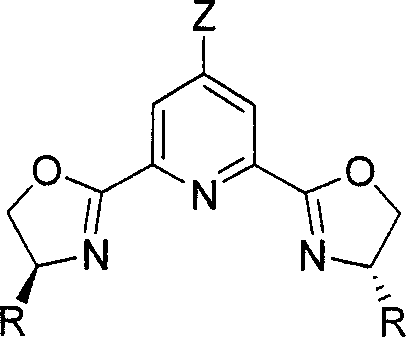
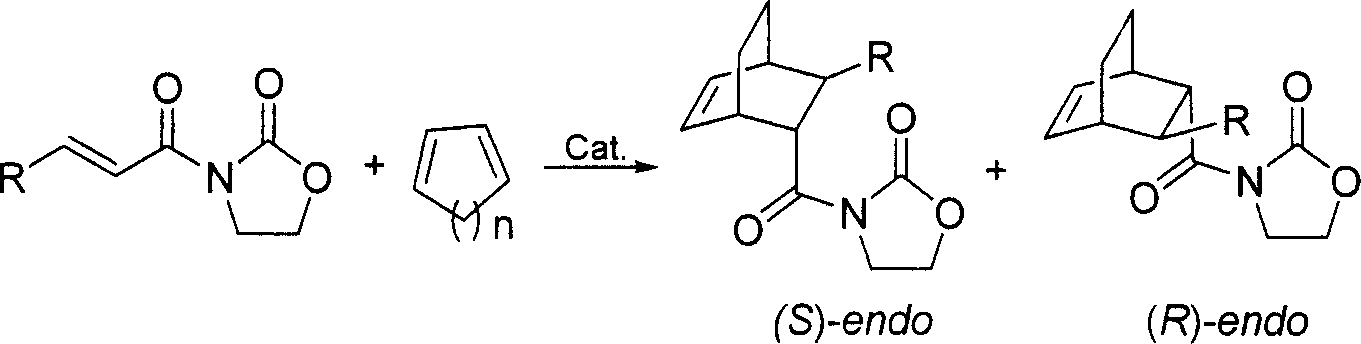
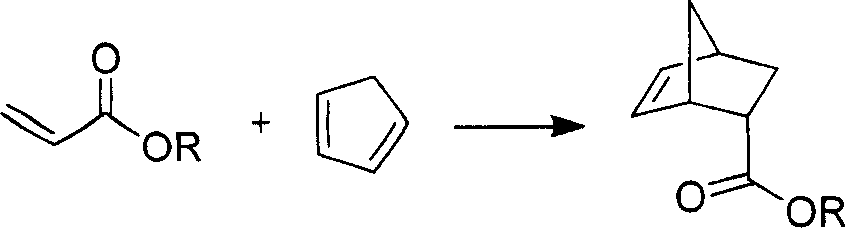
Chirality pyridine double-oxazoline catalyzer and method for preparing the same and application thereof
A pyridinebisoxazoline, catalyst technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of low activity and enantioselectivity, high enantioselectivity, Harsh reaction conditions and other problems, to achieve the effect of improving enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 4-Chloro-2,6-bis[4'(S)-tert-butyloxazoline-2'-]pyridine Synthesis
[0037] One drop of N,N-dimethylformamide was added to letronine (2.1 mmol, 422.1 mg), followed by the addition of thionyl chloride SOCl 2 11ml, reflux for 24h. Excess thionyl chloride was concentrated under reduced pressure.
[0038] Add 9ml of chloroform to (S)-tert-leucinol (4.3mmol, 503.9mg), cool to 0°C, add triethylamine (12.9mmol, 1.7ml), then add 15ml of acid chloride in chloroform, and stir at room temperature for one hour Days later, with 5% HCl and saturated NaHCO 3 Wash, anhydrous Na 2 SO 4 After drying and concentration, it was directly used in the next reaction. Add 20ml of chloroform to the concentrated substance, and directly add thionyl chloride SOCl 2 5.4ml, the mixture was refluxed for 9h, concentrated under reduced pressure, and purified by column chromatography (ethyl acetate / petroleum ether=1:4) to obtain 480mg of pure product with a yield of 52%.
Embodiment 2
[0041] 4-Bromo-2,6-bis[4'(S)-isopropyloxazoline-2'-]pyridine Synthesis
[0042] Phosphorus pentabromide (38.26mmol, 16.49mg) was added to leidronine (5.87mmol, 1.18g) and refluxed at 90°C for 3 hours. After the reaction was completed, it was lowered to room temperature, and 23ml of chloroform was added therein for dilution, and the resulting solution was filtered , the filtrate was cooled to 0°C, and then 33ml of methanol solution was slowly added dropwise to it, concentrated after the dropwise addition, and recrystallized in methanol to obtain 1.37g of crystalline 4-bromo-pyridine-2,6-dicarboxylic acid methyl ester, producing The rate is 85%.
[0043] Add 12ml of 5M aqueous sodium hydroxide solution to 4-bromo-pyridine-2,6-dicarboxylic acid methyl ester (2.50mmol, 685mg), reflux for 1 hour, and after cooling, the solution is acidified to pH=2, forming a white The precipitate was filtered and dried under vacuum at 60°C to obtain 584 mg of 4-bromo-pyridine-2,6-dicarboxylic a...
Embodiment 3
[0047] 4-Chloro-2,6-bis[4'(S)-benzyloxazoline-2'-]pyridine Synthesis
[0048] The synthetic method is the same as in Example 1, except that (S)-tert-leucinol is replaced by (S)-phenylalaninol, and finally 4-chloro-2,6-bis[4'(S)-benzyloxazoline The yield of -2'-]pyridine was 49%. 1 H NMR (400MHz, CDCl 3 )δ8.23(s, 2H), 7.34-7.22(m, 10H), 4.67-4.64(m, 2H), 4.47(t, J=8Hz, 2H), 4.26(t, J=8Hz, 2H), 3.28-3.23(m, 2H), 2.78-2.72(m, 2H); 13 C NMR (100 MHz, CDCl 3 )δ162.2, 148.3, 145.7, 137.8, 129.4, 128.9, 126.9, 126.2, 73.1, 68.4, 41.8; HRMS Calcd for C 25 h 22 N 3 o 2 Cl(M) 431.1401, found 431.1404.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com