Patents
Literature
124 results about "Tumor response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Structure and use of 5' phosphate oligonucleotides
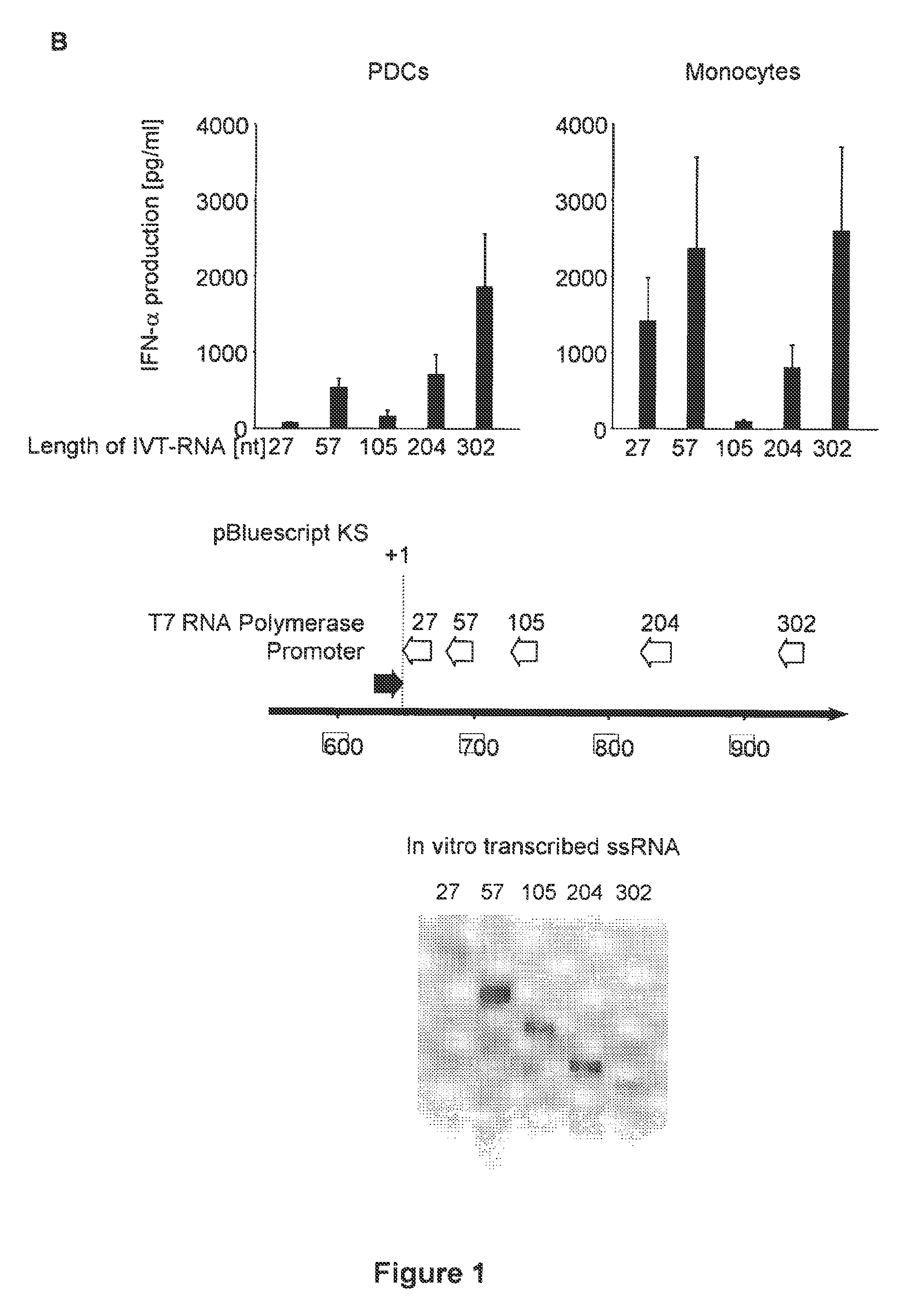
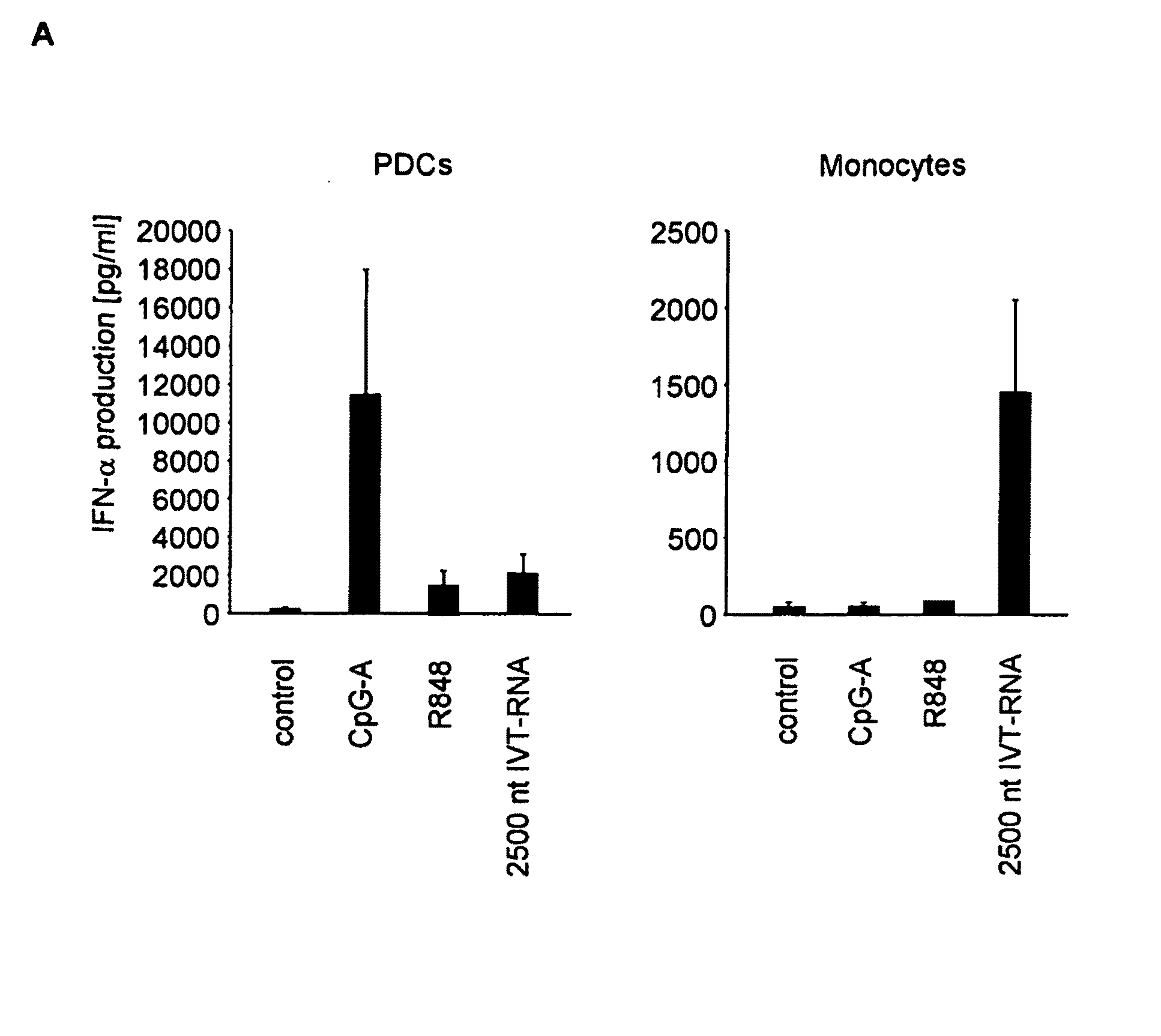
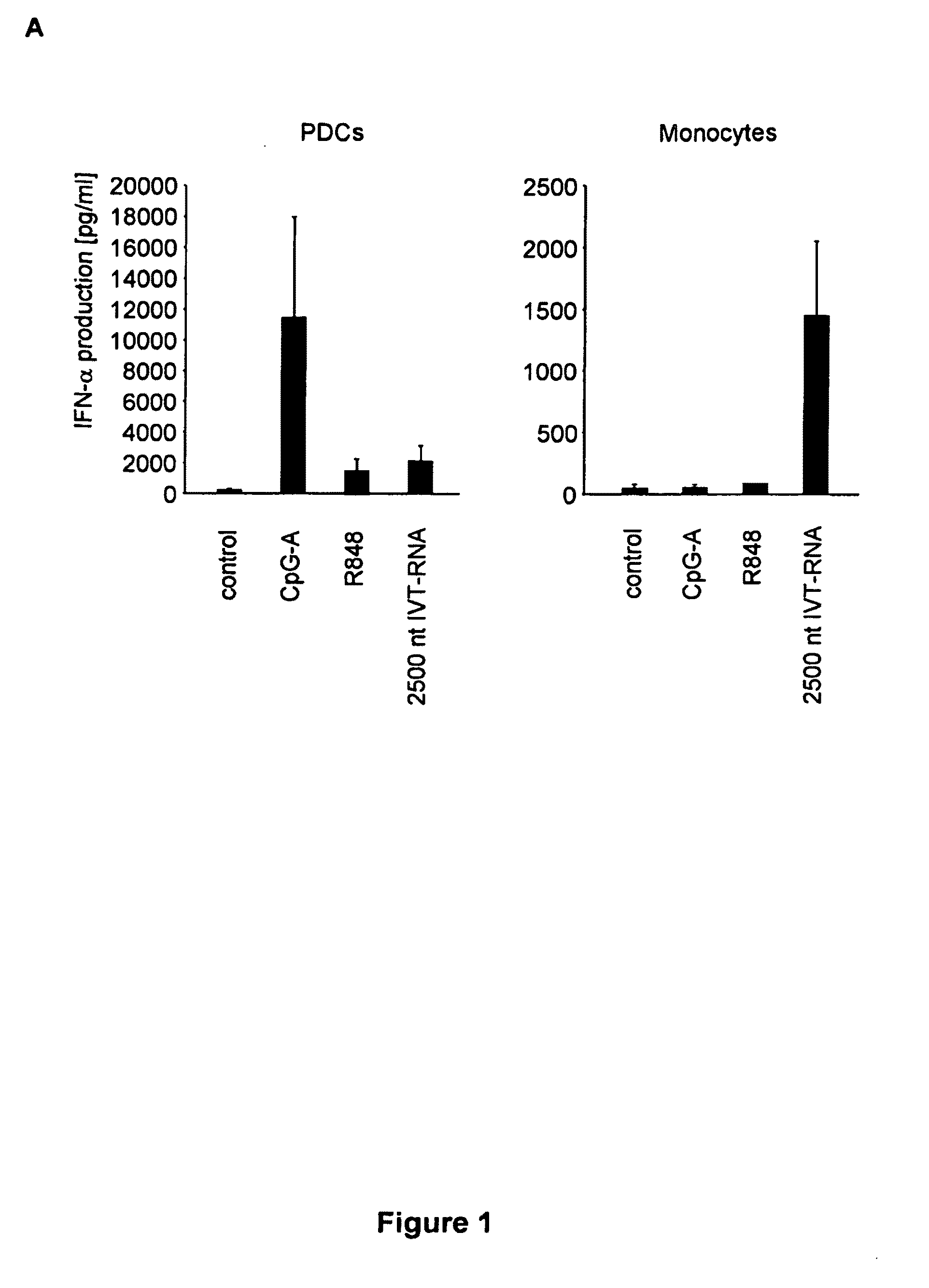
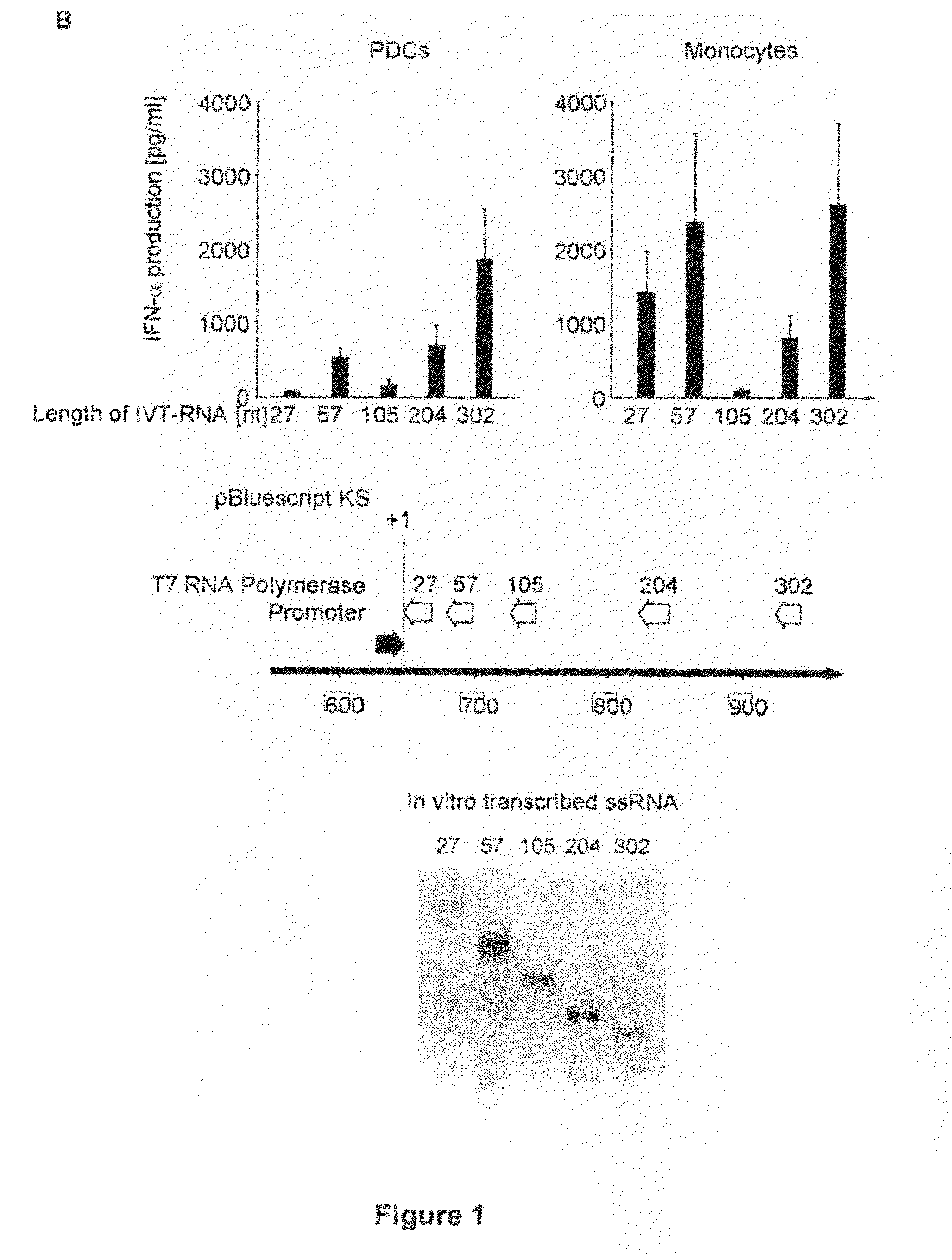
Oligonucleotides bearing free, uncapped 5′ phosphate group(s) are recognized by RIG-I, leading to the induction of type I IFN, IL-18 and IL-1β production. Bacterial RNA also induces type I IFN production. 5′ phosphate oligonucleotides and bacterial RNA can be used for inducing an anti-viral response or an anti-bacterial response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in vitro and in vivo and for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, tumors, allergies, autoimmune diseases, immunodeficiencies and immunosuppression. Single-stranded 5′ triphosphate RNA can be used for inducing an anti-viral response, an anti-bacterial response, or an anti-tumor response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in a target cell-specific manner.
Owner:UNIVERSITY OF BONN
Biomarkers for cancer treatment
ActiveUS20080131885A1Reduce healingAnalysis using chemical indicatorsSugar derivativesTumor responseWilms' tumor
The present invention provides identification of a thirty-five gene set that predicts the anticancer activity of inhibitors of the RAF / MEK / MAPK pathway, methods of qualifying cancer status in a subject, methods of identifying an anti-tumor response in a subject, methods of monitoring the efficacy of a therapeutic drug in a subject, and methods of identifying an agent useful in the treatment of a cancer based on expression of the thirty-five gene set.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
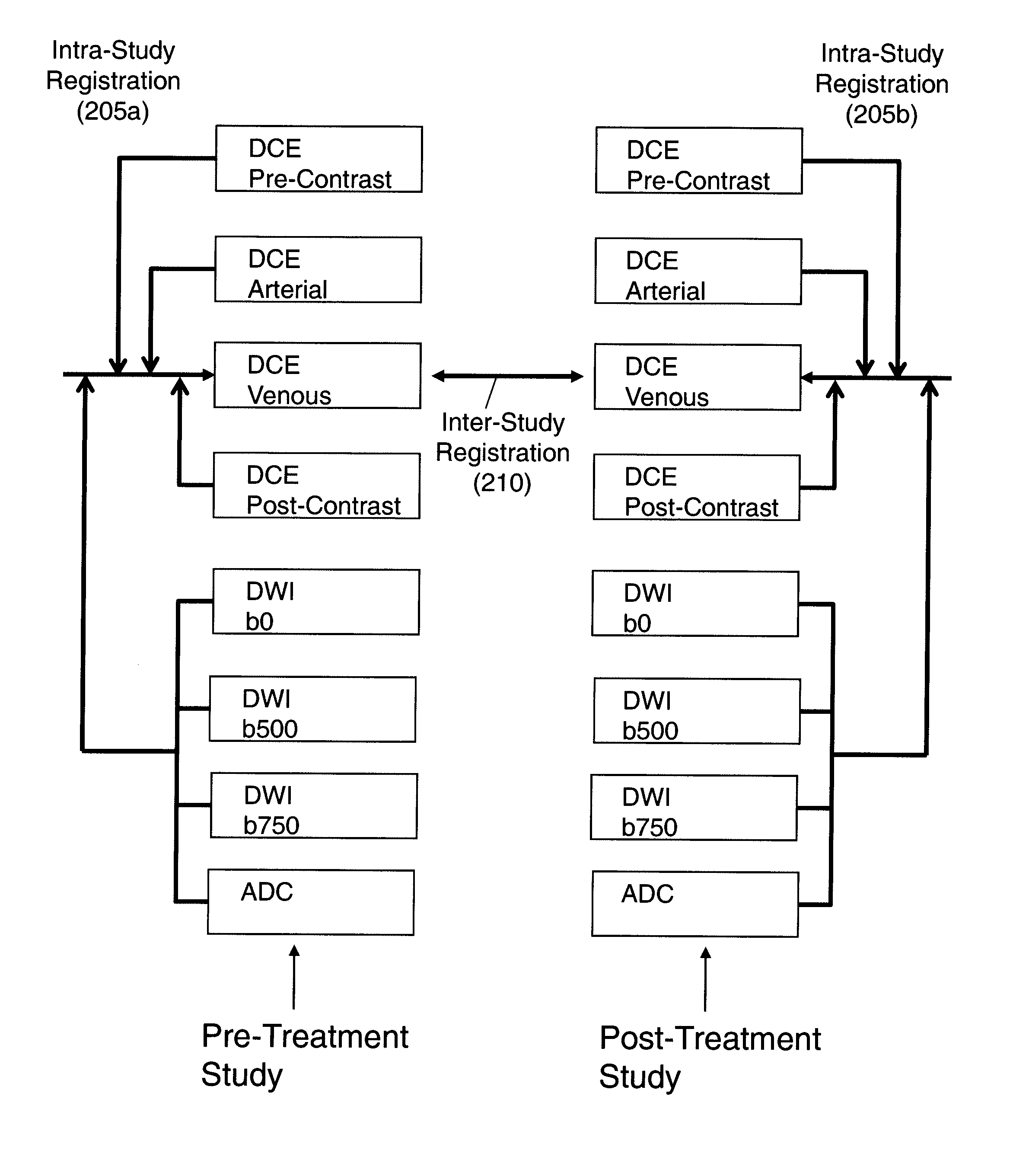
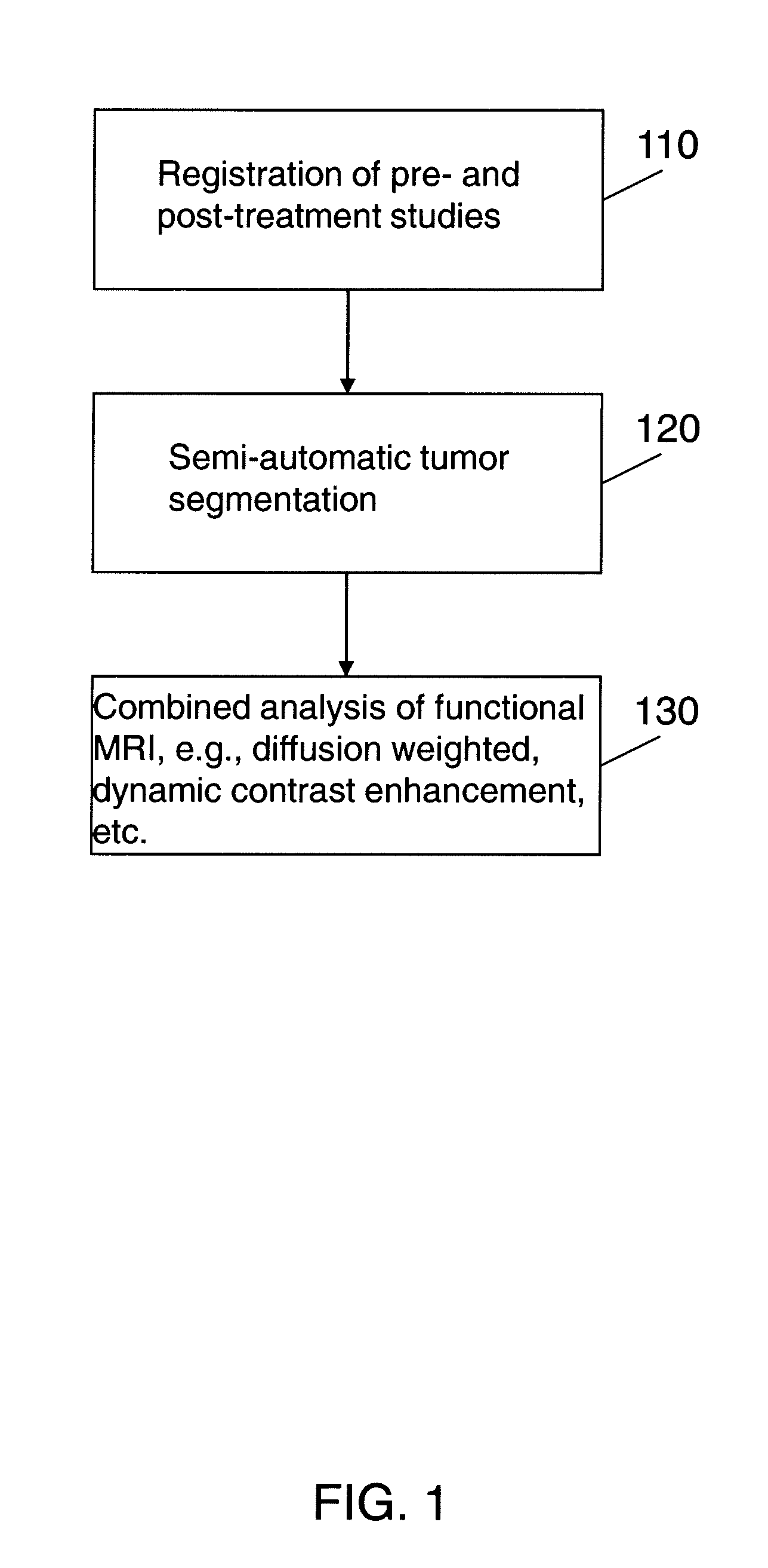
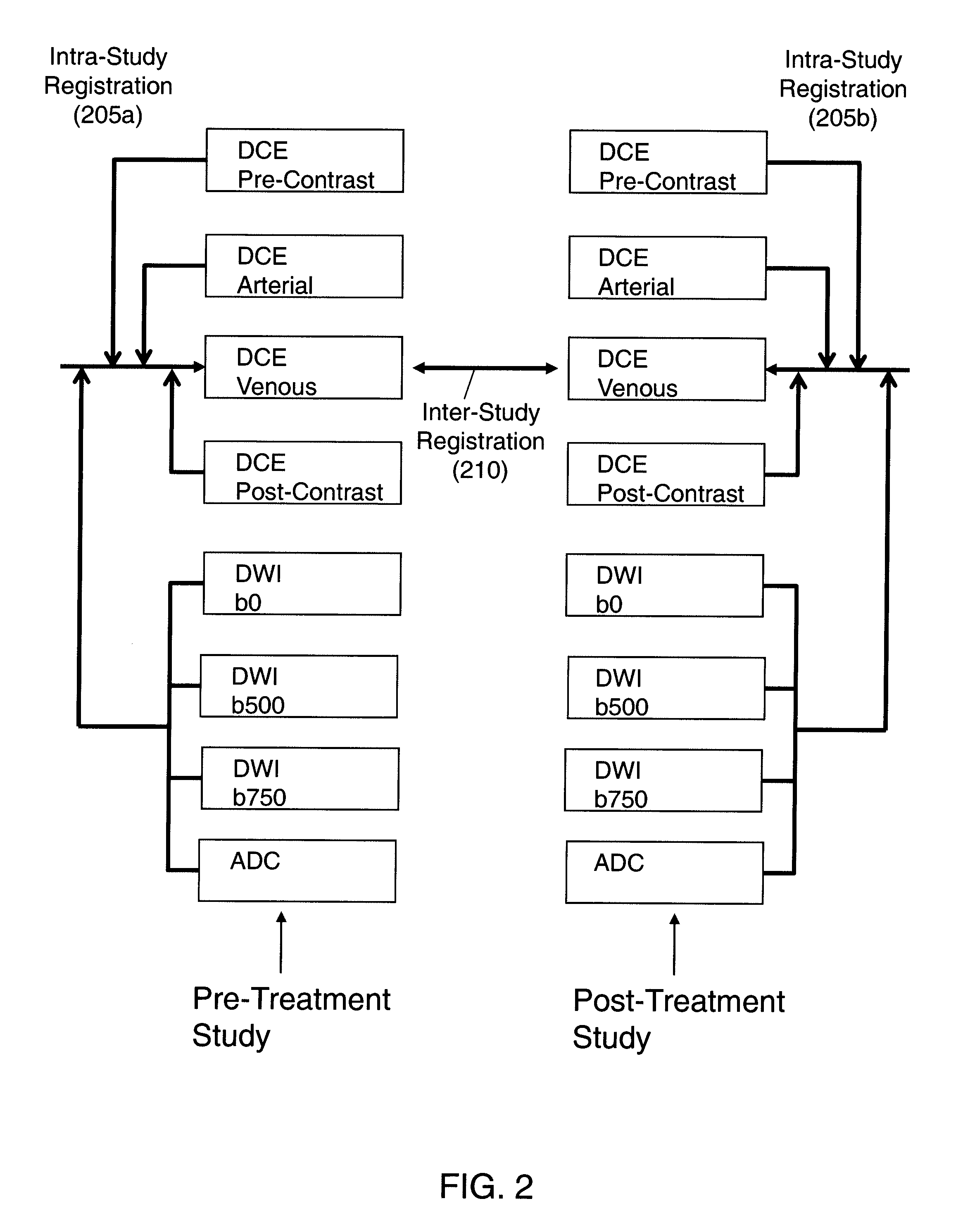
Automated method for assessment of tumor response to therapy with multi-parametric MRI
A method for assessing a tumor's response to therapy, includes providing images of a first study of a patient and images of a second study of the patient, the second study occurring after the first study and after the patient undergoes therapy to treat a tumor, each study comprising first and second types of functional magnetic resonance (fMR) images, performing a first registration in which the images within each study are registered, performing a second registration in which reference images from both studies are co-registered, segmenting the tumor in an image of each of the second registered studies; and determining that first and second fMR measure differences exist between the segmented tumor's of the first and second studies, the first fMR measure difference being obtained from the first type of fMR images, the second fMR measure difference being obtained from the second type of fMR images.
Owner:SIEMENS AG
Structure and use of 5'phosphate oligonucleotides
InactiveUS20100178272A1Antibacterial agentsOrganic active ingredientsImmunologic disordersAutoimmune condition
Owner:KLINISCHE PHARMAKOLOGIE
Pd-1 antibodies in combination with a cytokine-secreting cell and methods of use thereof
InactiveUS20100285013A1Good curative effectBiocidePeptide/protein ingredientsTumor responseSecreting cell
Owner:ER SQUIBB & SONS INC +1
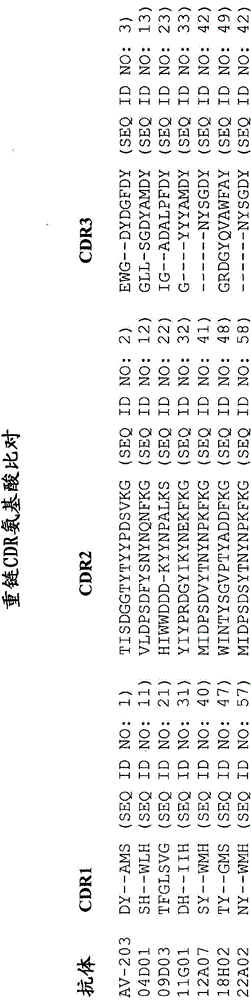
Immunoglobulin construct containing anti-mucin variable domain sequences for eliciting an anti-idiotype anti-tumor response
InactiveUS20040143101A1Improve expression levelImprove the level ofImmunoglobulin superfamilyAntibody mimetics/scaffoldsAntigenTumor response
The present invention provides a variant of an immunoglobulin variable domain including (A) at least one CDR region and (B) framework regions flanking the CDR region, wherein the variant also contains (a) a CDR region having added or substituted therein at least on binding sequence and (b) the flanking framework regions, wherein the binding sequence is heterelogous to the CDR and is an antigenic sequence from a MUC-1 binding sequence.
Owner:EURO-CELTIQUE SA
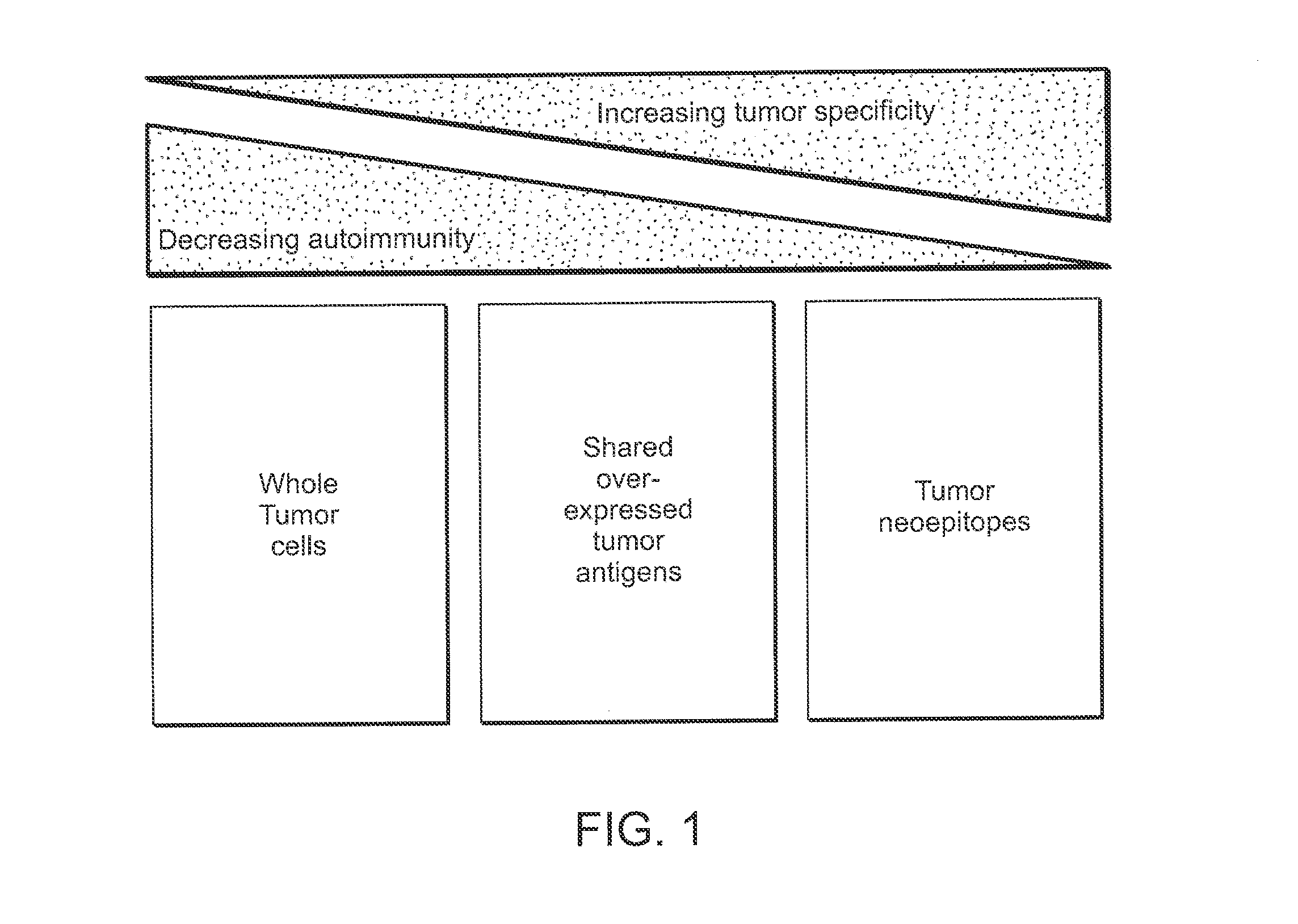
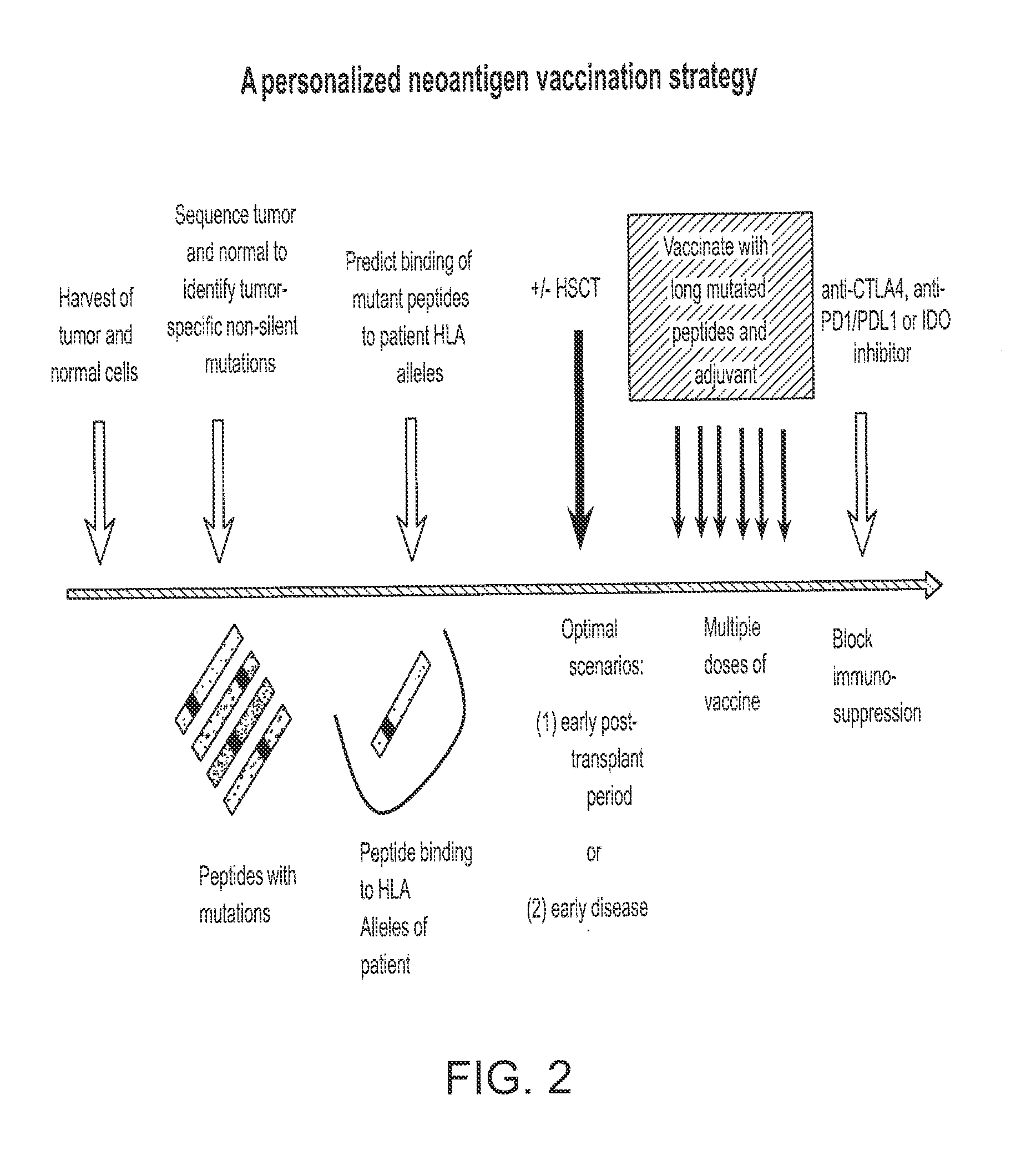
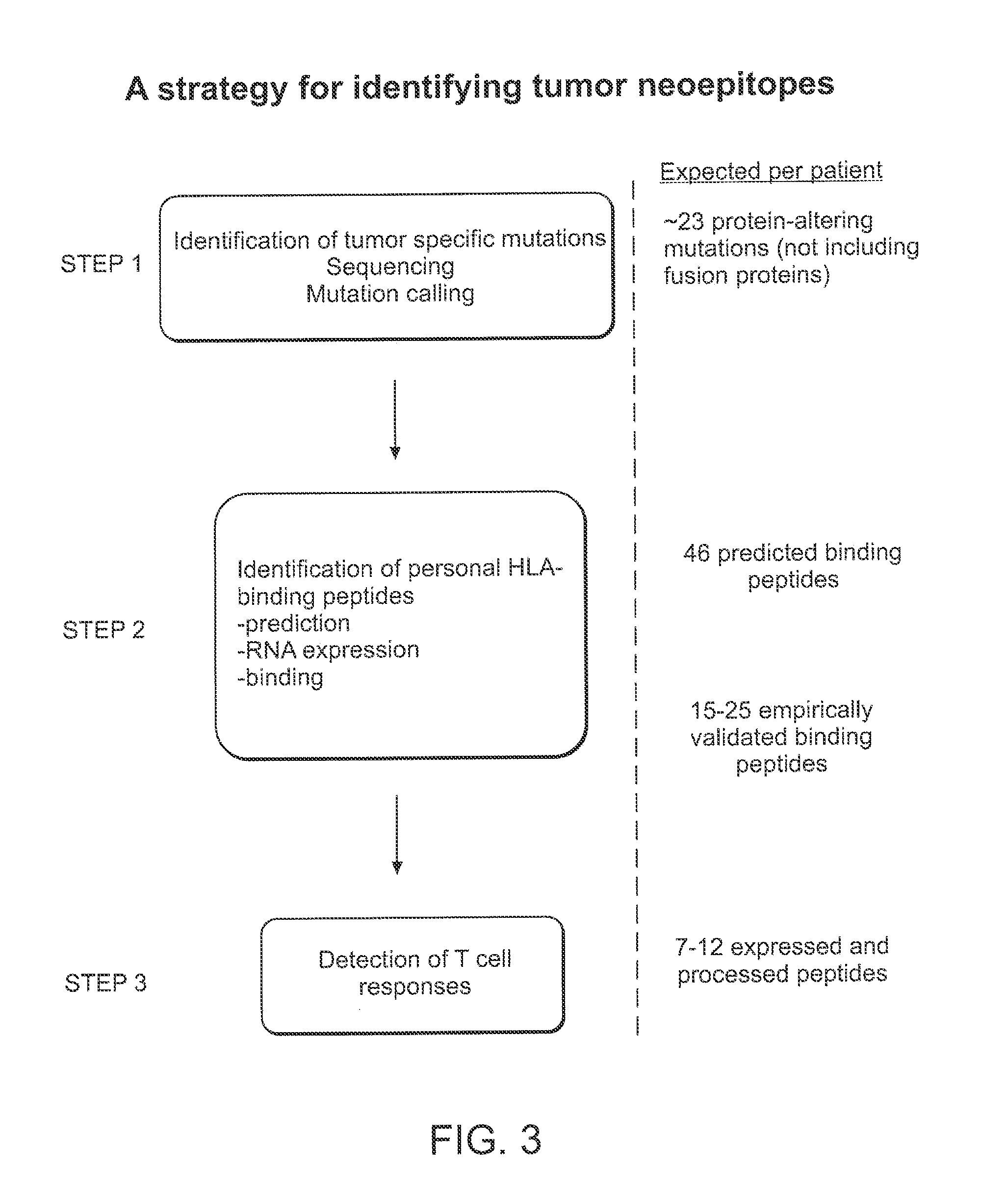
Compositions and methods of identifying tumor specific neoantigens
The present invention related to immunotherapeutic peptides and their use in immunotherapy, in particular the immunotherapy of cancer. Specifically, the invention provides a method of identifying tumor specific neoantigens that alone or in combination with other tumor-associated peptides serve as active pharmaceutical ingredients of vaccine compositions which stimulate anti-tumor responses.
Owner:THE GENERAL HOSPITAL CORP +1
Predicting tumor response to anti-ERBB3 antibodies
A diagnostic method for predicting quantitatively whether a human tumor will be sensitive or resistant to treatment with an ERBB3 inhibitor, e.g, an anti-ERBB3 antibody, is disclosed. The method is based on measurement of NRG1 expression at the RNA level, or at the protein level, in a tissue sample from the tumor.
Owner:AVEO PHARM INC
Cancer immunotherapy
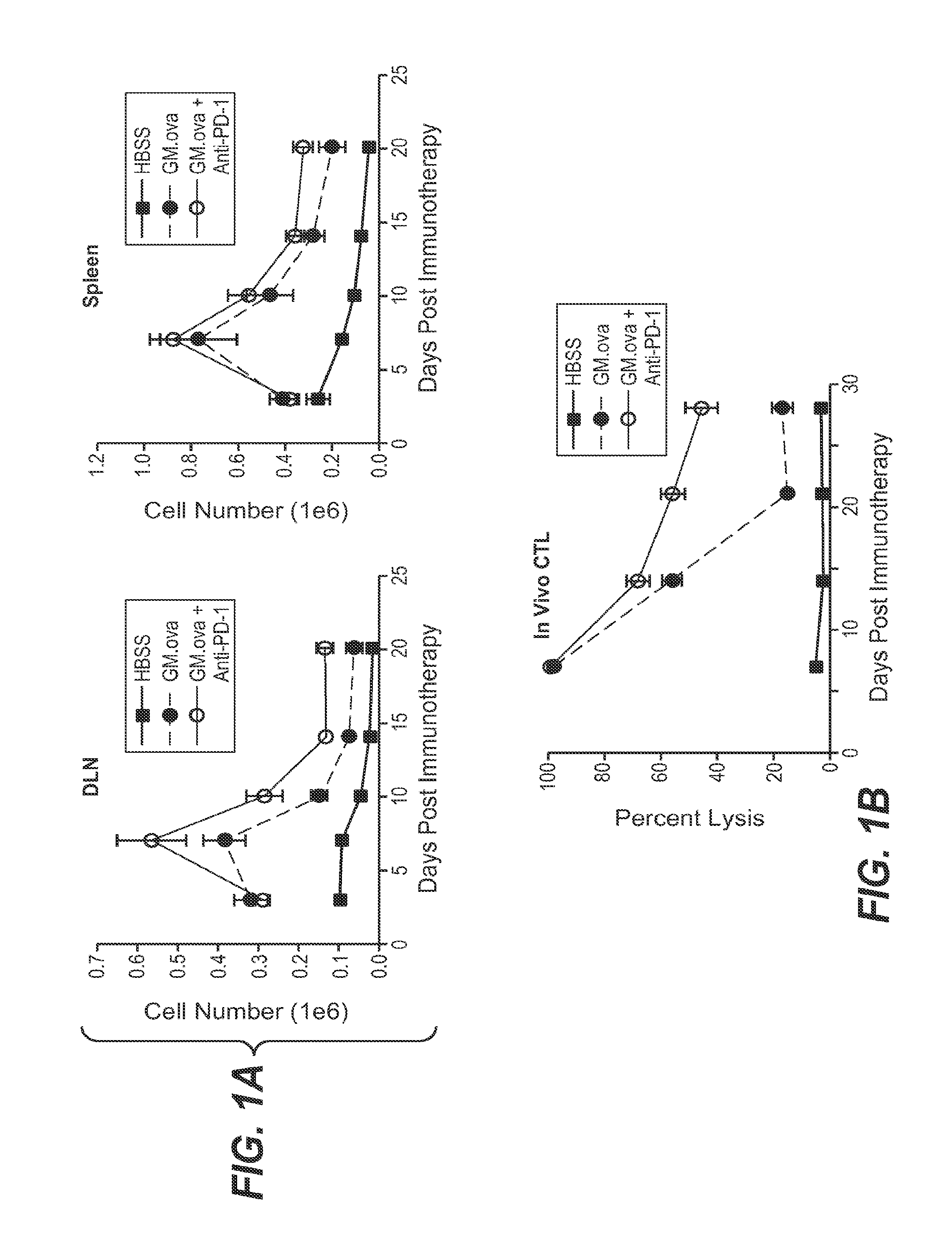
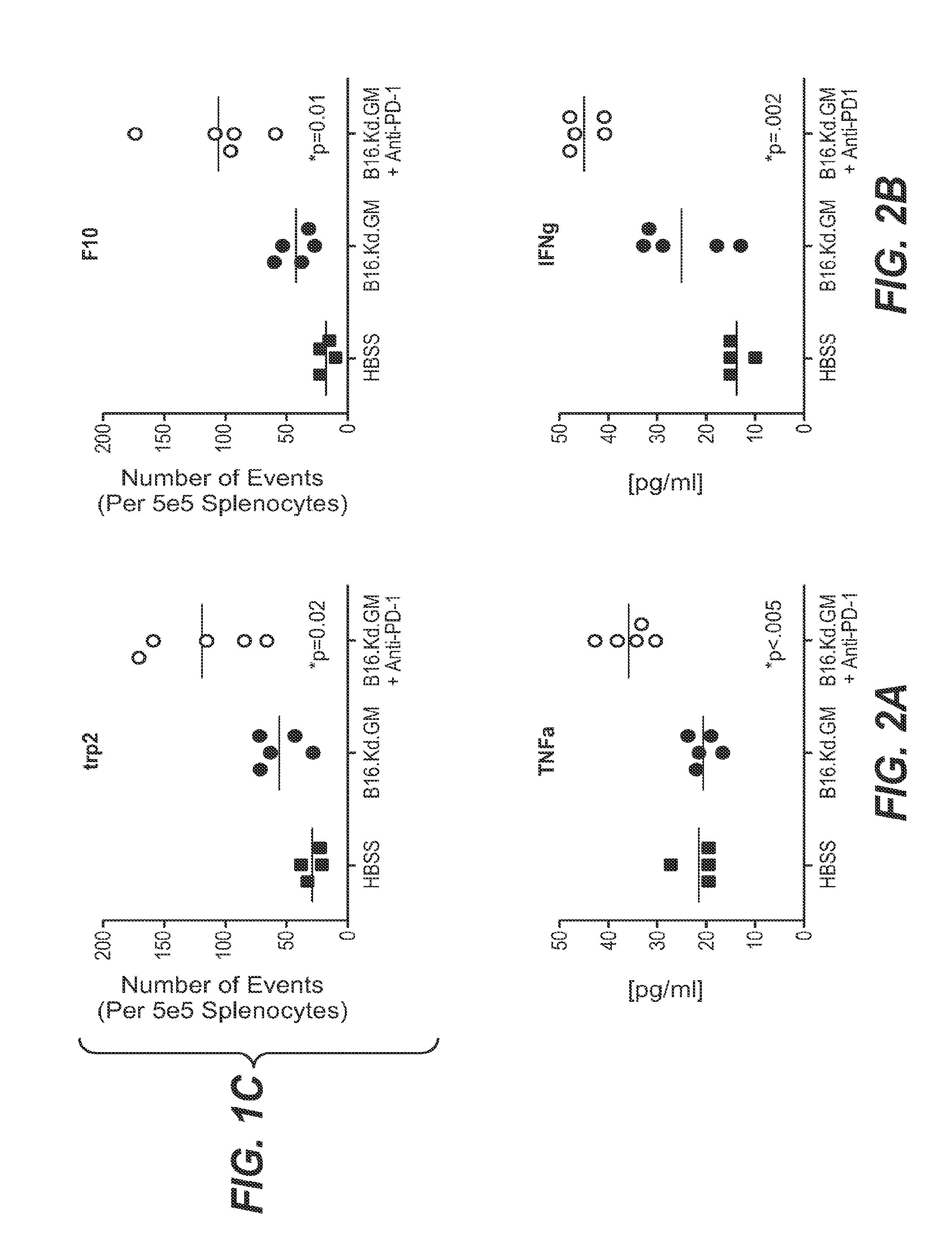
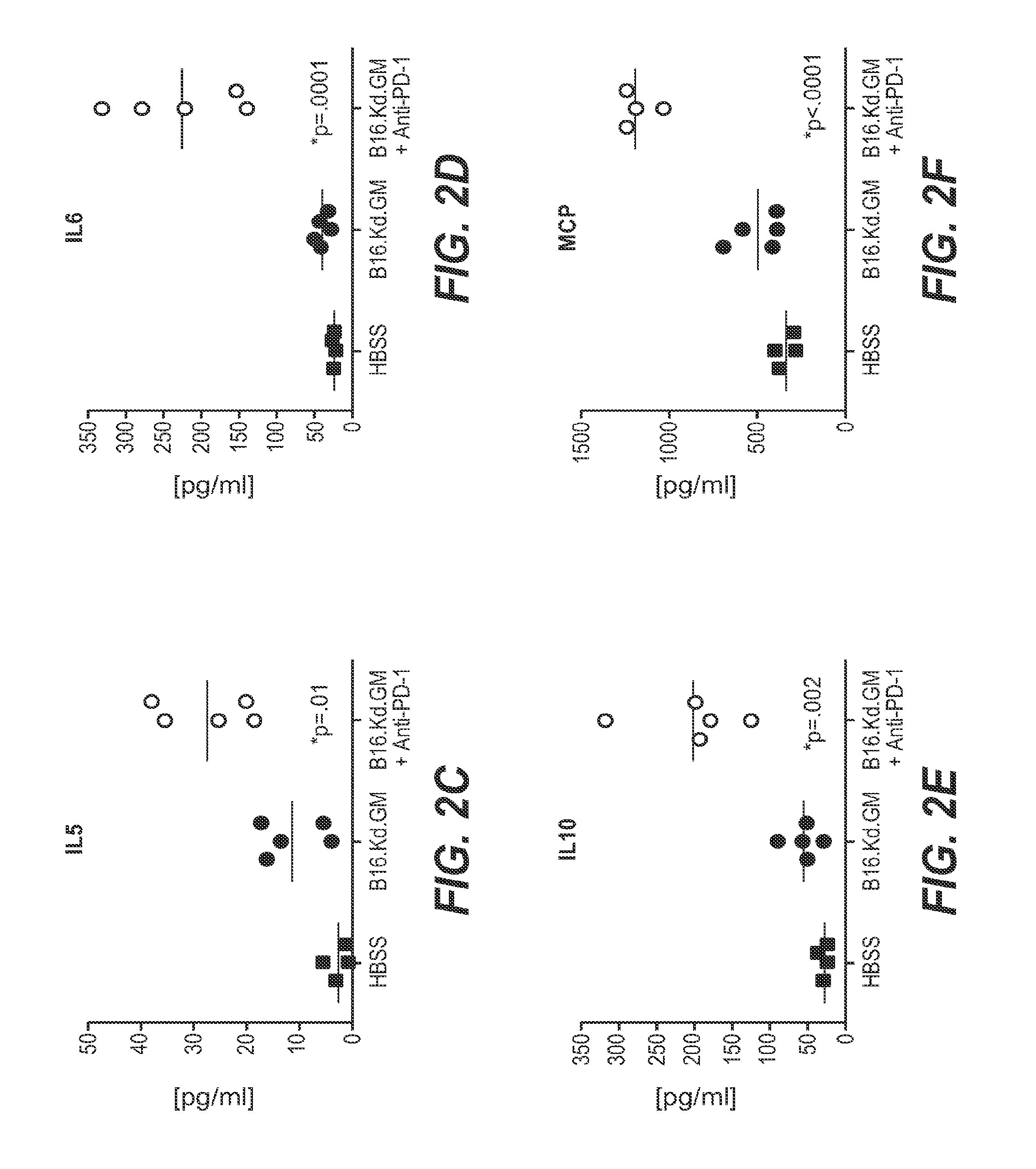
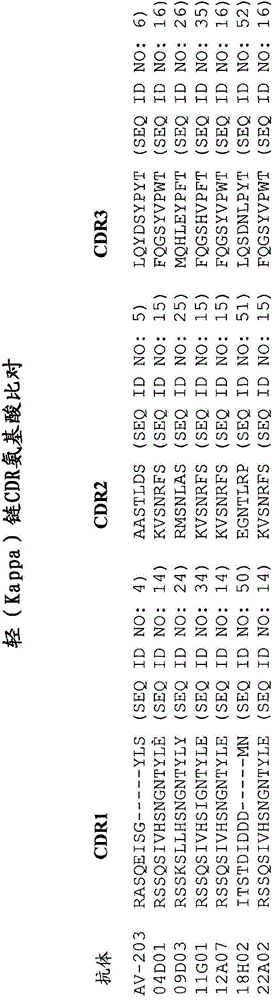
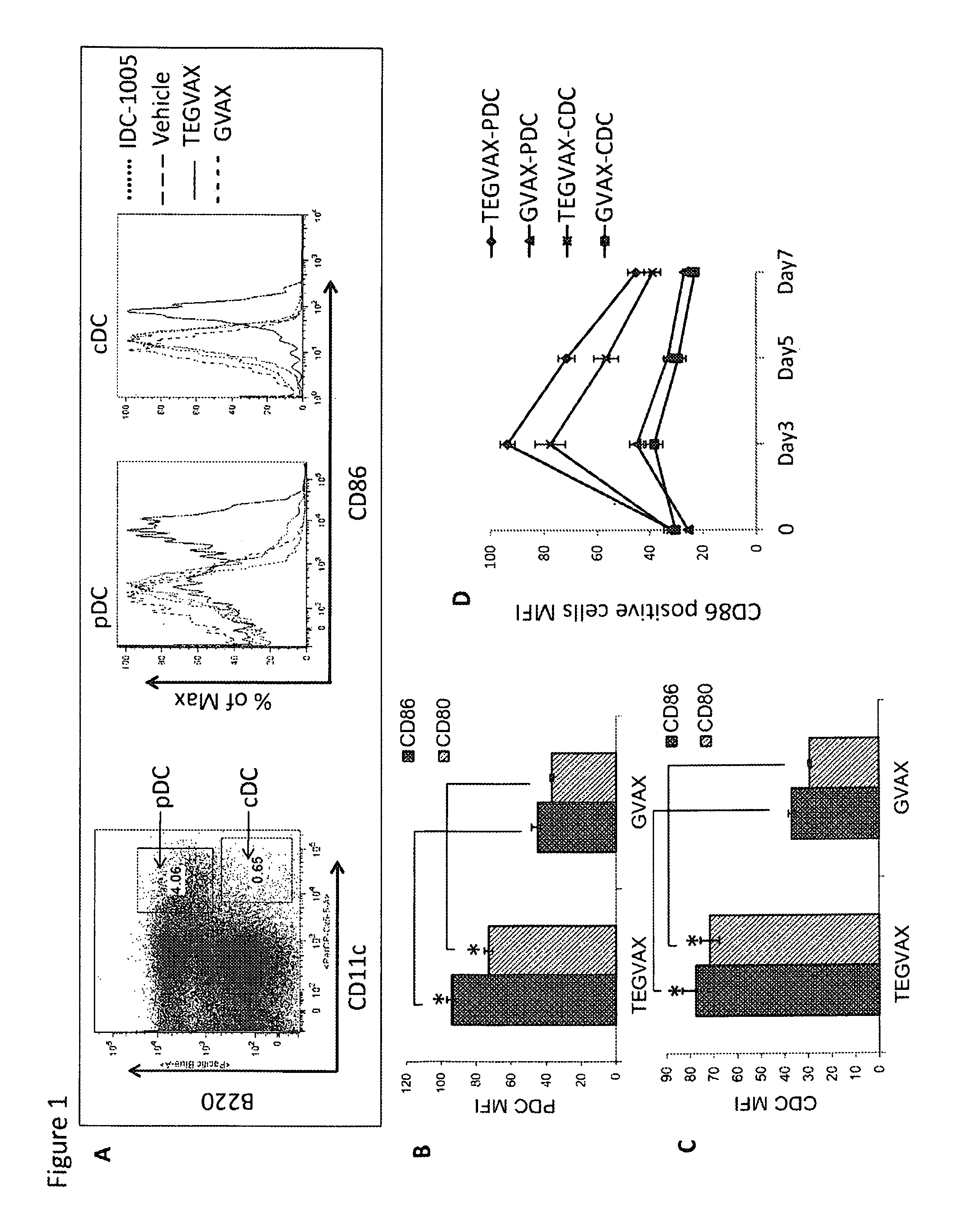
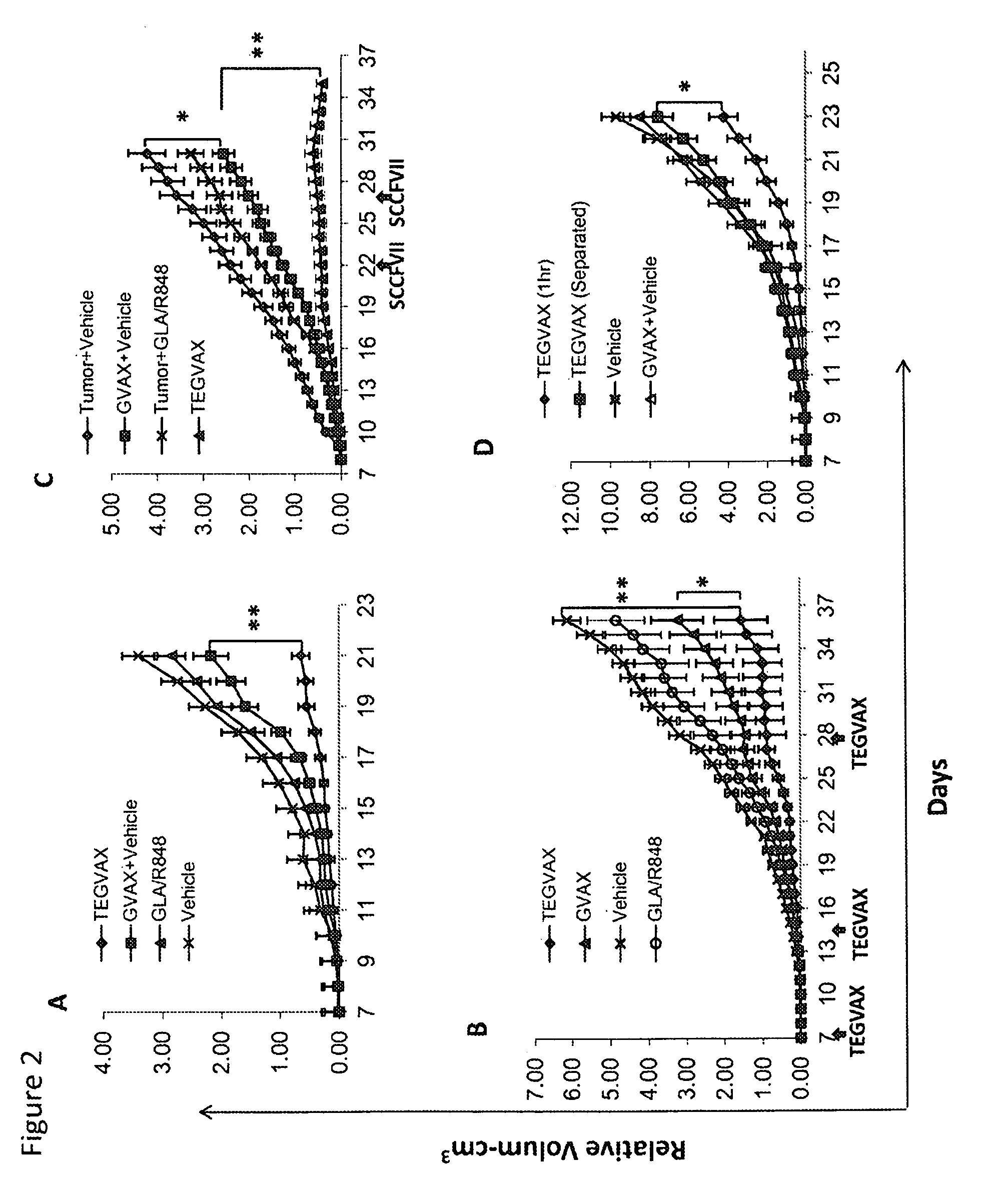
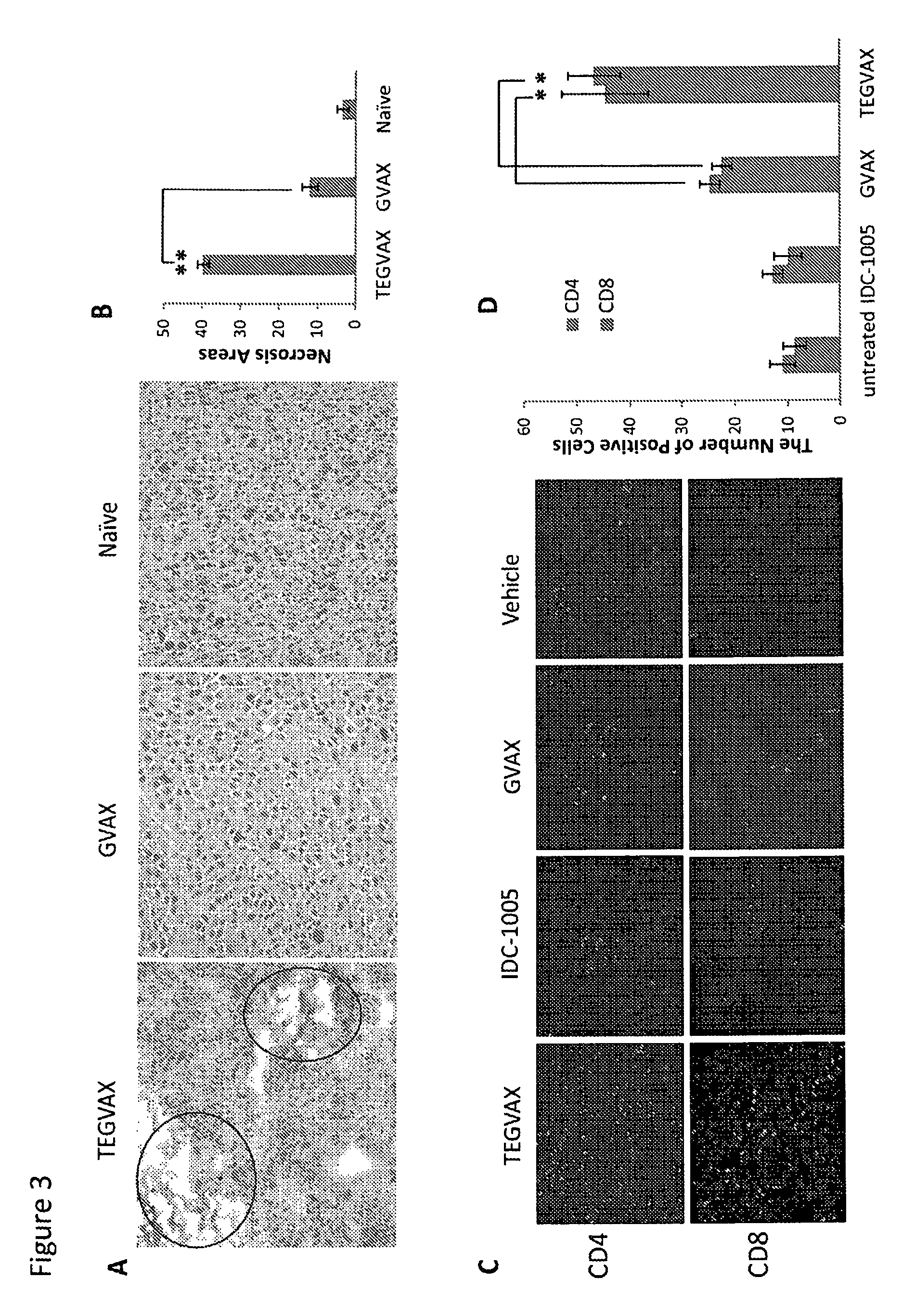
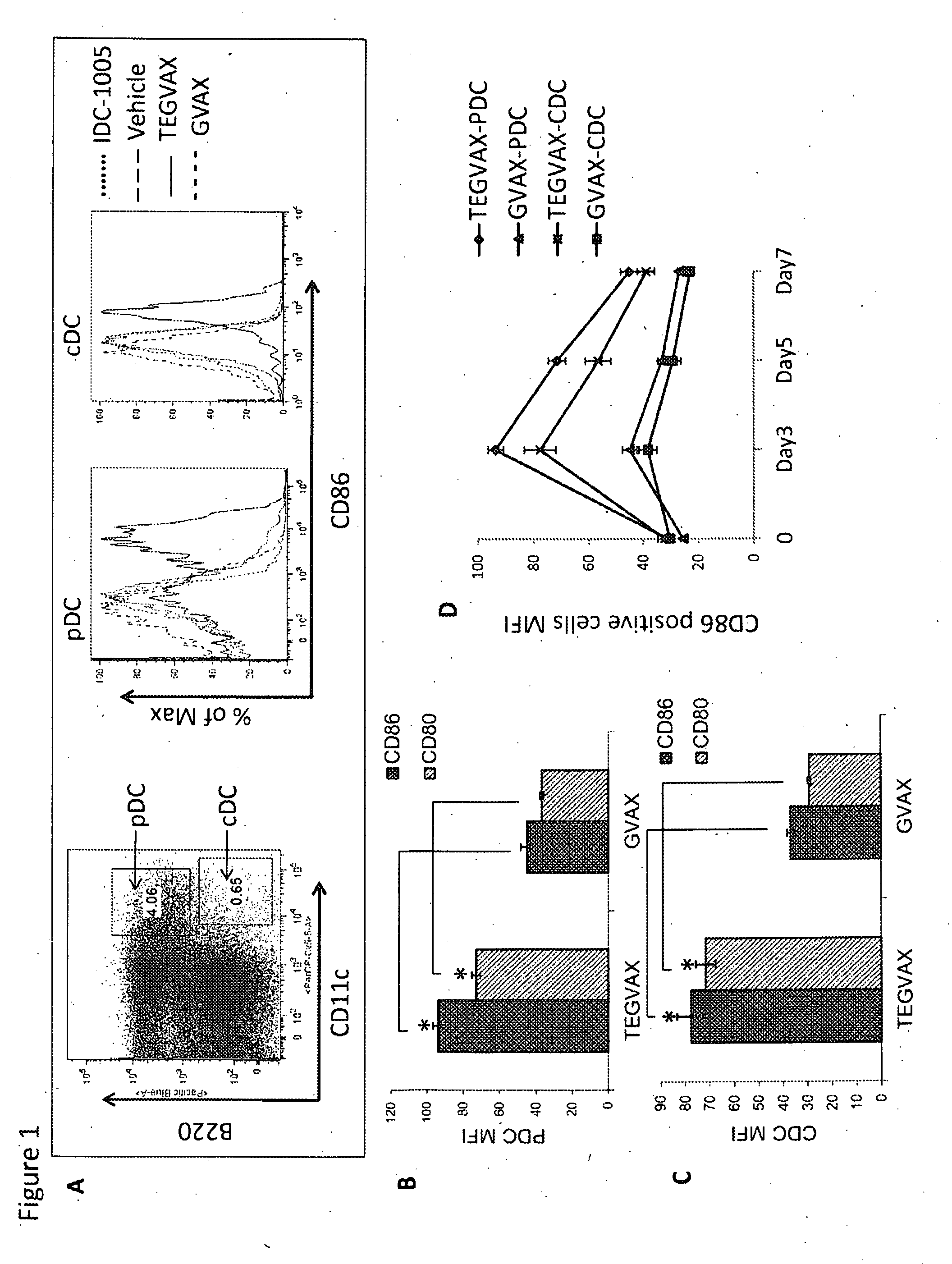
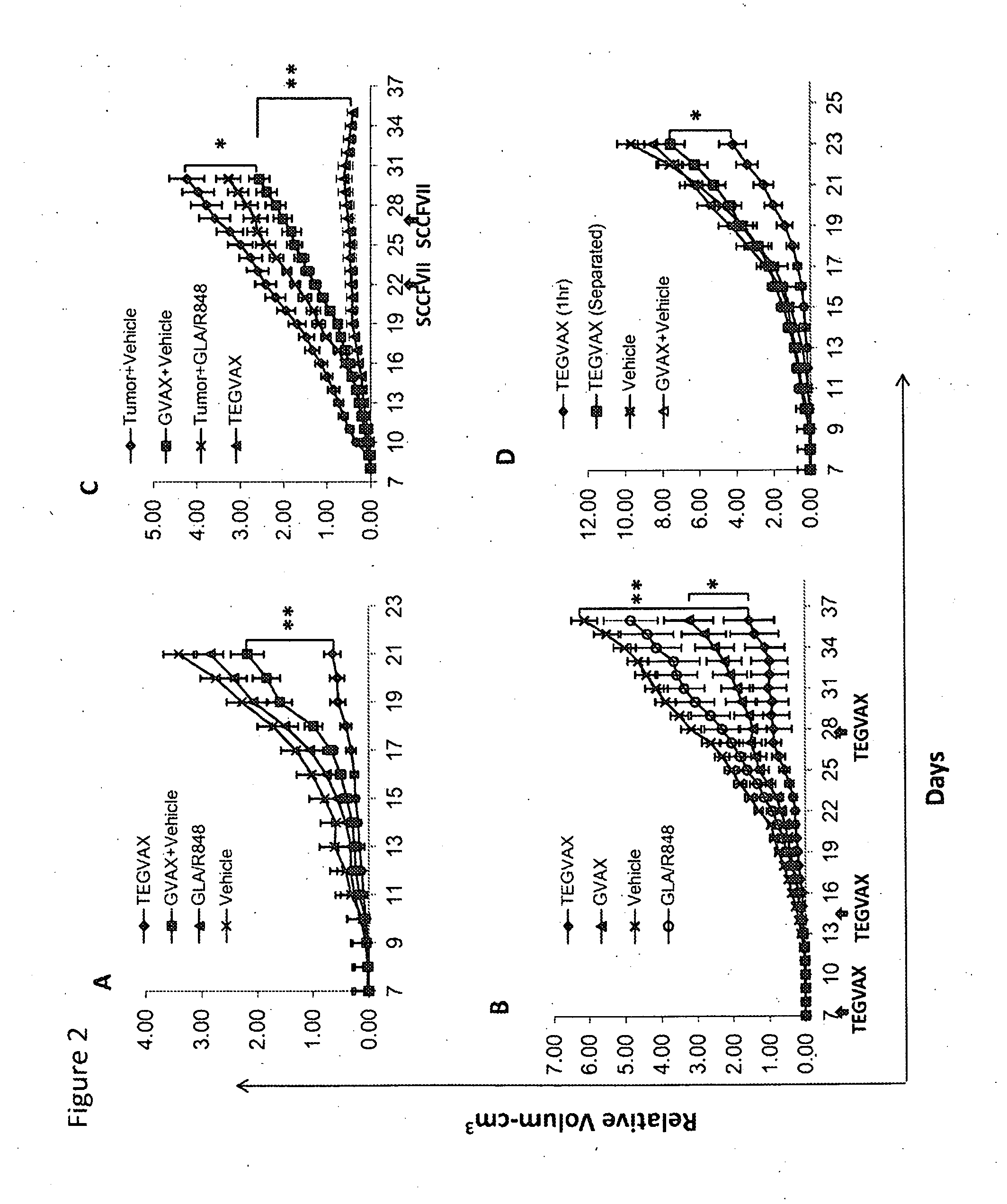
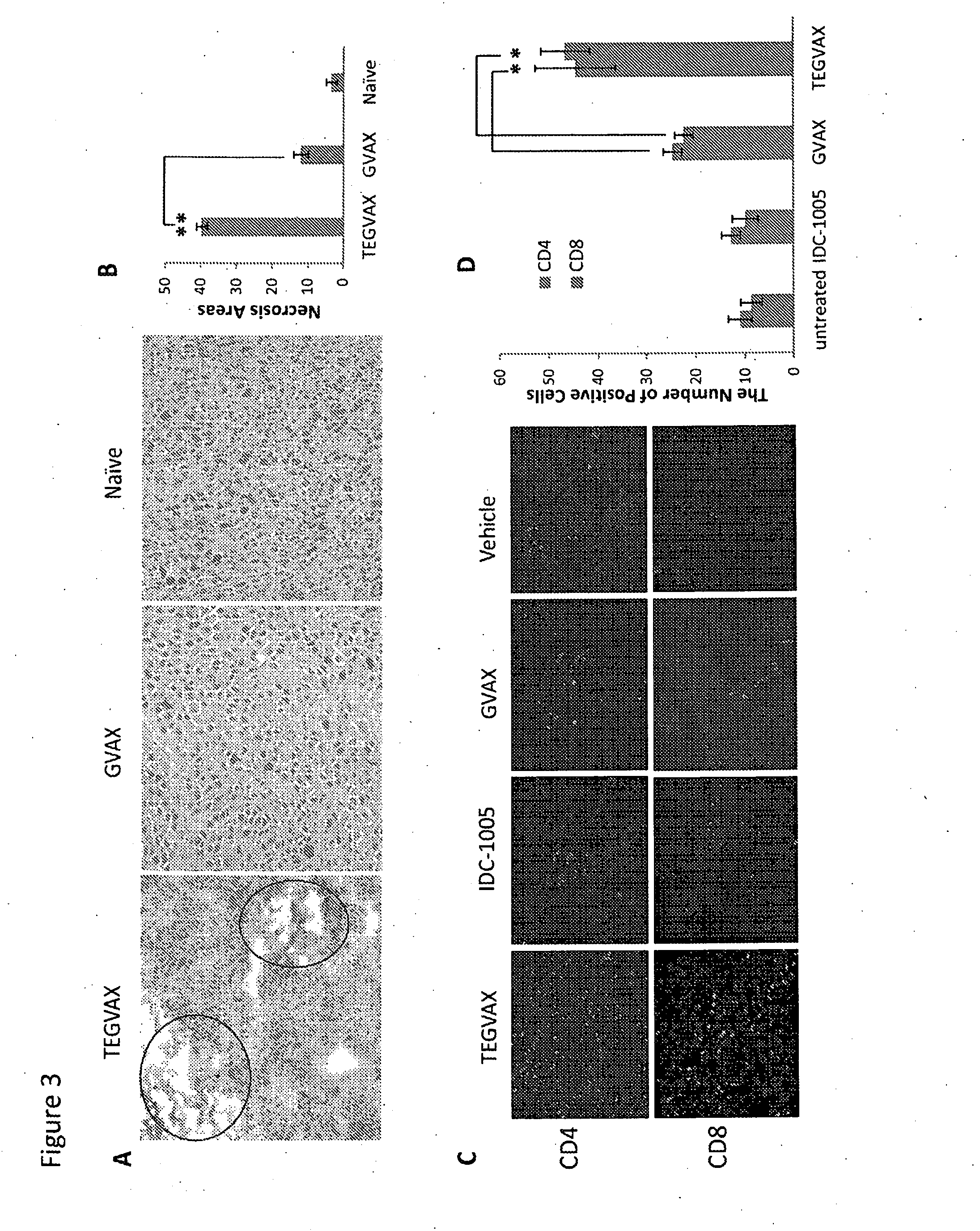
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Biomarkers for antibody-drug conjugate monotherapy or combination therapy
PendingUS20210093730A1Predict resistancePredict sensitivityMicrobiological testing/measurementOrganic non-active ingredientsDiseaseAnticarcinogen
The present invention relates to biomarkers of use in cancer therapy, wherein the therapy comprises treatment with anti-Trop-2, anti-CEACAM5 or anti-HLA-DR ADCs (antibody-drug conjugates), alone or in combination with and one or more anti-cancer agents, such as a DDR inhibitor, an ABCG2 inhibitor, a microtubule inhibitor, a checkpoint inhibitor, a PI3K inhibitor, an AKT inhibitor, a CDK 4 inhibitor, a CDK 5 inhibior, a tyrosine kinase inhibitor or a platinum-based chemotherapeutic agent. Preferably, the combination therapy has a synergistic effect on inhibiting tumor growth. The biomarkers are of use to predict efficacy and / or toxicity of ADC therapy, determine tumor response to treatment, identify minimal residual disease or relapse, determine prognosis, stratify patients for initial therapy or to optimize treatment for the patient, based on the specific biomarkers detected.
Owner:IMMUNOMEDICS INC
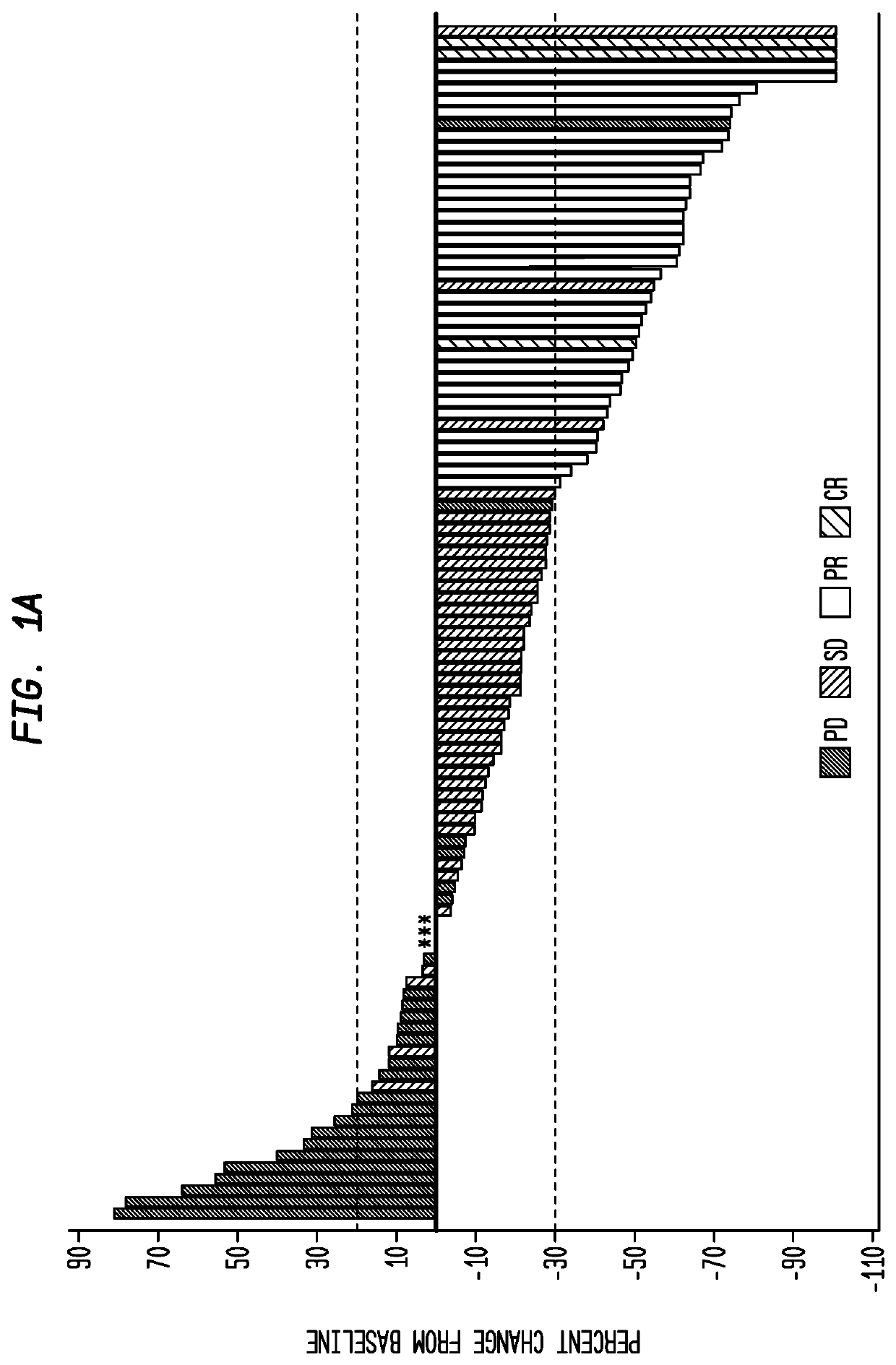
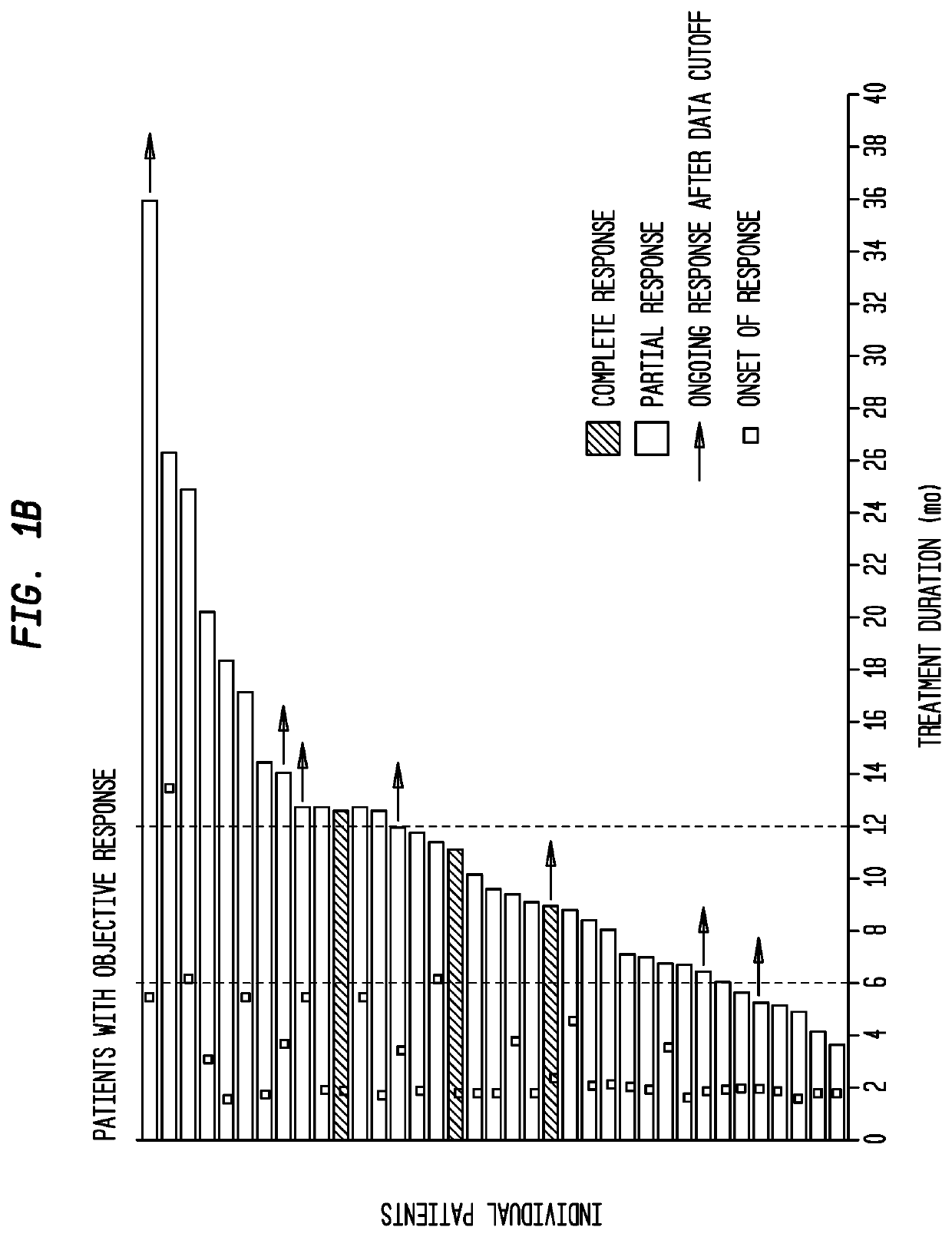
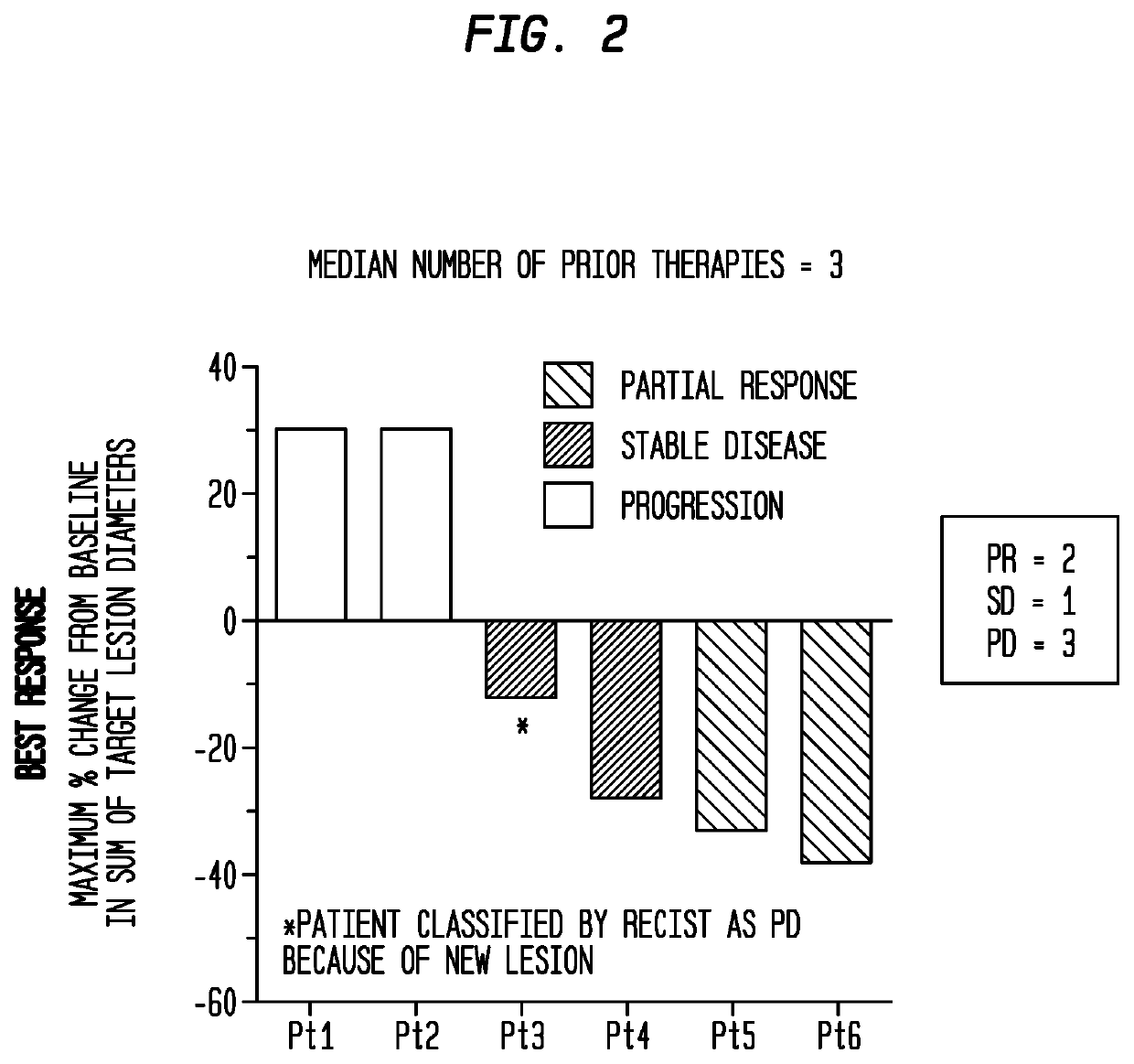
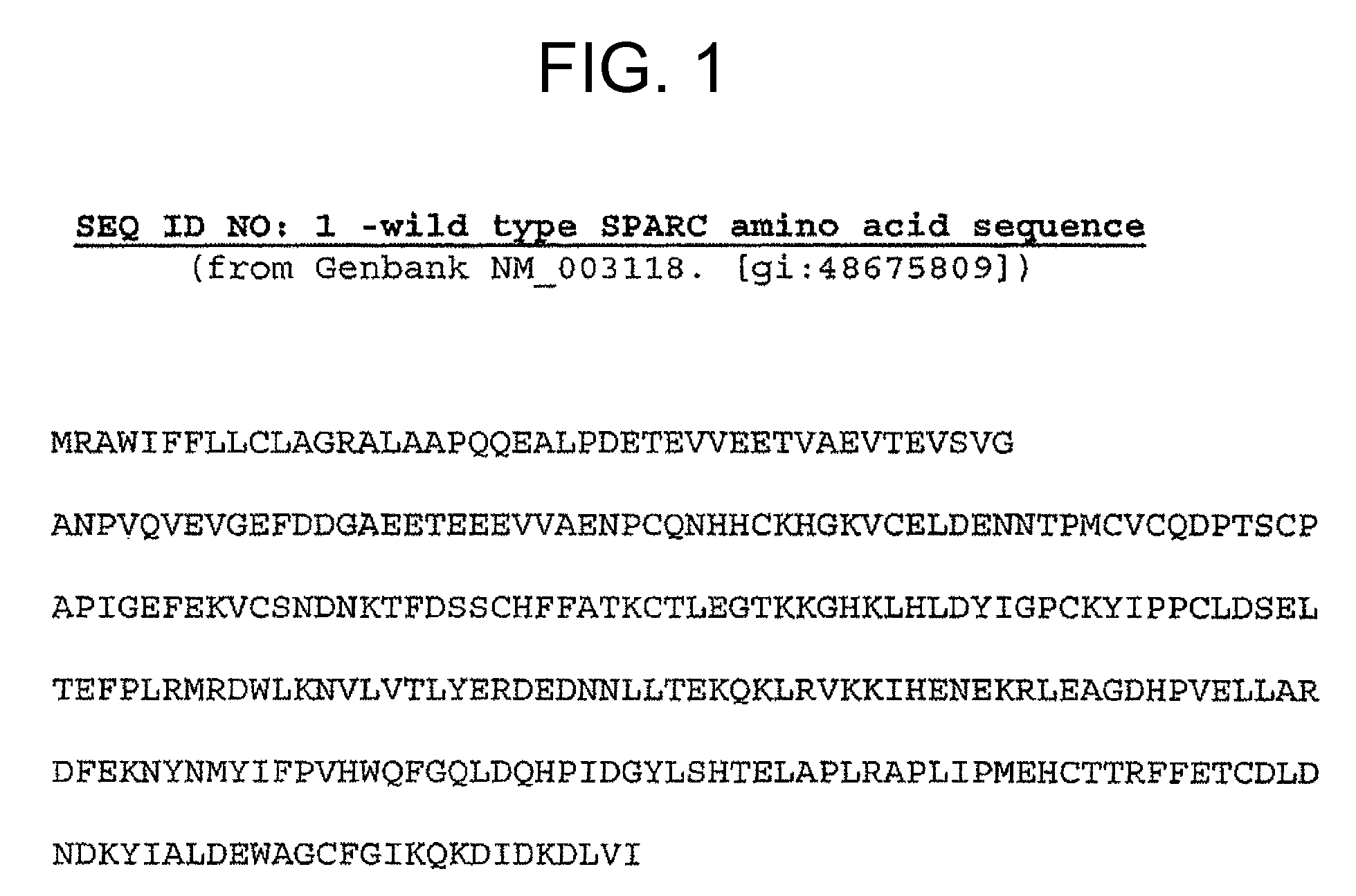
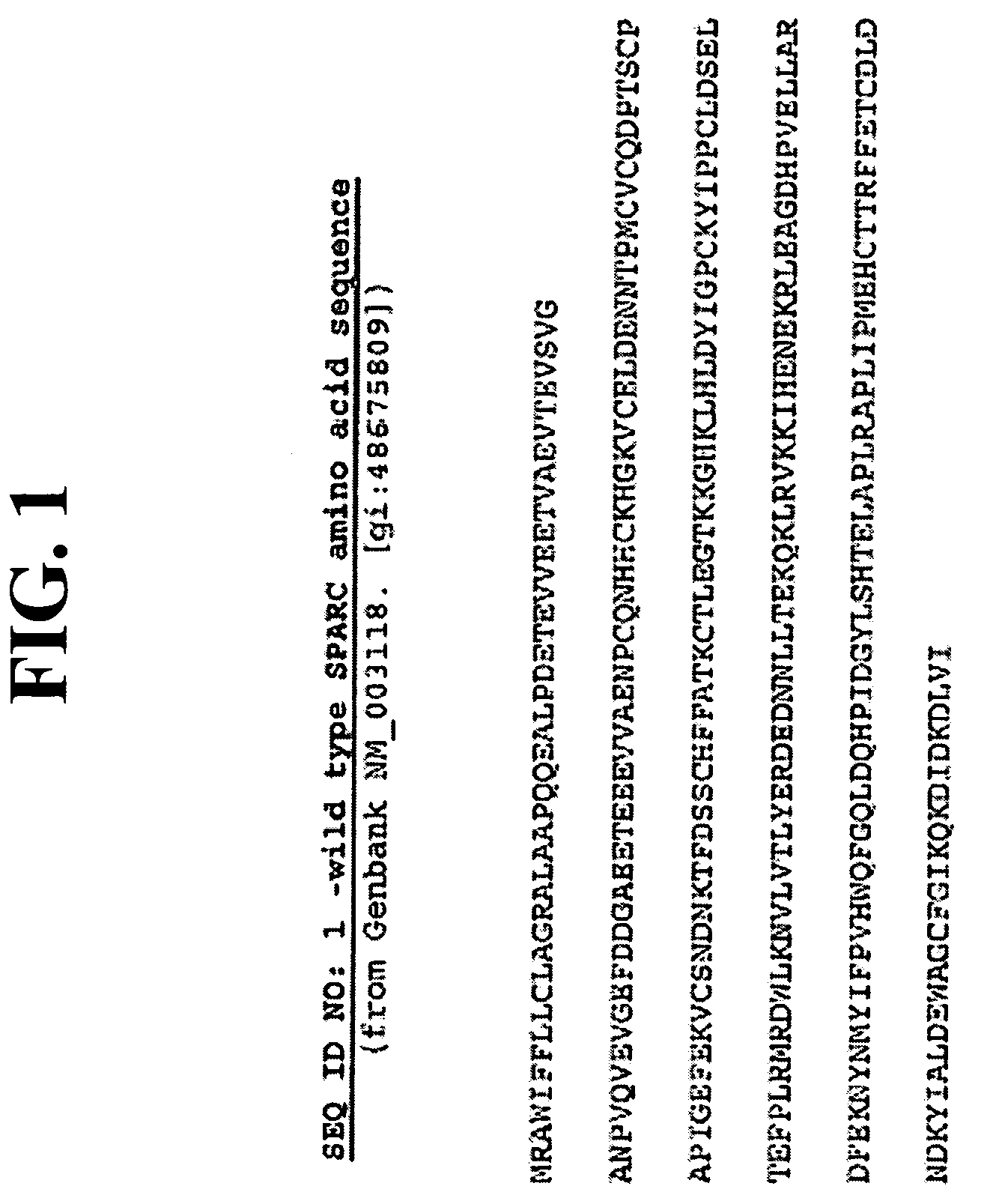
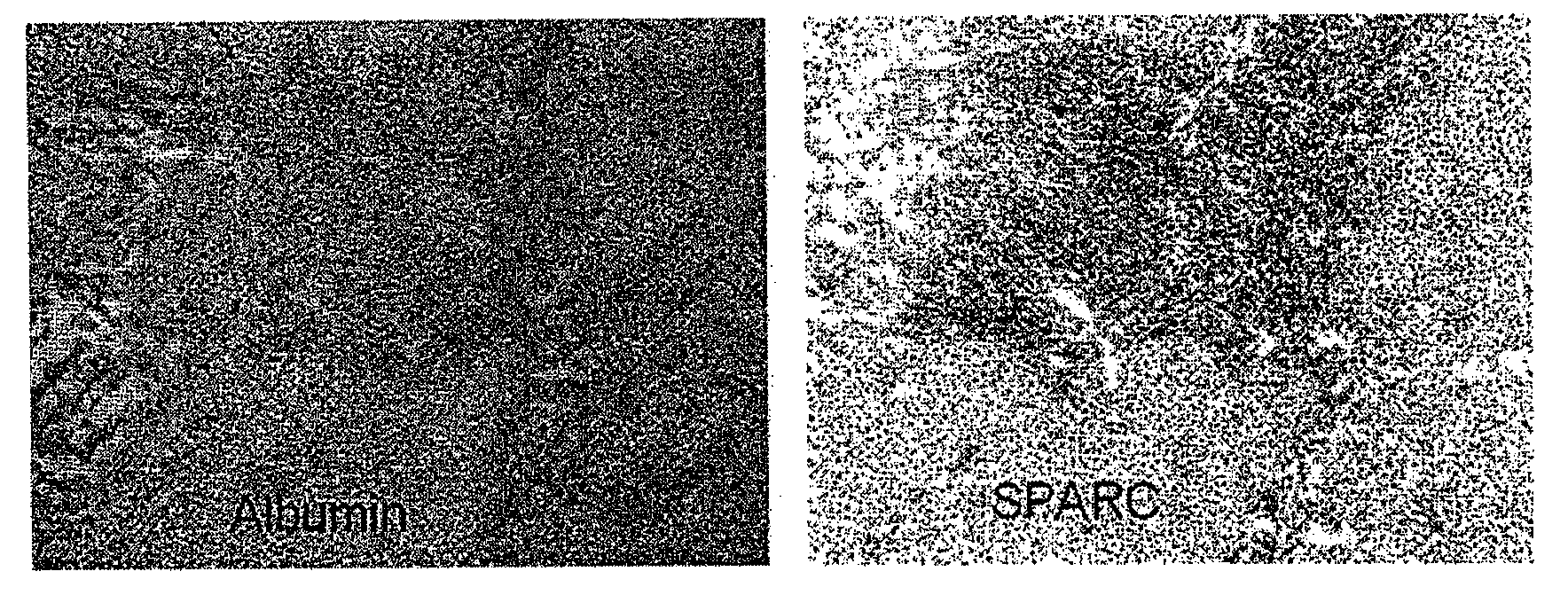
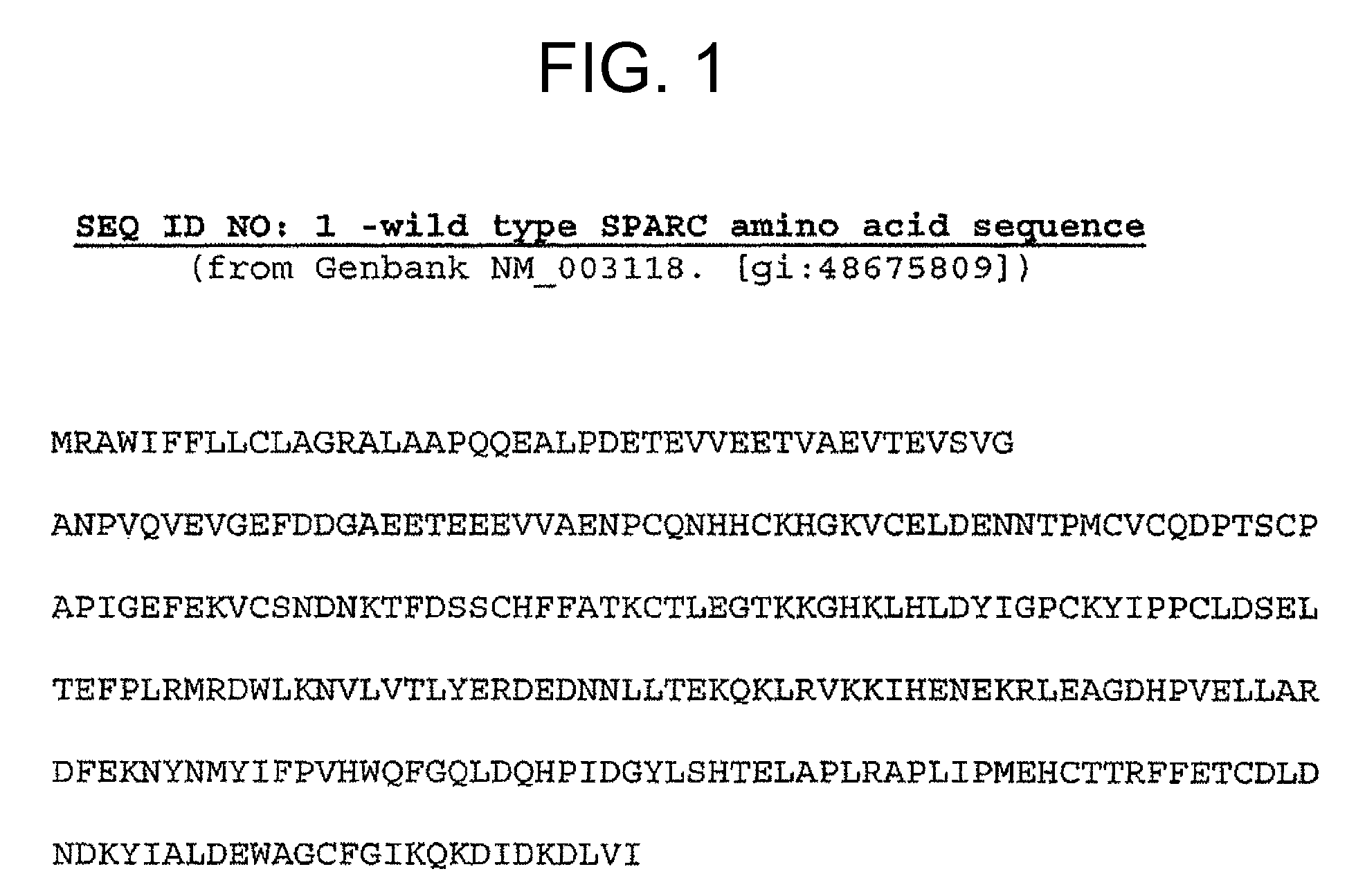
Sparc and methods of use thereof
The invention provides methods for predicting or determining the response of a mammalian tumor to a chemotherapeutic agent and for treating a mammalian tumor comprising detecting and quantifying the SPARC protein or RNA in a sample isolated from the mammal. The invention further provides kit for predicting the response of a mammalian tumor to a chemotherapeutic agent, comprising a means for the isolation of protein or RNA from the tumor, a SPARC protein or RNA detection and quantification means, control RNAs, and rules for predicting the response of the tumor based on the level of SPARC protein or RNA in tumor.
Owner:ABRAXIS BIOSCI LLC
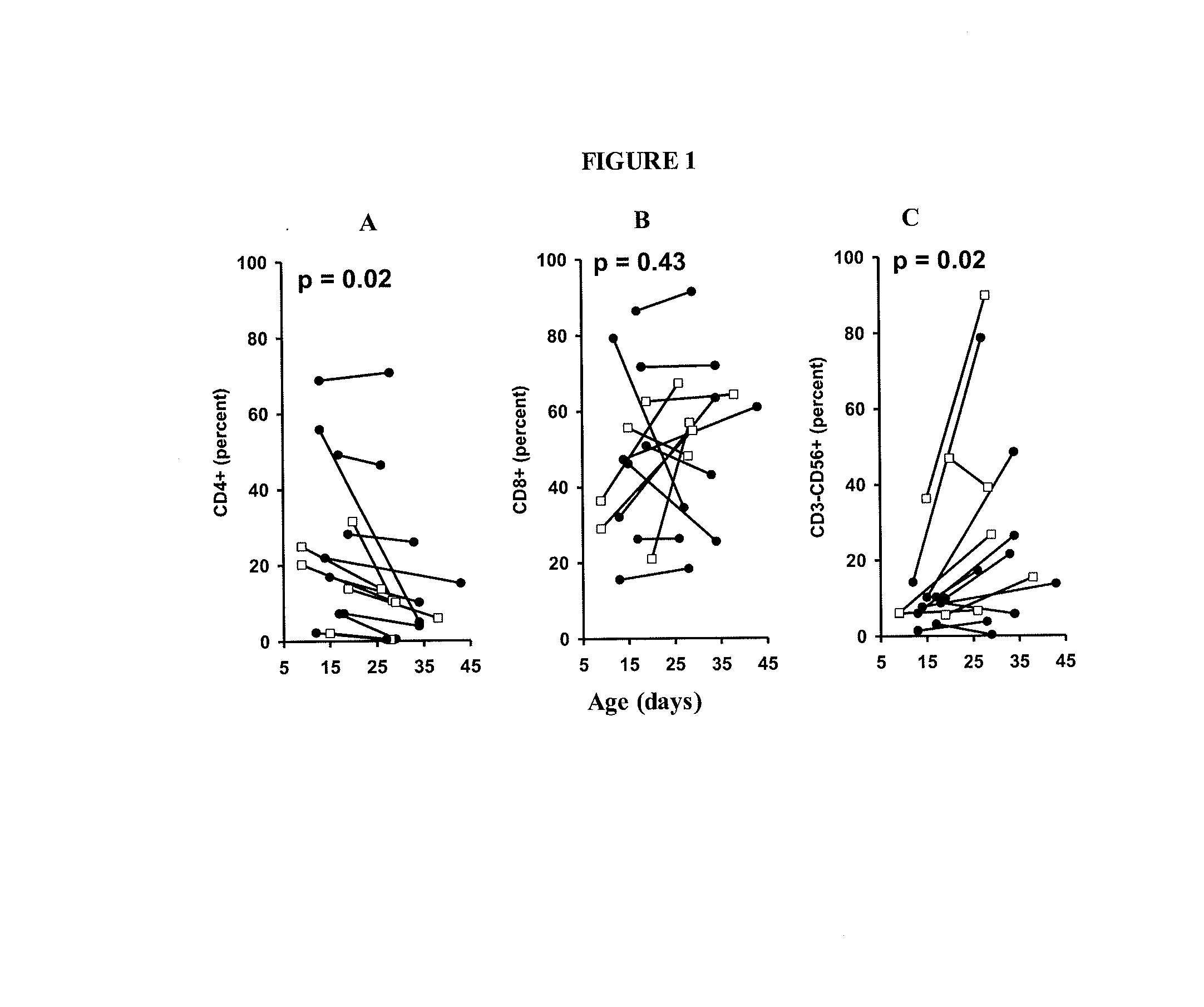
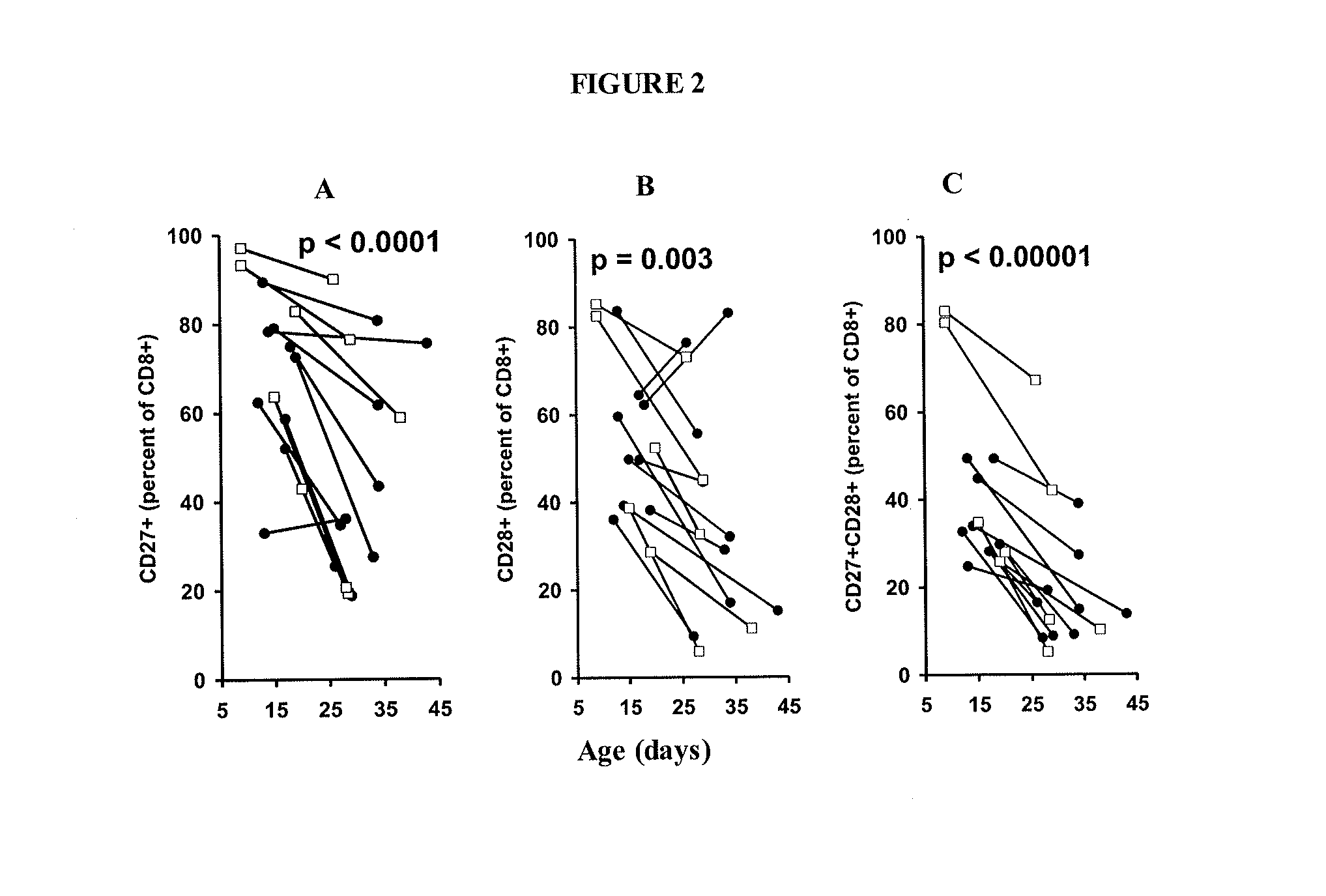
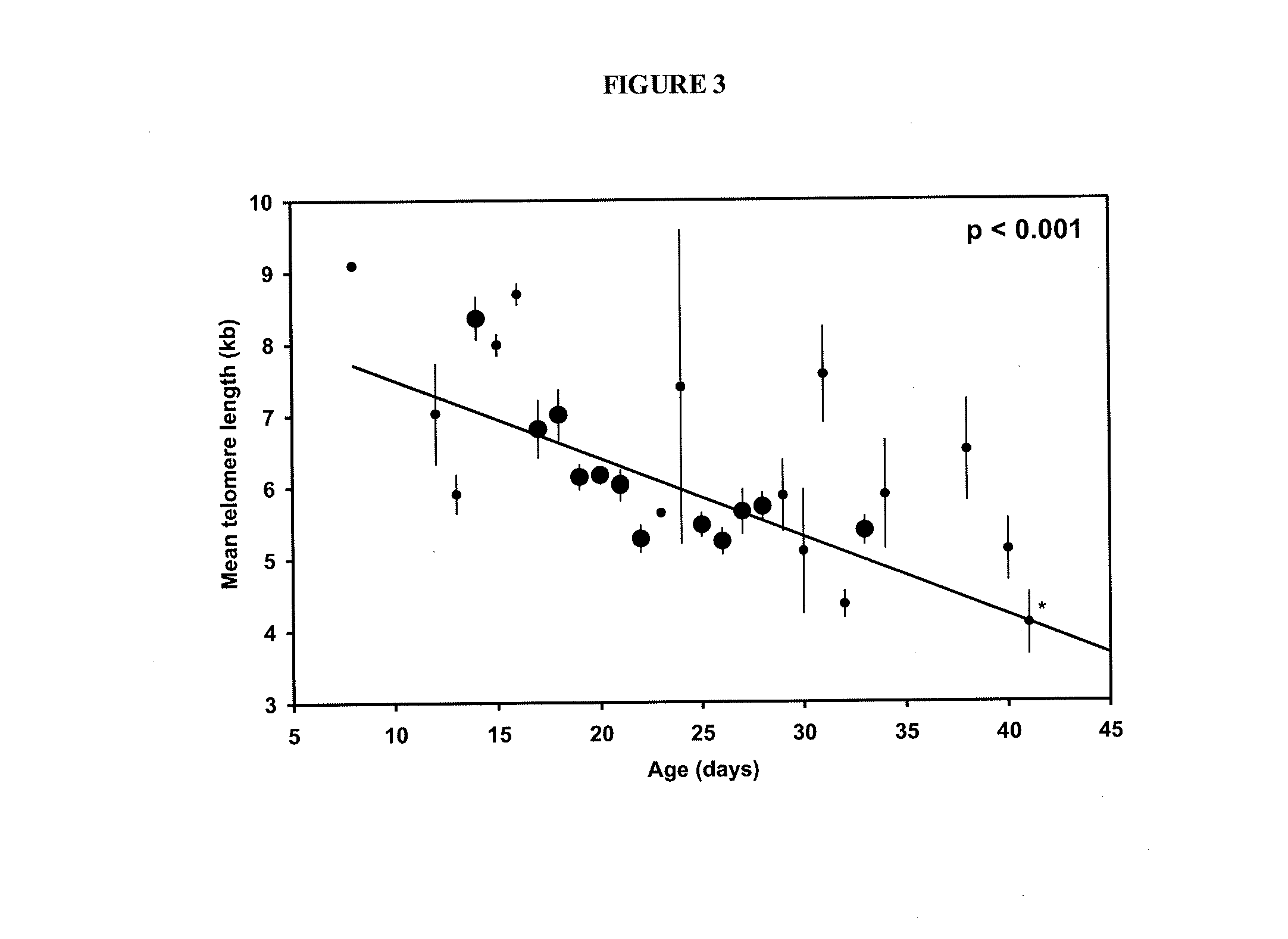
Adoptive cell therapy with young t cells
ActiveUS20140030806A1Organic active ingredientsPeptide/protein ingredientsAdoptive cellular therapyTumor response
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:UNITED STATES OF AMERICA
Methods for enhancing antigen-presenting cells and anti-tumor responses in a human patient
InactiveUS6838081B1Improve developmentReduce tumor burdenBiocidePeptide/protein ingredientsTumor responseCo administration
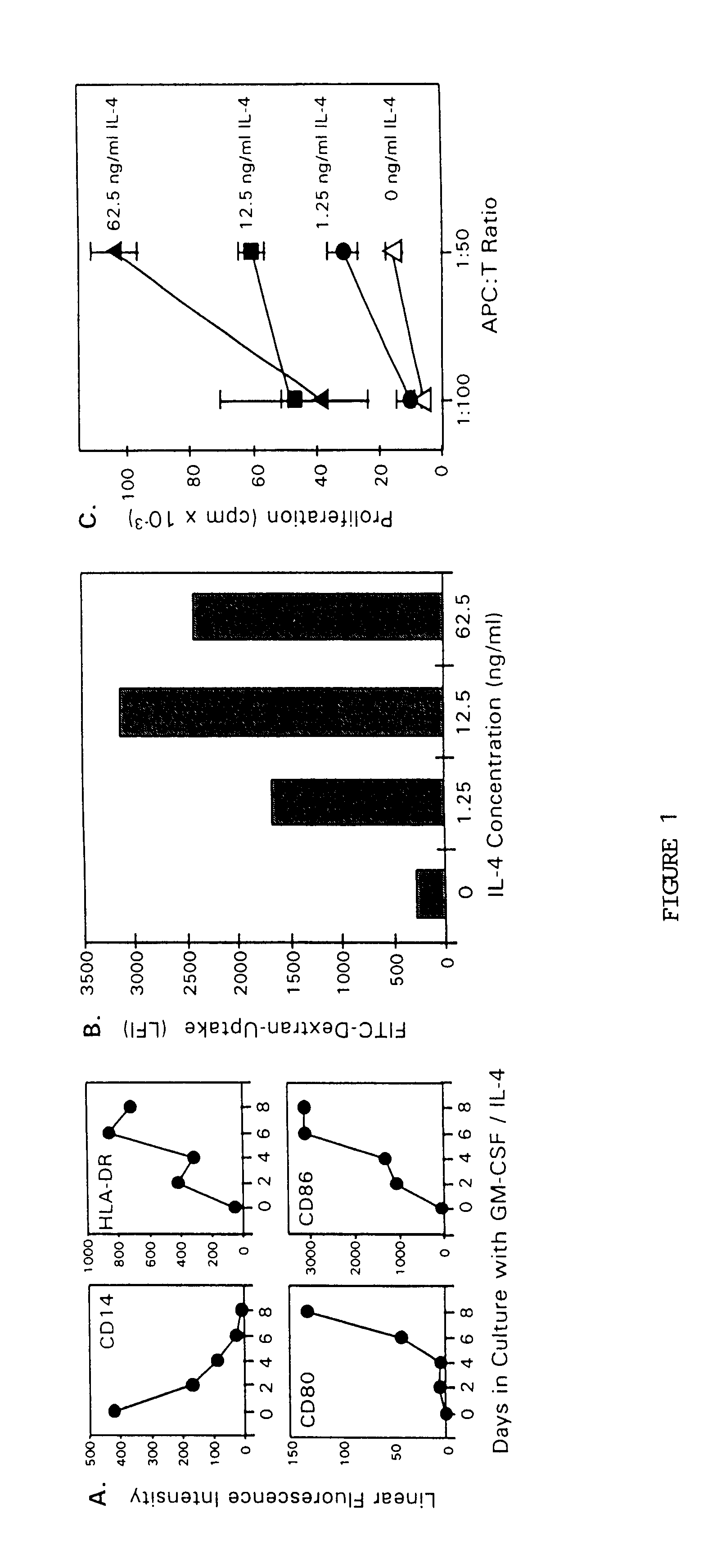
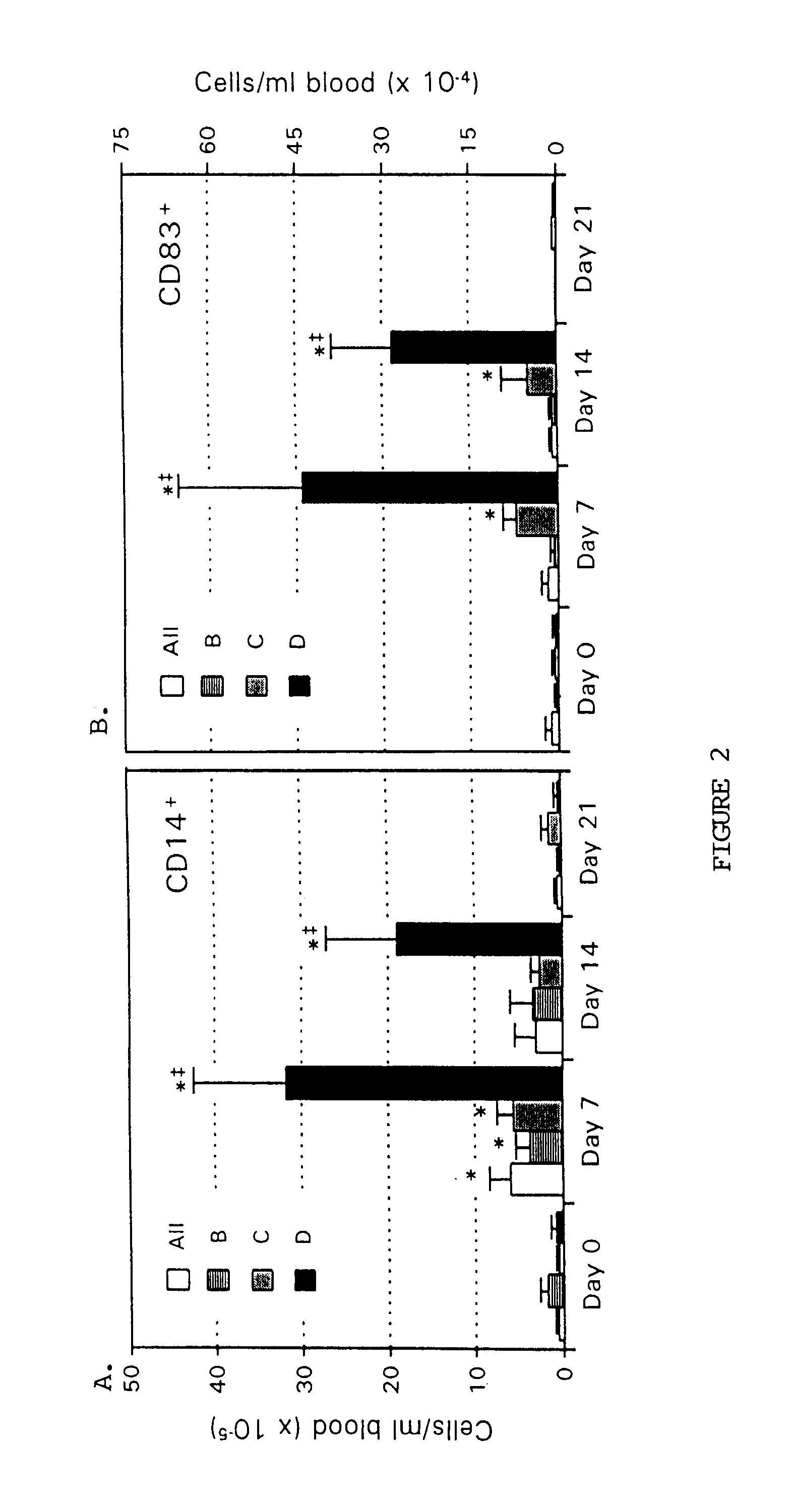
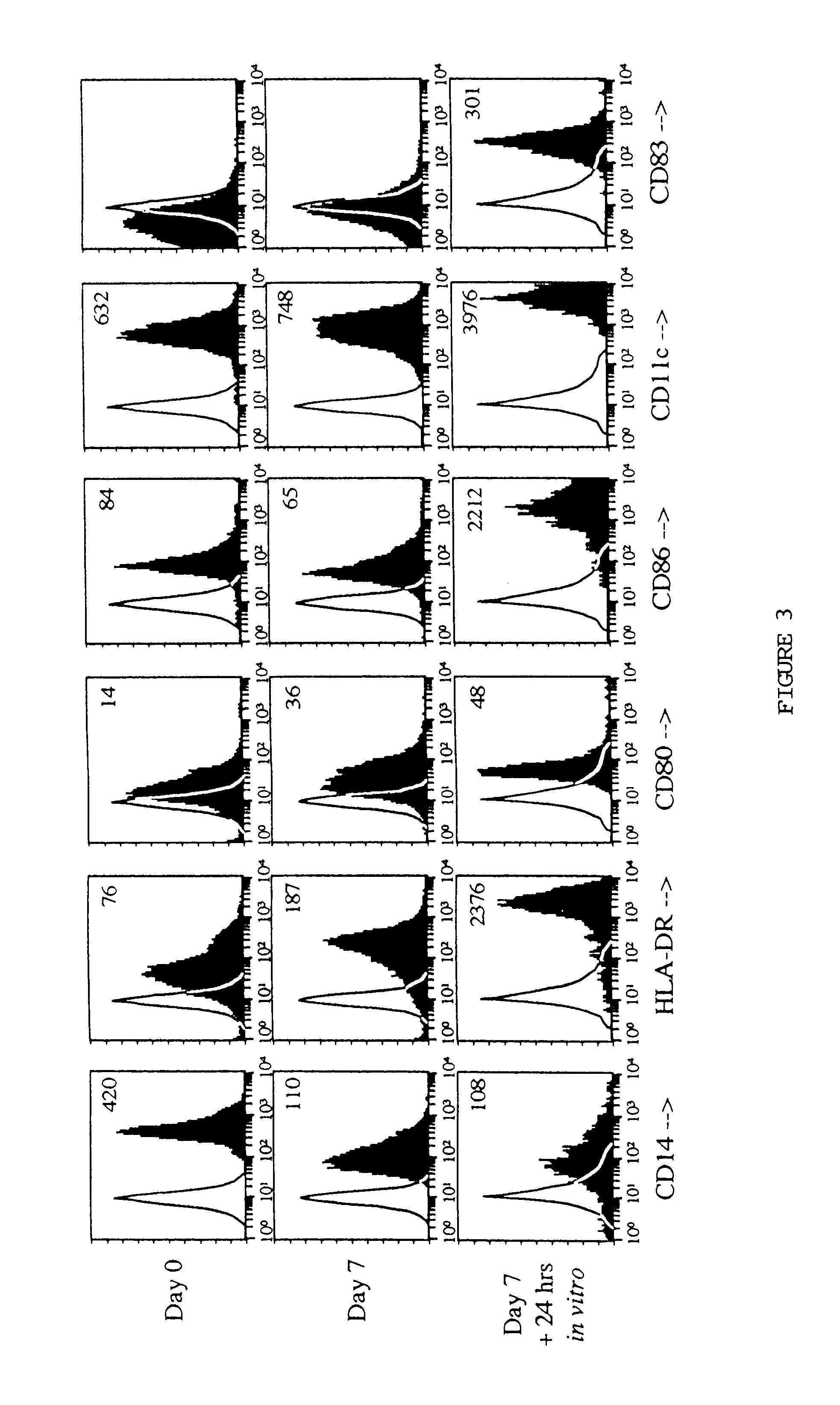
The present invention provides methods for enhancing the development of APC from precursor cells by administering a combination of GM-CSF and IL-4. The precursor cells include: cells contained in peripheral blood, CD14+ cells and precursors in bone marrow. Thus, administration of GM-CSF and IL-4 can be used as a form of cytokine immunotherapy. One embodiment of the present invention involves systemic administration of GM-CSF and IL-4. In this embodiment, APC are required to directly access tumor antigens as they exist in vivo within the patient. A further embodiment of the present invention involves co-administration of a tumor-associated or tumor-specific antigen, with GM-CSF and IL-4, to induce antigen-specific immunity mediated by APC. Yet another embodiment of the present invention describes systemic administration of GM-CSF and IL-4 to achieve reduced tumor burden.
Owner:RGT UNIV OF CALIFORNIA
Preparation method and application of ultrasonic control type anti-tumor drug delivery system
ActiveCN106267199AEfficient killingLittle side effectsInorganic non-active ingredientsEchographic/ultrasound-imaging preparationsErythrocyte membraneTumor response
The invention relates to a preparation method and application of an ultrasonic control type anti-tumor drug delivery system. The problems that existing tumor treatment drugs are low in drug loading capacity, are leaked when delivered, are low in tumor response sensitivity, slow in release and prone to causing drug resistance, and can not be controlled in vitro are solved effectively. The ultrasonic control type anti-tumor drug delivery system is established with hollow mesoporous titanium dioxide as the base body by being internally loaded with perfluorohexane and drug bleomycin, wrapped by an endogenous biological membrane-erythrocyte membrane and modified by tumor homing peptides; a nanoscale particle size distribution drug delivery system is prepared with hollow mesoporous titanium dioxide as the base body by being internally loaded with perfluorohexane and model drug bleomycin, wrapping surfaces of hollow mesoporous titanium dioxide by RBC, and being wrapped by an endogenous biological membrane-erythrocyte membrane; a tumor drug delivery system with a remote ultrasonic control function is established by modifying tumor homing peptide NGR tail ends with diacyl lipid.
Owner:ZHENGZHOU UNIV
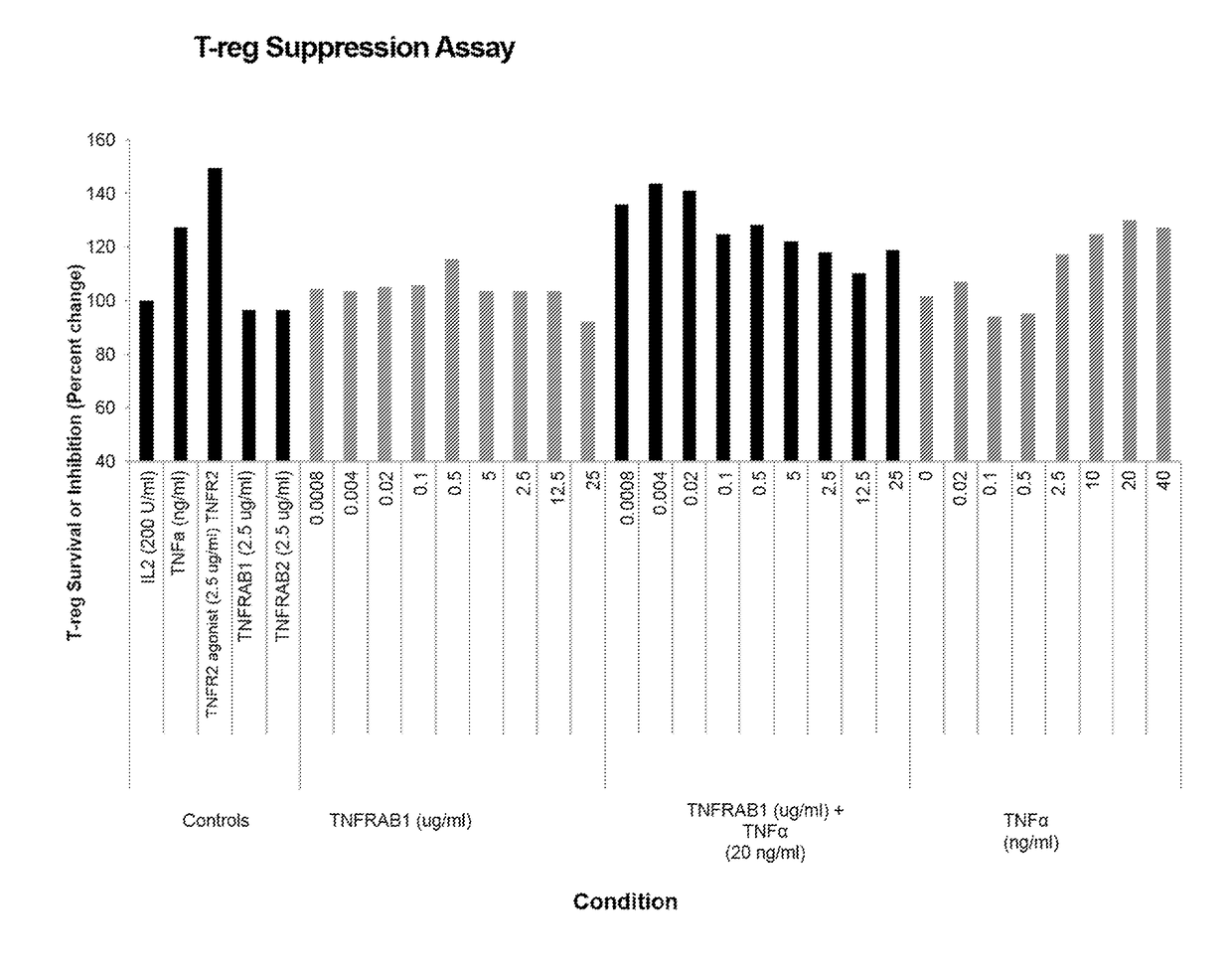
Antagonistic Anti-tumor necrosis factor receptor superfamily antibodies
ActiveUS20180194850A1Inhibit and reduce proliferationPromote apoptosisPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsRegulatory T cellTnfr superfamily
Antagonistic TNFR superfamily polypeptides, such as antibodies and antigen-binding fragments thereof, and the use of these polypeptides to inhibit the proliferation of regulatory T cells (T-regs). For example, antibodies of the invention include antagonistic TNFR2 antibodies and antigen-binding fragments thereof, and can be used to suppress the T-reg-mediated deactivation of tumor reactive T-lymphocytes, as well as to treat a wide variety of cancers and infectious diseases.
Owner:THE GENERAL HOSPITAL CORP
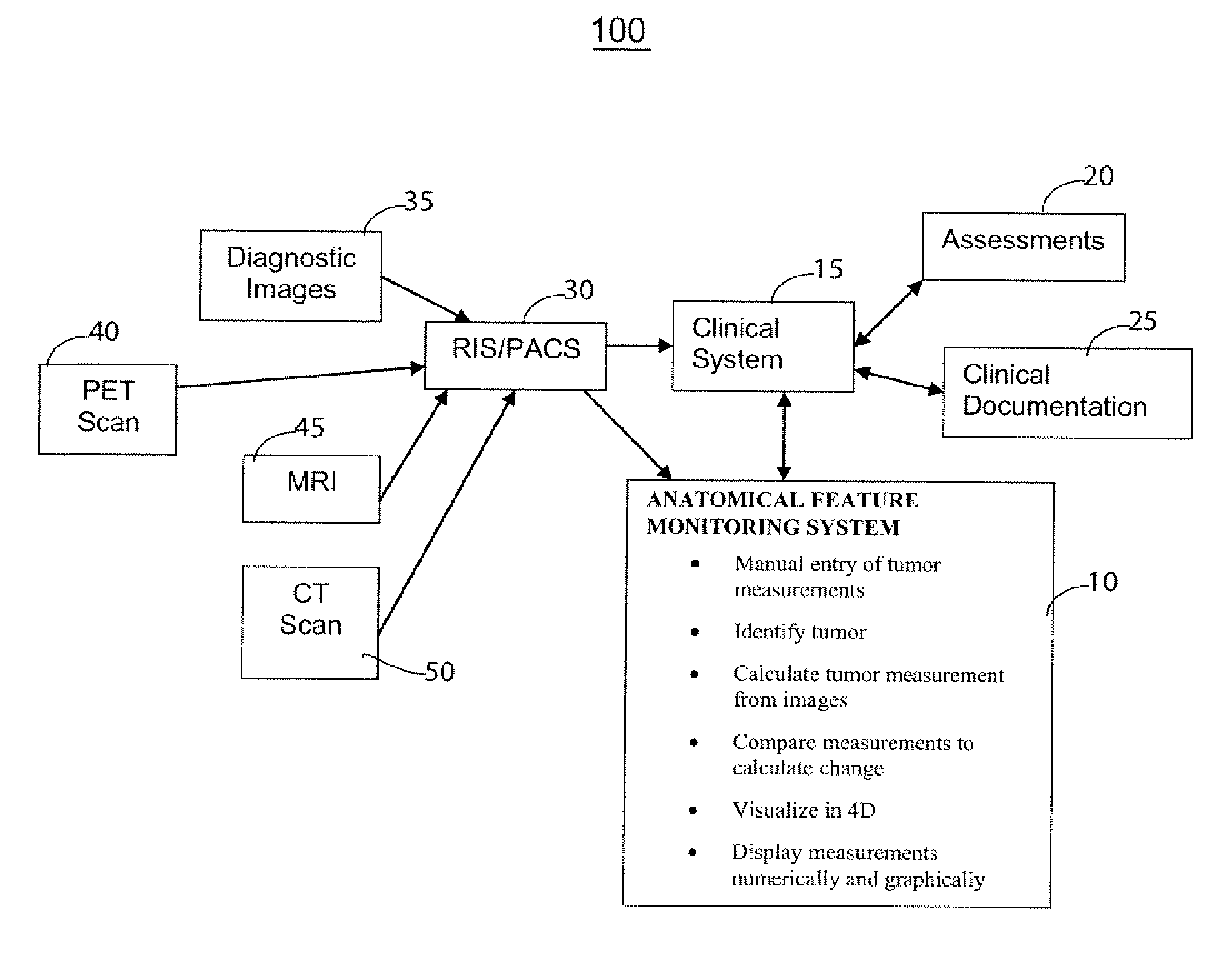
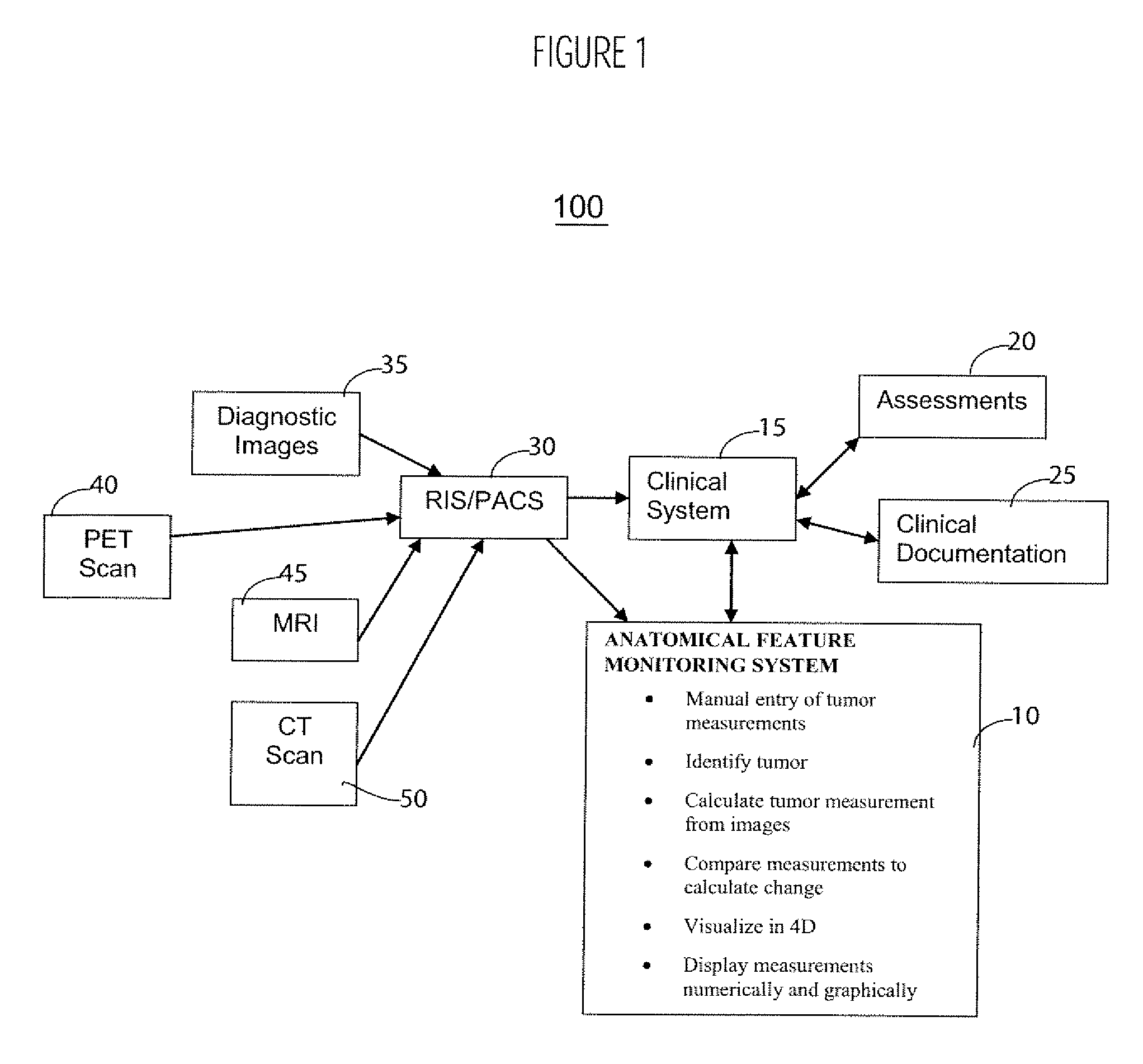
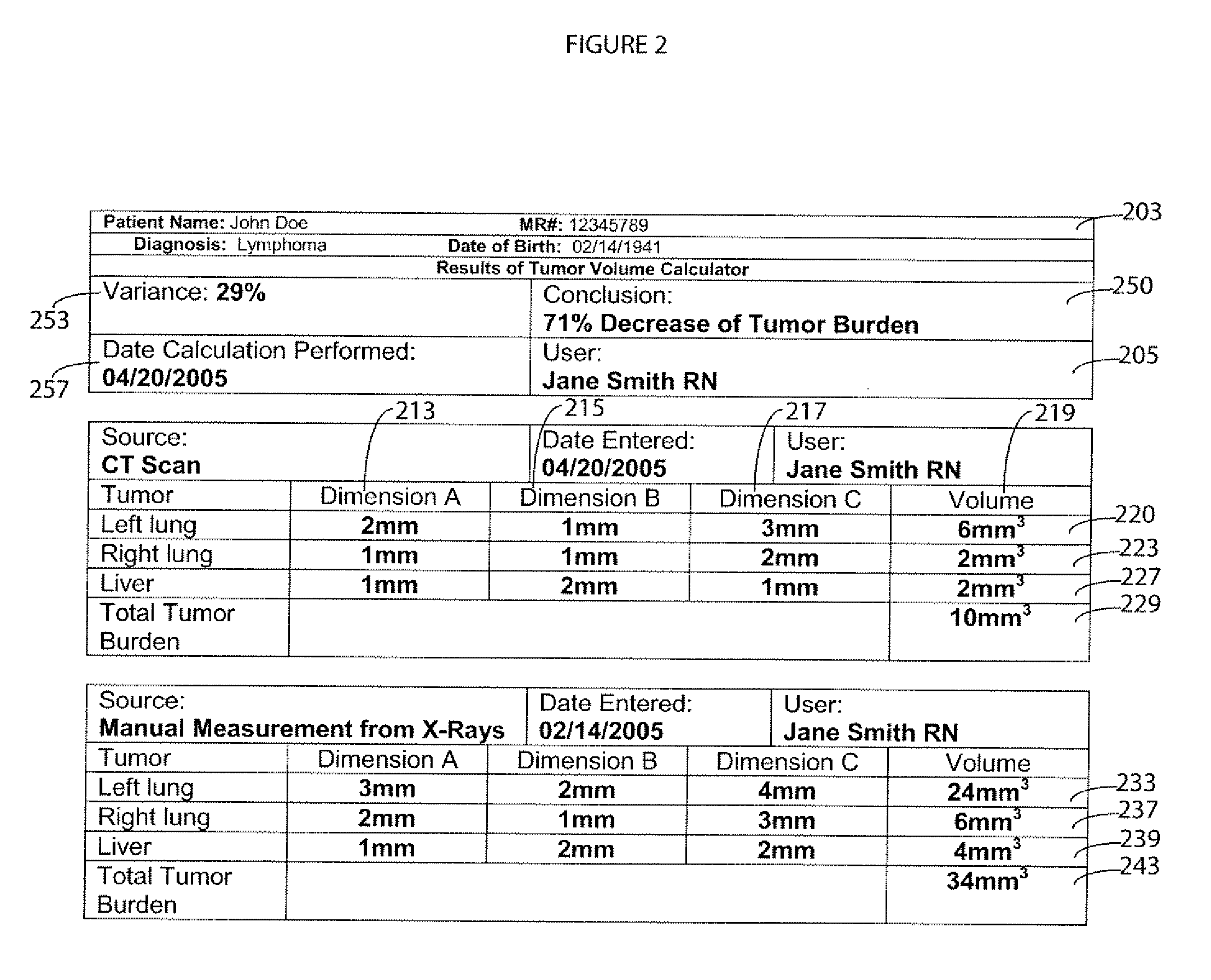
Anatomical Feature Tracking and Monitoring System
InactiveUS20070027408A1Person identificationMedical report generationAbnormal tissue growthTumor response
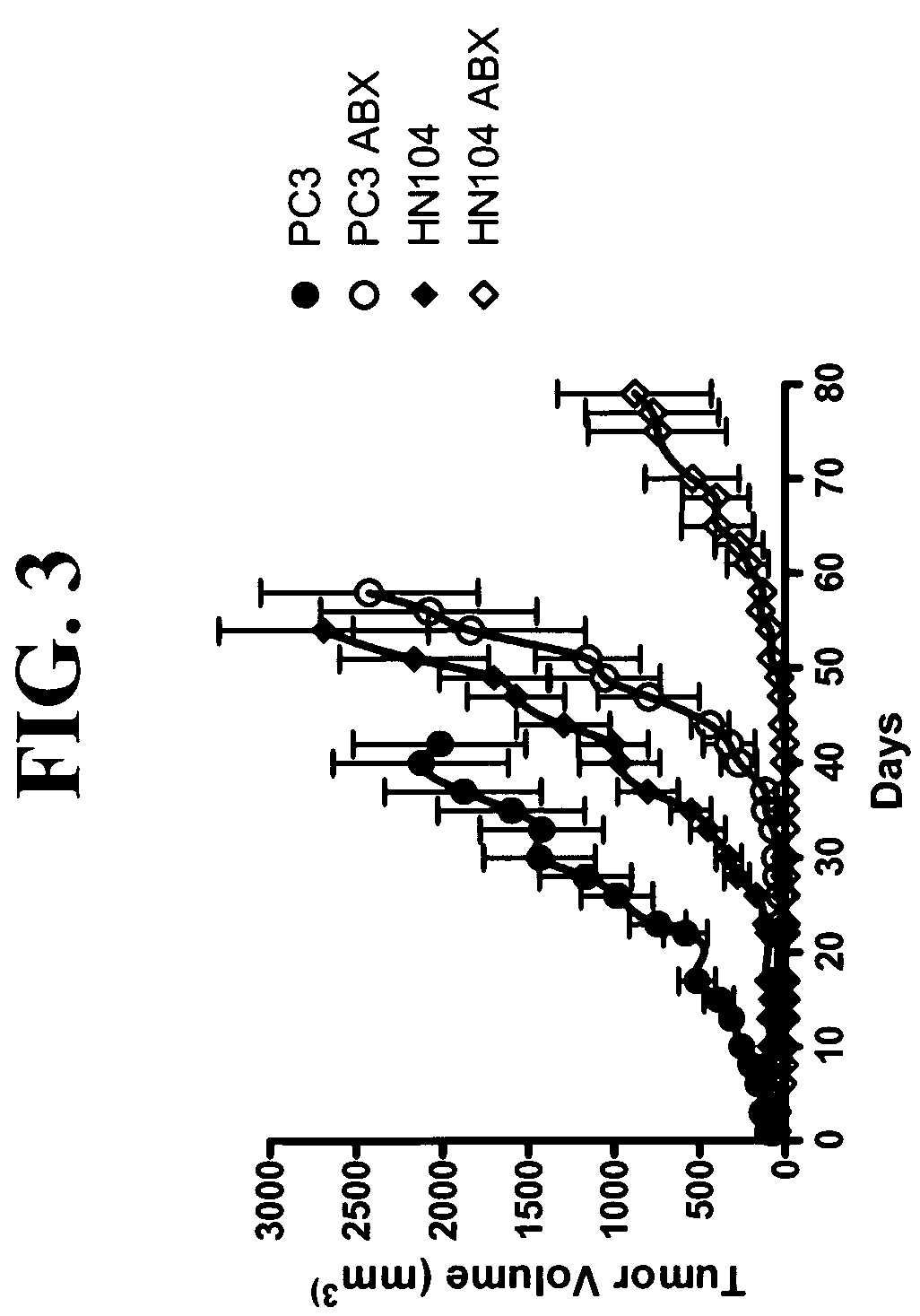
A system according to invention principles automatically calculates anatomical feature (e.g., tumor, body part, organ) size electronically to provide a clinician with useful information when assessing the tumor response during treatment. A system for monitoring change in patient anatomical features, includes a volumetric analyzer for automatically analyzing data derived from an imaging unit that represents a three dimensional view of a patient anatomical feature in order to determine spatial characteristics of the anatomical feature. A comparison processor automatically compares a first set of spatial characteristics of a patient anatomical feature derived at a first time with a second set of corresponding spatial characteristics of the patient anatomical feature derived at a subsequent second time, to provide change data representing change in spatial characteristics of the patient anatomical feature over a period of time. A repository stores change data for access by a user.
Owner:SIEMENS MEDICAL SOLUTIONS HEALTH SERVICES CORPORAT
SPARC and methods of use thereof
The invention provides methods for predicting or determining the response of a mammalian tumor to a chemotherapeutic agent and for treating a mammalian tumor comprising detecting and quantifying the SPARC protein or RNA in a sample isolated from the mammal. The invention further provides kit for predicting the response of a mammalian tumor to a chemotherapeutic agent, comprising a means for the isolation of protein or RNA from the tumor, a SPARC protein or RNA detection and quantification means, control RNAs, and rules for predicting the response of the tumor based on the level of SPARC protein or RNA in tumor.
Owner:ABRAXIS BIOSCI LLC
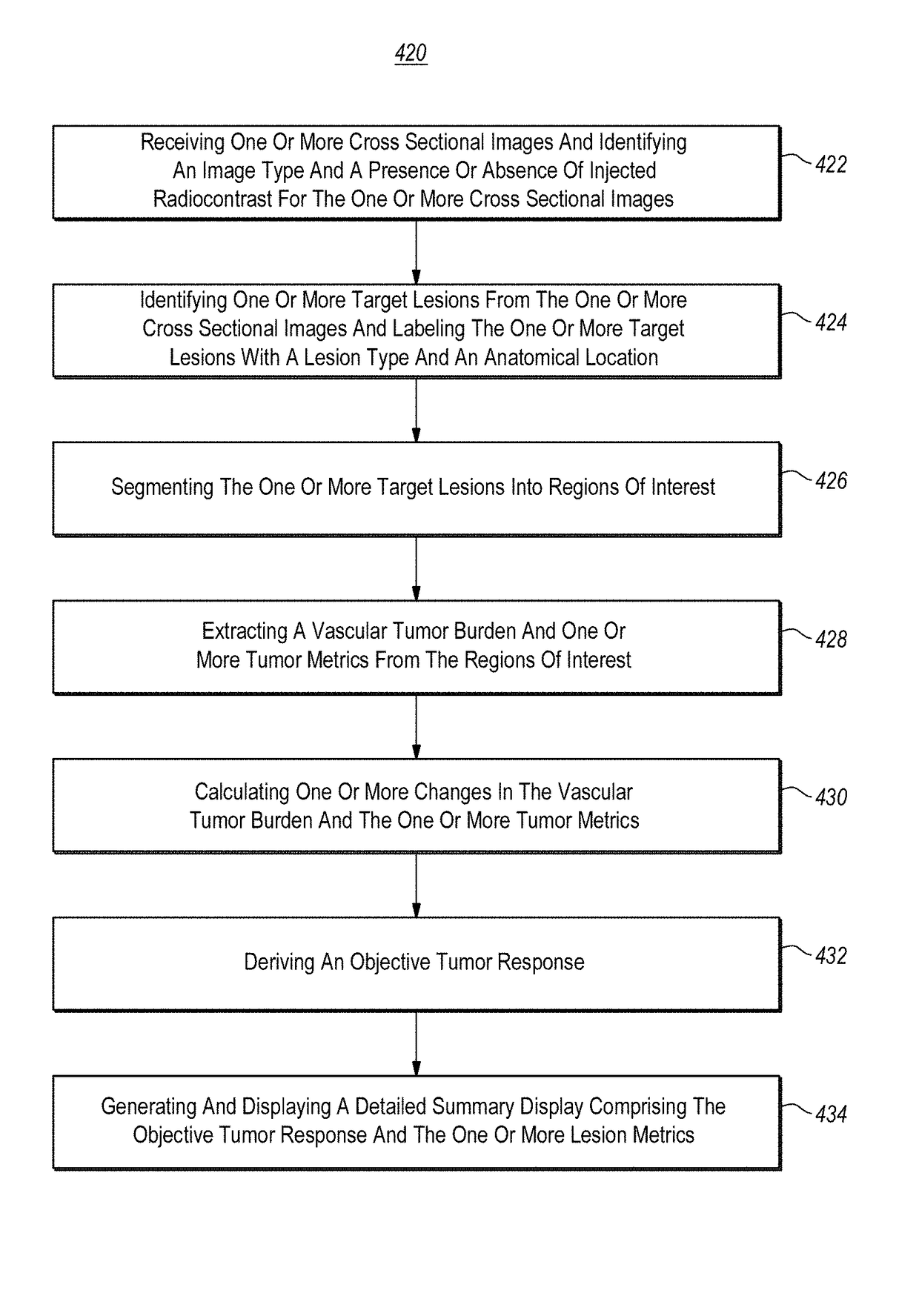
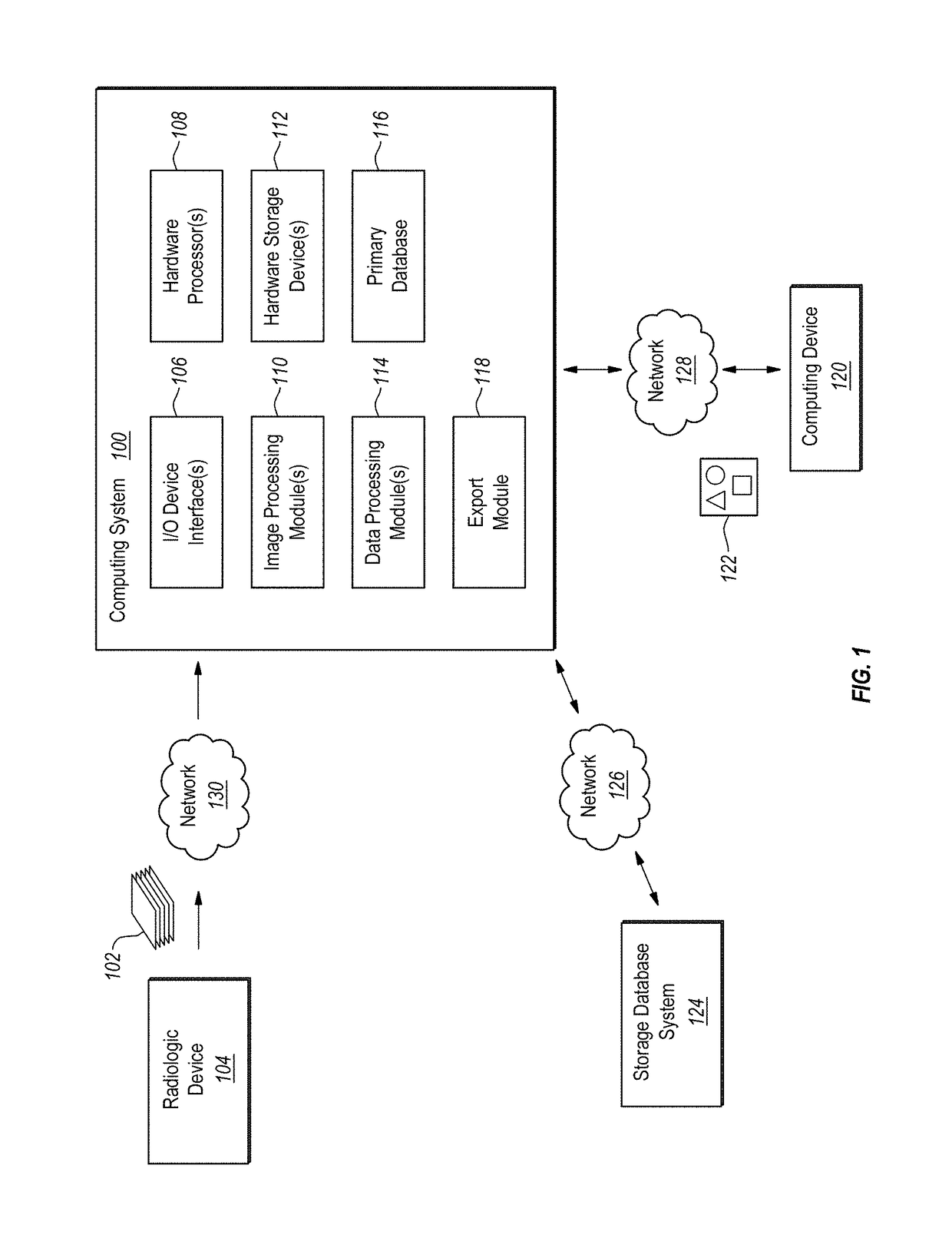
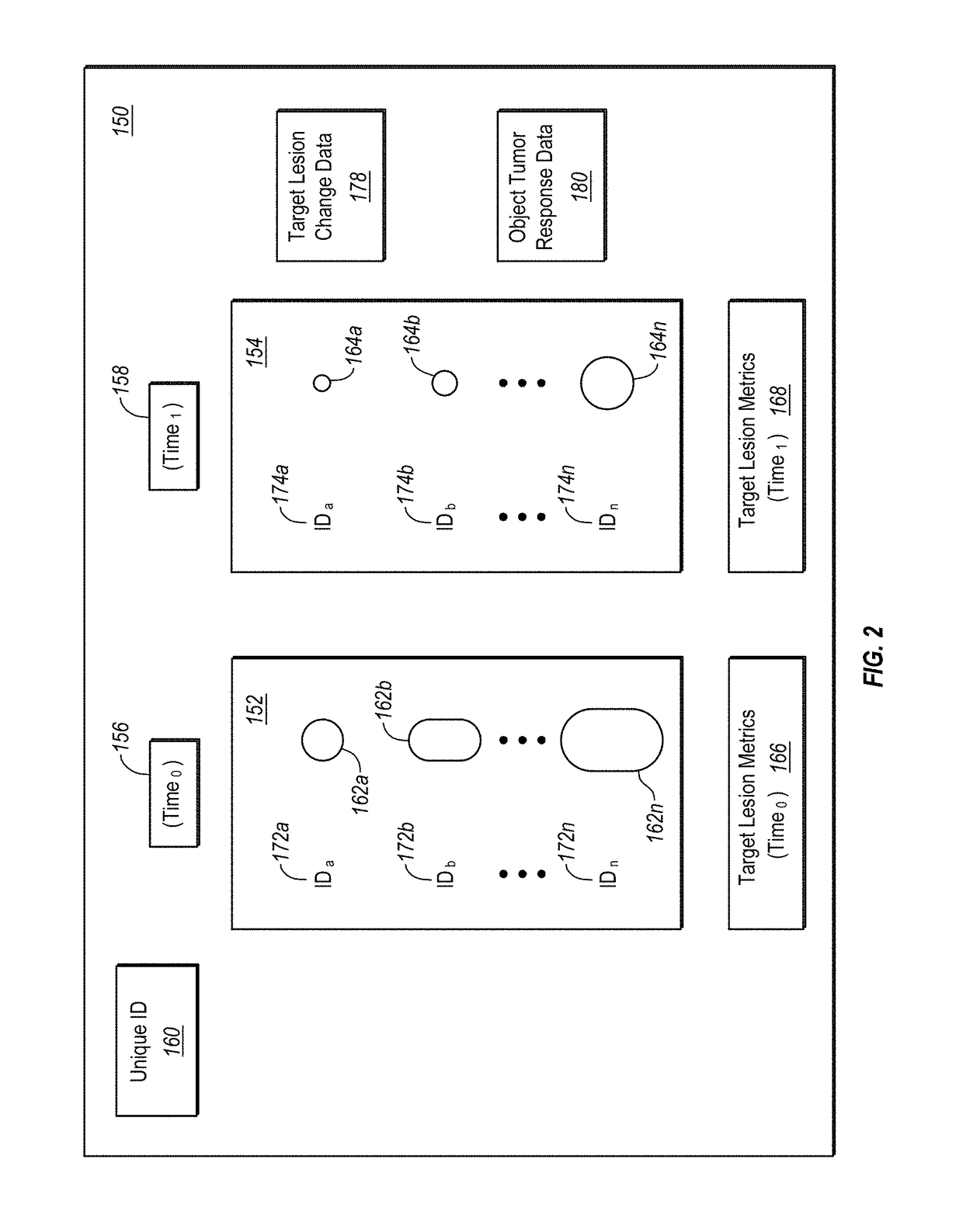
Computer-assisted tumor response assessment and evaluation of the vascular tumor burden
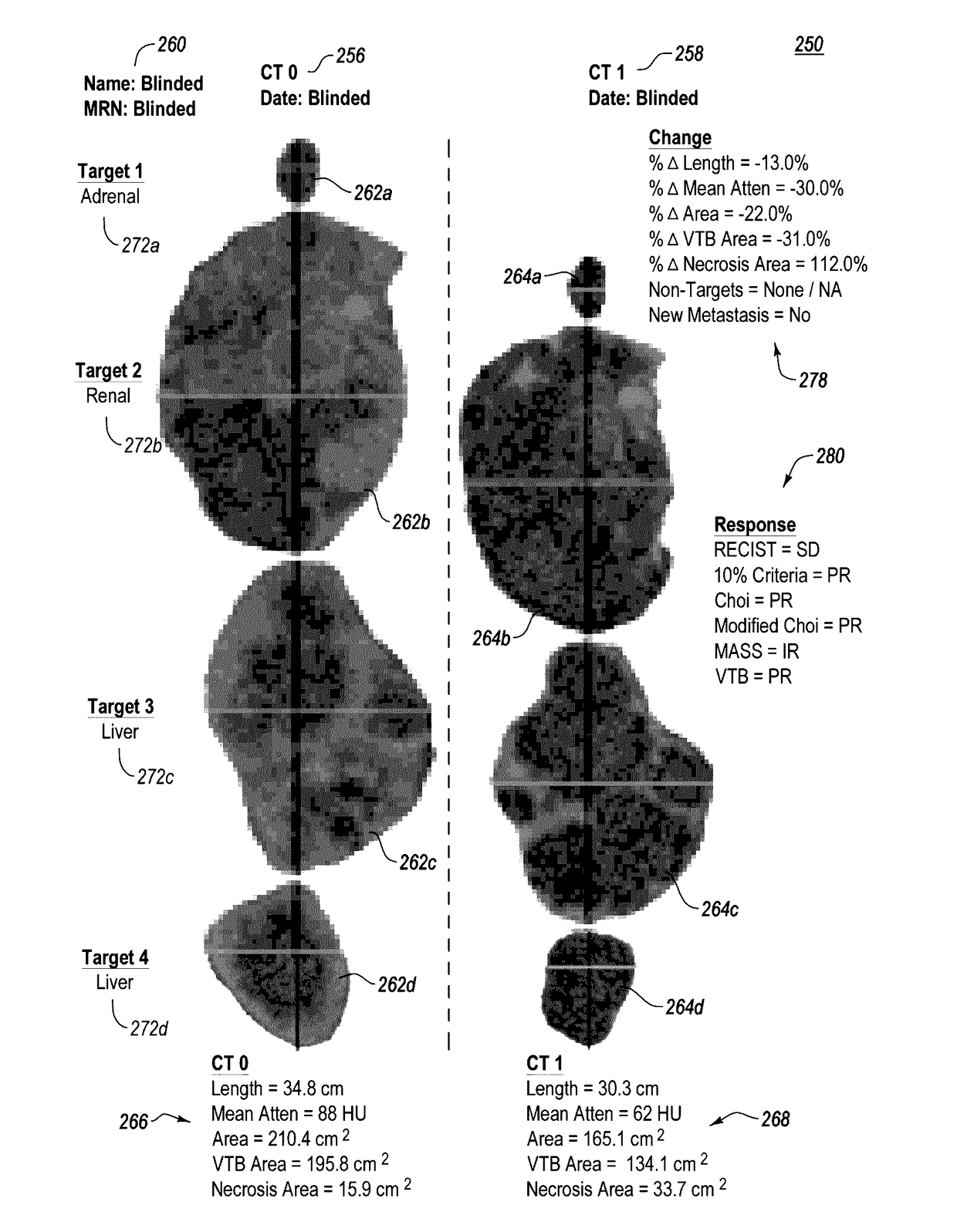
ActiveUS20170119334A1Accurately tumor responseEfficient responseImage enhancementImage analysisAbnormal tissue growthTumor response
A computerized method for determining an objective tumor response to an anti-cancer therapy using one or more cross-sectional images can comprise receiving one or more cross-sectional images that comprise one or more cross-sectional slices of digital medical image data and identifying one or more target lesions within the one or more cross-sectional images. The method can also comprise analyzing at least one of the target lesions with an image-processing module configured to identify a total range of pixel intensities and restrict the total range of pixel intensities to a first restricted range that corresponds to pixel intensities representative of vascularized tumor. Further still, the method can comprise deriving a vascular tumor burden for the at least one of the one or more target lesions and determining the objective tumor response for the at least one of the one or more target lesions based on the vascular tumor burden.
Owner:AI METRICS LLC
Cancer immunotherapy
InactiveUS20140341978A1Skin cancer vaccineMammal material medical ingredientsAbnormal tissue growthTumor response
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Sparc and methods of use thereof
The invention provides methods for predicting or determining the response of a mammalian tumor to a chemotherapeutic agent and for treating a mammalian tumor comprising detecting and quantifying the SPARC protein or RNA in a sample isolated from the mammal. The invention further provides kit for predicting the response of a mammalian tumor to a chemotherapeutic agent, comprising a means for the isolation of protein or RNA from the tumor, a SPARC protein or RNA detection and quantification means, control RNAs, and rules for predicting the response of the tumor based on the level of SPARC protein or RNA in tumor.
Owner:ABRAXIS BIOSCI LLC
Gene signature biomarkers of tumor response to pd-1 antagonists
InactiveUS20160312295A1Improve expression levelMicrobiological testing/measurementChemoinformaticsTumor responseTumor Sample
The present disclosure describes gene signature biomarkers that are useful for identifying cancer patients who are most likely to benefit from treatment with a PD-1 antagonist. The disclosure also provides methods and kits for testing tumor samples for the biomarkers, as well as methods for treating subjects with a PD-1 antagonist based on the test results.
Owner:MERCK SHARP & DOHME CORP
Method of preparation and composition of a water soluble extract of the bioactive component of the plant species uncaria for enhancing immune, anti-inflammatory, anti-tumor and DNA repair processes of warm blooded animals
InactiveUS20050176825A1Inhibit productionIncrease heightBiocideHydroxy compound active ingredientsDiseaseFluorescence
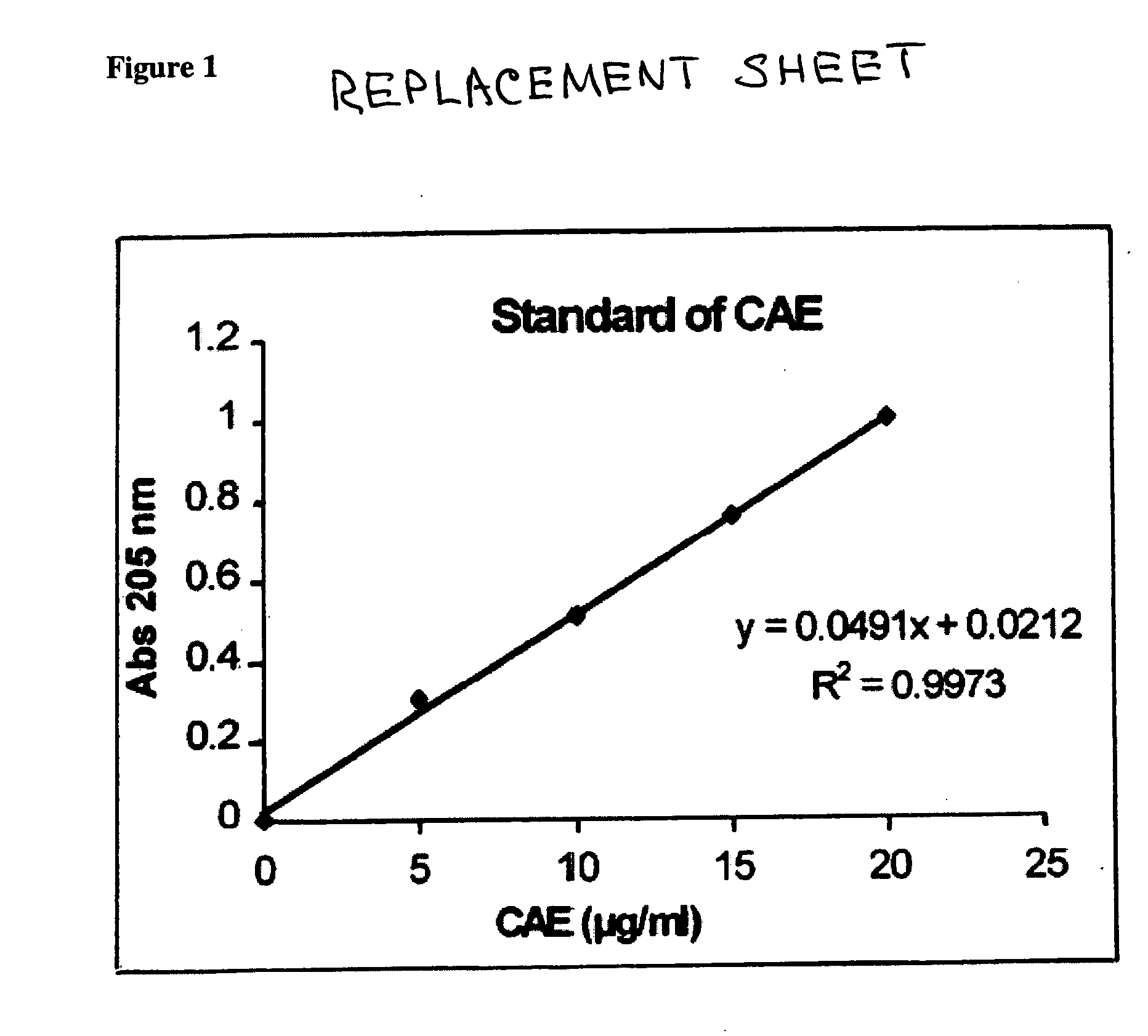
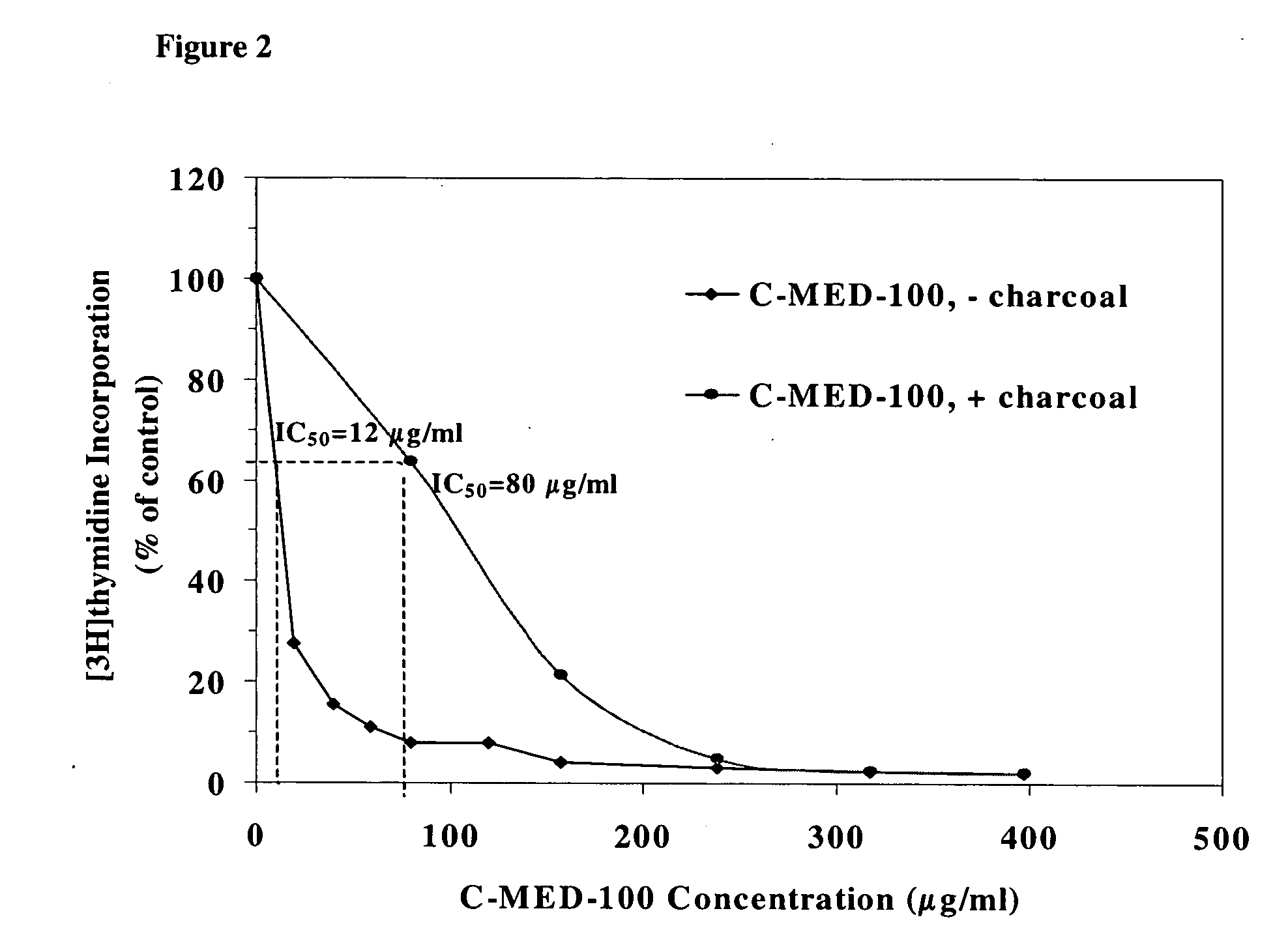
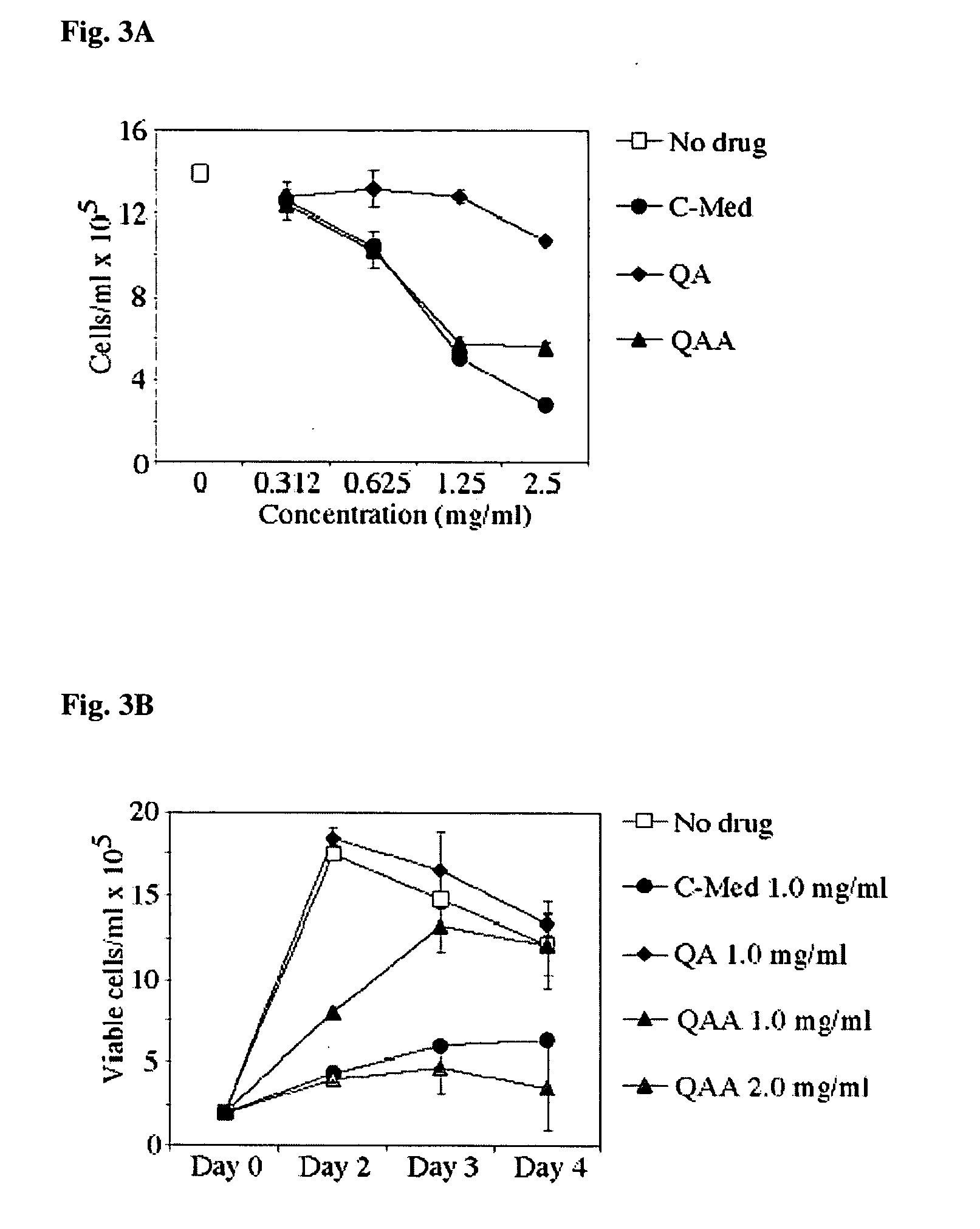
A method for isolating the bioactive component of the water-soluble extract of Uncaria tomentosa known as C-MED-100®, comprising (i) precipitating the spray drying carrier from C-MED-100®; (ii) using the resulting C-MED-100® to obtain a spotting mixture for thin layer chromatography (TLC); (iii) spotting the C-MED-100® spotting mixture on pre-run TLC plates and eluting the plates to obtain the fluorescing band with Rf=0.2-0.3; (iv) scraping off the Rf=0.2-0.3 band, eluting it in ammonia and freeze drying the eluted band to form a powder; and (v) extracting the powder with methanol to remove solubilized silica gel, concentrating the methanol solution and crystalizing the concentrated solution to obtain the bioactive component. The isolated bioactive component in vitro is a quinic acid analog, preferably quinic acid lactone. By contrast, the isolated bioactive component in vivo is quinic acid, whether as free acid or as a quinic acid salt, including quinic acid ammonium salt. A pharmaceutical composition comprising a pharmaceutically effective amount of the bioactive component and a nontoxic inert carrier or diluent. The bioactive component may be used to enhance immune competency, treat disorders associated with the immune system, inhibit the inflammatory response, treat disorders associated with the inflammatory response, enhance the anti-tumor response, and treat disorders associated with the response to tumor formation and growth, all in mammals.
Owner:OPTIGENEX INC
Rapid imaging method for ultra-wide band microwave detection based on Hilbert-huang transformation
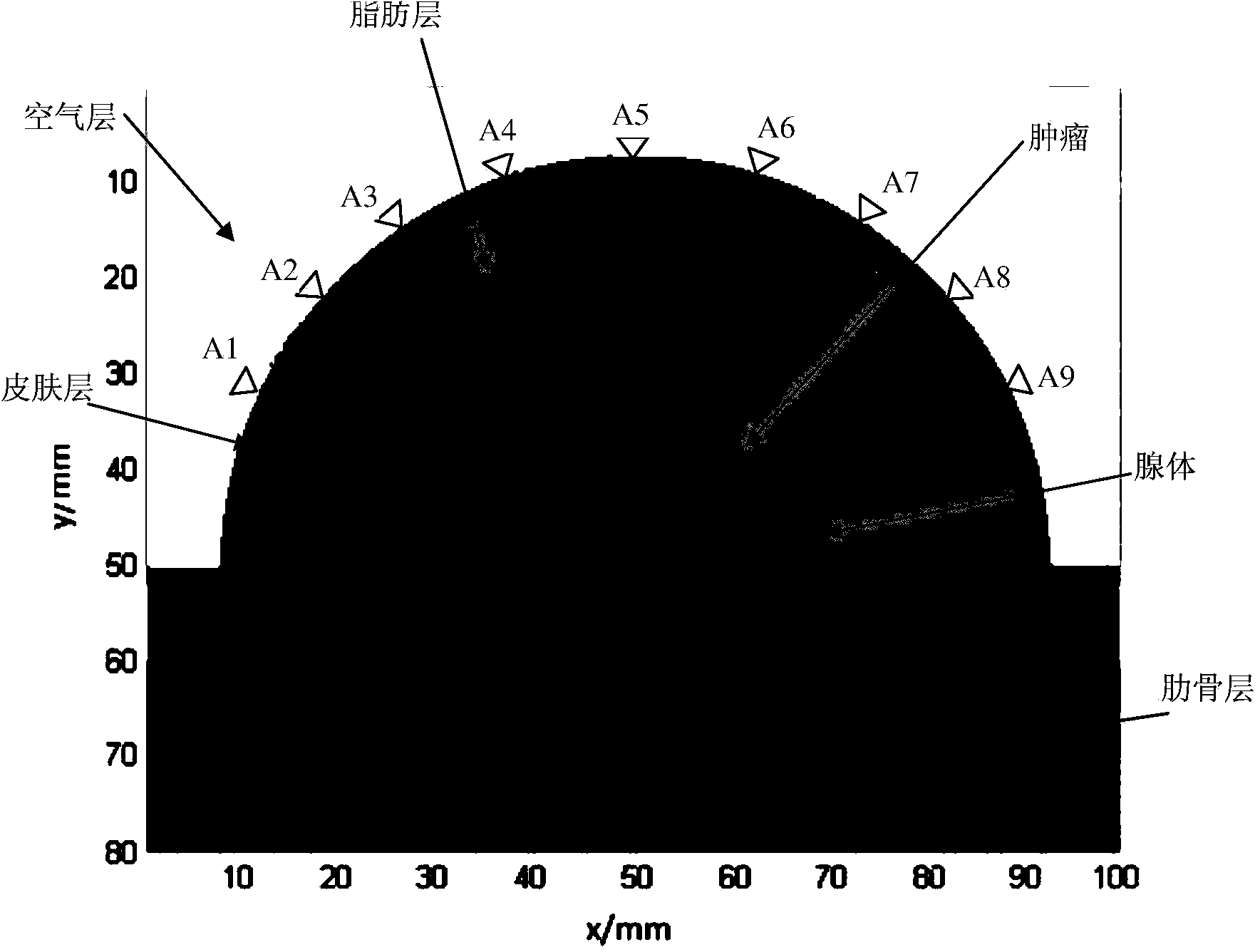
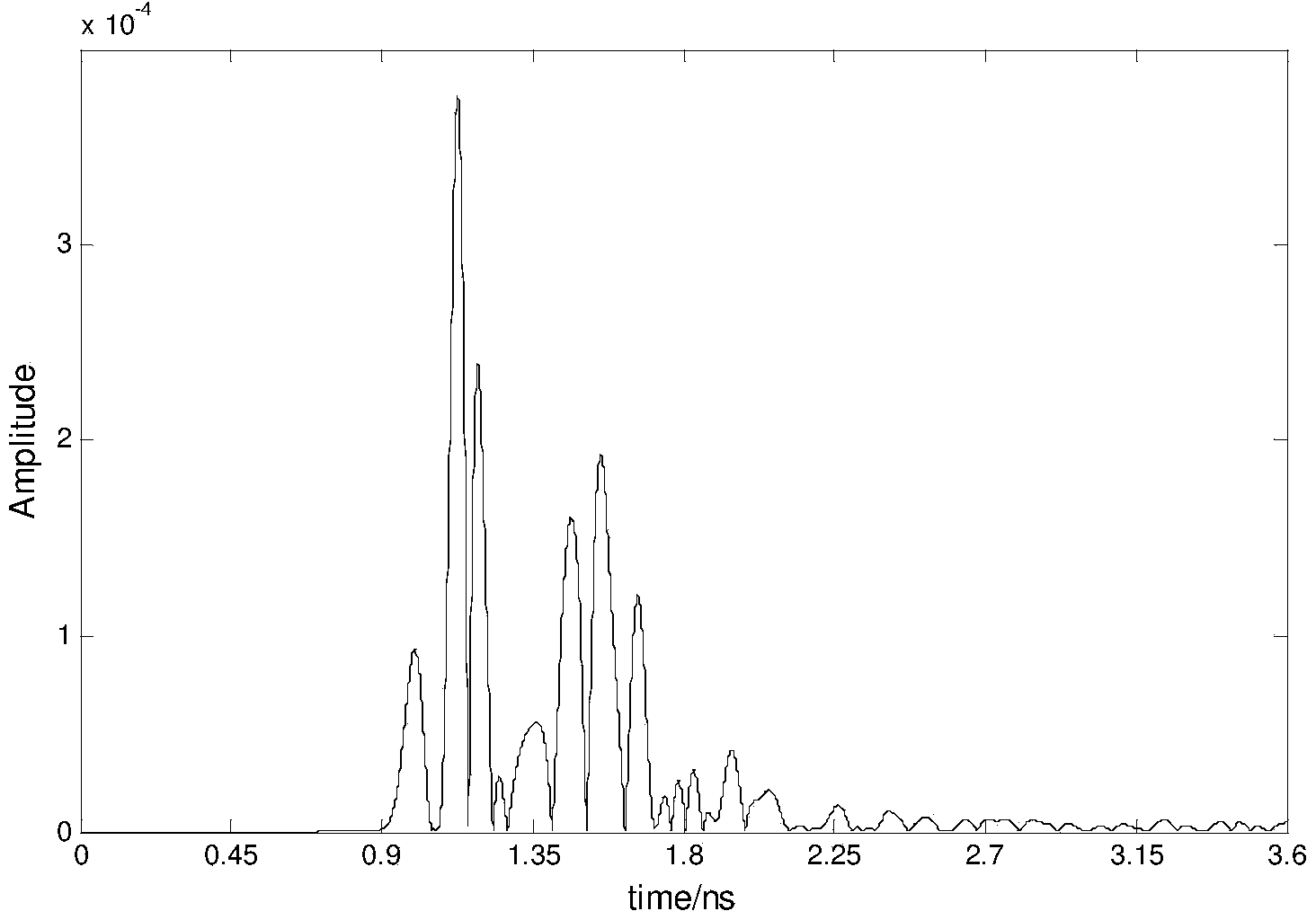
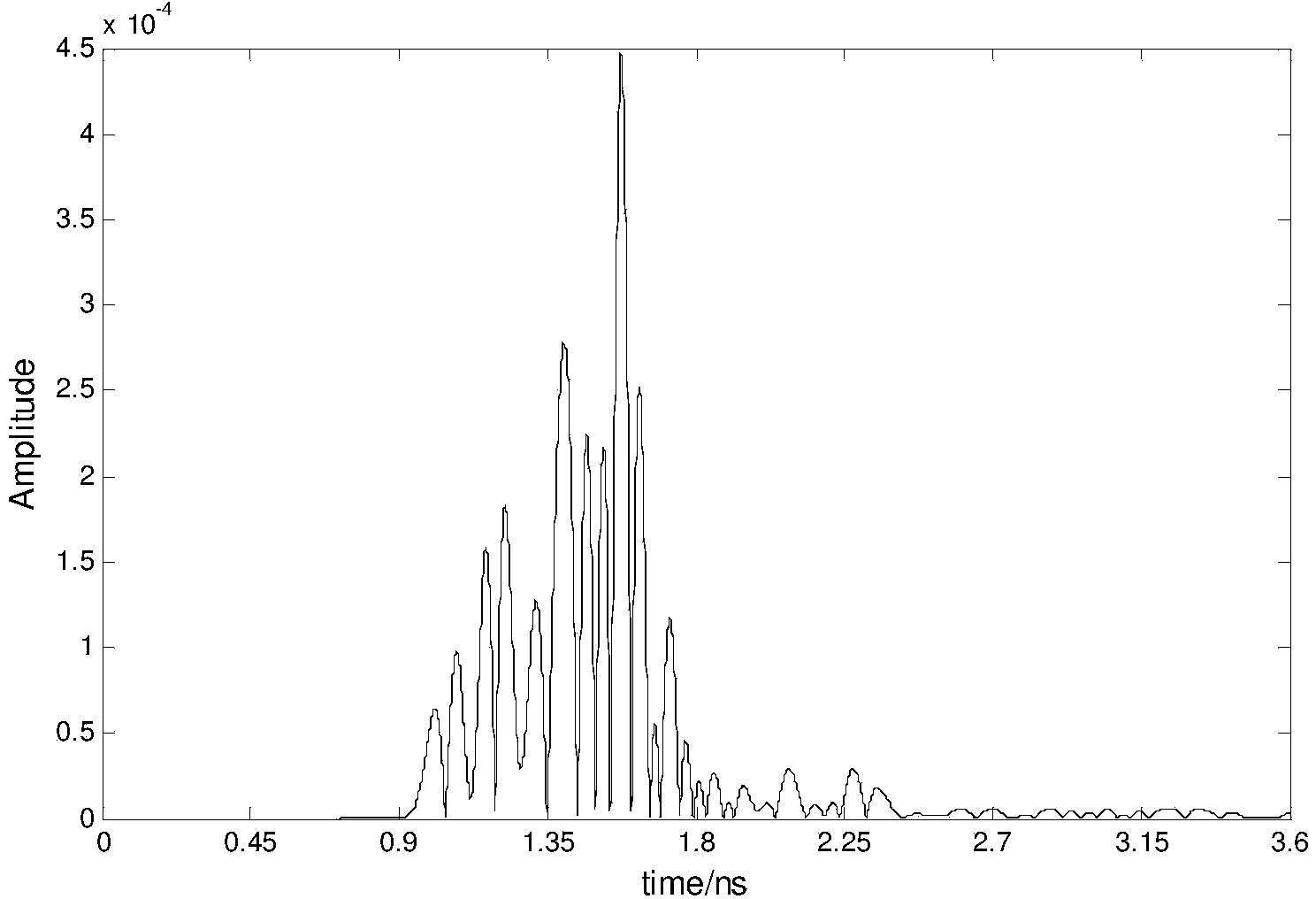
InactiveCN103799982AImprove the display effectDiagnostic recording/measuringSensorsRapid imagingTumor response
The invention relates to ultra-wide band microwave imaging, ultra-wide band wireless detection, biomedical detection equipment and microwave detection, and in particular to a rapid imaging method for ultra-wide band microwave detection based on Hilbert-huang transformation. According to the method, biological information can be displayed simply, conveniently, rapidly and obviously. According to the technical scheme, the rapid imaging method includes the following steps that an antenna Ai is used for emitting ultra-wide band microwave signals, and other antennas are used for receiving reflecting signals from the inner portion of one breast so as to obtain signals containing tumor information and signals containing no tumor information; tumor response signals are obtained by subtracting the signals containing no tumor information from the signals containing the tumor information, Hilbert transform is conducted on all the obtained tumor response signals, the signals are constructed into analytical signals, and then the instantaneous amplitude of the signals is obtained; for a breast area, scanning is conducted on each point in an imaging area through a confocal imaging method, and the position and size information of a tumor is obtained clearly. The rapid imaging method is mainly used for designing and manufacturing medical apparatuses and instruments.
Owner:TIANJIN UNIV
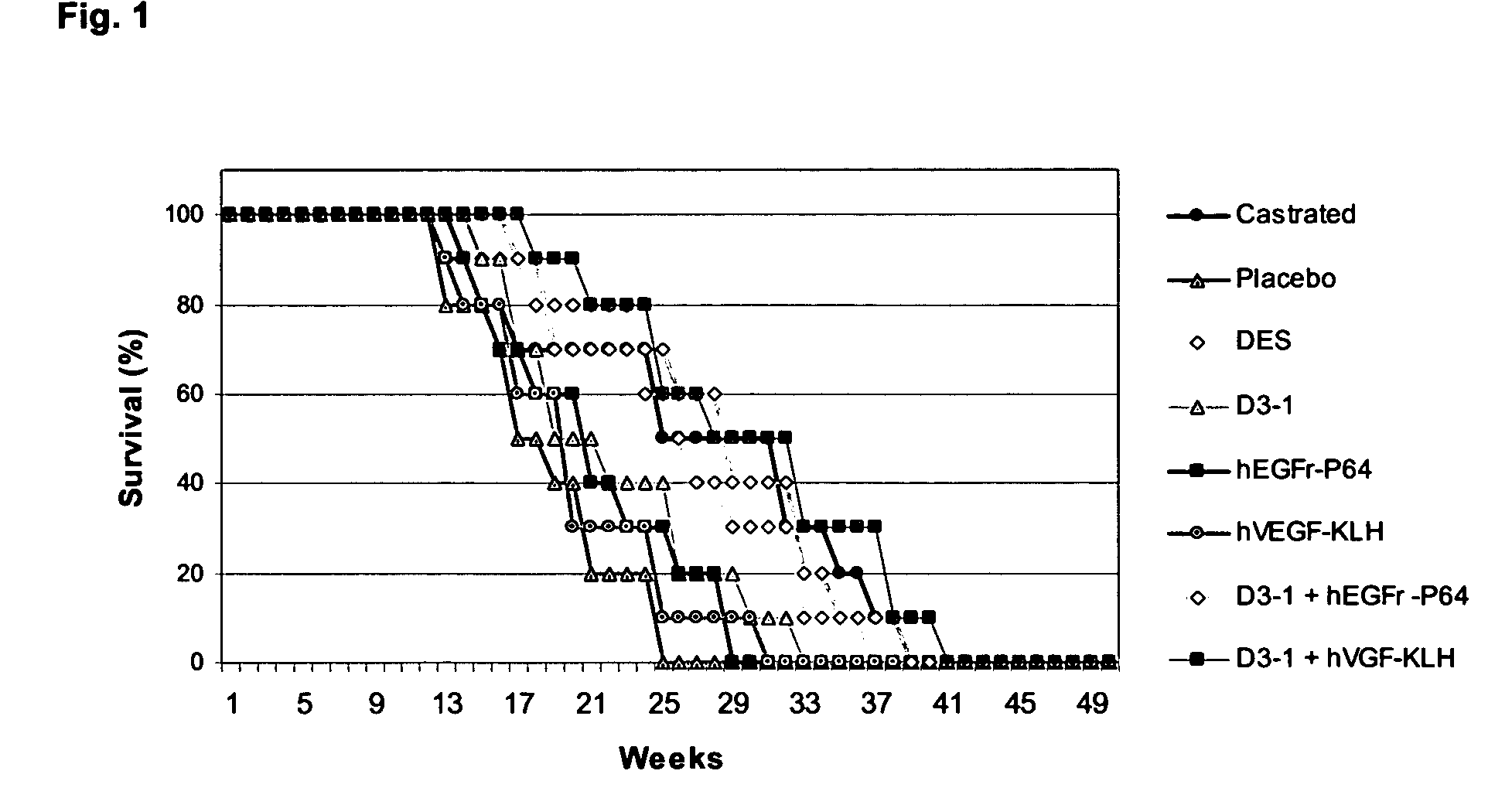
Combinations of growth-and hormone-regulating factors for the treatment of neoplasia
InactiveUS20060193853A1Reducing tumor massSynergic effectPeptide/protein ingredientsAntibody ingredientsAbnormal tissue growthTumor response
The invention relates to the field of immunology, endocrinology and oncology and, in particular, the generation of a combined immune response to determined growth factors and hormones. A synergic effect, outlined herein, between growth regulating factors (EGF, TGF and VEGF) and hormones involved in the sexual hormones release cascade or reproduction (GnRH, LH, FSH) stimulates the anti-tumor response which is expressed as a reduction in the tumor mass and an increase in the survival time.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Computer-assisted tumor response assessment and evaluation of the vascular tumor burden
ActiveUS9826951B2Effectively monitoring responseReduce the amount requiredImage enhancementImage analysisTumor responseImaging processing
A computerized method for determining an objective tumor response to an anti-cancer therapy using one or more cross-sectional images can comprise receiving one or more cross-sectional images that comprise one or more cross-sectional slices of digital medical image data and identifying one or more target lesions within the one or more cross-sectional images. The method can also comprise analyzing at least one of the target lesions with an image-processing module configured to identify a total range of pixel intensities and restrict the total range of pixel intensities to a first restricted range that corresponds to pixel intensities representative of vascularized tumor. Further still, the method can comprise deriving a vascular tumor burden for the at least one of the one or more target lesions and determining the objective tumor response for the at least one of the one or more target lesions based on the vascular tumor burden.
Owner:AI METRICS LLC
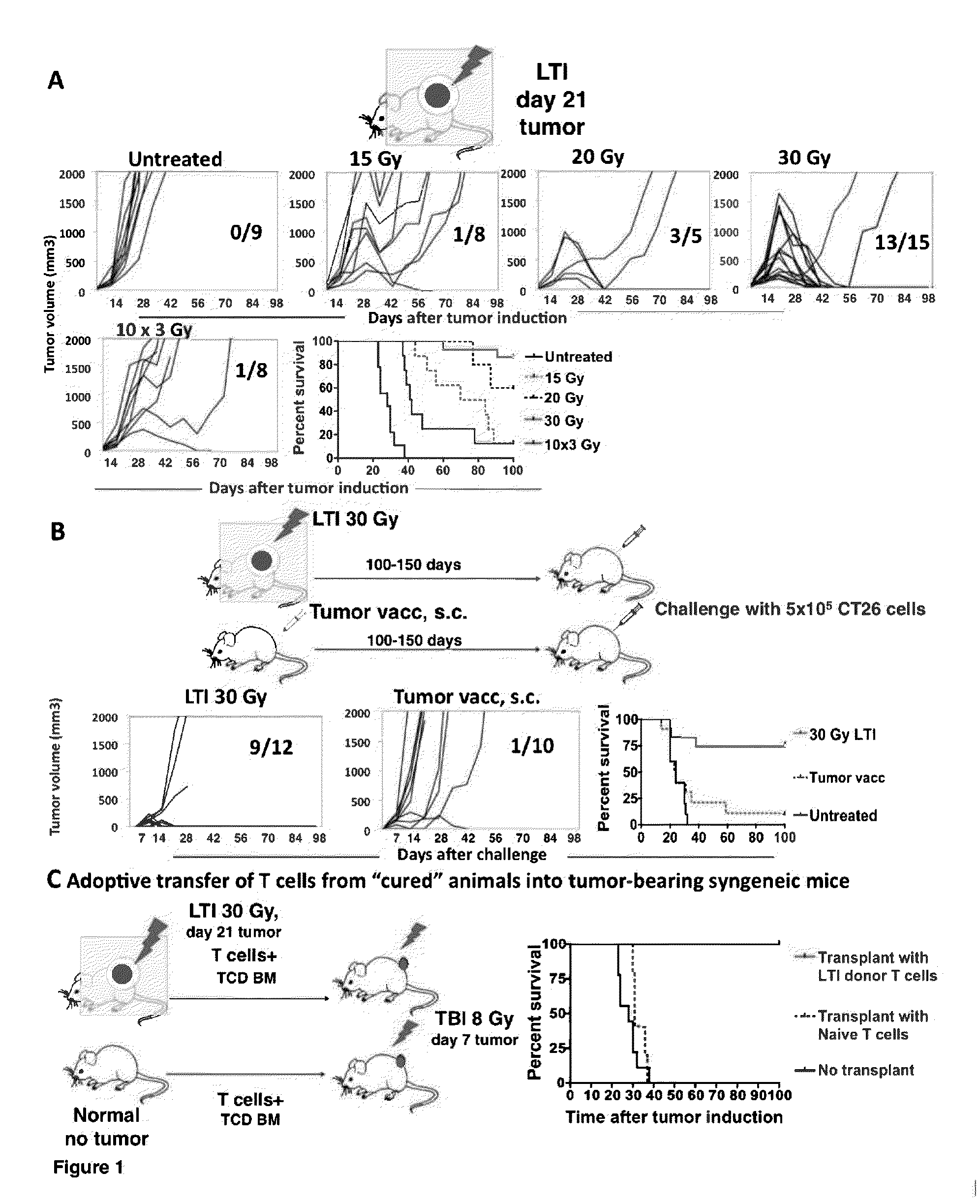
Anti-tumor T cell immunity induced by high dose radiation
ActiveUS9114157B2Growth inhibitionMammal material medical ingredientsBlood/immune system cellsTumor responseCancer therapy
Cancer treatment is provided, by irradiating an individual with a localized, high single dose or short course of doses at a primary tumor site; collecting T cells from the individual after a period of time sufficient activation of an anti-tumor response; treating the individual with an effective dose of dose of chemotherapy; and reintroducing the T cell population back to the individual.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
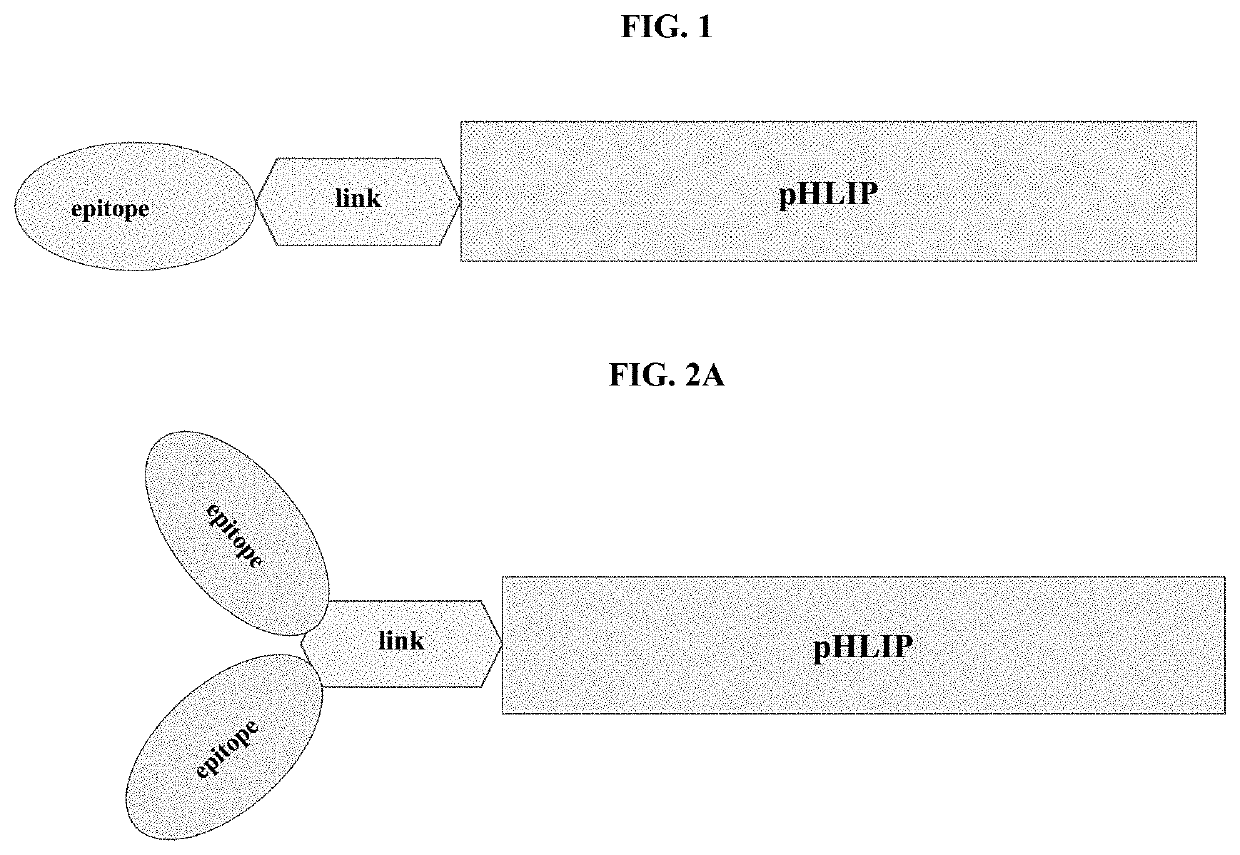
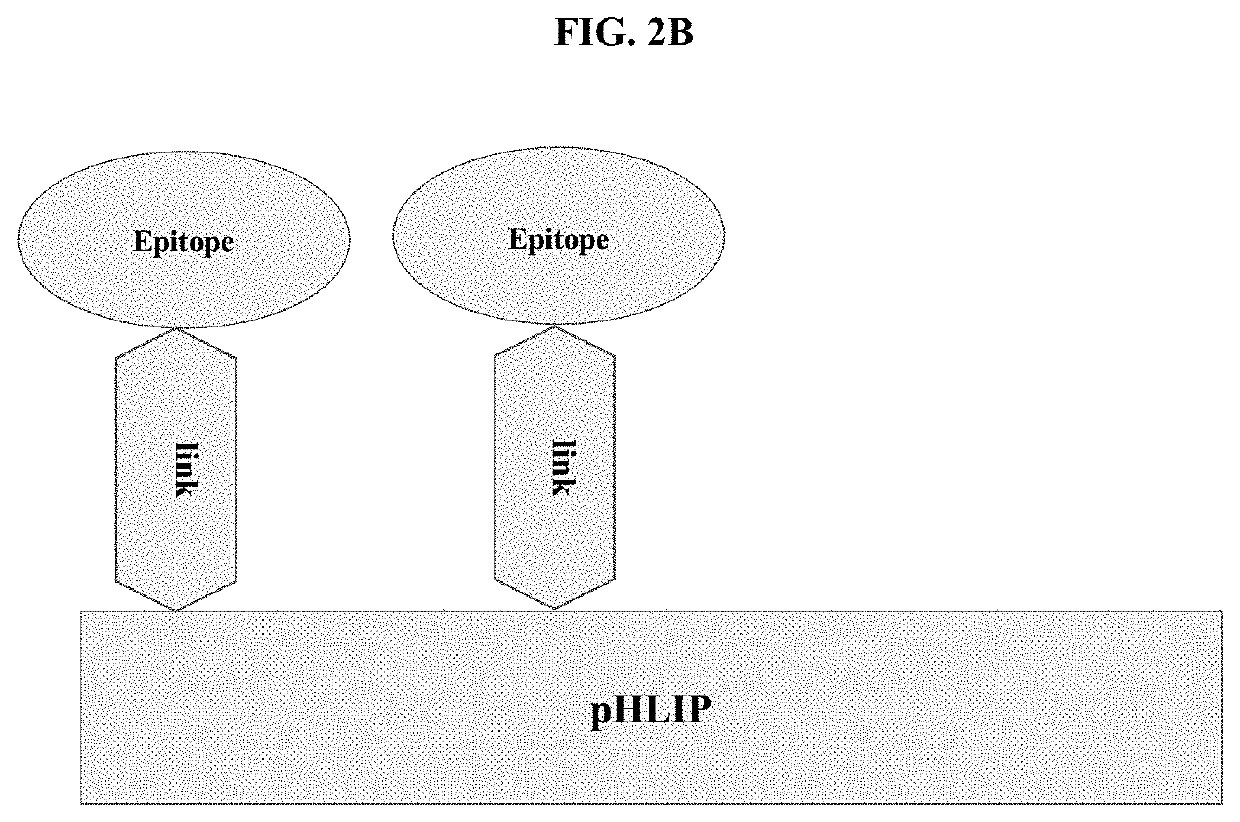
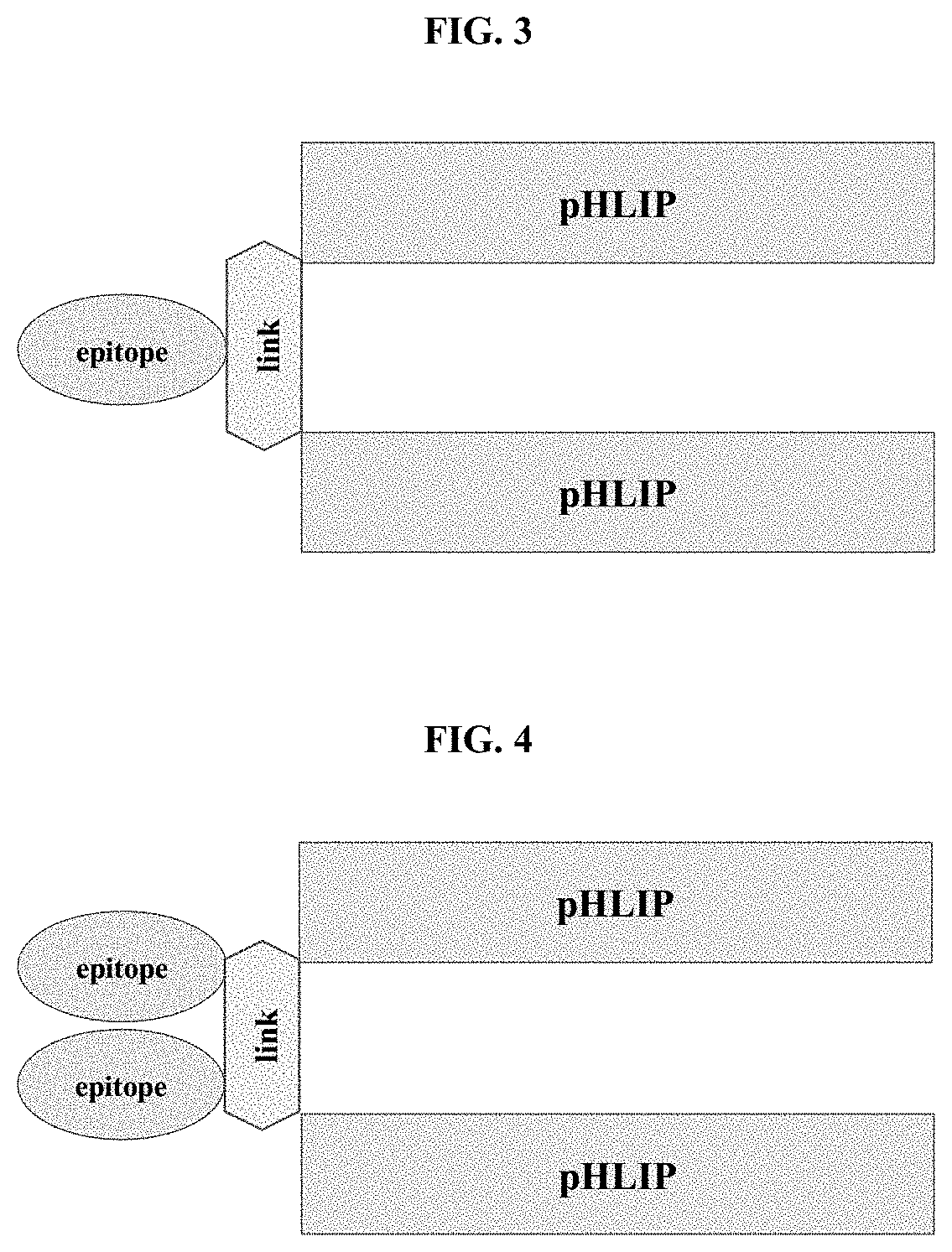
pHLIP® peptide-mediated epitope tethering at cell surfaces
PendingUS20200246420A1Enhances the recruitment of cellsEffective combinationPeptide/protein ingredientsPharmaceutical non-active ingredientsEpitopeTumor response
The invention features methods and compositions for eliciting an anti-tumor response in a subject comprising administering to the subject a pHLIP® construct comprising an antibody recruiting molecule linked to one or more pHLIP® peptides by a non-cleavable linker compound. The construct increases the amount of the antibody recruiting molecule on the surface of a diseased cell.
Owner:UNIV OF RHODE ISLAND BOARD OF TRUSTEES +1
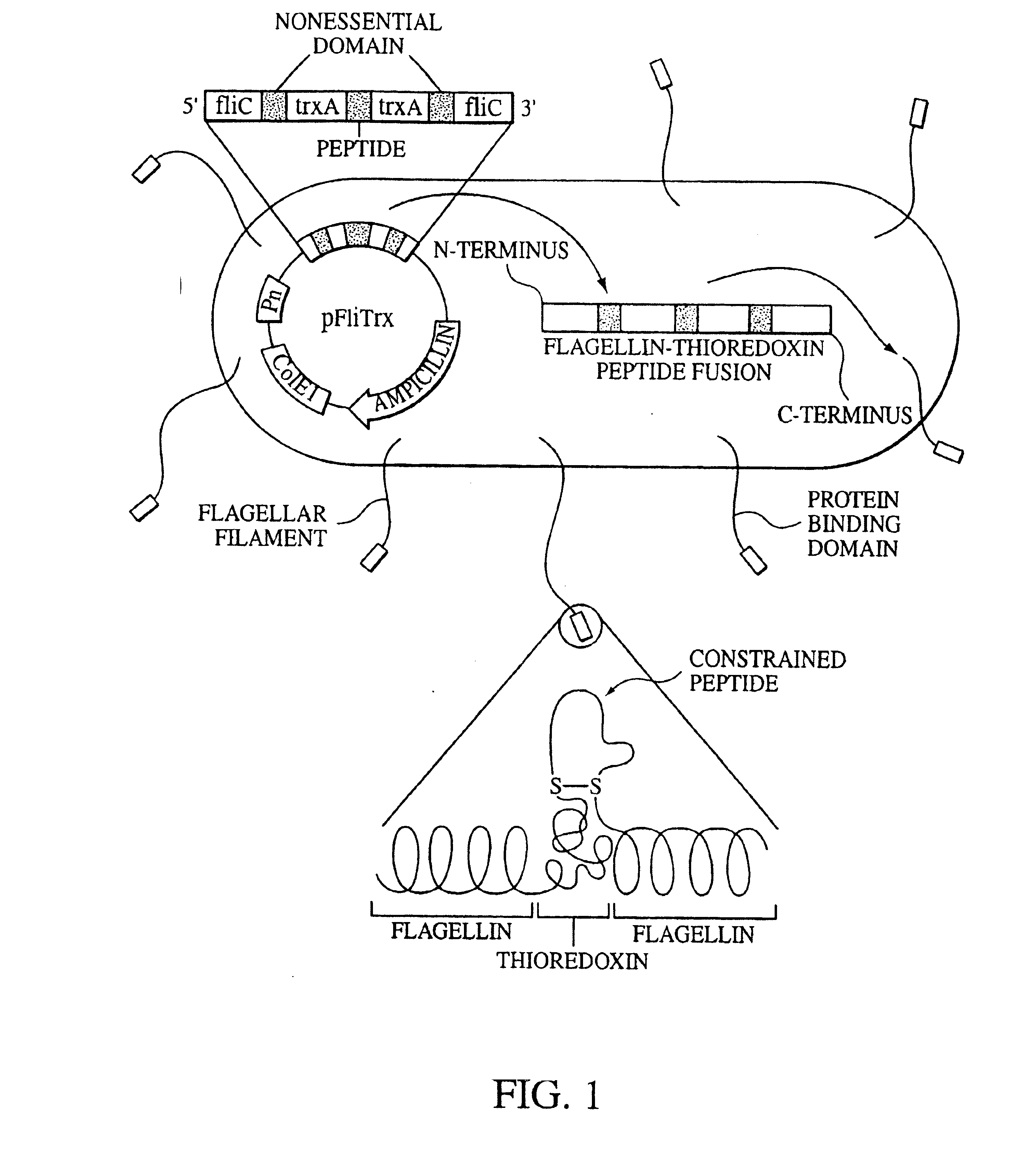
Endothelial specific targeting
InactiveUS6974791B2Easy to optimizeFacilitate anti-angiogenic therapiesIn-vivo radioactive preparationsPeptide/protein ingredientsTumor responseLymphatic Spread
Peptide motifs which define specificity of tumor-derived endothelial cells. These peptides possess a charge motif of positive-positive-hydrophobic which is important in determining the specificity of binding to tumor-derived endothelium. The specific molecular peptide motifs will facilitate diverse therapeutic and diagnostic applications including: anti-angiogenic therapies to be used in alone or in conjunction with standard therapies; imaging tools for both detection of very small metastasis that are undetectable by current techniques; for monitoring tumor response; for targeting and directing chemotherapy drugs to the tumor; for treatment of chronic inflammatory diseases such as rheumatoid arthritis and psoriasis, for treating some forms of blindness; as well as other diagnostic and therapeutic applications.
Owner:PITTSBURGH UNIV OF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com