Compositions and methods of identifying tumor specific neoantigens
a technology neoantigens, which is applied in the field of tumor specific neoantigens identification, to achieve the effect of efficient nucleotide infection and higher expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
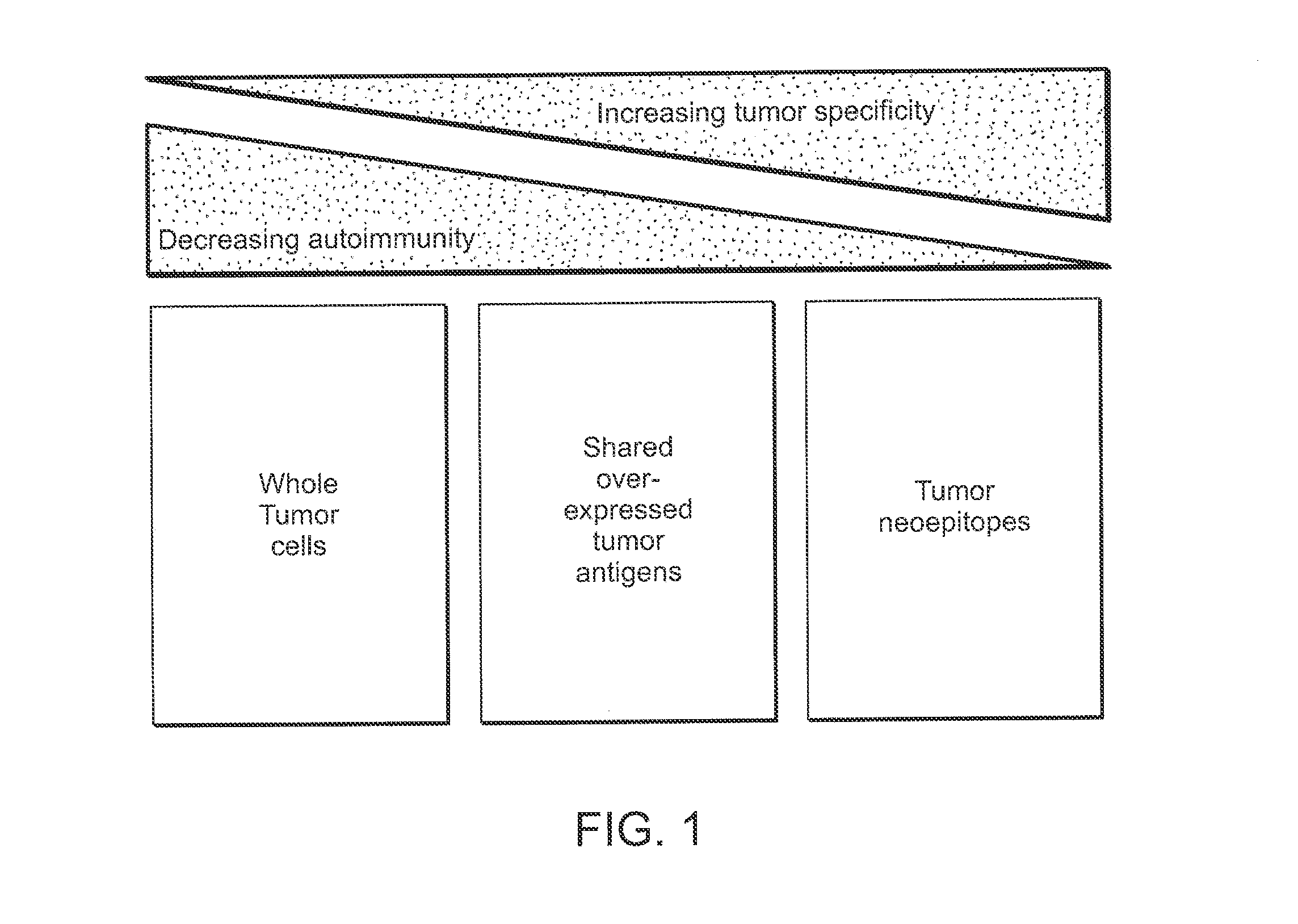
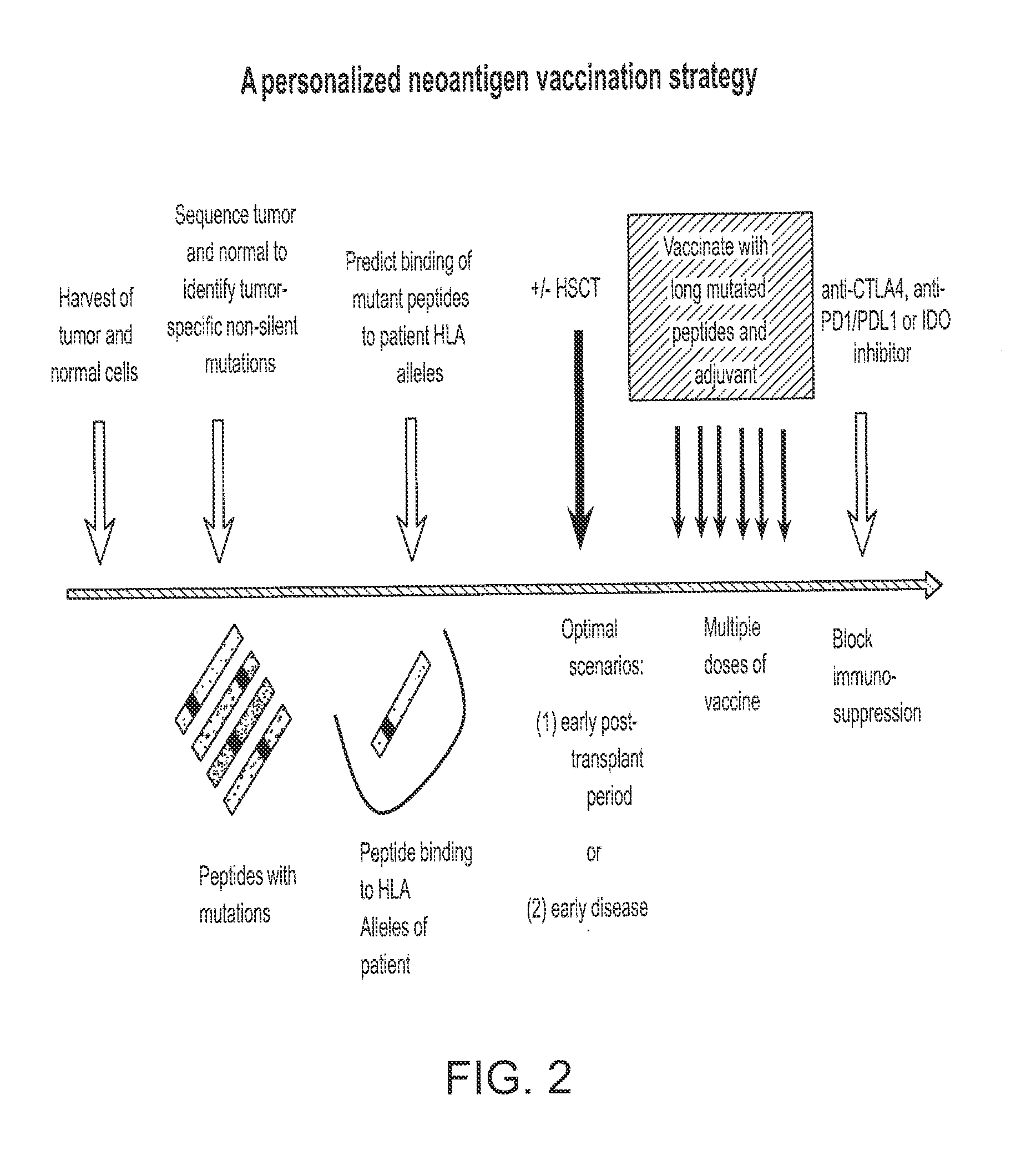
A Strategy to Identify Neoepitopes for Vaccination
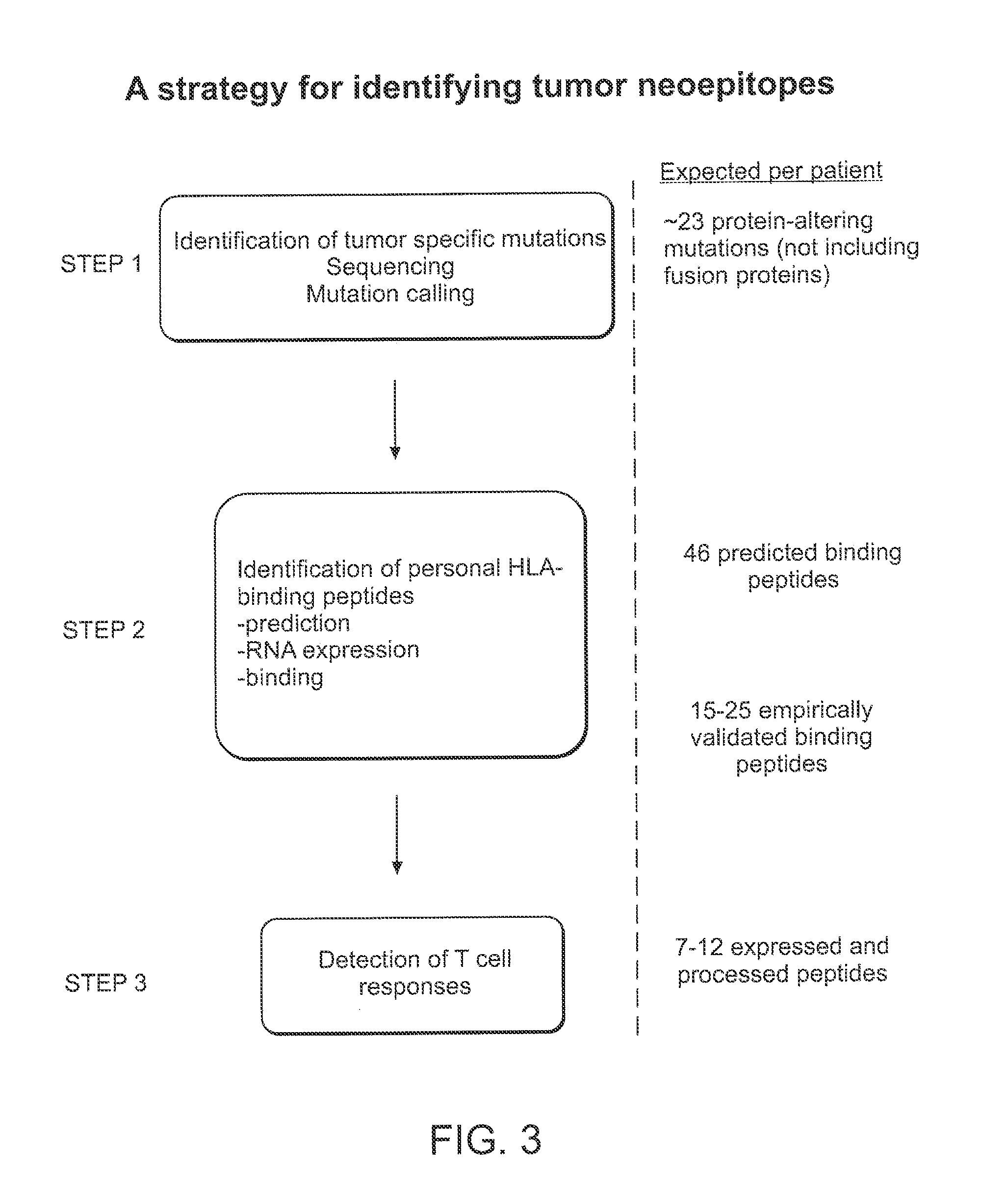
[0176]Our approach to identify tumor-specific neoepitopes involves 3 steps. (1) Identification of DNA mutations using whole genome or whole exome (i.e. only captured exons) sequencing of tumor versus matched germline samples from each patient. Our preliminary studies demonstrate that CLL cells contain many distinct genetic changes that alter amino acid sequence and could generate potential novel T cell epitopes. (2) Application of highly validated peptide-MHC binding prediction algorithms to generate a set of candidate T cell epitopes based on non-silent mutations present in tumors. We will confirm expression of mutated genes as RNA in CLL samples, and then confirm the peptide-HLA binding predictions using an experimental approach to quantify binding of candidate peptides to HLA alleles. (3) Generation of antigen-specific T cells against mutated peptides.
example 2
Tumor and Normal Genome Sequencing for the Identification of Mutated Genes in Tumors of Patients with Chronic Lymphocytic Leukemia (Step 1)
[0177]To detect tumor-specific mutations (that are not present in normal tissues), samples were collected from tumors and from normal tissues of each patient. For leukemias, tumors were purified using magnetic bead isolation or fluorescence-activated cell sorting using antibodies specific to tumor cells, e.g., the tumor cells of patients with chronic lymphocytic leukemia (CLL) express the CD5 and CD19 surface markers. Skin fibroblasts were used as a normal tissue control. DNA or RNA for sequencing was purified from isolated tumor or normal tissue cells. For melanoma, ovarian and other solid tumors (in which there is contamination with non-tumor cells), DNA and RNA were isolated from relatively homogeneous short-term cultures of tumor cells or from laser-captured tumor. PBMCs were used as normal control cells. For all samples, PBMCs were cryoprese...
example 3
Identification of HLA-Binding Peptides Derived from Expressed Proteins Harboring Tumor-Specific Mutations (Step 2)
[0180]The next question is whether mutated genes may generate peptides that can be presented by patient MHC / HLA proteins. First, several algorithms were used to predict 30 and 137 HLA-binding peptides with IC50 scores <500 nM from 10 missense mutations of Patient 1, and from 53 missense 1 indel and 2 gene fusions of Patient 2. An example for one missense mutation in a patient with 6 specific HLA alleles is shown with 2 predicted binding peptides out of 54 combinations of 9-mers peptides and HLA alleles (FIG. 6). To confirm that these genes are expressed in tumors, we measure RNA levels for the mutated genes (using several approaches that depend on the mutation class, FIG. 7), and found that 98% of mutated genes with HLA binding peptides were expressed.
[0181]The HLA binding capacity of all predicted peptides that pass RNA expression validation are then experimentally vali...
PUM
| Property | Measurement | Unit |
|---|---|---|
| incubation time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com