Process and device for preparing radiopharmaceutical products for injection
a radiopharmaceutical and process technology, applied in the direction of packaging/bundling articles, containers, packaging goods, etc., can solve the problems of increasing the level of automation, requiring a large amount of automation, and causing an appreciable cumulative exposur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
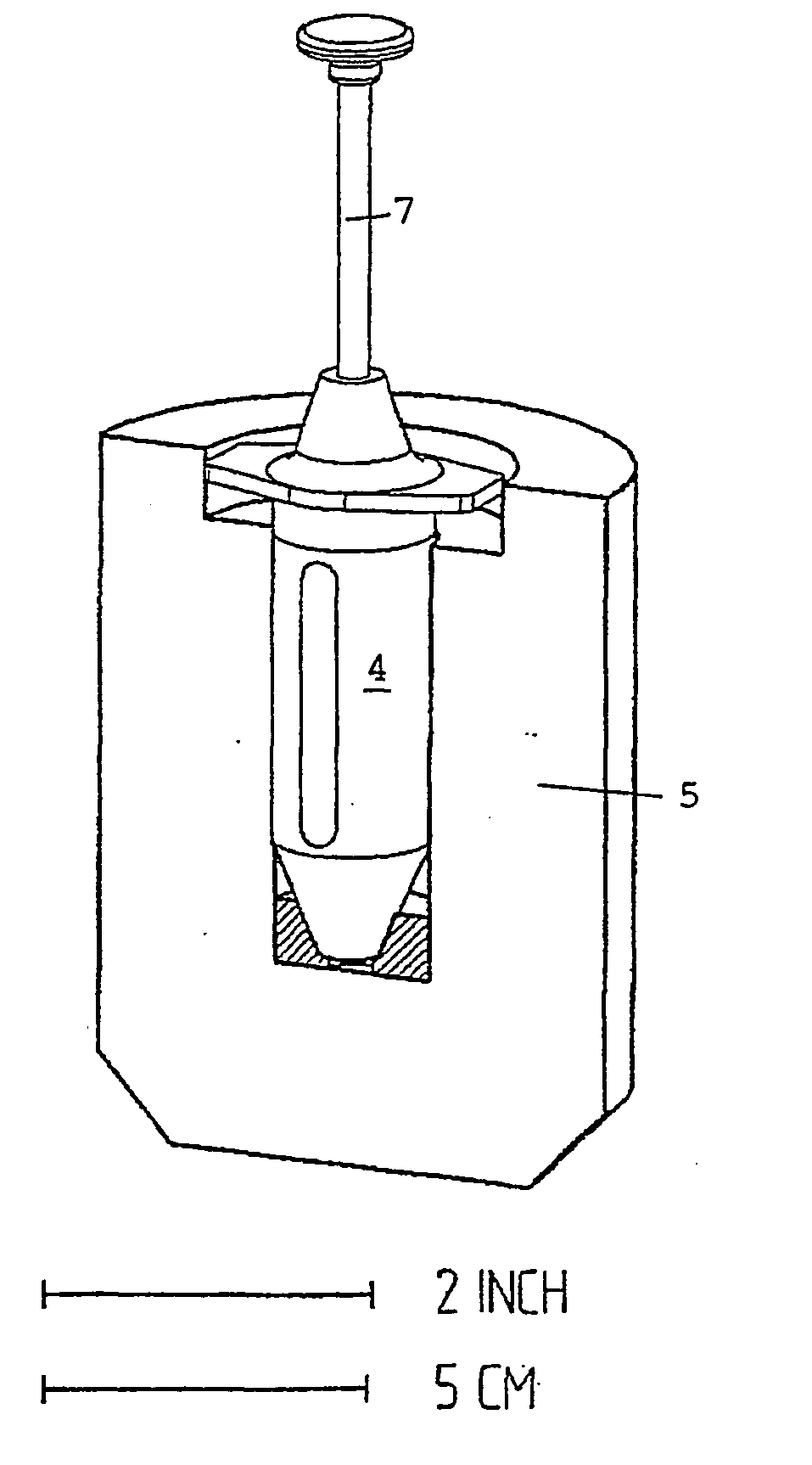
[0055] The present invention is related to a method and devices for preparing, packaging and handling individual doses of an injectable radiopharmaceutical compound. The method combines and incorporates the functions of filling (production) and transportation (packaging) and use while at the same time allowing the production to be easily automated.
[0056] The method of the invention comprises the following steps:
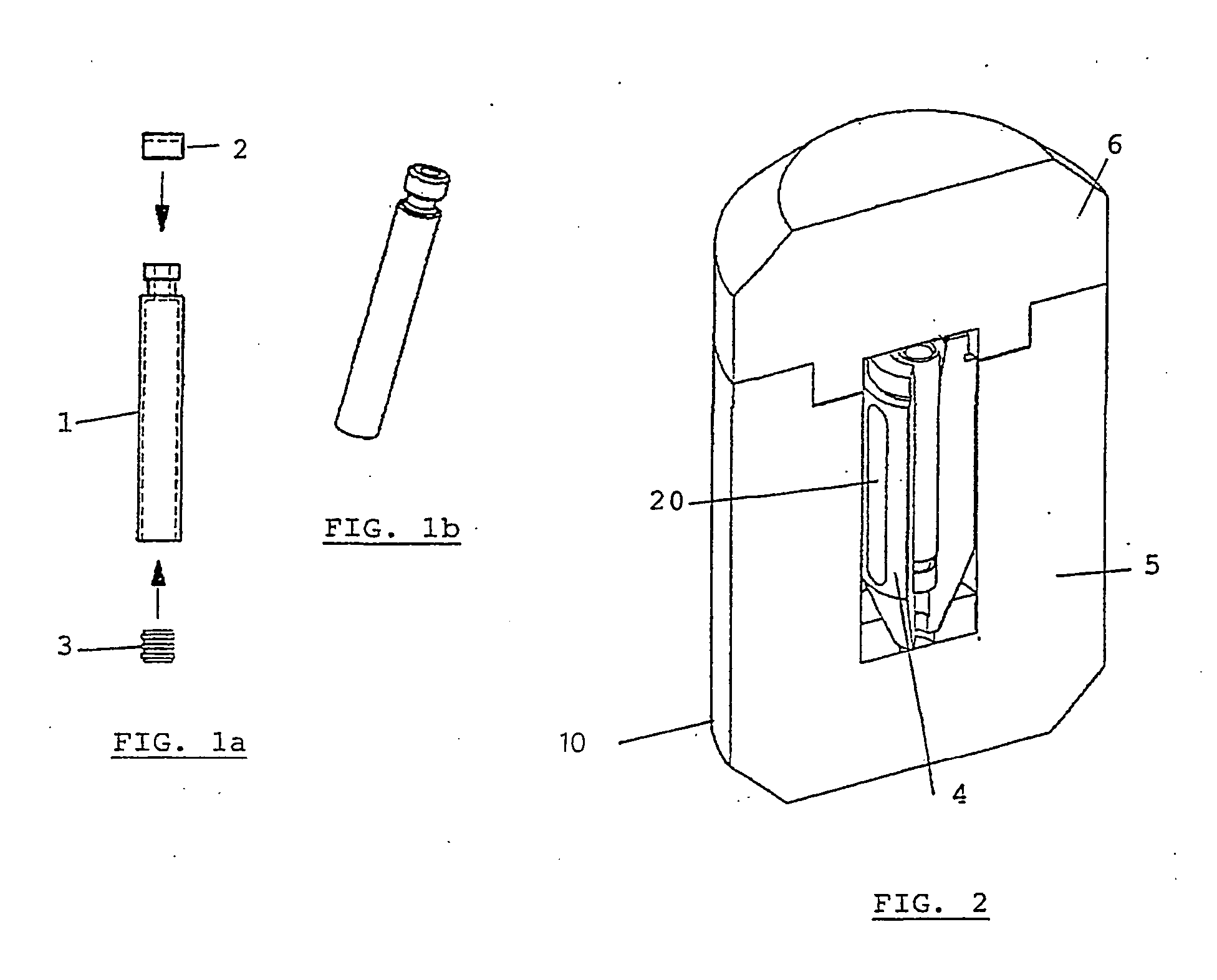
[0057] filling a "cartridge" 1 with an injectable substance of a radiopharmaceutical compound (FIG. 1);
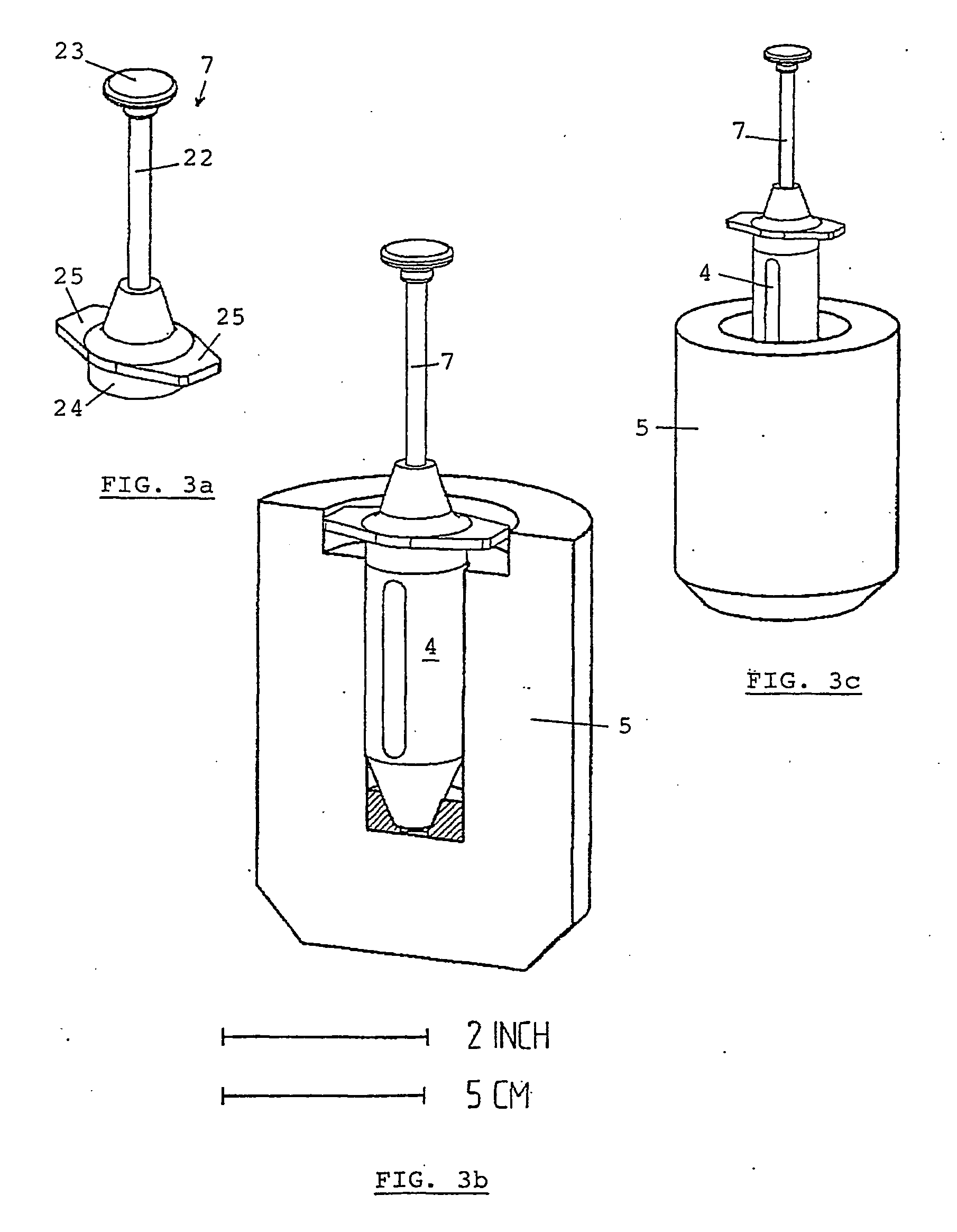
[0058] closing said cartridge 1 at one end by means of a setting elastomeric (rubber) stopper 2 to be capable of being pierced, such as a septum. The cartridge comprises a rubber piston 3 at the other end. The filling and closing may be performed automatically using a suitable dispensing device. A cartridge as is known to contain insulin may be used for this purpose;
[0059] placing the cartridge in a radiation shielding device 10 consisting of two concentric parts (FIG. 2): a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| Positron Emission Tomography | aaaaa | aaaaa |
| PET | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com