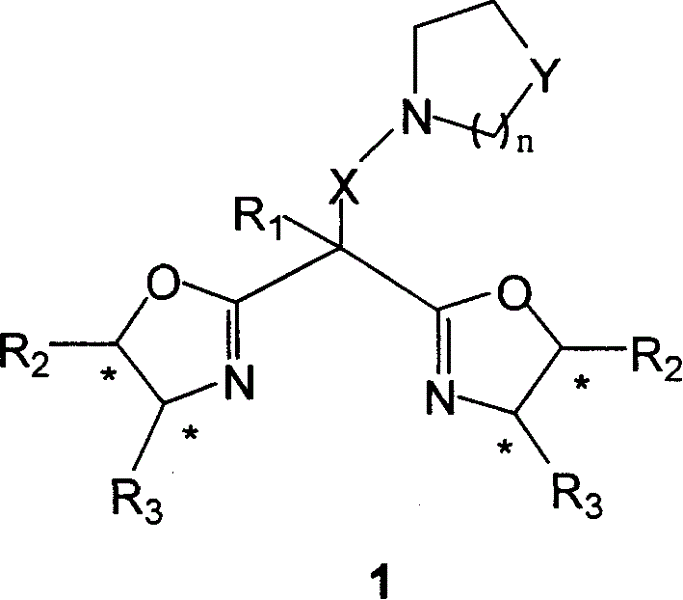
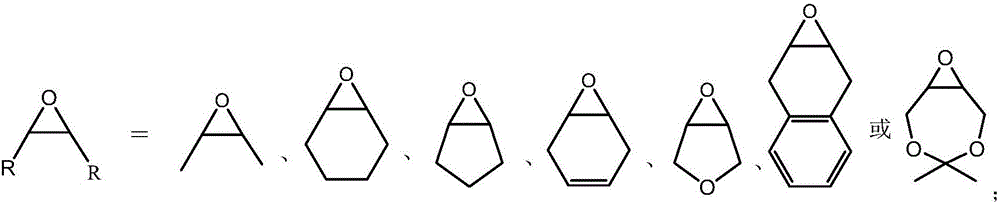
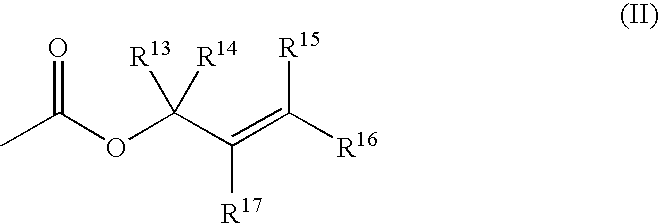
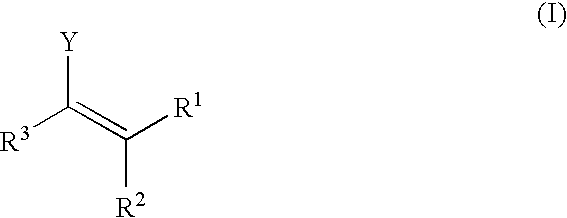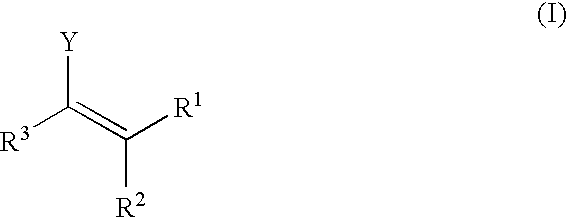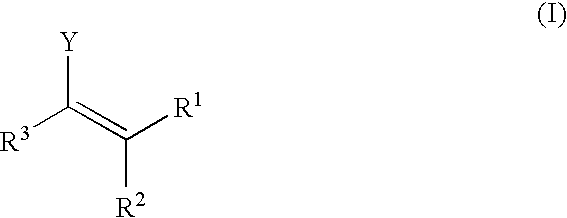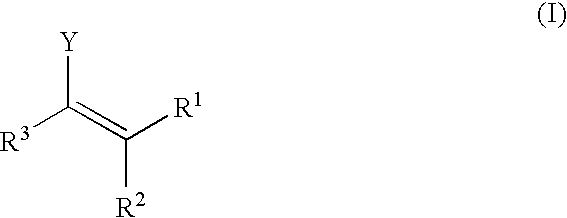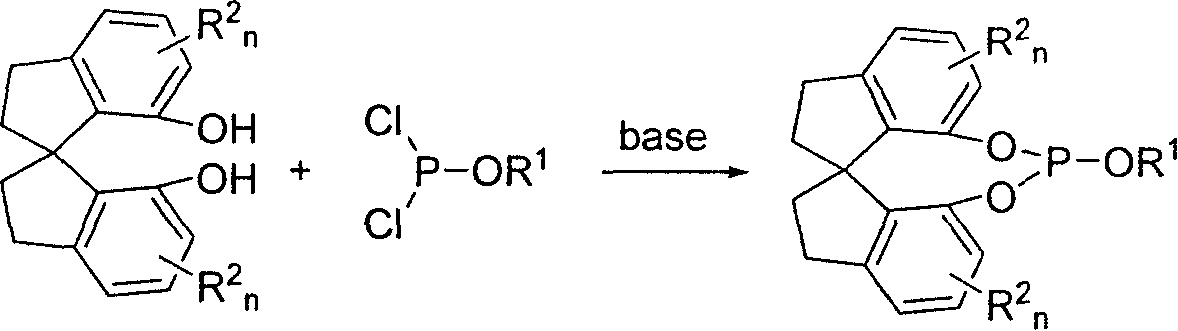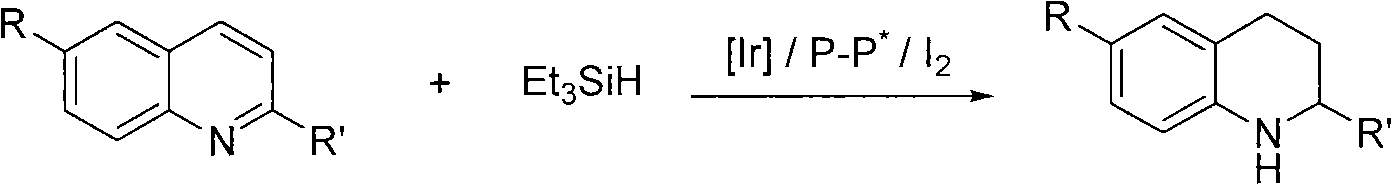Patents
Literature
621 results about "Chiral ligand" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
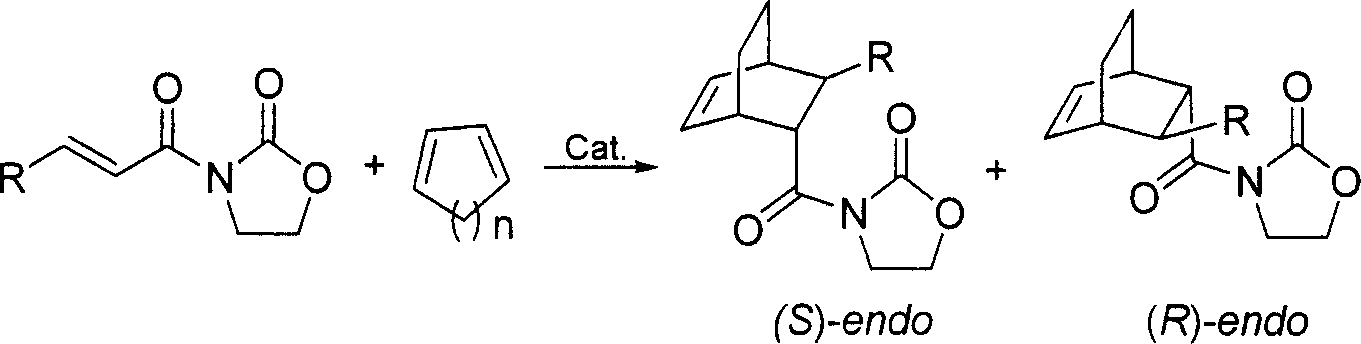
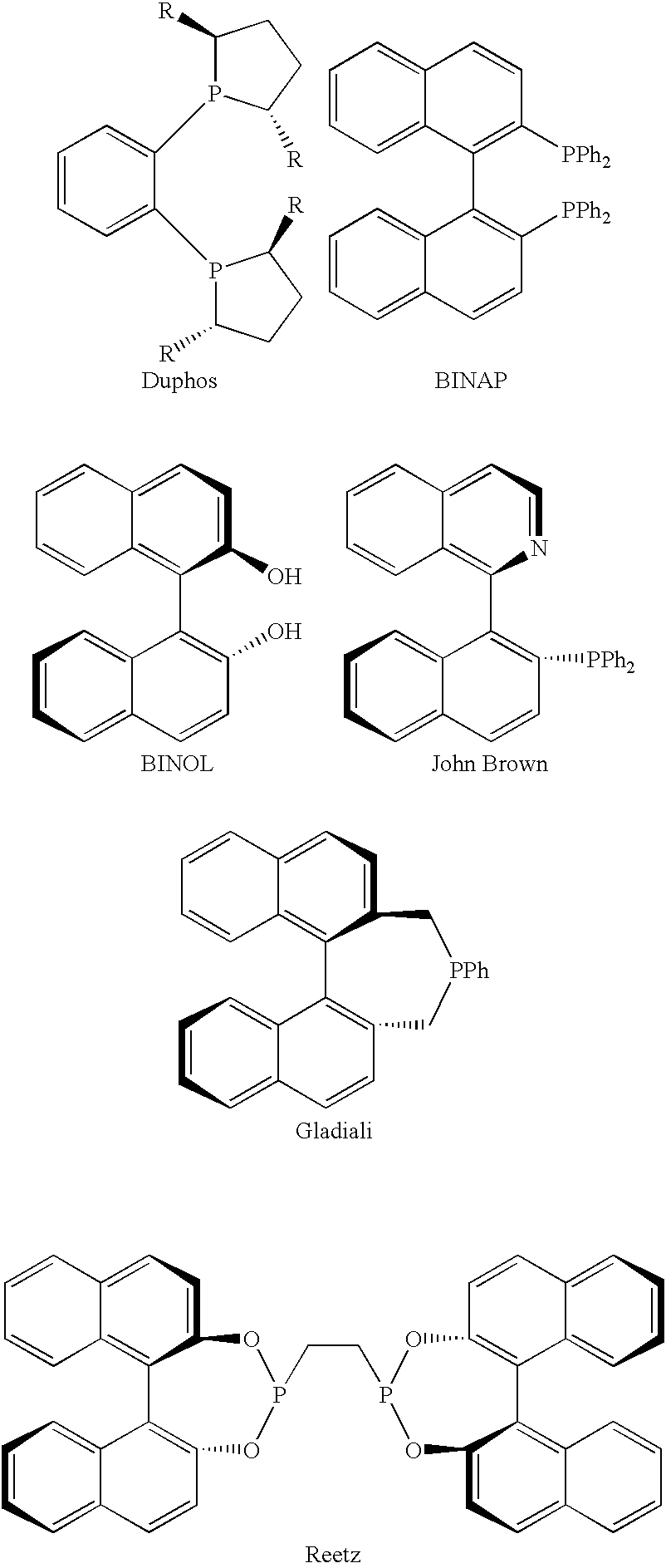
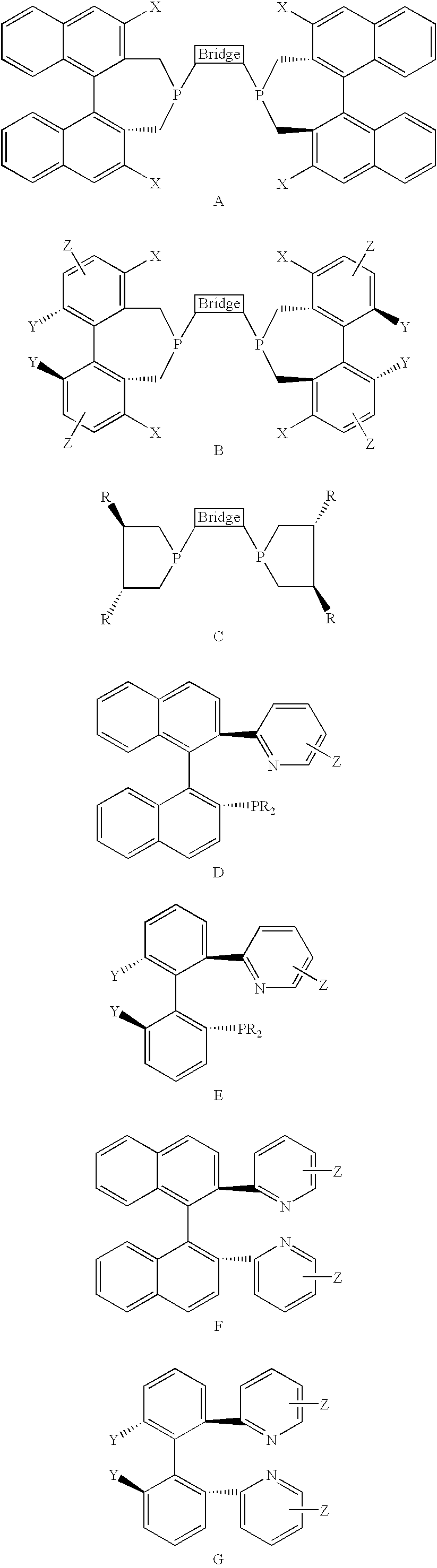
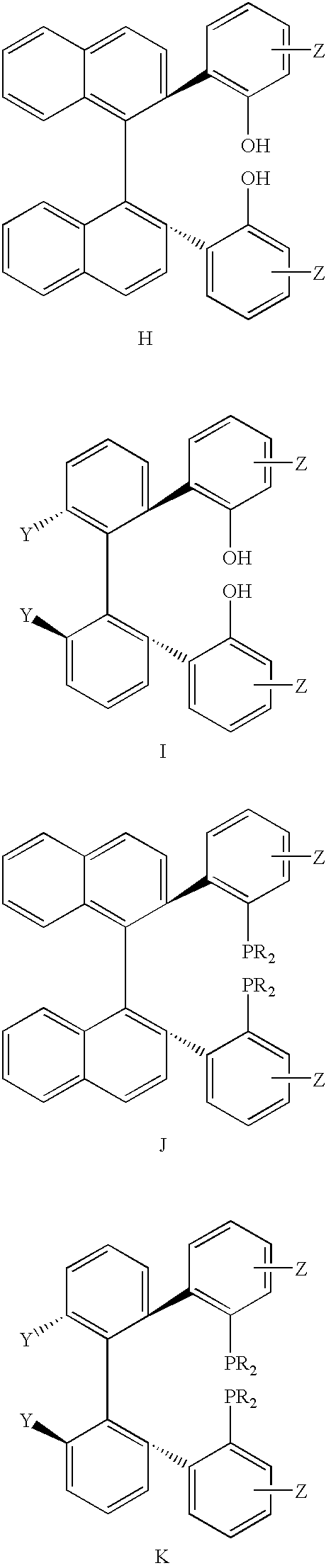
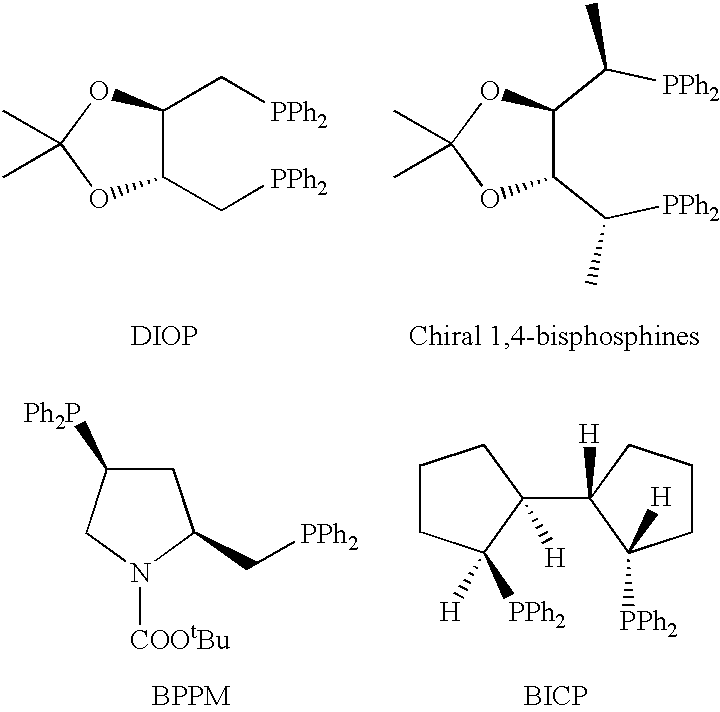
In homogeneous catalysis, a C₂-symmetric ligands usually describes bidentate ligands that are dissymmetric but not asymmetric by virtue of their C₂-symmetry. Such ligands have proven valuable in catalysis. With C2 symmetry, C₂-symmetric ligands limit the number of possible reaction pathways and thereby increase enantioselectivity, at least relative to asymmetrical analogues. Chiral ligands combine with metals to form chiral catalyst, which engages in a chemical reaction in which chirality is transfer to the reaction product. C₂ symmetric ligands are a subset of chiral ligands.
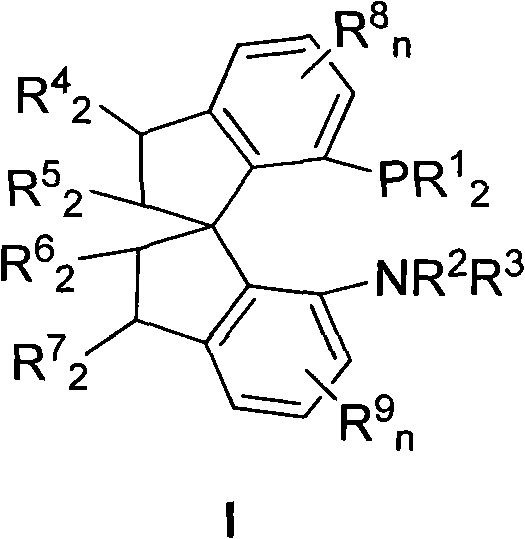
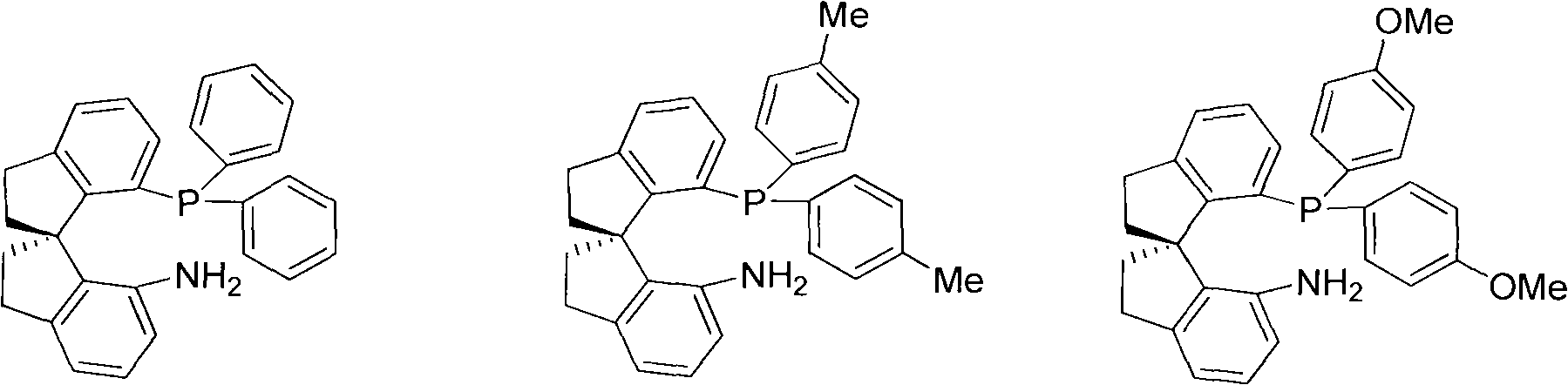
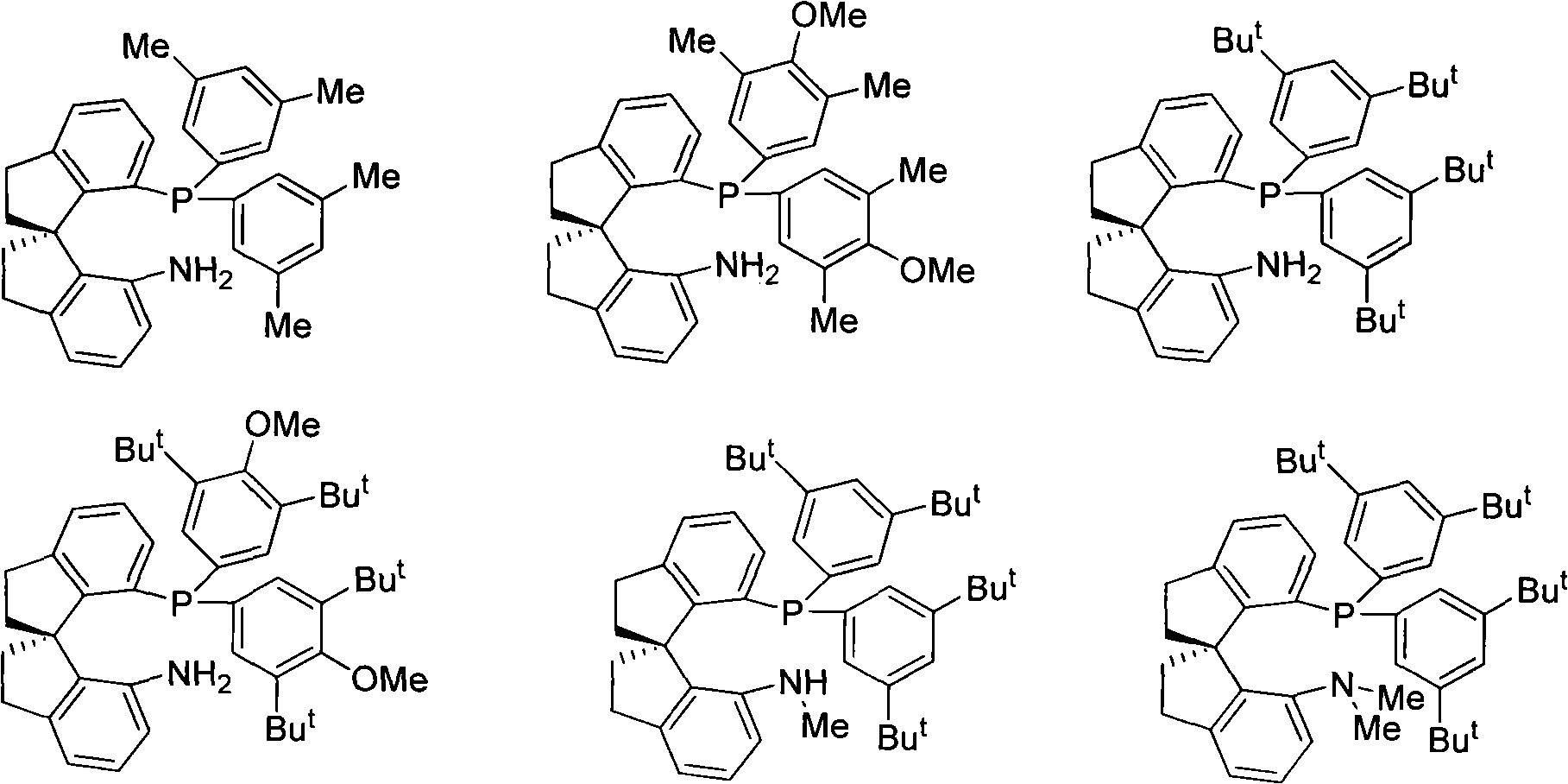
Chiral spiro aminophosphine ligand compound and synthesis method as well as application thereof
InactiveCN101671365AHigh reactivityHigh enantioselectivityOrganic compound preparationGroup 5/15 element organic compoundsIridiumSynthesis methods
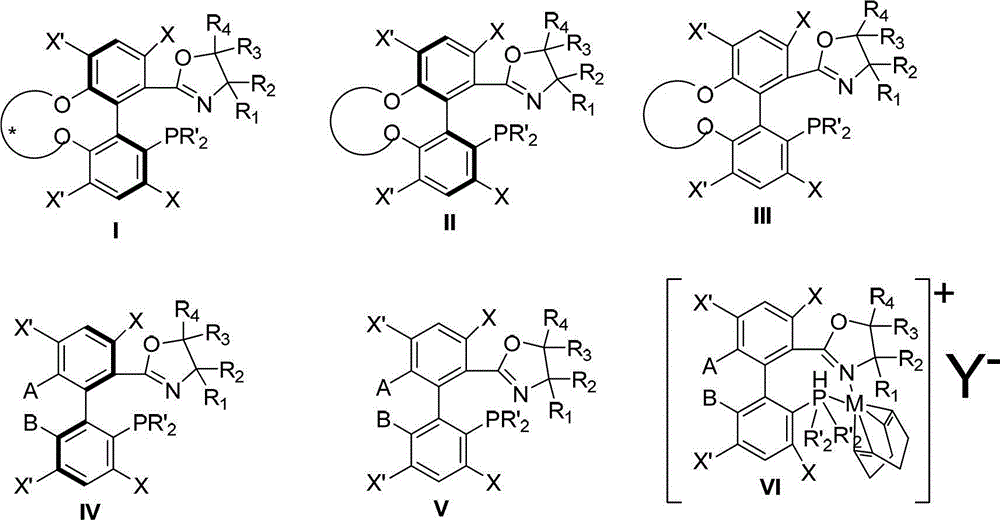
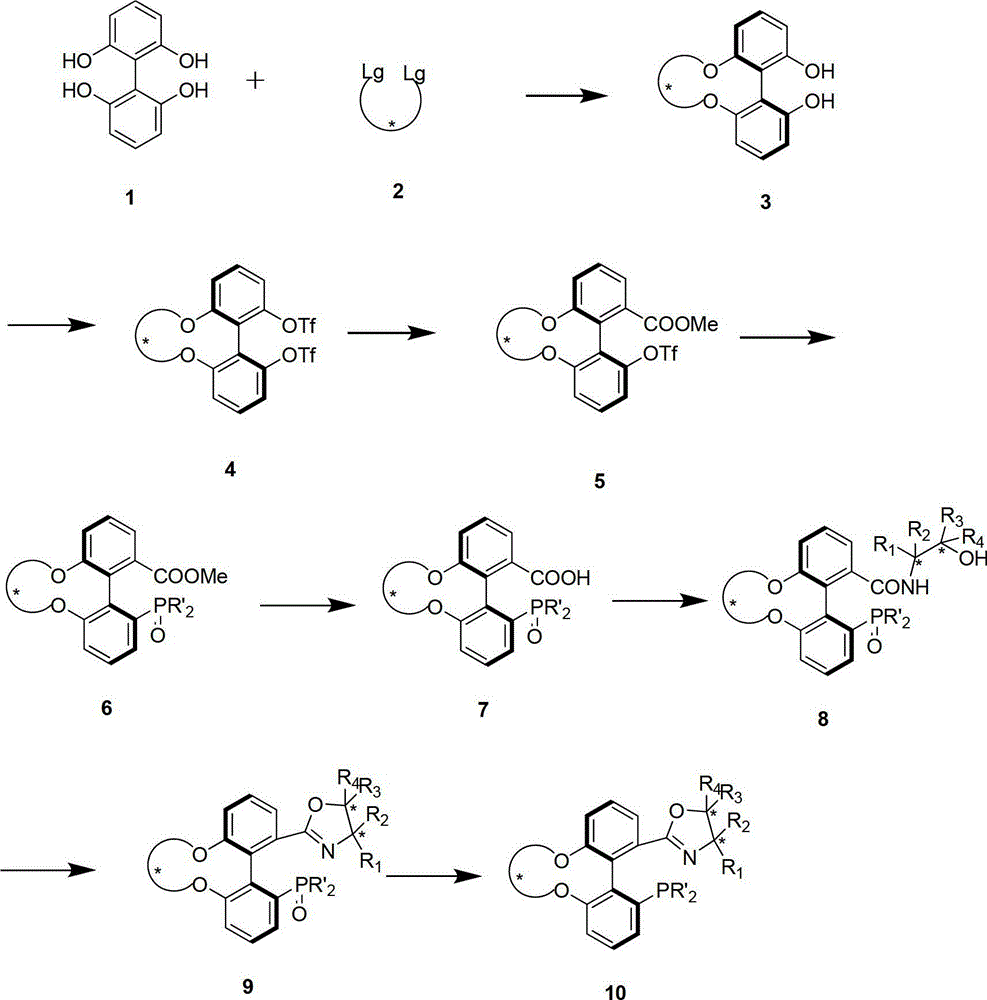
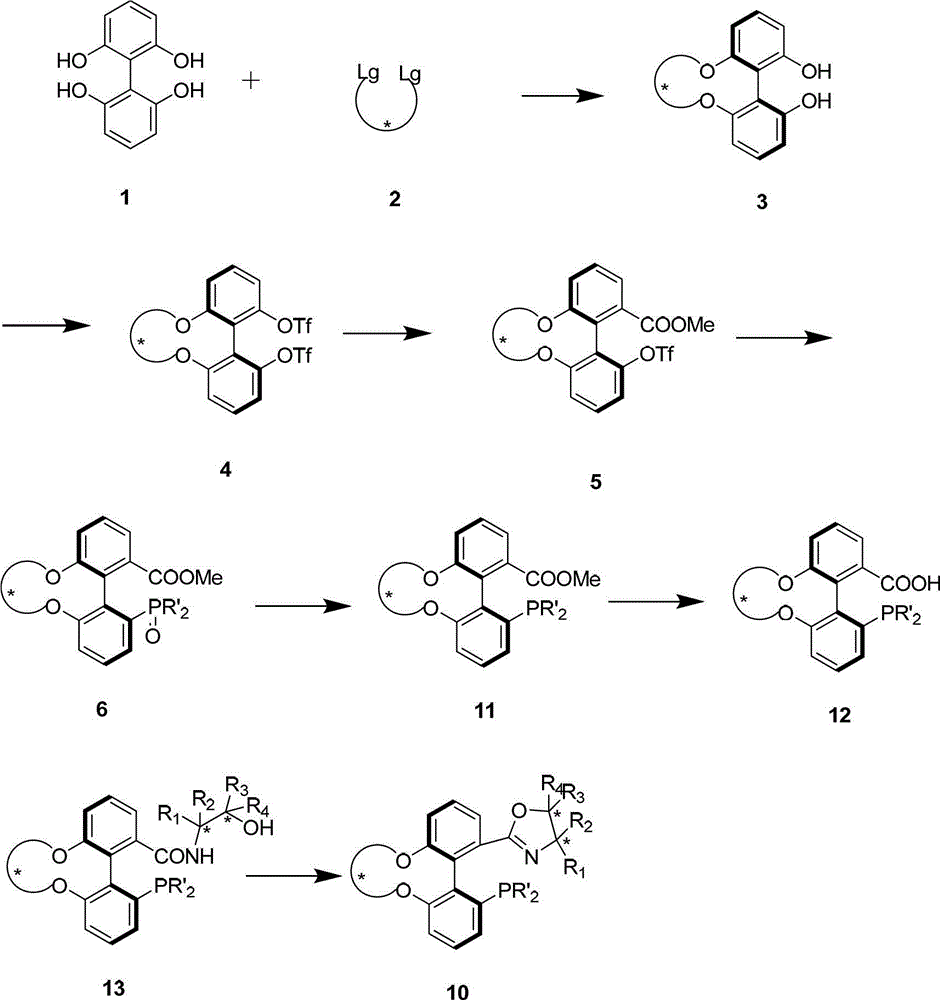
The invention relates to a chiral spiro aminophosphine ligand compound and a synthesis method as well as an application thereof. The chiral spiro aminophosphine compound contains a compound with a structure shown in I, or a racemic body or an optical isomer thereof, or a catalyzed acceptable salt thereof, wherein the main structure characteristic is with a chiral spirobiindane matrix. The chiral spiro aminophosphine compound can be synthesized by taking 7-diaryl / phosphito-7'-carboxy-1, 1'-spirobiindane or substituted 7-diaryl / phosphito-7'-carboxy-1, 1'-spirobiindane as chiral starting materials, which has optical activity and spiro matrix. The chiral spiro aminophosphine compound can be used as a chiral ligand for an asymmetrically catalyzed hydrogenation of an iridium catalyzed carboxy compound and achieves high yield and enantioselectivity (97%ee). The reactive activity is also high and the consumption of the catalyst can be reduced to 0.01% molar.
Owner:NANKAI UNIV
Heteroarylic-arylic diphosphines as chiral ligands
InactiveUS6153758ACarboxylic acid esters preparationNickel organic compoundsCarbon–carbon bondDiphosphines
PCT No. PCT / EP97 / 06358 Sec. 371 Date Apr. 18, 1999 Sec. 102(e) Date Apr. 18, 1999 PCT Filed Nov. 14, 1997 PCT Pub. No. WO98 / 22484 PCT Pub. Date May 28, 1998Diphosphines of a mixed heteroarylic-arylic type, wherein the phosphine group carrying backbone is constituted by the interconnection of a five-atom heteroaromatic ring and a carbocyclic aromatic ring, forming an atropoisomeric chiral system with a C1 symmetry. Said chiral diphosphines are advantageously used as ligands for the formation of chiral complexes with transition metals, in particular Ru, Rh, Pd, Ir, Ni. The so-obtained chiral complexes are used as chiral complexes are used as chiral catalysts for stereocontrolled reactions, in particular diastereo and enantioselective reduction reactions, hydroformylation reactions, hydrosilylation reactions, hydrocyanation reactions, double-bond isomerisation reactions, other reactions of carbon-carbon bond formation.
Owner:CHEMI SPA
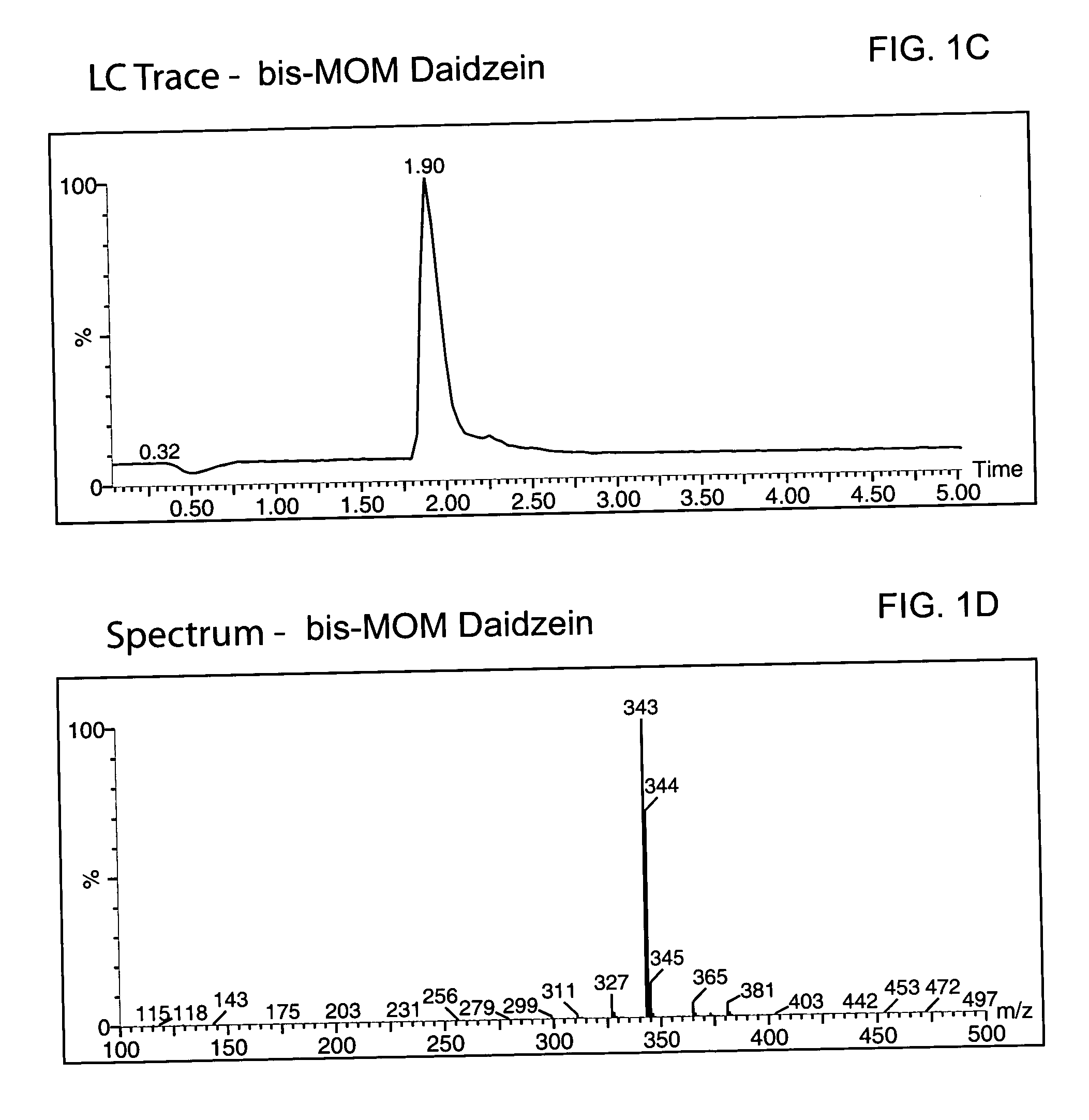
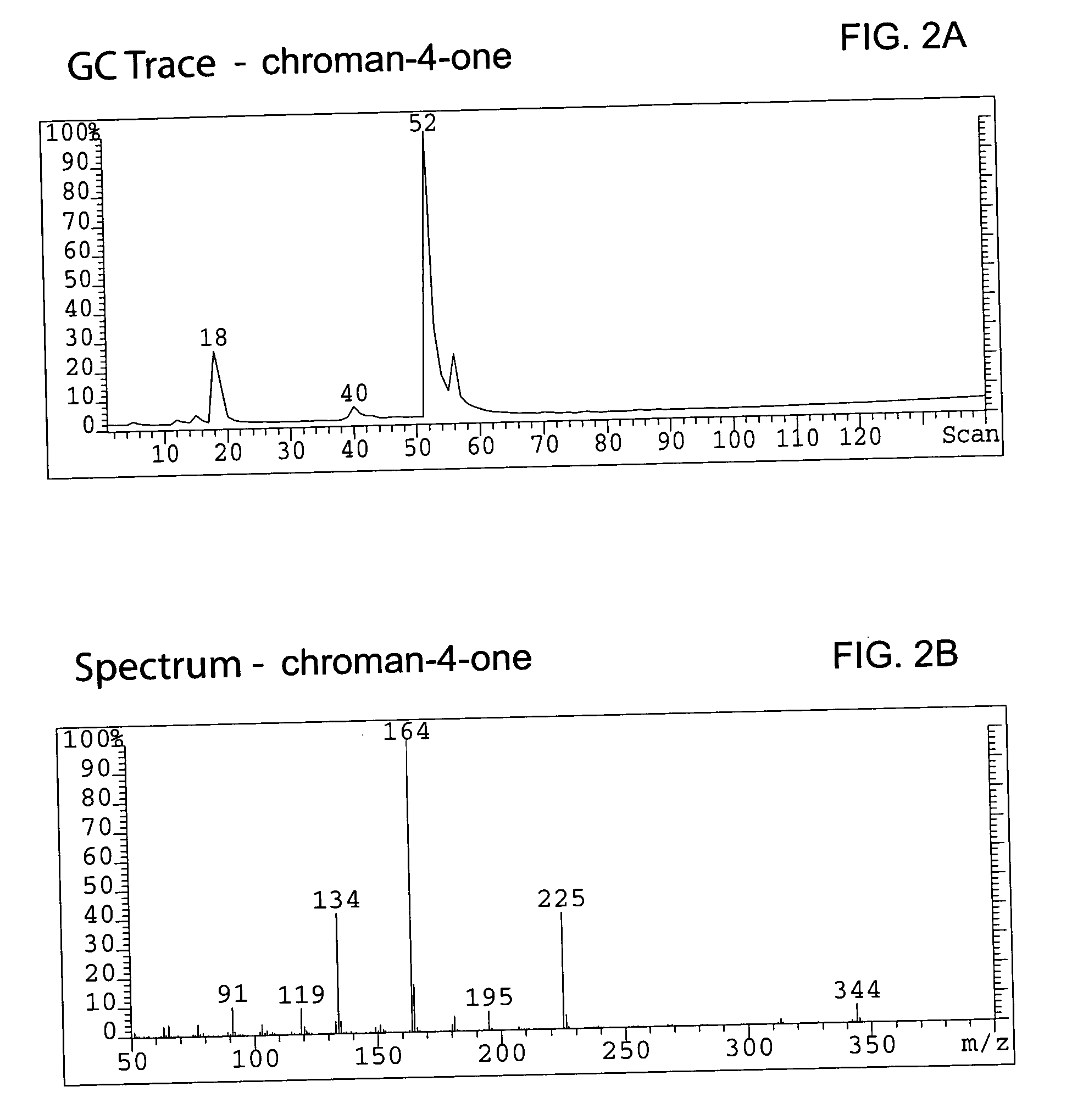
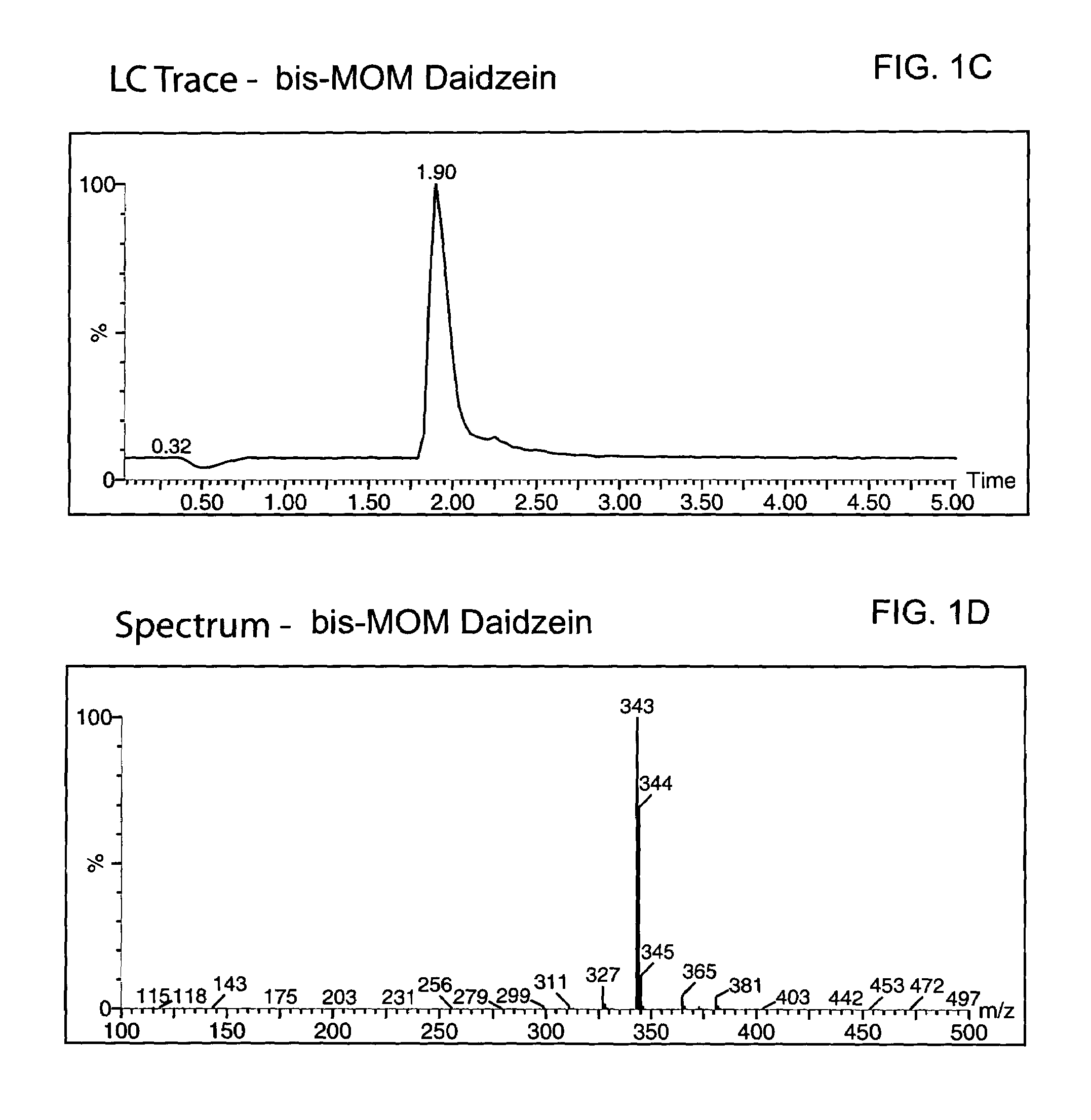
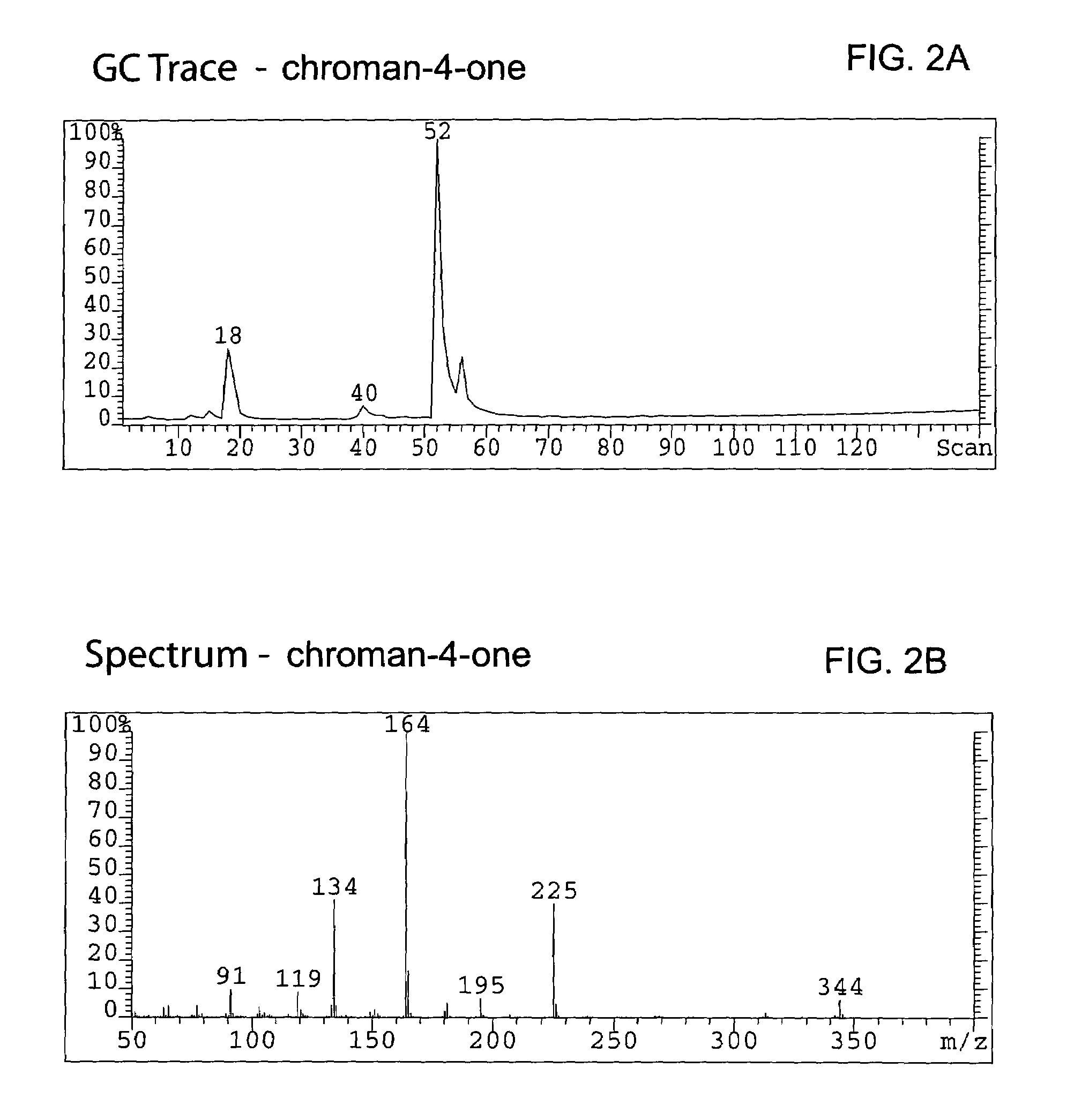
Method for enantioselective hydrogenation of chromenes
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Bimetallic catalyst for synthetizing vertical structure regular makrolon
ActiveCN103102480AMild reaction conditionsThe process is simple and convenientActivated carbonPolycarbonate
The invention relates to a bimetallic catalyst for synthetizing vertical structure regular makrolon through catalyzing and activating carbon dioxide to be copolymerized with internal compensation alkyleneoxide. The catalyst is a dual four-tooth or dual three-tooth Schiff alkali complex, of which two metal centers are connected through a biphenyl skeleton. Under the action of single or nucleophilicity co-catalyst, the catalyst can be used for catalyzing the carbon dioxide efficiently to be copolymerized with the internal compensation alkyleneoxide under mild condition and lower concentration of the catalyst to prepare the makrolon, the makrolon can be regulated when the catalyst efficiency is 104-106g polymer / mole catalyst and the polymer molecular weight is between 103 and 105, the makrolon can be regulated when the molecular weight distribution is less than 2 and the vertical structure regularity is between 60-100%, an alternate structure exceeds 98% and the makrolon can be degraded into a small molecular compound. The product selectivity and structure selectivity of the polymer compounds of the catalyst system using a chiral ligand are all above 98%, the enantiomer excess value of the mellow obtained by degradation reaches as high as 99%, so that the bimetallic catalyst provides a broad prospect for industrial application.
Owner:DALIAN UNIV OF TECH
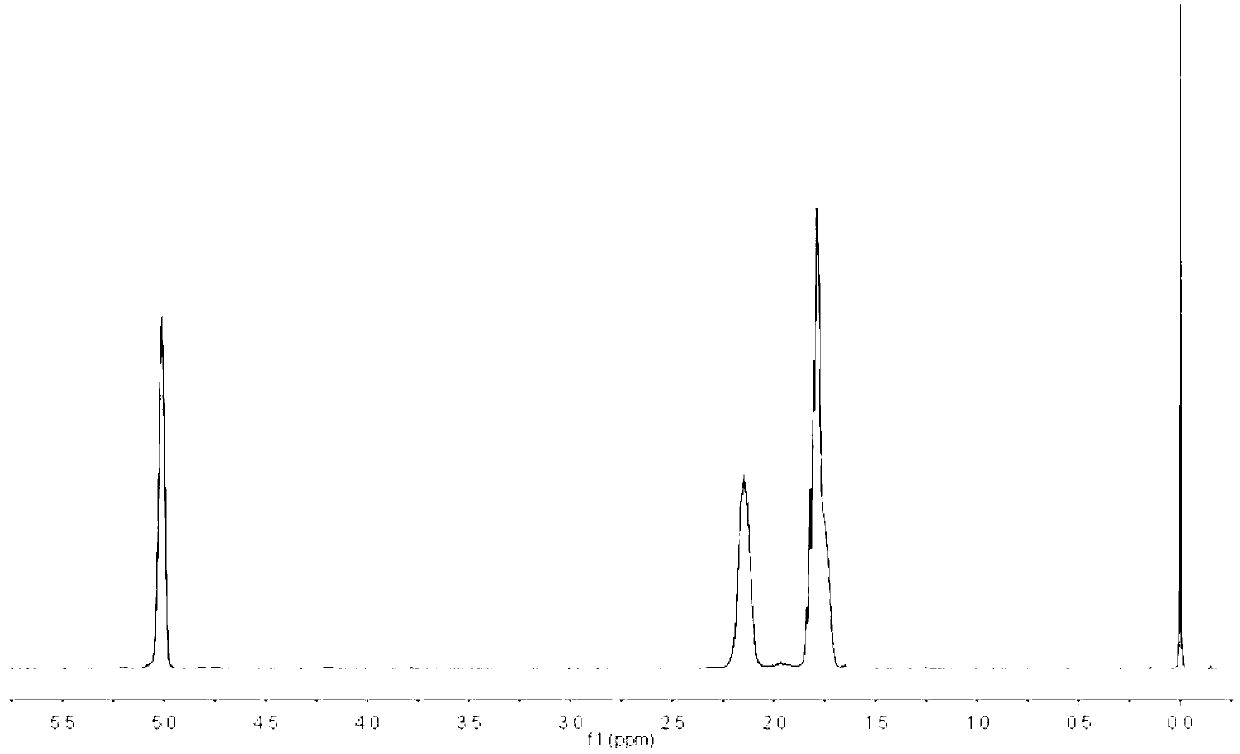
Preparation method of brivaracetam
ActiveCN106432030ARaw materials are easy to getLow priceOrganic chemistryBrivaracetamColumn chromatography
The invention provides a method for synthesizing brivaracetam. Raw materials used in the method are available and inexpensive, a preparation process avoids appearance of chiral isomers difficult to separate, purification means such as column chromatography purification and the like adverse to industrial amplification production is avoided, a synthesis process is not involved with expensive and toxic heavy metal and chiral ligands, and a product brivaracetam with high quality and high optical purity can be obtained.
Owner:SUZHOU PENGXU PHARM TECH CO LTD +1
Dual functions ligand compound of chirality dioxazoline, preparation and application
InactiveCN1626524AEasy to getEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical compoundSubstitution reaction
A bifunctional chiral bioxazoline ligand used as the catalyst for asymmetrical reactions, such as cyanosiliconizing reaction, is prepared from propylbinitrile or its derivative through substitution reaction and cyclizing reaction on chiral aminoalcohol, or from the bioxazoline methane through substitution reaction.
Owner:PEKING UNIV
Non-interpenetrating chiral MOF stationary phase, its preparation method and application in enantiomer separation in HPLC
InactiveCN103331151ARaw materials are cheap and easy to getEasy to operateAmino compound purification/separationOther chemical processesEnantiomerStructural formula
The invention relates to a non-interpenetrating chiral MOF (metal organic framework) stationary phase, its preparation method and application in enantiomer separation in HPLC (high-performance liquid chromatography). The stationary phase is a non-interpenetrating chiral three-dimensional porous framework complex with a structural formula as {[ZnL].H2O}n. An asymmetric structural unit {[ZnL].H2O} of the complex is composed of a Zn<2+>, an L ligand and a guest water molecule. The L ligand is -NH- containing chiral pyridine carboxylic acid, its chemical composition is [(N-(4-pyridylmethyl)-L-leucine.HBr)], and its molecular formula is C12H19BrN2O2. Chiral amino acid and 4-pyridylaldehyde are selected as raw materials to synthesize the-NH- containing pyridine carboxylic acid chiral ligand by a one-step process. The ligand and zinc acetate are adopted as raw materials to undergo room temperature diffusion so as to obtain the MOF stationary phase. The material provided in the invention has uniform chiral helical channel, uniform aperture and orifice, and can be used for separation of chiral drugs and other enantiomers. The separation is selectively dependent on the size of a separated enantiomer molecular size, but is not dependent on the functional group of the separated enantiomer. Thus, the non-interpenetrating chiral MOF stationary phase has the characteristics of traditional zeolite molecular sieve separation.
Owner:SHANDONG NORMAL UNIV
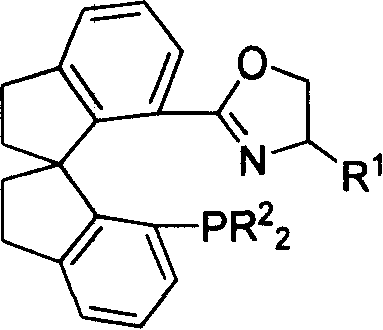
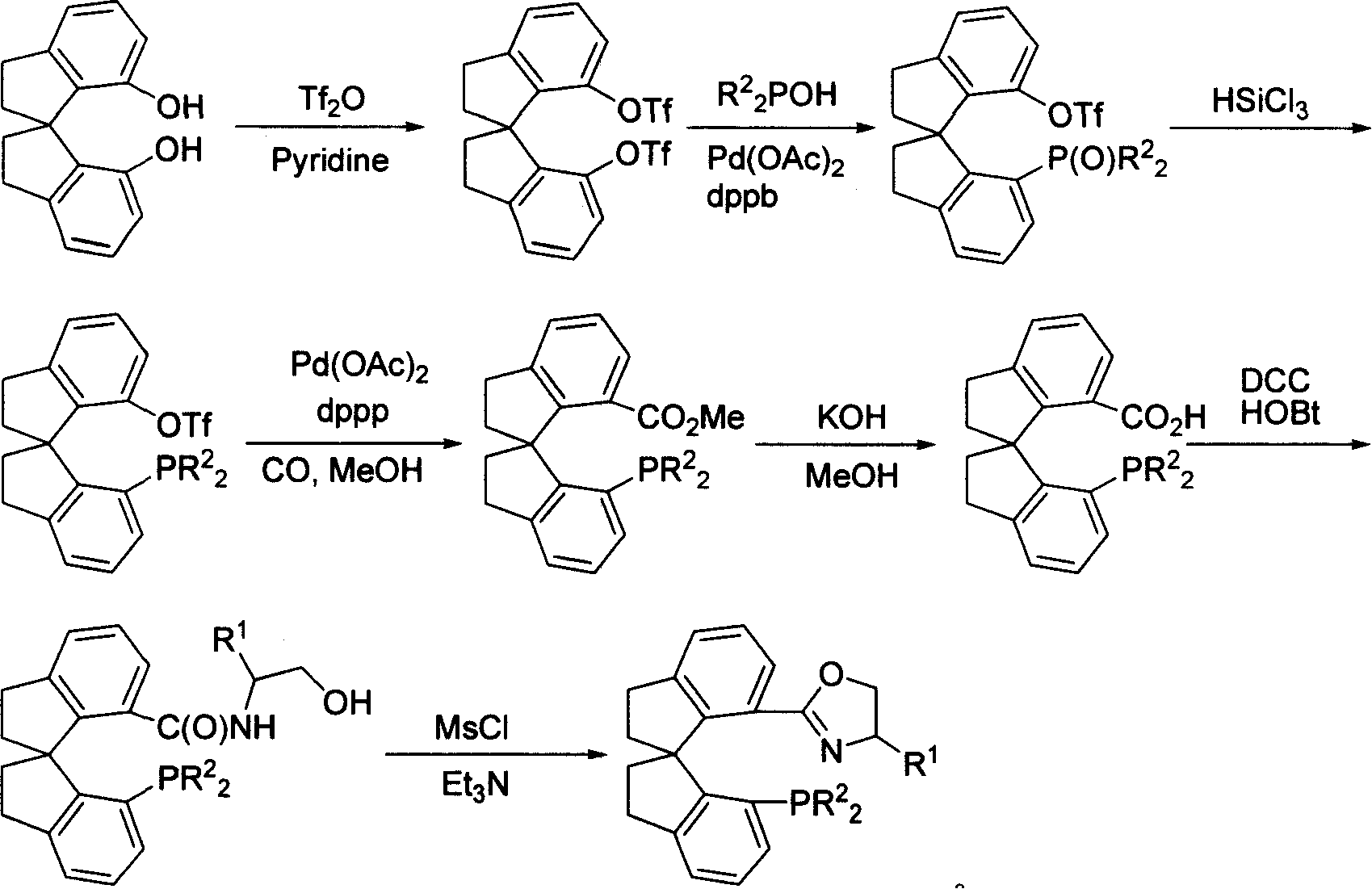
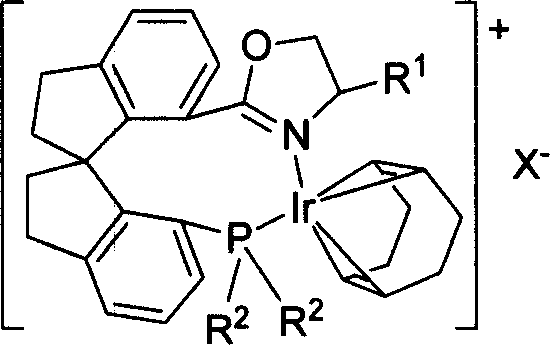
Phosphorus-oxazoline ligand with spiro backbone and its uses in asymmetrical catalytic hydrogenation
ActiveCN1884290AHigh stereoselectivityHigh reactivityGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsOrganic chemistryMedicinal chemistry
The invention relates to a new chiral toroid phosphine oxazoline ligand and its ionic iridium complex compound, and the application of said iridium complex compound in asymmetric catalytic hydrogenization for imines. The toroid phosphine oxazoline ligand is a widely used compound, for example, used as chiral ligand for asymmetric catalytic hydrogenization; especially said iridium complex compound is characterized by high stereoselectivity for asymmetric catalytic hydrogenization, the ee value reaches 97% and high reactive activity.
Owner:NANKAI UNIV
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
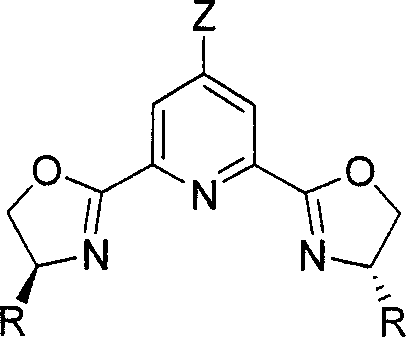
Chirality pyridine double-oxazoline catalyzer and method for preparing the same and application thereof
InactiveCN101116828AHigh activityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationOxazolidoneLanthanide
A catalyst of chiral bis-oxazolinylpyridine is provided, which comprises two parts including a 4-z-2, 6-bis [4'(s)-R oxazoline-2'-] pyridine as the chiral ligand of the bis-oxazolinylpyridine, and a Scandate, Yttrium and Lanthanide metal salt. The preparation method is to mix the metal salt, the ligand of bis-oxazolinylpyridin with the equal molar quantity, and a 4-molecular sieve together, the mixture is added with the needed solvent, and then stirred under the temperature of -78 to 25 DEG C to obtain the catalyst prepared in situ. The usual dosage of the catalyst used for catalyzing Diels-Alder reaction is 1percent to 5percent of the dosage of the substrate. The catalyst can catalyze Diels-Alder reaction of both alpha, beta-unsaturated N-acetyl oxazolidone and alpha, beta-unsaturated ester with high efficiency in high selectivity. Under condition of the normal pressure with the temperature of 0 to 25 DEG C, the catalyst can accomplish the conversion rate as high as 100 percent and the enantiomeric selectivity of 96 percent.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for manufacturing plasma chiral ligand sensor for mercury ions
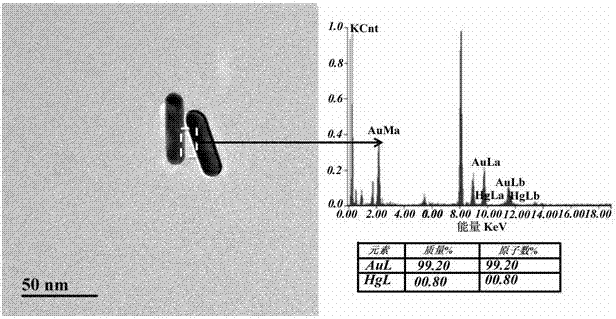
ActiveCN102879336AIncrease the number ofEasy to buildMaterial analysis by optical meansGold nanorodLinear relation
The invention provides a method for manufacturing a plasma chiral ligand sensor for mercury ions, and belongs to the technical field of analytical chemistry and the technical field of food safety. The method mainly comprises the following steps of: (1) synthesizing gold nanorods; (2) performing directional nucleic acid functionalization on surfaces of the gold nanorods; (3) constructing the plasma chiral ligand sensor by using the gold nanorods after the surfaces of the gold nanorods are subjected to the directional nucleic acid functionalization; (4) representing the plasma chiral ligand sensor; (5) measuring the specificity of the plasma chiral ligand sensor; and (6) measuring an actual sample of the plasma chiral ligand sensor. Along with increase of Hg2+ concentration, the number of the gold nanorods in a gold nanorods side surface assembly body is increased, and the Mohr ellipticity of the assembly body is also increased. The linear relation between the Mohr ellipticity and the concentration of the mercury ions is set up, and the plasma chiral ligand sensor which is simple and sensitive and is high in specificity is manufactured and is used for detecting the concentration of the mercury ions.
Owner:JIANGNAN UNIV
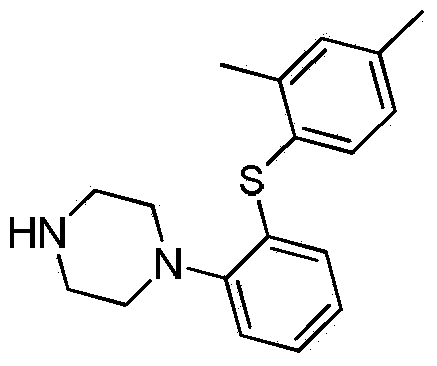
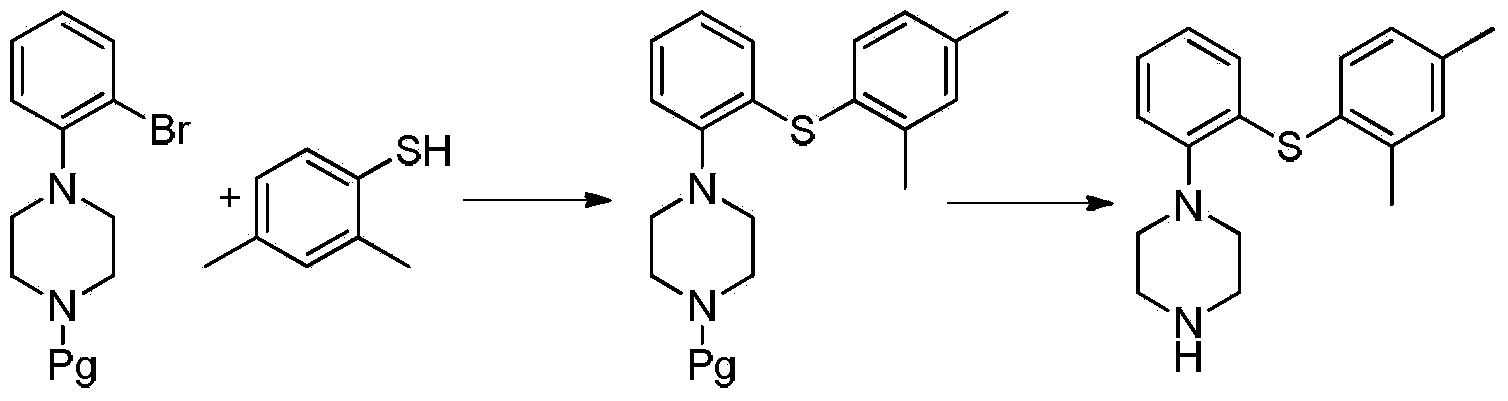
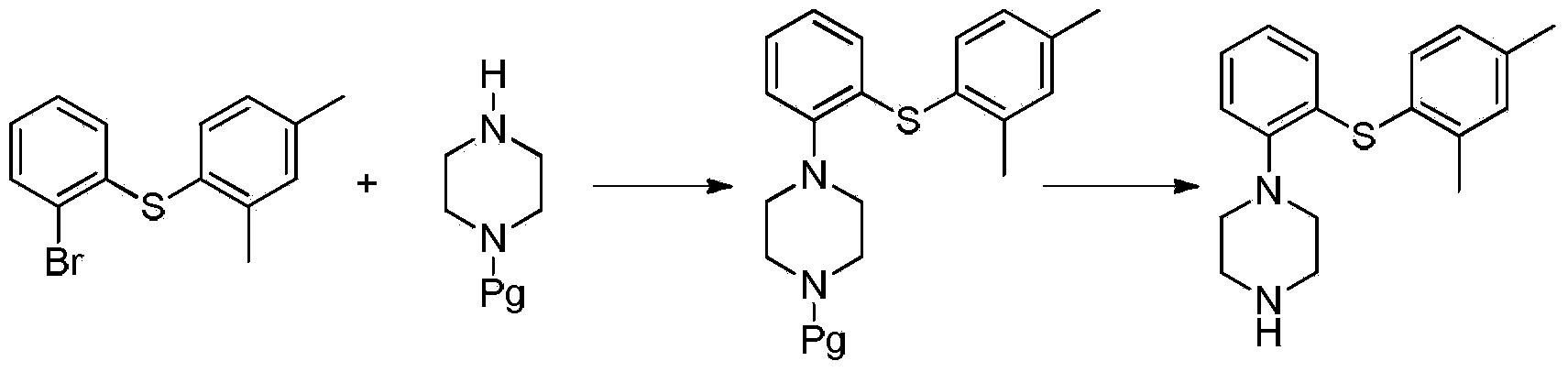
Preparation method of Vortioxetine
InactiveCN104098530ARaw materials are easy to getSimple processOrganic chemistryCopper iodideCoupling reaction
The invention relates to the preparation method of Vortioxetine. The preparation method is characterized by comprising the steps of: obtaining a compound (formula 2) by carrying out coupling reaction on compound 2,4-dimethylbenzenethiol (formula 4) and compound 2-bromoiodobenzene (formula 3) in the presence of copper iodide, chiral ligand and alkali; obtaining a compound (formula 1) through the reaction of compound in formula 2 and piperazine in the presence of copper iodide, chiral ligand and alkali; or operating the twp steps in one reactor by one-pot method; the preparation method is easy to obtain material, simple in technology, high in product purity, few in by-products and beneficial to industrial production of the bulk drug.
Owner:冯修武
Asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid
InactiveCN105481622AHigh catalytic activityHigh enantioselectivityOrganic reductionOrganic compound preparationEnkephalinase inhibitorAsymmetric hydrogenation
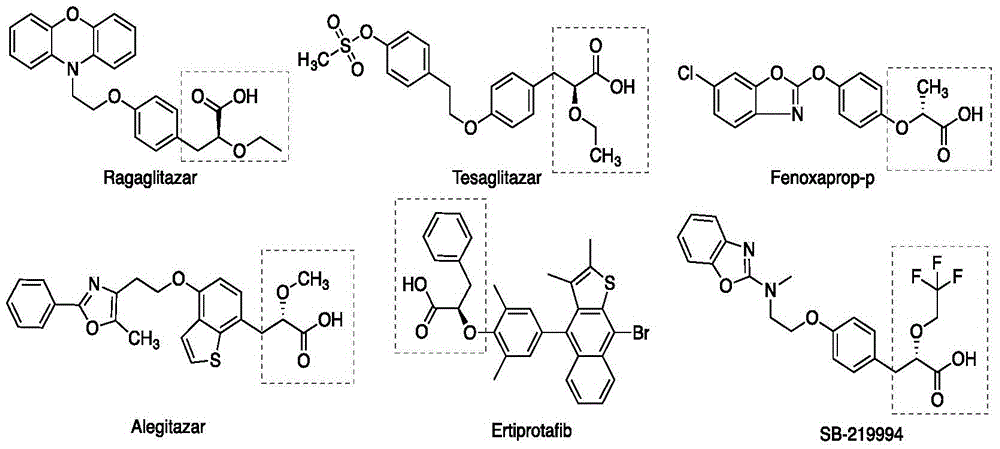
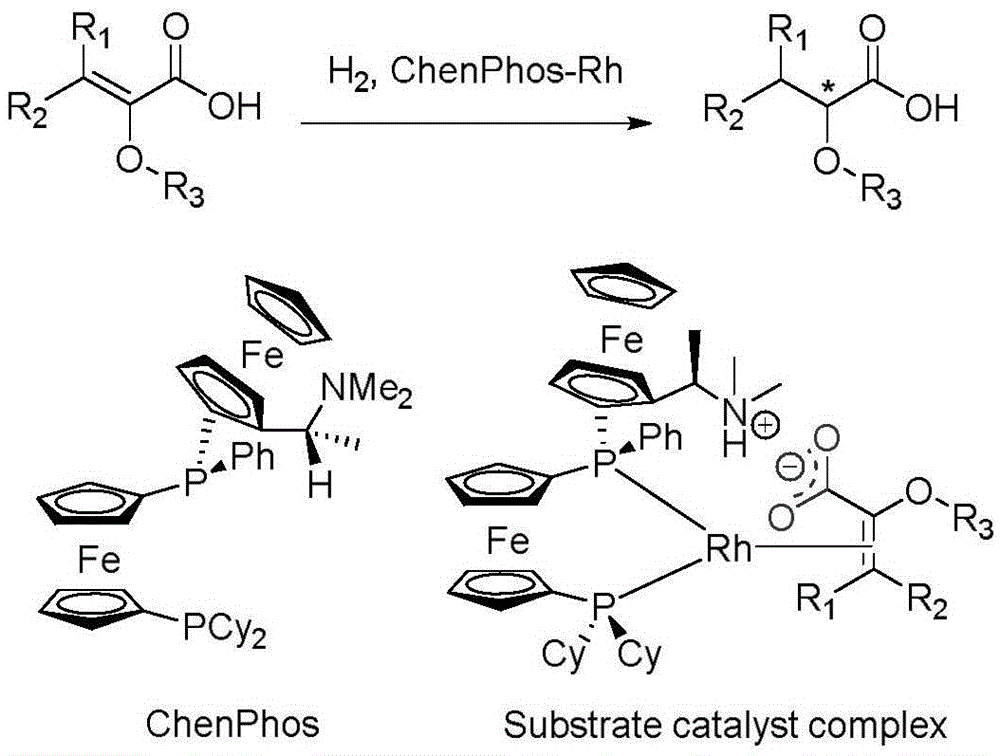
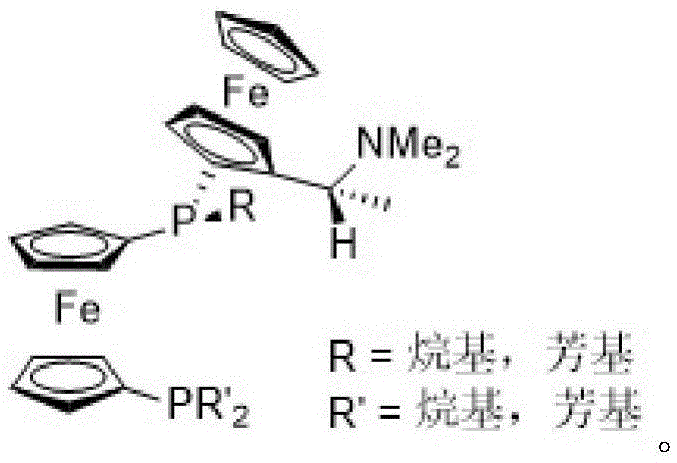
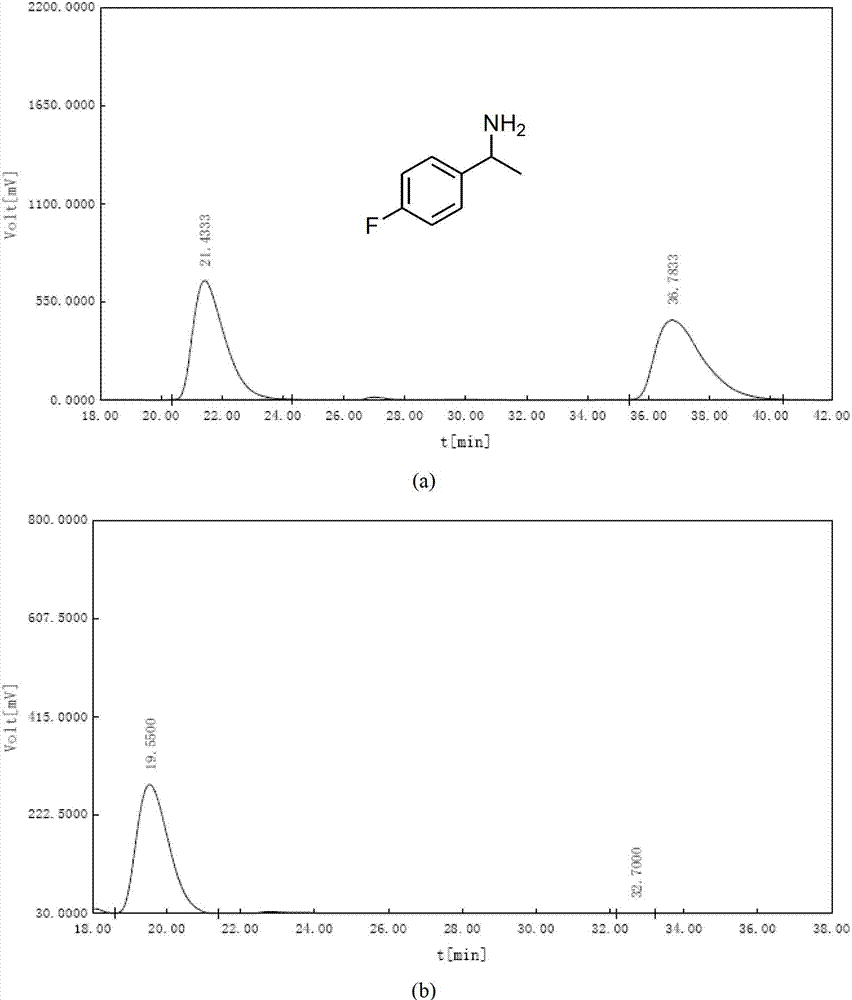
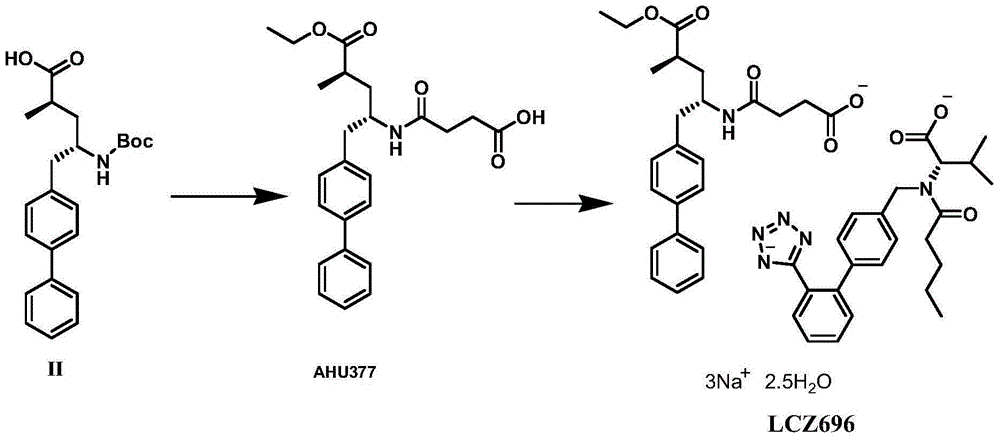
The invention relates to an asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid. A metal complex containing ChenPhos chiral ligand is a catalyst high in conversion efficiency, and particularly, the catalyst can be used for synthesizing a core framework in enkephalinase inhibitor Sacubitril through asymmetric hydrogenation. The inhibitor is one of components of medicine LCZ 696 approved by American Food and Drug Administration. The asymmetric hydrogenation method of the alpha-oxo-alpha, beta-unsaturated carboxylic acid is efficient, and the application range of substrate is wide.
Owner:WUHAN CATALYS TECH CO LTD
Applications of chiral polymer catalyst in asymmetric reaction
ActiveCN106853380AThe synthesis method is simpleShort reaction timeOrganic compound preparationOrganic chemistry methodsDispersityPorosity
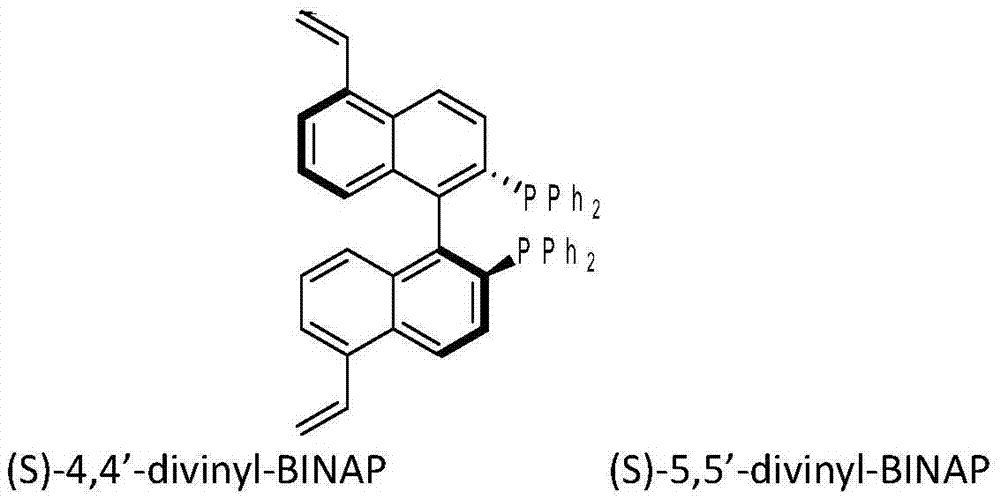
The invention discloses applications of a chiral polymer catalyst in asymmetric reaction, and belongs to the field of material synthesis and application. The phosphine-containing polymer is obtained via mixed polymerization of vinyl-containing chiral bidentate phosphine ligand BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) and derivative of vinyl-containing chiral bidentate phosphine ligand BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) with other vinyl comonomers. The vinyl polymer possesses relatively large specific surface area and porosity, and excellent thermal stability and chemical stability. In the heterogeneous catalyst, one or a plurality of elements selected from Ru, Rh, Ir, Pa, Au, and Cu are taken as active ingredients. The chiral ligand is uniformly embedded into and highly dispersed in a polymer skeleton, so that metal dispersion degree on the catalyst is relatively high, and relatively high catalytic activity is achieved. The heterogeneous catalyst is suitable for a plurality of reaction technology including intermittent still reaction, continuous fixed bed reaction, and trickle bed reaction. When the heterogeneous catalyst is used in catalytic kettle-type asymmetric hydrogenation, high target product yield is achieved, and enantioselectivity is higher than 96%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of mono-chiral metallic organic frame material with function of splitting chiral amine
InactiveCN103113411AShort reaction timeEasy to operateIon-exchange process apparatusOxygen-containing compound preparationManganesePhenyl group
The invention provides a preparation method of a mono-chiral metallic organic frame material with a function of splitting chiral amine. The method comprises the following steps of: dissolving manganese salt and mono-chiral ligand (S)-3,3'-di-tert-butyl-5,5'-di(3,5-dicarboxylphenyl)-6,6'-dimethyl-2,2'-dihydroxyl-1,1'-bipheny into methanol and N,N-dimethyl formamide according to certain proportions, stirring for 10 minutes and then heating up to 80-85 DEG C, reacting for about 24 hours to generate colorless needle-like crystals, and then washing with aether to obtain the structurally stable mono-chiral metallic organic frame material with the function of splitting the chiral amine. The preparation method provided by the invention has the characteristics that the preparation condition is mild, the operation is simple, the separation ee value of the chiral amine is higher than 90%, and the chiral amine is reusable.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method of NEP (neutral endopeptidase) inhibitor intermediate
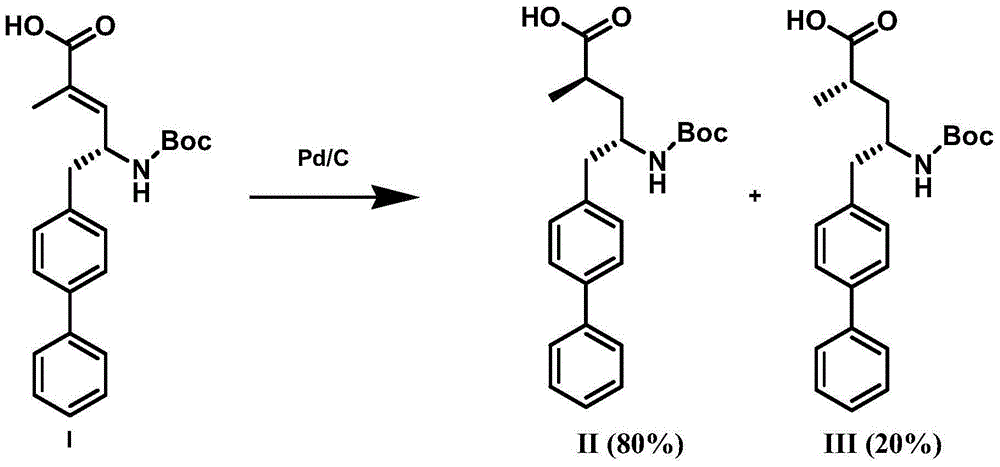
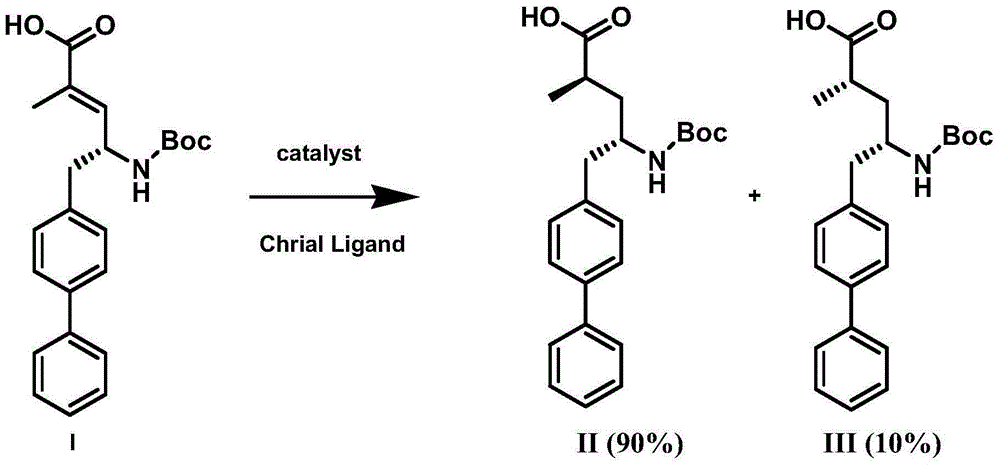
ActiveCN105061263ACarbamic acid derivatives preparationOrganic compound preparationHydrogenTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of an NEP (neutral endopeptidase) inhibitor intermediate, further discloses a selective reduction preparation method of the NEP inhibitor intermediate (2R, 4S)-5-([1,1'-biphenyl]-4-yl)-4-((t-butyloxycarboryl)amino)-2-methylpentanoic acid in presence of a chiral ligand, and particularly discloses a diastereoselective hydrogenation synthetic method with hydrogen under the condition of presence of a transition metal catalyst and the chiral ligand. The metal catalyst used in the method is cheap, the chiral ligand is obtained easily, high yield is realized, a high-purity product is produced preferably, and the product with the proportion of diastereoisomers being 90:10 is produced preferably.
Owner:苏州楚凯药业有限公司
Phosphine ligand and enantiomer or racemic body thereof and preparation methods thereof
ActiveCN102532196AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsIridiumSynthesis methods
The invention discloses a phosphine ligand and an enantiomer or a racemic body thereof and preparation methods and applications thereof. The structural formulae of the phosphine ligand and the enantiomer or the racemic body are shown in the specifications; a phosphine ligand compound has a novel framework; complete transfer of planar chirality to axial chirality in a synthesis process is realized through a desymmetrization reaction; a synthesis method is simple and economical; during preparation of a chiral ligand, common complex chiral splitting processes are avoided; and an obtained chiral ligand has the advantages of high reaction activity, high enantioselectivity and the like in a model reaction, can be applied to catalytic reactions of a plurality of metals such as palladium, rhodium, nickel, copper, iridium, ruthenium, iron, cobalt, gold, platinum and the like, and can have a very good catalytic effect.
Owner:SUN YAT SEN UNIV
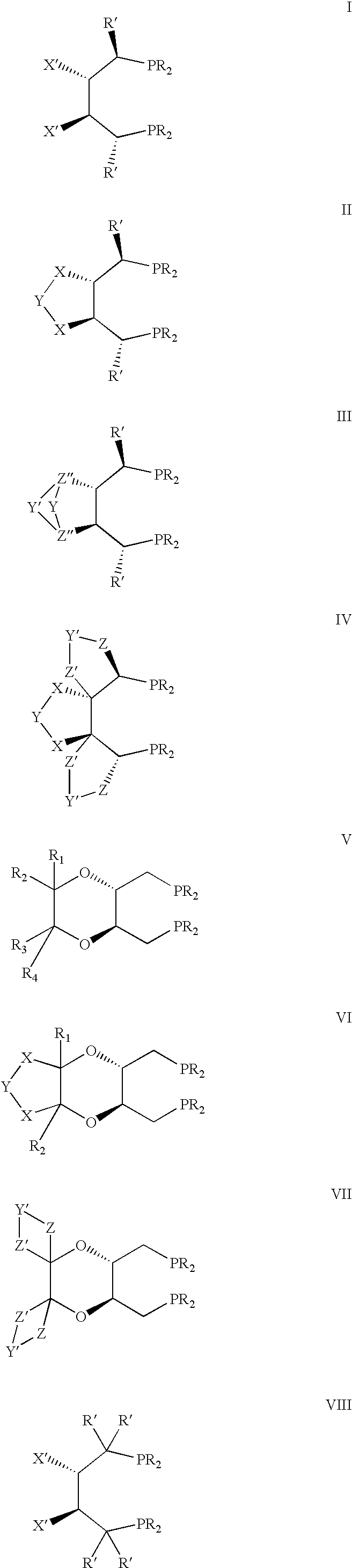
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6525210B1Carboxylic acid amides optical isomer preparationPreparation by carbon monoxide reactionIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
Rhodium/carbon nanotube catalyst and preparation method and application
InactiveCN104056622AEasy to operateEvenly dispersedOrganic compound preparationCarboxylic acid esters preparationFormylation reactionCarbon nanotube
The invention relates to a rhodium / carbon nanotube catalyst of rhodium nanoparticle supported in a tube chamber of a carbon nanotube used for an olefin hydrogen formylation reaction, the catalyst takes the carbon nanotube as a carrier, the rhodium nanoparticle can be dispersed in the tube chamber, the size is uniform and the particle size is 1-3nm; the rhodium / carbon nanotube catalyst appears higher activity by comparing with other carrier supported catalyst in the hydrogen formylation reaction of styrene, the highest TOF value can reach more than 300h<-1>, when an appropriate phosphite ester ligand is added in the reaction system, activity is increased, the highest TOF value can reach more than 1000h<-1> which is the highest intrinsic reaction activity obtained in the styrene hydrogen formylation reaction of a heterogeneous catalyst; the selectivity of product aldehyde generated by catalysis of the catalyst is high and can reach more than 99%, the selectivity for producing branched chain aldehyde can reach 94%; and 42% of enantioselectivity can be obtained in the presence of the chiral ligand.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method for stereoregularity polyester and bi-metallic catalyst
The invention relates to a synthesis method for stereoregularity polyester and a bi-metallic catalyst. The catalyst is a bi-nuclear metal complex formed by connecting two metal centers through a biphenyl or dinaphthalene framework. The catalyst can efficiently catalyze a cyclic anhydride and internal compensation epoxyalkane polymerization reaction under the mild condition and low catalyst concentration to prepare polyester under the effect of a nucleophilic co-catalyst, the catalysis efficiency is 103-106 g of a polymer per mole of the catalyst, the molecular weight of the polymer can be adjusted within the range of 103-105, the molecular weight distribution is smaller than 2, stereoregularity can be adjusted within the range of 60-100%, the alternative structure exceeds 98%, and polyester can be degraded into a small molecular compound under a certain condition. A chiral ligand catalytic system is used, the product selectivity and alternative structure selectivity of the polymer are both higher than 98%, the excess value of enantiomer of diol obtained after degradation is higher than 70%, and wide prospects are provided for industrial application.
Owner:DALIAN UNIV OF TECH
Enantioselective, catalytic allylation of ketones and olefins
Compounds containing a substituted or unsubstituted allyl group directly bound to a chiral carbon atom are prepared enantioselectively. Starting reactants are either chiral or achiral, and may or may not contain an attached allyloxycarbonyl group as a substituent. Chiral ligands are employed, along with transition metal catalysts. The methods of the invention are effective in providing enantioconvergent allylation of chiral molecules.
Owner:CALIFORNIA INST OF TECH
Enantioselective, catalytic allylation of ketones and olefins
Compounds containing a substituted or unsubstituted allyl group directly bound to a chiral carbon atom are prepared enantioselectively. Starting reactants are either chiral or achiral, and may or may not contain an attached allyloxycarbonyl group as a substituent. Chiral ligands are employed, along with transition metal catalysts. The methods of the invention are effective in providing enantioconvergent allylation of chiral molecules.
Owner:CALIFORNIA INST OF TECH
Method for enantioselective hydrogenation of chromenes
A method for preparing an enantiomeric chromane, by asymmetrically hydrogenating a chromene compound in the presence of an Ir catalyst having a chiral ligand. The method includes the enantioselective preparation of enantiomeric equol. A preferred Ir catalyst has a chiral phosphineoxazoline ligand. Enantiomeric chromanes of high stereoselective purity can be obtained.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
New type spirocyclic phosphic ester, preparation method and application in asymmetric addition reaction
A spirocyclosulphite is prepared from spirocyclodiphenol through three methods. Its spirodihydroindene structure has axial chirality, so said compound has two mutamers: levo- and dextro- spirocyclosulphite compounds. It can be used for the asymmetrical addition reaction of aldehyde and imine with high stereo activity.
Owner:NANKAI UNIV
Method of Chiral alkamine ligand used as catalyst of asymmetric addition process for terminal alkyne to fluoroalkylaryl ketone
InactiveCN1449865AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCarboxyl radical
The present invention provides a kind of chiral ligand (1R, 2R)-2-N,N-substituted amino-1-(4-substituted phenyl)-1-ethanol or its enantiomorph and a method using the above-mentioned chiral ligand or its enantiomorph as catalyst for asymmetric addition of acetylene copper or acetylene zinc to trifluoromethylarylketone. Said invention provides its structural general formula. Said invention adopts the asymmetric addition process so as to can high-effectively and high enantiomeric-selectively create the chiral quanternary carbon center in HIV transfrase high-activity inhibitor Efavirenz (Sustiva TM), so that it can high-effectively synthesize Efavirenz (Sustiva TM).
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Chiral ligand metal complex catalyst system, and its preparation method and use
InactiveCN1579627ALower synthesis costHigh stereoselectivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPhosphateHigh pressure
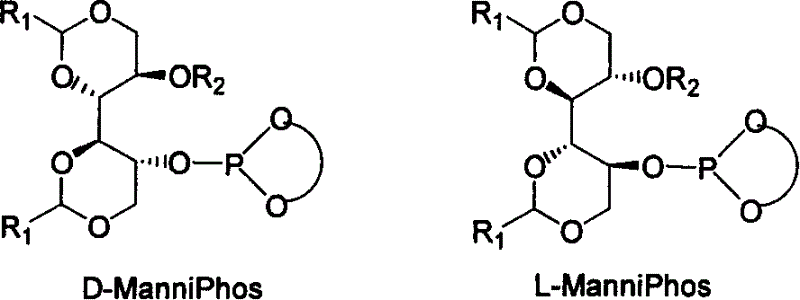
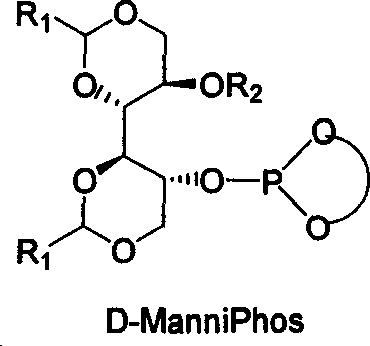
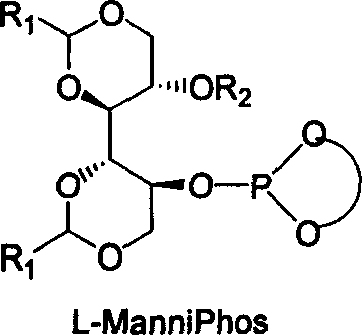
The invention relates to chirality phosphine ligand metal complex catalyse system, which is composed by complex formed by ligand and metal Rh, Ru, Ir, Pt or Pd or ligand and metal precursor in of 1-2 mol ratio. The phosphine ligand is a kind of effective chirality phosphate ligand composed by D-mannitol or L-mannitol after reaction. The catalyst composed by the chirality ligand and rhodium metallic compound in asymmetry hydrogenation reaction can work in room temperature. With a wide applied range, catalyst's activation and stereoselectivity is not influenced from normal pressure to high pressure. Reaction time is 1-24 hours. Mol ratio of ligand and metal rhodium compound is 1:1-2:1. Ratio of reactant and catalyst is 100-10,000.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Osmium oxide composition
The present invention provides an osmium oxide composition comprising an osmium oxide microencapsulated in an aromatic polyolefin (hereinafter abbreviated as MCOsOx), a method for preparation of MCOsOx, which comprises allowing an osmium oxide to contact with an aromatic polyolefin in an organic solvent, and precipitating MCOsOx, an oxidizing agent comprising MCOsOx, a method for preparing a chiral diol compound, which comprises reacting MCOsOx, a chiral ligand and an olefin compound with each other, and a method for preparing a chiral diol compound, which comprises oxidizing an olefin compound with MCOsOx wherein a chiral ligand further coordinates to an osmium oxide.
Owner:KOBAYASHI SHU +1
Chiral phosphines, transition metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6576772B1High enantioselectivityEnantioselectivity decreaseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationHydrosilylation
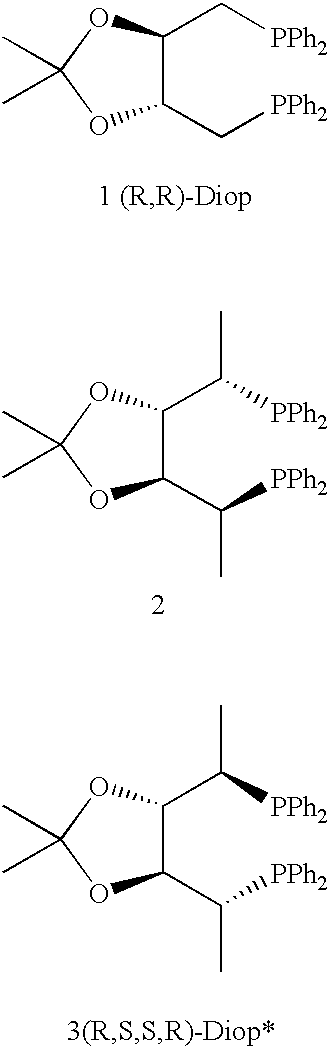
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include (R,S,S,R)-DIOP*. The ruthenium complex reduces enamide to the corresponding amine with up to 99% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation, hydride transfer, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, isomerization, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
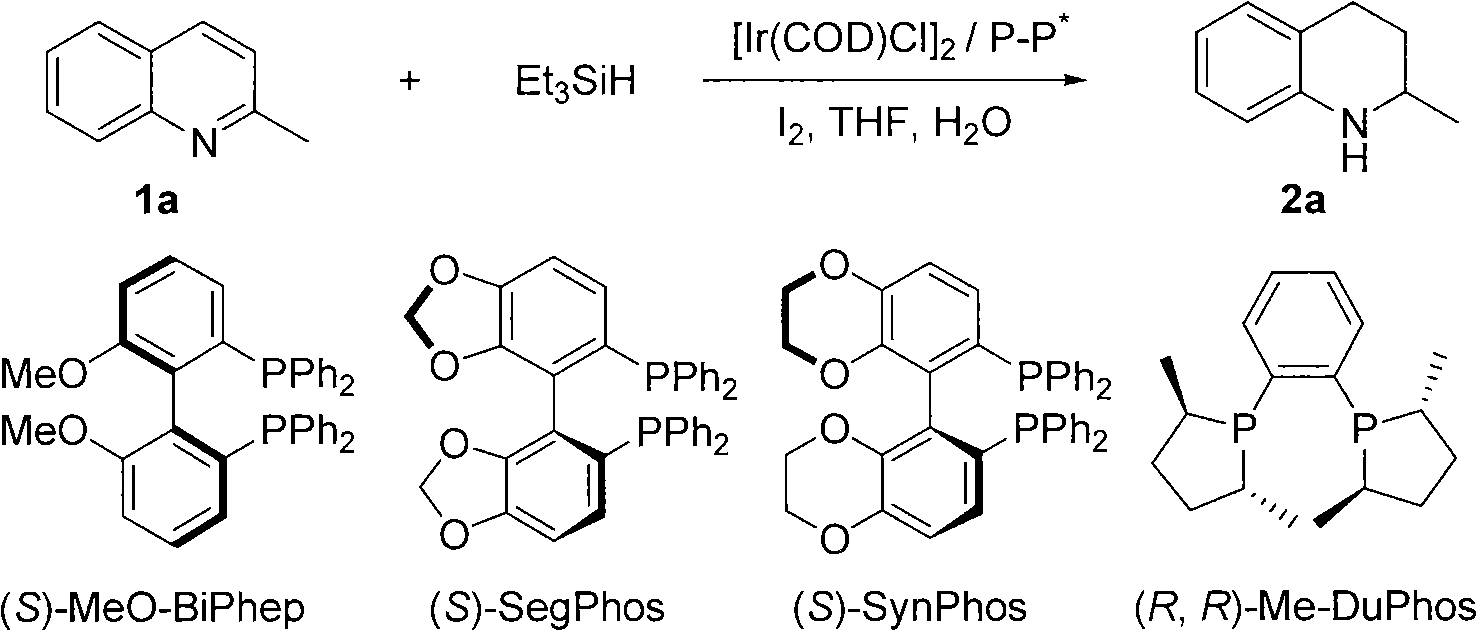
Method for synthesizing derivatives of chiral tetrahydroquinoline by catalyzing asymmetric hydrosilylation with iridium
InactiveCN101845016AReduce usageHigh reactivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIridiumQuinoline
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of high-optical purity bortezomib and intermediate of bortezomib
ActiveCN103304629AHigh optical purityReduce manufacturing costPeptidesGroup 3/13 element organic compoundsPharmaceutical drugProtecting group
The invention discloses a preparation method of high-optical purity bortezomib and an intermediate of bortezomib, and belongs to the field of pharmaceutical synthesis. The invention discloses a synthetic method of bortezomib or boric anhydride thereof and related intermediate of bortezomib or boric anhydride. The invention firstly discloses bortezomib intermediate shown in a formula A and a preparation method thereof, and further discloses a method for preparing bortezomib by the intermediate. The bortezomib, which is obtained by introducing novel chiral ligand in the method, is high optical purity, low in material cost, easily available, simple and convenient to operate in the process, capable of greatly lowering the production cost of the process, high in product yield, high in optical purity and suitable for the industrial production of bortezomib, wherein R is Cl, Br, NH2 or FORMULA; and R1 is hydrogen or an amino protecting group.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com