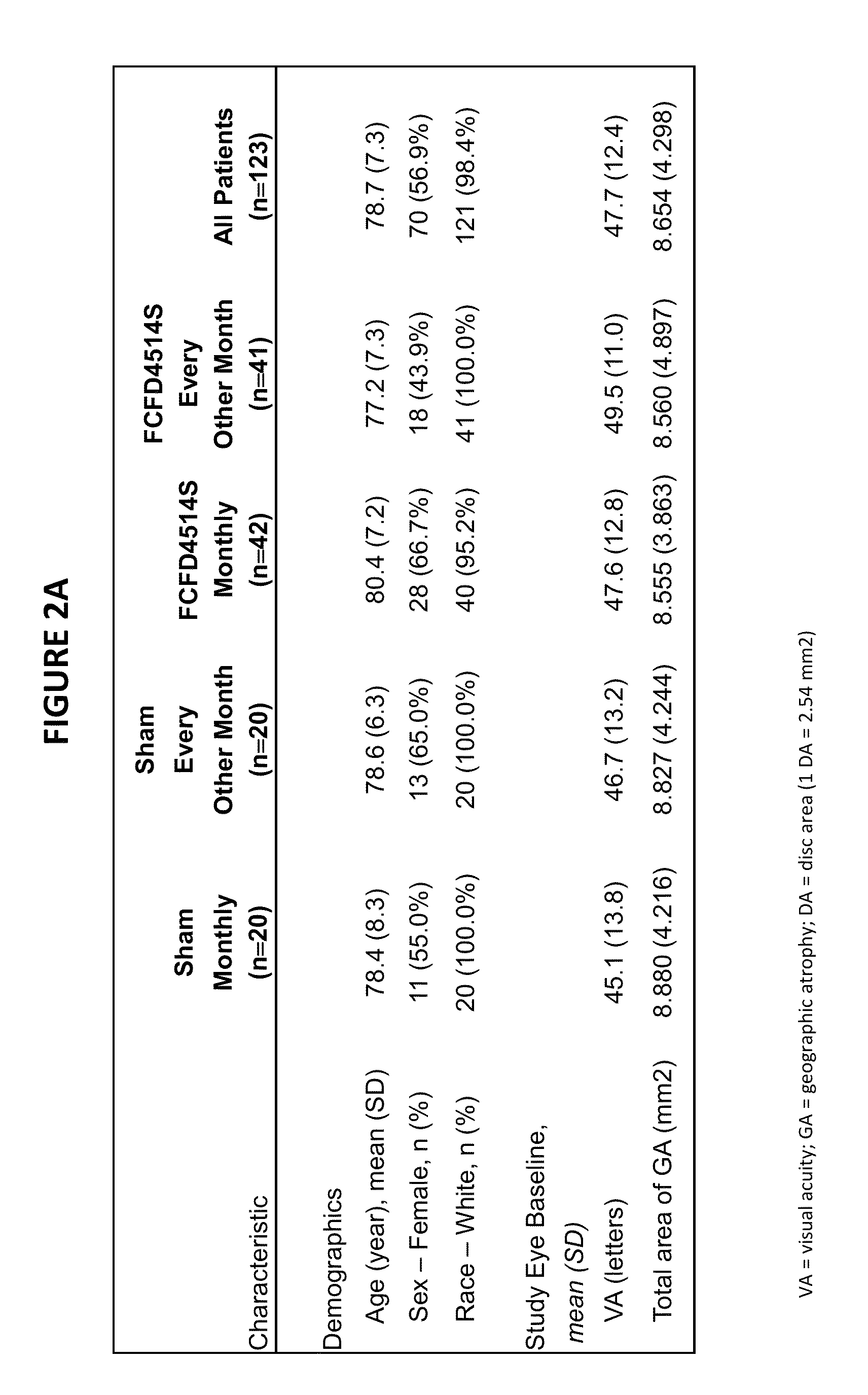
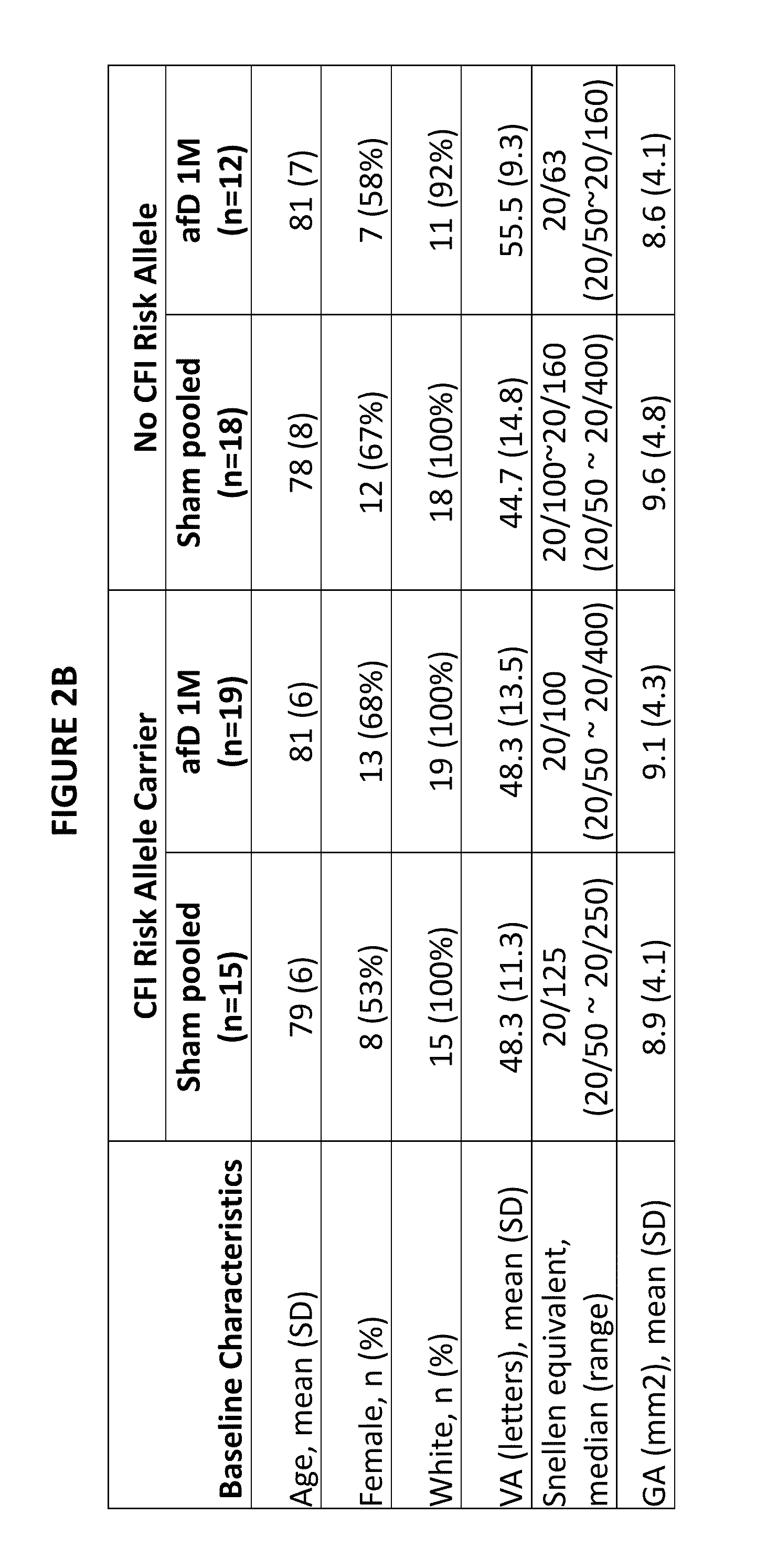
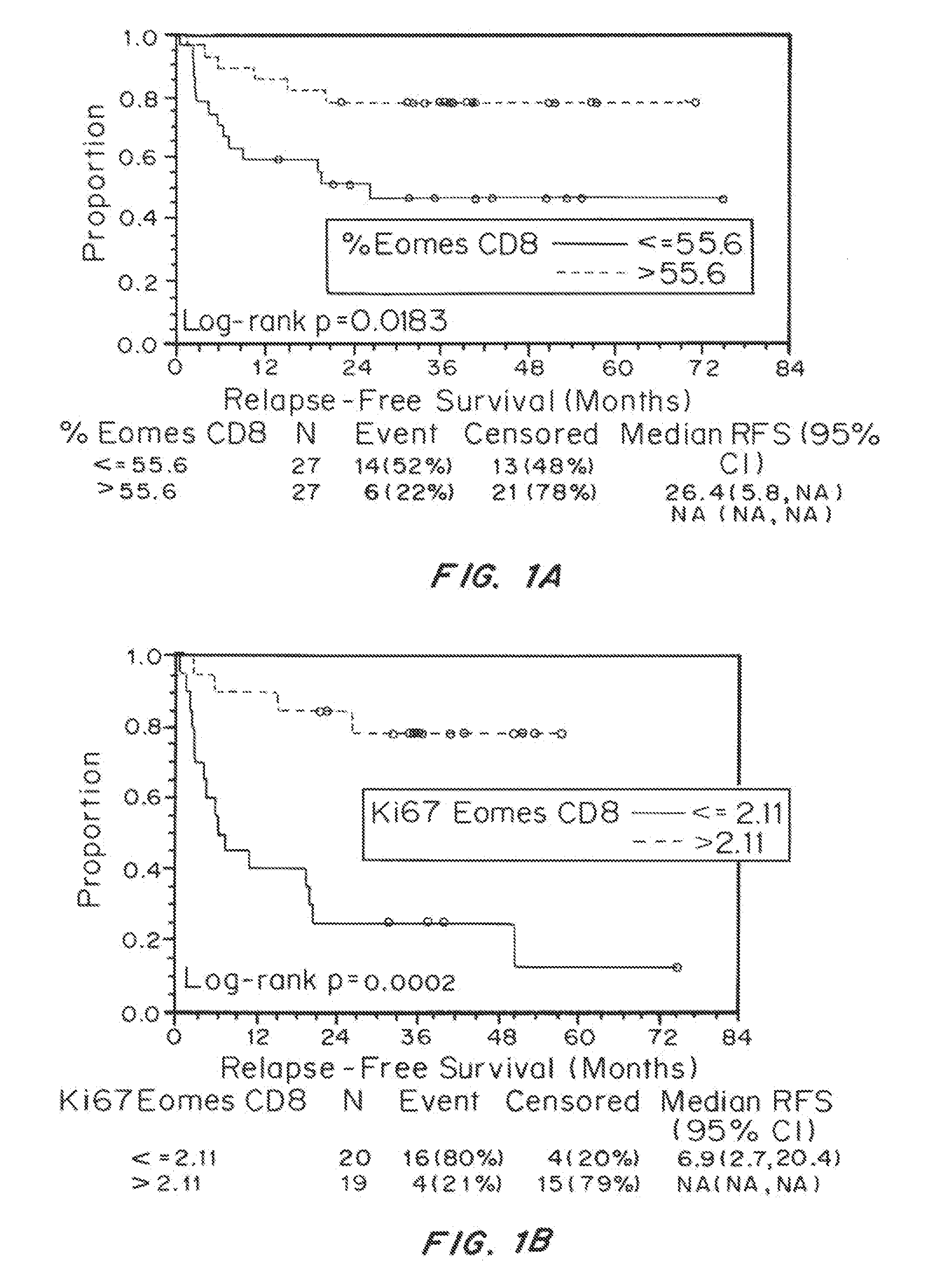
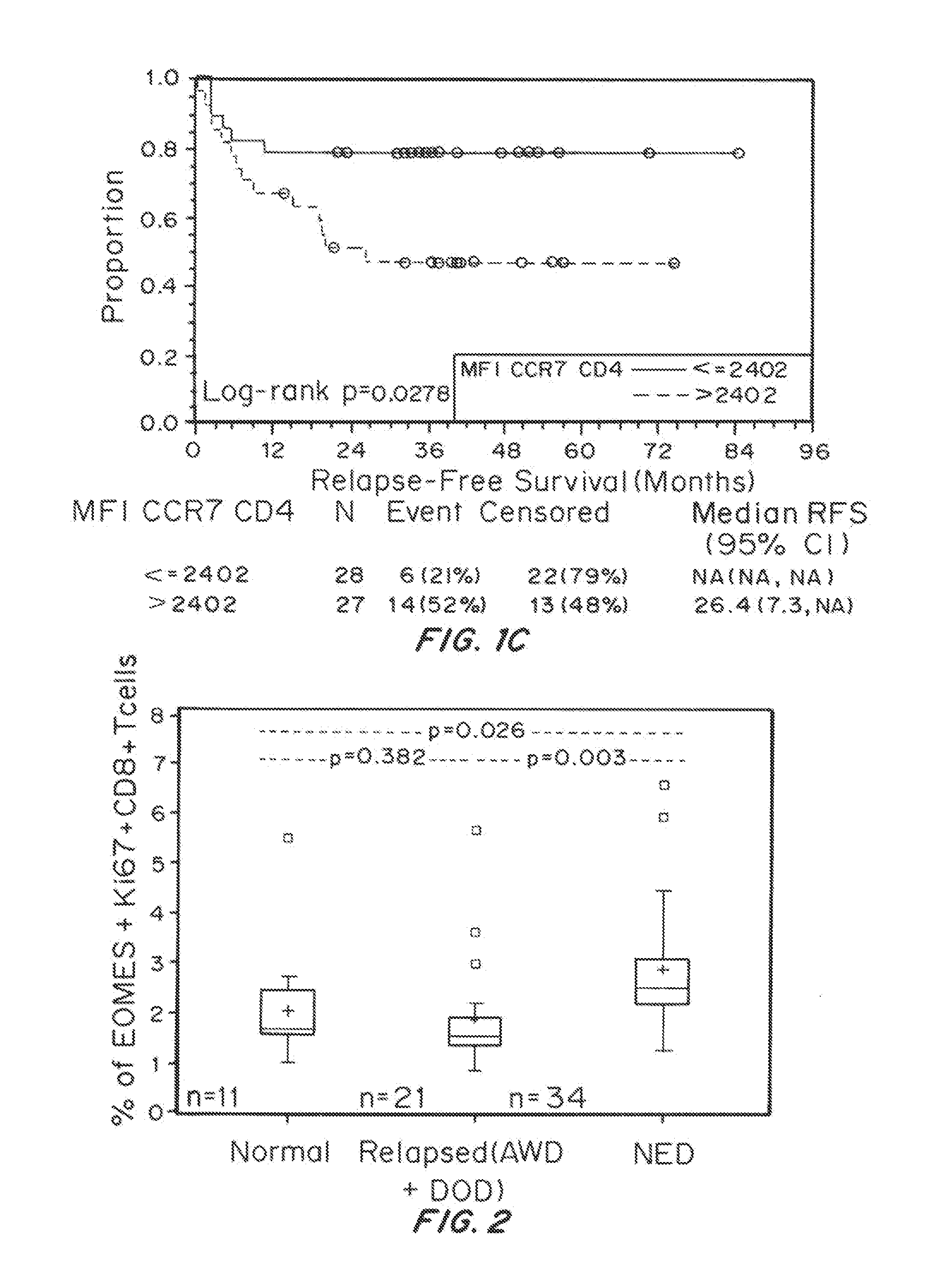
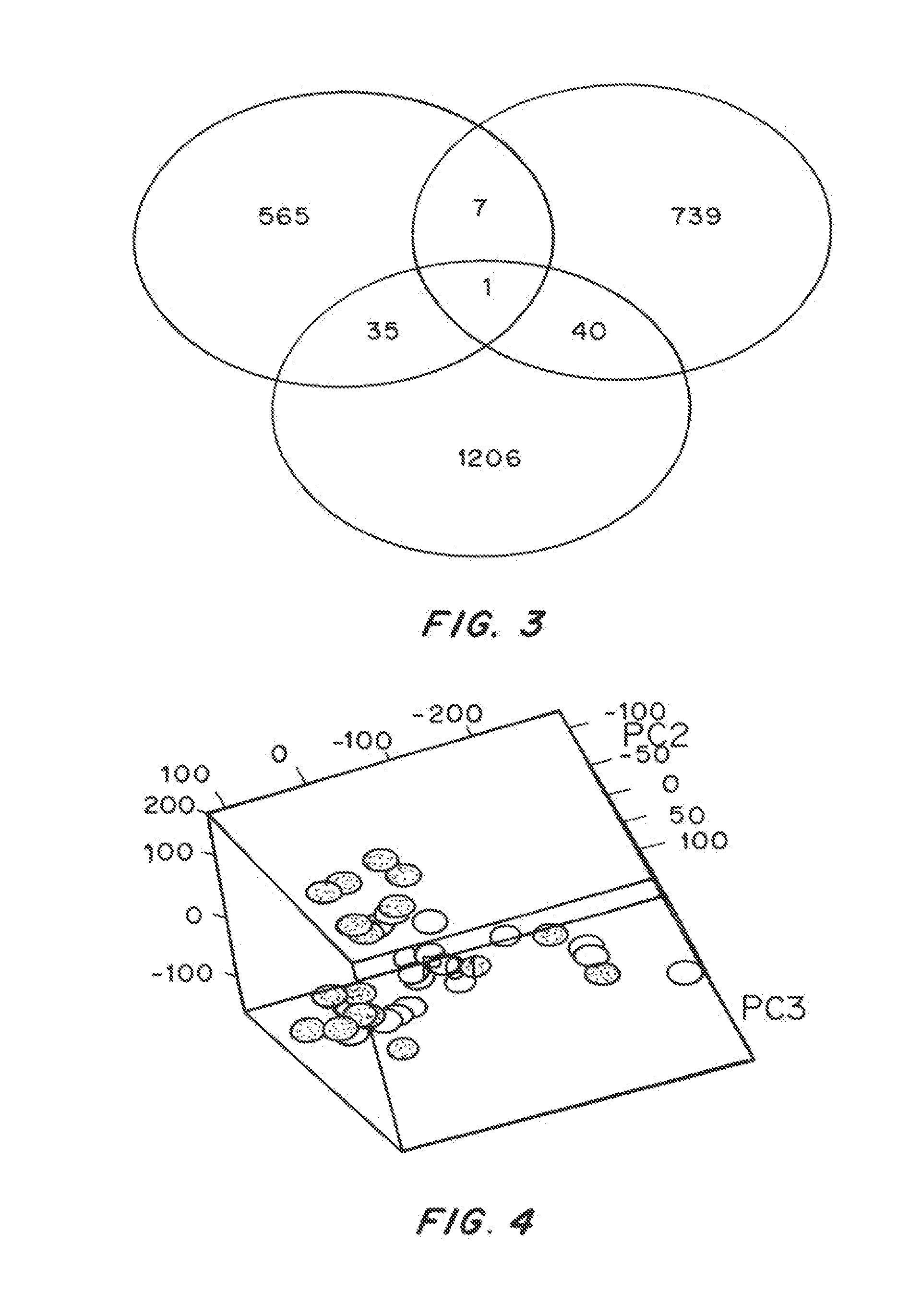
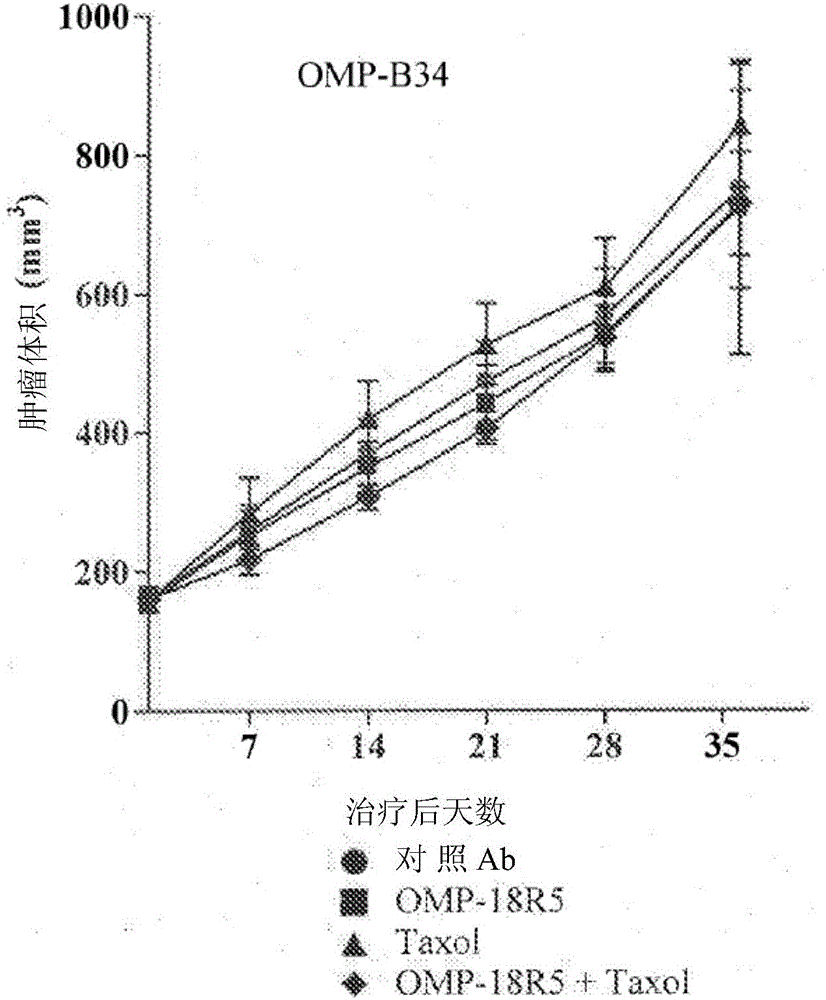
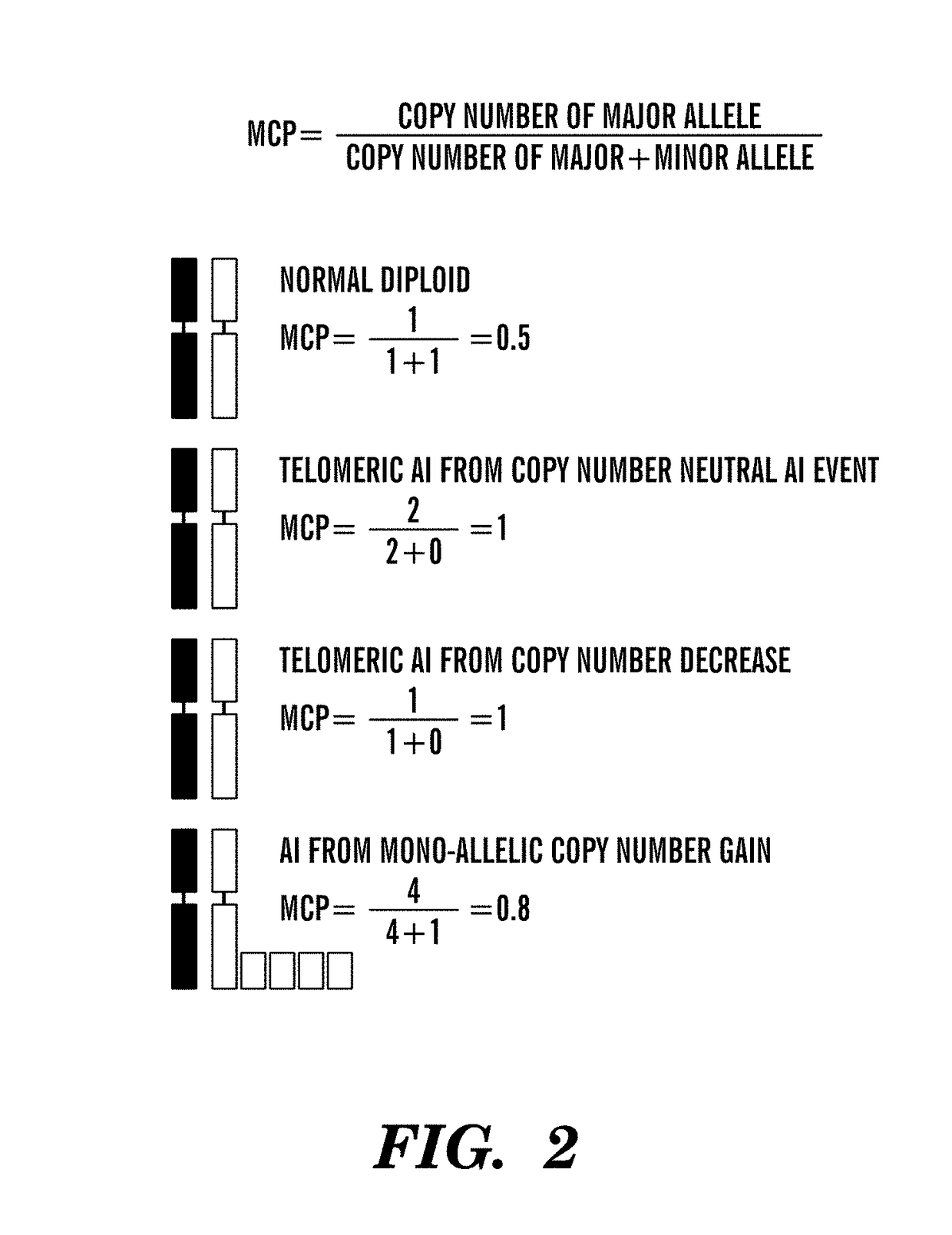
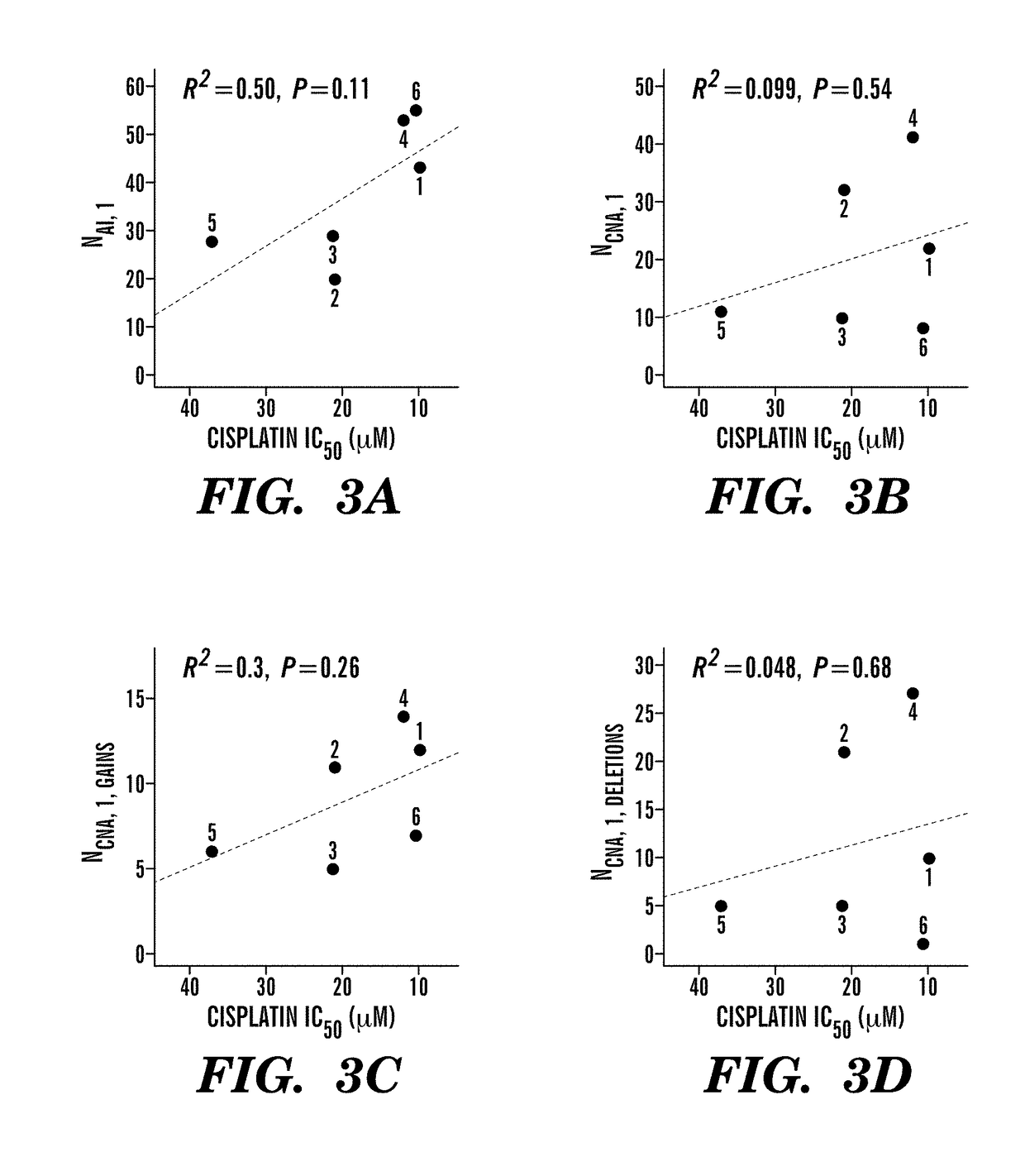
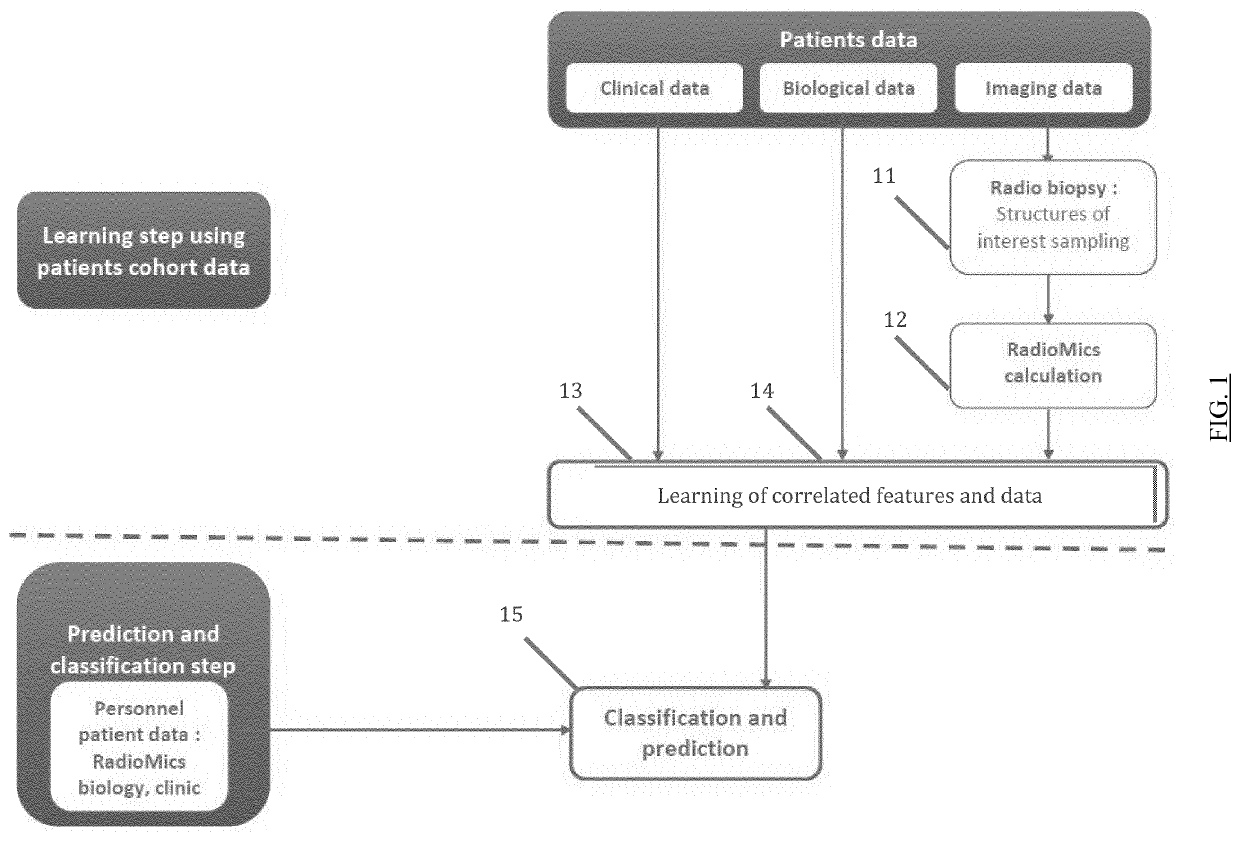
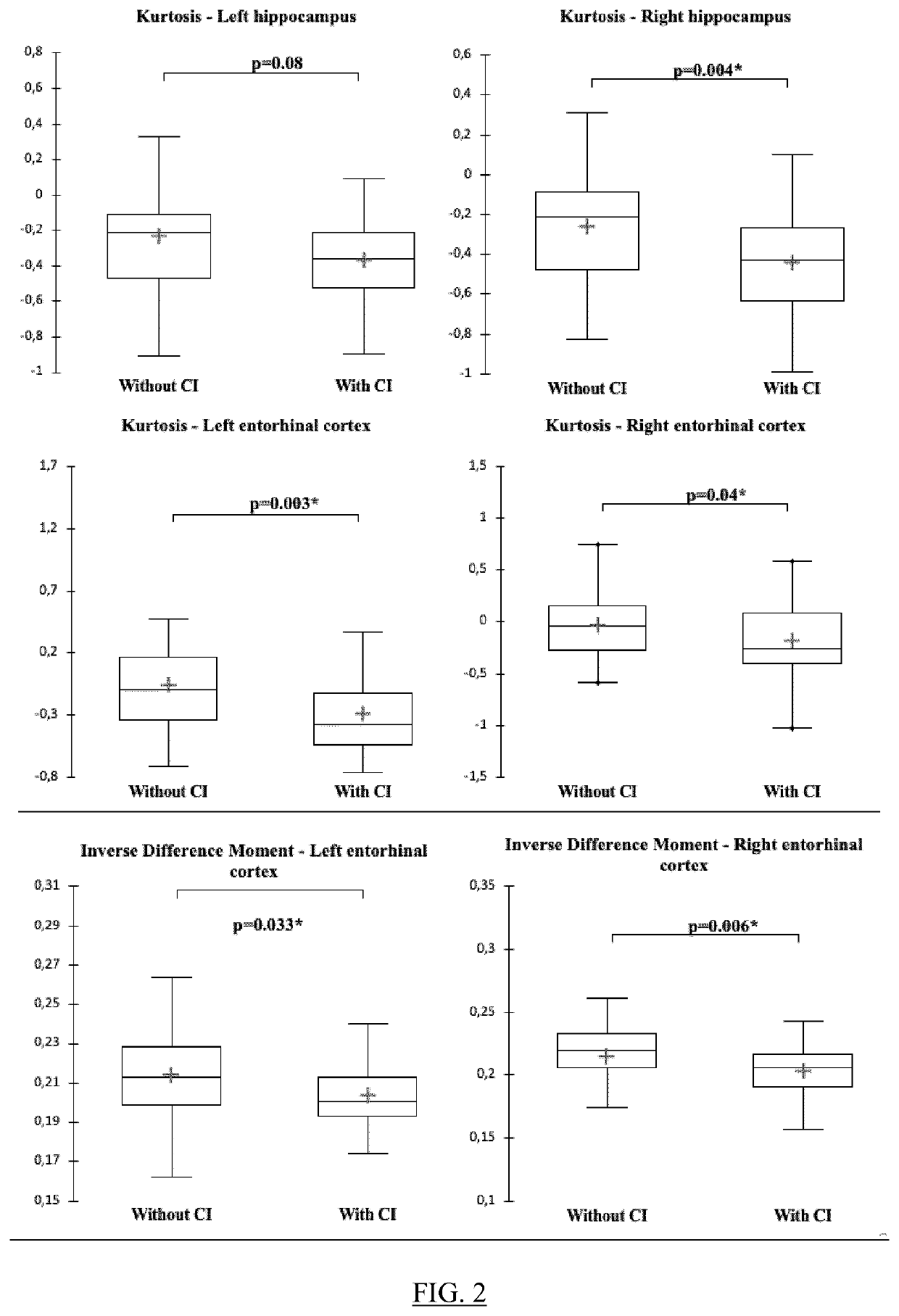
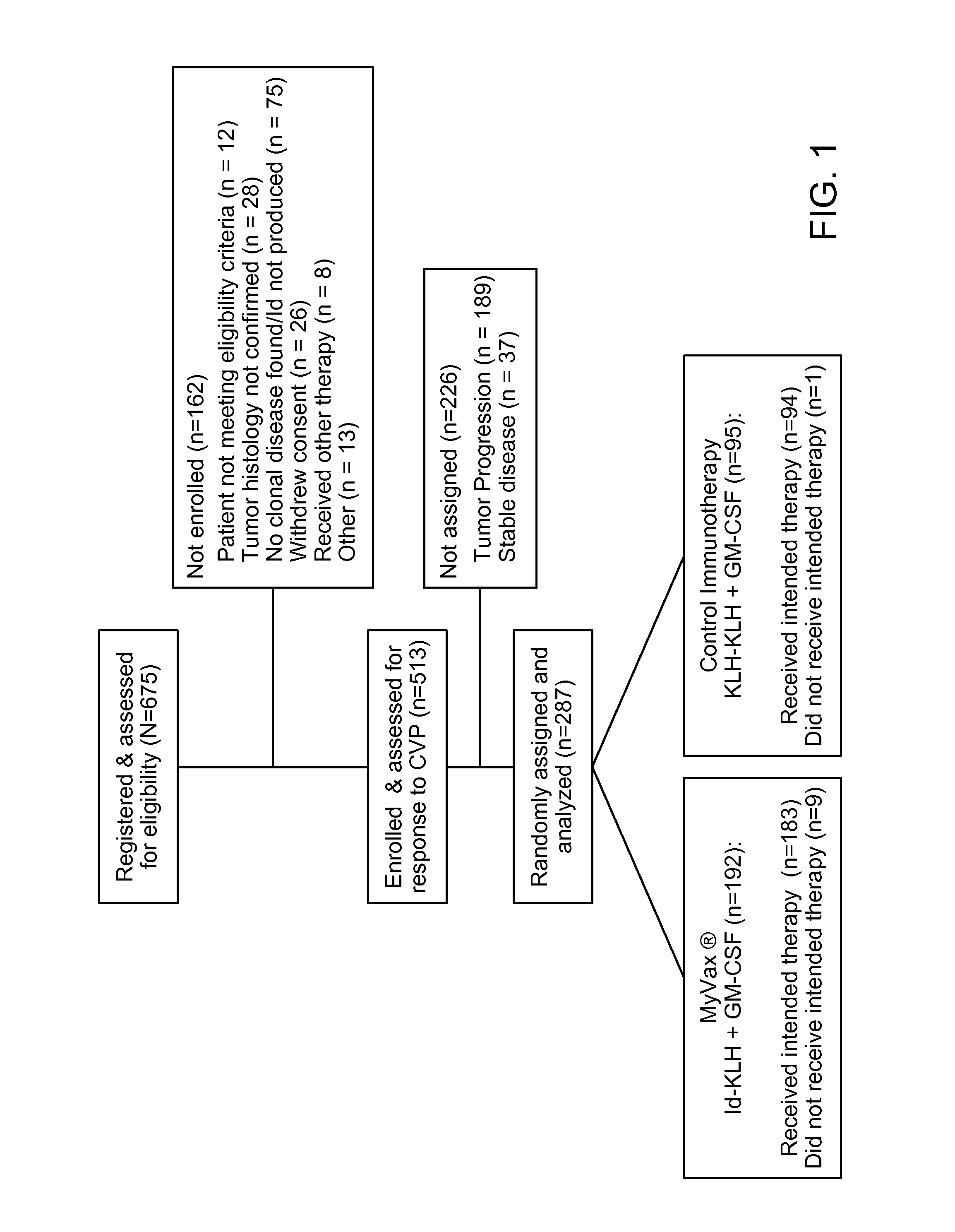
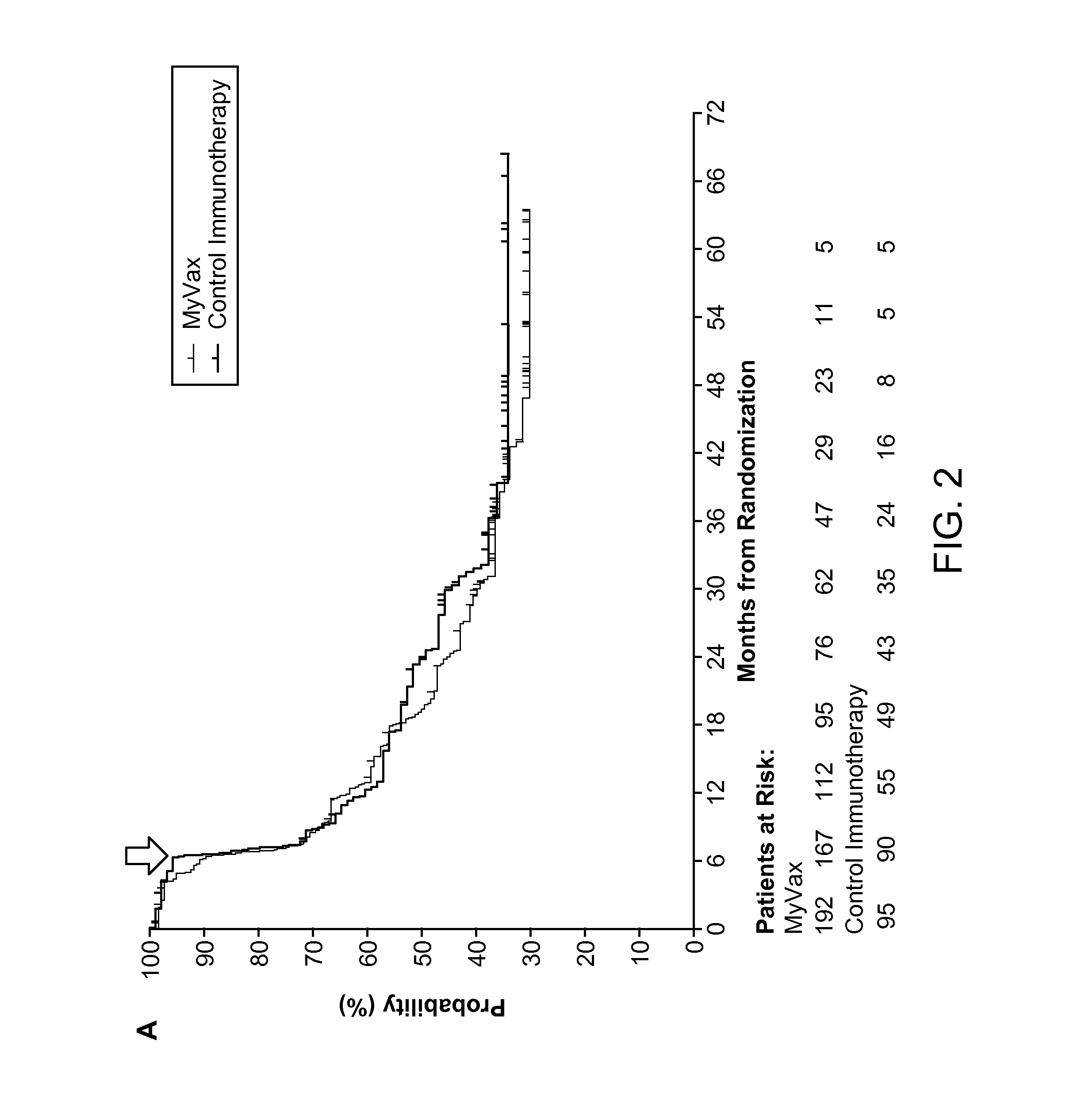
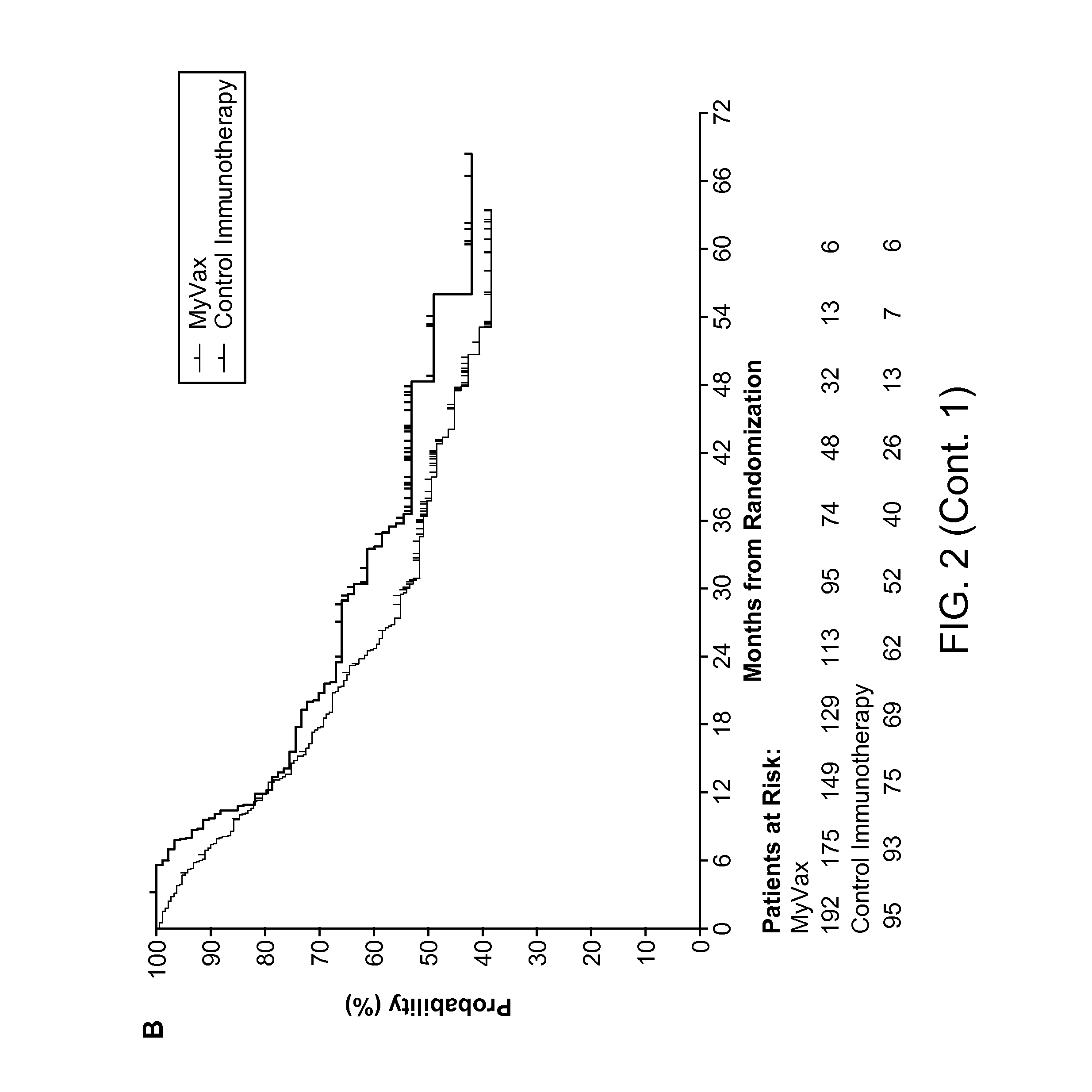
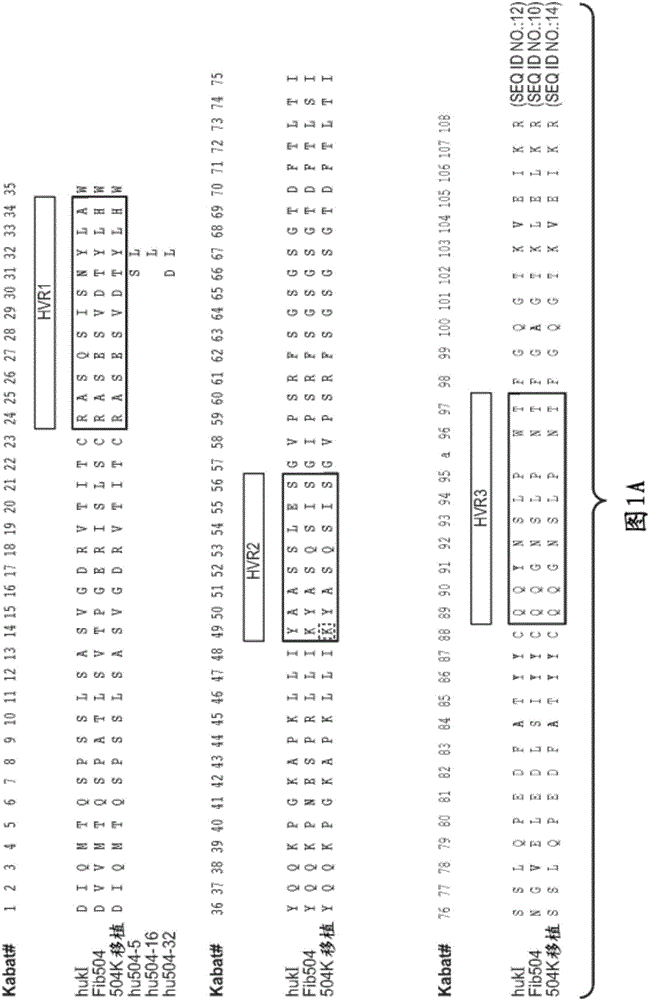
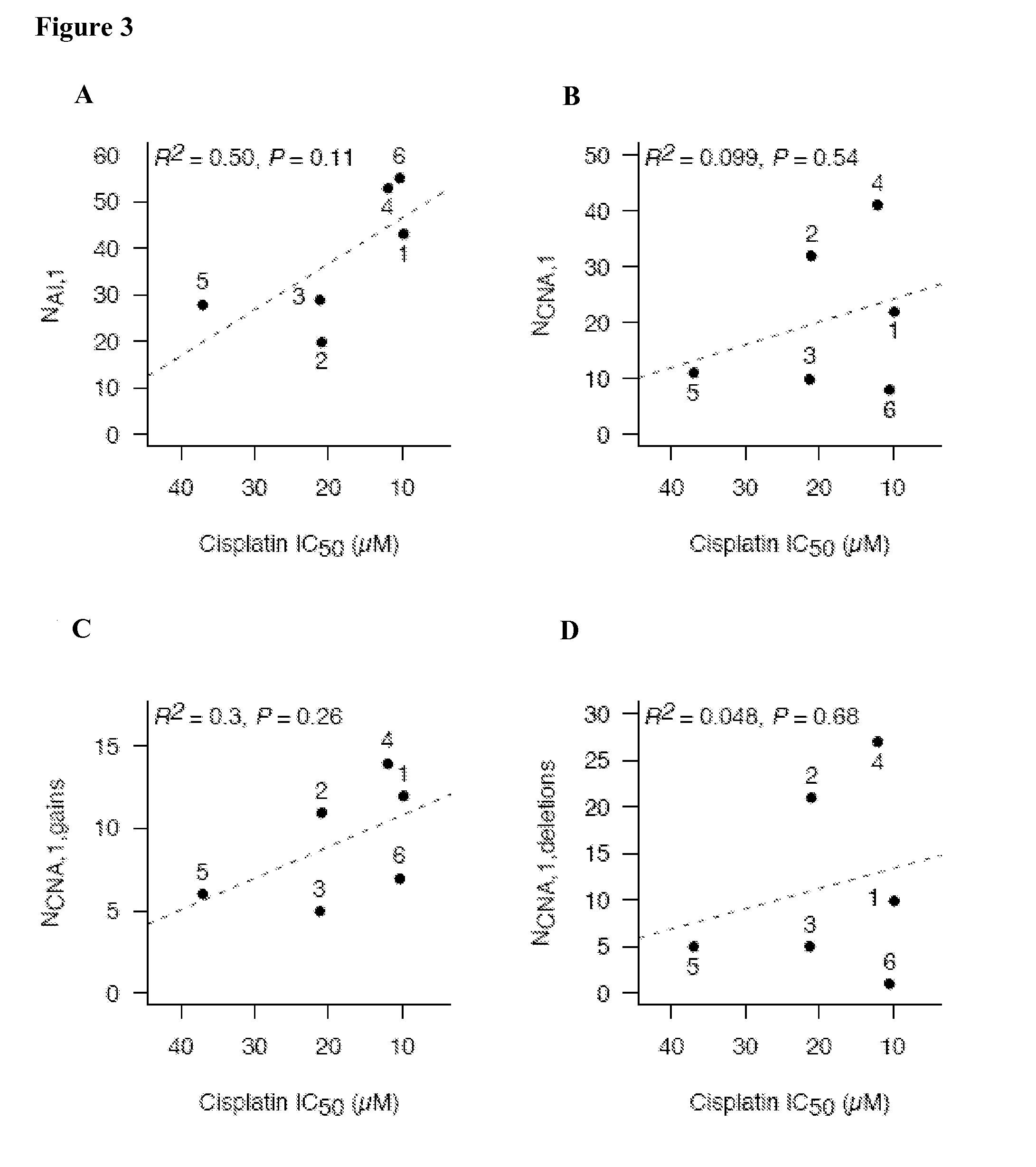Patents
Literature
114 results about "Predictive biomarker" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Predictive Biomarkers. Introduction. A predictive biomarker helps determine which patients are most likely to benefit from a specific treatment option. Predictive diagnostics can provide information about how well a treatment is likely to work in a particular patient or about the likelihood of that treatment causing an unwanted side effect.
Predictive biomarkers for chronic allograft nephropathy
InactiveUS20100022627A1Organic active ingredientsGenetic material ingredientsTransplant rejectionChronic allograft nephropathy
The invention relates to the analysis and identification of genes that are modulated in transplant rejection. This alteration of gene expression provides a molecular signature to accurately detect transplant rejection.
Owner:NOVARTIS AG
Diagnostic Methods Of Multiple Organ Amyloidosis
InactiveUS20080038192A1In-vivo radioactive preparationsMicrobiological testing/measurementElevated C-reactive proteinPredictive biomarker
Described are methods of assessing whether a subject has or is at risk of having multiple organ amyloidosis (MOA) The method includes detecting a diagnostically predictive collection of biomarkers of multiple organ amyloidosis, wherein the detection of a diagnostically predictive collection of biomarkers indicates the subject has or is at risk of having multiple organ amyloidosis Also described are methods of monitoring treatment of subjects with multiple organ amyloidosis and evaluating therapeutic compounds Representative biomarkers for use in the methods may be selected from variant serum amyloid A (SAA) allele, elevated SAA level, elevated C-reactive protein (CRP) level, depressed glycosammoglycan (GAG) level, elevated interleukin-18 (IL-18) level, elevated macrophage-colony stimulating factor (M-CSF) level, elevated hepatocyte growth factor (HGF) level, presence of an antibody against citrullmated vimentm (Sa), presence of a monoclonal immunoglobulin light chain, increased serum albumin, and increased creatinine clearance
Owner:NEUROCHEM INT
Predicitive biomarkers in cancer therapy
InactiveUS20070190583A1Increased pretreatment levelMicrobiological testing/measurementIn-vivo testing preparationsApoptosisPredictive biomarker
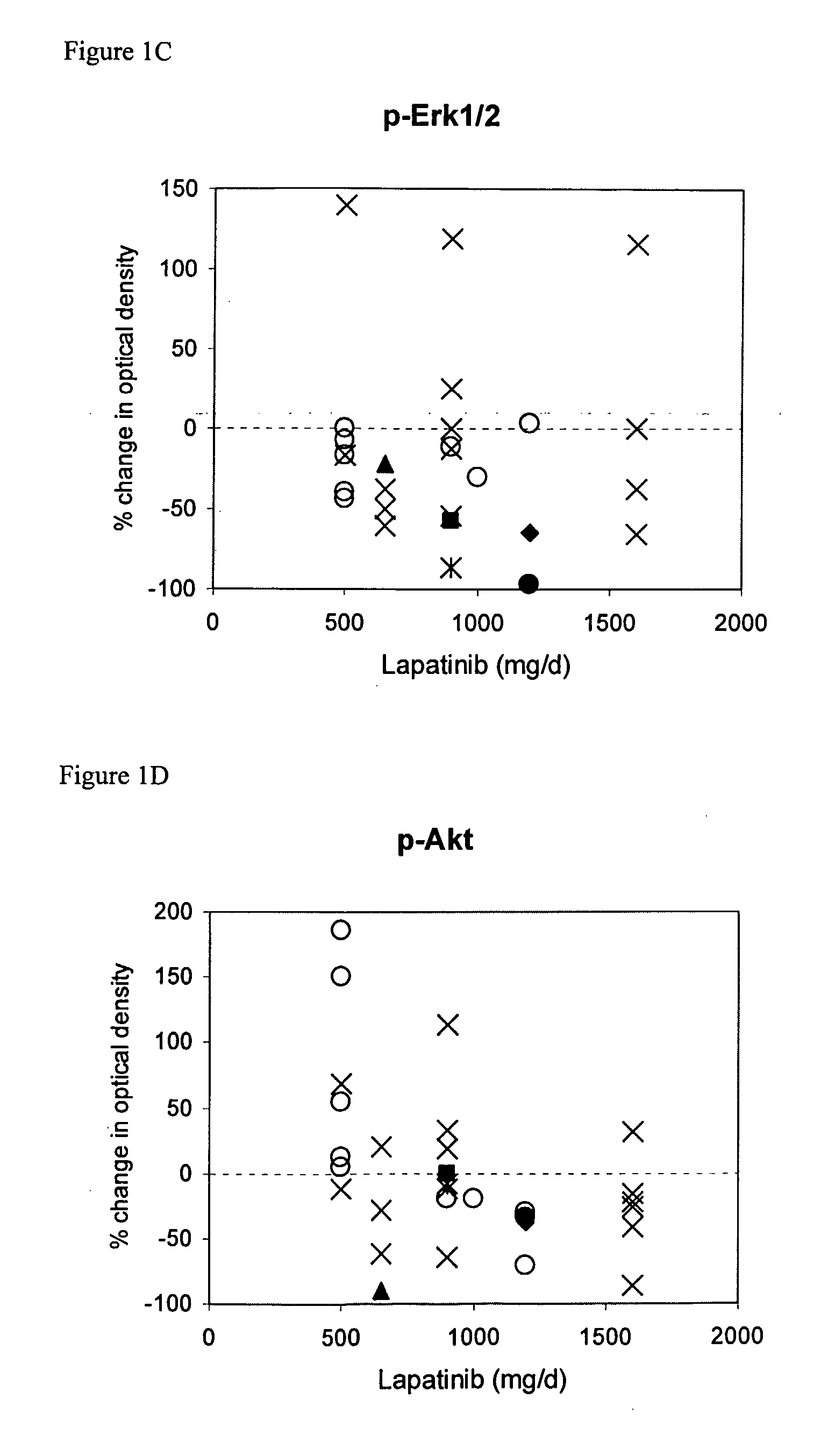
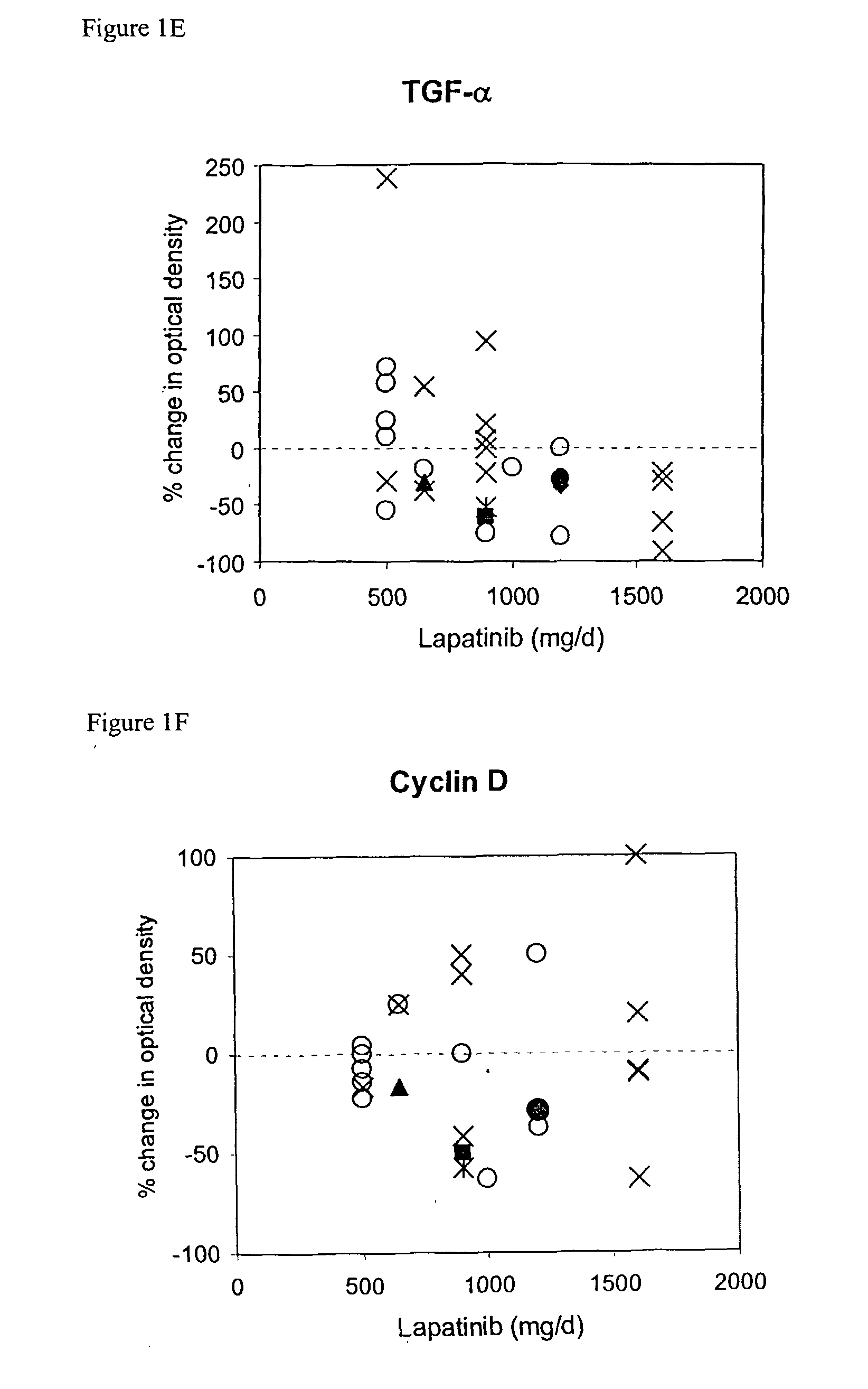
The use of various biomarkers to assess a subject's suitability for treatment with a EGFR / ErbB2 kinase inhibitor for a solid tumor are described. The biomarkers include TGFalpha, pS6, IGF-1R and levels of apoptosis occurring in tumor tissue.
Owner:NOVARTIS AG
Methods for predicting Anti-cancer response
ActiveUS20130281312A1Microbiological testing/measurementLibrary screeningPredictive biomarkerCancer research
The present invention is based, in part, on the identification of novel methods for defining predictive biomarkers of response to anti-cancer drugs.
Owner:DANMARKS TEKNISKE UNIV +3
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
InactiveUS20140322158A1Organic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
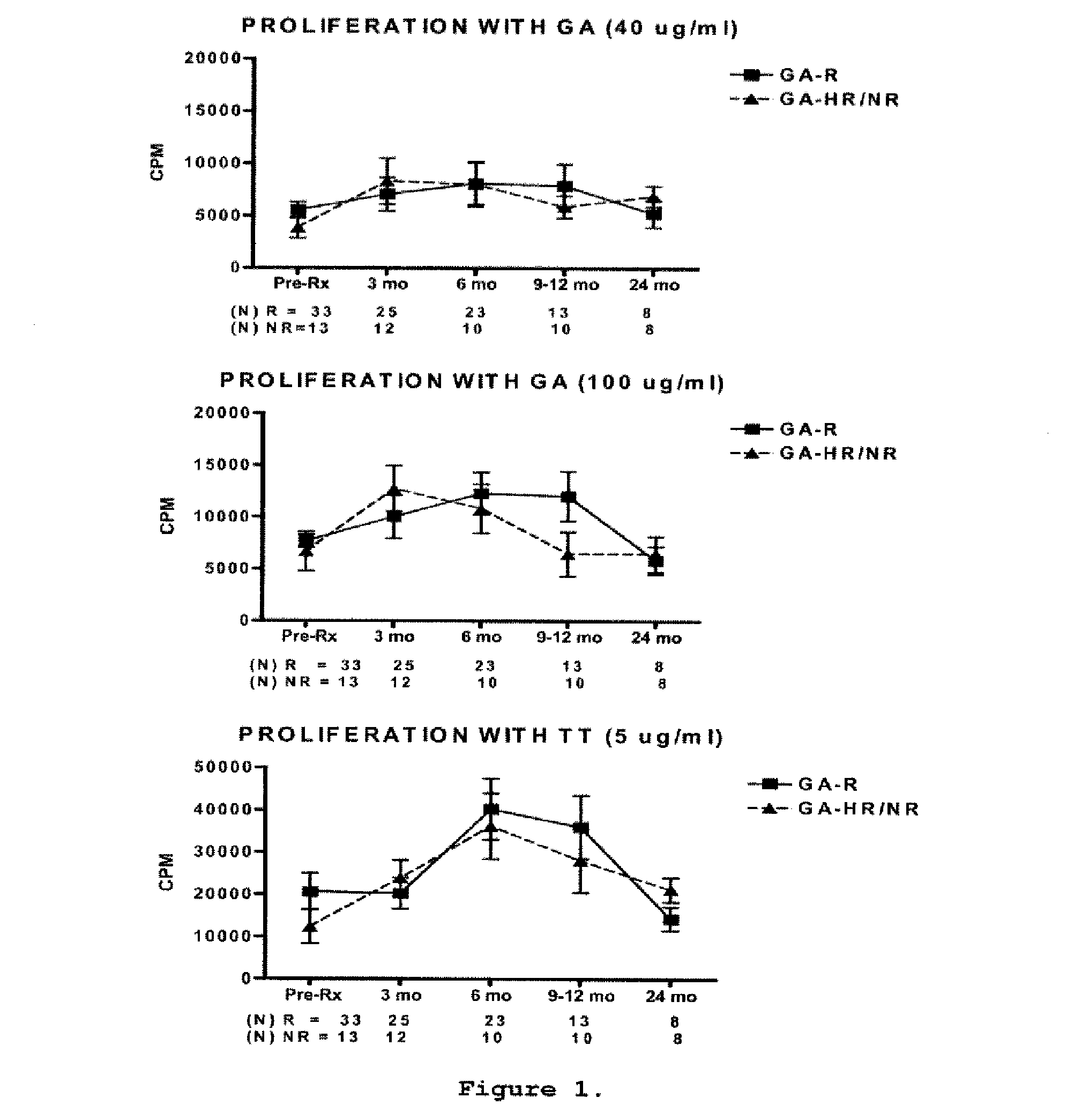
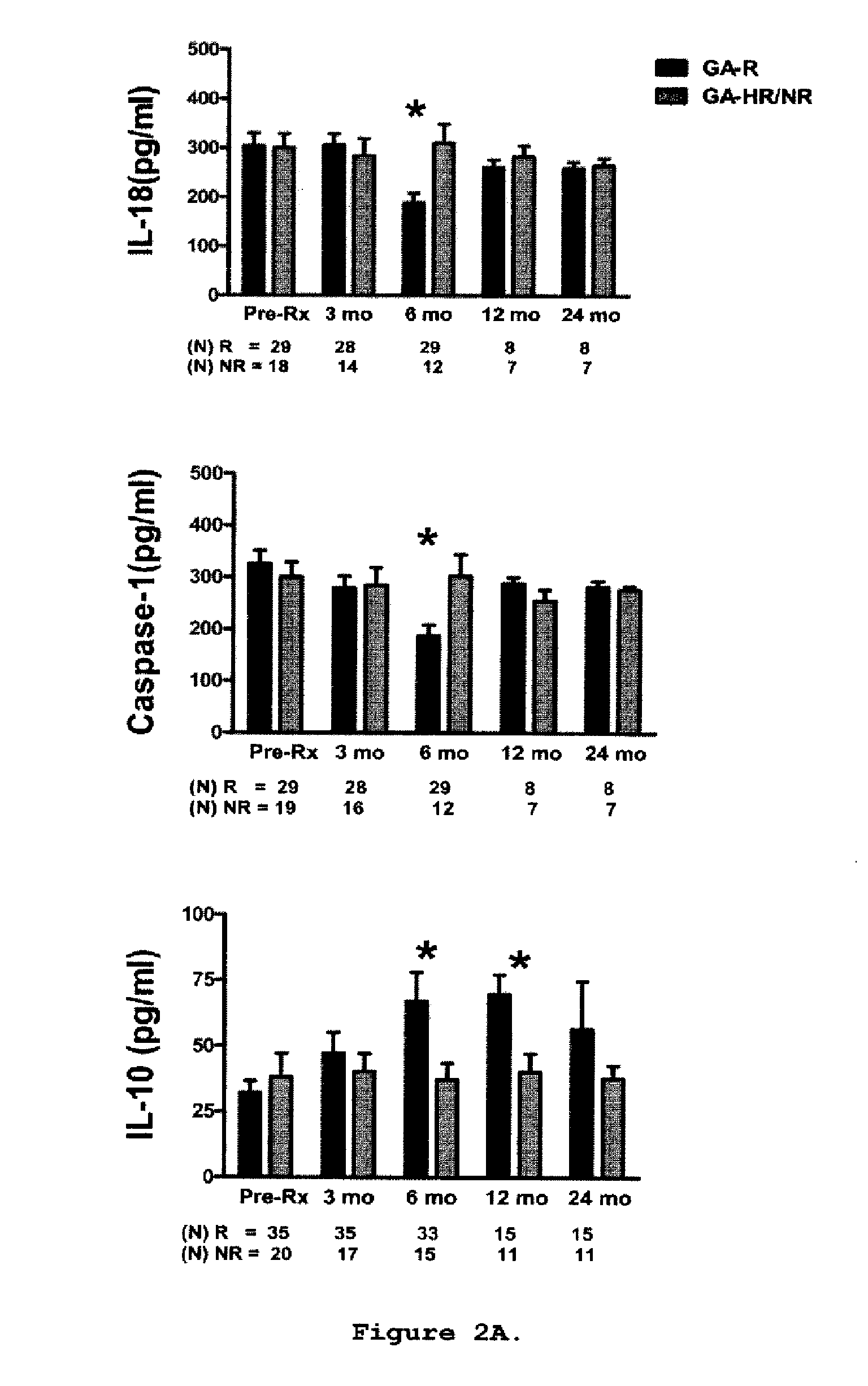
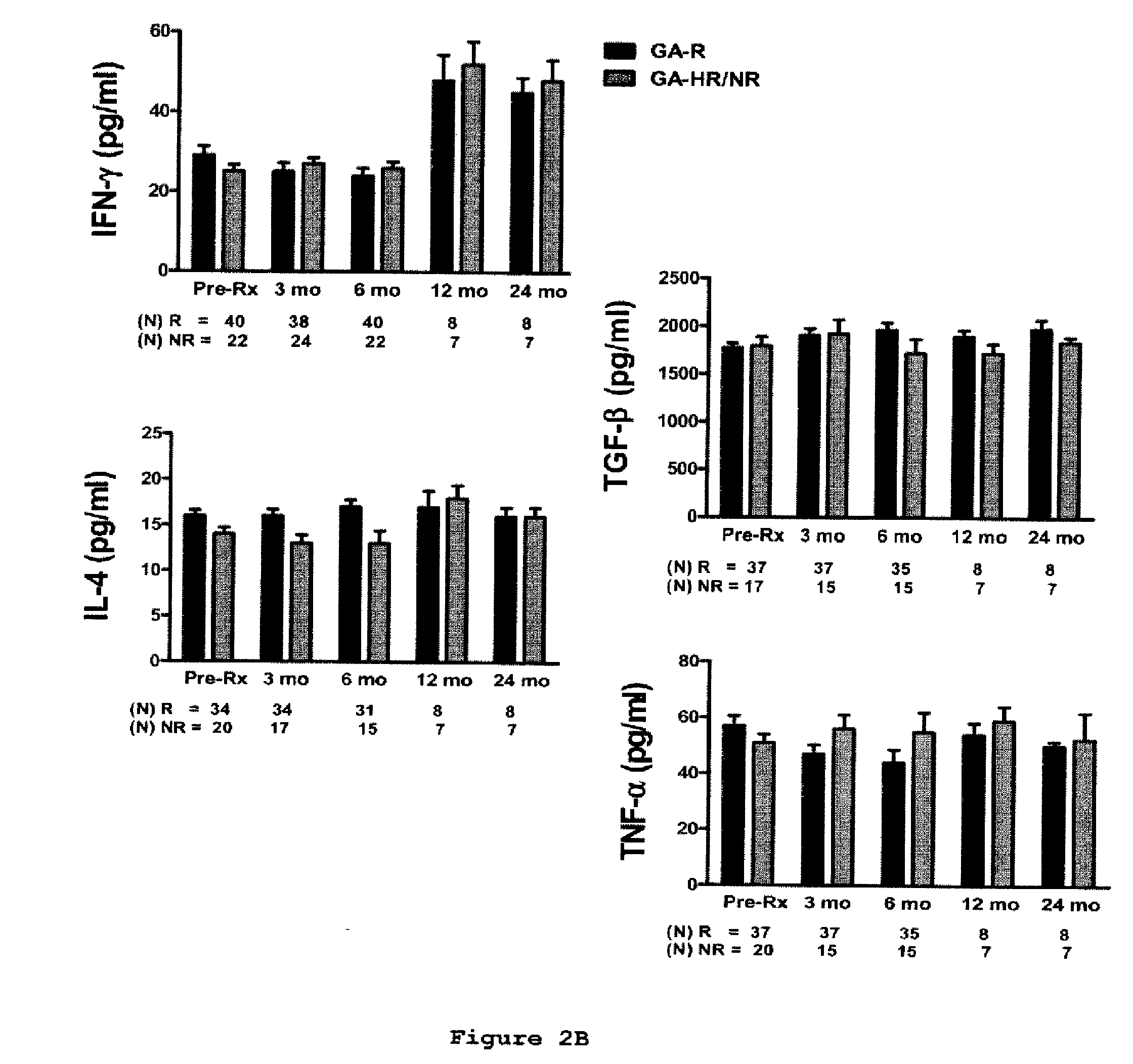
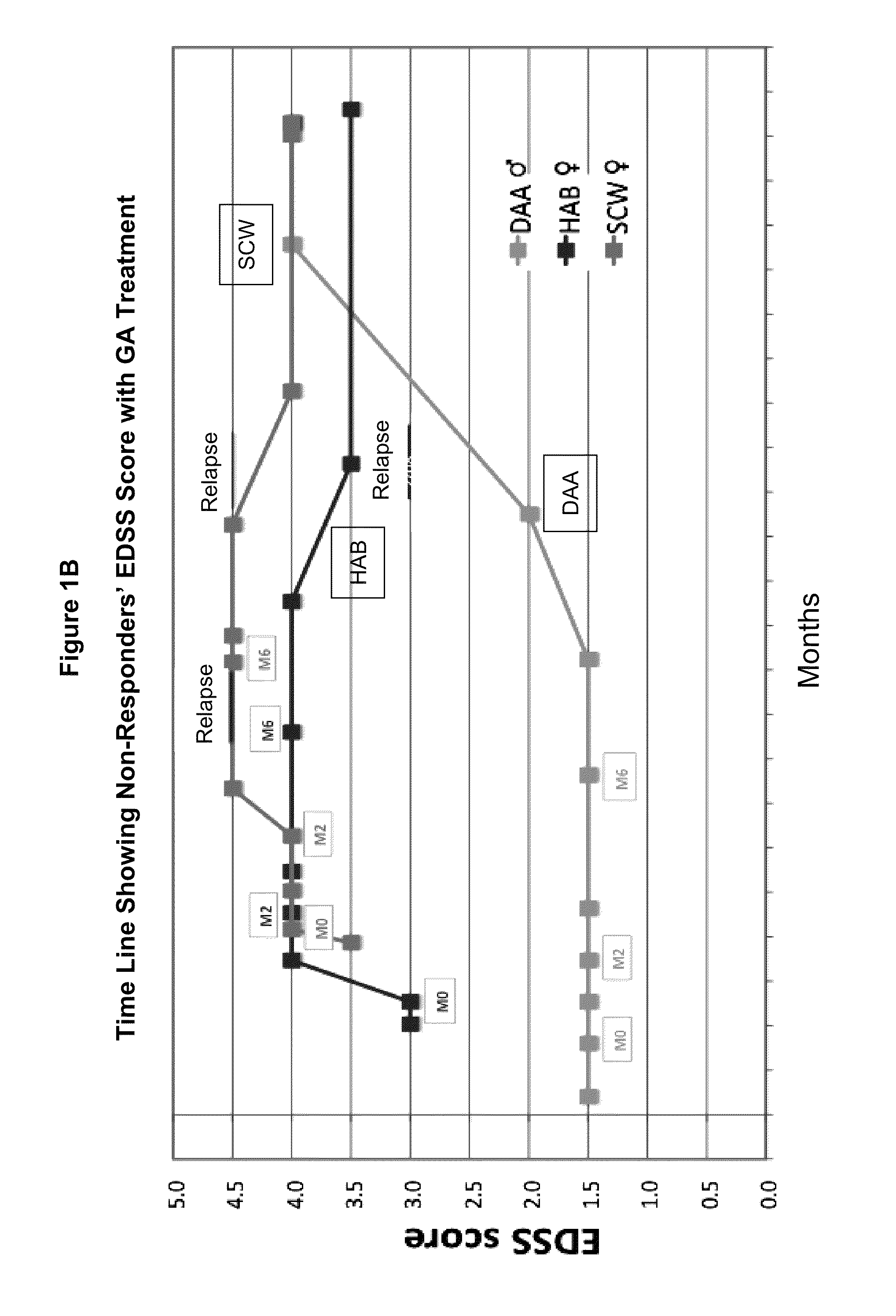
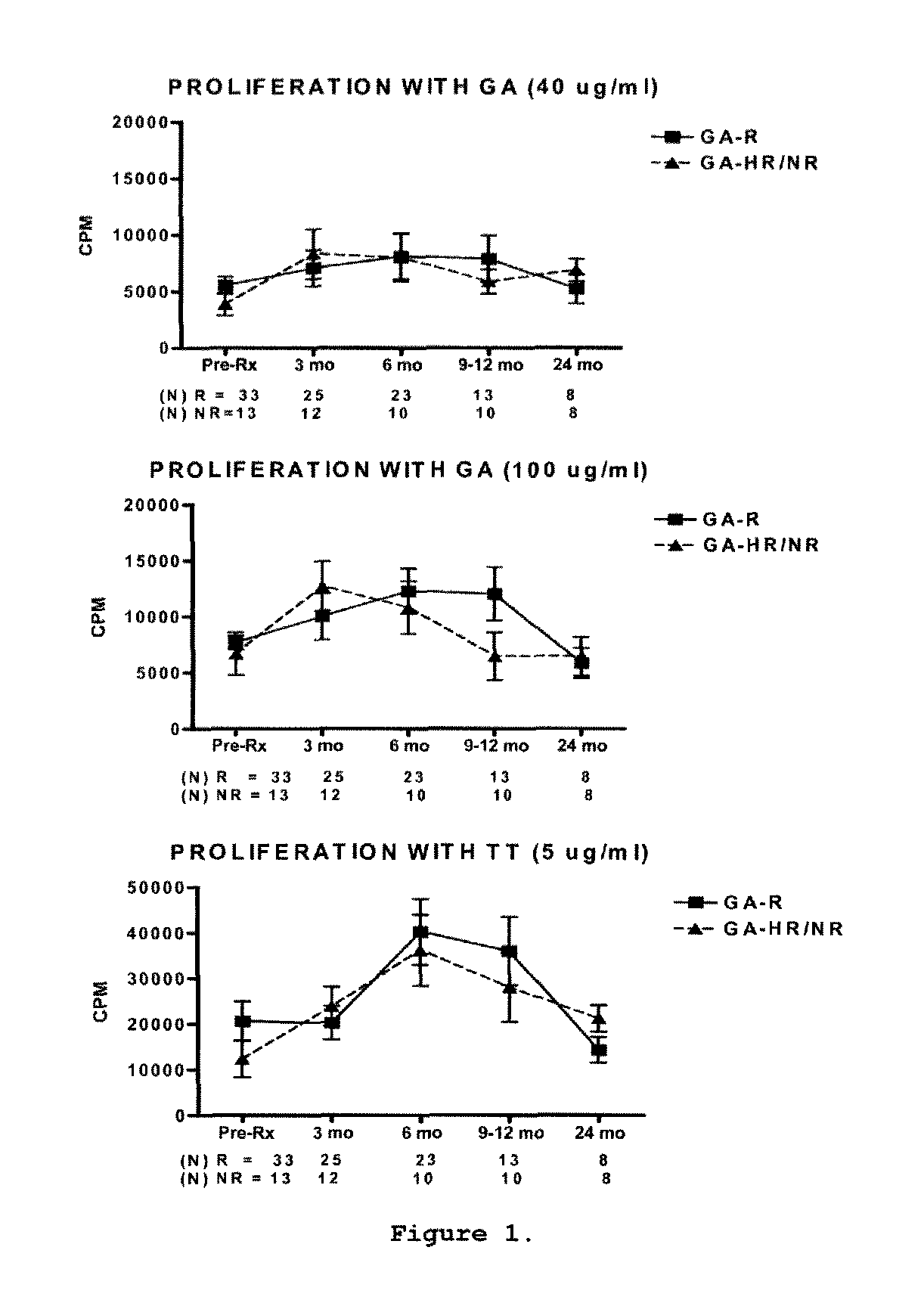
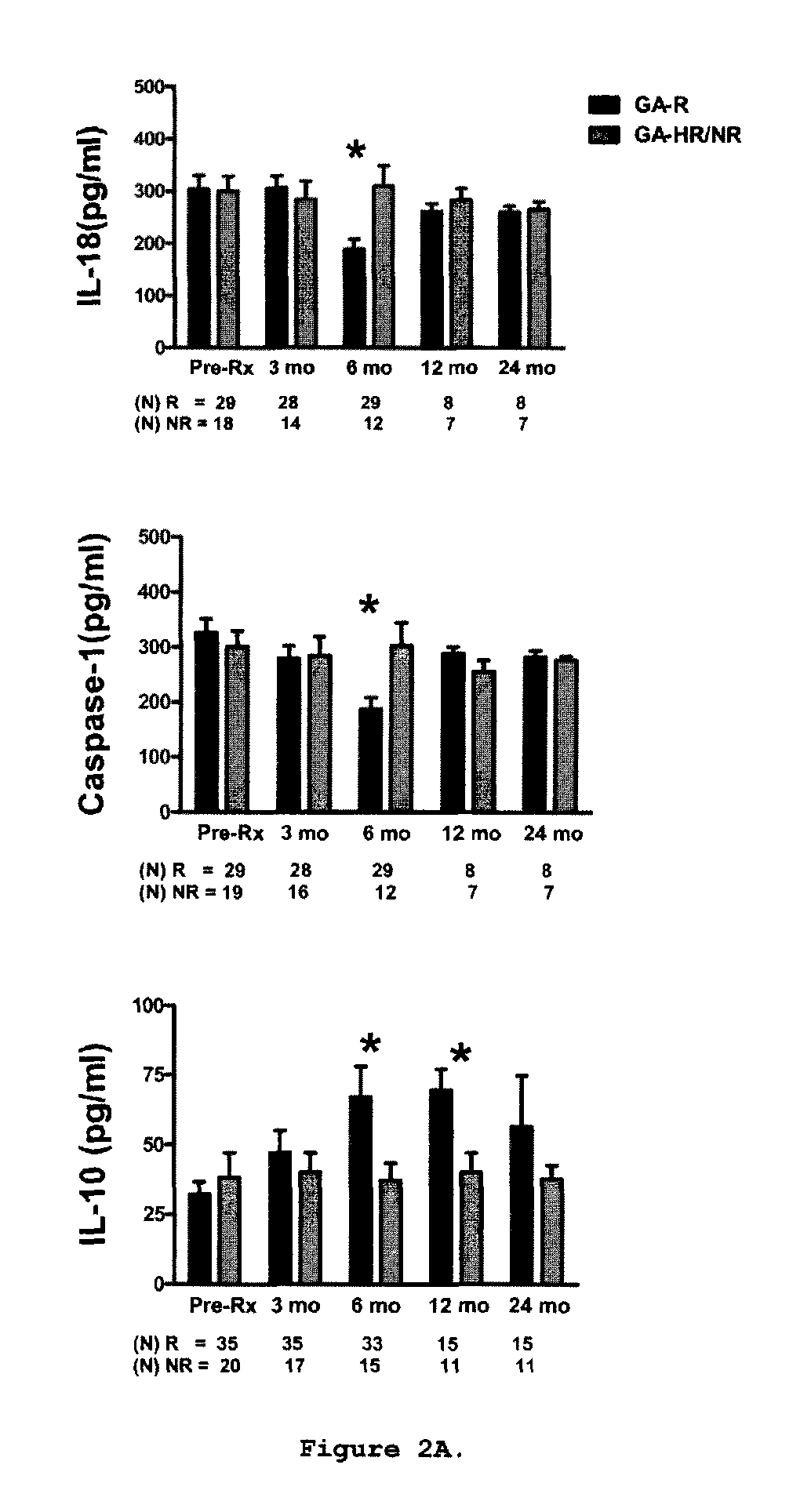
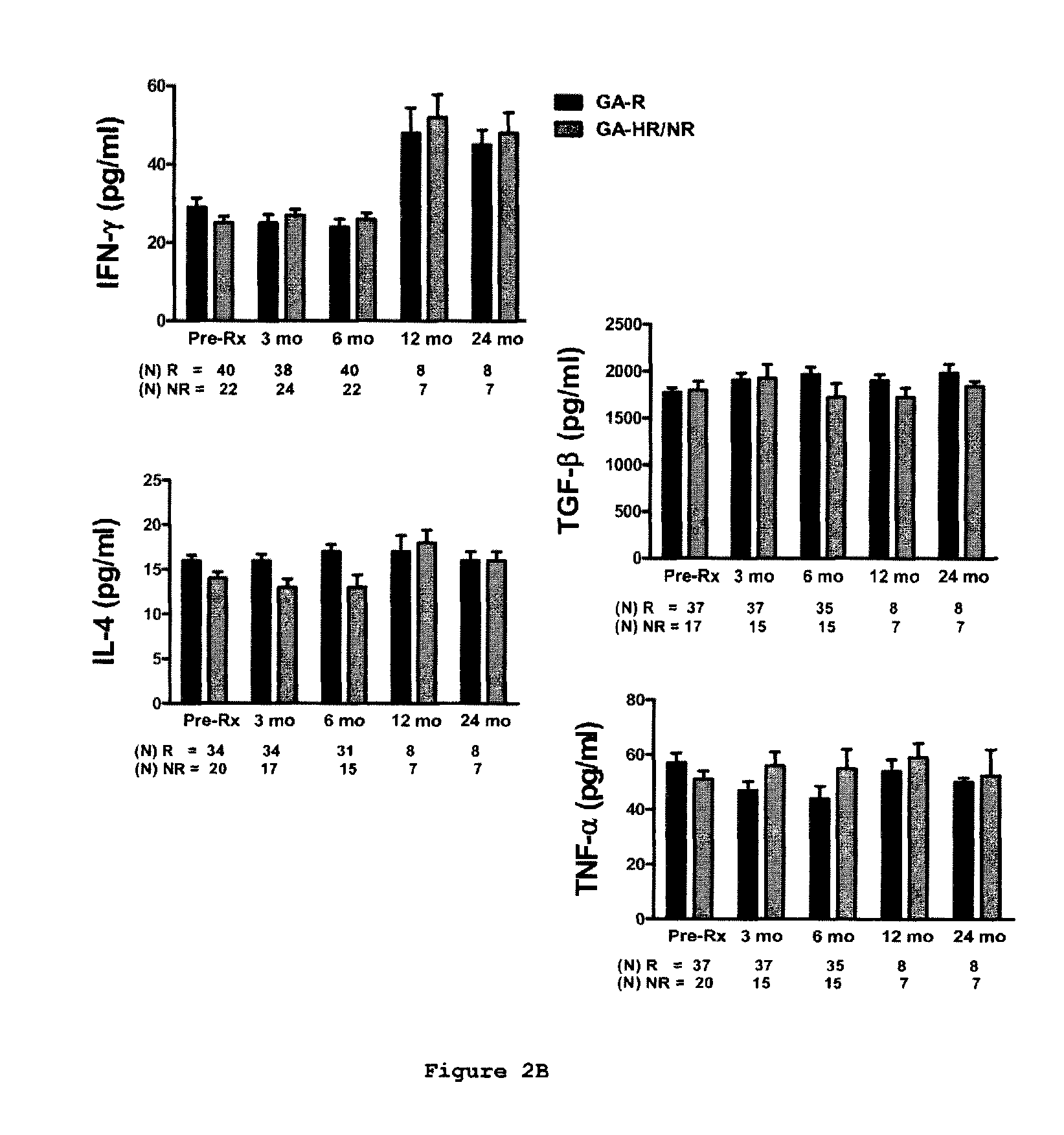
A method for treating a subject afflicted with an autoimmune disease with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of administering a therapeutic amount of the pharmaceutical composition to the subject, determining whether the subject is a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder by measuring the value of a biomarker selected from the group consisting of IL-10 concentration, IL-17 concentration, IL-18 concentration, TNF-α concentration, BDNF concentration, caspase-1 concentration, IL-10 / IL-18 ratio and IL-10 / IL-17 ratio in the blood of the subject, and comparing the measured value to a reference value for the biomarker to identify the subject as a glatiramer acetate responder or a glatiramer acetate hypo- / non-responder, and continuing the administration if the subject is identified as a glatiramer acetate responder, or modifying treatment of the subject if the subject is identified as a glatiramer acetate hypo- / non-responder.
Owner:TEVA PHARMA IND LTD
Predictive diagnostic workflow for tumors using automated dissection, next generation sequencing, and automated slide stainers
PendingUS20180340870A1Microbiological testing/measurementPreparing sample for investigationRegimenMedicine
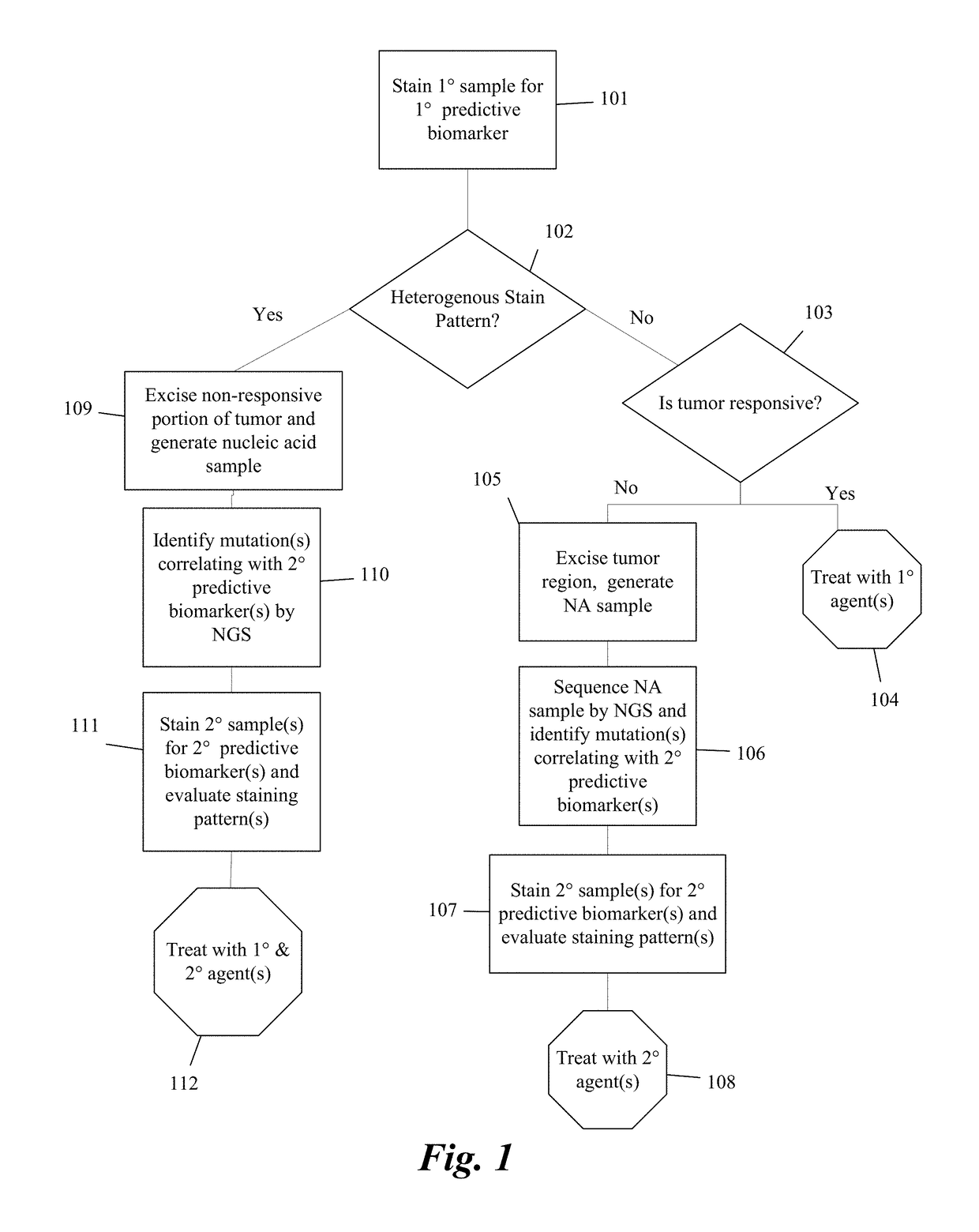
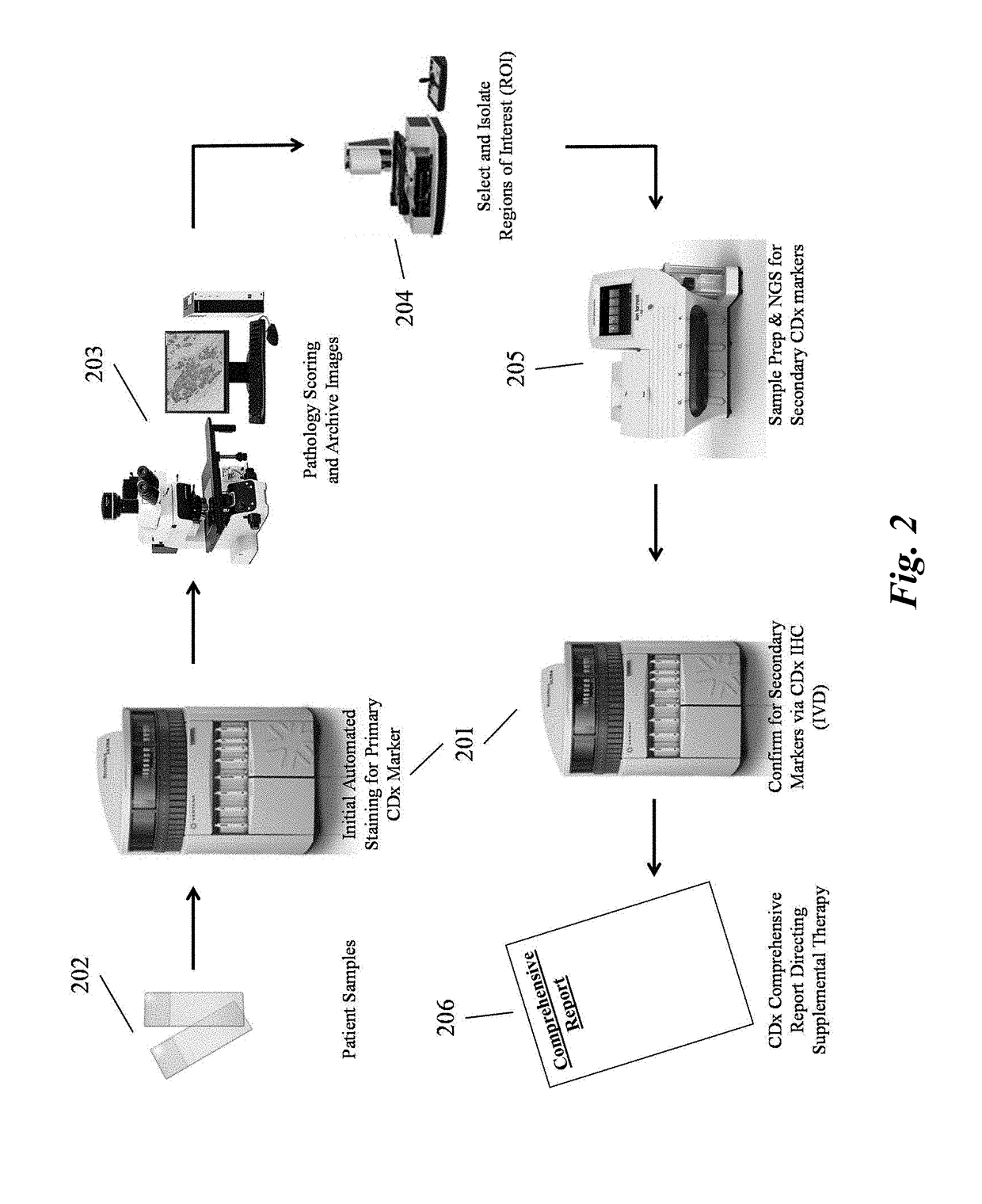
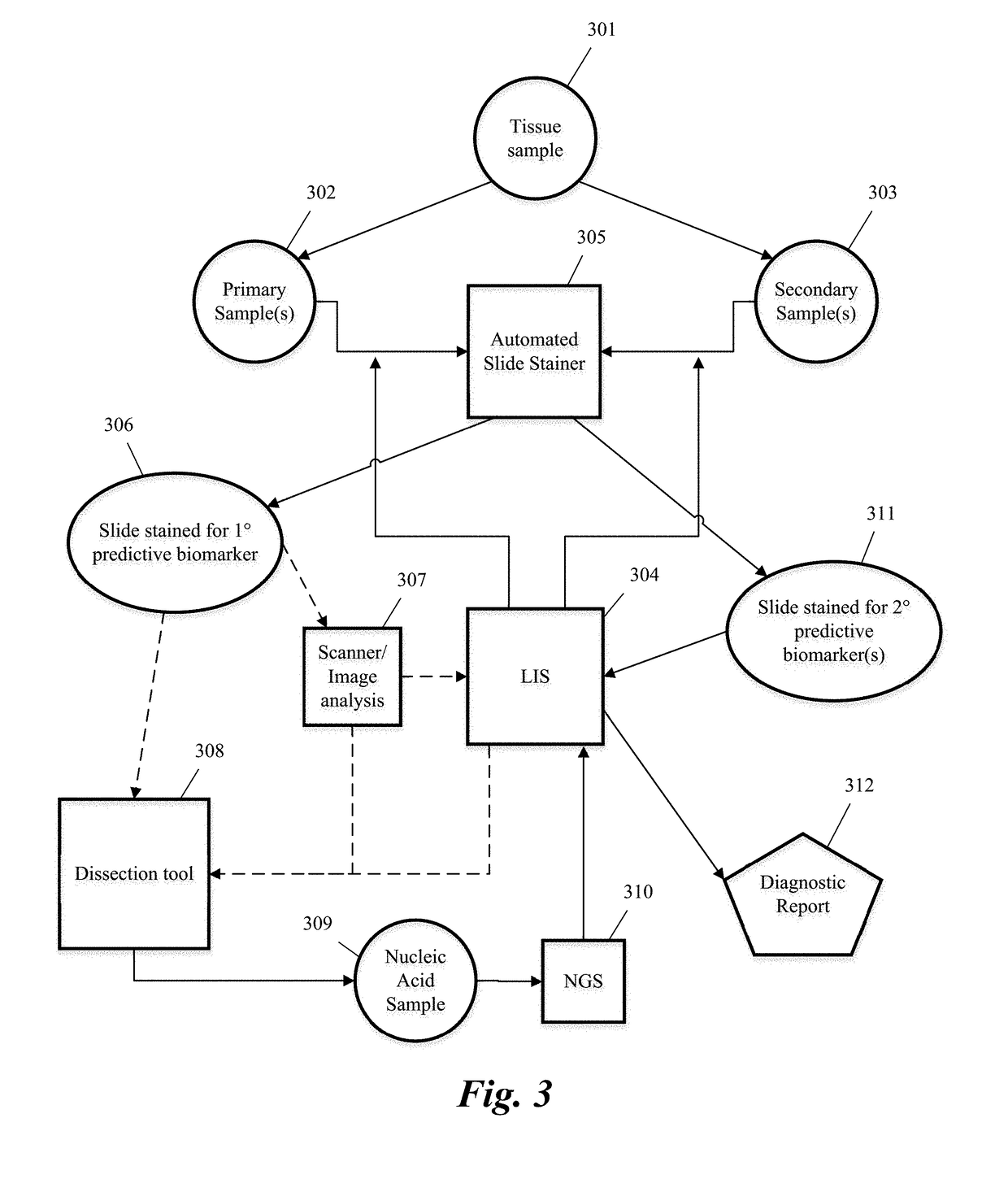
Systems and methods for selecting therapeutic agents for cancers using next generation sequencing, automated dissection, and / or automated slide stainers are disclosed. Non-responsive regions of a tumor sample having a heterogenous staining pattern for a predictive biomarker are excised using an automated dissection tool. Mutations linked to additional predictive biomarkers are identified in the excised portion of the sample by next generation sequencing. The relevance of the additional predictive biomarker(s) is confirmed by histochemical staining. Therapeutic courses may then be selected on the basis of the staining patterns of the predictive biomarkers.
Owner:VENTANA MEDICAL SYST INC
Cytokine biomarkers as predictive biomarkers of clinical response for Glatiramer acetate
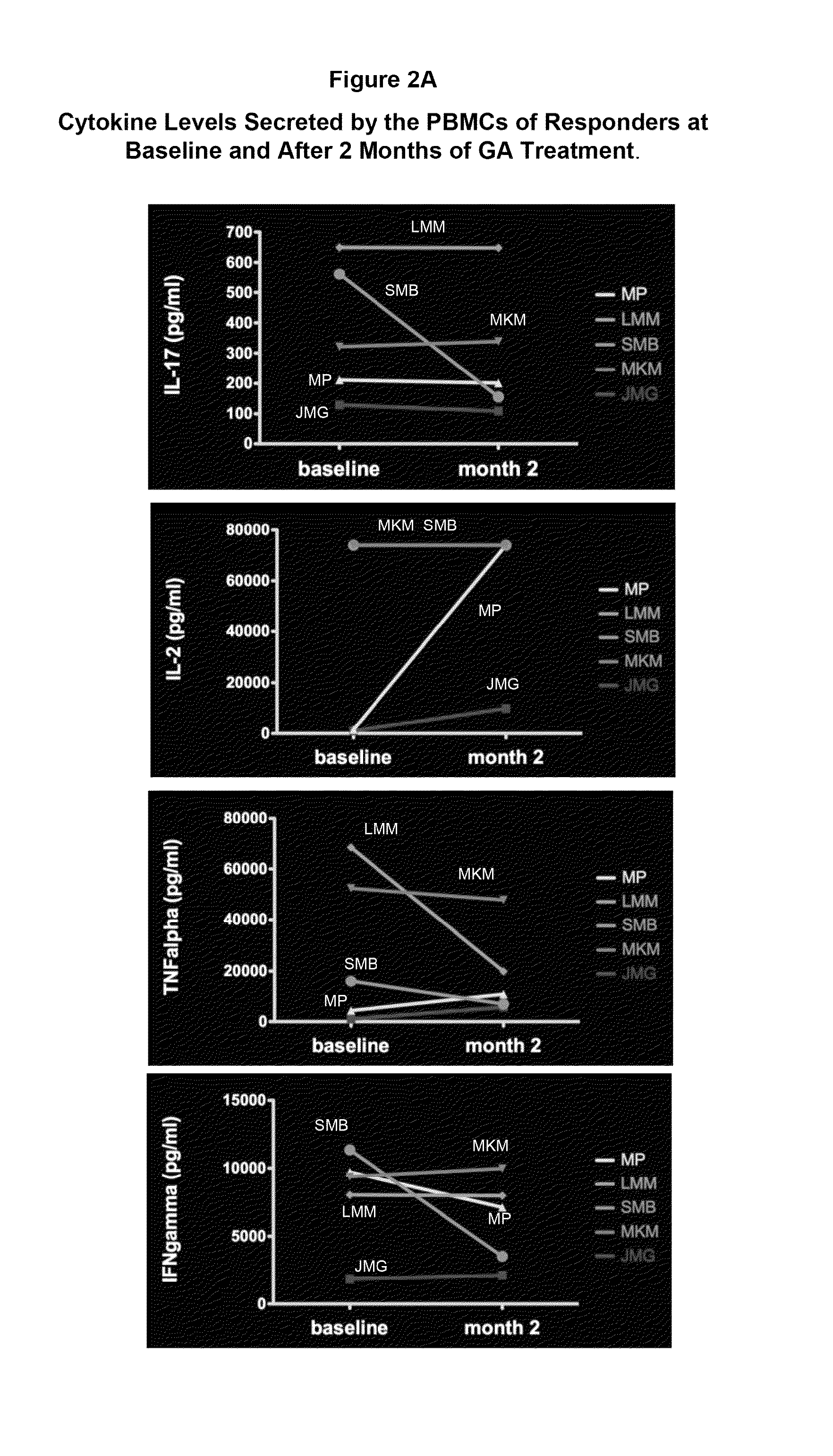
A method for treating a human subject afflicted with multiple sclerosis or a single clinical attack consistent with multiple sclerosis with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of determining whether the human subject is a glatiramer acetate responder by evaluating a biomarker selected from the group consisting of IL-17 concentration, TNF-α concentration, IL-2 concentration and IFN-γ concentration, or a combination thereof, in the blood of the human subject and administering the pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier to the human subject only if the human subject is identified as a glatiramer acetate responder.
Owner:TEVA PHARMA IND LTD
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
Owner:TEVA PHARMA IND LTD
Method to Predict Responsiveness of Breast Cancer to Polyamine-Type Chemotherapy
InactiveUS20110183336A1High expressionSugar derivativesMicrobiological testing/measurementPredictive biomarkerBiology
Methods of-identifying a basal or luminal phenotype of a cell, comprising detecting expression of one or more of a set of predictive biomarker genes or proteins that identify the cell as having a basal or luminal cancer subtype and compositions for treating identified basal or luminal cancers.
Owner:RGT UNIV OF CALIFORNIA
Predictive Markers For Cancer and Metabolic Syndrome
InactiveUS20130338027A1Microbiological testing/measurementLibrary screeningVascular diseasePredictive biomarker
Disclosed are predictive biomarkers and methods of use for the determination of insulin resistance and sensitivity, in addition to cardiovascular disease and risk associated with obesity. Methods for the stratification of patients along continuum of susceptibility to cardiometabolic risk, including prediction and progression to metabolic syndrome are also provided.
Owner:NUCLEA BIOMARKERS
Methods for predicting anti-cancer response
The present invention is based, in part, on the identification of novel methods for defining predictive biomarkers of response to anti-cancer drugs.
Owner:DANMARKS TEKNISKE UNIV +3
Compositions and method for treating compliment-associated conditions
InactiveUS20150044205A1Increased riskReduce riskSenses disorderSugar derivativesAntigenAntigen Binding Fragment
The invention provides methods and compositions for treating various degenerative diseases (e.g., AMD) with a factor D inhibitor (e.g., anti-factor D antibody or antigen-binding fragment thereof). Also provided are methods of selecting or identifying patients for treatment with a factor D inhibitor. Methods include the use of prognostic and / or predictive biomarkers.
Owner:GENENTECH INC
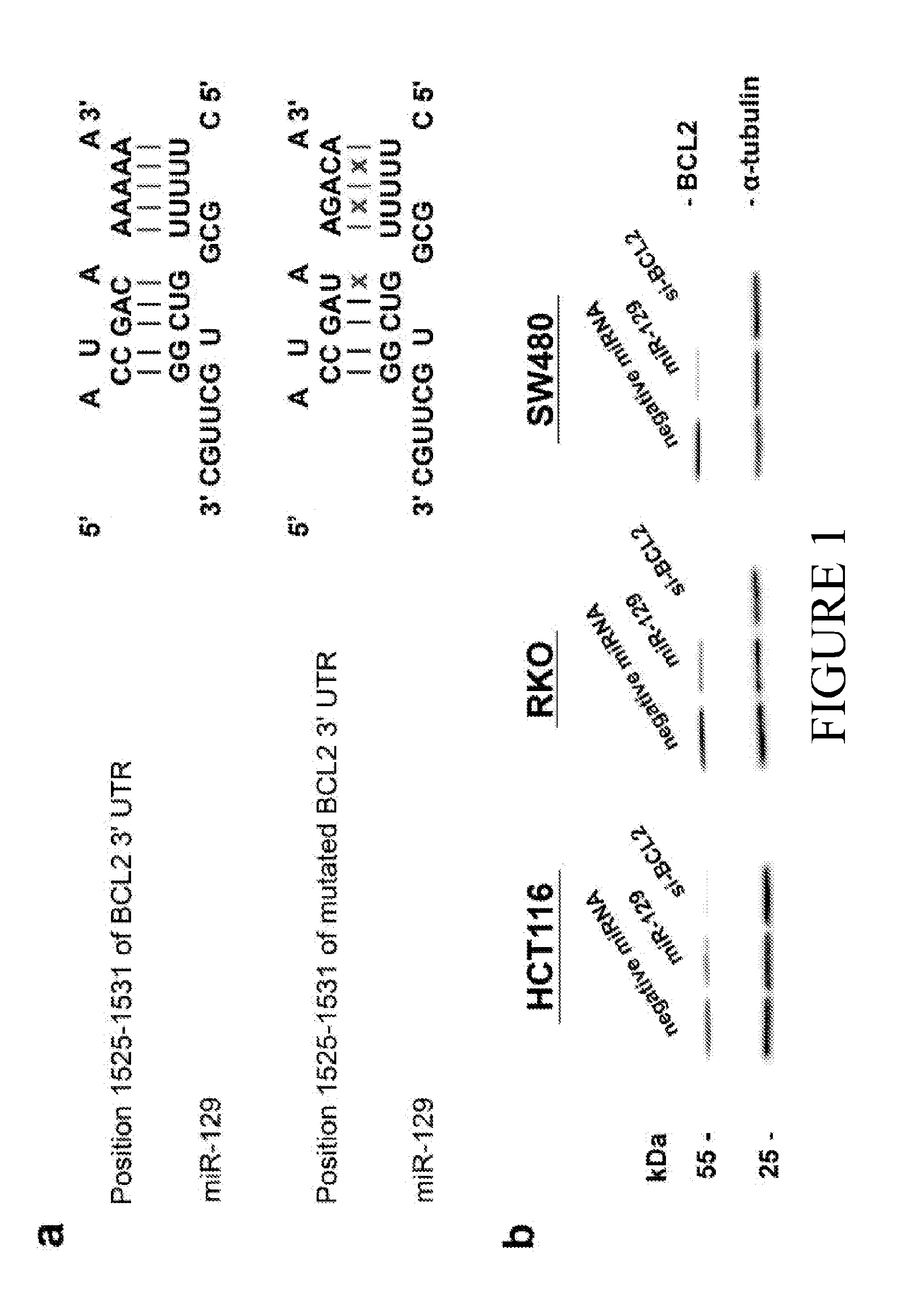
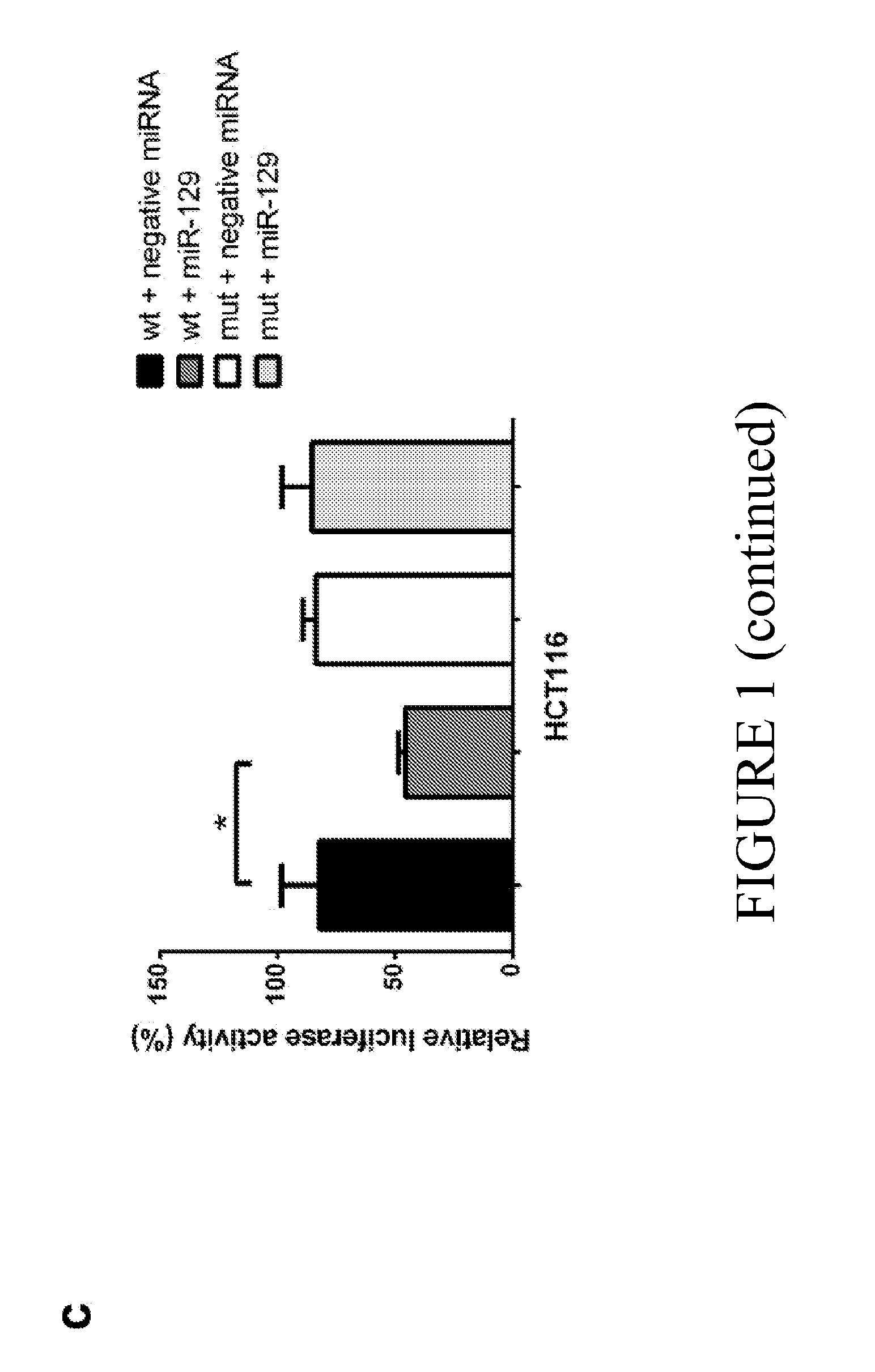
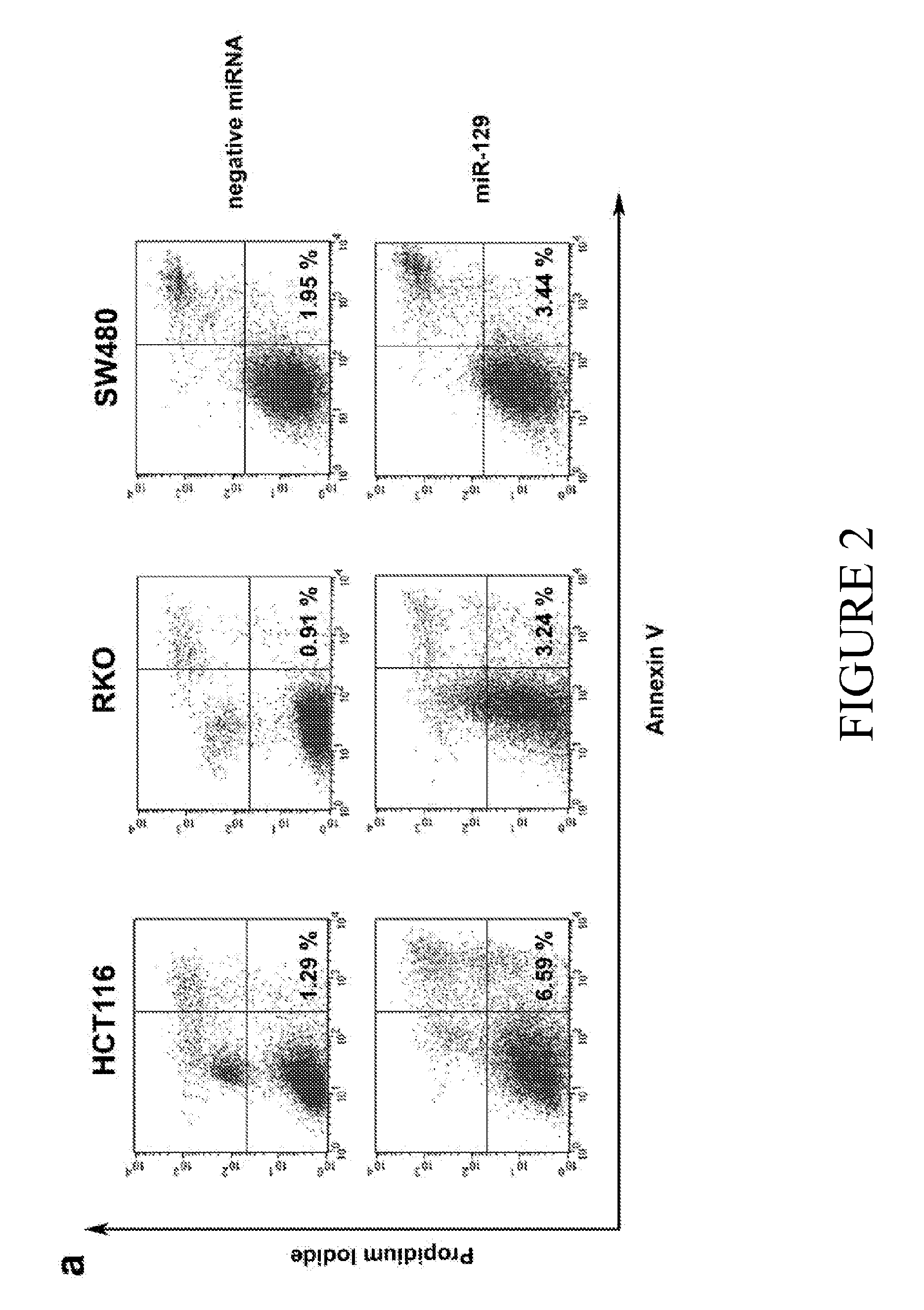
MicroRNA-129 AS A BIOMARKER FOR COLORECTAL CANCER
ActiveUS20160090636A1Improve responsivenessIncreased proliferationOrganic active ingredientsMicrobiological testing/measurementCancers diagnosisMicroRNA
The current disclosure describes methods for identifying subjects that would benefit from treatment with a chemotherapeutic agent. The disclosure is based in part on the observation that miR-129 expression levels are reduced in colorectal cancer. Accordingly, the current disclosure provides therapeutic compositions and methods for altering the expression of a miR-129 effector. Described herein are methods for characterizing the stage of colorectal cancer in a subject, based on the levels of miR-129 expression. The disclosure also identifies miR-129 as a predictive biomarker for cancer diagnosis and the subsequent treatment with directed therapeutic agents including but not limited to miR-129 nucleic acid molecules and / or a chemotherapeutic agent. The current disclosure also identifies novel therapeutic agents that modulate the level of BCL2, TS and / or E2F3 expression, as well as sensitize a subject to treatment with a chemotherapeutic agent.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
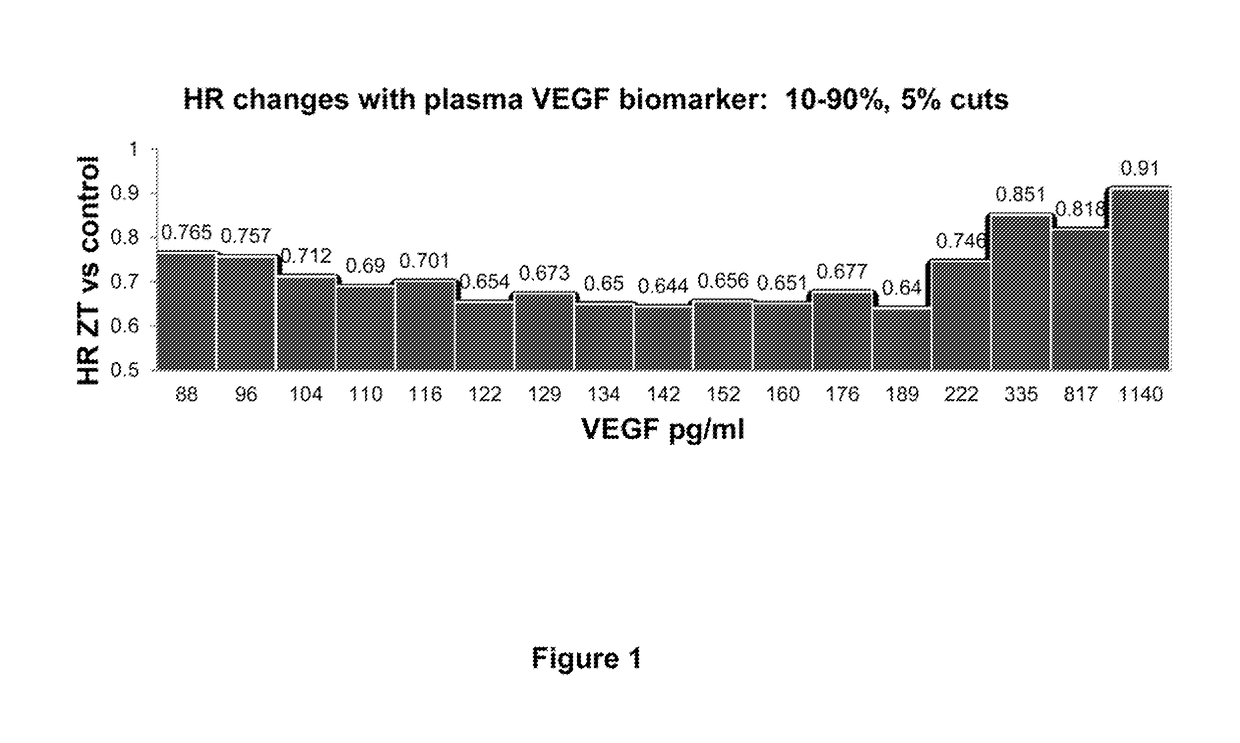
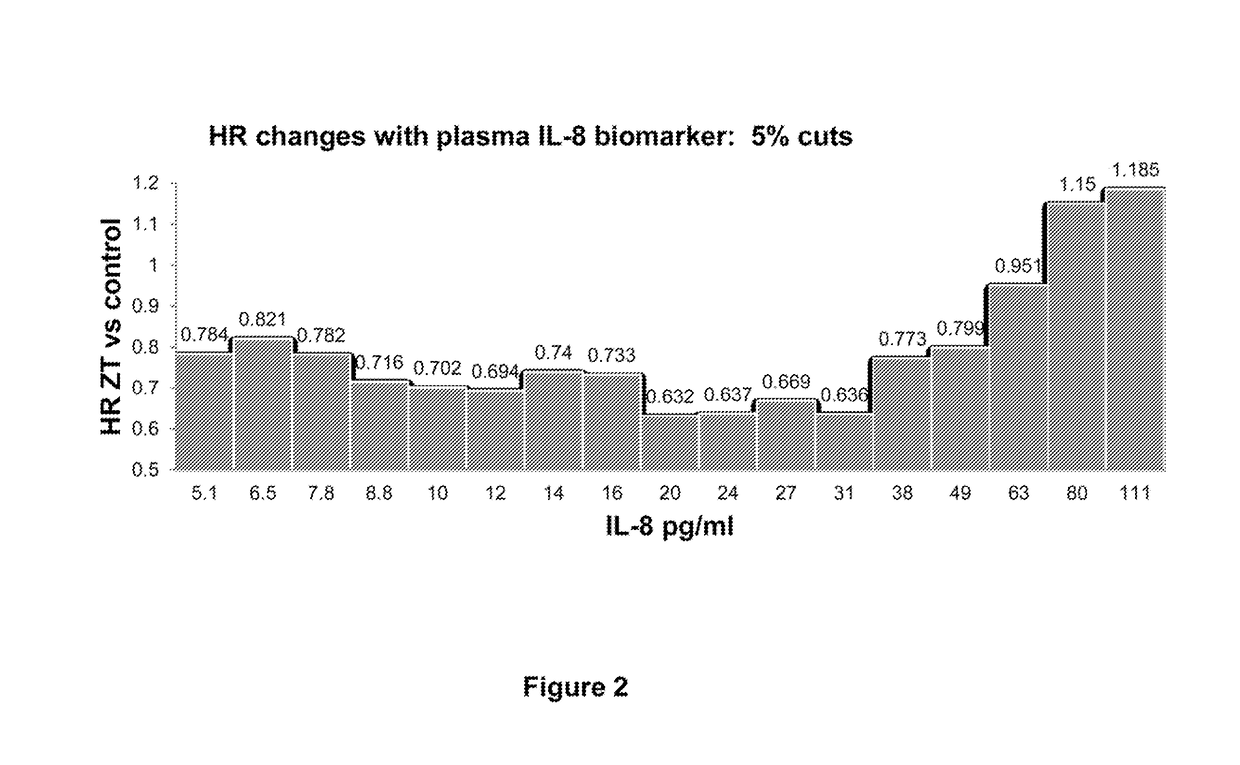
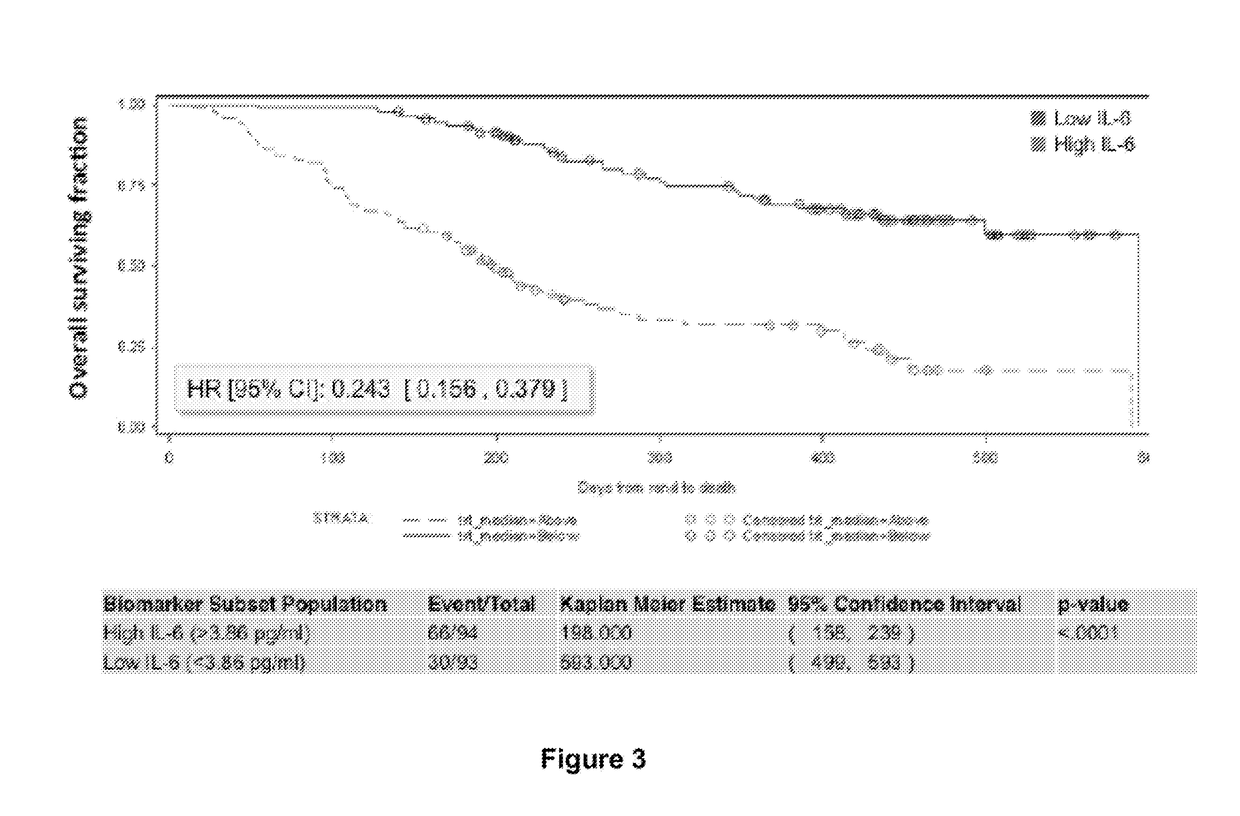
Predictive and prognostic biomarkers related to Anti-angiogenic therapy of metastatic colorectal cancer
ActiveUS20170281725A1Effective treatmentPeptide/protein ingredientsDisease diagnosisFAVORABLE RESPONSEPrognosis biomarker
The present invention provides methods for treating metastatic cancer comprising identifying subjects who will respond favorably to anti-VEGF therapy. According to certain aspects of the invention, subjects are identified based on their expression level of one or more predictive biomarkers. Favorable response to anti-VEGF therapy is indicated by high expression levels of certain biomarkers or by low expression levels of certain biomarkers. An exemplary predictive biomarker is VEGF-A. Also disclosed herein are prognostic biomarkers useful for identifying cancer-bearing subjects who are expected to have better relative survival outcomes.
Owner:REGENERON PHARM INC
Predictive Biomarkers for CTLA-4 Blockade Therapy and for PD-1 Blockade Therapy
Biomarkers are described for predicting the efficacy, risk of relapse, risk of an immune related adverse event (irAE), or combination thereof for a CTLA-4 blockade treatment, such as ipilimumab, in a subject with melanoma. Biomarkers are also described for predicting the efficacy and clinical benefit for a PD-1 blockade treatment, such as a PD-1 blocking antibody, in a subject with melanoma.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Identification of predictive biomarkers associated with Wnt pathway inhibitors
InactiveCN105829547APeptide/protein ingredientsMicrobiological testing/measurementPredictive biomarkerOncology
The invention relates to identification of predictive biomarkers associated with Wnt pathway inhibitors. According to the invention, biomarkers are provided for identifying tumors likely to respond to treatment with Wnt pathway inhibitors. Also provided are methods for identifying tumors and / or patients that are likely to be responsive or non-responsive to treatment with a Wnt pathway inhibitor. Methods for treating a patient with cancer are provided, wherein the cancer is predicted to respond to a Wnt pathway inhibitor.
Owner:ONCOMED PHARMA
Nlrc5 as a biomarker for cancer patients and a target for cancer therapy
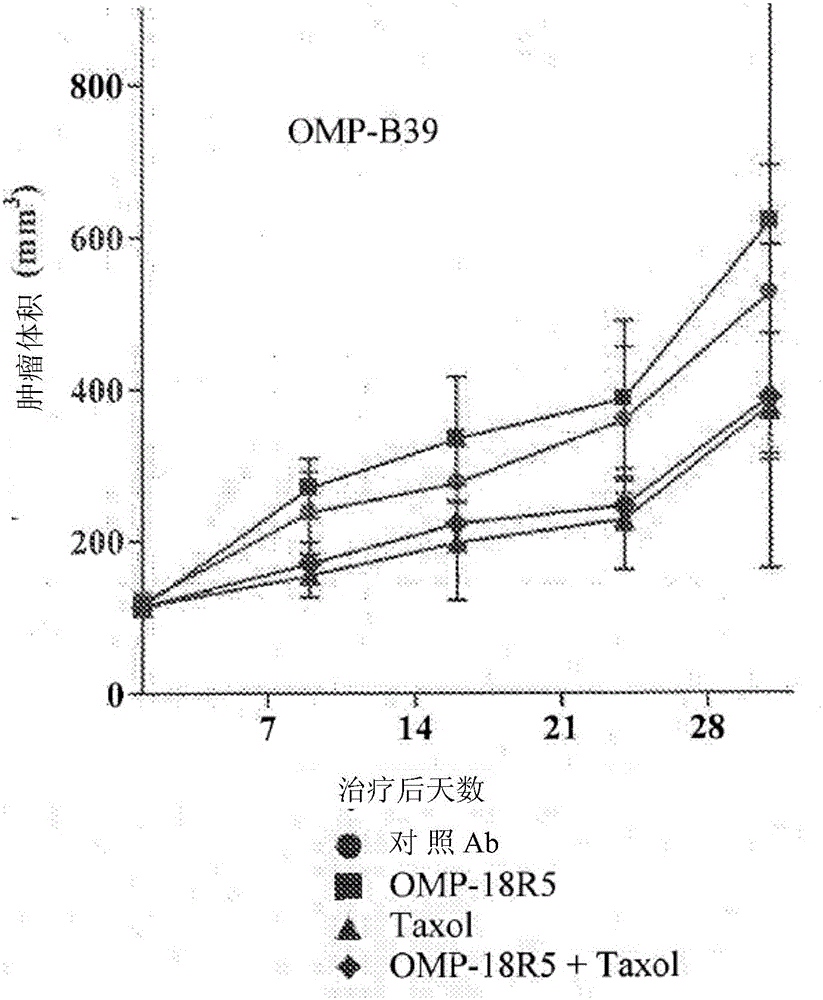
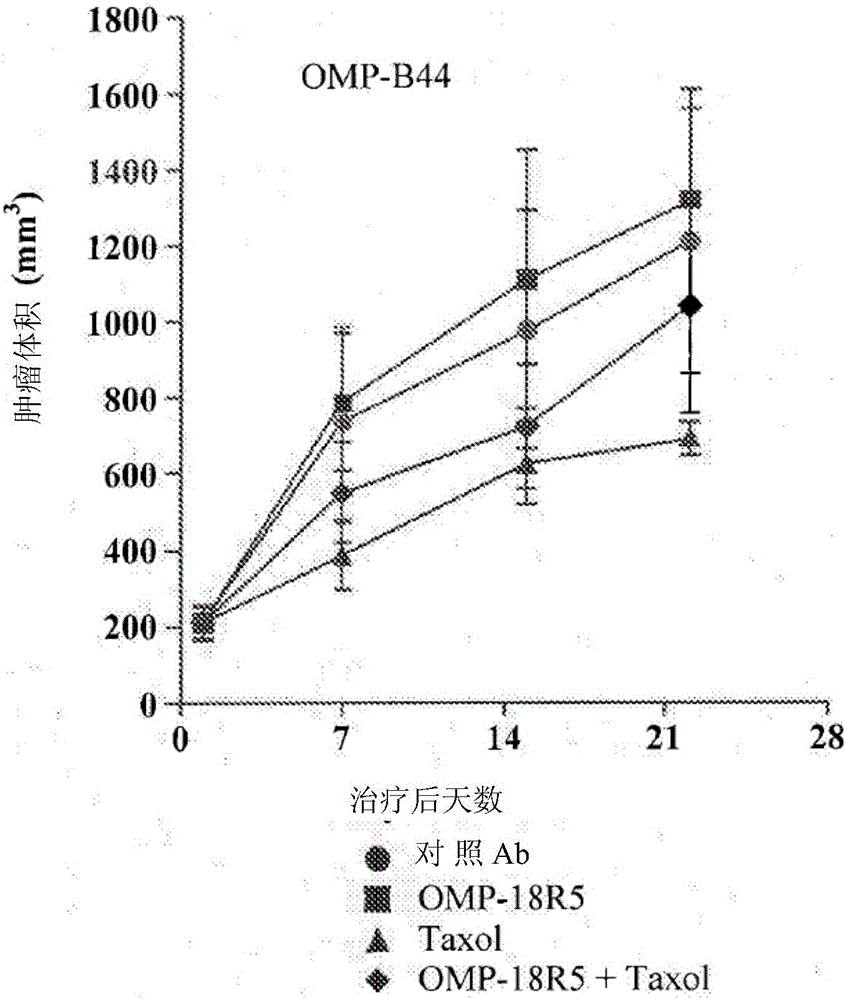
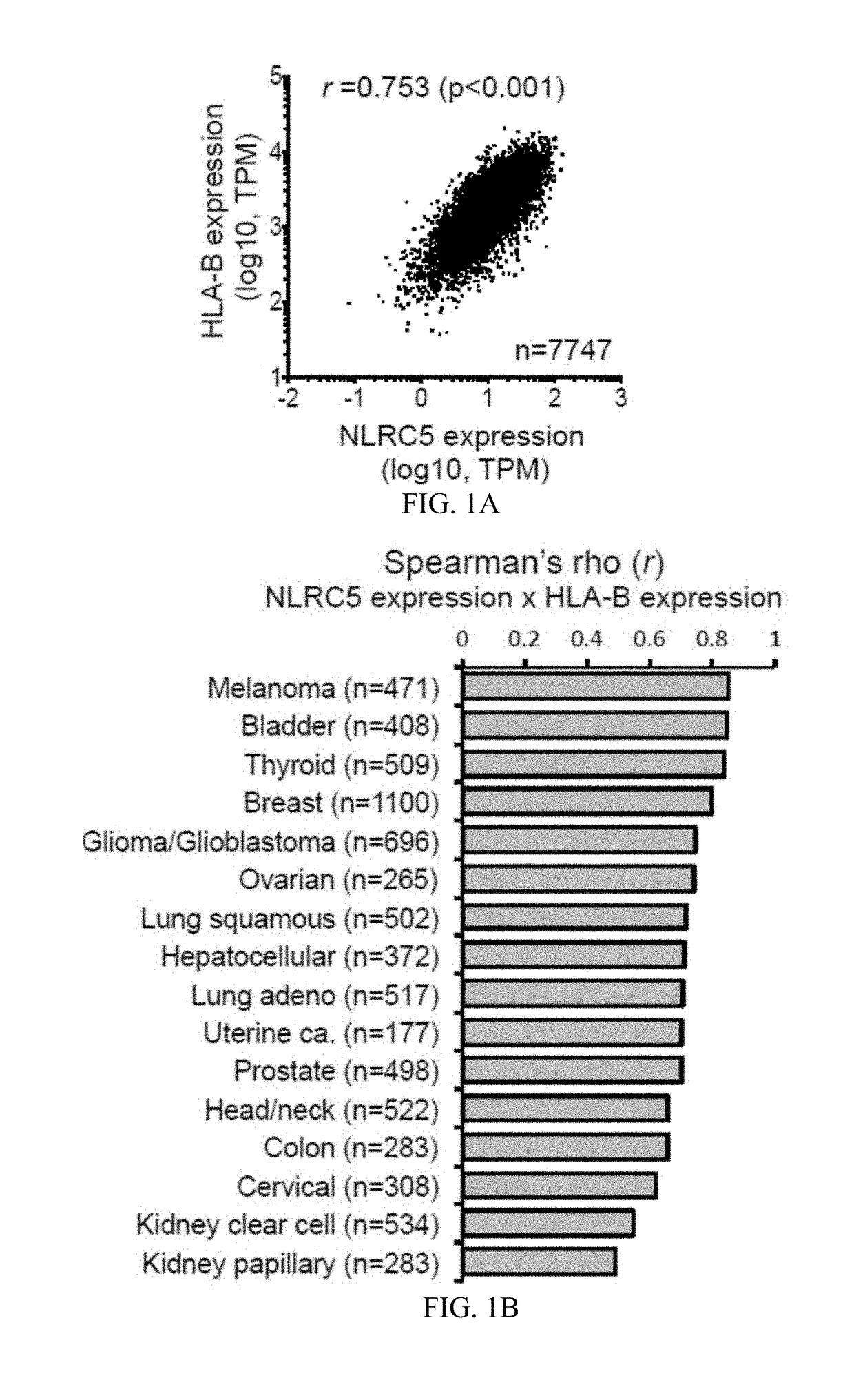
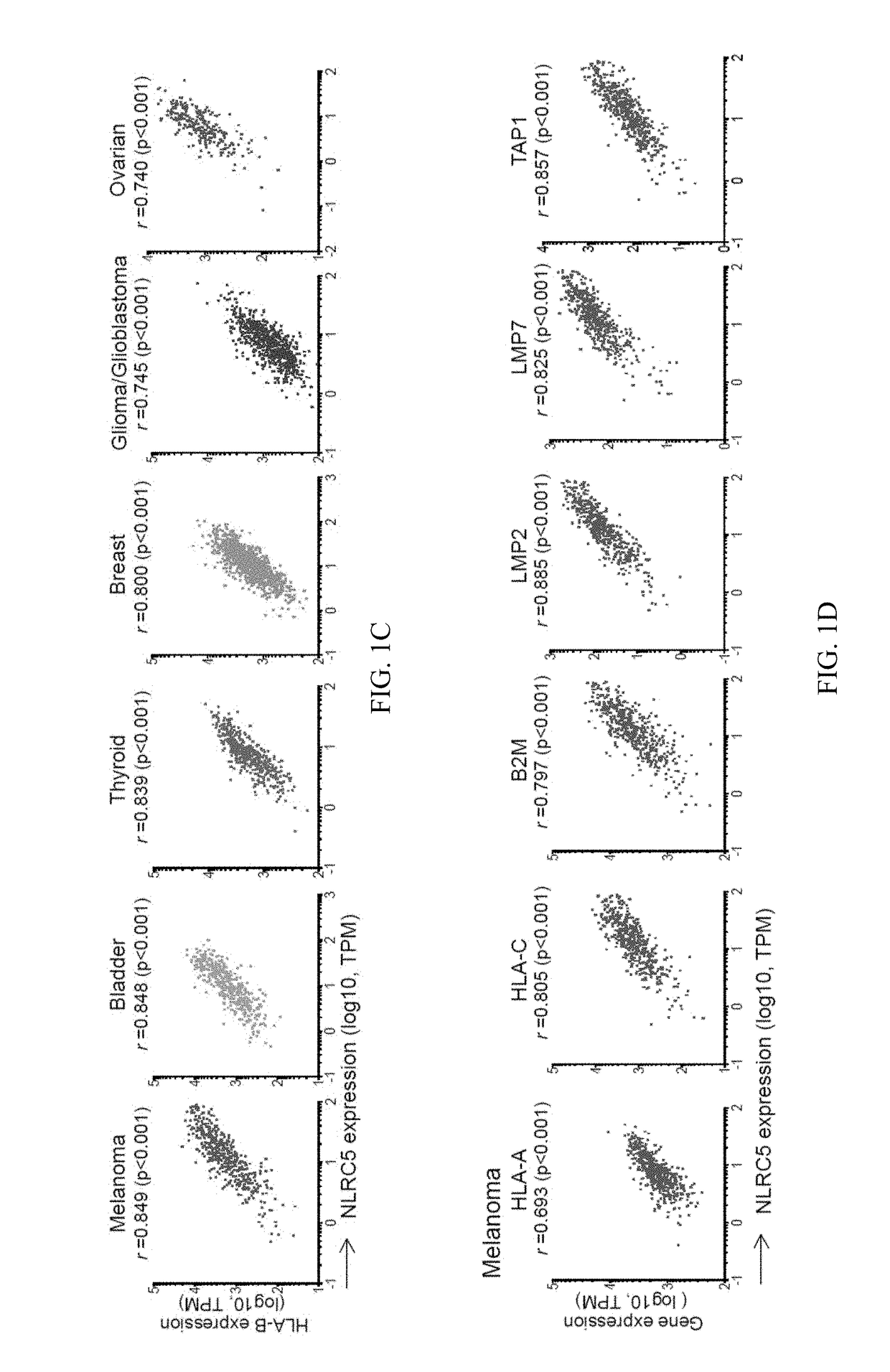
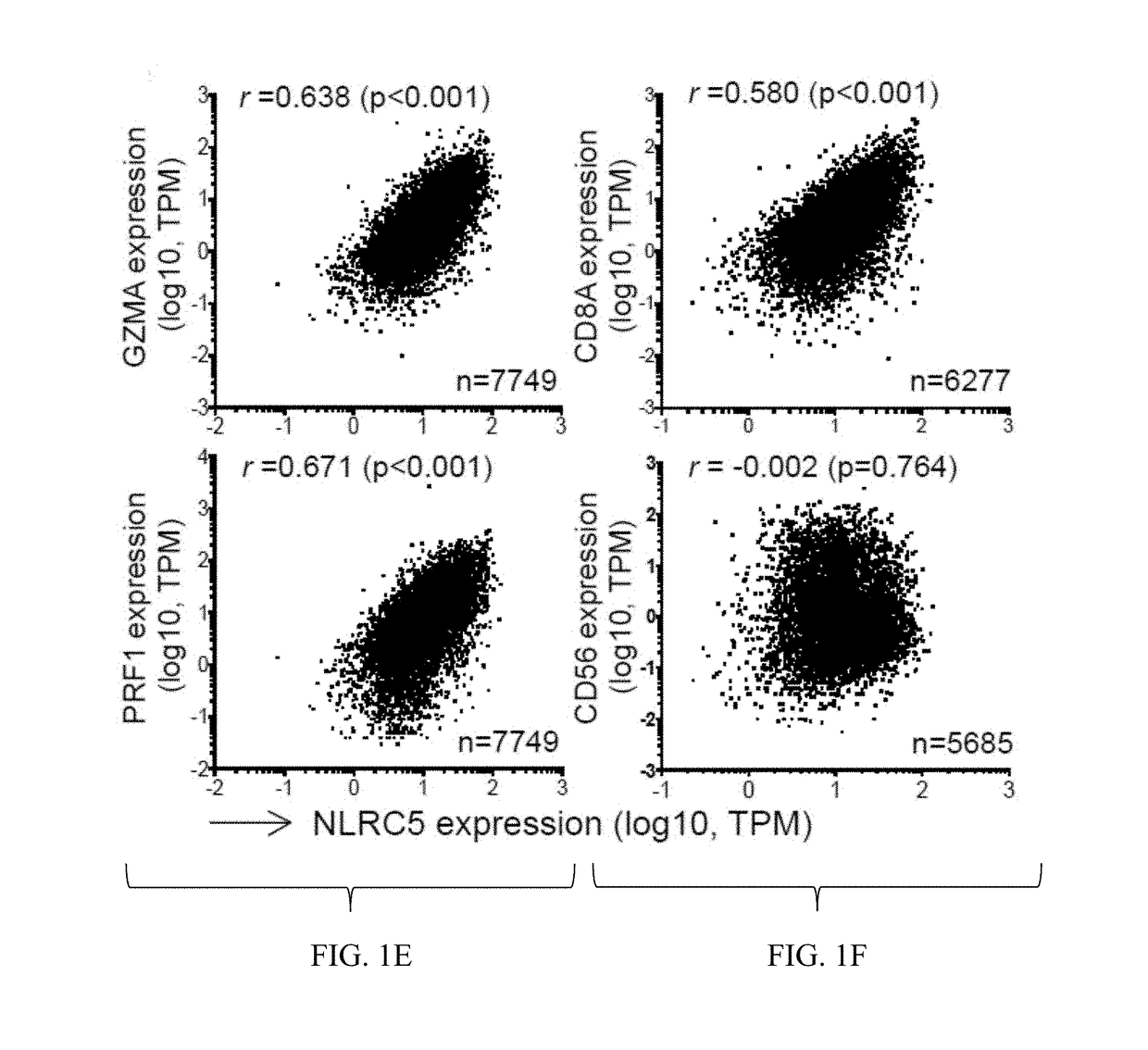
InactiveUS20170321285A1Reduce capacityHigh expressionMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellBiomarker identification
The invention pertains to biomarkers for identifying a cancer that is likely or not likely to evade the immune system of a subject, thus, is likely or not likely to show better prognosis (prognostic biomarker) and / or better responses to cancer therapies (predictive biomarker). The invention provides a method of identifying a subject as having a cancer that is likely to evade the immune system of the subject based on one or more of the following biomarkers in the cancer cells of the subject: a) reduced amount of NLRC5 mRNA or protein; b) reduced activity of NLRC5 protein; c) a mutation that reduces the activity of NLRC5 protein; d) increased methylation of nlrc5 or a portion thereof; and e) reduced copy number of nlrc5. These variables are useful to predict both patient survival (prognostic biomarker) and patient responses to immunotherapies (predictive biomarker). Furthermore, this invention provides a method of identifying a subject as having a cancer that is likely to evade the immune system of the subject with greater prediction power by utilizing multiple variables, in addition to above a)-e) variables, including neoantigen load, mutation number, or expression of genes involved in immune responses, including but not limited to CTLA4, PD1, PD-L1 and PD-L2. The invention also pertains to a method of treating a cancer likely to evade the immune system of the subject by administering an immunotherapy and a therapy designed to activate the MHC class I antigen presentation pathway by activating the expression and / or activity of NLRC5 protein.
Owner:TEXAS A&M UNIVERSITY
Predictive biomarker PD-L2 targeted polypeptide for tumor immunotherapy and application of predictive biomarker PD-L2 targeted polypeptide
ActiveCN107556367ATransport stableIncrease contentBiological testingMacromolecular non-active ingredientsClinical efficacyClinical value
The invention relates to a predictive biomarker PD-L2 targeted polypeptide for tumor immunotherapy and an application of the predictive biomarker PD-L2 targeted polypeptide. The general formula of thepolypeptide is: HX1RX2X3X4X5RIRN. The invention also relates to a DNA fragment encoding the polypeptide, an expression vector expressing the polypeptide, a host cell and a bivalent, a multivalent orpharmaceutical composition formed from the polypeptide. The polypeptide can be used for detecting the expression of PD-L2 in tumor cells and immune cells, can be taken as a detection index for predicting the clinical efficacy of anti-PD-L1 / PD-L2-PD-1 therapy and can specifically distinguish PD-L1 and PD-L2 protein with high homology, thereby having great clinical value and significance for optimization of therapeutic schedules and curative effect monitoring of individual patients.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Methods for predicting anti-cancer response
ActiveUS10190160B2Increased and decreasedOrganic chemistryPeptide/protein ingredientsMedicinePredictive biomarker
The present invention is based, in part, on the identification of novel methods for defining predictive biomarkers of response to anti-cancer drugs.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +3
Method for early prediction of neurodegenerative decline
PendingUS20210228079A1Easy to useImage enhancementMedical imagingEarly predictionPredictive biomarker
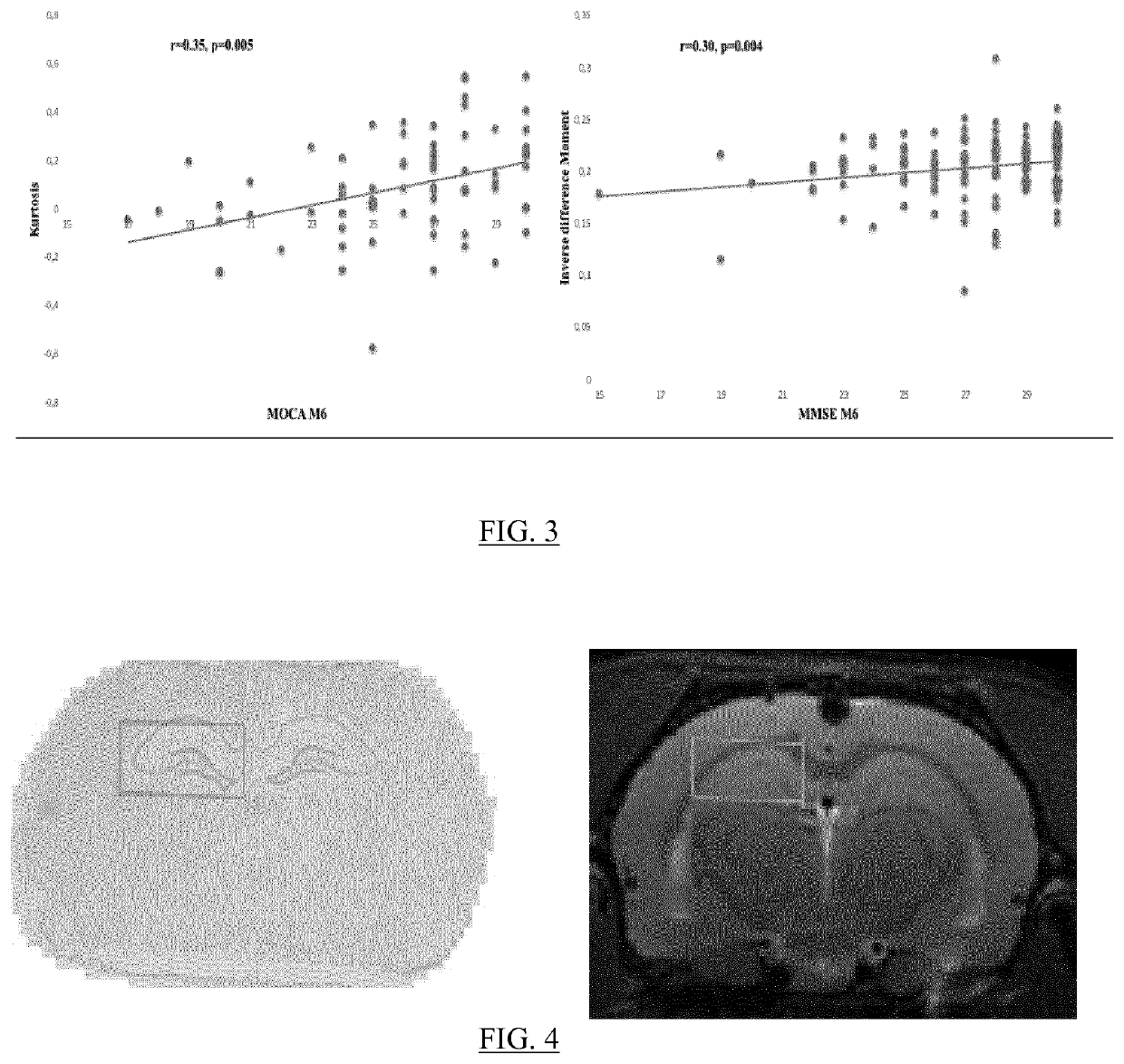
The present invention relates to a method for predicting neurodegenerative decline and / or its severity for a patient, especially of cognitive impairment (CI). Strokes and Parkinson's disease are frequently associated with occurrence of long-term cognitive impairment or dementia with still incompletely resolved mechanisms. The discovery of diagnostic and predictive biomarkers thus remains a major challenge. The method of the invention uses radiomics corresponding to texture features extracted from a plurality of previously-acquired medical brain images and correlated with previously-acquired clinical and / or biological data. A classifier is trained beforehand for learning these radiomics, and then operated on radiomics computed from at least one brain image of a patient to generate a score representative of its risks of neurodegenerative decline. By applying this method on a cohort of 160 MCI and non-MCI patients, the inventors show that MCI patients could be early predicted with a mean accuracy of 88%. In the same way, the method was able to discriminate very early stages of cognitive decline in a Parkinson's disease population of 100 patients.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Identification of Biomarkers Predictive of Dasatinib Effects in Cancer Cells
InactiveUS20100004257A1Rapid correlationTreatment to begin more readilyOrganic active ingredientsDisease diagnosisSrc inhibitorCancer cell
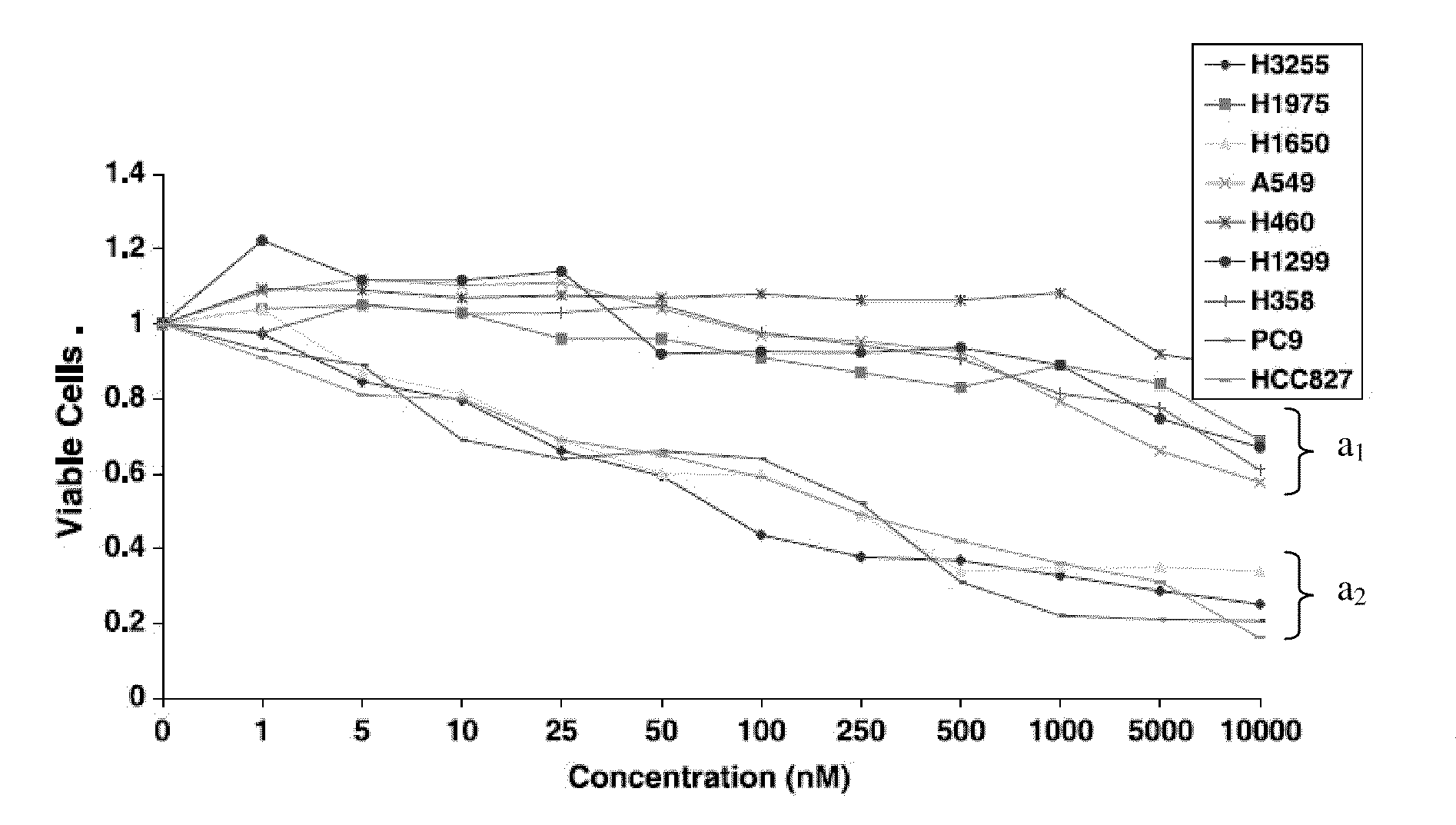
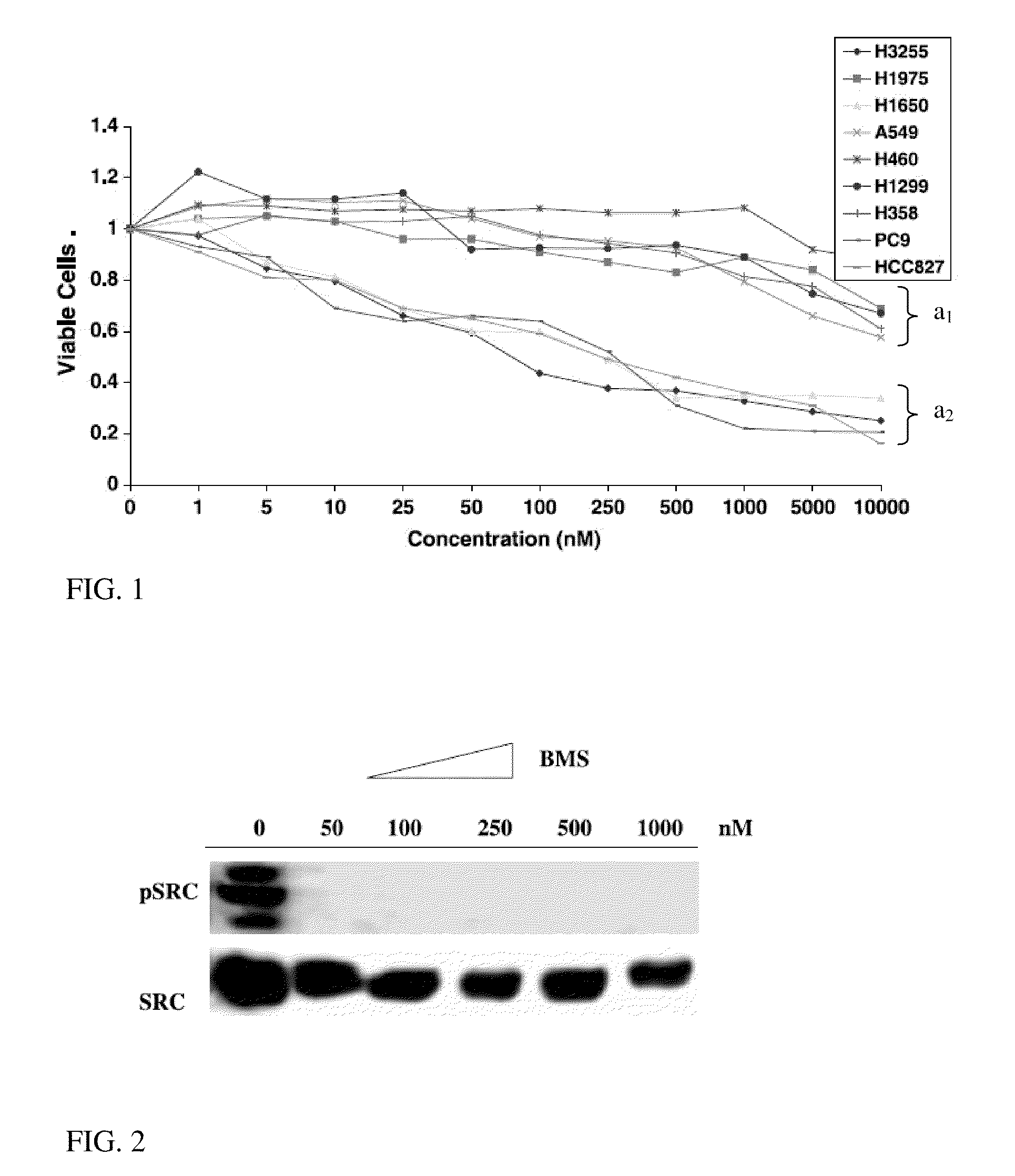
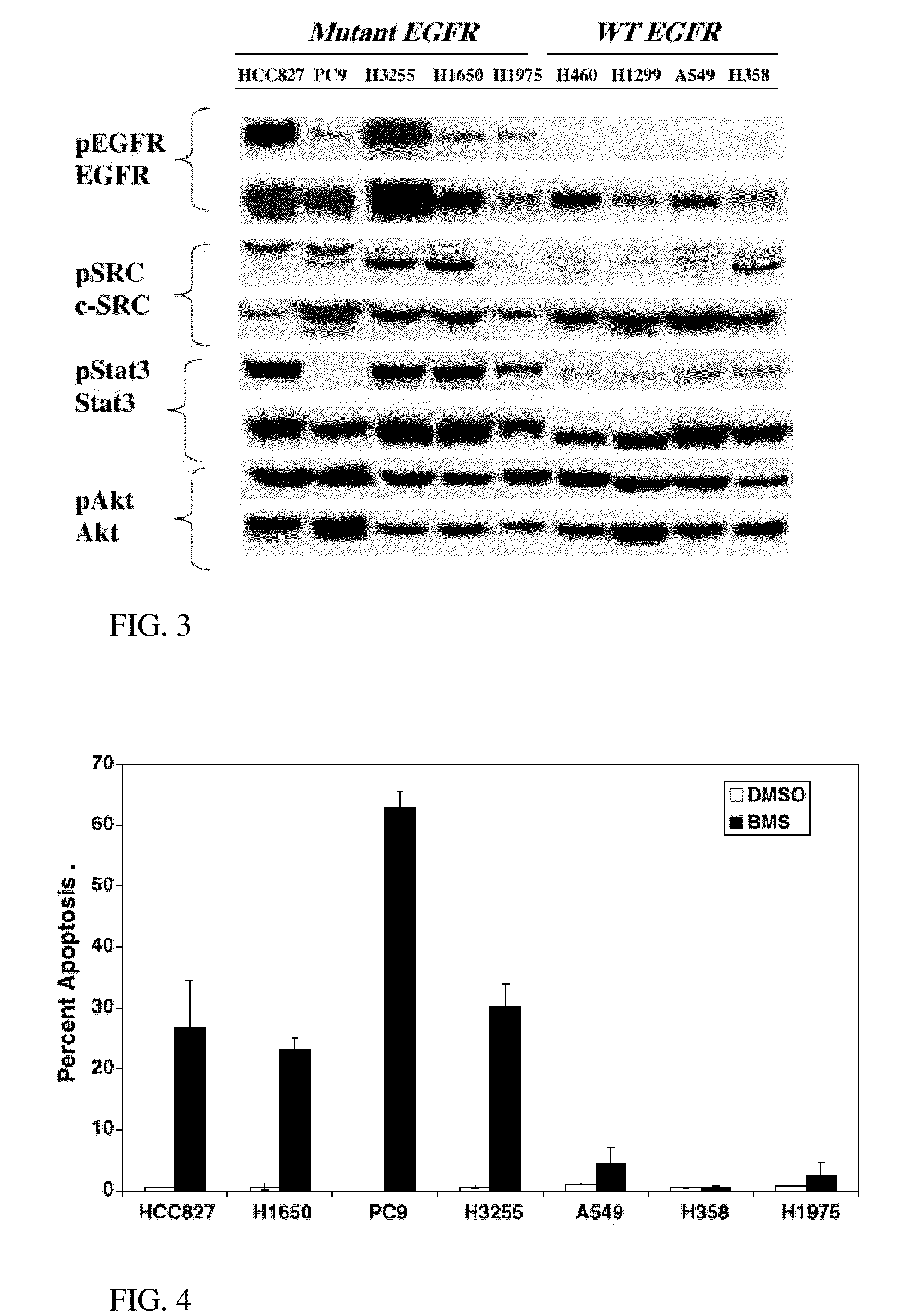
A method of predicting response to treatment with inhibitors of EGFR and SRC by screening for status of key biomarkers such as EGFR. Dasatinib is a drug that can inhibit a group of proteins called SRC proteins. In addition, other experiments have suggested that other important signaling proteins are affected by dasatinib. Early phase trials of dasatinib are ongoing in cancer patients. It will be important to determine which patients receive a clinical benefit of dasatinib. Predetermination of treatment benefit can be performed by assessing biomarkers in patients tumors prior to treatment with dasatinib or other inhibitors of EGFR and SRC. Patients that have positive biomarkers for treatment could then be treated with higher confidence of benefit while those not possessing these predictive biomarkers would avoid ineffective and potentially toxic therapy. Additionally, treatment can be tailored according to predetermined sensitivity by evaluating indicated biomarkers correlating with sensitivity to one or more agents.
Owner:UNIV OF SOUTH FLORIDA
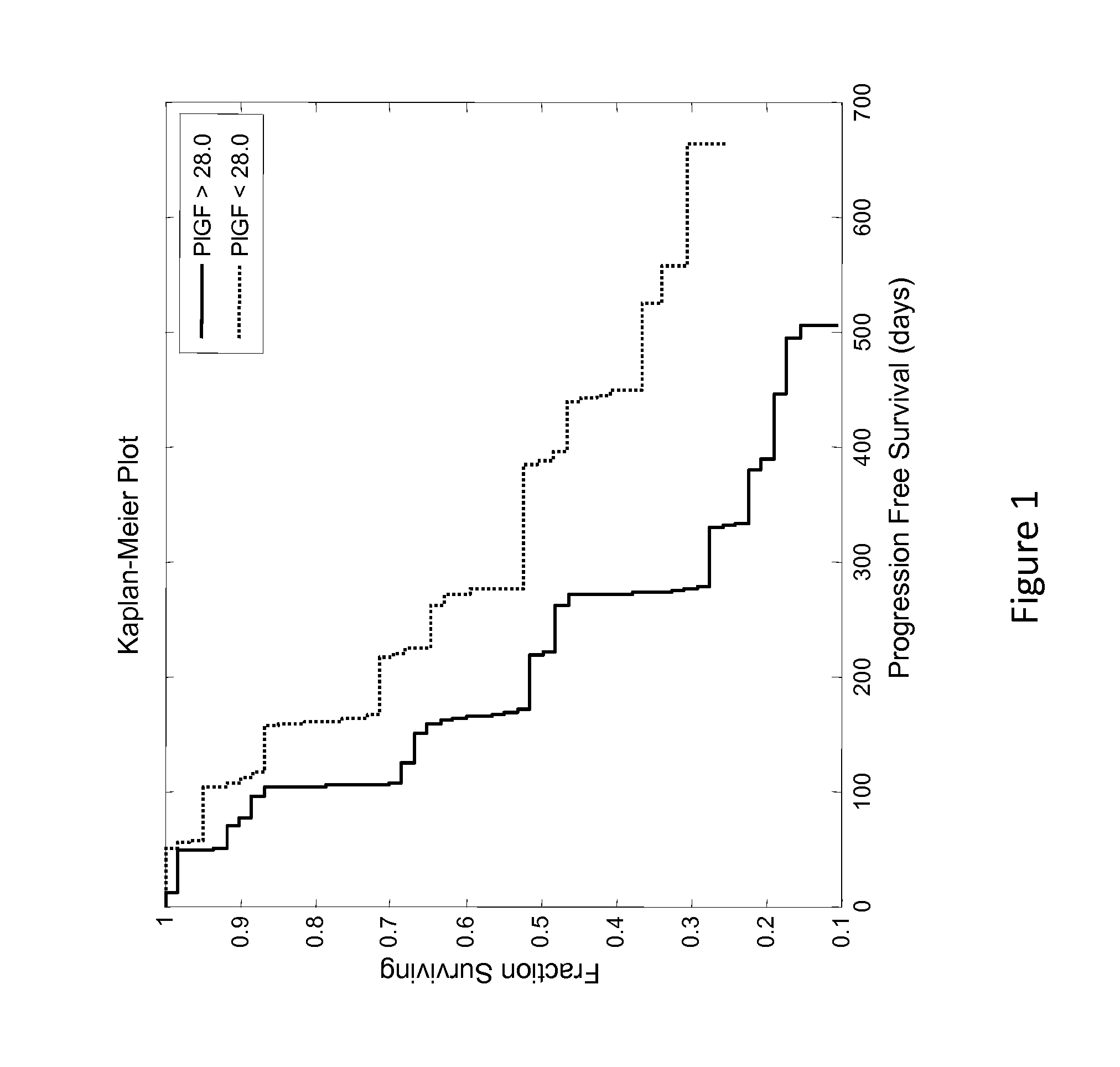
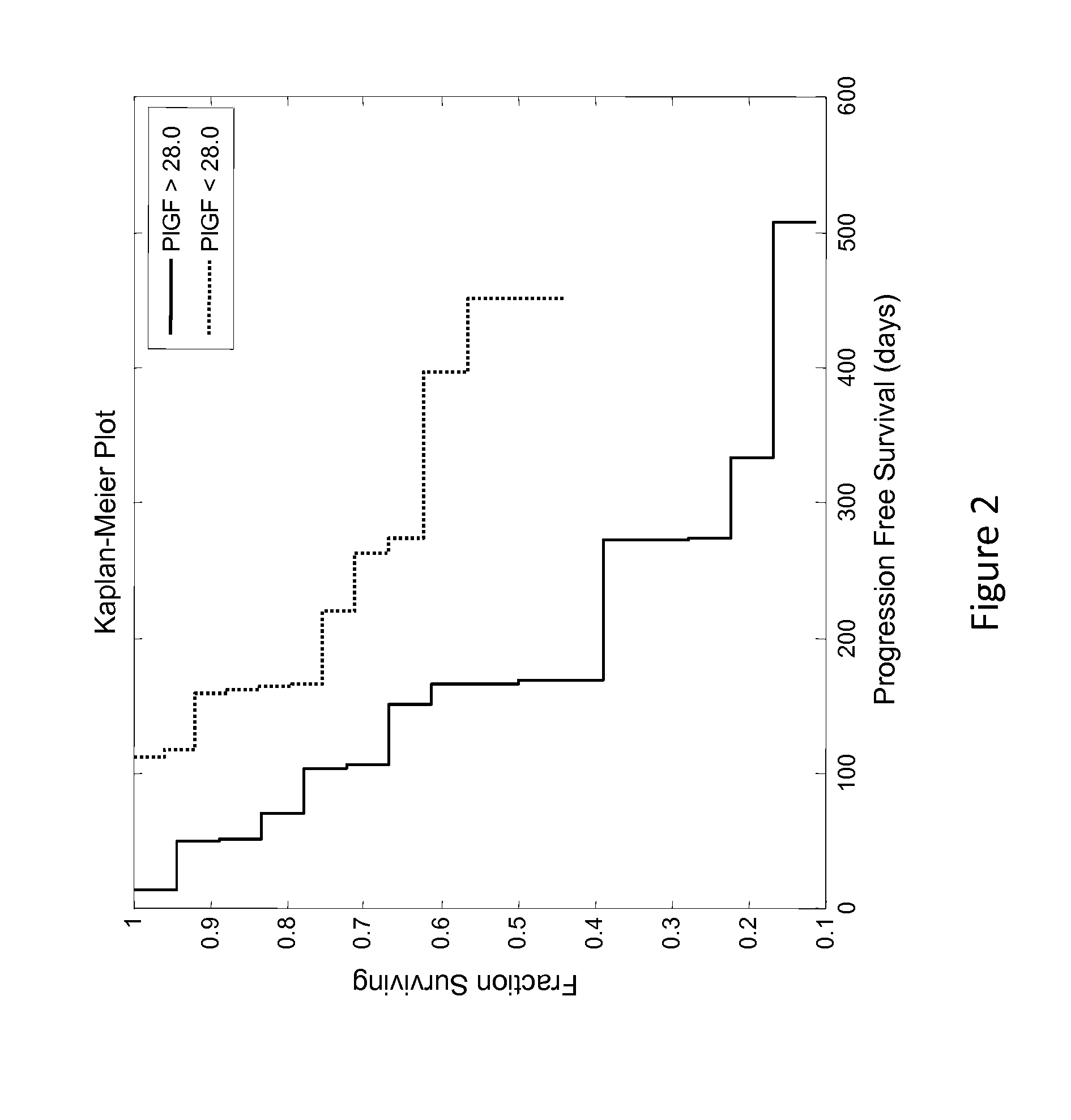
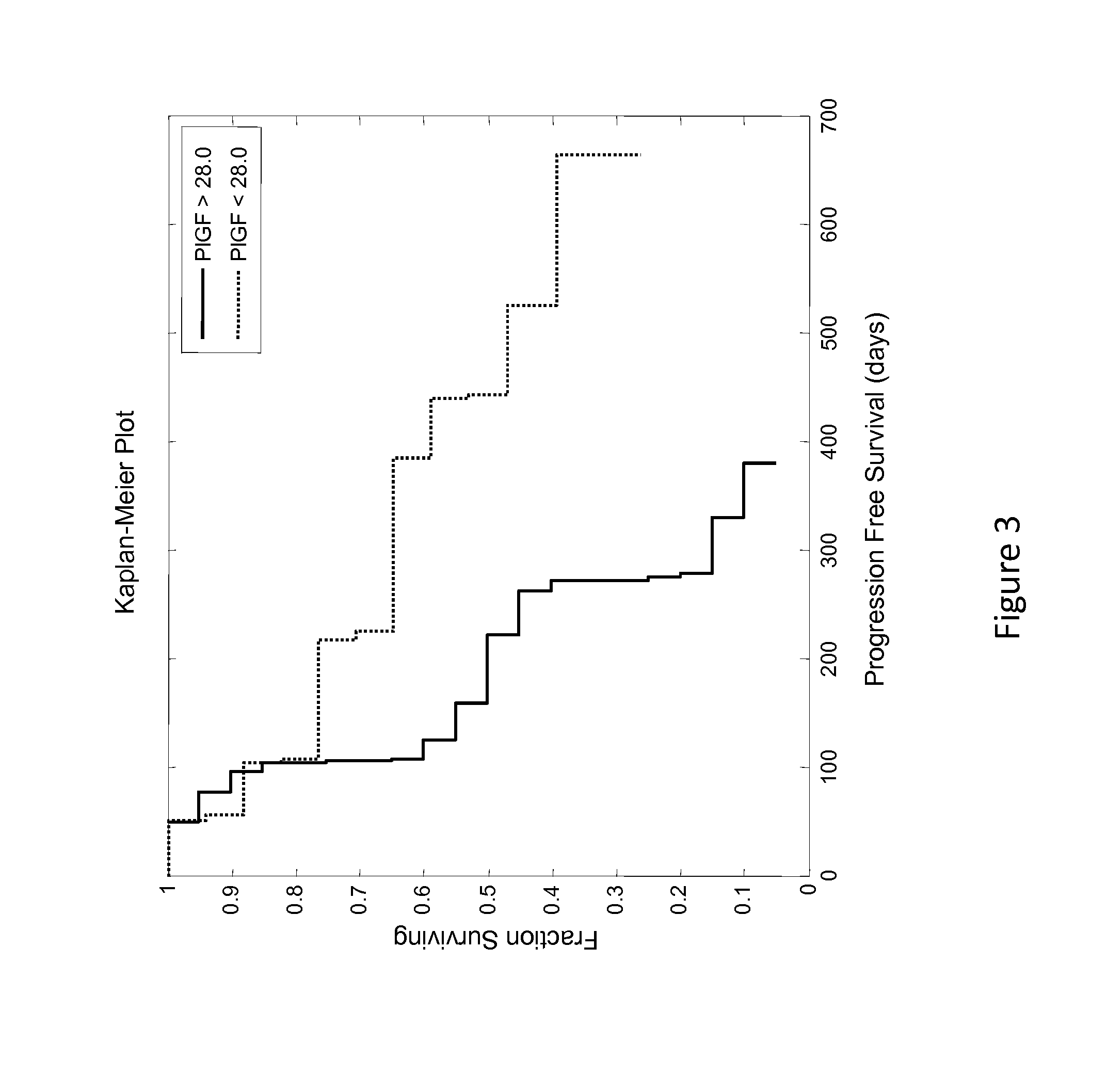
Predictive biomarker of survival in the treatment of renal cell carcinoma
ActiveUS20140348824A1Prolonged progression-free survivalAdequate levelPeptide/protein ingredientsLibrary screeningPredictive biomarkerOncology
Methods and compositions are disclosed for predicting and treating the clinical benefit to a human renal cell carcinoma patient prior to their treatment with a VEGFR inhibitor and an Ang2 inhibitor.
Owner:AMGEN INC
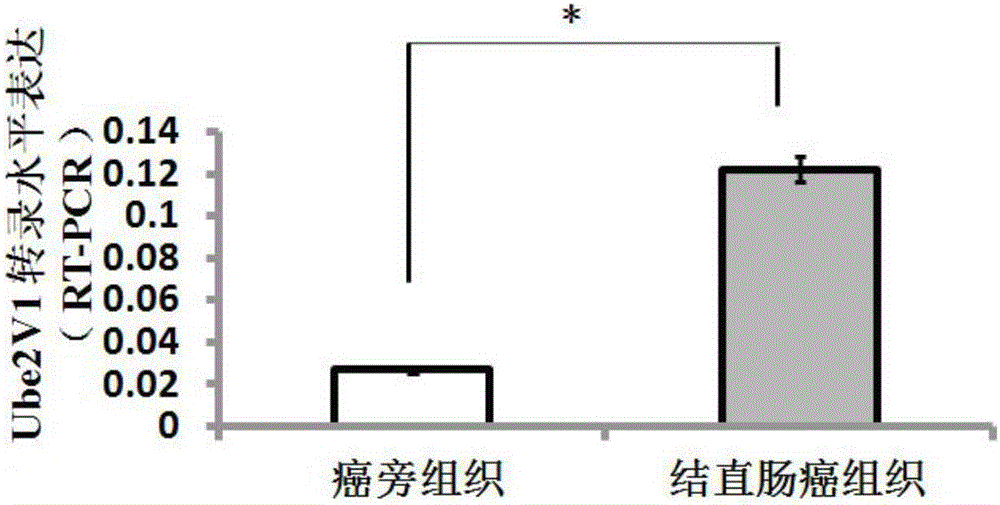
Predictive biomarker for human colorectal cancer or human colorectal cancer metastasis and applications of predictive biomarker
ActiveCN106191259AOrganic active ingredientsMicrobiological testing/measurementHistone deacetylasePredictive biomarker
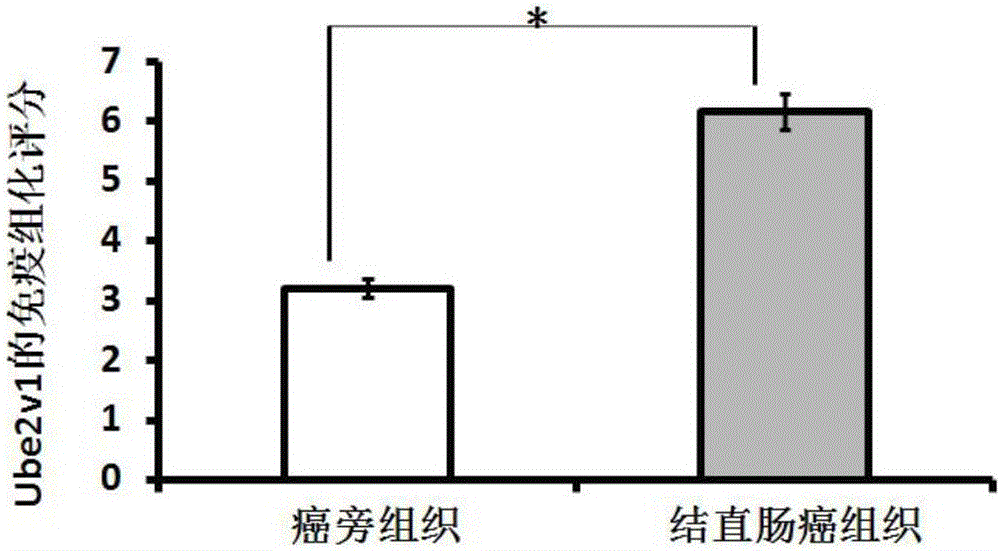
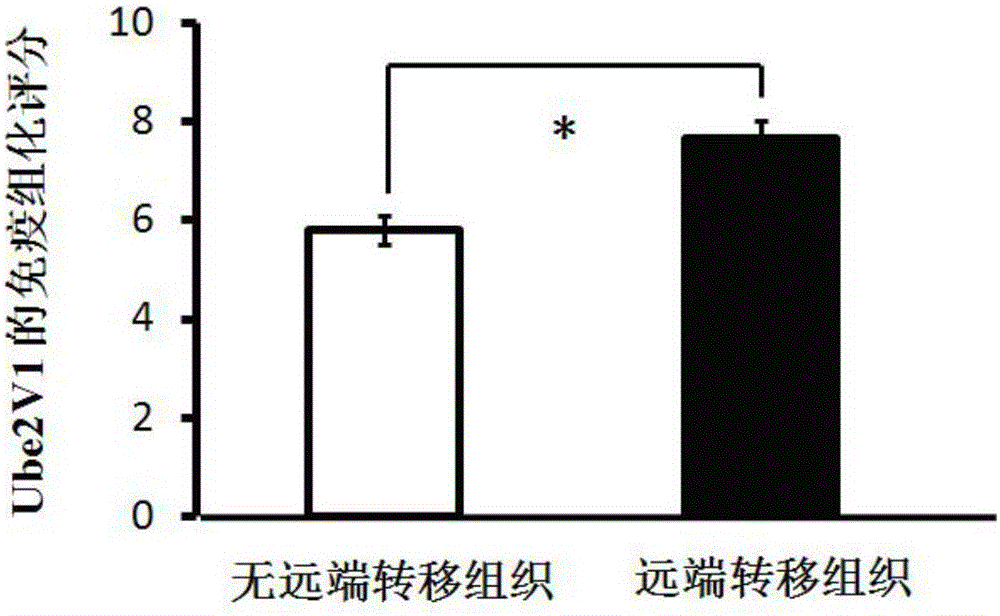
The invention discloses a predictive biomarker for human colorectal cancer or human colorectal cancer metastasis. The predictive biomarker comprises Ube2v1 protein. Based on the constructed colorectal cell strain with Ube2v1 over-expressed stably, the naked mouse experimental result shows that Ube2v1 can promote the growth and metastasis of tumor. Ube2v1 without functions is bonded to Ube2N for degrading histone deacetylase Sirt1, so that the deacetylation effect of Sirt1 for the H4K16 site of the histone is weakened, and the metastasis of colorectal cancer is promoted. The NSC697923 compound is added in the colorectal cancer cells, and the degradation effect of Ube2v1 for the histone deacetylase Sirt1 is obviously weakened. The result shows that Ube2v1 can be taken as the marker for the generation and metastasis of colorectal cancer, and in addition, NSC697923 can be used for preparing medicines for resisting colorectal cancer metastasis, thus having a significant clinical application value.
Owner:SUZHOU UNIV
Predictive biomarkers for prostate cancer
The invention relates to compositions and methods for detecting, screening, diagnosing or determining the progression of, regression of and / or survival from a proliferative disease or condition, specifically prostate cancer. The invention also provides new assays and kits for the staging or stratifying prostate cancer patients or patients suspected of having prostate cancer.
Owner:NUCLEA BIOMARKERS
Predictive Biomarkers for Detection of Organ Damage in Autoimmune Illnesses and Other Diseases
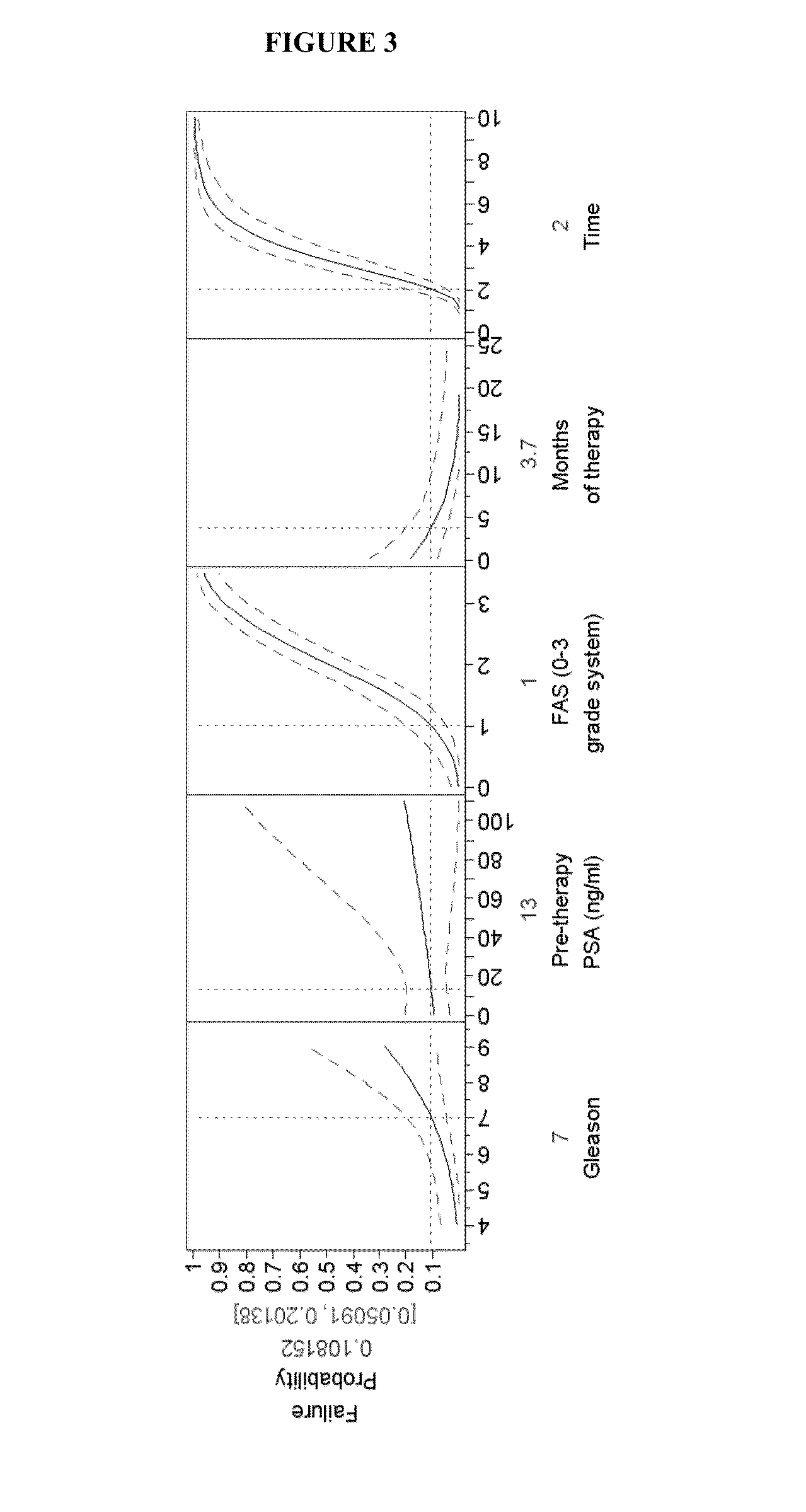
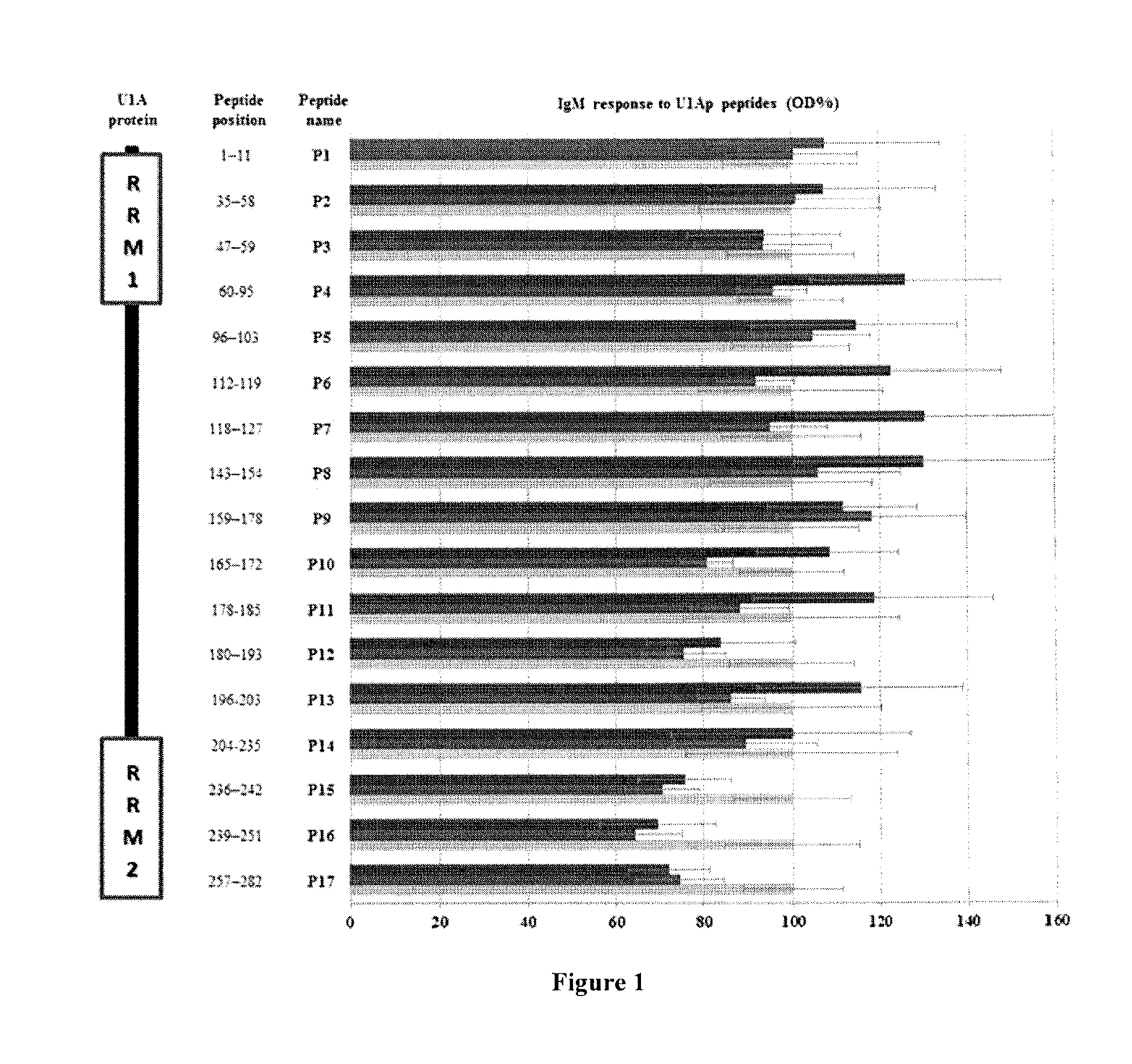
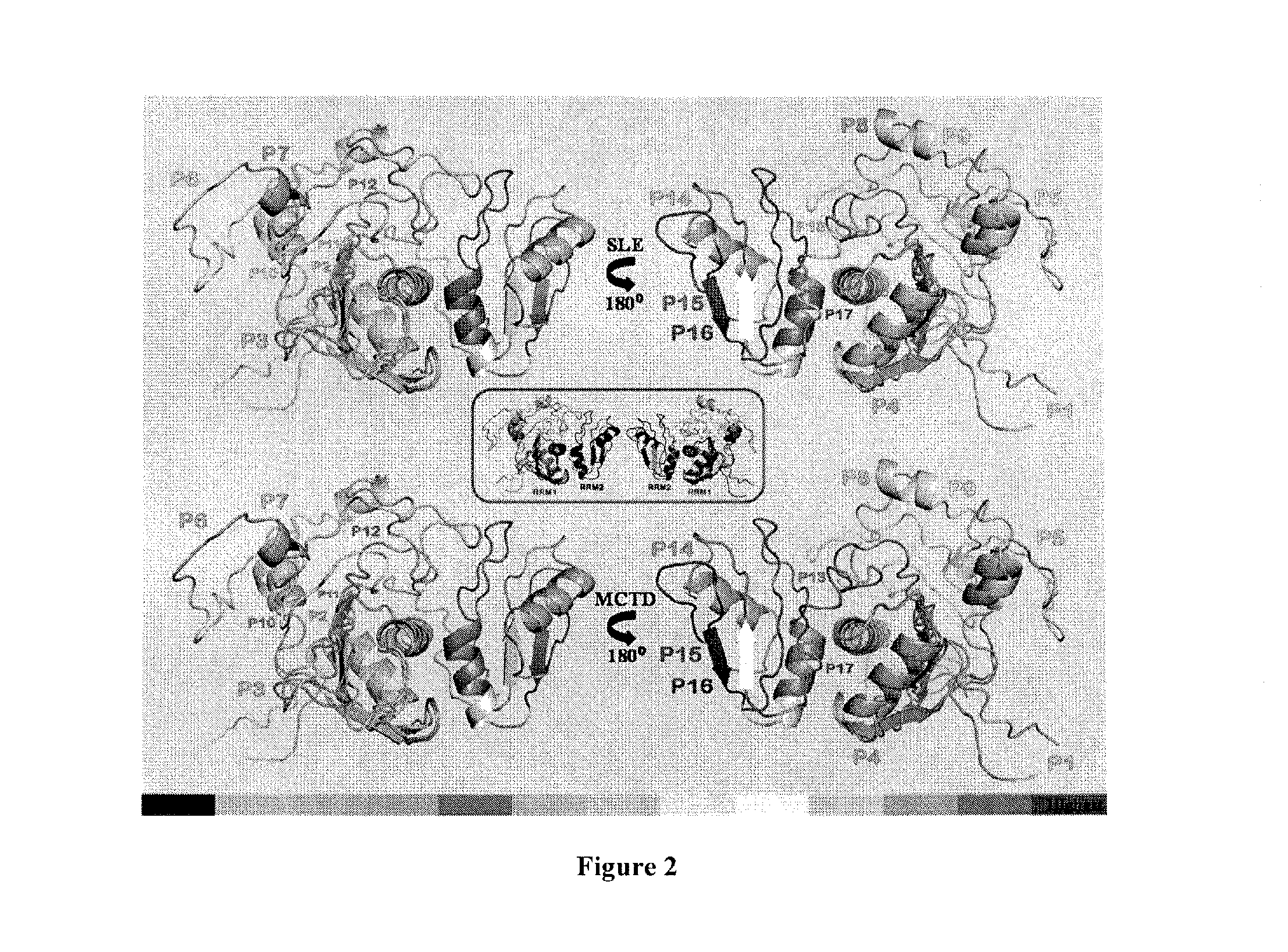
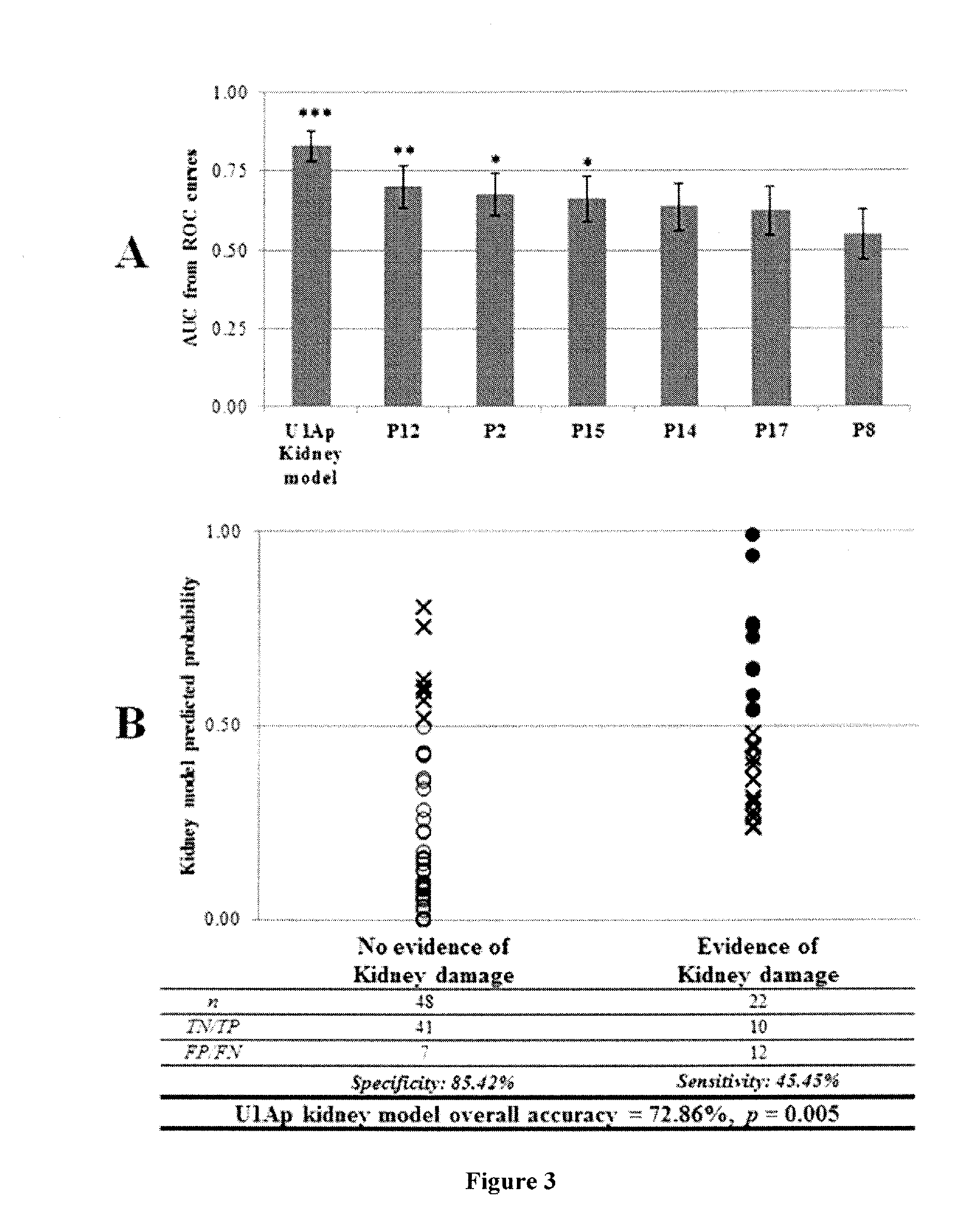
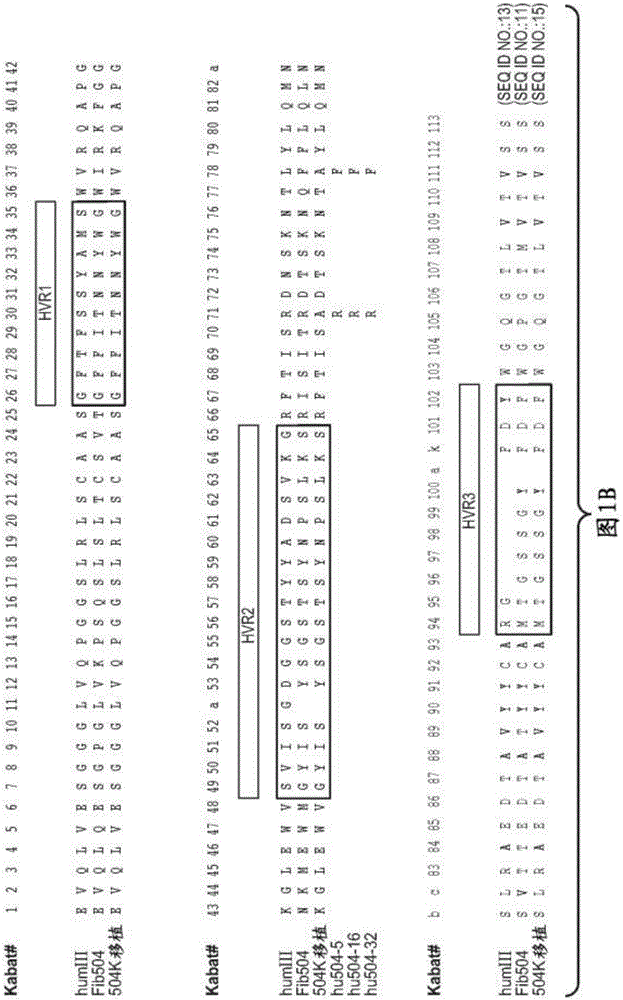
The invention provides methods for identifying the presence of, or an increased risk of developing, organ damage in a subject having an autoimmune disease, for example, Systemic Lupus Erythematosus (SLE) or Mixed Connective Tissue Disease (MCTD), or other disease in which the lungs and / or kidneys are involved. In one embodiment, a significantly increased combined IgM reactivity against the peptides having the sequences of SEQ ID NOs: 4, 9, 12, and 15 in a sample obtained from a patient compared to a healthy control indicates lung damage or an increased risk of developing lung damage in the subject. In another embodiment, a significantly increased combined IgM reactivity against the peptides having the sequences of SEQ ID NOs: 2, 8, 12, 14, 15, and 17, in a sample from a patient compared to a healthy control indicates kidney damage or an increased risk of developing kidney damage in the subject.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Method of Predicting Responsiveness of B Cell Lineage Malignancies to Active Immunotherapy
InactiveUS20140220562A1Microbiological testing/measurementDisease diagnosisTumor responseActive immunization
Predictive biomarkers identify those patients suffering from immunoglobulin positive (Ig+) B lineage malignancies that are responsive to active immunotherapy, where the active immunotherapy comprises vaccination with a tumor-specific idiotype-immunogen. It is shown herein that patient responsiveness to the idiotype-immunogen is dependent upon the sequence of the immunogen, where an immunogen having a low number of tyrosine residues in the CDR1 (herein termed CDR1-Y10) regions of one or both of the immunogen heavy and light chains is predictive of a positive anti-tumor response, while a high number of CDR1 tyrosine residues (herein termed CDR1-Yhi) is predictive of a low anti tumor response.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods for diagnosing and treating inflammatory bowel disease
ActiveCN106102767APeptide/protein ingredientsMicrobiological testing/measurementUlcerative colitisAnti-Beta7
Biomarkers predictive of responsiveness to integrin beta7 antagonists, including anti-beta7 integrin subunit antibodies, and methods of using such biomarkers are provided. In addition, methods of treating gastrointestinal inflammatory disorders such as inflammatory bowel diseases including ulcerative colitis and Crohn's disease are provided. Also provided are methods of using such predictive biomarkers for the treatment of inflammatory bowel diseases including ulcerative colitis and Crohn's disease.
Owner:F HOFFMANN LA ROCHE & CO AG
Use of masitinib for treatment of cancer in patient subpopulations identified using predictor factors
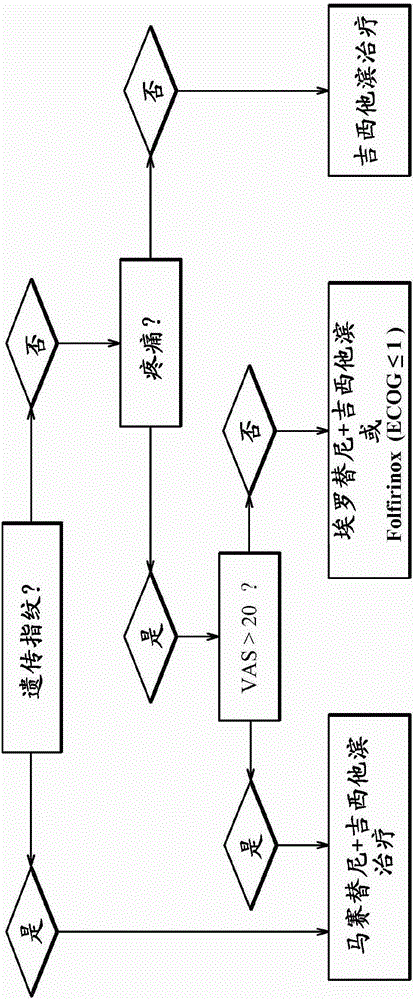
A method for treating patients afflicted with cancer, wherein the patients are treated with a compound including tyrosine kinase inhibitor, mast cell inhibitor or c-Kit inhibitor, in particular masitinib, optionally in combination with at least one antineoplastic agent. The tyrosine kinase inhibitor, mast cell inhibitor or c-Kit inhibitor, and the optional at least one antineoplastic agent, are administered in a dosage regimen that includes a therapeutically effective amount. Also described are methods for predicting therapeutic response to the treatment in a given patient and therefore identification of applicable patient subpopulations based upon these predictor factors; sometimes referred to as biomarkers. One method is based upon the clinical marker of pain intensity. Another method is based upon gene expression predictive biomarkers assessed via RNA expression in peripheral blood cell samples collected prior to treatment with the compound, which is also used for treating patients afflicted with pancreatic cancer.
Owner:AB SCIENCE
Method for determining radiosensitivity
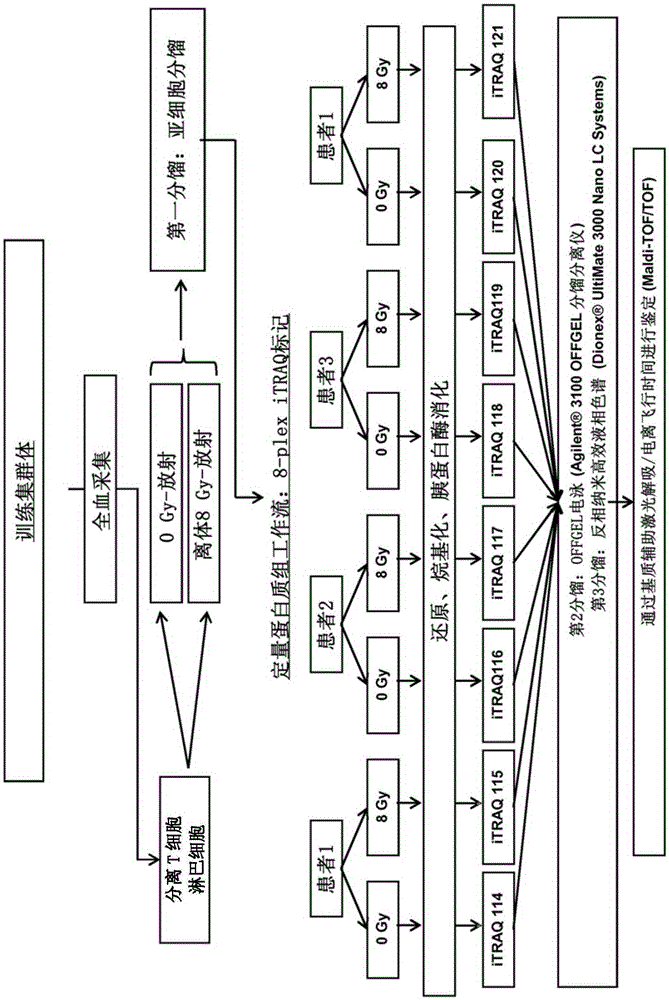
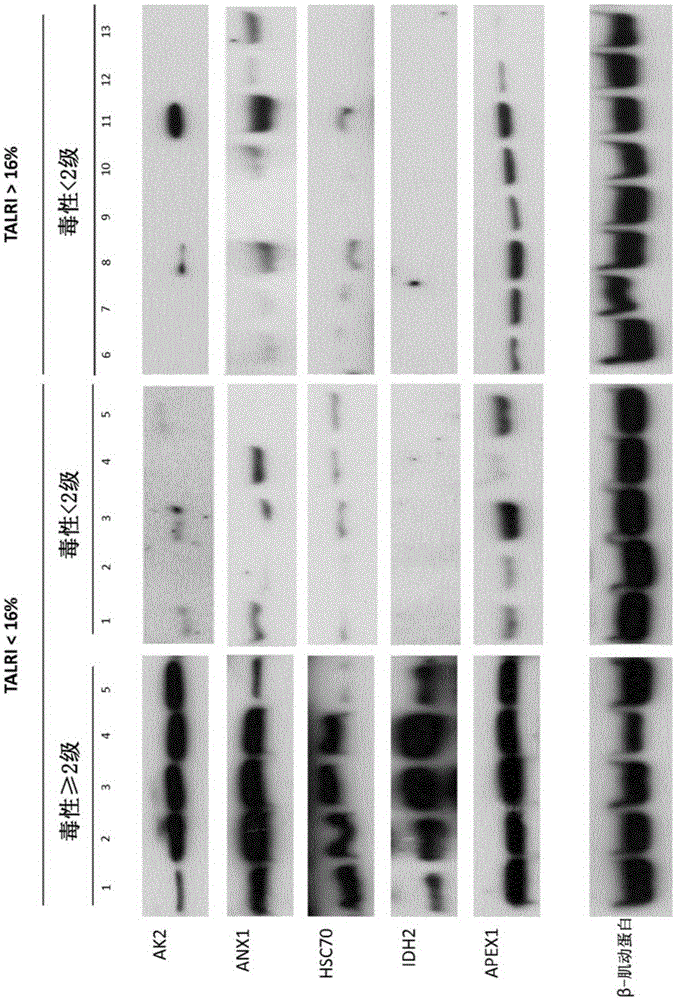
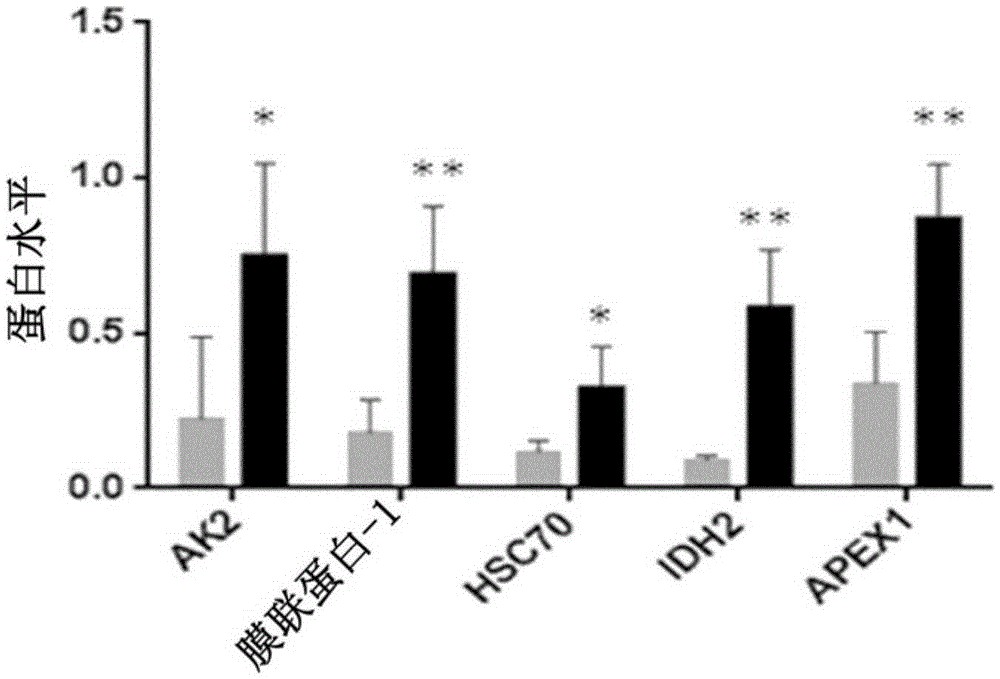
InactiveCN105263578ADisease diagnosisX-ray/gamma-ray/particle-irradiation therapyReference samplePredictive biomarker
The present invention relates to a method for the in vitro determination of the radiosensitivity of a subject. More particularly, the invention relates to a method comprising a step of inducing an exogenous stress on a biological sample from a subject, followed by the comparison of the presence or level of at least one compound chosen in a group of defined compounds, in said biological sample and in a reference sample. The present invention also relates to the use of said at least one compound as predictive biomarker of the radiosensitivity of a subject. The invention also relates to a kit for the detection of the presence or level of at least one of said compounds, usable in a method according to the invention.
Owner:蒙彼利埃大学医疗中心 +2
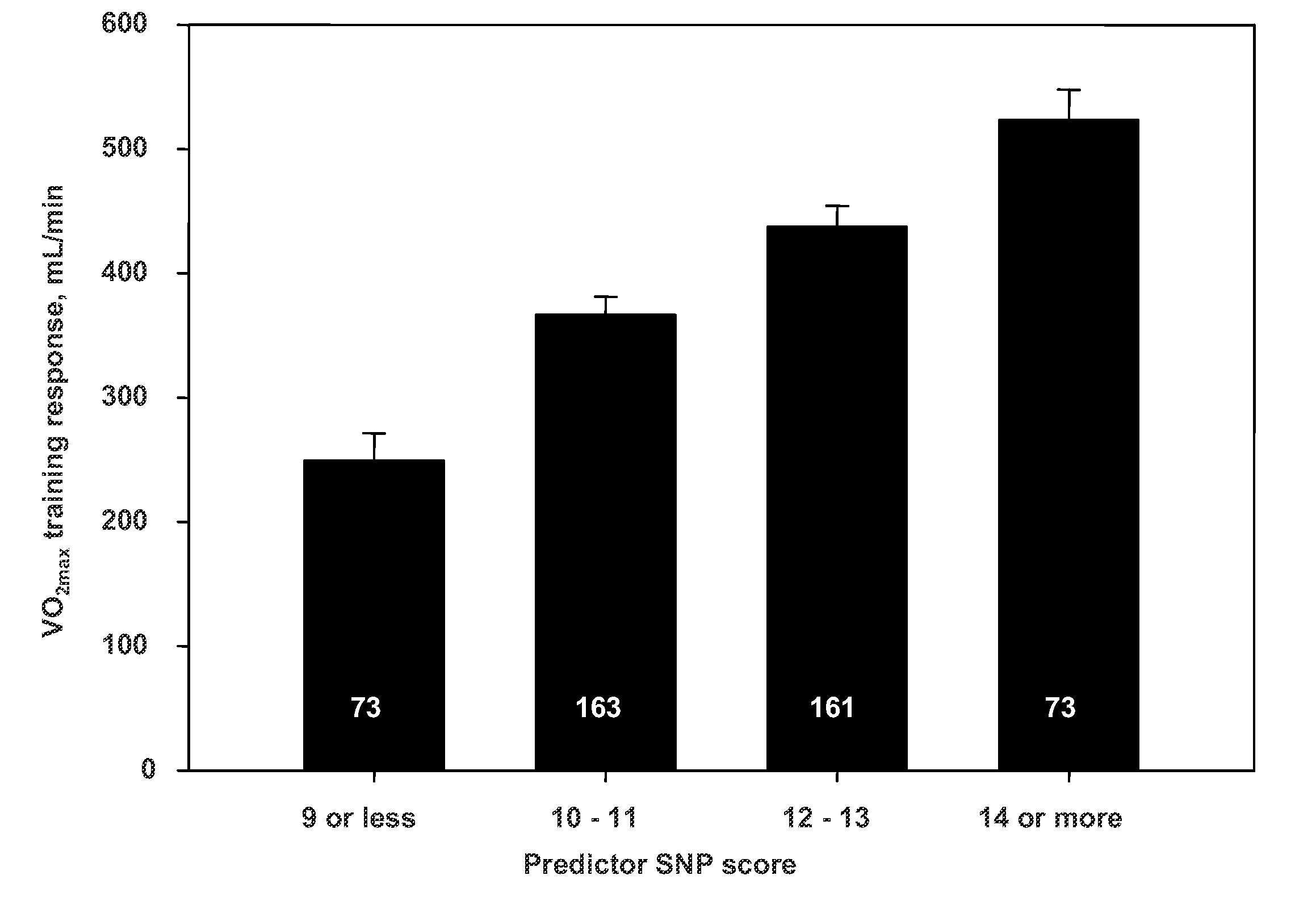
Predictive Biomarkers for Response to Exercise
InactiveUS20110195412A1Increase capacityImprove responsivenessSugar derivativesMicrobiological testing/measurementVascular diseaseMedicine
A set of biomarkers have been identified that allows one to predict subjects who will respond to an exercise regime in term of cardiorespiratory fitness as assessed by maximal oxygen uptake. These predictions may be used, for example, to predict risk of cardiovascular disease, to design a more effective program for cardiac rehabilitation, to predict capacity for athletic performance or physically demanding occupation, and to predict ability to maintain functional capacity with aging using exercise.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com