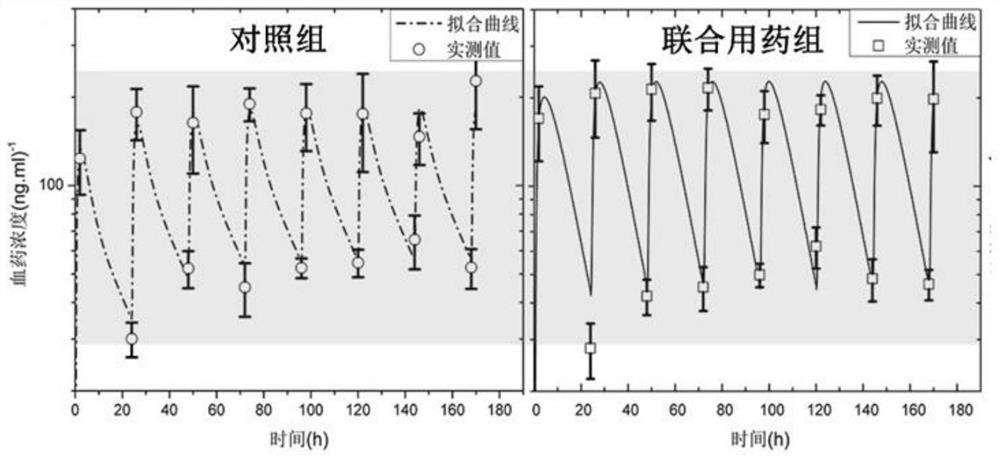
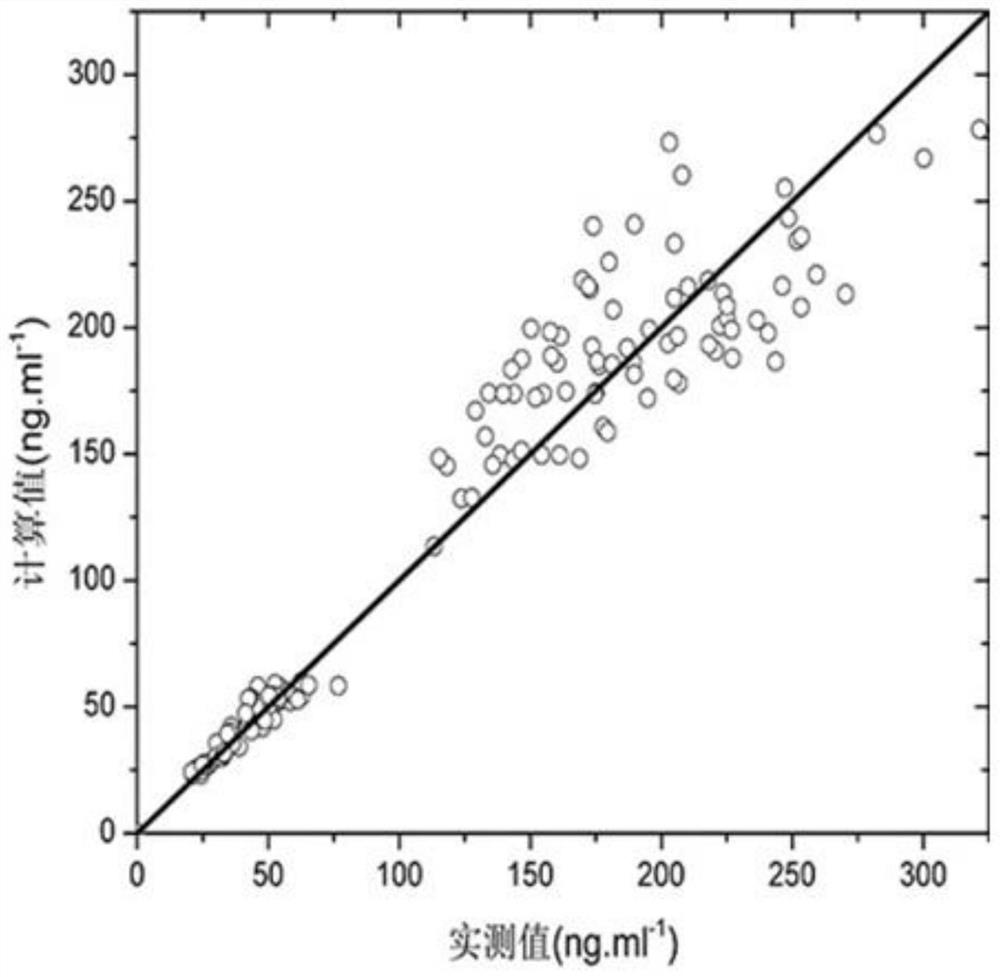
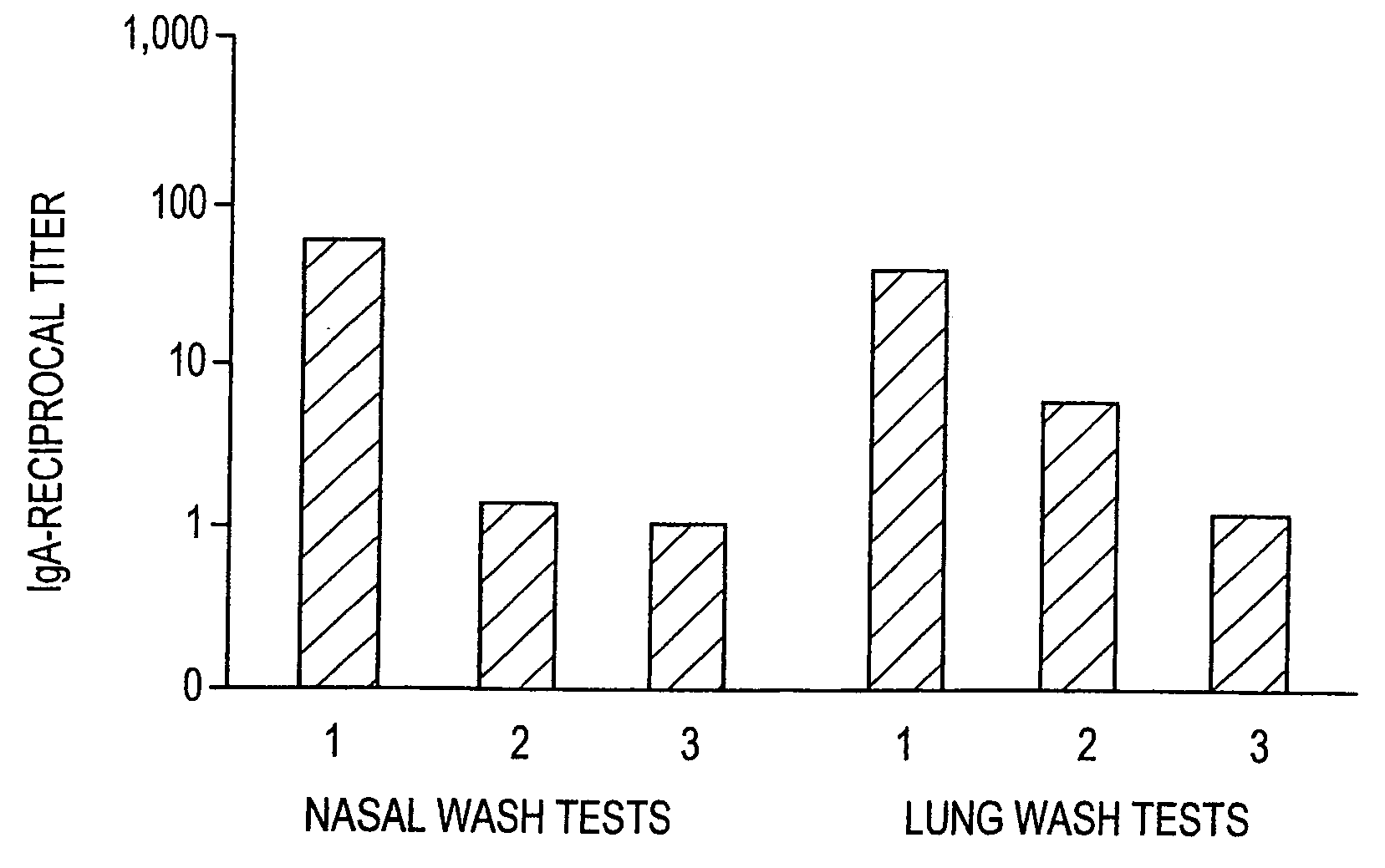
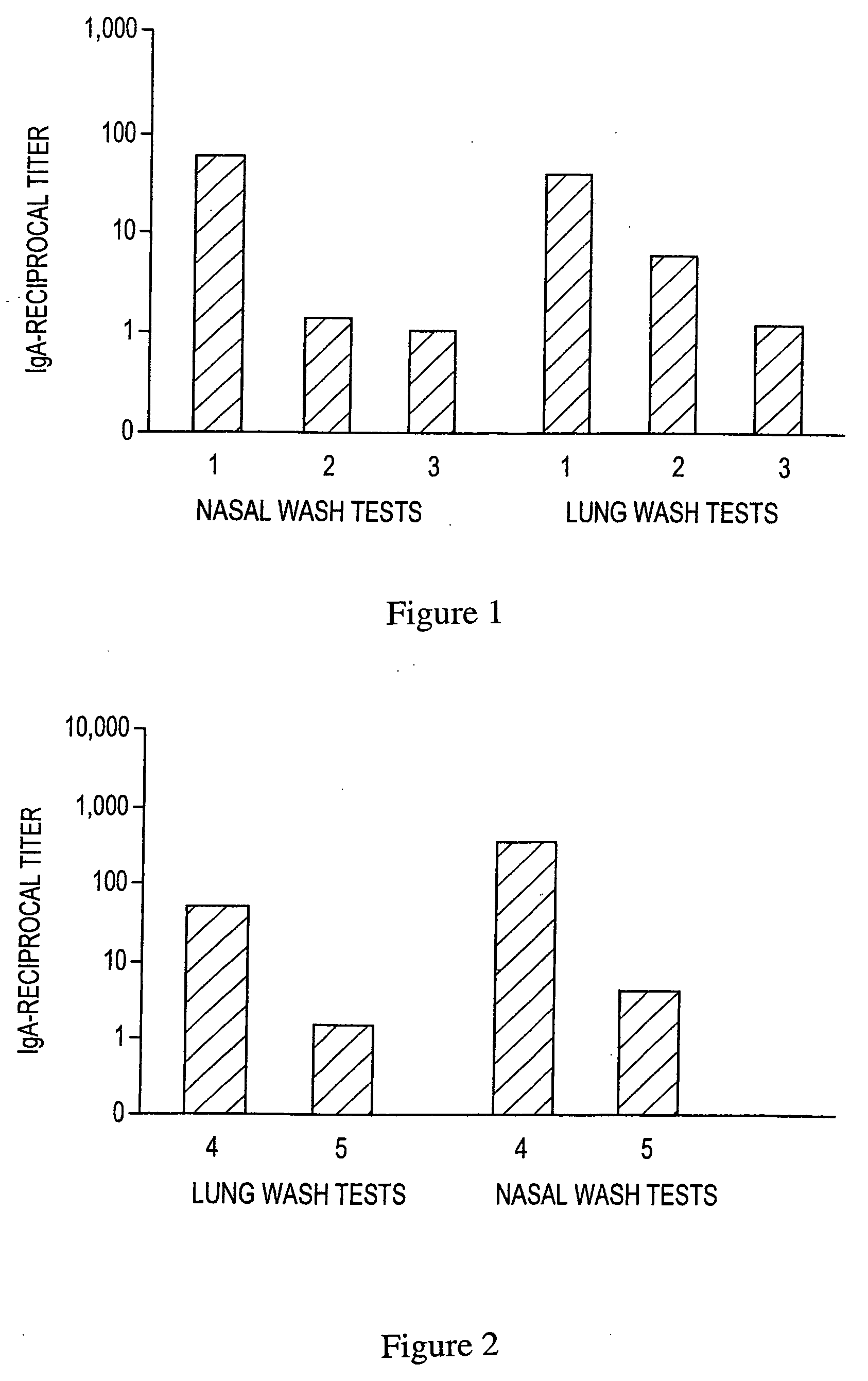
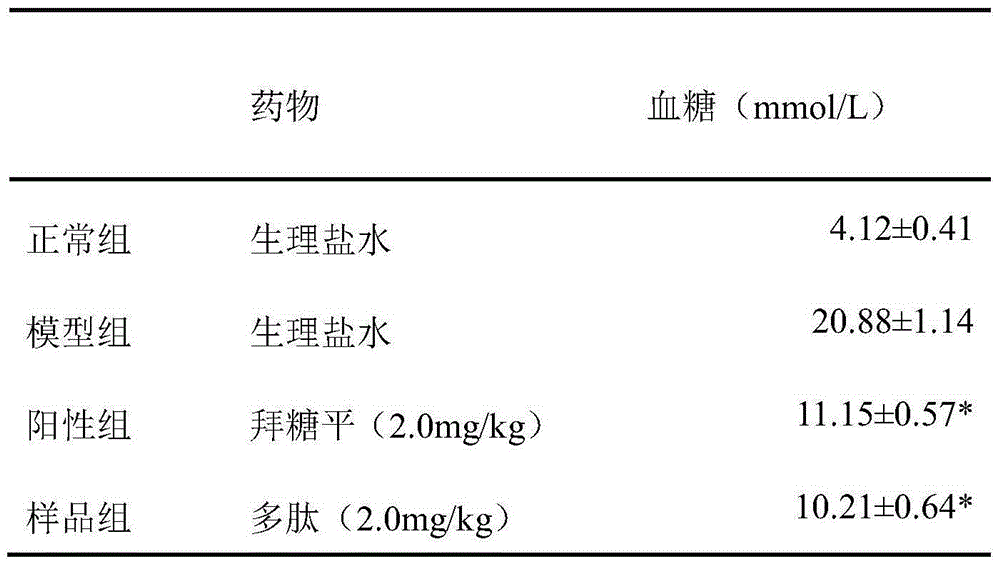
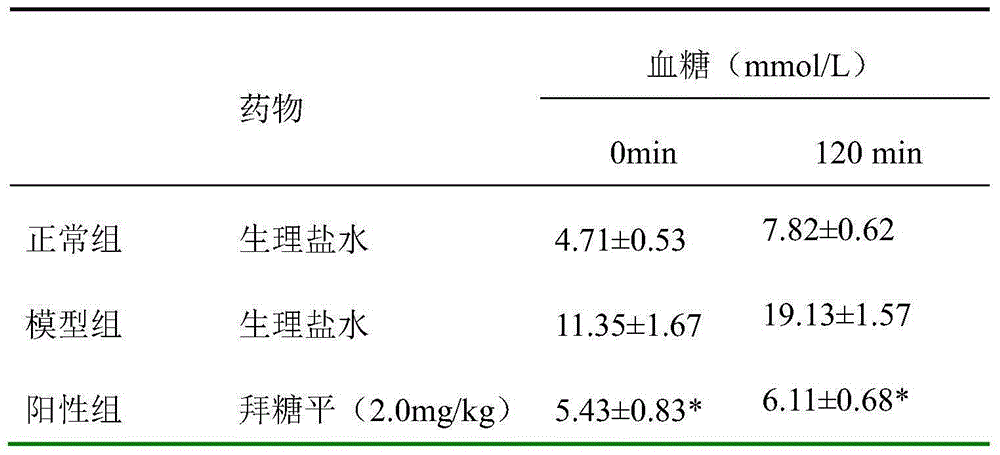
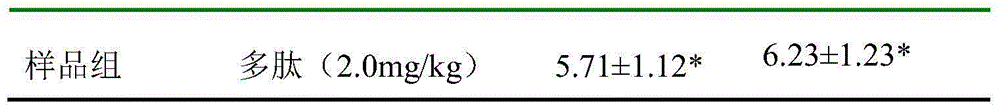
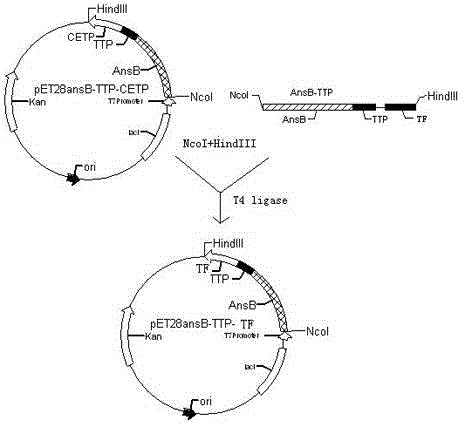
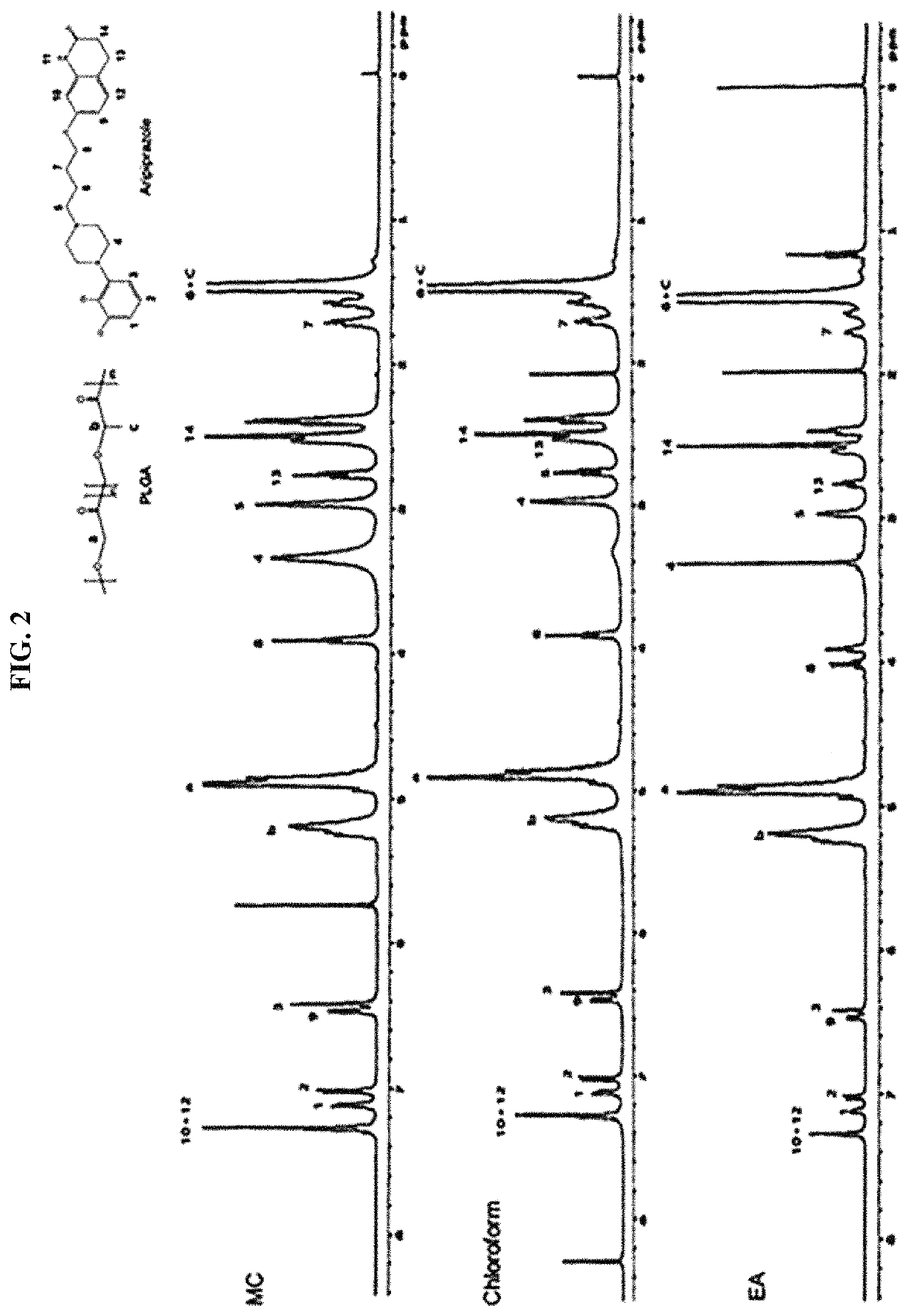
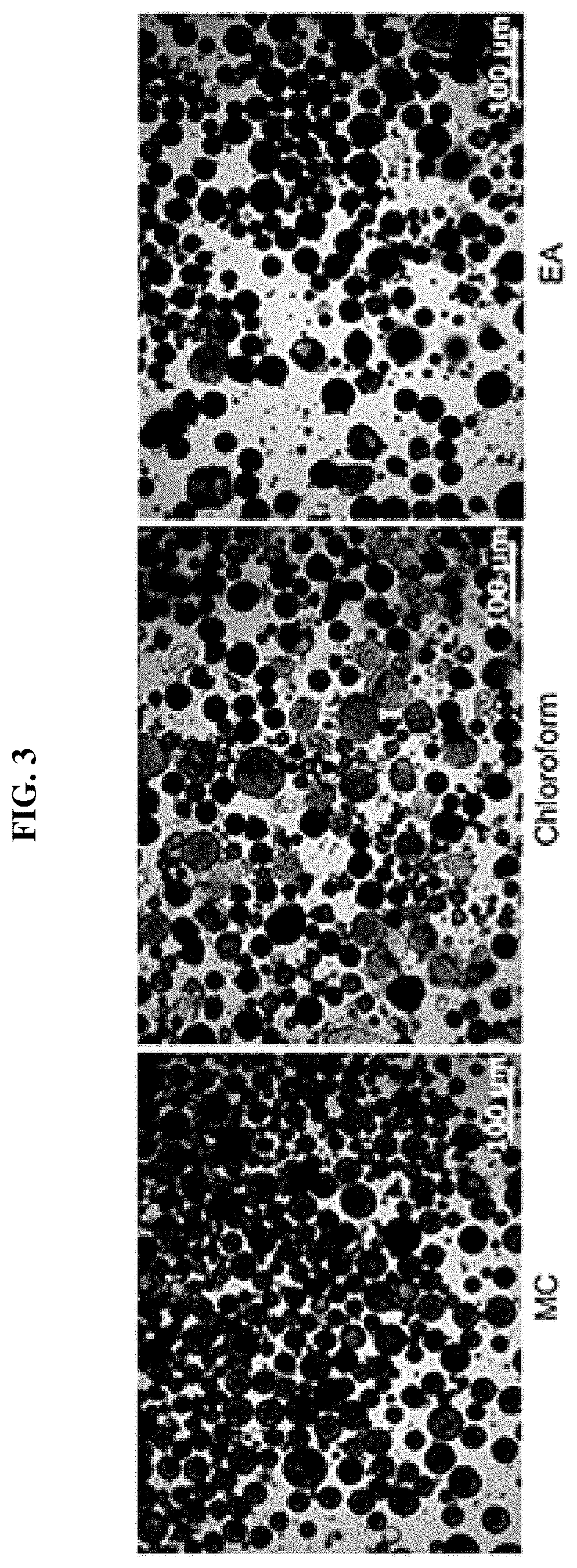
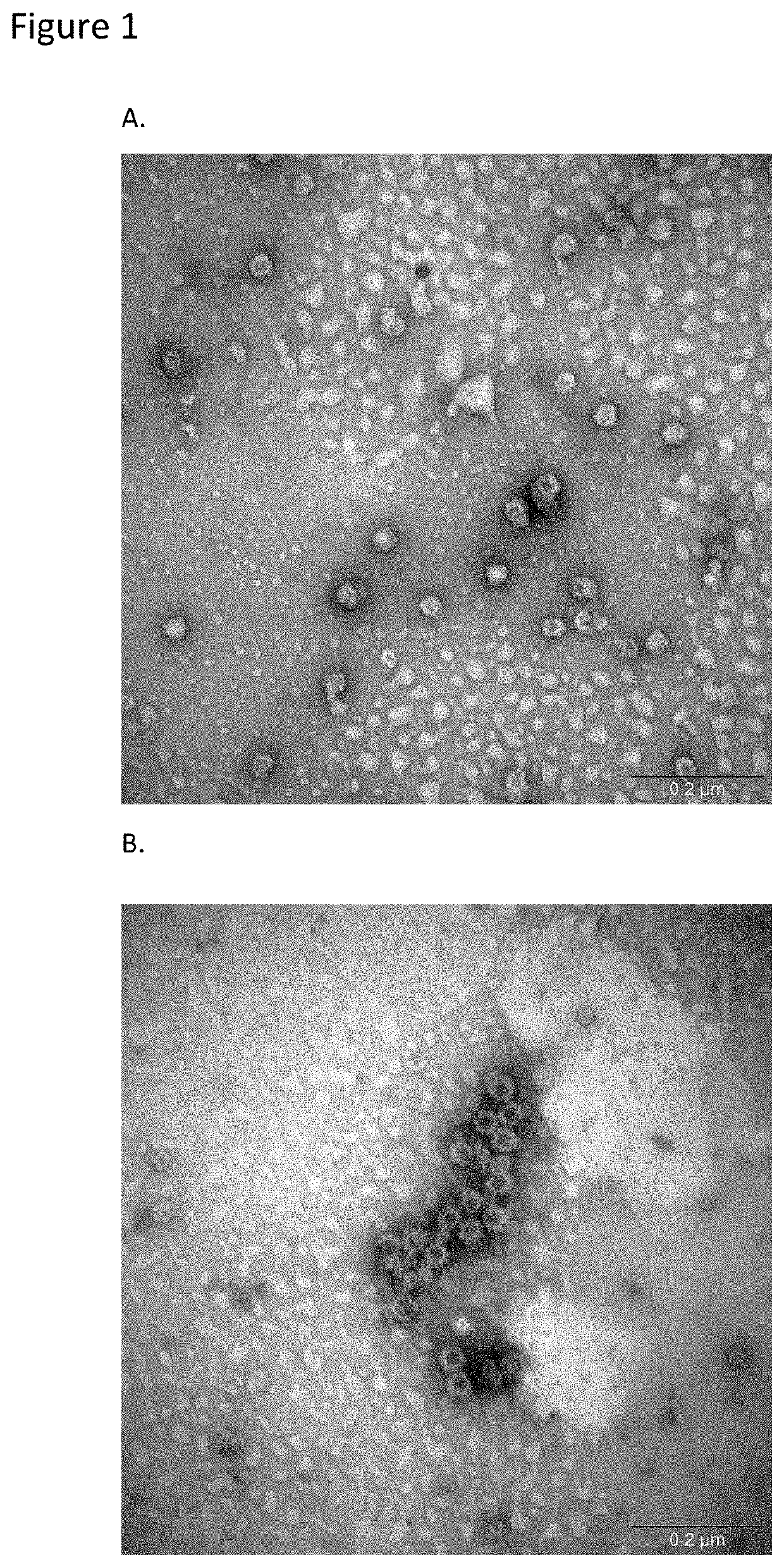
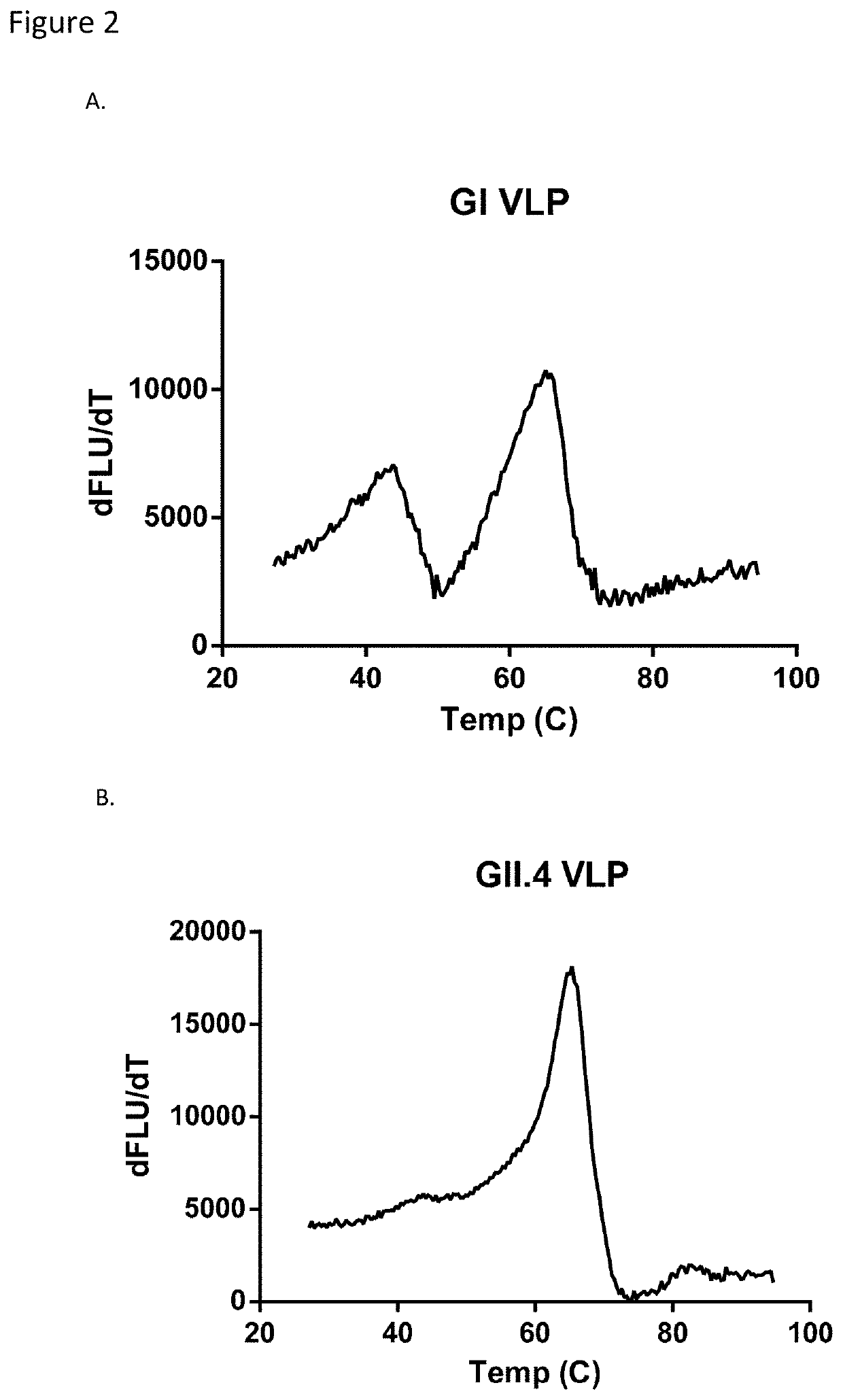
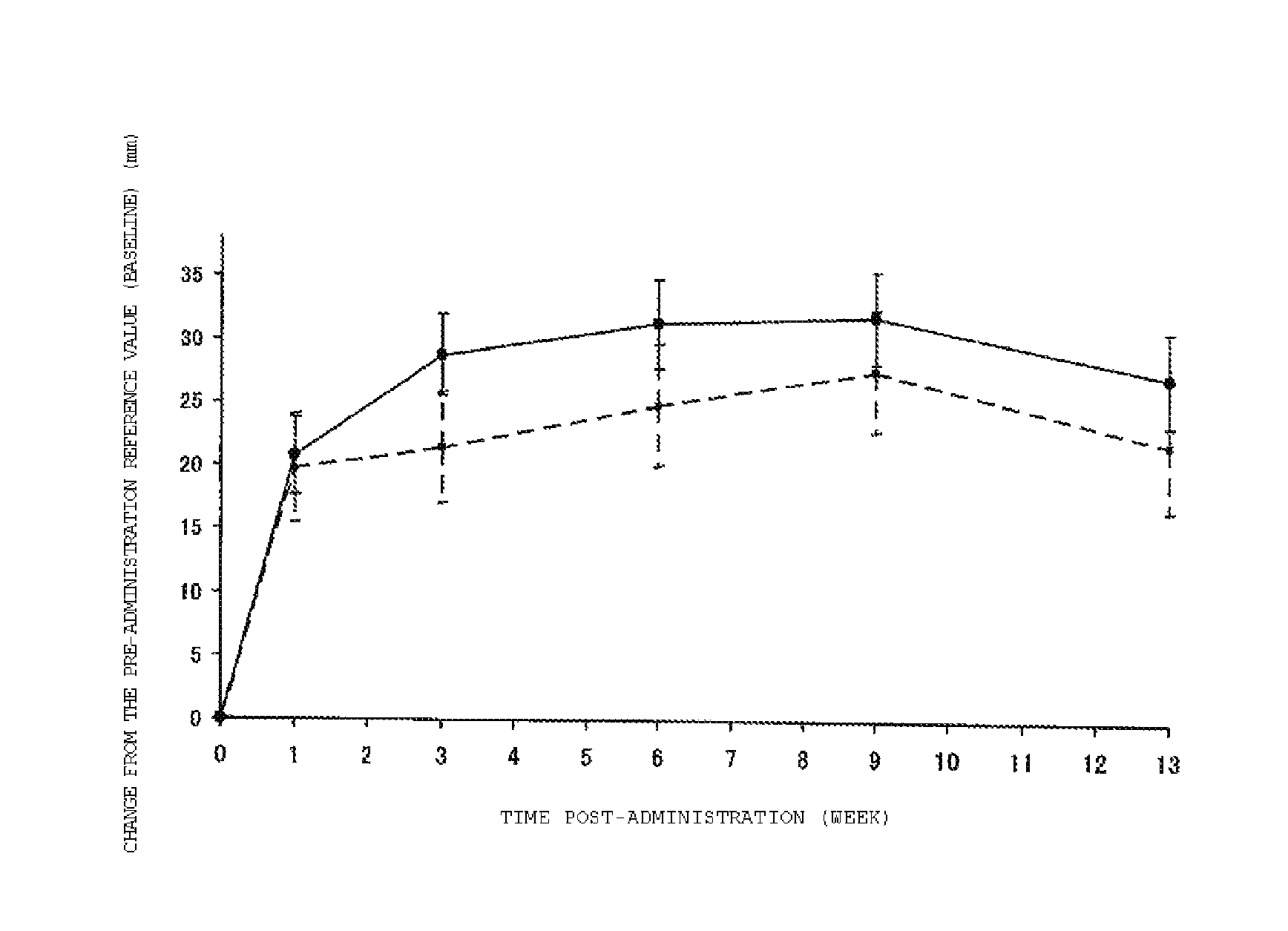
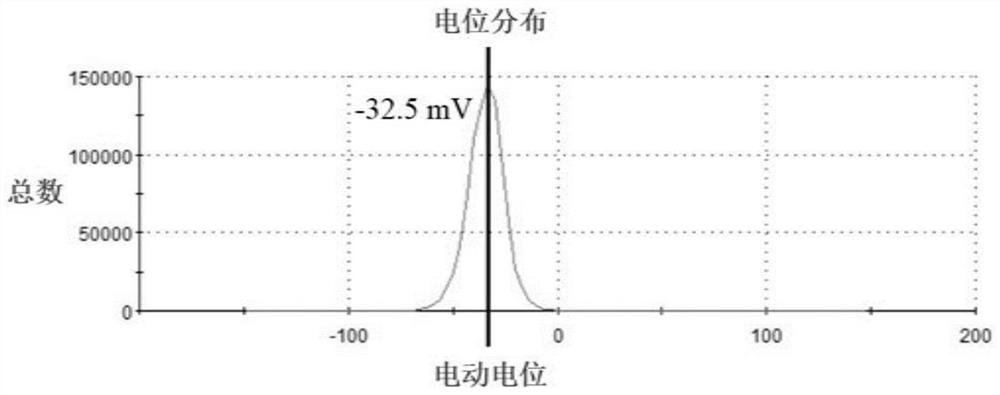
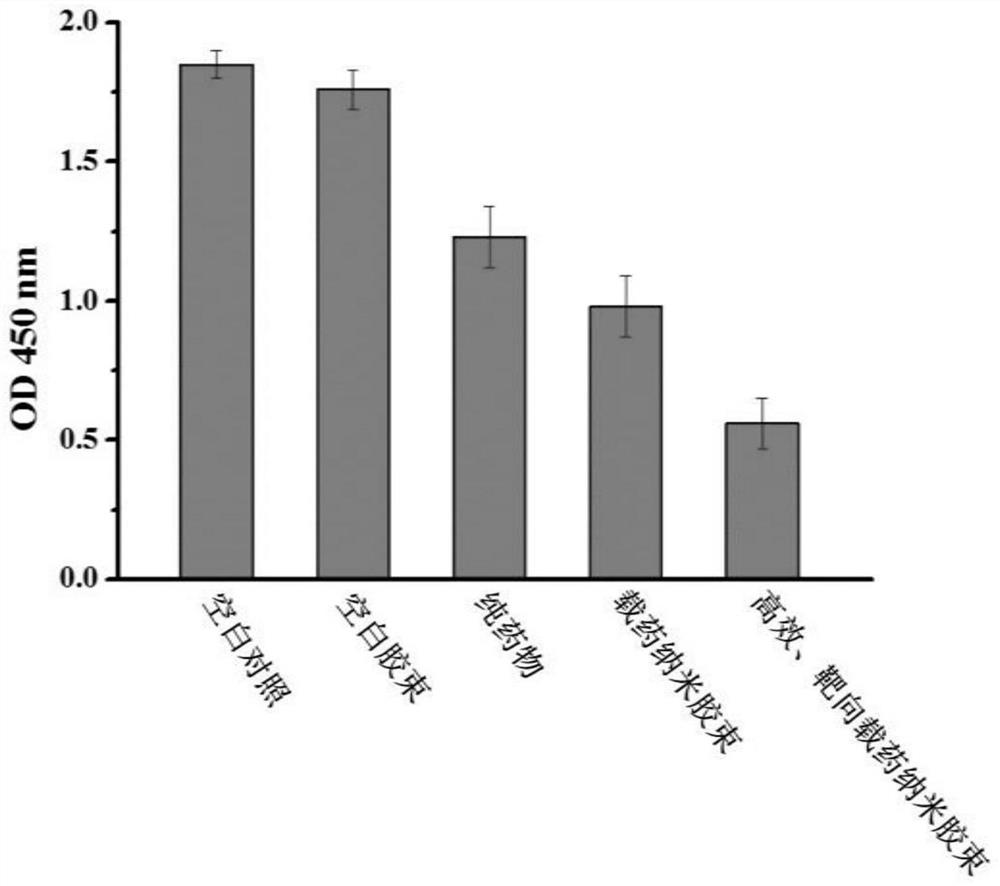
Patents
Literature
39 results about "Multiple administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
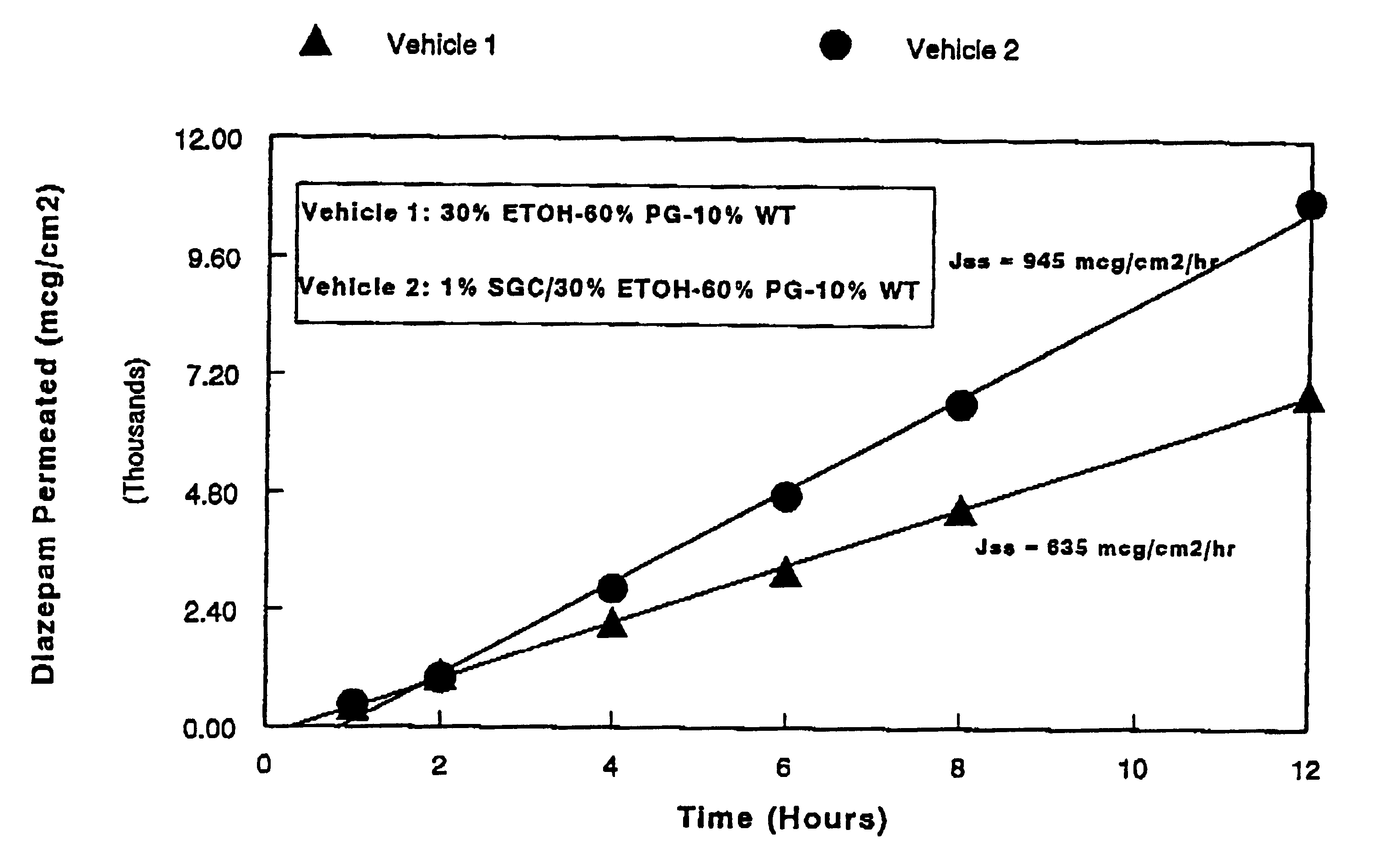
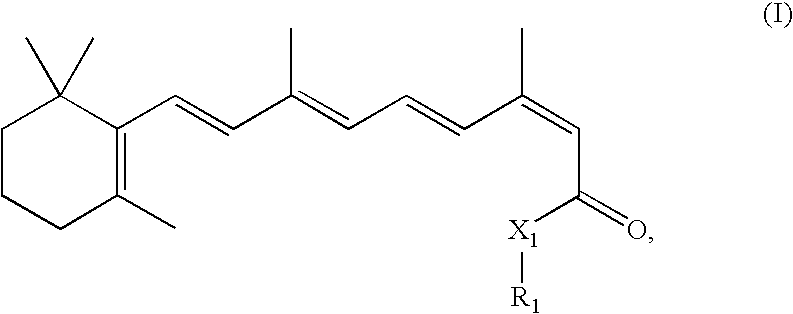
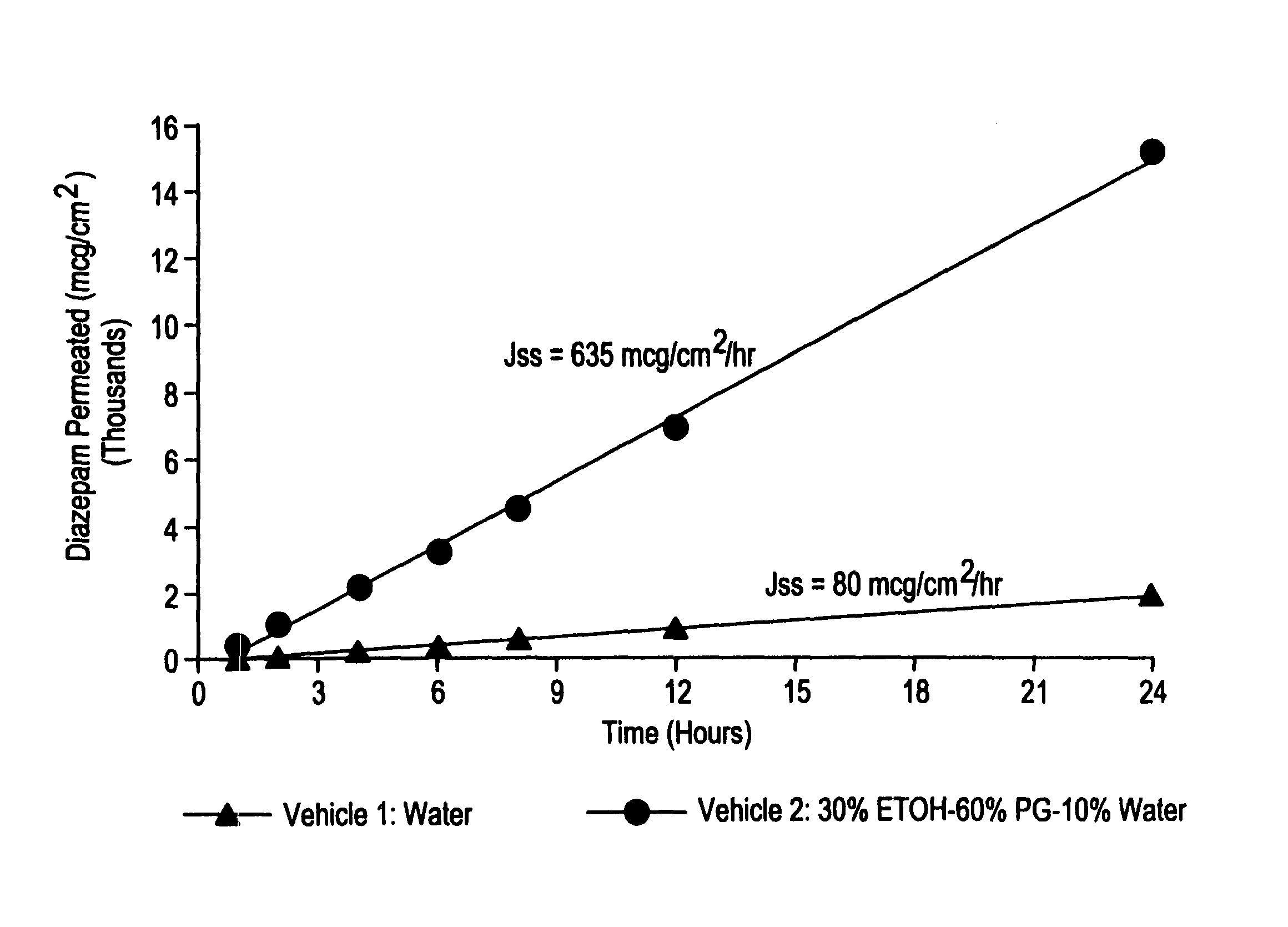
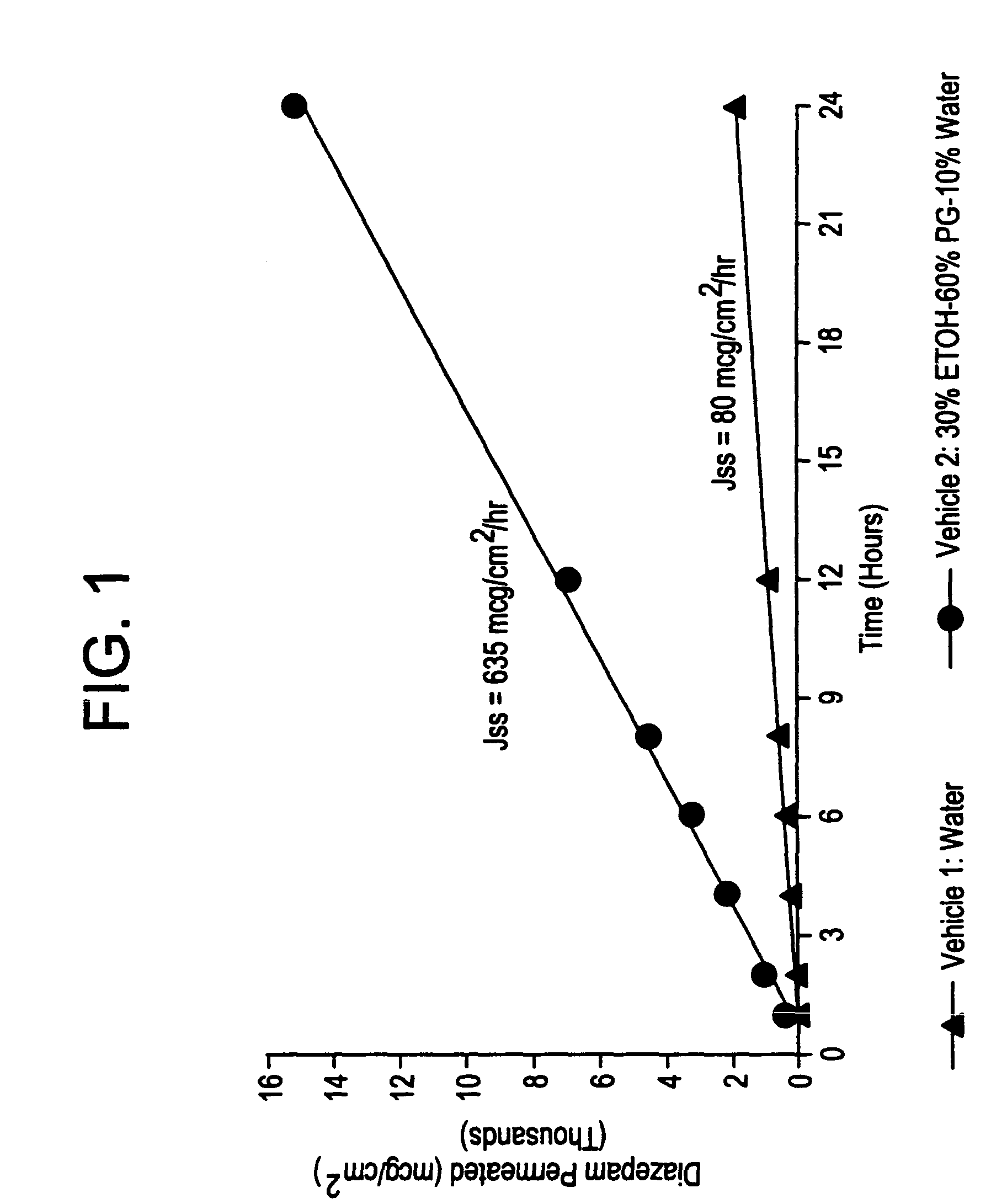
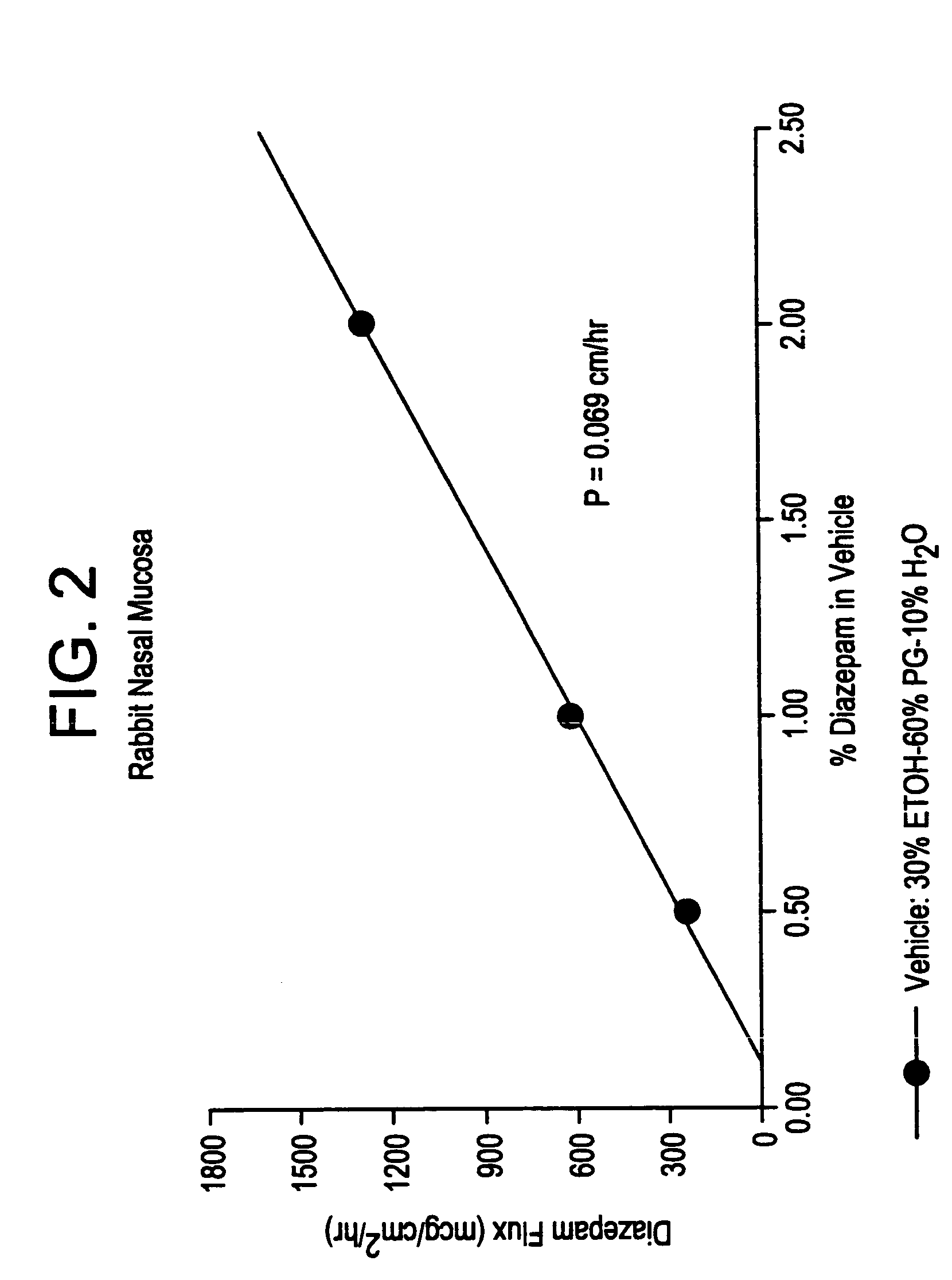
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
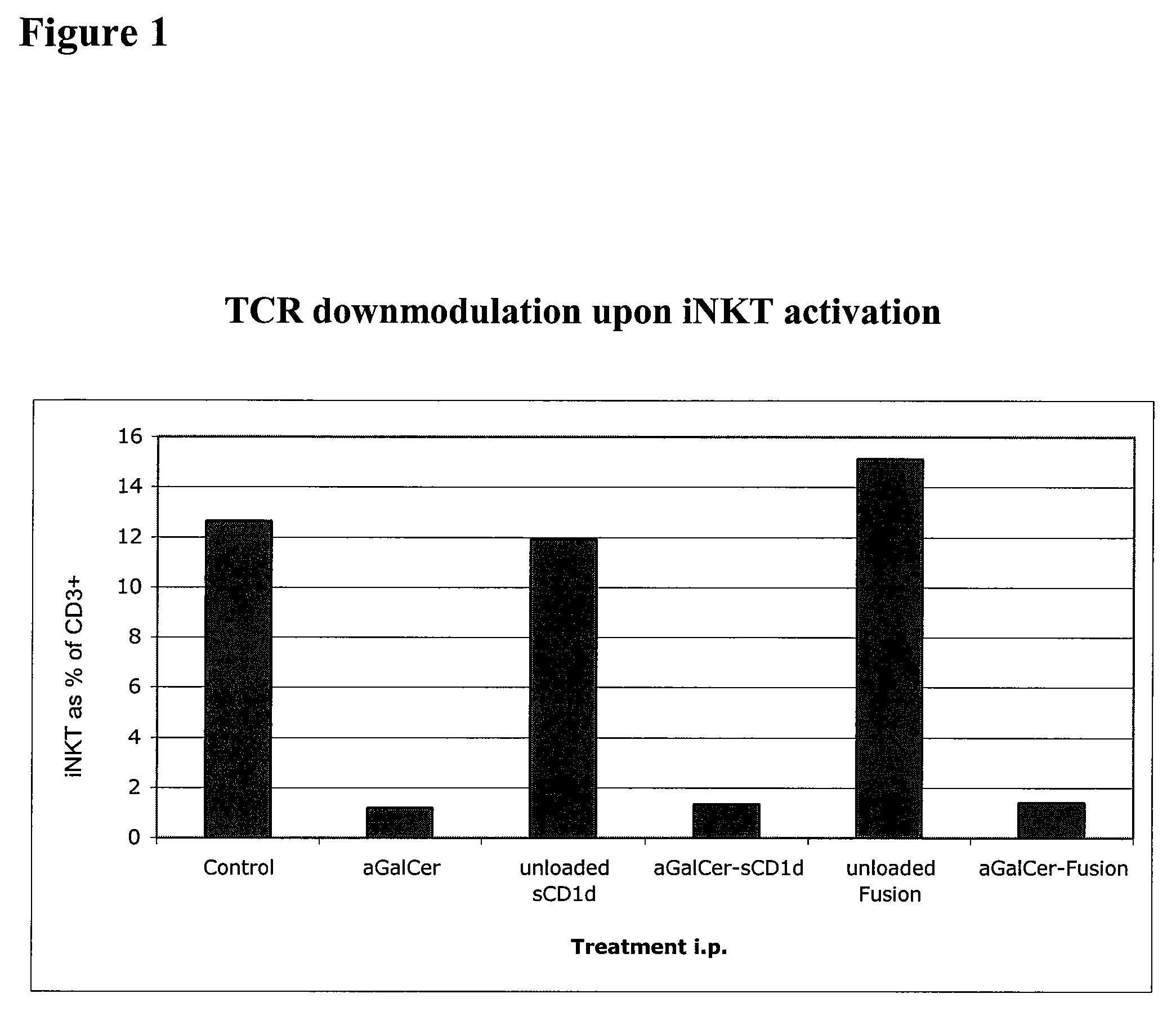
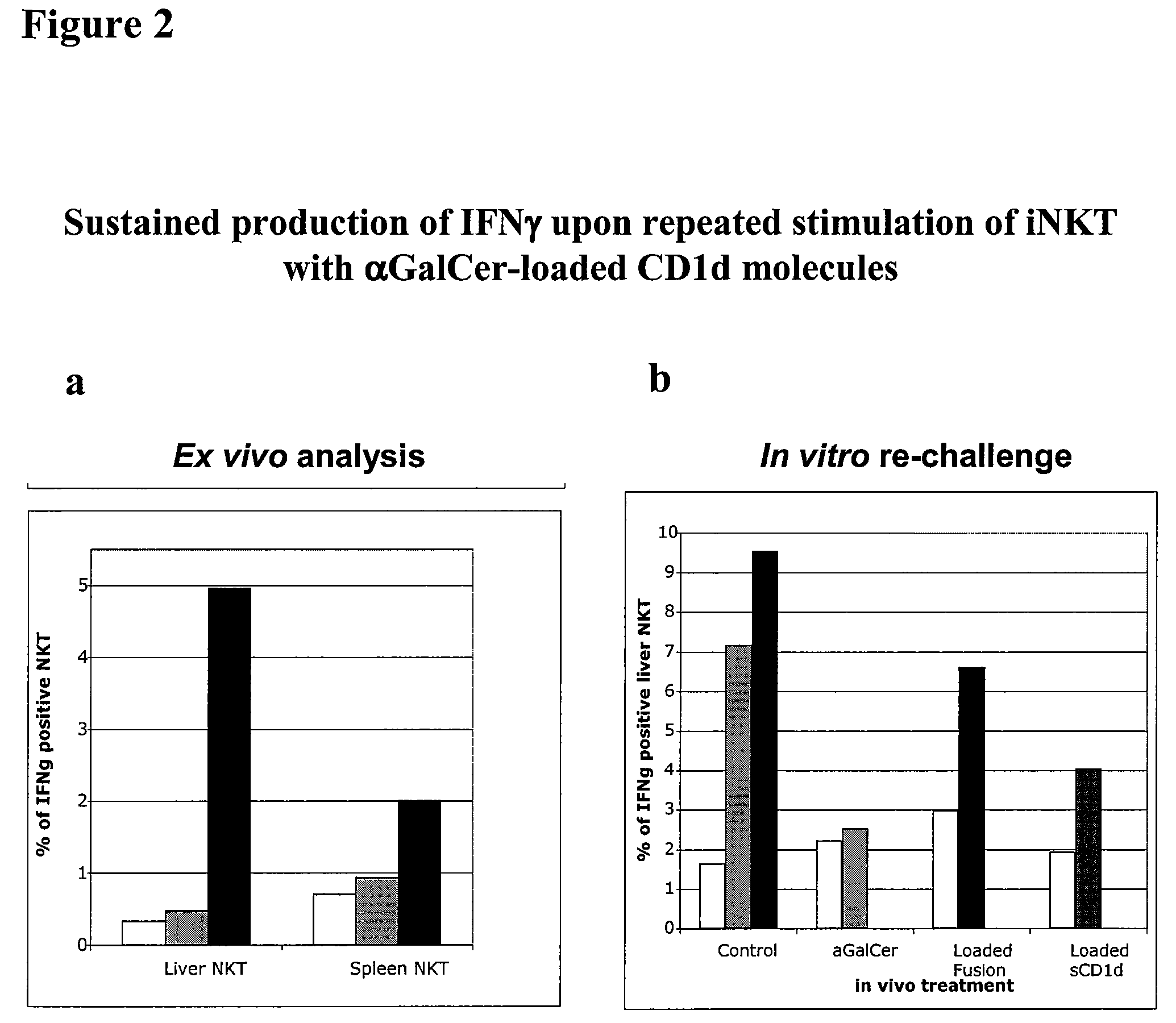
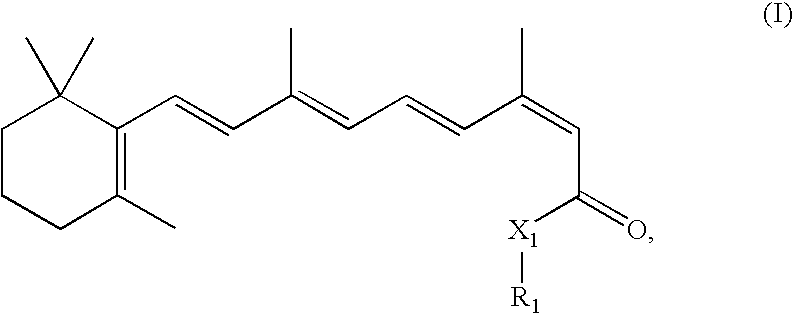
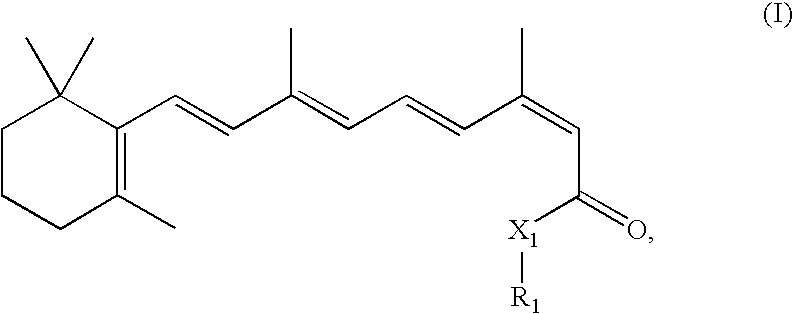
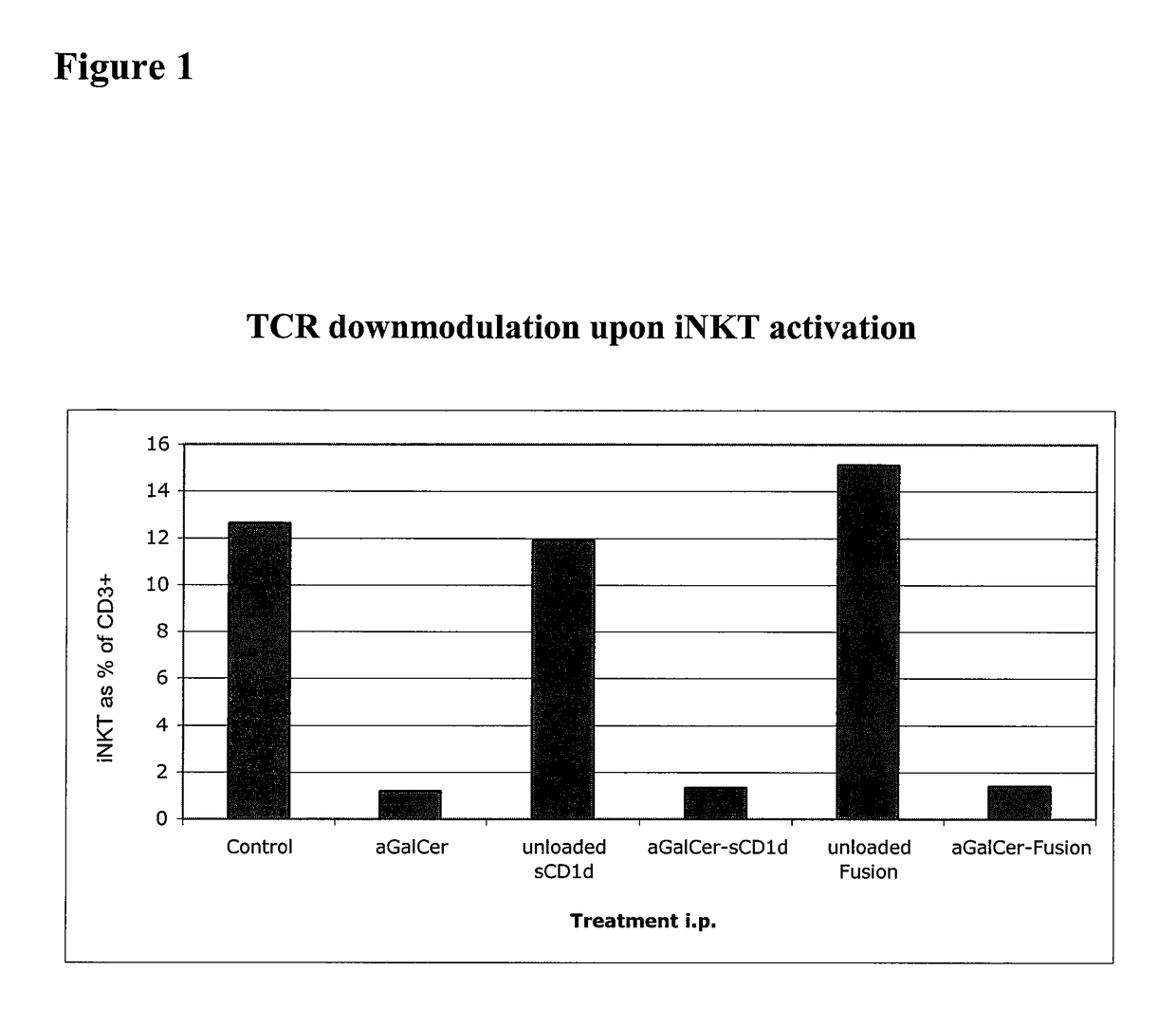
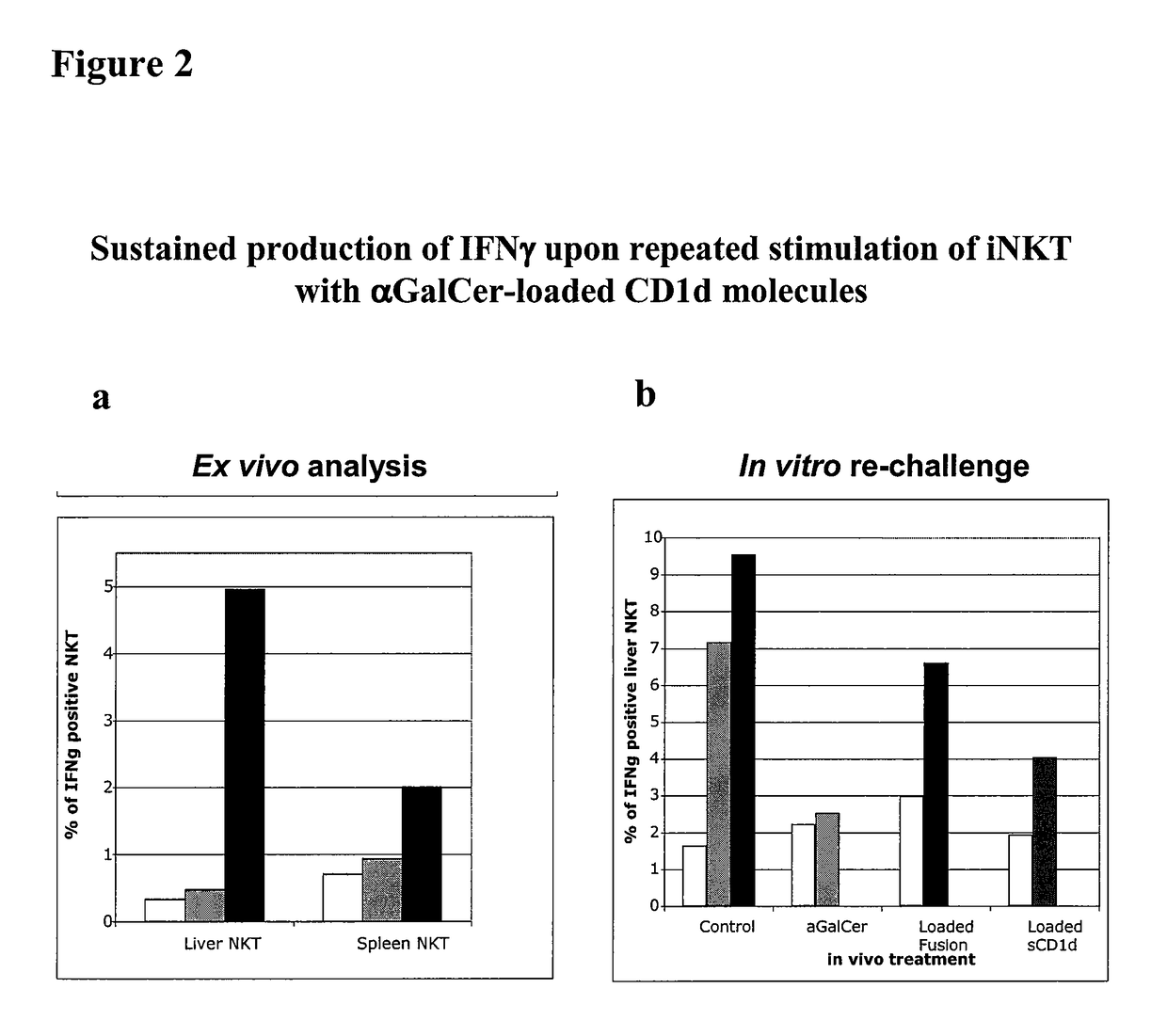
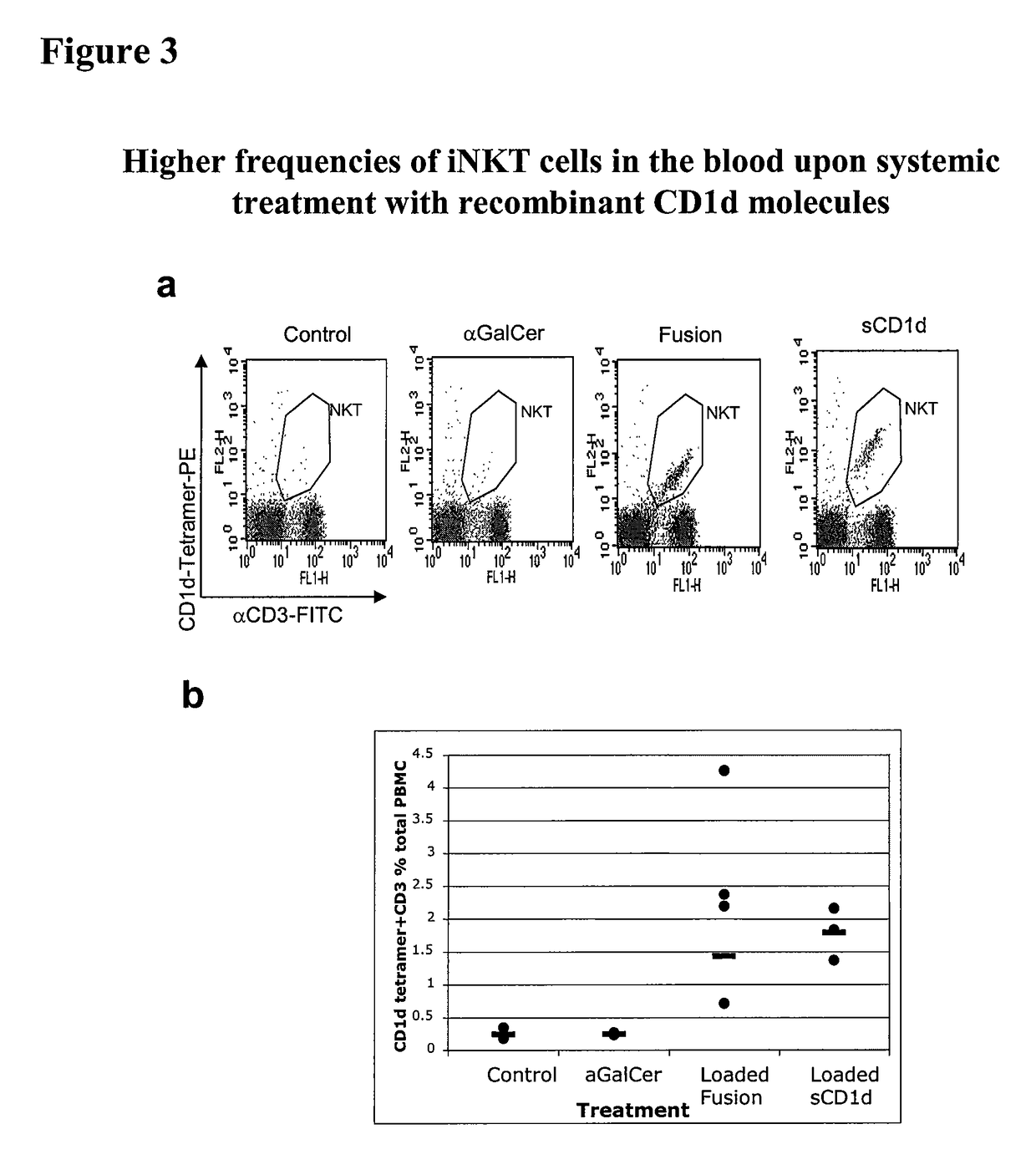
Modulation of NKT Cell Activity with Antigen-Loaded CD1d Molecules
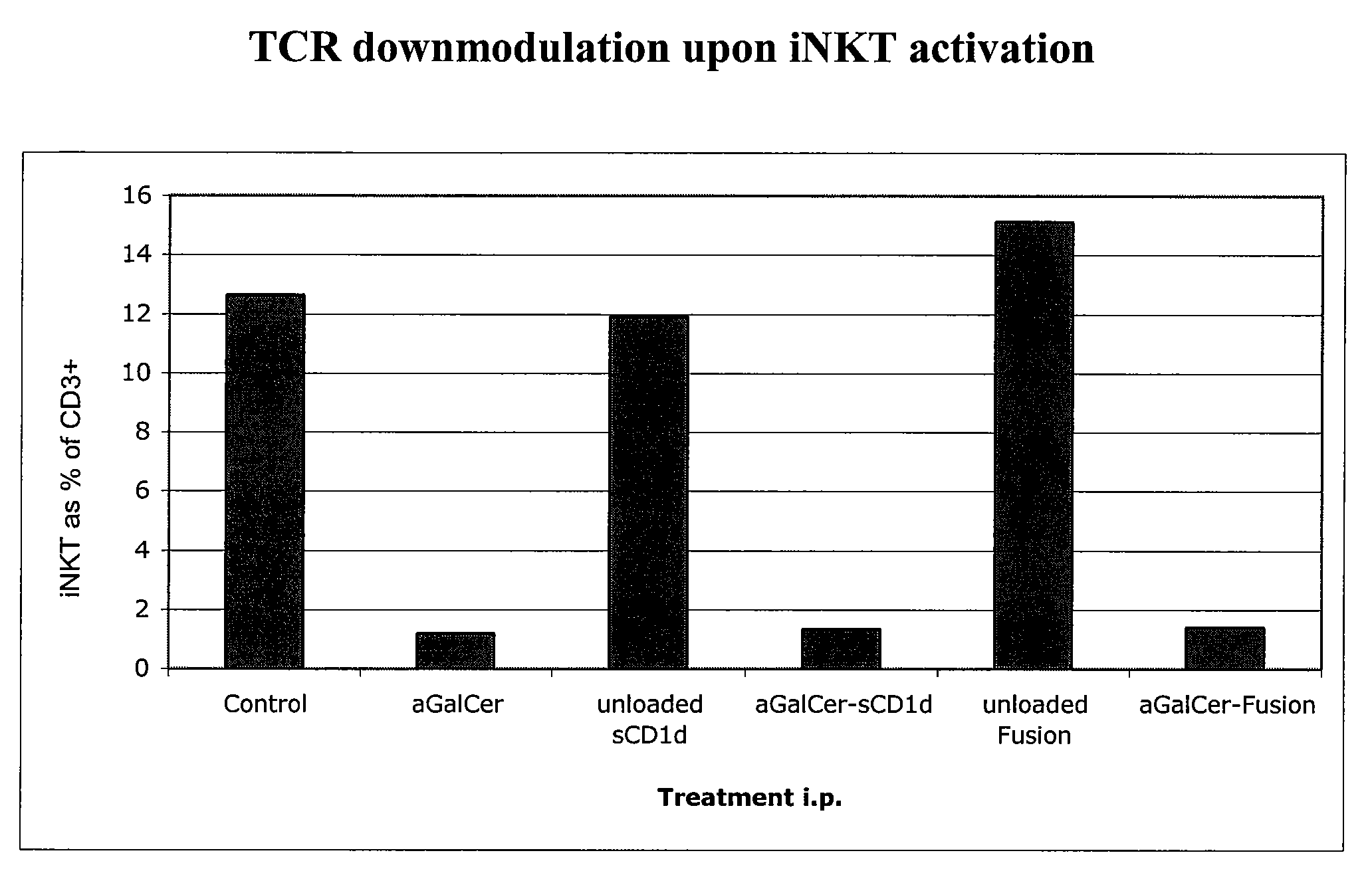
The invention is directed to methods of modulating an immune response in an animal, comprising administering a composition comprising one or more soluble CD1d complexes, in particular non-specific soluble CD1d complexes. Soluble CD1d complexes comprise a soluble CD1d polypeptide, a β2-microglobulin polypeptide, and a ceramide-like glycolipid antigen bound to the CD1d antigen binding groove, and in certain embodiments, an immunogen. The administration of compositions of the present invention affects the activity of CD1d-restricted NKT cells, and in particular, allows for multiple administrations without causing CD1d-restricted NKT cell anergy.
Owner:VACCINEX
Combination Methods and Therapies for Treating Opthalmic Conditions with 13-Cis-Retinyl Derivatives
InactiveUS20080254140A1Reduce formationPrevent macular degenerationBiocideSenses disorderDiseaseAntioxidant
Described herein are combination methods, compositions and therapies for treating ophthalmic conditions or diseases arising from, associated with or leading to the overproduction of waste products in the visual cycle. Agents included within these combinations are 13-cis-retinyl derivatives; other agents included within these combinations are selected from vitamins, antioxidants, minerals, inducers of nitric oxide production, anti-inflammatory agents, and negatively-charged phospholipids. Such combination methods may be used as single or multiple administration therapies, or in combination with other agents or therapies.
Owner:REVISION THERAPEUTICS INC
Transnasal anticonvulsive compositions and modulated process
InactiveUS7132112B2Promote absorptionIncrease permeationNervous disorderAerosol deliveryNasal cavityCo administration
A novel method of vehicle modulated administration of an anticonvulsive agent to the mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol (10–80%) or a glycol (10–80%), and their combinations with a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
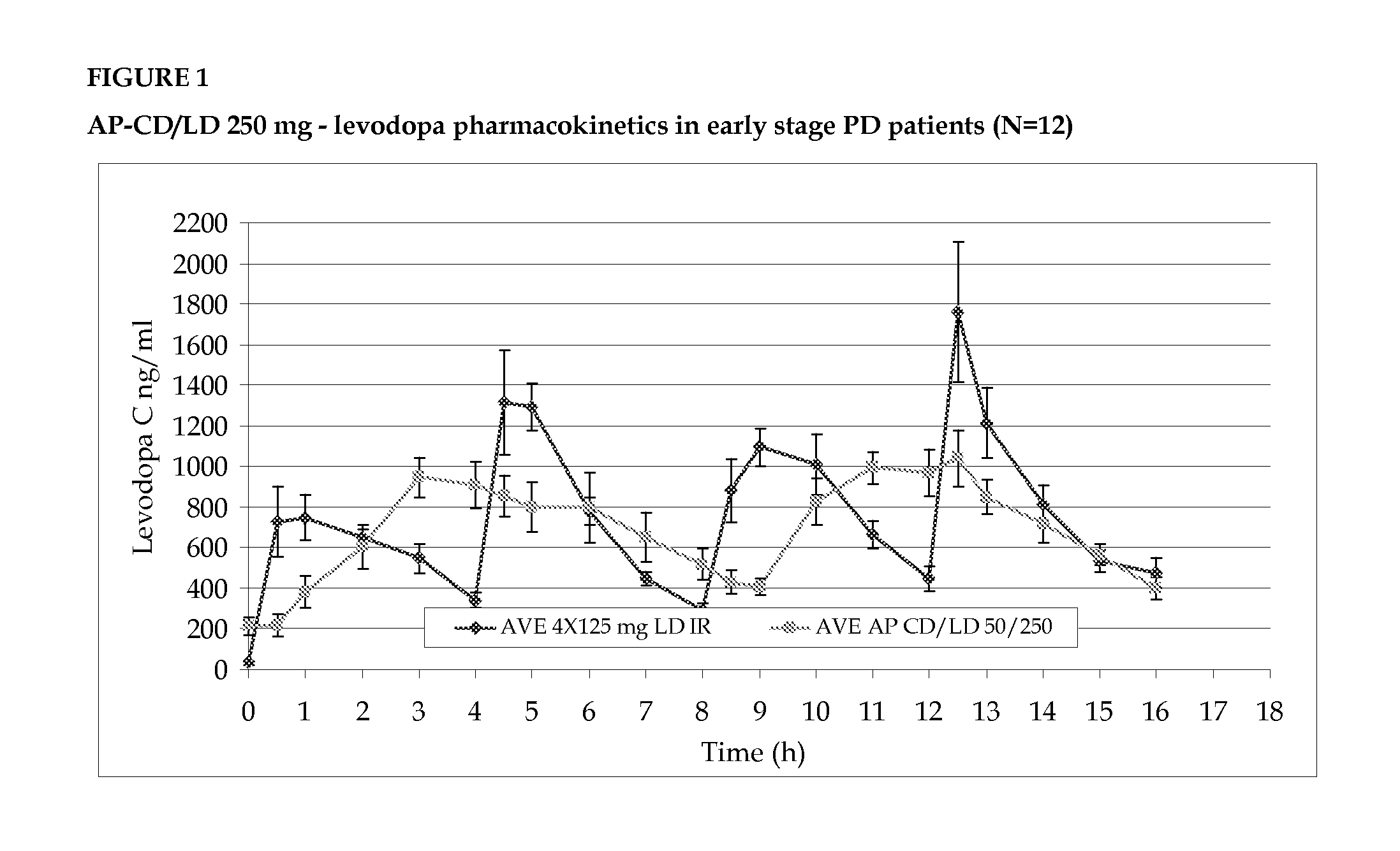
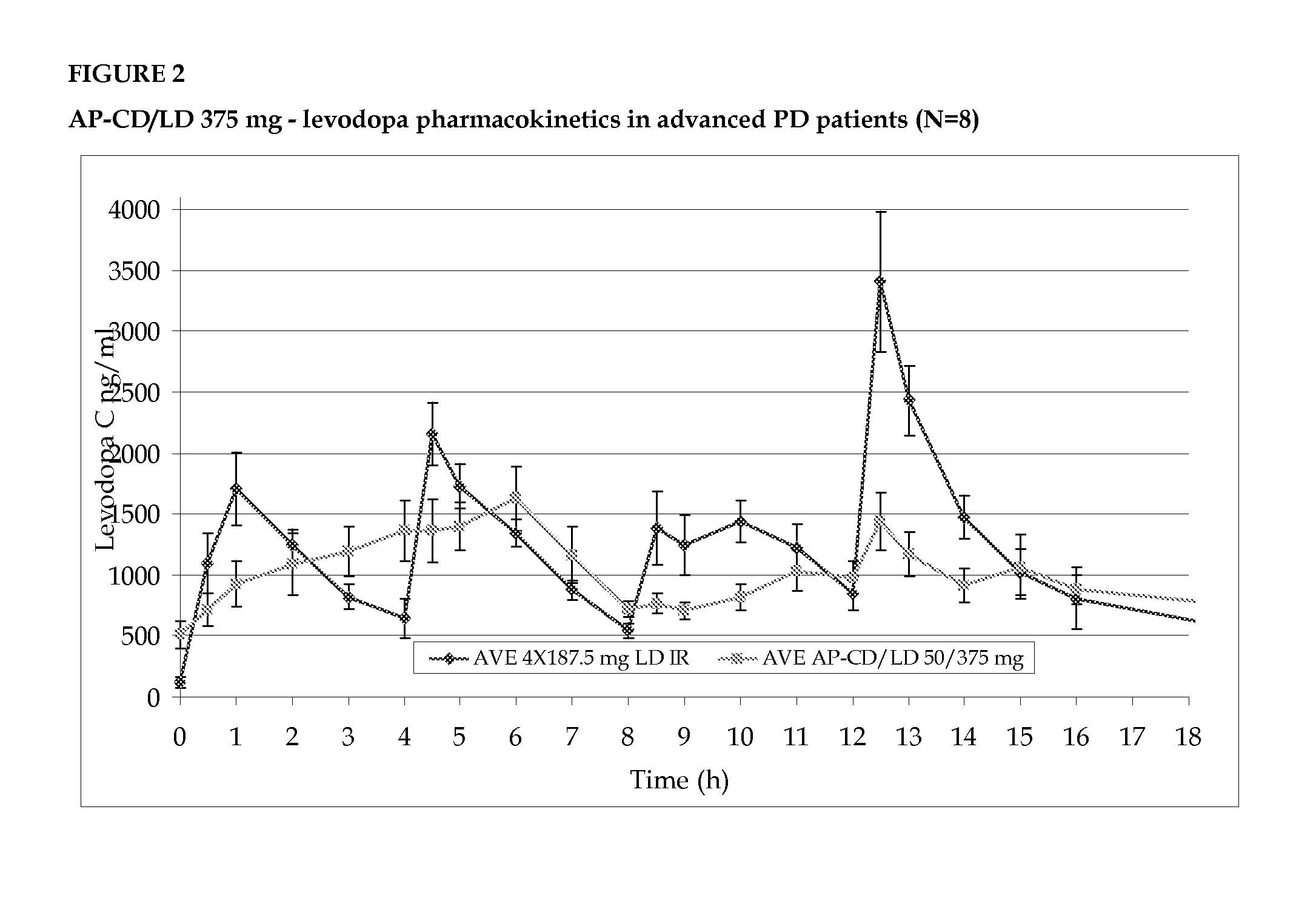
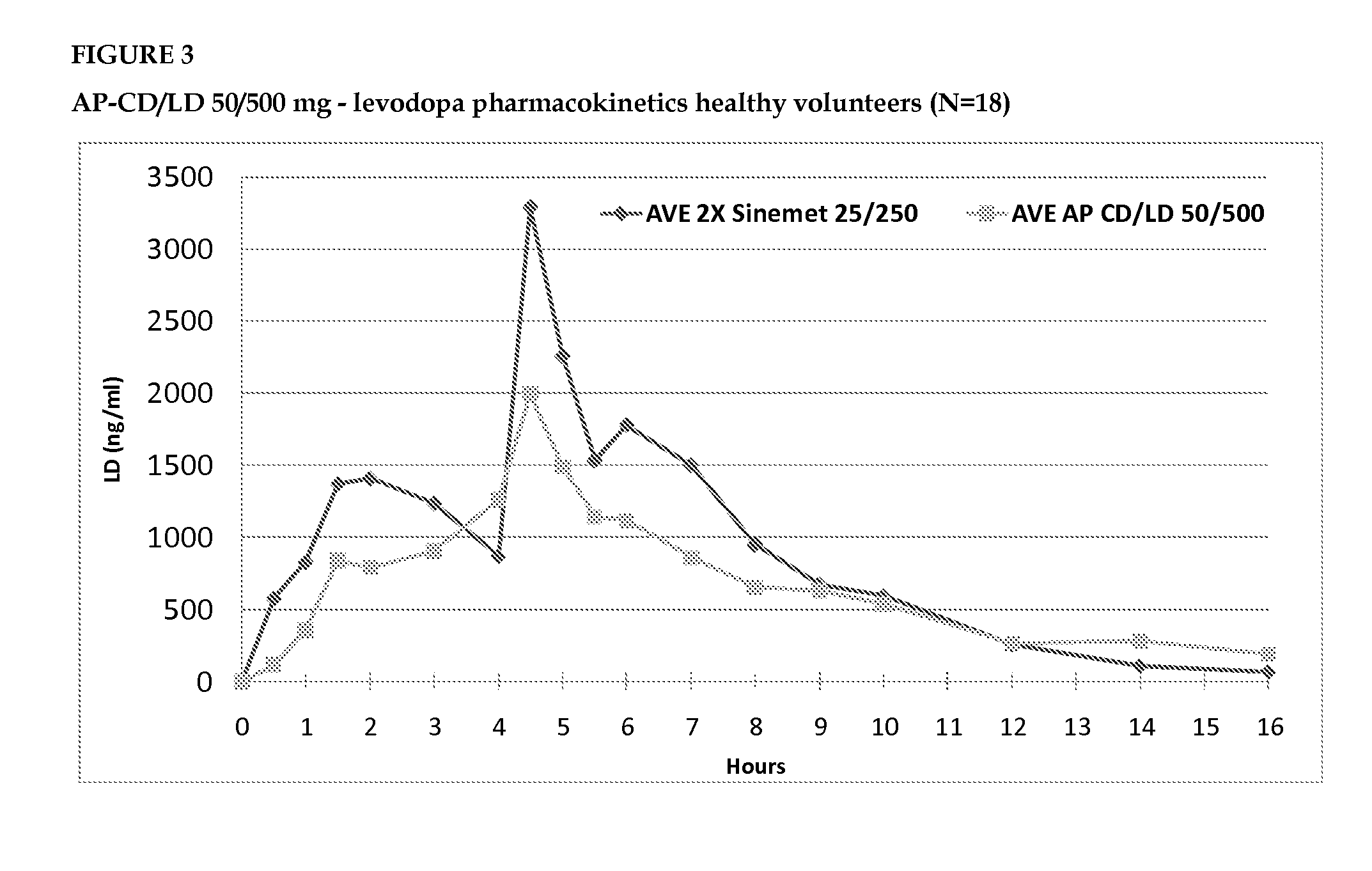
Accordion pill comprising levodopa for an improved treatment of parkinson's disease symptoms
InactiveUS20140017303A1Improve sleep qualityAlleviates eliminates symptomOrganic active ingredientsBiocideRegimenMultiple administration
The invention provides for the use of an accordion pill comprising levodopa for the treatment of symptoms of Parkinson's disease in a subject in need thereof over a 24 hour period, to be administered to the subject in a twice daily administration regimen, with an interval of about 8 to about 10 hours between the first dose and the second dose, and with an interval of about 14 to about 16 hours between the second dose and the first dose of the following day. The twice daily administration regimen provides a stable blood plasma level of levodopa in the subject after multiple administrations and is effective in treating the symptoms of Parkinson's disease over a 24 hour period.
Owner:INTEC PHARMA
Pharmaceutical composition for relieving pain
ActiveUS20120142629A1Early onsetRapid pain improvement effectOrganic active ingredientsBiocideCross-linkDisease
The present invention relates to a pharmaceutical composition for relieving pain in a joint disease, including a hyaluronic acid and a pharmaceutically acceptable carrier, in which the hyaluronic acid is cross-linked by cyclizing a double bond in the moiety of a cinnamic acid in a partially amidated hyaluronic acid represented by Formula (1): [Ar—CH═CH—COO—(CH2)n—NH-]m-HA, to form a cycloubutane ring, in which Ar represents an optionally substituted phenyl group, n represents an integer of 2 or 3, HA represents a carboxy residue of the hyaluronic acid, and m represents an amidation ratio of the hyaluronic acid to the total carboxyl group and is in the range of 3 to 50% relative to the total carboxyl group.The pharmaceutical composition of the present invention is an intra-articular formulation that exerts rapid analgesic effects after administration, and shows extremely long durable effects for a human joint disease with only a single administration rather than multiple administrations of a conventional way.
Owner:SEIKAGAKU KOGYO CO LTD
Modulation of NKT cell activity with antigen-loaded CD1d molecules
The invention is directed to methods of modulating an immune response in an animal, comprising administering a composition comprising one or more soluble CD1d complexes, in particular non-specific soluble CD1d complexes. Soluble CD1d complexes comprise a soluble CD1d polypeptide, a β2-microglobulin polypeptide, and a ceramide-like glycolipid antigen bound to the CD1d antigen binding groove, and in certain embodiments, an immunogen. The administration of compositions of the present invention affects the activity of CD1d-restricted NKT cells, and in particular, allows for multiple administrations without causing CD1d-restricted NKT cell anergy.
Owner:VACCINEX
Nano-suspension of Hsp90 inhibitor by using benzamide as basic skeleton and preparation method of nano-suspension
ActiveCN104173283AHigh drug contentImprove stabilityOrganic active ingredientsSolution deliveryDrug contentActive agent
The invention discloses a nano-suspension of an Hsp90 inhibitor by using benzamide as a basic skeleton and a preparation method of the nano-suspension. In the nano-suspension, the weight ratio of the Hsp90 inhibitor by using benzamide as the basic skeleton to a surfactant is 1 to 0.1-1 to 10. The nano-suspension has the characteristics of high drug content, small auxiliary dose, high stability, stable dissolution rate and the like, is applicable to multiple administration routes, in particular injection, is capable of overcoming the defects of poor water solubility, difficult administration, low bioavailability, strong toxic and side effects and the like, realizes technical breakthrough in the field, and provides a novel administration preparation with high drug content, high bioavailability and passive targeting to the Hsp90 inhibitors for the first time so as to accelerate the progress of the nano-suspension in clinical application.
Owner:广州少伯控股集团有限公司 +1
Docetaxel polymer nano-micelle injection, and preparation method and application thereof in preparation of tumor drugs
InactiveCN106983719AGood water solubilityExtend cycle timePowder deliveryOrganic active ingredientsSolubilitySide effect
The invention provides docetaxel polymer nano-micelle injection, and a preparation method and application thereof in preparation of tumor drugs; the docetaxel polymer nano-micelle injection comprises, by weight, 1 part of docetaxel and 10-100 parts of a nano-micelle polymer; the docetaxel nano-micelles are uniform in particle size and have 20-200 nm in average particle size. The docetaxel polymer nano-micelle injection can provide greatly improved water-solubility of docetaxel and has greatly improved stability, the cycle time of docetaxel in blood is extended, and toxic and side effects of commercially available preparations are decreased; docetaxel is passively targeted to a tumor part through EPR (enhanced permeability and retention) effect, anti-tumor effect of docetaxel is thereby improved, and the case that polymer nano-micelle preparations using PEG (polyethylene glycol) as a shell layer are not applicable (such as to the treatment of a patient having PEG antibodies or a patient having PEG antibodies generated in multiple administration) can be avoided.
Owner:江苏富泽药业有限公司
Moldavica dragonhead total flavone sustained release tablet and preparation method thereof
InactiveCN102048811AShort development cycleGood drug releasePharmaceutical delivery mechanismRespiratory disorderSustained Release TabletSide effect
The invention discloses a moldavica dragonhead total flavone sustained release tablet and a preparation method thereof. The moldavica dragonhead total flavone sustained release tablet is prepared by mixing, granulating and tabletting moldavica dragonhead total flavone, a framework material, filler, a caking agent and a lubricating agent. Compared with the prior art, the moldavica dragonhead total flavone sustained release tablet prepared by the method disclosed by the invention has the advantages of short development period, simple and easy production process, suitability for bulk production, favorable medicine release performance, favorable safety performance, simpler prescription, low research and development cost, convenience for taking, easy control for quality and the like. In addition, after the medicine is administrated, the slow release at non constant-speed can be realized, the blood-medicine concentration is more stable, the peak valley fluctuation is smaller and the toxic or side effects due to multiple administrations with the result that the blood-medicine concentration exceeds the treatment range can be avoided. Meanwhile, the moldavica dragonhead total flavone sustained release tablet can be maintained in the effective concentration range for a longer time so as to maintain the curative effect and improve the safety, the effectiveness and the adaptability of the medicine; and the medicine administration frequency each day is reduced to 1-2 times from 3-4 times, thus the moldavica dragonhead total flavone sustained release tablet is greatly convenient for patients and particularly patients required to administrate the medicine for a long term.
Owner:XINJIANG INST OF MATERIA MEDICA
Isosorbide mononitrate micro-porous osmotic pump tablet and preparation method thereof
InactiveCN102423305AEffect of drug release rateSmall individual differencesDrageesCardiovascular disorderDrug release rateSide effect
The invention relates to an isosorbide mononitrate micro-porous osmotic pump tablet and a preparation method thereof. The preparation can release isosorbide mononitrate at a constant drug release rate. The isosorbide mononitrate micro-porous osmotic pump tablet is mainly composed of a tablet core, a semipermeable coating film and drug release pores, wherein the tablet core consists of isosorbide mononitrate, an osmotic pressure active substance, filler and other pharmaceutical auxiliary materials; and the semipermeable coating film consists of cellulose acetate, a plasticizer and a pore former. The osmotic pressure between the outside and inside of the semipermeable film is used as the driving force and the osmotic pump tablet releases the drug at a zero order release rate, thus the tablet has the characteristics that the blood concentration is stable, the in-vivo drug action time is proper, the side effects are little and the drug resistance is hardly caused after multiple administration.
Owner:CHINA PHARM UNIV
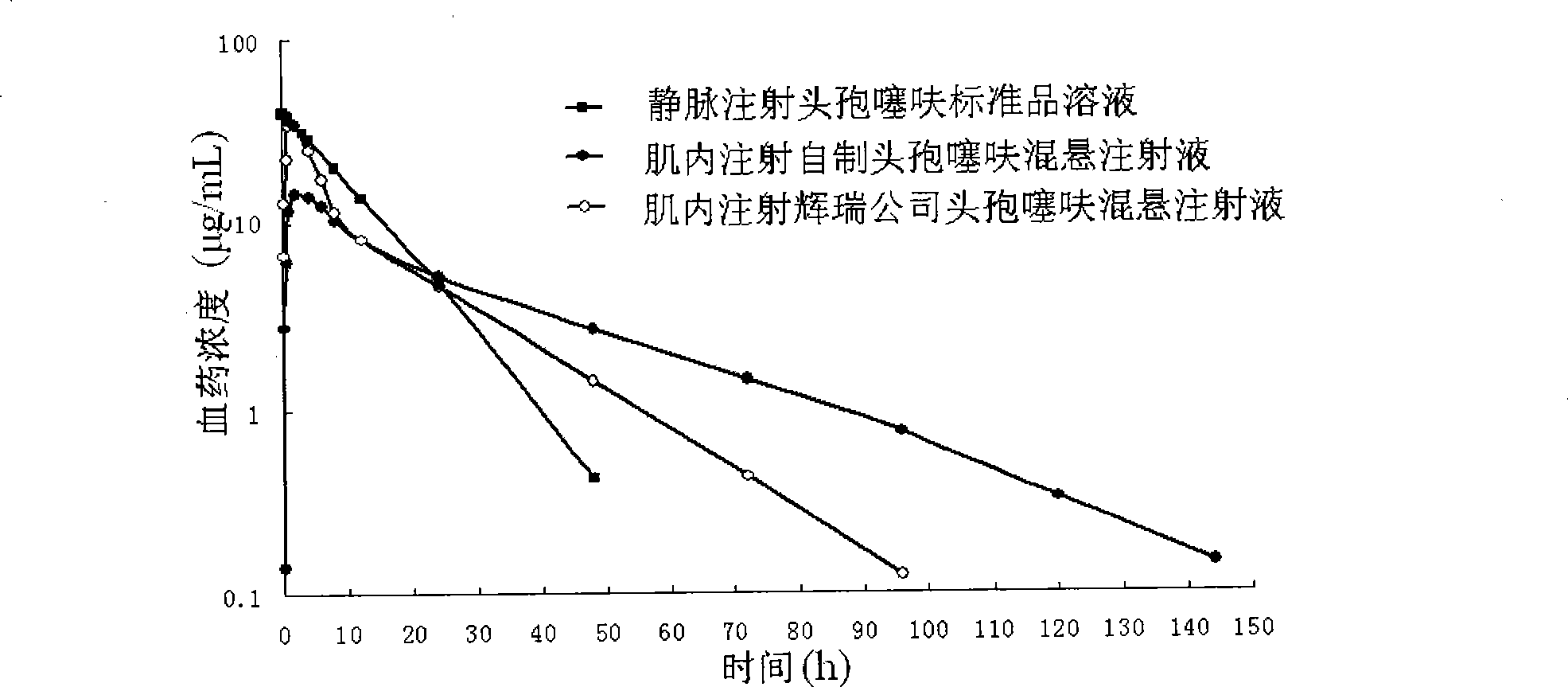
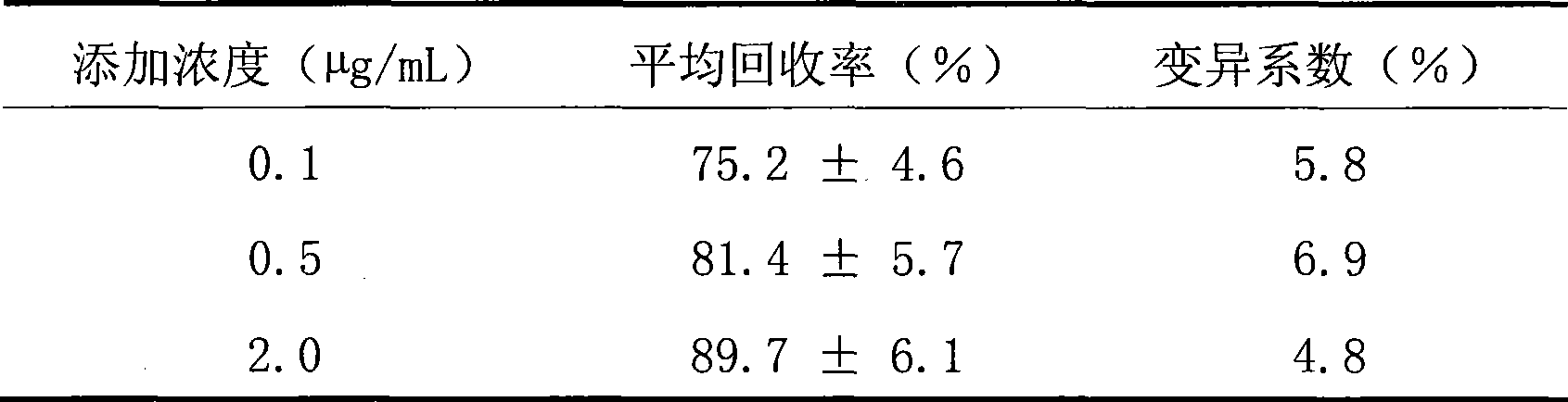
Ceftiofur long-acting injection and preparation method thereof
InactiveCN101401787BAccording to the law of pharmacokineticsDecreased peak plasma concentrationAntibacterial agentsOrganic active ingredientsVegetable oilVeterinary Drugs
The invention discloses a ceftiofur long-acting injection and a method for preparing the same. The ceftiofur long-acting injection is a suspension injection containing ceftiofur hydrochloride, and the main compositions of the solvent system of the drug are vegetable oil for injection and grease. The preparation process of the ceftiofur long-acting injection has simple operation, and the prepared suspension injection meets the requirements and has obvious long-acting effect after intramuscular injection, so that the therapeutic effect of the drug is improved and the disadvantages that a respiratory disease has a long course and needs multiple administrations in treatment are overcome, and the waste of human resources in a farm caused by frequent administrations and the animal stress reaction caused by multiple administrations are reduced, thus the ceftiofur long-acting injection is a novel veterinary drug preparation with long-acting effect on respiratory diseases of livestock and poultry, and has very high popularization and application value.
Owner:北京中农大动物保健品技术研究院
Degarelix acetate injectable sustained release implant
InactiveCN107773528AEasy to prepareEase of mass productionPeptide/protein ingredientsAerosol deliveryDegarelix AcetateSolvent
The invention relates to an injectable sustained release implant comprising degarelix acetate and a preparation method of the injectable sustained release implant. The implant is a liquid preparationprepared by dissolving degarelix acetate and a biodegradable high-molecular polymer into an appropriate organic solvent. The liquid preparation is injected into the human body through local subcutaneous or muscular injection, the solvent is rapidly dispersed into the surrounding tissue water, the polymer is not soluble in water, is solidified on the administration part, wraps the medicine to forma semi-solid or solid medicine storage reservoir, the medicine is slowly released along with the degradation of the polymer, so that the effect of long-acting controlled release is achieved. The implant has the advantages that the troublesome of multiple administration for a patient is reduced, the untoward effect of the medicine is reduced and the compliance is improved.
Owner:南京星银药业集团有限公司 +1
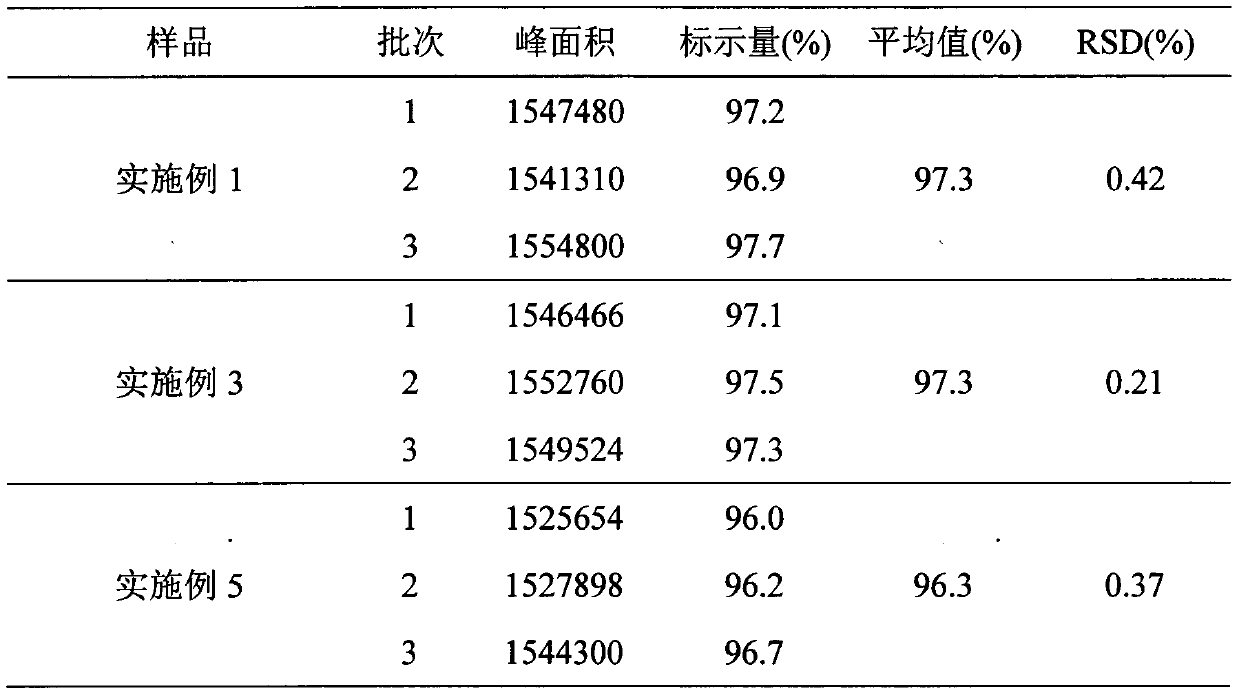
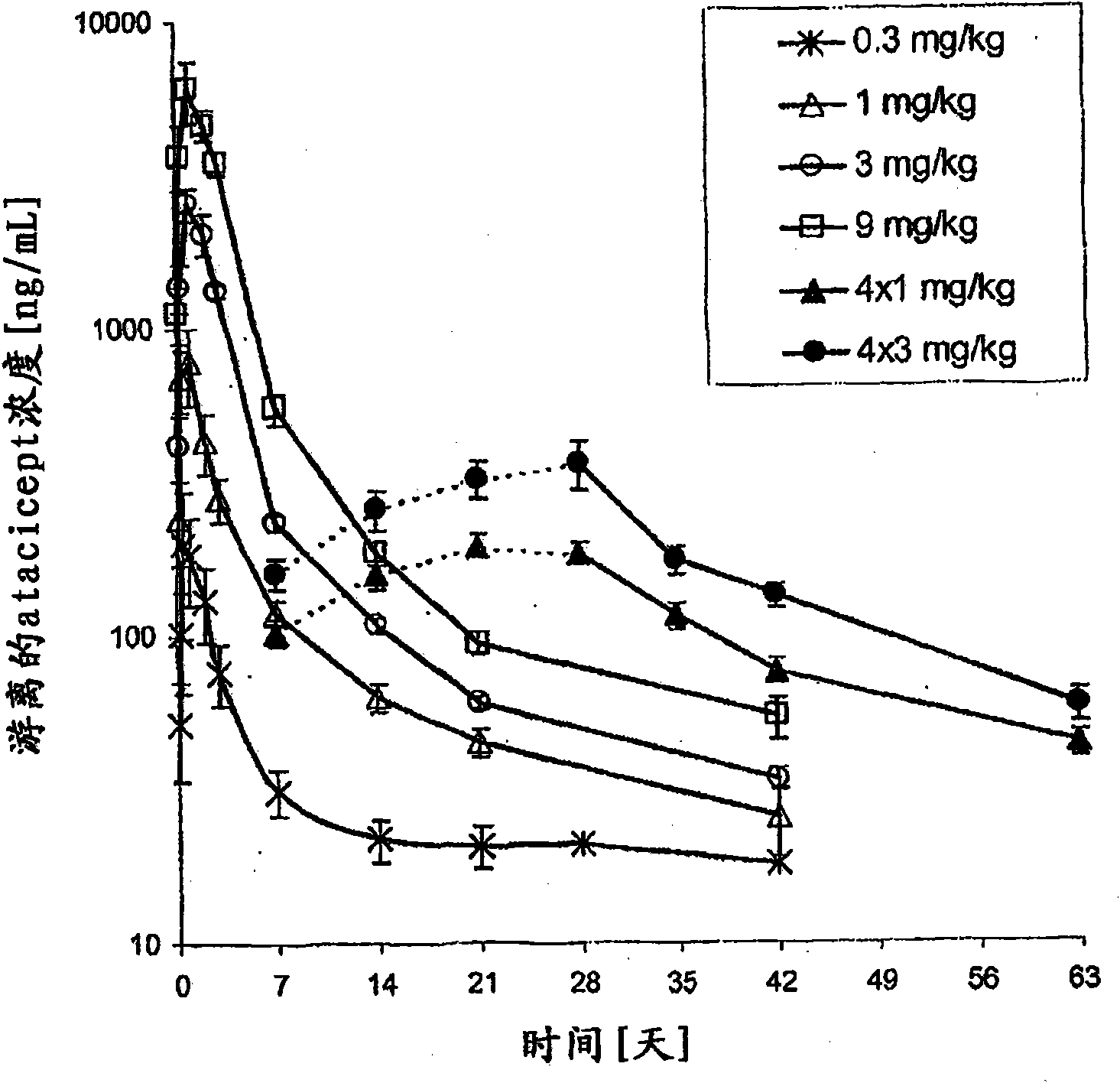
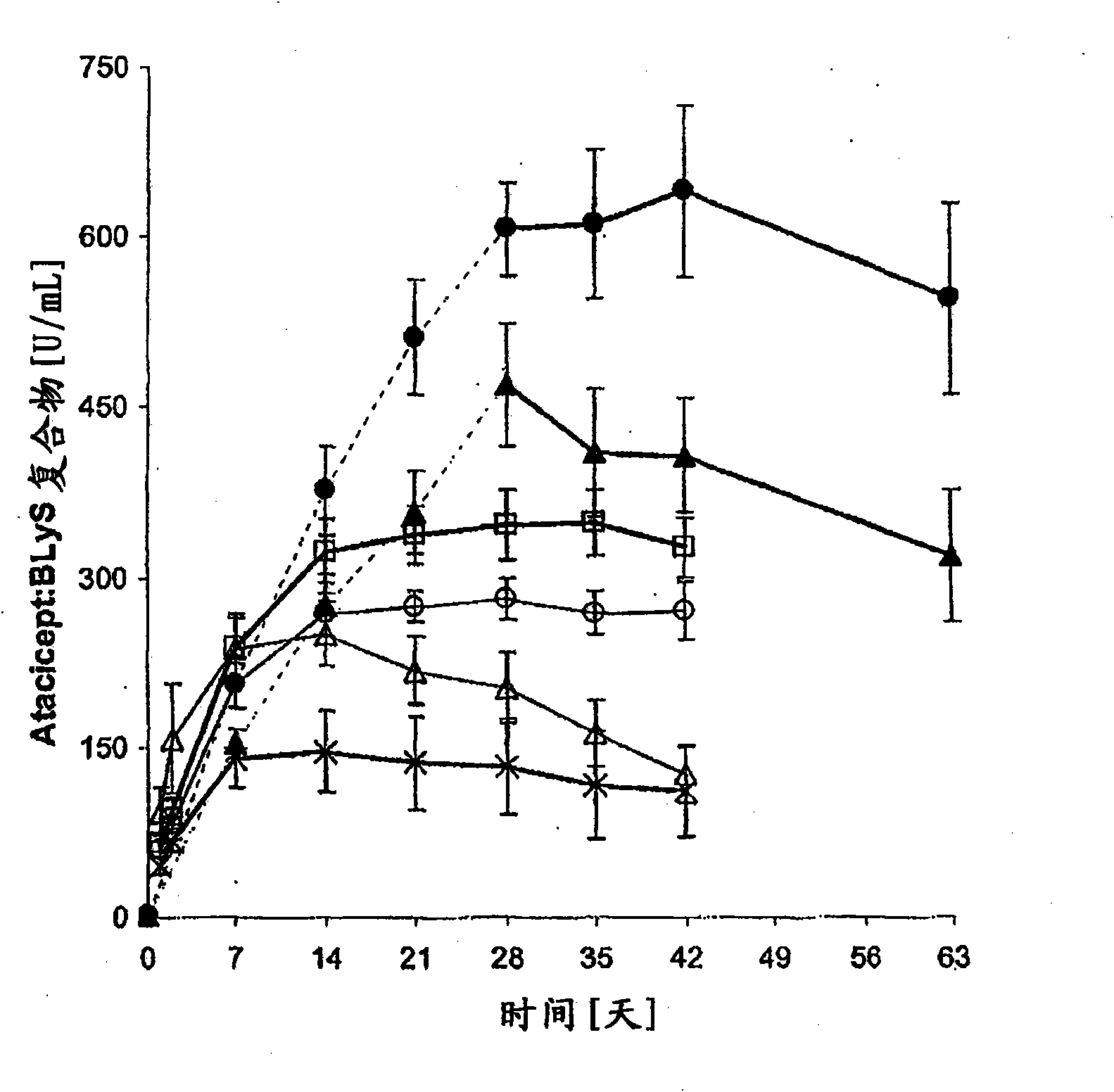
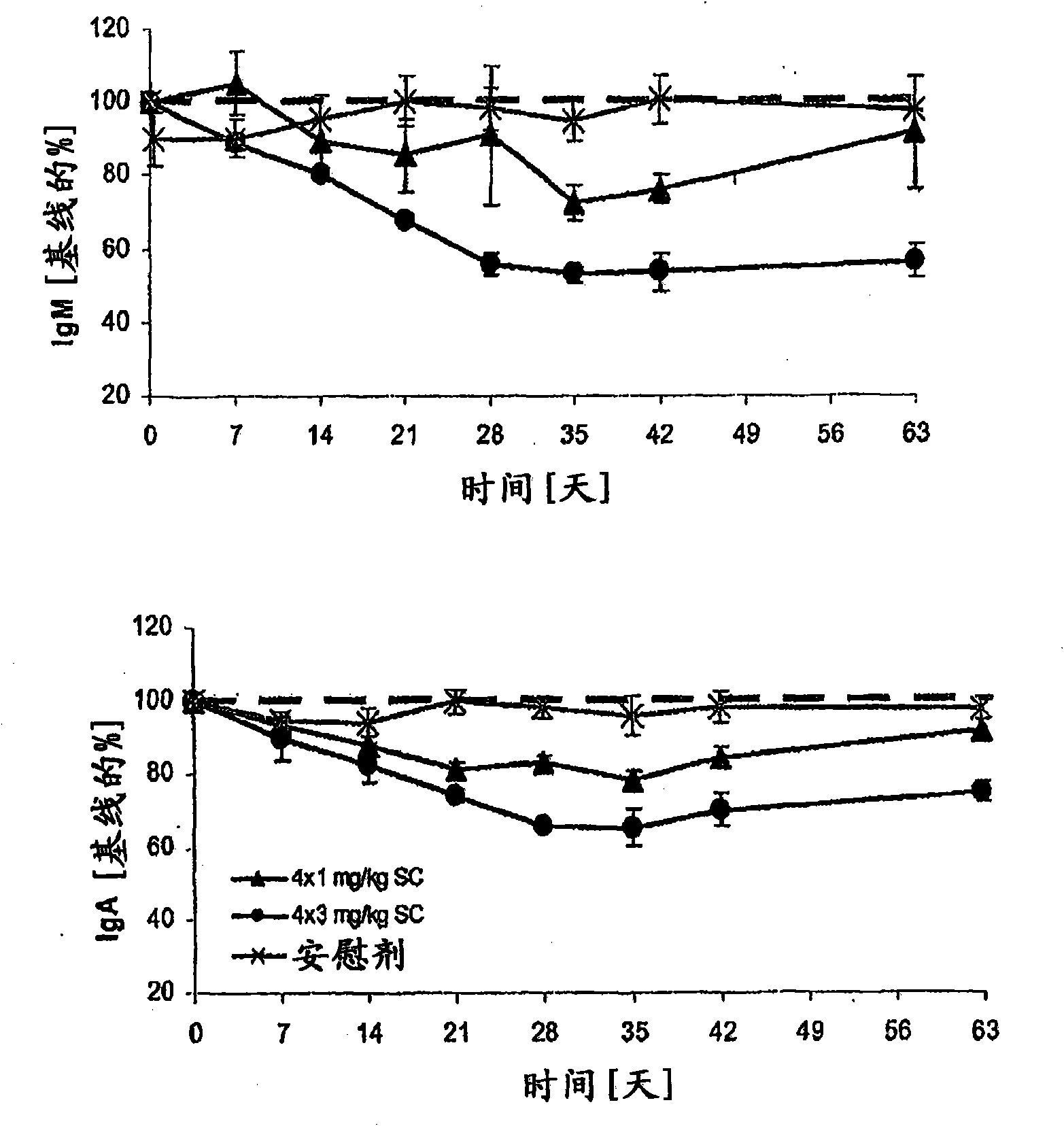
Dosing methods for treating autoimmune diseases using a taci-ig fusion protein such as atacicept
In various embodiments, the present invention provides methods, compositions, dosing, and administration schedules for treatment of autoimmune diseases, including systemic erythematosus (SLE), for example, comprising administering to a patient in need of such treatment a TACI-Ig fusion molecule such as atacicept. In one embodiment, the TACI-Ig fusion molecule is administered in amount sufficient to slow, suppress or inhibit proliferation-inducing functions of BLyS and APRIL, in particular the use of multiple administrations of the fusion molecule at relatively low dose over the course of the treatment.
Owner:ZYMOGENETICS INC +1
NOD1 protein inhibiting polypeptide and application thereof
InactiveCN105017388ASignificant effect in treating rheumatoid arthritisPeptide/protein ingredientsAntipyreticDiseaseProtein C
The invention relates to the field of medicines, in particular to a polypeptide which has an NOD1 protein inhibiting function and can prevent or treat rheumatoid arthritis. The sequence of the polypeptide is MKNMWKSARKIDAFQHM and is brand-new; the polypeptide can be used for treating and preventing rheumatoid arthritis diseases in multiple administration modes including subcutaneous or intramuscular injection, intravenous injection or drip and oral administration in modes of pills, capsules, nasal spray and the like. The designed NOD1 protein inhibiting polypeptide is scientific, reasonable, feasible and effective, and can be used as the medicine for treating or preventing rheumatoid arthritis.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
Composite medicine as well as preparation method and application thereof
PendingCN113318088AImprove the immunityGood sustained release effectOrganic active ingredientsPharmaceutical non-active ingredientsPharmacyBlood concentration
The invention belongs to the technical field of pharmacy, and particularly relates to a composite medicine and a preparation method and application thereof. The invention relates to a composite medicine, the composite medicine at least comprises red blood cells and artesunate, and the artesunate is entrapped in the red blood cells. Compared with the traditional artesunate injection, the composite medicine disclosed by the invention can realize slow release of the medicine after entering the body, the medicine effect can be maintained for about 9 days, the composite medicine has an obvious slow release effect, and the problems of rapid reduction of the blood concentration of the traditional artesunate injection and side effects caused by short-time multiple administration can be solved, so that malaria can be effectively prevented or treated.
Owner:SHANGHAI JIAO TONG UNIV
Norovirus vaccine
InactiveUS20180243397A1Improves mucosal residence timeMaximize both mucosal and systemic immune responsesPowder deliverySsRNA viruses positive-senseAntigenParenteral nutrition
Owner:RESILIENCE GOVERNMENT SERVICES INC
Method for evaluating bioavailability in combined application of fuke qianjin tablets and antibiotics
ActiveCN107505403ARationality of evaluationSimple and fast operationComponent separationBiological testingHplc esi msStatistical analysis
The invention discloses a method for evaluating the bioavailability in combined application of fuke qianjin tablets and antibiotics. According to the method, the concentration of a drug to be detected in a blood plasma sample is detected, the pharmacokinetic parameters are calculated by using an ADAPT 5.1 software inverse Gaussian model, and each group of the parameters are subjected to statistical analysis with SPSS software so as to complete the evaluation, wherein the blood plasma sample is obtained after the single administration of antibiotic drugs or the combined administration of fuke qianjin tablets and antibiotics, the concentration of the drug to be detected is the concentration of the antibiotic drug, and the method for detecting the concentration of the drug to be detected in the blood plasma sample is a HPLC-ECD method or HPLC-ESI-MS method. According to the present invention, the pharmacokinetic evaluation method for the multiple administration of fuke qianjin tablets and azithromycin in chronic pelvic inflammatory disease rats is established, and the reliable method for evaluating the rationality of the combined administration of fuke qianjin tablets and azithromycin is achieved; and with the evaluation method, the pharmacokinetic rule of fuke qianjin tablets and azithromycin in chronic pelvic inflammatory disease rats can be successfully obtained, the scientific basis can be provided for the reasonable clinical application of fuke qianjin tablets and azithromycin, and the reference can be provided for the subsequent related research.
Owner:ZHUZHOU QIANJIN PHARMA
MYC cancer gene inhibition polypeptide and application thereof
InactiveCN106749549APrevent proliferationInhibition of telomerase activityPeptide/protein ingredientsDepsipeptidesTelomeraseFhit gene
The invention relates to the field of medicines, and in particular relates to polypeptide which can inhibit MYC carcinogenic protein and treat tumor. The sequence of the polypeptide is as shown in SEQ ID NO.1. The polypeptide has the application of tumor treatment. The application includes tumor treatment in multiple administration modes, including subcutaneous or intramuscular injection, intravenous injection or intravenous drip, and the like. The polypeptide has the beneficial effects that in-vitro proliferation activity of multiple tumor cells can be inhibited, MYC protein expression in tumor cells can be promoted, telomerase activity of a tumor cell can be inhibited, growth of tumor inside tumor model naked mice can be inhibited, and the survival rate of tumor-bearing mice can be increased. An effect of treating tumor can be achieved.
Owner:罗瑞雪
Evaluation Method of Bioavailability of Fukeqianjin Tablets Combined with Antibiotics
ActiveCN107505403BSimple and fast operationRationality of evaluationComponent separationBiological testingStatistical analysisBlood plasma
The invention discloses a method for evaluating the bioavailability in combined application of fuke qianjin tablets and antibiotics. According to the method, the concentration of a drug to be detected in a blood plasma sample is detected, the pharmacokinetic parameters are calculated by using an ADAPT 5.1 software inverse Gaussian model, and each group of the parameters are subjected to statistical analysis with SPSS software so as to complete the evaluation, wherein the blood plasma sample is obtained after the single administration of antibiotic drugs or the combined administration of fuke qianjin tablets and antibiotics, the concentration of the drug to be detected is the concentration of the antibiotic drug, and the method for detecting the concentration of the drug to be detected in the blood plasma sample is a HPLC-ECD method or HPLC-ESI-MS method. According to the present invention, the pharmacokinetic evaluation method for the multiple administration of fuke qianjin tablets and azithromycin in chronic pelvic inflammatory disease rats is established, and the reliable method for evaluating the rationality of the combined administration of fuke qianjin tablets and azithromycin is achieved; and with the evaluation method, the pharmacokinetic rule of fuke qianjin tablets and azithromycin in chronic pelvic inflammatory disease rats can be successfully obtained, the scientific basis can be provided for the reasonable clinical application of fuke qianjin tablets and azithromycin, and the reference can be provided for the subsequent related research.
Owner:ZHUZHOU QIANJIN PHARMA
Vaccine delivery
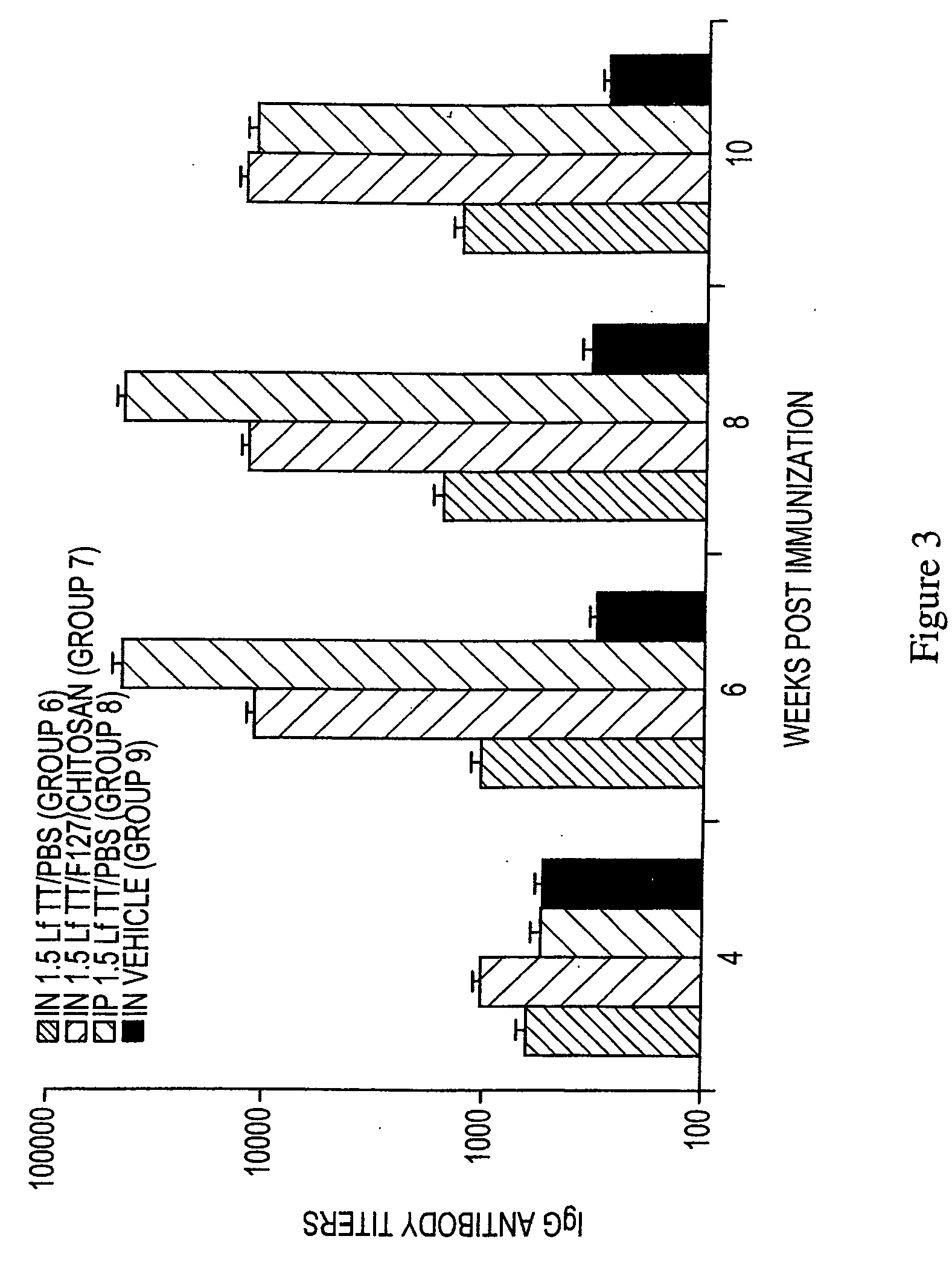
InactiveUS20070116719A1Enhance antibody responseReduce deliveryBacterial antigen ingredientsDispersion deliveryVaccine deliveryMultiple administration
The present invention provides an immunogen composition and methods for using the same for the development of immunity. In one method, the immunogen composition is administered to prepare a person for a later booster administration. This immunogen composition includes an antigen and a polyoxyalkylene block copolymer. In another method the booster administration is given to a person previously prepared for the booster through prior administration of the immunogen composition. Another method includes multiple administrations, with a later administration boosting the immune response from a prior administration.
Owner:RXKINETIX
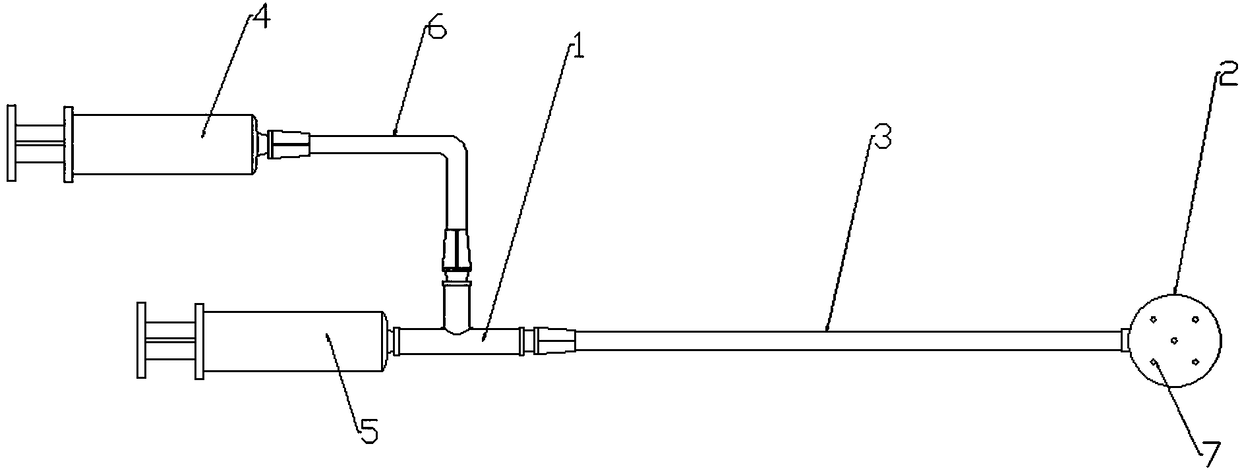
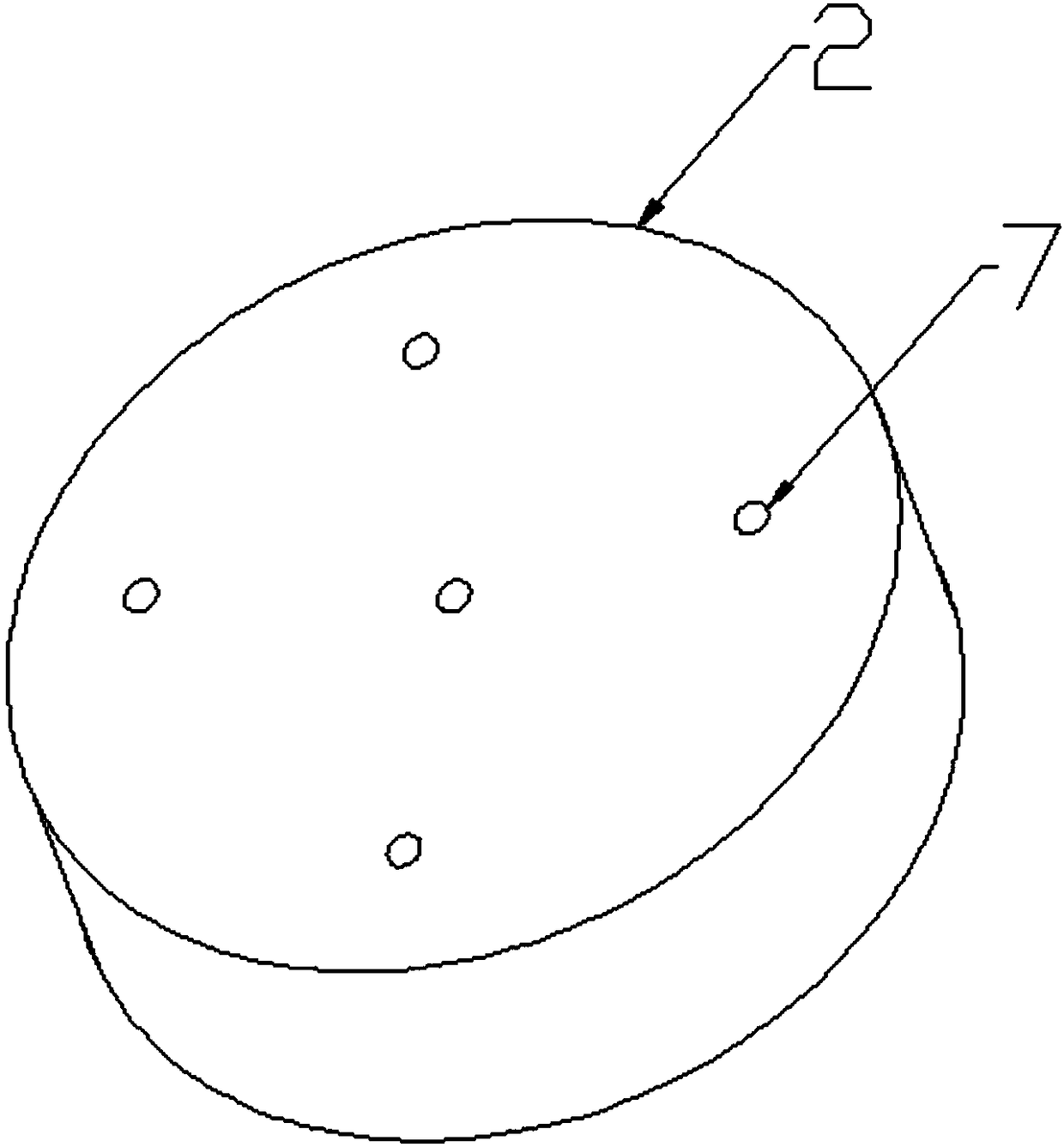
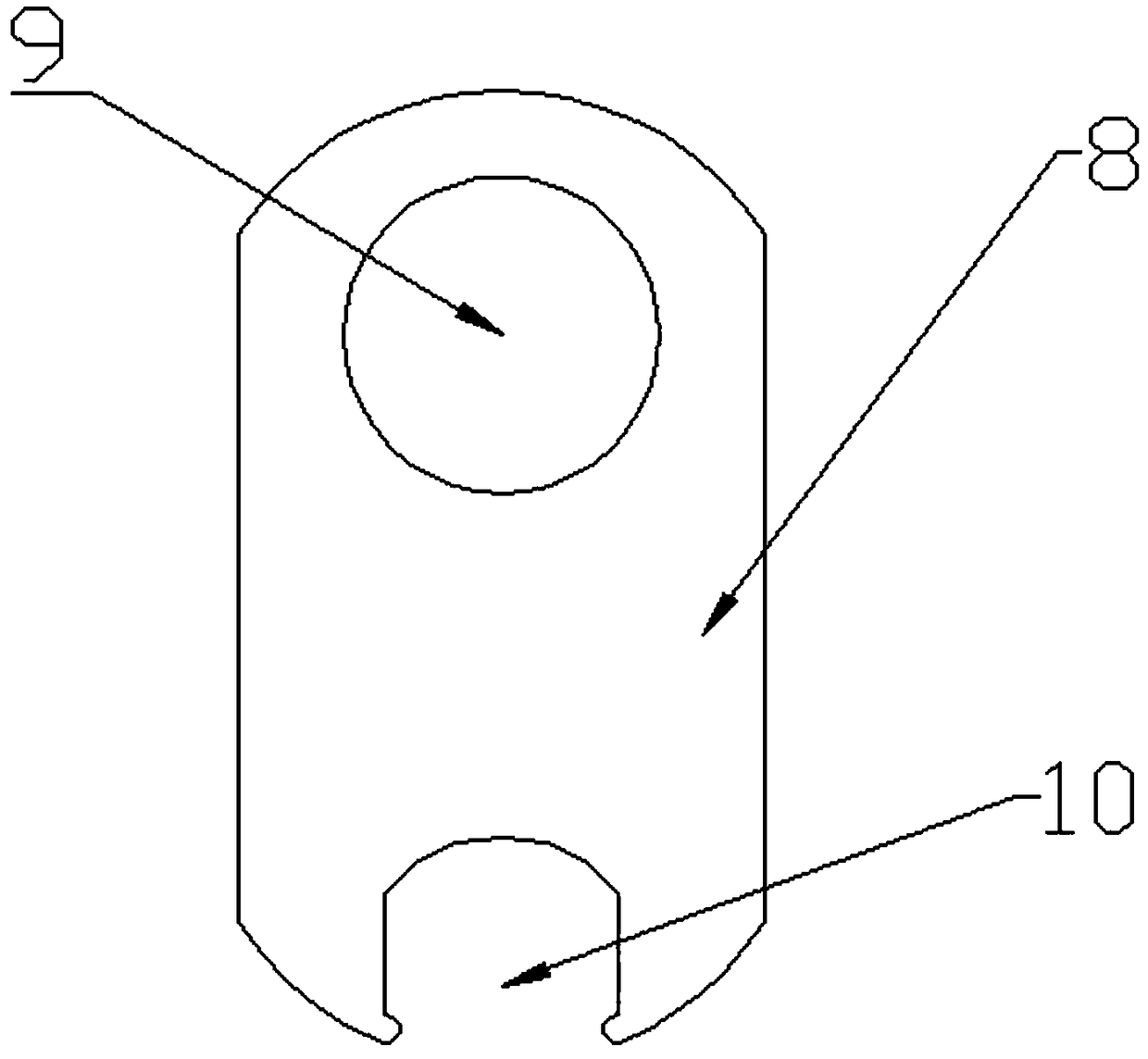
Disposable oral administration device and administration method
The invention discloses a disposable oral administration device and an administration method, and belongs to the technical field of medical instruments. The oral administration device comprises a three-way connecting tube, an oral administration end and a first extension tube. A first connecting port and a second connecting port of the three-way connecting tube are separately connected with a first injector and a second injector. One end of the first extension tube is fixedly connected with the oral administration end to form a whole, and the other end of the first extension tube is detachablyconnected with a third connecting port of the three-way connecting tube. The oral administration end is designed as a cylindrical housing, the first extension tube communicates with the inside of thecylindrical housing, multiple administration holes are evenly distributed on both end surfaces of the cylindrical housing, so that the comfort of use of the oral administration device is improved, and the oral administration device is conveniently washed to reduce residues of radioactive drugs, and can be quickly replaced to avoid radioactive contamination.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Polypeptide for inhibiting mTOR and application thereof
InactiveCN105085630AInhibitory activityLower blood sugar in the bodyPeptide/protein ingredientsMetabolism disorderDiabetes mellitusIntramuscular injection
The invention relates to the field of medicines, in particular relates to polypeptide which can inhibit mTOR and prevent and treat diabetes. The sequence of the polypeptide is EIQATWYEKLHEW. The application of the polypeptide in preventing or treating the diabetes can be realized by multiple administration methods including subcutaneous injection or intramuscular injection, intravenous injection or intravenous drip, oral administration such as pills or capsules, nasal sprays and the like. The polypeptide for inhibiting mTOR and application thereof have the beneficial effects that the mTOR can be inhibited in a targeted manner, the physiological and pathological effects generated by an mTOR receptor can be inhibited, and the effects of preventing and treating the diabetes can be achieved.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
Tumor polypeptide vaccine based on tissue factor, preparation method and application thereof
Owner:JIANGSU HIGH WIT BIOTECH CO LTD
Pharmaceutical composition for relieving pain
ActiveUS20140350239A1Early onsetQuick effectOrganic active ingredientsSugar derivativesCross-linkDisease
The present invention relates to a pharmaceutical composition for relieving pain in a joint disease, including a hyaluronic acid and a pharmaceutically acceptable carrier, in which the hyaluronic acid is cross-linked by cyclizing a double bond in the moiety of a cinnamic acid in a partially amidated hyaluronic acid represented by Formula (1): [Ar—CH═CH—COO—(CH2)n-NH-]m-HA, to form a cycloubutane ring, in which Ar represents an optionally substituted phenyl group, n represents an integer of 2 or 3, HA represents a carboxy residue of the hyaluronic acid, and m represents an amidation ratio of the hyaluronic acid to the total carboxyl group and is in the range of 3 to 50% relative to the total carboxyl group.The pharmaceutical composition of the present invention is an intra-articular formulation that exerts rapid analgesic effects after administration, and shows extremely long durable effects for a human joint disease with only a single administration rather than multiple administrations of a conventional way.
Owner:SEIKAGAKU KOGYO CO LTD
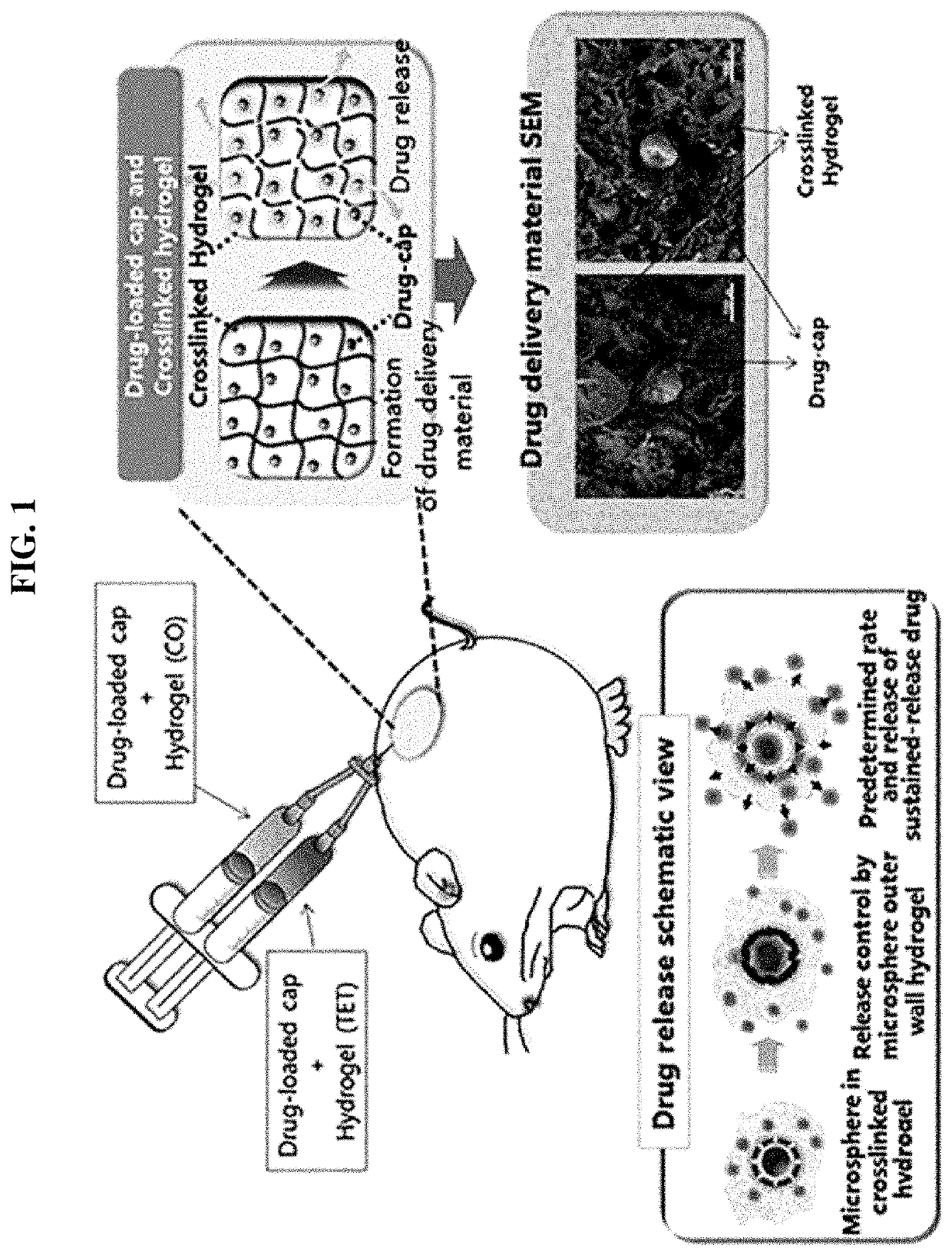
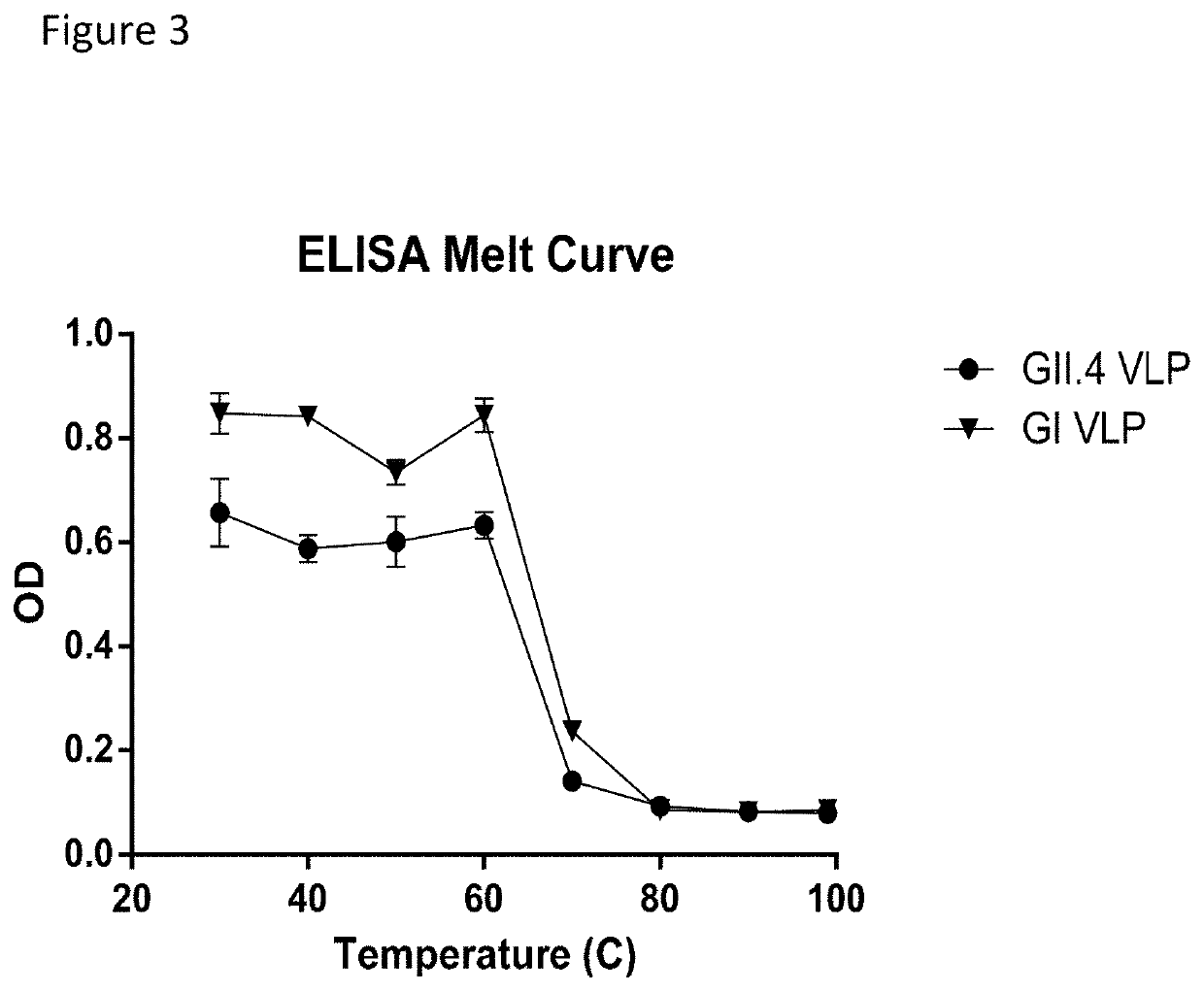
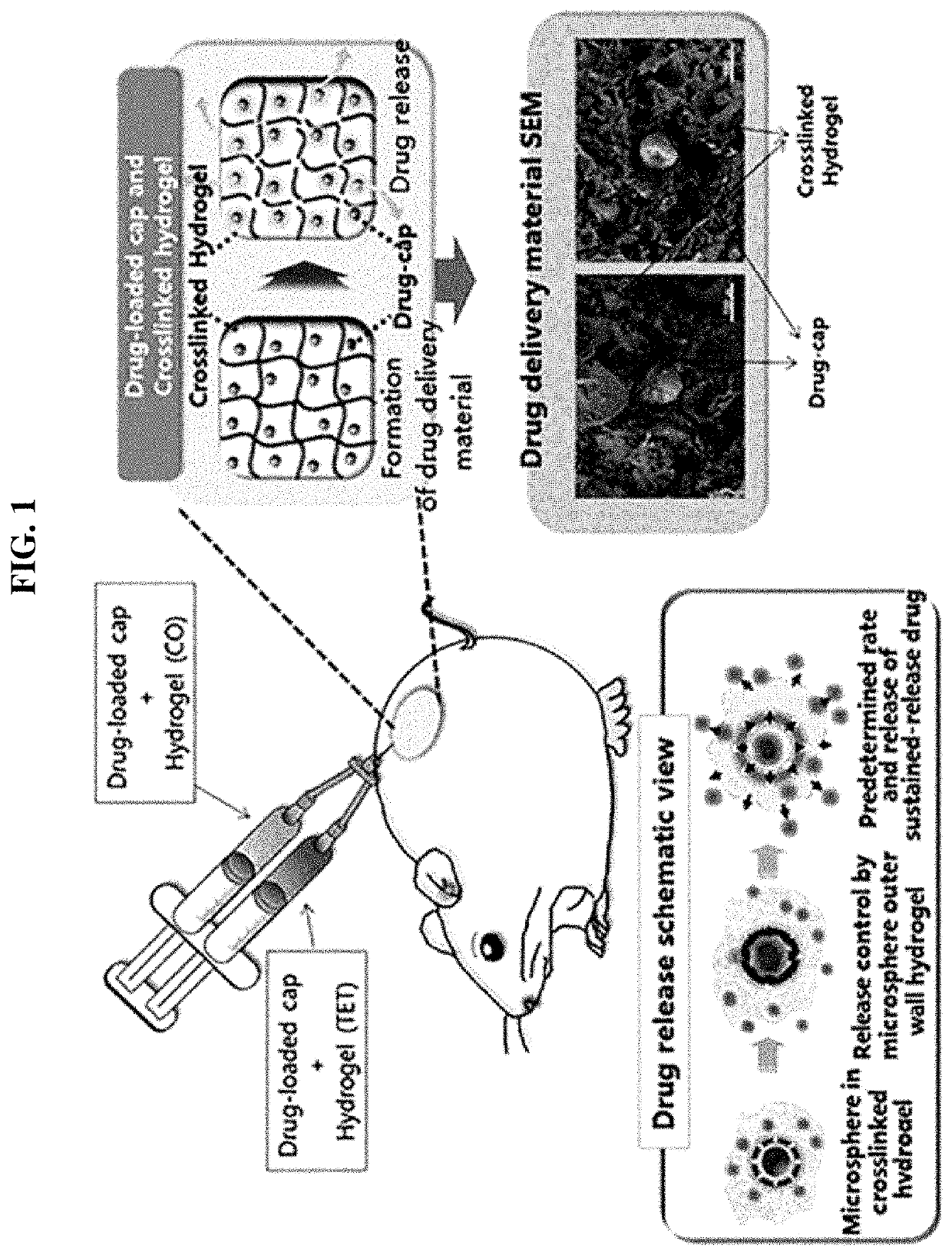
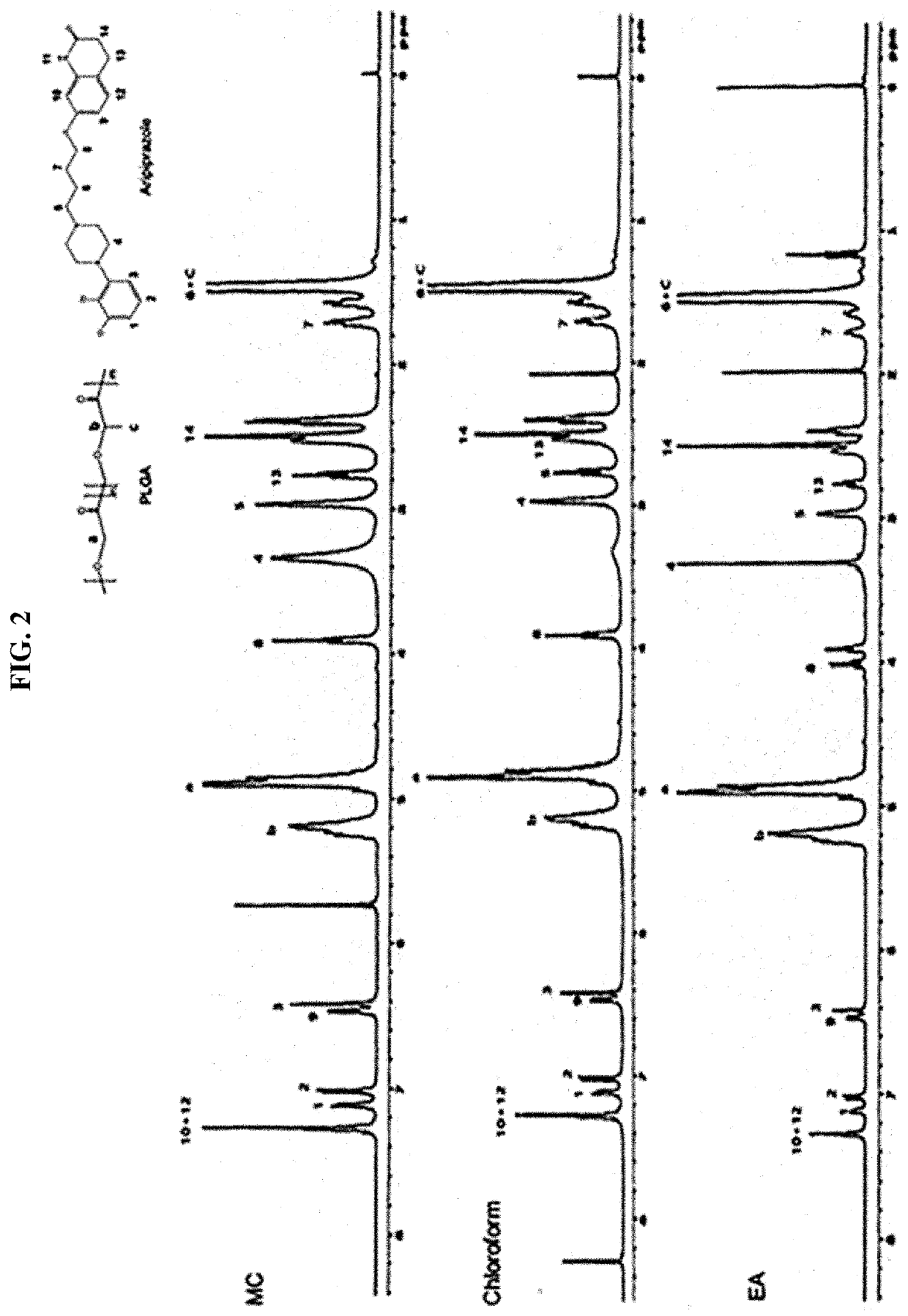
Drug delivery formulation for treating mental illness or central nervous system disorder
ActiveUS11337929B2Excessive drug releaseEasy to getOrganic active ingredientsSolution deliveryDrug administrationDrug release
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Norovirus vaccine
ActiveUS20200345828A1Improves mucosal residence timeMaximize both mucosal and systemic immune responsesPowder deliverySsRNA viruses positive-senseAntigenMultiple administration
A dry powder norovirus vaccine is provided, which comprises at least two norovirus antigens representing different genogroups. The vaccine may be produced by formulation with a mixture of different antigens or combination of monovalent powders with each containing one antigen. The formulated vaccine is suitable for mucosal administration and soluble in aqueous solutions for parenteral administration. A method of immunization is also provided, which comprises at least one administration of the vaccine via mucosal and / or parental route. The immunization may have multiple administrations of the vaccine, i.e., one or more immunizations via a mucosal route followed by one or more immunizations via a parenteral route or vice versa, to maximize both mucosal and systemic immune responses and protection against norovirus infections.
Owner:RESILIENCE GOVERNMENT SERVICES INC
Drug delivery formulation for treating mental illness or central nervous system disorder
ActiveUS20190374476A1Excessive drug releaseShorten the timeOrganic active ingredientsSolution deliveryDiseaseDrug administration
A drug delivery formulation for treating a mental illness or a central nervous system disorder is disclosed. The drug delivery formulation includes a microcapsule containing a biodegradable polymer and a drug and a crosslinked hydrogel and a method for preparing the same. The drug delivery formulation can slowly release a drug at a constant rate without a burst of drug release in the early stage of drug administration to maintain a constant blood drug level over a long period of time and thus enjoys the advantage of preventing excessive initial release of drug, thereby obtaining a desirable sustained release profile by single administration without necessity for multiple administrations divided at regular time intervals.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Pharmaceutical composition for relieving pain
The present invention relates to a pharmaceutical composition for relieving pain in a joint disease, including a hyaluronic acid and a pharmaceutically acceptable carrier, in which the hyaluronic acid is cross-linked by cyclizing a double bond in the moiety of a cinnamic acid in a partially amidated hyaluronic acid represented by Formula (1):[Ar—CH═CH—COO—(CH2)n—NH-]m-HA,to form a cycloubutane ring, in which Ar represents an optionally substituted phenyl group, n represents an integer of 2 or 3, HA represents a carboxy residue of the hyaluronic acid, and m represents an amidation ratio of the hyaluronic acid to the total carboxyl group and is in the range of 3 to 50% relative to the total carboxyl group.The pharmaceutical composition of the present invention is an intra-articular formulation that exerts rapid analgesic effects after administration, and shows extremely long durable effects for a human joint disease with only a single administration rather than multiple administrations of a conventional way.
Owner:SEIKAGAKU KOGYO CO LTD
A targeted drug-loaded nano-micelle and its preparation method and application
ActiveCN108339125BGood monodispersityHigh drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsAntileukemic agentCell Surface Antigens
The invention discloses a high-efficiency, targeted drug-loaded nano-micelle and its preparation method and application. The preparation steps are: (1) dissolving the amphiphilic block copolymer in an organic solvent, and then adding a small-molecule anti-leukemia drug Hydrochloride, after fully dissolved and mixed, add triethylamine to remove hydrochloric acid to form a small molecule anti-leukemia drug, and then add it to PBS buffer to form nanomicelles with inner core encapsulated drug and outer shell hydrophilic; after dialysis, dry , to obtain drug-loaded nanomicelles; the polydopamine modification method is used to modify the specific antibody against the leukemia cell surface antigen to achieve targeting. The particle size of the drug-loaded nano-micelle prepared by the invention is 50-100nm, showing good monodispersity, high drug-loading capacity and encapsulation efficiency, simple targeting modification steps, and good targeting leukemia cell killing effect , while for normal cells, it has high biological safety and can achieve slow release, avoiding the disadvantages of long-term and multiple administrations.
Owner:WENZHOU INST OF BIOMATERIALS & ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com