Patents
Literature
27824results about "Large containers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
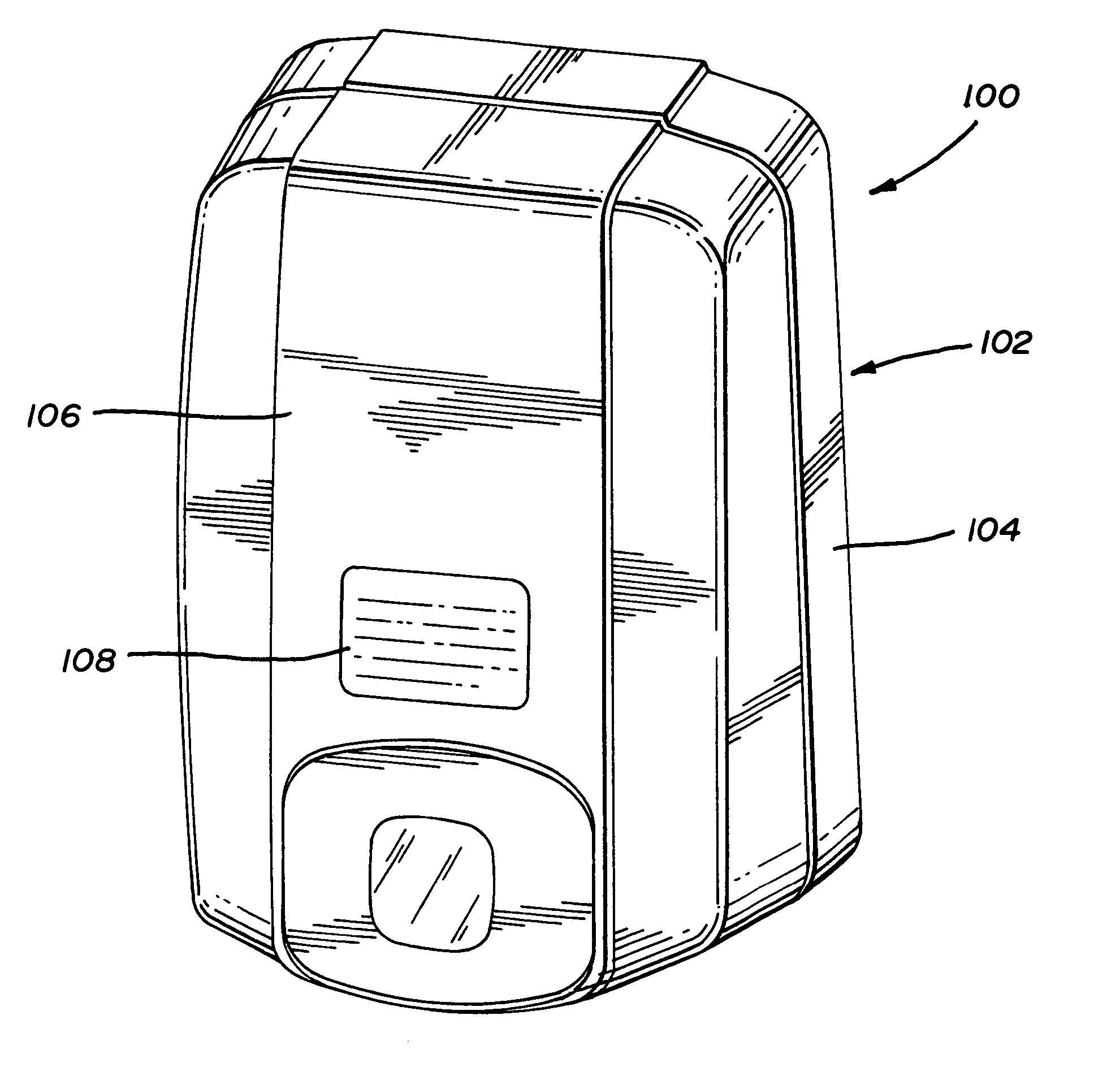
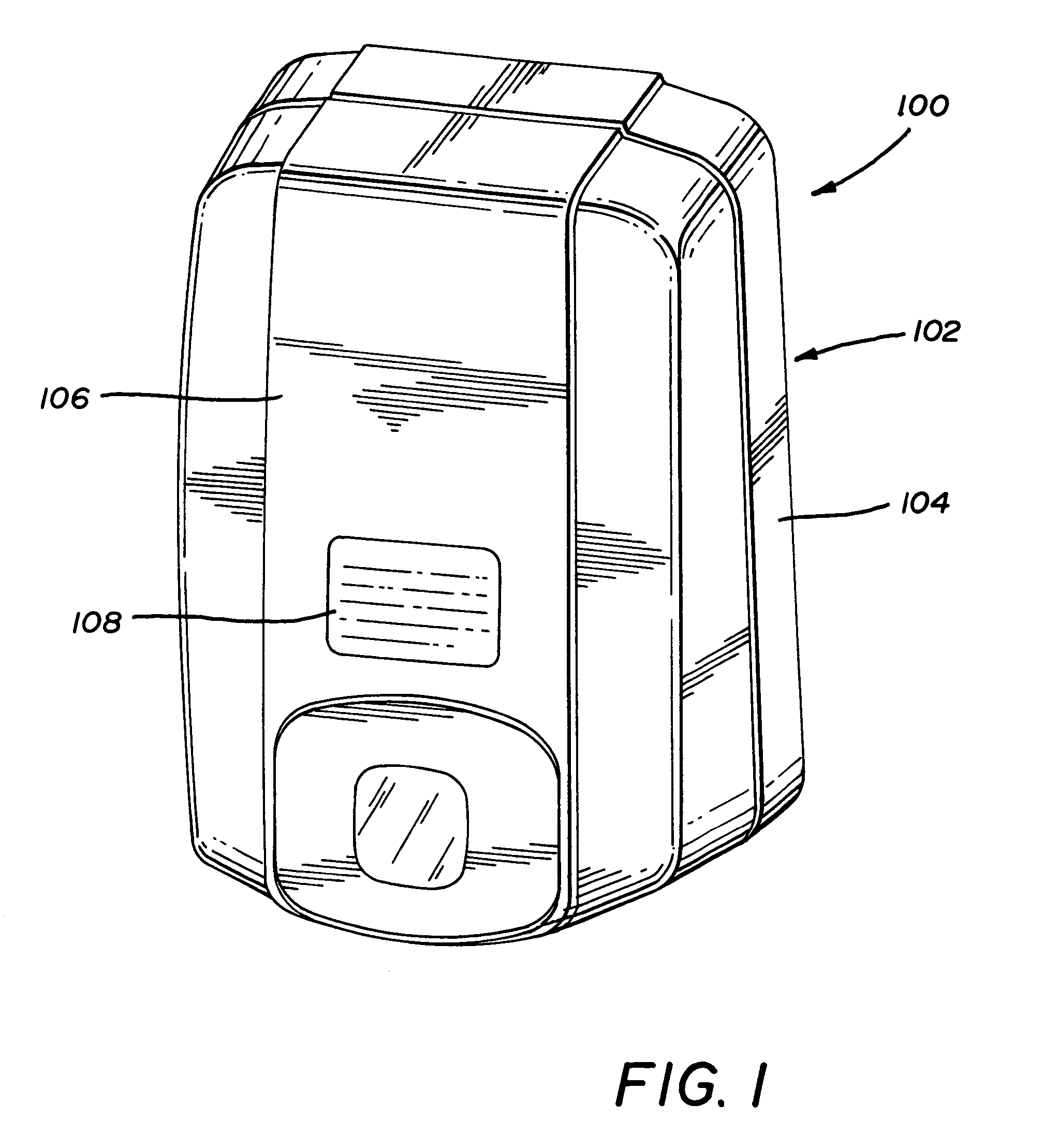
Two component fluid dispenser
InactiveUS6047861AEnhanced couplingSecurely holdLiquid surface applicatorsSurgeryEngineeringViscosity
A two-component fluid dispenser which can accurately mix two liquids of varying viscosity and then precisely deliver the mixture formed in discrete amounts. The mixture of the two components is delivered from a single delivery tube in a manner such that none of the mixture remains within the delivery tube at the completion of each mixing and delivery cycle. In one form of the apparatus, the single delivery tube of the apparatus is operably coupled with conventional hypodermic syringes of various sizes so that different fluids can be mixed in different ratios.
Owner:BAXTER INT INC
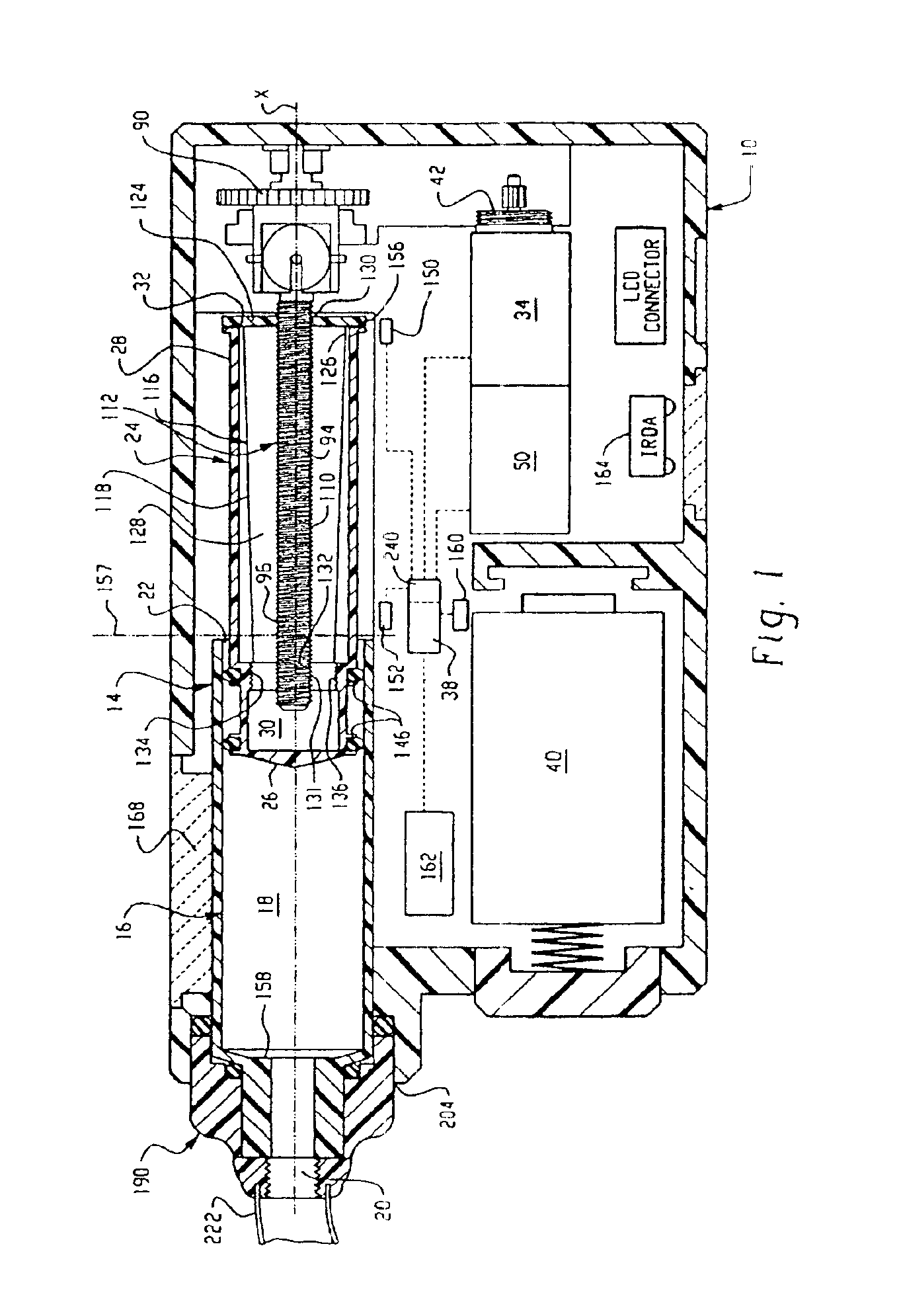
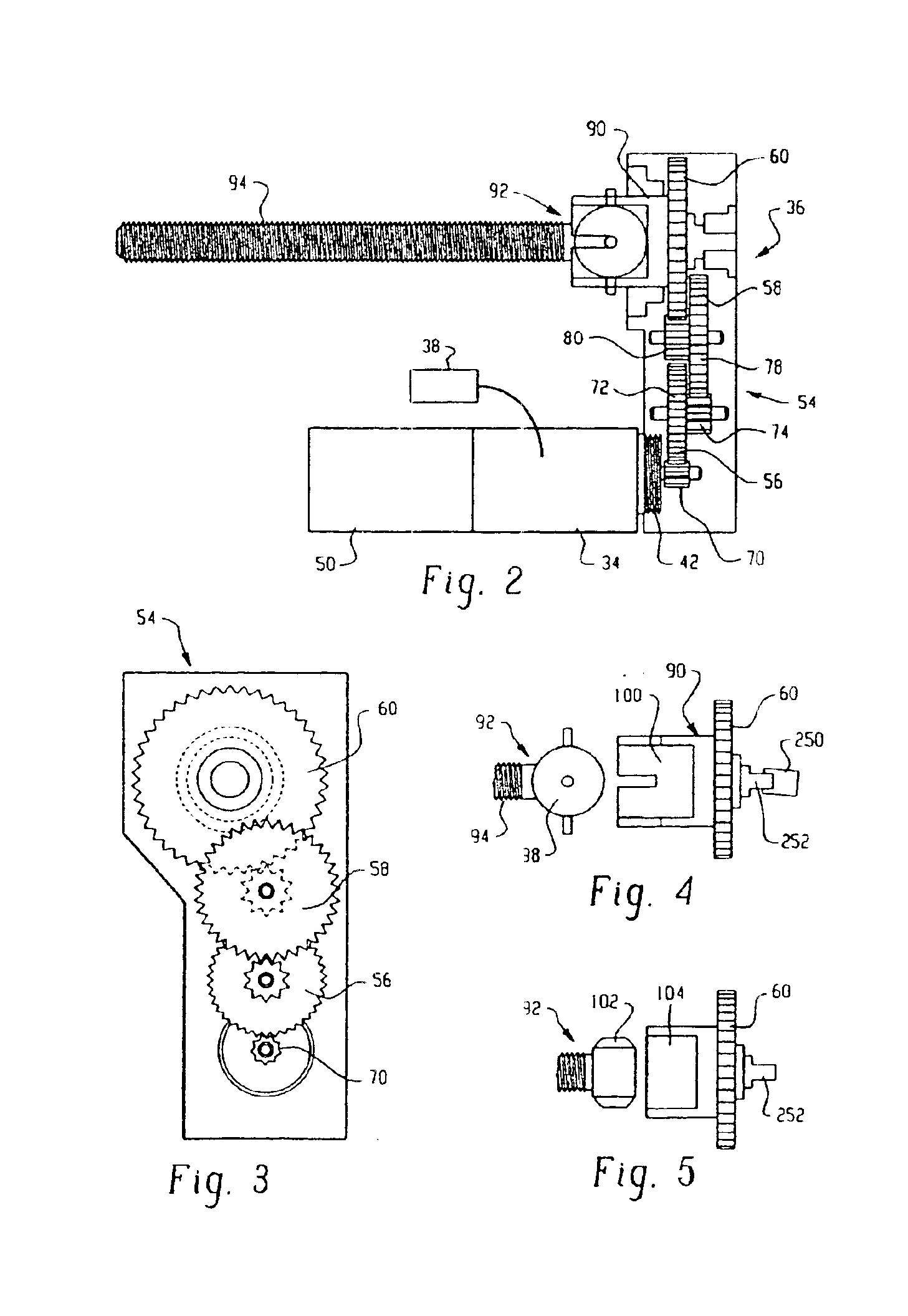
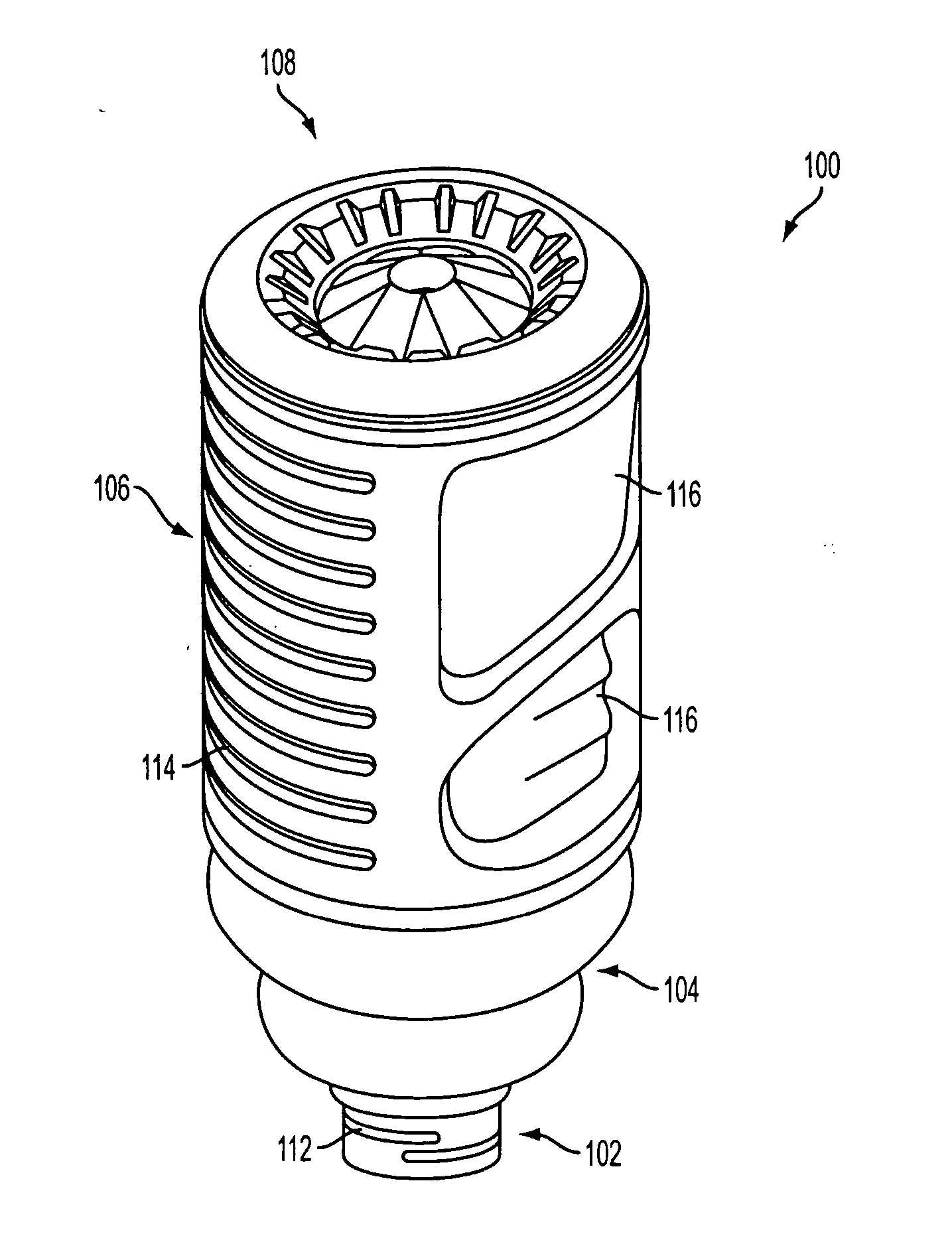
Drive system for an infusion pump
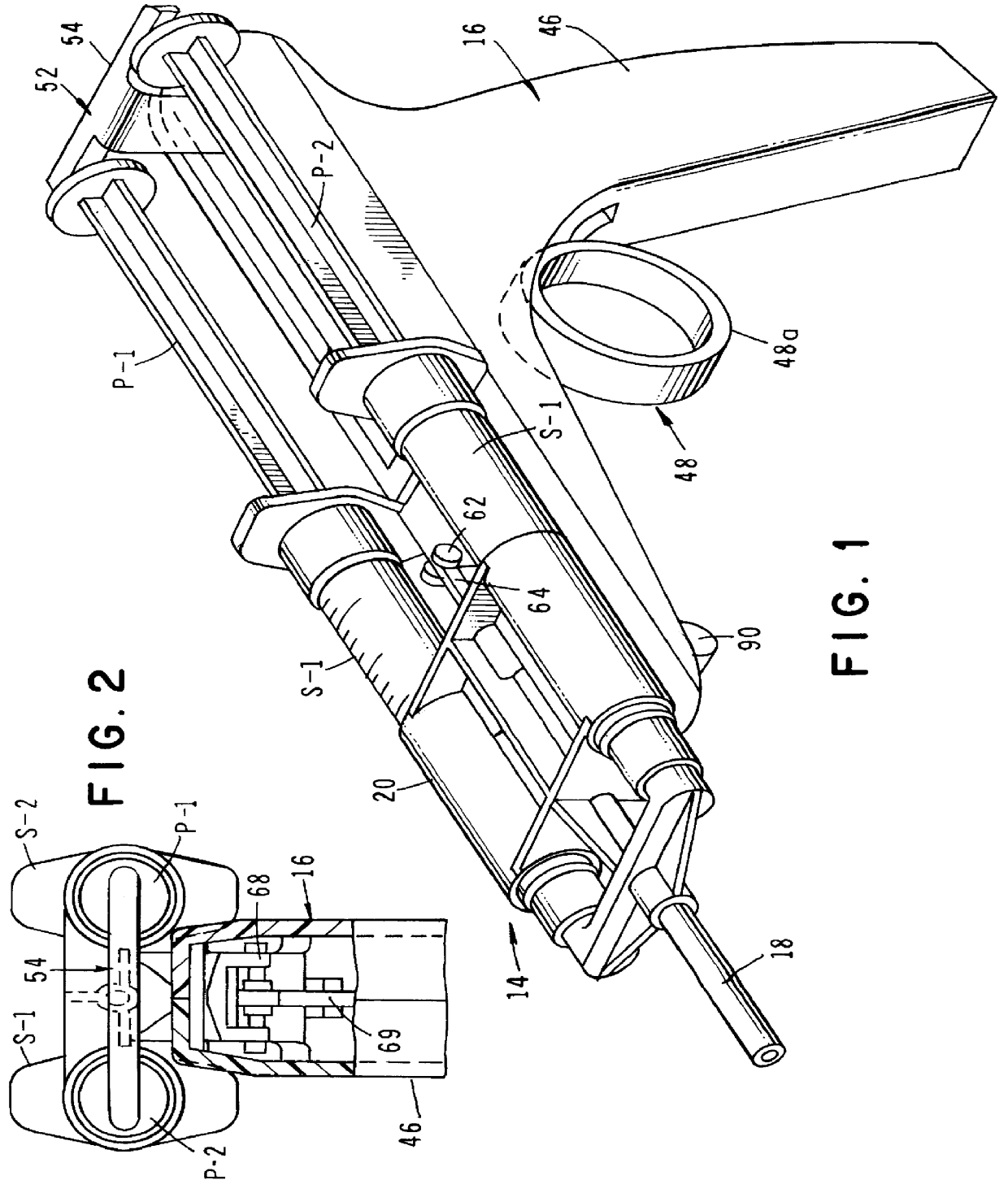
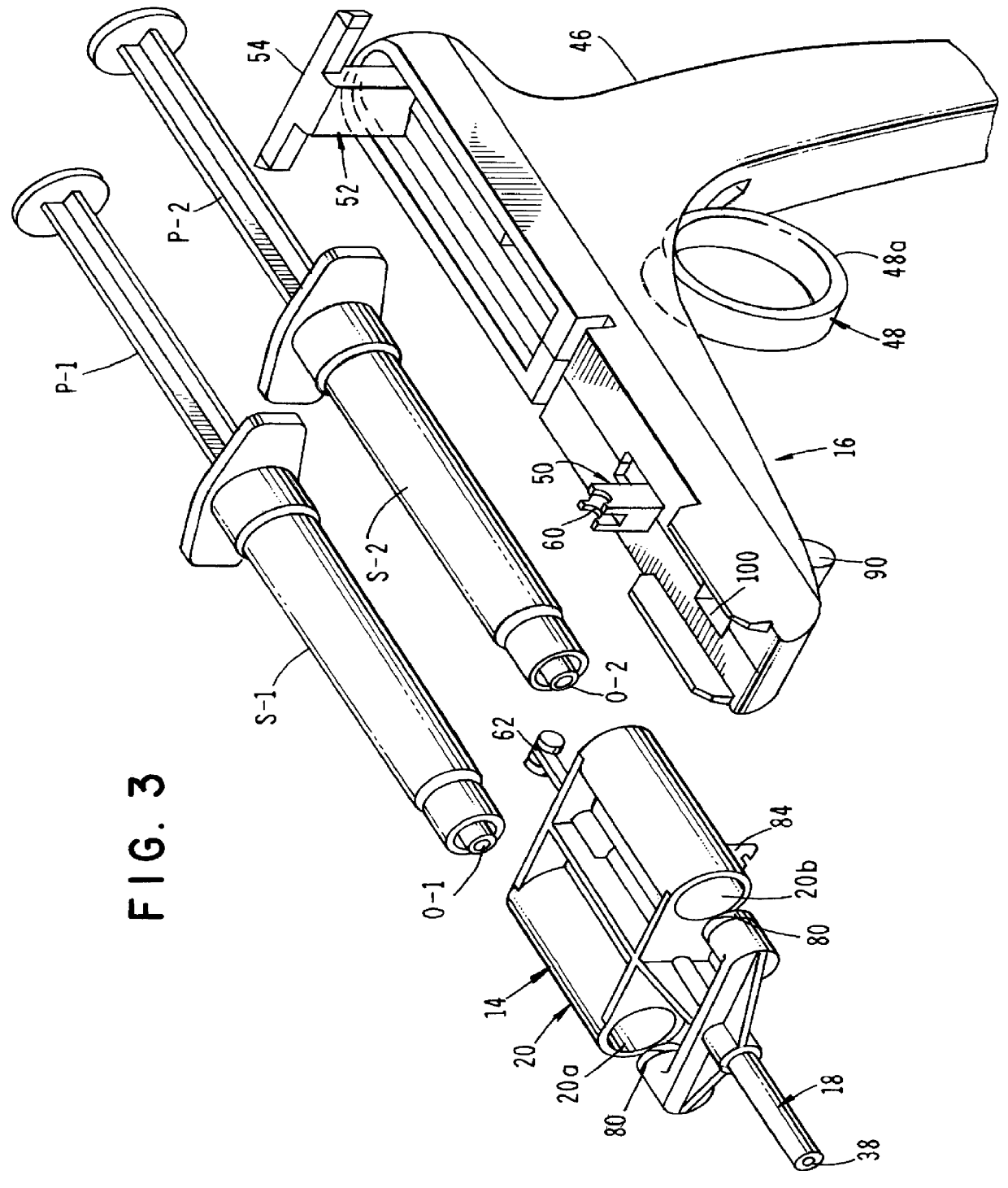
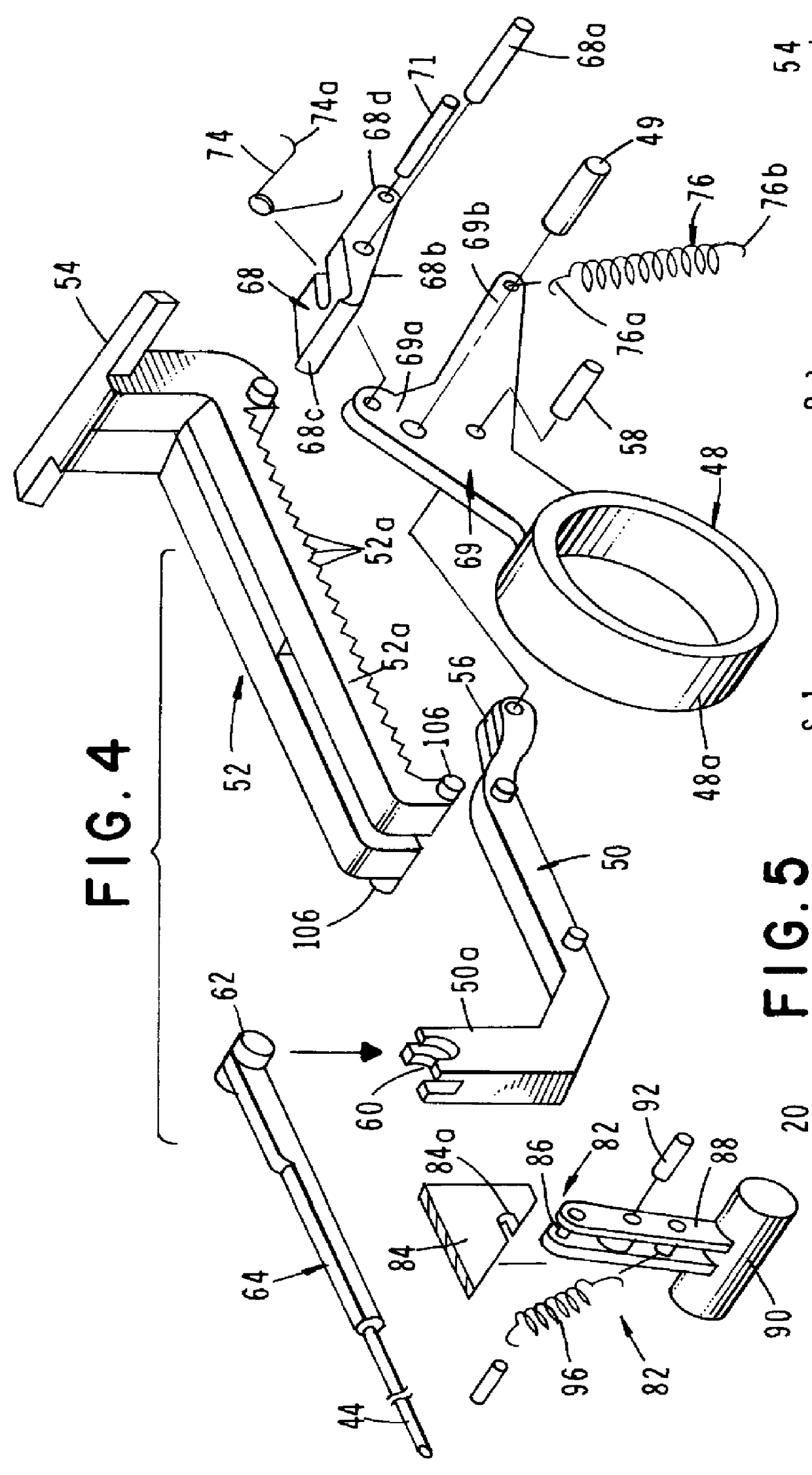
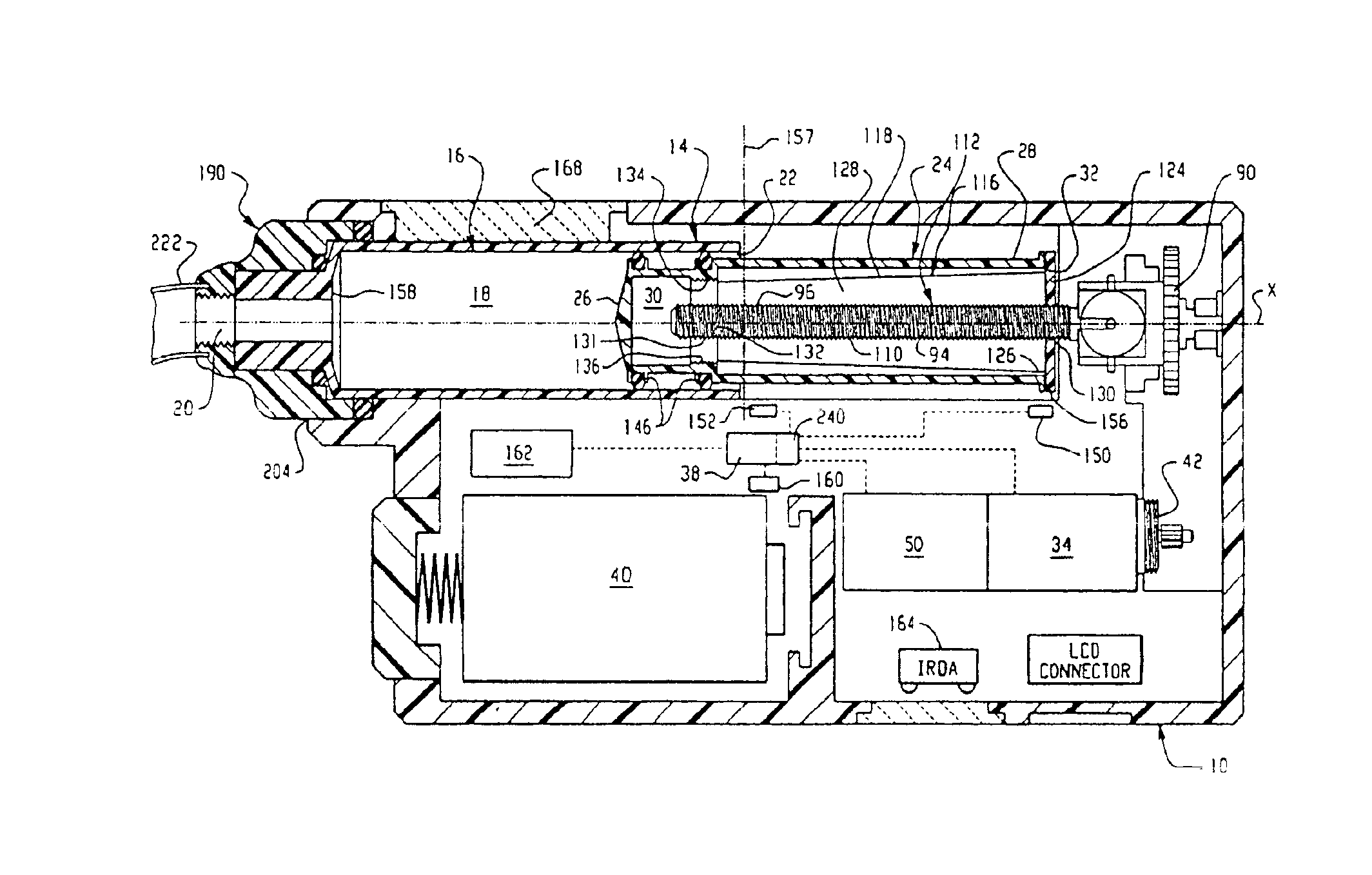
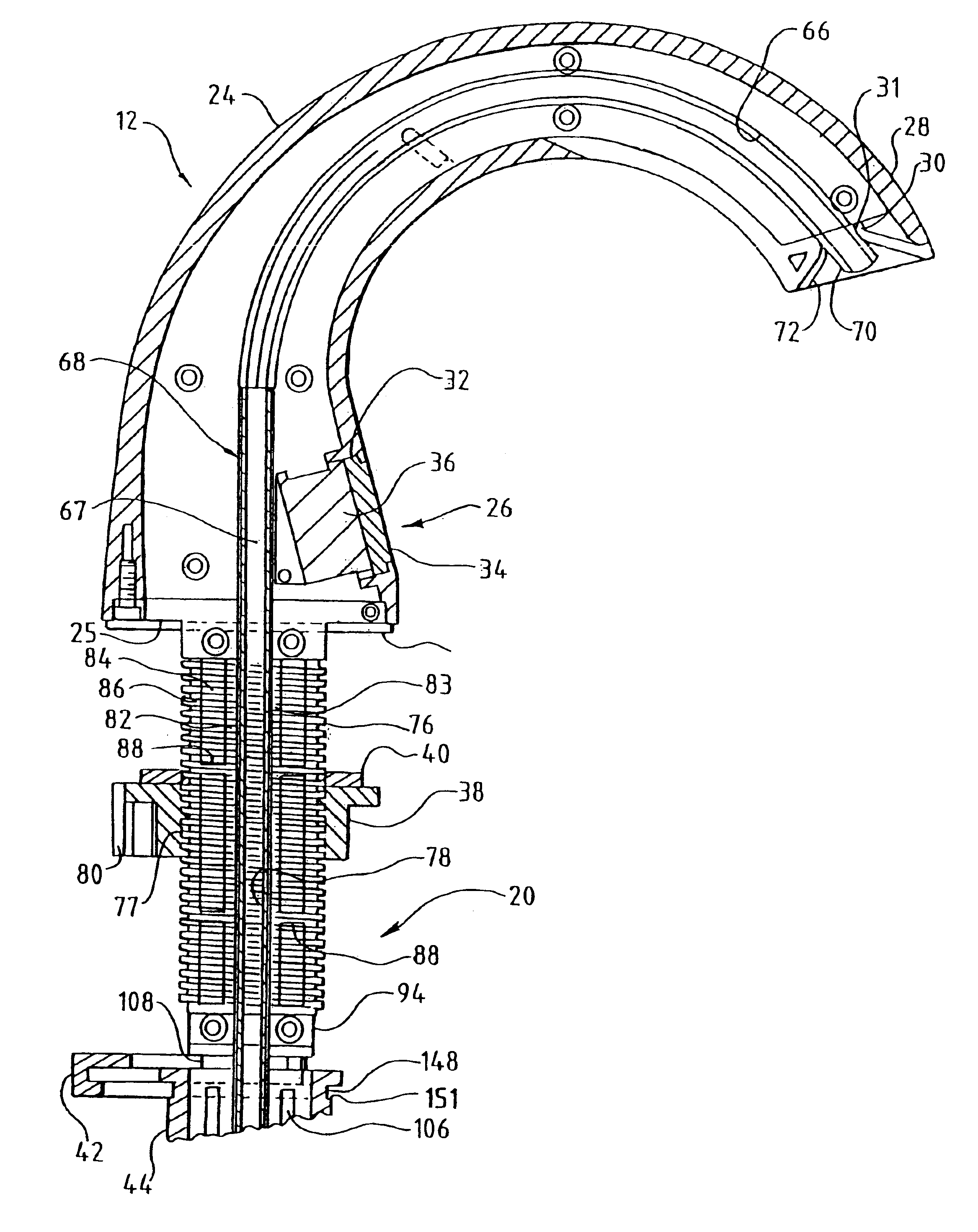
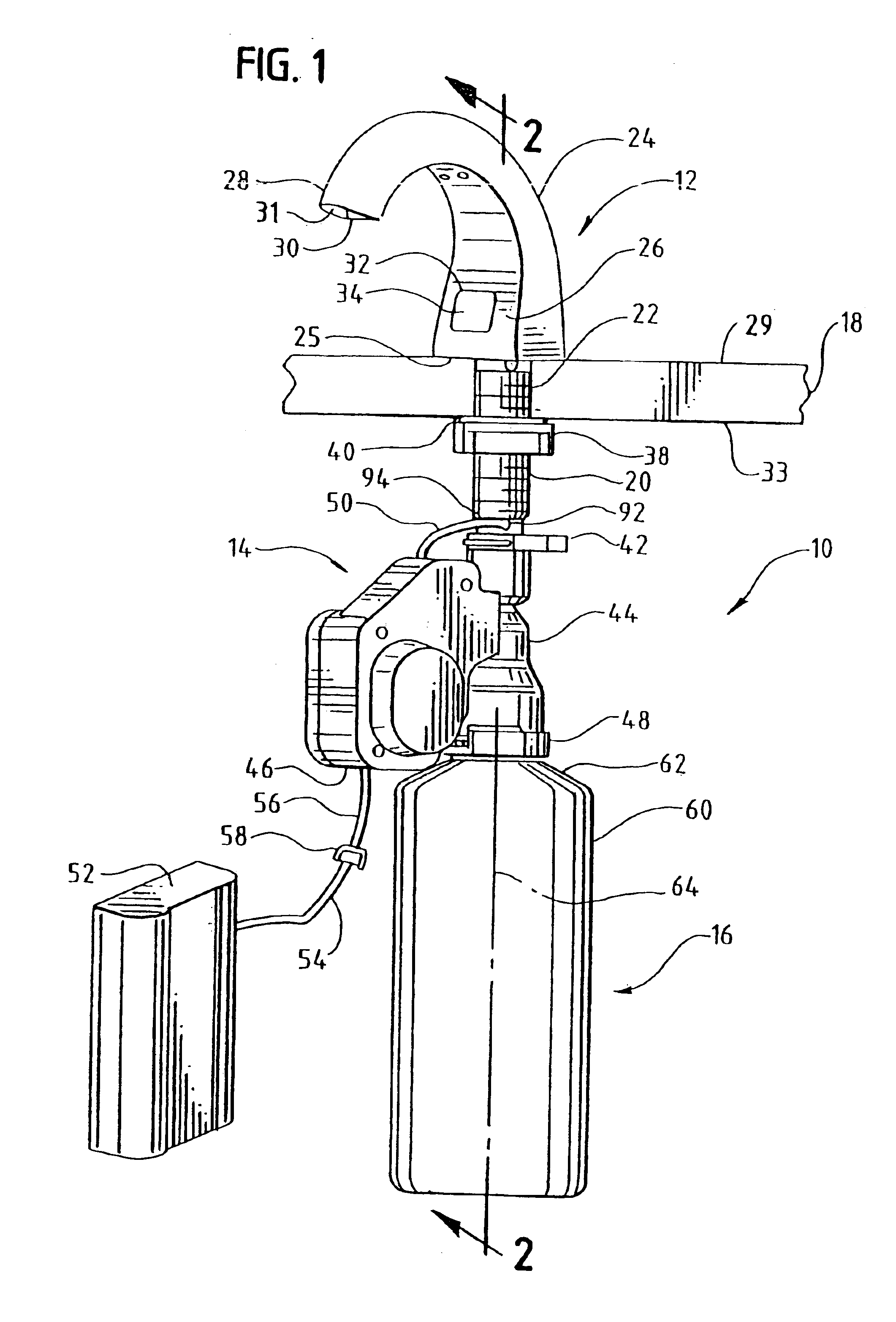
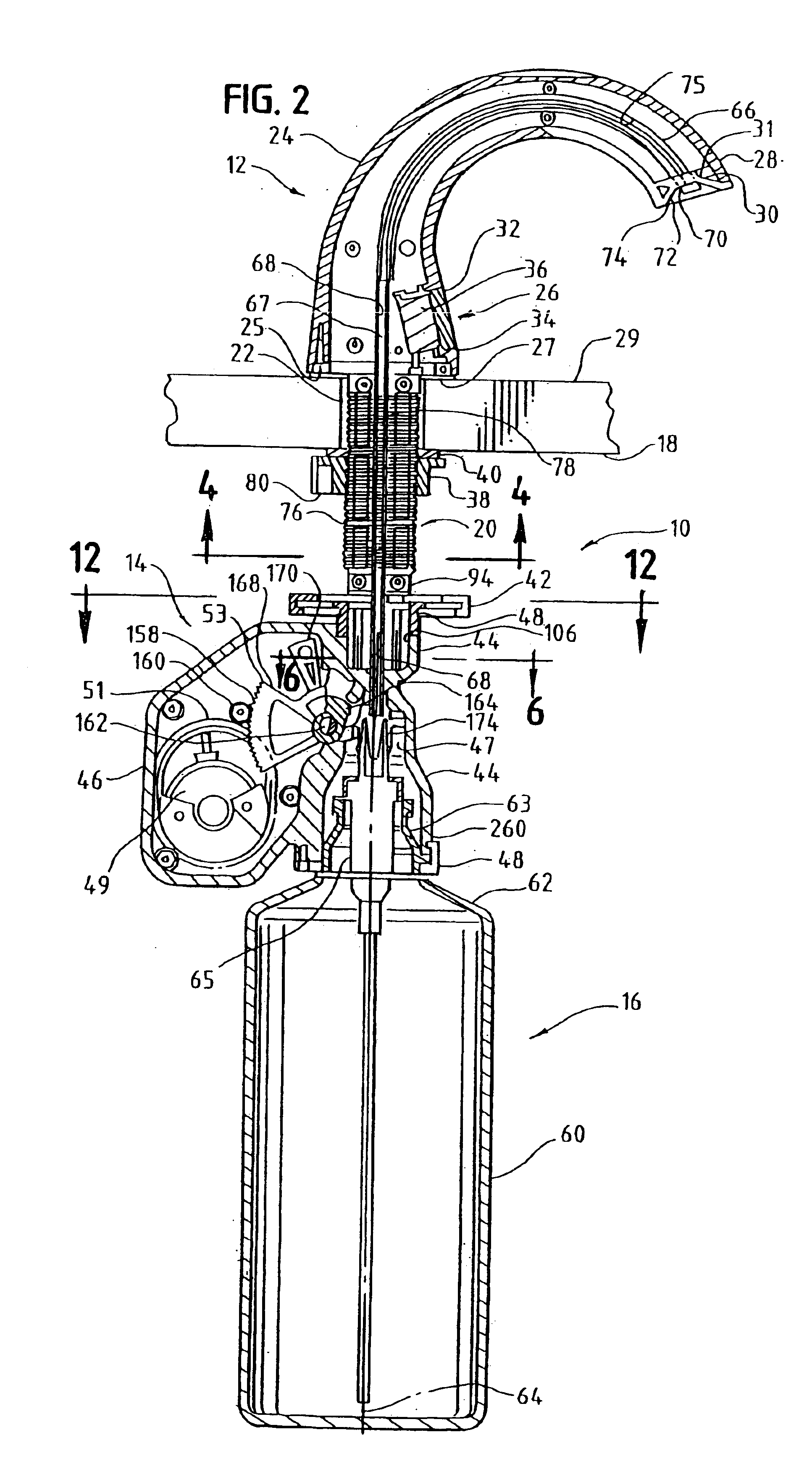
InactiveUS6854620B2Small sizeImprove portabilityClosure using stoppersLarge containersLinear motionProximity sensor
A pump system for an infusion system includes a linear drive (36, 36′) which minimizes the space occupied by the pump components in a portable housing (10, 10′). A motor (34) and a motor drive shaft (42) are arranged in parallel with, and adjacent to a syringe (14, 14′) and lead screw (94, 94′). A gear box (54) connects the drive shaft and lead screw to transfer rotational movements between them. A piston driving member, such as a cone (116) or drive nut (116′) converts the rotational movement of the lead screw into linear motion of a syringe piston (24). Sensors (150, 152) detect when the piston or cone is in a “home” position and in an “end” position, respectively. Optionally, a proximity sensor (170) is used to ensure that the cone and the piston (24) are abutting during dispensing. Alternatively, a clamping member (350) selectively clamps the lead screw (94′) against linear motion in at least a dispensing direction.
Owner:TRIVIDIA HEALTHCARE SYST LLC
Aggregate Delivery Unit
A delivery unit for providing aggregate to a worksite, such as a wellsite location. The unit may include a mobile chassis for accommodating a plurality of modular containers which in turn house the aggregate. As such, a weight measurement device may be located between each container and the chassis so as to monitor aggregate levels within each container over time. The units may be particularly well suited for monitoring and controlling aggregate delivery during a fracturing operation at an oilfield. The modular containers may be of an interchangeable nature. Furthermore, a preferably wireless control device may be provided for monitoring and directing aggregate delivery from a relatively remote location.
Owner:SCHLUMBERGER TECH CORP
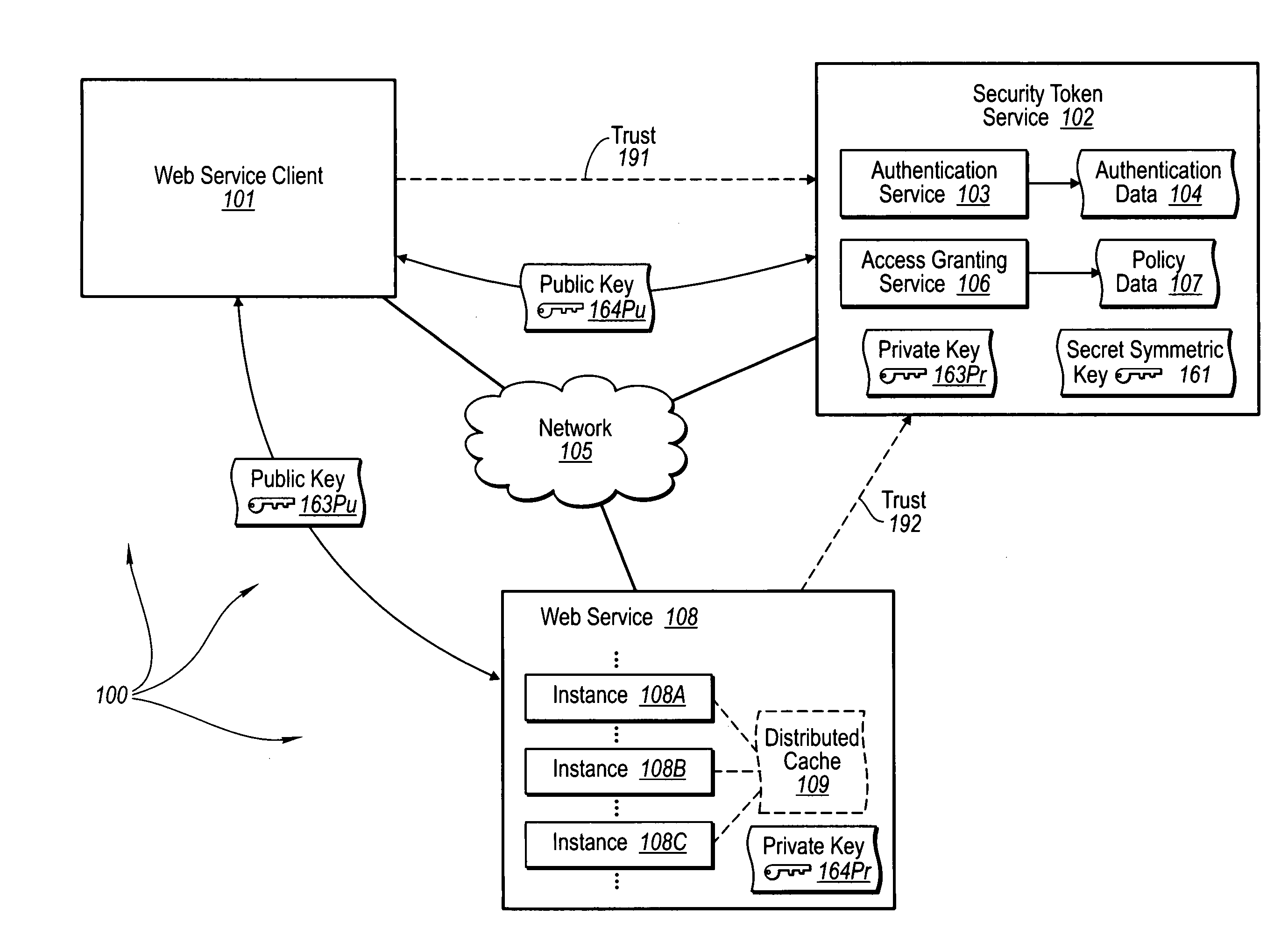
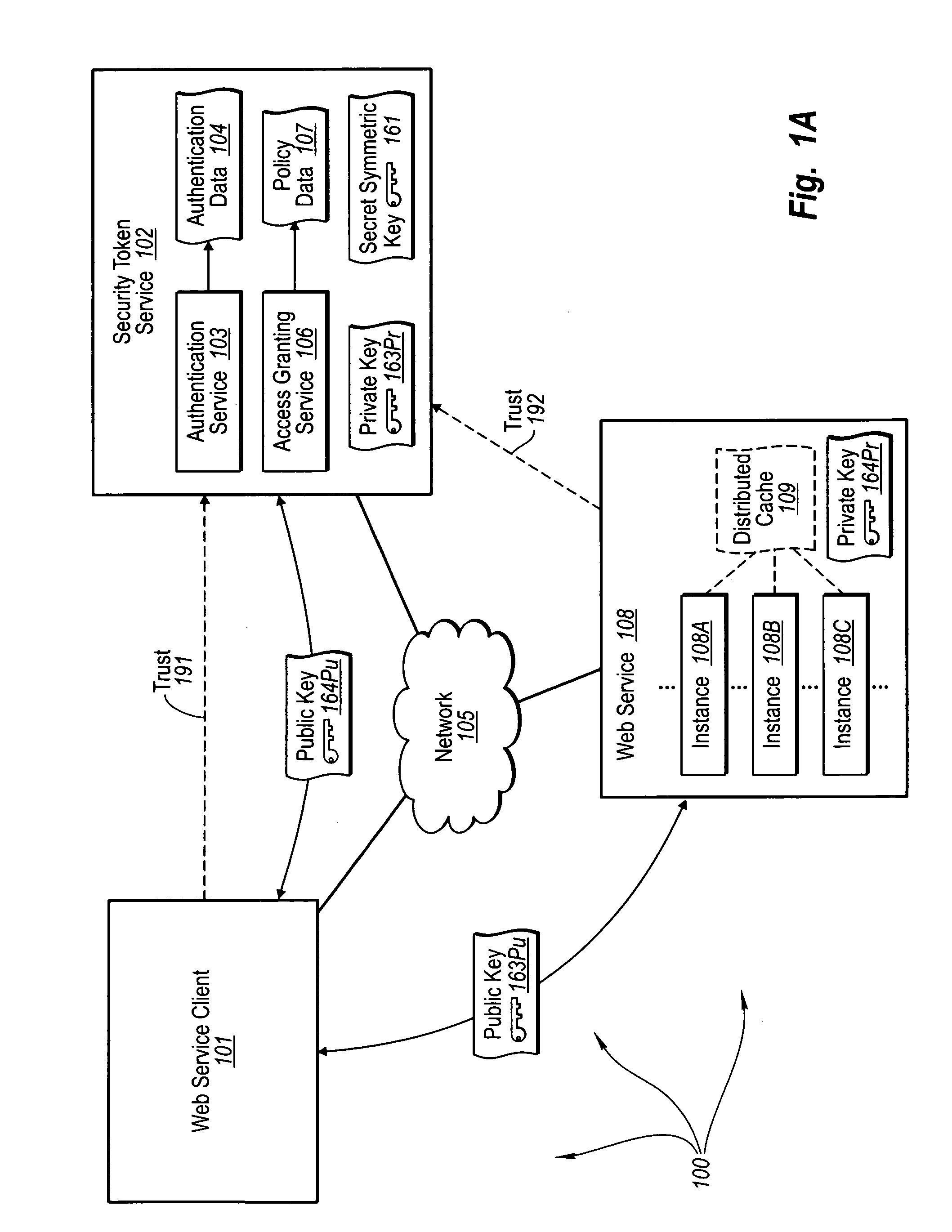
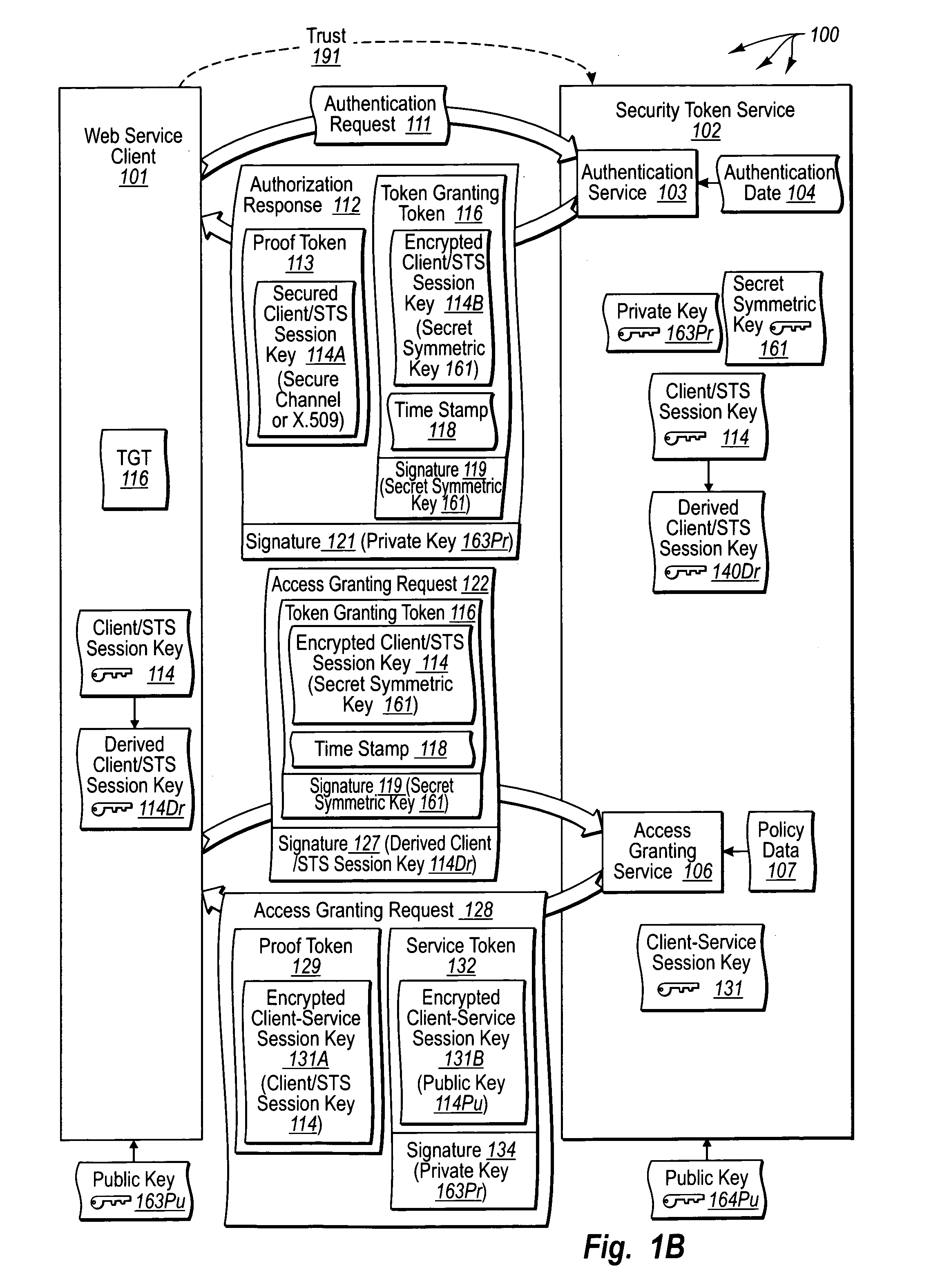
Trusted third party authentication for web services
InactiveUS20060206932A1Communication securityDigital data processing detailsUser identity/authority verificationWeb serviceInternet privacy
The present invention extends to trusted third party authentication for Web services. Web services trust and delegate user authentication responsibility to a trusted third party that acts as an identity provider for the trusting Web services. The trusted third party authenticates users through common authentication mechanisms, such as, for example, username / password and X.509 certificates and uses initial user authentication to bootstrap subsequent secure sessions with Web services. Web services construct user identity context using a service session token issued by the trusted third party and reconstruct security states without having to use a service-side distributed cache.
Owner:MICROSOFT TECH LICENSING LLC
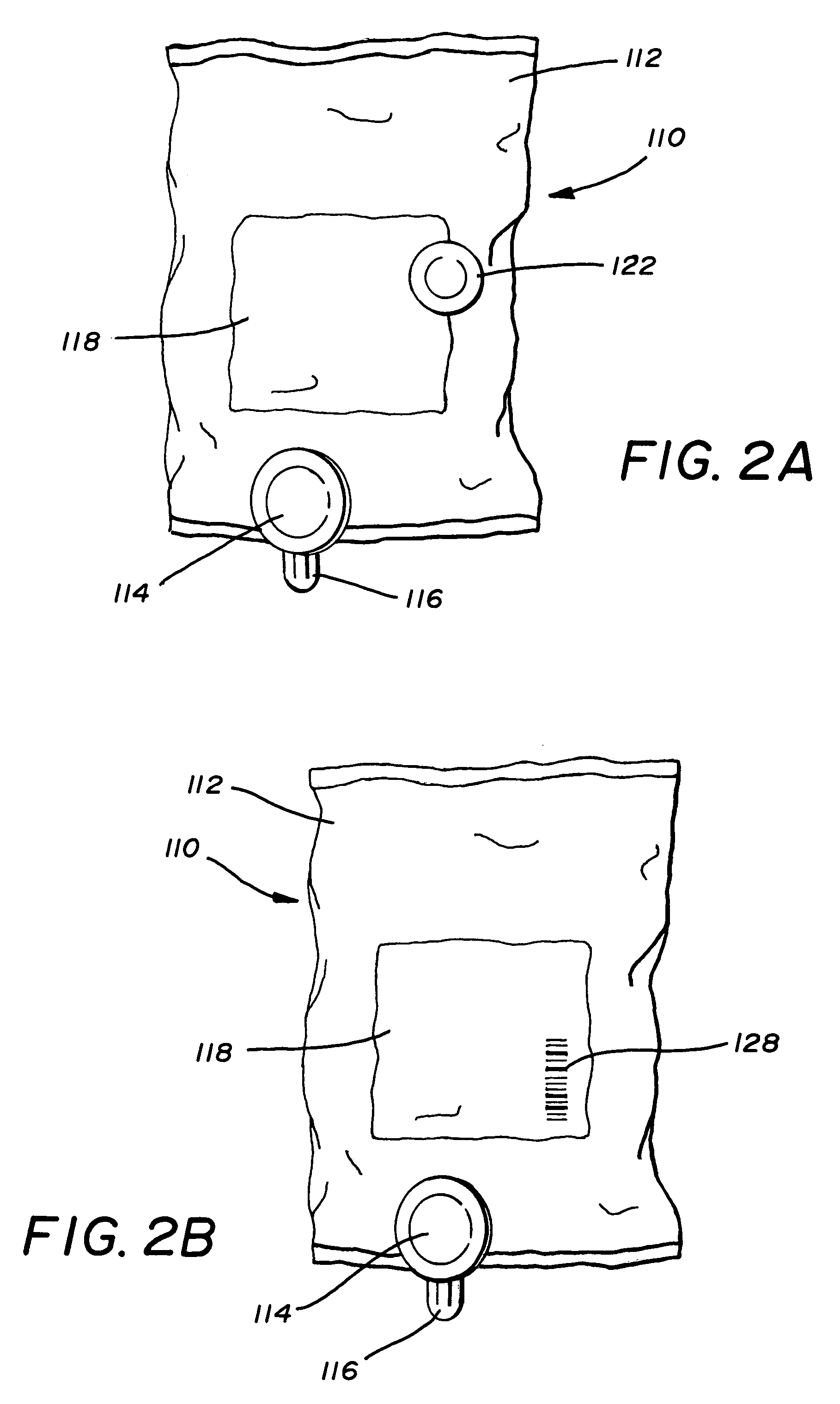
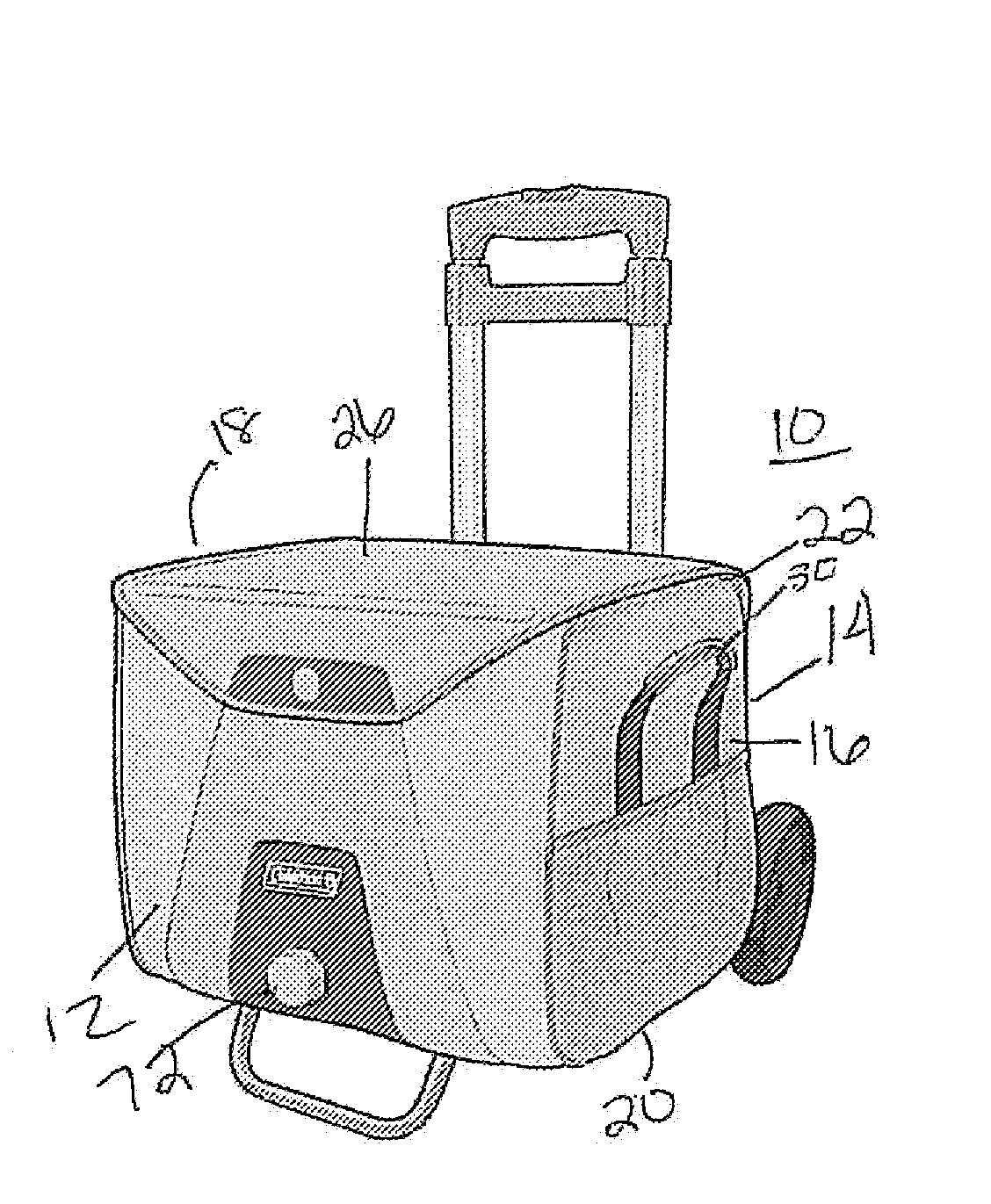
Electronically keyed dispensing systems and related methods of installation and use
Dispensing systems are disclosed which utilize electronically powered key devices and / or identification codes associated with a refill container to preclude the need for mechanical keys. A first embodiment of the device utilizes a matching code stored in a radio frequency identification tag or bar code associated with a fluid refill container and an identification code associated with the dispenser housing. Matching of the codes by a controller allows for continued use of the dispenser via some type of operational mechanism. Another embodiment employs a key which carries the matching code wherein matching of the codes allows for actuation of a motor actuated pumping device. Yet another embodiment employs a blocking mechanism to prevent use of a dispenser's push bar if a key and dispenser housing do not have matching codes. And yet another embodiment requires the use of a key that has a matching code that matches the dispenser's identification code in order to permit initial access to the dispenser housing.
Owner:JOSEPH S KANFER
Rigid collapsible liner for insulated container
InactiveUS20150175338A1Domestic cooling apparatusLighting and heating apparatusEngineeringMechanical engineering
Owner:THE COLEMAN CO INC
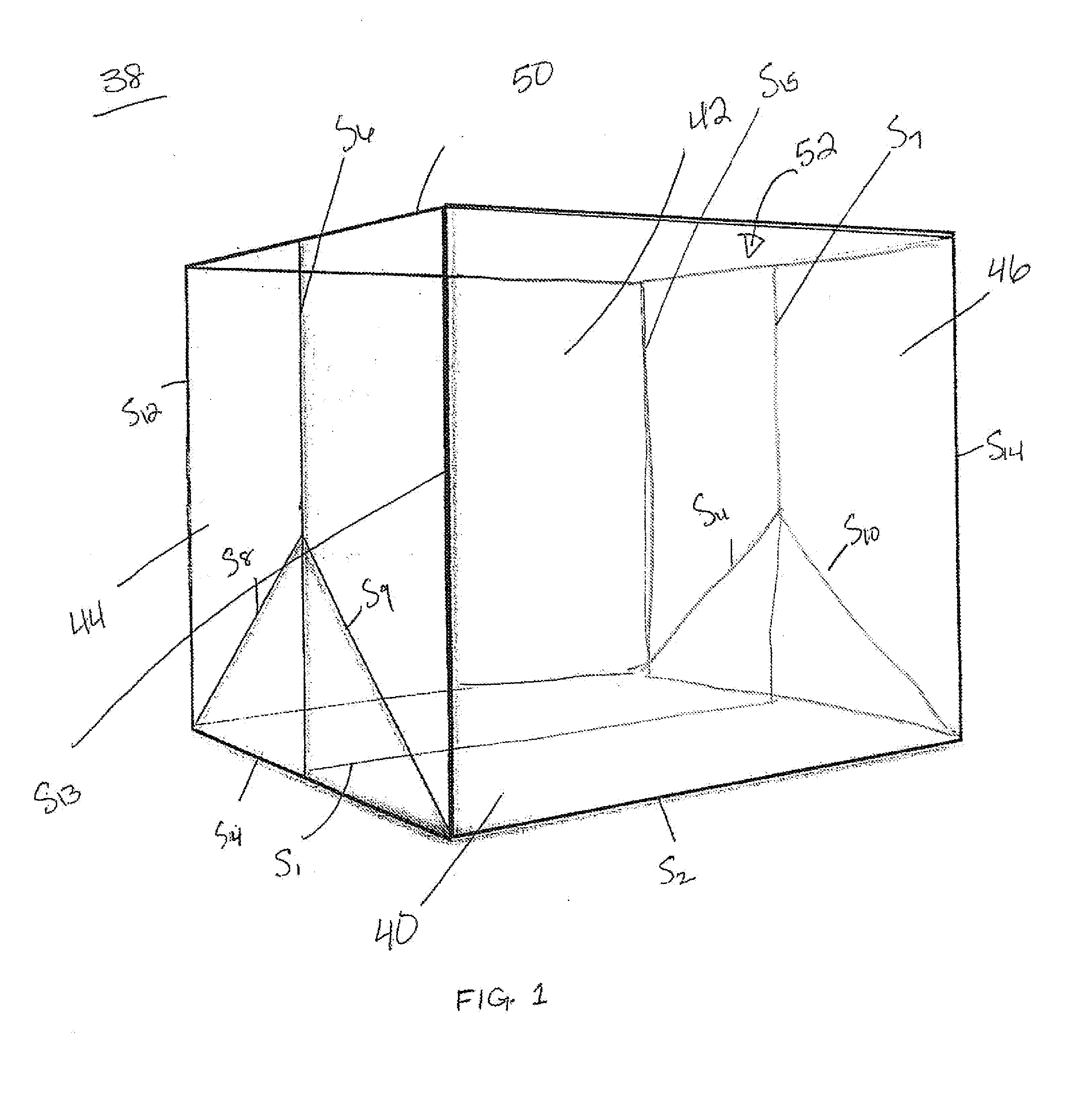
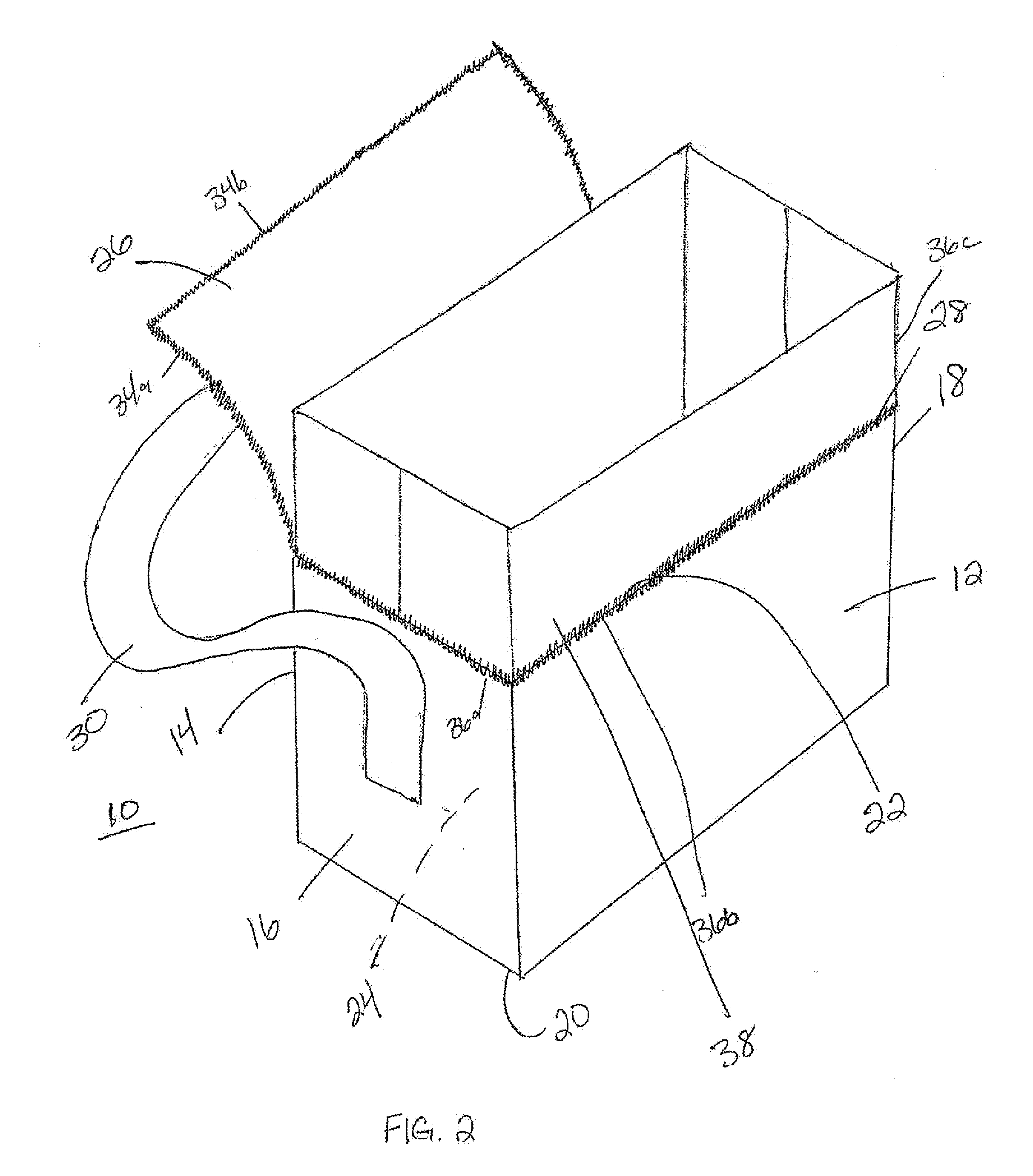
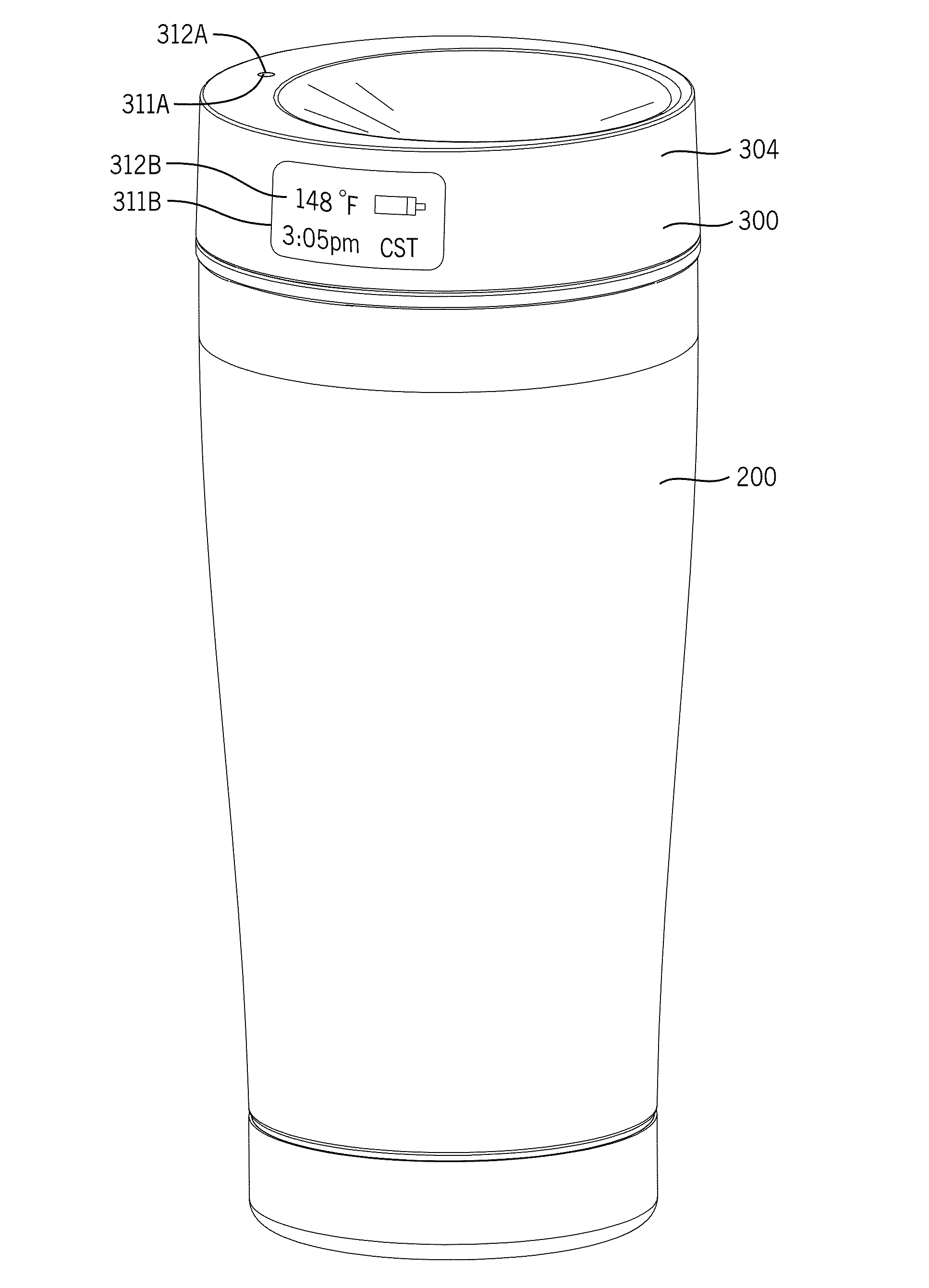
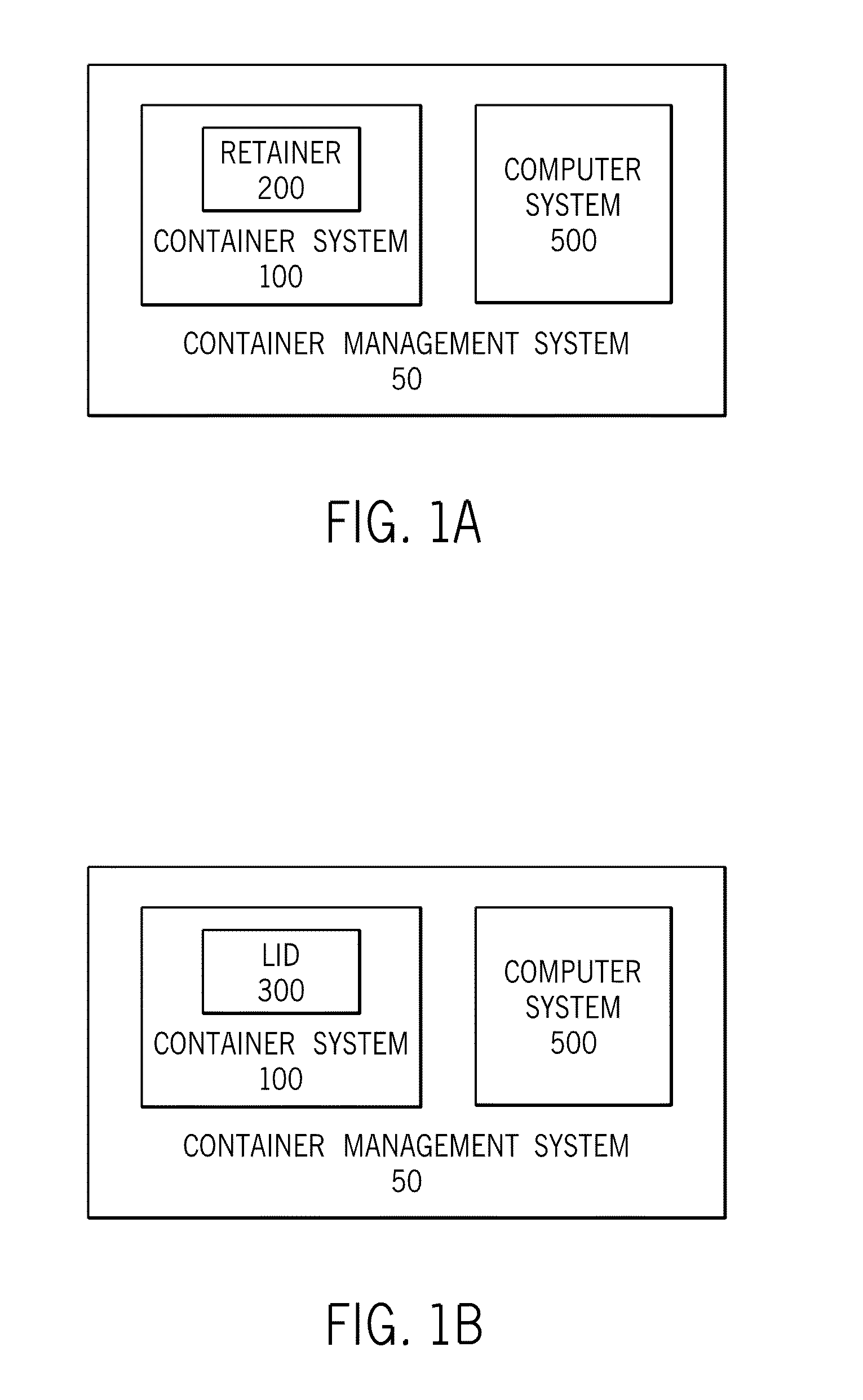
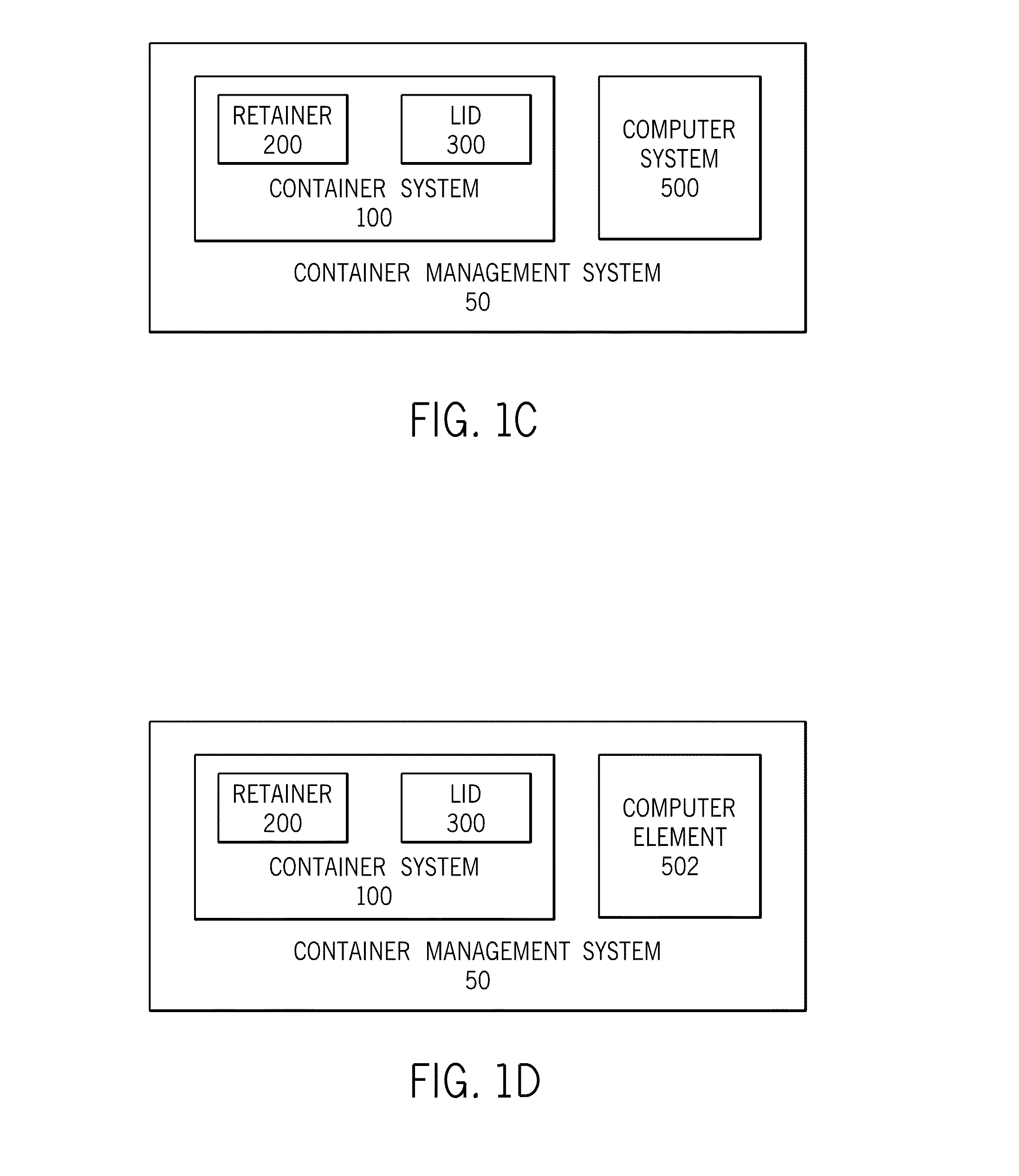
System and methods for managing a container or its contents
ActiveUS20150122688A1Reduce leakageMinimize spillCapsClosure with auxillary devicesComputerized systemEngineering
Certain embodiments of the present invention include a retainer, a lid, and a sensor, where the sensor is configured to detect information about the retainer, the lid, or the contents in the retainer. The sensor also may be configured to communicate with an internal or external computer system, thereby facilitating showing the detected information as a representation via a display element. In certain embodiments, the system may include an action element such as an open / close lid opening assembly configured to permit automatically or manually opening or closing a drink aperture or another type of dispensing aperture.
Owner:THERMOS LLC
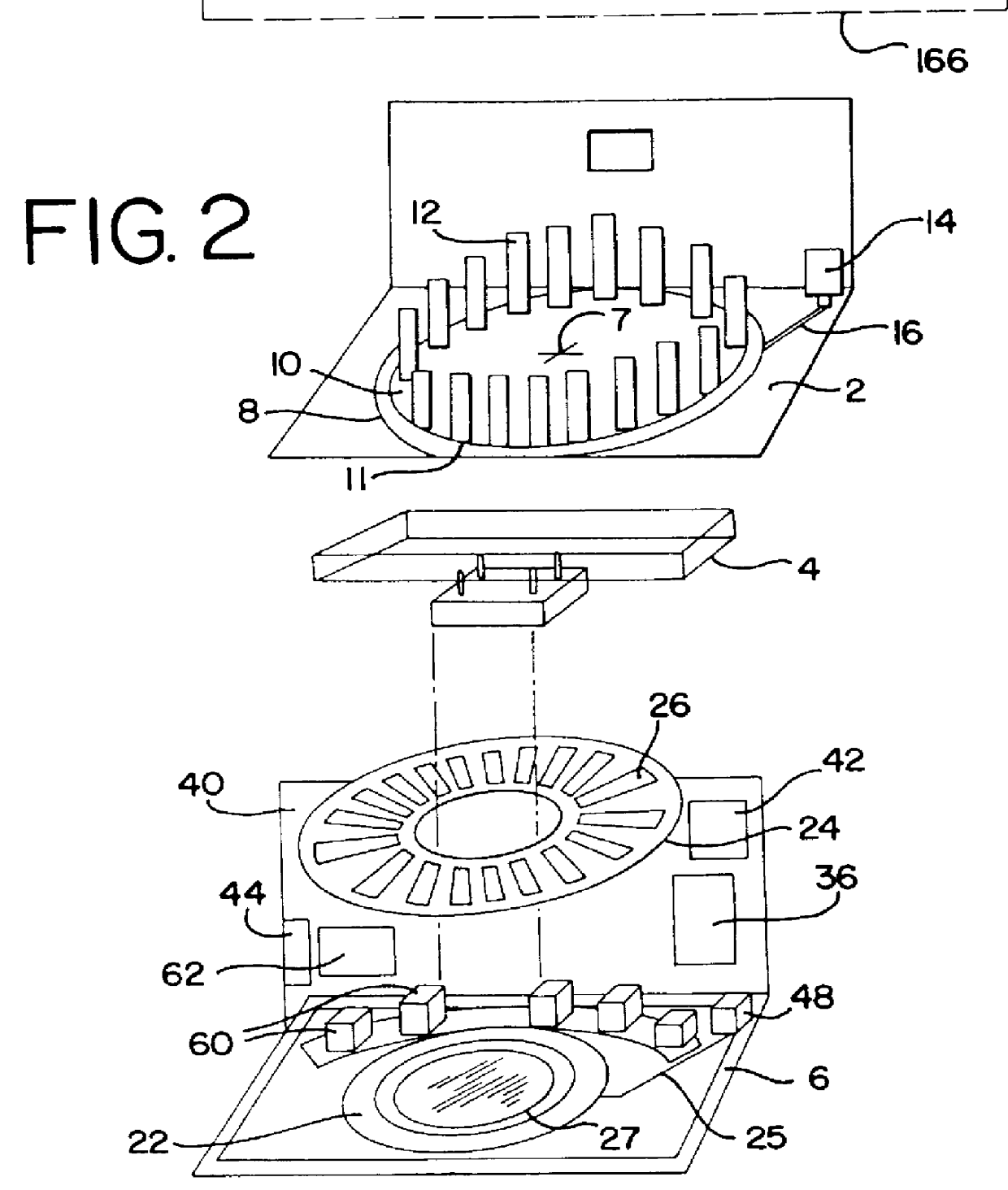
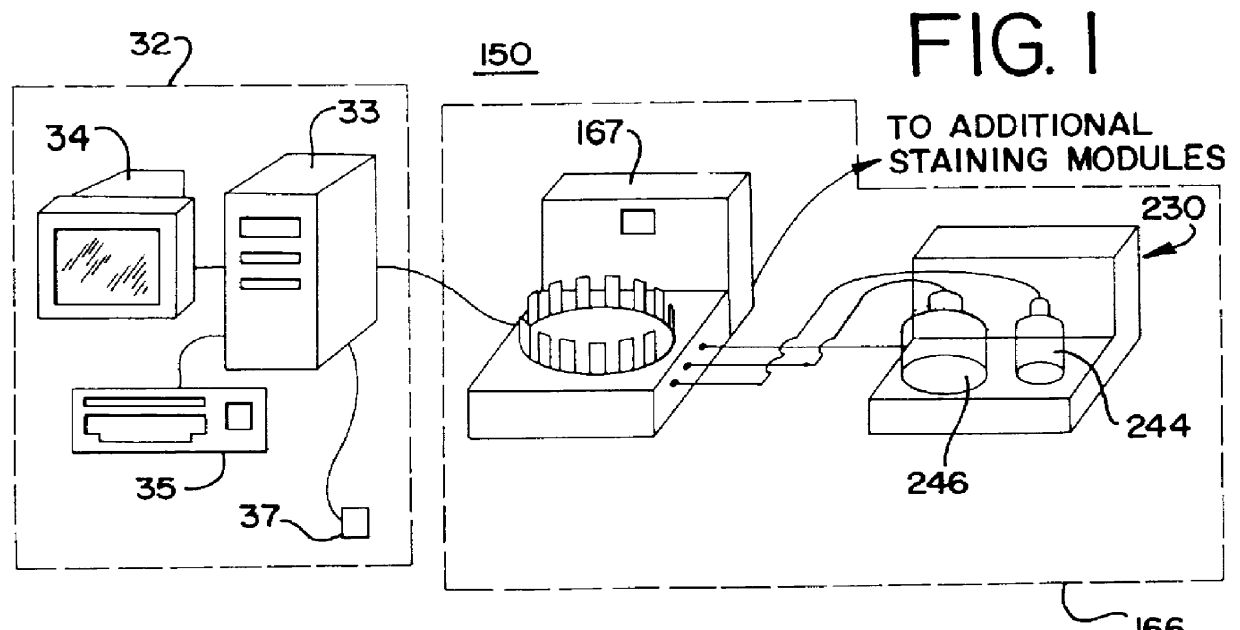
Fluid dispenser
InactiveUS6045759AEfficient and reliableEasy to manufactureCheck valvesLarge containersModularityReactive system
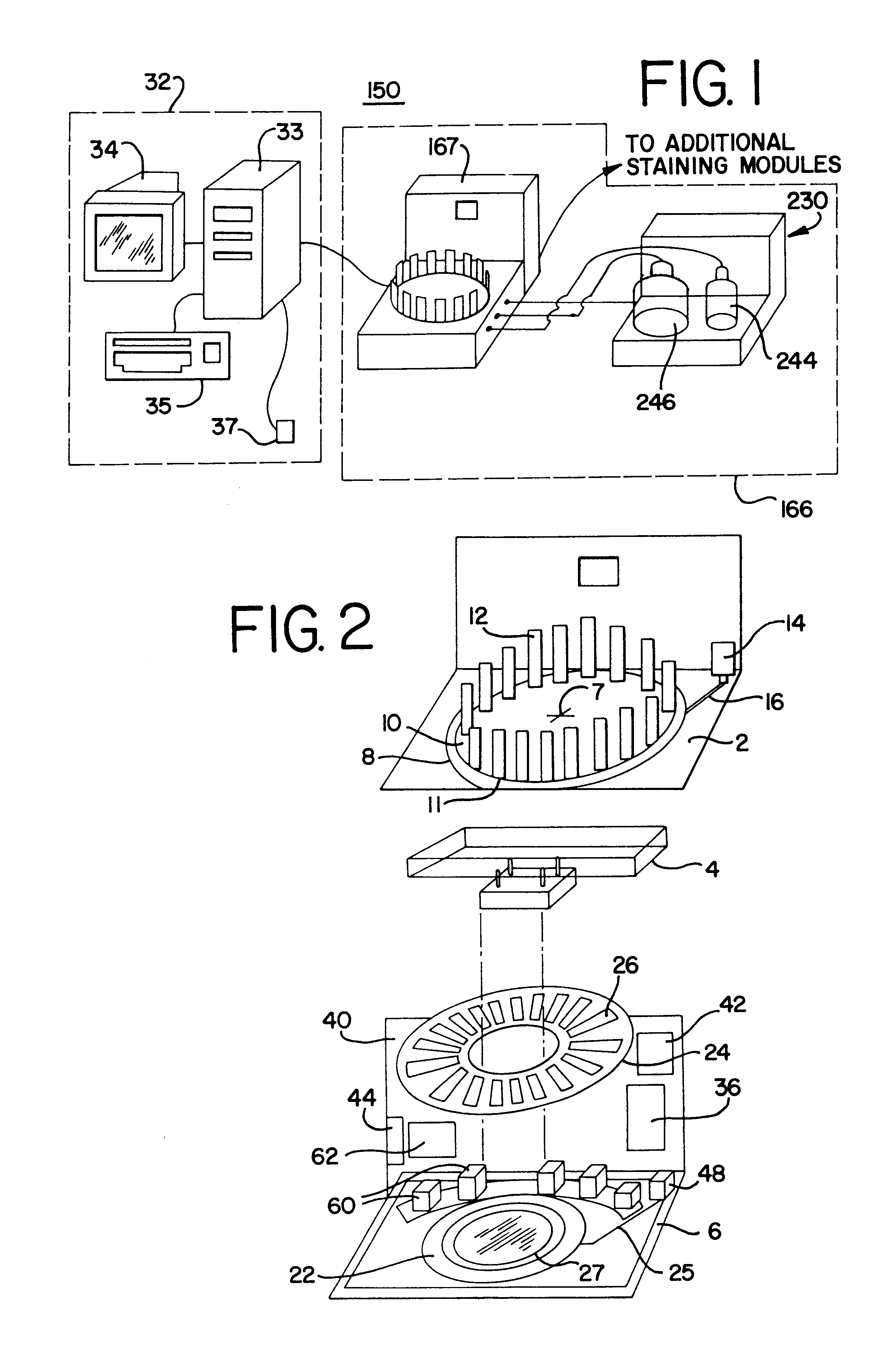
A method and apparatus for an automated biological reaction system is provided. In the processing of a biological reaction system, there is a need for consistently placing an amount of fluid on a slide. In order to accomplish this, several methods are used including a consistency pulse and a volume adjust means. Moreover, in order to reliably operate an automated biological reaction system, the dispenser must be reliable, easy to assemble and accurate. Among other things, in order to accomplish this, the dispense chamber is substantially in line with the reservoir chamber, the reservoir chamber piston is removed, and the flow of fluid through the dispenser is simplified. Further, in order to operate the automated biological reaction system more reliably, the system is designed in modular pieces with higher functions performed by a host device and the execution of the staining operations performed by remote devices. Also, to reliably catalog data which is used by the automated biological reaction system, data is loaded to a memory device, which in turn is used by the operator to update the operator's databases. The generation of the sequence of steps for the automated biological reaction device based on data loaded by the operator, including checks to determine the ability to complete the run, is provided.
Owner:VENTANA MEDICAL SYST INC
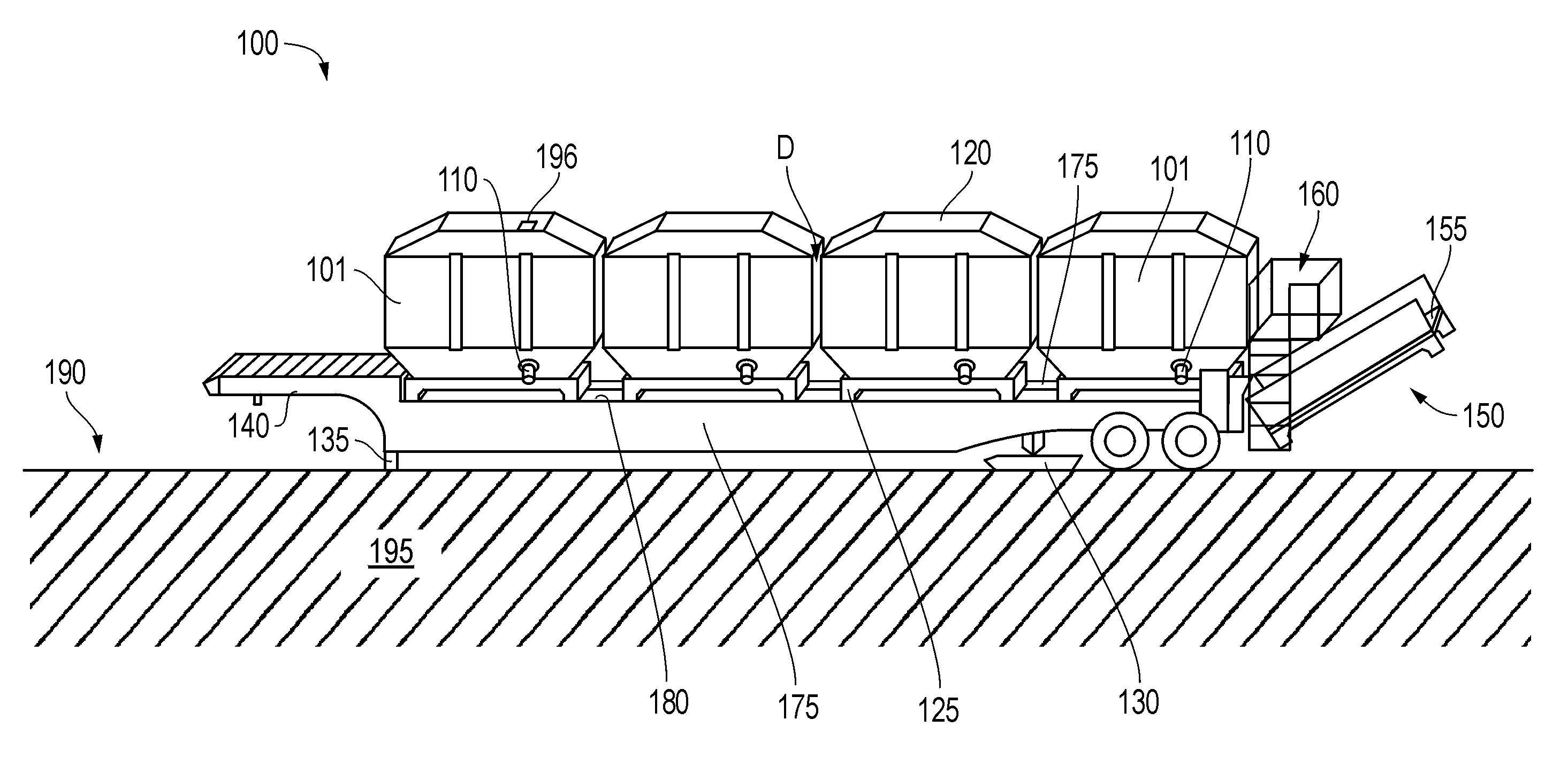
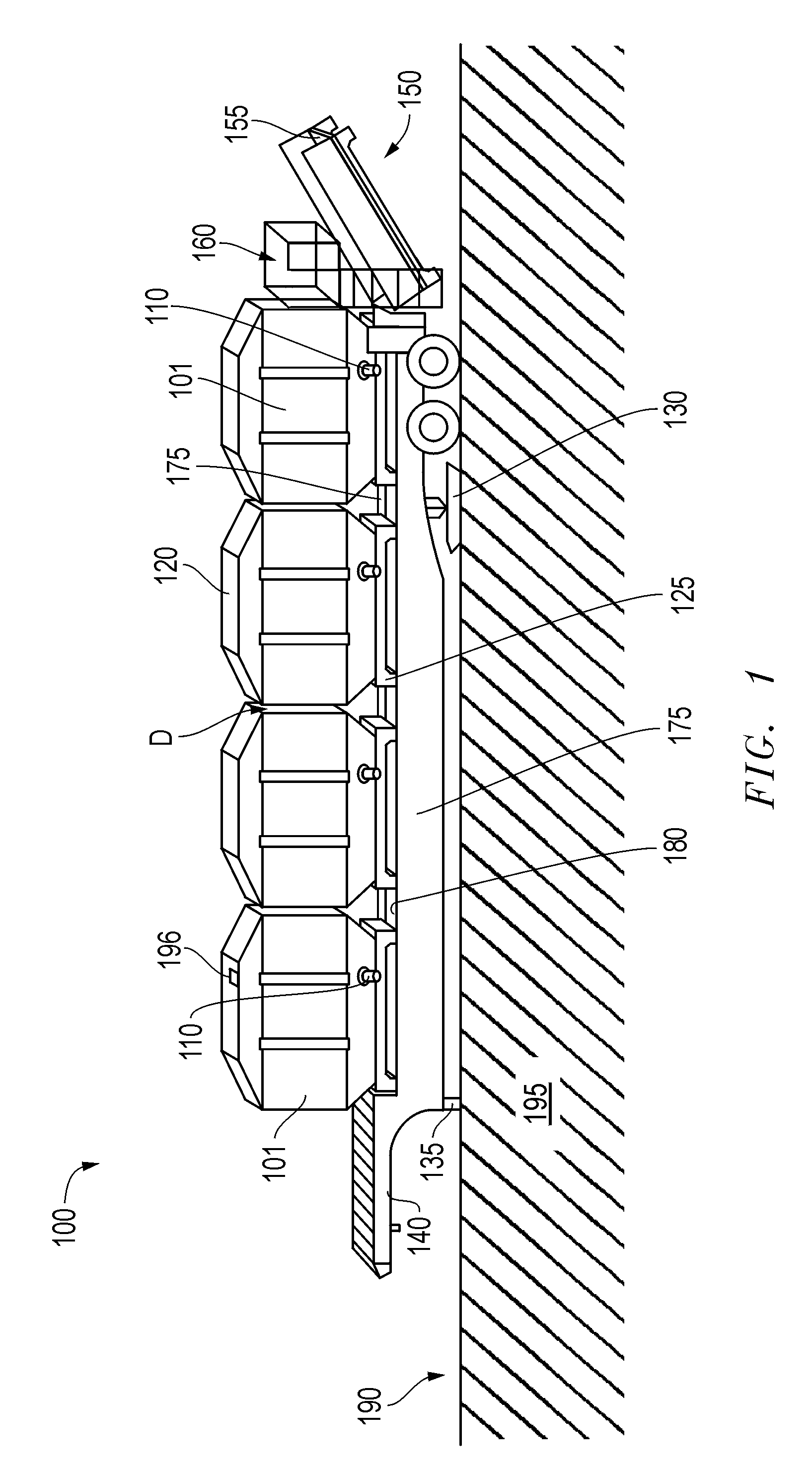
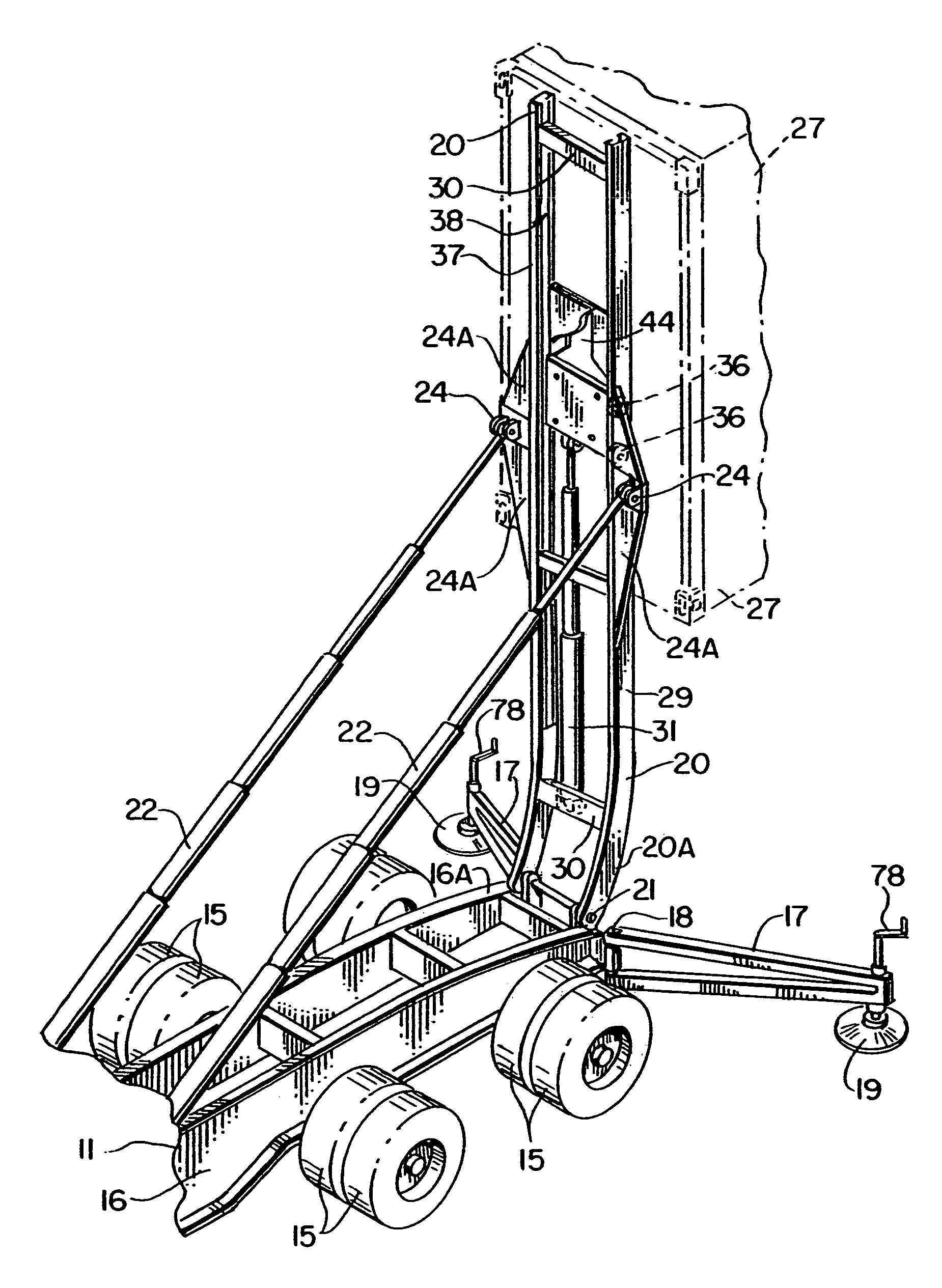
Method and apparatus for supplying bulk product to an end user
InactiveUS7214028B2Eliminate needEfficient transportTank vehiclesMixing operation control apparatusMarine engineeringEngineering
A method and apparatus for supplying bulk material to an end user includes the step of providing a bulk material source that is at a location distant from the end user and a specially configured vessel and trailer apparatus for transporting the bulk material to the end user. The vessel is filled with bulk material at the bulk material source and then transported with a specially configured trailer. Alternatively, at source or destination, the vessel can remain as a temporary storage device, free-standing from the trailer. During transport between the bulk material source and the end user, the vessel is filled or partially filled with a selected bulk material. During transport, the vessel is in a generally horizontal position, supported by the trailer and a specially configured elevator. The vessel is unloaded from the trailer by moving the vessel longitudinally along the trailer and by transferring the vessel from a generally horizontal position upon the trailer to an elevated upright position.
Owner:TILT TANK L L C
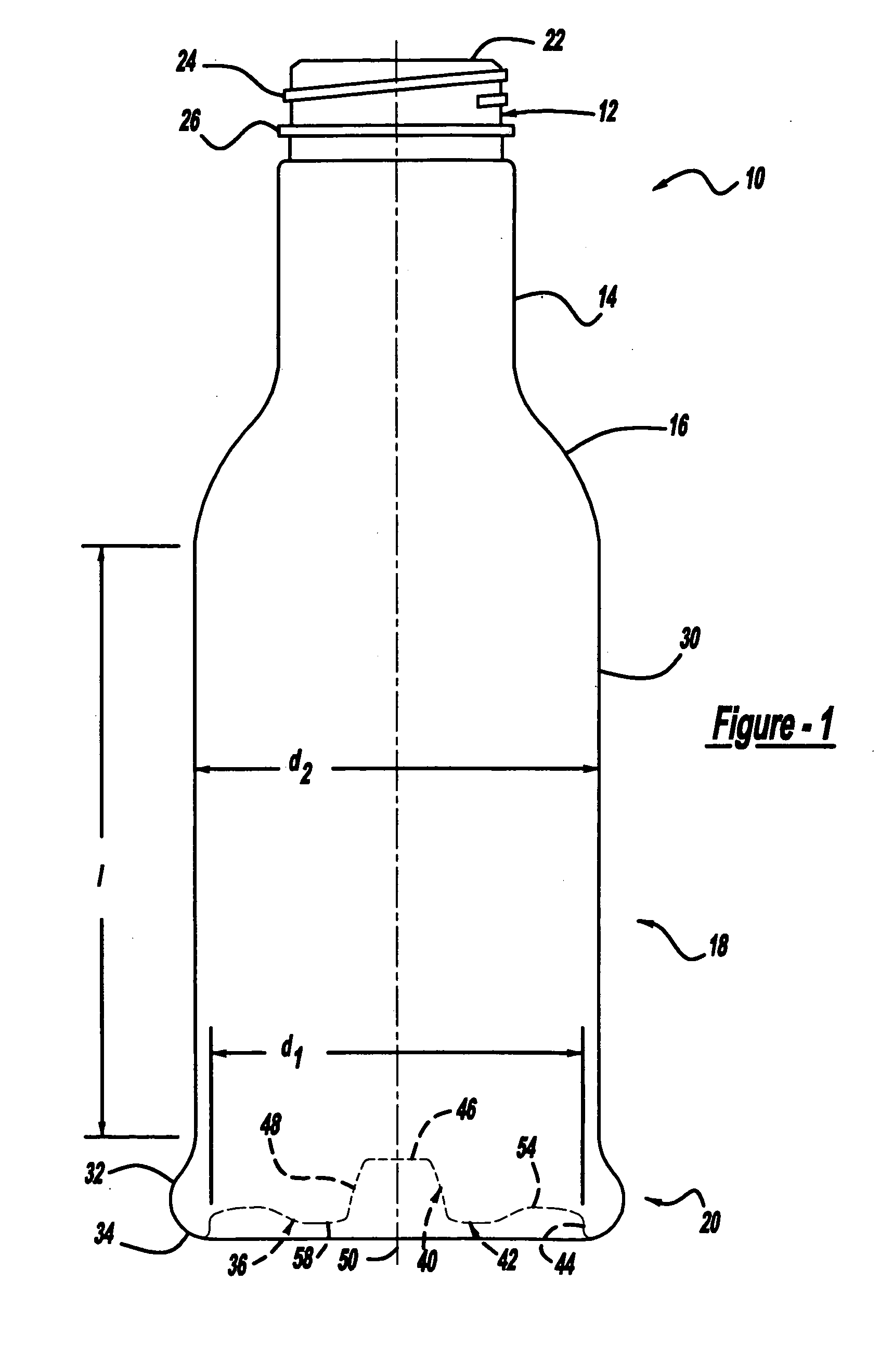
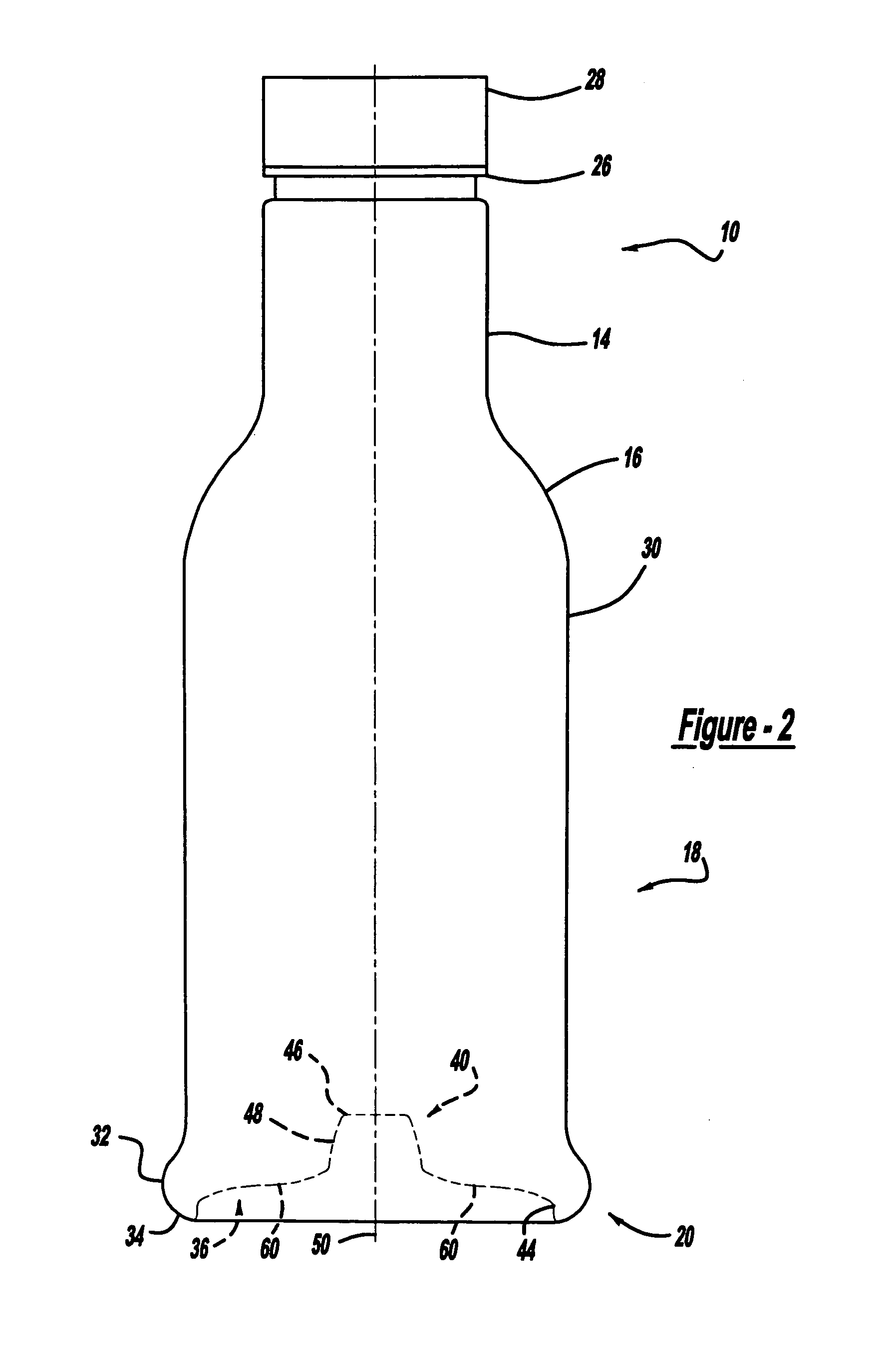
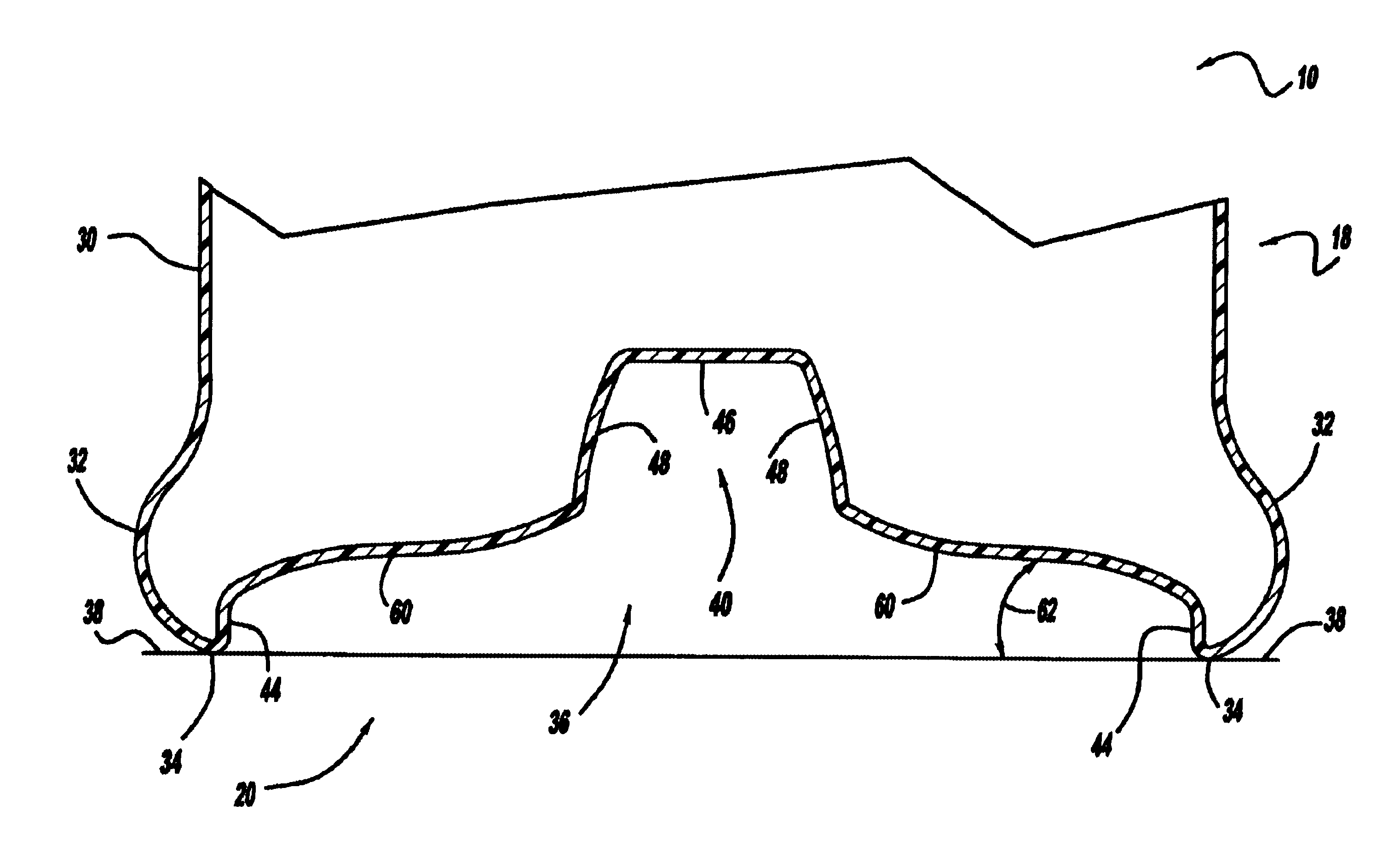
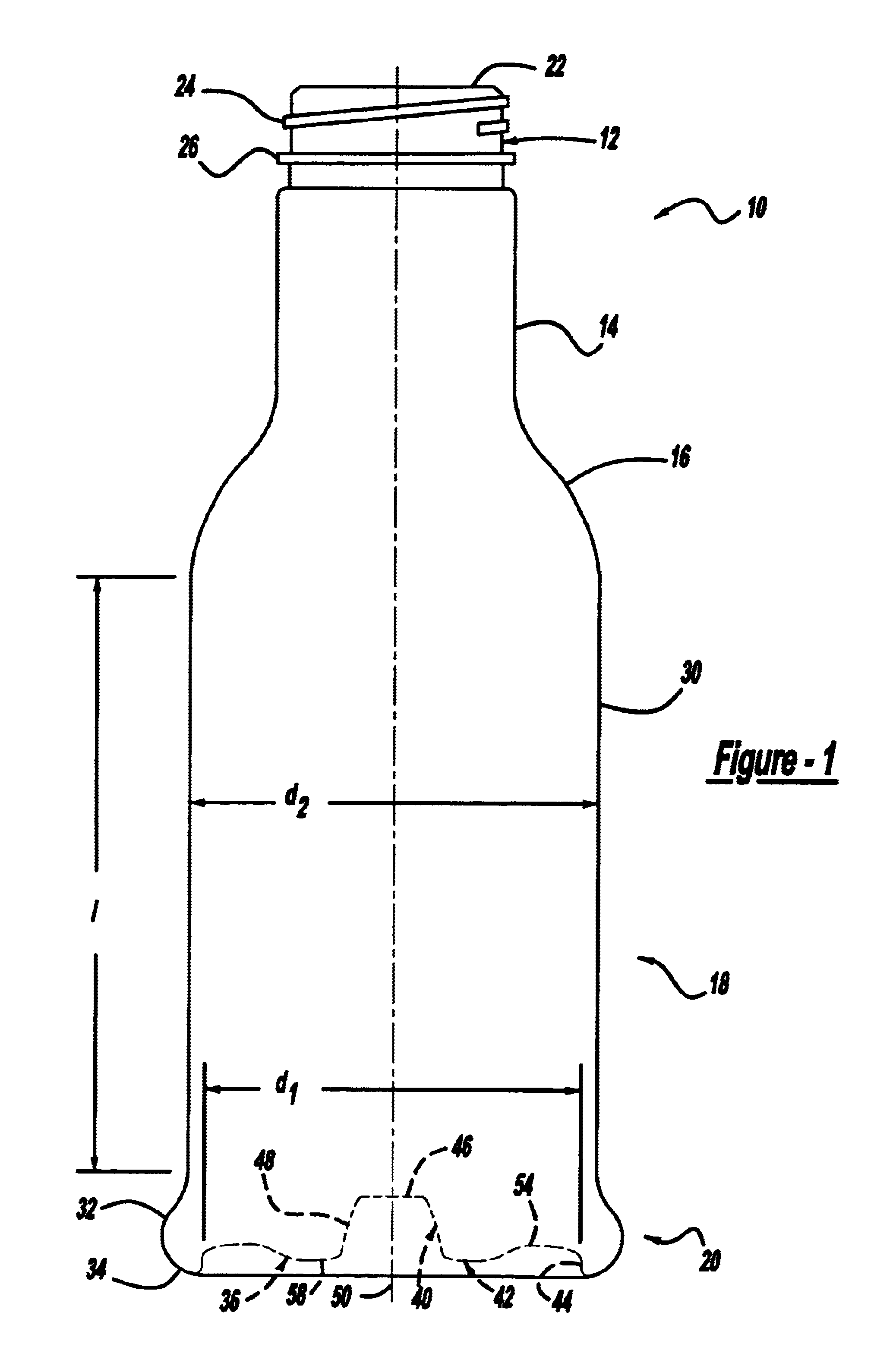
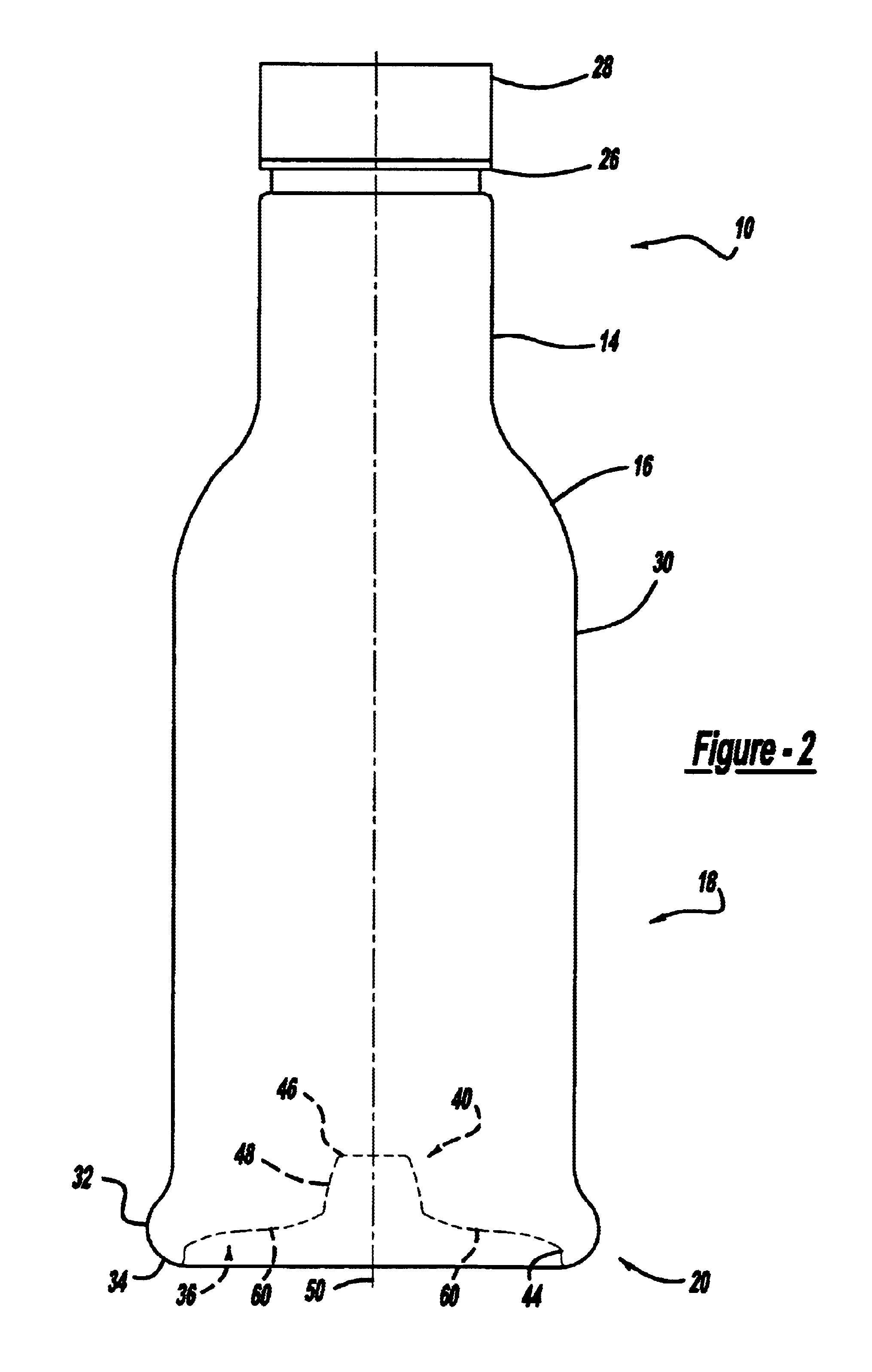
Repositionable base structure for a container
A base of a container including a bearing surface, a hinge, a first wall, and a second wall. The first wall sloping in a first direction from the bearing surface to the hinge, and the second wall sloping in a second direction away from the hinge. The second wall is adapted to be repositioned about the hinge with substantially no movement of the first wall.
Owner:CO2 PAC
Industrial hopper with support
ActiveUS7475796B2Efficient configurationInexpensive materialsLarge containersLiquid transferring devicesSupporting systemEngineering
An industrial hopper and support system includes a hopper having a plurality of receivers complementally configured to receive the top ends of legs of a support. The hopper is particularly designed to receive and discharge both solid and liquid material by the mounting of a selected valve, and the receivers include recesses therein which inhibit the spread of the legs and provide a structural connection between the legs of the support when the hopper is mounted thereon. The hopper may be mounted to the legs without the use of tools, but a separate fastener may be used when it is desired to lift both the hopper and its support from above. The support is provided with openings through primary tubular members which align with crossmembers so that forks inserted into the openings pass longitudinally through the crossmembers.
Owner:TANK HLDG CORP
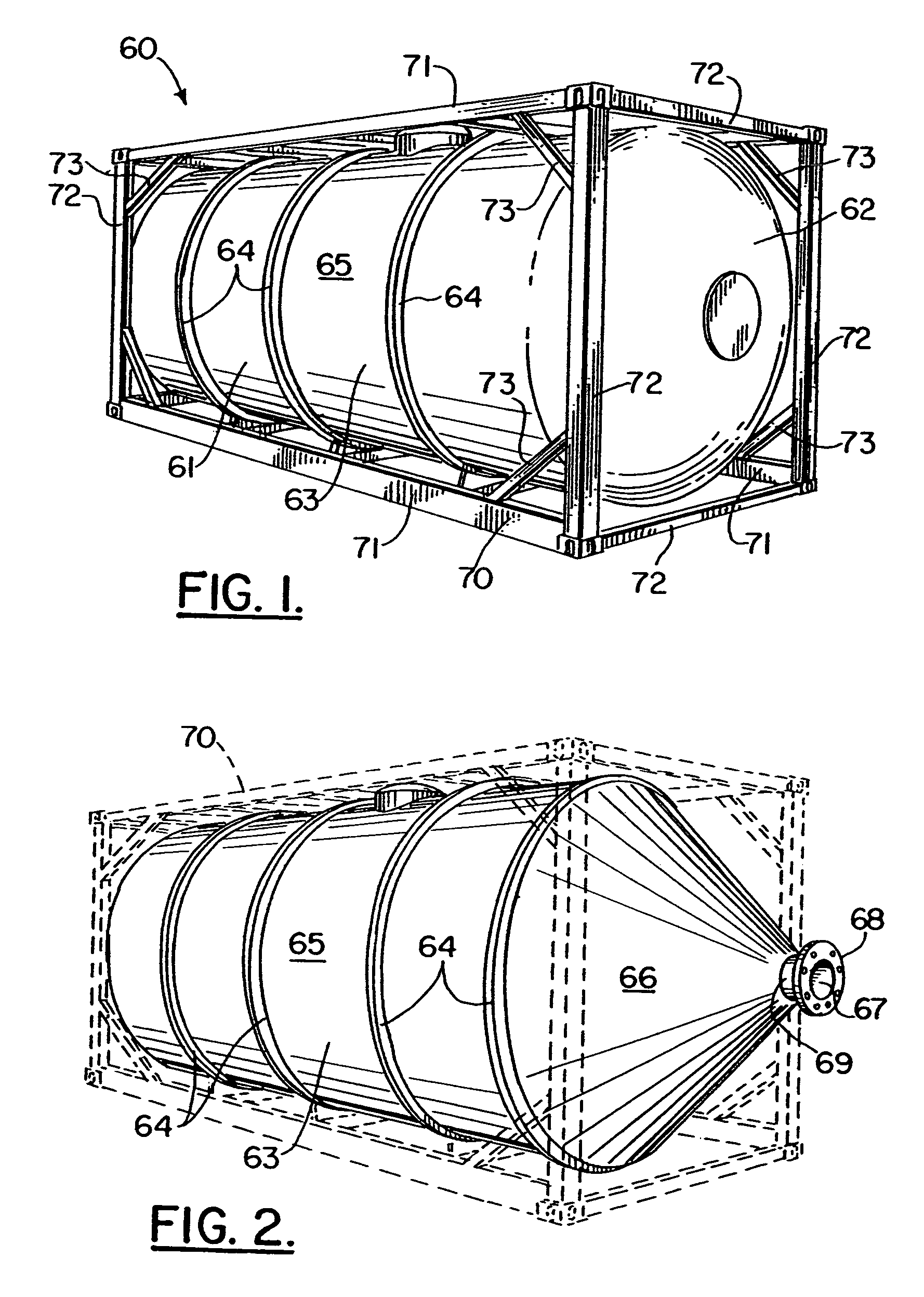
Containerised handling of bulk materials and apparatus therefor
InactiveUS7252309B2Advantageously producedFlow mixersLarge containersParticulate pollutionEngineering
A freight container (10) has a base (20) or a portion thereof that can be opened to discharge its contents. This container (10) has a top which can be opened in similar manner as the base. Another container having at least one compartment, each compartment having a lower section with a reducing cross-sectional area and a pivotably operable closure assembly, with several such closure assemblies being linked together by means of bars. Such a container is used to contain raw building materials for stockpiling of these materials at a container port. It is also used to supply materials to a concrete production plant whee pollution control containers are provided below the supply container and above the scaling and mixing stations to reduce particulate pollution. A pair of slewing apparatuses is also provided to engage both ends of a container and turn the container over about its longitudinal axis, thereby emptying its contents.
Owner:ONG BEE KIM
Collapsible container
ActiveUS20050127073A1Compact configurationMaximize available spaceLarge containersVariable capacity containersEngineeringMechanical engineering
Owner:DART IND INC
Uses and compositions for treatment of juvenile rheumatoid arthritis
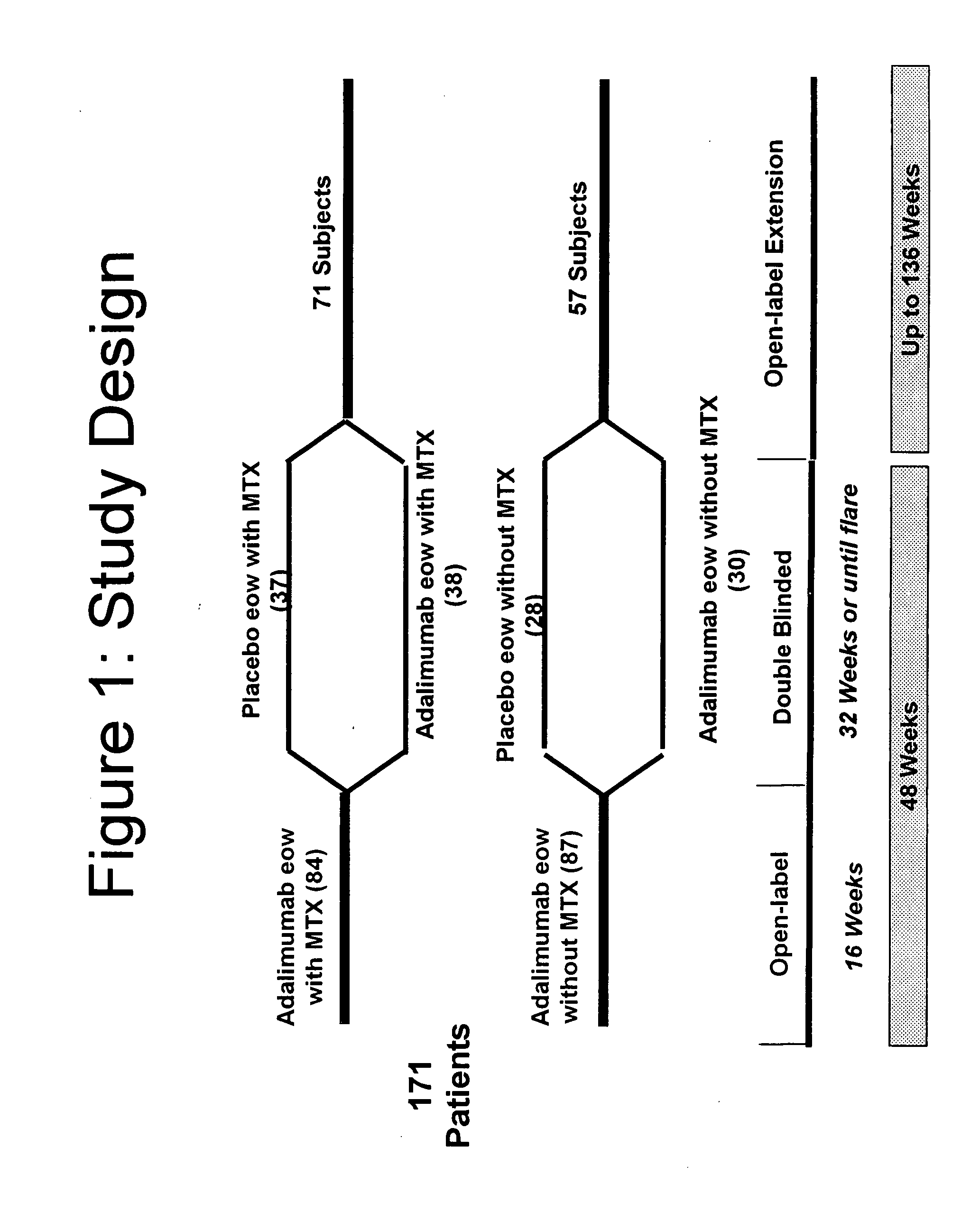
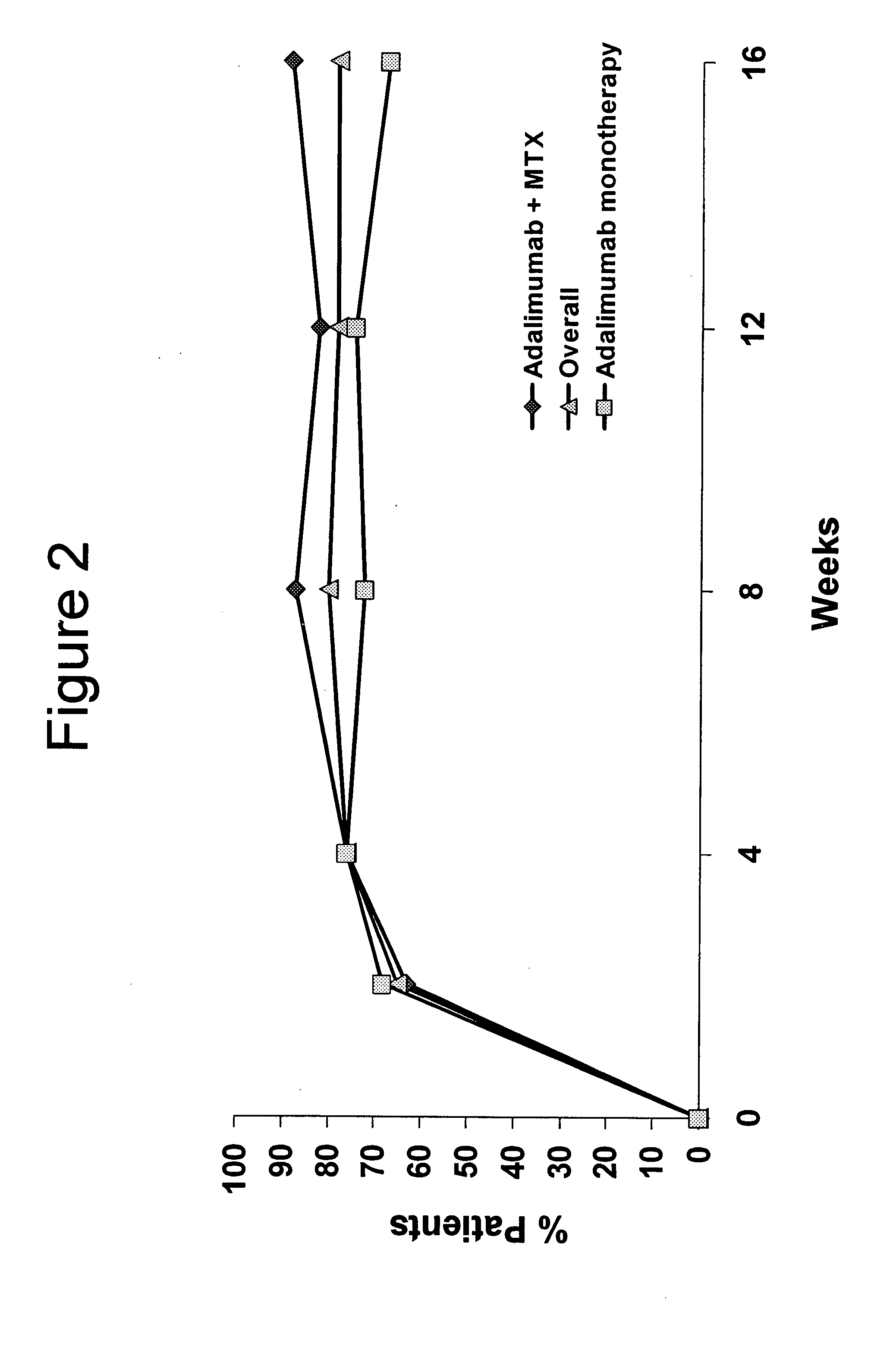
InactiveUS20080118496A1Prevent outbreakTreatment safetyLarge containersAntibody ingredientsMedicineAntigen binding
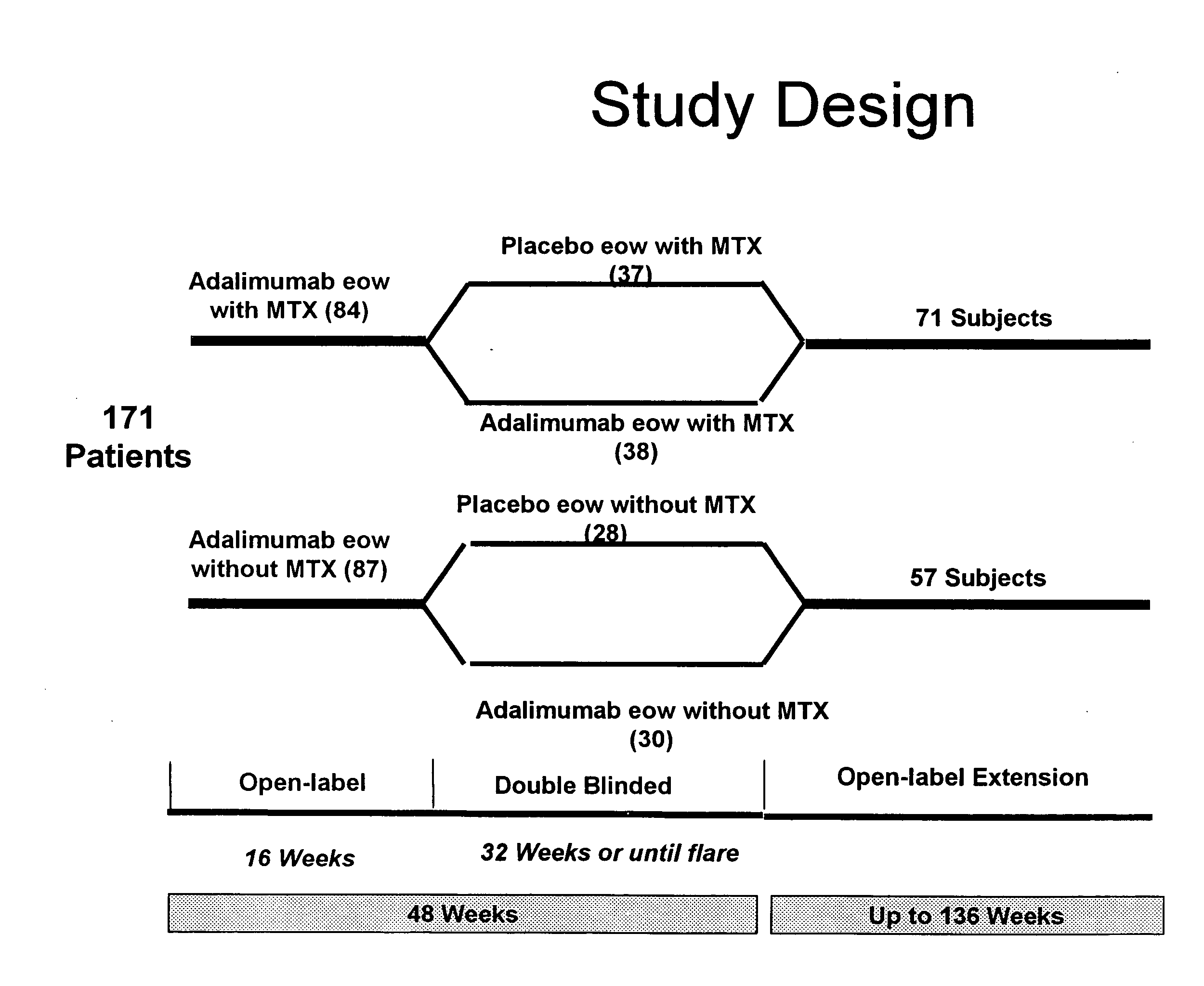
The invention provides methods, uses and compositions for the treatment of juvenile rheumatoid arthritis (JRA). The invention describes methods and uses for treating JRA, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to prevent flare-ups associated with JRA. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of JRA in a subject.
Owner:MEDICH JOHN R +4
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS6889690B2Easy to optimizeLimit amount of resistanceSmall article dispensingLiquid surface applicatorsPowder InhalerInhalation
The present invention includes dry powder inhalers and associated multi-dose dry powder packages for holding inhalant formulated dry powder substances and associated fabrication and dispensing methods. The multi-dose package can include a platform body comprising at least one thin piezoelectric polymer material layer defining at least a portion of a plurality of spatially separated discrete elongate dry powder channels having an associated length, width and height; and a metallic material attached to selected portions of the piezoelectric polymer material including each of the regions corresponding to the elongate dry powder channels to, in operation, define active energy releasing vibratory channels. In operation, the elongate channels can be selectively individually activated to vibrate upon exposure to an electrical input.The dry powder inhaler includes an elongate body having opposing first and second outer primary surfaces with a cavity therebetween and having opposing top and bottom end portions and a multi-dose sealed blister package holding a plurality of discrete meted doses of a dry powder inhalable product located in the cavity of the elongate body. The inhaler also includes an inhalation port formed in the bottom end portion of the elongate body, the inhalation port configured to be in fluid communication with at least one of the discrete meted doses during use and a cover member that is pivotably attached to the elongate body so that it remains attached to the body during normal operational periods of use and moves to a first closed position to overlie the inhalation port at the bottom end portion of the body during periods of non-use and moves to a second open position away from the inhalation port during periods of use to allow a user to access the inhalation port.
Owner:ORIEL THERAPEUTICS INC
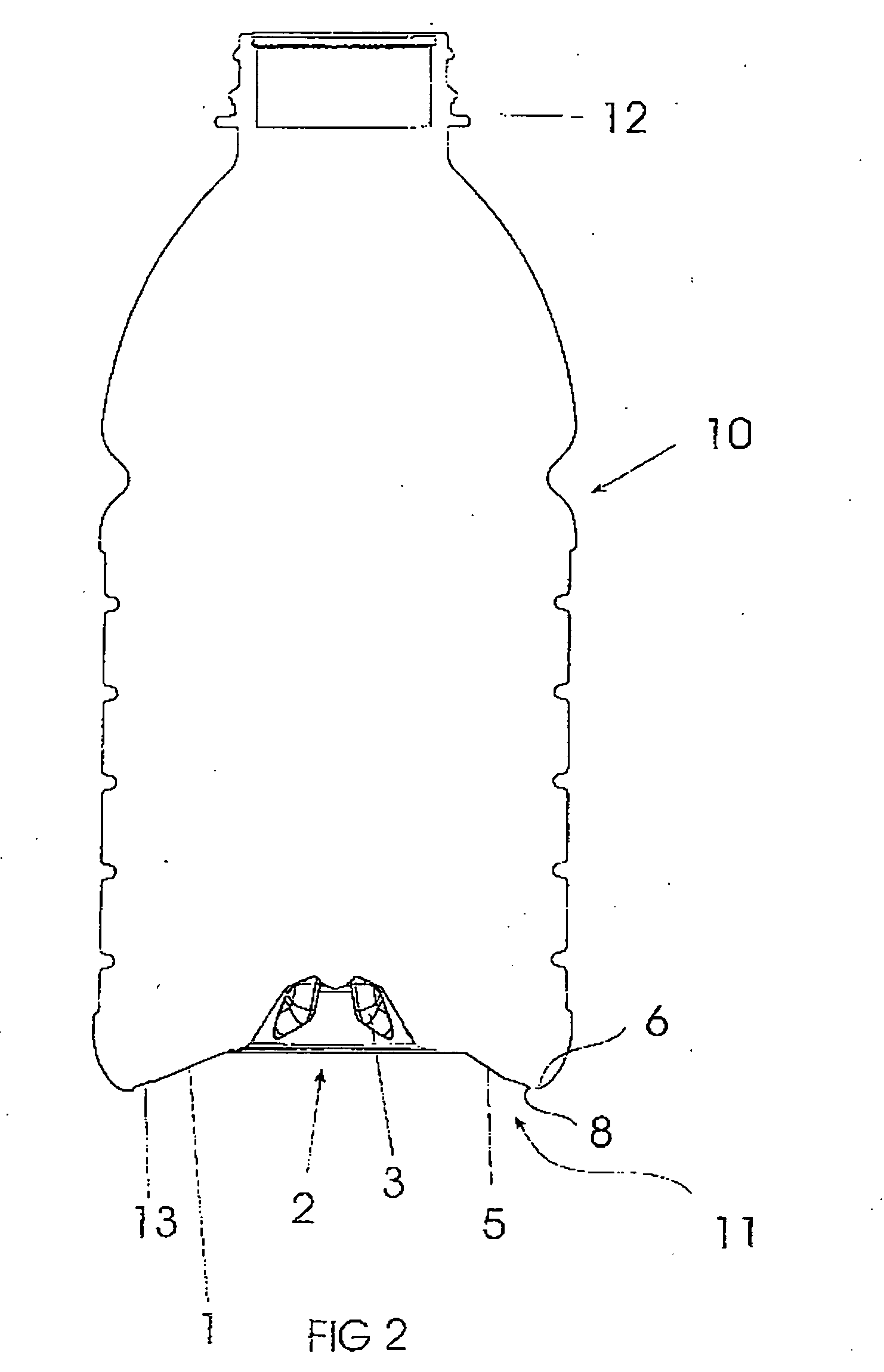
Pressurized plastic bottle for dispensing an aerosol
InactiveUS7028866B2Deformation MinimizationUniform changeLarge containersLiquid dispensingEngineeringAerosol composition
A pressure resistant plastic bottle for containing and dispensing an aerosol composition. The plastic bottle is comprised of a hollow elongate body having a central portion, a top portion and an opposite bottom portion with the central portion having an inwardly concave configuration extending along its longitudinal direction. The bottom portion of the elongate body is integral with the central portion and defines an outwardly projecting convexly shaped configuration. The top portion of the bottle is integral with the central portion and has an outwardly convex configuration extending along its longitudinal direction and defines a neck having an opening for receiving and dispensing the aerosol composition. A closure covers the opening and is sealingly attached to the neck to contain the aerosol within the plastic bottle.
Owner:SC JOHNSON & SON INC
Insulated water-tight container
InactiveUS6296134B1Easy and inexpensive to manufacturePreserving spaceDomestic cooling apparatusLighting and heating apparatusEngineeringWater resistant
An insulated container for shipping, transporting, or storing warm or cold items is disclosed, useful for maintaining temperature of items stored or shipped within the container, the container assembly consisting of at least one layer of rigid or semi-rigid material, and at least one layer of flexible, thermally insulating, water-resistant material, in the form of a pouch, which pouch is secured to the rigid material at areas which allow easy reconfiguration of the container to form a finished container having desirable insulating and water-resistant characteristics.
Owner:THERMAL SHIPPING SOLUTIONS
Container base structure responsive to vacuum related forces
A plastic container having a base portion adapted for vacuum pressure absorption. The base portion including a contact ring that supports the container, an upstanding wall, and a central portion. The upstanding wall being adjacent to and generally circumscribing the contact ring. The central portion defined in at least part by a pushup and an inversion ring that generally circumscribes the pushup. The pushup and the inversion ring being moveable to accommodate vacuum related forces generated within the container.
Owner:AMCOR RIGID PLASICS USA LLC
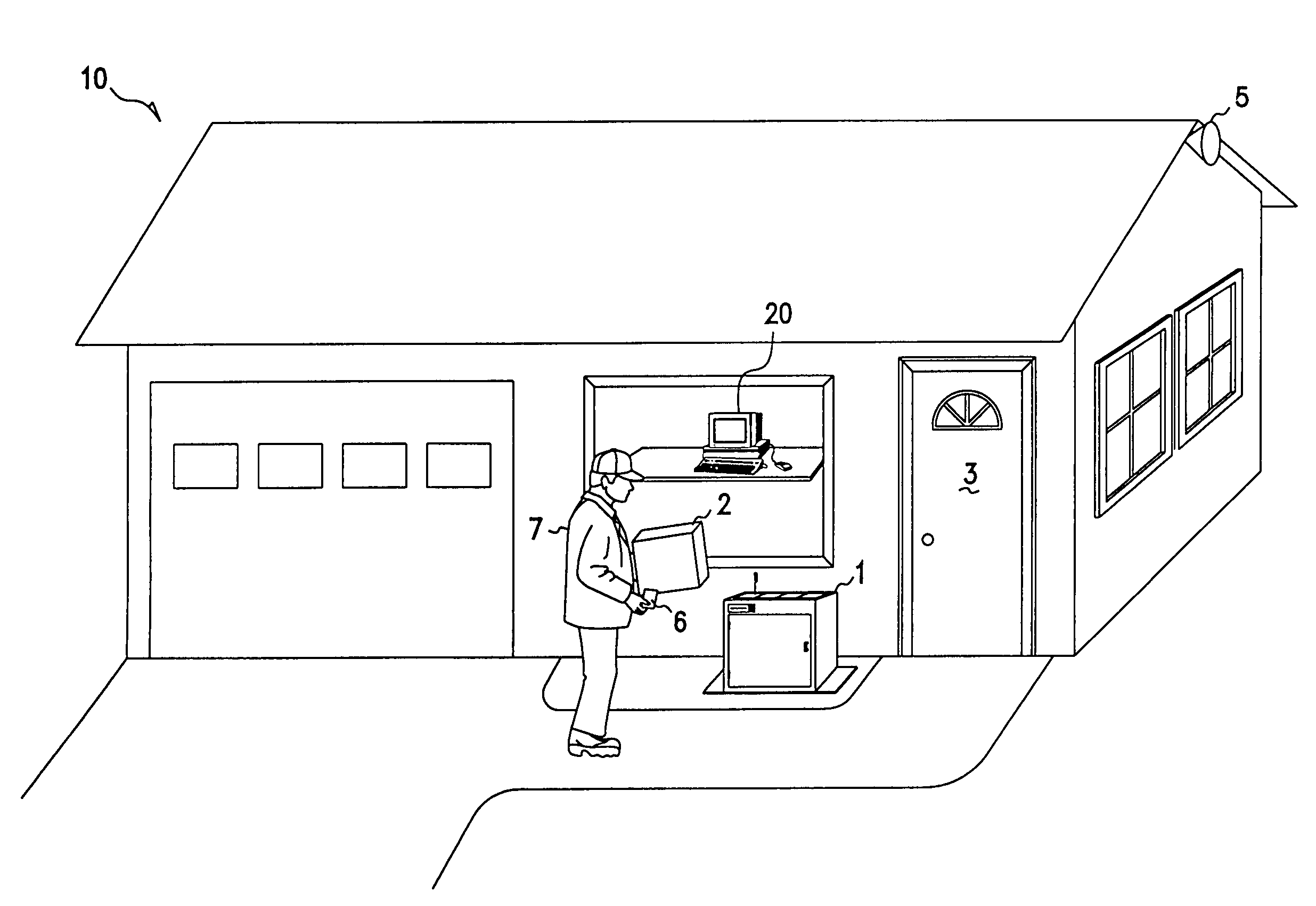
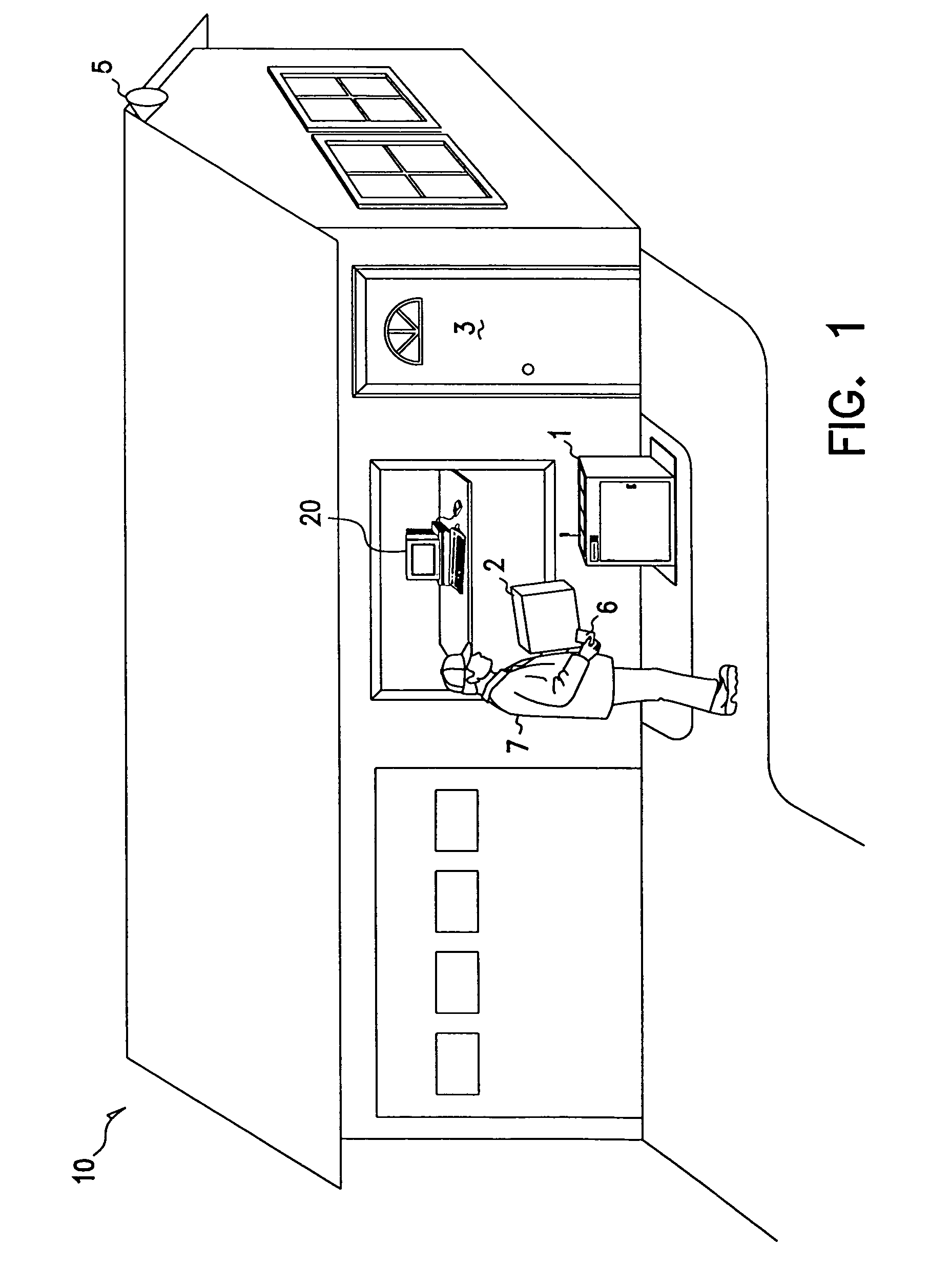
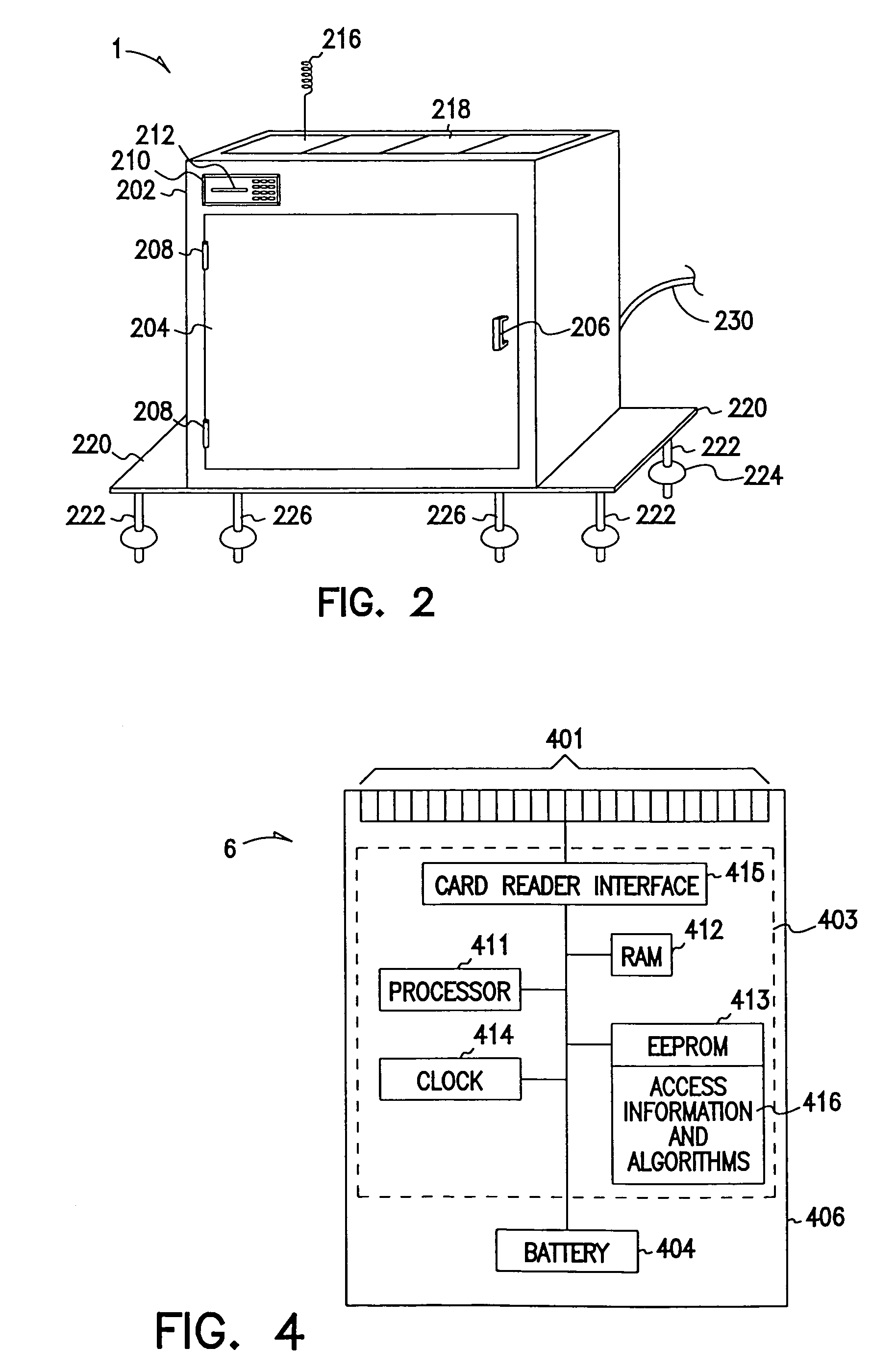
Methods and apparatus for unattended pickups and deliveries
A secure pickup and delivery container includes a lockable door, a control unit, an access element, and an anchoring element, according to one embodiment. The control unit includes a processing element and a memory that can be programmed either on-site or remotely with access privilege information such as identity (e.g. of container, delivery person, etc.), location, date, time, frequency of access, and / or package-specific information. In one embodiment, access privilege information is programmed when an intended recipient of a delivery consummates a point of sale transaction, for example over the Internet or telephone. The access element can be a keypad, a biometric scanner, a card reader, a bar-code reader, and / or a wireless control element to read a programmable token such as a smart card. Delivery personnel can enter access request information into the access element, and if it favorably compares with the access privilege information, the control unit unlocks the door. Notification can be concurrently made via wireline or wireless communications to the intended recipient, who may be situated remote from the secure container. The recipient can optionally return a delivery acknowledgment to the delivery personnel. Details of the delivery transaction can also be recorded electronically on the delivery personnel's token, within the container, or at a remote location.
Owner:INTEL CORP
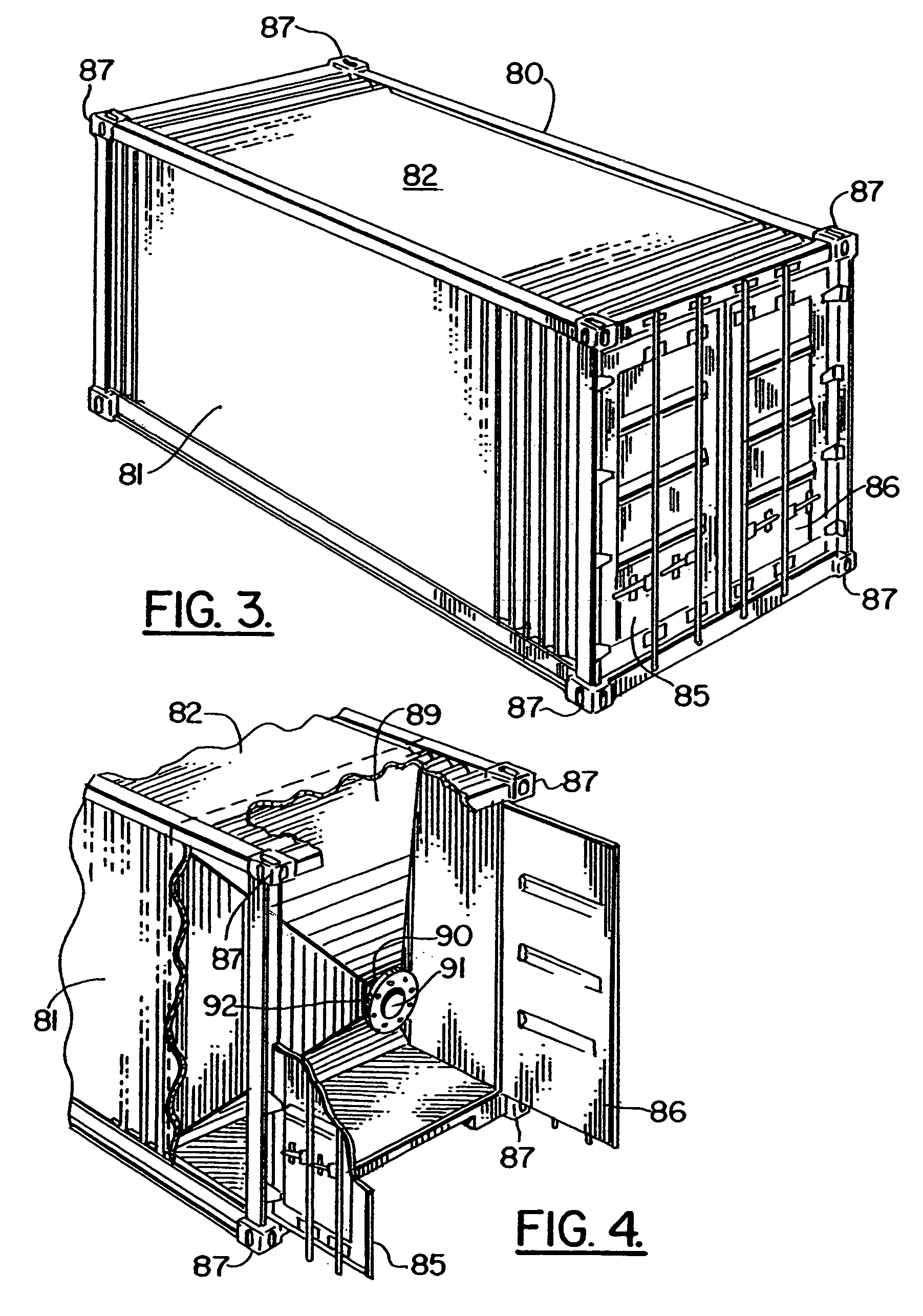
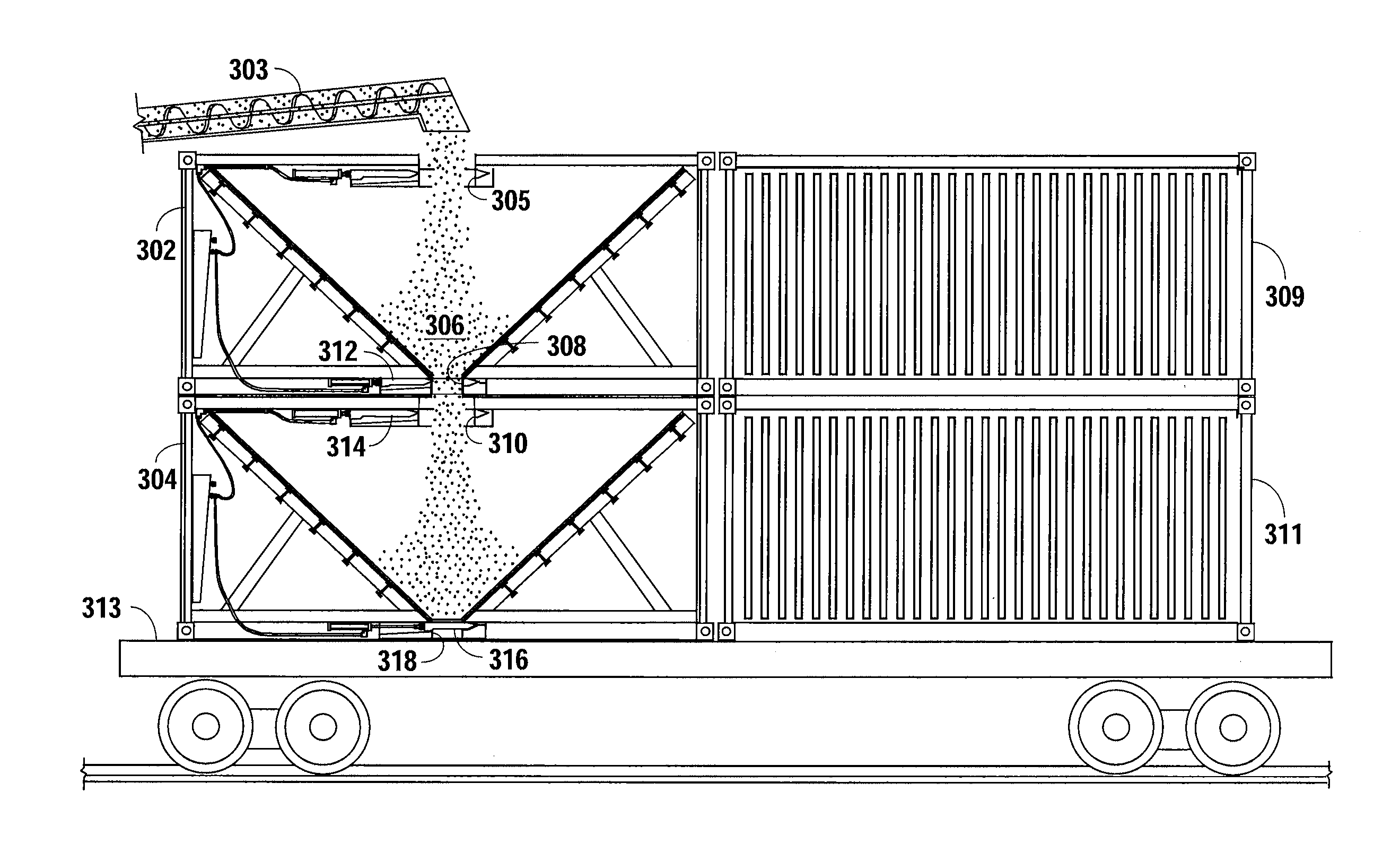
Method and Apparatus for Modifying a Cargo Container to Deliver Sand to a Frac Site
A cargo container is modified to carry a fracing proppant such as sand from a quarry or source to the frac site. Openings are cut in the top and bottom of a cargo container and hydraulically operated sliding doors are placed there under. A hopper module with the walls being inclined to approximately the angle of repose for the proppant is installed inside the cargo container. The hopper module is sealed inside the cargo container so that a proppant enters through the top opening at the quarry and flows out through the bottom opening at the fracing site.
Owner:SANDCAN INC
Container base structure responsive to vacuum related forces
A plastic container having a base portion adapted for vacuum pressure absorption. The base portion including a contact ring upon which the container is supported, an upstanding wall and a central portion. The upstanding wall being adjacent to and generally circumscribing the contact ring. The central portion being defined in at least part by a central pushup and an inversion ring which generally circumscribes the central pushup. The central pushup and the inversion ring being moveable to accommodate vacuum forces generated within the container.
Owner:AMCOR RIGID PLASICS USA LLC
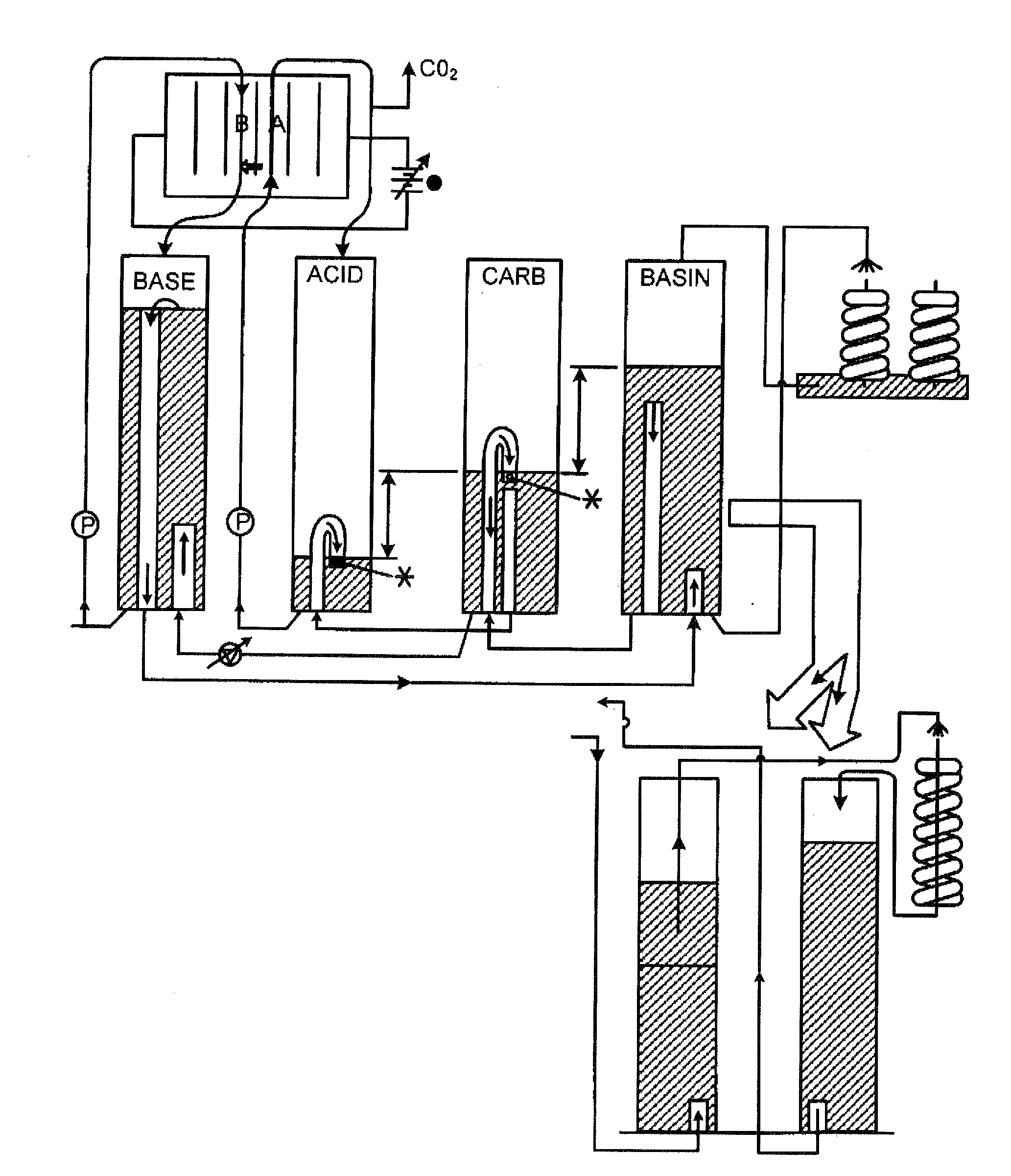
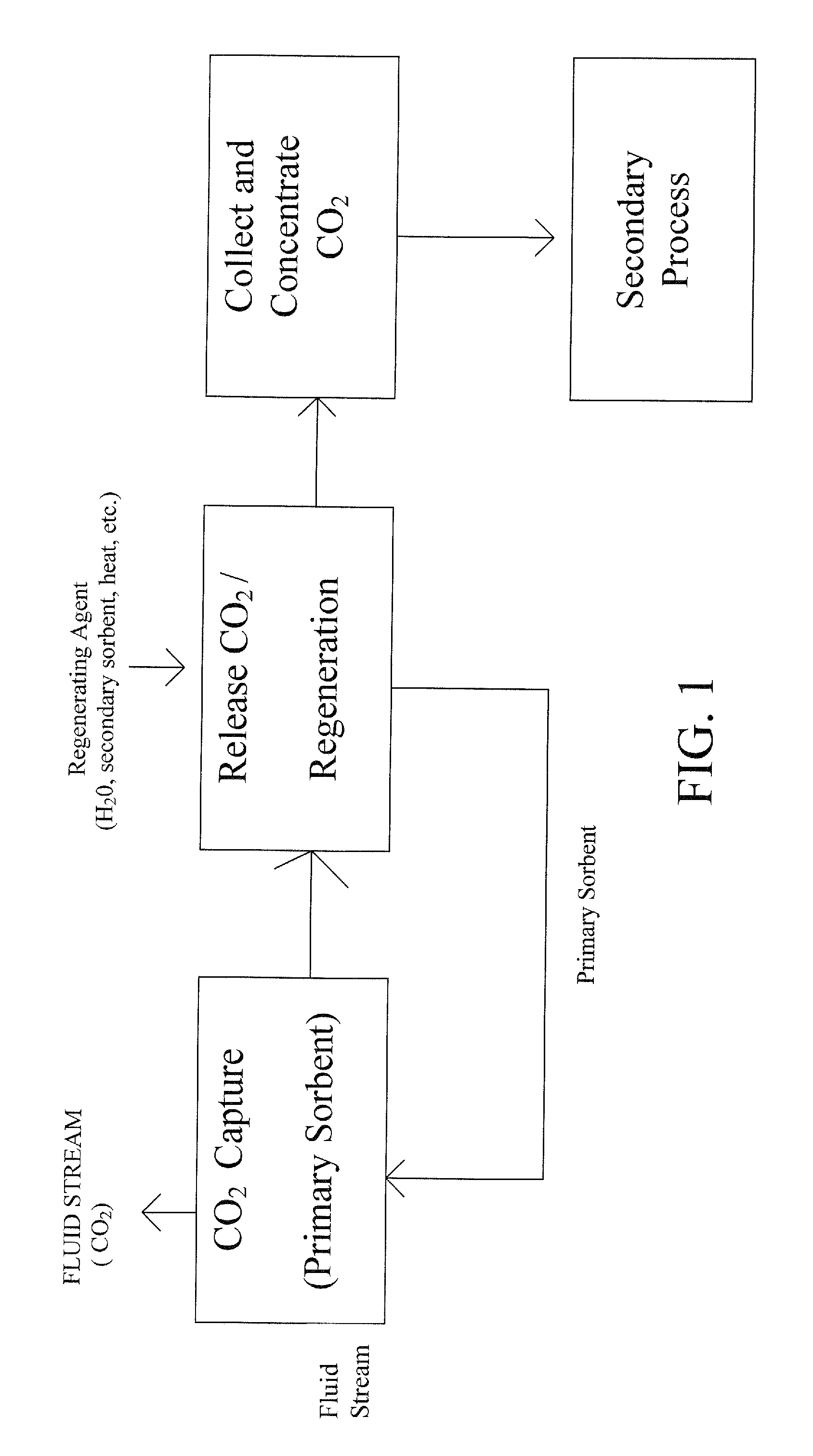
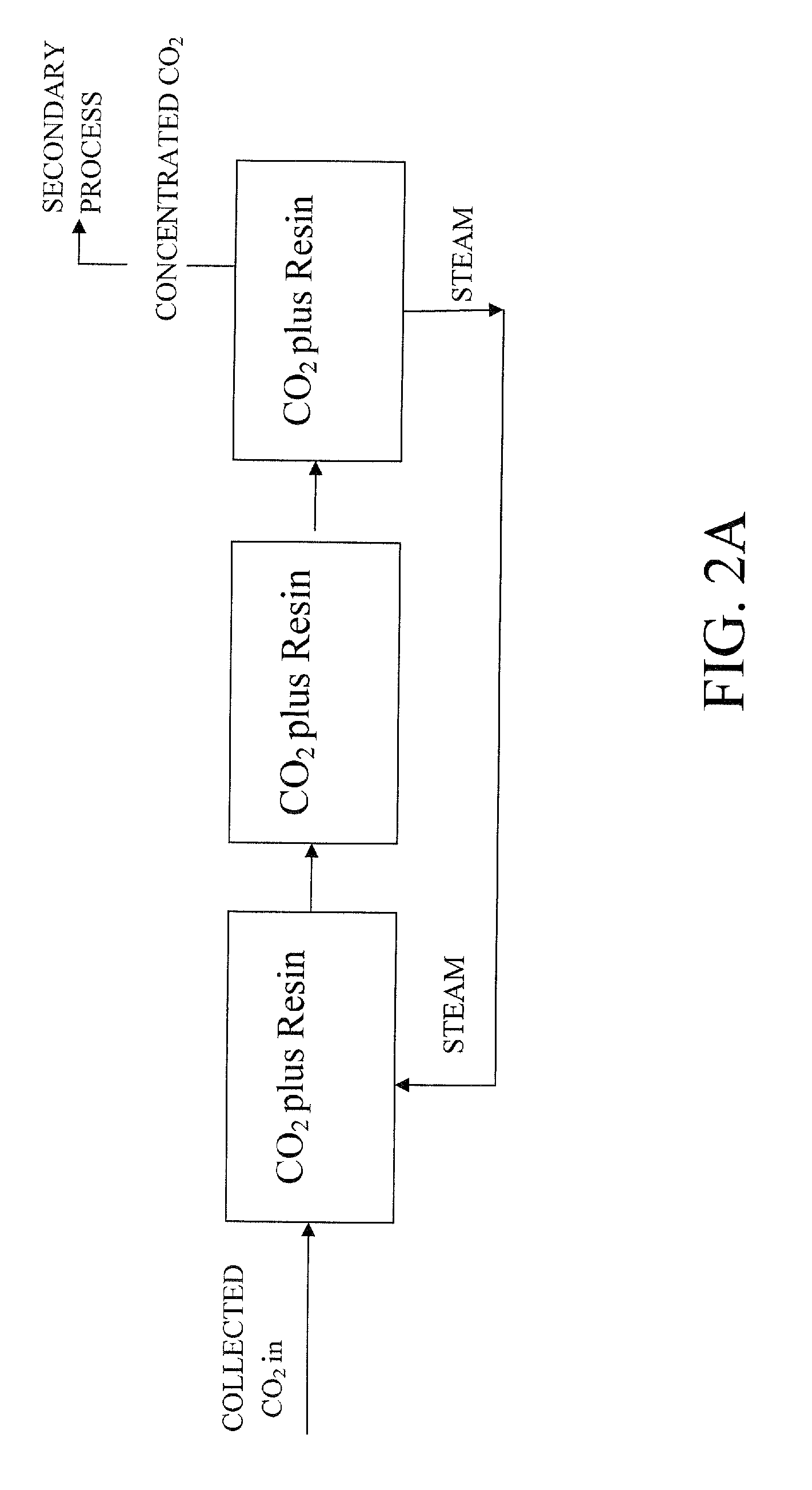
Extraction and sequestration of carbon dioxide
The present disclosure provides a method and apparatus for extracting carbon dioxide (CO2) from a fluid stream and for delivering that extracted CO2 to controlled environments for utilization by a secondary process. Various extraction and delivery methods are disclosed specific to certain secondary uses, included the attraction of CO2-sensitive insects, the ripening and preservation of produce, and the neutralization of brine.
Owner:KILIMANJARO ENERGY
Container structure for removal of vacuum pressure
A hot-fill PET container or bottle (10) filling with a liquid at an elevated temperature has a side wall (9) extending to a lower portion including a pressure panel (11) and a base (21) in its unfolded or pre-fill position. The panel (11) is transversely oriented and has a decoupling or hinge structure (13), an initiator portion (1) and control portion (5) of a steeply angled inverting conical section between 30 and 45 degrees. The control portion enables the inversion of the panel (11) into the container (10) to compensate for vacuum or reduced pressure induced within the container as the liquid cools down. The base (2) can also have a plurality of reinforcing ribs (3).
Owner:CO2 PAC
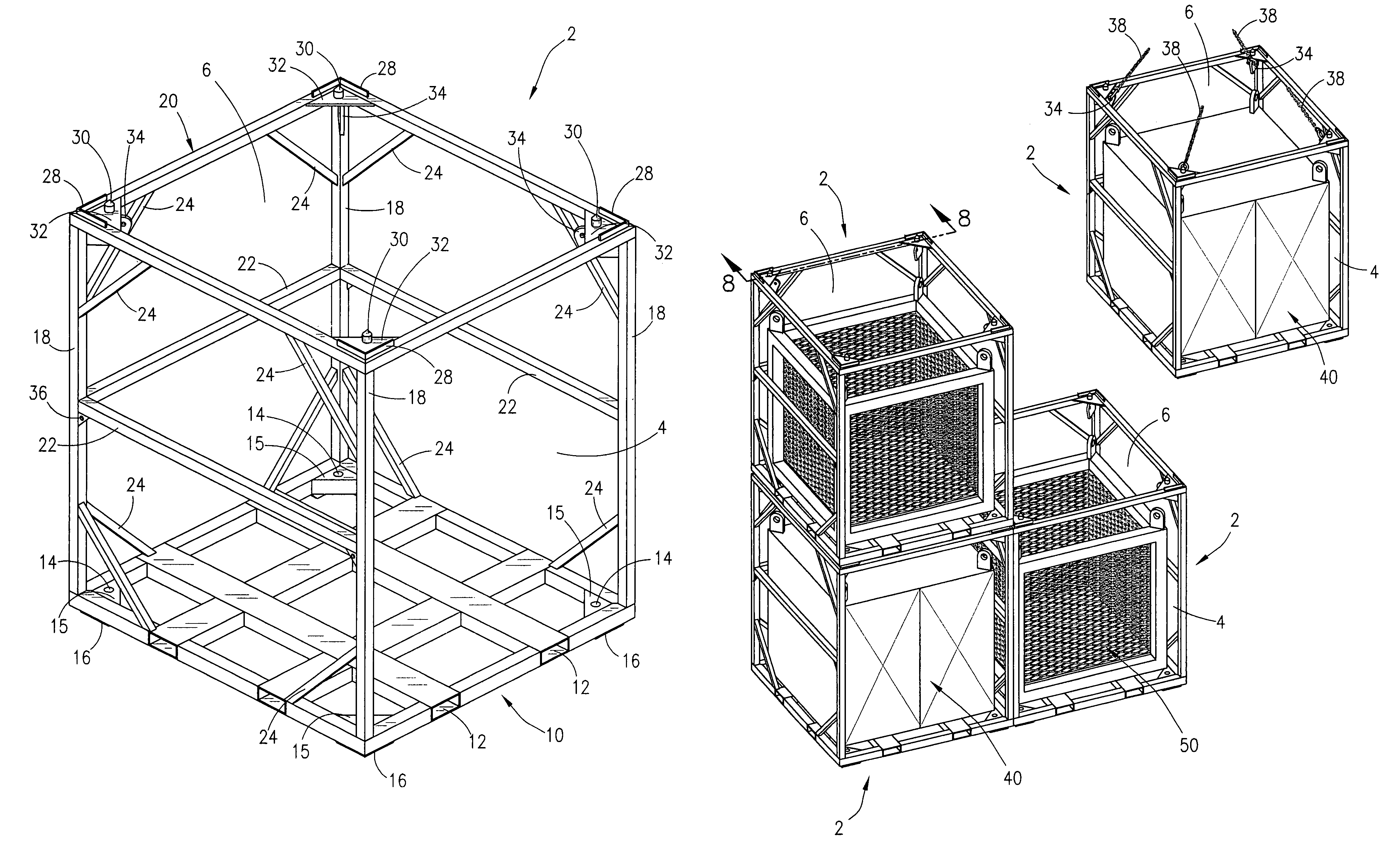
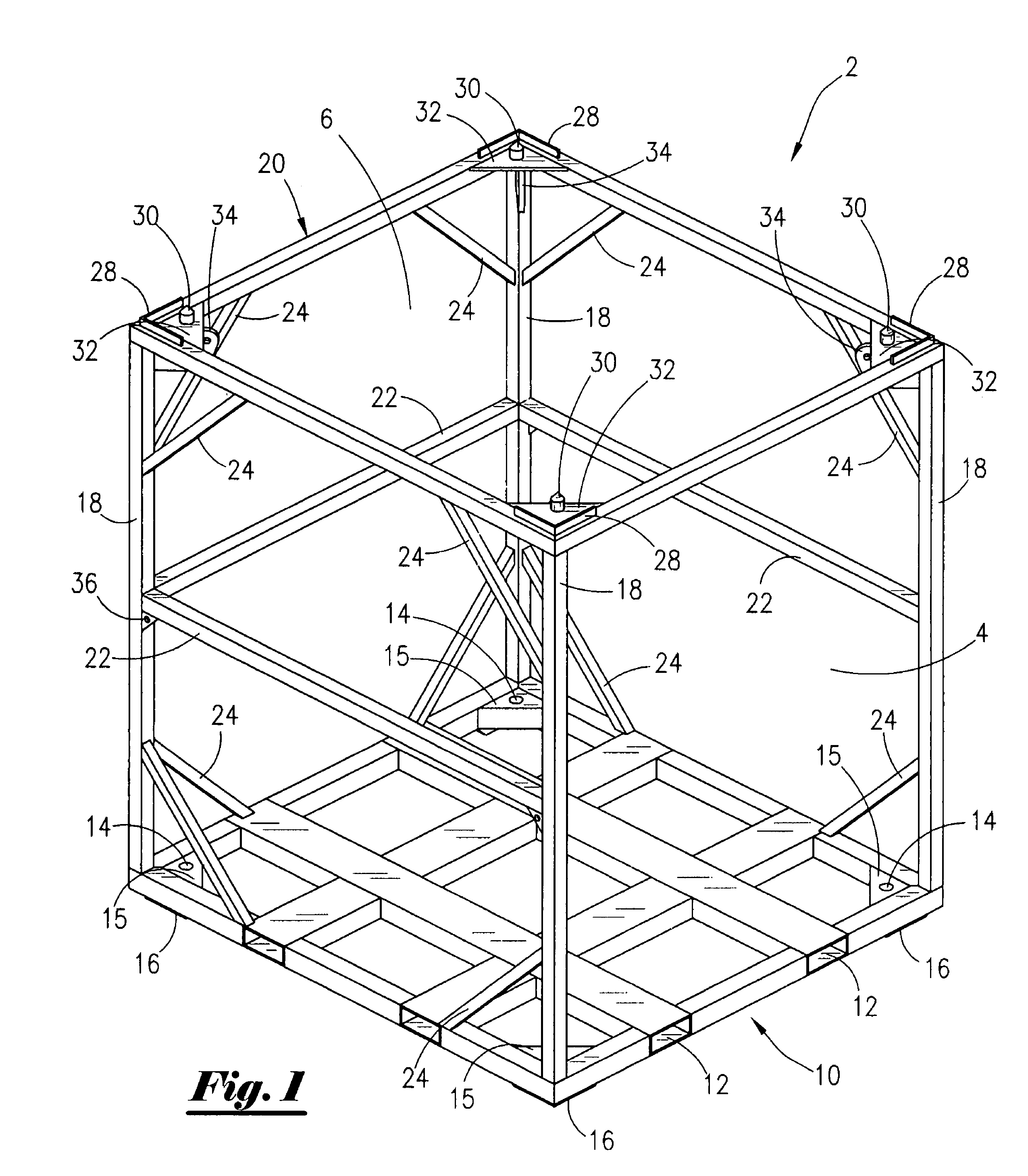
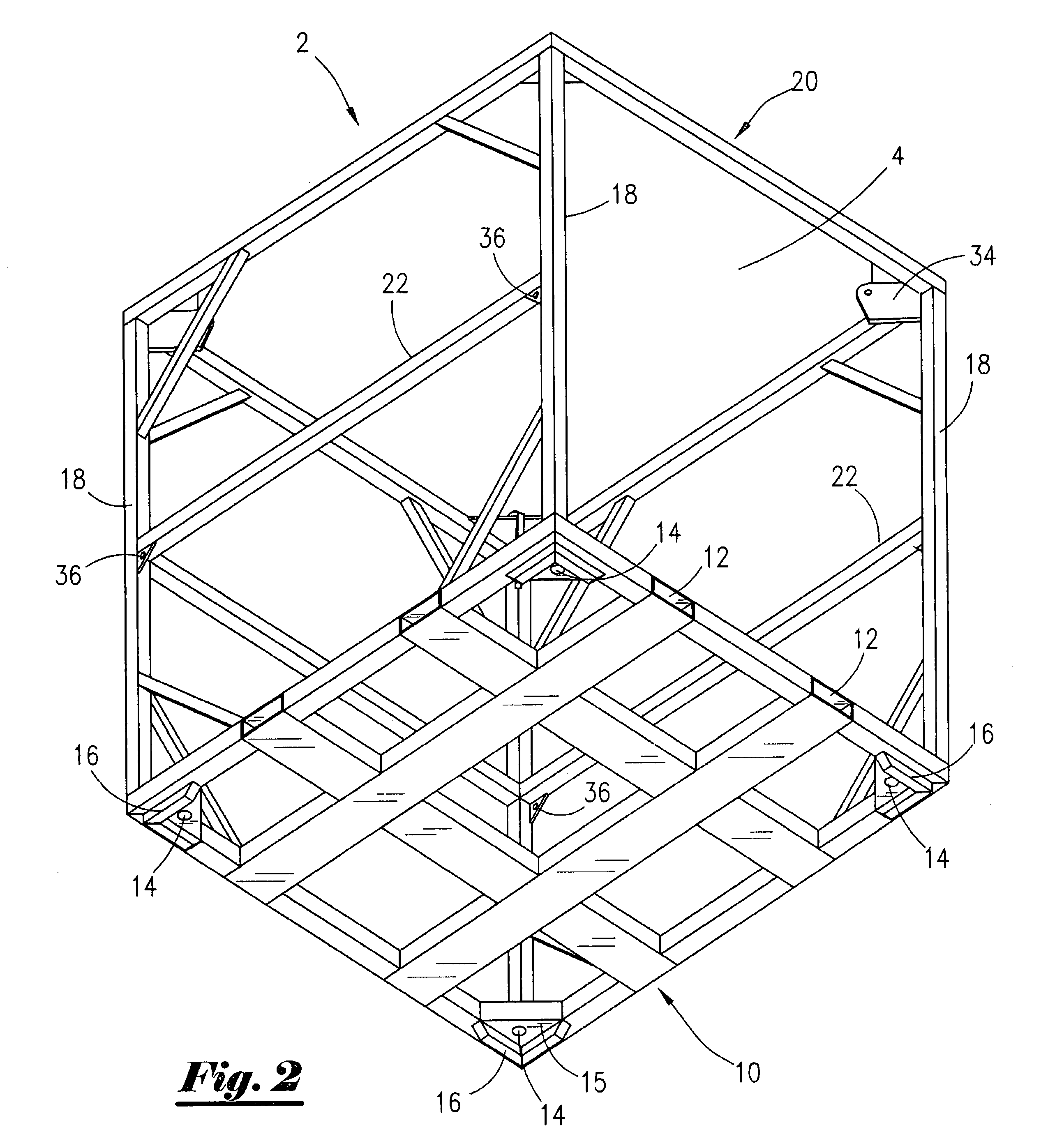
Cargo container cradle
A modular cradle for reinforcing, transporting, and stacking cargo containers used in the transportation and storage of bulk cargo in the oil and gas industry so as to allow compliance with construction and shipping standards adopted by the oil and gas industry is disclosed. The cargo cradle is comprised of a rectangular frame for holding and supporting a cargo container. The cradle has attached lifting lugs positioned within the interior of the cargo cradle for attachment of lifting cables. The lifting lugs are positioned so as to allow cradles to be stacked one upon the other. The container cradles interlock when stacked.
Owner:R3G
Data mining techniques for improving search engine relevance
InactiveUS20060224579A1Facilitate efficient searching and retrieval and analysisSimple processWeb data indexingSolid waste disposalLearning dataInformation retrieval
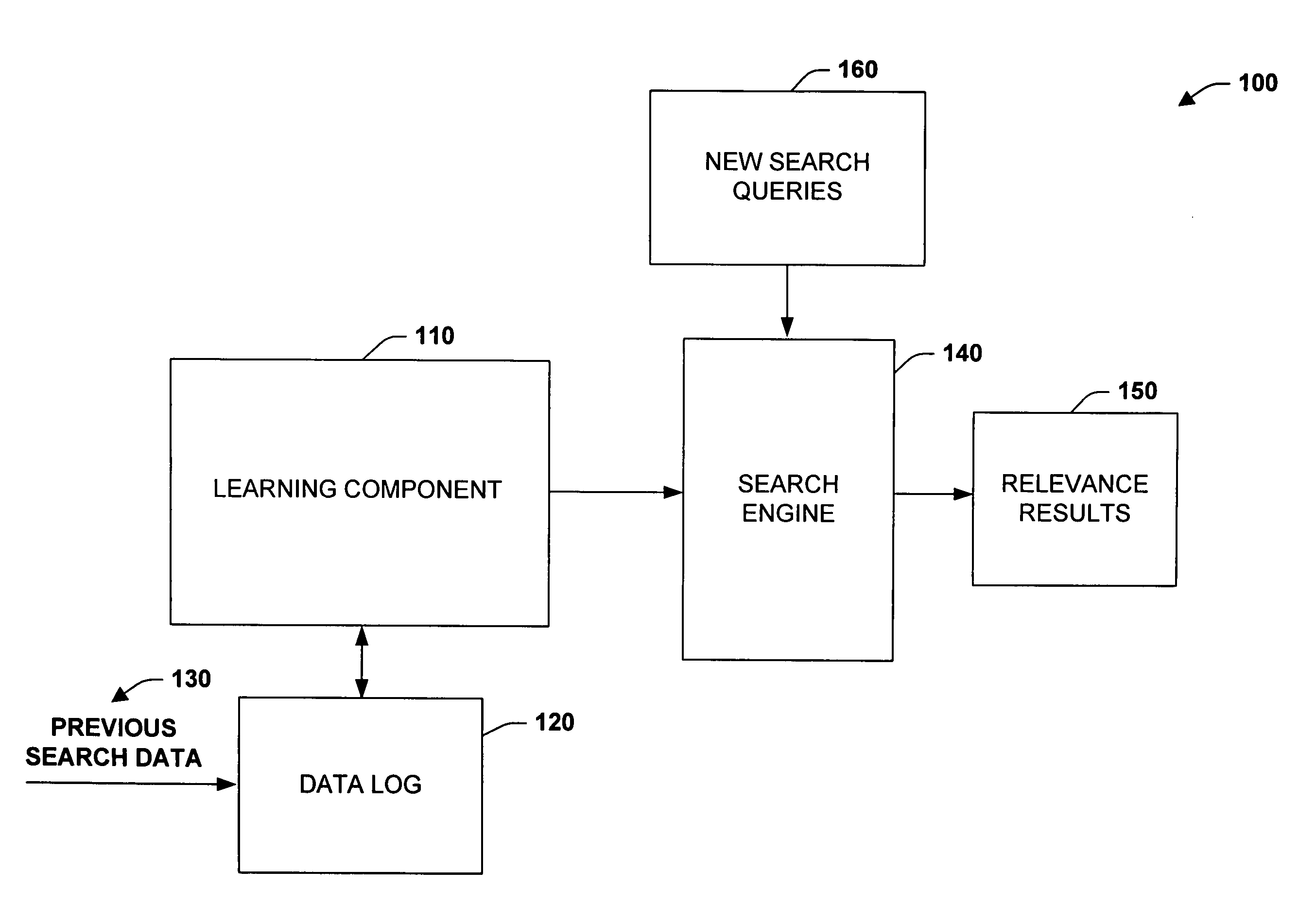
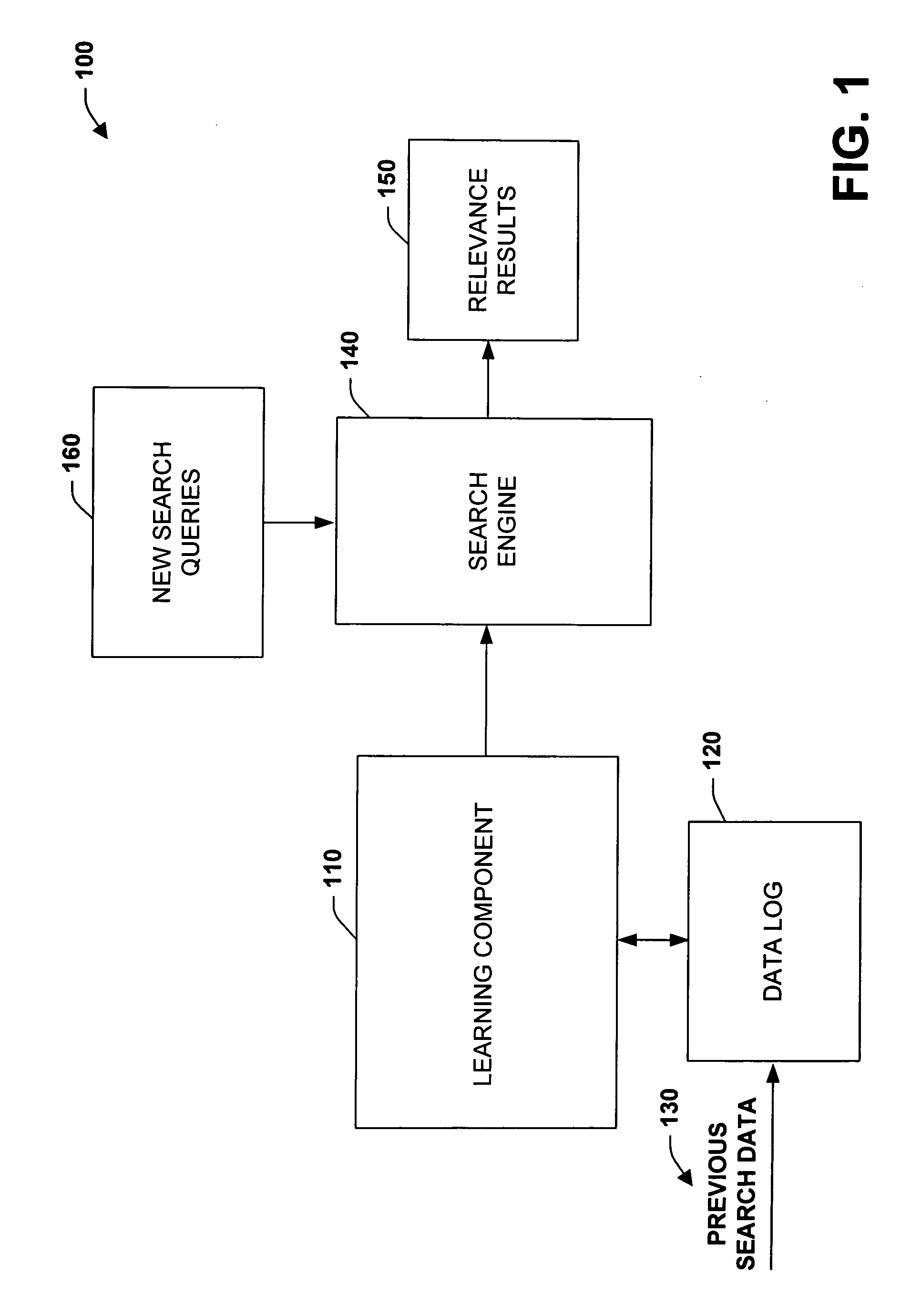
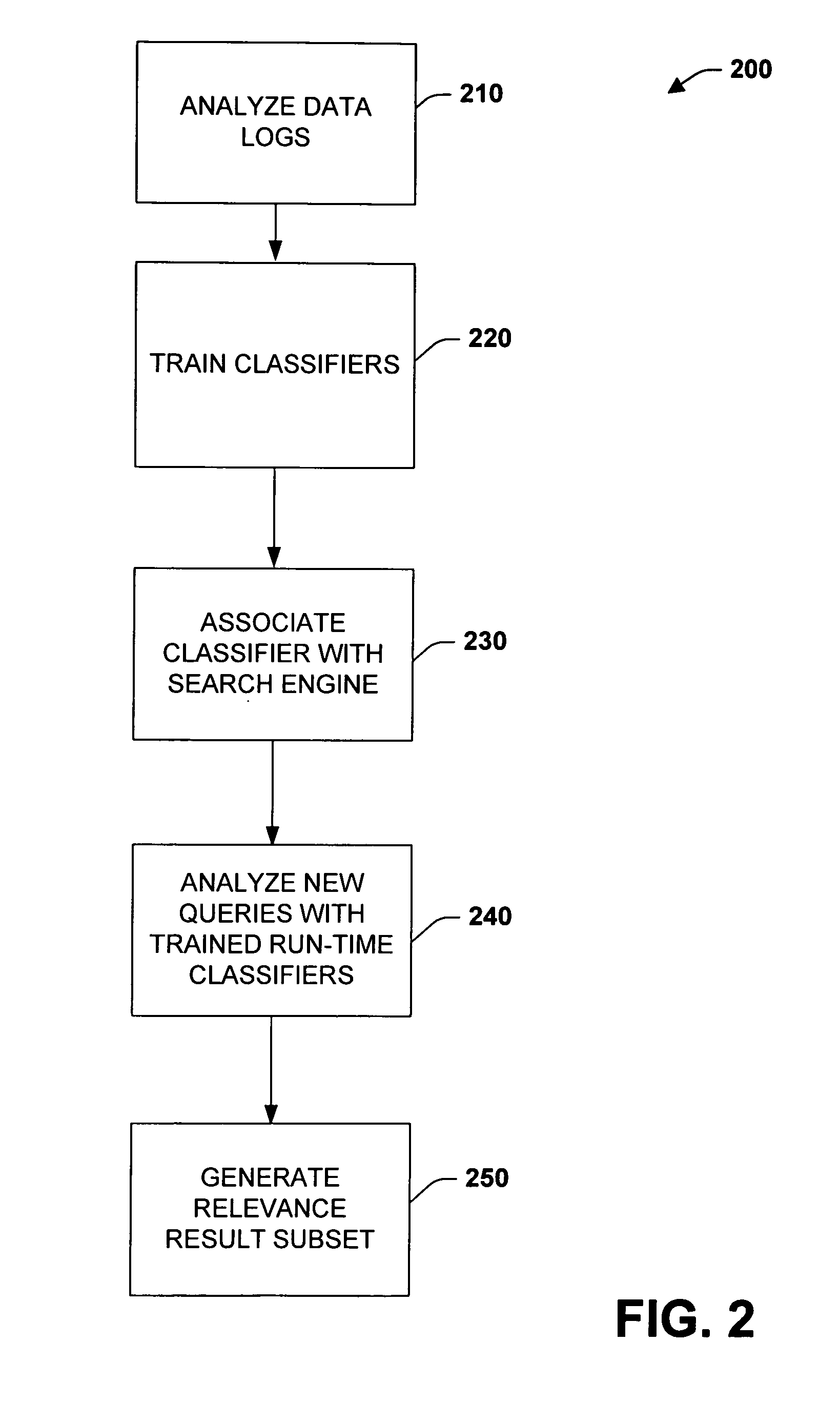
The subject invention relates to systems and methods that automatically learn data relevance from past search activities and apply such learning to facilitate future search activities. In one aspect, an automated information retrieval system is provided. The system includes a learning component that analyzes stored information retrieval data to determine relevance patterns from past user information search activities. A search component employs the learning component to determine a subset of current search results based at least in part on the relevance patterns, wherein numerous variables can be processed in accordance with the learning component to efficiently generate focused, prioritized, and relevant search results.
Owner:MICROSOFT TECH LICENSING LLC
Fluid dispenser
InactiveUS6192945B1Efficient and reliableEasy to manufacturePreparing sample for investigationLiquid flow controllersModularityReactive system
A method and apparatus for an automated biological reaction system is provided. In the processing of a biological reaction system, there is a need for consistently placing an amount of fluid on a slide. In order to accomplish this, several methods are used including a consistency pulse and a volume adjust means. Moreover, in order to reliably operate an automated biological reaction system, the dispenser must be reliable, easy to assemble and accurate. Among other things, in order to accomplish this, the dispense chamber is substantially in line with the reservoir chamber, the reservoir chamber piston is removed, and the flow of fluid through the dispenser is simplified. Further, in order to operate the automated biological reaction system more reliably, the system is designed in modular pieces with higher functions performed by a host device and the execution of the staining operations performed by remote devices. Also, to reliably catalog data which is used by the automated biological reaction system, data is loaded to a memory device, which in turn is used by the operator to update the operator's databases. The generation of the sequence of steps for the automated biological reaction device based on data loaded by the operator, including checks to determine the ability to complete the run, is provided.
Owner:VENTANA MEDICAL SYST INC
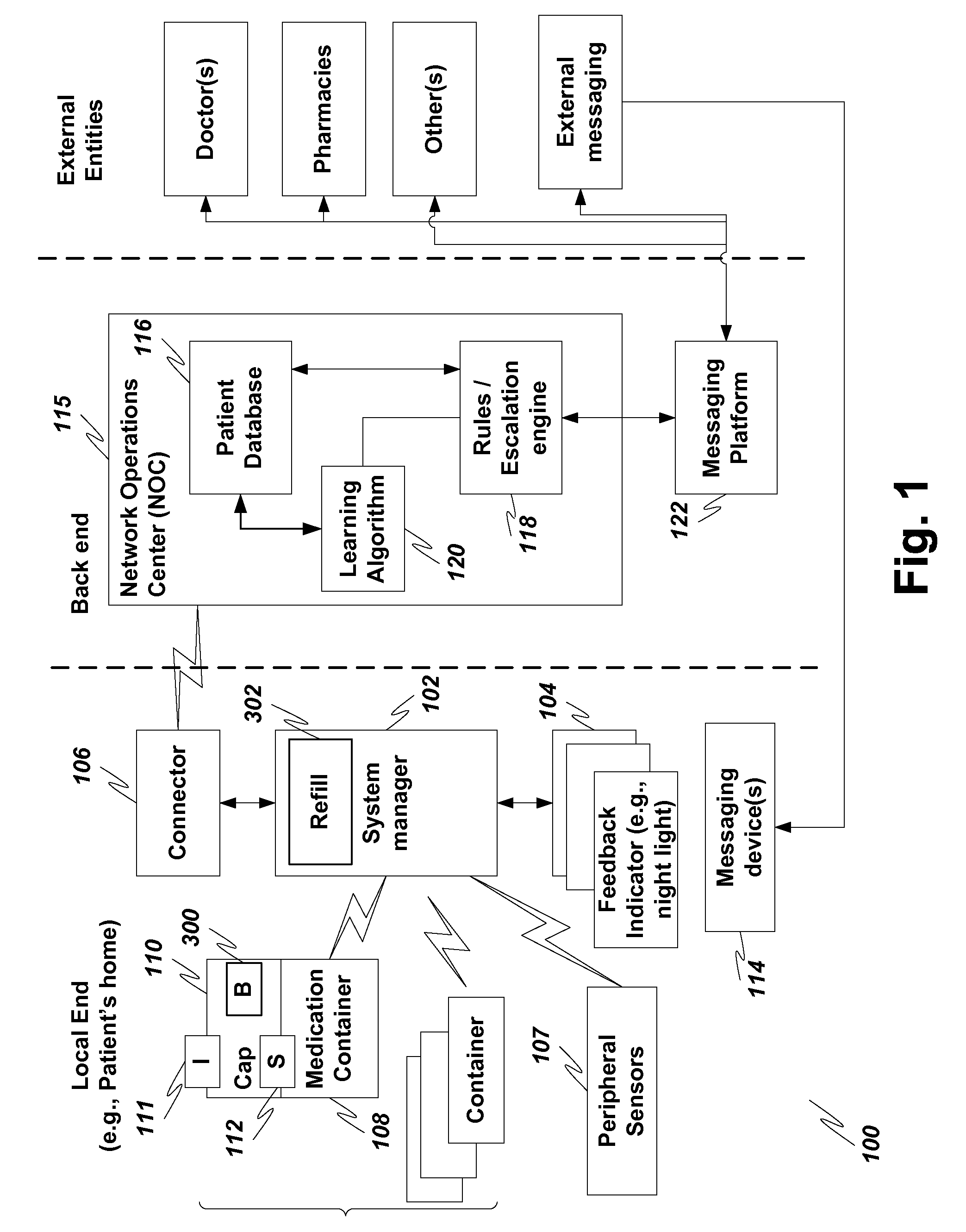
Medication dispenser with automatic refill
A device for aiding medication compliance includes a cap for a medicine container. The cap has a multi-color light-emitting diode; for determining when said cap is opened; and a button, operatively connected to the circuitry, for triggering a signal to an external dispenser to request a refill of medication when pressed.
Owner:NANT HEALTH
Electronically keyed dispensing systems and related methods utilizing near field frequency response
ActiveUS20060124662A1Optimize quantityAcutation objectsIndication apparatusEngineeringMechanical engineering
A dispensing system is disclosed which utilizes an electronically powered key device and / or identification code associated with a refill container to preclude the need for mechanical keys. The system utilizes a near field frequency response to determine whether a refill container is compatible with a dispensing system. In particular, the refill container is provided with a coil terminated by one of a number of capacitors. The container is received in a housing that provides a pair of coils that are in a spatial relationship with the installed refill container's coil. By energizing one of the housing's coils, the other coil detects a unique electronic signature generated by the container's coil. If the signature is acceptable, the dispensing system is allowed to dispense a quantity of material. The system also provides a unique latching mechanism to retain the container and ensure positioning of all the coils.
Owner:KANFER JOSEPH
System and method for dispensing soap
InactiveUS6929150B2Smooth rotationEasy to movePower operated devicesLarge containersBiomedical engineeringSOAP
A method of dispensing soap from a fluid dispensing system is disclosed. The method includes the steps of presenting a tube having a tube end disposed at a first position within an indented portion of a spout of the fluid dispensing system, sensing an object below the tube end, in response to sensing the object, expelling said soap from the tube end by drawing the tube end further within the indented portion to a second position, and returning the tube end to the first position.
Owner:RUBBERMAID COMMERCIAL PRODUCTS
Thermal Containment System Providing Temperature Maintaining Shipping Package with Segmented Flexible PCM Panels
InactiveUS20100314397A1Domestic cooling apparatusHeat storage plantsEngineeringPhase-change material
A packaging system having an outer container and a segment panel defining a plurality of phase change material (PCM) segments, with said segments being aligned with sides of the outer container interior upon wrapping around a payload and insertion into the outer container. The segmented panels may include edge tapers which facilitate the wrapping process with adjacent edge tapers being brought together during the wrapping process. A method of packaging using PCM-containing segments which are wrapped around a portion of a payload is also disclosed.
Owner:WILLIAMS PRESTON NOEL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com