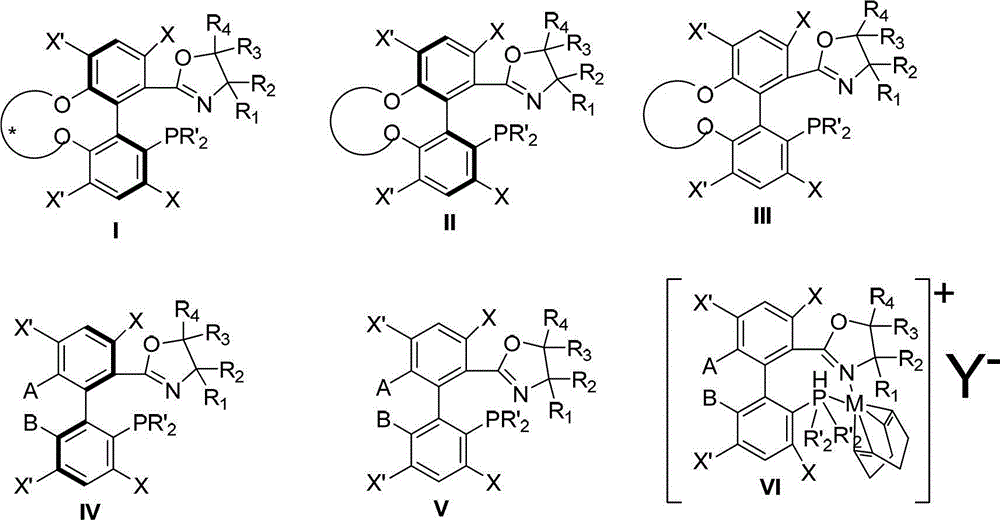
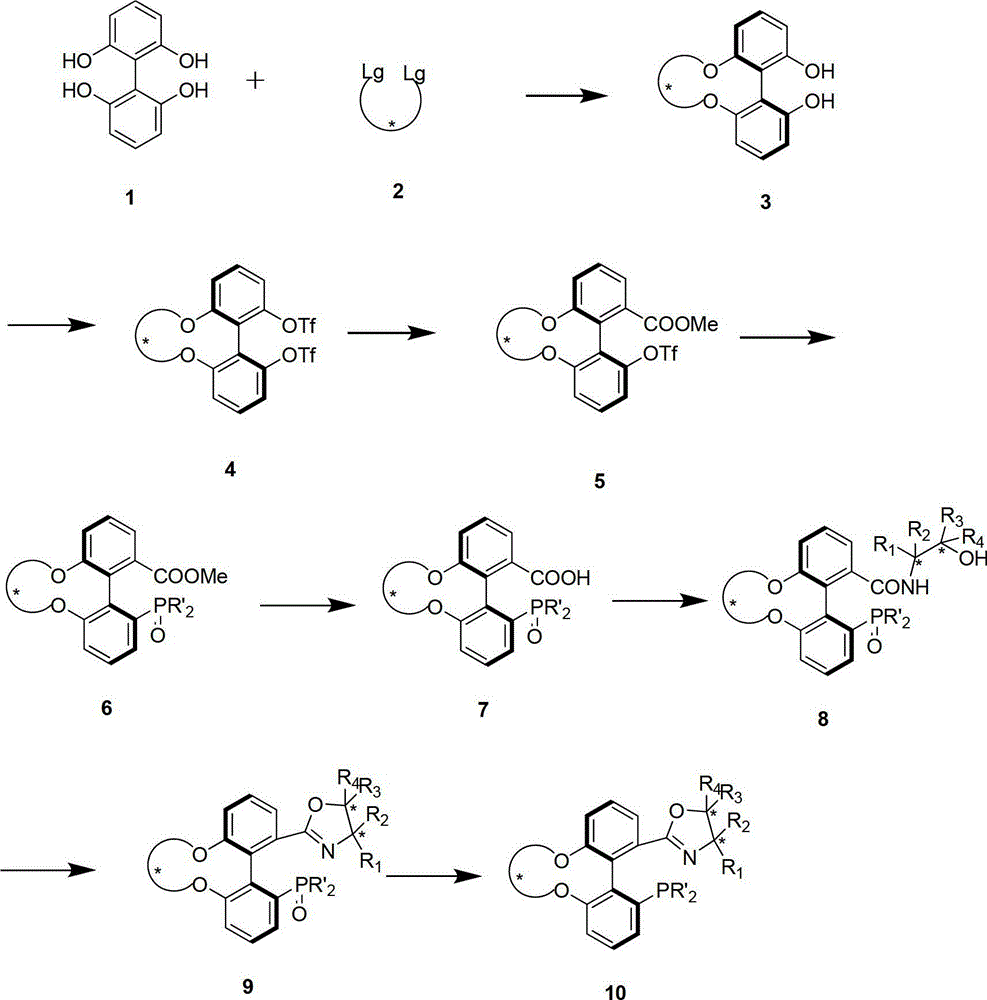
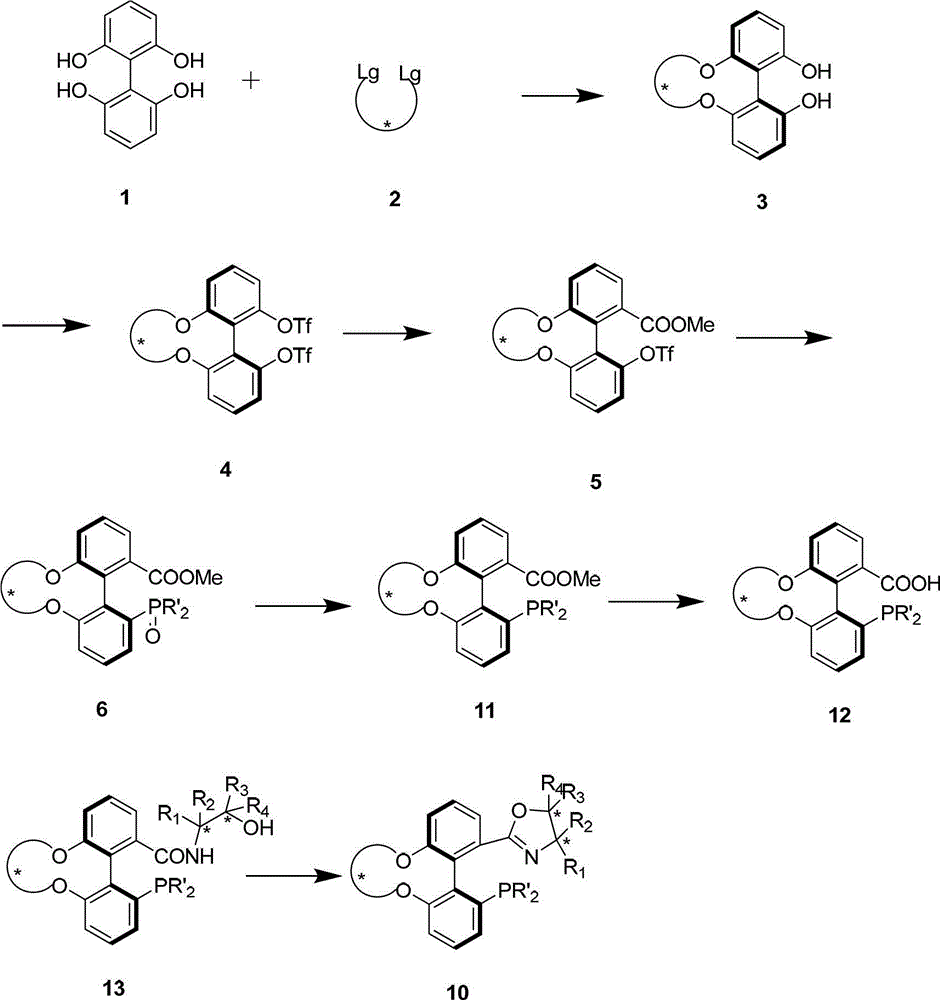
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
A metal complex and oxazoline technology, which is applied in the field of preparation of chiral phosphine-oxazoline ligand ionic complexes, can solve the problems of substrate limitation and single electronic effect of the skeleton
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of (R)-[6,6'-(S,S)-2,3-butanedioloxy)]-2,2'-dihydroxybiphenyl
[0065] Under nitrogen protection, suspend 0.88 g (4.04 mmol) of 2,2',6,6'-tetrahydroxybiphenyl 1, 3.06 g (9.39 mmol) of cesium carbonate in 120 mL of dry N,N-dimethylformamide (DMF), the temperature was raised to 80°C, and stirred for 1 hour. Then 1.0 g (4.06 mmol) of (2R,3R)-2,3-butanediol methanesulfonate was dissolved in 40 mL of dry DMF, and slowly added dropwise to the suspension of 1 and cesium carbonate within 4 hours middle. The suspension was stirred at 80°C for 12 hours, and DMF was evaporated under reduced pressure. The reaction residue was poured into 1mol / L hydrochloric acid, extracted 3 times with ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, and the crude product was purified by column chromatography to obtain 0.57g product (R)-[ 6,6'-((S,S)-2,3-butanedioloxy)]-2,2'-dihydroxybiphenyl, yield 52%.
[0066] Product Analysis Re...
Embodiment 2
[0067] Example 2: Preparation of (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2-bis(trifluoromethanesulfonyloxy)biphenyl
[0068] (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2,2-dihydroxybiphenyl 2.72g (10.0mmol) was dissolved in 80mL of anhydrous pyridine, Under ice cooling, 6.00 g (20.0 mmol) of trifluoromethanesulfonic anhydride was slowly added. After the dropwise addition, react under ice bath for 1 hour, slowly raise the temperature to room temperature, and continue the reaction overnight. Washed with sodium and saturated brine, the organic phase was dried with anhydrous magnesium sulfate, and the crude product was purified by column chromatography after evaporating the solvent to obtain 5.41 g of the product (R)-[6,6'-((S,S)-2 ,3-Butanedioloxy)]-2,2'-bis(trifluoromethanesulfonyloxy)biphenyl, the yield was 95%.
[0069] Product Analysis Results: 1 H NMR (300MHz, CDCl 3 ):δ1.41(d,J=6.0Hz,6H),3.86-3.96(m,2H),7.22(d,J=8.1Hz,4H),7.50(dd,J=8.1Hz,J=8.4Hz ,2H). 13 CNMR (75MHz, CDCl 3 )...
Embodiment 3
[0070] Example 3: (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2-methoxyl-2'-trifluoromethanesulfonyloxybiphenyl preparation of
[0071] Add MeOH (37mL), DMSO (55mL) and Et 3 N (3.7 mL), after mixing well, degas three times under CO atmosphere. Add (R)-[6,6'-((S,S)-2,3-butanedi Alcoholoxy)]-2,2'-bis(trifluoromethanesulfonyloxy)biphenyl (1.07g, 2.0mmol), Pd(OAc) 2 (44.9mg, 0.2mmol) and dppp (82.5mg, 0.2mmol), replace the system with CO atmosphere, and immediately use a double-ended needle to carefully transfer the degassed solution into it. Heat the oil bath to 70°C, stir and react for 2-6h, the system turns from light green to wine red. TLC tracking detection until the reaction is complete. After washing with water and extracting with ethyl acetate, the solvent was removed under reduced pressure, and the residue was washed with CH 2 Cl 2 Redissolved, silica gel column chromatography, ethyl acetate / petroleum ether as eluent, to obtain compound (R)-[6,6'-((S,S)-2,3-butanedioloxy)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com