Sustained release composition
A composition and sustained-release technology, applied in drug combination, drug delivery, food science, etc., can solve problems such as treatment and response time lag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Study on the release of deslorelin acetate from solid sustained-release implants in pigs
[0198] Study Number: DSL-P-03-001
[0199] Research site: Pig Research and Training Center (Pig Research and TrainingCentre, PRTC)
[0200] Victorian Institute of Animal Science
[0201] Department of Primary Industries
[0202] 600 Sneydes Road
[0203] Werribee Victoria Australia 3030
[0204] Implants:
[0205] Three different implant formulations were evaluated.
[0206] Dosage Form No. 1 consists of deslorelin acetate and DOC and mannitol as additives.
[0207]Formulation No. 2 consists of deslorelin acetate and NaCl and dextran as additives.
[0208] Dosage form number 3 consists of deslorelin acetate without additives.
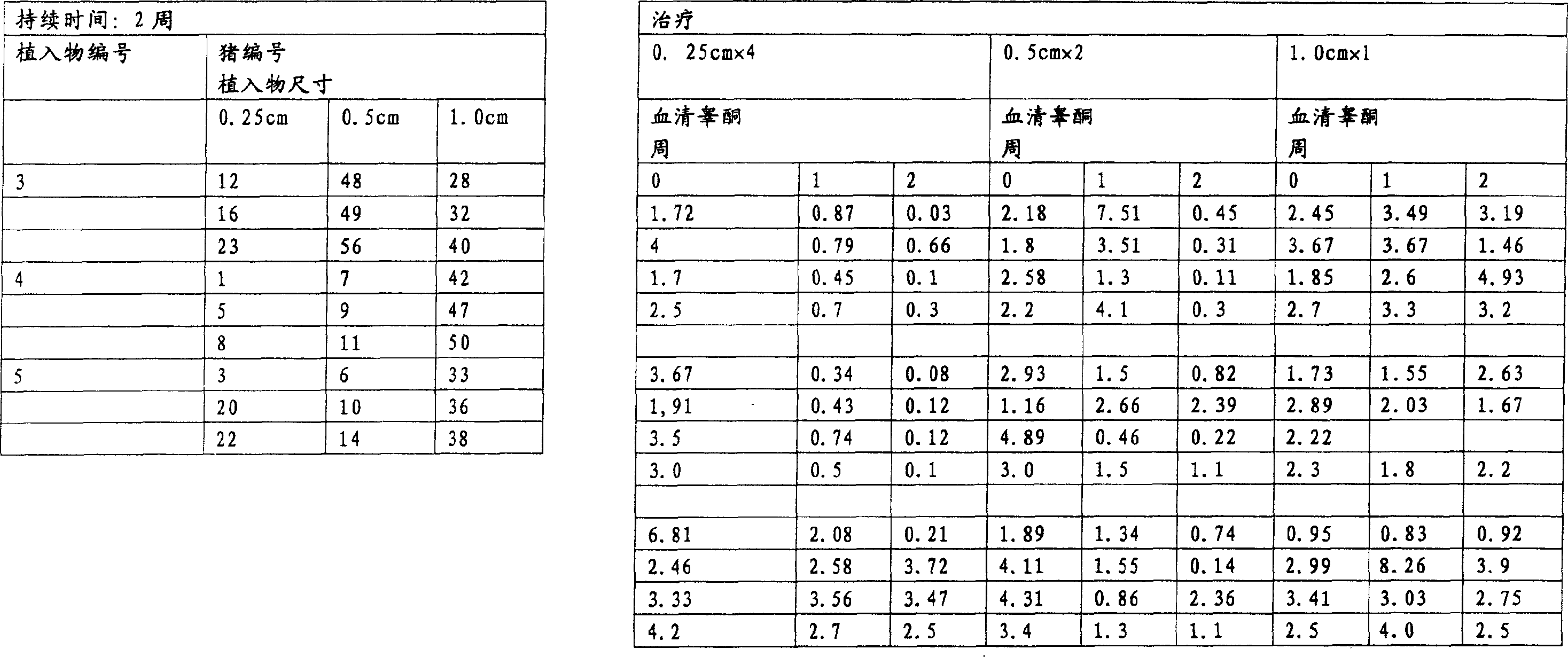
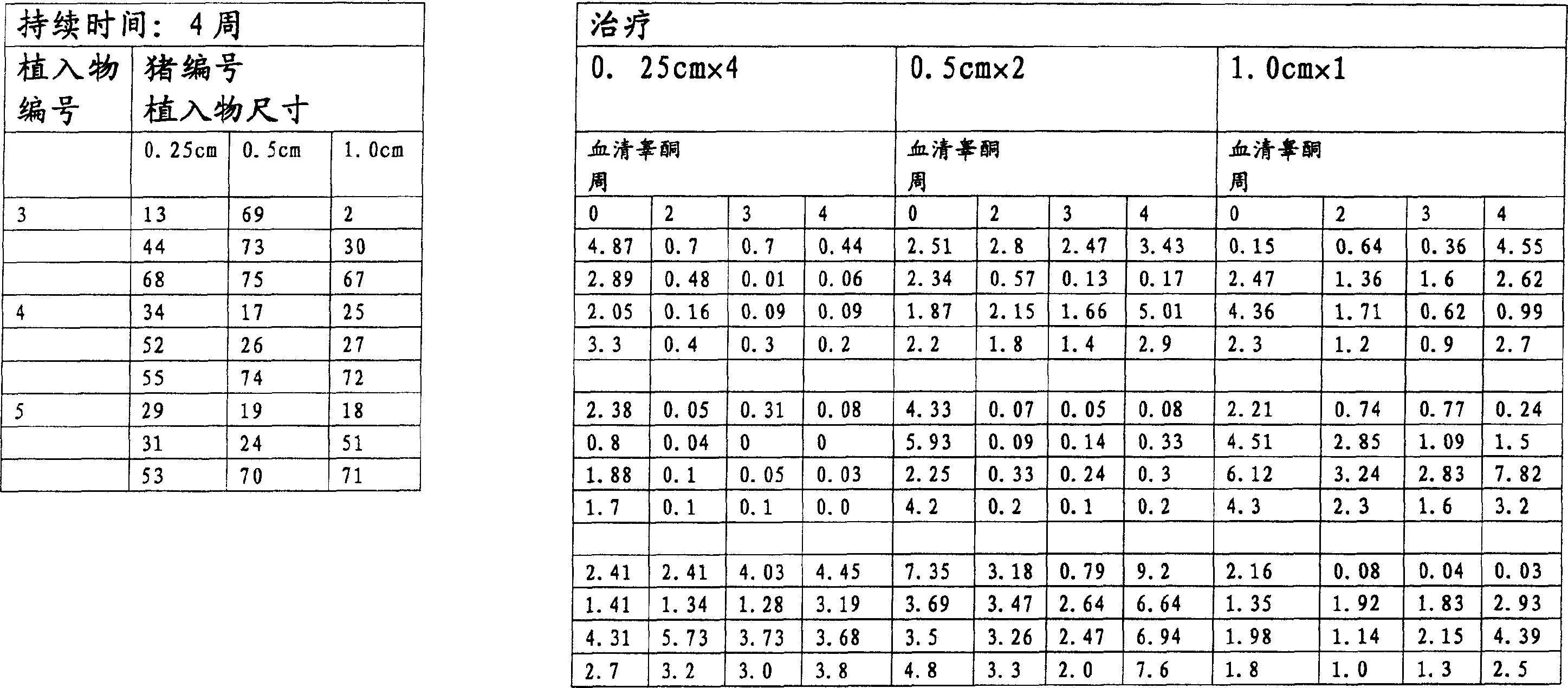
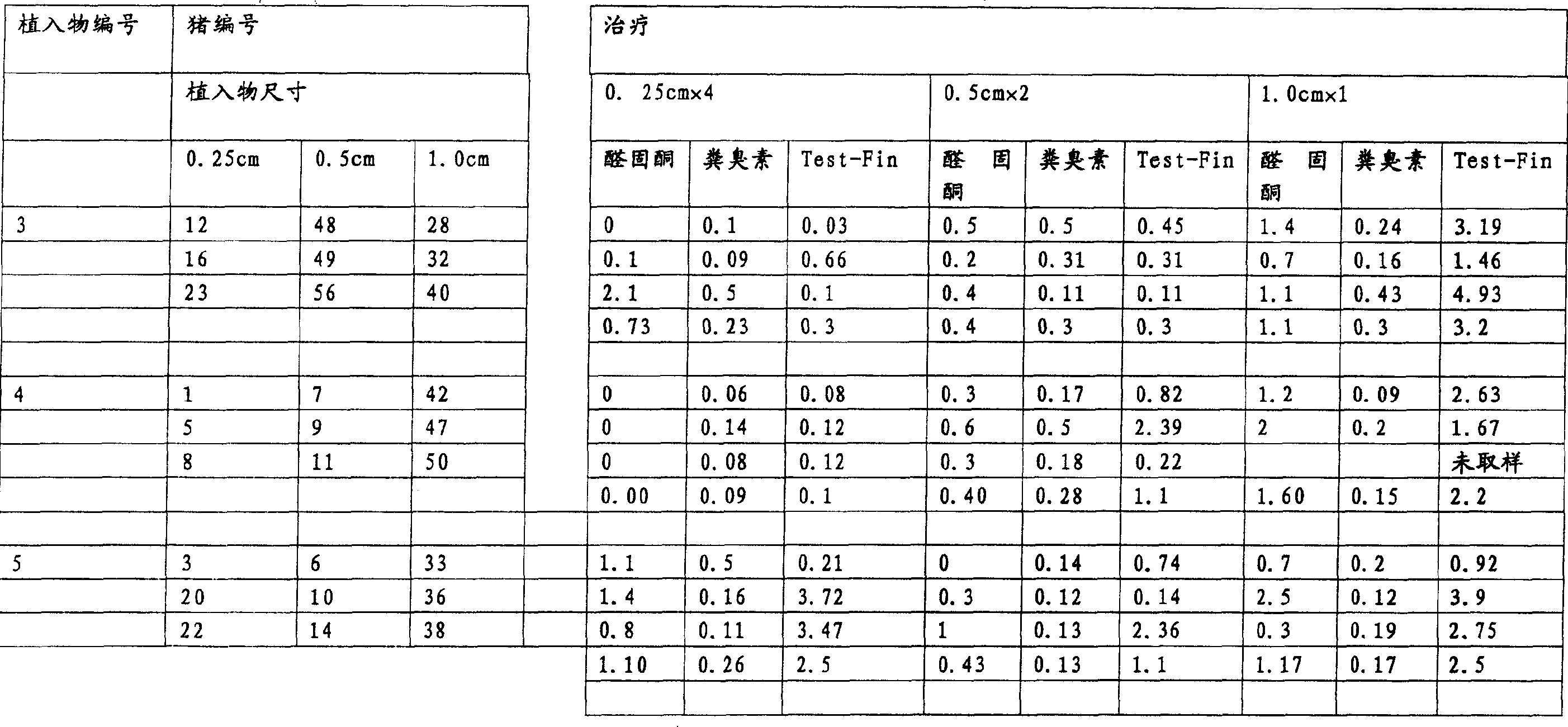
[0209] Implant size and duration of treatment:
[0210] Groups of pigs were treated with implants of different lengths and for different durations.
[0211] Pigs were treated with implants 1 cm, 0.5 cm or 0.25 cm in length. All implants have a di...
Embodiment 2
[0280] Study on the release of deslorelin acetate from porcine sustained-release implants
[0281] Study Number: DSL-P-04-001
[0282] Research site: Pig Research and Training Center (Pig Research and TrainingCentre, PRTC)
[0283] Victorian Institute of Animal Science
[0284] Department of Primary Industries
[0285] 600 Sneydes Road
[0286] Werribee Victoria Australia 3030
[0287] Implants:
[0288] Three different implants were evaluated.
[0289] Implant No. 3 consisted of 36% deslorelin acetate without additives and had an outer diameter of 1.5 mm.
[0290] Implant No. 4 consisted of 54% deslorelin acetate without additives and had an inner diameter of 1.5 mm.
[0291] Implant No. 5 consisted of 36% deslorelin acetate without additives and had an outer diameter of 2.0 mm.
[0292] Implant size and duration of treatment:
[0293] Groups of pigs were treated with implants of varying lengths, but all treatments totaled 1 cm total implants. Pigs were treated for ...
Embodiment 3
[0324] Study on the release of deslorelin acetate from porcine sustained-release implants
[0325] Study Number: DSL-P-04-002
[0326] Research site: Pig Research and Training Center (Pig Research and TrainingCentre, PRTC)
[0327] Victorian Institute of Animal Science
[0328] Department of Primary Industries
[0329] 600 Sneydes Road
[0330] Werribee Victoria Australia 3030
[0331] Implants:
[0332] The various implants were evaluated as follows.
[0333] Implant type 2 consisted of 54% deslorelin acetate and had an inner diameter of 1.4 mm and an outer diameter of 1.6 mm.
[0334] Implant size and duration of treatment:
[0335] Three groups of pigs were treated as follows.
[0336] · Group 1 - 10 boars - untreated (control)
[0337] · Group 2 - 5 boars - 9.6 mg deslorelin - treatment for 2 weeks
[0338] · Group 3 - 5 boars - 9.6 mg deslorelin - treatment for 4 weeks
[0339] Pigs were treated with 1 cm (ie 4 x 0.25 cm) implants cut to 0.25 cm length.
[034...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com