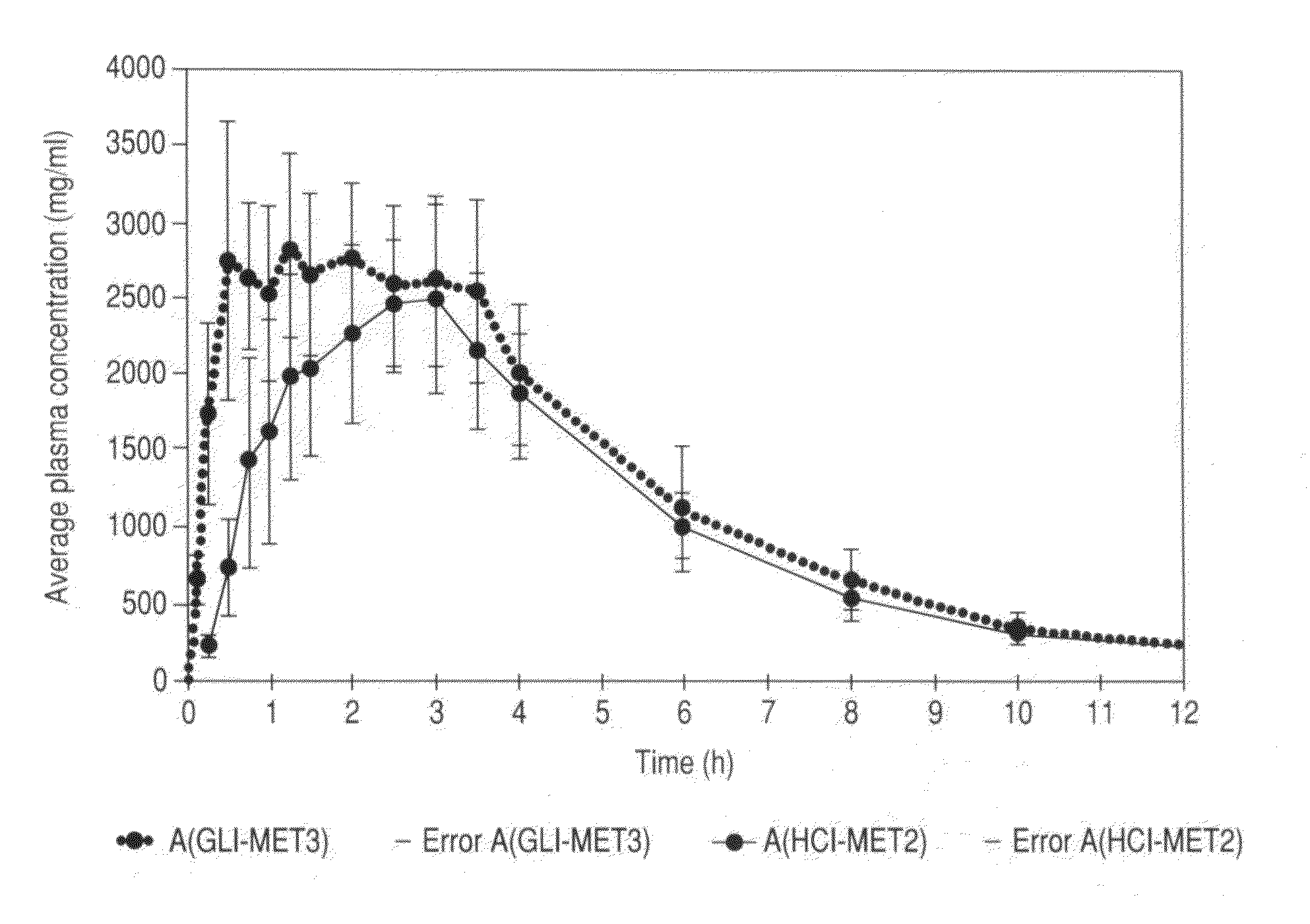
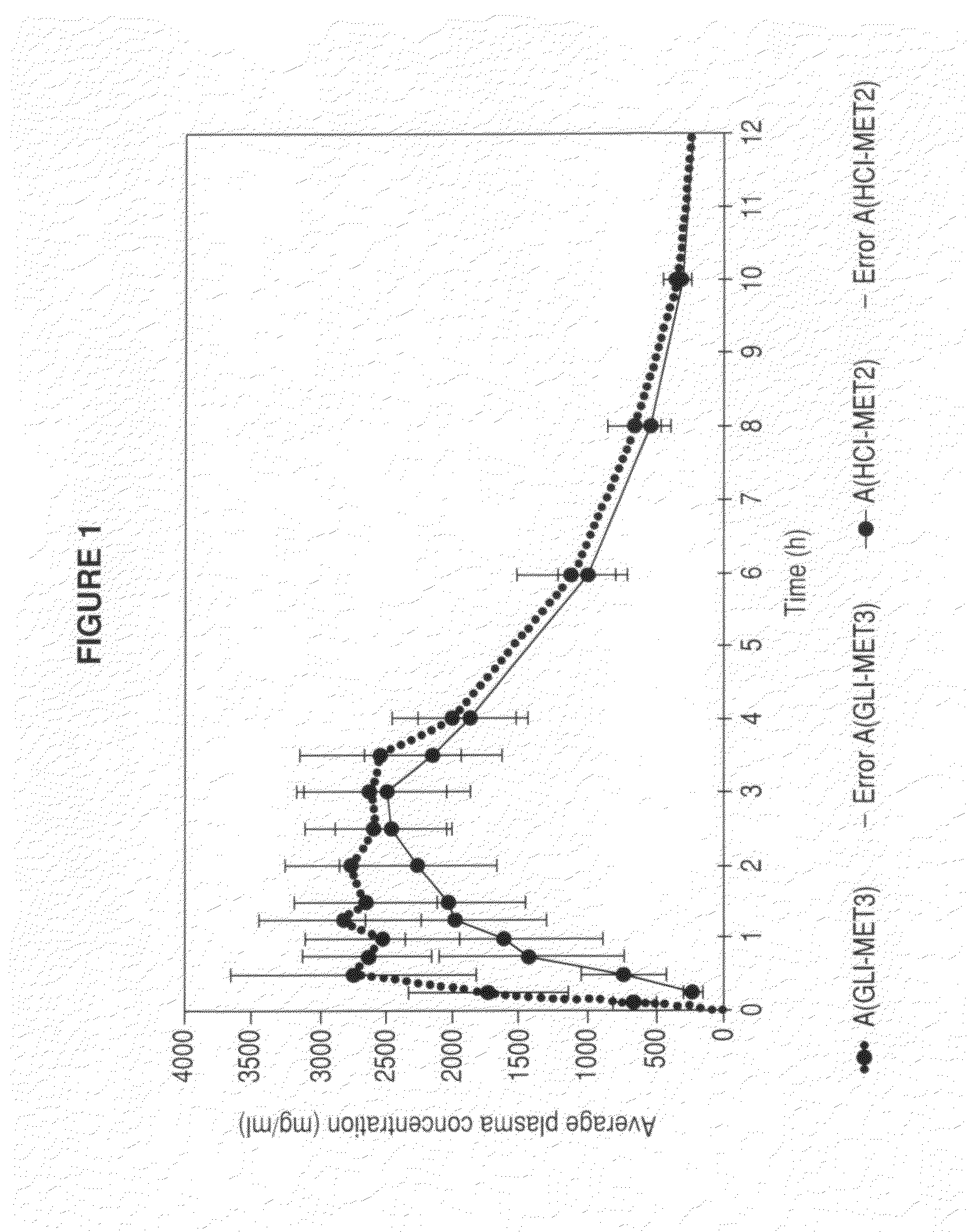
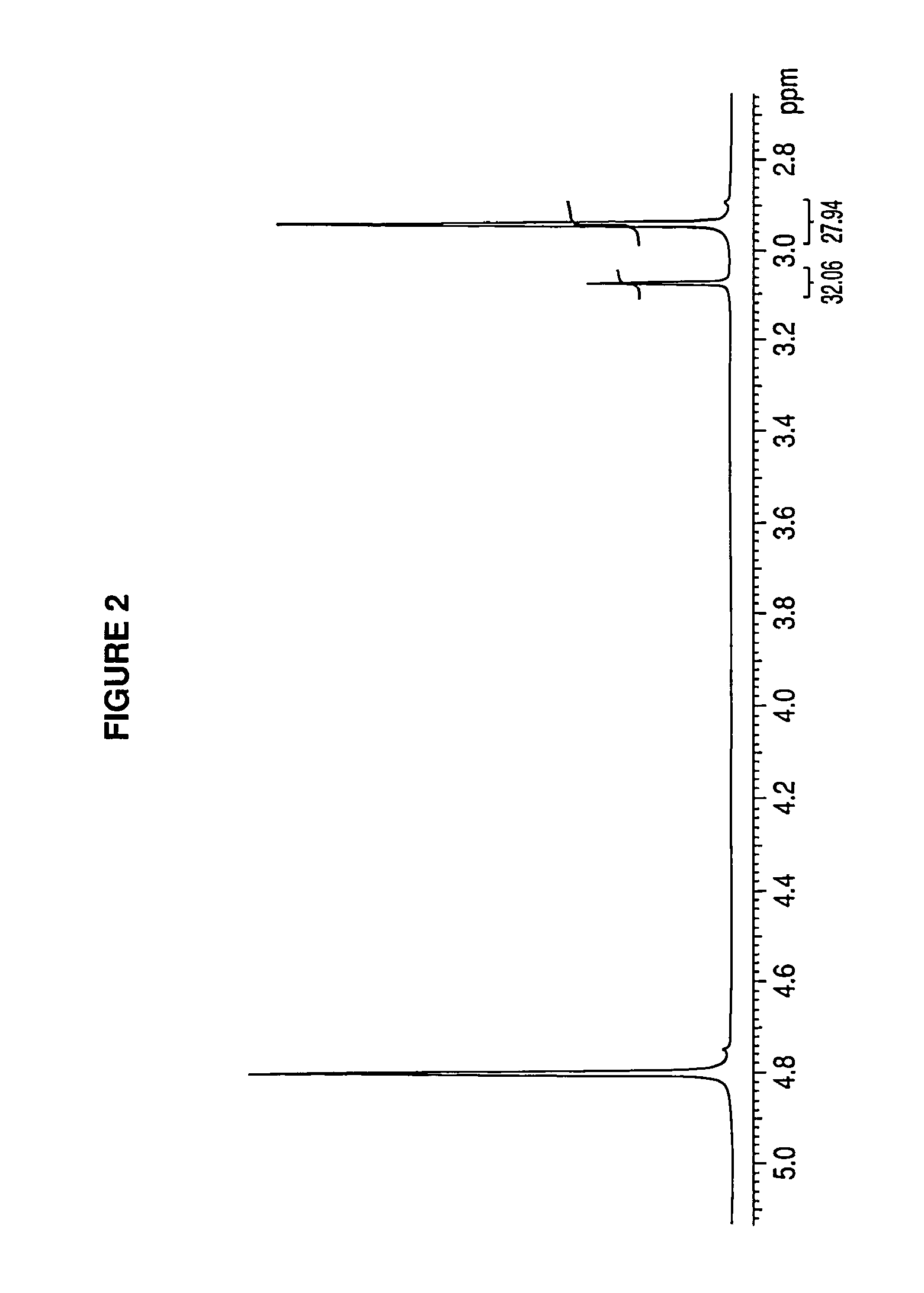
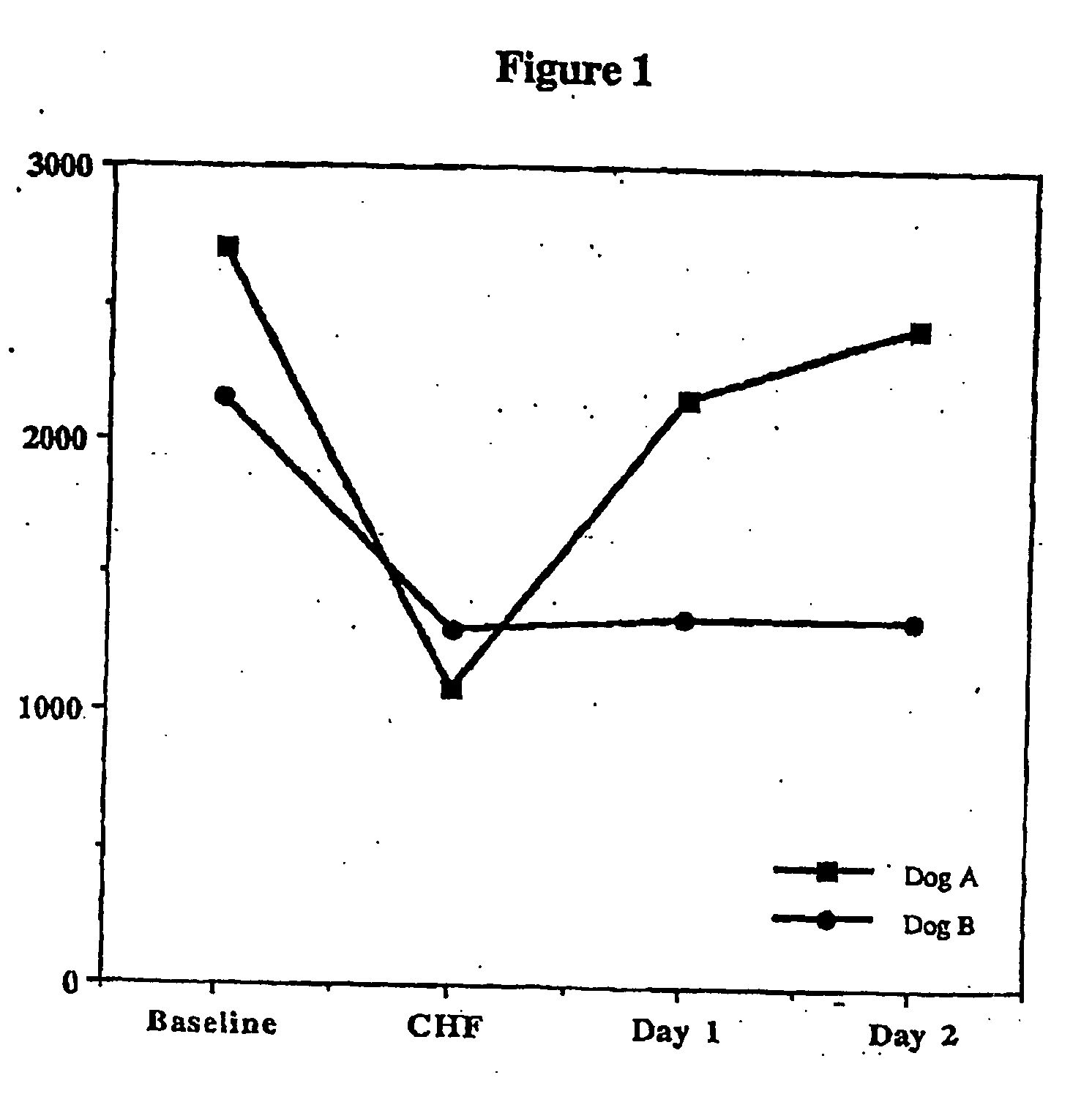
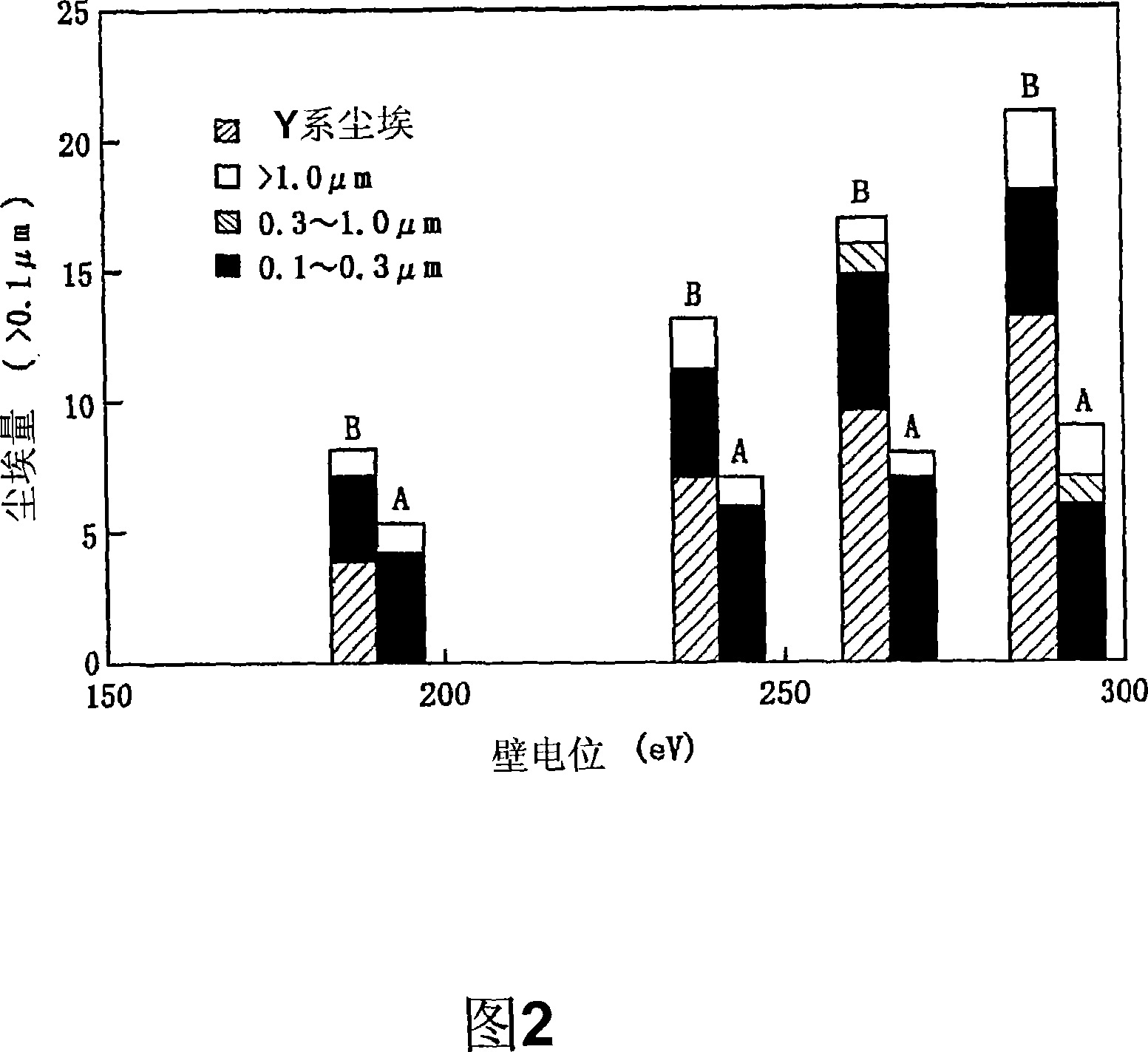
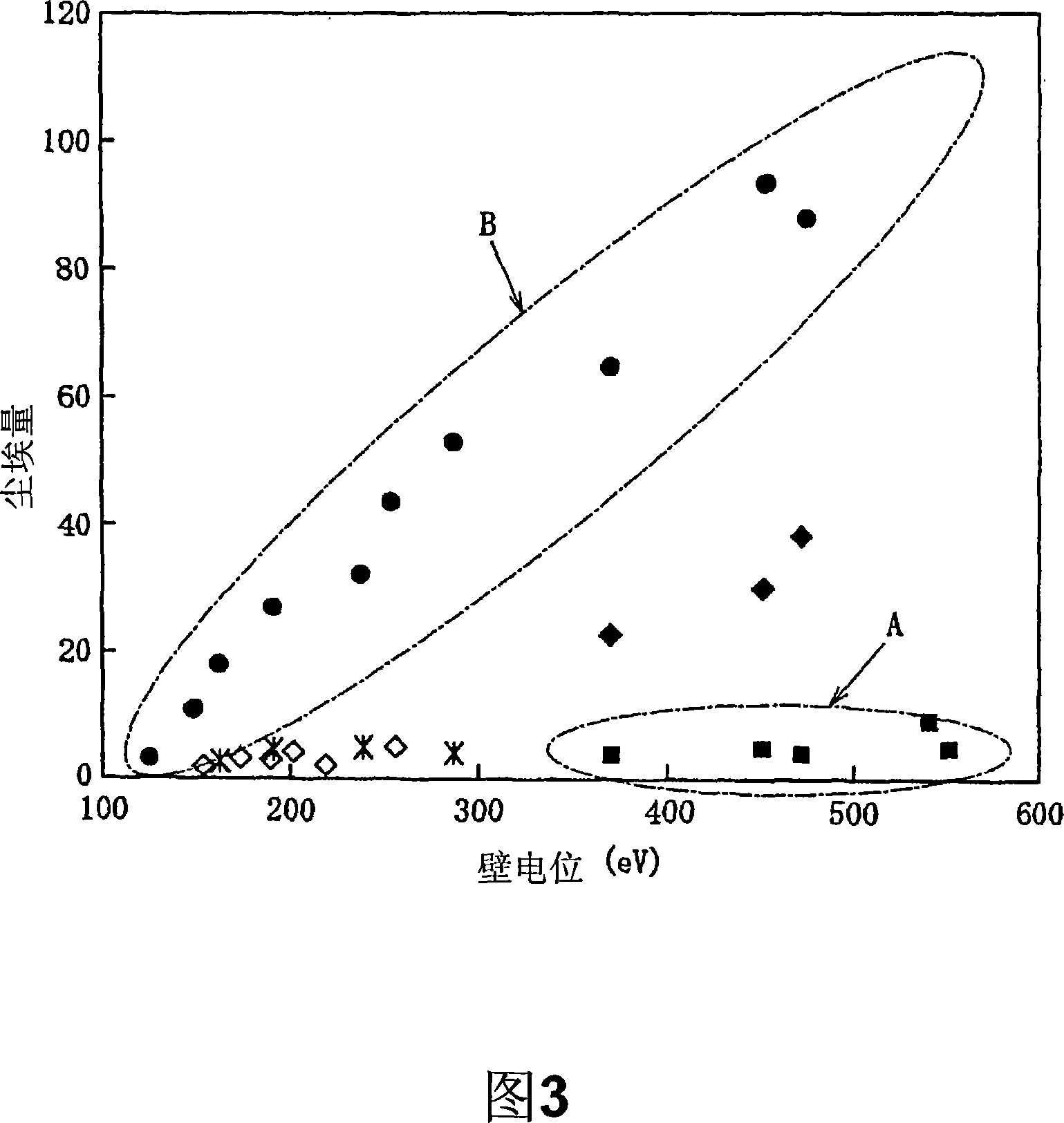
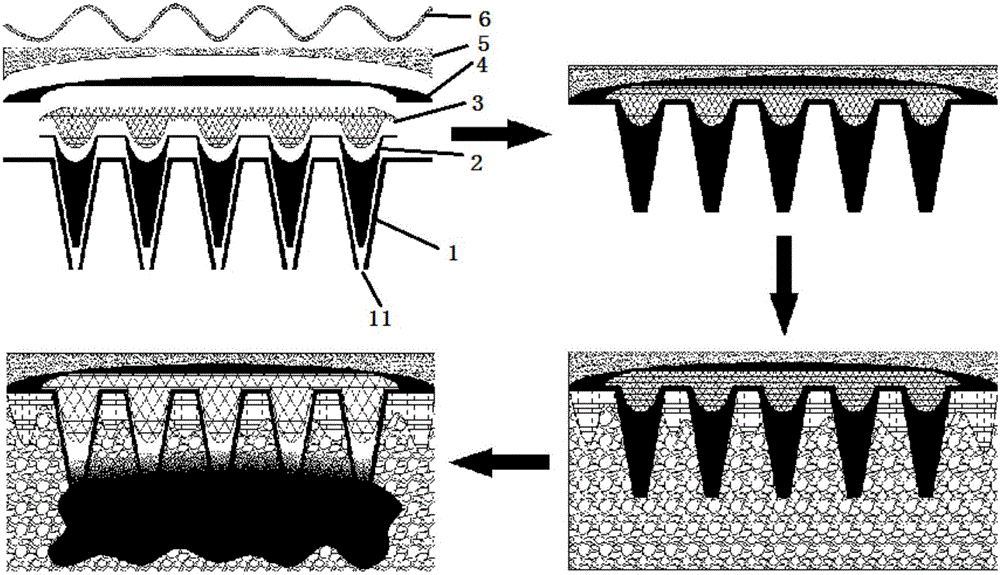
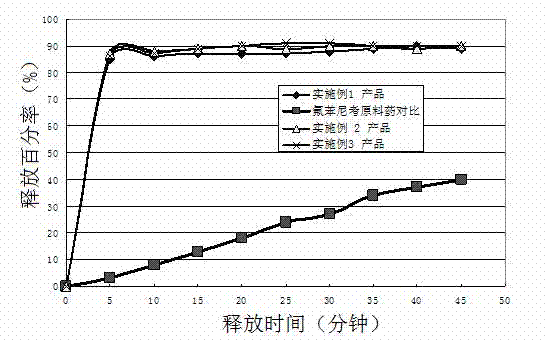
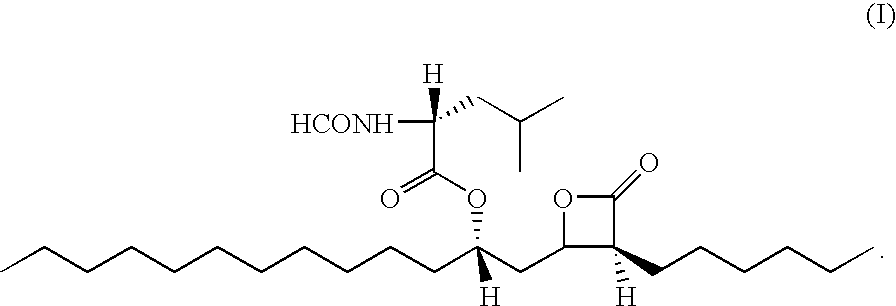
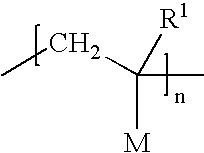
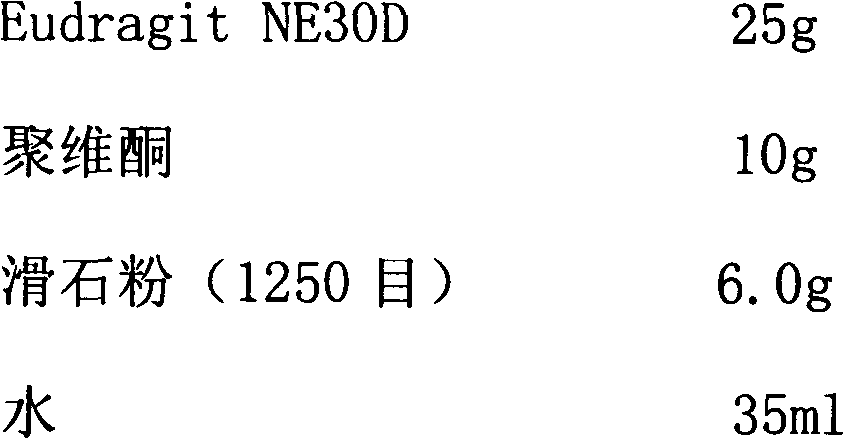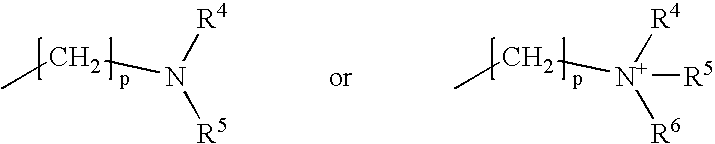Patents
Literature
91 results about "High plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A high level of plasma proteins in the blood is caused by hyperproteinemia, which can be a sign of many illnesses, both mild and serious, such as infection, dehydration, and lymphocytic leukemia. A high level of protein is usually a signal for more tests and examination to determine the underlying illness or disease.
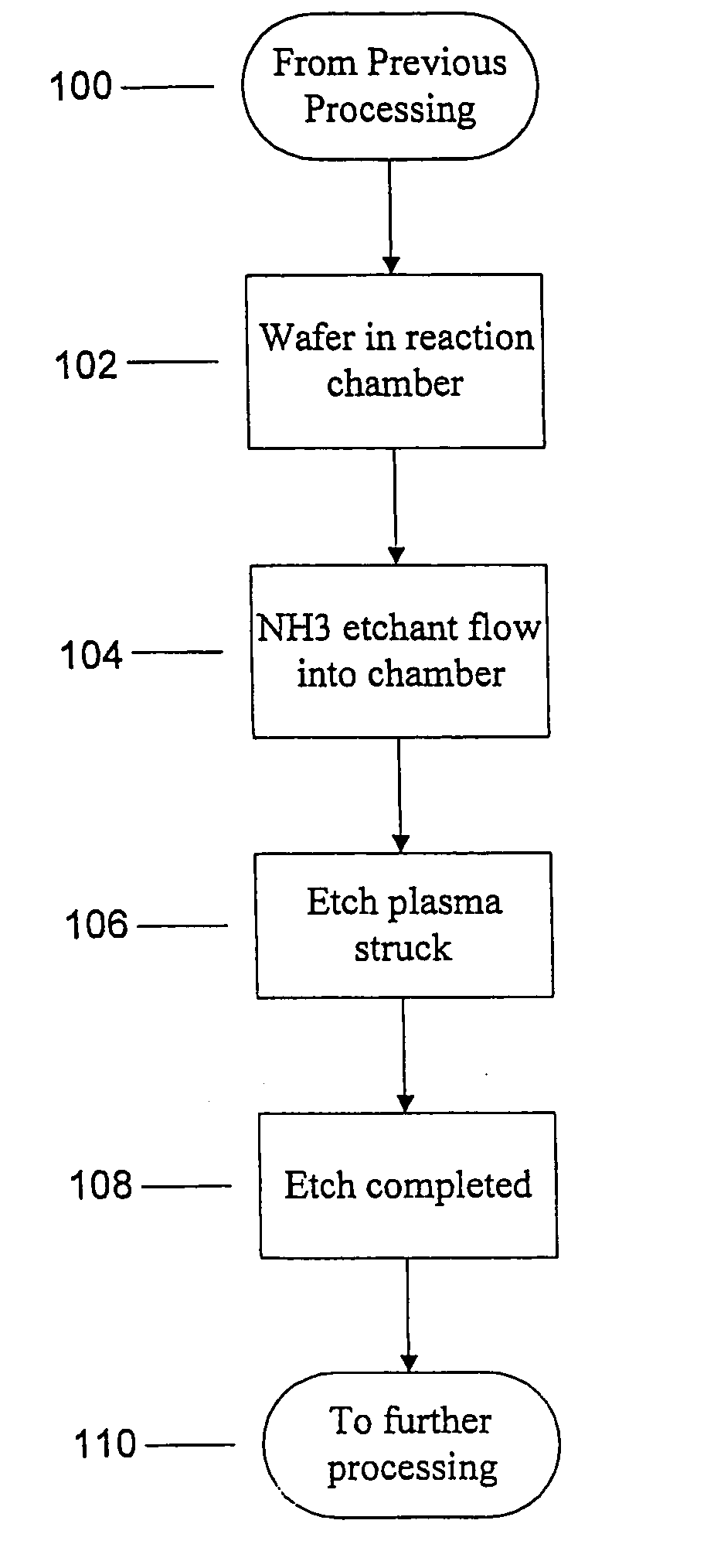
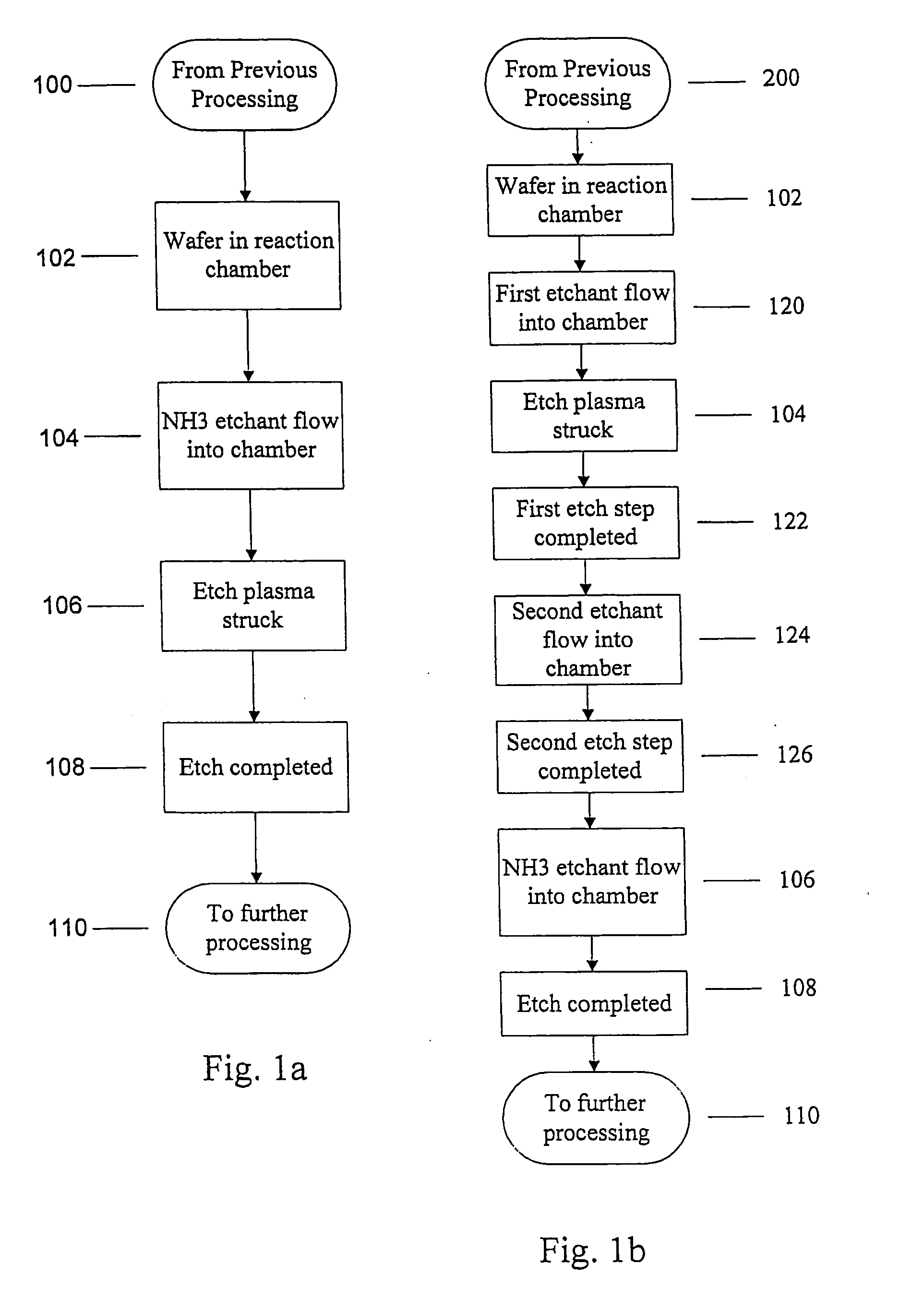
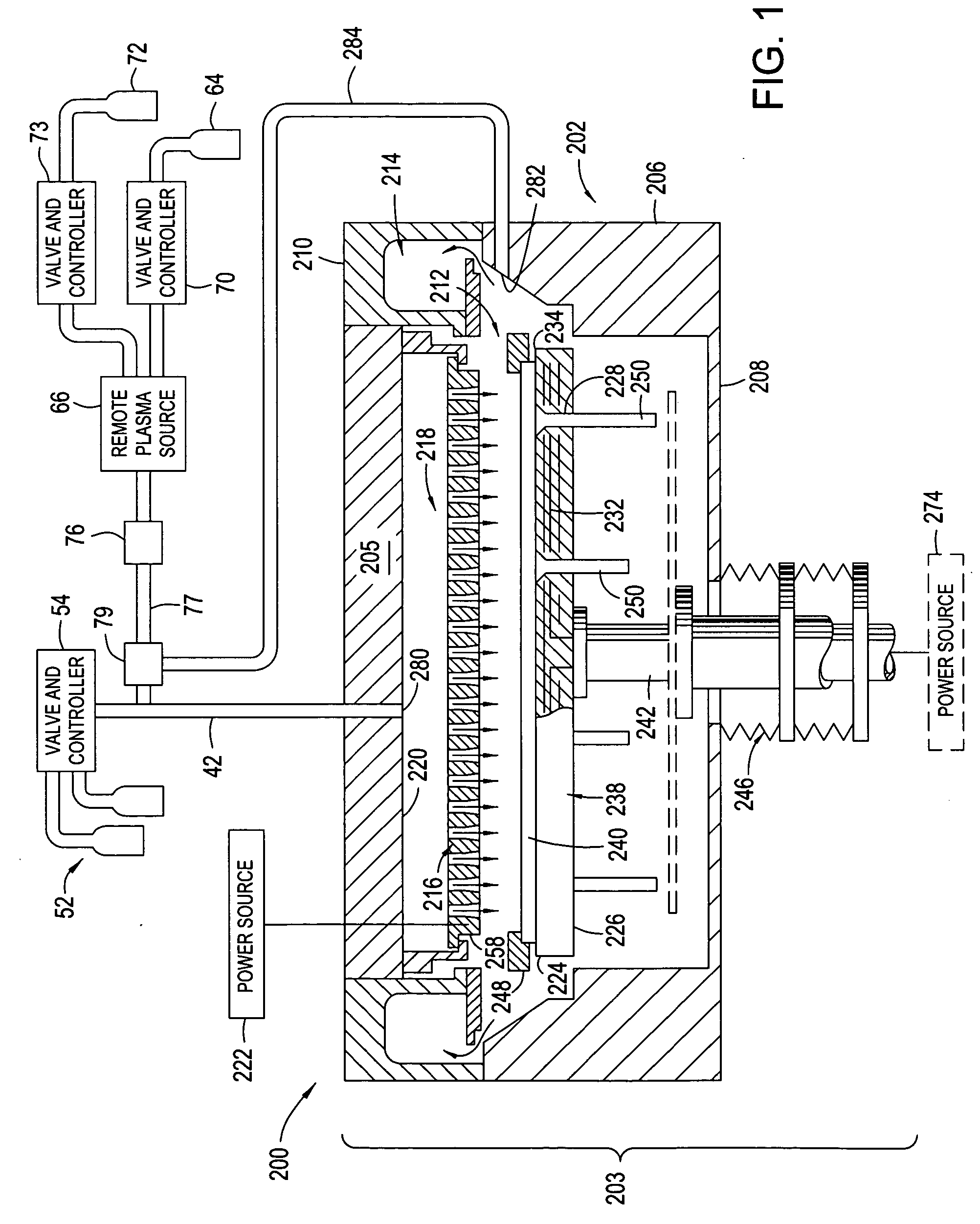
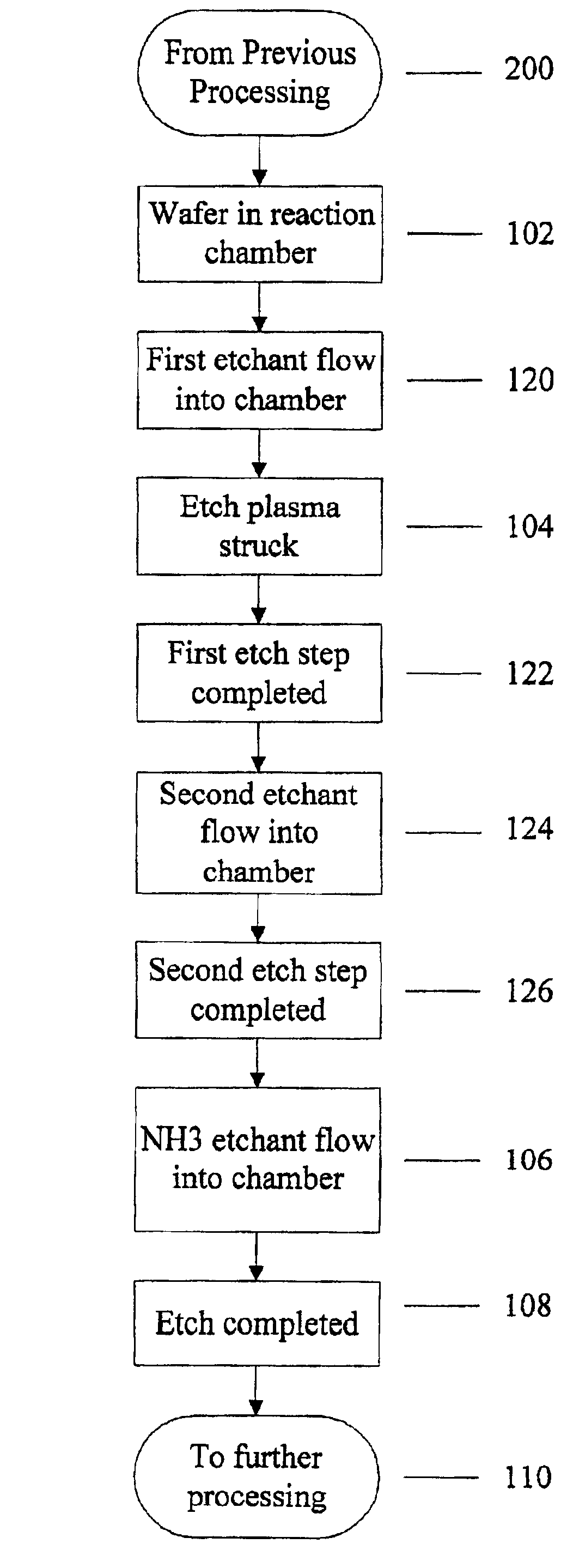
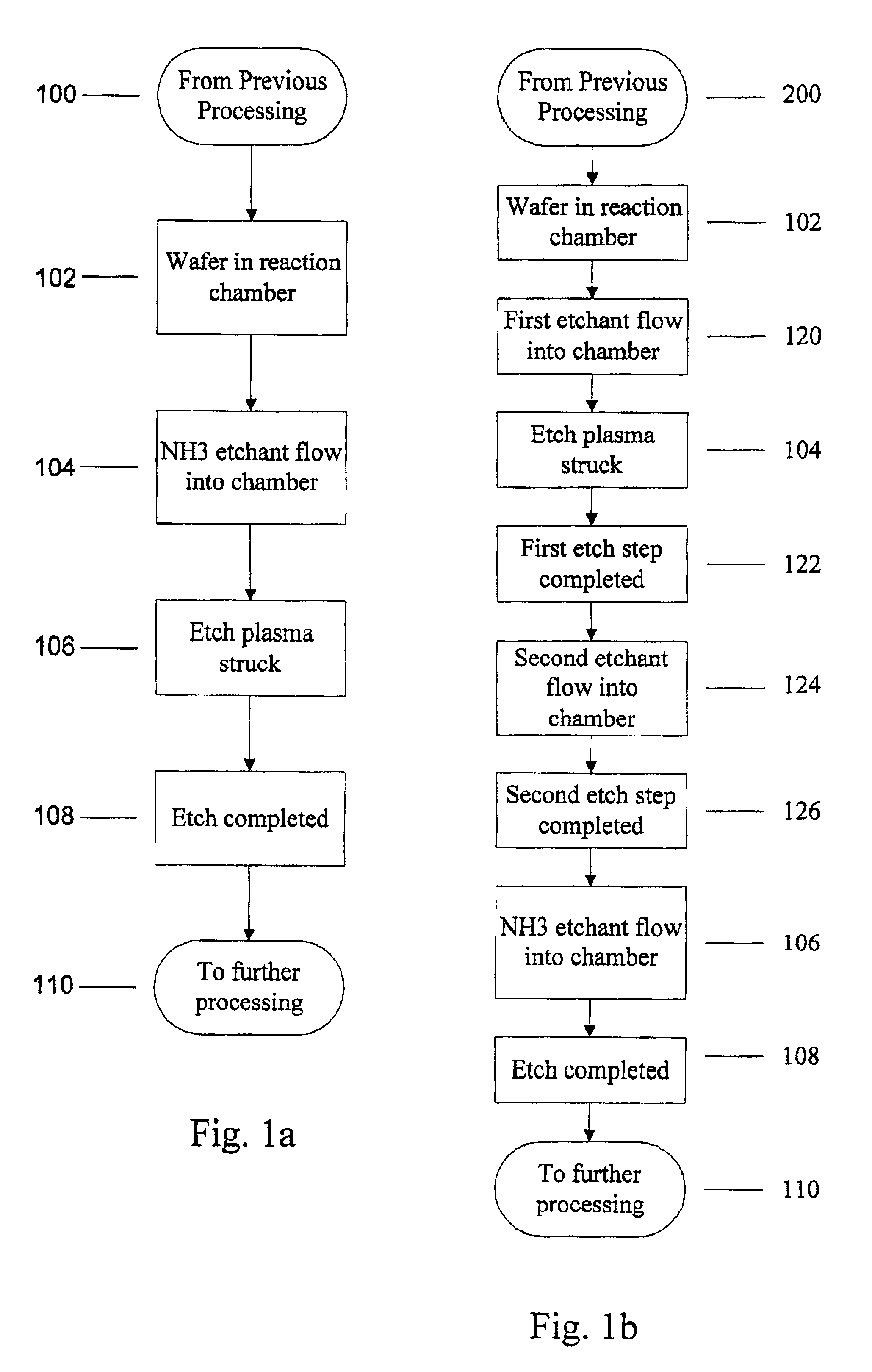
Use of ammonia for etching organic low-k dielectrics
InactiveUS20050003676A1Increase etch rateHigh selectivityDecorative surface effectsSemiconductor/solid-state device manufacturingProcess chemistryElectricity
Method for etching organic low-k dielectric using ammonia, NH3, as an active etchant. Processes using ammonia results in at least double the etch rate of organic low-k dielectric materials than processes using N2 / H2 chemistries, at similar process conditions. The difference is due to the much lower ionization potential of NH3 versus N2 in the process chemistry, which results in significantly higher plasma densities and etchant concentrations at similar process conditions.
Owner:LAM RES CORP
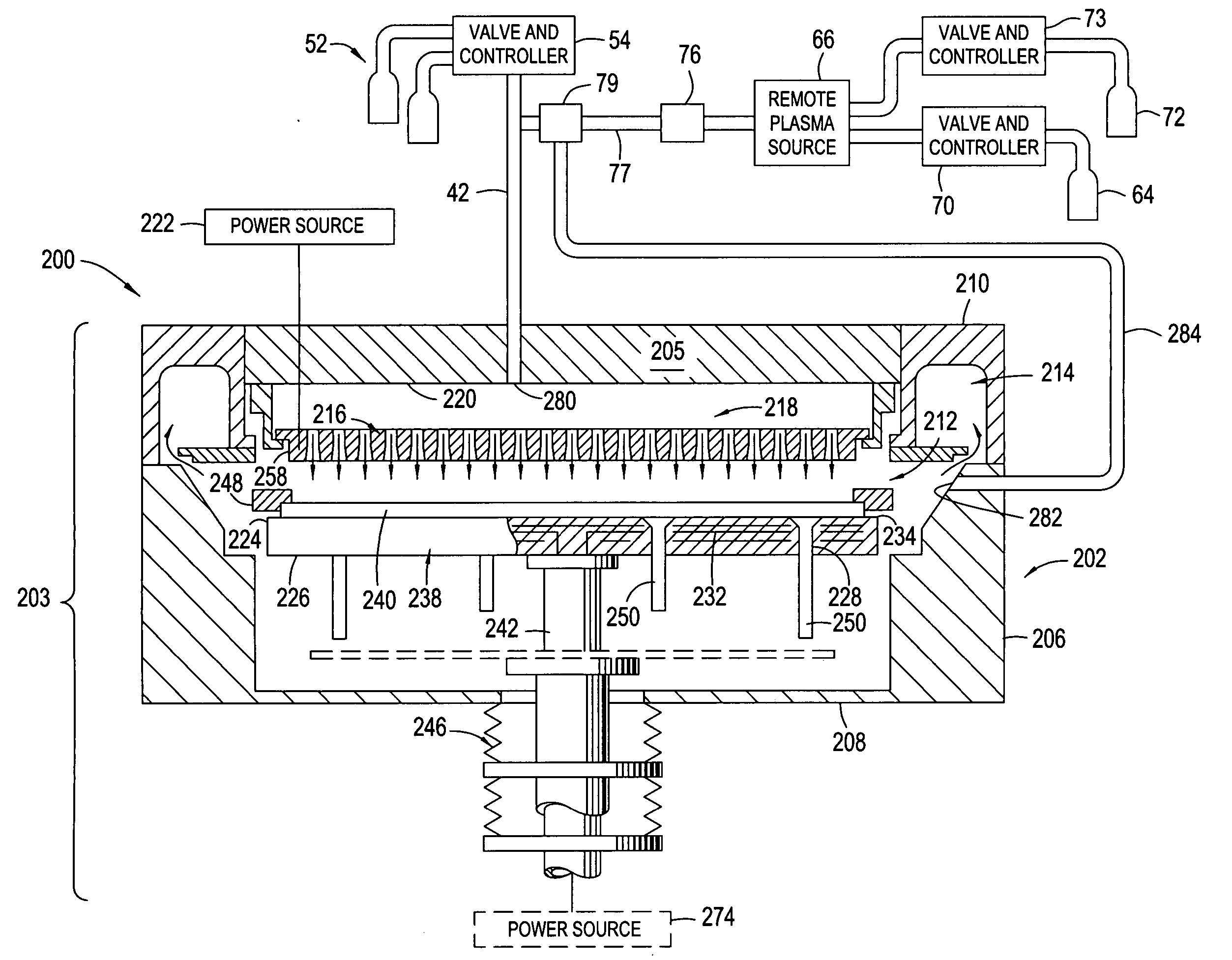
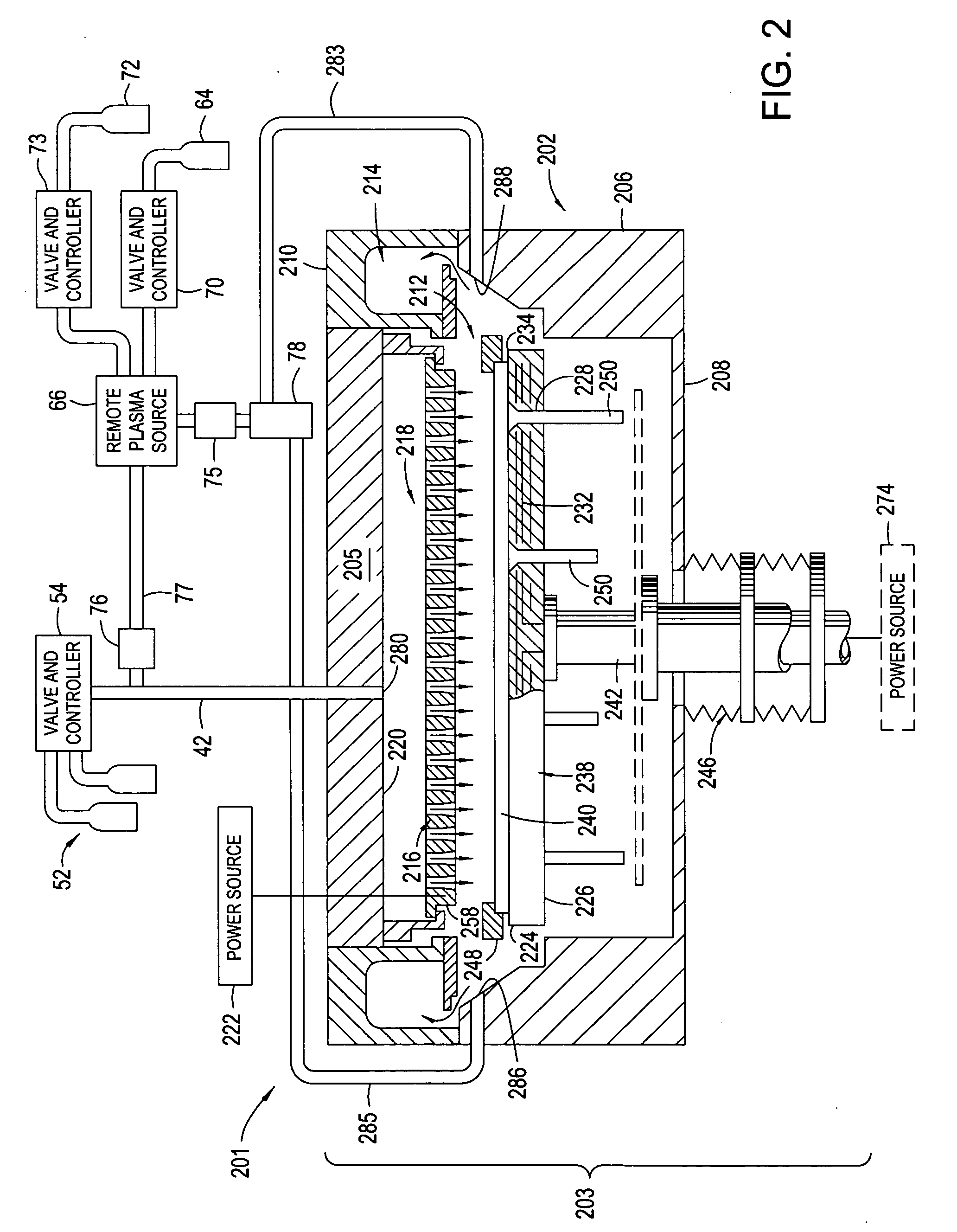
High plasma utilization for remote plasma clean
InactiveUS20060266288A1Tube/lamp screens manufactureElectric discharge tubesRemote plasmaHigh plasma
A method and apparatus for cleaning a chemical vapor deposition chamber are provided. The chemical vapor deposition chamber includes an inlet that introduces reactive species into the chamber from a remote plasma source while bypassing a gas distribution assembly of the chamber and an inlet that introduces reactive species from a remote plasma source into the chamber via the gas distribution assembly.
Owner:APPLIED MATERIALS INC
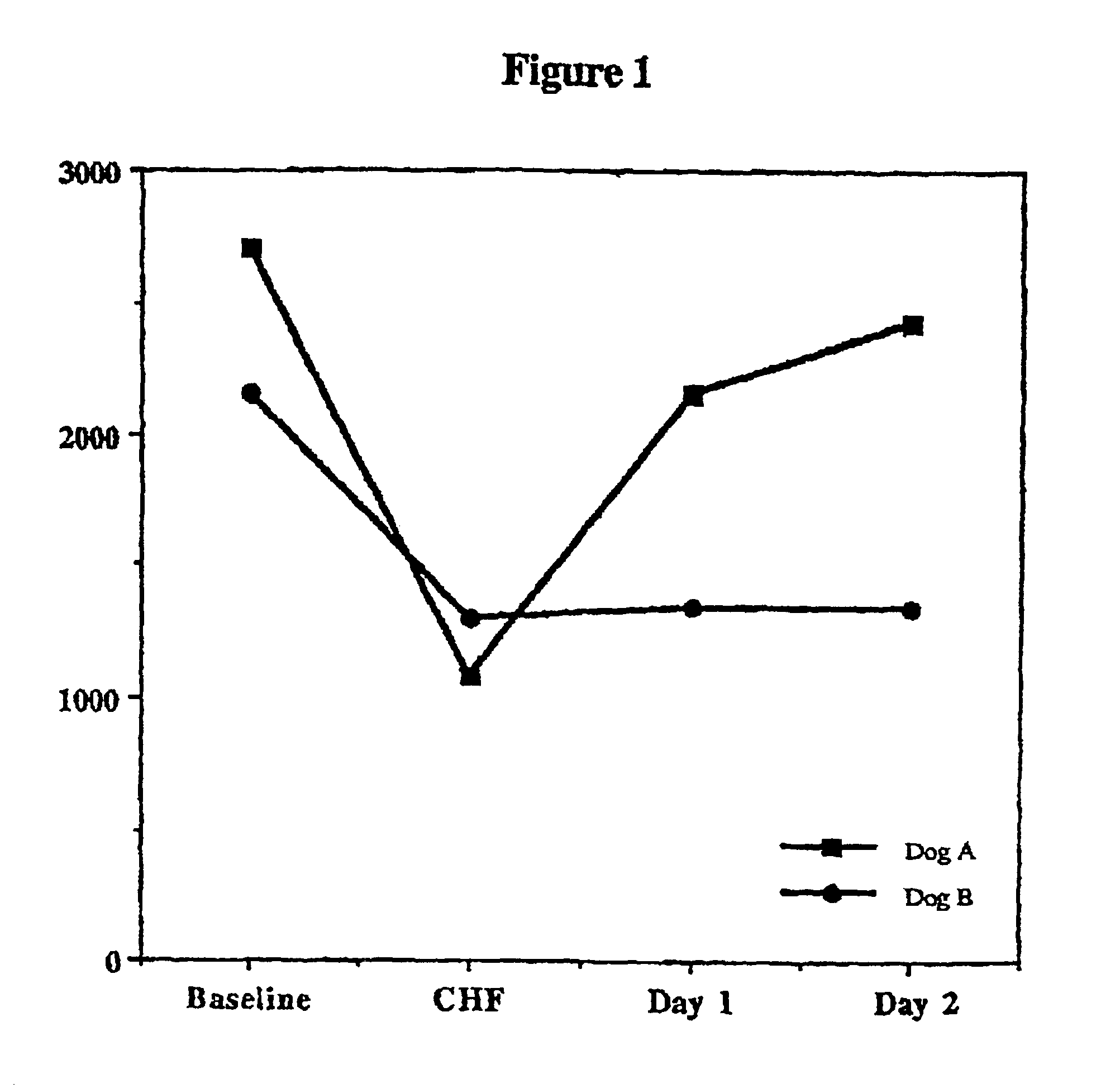
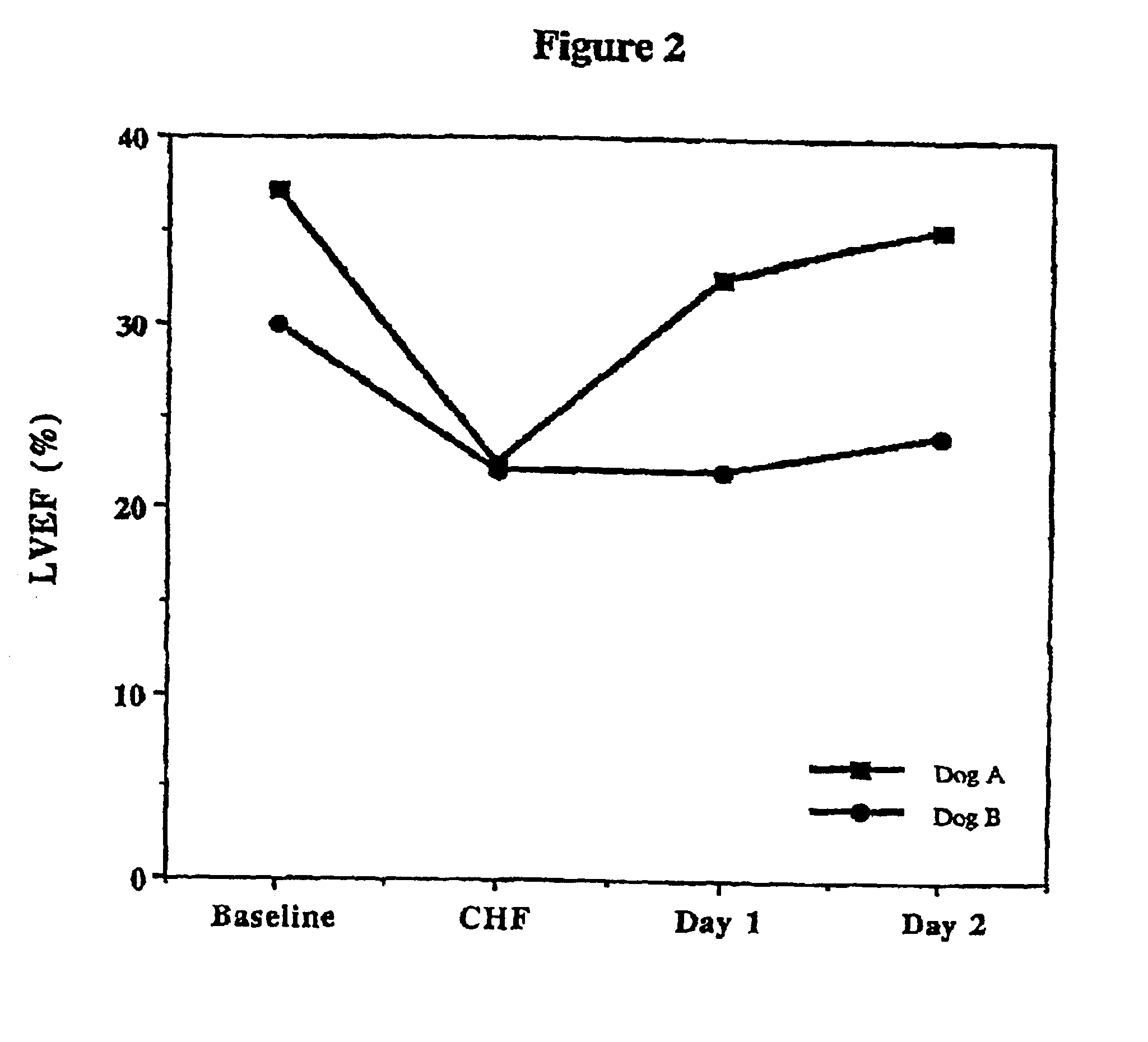
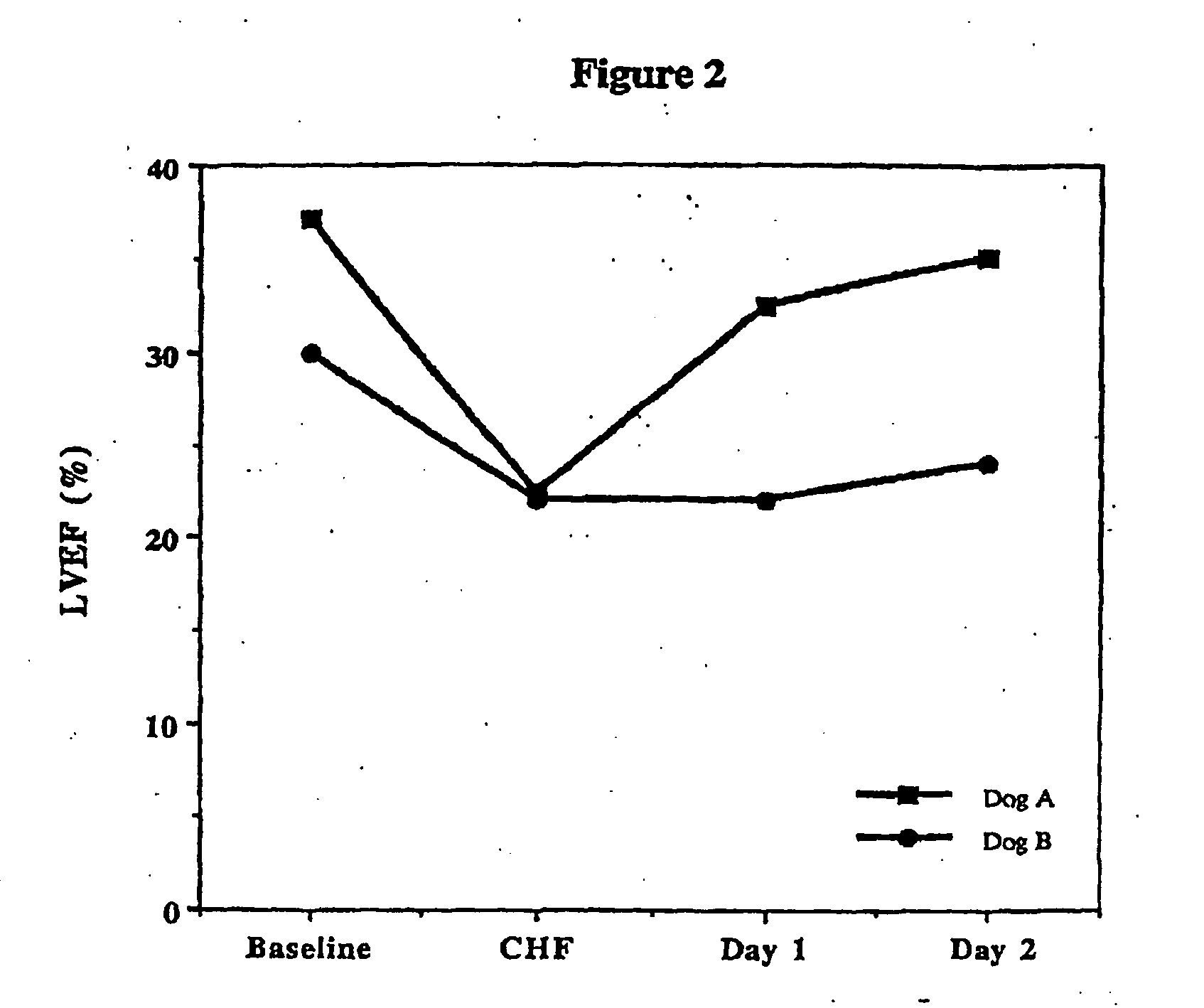
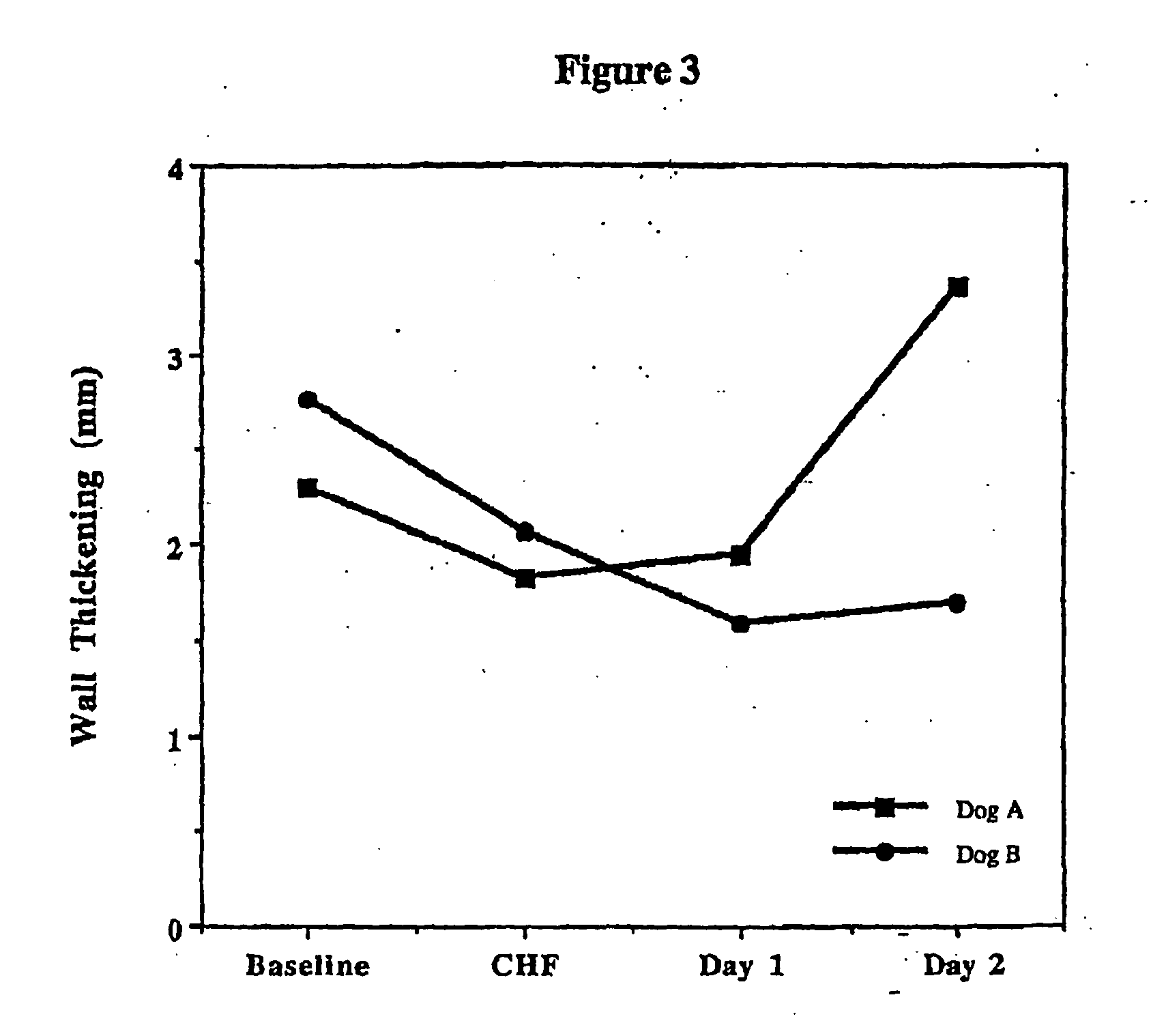
Treatment of hibernating myocardium and diabetic cardiomyopathy with a GLP-1 peptide
InactiveUS6894024B2Suppress plasma blood levelReducing norepinepherine levelPeptide/protein ingredientsMetabolism disorderHigh energyMortality rate
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:ASTRAZENECA PHARMA LP
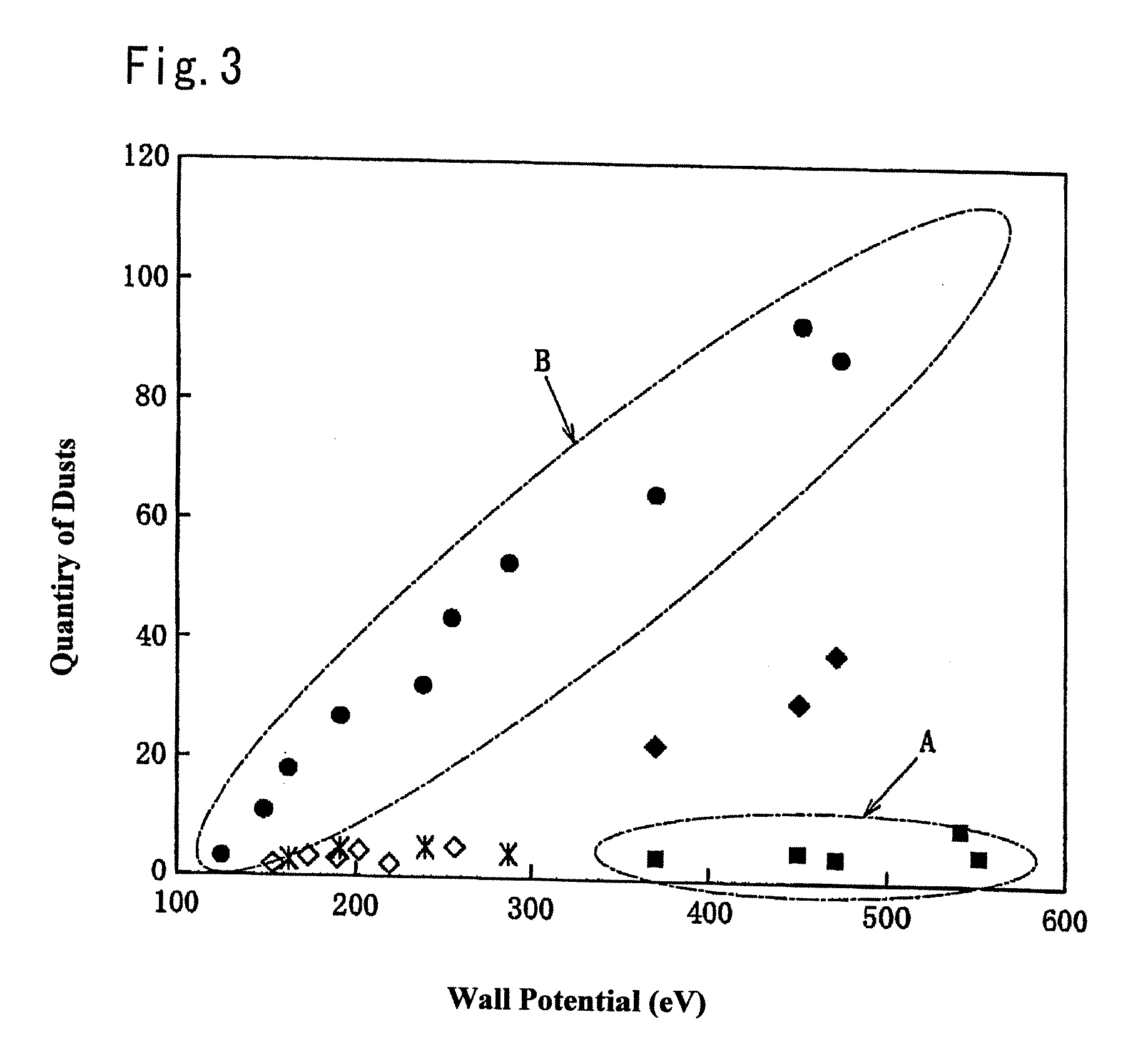
High pressure high non-reactive diluent gas content high plasma ion density plasma oxide etch process
InactiveUS6238588B1Increase pressureHigh strengthElectric discharge tubesDecorative surface effectsHigh plasmaOxygen
The invention is embodied in a method of processing a semiconductor workpiece in a plasma reactor chamber, including supplying a polymer and etchant precursor gas containing at least carbon and fluorine into the chamber at a first flow rate sufficient of itself to maintain a gas pressure in the chamber in a low pressure range below about 20 mT, supplying a relatively non-reactive gas into the chamber at second flow rate sufficient about one half or more of the total gas flow rate into the chamber, in combination with the first flow rate of the precursor gas, to maintain the gas pressure in the chamber in a high pressure range above 20 mT, and applying plasma source power into the chamber to form a high ion density plasma having an ion density in excess of 1010 ions per cubic centimeter. In one application of the invention, the workpiece includes an oxygen-containing overlayer to be etched by the process and a non-oxygen-containing underlayer to be protected from etching, the precursor gas dissociating in the plasma into fluorine-containing etchant species which etch the oxygen-containing layer and carbon-containing polymer species which accumulate on the non-oxygen-containing underlayer. Alternatively, the high pressure range may be defined as a pressure at which the skin depth of the inductive field exceeds {fraction (1 / 10)} of the gap between the inductive antenna and the workpiece.
Owner:APPLIED MATERIALS INC
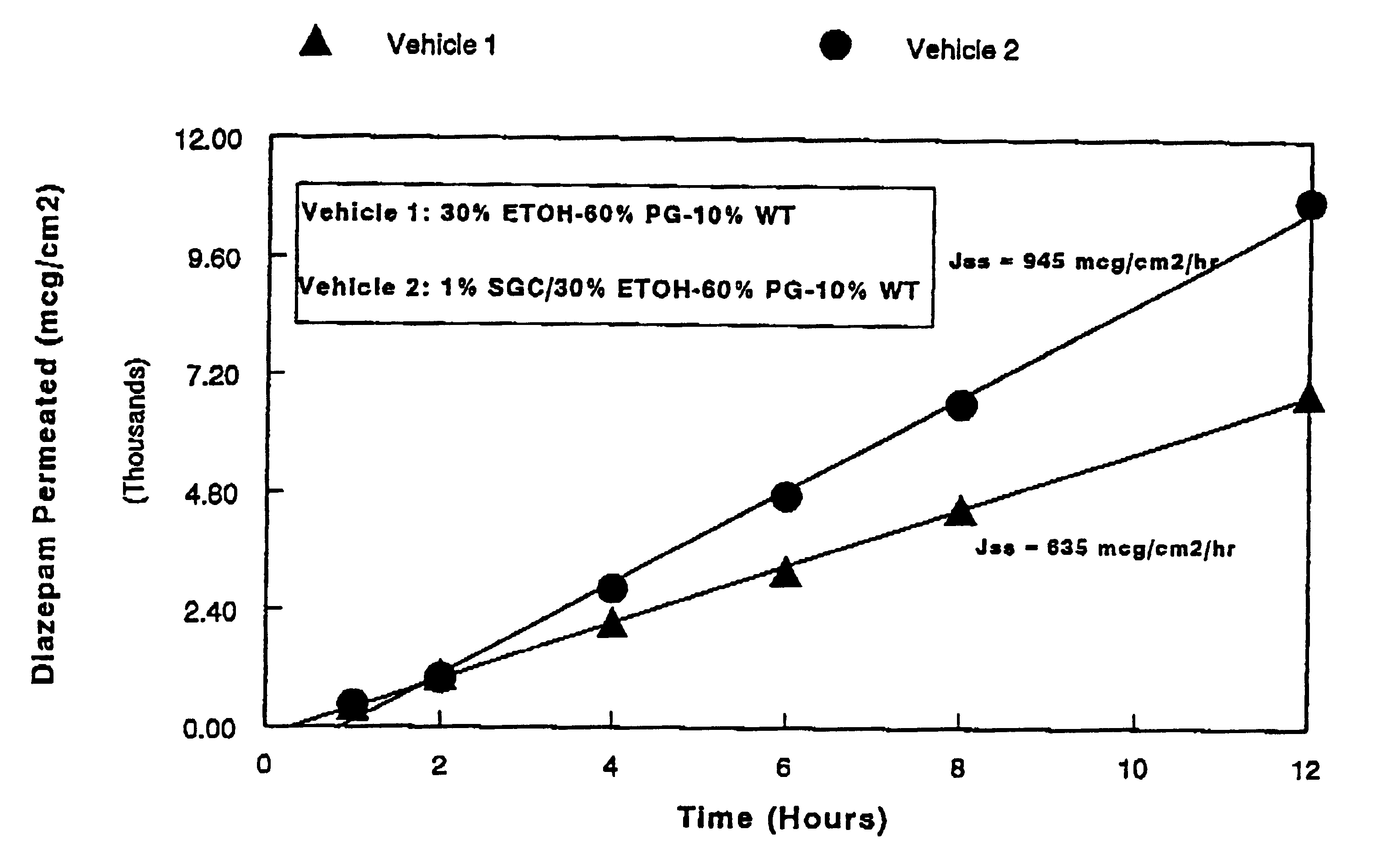
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
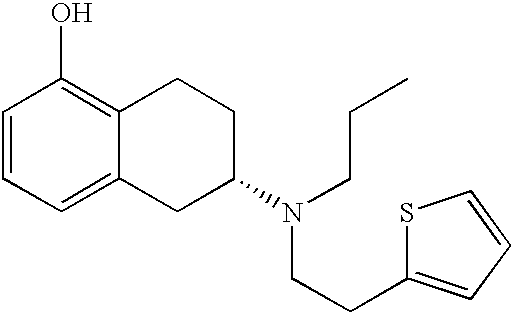
Transdermal therapeutic system for parkinson's disease inducing high plasma levels of rotigotine
This invention provides the use of a silicone-based transdermal therapeutic system having an area of 10 to 40 cm2 and containing 0.1 to 3.15 mg / cm2 of rotigotine as active ingredient, for the preparation of an anti-Parkinson medicament which induces a mean plasma concentration of rotigotine in the range of 0.4 to 2 ng / ml 24 h after administration.
Owner:UCB SA +1
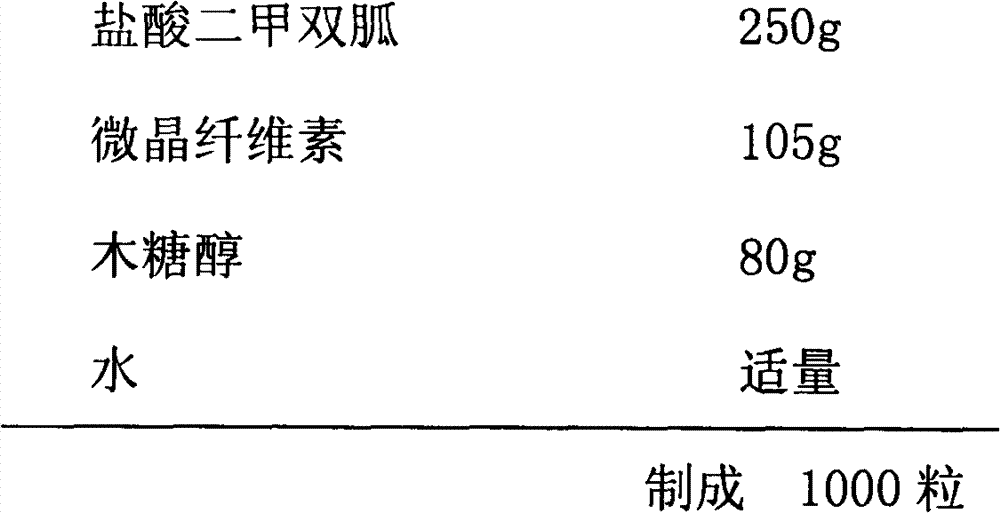
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
Treatment of hibernating myocardium with a GLP-1 peptide
InactiveUS20050096276A1Suppress plasma blood levelEase ischemic stressPeptide/protein ingredientsMetabolism disorderCatecholamineHigh energy
Hibernating myocardium is characterized by viable myocardium with impaired function due to localized reduced perfusion. Hibernating myocytes retain cellular integrity, but cannot sustain high-energy requirements of contraction. High plasma levels of catecholamines, such as norepinepherine, are believed to be predictive of mortality from hibernating myocardium. Likewise, high levels of catecholamines lead to cardiomyopathy in patients with diabetes. GLP-1 reduces plasma norepinepherine levels, and it thus is useful in a method of treating hibernating myocardium or diabetic cardiomyopathy.
Owner:COOLIDGE THOMAS +1
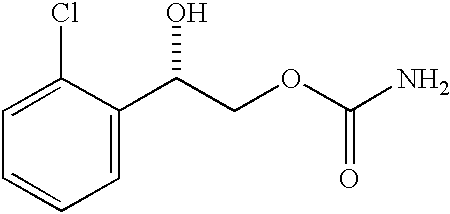
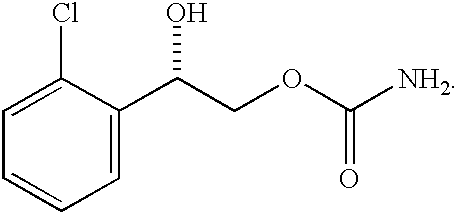
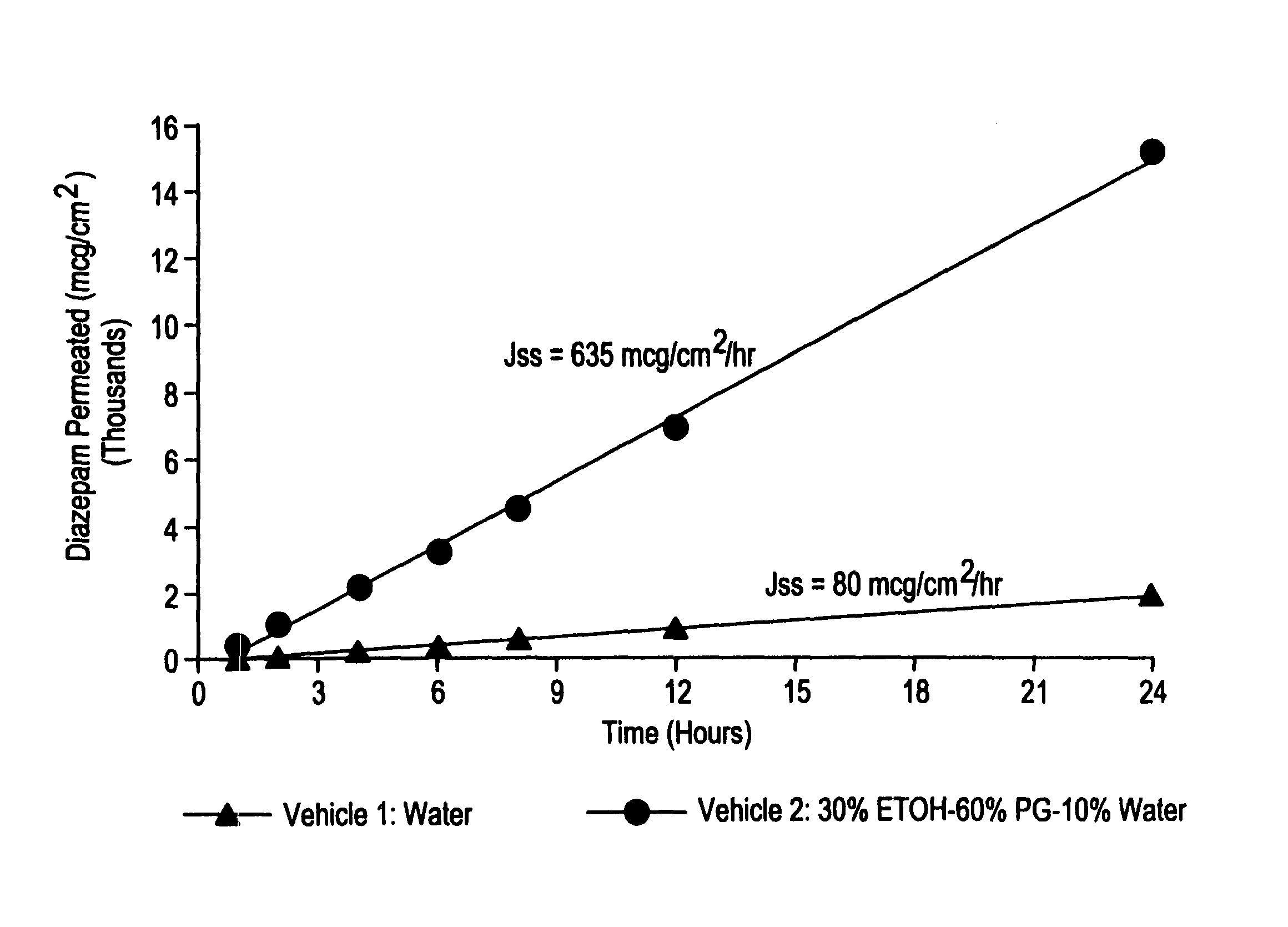
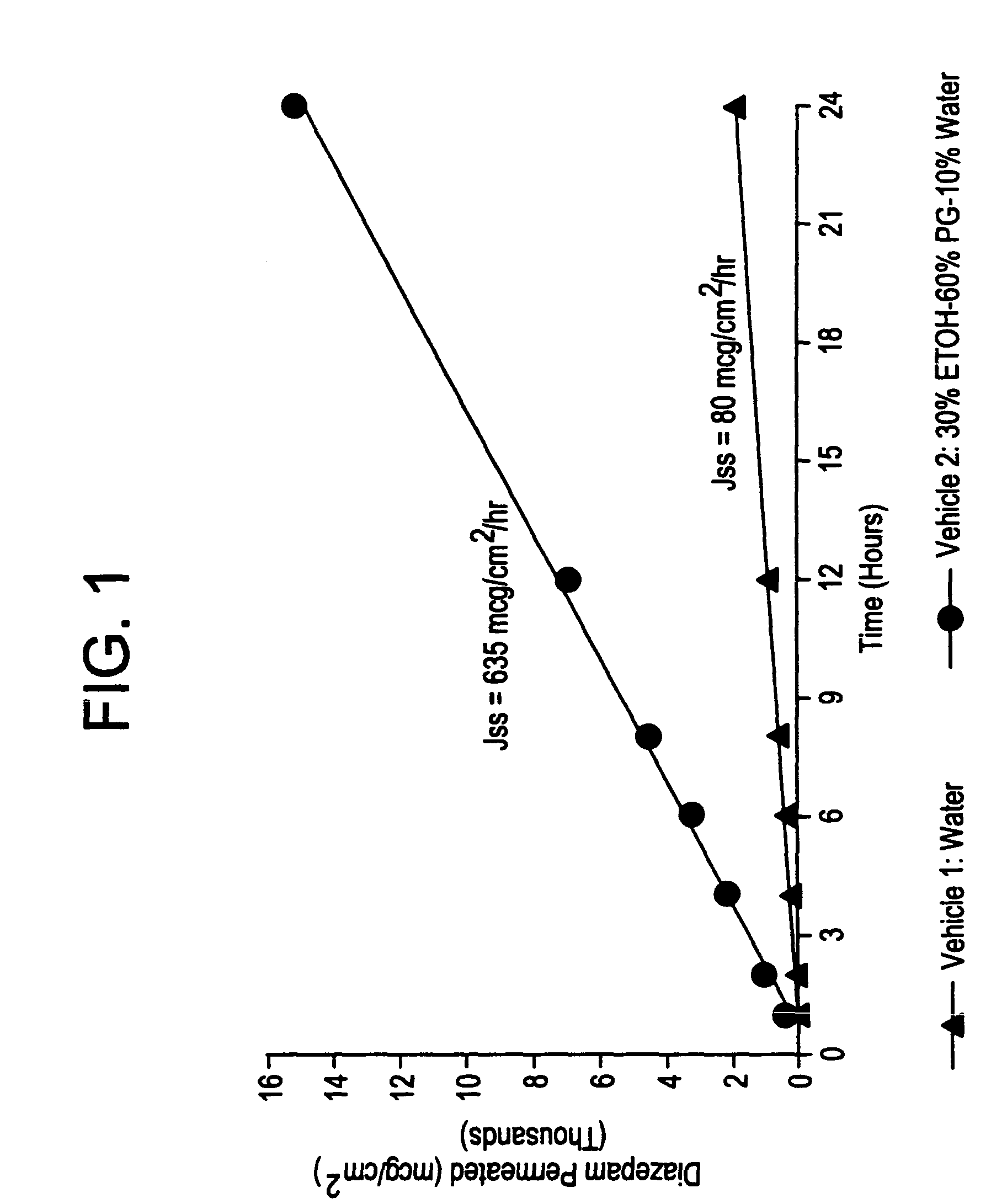
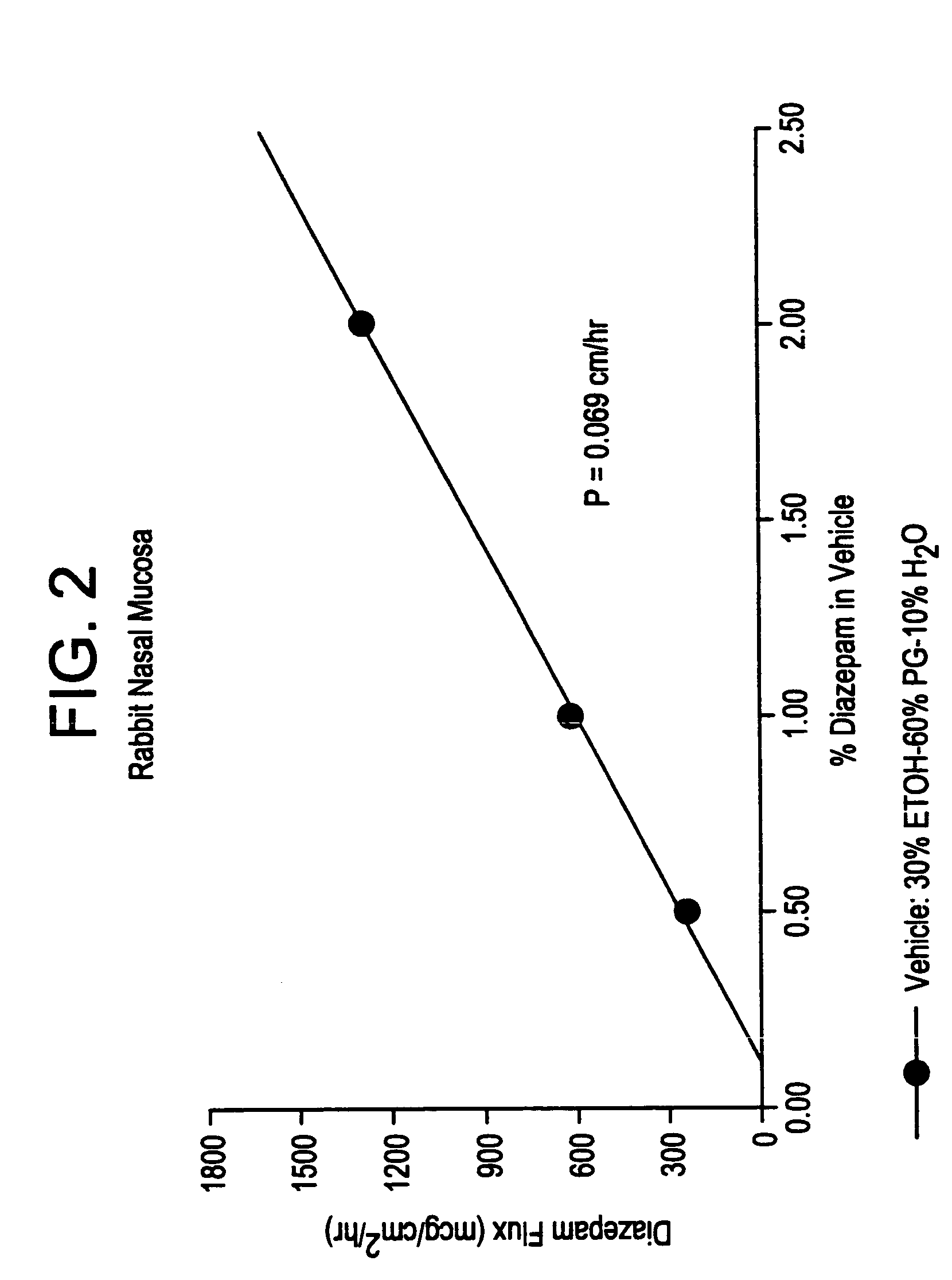
Transnasal microemulsions containing diazepam
InactiveUS20050002987A1High plasma concentrationHigh concentrationPowder deliveryNervous disorderNasal cavityBlood plasma
Diazepam is administered intranasally in the form of specific microemulsions having advantageous properties. The microemulsions are comprised of about equal quantities of a fatty acid and water with the remainder being a hydrophilic surfactant, a polar solvent and an alcohol in a weight ratio such that alcohol is present in a greater quantity by weight than either of the other two. Nasal administration of the subject microemulsions produces a high plasma concentration of diazepam nearly as fast as intravenous administration. The present microemulsions are particularly suitable for a prompt and timely treatment of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:SK
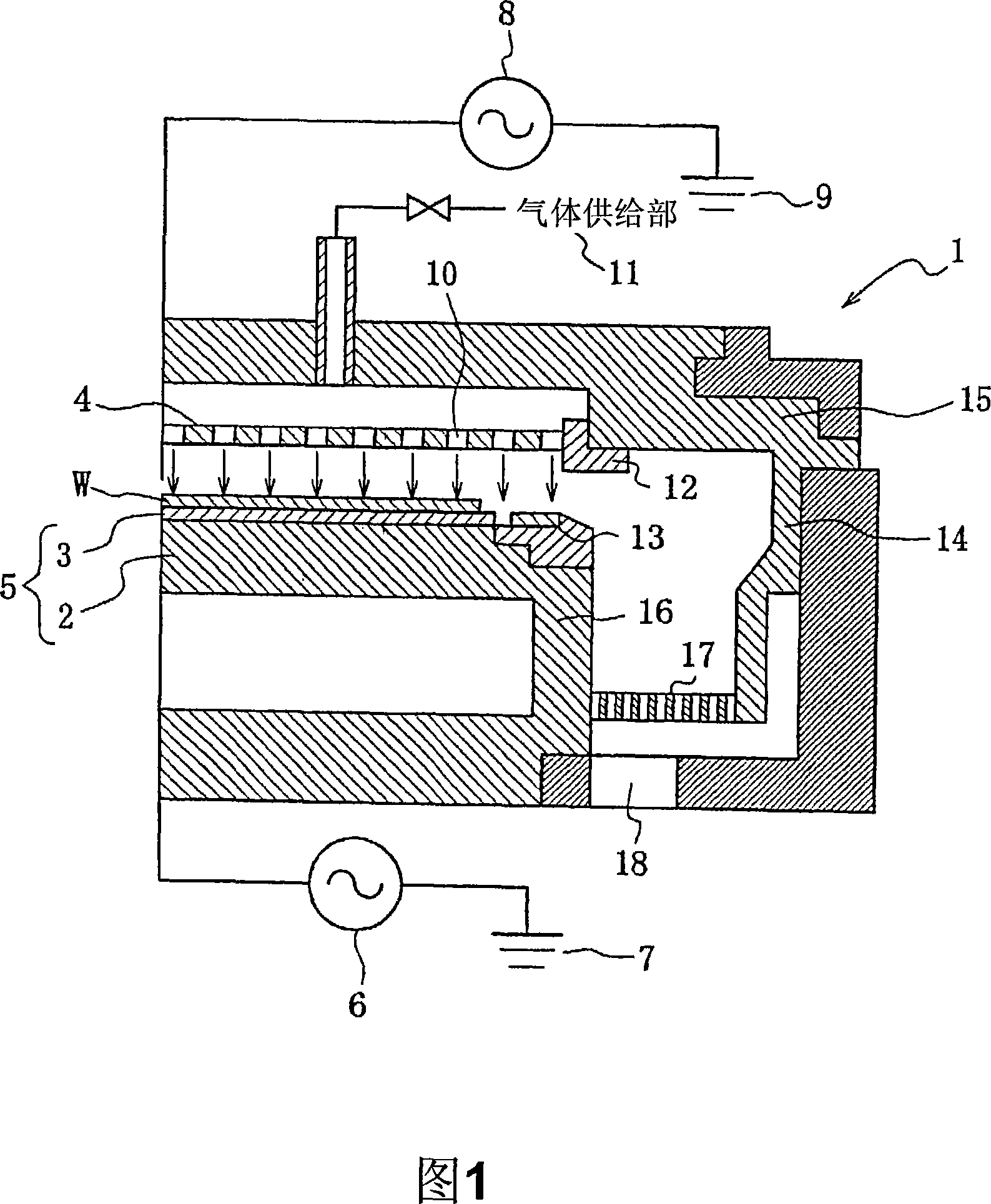
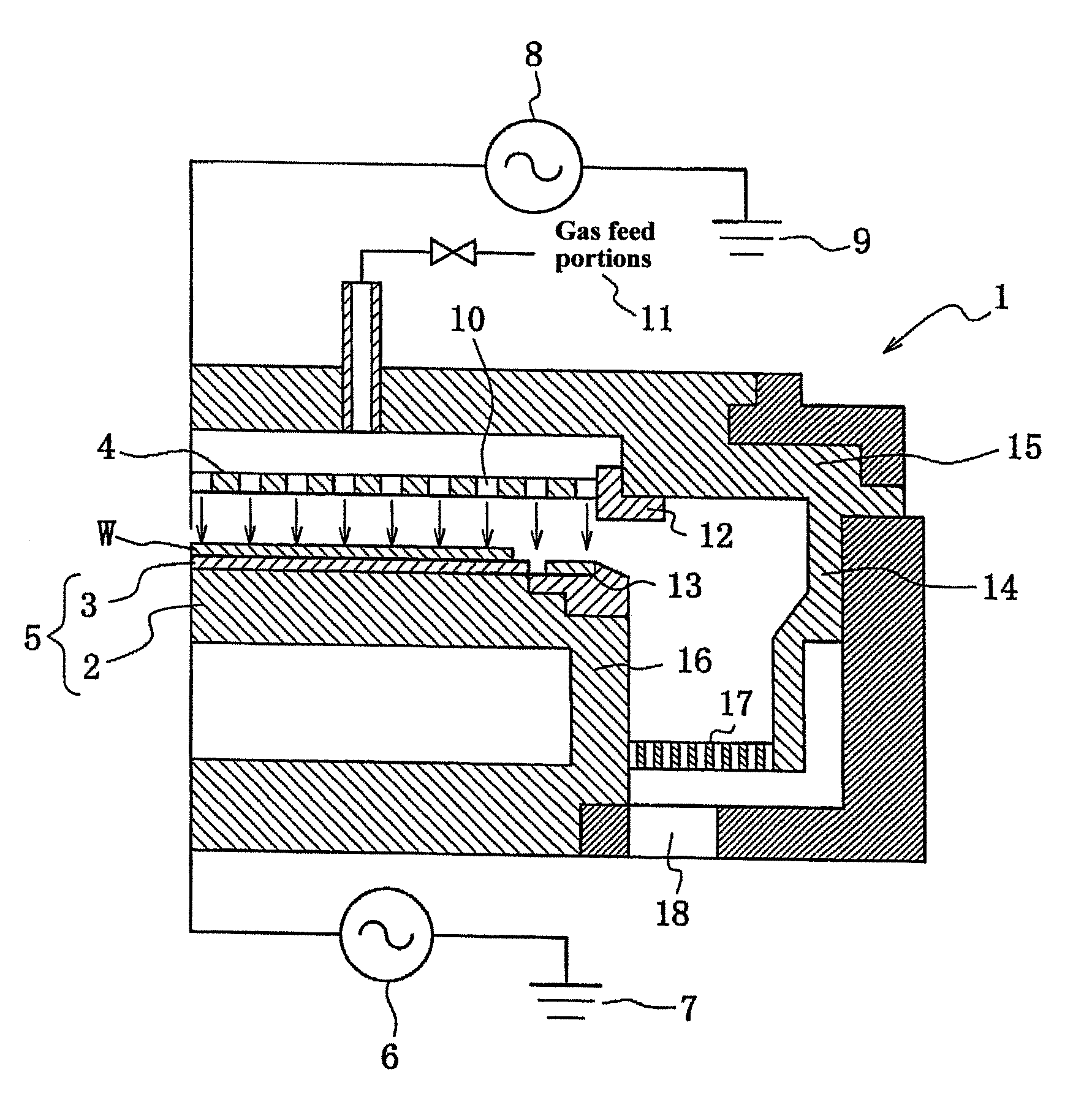
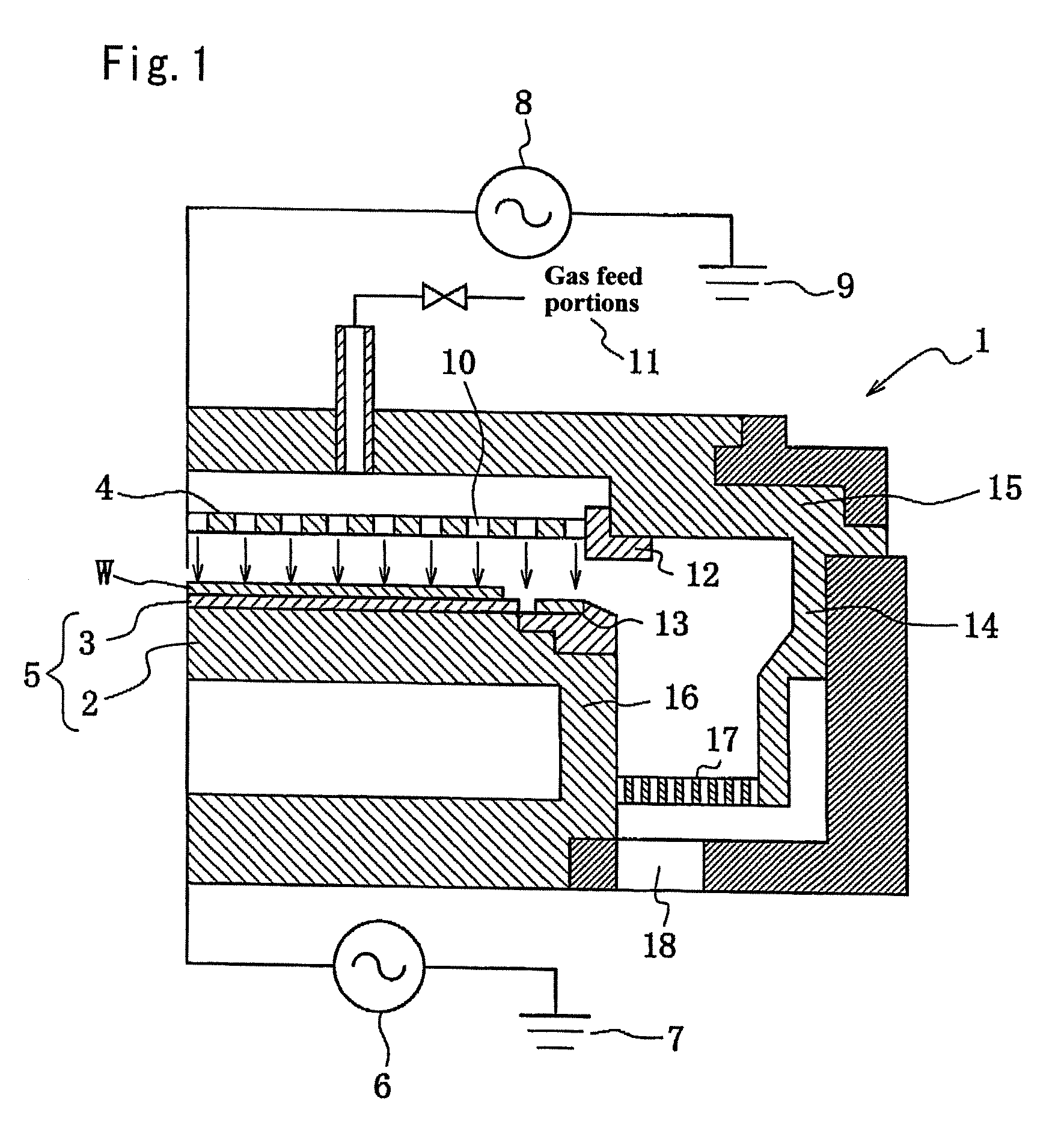
Plasma treating apparatus and plasma treating method
ActiveUS20070215283A1High energyElectric discharge tubesDecorative surface effectsHigh plasmaMaterials science
There are proposed a plasma treating apparatus and a plasma treating method using the same capable of improving the durability of site, member and parts in a chamber used for plasma etching in a corrosive gas atmosphere, which are exposed to the plasma atmosphere, and improving the resistance to plasma erosion of a coating formed on the surface of the member or the like in the corrosive gas atmosphere and preventing the occurrence of particles of a corrosion product even under a high plasma power. As a means therefore, in a plasma treating apparatus wherein a surface of a body to be treated in a chamber is subjected to a plasma treatment with an etching gas, at least surfaces of sites of the chamber itself exposing to the plasma atmosphere, or surfaces of a member or parts accommodated in the chamber are covered with a composite layer including a porous layer made from a metal oxide and a secondary recrystallized layer of the metal oxide formed on the porous layer.
Owner:TOKYO ELECTRON LTD
Integral micro-discharge cold plasma cosmetic instrument
The invention discloses an integral micro-discharge cold plasma cosmetic instrument mainly comprising an integrated case and a handheld electrode. According to the surface micro-discharge technology and the principle of dielectric barrier discharge, cold plasma is generated continuously stably, reaction with the chromophoric group is omitted, tissue vaporizing is not needed, separated skin is preserved completely and used as a natural dressing, and thus wound healing can be improved to facilitate recovery of patients. The integral micro-discharge cold plasma cosmetic instrument has the advantages of convenience in operation, high plasma generation efficiency, no gas supply device, and the like.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Use of ammonia for etching organic low-k dielectrics
InactiveUS6893969B2High selectivityIncrease etch rateDecorative surface effectsSemiconductor/solid-state device manufacturingProcess chemistryHigh plasma
Method for etching organic low-k dielectric using ammonia, NH3, as an active etchant. Processes using ammonia results in at least double the etch rate of organic low-k dielectric materials than processes using N2 / H2 chemistries, at similar process conditions. The difference is due to the much lower ionization potential of NH3 versus N2 in the process chemistry, which results in significantly higher plasma densities and etchant concentrations at similar process conditions.
Owner:LAM RES CORP
Plasma treating apparatus and plasma treating method
ActiveCN101042996ADurableSmall particlesMolten spray coatingElectric discharge tubesPorous layerProduct gas
Provided is a plasma processing apparatus wherein durability of a part, a member and a component, which are exposed to plasma atmosphere in a chamber used for performing plasma etching in a corrosion resistant gas atmosphere is improved, resistance to plasma erosion of a film formed on the surface of the member and the like in the corrosion resistant gas atmosphere is improved, and furthermore, generation of particles of corrosion resistant products even under high plasma output is prevented. A plasma processing method using such plasma processing apparatus is also provided. In the plasma processing apparatus for processing the surface of a subject which is stored in the chamber to be processed is processed by etching process gas plasma. The part exposed to the plasma generating atmosphere in the chamber or the member arranged inside the chamber or the surface of the component is coated with at least a porous layer composed of a metal oxide and a secondary recrystallized layer of the metal oxide formed on the porous layer.
Owner:TOKYO ELECTRON LTD
Transnasal anticonvulsive compositions and modulated process
InactiveUS7132112B2Promote absorptionIncrease permeationNervous disorderAerosol deliveryNasal cavityCo administration
A novel method of vehicle modulated administration of an anticonvulsive agent to the mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol (10–80%) or a glycol (10–80%), and their combinations with a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Porous film material fixed with heparin on surface, its preparing method and use
InactiveCN101024150ALow costWide variety of sourcesSemi-permeable membranesOther chemical processesBiocompatibility TestingBlood purification
This invention aims at providing a plasma lipid composition selective adsorption separation porous membrane carrier materials, preparation methods and related applications. The porous membrane carrier used the average pore size of 0.05 m ~ 100 u medical nonwoven polymer as the starting raw materials, through a total of 60 Co-ray irradiation grafting copolymerization polyacrylic acid surface modification, it further fixed the cholesterol ligand by carboxyl of 1 - (3 - dimethyl amino-propyl ) -3 - ethyl activated carbon Diimide and biocompatibility ligand adsorption of heparin covalent coupling, finally get the polymer porous membrane carrier materials of plasma lipid selective adsorption separation ability. The invention related to the preparation method is simple, safe, effective and easy to promote large-scale production. With good material blood compatibility, it can be used as a plasma lipid composition adsorption separation of hyperlipidemia, dynamic perfusion of clinic high plasma patients' blood purification and waste blood separation renewable auxiliary materials.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Metformin glycinate salt for blood glucose control
The present invention relates to metformin glycinate salt and pharmaceutical compositions thereof for the treatment of diabetes mellitus. The method includes administration of the metformin glycinate salt by various routes selected from oral, intravenous injectable, intramuscular injectable, nasal, intraperitoneal, or sublingual, in order to achieve a reduction in blood glucose levels. The invention further relates to the synthesis of a new 1,1-dimethylbiguanide glycinate salt, called Metformin Glycinate. The resulting salt exhibits advantages over other metformin salts. These advantages are due, in the first place, to the fact that the glycine counterion exhibits hypoglycemic effects by itself. Moreover, the salt exhibits more rapid absorption, reaching higher plasma concentrations than those produced with metformin hydrochloride.
Owner:LAB SILANES S A DE
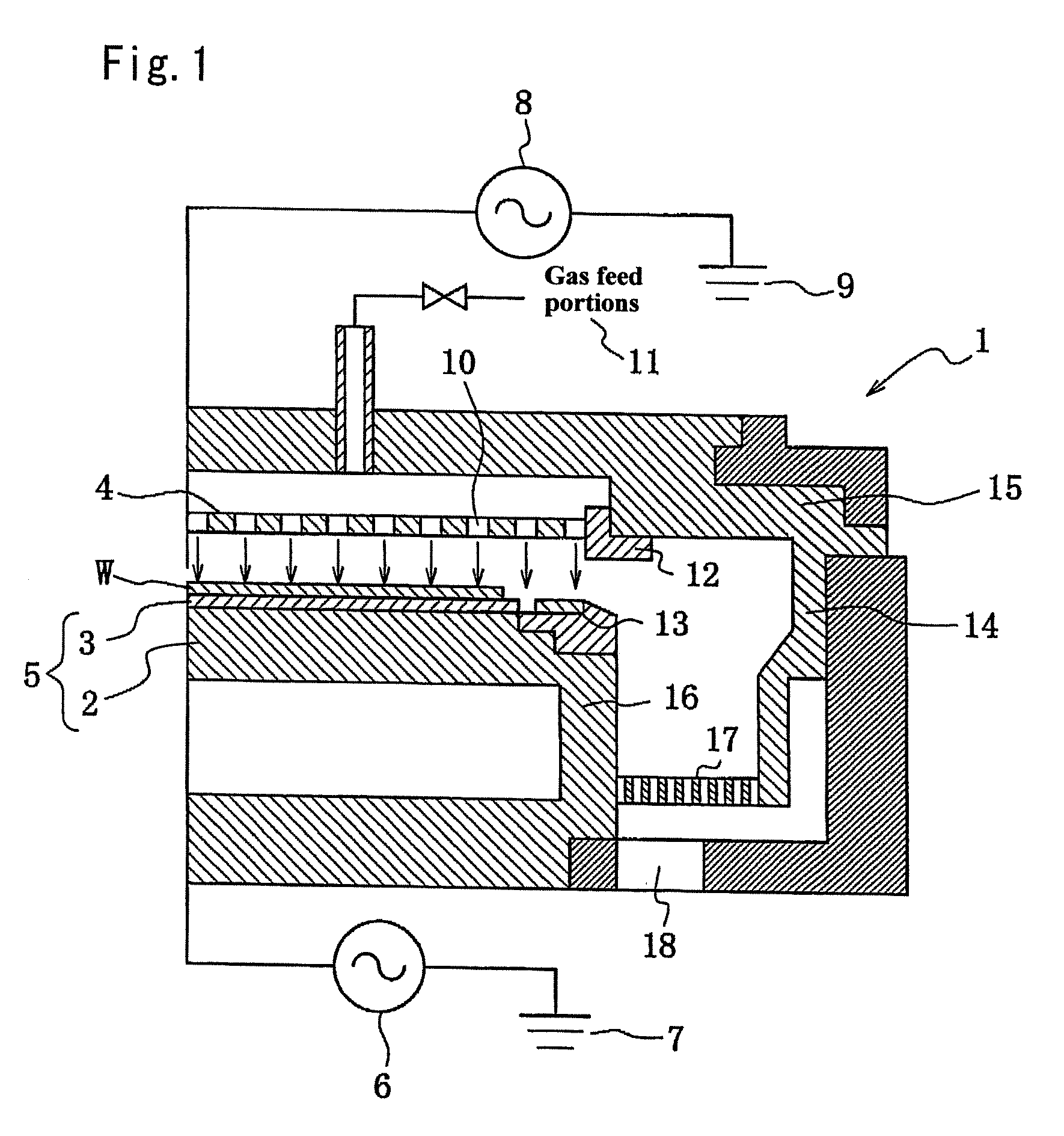
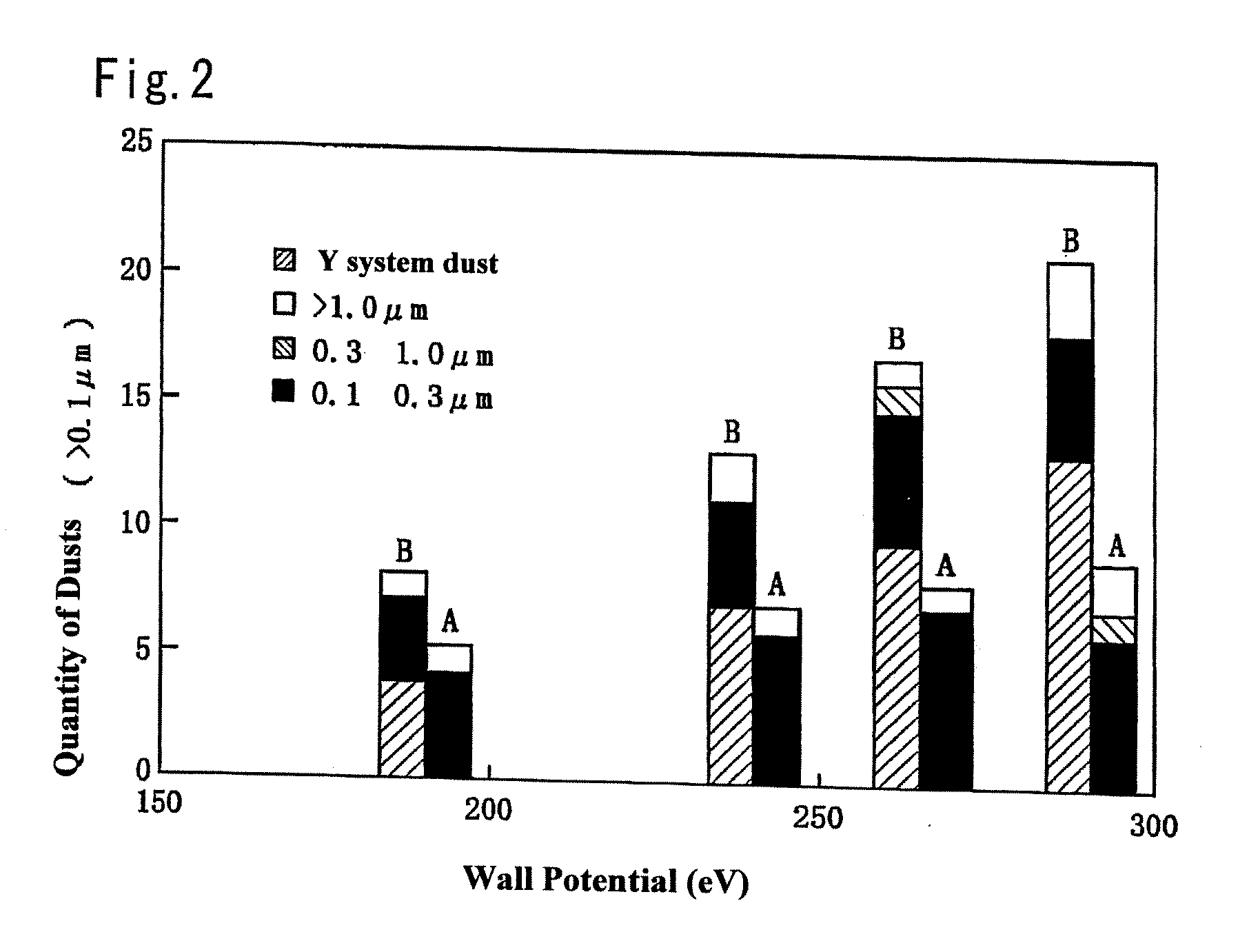
Member for plasma etching device and method for manufacture thereof
A member for a plasma etching device, which comprises a device substrate comprising quartz glass, aluminum, alumite or a combination thereof and, formed on the surface thereof, a coating film of yttrium oxide or YAG having a film thickness of 10 μm or more and a variation in the thickness of 10% or less, and preferably a surface roughness (Ra) of 1 μm or less; and a method for manufacturing the member for a plasma etching device, which comprises a step of plasma-spraying yttrium oxide or YAG to the surface of said device substrate or a step of fusing yttrium oxide or YAG with an oxyhydrogen flame, followed by coating the surface with the fused product, or a step of applying a solution containing yttrium, a yttrium compound or YAG on the above surface, followed by heating to fuse the resultant coating, or a combination of the above steps, thereby forming a coating film of yttrium oxide or YAG having a film thickness 10 μm or more and a variation in the thickness of 10% or less, and preferably a surface roughness (Ra) of 1 μm or less. The member for a plasma etching device is capable of retaining high plasma resistance for a long period of time, is free from the occurrence of the abnormal etching owing to partial change of electric characteristics, and thus can be used for a long time, in particular, even in the treatment of a large semiconductor device of a 12 inch silicon wafer.
Owner:SHIN ETABU QUARTZ PRODS
Dasatinib liposome preparation, and preparation method thereof
ActiveCN107260680AAchieve slow and controlled releaseImprove lipophilicityOrganic active ingredientsInorganic non-active ingredientsDasatinibBiocompatibility Testing
The invention relates to a dasatinib liposome preparation, and a preparation method thereof. The dasatinib liposome preparation is high in biocompatibility; target modification can be carried out; sustained and controlled release of dasatinib can be realized; and it is beneficial for maintenance of relatively high plasma drug concentration in a long term, improvement of drug distribution, and increasing of drug bioavailability. The dasatinib liposome preparation possesses excellent lipophilic performance, is capable of passing through phospholipid bilayer in a molecular form and entering into inner water phase; pH value of liposome inner water phase is relatively low, the dasatinib moleculars are capable of bonding with hydrogen ions so as to form dasatinib ions, and combination with anions in an ammonium salt solution and forming of insoluble salts are realized, diffusion of dasatinib in the inner water phase into an outer water phase is inhibited, dasatinib is coated by the liposome inner water phase steadily, encapsulation efficiency and storage stability are improved, and excellent in-vitro slow release effect is achieved.
Owner:SHANGHAI JIAO TONG UNIV
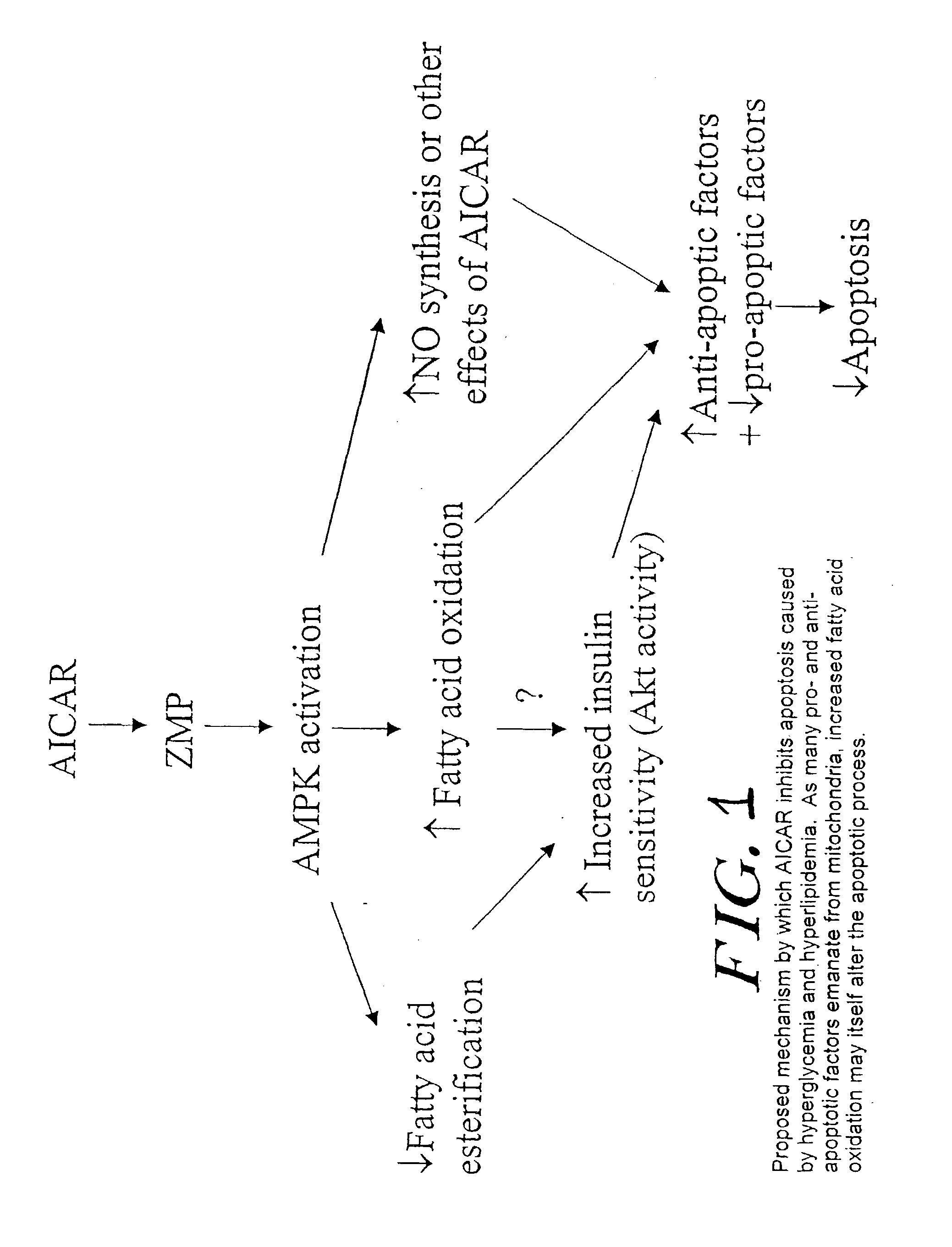
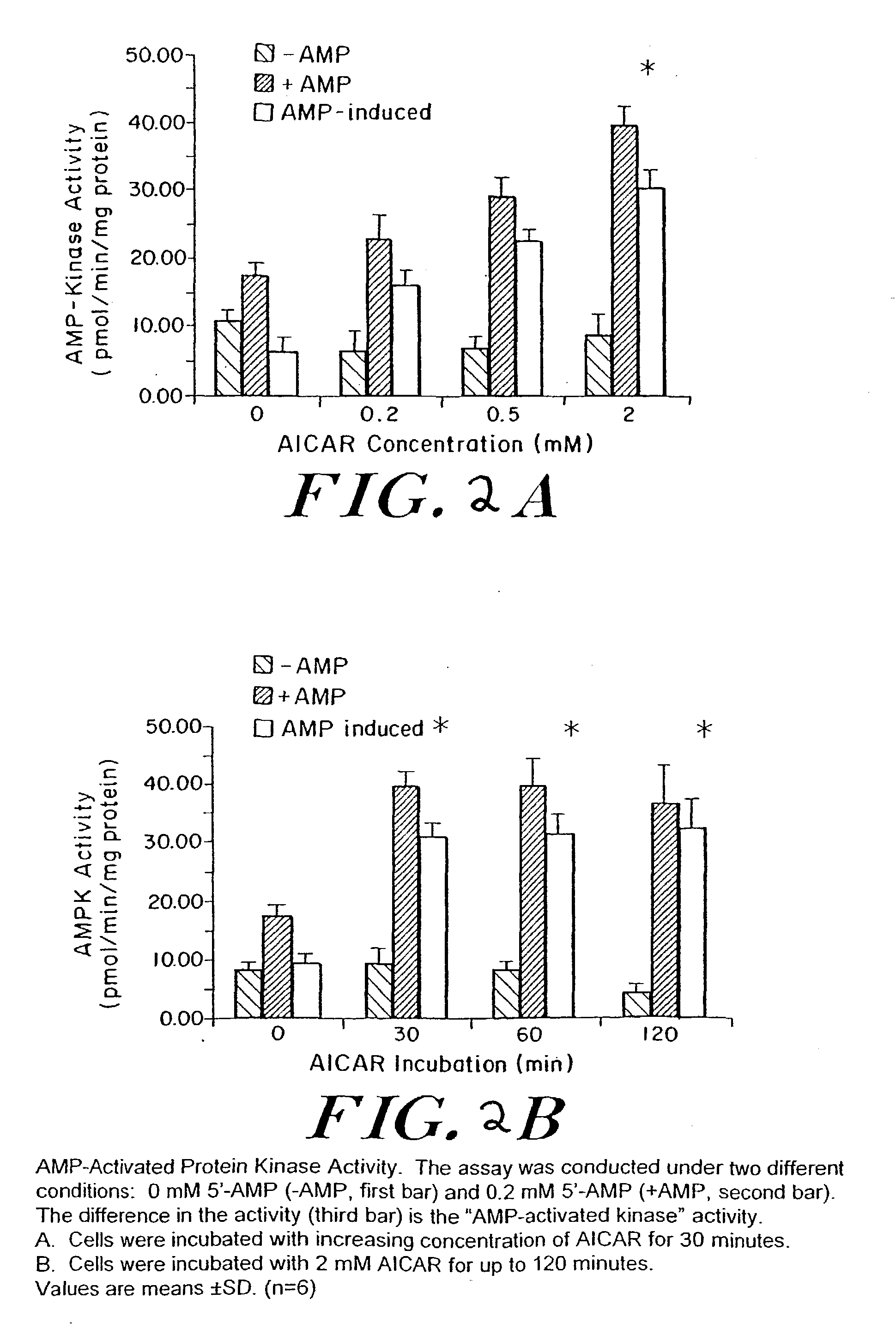
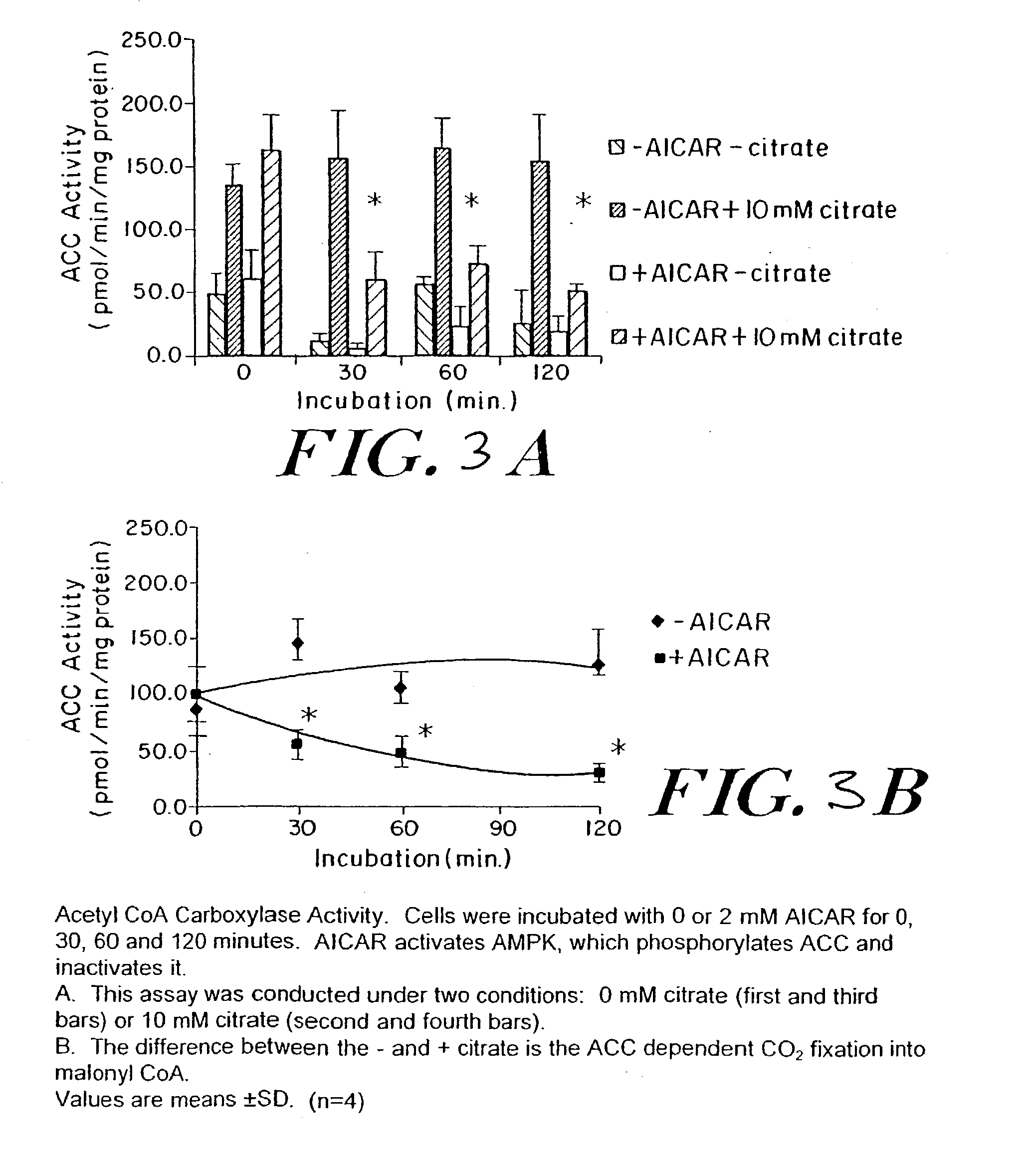
Methods fo treating conditions associated with insulin resistance with aicar, (5-amino-4-imidazole carboxamide riboside) and related compounds
InactiveUS20030212014A1Positive impact in reducing obesityIncrease insulin sensitivityBiocideCompound screeningMammalDisease cause
The long-term usage of AICR (5-aminio, 4-imidazole carboxamide riboside) to produce sustained metabolic and biological changes in mammals that overcome insulin resistance, i.e., increase insulin sensitivity, and result in benefits in diseases and conditions such as diabetes, hypertension, atherosclerosis, polycystic ovary syndrome and gallstones is described long-term usage of AICAR, particularly intermittent administration, e.g., three days per week, appears to have some of the positive effects of exercise, having an impact on the amount Of food consumed by a subject and resulting in reduced fat build-up and increase in muscle mass. Therefore, AICAR administration has a positive impact in reducing obesity. AICAR can also Prove useful in preventing or treating vascular diseases associated with hyperglycemia, high plasma levels of free fatty acids (FFA) and triglyceride, and insulin resistance by virtue of the fact that this agent activates fatty acid oxidation. Animal tests have Shown that chronic intermittent treatment with AICAR has not resulted in any noticeable toxic effects. AICAR and related compounds are activators of AMP-activated protein kinase (AMPK) and, furthermore, are effective at decreasing malonyl CoA levels in the animal.
Owner:UNIV BOSTON TRUSTEES OF THE +1
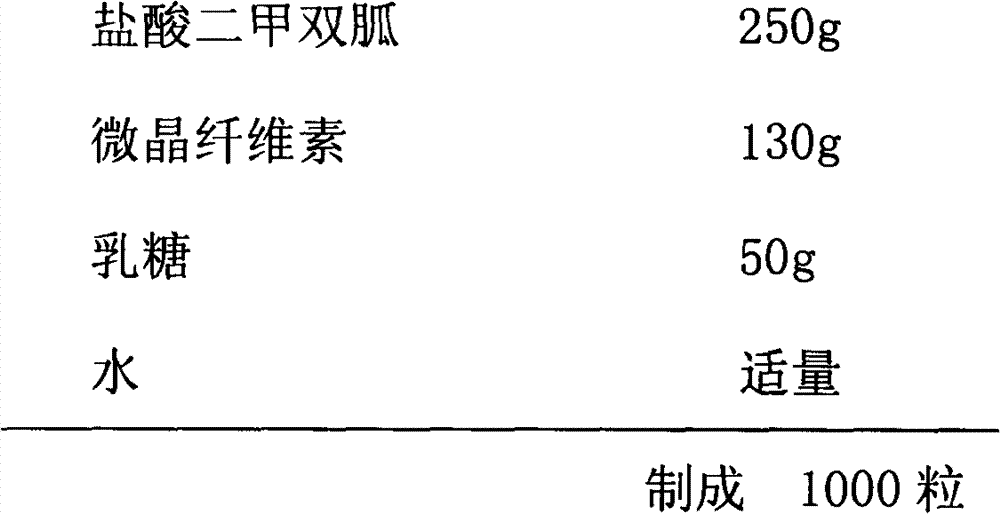
Metformin hydrochloride sustained-release capsule and its preparation method
InactiveCN103239424AEasy to swallowAvoid disintegrationOrganic active ingredientsMetabolism disorderSide effectPatient compliance
The invention relates to a metformin hydrochloride sustained-release capsule and its preparation method. The metformin hydrochloride sustained-release capsule is prepared by steps of coating a metformin hydrochloride granule sustained-release material and putting into an enteric capsule. In comparison with the prior art, sustained-release granules and enteric capsule filling technology are combined together to prepare the new dosage form of metformin hydrochloride sustained-release (enteric-coated) capsule. As the sustained-release granule coating and enteric capsule filing technology is adopted, metformin hydrochloride will not be disintegrated and will not stimulates gastric mucosa, and adverse reactions such as nausea, stomachache, diarrhoea and the like caused by medication can be avoided. Meanwhile, metformin hydrochloride will not be damaged by gastric juice, and bioavailability of metformin hydrochloride is raised. In addition, the product provided by the invention is also a sustained-release enteric-coated preparation. The medicine can be stably released in a body, effective plasma concentration is maintained for a long time, and toxic and side effect which might be caused by higher plasma concentration within a short time are avoided. The frequency for taking the medicine is reduced, and patient compliance is also raised.
Owner:BOSEN BIO PHARMA SHANXI PROVINCE
Plasma treating apparatus and plasma treating method
There are proposed a plasma treating apparatus and a plasma treating method using the same capable of improving the durability of site, member and parts in a chamber used for plasma etching in a corrosive gas atmosphere, which are exposed to the plasma atmosphere, and improving the resistance to plasma erosion of a coating formed on the surface of the member or the like in the corrosive gas atmosphere and preventing the occurrence of particles of a corrosion product even under a high plasma power. As a means therefore, in a plasma treating apparatus wherein a surface of a body to be treated in a chamber is subjected to a plasma treatment with an etching gas, at least surfaces of sites of the chamber itself exposing to the plasma atmosphere, or surfaces of a member or parts accommodated in the chamber are covered with a composite layer including a porous layer made from a metal oxide and a secondary recrystallized layer of the metal oxide formed on the porous layer.
Owner:TOKYO ELECTRON LTD
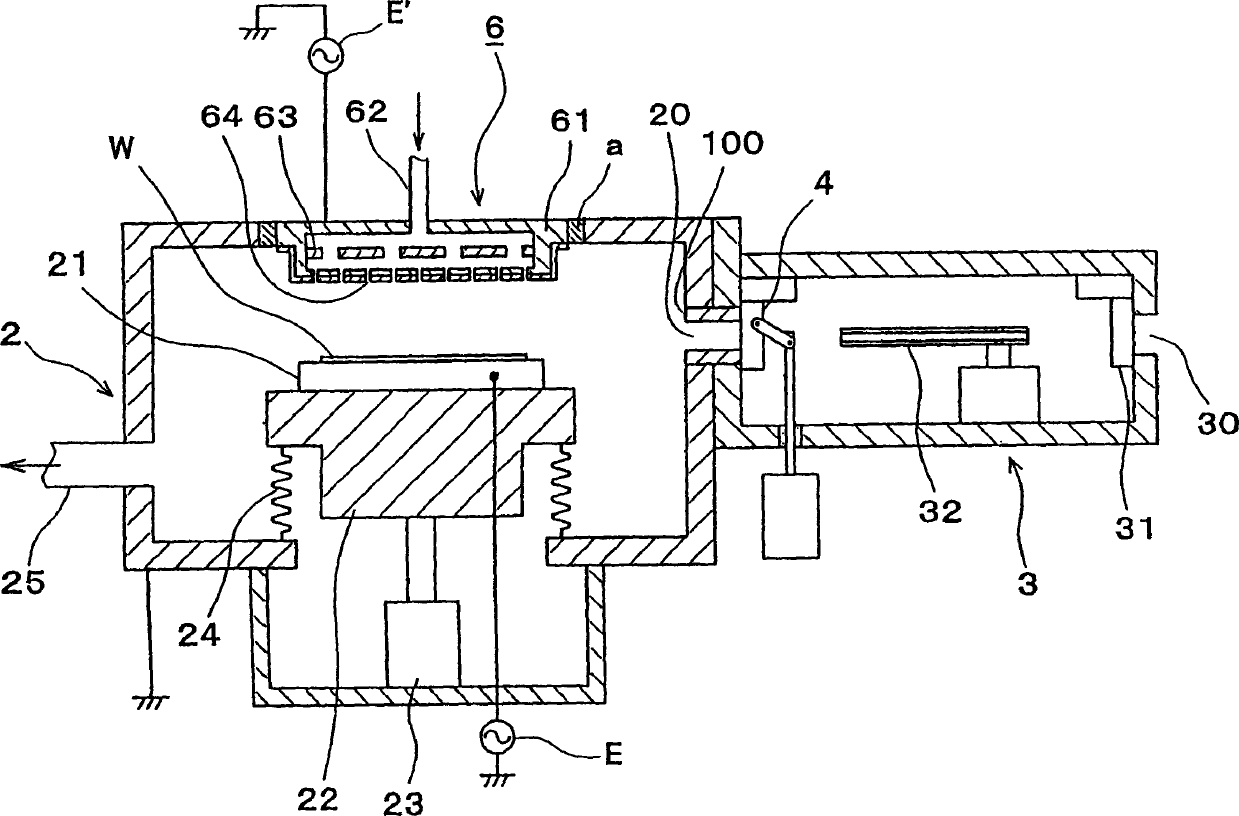
Vacuum processing device
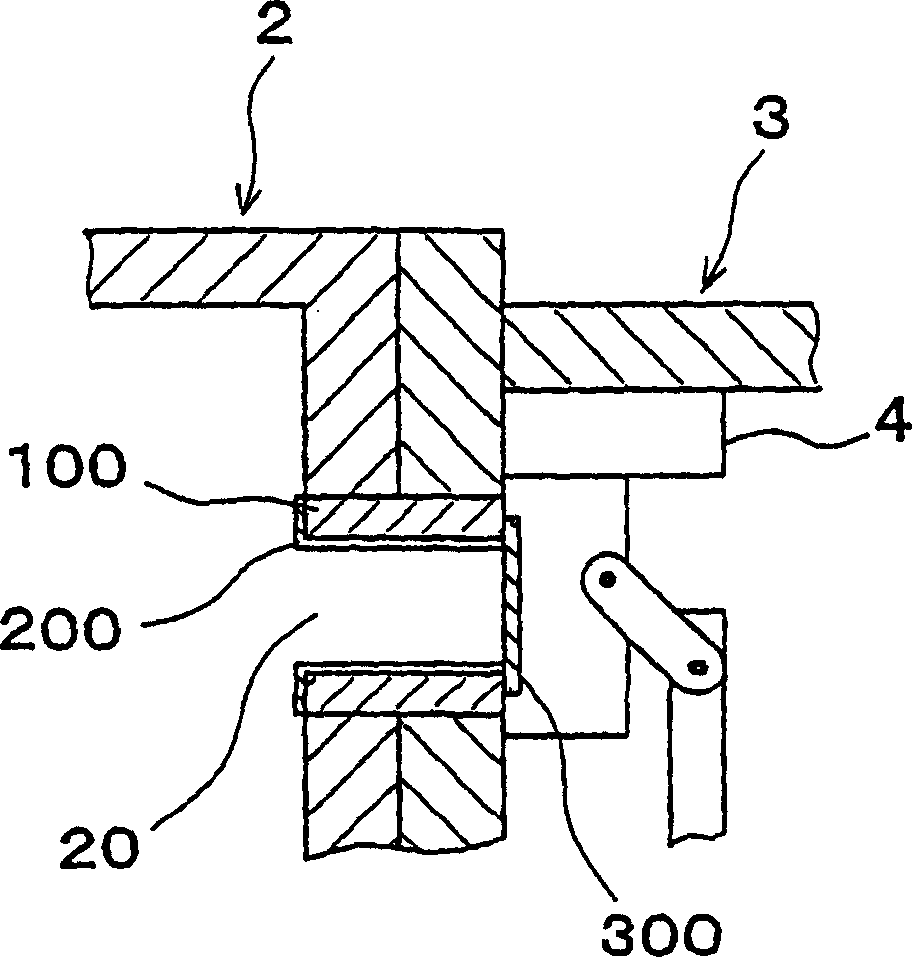
InactiveCN1468444AShorten the timeIncrease productivityElectric discharge tubesSemiconductor/solid-state device manufacturingRare earthEngineering
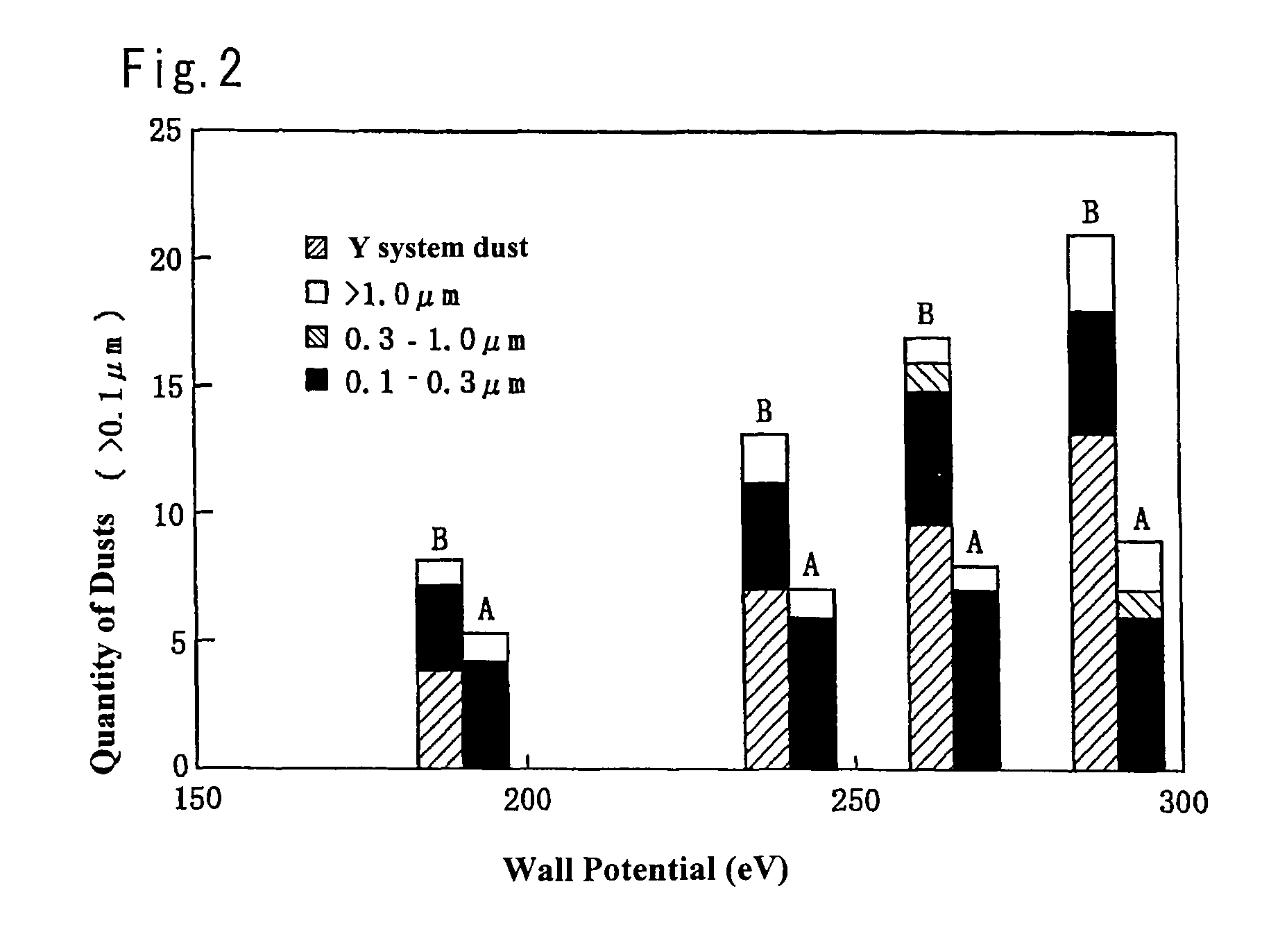
Maintenance work on an apparatus is facilitated, the maintenance cycle is extended and an improvement in throughput is achieved. A processing chamber 2 and an auxiliary vacuum chamber 3 are connected via a transfer port 20 formed through their wall surfaces. At the inner wall of the transfer port 20, a detachable gate liner 100 constituted of a plurality of members is installed. The maintenance work at the inner wall of the transfer port is facilitated since the gate liner 100 alone simply needs to be disengaged to be washed, replaced or the like. Insulating films 200 and 300 constituted of a rare earth oxide spray-deposit film with high plasma erosion resistance are used to coat the surface of the gate liner 100 and the surface of a gate valve 4 over the area covering the transfer port 20. As a result, damage attributable to plasma does not occur readily at these surfaces, and the extent of metal contamination and dust generation is lowered.
Owner:TOKYO ELECTRON LTD
Blank pipe fibroin microneedle drug administration system and preparation method thereof
PendingCN105771082AFacilitated releaseFacilitated DiffusionMicroneedlesMedical devicesHigh plasmaDrug administration
The invention belongs to the field of medical beauty microneedles and particularly relates to a blank pipe fibroin microneedle drug administration system and a preparation method thereof. The blank pipe fibroin microneedle drug administration system comprises a microneedle casing, a medicine, a swelling interlayer, a coating, a pressure-sensitive adhesive coating and a backing layer. The surface of the microneedle casing is provided with a plurality of evenly arranged needle-shaped protrusions, needle-shaped cavities are positioned in the needle-shaped protrusions, the volume of the needle-shaped protrusions accounts for 40-90% that of the needle-shaped protrusions, tips of the needle-shaped protrusions are provided with through holes, and the diameters of the through holes are 10-50 [mu]m. Medicines are placed in the needle-shaped cavities. The microneedle drug administration system is simple and short in manufacturing process, mild in processing condition, simple in finished product structure, flexible in medicine loading mode, higher in drug loading rate, suitable for symptoms needing quick drug release and high plasma concentration and suitable for large-scale production.
Owner:NANTONG TEXTILE & SILK IND TECH RES INST
Efficient florfenicol powder composition and preparation method thereof
InactiveCN102885777ARapid dissolutionEasy to prepareAntibacterial agentsOrganic active ingredientsOrganic solventPharmaceutical drug
The invention discloses an efficient florfenicol powder composition and a preparation method thereof. The powder composition comprises the following components in percentage by weight: 1-50% of florfenicol, 46-98.9% of a florfenicol-absorbed carrier and 0.05-4% of a florfenicol absorption promoter. The method comprises the following steps of: preparing the raw materials according to the above components; preparing a florfenicol organic solvent solution; dissolving the florfenicol absorption promoter and the florfenicol-absorbed carrier into water to obtain a mixed water solution; and adding the florfenicol organic solvent solution into the mixed water solution, and rapidly spray-drying. The preparation method disclosed by the invention is simple and low in cost. The product disclosed by the invention has the advantages of extrinsic quick release, esoteric quick absorption, high plasma peak concentration, thorough drug absorption and less antibiotic residue. The product disclosed by the invention can be applied to the treatment of infection on all parts of domestic animals caused by bacteria.
Owner:上海明磊邦森生物科技(登封)有限公司
Method of treating high plasma cholesterol levels
InactiveUS20060189574A1Reduce plasma cholesterol levelEliminate side effectsBiocideMetabolism disorderLipase inhibitorsBlood plasma
A method prevents or treats diseases associated with high plasma cholesterol levels. In addition, this method reduces plasma cholesterol levels. The method comprises administering a lipase inhibitor, e.g. orlistat, and a pharmaceutically acceptable bile acid sequestrant.
Owner:HADVARY PAUL +2
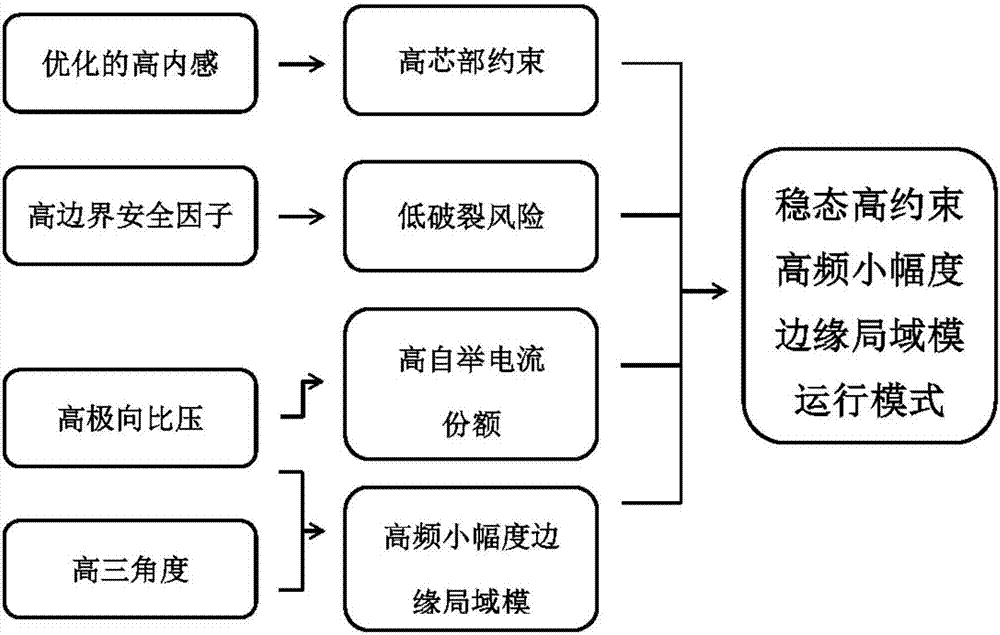
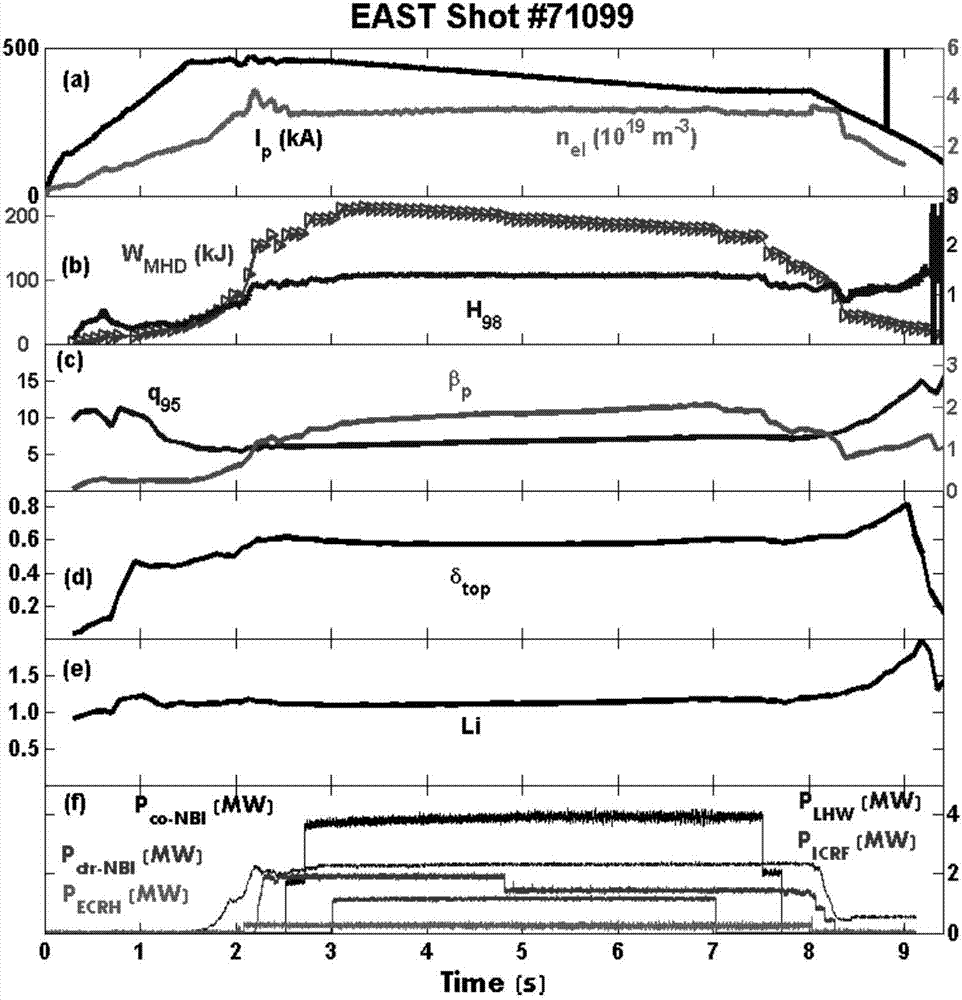
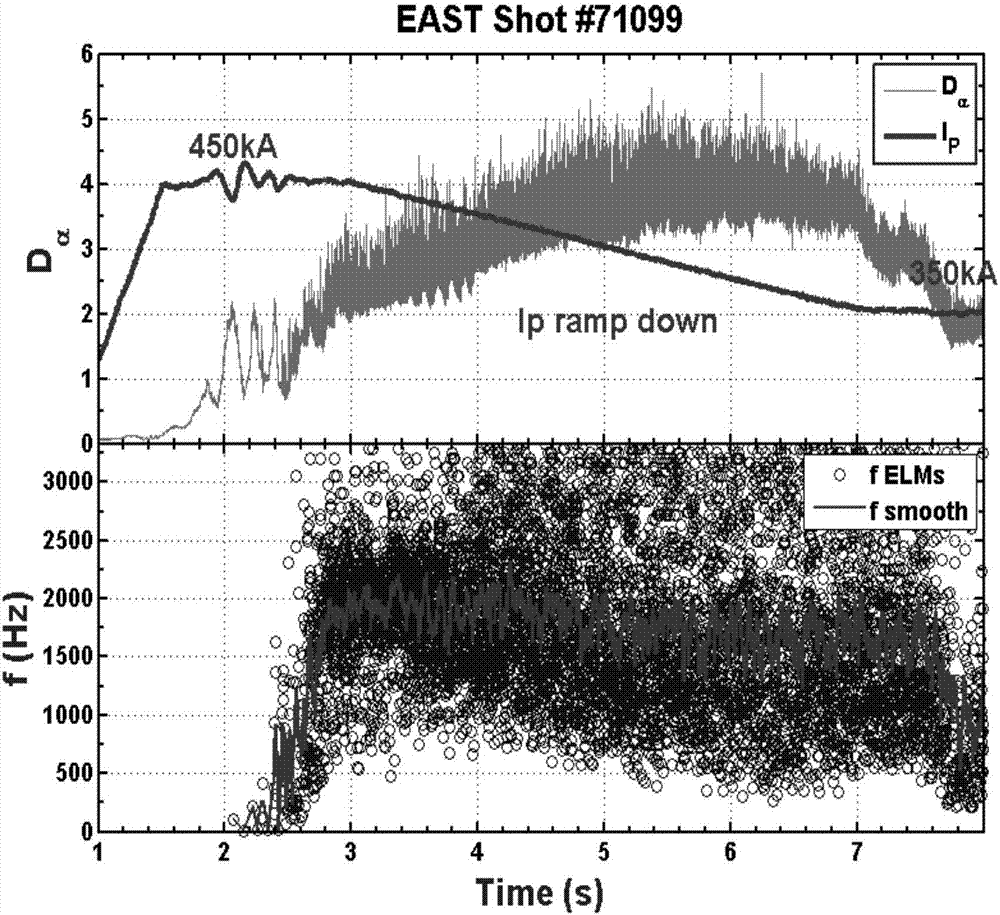
Stable high-restraint high-frequency small-amplitude boundary local die operation method suitable for fusion reactor
PendingCN107146640AHigh energy storageReduced risk of ruptureNuclear energy generationThermonuclear fusion reactorSmall amplitudeTriangulation
The invention discloses a stable high-restraint high-frequency small-amplitude boundary local die operation method suitable for a fusion reactor. On the condition of optimized high inner resistance, high boundary safety factor, high polarized specific voltage and high triangulation, high plasma energy storage and high plasma core restraint are obtained, and small-amplitude high-frequency boundary local die is obtained. The high core restraint facilitates sustaining of high parameter of the plasma core part and facilitates realization of a fusion reaction. A high-frequency small-amplitude boundary local die facilitates settlement of a first wall transient heat load problem, and furthermore has relatively high particle eliminating capability, thereby preventing a plasma impurity core polymerization problem. The operation parameter interval which is required by the method is compatible with the operation interval of a future fusion reactor. Furthermore stable state operation of the plasma can be realized. The stable high-restraint high-frequency small-amplitude boundary local die operation method has low breakage risk, excellent robustness and high repeatability. The stable high-restraint high-frequency small-amplitude boundary local die operation method has a relatively ideal operation mode which may be used for the reactor grade.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Plasma processing method and plasma processing device
InactiveUS20070056928A1Increase productionReduce the amount requiredElectric discharge tubesDecorative surface effectsForeign matterDevice material
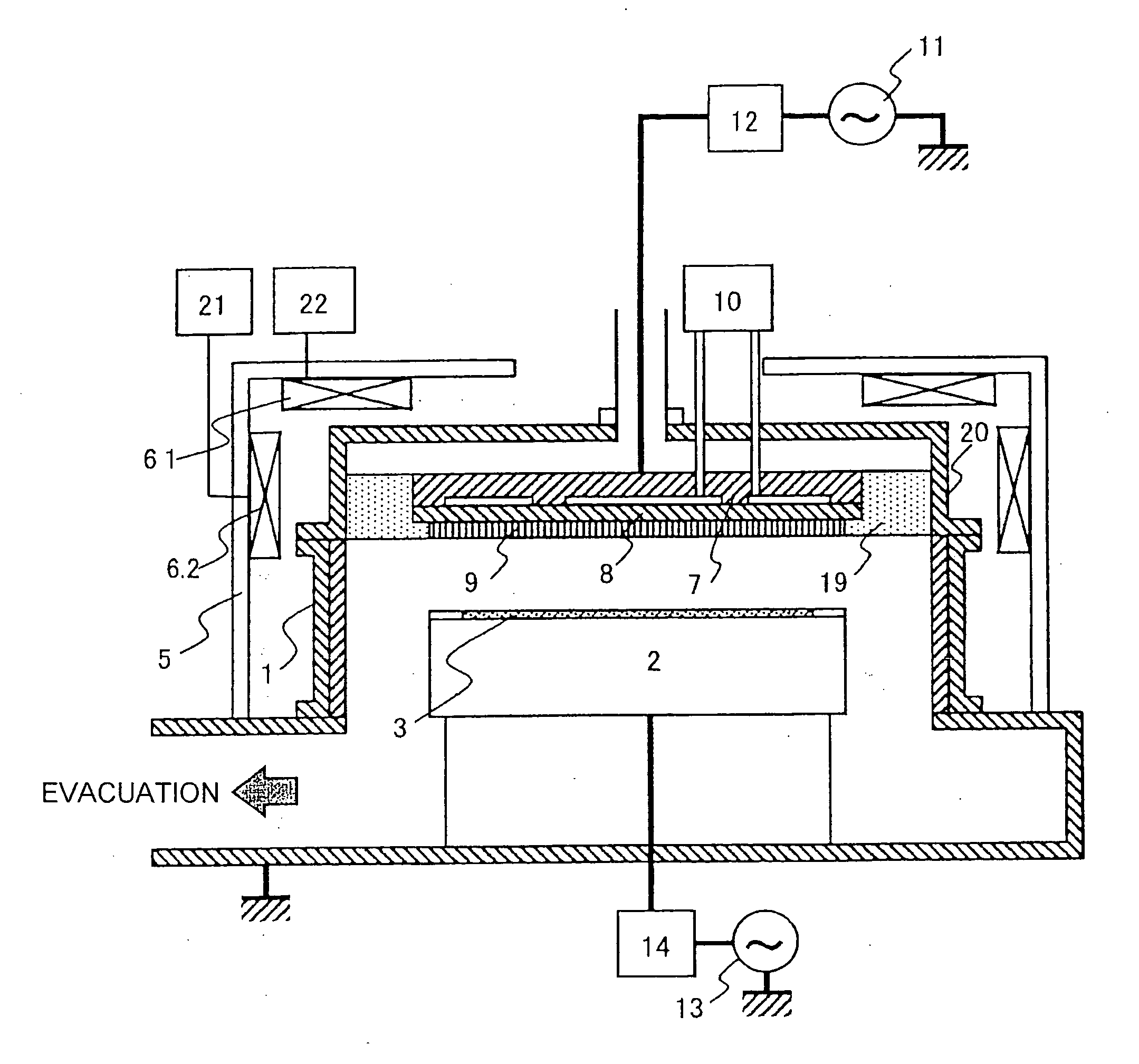
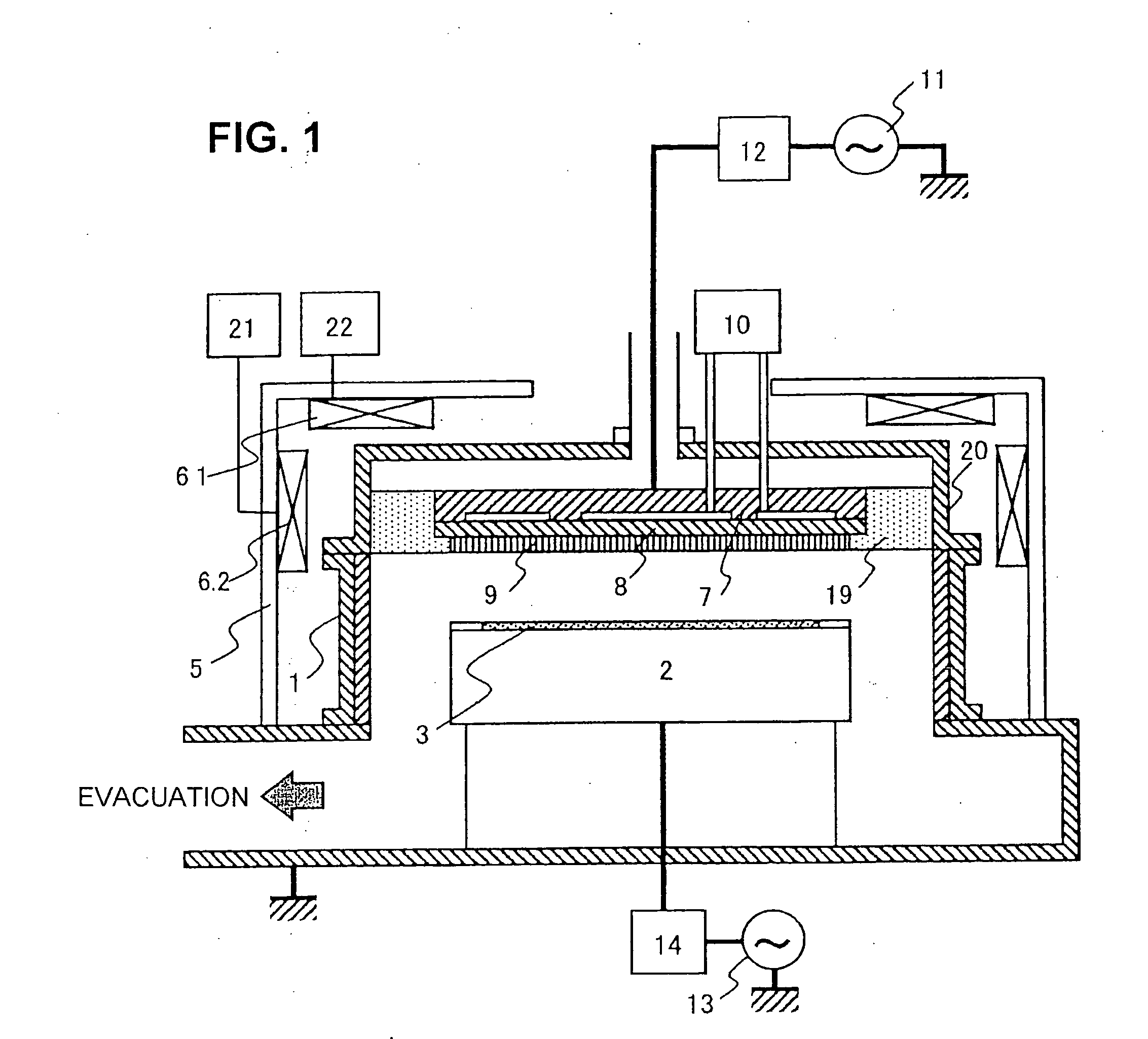
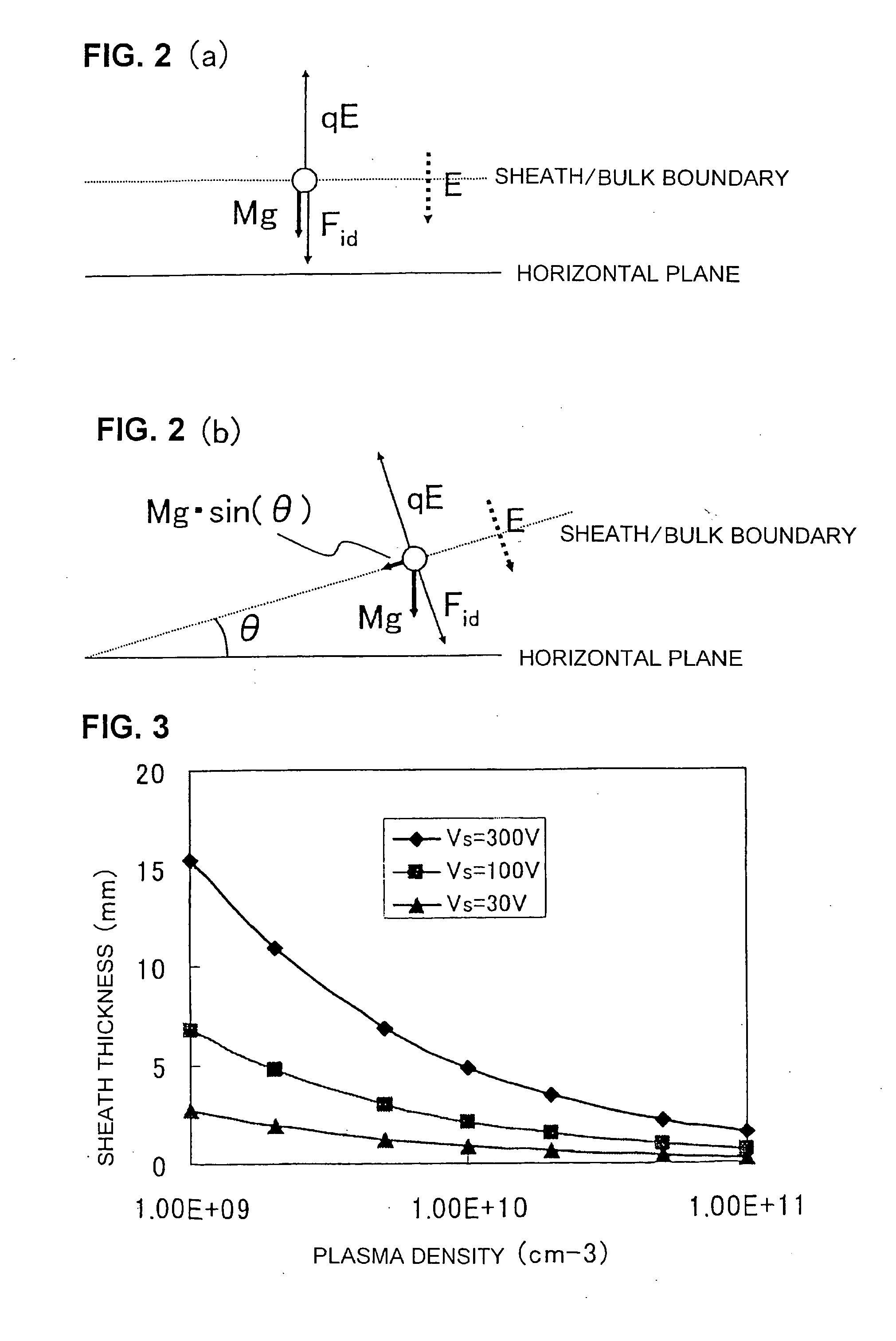
The invention provides a plasma processing method and plasma processing device for manufacturing semiconductor devices in which the number of foreign particles being adhered to the wafer is reduced greatly and the yield is improved. In a plasma processing device having a plasma source capable of controlling plasma distribution, the shape of a sheath / bulk boundary above the wafer is controlled to a convexed shape when the plasma is turned on and off. By adding a step of applying a low source power and wafer bias power when the plasma is turned on and off in order to realize an out-high plasma distribution, it is possible to form a sheath that is thicker near the center of the wafer and thinner at the outer circumference portion thereof.
Owner:HITACHI HIGH-TECH CORP
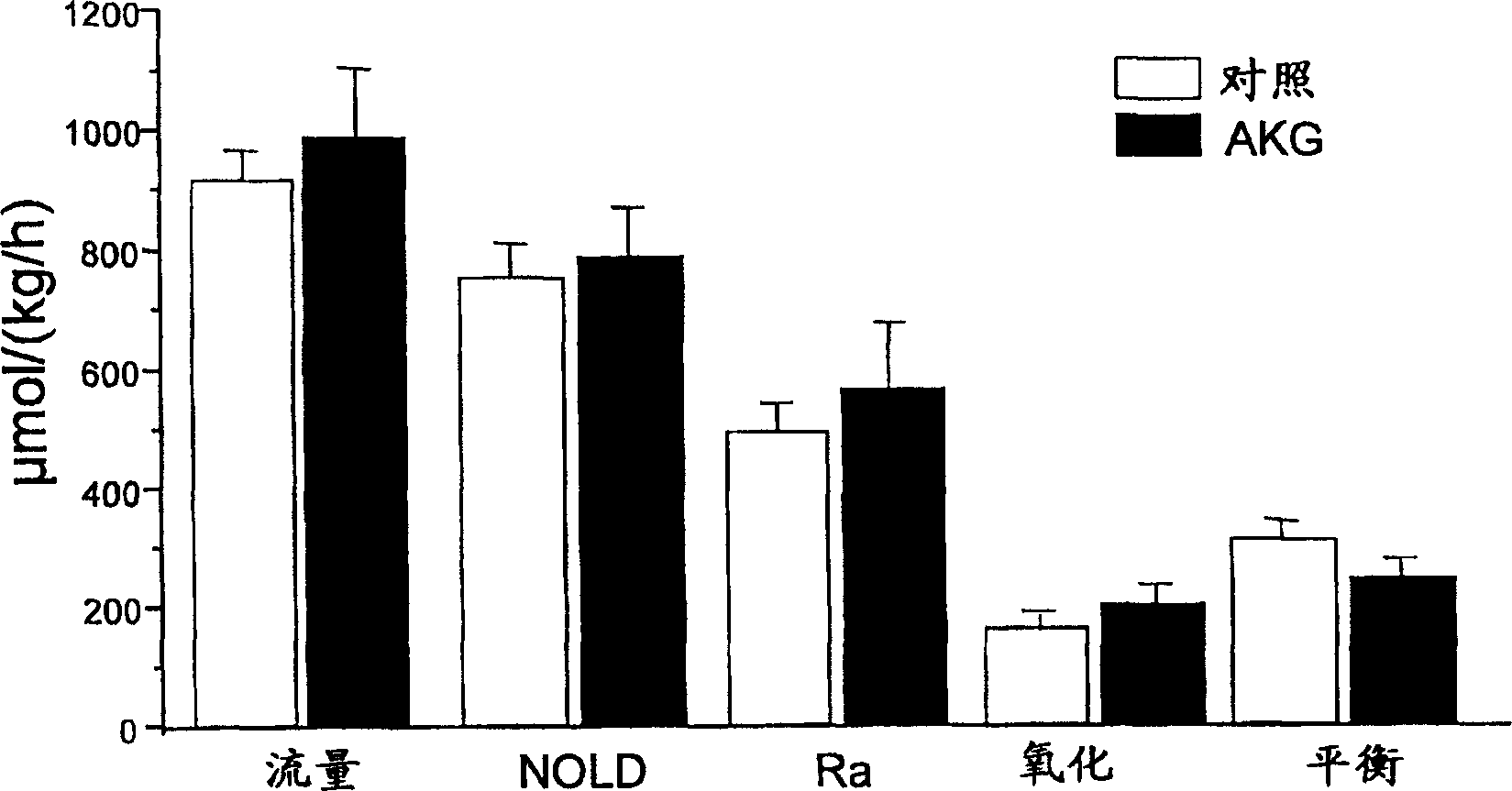
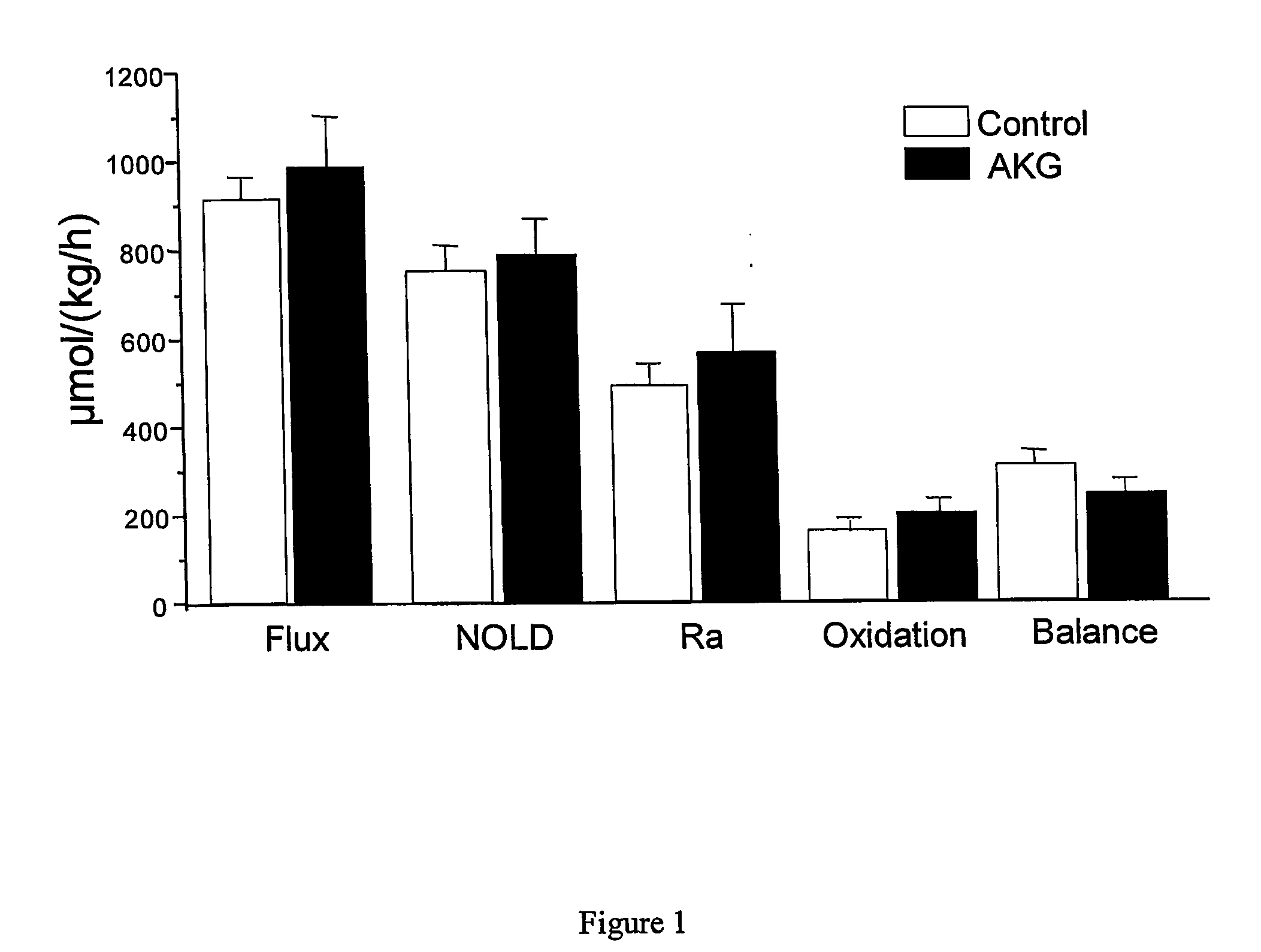
Use of alpha-ketoglutaric acid for the treatment of malnutrition or high plasma glucose condition
A method for improving adsorption of amino acids in a vertebrate, including mammal and bird, is included. The method comprises administering to a vertebrate, including mammal and bird, in a sufficient amount and / or at a sufficient rate to enable desire effect. AKG, AKG derivatives or metabolites, AKG analogues or mixture thereof. Also conteplated is a method for decreasing adsorption of glucose in a vertebrate, including mammal and bird, in the need thereof, AKG, AKG derivates or metabolites, AKG analogues or mixture thereof, for decreasing glucose adsorption as well as compositions for use in treatment.
Owner:ESSENTYS AB
Methods and apparatus for plasma-based deposition
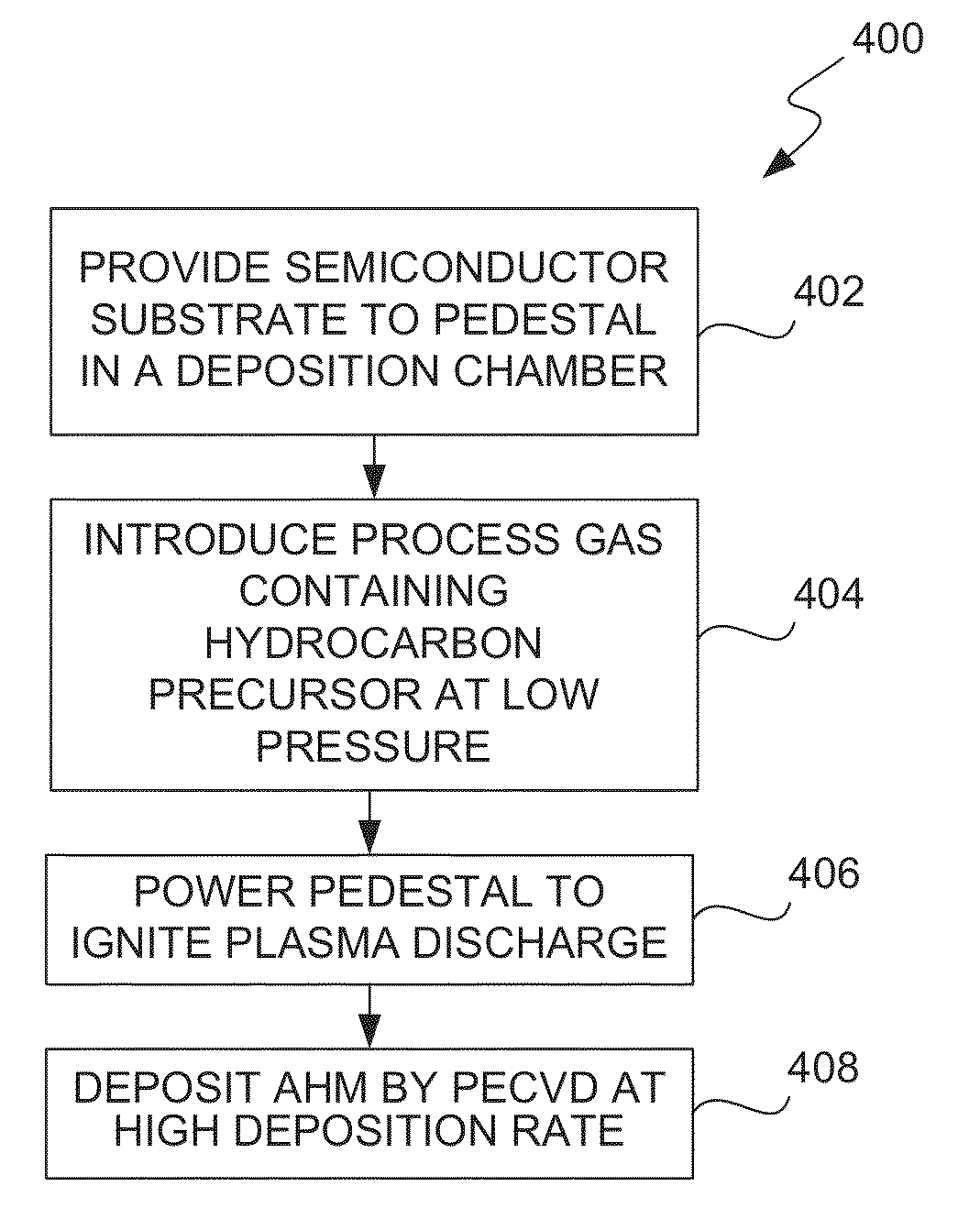
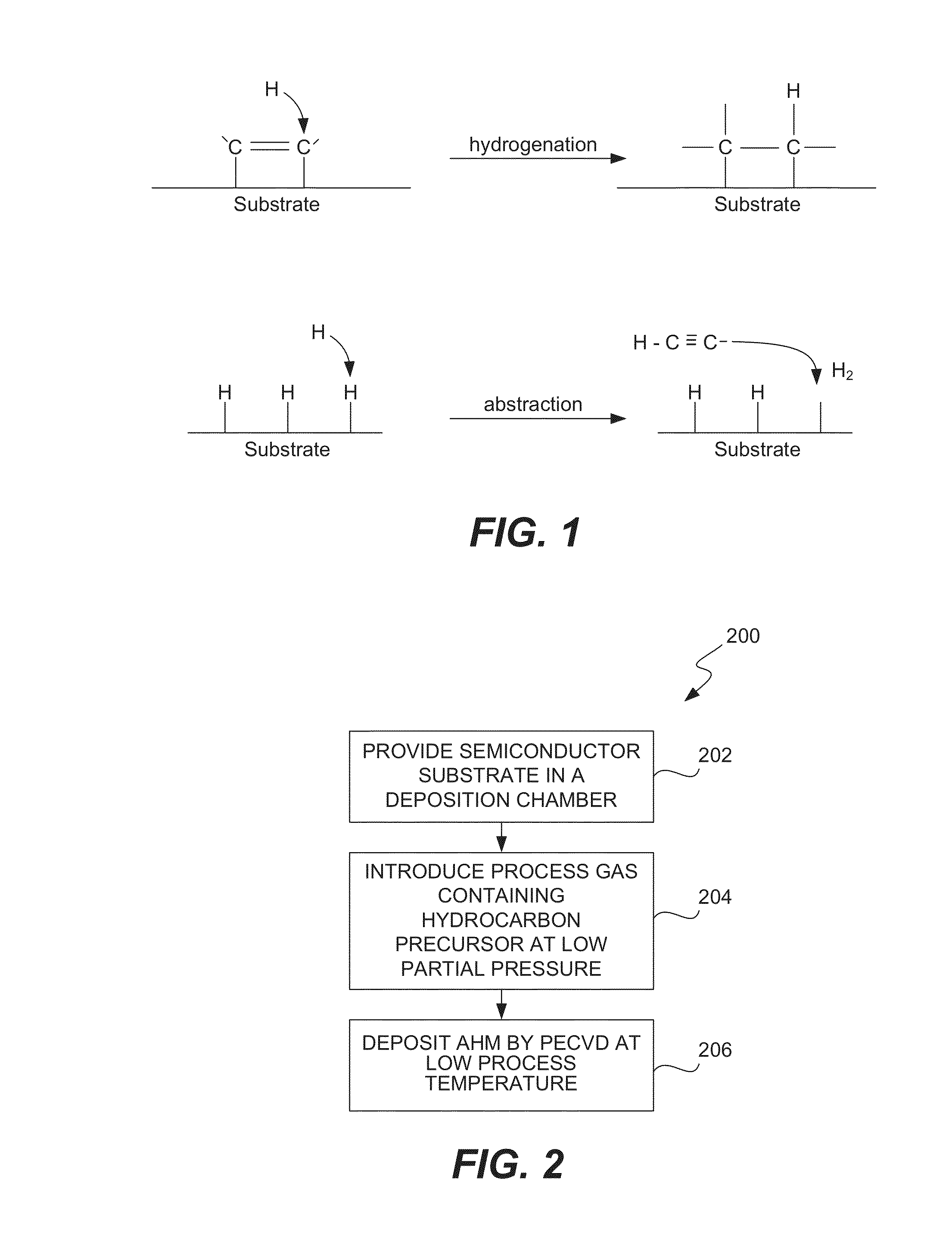
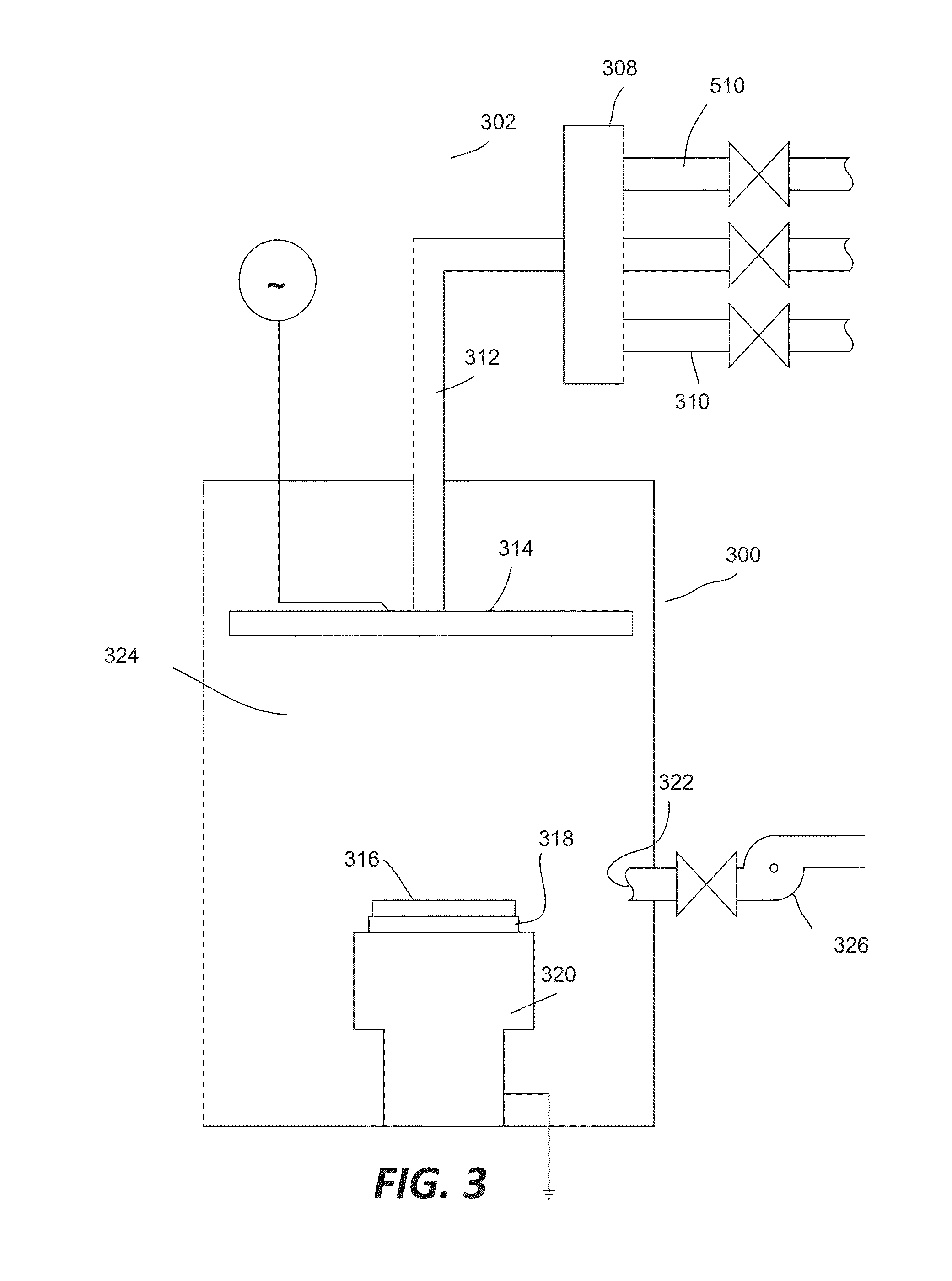
ActiveUS8962101B2Improve etch selectivityReduction of overall thermal budgetElectric discharge tubesSemiconductor/solid-state device manufacturingEngineeringHigh plasma
High-deposition rate methods for forming transparent ashable hardmasks (AHMs) that have high plasma etch selectivity to underlying layers are provided. The methods involve placing a wafer on a powered electrode such as a powered pedestal for plasma-enhanced deposition. According to various embodiments, the deposition is run at low hydrocarbon precursor partial pressures and / or low process temperatures. Also provided are ceramic wafer pedestals with multiple electrode planes embedded with the pedestal are provided. According to various embodiments, the pedestals have multiple RF mesh electrode planes that are connected together such that all the electrode planes are at the same potential.
Owner:NOVELLUS SYSTEMS
Use of alpha-ketoglutaric acid for the treatment of malnutrition or high plasma glucose condition
InactiveUS20060247207A1Reduce absorptionBiocidePeptide/protein ingredientsMetaboliteGlucose polymers
A method for improving adsorption of amino acids in a vertebrate, including mammal and bird, is included. The method comprises administering to a vertebrate, including mammal and bird, in a sufficient amount and / or at a sufficient rate to enable desire effect. AKG, AKG derivatives or metabolites, AKG analogues or mixture thereof. Also conteplated is a method for decreasing adsorption of glucose in a vertebrate, including mammal and bird, in the need thereof, AKG, AKG derivates or metabolites, AKG analogues or mixture thereof, for decreasing glucose adsorption as well as compositions for use in treatment.
Owner:ESSENTYS AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com