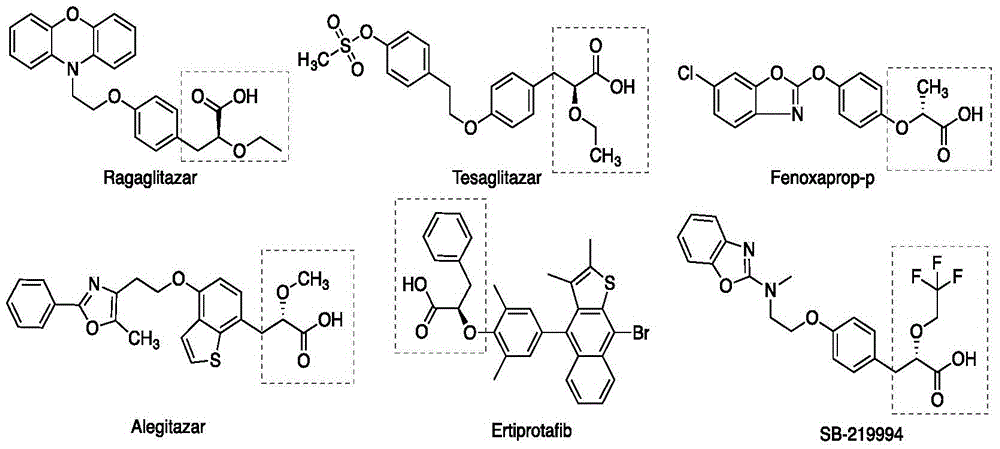
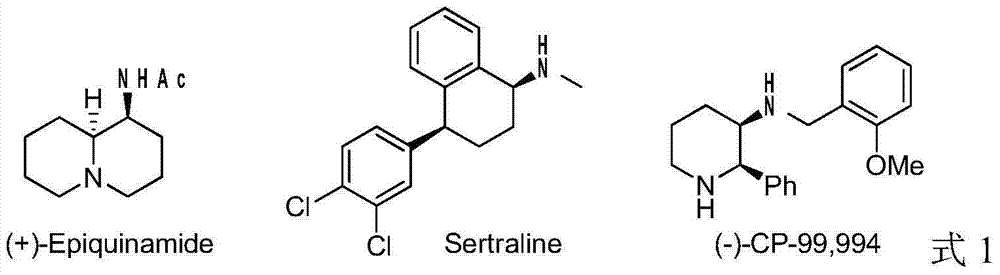
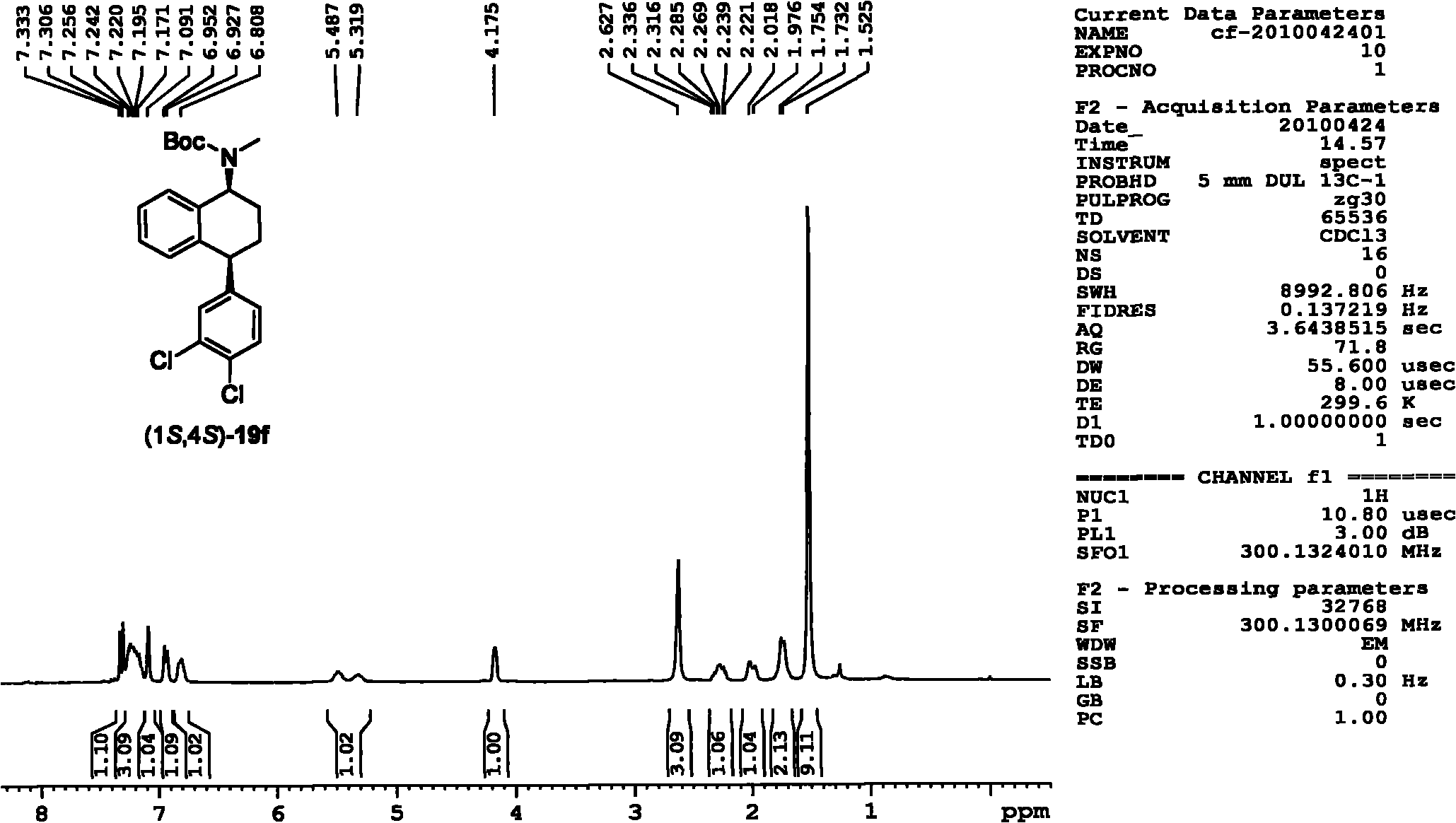
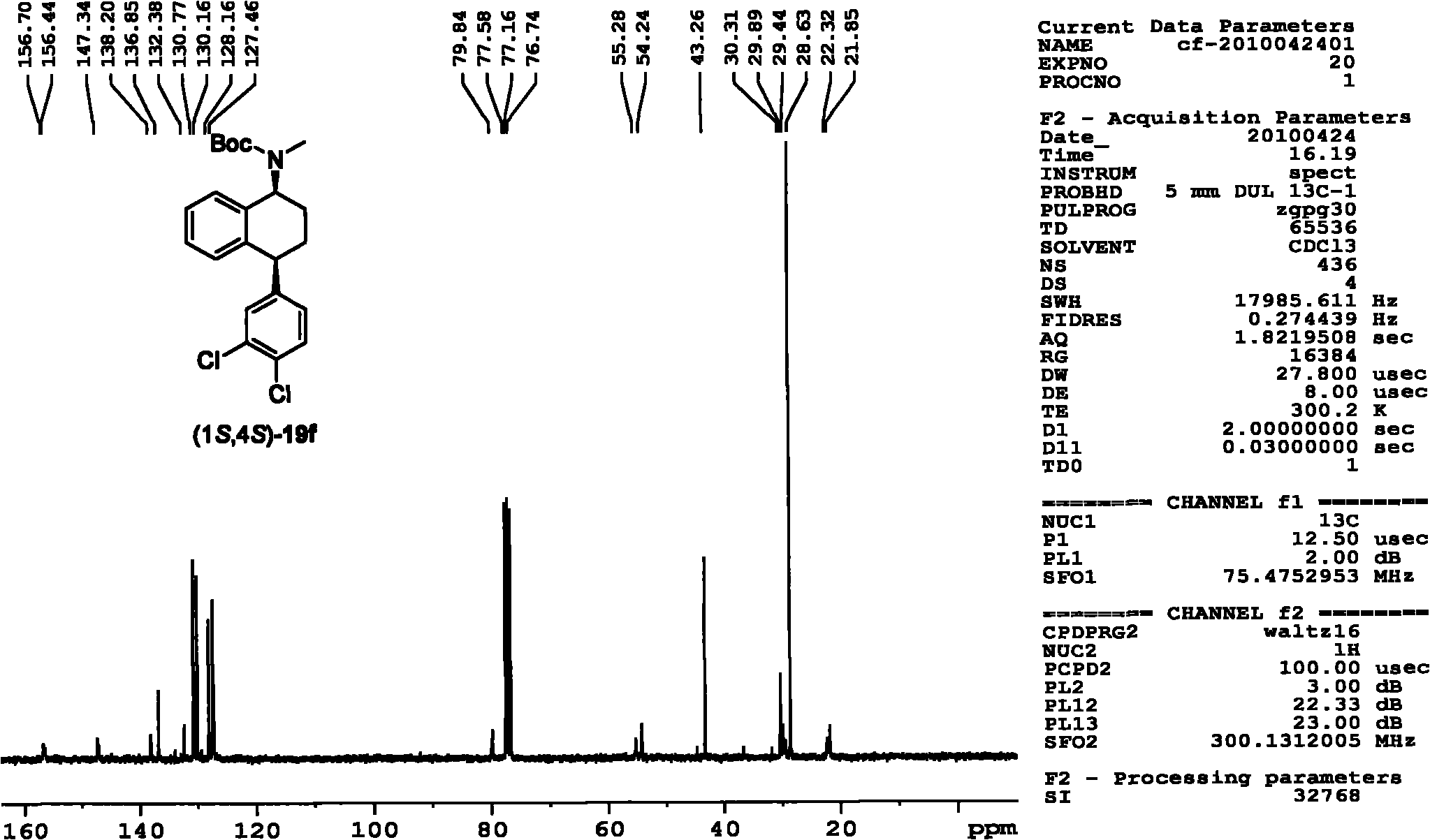
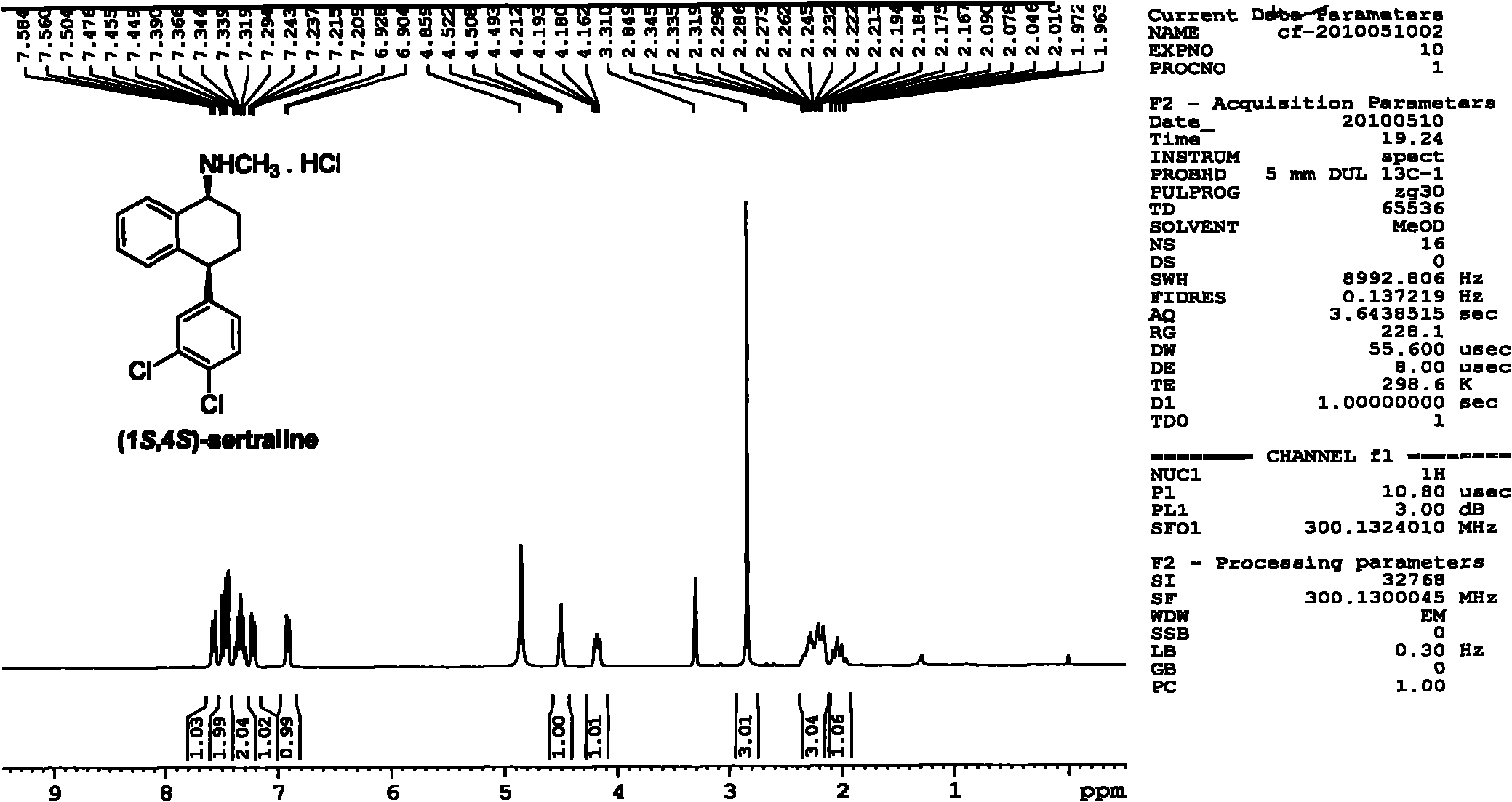
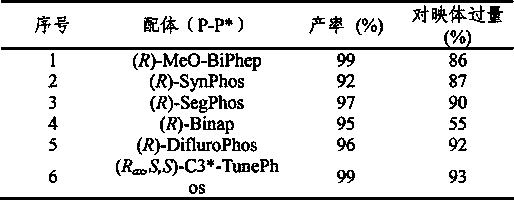
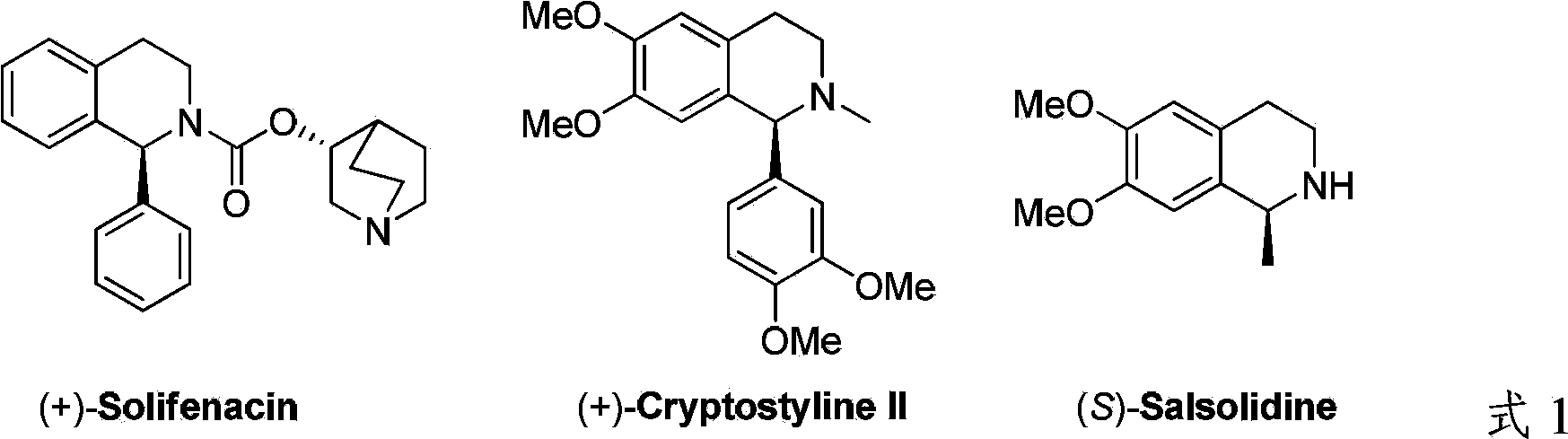
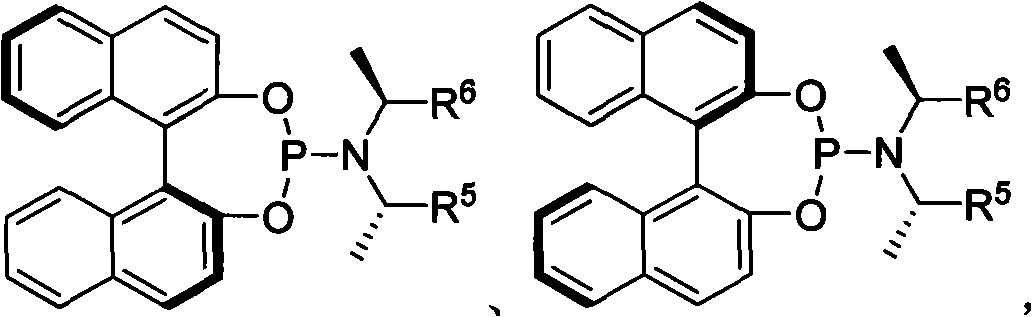
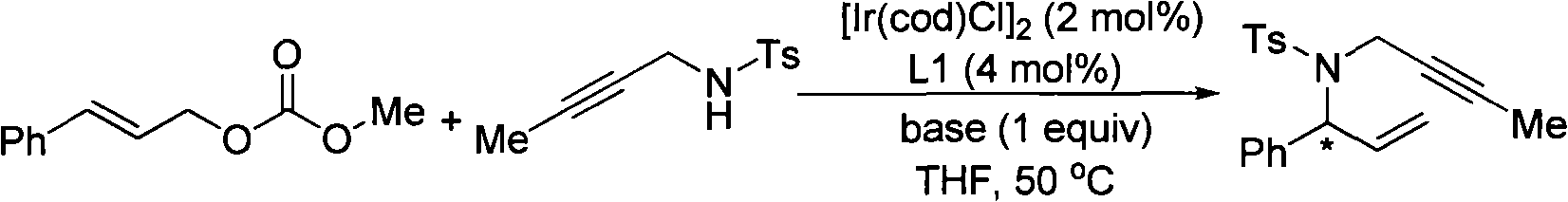
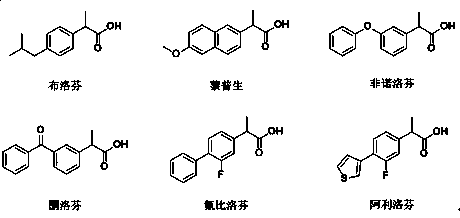
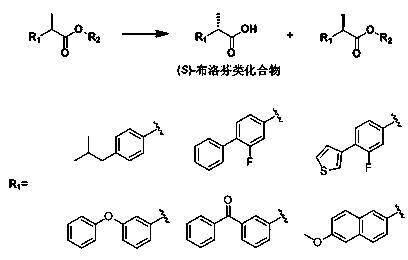
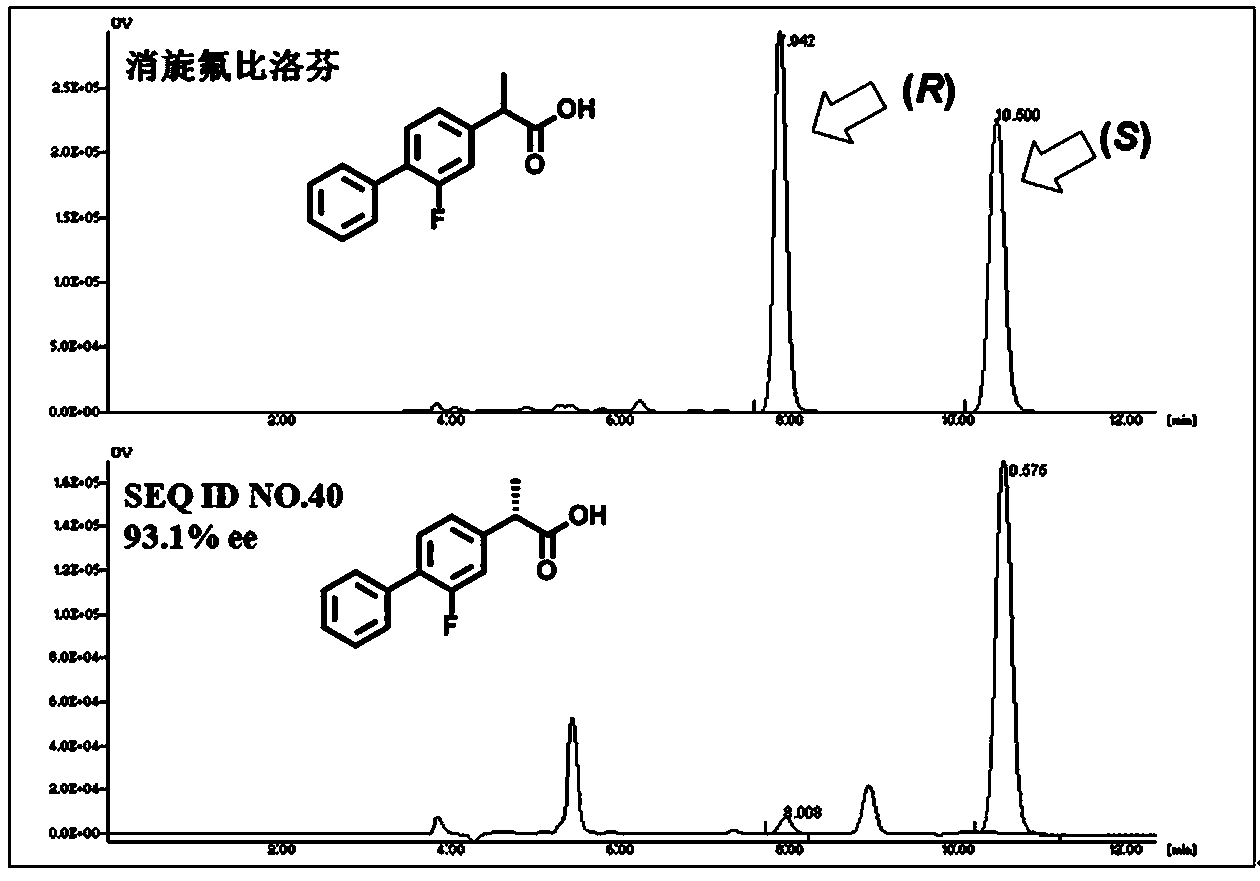
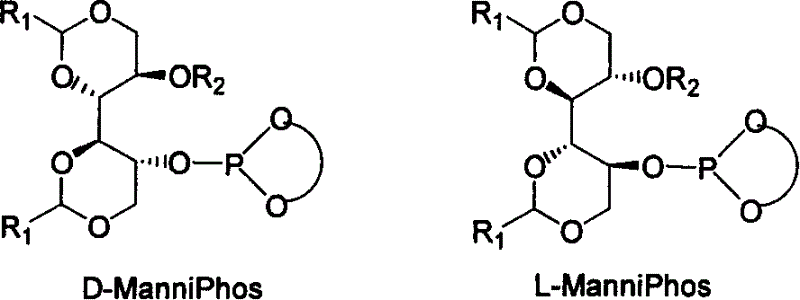
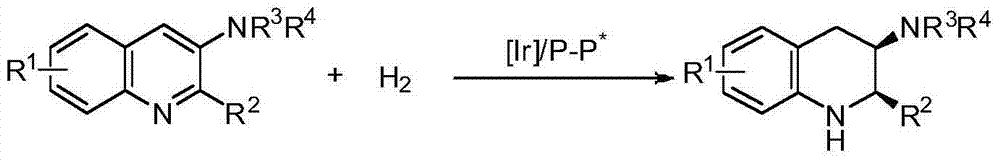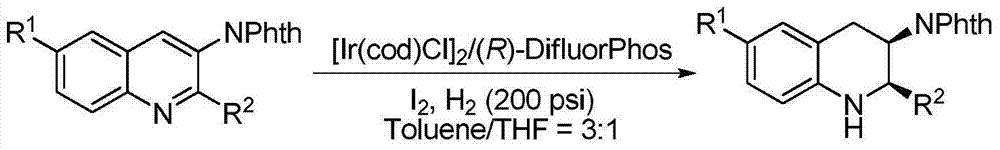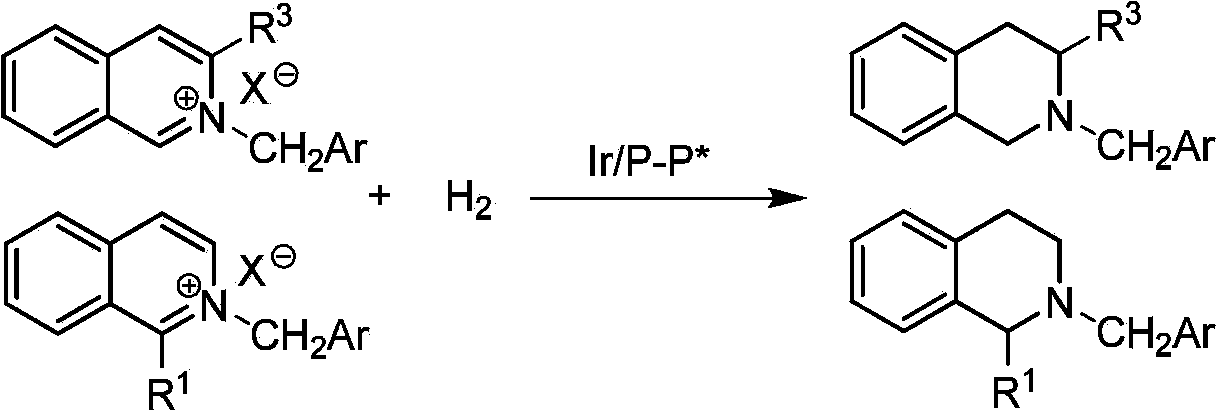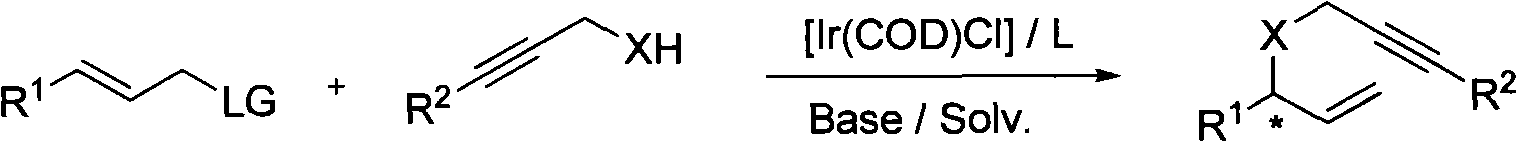Patents
Literature
902results about How to "High enantioselectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stereoselective alternating copolymerization of epoxide with carbon dioxide
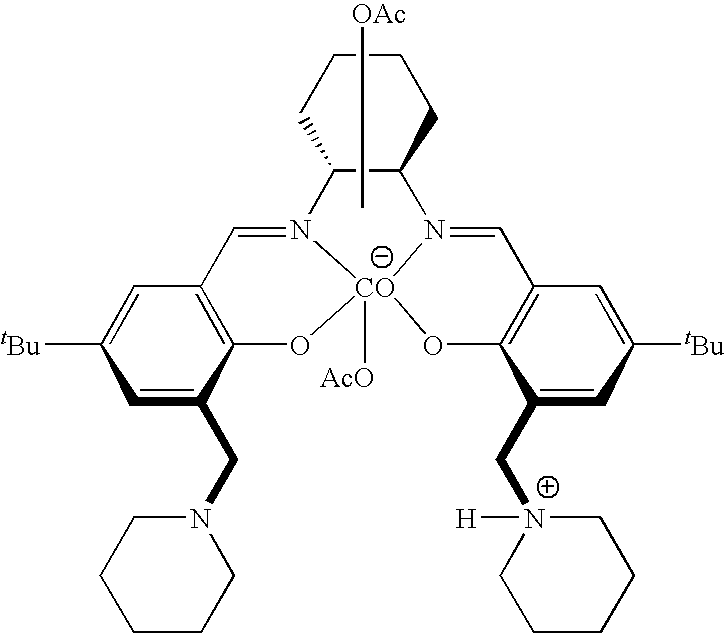
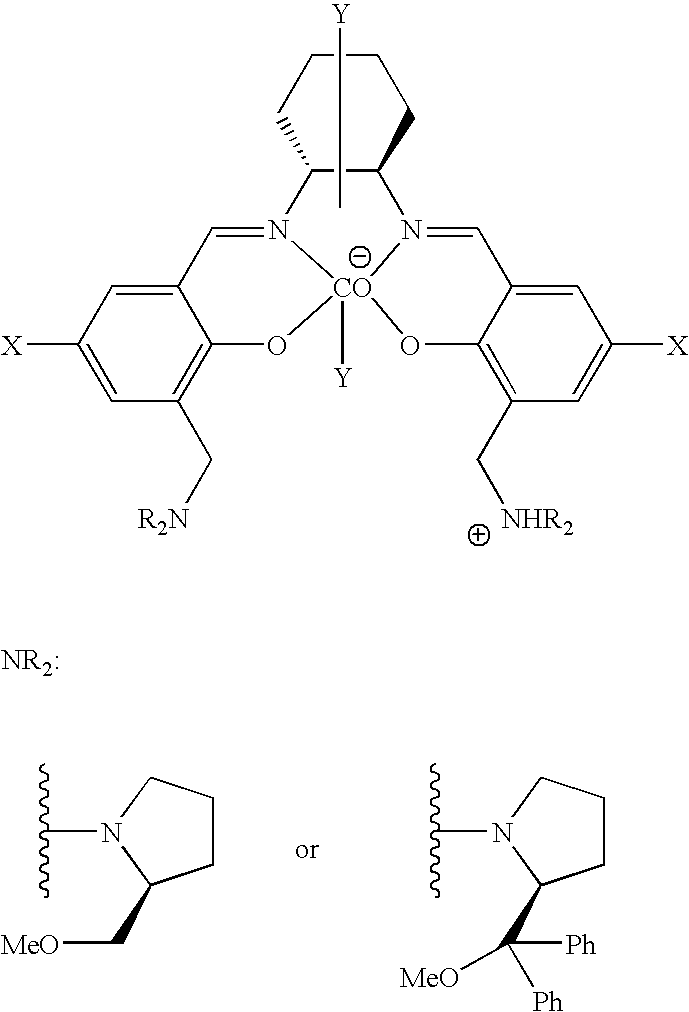
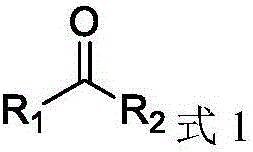
The present invention provides a method for manufacturing polycarbonate, which has a high conversion rate of a propylene oxide material into a polymer and can control stereoregularity of the macromolecular structure, and a catalytic compound for the manufacturing method.The manufacturing method of the present invention is a method for manufacturing a polycarbonate copolymer by copolymerizing an epoxide compound as a monomer with carbon dioxide in the presence of a planar tetracoordinate-type cobalt-Schiff base complex, wherein a ligand of the Schiff base is N,N′-bis(2-hydroxybenzylidene)ethylenediamine, N,N′-bis(2-hydroxybenzylidene)phenylenediamine, or a derivative thereof, and a methyl group substituted with an amino group having an asymmetrical carbon atom or an asymmetrical axis is introduced to the 3- and / or 3′-position of the benzene ring derived from the salicyl group. In addition, the catalytic compound of the present invention is a cobalt-Schiff base complex, wherein a methyl group substituted with an amino group having an asymmetrical carbon atom or an asymmetrical axis is introduced to the 3- and / or 3′-position of the salicyl group.
Owner:THE UNIV OF TOKYO
Spiro-diphosphine ligand
InactiveCN1439643AHigh stereoselectivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideDiphosphines
A spirocyclo-biphosphine ligand is prepared from spirocyclo-biphenol through esterifying by trifluoro methylsulfonic acid anhydride, coupling with diaryloxyphosphine under catalysis of Pd, and reduction reacting on trichlorosilane. It includes d-and levo-spricyclo-biphosphine ligands, whose mixture is dl-spirocyclo-biphosphine ligand. It can be used for asymmetrical catalytic hydrogenation reaction of latent chiral ketone with high stereo selectivity and e.e. value up to 99.5%.
Owner:NANKAI UNIV
Method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis
ActiveCN102559520AHigh excessLess side effectsFungiMicroorganism based processesPhosphateReaction temperature
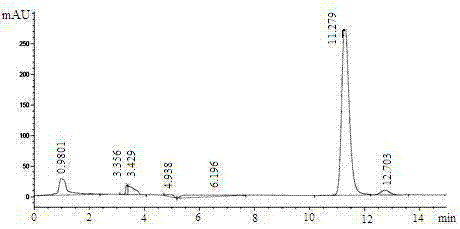
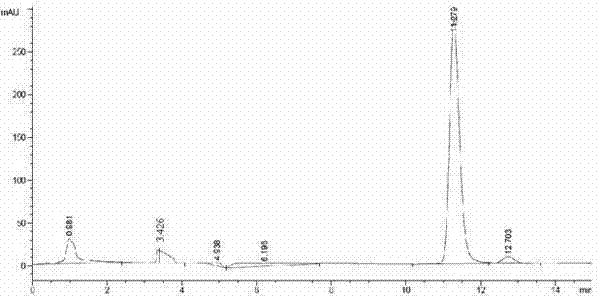
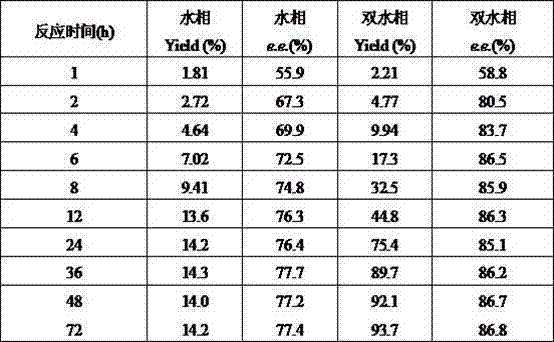
The invention relates to a method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis, and belongs to the technical field of biological catalysis. According to the method, 4-chlorphenyl-(pyridine-2-yl)-ketone is subjected to asymmetrical reduction by utilizing kluyveromycessp (CCTCCM 2011385) whole cells to synthesize the (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol. The method comprises the following steps of: sieving a microbe which has high-stereoselectivity carbonyl reductase activity on a prochiral ketone substrate, determining a series of conditions of asymmetrical reduction reaction, such as reaction temperature, pH, the concentration of cells, the concentration of the substrate and reaction time and additives (including various secondary solvents, polyethyleneglycol (PEG), phosphates and the like), wherein the enantiomer excess value and yield of the (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol serving as a product can reach 86.7 percent e.e and 92.1. The product is separated and extracted initially by silicagel column chromatography, so that the purity of the separated product is 99.2 percent, and the extraction yield is 56.7 percent.
Owner:JIANGNAN UNIV
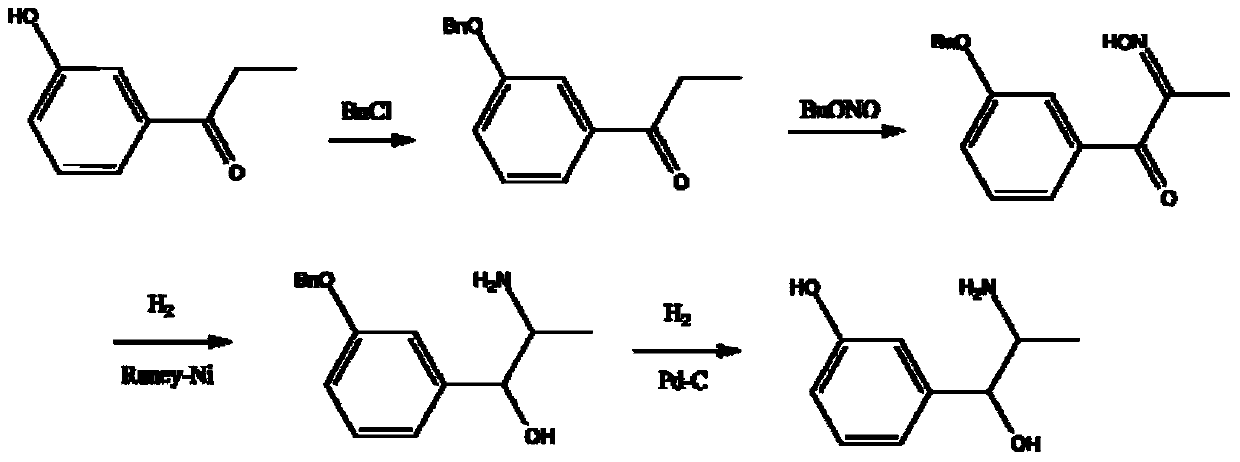
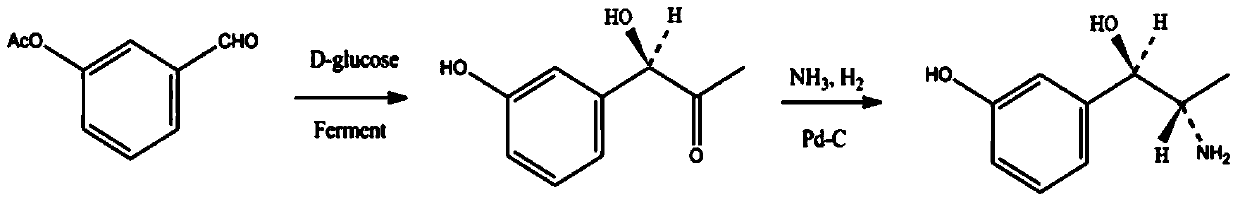
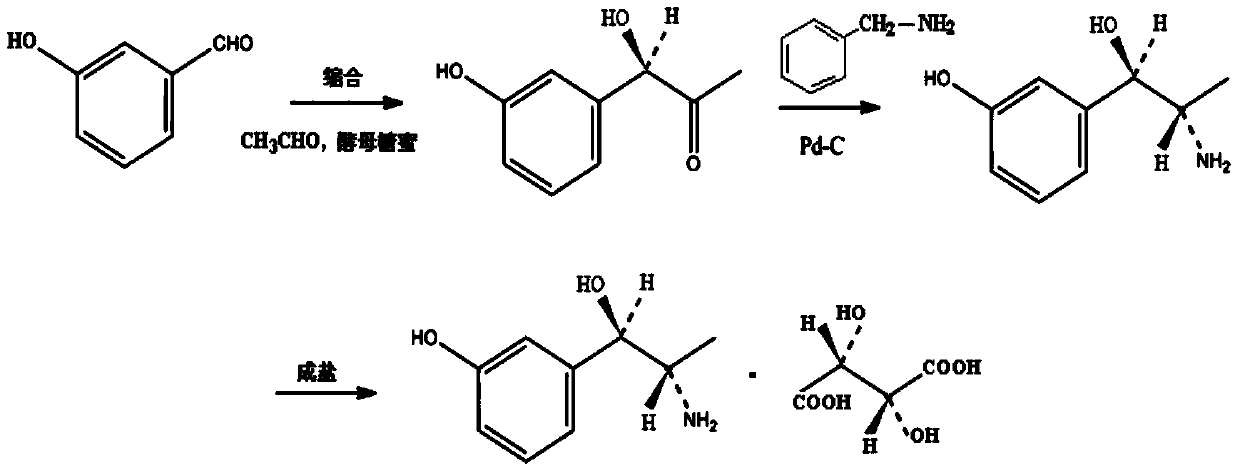
Synthesis method of metaraminol bitartrate
ActiveCN103739504AEasy to controlFew synthetic stepsOrganic compound preparationCarboxylic acid salt preparationSynthesis methodsSpatial configuration
The invention discloses a synthesis method of metaraminol bitartrate, and in particular provides a method for synthesizing metaraminol bitartrate by using a chiral catalysis method. The synthesis method comprises the steps: catalyzing a chiral addition reaction of hydroxybenzaldehyde and nitroethane by using a chiral catalyst system consisting of cinchona alkaloid, copper acetate hydrate and less imidazole to obtain an addition product with a dominant required spatial configuration, and then reducing nitro by using hydrogen in the presence of Pd-C to obtain amine to obtain aramine, and salifying the aramine with L(+)-tartaric acid to obtain a final product metaraminol bitartrate. According to the synthesis method, an enzyme catalyst is prevented from being used, a raw material of the synthesis reaction is easily available, the chiral catalyst is easily purchased or prepared self, the synthesis steps are relatively less, the chiral control efficiency is higher, the enantioselectivity is high, the yield is good, the reaction operation is easily controlled, and is safe and reliable, and the foundation is laid for the later industrialized amplification production.
Owner:广州普星药业有限公司
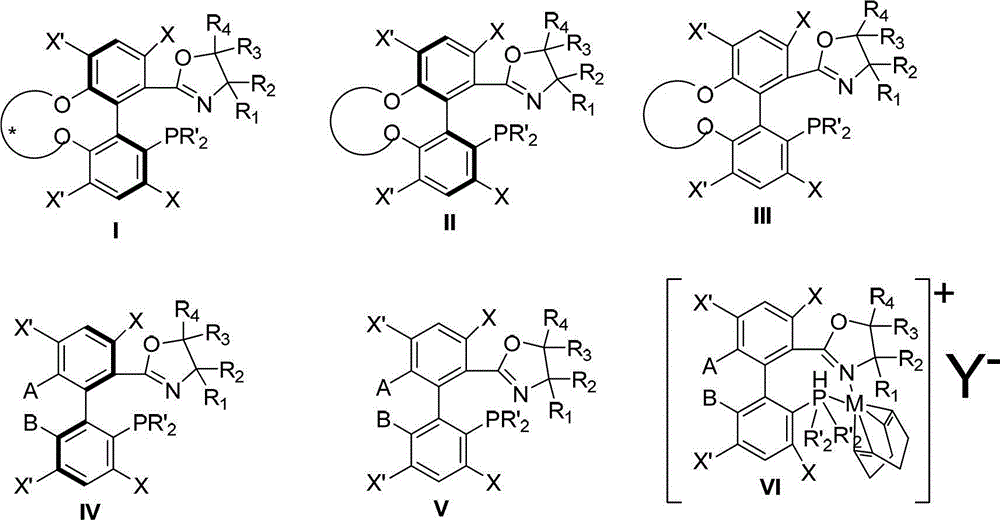
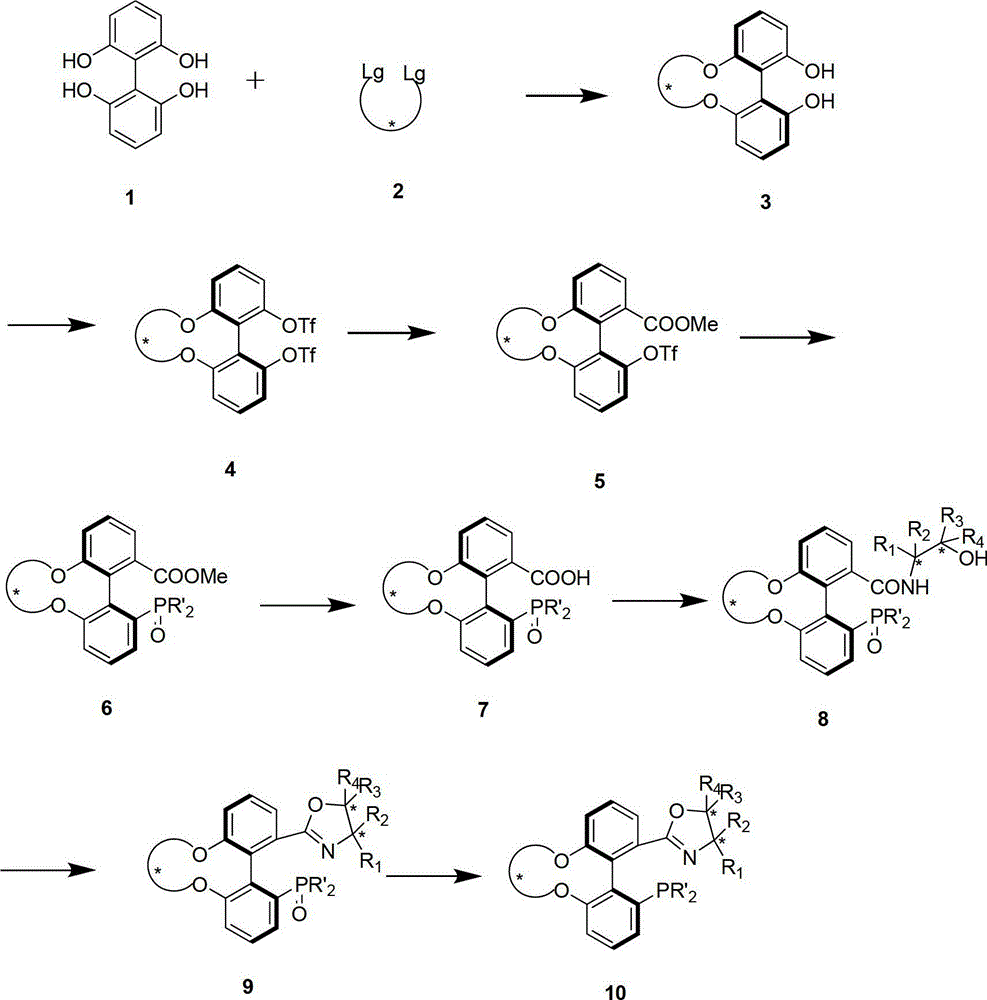
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
Spiro phosphine-oxazoline and preparation method and application thereof
ActiveCN101565434AHigh activityHigh enantioselectivityRuthenium organic compoundsIndium organic compoundsIridiumIon exchange
The invention belongs to spirophosphine-oxazoline and a preparation method and an application thereof, in particular to novel spirophosphine-oxazoline and a preparation method of iridium complex thereof. The novel spirophosphine-oxazoline is synthesized through two reactions by using substitutional 7-diarylphosphino-7'-carboxyl-1, 1'-spirobiindane as starting raw materials. Complexation and ion exchange are performed for the novel spirophosphine-oxazoline and iridium precursor, so as to obtain iridium / spirophosphine-oxazoline complex compound containing different anions. The invention overcomes the defects of the prior art, synthesizes the novel spirophosphine-oxazoline without substitutional group on oxazoline 4- position by using cheap aminoethanol as raw materials, can catalyze asymmetric hydrogenation reaction of Alpha-substituting acrylic acid, expresses higher activity and enantioselectivity and has higher research value and industrial prospect.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
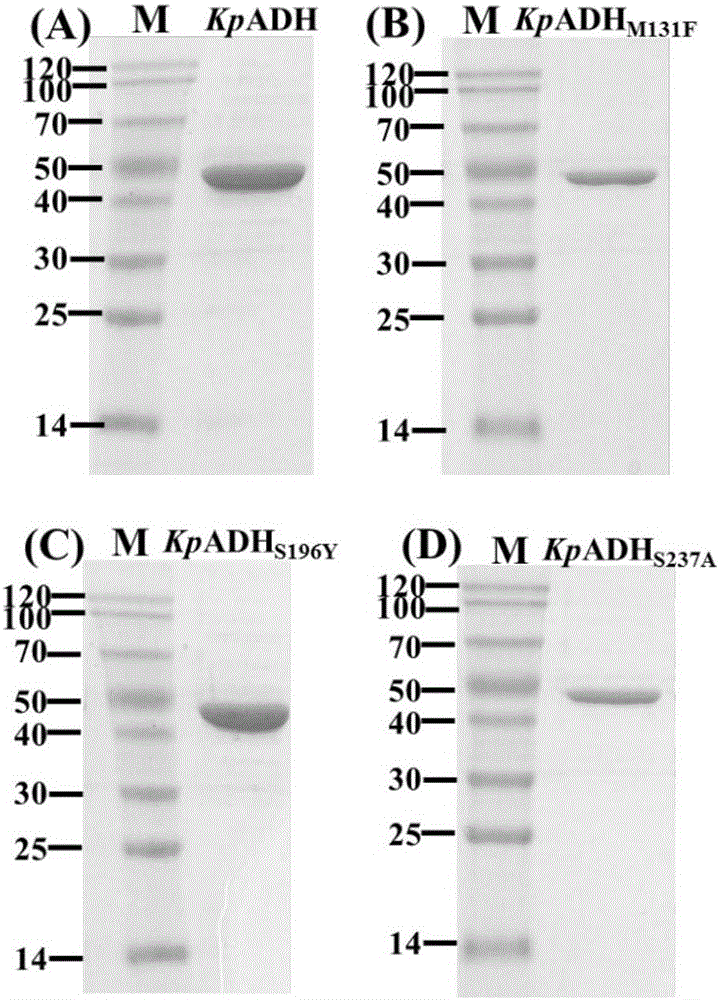
Alcohol dehydrogenase mutant, gene thereof, and application thereof in preparation of chiral diaryl alcohol
InactiveCN105936895AHigh catalytic activityHigh enantioselectivityOxidoreductasesGenetic engineeringReduction ActivityKluyveromyces sp.
The invention discloses an alcohol dehydrogenase mutant, an encoding gene thereof, and an application thereof in the preparation of chiral diaryl alcohol. The mutant is obtained through substituting serine in the 237th position of alcohol dehydrogenase from Kluyveromyces sp.CCTCC M2011385 and with the amino acid sequence represented by SEQ ID No.1 with alanine. The alcohol dehydrogenase mutant has greatly higher reduction activity and stereoselectivity than wild enzymes. The mutant is especially suitable for asymmetric reduction of diaryl ketone to prepare chiral diaryl alcohol, and can be used to synthesize various antihistamine medicines. The alcohol dehydrogenase mutant has good industrial application prospect.
Owner:JIANGNAN UNIV
Phosphine ligand and enantiomer or racemic body thereof and preparation methods thereof
ActiveCN102532196AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsIridiumSynthesis methods
The invention discloses a phosphine ligand and an enantiomer or a racemic body thereof and preparation methods and applications thereof. The structural formulae of the phosphine ligand and the enantiomer or the racemic body are shown in the specifications; a phosphine ligand compound has a novel framework; complete transfer of planar chirality to axial chirality in a synthesis process is realized through a desymmetrization reaction; a synthesis method is simple and economical; during preparation of a chiral ligand, common complex chiral splitting processes are avoided; and an obtained chiral ligand has the advantages of high reaction activity, high enantioselectivity and the like in a model reaction, can be applied to catalytic reactions of a plurality of metals such as palladium, rhodium, nickel, copper, iridium, ruthenium, iron, cobalt, gold, platinum and the like, and can have a very good catalytic effect.
Owner:SUN YAT SEN UNIV
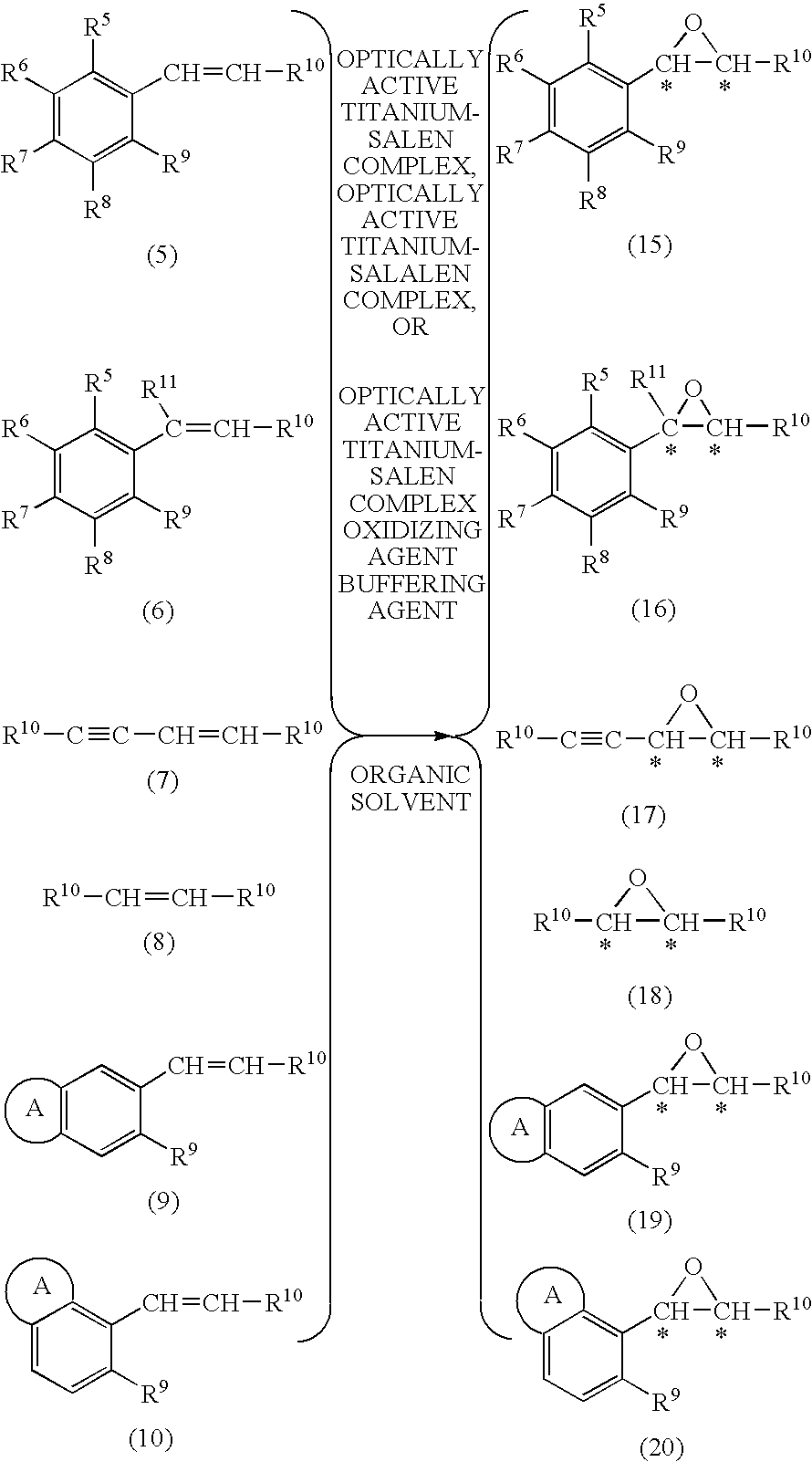
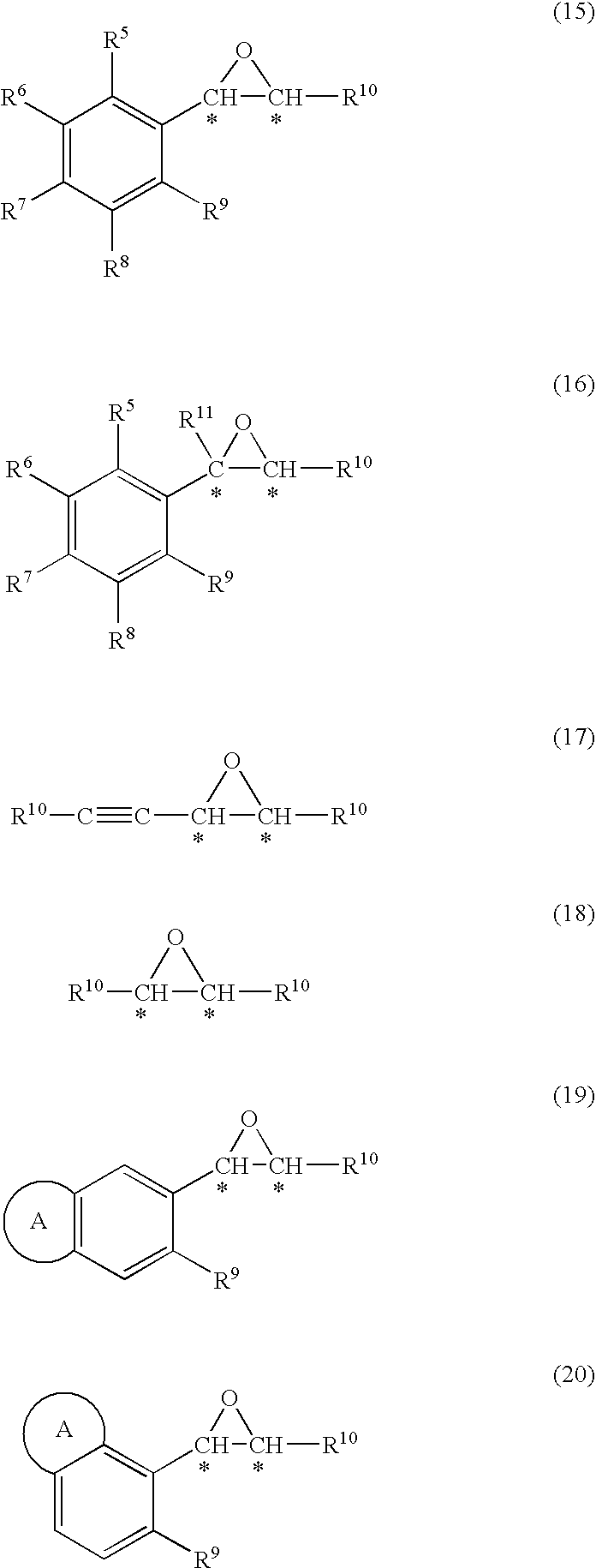
Process for production of optically active epoxy compound
InactiveUS20100081808A1Reduce the amount requiredHigh catalytic efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsState of artCatalyst degradation
[Problems] To provide an efficient process for producing an optically active epoxy compound.[Means For Solving Problems] The process for producing an optically active epoxy compound comprises asymmetrically epoxidizing an unsaturated compound with an oxidizing agent in the presence of an optically active titanium-salen complex, an optically active titanium-salalen complex or an optically active titanium-salan complex, with addition of a buffering agent or a buffer solution. The process can inhibit catalyst degradation, reduce the amount of the catalyst used in the reaction, and inhibit a by-product, compared with the prior art, and can provide an optically active epoxy compound in high chemical yield and optical yield and with high quality, and therefore is an industrially useful process.
Owner:NISSAN CHEM IND LTD
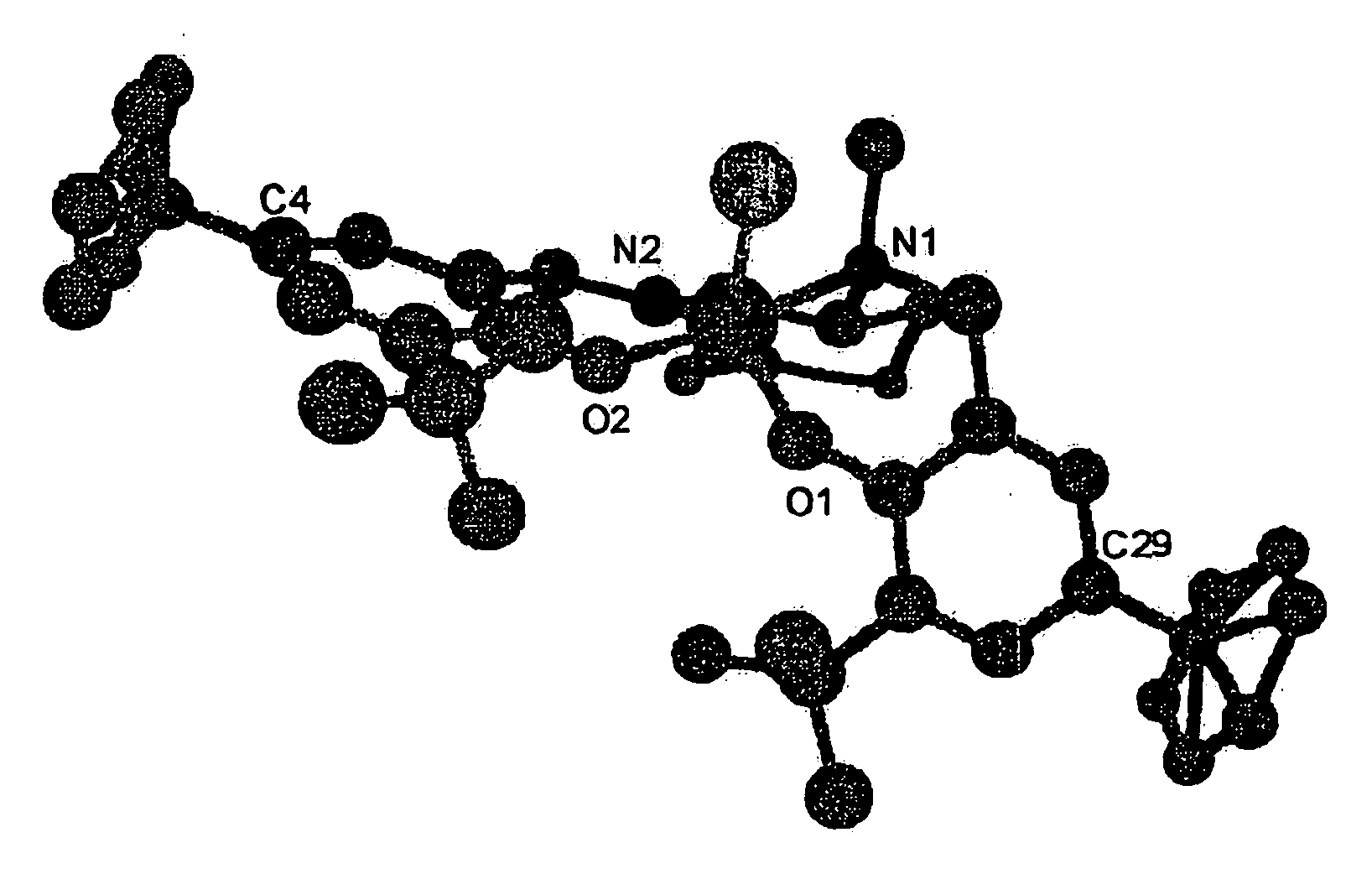
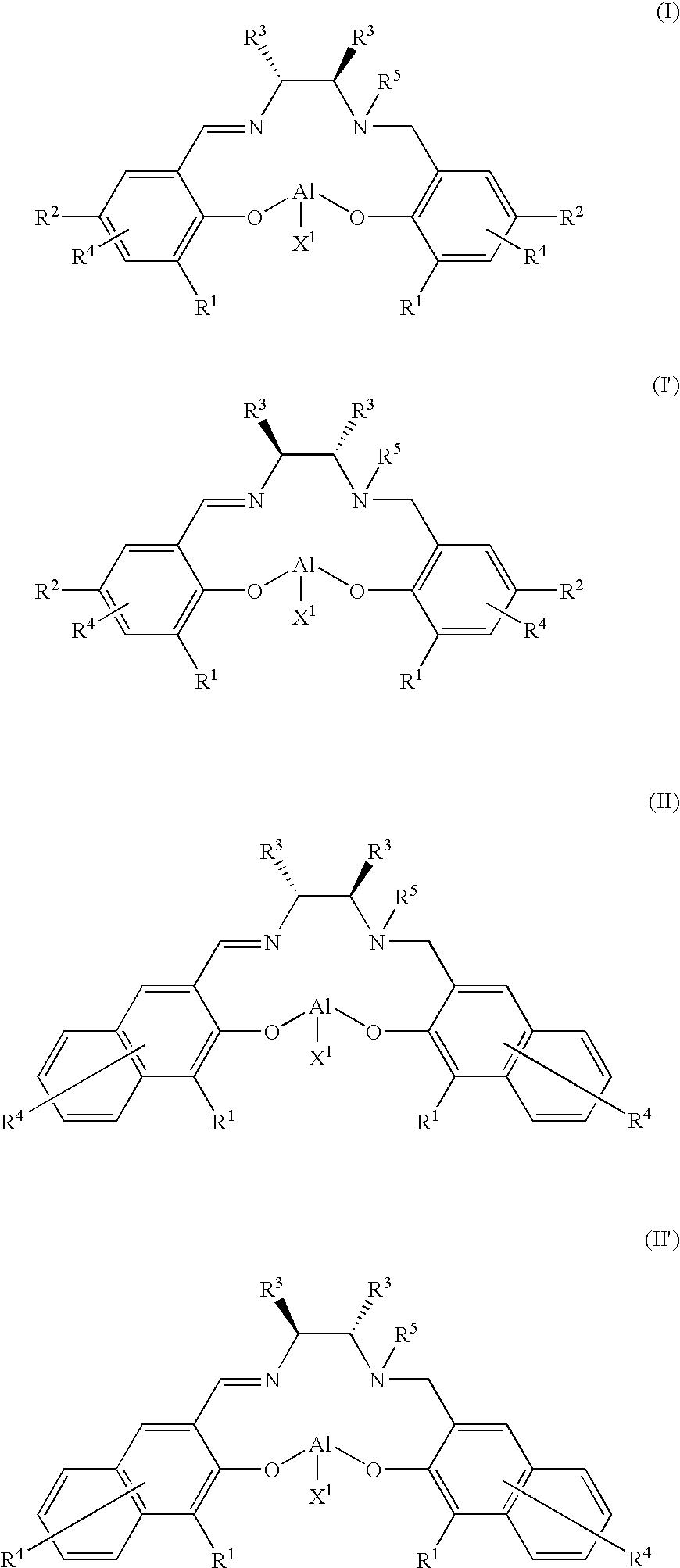
Optically Active Alpha-Hydroxyphosphonic Acid, Its Derivatives and Production Method thereof, Optically Active Aluminum (Salalen) Complex and Production Method Thereof, and Production Method of Salalen Ligand
InactiveUS20090099381A1High enantioselectivityEfficient CatalysisIsocyanic acid derivatives preparationOrganic compound preparationTosylhydrazoneEnantio selectivity
The present invention relates to a production method capable of producing an optically active α-hydroxyphosphonic acid and its derivatives with sufficiently high enantioselectivity not only for aromatic aldehydes but also for aliphatic aldehydes, and more specifically to a method of producing an optically active α-hydroxyphosphonic acid and its derivatives, characterized in that an optically active aluminum(salalen) complex represented by any one of the following formulae (I), (I′), (II) and (II′):[wherein R1s are each alkyl group or aryl group independently; R2s are each alkyl group or aryl group independently; R3s are each alkyl group or aryl group independently, and two R3s may bond with each other to form a ring; R4s are each hydrogen atom, halogen atom, alkyl group, alkoxy group, nitro group, or cyano group independently; R5 is alkyl group; and X1 is halogen atom, alkyl group, alkoxy group, acetoxy group or toluenesulfonyloxy group] is used as a catalyst to asymmetrically hydrophosphonylate an aldehyde with phosphonic acid or its derivatives.
Owner:JAPAN SCI & TECH CORP
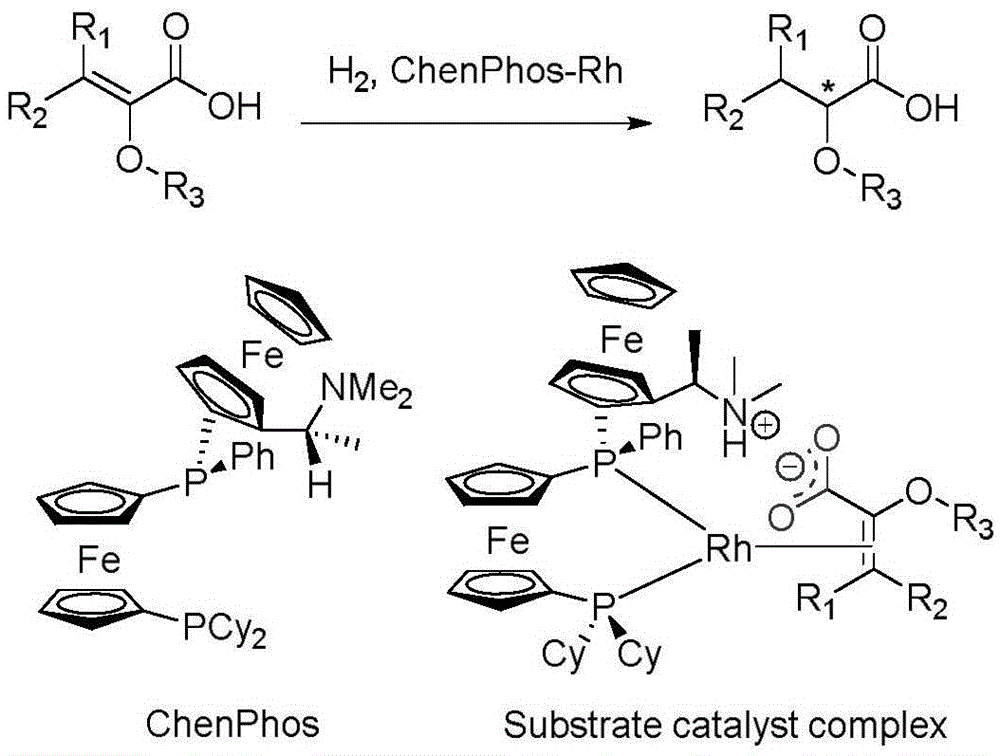
Asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid
InactiveCN105481622AHigh catalytic activityHigh enantioselectivityOrganic reductionOrganic compound preparationEnkephalinase inhibitorAsymmetric hydrogenation
The invention relates to an asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid. A metal complex containing ChenPhos chiral ligand is a catalyst high in conversion efficiency, and particularly, the catalyst can be used for synthesizing a core framework in enkephalinase inhibitor Sacubitril through asymmetric hydrogenation. The inhibitor is one of components of medicine LCZ 696 approved by American Food and Drug Administration. The asymmetric hydrogenation method of the alpha-oxo-alpha, beta-unsaturated carboxylic acid is efficient, and the application range of substrate is wide.
Owner:WUHAN CATALYS TECH CO LTD
Chiral spiro phosphine-nitrogen-sulfur tridentate ligand and preparation method and application thereof
ActiveCN104892672AHigh catalytic activityHigh enantioselectivityCarbamic acid derivatives preparationIndium organic compoundsIridiumSulfur
The invention relates to a chiral spiro phosphine-nitrogen-sulfur tridentate ligand and a preparation method and application thereof. The chiral spiro phosphine-nitrogen-sulfur tridentate ligand is a compound with a formula I or formula II, or a racemate or optical isomer thereof, or a catalytically-acceptable salt thereof, and has the main structure characteristic of having a chiral spiro indan skeleton and a sulfoether group. The chiral spiro phosphine-nitrogen-sulfur tridentate ligand can be synthesized from chiral starting materials of 7-diaryl / alkyl phosphino-7'-amino-1,1'-spiro indan compound with a spiro skeleton. The chiral spiro phosphine-nitrogen-sulfur tridentate ligand and transition metal salt form a complex, which can be used in catalysis of an asymmetric catalytic hydrogenation reaction of a carbonyl compound. Especially, the iridium complex shows high catalytic activity (catalyst amount of 0.0002% mol) and enantioselectivity (up to 99.9%ee) in asymmetric hydrogenation reaction of beta-alkyl-beta-keto ester, and has practical value.
Owner:ZHEJIANG RAYBOW PHARM CO LTD
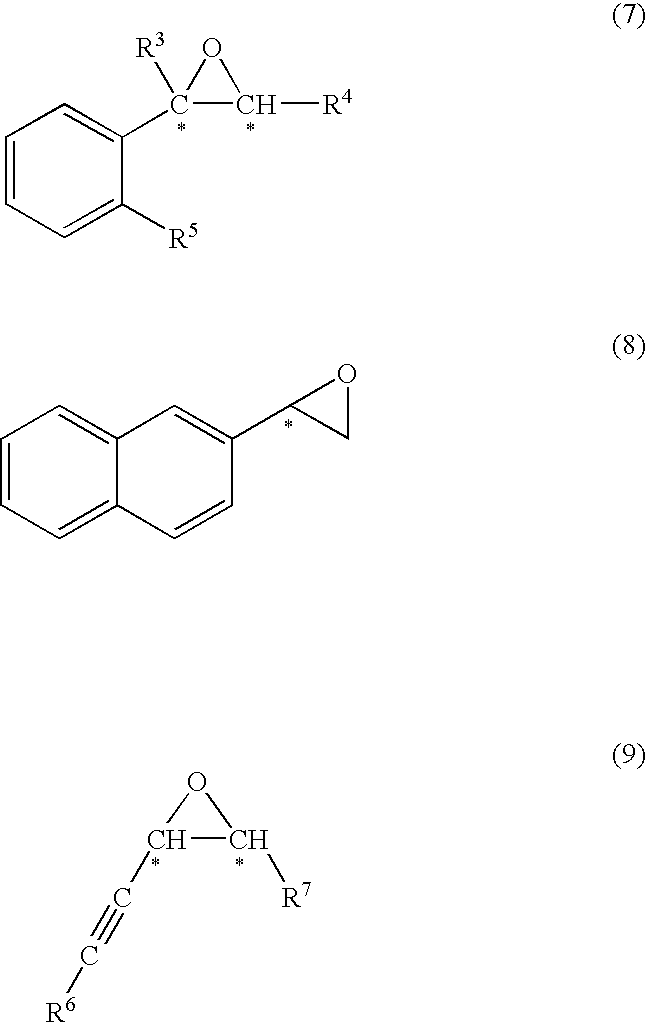
Production method of optically active epoxy compound, complex used for the method and production method of the complex
InactiveUS7812184B2High enantioselectivityOrganic compound preparationTitanium organic compoundsDouble bondTitanium
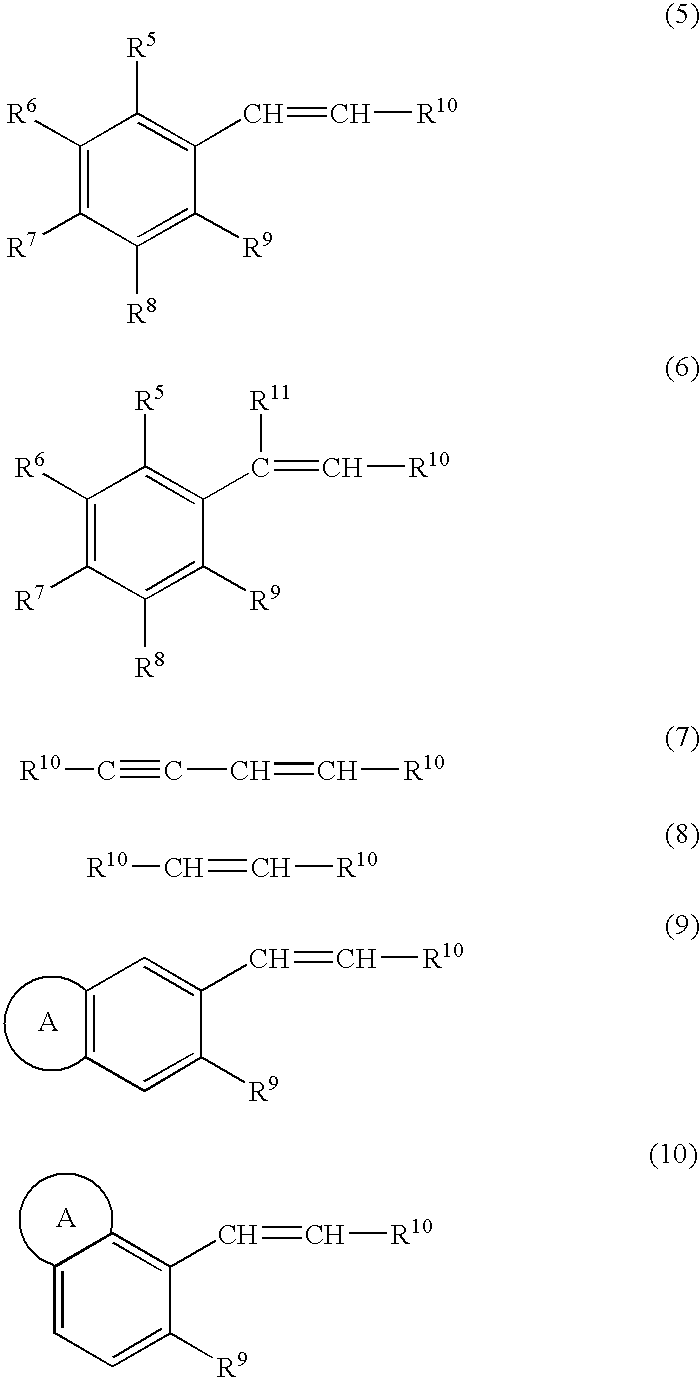
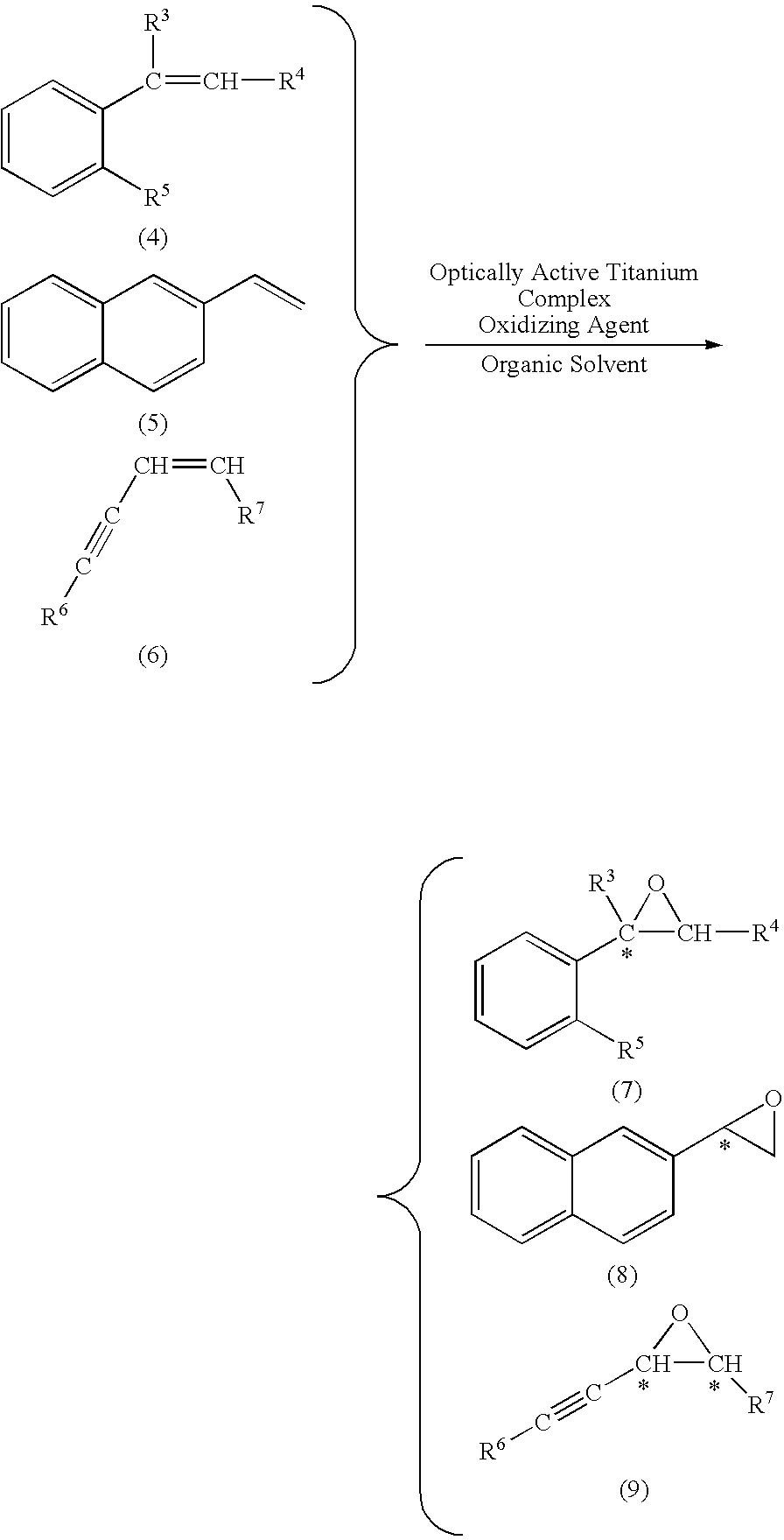
[Problem] To provide a production method of optically active epoxy compound, and a complex used for the production method and a production method of the complex.[Means for solving the problem] The skeleton that is necessary for expressing a high catalyst activity of optically active titanium salan complex of formulae (1) and (1′)and the substituent that is useful therefor and the position of the substituent are identified, and it is found that optically active epoxy compounds can be produced with a high enantioselectivity and a high chemical yield compared with a case where the prior optically active titanium salan complex is used. The production method comprises subjecting a prochiral compound (formula (4), (5) or (6)) having carbon-carbon double bond in the molecule to asymmetric epoxidation to produce an optically active epoxy compound (formula (7), (8) or (9)). The present invention relates also to a complex used for the production method and a production method of the complex.
Owner:NISSAN CHEM IND LTD
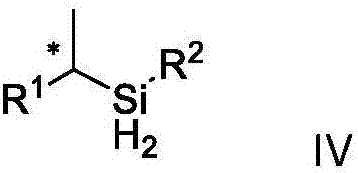
Chirality dihydrogen silane compound and synthetic method and application thereof
ActiveCN107235995AHigh selectivityHigh efficiency and high selectivitySilicon organic compoundsPreparation by oxidation reactionsSilanesEnantio selectivity
The invention discloses a chirality dihydrogen silane compound. The chirality dihydrogen silane compound is as shown in the formula IV. In the formula IV, X represents a chiral carbon atom. The invention further discloses a synthetic method for the chirality dihydrogen silane compound. The method comprises the following steps: using olefin shown in the formula I and silane shown in the formula II as raw materials, and using a chiral CoX2-OIP complex compound as a catalyst, in the existence of a reducing agent, reacting to obtain the chirality dihydrogen silane compound shown in the formula IV. The synthetic method is suitable for different types of the olefins, the reaction condition is moderate, the operation is simple and convenient, and the atomic economy is high. The reaction does not need to be added with any other toxic transition metal ions, the reaction yield is better and is 53%-97% generally, and the enantio-selectivity is higher and is 81%-99% and gt generally. The provided chirality dihydrogen silane compound shown in the formula IV can be used for synthesizing a chiral alcohol compound, a chiral silicon alcohol compound, a chiral polysubstituted silane compound and so on.
Owner:ZHEJIANG UNIV
Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium
ActiveCN104710406AEasy to separateHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDiphosphinesIridium chloride
A catalysis system used by a method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by iridium is a chiral diphosphine complex of iridium. A reaction is carried out under the following conditions: the temperature is 25-70DEG C; a solvent is a toluene / tetrahydrofuran mixed solvent (V / V=3:1); the pressure is 2-14atm; a ratio of a substrate to a catalyst is 25:1; and the catalyst is a (1,5-cyclooctadiene)iridium chloride dimer and chiral diphosphine ligand complex. A corresponding chiral cyclic amine derivative is prepared from quinolin-3-amine, and the enantiomeric excess value can reach 94%. The method has the advantages of simple and practical operation, easily available raw material, high enantioselectivity, good yield, and green, atom-economic and environmentally-friendly reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
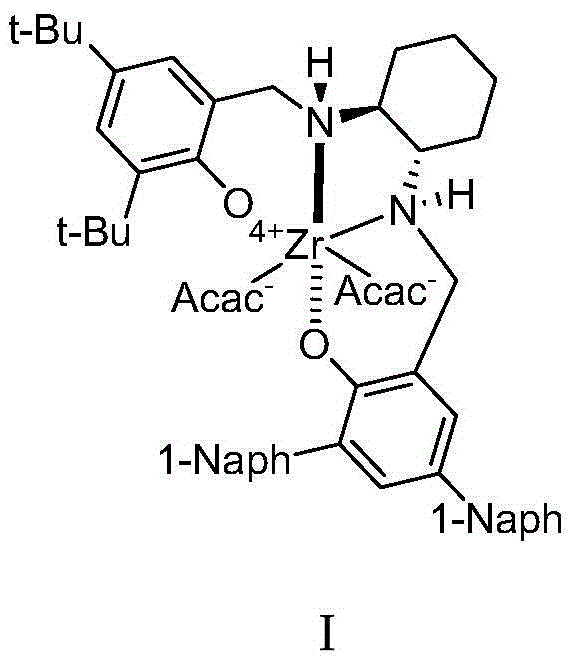
Zirconium catalyst and method for preparing chiral alpha-hydroxy-beta-keto ester compound by use of zirconium catalyst
ActiveCN105521826AEfficient manufacturingEasy to synthesizeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperatureSolvent
The invention relates to a method for preparing a chiral alpha-hydroxy-beta-keto ester compound by the application of a zirconium catalyst with a chiral diaminocyclohexane derivative as a ligand. The method comprises the following process: in the presence of a zirconium complex with a diaminocyclohexane derivative as a ligand, a beta-keto ester compound and an oxidizing agent are contacted with each other in an inert solvent, wherein dosage of a catalyst is 0.5-50 mol% of dosage of the beta-keto ester compound; dosage of the oxidizing agent is 100-2000 mol% of dosage of the beta-keto ester compound; and reaction temperature is 0-100 DEG C. yield of the alpha-hydroxy-beta-keto ester compound reaches up to 99%, and ee value reaches up to 98%. According to the invention, the zirconium catalyst with the diaminocyclohexane derivative as a ligand that is easy to synthesize, is low-cost and has stable performance is used, and then the alpha-hydroxy-beta-keto ester compound can be effectively prepared. By the method, very high yield and good enantioselectivity can be gained. The method of the invention is simple to operate, is low-cost and is suitable for industrialization.
Owner:DALIAN UNIV OF TECH
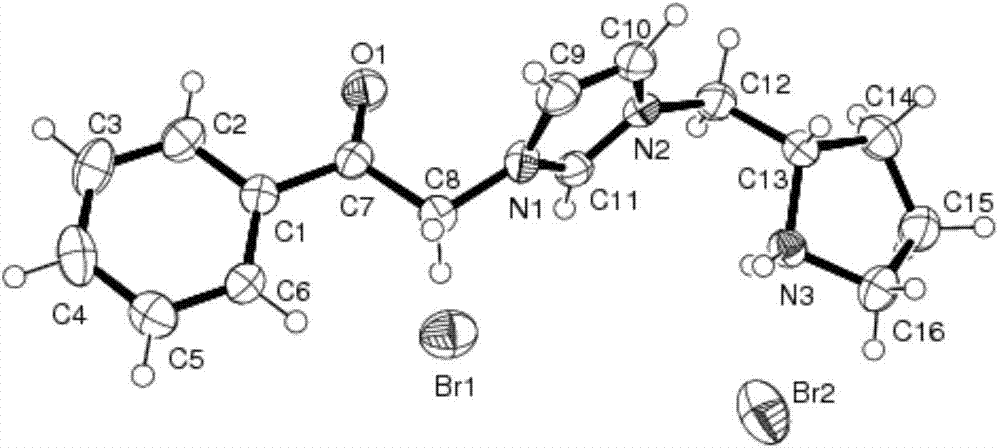
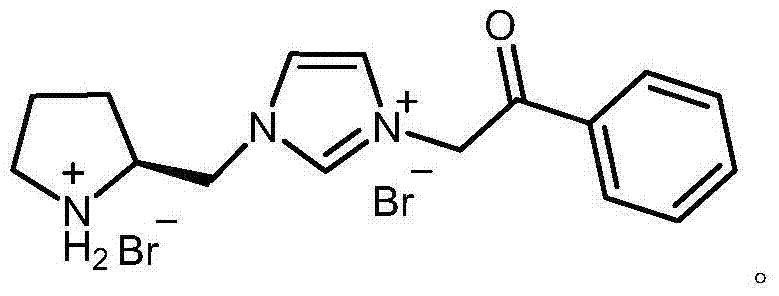
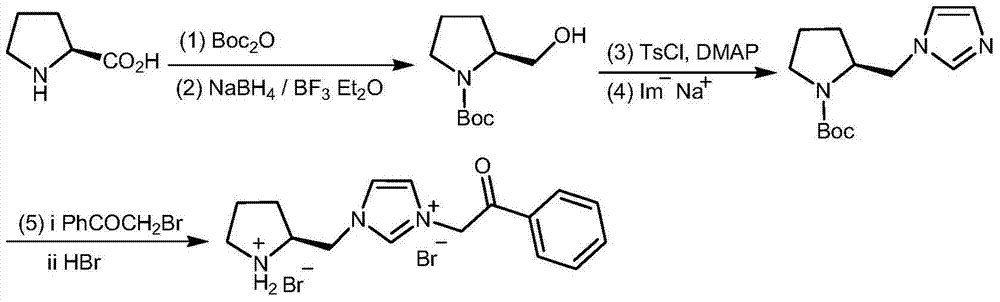
Chiral pyrrolidine functionalized imidazolium salt, and preparation method and application thereof
InactiveCN103570691ARaw materials are easy to getEasy to manufactureOrganic chemistryOrganic compound preparationCyclohexanoneHydrobromide
The invention provides a chiral pyrrolidine functionalized imidazolium salt, and a preparation method and an application thereof. The chiral pyrrolidine functionalized imidazolium salt is bromized 1-[2-(S)-(pyrrolidyl)methyl]-3-benzoyl methyl imidazole hydrobromide. The preparation method of the chiral pyrrolidine functionalized imidazolium salt comprises the following steps of: by taking natural amino acid L-proline as the starting raw material, obtaining the chiral pyrrolidine functionalized imidazolium salt through a plurality of conventional organic synthetic reactions such as Boc acylation, carboxylic acid reduction, hydroxyl sulfonylation, nucleophilic substitution of imidazole anions, quaternization of halohydrocarbon and de-Boc protection. The chiral pyrrolidine functionalized imidazolium salt provided by the invention is capable of catalyzing asymmetric Michael addition reaction of cyclohexanone and nitroolefin, and has extremely high diastereoselectivity and enantioselectivity.
Owner:SHANXI UNIV
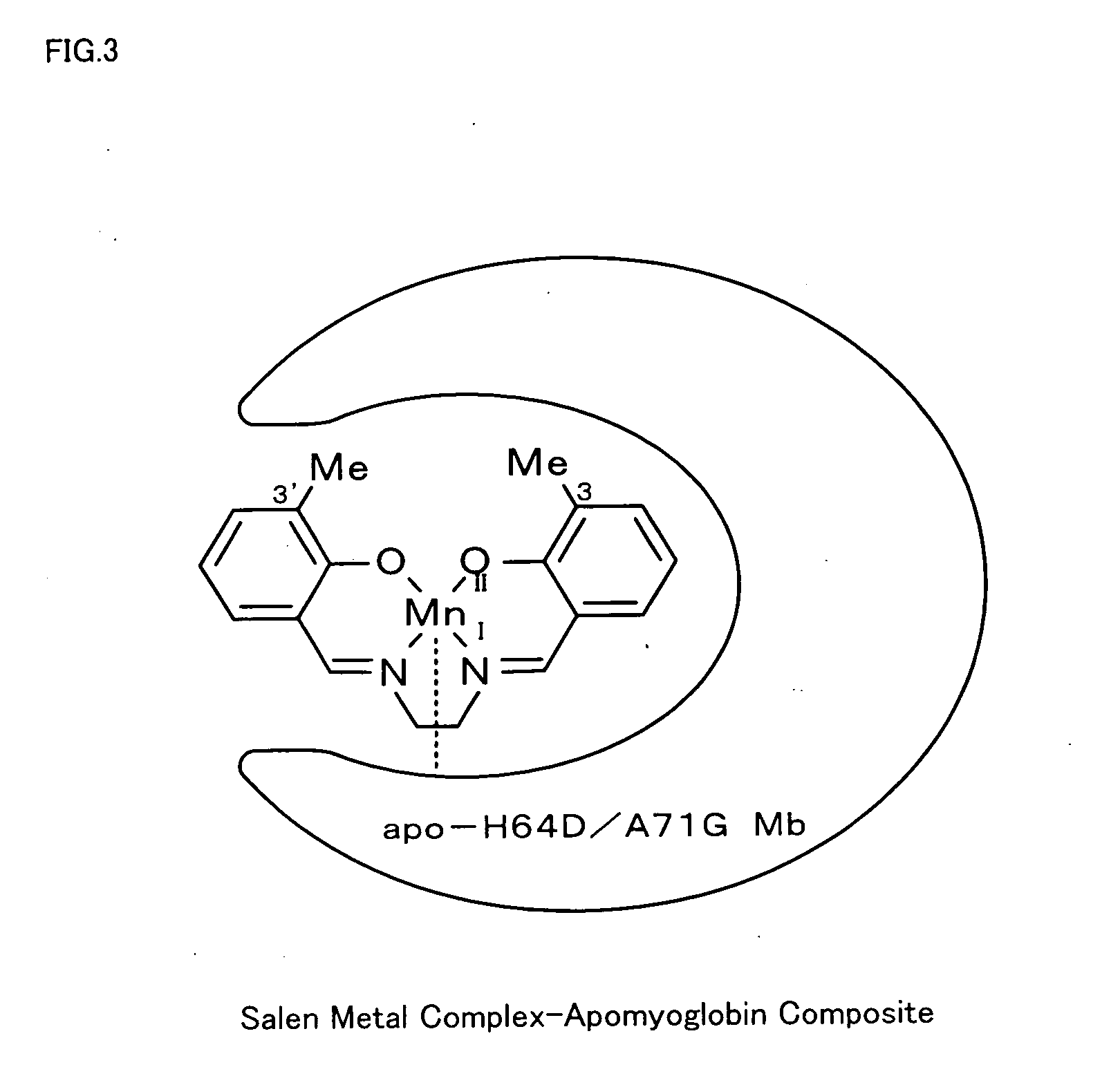
Metal complex-protein composite and oxidation catalyst
InactiveUS20050096260A1Improve responseHigh enantioselectivityPeptide/protein ingredientsOrganic-compounds/hydrides/coordination-complexes catalystsHalogenHydrogen atom
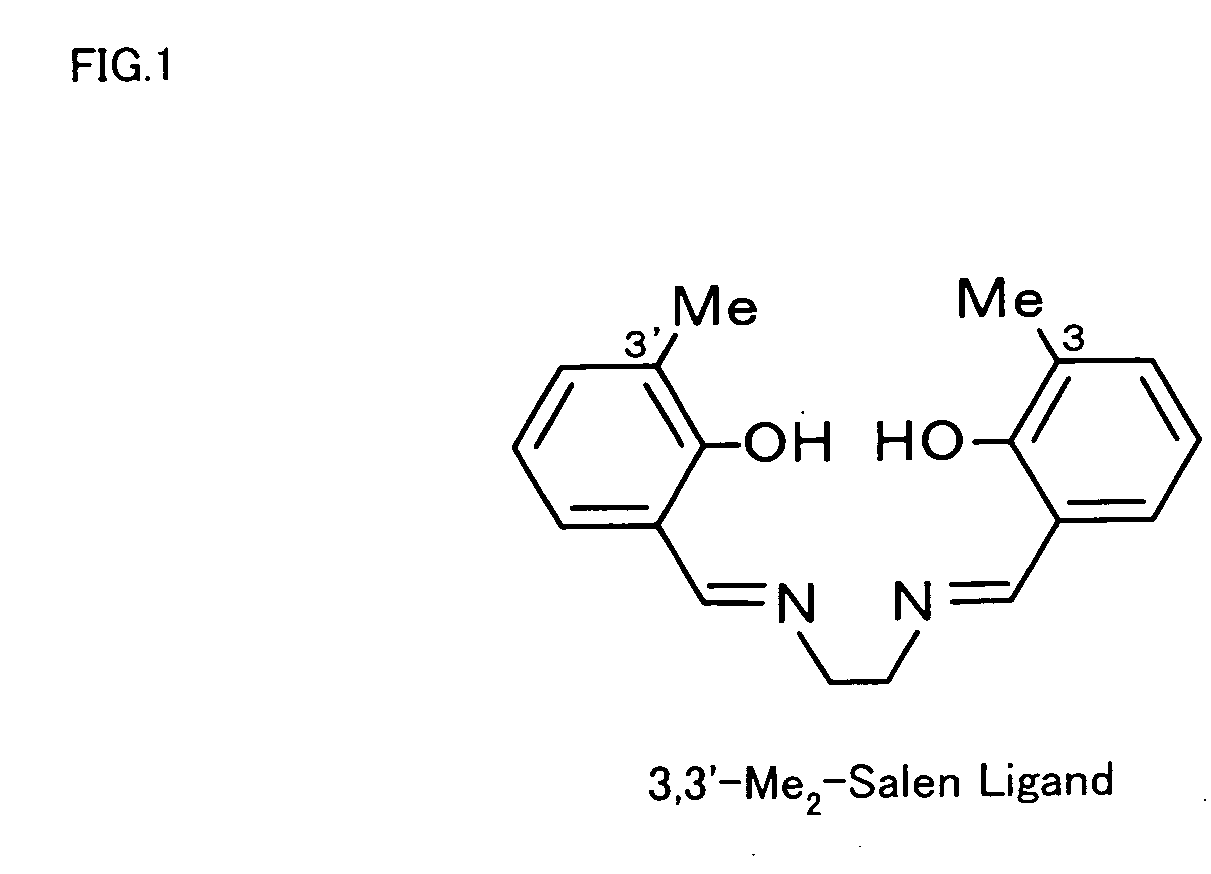
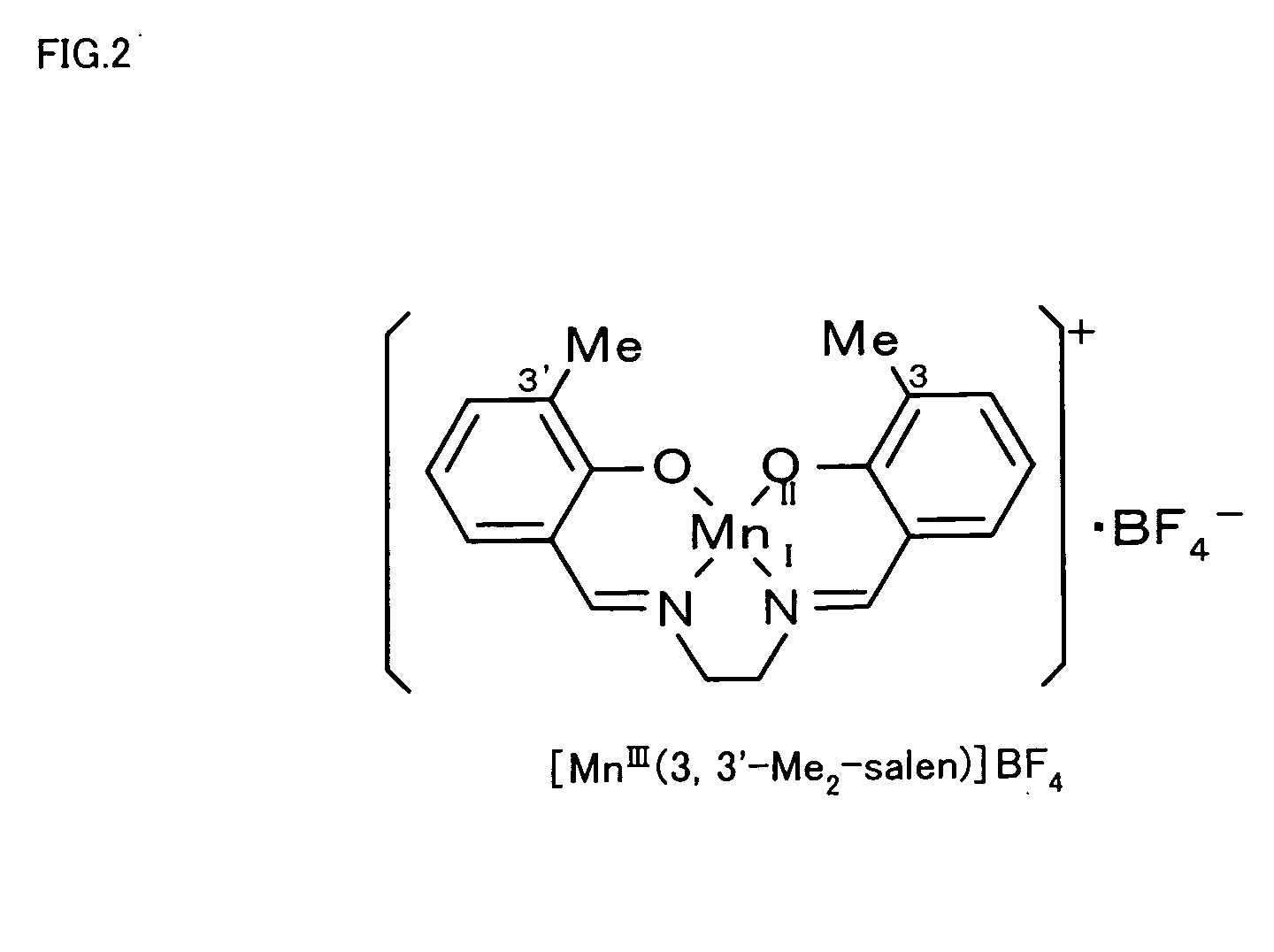
The metal complex-protein composite of the invention has a specific structure that a salen metal complex expressed by Formula (1) given below is received in a cavity of a protein: where M in Formula (1) represents a metal ion, and R1 through R10 independently represent any of hydrogen atom, halogen atoms, alkyl groups containing 1 to 5 carbon atoms, and alkoxy groups containing 1 to 5 carbon atoms.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST
Carbonyl reductase gene, enzyme, vector and engineering bacterium as well as application of carbonyl reductase gene in asymmetrically reducing prochiral carbonyl compounds
ActiveCN104630243AHigh enantioselectivityImprove conversion rateBacteriaOxidoreductasesKetoneCarbonyl group
The invention discloses a recombinant carbonyl reductase sourced from Burkholderia gladioli ZJB-12126 as well as an encoding gene of the recombinant carbonyl reductase, a recombinant vector containing the gene, a recombinant gene engineering bacterium obtained by transforming the recombinant vector, and an application of the recombinant carbonyl reductase in asymmetrically reducing prochiral carbonyl compounds. According to the recombinant carbonyl reductase disclosed by the invention, by respectively taking 2-benzamidomethyl-3-ketone butyrate, 4-chloroacetoacetic acid ethyl ester (COBE), (R)-6-cyano-5-hydroxyl-3-carbonyl caproate tert-butyl ester and (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone as substrates, (2S, 3R)-2-benzamidomethyl-3-polyhydroxybutyrate, (S)-4-chloro-3-ethyl hydroxybutyrate, 6-cyano-(3R, 5R)-dihydroxyhexanoic acid tert-butyl ester and (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxyl valeryl]-4-phenyl-1,3-oxo-azacyclopentane-2-ketone with high optical purity are prepared by virtue of biocatalytic reaction, wherein the biocatalytic reaction can be performed by using recombinant escherichia coli as a biocatalyst, and thus optional new enzyme sources can be provided for biocatalytic synthesis of chiral intermediates of medicines.
Owner:ZHEJIANG UNIV OF TECH
Asymmetric catalytic hydrogenation method for ketone-derived N-alkylimine
InactiveCN102050688AHigh enantioselectivityCarbamic acid derivatives preparationOrganic compound preparationAsymmetric hydrogenationKetone
The invention discloses a method for performing the asymmetric catalytic hydrogenation of ketone-derived N-alkylimine. In the method disclosed by the invention, the catalytic hydrogenation of the ketone-derived N-alkylimine is performed in the presence of a chiral catalyst formed by a chiral diamine ligand and a transitional metal to obtain a chiral amine product with high yield and high enantioselectivity. The enantiomeric excess of a chiral amine product formed by the hydrogenation of an acyclic N-alkylimine substrate may reach 98 percent ee; and the enantiomeric excess of a chiral amine product formed by the hydrogenation of an exocyclic N-alkylimine substrate may reach over 99 percent ee. The method disclosed by the invention realizes the asymmetric catalytic hydrogenation of ketone-derived N-alkylimine with high enantioselectivity, and the obtained chiral amine product is an important medicinal and material intermediate and has a bright actual application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for synthesizing chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of iridium catalyst
The invention discloses a method for synthesizing a chiraltetrahydro naphthalenederivate through asymmetric hydrogenation on isoquinoline by means of an iridium catalyst. A catalysis system applied in the method is a chiral bi-phosphine complex of metal iridium. The reaction is carried out under the conditions that the temperature is 25-60 DEG C; the volume ratio of tetrahydrofuran to dichloromethane in a solvent, namely a mixed solvent of tetrahydrofuran and dichloromethane is 1:1; the pressure is 13-50 Mpa; the ratio of a substrate to a catalyst is 50:1; the catalyst is a coordination compound of a (1,5-cyclooctadiene) iridium chloridedipolymer and a chiral bi-phosphine ligand. The corresponding chiral 1-position or 3-position substituted tetrahydro naphthalenederivate through hydrogenation on isoquinoline, and the enantiomeric excess of the derivate can reach 96%. The method is simple and practical in operation, raw materials are easy to obtain, the enantioselectivity is high, the yield is high, the reaction has atom economy, and the environment is friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing 1,6-eneyne compounds
InactiveCN101591275AHigh enantioselectivityMild reaction conditionsGroup 4/14 element organic compoundsOrganic compound preparationIridiumRegioselectivity
The invention provides a method for synthesizing 1,6-eneyne compounds. The method is an effective method which synthesizes the optically active 1,6-eneyne compounds with high regioselectivity and enantioselectivity by using iridium complex as a catalyst as well as allyl carbonate compounds and a propargyl nucleophilic reagent as raw materials. The method has the advantages of readily available catalyst, high catalytic activity, mild reaction temperature, wide substrate application range and high product regioselectivity and enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Lipase mutant and uses thereof
The invention relates to the technical field of biology, and relates to a lipase mutant and uses thereof. The mutant is obtained from wild type candida antarctica lipase B (CALB) through mutation. The invention particularly relates to the lipase mutant, a preparing method thereof, and a method of preparing S-configuration ibuprofen type compounds with optical activity through catalysis of hydrolysis of ester derivatives of the ibuprofen type compounds by utilization of the lipase mutant and through kinetic resolution. The wild type candida antarctica lipase B is subjected to mutation to obtain the modified lipase. The lipase is expressed in pichia pastoris engineering bacteria. The lipase comprises an amino acid sequence having at least 85% of identity with the SEQ ID NO.02. The 189 site residue corresponding to the SEQ ID NO.02 of the lipase is an aromatic amino acid residue and the 190 site residue corresponding to the SEQ ID NO.02 of the lipase is a nonpolar amino acid residue. By subjecting the wild type candida antarctica lipase B to mutation, the enantioselectivity of the lipase to the ibuprofen type compounds is improved.
Owner:SHENYANG PHARMA UNIVERSITY
Chiral ligand metal complex catalyst system, and its preparation method and use
InactiveCN1579627ALower synthesis costHigh stereoselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPhosphateHigh pressure
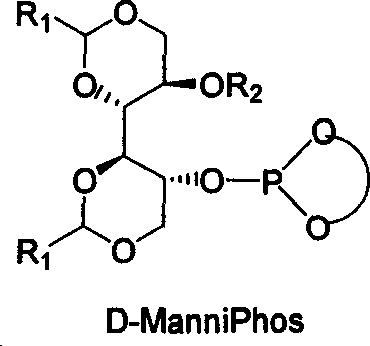
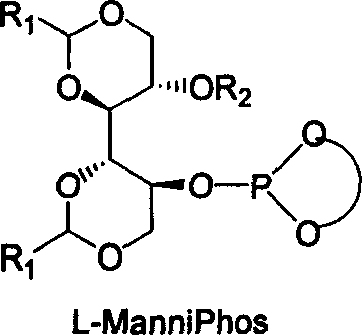
The invention relates to chirality phosphine ligand metal complex catalyse system, which is composed by complex formed by ligand and metal Rh, Ru, Ir, Pt or Pd or ligand and metal precursor in of 1-2 mol ratio. The phosphine ligand is a kind of effective chirality phosphate ligand composed by D-mannitol or L-mannitol after reaction. The catalyst composed by the chirality ligand and rhodium metallic compound in asymmetry hydrogenation reaction can work in room temperature. With a wide applied range, catalyst's activation and stereoselectivity is not influenced from normal pressure to high pressure. Reaction time is 1-24 hours. Mol ratio of ligand and metal rhodium compound is 1:1-2:1. Ratio of reactant and catalyst is 100-10,000.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Pronucleus expression of epoxide hydrolase gene (EH-B) and preparation of chiral epichlorohydrin
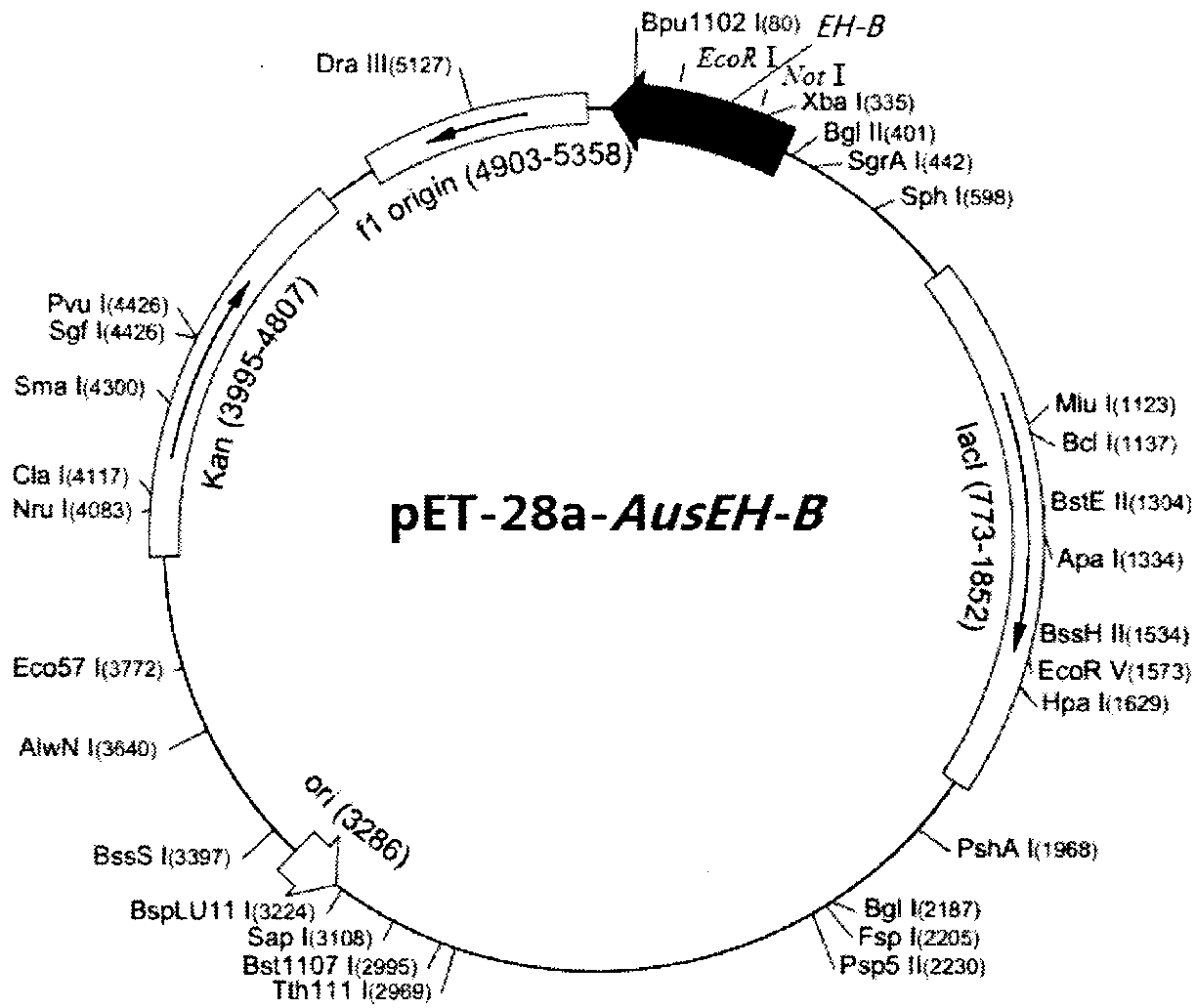
InactiveCN102994470AHigh enzyme activityHigh enantioselectivityHydrolasesMicroorganism based processesEpoxide hydrolaseAspergillus usamii
The invention provides a clone and a pronucleus expression method of a novel B class epoxide hydrolase gene mature peptide cDNA sequence from Aspergillus usamii E001. The nucleotide sequence of the novel B class epoxide hydrolase gene mature peptide cDNA sequence is SEQ ID NO:1, the corresponding amino acid sequence is SEQ ID NO:2, and the corresponding gene is named Aus EH-B. According to the invention, good stereoselectivity is achieved for (R)-epichlorohydrin through chiral gas chromatography analysis rEH, and a produced (S)-epichlorohydrin antipode excess value achieves 99%. The pronucleus expression method provided by the invention lays the foundation for the industrialized production of epoxide hydrolase and provides the basis for preparing chiral epichlorohydrin by researching an EH enzyme kinetic resolution method for biological catalysis technology industrialization.
Owner:JIANGNAN UNIV
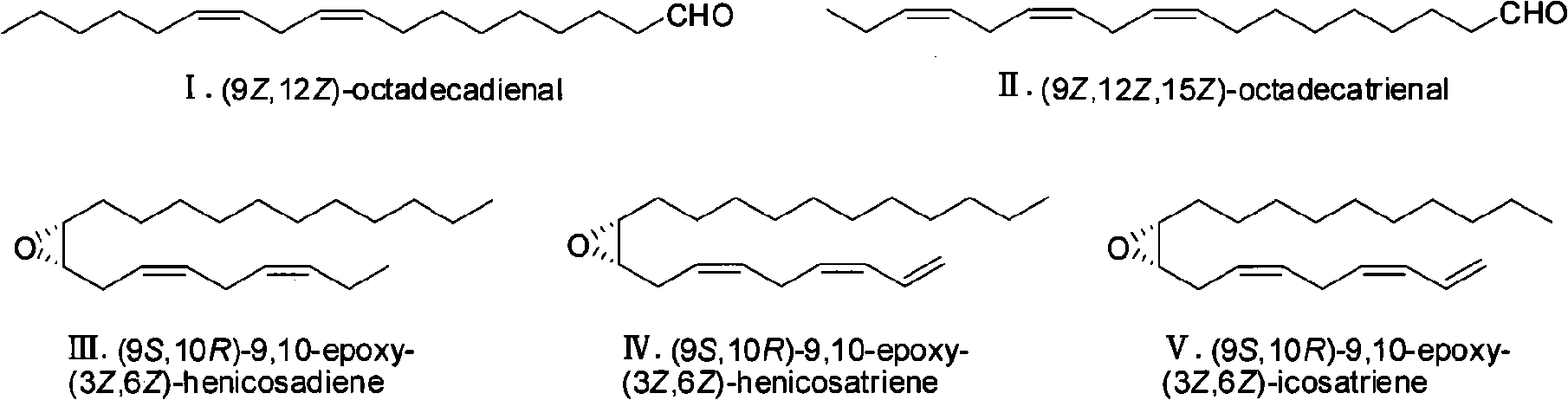
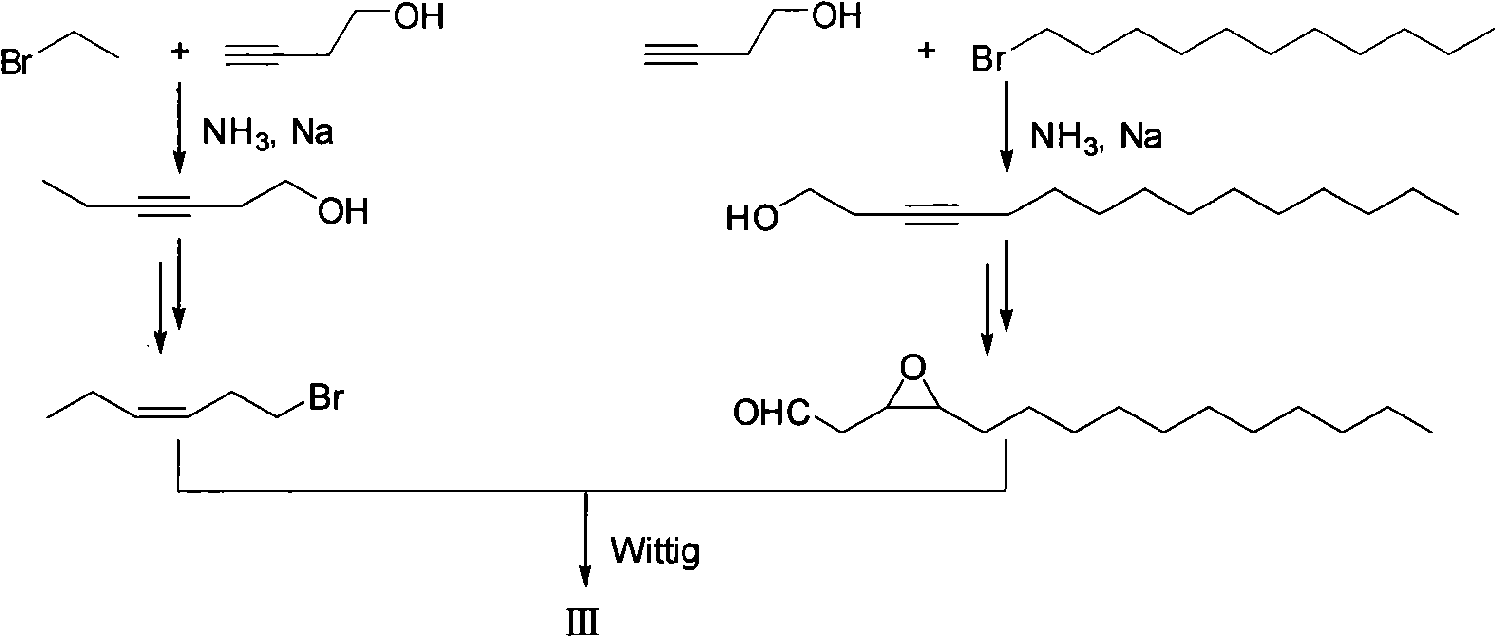
Fall webworm sex pheromone synthesizing method
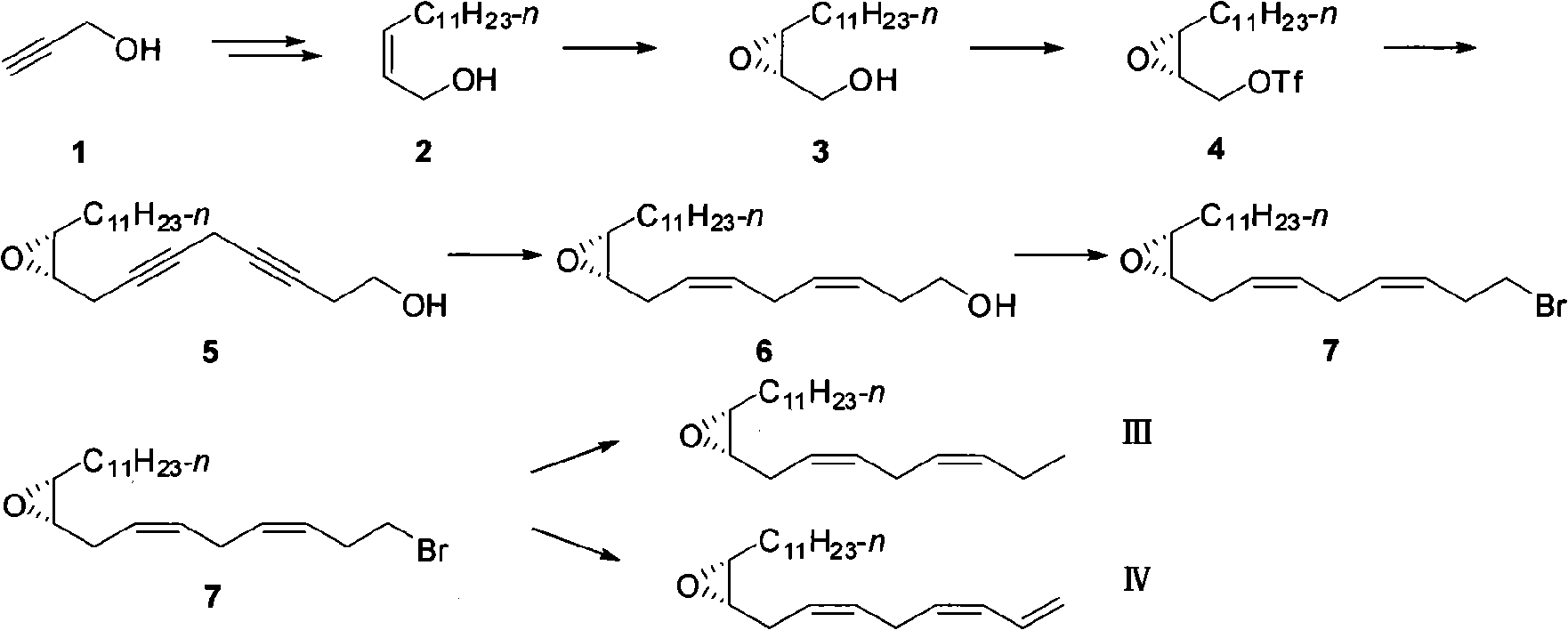
The invention provides a fall webworm sex pheromone synthesizing method, which relates to an epoxypropane coumpound. The method takes (Z)-2-acetylene tetradecanol synthesized by cheap and easy obtained 2-propargyl alcohol as raw materials, to synthesize fall webworm sex pheromone 3 [(9S, 10R) -9,10 - epoxy - (3Z, 6Z) -3,6-heneidecadienol] and 4 [(9S, 10R) -9,10 - epoxy - (3Z, 6Z) -1,3 ,6-heneidecatrienol] with high efficiency, high enantioselectivity and high yield. Step 6, 25% of the total yield is synthesized into (9S, 10R) -3; 6-step, 21% of the total yield is synthesized into (9S, 10R)-4; and the antipode excess e. e is more than 99%. The separation of the various steps of the invention is simple; the used reagent are all common reagents, cheap and easy to get; the invention has high yield and a shorter route, and is suitable for requirement o f the industrial production.
Owner:XIAMEN UNIV
Recombinant halohydrin dehalogenase, and mutant and engineering strain and applications thereof
ActiveCN104745556AEnhanced asymmetric dehalogenation synthesisHigh enantioselectivityBacteriaHydrolasesEpoxyAlcohol
The invention discloses a recombinant halohydrin dehalogenase and a mutant, an engineering strain and applications thereof. The amino acid sequence of the recombinant halohydrin dehalogenase is shown in SEQ ID No: 2. The invention also discloses an application of the recombinant halohydrin dehalogenase and the mutant of recombinant halohydrin dehalogenase in catalyzing asymmetric dehalogenation of 1,3-dichloropropanol so as to synthesize (S)-epoxy chloropropane and in preparing other chiral epoxides and beta-substituted alcohol. Compared with other halohydrin dehalogenases, the halohydrin dehalogenase obtained according to the invention and the mutant thereof have higher enantioselectivity and have an extremely good industrial application prospect.
Owner:ZHEJIANG UNIV OF TECH
Nanogel modified by ionic liquid and loaded with chiral catalyst and preparing method and application thereof
InactiveCN105131170AHigh selectivityGood dispersionOrganic chemistryOrganic compound preparationFunctional monomerSolvent
The invention discloses nanogel modified by ionic liquid and loaded with a chiral catalyst and a preparing method and application thereof. The nanogel is characterized in that polymerization of functional monomers, a crosslinking agent, sodium dodecyl sulfate, chiral monomers and the imidazolium ionic liquid is initiated by initiator ammonium persulfate in solvent water to form a crosslinking copolymer, and the nanogel modified by the ionic liquid and loaded with the chiral catalyst is obtained through dialysis. The invention further discloses the preparing method of the nanogel modified by the ionic liquid and loaded with the chiral catalyst and application of the nanogel to an asymmetric Aldol reaction. The ionic liquid is introduced into preparation of chiral nanometer hydrogel, and a simple and effective path is provided for preparing a novel chiral catalyst which is high in catalytic activity and selectiveness, easy to separate, capable of being used repeatedly in an aqueous solution.
Owner:HENAN NORMAL UNIV
Alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol
InactiveCN108384765AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesFormate dehydrogenase HGlycol synthesis
The invention discloses an alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol and belongs to the technical field of biological engineering. The alcohol dehydrogenase mutant disclosed by the invention has good catalytic activity and stereoselectivity, and a series of chiral diaryl alcohol with R- and S- configurations can be prepared through efficient catalysis. According to the alcohol dehydrogenase mutant, alcohol dehydrogenase is coupled with glucose dehydrogenase or formate dehydrogenase, and can be used for synthesizing various antihistamine drugs, i.e., a chiral diaryl alcohol intermediate. Compared with an existing report, a method for preparing the diaryl chiral alcohol through asymmetric catalysis of the alcohol dehydrogenase has the advantages of simplicity in operation, high substrate concentration, complete reaction and high product purity and has a very good industrial application prospect.
Owner:JIANGNAN UNIV
Chiral 3,3-disubstituted oxoindole derivative, and synthetic method and application thereof
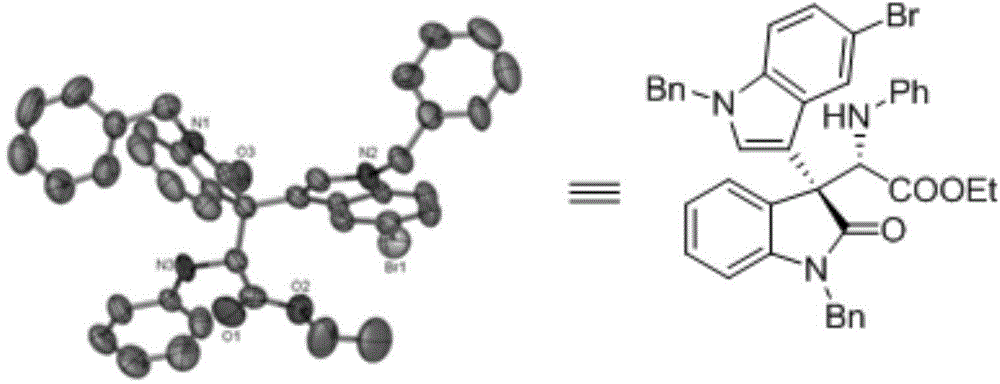
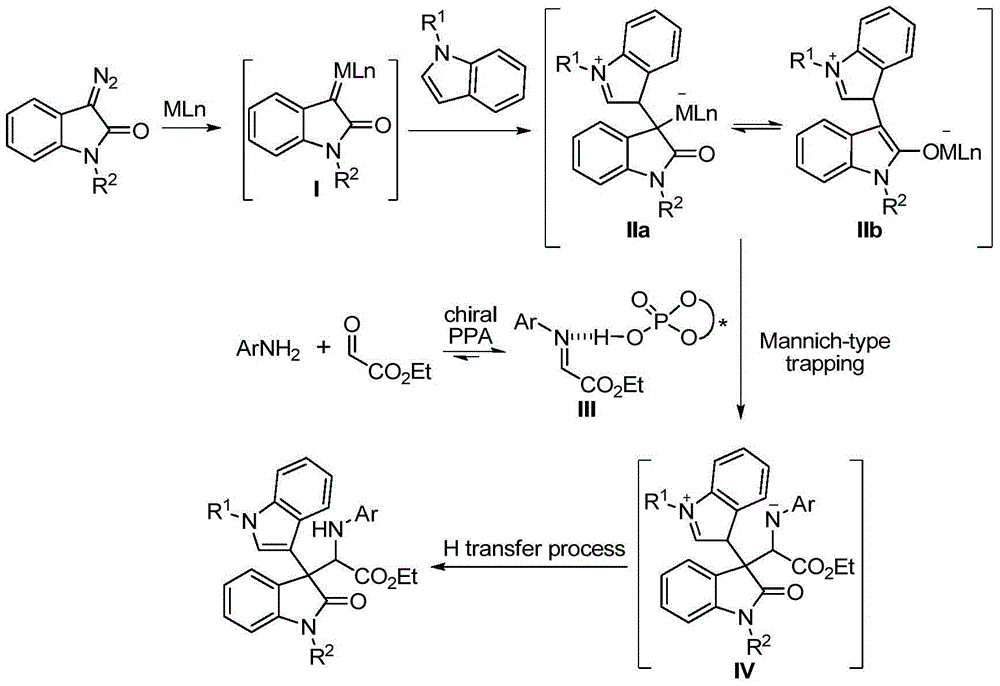
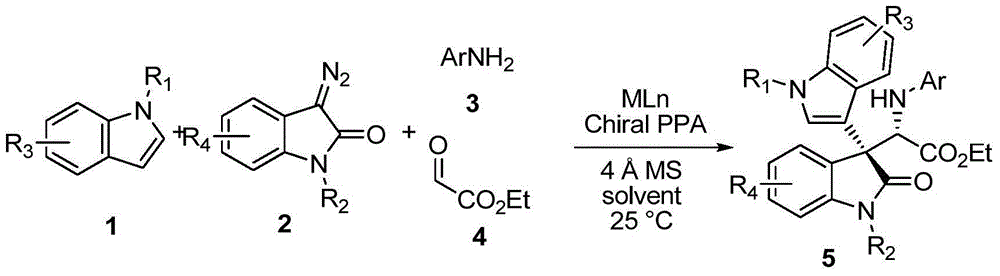
ActiveCN104693092AHigh yieldGood diastereoselectivityOrganic chemistryMetabolism disorderChemical synthesisSynthesis methods
The invention discloses a chemical synthetic method of a novel chiral 3,3-disubstituted oxoindole derivative with PTP1B inhibiting action. With diazoisatin, indole, arylamine and aldehyde ester as raw materials, a metal catalyst as catalyst, chiral phosphoric acid as a co-catalyst, an organic solvent as a solvent, and molecular sieve shown in the specification as an additive, a one-step reaction is performed under the condition of 25 degrees centigrade, and the product undergoes column chromatography purification to obtain the target product. The synthetic method has the advantages of high step economy, strong atom economy, high diastereoselectivity and enantioselectivity, high yield, mild reaction condition, and simple and safe operation. The optically pure chiral 3,3-disubstituted oxoindole derivative with two chiral centers synthesized in the invention is an important intermediate in chemical engineering, chemistry and medicines, and has a wide application prospect in the fields of medicines and the chemical engineering.
Owner:广东和博制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com