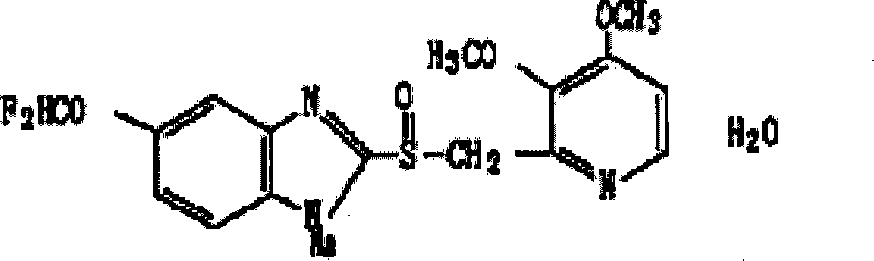
Patents
Literature
2829 results about "MANNITOL/SORBITOL" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mannitol is classified as a sugar alcohol; that is, it can be derived from a sugar (mannose) by reduction. Other sugar alcohols include xylitol and sorbitol. Mannitol and sorbitol are isomers, the only difference being the orientation of the hydroxyl group on carbon 2.
Sweetener containing d-psicose and foods and drinks obtained by using the same
Providing a D-psicose-containing sweetener with the modification of the taste of D-psicose, comprising D-psicose, a sugar alcohol and / or a high intensity sweetener, preferably containing D-psicose as the main component, particularly a low-calorie sweetener and / or a sweetener giving refreshing feel in the oral cavity, as well as foods and drinks obtained by using the D-psicose-containing sweetener with the modification of the taste of D-psicose, and other products given with sweetness. The sugar alcohol is one or more sugar alcohols selected from the group consisting of sorbitol, mannitol, lactitol, maltitol, xylitol and erythritol, while the high intensity sweetener is one or more high intensity sweeteners as selected from aspartame, acesulfame K, sodium cyclamate, sodium saccharin, Sucralose (under trade name), stevia sweetener, dulcin, taumatin, neotame and monellin.
Owner:MATSUTANI CHEM INDS CO LTD +1
Lyophilized beads containing mannitol
Mannitol in certain weight percentages can be used to produce lyophilized beads of consistent size, consistent morphology, and reduced moisture content. These mannitol-containing lyophilized beads are useful in a variety of biological applications where precise reagent amounts are required or moisture-sensitive components are utilized. PCR technologies represent one biological application where mannitol-containing lyophilized beads can be used.
Owner:CEPHEID INC
Anti a beta antibody formulation
ActiveUS20060193850A1Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
Stabilized liquid polypeptide formulations
InactiveUS20060210557A1Provide stabilityMaintain biological activityBiocideOrganic active ingredientsMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of polypeptides, in particular, therapeutic antigen-binding polypeptides such as antibodies and the like, for example, anti-Aβ antibodies. The formulations generally include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine, and thus, the formulations are suitable for several different routes of administration.
Owner:WYETH LLC
Sludge curing agent and application thereof
ActiveCN101081718AGood boardIncreased durabilitySludge treatment by de-watering/drying/thickeningSolid waste managementSludgeSlag
The present invention is sludge curing agent and its application, and belongs to the field of soil treating chemicals technology. The sludge curing agent includes powdered components and liquid components, the powdered components include cement clinker 30-60 weight portions, slag 30-60 weight portions, lime 3-8 weight portions, gypsum 1-7 weight portions and other sulfates 1-7 weight portions; and the liquid components include polyacrylamide 5-30 weight portions, polyaluminum chloride 0-20 weight portions, mannitol 0-30 weight portions, lignosulfonate 20-80 weight portions, lignosulfonate-iron or chromium ion complex 0-30 weight portions, alkylphenol ethoxylate 0.2-2 weight portions, tannin 0-10 weight portions, humate 0-10 weight portions, and alpa-olefin sulfonate 0.2-2.5 weight portions. The sludge curing agent has low cost, small consumption, high cumulate strength and high cumulate water tolerance, and may be applied widely.
Owner:BEIJING ZHONGYONGJI FIRMING AGENT TECH DEV
Ethanol production in fermentation of mixed sugars containing xylose
Xylose-utilizing Z. mobilis strains were found to have improved ethanol production when grown in medium containing mixed sugars including xylose if sorbitol or mannitol was included in the medium. The effect was seen in concentrations of mixed sugars where no growth lag period occurs, as well as in higher sugars concentrations.
Owner:SUSTAINABLE TECH CORP +1
Reduced-carbohydrate and nutritionally-enhanced frozen desserts and other food products
InactiveUS20060286248A1Mitigate laxation effectReduce impactFrozen sweetsConfectioneryTagatoseMaltitol
A reduced carbohydrate ice cream or other frozen dessert product that contains a low-digestible sweetener system and a fermentable fiber material. The a low-digestible sweetener system consists of one or more low-digestible sweeteners having a molecular weight of from about 90 to about 190; and is typically a low molecular weight saccharide or a polyol. Typical low-digestible sweeteners include mannitol, maltitol, sorbitol, lactitol, erythritol, xylitol, isomalt, glycerin, talitol, mannose, tagatose, fructose, arabinose, fucose, lycose, ribose, sorbose, talose, and xylose, and mixtures thereof. The low-digestible sweetener replaces the digestible sugars to provide the appropriate freezing point depression of the product. The level of fermentable fiber is sufficient to mitigate a Taxation effect that can be caused by ingestion of the amount of the low-digestive sweetener. The fermentable fiber can be an inulin, a maltodextrin resistant to human digestion, an oligofructose, a fructooligosaccharide, a high water binding fermentable fiber, and a mixture thereof.
Owner:TECHCOM GRP LLC
Well treatment composition crosslinkers and uses thereof
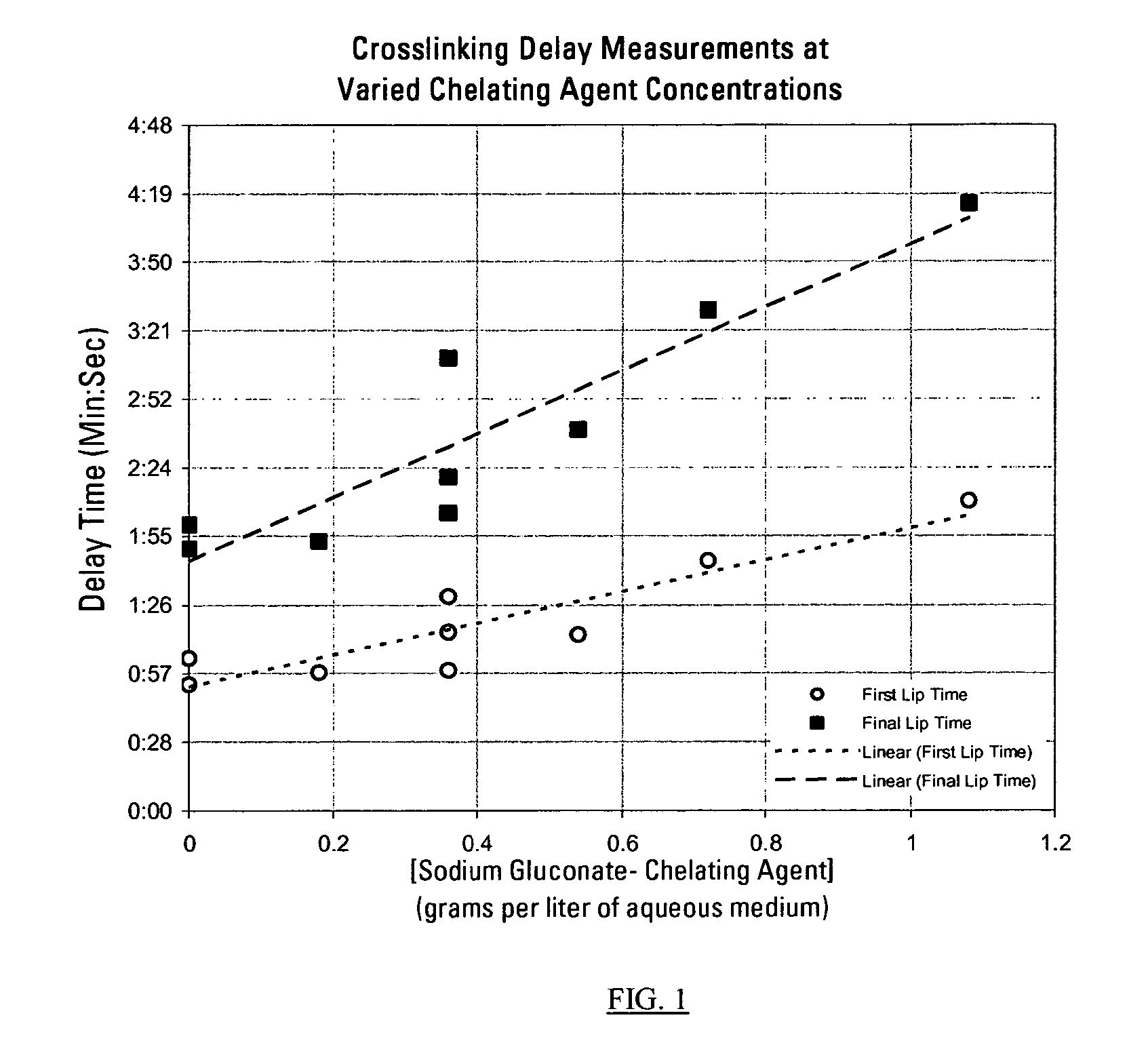
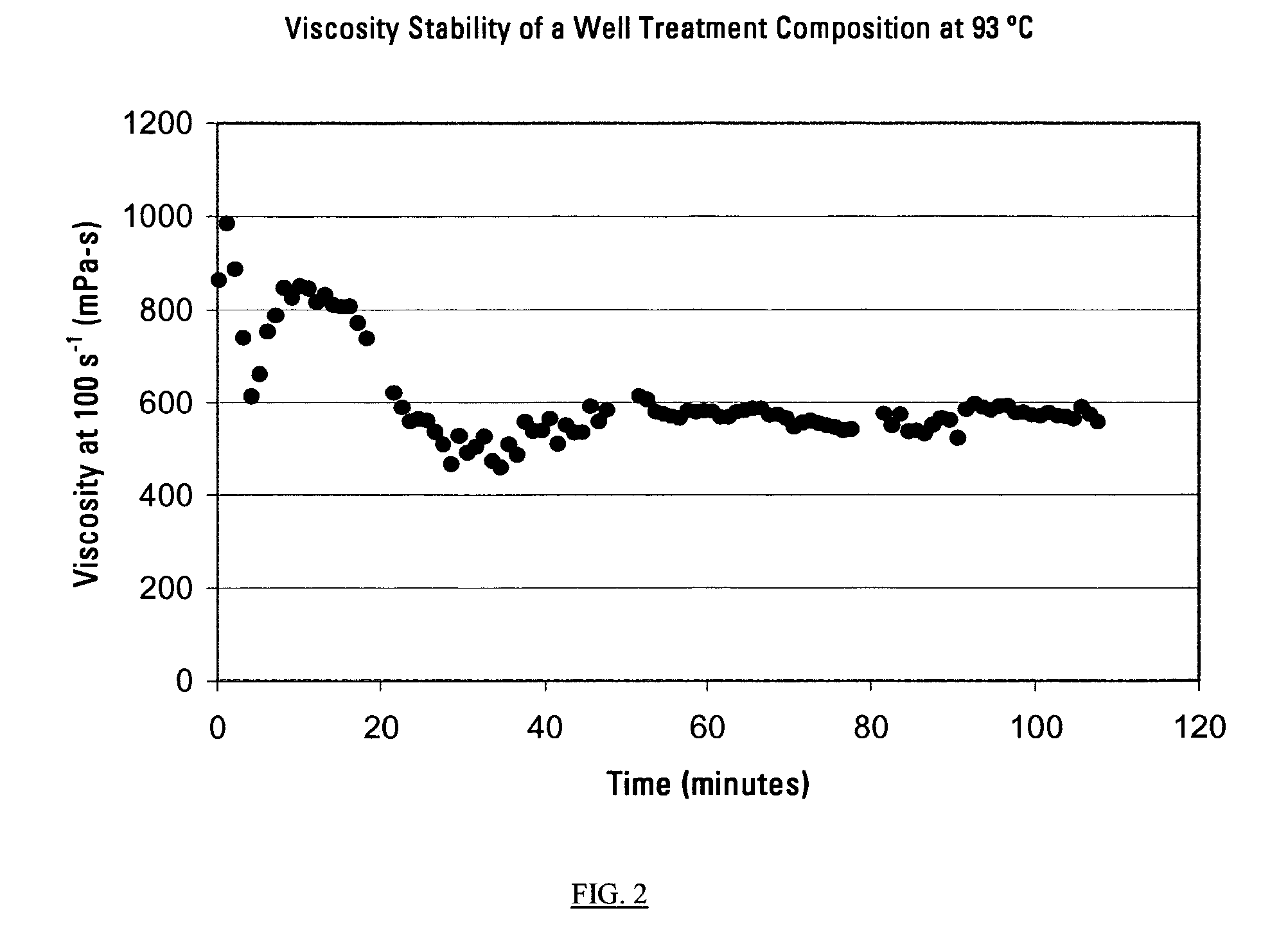
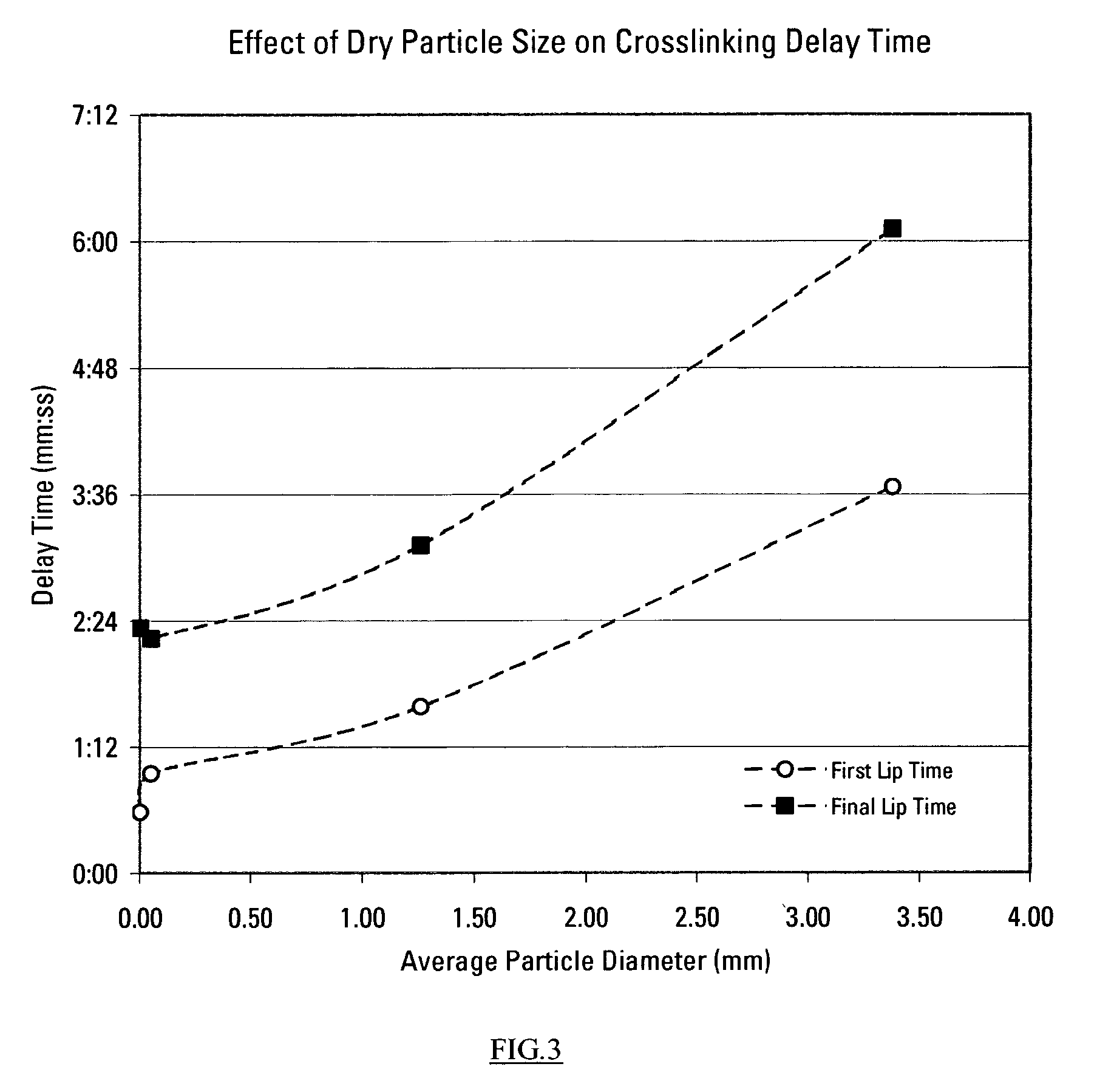
This invention relates to compositions used in treating subterranean formations, which include a hydrated polymer, and a dry blended multi-functional component. The hydrated polymer and dry blended multi-functional component are mixed at the ground surface of a wellsite, and subsequently injected into the formation providing controlled delay in crosslinking to achieve targeted fluid viscosity properties. The hydrated polymer may be a guar, hydroxypropyl guar, carboxymethyl guar, carboxymethylhydroxypropyl guar, synthetic polymers, and guar-containing compounds. The dry blended multi-functional component may include a crosslinker and a chelating agent, and the well treatment fluid may further include an activator mixed with the hydratable polymer. The chelating agent may be a polyols, gluconate, sorbitol, mannitol, carbonate, or any mixtures thereof. The crosslinker may be any source of boron, alkaline earth metal borates, alkali metal borates, zirconium compounds, titanium compounds, or any combination thereof, while the activator may be a caustic soda or magnesium oxide compound. The invention further provides methods for producing a well treatment composition including providing a hydrated polymer, and providing a dry blended multi-functional component. Also, methods of hydraulically fracturing a subterranean formation, as well as cleanup operations and gravel packing a wellbore are provided as well.
Owner:SCHLUMBERGER TECH CORP
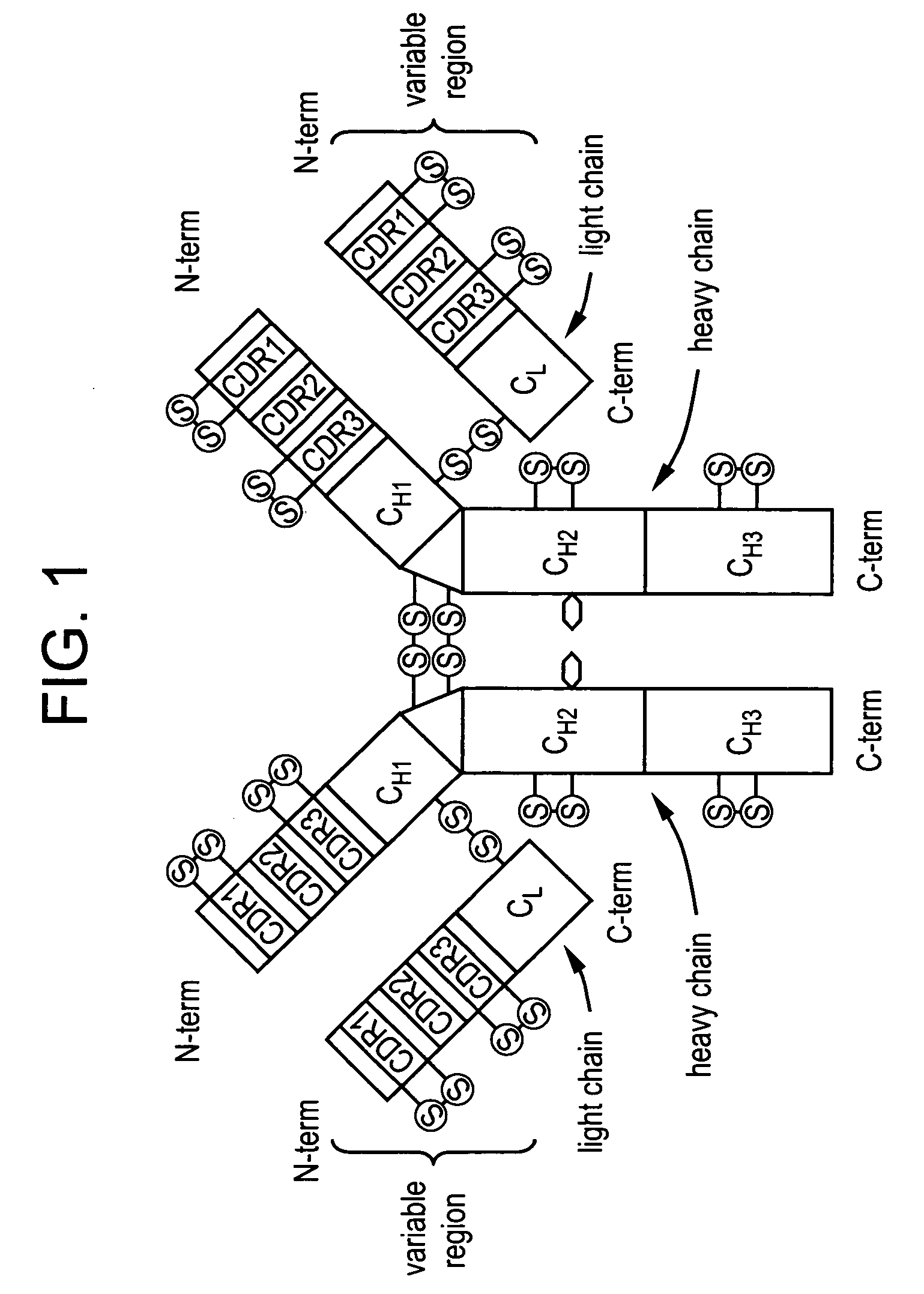
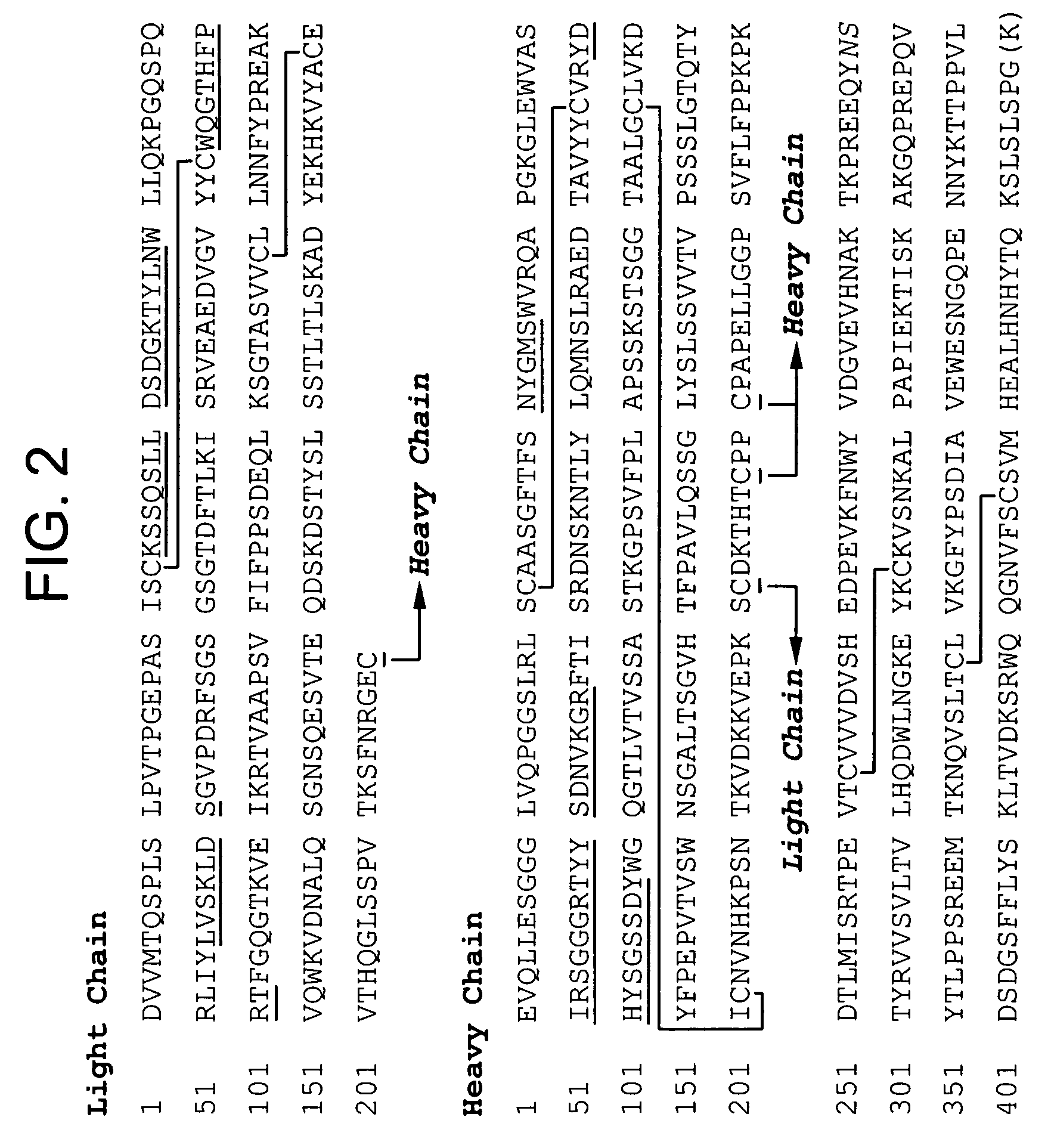
Stable lyophilized pharmaceutical formulation of IgG antibodies
InactiveUS7592004B2Process stabilityAvoid formingPowder deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-IL2 ReceptorHigh concentration
Owner:ABBVIE BIOTHERAPEUTICS
Polymeric films
ActiveUS20090312462A1Excellent oxygen barrier propertiesSuitable for usePaper coatingDomestic articlesMolten statePolymer science
A polymer composition and its use for thin film packaging applications including on a dry basis: a) from 45 to 90% by weight of starch; b) from 0.1 to 15% by weight of a water soluble polymer selected from polyvinyl alcohol, polyvinylacetate, and copolymers of ethylene and vinyl alcohol which have a melting point compatible with the molten state of the starch component; and c) from 5 to 45% by weight of one or more plasticizers having a molecular weight in the range of 50-6000, more preferably 50-2500 and more preferably still 100-400 and desirably selected from the group consisting of sorbitol, glycerol, maltitol, xylitol, mannitol, erythritol, glycerol trioleate, tributyl citrate, acetyl tri-ethyl citrate, glyceryl triacetate, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, polyethylene oxide, ethylene glycol, diethylene glycol or polyethylene glycol.
Owner:PLANTIC TECH
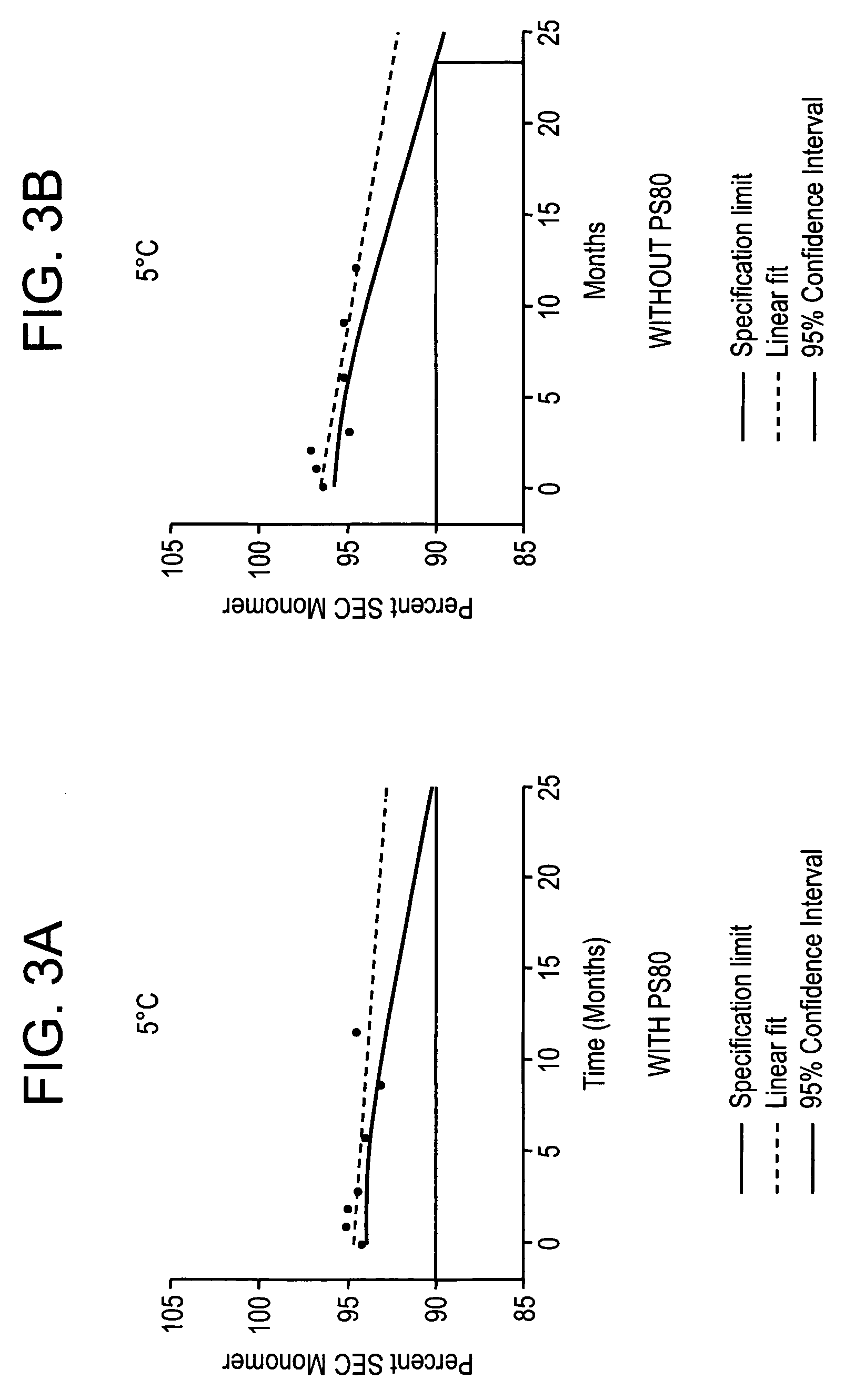
Pharmaceutical Formulation and Process
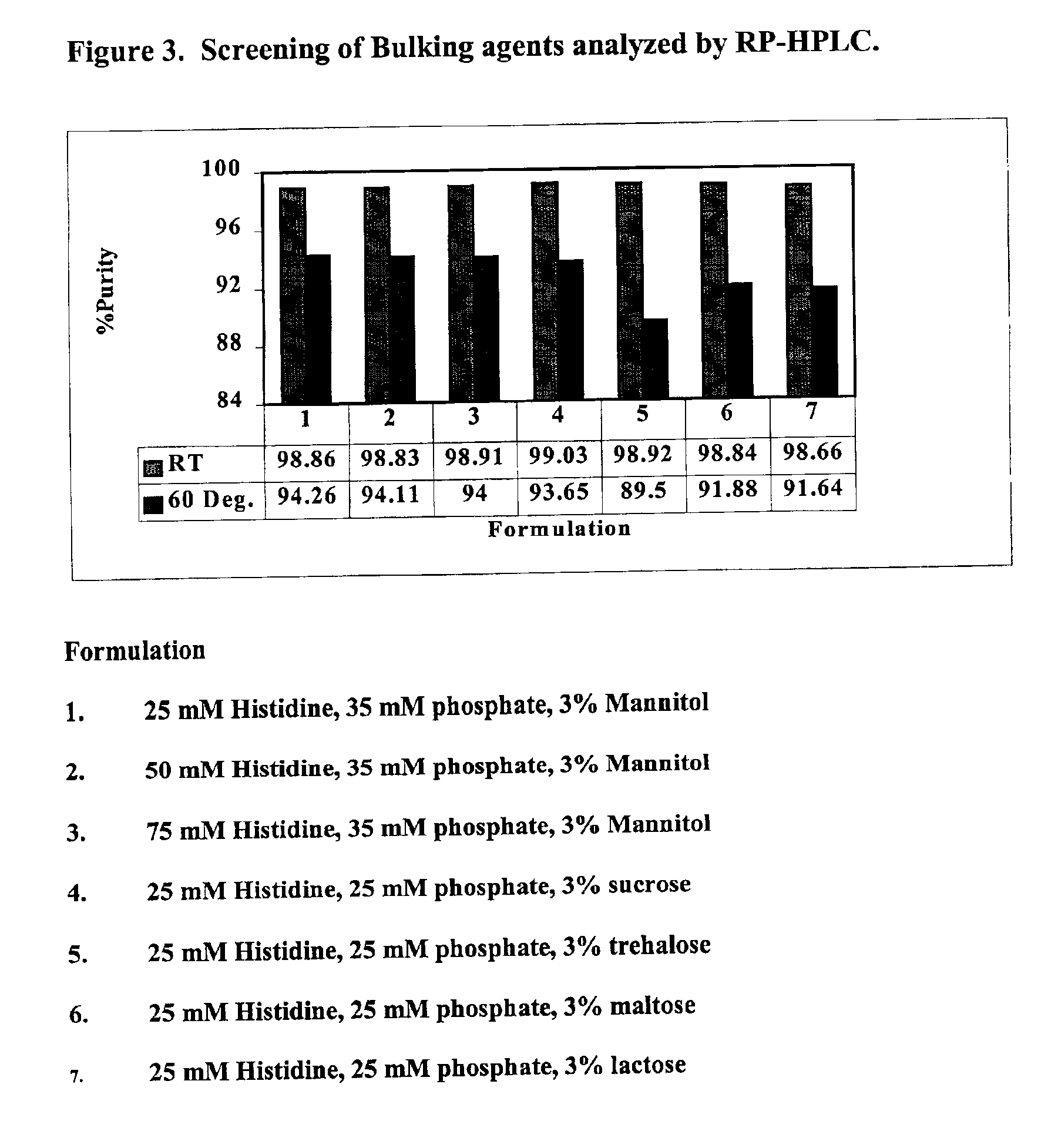
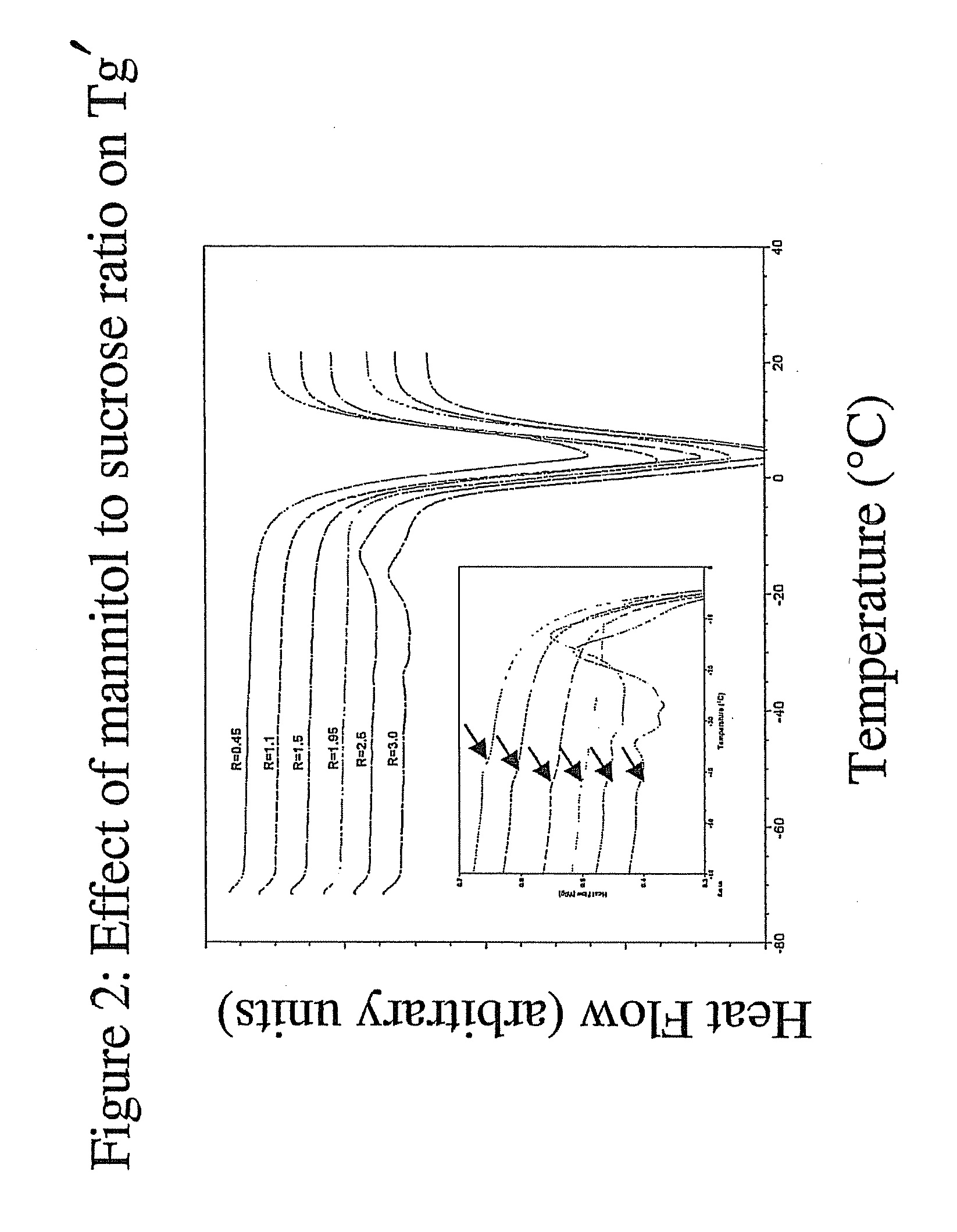
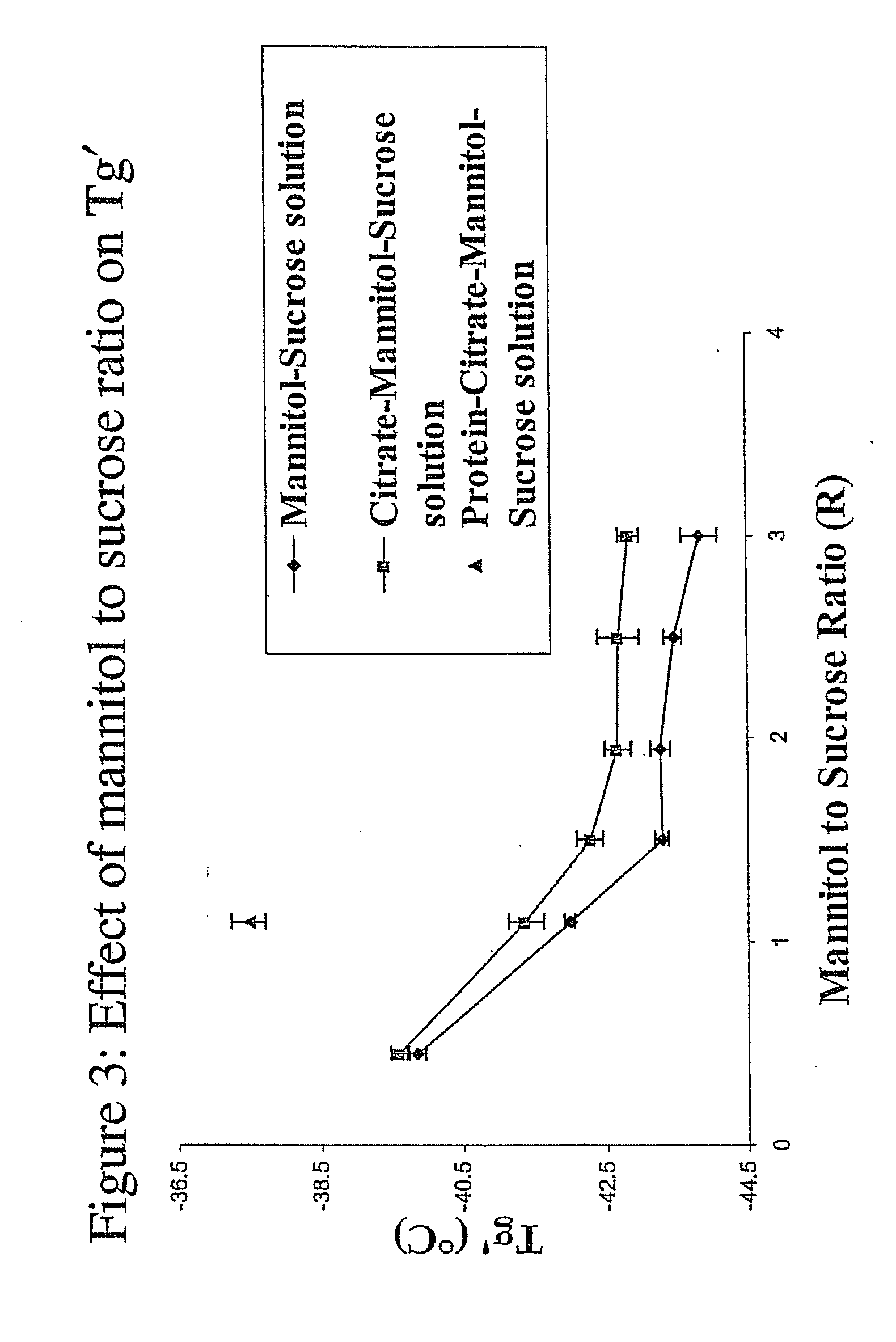
InactiveUS20070196364A1Increased Tg′Less-dramatic effectOrganic active ingredientsBiocideSucroseActive agent
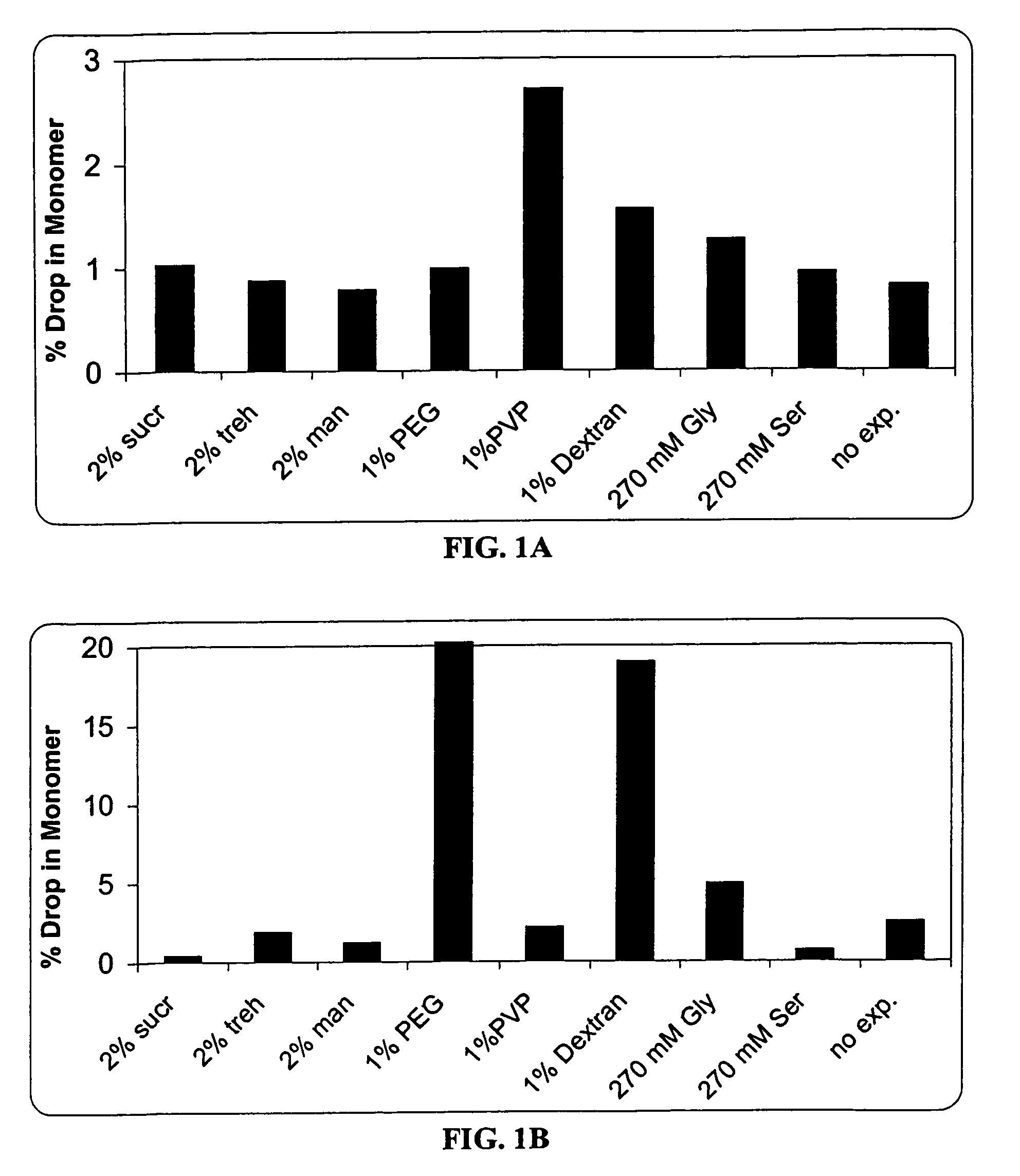
A process for lyophilization or freeze-drying of a pharmaceutical product is provided and a liquid formulation suitable for lyophilization. In particular, a process for lyophilization or freeze-drying a liquid formulation that includes a protein active agent, a bulking agent and a saccharide stabilizing agent is provided. The saccharide to bulking agent ratio and the protein concentration of the formulation are important factors that affect crystallization of the bulking agent during lyophilization and storage as are some processing conditions. In one embodiment, the saccharide is a disaccharide, such as sucrose and the crystalline bulking agent is mannitol. The protein can be an antibody or a non-antibody protein.
Owner:HUMAN GENOME SCI INC
Polyol-based polymers
ActiveUS20110008277A1Cheap preparationOrganic active ingredientsProsthesisMethacrylatePolymer science
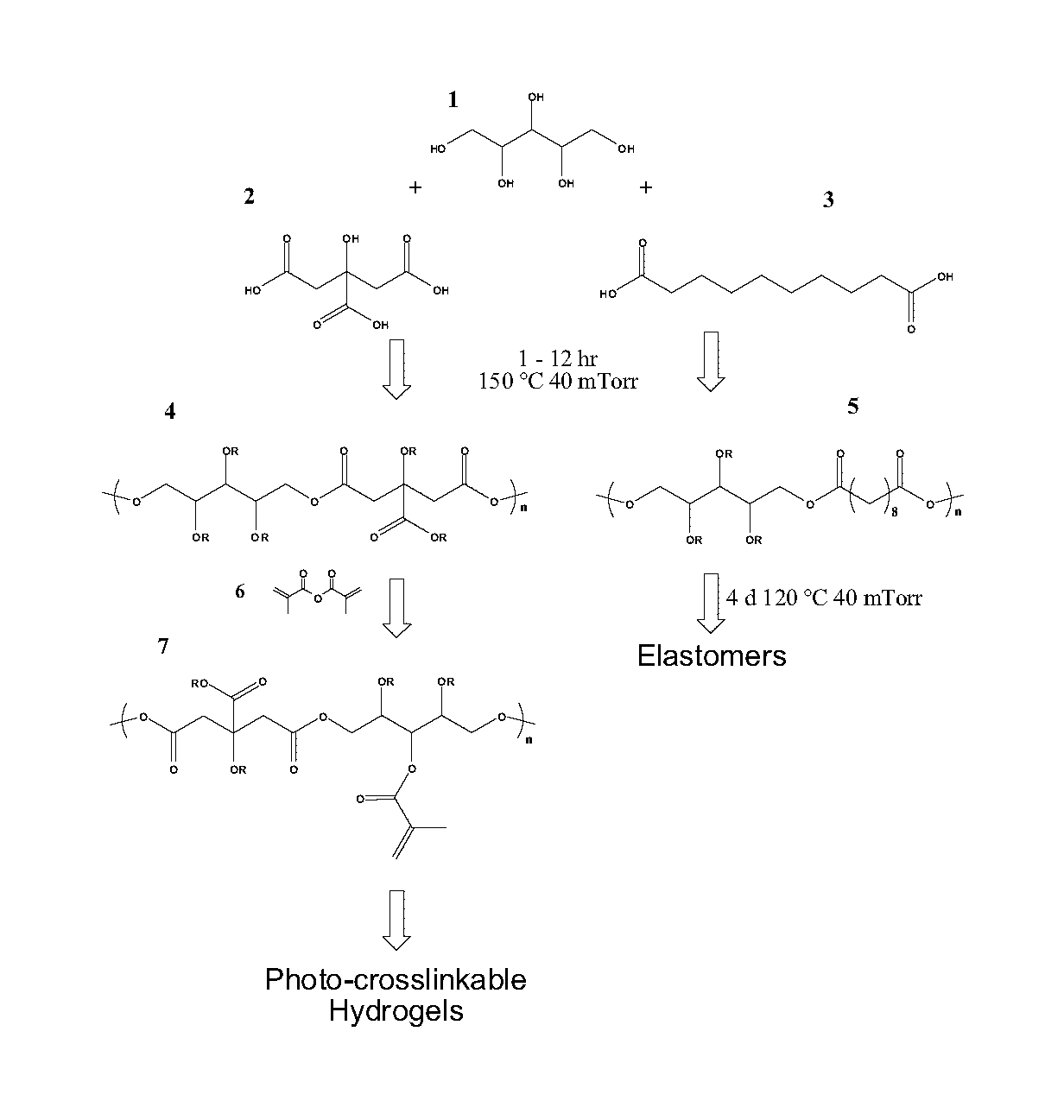
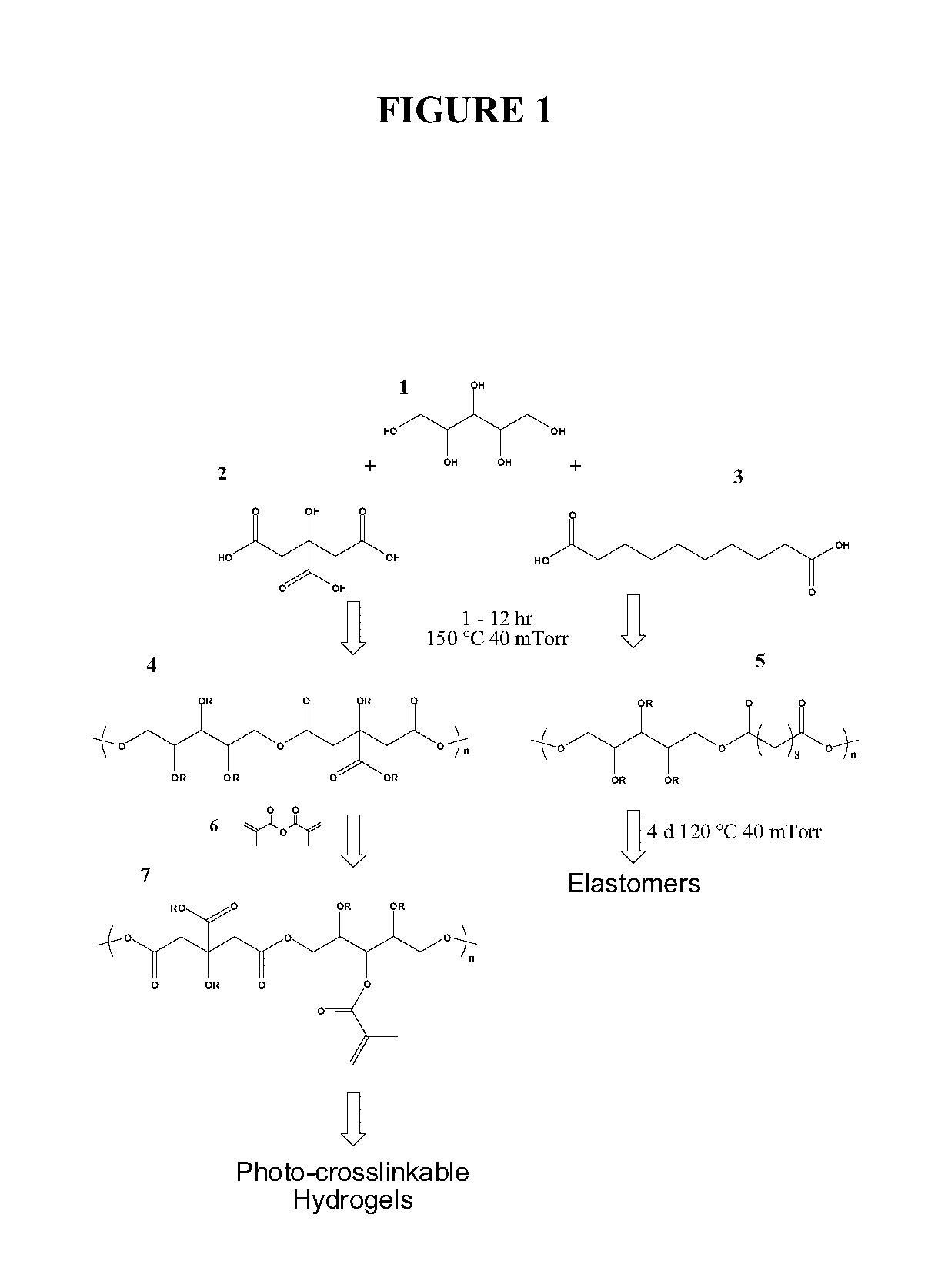
The present invention provides inventive polyol-based polymers, materials, pharmaceutical compositions, and methods of making and using the inventive polymers and materials. In certain aspects of the invention, an inventive polymer corresponds to a polymer depicted below. Exemplary inventive polymers includes those prepared using polyol units (e.g., xylitol, mannitol, sorbitol, or maltitol) condensed with polycarboxylic acid units (e.g., citric acid, glutaric acid, or sebacic acid). The inventive polymers may be further derivatized or modified. For example, the polymer may be made photocrosslinkable by adding methacrylate moieties to the polymer.
Owner:THE GENERAL HOSPITAL CORP +1
Pharmaceutical Compositions and Methods of Treating Neurological Insults
InactiveUS20110318431A1Sufficient amountPromote functional recoveryBiocideNervous disorderMANNITOL/SORBITOLMagnesium salt
A pharmaceutical composition containing a magnesium salt and an osmotic hypertonic agent, like a mannitol, is disclosed. Also disclosed are methods of treating individuals who have suffered a neurological insult, such as traumatic brain injury.
Owner:ENDOGENX
Raman spectroscopy for bioprocess operations
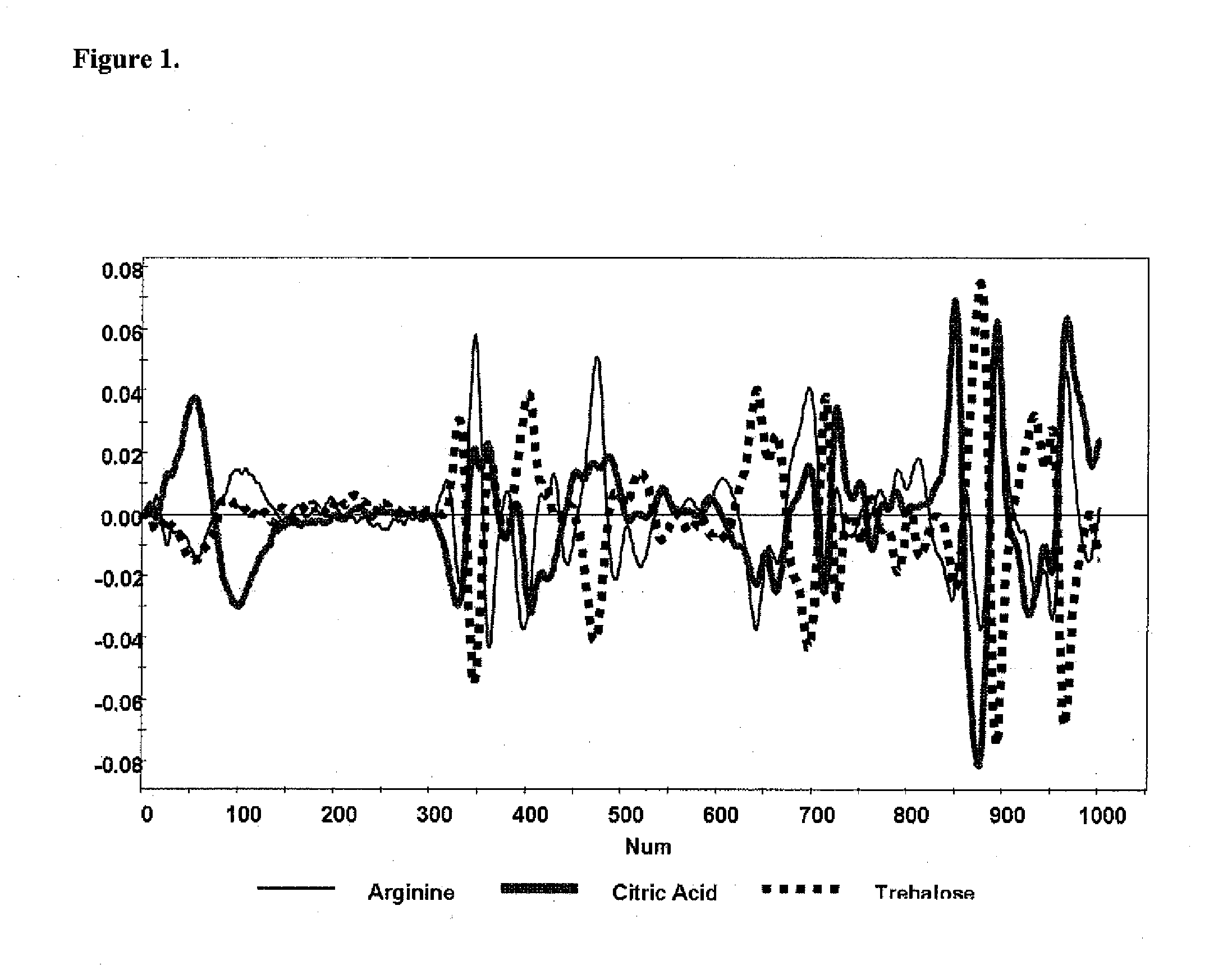
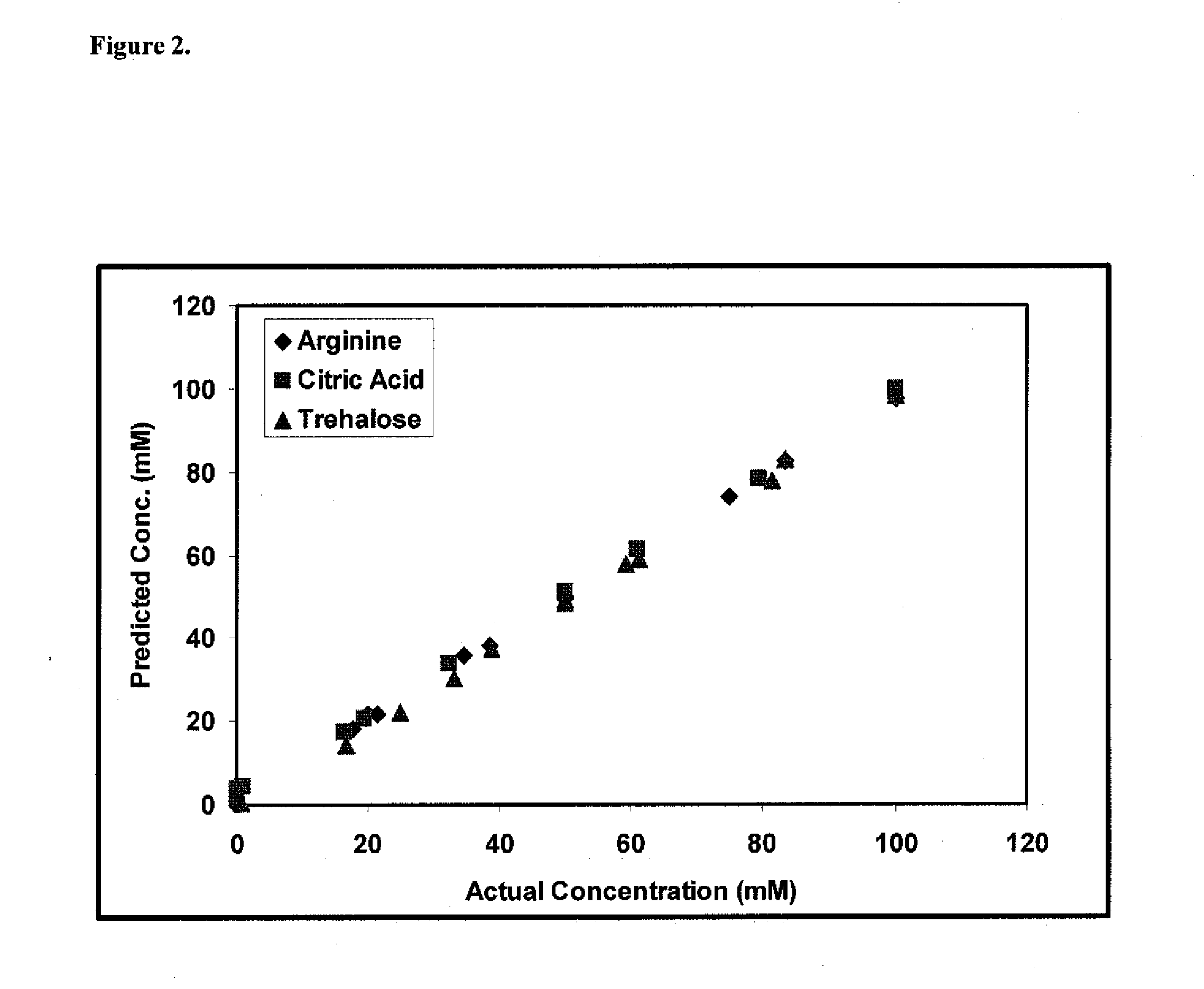
A method of characterizing a multi-component mixture for use in a bioprocess operation that includes providing a multi-component mixture standard with pre-determined amounts of known components; performing a Raman Spectroscopy analysis on the multi-component mixture standard; providing a multi-component test mixture from the bioprocess operation; performing a Raman Spectroscopy analysis on the multi-component test mixture; and comparing the analysis of the multi-component mixture standard and the multi-component test mixture to characterize the multi-component test mixture. In one embodiment, the multi-component mixture standard and the multi-component test mixture both comprise one or more of, at least two, at least three of, or each of, a polysaccharide (e.g. sucrose or mannitol), an amino acid (e.g., L-arginine, L-histidine or L-ornithine), a surfactant (e.g. polysorbate 80) and a pH buffer (e.g., a citrate formulation).
Owner:ABBVIE INC
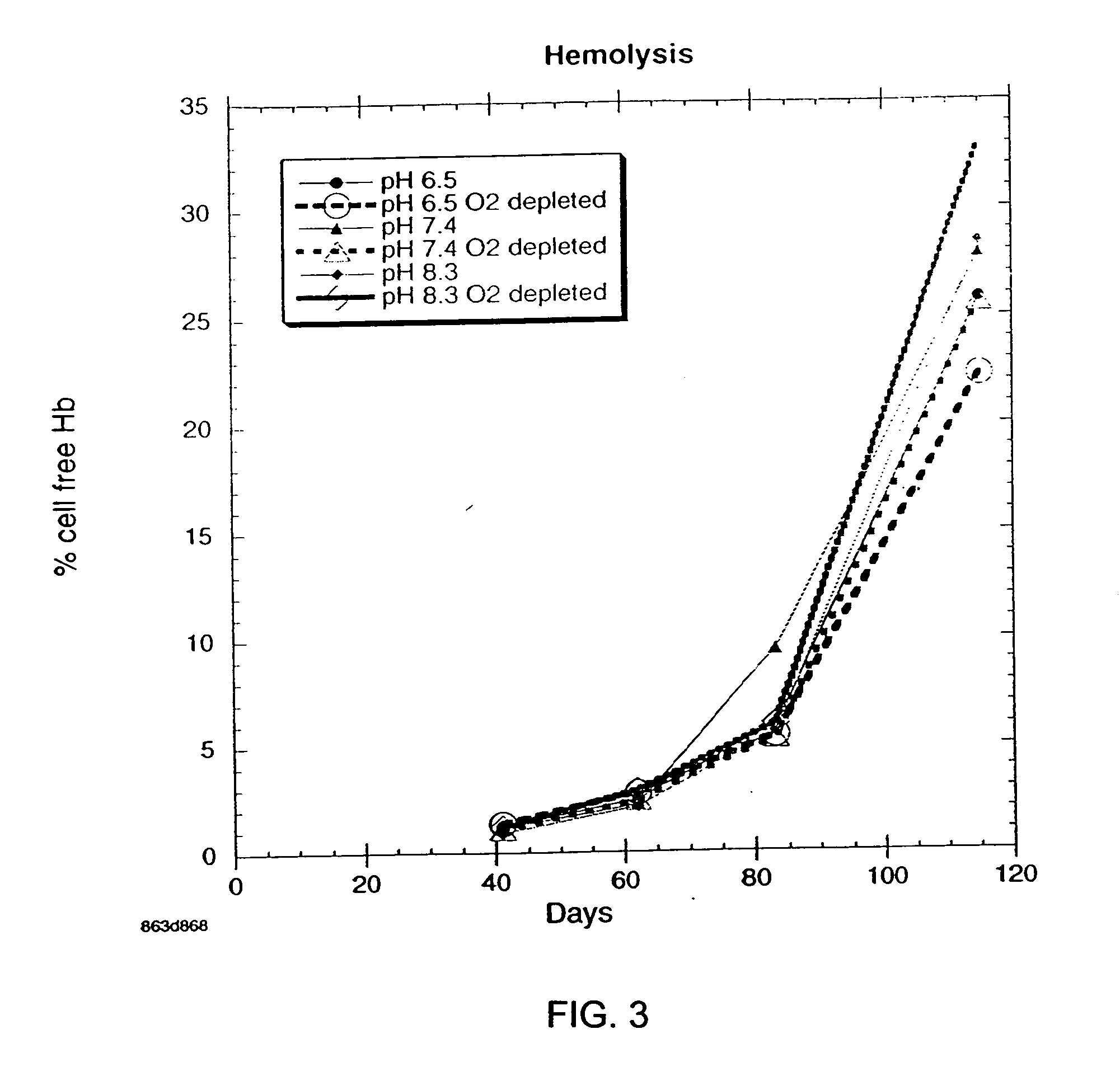
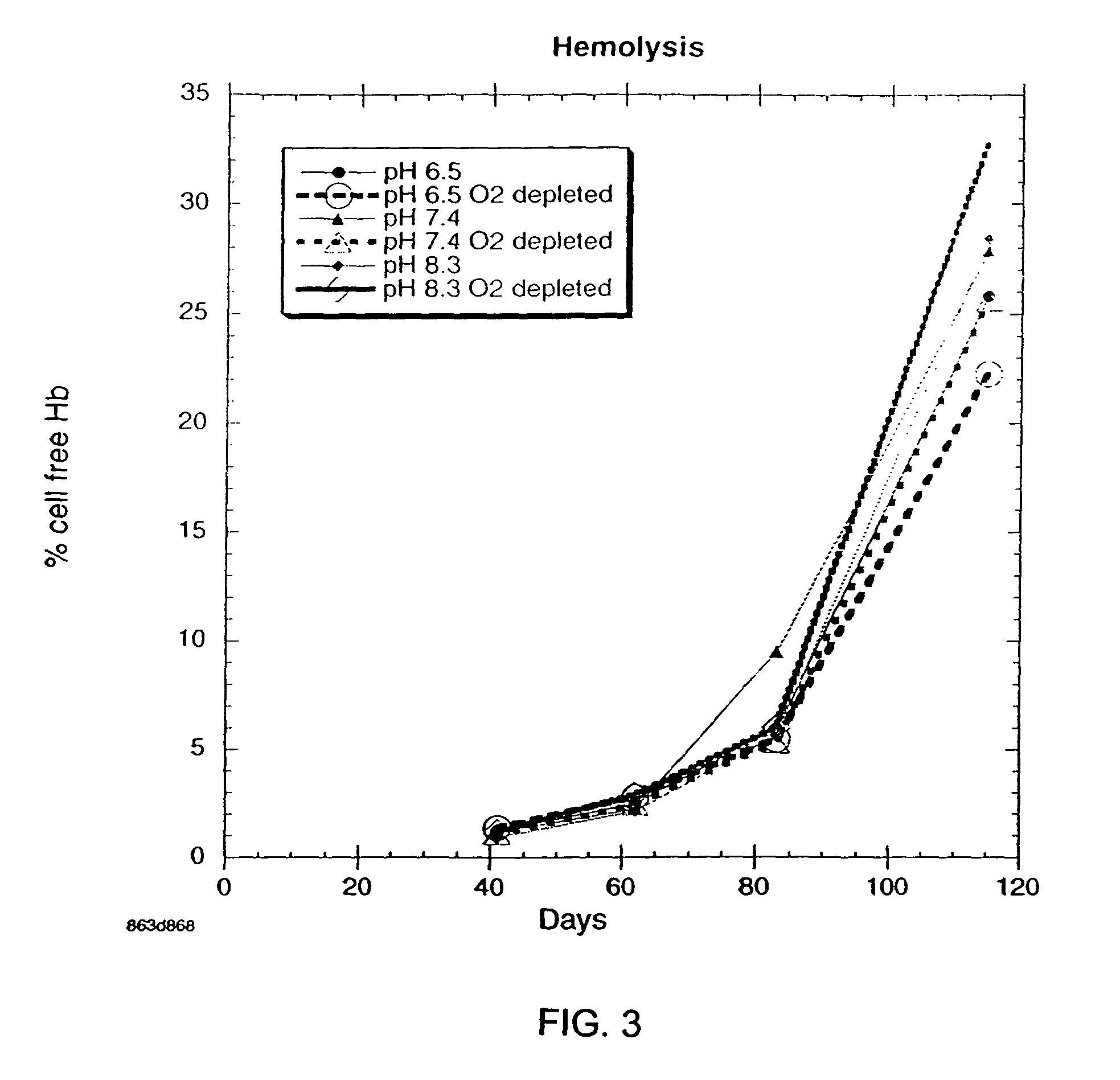
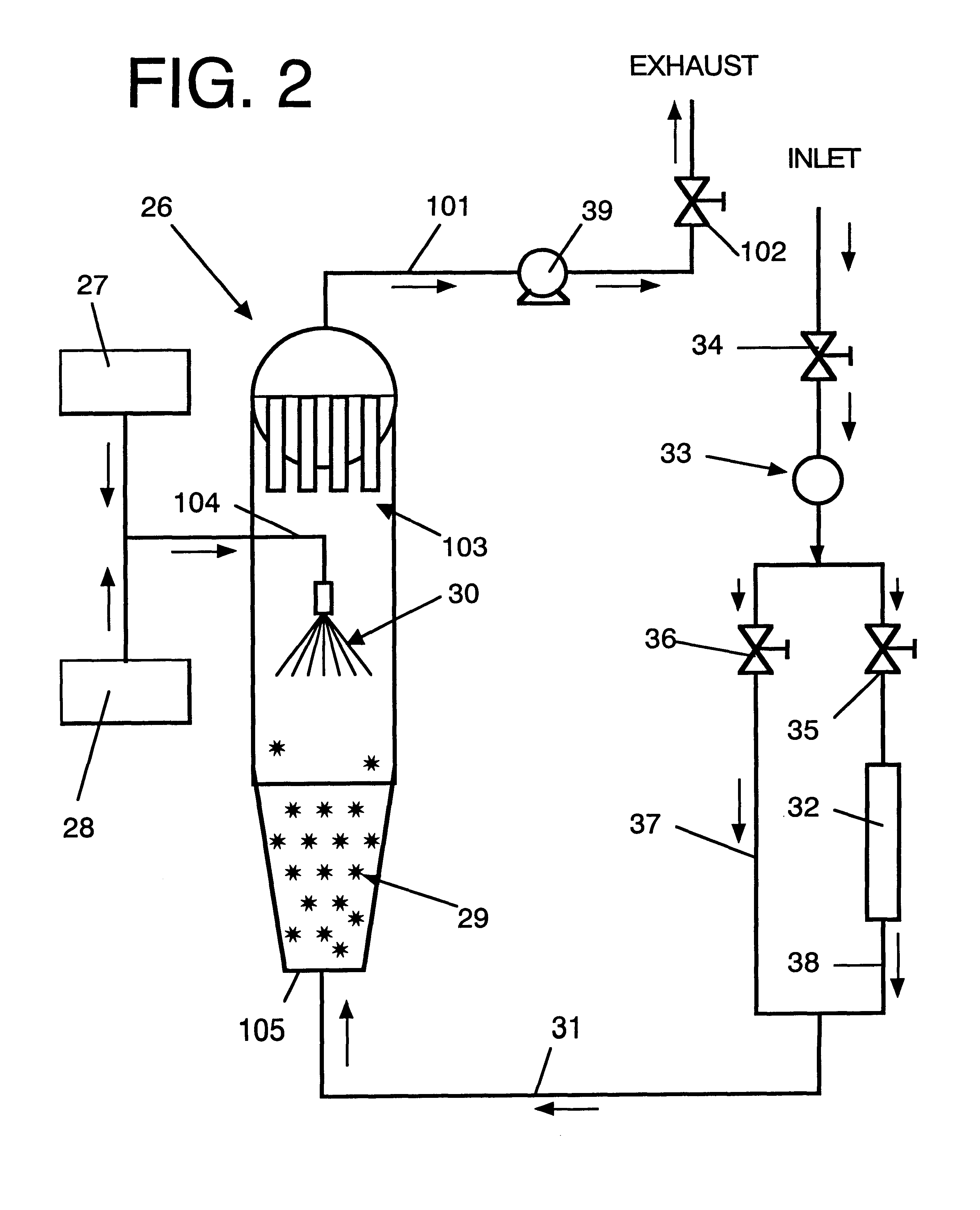
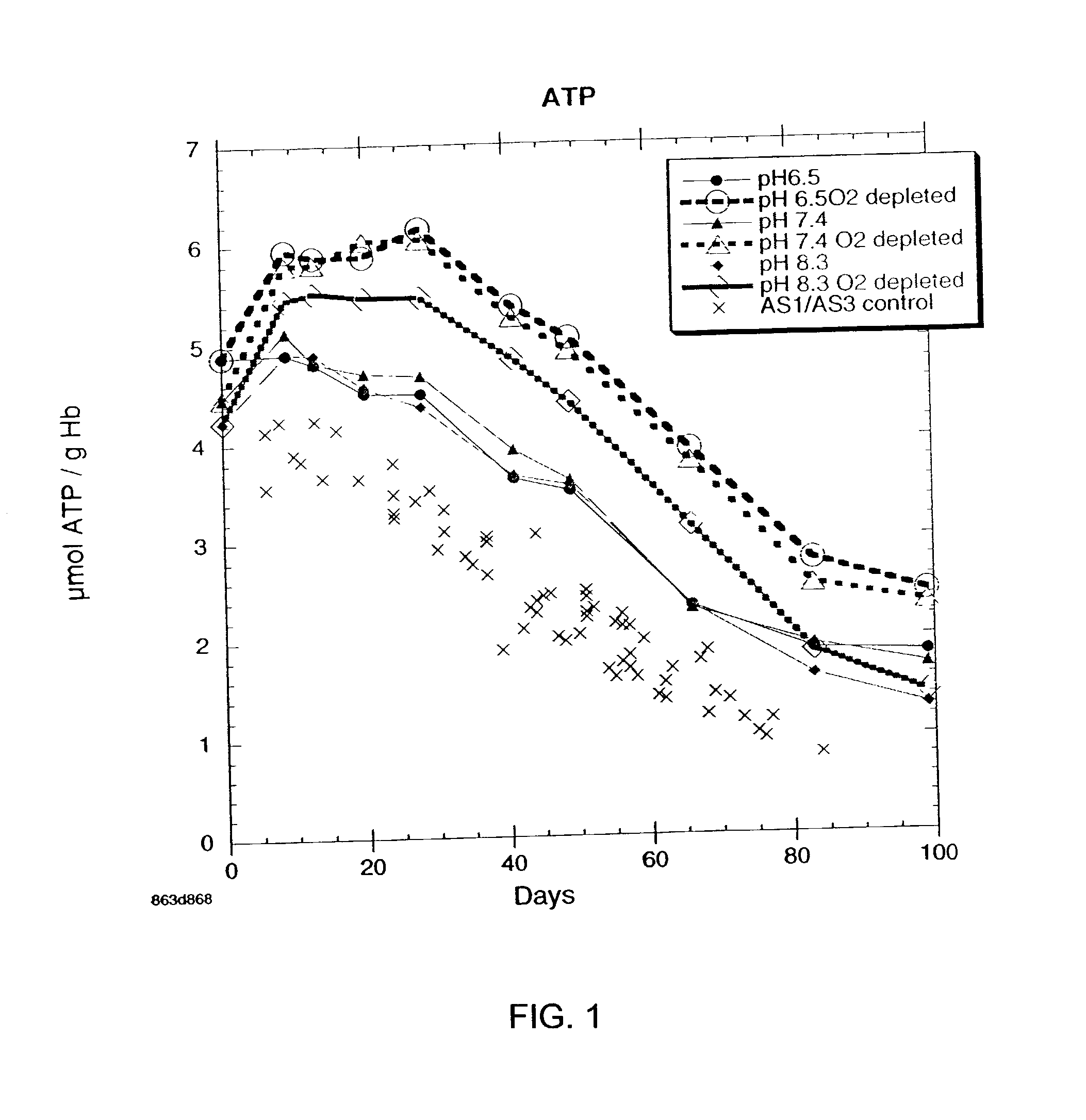
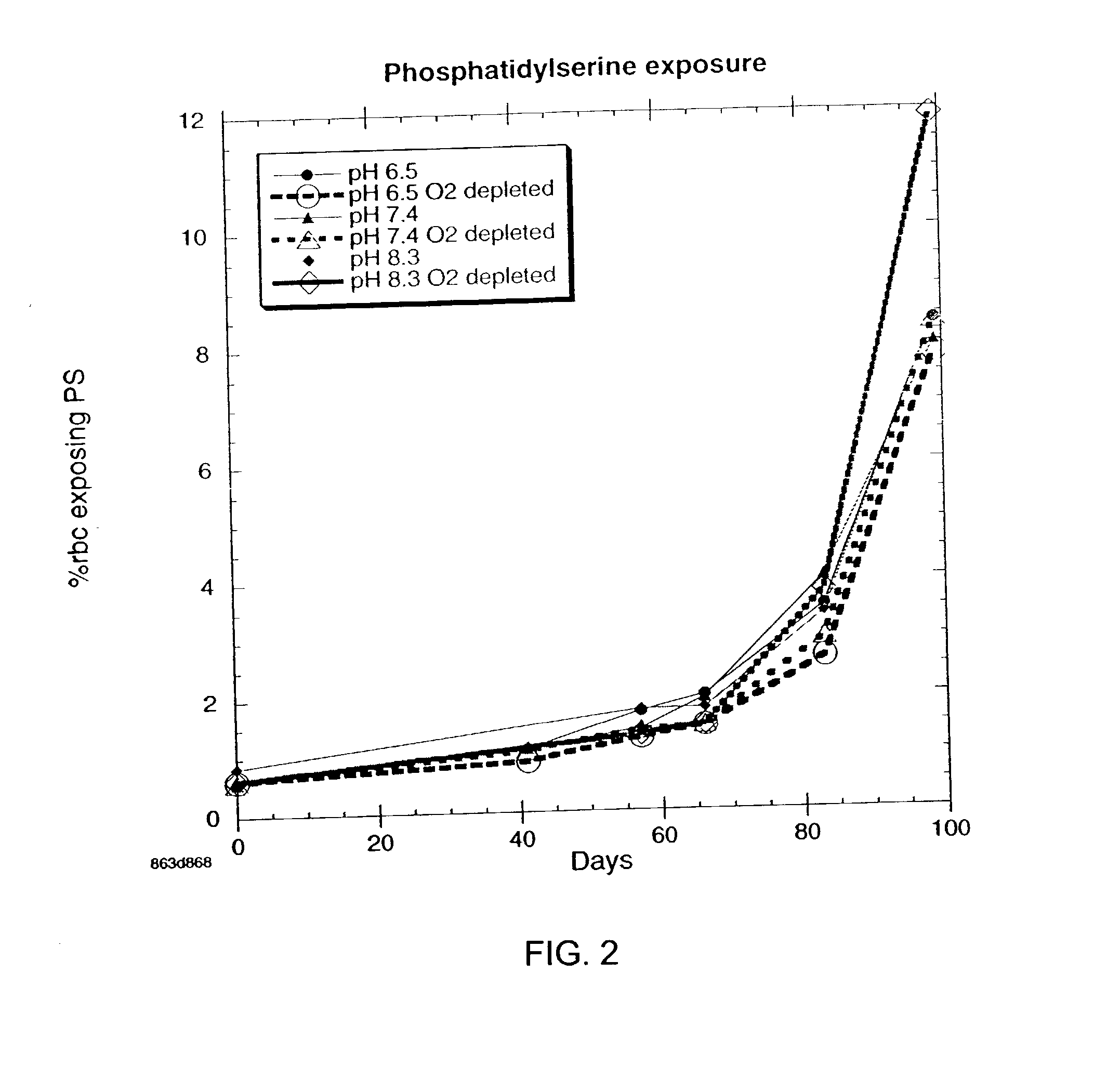
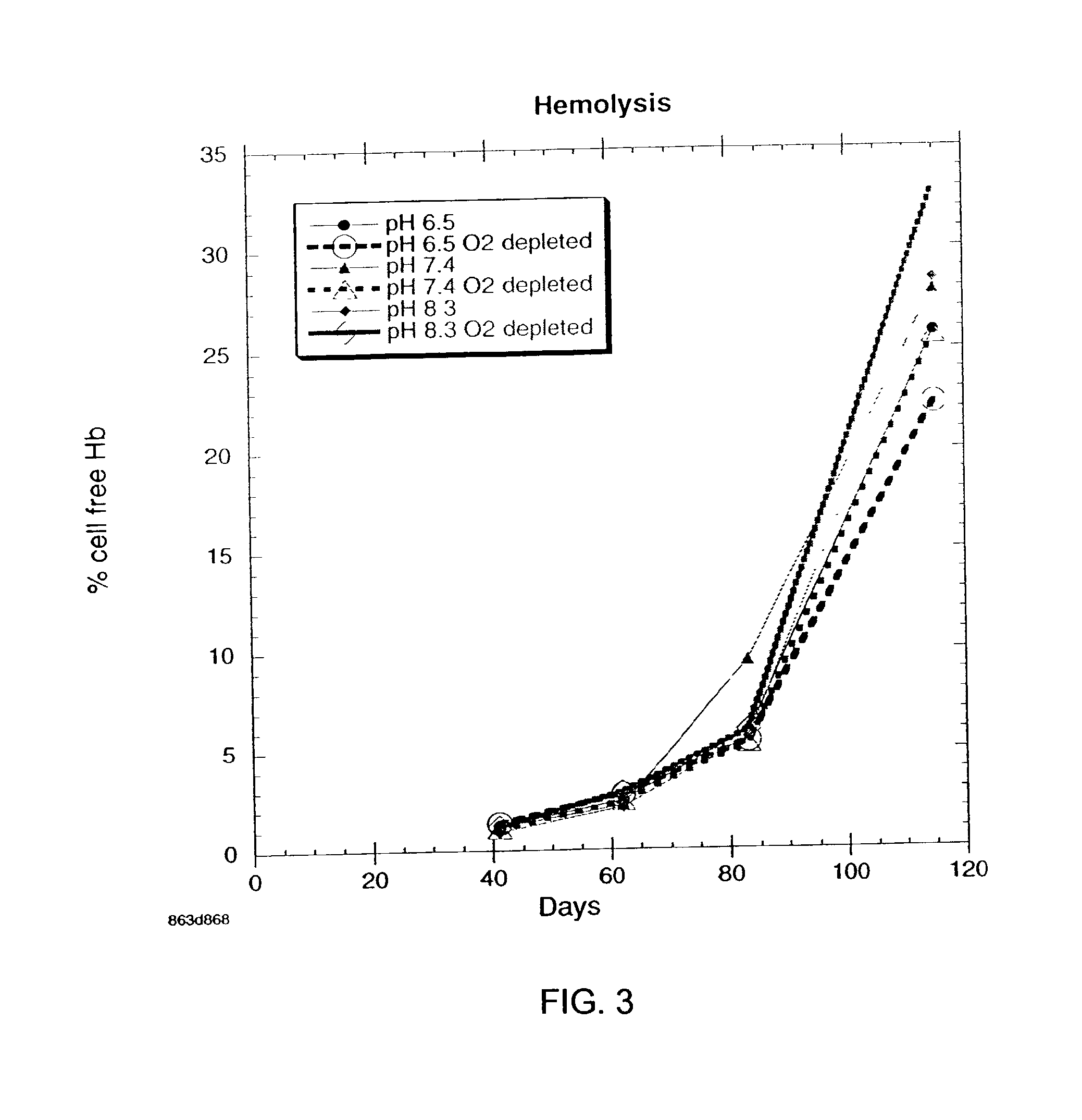
Additive solution for blood preservation
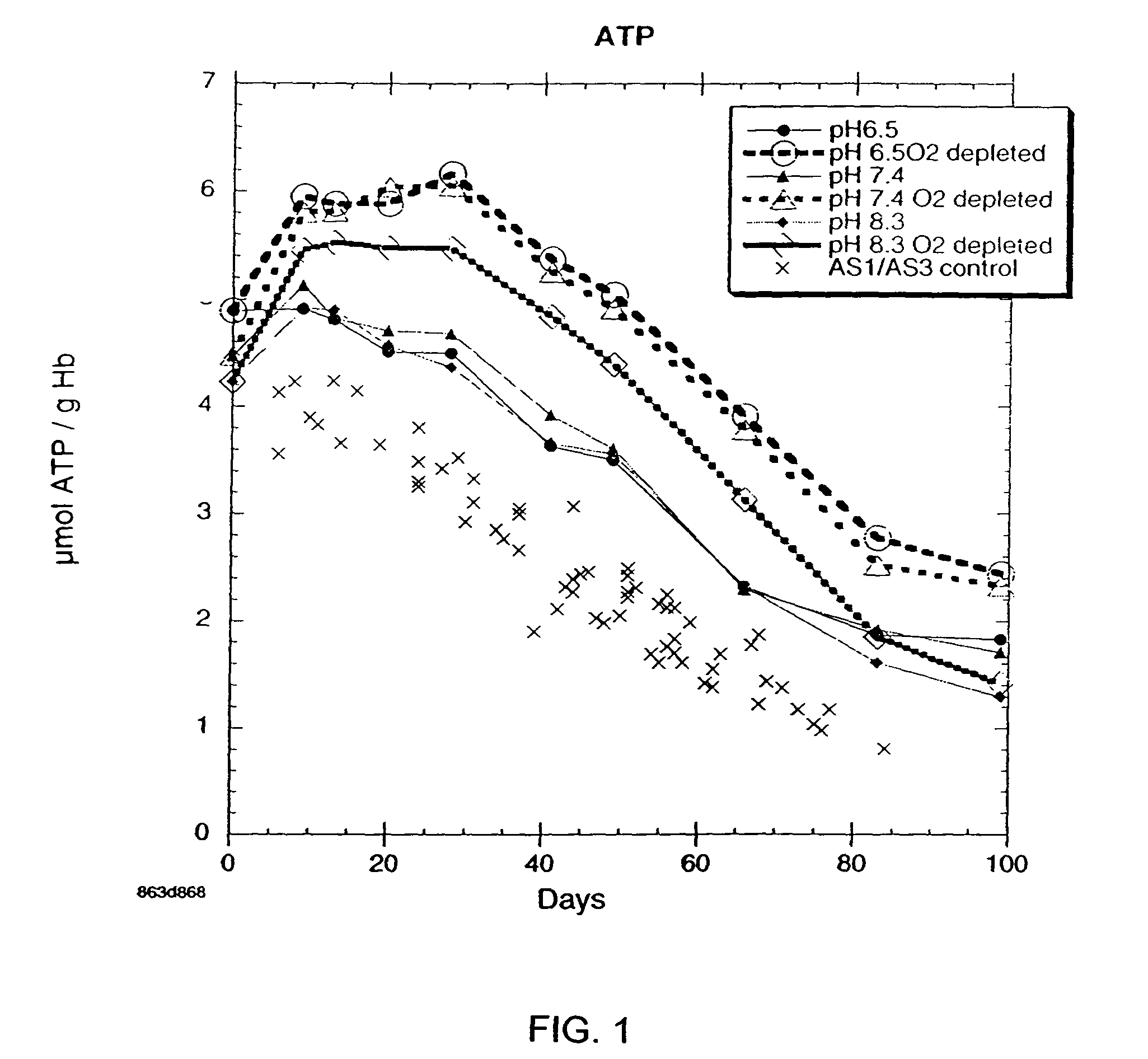
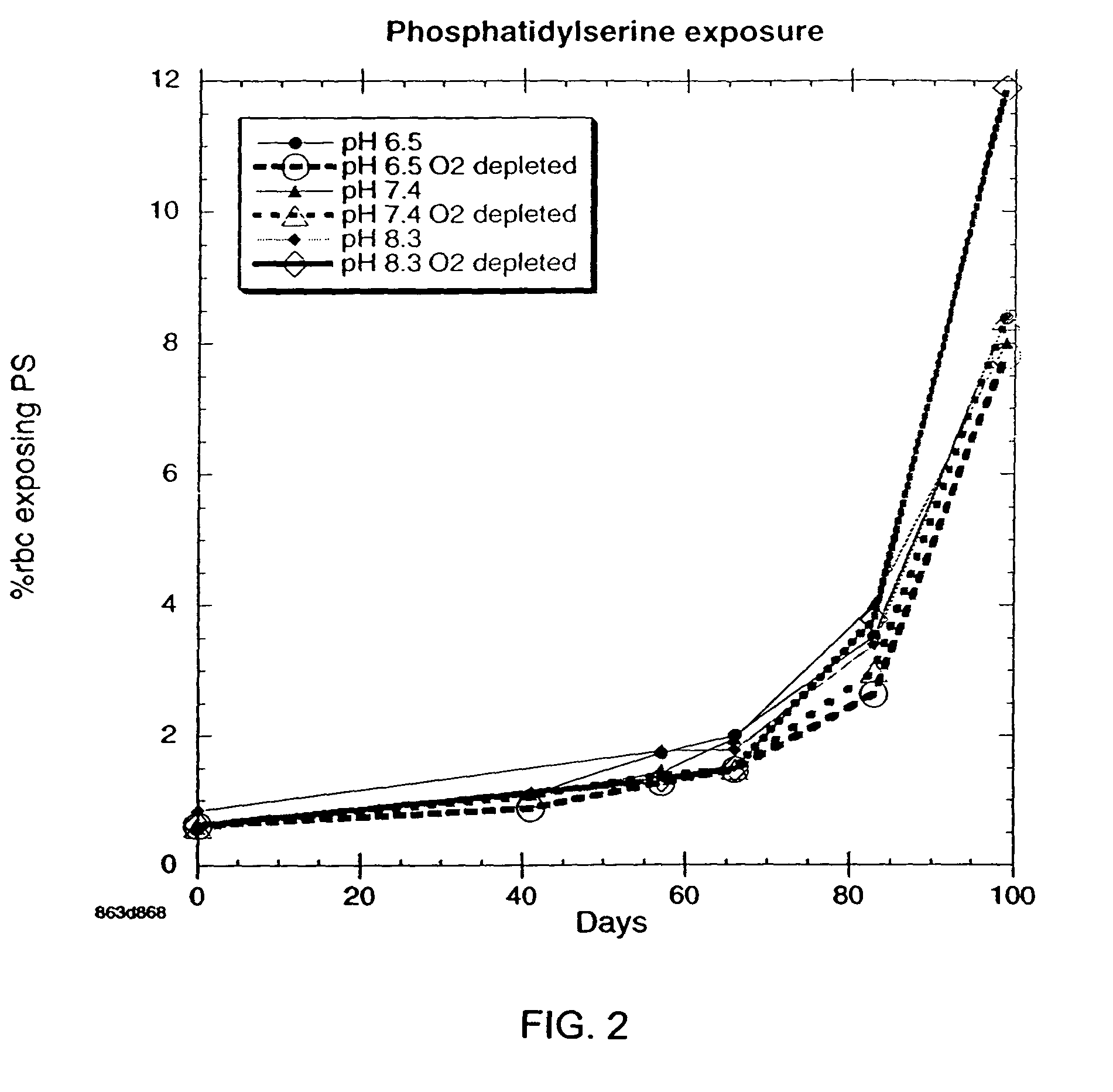
InactiveUS20050208462A1Dead animal preservationBlood/immune system cellsMANNITOL/SORBITOLRed blood cell
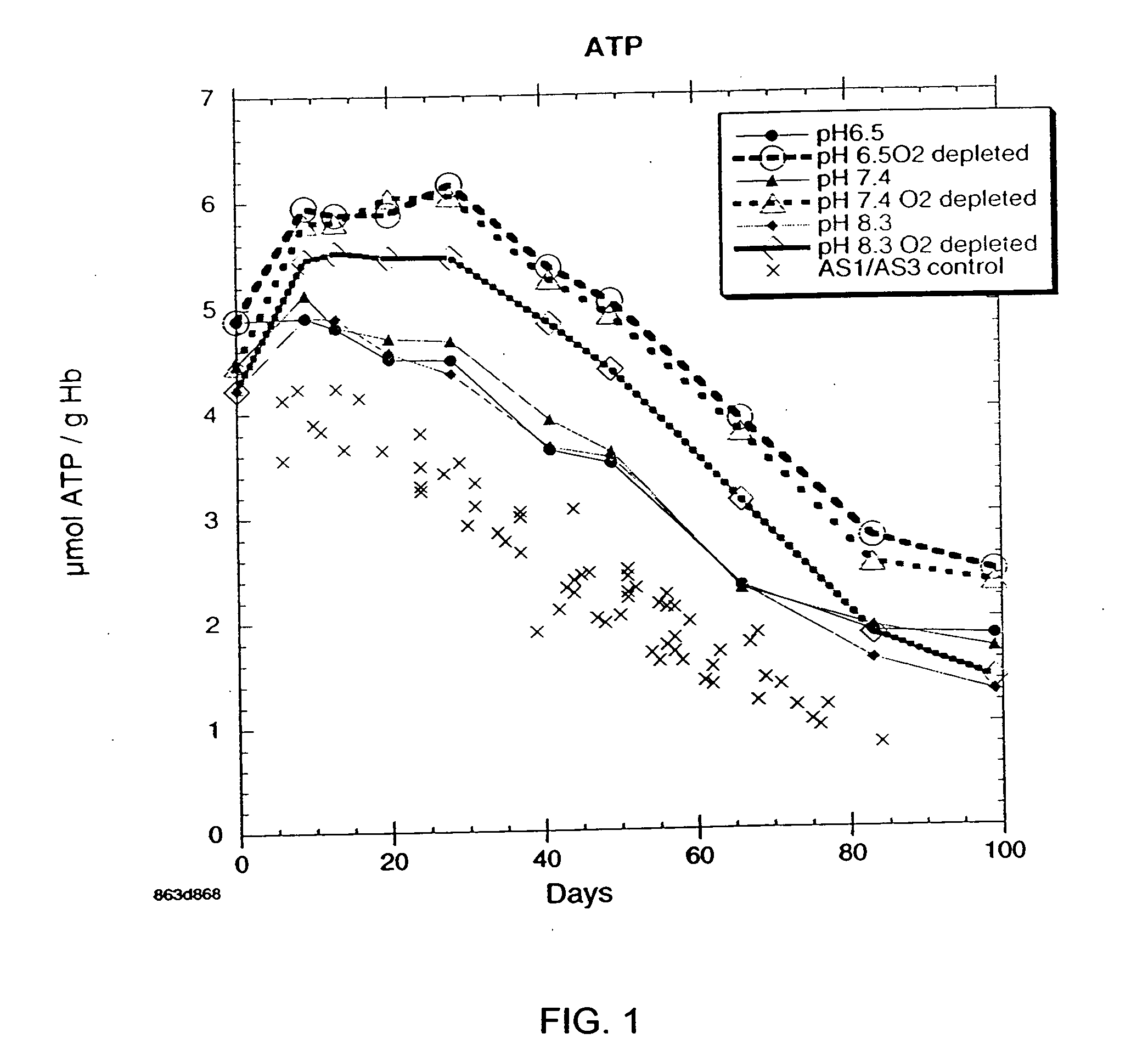
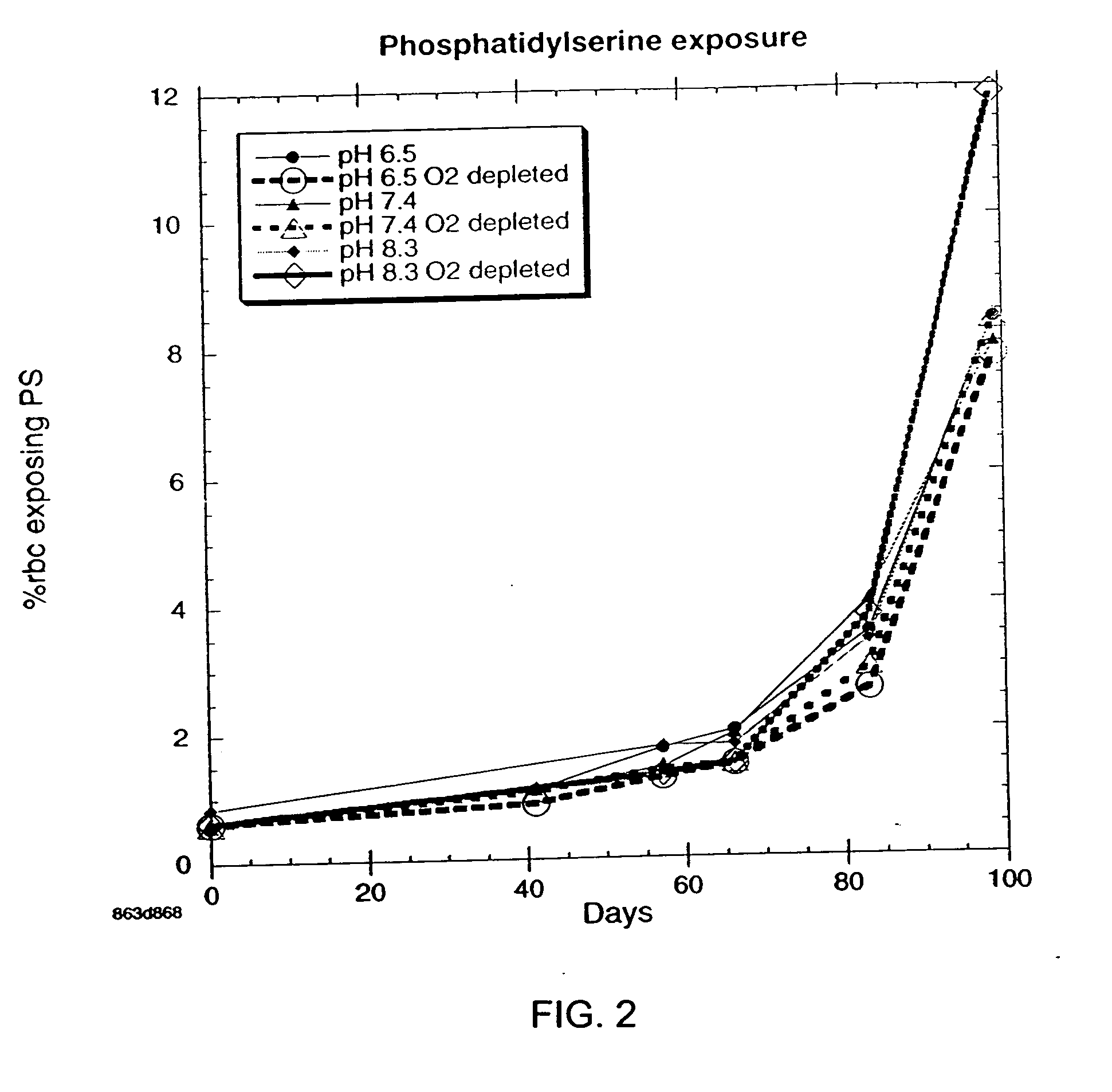
There is provided compositions and methods for the storage of red blood cells. The compositions are additive solutions comprising adenine, dextrose, mannitol, NaH2PO4, and optionally NaCl and / or N4HCl. Composition are preferably used with oxygen-depletion refrigerated storage of red blood cells and may optionally be employed with nutrient supplements extending the useful shelf life of stored blood.
Owner:TRUSTEES OF BOSTON UNIV
High-intensity sweetener-polyol compositions
InactiveUS20050196503A1Improve sweetness qualityImprove taste qualityConfectionerySweetmeatsCyclamatesMANNITOL/SORBITOL
The present invention provides a sweetener composition and methods for improving the taste of a sweetener composition. The sweetener composition includes a mixture of a high-intensity sweetener such as aspartame, encaspsulated aspartame, neotame, encapsulated neotame, cyclamate, sucralose, saccharin or Acesulfame-K, with polyols such as maltitol, sorbitol, mannitol, erythritol, xylitol, lactitol, or palatinit, wherein the high-intensity sweetener is present in the mixture in an amount from about 0.0001% to 15% by weight.
Owner:RICHMOND CHEM CORP
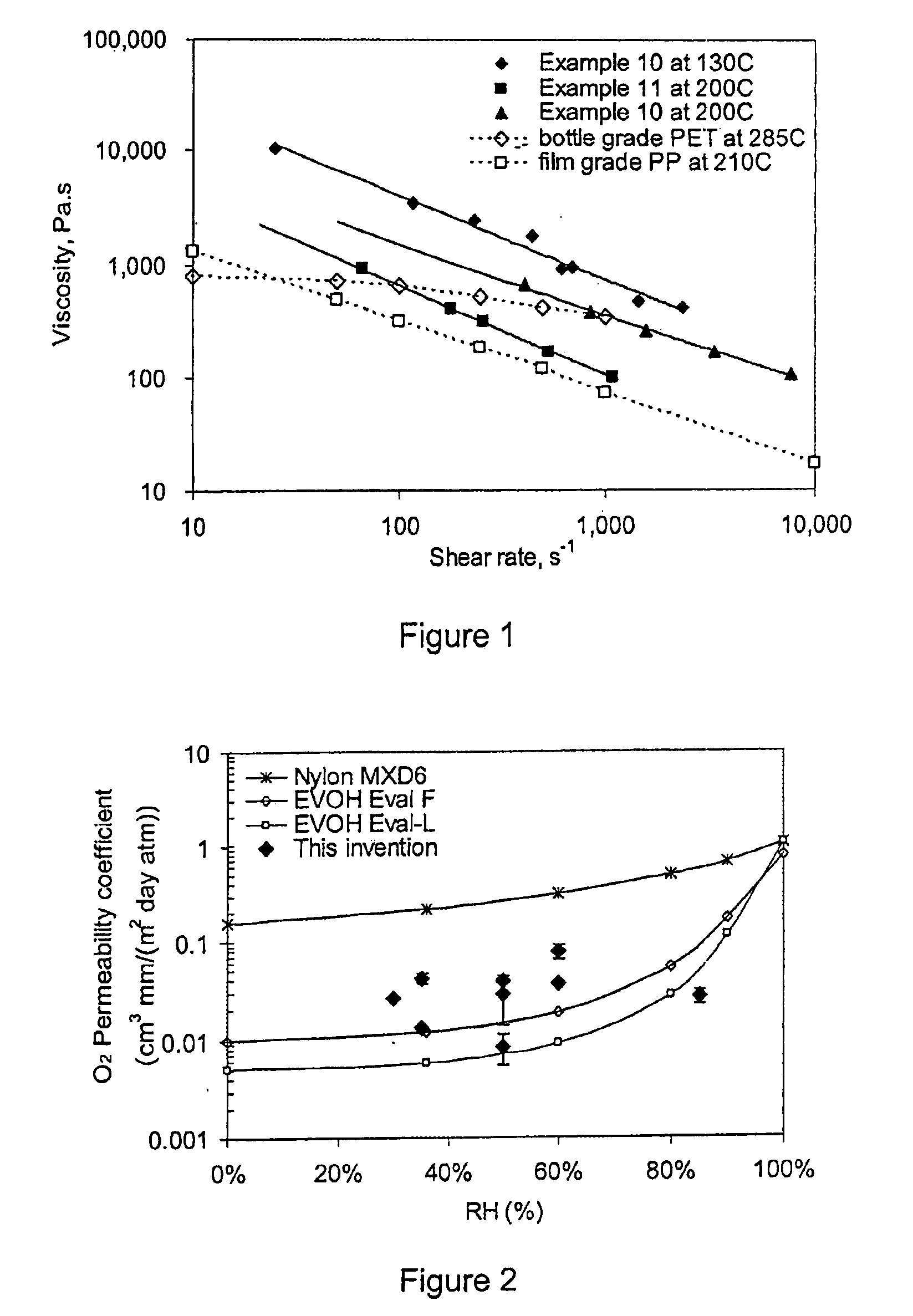
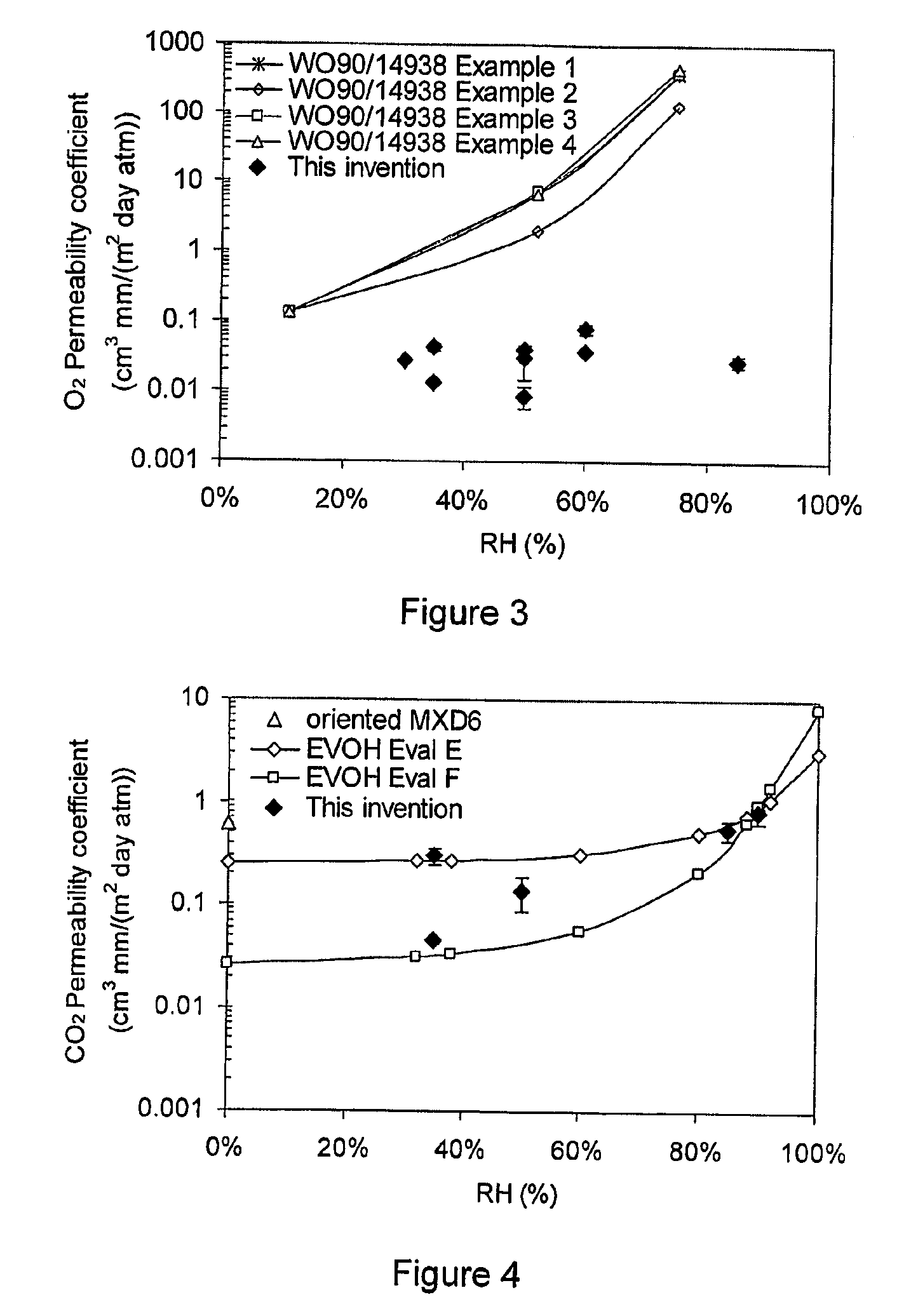
Barrier film
InactiveUS20090110942A1Stable mechanical propertiesImprove homogeneityFireproof paintsFibre treatmentMolten statePolyethylene oxide
A barrier composition which is injection mouldable and able to be made into a transparent film or incorporated (by co-extrusion and / or lamination) into multi-layer film products, the composition on dry basis: a) from 45 to 90% by weight of a starch and / or a modified starch selected from starches modified by reaction with a hydroxyl alkyl group, an acetate or a dicarboxylic acid anhydride or a grafting polymer; b) from 4 to 12% by weight of a water soluble polymer selected from polyvinyl alcohol, polyvinylacetate, and copolymers of ethylene and vinylalcohol which have a melting point compatible with the molten state of the starch components c) from 5 to 45% by weight of a non-crystallising mixture of sorbitol and at least one other plasticizer selected from glycerol, maltitol, xylitol, mannitol, glycerol trioleate, epoxidised linseed or soybean oil, tributyl citrate, acetyl tri-ethyl citrate, glyceryl triacetate, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate; polyethylene oxide or polyethylene glycol; d) from 0.3 to 2.5 % by weight of a C12-22 fatty acid or salt; e) from 0.25% to 3% of an emulsifier system having a hydrophilic lipophilic balance value between 2 and 10. The barrier film may be co-injection moulded with polyethylene terephthalate (PET) or polylactic acid (PLA) for blow moulding into beverage bottles, with polyethylene (PE) or polypropylene (PP) or biodegradable polymers for high gas-barrier containers or closures, or may be co-extruded with polyethylene, polypropylene or polylactic acid for thin film packaging applications or for blow-moulded containers.
Owner:PLANTIC TECH
Ethanol production in fermentation of mixed sugars containing xylose
Xylose-utilizing Z. mobilis strains were found to have improved ethanol production when grown in medium containing mixed sugars including xylose if sorbitol or mannitol was included in the medium. The effect was seen in concentrations of mixed sugars where no growth lag period occurs, as well as in higher sugars concentrations.
Owner:SUSTAINABLE TECH CORP +1
Method of storing red blood cells with an acidic additive solution under oxygen depletion
There is provided compositions and methods for the storage of red blood cells. The compositions are additive solutions comprising adenine, dextrose, mannitol, NaH2PO4, and optionally NaCl and / or NH4Cl. Composition are preferably used with oxygen-depletion refrigerated storage of red blood cells and may optionally be employed with nutrient supplements extending the useful shelf life of stored blood.
Owner:TRUSTEES OF BOSTON UNIV
Freeze-drying protective agent of nucleic acid amplification reaction reagents and freeze-drying method
InactiveCN103911367AHigh glass transition temperatureLess internal hydrogen bondsMicrobiological testing/measurementDNA preparationMANNITOL/SORBITOLFreeze-drying
The invention relates to a freeze-drying protective agent of nucleic acid amplification reaction reagents and a freeze-drying method. The invention provides a composition for freeze-drying protection, which is the following 1) or 2): 1) the composition comprises mycose, mannitol, bovine serum albumin, and water with a ratio of 4.0-25g:1-7g:1.0-7g:49-50ml; 2) the composition comprises mycose, mannitol, bovine serum albumin, Tween, Tris-HCl and water with a ratio of 4.0-25g:1-7g:1.0-7g:0.1-0.25ml:2.5*10<-3>-5.0*10<-3>mol:49-50ml. The freeze-drying protective agent of the invention is low in raw material cost, simple in operation, and suitable for large-scale production, can reduce production cost, and can effectively prolong the storage life of nucleic acid amplification reaction reagents.
Owner:CHINA AGRI UNIV
Anti Abeta antibody formulation
ActiveUS7635473B2Reduce by-product formationProvide stabilityOrganic active ingredientsBiocideMANNITOL/SORBITOLAntioxidant
The present invention provides formulations for maintaining the stability of Aβ binding polypeptides, for example, Aβ antibodies. Exemplary formulations include a tonicity agent such as mannitol and a buffering agent or amino acid such as histidine. Other exemplary formulations include an antioxidant in a sufficient amount as to inhibit by-product formation, for example, the formation of high molecular weight polypeptide aggregates, low molecular weight polypeptide degradation fragments, and mixtures thereof. The formulations of the invention optionally comprise a tonicity agent, such as mannitol, and a buffering agent or amino acid such as histidine. The formulations are suitable for several different routes of administration.
Owner:WYETH LLC +1
Topical compositions for treatment of irritation of mucous membranes
ActiveUS20170021023A1Effect can be damagingEffective strikeCosmetic preparationsToilet preparationsIrritationNose
A topical pharmaceutical or cosmetic composition for treatment of irritation of mucous cells such as mucosal cells of the eye, nose, and vagina is disclosed. The composition comprises an aqueous solution of at least one polyol selected from the group consisting of xylitol, myoinositol and mannitol and at least one substance selected from the group consisting of glycerol and urea. The composition contains less than 0.01% inorganic salt and is free of any oil-in-water or wax-in-water emulsion. In particularly preferred embodiments, the composition comprises an aqueous solution containing 1.5-5% (w / v) xylitol and 0.9-2.0% (w / v) glycerol.
Owner:RESDEVCO RES & DEV
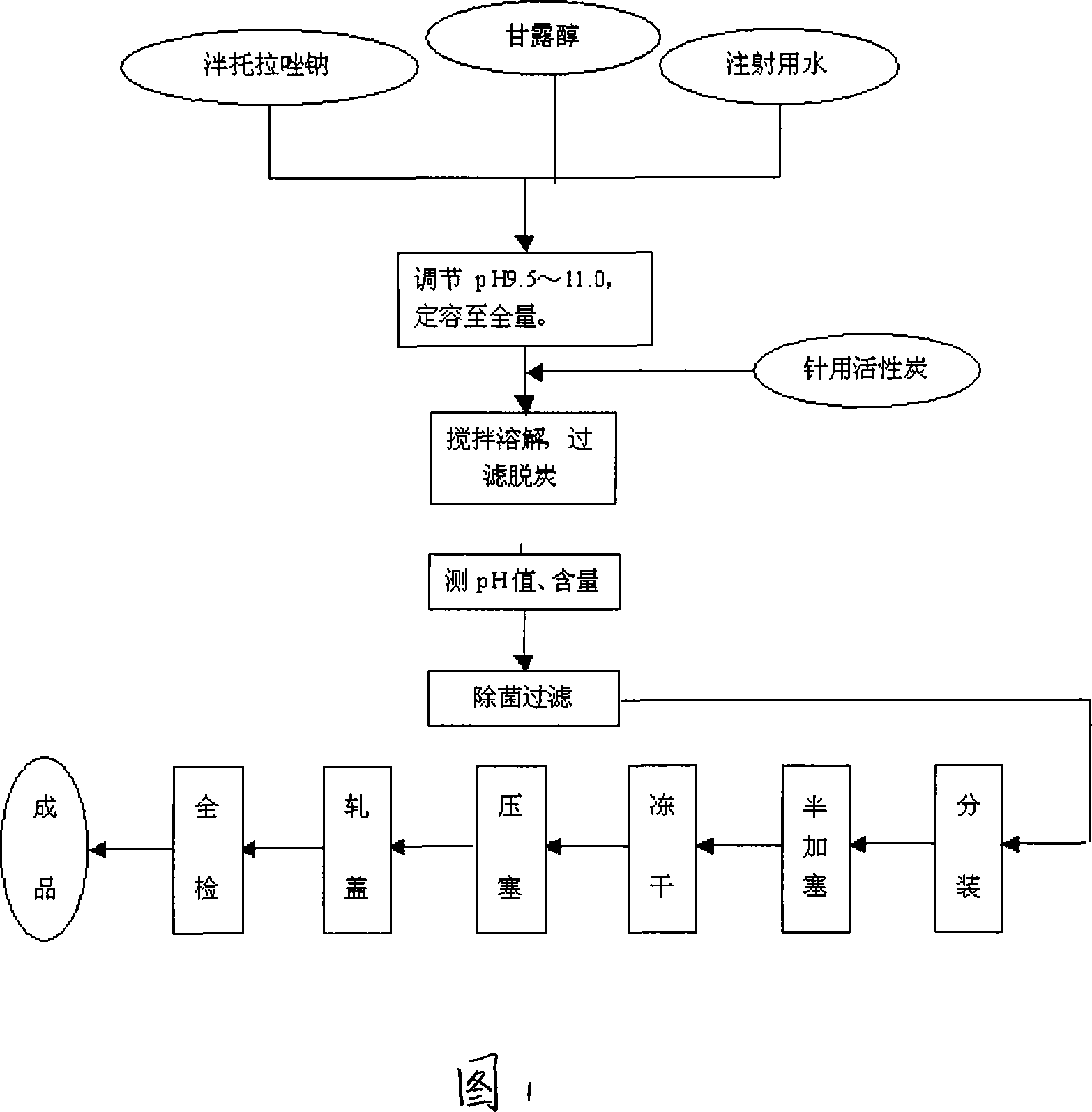
Pantoprazole sodium freeze-dried powder injection and preparing method thereof
ActiveCN101229138ASimple recipeLittle side effectsPowder deliveryOrganic active ingredientsSolubilityMANNITOL/SORBITOL
The invention aims at providing a pantoprazole sodium freeze-dried powder injection and comprises pantoprazole sodium and mannitol with the weight ratio of 1: 2 to 5. The invention is simple in formula and little in side effect; products prepared by the method are plump in appearance, good in complex solubility and excellent in quality with the adoption of an advanced freezing and drying process.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Non-crystallizing syrups containing sorbitol and their use in chewing gum
ActiveUS20070196534A1Low costImproved chewing gum compositionContainers for annular articlesChewing gumMaltitolGlycerol
An aqueous syrup for use in chewing gum, particularly pellet gum, comprises, on a dry basis, greater than about 98% polyols, of which i) about 50% to about 90% is sorbitol, ii) about 3% to about 30% is maltitol, iii) about 2% to about 20% are polyols, other than sorbitol and maltitol, with a degree of polymerization (DP) of 1 or 2, and iv) less than about 20% (and perhaps less than about 12%) are polyols with a DP of 3 or greater. The syrup is made with less than about 1.0% plasticizing agent, such as glycerin or propylene glycol. The syrup contains less than 5% water. The syrup can be made by evaporating a mixture of a sorbitol solution, a maltitol syrup and one or more polyols selected from the group consisting of mannitol, xylitol, lactitol, erythritol, hydrogenated isomaltulose, and combinations thereof. Methods of using the syrup to make chewing gum, and chewing gum products containing the syrup are also provided.
Owner:WM WRIGLEY JR CO
Probiotic microcapsules as well as preparation method and application thereof
ActiveCN105310080AImprove the situation of low freeze-drying survival rateImprove stabilityFood freezingFood shapingFreeze-dryingK carrageenan
The invention relates to probiotic microcapsules as well as a preparation method and application thereof. The probiotic microcapsules comprise a core material and a wall material, wherein the core material is probiotics; the outer layer of the wall material is coated with chitosan; the wall material is prepared from an aqueous solution containing a natural polymer material and a freeze-drying protection agent; the freeze-drying protection agent comprises one or more of glucose, fructose, sucrose, lactose, trehalose, soluble starch, glycerin, mannitol, Arabic gum, dextran 40 and skim milk; the natural polymer material comprises one or more of gellan gum, xanthan gum, k-carrageenan, sodium alginate, cellulose acetate phthalate or gelatin; in the aqueous solution, the volume fraction of the freeze-drying protection agent is 4.0%-20.0% and the volume fraction of the polymer material is 0.5%-5.0%. The probiotic microcapsules can keep excellent acid resistance and storage stability before and after being freeze-dried.
Owner:SUN YAT SEN UNIV
Medicinal preparation containing exenatide
The invention provides a medicinal preparation containing exenatide suitable for multi-administration, which contains exenatide, buffer solution, pharmaceutically acceptable accessory and preservative, wherein the buffer solution can keep the pH value of the preparation in an aqueous solution state at 3.0 to 7.0; the accessory may be one or combination of glucose, sucrose, methionine, mannitol or glycine; and the preservative is selected from benzoic acid, sodium benzoate, potassium sorbate or acetone chloroform. The medicinal preparation has the advantages that the stability of physicochemical and biological activities of the exenatide is enhanced by adding a few components capable of being accepted by the human body, and then a preparation suitable for clinical use, particularly injection use is prepared.
Owner:HANGZHOU JIUYUAN GENE ENG
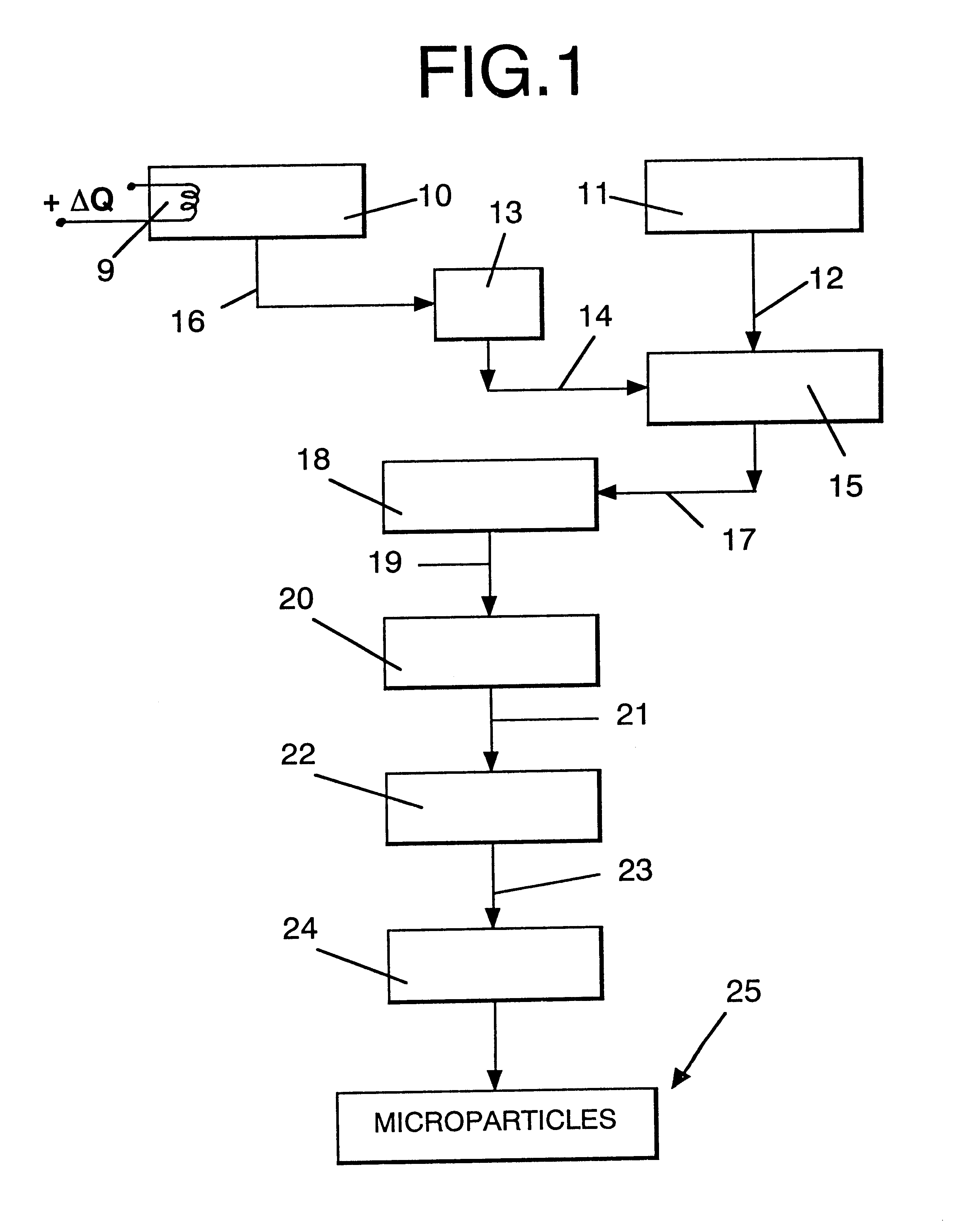
Microparticles which controllably release olfactorily active substances, methods of using same and processes for preparing same
InactiveUS6235274B1Increase the fragranceAugmenting and enhancing and imparting aroma and tasteCosmetic preparationsPowder deliveryParticulatesFlavor
Described are flavor composition, flavor component, perfume composition and perfume component-containing microparticles which are particulate matrices composed of:(a) an olfactorily active component (e.g., perfume component);(b) silica; and(c) a saccharide composition which is a mixture of mannitol and maltose.The microparticles are useful in augmenting, enhancing and / or imparting aroma and / or taste (over relatively long periods of time in a controllably releasable manner) to perfume compositions, perfumed articles (e.g., deodorancy and antiperspirant sticks), foodstuffs, chewing gums, beverages and the like. Also described is a process for preparing the above-mentioned microparticles using, in sequence, (1) adsorption of the olfactorily active material onto silica followed by (2) a blending / extrusion step followed by (3) at least one particularization step.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Additive solution for blood preservation
There is provided compositions and methods for the storage of red blood cells. The compositions are additive solutions comprising adenine, dextrose, mannitol, NaH2PO4, and optionally NaCl and / or NH4Cl. Composition are preferably used with oxygen-depletion refrigerated storage of red blood cells and may optionally be employed with nutrient supplements extending the useful shelf life of stored blood.
Owner:TRUSTEES OF BOSTON UNIV +1
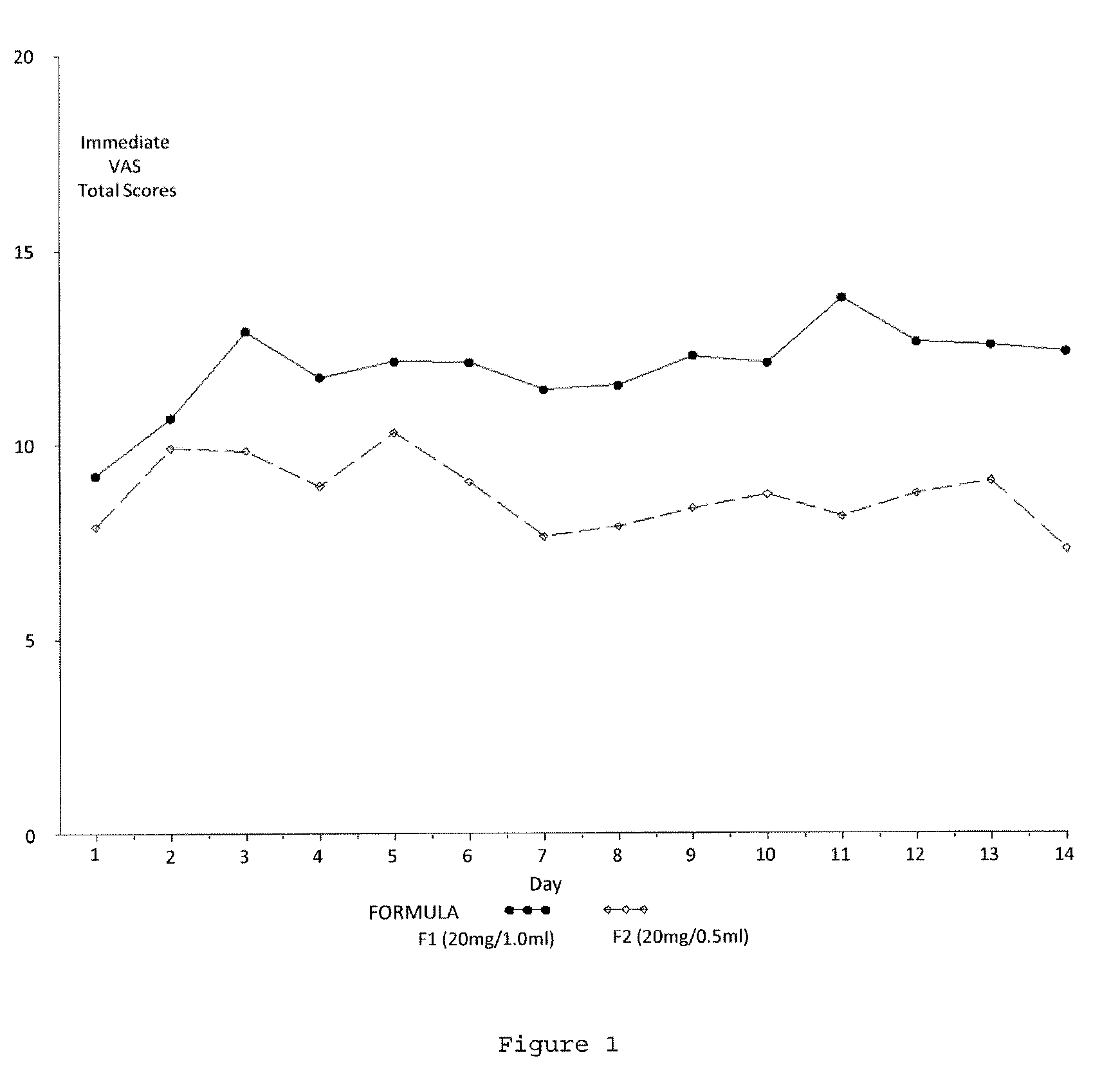
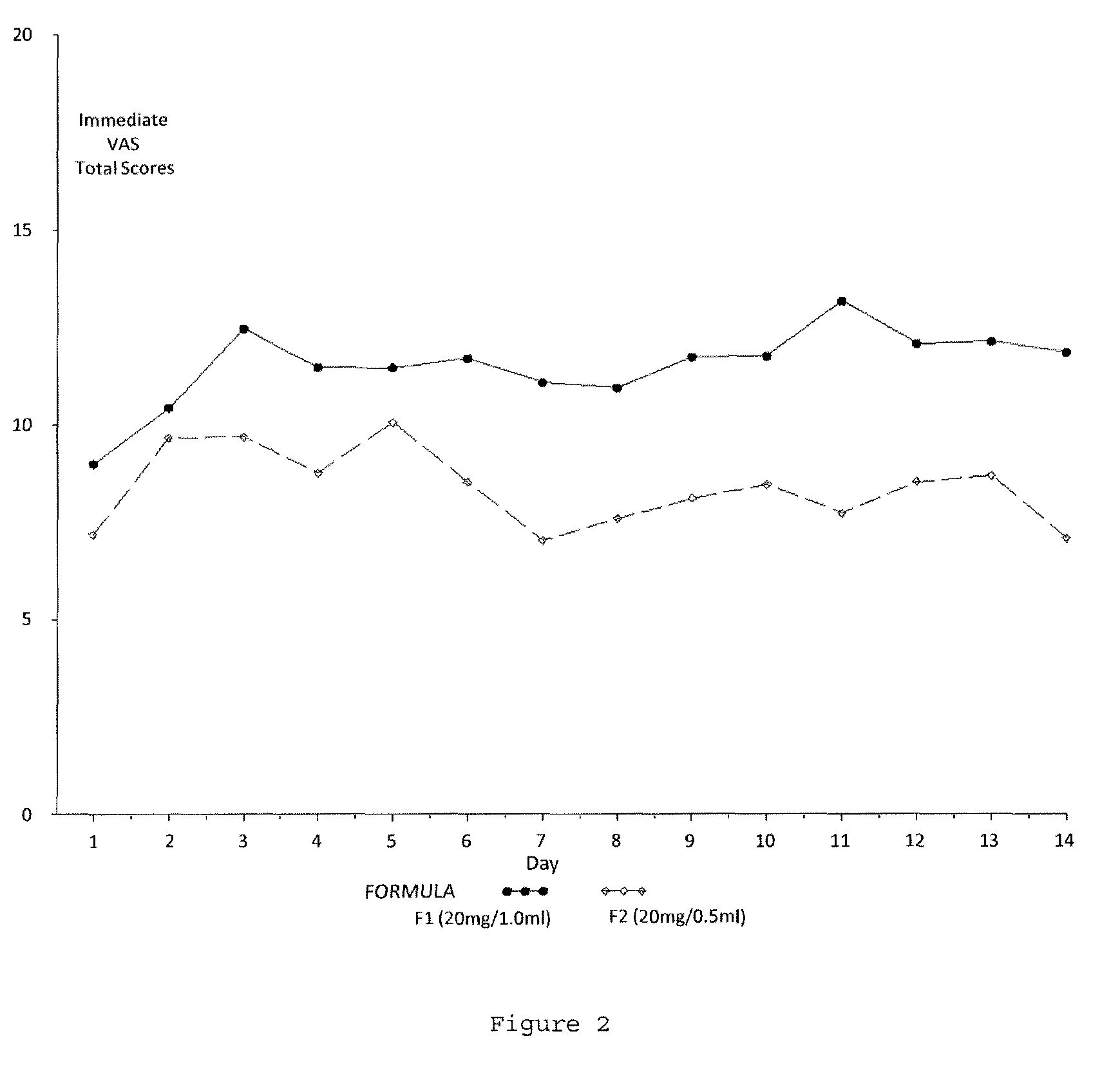
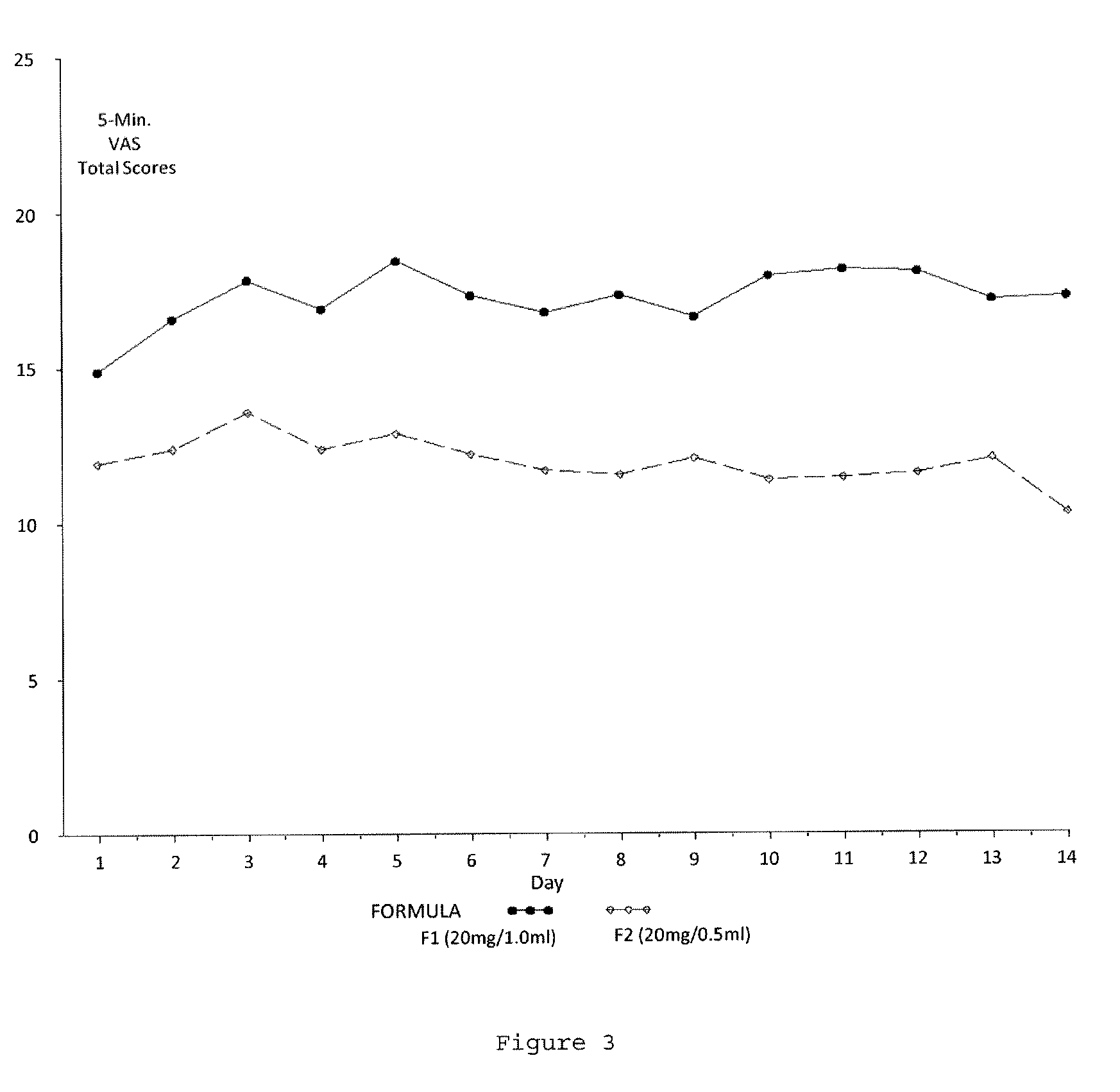
Reduced volume formulation of glatiramer acetate and methods of administration
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com