Patents
Literature
365results about "Multiple wrapper application" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
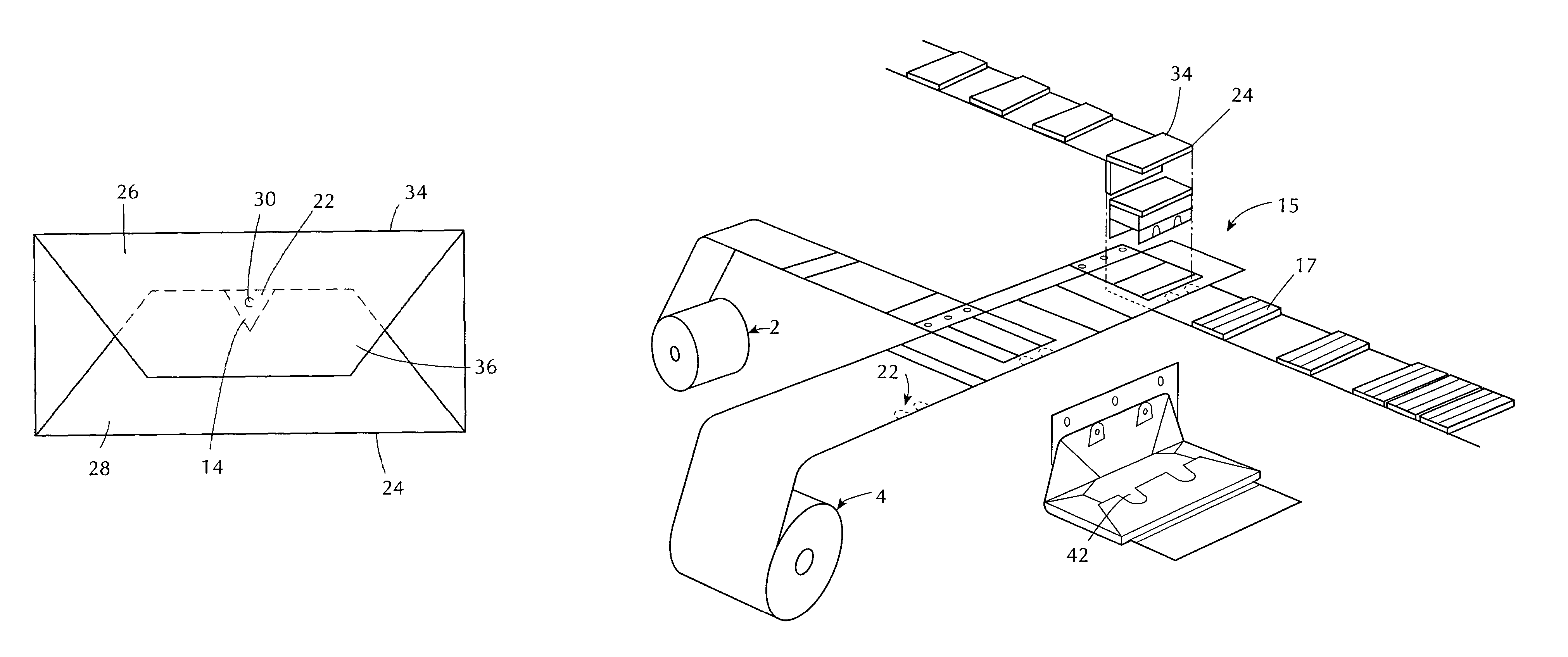
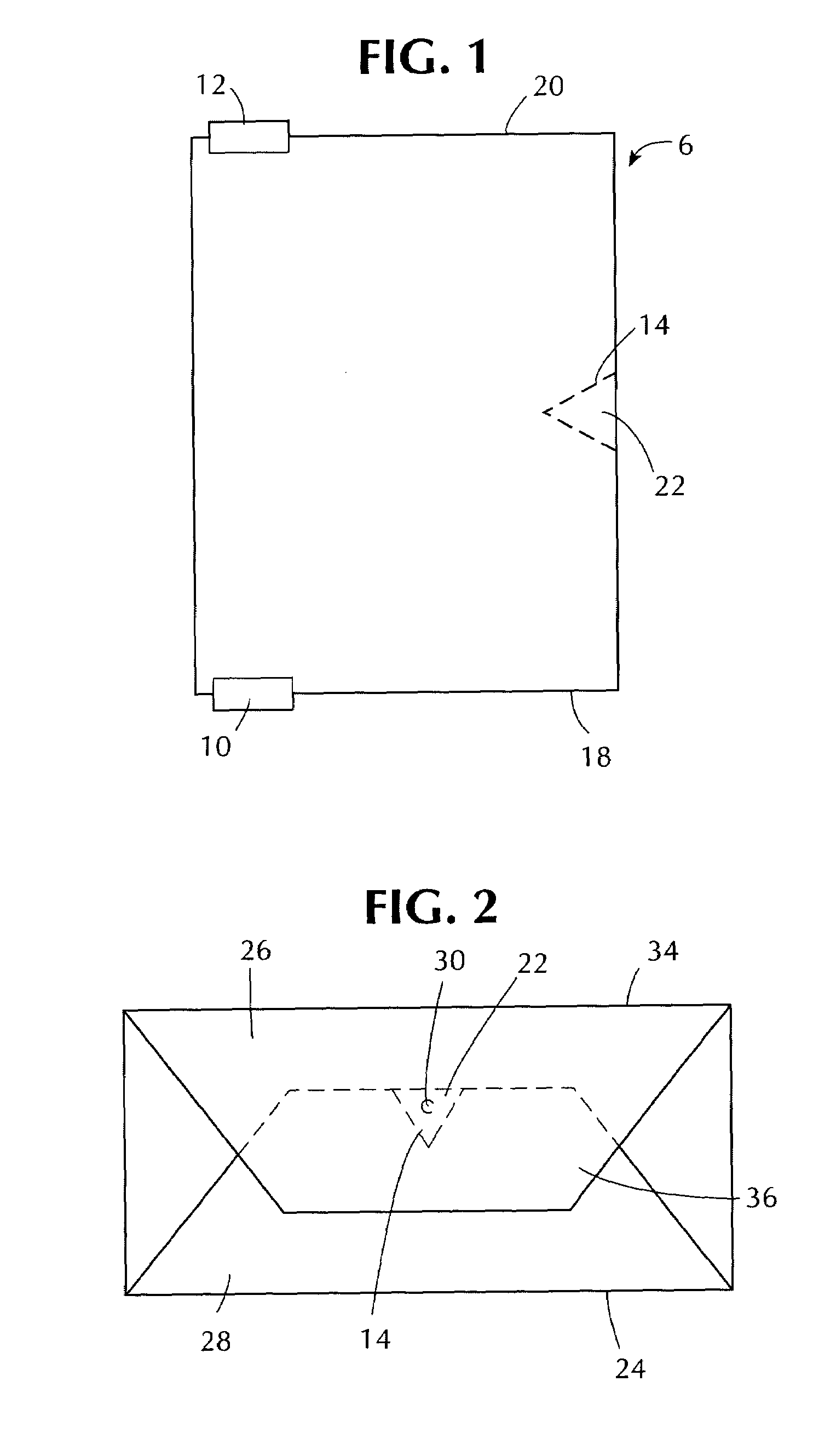
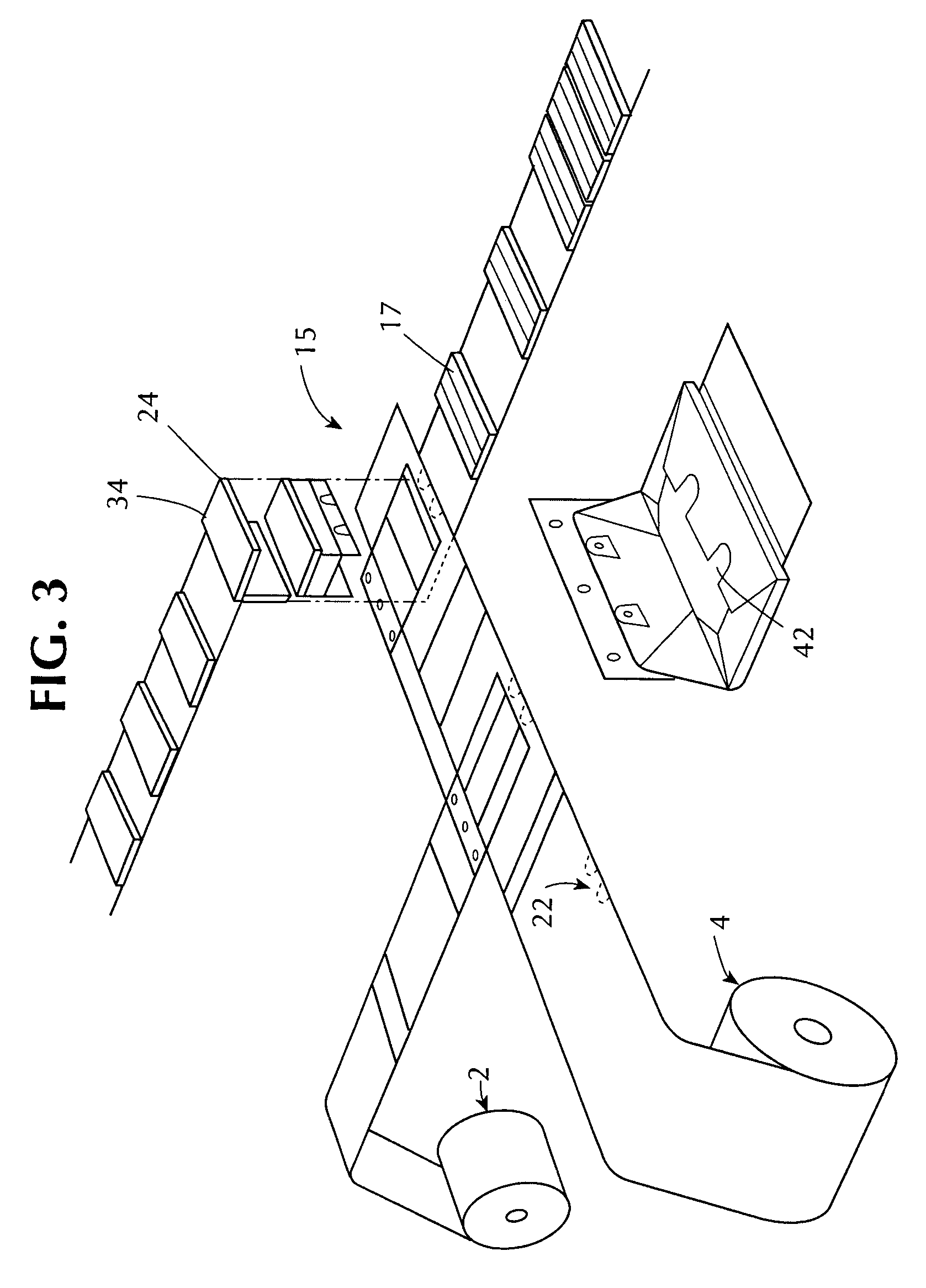
Stretch wrap films
InactiveUS6093480AImprove clingImproved maximum stretchWrappers shrinkageSynthetic resin layered productsStretch wrapGram
A multilayer, thermoplastic stretch wrap film containing at least three polymeric film layers and comprised of a first layer and a second layer. The first and second layers may comprise a polymer of two or more monomers, wherein a first monomer is ethylene, in a major amount by weight, and a second monomer is an alpha olefin of from about 3 to about 12 carbon atoms, in a minor amount by weight. If the first and second layers are outer layers, they have a cling force to each other of at least about 140 grams / inch. The stretch wrap film also has at least one inner polymeric layer, located between the first and second layers. The inner polymeric layer comprises a low polydispersity polymer having a polydispersity of from about 1 to about 4, a melt index (I.sub.2) of from about 0.5 to about 10 g / 10 min., and a melt flow ratio (I.sub.20 / I.sub.2) of from about 12 to about 22. The inner layer(s) comprise(s) from about 5 wt. % to about 40 wt. % of the stretch wrap film so as to produce a film having a maximum stretch of at least 340%, a F-50 dart drop value of at least about 130 g / mil, a machine directional tear resistance of at least about 125 g / mil and a transverse directional tear resistance of at least about 500 g / mil. It is contemplated that additional outer layers may be added such an outer high cling layer or an outer slip layer, as well as additional inner layers.
Owner:BERRY PLASTICS CORP
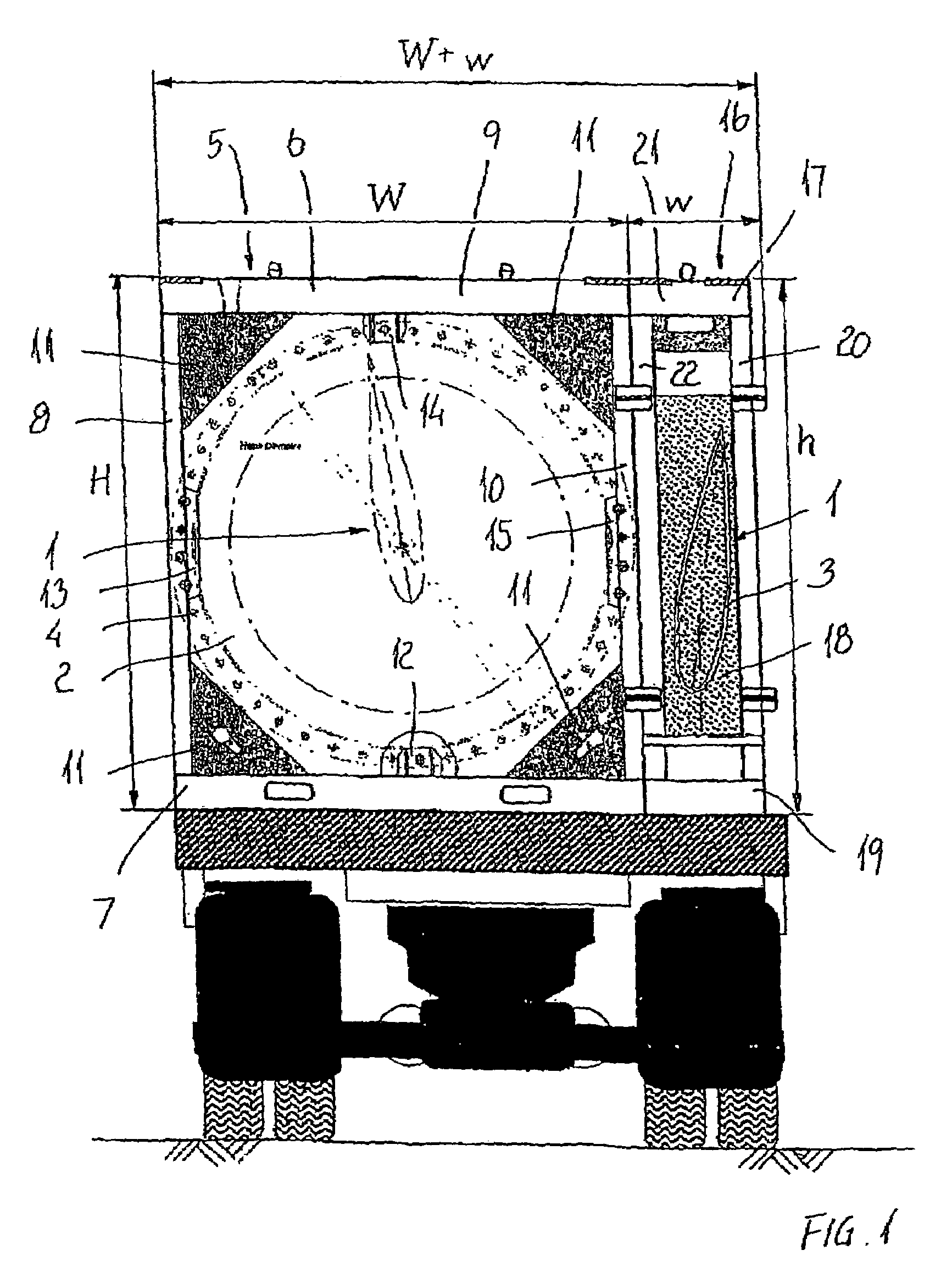
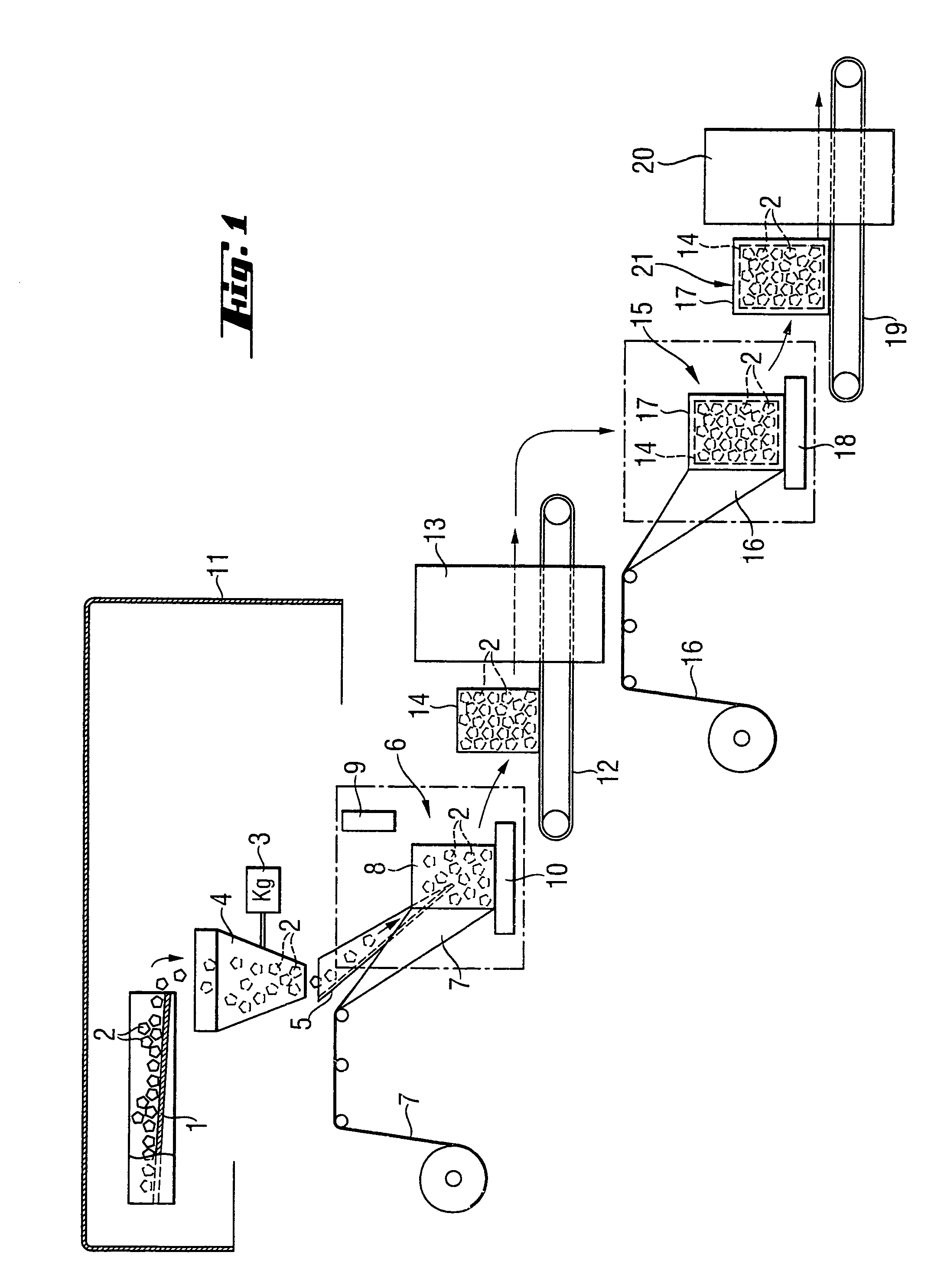
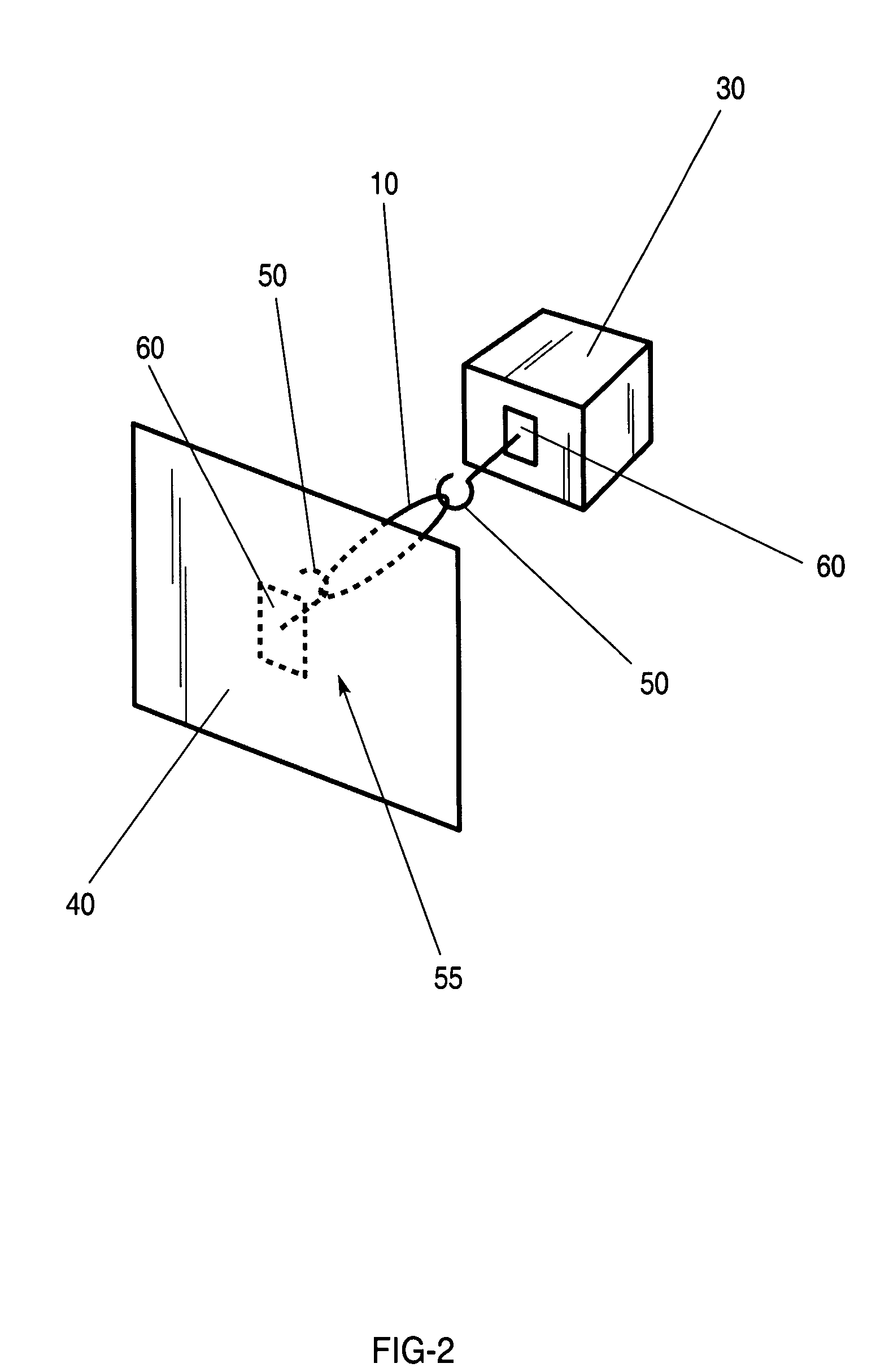
Method and apparatus for packaging panel products
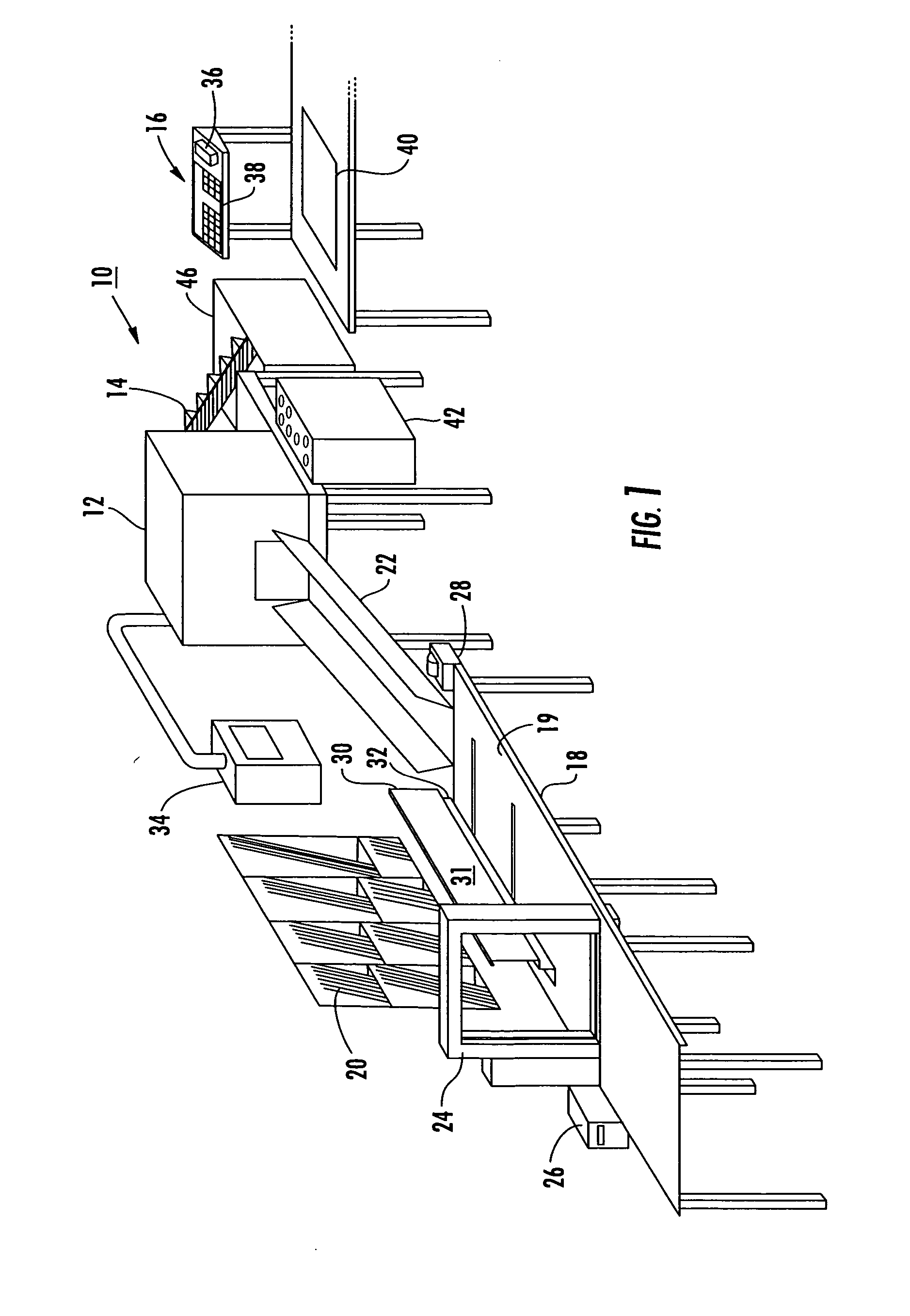
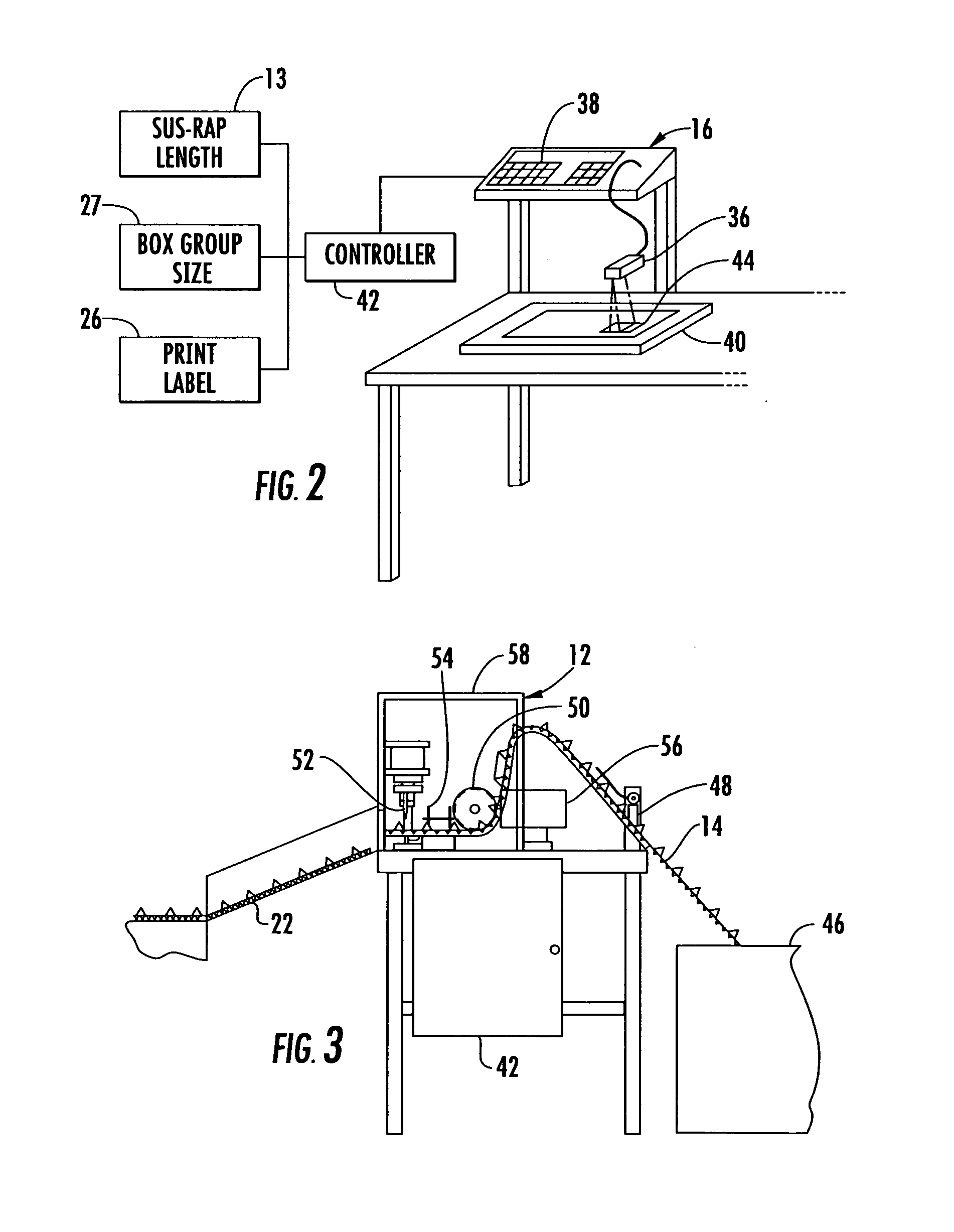
ActiveUS20050115202A1Easy to foldAdjustable sizeWrapping material feeding apparatusMultiple wrapper applicationBiomedical engineeringData entry
A system for packaging a flat panel product includes a dispensing device for dispensing a length of inner protective packaging material, a folding table for wrapping the inner protective packaging material around the product and folding an outer protective packaging material around the product, and a binding device for binding the outer packaging material, inner packaging material, and product together, suspending the product relative to at least one of the walls of the sidewall and supporting the product laterally. A data entry device provides data related to the length of inner protective packaging material required, and this data is fed to a controller, which controls the dispensing device to provide an appropriate length of material. The data entry device can also provide shipping information, fed to a printer for printing a shipping label.
Owner:SIGNODE IND GRP
Process and apparatus for the cost-effective packaging of polysilicon fragments
InactiveUS20050034430A1Cost-effectiveHigh purityMultiple wrapper applicationSolid materialBiomedical engineeringPolycrystalline silicon
Owner:WACKER CHEM GMBH
Process and device for preparing radiopharmaceutical products for injection
InactiveUS20040084340A1Reduce needAmpoule syringesDispensing apparatusRadiopharmaceutical CompoundChemical compound
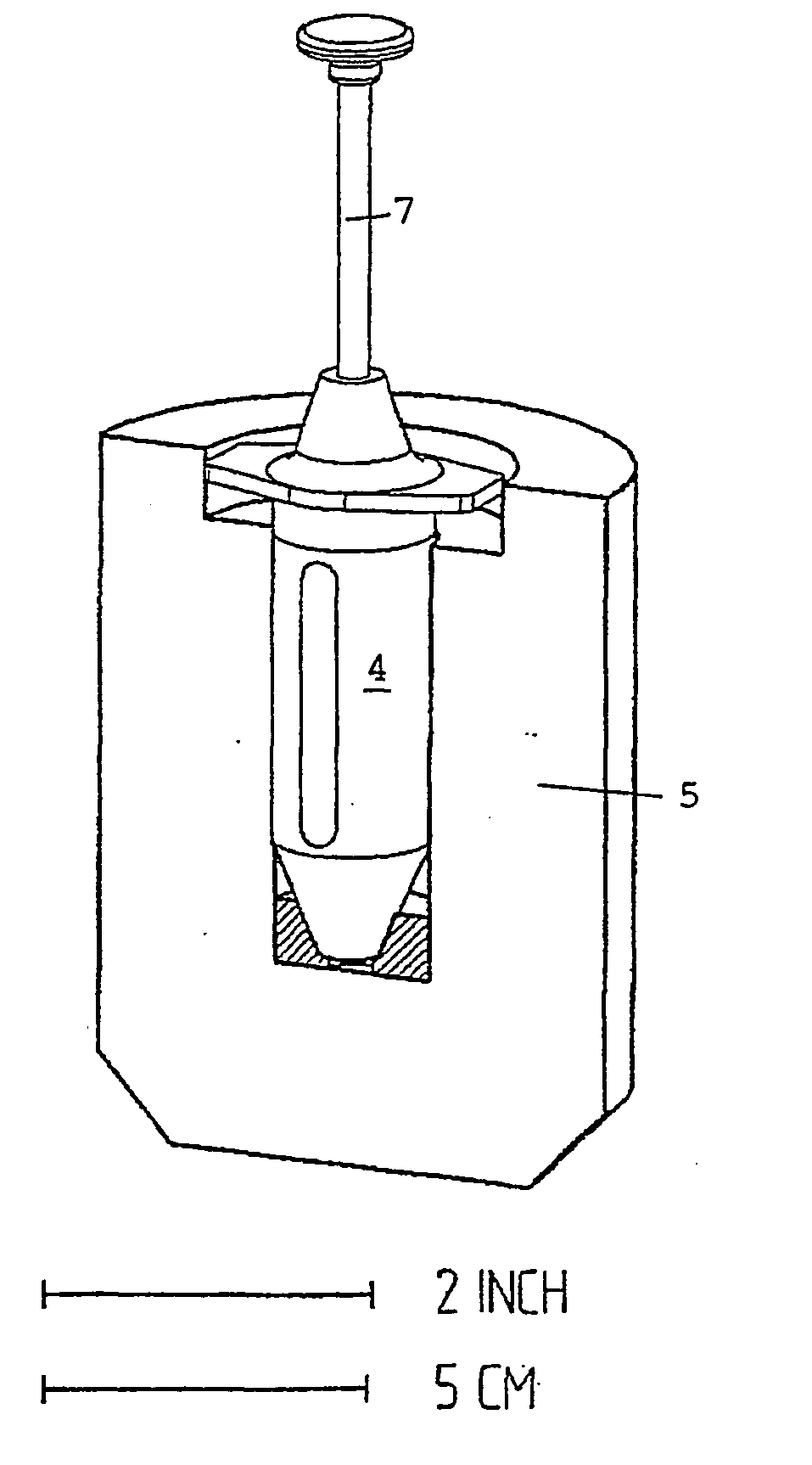
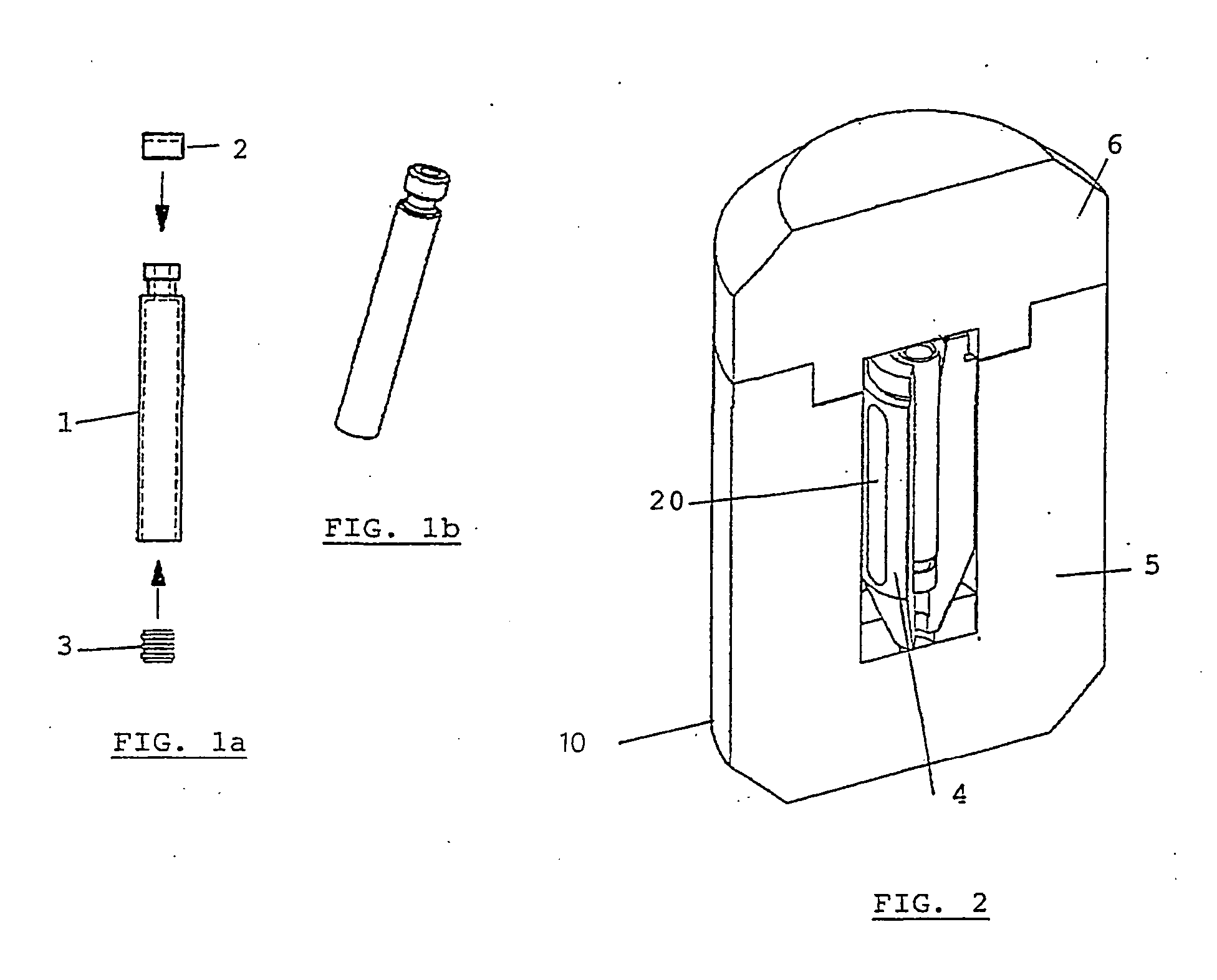
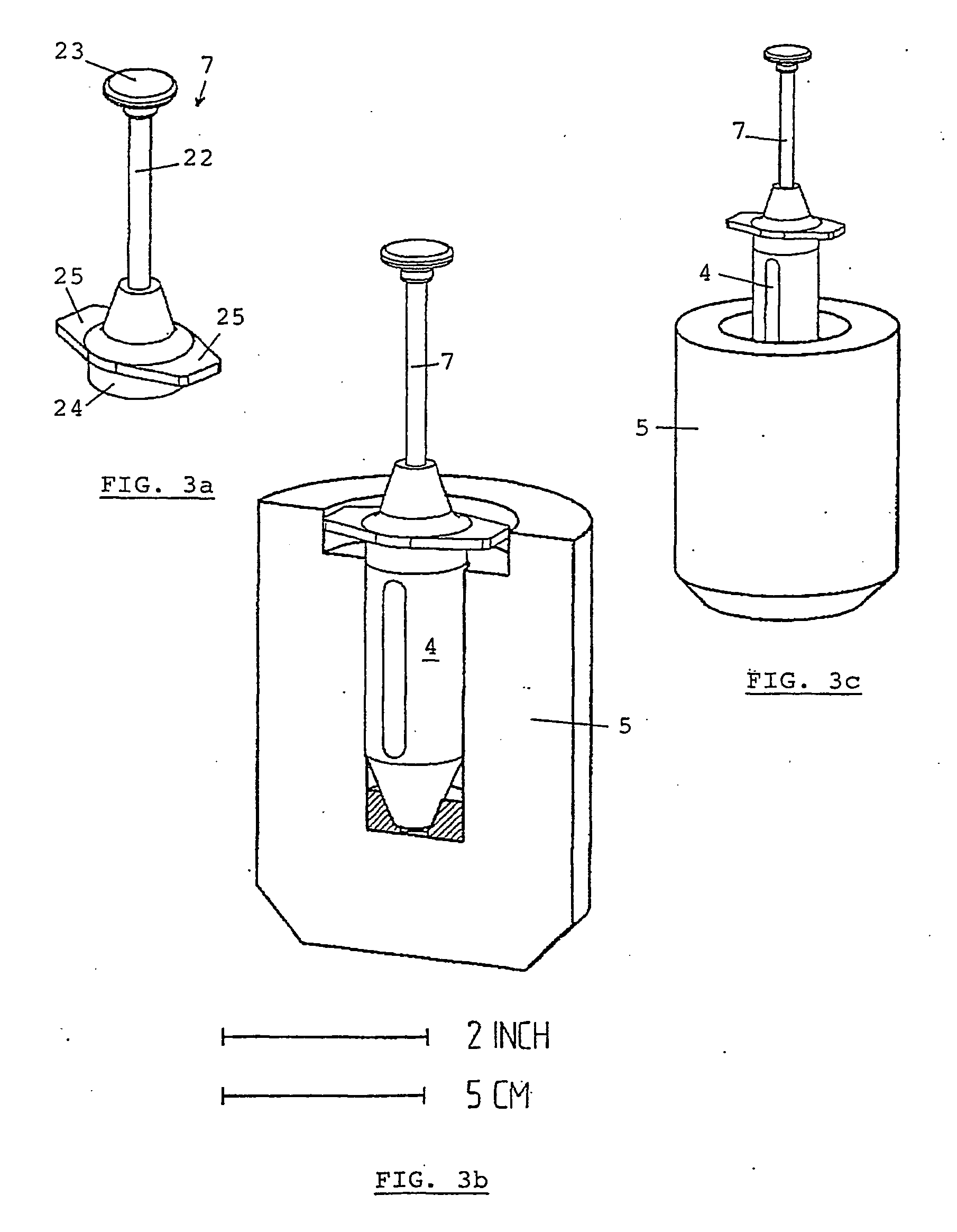
Method for preparing, packaging and handling an individual dose of a radiopharmaceutical compound, comprising the following steps: filling a cartridge (1) with said dose of radiopharmaceutical compound via a first end, the second end being closed by means of a component serving as a piston (3); closing said cartridge (1) at said first end by means of a closure device (2); placing said cartridge (1) in a radiation shielding device (10), comprising an inner part (4) and an outer part (5), said inner part serving as radiation shielding for an operator and said outer part serving as a transportation shielding container; closing said container by means of an appropriate shielding lid (6); transporting said container up to the place at which an injection of said radiopharmaceutical compound will take place; removing the shielding lid (6) of the container; fixing a plunger (7) to the cartridge piston (3); extracting the cartridge and the inner part (4) of the radiation shielding device (10) from the outer part (5) serving as a container, and placing injection means (30) on the cartridge end which has the setting closure device (2).
Owner:MORELLE JEAN LUC
Method for transporting a set of large longitudinal items, a package system to be used by the method and use of such a package system
InactiveUS6983844B2Easy to useEasy to transportLarge containersMultiple wrapper applicationTurbine bladeLateral extension
The invention relates to a transportation method and a package system for transporting a set of large longitudinal items such as blades for a wind turbine (1) or a tower for a wind turbine. Taking wind turbine blades (1) as an example, the advantages of the invention reside in packaging a tip (3) of one blade and a base (2) of a second blade in the one and same package (5) such that two blades (1) may be transported in packages (5, 16) having an overall lateral extension, approximately the same as the base of only one blade (1) for the wind turbine. Thereby a very compact but also a very easy means of transportation of large longitudinal items such as wind turbine blades (1) is provided.
Owner:VESTAS WIND SYST AS
Packaging material profiling for containment force-based wrapping
Packaging material may be profiled to generate an incremental containment force per revolution (ICF) attribute that is represented by a function that is variable as a function of wrap force. Moreover, the performance of different packaging materials, e.g., in terms of speed or cost, may be compared for a particular load through simulation of wrap operations based upon dimensions of the load and a desired load containment force requirement for the load.
Owner:LANTECH COM
Method of providing a therapeutic regimen and prefabricated container therefor
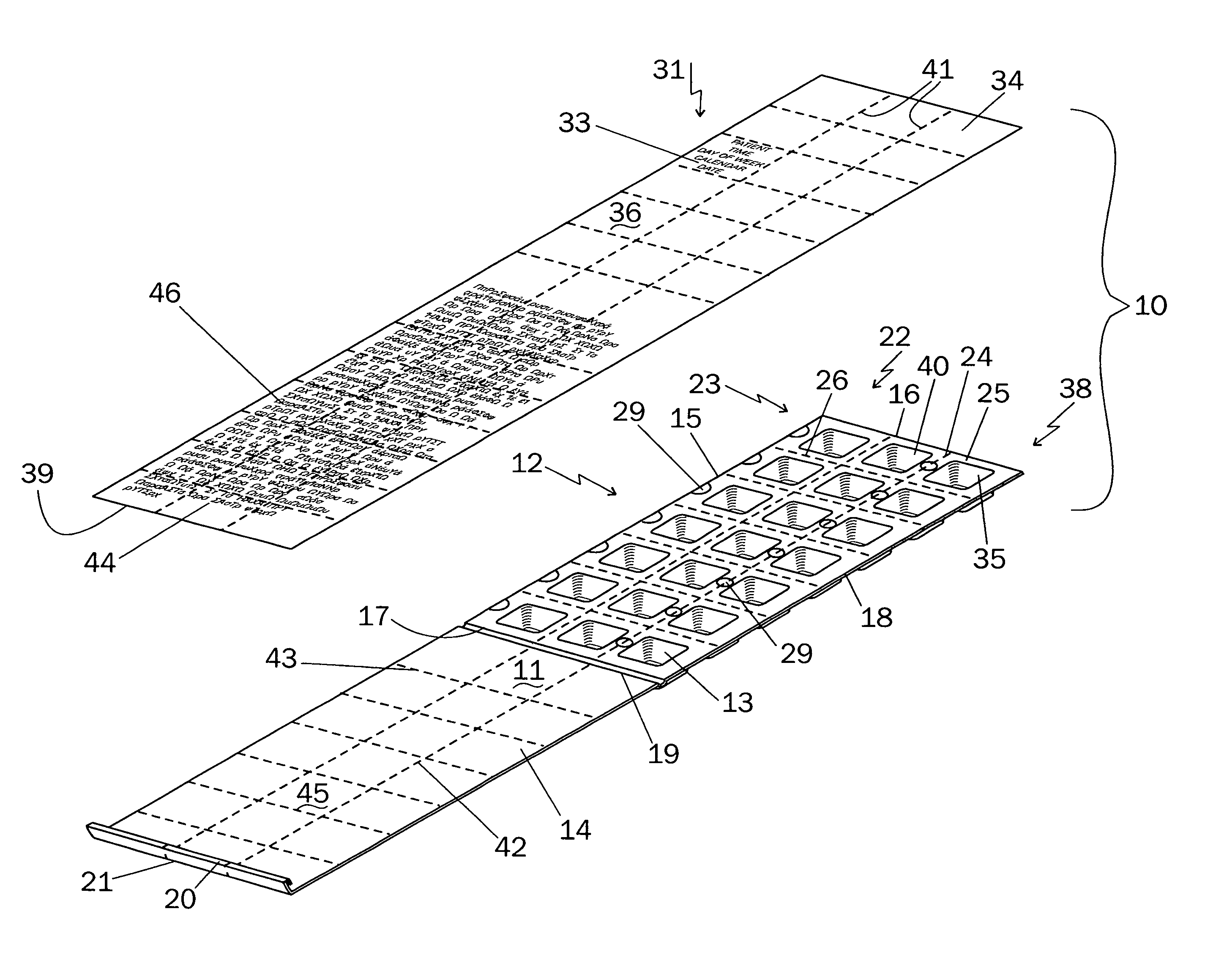
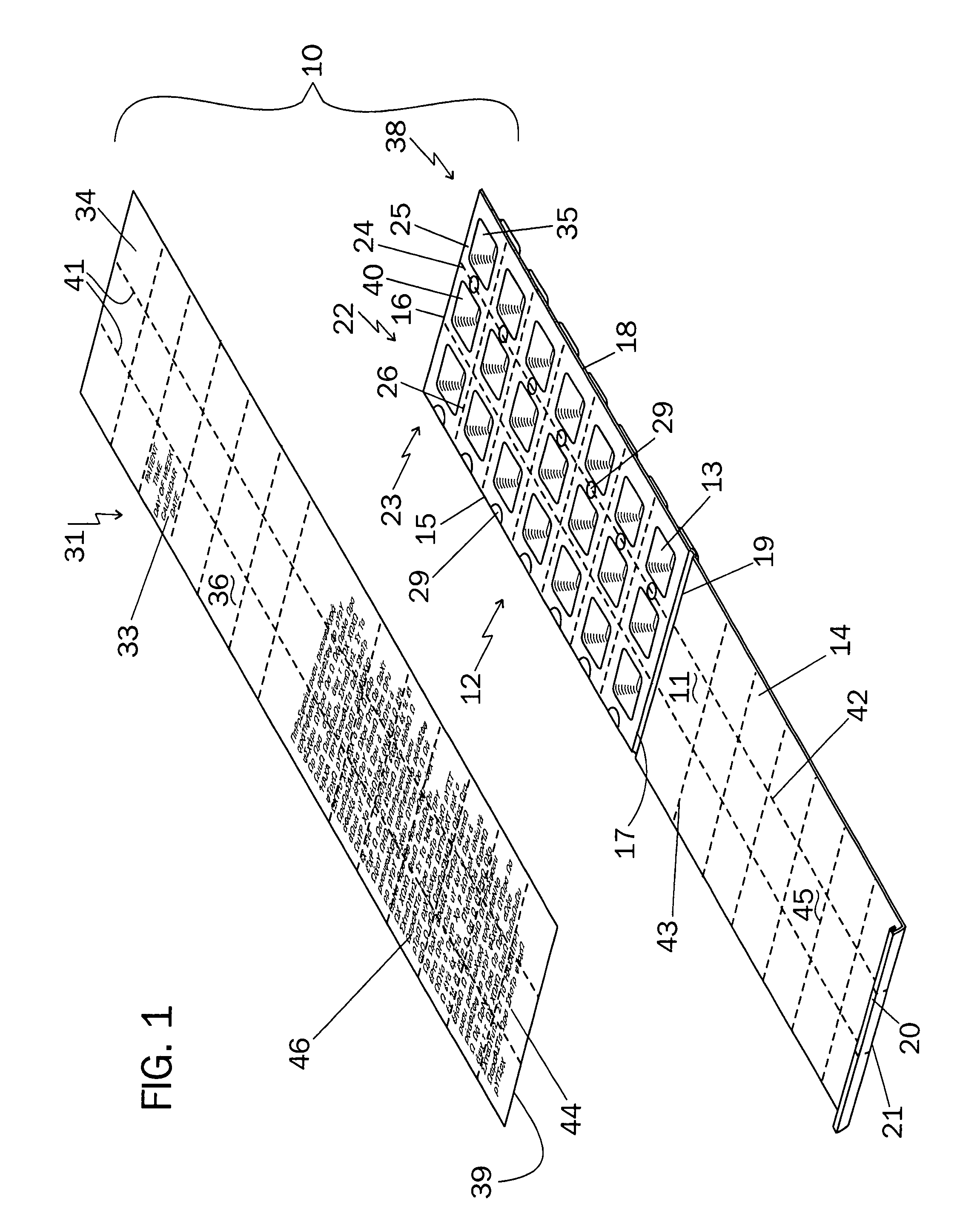
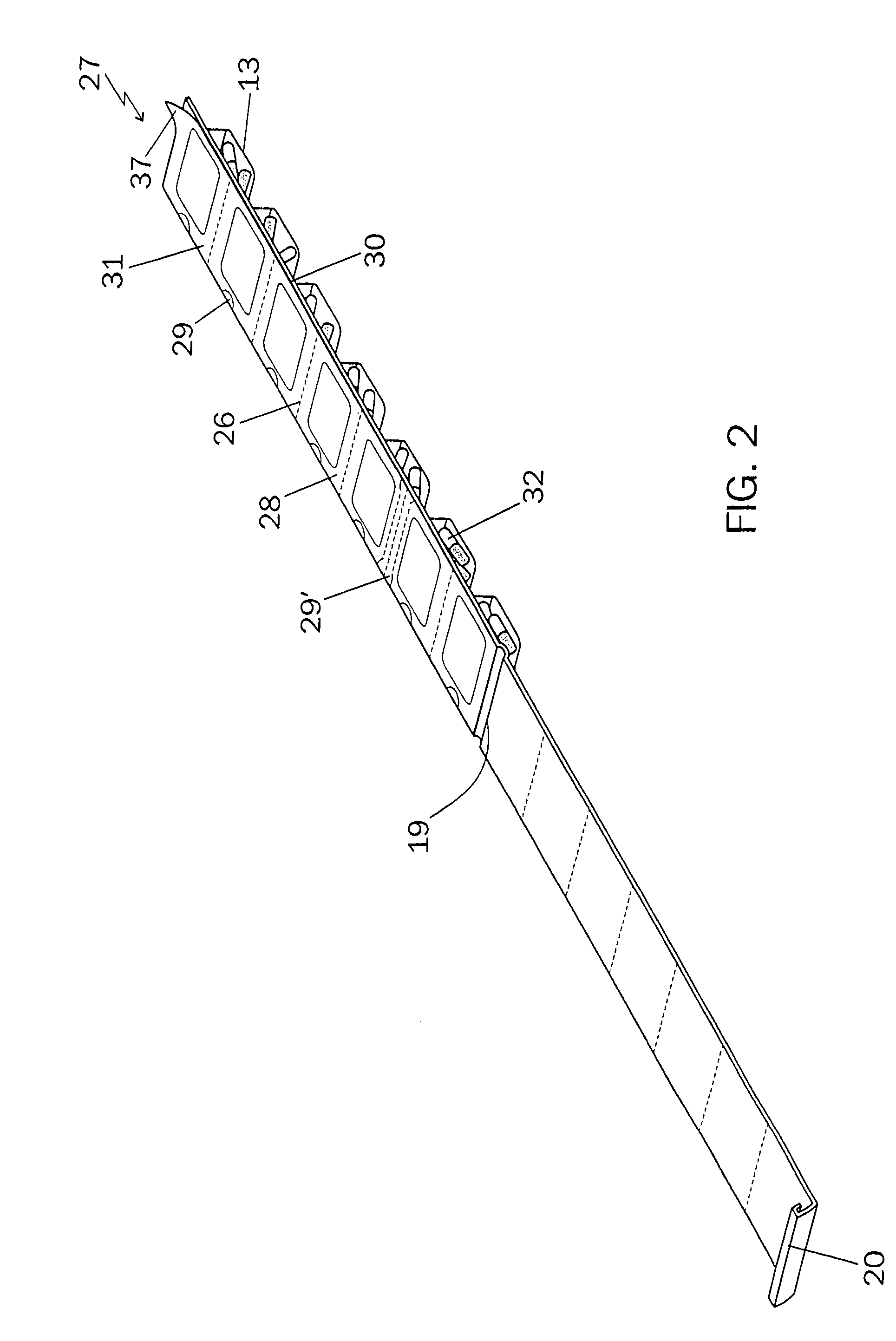
An integrated medicament package comprises a prefabricated medicament container having a plurality of initially open top blisters, a closure cover affixed to one marginal edge thereof by at least one living hinge, the closure cover having at least one clasp on an open end of the closure cover opposite the living hinge, the blisters arranged in rows and columns separated by longitudinal and transverse shoulders, at least one of the longitudinal and the transverse shoulders having perforations therethrough wherein the prefabricated medicine container is adapted to be separated along the perforations into a plurality of units, and a sealing sheet with medicament information at a location corresponding to the at least one said plurality of blister such that the medicament information on the outside surface of the sealing sheet corresponds with the medicament in the blisters, the medicament information comprising the name of the patient, the name of the medicament, the dosage of the medicament, the time of day, the day of week and the calendar date for the patient to take the medicament in the blister, the sealing sheet when removed from the location indicates to the patient that the medicament has been taken.
Owner:LEWIS GRAHAM L
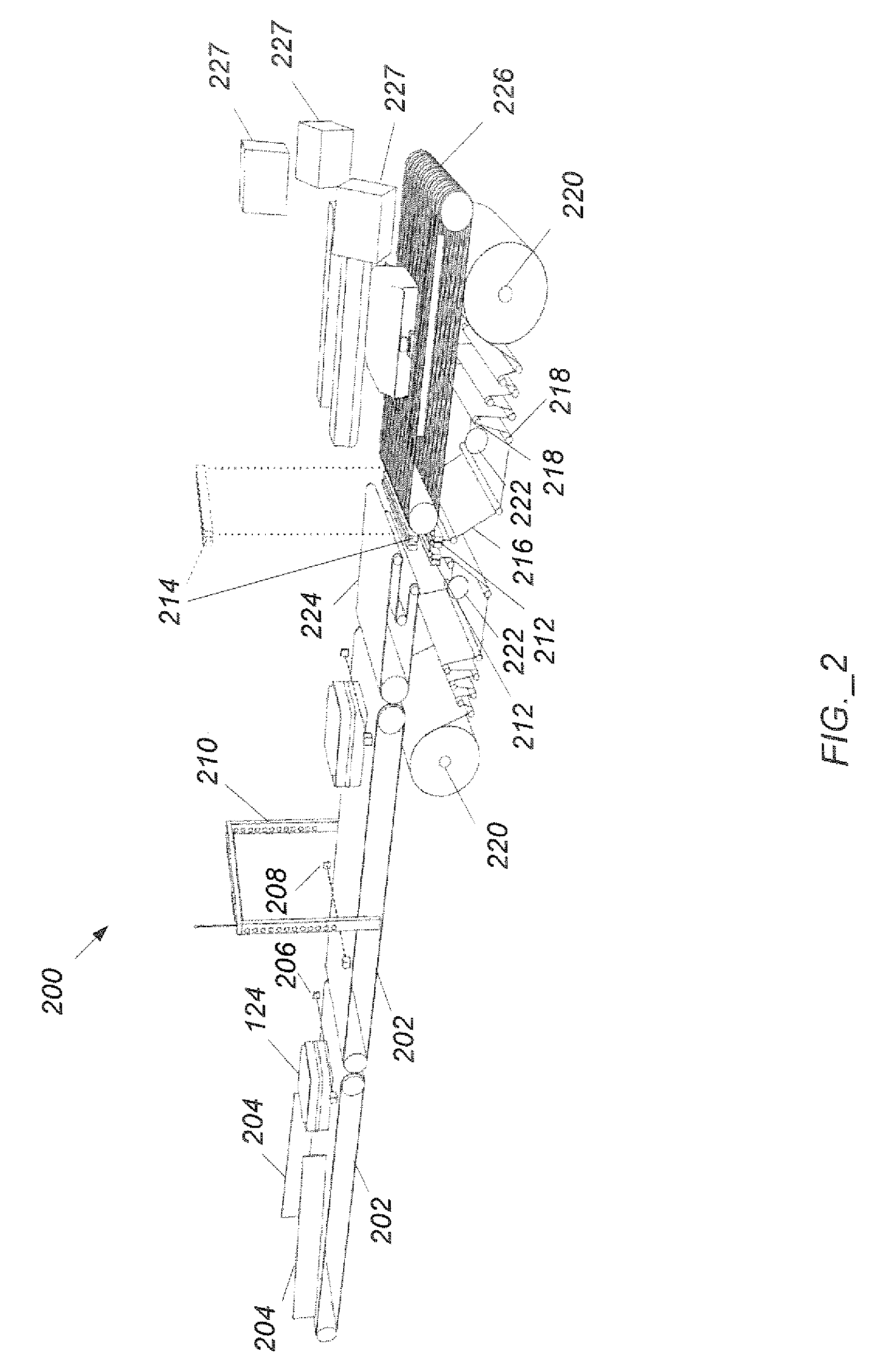
Method of wrapping a round bale compacted by a round baler, film-wrapping device and round baler that is provided with such a film-wrapping device
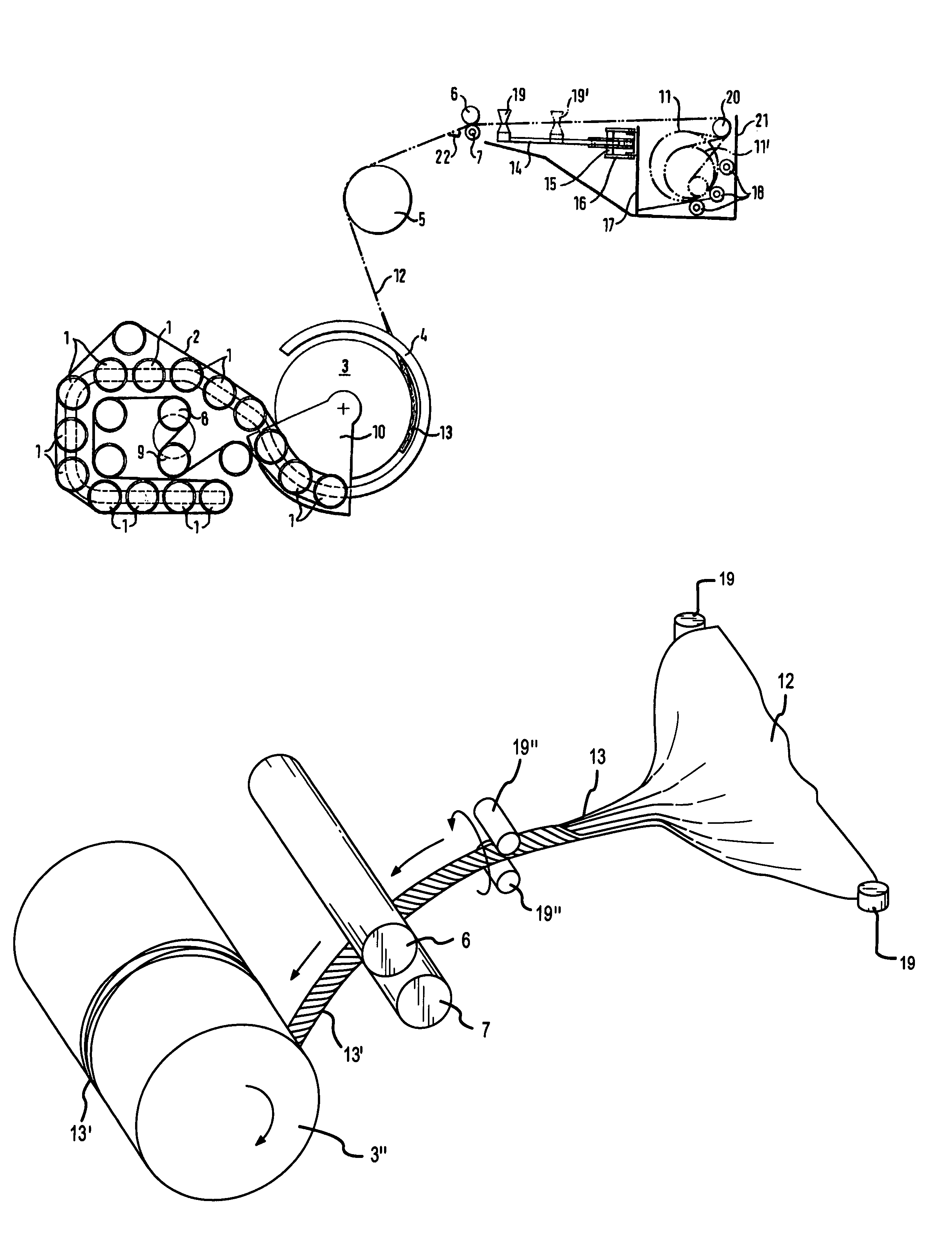
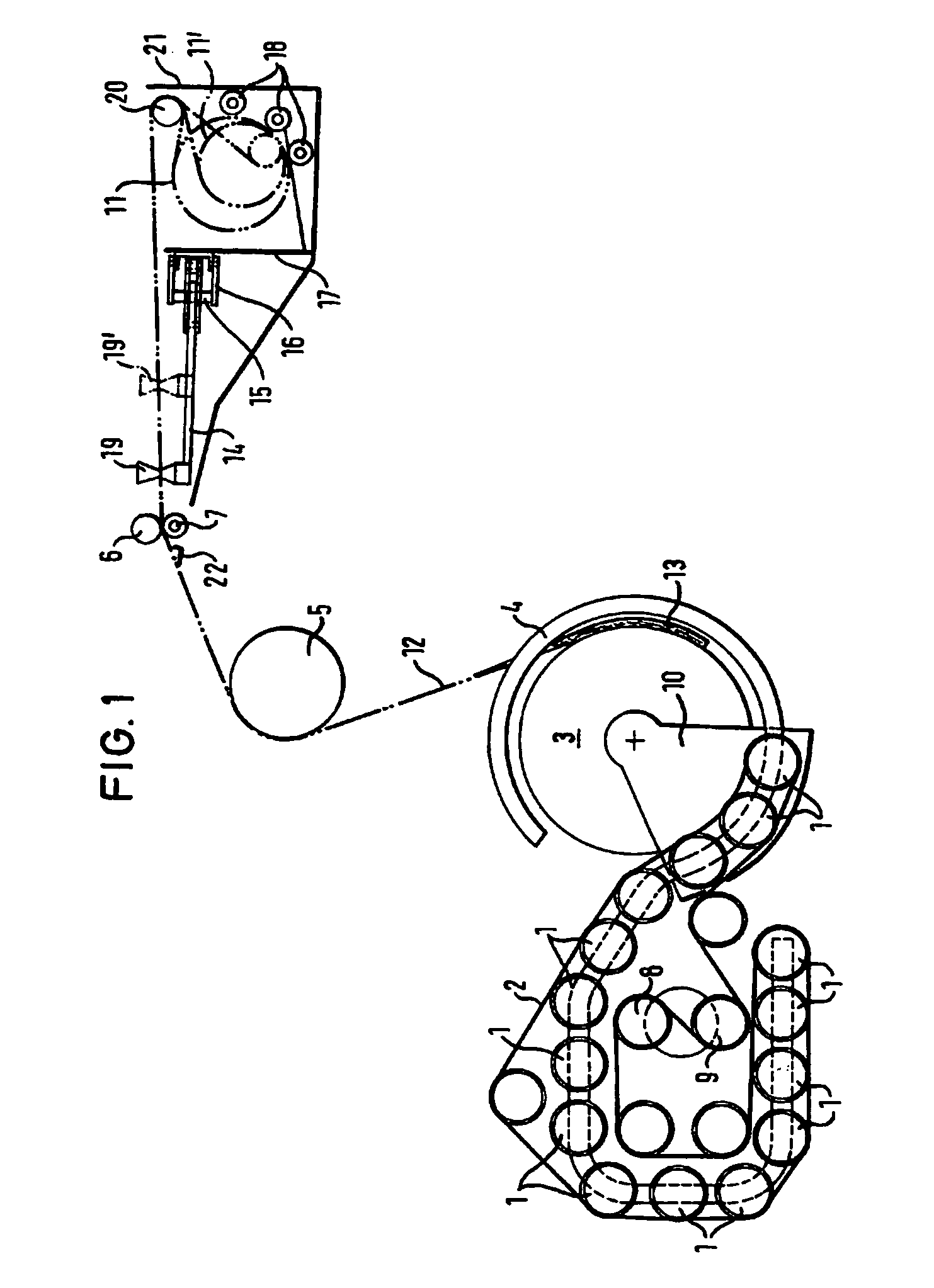
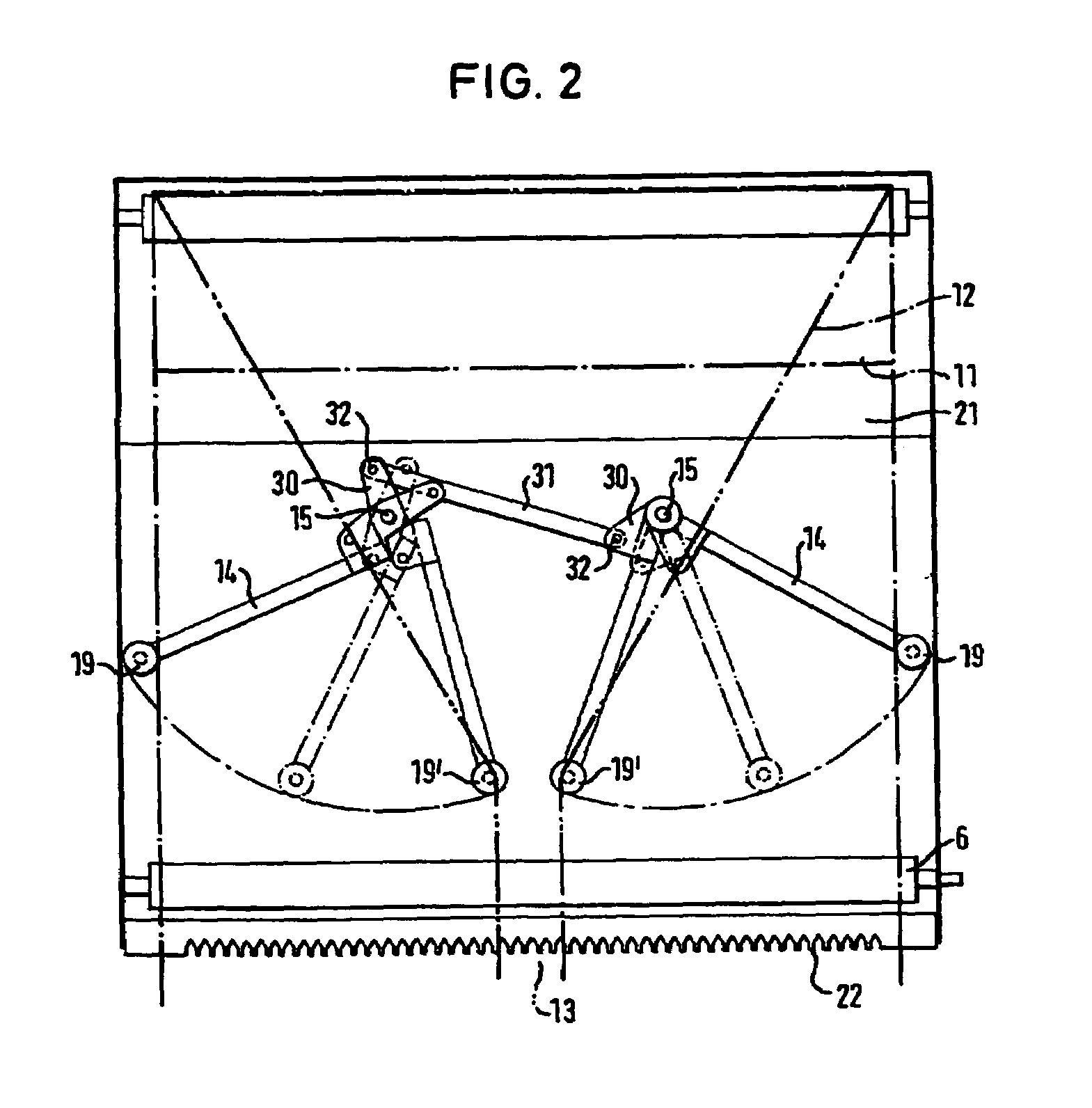
The invention relates to a method and a device for wrapping a round bale pressed in a round bale press at least about its cylindrical surface area with an at least unilaterally adhesive film (12). With the inventive method, the film (12) is pulled off by means of a pulling-off device (6, 7) from a film roll (11) in its entire width. During a predetermined space o time of said pulling-off operation, a film rope (13) is formed from the film (12). Said film rope (13) is introduced into the gap between the round bales (3) to be wrapped and a device forming the circumferential press chamber wall (2). By setting the round bale (3) into rotation, the film rope (13) present in the gap is carried along. The round bale (3) is so long rotated until the desired number of film layers has formed on the surface area of the round bale.
Owner:RPP AMERICA
Containment force-based wrapping
ActiveUS20140223864A1Reduce the amount requiredWeb rotation wrappingMultiple wrapper applicationUser inputControl system
Control of a wrapping apparatus is facilitated by enabling an operator to input a load containment force requirement and / or a minimum number of layers of packaging material to be applied to a load, with a wrap control system automatically determining wrap force and other parameters required to meet user input requirements and / or parameters to minimize the expertise required of an operator and to provide more consistent and reliable wrapping of loads. In addition, a wrapping apparatus may be controlled to apply at least a minimum number of layers of packaging material to a load throughout a contiguous region thereof.
Owner:LANTECH COM
Process for producing a water soluble package
InactiveUS6363693B1Improve impact resistanceEasy to handlePackage recyclingWrappersWater solubleMoisture barrier
A process for producing a package comprising a composition contained within a closed water soluble envelope comprises the initial steps of moulding a first sheet of water soluble material to form at least one recess adapted to contain the composition, placing the composition in the at least one recess, placing a second sheet of water soluble material across the recess, and sealing the first sheet to the second sheet to form a continuous closed seal around the recess thereby closing the package. The or each closed package is conditioned in an environment of raised relative humidity for a period of time prior to or during the packaging of the package within a secondary pack. The secondary pack includes a moisture barrier to maintain the package within the secondary pack in a conditioned form.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C +1
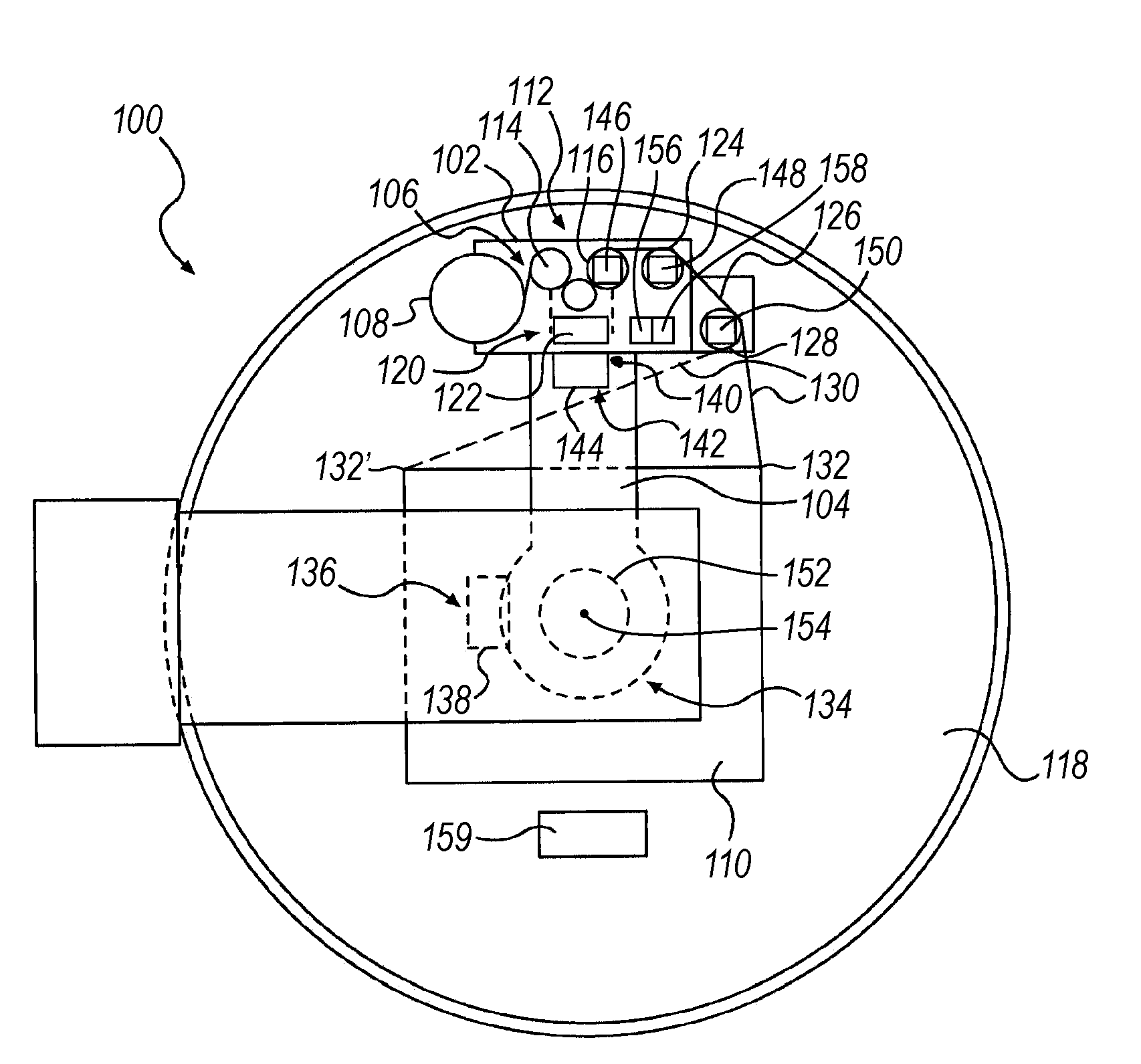
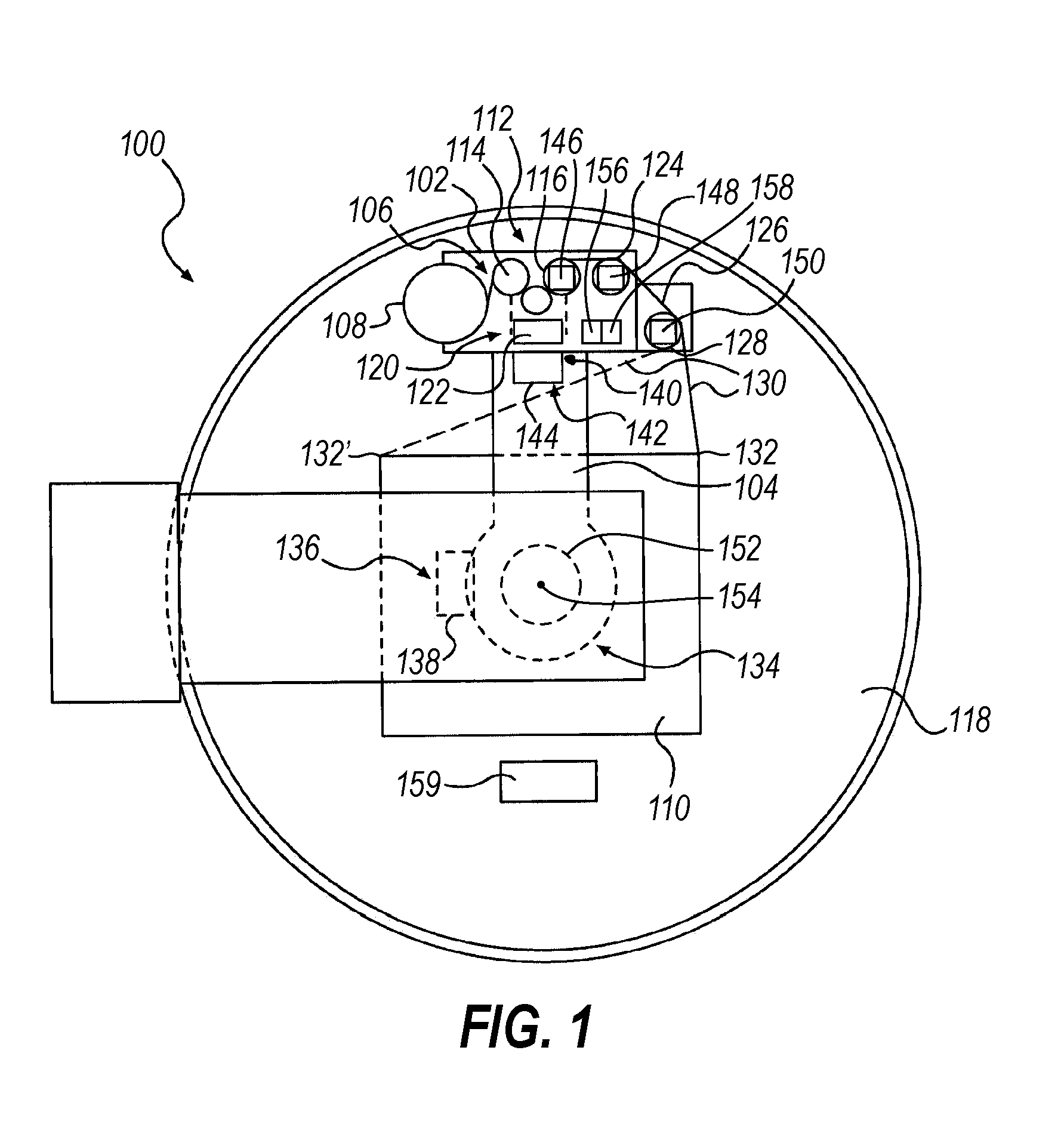
Computerized, monitored, temperature affected, delivery system for perishable goods
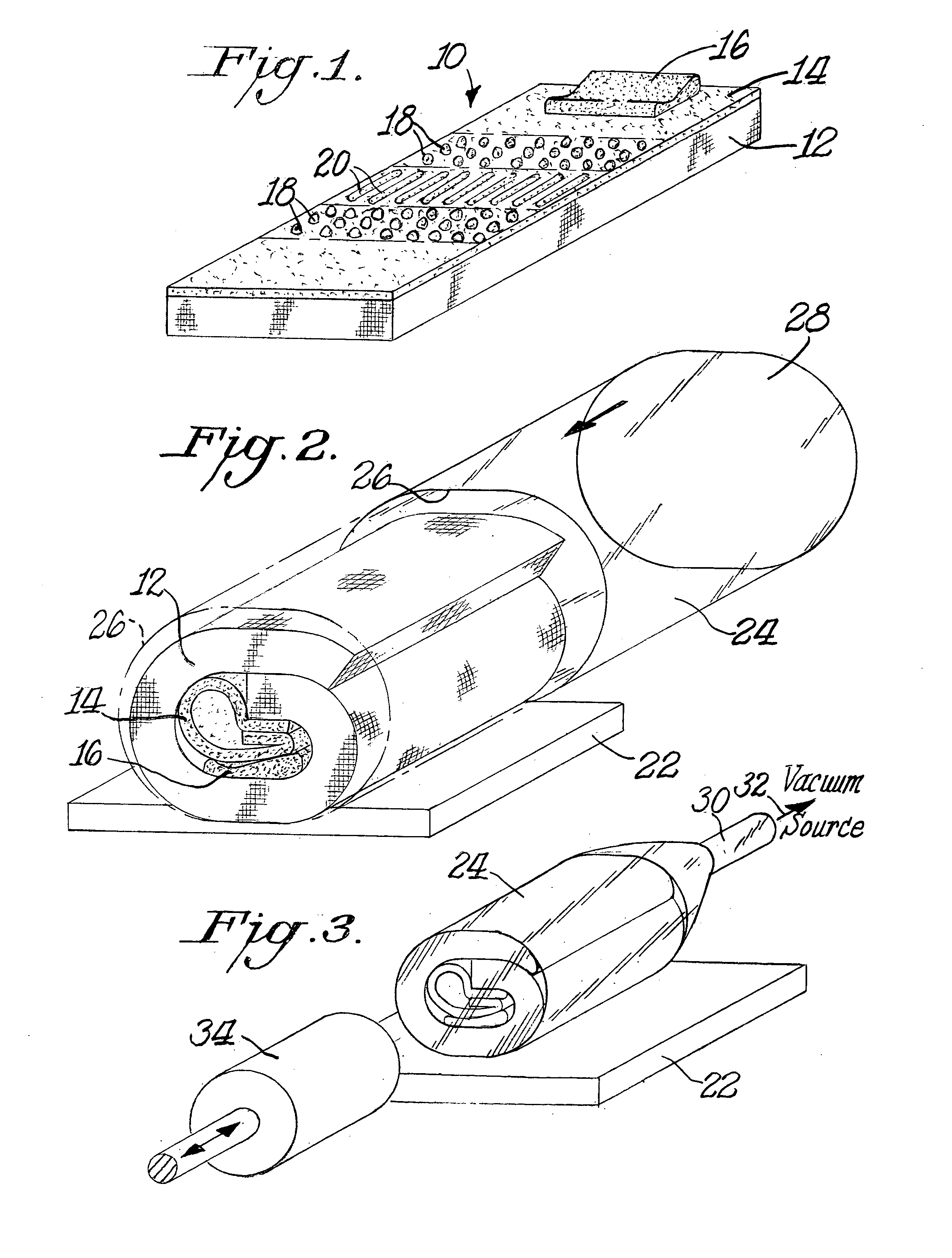
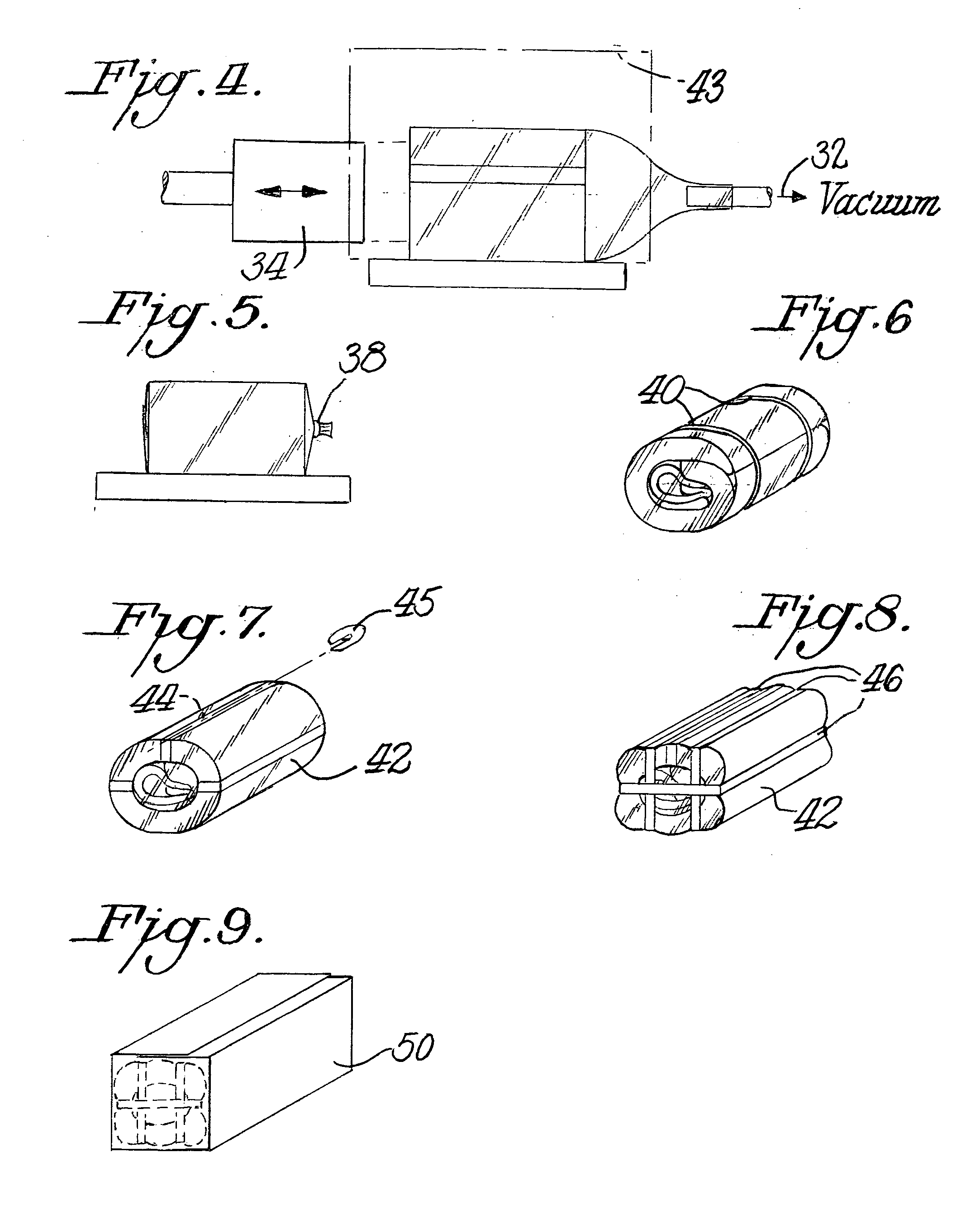
InactiveUS6536189B1Keep the heatSlow heatingPackage sterilisationMultiple wrapper applicationSuper absorbentBubble wrap
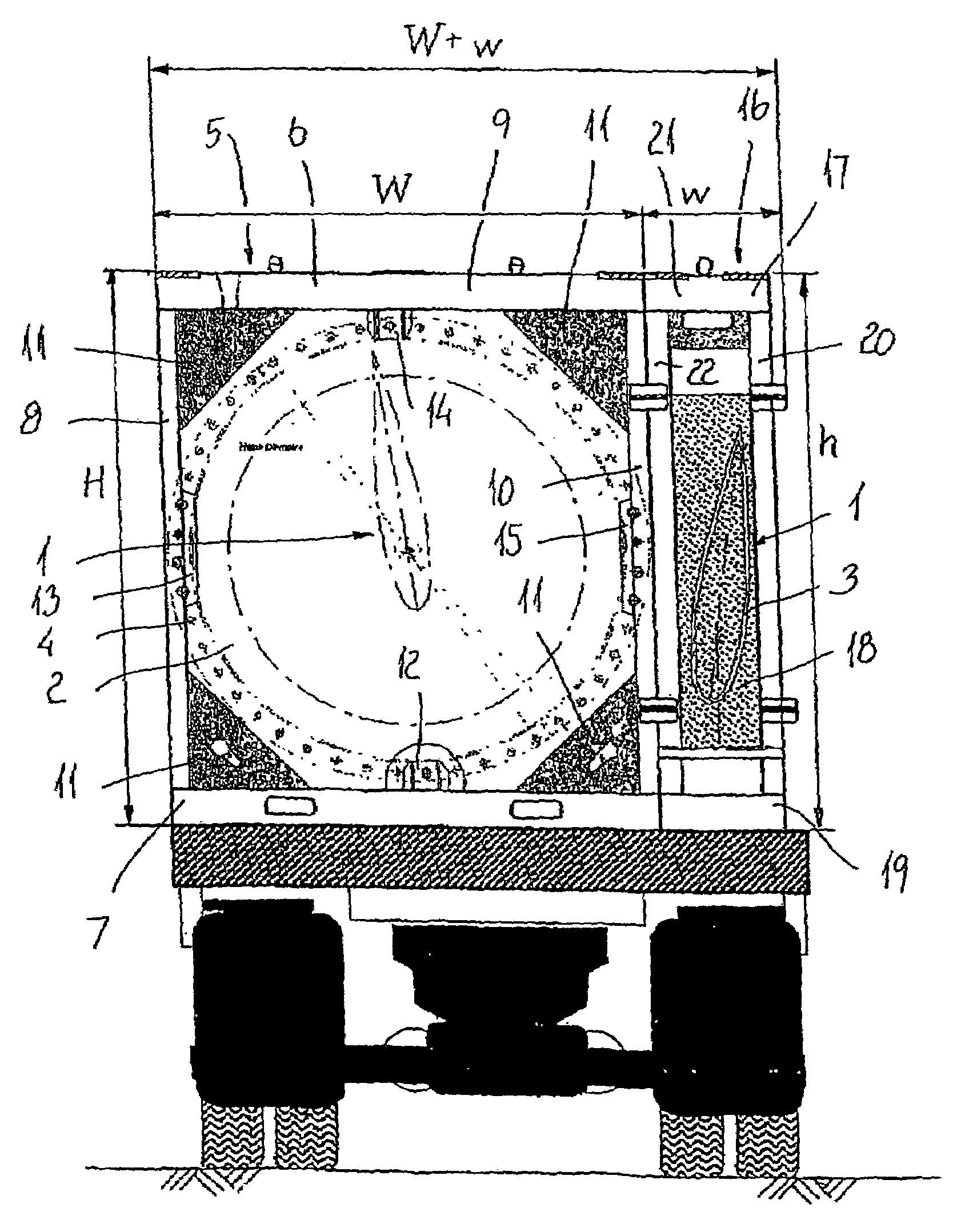
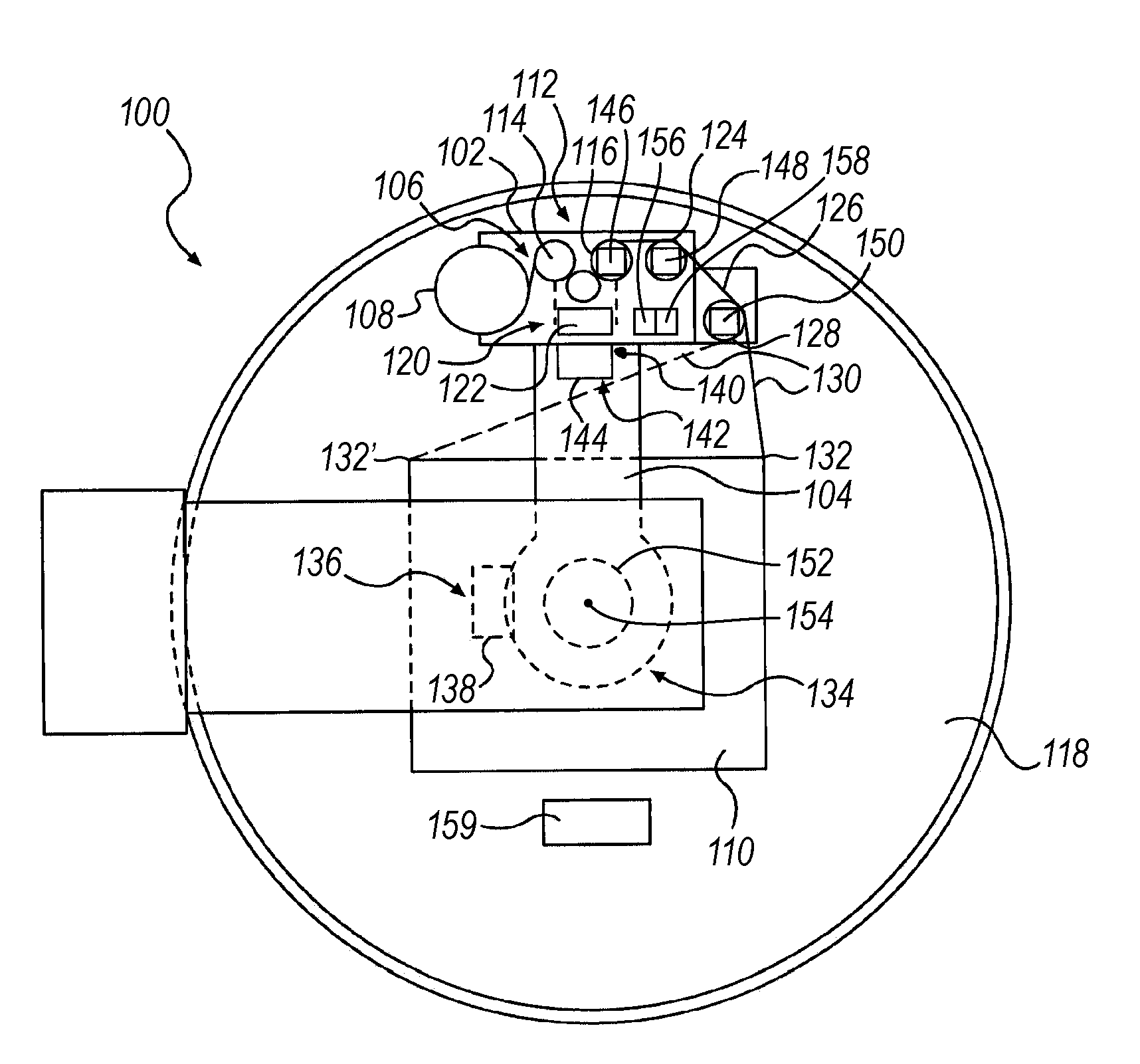
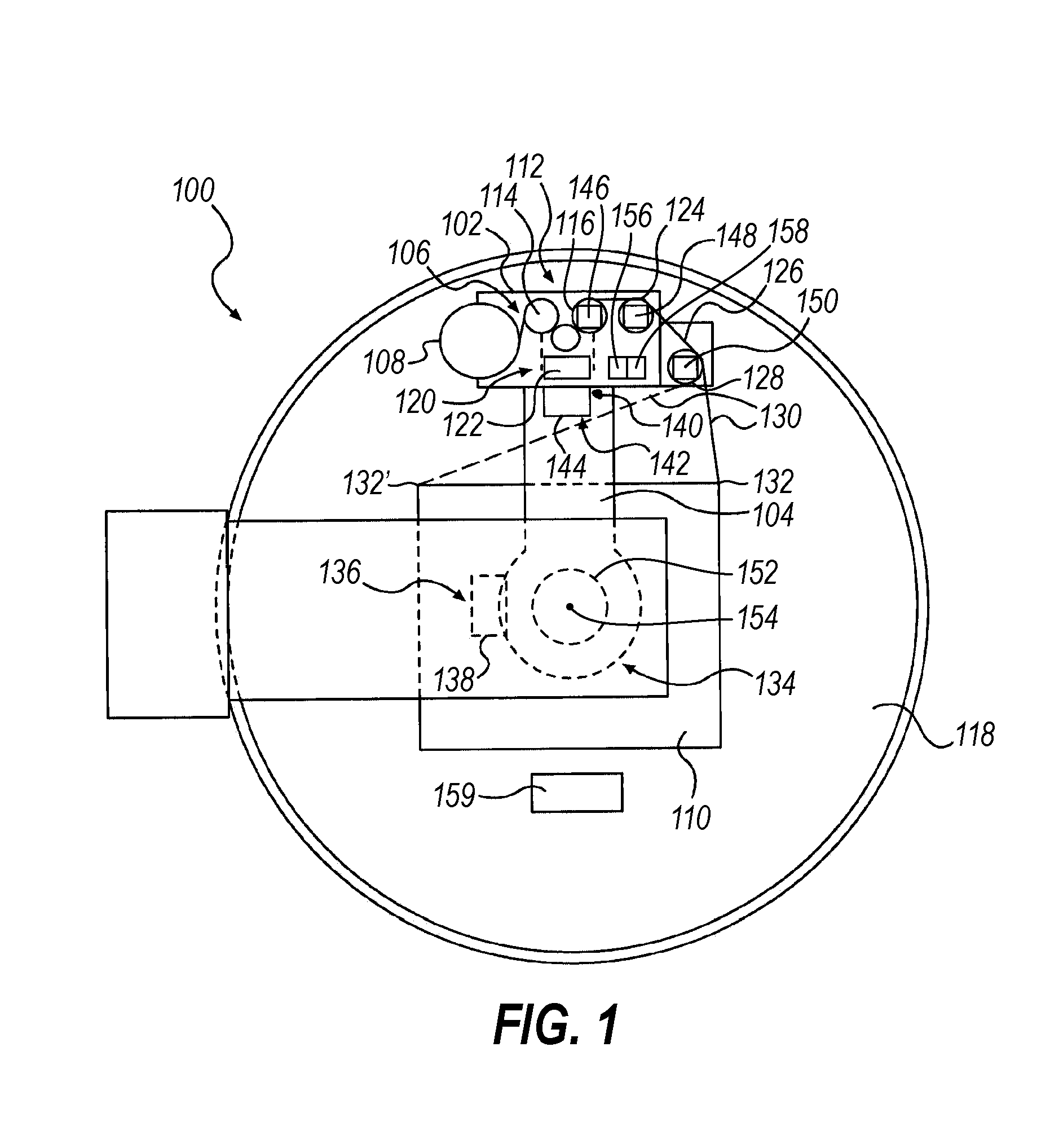
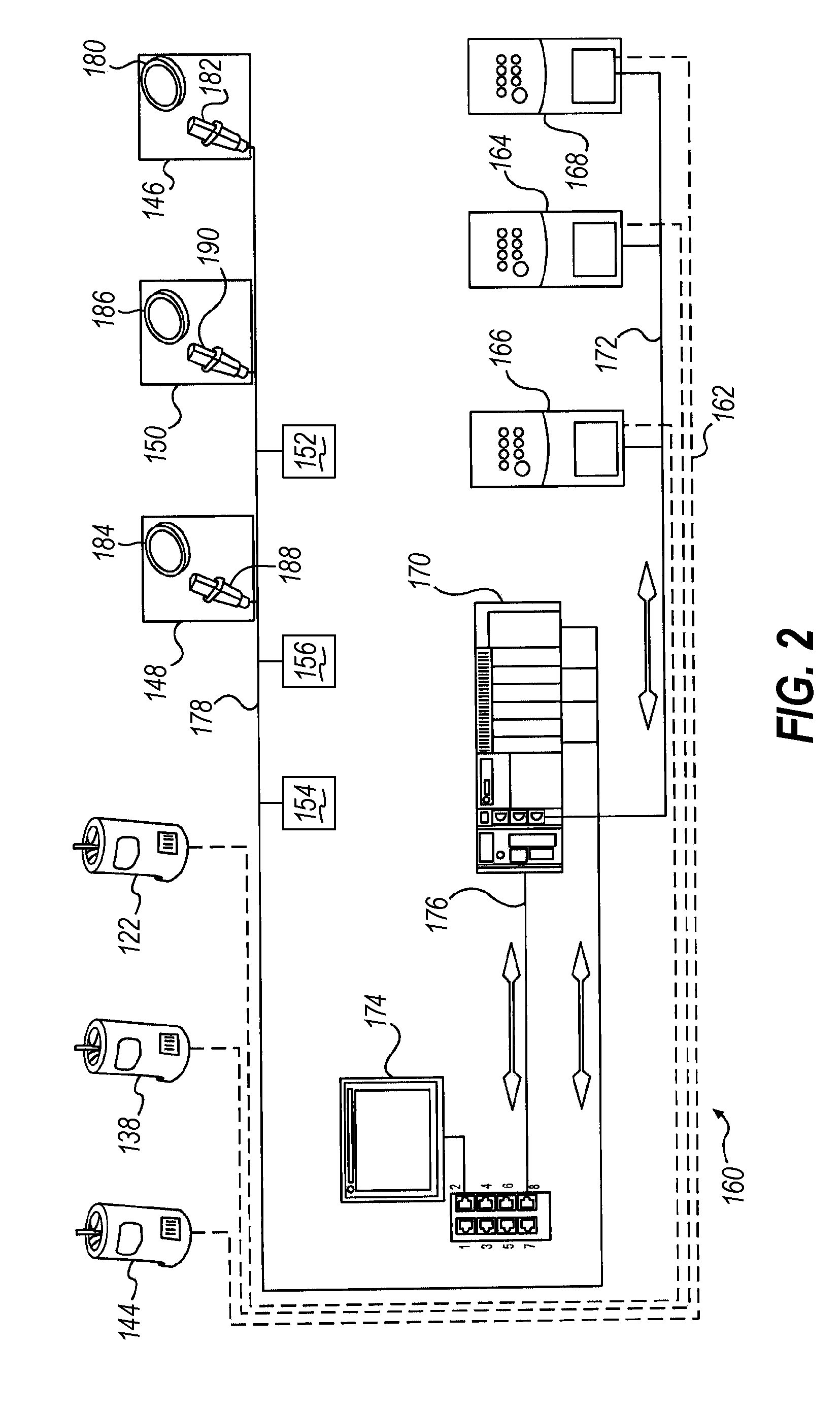
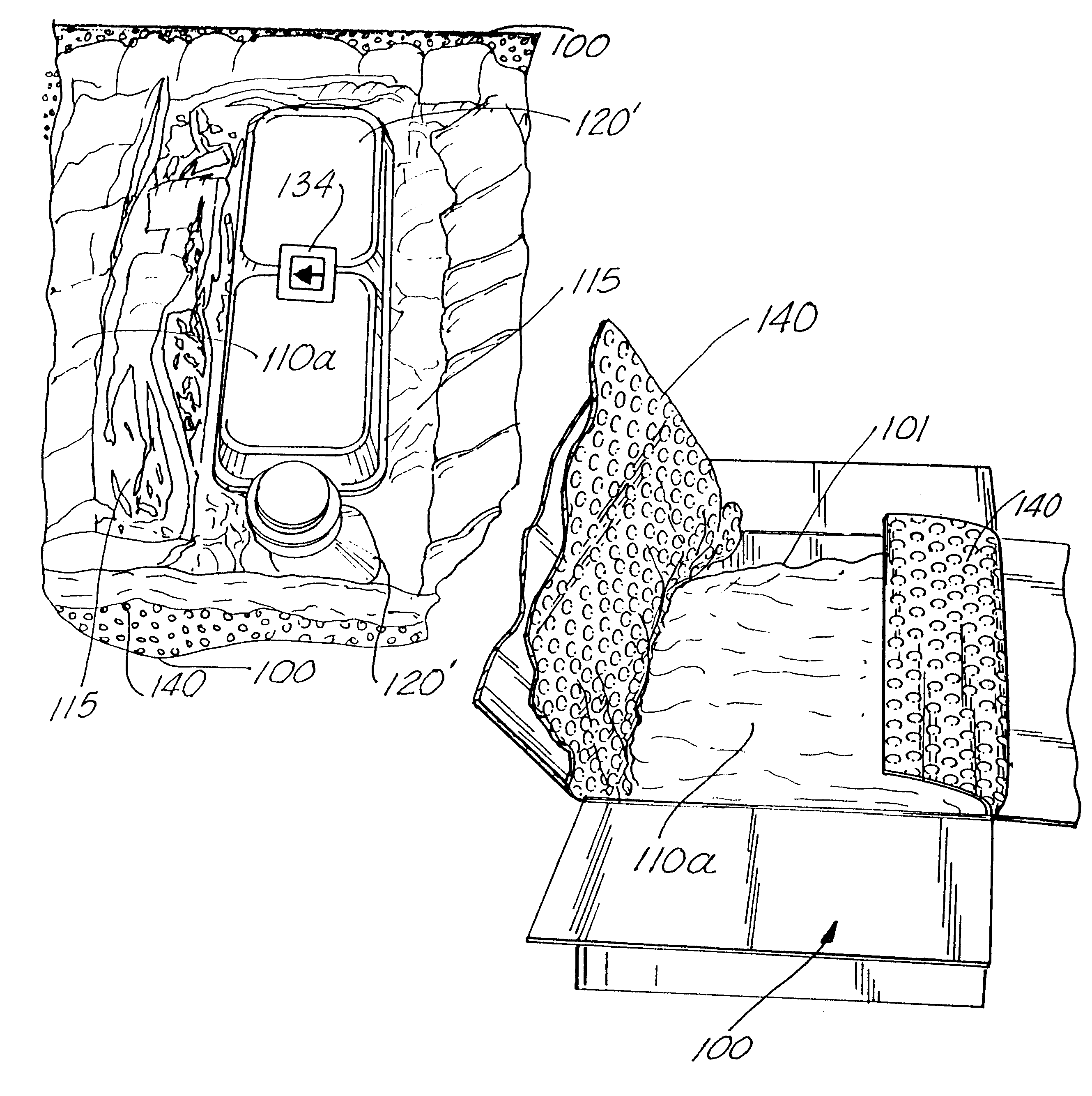
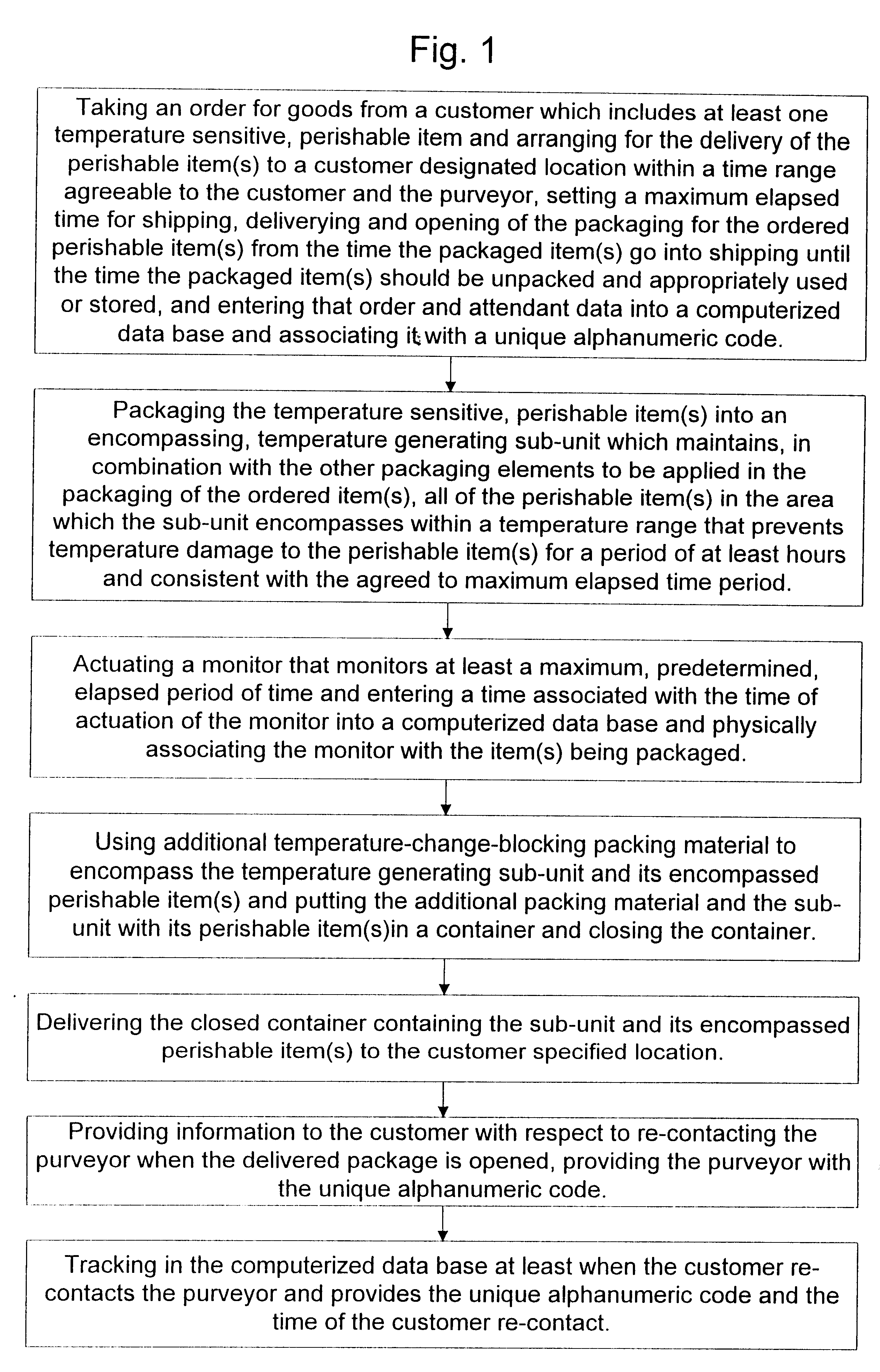
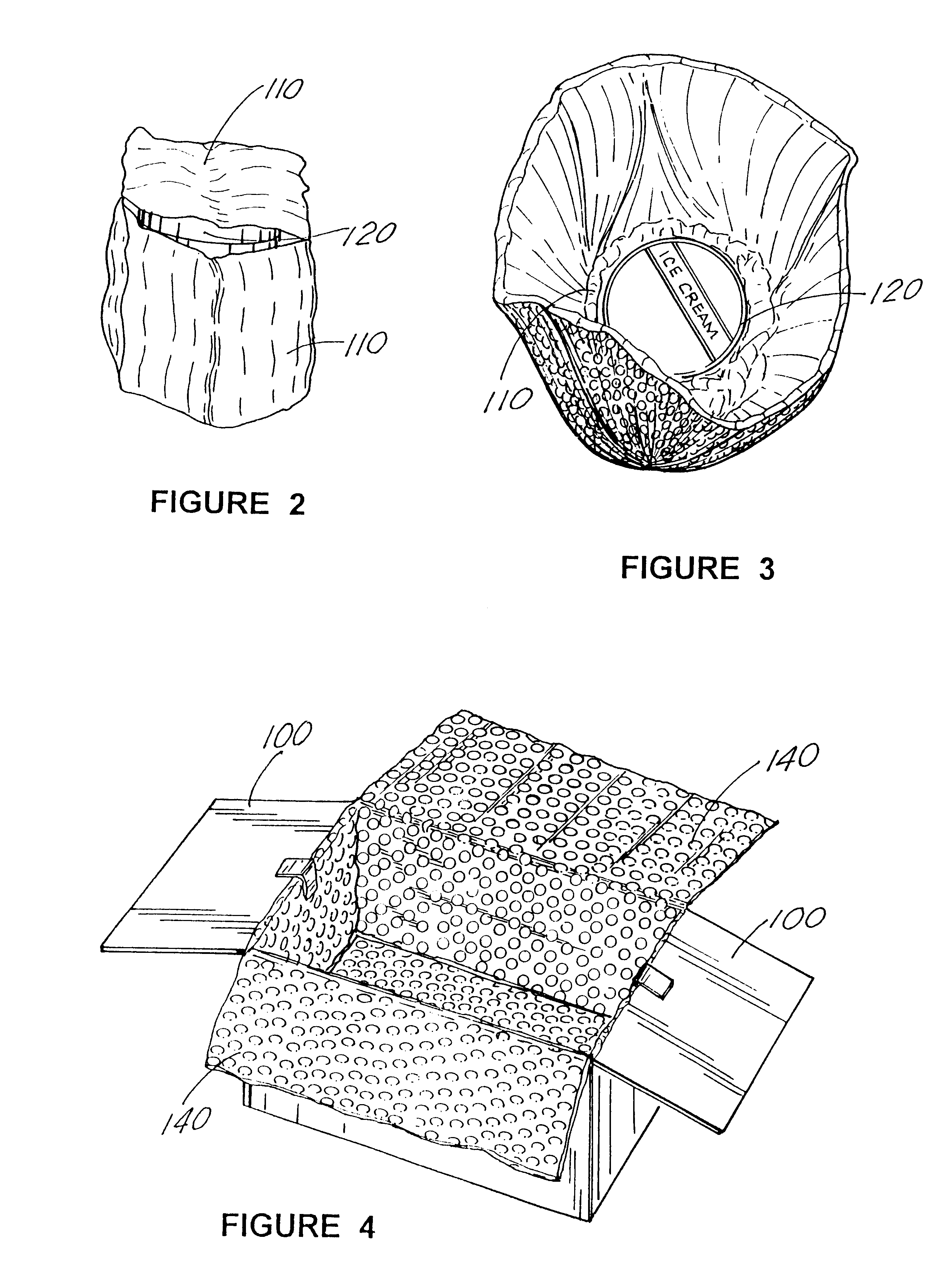
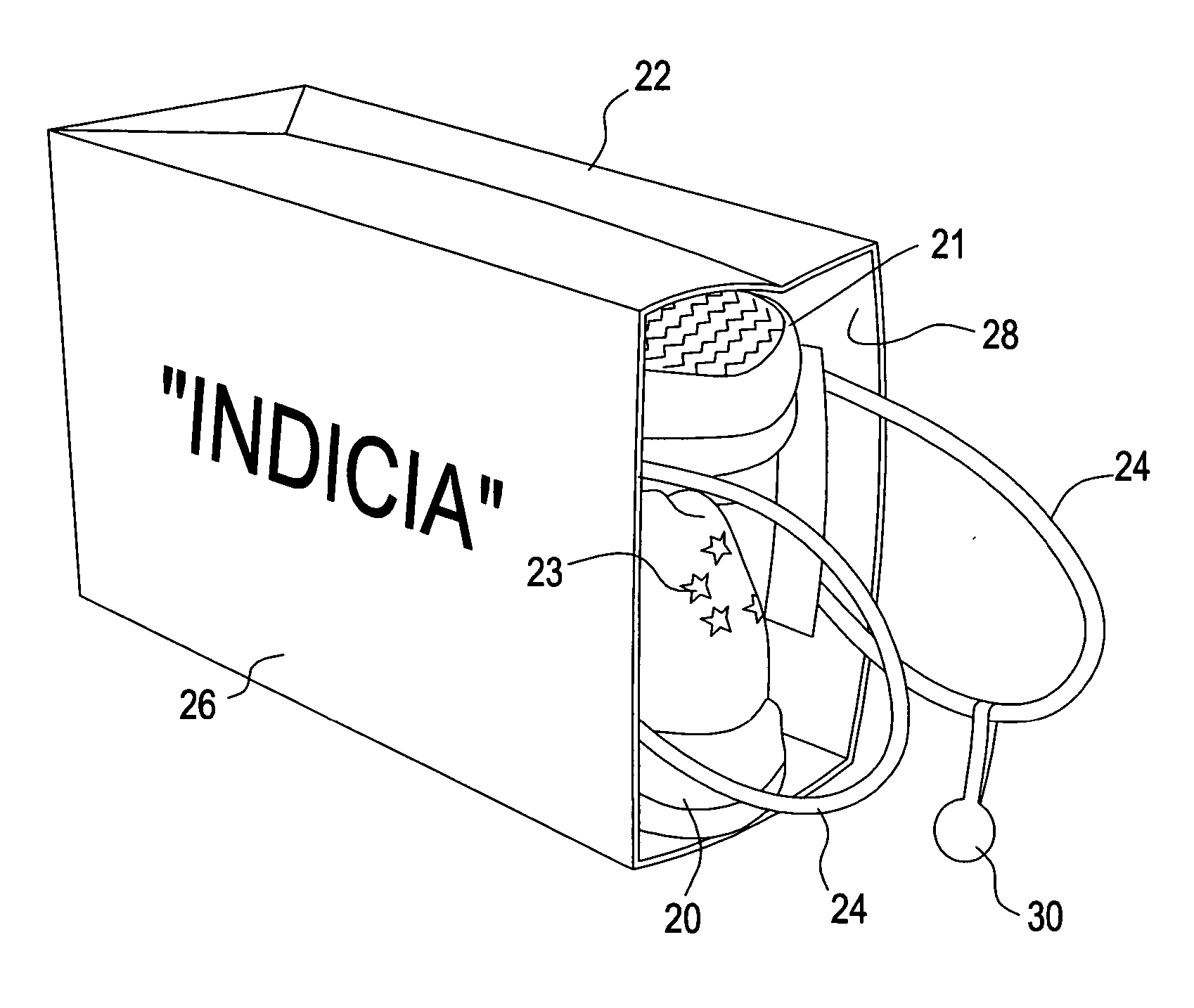
A "safe delivery"SM system for delivering perishable groceries (120 / 120'), including an inexpensive, corrugated cardboard box (100); a source of cold (or heat as needed) maintaining the temperature inside the box within a desired temperature range for hours, using an all encompassing pouch of packet material (110 / 10), used individually (FIGS. 2 & 3) or collectively (FIGS. 5 & 6), with each packet (17) containing a super-absorbent polymer (14, FIG. 12) which is hydrated (14', FIG. 12A) and then either frozen (e.g., in a freezer) or heated (e.g., in a microwave), without producing moisture as the polymer returns to its natural state; a protective cover (130) protecting the box and its contents from heat radiation (e.g., sunlight). Other components (e.g., bubble wrap 140, sealing tape 133) prevent heat attacking convection and / or conduction, with the cover having multiple plies with an outer metallized surface (131); a time / temperature monitoring alert (134) indicating when either a maximum predetermine temperature or a maximum allowed, elapsed time from packing to opening has been exceeded. If so, the customer knows that the perishable items are not warranted to be safe, and the customer is responsible for contacting the purveyor for a return of the goods. A computerized methodology (FIG. 1) insures that the purveyor knows at least approximately when the customer has opened the packed groceries, etc., using a predictive calculator and an automated tracking system, in which the customer is obligated to transmit a unique code, preferably through an automated telephonic or Internet system, when the package is opened.
Owner:THERMAFREEZE PRODS CORP
Protective Box for Surgery
ActiveUS20140346072A1Readily availableContamination damageSurgical furnitureDiagnosticsSurgical GraftSTERILE FIELD
The present invention is directed to providing a sterile container protecting various surgical grafts, implants and devices from incidental contamination and damage during operative procedures. The design includes an inner box to contain the items, and an outer box to protect the exterior of the inner box from contamination in the event of fall or other contamination, such as from airborne particles or liquids, fall from the sterile field, or by non-sterile handling. In the event of fall or contamination of the outer box, the surgical staff will be able to recover the inner box and its contents in sterile and unspoiled condition without difficulty.
Owner:JACOBSON DANIEL R
System and method for footwear packaging
Owner:NIKE INC
Process and apparatus for the cost-effective packaging of polysilicon fragments
InactiveUS7013620B2Cost-effectiveHigh purityMultiple wrapper applicationSolid materialPolycrystalline siliconElectrical and Electronics engineering
Owner:WACKER CHEM GMBH
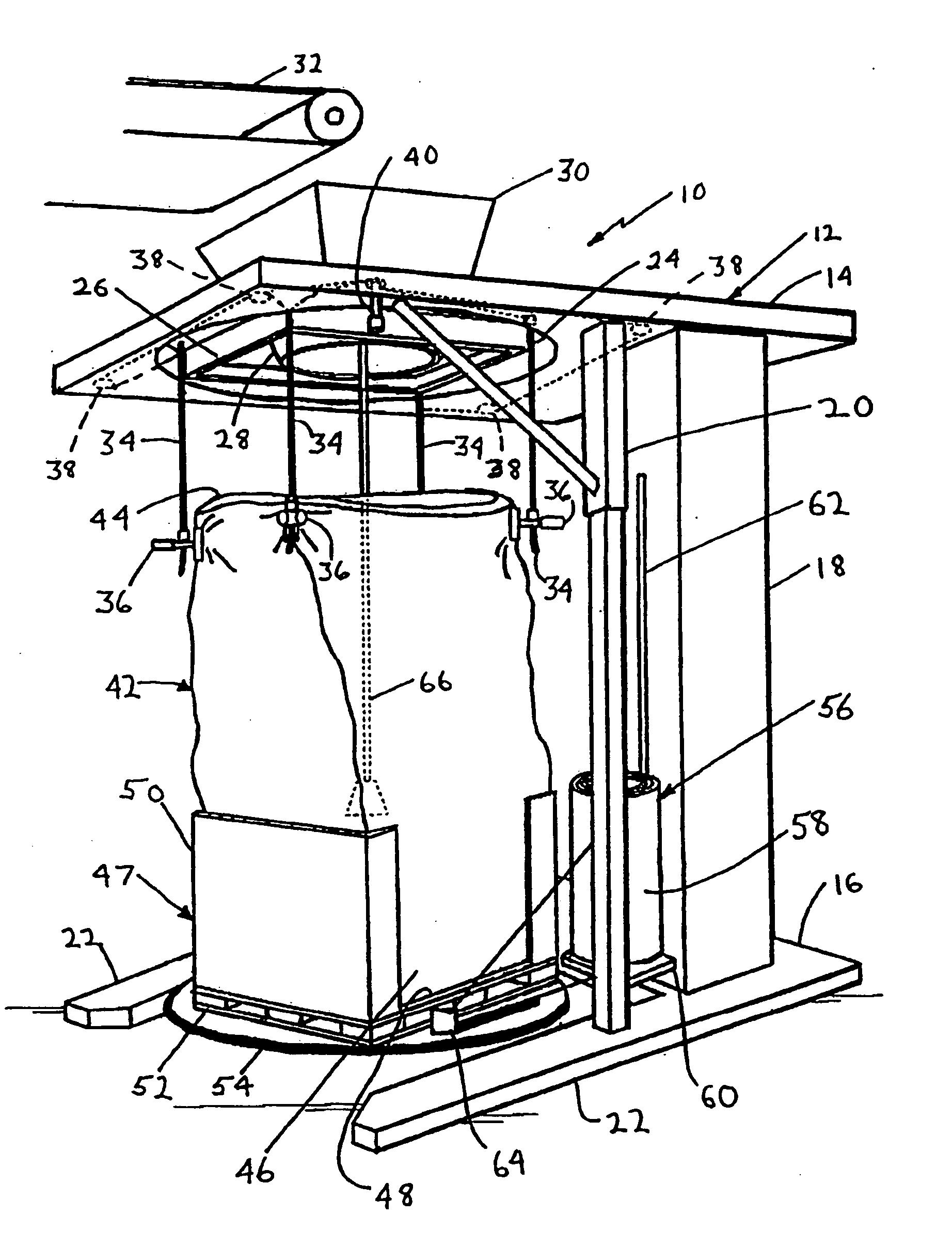
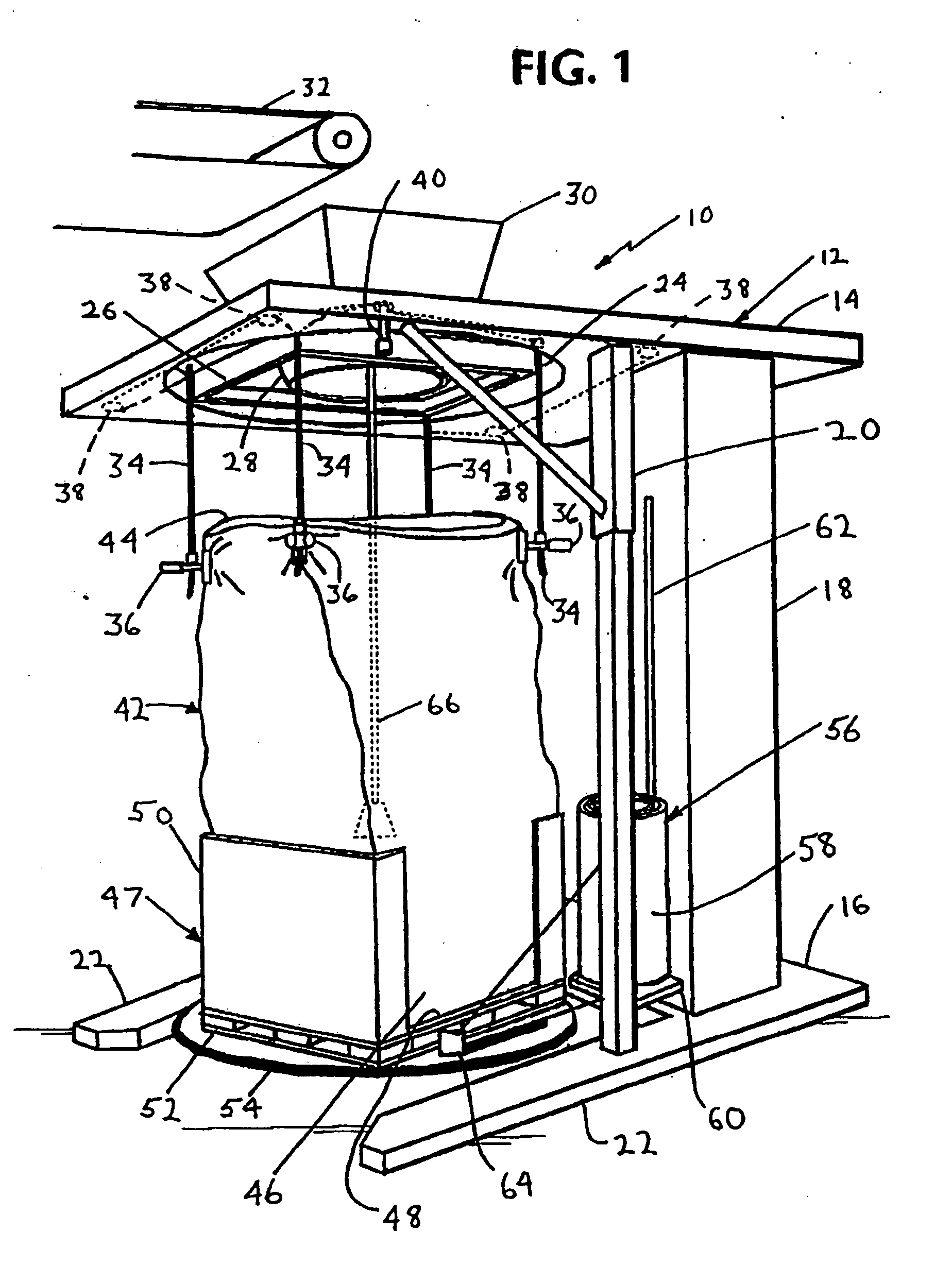
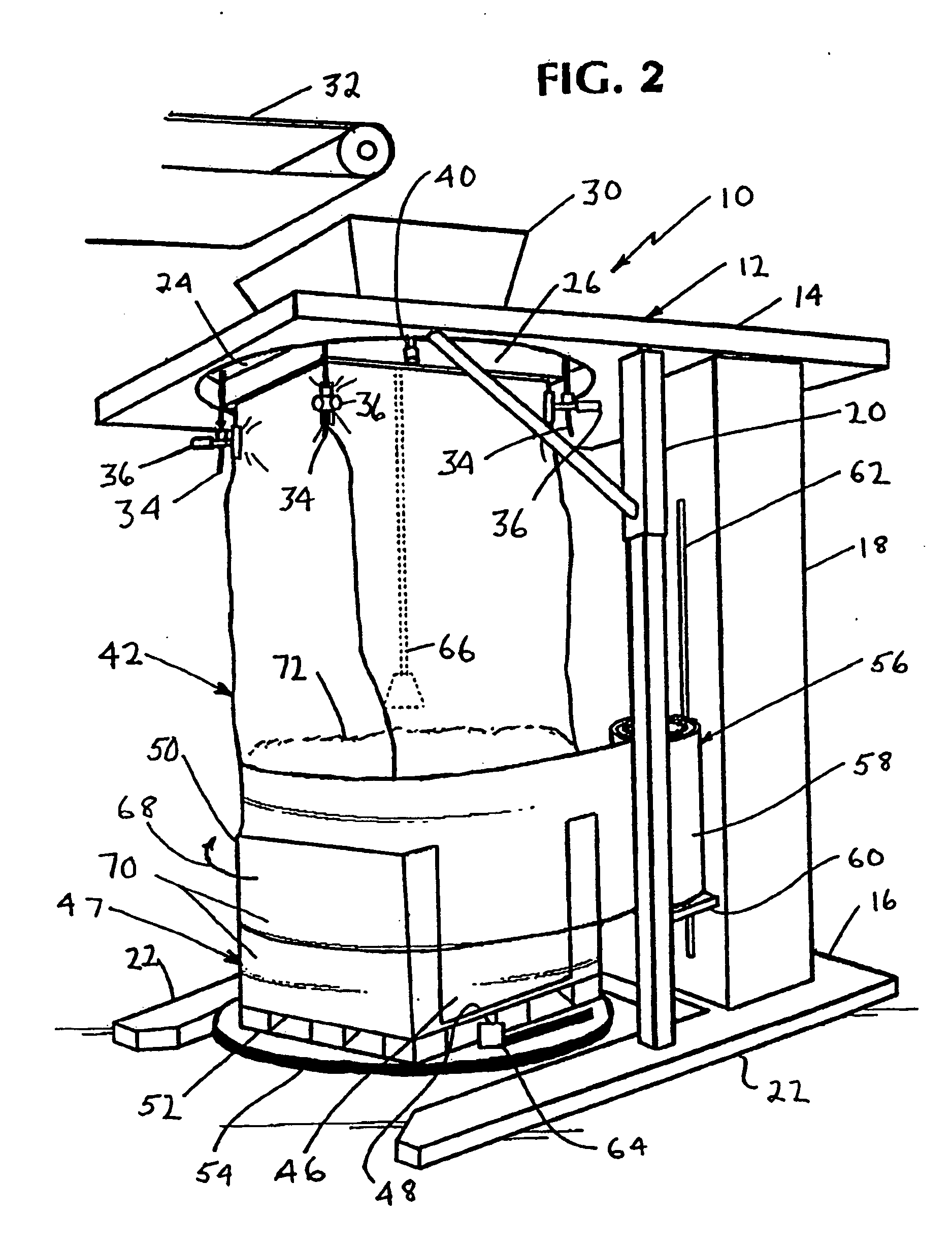
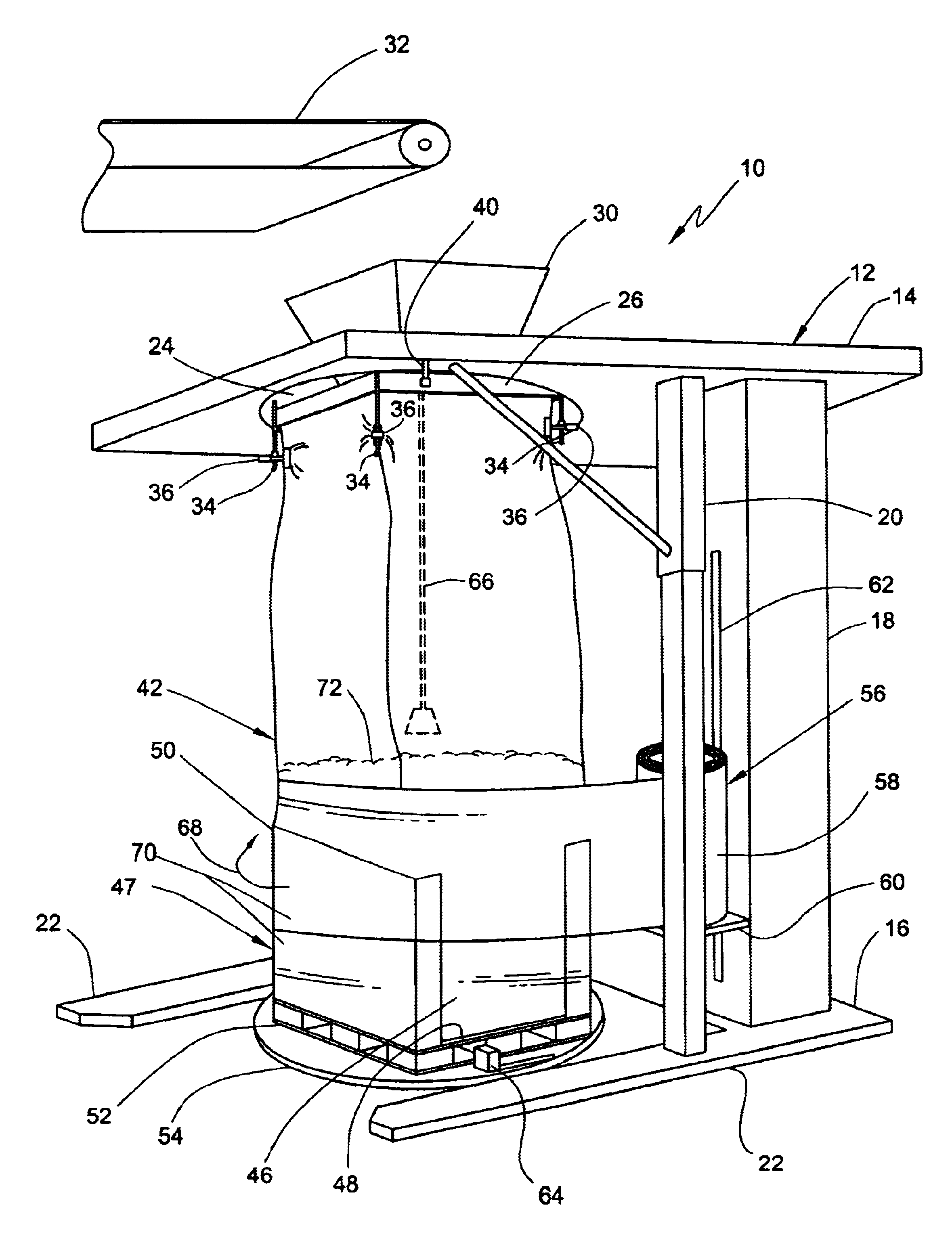
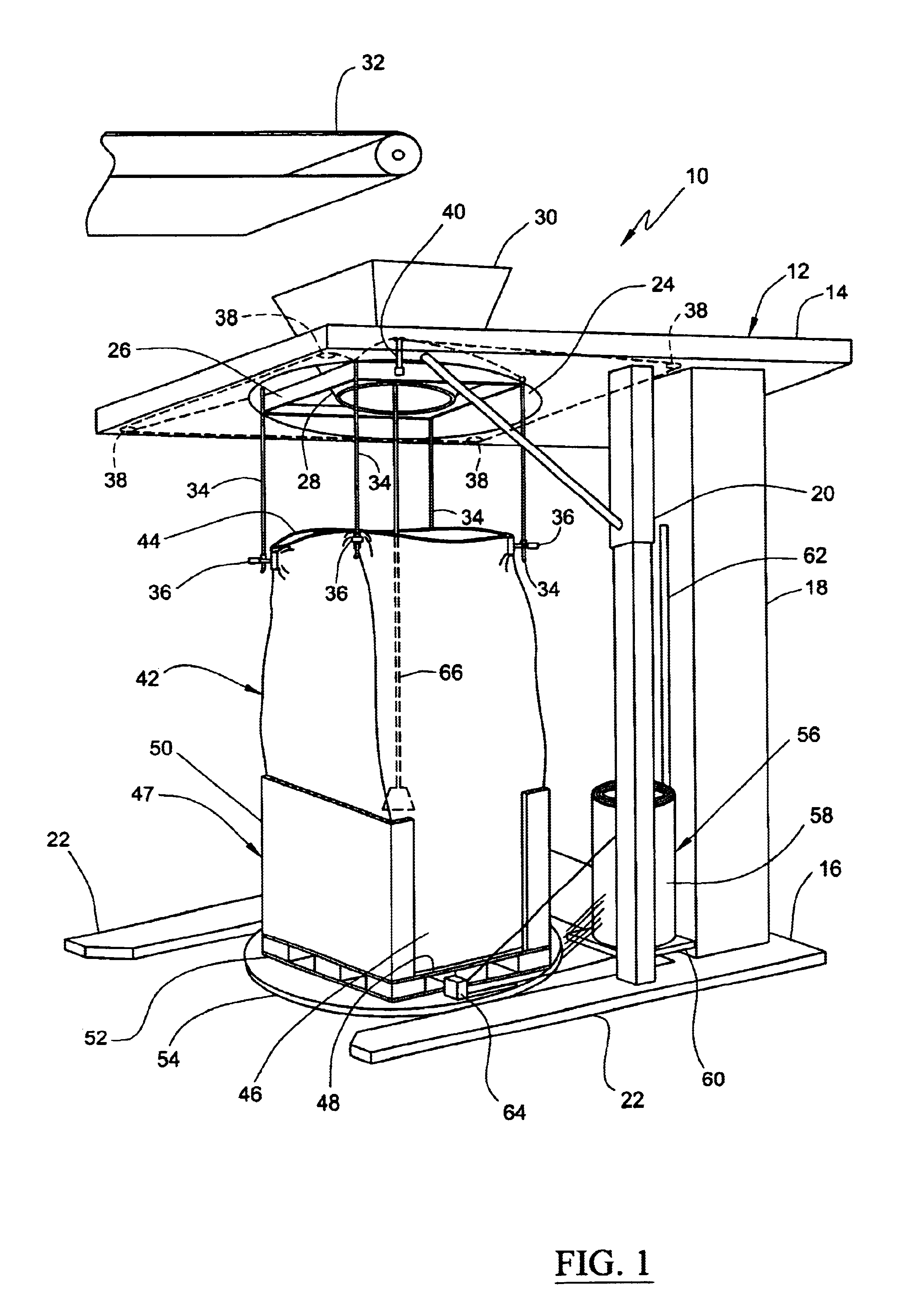
Method for forming a transportable container for bulk goods
InactiveUS20060151059A1Cut large diameterLiquid fillingMultiple wrapper applicationEngineeringVolumetric Mass Density
The invention provides a method for filling a container with a plurality of particles. The method includes the step of filling a radially flexible container through a large diameter with a plurality of particles to a fill level. The method also includes the step of reducing the large diameter of the radially flexible container to a smaller fill diameter in vertical relationship to the fill level as the fill level rises during filling of the flexible container. The method also includes the step of varying the vertical relationship between the fill level and the smaller fill diameter in response to the density of the particles.
Owner:KELLOGG CO
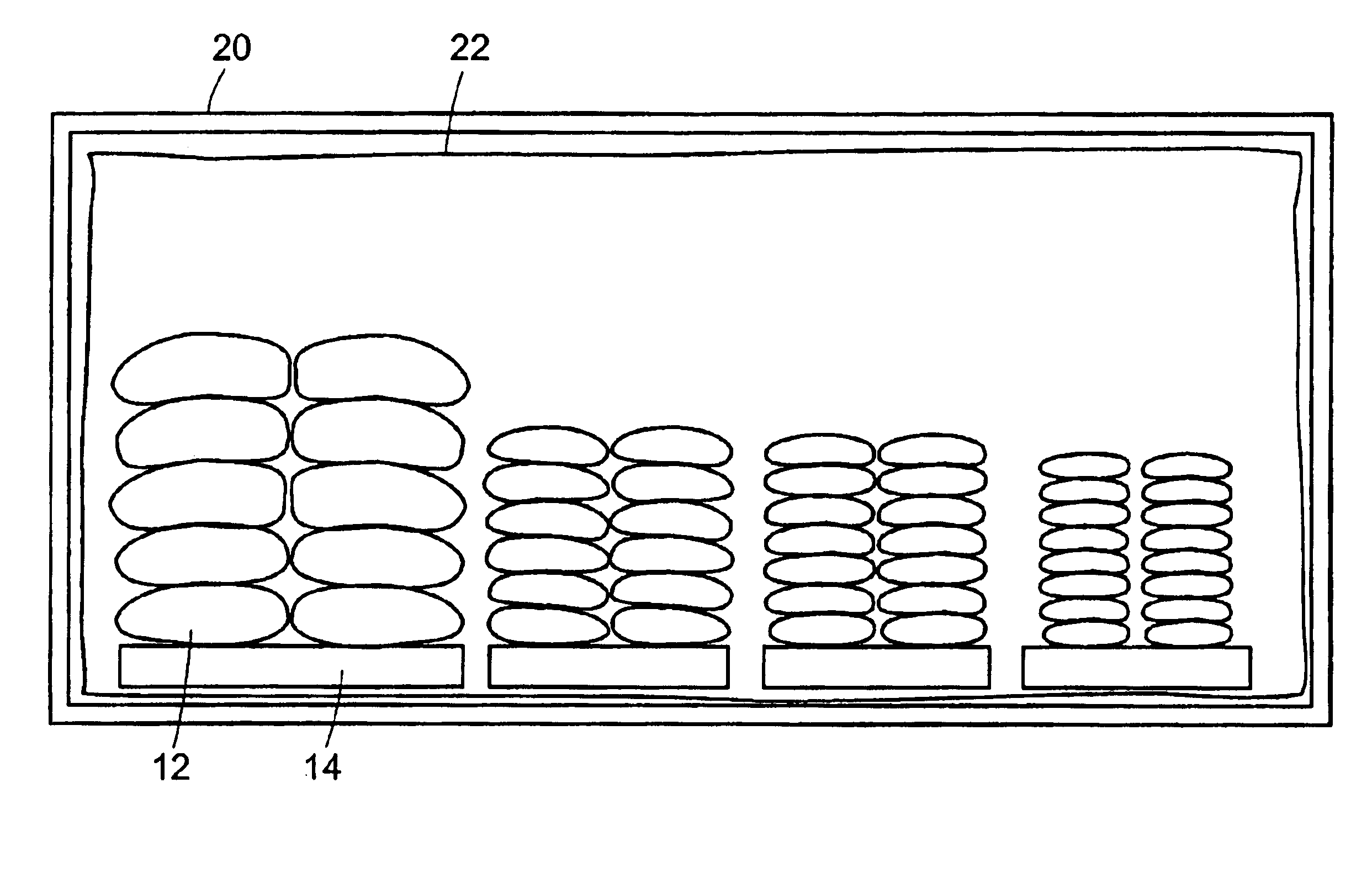
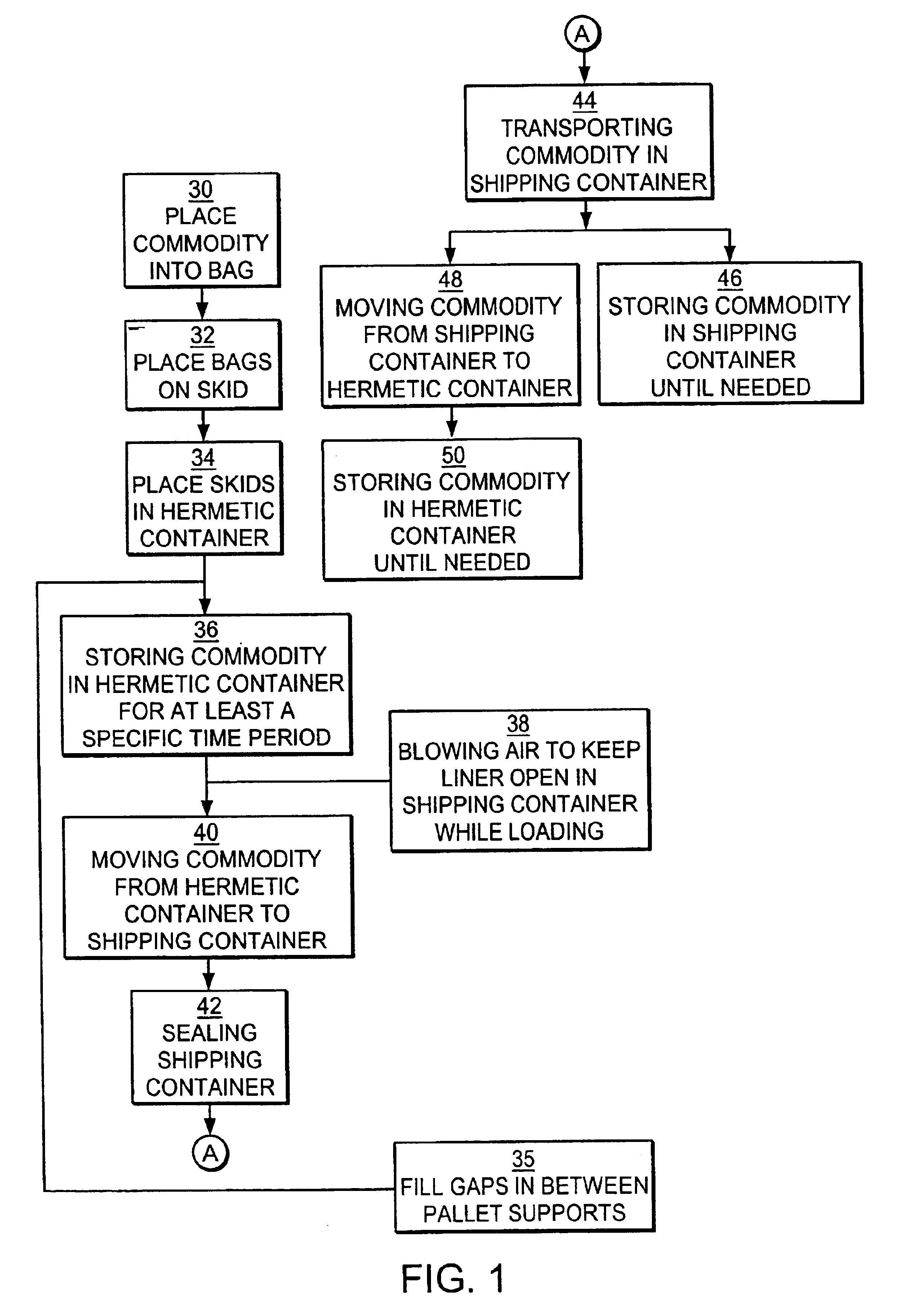
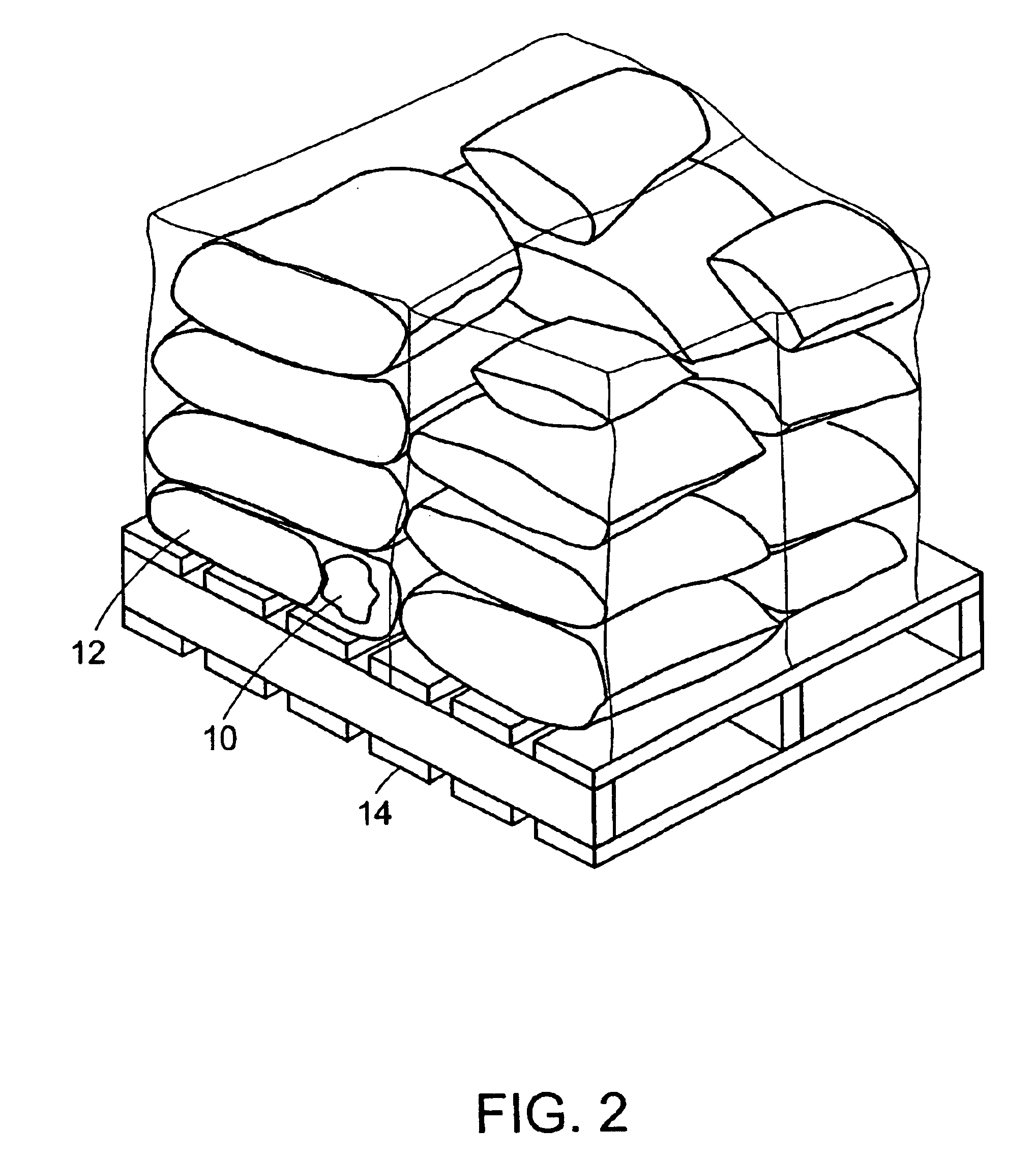
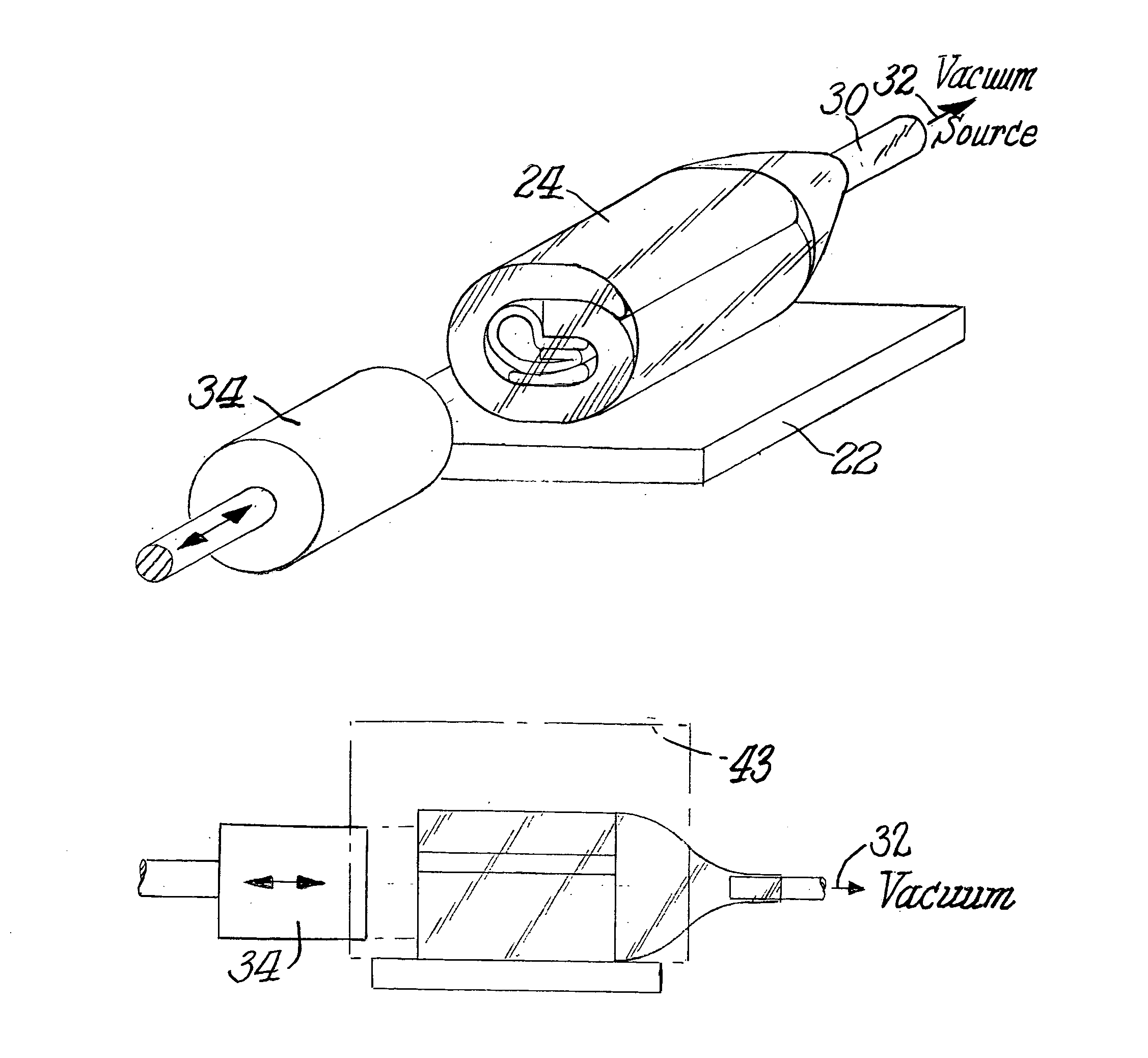
Method and system for transporting and storing commodities
InactiveUS6941727B2Shorten the time periodReduce the amount requiredWrapping material feeding apparatusFood preservationSufficient timeProduct gas
A method and a system of transporting a commodity includes the step of placing the commodity in a flexible hermetic container. The commodity is stored in the flexible hermetic container for at least a specific time period. The commodity is then moved from the flexible hermetic container to a shipping container having an insect barrier liner. The commodity is transported in the shipping container. The specific time period is a sufficient time period to kill substantially all of the insects that have infested the commodity prior to placement in the flexible hermetic container. In one embodiment, a vacuum is drawn on the flexible hermetic container to reduce the specific time period and an optional small amount of pesticide can be injected into the flexible hermetic container to which a vacuum has been applied to further reduce the specific time period. An inert gas is used to reduce the specific time period.
Owner:GRAINPRO +1
Method for packaging multi-component bedding assembly
Owner:FOAMEX LP
Method and apparatus for preventing luggage mishandling in public transportation systems
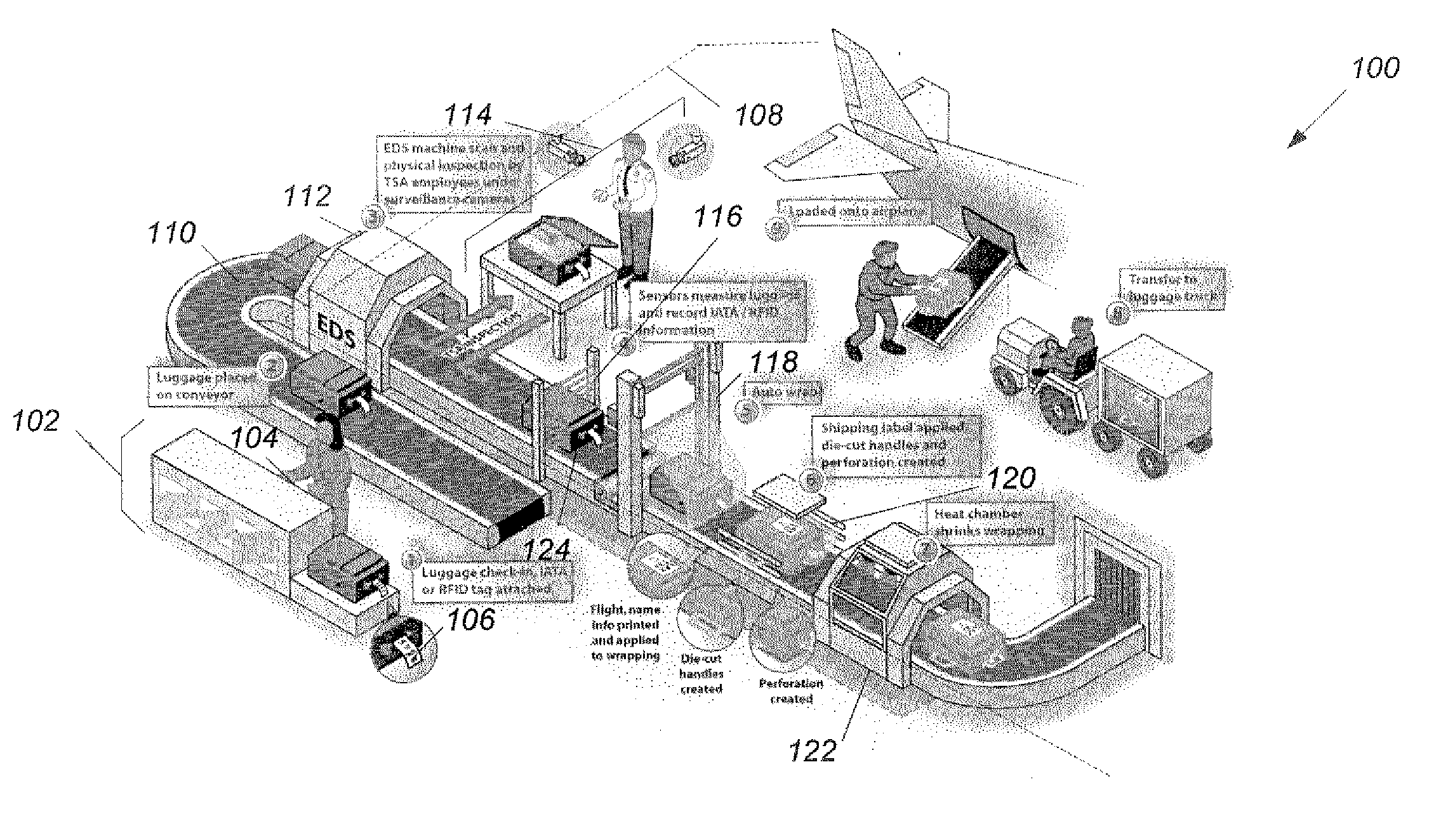
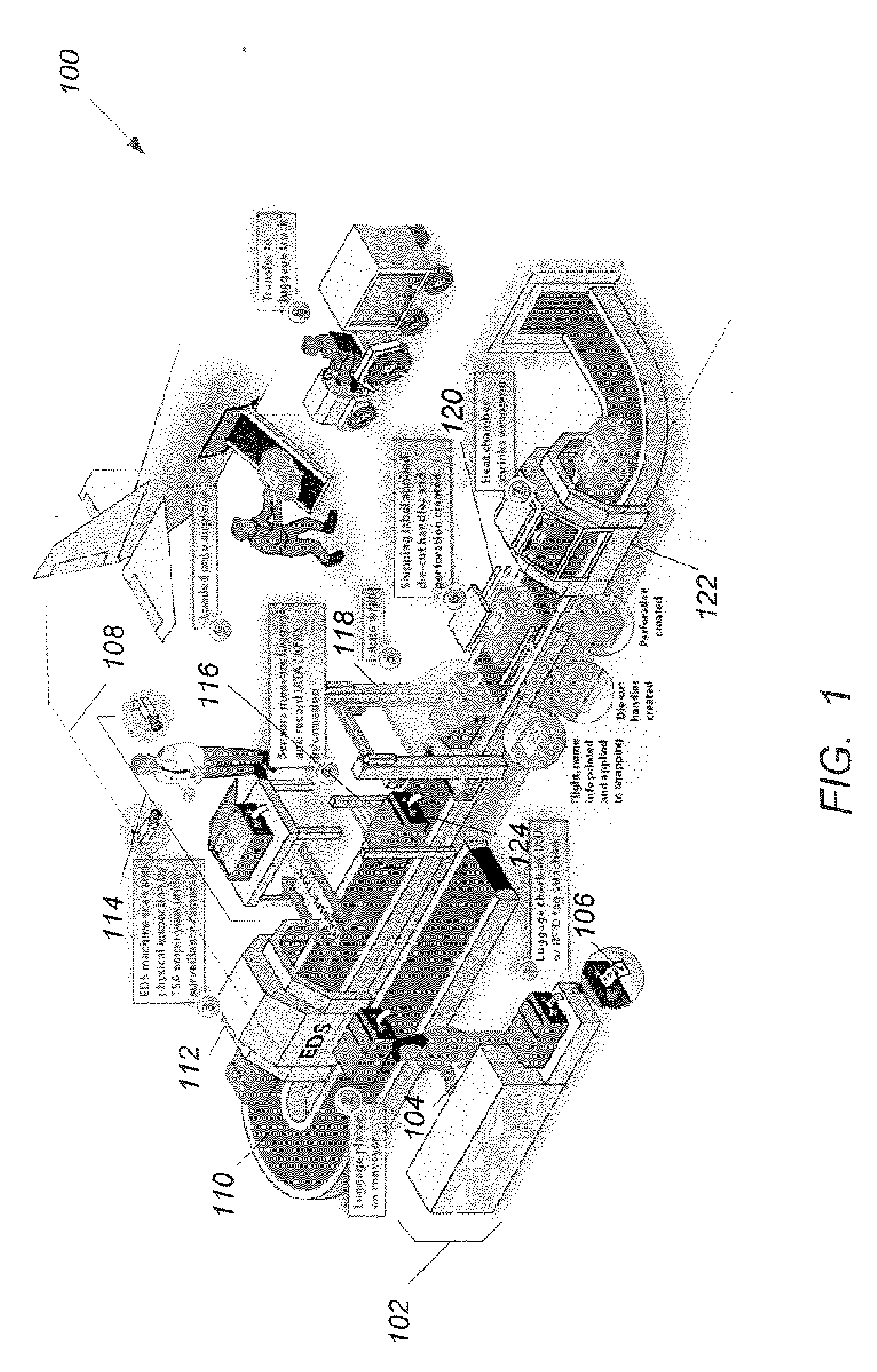
InactiveUS20070109127A1Wrapper folding/bending apparatusMultiple wrapper applicationEngineeringPublic transport
A method of protecting luggage from tampering includes steps of receiving a piece of luggage at a baggage check-in station (1302), and encoding passenger and routing information at the baggage check-in station onto an identification tag (1304). The identification tag is attached to the piece of luggage (1306). The piece of luggage is placed on a baggage conveyor and conveyed to a security inspection station inside a secure area that excludes all unauthorized s personnel (1308). The piece of luggage is conveyed on the baggage conveyor to a scanning station to detect and decode the passenger and routing information on the identification tag (1310). The piece of luggage is conveyed on the baggage conveyor to a wrapping station (1312). When an identification tag is detected, the method continues from step (1316), otherwise from step (1322). When the identification tag indicates that the piece of luggage is to be wrapped, the method continues from step (1320), otherwise from step (1322). The piece of luggage is wrapped automatically (1320). The piece of luggage is conveyed through the wrapping station to a baggage receiving station (1322).
Owner:JOHNSON JEFFREY DOUGLAS
Process and packaging for a garment having a desired sterility assurance level
InactiveUS20070084145A1Reduces and limit bioburdenReduces and limit and diversityPackage sterilisationLavatory sanitoryCartonSterility assurance level
A packaging for a garment and the process for forming same is disclosed. The process reduces or limits bioburden and diversity of genome on a garment. At least one non-sterile garment is placed in a heat sealable bag, a vacuum is formed in the bag and thereafter, the bag is sealed by heat sealing. The bag is then placed in a carton liner and the carton liner is closed. This defines an assembly. The assembly is placed in a carton, and the carton is closed. Thereafter, the carton containing said assembly therein is irradiated to a desired Sterility Assurance Level. All of the steps may be performed in a clean room, or the steps prior to forming the assembly are performed in the clean room and the steps after assembly are performed outside of the clean room.
Owner:ALPHA PRO TECH
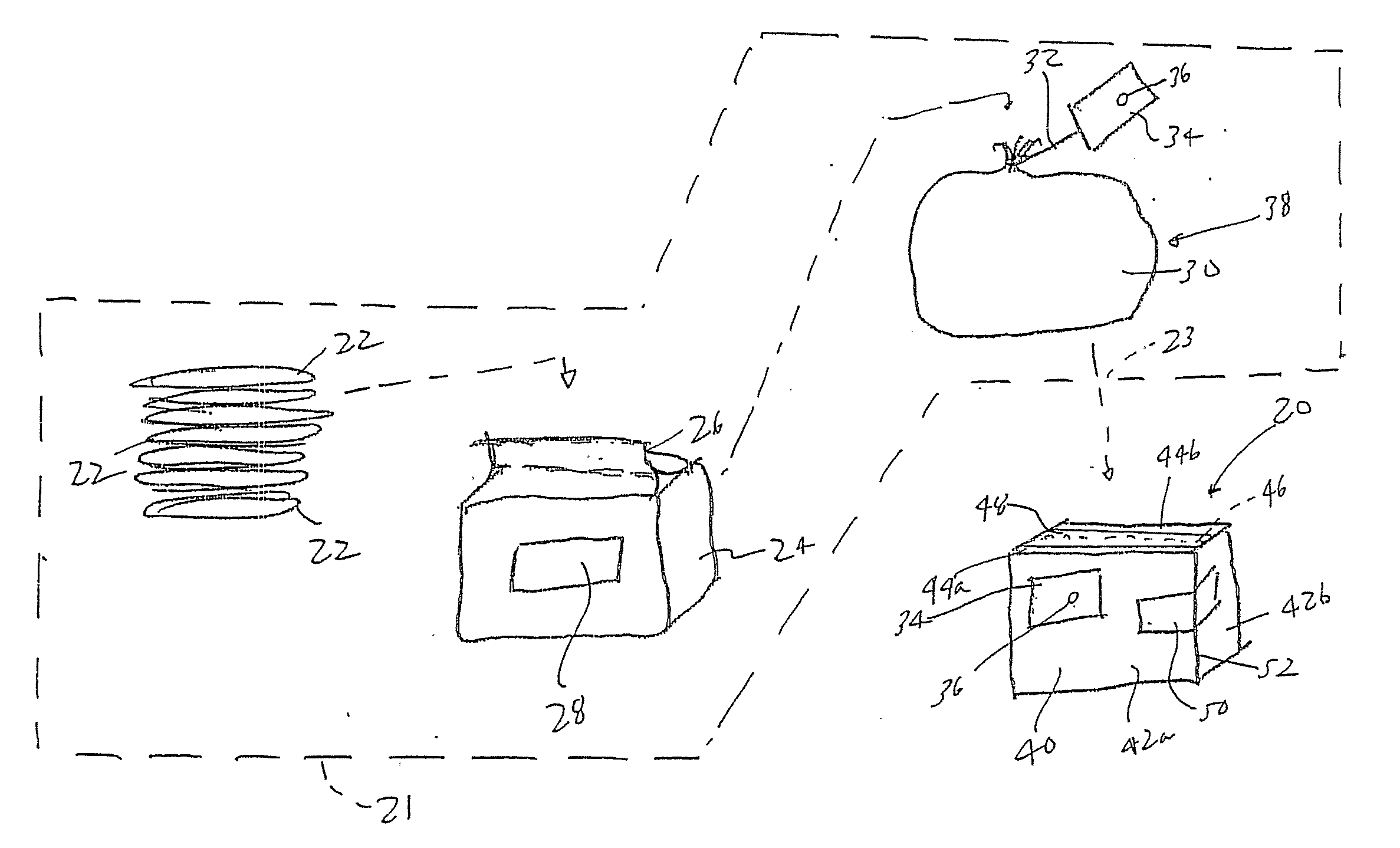
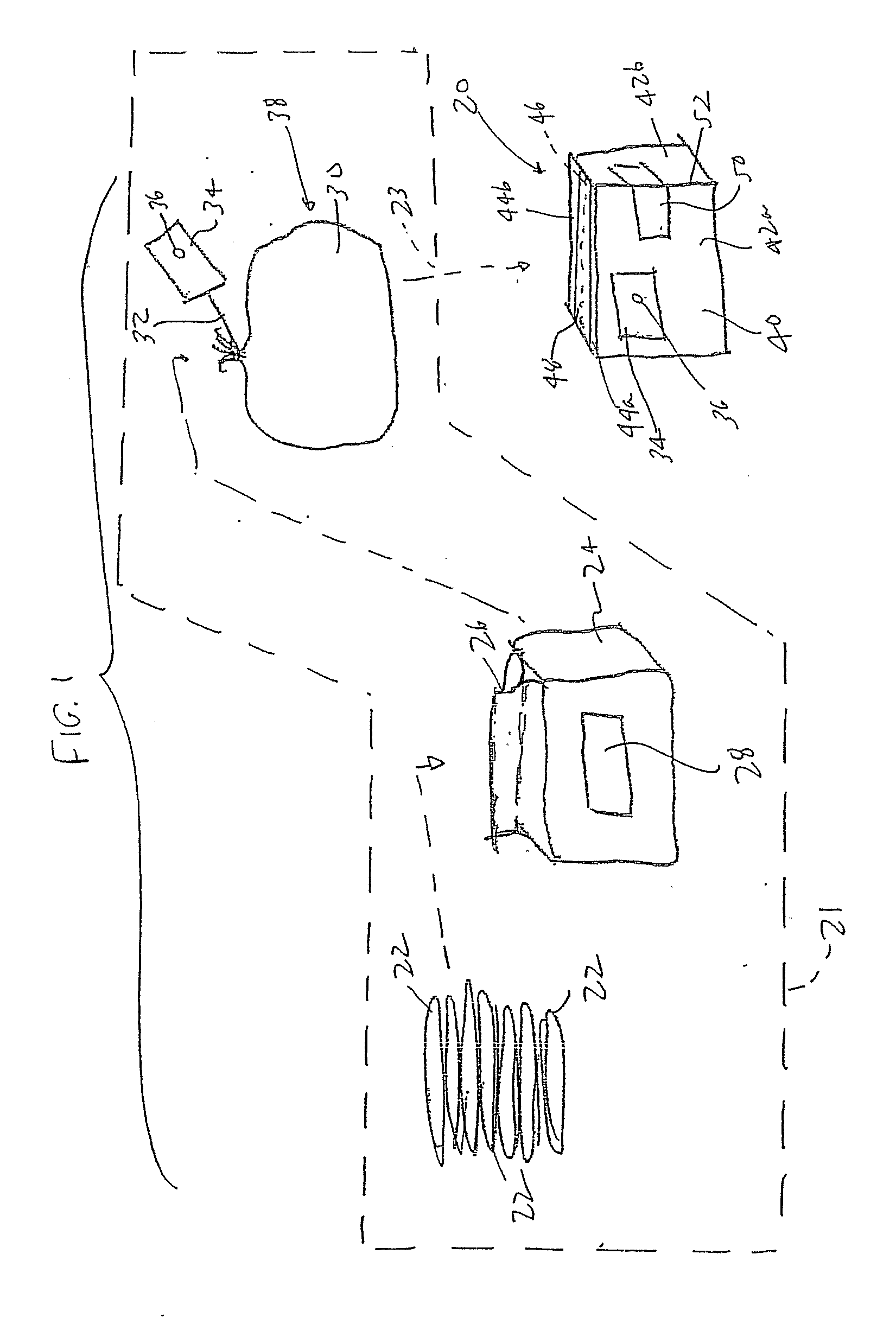
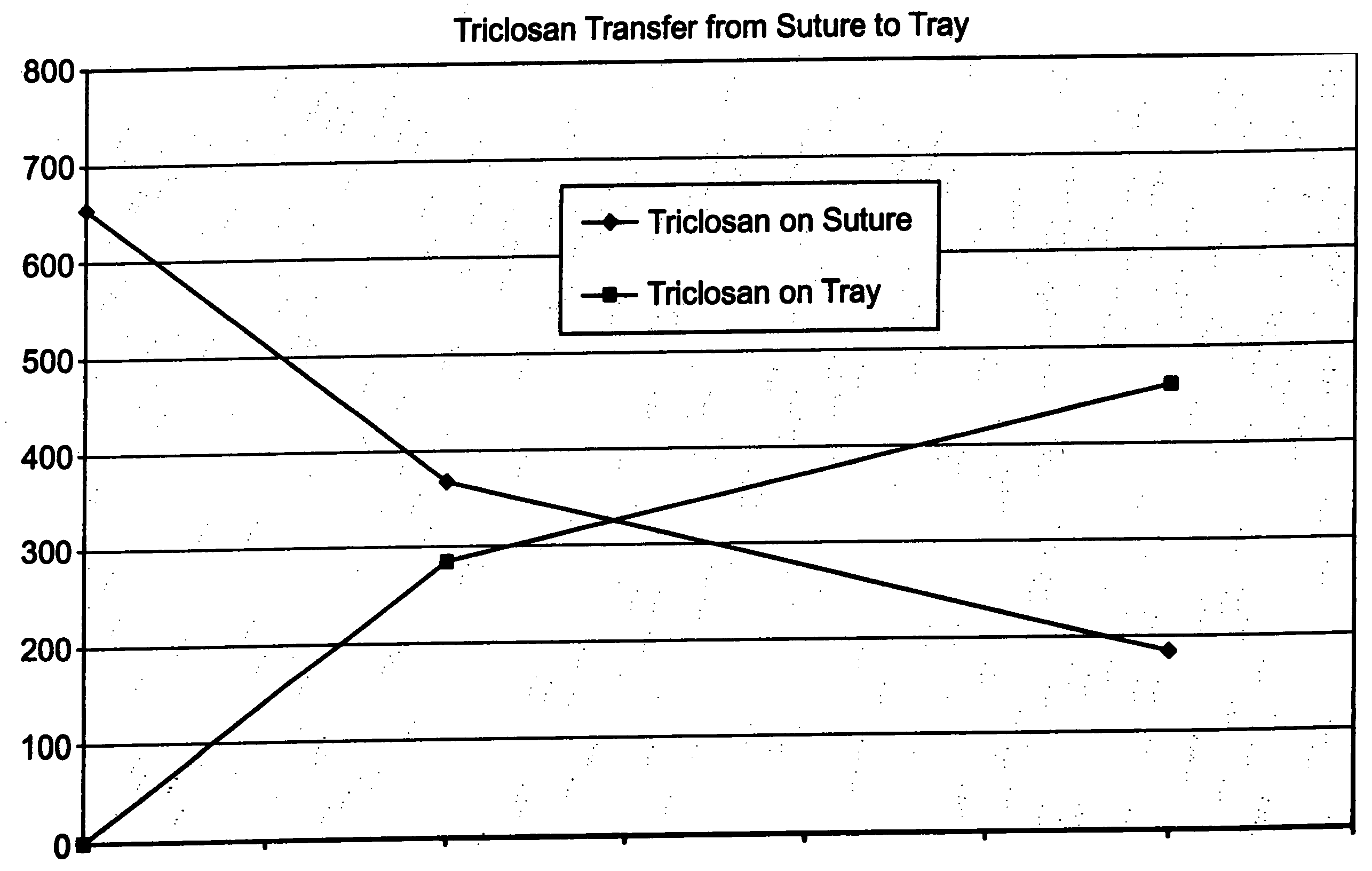
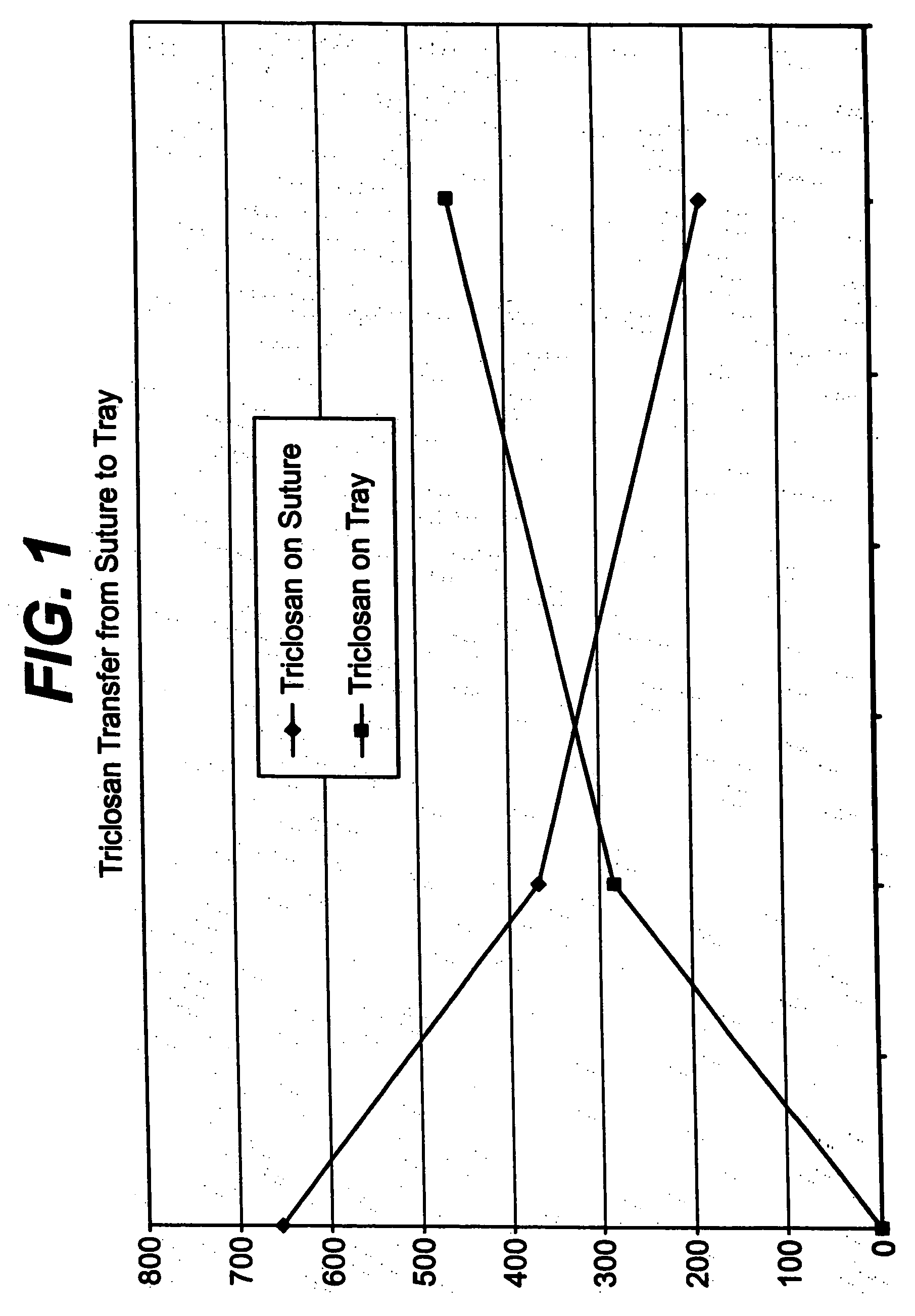
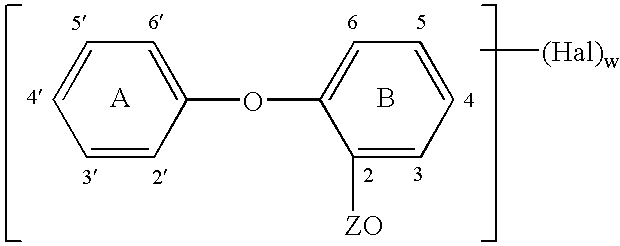
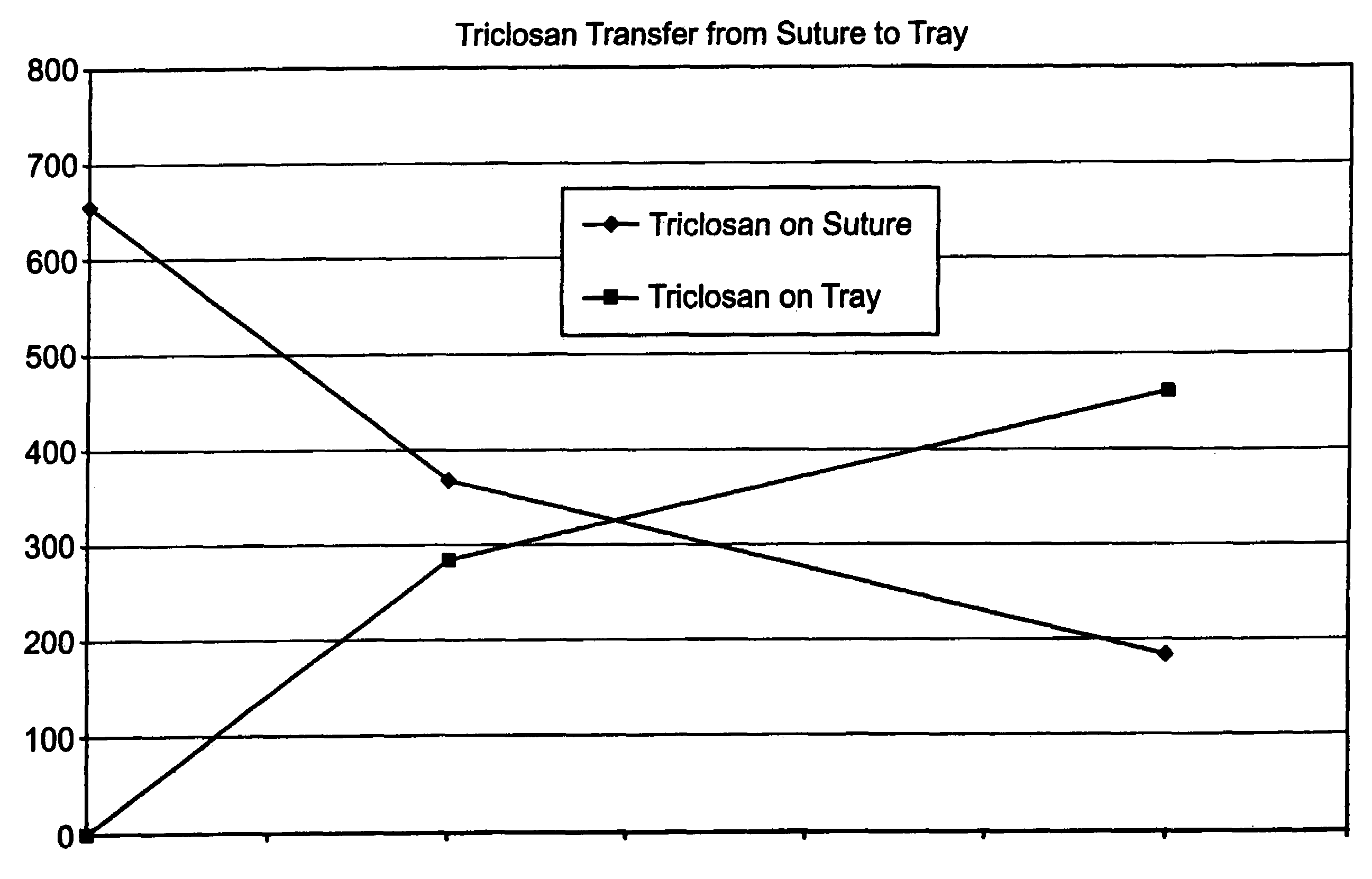
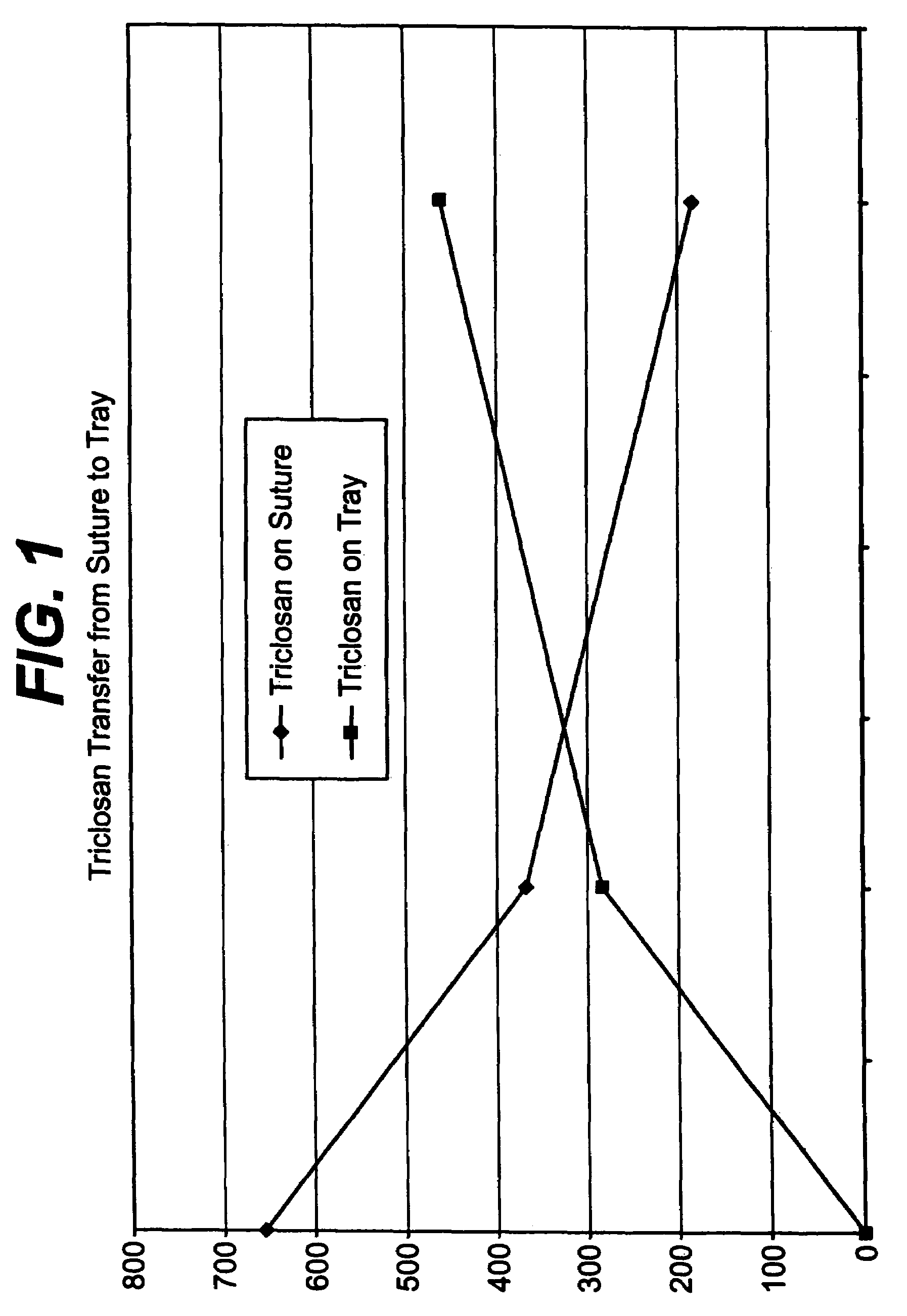
Method of preparing an antimicrobial packaged medical device
InactiveUS20060091034A1Prevent bacterial growthInhibit bacterial colonizationSuture equipmentsSurgical furnitureEtherBacterial colonization
A method for making an antimicrobial suture comprising the steps of positioning an antimicrobial agent source within a package comprising an inner surface, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; positioning a medical device within the package; and subjecting the package, the antimicrobial agent source and the medical device to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device, thereby substantially inhibiting bacterial colonization on the medical device. Alternatively, the packaged medical device is produced according to the steps of positioning a medical device within a package; exposing the package having the medical device to an antimicrobial agent source; and subjecting the package having the medical device and the antimicrobial agent source to time, temperature and pressure conditions sufficient to transfer an effective amount of the antimicrobial agent from the antimicrobial agent source to the medical device within the package, thereby substantially inhibiting bacterial colonization on the medical device.
Owner:ETHICON INC
Method of preparing a packaged antimicrobial medical device
A method of making a packaged antimicrobial suture comprising the steps of providing a containment compartment that is substantially free of an antimicrobial agent; positioning a suture within the containment compartment, said suture comprising one or more surfaces having an antimicrobial agent disposed thereon, said antimicrobial agent being selected from the group consisting of halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof; placing the containment compartment having the suture in an outer package; and subjecting the outer package, the containment compartment and the suture to time, temperature and pressure conditions sufficient to vapor transfer an effective amount of the antimicrobial agent from the suture to the containment compartment, while retaining an effective amount of said antimicrobial agent on the suture, thereby substantially inhibiting bacterial colonization on the suture and the containment compartment.
Owner:ETHICON INC
Tamper evident food packaging
Owner:MARS INC
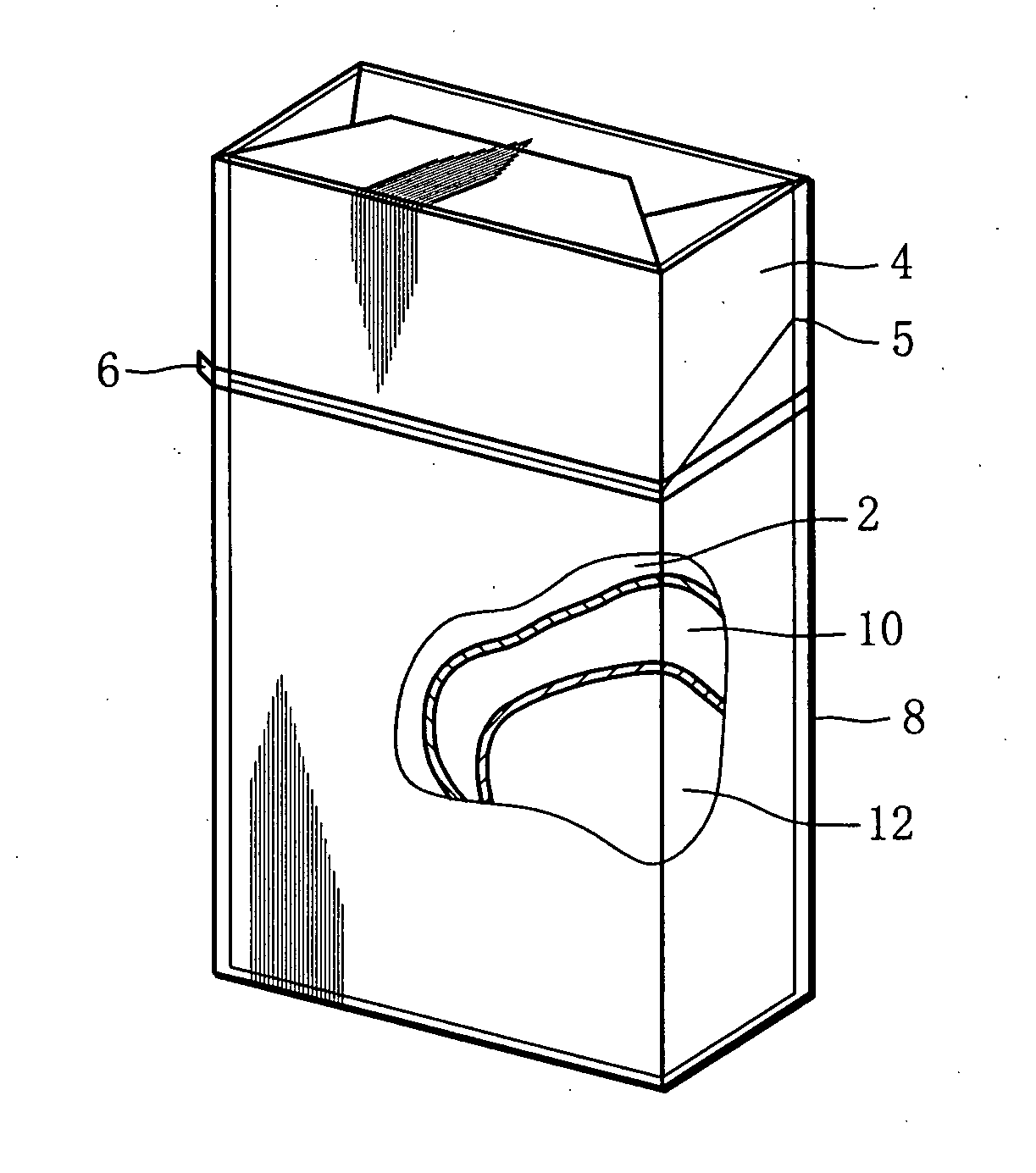
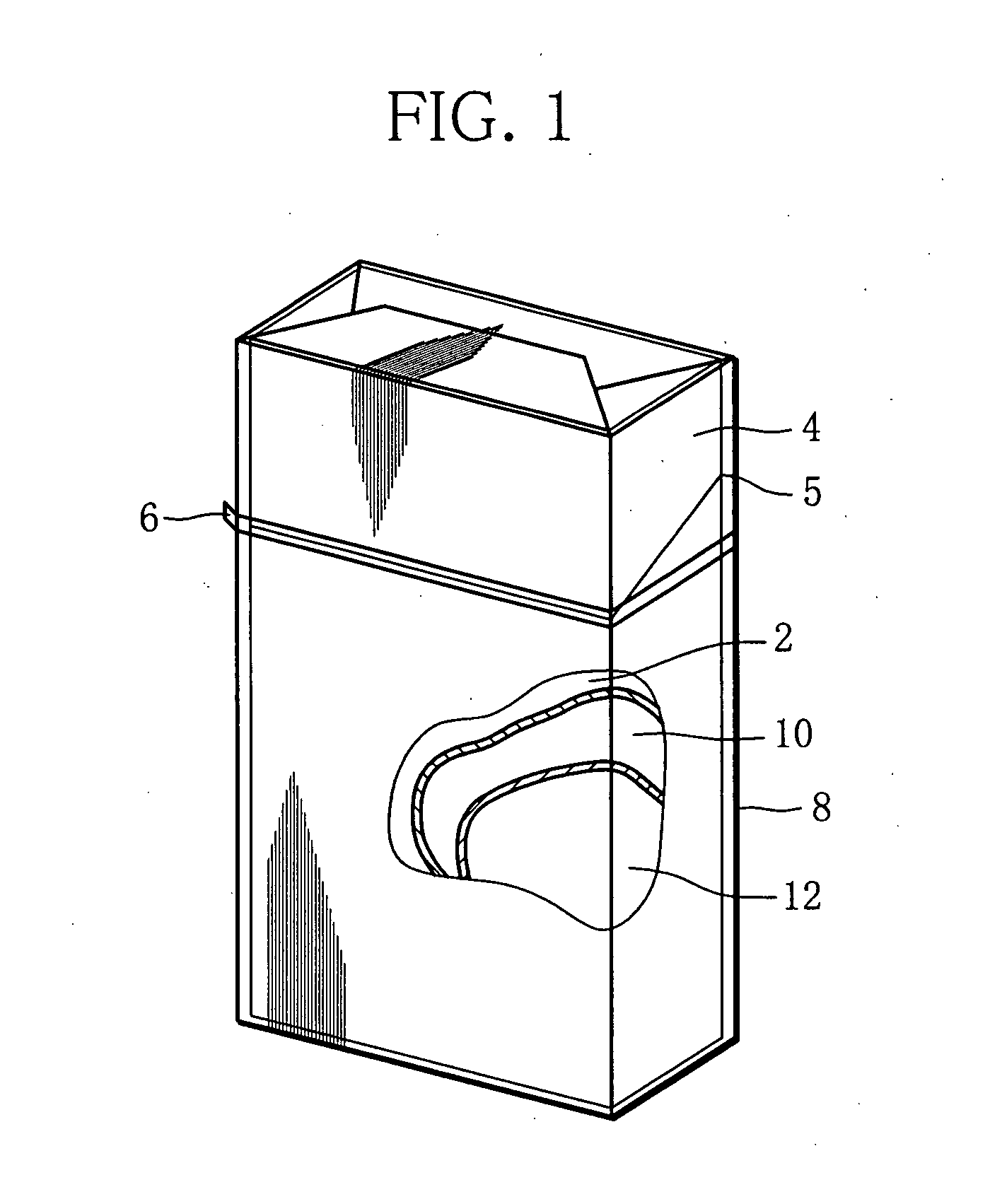
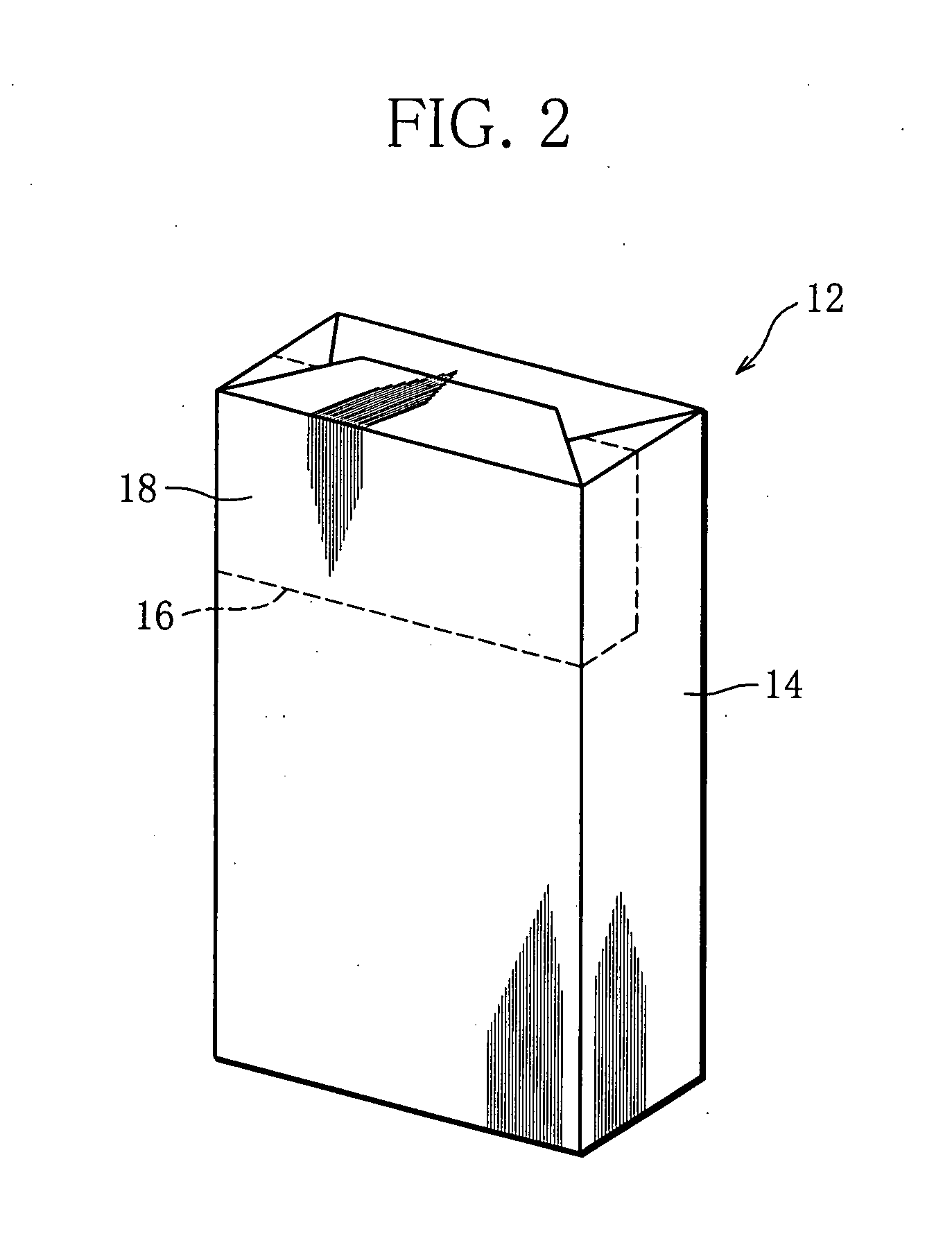
Cigarette package and method of fabricating the same
Owner:JAPAN TOBACCO INC
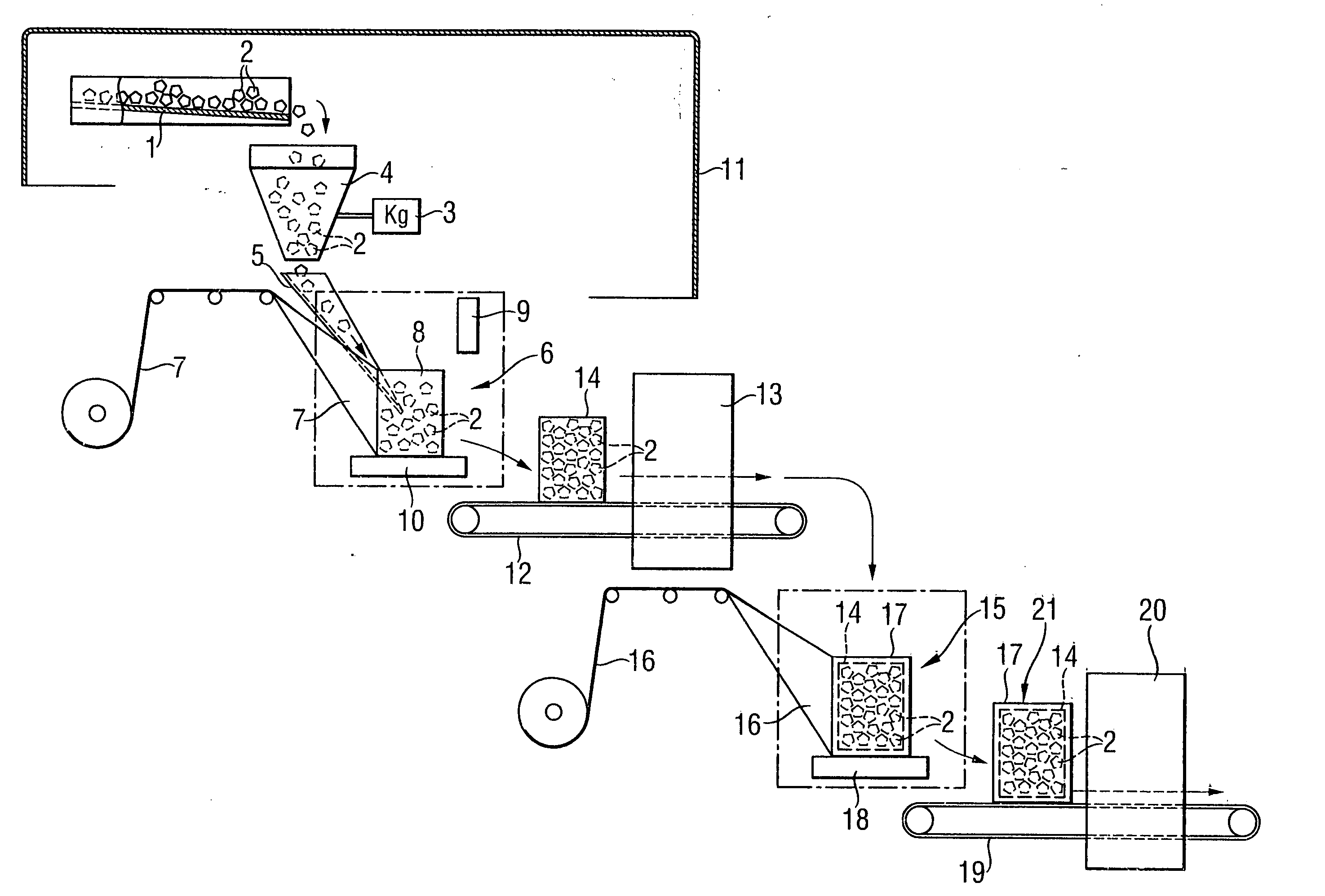
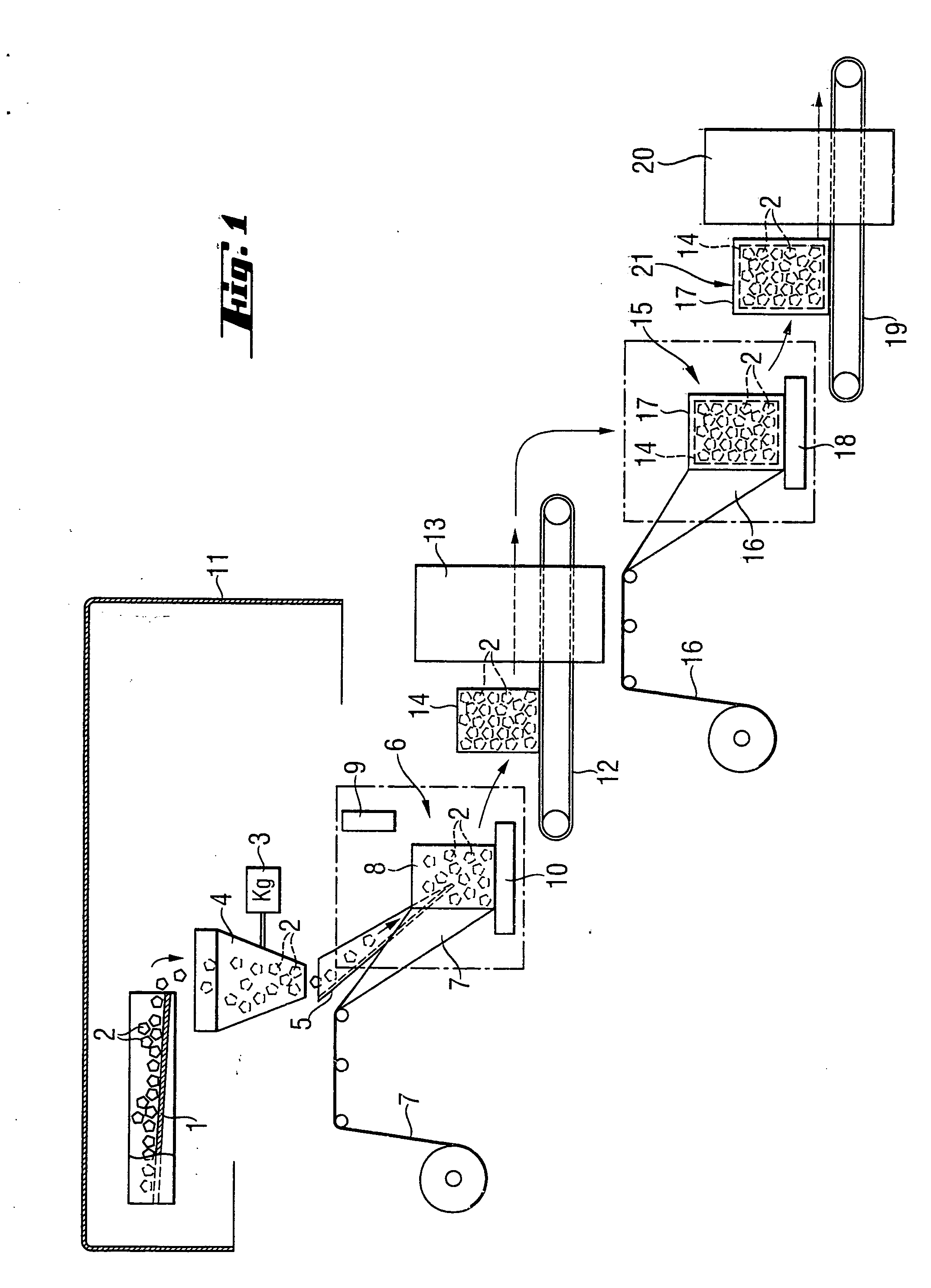
Transportable container for bulk goods and method for forming the container
InactiveUS6918225B2Avoid damaging the materialStabilizing and compressingPackaging cigaretteWrapper folding/bending apparatusEngineeringParticulate material
A transportable container for bulk goods and a method for forming the container are disclosed. The transportable container is formed from a bag having a closed base and an open top. The open top is in a folded over position and a bottom support is located adjacent to the closed base. A particulate material is filled into the bag and an outer wrap is spirally wrapped around the bottom support and the bag such that the outer wrap secures the bag to the bottom support and secures the open top in the folded over position. The method for forming the transportable container includes the steps of securing the open top of the bag in an opened position and supporting a base of the bag. The bag is filled to a predetermined level with a particulate material and while being filled the fill level of the particulate material in the bag is monitored. Simultaneously with filling of the bag, an outer wrap is spirally wrapped around the bag in an upward direction to a predetermined fill level. Once the bag is completely filled the open top of the bag is released and moved into a folded over position whereupon the outer wrap is spirally wound around the bag in a downward direction to secure the open top in the folded over position thereby forming the transportable container.
Owner:KELLOGG CO
Pre-filled personal hydration reservoir
InactiveUS6837026B2Without riskEasy to useTravelling sacksTravelling carriersEngineeringWater reservoir
A beverage reservoir for a personal hydration device is filled with a beverage, e.g., water, and sealed in a tamper-evident manner. As a result, the reservoir can be sold pre-filled and the user can use the reservoir confident that the beverage contains no mold, fungus, or residue from previously stored and consumed beverage. The reservoir can also include a port for re-filling such that the user can use the reservoir in a conventional manner after consumption of the previously sealed-in beverage.
Owner:SETTON DAVID
Packaging system
InactiveUS20050223679A1Little or no shelf lifeSurgical furnitureDiagnosticsEngineeringMechanical engineering
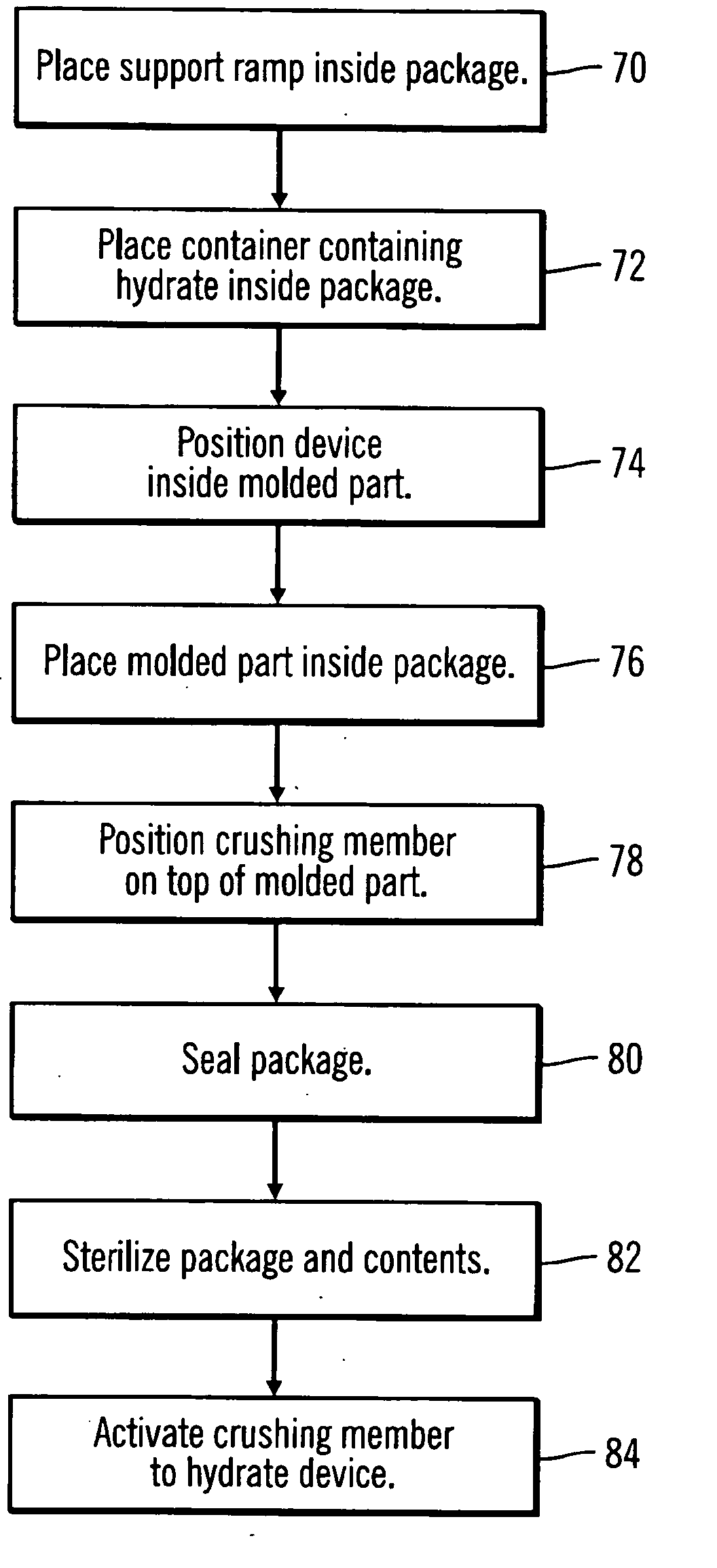
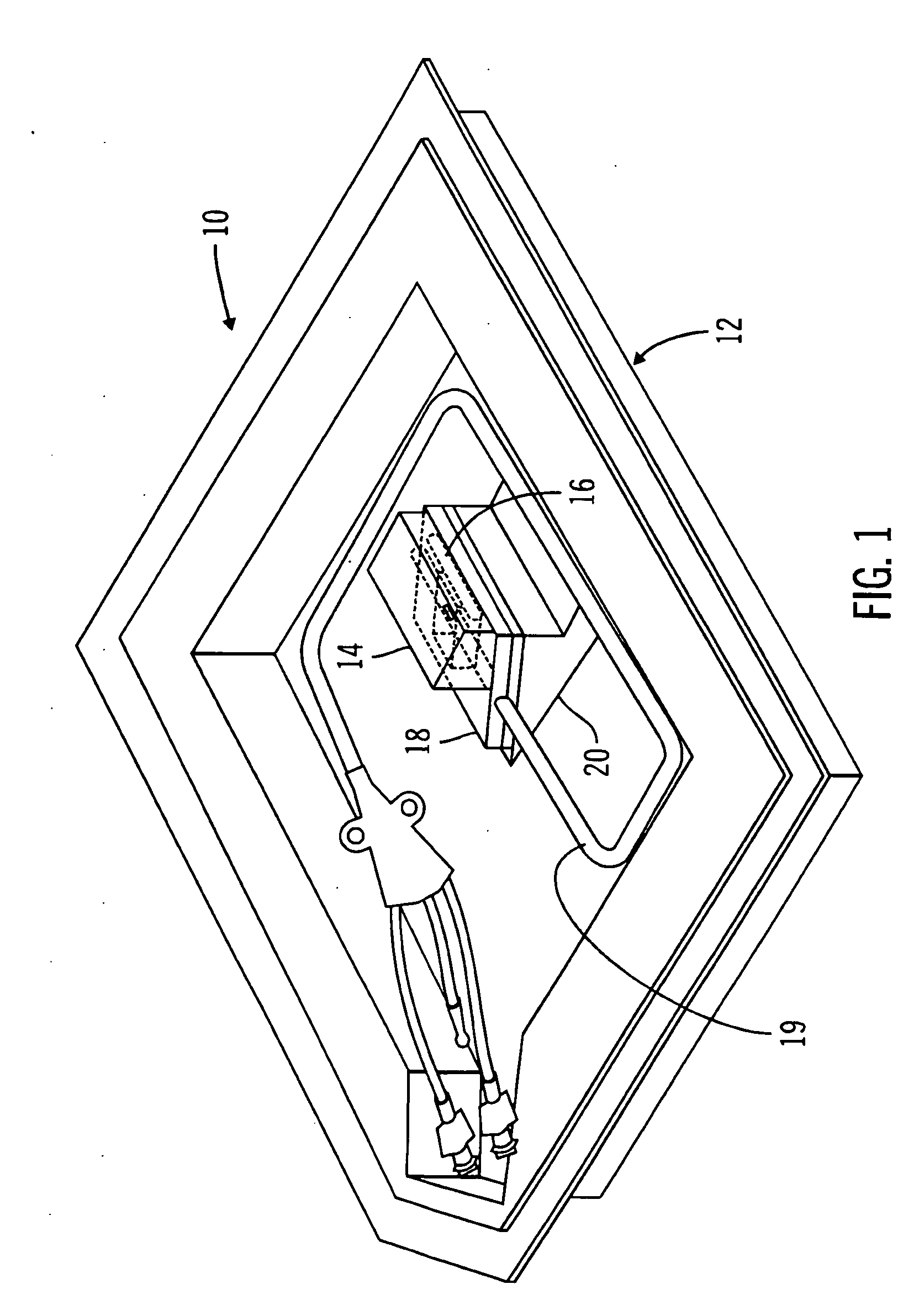
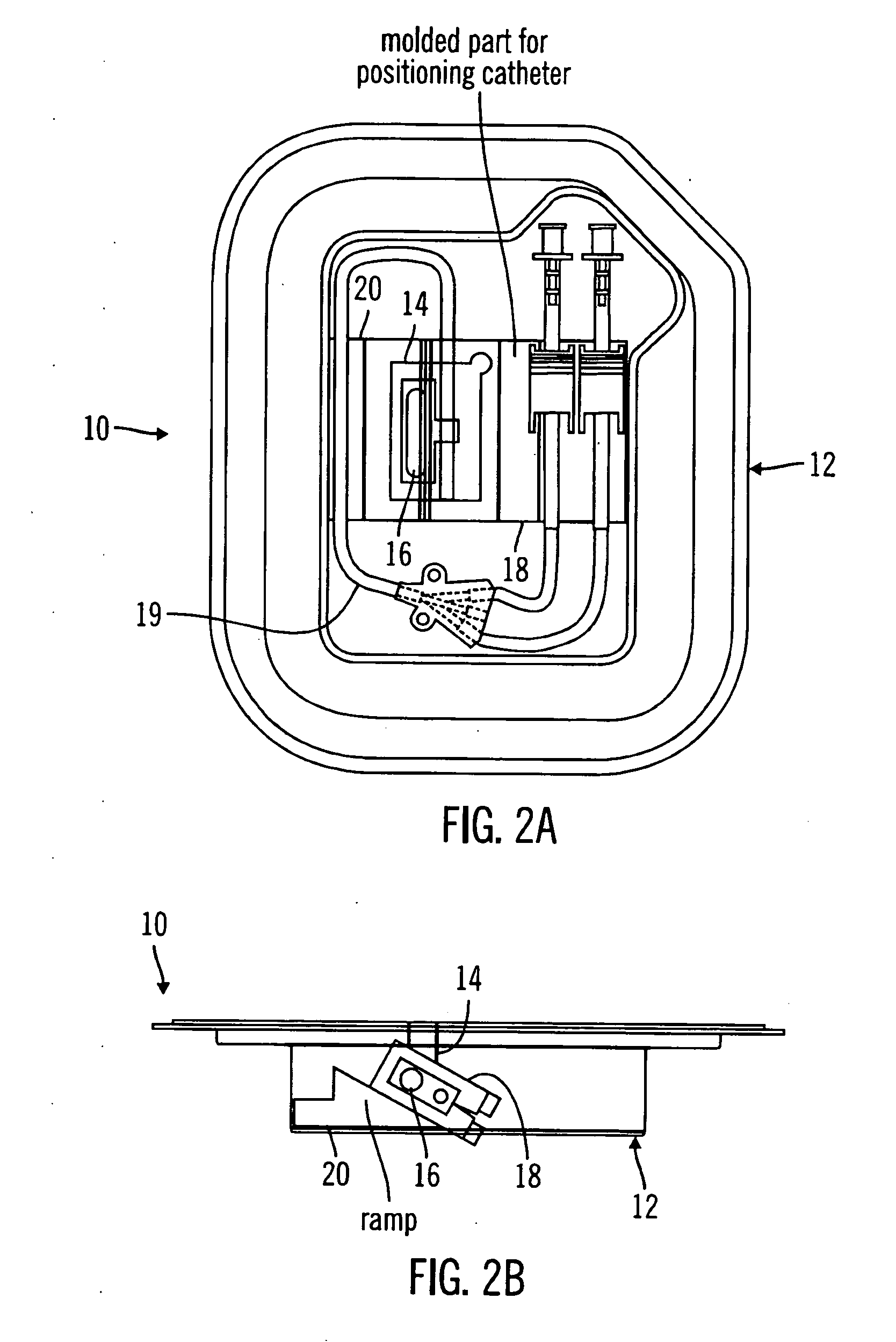
A packaging system for hydrating sterile devices without comprising the integrity of the sterilization. The packaging system may include an enclosure for enclosing a device requiring hydration, a container containing a hydrate, a base located within the interior of the enclosure and an activating member located within the interior of the enclosure. The container and the device may be located within a receptacle. The receptacle may rest on the base and the activating member may be affixed on top of the receptacle. A force may be exerted on an exterior portion of the enclosure such that the activating member pushes on the receptacle and crushes or ruptures the container. The hydrate located within the container is then released to the device, thereby hydrating the device without breaking the seal of the enclosure. The sterilized environment is therefore maintained and the device is hydrated.
Owner:MEDTRONIC MIMIMED INC
System and method for footwear packaging
Owner:NIKE INC
Method and apparatus for shock-absorbing packaging
Owner:ROOSE LARS D
Method of lap sealing a molten cheese product with non-wax film
A method of sealing a non-wax-coated film pouch contained within an enclosure includes forming an enclosure, positioning a piece of non-wax-coated film within the enclosure to form a floor and side walls of a pouch within the enclosure, dispensing a flowable dairy-based product into the pouch, overlapping portions of the film onto one another to produce a top wall over the dairy-based product, and heat sealing the overlapping portions of the top wall to each other.
Owner:LAND O'LAKES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com