Chiral ligand metal complex catalyst system, and its preparation method and use
A metal complex, catalytic system technology, applied in the direction of organic compound/hydride/coordination complex catalyst, catalytic reaction, physical/chemical process catalyst, etc., can solve the narrow application scope and limited application of ligand substrates problem, to achieve the effect of low synthesis cost, wide application range and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Add vitriol oil (20ml) in the there-necked flask with mechanical stirring of 1000ml, D-mannitol (100g), benzaldehyde (120ml) and solvent DMF (300ml), after this reaction mixture was stirred at room temperature for 3 days, the reaction The mixture was poured into ice water containing 30 g of potassium carbonate and 500 ml of petroleum ether and stirred vigorously until the ice melted. The solid was filtered and washed with petroleum ether. The solid was extracted twice with 400 ml of boiling chloroform, and the filtered solid was recrystallized from methanol. The product 1 (86 g, 42%) was obtained as a white solid.
[0045] In a 250ml round-bottomed flask, add sodium hydride (12mmol, content 80% sodium hydride dissolved in mineral oil), the compound 1 (10mmol) synthesized in the previous step, solvent DMF (40ml), and the reaction mixture was heated at room temperature After stirring the reaction for 1 hour, iodomethane (10 mmol) was added to continue the reaction at room ...
Embodiment 2
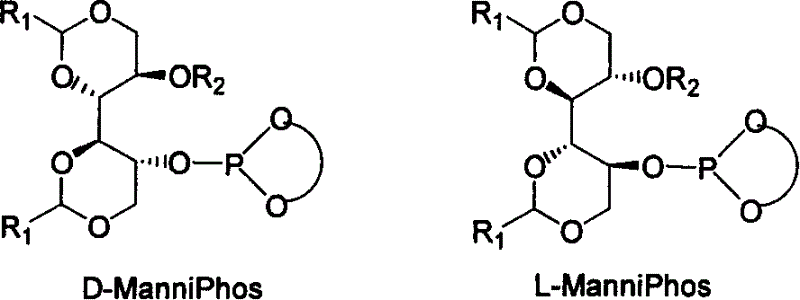
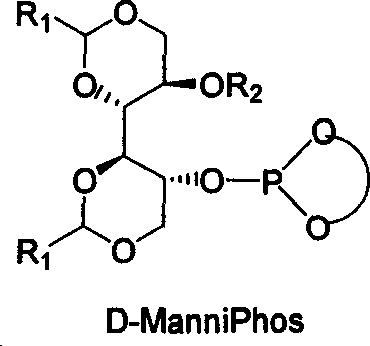
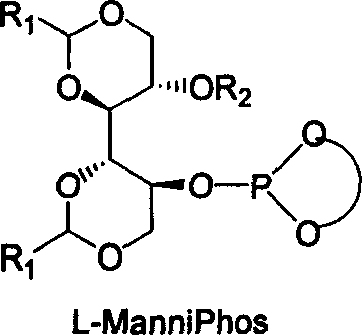
[0051] Under nitrogen protection, 2.0mg (0.005mmol) [Rh(COD) 2 ] BF 4 , with the above-mentioned synthesized chiral ligand ManniPhos (0.010mmol), solvent dichloromethane (1ml) in a 10ml reactor at room temperature, reacted for 10-30 minutes to make a catalyst, and the hydrogenation reaction substrate 2-acetyl Aminocinnamic acid methyl ester (0.5mmol) is transferred in this reactor, after hydrogen displacement 3 times, continue to pass into hydrogen, maintain normal pressure reaction after 30 minutes and stop reaction, filter with short silica gel column, after the filtered gained filtrate is concentrated, use The content and optical purity were determined by GC, and the yield of methyl S-acetamidophenylpropionate was 100% (calculated as methyl 2-acetamidocinnamate), and the enantiomeric excess was 98.0% ee.
Embodiment 3
[0053] Under nitrogen protection, 2.0mg (0.005mmol) [Rh(COD) 2 ] BF 4 , with the chiral ligand ManniPhos (0.010mmol) of above-mentioned synthesis, solvent methylene chloride (1ml) in the reactor of 10ml at room temperature, react 10-30 minute and make catalyst, the hydrogenation reaction substrate dopa The precursor (0.5 mmol) was transferred to the reactor, hydrogen was replaced 3 times, and hydrogen was continuously introduced, and the reaction was terminated after maintaining normal pressure for 30 minutes, filtered through a short silica gel column, and the filtered filtrate was concentrated, and the content was determined by GC. And optical purity measurement, the yield of L-dopa was 100% (based on the precursor of dopa), and the enantiomeric excess was 98.0% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com