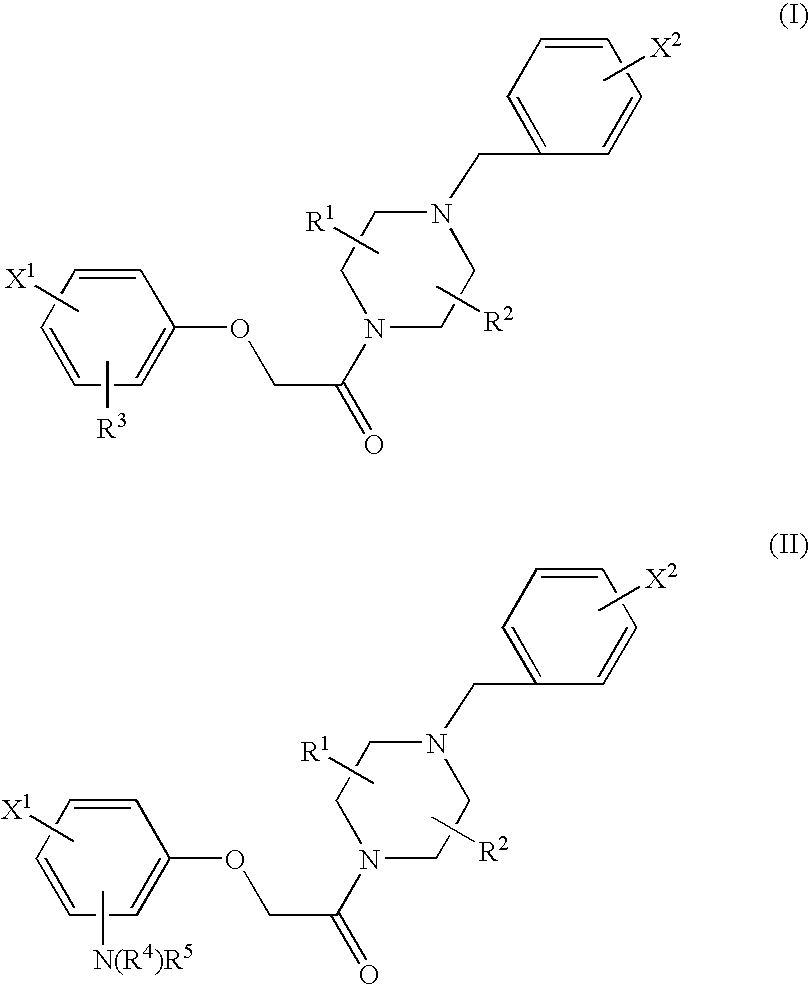
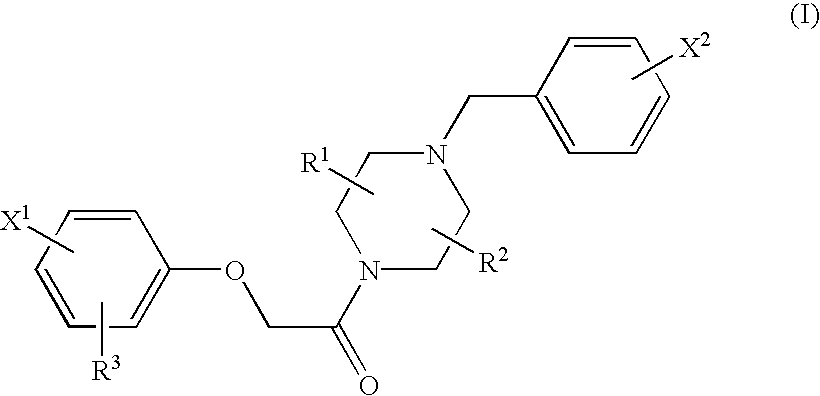
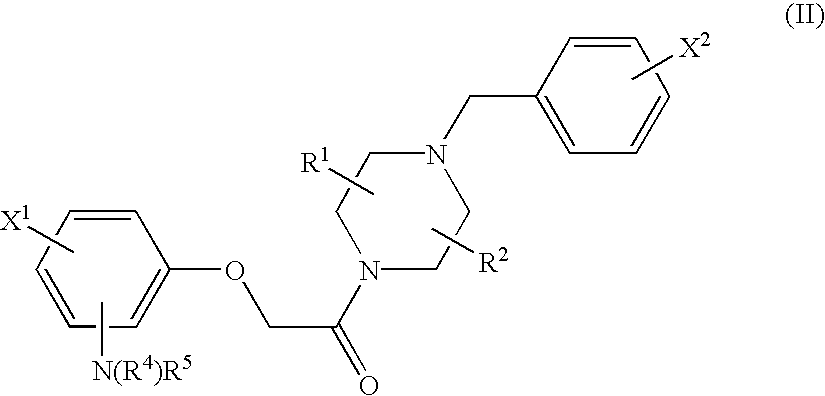
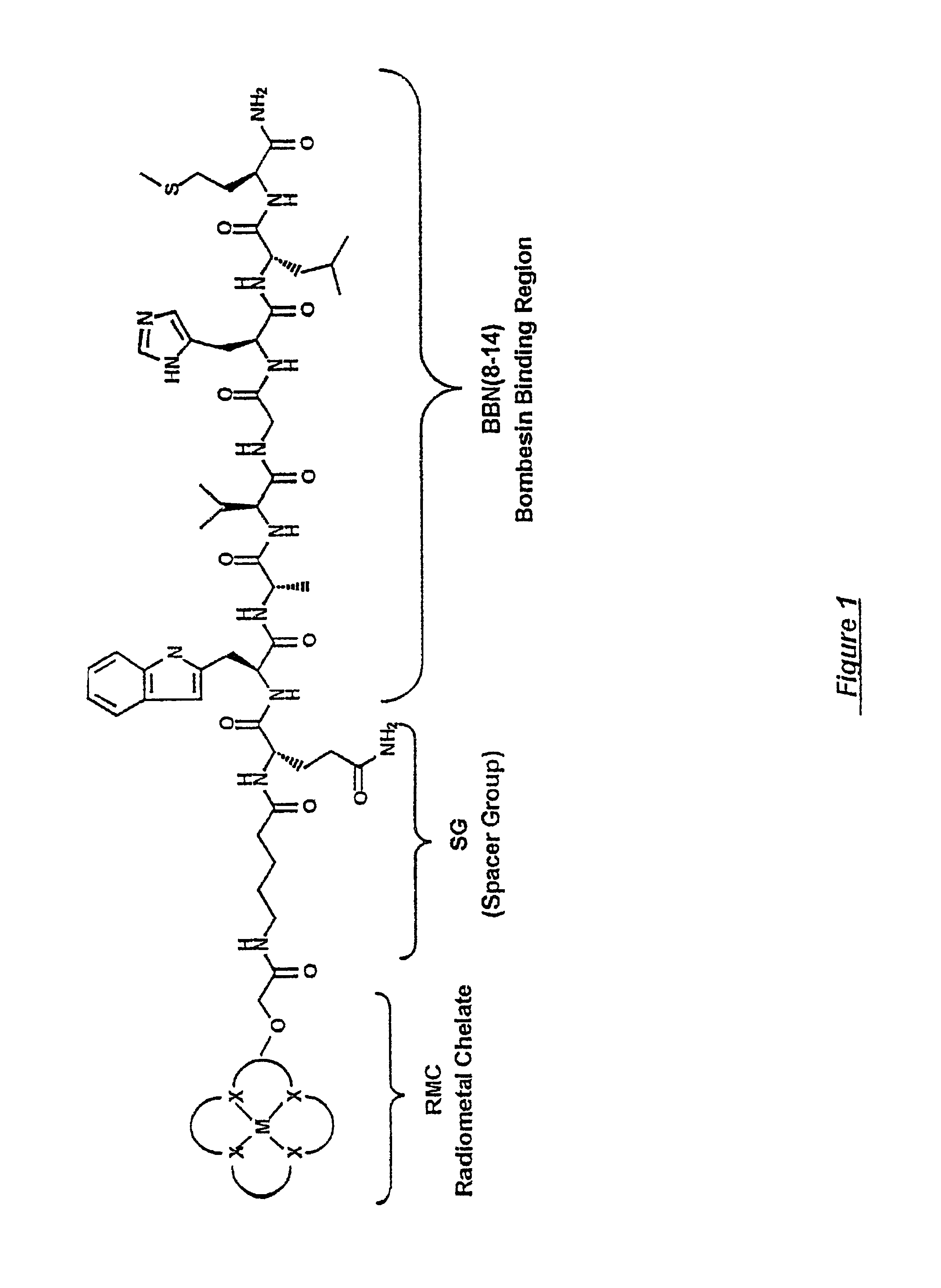
Patents
Literature
428 results about "Radioactive drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Radioactive drug: A drug containing a radioactive substance that is used in the diagnosis and treatment of cancer and in pain management of bone metastases. Also called a radiopharmaceutical.
Multi-dimensional image reconstruction
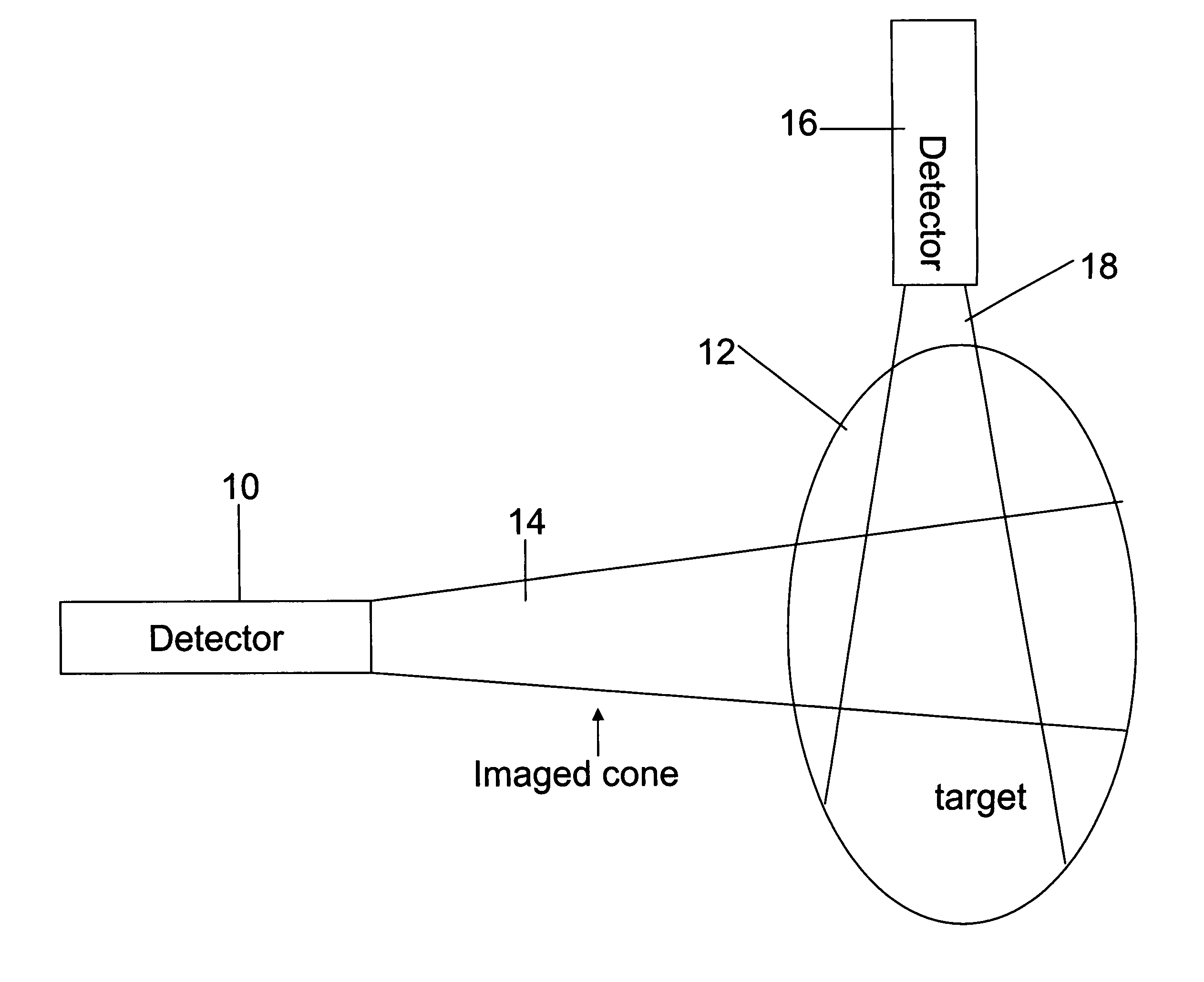
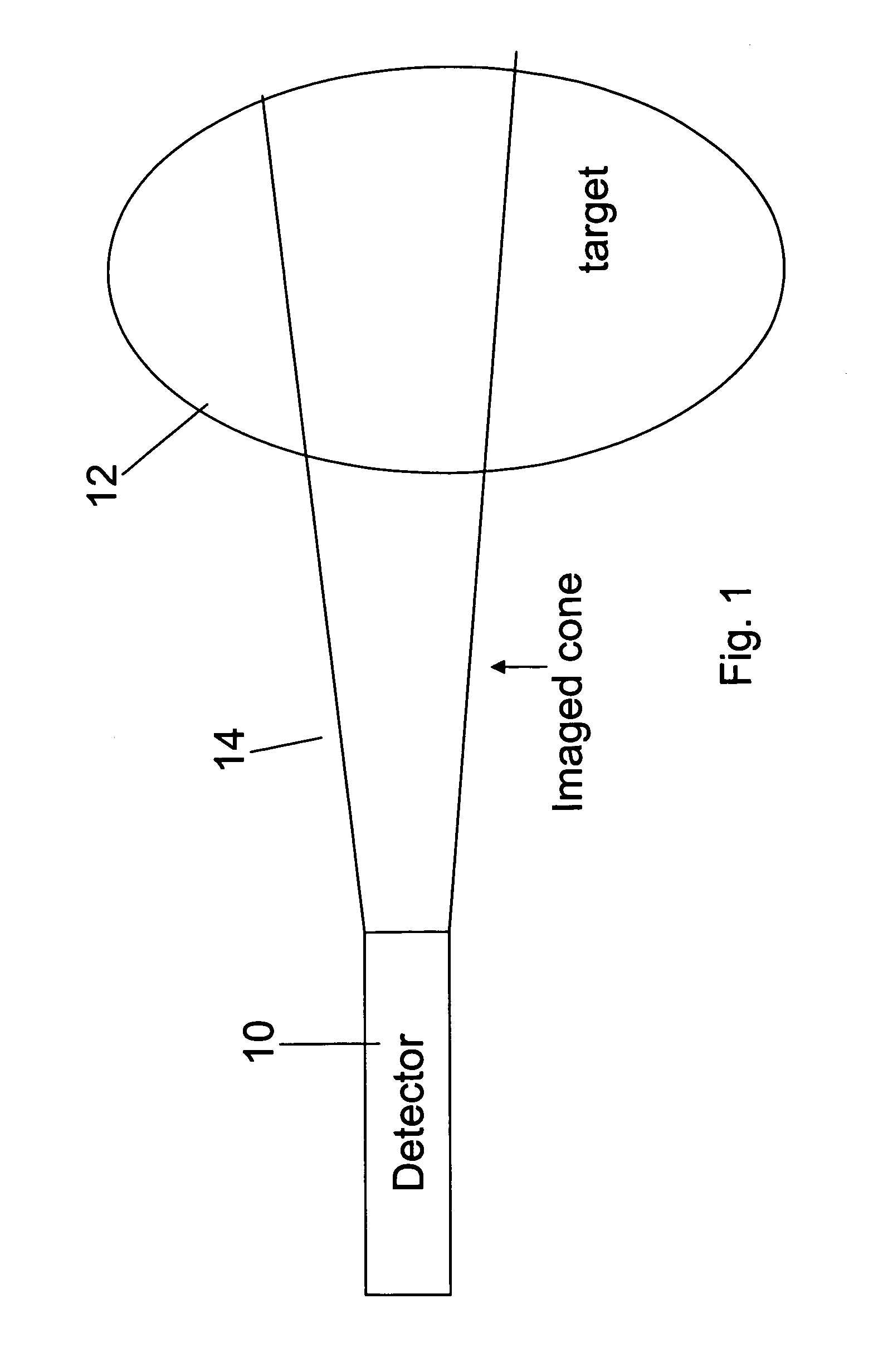
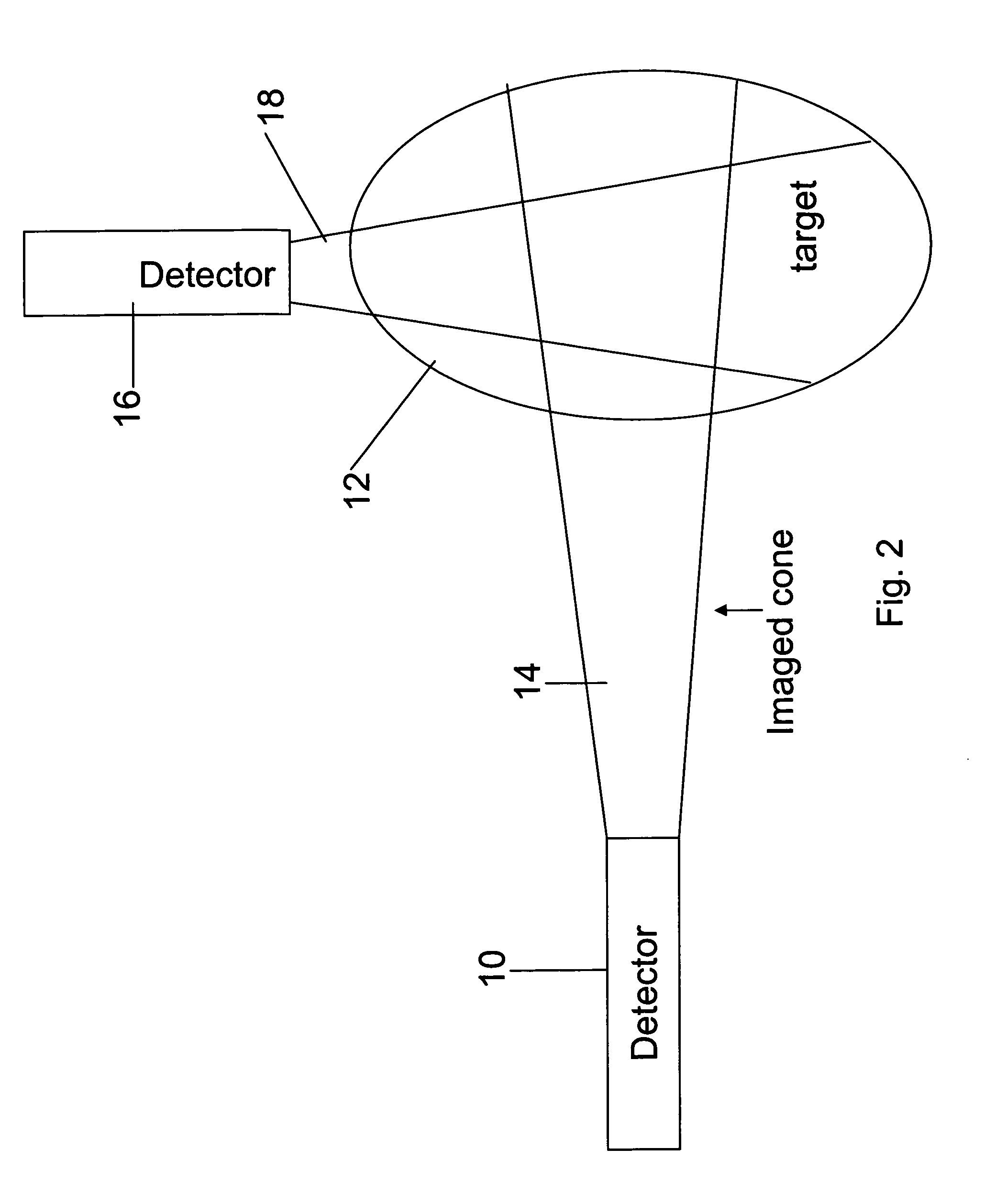
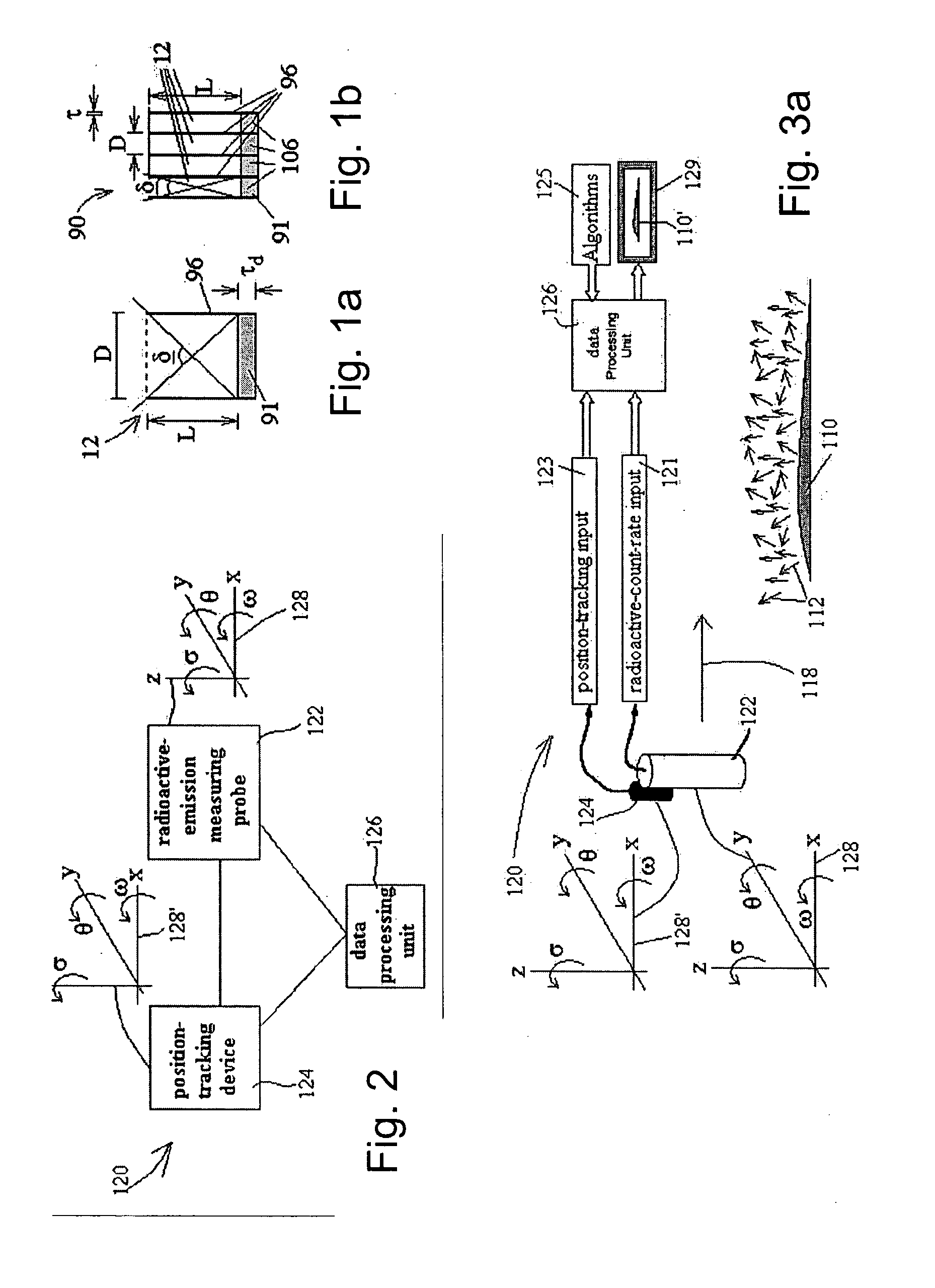
Apparatus for radiation based imaging of a non-homogenous target area having distinguishable regions therein, comprises: an imaging unit configured to obtain radiation intensity data from a target region in the spatial dimensions and at least one other dimension, and an image four-dimension analysis unit analyzes the intensity data in the spatial dimension and said at least one other dimension in order to map the distinguishable regions. The system typically detects rates of change over time in signals from radiopharmaceuticals and uses the rates of change to identify the tissues. In a preferred embodiment, two or more radiopharmaceuticals are used, the results of one being used as a constraint on the other.
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Radioimaging
ActiveUS20080042067A1Unprecedented sensitivityAvoid saturationHandling using diaphragms/collimetersMaterial analysis by optical meansNuclear medicineRadioactive drug
Owner:SPECTRUM DYNAMICS MEDICAL LTD
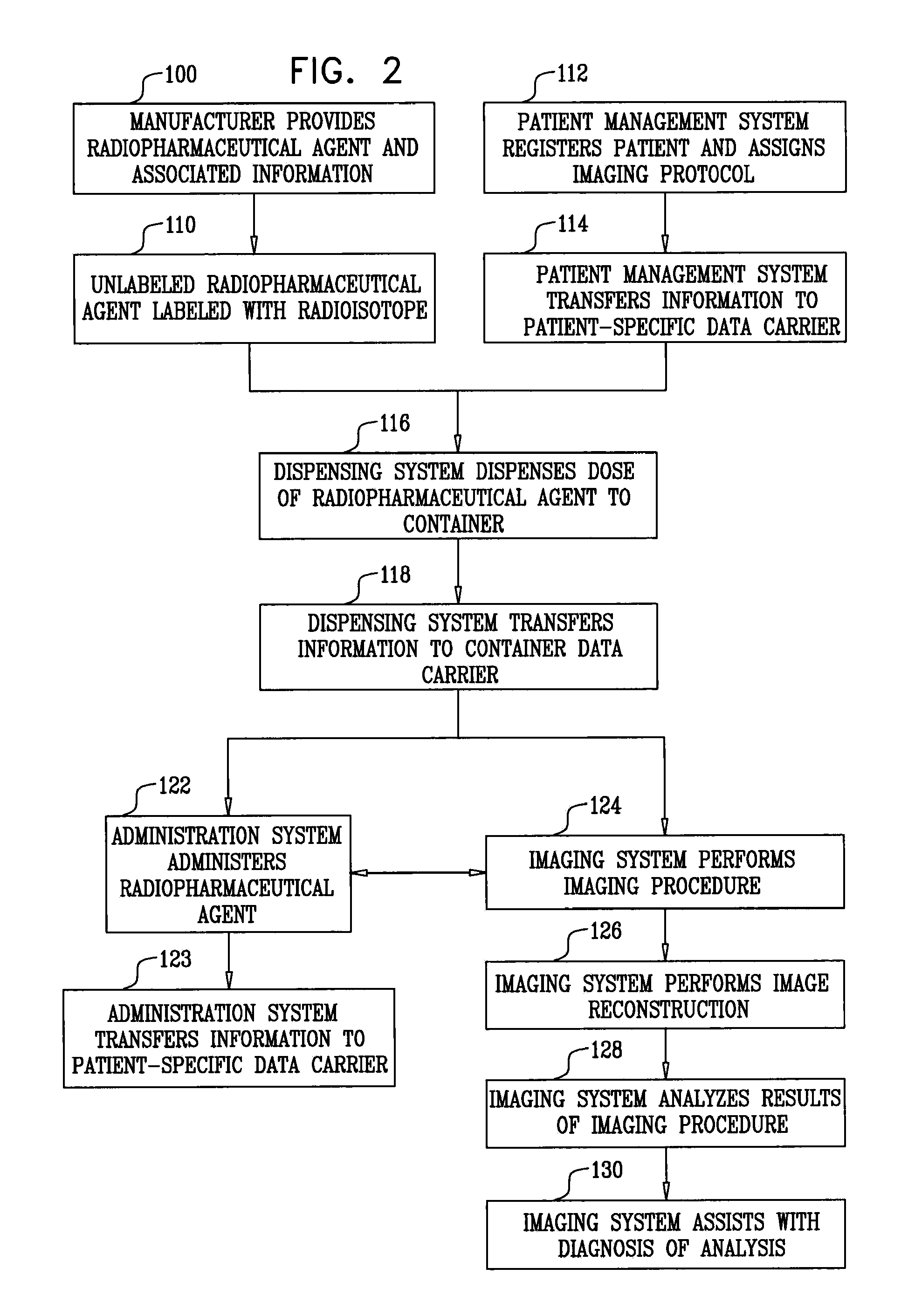
Radiopharmaceutical dispensing, administration, and imaging
ActiveUS20080131362A1Improve securityDrug and medicationsSurgeryComputer scienceRadiopharmaceutical agent
Apparatus is provided for use with at least one labeled radiopharmaceutical agent, the apparatus including a container (22) containing the at least one labeled radiopharmaceutical agent, and a portable computer-communicatable data carrier (120, 24) associated with the container (22), the data carrier (120, 24) containing imaging protocol information for use with the at least one labeled radiopharmaceutical agent. Other embodiments are also described.
Owner:SPECTRUM DYNAMICS MEDICAL LTD
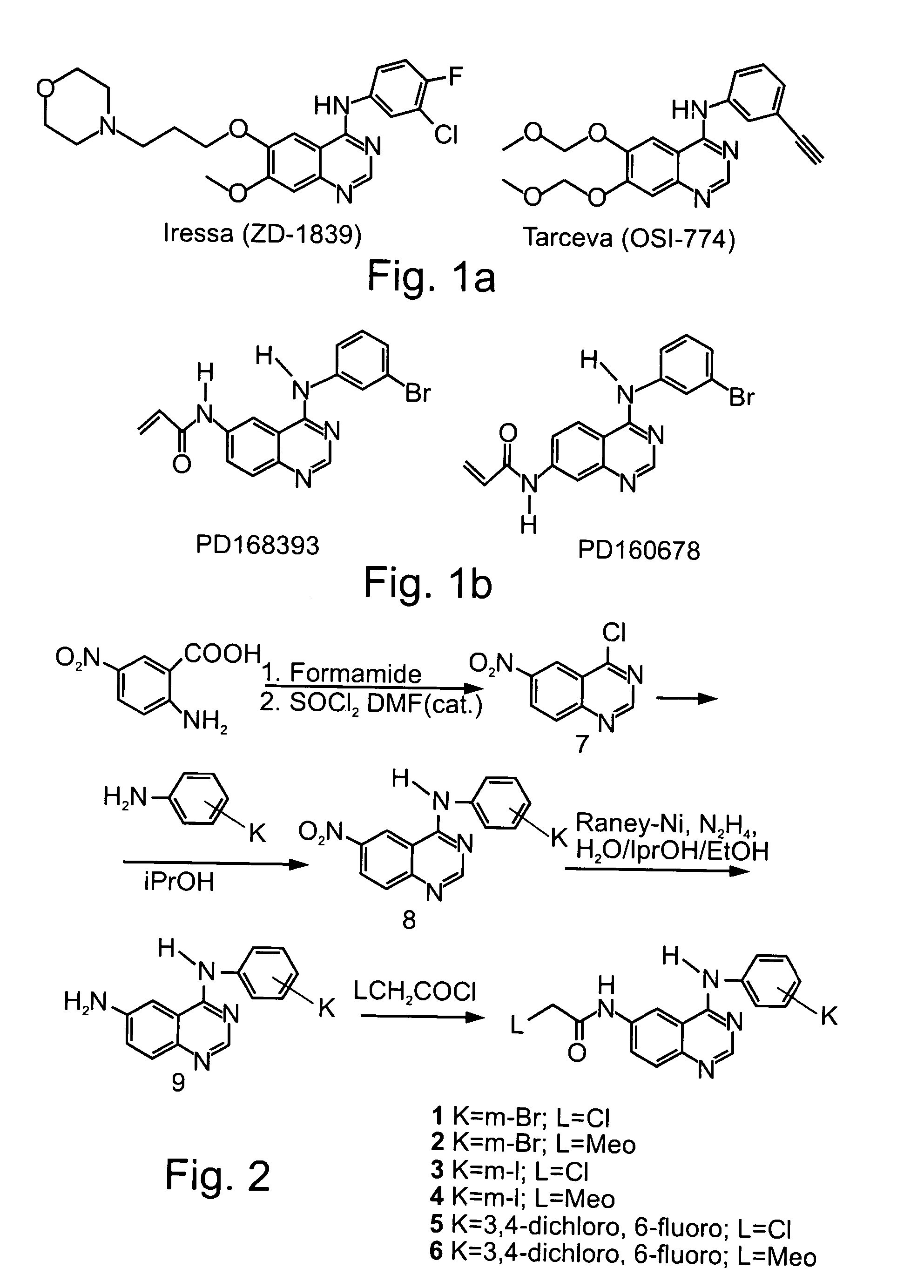
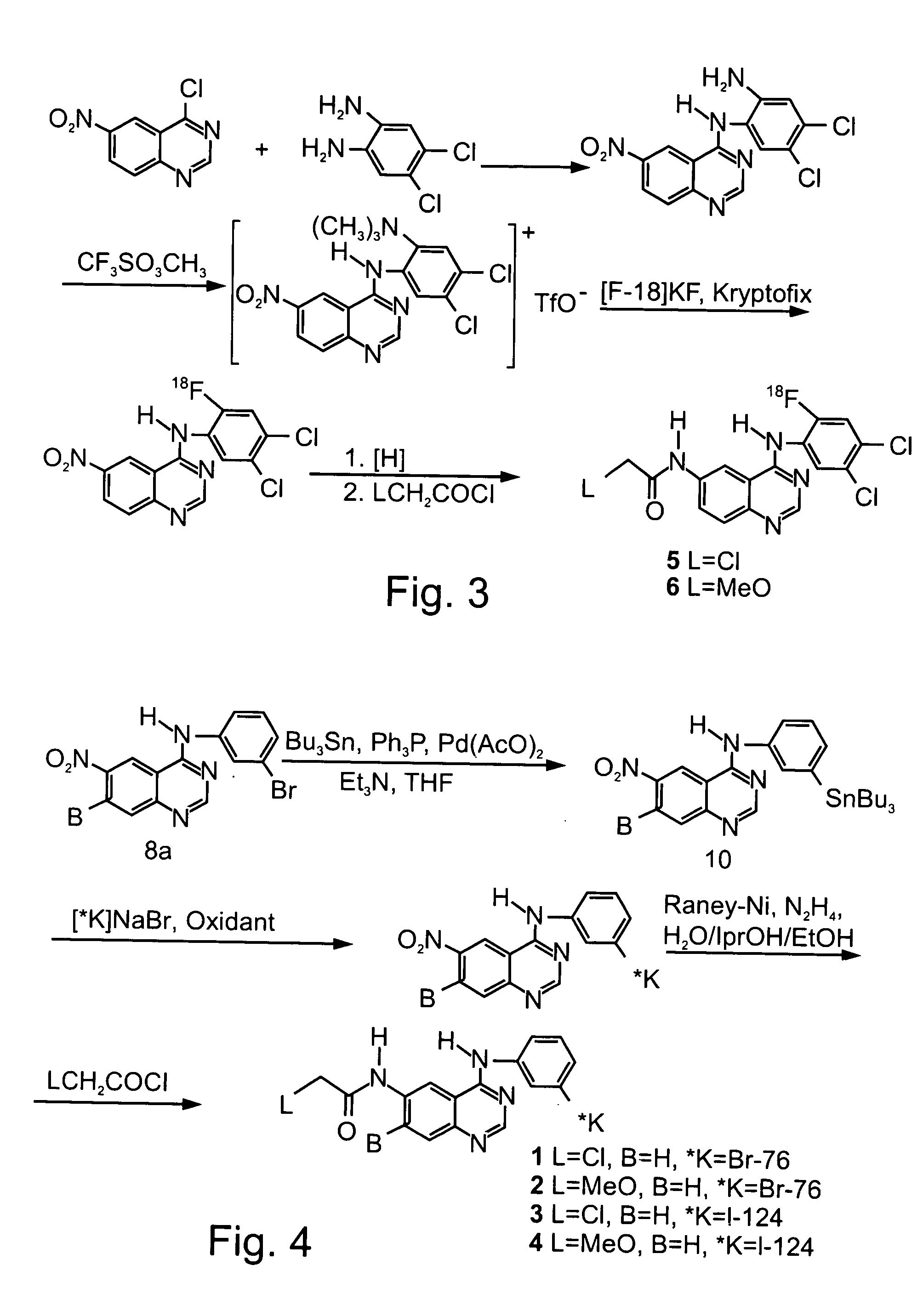
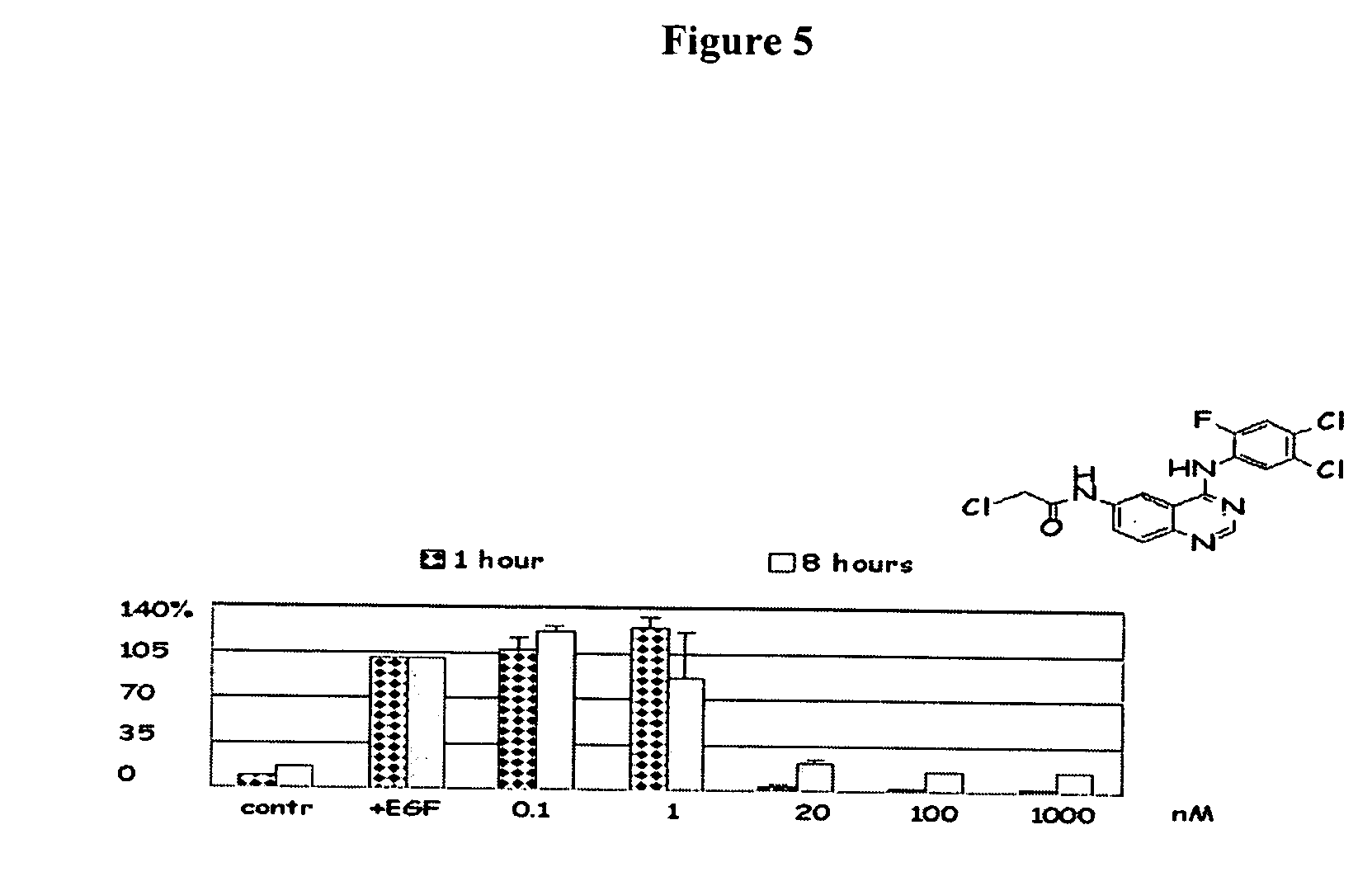
Radiolabeled irreversible inhibitors of epidermal growth factor receptor tyrosine kinase and their use in radioimaging and radiotherapy
InactiveUS6562319B2BiocideOrganic chemistryPositron emission tomographyEpidermal growth factor receptor tyrosine kinase
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Radiolabeled irreversible inhibitors of epidermal growth factor receptor tyrosine kinase and their use in radioimaging and radiotherapy
InactiveUS20020128553A1BiocideOrganic chemistryPositron emission tomographyEpidermal growth factor receptor tyrosine kinase
Radiolabeled epidermal growth factor receptor tyrosine kinase (EGFR-TK) irreversible inhibitors and their use as biomarkers for medicinal radioimaging such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) and as radiopharmaceuticals for radiotherapy are disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Method and apparatus for dispensing radioactive liquid
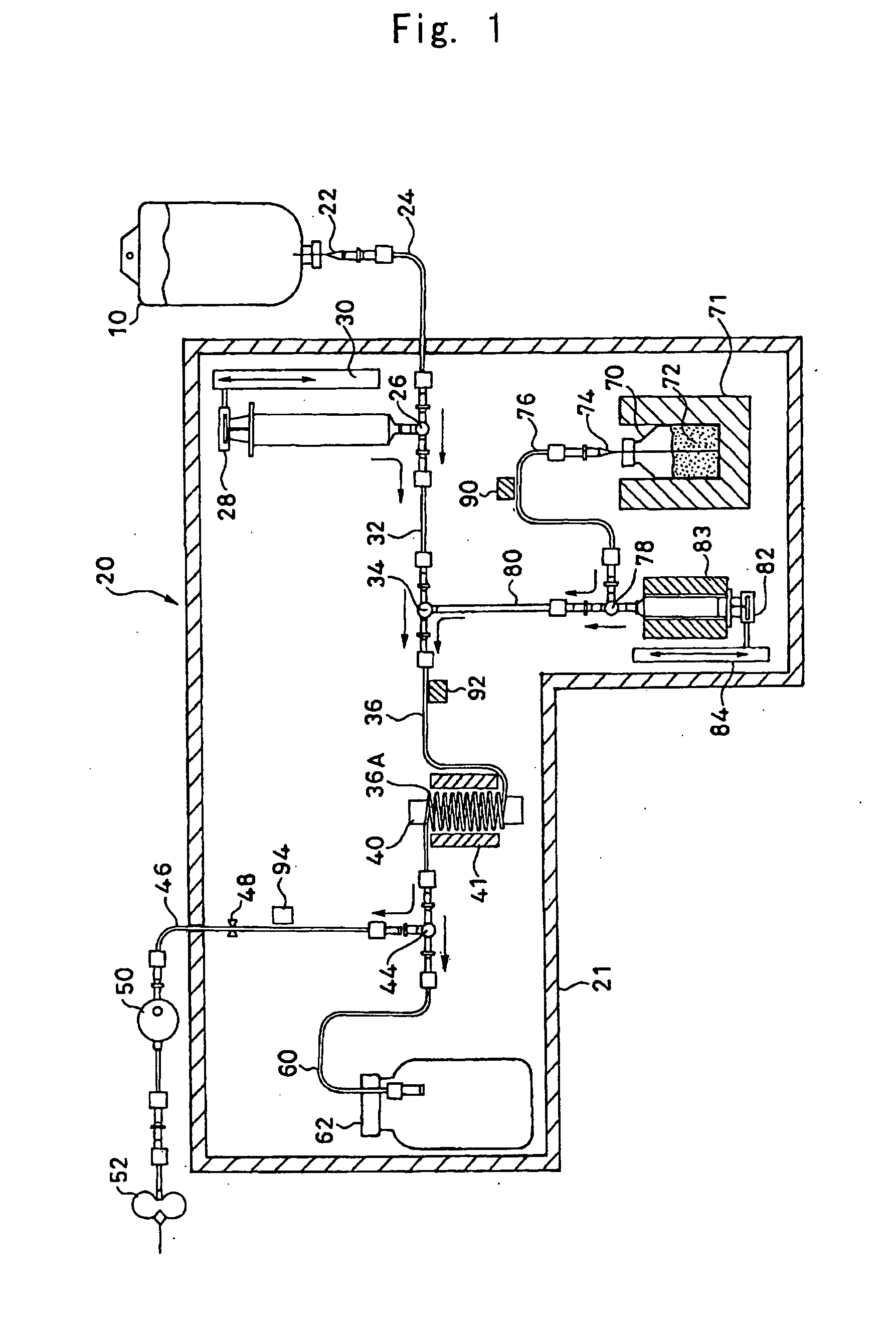
InactiveUS20050085682A1Reduce radiation exposureConvenient and accurateIn-vivo radioactive preparationsInfusion devicesRadioactive drugMedicine
A method and apparatus which frees the operator from dispensing operations with a reduction in exposure. A necessary amount of radioactive drug solution is dispensed by measuring radioactivity concentration of the radioactive drug solution passing through a tube constituting the channel of the radioactive drug solution, and controlling the amount of dispensation.
Owner:SUMITOMO HEAVY IND LTD
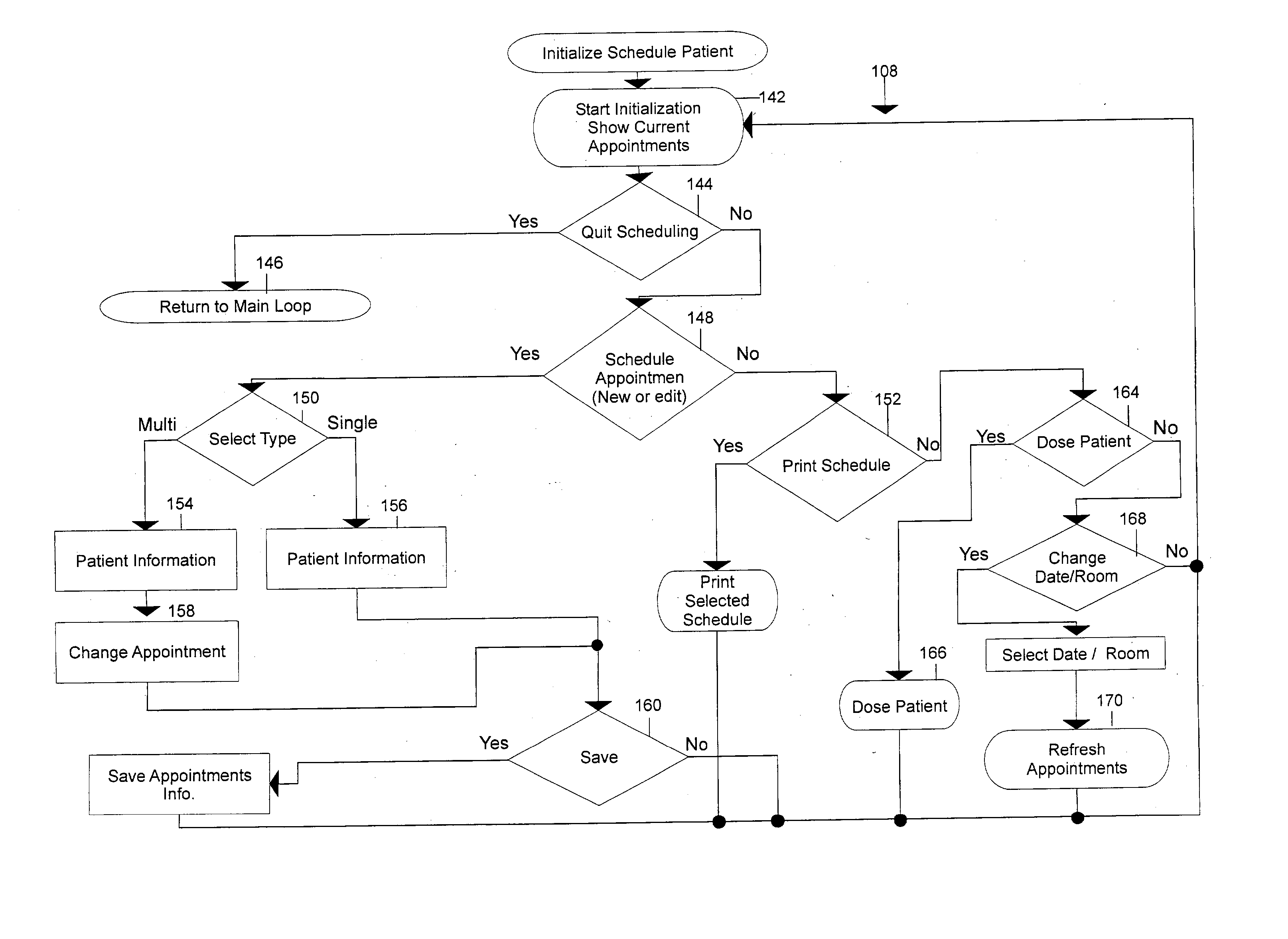
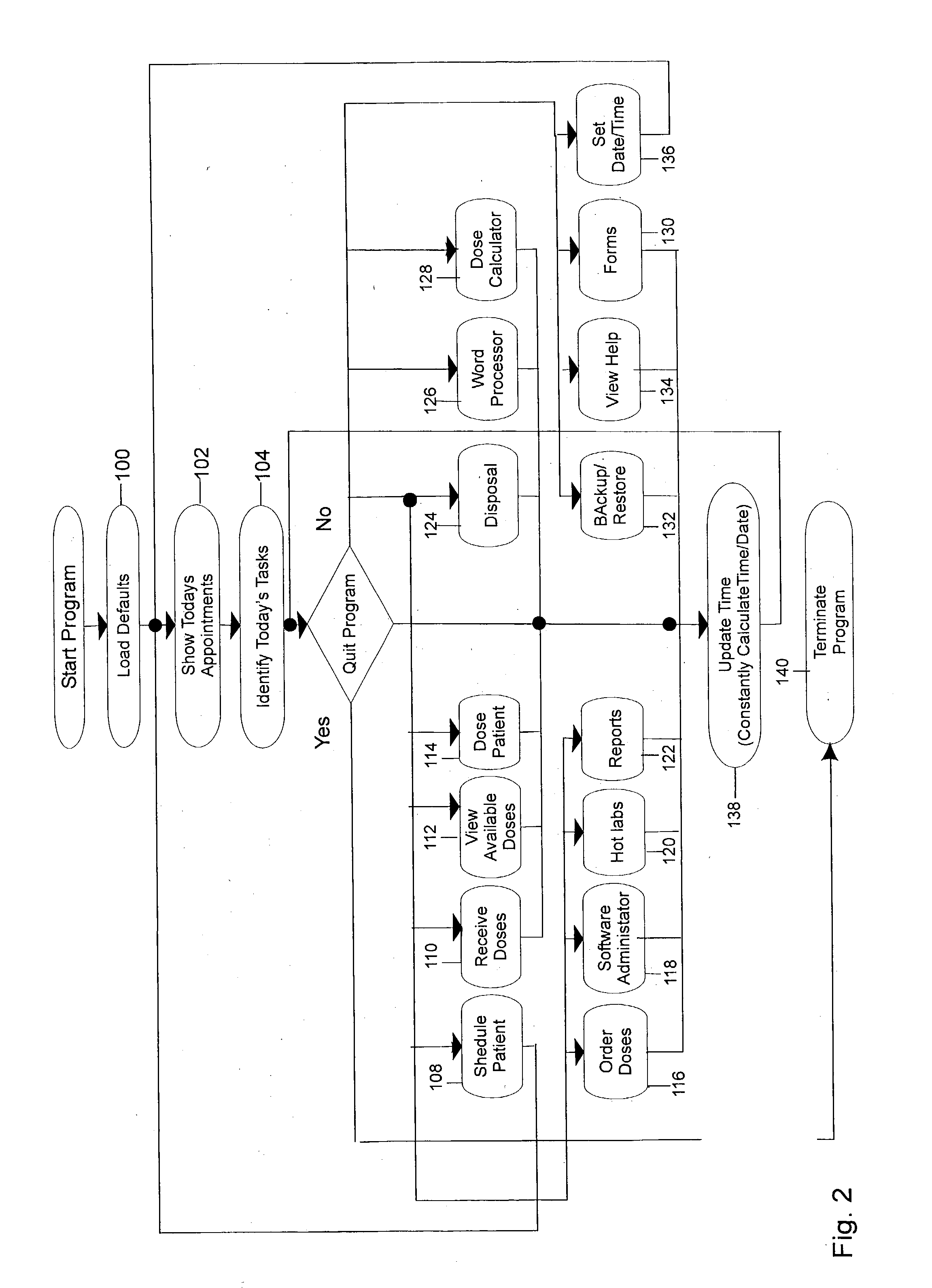
Algorithm and program for the handling and administration of radioactive pharmaceuticals
ActiveUS20030139640A1Automatically and easily addressAvoidingData processing applicationsDrug and medicationsAlgorithmRadioactive drug
An algorithm and associated program for performing method steps in the maintaining of records and generating of reports used in the processing of radioactive pharmaceuticals. The algorithm is used as the basis of a program which can accomplish this method automatically. The method involves the determination of dose, the acquisition of the materials, scheduling for the issuance of doses and for future doses, the actual monitoring and control of material and equipment disposal. The algorithm and method are also adapted for the generation of reports on a periodic basis. In short, the method performed by the algorithm allows for an automation through a computer system and this, in turn, allows for the automatic processing of the steps performed and the controls involved in the dispensing of radioactive pharmaceuticals and automatically allows for the generation of governmental and other reports therefor.
Owner:EC2 SOFTWARE SOLUTIONS LLC
Pharmaceutical Dosing Method
InactiveUS20080166292A1Accurately determineMedical simulationIn-vivo radioactive preparationsTime factorTime standard
Systems, devices, and methods for more accurately determining a radiopharmaceutical dose administered to a patient by relying on a time factor. Particularly, broadly contemplated herein is the administration of a dose on the basis of an elapsed time from when a dose was last accurately measured in the past to when it is injected into the patient. As such, when a dose is first measured, that timepoint is preferably recorded whereupon the time of injection or administration into a patient is also recorded. Based on the original measured dose, the radionuclide (and thus its known decay rate) and the time elapsed, the dose is calculated and not directly measured on injection. The clocks on the filling station and the transport cart are synchronized to each other or to a known and accepted time standard. In this manner, there is temporal continuity and no inaccuracies of time or loss of time occurs between measurement and injection.
Owner:MEDRAD INC.
Fully-automated microfluidic system for the synthesis of radiolabeled biomarkers for positron emission tomography
InactiveUS20080233018A1Isotope introduction to sugar derivativesSugar derivativesChemical synthesisConfocal
The present application relates to microfluidic devices and related technologies, and to chemical processes using such devices. More specifically, the application discloses a fully automated synthesis of radioactive compounds for imaging, such as by positron emission tomography (PET), in a fast, efficient and compact manner. In particular, this application describe an automated, stand-alone, microfluidic instrument for the multi-step chemical synthesis of radiopharmaceuticals, such as probes for PET and a method of using such instruments.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Efficient single photon emission imaging
InactiveUS7026623B2Shorten the timeImprove image qualityReconstruction from projectionMaterial analysis by optical meansRadioactive drugRadiology
Owner:ULTRASPECT
Radioimaging
ActiveUS20080230702A1Avoid saturationLarge diameterMaterial analysis by optical meansDiagnostic recording/measuringRadioactive drugRadiation imaging
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Process and device for preparing radiopharmaceutical products for injection
InactiveUS20040084340A1Reduce needAmpoule syringesDispensing apparatusRadiopharmaceutical CompoundChemical compound
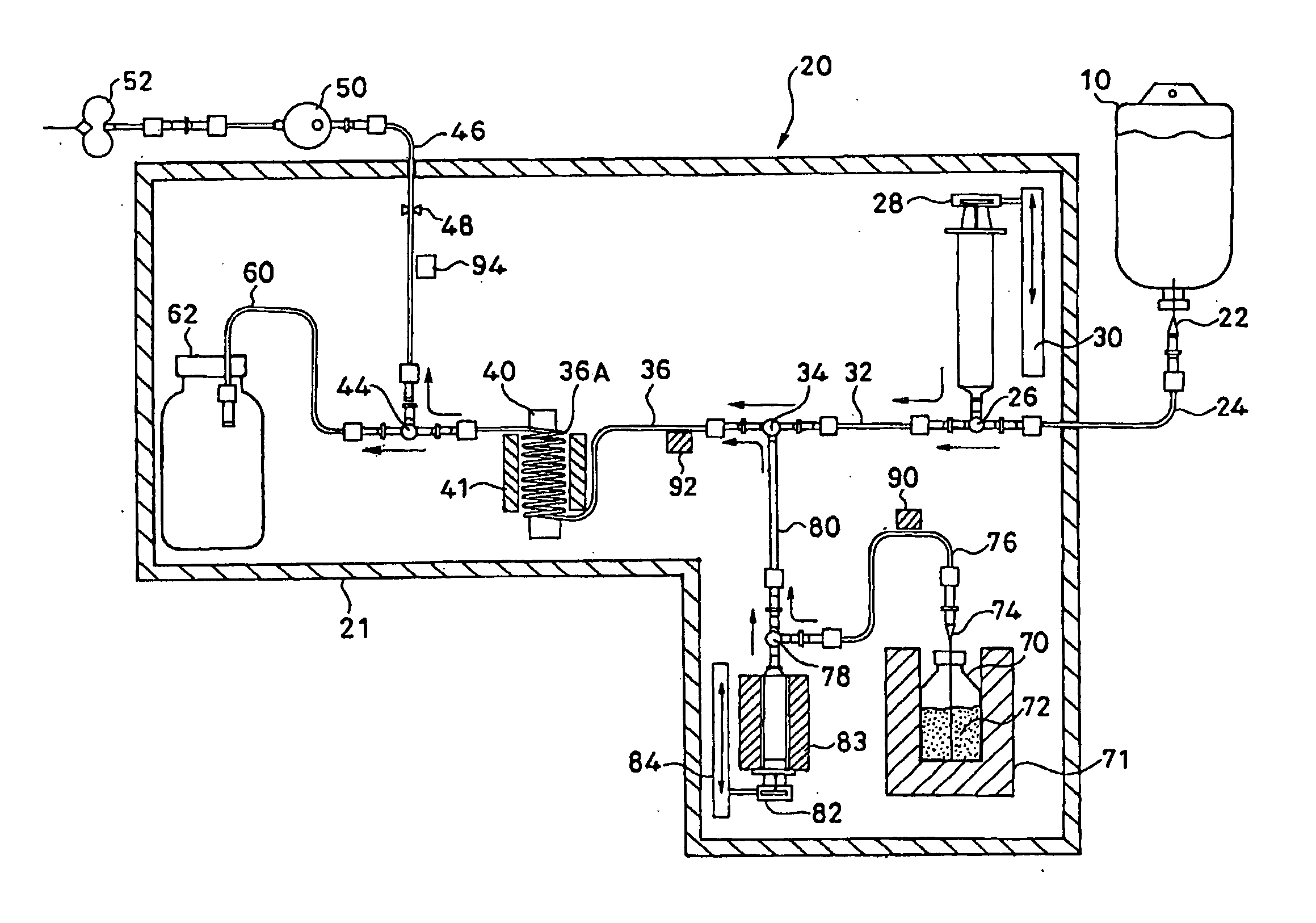
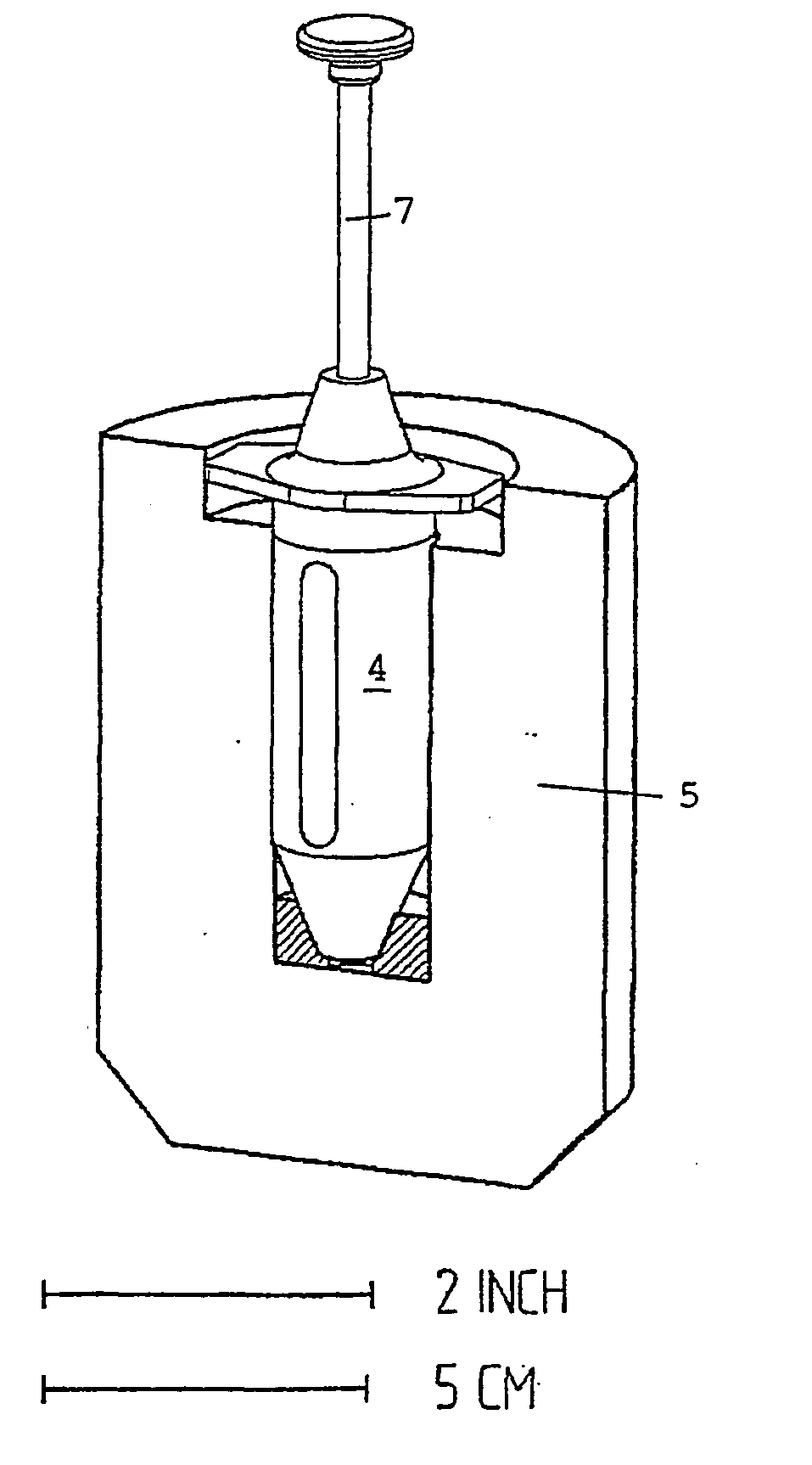
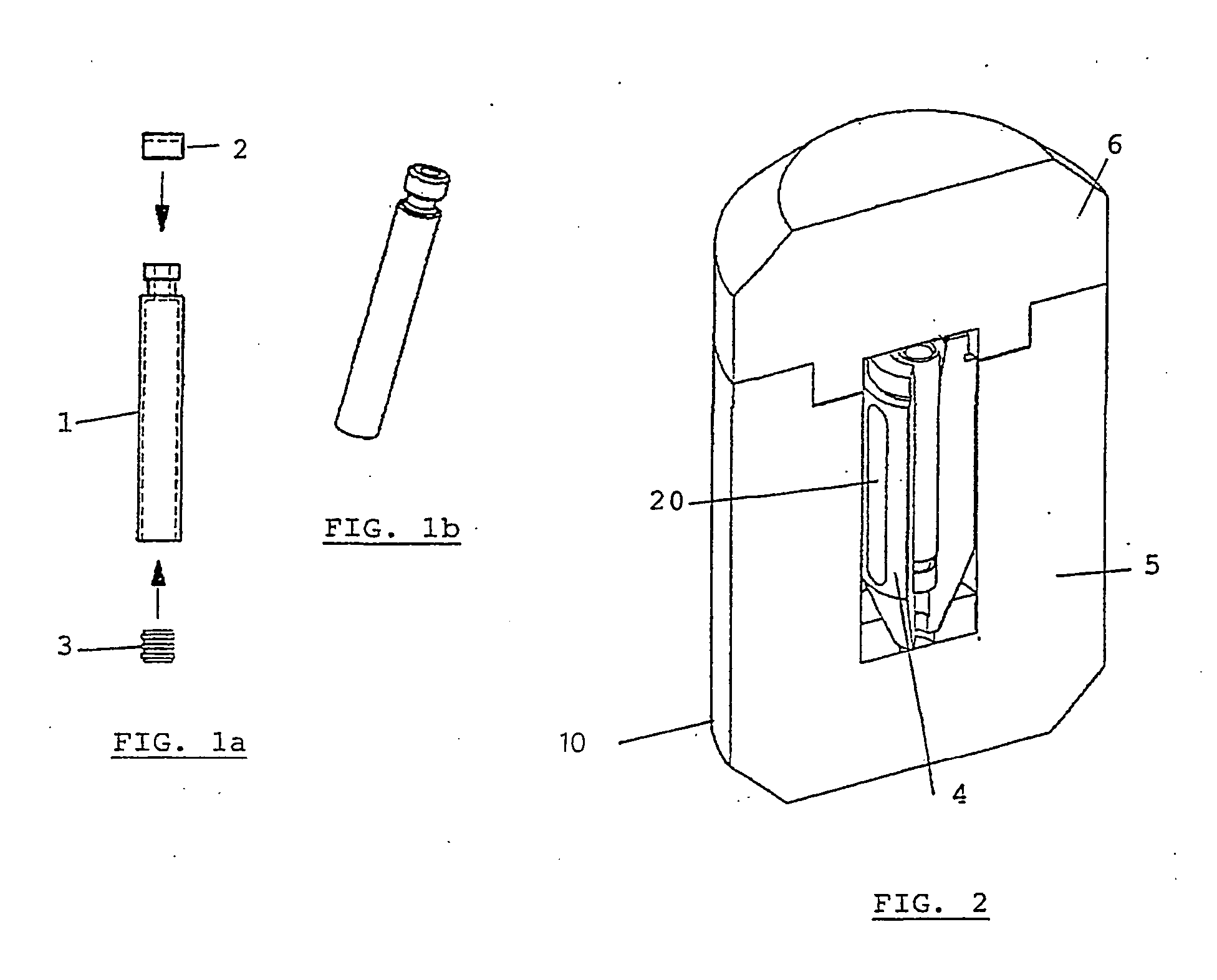
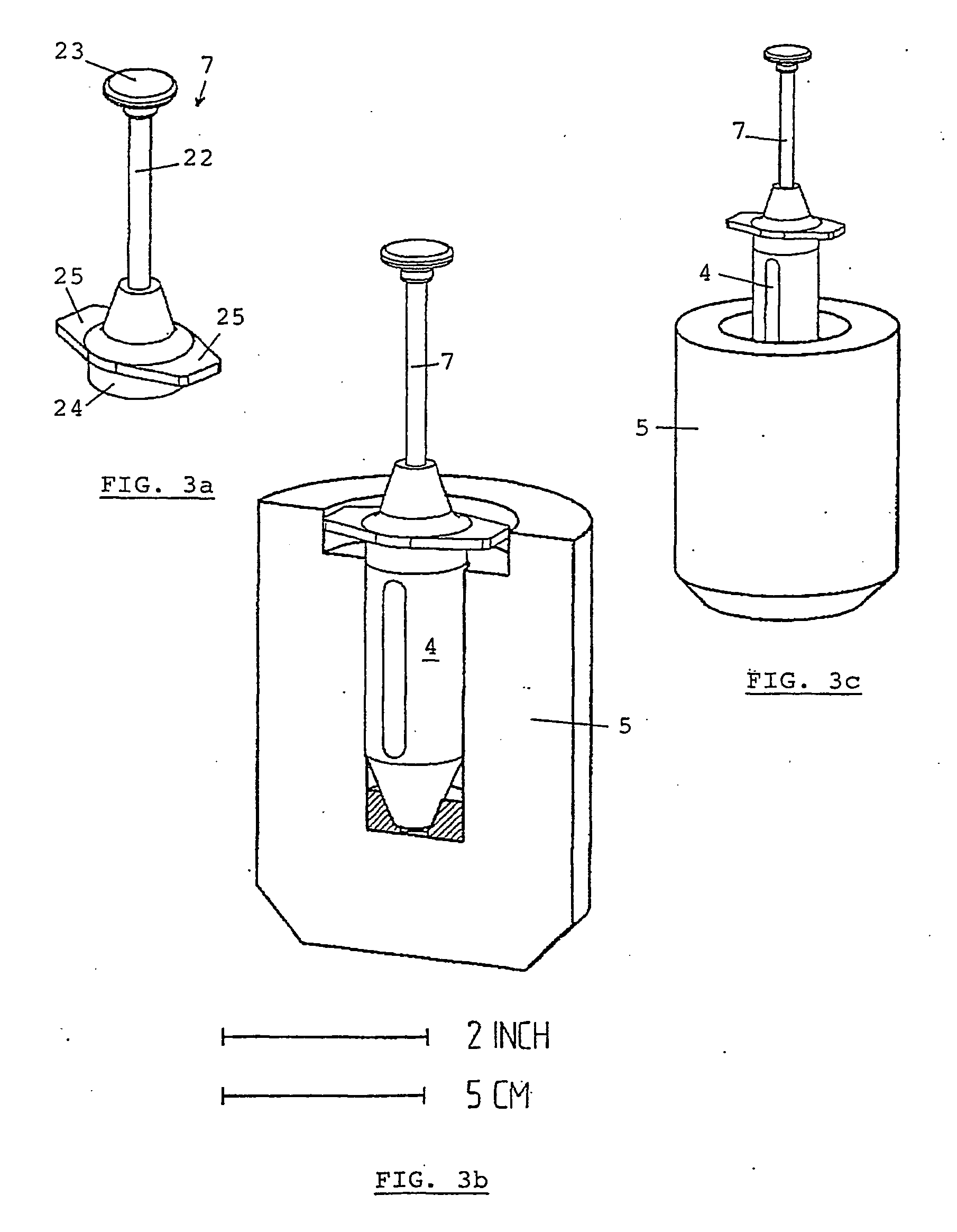
Method for preparing, packaging and handling an individual dose of a radiopharmaceutical compound, comprising the following steps: filling a cartridge (1) with said dose of radiopharmaceutical compound via a first end, the second end being closed by means of a component serving as a piston (3); closing said cartridge (1) at said first end by means of a closure device (2); placing said cartridge (1) in a radiation shielding device (10), comprising an inner part (4) and an outer part (5), said inner part serving as radiation shielding for an operator and said outer part serving as a transportation shielding container; closing said container by means of an appropriate shielding lid (6); transporting said container up to the place at which an injection of said radiopharmaceutical compound will take place; removing the shielding lid (6) of the container; fixing a plunger (7) to the cartridge piston (3); extracting the cartridge and the inner part (4) of the radiation shielding device (10) from the outer part (5) serving as a container, and placing injection means (30) on the cartridge end which has the setting closure device (2).
Owner:MORELLE JEAN LUC
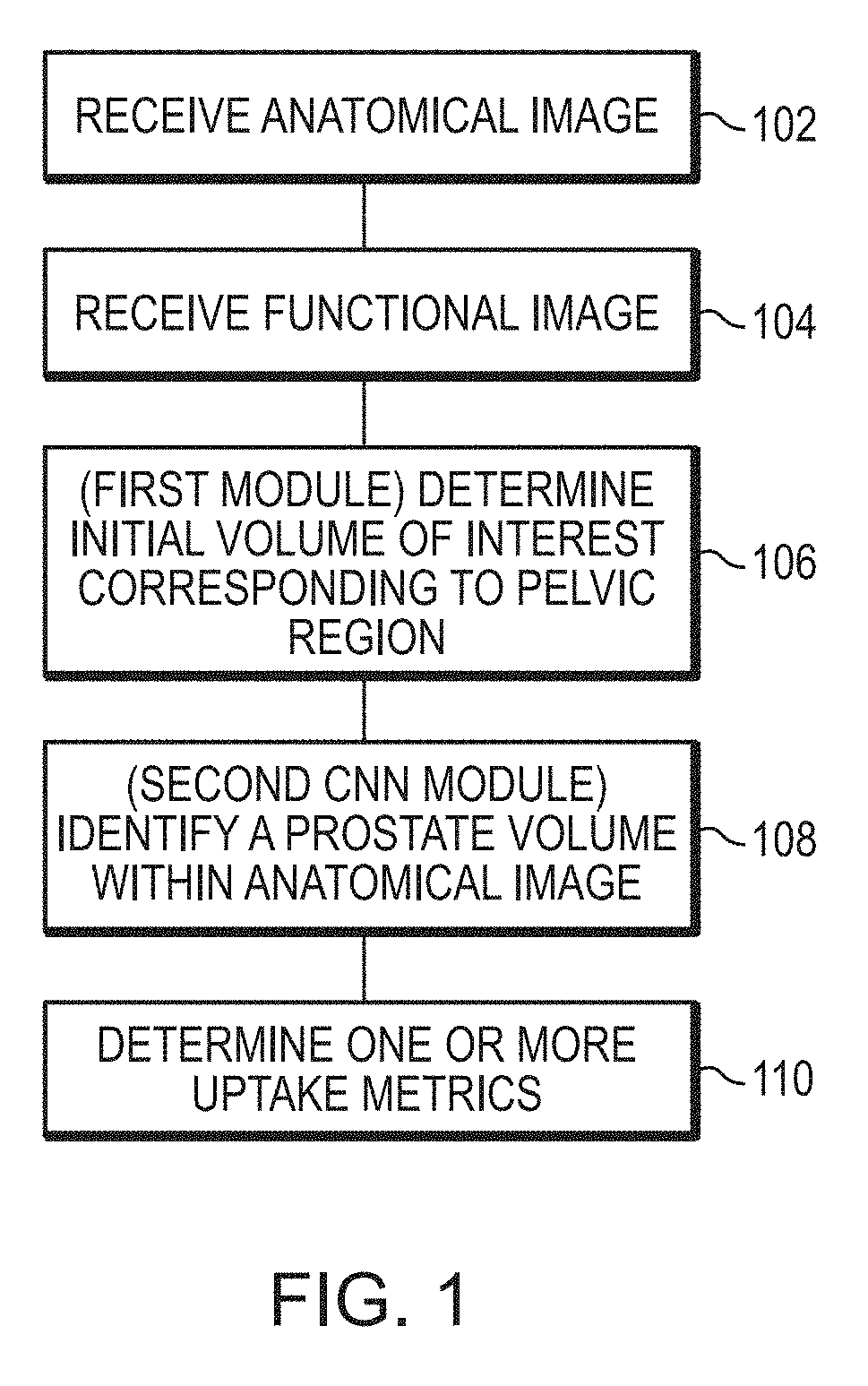
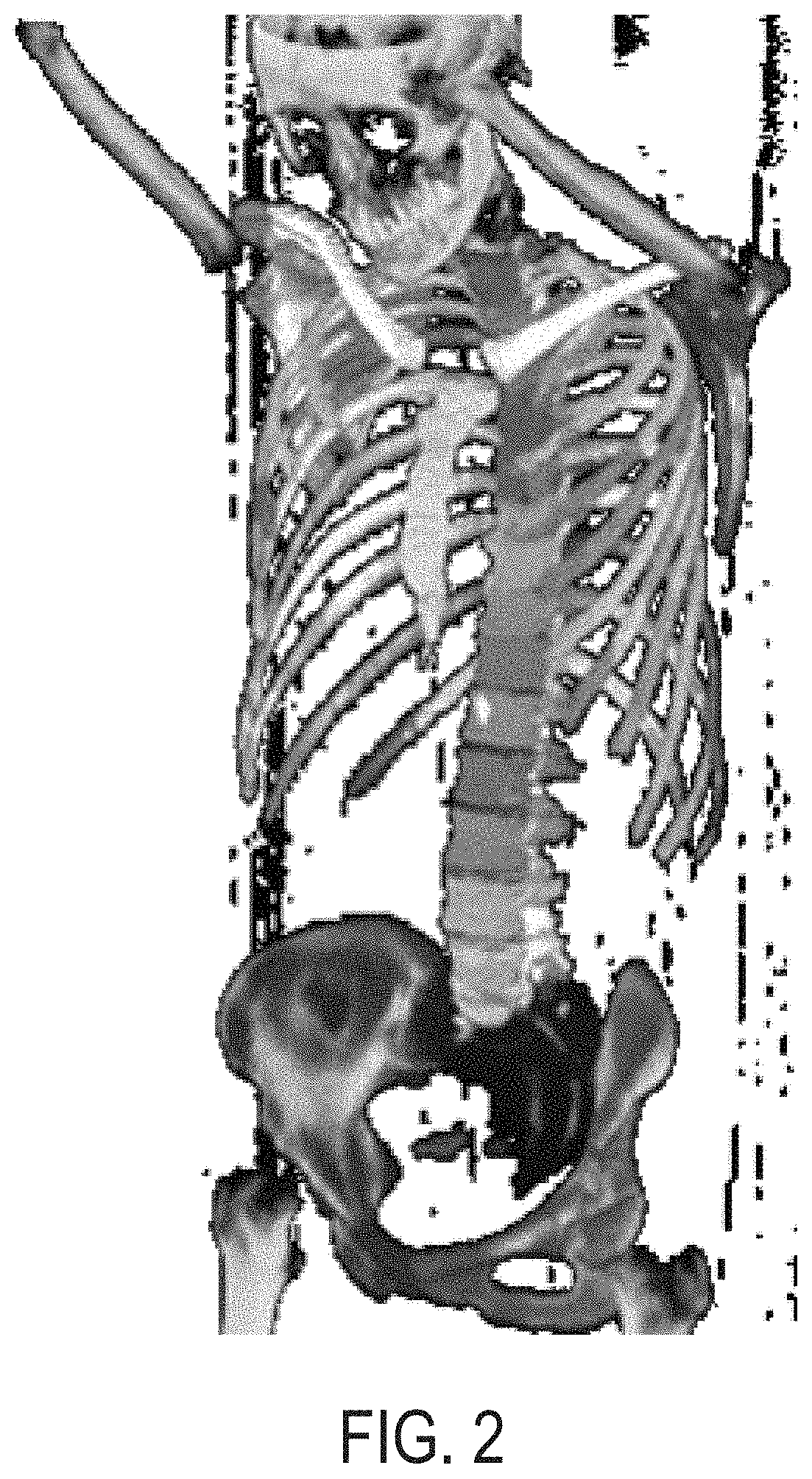
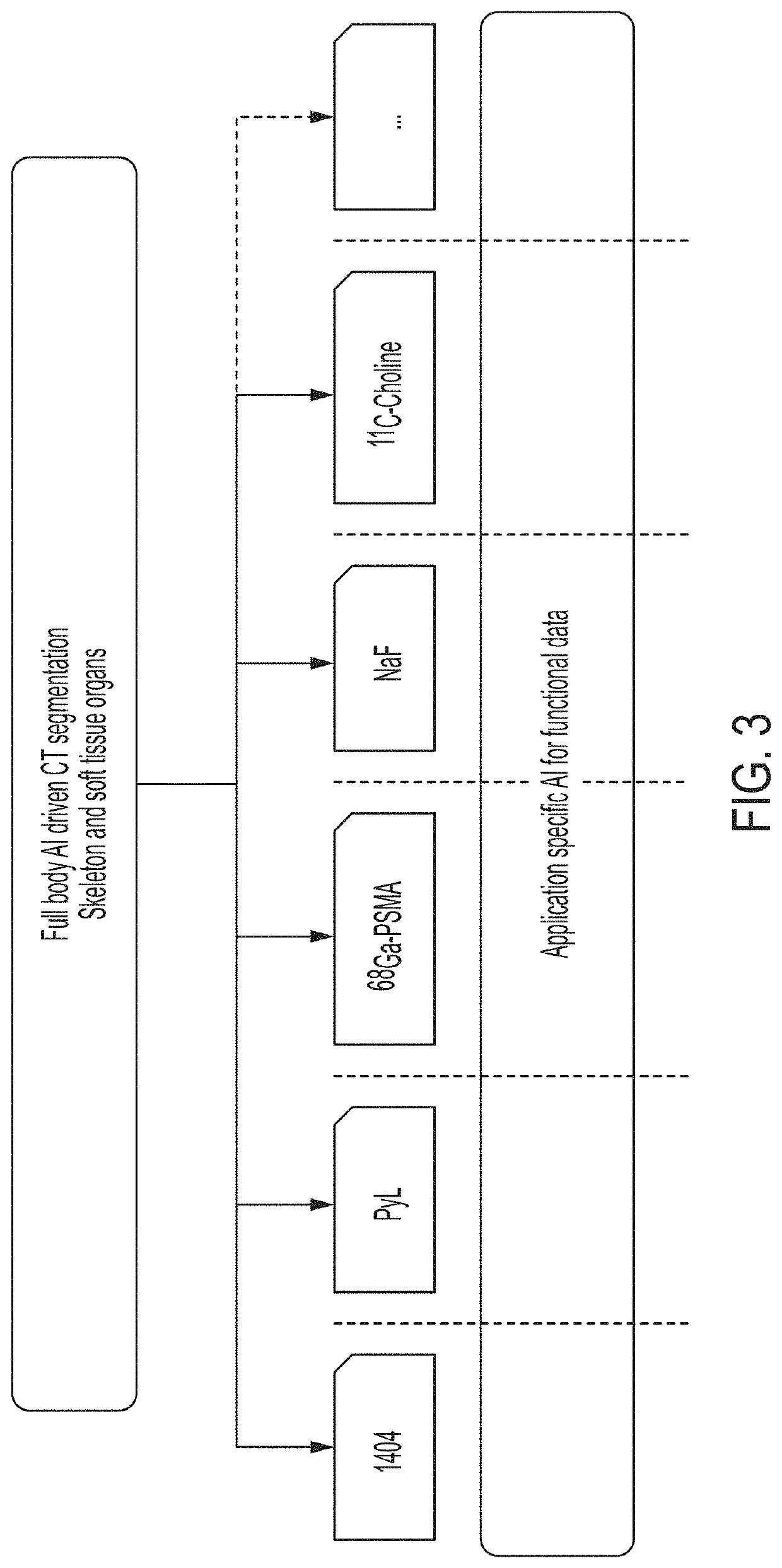
Systems and methods for rapid neural network-based image segmentation and radiopharmaceutical uptake determination
ActiveUS20190209116A1Improve computing efficiencyPrecise processingImage enhancementImage analysisDisease3d image
Presented herein are systems and methods that provide for automated analysis of three-dimensional (3D) medical images of a subject in order to automatically identify specific 3D volumes within the 3D images that correspond to specific organs and / or tissue. In certain embodiments, the accurate identification of one or more such volumes can be used to determine quantitative metrics that measure uptake of radiopharmaceuticals in particular organs and / or tissue regions. These uptake metrics can be used to assess disease state in a subject, determine a prognosis for a subject, and / or determine efficacy of a treatment modality.
Owner:EXINI DIAGNOSTICS +1
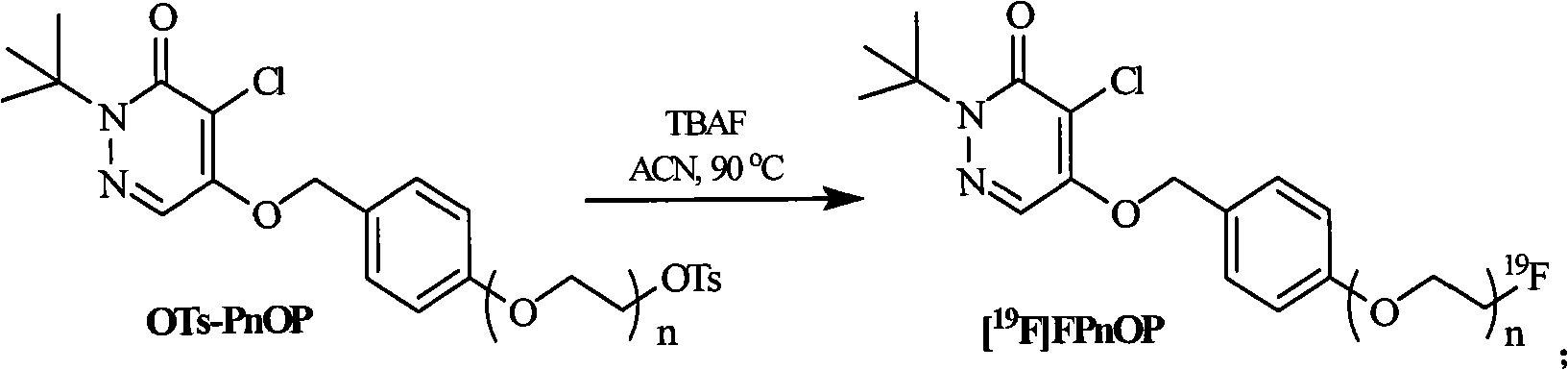
Pyridazinone compound marked by fluorine-18, preparation method and applications
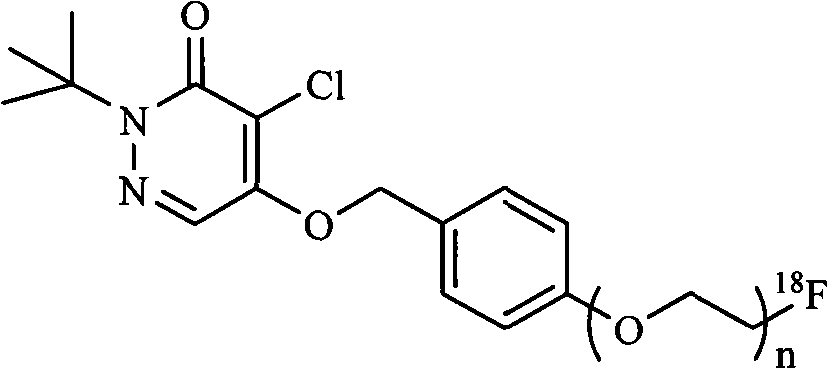
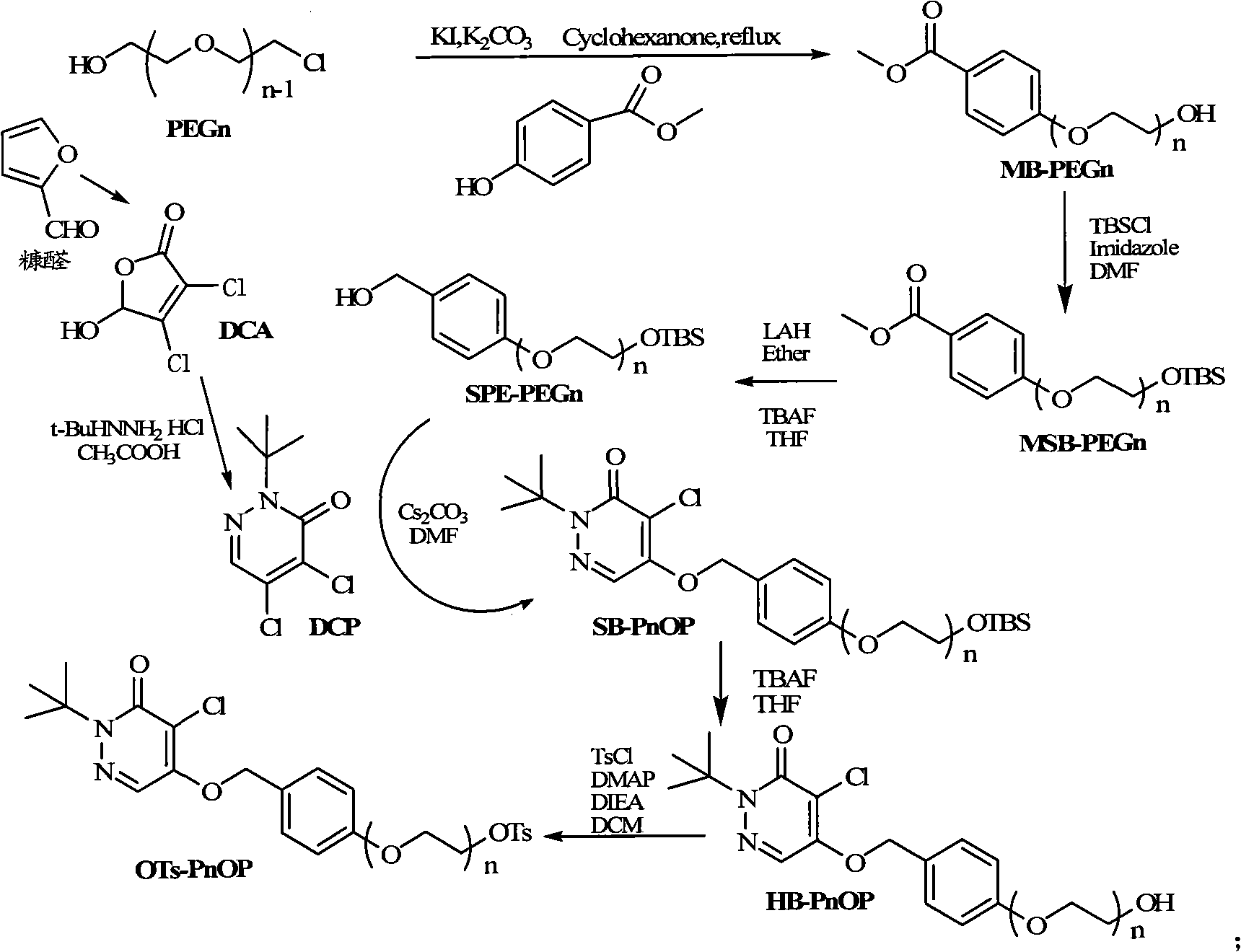
InactiveCN101555232AHigh radiochemical purityGood biological propertiesOrganic chemistryRadioactive preparation carriersBiological propertyRadioactive drug
The invention discloses a pyridazinone compound marked by fluorine-18 with a molecular formula of FFPnOP and a preparation method and applications; in the formula, n is equal to 1, 2 or 3. By technical synthesis to ligand OTs-PnOP, the pyridazinone compound FFPnOP marked by radioactive fluorine-18 and stable reference compound FFPnOP are obtained by synthesis, wherein the stable reference compound is used for confirming the structure of the compound with radioactive marks; the compound has high radiochemical purity, good biological properties, high initial uptake value, simple preparation and low use cost, and is applied in the technical fields of radioactive drug chemistry and clinical nuclear medicine as a novel myocardial perfusion imaging agent marked by fluorine-18.
Owner:BEIJING NORMAL UNIVERSITY +1
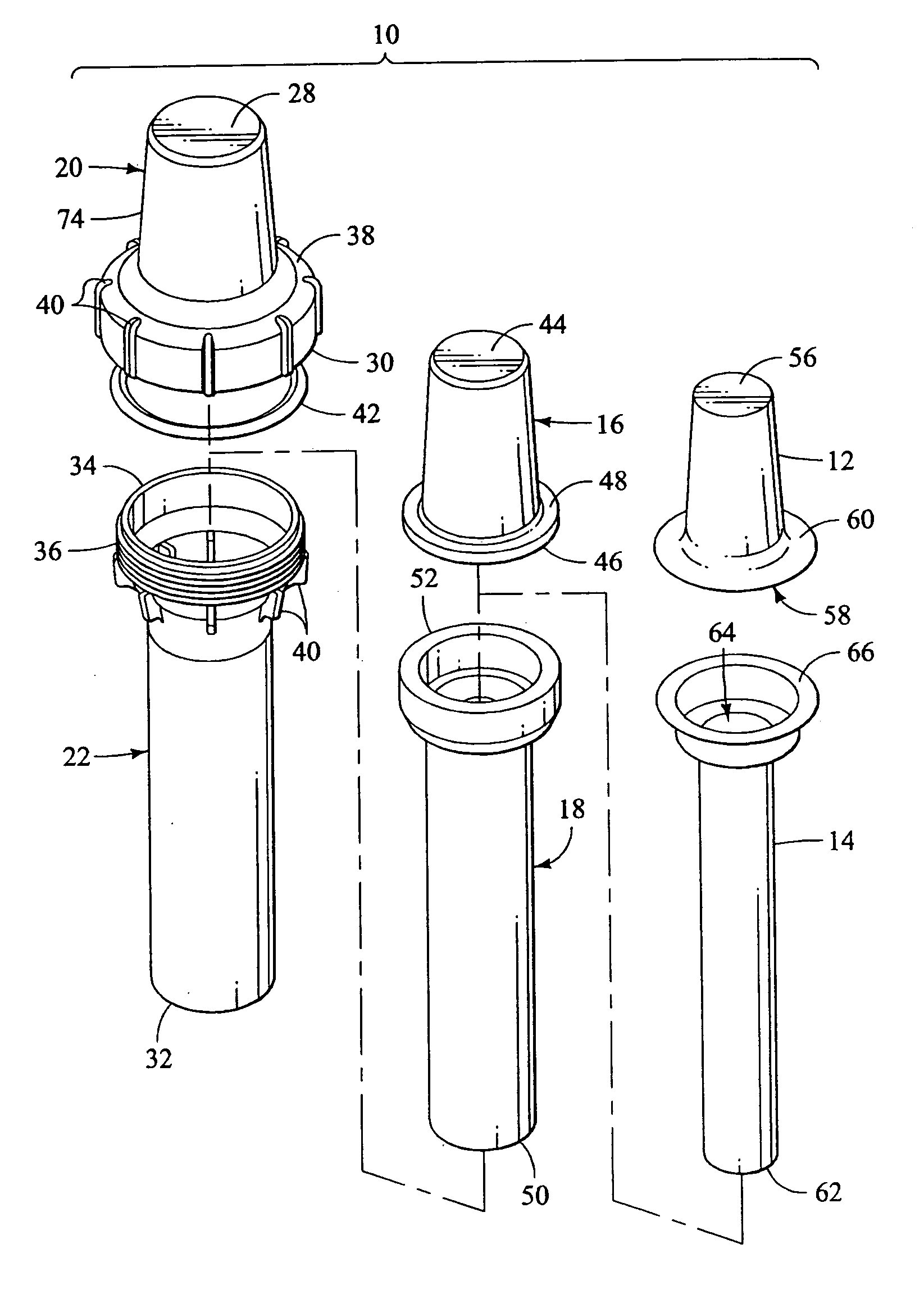
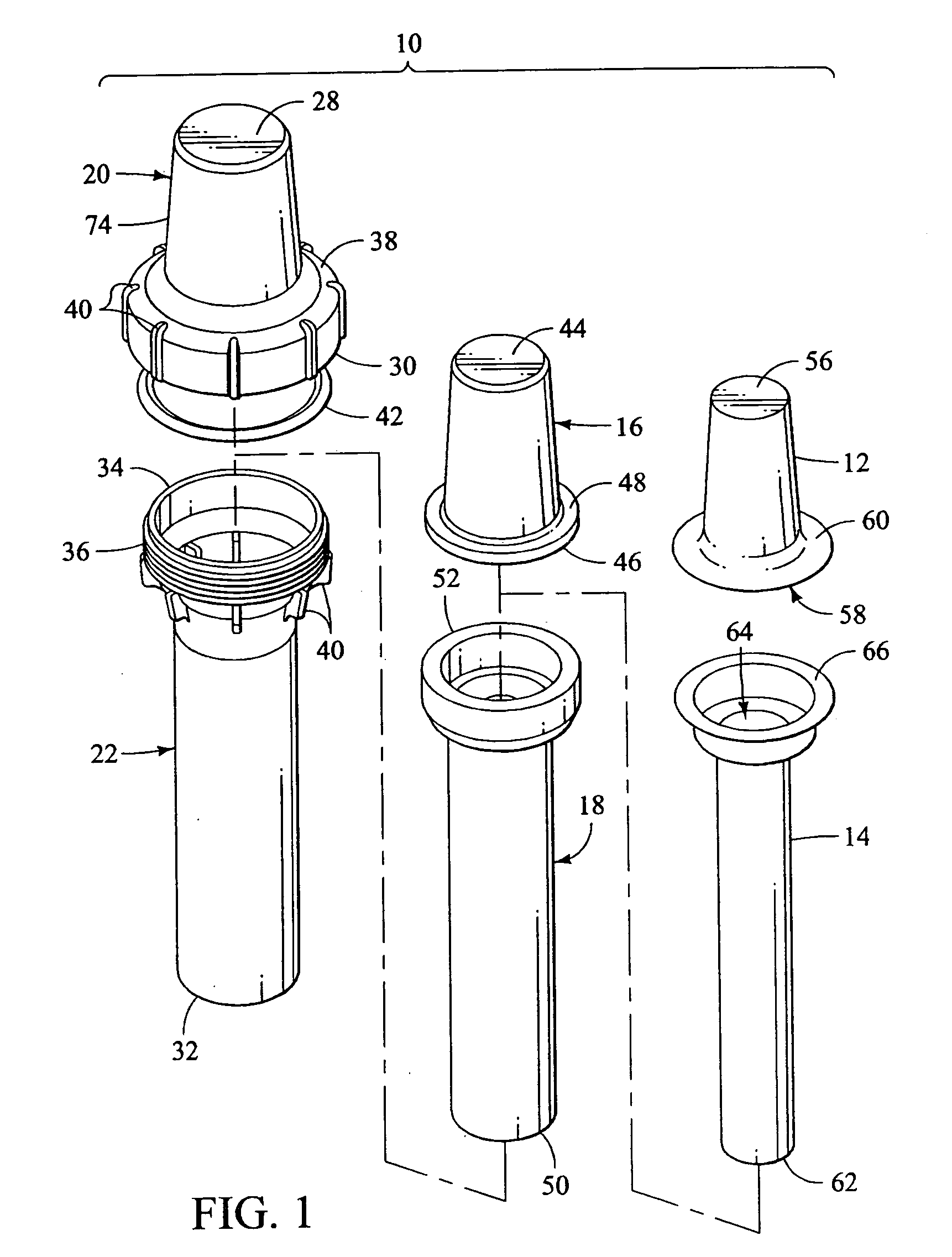
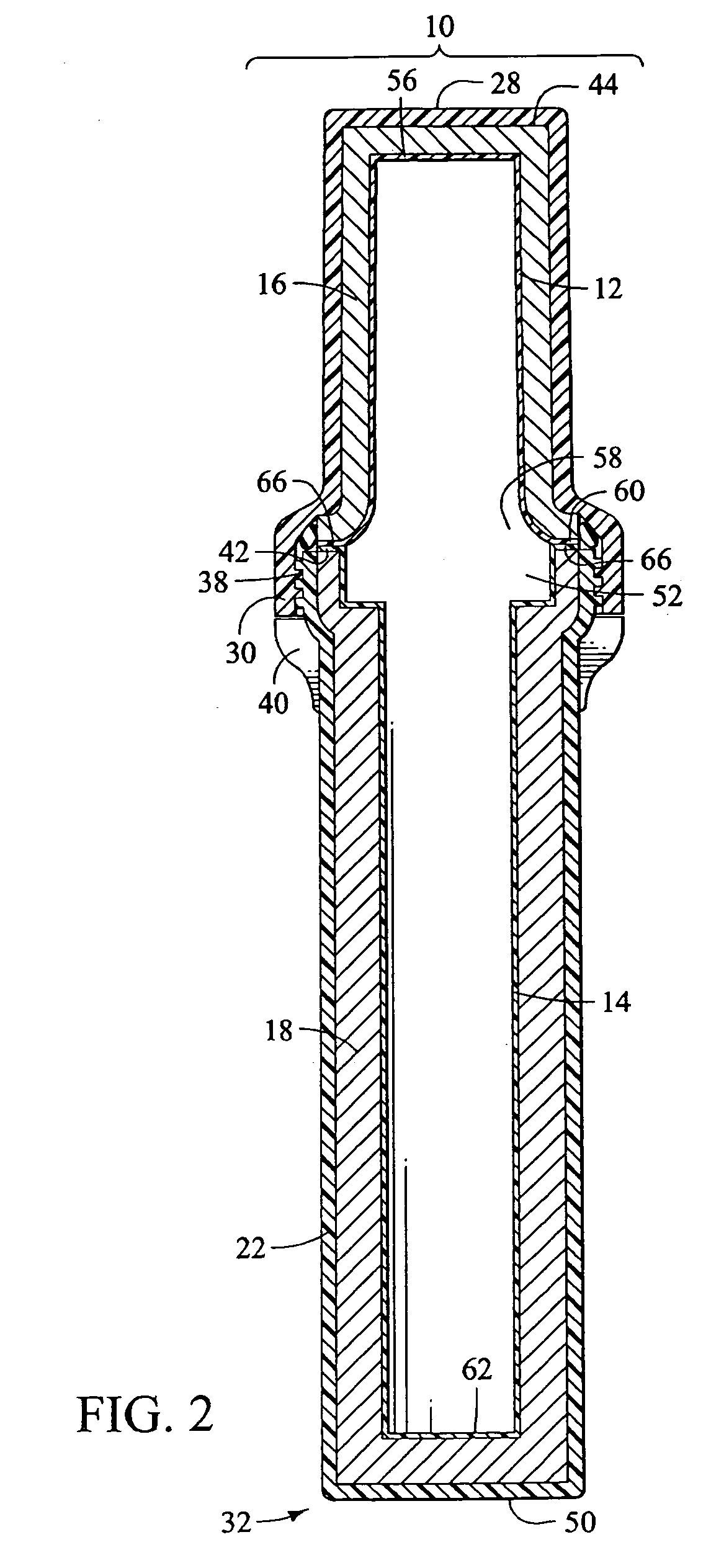
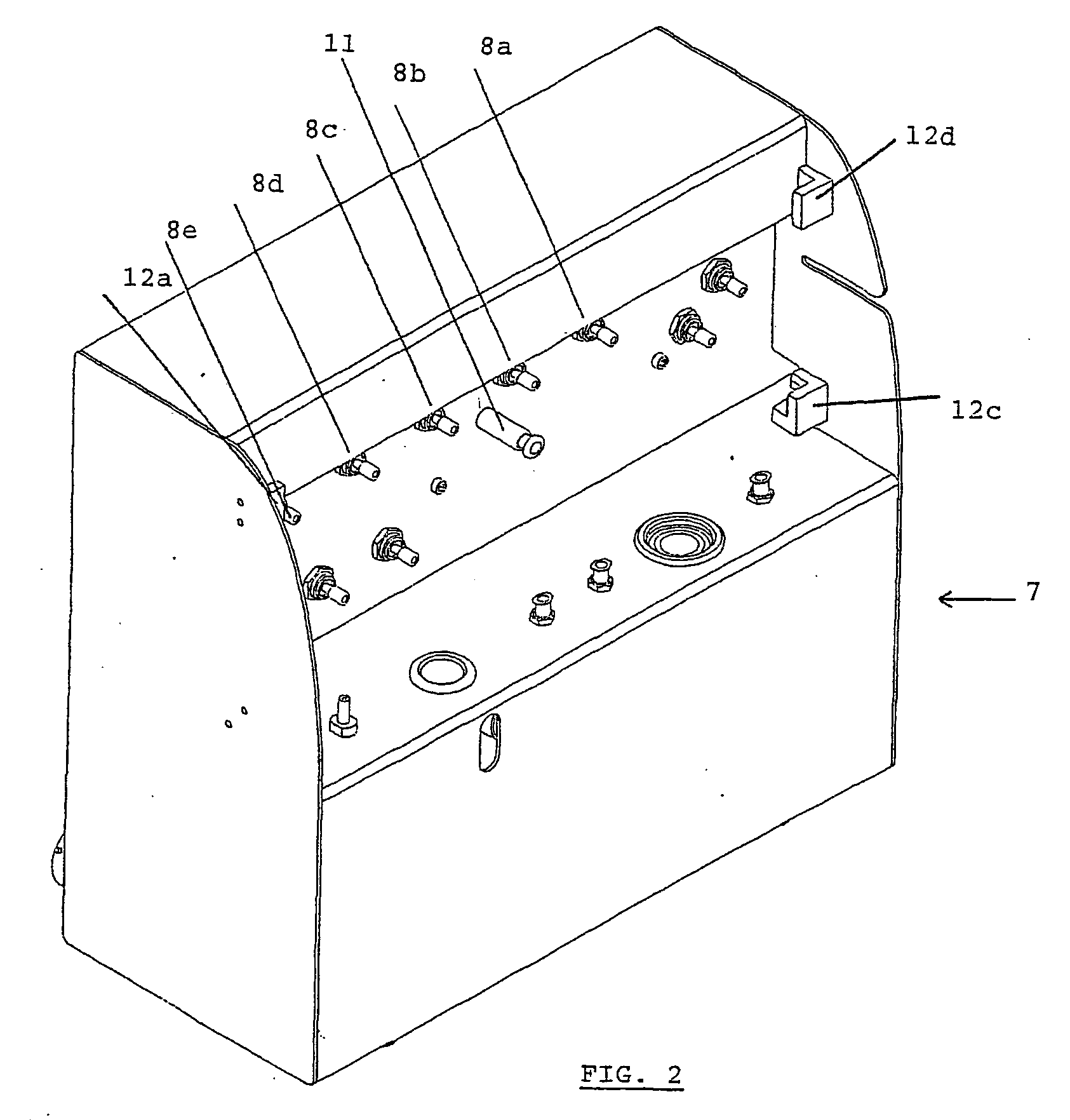
Apparatus and method for transporting radiopharmaceuticals
InactiveUS20050247893A1Quickly and inexpensively cleanedAvoid pollutionDispensing apparatusIntravenous devicesEngineeringRadiation shield
A method and apparatus for transporting radiopharmaceuticals. Typically, the apparatus is a two-part assembly, each part having an exterior shell, a radiation shield and a non-porous lining. Additionally, the assembled apparatus has a sealed internal chamber suitable for carrying a syringe or a sharps container containing a syringe. The internal chamber of the radiopharmaceutical pig is lined with a non-porous lining, typically a durable plastic, that prevents contamination of the radiopharmaceutical doses, the radiation shield, or the environment. Additionally, the non-porous lining can be quickly and easily cleaned and sterilized, avoiding the often difficult, to impossible, task of cleaning and sterilizing the radiation shield of the radiopharmaceutical pig. The non-porous lining is surrounded by a radiation shield that is typically comprised of elemental lead. The radiation shield prevents radiation from the radiopharmaceutical from contaminating the user or environment. The radiation shield is surrounded by an exterior shell that absorbs impact and prevents the radiopharmaceutical pig from breaking. Additionally, the exterior shell prevents environmental exposure to the potentially hazardous material of the radiation shield. Generally, a method of transporting a radiopharmaceutical by filling the container with a radiopharmaceutical, inserting the container into the internal chamber of the radiopharmaceutical pig having a non-porous lining, and assembling the radiopharmaceutical pig so the that the container is in the internal chamber and is encapsulated by the radiation shield, is also provided.
Owner:CARDINAL HEALTH INC
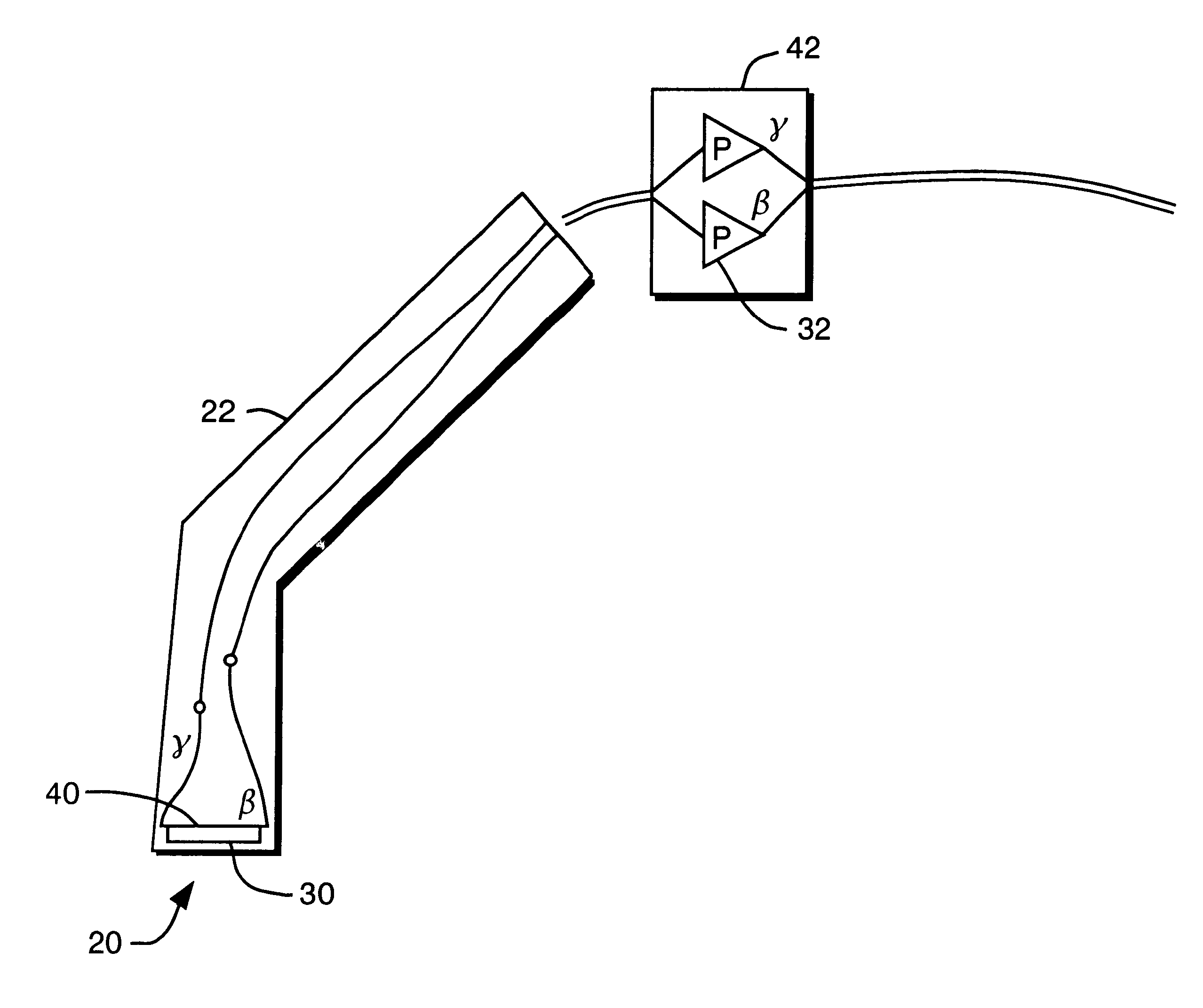
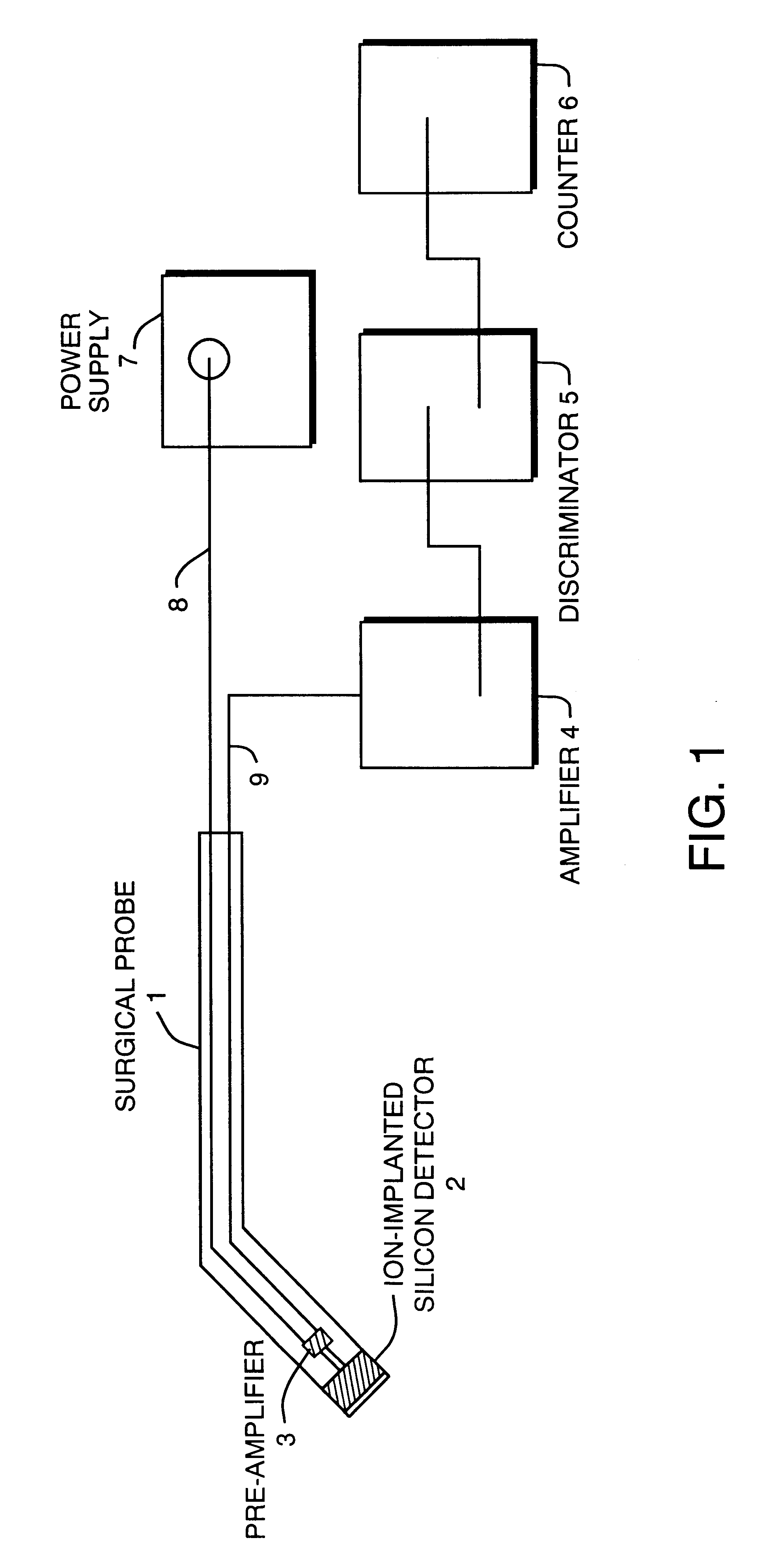
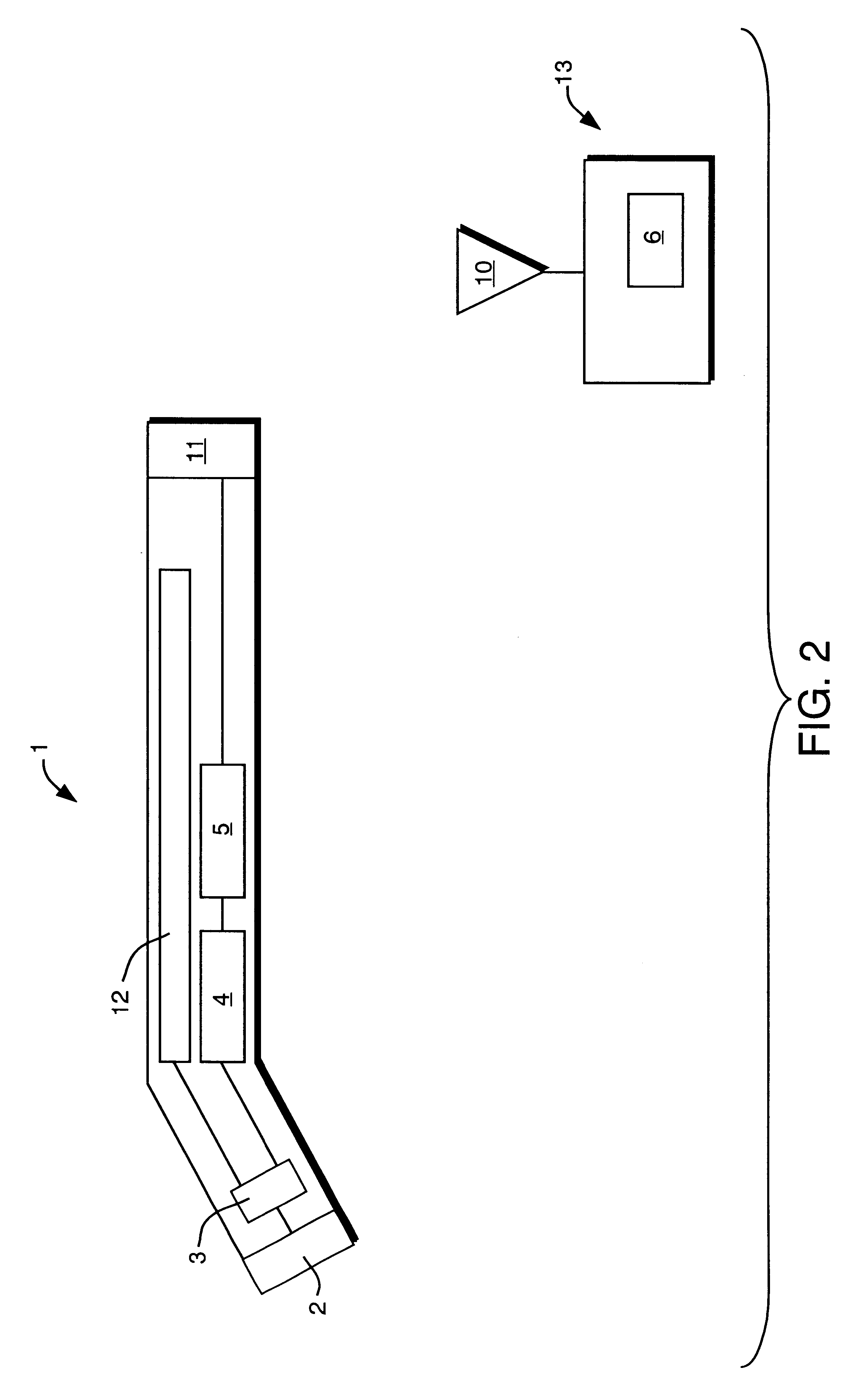
Solid state beta-sensitive surgical probe
InactiveUS6456869B1Sensitive highMinimizing requisite sizeSolid-state devicesMaterial analysis by optical meansInfraredAudio power amplifier
An intraoperative probe system for preferentially detecting beta radiation over gamma radiation emitted from a radiopharmaceutical is described. In one embodiment, the probe system of the present invention is a probe having an ion-implanted silicon charged-particle detector for generating an electrical signal in response to received beta particles. In such an embodiment, a preamplifier may be located in close proximity to the detector filters and amplifies the electrical signal. Furthermore, a wire may be used to couple the probe to a processing unit for amplifying and filtering the electrical signal, and a counter may be utilized to analyze the resulting electrical signal to determine the number of beta particles being received by the detector. Alternatively, the wire can be replaced with an infrared or radio transmitter and receiver for wireless operation of the probe.
Owner:RGT UNIV OF MICHIGAN
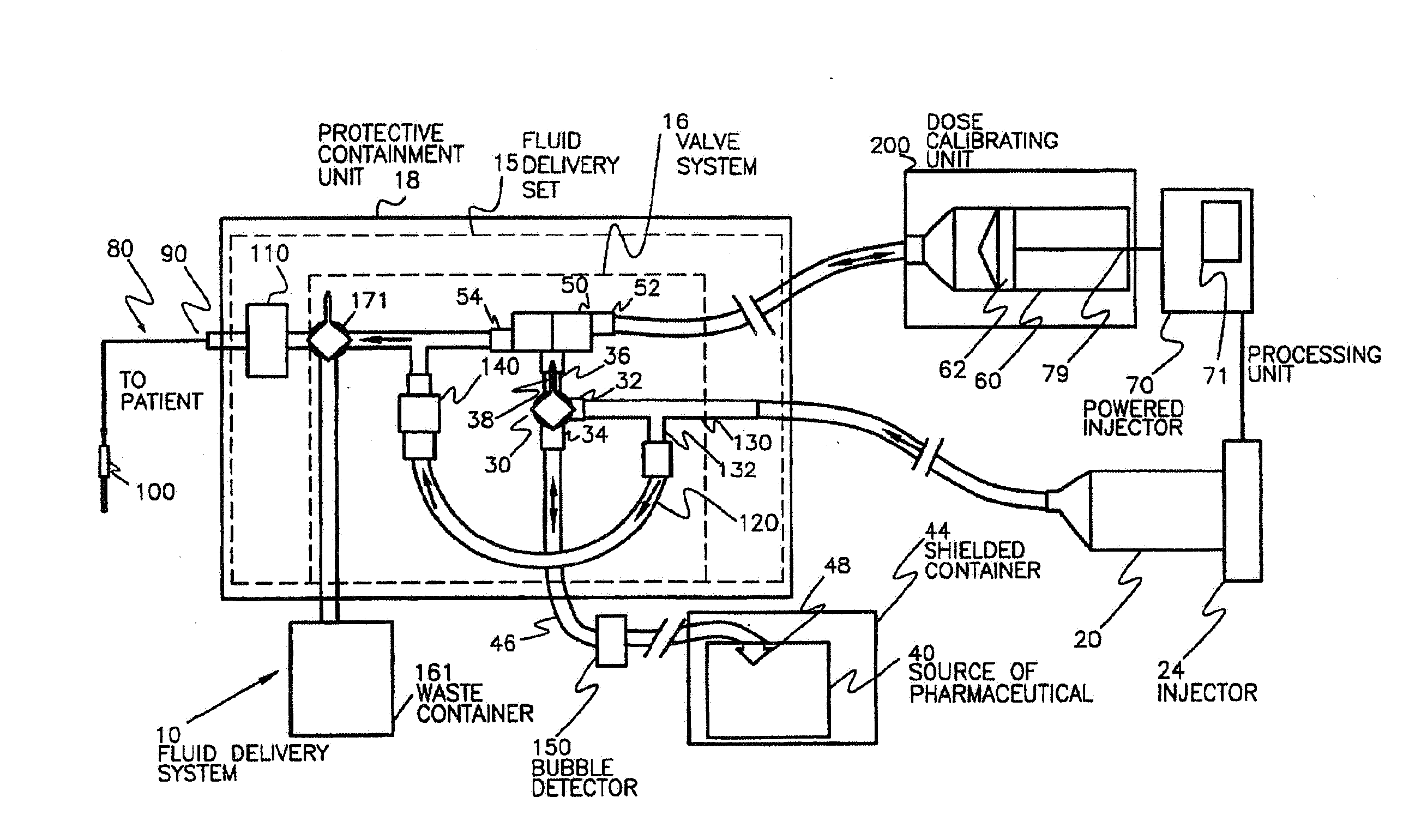
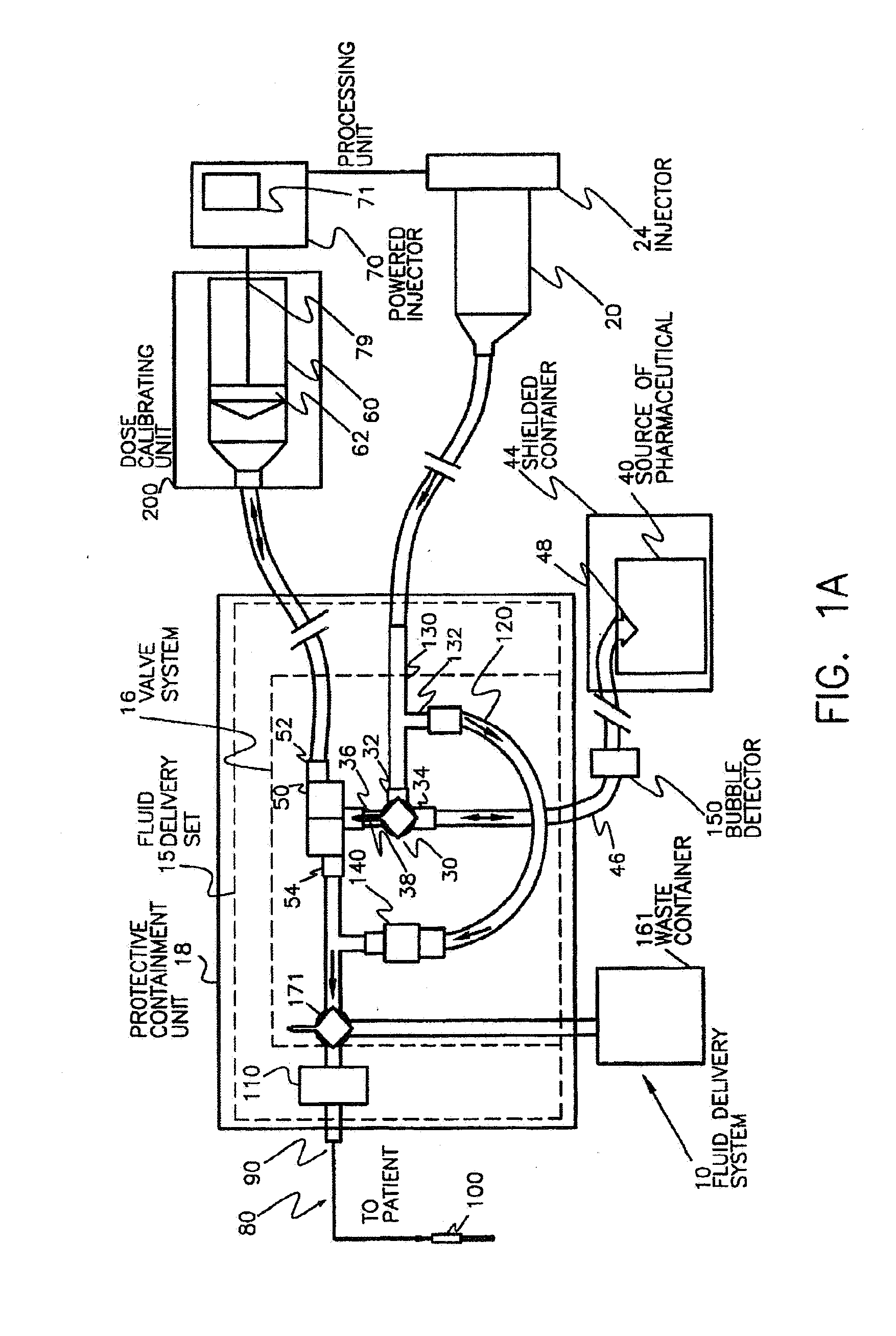
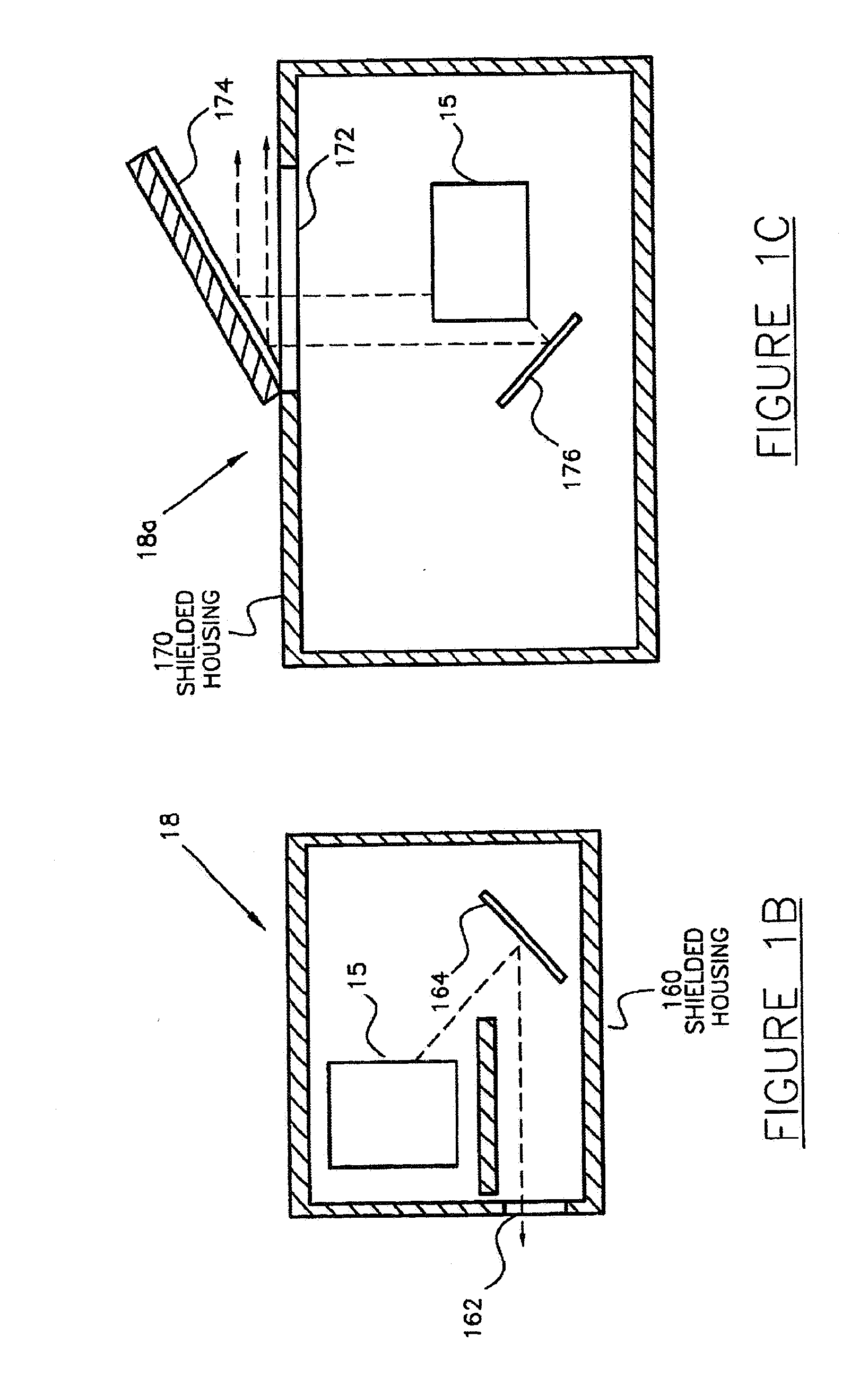
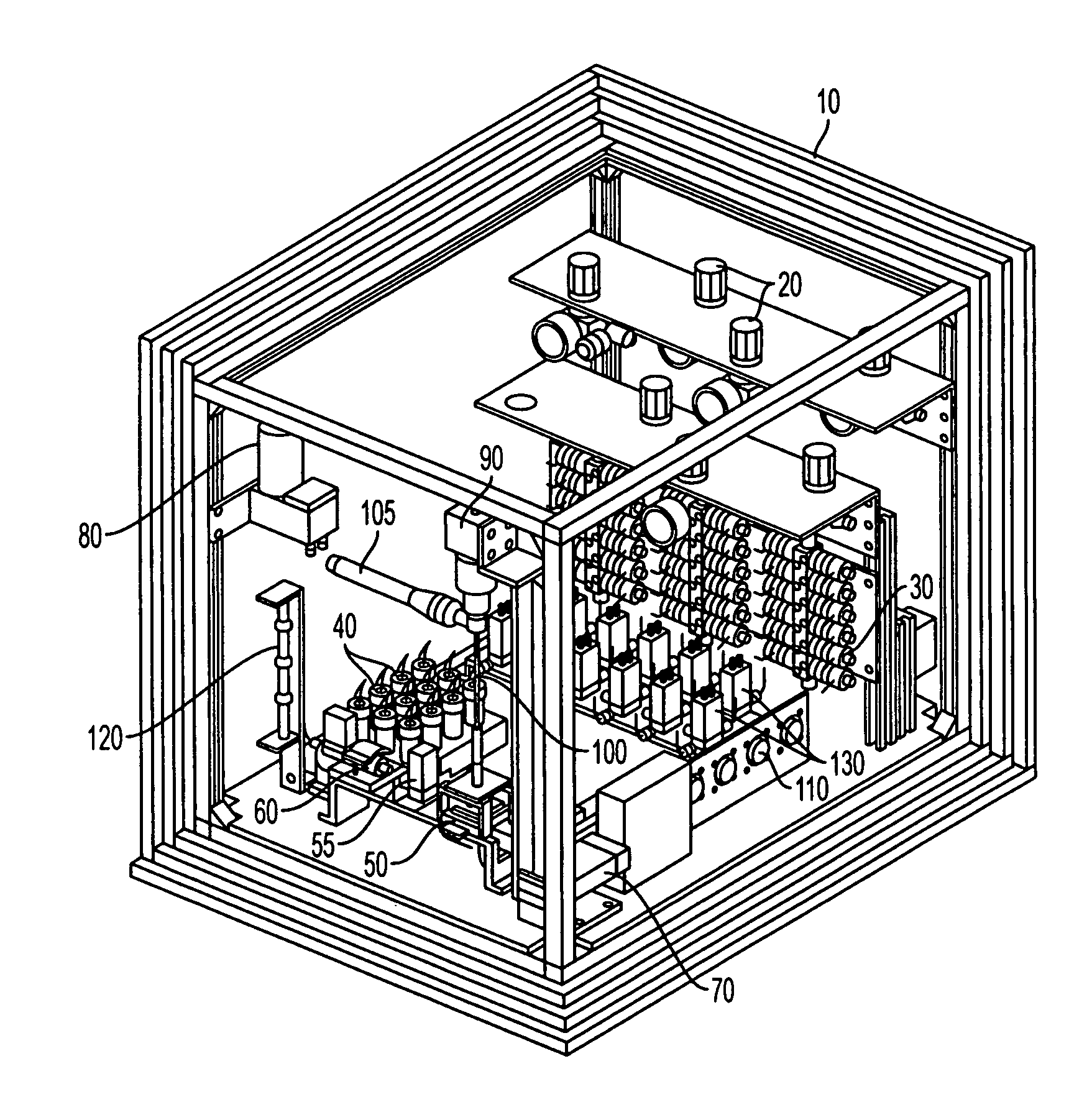
Activity delivery progress monitor
ActiveUS20130079581A1Mechanical/radiation/invasive therapiesIsotope delivery systemsRadiopharmaceutical ActivityDelivery system
A system and method for monitoring progress of a radiopharmaceutical injection procedure includes: measuring and monitoring radiopharmaceutical activity of a radiopharmaceutical remaining in at least a portion of a disposable administration set used with a radiopharmaceutical fluid delivery system; and displaying the radiopharmaceutical activity remaining in at least the portion of the disposable administration set to an operator.
Owner:BAYER HEALTHCARE LLC
Stabilized and Lyophilized Radiopharmaceutical Agents For Destroying Tumors
InactiveUS20070248533A1Easy to refactorReduces predictabilityPowder deliveryNervous disorderAbnormal tissue growthDiagnostic radiopharmaceuticals
A novel method is set out of preparation of radioactive diagnostic radiopharmaceutical in a stable, shippable, lyophilized form by an apparatus designed to rapidly flash freeze and dehydrate a radiopharmaceutical composition to minimize auto radiolysis. The method proposes rapid cooling and removal of ambient vapor, and then ultra cold removal when the potential of explosive liquid oxygen is eliminated. The radioactive diagnostic radiopharmaceutical requires no further cold or refrigerated storage, including with respect to shipping, subsequent to stabilization. The preferred composition can be reconstituted “on site” by the addition of a suitable diluent to bring the radiopharmaceutical complex into solution at a desired concentration.
Owner:KUPERUS JOHN H +2
Device for synthesis of radiopharmaceutical products
ActiveUS20040028573A1Short half-lifeEasy to useRadiation measurementPretreated surfacesComputer moduleProduct base
Owner:ION BEAM APPL
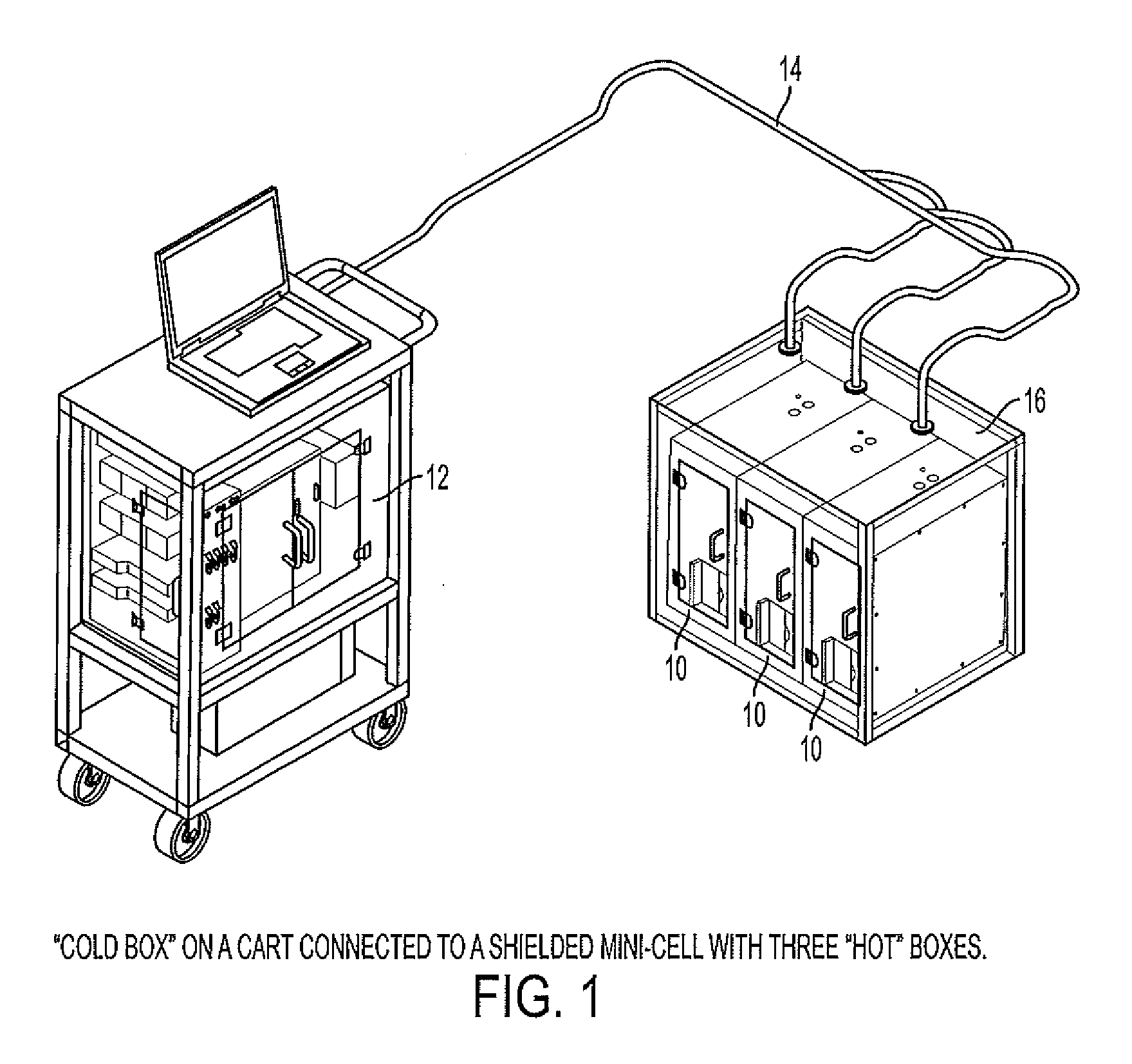
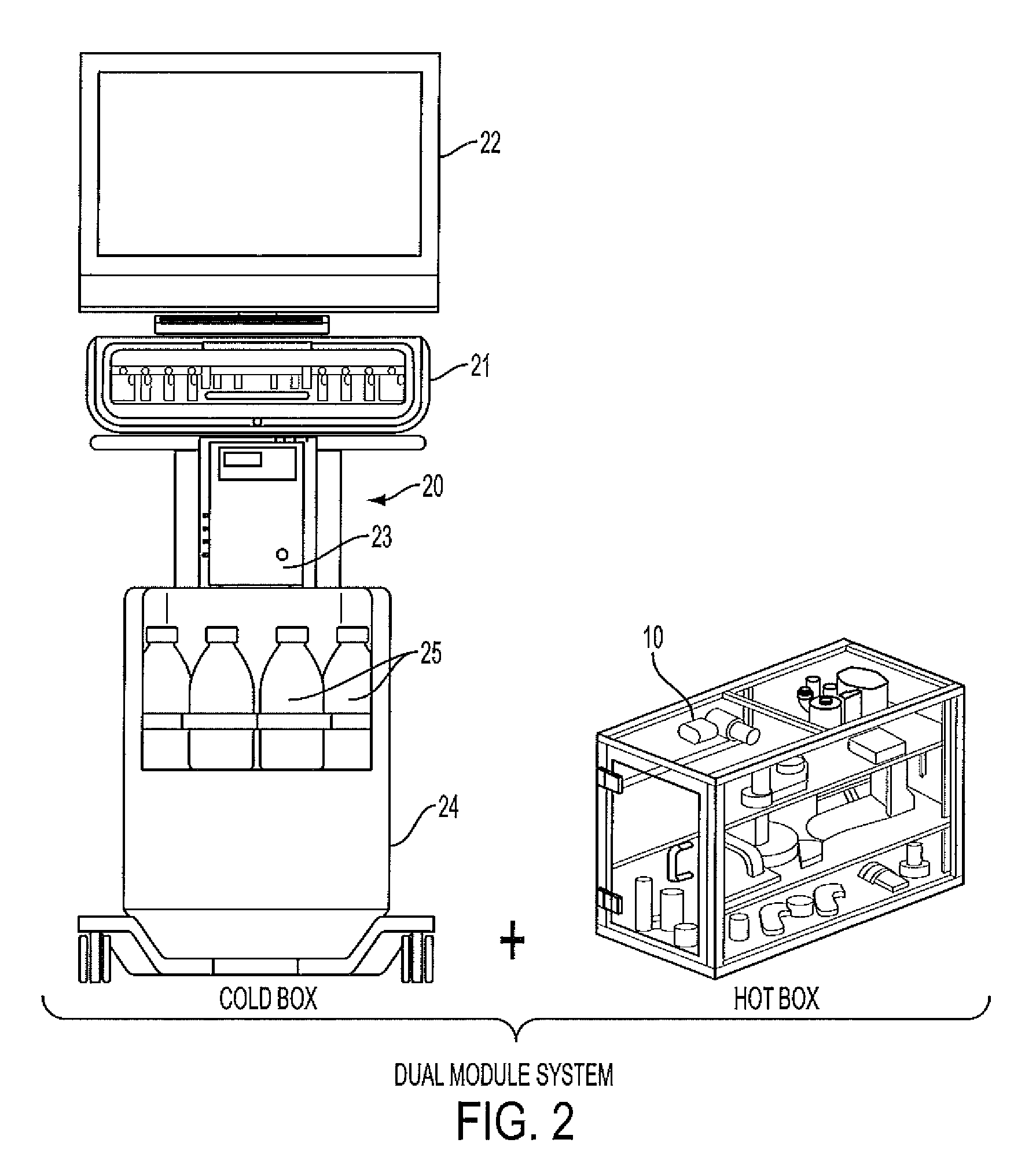
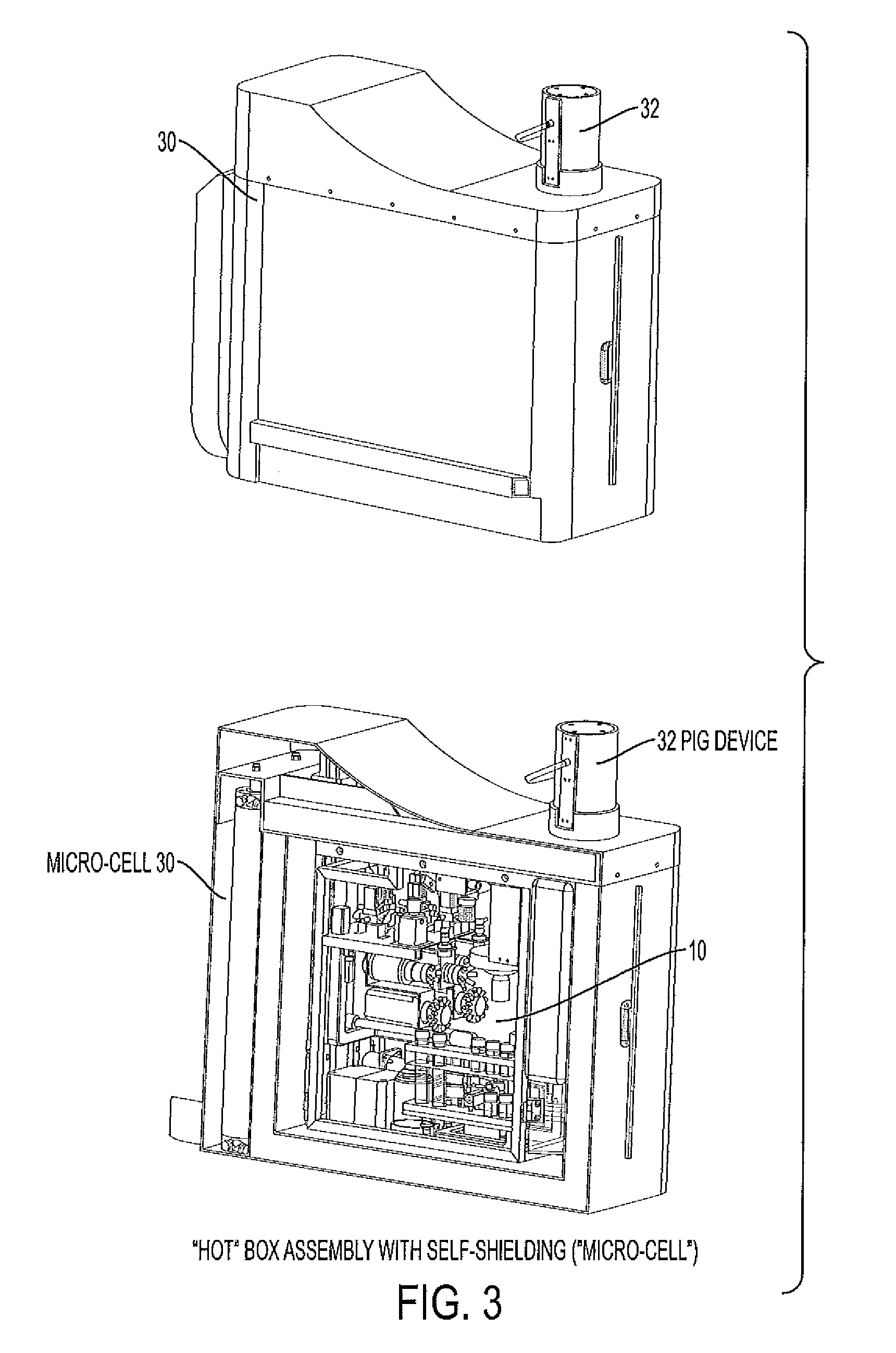
Modular system for radiosynthesis with multi-run capabilities and reduced risk of radiation exposure
ActiveUS20110008215A1Fast and efficientFast and efficient and compact and safe to operatorGaseous chemical processesLiquid-gas reaction of thin-film typeModularityChemical process
Macro- and microfluidic devices and related technologies, and chemical processes using such devices. More specifically, the devices may be used for a fully automated synthesis of radioactive compounds for imaging, such as by positron emission tomography (PET), in an efficient, compact and safe to the operator manner. In particular, embodiments of the present invention relate to an automated, multi-run, microfluidic instrument for the multi-step synthesis of radiopharmaceuticals, such as PET probes, comprising a remote shielded mini-cell containing radiation-handing components.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Novel irreversible inhibitors of epidermal growth factor receptor tyrosine kinase and uses thereof for therapy and diagnosis
InactiveUS20060025430A1Improve bioavailabilityImprove biostabilityBiocideOrganic active ingredientsRadiation therapyPositron emission tomography
Novel epidermal growth factor receptor tyrosine kinase (EGFR-TK) irreversible inhibitors, pharmaceutical compositions including same and their use in the treatment of EGFR-TK related diseases or disorders are disclosed. Novel radiolabeled EGFR-TK irreversible inhibitors as their use as biomarkers for medicinal radioimaging such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) and as radiopharmaceuticals for radiotherapy are further disclosed.
Owner:T K SIGNAL
Systems and methods for platform agnostic whole body image segmentation
ActiveUS20200245960A1Consistent and efficient and accurate detectionDetermining prognosisMedical simulationImage enhancement3d imageRadioactive drug
Presented herein are systems and methods that provide for automated analysis of three-dimensional (3D) medical images of a subject in order to automatically identify specific 3D volumes within the 3D images that correspond to specific anatomical regions (e.g., organs and / or tissue). Notably, the image analysis approaches described herein are not limited to a single particular organ or portion of the body. Instead, they are robust and widely applicable, providing for consistent, efficient, and accurate detection of anatomical regions, including soft tissue organs, in the entire body. In certain embodiments, the accurate identification of one or more such volumes is used to automatically determine quantitative metrics that represent uptake of radiopharmaceuticals in particular organs and / or tissue regions. These uptake metrics can be used to assess disease state in a subject, determine a prognosis for a subject, and / or determine efficacy of a treatment modality.
Owner:EXINI DIAGNOSTICS
Systems and methods for rapid neural network-based image segmentation and radiopharmaceutical uptake determination
PendingUS20200342600A1Improvement in accuracy and consistency and reproducibilityReduce effortImage enhancementReconstruction from projection3d imageRadioactive drug
Presented herein are systems and methods that provide for automated analysis of three-dimensional (3D) medical images of a subject in order to automatically identify specific 3D volumes within the 3D images that correspond to specific organs and / or tissue. In certain embodiments, the accurate identification of one or more such volumes can be used to determine quantitative metrics that measure uptake of radiopharmaceuticals in particular organs and / or tissue regions. These uptake metrics can be used to assess disease state in a subject, determine a prognosis for a subject, and / or determine efficacy of a treatment modality.
Owner:PROGENICS PHARMA INC +1
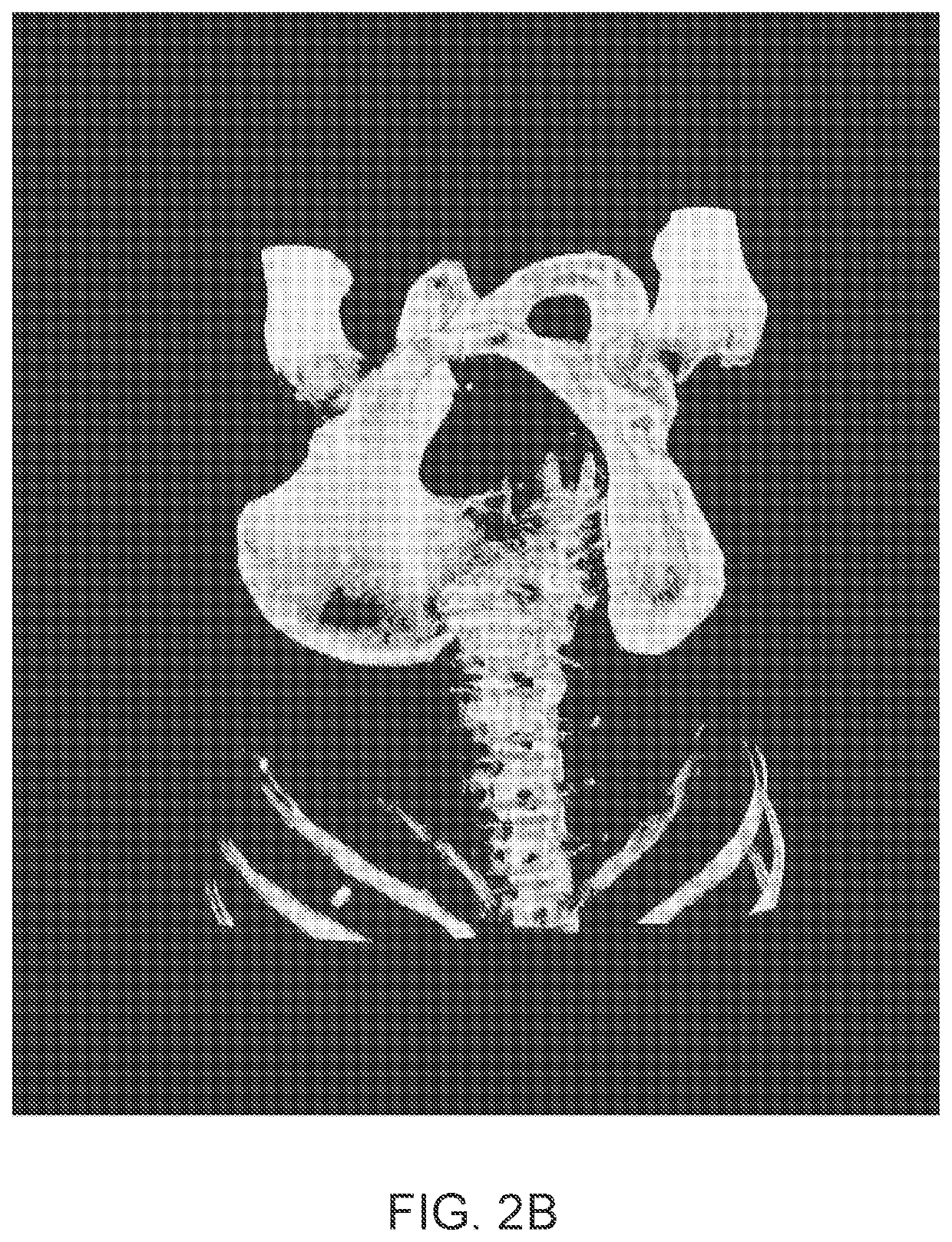
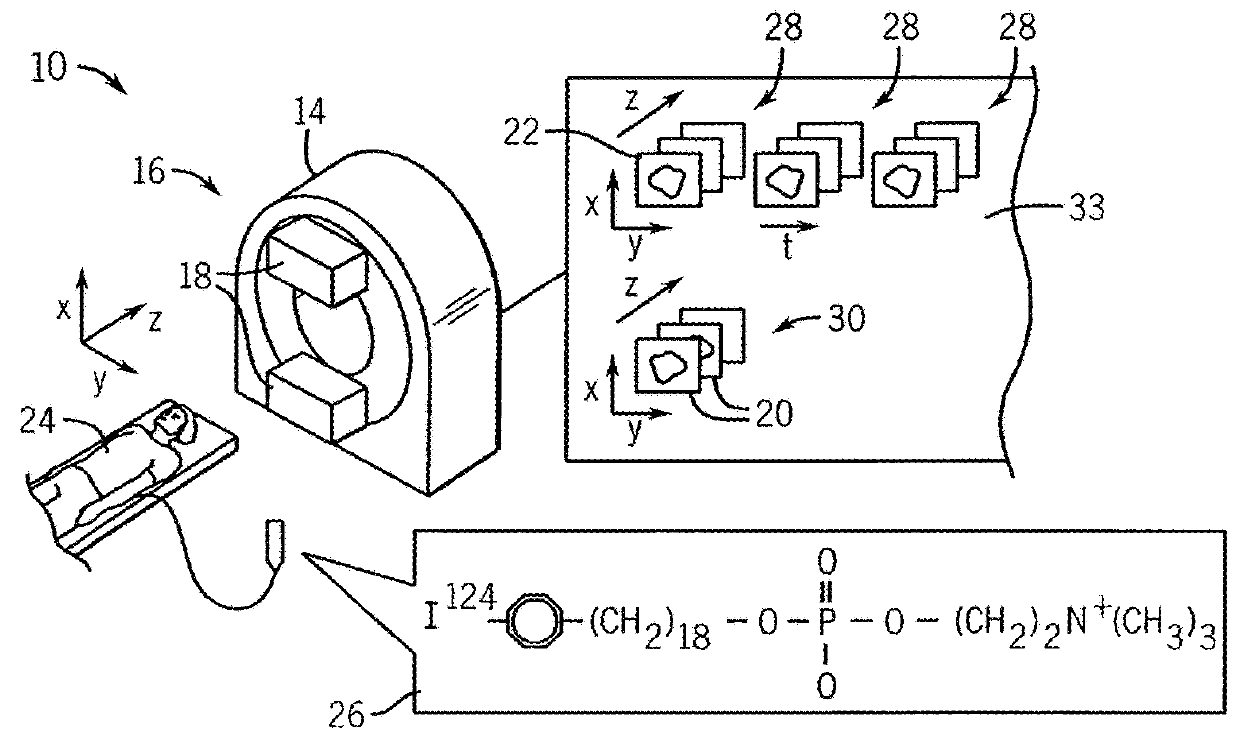
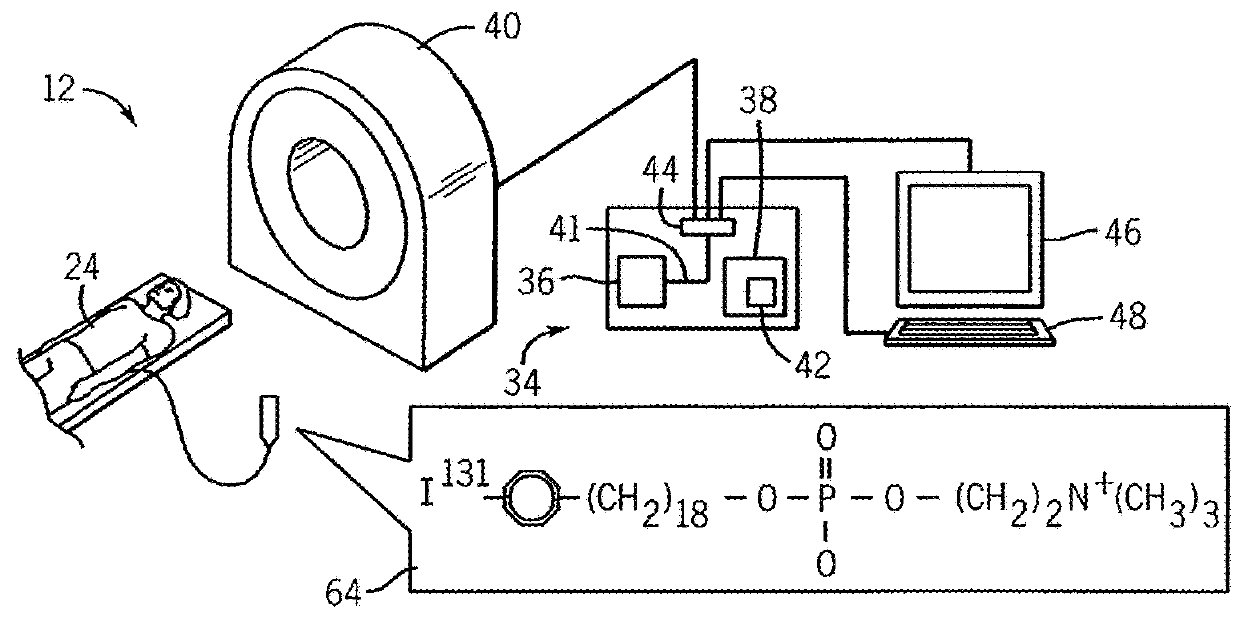
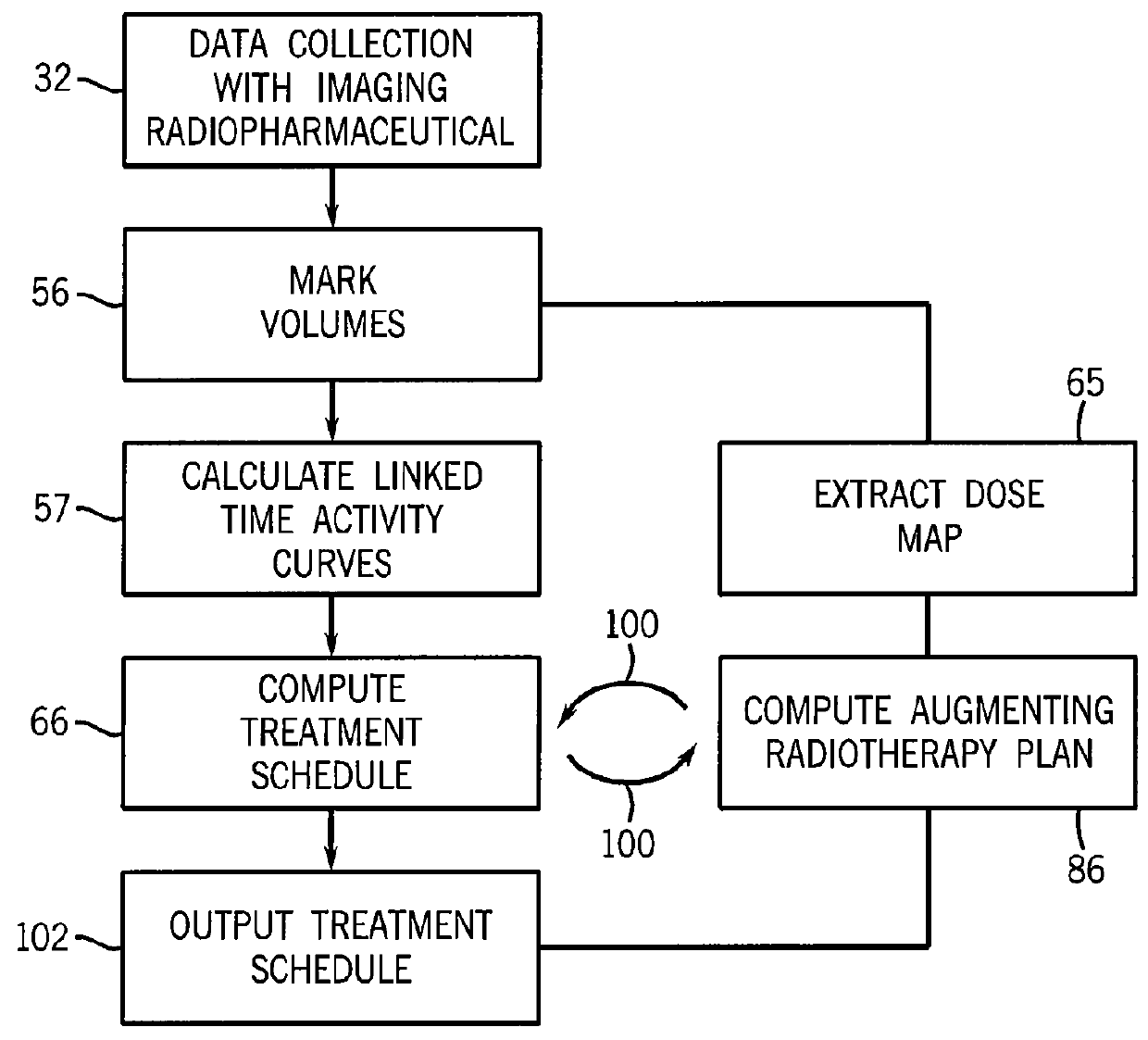
Treatment planning system for radiopharmaceuticals
ActiveUS10007961B2Solve the lack of precisionAccurate time differenceData processing applicationsMechanical/radiation/invasive therapiesTreatment ScheduleVolume of interest
A treatment schedule for radiopharmaceuticals is developed by collecting a volumetric history of tissue uptake in identified volumes of interest using emitted-radiation scans and relating this data to a treatment-radiopharmaceutical to develop a quantitatively accurate radiation treatment schedule of delivery amounts and delivery times of the treatment-radiopharmaceutical. This data may also be used to model biological effective dose and to prepare augmenting external radiation beam treatment schedules.
Owner:WISCONSIN ALUMNI RES FOUND
Radiolabeled irreversible inhibitors of epidermal growth factor receptor tyrosine kinase and their use in radioimaging and radiotherapy
InactiveUS20040265228A1Organic active ingredientsRadiation applicationsSingle photon emission computerized tomographyComputed tomography
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
Radiotracer compositions
InactiveUS20130209358A1In-vivo radioactive preparationsRadiation therapyRadioactive tracerMethod of images
Owner:GE HEALTHCARE LTD
Gastrin receptor-avid peptide conjugates
InactiveUS20060067886A1Peptide/protein ingredientsRadioactive preparation carriersDiseaseGastrin-releasing peptide receptor
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:HOFFMAN TIMOTHY J +4
RGD polypeptide radiopharmaceuticals and preparation method thereof
ActiveCN101428148AIncrease intakeHigh binding affinityIn-vivo radioactive preparationsAntineoplastic agentsDimerCyclic peptide
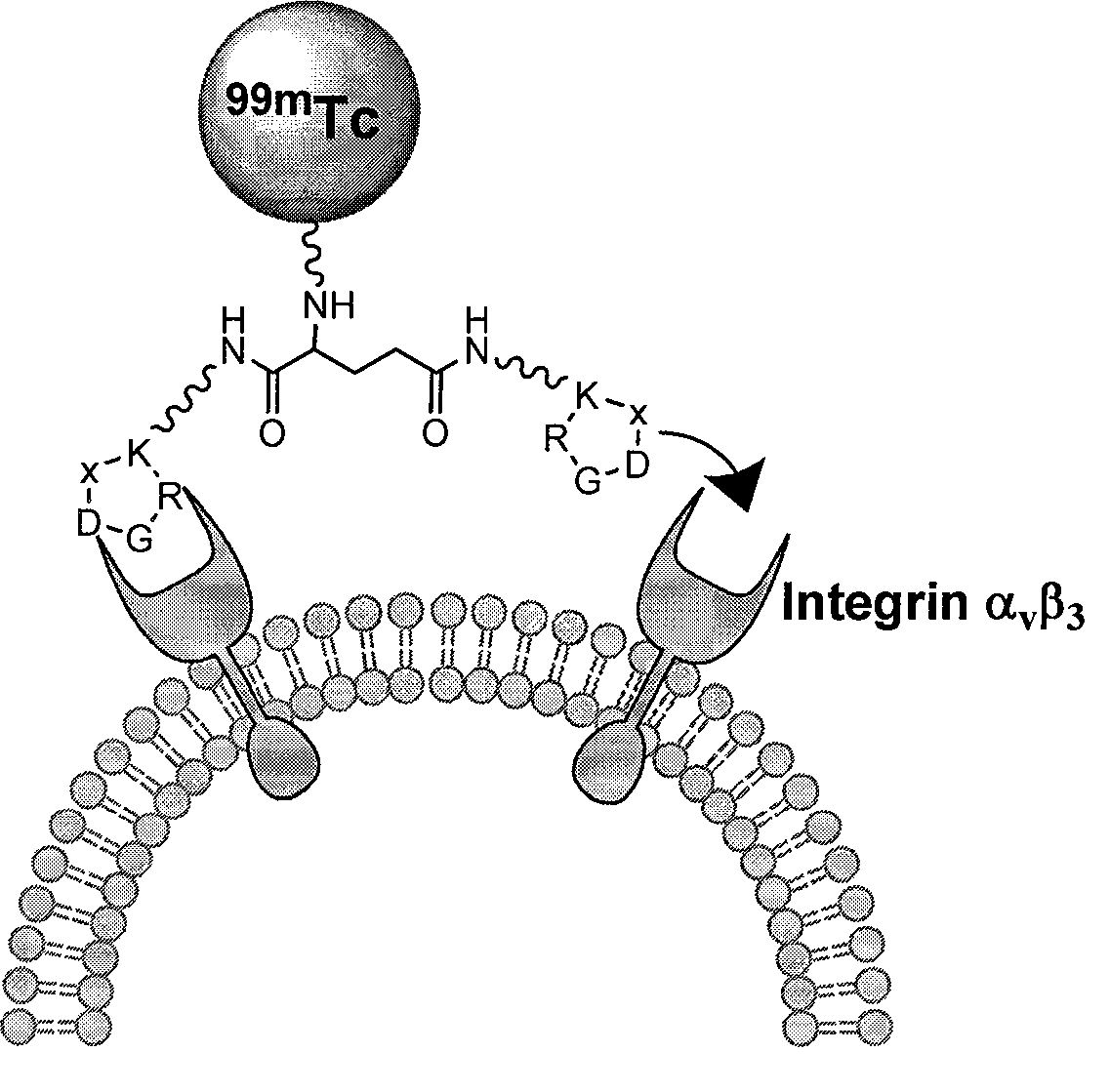
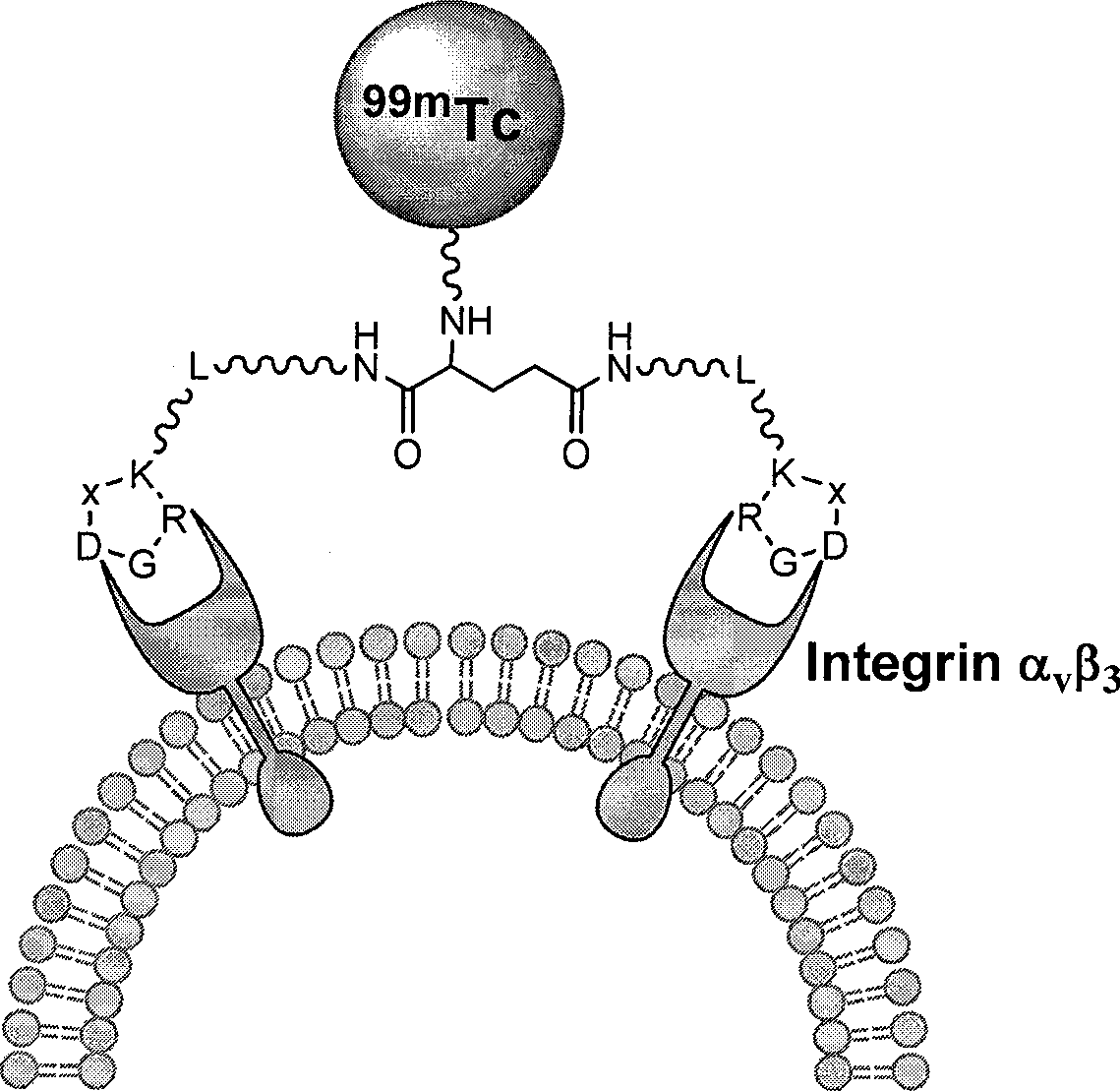
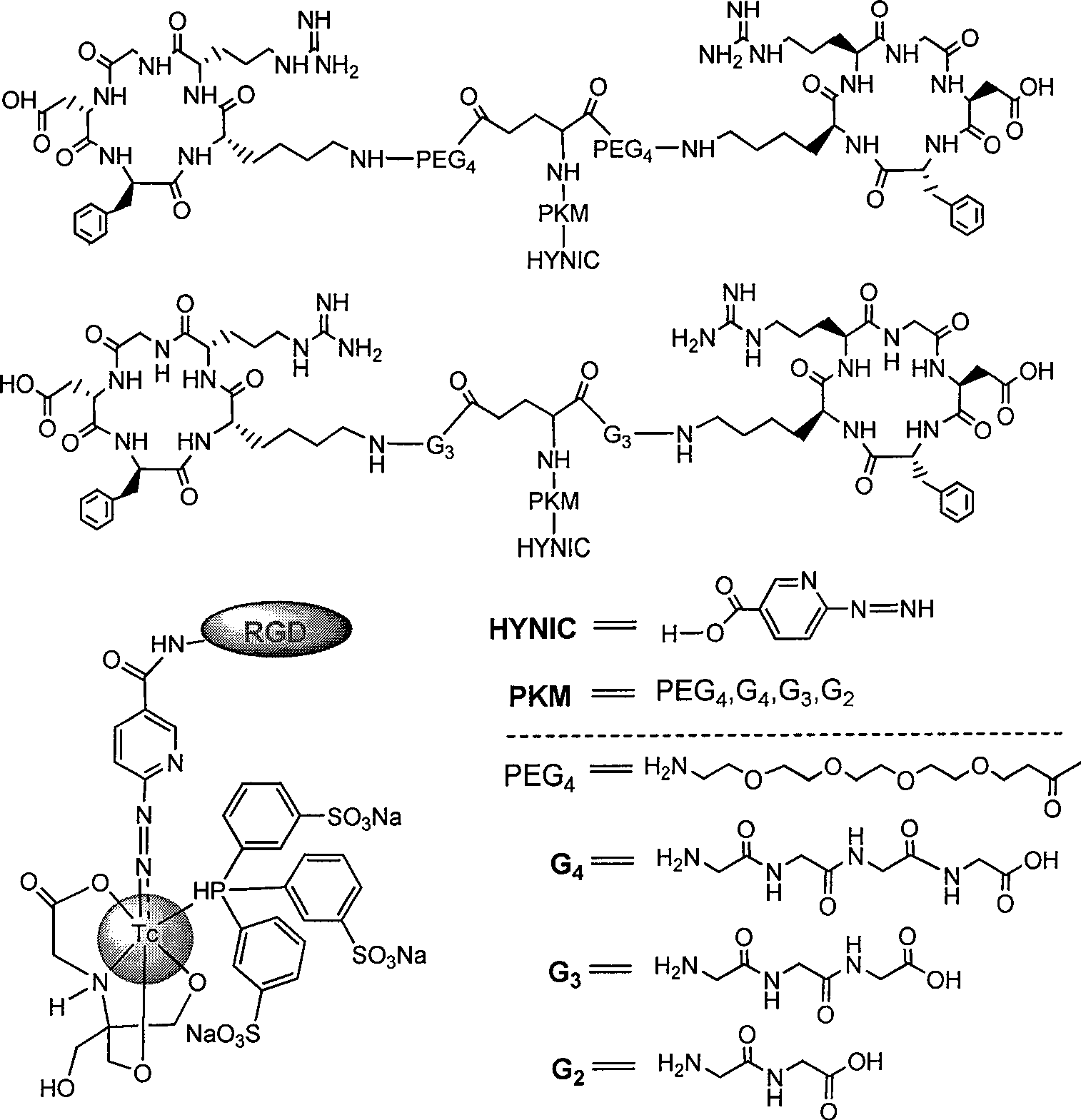
The invention relates to an RGD polypeptide radiopharmaceutical and a preparation method thereof. The RGD polypeptide radiopharmaceutical includes an RGD polypeptide and a radionuclide<99m>Tc, wherein, the RGD polypeptide radiopharmaceutical is an RGD cyclopeptide dimmer, that is, E(L-cRGDxK)2 which is synthesized by dimerizing two RGD polypeptide monomers connected with coupling agent L. The radionuclide<99m>Tc serves to mark the RGD cyclopeptide dimmer through a bifunctional chelating agent HYNIC. A pharmacokinetics modified molecule PKM is further connected between the RGD cyclopeptide dimmer and the bifunctional chelating agent. The RGD polypeptide radiopharmaceutical is <99m>Tc-HYNIC-PKM-E(L-cRGDxK)2. The RGD polypeptide radiopharmaceutical is colorless and transparent liquid injection. The RGD polypeptide radiopharmaceutical provided by the invention has the advantages of further reinforcing the binding affinity and the ingestion of drugs by tumor, and achieving better diagnosis effect.
Owner:广东瑞迪奥科技有限公司
Radiopharmaceuticals for diagnosing Alzheimer's disease
InactiveUS6676926B2Organic active ingredientsIsotope introduction to heterocyclic compoundsDiseaseCCR1
Owner:BAYER SCHERING PHARMA AG
Gastrin receptor-avid peptide conjugates
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a-moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:UNIVERSITY OF MISSOURI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com