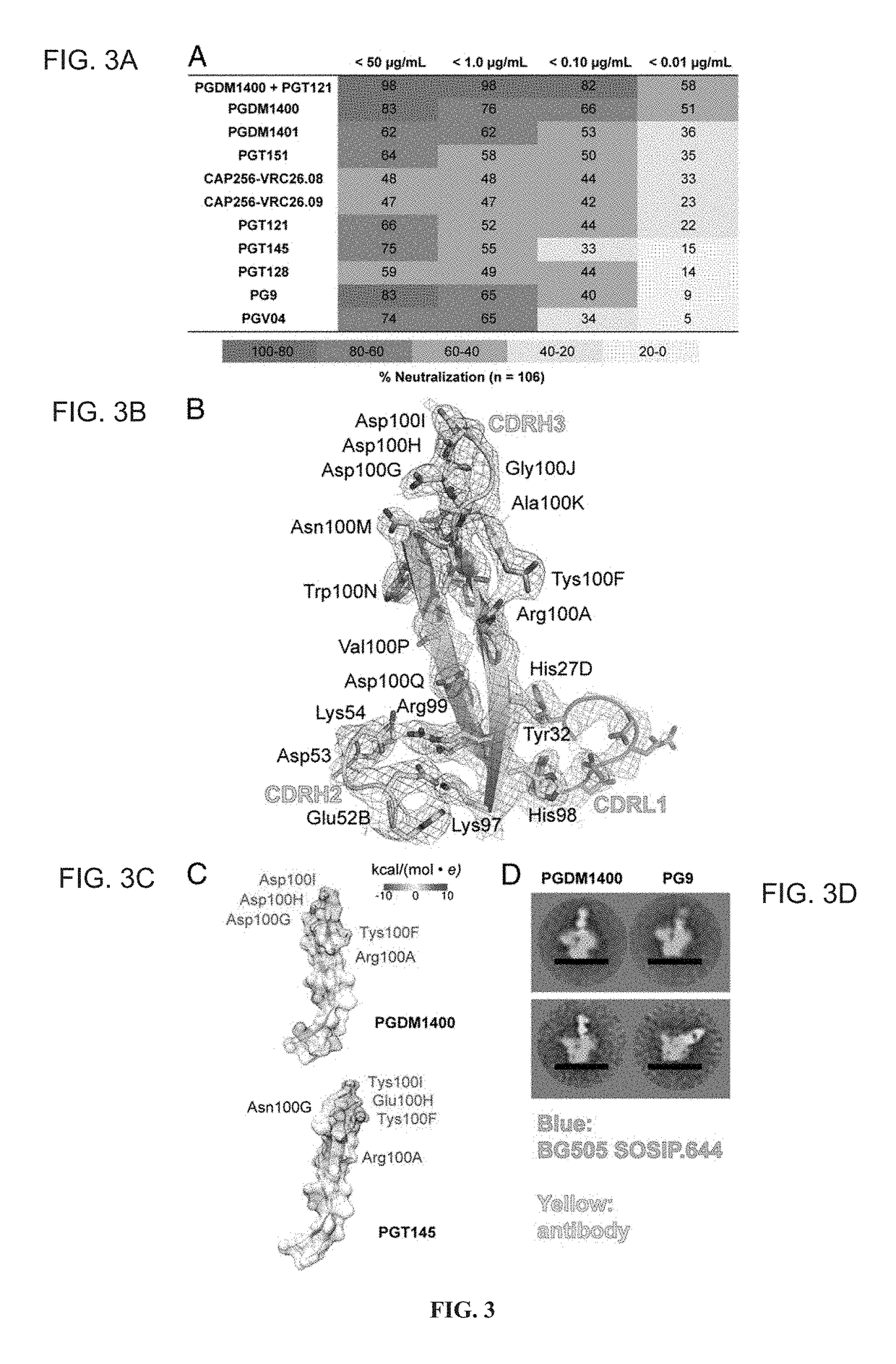
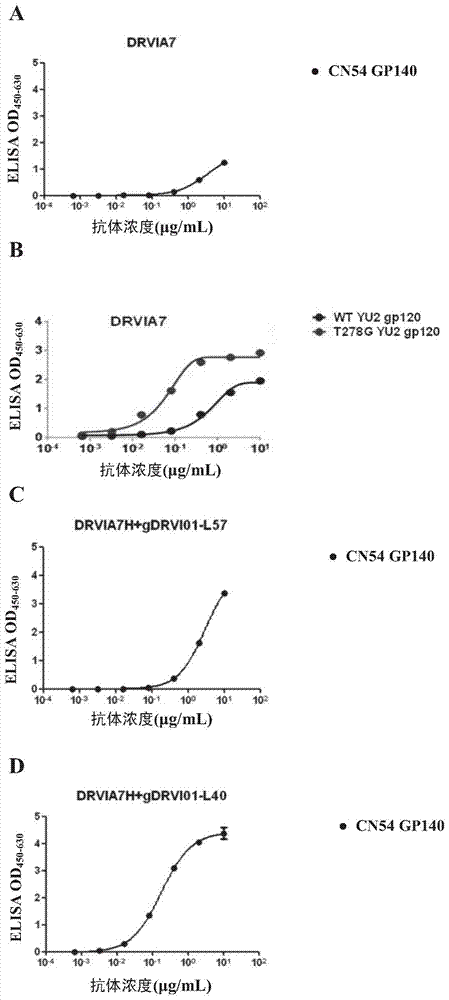
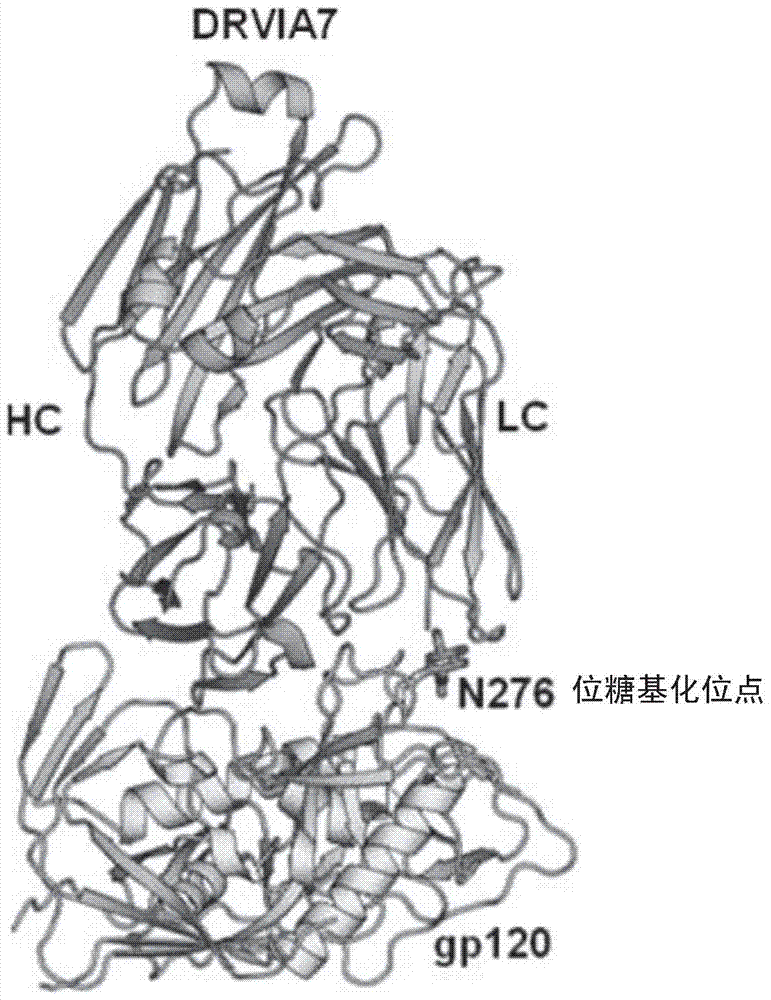
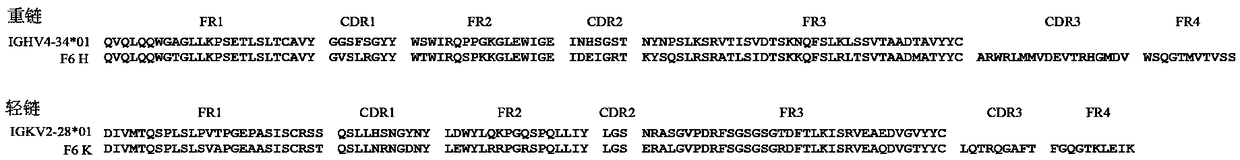
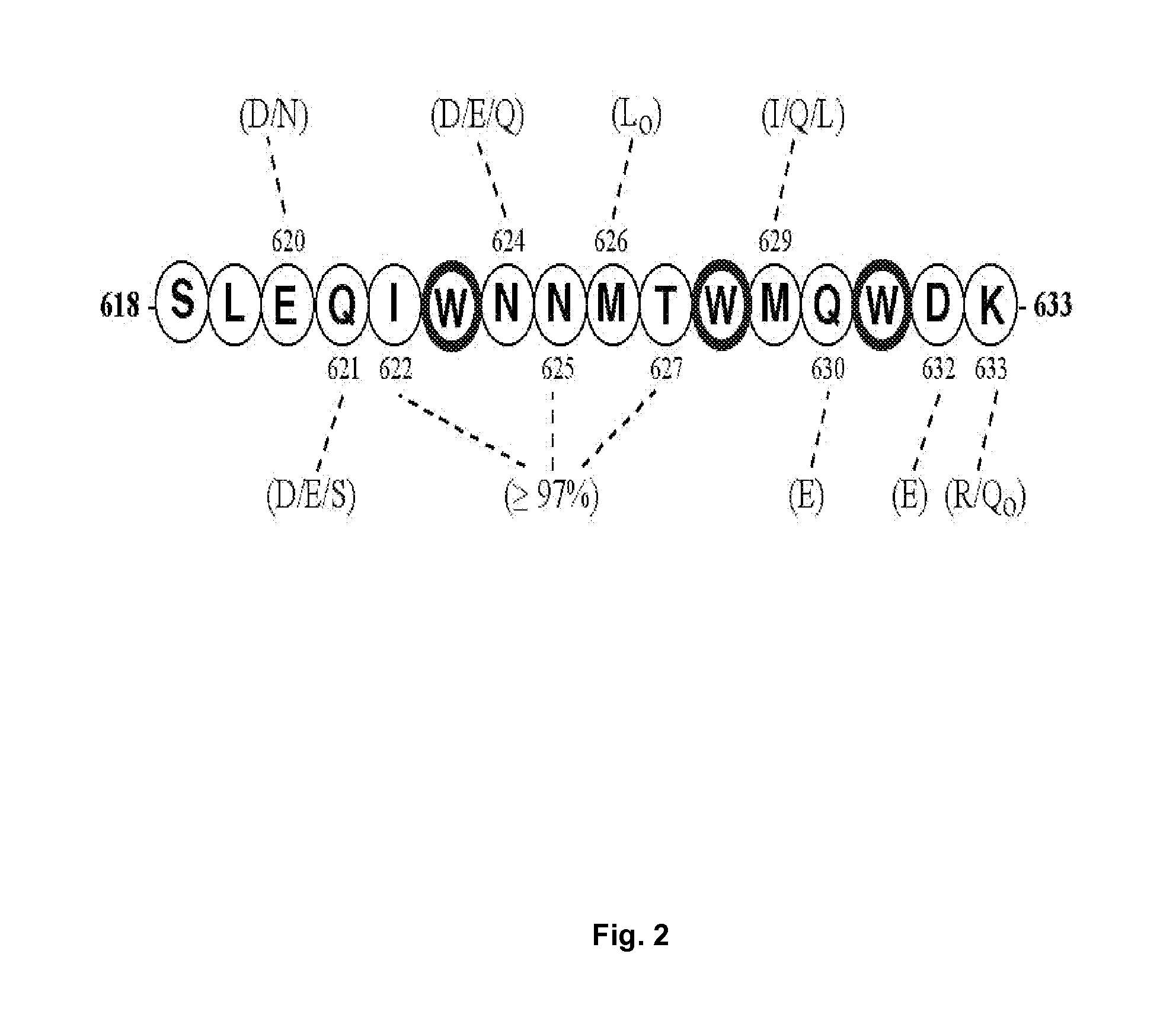
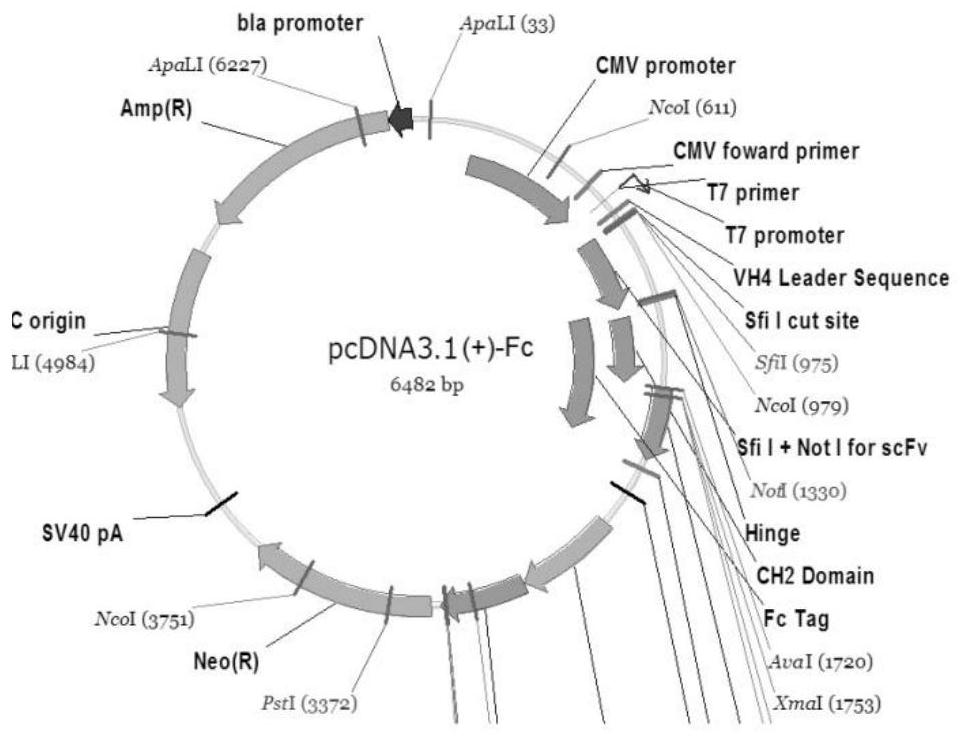
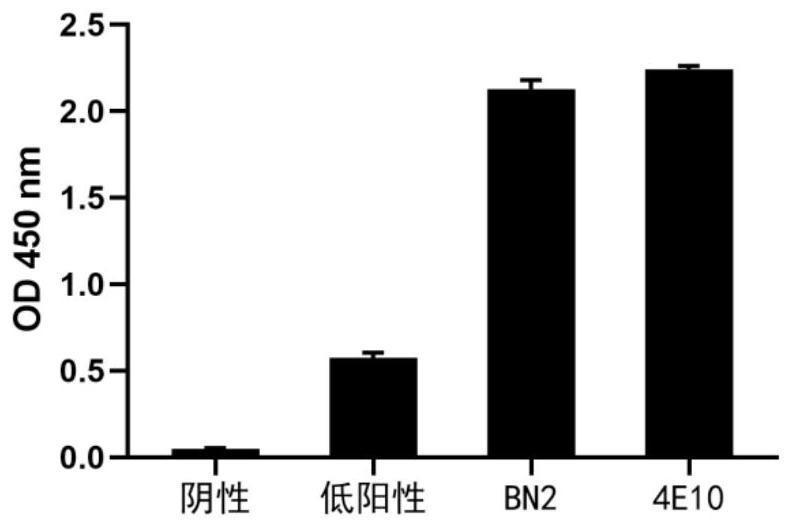
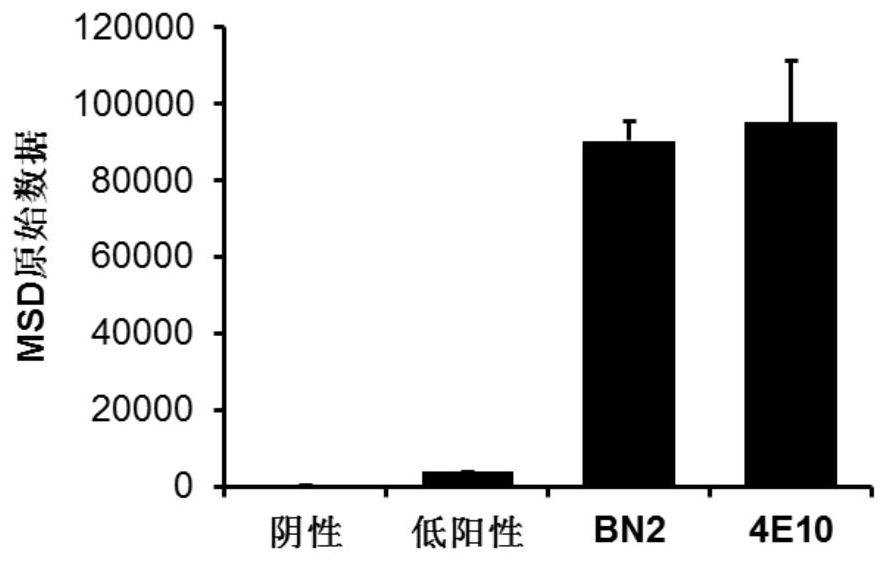
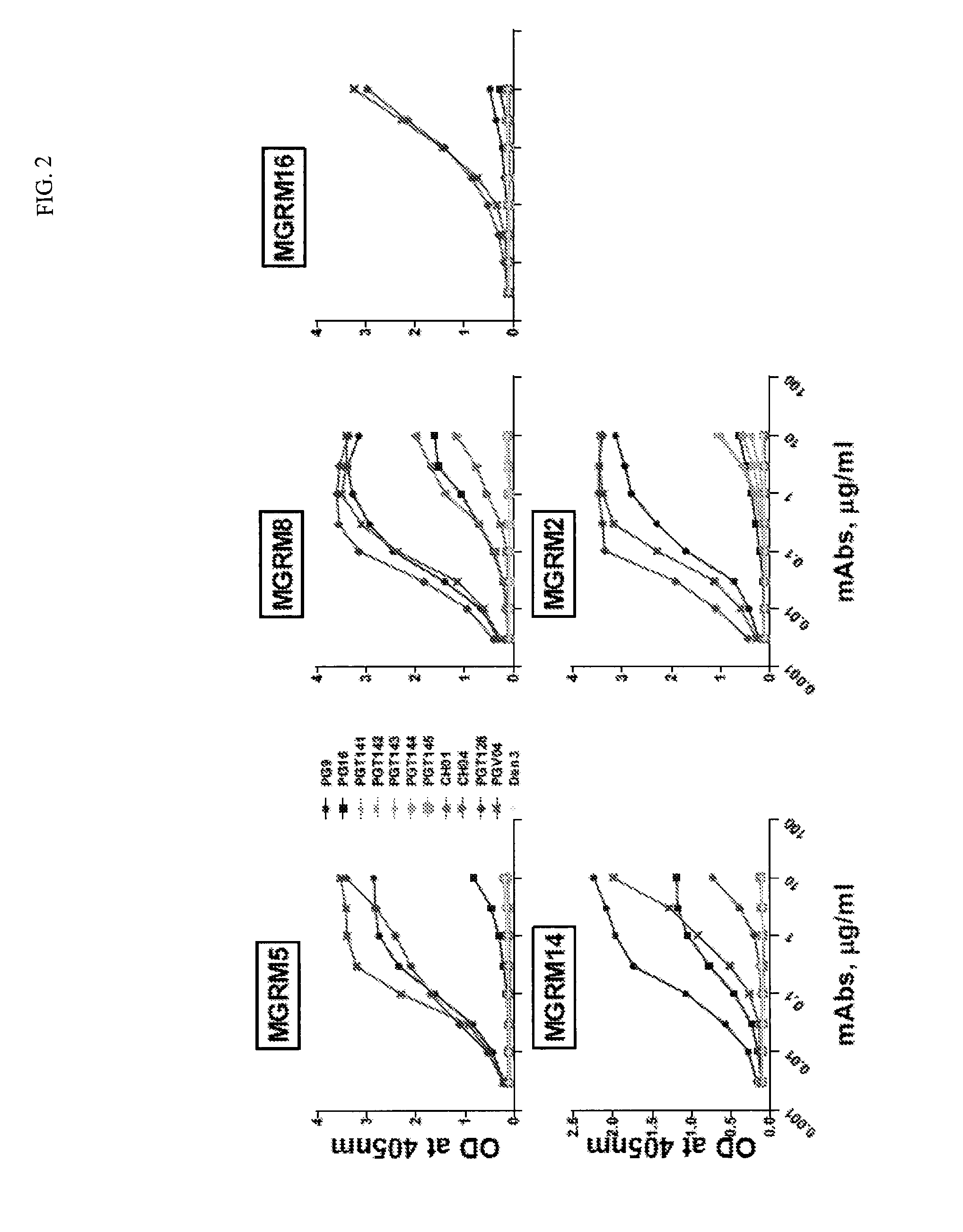
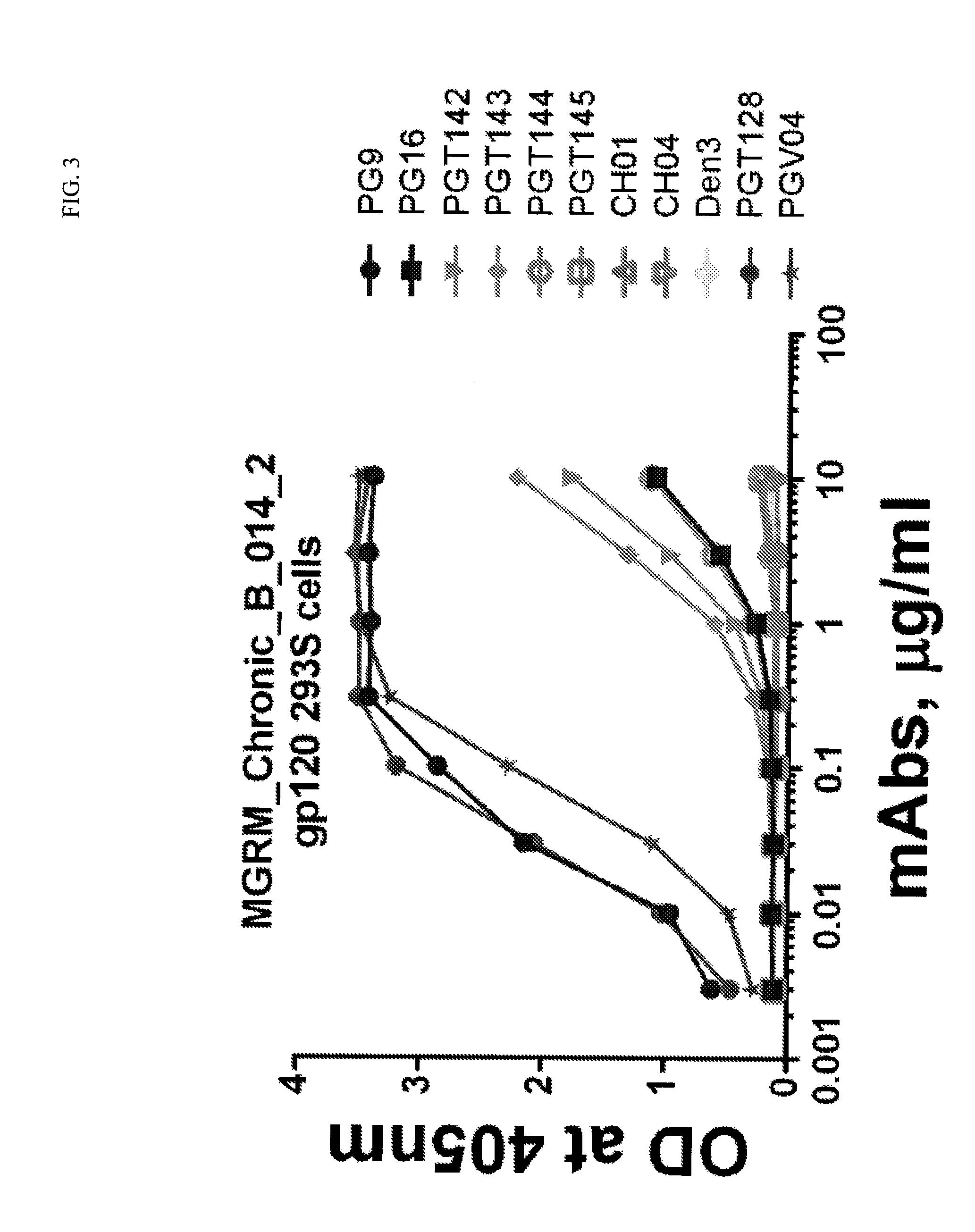
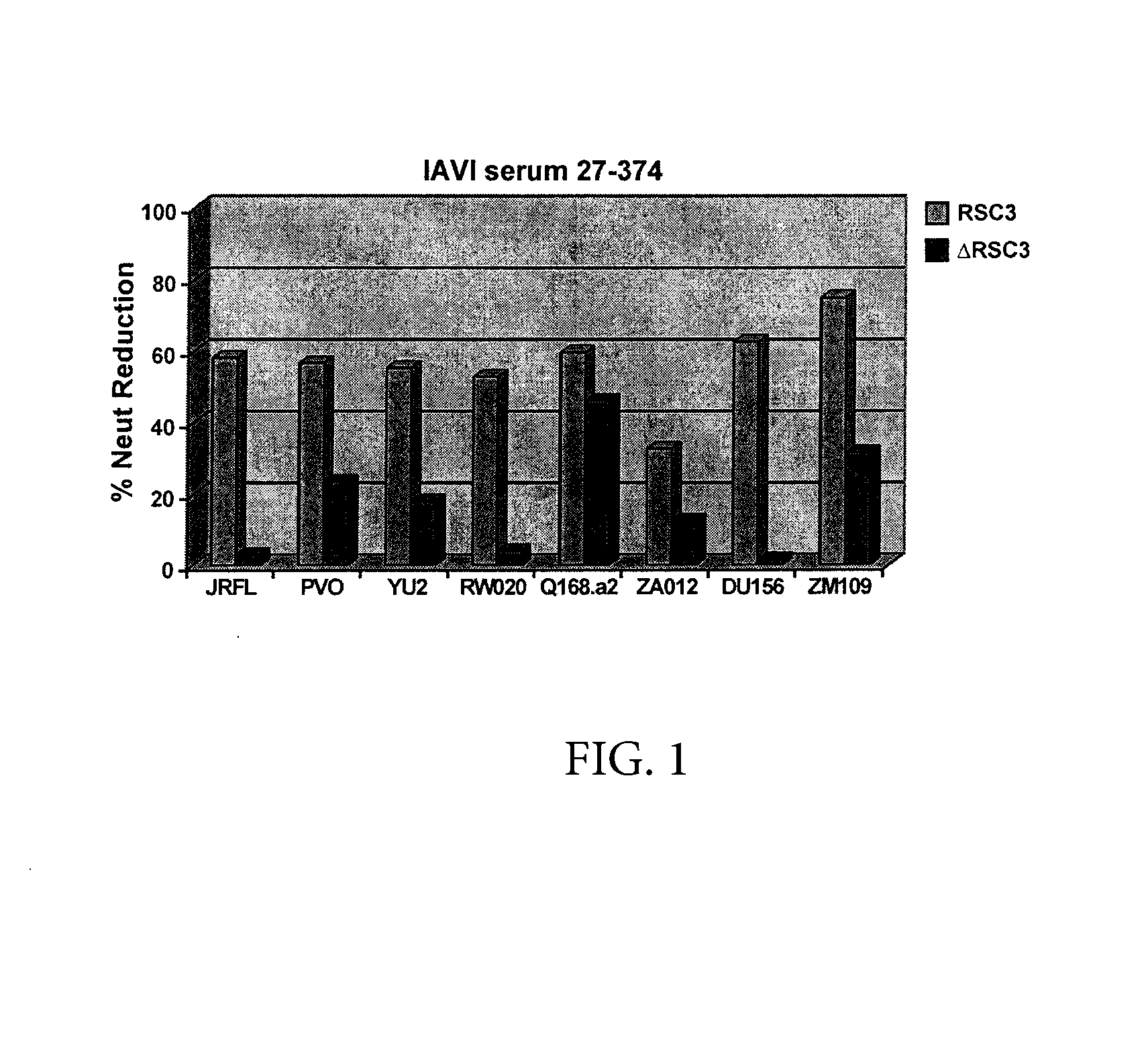
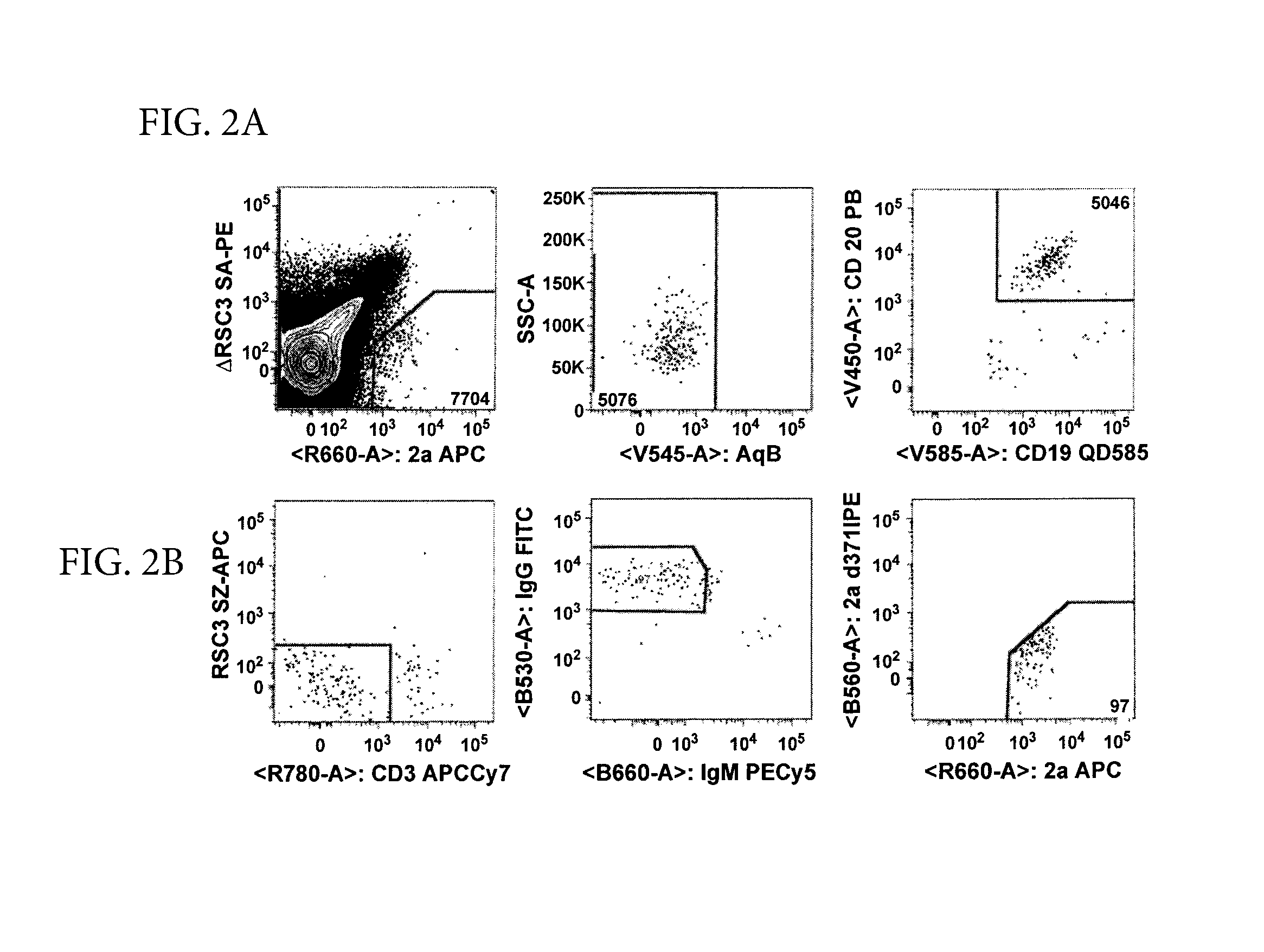
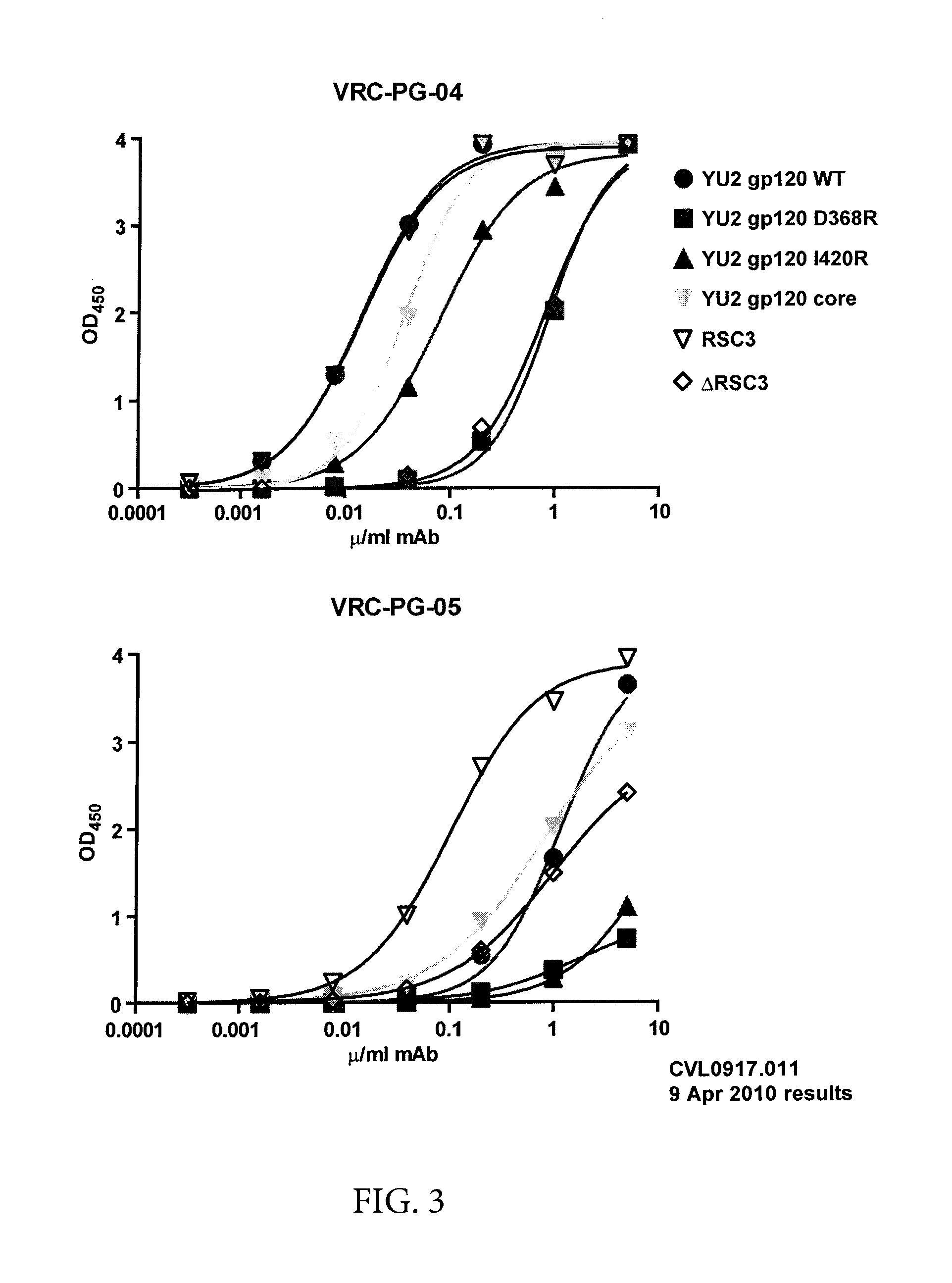
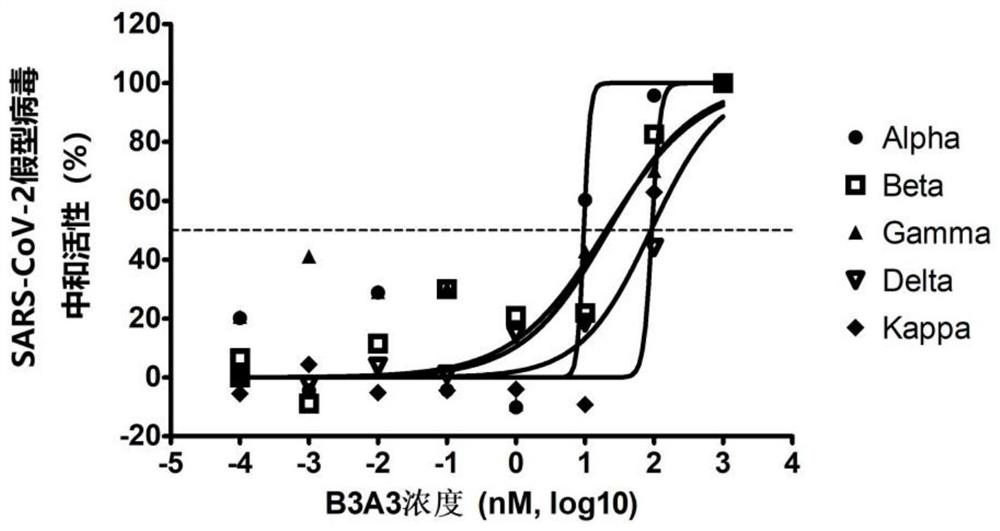
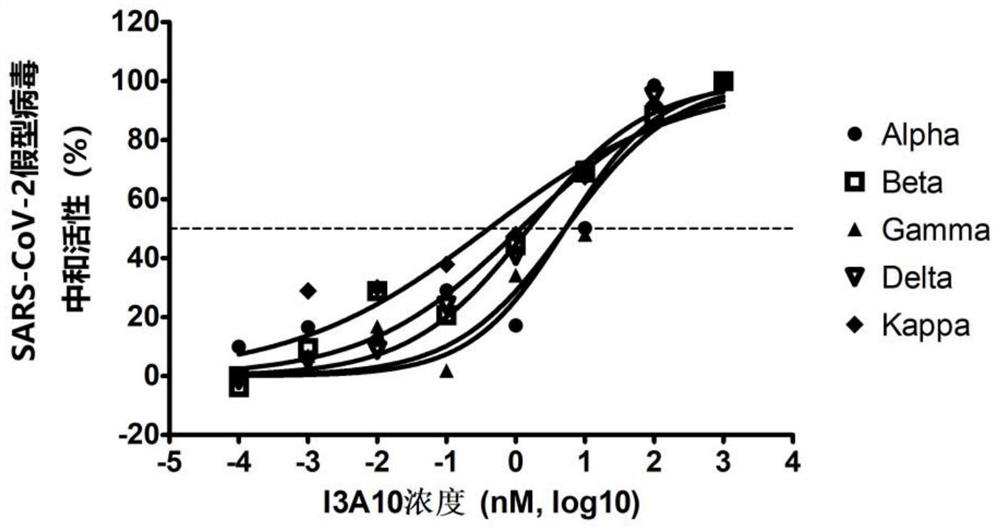
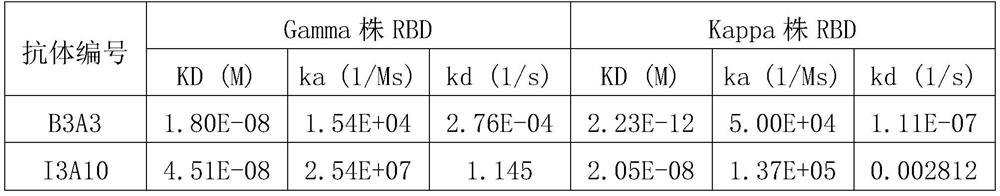
Patents
Literature
70 results about "Broadly neutralizing antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Broadly Neutralizing Antibodies (bNAbs) A type of antibody that can recognize and block many types of HIV from entering healthy cells.
Novel HIV -1 broadly neutralizing antibodies
ActiveUS20130251726A1Reduce the impactMicrobiological testing/measurementImmunoglobulins against virusesHeavy chainNeutralising antibody
The present application relates novel HIV-1 broadly neutralizing antibodies. The antibodies of the present invention are further characterized by their ability to bind epitopes from the Env proteins. The invention also provides light and heavy chain variable region sequences. Compositions for prophylaxis, diagnosis and treatment of HIV infection are provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +2
Human immunodeficiency virus (HIV)-neutralizing antibodies
ActiveUS20110044994A1Sugar derivativesImmunoglobulins against virusesVaccinationImmunodeficiency virus
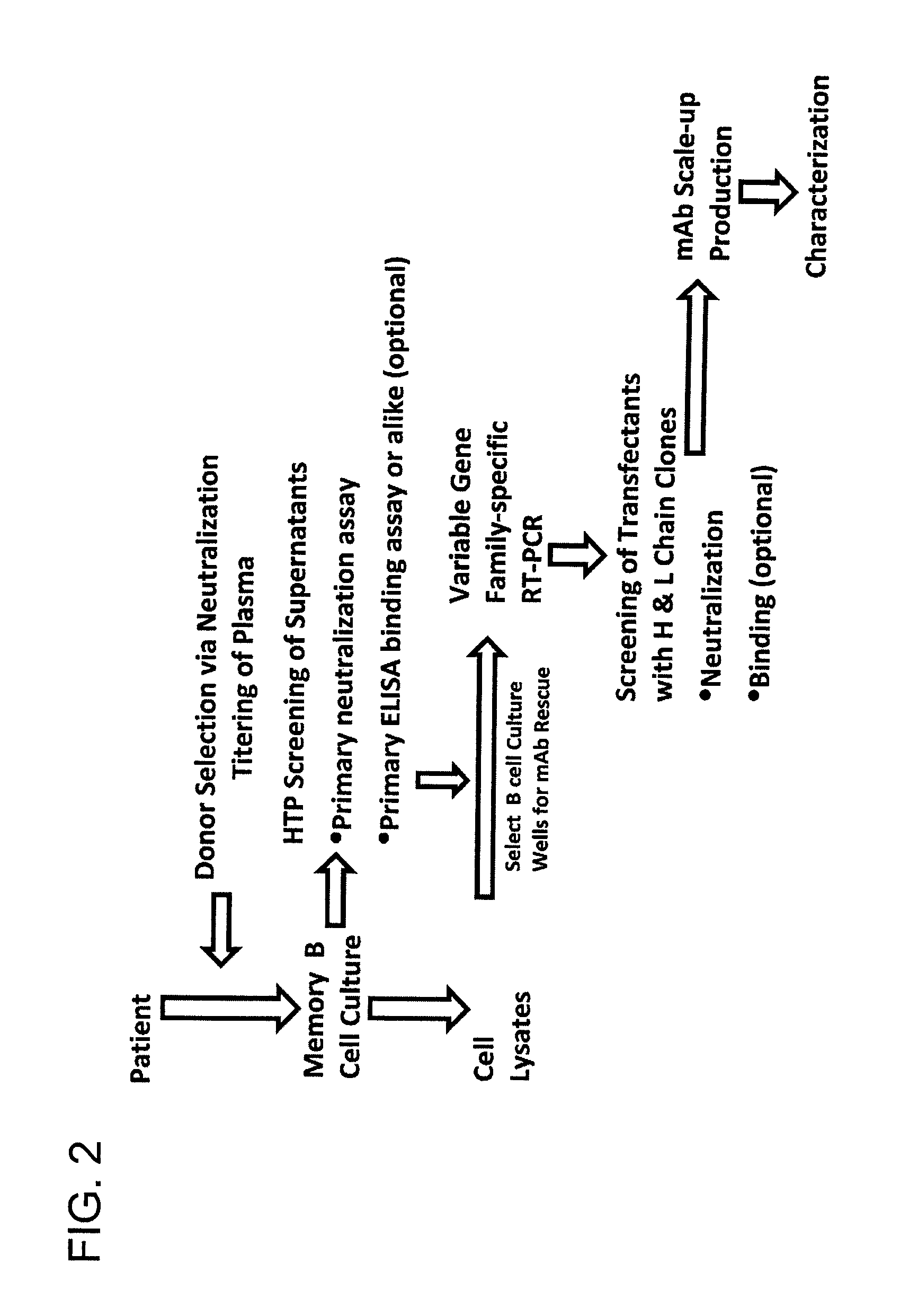
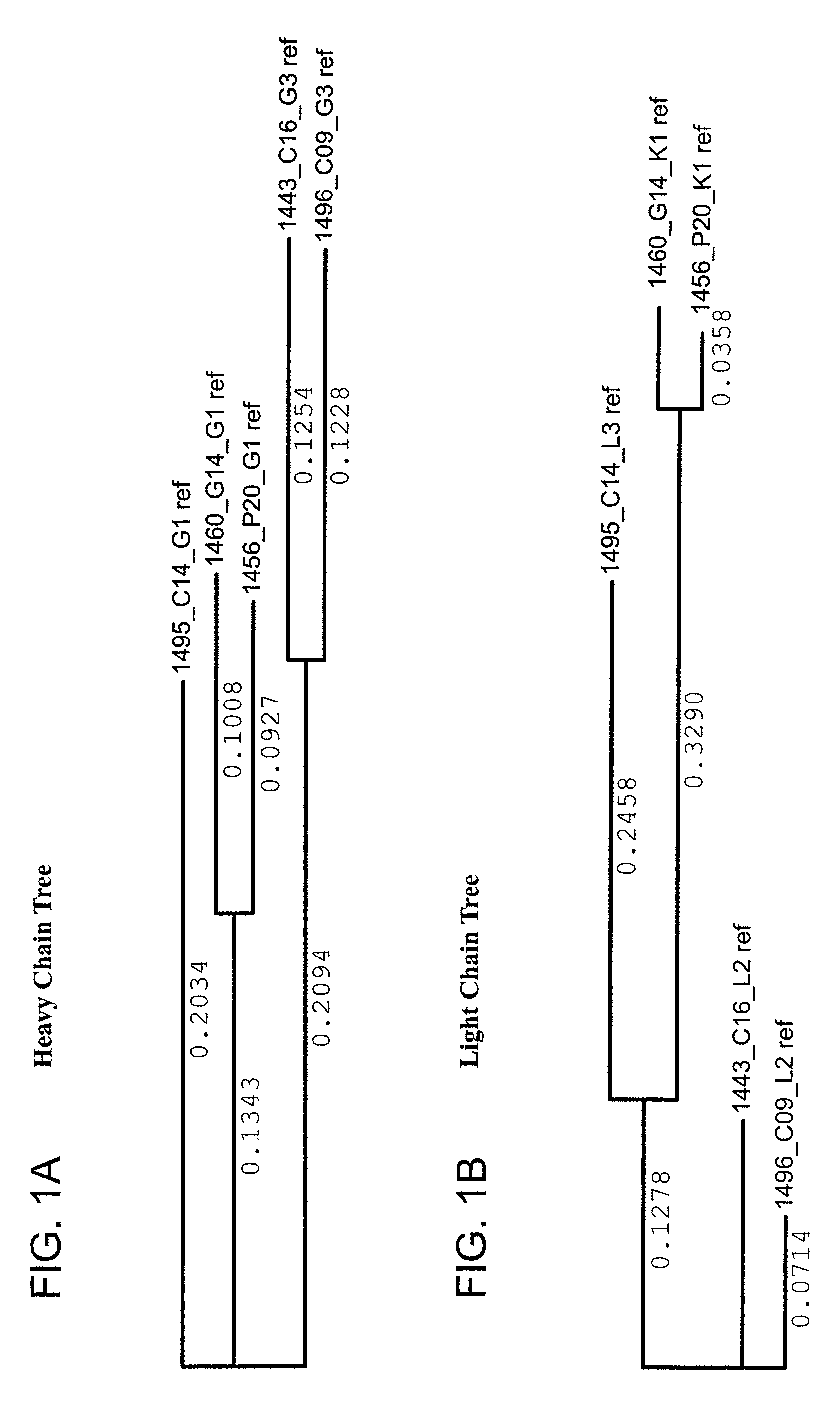
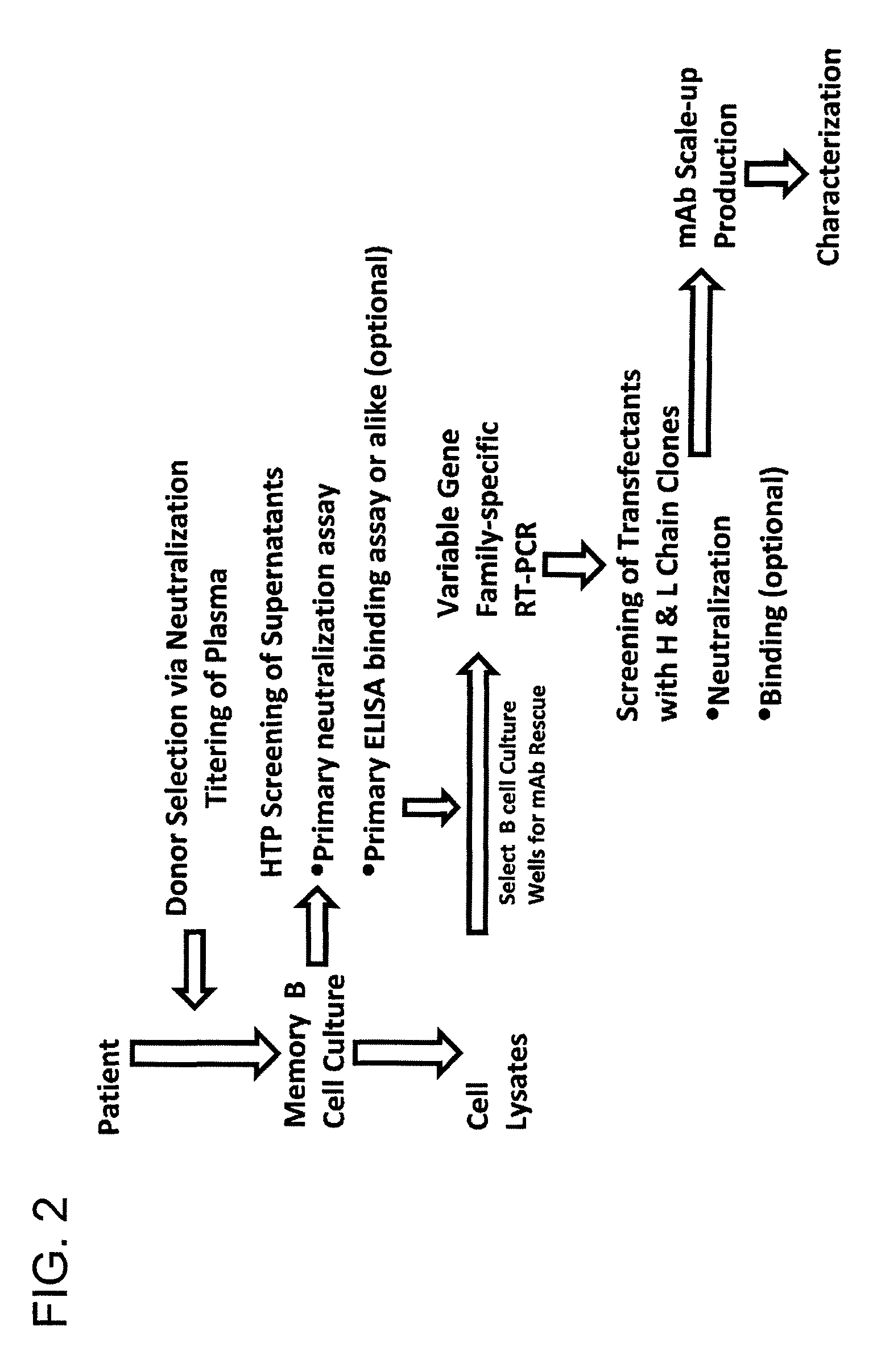
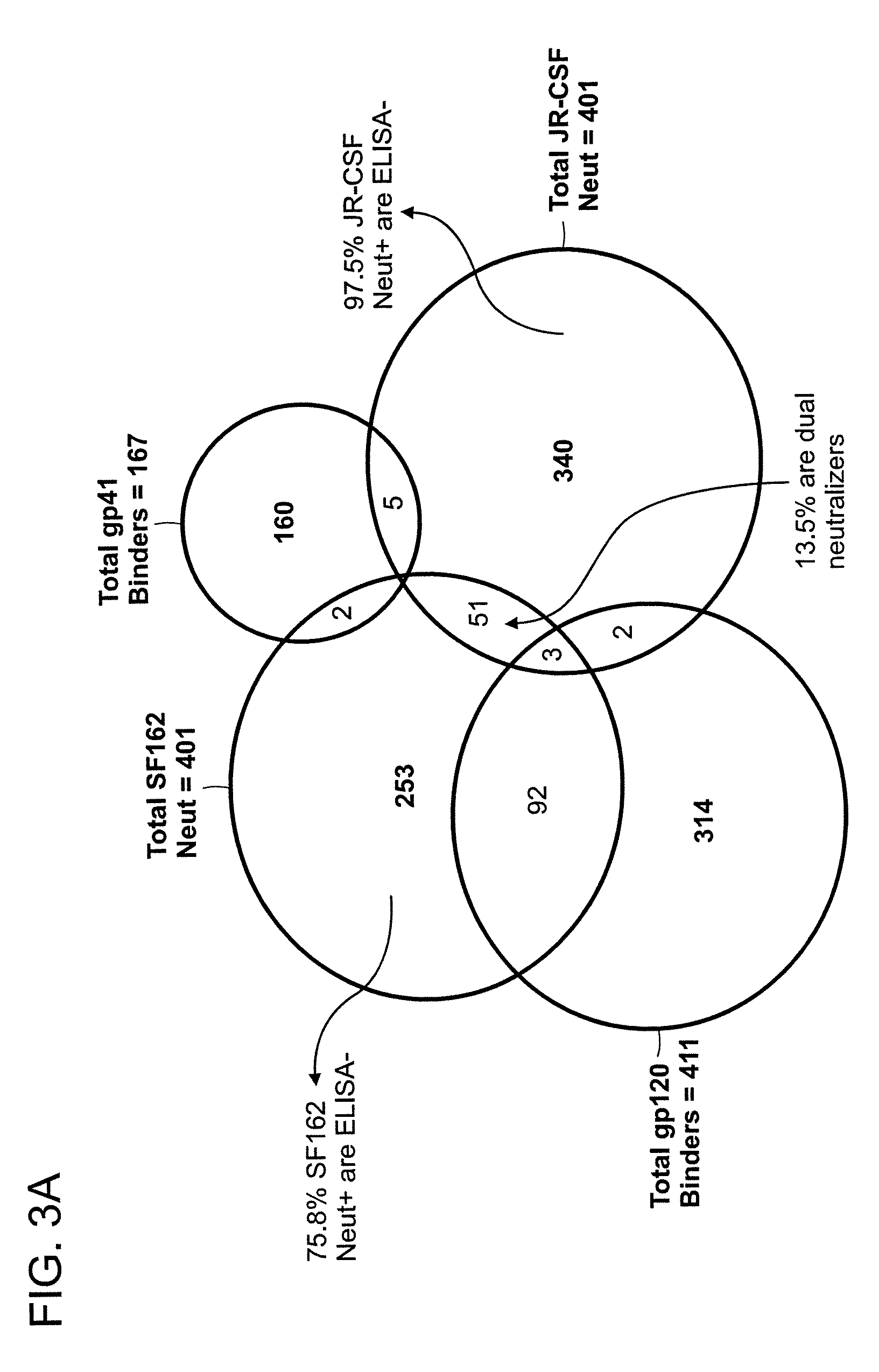
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
Immunoselection of recombinant vesicular stomatitis virus expressing hiv-1 proteins by broadly neutralizing antibodies
InactiveUS20130189754A1Simplifies enrichmentIncrease exposureViral/bacteriophage medical ingredientsElectrical/wave energy microorganism treatmentImmunogenicityVesicular stomatitis virus
The present relation relates to recombinant vesicular stomatitis virus for use as prophylactic and therapeutic vaccines for infectious diseases of AIDS. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Antigenic mimics of discontinuous epitopes of pathogen recognized by broadly neutralizing antibodies
InactiveUS20130039927A1Antibacterial agentsOrganic active ingredientsPeptide sequenceDiscontinuous epitope
The present invention relates to an anti-idiotypic polypeptide scaffold that includes two or more peptide sequences that mimic a discontinuous epitope of a pathogen that is recognized by or induces formation of a broadly neutralizing antibody. Using a fibronectin FNfn10 scaffold bearing two or more modified discontinuous loops, scaffolds that recognize broadly neutralizing antibodies in vitro and from patient serum have been identified. These scaffolds should induce an immune response or mobilize germline specificities to initiate their affinity maturation.
Owner:UNIVERSITY OF ROCHESTER
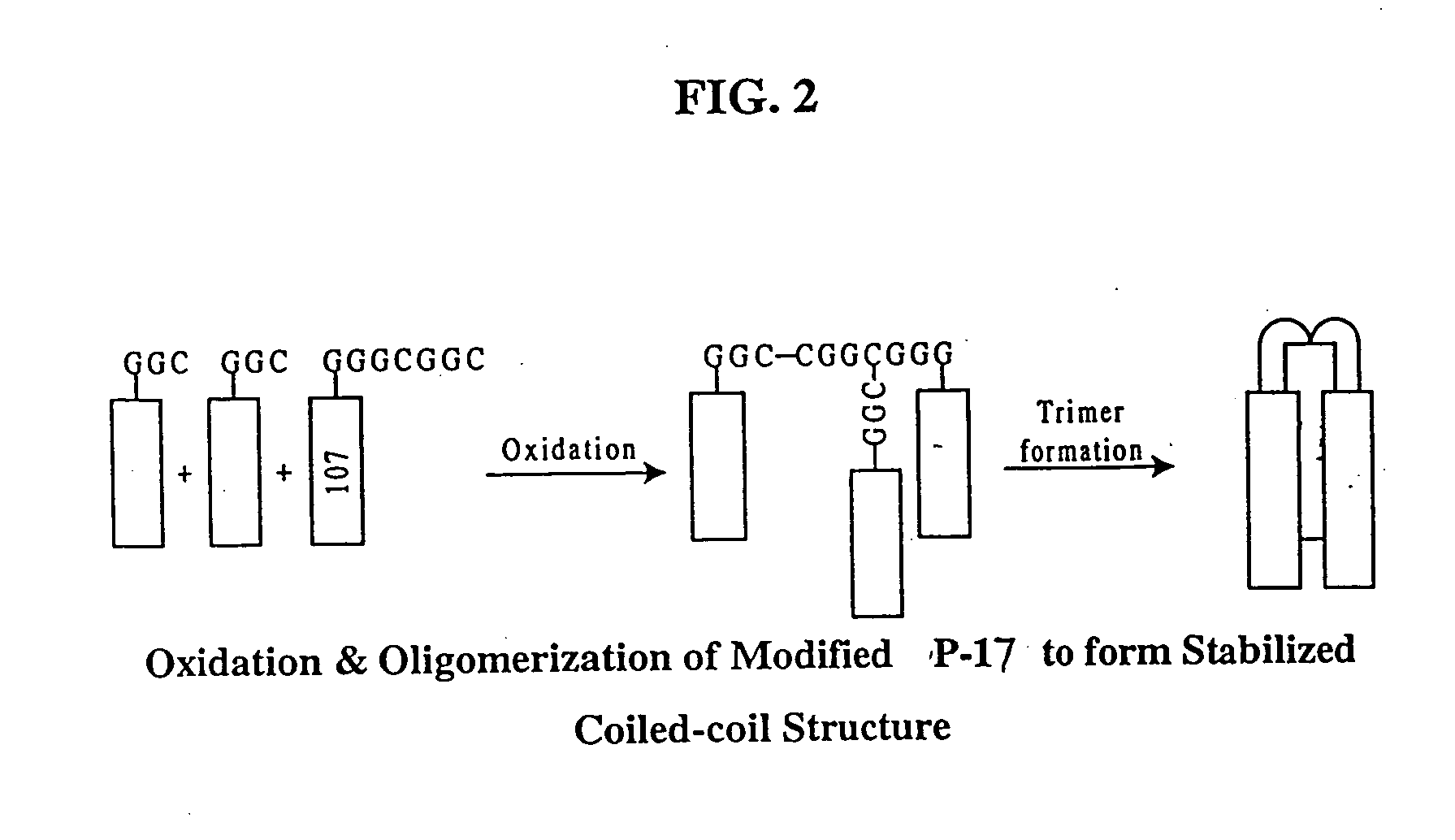
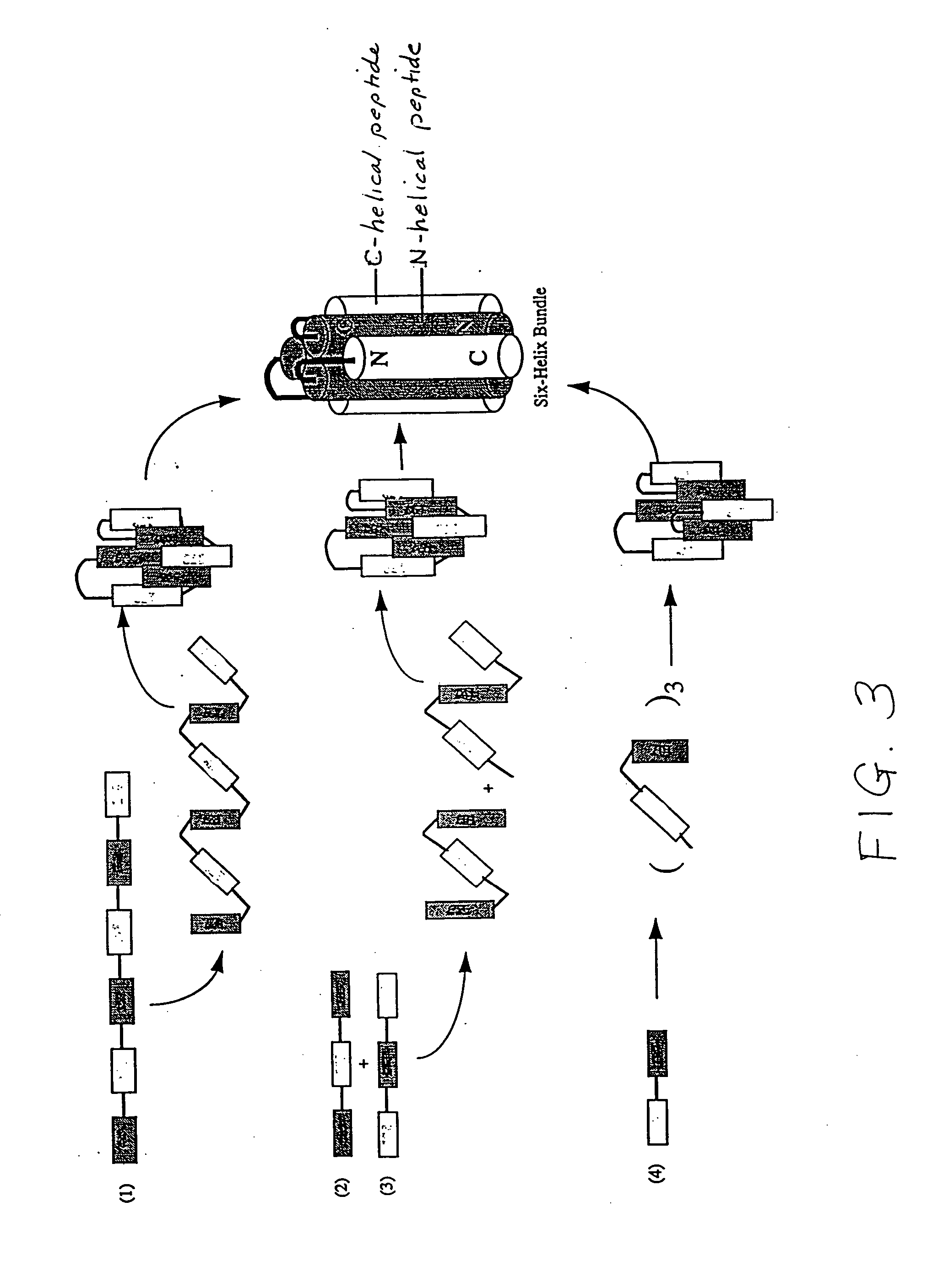
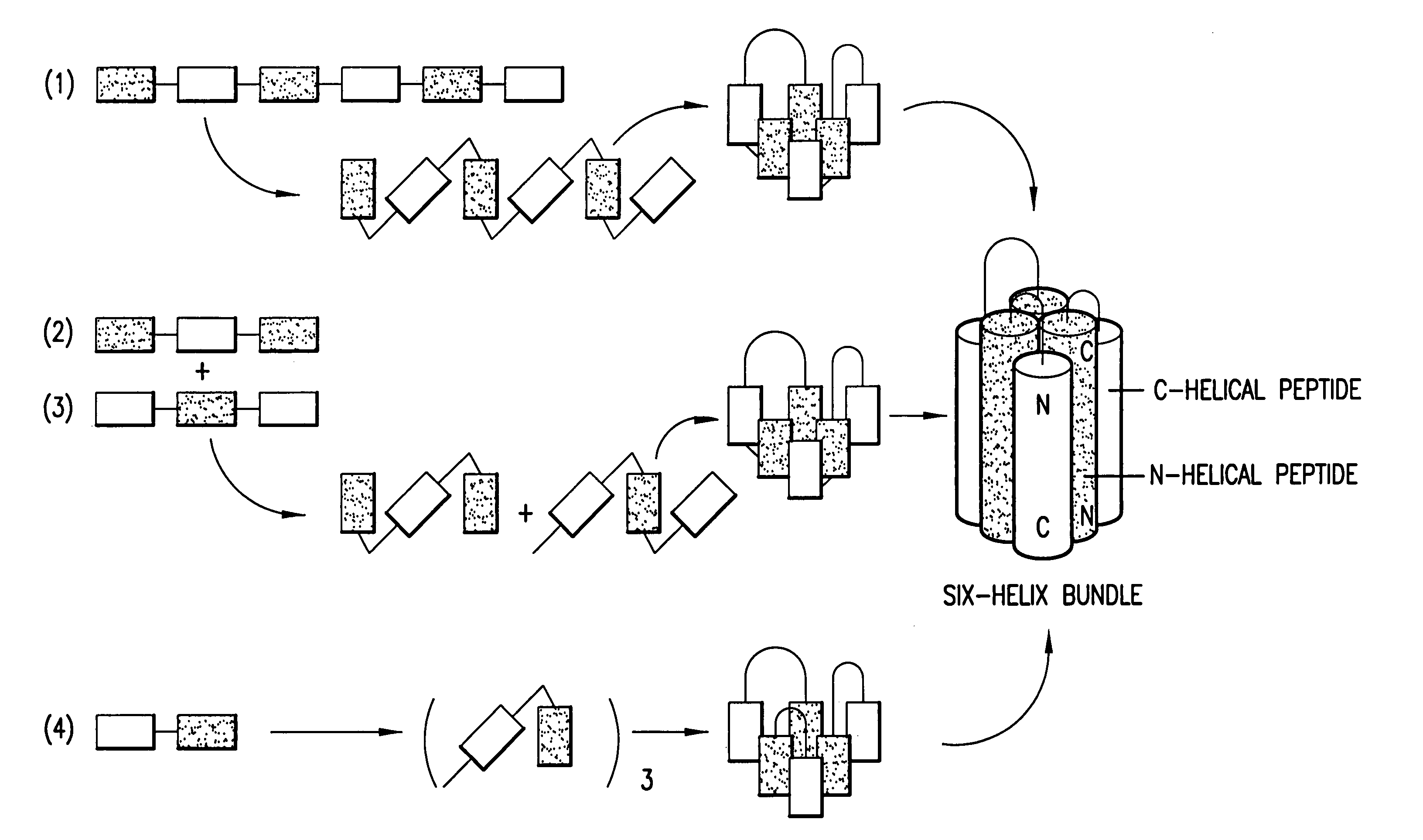
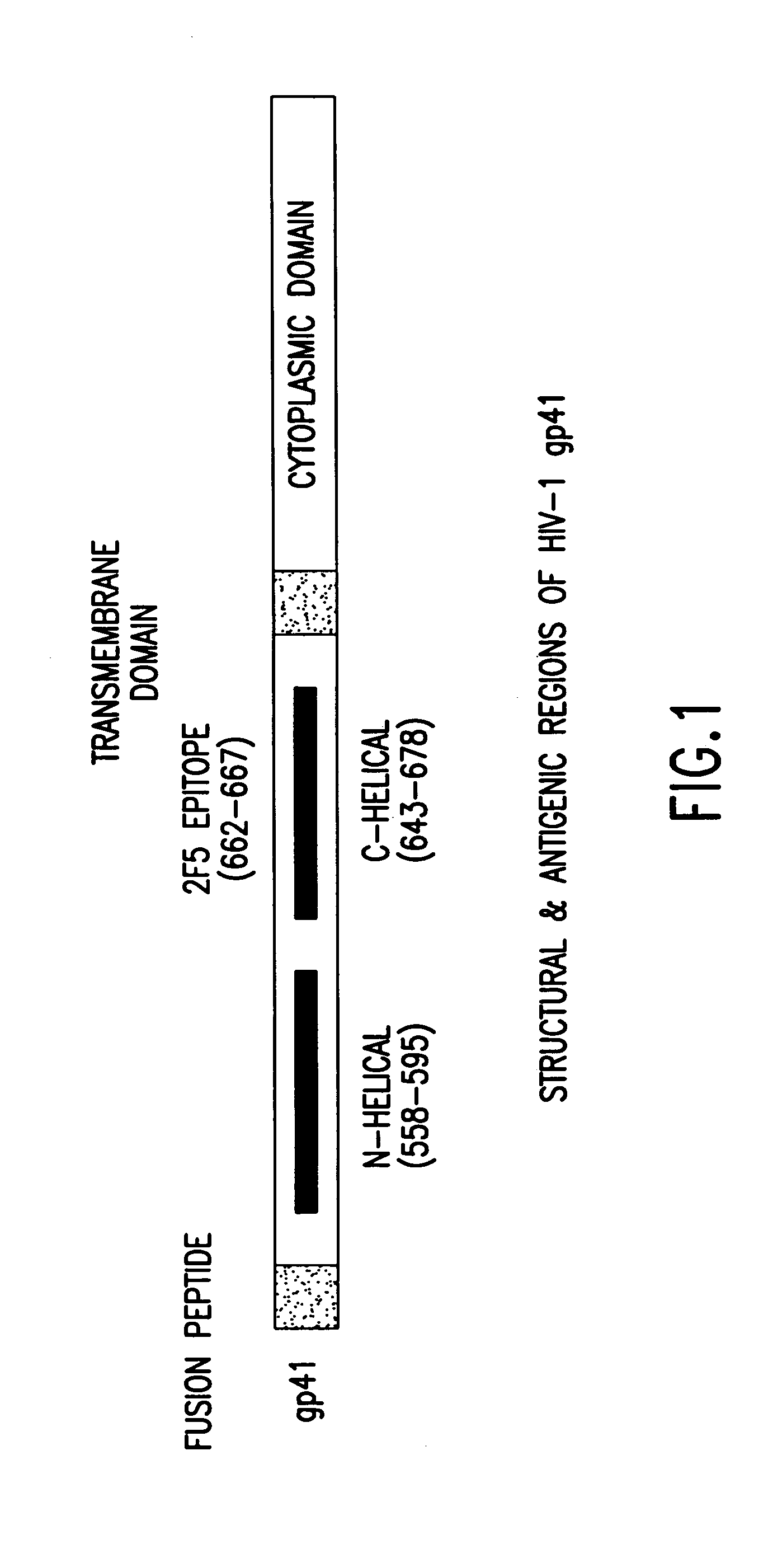
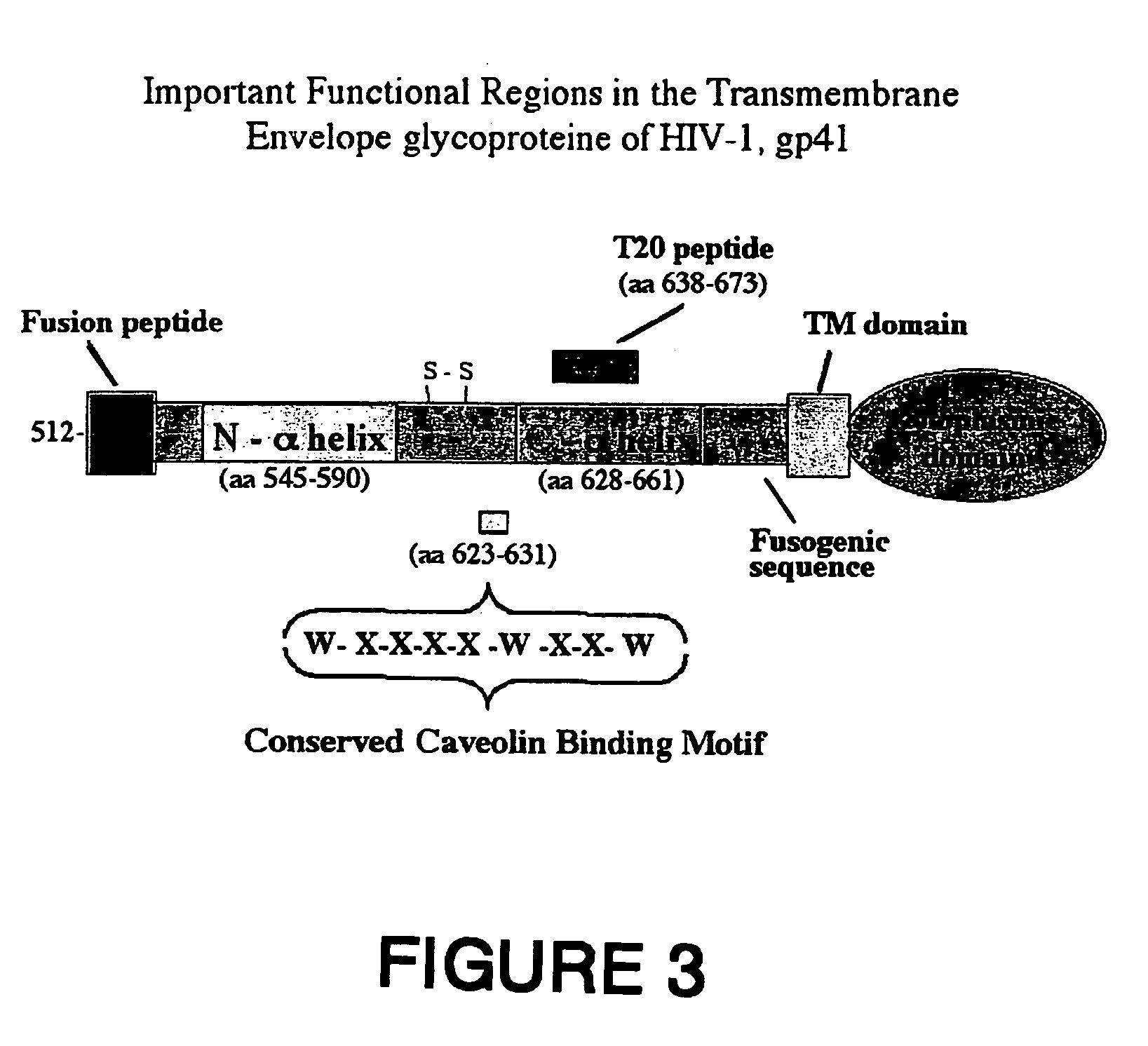
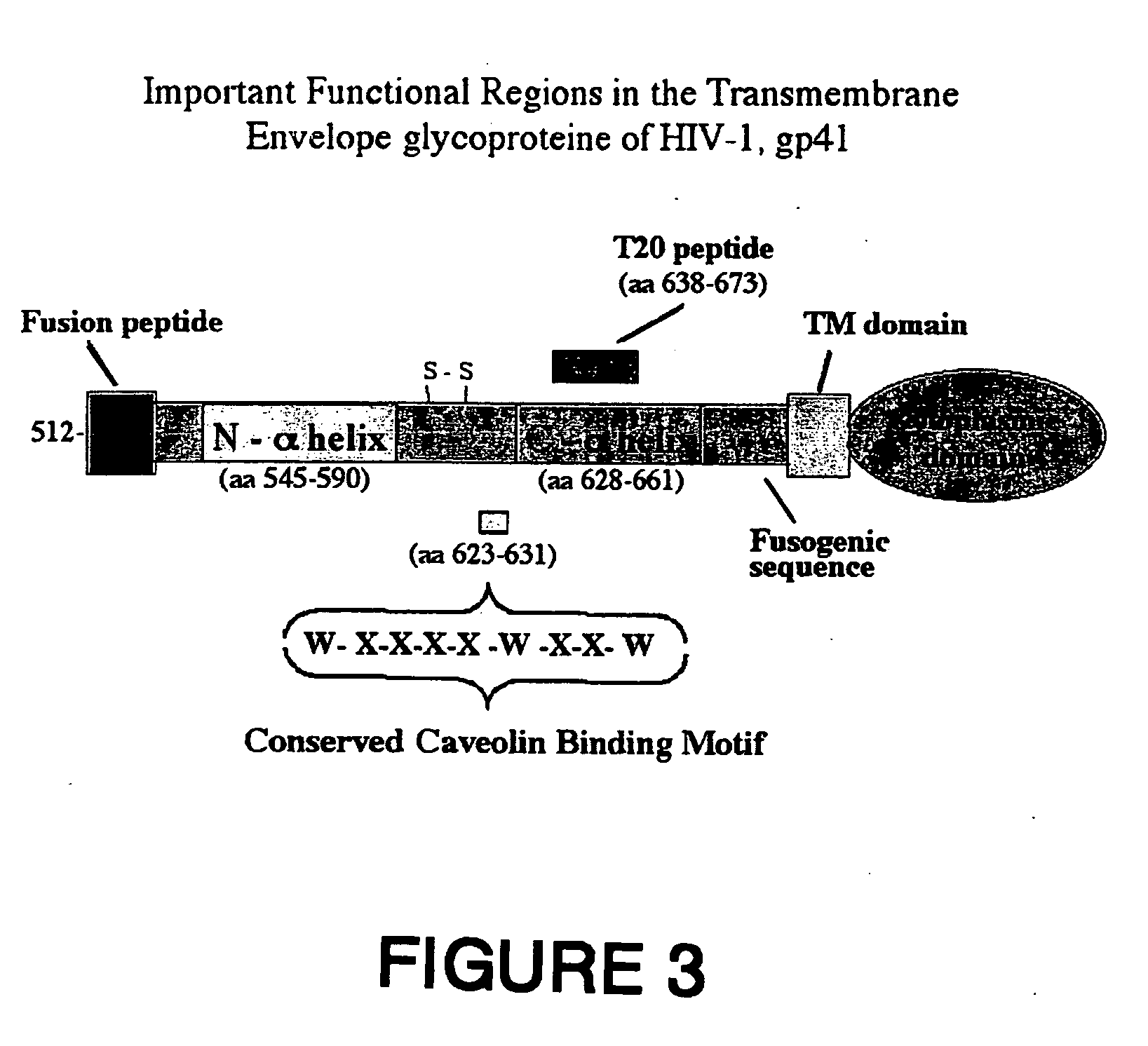
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting "entry-relevant" gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against "entry relevant" gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
Methods of eliciting broadly neutralizing antibodies targeting HIV-1 gp41
The present invention is directed to the induction and characterization of a humoral immune response targeting “entry-relevant” gp41 structures. In its broadest aspect, the present invention is directed to methods of raising a neutralizing antibody response to a broad spectrum of HIV strains and isolates. The present invention targets particular molecular conformations or structures that occur at the cell surface of HIV during viral entry into host cells. Such a humoral response can be generated in vivo as a prophylactic measure in individuals to reduce or inhibit the ability of HIV to infect uninfected cells in the individual's body. Such a response can also be employed to raise antibodies against “entry relevant” gp41 structures. These antibodies can be employed for therapeutic uses, and as tools for further illuminating the mechanism of HIV cell entry.
Owner:UNITED STATES OF AMERICA +1
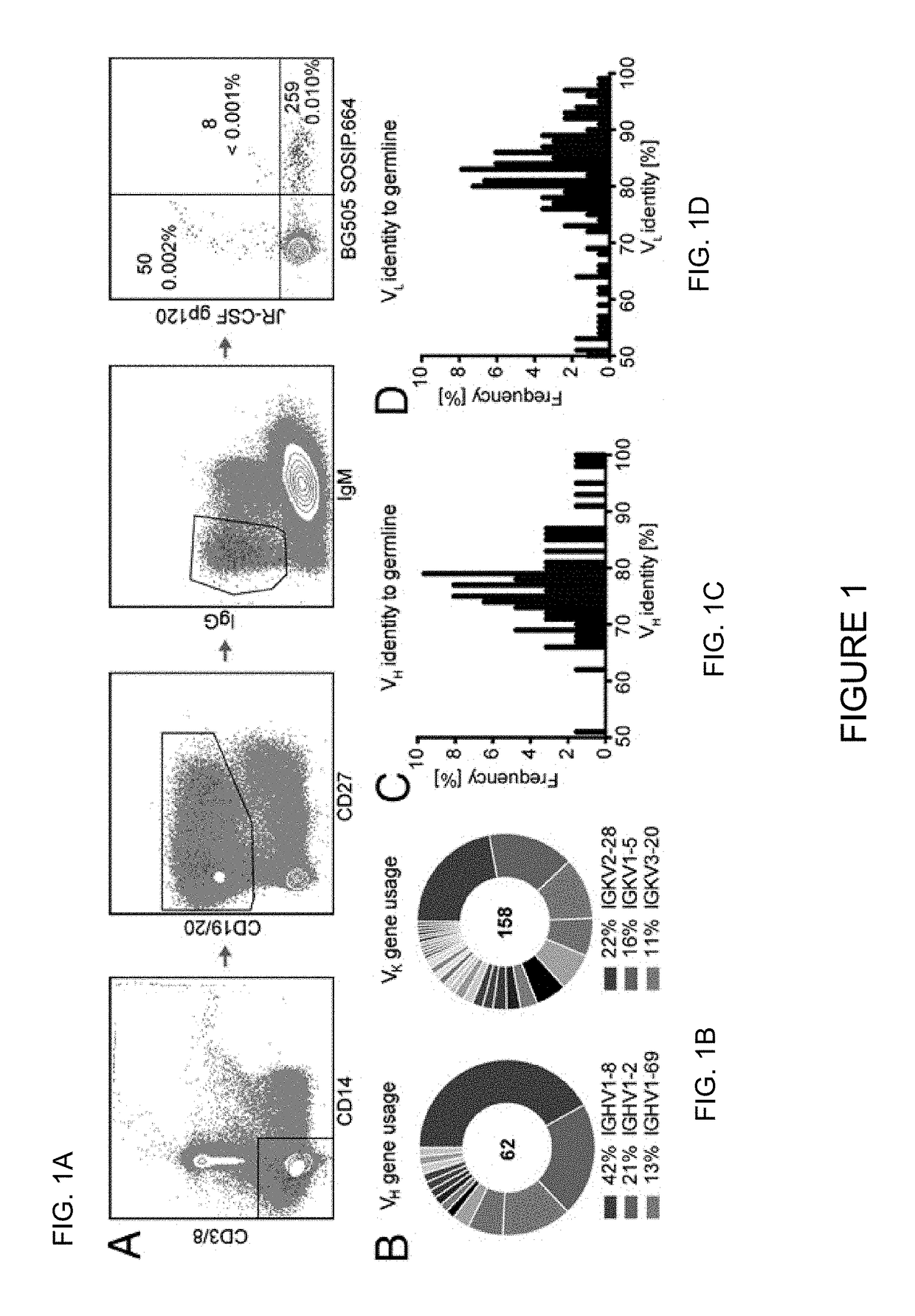
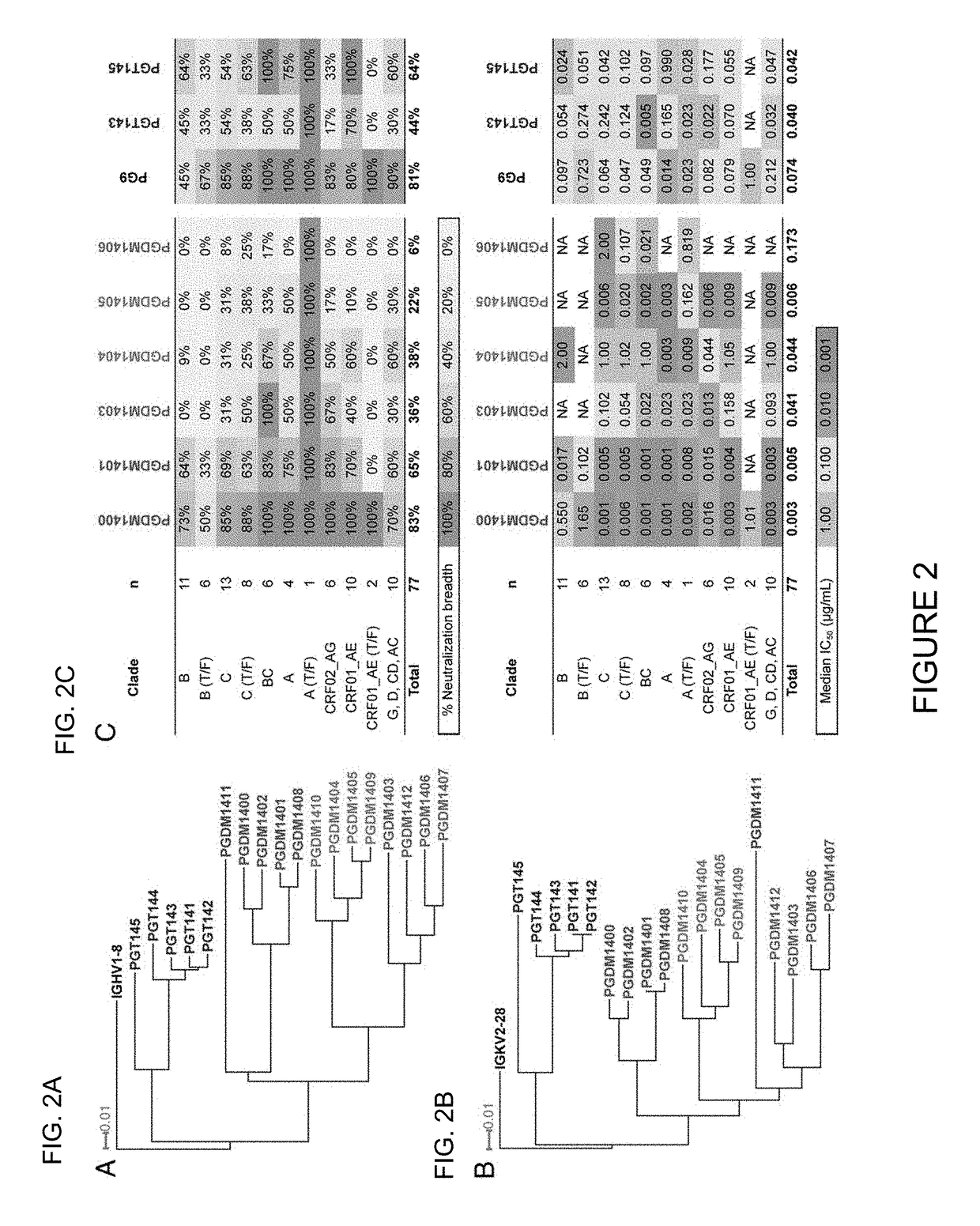
Broadly neutralizing antibody and uses thereof
ActiveUS20150361160A1RangeGenetic material ingredientsImmunoglobulins against virusesNeutralizing antibodyVirology
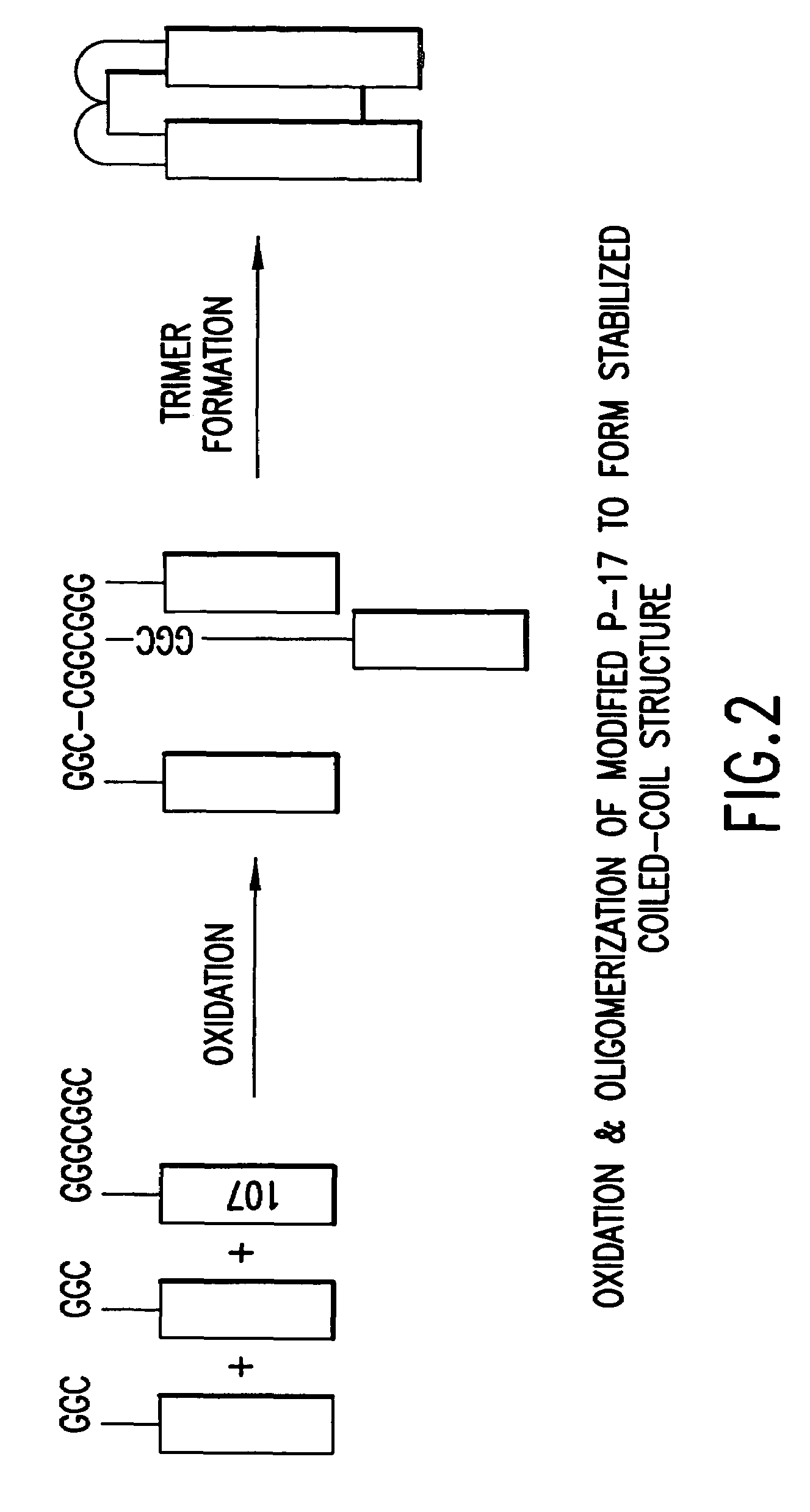
The present invention relates to an exceptionally broad and potent neutralizing antibody which may comprise cross-clade neutralizing coverage of 83% at a median IC50 of 0.003 μg / ml, compositions containing the same and uses thereof.
Owner:CORNELL UNIVERSITY +2
Monoclonal antibodies directed against trimeric forms of the HIV-1 envelope glycoprotein with broad and potent neutralizing activity
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
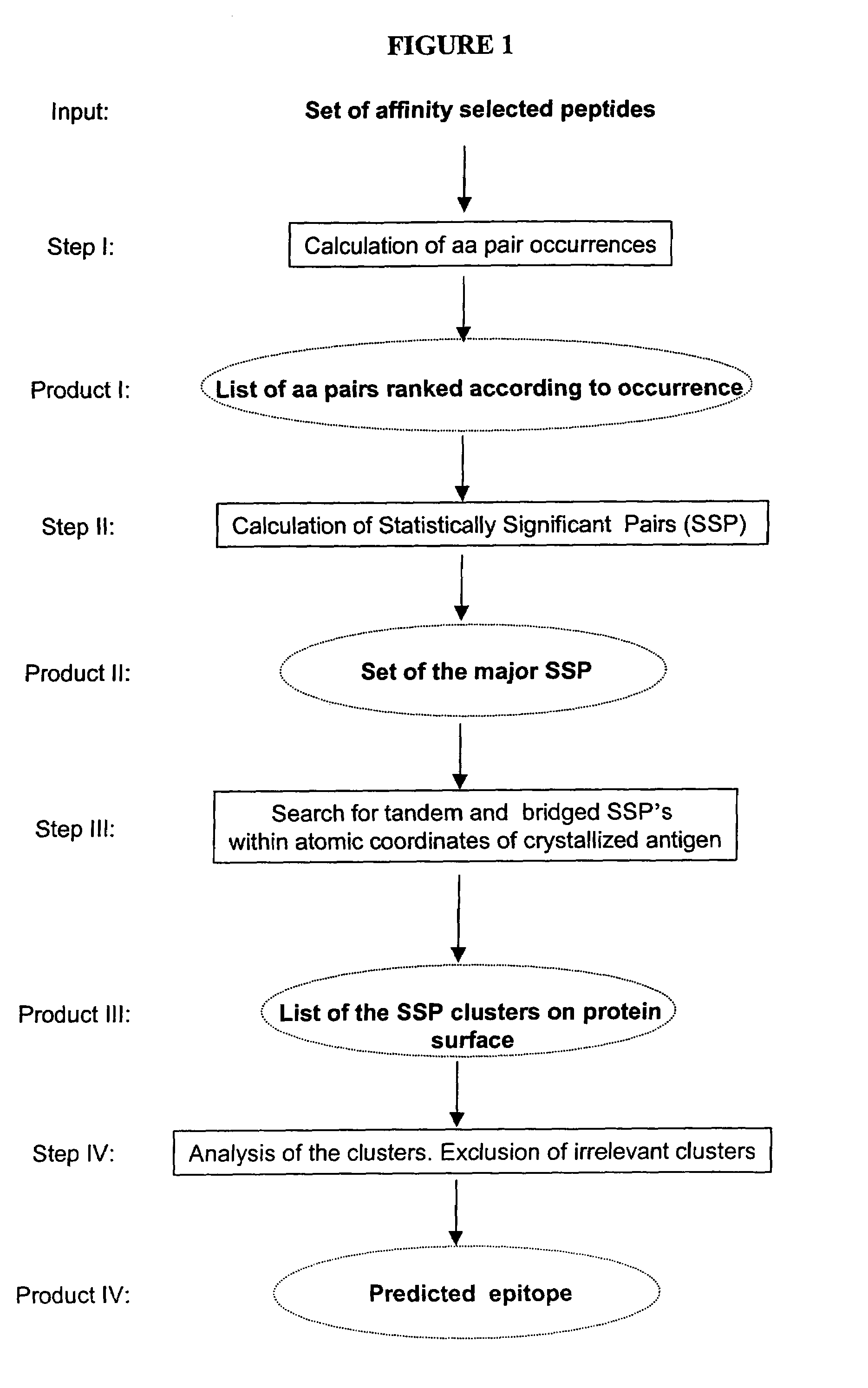
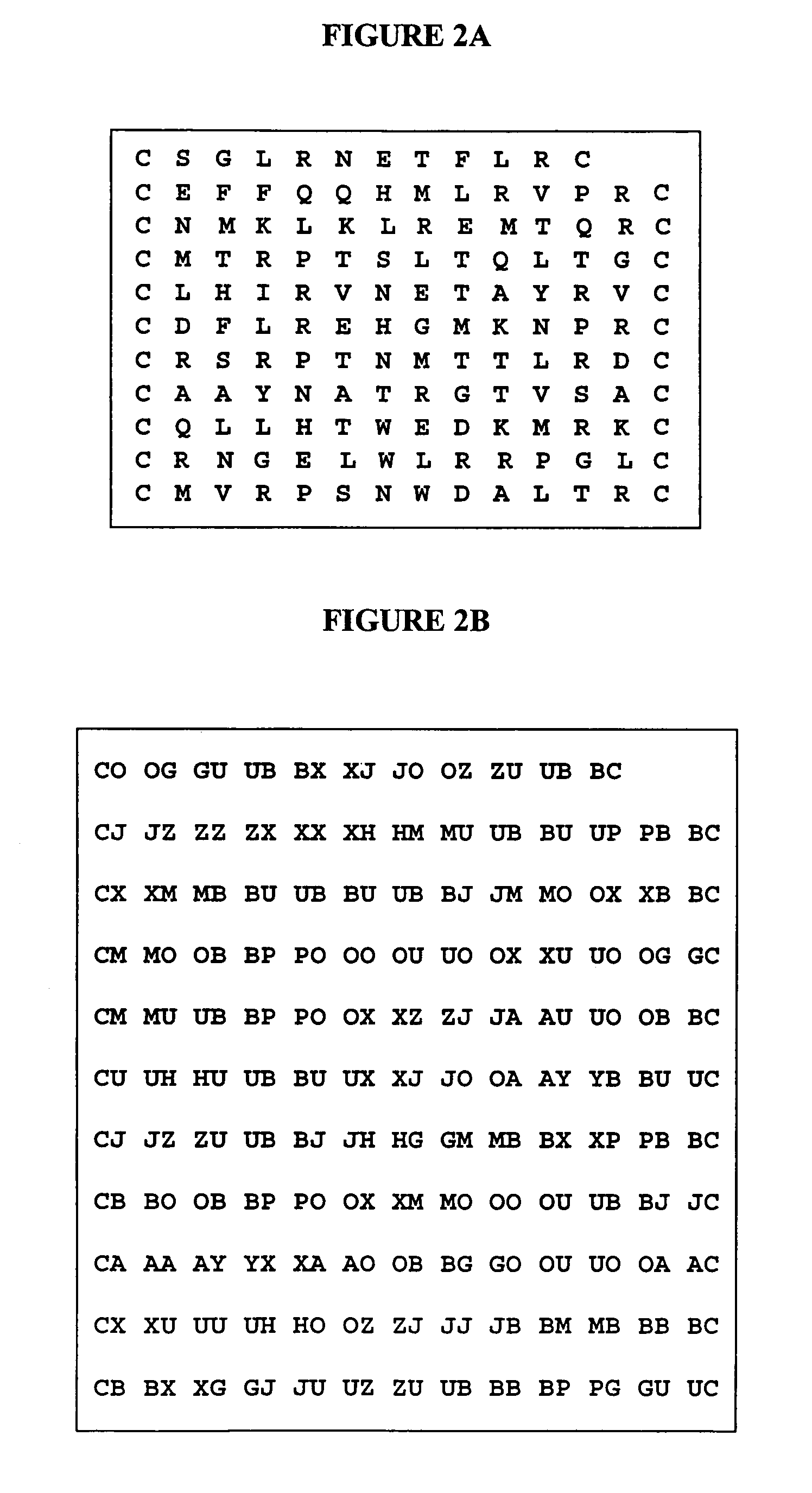
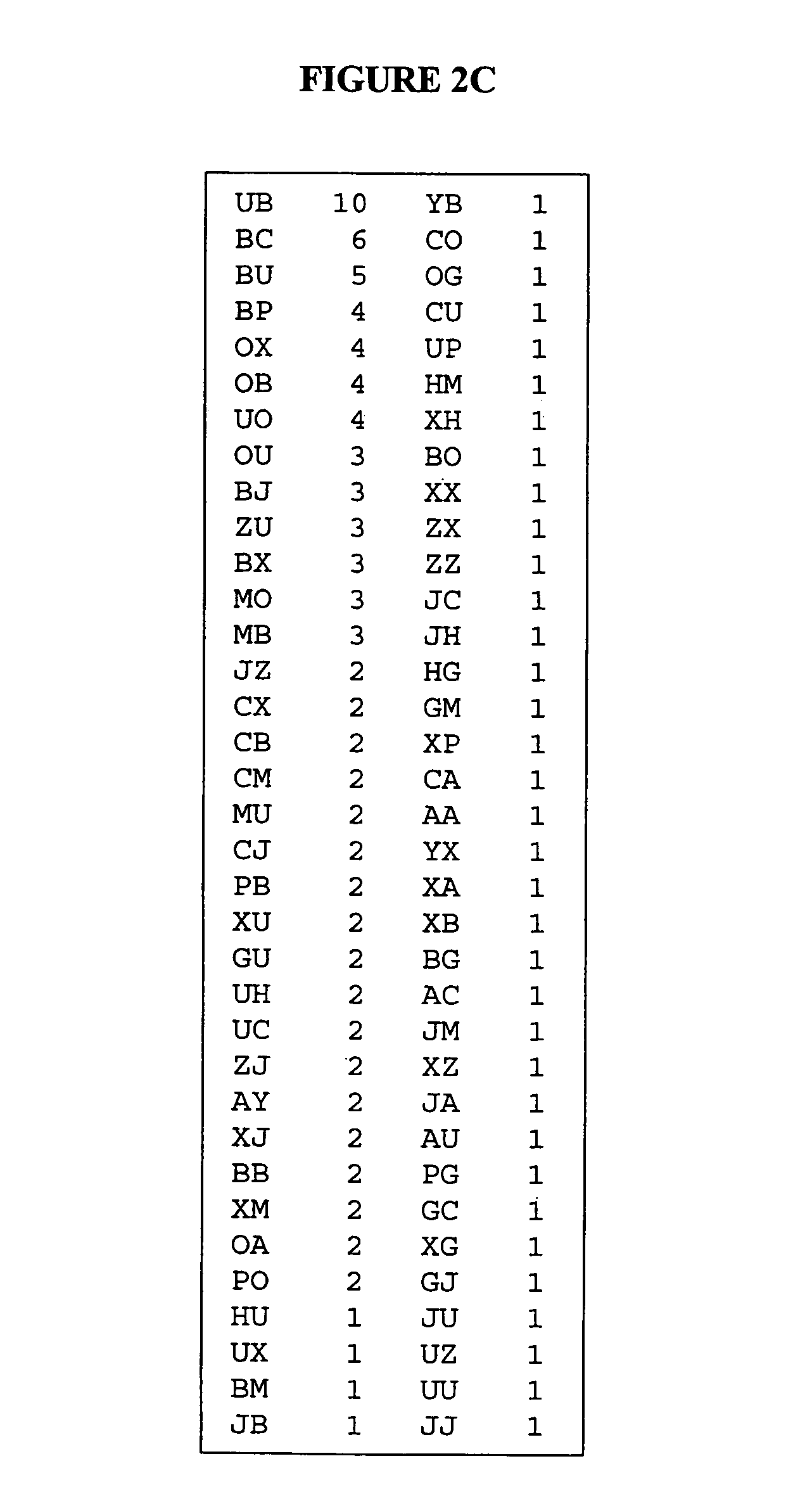
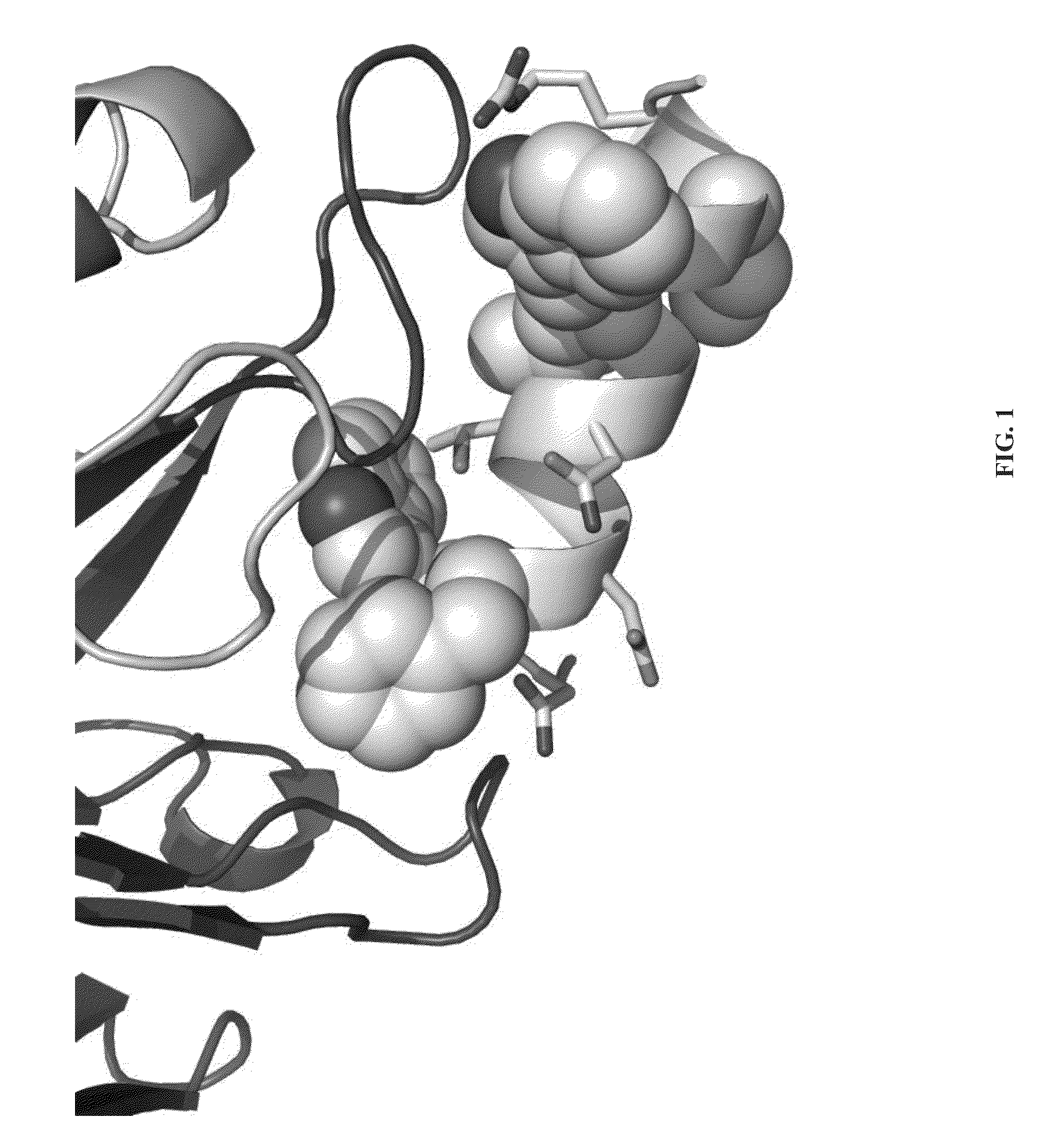
Mapping and reconstitution of a conformational discontinuous binding surface
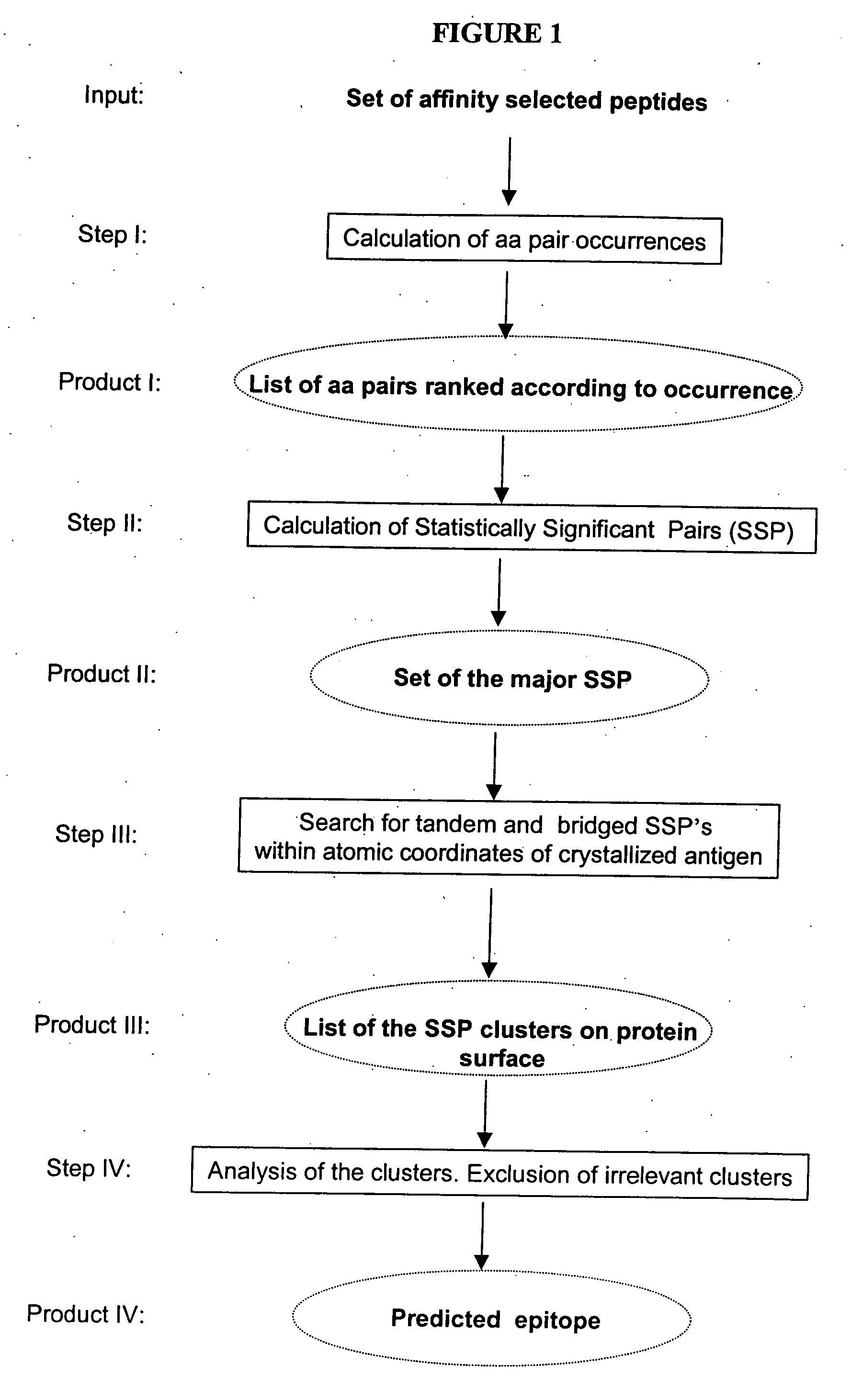
ActiveUS20070005262A1Keep the distancePeptide/protein ingredientsMicrobiological testing/measurementMonoclonal antibodyCrystal structure
The structure of conformational, discontinuous binding surfaces that associate with a binding molecule, preferably the epitopes of monoclonal antibodies (mAbs) may be discovered. The binding molecule is used to select specific peptides from a peptide library that, in turn, are used as a binding surface (epitope) defining database that is applied via a novel computer algorithm to analyze the crystalline-structure of the original binding surface (antigen). An antigenic epitope-mimetic that is recognized by its original mAb may be reconstituted based on the segments of the epitope identified in the prediction. The basic elements of the binding domain on gp120 that is recognized by broadly neutralizing antibody b12 are disclosed, as in their use in making vaccines for preventing or treating HIV.
Owner:RAMOT AT TEL AVIV UNIV LTD
Synthetic peptide vaccines for HIV: the CBD epitope as an effective immunogen to elicit broadly neutralizing antibodies against HIV
Owner:INST PASTEUR +1
Human immunodeficiency virus neutralizing antibodies adn methods of use thereof
ActiveCN103797029AImmunoglobulins against virusesAntiviralsImmunodeficiency virusHuman immunodeficiency
The invention provides broadly neutralizing antibodies directed to epitopes of Human Immunodeficiency Virus, or HIV. The invention further provides compositions containing HIV antibodies used for prophylaxis, and methods for diagnosis and treatment of HIV infection.
Owner:THE ROCKEFELLER UNIV +1
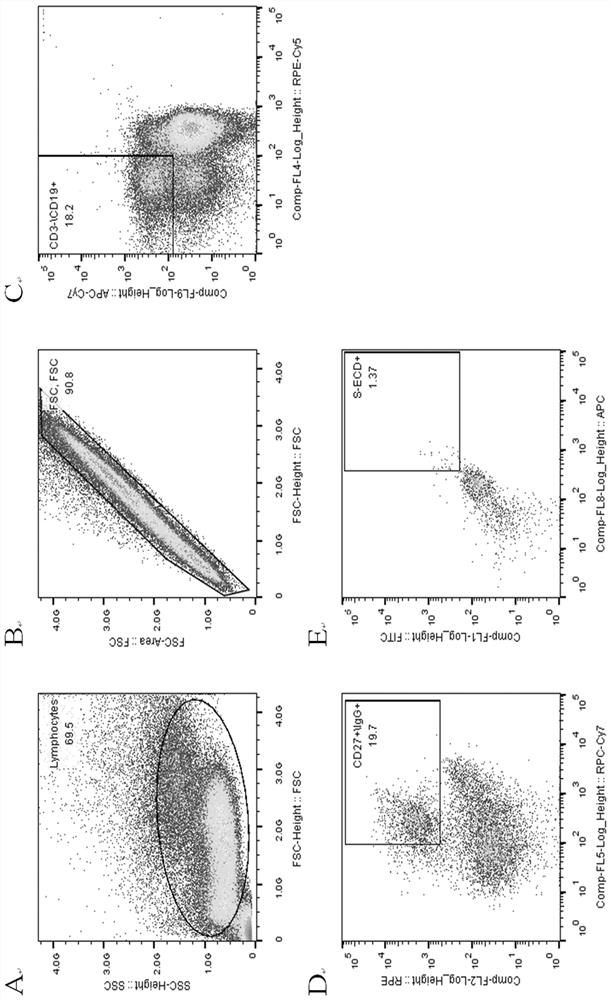
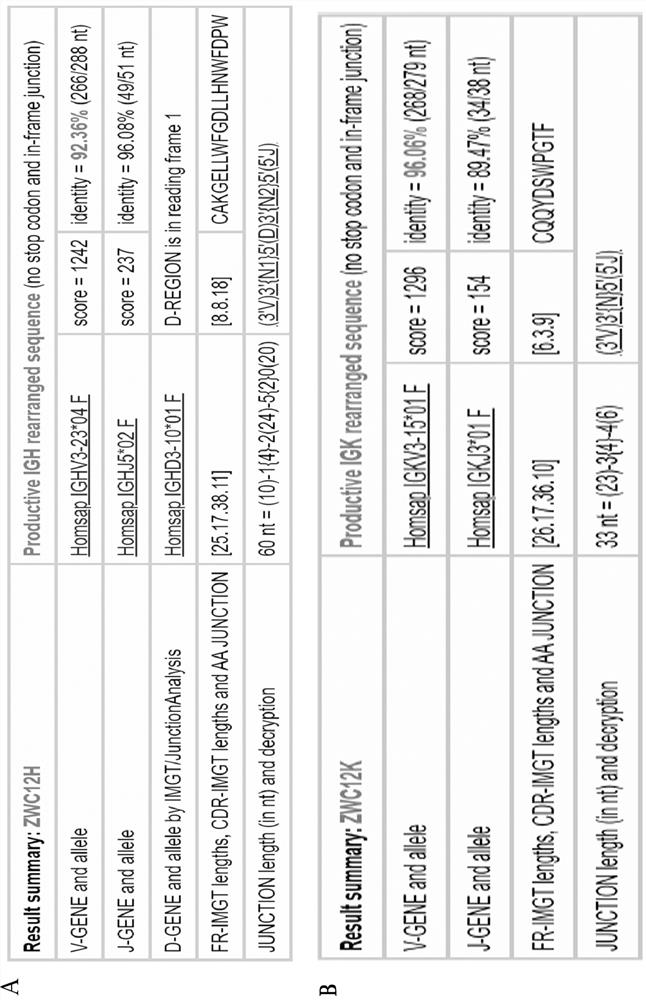
Anti-SARS-CoV-2 completely-humanized broadly-neutralizing antibody ZWC12 and application thereof
ActiveCN114044821AUnique CDR PartitionHigh expressionImmunoglobulins against virusesAntiviralsWild typeVariant strain
The invention discloses an anti-SARS-CoV-2 completely-humanized monoclonal antibody ZWC12. The antibody has a unique CDR partition, and the antigen recognition epitope of the antibody is located in the RBD region of S1 protein. The EC50 of the antibody for neutralizing novel coronavirus wild type pseudoviruses, Alpha variant pseudoviruses, Beta variant pseudoviruses, Gamma variant pseudoviruses, Delta variant pseudoviruses and Omicro variant pseudoviruses is 1.041 [mu]g / mL, 0.124 [mu]g / mL, 0.162 [mu]g / mL, 0.136 [mu]g / mL, 0.411 [mu]g / mL and 0.093 [mu]g / mL respectively, and the antibody has broad-spectrum efficient neutralizing activity on current main variants. The antibody also has the characteristics of high expression, full human source and good stability, is suitable for industrial production, and has important application value for coping with outbreak and prevalence caused by novel coronavirus variants.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Novel synthetic peptide vaccines for HIV: the CBD epitope as an effective immunogen to elicit broadly neutralizing antibodies against HIV
Owner:INST PASTEUR +1
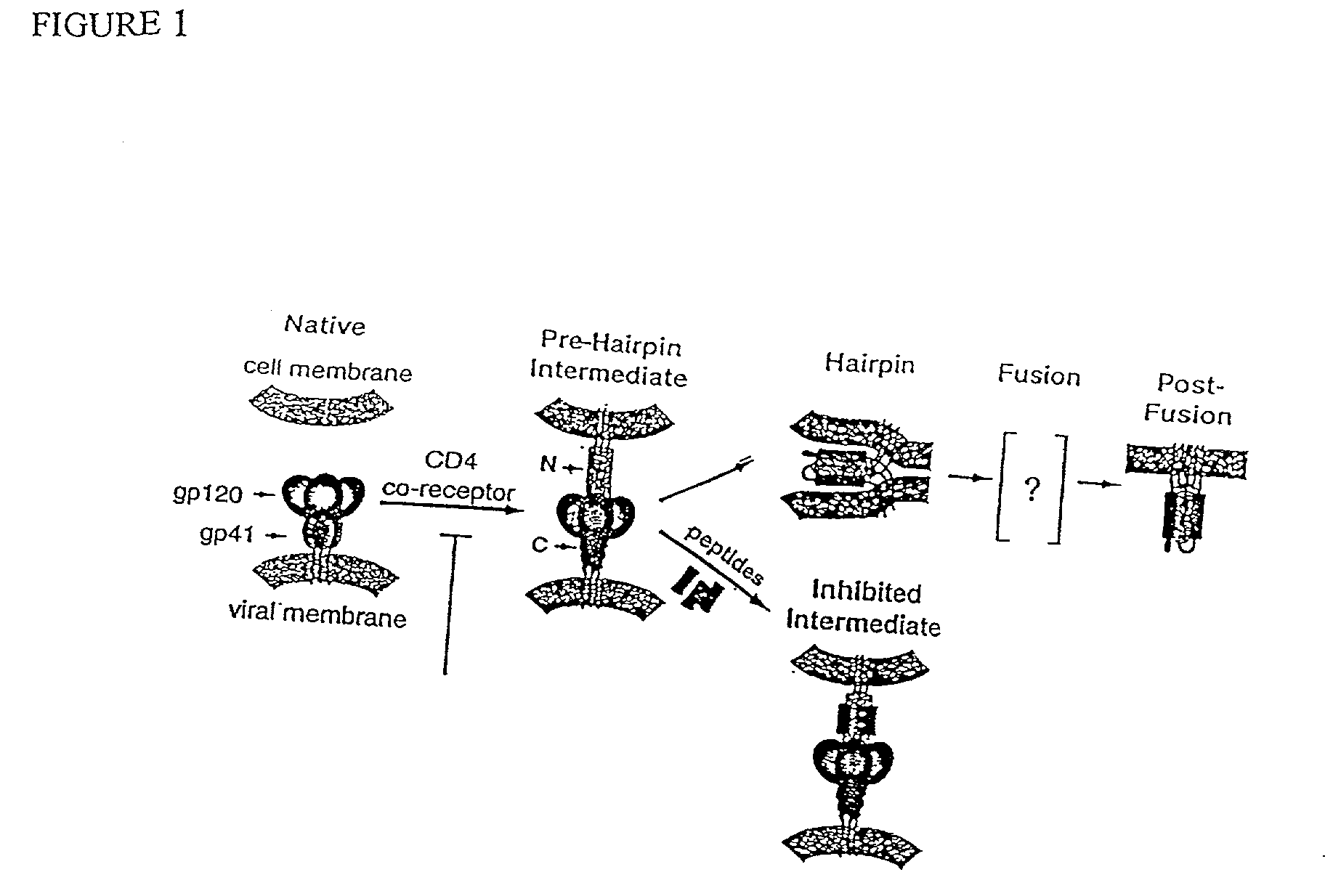
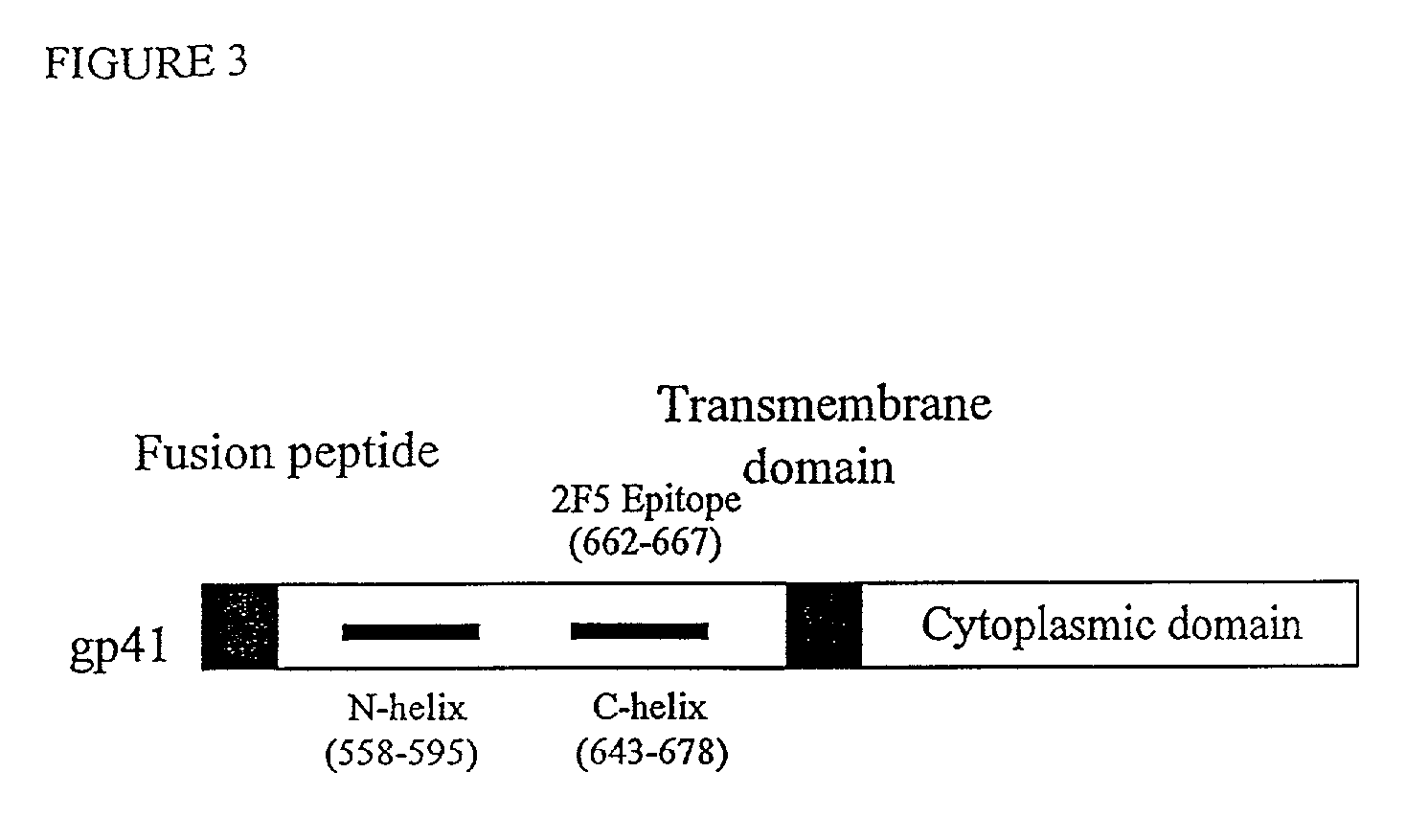
Method for generating immunogens that elicit neutralizing antibodies against fusion-active regions of HIV envelope proteins
The current invention relates to methods of generating immunogens that elicit broadly neutralizing antibodies which target regions of viral envelope proteins such as the gp 120 / gp41 complex of HIV-1. More specifically, the current invention involves using stabilizing peptides modeling the alpha-helical regions of the ectodomain of the HIV-1 transmembrane protein to stabilize fusion-active intermediate structures which can be used as vaccine immunogens.
Owner:PANACOS PHARMACEUTICALS INC
Mapping and reconstitution of a conformational discontinuous binding surface
ActiveUS7587281B2Peptide/protein ingredientsMicrobiological testing/measurementMonoclonal antibodyBinding domain
The structure of conformational, discontinuous binding surfaces that associate with a binding molecule, preferably the epitopes of monoclonal antibodies (mAbs) may be discovered. The binding molecule is used to select specific peptides from a peptide library that, in turn, are used as a binding surface (epitope) defining database that is applied via a novel computer algorithm to analyze the crystalline-structure of the original binding surface (antigen). An antigenic epitope-mimetic that is recognized by its original mAb may be reconstituted based on the segments of the epitope identified in the prediction. The basic elements of the binding domain on gp120 that is recognized by broadly neutralizing antibody b12 are disclosed, as in their use in making vaccines for preventing or treating HIV.
Owner:RAMOT AT TEL AVIV UNIV LTD
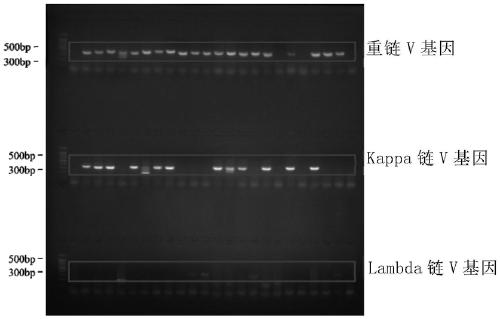
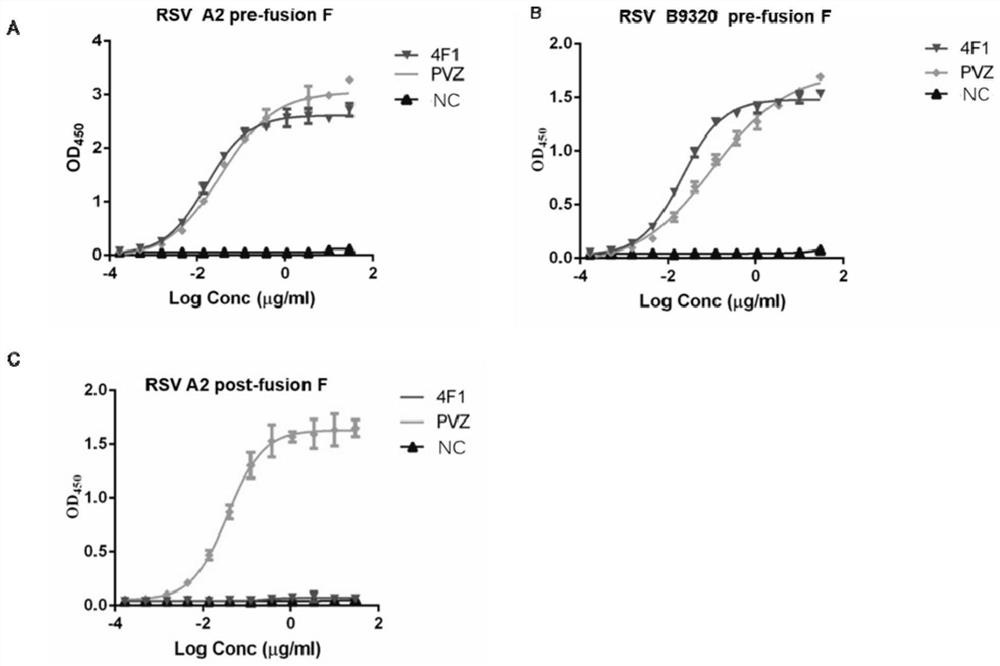
Anti-respiratory syncytial virus fully human broad-spectrum neutralizing antibody 4F1 and application thereof
ActiveCN111606993AHigh neutralizing activityInhibit or prevent infestationImmunoglobulins against virusesAntiviralsAntibody fragmentsF protein
The invention discloses a fully-human neutralizing antibody of anti-respiratory syncytial virus fusion protein and application of the fully-human neutralizing antibody. Specifically, the invention discloses a fully human monoclonal antibody 4F1 aiming at respiratory syncytial virus fusion protein (F protein) and pre-fusion F protein (preF protein), a nucleic acid sequence for encoding an antibodyand an antibody fragment, and a preparation method of the nucleic acid sequence. In-vitro and in-vivo experiments prove that the 4F1 antibody can effectively prevent and control RSV infection, has lowimmunogenicity for a human body, can avoid antibody-mediated immunological rejection of human anti-mouse and other species sources, and can be clinically used for preventing and treating respiratorysyncytial virus infection.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI +1
Broadly neutralizing antibody and uses thereof
The present invention relates to an exceptionally broad and potent neutralizing antibody which may comprise cross-clade neutralizing coverage of 83% at a median IC50 of 0.003 μg / ml, compositions containing the same and uses thereof.
Owner:CORNELL UNIVERSITY +2
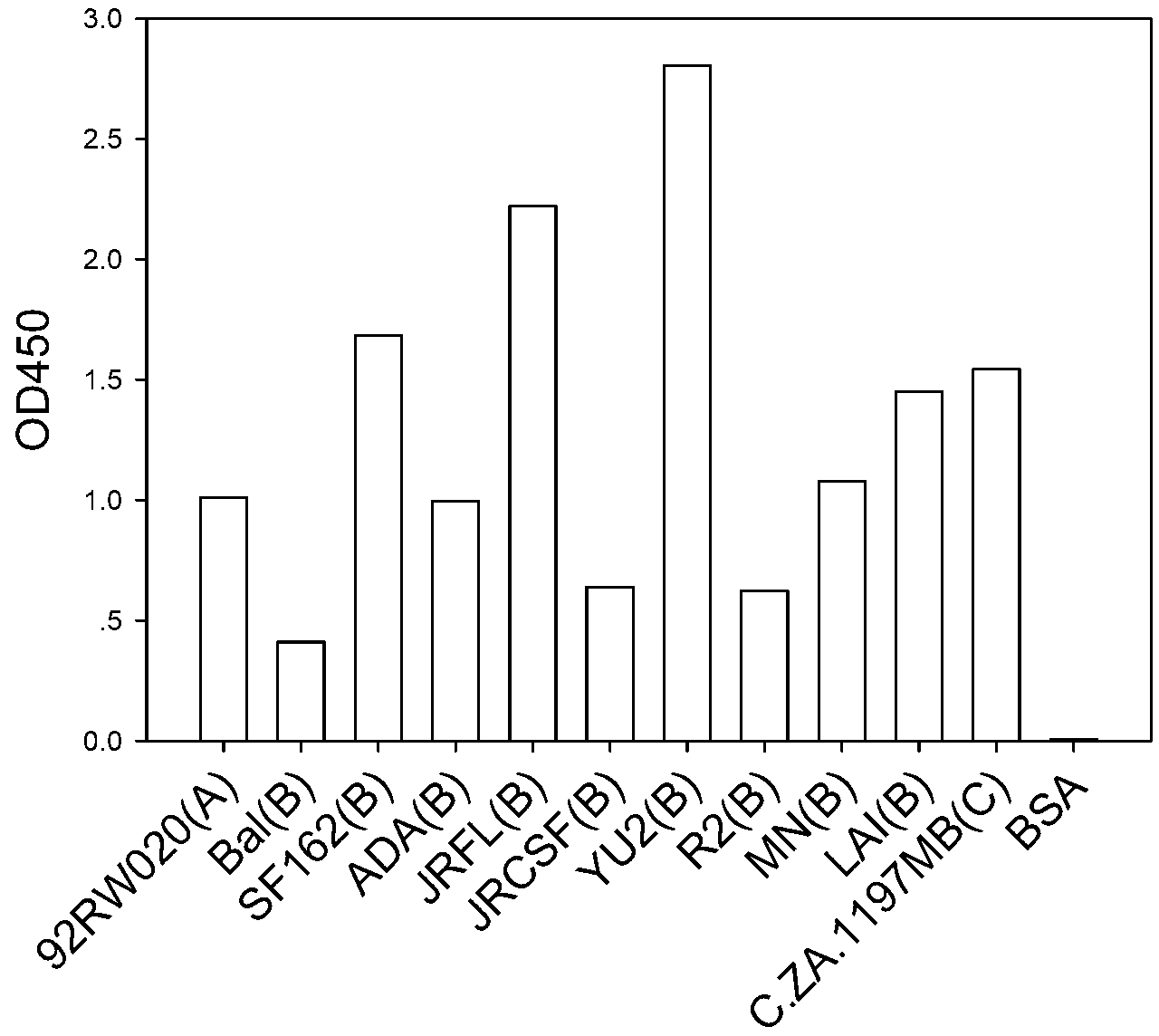
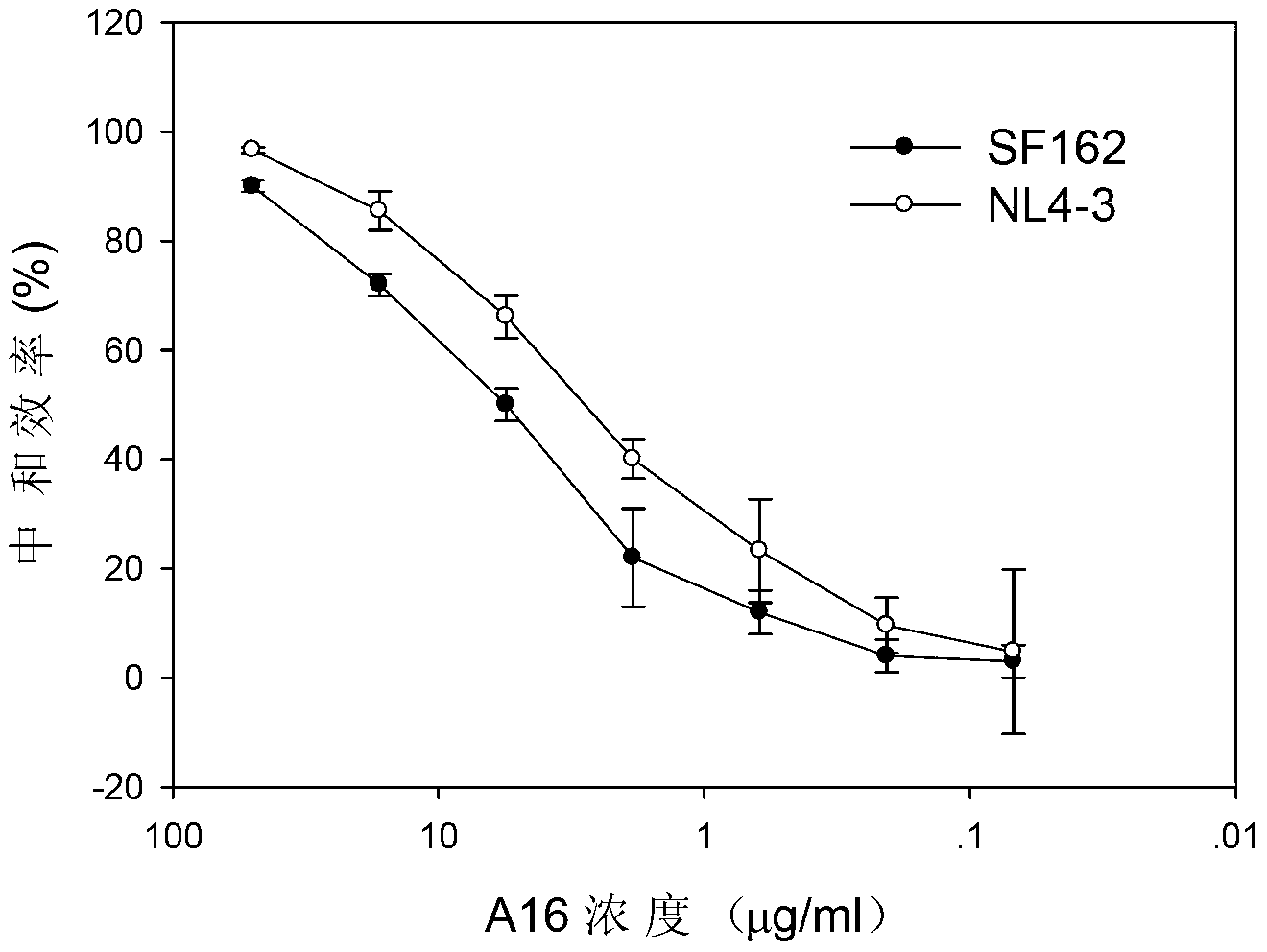
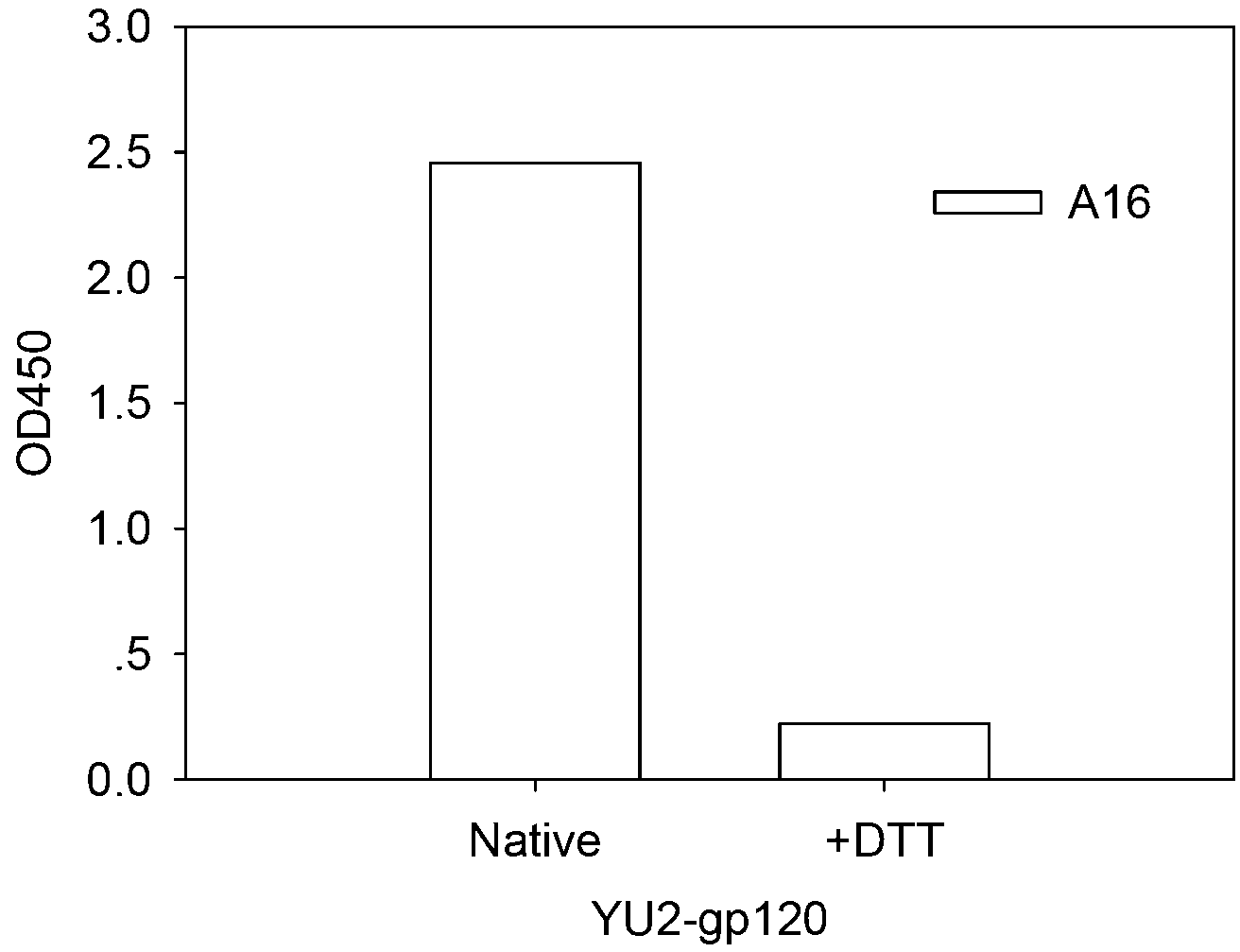
Human HIV broad spectrum neutralization antibody A16, associated biological material and applications thereof
The invention discloses human HIV broad spectrum neutralization antibody A16, an associated biological material and applications thereof. The A16 comprises an antibody heavy chain Fd fragment and an antibody light chain, wherein the heavy chain Fd fragment comprises a variable region VH and a constant region subunit CH1, the VH and the VL comprise determinant complementarity regions and framework regions, the determinant complementarity region comprises CDR1, CDR2 and CDR3, amino acid sequences of the CDR1, the CDR2 and the CDR3 of the VL are respectively represented by sites 26-31, sites 49-51 and sites 88-98 of a sequence 3, and amino acid sequences of the CDR1, the CDR2 and the CDR3 of the VH are respectively represented by sites 26-33, sites 51-58 and sites 97-115 of a sequence 4. The A16 can be adopted to prepare drugs, vaccines and diagnostic reagents for treatments, preventions and diagnosis of HIV infection and AIDS.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
HIV-1 broadly neutralizing antibody and use thereof
ActiveCN107022027ABiological material analysisImmunoglobulins against virusesNeutralizing antibodyVirology
The present invention relates to a HIV-1 broadly neutralizing antibody which can be specifically bonded with HIV-1gp120. The present invention also relates to a preparation method and use of the HIV-1 broadly neutralizing antibody.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION +1
HIV-1 broadly neutralizing antibody and use thereof
ActiveCN109251246AWidely neutralizedImmunoglobulins against virusesAntiviralsBroadly neutralizing antibodyHeavy chain
The present invention relates to a HIV-1 broadly neutralizing antibody and use thereof, and relates to HIV-1 broadly neutralizing antibodyies. The HIV-1 broadly neutralizing antibody comprises a heavychain variable region and a light chain variable region, and can be specifically bonded with HIV-1gp120. The present invention also relates to a preparation method, and the use of the HIV-1 broadly neutralizing antibody.
Owner:NANKAI UNIV +1
HIV-1 envelope proteins and fragments thereof that possess epitopes recognized by broadly neutralizing antibodies
ActiveUS20150246111A1Maximum efficiencyHigh strengthViral antigen ingredientsVirus peptidesHiv 1 envelopeVirology
Owner:RGT UNIV OF CALIFORNIA
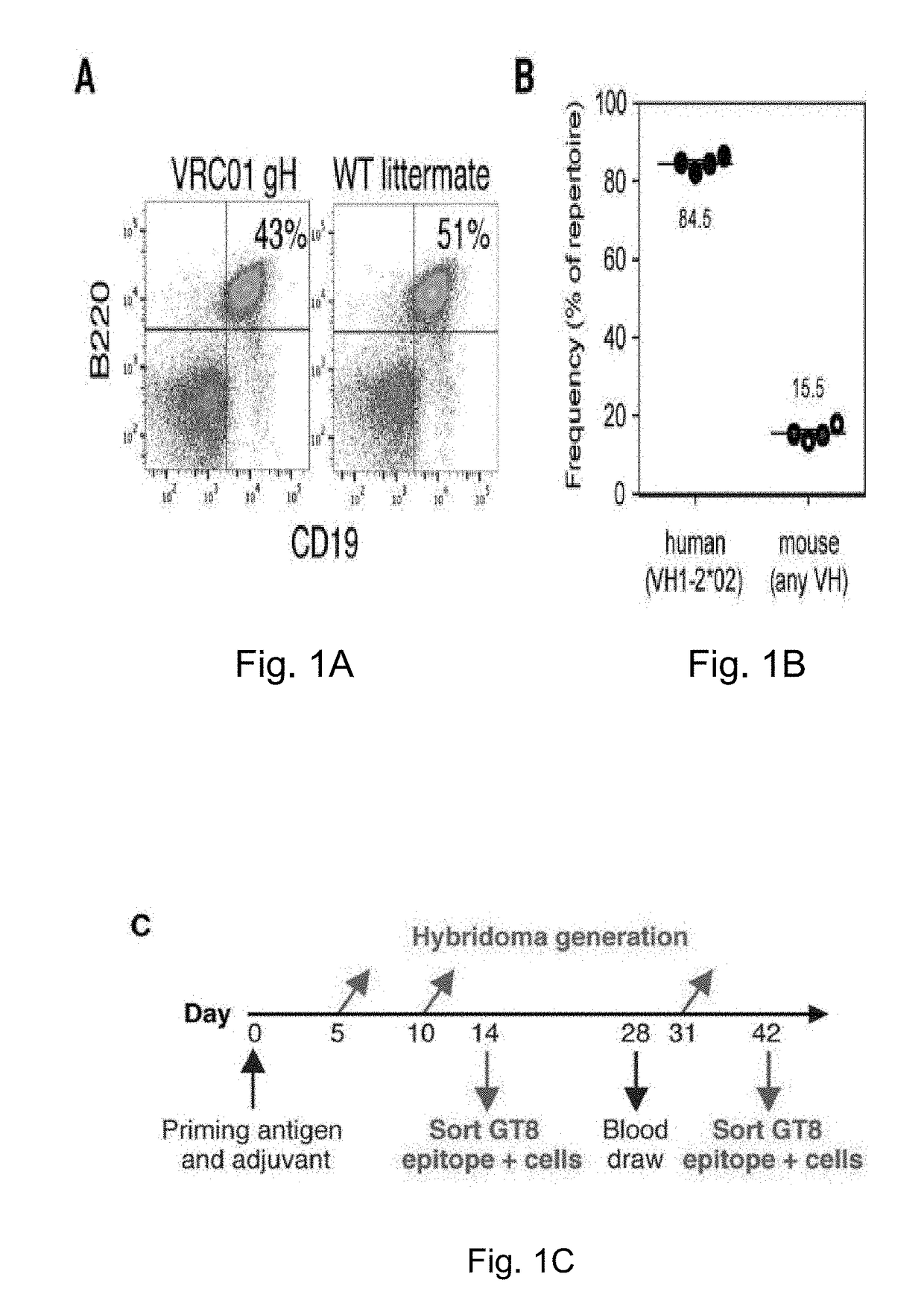
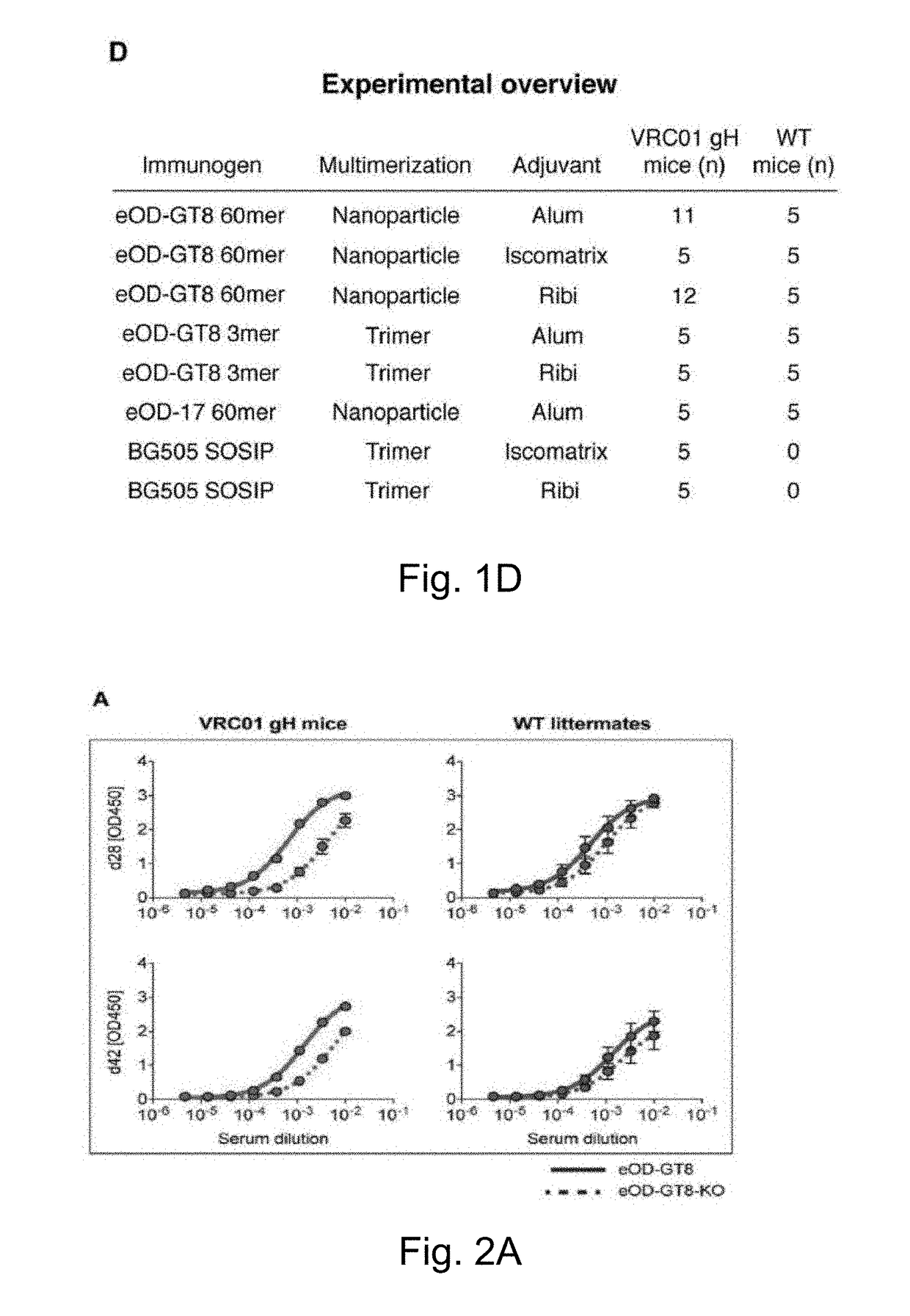
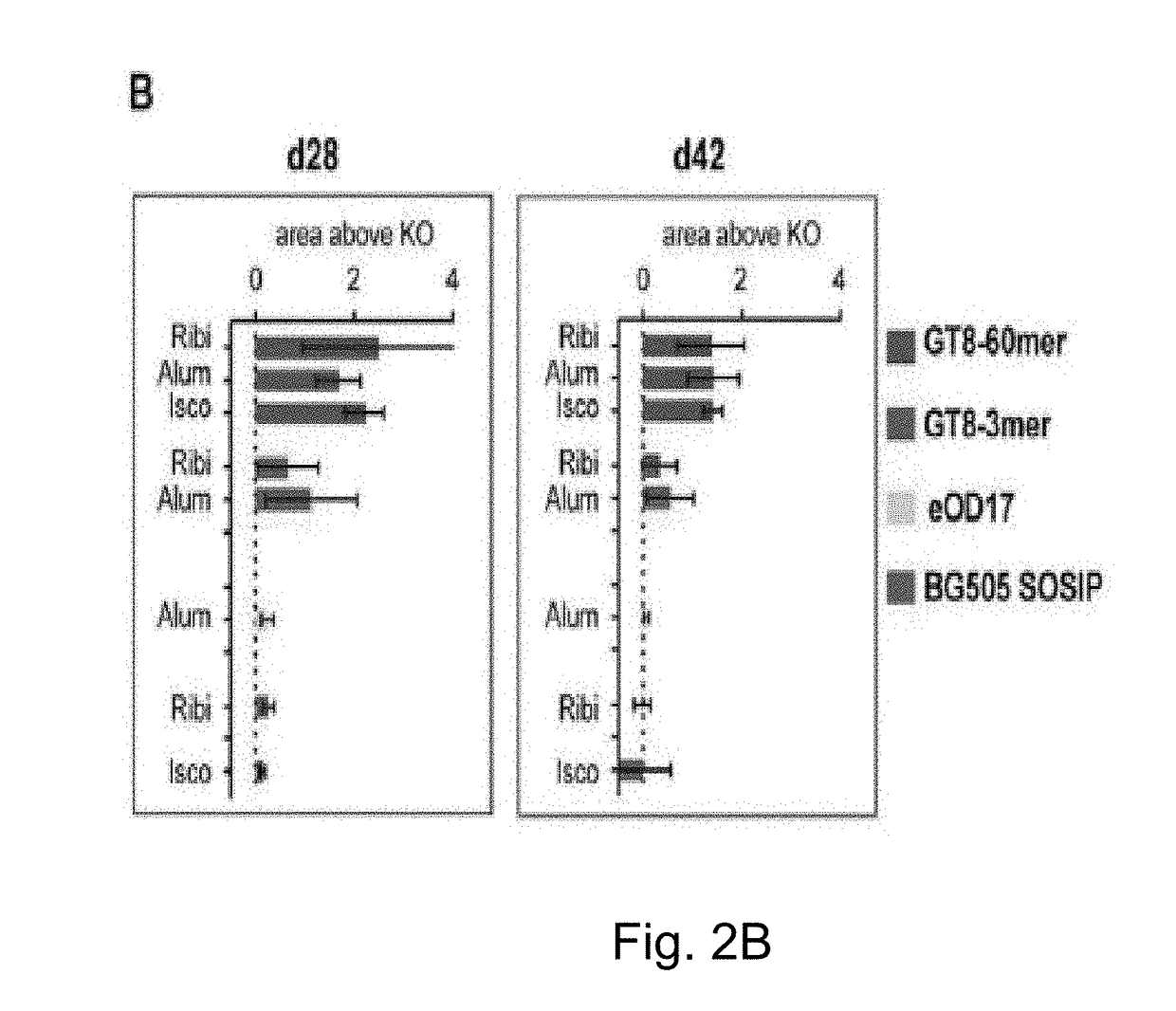
Engineered outer domain (EOD) of HIV gp120, mutants and use thereof
ActiveUS20180194809A1Elicit immune responseCompounds screening/testingViral antigen ingredientsHeavy chainMutant
The present invention relates to engineered outer domain (eOD) immunogens of HIV gp120 and mutants thereof and methods of making and using the same. The present invention also includes fusions of eOD to various protein multimers to enhance immunogenicity. The mutant eODs bind to neutralizing antibody precursors. The mutant eODs can activate germline precursors on the pathway to eliciting a broadly neutralizing antibody (bnAb) response. The invention also relates to immunized knock-in mice expressing germline-reverted heavy chains. Induced antibodies showed characteristics of bnAbs and mutations that favored binding to near-native HIV-1 gp120 constructs. In contrast, native-like immunogens failed to activate precursors. The invention also relates to rational epitope design that can prime rare B cell precursors for affinity maturation to desired targets.
Owner:INT AIDS VACCINE INITIATIVE +1
Immunogens of hiv-1 broadly neutralizing antibodies, methods of generation and uses thereof
ActiveUS20150238594A1Sugar derivativesViral antigen ingredientsNeutralizing antibodyHuman immunodeficiency virus (HIV)
The present application relates to immunogens of broadly neutralizing monoclonal antibodies specific for HIV-1, such as broad and potent neutralizing monoclonal antibodies specific for HIV-1 and their gernation and methods of use. Broad neutralization suggests that the antibodies can neutralize HIV-1 isolates from different individuals. Immunogens or vaccines which may elicit such antibody associated responses are useful in pharmaceutical compositions for the prevention and treatment of HIV, and for the diagnosis and monitoring of HIV infection.
Owner:INT AIDS VACCINE INITIATIVE +1
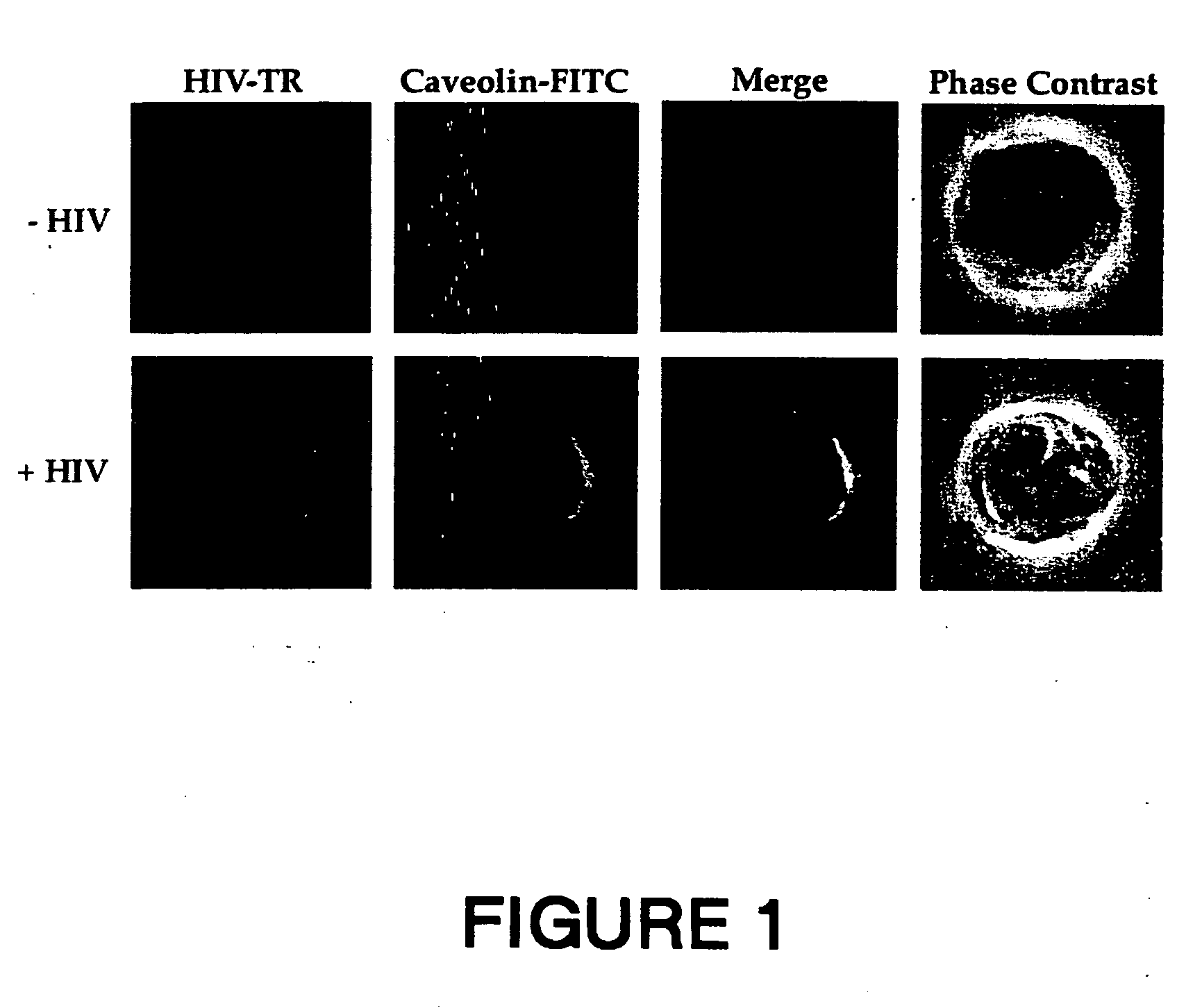
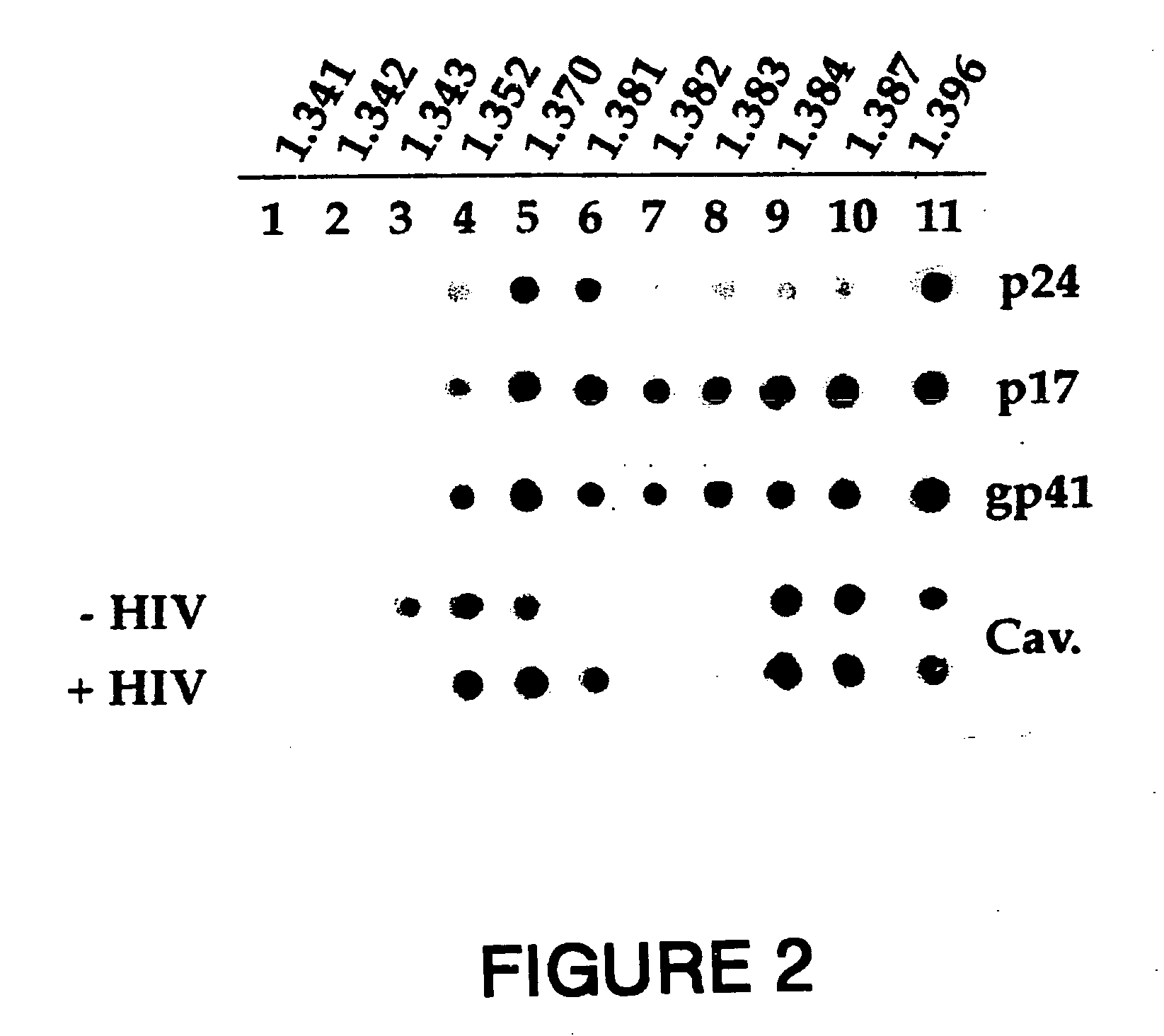
Synthetic peptides corresponding to overlapping neutralizing determinants in the cbd1 epitope induce broadly neutralizing antibodies
InactiveUS20110217307A1Peptide/protein ingredientsViral antigen ingredientsNeutralizing antibodyHiv 1 gp41
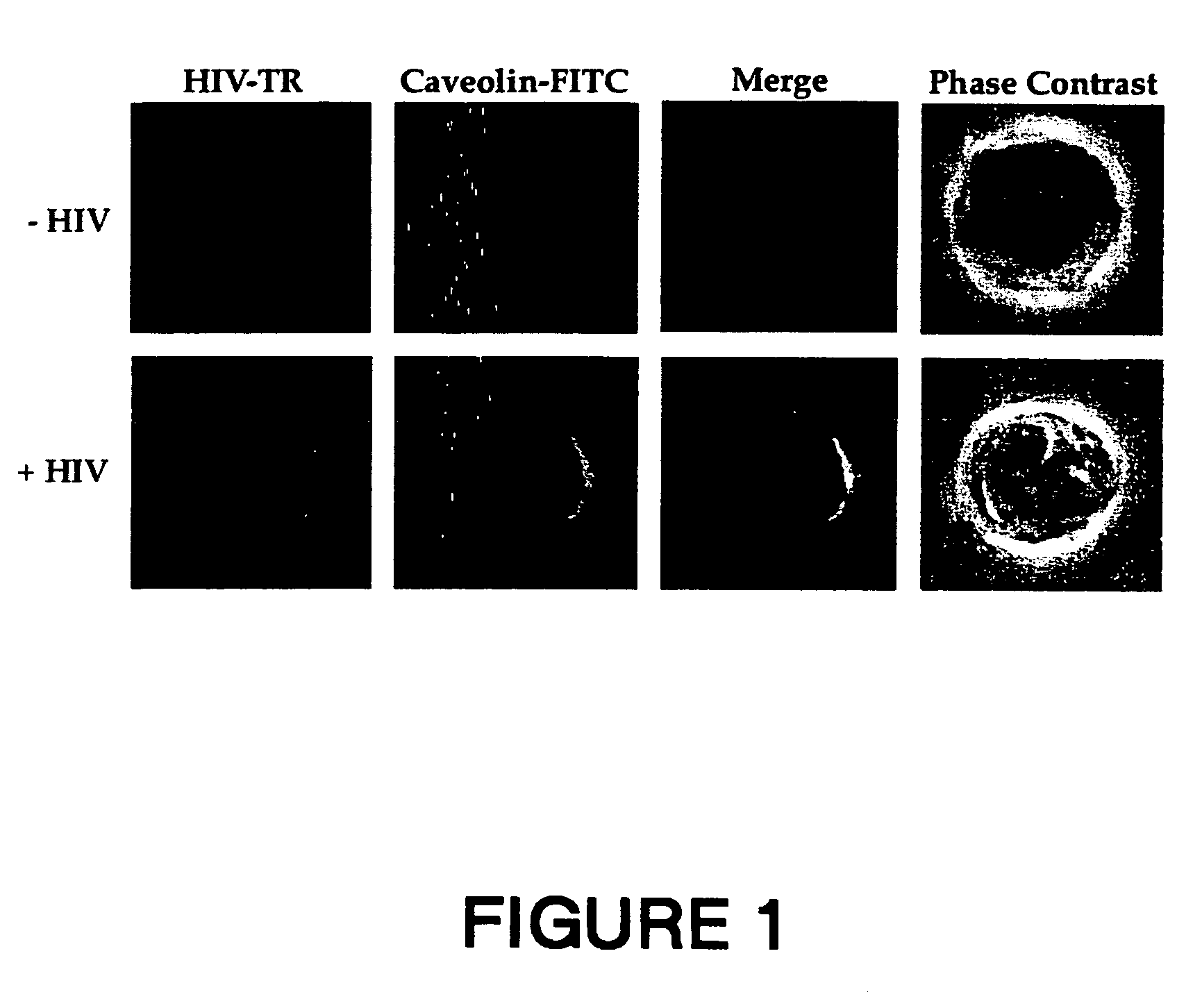
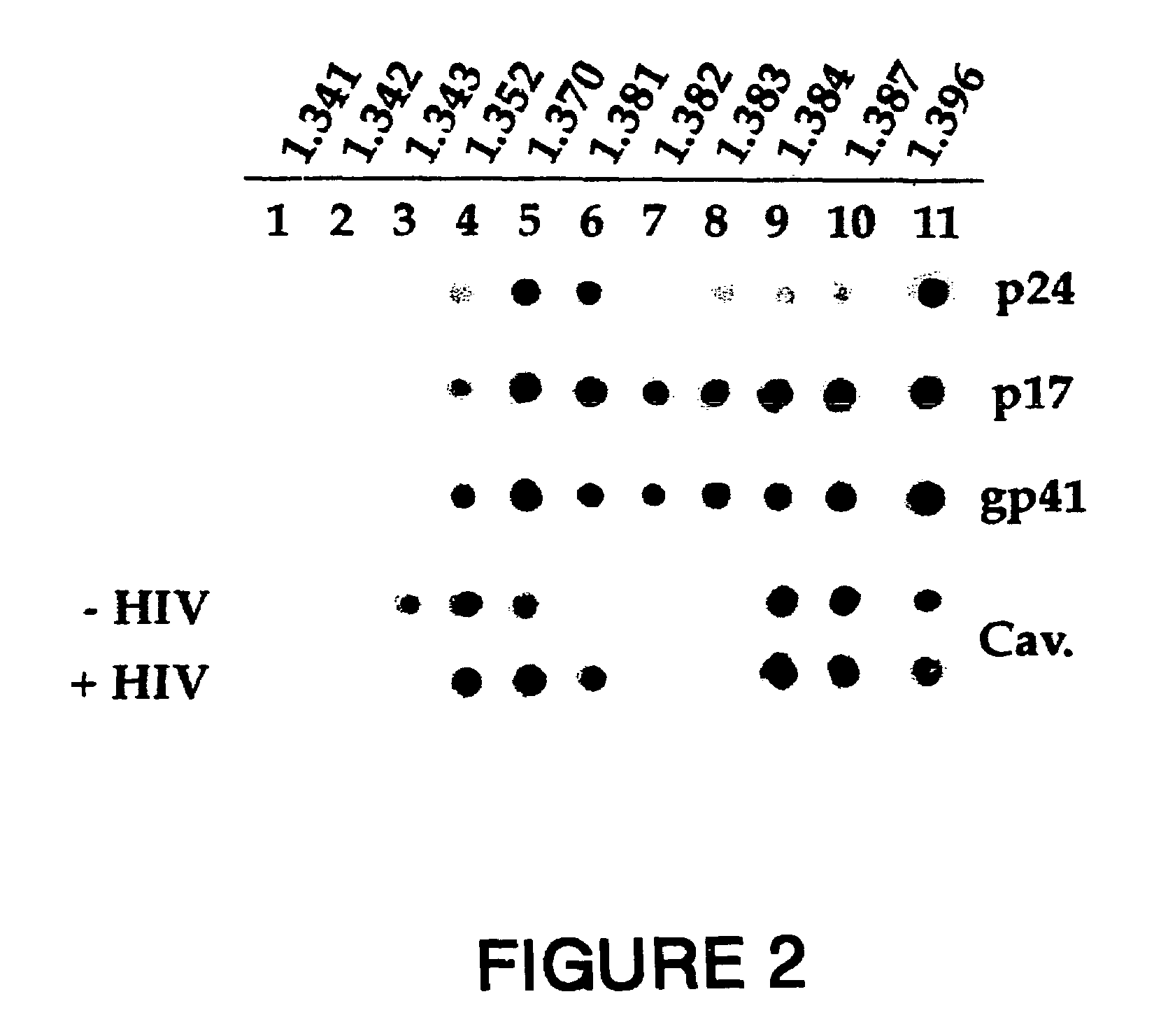
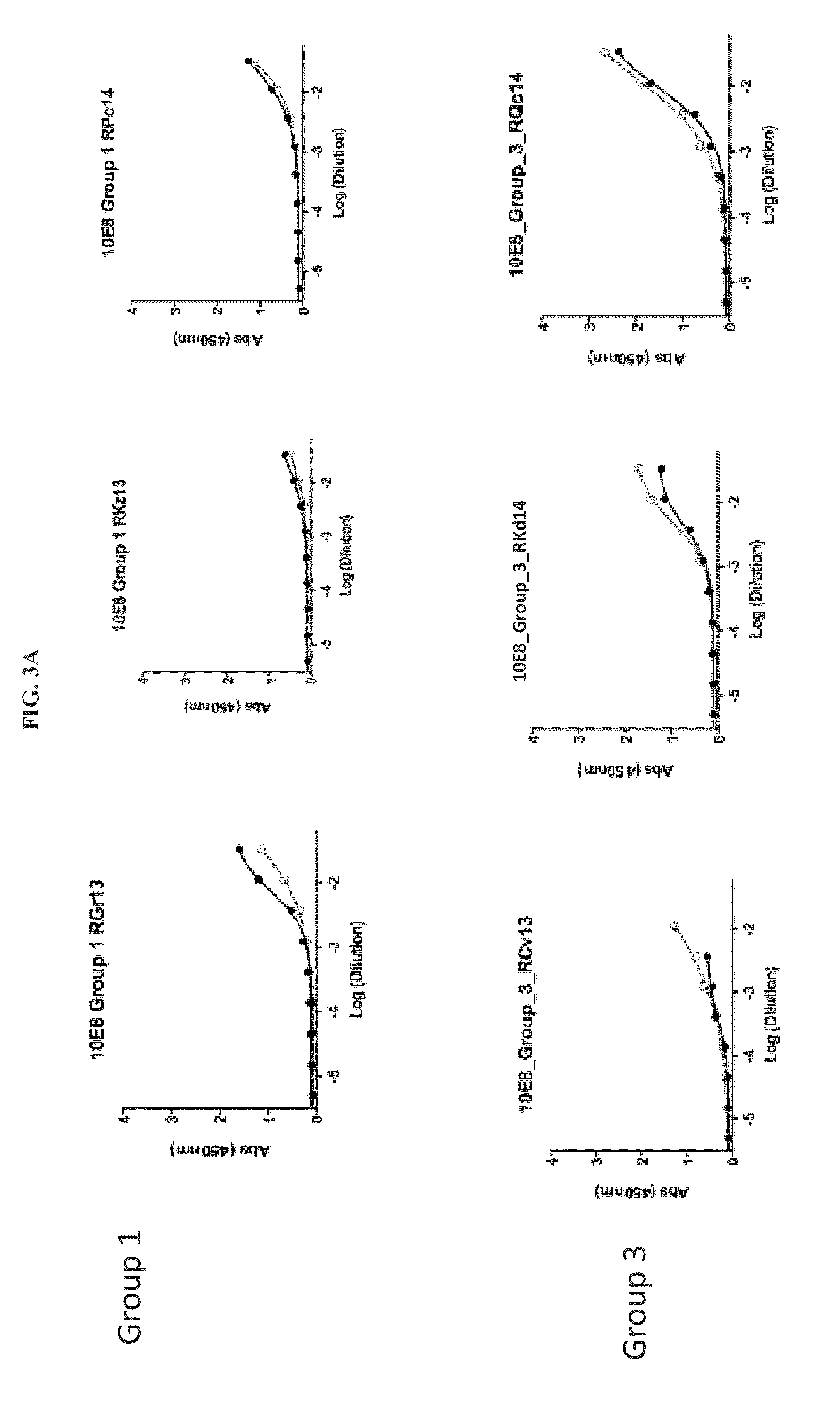
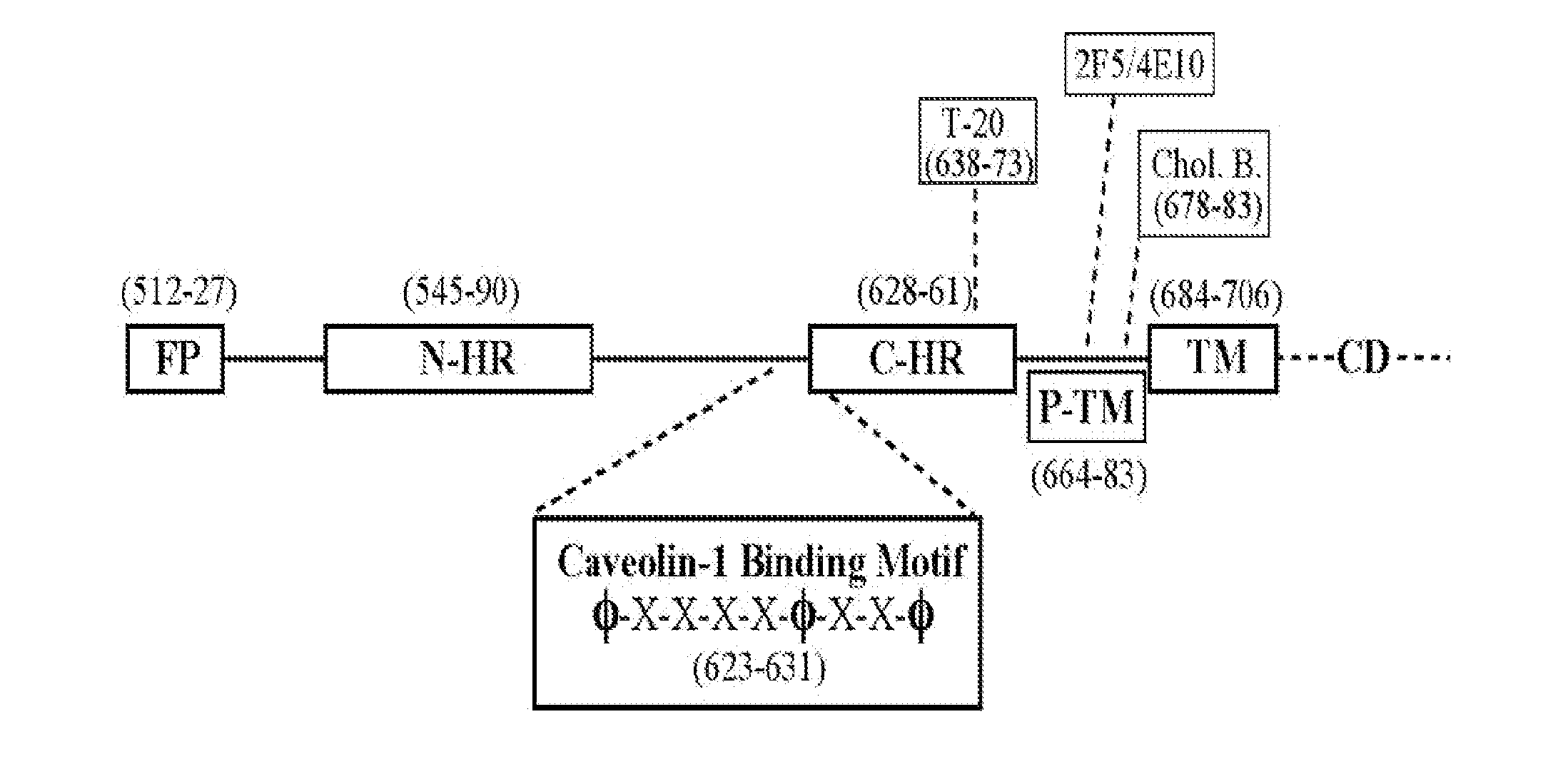
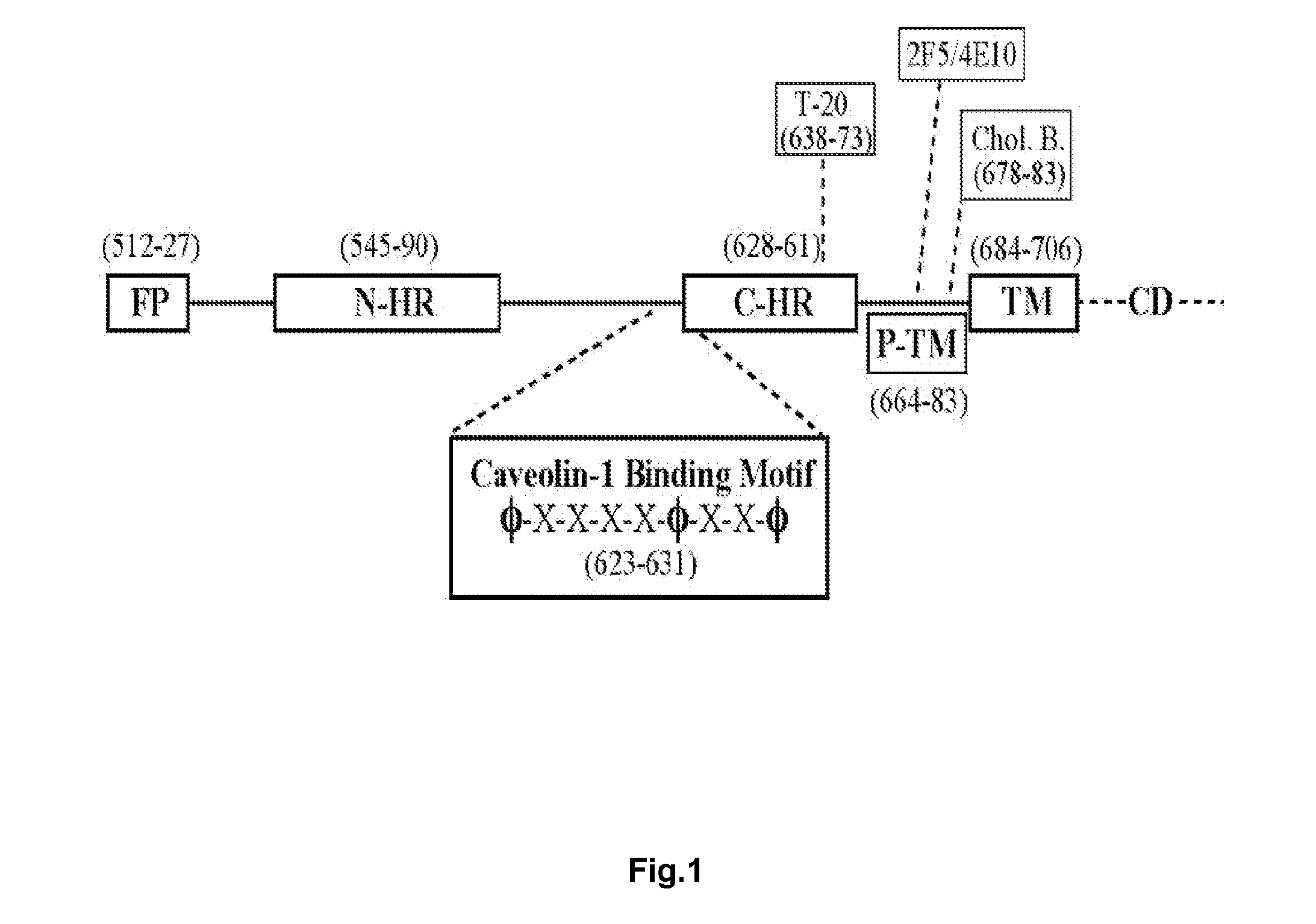
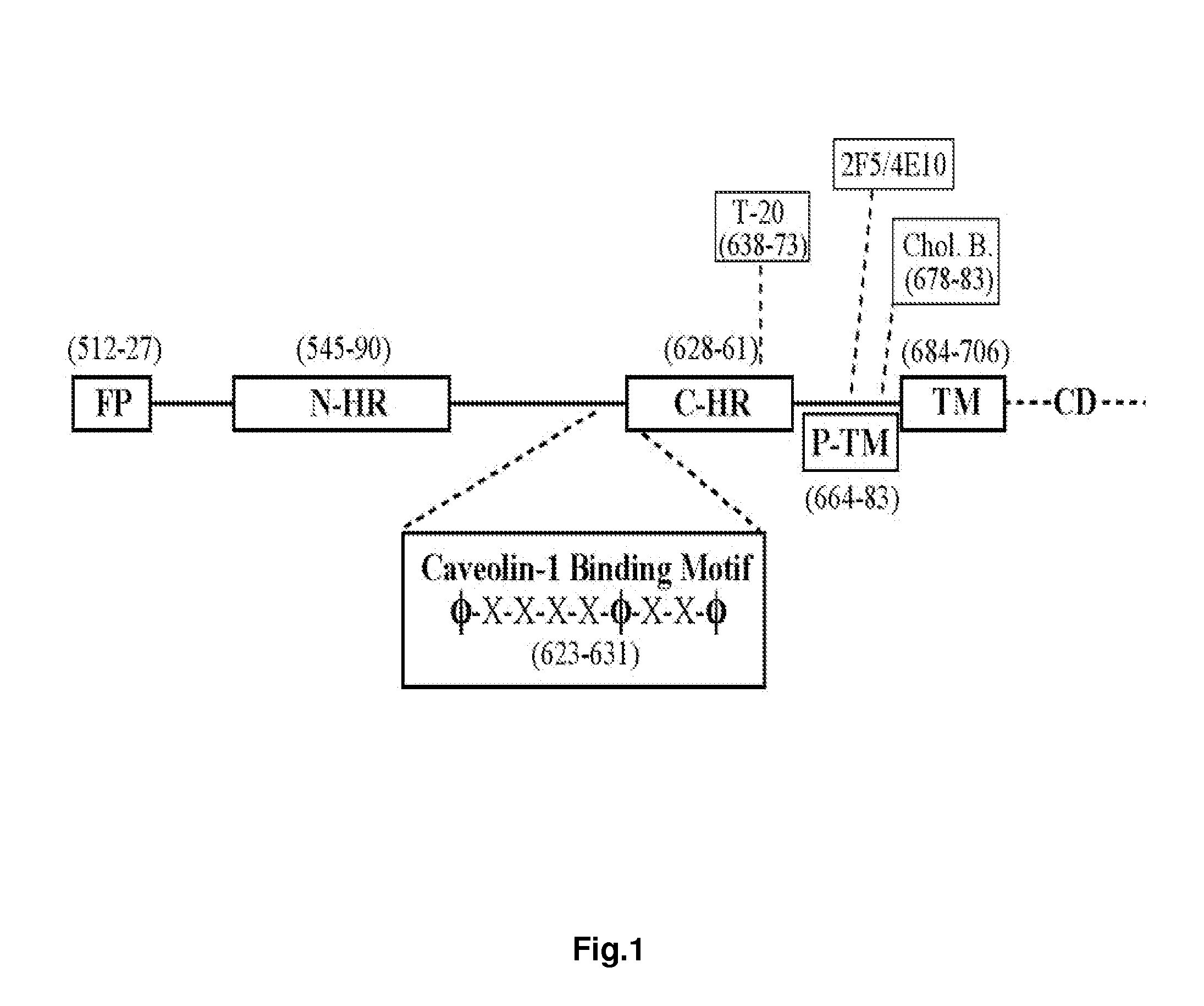
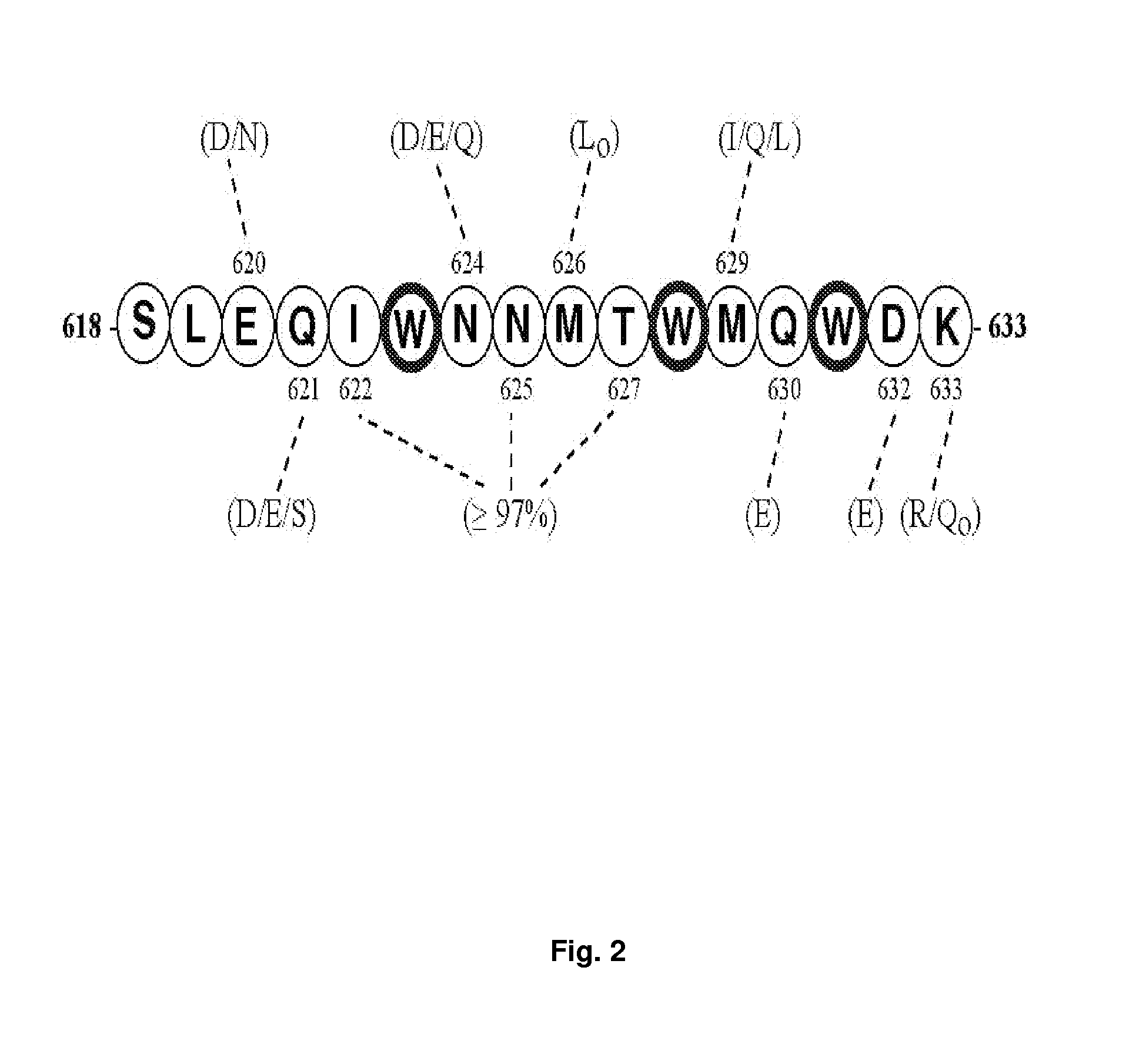
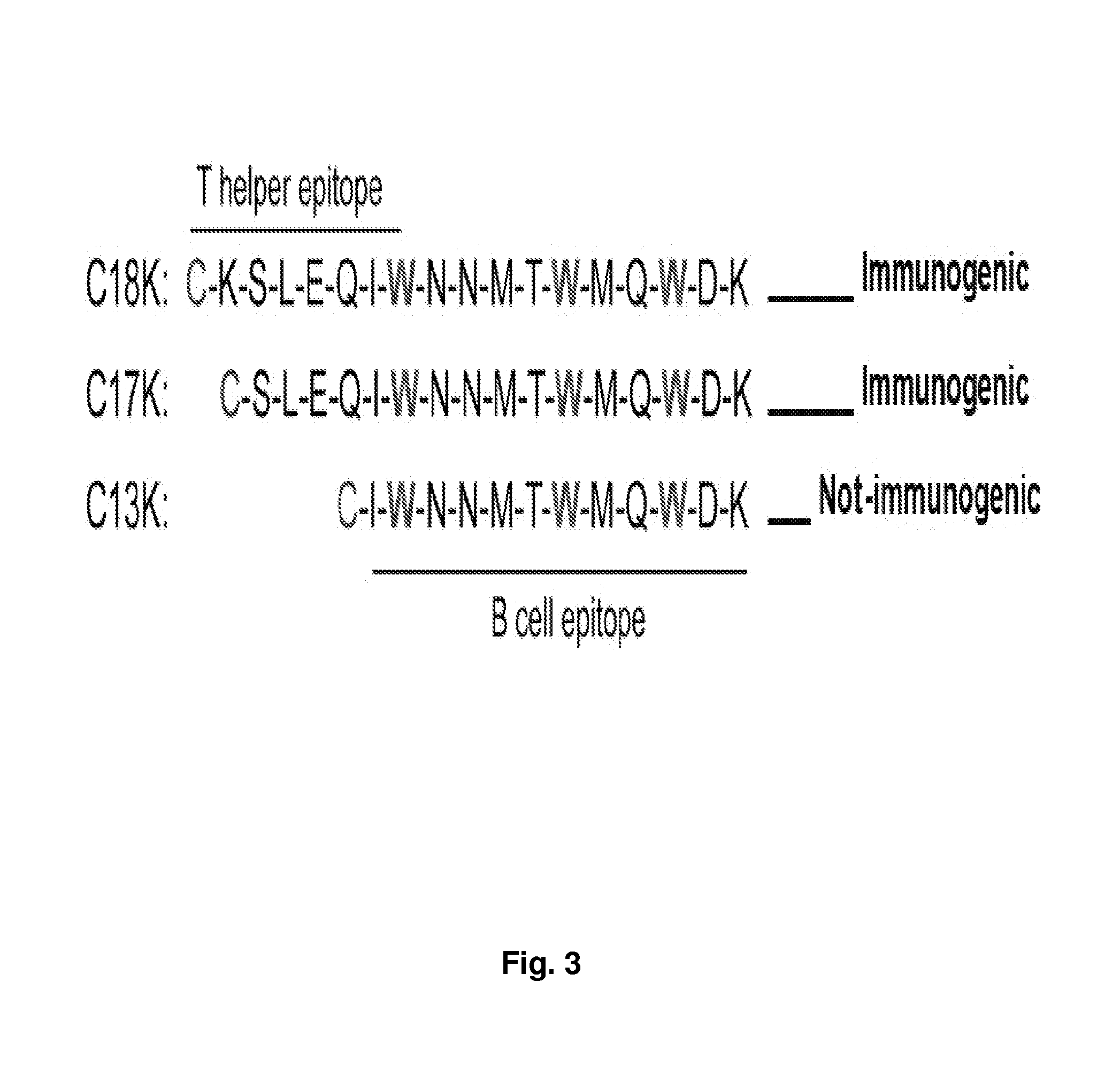
The present invention relates to chimeric peptides having a caveolin-1 binding domain of an HIV-1 gp41 (CBD1) peptide or a variant of said CBD1, fused to a T helper epitope. In one aspect, the T epitope is from a peptide selected from the group consisting of a tetanus toxin, an HIV-1 Gag p24 and an HIV-1 Env-gp120. Compositions containing these chimeric peptides and pharmaceutical and immunogenic compositions as well as vaccines comprising these chimeric peptides also are part of the present invention. Methods to induce neutralizing antibodies against HIV-1 activity and uses of the chimeric peptides to treat or to prevent HIV-1 infection are also disclosed.
Owner:CENT NAT DE LA RECHERCHE SCI
Broad-spectrum neutralizing antibody against HIV as well as preparation method and application thereof
ActiveCN112266416AThe effect of variation is smallIncrease success rateImmunoglobulins against virusesAntiviralsDiseaseNeutralizing antibody
The invention discloses a broad-spectrum neutralizing antibody against HIV as well as a preparation method and application thereof. Specifically, the invention relates to a heavy chain variable regions CDR-H1, CDR-H2 and CDR-H3 of amino acid sequences shown as SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3, and a light chain variable regions CDR-L1, CDR-L2 and CDR-L3 of amino acid sequences as shown asSEQ ID NO.5, SEQ ID NO.6 and SEQ ID NO.7. The invention also relates to nucleic acids encoding the antibody, vectors containing the nucleic acids, cells containing the vectors and pharmaceutical compositions. The invention also relates to the use of the antibody for the detection and treatment of HIV infection and HIV-related diseases.
Owner:SUZHOU HEALTH COLLEGE
Mammalian protein co-recognition by broadly neutralizing antibodies as modified immunogens for re-elicitation
The present invention relates to using HIV-1 broadly neutralizing antibodies to screen for glycan-dependent or protein-dependent self reactivities inherent in these mutated antibodies, and defining this cross recognition at the molecular level, and utilizing the information to re-elicit trimer-specific and / or N-glycan-dependent or protein-surface-dependent broadly neutralizing antibodies and therapeutic applications thereof.
Owner:INT AIDS VACCINE INITIATIVE +1
Methods of identifying novel HIV-1 immunogens
The present application relates to identifying one or more components of HIV envelope glycoprotein which bind to broadly neutralizing antibodies, which may be utilized as research tools for developing HIV-1 vaccine immunogens, antigens for crystallization and / or for identifying of broad neutralizing antibodies.
Owner:INT AIDS VACCINE INITIATIVE +1
Novel HIV -1 broadly neutralizing antibodies
The present application relates novel HIV-1 broadly neutralizing antibodies. The antibodies of the present invention are further characterized by their ability to bind epitopes from the Env proteins. The invention also provides light and heavy chain variable region sequences. Compositions for prophylaxis, diagnosis and treatment of HIV infection are provided.
Owner:UNITED STATES OF AMERICA +2
Novel coronavirus SARS-CoV-2 broad-spectrum neutralizing antibody and application thereof
ActiveCN113861288AHigh affinityEnhanced inhibitory effectBacteriaImmunoglobulins against virusesPhage antibodiesSingle-domain antibody
The invention discloses a novel coronavirus SARS-CoV-2 broad-spectrum neutralizing antibody and application thereof, and particularly discloses a single-domain antibody capable of effectively inhibiting various novel coronavirus SARS-CoV-2 mutant strains. By means of a phage antibody library technology, broad-spectrum neutralizing single-domain antibodies B3A3 and I3A10 specifically combined with SARS-CoV-2 spike protein RBD are successfully obtained. The single-domain antibody is high in antigen affinity, has an obvious inhibiting effect on SARS-CoV-2 main epidemic strains, is a neutralizing antibody with a broad-spectrum effect, and is good in broad spectrum and neutralizing activity. The single-domain antibody can be prepared into specific antibody medicine, SARS-CoV-2 diagnostic reagents or kits and the like clinically used for preventing and treating novel coronavirus pneumonia (COVID-19), and has very wide prospects and important significance in the fields of medicine application, clinical diagnosis and the like.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Synthetic peptides corresponding to overlapping neutralizing determinants in the CBD1 epitope induce broadly neutralizing antibodies
The present invention relates to chimeric peptides having a caveolin-1 binding domain of an HIV-1 gp41 (CBD1) peptide or a variant of said CBD1, fused to a T helper epitope. In one aspect, the T epitope is from a peptide selected from the group consisting of a tetanus toxin, an HIV-1 Gag p24 and an HIV-1 Env-gp120. Compositions containing these chimeric peptides and pharmaceutical and immunogenic compositions as well as vaccines comprising these chimeric peptides also are part of the present invention. Methods to induce neutralizing antibodies against HIV-1 activity and uses of the chimeric peptides to treat or to prevent HIV-1 infection are also disclosed.
Owner:CENT NAT DE LA RECHERCHE SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com