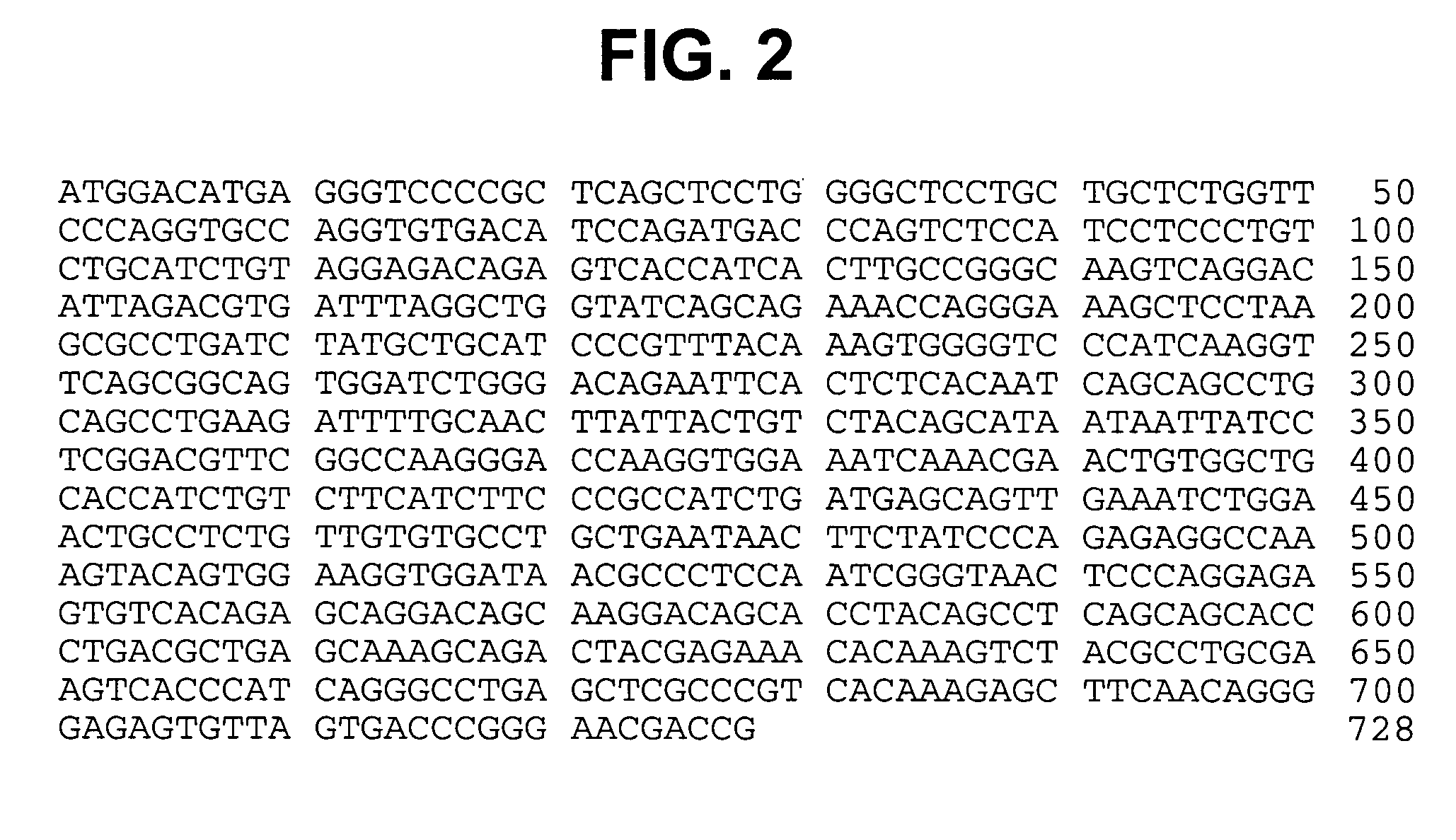
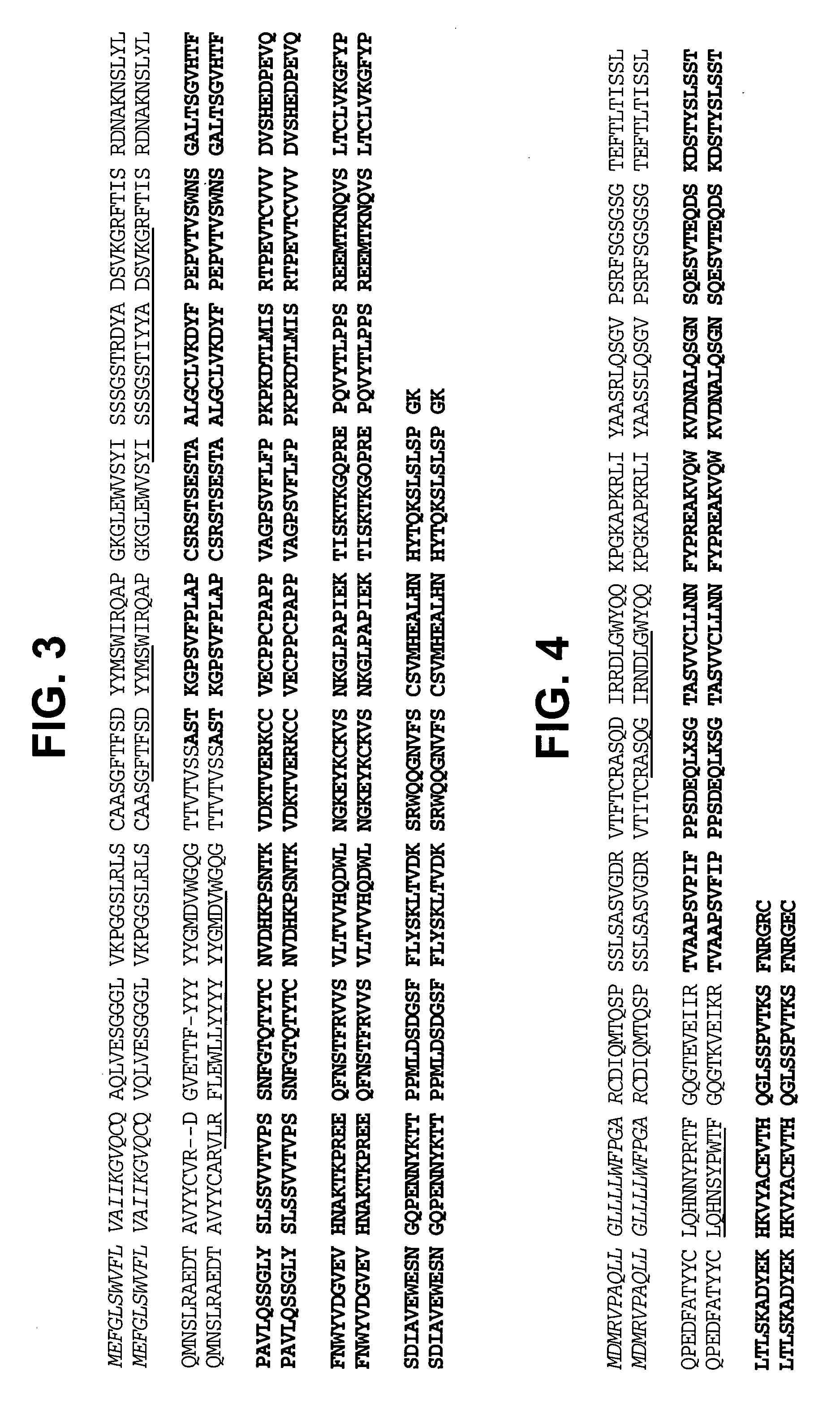
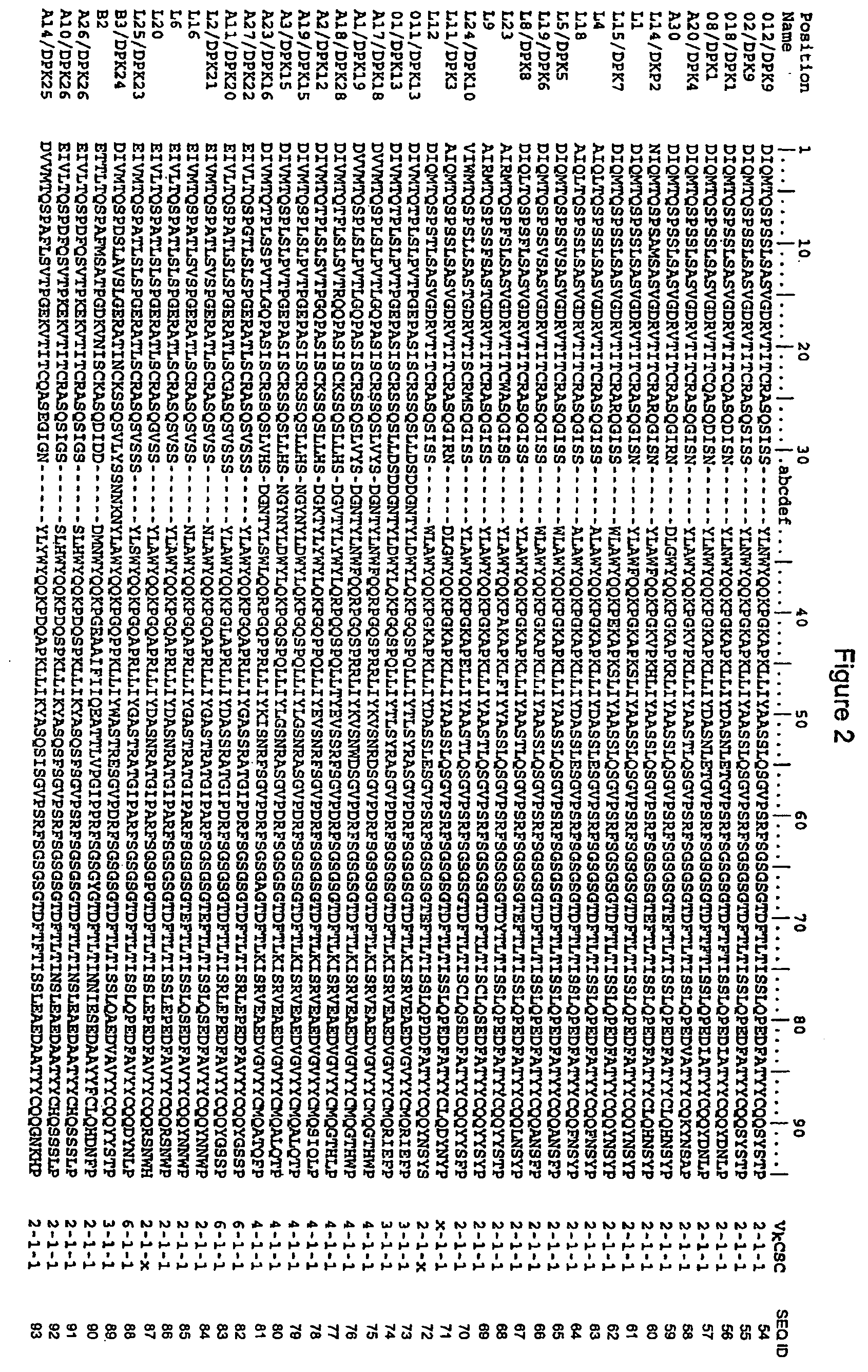
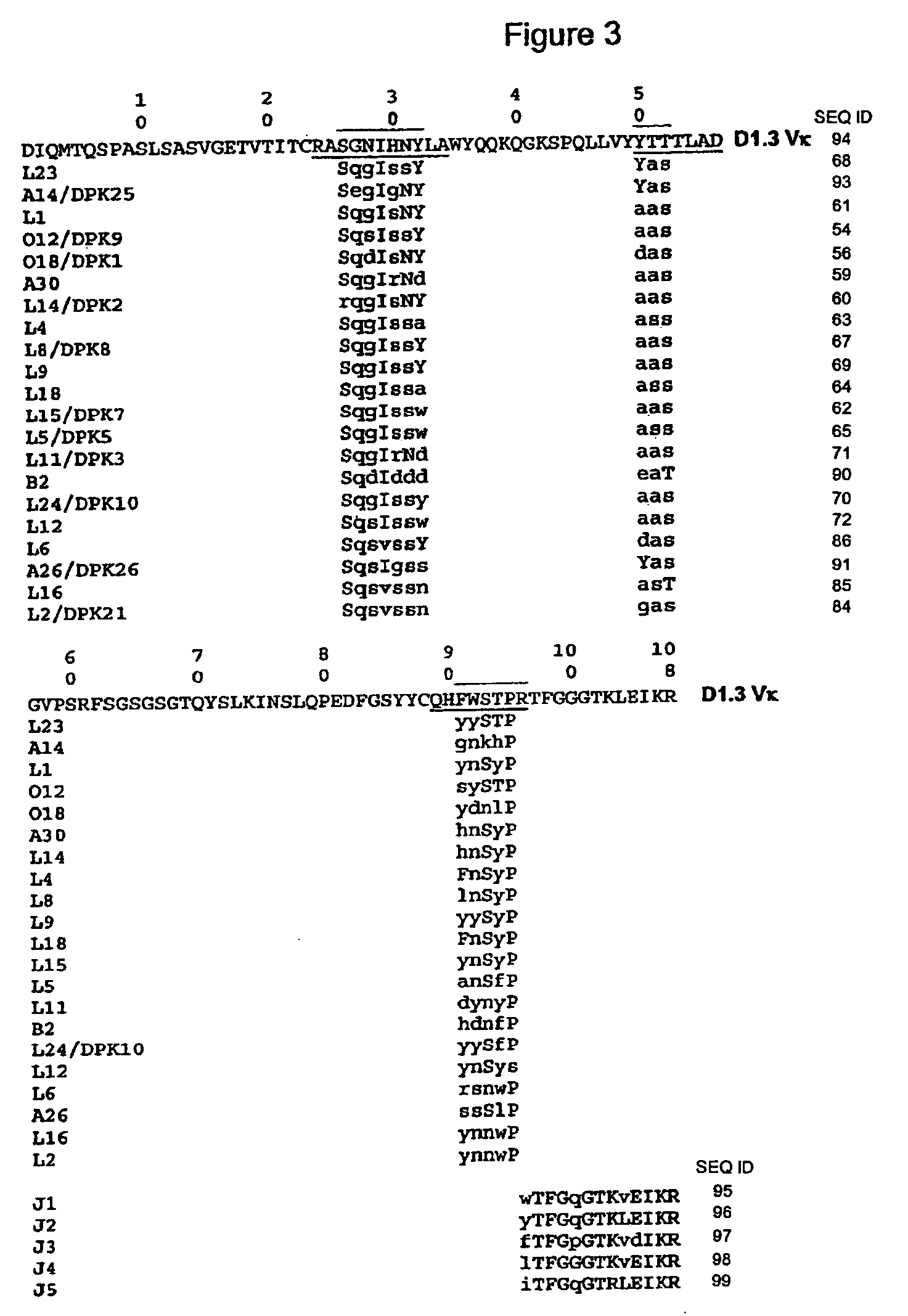
Patents
Literature
229 results about "Germline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In biology and genetics, the germline in a multicellular organism is the population of its bodily cells that are so differentiated or segregated that in the usual processes of reproduction they may pass on their genetic material to the progeny.
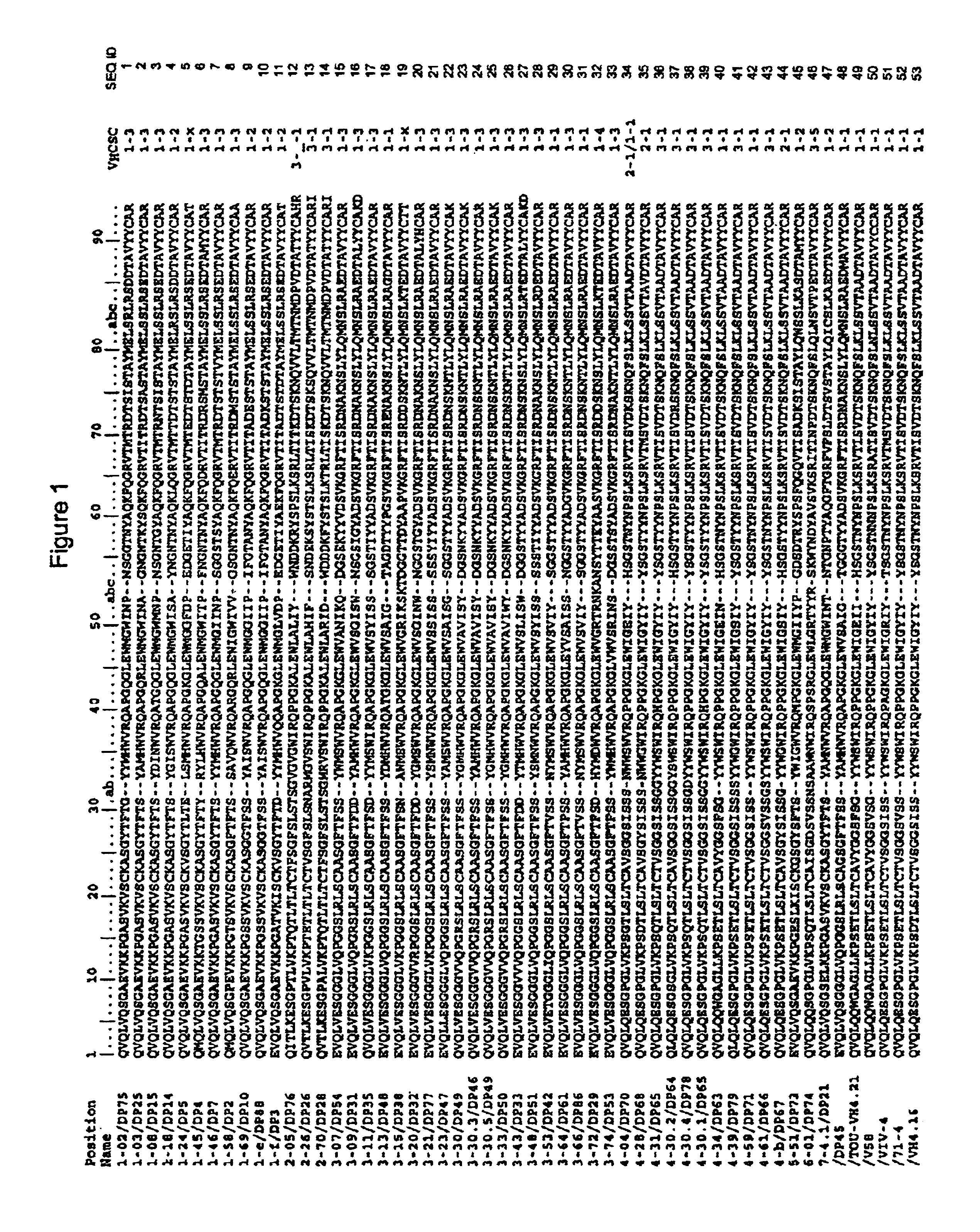
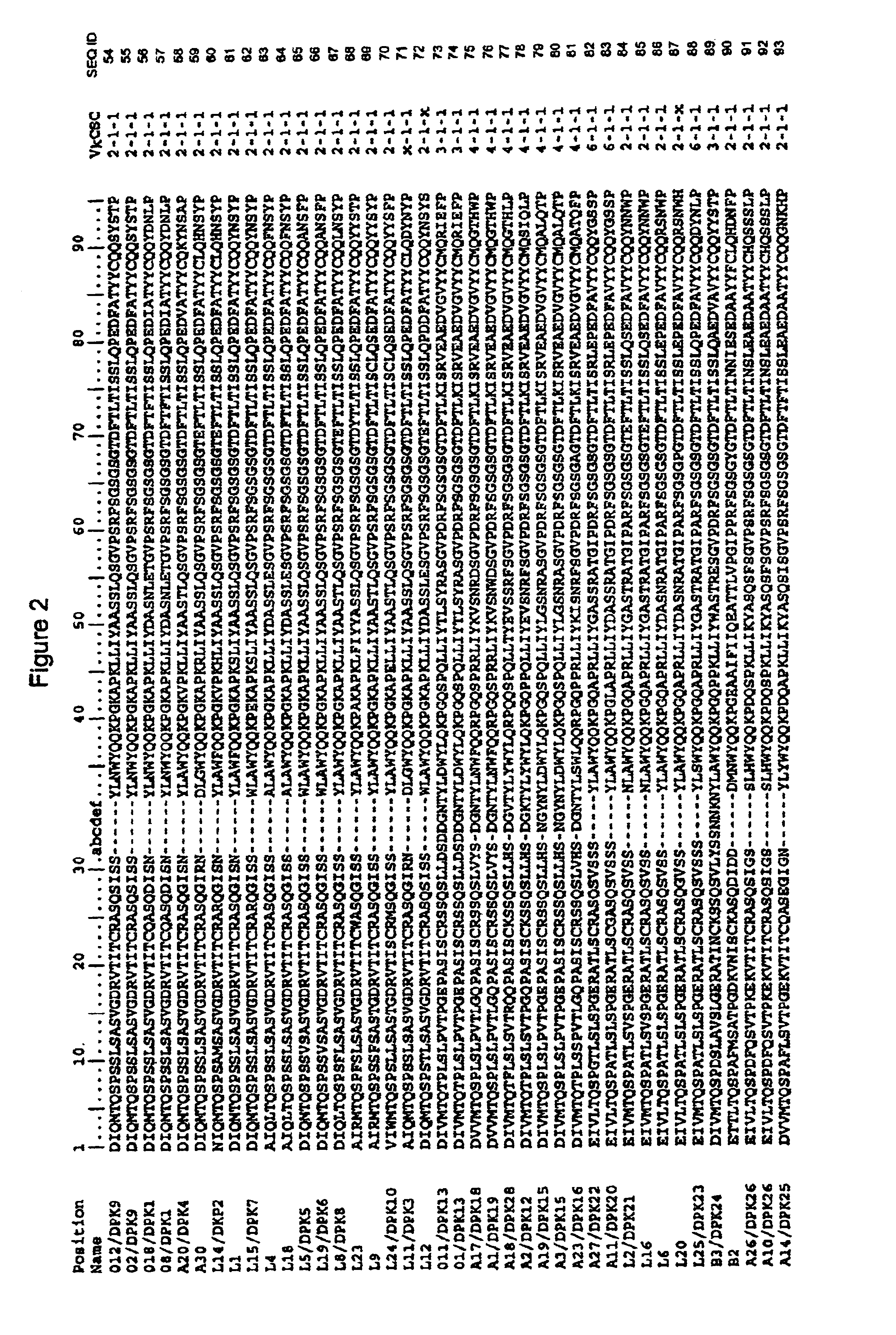
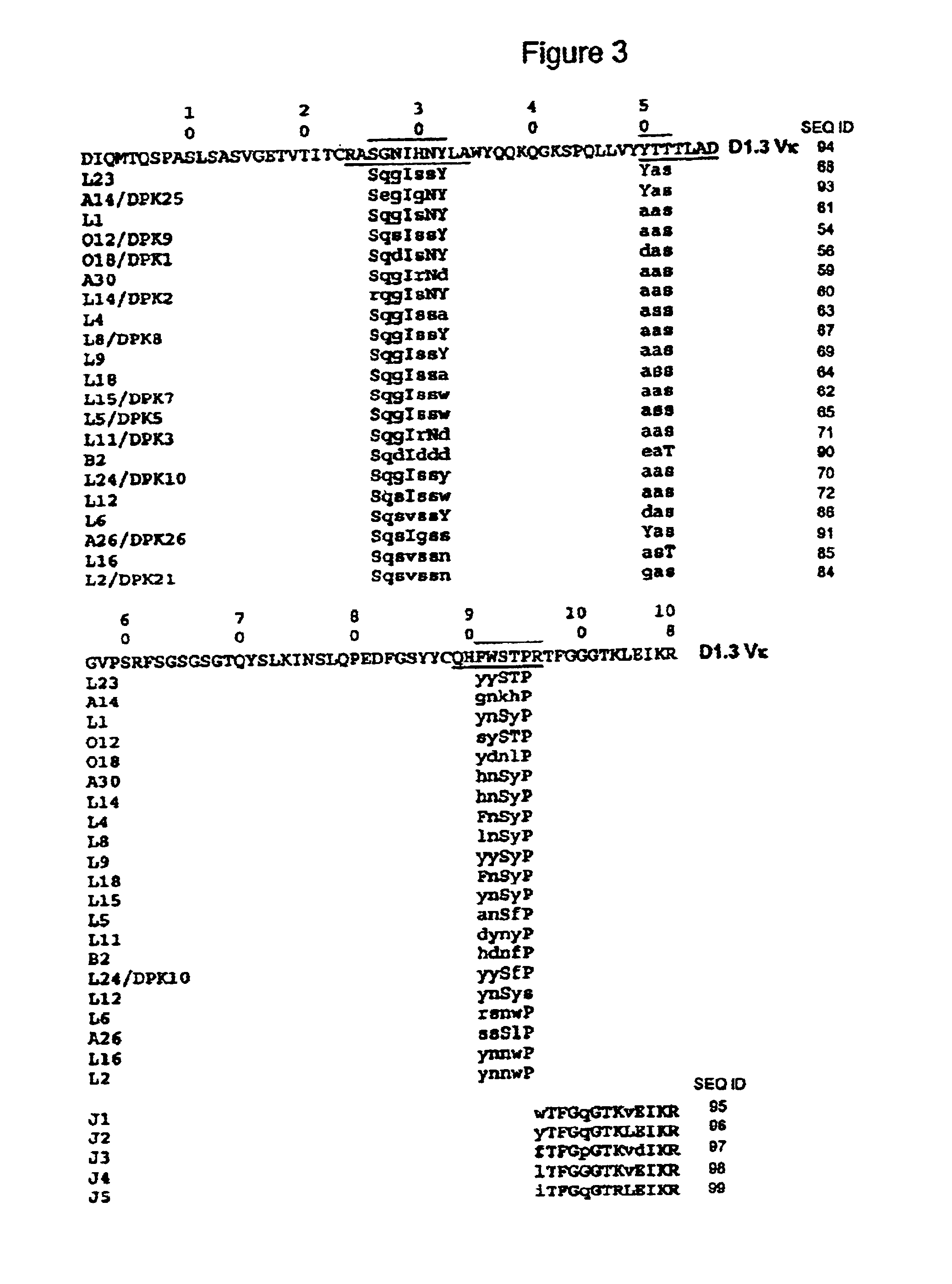
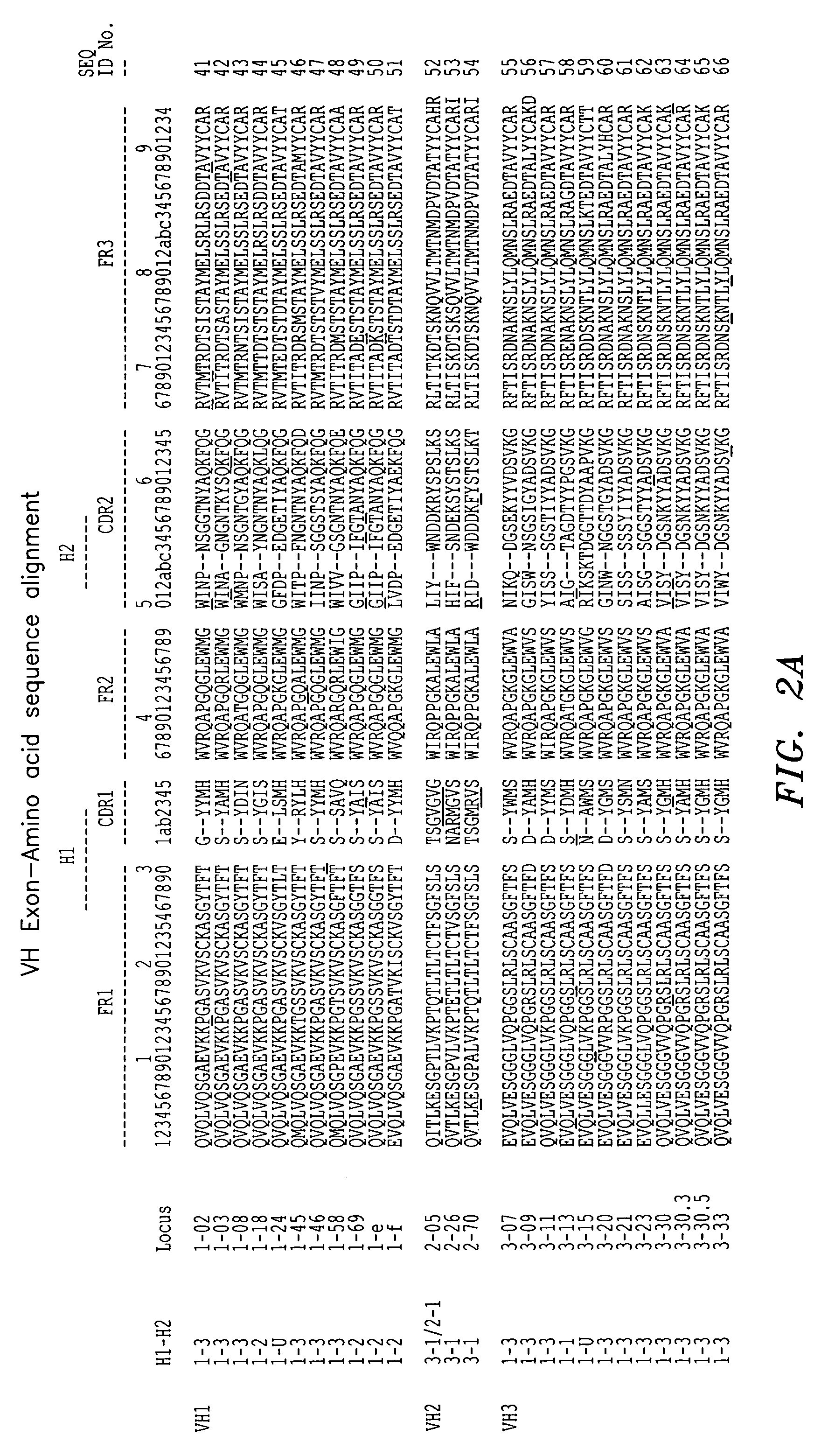
Super humanized antibodies
InactiveUS6881557B2Antibody mimetics/scaffoldsAnalogue computers for chemical processesHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
Targeted modification of rat genome
Compositions and methods are provided for modifying a rat genomic locus of interest using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Compositions and methods for generating a genetically modified rat comprising one or more targeted genetic modifications in their germline are also provided. Compositions and methods are provided which comprise a genetically modified rat or rat cell comprising a targeted genetic modification in the rat interleukin-2 receptor gamma locus, the rat ApoE locus, the rat Rag2 locus, the rat Rag1 locus and / or the rat Rag2 / Rag1 locus. The various methods and compositions provided herein allows for these modified loci to be transmitted through the germline.
Owner:REGENERON PHARM INC
Methods and Compositions for the Targeted Modification of a Genome
Compositions and methods are provided for modifying a genomic locus of interest in a eukaryotic cell, a mammalian cell, a human cell or a non-human mammalian cell using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Further methods combine the use of the LTVEC with a CRISPR / Cas system. Compositions and methods for generating a genetically modified non-human animal comprising one or more targeted genetic modifications in their germline are also provided.
Owner:REGENERON PHARM INC
Targeted modification of rat genome
InactiveUS20140309487A1Enhance homologous recombinationAnimal reproductionApolipeptidesRAG2Nucleic acid sequencing
Compositions and methods are provided for modifying a rat genomic locus of interest using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Compositions and methods for generating a genetically modified rat comprising one or more targeted genetic modifications in their germline are also provided. Compositions and methods are provided which comprise a genetically modified rat or rat cell comprising a targeted genetic modification in the rat interleukin-2 receptor gamma locus, the rat ApoE locus, the rat Rag2 locus, the rat Rag1 locus and / or the rat Rag2 / Rag1 locus. The various methods and compositions provided herein allows for these modified loci to be transmitted through the germline.
Owner:REGENERON PHARM INC
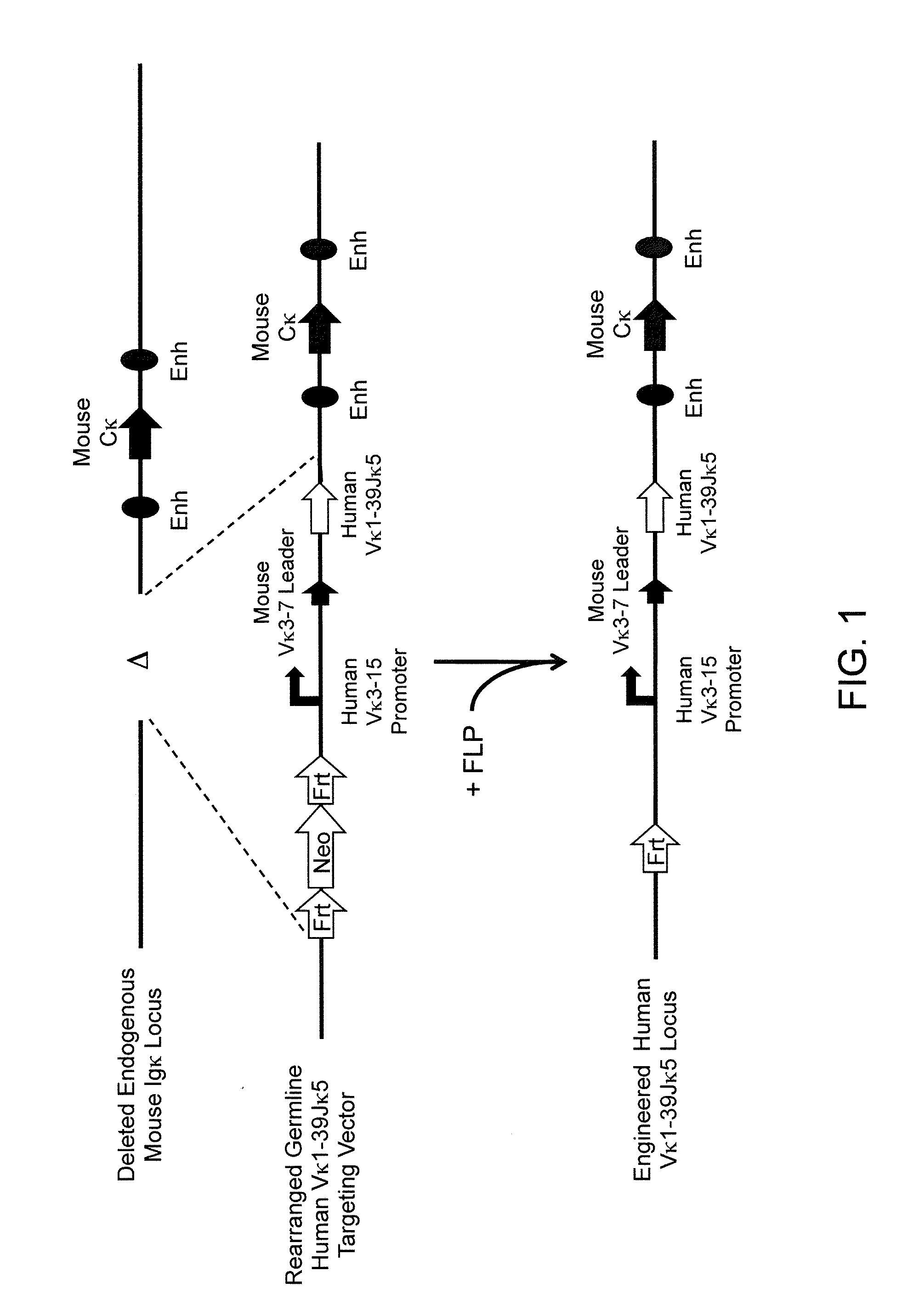
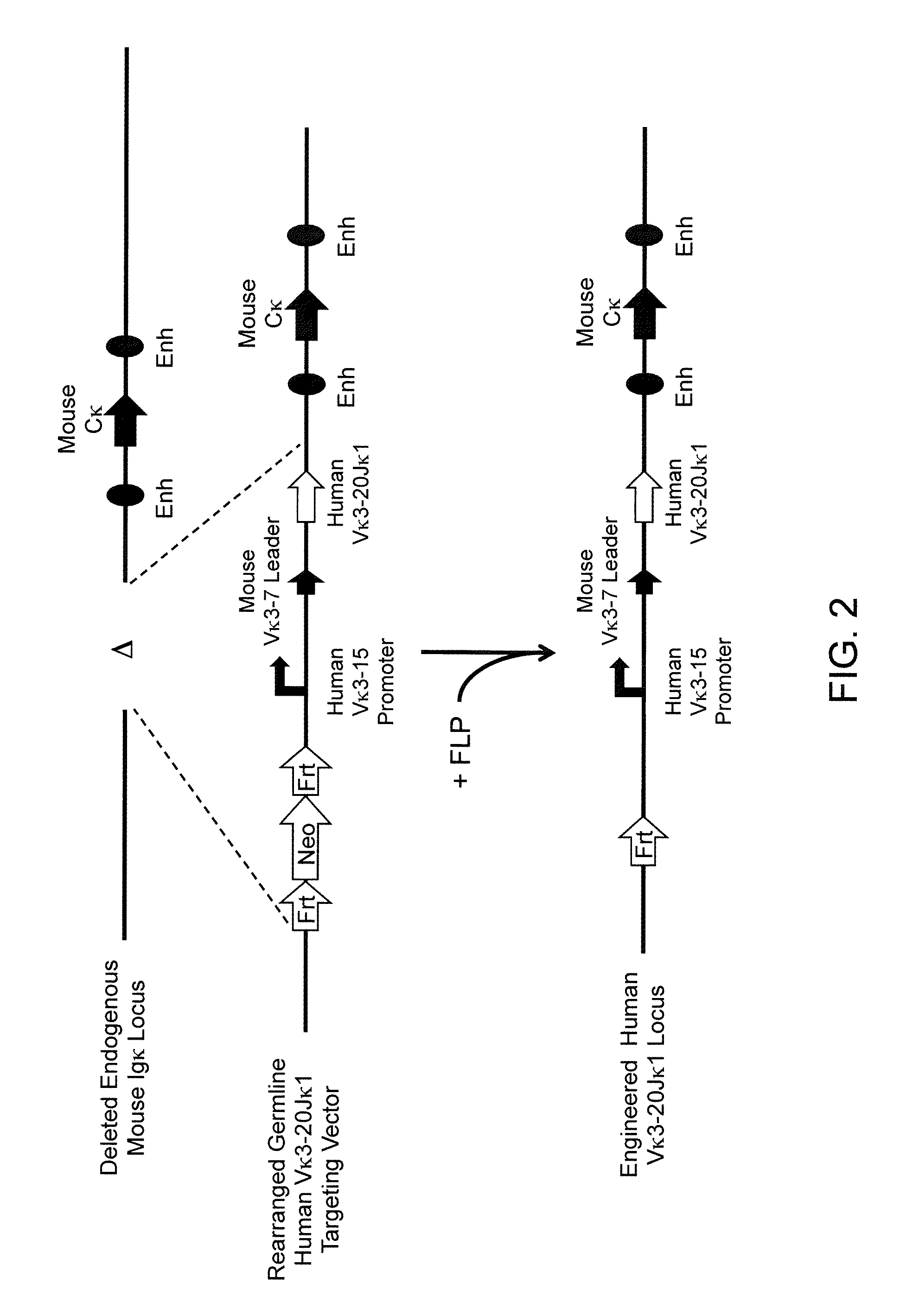
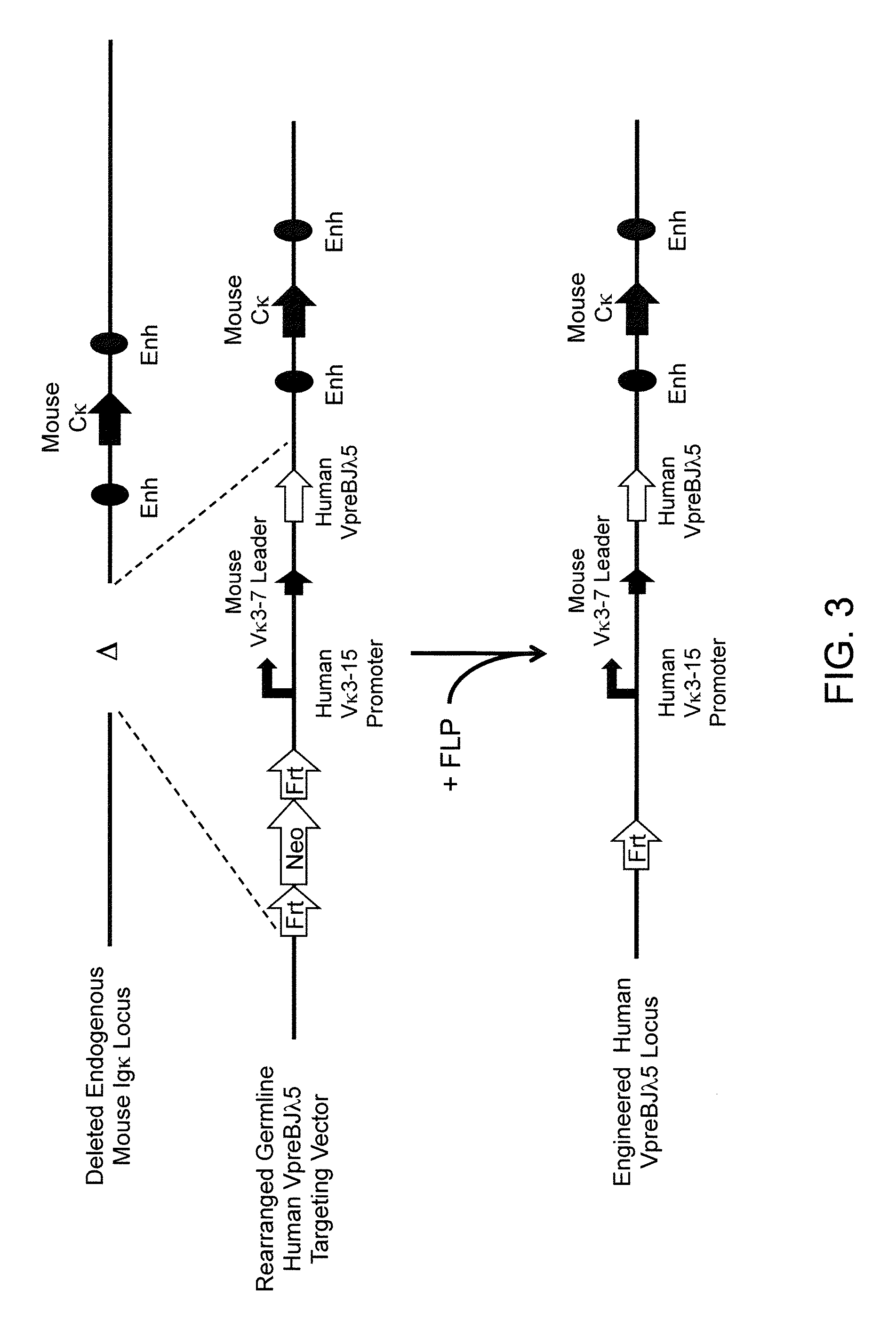
Methods For Making Fully Human Bispecific Antibodies Using A Common Light Chain
InactiveUS20130045492A1Reduce in quantitySimple methodAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeProtein insertion
A genetically modified mouse is provided, wherein the mouse expresses an immunoglobulin light chain repertoire characterized by a limited number of light chain variable domains. Mice are provided that express just one or a few immunoglobulin light chain variable domains from a limited repertoire in their germline. Methods for making bispecific antibodies having universal light chains using mice as described herein, including human light chain variable regions, are provided. Methods for making human variable regions suitable for use in multispecific binding proteins, e.g., bispecific antibodies, and host cells are provided. Bispecific antibodies capable of binding first and second antigens are provided, wherein the first and second antigens are separate epitopes of a single protein or separate epitopes on two different proteins are provided.
Owner:REGENERON PHARM INC
Methods and compositions for the targeted modification of a genome
Compositions and methods are provided for modifying a genomic locus of interest in a eukaryotic cell, a mammalian cell, a human cell or a non-human mammalian cell using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Further methods combine the use of the LTVEC with a CRISPR / Cas system. Compositions and methods for generating a genetically modified non-human animal comprising one or more targeted genetic modifications in their germline are also provided.
Owner:REGENERON PHARM INC
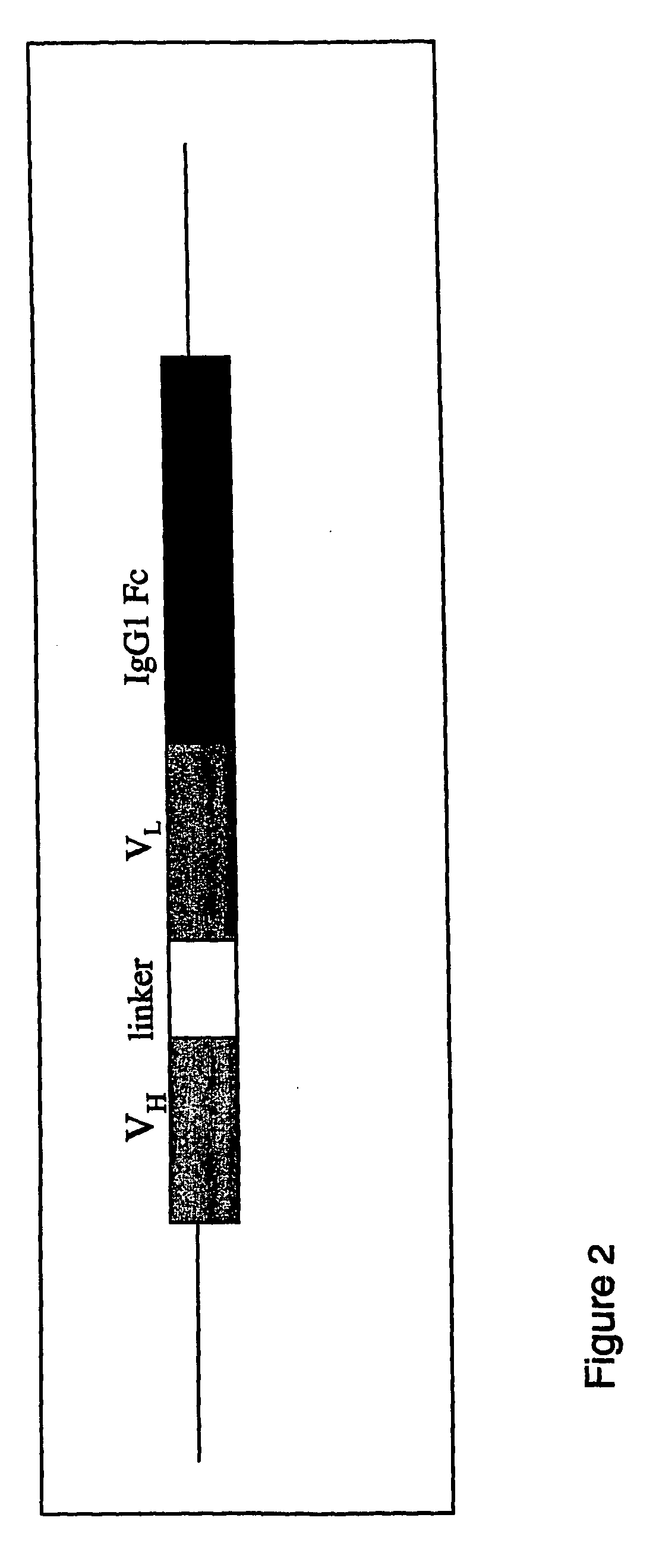
Hybrid antibodies
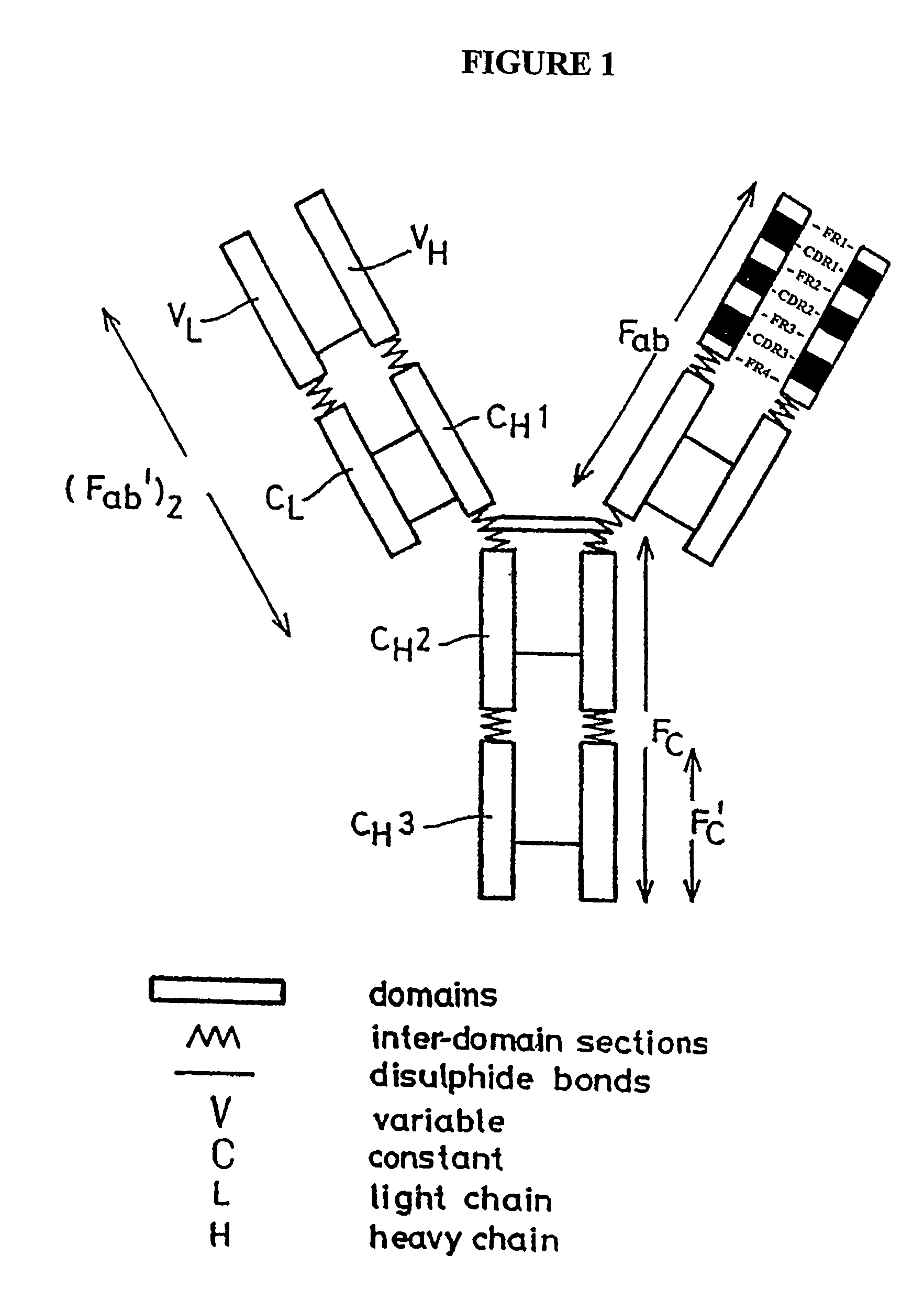
Hybrid antibodies and / or hybrid antibody fragments and methods of making them are provided. In one embodiment the hybrid antibodies and / or hybrid antibody fragments contain heavy and / or light variable regions that contain two or more framework regions derived from at least two antibodies. In another embodiment, at least two of the framework regions are classified in the same germline gene family. In one embodiment, at least two framework regions are classified in the same germline gene family member. The hybrid antibodies or hybrid antibody fragments may contain human framework regions and nonhuman CDRs.
Owner:ALEXION PHARMA INC
Methods and compositions for the targeted modification of a genome
Compositions and methods are provided for modifying a genomic locus of interest in a eukaryotic cell, a mammalian cell, a human cell or a non-human mammalian cell using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Further methods combine the use of the LTVEC with a CRISPR / Cas system. Compositions and methods for generating a genetically modified non-human animal comprising one or more targeted genetic modifications in their germline are also provided.
Owner:REGENERON PHARM INC
Methods and compositions for the targeted modification of a genome
Compositions and methods are provided for modifying a genomic locus of interest in a eukaryotic cell, a mammalian cell, a human cell or a non-human mammalian cell using a large targeting vector (LTVEC) comprising various endogenous or exogenous nucleic acid sequences as described herein. Further methods combine the use of the LTVEC with a CRISPR / Cas system. Compositions and methods for generating a genetically modified non-human animal comprising one or more targeted genetic modifications in their germline are also provided.
Owner:REGENERON PHARM INC
Method for Improved Transgene Expression
The present invention provides an improved method for achieving efficient transcription and translation of modified transgene constructs in vector systems. The vector may be a lentiviral vector. Such a method facilitates the production of viral vector genomes with intact functional transgene sequences allowing stable integration of a transgene-containing viral vector genome into the germline of an animal such as a transgenic avian. The subsequent expression of the transgene results in a recombinant protein product being produced, which, in the case of a transgenic avian can result in the targeted production of the protein into the egg of the transgenic bird.
Owner:AVIGENICS
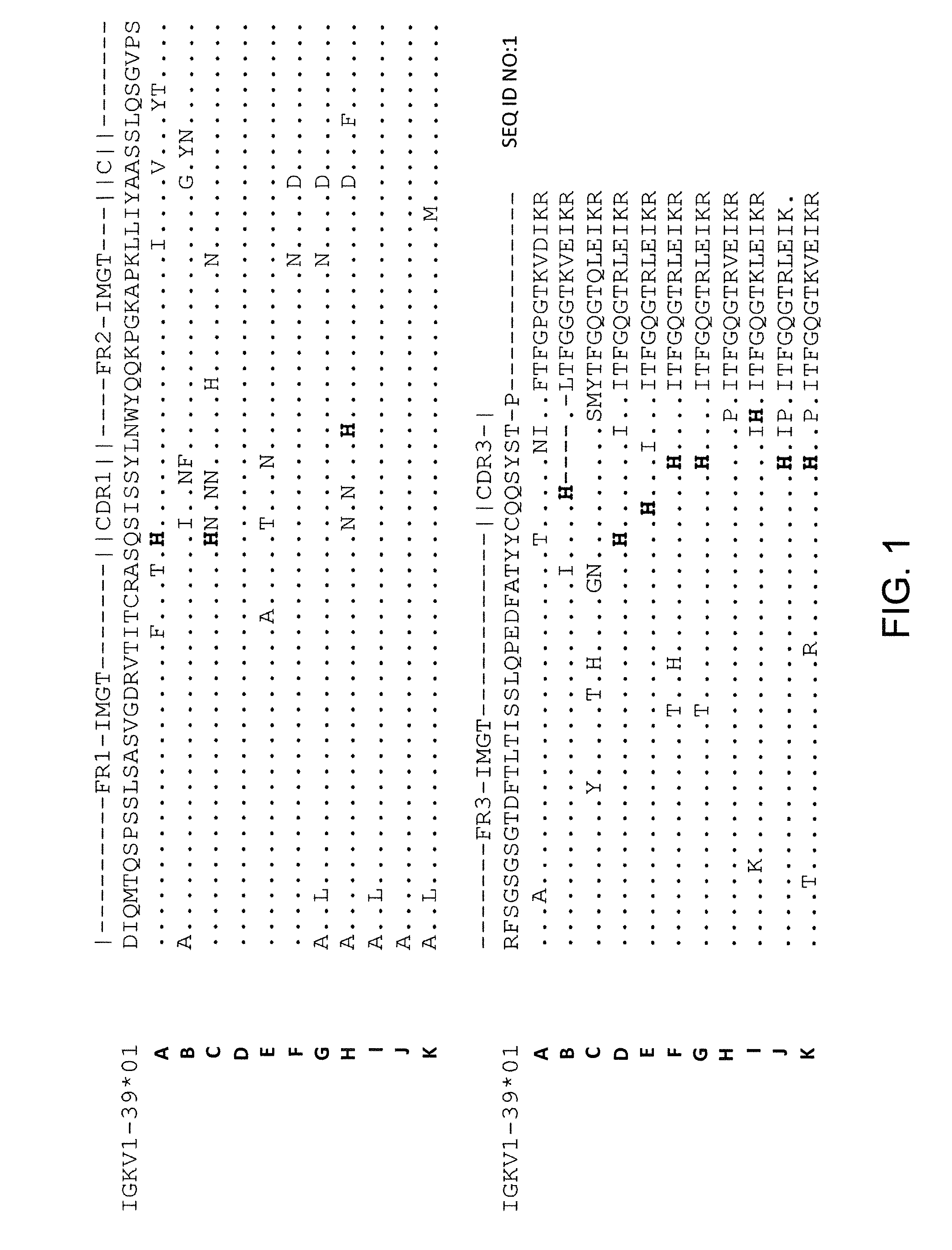
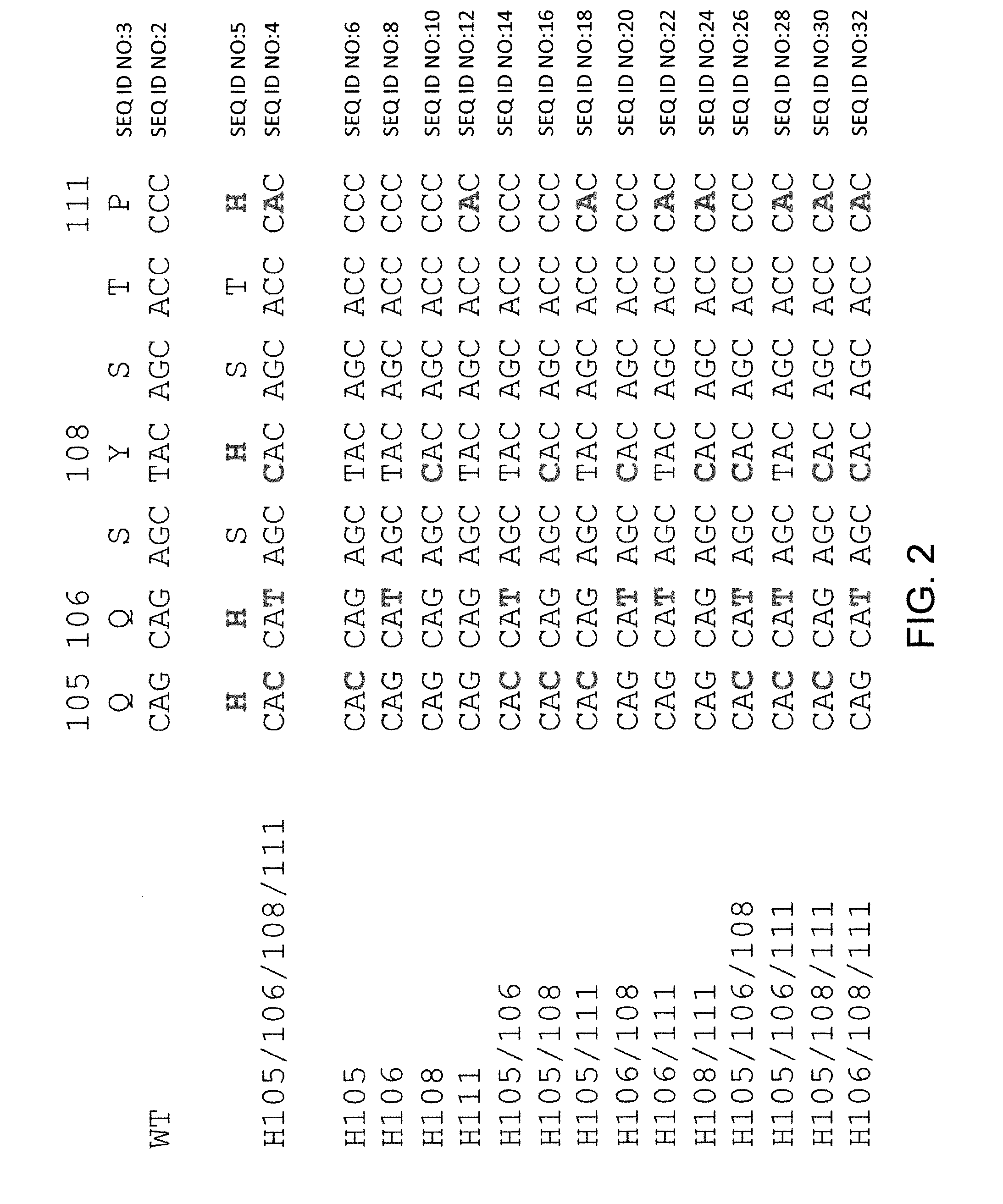
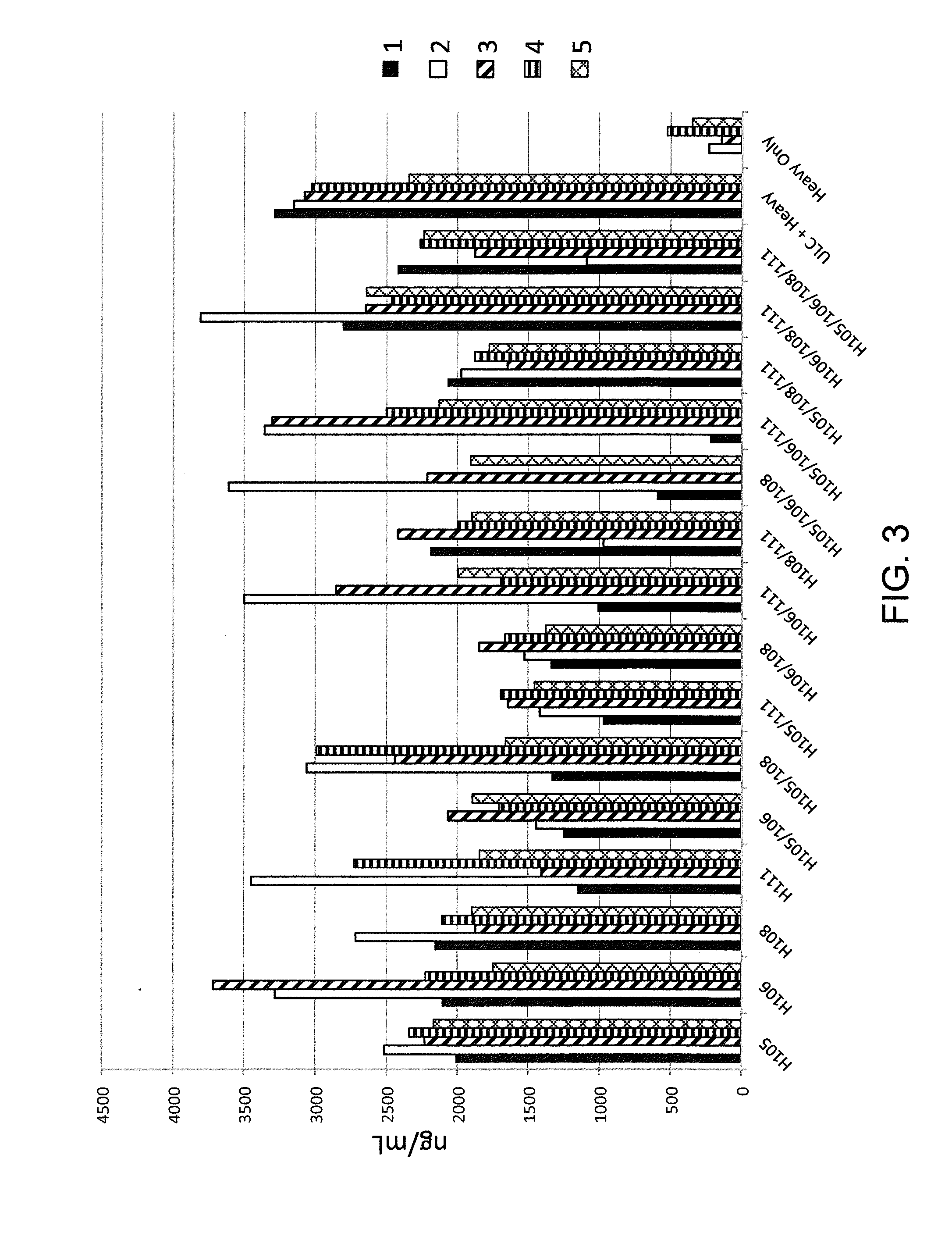
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
InactiveUS20140013456A1Reduce the binding forceNucleic acid vectorImmunoglobulinsNucleotideGenetically modified crops
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses human immunoglobulin light chain variable domains derived from a limited repertoire of human immunoglobulin light chain variable gene segments that comprise histidine modifications in their germline sequence. Methods of making non-human animals that express antibodies comprising histidine residues encoded by histidine codons introduced into immunoglobulin light chain nucleotide sequences are provided.
Owner:REGENERON PHARM INC
Modified human IGF-IR antibodies
InactiveUS20050069539A1Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsVaccine ImmunogenicityFramework region
The present invention relates to antibodies, which are directed to the human IGF-1 receptor (IGF-1R) and are to be administered for the treatment of cancer. The antibodies of the present invention have been altered to comprise antibodies with one or more selected germlne framework amino acid residues which replace one or more corresponding somatically mutated residues in the variable region of the unaltered antibody. The modification results in the framework region mutations converted to germline. The modification results in a reduced propensity for the antibody to elicit an immune response (reduced immunogenicity) following administration to a human subject.
Owner:PFIZER INC +1
Super humanized antibodies
InactiveUS20050261480A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
Combinatorial DNA Screening
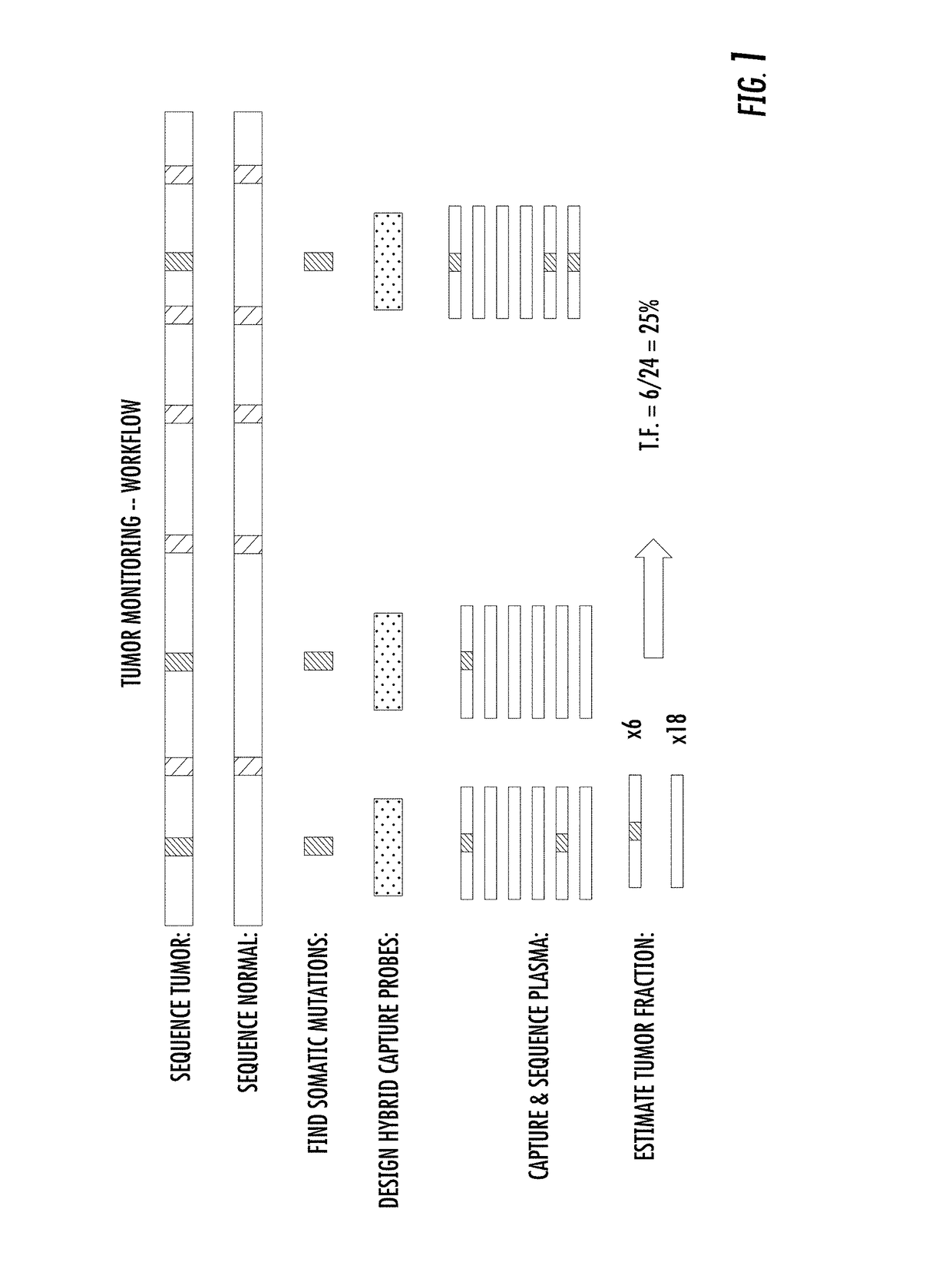
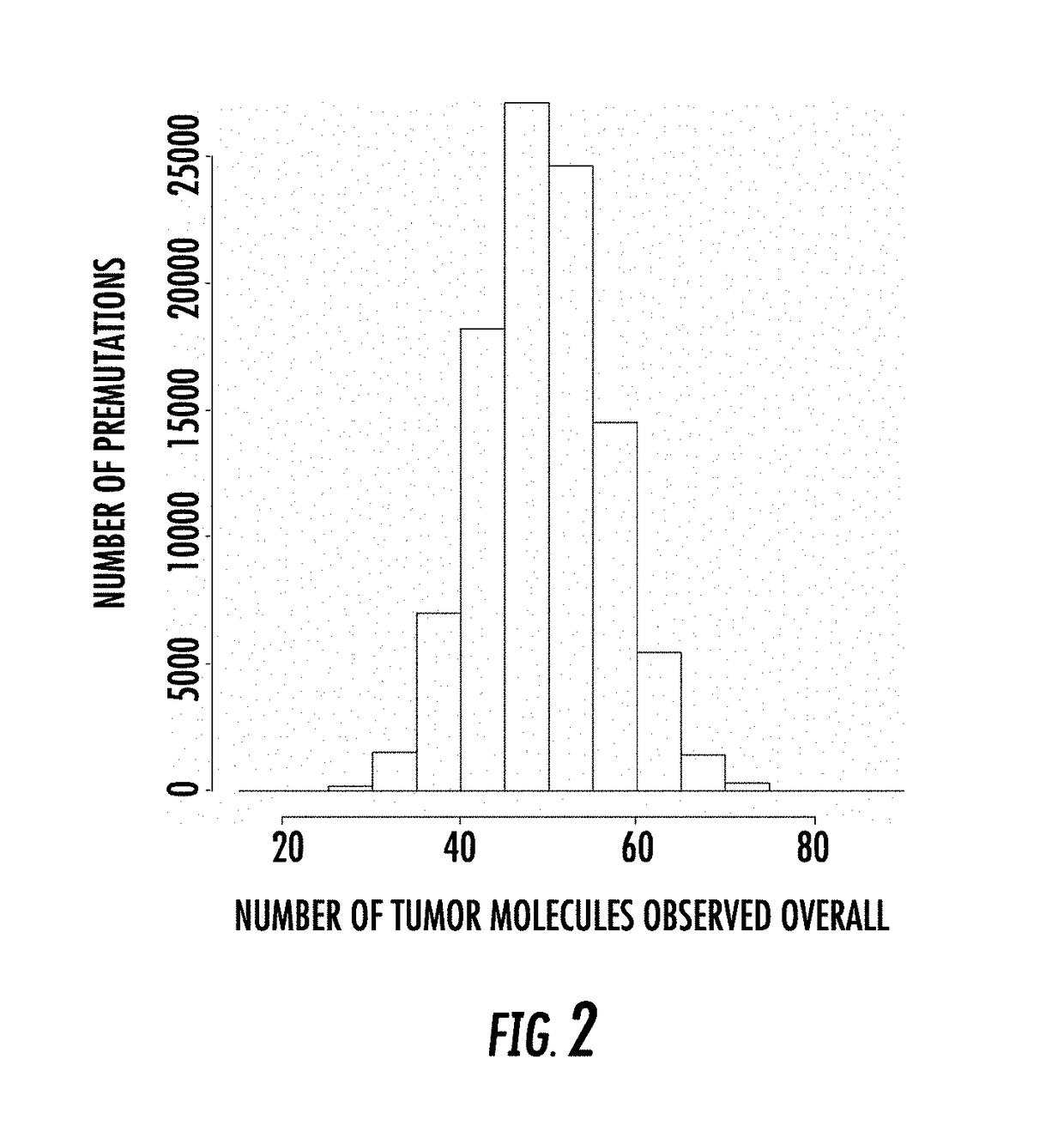
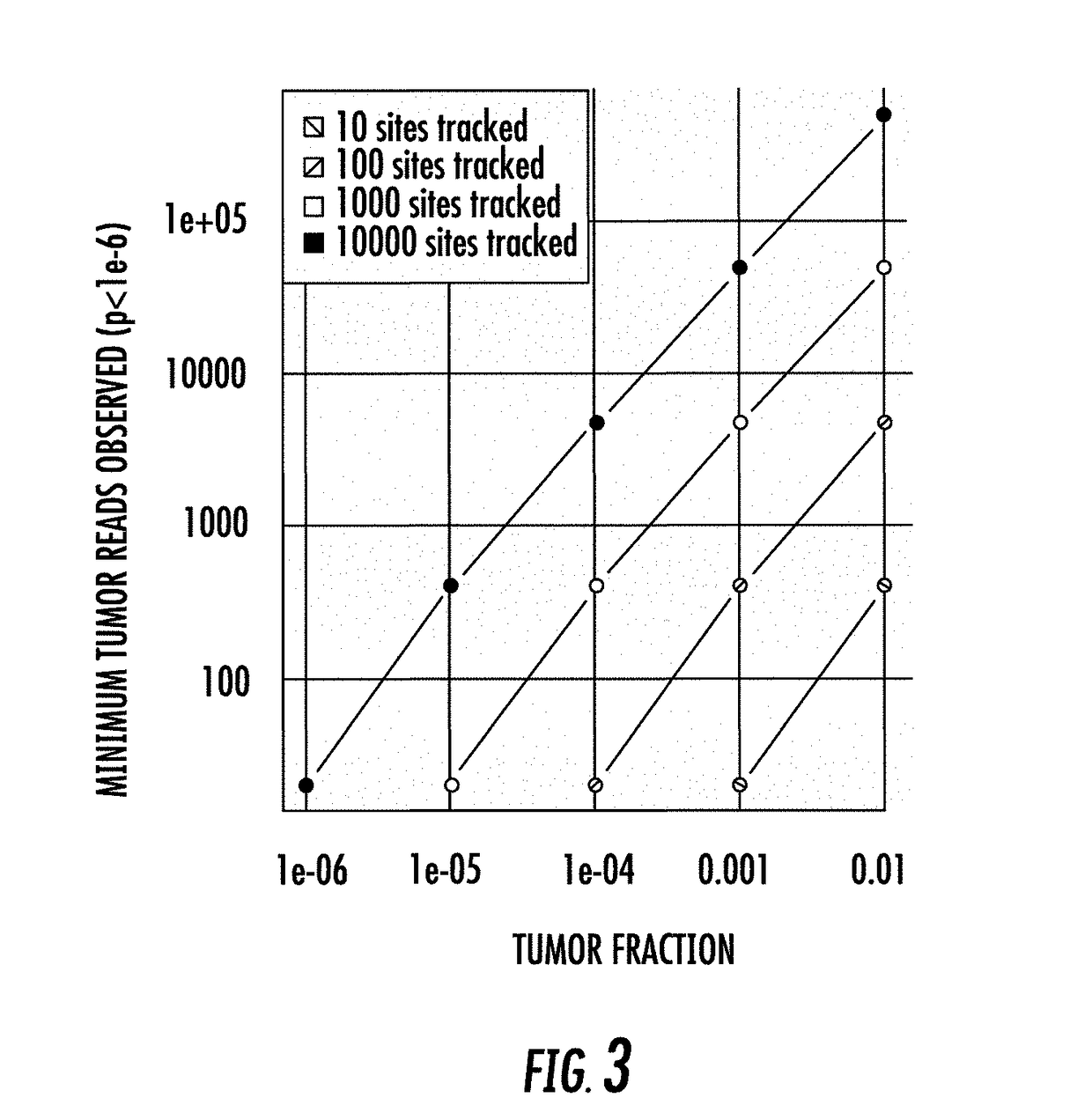
The present disclosure relates to methods for detecting unique genetic signatures derived from markers such as, for example, mutations, somatic or germ-line, in nucleic acids obtained from biological samples. The sensitivity of the methods provides for detection of mutations associated with a disease, e.g., cancer mutations, or with inherited disease, e.g., an autosomal recessive disease, in a noninvasive manner at ultra-low proportions of sequences carrying mutations to sequences carrying normal, e.g., non-cancer sequences, or a reference sequence, e.g., a human reference genome.
Owner:MYRIAD WOMENS HEALTH INC
Depletion of endogenous primordial germ cells in avian species
InactiveUS20060095980A1Decrease in primordial germ cell numberIncrease in primordial germ cell numberVertebrate antigen ingredientsFermentationAntigenHigh concentration
Methods for modulating primordial germ cell (PGC) numbers and / or development in avians are provided. In one embodiment, the presently disclosed subject matter provides a method for modulating primordial germ cells numbers in an avian embryo comprising immunizing a female bird with an antigen associated with primordial germ cells, whereby an egg produced by the female bird comprises a sufficiently high concentration of antibodies specific for the antigen to modulate numbers of endogenous PGCs in an avian embryo present within in the egg. Also provided are methods for producing chimeric avians, methods for increasing the proportion of male birds in a plurality of eggs, methods of producing avian gametes, and methods for enhancing germ line transmission of nucleic acids in birds.
Owner:NORTH CAROLINA STATE UNIV
Systems for gene targeting and producing stable genomic transgene insertions
InactiveUS20090083870A1Improve stabilityStable introduction of DNANucleic acid vectorInstabilityTransgene
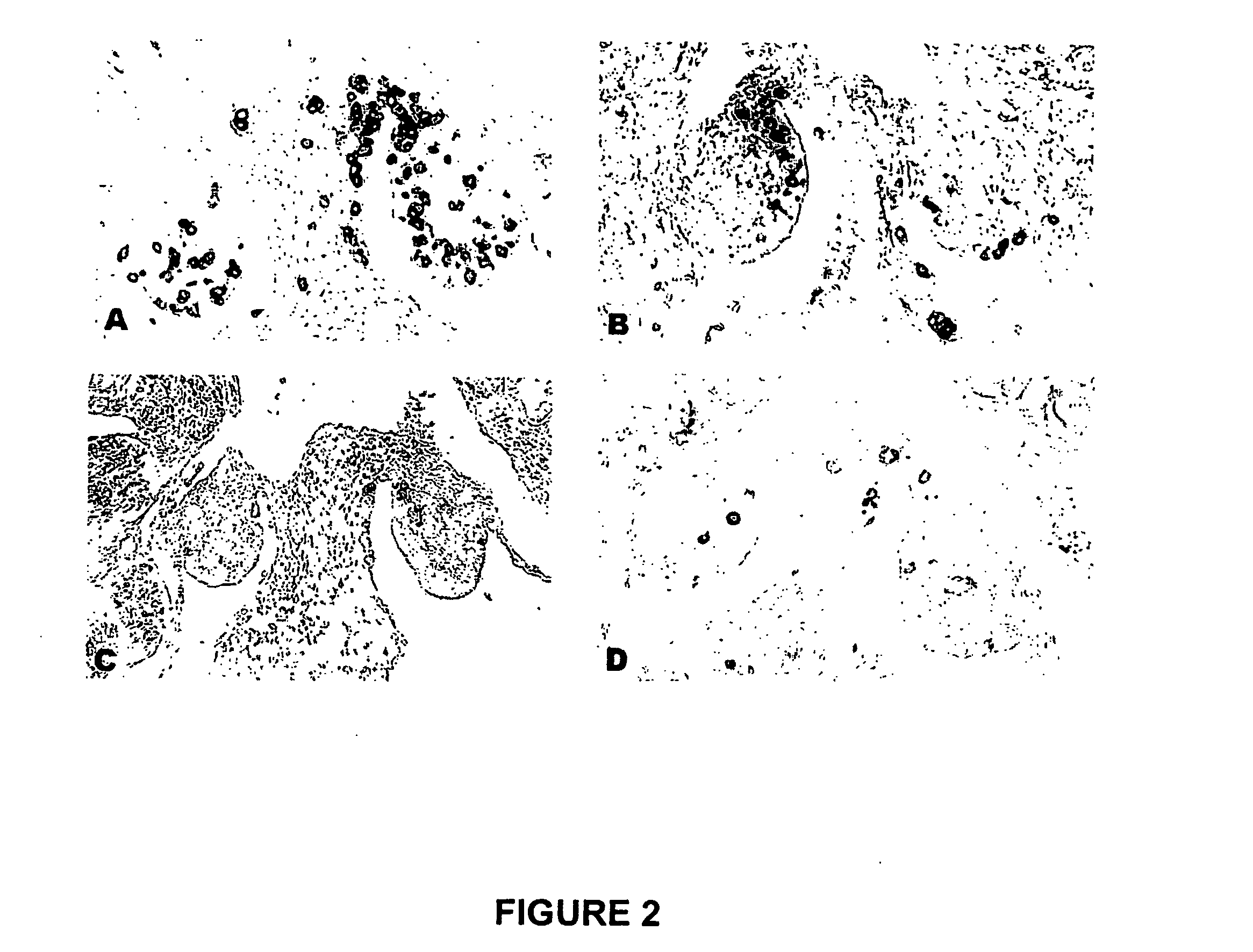
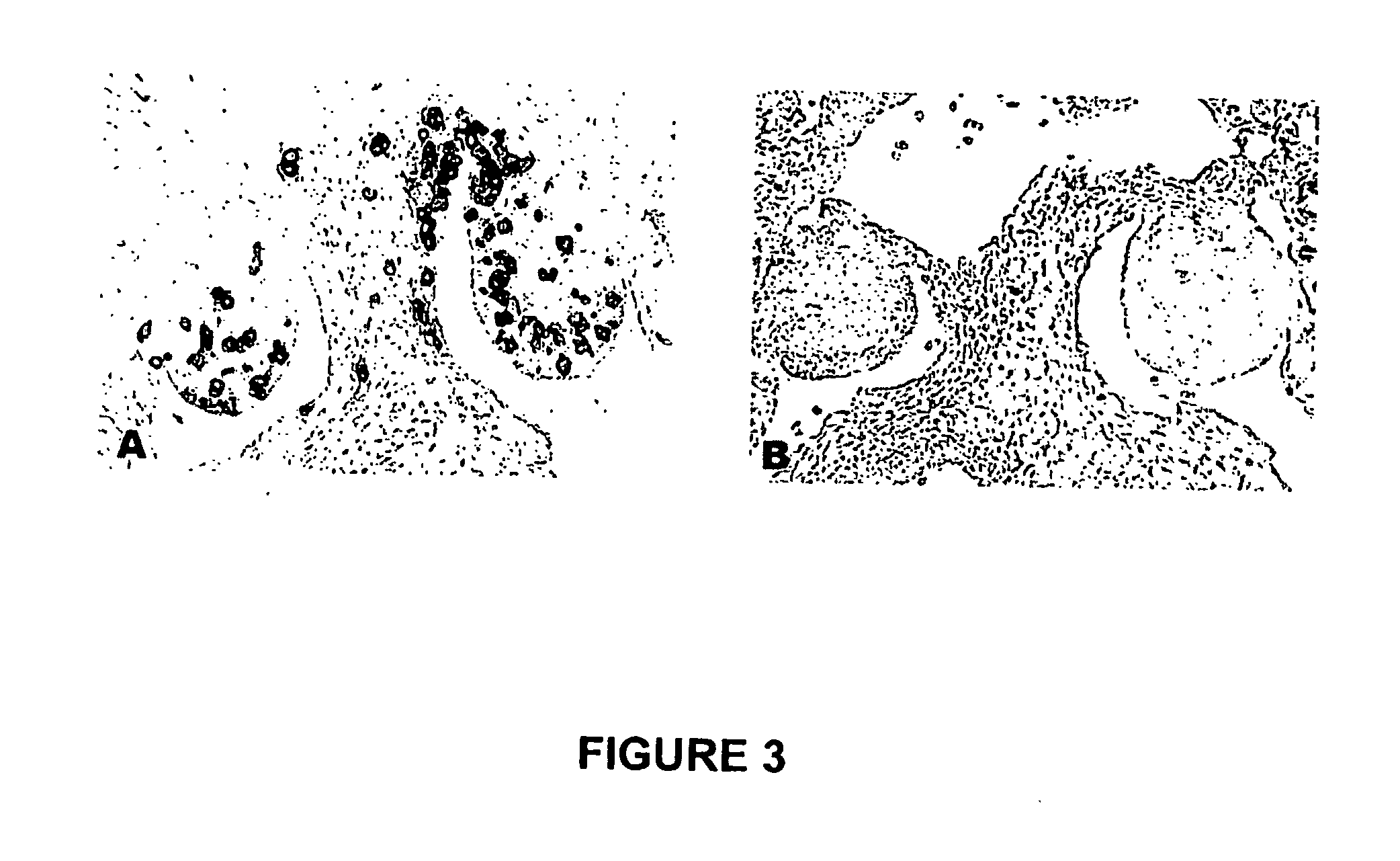
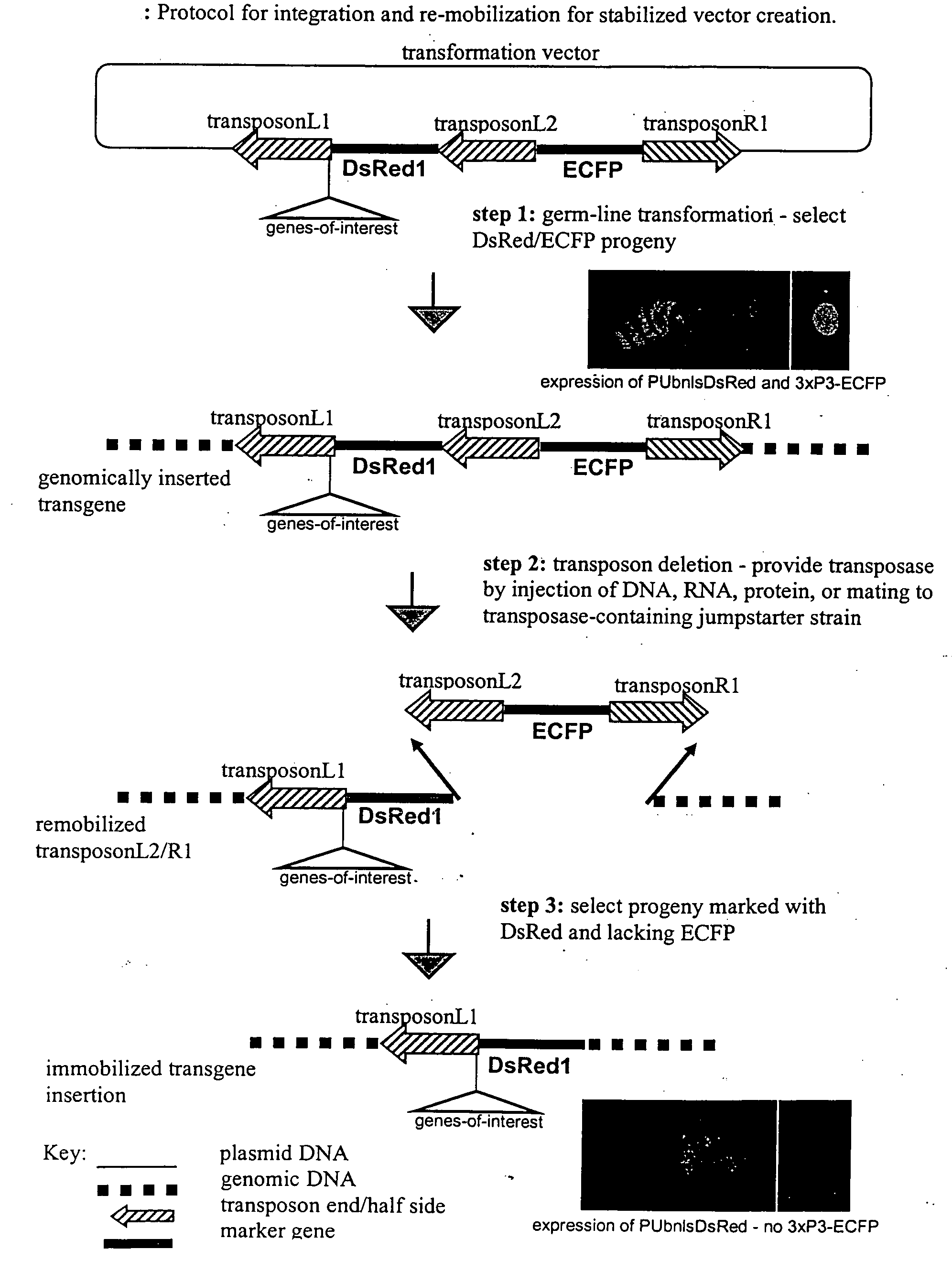
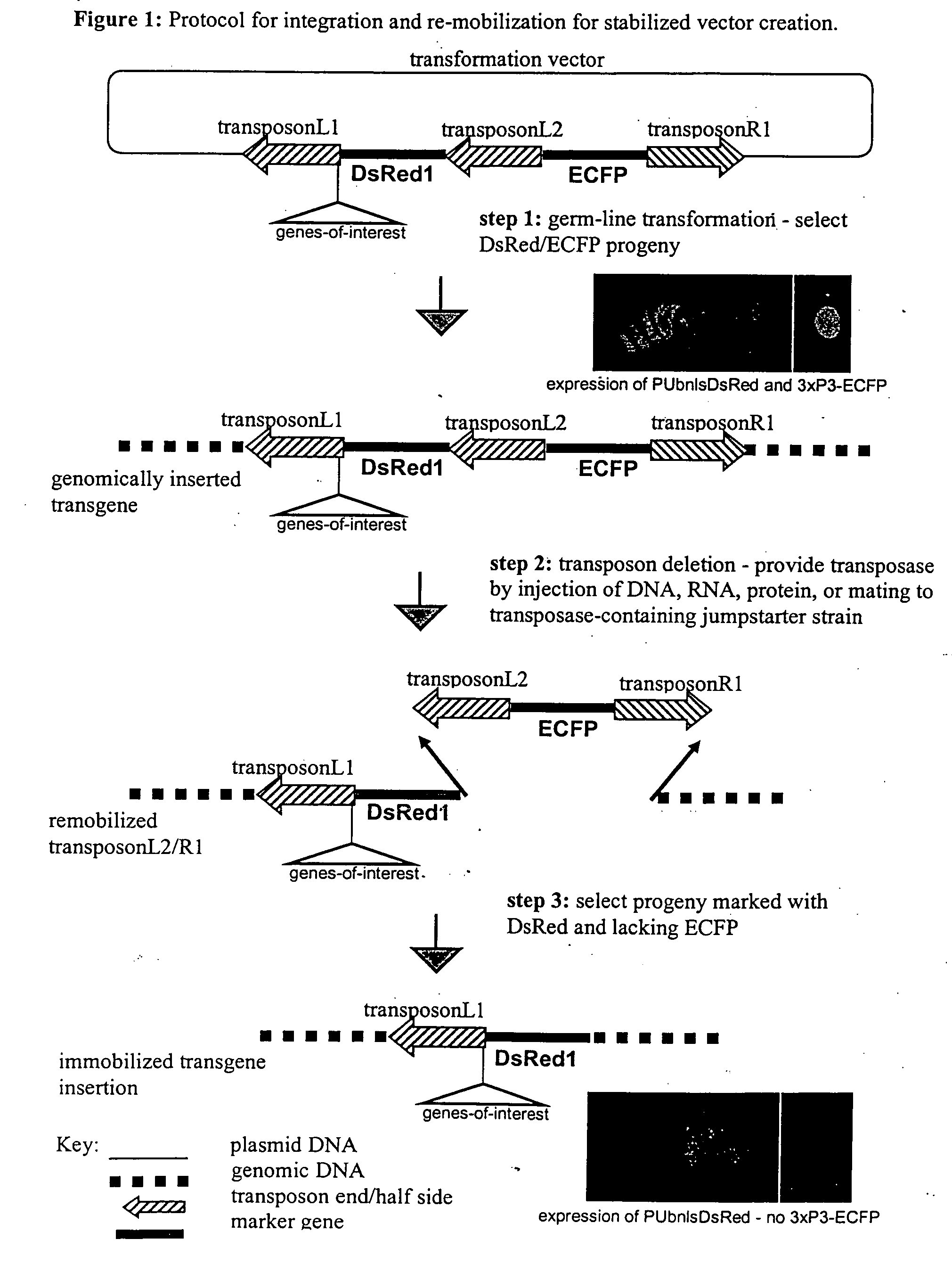
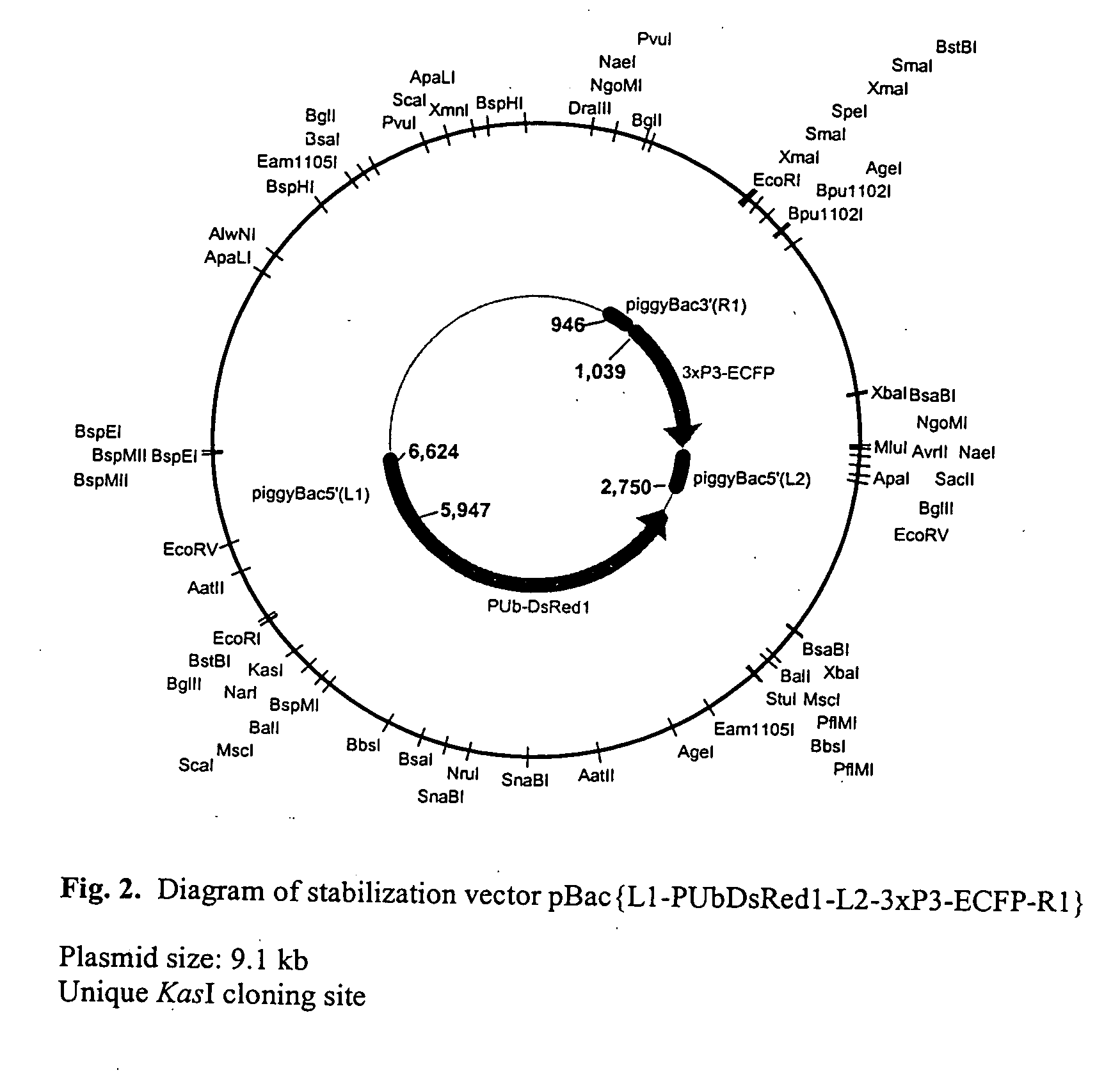
The novel germ-line transformation systems disclosed in this patent application allow the physical deletion of transposon DNA following the transformation process, and the targeting of transgene integrations into predefined target sites. In this way, transposase-mediated mobilization of genes-of-interest is excluded mechanistically and random genomic integrations eliminated. In contrast to conventional germ-line transformation technology, our systems provide enhanced stability to the transgene insertion. Furthermore, DNA sequences required for the transgene modification (e.g. transformation marker genes, transposase or recombinase target sites), are largely removed from the genome after the final transgene insertion, thereby eliminating the possibility for instability generated by these processes. The RMCE technology, which is disclosed in this patent application for invertebrate organisms (exemplified in Drosophila melanogaster) represents an extremely versatile tool with application potential far beyond the goal of transgene immobilization. RMCE makes possible the targeted integration of DNA cassettes into a specific genomic loci that are pre-defined by the integration of the RMCE acceptor plasmid. The loci can be characterized prior to a targeting experiment allowing optimal integration sites to be pre-selected for specific applications, and allowing selection of host strains with optimal fitness. In addition, multiple cassette exchange reactions can be performed in a repetitive way where an acceptor cassette can be repetitively exchanged by multiple donor cassettes. In this way several different transgenes can be placed precisely at the same genomic locus, allowing, for the first time, the ability to eliminate genomic positional effects and to comparatively study the biological effects of different transgenes.
Owner:HORN CARSTEN +1
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
ActiveUS20130247234A1Reduce the binding forceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman animalVariable domain
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses a single light chain variable domain derived from a single rearranged light chain variable region gene in the germline of the non-human animal, wherein the single rearranged light chain variable region gene comprises a substitution of at least one non-histidine encoding codon with a histidine encoding codon. Methods of making non-human animals that express antibodies comprising a histidine-containing universal light chain are provided.
Owner:REGENERON PHARM INC
Site-specific recombination in eukaryotes and constructs useful therefor
InactiveUS7135608B1Effective recombinationEfficient productionEnzymesFermentationMammalSite-specific recombination
Owner:SALK INST FOR BIOLOGICAL STUDIES
Methods of performing homologous recombination based modification of nucleic acids in recombination deficient cells and use of the modified nucleic acid products thereof
A simple method for modifying genes in a recombination deficient host cell is disclosed. Such modifications include generating insertion, deletions, substitutions, and / or point mutations at any chosen site in the independent origin based cloning vector. The modified gene can be contained in an independent origin based cloning vector that is used to introduce a modified heterologous gene into a cell. Such a modified vector may be used in the production of a germline transmitted transgenic animal, or in gene targeting protocols in eukaryotic cells.
Owner:THE ROCKEFELLER UNIV
CD20 binding molecules
ActiveUS8153125B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Heavy chain
The present invention relates to CD20 binding molecules and nucleic acid sequences encoding CD20 binding molecules. In particular, the present invention relates to CD20 binding molecules with a high binding affinity, and a low dissociation rate, with regard to human CD20. Preferably, the CD20 binding molecules of the present invention comprise light and / or heavy chain variable regions with fully human frameworks (e.g. human germline frameworks).
Owner:APPLIED MOLECULAR EVOLUTION
Hco32 and hco27 and related examples
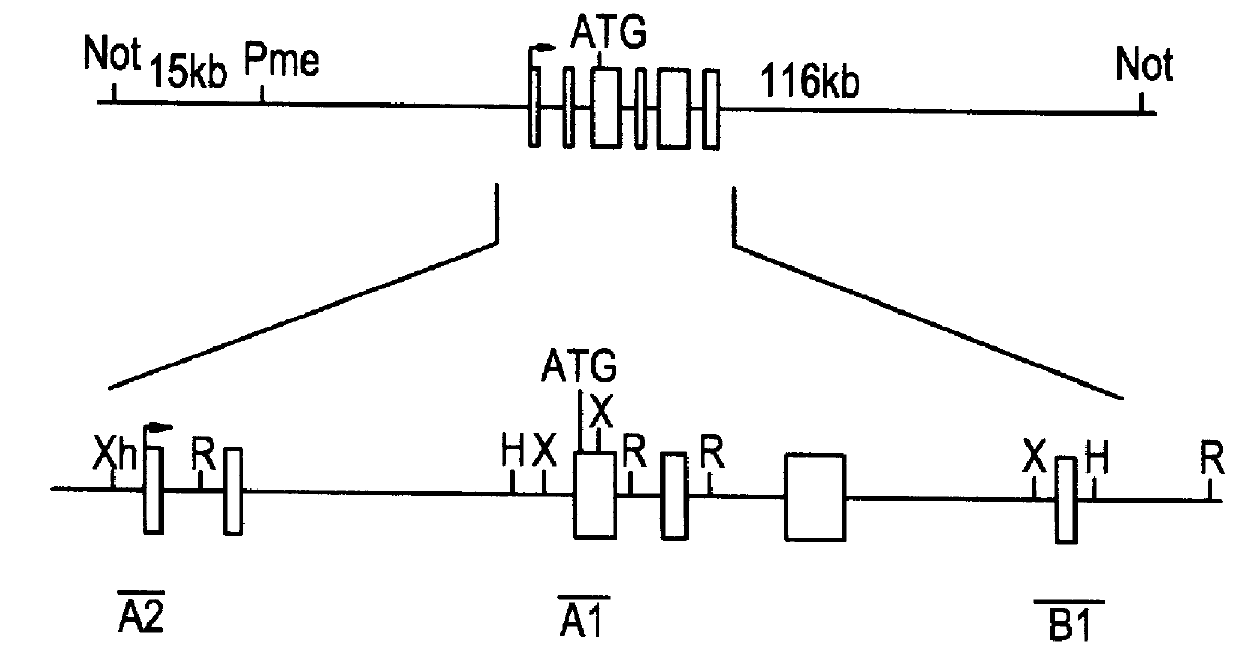
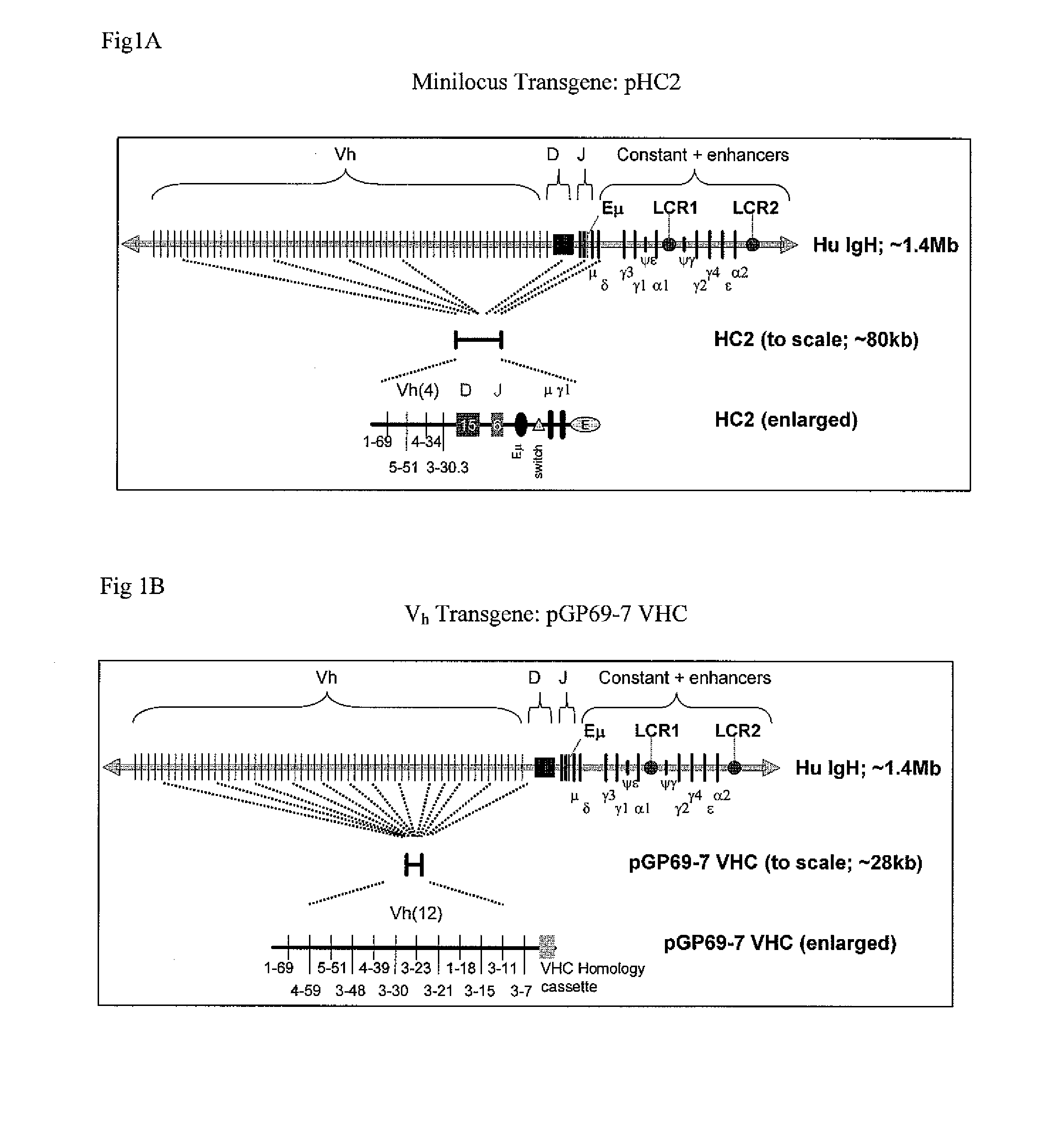
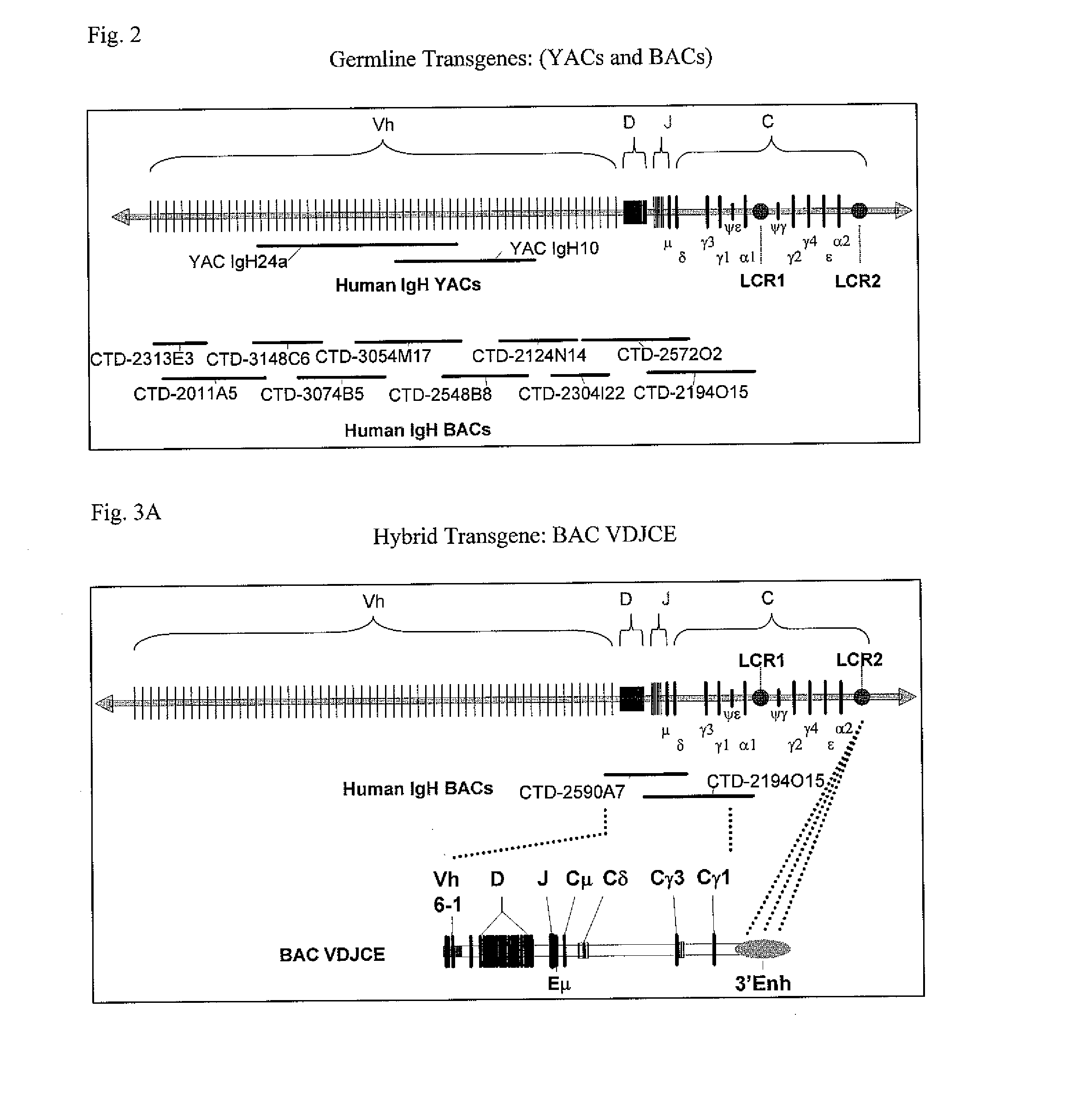
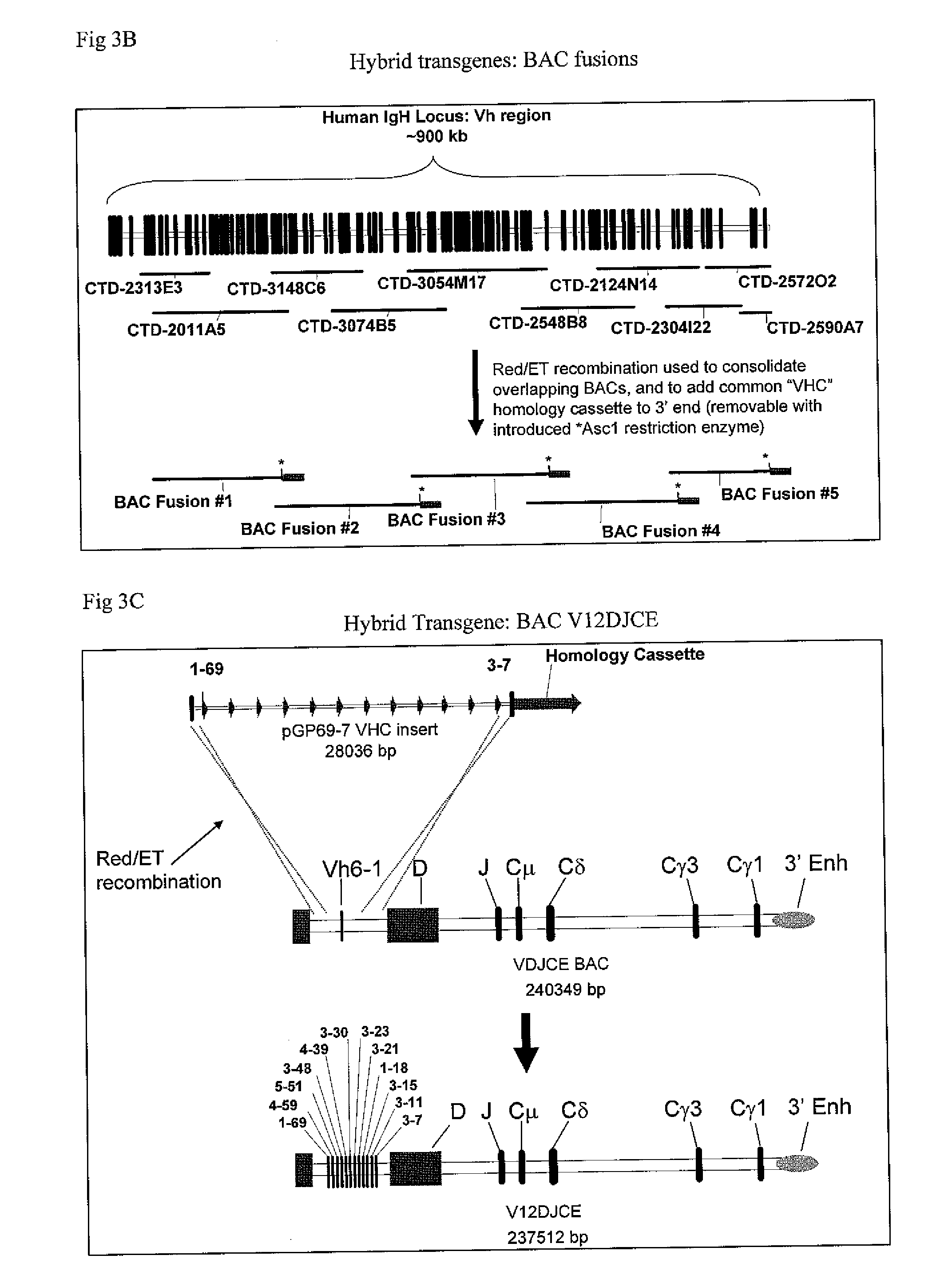
The instant invention relates to transgenic non-human animals capable of producing heterologous antibodies, transgenes used to produce such transgenic animals, transgenes capable of functionally rearranging a heterologous D gene in V-D-J recombination, immortalized B-cells capable of producing heterologous antibodies, methods and transgenes for producing heterologous antibodies of multiple isotypes, methods and transgenes for producing heterologous antibodies wherein a variable region sequence comprises somatic mutation as compared to germline rearranged variable region sequences, transgenic nonhuman animals which produce antibodies having a human primary sequence and which bind to human antigens, hybridomas made from B cells of such transgenic animals, and monoclonal antibodies expressed by such hybridomas.
Owner:ER SQUIBB & SONS INC
Modified human IGF-IR antibodies
InactiveUS7371378B2Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsVaccine ImmunogenicityAntibody
The present invention relates to antibodies, which are directed to the human IGF-1 receptor (IGF-1R) and are to be administered for the treatment of cancer. The antibodies of the present invention have been altered to comprise antibodies with one or more selected germlne framework amino acid residues which replace one or more corresponding somatically mutated residues in the variable region of the unaltered antibody. The modification results in the framework region mutations converted to germline. The modification results in a reduced propensity for the antibody to elicit an immune response (reduced immunogenicity) following administration to a human subject.
Owner:PFIZER INC +1
Bovine germline D-genes and their application
ActiveUS7196185B2Large capacitySugar derivativesMicrobiological testing/measurementDiseaseImmunocompetence
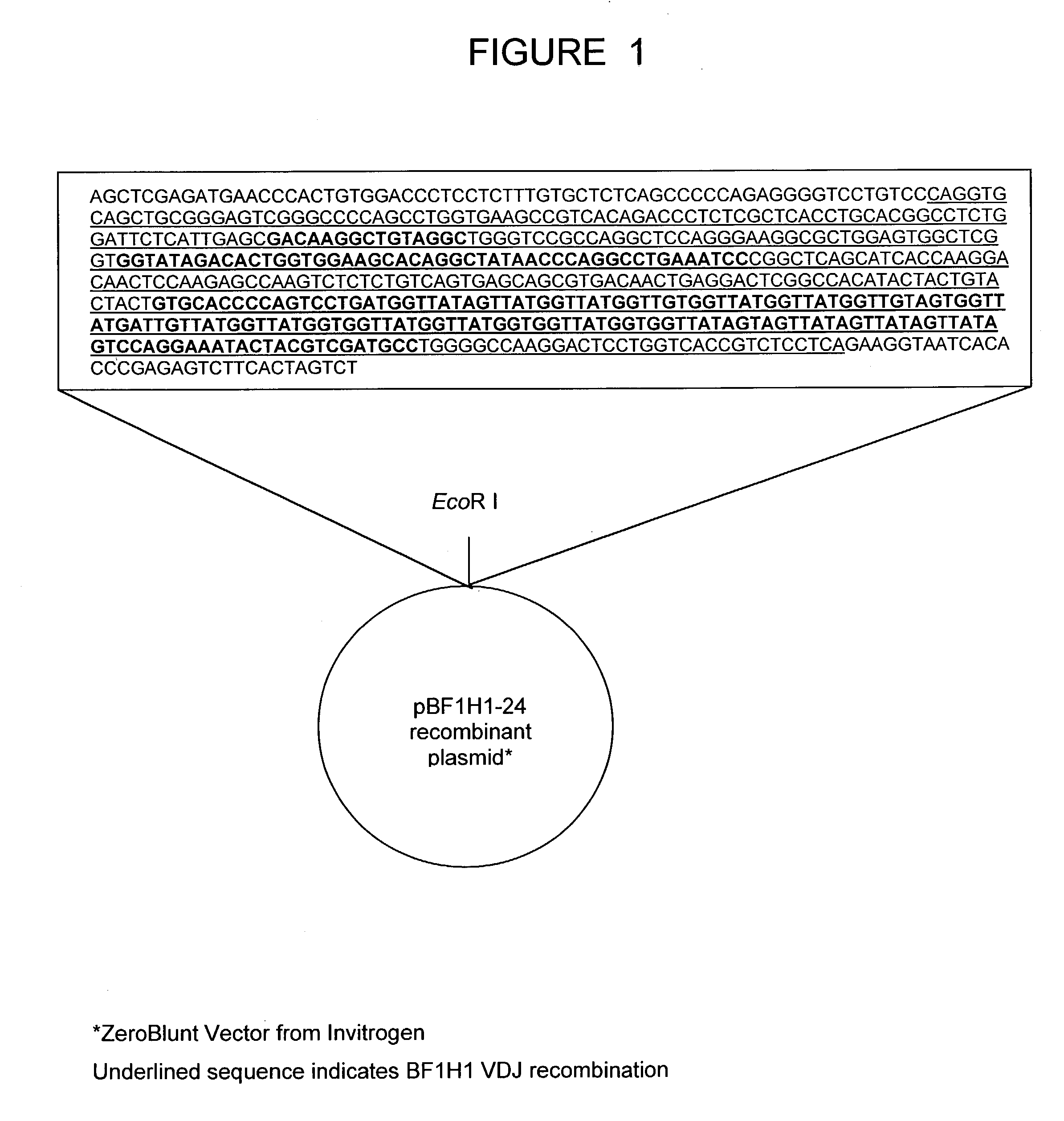
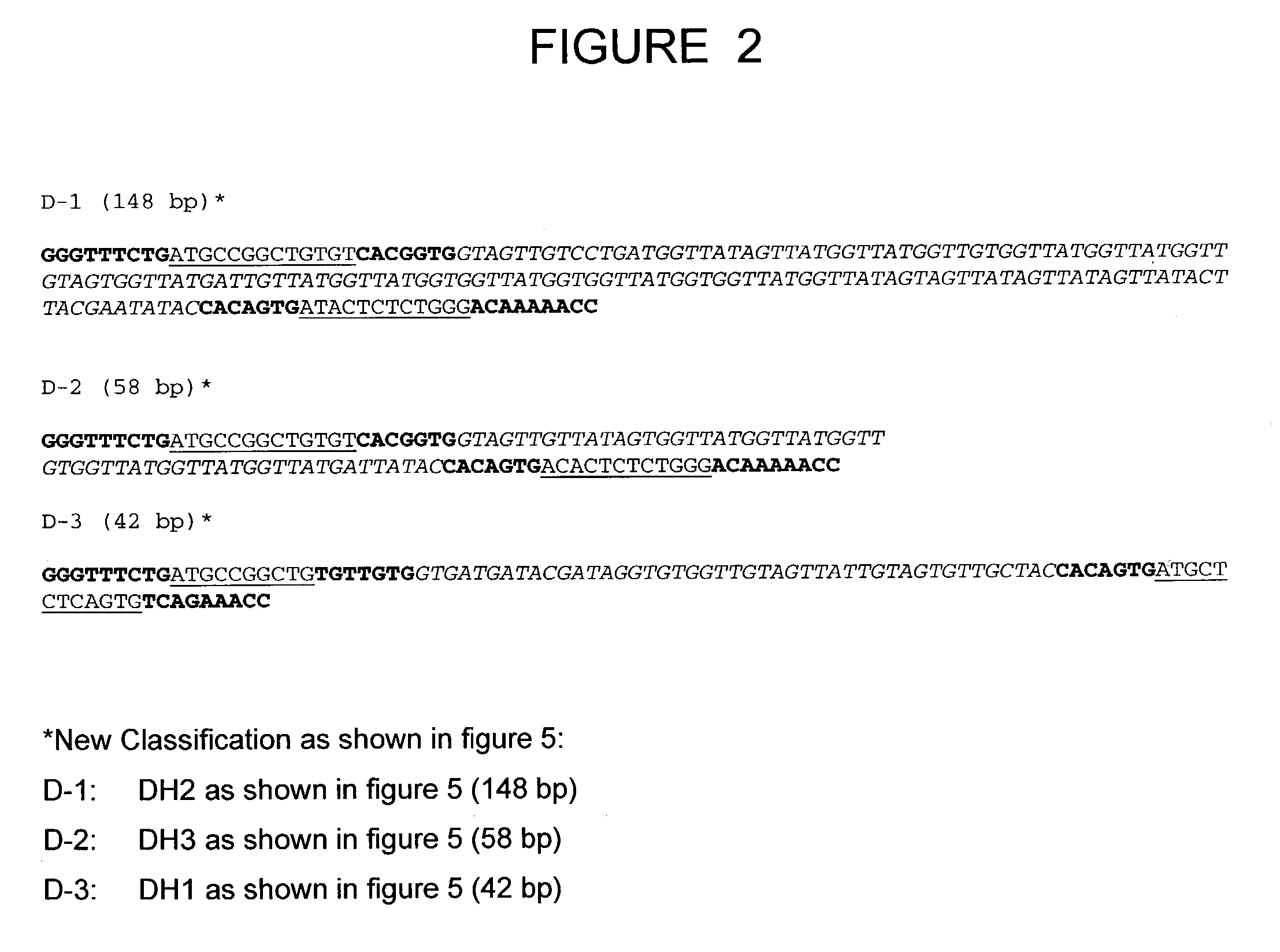
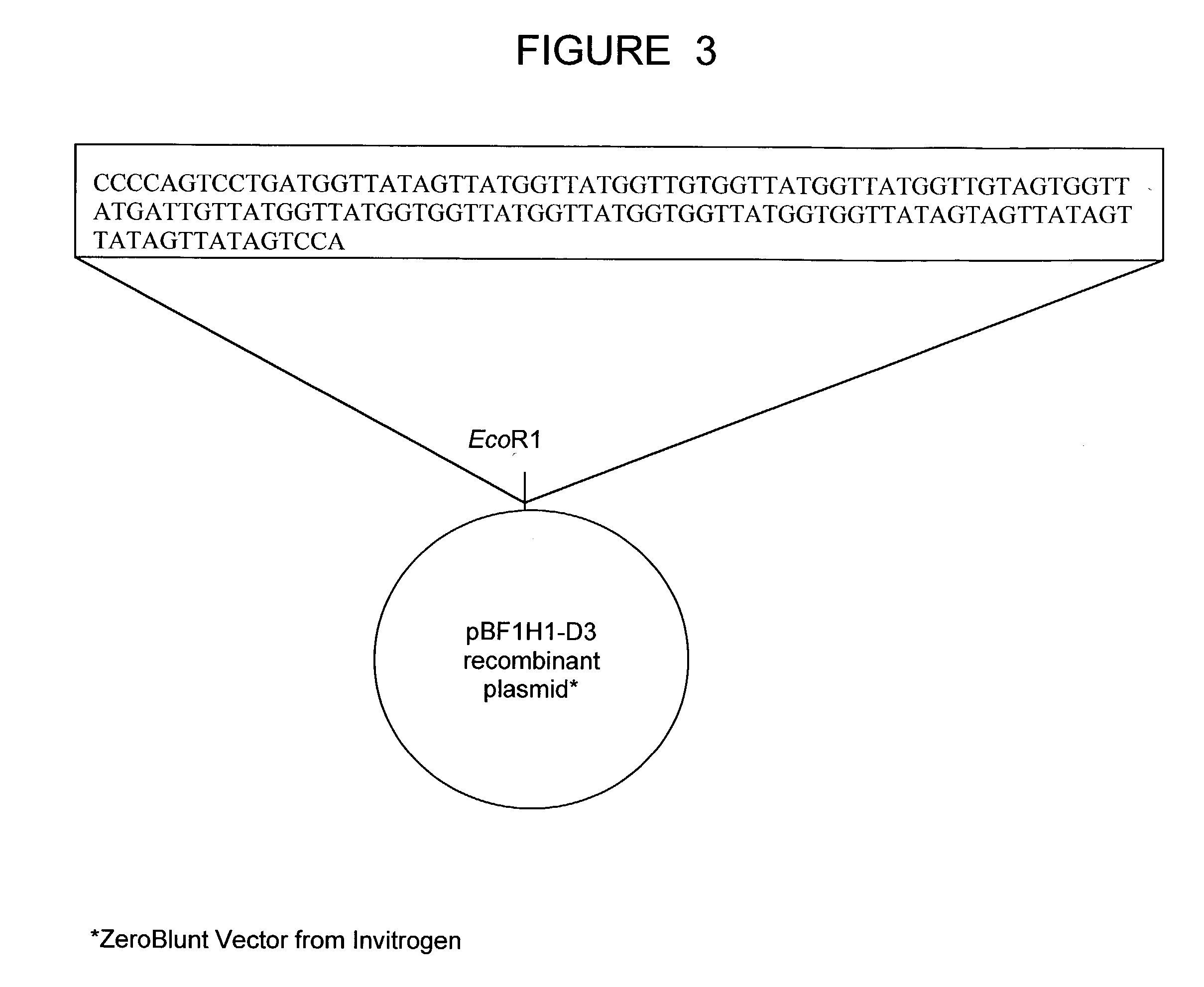
The present invention relates to a bovine VDJ cassette (BF1H1) that provides the novel ability to develop chimeric immunoglobulin molecule capable of incorporating both linear T cell epitope(s) (CDR1H and CDR2H) as well as conformational B cell epitope(s) (exceptionally long CDR3H). Further, multiple epitopes can be incorporated for development of multivalent vaccine by replacing at least a portion of an immunoglobulin molecule with the desired epitope such that functional ability of both epitope(s) and parent VDJ rearrangement is retained. The antigenized immunoglobulin incorporating both T and B epitopes of interest is especially useful for development of oral vaccines for use in humans apart from other species including cattle. The long CDR3H in BF1H1 VDJ rearrangement originates from long germline D-genes. The novel bovine germline D-genes provide unique molecular genetic marker for sustaining the D-gene pool in cattle essential for immunocompetence via selective breeding. D-gene specific DNA probe permits typing and selection of breeding cattle stock for maximum gemline D gene pool for better health and disease prevention. The bovine D-genes are unique to cattle and, therefore, provide sensitive and specific forensic analytical tool using molecular biology techniques to determine tissues suspected of bovine origin.
Owner:KAUSHIK AZAD KUMAR +2
Antigenic mimics of discontinuous epitopes of pathogen recognized by broadly neutralizing antibodies
InactiveUS20130039927A1Antibacterial agentsOrganic active ingredientsPeptide sequenceDiscontinuous epitope
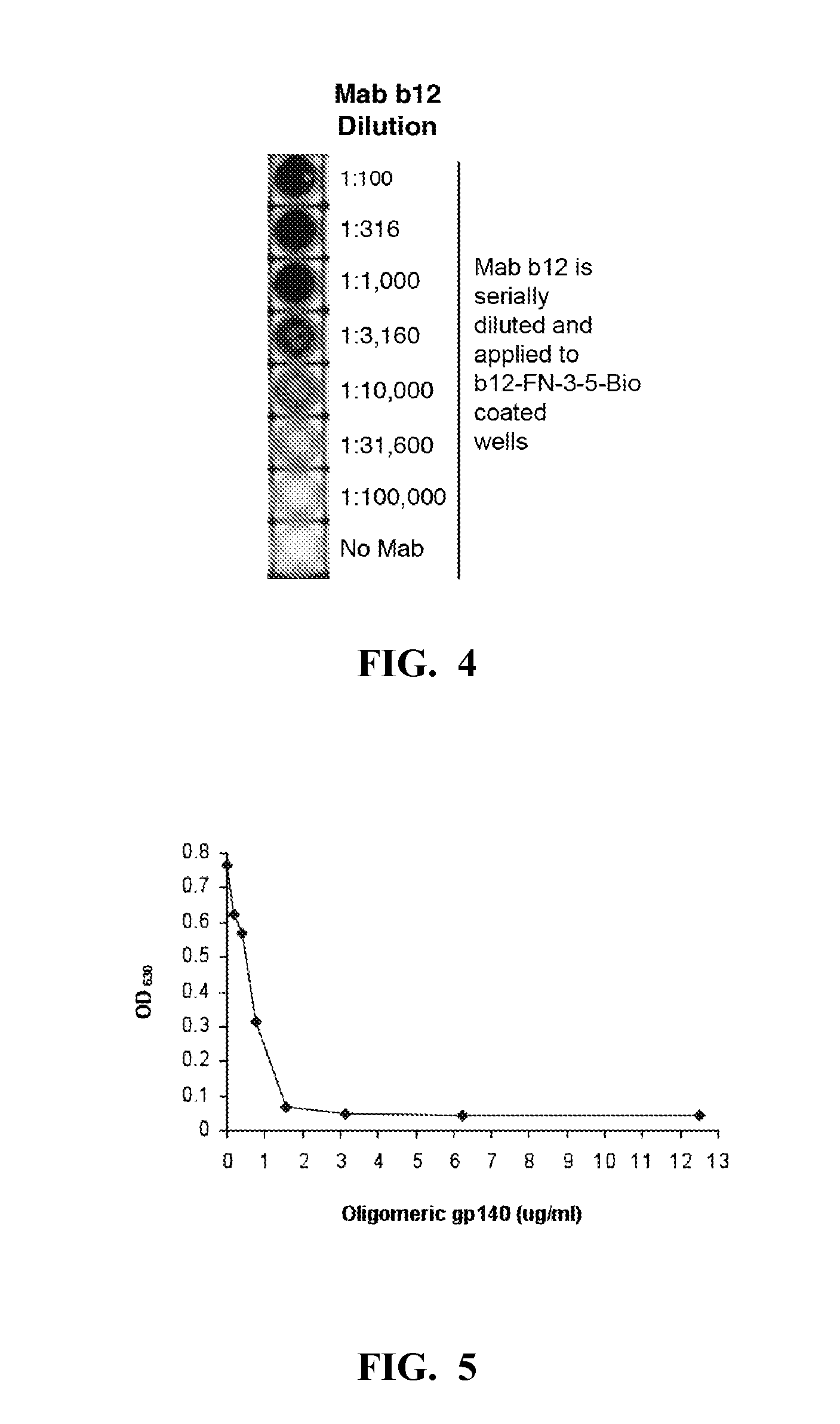
The present invention relates to an anti-idiotypic polypeptide scaffold that includes two or more peptide sequences that mimic a discontinuous epitope of a pathogen that is recognized by or induces formation of a broadly neutralizing antibody. Using a fibronectin FNfn10 scaffold bearing two or more modified discontinuous loops, scaffolds that recognize broadly neutralizing antibodies in vitro and from patient serum have been identified. These scaffolds should induce an immune response or mobilize germline specificities to initiate their affinity maturation.
Owner:UNIVERSITY OF ROCHESTER
Transgenic chickens with an inactivated endogenous gene locus
InactiveUS20100138946A1Convenient treatmentGood curative effectVirusesImmunoglobulins against cell receptors/antigens/surface-determinantsFowlIntegrases
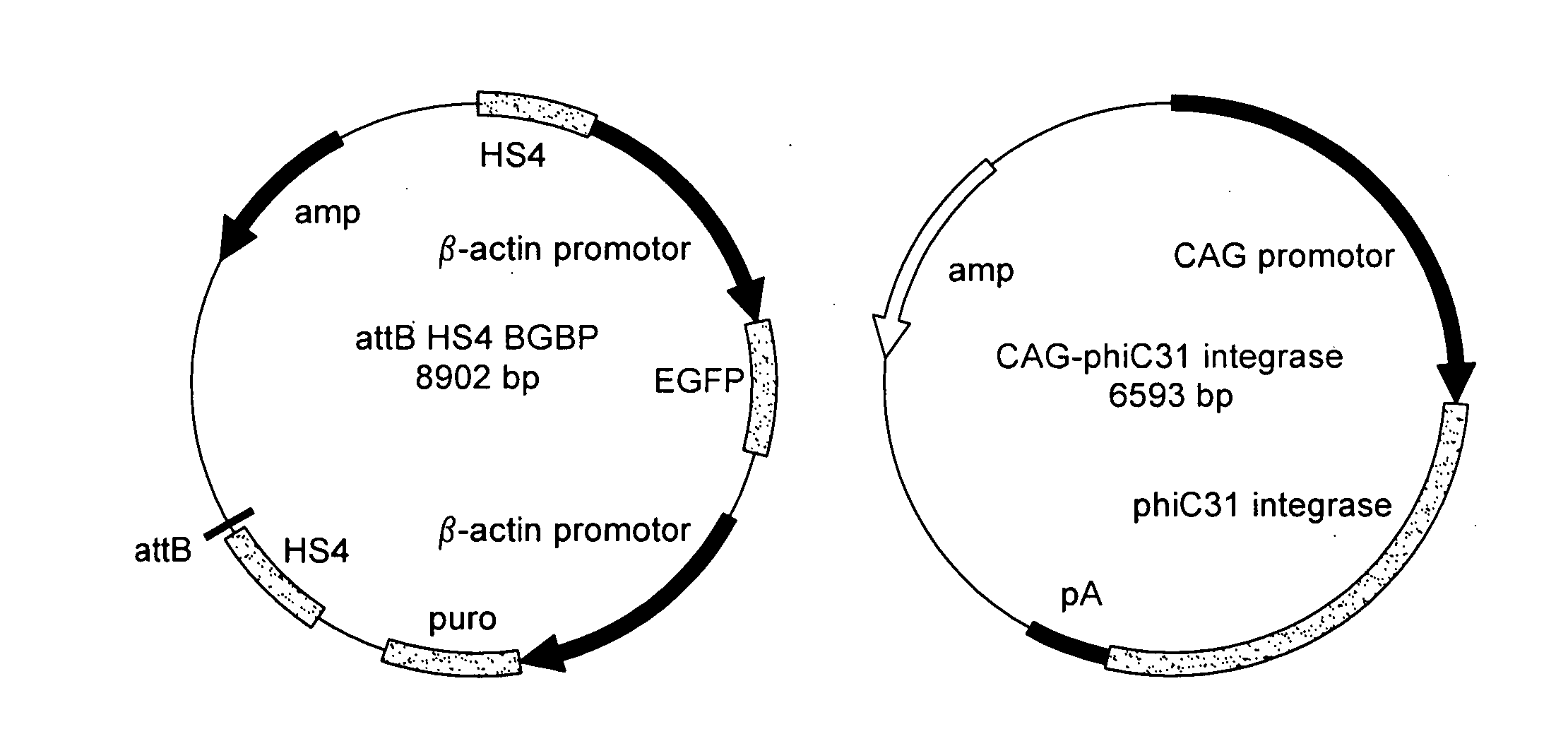
The present invention is transgenic chickens obtained from long-term cultures of avian PGCs and techniques to produce and transgenic birds derived from prolonged PGC cultures. In some embodiments, these PGCs can be transfected with genetic constructs to modify the DNA of the PGC, specifically to introduce a transgene encoding an exogenous protein. When combined with a host avian embryo by known procedures, those modified PGCs are transmitted through the germline to yield transgenic offspring. This invention includes compositions comprising long-term cultures of PGCs and offspring derived from them that are genetically modified. The genetic modifications introduced into PGCs to achieve the gene inactivation may also include, but are not restricted to, random integrations of transgenes into the genome, transgenes inserted into the promoter region of genes, transgenes inserted into repetitive elements in the genome, site specific changes to the genome that are introduced using integrase, site specific changes to the genome introduced by homologous recombination, and conditional mutations introduced into the genome by excising DNA that is flanked by lox sites or other sequences that are substrates for site specific recombination.
Owner:ORIGEN THERAPEUTICS +1
Protection of the female reproductive system from natural and artificial insults
InactiveUS20030157086A1Promising therapeutic effectPreserving fertilityPeptide/protein ingredientsPhosphorous compound active ingredientsObstetricsPhysiology
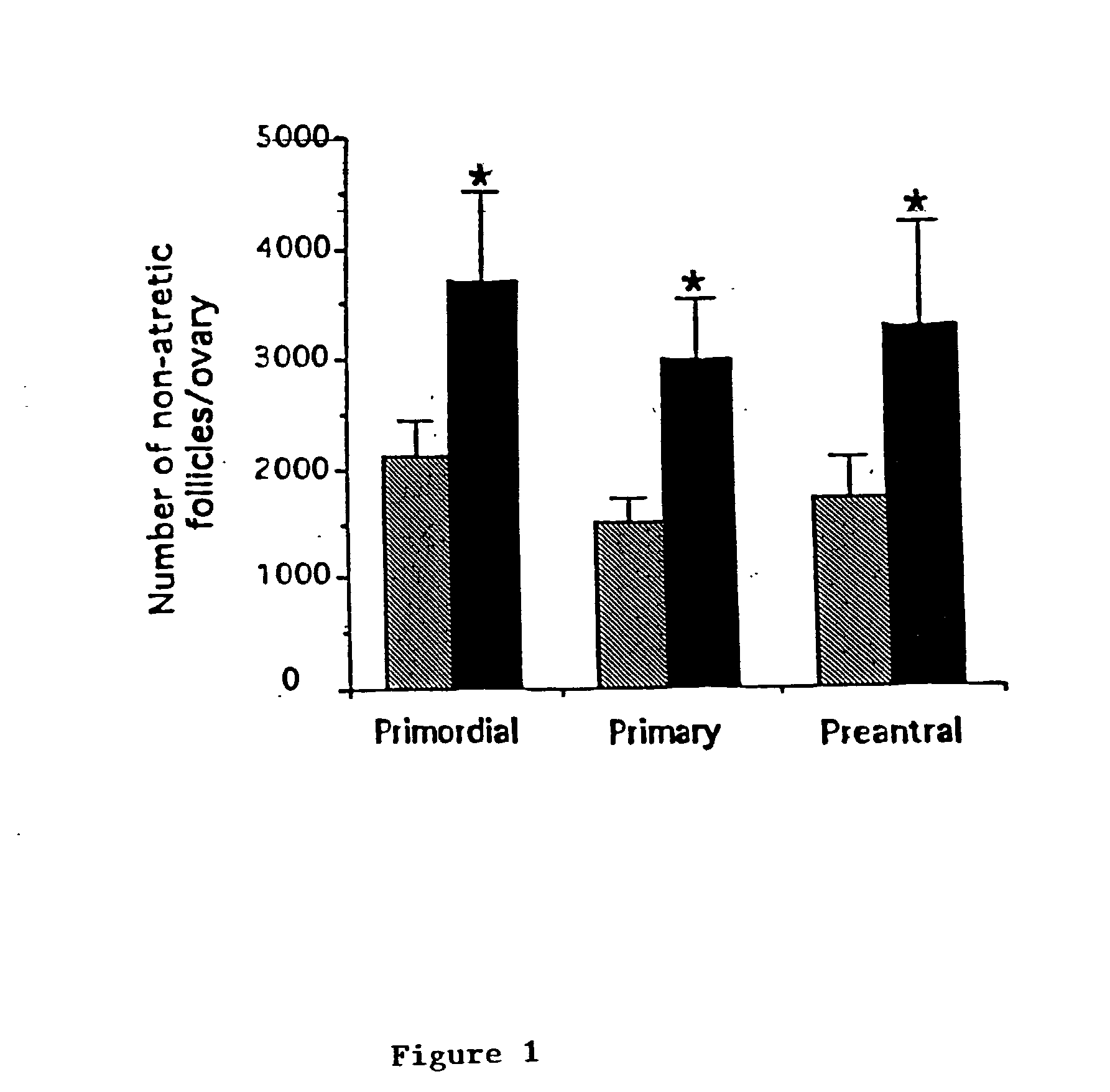
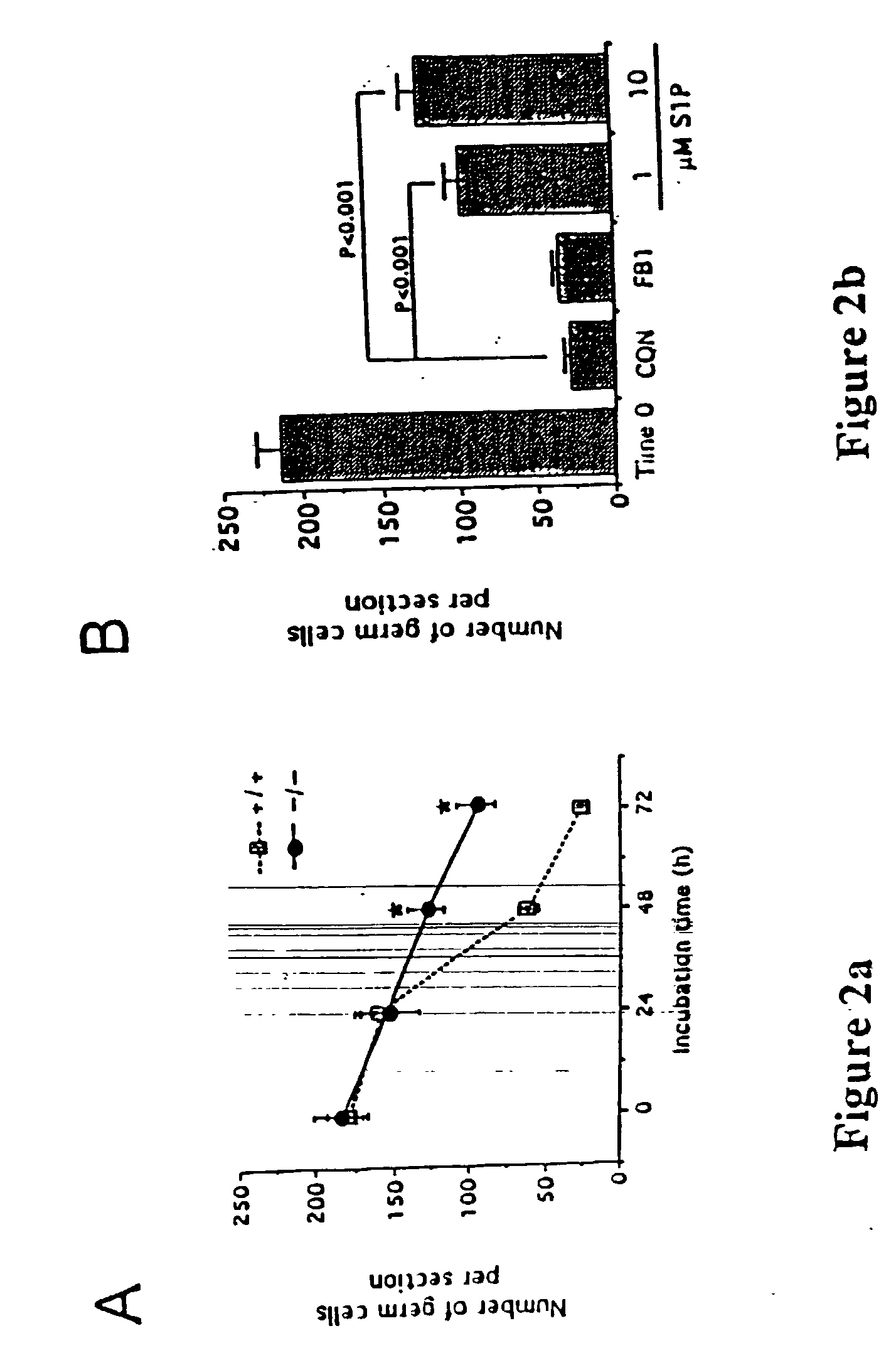
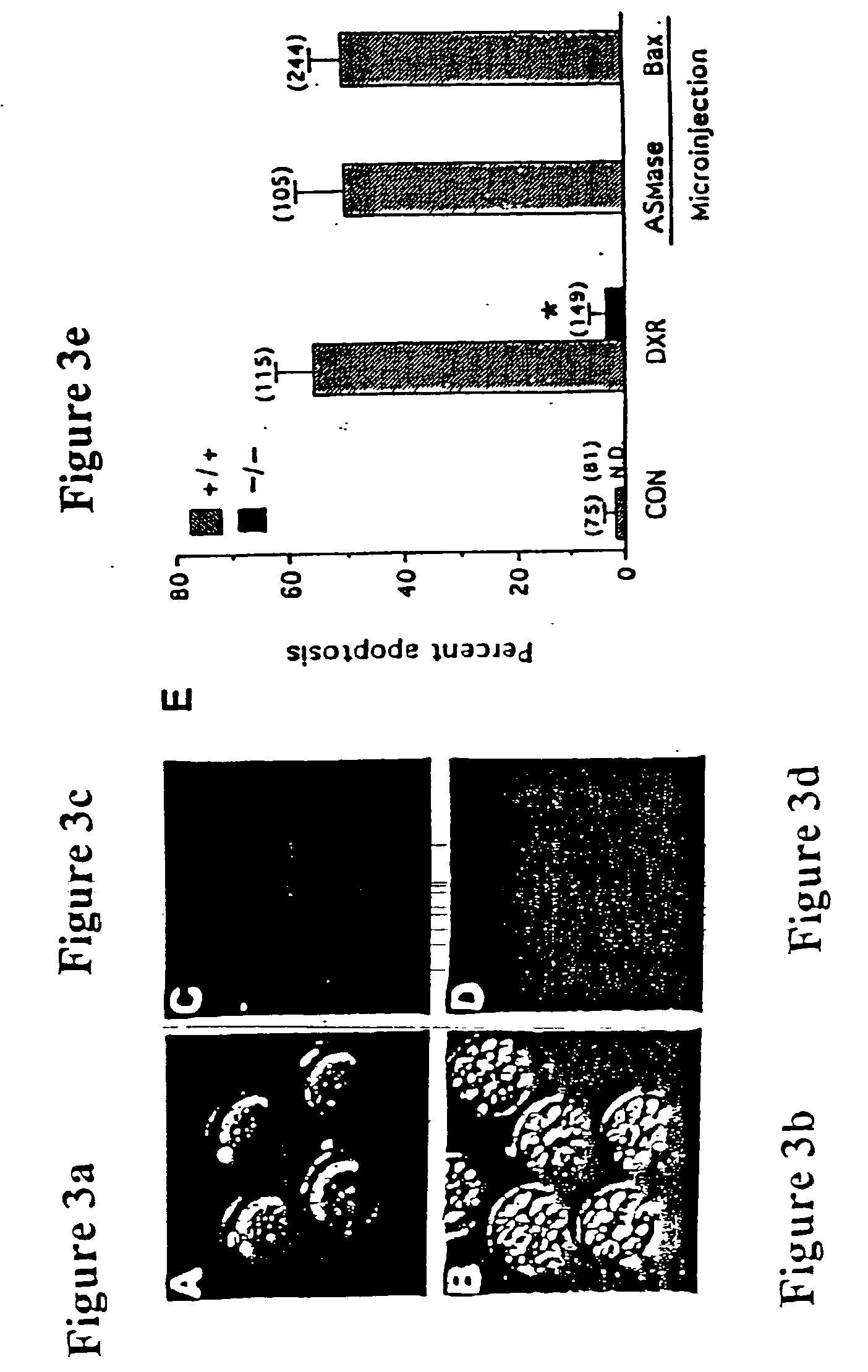
Described are methods for protecting the female reproductive system against natural and artificial insults by administering to women a composition comprising an agent that antagonizes one or more acid sphingomyelinase (ASMase) gene products. Specifically, methods disclosed herein serve to protect women's germline from damage resulting from cancer therapy regimens including chemotherapy or radiotherapy. In one aspect, the method preserves, enhances, or revives ovarian function in women, by administering to women a composition containing sphingosine-1-phosphate, or an analog thereof. Also disclosed are methods to prevent or ameliorate menopausal syndromes and to improve in vitro fertilization techniques.
Owner:THE GENERAL HOSPITAL CORP +1
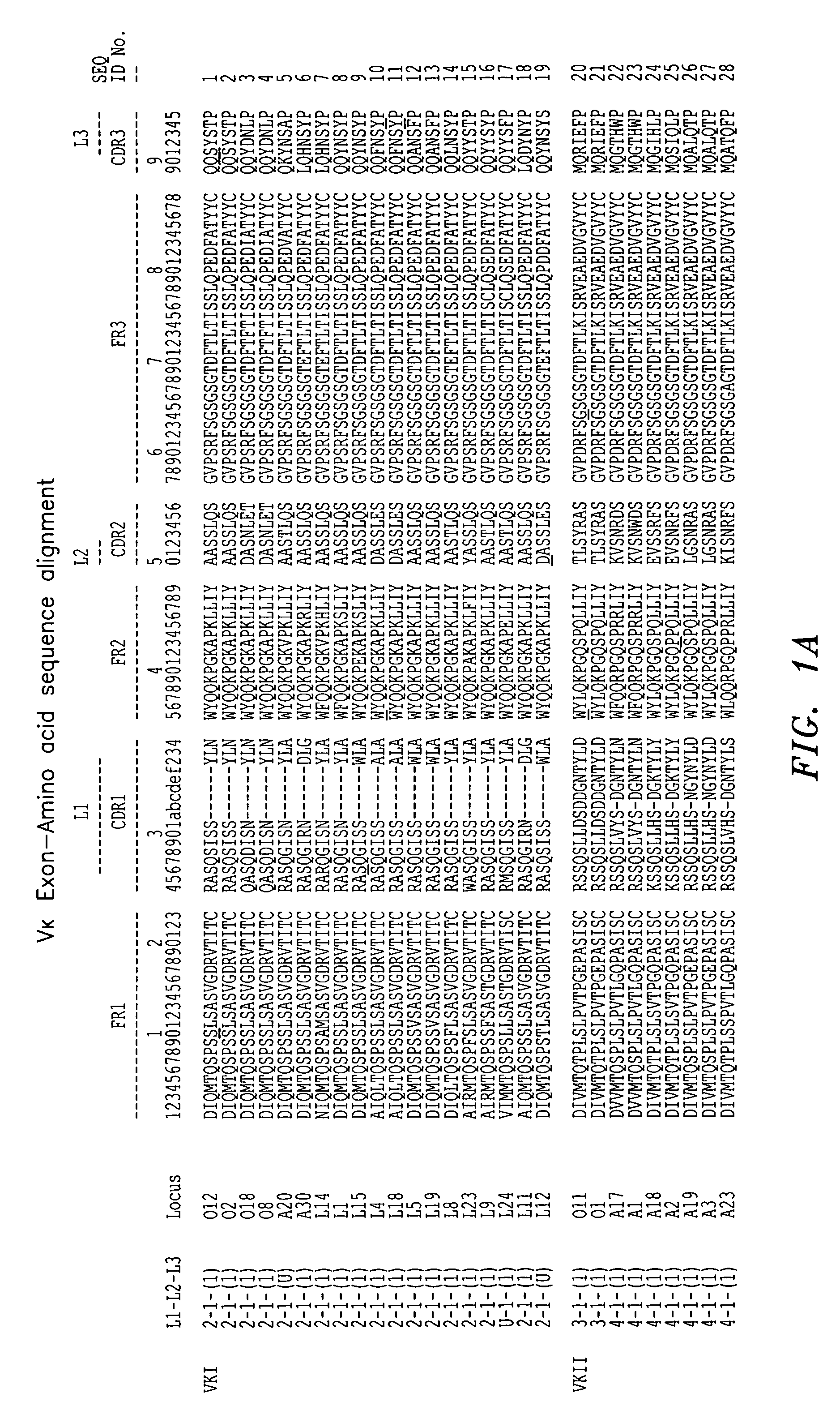
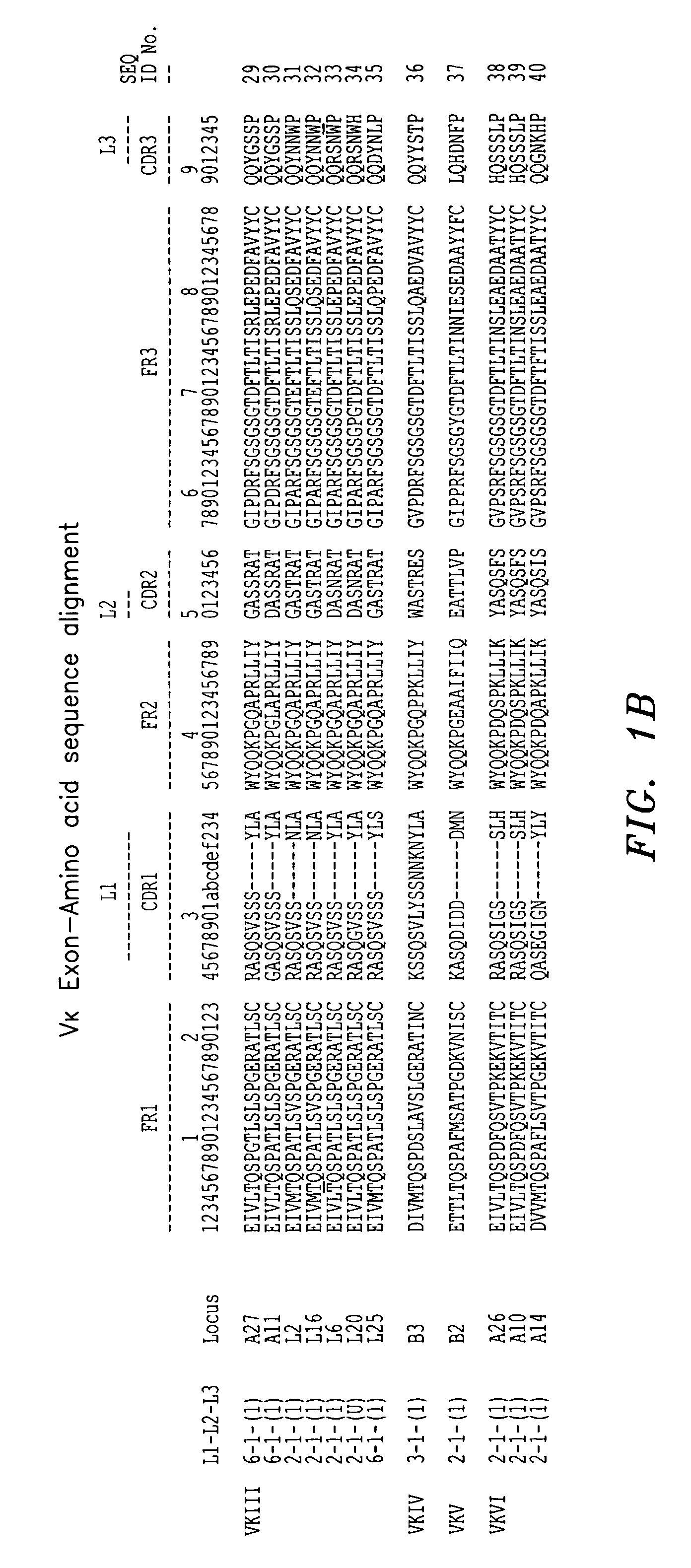
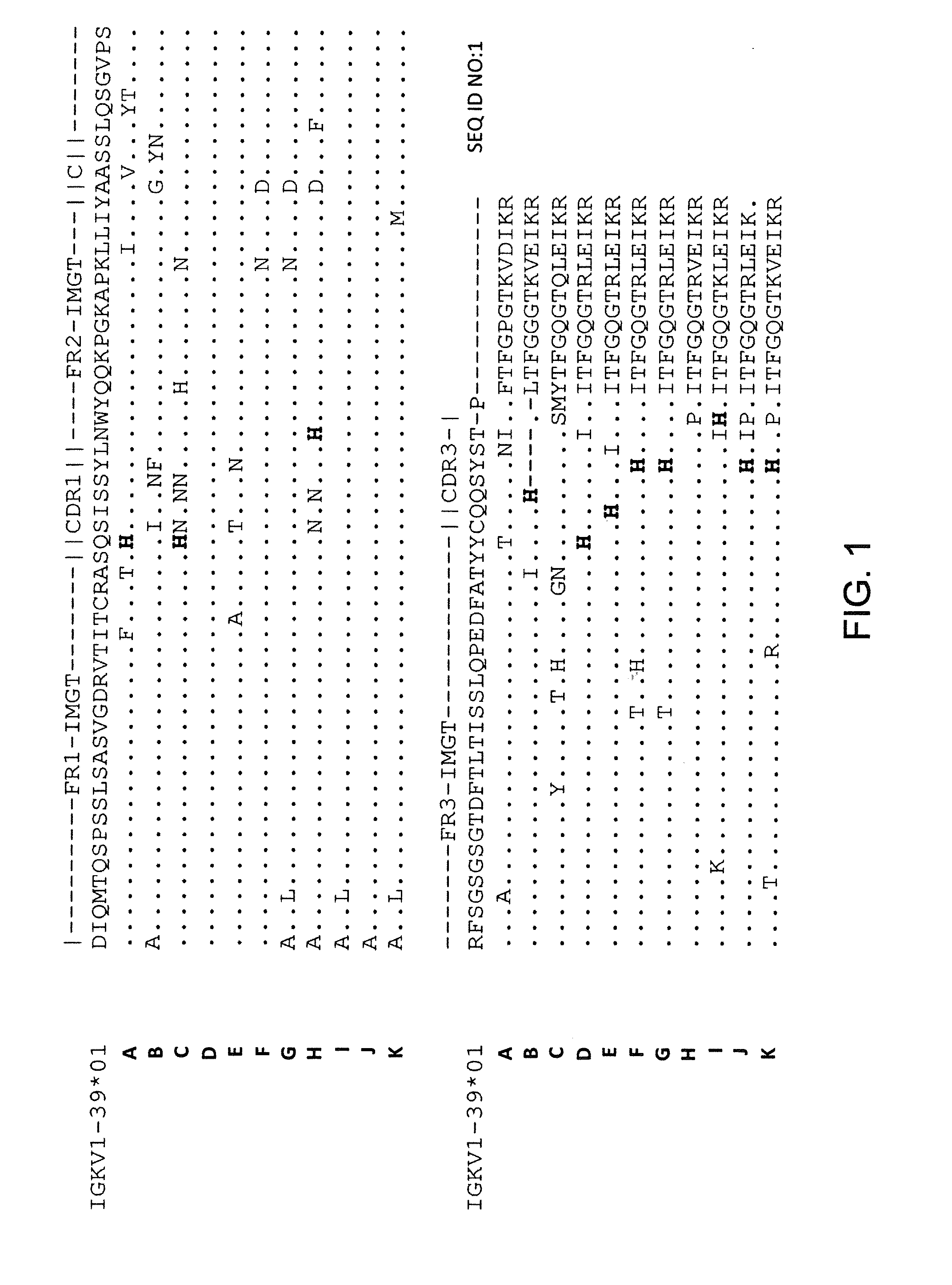
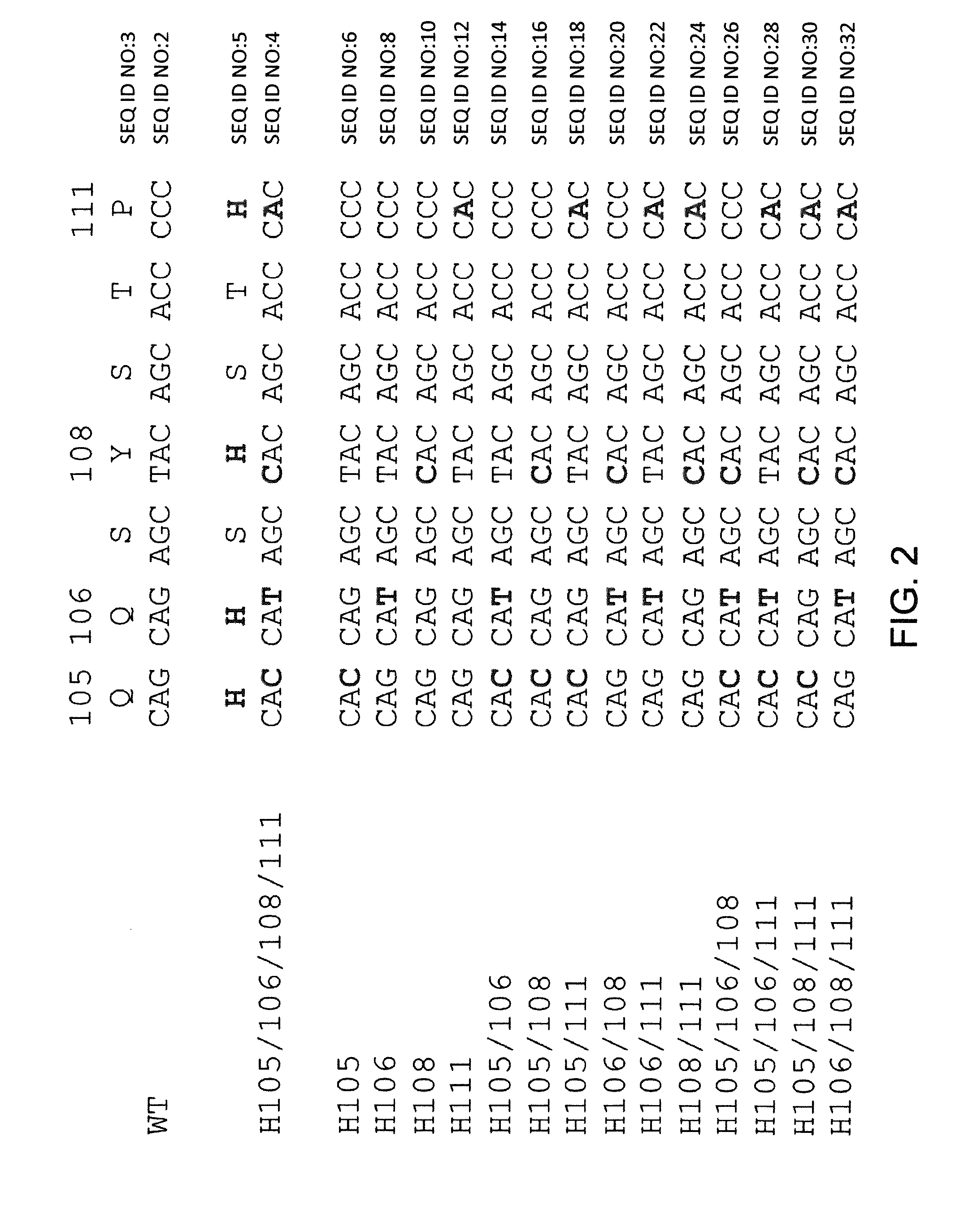
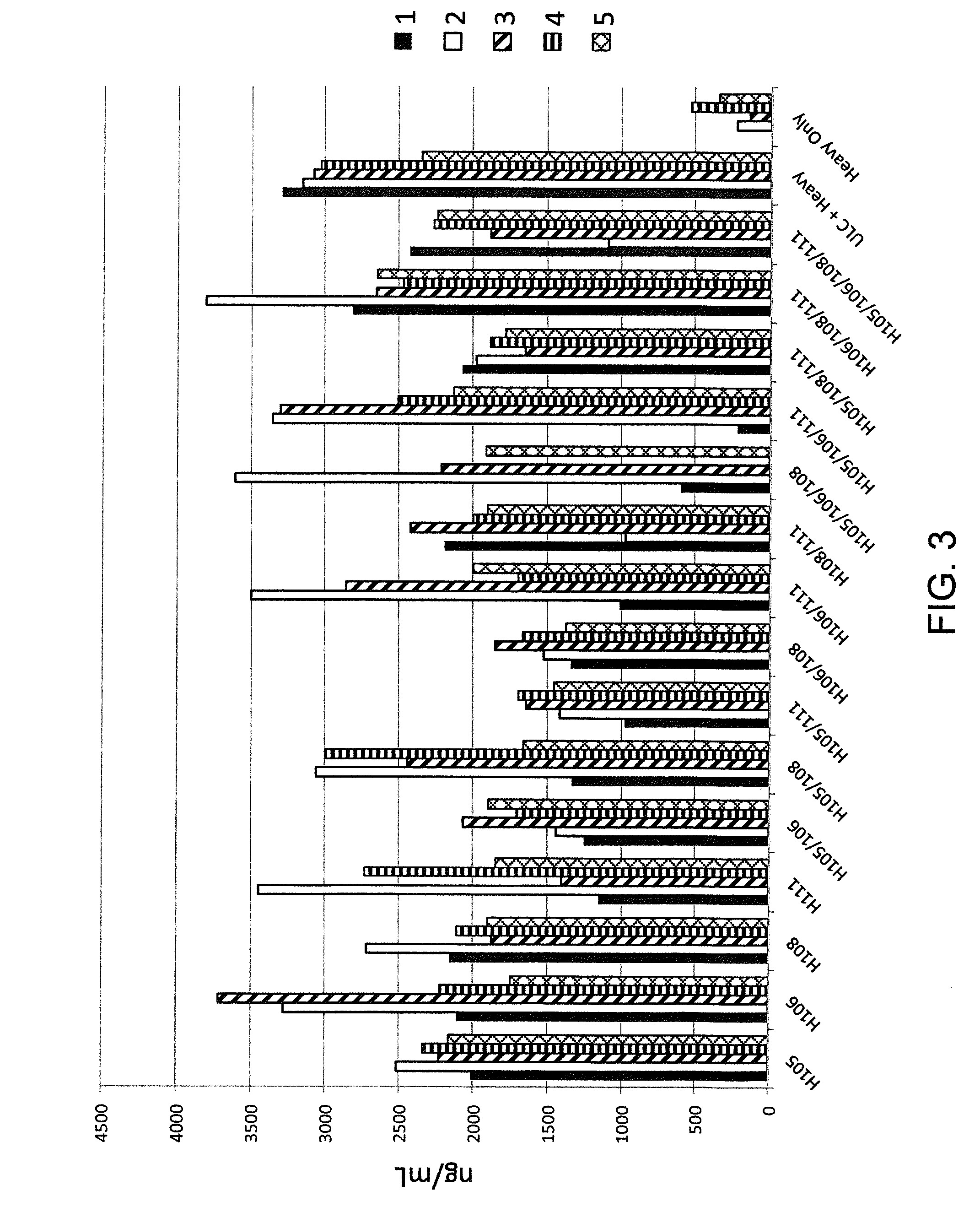
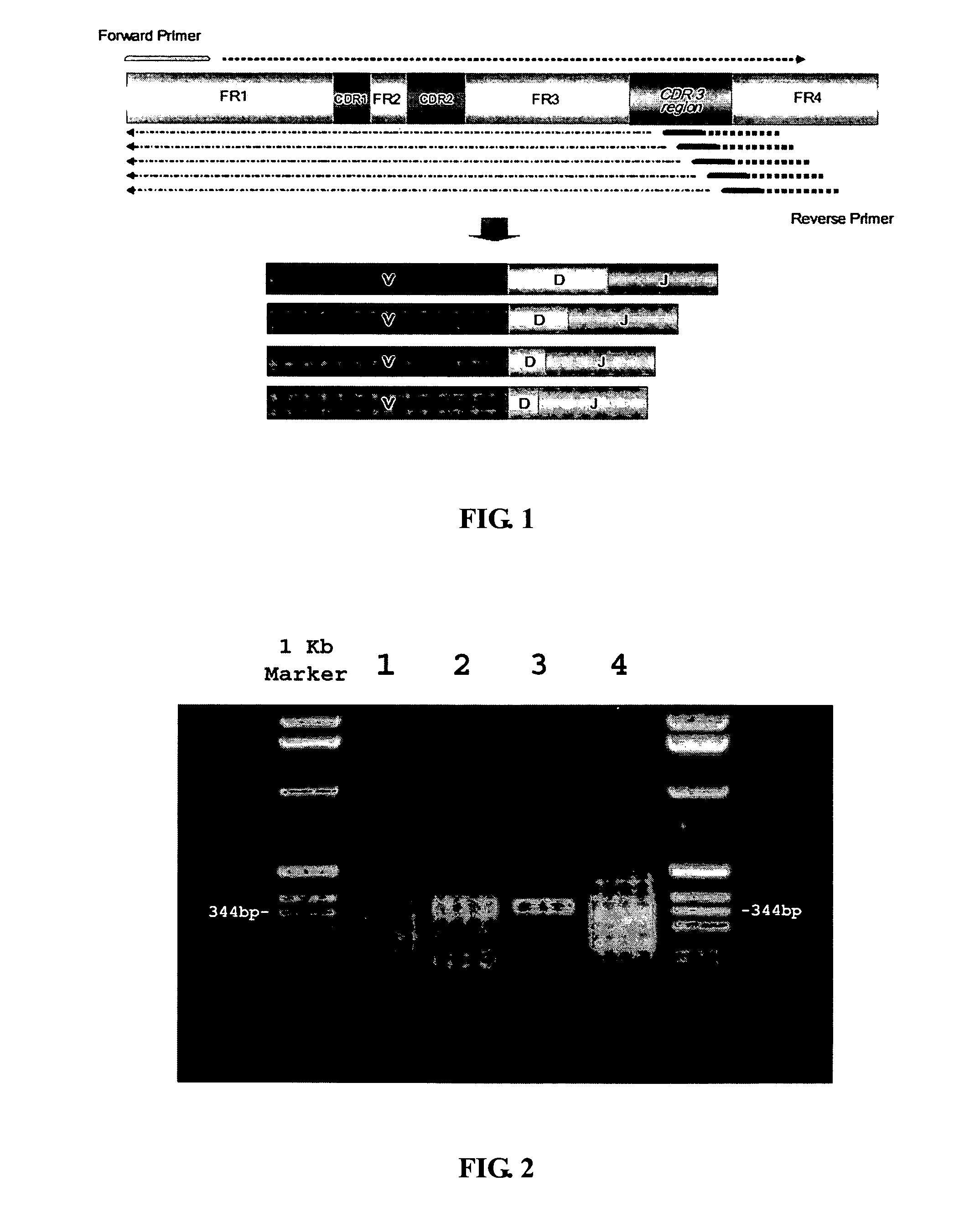
Frame-shifting PCR for germline immunoglobulin genes retrieval and antibody engineering
A method for preparing an antigen-specific antibody by constructing a library of phage-displayed single chain variable fragment of an antibody with a novel frame-shifting PCR is disclosed. Also disclosed is a method for preparing a clone for producing an antigen-specific antibody.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Soybean transformation for efficient and high-throughput transgenic event production
A method is disclosed for the Agrobacterium-mediated germline transformation of soybean, comprising infecting split soybean seeds, with a portion of the embryonic axis, with Agrobacterium tumefaciens containing a transgene. The method can further comprise regenerating the explants produced from the transformation of the split soybean seeds comprising a portion of embryonic axis in vitro on selection medium.
Owner:DOW AGROSCIENCES LLC
Targeted-panel tumor mutational burden calculation systems and methods
PendingUS20200258601A1Adapt quicklyLarge adaptabilityBiostatisticsMedical automated diagnosisGenomic sequencingCell Fraction
A method and system for conducting genomic sequencing, the system comprising a first microservice for receiving an order from a physician, the order to initiate an NGS of a patient's germline specimen and somatic specimen using a targeted-panel, a second microservice for executing an NGS of the patient's germline specimen to identify sequences of nucleotides in the germline specimen using the targeted-panel to generate germline sequencing results, a third microservice for executing an NGS of the patient's somatic specimen to identify sequences of nucleotides in the somatic specimen using the targeted-panel to generate somatic sequencing results, a fourth microservice for executing quality control (QC) testing on the germline sequencing results to generate a germline QC score and on the somatic sequencing results to generate a somatic QC score, a fifth microservice for generating at least one clinical report, and a sixth microservice for providing the at least one clinical report to the physician, the at least on clinical report comprising the patient's TMB status.
Owner:TEMPUS LABS
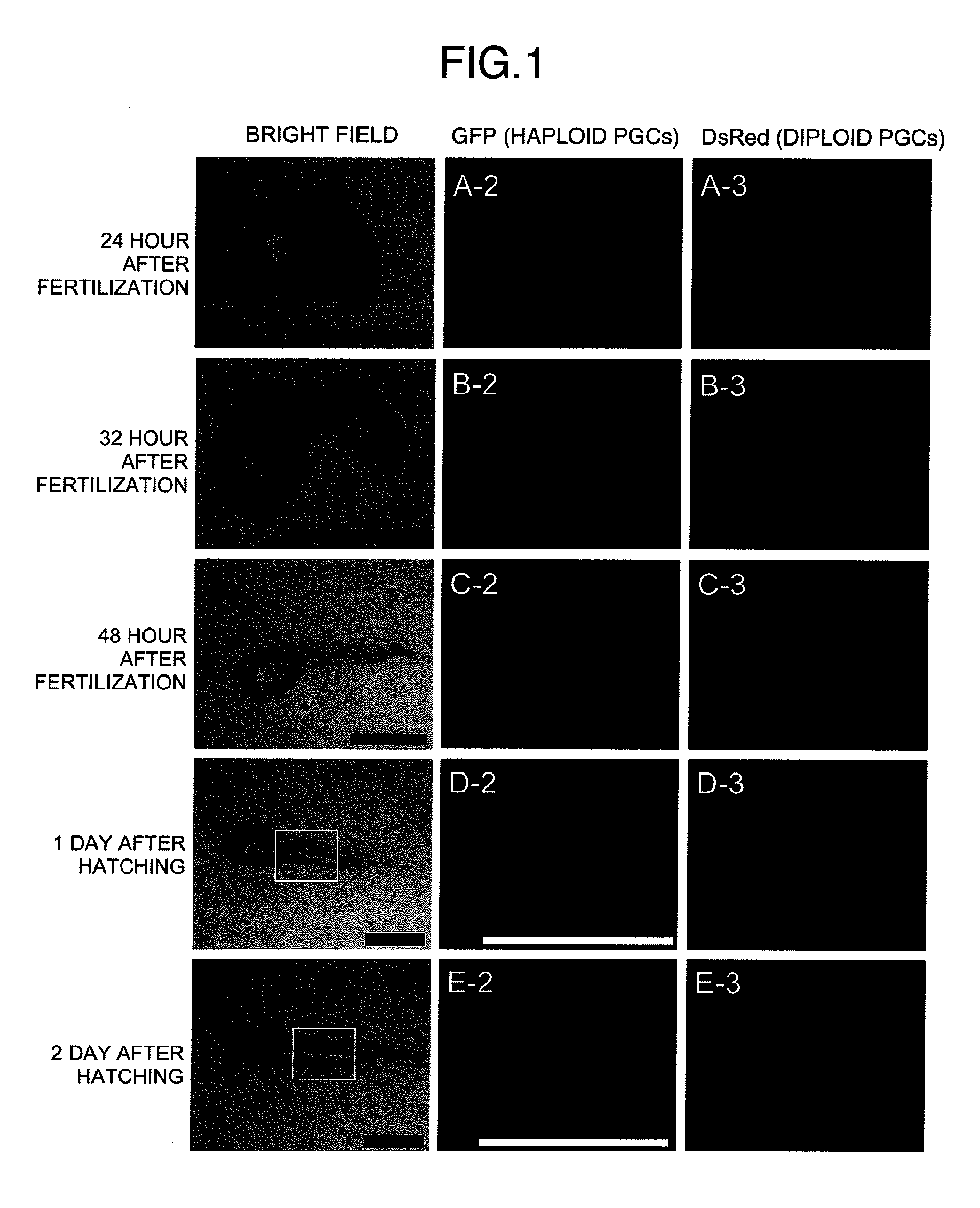
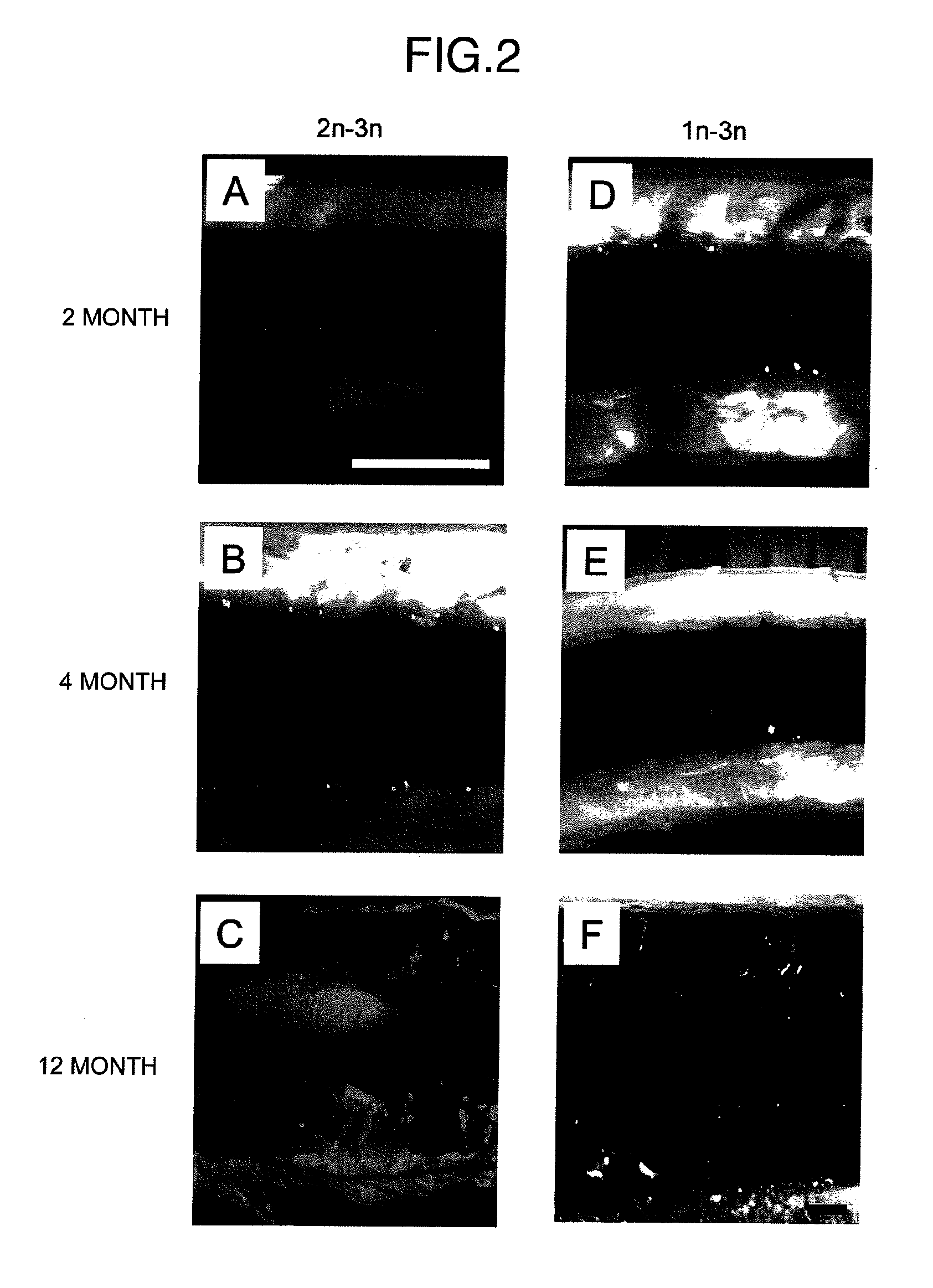
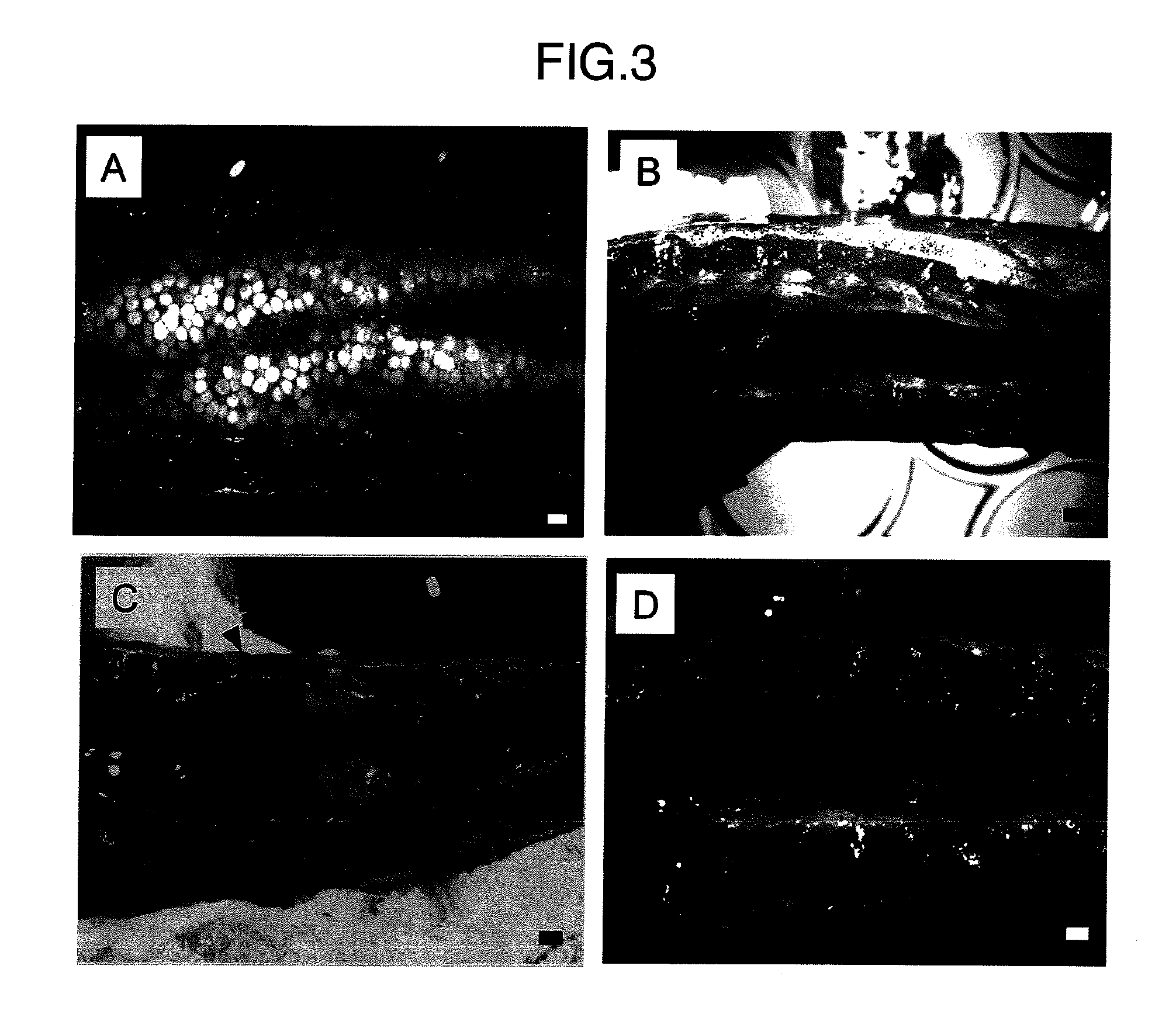
Method for acquiring genetically identical gamete from lethal fish haploid-derived germ cell via germ line chimera
InactiveUS20120304322A1Short timeEasy to useRecombinant DNA-technologyFermentationPlant Germ CellsPlant Gametes
Disclosed are a method for acquiring a germ line chimeric fish having fish haploid germ cells, a germ line chimeric fish having haploid germ cells obtained by the aforesaid method, and a genetically identical gamete, said gamete having been derived from a donor haploid germ cell, produced by a germ line chimeric fish obtained by the aforesaid method.
Owner:HOKKAIDO UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com