Novel coronavirus SARS-CoV-2 broad-spectrum neutralizing antibody and application thereof
A sars-cov-2 and single-domain antibody technology, applied in the field of biomedicine, can solve problems such as changes in pathogenicity, failure of monoclonal antibody drugs or reduction of neutralization ability, and decline in vaccine protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Screening and preparation of single-domain antibodies that specifically bind to the RBD region of the SARS-CoV-2 spike protein
[0066] 1. Phage library screening
[0067] The basic principle and basic operation process of phage library screening refer to "Biological Library Technology-Phage Display and SELEX Technology" written by Shao Ningsheng et al. The screening steps are briefly described as follows: First, 50 μg of SARS-CoV- 2 Recombinant protein in the RBD region of the spike protein (Beijing Yiqiao Shenzhou Technology Co., Ltd., catalog number: 40592-V08H86, 40592-V08H88, referred to as RBD protein) antigen using the commercial kit EZ-Link of Thermo Fisher Scientific TM Sulfo NHS-LC-LC-Biotin was labeled with biotin and desalted and purified (the desalting column was purchased from Thermo Fisher Scientific). The first round of screening was liquid-phase selection, firstly, 12 μg of biotin-labeled RBD protein was mixed with 1 mL (10 12 -10 13 ) pha...
Embodiment 2
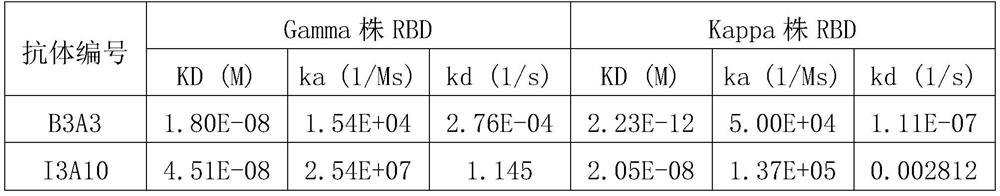
[0091] Example 2 Affinity Determination of Anti-RBD Single Domain Antibody
[0092] Antibodies to be tested: two RBD single domain antibodies B3A3 and I3A10 purified in Example 1.
[0093] BIAcore T-200 biomolecular interaction instrument (GE Life Sciences) was used to measure the affinity of antibodies. This technology was developed based on surface plasmon resonance (SPR) technology, which can be used for label-free and rapid detection of interactions between biomacromolecules. , Real-time, automatic operation and detection.
[0094] When measuring the interaction between the recombinant RBD protein and its corresponding single domain antibody, first coat the antigen RBD protein (Beijing Yiqiao Shenzhou Technology Co., Ltd., Gamma strain RBD product number: 40592-V08H86, Kappa strain RBD product number: 40592-V08H88) on On the sensor chip, the association constant, dissociation constant and affinity constant were determined using the single domain antibody as the mobile pha...
Embodiment 3
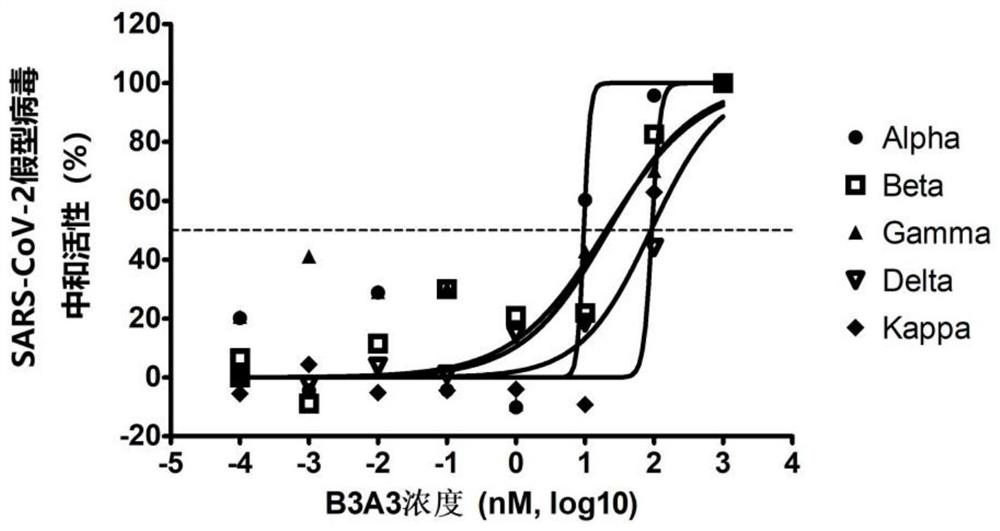
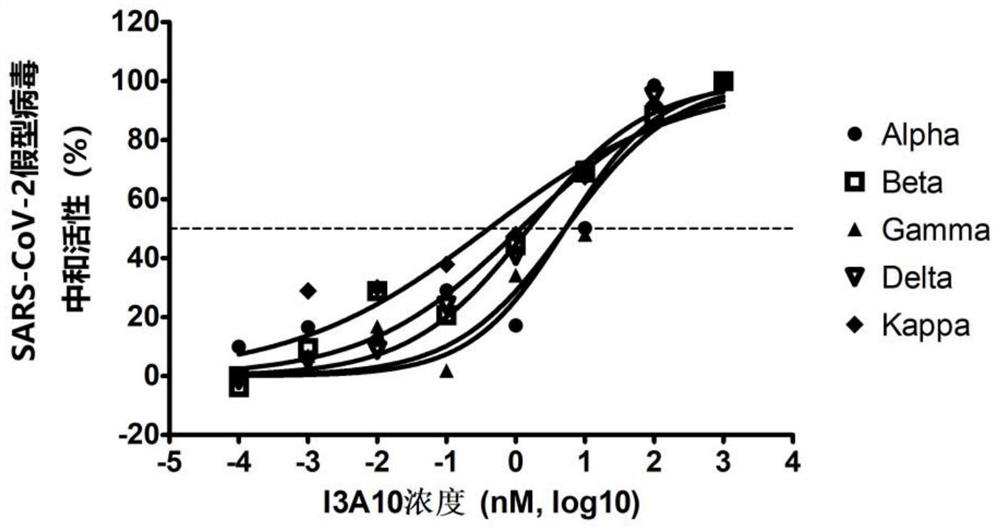
[0101] SARS-CoV-2 pseudotyped virus neutralizing activity assay of embodiment 3 anti-RBD single domain antibody
[0102] Antibodies to be tested: two single domain antibodies B3A3 and I3A10 purified in Example 1.
[0103] SARS-CoV-2 pseudotyped virus is a new type of virus particle formed by assembling the replication core element of retrovirus and the envelope spike glycoprotein (ie, S protein) of SARS-CoV-2 virus. Compared with true viruses, pseudotyped viruses can only infect cells at one time, have a wide host range, high titers, and are not easily inactivated by serum complement, so they can replace true viruses for neutralization detection. The ability of pseudotyped virus to infect cells depends on the type and characteristics of the glycoprotein wrapped outside it, which is an ideal tool for studying the inhibition efficiency of neutralizing antibodies, receptor utilization and invasion infection mechanism of SARS-CoV-2.
[0104] 1. Packaging preparation of SARS-CoV-2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com