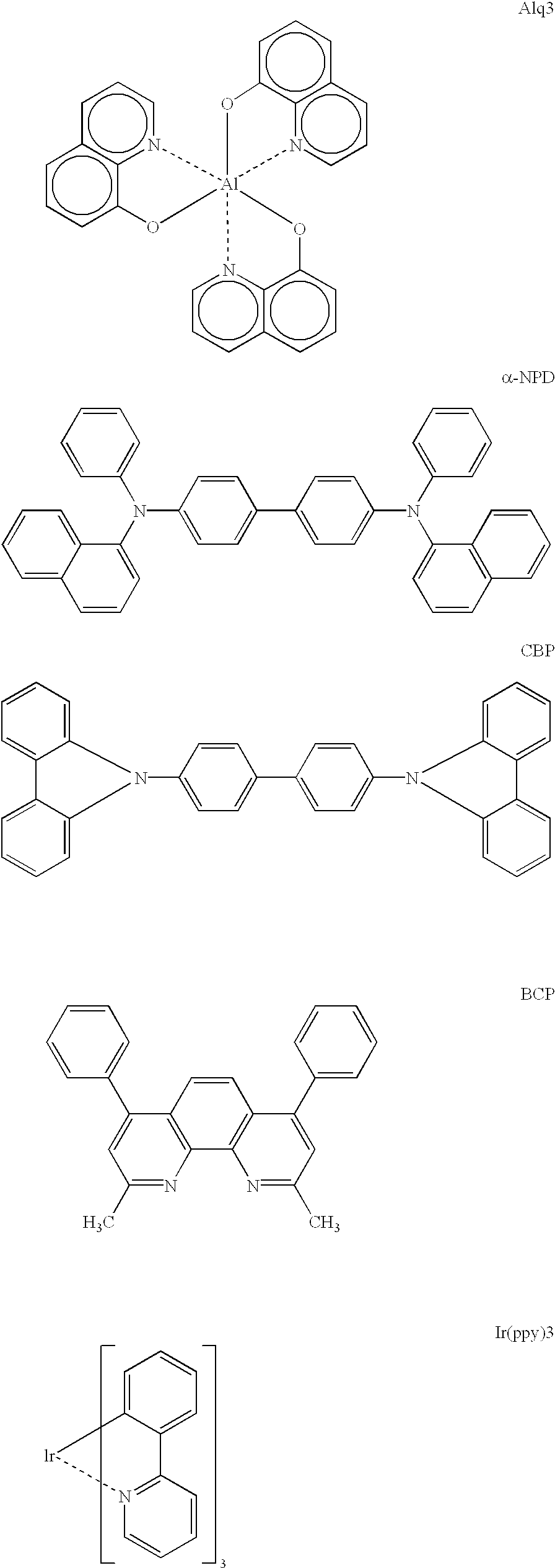
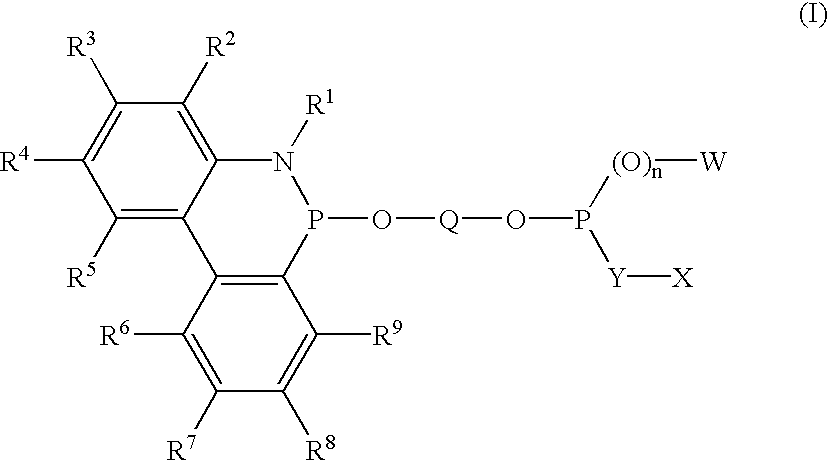
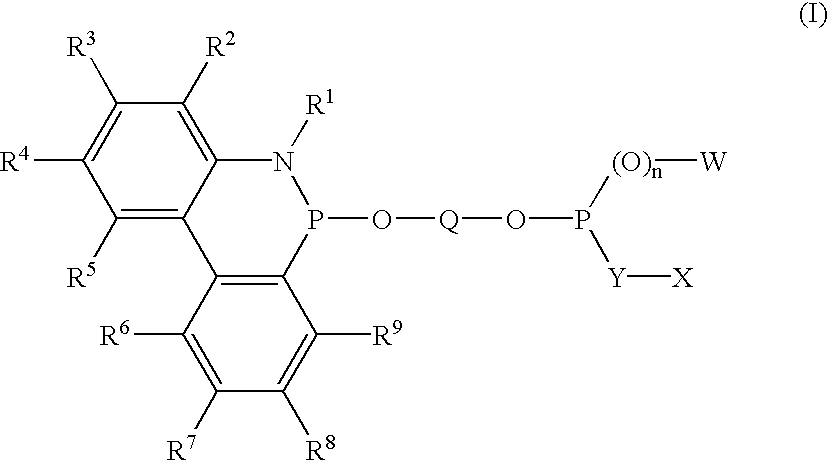
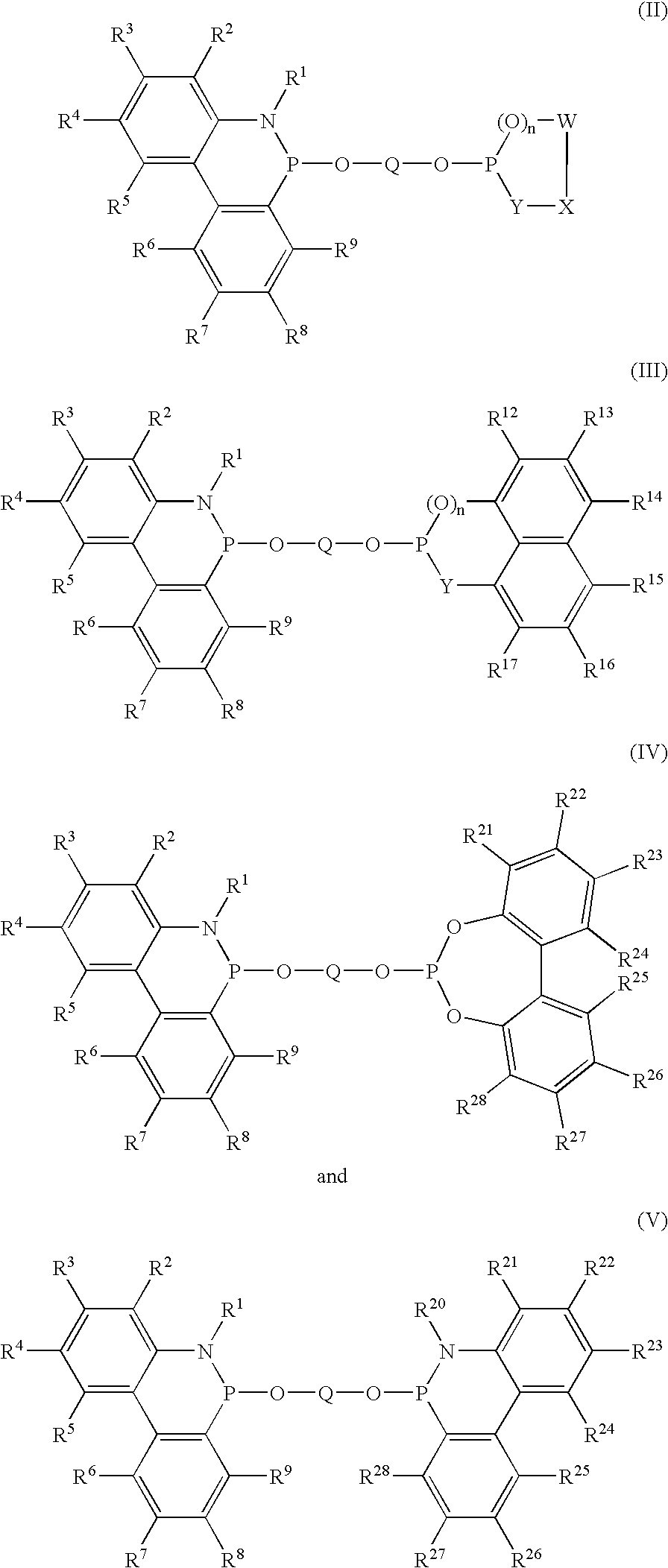
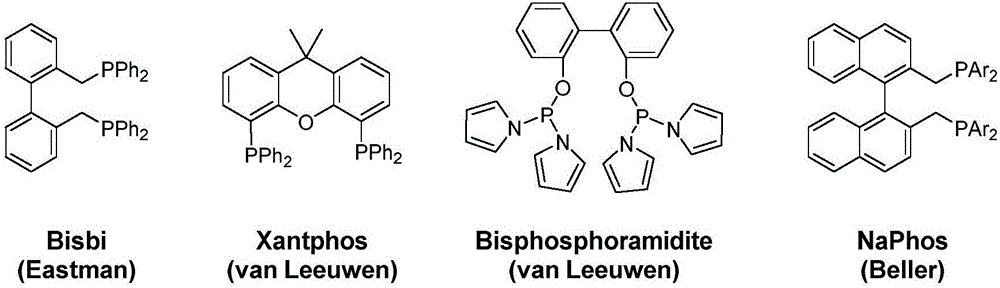
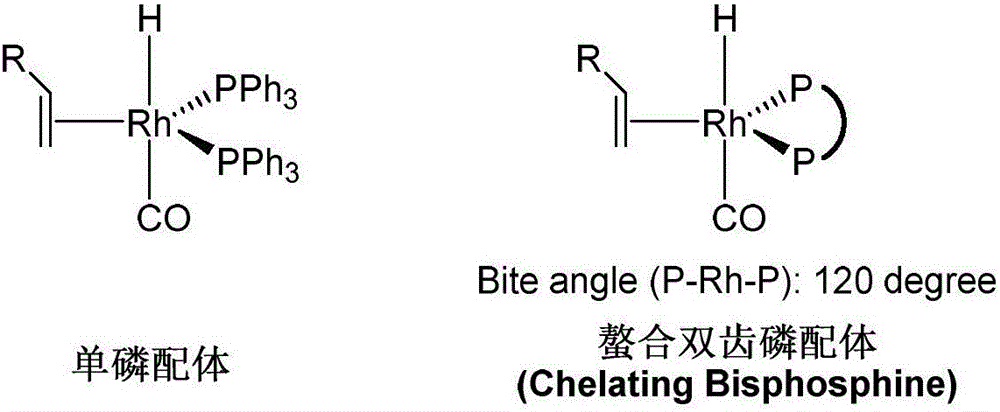
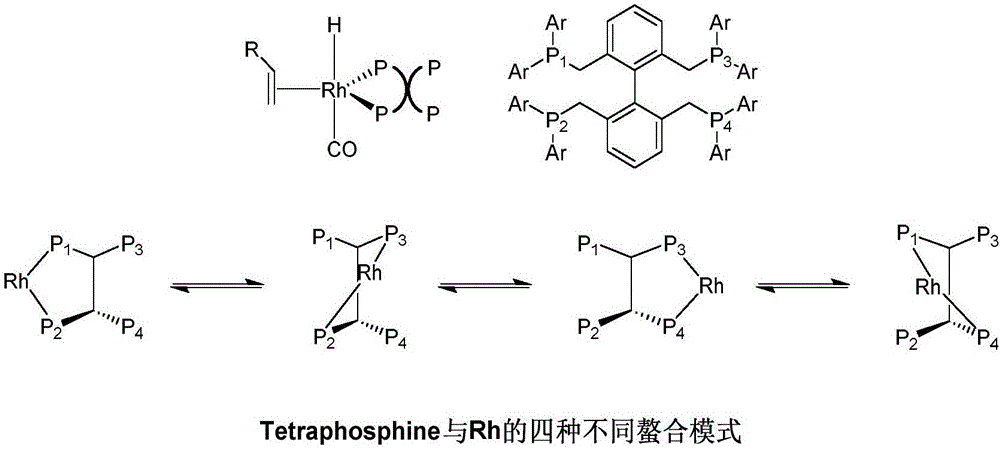
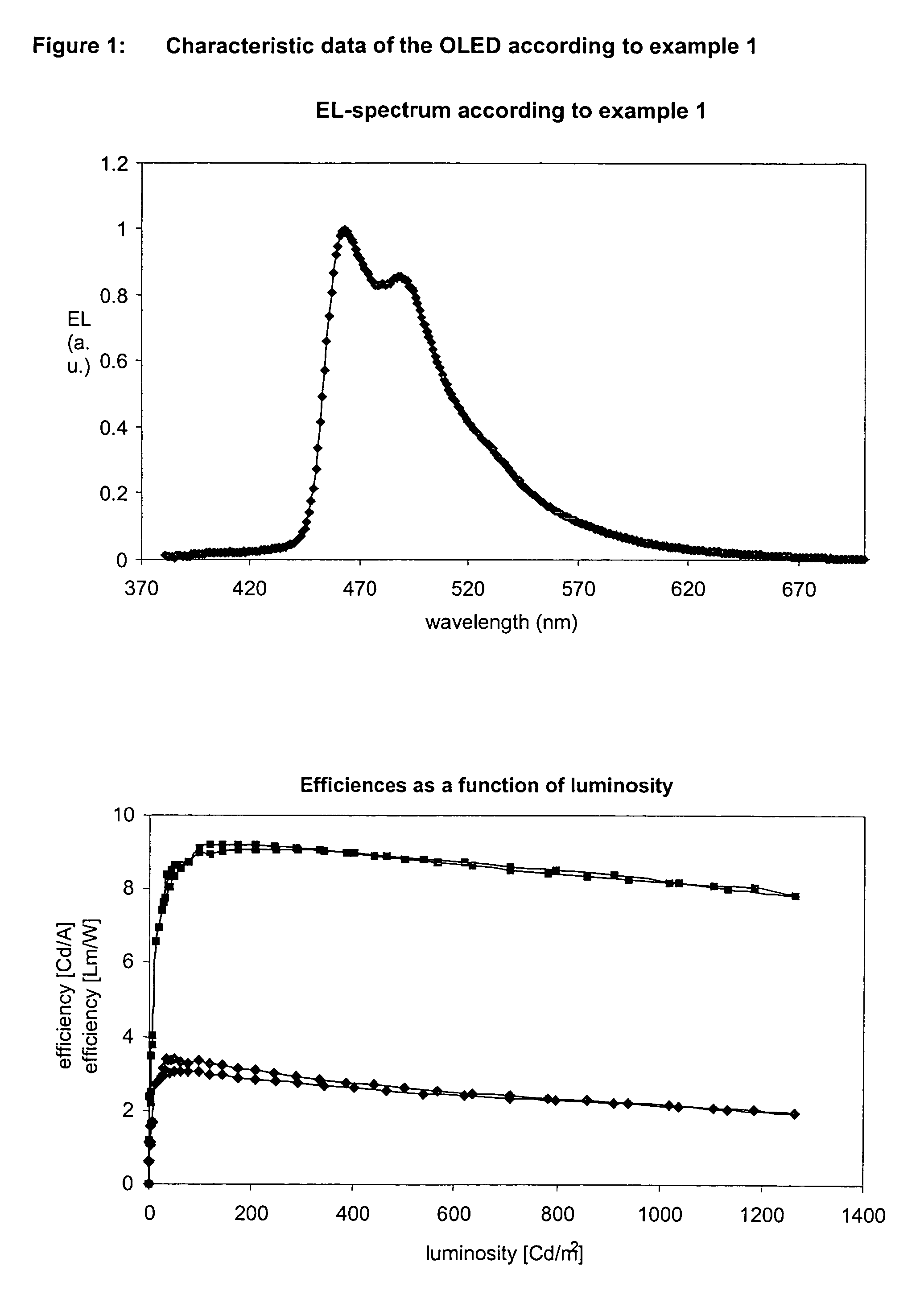
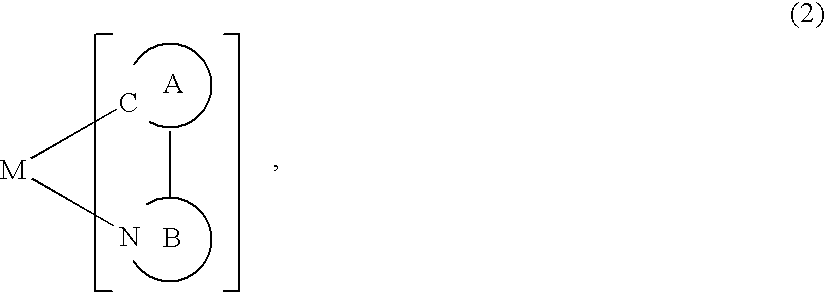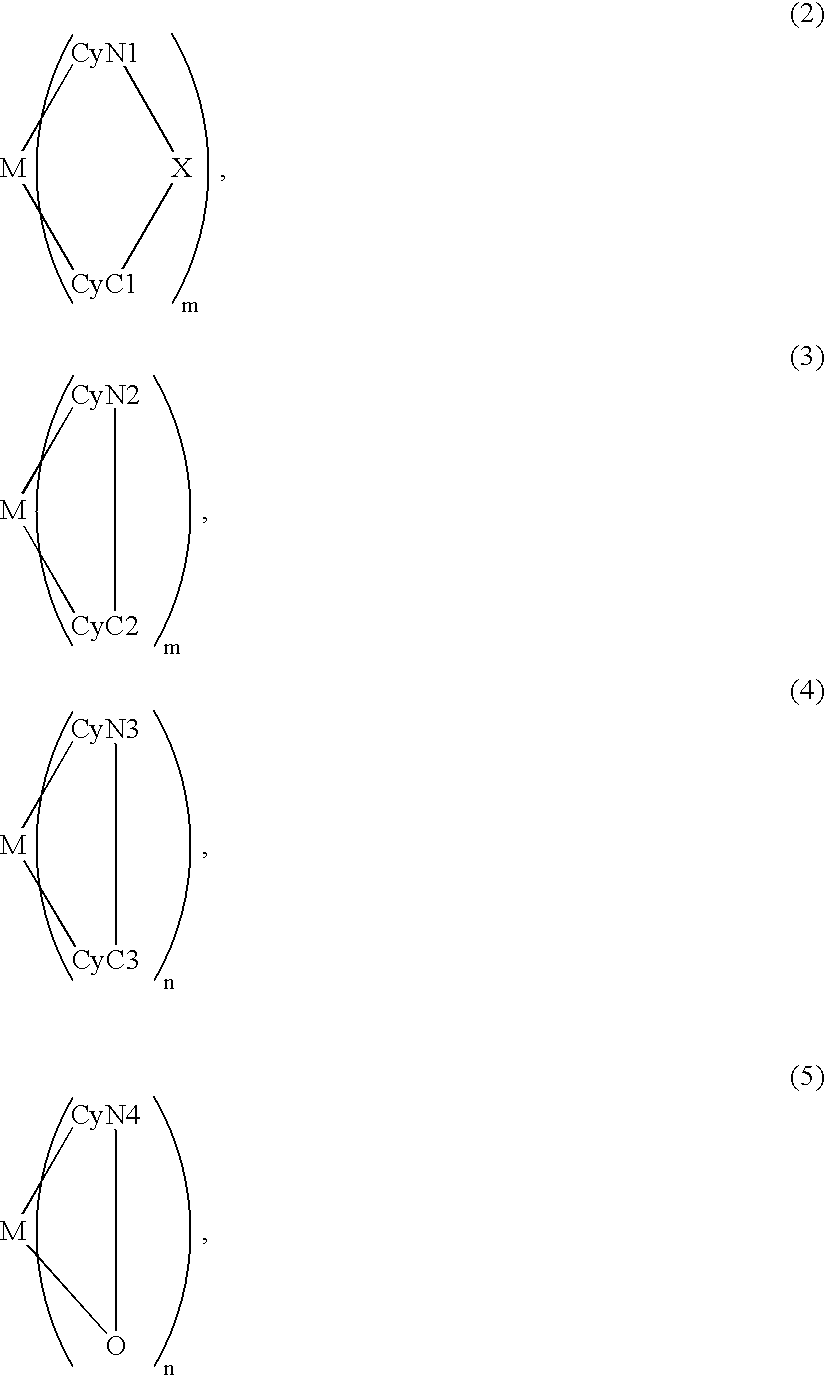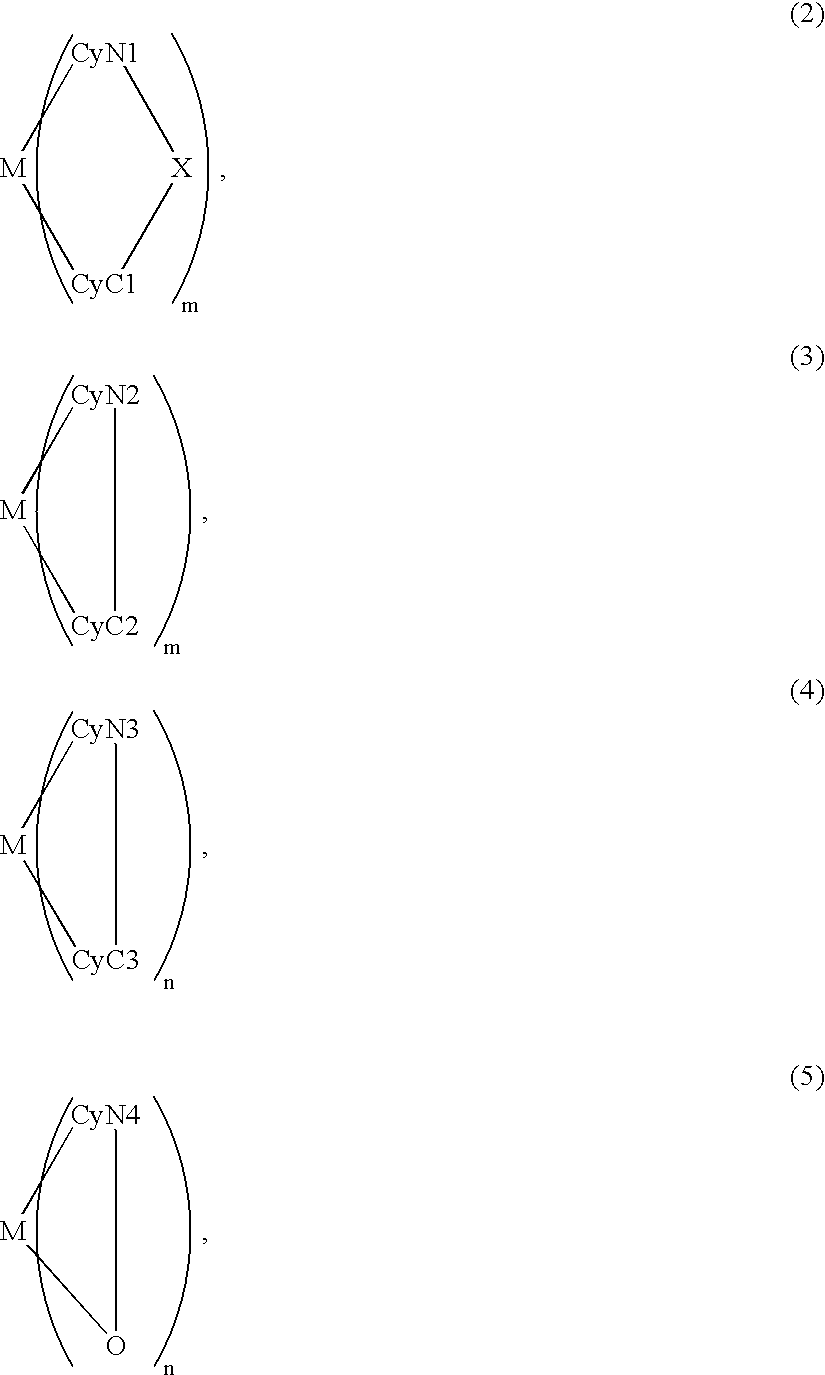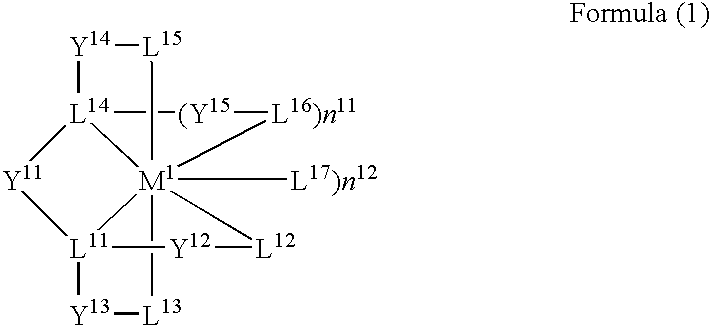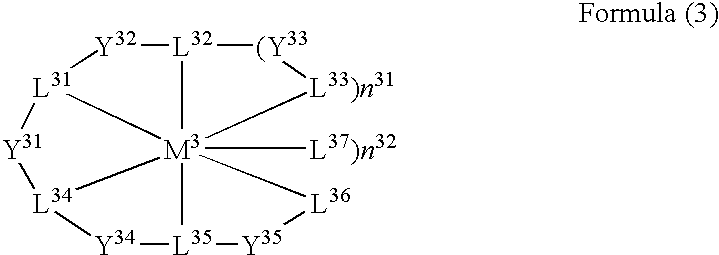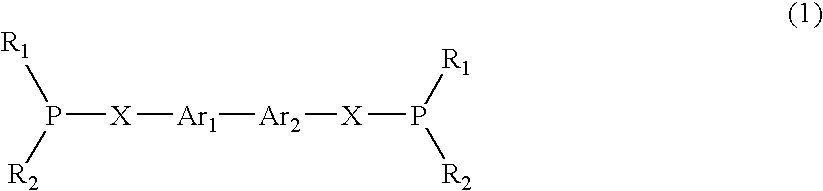Patents
Literature
355results about "Rhodium organic compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
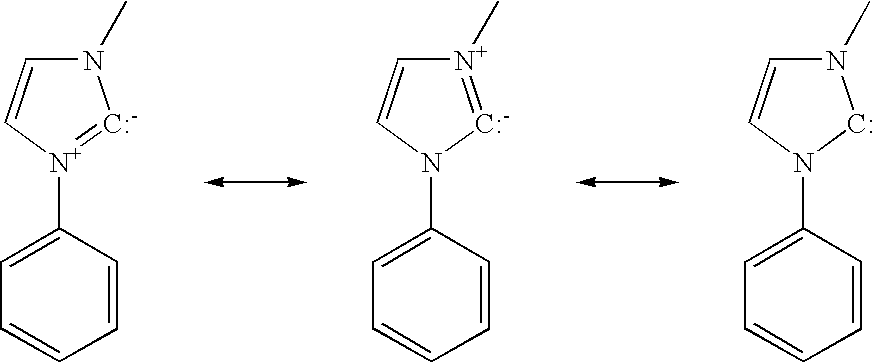
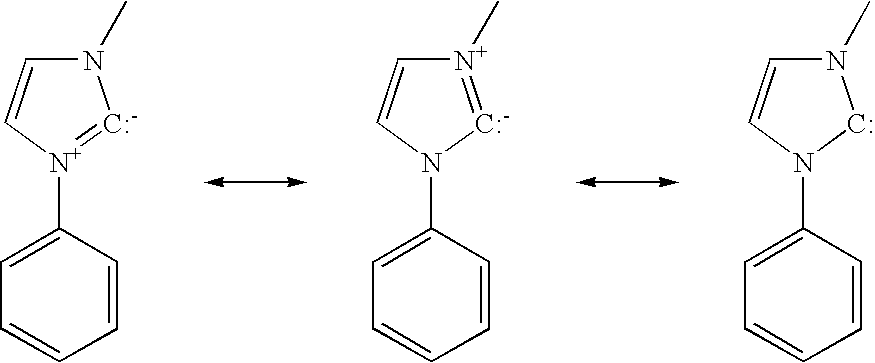
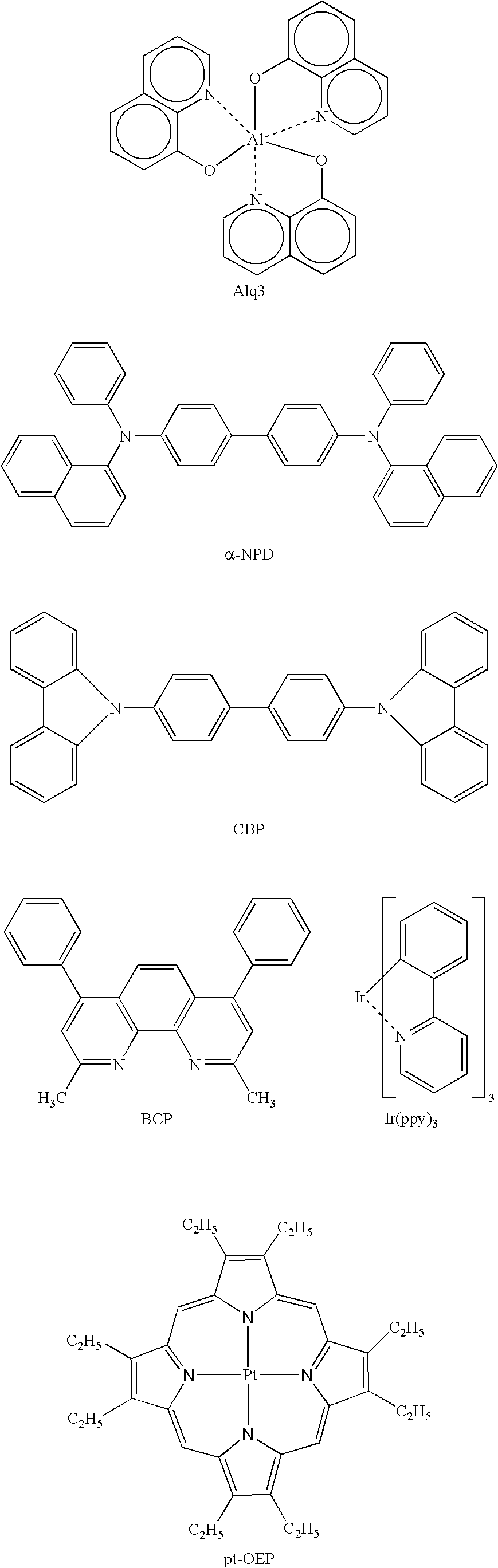
Cationic metal-carbene complexes
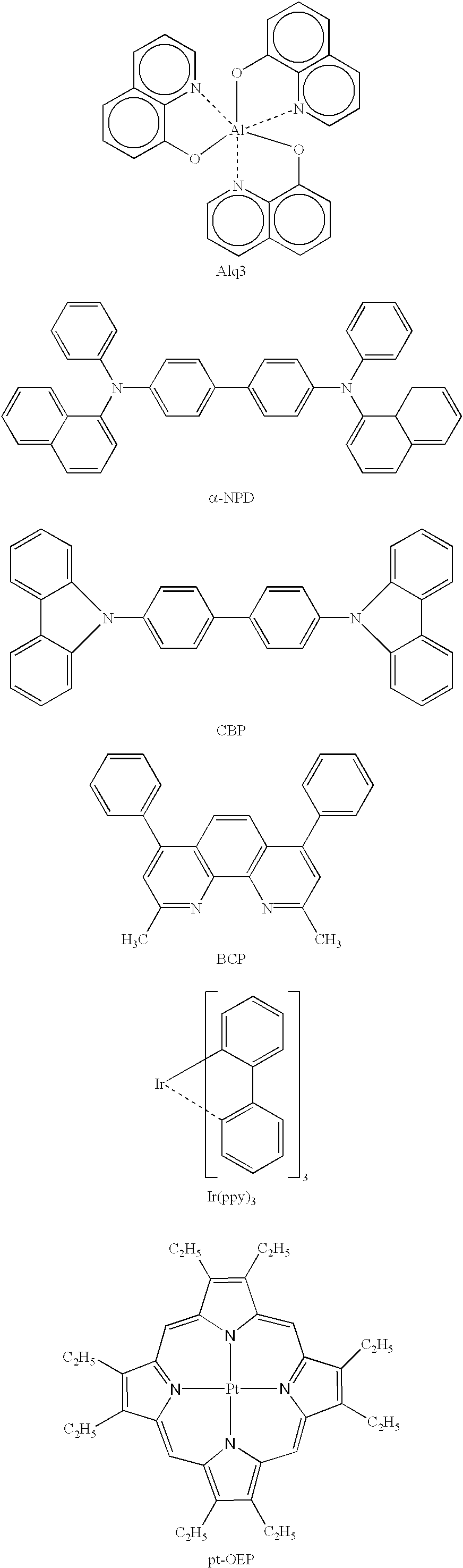
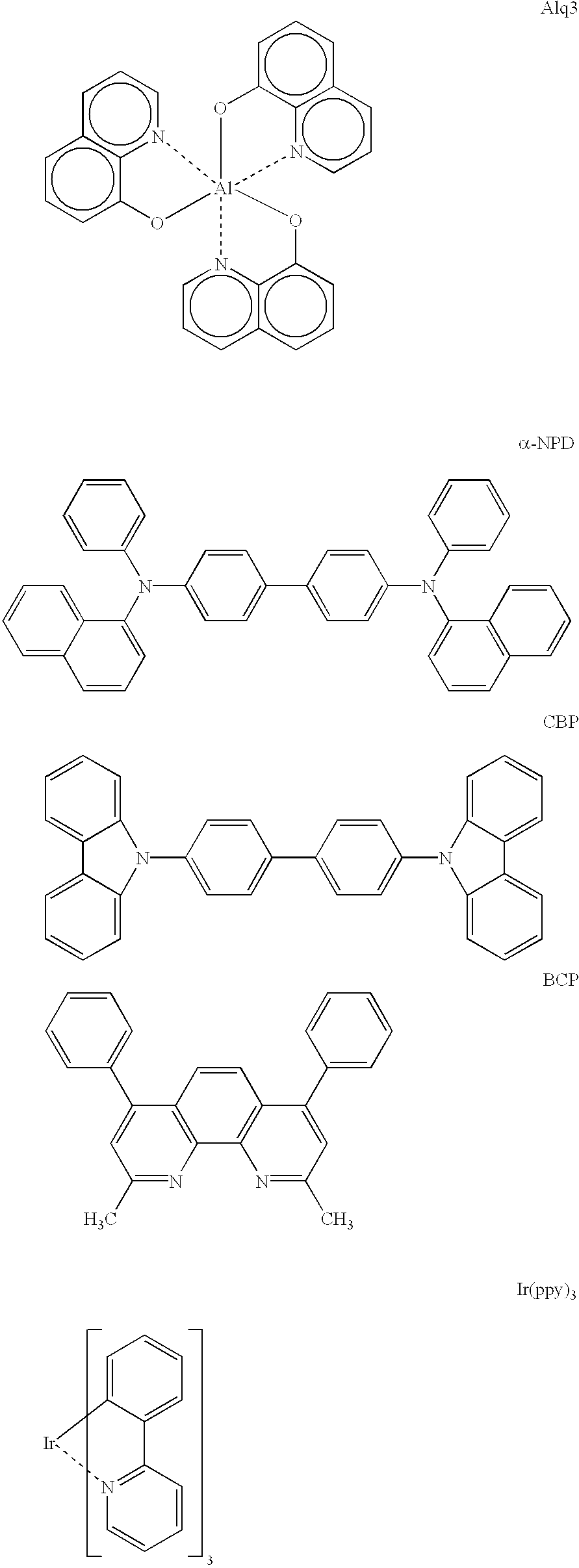
ActiveUS7445855B2Solid-state devicesSemiconductor/solid-state device manufacturingOrganic light emitting deviceCarbene
Owner:UNIVERSAL DISPLAY +1
Luminescence device and display apparatus
InactiveUS20030068526A1High efficiency luminescenceExtend device lifeIndium organic compoundsDischarge tube luminescnet screensHigh luminanceLight emitting device
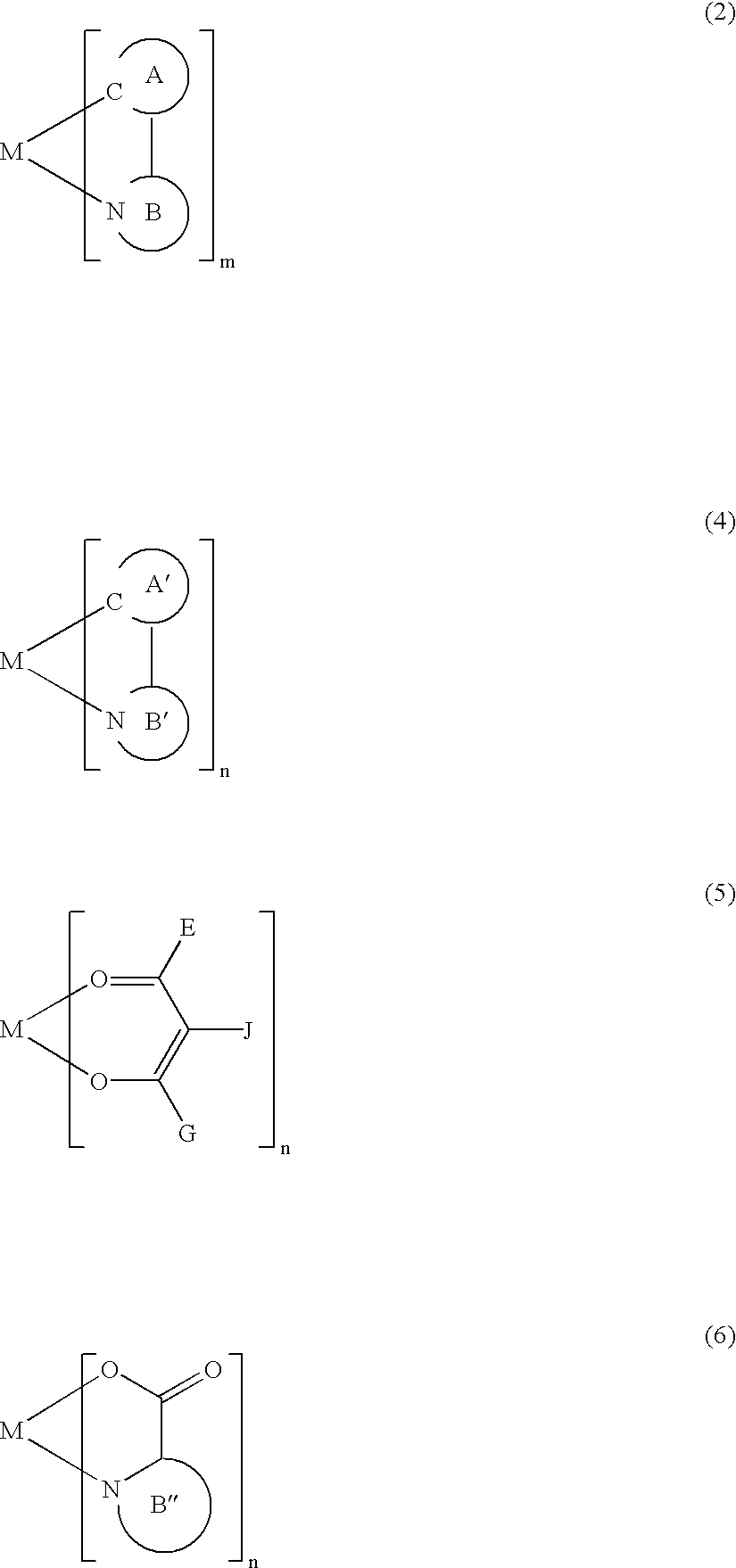
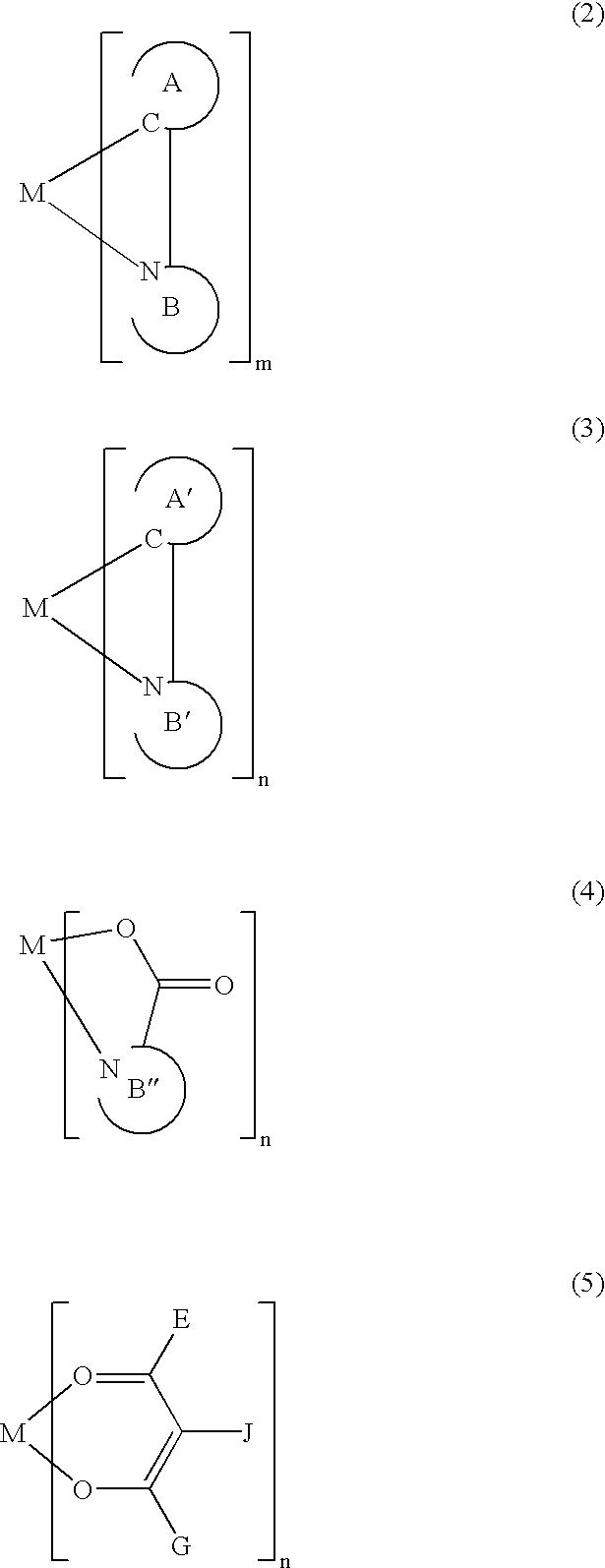
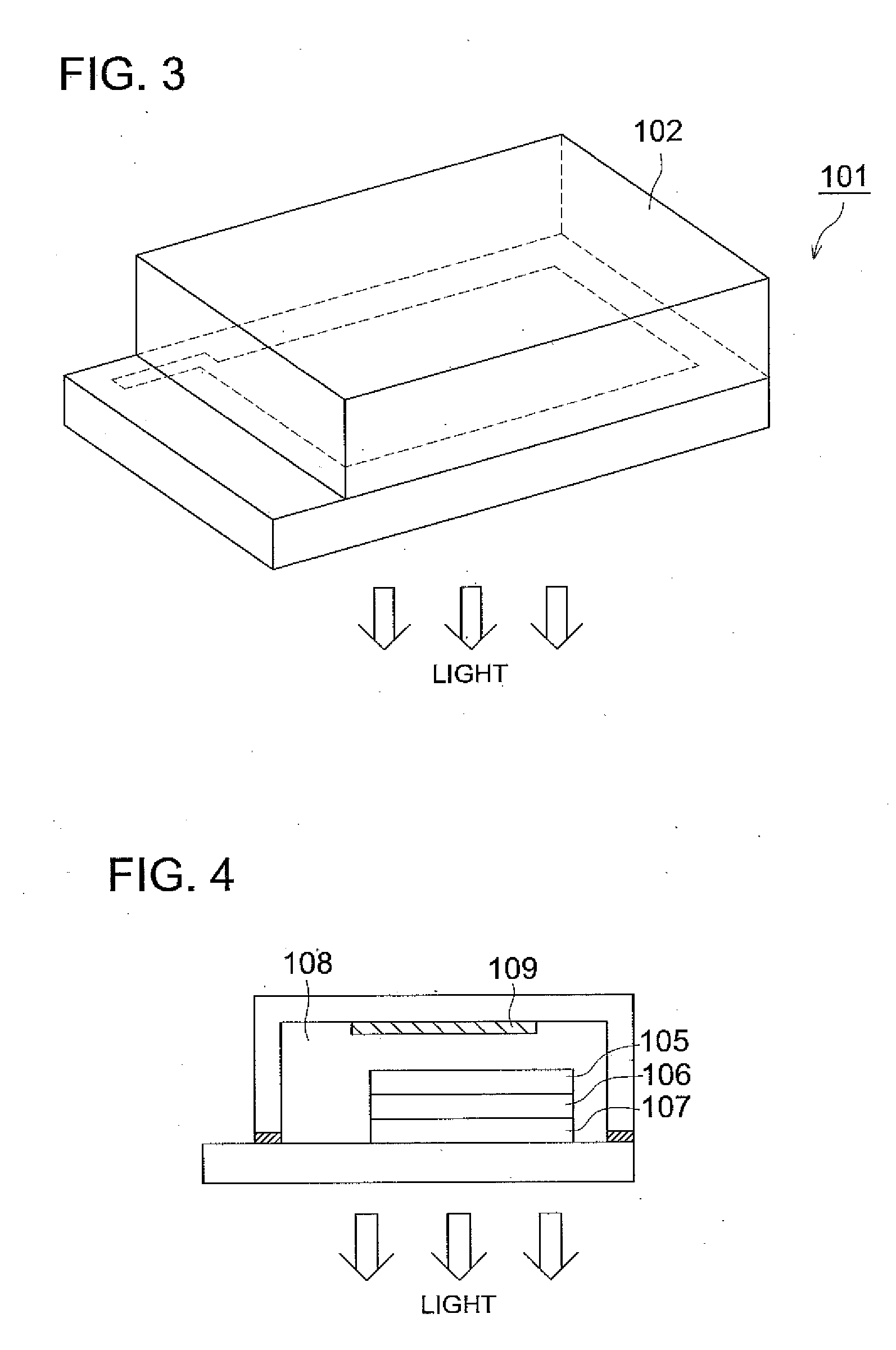
A luminescence device having a layer containing a metal coordination compound which has a partial structure MLm of formula (2) below and is preferably entirely represented by formula (3) below:MLmL'n (3),wherein M denotes a metal atom of Ir, Pt, Rh or Pd; represent mutually different bidentate ligands; m is 1 or 2 or 3; n is 0 or 1 or 2 with the proviso that m+n=2 or 3; the partial structure MLm is represented by formula (2) below (wherein B is an isoquinolyl group bonded to the metal M with its N and including a position-1 carbon atom bonded to a cyclic group A which includes the C bonded to the metal M), and the partial structure ML'n is represented by formula (4), (5) or (6) shown below. There is provided a luminescence device capable of high-efficiency luminescence and long-term high luminance and adapted to red luminescence.
Owner:CANON KK
Luminescence device and display apparatus
InactiveUS20030059646A1High luminous intensityIndium organic compoundsDischarge tube luminescnet screensOrganic filmHigh concentration
In a luminescence device formed of one or plural layers of organic film between a cathode and an anode, at least one layer is a luminescence layer, and a luminescence molecule of a metal coordination compound having a basic structure represented by formula (1) below and having a substituent on at least one of cyclic groups A and B is incorporated as a guest in a host material at a concentration of at least 8 wt. %, which is higher than a concentration at which a luminescence molecule of a similar structure but having no substituent exhibits a maximum luminescence efficiency to form the luminescence layer. As a result, a high-efficiency luminescence device is provided, which is less liable to cause concentration extinction even when a luminescence molecule is contained at a high concentration relative to the host material in the luminescence layer.
Owner:CANON KK
Metal coordination compound, luminescence device and display apparatus
InactiveUS20020094453A1Solid-state devicesSemiconductor/solid-state device manufacturingHydrogen atomNitrogen
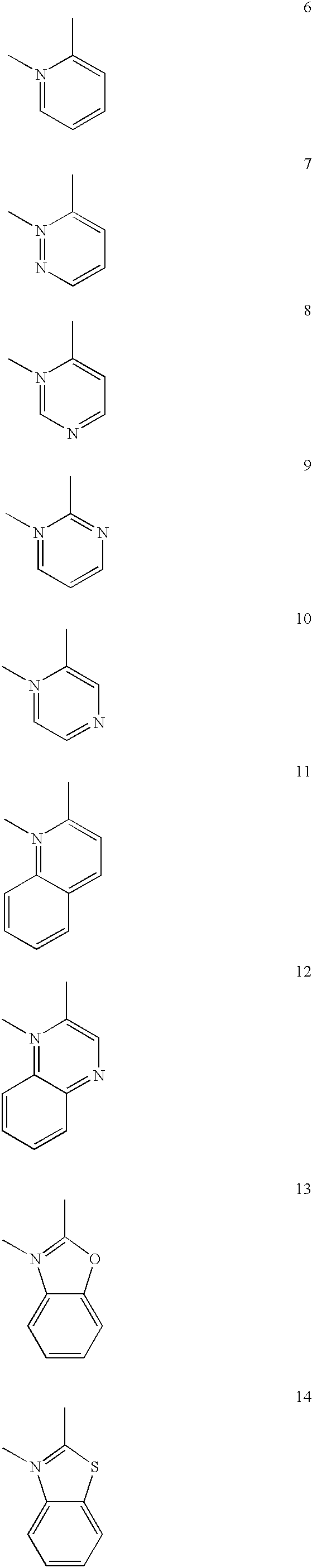
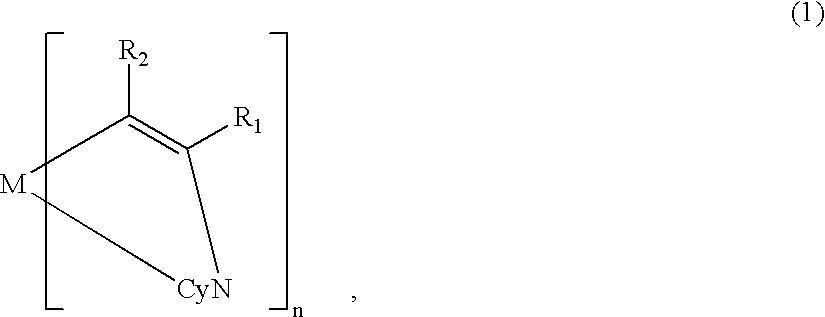
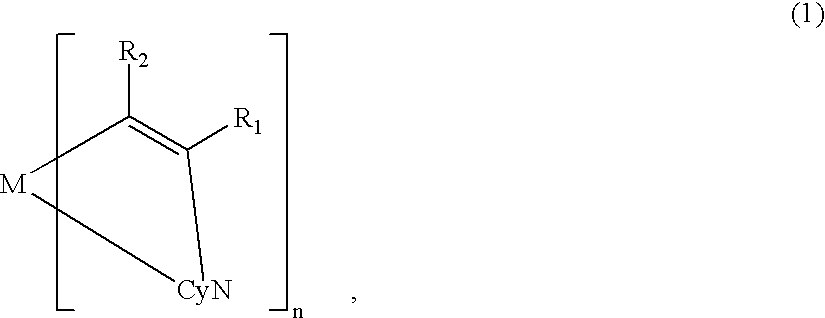
A metal coordination compound suitable as an organic material for a luminescent device is represented by the following formula (1): wherein M denotes Ir, Pt, Rh or Pd; n is 2 or 3; Y denotes an alkylene group having 2-6 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S- or -CO- and capable of including hydrogen atom which can be replaced with a linear or branched alkyl group which has 1-10 carbon atoms and is capable of including hydrogen atom which can be replaced with fluorine atom; and CyN denotes a cyclic group containing nitrogen atom connected to M and capable of having a substituent selected from the group consisting of halogen atom; nitro group; phenyl group; trialkylsilyl group having 1-8 carbon atoms; and a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom.
Owner:CANON KK
Metal coordination compound, luminescence device and display apparatus
InactiveUS20030054198A1Group 5/15 element organic compoundsSolid-state devicesQuinolinePerylene derivatives
An organic EL device includes a luminescence layer containing, as a luminescent material allowing a high-luminescence and high-efficiency luminescence for a long period of time, a metal coordination compound represented by the following formula (1): LmML'n, wherein M denotes Ir, Pt, Ph or Pd; L denotes a bidentate ligand; L' denotes a bidentate ligand different from L; m is an integer of 1, 2 or 3; and n is an integer of 0, 1 or 2 with the proviso that the sum of m and n is 2 or 3. The partial structure MLm is represented by a formula (2) or a formula (3) shown below, and the partial structure ML'n is represented by a formula (4) or a formula (5) shown below: wherein CyN1, CyN2 and CyN3 independently denote a substituted or unsubstituted cyclic group containing a nitrogen atom connected to M; CyN4 denotes a cyclic group containing 8-quinoline or its derivative having a nitrogen atom connected to M; CyC1, CyC2 and CyC3 independently denote a substituted or unsubstituted cyclic group containing a carbon atom connected to M, with the proviso that the metal coordination compound is represented by the formula (2) when n is 0.
Owner:CANON KK
Metal coordination compound, luminescence device and display apparatus
A metal coordination compound suitable as an organic material for a luminescent device is represented by the following formula (1): wherein M denotes Ir, Pt, Rh or Pd; n is 2 or 3; R1 and R2 independently denote a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom; and CyN denotes a cyclic group containing nitrogen atom connected to M and capable of having a substituent selected from the group consisting of halogen atom; nitro group; phenyl group; trialkylsilyl group having 1-8 carbon atoms; and a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including hydrogen atom which can be replaced with fluorine atom.
Owner:CANON KK
Luminescence device, display apparatus and metal coordination compound
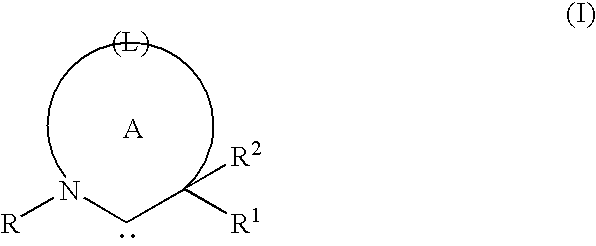
A luminescence device that has an organic compound layer disposed between a pair of electrodes. This organic compound layer contains a metal coordination compound represented by the following formula (1)wherein M is Ir, Rh or Pd; n is 2 or 3; CyN is a substituted or unsubstituted cyclic group containing a nitrogen atom connected to M and capable of containing another nitrogen atom and / or sulfur atom; and CyC is a substituted or unsubstituted cyclic group containing a carbon atom connected to M and capable of containing a nitrogen atom and / or a sulfur atom.
Owner:CANON KK
Cationic metal-carbene complexes
ActiveUS20050260446A1Solid-state devicesSemiconductor/solid-state device manufacturingOrganic light emitting deviceOrganic chemistry
The present invention relates to organic light emitting devices (OLEDs), and more specifically to phosphorescent organic materials used in such devices. More specifically, the present invention relates to emissive phosphorescent material comprising a carbene ligand bound to a metal center, wherein the resulting metal-carbene complex is cationic.
Owner:UNIVERSAL DISPLAY +1
Limuninescence device, display apparatus and metal coordination compound
InactiveUS20020063516A1Indium organic compoundsDischarge tube luminescnet screensOrganic compoundLuminescence
A luminescence device is principally constituted by a pair of electrodes and an organic compound layer disposed therebetween. The layer contains a metal coordination compound represented by the following formula (1): wherein M denotes Ir, Rh or Pd; n is 2 or 3; CyN denotes a substituted or unsubstituted cyclic group containing a nitrogen atom connected to M and capable of containing another nitrogen atom and / or a sulfur atom; and CyC denotes a substituted or unsubstituted cyclic group containing a carbon atom connected to M and capable of containing a nitrogen atom and / or a sulfur atom, CyN and CyC being connected to each other via a covalent bond, and each of substituents for CyN and CyC being selected from the group consisting of a halogen atom; nitro group; a trialkylsilyl group containing three linear or branched alkyl groups each independently having 1-8 carbon atoms; and a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -CO-, -CO-O-, -O-CO-, -CH=CH- or -C=C- and capable of including a hydrogen atom which can be replaced with a fluorine atom; with the proviso that a sum of nitrogen atom and sulfur atom present in ring structures of CyN and CyC is at least 2.
Owner:CANON KK
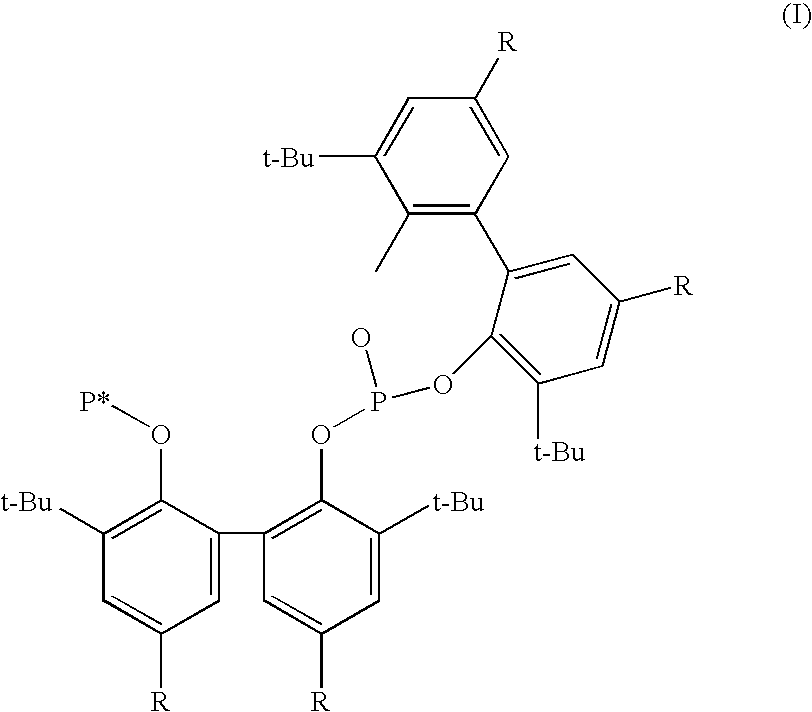
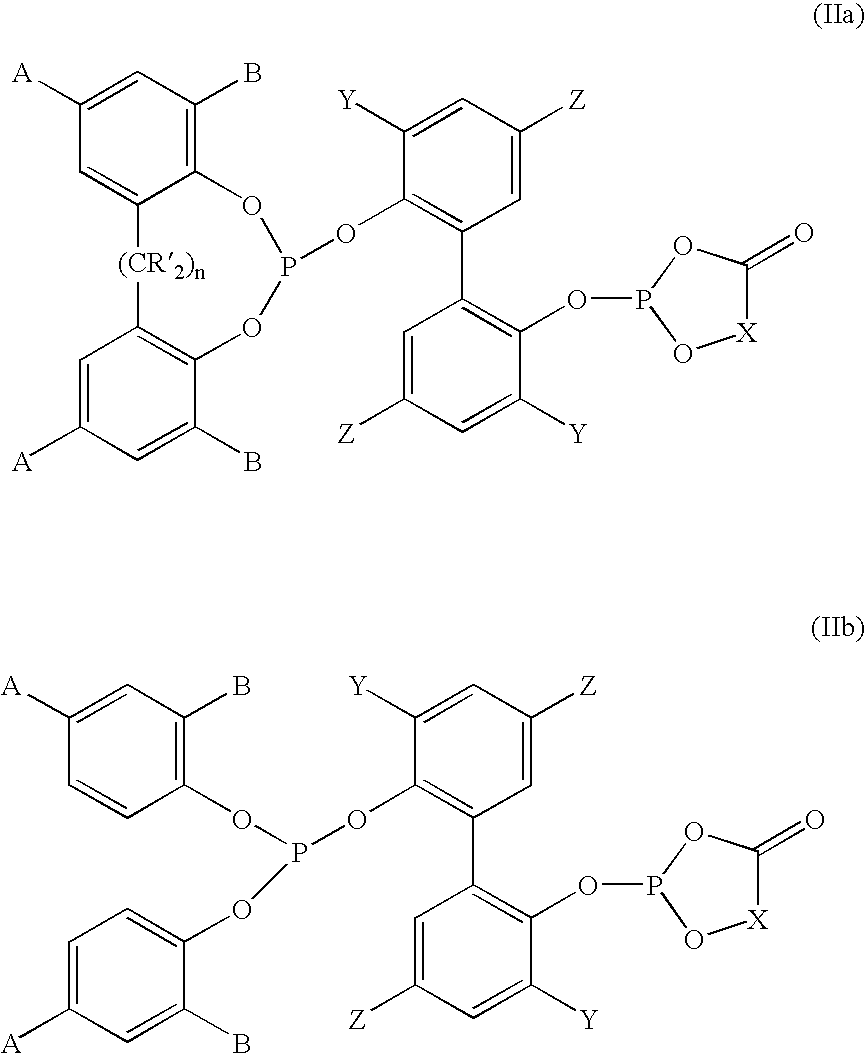
Phosoxophite ligands and use thereof in carbonylation processes
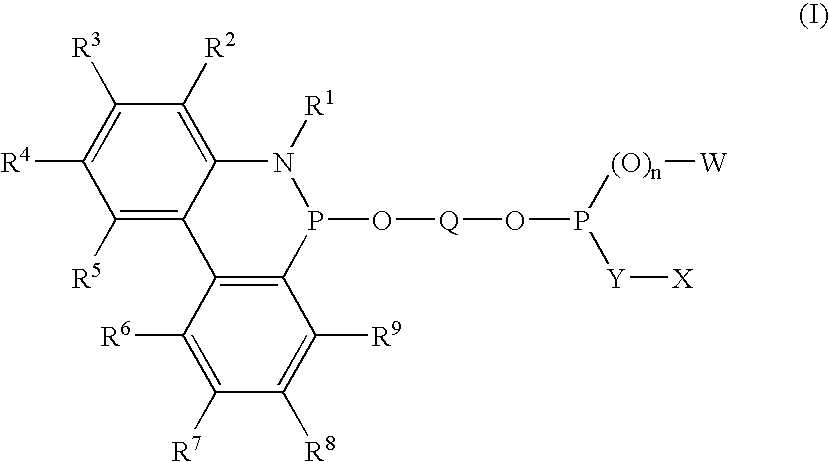
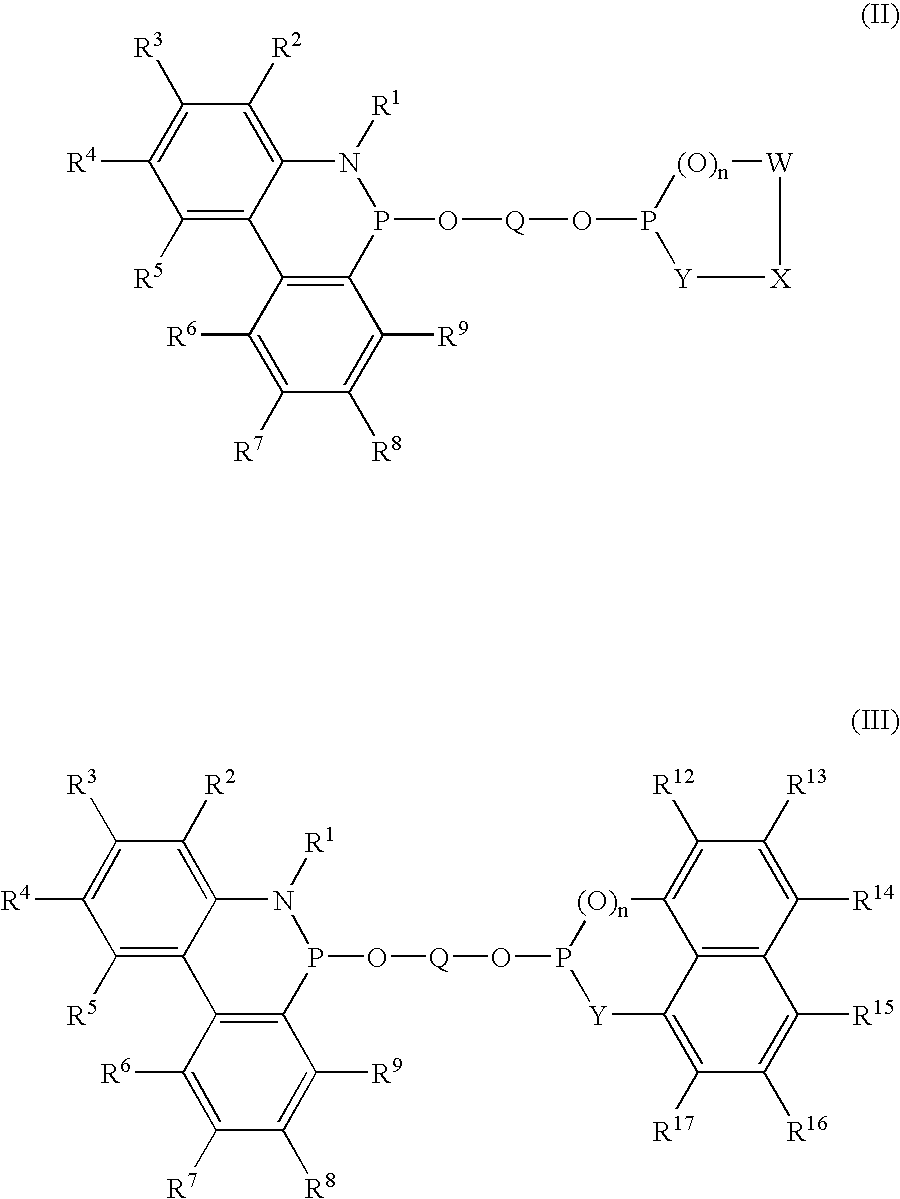
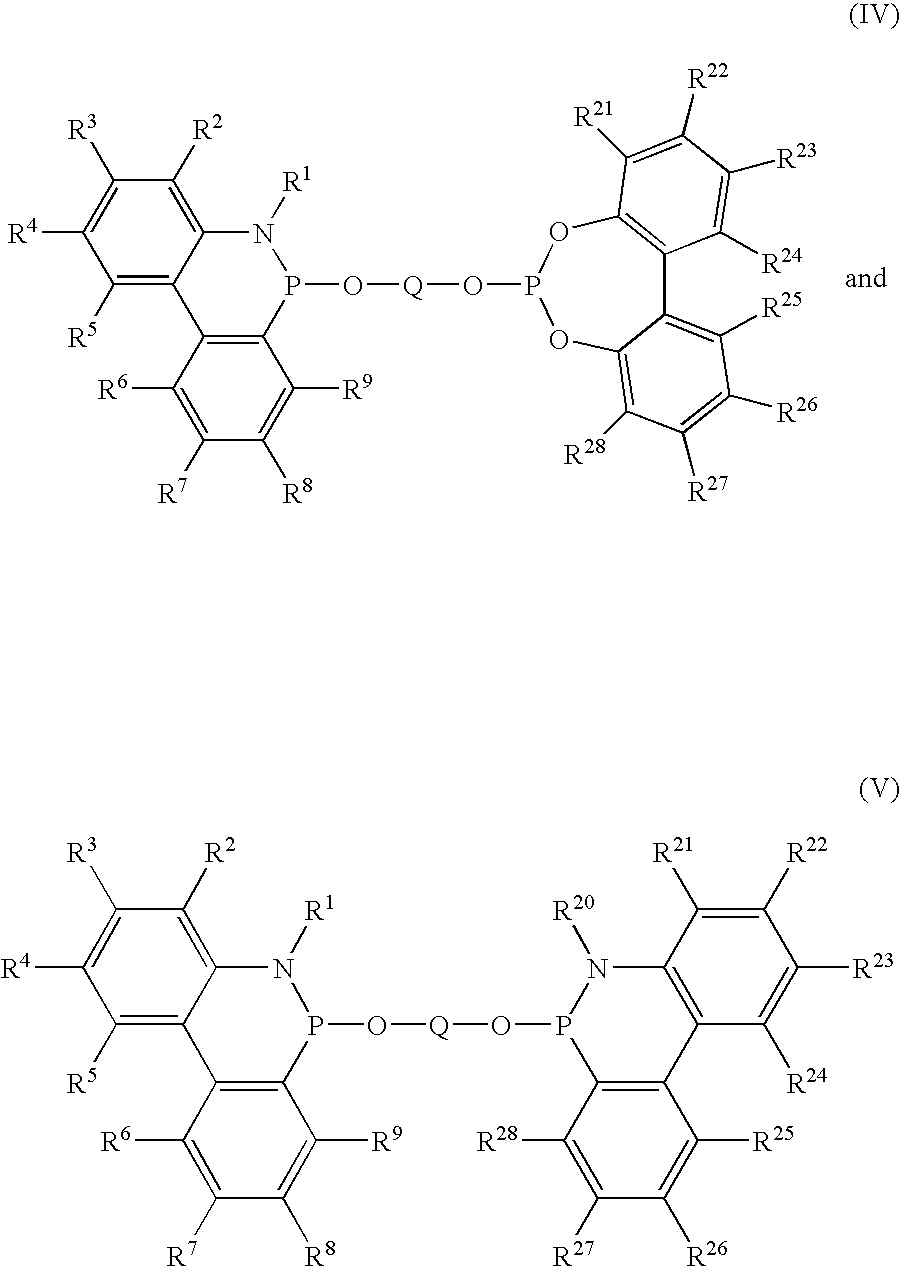
ActiveUS7196230B2High activityHigh selectivitySilicon organic compoundsRhodium organic compoundsCarbonylationProtein carbonyl
A novel organophosphorus composition and synthesis thereof, the composition being characterized by one phosphite moiety, one phosoxophite moiety, and a plurality of sterically bulky substituents. The novel composition finds utility as a ligand in Group VIII transition metal phosoxophite complex catalysts and complex catalyst precursors that are used in carbonylation processes, preferably, hydroformylation processes. Additionally, there is disclosed a novel method of preparing a phosphoromonochloridite composition that finds utility as a precursor to the novel phosoxophite composition.
Owner:DOW TECH INVESTMENTS
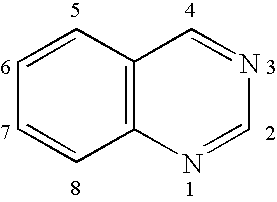
Electroluminescent device with quinazoline complex emitter
ActiveUS20070122655A1Useful emissionUseful stabilityGroup 5/15 element organic compoundsSolid-state devicesLuminophoreHost material
An OLED device comprises a cathode, an anode, and located therebetween a light-emitting layer containing a host material and a tris-CˆN-cyclometallated complex of Ir or Rh wherein at least one of the ligands comprises a substituted quinazoline moiety. The device provides useful emission and stability attributes.
Owner:GLOBAL OLED TECH
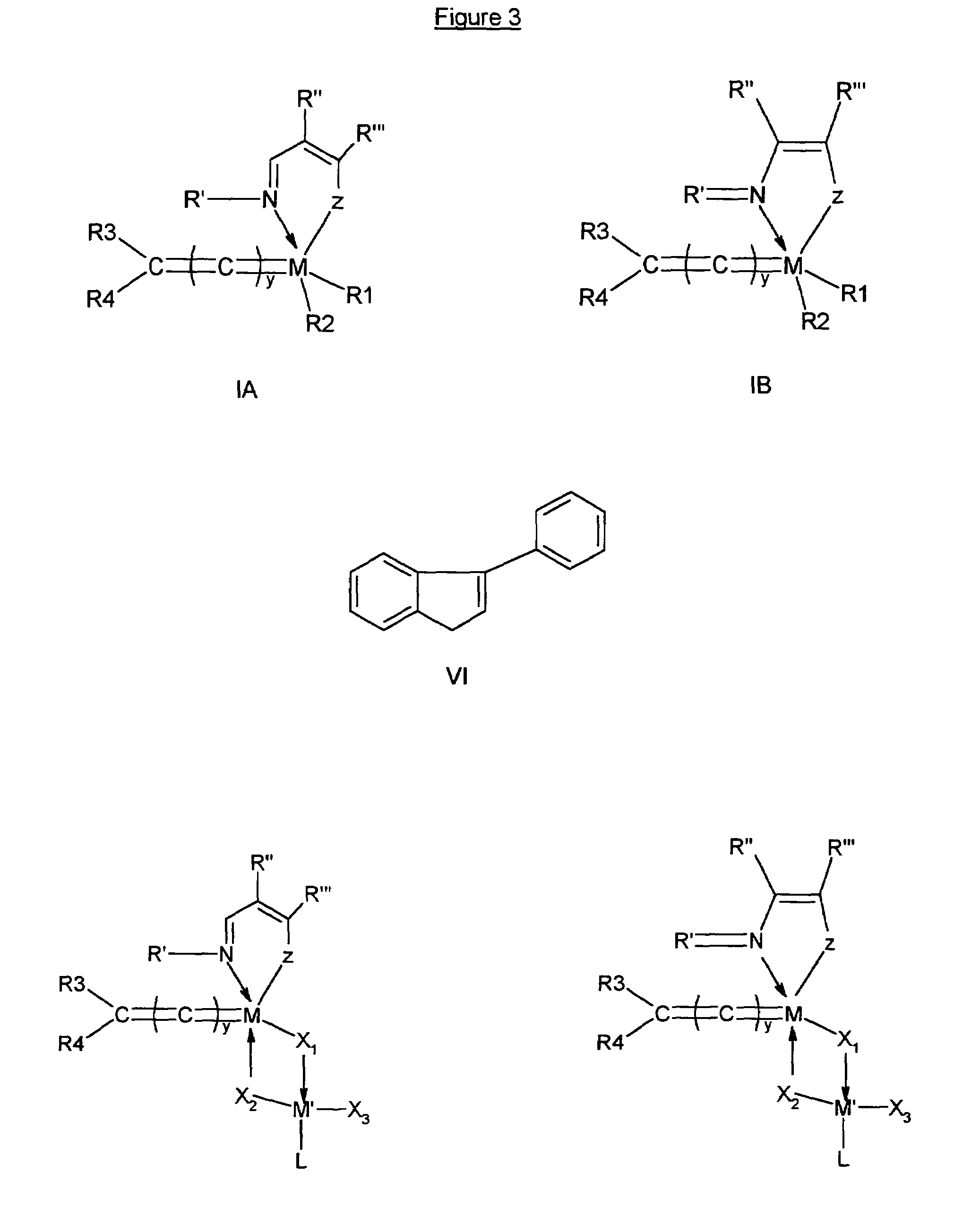
Metal complexes useful in metathesis and other reactions
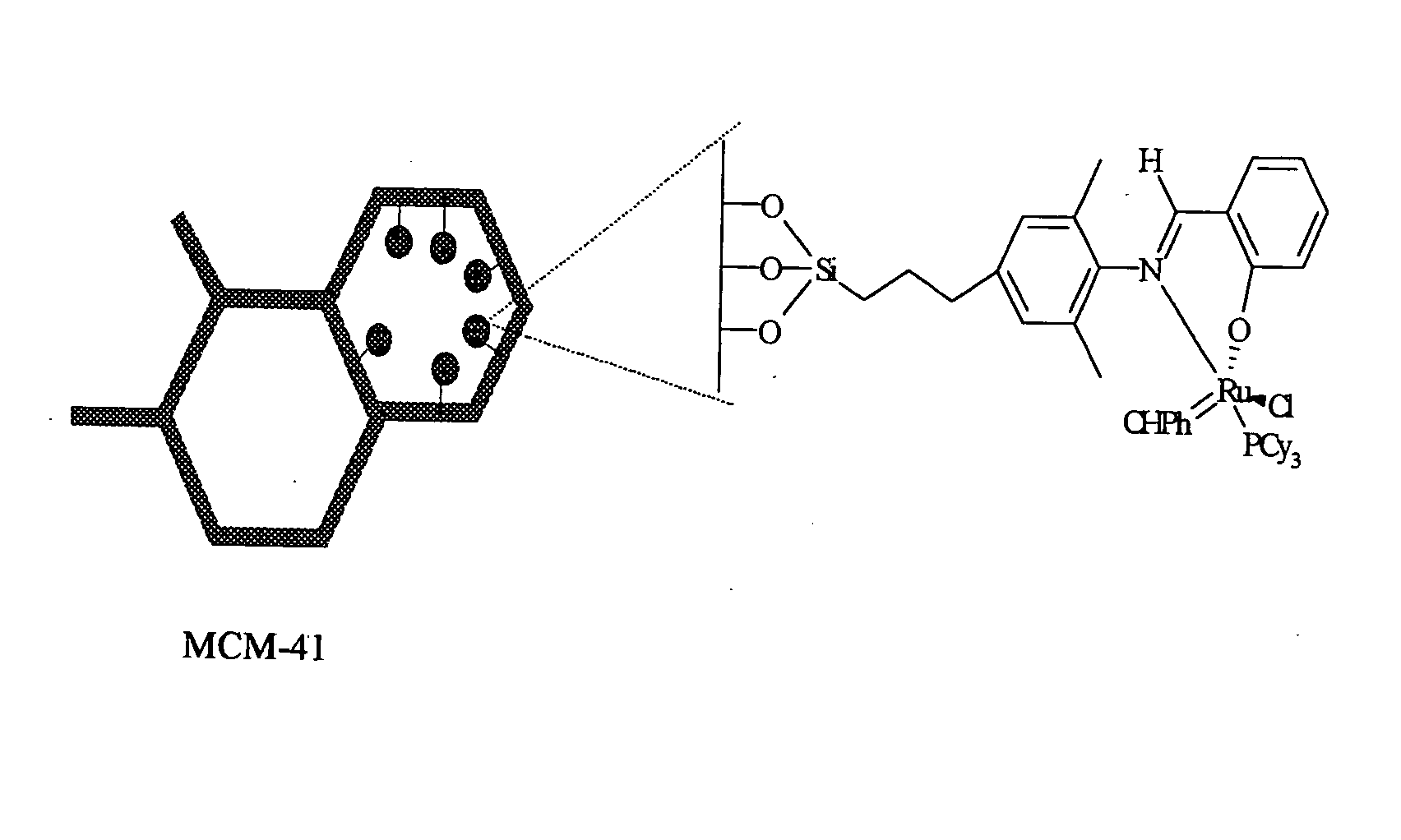
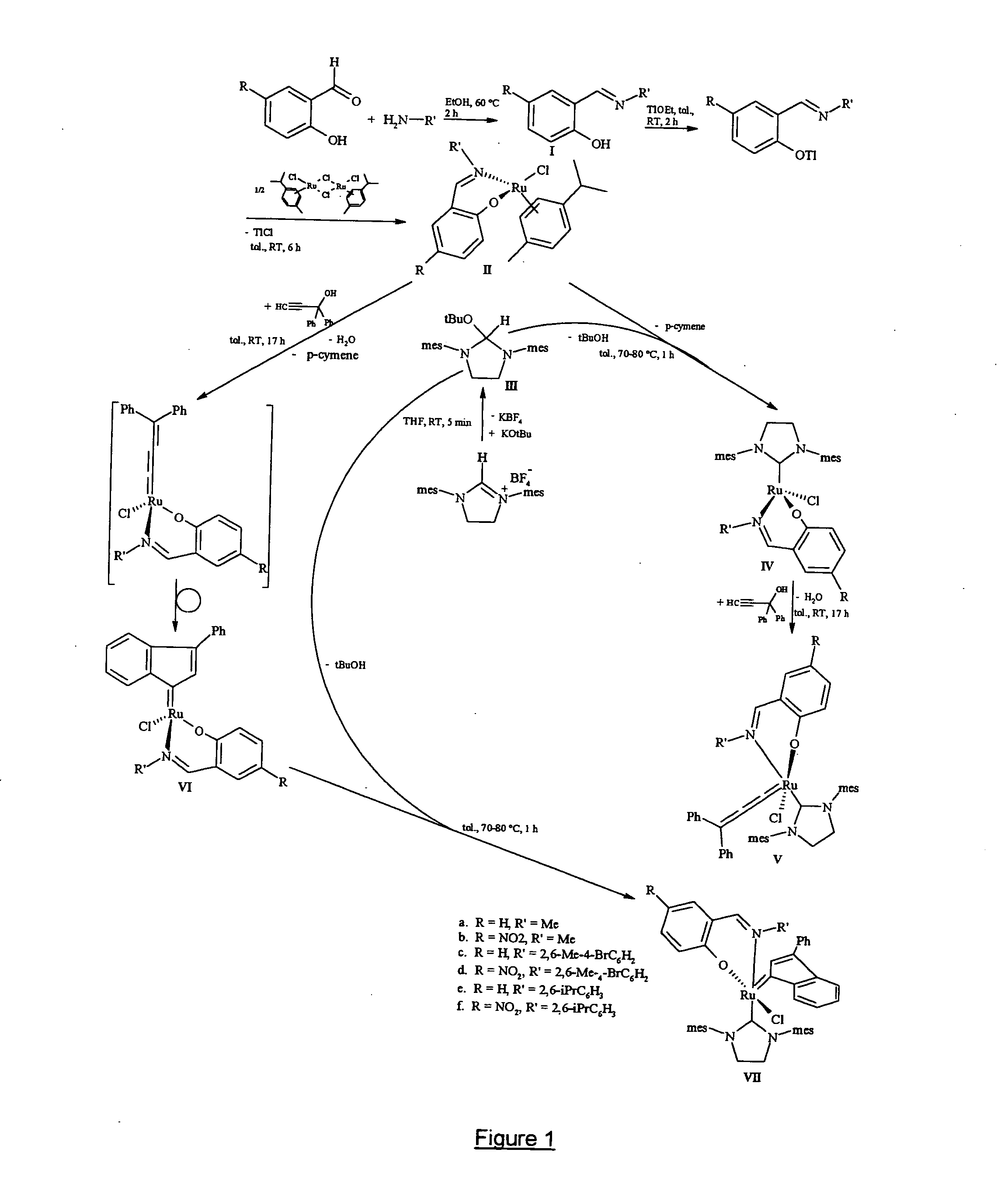
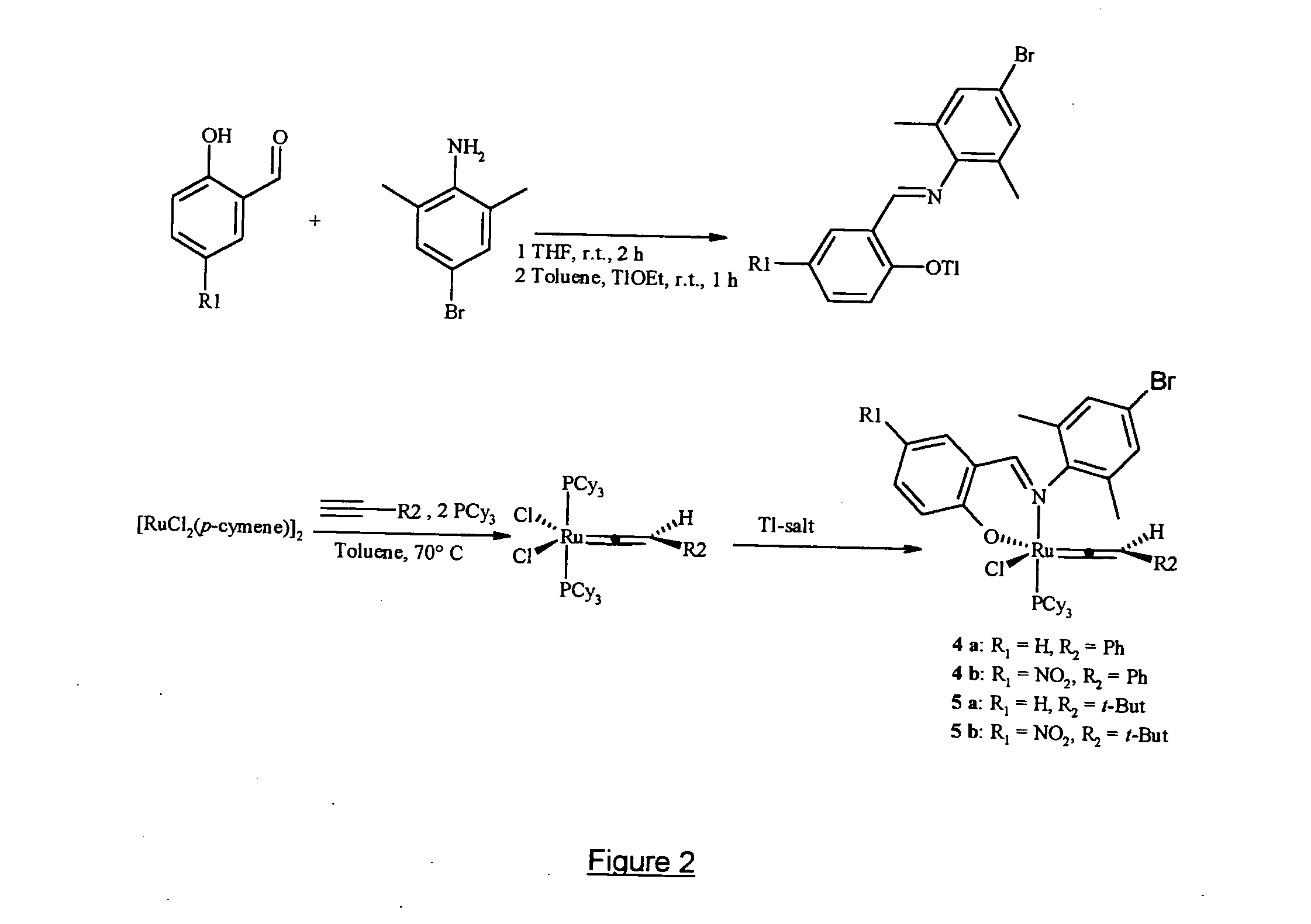
InactiveUS20050043541A1Group 5/15 element organic compoundsOrganic chemistry methodsFuranHigh activity
This invention provides metal complexes being useful as catalyst components in metathesis reactions and in reactions involving the transfer of an atom or group to an ethylenically or acetylenically unsaturated compound or another reactive substrate and, with respect to a sub-class thereof, for the polymerisation of α-olefins and optionally conjugated dienes, with high activity at moderate tempera-tures. It also provides methods for obtaining polymers with very narrow molecular weight distribution by means of a living reaction. It also provides methods for making said metal complexes and novel intermediates involved in such methods. It further provides derivatives of said metal complexes which are suitable for covalent bonding to a carrier, the product of such covalent bonding being useful as a supported catalyst for heterogeneous catalytic reactions. It also provides a direct one-step synthesis of pyrrole, furan and thiophene compounds from diallyl compounds.
Owner:RIMTEC CORP
Metal complexes, methods, and uses thereof
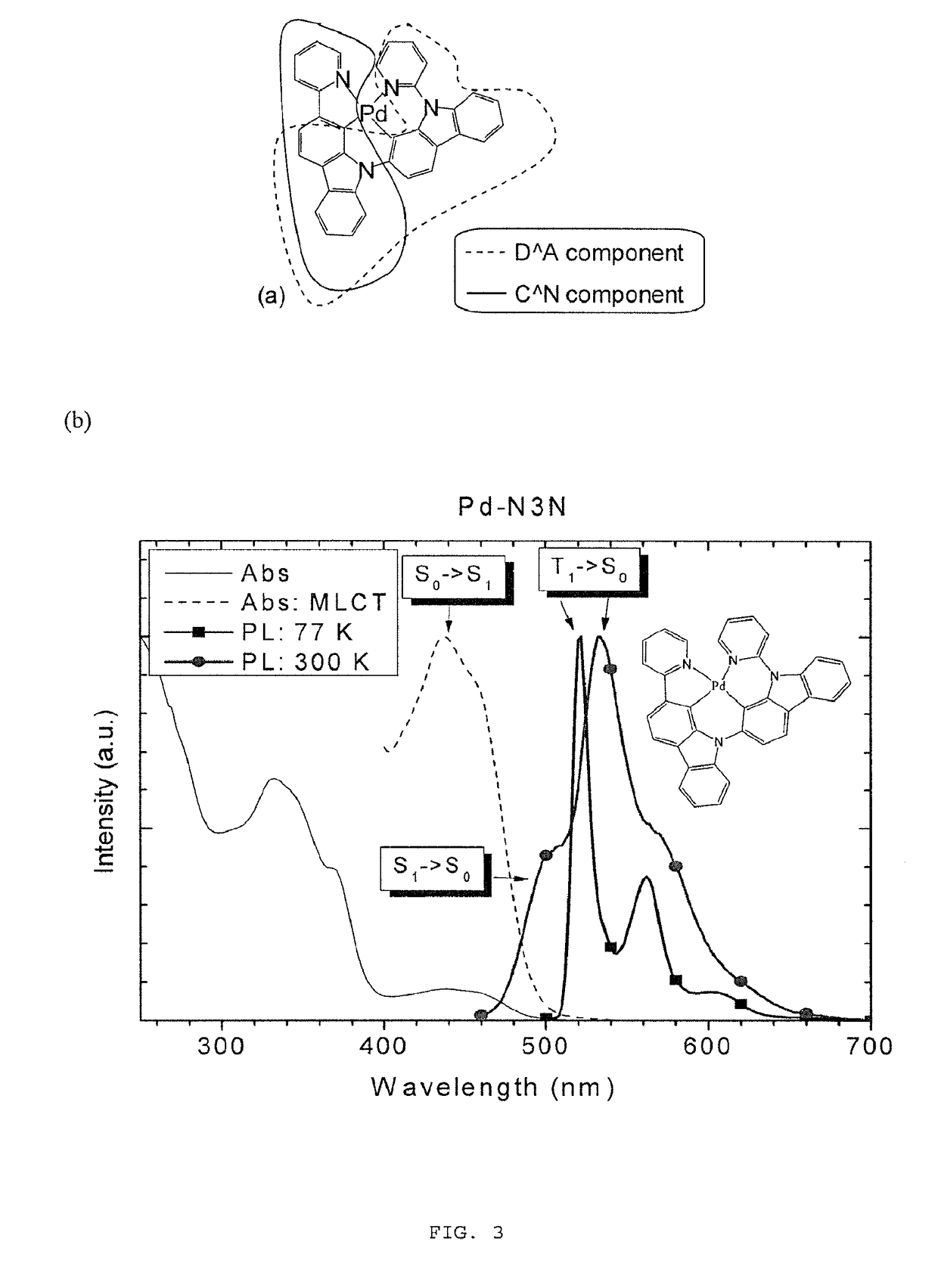
Metal complexes that exhibit multiple radiative decay mechanisms, together with methods for the preparation and use thereof.
Owner:ARIZONA STATE UNIVERSITY
Metal complexes useful in metathesis and other reactions
This invention provides metal complexes being useful as catalyst components in metathesis reactions and in reactions involving the transfer of an atom or group to an ethylenically or acetylenically unsaturated compound or another reactive substrate and, with respect to a sub-class thereof, for the polymerisation of α-olefins and optionally conjugated dienes, with high activity at moderate tempera-tures. It also provides methods for obtaining polymers with very narrow molecular weight distribution by means of a living reaction. It also provides methods for making said metal complexes and novel intermediates involved in such methods. It further provides derivatives of said metal complexes which are suitable for covalent bonding to a carrier, the product of such covalent bonding being useful as a supported catalyst for heterogeneous catalytic reactions. It also provides a direct one-step synthesis of pyrrole, furan and thiophene compounds from diallyl compounds.
Owner:RIMTEC CORP
Metal coordination compound, luminescence device and display apparatus
InactiveUS6733905B2Solid-state devicesSemiconductor/solid-state device manufacturingHydrogen atomPhenyl group
A metal coordination compound suitable as an organic material for a luminescent device is represented by the following formula (1):wherein M denotes Ir, Pt, Rh or Pd; n is 2 or 3; Y denotes an alkylene group having 2-6 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -C(O)-, -C(O)-O- or -O-C(O)- and capable of including a hydrogen atom which can be replaced with a linear or branched alkyl group which has 1-10 carbon atoms and is capable of including a hydrogen atom which can be replaced with fluorine atom; and CyN denotes a cyclic group containing a nitrogen atom connected to M and capable of having a substituent selected from the group consisting of a halogen atom; a nitro group; a phenyl group; a trialkylsilyl group having 1-8 carbon atoms; and a linear or branched alkyl group having 1-20 carbon atoms capable of including one or at least two non-neighboring methylene groups which can be replaced with -O-, -S-, -C(O)-, -C(O)-O-, -O-C(O)-, -CH=CH- or -C=C- and capable of including a hydrogen atom which can be replaced with a fluorine atom.
Owner:CANON KK
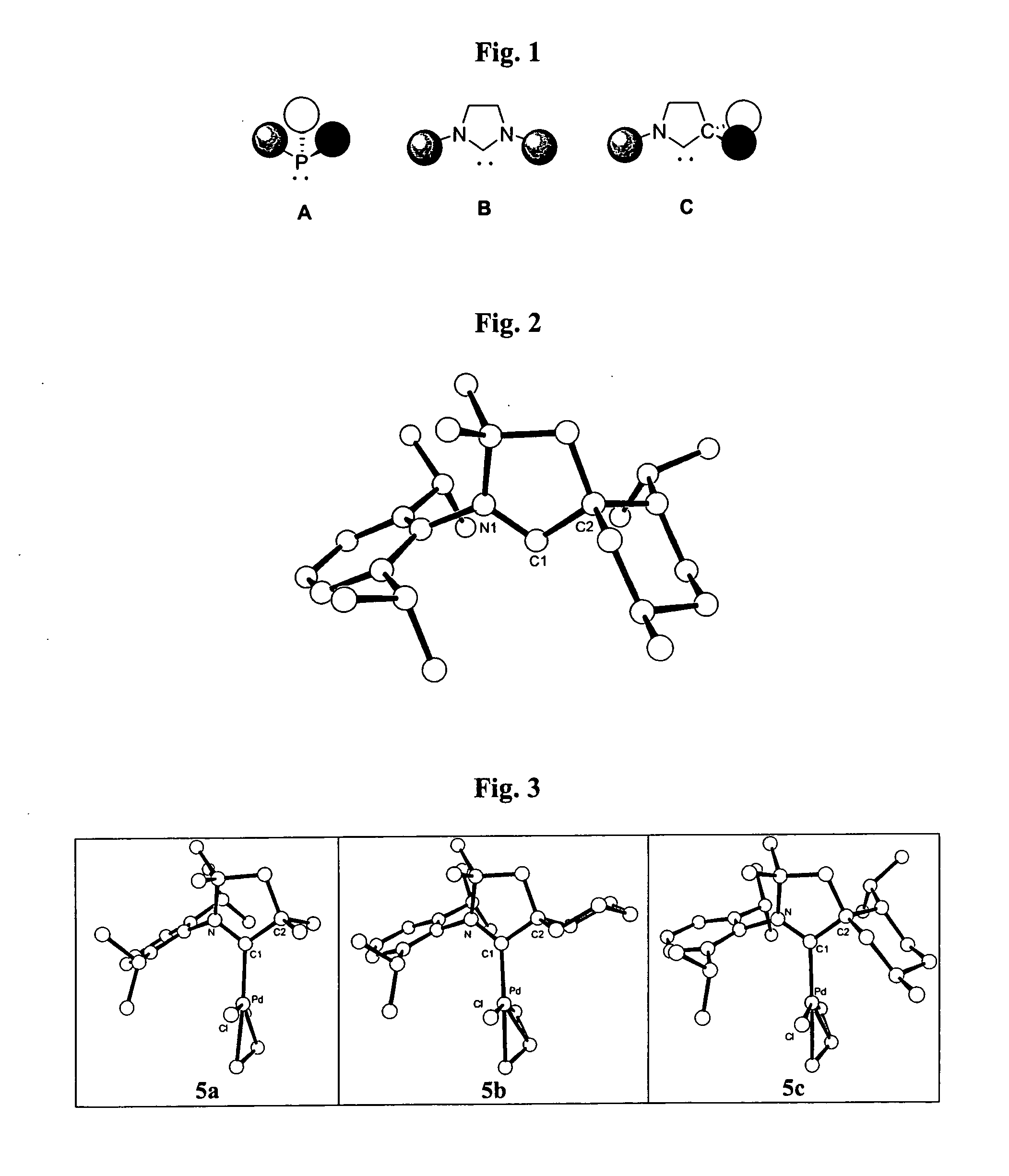
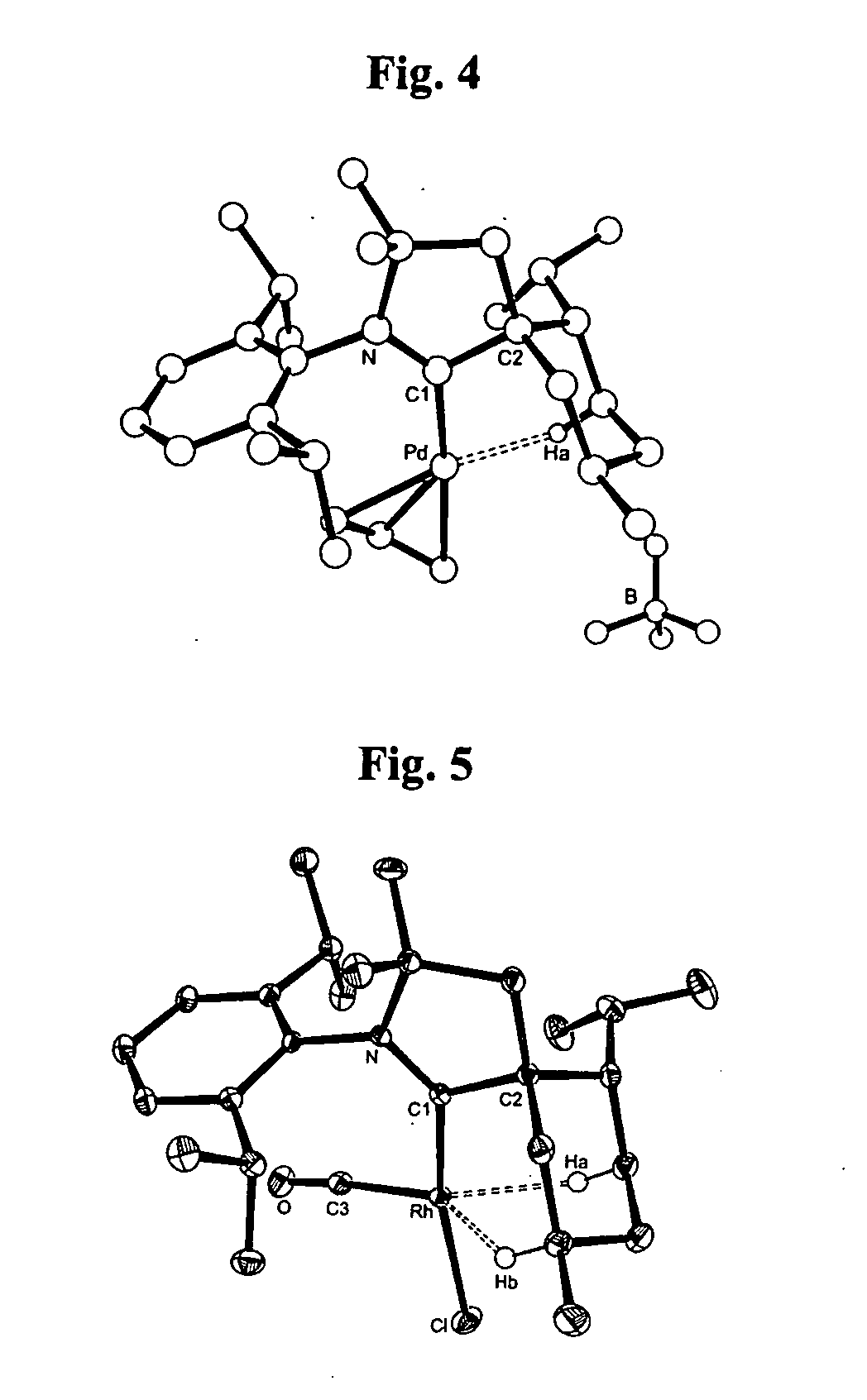
Stable cyclic (alkyl)(amino) carbenes as ligands for transition metal catalysts
Stable carbene ligands are provided having a carbene center flanked by a quaternary carbon and an amino group, and having utility in the preparation of various transition metal complexes.
Owner:RGT UNIV OF CALIFORNIA
Metal Complexes, Methods, and Uses Thereof
Metal complexes that exhibit multiple radiative decay mechanisms, together with methods for the preparation and use thereof.
Owner:ARIZONA STATE UNIVERSITY
Phosphinine compounds and metal complexes thereof
InactiveUS6818770B2Ruthenium organic compoundsOrganic compound preparationPhosphorineCoordination complex
Phosphinines of formula (I) can be combined with metal salts to prepare hydroformylation catalysts. The phosphinine complexes have two phosphorus centers that may be substituted with a variety of hetero atoms or alkyl substituents to modify the ligand characteristics of the phosphinine. Phosphinine metal complexes are employed under normal hydroformylation reaction conditions. The preparatory routes to the phosphinine ligands of formula (I) allow for their convenient synthesis.
Owner:EVONIK OXENO GMBH (DE)
Organic electroluminescent device
InactiveUS20060073360A1Indium organic compoundsRhodium organic compoundsOrganic layerOrganic electroluminescence
Disclosed is an organic electroluminescent device including at least one organic layer containing a luminescent layer between a pair of electrodes, wherein the organic layer contains at least one metal complex having a ligand with five or more coordination atoms.
Owner:UDC IRELAND
Stable cyclic (alkyl)(amino) carbenes as ligands for transition metal catalysts
Stable carbene ligands are provided having a carbene center flanked by a quaternary carbon and an amino group, and having utility in the preparation of various transition metal complexes.
Owner:RGT UNIV OF CALIFORNIA
Metal coordination compound, luminescence device and display apparatus
InactiveUS6797980B2Indium organic compoundsRhodium organic compoundsLuminescenceLight emitting device
A metal coordination compound suitable as an organic material for a luminescent device is represented by the following formula (1):
Owner:CANON KK
Heteroarylic-arylic diphosphines as chiral ligands
InactiveUS6153758ACarboxylic acid esters preparationNickel organic compoundsCarbon–carbon bondDiphosphines
PCT No. PCT / EP97 / 06358 Sec. 371 Date Apr. 18, 1999 Sec. 102(e) Date Apr. 18, 1999 PCT Filed Nov. 14, 1997 PCT Pub. No. WO98 / 22484 PCT Pub. Date May 28, 1998Diphosphines of a mixed heteroarylic-arylic type, wherein the phosphine group carrying backbone is constituted by the interconnection of a five-atom heteroaromatic ring and a carbocyclic aromatic ring, forming an atropoisomeric chiral system with a C1 symmetry. Said chiral diphosphines are advantageously used as ligands for the formation of chiral complexes with transition metals, in particular Ru, Rh, Pd, Ir, Ni. The so-obtained chiral complexes are used as chiral complexes are used as chiral catalysts for stereocontrolled reactions, in particular diastereo and enantioselective reduction reactions, hydroformylation reactions, hydrosilylation reactions, hydrocyanation reactions, double-bond isomerisation reactions, other reactions of carbon-carbon bond formation.
Owner:CHEMI SPA
Novel phosphinine compounds and metal complexes thereof
Phosphinines of formula (I) can be combined with metal salts to prepare hydroformylation catalysts. The phosphinine complexes have two phosphorus centers that may be substituted with a variety of hetero atoms or alkyl substituents to modify the ligand characteristics of the phosphinine. Phosphinine metal complexes are employed under normal hydroformylation reaction conditions. The preparatory routes to the phosphinine ligands of formula (I) allow for their convenient synthesis.
Owner:EVONIK OXENO GMBH (DE)
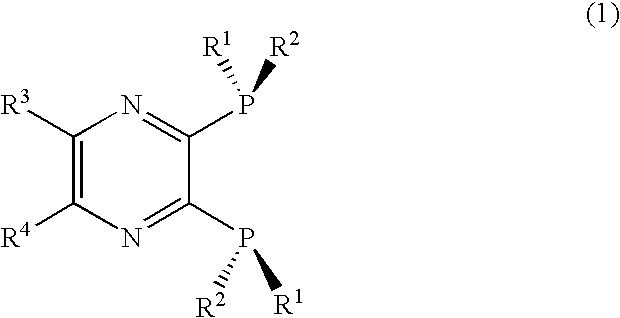
2, 3-Bis(dialkylphosphino)pyrazine derivative, process of producing the same, and metal complex having the same as ligand
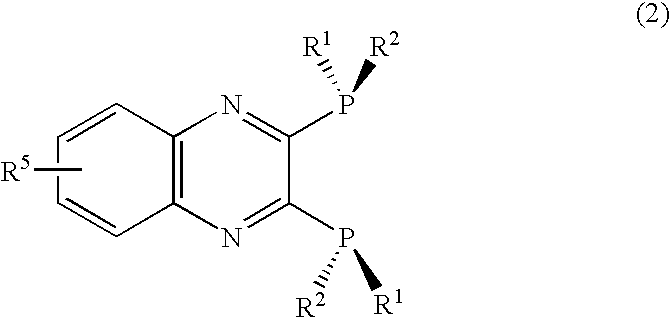
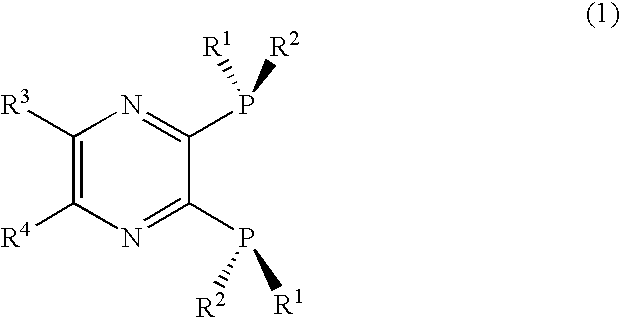
ActiveUS20070021610A1Silicon organic compoundsGroup 1/11 element organic compoundsQuinoxalineHydrogen atom
An optically active 2,3-bis(dialkylphosphino)pyrazine derivative represented by formula (1) is disclosed. The pyrazine derivative is preferably a quinoxaline derivative represented by formula (2). In formula (1) and (2), R1 is preferably a t-butyl or adamantyl group, and R2 is preferably a methyl group. wherein R1 is a substituted or unsubstituted, straight chain or branched alkyl group having 2 to 10 carbon atoms; R2 is a substituted or unsubstituted, straight chain or branched alkyl group having fewer carbon atoms than R1; and R3 and R4, which may be the same or different, are each a hydrogen atom or an alkyl group having 1 to 6 carbon atoms, or R3 and R4 are taken together to form a saturated or unsaturated ring. wherein R1 and R2 are as defined above; and R5 is a monovalent substituent.
Owner:CHIBA UNIVERSITY +1
Luminescence device and display apparatus
InactiveUS20060177694A1High efficiency luminescenceExtend device lifeIndium organic compoundsDischarge tube luminescnet screensLuminescenceHigh luminance
A luminescence device having a layer containing a metal coordination compound which has a partial structure. MLm of formula (2) below and is preferably entirely represented by formula (3) below: MLmL′n (3) wherein M denotes a metal atom of Ir, Pt, Rh or Pd; represent mutually different bidentate ligands; m is 1 or 2 or 3; n is 0 or 1 or 2 with the proviso that m+n=2 or 3; the partial structure MLm is represented by formula (2) below (wherein B is an isoquinolyl group bonded to the metal M with its N and including a position-1 carbon atom bonded to a cyclic group A which includes the C bonded to the metal M), and the partial structure ML′n is represented by formula (4), (5) or (6) shown below. There is provided a luminescence device capable of high-efficiency luminescence and long-term high luminance and adapted to red luminescence.
Owner:CANON KK
Phosphorus-containing catalyst composition and hydroformylation process using the same
ActiveUS20070123735A1High catalytic activityControl N/I selectivityOrganic compound preparationRhodium organic compoundsAldehydeAlkene
Provided are a catalyst composition comprising a bidentate ligand, a monodentate ligand, and a transition metal catalyst and a process of hydroformylation of olefin compounds, comprising reacting the olefin compound with a gas mixture of hydrogen and carbon monoxide while being stirred at elevated pressures and temperatures in the presence of the catalyst composition to produce an aldehyde. The catalytic composition demonstrates the high catalytic activity and option control of selectivity to normal aldehyde or iso aldehyde (N / I selectivity) to a desired value.
Owner:LG CHEM LTD
Compound, electroluminescent element containing the same, illuminating device and display device
ActiveUS20090102370A1High external extraction quantum yieldLong life-timeGroup 5/15 element organic compoundsSolid-state devicesQuantum efficiencyDisplay device
Provided are a novel compound, an organic EL element which contains such novel compound and has a high external extraction quantum efficiency and a long service life, an illuminating apparatus and a display apparatus.
Owner:UDC IRELAND
Novel chiral phosphorus ligands
InactiveUS20120277455A1High enantioselectivityHigh reactivityRhodium organic compoundsPhosphorus organic compoundsAsymmetric hydrogenationCoordination complex
Owner:BOEHRINGER INGELHEIM INT GMBH
Hydroformylation reaction method by using rhodium-ruthenium bimetal and quadridentate phosphine ligand and catalyst
ActiveCN106824282APositive and lowPromote generationRhodium organic compoundsOrganic compound preparationIsomerizationRuthenium
The invention discloses a long-chain internal olefin isomerization / hydroformylation homogeneous catalytic reaction method and a catalyst. According to the method, a rhodium-ruthenium bimetal complex is used as the catalyst, and the ligand adopts a quadridentate phosphine ligand. The catalytic system can be used for homogeneous internal olefin isomerization and hydroformylation reaction under the conditions of certain temperature and pressure. The method is suitable for long-chain internal olefins (>=C8) and internal olefins (<C8), and is a high-normal / isomeric ratio homogeneous bimetal-catalyzed reaction method.
Owner:WUHAN CATALYS TECH CO LTD
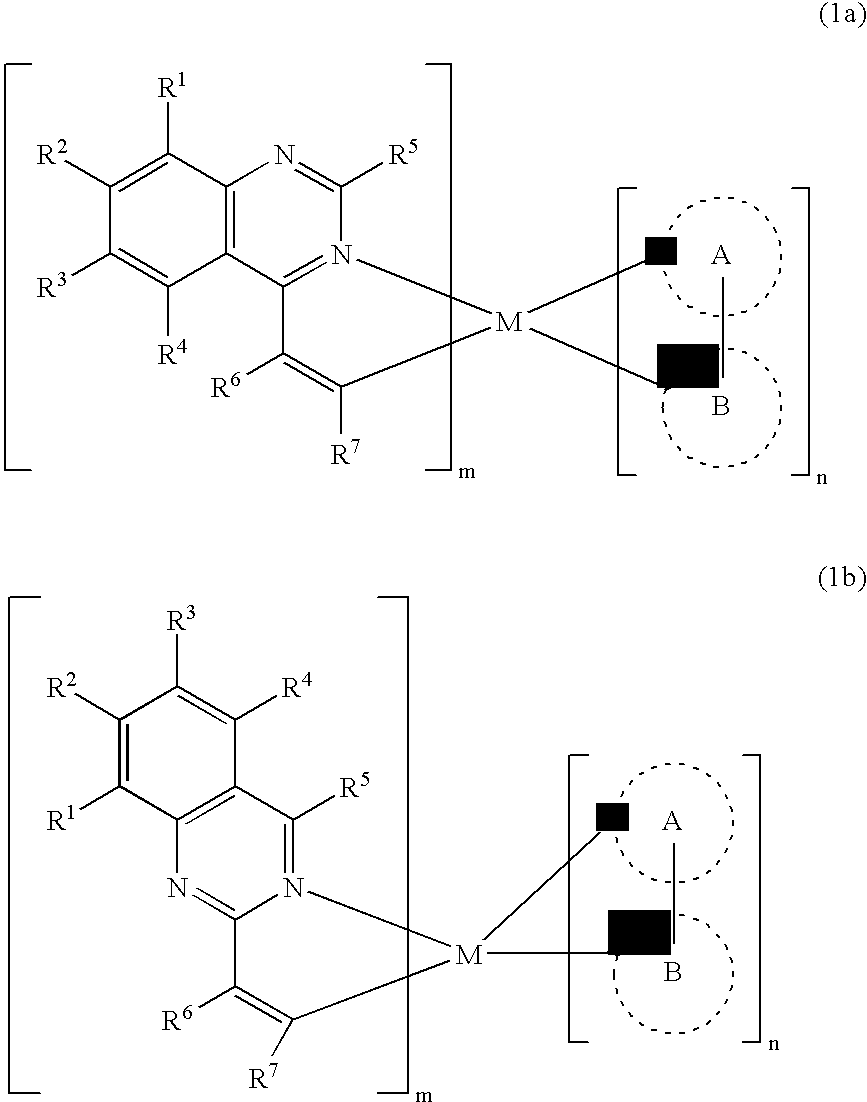
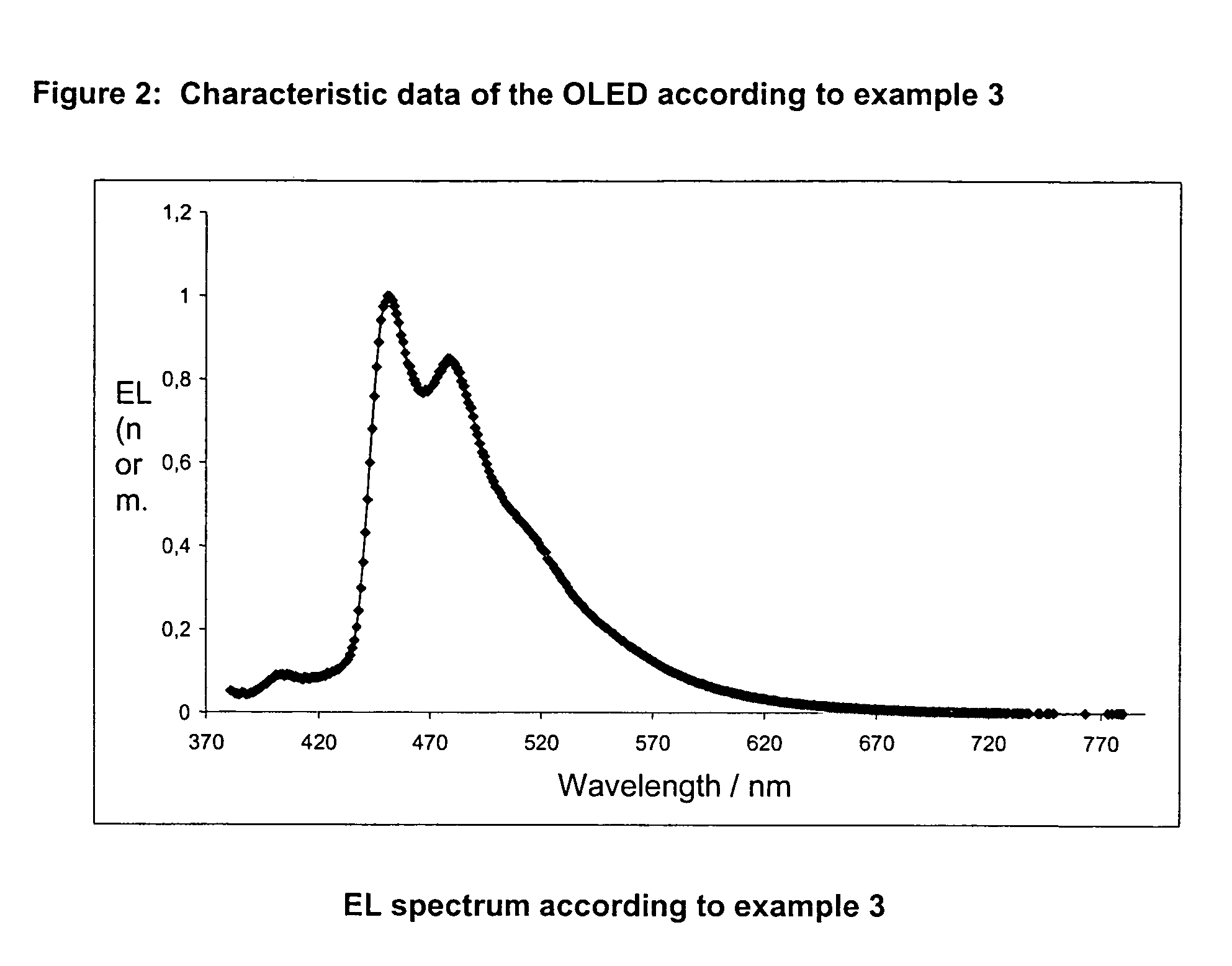
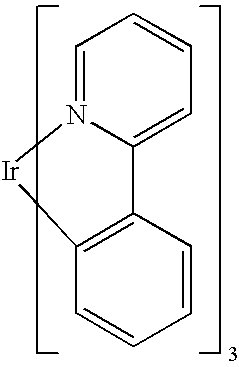
Rhodium complexes and iridium complexes
The present invention describes novel organometallic compounds which are phosphorescence emitters. Such compounds can, as active constituents (functional materials), be used in a number of different applications, which in the broadest sense can be classed as belonging to the electronics industry. The compounds according to the invention are described by the formulas (I), (Ia), (II), (IIa), (III), (IIIa), (IV) and (IVa).
Owner:MERCK PATENT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com