Patents
Literature
8514results about "Flow control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
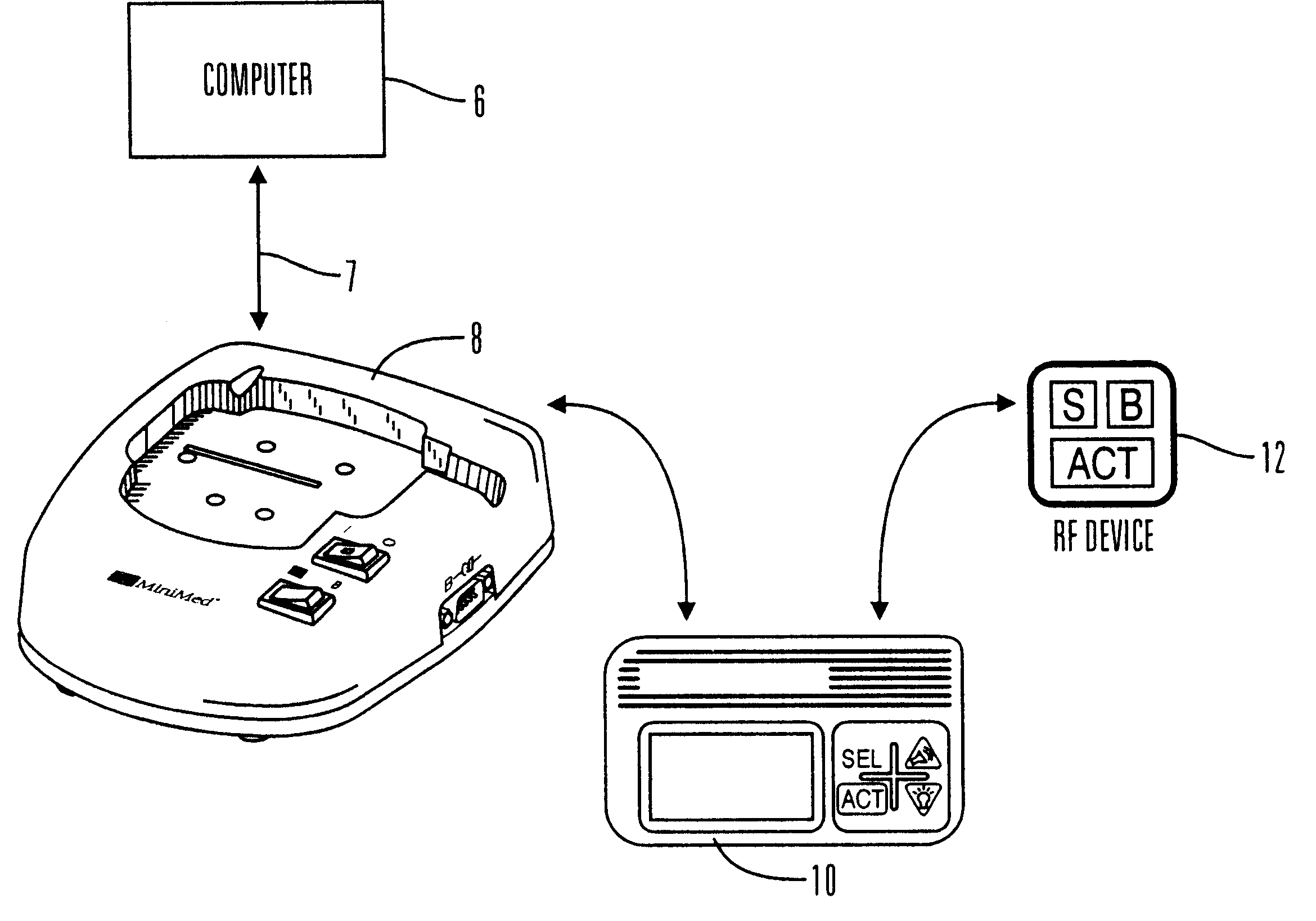
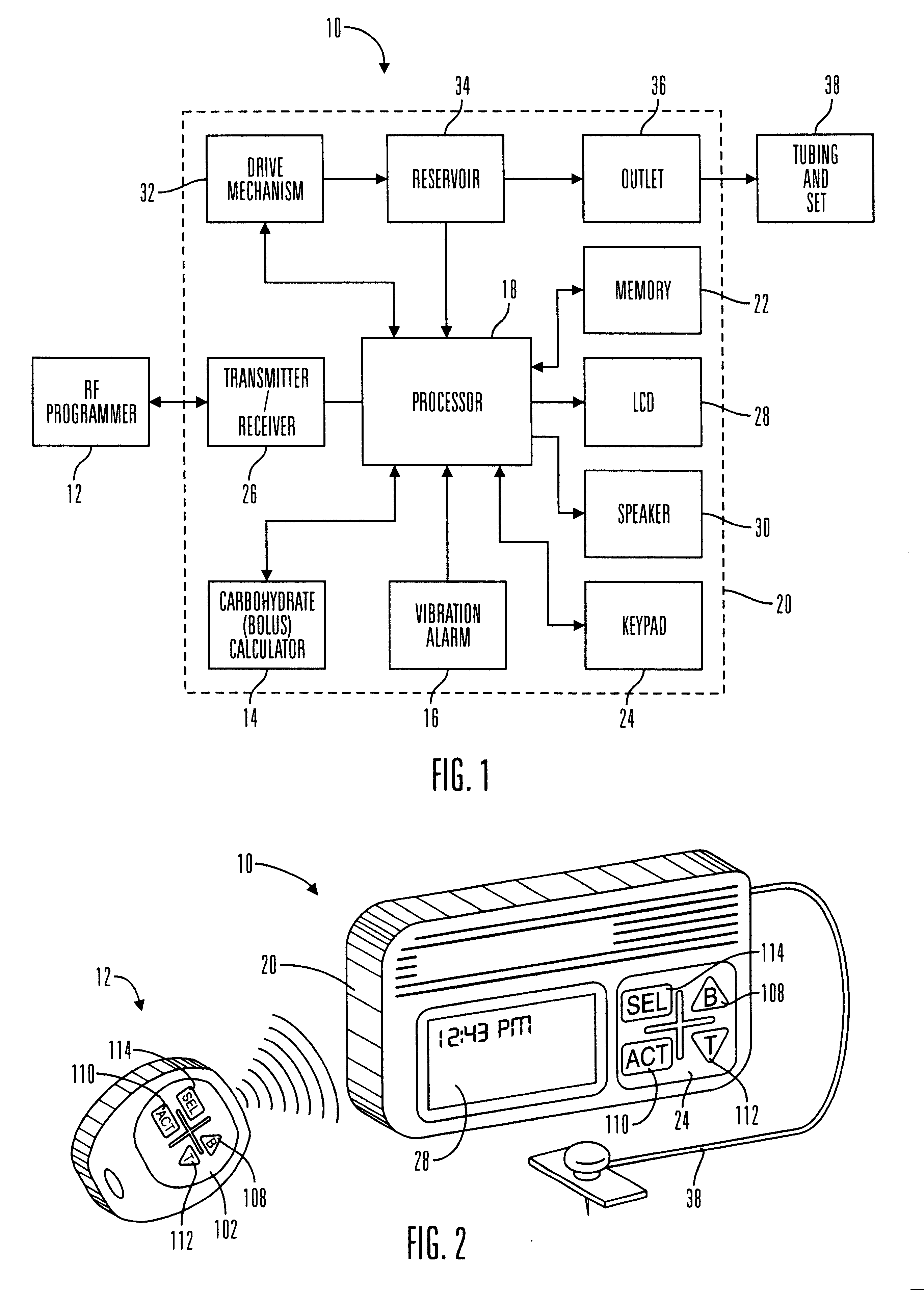
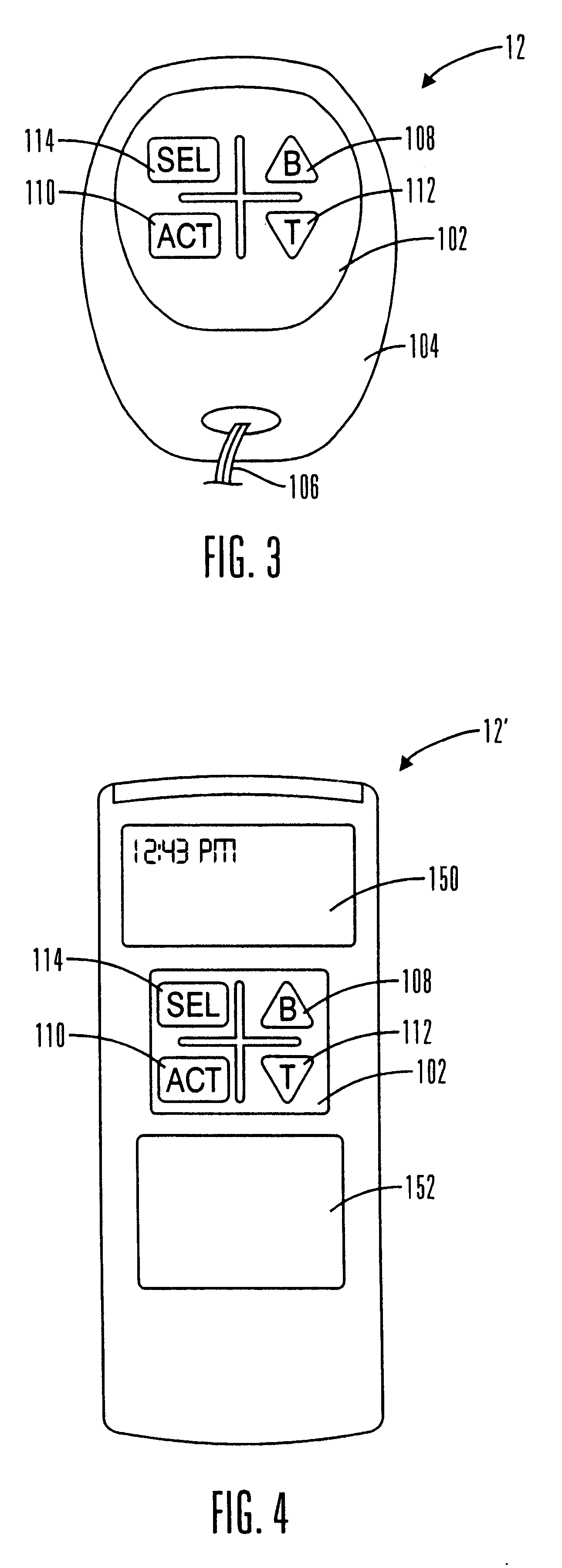
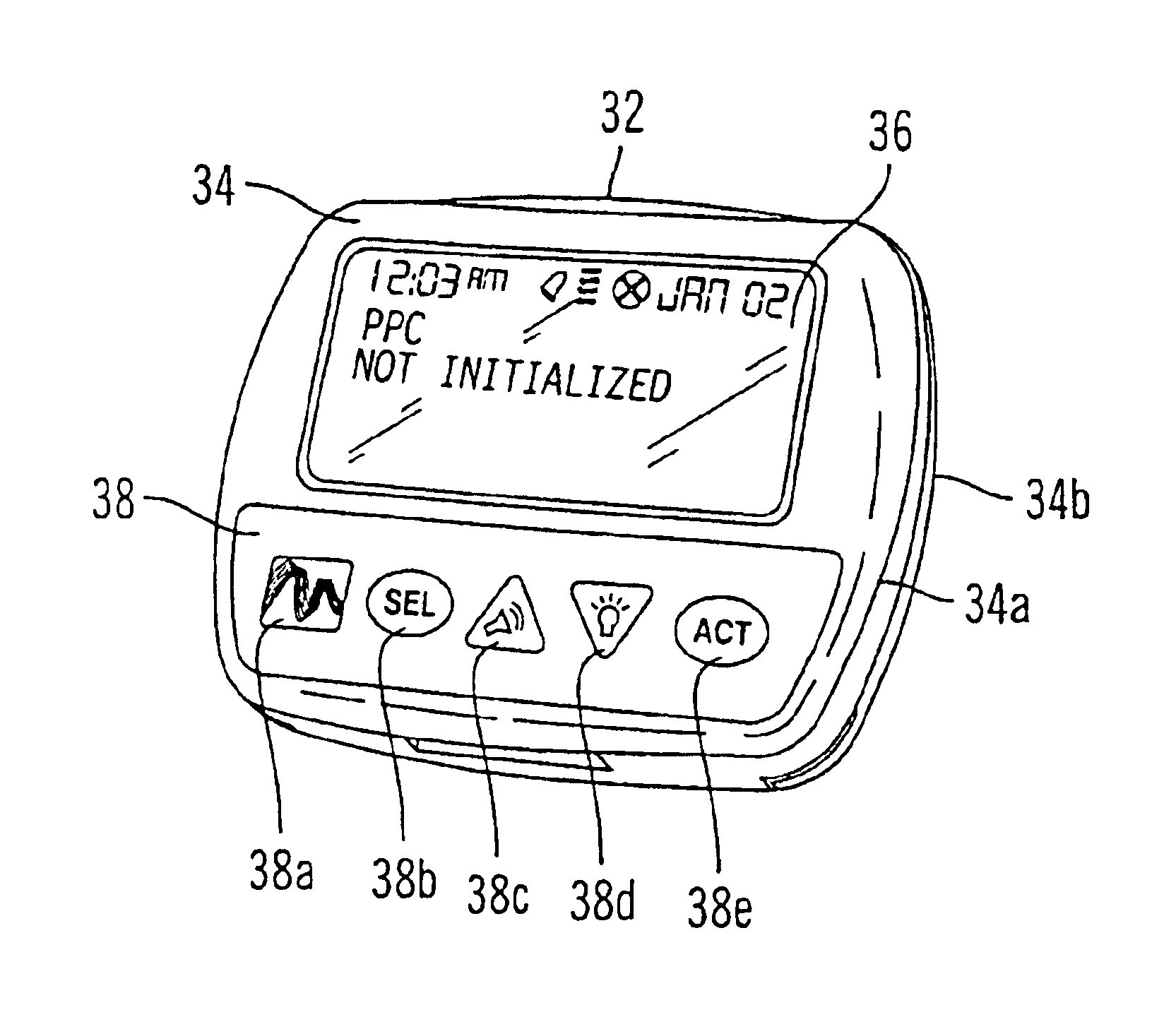
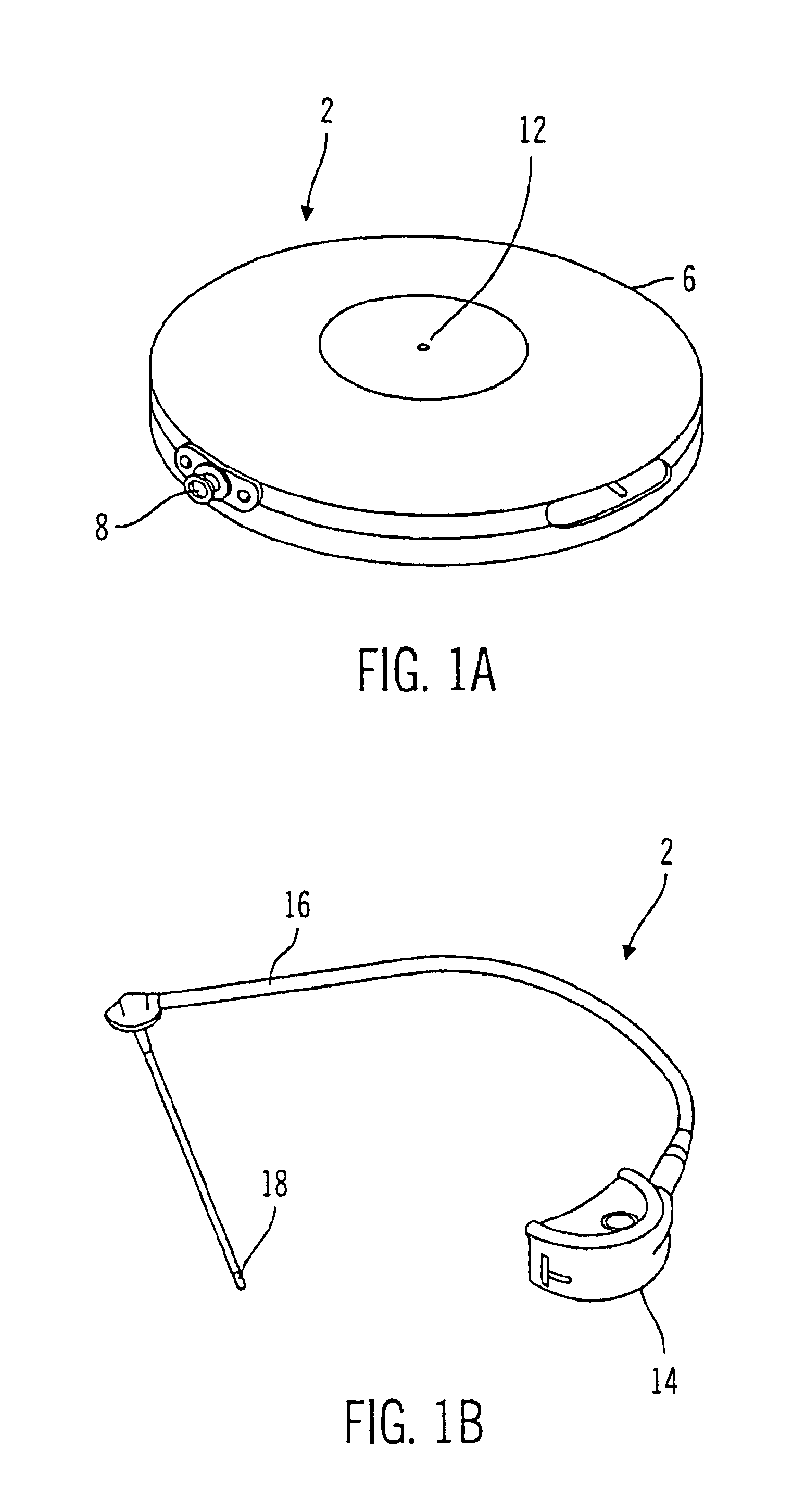
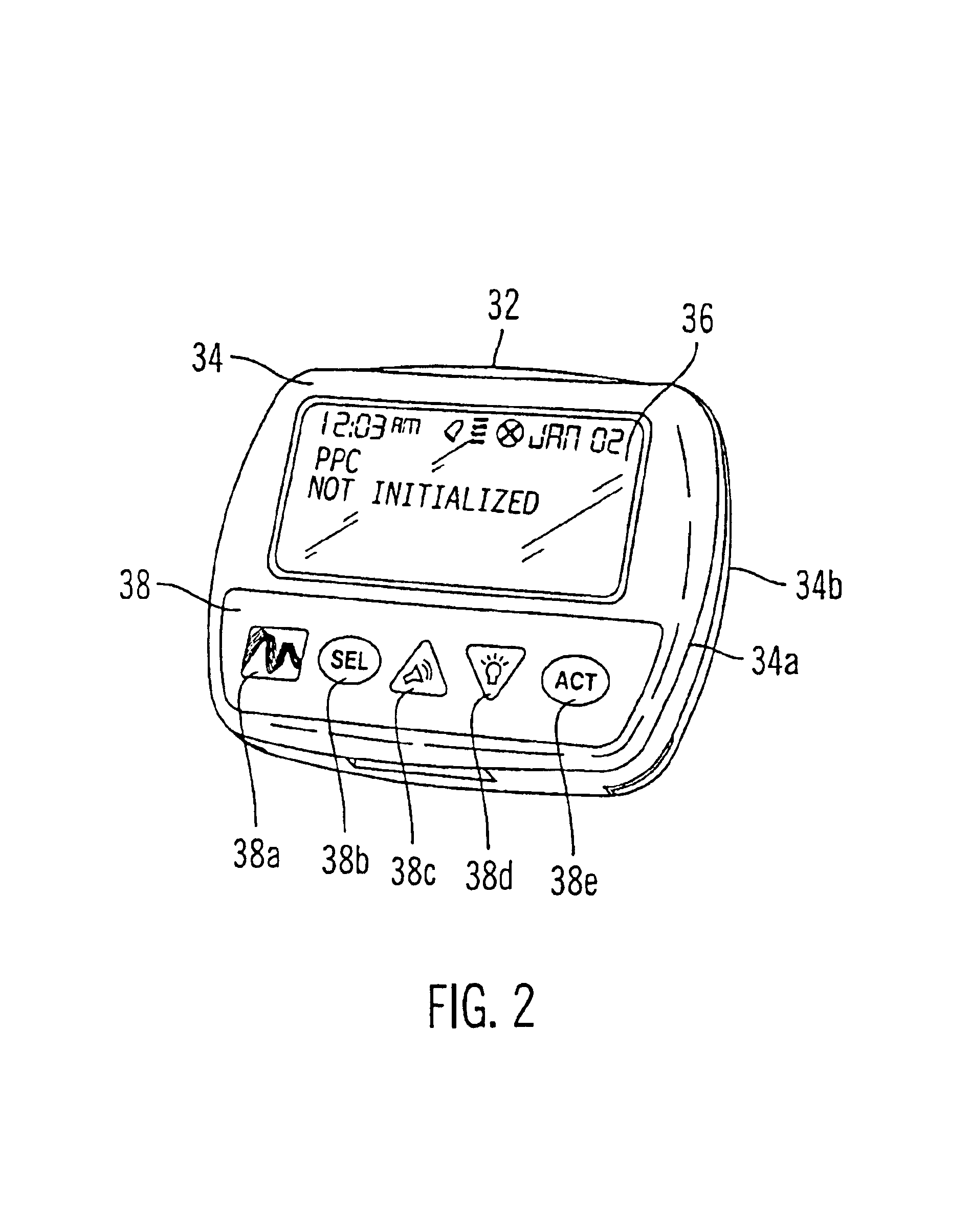
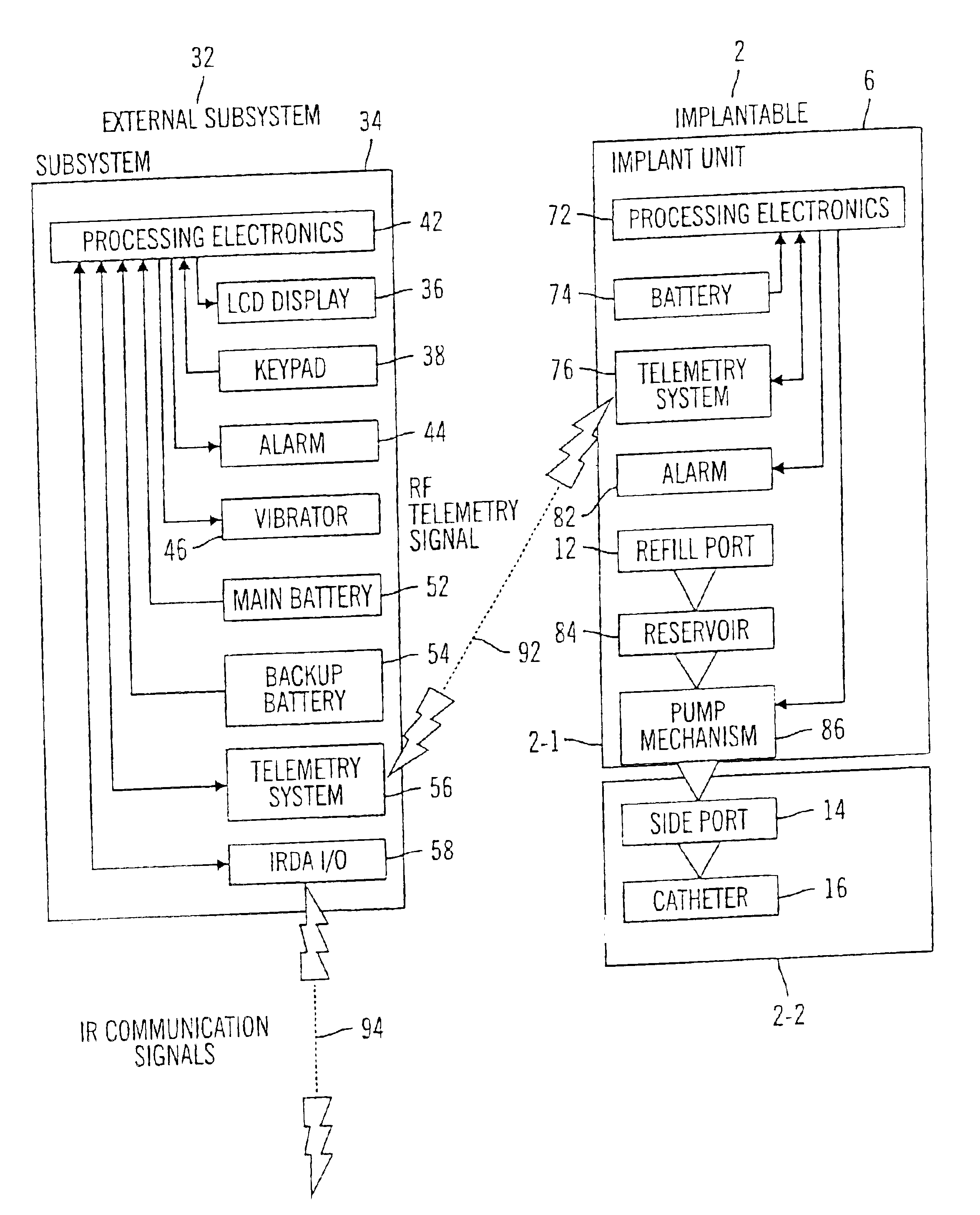
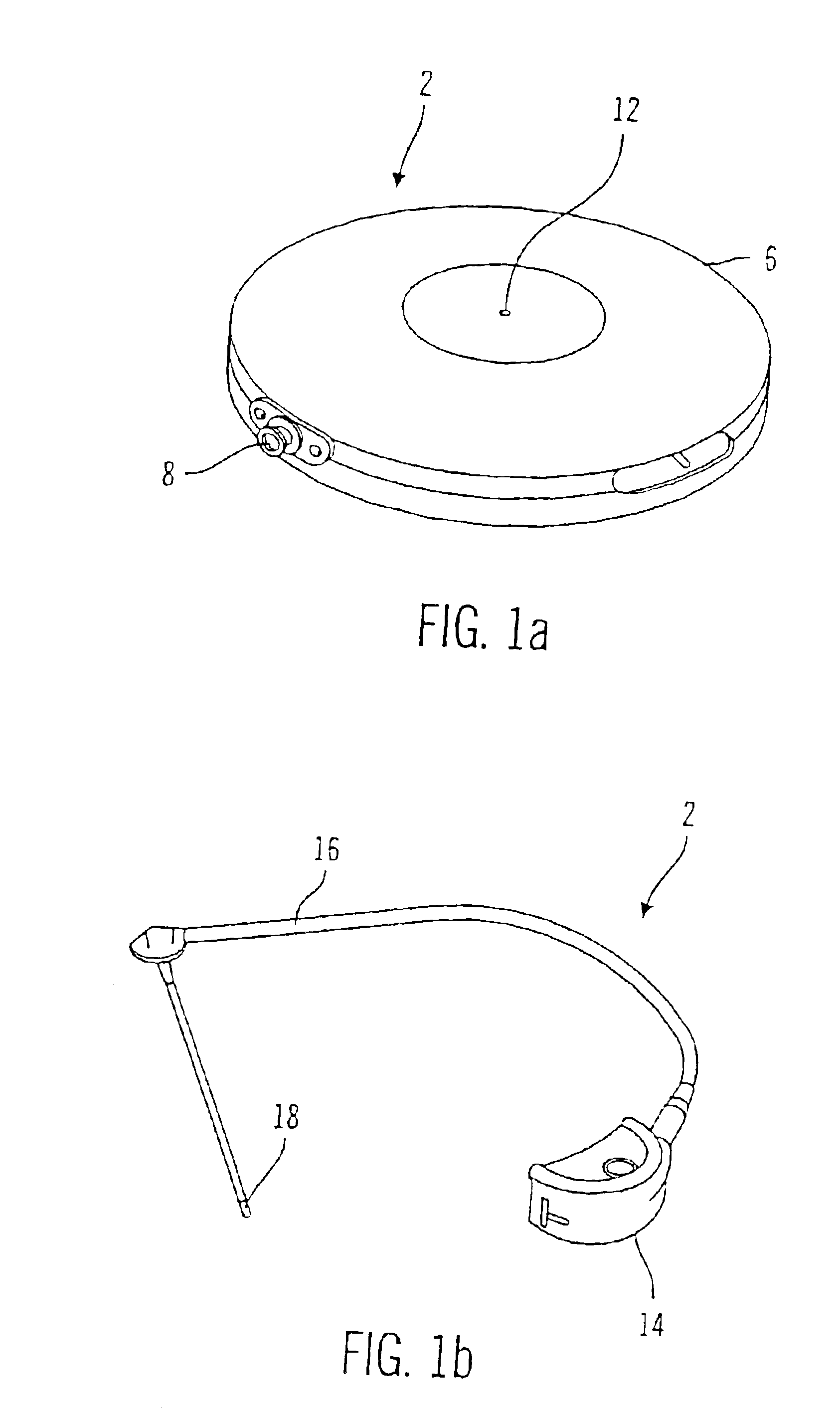
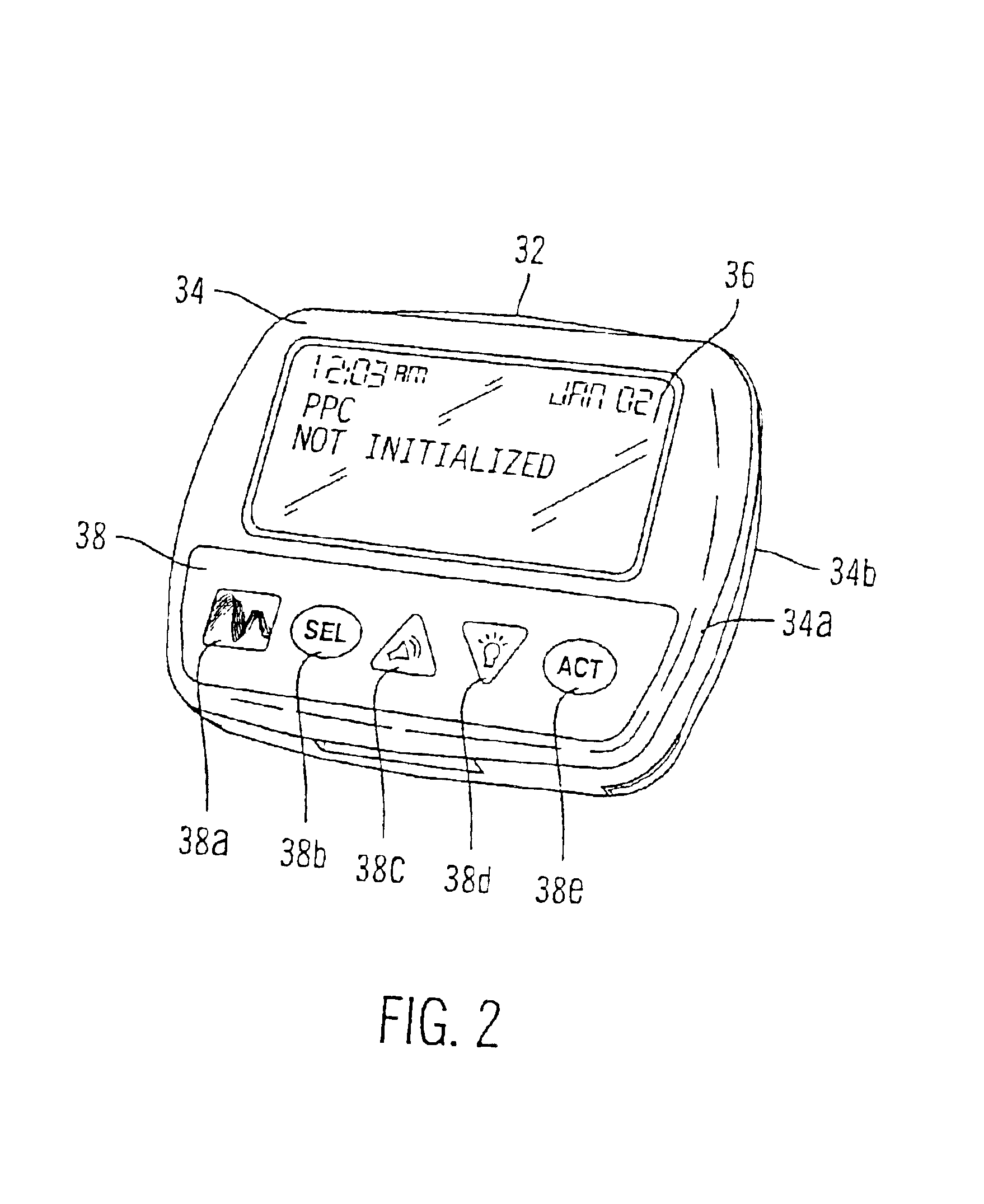
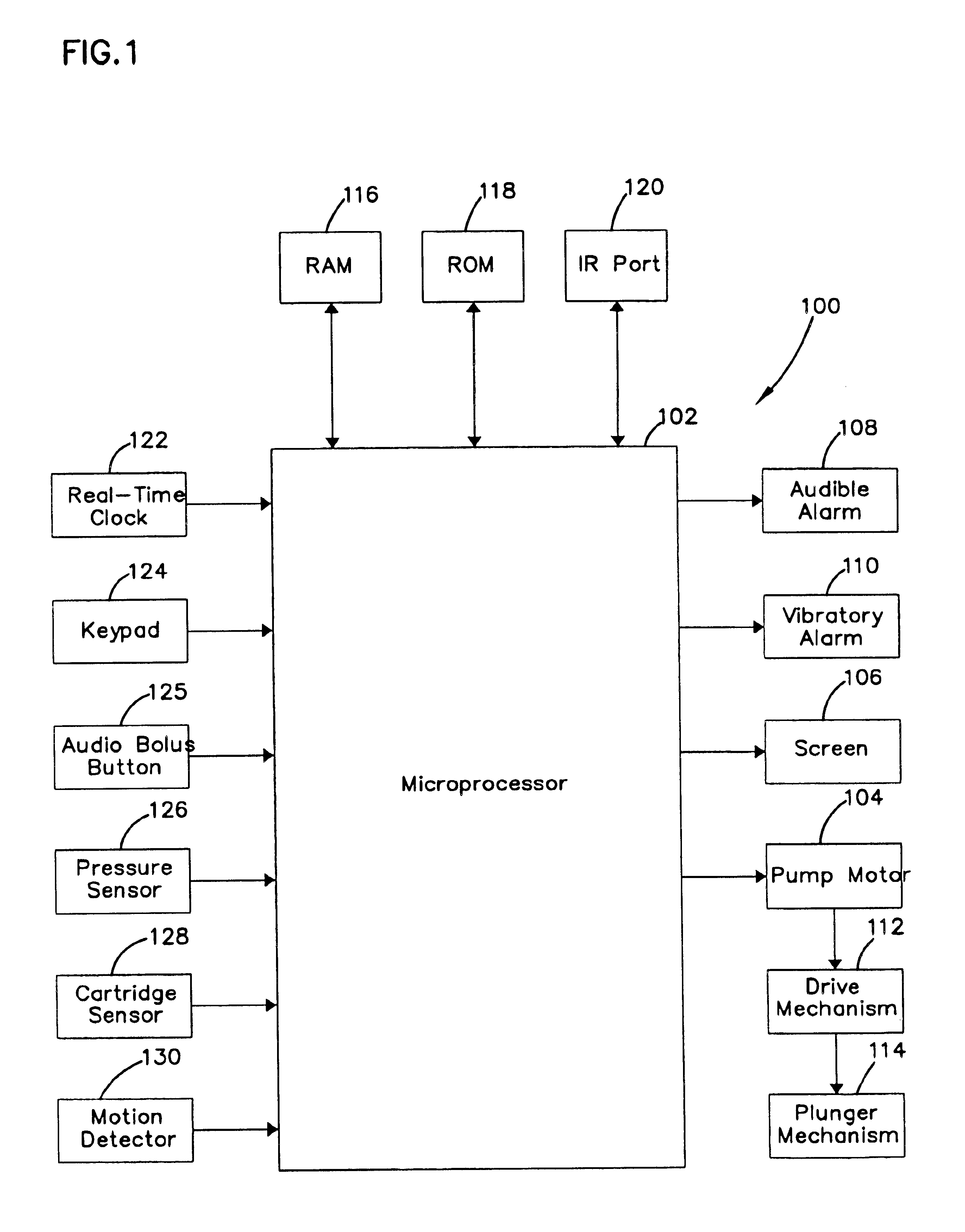
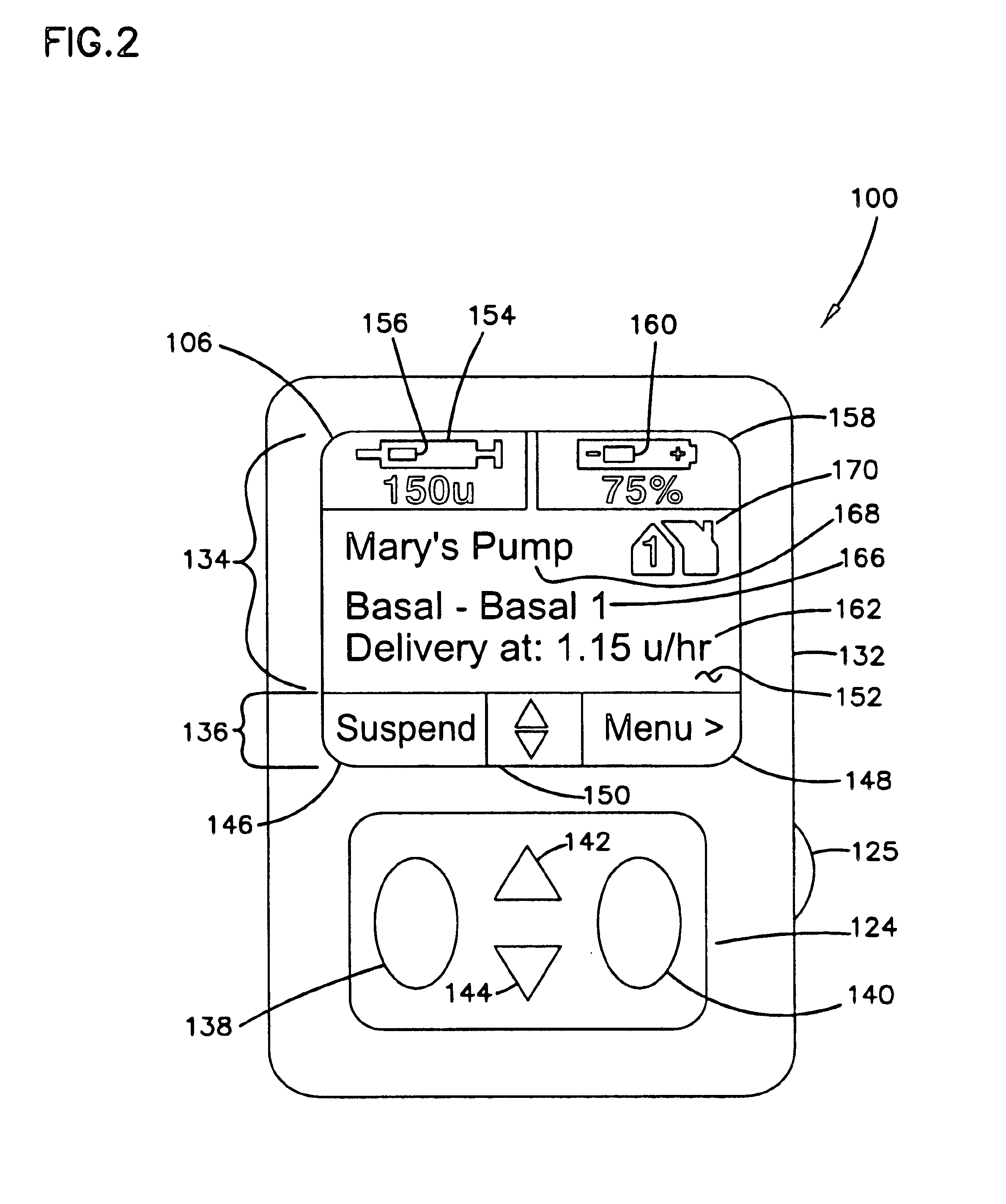
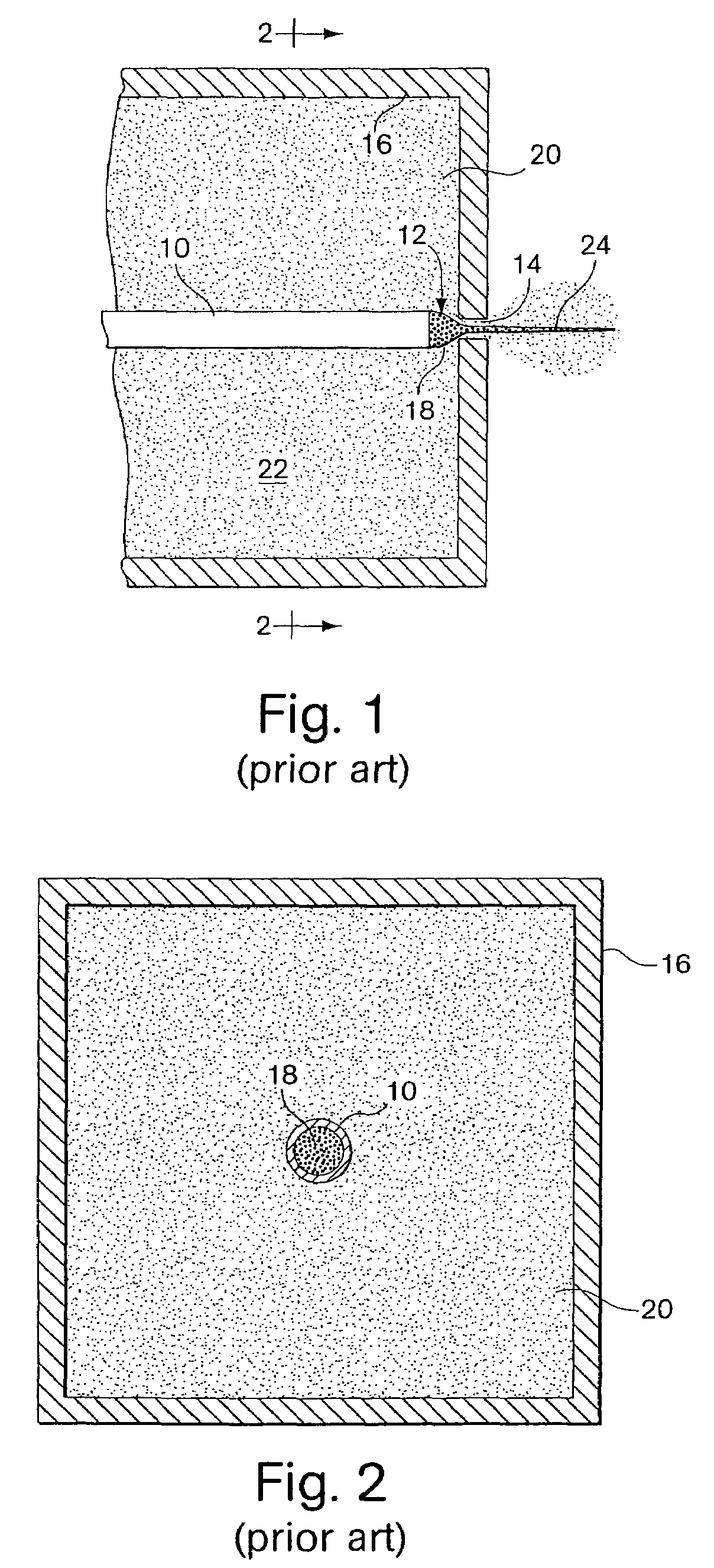
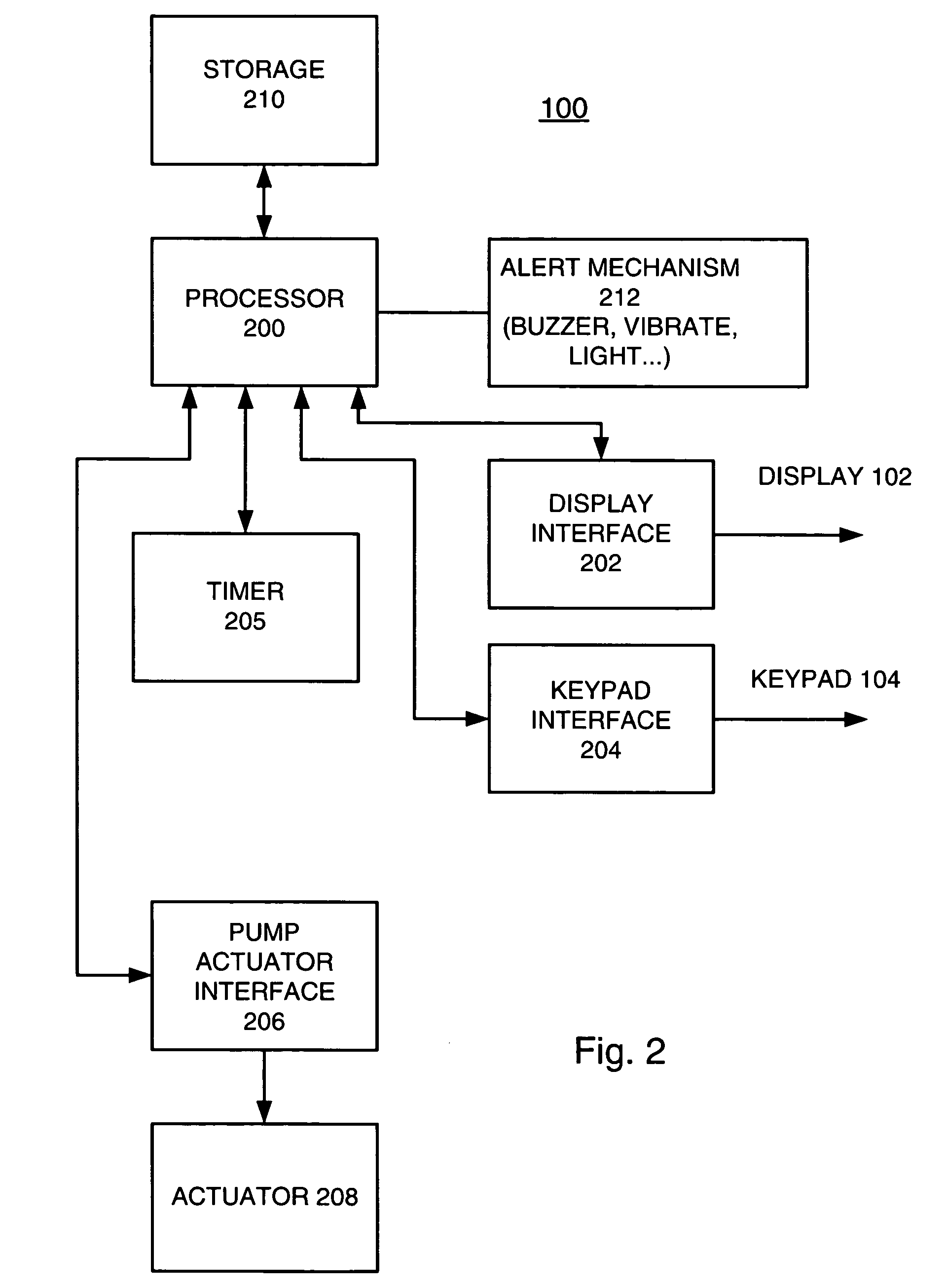
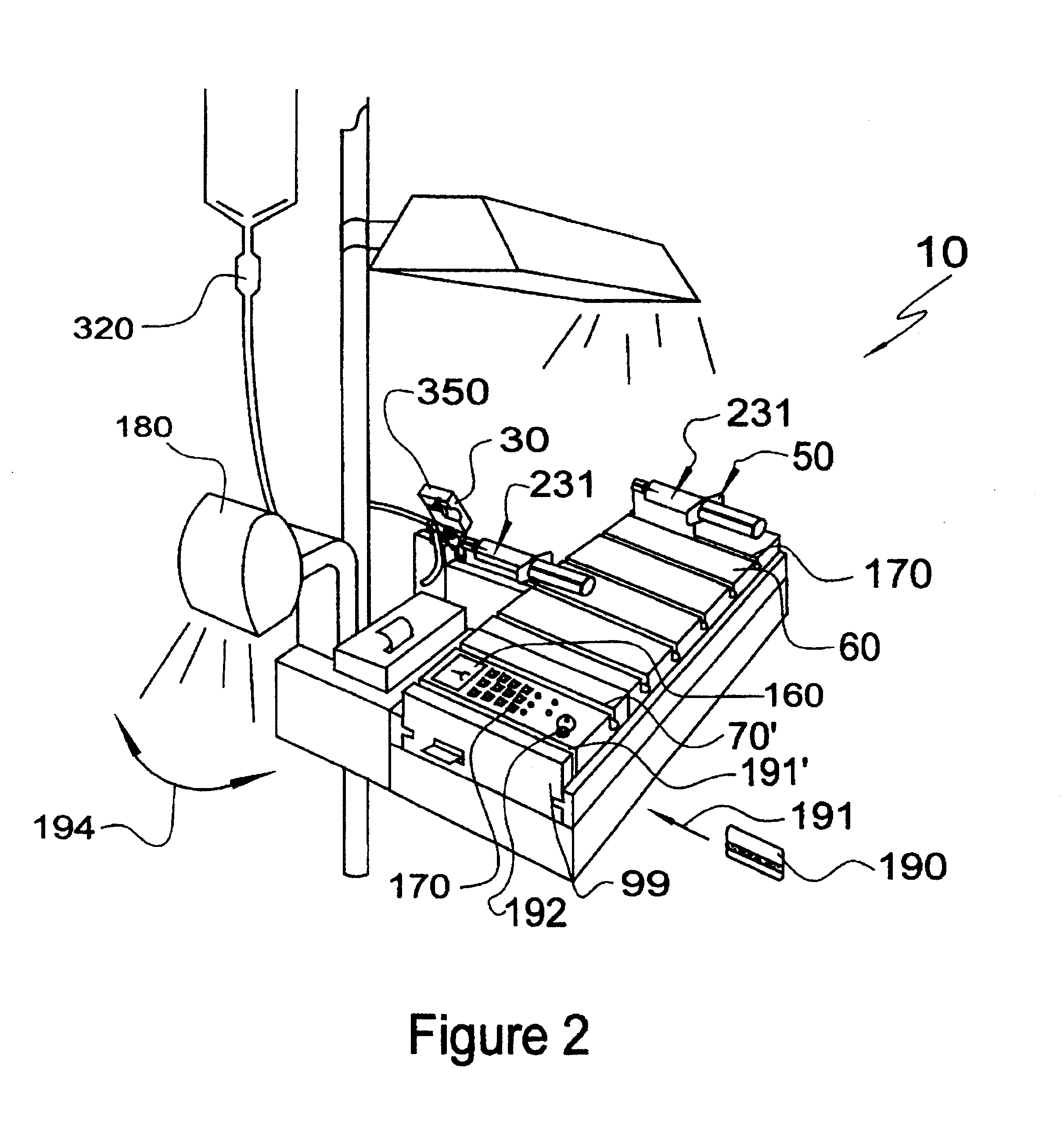
External infusion device with remote programming bolus estimator and/or vibration alarm capabilities
InactiveUS6551276B1Short and long period of timeTime indicationAutomatic syringesIntensive care medicineTransmitter
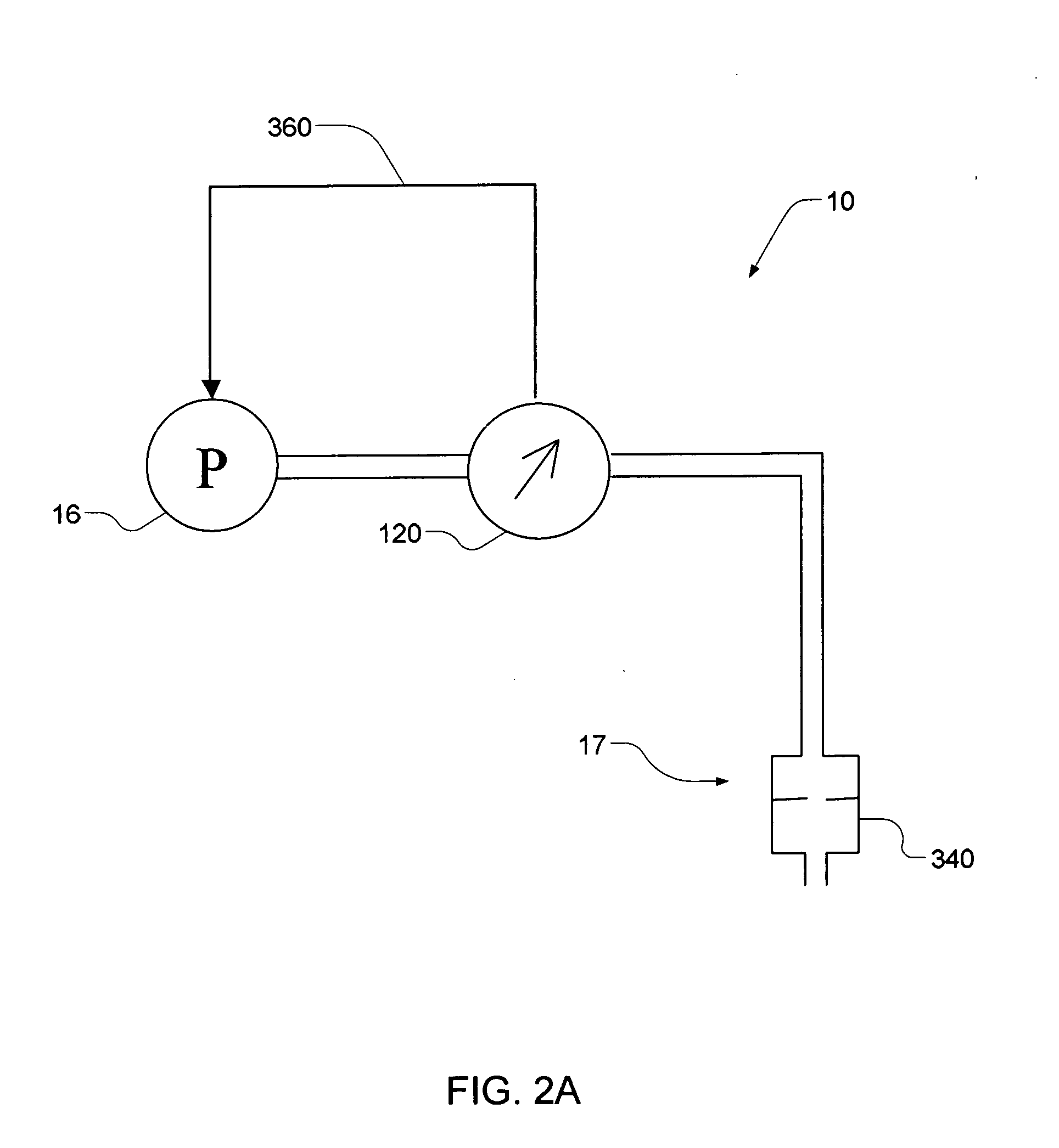
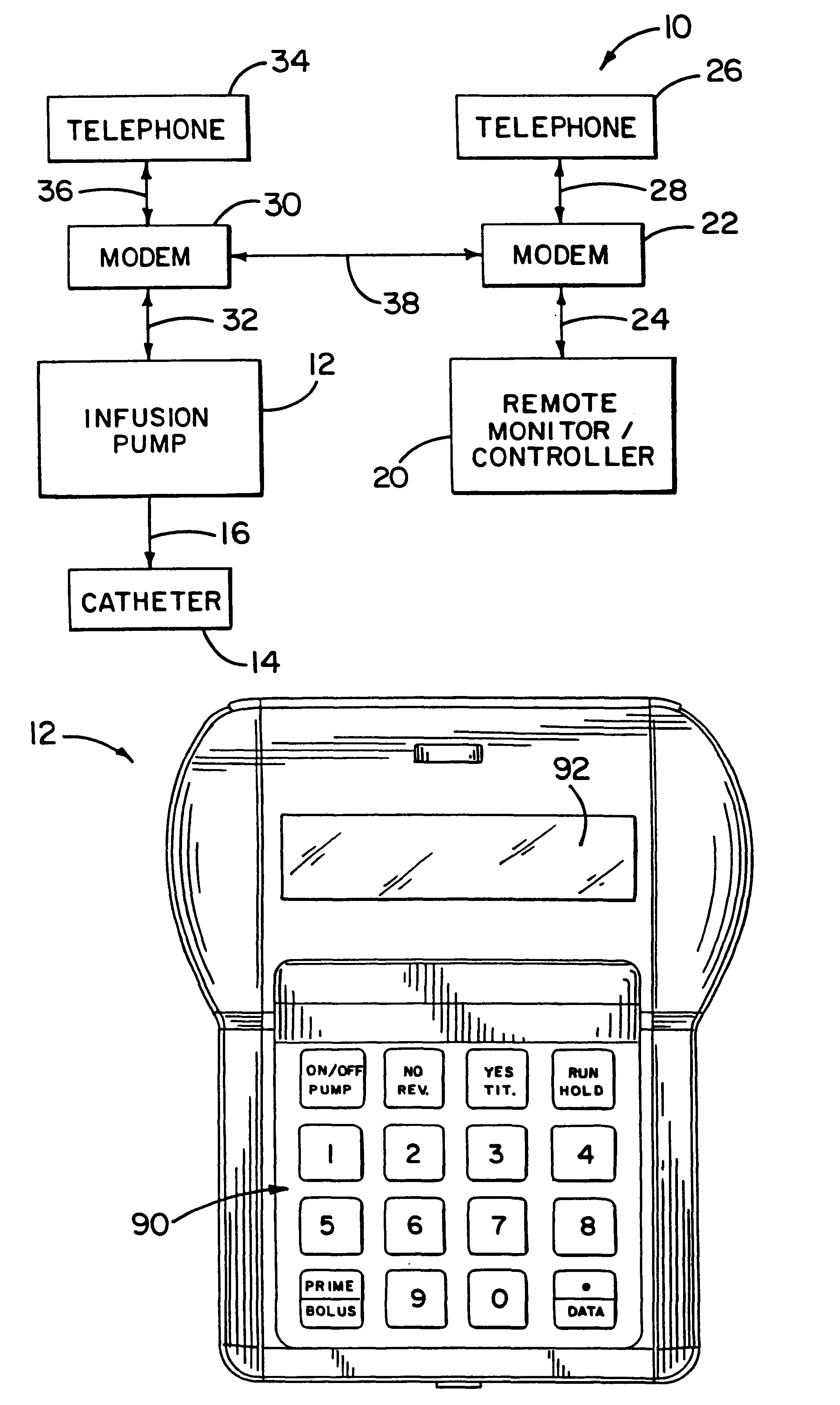
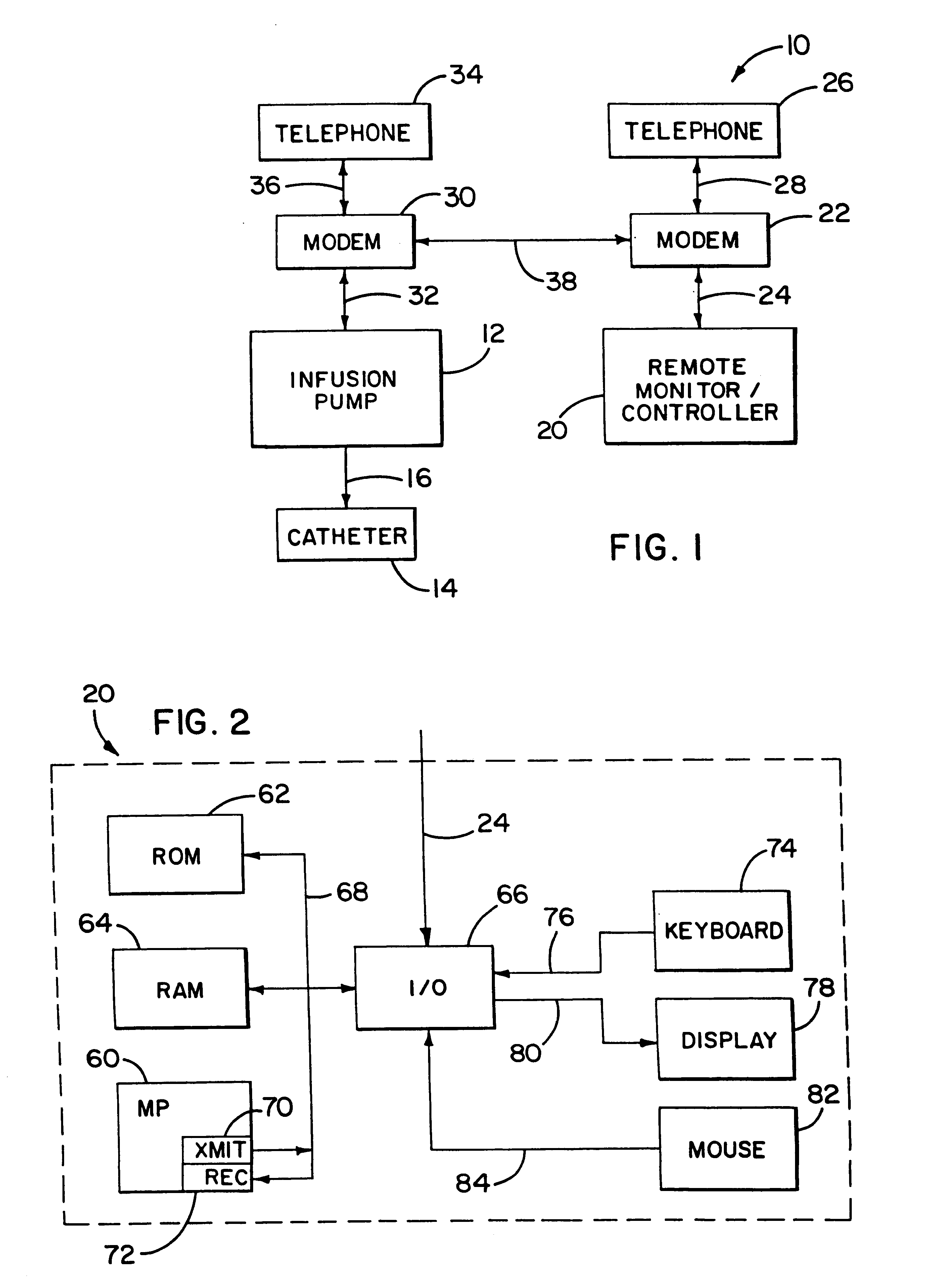
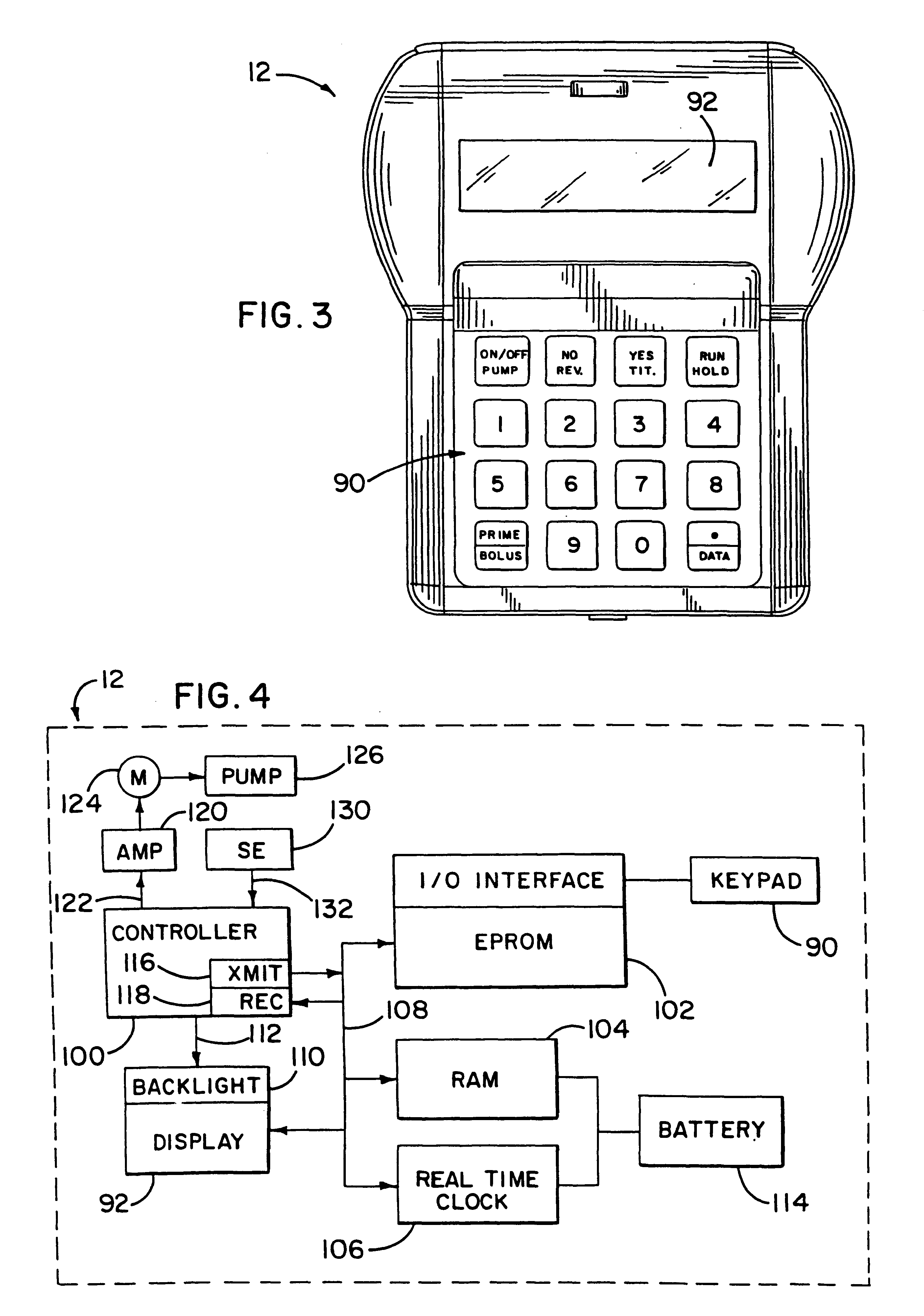
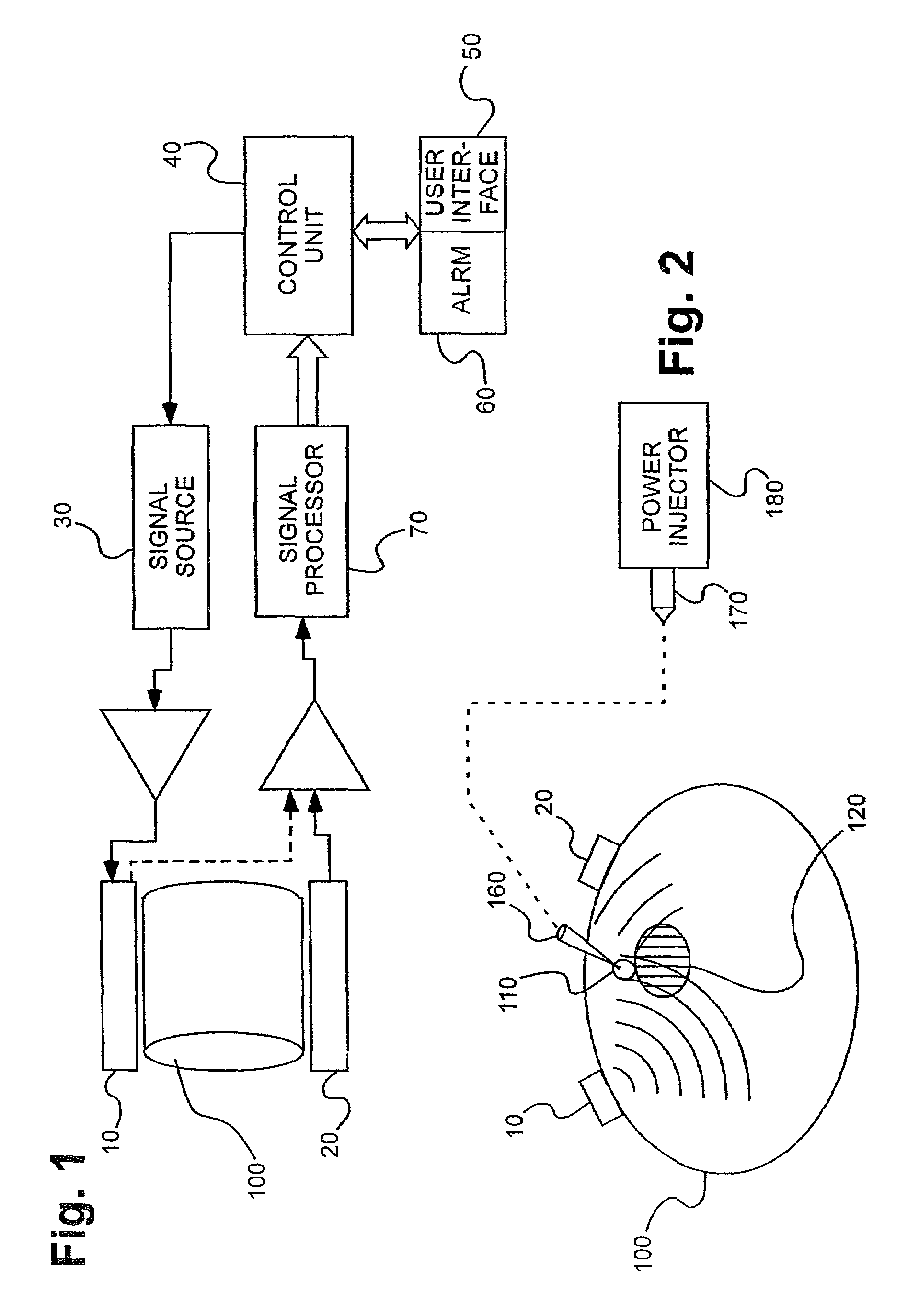
An infusion system for infusing a liquid into a body includes an external infusion device and a remote commander. The external infusion device includes a housing, a receiver, a processor and an indication device. The receiver is coupled to the housing and for receiving remotely generated commands. The processor is coupled to the housing and the receiver to receive remotely generated commands and to control the external infusion device in accordance with the commands. The indication device indicates when a command has been received and indicates when the command is being utilized to control the external infusion device so that the external infusion device is capable of being concealed from view when being remotely commanded. The remote commander includes a commander housing, a keypad for transmitting commands, and a transmitter for transmitting commands to the receiver of the external infusion device.
Owner:MEDTRONIC MIMIMED INC
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
Ambulatory medical apparatus and method using a telemetry system with predefined reception listening
InactiveUS6950708B2Reduce power consumptionConsuming and burdensomeEnergy efficient ICTElectrotherapyAmbulatoryStart time
An implanted medical device (e.g. infusion pump) and an external device communicate with one another via telemetry messages that are receivable only during windows or listening periods. Each listening period is open for a prescribed period of time and is spaced from successive listening periods by an interval. The prescribed period of time is typically kept small to minimize power consumption. To increase likelihood of successful communication, the window may be forced to an open state, by use of an attention signal, in anticipation of an incoming message. To further minimize power consumption, it is desirable to minimize use of extended attention signals, which is accomplished by the transmitter maintaining an estimate of listening period start times and attempting to send messages only during listening periods. In the communication device, the estimate is updated as a result of information obtained with the reception of each message from the medical device.
Owner:MEDTRONIC MIMIMED INC
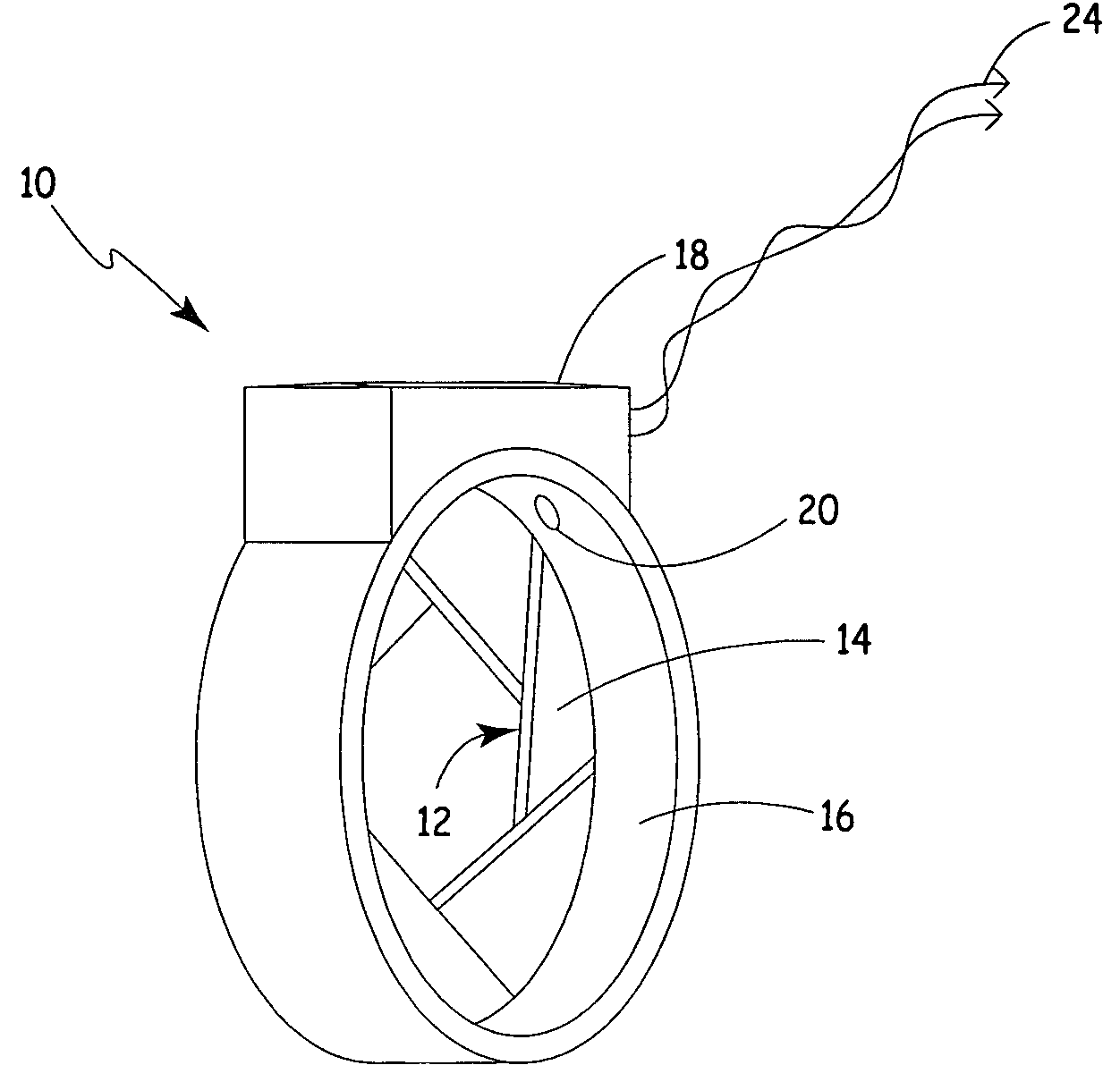
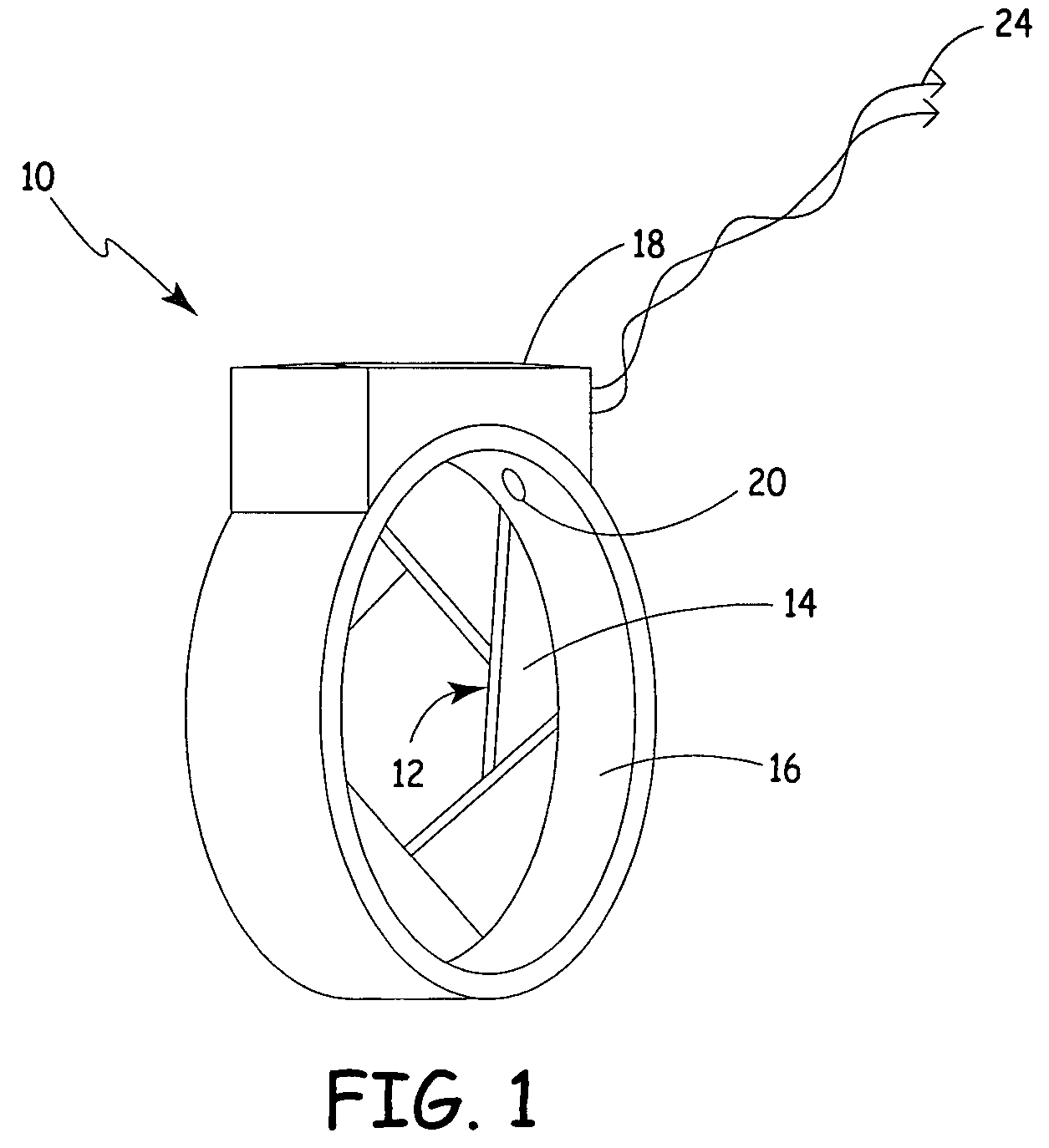
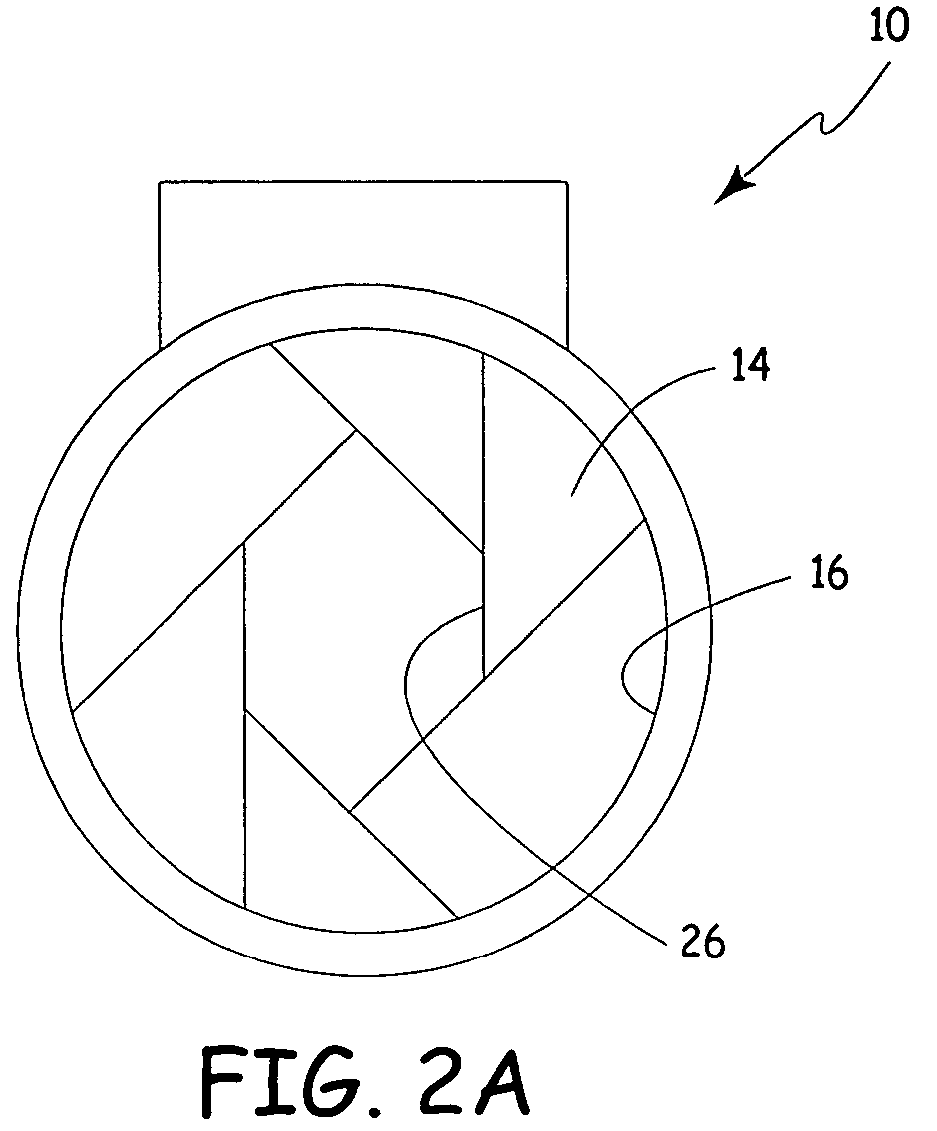
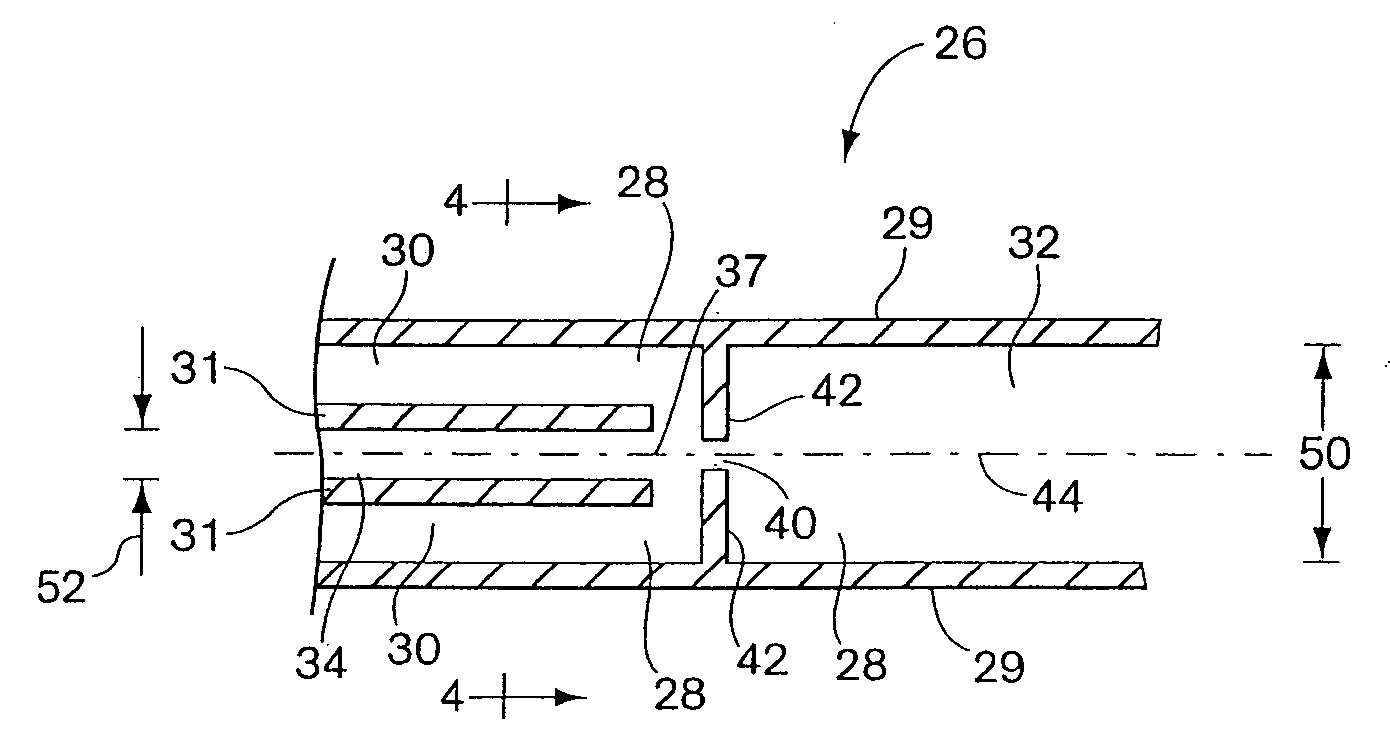
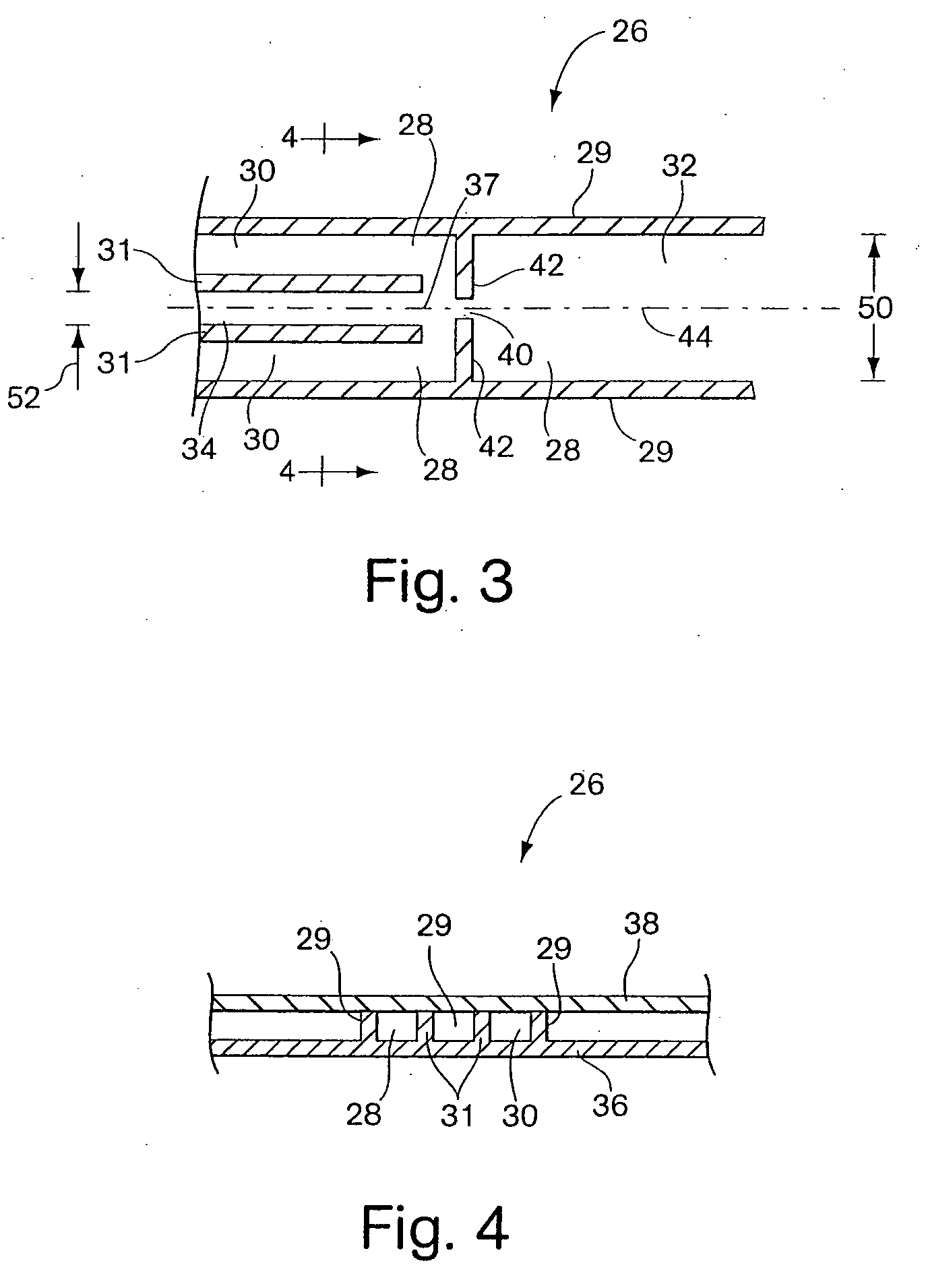
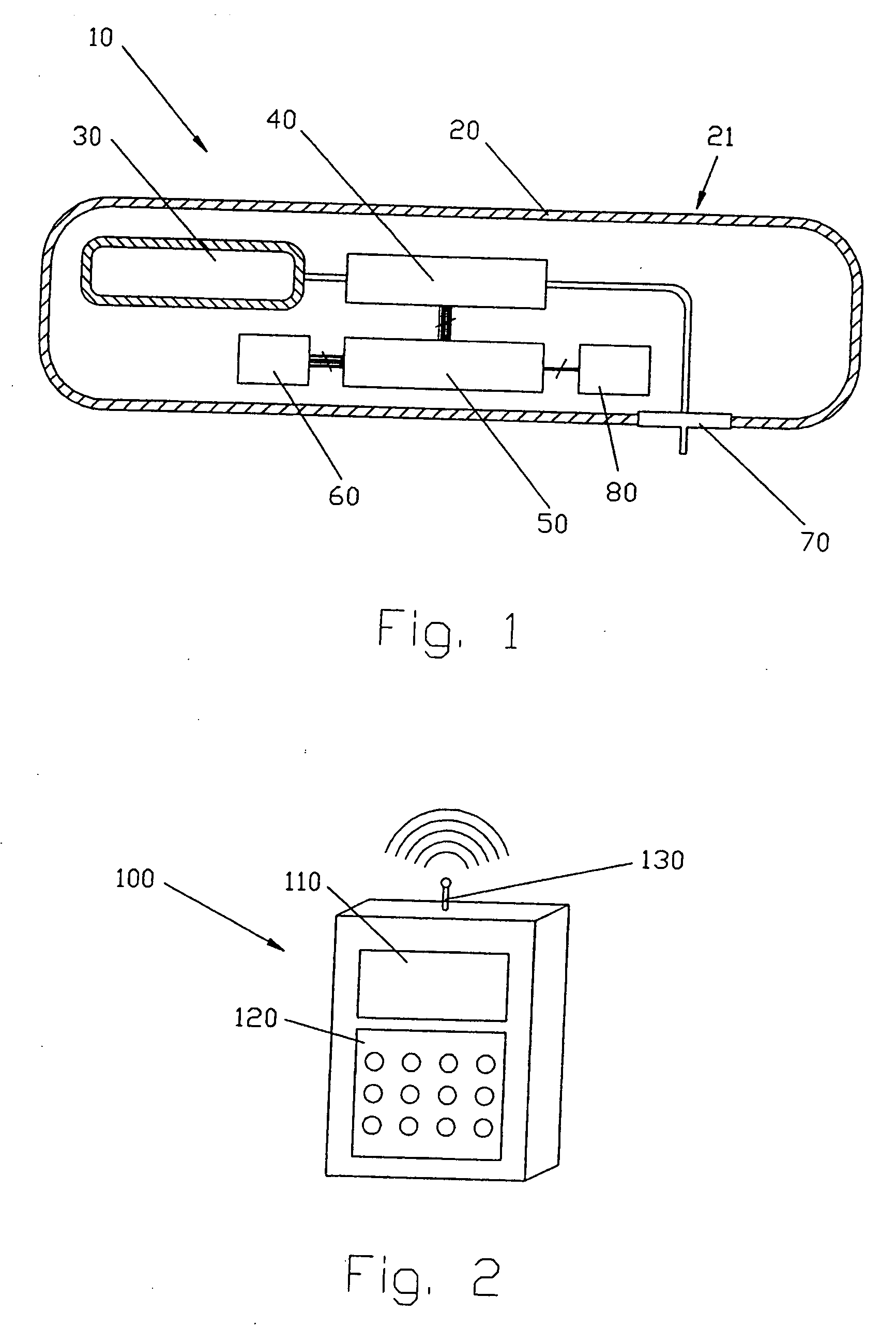
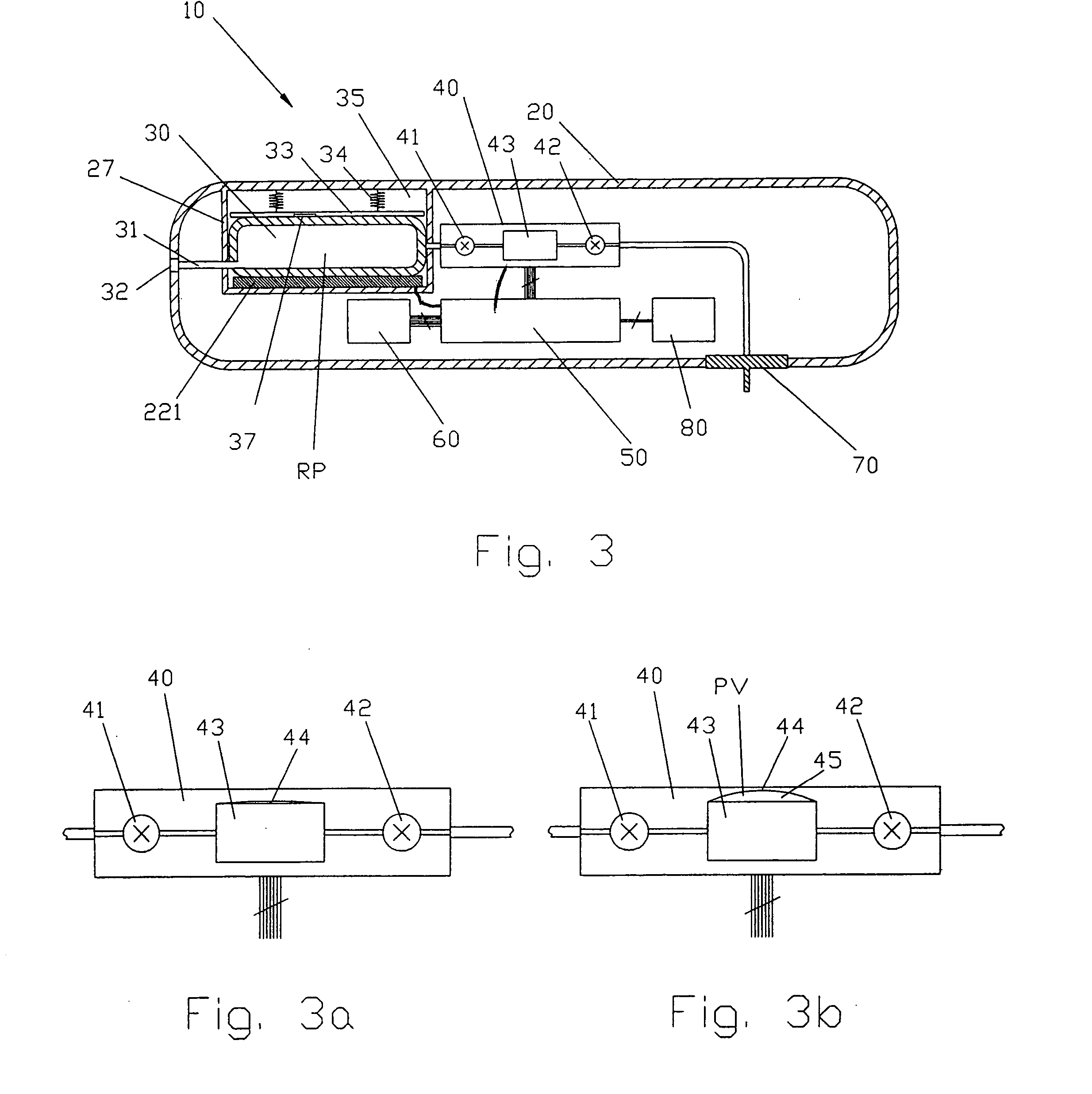
Process fluid flow device with variable orifice
A process device providing total fluid flow control is provided. The device includes a closure mechanism disposed in a flow conduit. The closure mechanism, which is preferably an iris-type diaphragm, provides a variable internal diameter. The device includes a differential pressure sensor for sensing the differential pressure on opposite sides of the diaphragm. A controller receives an indication of differential pressure and generates a control signal to an actuator that actuates the closure mechanism. The closure mechanism, differential pressure sensor and controller create a closed-loop flow controller in a single process device.
Owner:ROSEMOUNT INC
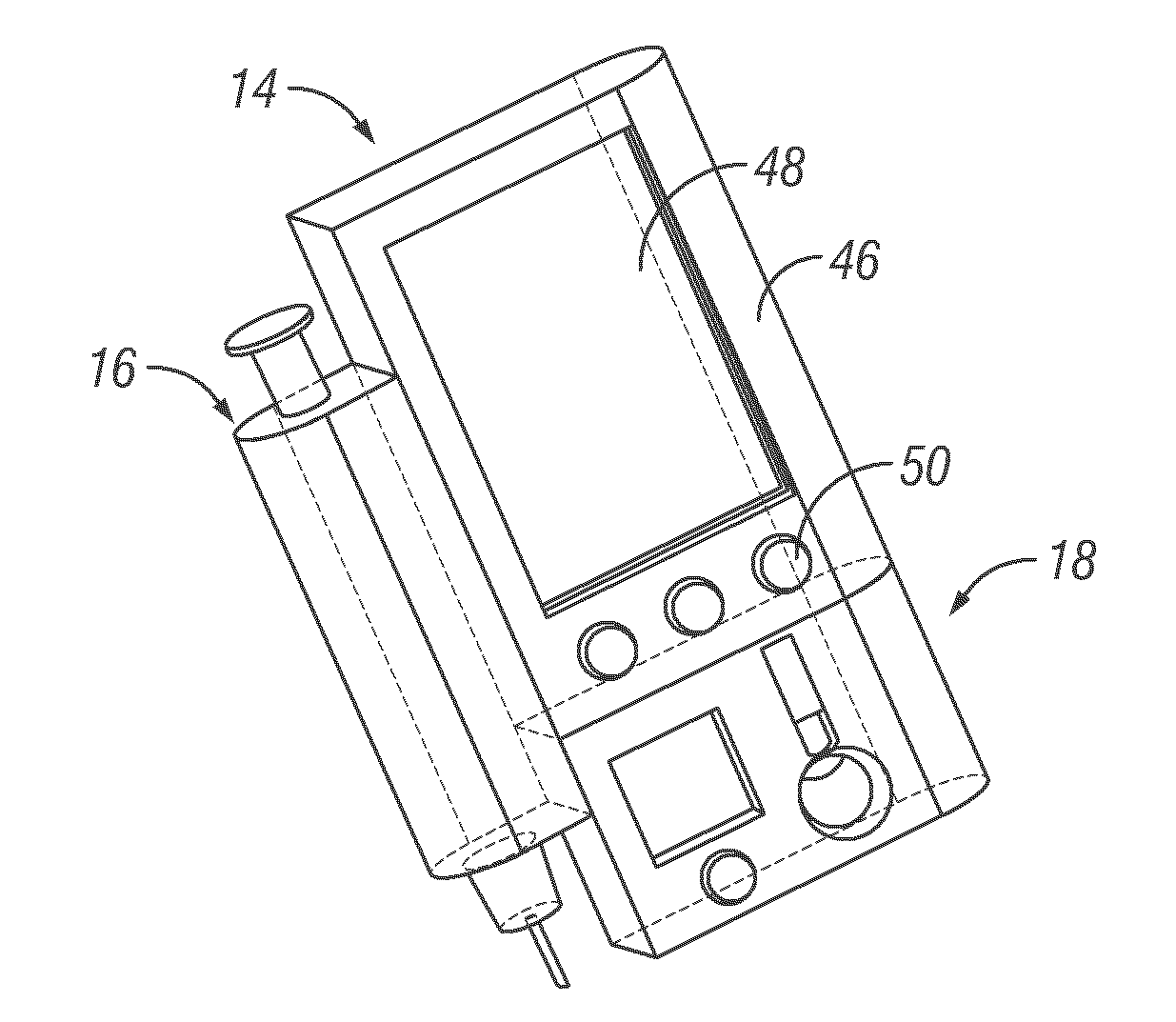
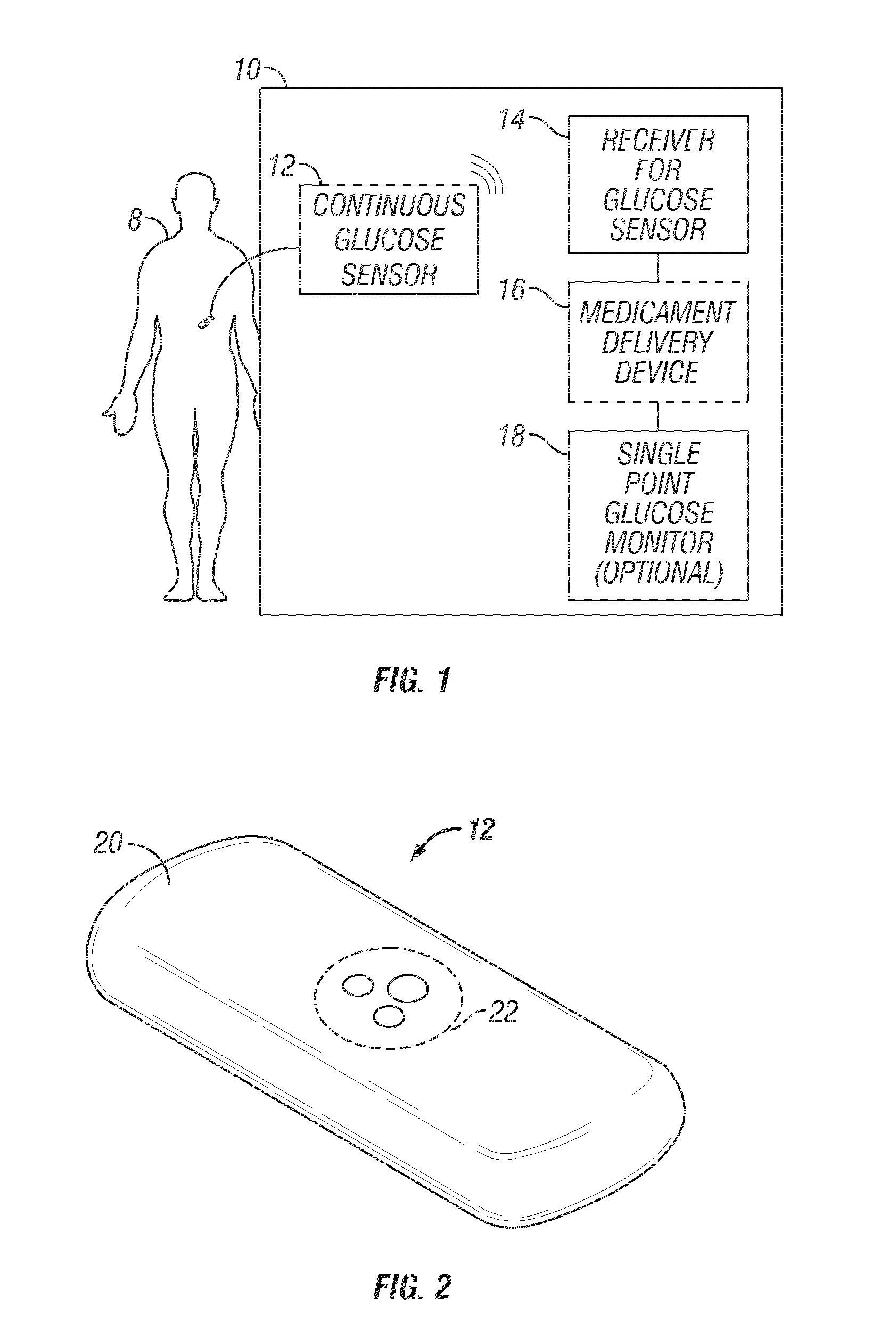
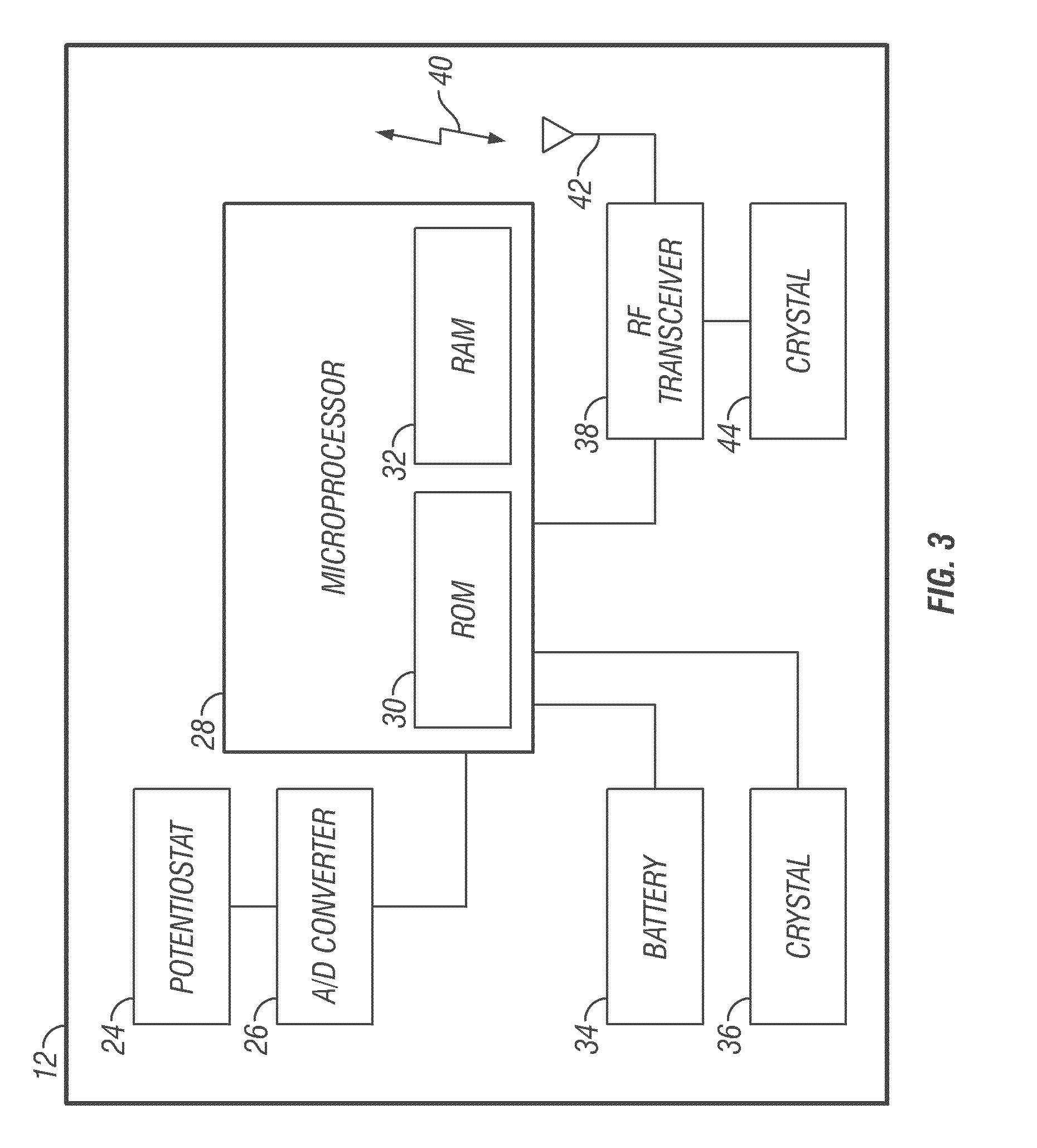
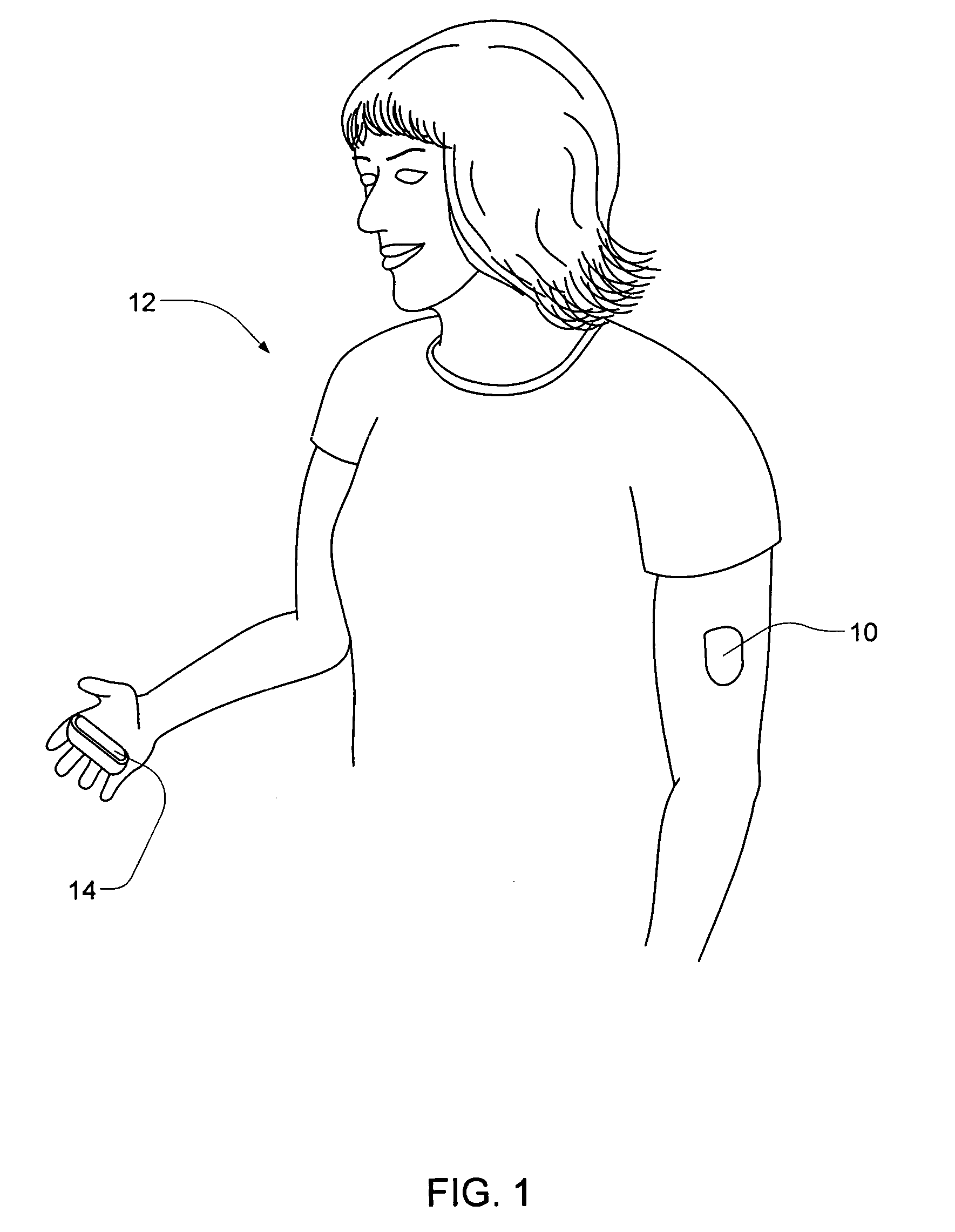
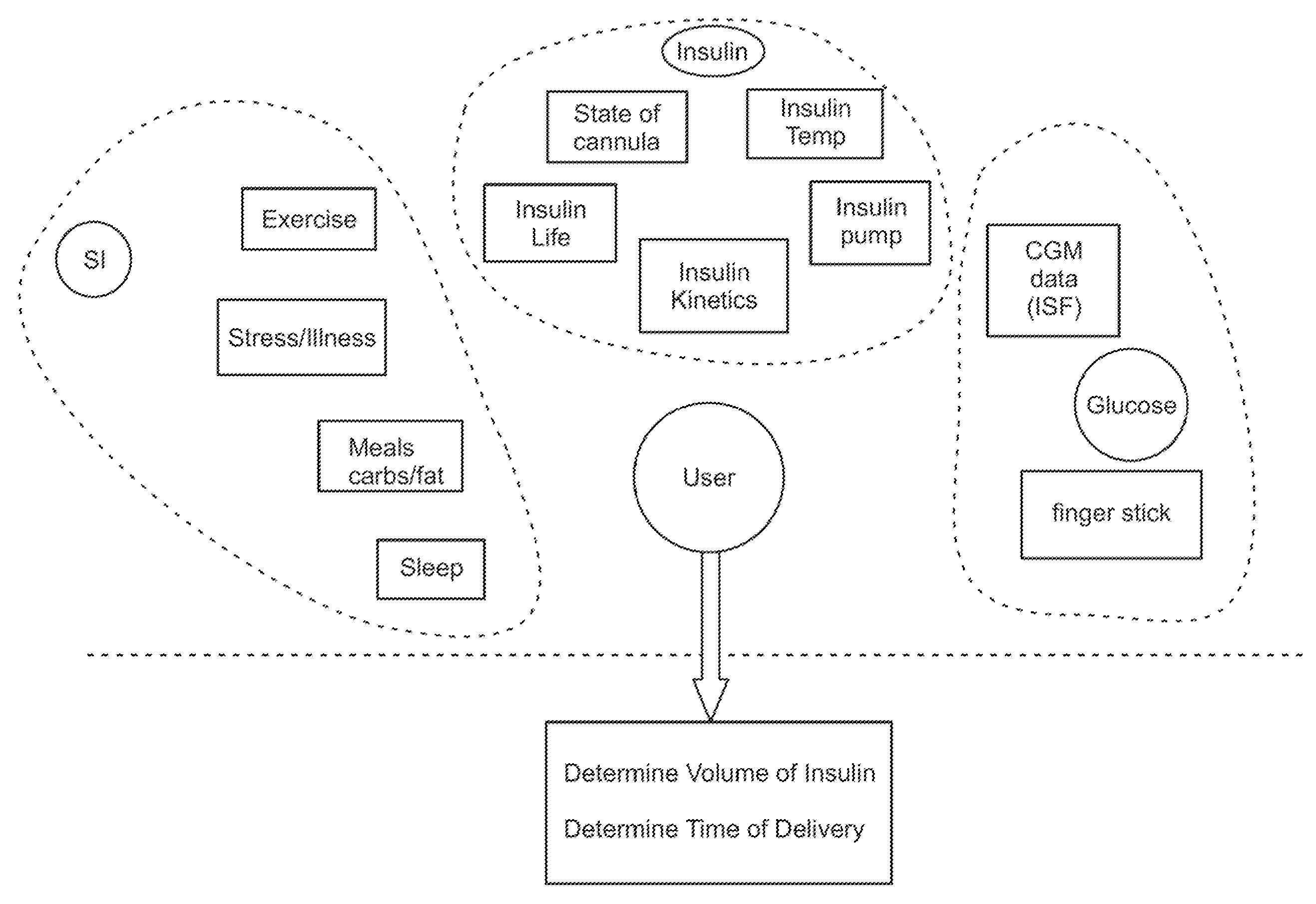
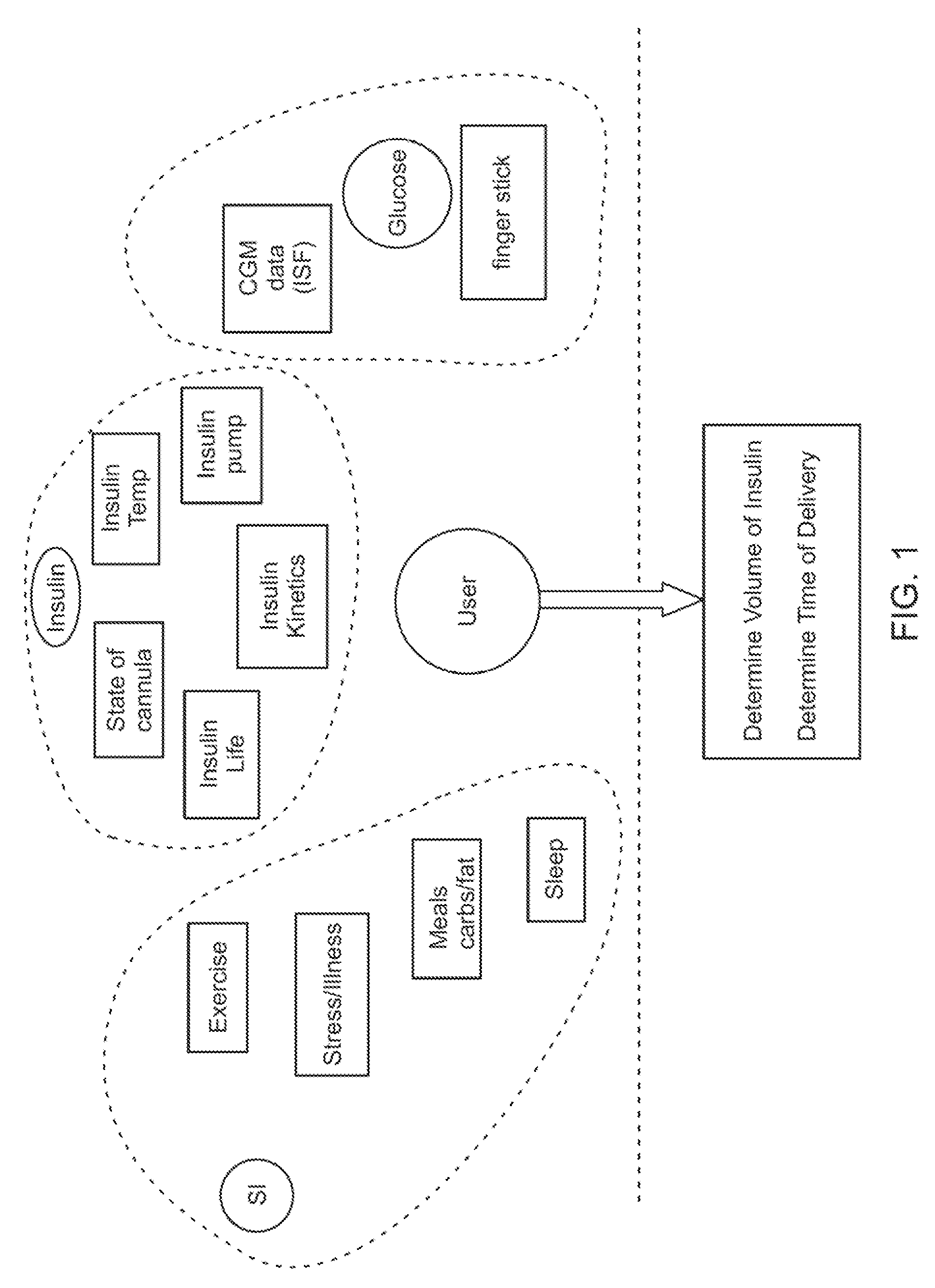
Integrated delivery device for continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM INC
Programmable insulin pump
Owner:TANDEM DIABETES CARE INC
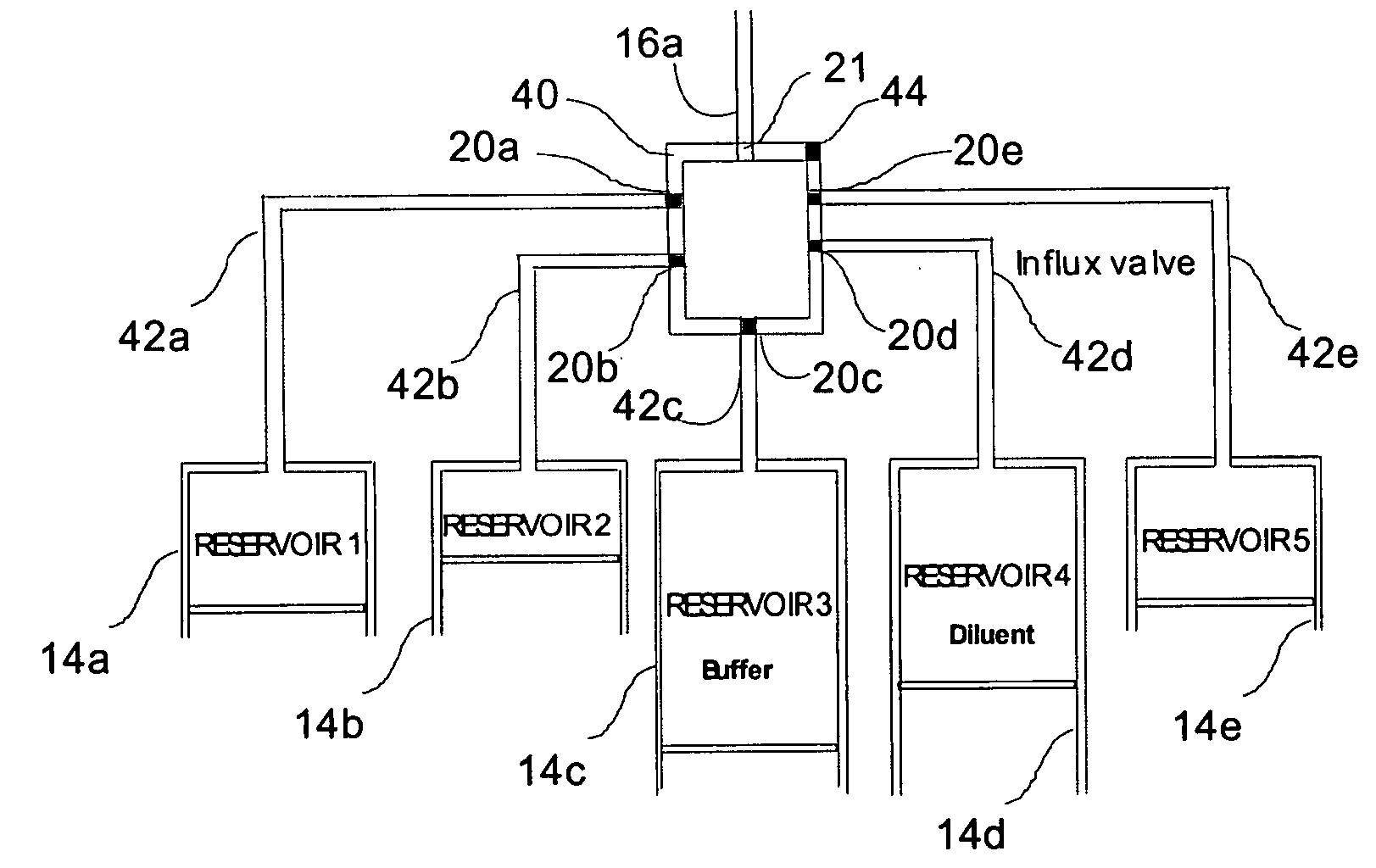
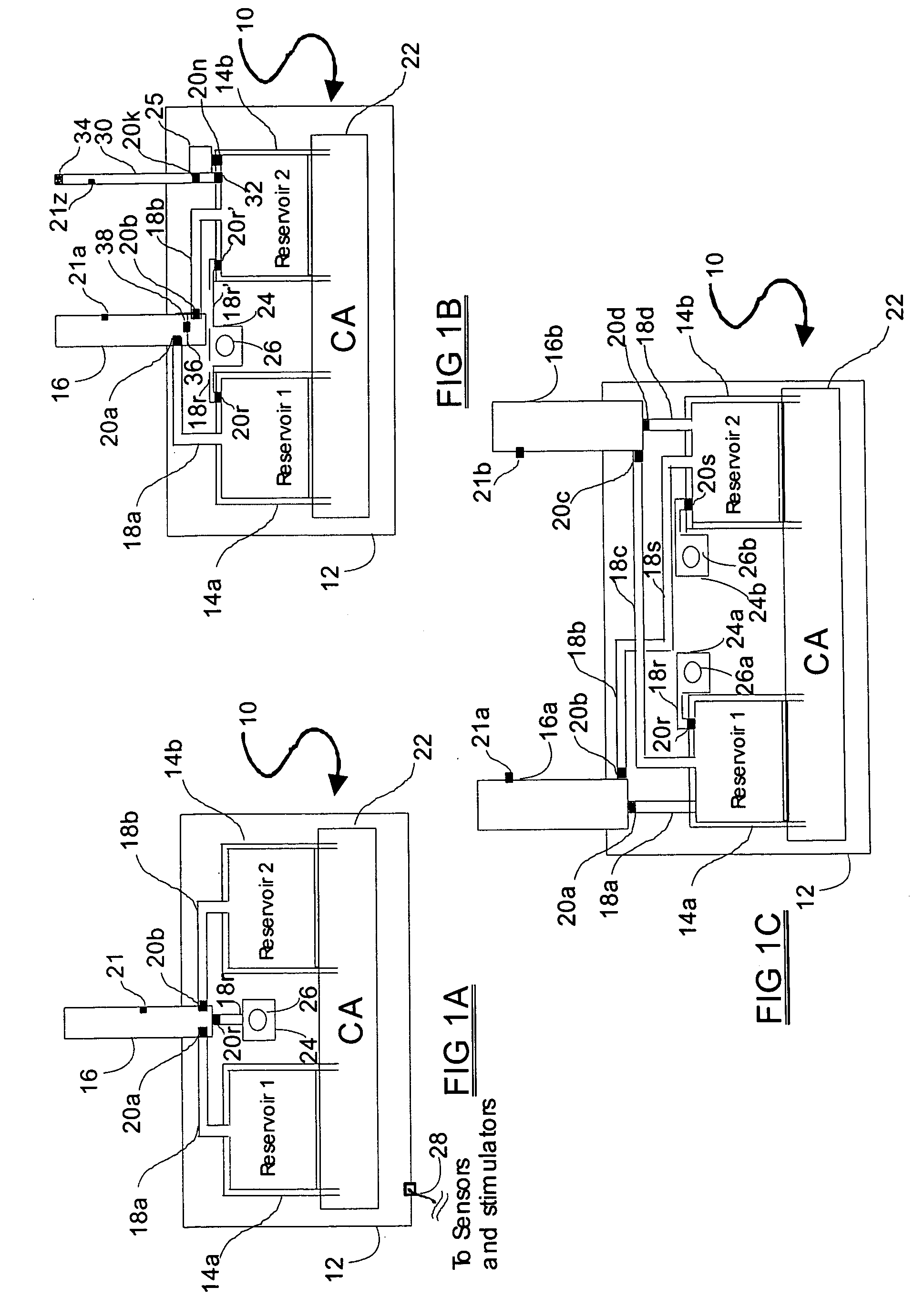
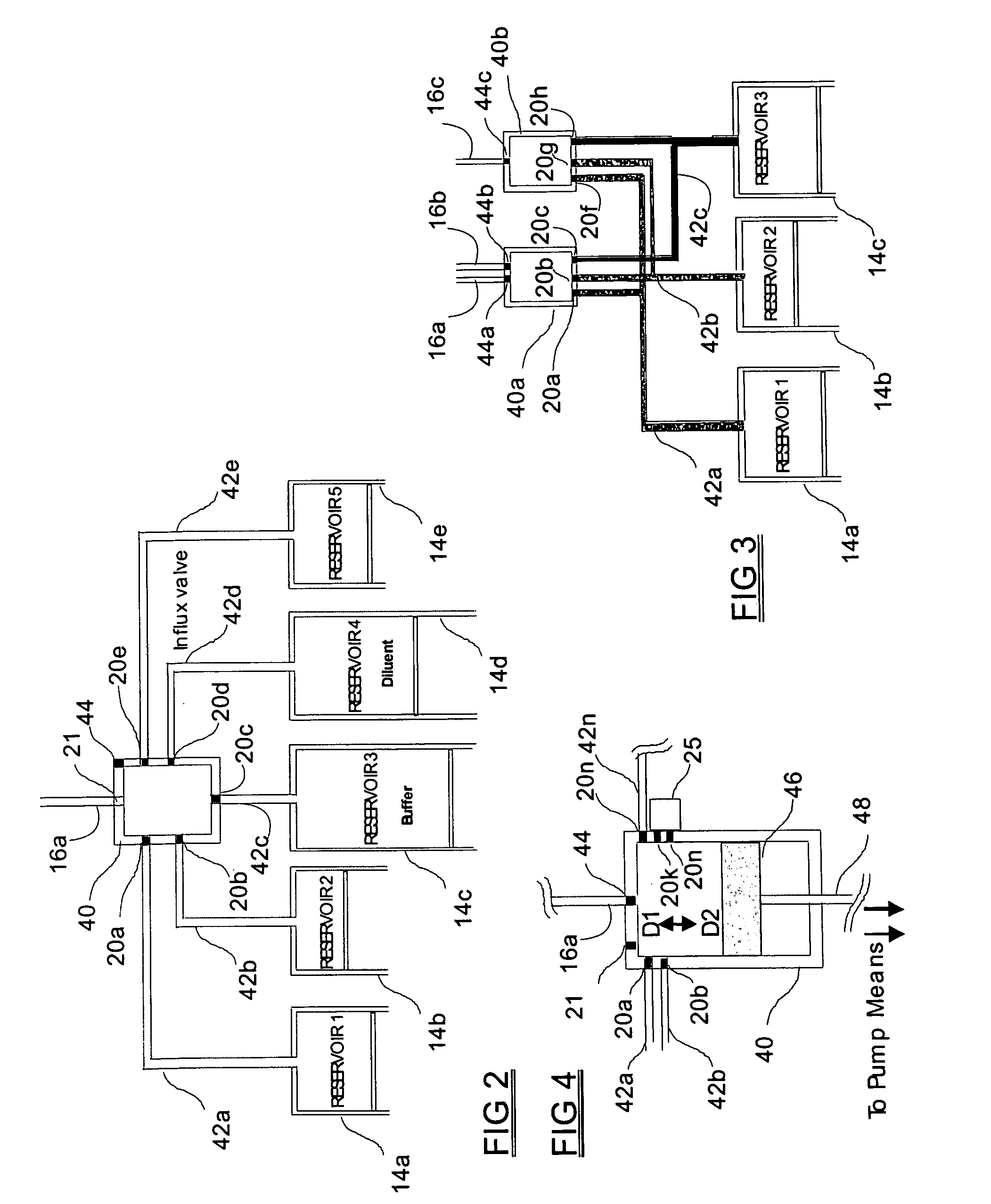
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS20050277912A1Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
Devices, systems and methods for patient infusion
InactiveUS7029455B2Easy to carryReduce financial burdenDrug and medicationsPharmaceutical delivery mechanismUser inputRemote control
A device for delivering a fluid to a patient, including an exit port, a dispenser for causing fluid from a reservoir to flow to the exit port, a local processor programmed to cause a flow of fluid to the exit port based on flow instructions from a separate, remote control device, and a wireless receiver connected to the local processor for receiving the flow instructions. The device also includes a housing free of user input components for providing flow instructions to the local processor, in order to reduce the complexity and costs of the device so that the device lends itself to being disposable in nature. A system and a kit are also described that include the fluid delivery device, a separate, remote control device, and accessories for transcutaneous delivery of fluid medications. Methods of utilizing the fluid delivery device to infuse fluid medications are additionally disclosed.
Owner:INSULET CORP
Delivery of agents such as cells to tissue
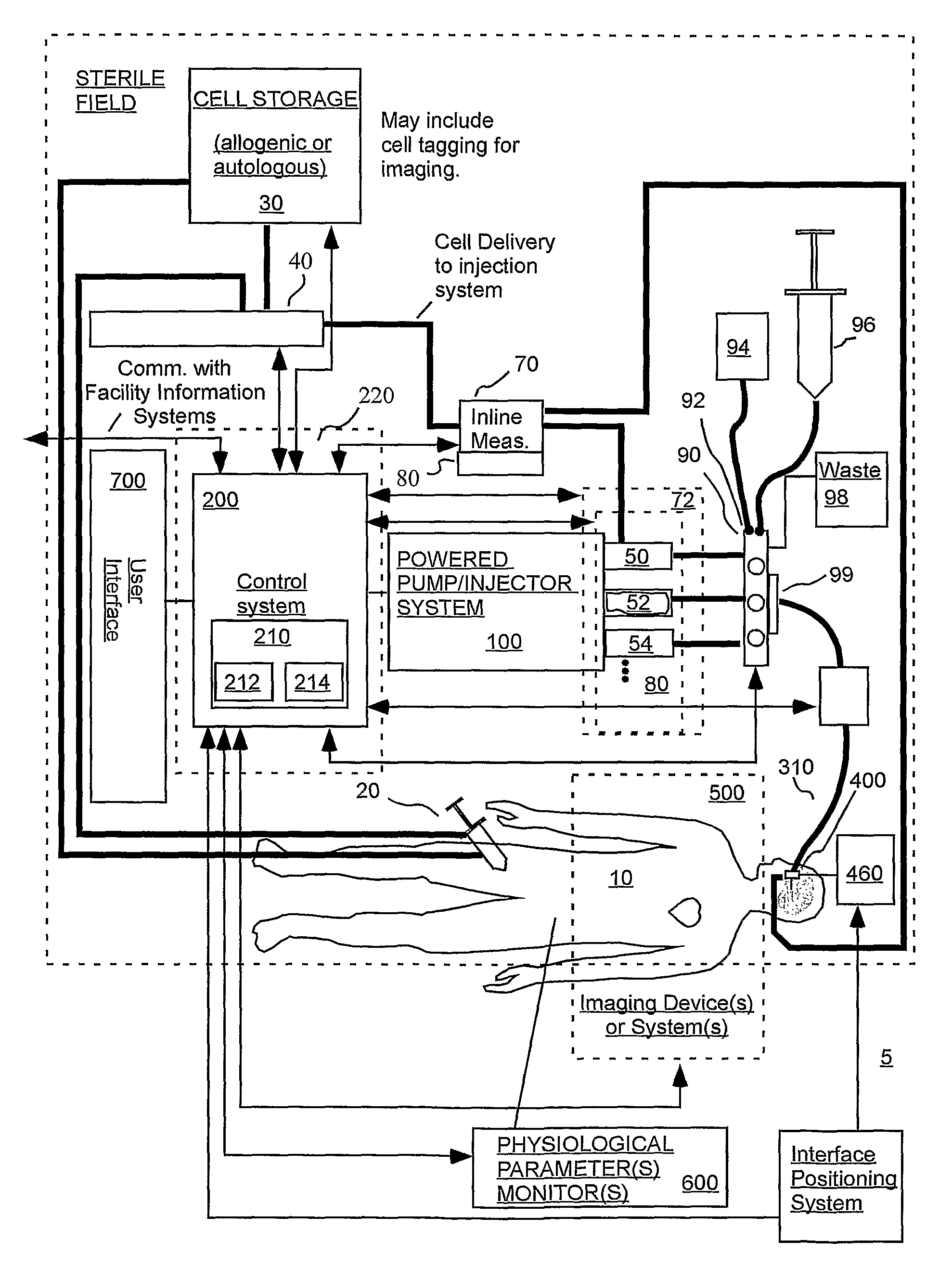
A system for delivering a fluid comprising cells to tissue of a patient includes a container holding an injection fluid and a powered drive. A sensor system provides a measurement indicative of at least shear forces on the cells to a control system. Based at least in part on this measurement, the control system is adapted to transmit a control signal to the powered drive for pressurizing the contents of the container to deposit cells within the tissue of a patient via a fluid path and a patient interface. As an example, the cells can be pregenitor or stem cells.
Owner:BAYER HEALTHCARE LLC
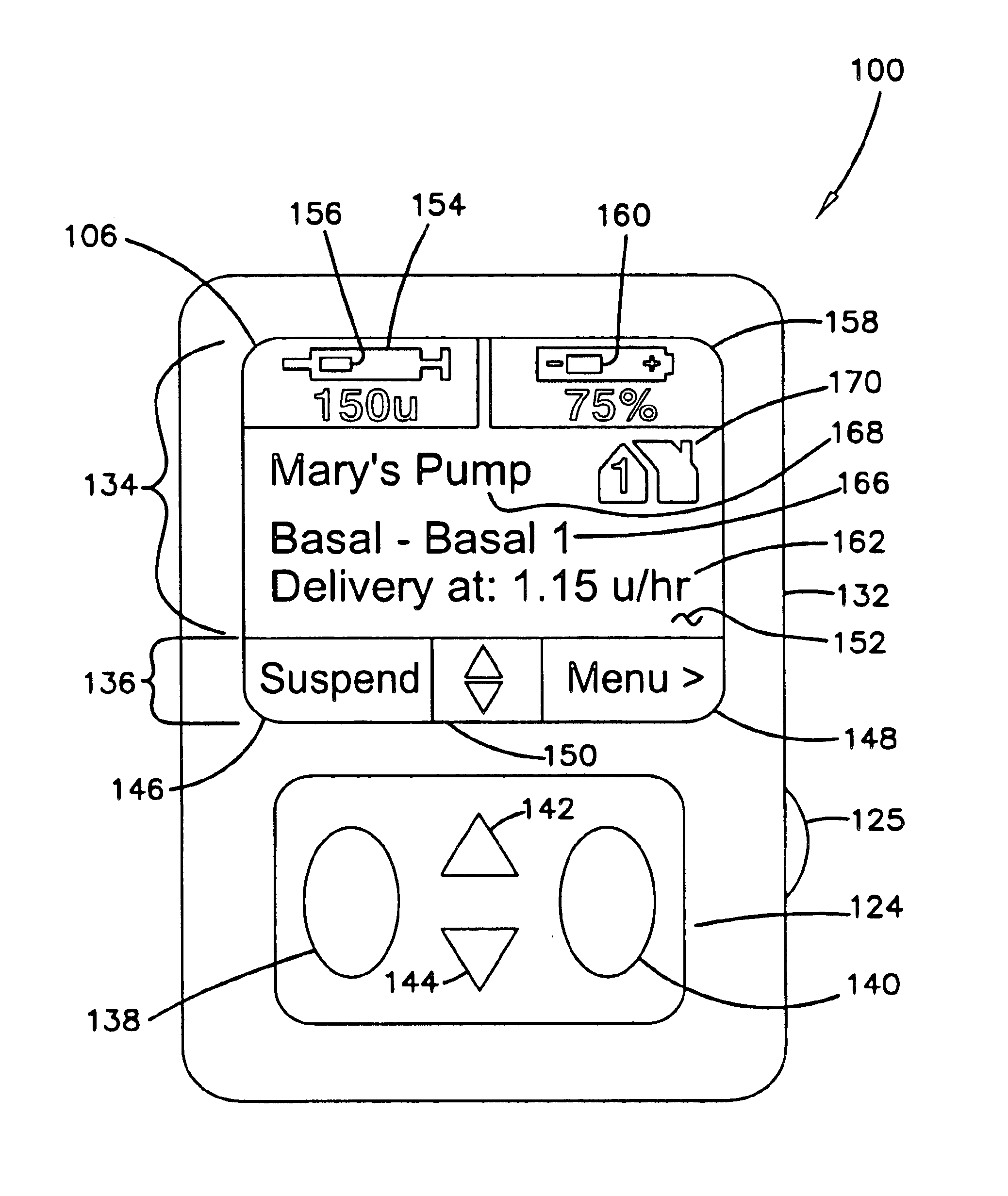
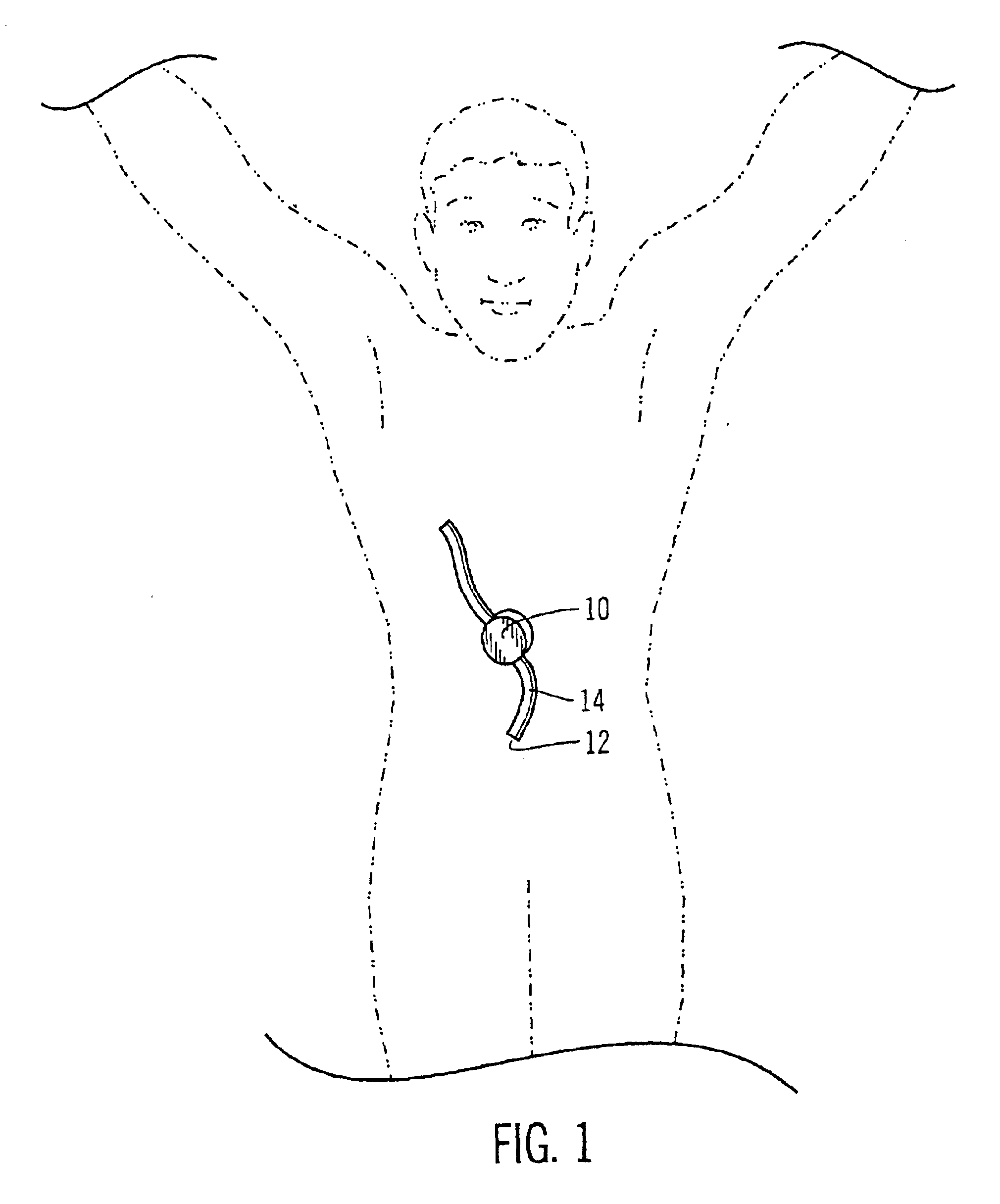
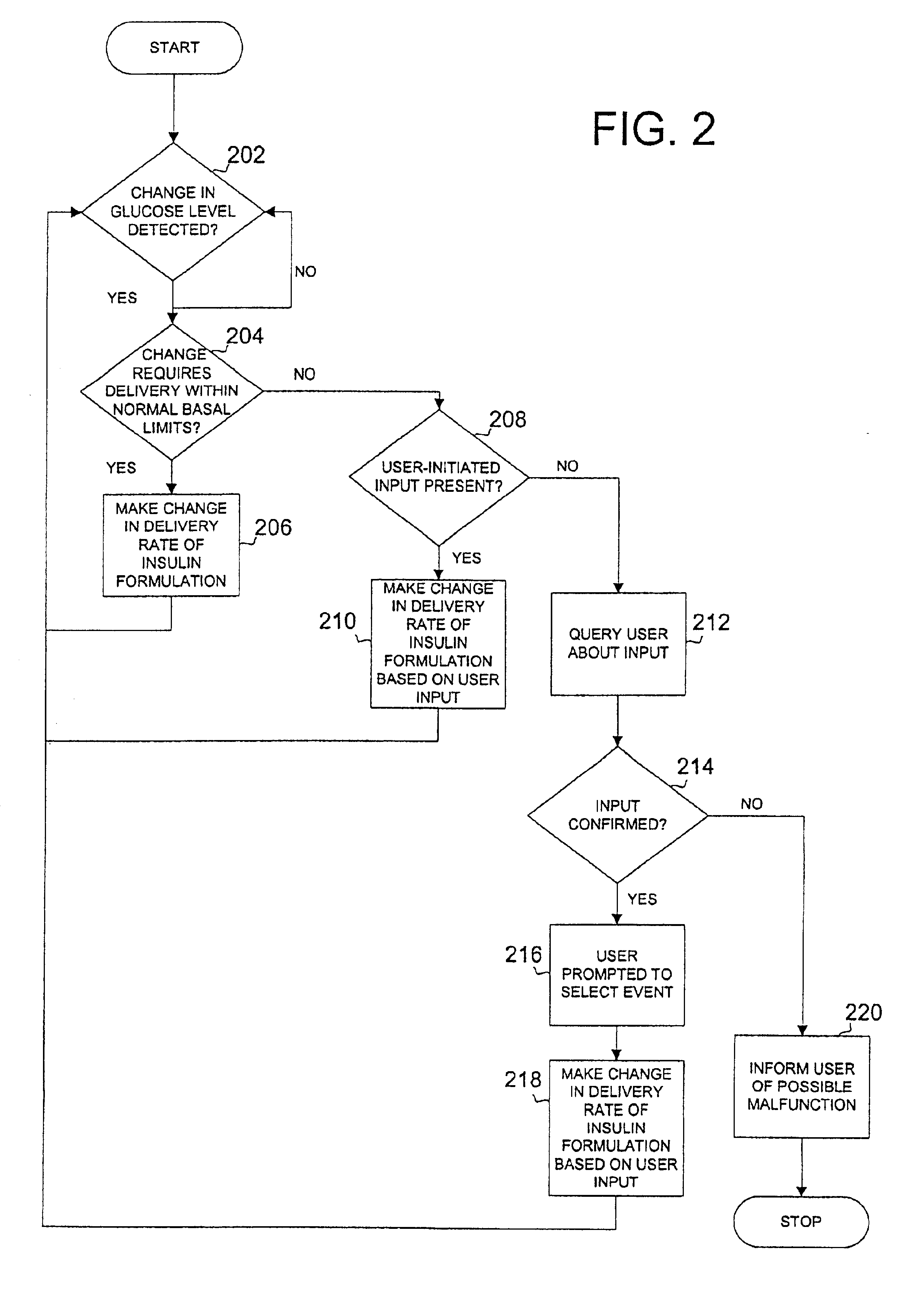
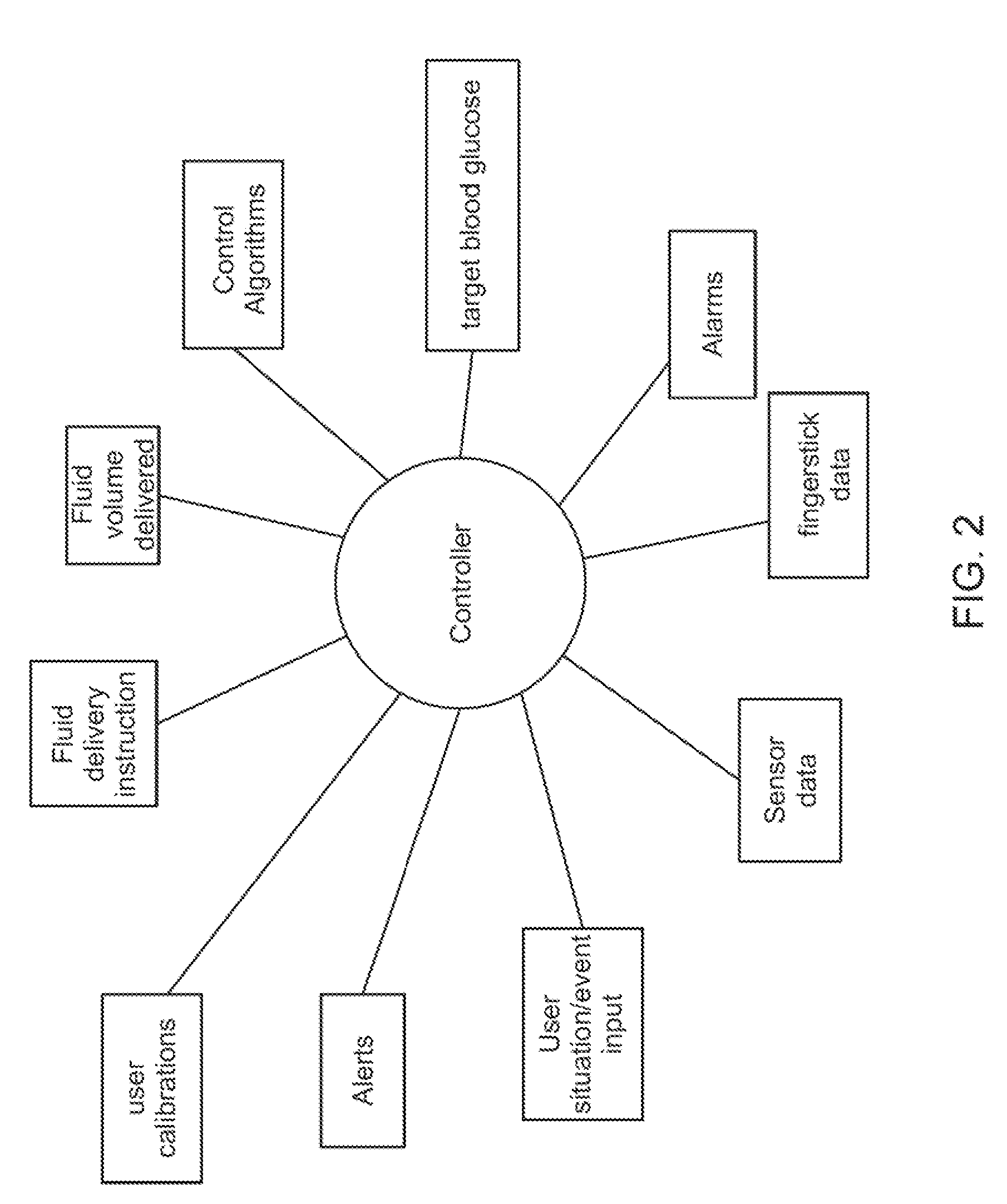
Safety limits for closed-loop infusion pump control
A system and process for providing safety limits on the delivery of an infusion formulation by an infusion pump system in response to a sensed biological state. The safety limits may comprise user-initiated event signals corresponding to events that may significantly affect the biological state. The safety limits may further comprise user-initiated event ranking signals for respective events which specify a degree, quantity, or measure for the respective event. The user-initiated event and event ranking signals may be communicated to a computing element associated with the infusion pump by an associated communication device having a user interface which comprises a plurality of user-selectable operators for entering information about the events and event rankings.
Owner:MEDTRONIC MIMIMED INC
Minimally-invasive system and method for monitoring analyte levels
InactiveUS6952604B2Improve responseImprove signal and performanceCatheterSensorsAnalyteStratum corneum
A minimally-invasive analyte detecting device and method for using the same. The system and method employ a device having an active electrode optionally coated with a substance, and a counter-electrode that is configured at least partially surround the active electrode. The configuration of the auxiliary electrode and active electrode improves the current flow through the device and increases the sensitivity of the device. When the device is placed against the patient's skin, the active electrode is adapted to enter through the stratum corneum of a patient to a depth less than a depth in the dermis at which nerve endings reside. An electric potential is applied to the active electrode and the analyte level is determined based on the amount of current or charge flowing through the device.
Owner:BECTON DICKINSON & CO
Infusion pump with a sealed drive mechanism and improved method of occlusion detection
InactiveUS20020128594A1Error minimizationImprove accuracyFlexible member pumpsSurgeryOcclusion detectionImproved method
A piston-type infusion pump is provided having an improved method of occlusion detection. The infusion pump includes processing circuitry for controlling the drive mechanism to infuse medication to a patient, including a sensor to track the position of the syringe plunger, thereby metering the amount of medication dispensed to the patient. The processing circuitry also includes a force sensor for providing signals indicative of the presence of occlusions along the infusion path. The operation of the drive mechanism causes delivery of medication to the patient. The infusion pump is constructed to be watertight.
Owner:LIFESCAN IP HLDG LLC
Method and apparatus for fluid dispersion
Owner:THE GOVERNINIG COUNCIL OF THE UNIV OF TORANTO +1
Devices, systems and methods for patient infusion
InactiveUS20050171512A1Easy to carryReduce financial burdenDrug and medicationsPharmaceutical delivery mechanismIntensive care medicineInfusion pump
A method for transcutaneously delivering fluid to a patient including providing at least one disposable infusion pump, wherein the pump includes a housing adapted to be mounted on a patient's skin, a transcutaneous patient access tool for extending through the housing and providing transcutaneous access to the patient, a reservoir prefilled with a therapeutic fluid, a dispenser for causing fluid from the reservoir to flow to the transcutaneous patient access tool, a processor connected to the dispenser and programmed to cause a flow of fluid from the reservoir to the transcutaneous patient access tool based on flow instructions, and a wireless receiver connected to the processor for receiving flow instructions from a remote controller and for delivering the flow instructions to the processor.
Owner:INSULET CORP
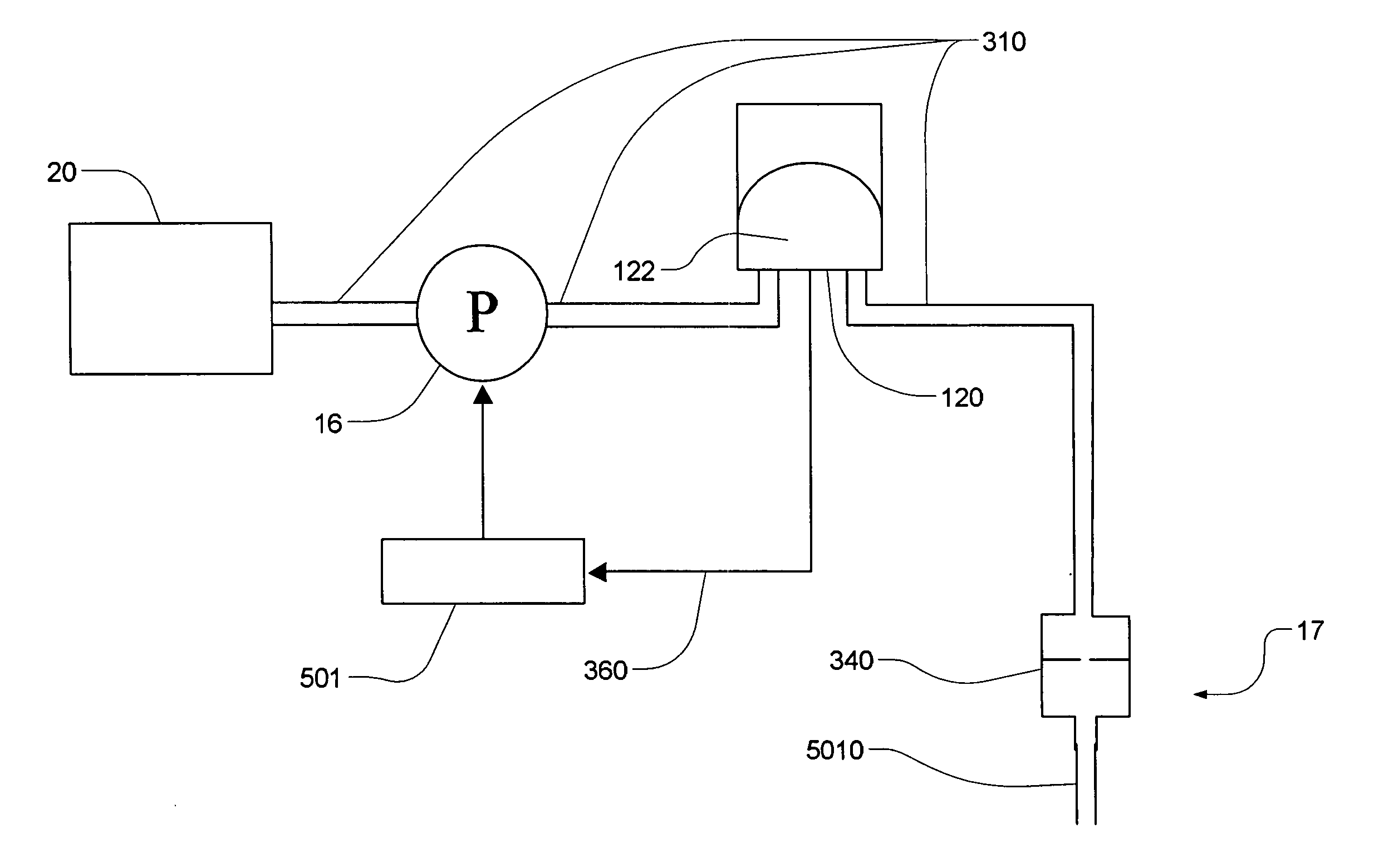
Fluid delivery systems and methods
ActiveUS20070228071A1Great degreePrevent backflowFlexible member pumpsMedical devicesDelivery systemBiomedical engineering
A method of dispensing fluid includes three processes. A first one of these processes includes pumping fluid into a resilient variable-volume dispensing chamber. The dispensing chamber is in series with a normally present finite fluid impedance and an output. The impedance is sufficient so as to cause expansion of the dispensing chamber as it receives pumped fluid even while some fluid flows through the output. Another one of these processes includes repeatedly measuring a parameter related to volume of the dispensing chamber over time. A third one of these processes includes controlling the pumping of fluid based on repeated measurements of the parameter to produce a desired fluid flow through the output. A corresponding system for dispensing fluid implements these processes.
Owner:DEKA PROD LLP
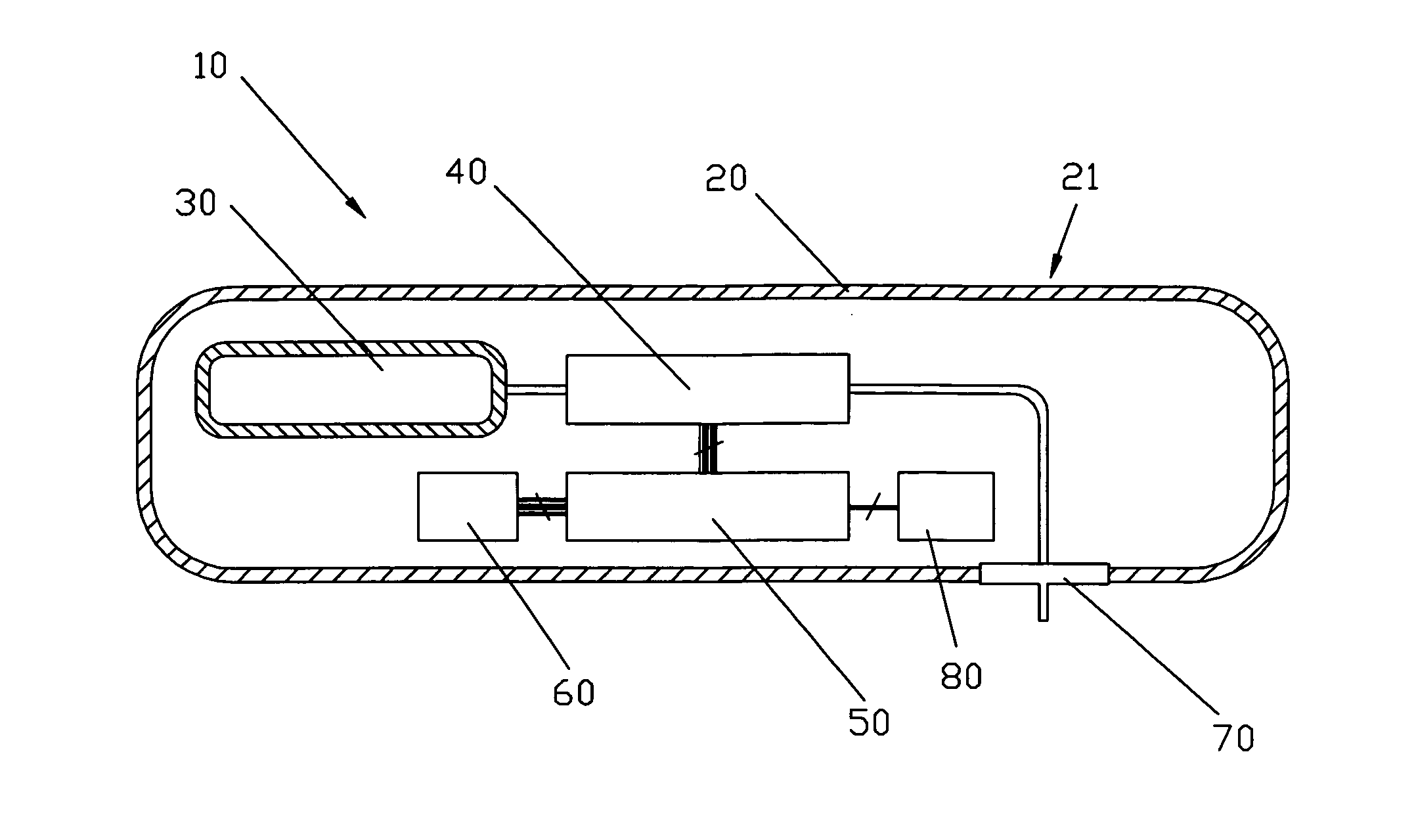
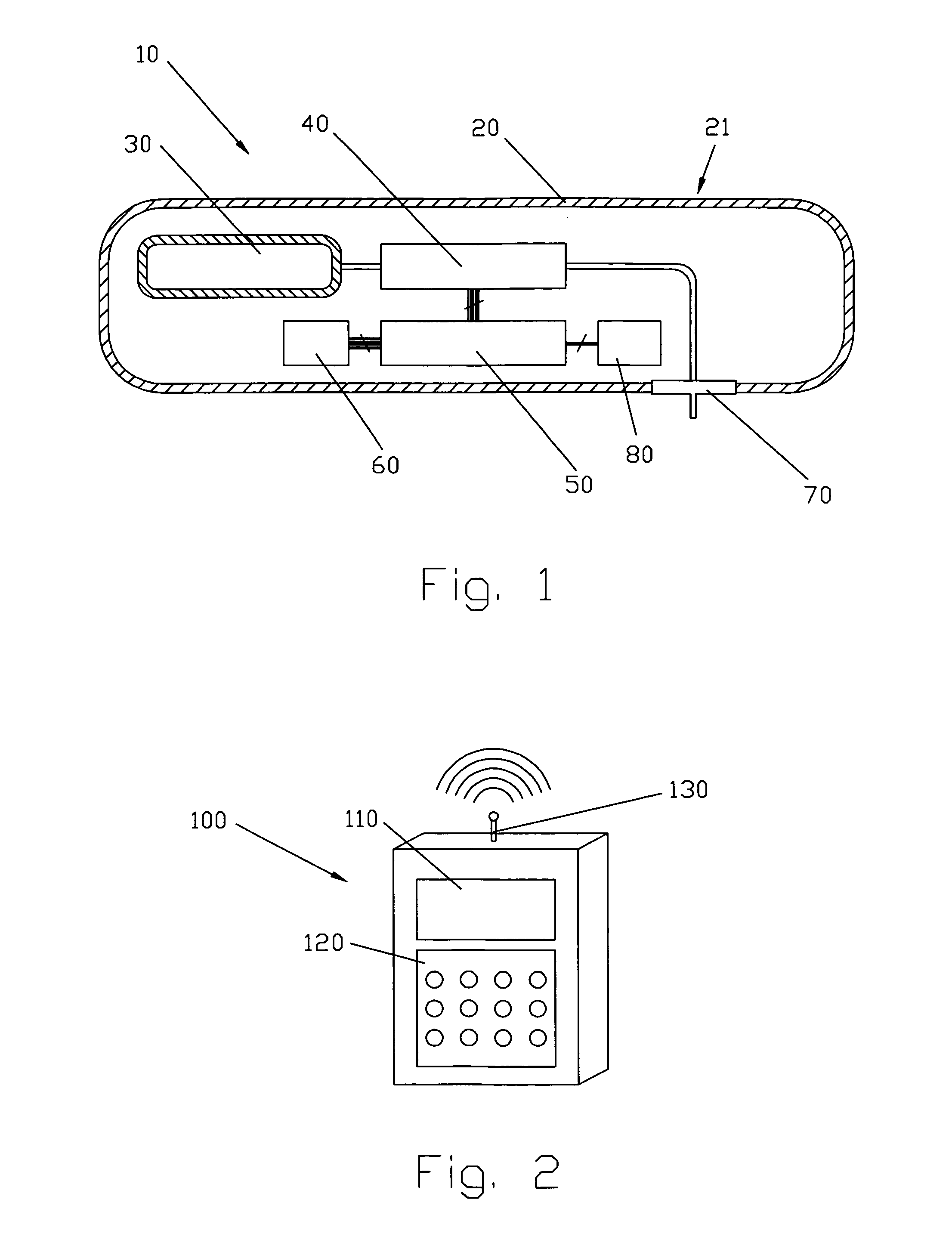
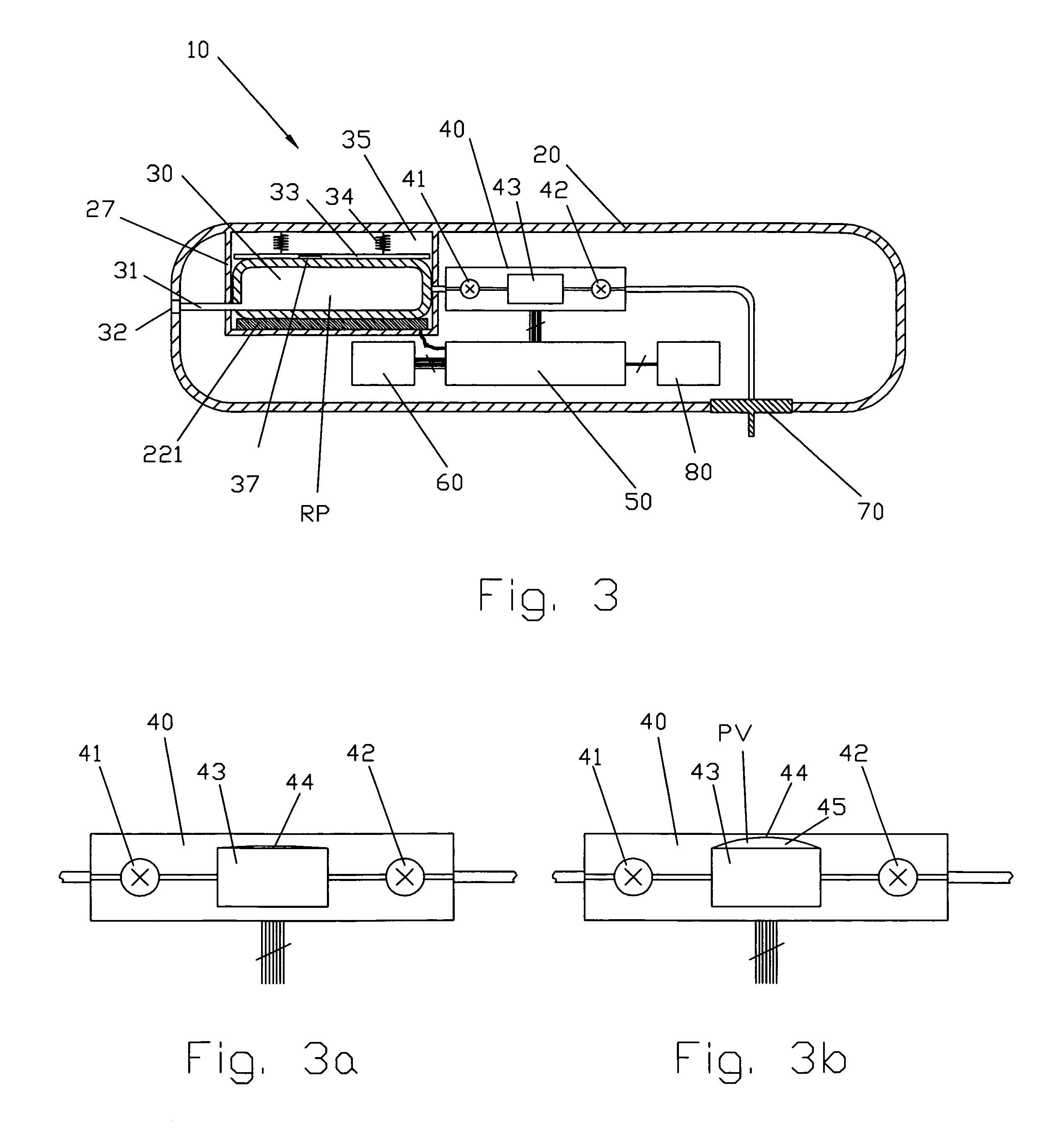
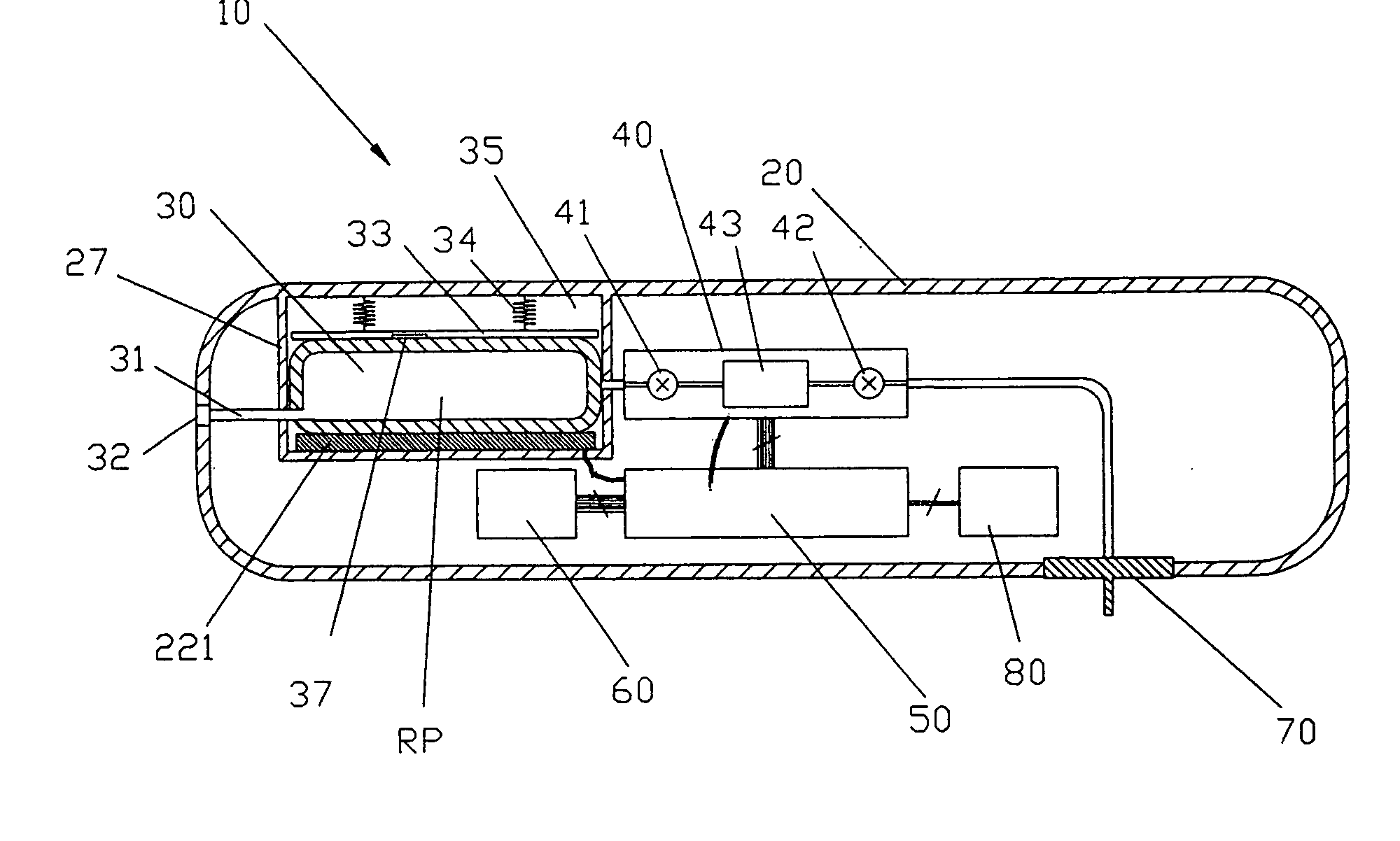
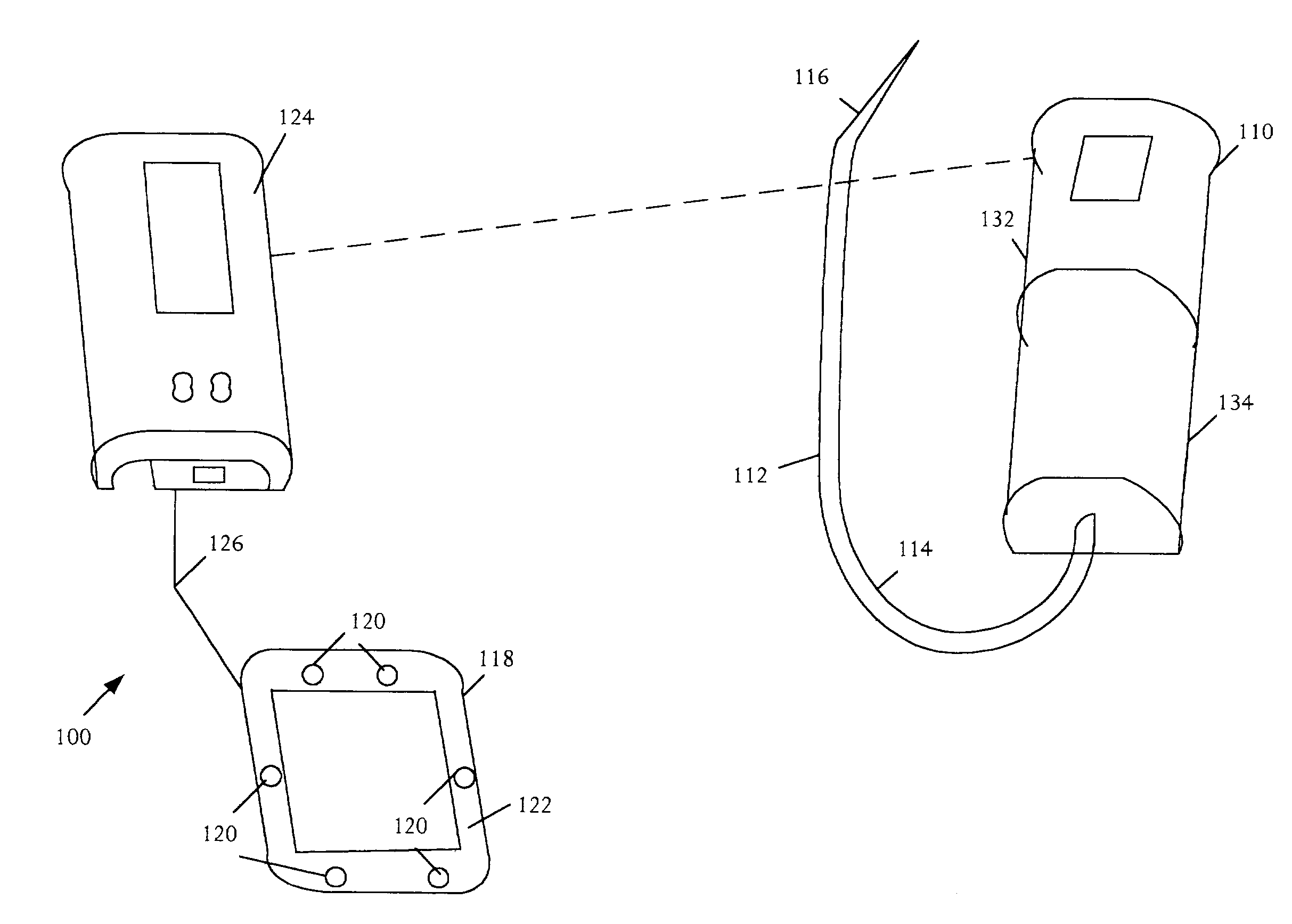
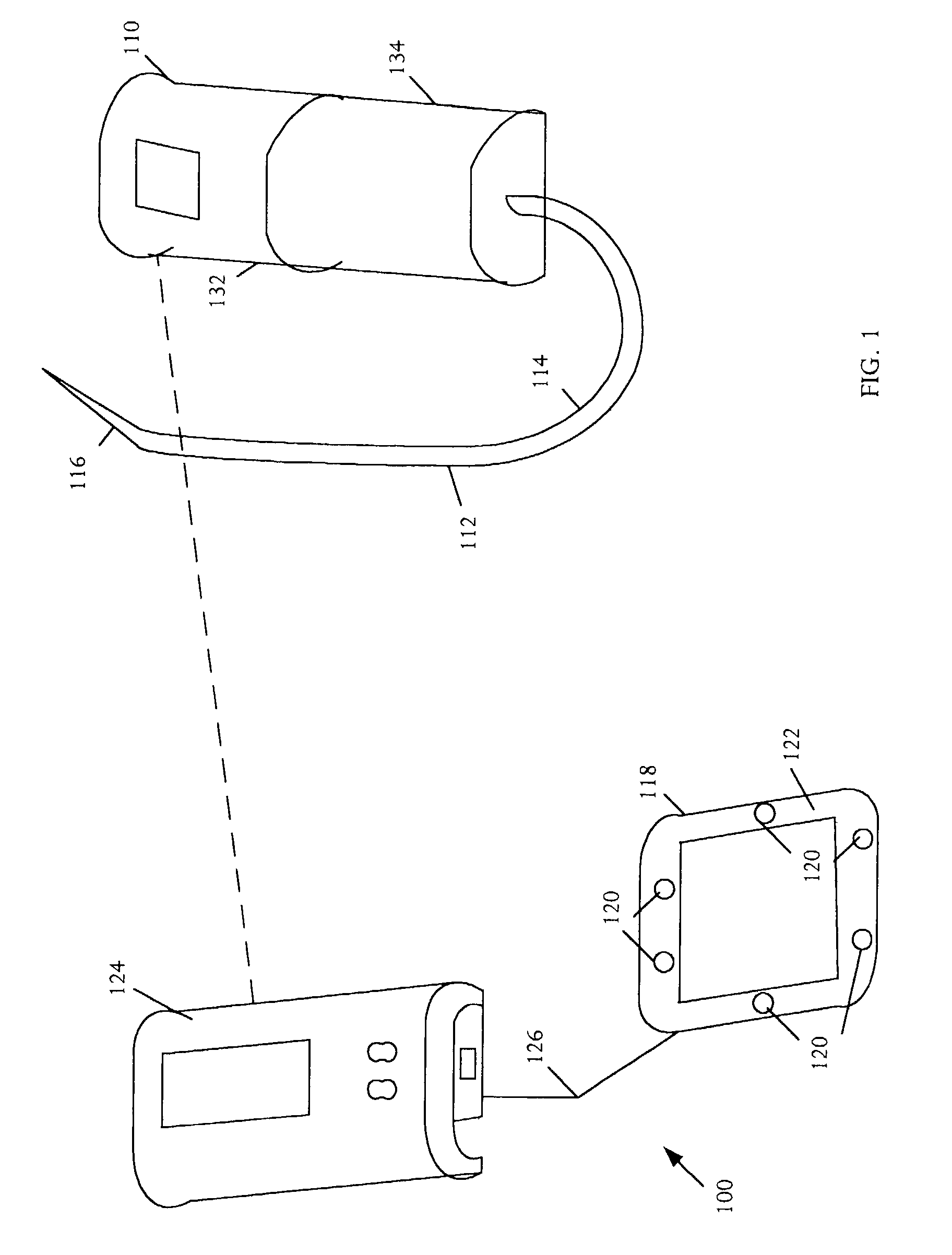
Medical apparatus with remote control
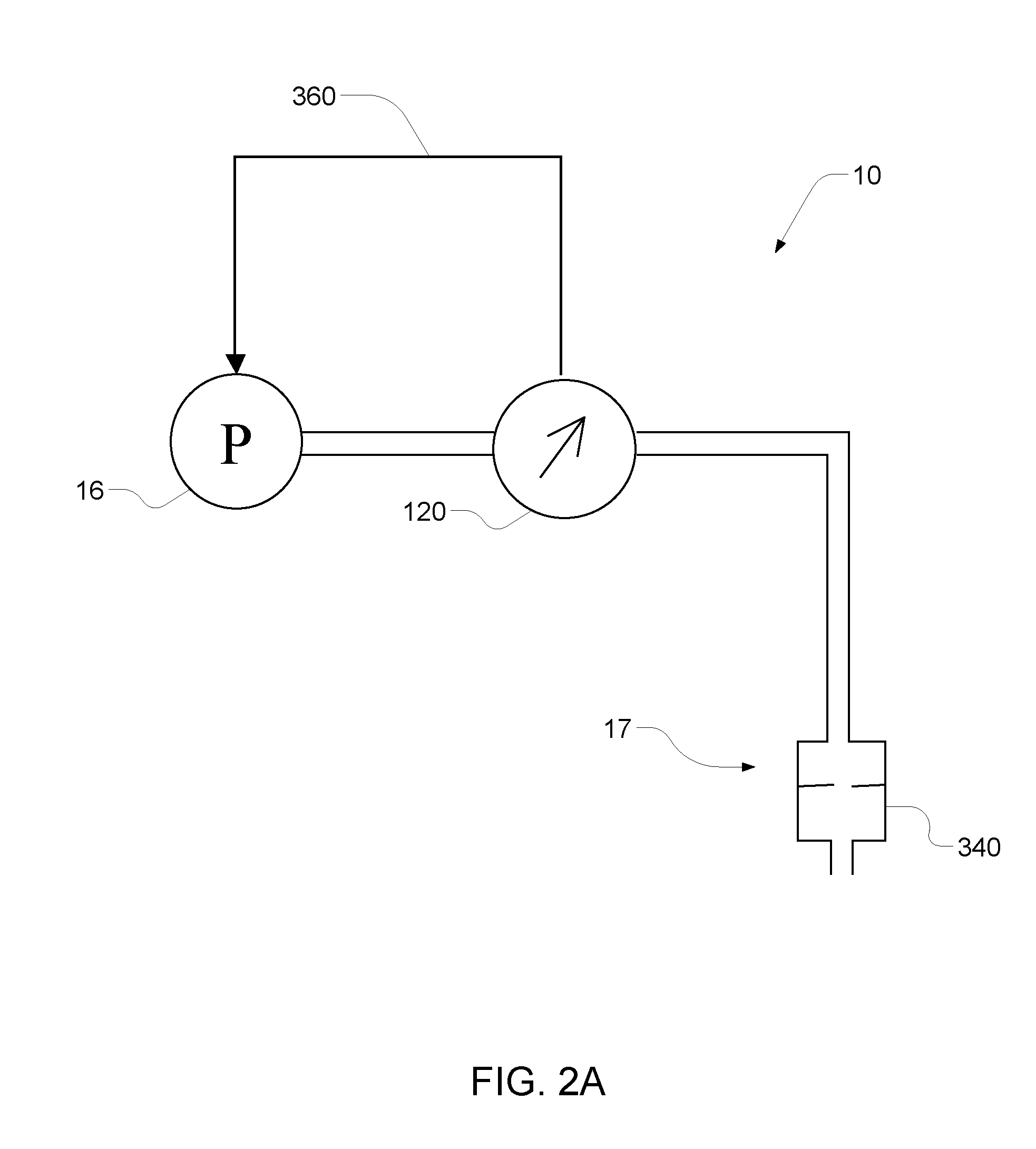
A medical treatment administration system 610 for delivering a medical treatment to a patient 618. The system 610 has a medical device 612 disposed in a first location, an electronic processor 628 coupled to the medical device 612, a sensor 616 coupled to the processor 628, and a remote controller 646 disposed at a second location remote from the first location. The remote controller 646 has an input device to control operation of the electronic processor 628. The sensor 616 receives one or more signals which it transfers 624 to the processor 628. The signals can be derived from the patient's physiological condition and / or the environment of the patient. The processor 628 receives the signals and performs a calculation 630 of the signal. Based on the result of the calculation, the processor 628 regulates the distribution of medical treatment to the patient 618 over a period of time.
Owner:BAXTER INT INC
Drive system for an infusion pump
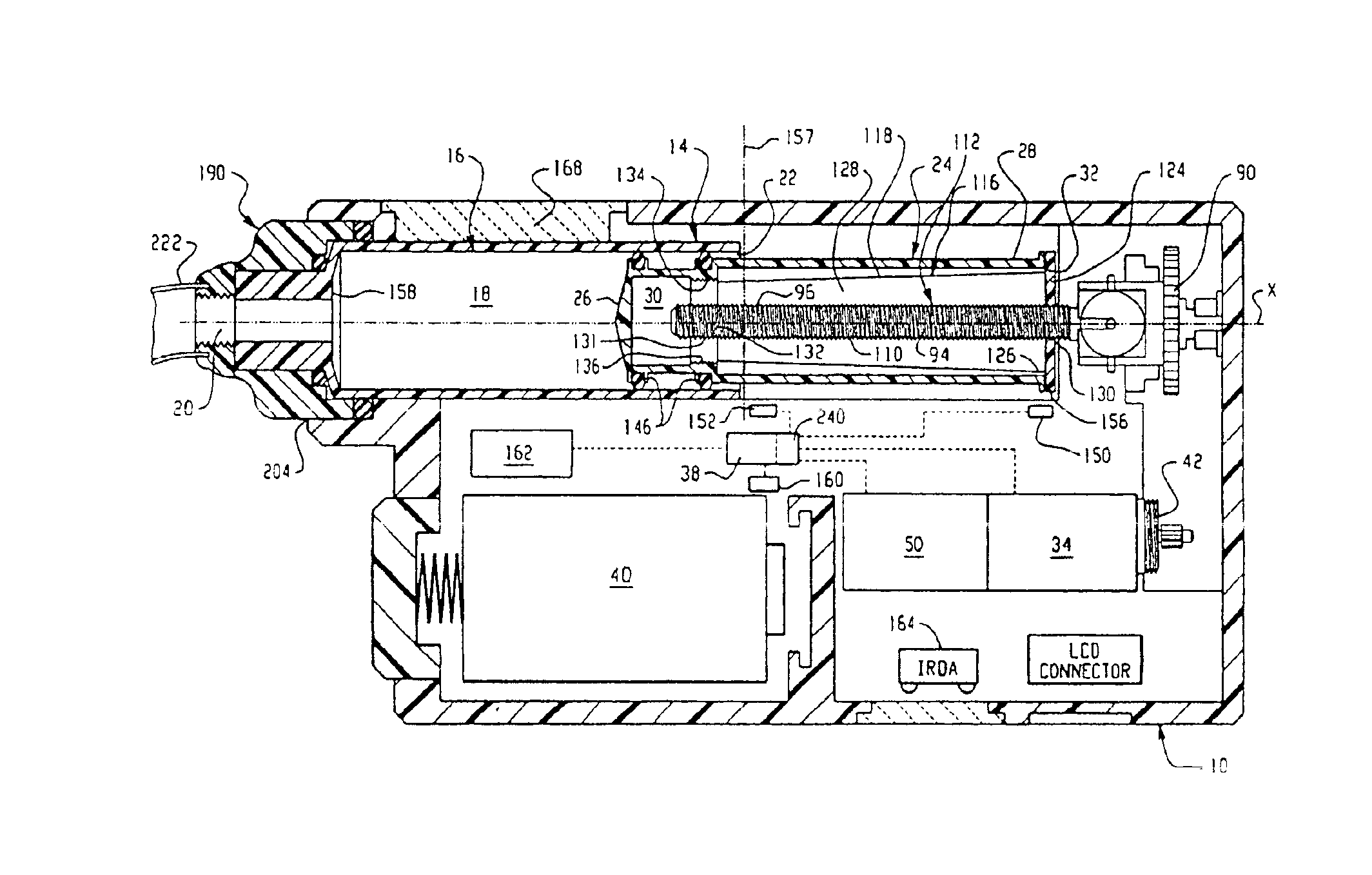
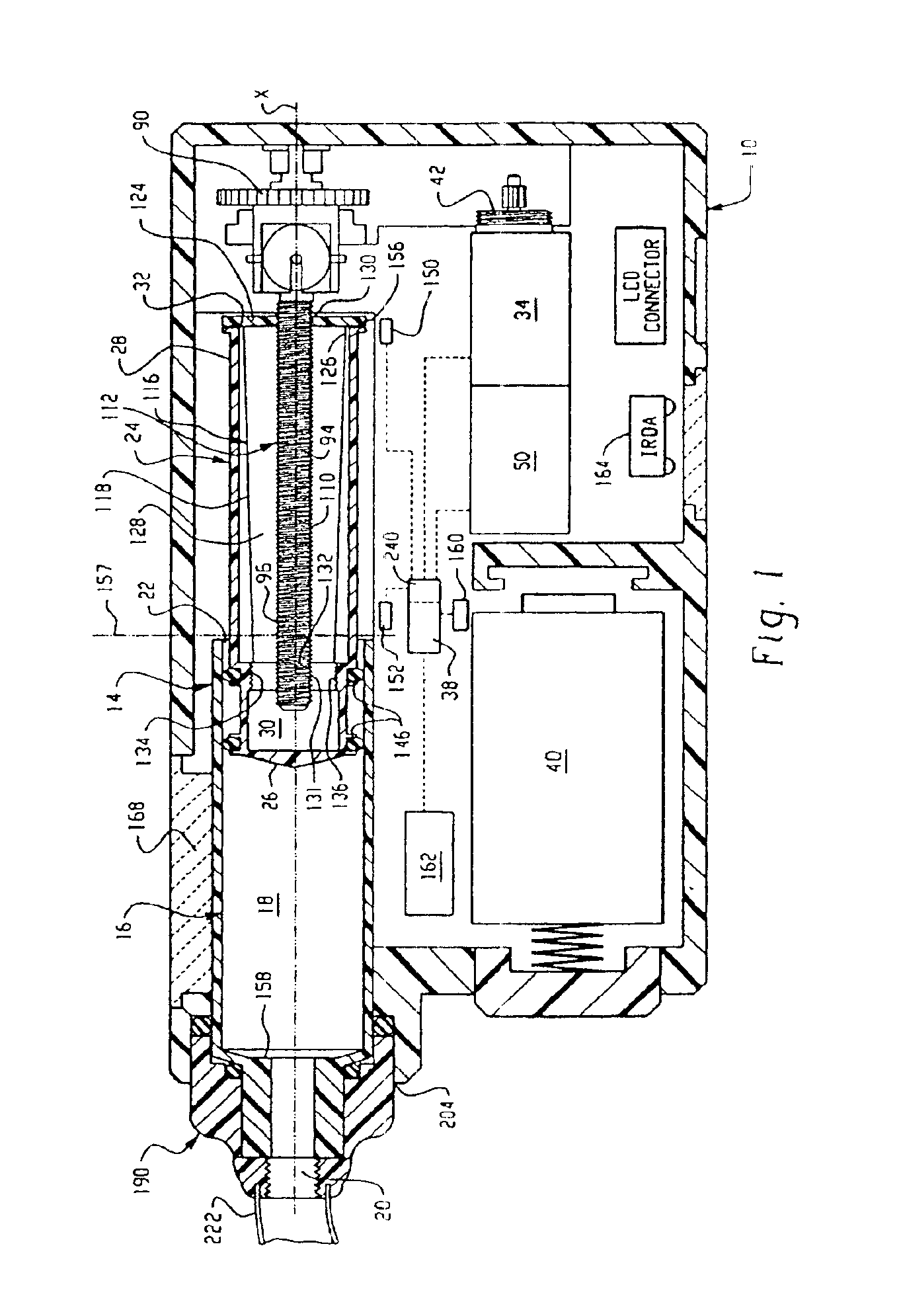
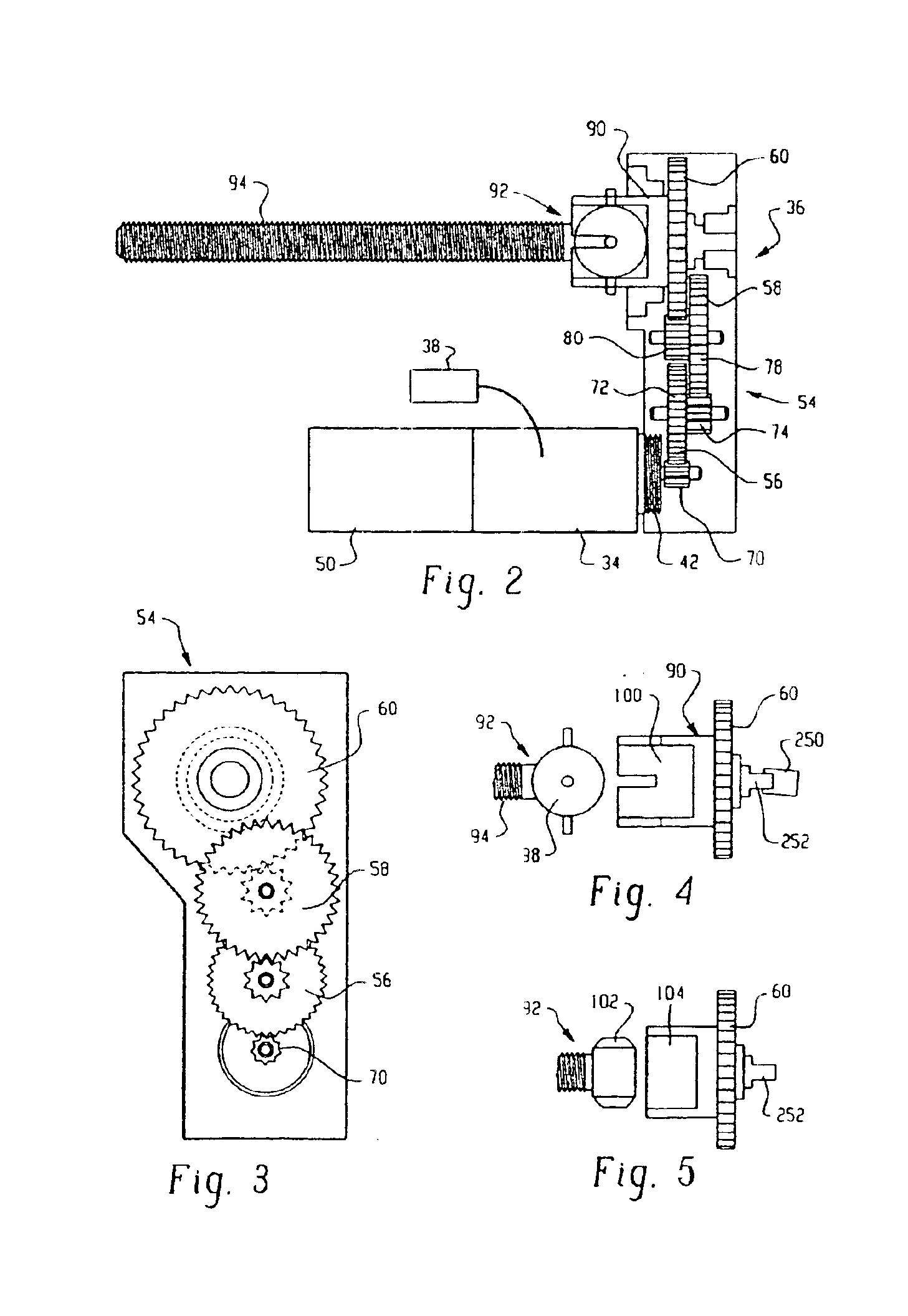
InactiveUS6854620B2Small sizeImprove portabilityClosure using stoppersLarge containersLinear motionProximity sensor
A pump system for an infusion system includes a linear drive (36, 36′) which minimizes the space occupied by the pump components in a portable housing (10, 10′). A motor (34) and a motor drive shaft (42) are arranged in parallel with, and adjacent to a syringe (14, 14′) and lead screw (94, 94′). A gear box (54) connects the drive shaft and lead screw to transfer rotational movements between them. A piston driving member, such as a cone (116) or drive nut (116′) converts the rotational movement of the lead screw into linear motion of a syringe piston (24). Sensors (150, 152) detect when the piston or cone is in a “home” position and in an “end” position, respectively. Optionally, a proximity sensor (170) is used to ensure that the cone and the piston (24) are abutting during dispensing. Alternatively, a clamping member (350) selectively clamps the lead screw (94′) against linear motion in at least a dispensing direction.
Owner:TRIVIDIA HEALTHCARE SYST LLC
Method and apparatus for fluid dispersion
Owner:THE GOVERNING COUNCIL OF THE UNIV OF TORONTO +1
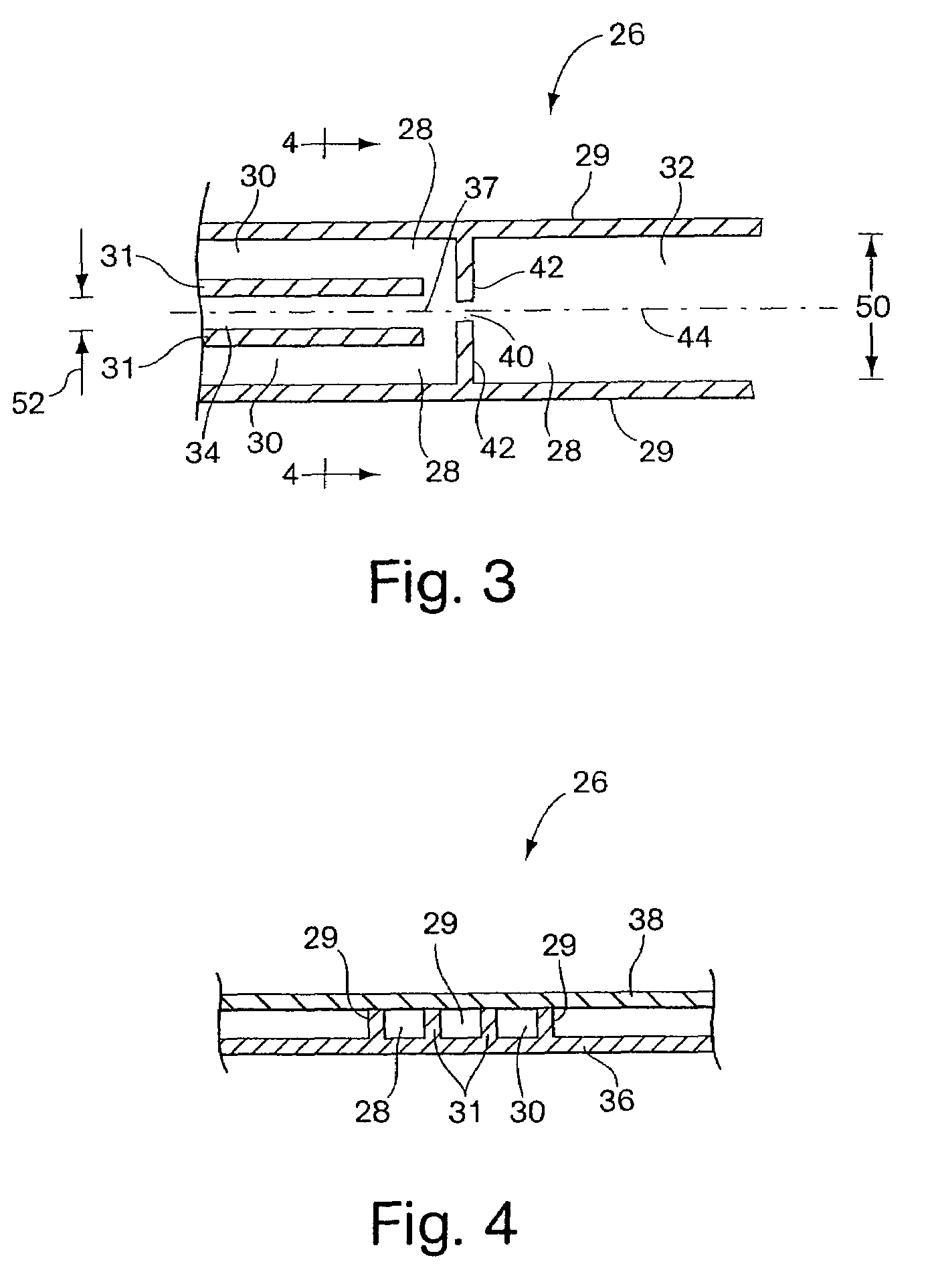
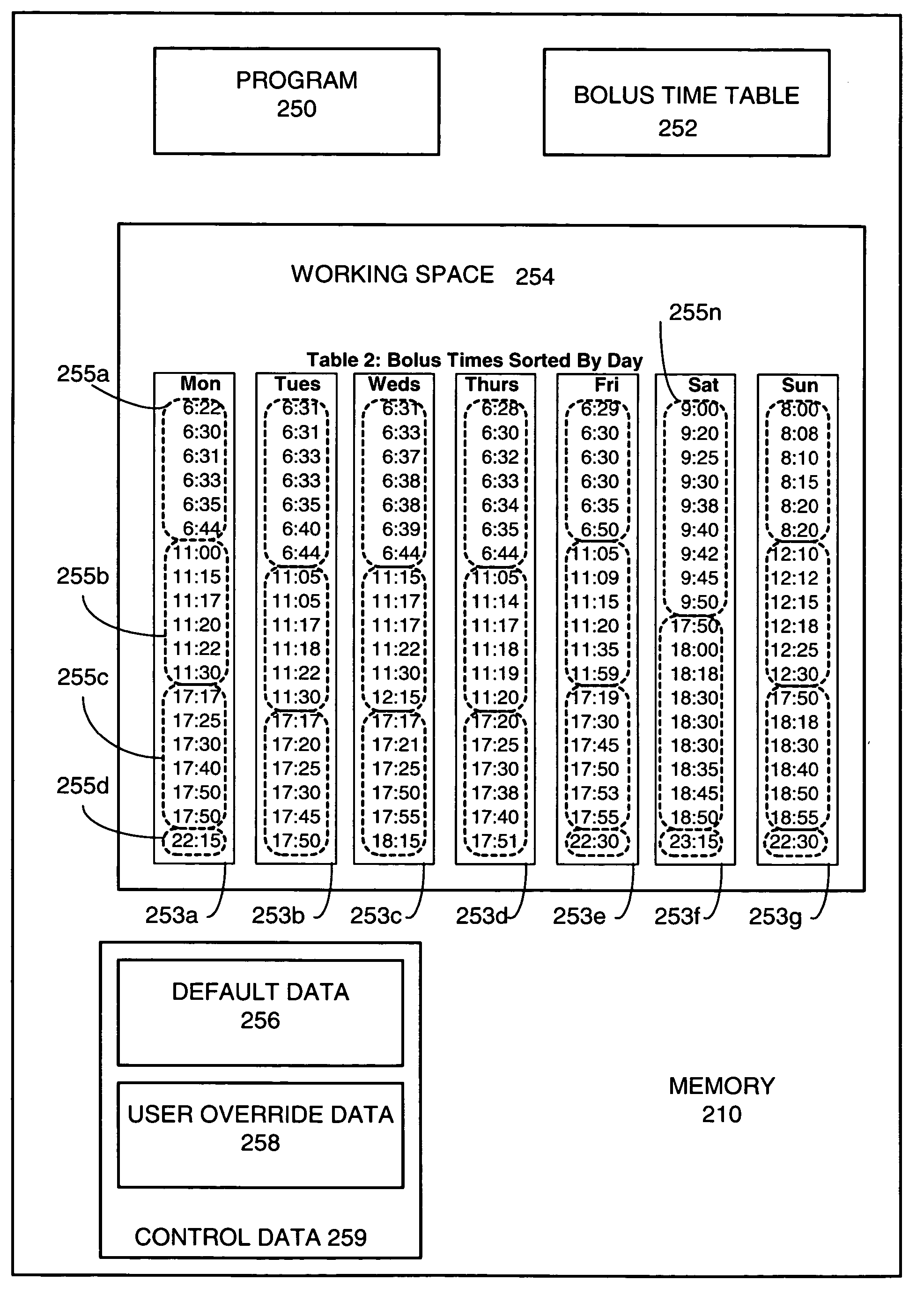
Medical infusion pump capable of learning bolus time patterns and providing bolus alerts
ActiveUS6999854B2Drug and medicationsPharmaceutical delivery mechanismInsulin pumpEmergency medicine
An apparatus and method are disclosed for improving a medical infusion pump. Users of medical infusion pumps, such as insulin pumps, require a bolus of a medication at predicable times of the day, such as at or near mealtimes for insulin pumps. The disclosed medical infusion pump determines bolus time intervals during which boluses are usually taken, and, alerts the user at one or more calculated alert times during an active bolus time interval when a bolus has not yet been delivered during the active bolus time interval. Advantageously, a different set of bolus time intervals are determined by day of week, to accommodate, for example, different bolus patterns during weekends versus weekdays.
Owner:TANDEM DIABETES CARE INC
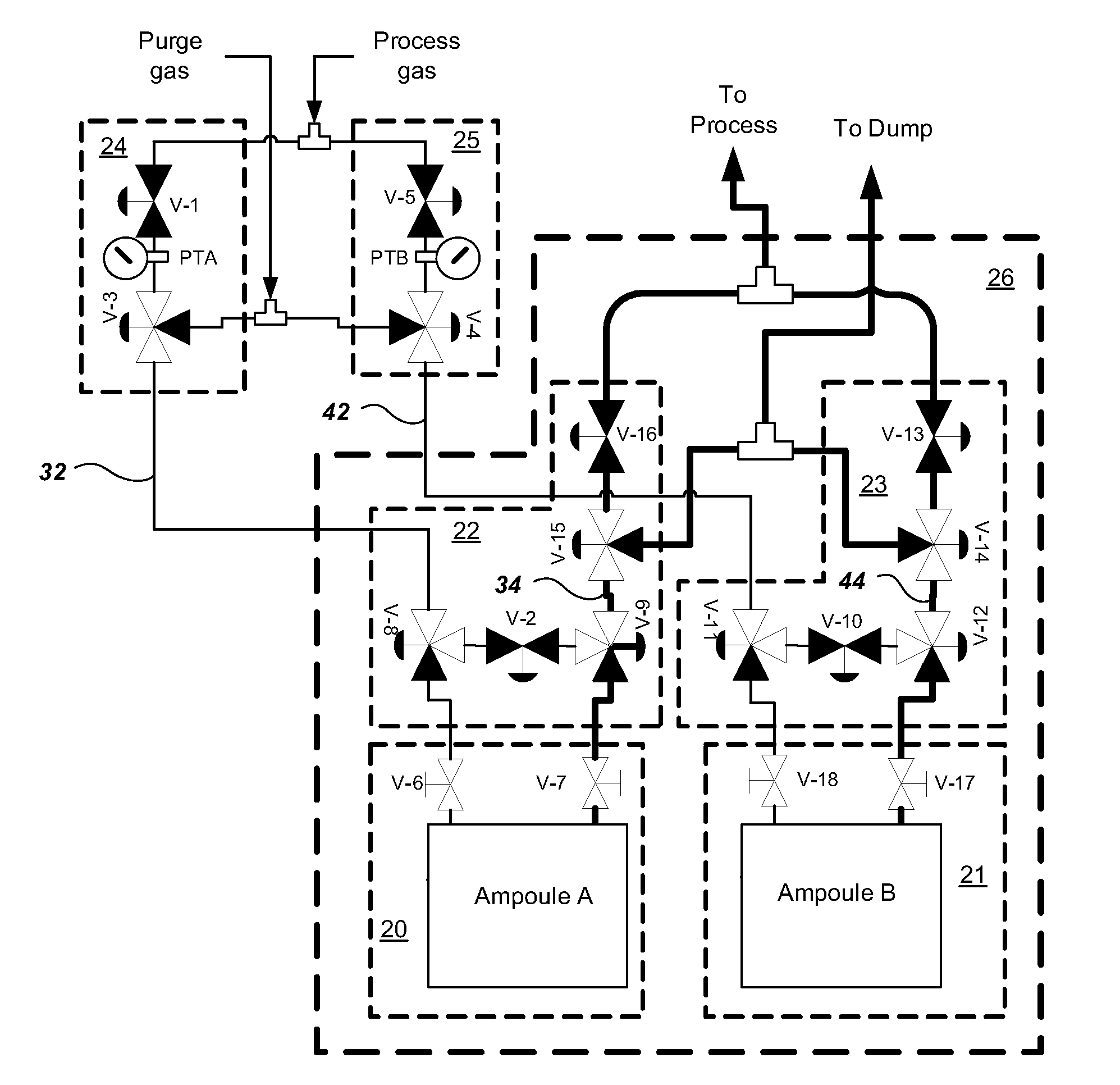
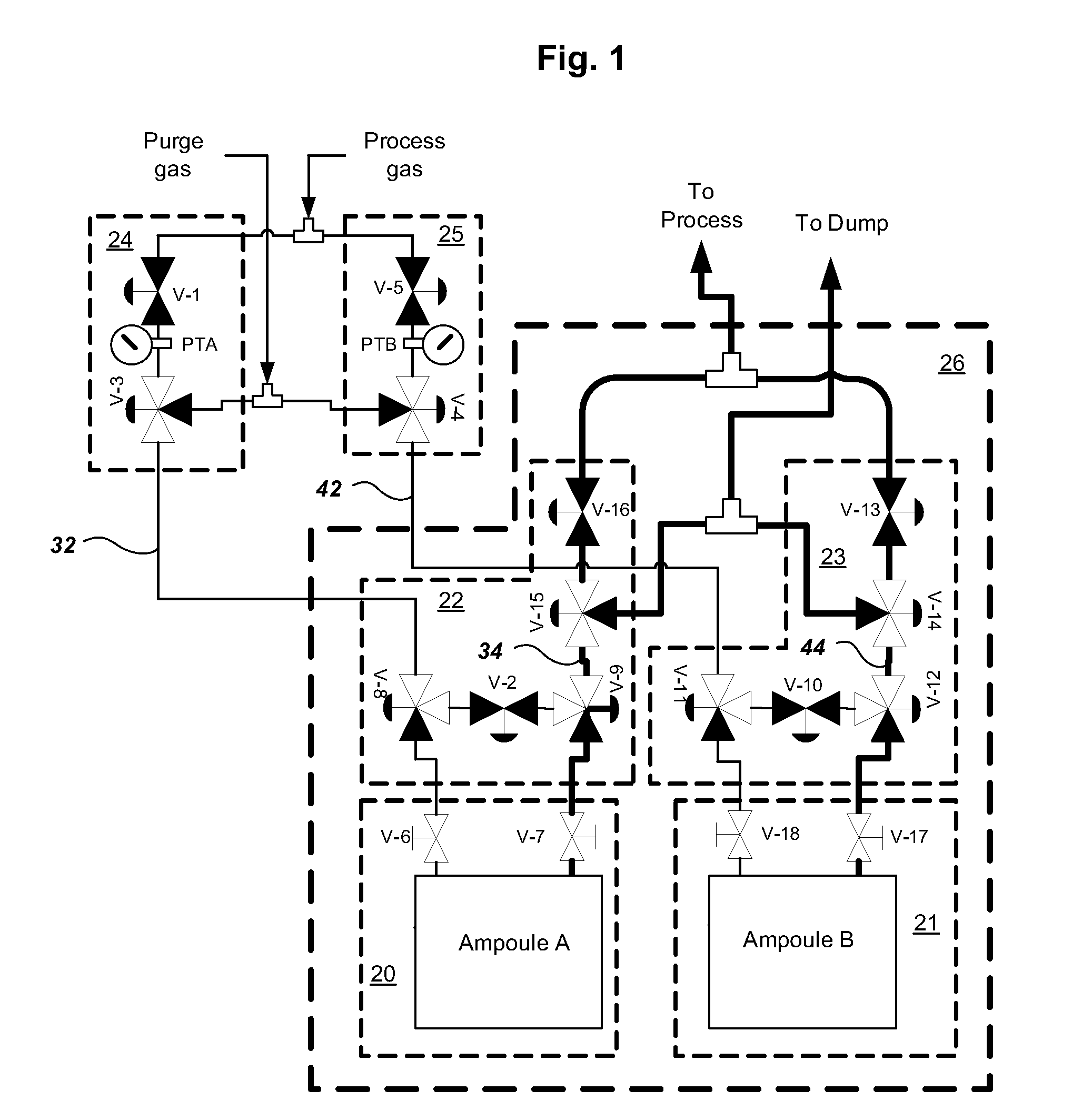
Multiple ampoule delivery systems
InactiveUS20090211525A1Easy to useReduce wasteLiquid surface applicatorsPipeline systemsSemiconductor materialsDelivery system
This invention relates to an integrated vapor or liquid phase reagent dispensing apparatus having a plurality of vessels and a plurality of carrier or inert gas feed / vapor or liquid phase reagent delivery manifolds, that may be used for continuously dispensing vapor or liquid phase reagents such as precursors for deposition of materials in the manufacture of semiconductor materials and devices.
Owner:PRAXAIR TECH INC
Internal drug dispenser capsule medical device
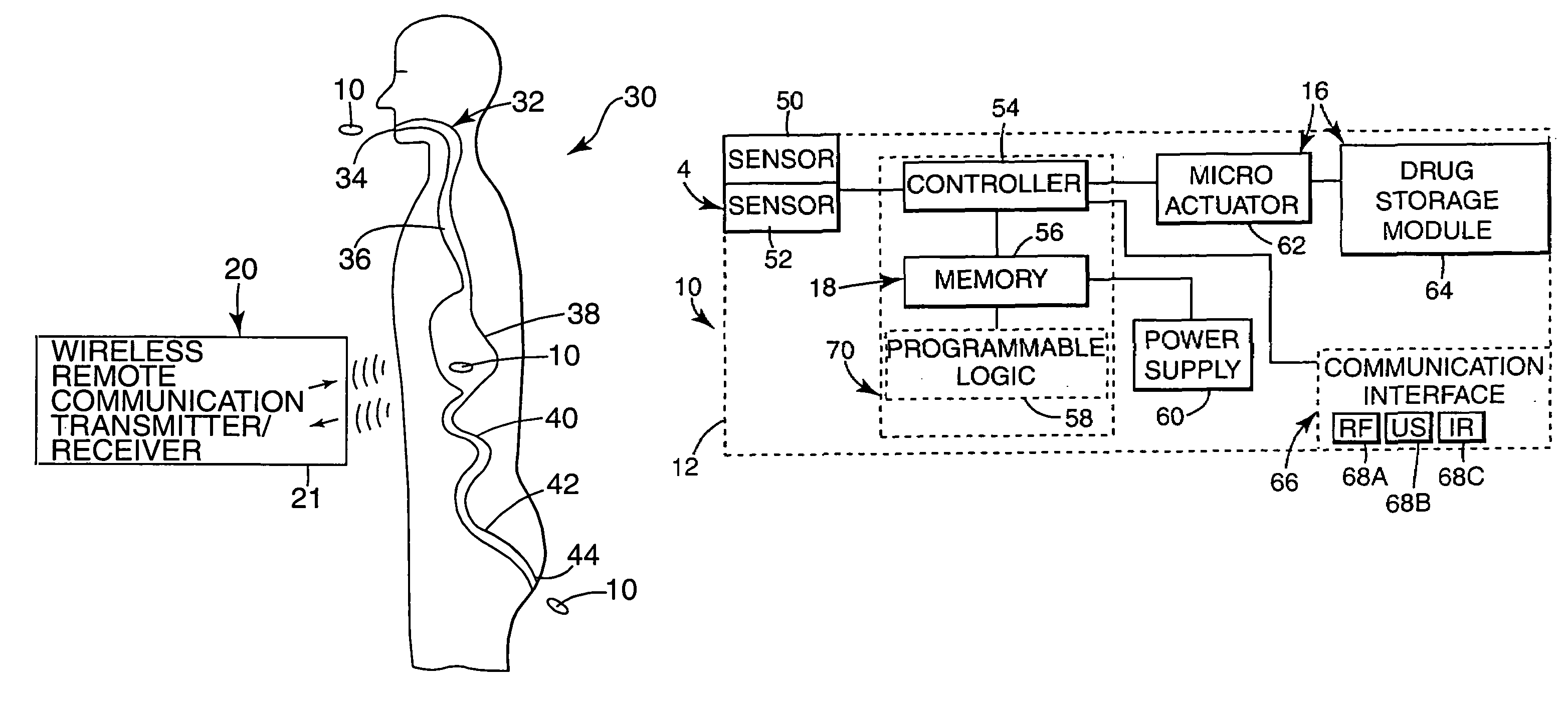
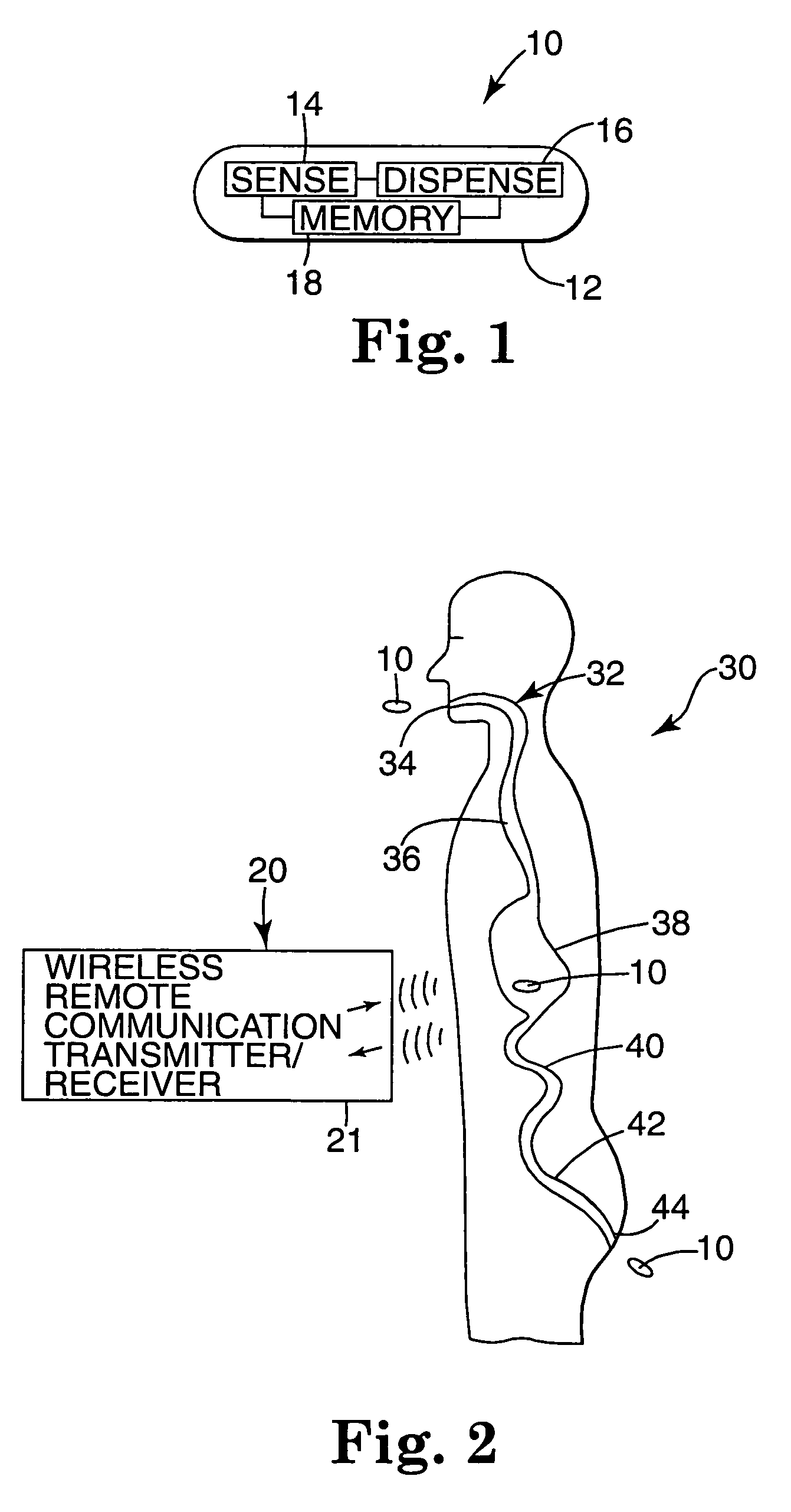
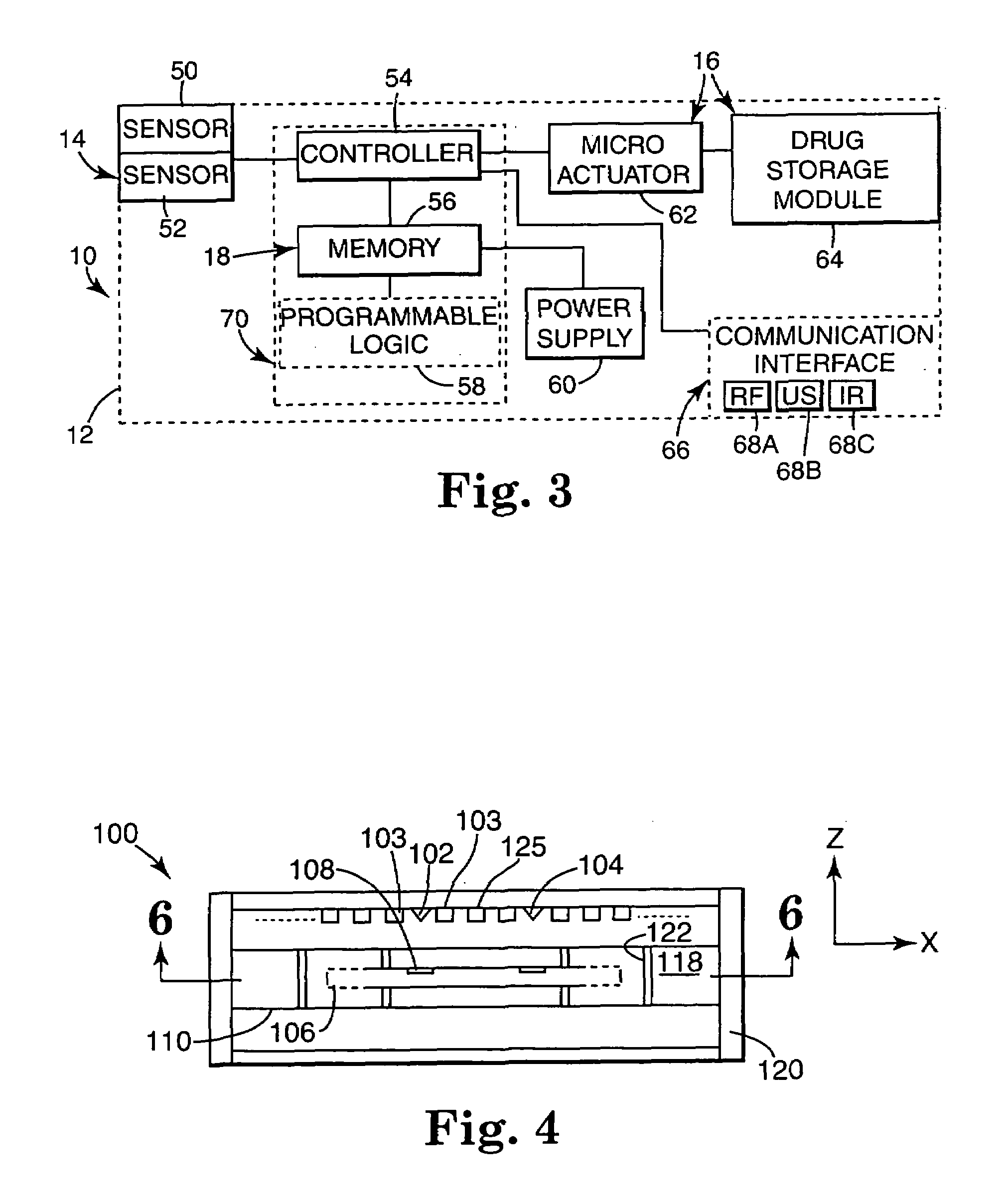
The present invention provides a swallowable internal drug medical device. The device includes a swallowable capsule. A sensing module is disposed in the capsule. A bioactive substance dispenser is disposed in the capsule. A memory and logic component is disposed in the capsule and in communication with the sensing module and the dispenser.
Owner:HEWLETT PACKARD DEV CO LP
Pumping fluid delivery systems and methods using force application assembly
ActiveUS20070219496A1Easy to liftGreat mechanical advantageFlexible member pumpsMedical devicesPump chamberEngineering
A method of dispensing a therapeutic fluid from a line includes providing an inlet line connectable to an upstream fluid source. The inlet line is in downstream fluid communication with a pumping chamber. The pumping chamber has a pump outlet. The method also includes actuating a force application assembly so as to restrict retrograde flow of fluid through the inlet while pressurizing the pumping chamber to urge flow through the pump outlet. A corresponding system employs the method.
Owner:DEKA PROD LLP
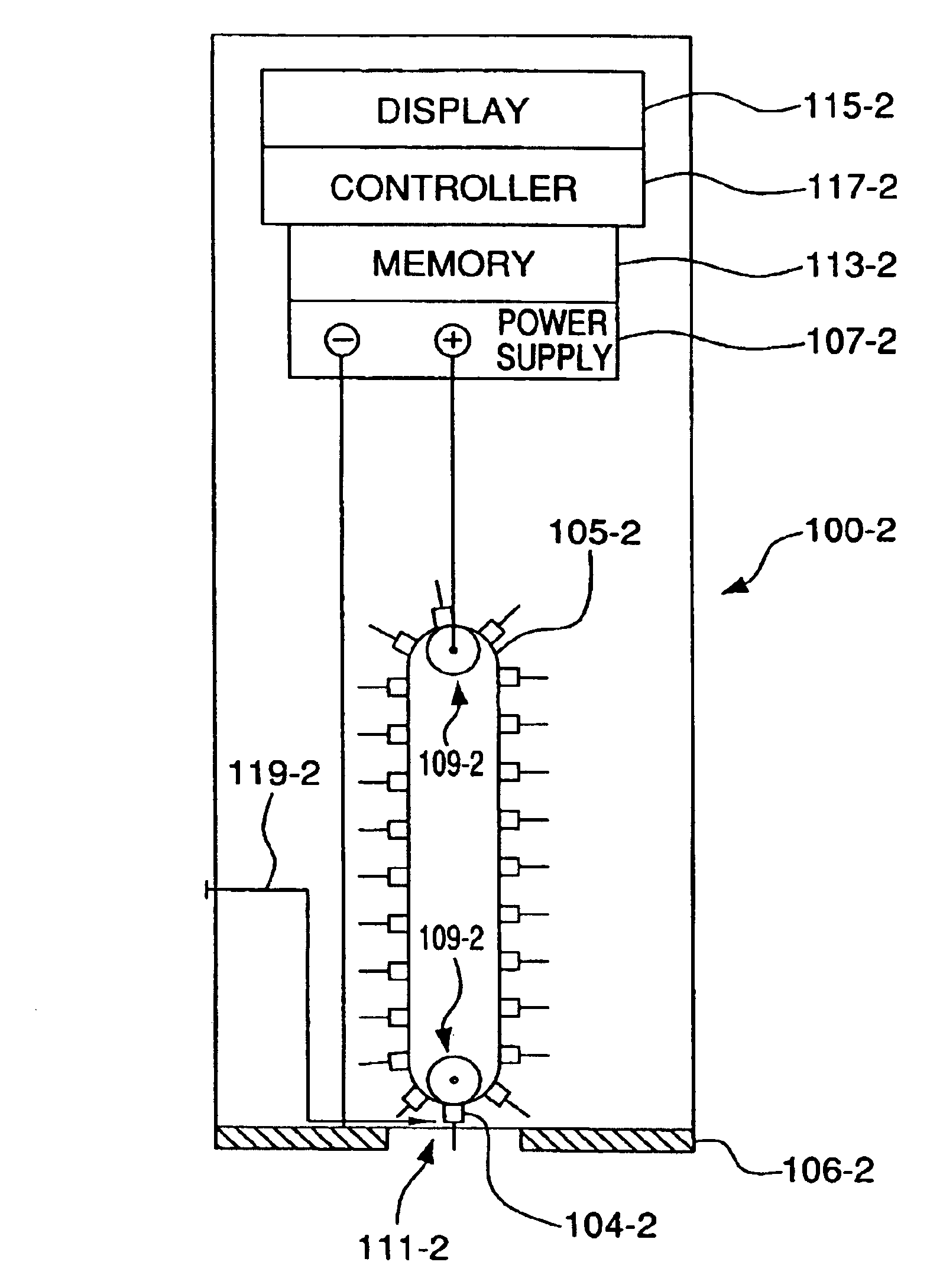
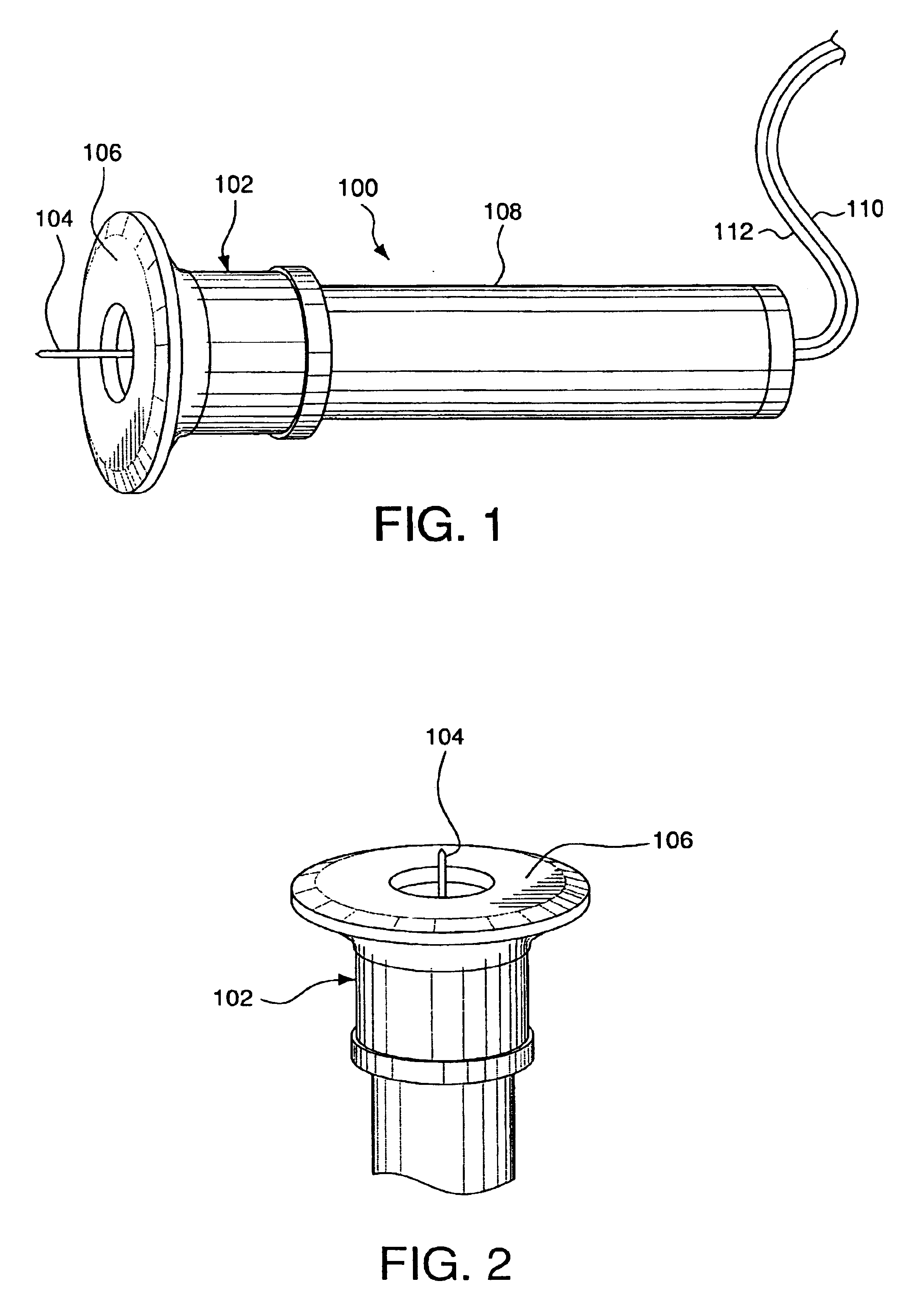
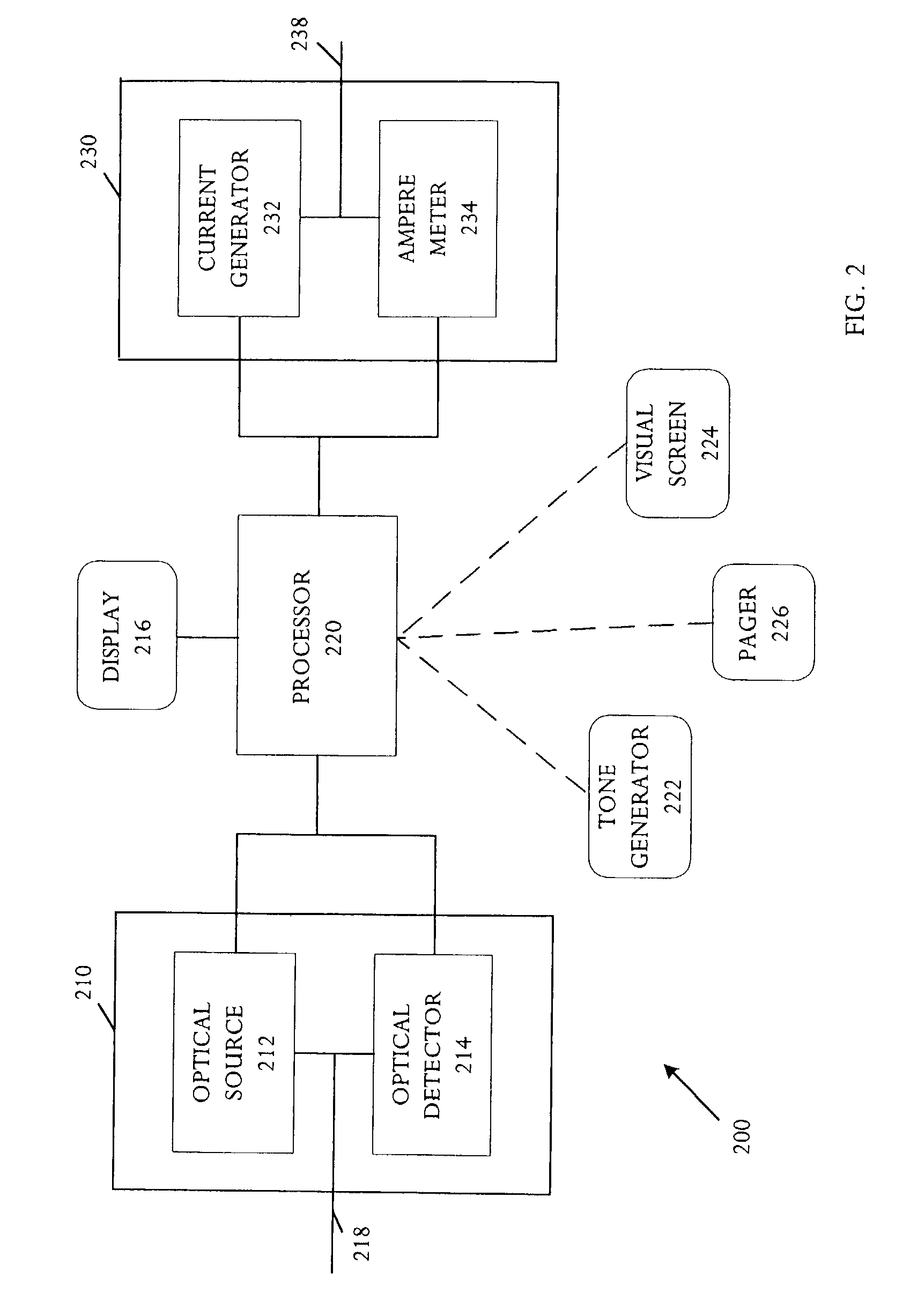
Detection of fluids in tissue
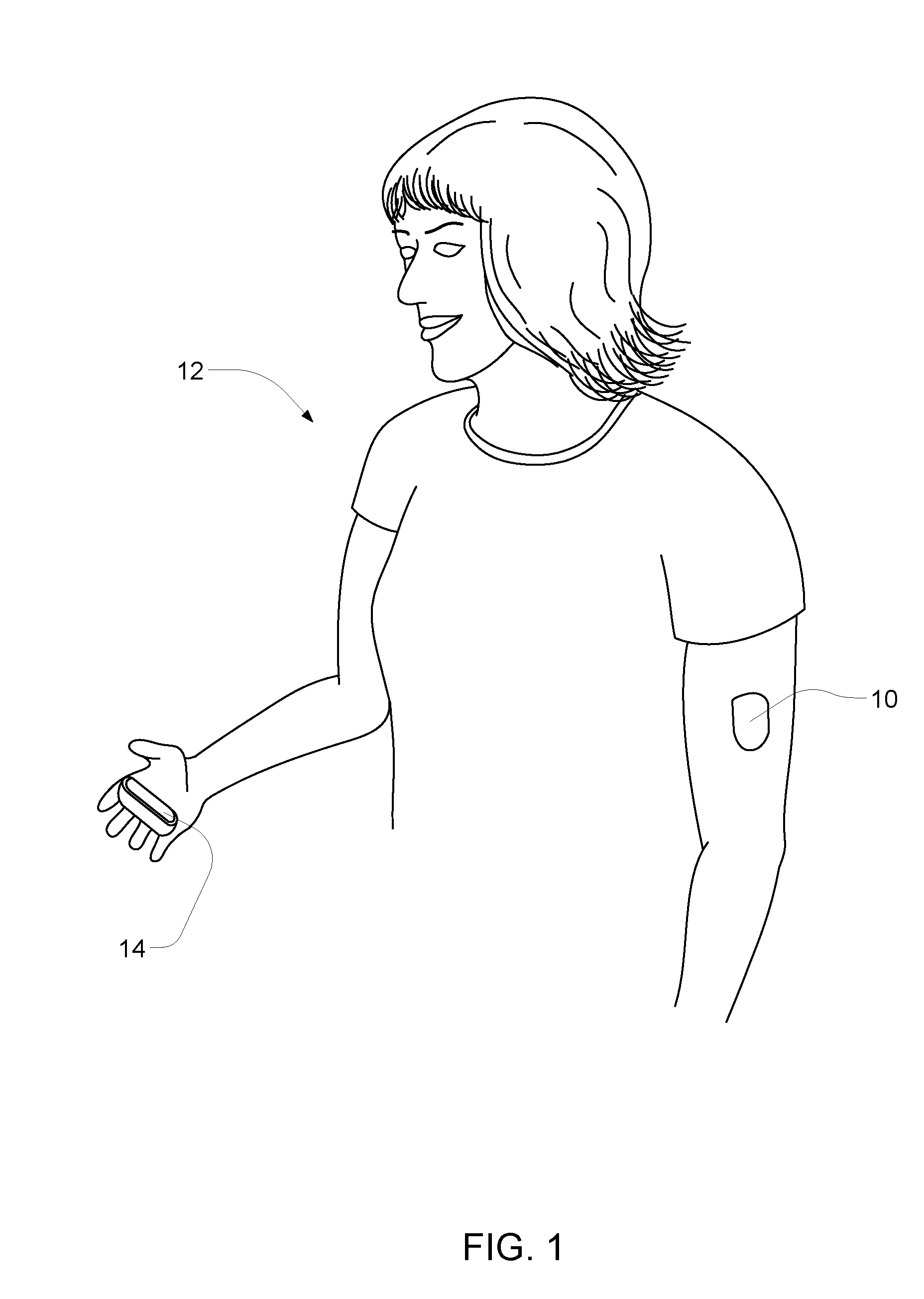
InactiveUS7122012B2Easy to detectLow profileElectrotherapyMaterial analysis using microwave meansMedicineFluid level
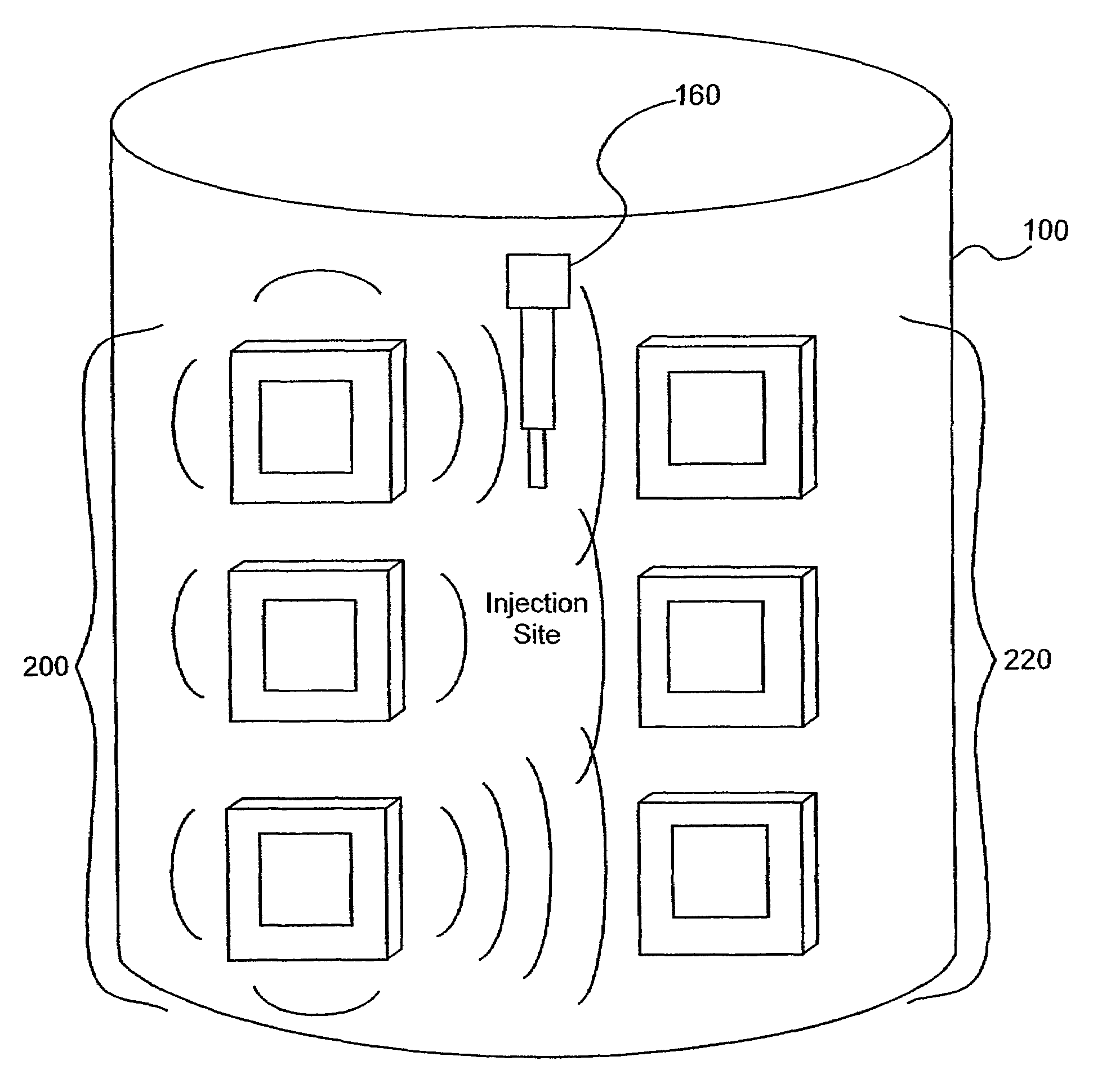
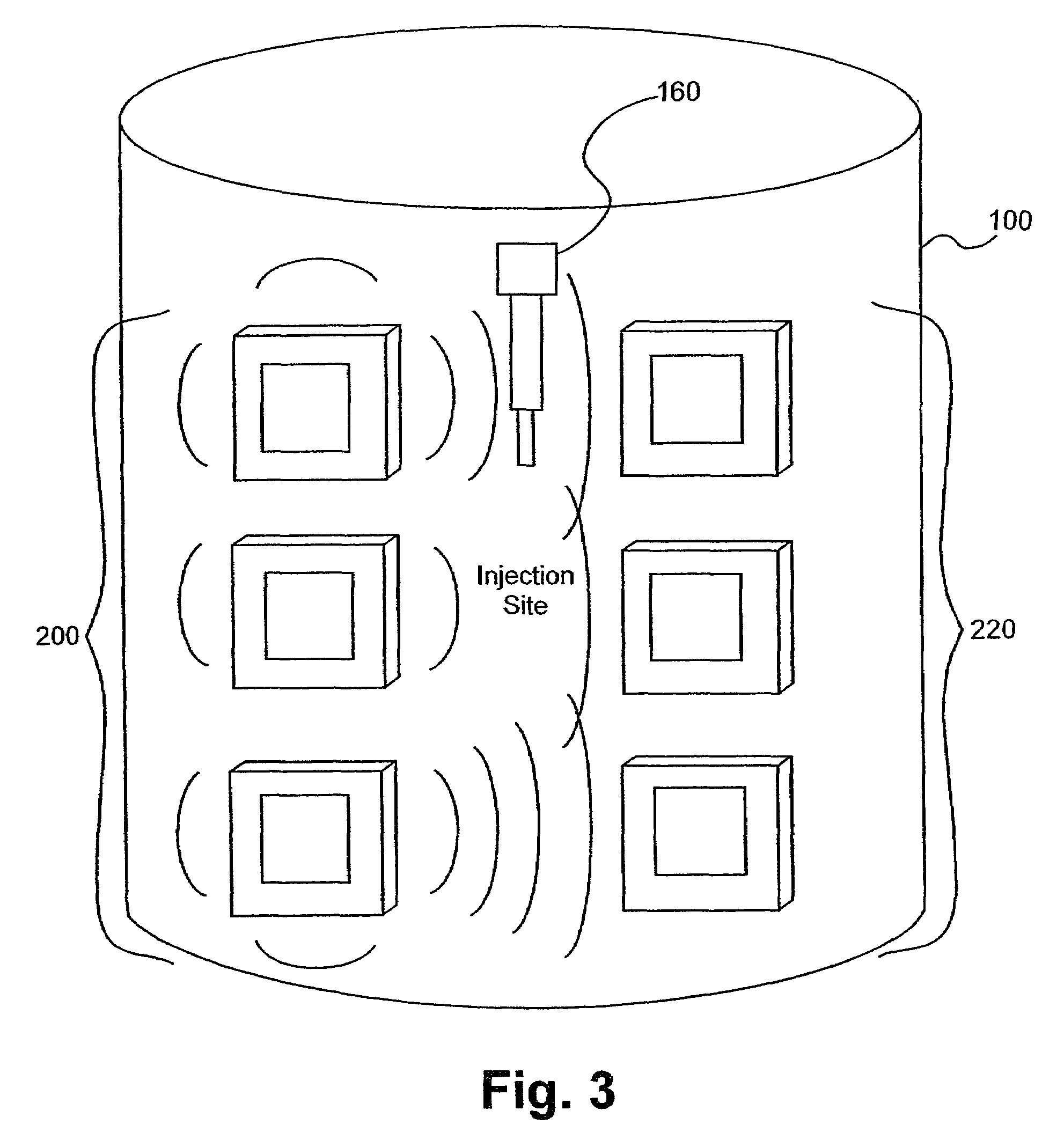
A method of detecting a change (that is, an increase or a decrease) in the level of fluid in tissue in a first area of a body includes the steps of: applying electromagnetic energy, preferably in the frequency range of approximately 300 MHz to approximately 30 GHz, to a first volume of the body; measuring a resultant or returned signal; comparing the signal to a reference signal to determine if the fluid level in the tissue has changed. In one embodiment, the method detects changes in the level of fluid in tissue of a body by applying electromagnetic energy to a first volume of the body over a period of time (for example, using an antenna or antennae); measuring a resultant signal or a signal returned from the tissue; and comparing the signal to a reference signal to determine if a level of fluid in the tissue has changed during the period of time.
Owner:BAYER HEALTHCARE LLC
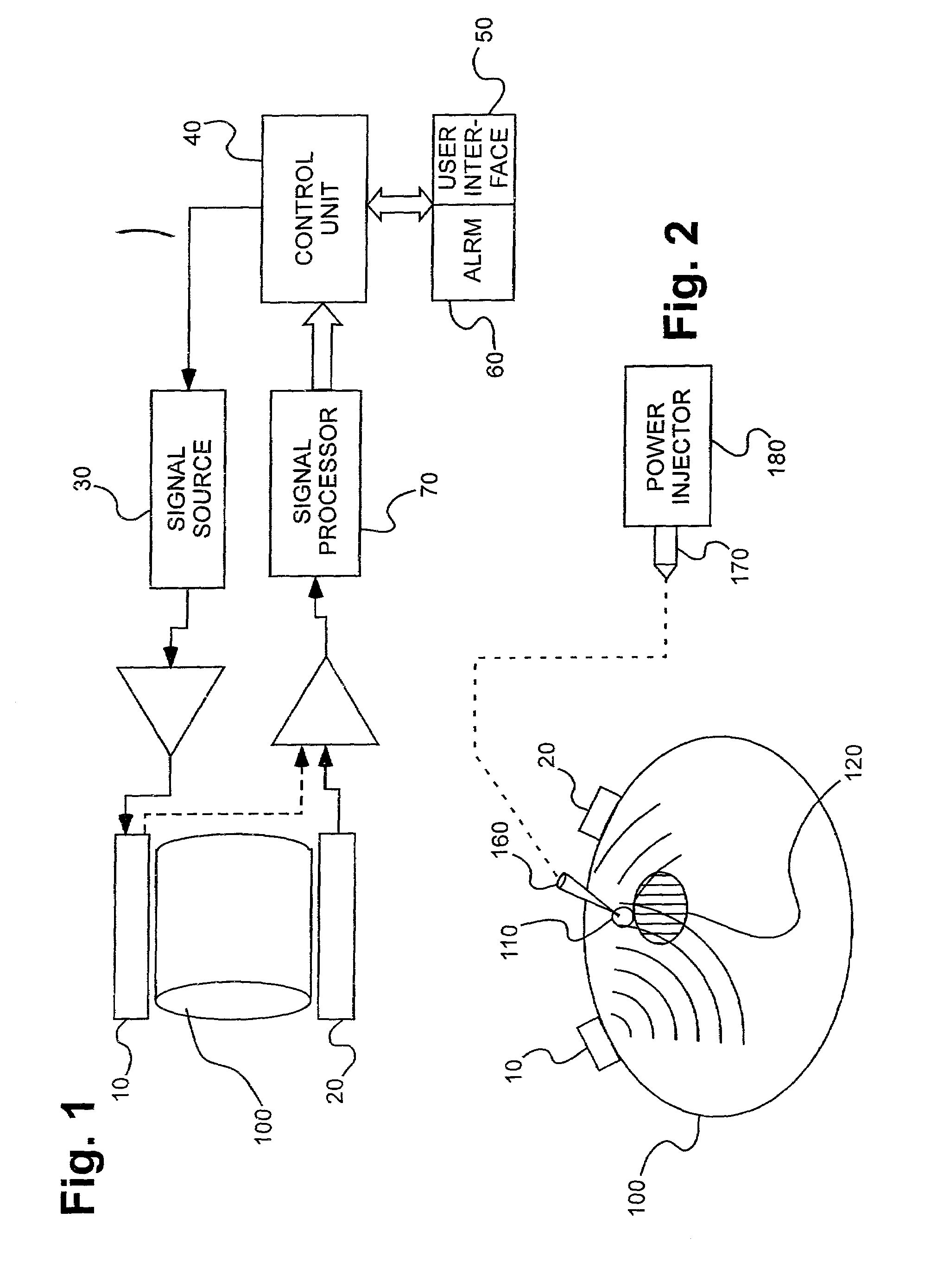
Device for diagnosing physiological state and device for controlling the same
PCT No. PCT / JP96 / 01254 Sec. 371 Date Apr. 2, 1997 Sec. 102(e) Date Apr. 2, 1997 PCT Filed May 13, 1996 PCT Pub. No. WO96 / 35368 PCT Pub. Date Nov. 14, 1996The present invention relates to a device for diagnosing physiological state based on blood pulse waves detected in the body. It is the objective of the present invention to provide a device which correctly diagnoses the current physiological state based on changes in physiological state measured over a specified period of time in the past while taking into consideration the cyclical variation exhibited in physiological state. In order to realize this objective, the device according to the present invention has as its main components: blood pulse wave detector 381 and stroke-volume-per-beat measurer 382 which respectively detect blood pulse wave and stroke volume in the body; blood pulse wave extraction memory 386 which extracts characteristic information from the detected blood pulse wave; memory 383 in which the physiological state calculated from the stroke volume and this characteristic information is stored; output portion 385 which outputs an alarm; and microcomputer 387 which controls each part inside the device. The microcomputer calculates the circulatory parameters based on characteristic information obtained from the waveform extraction memory, and stores the parameters in memory at specified time intervals. At these times, microcomputer 387 calculates the circulatory parameters from the stroke volume per beat and the characteristic information of the blood pulse wave at specified time intervals, and stores the parameters in memory 383. Further, microcomputer 387 reads out from memory 383 the circulatory parameters from a specified time interval in the past, and calculates the average value and standard deviation. Microcomputer 387 then determines whether or not the current circulatory parameters are within a specified range determined by their average value and standard deviation. When the circulatory parameters are determined to be outside this range, microcomputer 387 controls output portion 385 to sound an alarm.
Owner:SEIKO EPSON CORP
Conductivity reconstruction based on inverse finite element measurements in a tissue monitoring system
InactiveUS7169107B2Low and high conductivityReduce conductivityUltrasonic/sonic/infrasonic diagnosticsMedical devicesElectrical impedance tomographyEngineering
An impedance model of tissue is useful for describing conductivity reconstruction in tissue. Techniques for determining and mapping conductivity distribution in tissue supply useful information of anatomical and physiological status in various medical applications. Electrical Impedance Tomography (EIT) techniques are highly suitable for analyzing conductivity distribution. Electrical characteristics of tissue include resistive elements and capacitive elements. EIT techniques involve passing a low frequency current through the body to monitor various anatomical and physiological characteristics. The system can interrogate at multiple frequencies to map impedance. Analytical techniques involve forward and inverse solutions to boundary value analysis to tissue characteristics.
Owner:INOTECH MEDICAL SYST
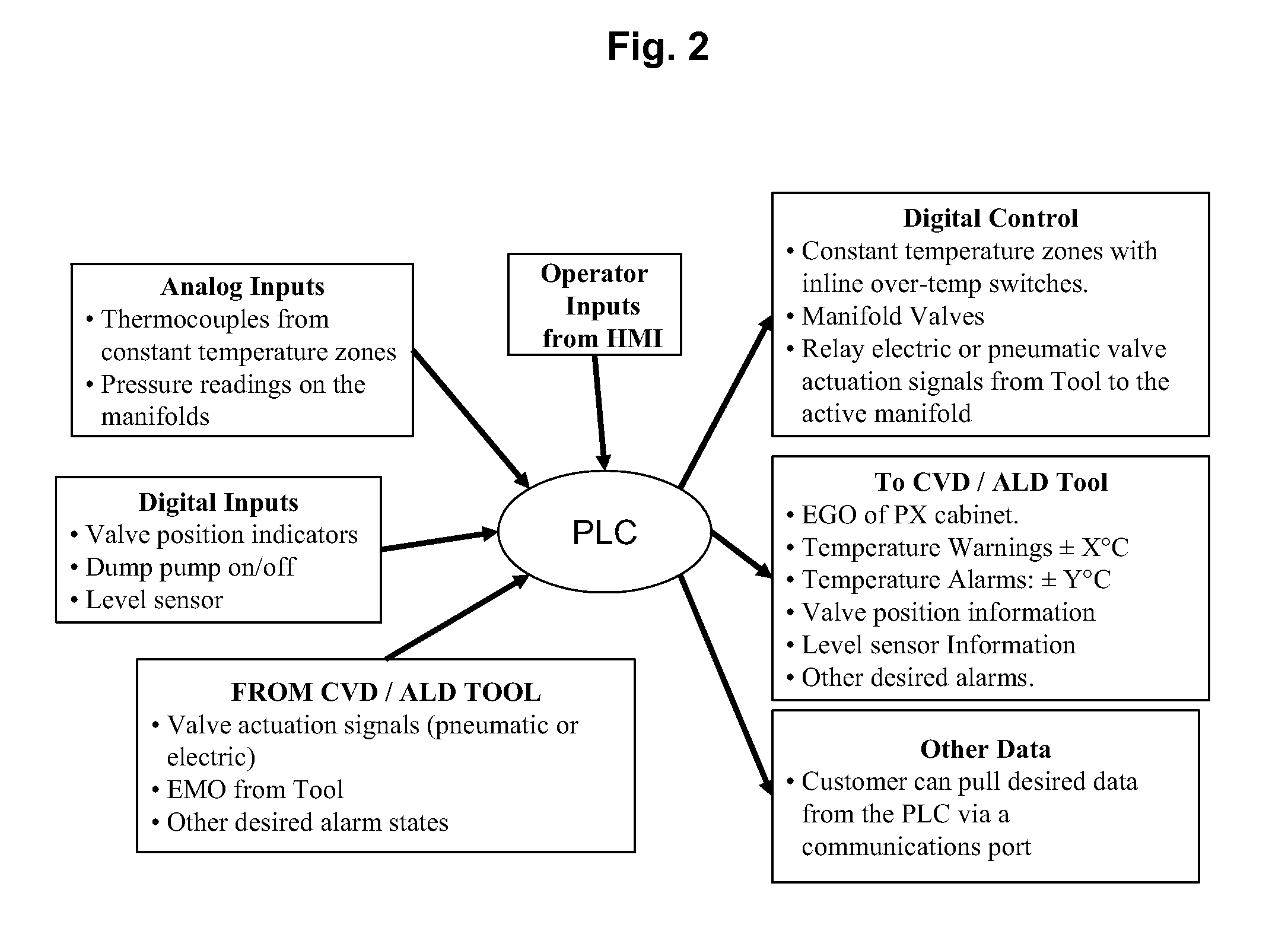
Code replacement for irrigation controllers
ActiveUS20080027587A1Not allowEasy programmingProgramme controlWatering devicesComputer moduleModularity
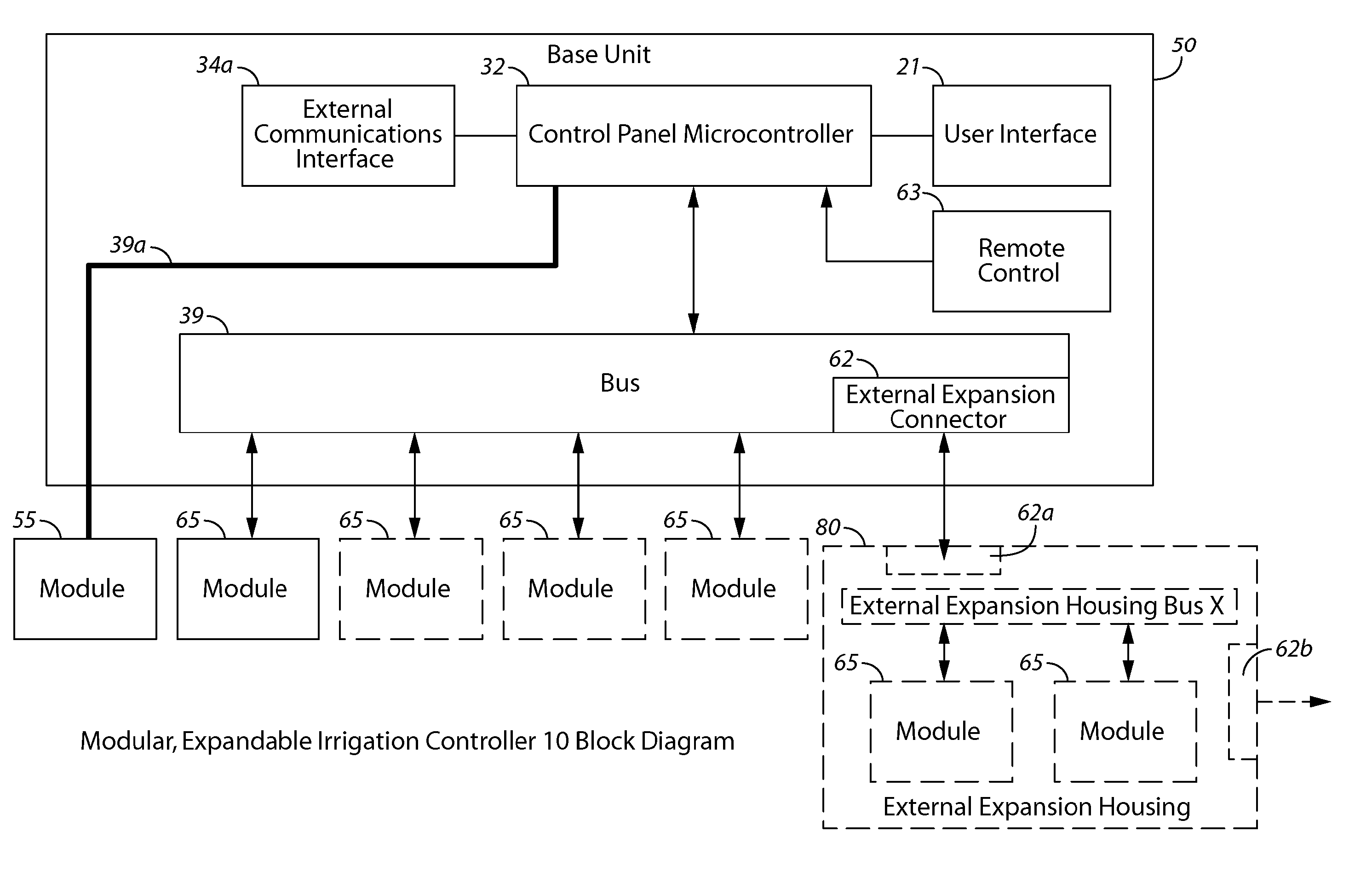
Described herein are several embodiments relating to modular irrigation controllers. In many implementations, methods of implementing irrigation control are provided that detect a presence of a first module coupled with a control unit of an irrigation controller, the control unit operating in accordance with a bootloader set of code and a first set of code to implement irrigation control, identify that the first module stores a second set of code, and activate the bootloader set of code to replace the first set of code with the second set of code. Also described are various different types of modular controllers, expansion modules that may be coupled to the modular controller, having as variety of functions and features, as well as related methods of use and configuration of the controller and these modules in the controller.
Owner:RAIN BIRD CORP
Microfluidic system
ActiveUS20060094119A1Rapid and economical reactionIncrease rangePolycrystalline material growthAnalysis using chemical indicatorsFemtoliterEngineering
Owner:CHICAGO UNIV OF
Medication delivery and monitoring system and methods
InactiveUSRE38189E1Low costLimited accessMedical devicesPressure infusionOperational systemDrugs preparations
A medication delivery and monitoring system and methods whereby drugs are safely delivered to a patient, monitored in real-time during delivery and crucial events are recorded during delivery to provide real-time, on-line information and detail for an audit trail. A novel safety label cradle unit is disclosed. Safety label cradles (SLC's) are provided in a plurality of sizes to match varying sizes of syringes which are disposed on a cradle of the SLC to provide a constant needle height on the SLC unit independent of syringe volume (barrel diameter). A selected SLC is securely affixed to a syringe by an adhesively backed label wrapping. The label is preprinted to provide drug identification indicia and drug preparation information. The information is automatically read into the system from the label. A novel delivery station of the system monitors drug delivery as a plunger of the syringe is pushed to deliver a drug to a patient. A smart tray in cooperation with a slider portion of the SLC is used to selectively deliver drugs to a port in the IV set. The smart tray comprises a first portion for carrying SLC units, an attachable second portion having a control panel for operating the system and a cover for lockably affixing the SLC units to the tray.
Owner:IBM CORP
Systems and methods for fluid delivery
A system for at least partial closed-loop control of a medical condition is disclosed. The system includes at least one medical fluid pump. The medical fluid pump including a sensor for determining the volume of fluid pumped by the pump. Also, at least one continuous analyte monitor, and a controller. The controller is in communication with the medical fluid pump and the at least one continuous analyte monitor. The controller includes a processor. The processor includes instructions for delivery of medical fluid based at least on data received from the at least one continuous analyte monitor.
Owner:DEKA PROD LLP
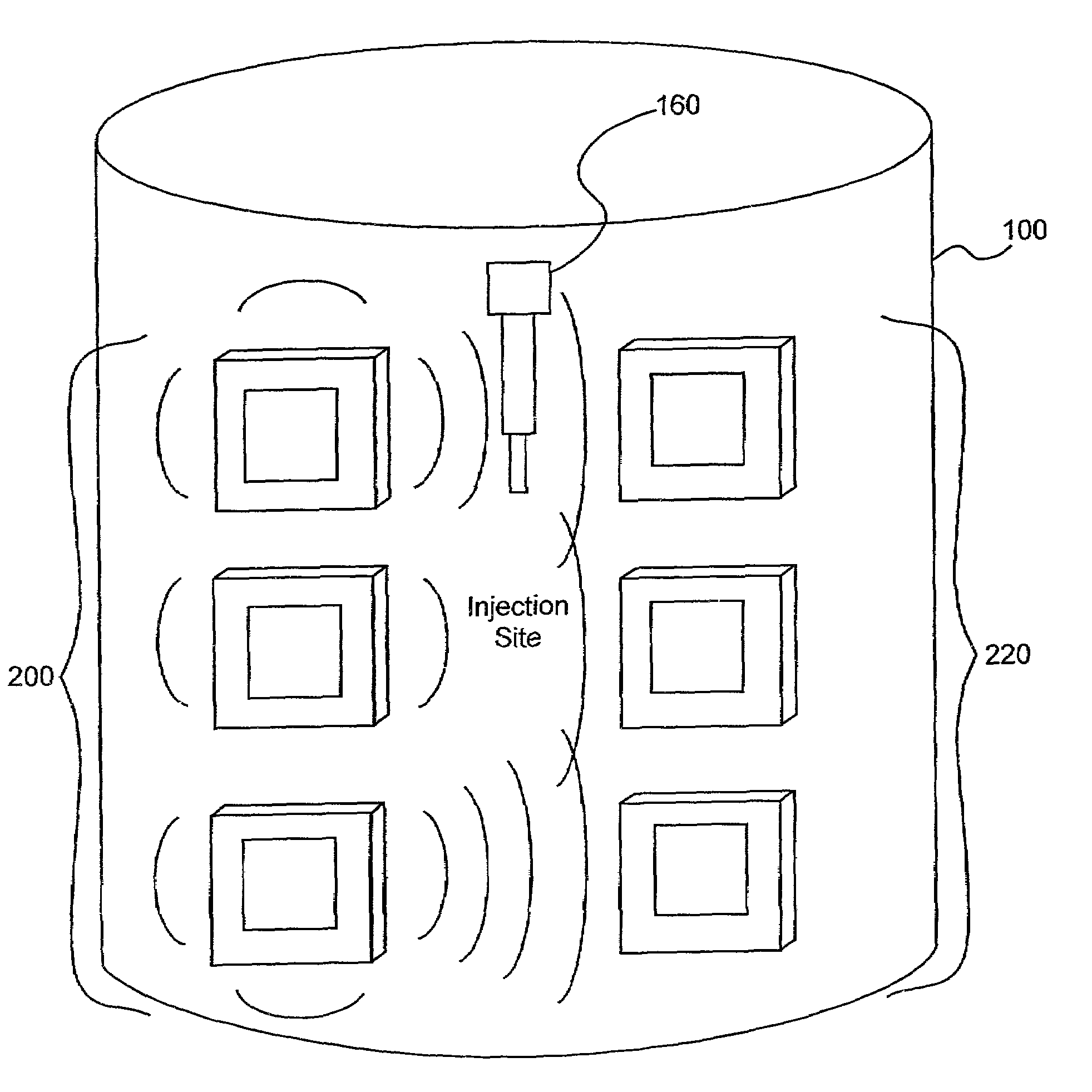
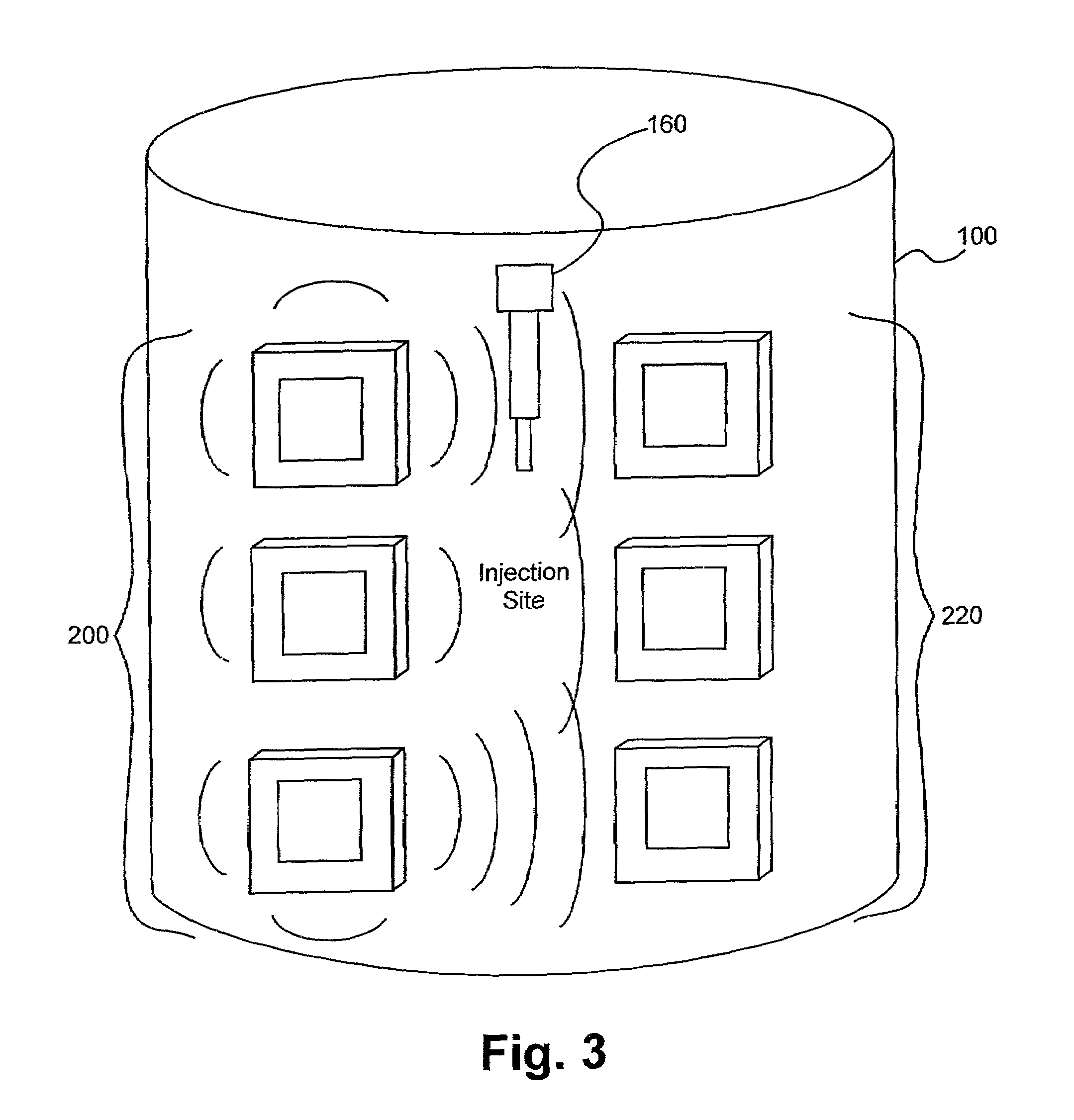
Electromagnetic sensors for biological tissue applications and methods for their use
ActiveUS7591792B2Improves signal couplingImproves resulting measurementElectrotherapyAntenna supports/mountingsMeasurement pointEngineering
Tissue sensors house one or more sensor elements. Each element has a housing mounted substrate and a superstrate with a planar antenna between. A transitional periphery (TP) of a superstrate outer surface interconnects a base to a plateau. At least some of the TP has a generally smooth transition. Plural elements are spaced by the housing. Alternately, the superstrate TP is flat, the housing extends to the outer superstrate surface and a shield surrounds the element. The housing is flush with or recessed below the superstrate and defines a TP between the housing and superstrate. A method converts a reference signal to complex form; plots it in a complex plane as a reference point (RP); converts a measurement signal to complex form; plots it in the complex plane as a measurement point (MP); determine a complex distance between the MP and the RP; and compares complex distance to a threshold.
Owner:BAYER HEALTHCARE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com