Photosensitive fluorosilicone segmented urethane acrylate oligomer and preparation method thereof
A polyurethane acrylate and fluorosilicone block technology, which is applied in leather surface treatment, coating, animal husbandry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Synthesis of N,N-dihydroxyethyl-3-alanine hexafluorobutyl acrylate, adding hexafluorobutyl acrylate in equal amount to a four-necked flask equipped with a condenser tube, a thermometer, a magnetic stirrer and a dropping funnel 10.00g and diethanolamine 4.45g, 5.00g methanol as solvent. mixture at room temperature and pass N 2 After stirring for 20 minutes under certain conditions, the temperature was raised to 40°C for 5 hours, and then distilled under reduced pressure to remove methanol to obtain light yellow and transparent N, N-dihydroxyethyl-3-aminopropionic acid hexafluorobutyl ester, the reaction formula is as follows:
[0121]
Embodiment 2
[0123] The synthesis of N,N-dihydroxyethyl-3-aminomethyl propionate dodecafluoroheptyl ester, add the same amount of methyl alcohol into a four-necked flask equipped with a condenser tube, a thermometer, a magnetic stirrer and a dropping funnel 10.00 g of dodecafluoroheptyl acrylate, 2.63 g of diethanolamine, and 3.00 g of methanol were used as solvents. mixture at room temperature and pass N 2 After stirring for 20 minutes under certain conditions, the temperature was raised to 40 ° C for 6 hours, and then distilled under reduced pressure to remove methanol to obtain light yellow transparent N, N-dihydroxyethyl-3-aminomethyl propionate hexafluorobutyl ester, the reaction formula is as follows :
[0124]
Embodiment 3
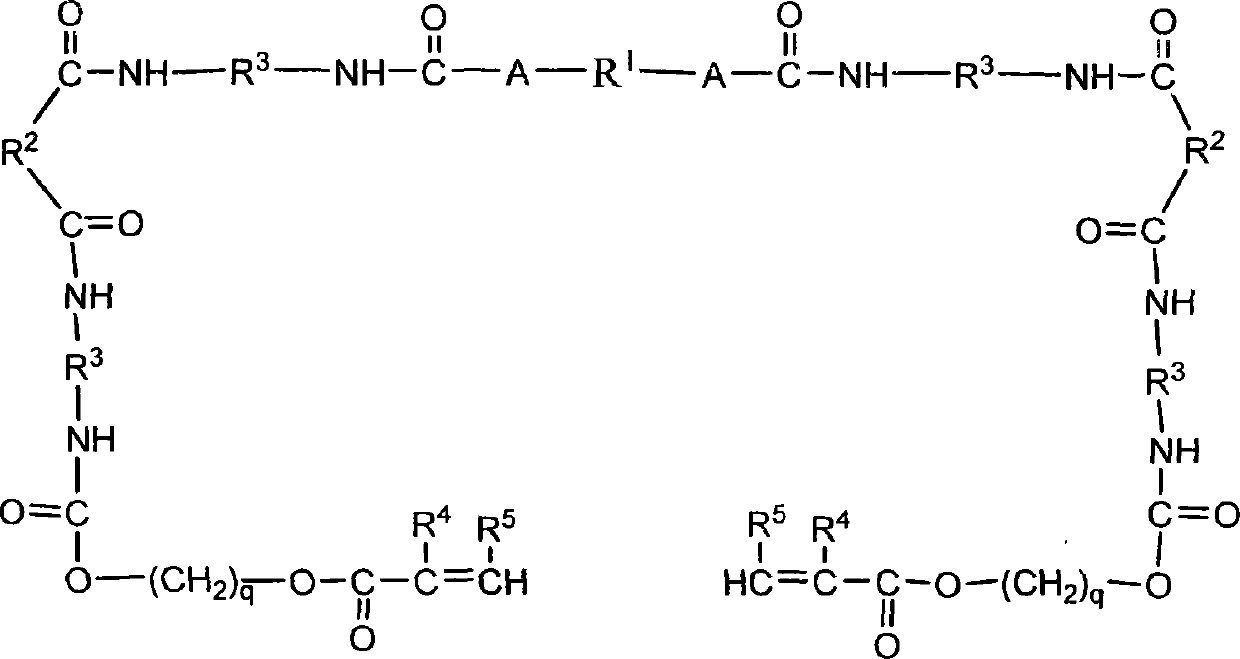
[0126] In a 100mL four-neck flask equipped with a stirrer, dropping funnel, condenser and thermometer, add 15.00g of Q4-3667, 0.03g of catalyst dibutyltin dilaurate (DBTDL), and dropwise add 4.17g of isophorone Diisocyanate (IPDI), react at 45°C, use the di-n-butylamine-acetone method to measure the isocyanate content, monitor the progress of the reaction, when the reaction reaches 50% of the initial value of the isocyanate, add 5.89gN,N -Dihydroxyethyl-3-aminopropionic acid hexafluorobutyl monomer, react at 45°C until the NCO value is 0; then add 4.17g of isophorone diisocyanate (IPDI), and wait for the NCO to react by 50% , then add 2.18g of hydroxyethyl acrylate (HEA) and 0.03g of inhibitor p-methoxyphenol, react until the NCO content is 0 to finish the reaction, and obtain the photosensitive fluorosilicone block polyurethane acrylate oligomer, react The formula is as follows:
[0127]
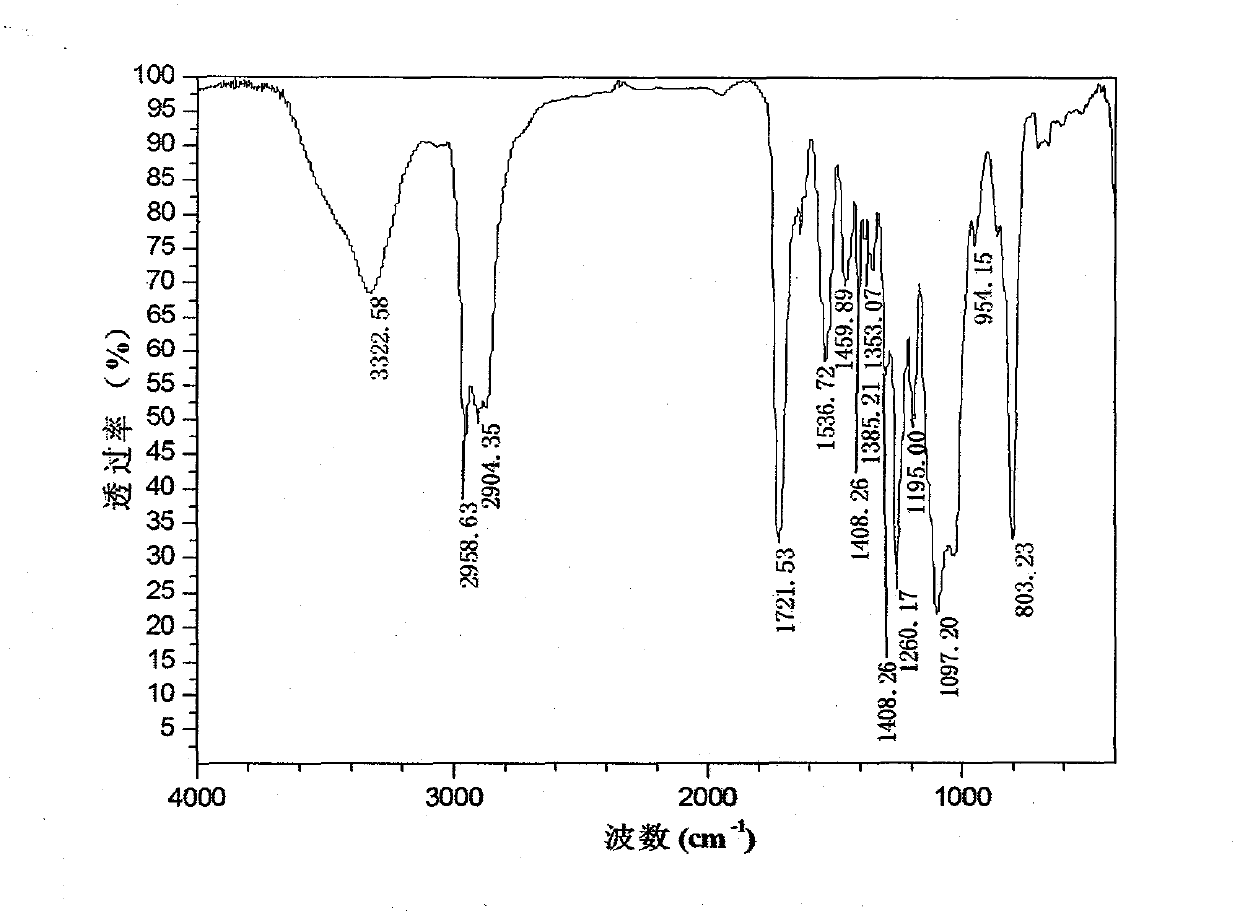
[0128] Wherein the infrared spectrogram of final product sees appendix figure 1 , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com