Patents
Literature
117 results about "Glatiramer acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
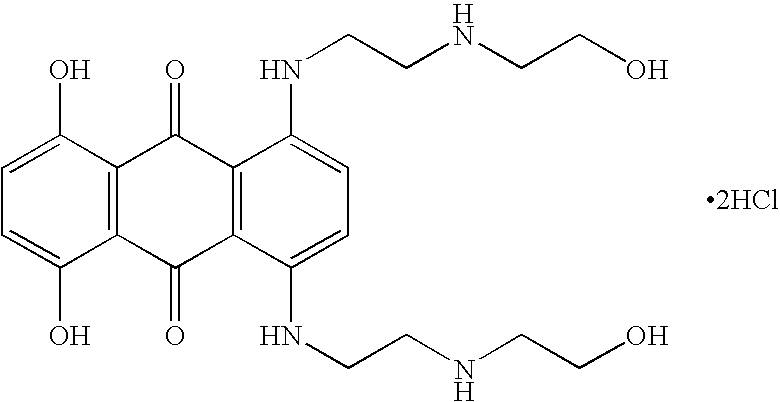
Glatiramer acetate (also known as Copolymer 1, Cop-1, or Copaxone) is an immunomodulator medication currently used to treat multiple sclerosis. Glatiramer acetate is approved in the United States to reduce the frequency of relapses, but not for reducing the progression of disability. Observational studies, but not randomized controlled trials, suggest that it may reduce progression of disability. While a conclusive diagnosis of multiple sclerosis requires a history of two or more episodes of symptoms and signs, glatiramer acetate is approved to treat a first episode anticipating a diagnosis. It is also used to treat relapsing-remitting multiple sclerosis. It is administered by subcutaneous injection.
Method of treating multiple sclerosis
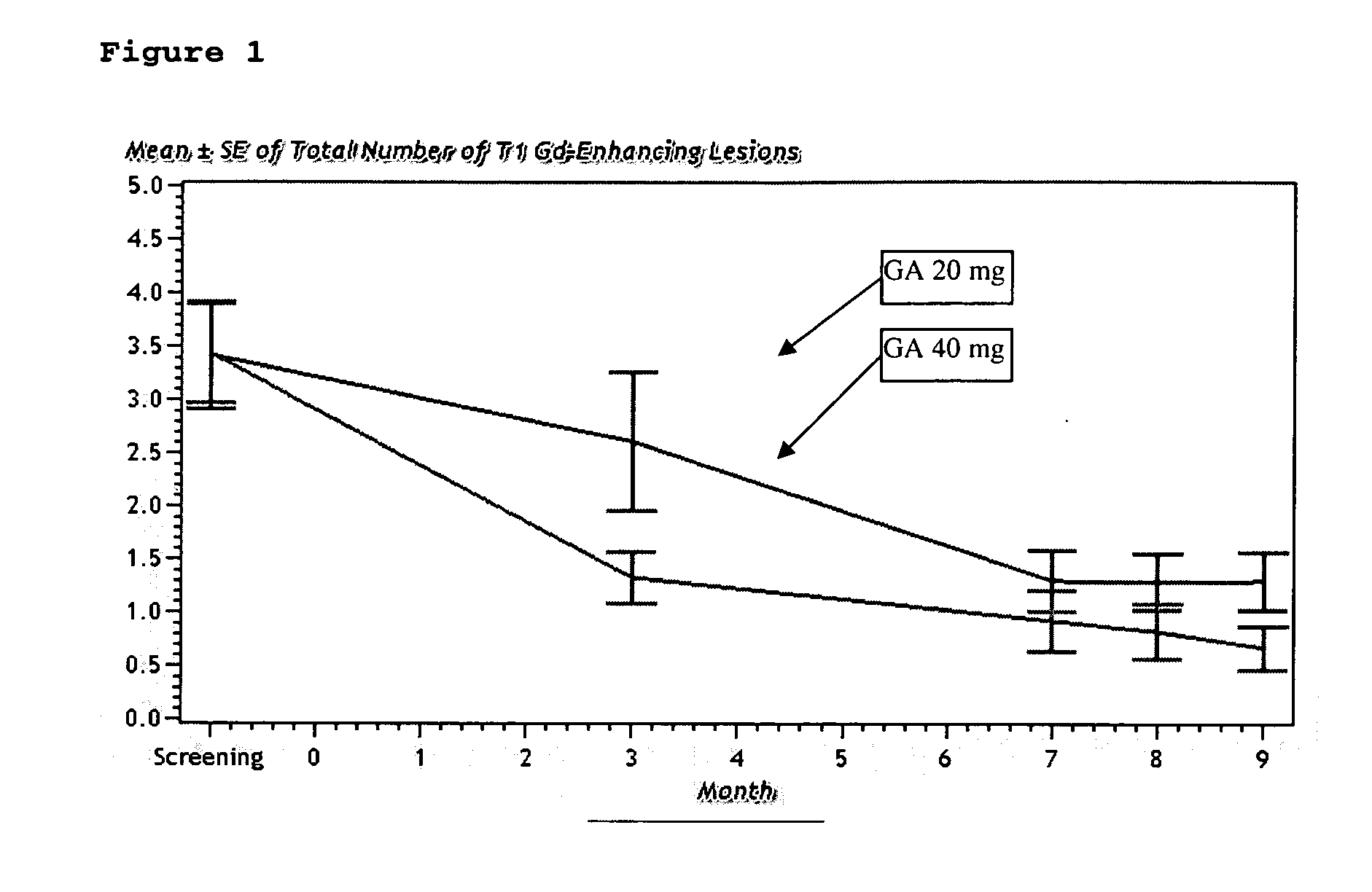
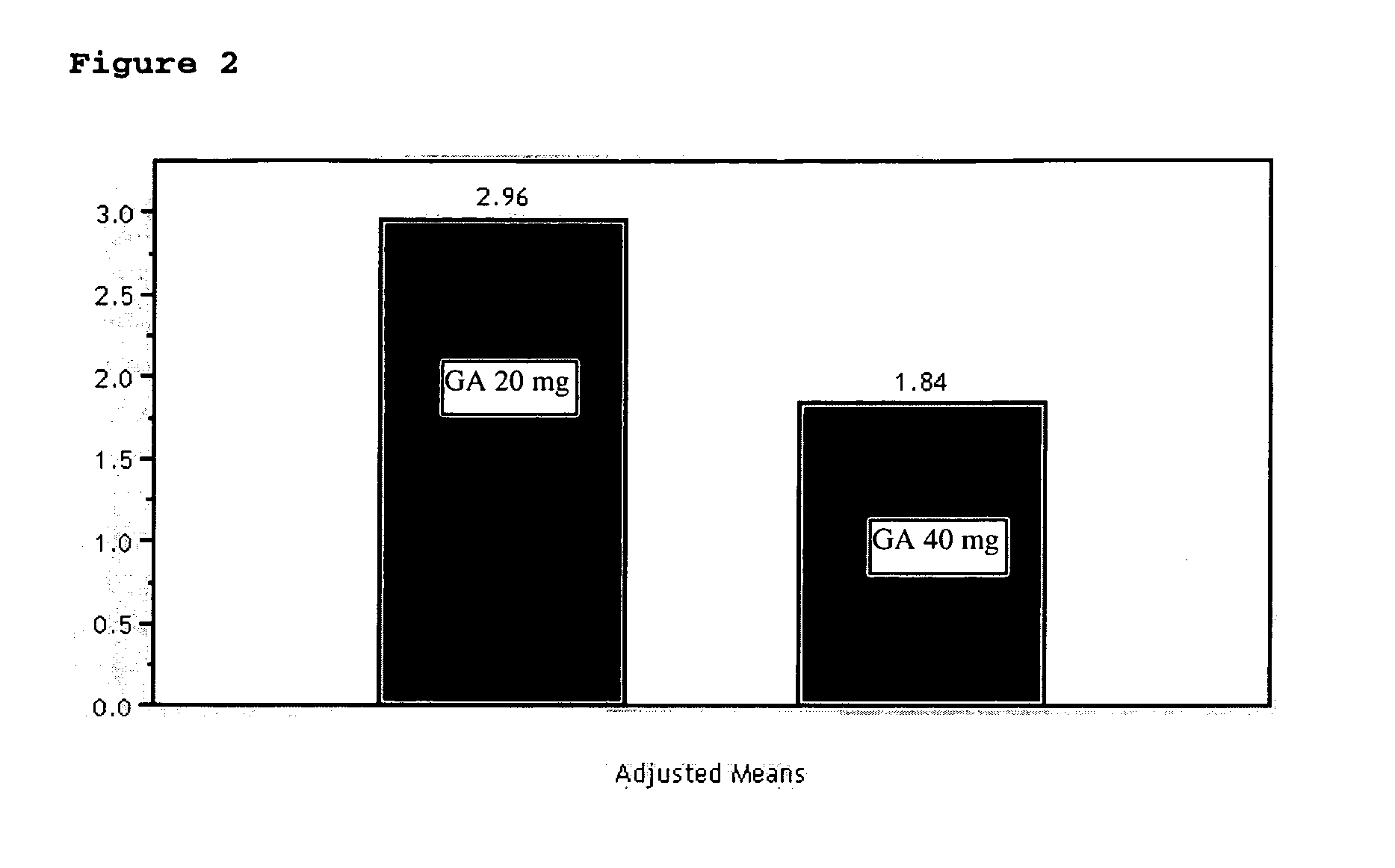
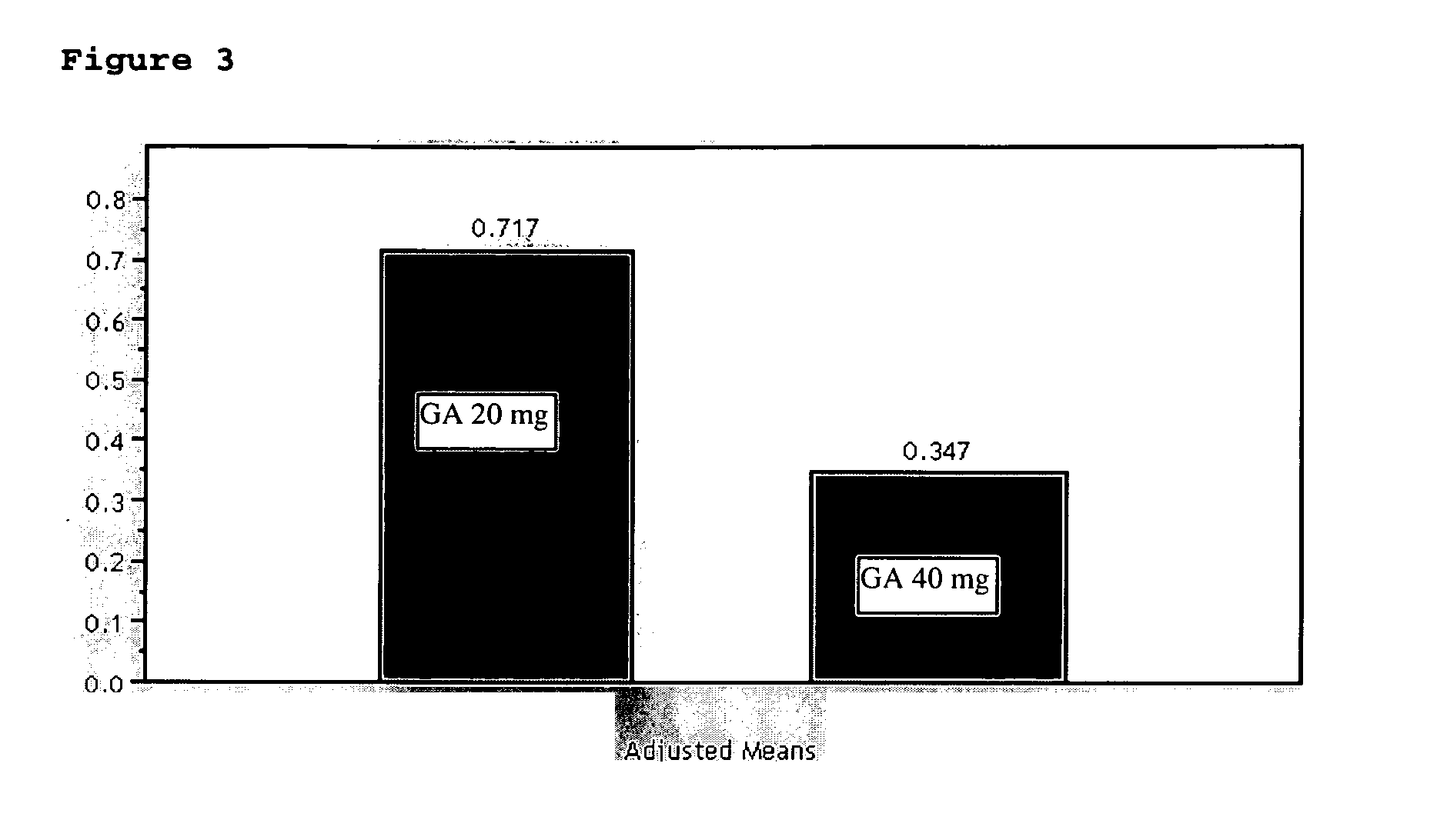
InactiveUS20070161566A1Relieve symptomsReducing MRI-monitored disease activity and burden of a patientTetrapeptide ingredientsDepsipeptidesEnhancing LesionSubcutaneous injection
This invention provides a method of alleviating a symptom of a patient suffering from a relapsing form of multiple sclerosis which comprises periodically administering to the patient by subcutaneous injection a single dose of a pharmaceutical composition comprising 40 mg of glatiramer acetate so as to thereby alleviate the symptom of the patient. This invention also provides a method of reducing Gd-enhancing lesions in the brain and a pharmaceutical composition in a unit dosage.
Owner:TEVA PHARMA IND LTD
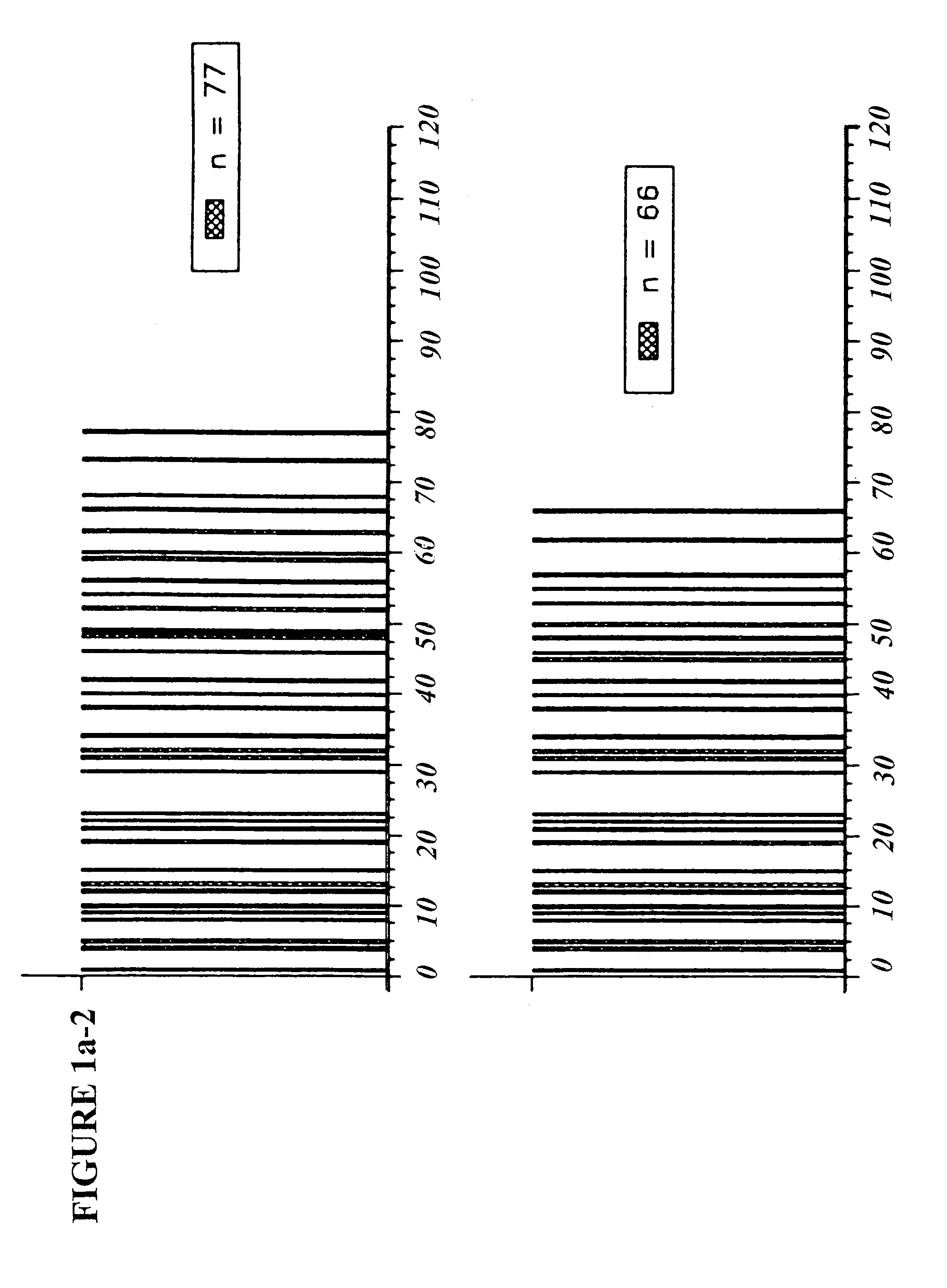
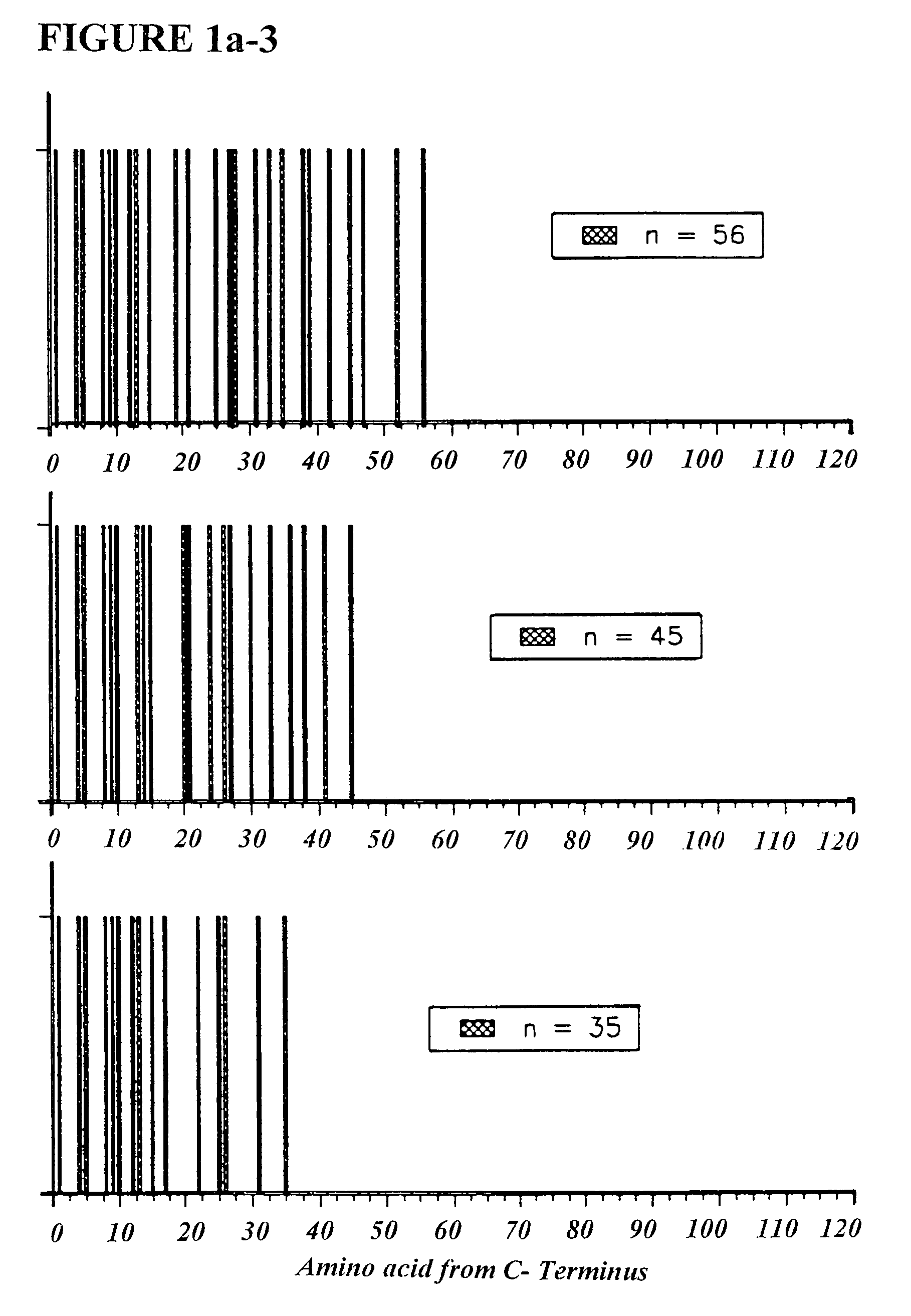
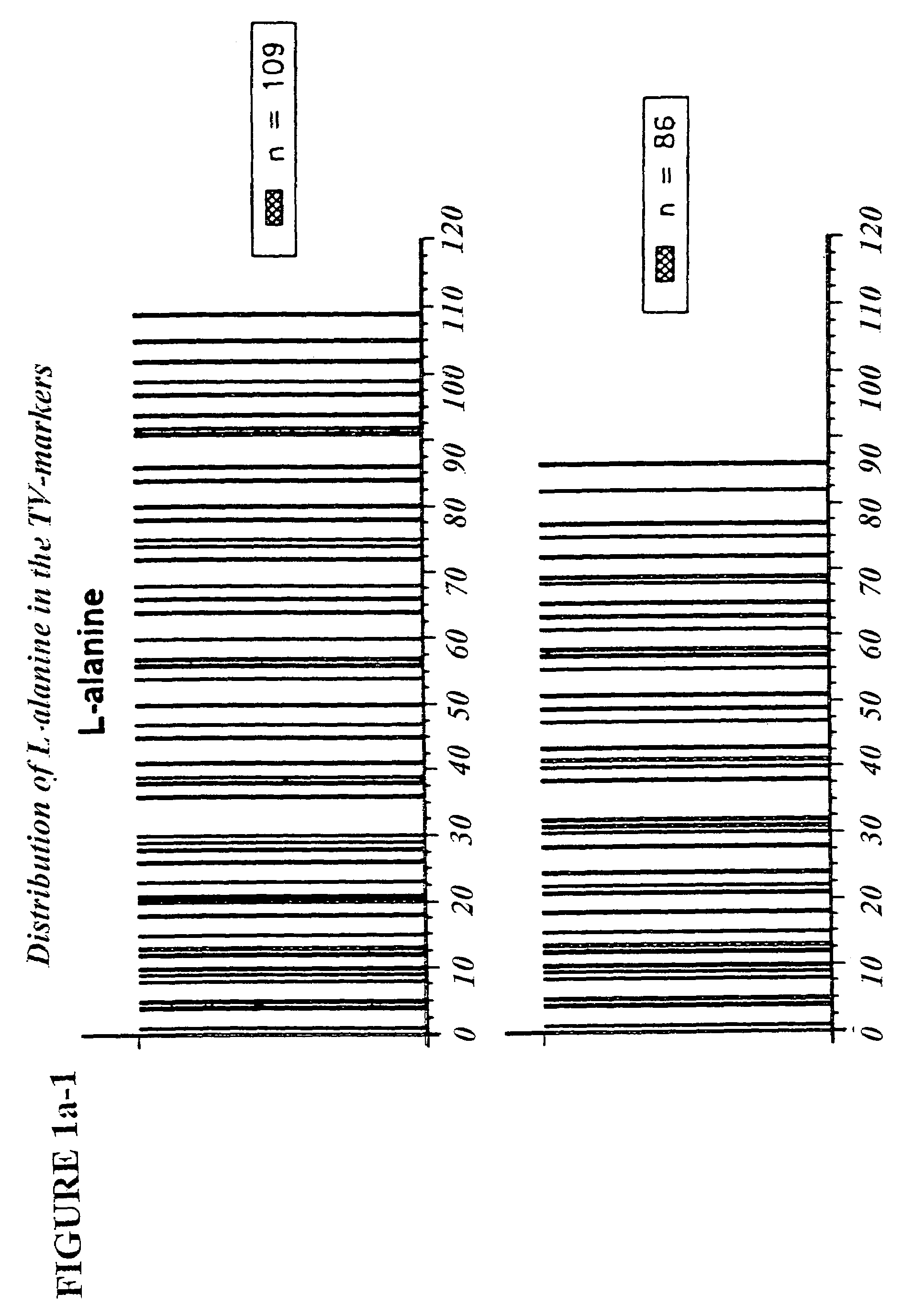
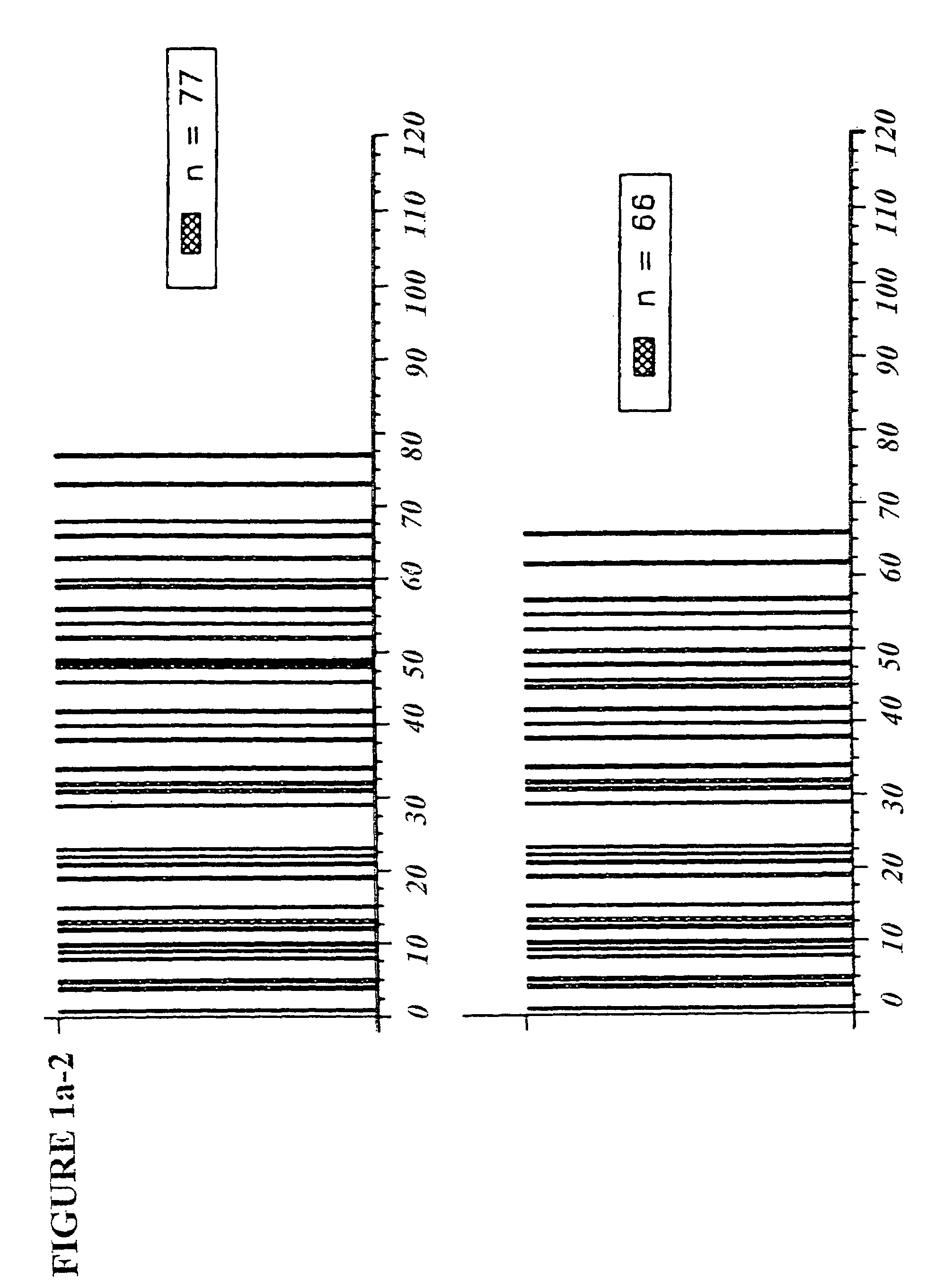
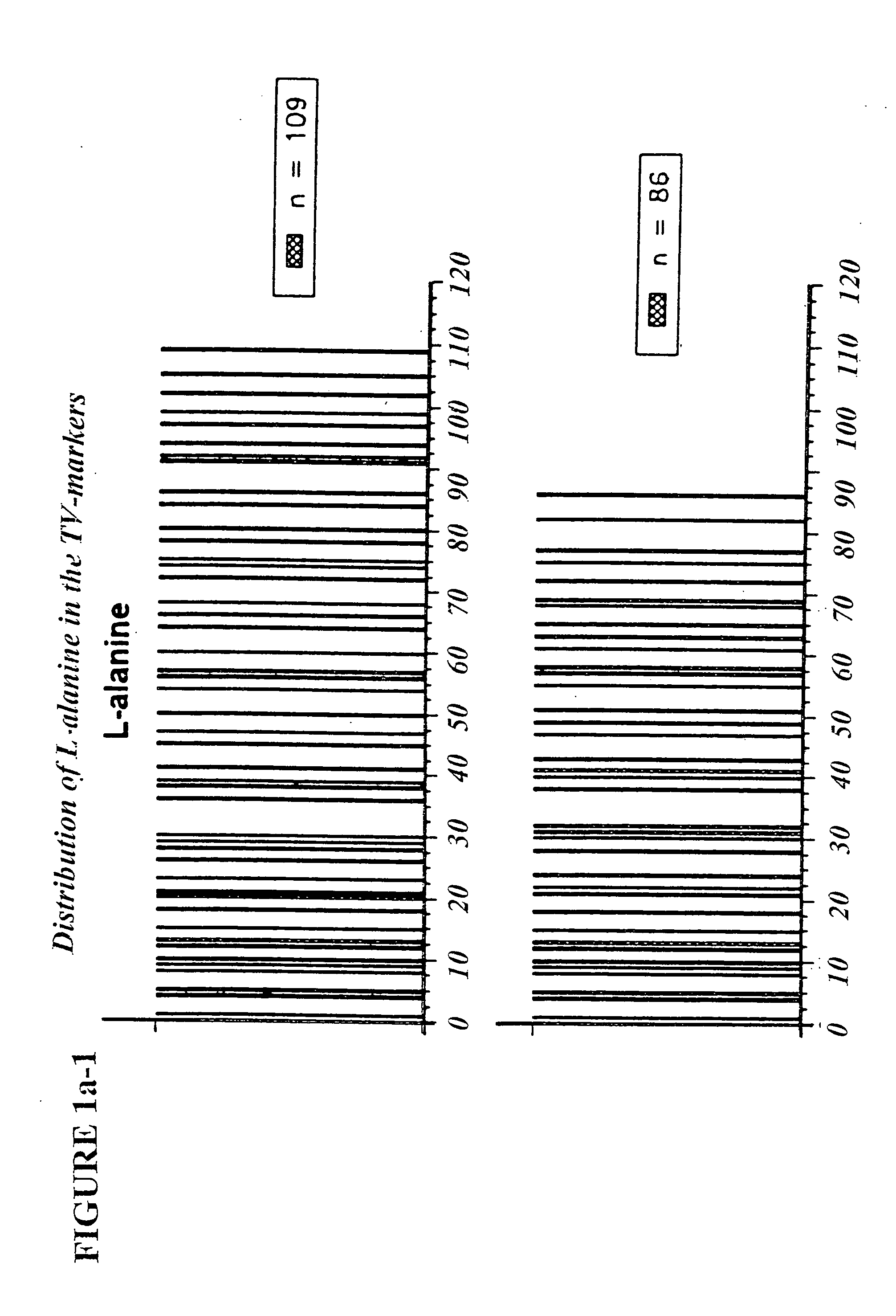
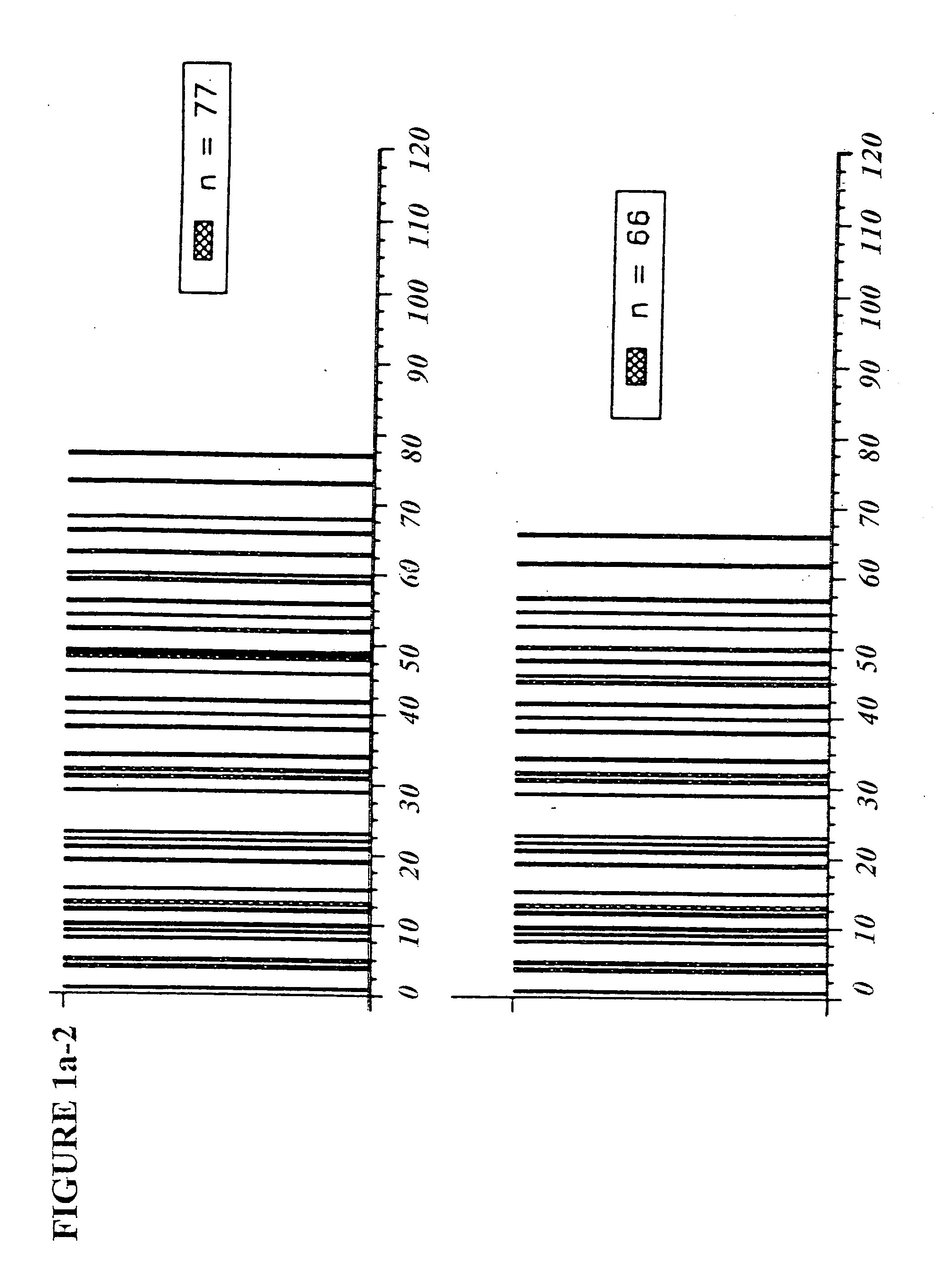
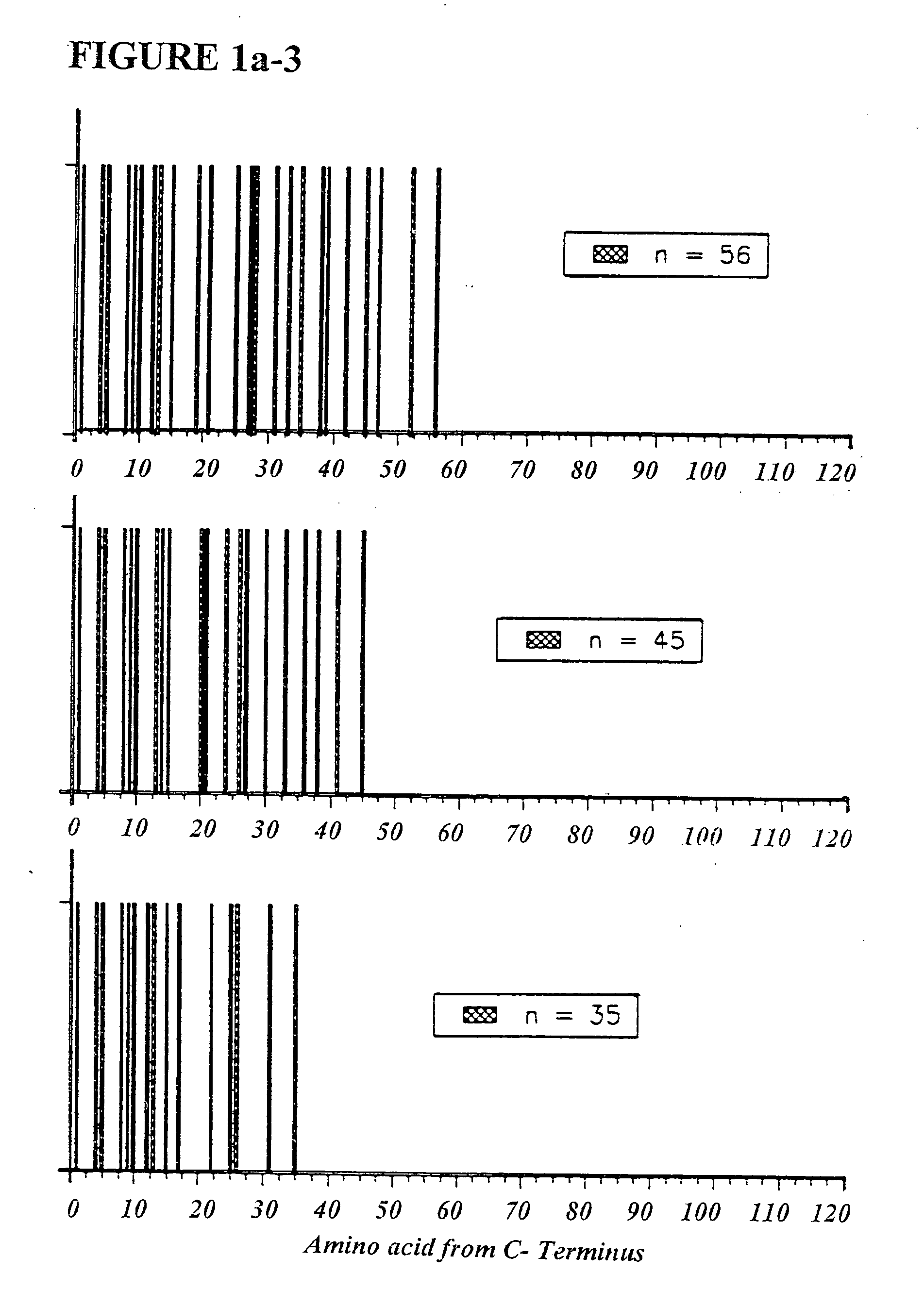
Copolymer 1 related polypeptides for use as molecular weight markers and for therapeutic use
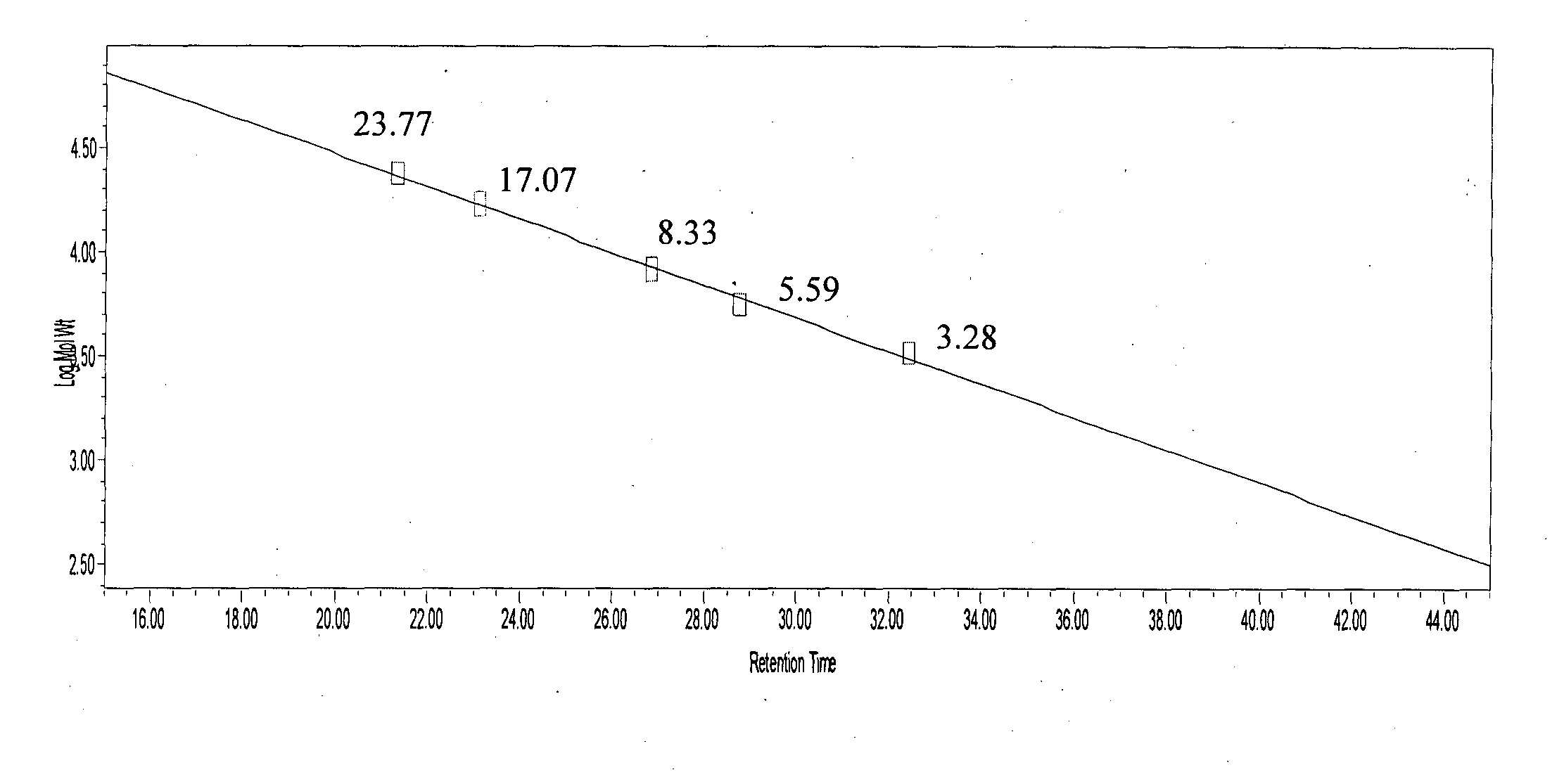
InactiveUS7074580B2Accurate and robust calibration setTherapeutic utilityPeptide/protein ingredientsDisease diagnosisRetention timeLinear relationship
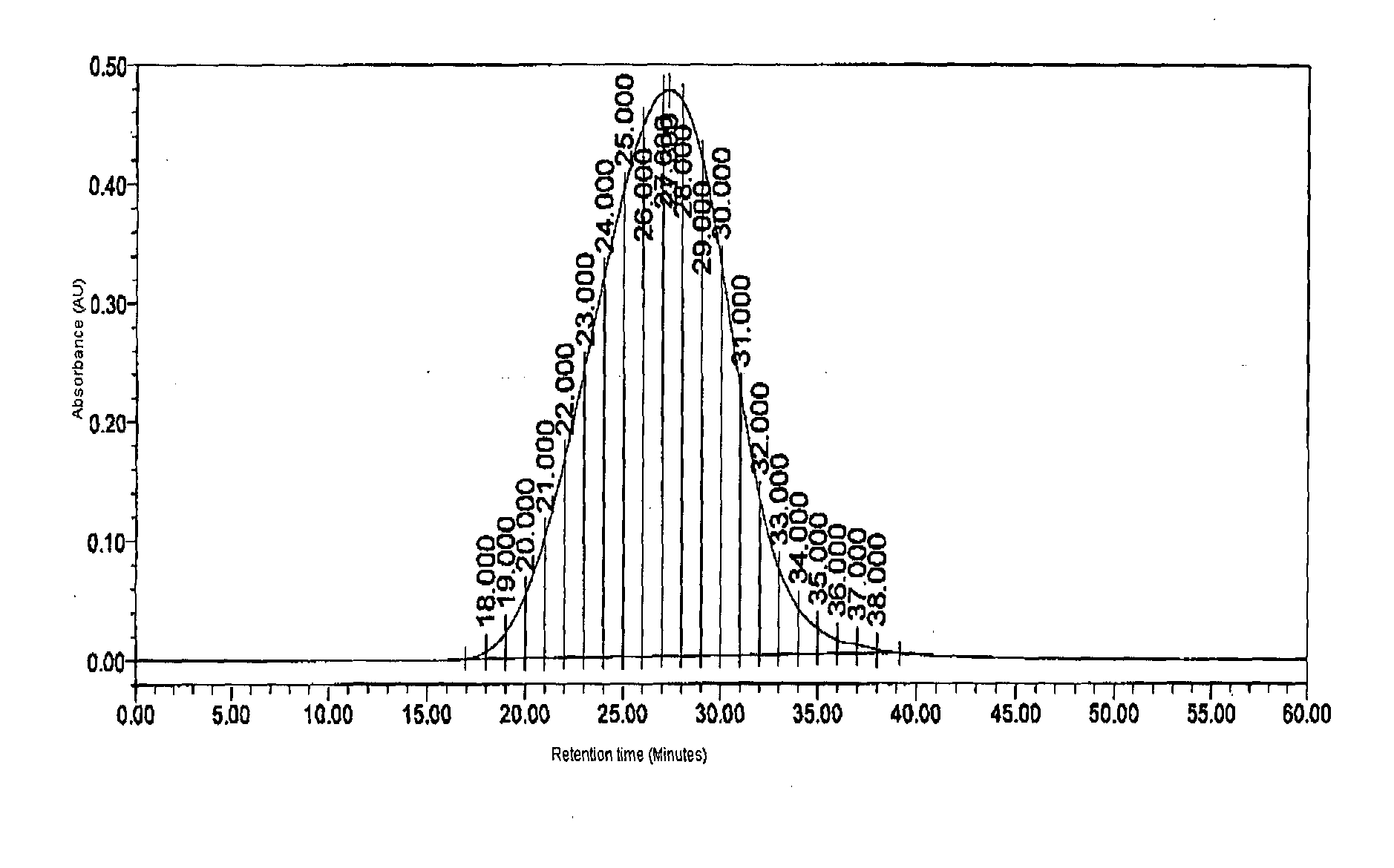
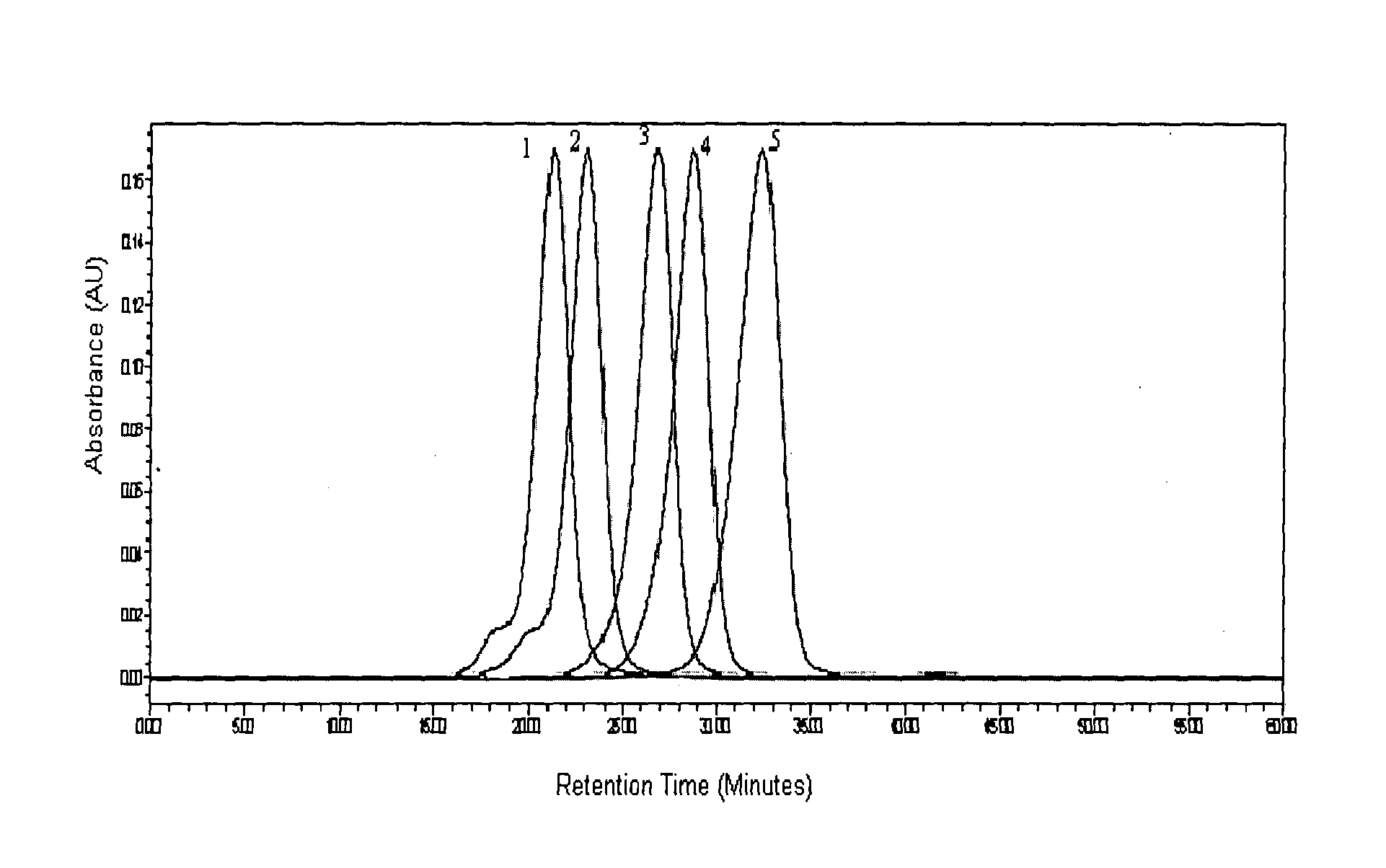
The present invention provides processes for determining the molecular weight of glatiramer acetate and other copolymers using molecular weight markers. The present invention further provides a plurality of molecular weight markers for determining the molecular weight of glatiramer acetate and other copolymers which display linear relationships between molar ellipticity and molecular weight, and between retention time and the log of the molecular weight. The molecular weight markers also optimally demonstrate biological activity similar to glatiramer acetate or corresponding copolymers and can be used for treating or preventing various immune diseases.
Owner:YEDA RES & DEV CO LTD
Copolymer 1 related polypeptides for use as molecular weight markers and for therapeutic use
InactiveUS7163802B2Accurate and robust calibration setTherapeutic utilityPeptide/protein ingredientsDisease diagnosisRetention timeLinear relationship
The present invention provides processes for determining the molecular weight of glatiramer acetate and other copolymers. The present invention further provides a plurality of molecular weight markers for determining the molecular weight of glatiramer acetate and other copolypmers which display linear relationships between molar ellipticity and molecular weight, and between retention time and the log of the molecular weight. The molecular weight markers also optimally demonstrate biological activity similar to glatiramer acetate or corresponding copolymers and can be used for treating or preventing various immune diseases. In addition, the subject invention provides pharmaceutical compositions for the treatment of immune diseases comprising a polypeptide having an identified molecular weight and an amino acid composition corresponding to glatiramer acetate or a terpolymer.
Owner:YEDA RES & DEV CO LTD
Markers associated with the therapeutic efficacy of glatiramer acetate
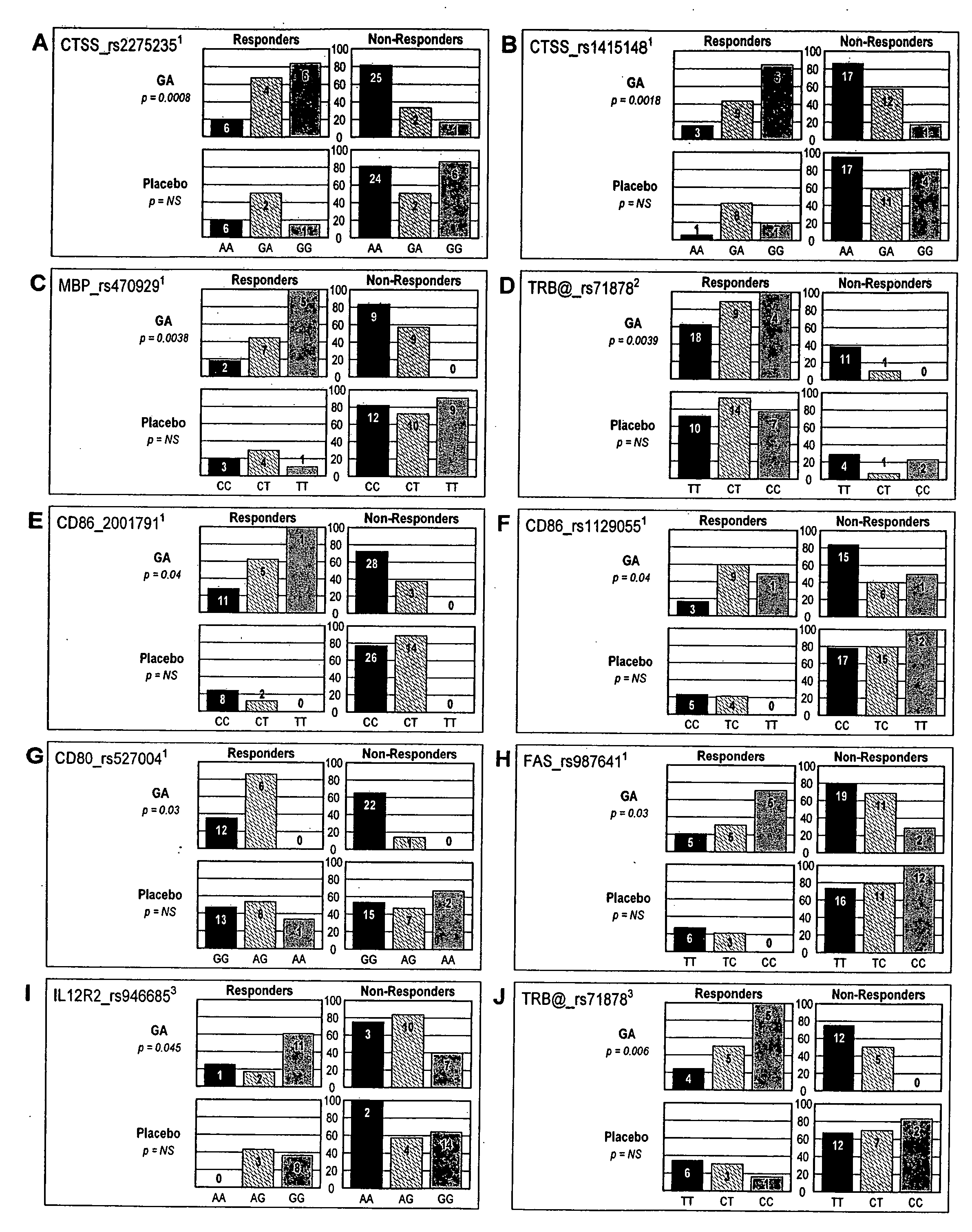
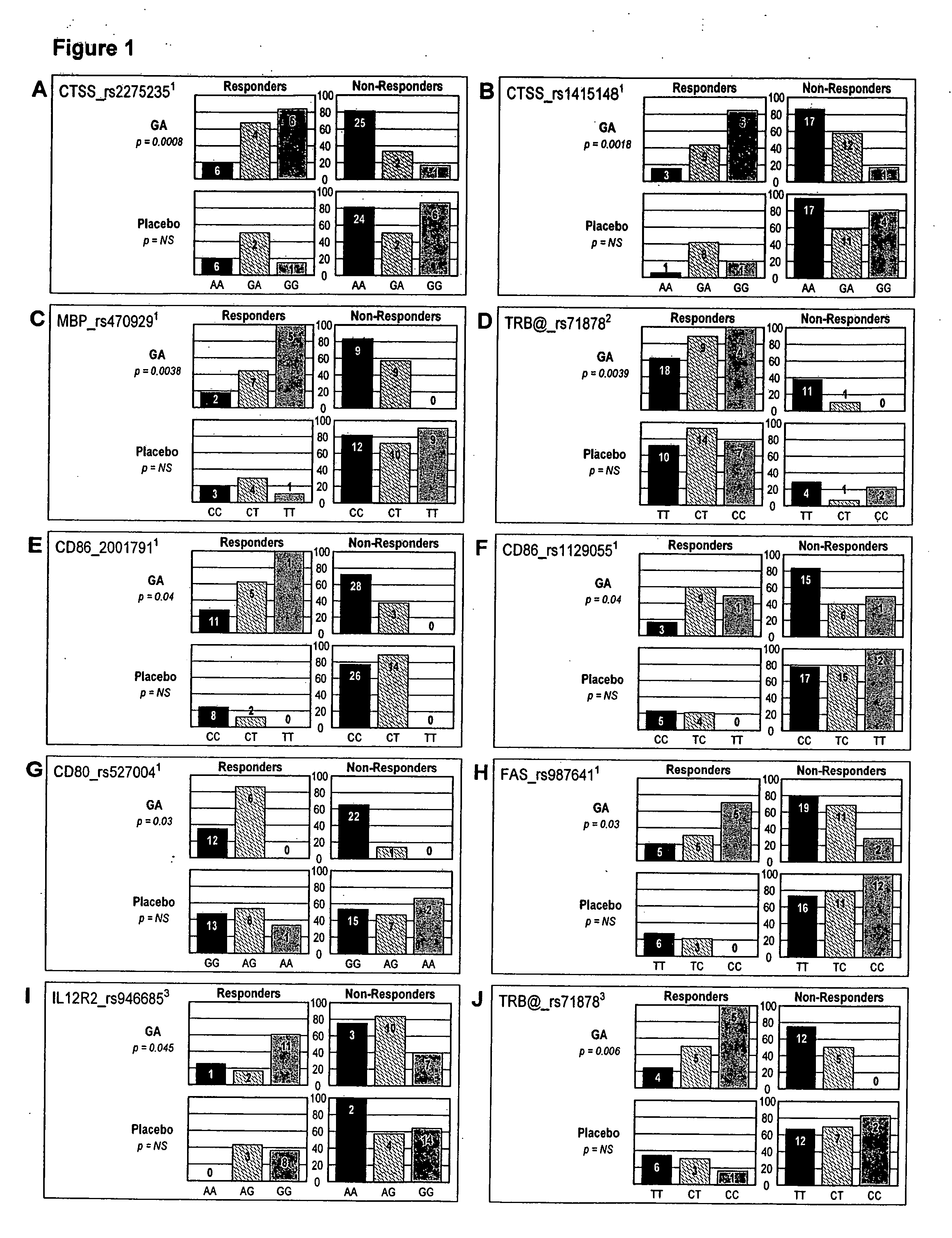
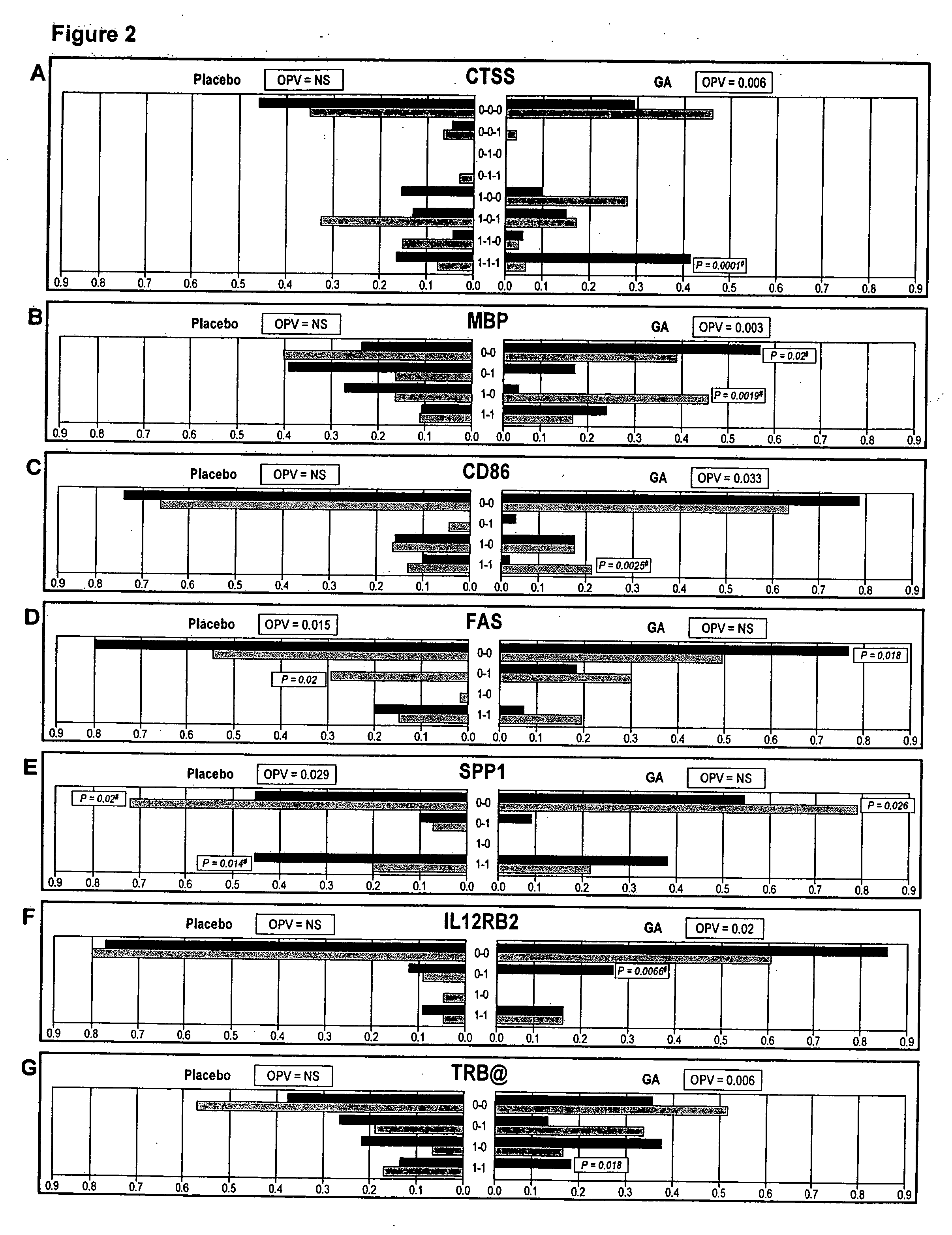
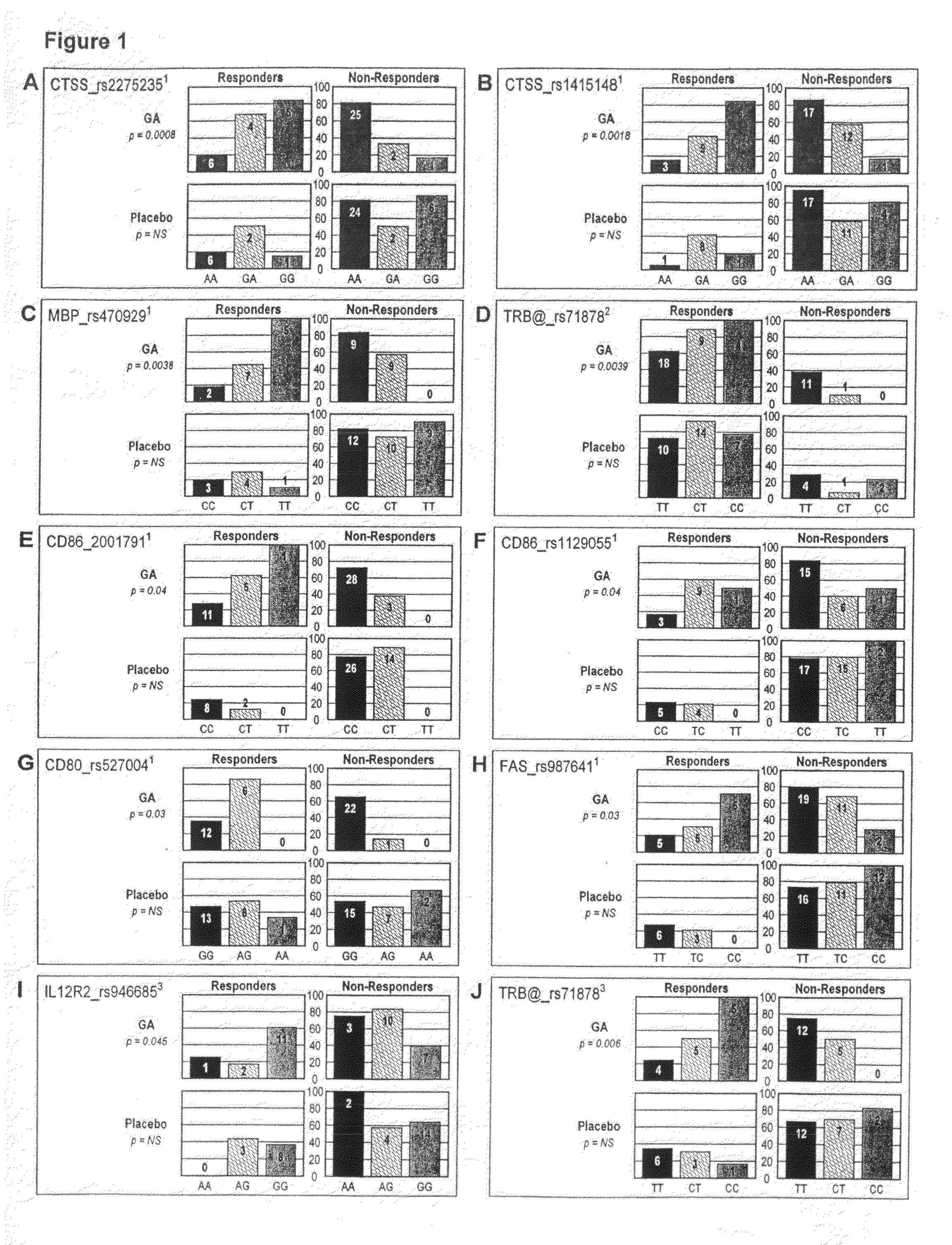
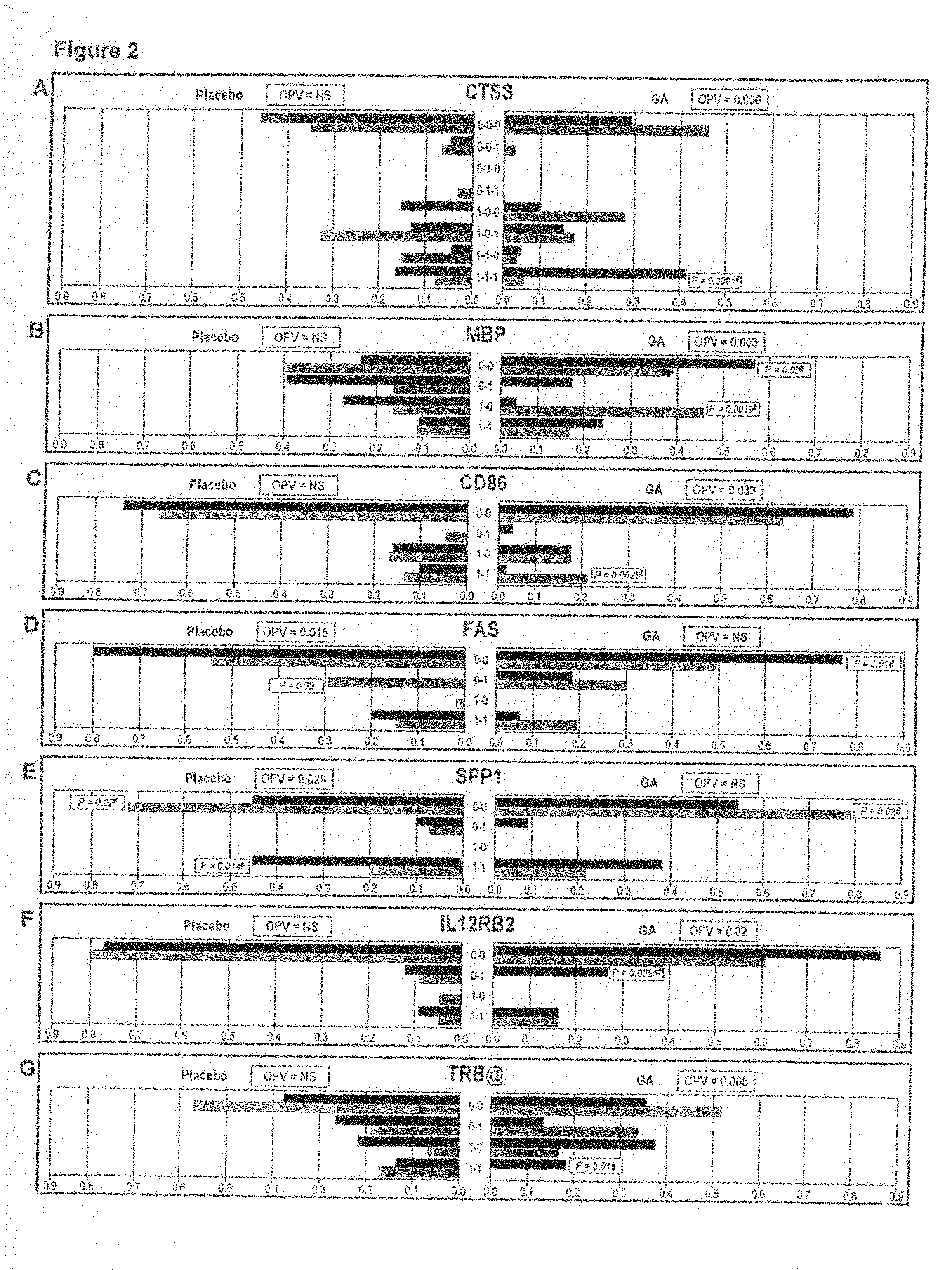
The present invention is directed to methods and kits based, at least in part, on the identification of allele-specific responsiveness or non-responsiveness to glatiramer acetate for the treatment of immune disorders, such as relapsing-remitting multiple sclerosis. The allele-specific responsiveness or non-responsiveness is based on polymorphisms in the following genes, CTSS, MBP, TCRB, CD95, CD86, IL-1R1, CD80, SCYA5, MMP9, MOG, SPP1 and IL-12RB2.
Owner:RAPPAPORT FAMILY INSTITUTE FOR RESEACH IN THE MEDICAL SCIENCES +2
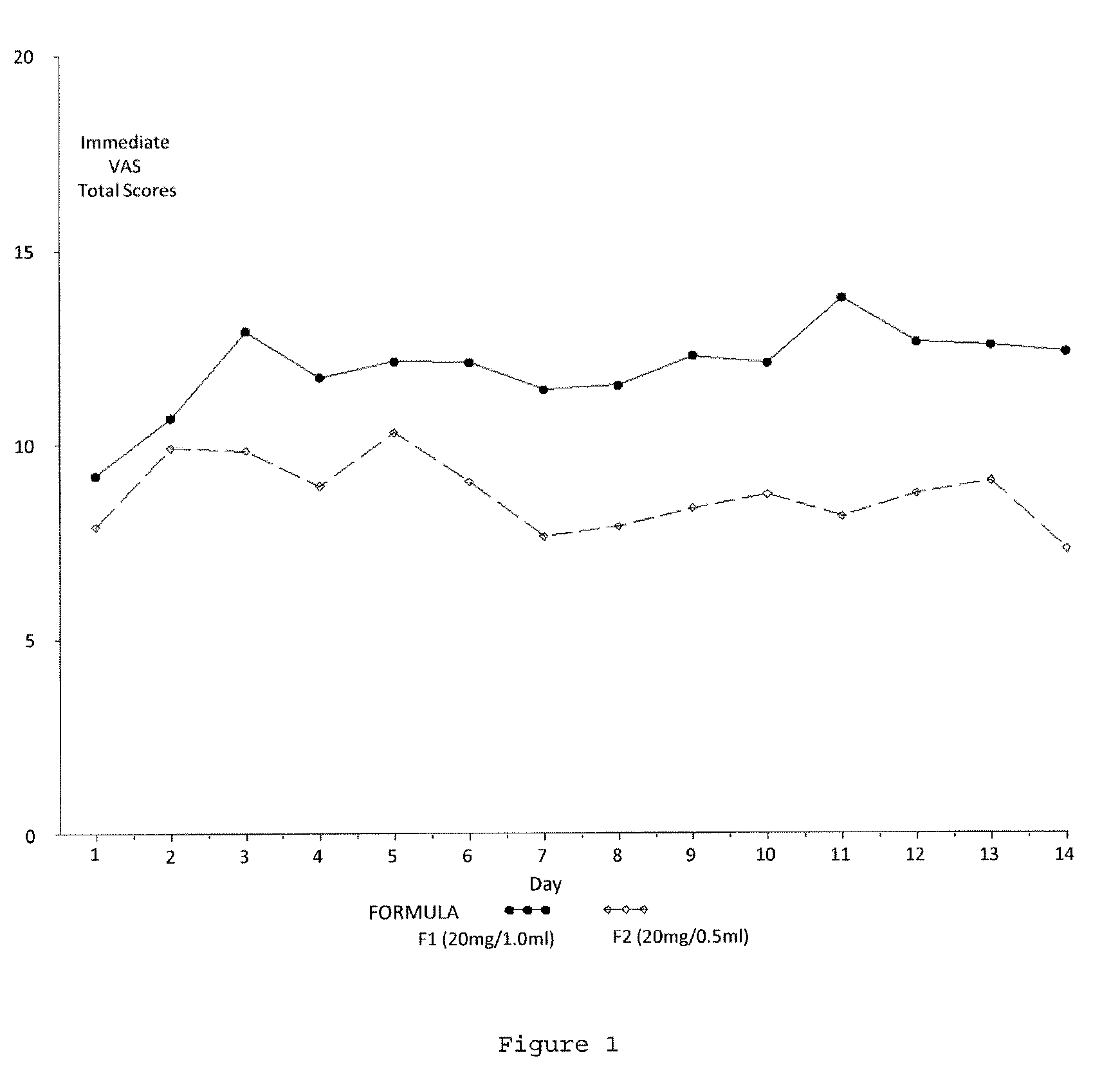
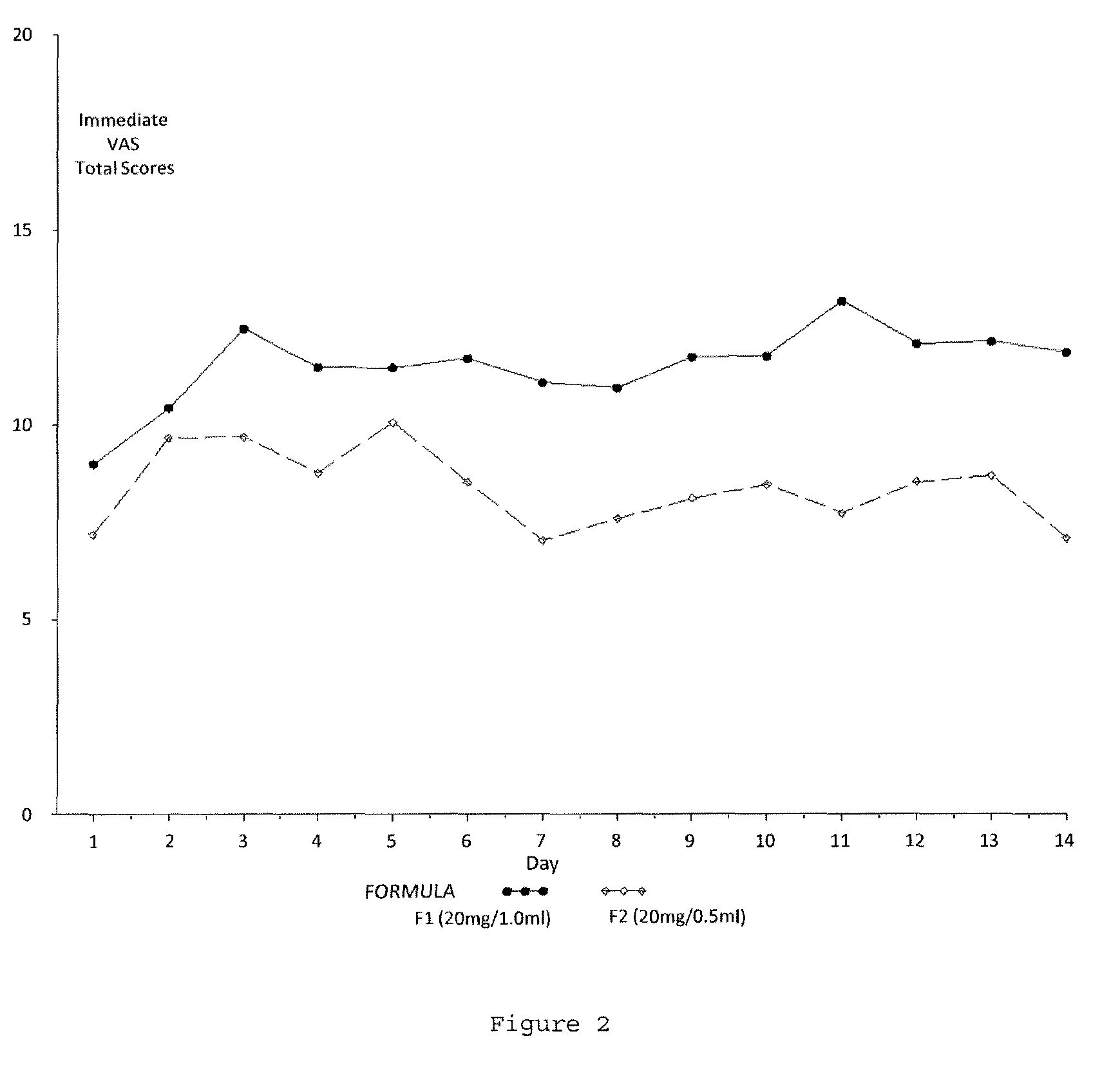
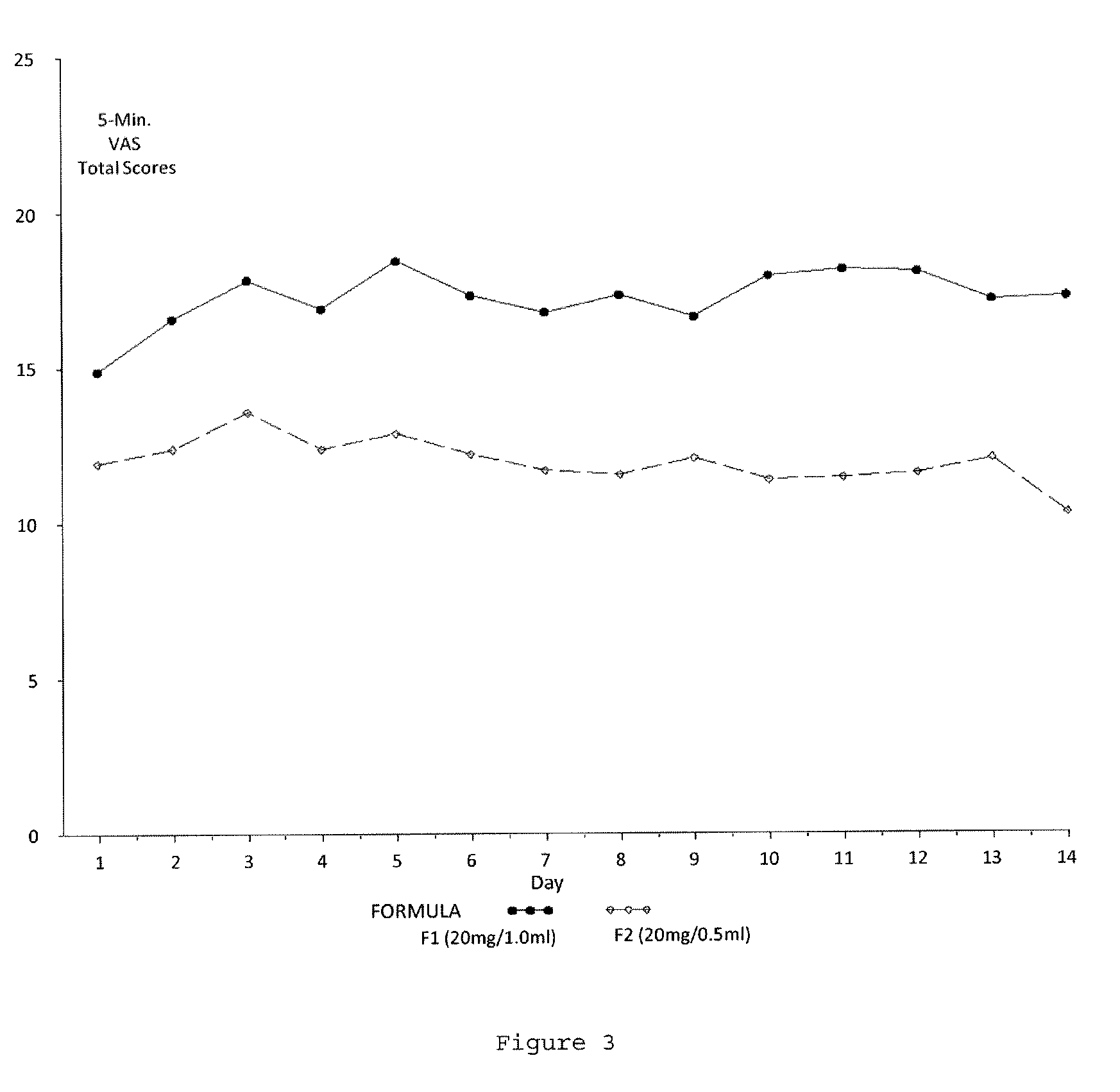
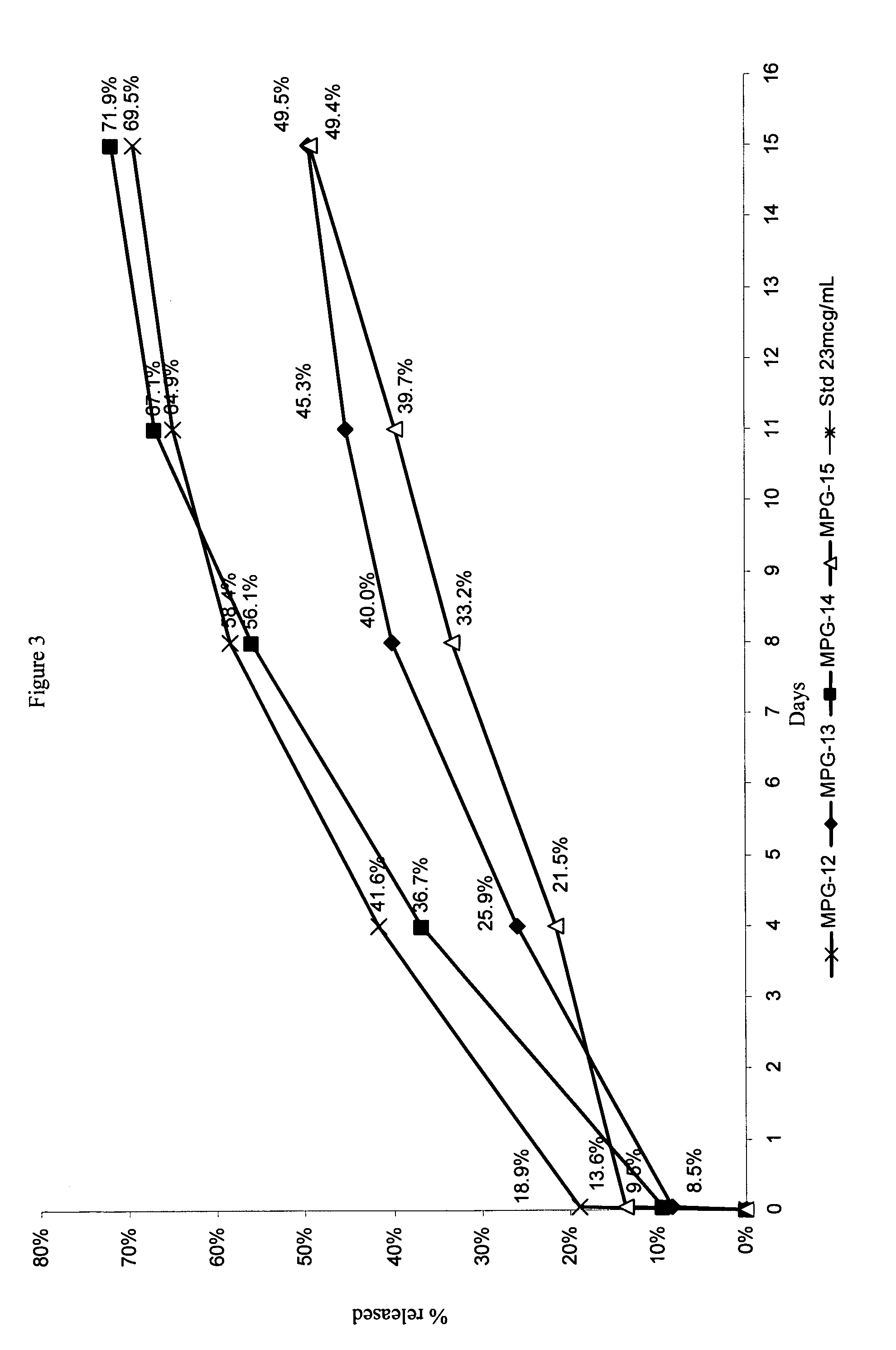
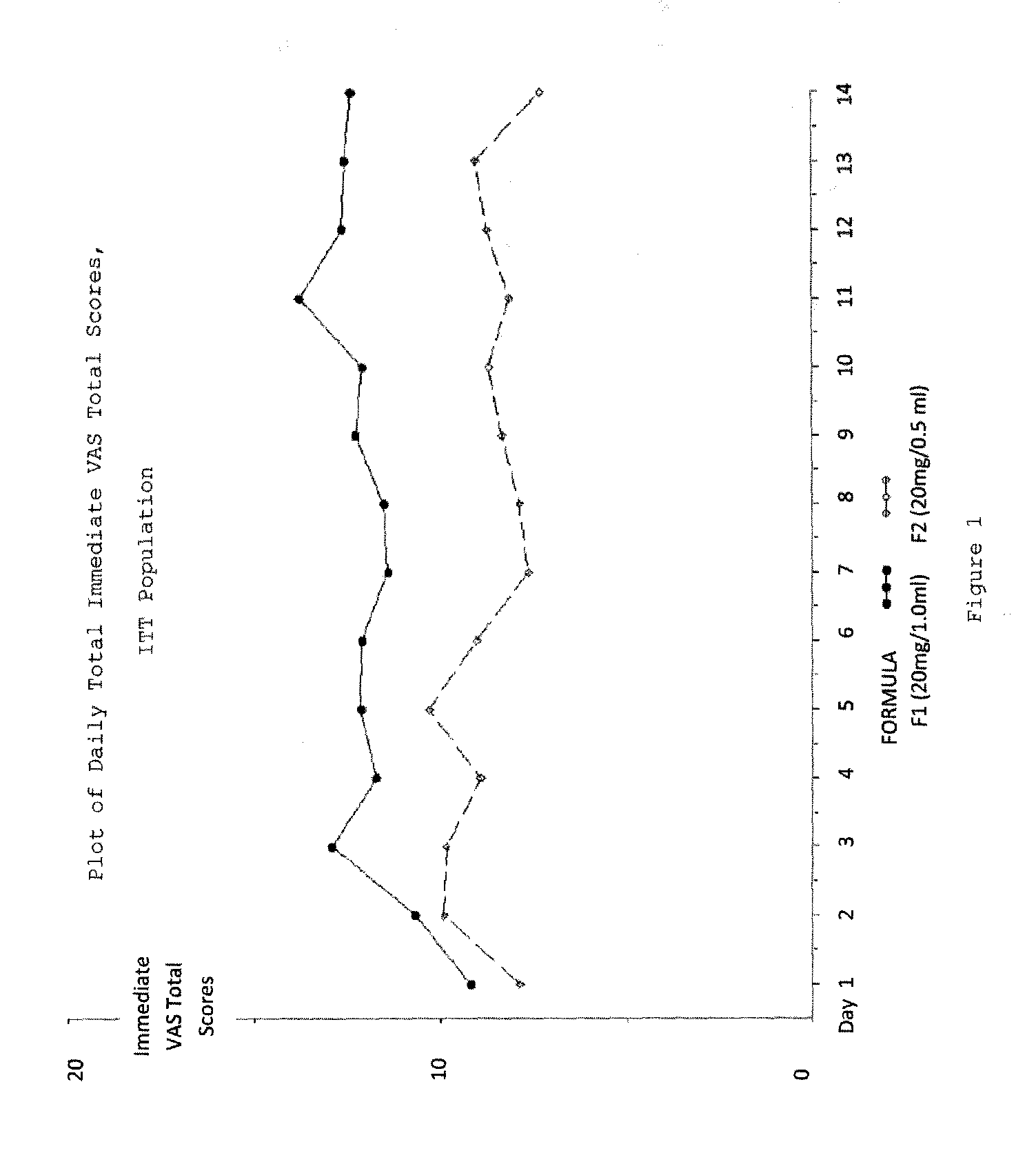
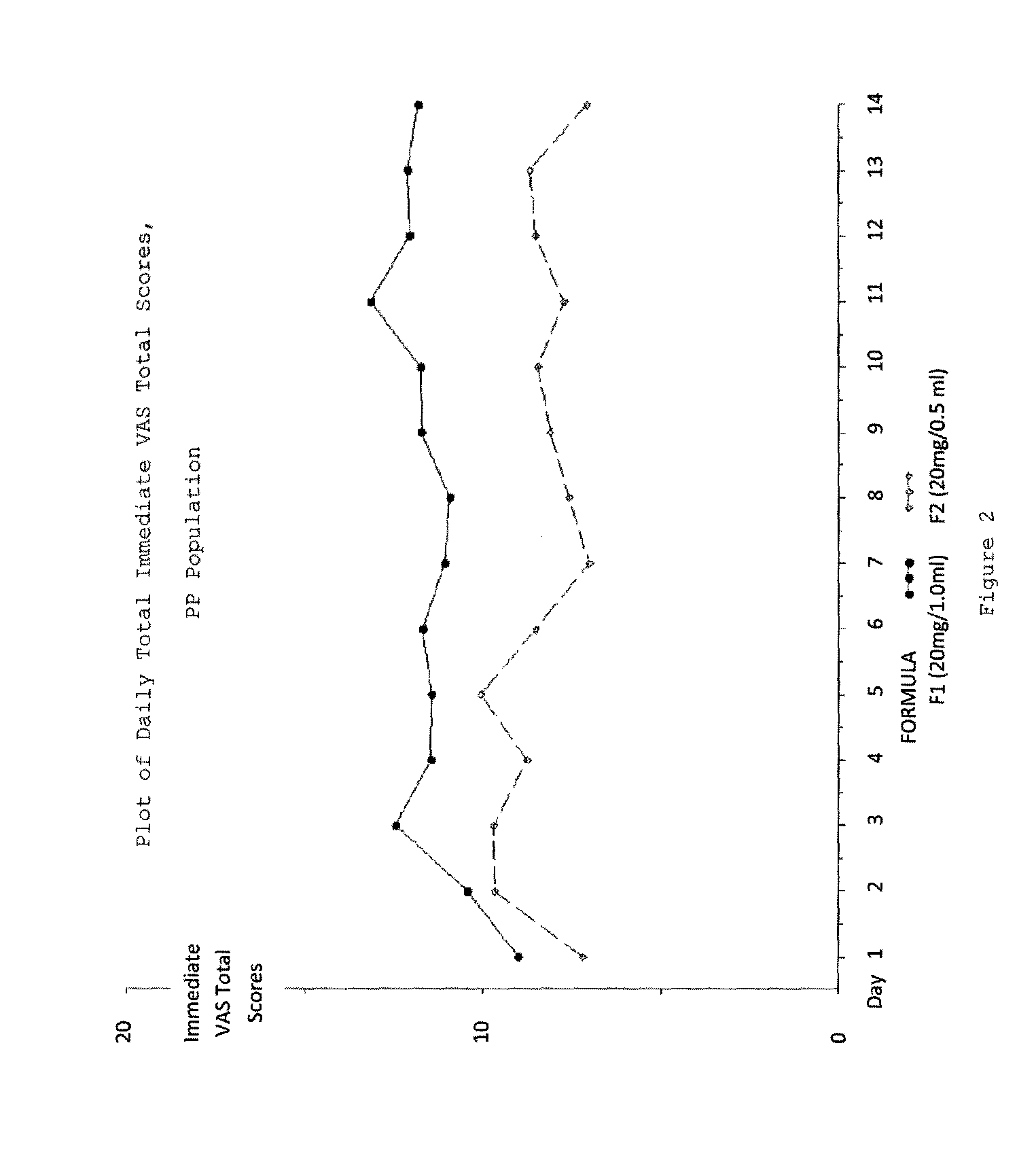
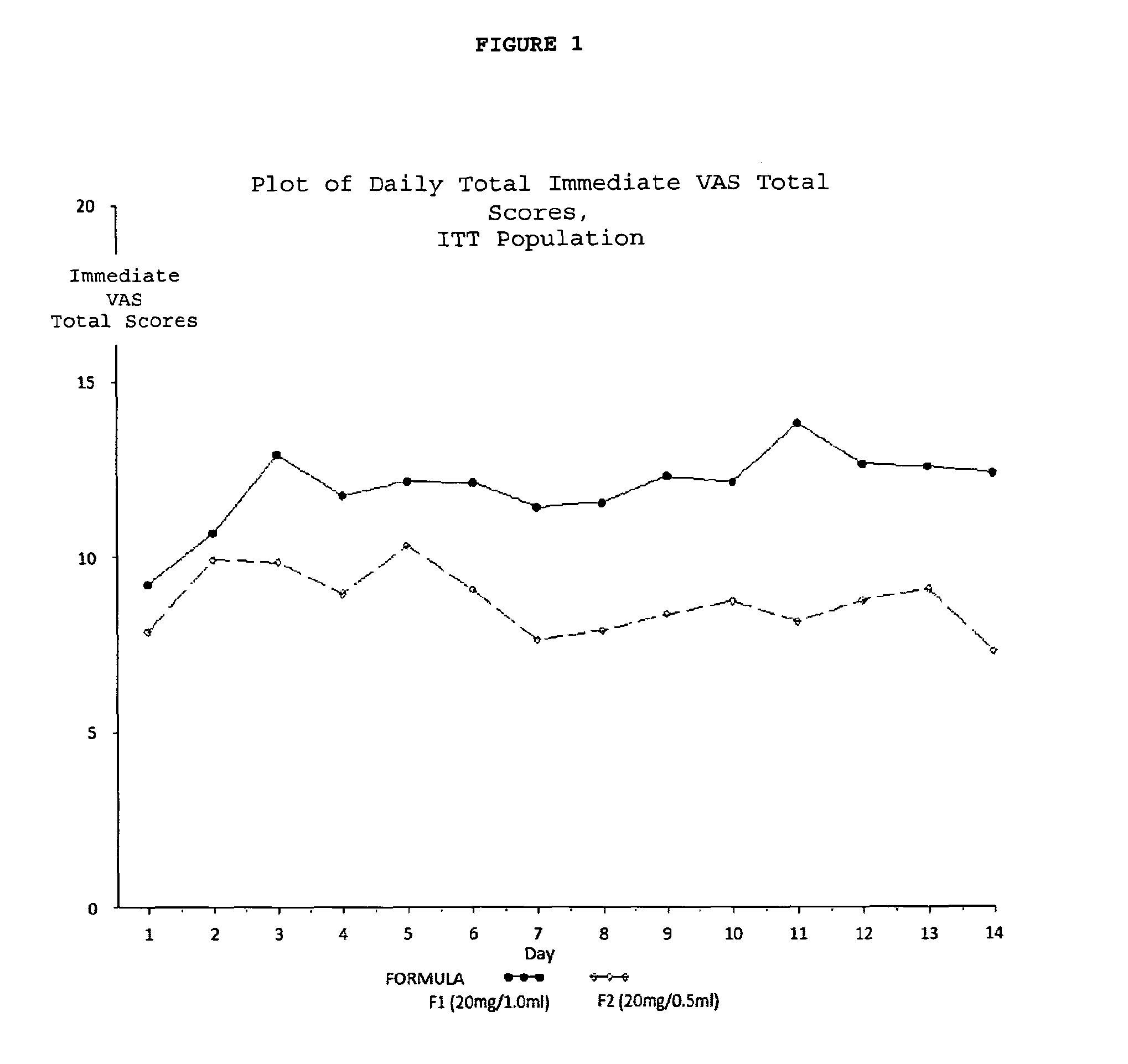
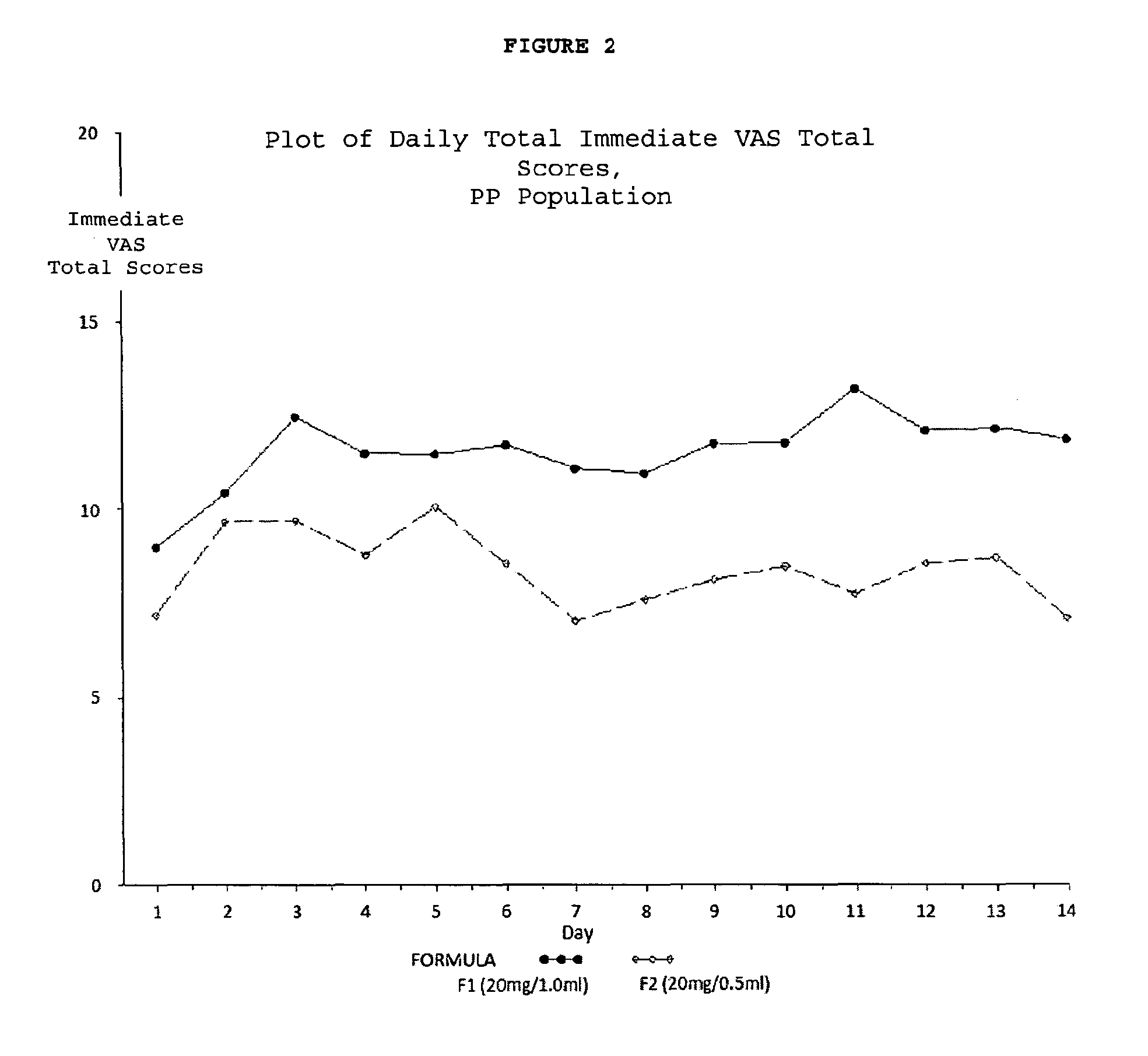
Reduced volume formulation of glatiramer acetate and methods of administration
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
Compositions and methods for treating neurological disorders
InactiveUS20060229233A1Preventing new amyloid plaqueMaintaining current amyloid plaque levelOrganic active ingredientsBiocideEmulsionCell Aggregations
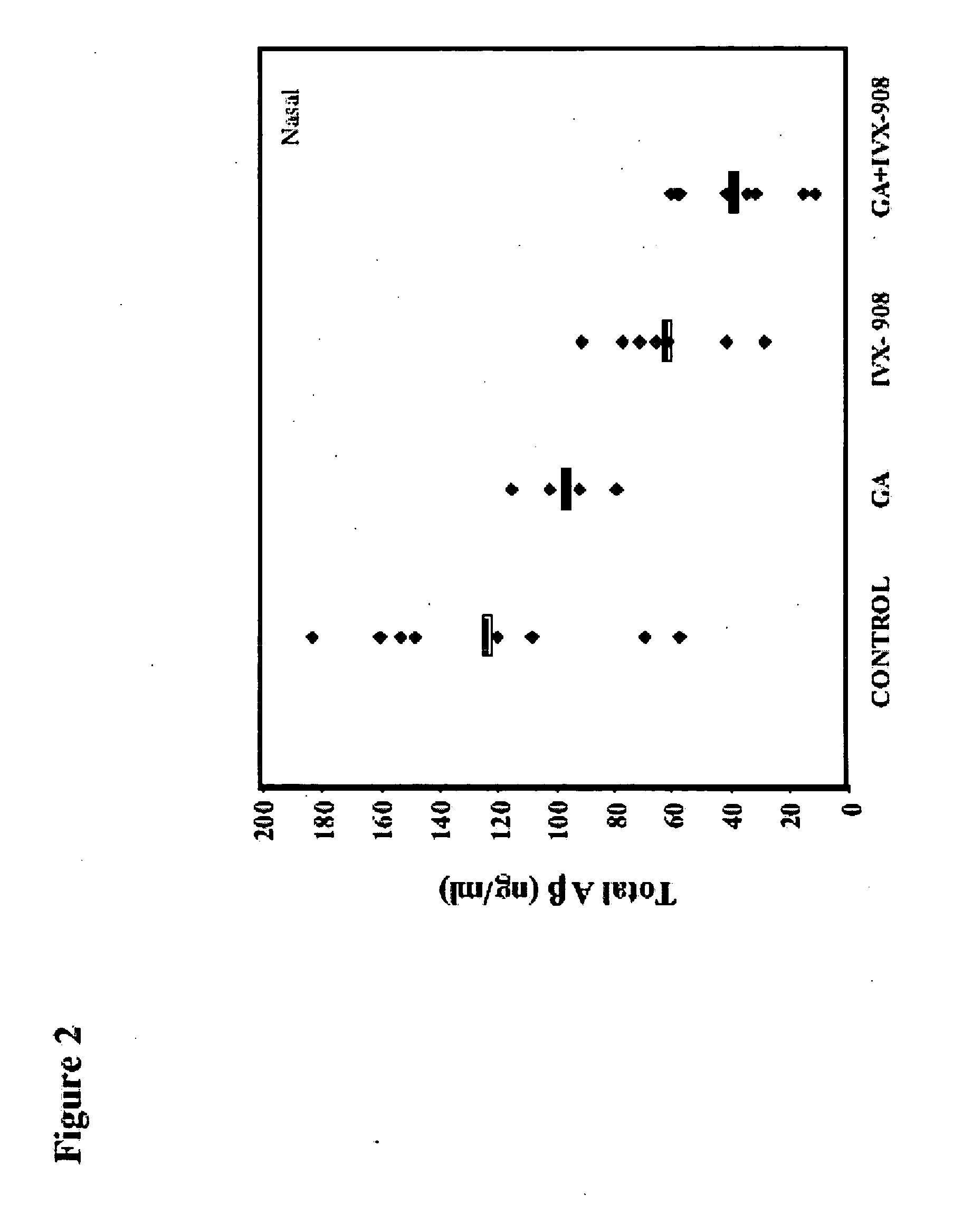
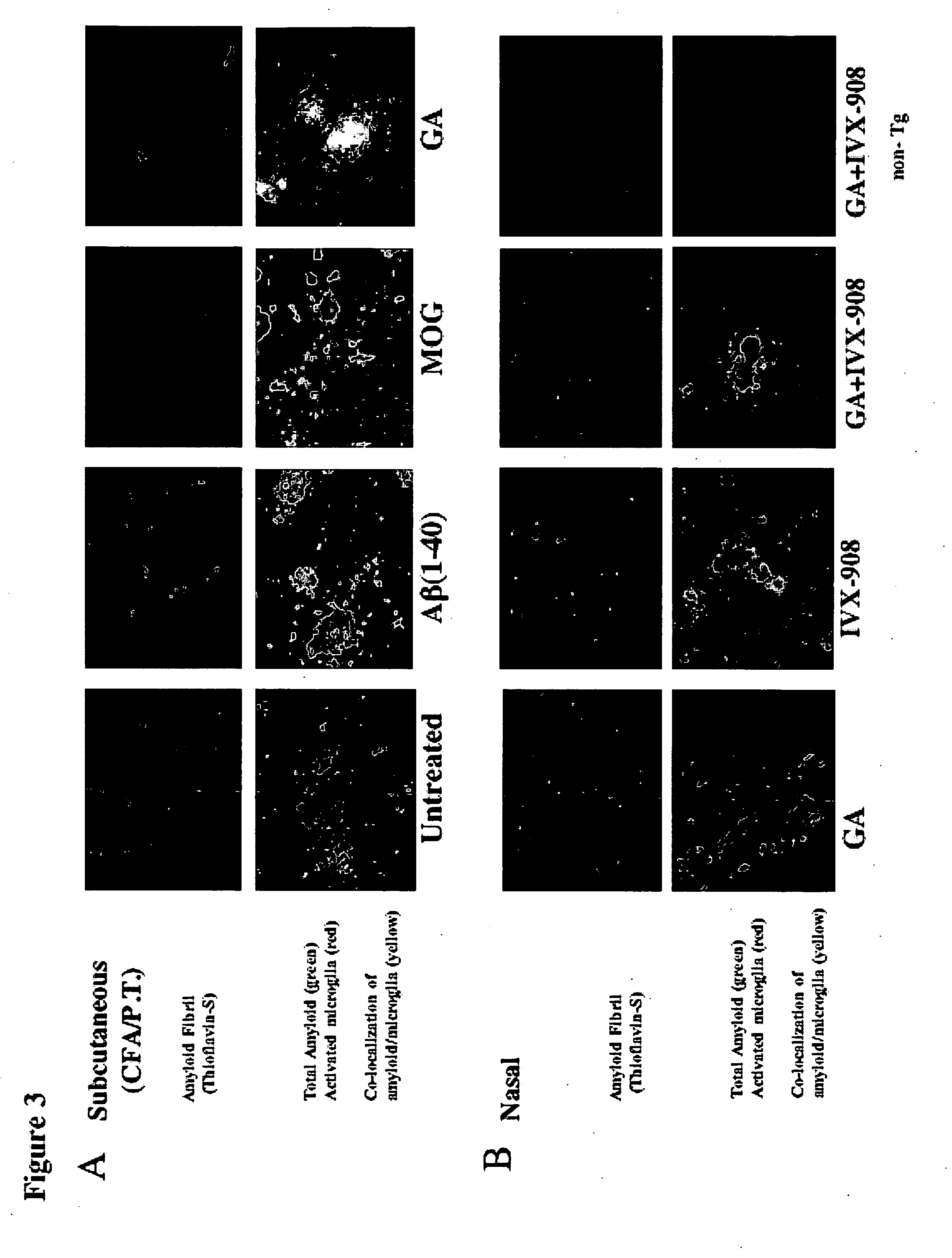
Compositions useful for treating neurological disorders including neurodegenerative disorders associated with deleterious protein aggregation, aberrant protein folding, such as brain amylogenic diseases, and / or neurodegenerative autoimmune disorders are described. Methods of using said compositions also are described. In particular, methods to treat a neurodegenerative disorder such as Alzheimer's disease and a neurodegenerative autoimmune disorder such as Multiple Sclerosis are contemplated utilizing proteosomes and / or Glatiramer Acetate, wherein the GA is in a submicron emulsion or a nanoemulsion.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
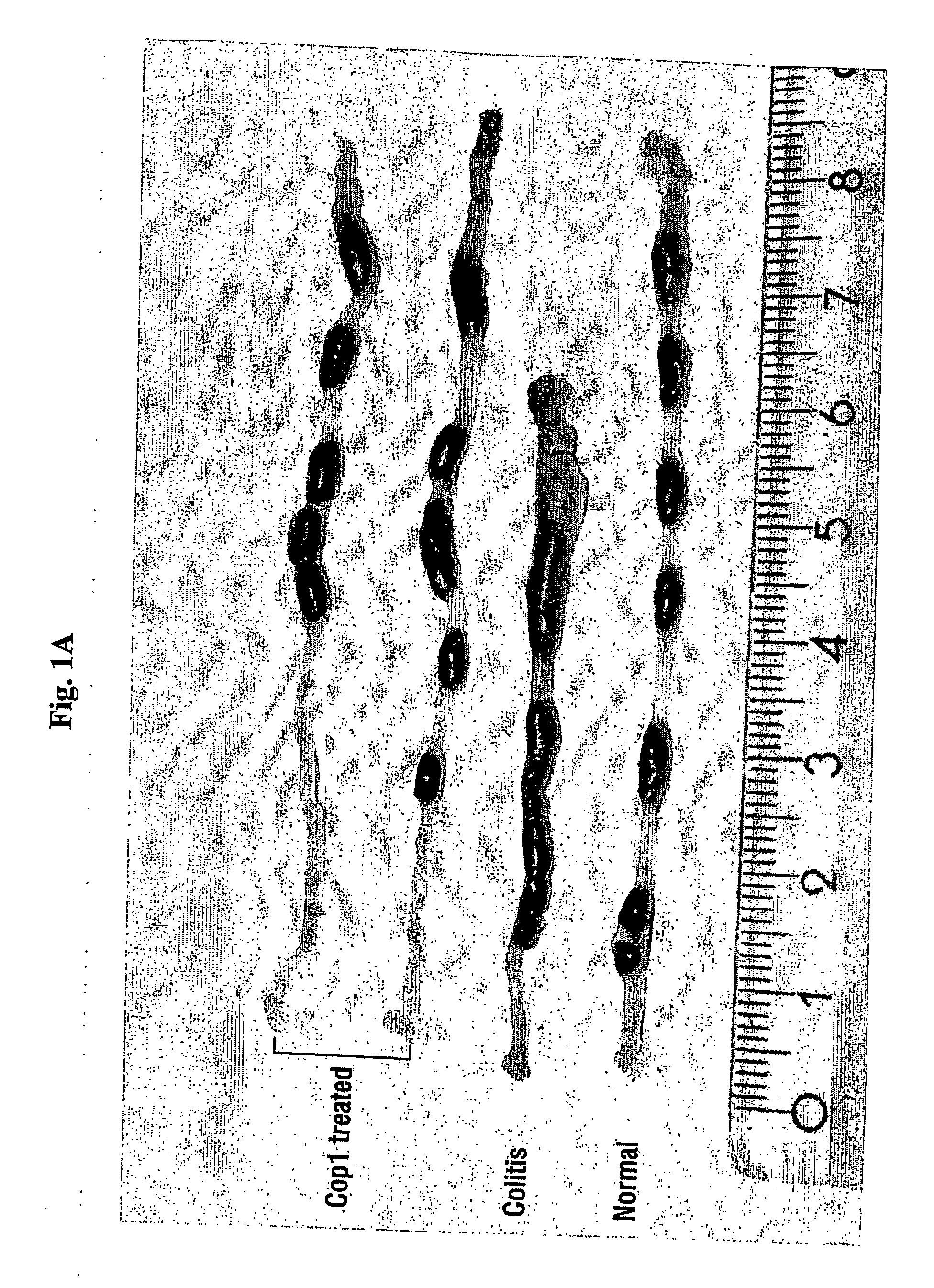
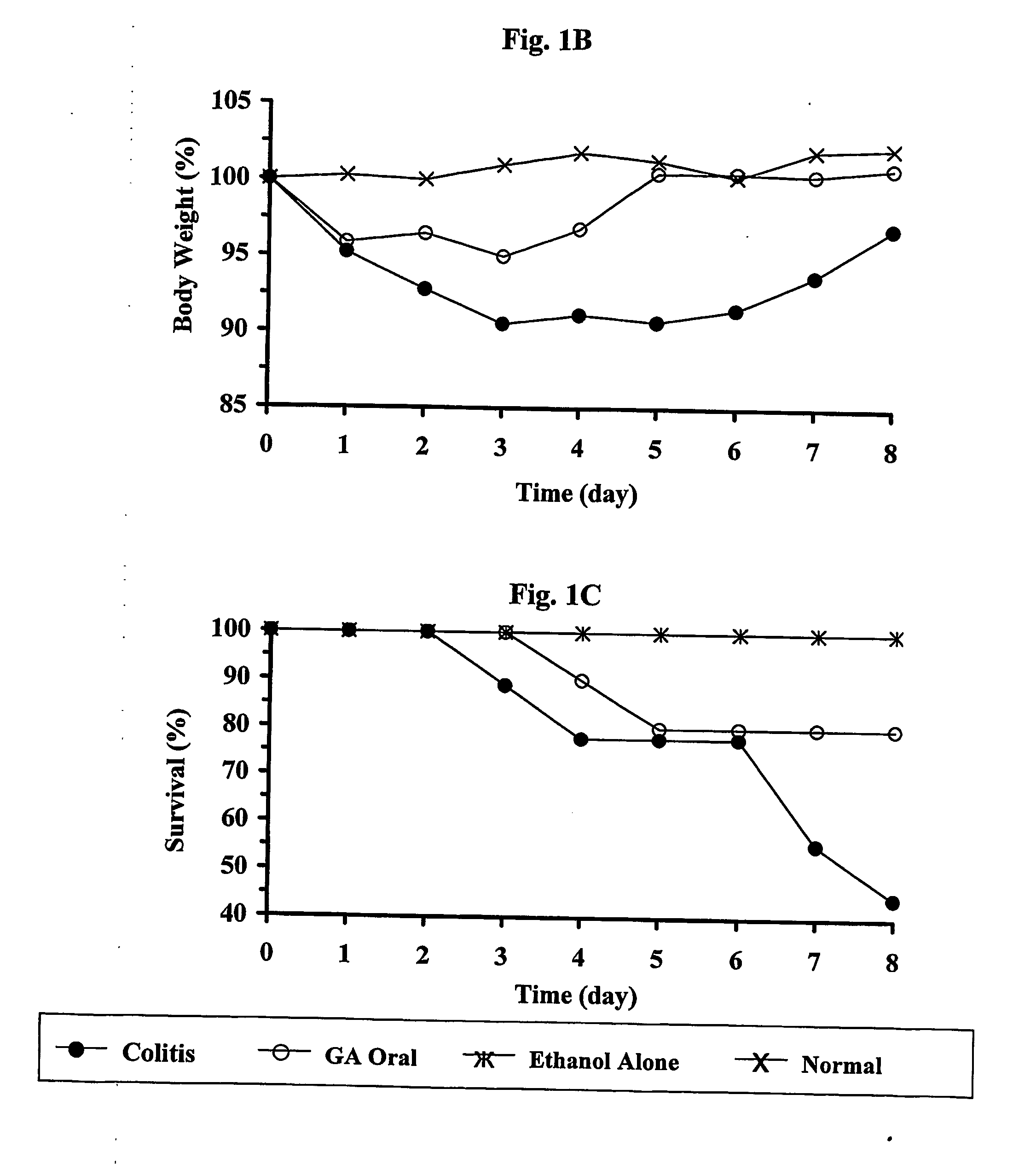
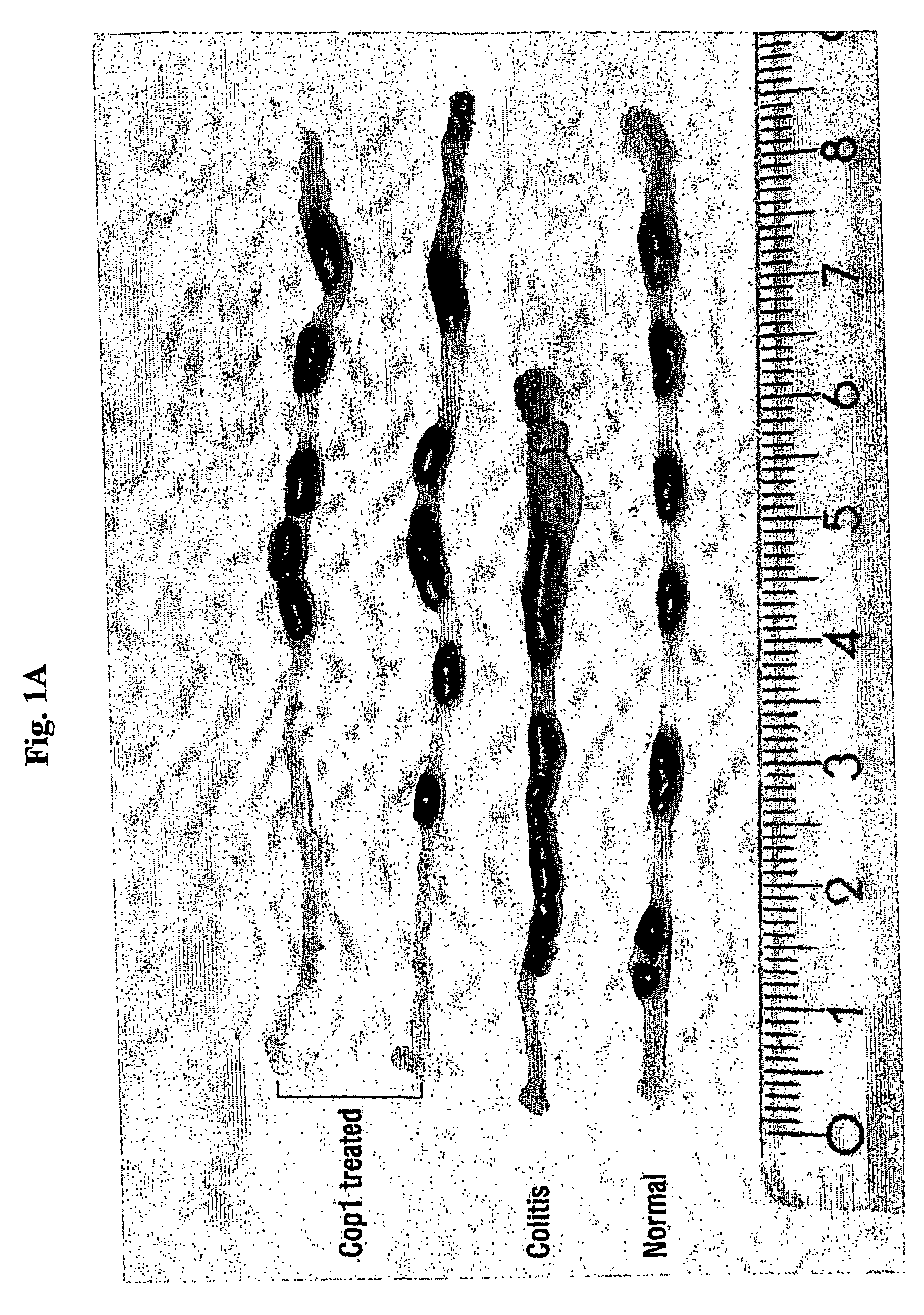
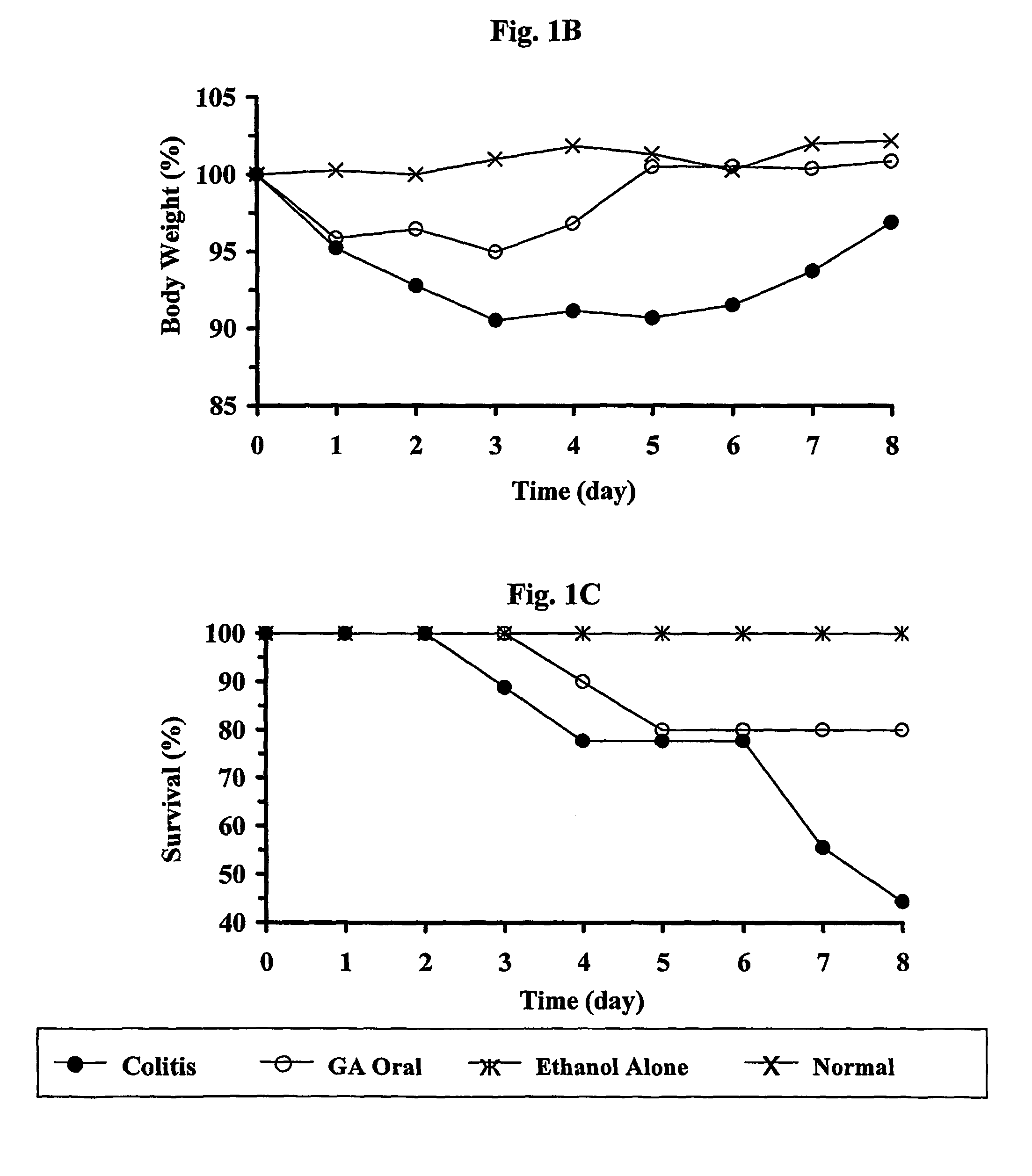
Cop 1 for treatment of inflammatory bowel diseases
The present invention relates to the use of Copolymer 1 (glatiramer acetate), a Copolymer 1-related polypeptide, or a Copolymer 1-related peptide, for the treatment of inflammatory bowel diseases such as Crohn's disease and ulcerative colitis.
Owner:YEDA RES & DEV CO LTD
Markers associated with the therapeutic efficacy of glatiramer acetate
InactiveUS20100285600A1Microbiological testing/measurementBiological testingImmunologic disordersCD86
The present invention is directed to methods and kits based, at least in part, on the identification of allele-specific responsiveness or non-responsiveness to glatiramer acetate for the treatment of autoimmune disorders, such as relapsing-remitting multiple sclerosis. The allele-specific responsiveness or non-responsiveness is based on polymorphisms in the following genes, CTSS, MBP, TCRB, CD95, CD86, IL-1R1, CD80, SCYA5, MMP9, MOG, SPP1 and IL-12RB2.
Owner:TEVA PHARMA IND LTD
Copolymer-1, process for preparation and analytical methods thereof
InactiveUS20130323771A1Simple and cost-effectiveConsiderable timePeptide-nucleic acidsNervous disorderAnalysis methodCopolymer
The present invention relates to analytical methods such as molecular weight determination of polypeptide, in particular Glatiramer acetate. The present invention further relates to an improved process for preparation of polypeptides or pharmaceutically acceptable salts thereof, particularly Glatiramer acetate also known as Copolymer-1. The present invention further relates to characterization of Glatiramer acetate by peptide mapping.
Owner:USV LTD
Combination therapy with glatiramer acetate and rasagiline for the treatment of multiple sclerosis
InactiveUS20100167983A1Relieve symptomsNervous disorderPeptide/protein ingredientsMedicineCombination therapy
The subject invention provides a method of treating a subject afflicted with a form of multiple sclerosis comprising periodically administering to the subject an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, wherein the amounts when taken together are effective to alleviate a symptom of the form of multiple sclerosis in the subject so as to thereby treat the subject. The subject invention also provides a package comprising glatiramer acetate, rasagiline or the pharmaceutically acceptable salt thereof and instructions for use of the together to alleviate a symptom of a form of multiple sclerosis in a subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, which combination is useful to alleviate a symptom of a form of multiple sclerosis in a subject.
Owner:TEVA PHARMA IND LTD
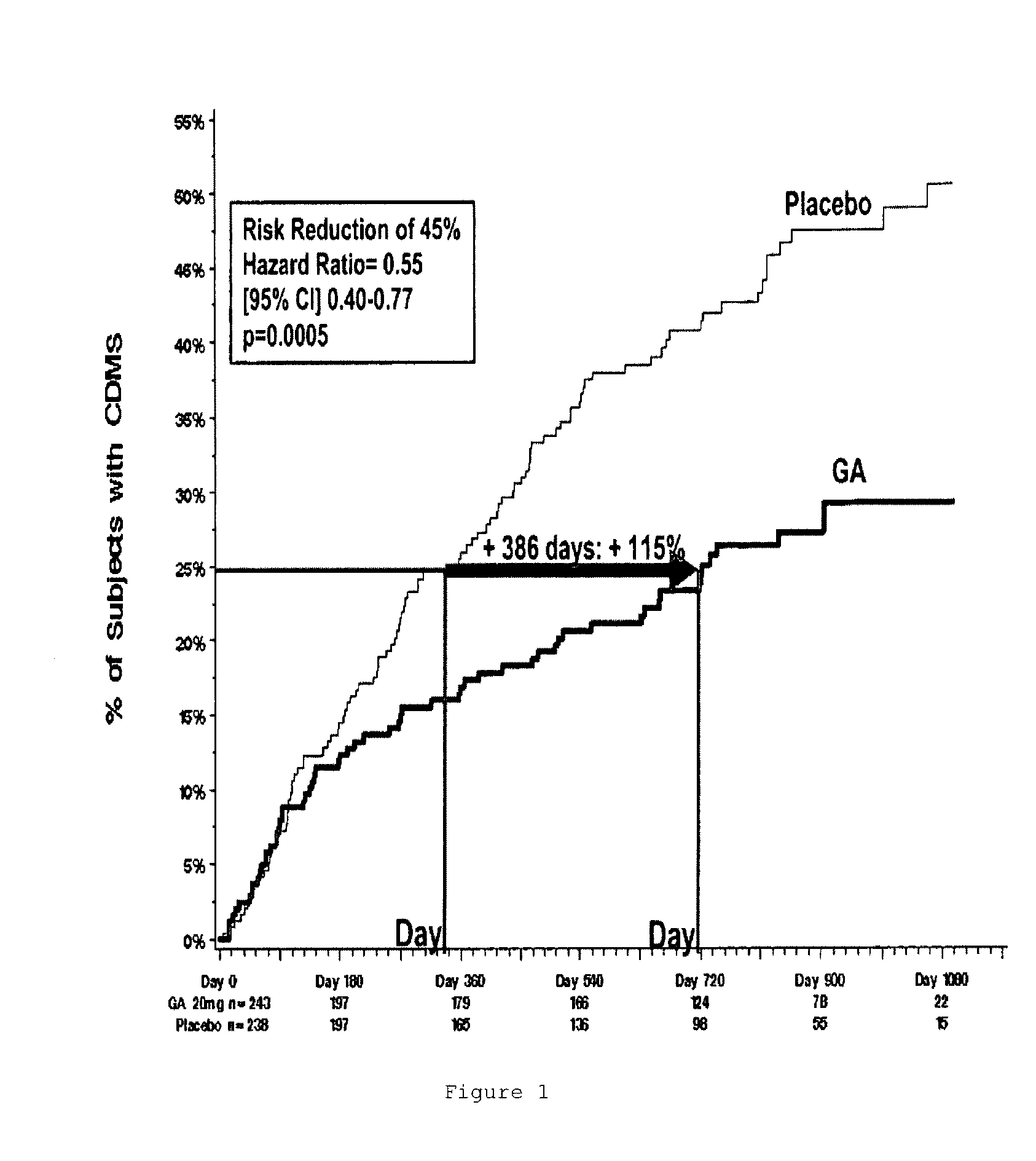
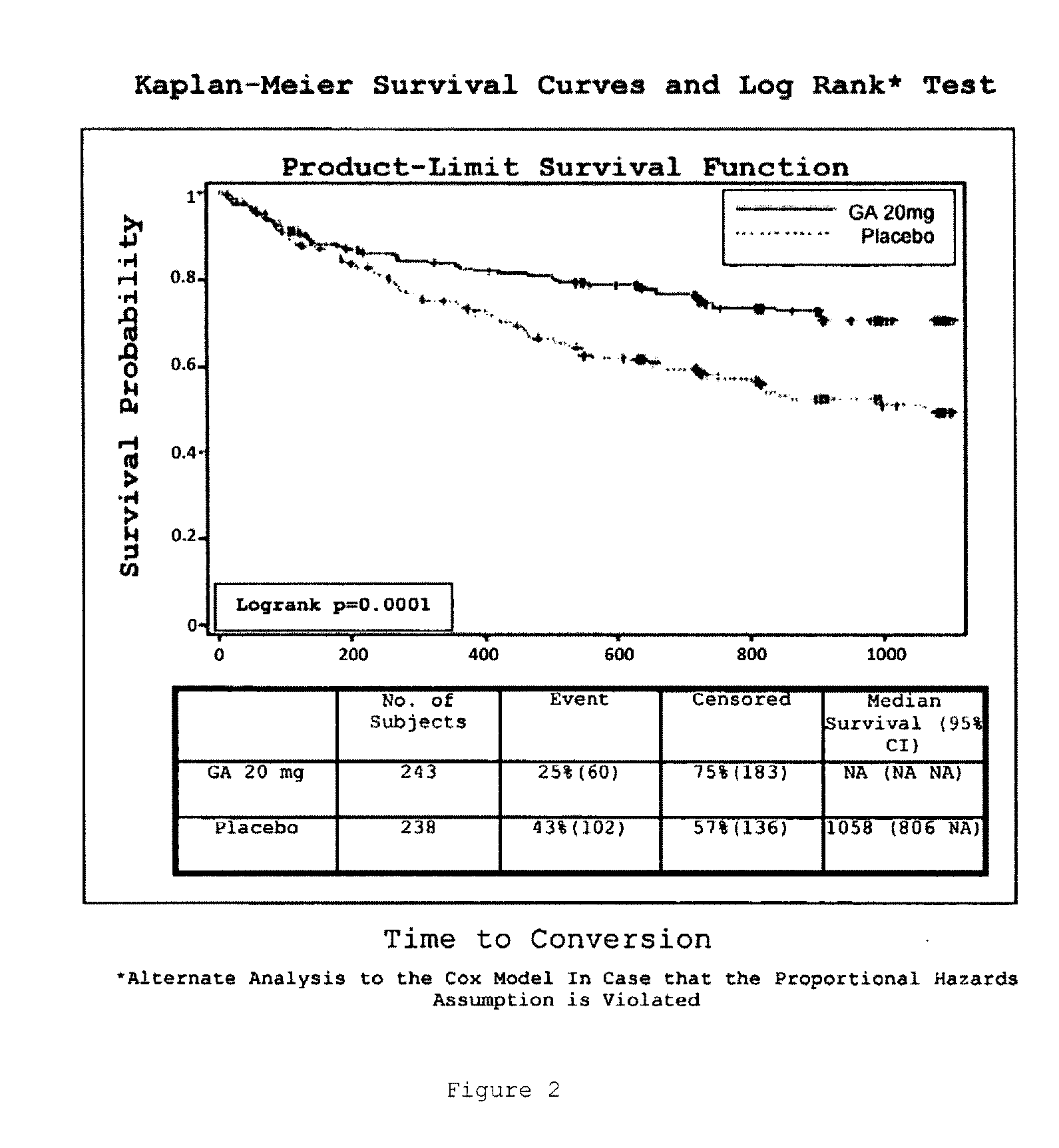
Method of Delaying The Onset of Clinically Definite Multiple Sclerosis
InactiveUS20100305023A1Prolonged recurrence timeReduce frequencyOrganic active ingredientsNervous disorderGlatiramer acetatePathology
A method for delaying the onset of clinically definite multiple sclerosis in a patient at risk of developing clinically definite multiple sclerosis and retard long-term progression of multiple sclerosis and its symptoms, the method comprising periodically administering a pharmaceutical composition comprising a therapeutically effective amount of glatiramer acetate to the patient, thereby delaying onset of clinically definite multiple sclerosis in the patient and retarding long-term progression of multiple sclerosis and its symptoms.
Owner:YEDA RES & DEV CO LTD
Cop 1 for treatment of inflammatory bowel diseases
The present invention relates to the use of Copolymer 1 (glatiramer acetate), a Copolymer 1-related polypeptide, or a Copolymer 1-related peptide, for the treatment of inflammatory bowel diseases such as Crohn's disease and ulcerative colitis.
Owner:YEDA RES & DEV CO LTD
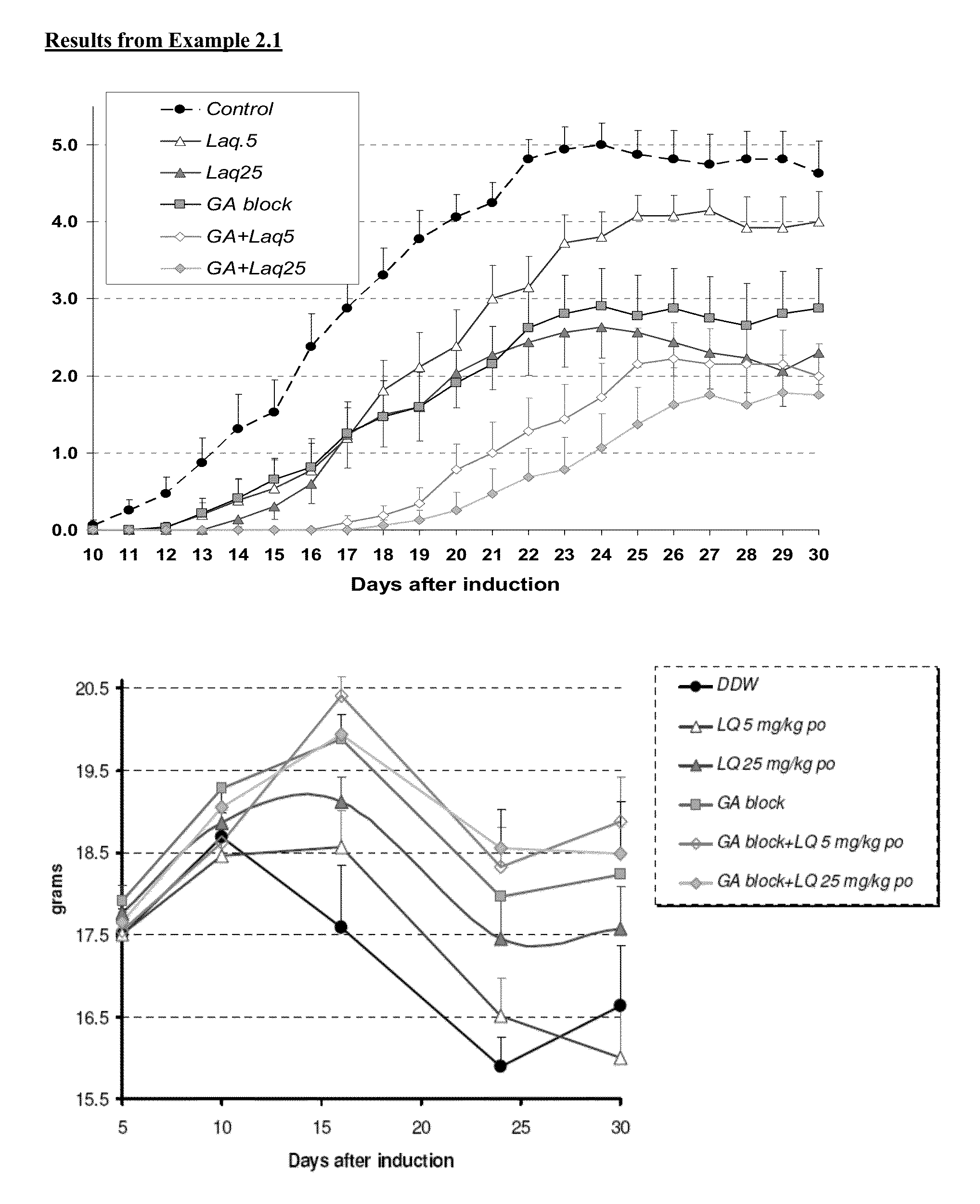
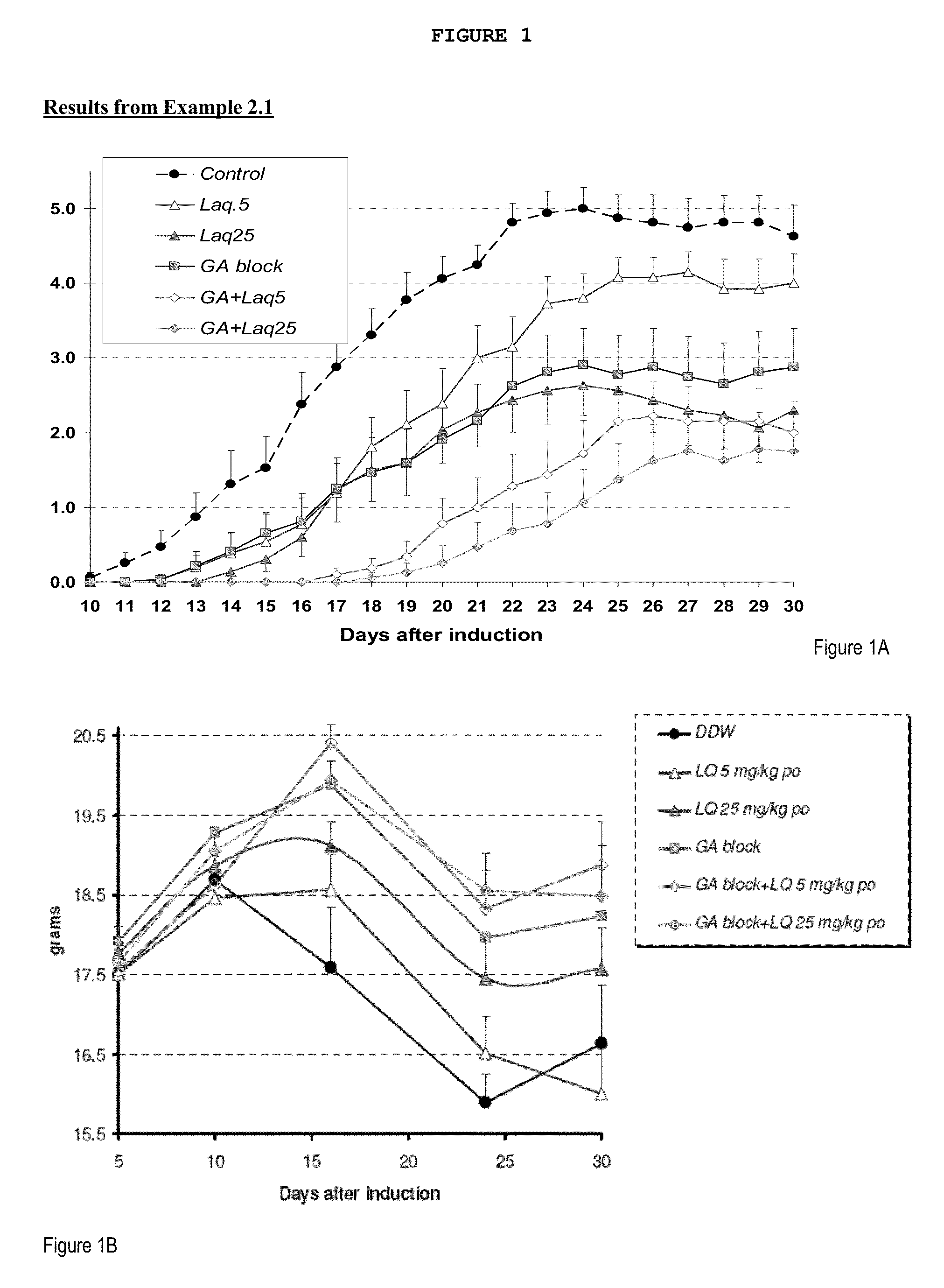
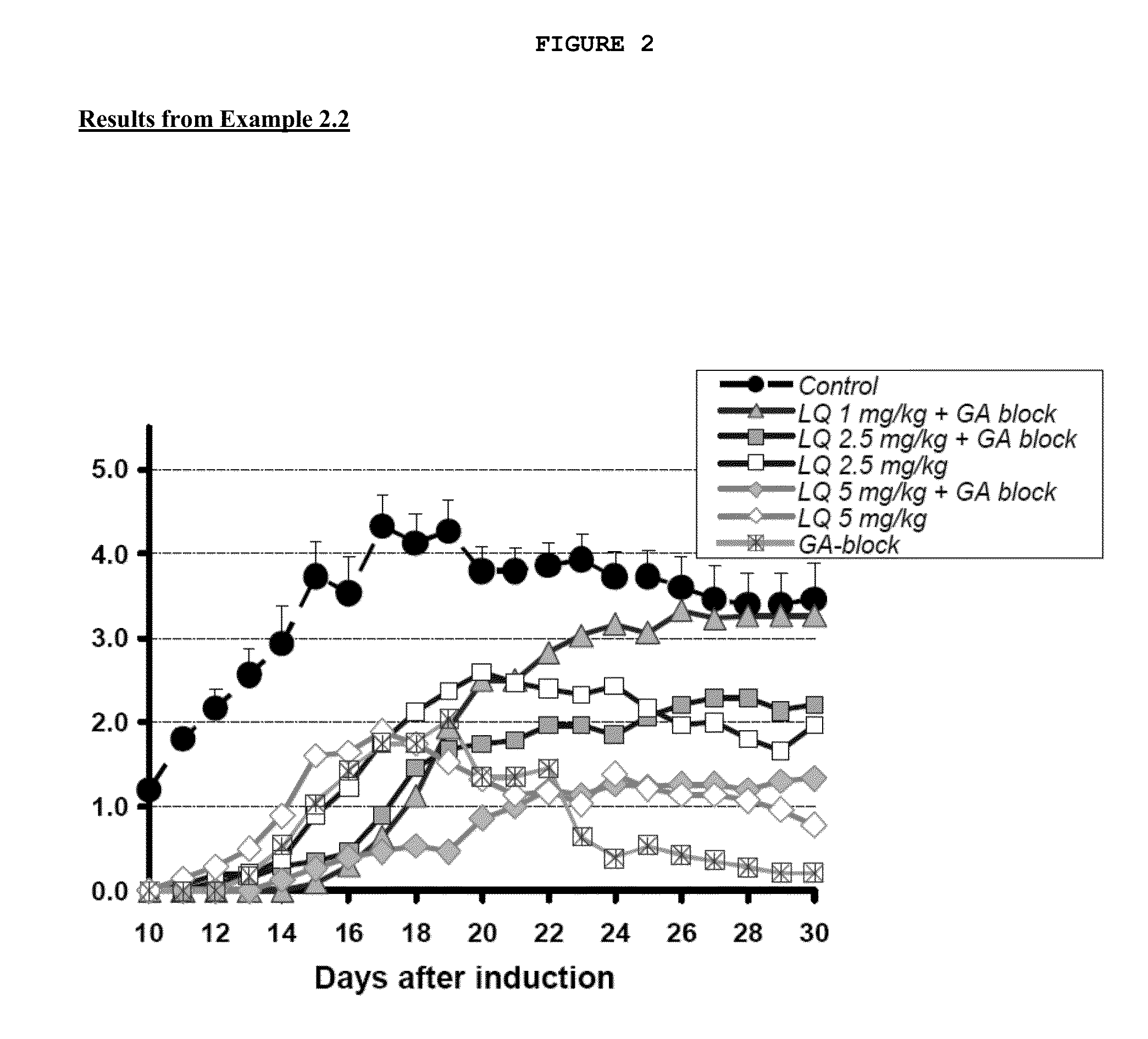
Treatment of multiple sclerosis with combination of laquinimod and glatiramer acetate
InactiveUS20130029916A1Effective treatmentPowder deliverySenses disorderLaquinimodGlatiramer acetate
This invention provides a method of treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome comprising administering to the patient laquinimod as an add-on therapy to or in combination with glatiramer acetate. This invention also provides a package and a pharmaceutical composition comprising laquinimod and glatiramer acetate for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention also provides laquinimod for use as an add-on therapy or in combination with glatiramer acetate in treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome. This invention further provides use of laquinimod and glatiramer acetate in the preparation of a combination for treating a patient afflicted with multiple sclerosis or presenting a clinically isolated syndrome.
Owner:TEVA PHARMA IND LTD
Depot systems comprising glatiramer or a pharmacologically acceptable salt thereof
ActiveUS20120015891A1Equal and superior therapeutic efficacyReduce incidencePeptide-nucleic acidsNervous disorderMedicineGlatiramer acetate
The present invention provides long acting parenteral pharmaceutical compositions comprising a therapeutically effective amount of glatiramer. In particular, the present invention provides a long acting pharmaceutical composition comprising a therapeutically effective amount of glatiramer acetate in depot form suitable for administering at a medically acceptable location in a subject in need thereof. The depot form is suitable for subcutaneous or intramuscular implantation or injection.
Owner:MAPI PHARMA
Combination Therapy with Glatiramer Acetate and Rasagiline for the Treatment of Multiple Sclerosis
InactiveUS20080261894A1Relieve symptomsOrganic active ingredientsNervous disorderCombination therapyGlatiramer acetate
The subject invention provides a method of treating a subject afflicted with a form of multiple sclerosis comprising periodically administering to the subject an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, wherein the amounts when taken together are effective to alleviate a symptom of the form of multiple sclerosis in the subject so as to thereby treat the subject. The subject invention also provides a package comprising glatiramer acetate, rasagiline or the pharmaceutically acceptable salt thereof and instructions for use of the together to alleviate a symptom of a form of multiple sclerosis in a subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of rasagiline or the pharmaceutically acceptable salt thereof, which combination is useful to alleviate a symptom of a form of multiple sclerosis in a subject.
Owner:TEVA PHARMA IND LTD
Reduced Volume Formulation of Glatiramer Acetate and Methods of Administration
InactiveUS20110060279A1Reduce frequencyNervous disorderPeptide/protein ingredientsMANNITOL/SORBITOLHuman patient
A method for reducing frequency of relapses in a human patient afflicted with relapsing-remitting multiple sclerosis (RRMS) comprising administering to the patient 0.5 ml of an aqueous pharmaceutical solution of 20 mg glatiramer acetate and 20 mg mannitol.
Owner:TEVA PHARMA IND LTD
Copolymer 1 related polypeptides for use as molecular weight markers and for therapeutic use
InactiveUS20050256046A1Accurate and robust calibration setTherapeutic utilitySenses disorderPeptide/protein ingredientsRetention timeLinear relationship
Owner:YEDA RES & DEV CO LTD
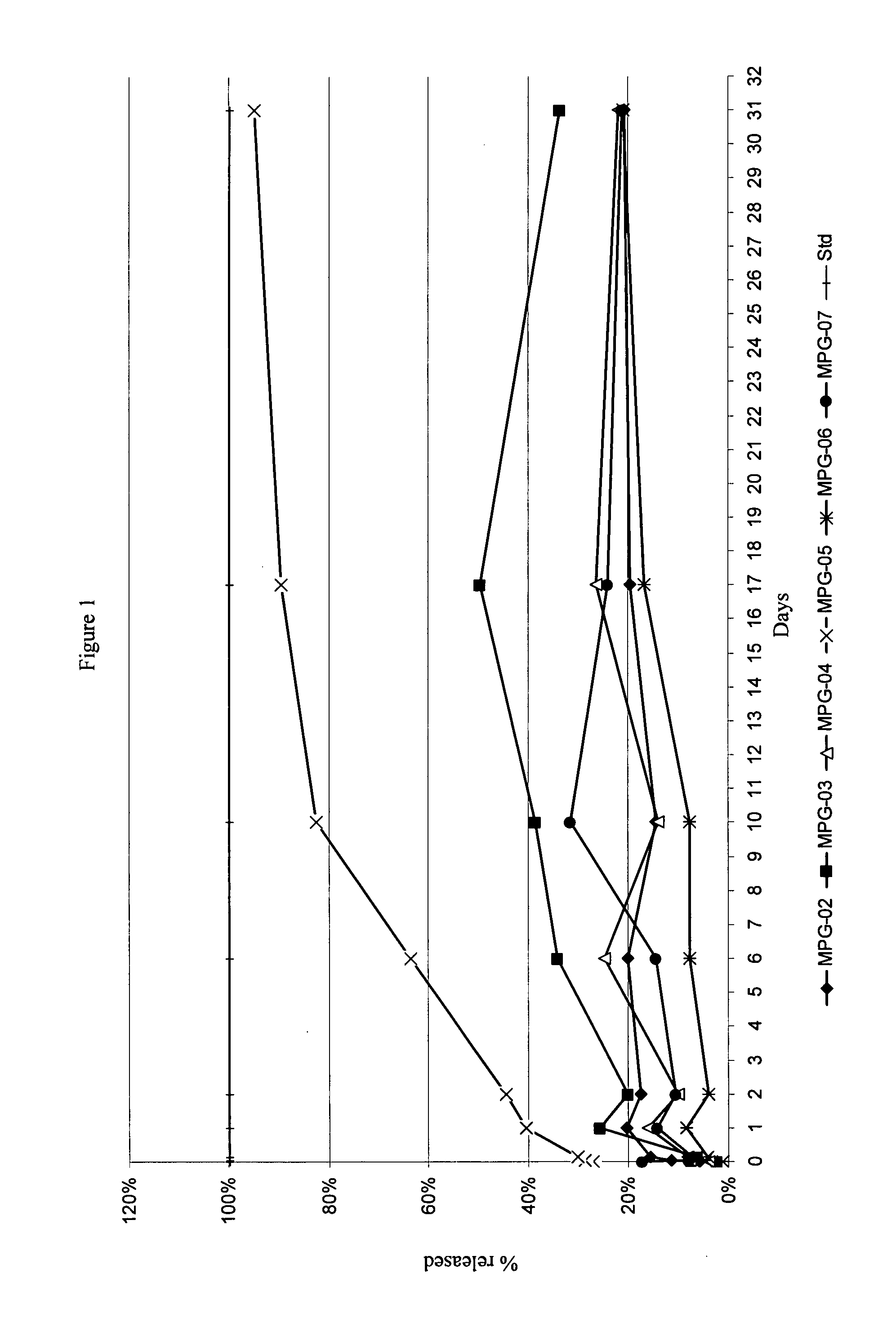
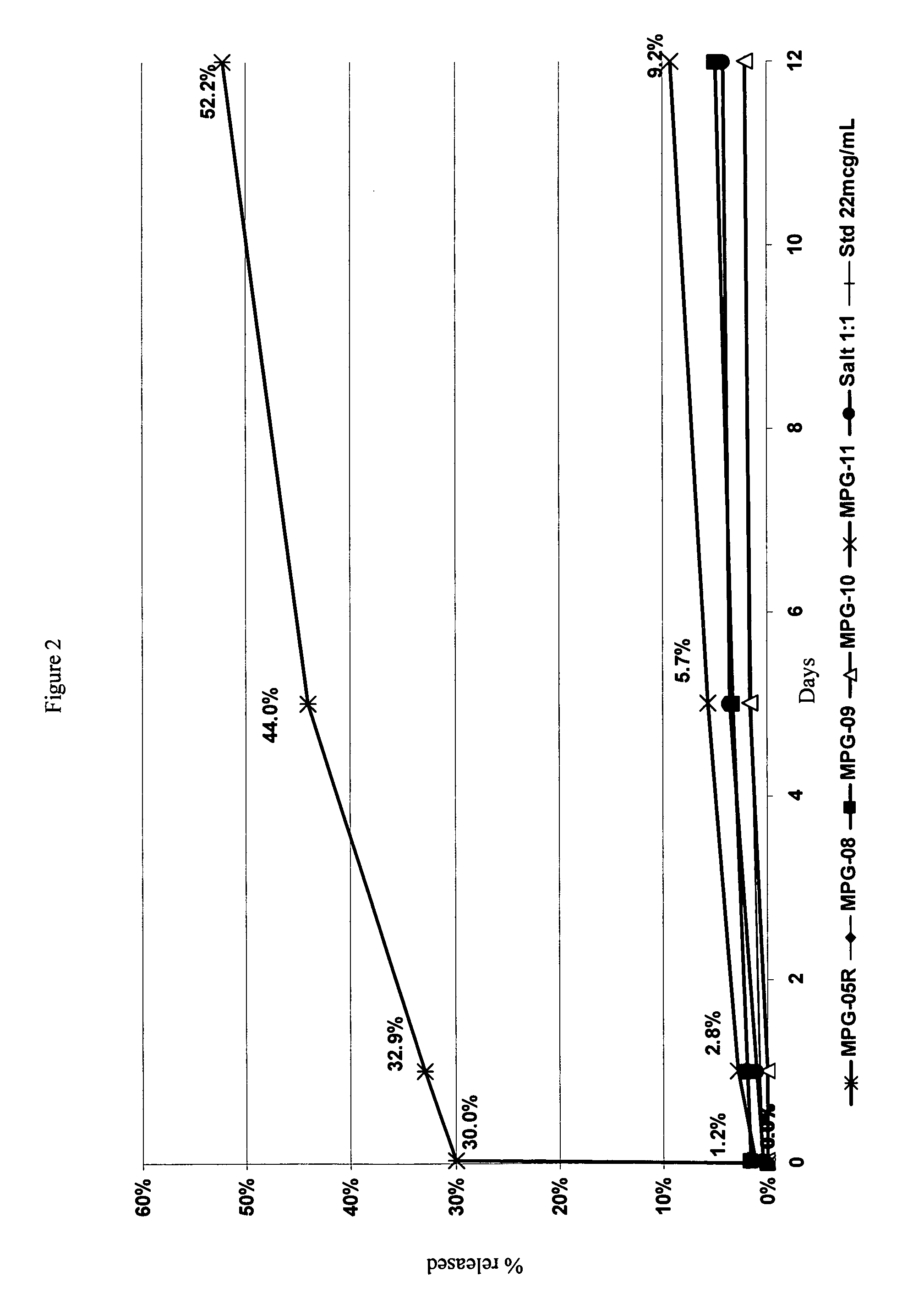
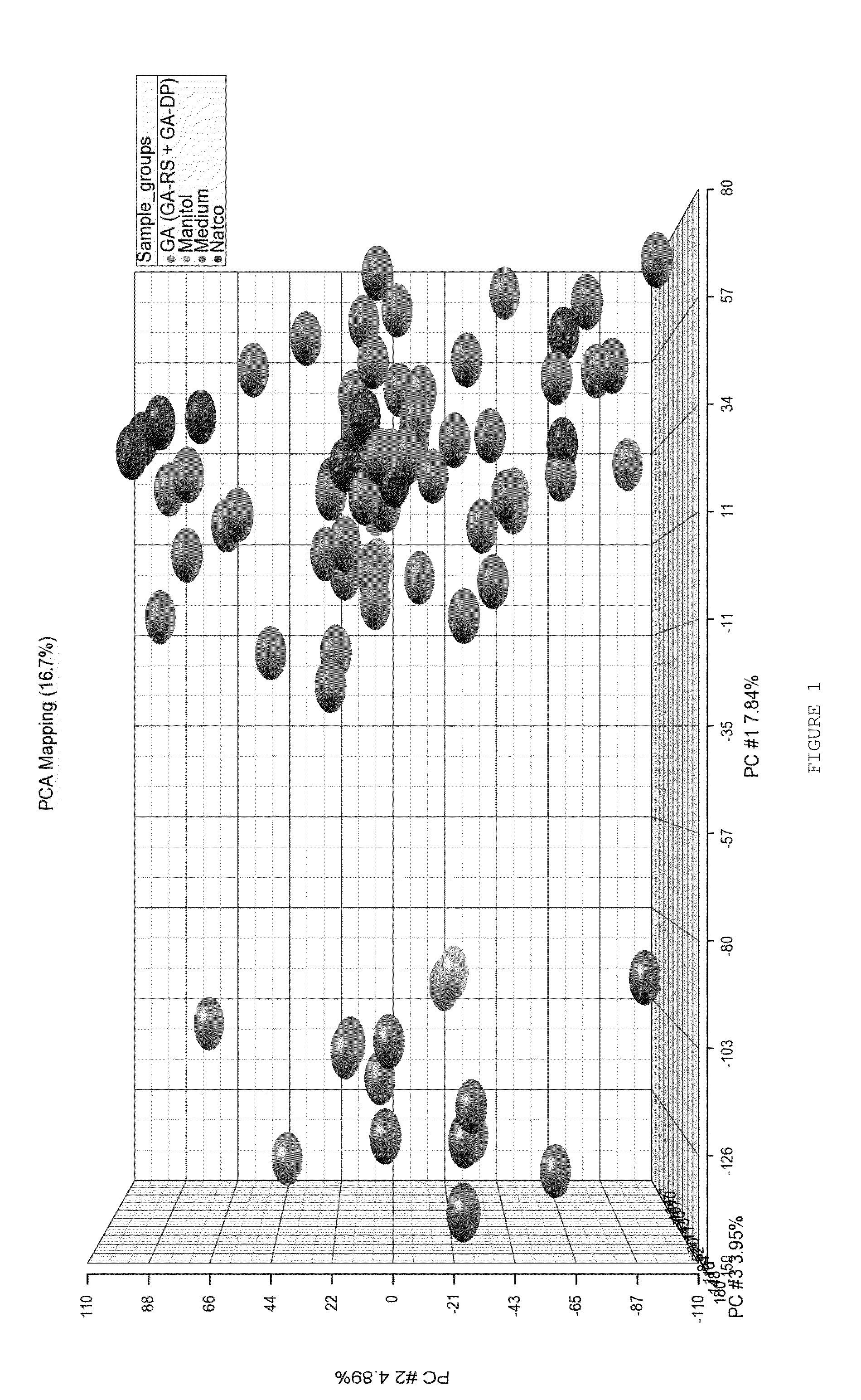
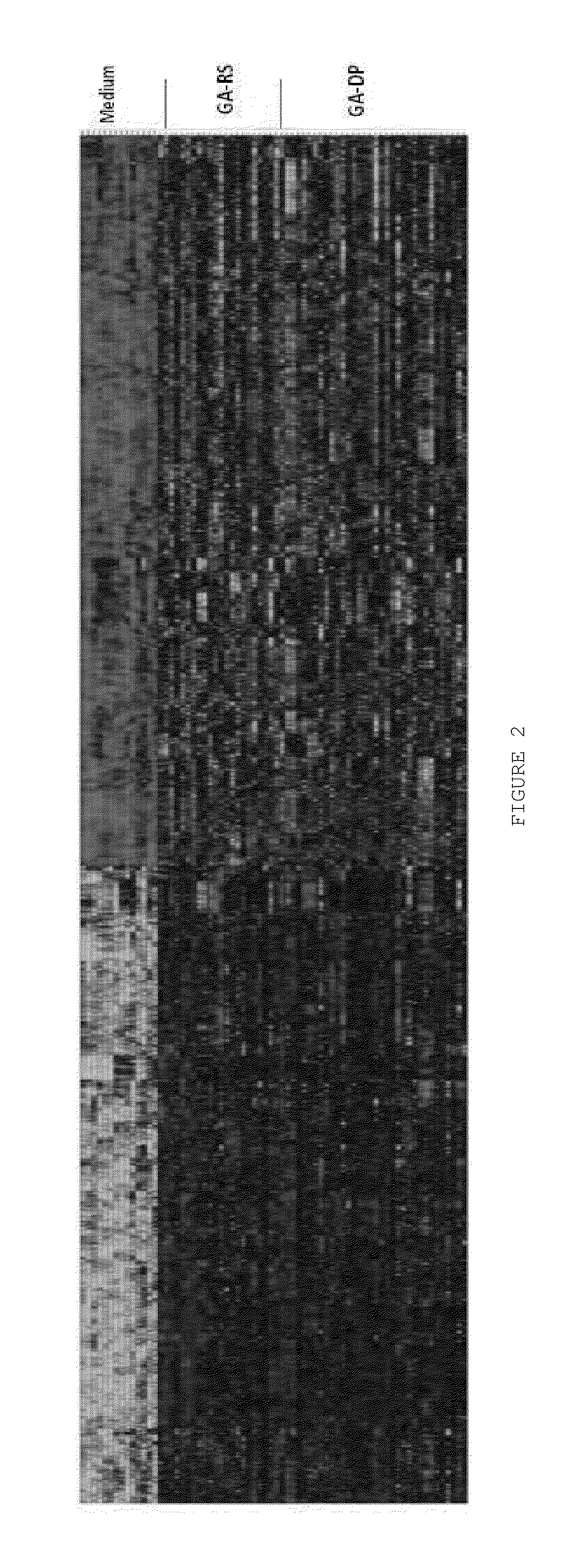
Characterizing a glatiramer acetate related drug product
InactiveUS20140193827A1Safe and effectiveReduce recurrenceMicrobiological testing/measurementBiological testingCombinatorial chemistryDrug product
The present invention provides a process for characterizing a glatiramer acetate related drug substance or drug product comprising the steps of:a) obtaining a batch of the glatiramer acetate related drug substance or drug product;b) immunizing a mammal with a predetermined amount of a glatiramer acetate related drug substance or drug product;c) preparing a culture of cells from the mammal of step b) at a predetermined time after immunization;d) incubating cells from the culture of step c) with a predetermined amount of the glatiramer acetate drug related substance or drug product of step a); ande) determining the level of expression of at least one gene disclosed herein or determining the level of biological activity of the cells of step c) as disclosed herein,thereby characterizing the glatiramer acetate related drug substance or drug product of step a).
Owner:TEVA PHARMA IND LTD
Combination Therapy With Glatiramer Acetate and Riluzole
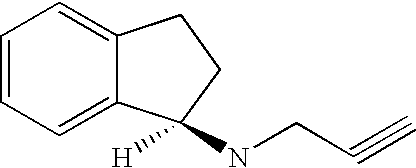
The subject invention provides a method of providing neuroprotection to the central or peripheral nervous system of a subject in need of such neuroprotection comprising periodically administering to the subject an amount of glatiramer acetate and an amount of 2-amino-6-trifluoromethoxybenzathiazole, wherein the amounts when taken together are effective to provide neuroprotection to the central or peripheral nervous system of the subject. The subject invention also provides a package comprising glatiramer acetate, 2-amino-6-trifluorormethoxybenzothiazole and instructions for use together to provide neuroprotection to the central or peripheral nervous system of a subject in need of such neuroprotection. Additionally, the subject invention provides a pharmaceutical composition comprising an amount of glatiramer acetate and an amount of 2-amino-6-trifluorormethoxybenzothiazole, wherein the amounts when taken together are effective to provide neuroprotection to the central or peripheral nervous system of the subject. The subject invention further provides a pharmaceutical combination comprising separate dosage forms of an amount of glatiramer acetate and an amount of 2-amino-6-trifluorormethoxybenzothiazole, which combination is useful to provide neuroprotection to the central or peripheral nervous system of the subject. In addition, the combination therapy may be used to treat a subject afflicted with multiple sclerosis or one afflicted with amyotrophic lateral sclerosis.
Owner:TEVA PHARM USA INC
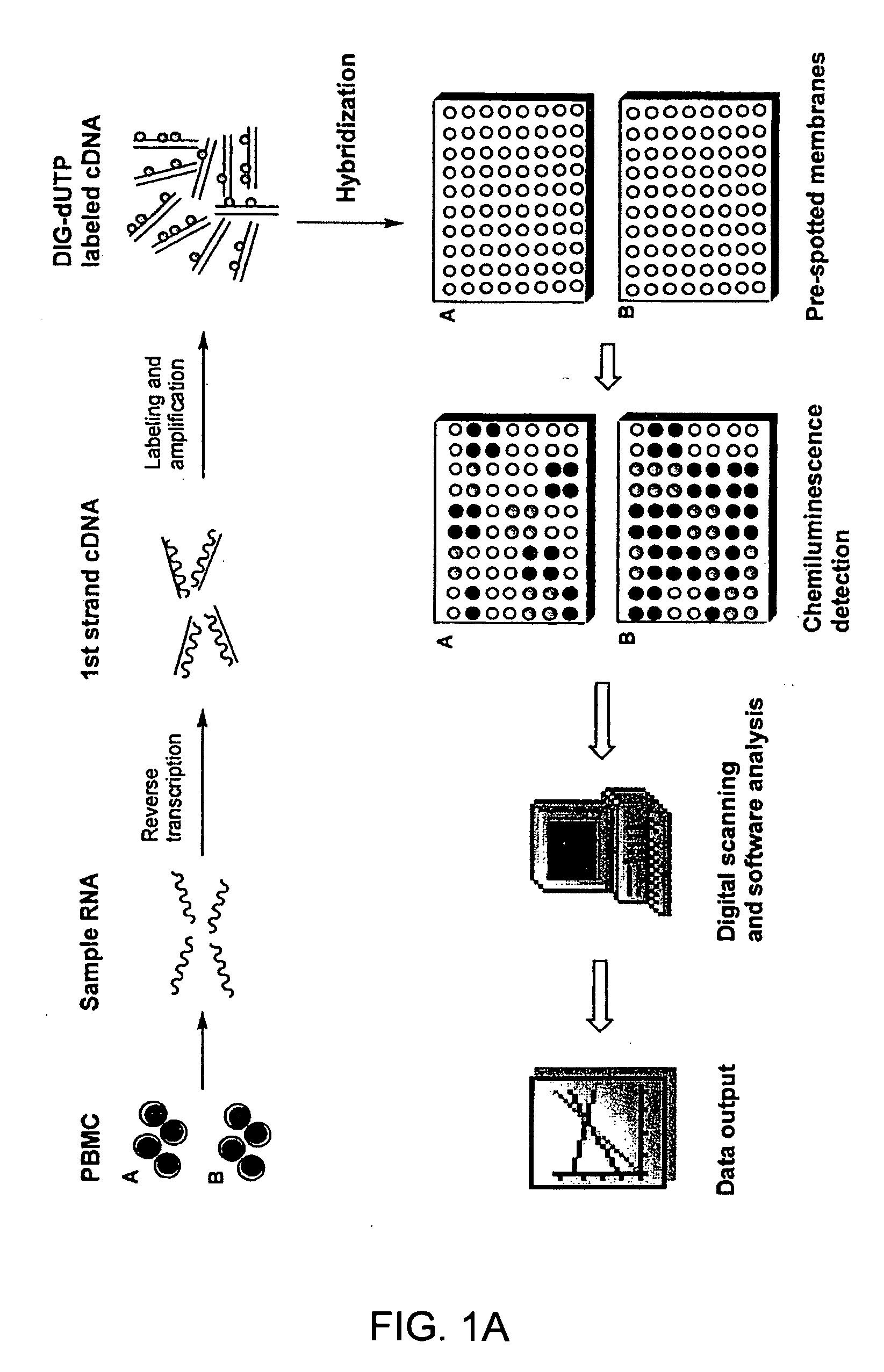
Gene expression profiling technology for treatment evaluation of multiple sclerosis
InactiveUS20050064483A1Microbiological testing/measurementNeutralizing antibodyGene expression profiling
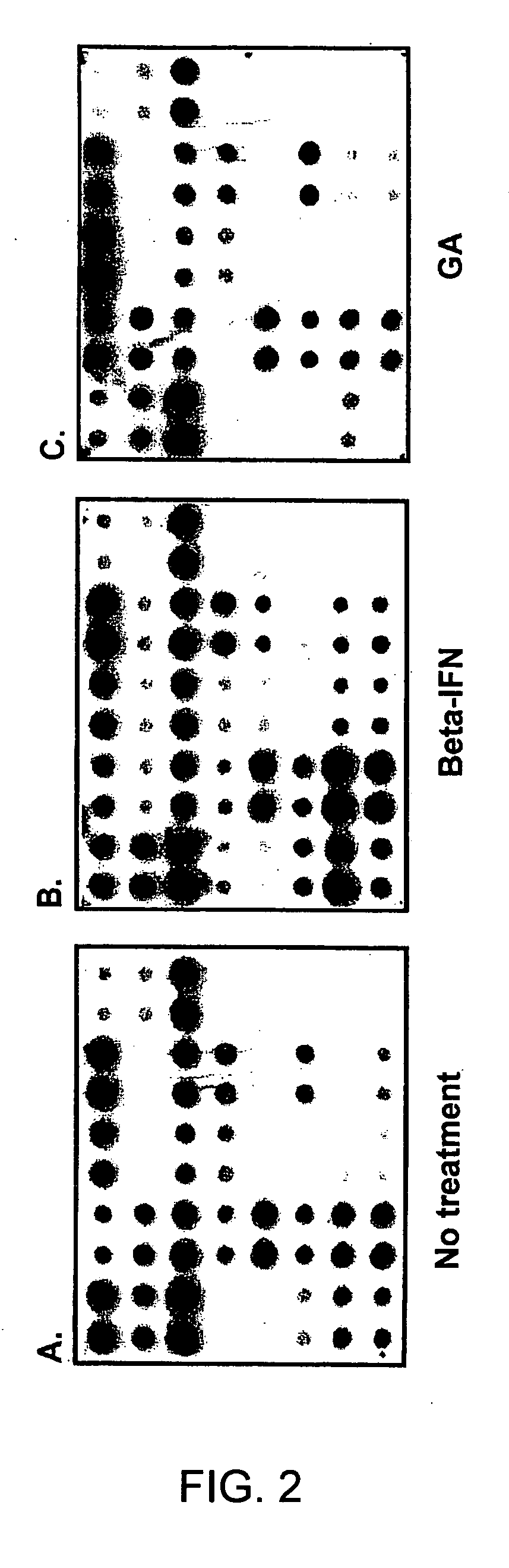
The invention relates to gene expression profiling technology to quantitatively measure the expression profiles of genes selected based on their role in inflammation and their susceptibility to regulation by current multiple sclerosis (MS) treatment agents, beta-interferon (IFN) and glatiramer acetate (GA). The invention also provides an assay for detection of beta-IFN neutralizing antibody based on the blocking effect of serum antibodies on the known regulatory properties of beta-IFN on PBMC and evaluation of treatment responses in MS patients.
Owner:BAYLOR COLLEGE OF MEDICINE
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
InactiveUS20140322158A1Organic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
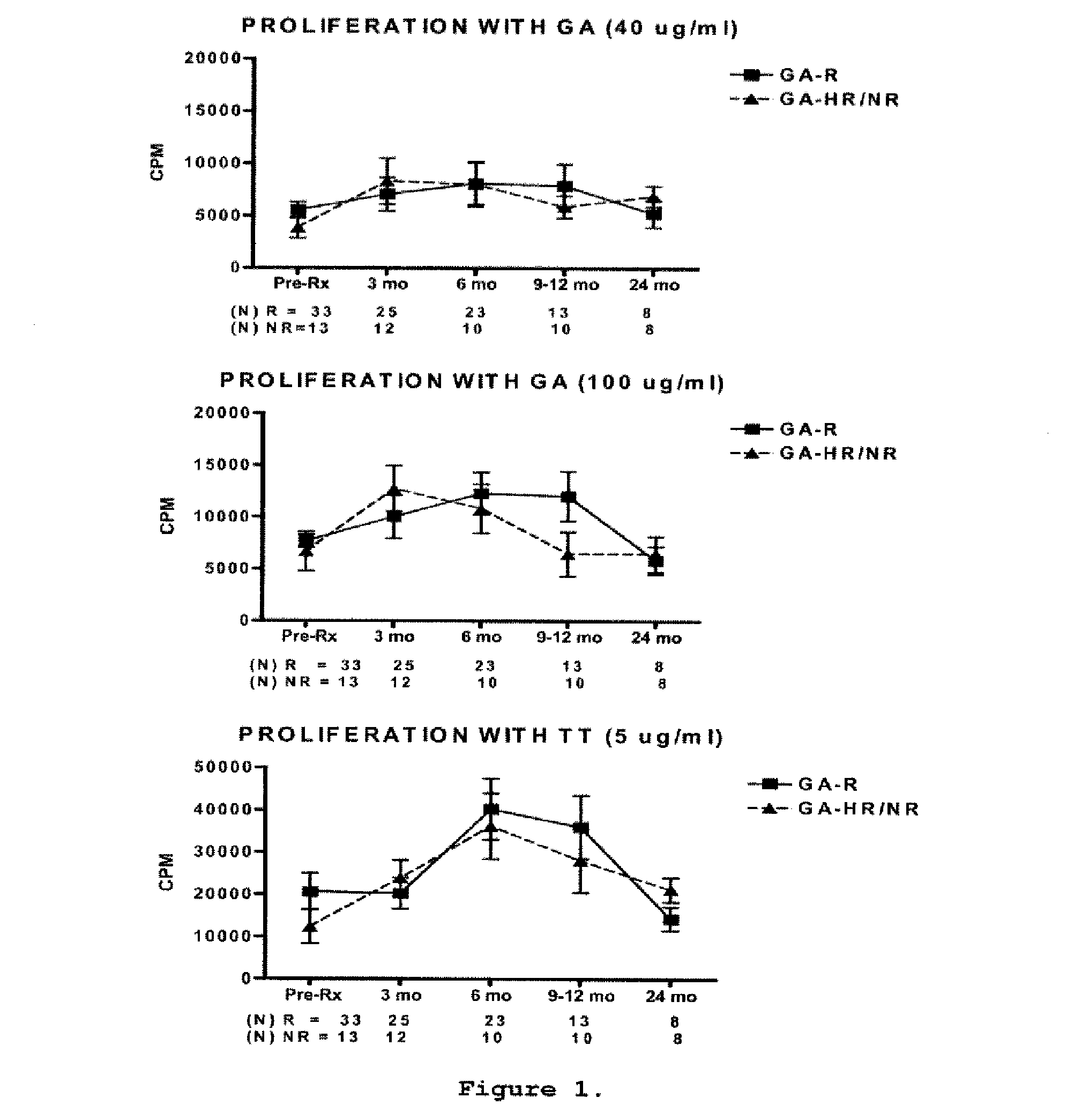
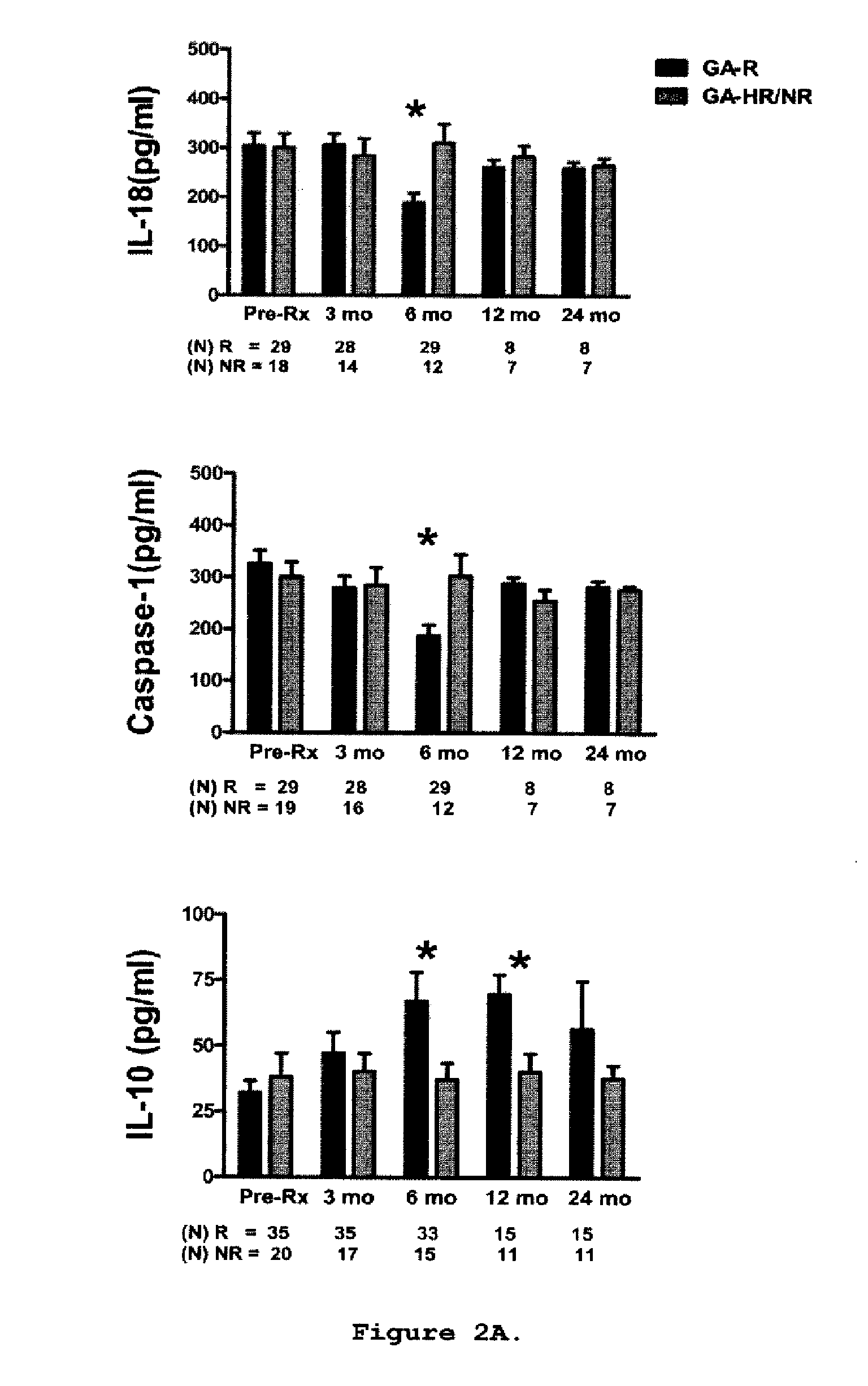
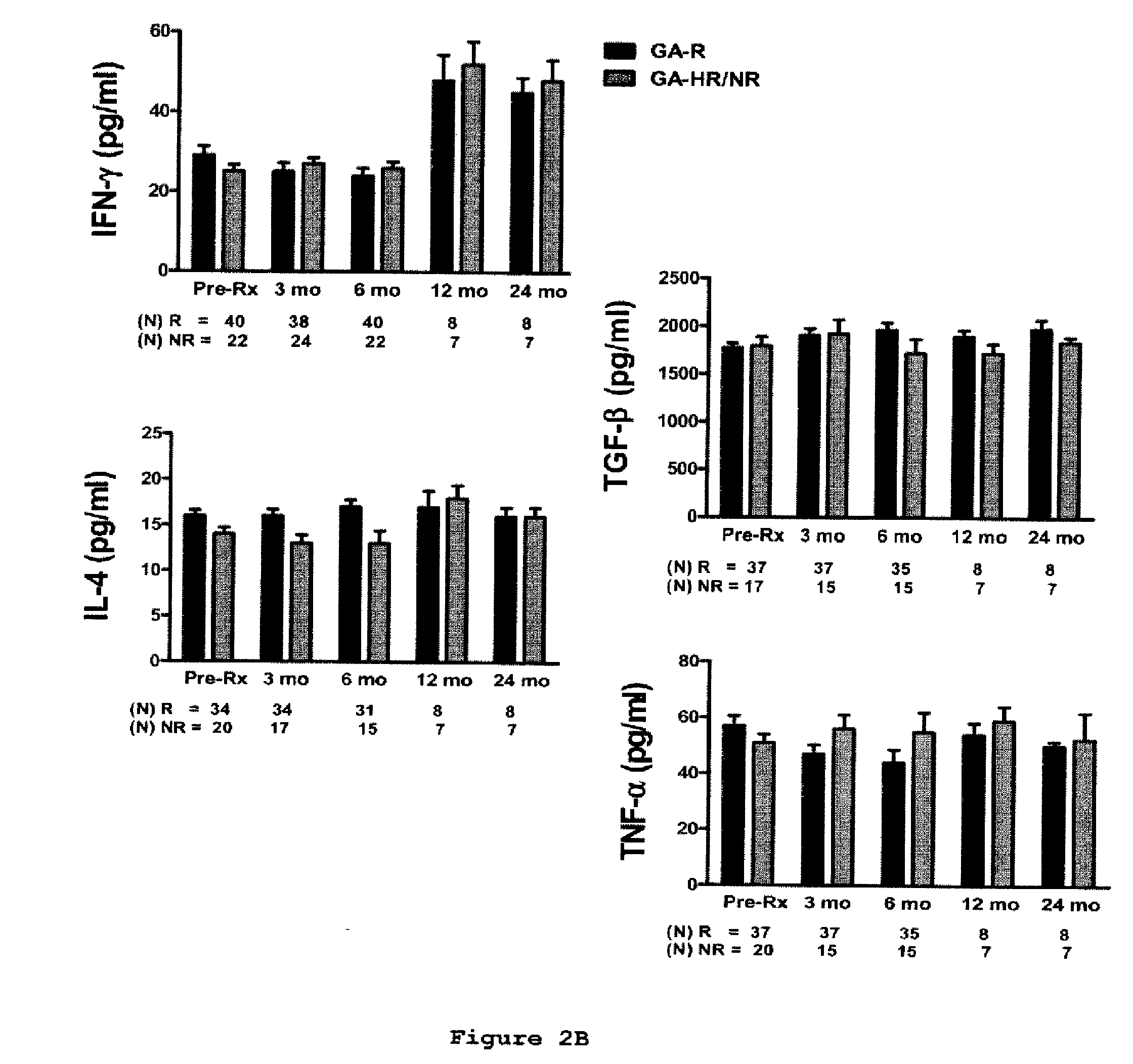
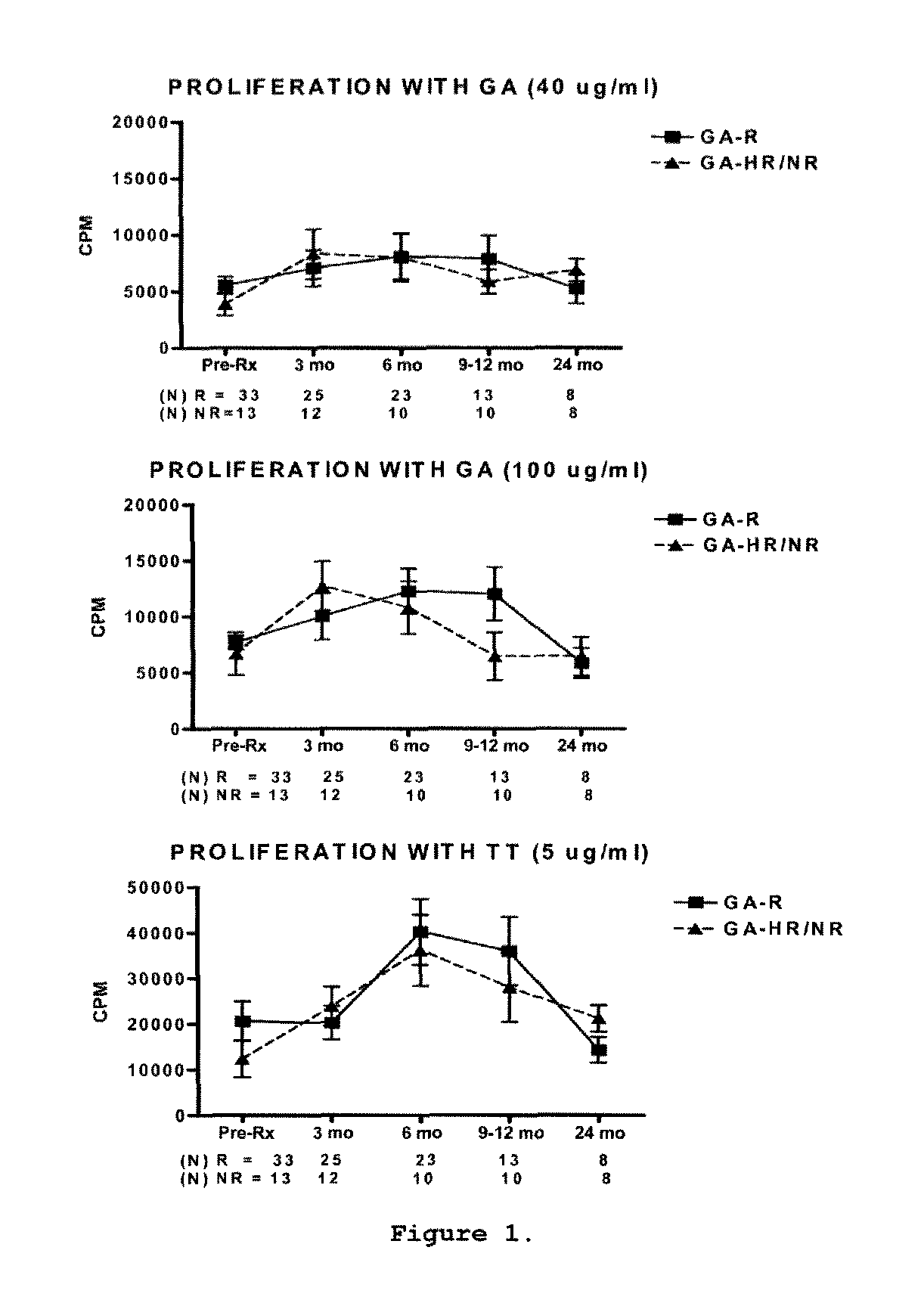
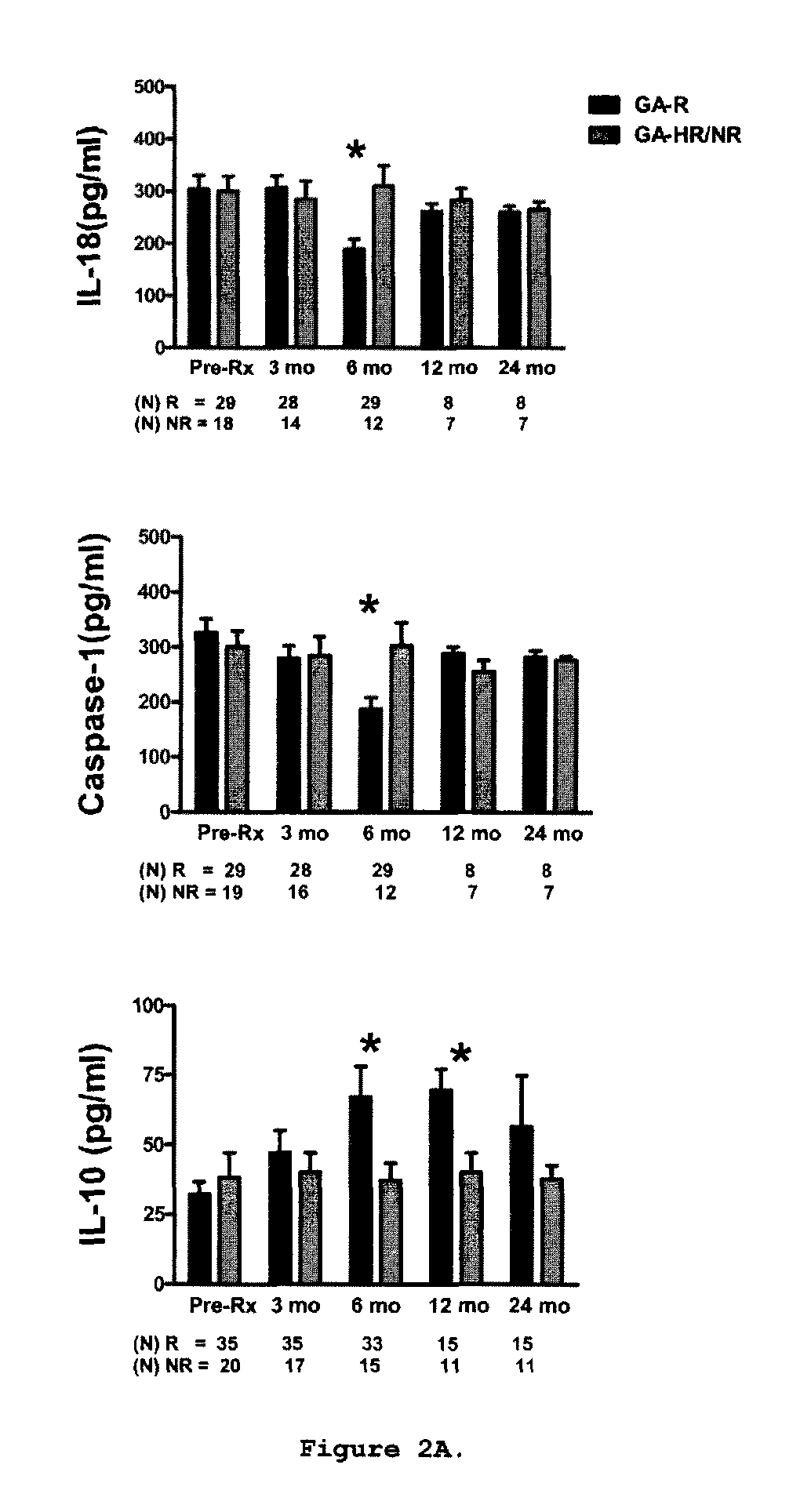
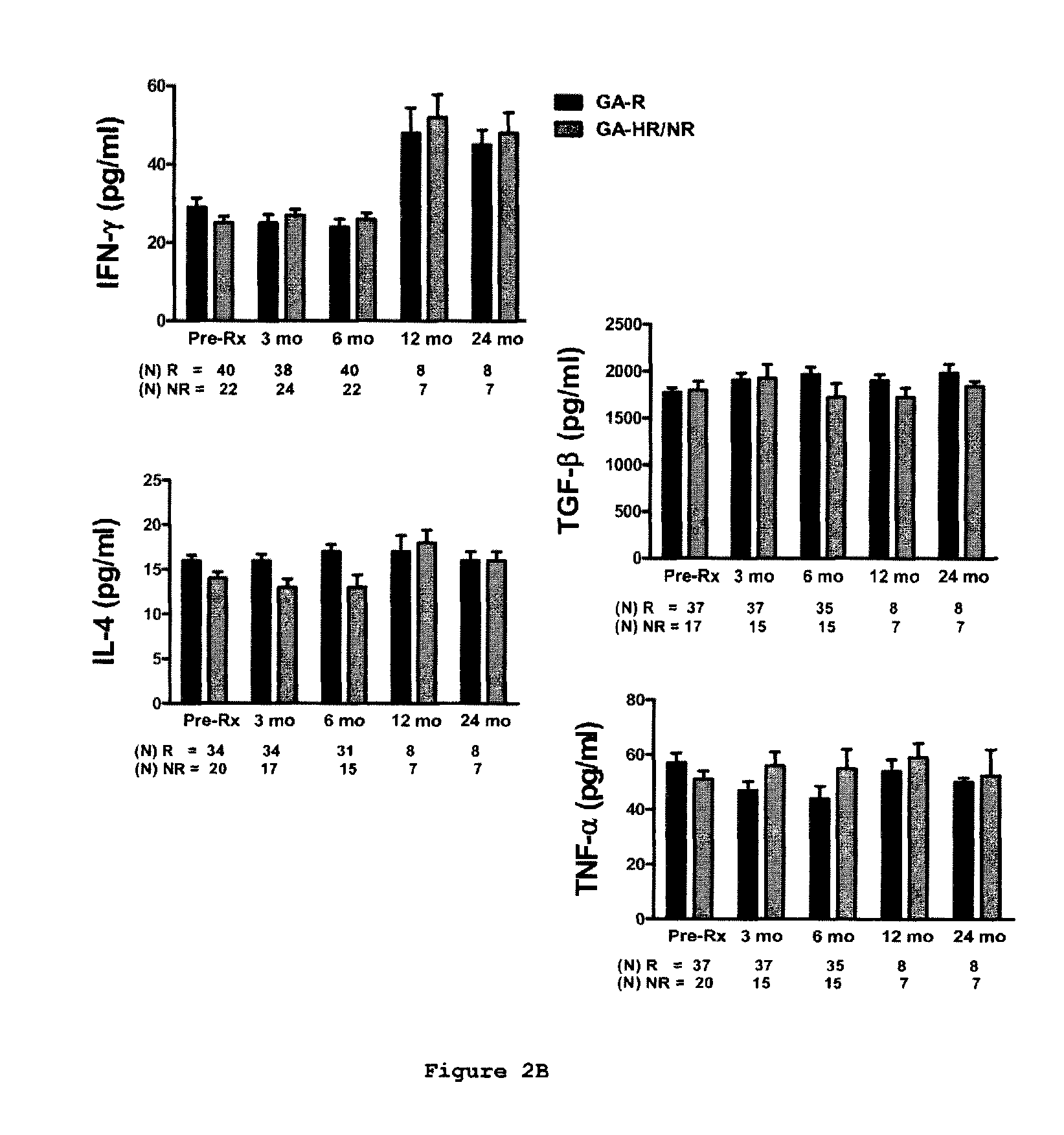
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
Glatiramer acetate for use as an immuno-modulatory agent
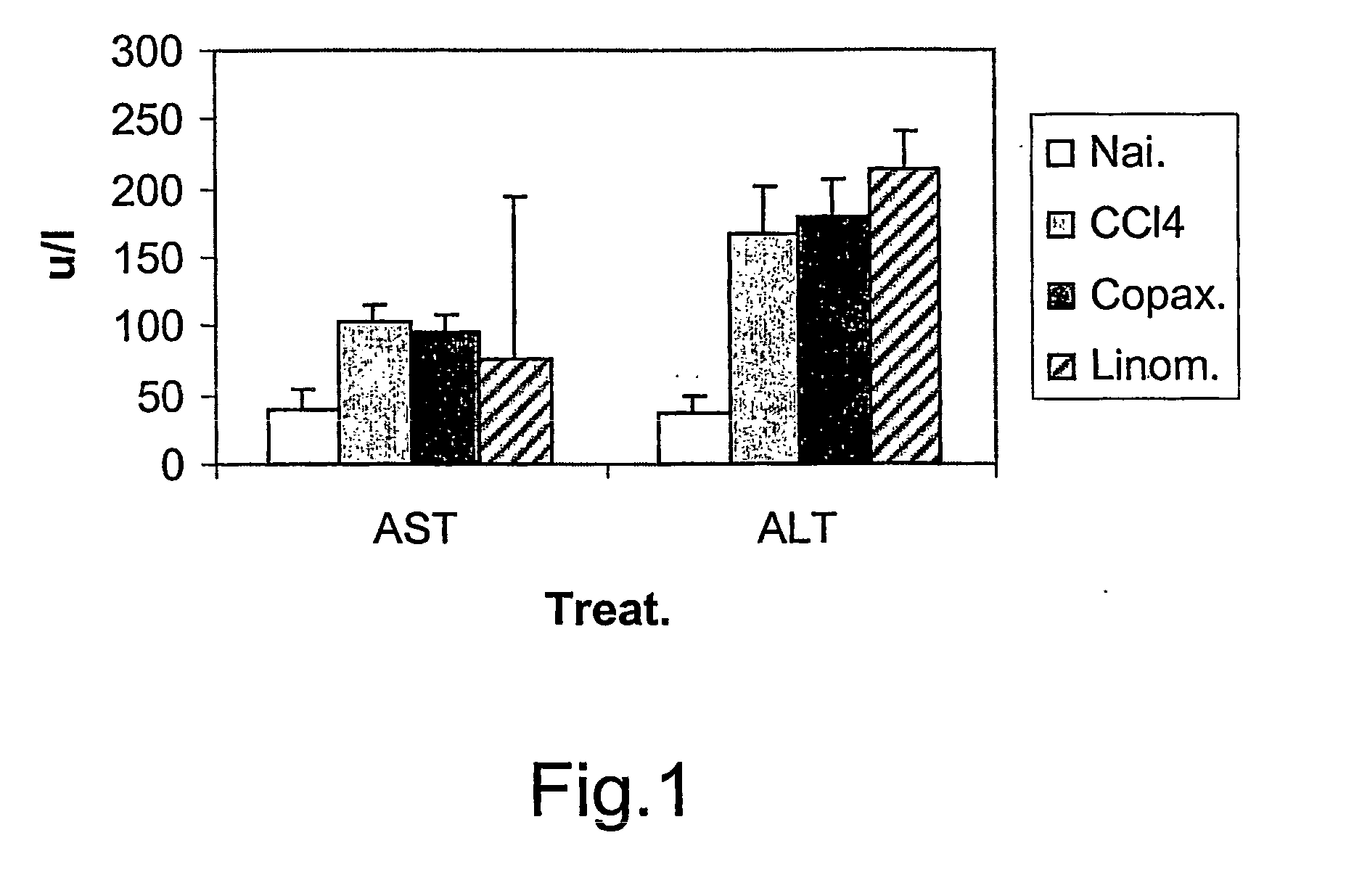
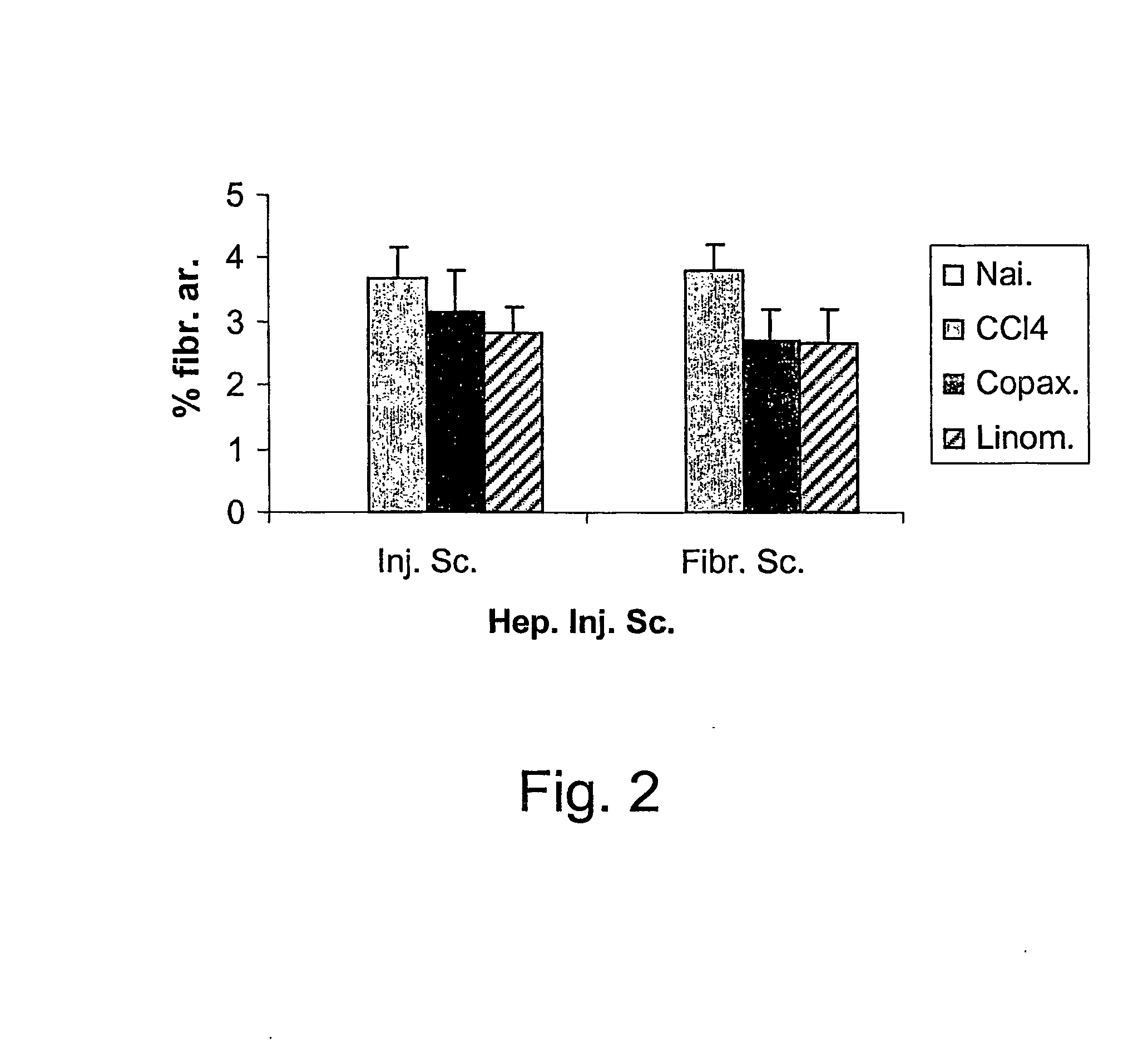
InactiveUS20070197424A1Increase the number ofIncrease the number of cellsDigestive systemTetrapeptide ingredientsHepatocellular carcinomaHepatic fibrosis
A method for the treatment of hepatic fibrotic injuries caused by various diseases, viral infections or toxic agents which involves the use of glatiramer acetate as an immuno-modulatory agent. The diseases to be treated are hepatic fibrosis and hepatic cellular carcinomas. Also disclose is the use of glatiramer acetate in the treatment of inflammatory bowel diseases. Additionally, disclosed are methods for screening for immuno-modulatory agents which are useful in the treatment of hepatic fibrosis, hepatic cellular carcinomas and inflammatory bowel diseases.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
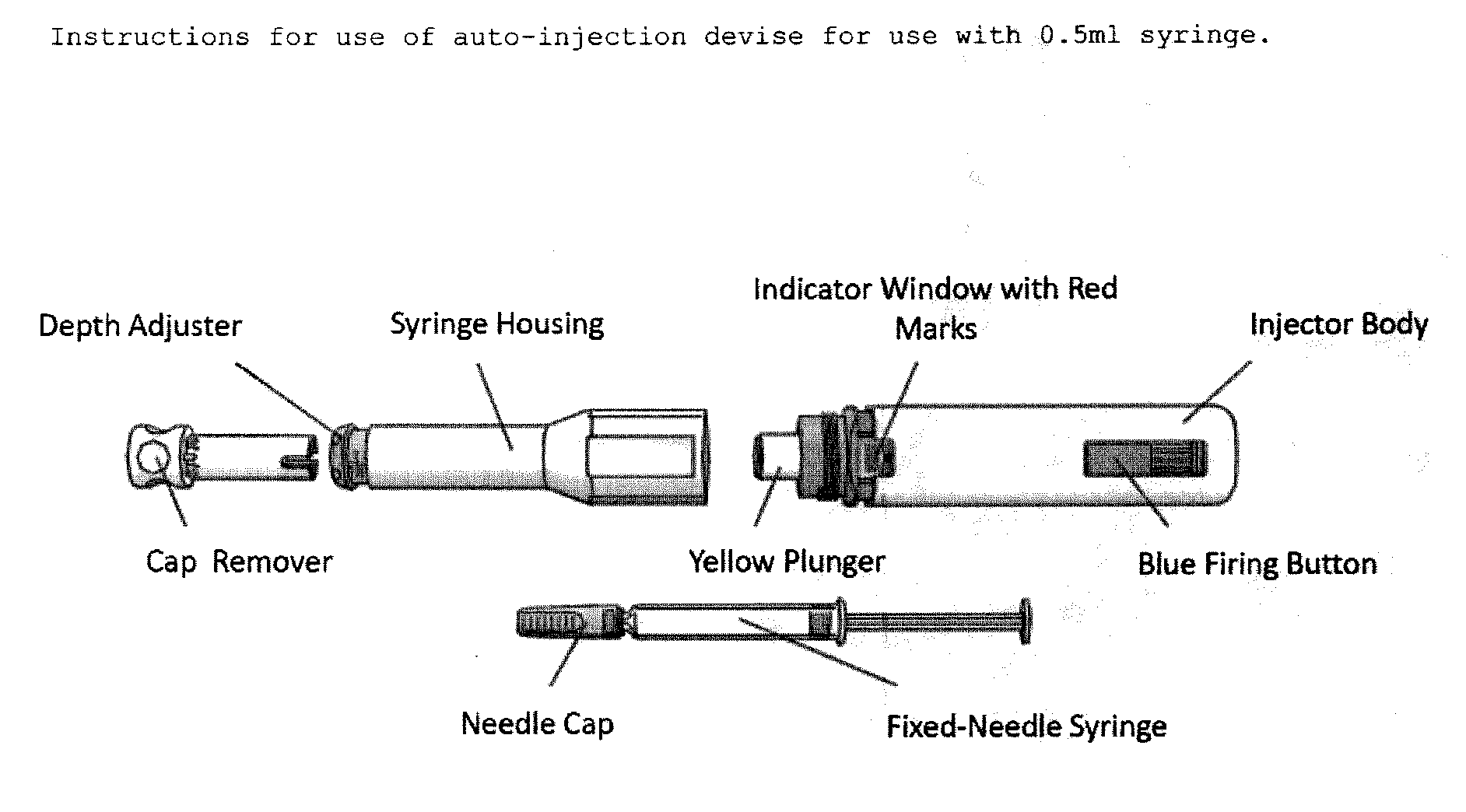
Reduced volume formulation of glatiramer acetate and methods of administration
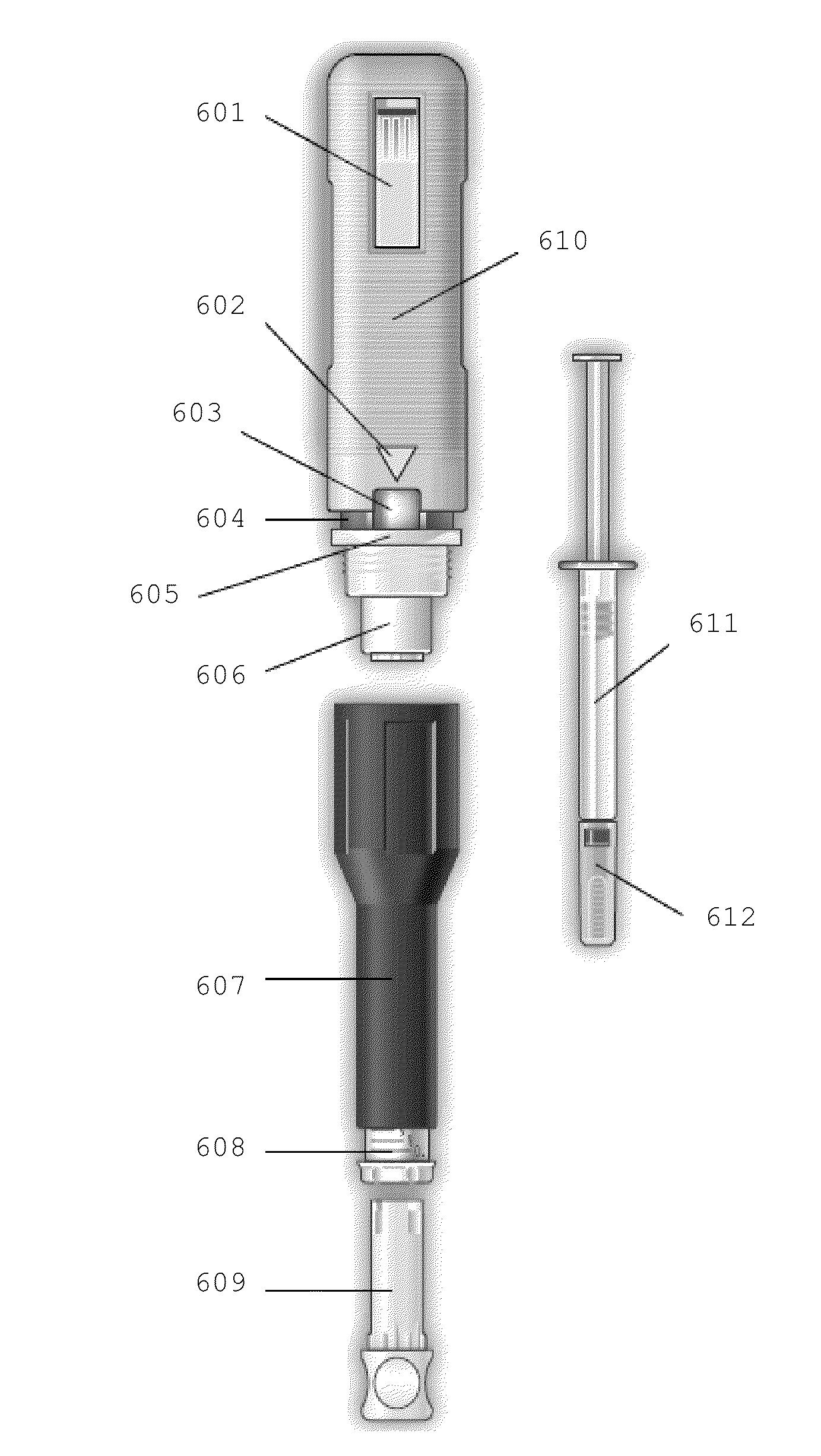
InactiveUS8920373B2Reduce frequencyNervous disorderPeptide/protein ingredientsInjection lockedGlatiramer acetate
The present invention provides an improved injection assisting device wherein the improvement comprises an injection lock indicator located between the first and the second outer shell and the outside of the indicator window, configured to indicate to a user whether the injection locking member is in a locked state, and configured to substantially contrast in color with both the first outer shell and the second outer shell, which first and second outer shells contrast in color with each other, and configured to be substantially shielded from a view of a user in the presence of the predetermined amount of compression force on the second outer shell.
Owner:TEVA PHARMA IND LTD
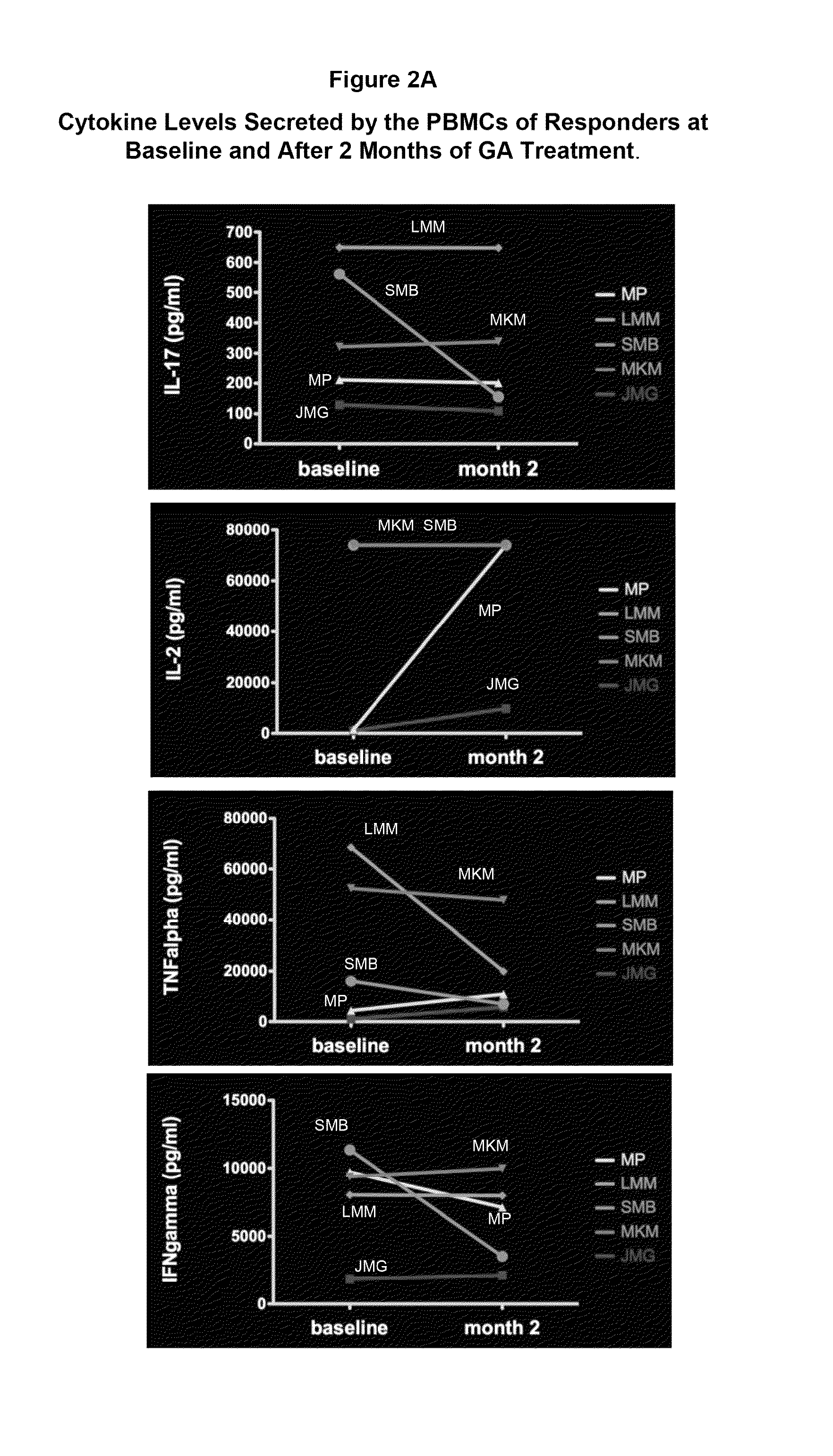
Cytokine biomarkers as predictive biomarkers of clinical response for Glatiramer acetate
A method for treating a human subject afflicted with multiple sclerosis or a single clinical attack consistent with multiple sclerosis with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of determining whether the human subject is a glatiramer acetate responder by evaluating a biomarker selected from the group consisting of IL-17 concentration, TNF-α concentration, IL-2 concentration and IFN-γ concentration, or a combination thereof, in the blood of the human subject and administering the pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier to the human subject only if the human subject is identified as a glatiramer acetate responder.
Owner:TEVA PHARMA IND LTD
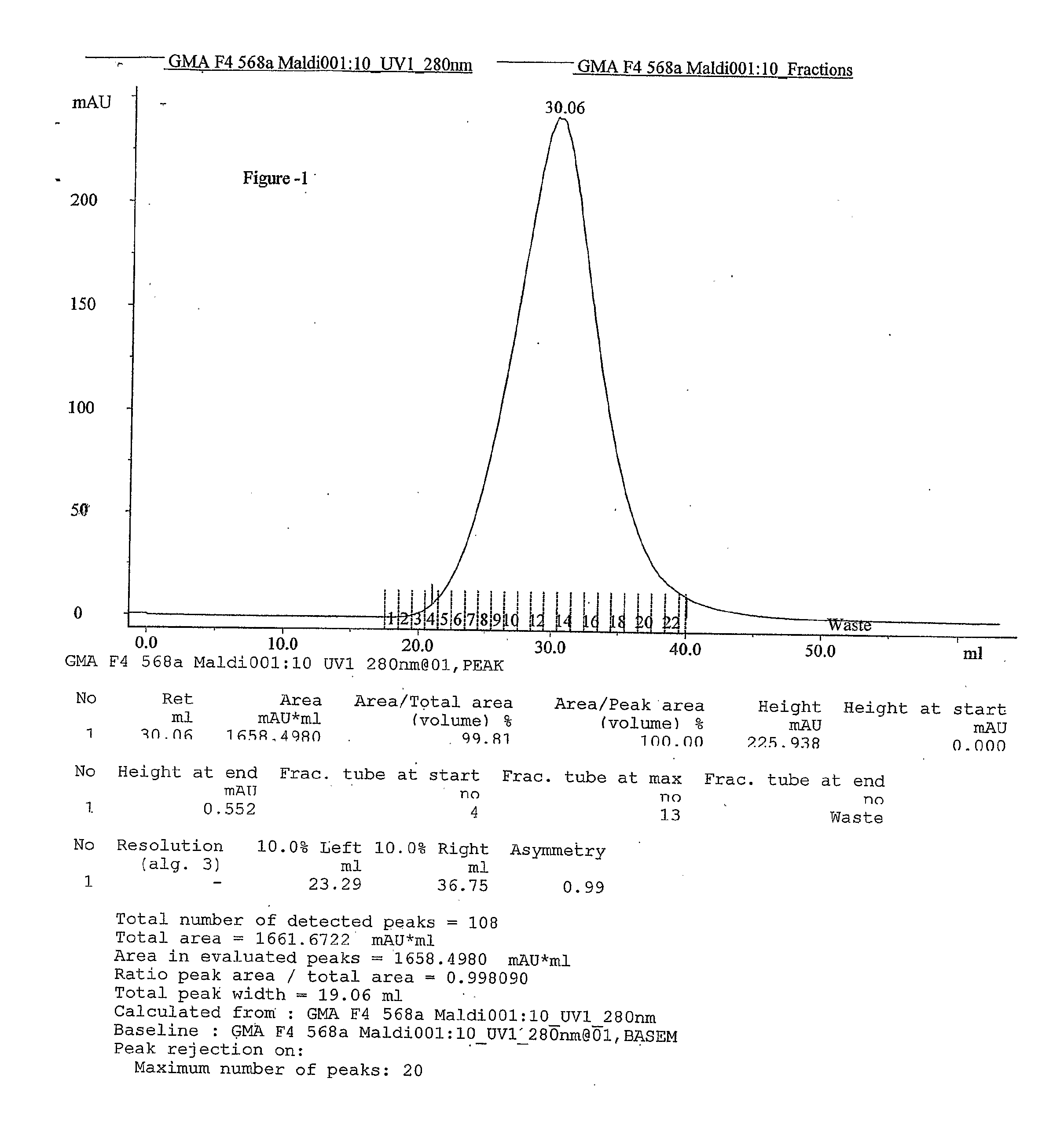
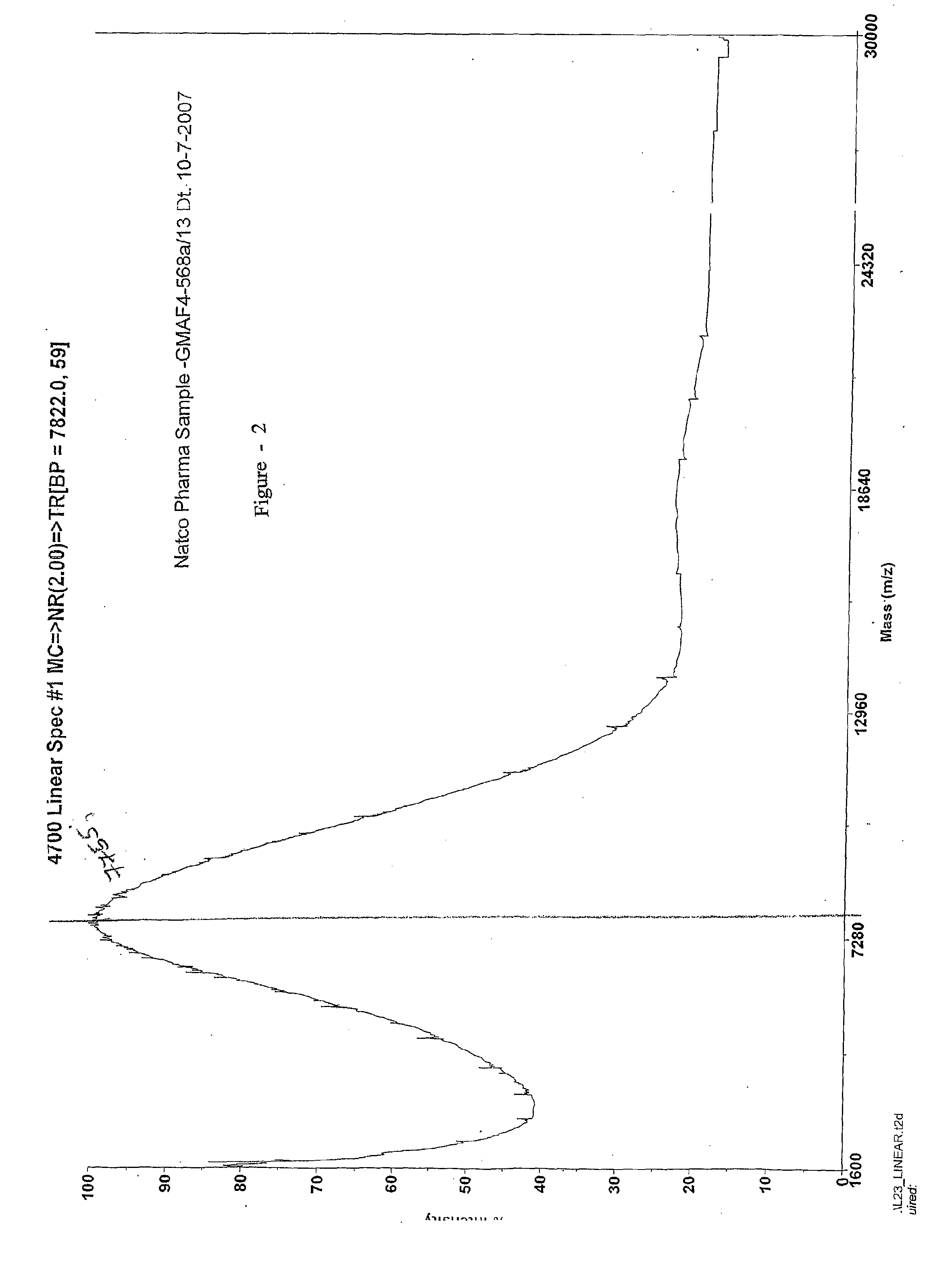
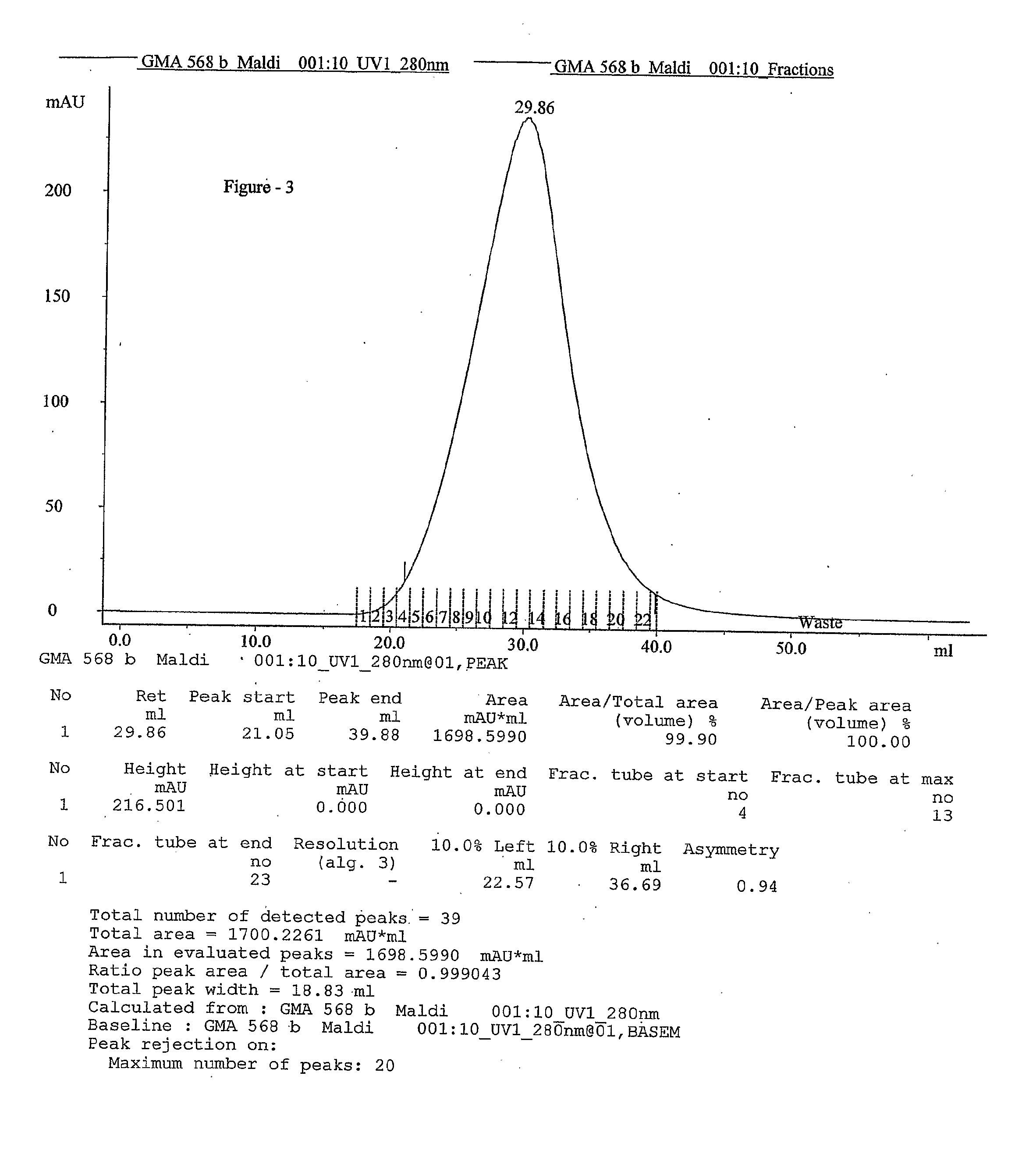
Process for the preparation glatiramer acetate (copolymer-1)
InactiveUS20100324265A1Easy to optimizeShort reaction timePeptide-nucleic acidsNervous disorderBenzoyl bromideTyrosine
This invention relates to a convenient and improved process for preparation of glatiramer acetate (copolymer-1) of pharmaceutical grade. The process involves polymerizing N-carboxyanhydrides of tyrosine, alanine, y-benzyl glutamate and &egr;-N-trifluoroacetyllysine in dioxane with diethylamine as initiator to afford protected copolymer-1. Treatment with hydrogen bromide in acetic acid at 35° C. for 3-5 h cleaves benzyl group to produce trifluoroacetyl copolymer-1. The trifluoroacetyl copolymer-1 is washed with an organic solvent to remove reactive benzyl bromide generated during debenzylation. Deprotection with aqueous piperidine, followed by dialysis offers glatiramer acetate (copolymer-1) of Molecular weight 5000-9000 daltons.
Owner:NATCO PHARMA LTD
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
Owner:TEVA PHARMA IND LTD
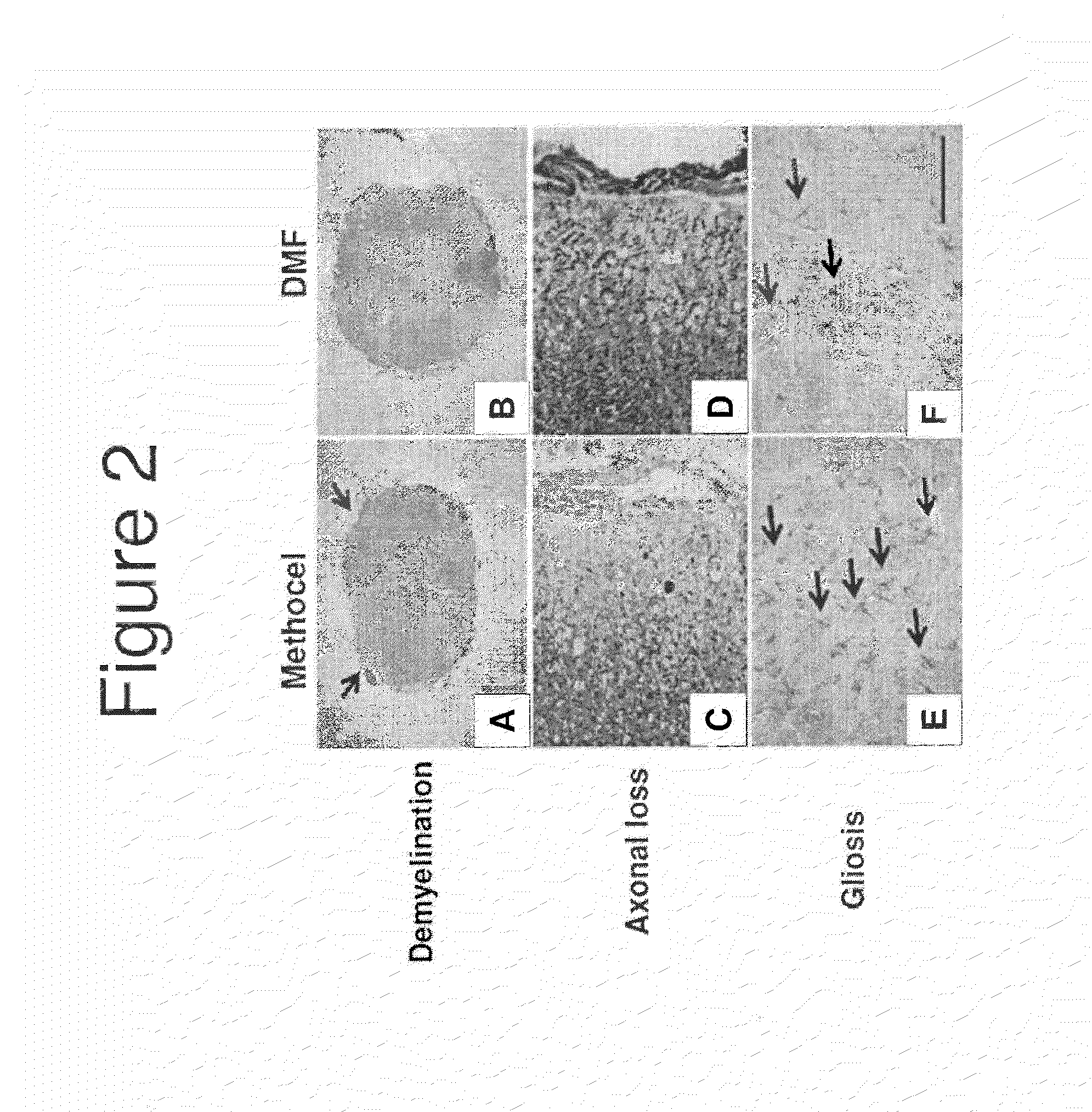
Neuroprotection in Demyelinating Diseases
InactiveUS20130287732A1Reduce demyelinationReduce axonal deathOrganic active ingredientsNervous disorderNervous systemDemyelinating disease
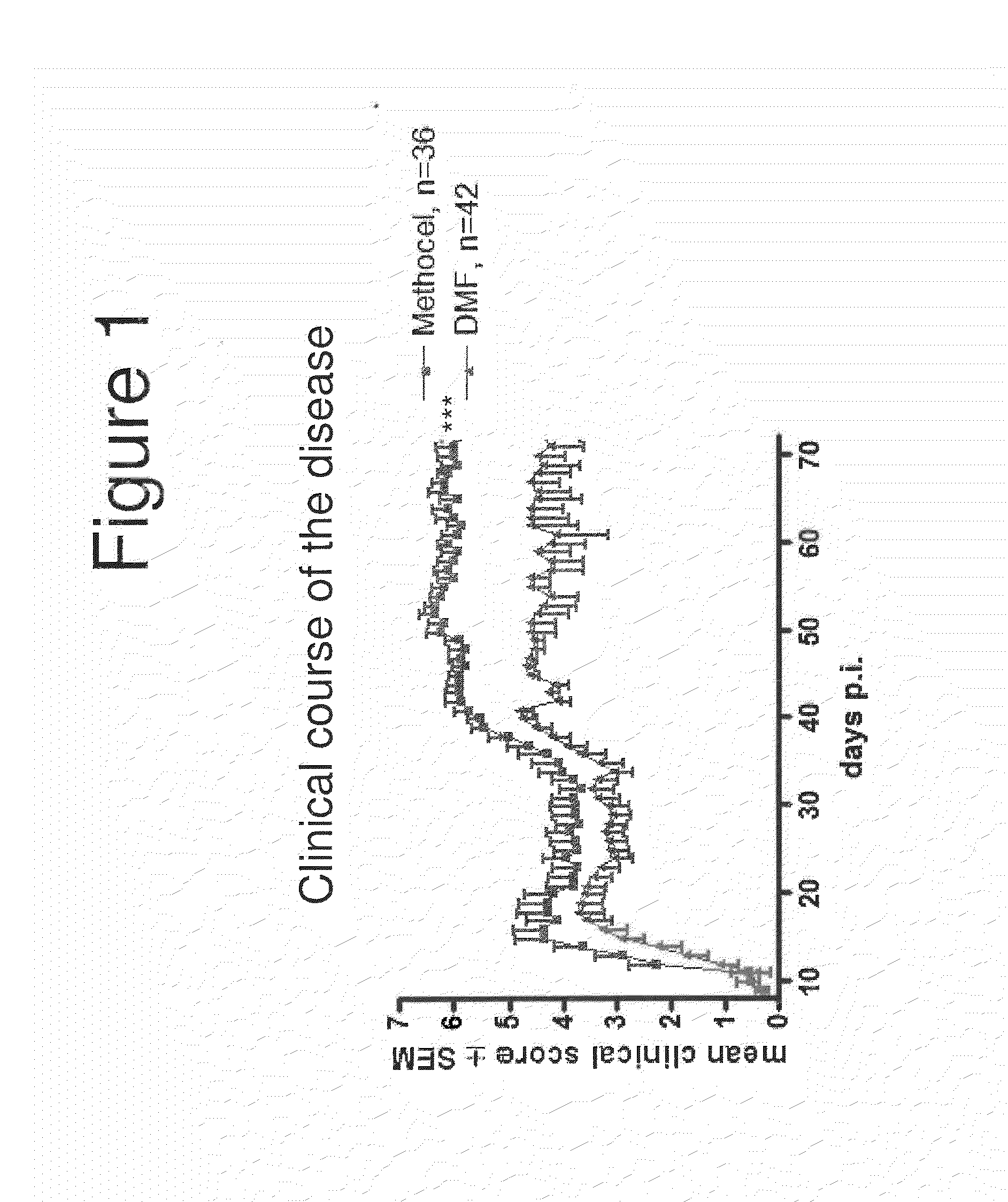
Methods of treating neurological disorders, e.g., those characterized by demyelination and / or axonal loss (e.g., MS), are provided. The methods comprise administration of a therapeutically effective amount of at least one compound of Formula I:wherein R1 and R2 are independently selected from OH, O−, and (C1-6)alkoxy, or a pharmaceutically acceptable salt thereof; and either glatiramer acetate or interferon-beta.
Owner:BIOGEN MA INC
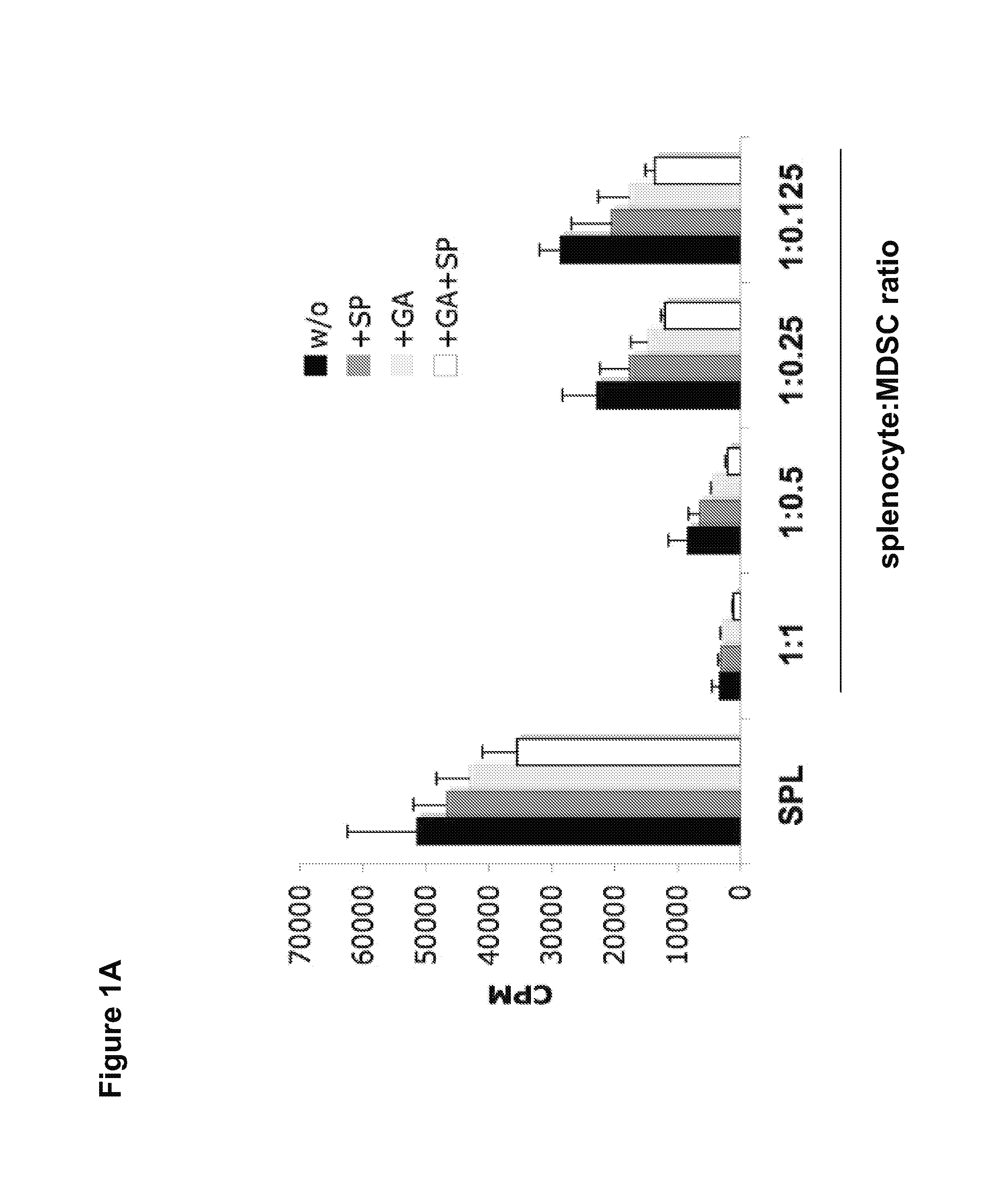
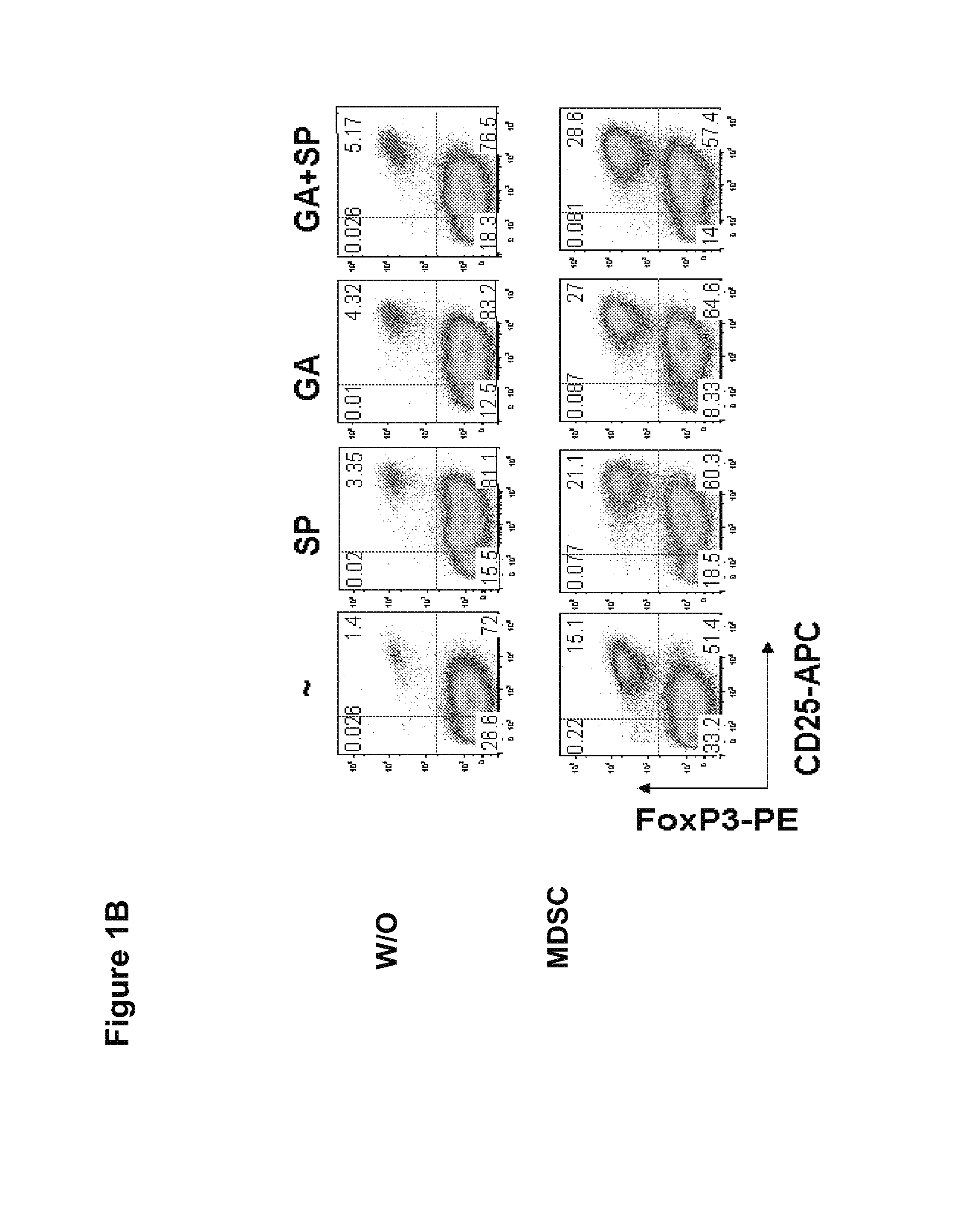
Methods of using small compounds to enhance myeloid derived suppressor cell function for treating autoimmune diseases
InactiveUS20130108579A1Sure easyLittle to toxicityOrganic active ingredientsPeptide/protein ingredientsAutoimmune diseaseMitogen-activated protein
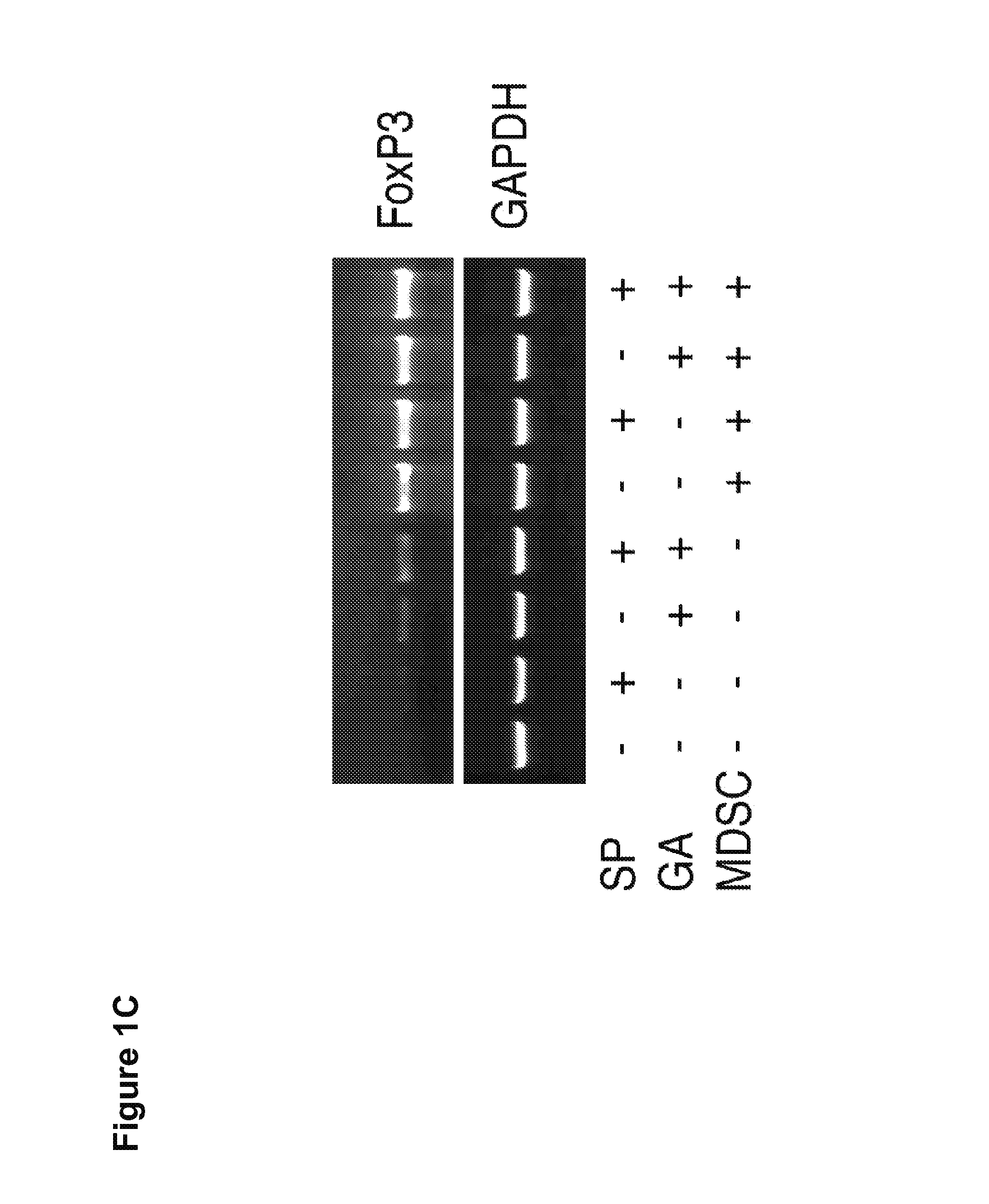
Methods for enhancing the suppressive function of myeloid derived suppressor cells (MDSCs) for the treatment of autoimmune diseases using small compounds are disclosed. In certain aspects, the small compounds are glatiramer acetate and mitogen activated protein (MAP) kinase inhibitors. In other aspects, these methods include the administration of exogenous MD-SCs or the use of endogenous MDSCs mobilized using stem cell mobilizers. In yet other aspects, compositions containing MDSCs and small compounds of the invention are provided.
Owner:MT SINAI SCHOOL OF MEDICINE
Combination therapy with glatiramer acetate and mitoxantrone for the treatment of multiple sclerosis
Owner:TEVA PHARMA IND LTD
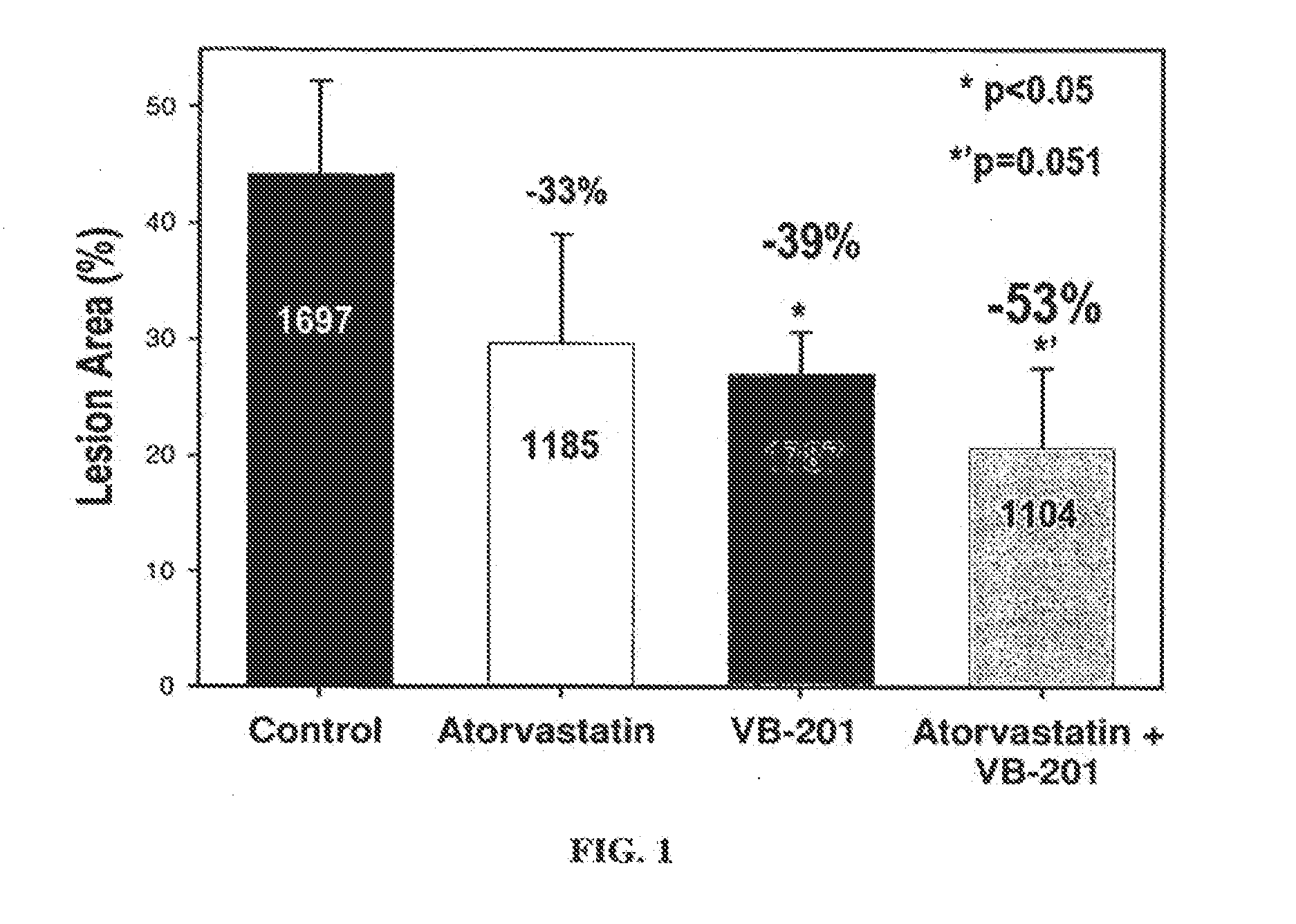
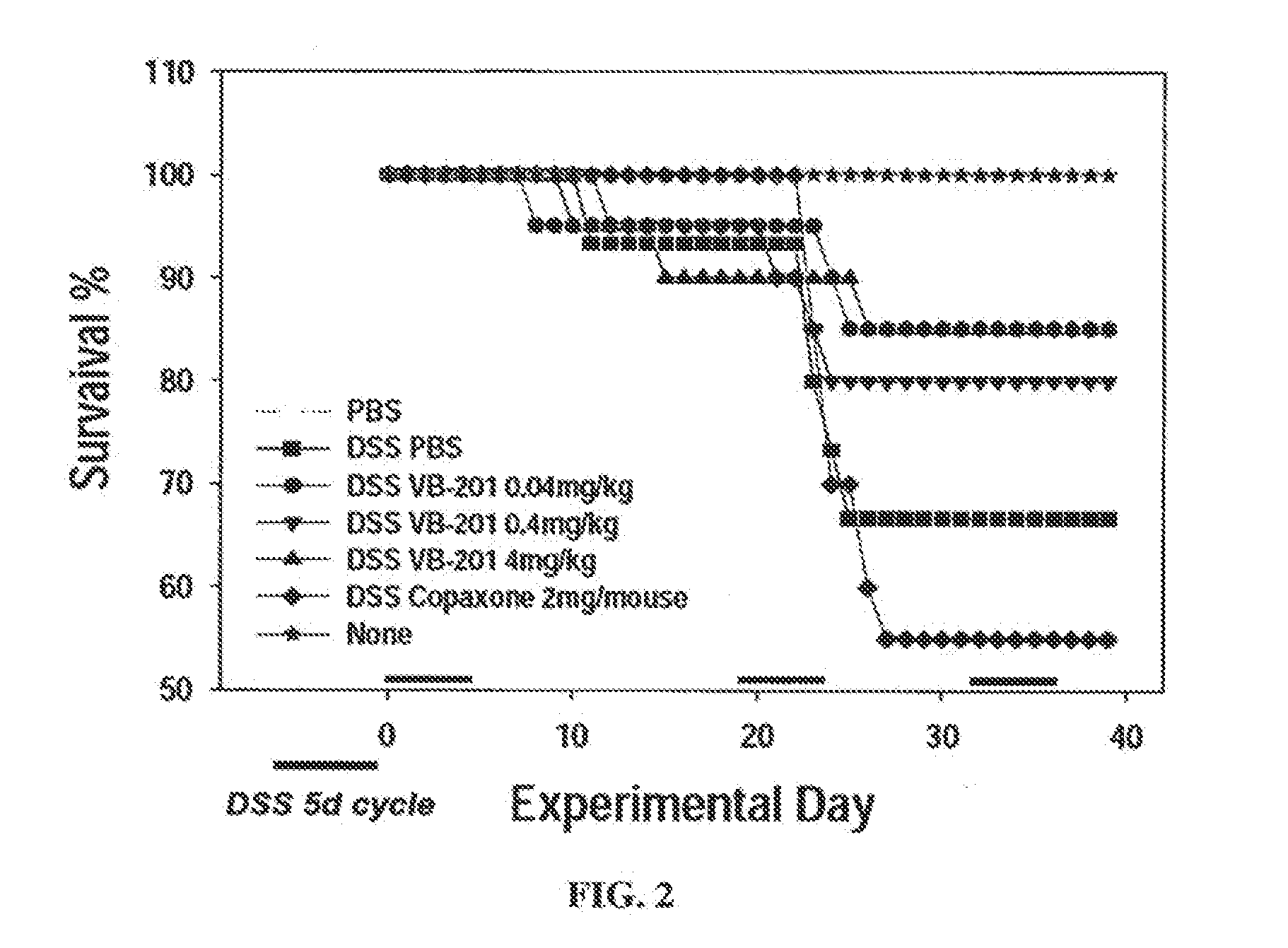
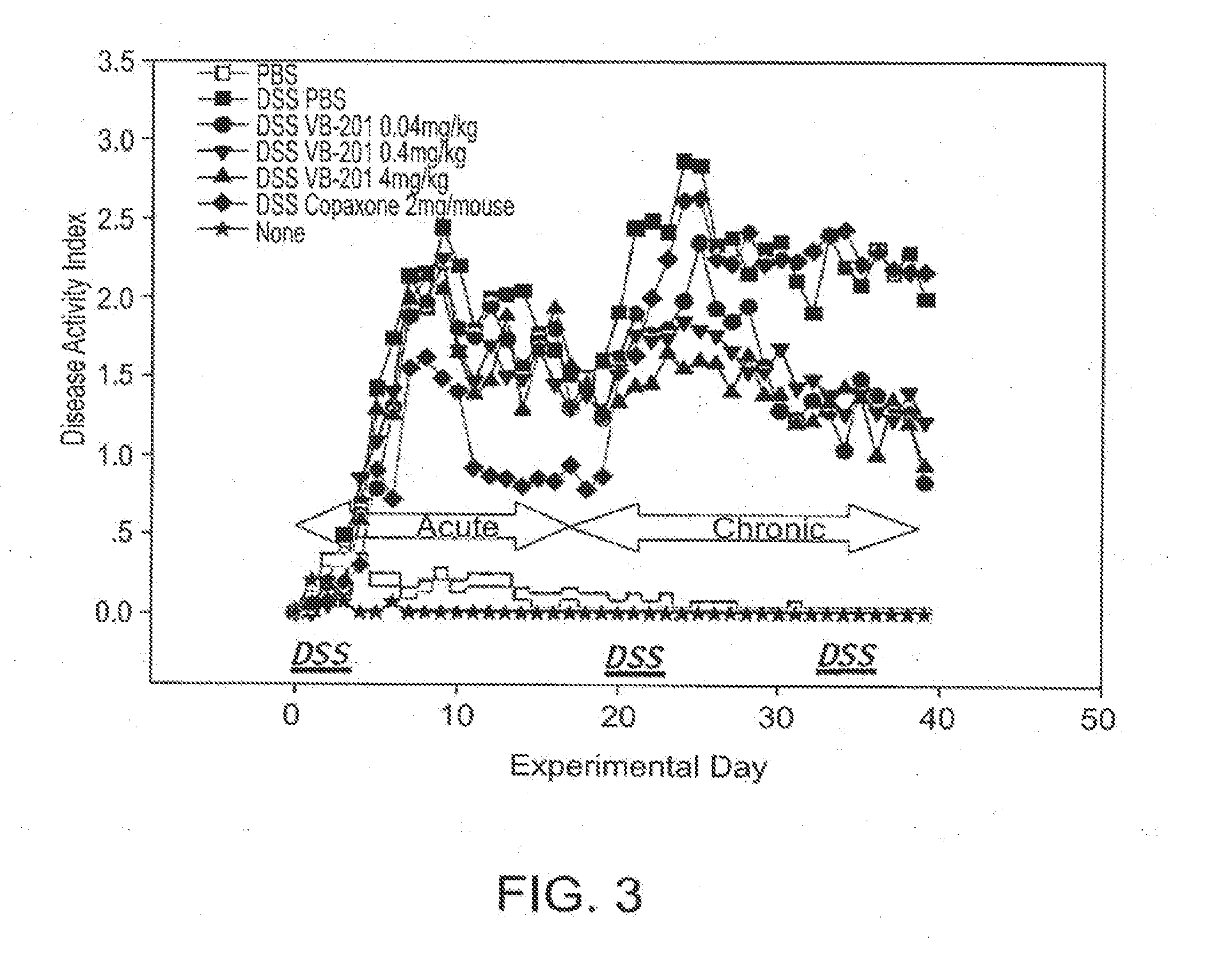
Combined Treatment Utilizing VB-201
Methods of treatment which utilize co-administration of the oxidized lipid VB-201 with an additional therapeutically active agent are described herein. Methods of treating a cardiovascular disease are described herein, comprising co-administration of VB-201 and a statin to a subject who is not fully responsive to the statin, as well as methods of treating an inflammatory disease or disorder, comprising co-administration of VB-201 and glatiramer acetate. A pharmaceutical composition comprising VB-201, identified for use in combination with glatiramer acetate, is also described herein. Methods of determining a therapeutically effective amount of VB-201 in a subject and of determining a therapeutically effective amount of VB-201 for co-administration with an additional therapeutically active agent are also described. Novel unit dosage forms of VB-201 and methods utilizing same are also disclosed.
Owner:VASCULAR BIOGENICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com