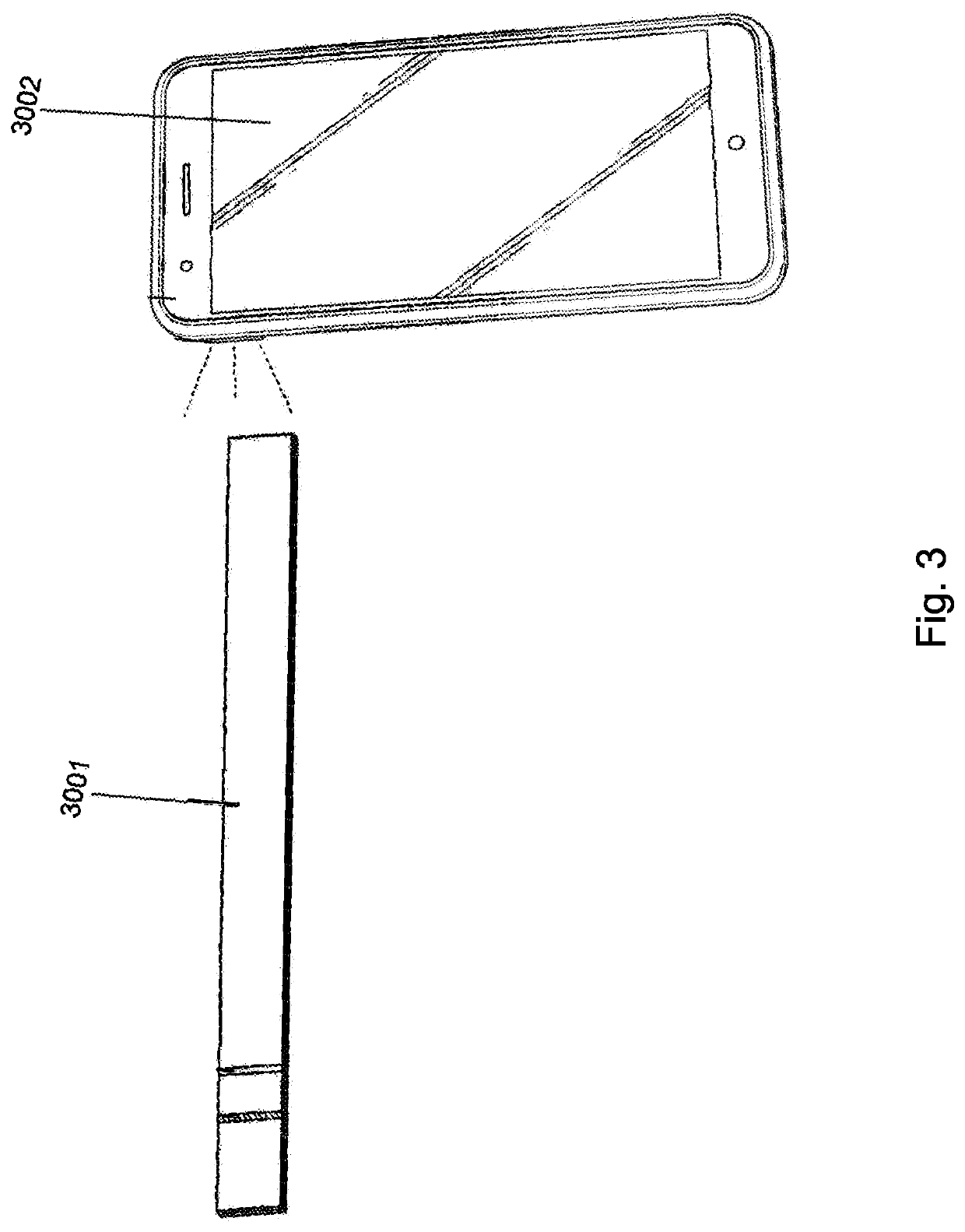
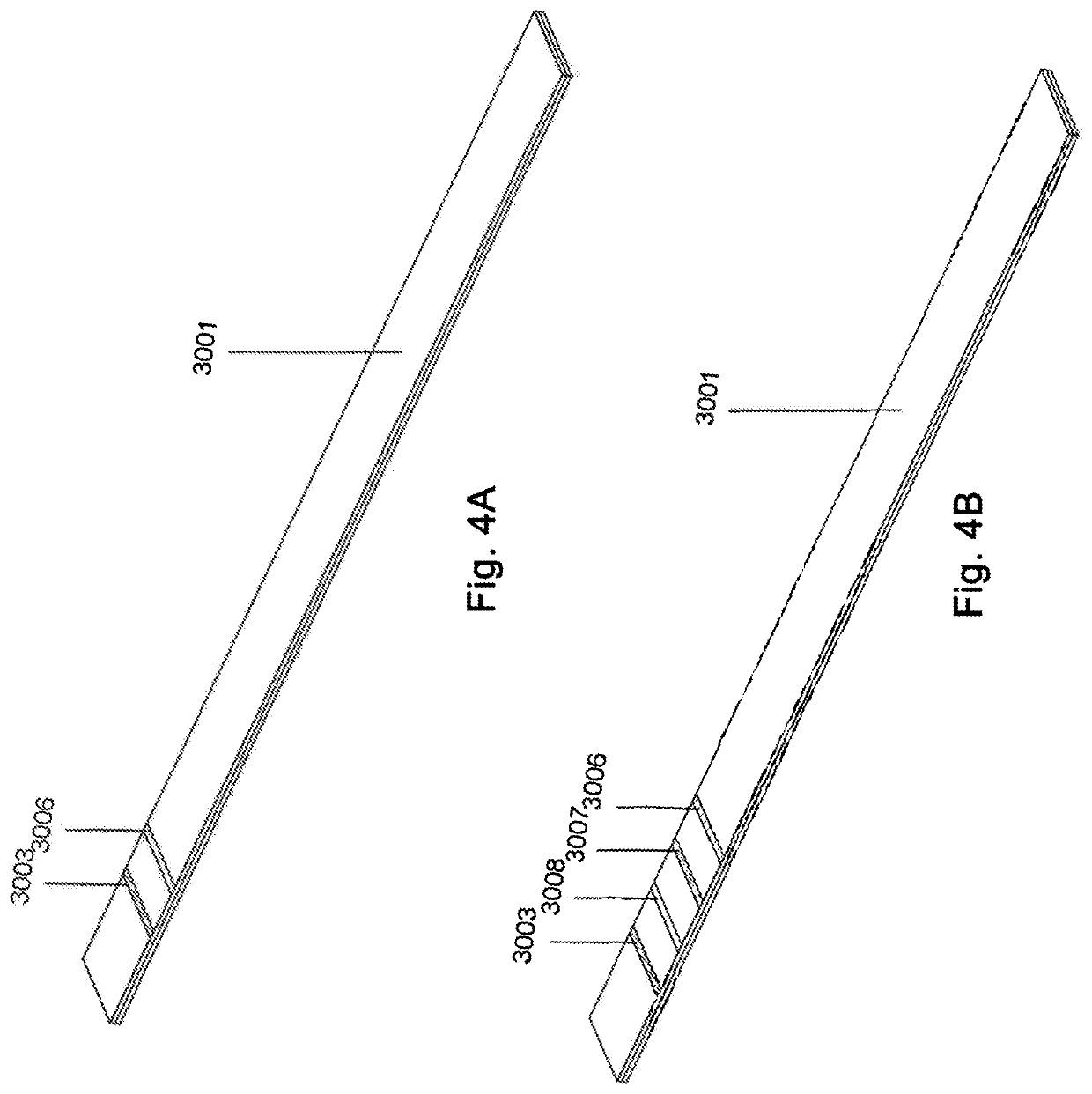
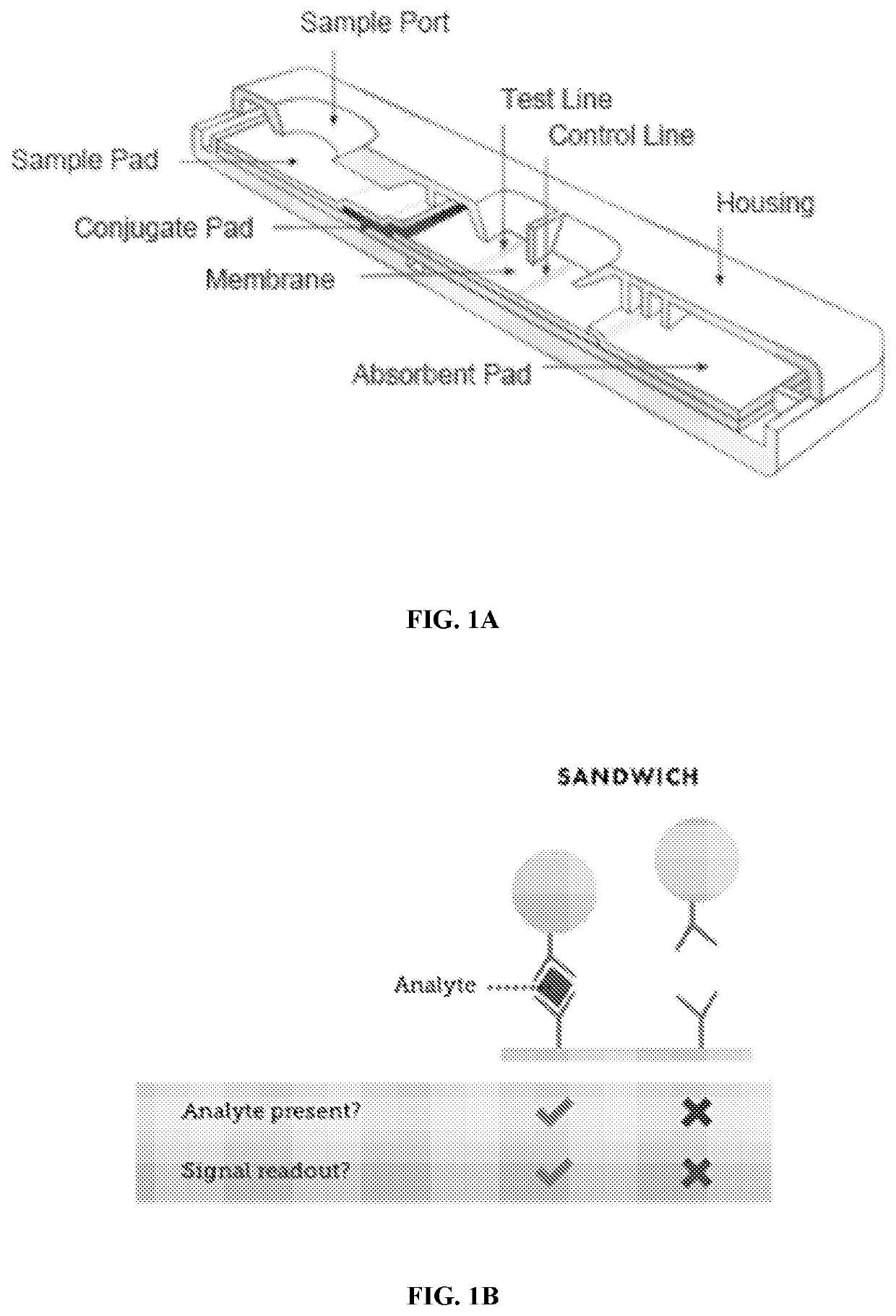
Methods, devices, and systems for detecting analyte levels
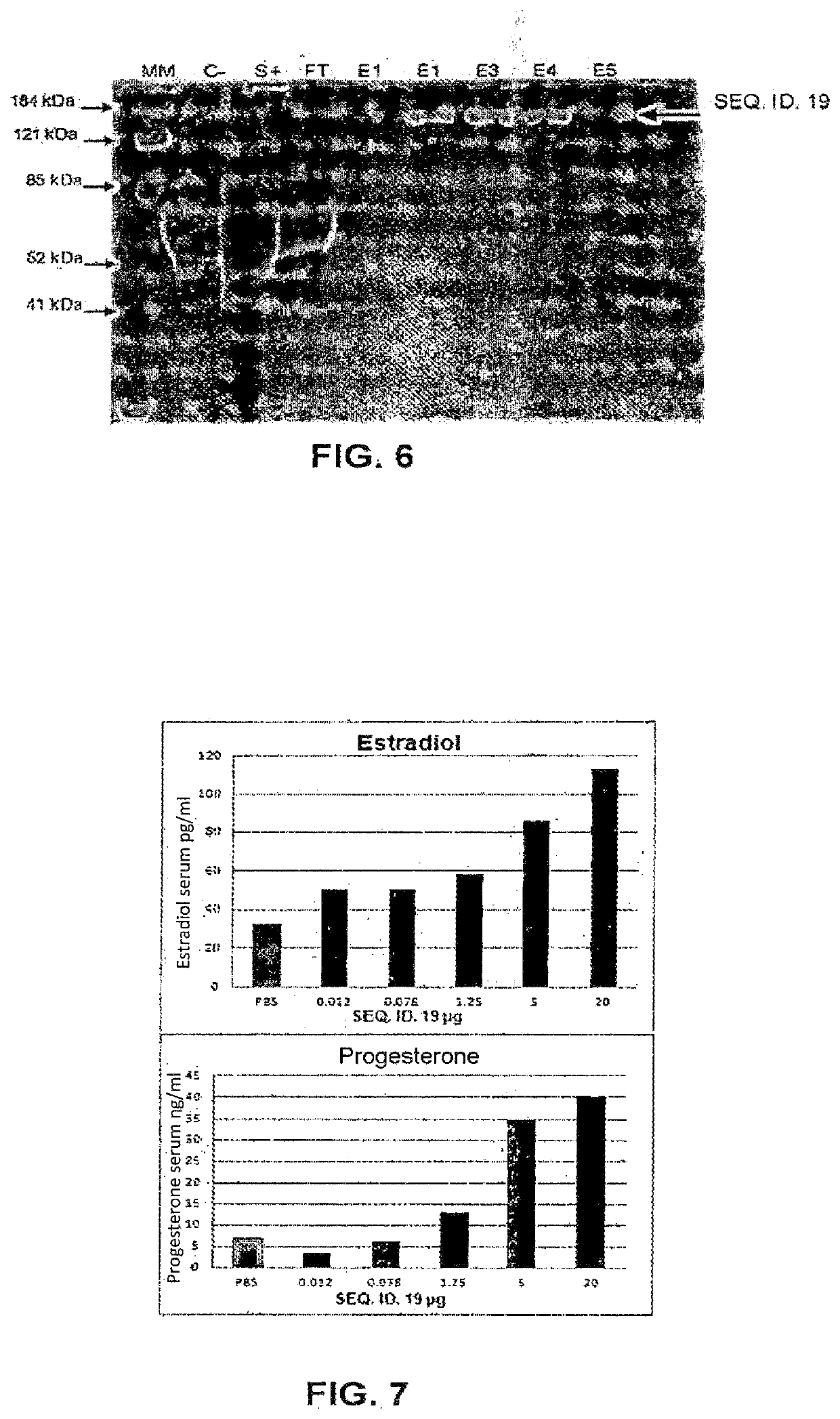
The present disclosure describes a method of quantifying analyte levels using gold nanoparticles. The present disclosure also describes a device to quantify analyte levels using gold nanoparticles and image processing of pixels isolated from a single vector. The methods, devices, and systems of the disclosure can be used to quantify analytes such as luteinizing hormone, progesterone, estradiol, or testosterone.
Owner:OOVA INC
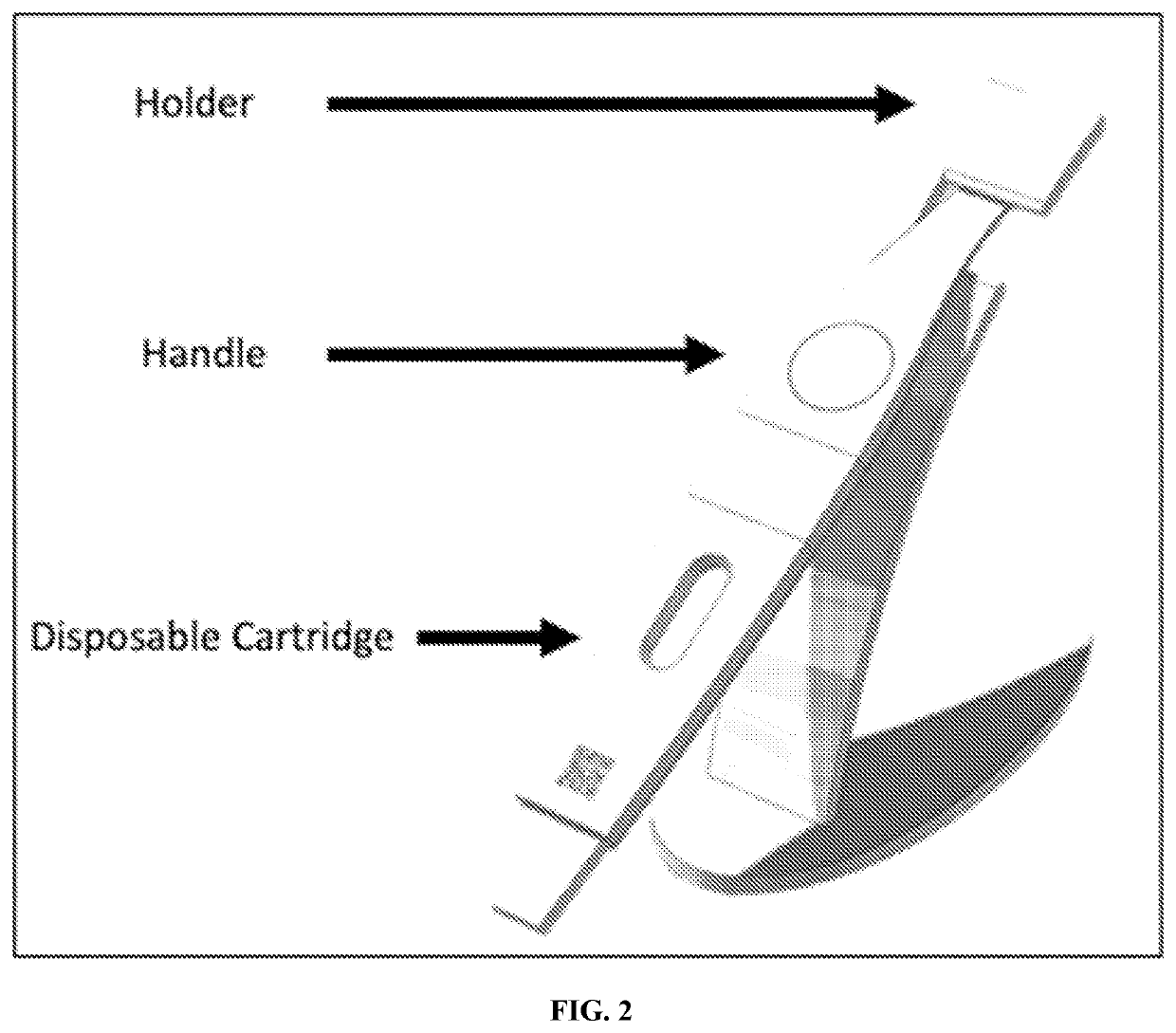
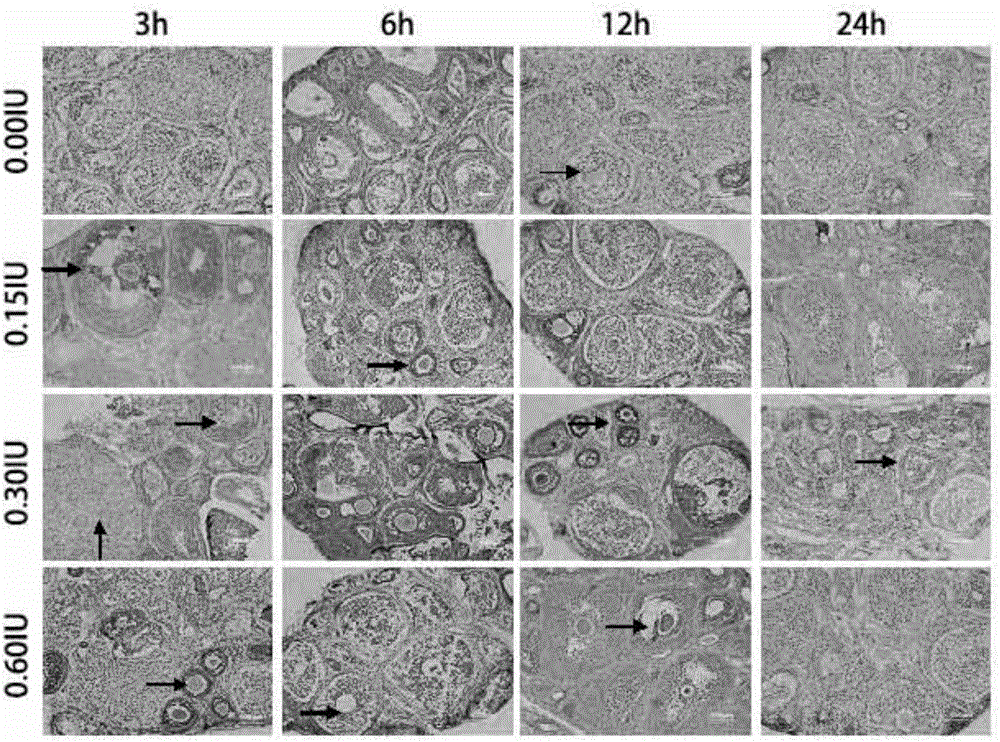
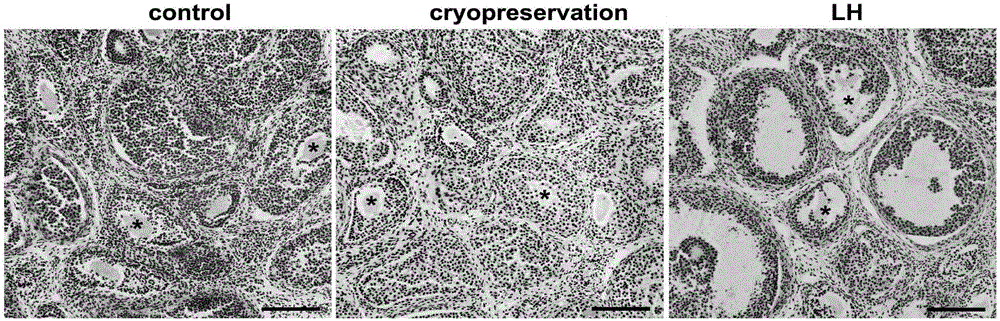
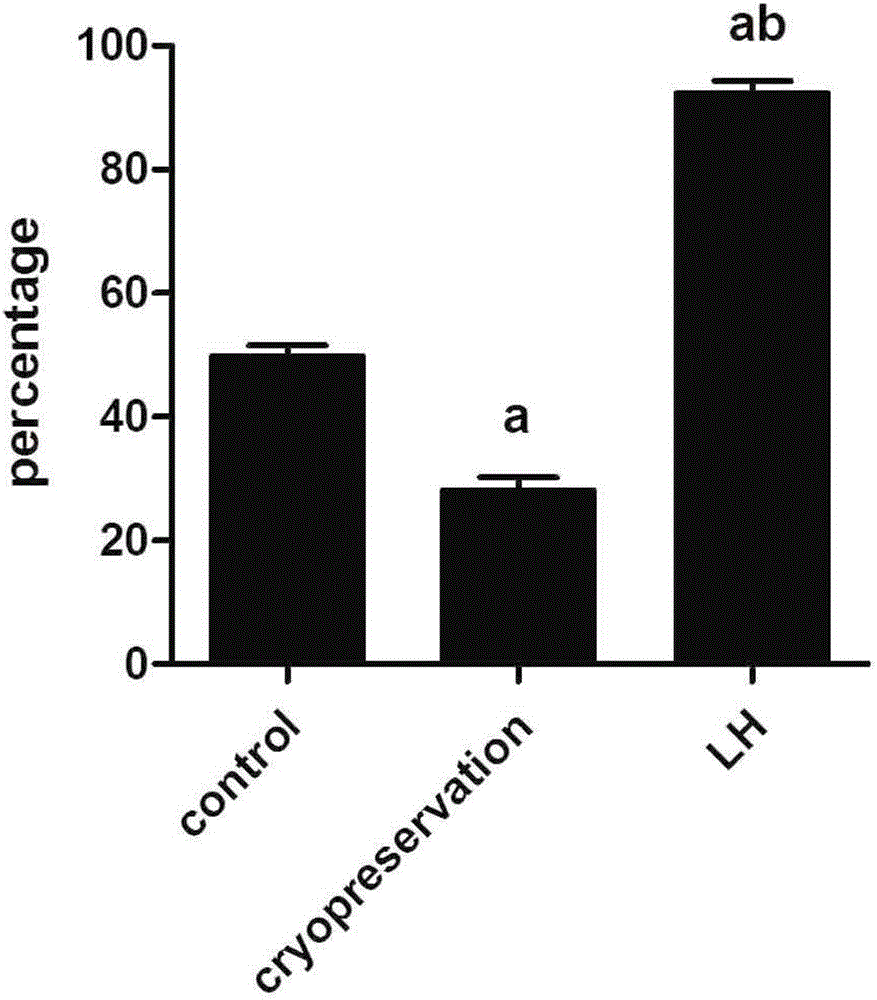
Ovary vitrification cryopreservation method under intervention of luteinizing hormone
InactiveCN106172376AGood functionGood endocrine functionDead animal preservationHigh concentrationSucrose
The invention discloses an ovary vitrification cryopreservation method under intervention of luteinizing hormone. The ovary vitrification cryopreservation method comprises the following steps: soaking an in-vitro ovary into a culture solution, putting into a constant-temperature incubator with 5 percent of CO2 at the temperature of 37 DEG C for culture, then transferring into a permeable cryoprotective agent-containing ethylene glycol pre-equilibrium solution for soaking, after then, transferring into a high-concentration cryoprotective agent-containing vitrified cryopreservation fluid for continuous permeable equilibrium, and finally transferring into liquid nitrogen for soaking for at least 24 hours. A thawing process comprises the steps of taking out the cryopreserved ovary from the liquid nitrogen, placing the ovary into 0.50 mol / L, 0.25 mol / L and 0.125 mol / L of thawing solutions which are preheated at the temperature of 37 DEG C and has the sucrose concentration gradually decreased in sequence for 10 min, then transferring the ovary into the culture solution, and putting into the constant-temperature incubator with 5 percent of CO2 at the temperature of 37 DEG C for culture; the culture solution, the pre-equilibrium solution, the vitrified cryopreservation fluid and the thawing solution contain the luteinizing hormone, so that the survival rate of the cryopreserved ovary can be increased, and the reproductive function and the endocrine function can be retained to a relatively large extent.
Owner:NINGXIA MEDICAL UNIV
Estrogen receptor ligands and methods of use thereof
The present invention relates to methods for reducing testosterone levels by reduction of luteinizing hormone (LH) or independent of LH levels in a male subject and methods of treating, suppressing, reducing the incidence, reducing the severity, or inhibiting prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC), metastatic castration resistant prostate cancer (mCRPC) and palliative treatment of prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC), and methods of reducing high or increasing PSA levels and / or increasing SHBG levels in a subject suffering from prostate cancer, advanced prostate cancer, castration resistant prostate cancer (CRPC) and metastatic castration resistant prostate cancer (mCRPC). The compounds of this invention suppress free or total testosterone levels despite castrate levels secondary to ADT and reduce high or increasing PSA levels. This reduction in testosterone levels may be used to treat prostate cancer, advanced prostate cancer, CRPC and mCRPC without causing bone loss, decreased bone mineral density, increased risk of bone fractures, increased body fat, hot flashes and / or gynecomastia.
Owner:GTX INCORPORATED
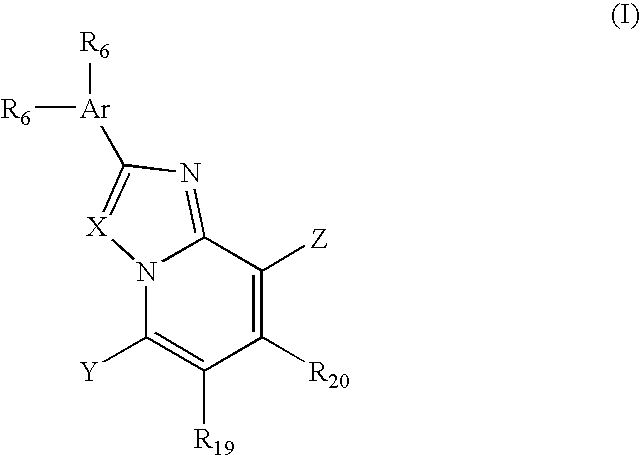
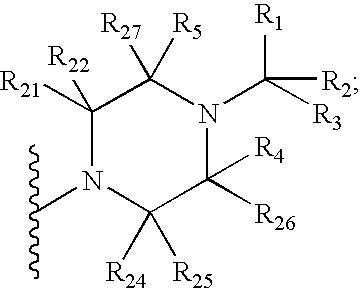
Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor
InactiveUS7534796B2BiocideOrganic chemistryGonadotropin-releasing hormone receptorHormones regulation
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
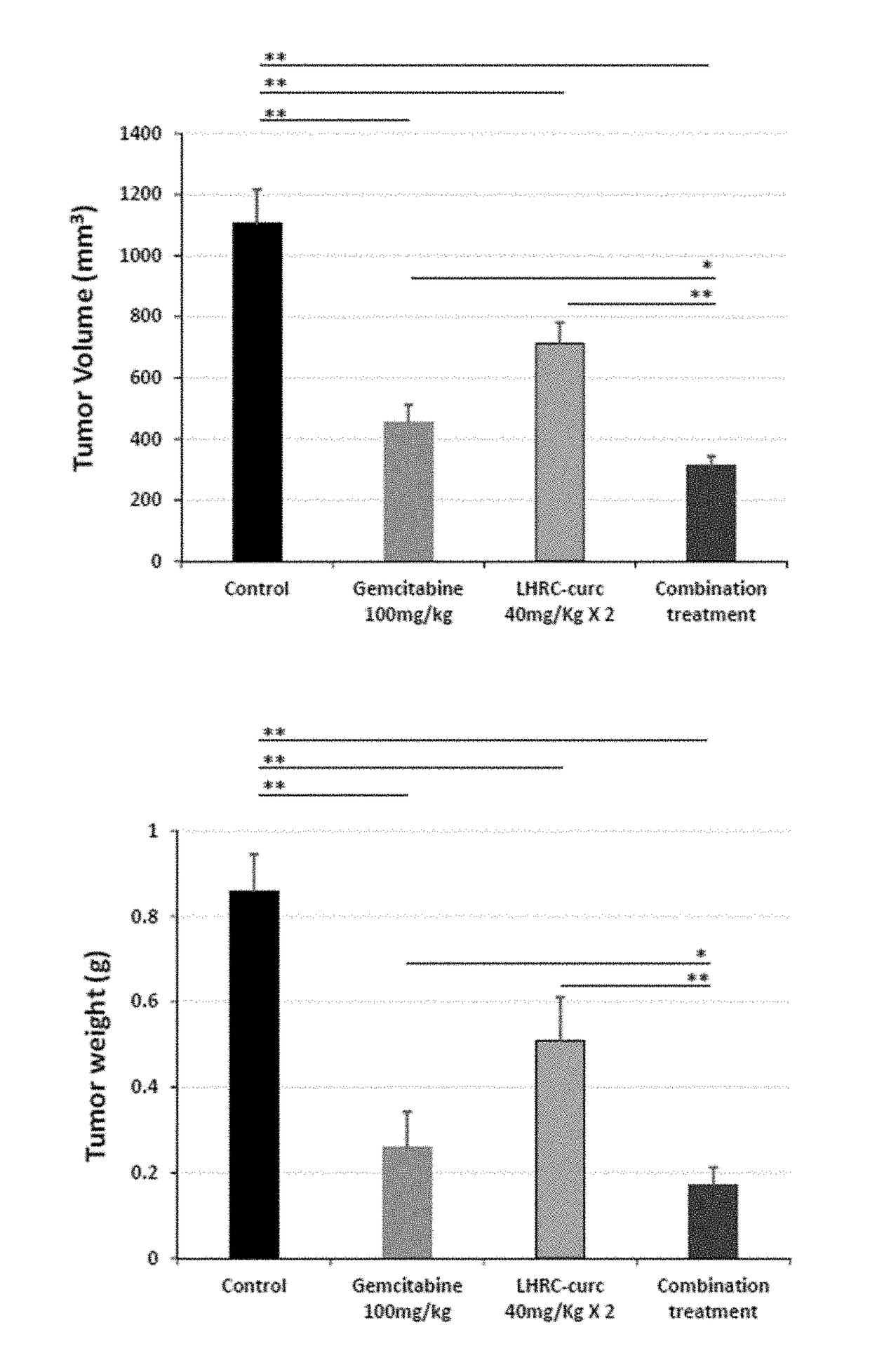
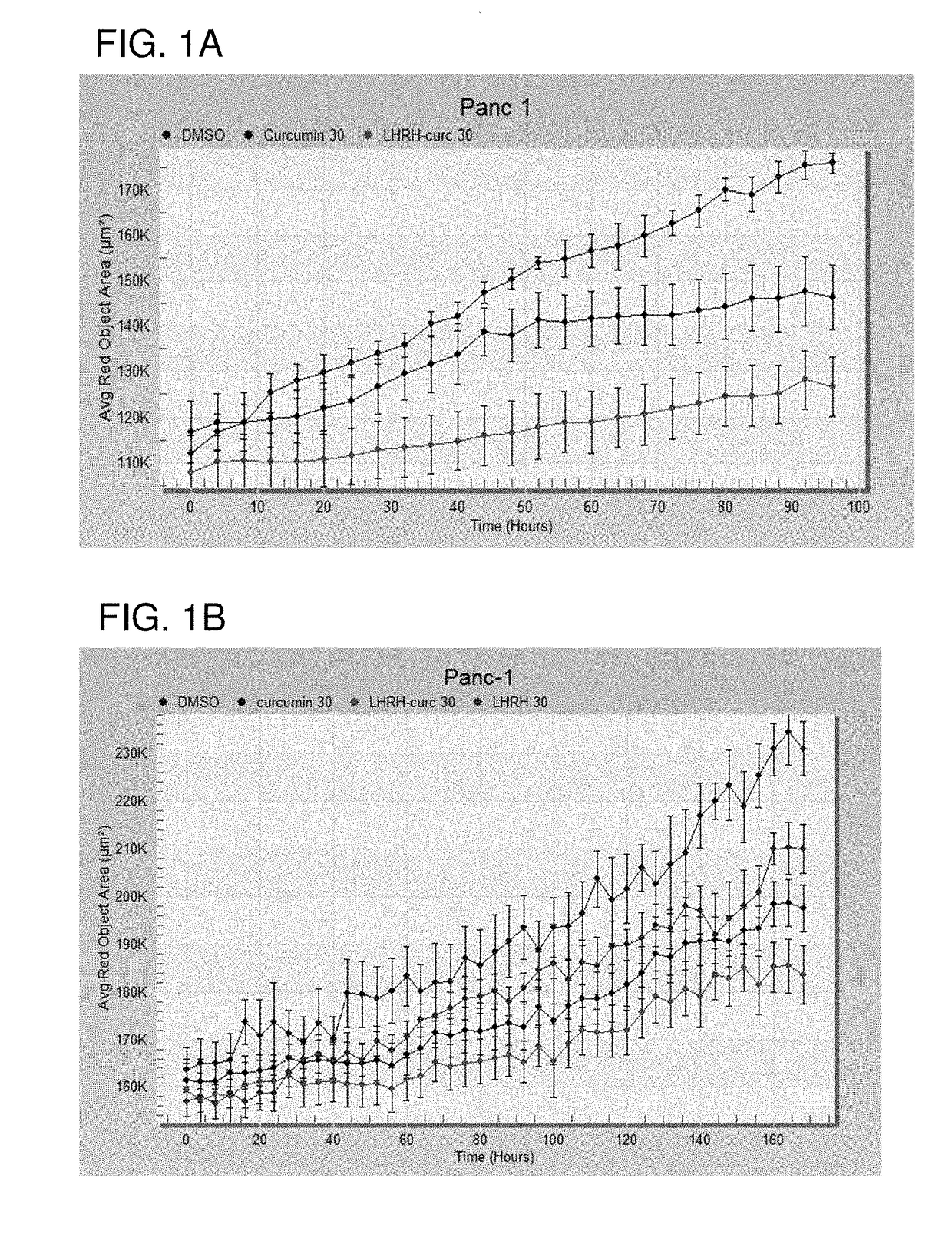
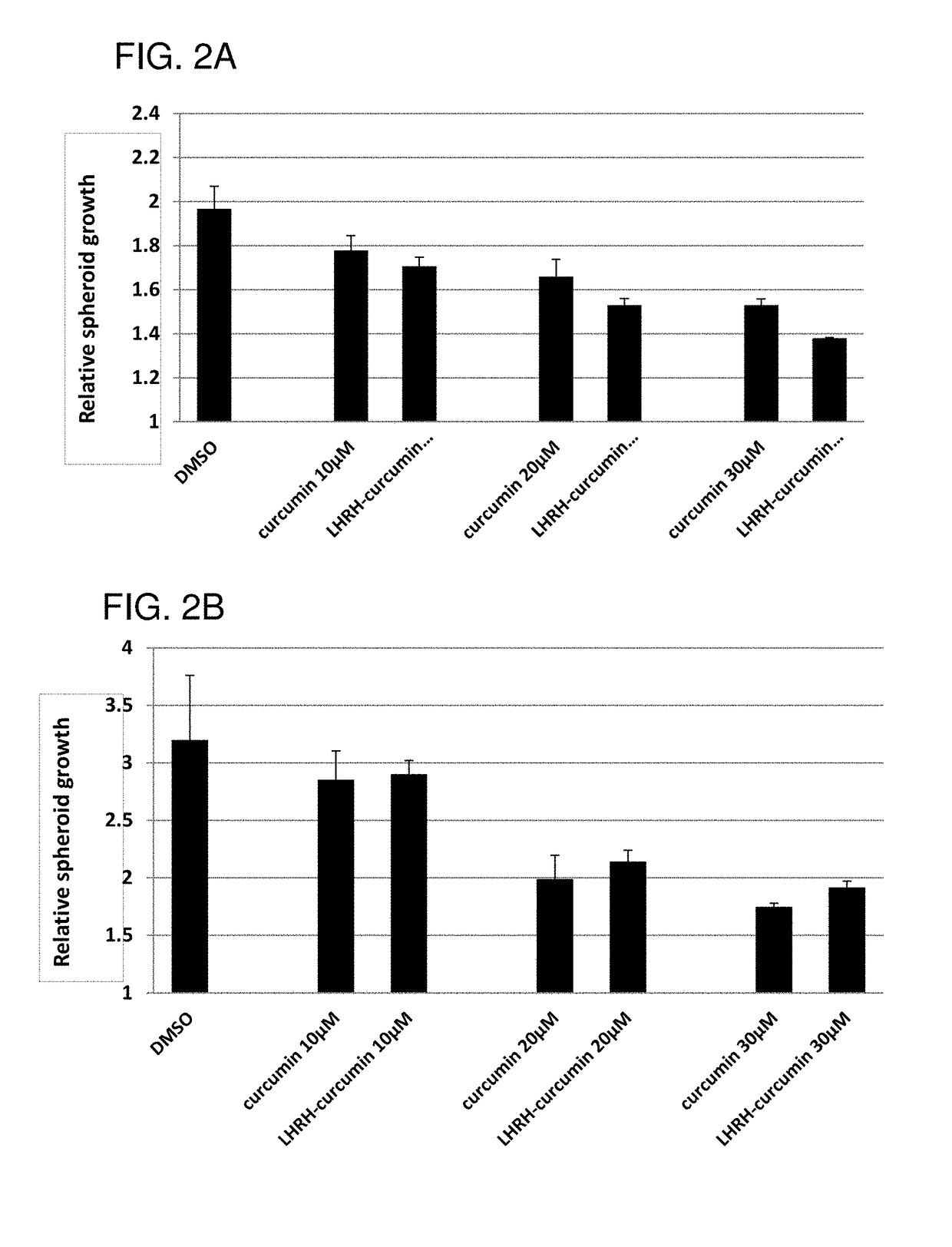
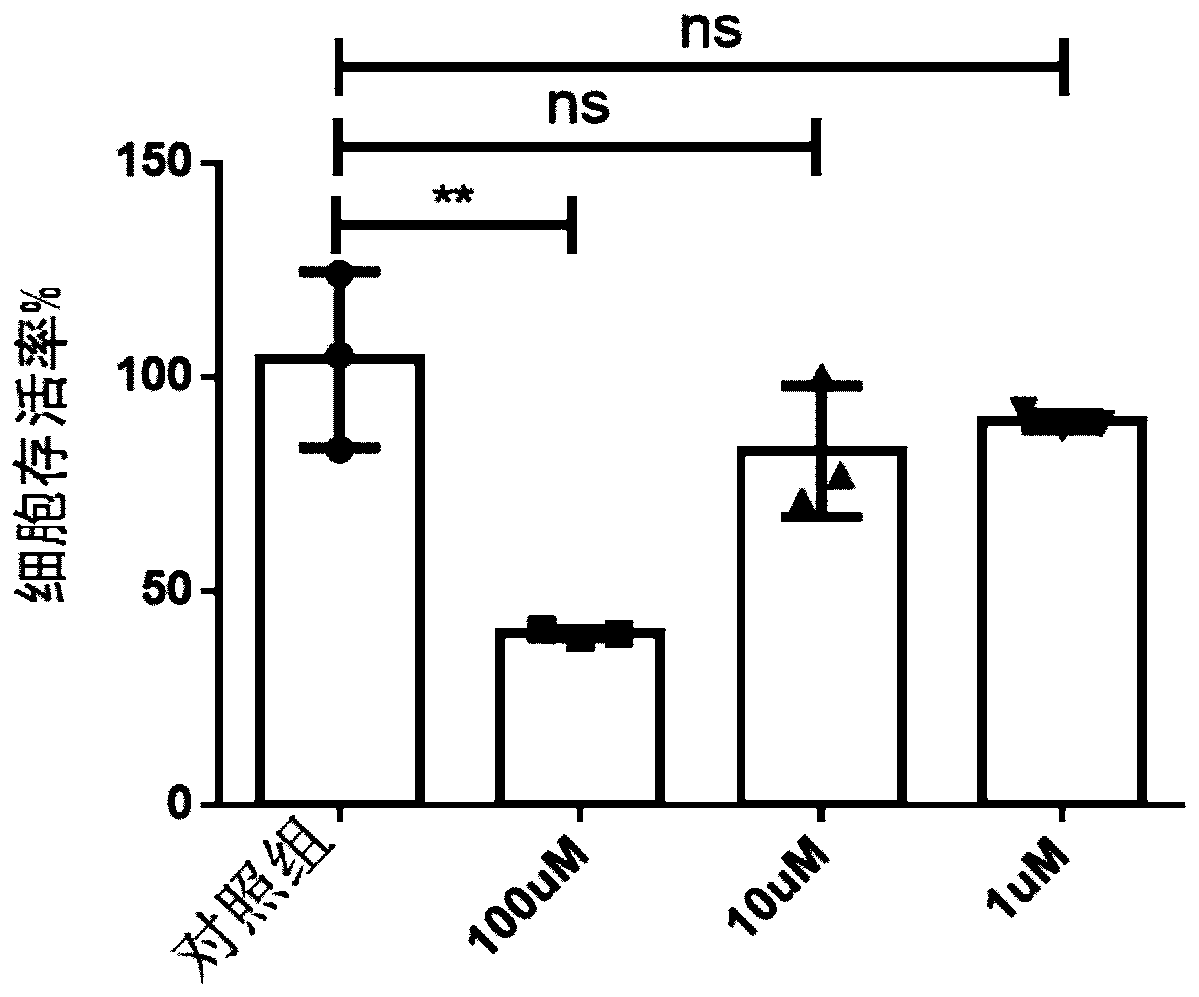
Cancer treatment combination compositions, methods and uses
ActiveUS20170258929A1Reduced drug cytotoxicityEffective therapyKetone active ingredientsCyclic peptide ingredientsMalignancyCancer treatment
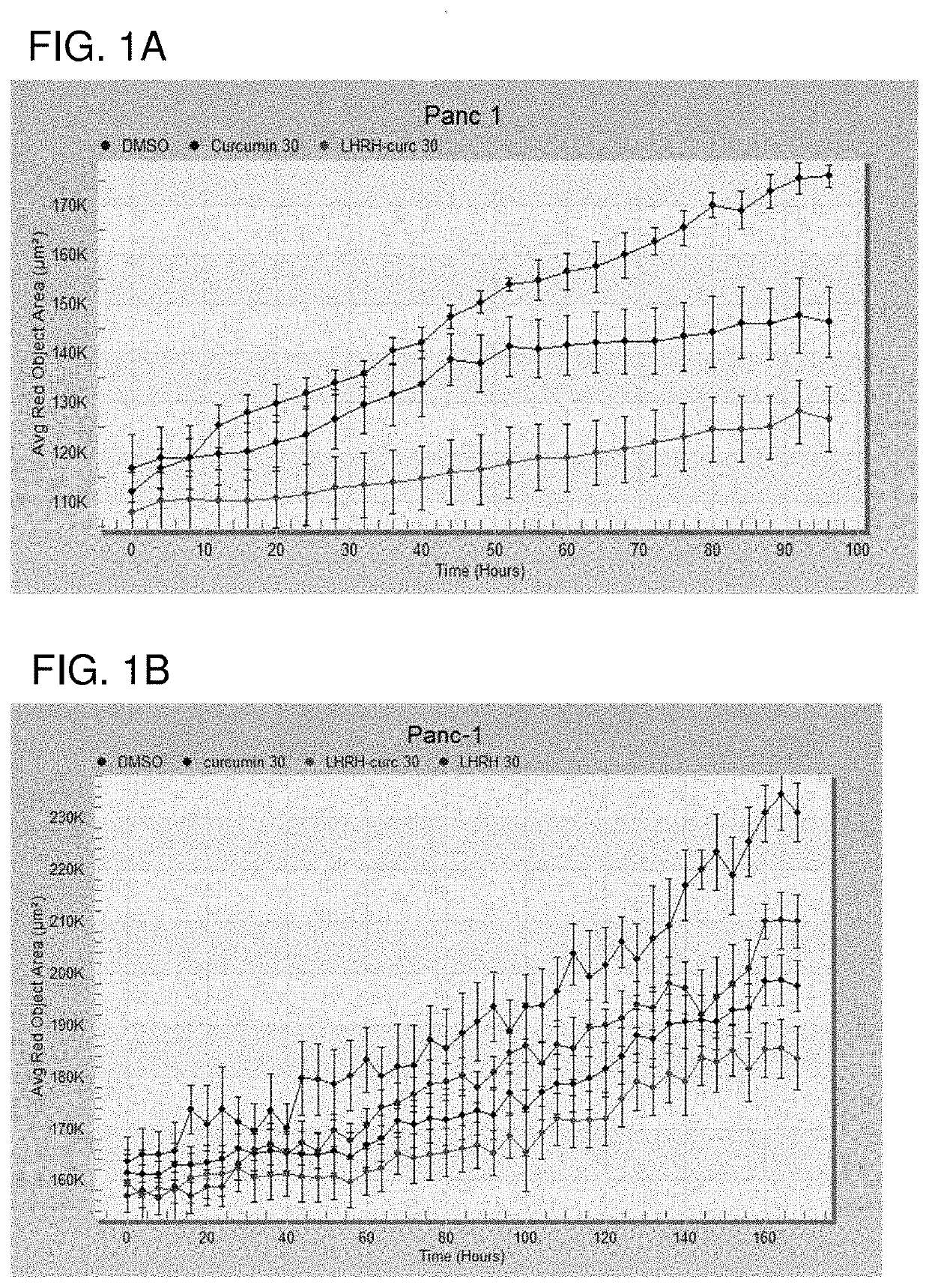
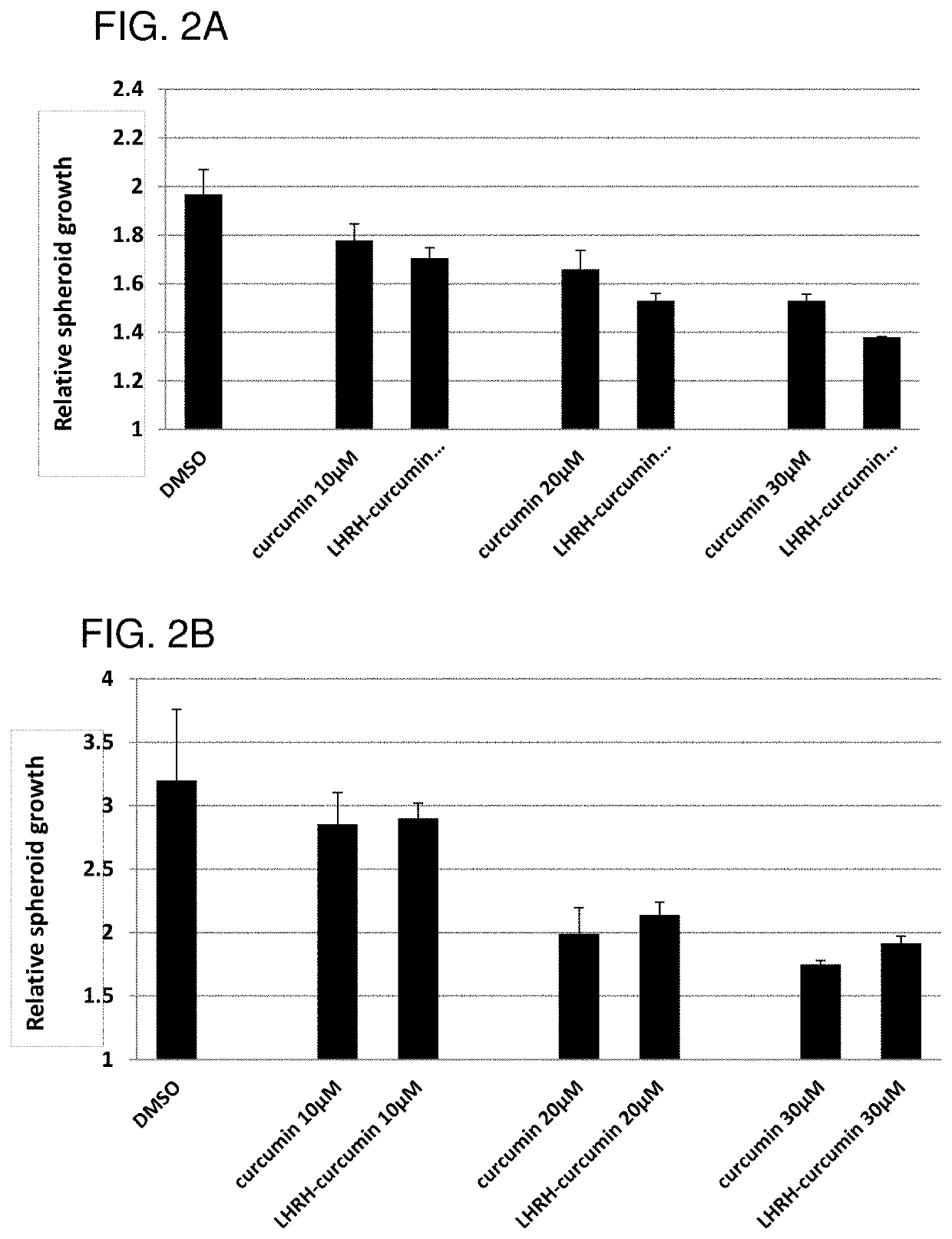
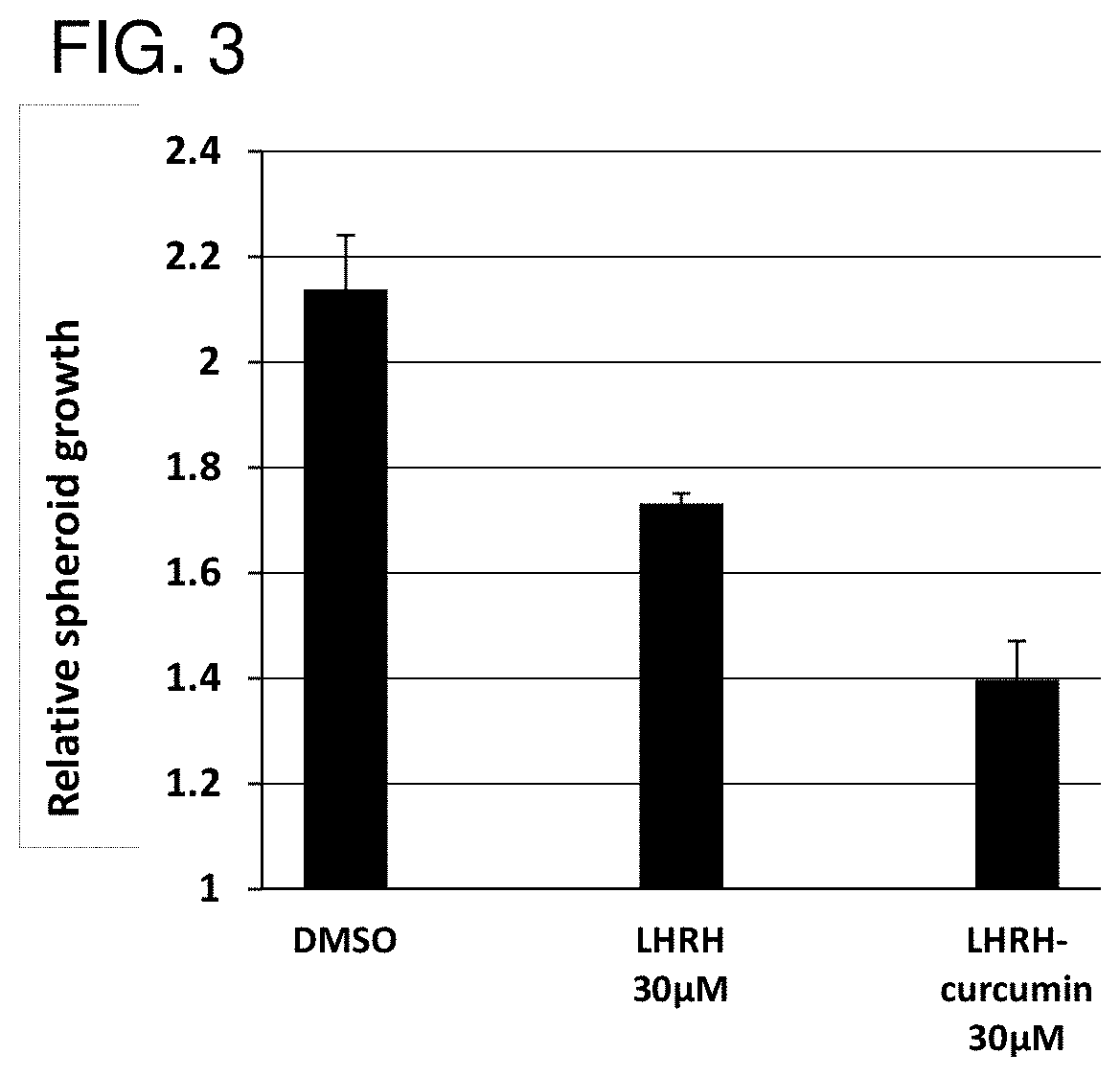
The invention provides combinations and formulations including a luteinizing hormone-releasing hormone (LHRH) or a LHRH analog; and curcumin or a curcumin analog. LHRH or LHRH analog can be fused or conjugated to a curcumin or curcumin analog. Invention combinations and formulations can also include an anti-cell proliferative drug. Invention combinations and formulations can be used for inhibiting proliferation of a cell; treating a hyperproliferative disorder; and treating a neoplasia, tumor, cancer or malignancy.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
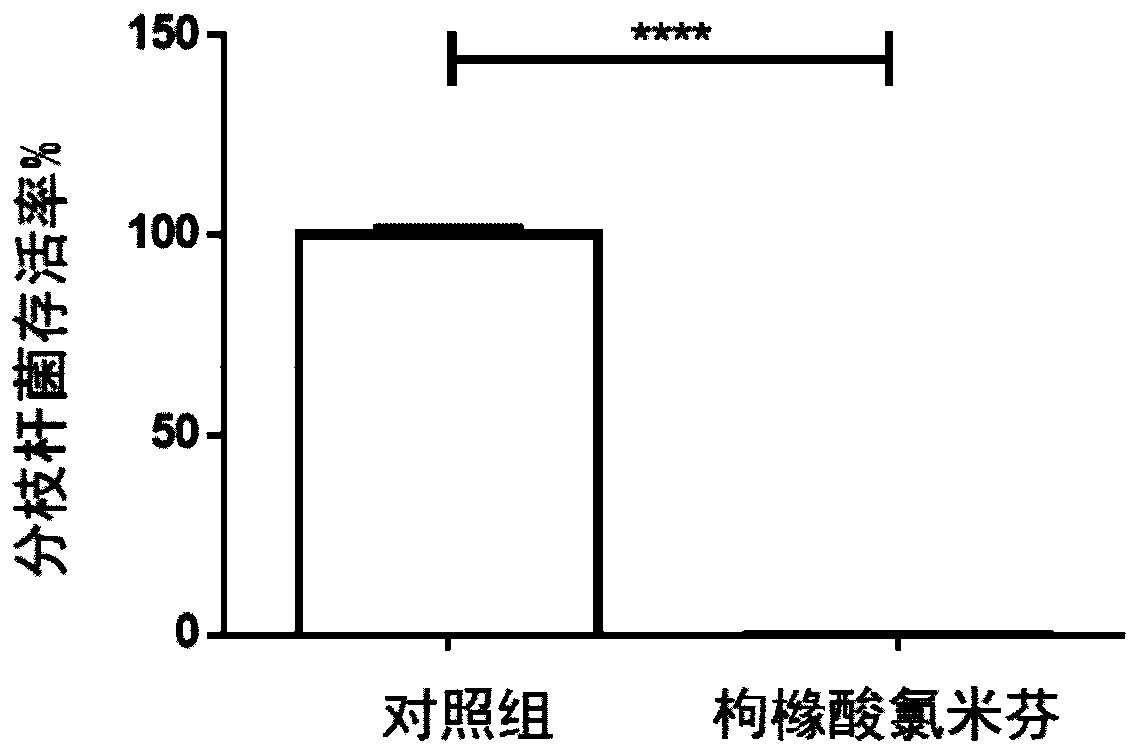
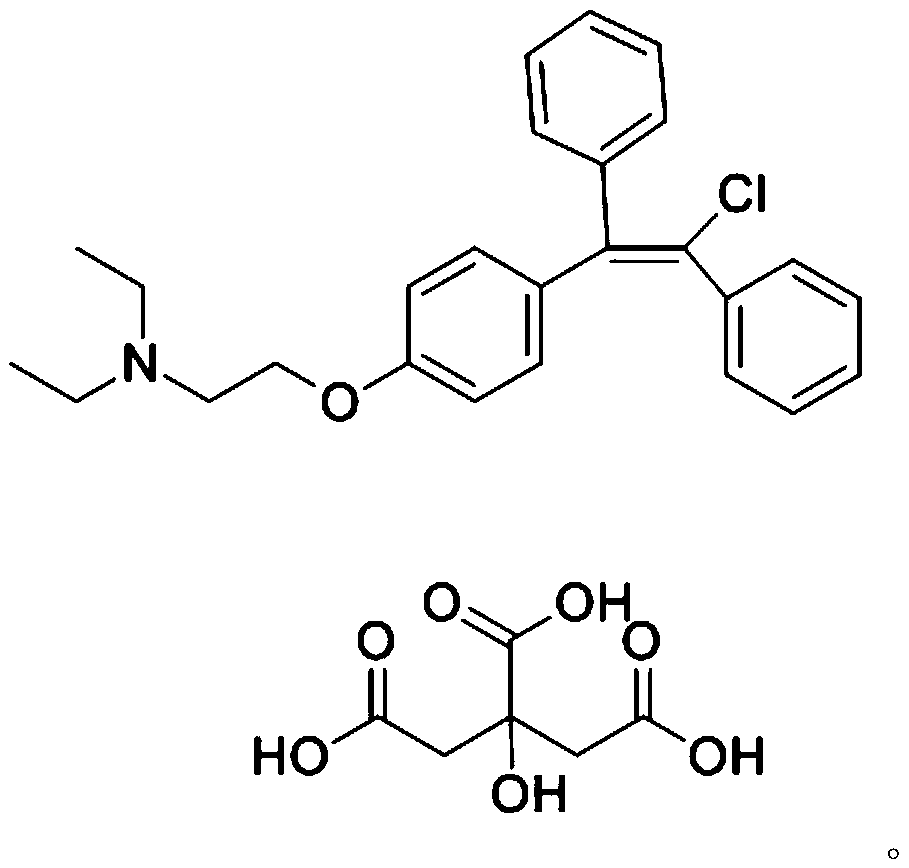
Application of clomifene citrate to anti-mycobacterium tuberculosis medicines
InactiveCN110354108AHas an anti-tuberculosis effectGood development valueAntibacterial agentsOrganic active ingredientsClomifene citrateOvarian follicle
The invention relates to application of clomifene citrate to anti-mycobacterium tuberculosis medicines. Clomifene citrate has estrogenic and antiestrogenic properties that appear to prevent the release of gonadotropins, follicle-stimulating hormone and luteinizing hormone, thus leading to follicular development and maturation, ovulation and subsequent corpus luteum development and function and then resulting in pregnancy. It is found through researches that the clomifene citrate has an antitubercular effect and has good development value. The application of the clomifene citrate to anti-mycobacterium tuberculosis medicines and the obvious anti-mycobacterium tuberculosis effect of the clomifene citrate are disclosed for the first time.
Owner:SHENZHEN UNIV
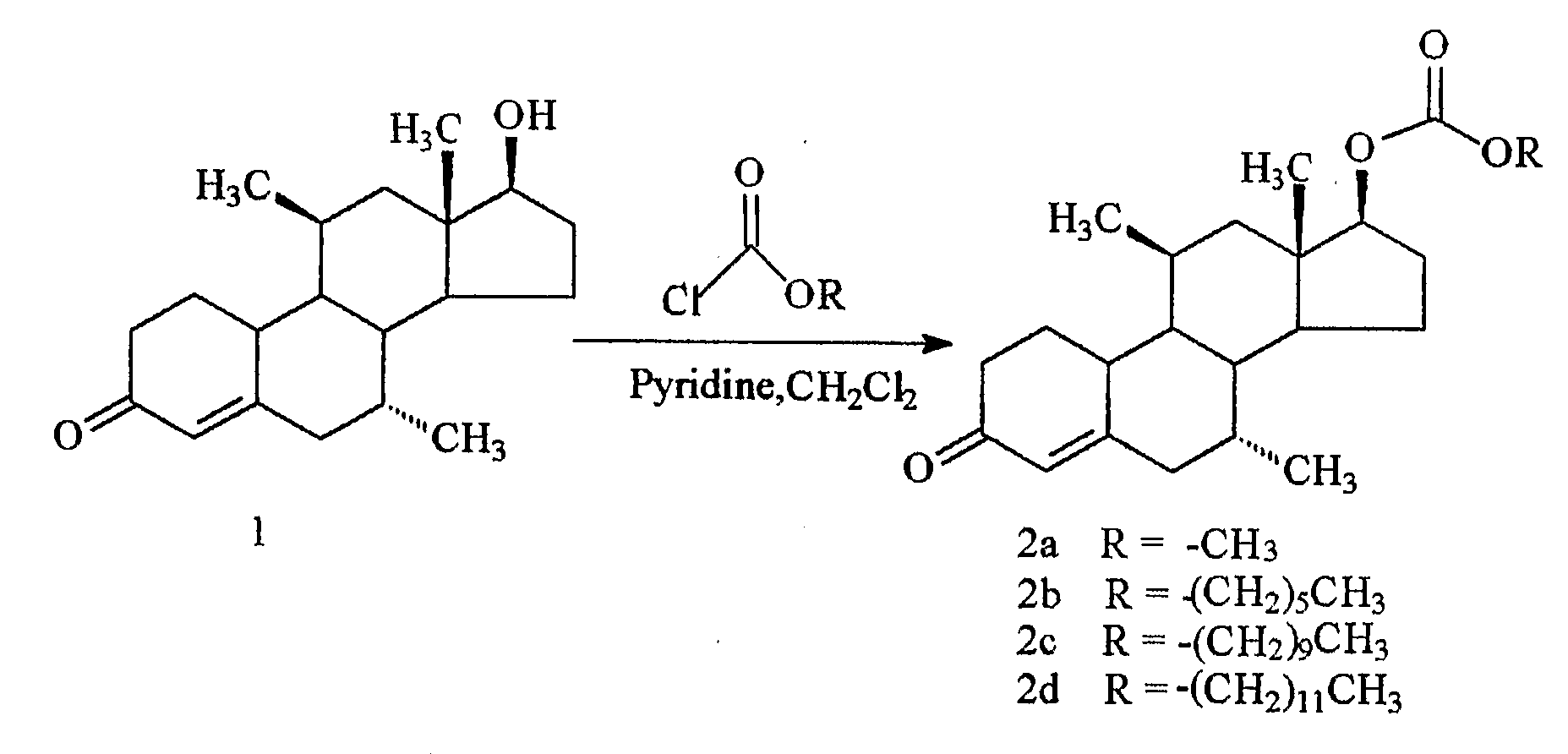
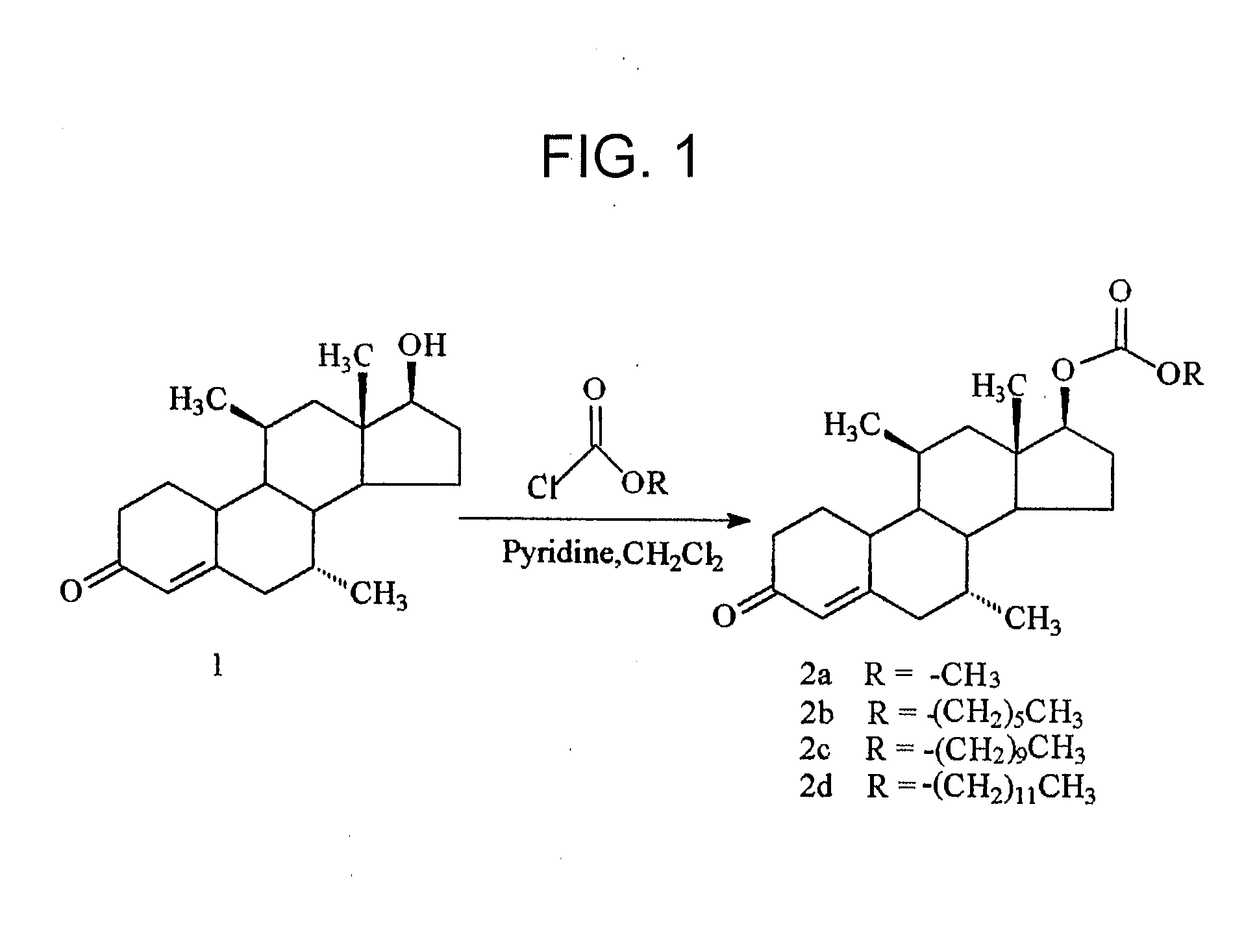
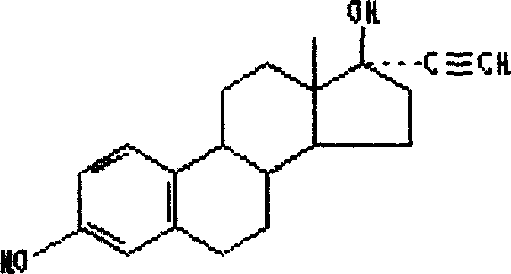
Nandrolone 17 Beta-Carbonates
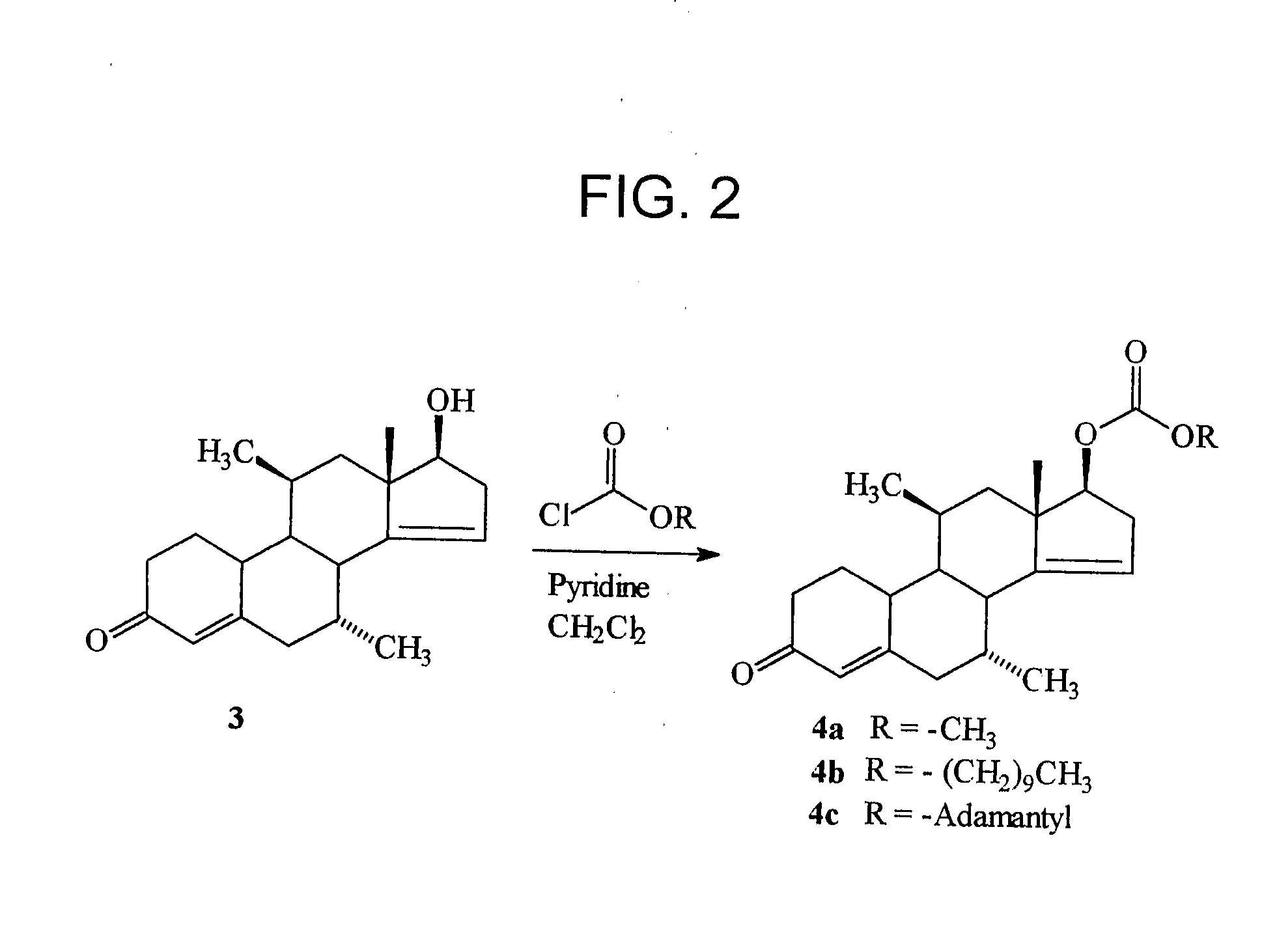
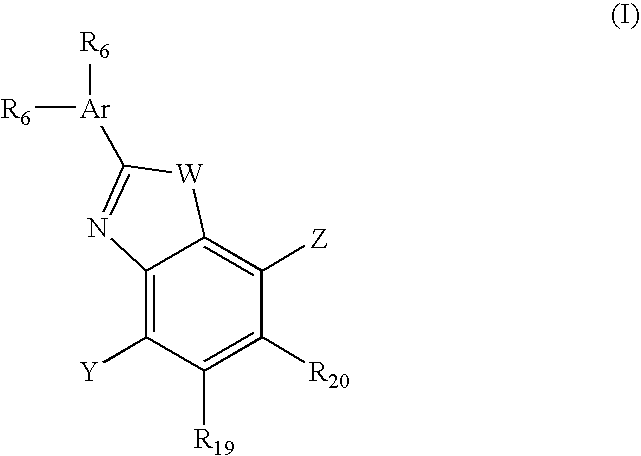
ActiveUS20080167283A1Inhibition releaseOrganic active ingredientsMuscular disorderLutenizing hormoneAnabolic Agents
Disclosed are compounds of the formula (I) wherein R is C1-C30 alkyl, which may be optionally further substituted with one or more C5-C8 cycloalkyl groups, or a C5-C12 cycloalkyl, which may be optionally substituted with one or more C1-C30 alkyl groups, R′ is hydrogen or lower alkyl, R″ is a C1-C30 alkyl or halo, and the bond between C14 and C15 can be a single bond or double bond. Also disclosed are pharmaceutical compositions comprising such compounds and methods of use thereof. These compounds can find use in treating a number of diseases or conditions such as hypogonadism, hypergonadism, osteoporosis, and anemia, in providing hormonal therapy and contraception, as an anabolic agent, and in suppressing the release of hormones such as the luteinizing hormone.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
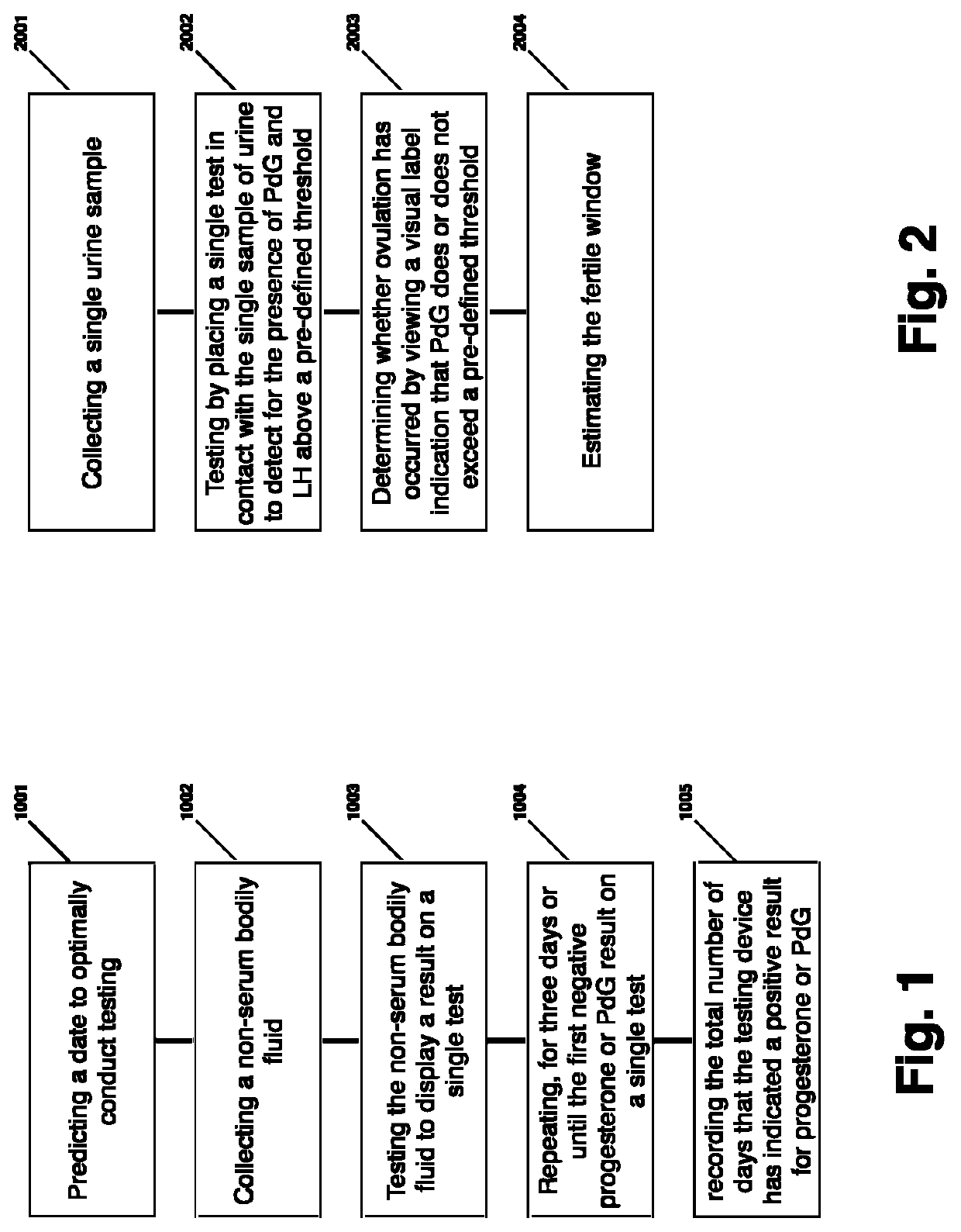
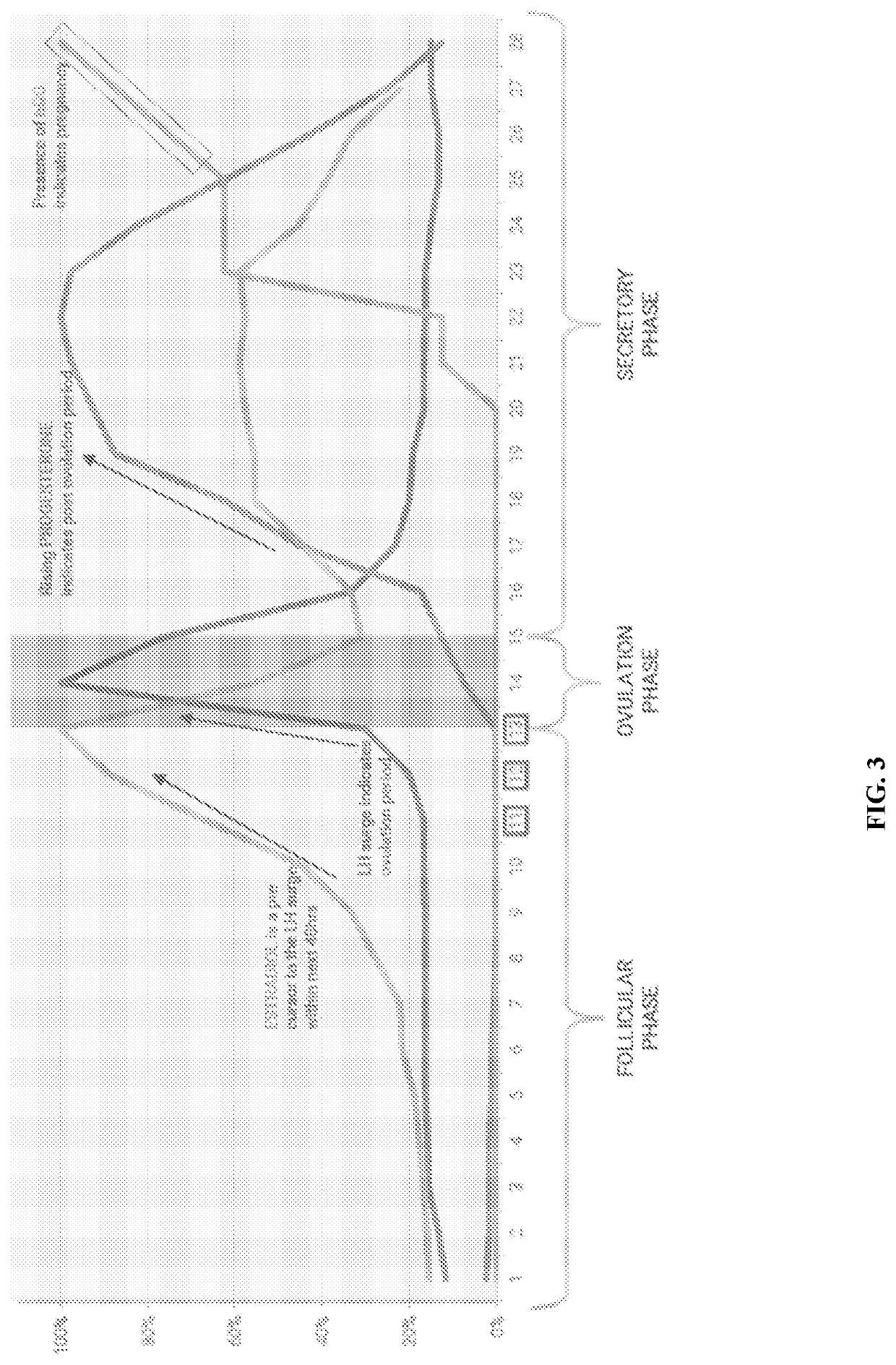
Method for evaluating urine of a subject to estimate the fertile window by evaluating for the presence of analytes of estrogen and progesterone
Disclosed herein are devices, systems, methods and kits for performing immunoassay tests to detect for at least progesterone or analytes of progesterone on a sample in association with diagnosing problems and issues associated with corpus luteum functionality. The immunoassay devices and methods may be used in conjunction with diagnostic reader systems and / or a base unit for obtaining a sensitive readout of the immunoassay results. The methods disclosed herein may also incorporate steps associated with evaluating the urine of a sample for the presence of an estrogen metabolite and / or luteinizing hormone.
Owner:MFB FERTILITY INC
Sustained release composition
InactiveCN101001640APeptide/protein ingredientsMetabolism disorderLHRH AgonistLuteinising hormone releasing hormone
A sustained release apparatus including at least one sustained release mini-implant or pellet; the or each mini-implant or pellet including: a sustained release support material; and a pharmaceutical composition including a Luteinising Hormone Releasing Hormone (HLRH) agonist and / or antagonist component the size and / or number and / or payload of mini-implant(s) or pellet(s) providing, release of LHRH agonist and / or antagonist at, or above, a desired threshold level for treatment of a selected indication, the apparatus providing approximately zero order release of the LHRH agonist and / or antagonist.
Owner:SMART DRUG SYST
Benzooxazole and benzothiazole antagonists of gonadotropin releasing hormone receptor
InactiveUS7531542B2Organic active ingredientsOrganic chemistryGonadotropin-releasing hormone receptorBenzoxazole
The present invention relates to Gonadotropin Releasing Hormone (GnRH, also known as Luteinizing Hormone Releasing Hormone) receptor antagonists.
Owner:WYETH LLC
Method for the treatment of prostate cancer
A method for the treatment of advanced prostate cancer comprises administering to a patient suffering from advanced prostate cancer an androgen suppressing amount of a luteinizing hormone releasing hormone agonist analog and an amount of calcitriol sufficient to enhance the effectiveness of the luteinizing hormone releasing hormone agonist analog against the cancer relative to treatment with the luteinizing hormone releasing hormone agonist analog alone. Preferably the calcitriol is in the form of a stabilized, injectable solution of calcitriol in isotonic saline containing about 1 to about 30 milligrams per milliliter of calcitriol and a sufficient quantity of nonionic surfactant to solubilize the calcitriol therein. Preferably the a luteinizing hormone releasing hormone agonist analog is a nonapeptide or decapeptide agonist, such as leuprolide, goserelin or salts thereof. The method of the present invention affords a surprisingly improved efficacy for treatment of advanced prostate cancer such as androgen-independent prostate cancer (AIPC) or hormone refractory prostate cancer (HRPC) in comparison to treatment with a luteinizing hormone releasing hormone agonist analog alone.
Owner:GENIX THERAPEUTICS
Method for producing and purifying hybrid or non-hybrid recombinant glycoprotein hormones, hybrid or non-hybrid recombinant glycoprotein hormones, expression vectors and uses of the recombinant glycoprotein hormones
PendingUS20220267401A1Hormone peptidesPeptide/protein ingredientsEquine chorionic gonadotropinPhysiology
Disclosed is a method for producing hybrid or non-hybrid recombinant glycoprotein hormones, for example the recombinant equine chorionic gonadotropin (r-eCG), the hybrid recombinant chorionic gonadotropin, the recombinant thyroid-stimulating hormone (r-TSH), the recombinant luteinising hormone (r-LH), the luteinising hormone and the recombinant follicle-stimulating hormone (r-FSH). In addition, the present invention relates to the recombinant glycoprotein hormones comprising the equine α and β subunits, inter alia, the α subunit of mammals and equine β subunit, where the two subunits are fused in a simple chain, and chain-modifying agents, which hormones are easier to purify, more homogeneous, easier to produce on an industrial scale without using animals, in comparison with the wild glycoprotein hormone The hormones are useful for inducing animal reproduction, ovulation induction, superovulation induction, follicle growth, oestrus induction, anoestrus reversal, puberty induction in animals, both with and without commercial interest.
Owner:UNIV DE SAO PAULO +1
Use of Euycoma longifolia extract in alleviating symptoms and/or conditions associated with hormonal imbalance in females
ActiveUS11058737B2Safe and effective and of symptomSafe and effective treatmentEndocrine system disorderPlant ingredientsHormonal imbalanceLutenizing hormone
The present invention is directed to a new kind of medicinal value or health care function of Eurycoma longifolia extracts, particularly in the treatment or alleviation symptoms and / or conditions associated to hormonal imbalance in females, including menopause and its related symptoms. In one aspect, the present invention discloses the use of a composition comprising a therapeutically effective amount of Eurycoma longifolia extract in the manufacture of a medicament for the alleviation of symptoms and / or conditions associated to hormonal imbalance in females, including menopause and its related symptoms. The alleviation of the symptoms and / or conditions according to the present invention is characterised by inducing a change in the hormonal contents of estrogen, progesterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH). Also disclosed is the use of a pharmaceutical composition comprising the therapeutically effective amount of Eurycoma longifolia extract.
Owner:BIOTROPICS MALAYSIA BERHAD
Use of euycoma longifolia extract in alleviating symptoms and/or conditions associated with hormonal imbalance in females
ActiveUS20200129573A1Reduce productionSafe and effective and of symptomEndocrine system disorderPlant ingredientsHormonal imbalanceLutenizing hormone
The present invention is directed to a new kind of medicinal value or health care function of Eurycoma longifolia extracts, particularly in the treatment or alleviation symptoms and / or conditions associated to hormonal imbalance in females, including menopause and its related symptoms. In one aspect, the present invention discloses the use of a composition comprising a therapeutically effective amount of Eurycoma longifolia extract in the manufacture of a medicament for the alleviation of symptoms and / or conditions associated to hormonal imbalance in females, including menopause and its related symptoms. The alleviation of the symptoms and / or conditions according to the present invention is characterised by inducing a change in the hormonal contents of estrogen, progesterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH). Also disclosed is the use of a pharmaceutical composition comprising the therapeutically effective amount of Eurycoma longifolia extract.
Owner:BIOTROPICS MALAYSIA BERHAD
A kind of luteinizing hormone analogue and preparation method thereof
ActiveCN107827987BGood practicality and commercial valueNucleic acid vectorHybrid peptidesHormone analogLutenizing hormone
Owner:HUAQIAO UNIVERSITY +1
3',3'-N-substituted macrolide LHRH antagonists
Disclosed are 3',3'-N-bisdesmethyl-3',3'-N-bis-substituted-6-O-methyl-11-deoxy-11,12 -cyclic carbamate erythromycin A derivatives of formula (I) which are antagonists of lutenizing hormone-releasing hormone (LHRH). Also disclosed are pharmaceutical compositions comprising the compounds, methods of using the compounds and the process of making the same.
Owner:ABBOTT LAB INC
Cancer treatment combination compositions, methods and uses
ActiveUS10603335B2Effective therapyLow cytotoxicityKetone active ingredientsCyclic peptide ingredientsDiseaseLutenizing hormone
The invention provides combinations and formulations including a luteinizing hormone-releasing hormone (LHRH) or a LHRH analog; and curcumin or a curcumin analog. LHRH or LHRH analog can be fused or conjugated to a curcumin or curcumin analog. Invention combinations and formulations can also include an anti-cell proliferative drug. Invention combinations and formulations can be used for inhibiting proliferation of a cell; treating a hyperproliferative disorder; and treating a neoplasia, tumor, cancer or malignancy.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
LH liquid formulations
ActiveUS8664369B2Peptide/protein ingredientsPharmaceutical delivery mechanismPharmaceutical formulationLuteinizing hormone
Owner:MERCK SERONO SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/f53277eb-a339-4cb4-aff5-8085c35a42e7/US20060189618A1-20060824-C00001.png)
![4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/f53277eb-a339-4cb4-aff5-8085c35a42e7/US20060189618A1-20060824-C00002.png)
![4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-Substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/f53277eb-a339-4cb4-aff5-8085c35a42e7/US20060189618A1-20060824-C00003.png)
![4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/29818a27-cdfa-43ea-85b2-c51463c1e757/US07538113-20090526-C00001.png)
![4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/29818a27-cdfa-43ea-85b2-c51463c1e757/US07538113-20090526-C00002.png)
![4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor 4-substituted imidazo[4,5-c]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/29818a27-cdfa-43ea-85b2-c51463c1e757/US07538113-20090526-C00003.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/0928bf2c-5b7a-464d-a1f5-c15fce86653f/US07534796-20090519-C00001.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/0928bf2c-5b7a-464d-a1f5-c15fce86653f/US07534796-20090519-C00002.png)
![Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor Imidazo[4,5-b]pyridine antagonists of gonadotropin releasing hormone receptor](https://images-eureka.patsnap.com/patent_img/0928bf2c-5b7a-464d-a1f5-c15fce86653f/US07534796-20090519-C00003.png)