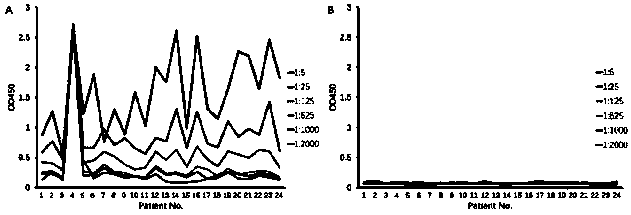
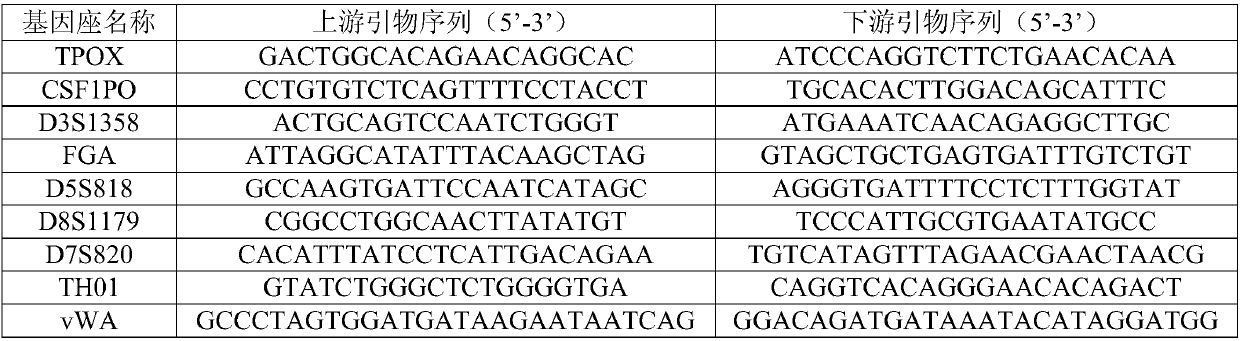
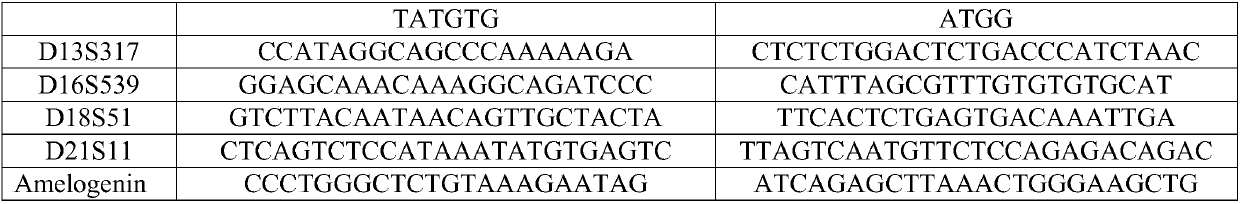
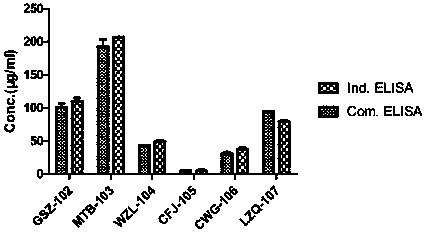Patents
Literature
53 results about "Commercial kit" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
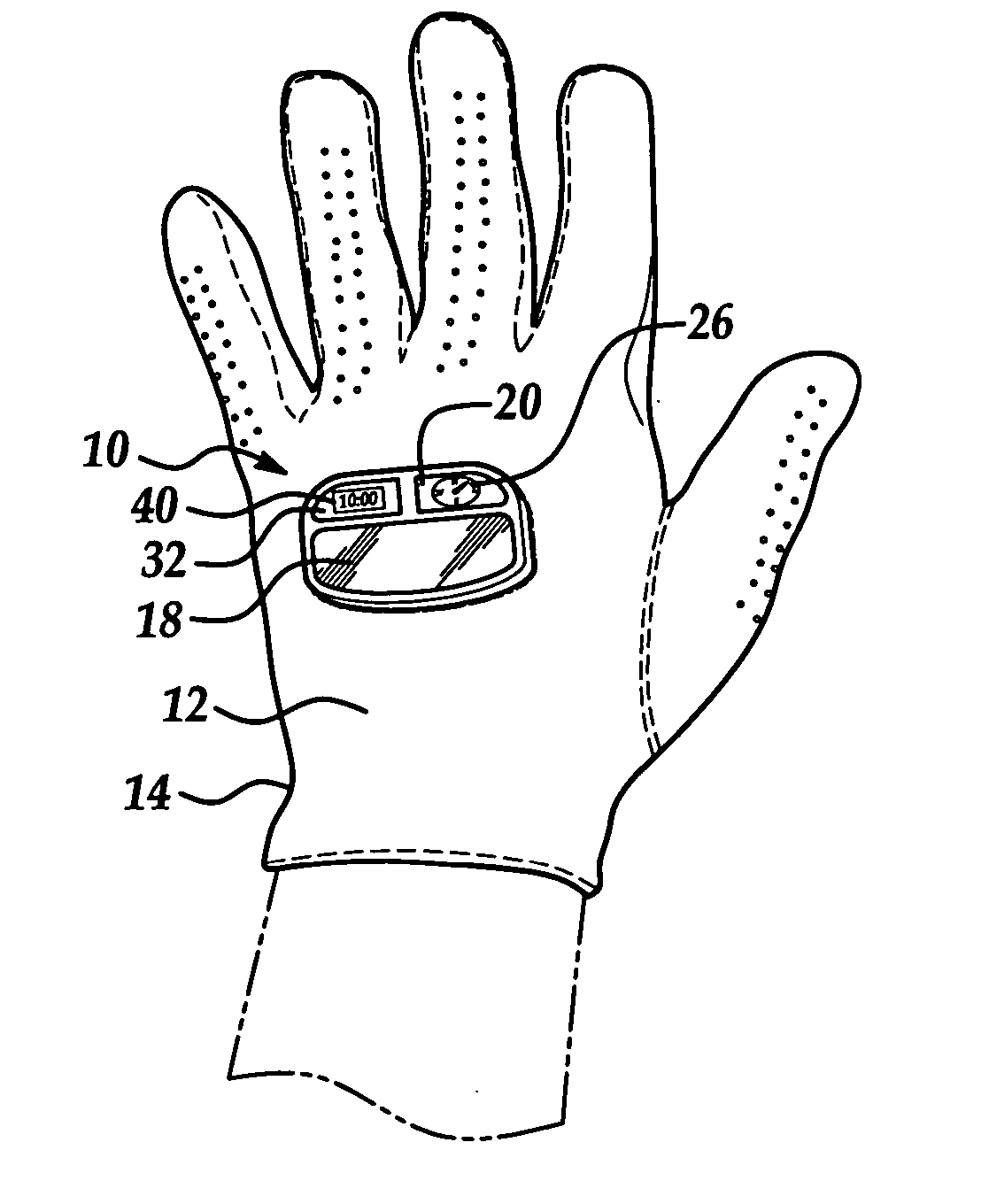
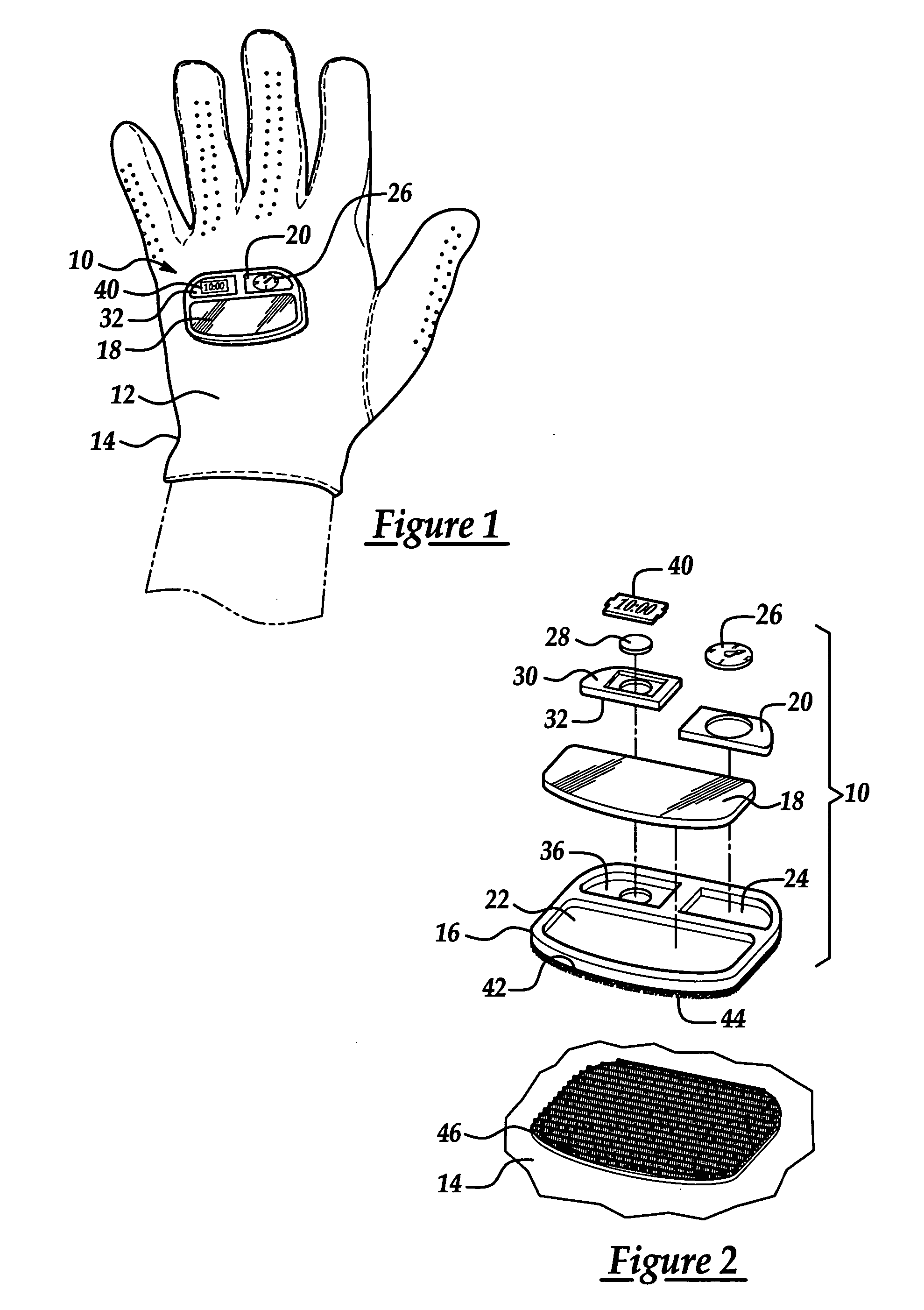
Outdoor activity accessory hand wear
Owner:EISENBRAUN KENNETH D
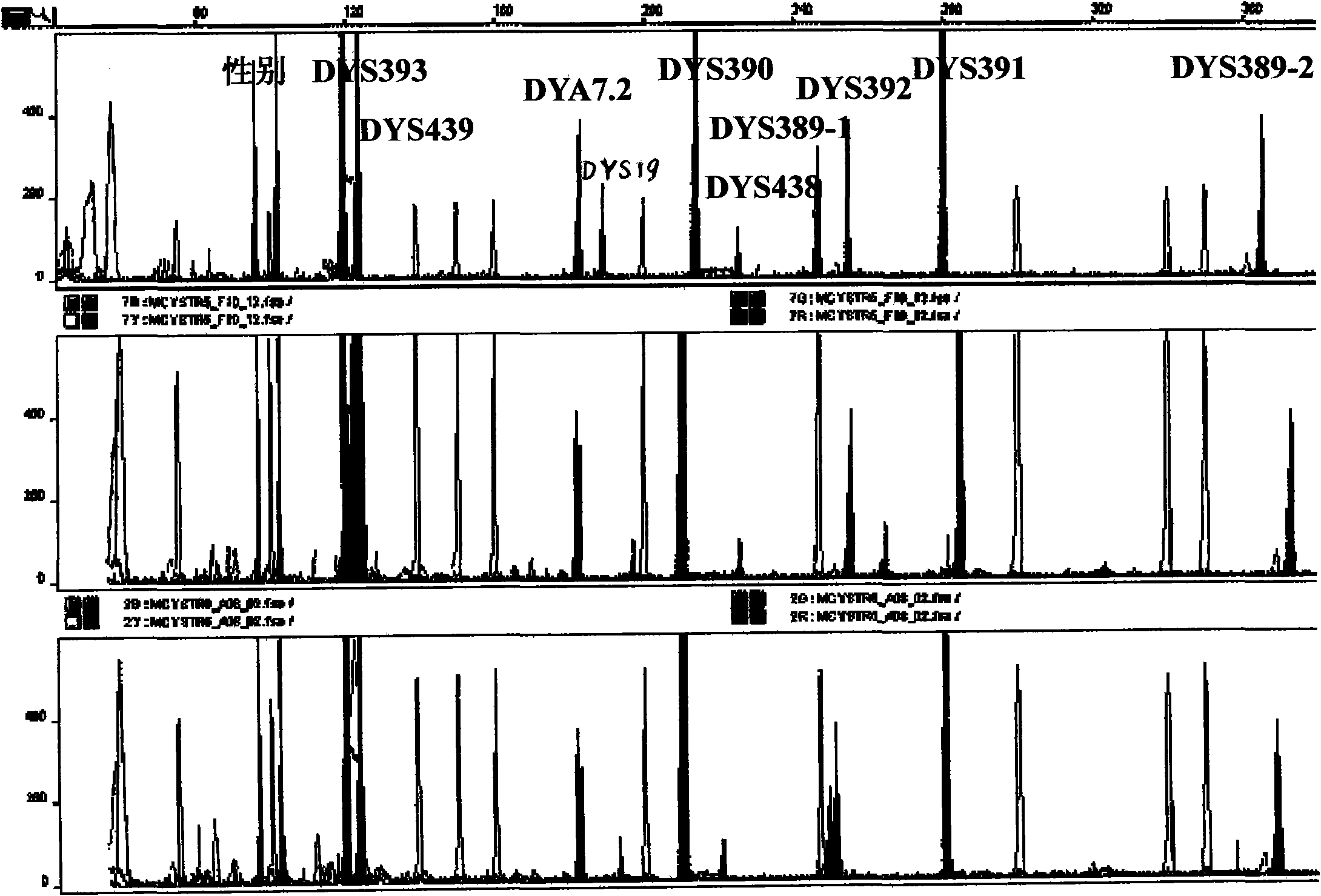
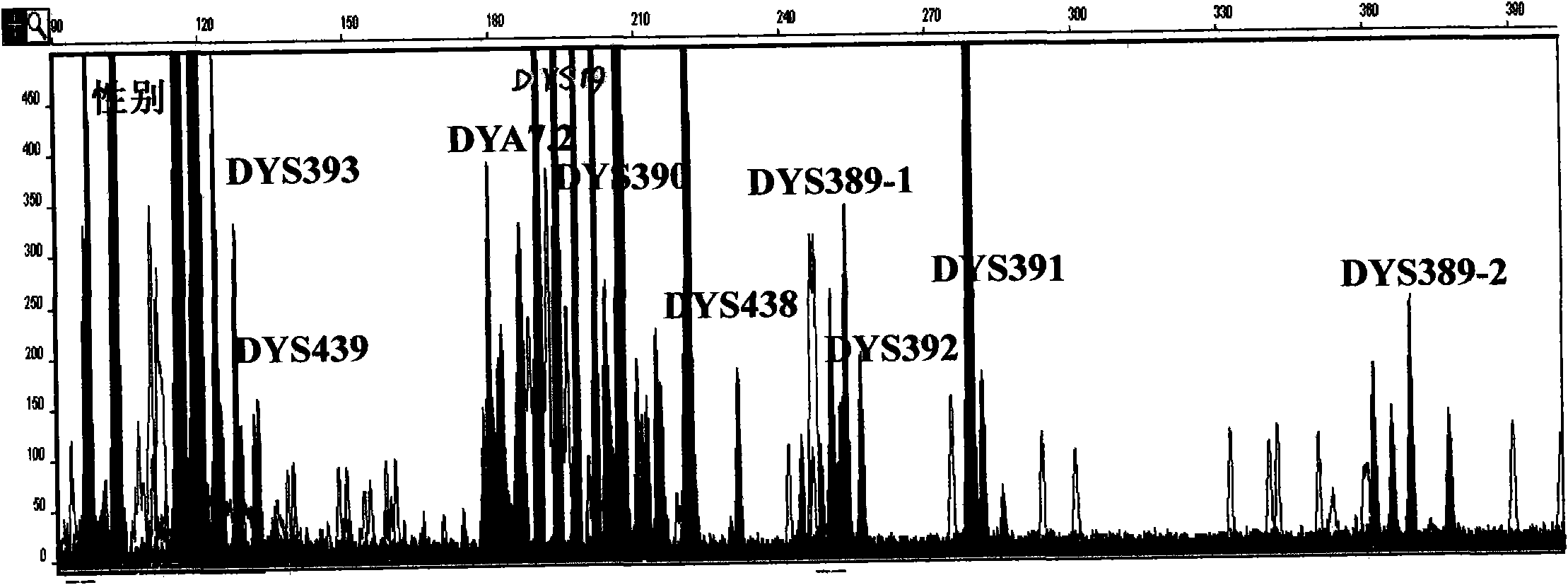
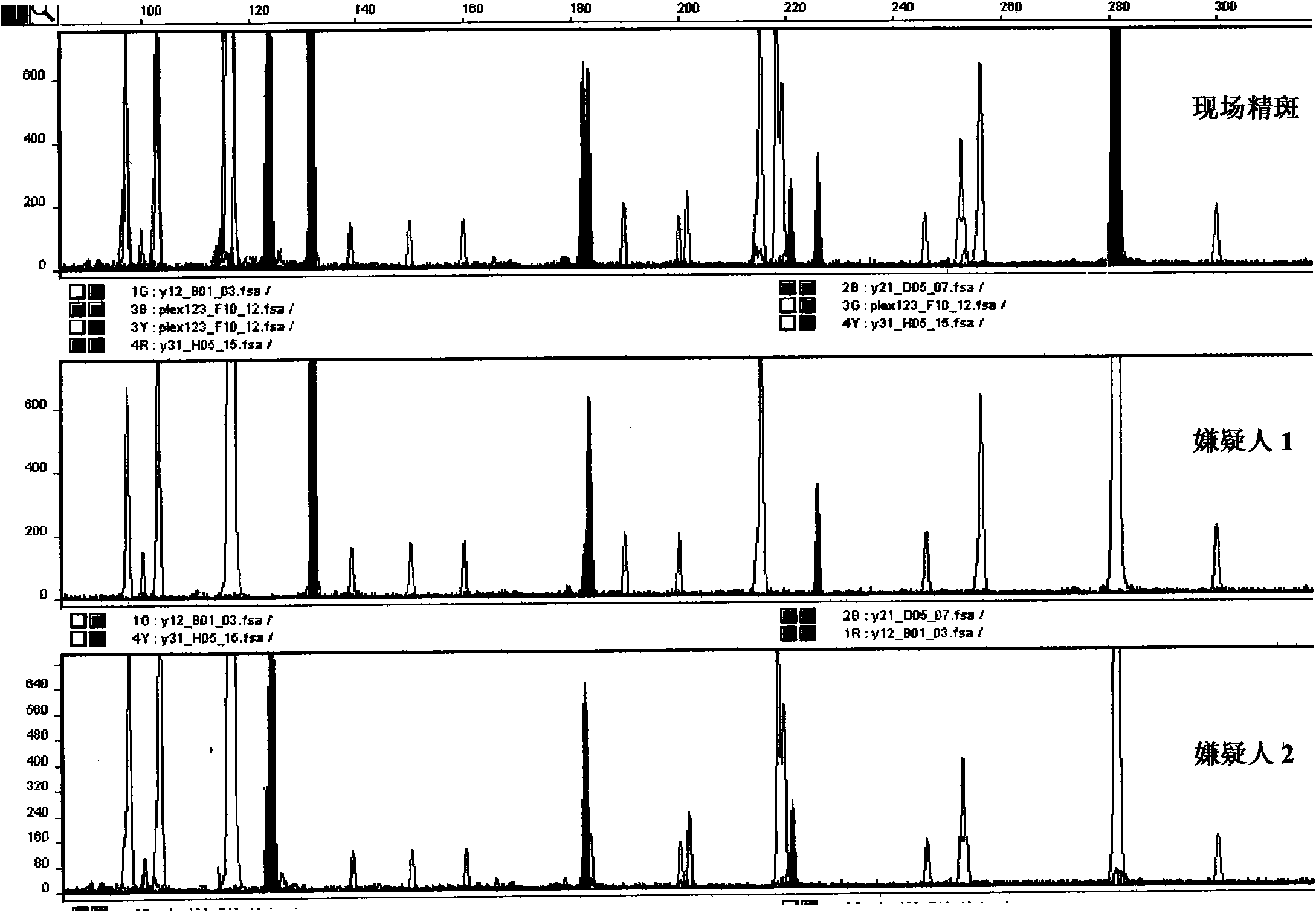
Y-STR locus fluorescent label multiplex amplification system and application thereof
InactiveCN102433374AHigh genetic polymorphismImprove individual recognitionMicrobiological testing/measurementFluorescence/phosphorescenceHuman DNA sequencingFluorescence
The invention relates to a Y-STR locus fluorescent label multiplex amplification system and an application thereof, and belongs to the field of polymorphic marker in detected human genome. The multiplex amplification system can simultaneously amplify loca of DYS19, DYS389-1, DYS389-2, DYS390, DYS391, DYS392, DYS393, DYS438, DYS439, DYA7.2 and Amelogenin. In the meanwhile, through the design of specific primers and a unique locus combination fluorescent label mode, only three marks can be adopted to simultaneously amplify the above loca, and amplified products are all controlled between 100bp to 400bp with very high sensitivity. A kit designed according to the amplification system is simple and rapid to operate, has high sensitivity, is high efficient, saves templates, has high individual identification capability, and reaches the level of foreign commercial kits of the same type.
Owner:辽宁省刑事科学技术研究所 +1
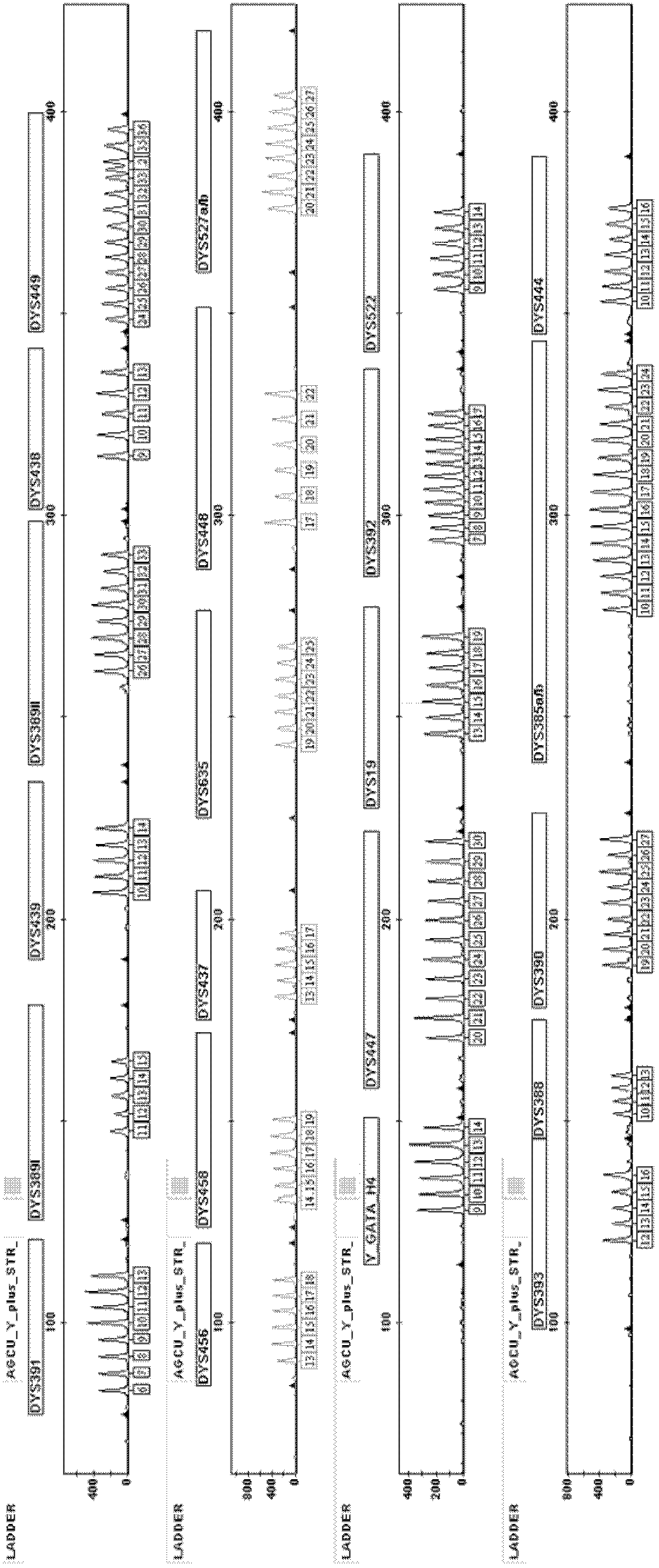
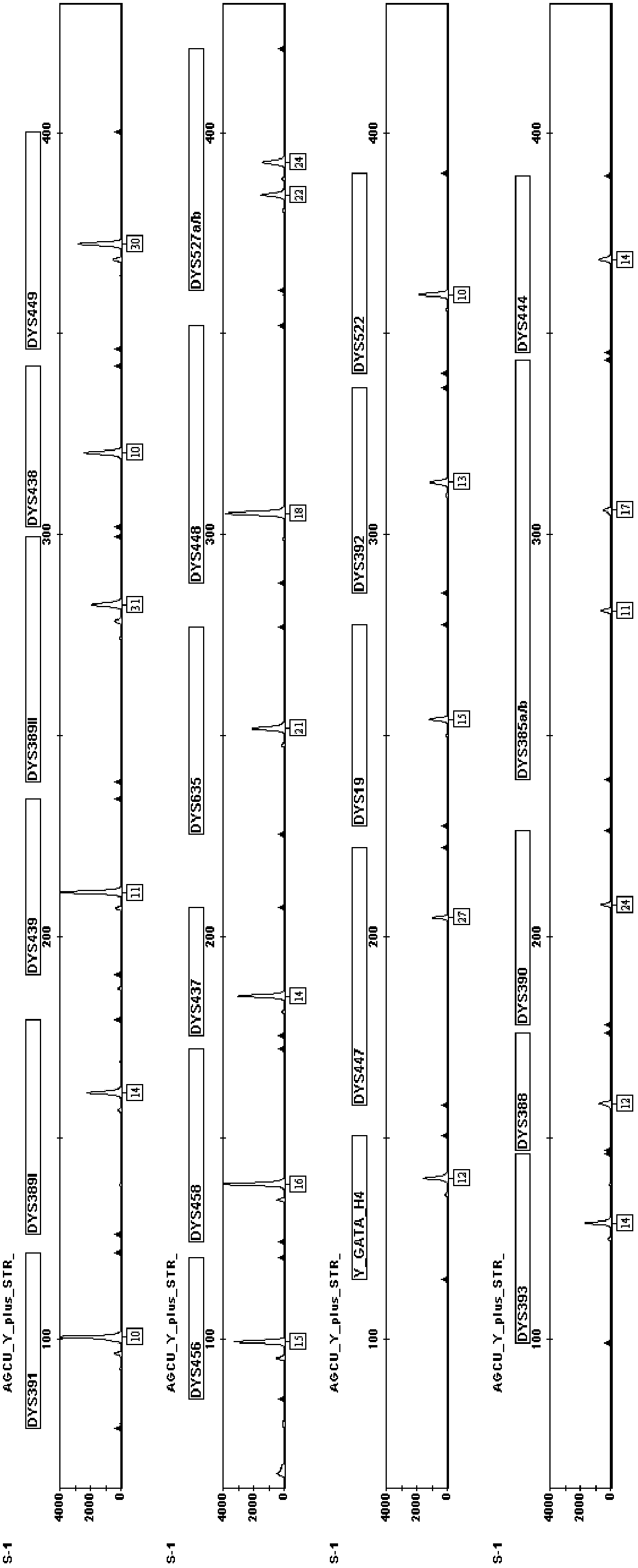
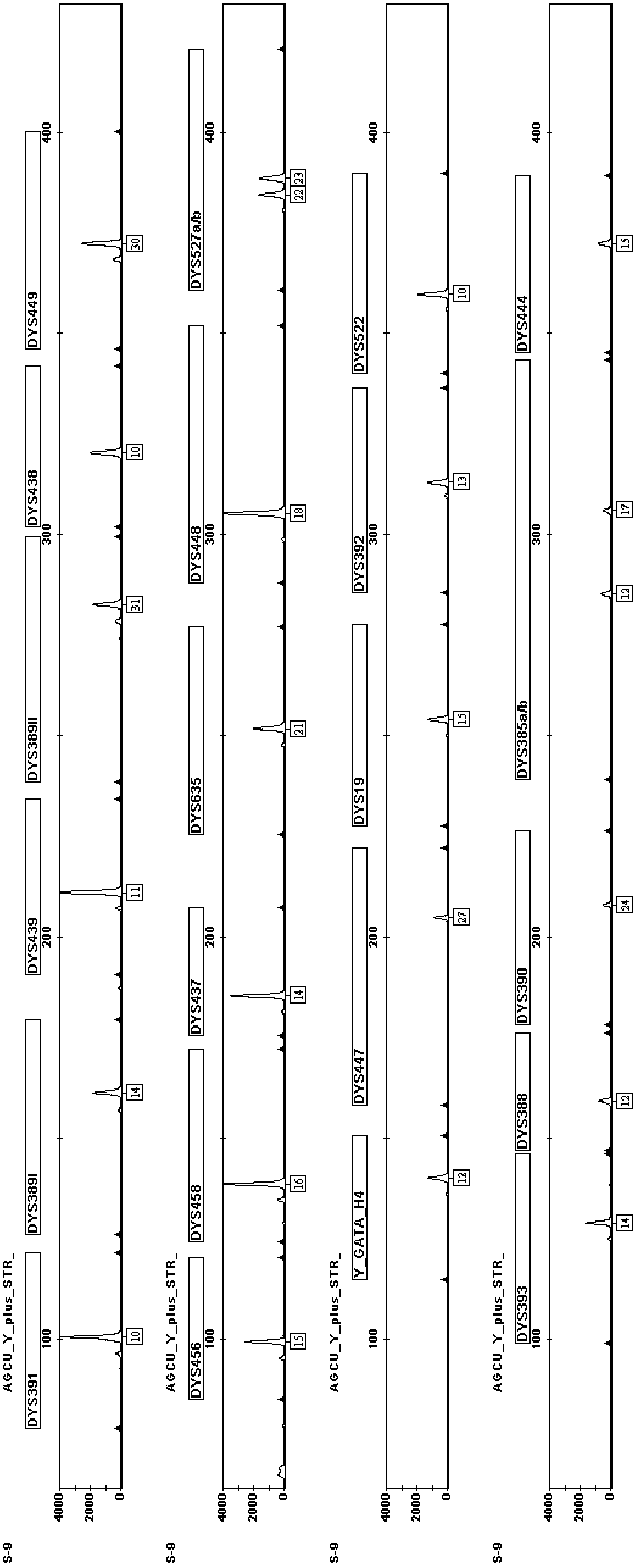
Fluorescence-labeled composite amplification kit for Y chromosome STR (short tandem repeat) gene loci capable of improving distinguishing capability and application thereof
ActiveCN102703583AEasy to identifyHigh polymorphismMicrobiological testing/measurementFluorescenceCommercial kit
The invention provides a fluorescence-labeled composite amplification kit for Y chromosome STR (short tandem repeat) gene loci capable of improving distinguishing capability. When the kit is used for detecting DNA (deoxyribonucleic acid) gene, not only can 17 STR gene loci of DYS391, DYS389I / II, DYS439, DYS438, DYS456, DYS458, DYS437, DYS635, DYS448, Y-GATA-H4, DYS19, DYS392, DYS393, DYS390 and DYS385a / b, which can be analyzed by the commercial kit, be amplified and analyzed, but also at least one of STR gene loci of DYS449, DYS527a / b, DYS522, DYS388, DYS447 and DYS444 can be simultaneously amplified and analyzed, so that the accumulative individual distinguishing capability and cumulative probability of exclusion of the system are improved, and the individual distinguishing capability is improved overall.
Owner:AGCU SCIENTECH
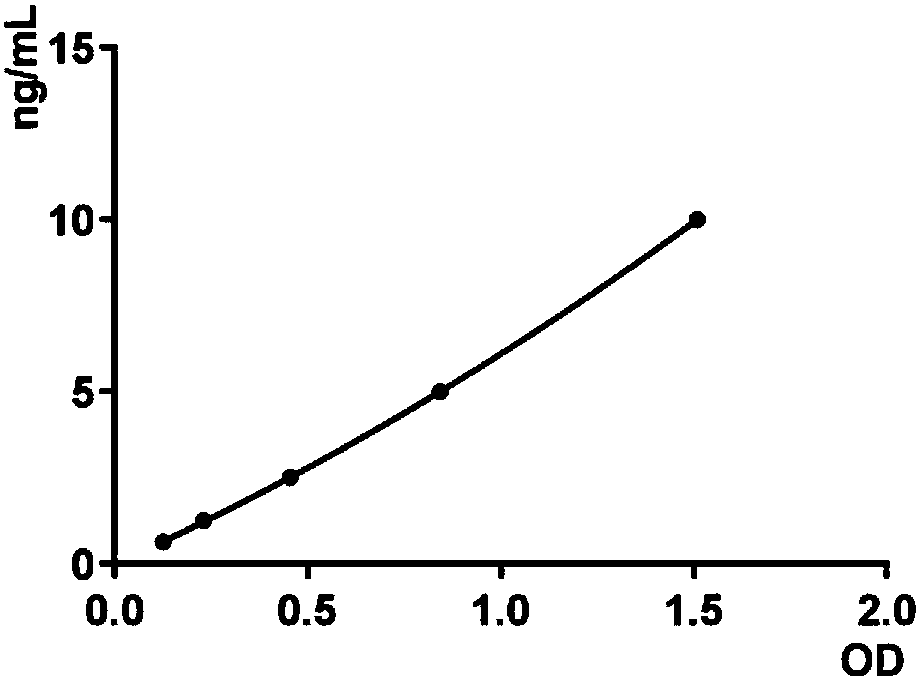
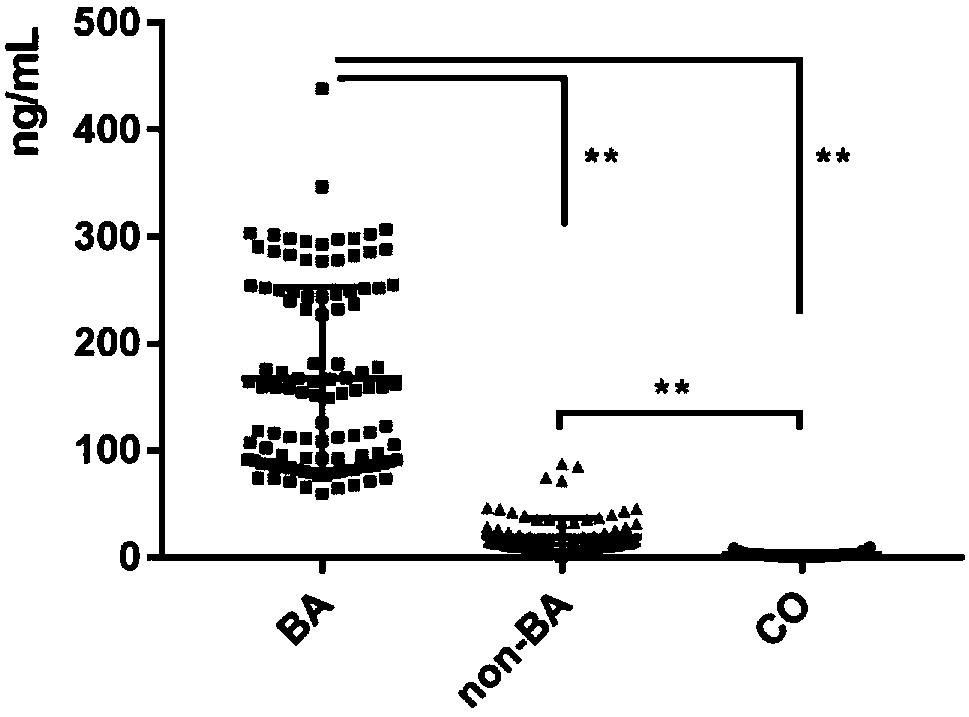
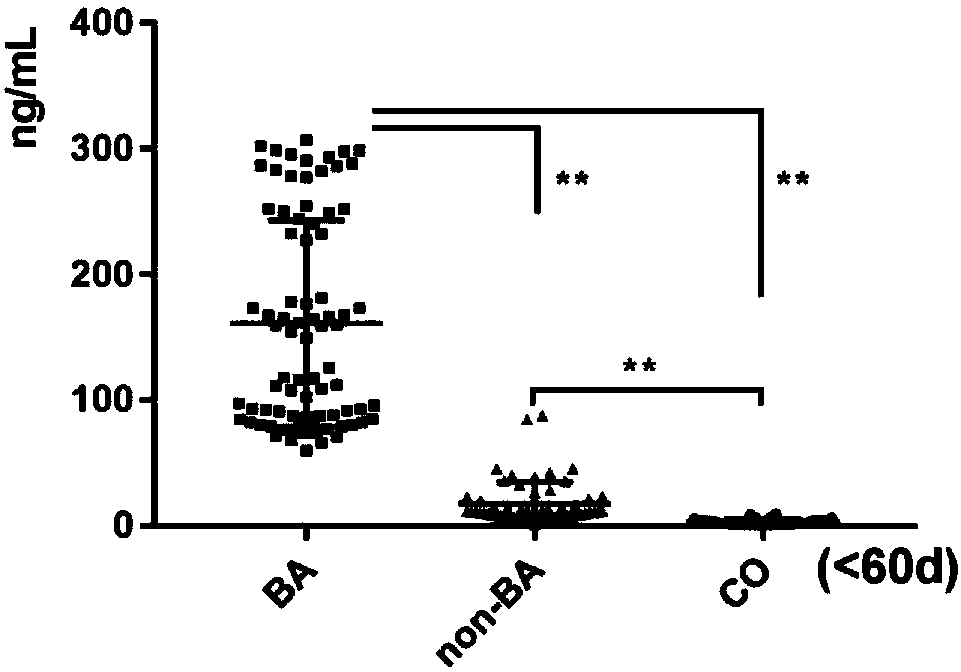
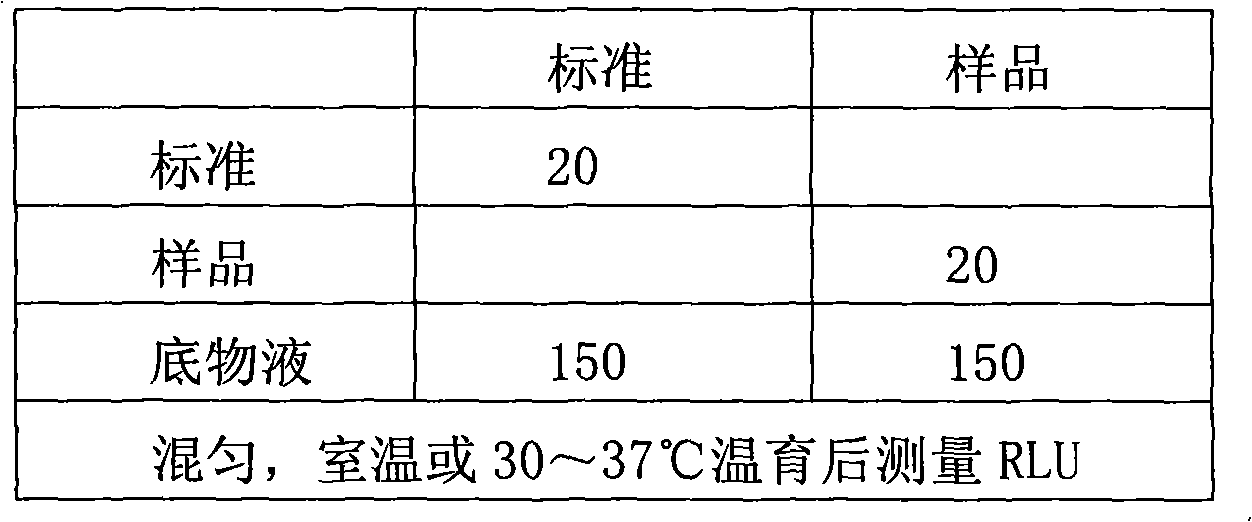
Serum marker MMP-7-based biliary atresia diagnosis kit
InactiveCN108267585AEasy to operateAid in early diagnosisDisease diagnosisPositive controlPRIMARY BILIARY ATRESIA
The invention relates to a serum marker MMP-7-based biliary atresia diagnosis kit. The diagnosis kit comprises an anti-human MMP-7 monoclonal antibody coated ELISA plate, a negative control solution,a positive control solution, an enzyme labeling reagent, an enzyme substrate solution, a blocking solution, a sample diluent, a washing solution and a stopping solution. The diagnosis kit is a new sensitive, safe, reliable and easily-operated commercial kit. Quantitative detection of the level of MMP-7 in human serum is helpful for early diagnosis of BA; the BA diagnosis sensitivity of the serum biomarker protein MMP-7 is 100%, and the BA diagnosis specificity is 95.6%, so the kit has the characteristics of high specificity and high sensitivity; and the kit improves the early diagnosis rate ofBA, reduces misdiagnosis, and improves the self liver survival rate of the BA.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
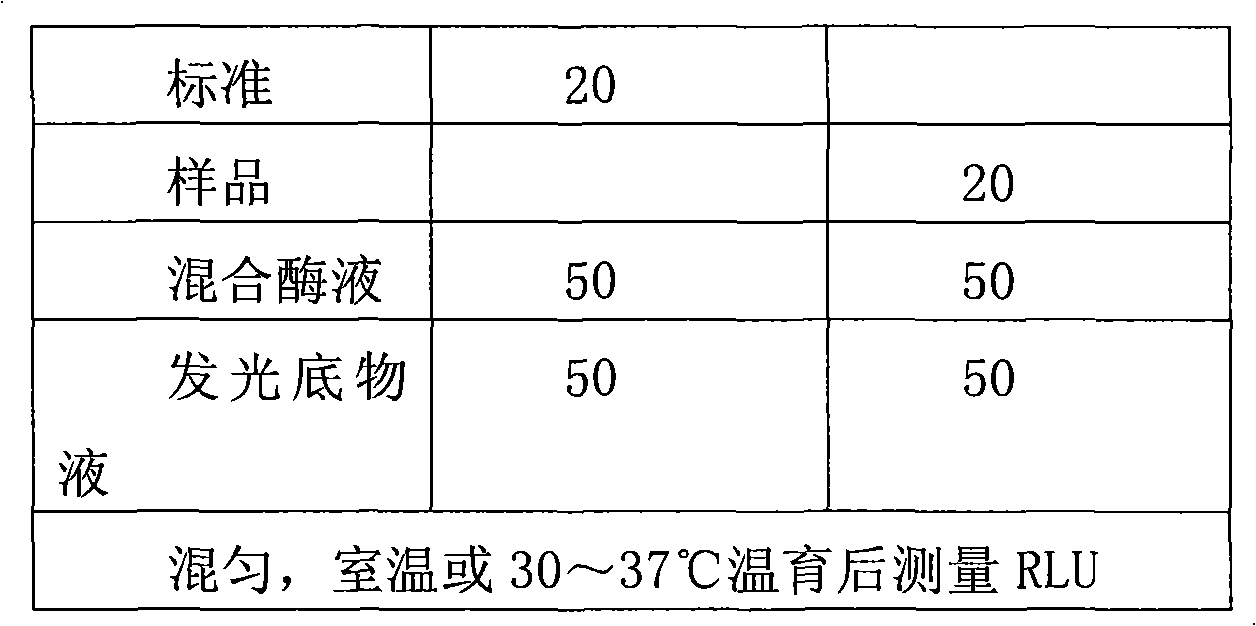
Method for detecting blood lactic acid in vitro by using chemiluminescence method
InactiveCN101639446AHigh sensitivityMinimum minimumMicrobiological testing/measurementChemiluminescene/bioluminescenceO-Phosphoric AcidGlycerol
The invention discloses a method for detecting blood lactic acid in vitro by using a chemiluminescence method, which is characterized in that LD is utilized to catalyze L-lactic acid and NAD<+> to generate pyruvate and NADH, the pyruvate and ADP generate ATP under the catalysis of pyruvate kinase (PK), ATP and glycerol generate glycerol-3-phosphoric acid and ADP under the catalysis of GK, the glycerol-3-phosphoric acid is acted by GPO to obtain H2O2, and H2O2 is catalyzed by HPR to enable luminol to emit light; the size of light signals is in positive correlation with the concentration of thepyruvate, i.e. the bigger the concentration of the lactic acid is, the stronger the emitted light signals are; the concentration of the lactic acid can be conjectured by recording the light signals; and the lactic acid with the known concentration is used for detecting the light signals to make a dose-response curve, and the content of the lactic acid of an unknown sample can be calculated throughthe curve. In the invention, a chemiluminescence substance replaces a colored substance to achieve the purposes of sensitivity, stability, wide range and safety. The method can be used for preparinga corresponding commercial kit for quantitatively detecting the lactic acid in body fluids such as whole blood, plasma, cerebrospinal fluid, urine, gastric juice and the like.
Owner:福建省洪诚生物药业有限公司
Genetic marker for human individual recognition and/or paternity identification and detection method thereof and kit
ActiveCN107541554ABroad multi-racial adaptabilityThe test result is accurateMicrobiological testing/measurementDNA/RNA fragmentationAllele frequencyCommercial kit
The invention discloses a genetic marker combination for human individual recognition and / or paternity identification and a detection method thereof. By conducting whole-genome SNPs unbiased scanningon multiple races, a SNPs site combination widely applicable to the multiple races is screened, and 116 autosome SNPs sites which come from 37 groups in different regions in the whole world, have thehypermorph allel frequency and low difference and achieve independent inheritance and 12 X chromosome SNPs sites which come from 37 groups in different regions in the whole world, have the hypermorphallel frequency and low difference and achieve independent inheritance are screened from 25,580,678 SNPs sites in a genome-wide scale. Compared with an existing commercial kit, the SNPs site combination has higher and wider multiracial adaptation, the cumulative individual recognition probability and the cumulative probability of exclusion are both significantly superior to those of the commercialkit, the detection result is more accurate, and a great application prospect is achieved.
Owner:SUN YAT SEN UNIV
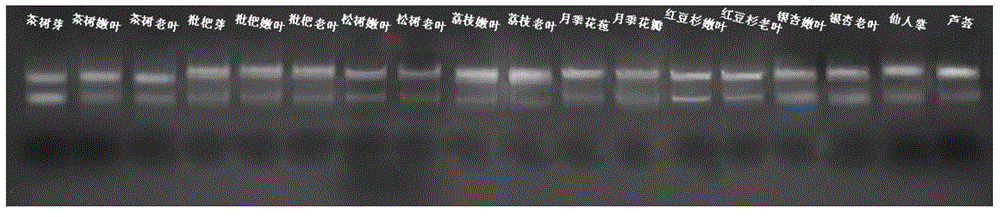
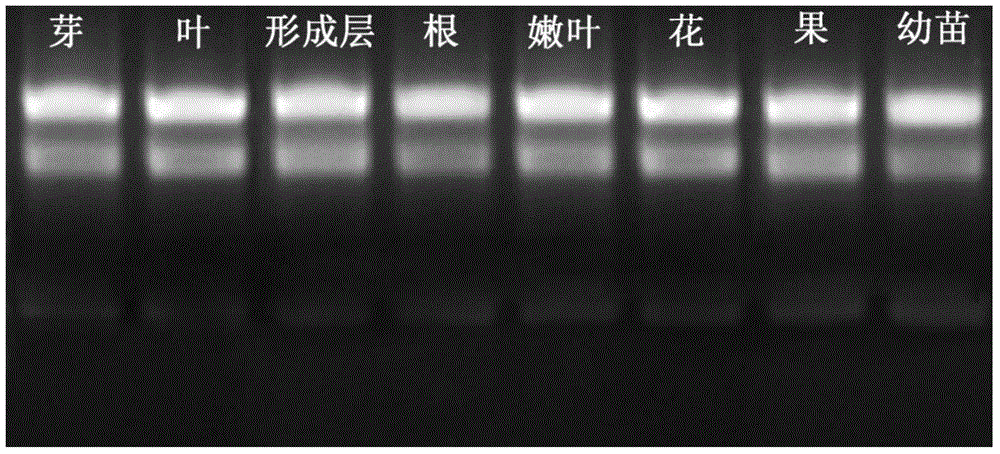
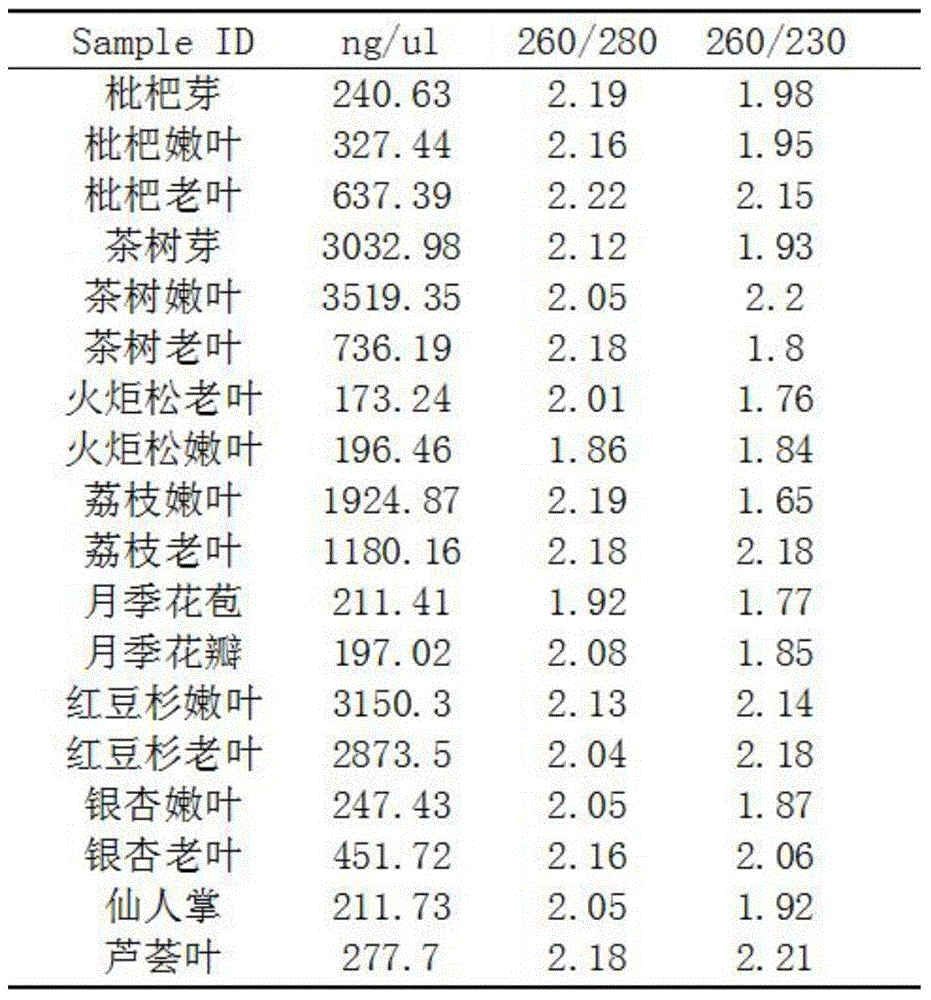
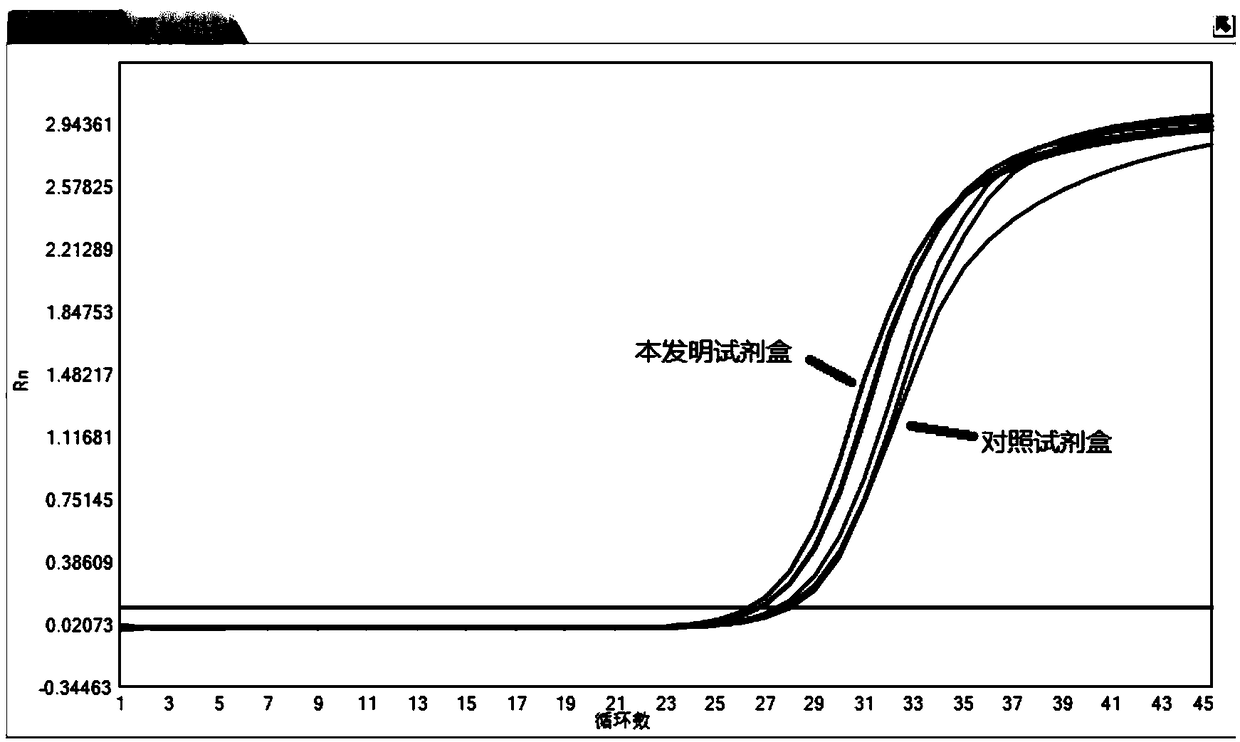
High-quality high-efficiency polyphenol polysaccharide plant sample RNA (ribonucleic acid) extraction method
The invention relates to a polysaccharide polyphenol plant sample RNA (ribonucleic acid) extraction method. By combining a CTAB (cetyltrimethylammonium bromide) process and a commercial kit provided by an OMEGA kit, the invention develops a high-efficiency high-quality RNA extraction method. The inspection proves that the method is applicable to various tissues of Neolamarckia cadamba, Camellia sinensis L., Eriobotrya japonica Lindl., Rosa chinensis, Litchi chinensis Sonn., Ginkgo biloba L., Opuntia ficus-indica L., Pinus taeda L., Aloe barbadensis Mill., Taxus media and other plants, and provides a favorable option for RNA extraction and preparation in molecular biology experiments in future, and the effect is good.
Owner:SOUTH CHINA AGRI UNIV
Kit and method for extracting viral nucleic acid
InactiveCN108642044AHigh recovery rateEasy to operateMicrobiological testing/measurementDNA preparationMagnetic beadProteinase K
The invention discloses a kit and method for extracting viral nucleic acid from a blood screening mixed detection sample. The kit comprises a lysate, a binding solution, a first rinsing solution, a second rinsing solution, an eluent, silicon dioxide magnetic beads, proteinase K and nucleic acid settling agent, wherein the first rinsing solution contains the binding solution and sodium citrate. Theinvention further discloses a method for rapidly detecting viruses in plasma / serum. With the kit and the method provided by the invention, trace of viral nucleic acid can be easily captured from thesample under the specific pH, and the recovery rate of the obtained nucleic acid is high; the operation procedure is concise, the extraction and purification of viral nucleic acid can be completed within 30min, and thus the kit is more time-saving and labor-saving than the existing majority of commercial kits; the aimed sample volume dose is large, combined extraction can be carried out on small-volume samples, thus time is saved, and the reagent is saved.
Owner:GUANGZHOU YIXIN BIOTECH CO LTD
Soluble expression method of recombinantpeste des petits ruminant virus H-F fusion protein
ActiveCN107236047AImprove expression levelEasy to monitorSsRNA viruses negative-senseAntibody mimetics/scaffoldsMicroorganismCommercial kit
The invention discloses a soluble expression method of a recombinantpeste des petits ruminant virus H-F fusion protein. The solubleexpressionmethod comprises a step of expressing an encoding gene of a protein in a living thing, thereby obtaining the protein, wherein the living thing is a microorganism, a plant or a non-human animal; the protein is a protein of a) or b), namely a) a protein consisting of amino acid sequences of SEQ ID No.2, and b) a soluble protein generated by substituting and / or deleting and / or adding one or more amino acid residues into an amino acid sequence of SEQ ID No.2. The recombinantpeste des petits ruminant virus H-F fusion protein treated with the solubleexpression method of the recombinantpeste des petits ruminant virus H-F fusion protein disclosed by the invention is high in expression level and low in production cost, and a basis is made for furtherdevelopment of commercial kits.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Foot-and-mouth disease virus 2C3ABC recombinant protein as well as preparation method and application thereof
InactiveCN104788547AShow detection specificityShorten the emergency response time for prevention and controlSsRNA viruses positive-senseVirus peptidesBacteroidesSolubility
The invention discloses a foot-and-mouth disease virus (FMDV) 2C3ABC recombinant protein as well as a preparation method and application thereof, and belongs to the field of pharmaceutical biotechnology. According to the invention, the mutation of the following sites is performed on the basis of an original FMDV 3C protease gene: Cys142-Ser, Cys163-Gly. FMDV recombinant protein 2C3ABC is expressed as an inclusion body in the bacterial cytoplasm, and is subjected to separation, denaturation, renaturation and multi-step purification, to obtain a complete and enzymolysis-free FMDV nonstructural protein mu2C3ABC, wherein the protein has solubility and complete antigenicity, and has a molecular weight of 72 kDa. The protein, as a diagnostic antigen, is prepared into chromatographic strips, has sensitivities of 98.4% and 100% respectively in the detection of FMDV experimentally infected pigs and naturally infection-free pigs, and has specificities of 100% and 98% respectively in the detection of naturally infection-free pigs and vaccine-immunized pigs. Compared with commercial kits Ceditest and UBI, the FMDV 2C3ABC recombinant protein has a high degree of consistency, can be used to distinguish infected animals and immunized animals, wherein K = 0.823 (p is smaller than 0.05).
Owner:吕宏亮 +2
Protein chip antibody detection kit for avian infectious bronchitis virus and application thereof
The invention discloses a protein chip antibody detection kit for an avian infectious bronchitis virus and application thereof. The kit comprises (1) an IBV nsp5 protein chip prepared from a recombinantly expressed IBV non-structural protein 5; (2) an IgY enzyme-labeled antibody solution diluted by using an antibody diluent; (3) a 20*TBST concentrated washing solution; (4) diluent serum; (5) a positive control sample, i.e., anti-IBV nsp5 chicken serum; (6) a negative control sample, i.e., negative SPF chicken serum; (7) chemiluminescent substrate liquid including chemiluminescent liquid A andluminescent substrate liquid B; and (8) a TMB color-developing solution. The IBV protein chip antibody detection kit provided by the invention uses the non-structural protein nsp5 as an antigen, has the characteristic of high coincidence rate with imported commercial kits and is substantially reduced in cost, faster in detection, higher in sensitivity and simple to operate.
Owner:NANJING AGRICULTURAL UNIVERSITY
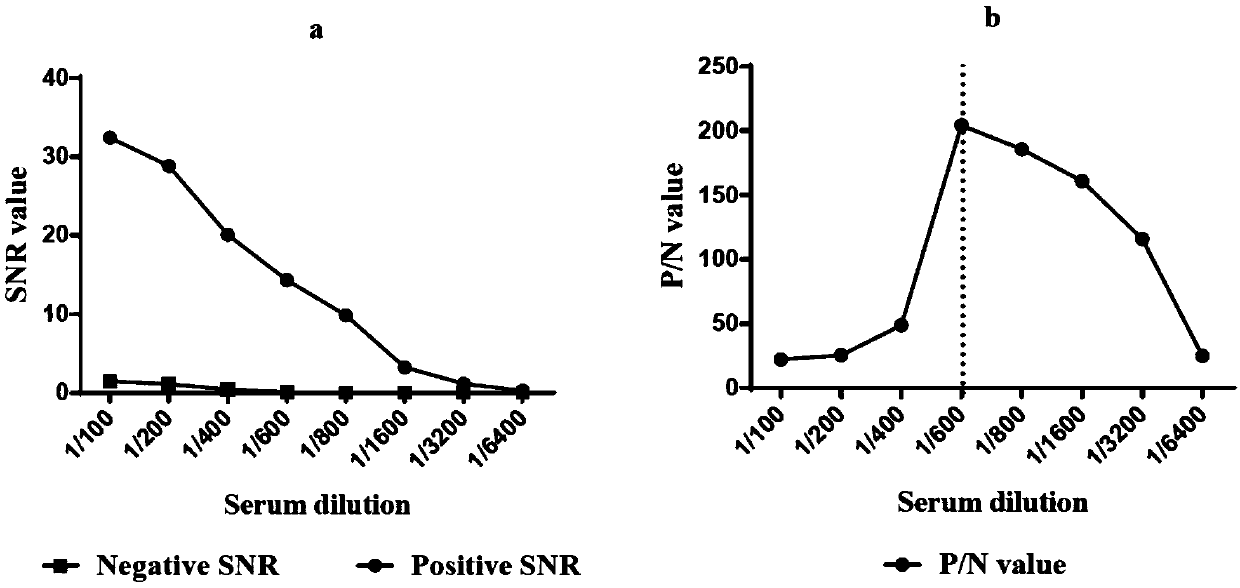
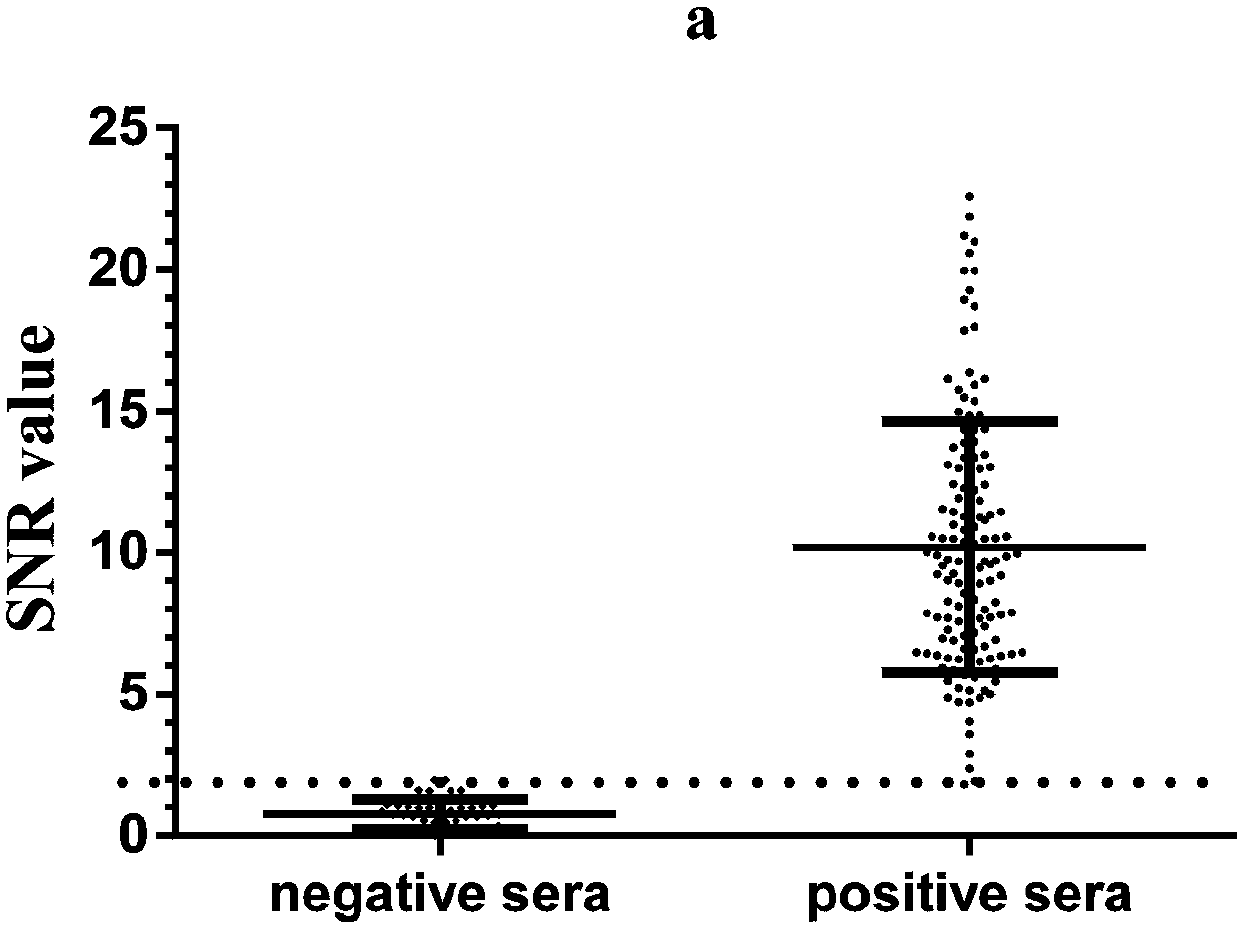
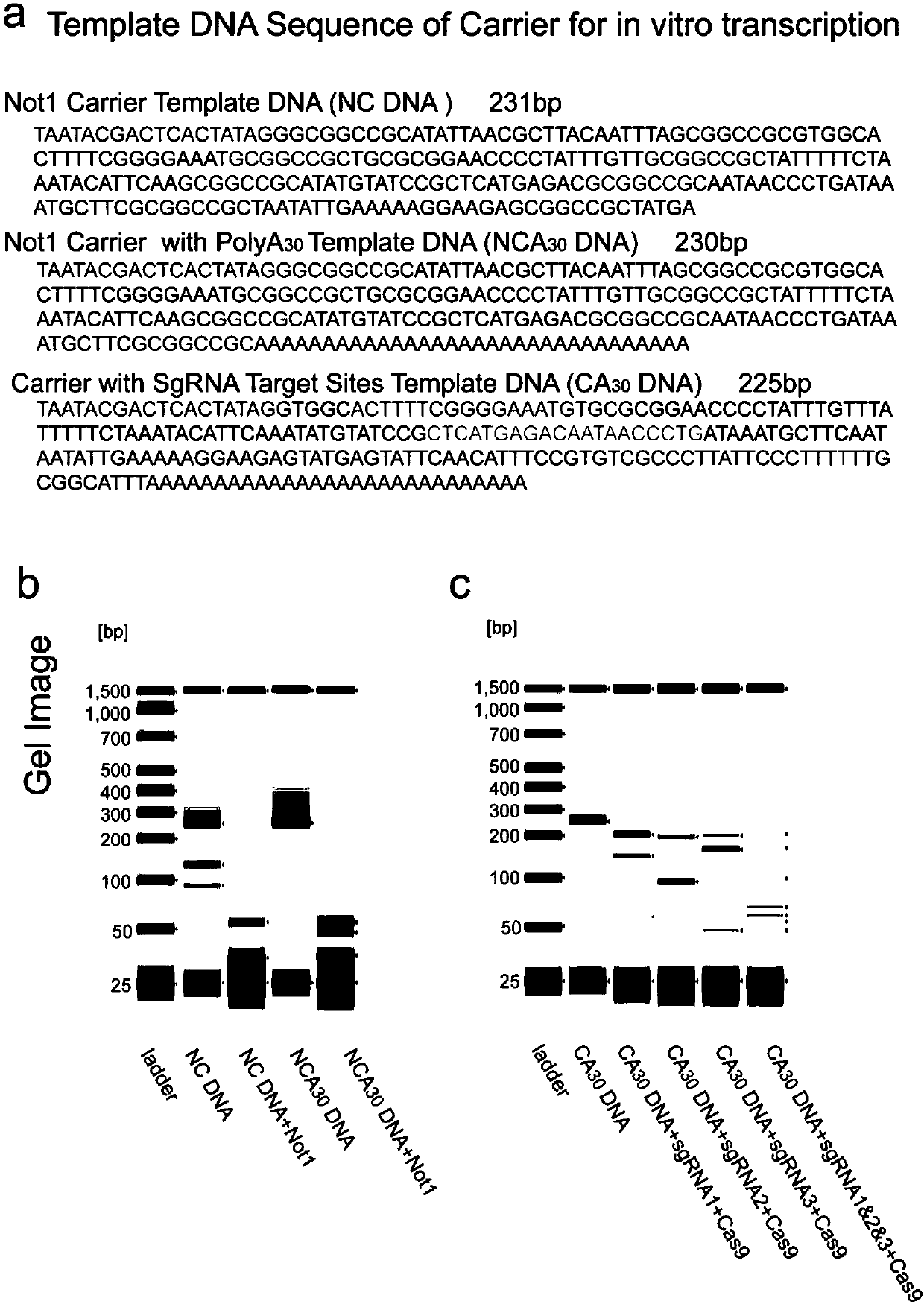
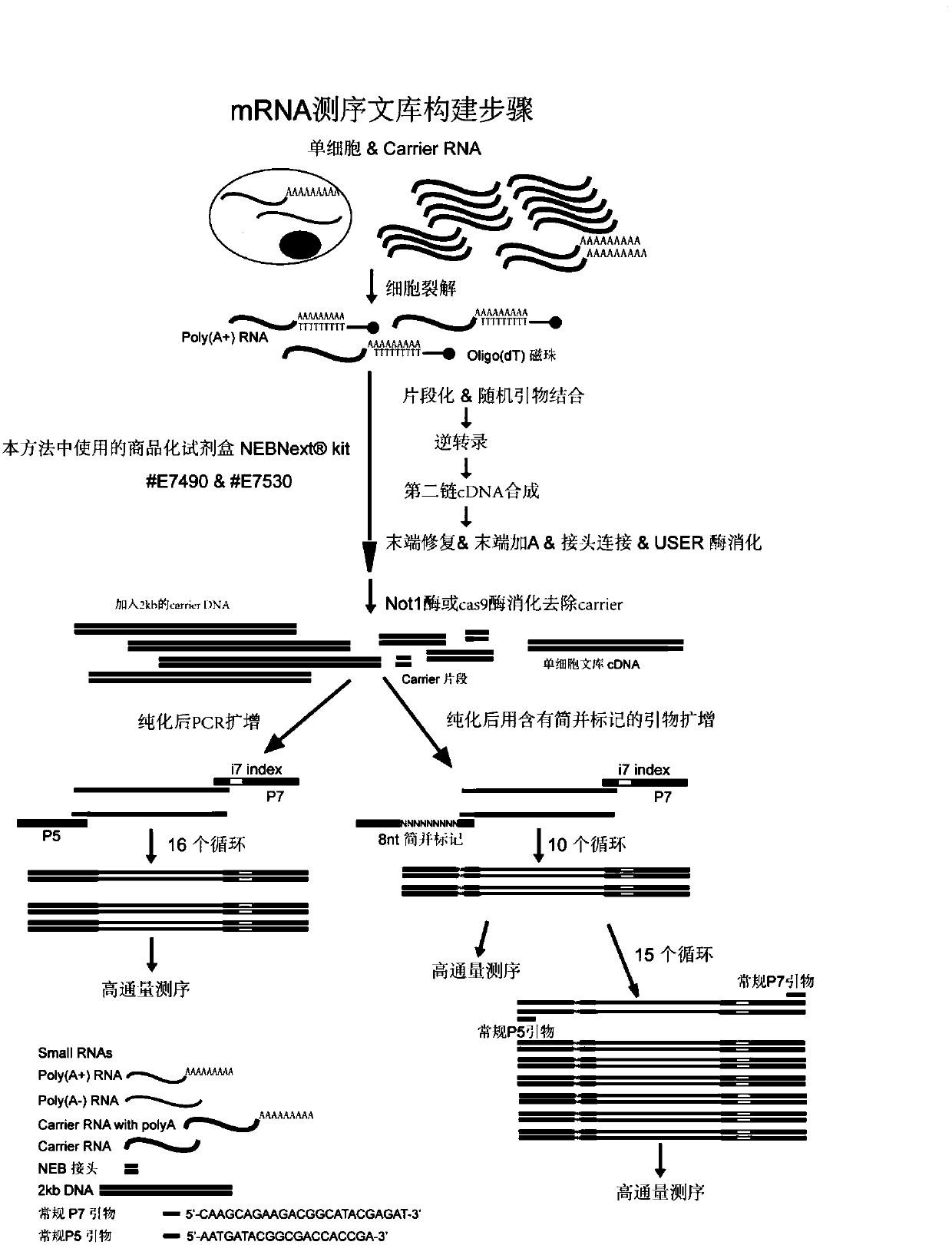
Construction method of single cell RNA sequencing library
ActiveCN110157785AWon't interfereLower Sequencing CostsMicrobiological testing/measurementLibrary creationDigestionCommercial kit
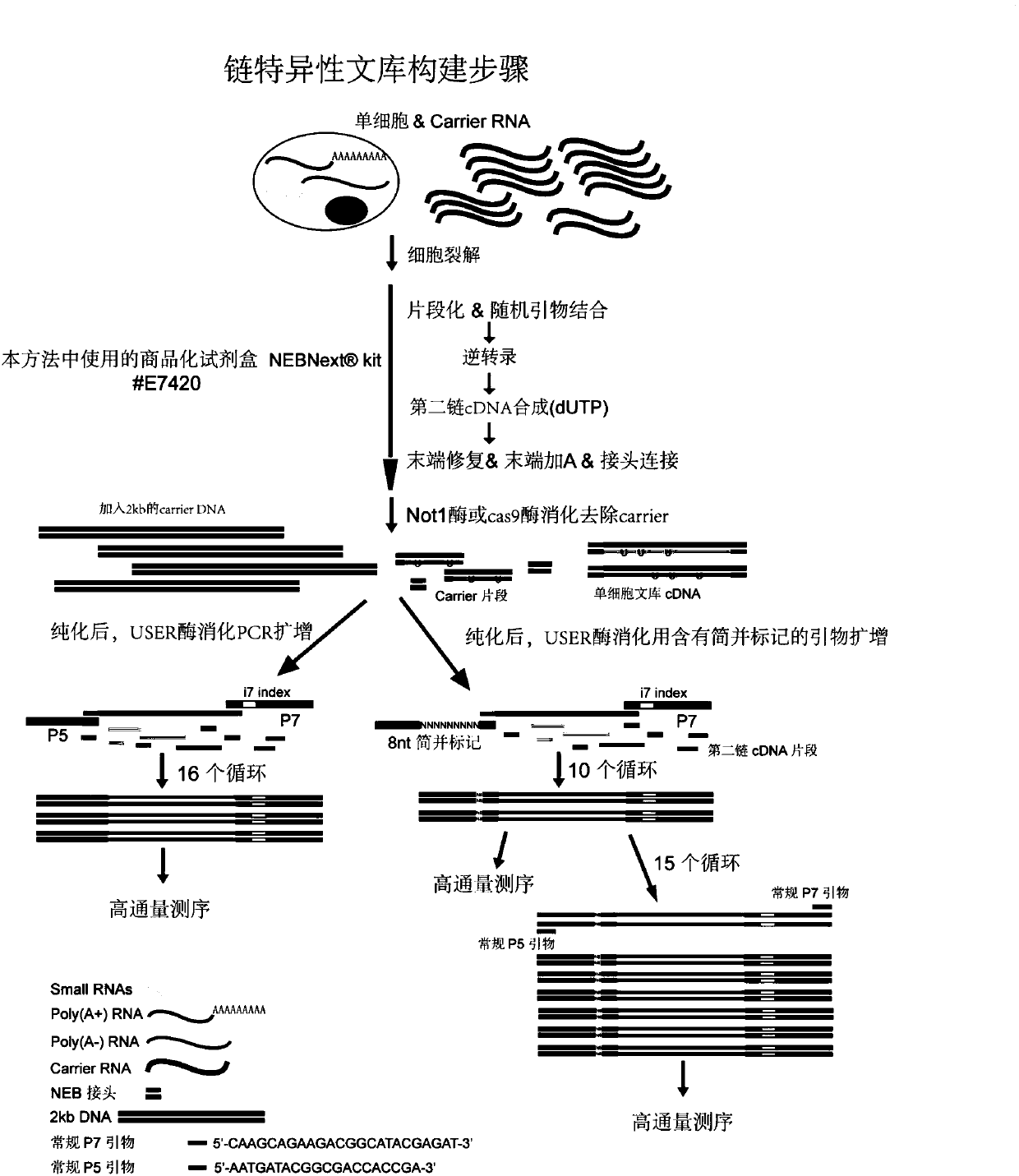
The invention relates to the field of biotechnology and provides a construction method of a single-cell RNA sequencing library. The method includes the following steps that 1, carrier RNA is added ina single cell lysis system to ensure the RNA quantity required by library construction without pre-amplification of full-length cDNA; 2, the library is constructed by using a commercial kit or RNA sequencing library construction method; 3, DNA formed through carrier RNA transcription is removed from the library; 4, high-throughput sequencing is performed. The pre-amplification step of the full-length cDNA in an existing method is omitted, in the library construction process, the added RNA with the known sequence is subjected to digestion removal and prevented from causing interference to sequencing, and the sequencing cost is greatly reduced.
Owner:ZHEJIANG UNIV
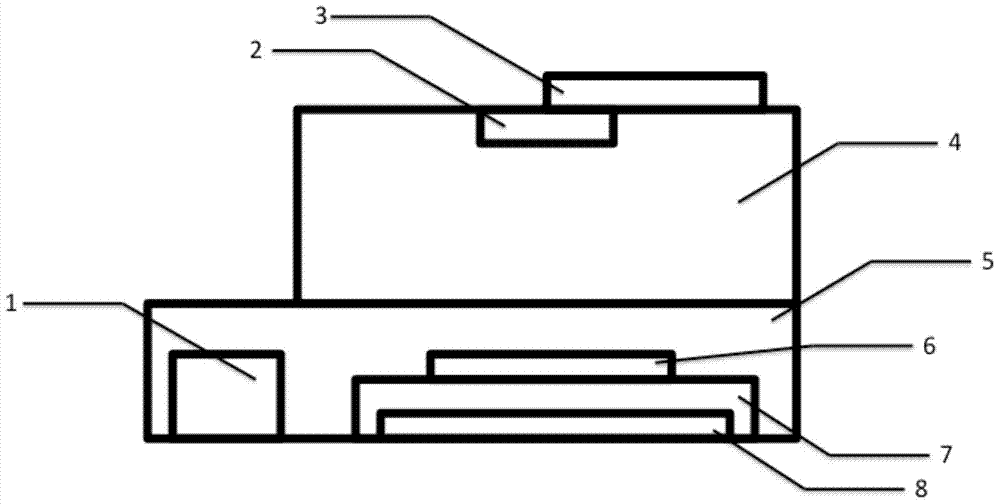
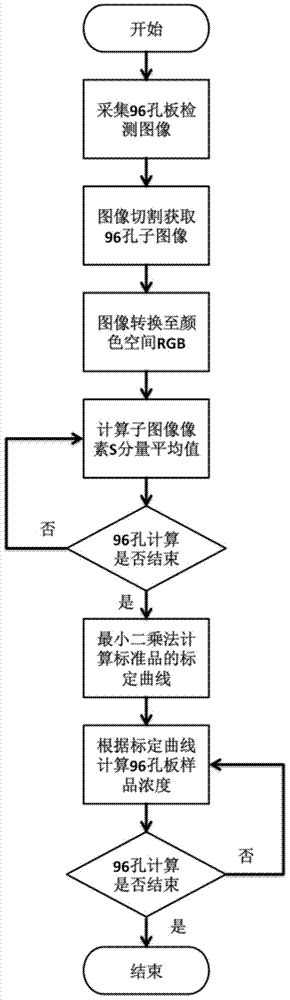
Diarrhetic shellfish toxin high throughput detection device and method based on image analysis
The invention discloses a diarrhetic shellfish toxic high throughput detection device and method based on image analysis. The device comprises a power supply adapter, a wide-angle lens, an intelligent mobile facility, a first darkroom, a second darkroom, a commercial reagent detection plate, a detection plate loading stage, and an electroluminescent panel. The method comprises the following steps: preparing a sample solution of diarrhetic shellfish toxin, then preparing a standard solution, using a commercial kit to prepare a detection plate to be detected; collecting and analyzing detection images to calculate the color ratio (CR) of R component of RGB space in a detection plate hole image; drawing a calibration curve of the commercial kit to detect the diarrhetic shellfish toxin through a least square method; obtaining the hole image CR value of the sample solution, and substituting the CR value of the sample solution into the calibration curve to calculate the concentration of the diarrhetic shellfish toxic of the sample solution. The provided device achieves the high throughput quantitative detection of diarrhetic shellfish toxic, and has the advantages of simple operation steps, low cost, and suitability for on-site detection.
Owner:ZHEJIANG UNIV
DNA methylation detection method of specific gene segment
InactiveCN105368947AAccurate analysisAvoid cumbersome stepsMicrobiological testing/measurementDNA methylationA-DNA
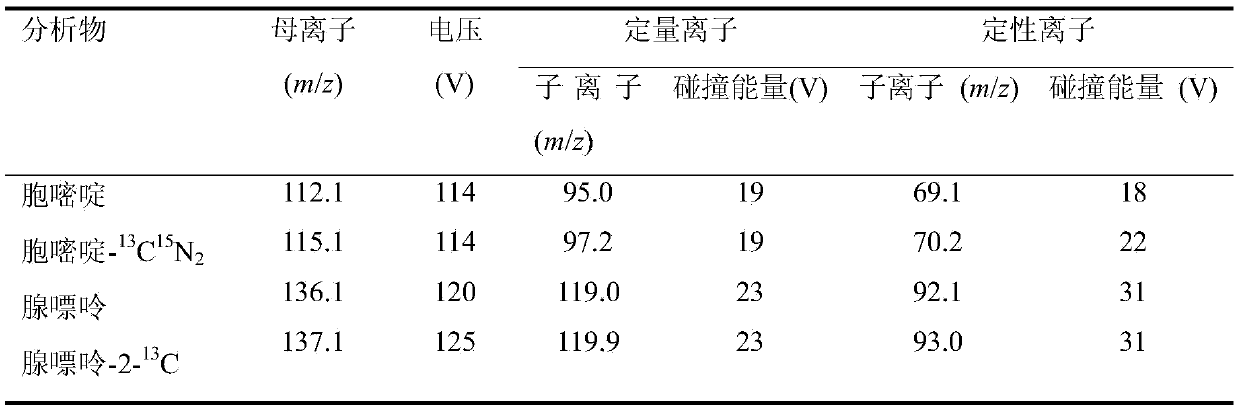
The invention discloses a DNA methylation detection method of a specific gene segment. The method is characterized in that genome DNA of a biological sample is extracted through a commercial kit method, and the specific gene segment is obtained by using the polymerase chain reaction technology after the genome DNA is subjected to bisulfite conversion; a chemical splitting method or enzymolysis method is used to process the target gene segment product to allow the same to be split from a chain sequence into single basic groups or nucleosides; an internal standard method is used to detect the contents of cytosine and adenine or the nucleosides of the adenine through liquid-phase tandem mass spectrum (LC-MS / MS), and the methylation rate of the target gene segment is calculated according to a formula. By the method, the DNA methylation level of the specific gene segment can be detected fast, efficiently and accurately in a quantified manner.
Owner:GUANGDONG MEDICAL UNIV
Few-sample whole genome DNA (deoxyribonucleic acid) methylation detection method and kit
InactiveCN108796057AThe operation method is simple and efficientLow costMicrobiological testing/measurementDNA methylationImmunoprecipitation
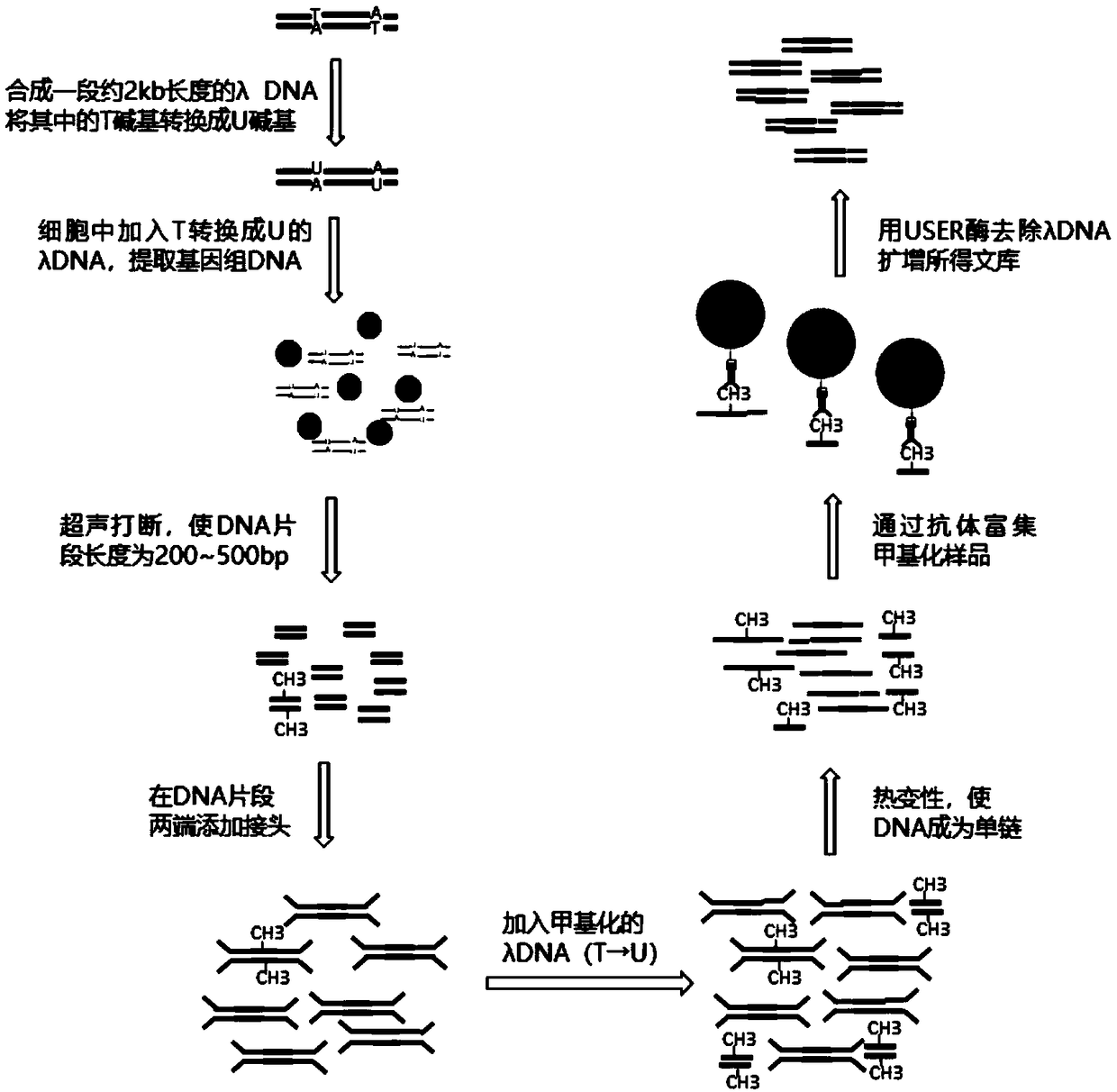
The invention discloses a few-sample whole genome DNA (deoxyribonucleic acid) methylation detection method, and relates to a method for detecting less-sample (300pg DNA or 50 cells) whole genome DNA methylation by an improved MeDIP-Seq technique based on high-throughput sequencing. According to the method, lambda DNA containing dUTP is added into a small number of initial samples to reduce sampleloss, base-building and immuno-precipitation efficiency is improved, MeDIP-DNA is digested by USER enzyme, so that added methylation lambda DNA is removed, and a methylation sample sequencing libraryto be detected cannot be polluted by the methylation lambda DNA. According to the method, needed sample amount is 300pg DNA or 50 cells, the method is more applicable to MeDIP-Seq analysis of a smallnumber of source samples, operation method of a MeDIP experimental technique and a kit matched with the experimental technique is simple and convenient, specific commercial kits and experimental devices are omitted, cost is low, and the method has wide applicability and universality.
Owner:SHANGHAI JIAO TONG UNIV
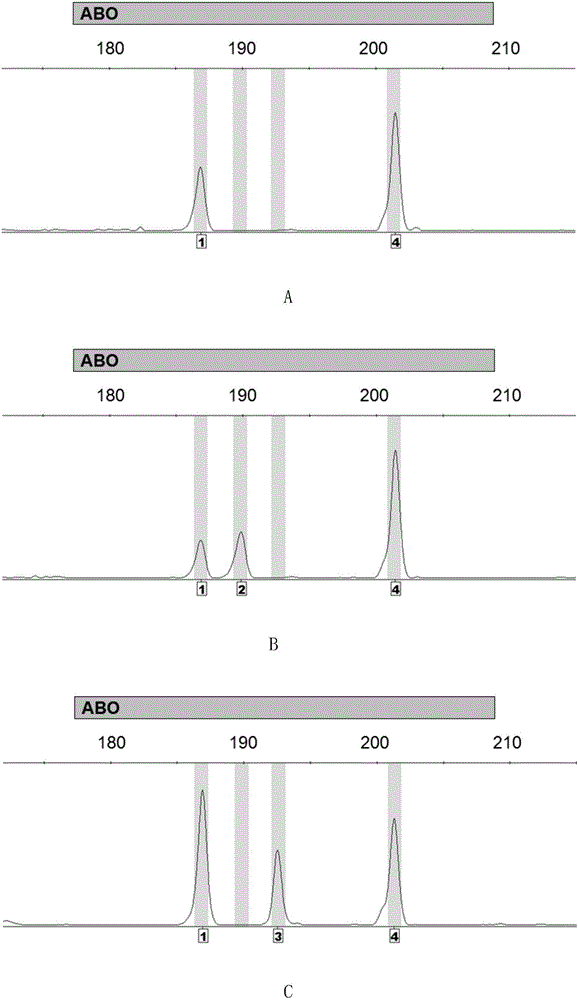
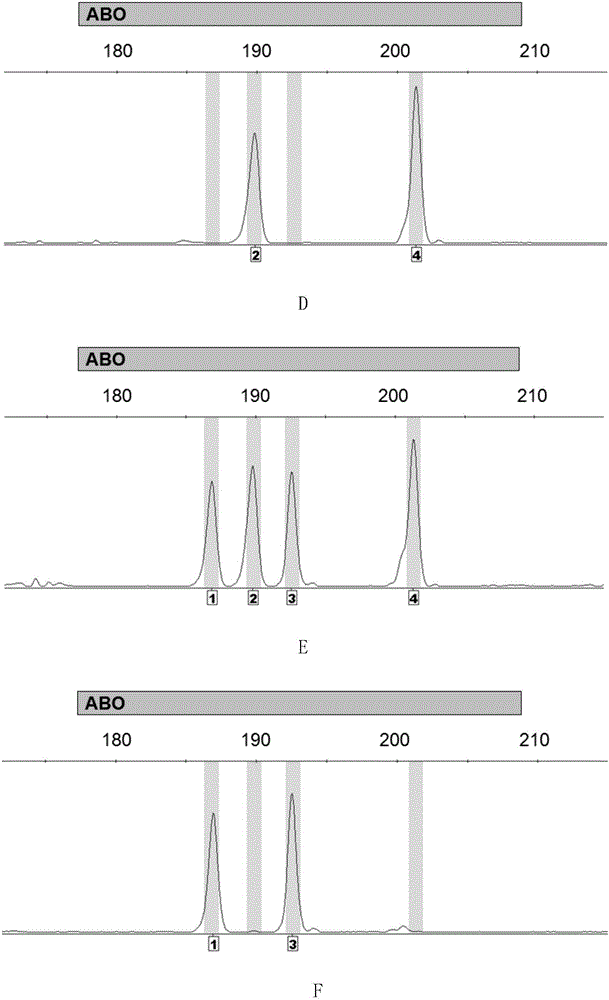
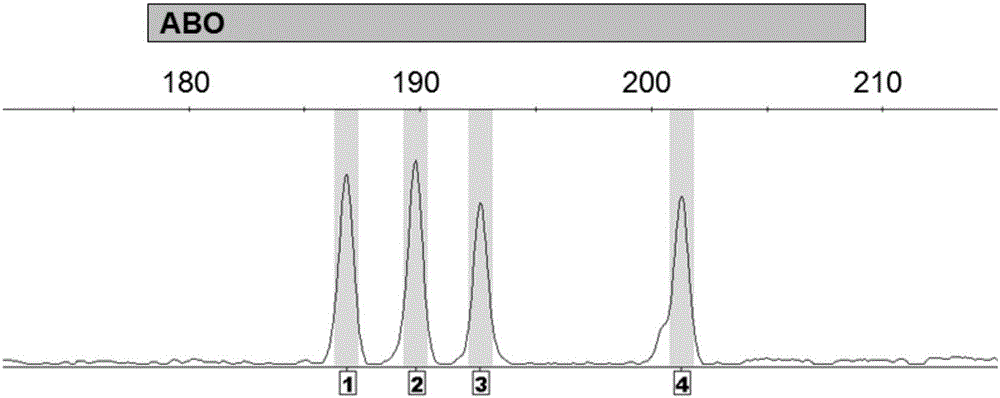
Method for detecting ABO blood-group genotype and template for allelic ladder of ABO blood-group gene locus
ActiveCN106834483AMicrobiological testing/measurementVector-based foreign material introductionGenotypeAllele
The invention discloses a method for detecting the ABO blood-group genotype and a template for an allelic ladder of an ABO blood-group gene locus. The method comprises the following steps: taking a genome DNA or general DNA of a to-be-detected sample as a template to perform PCR amplification by adopting primer combination to obtain a PCR amplified product; then, determining which alleles are contained, and further determining the ABO blood-group genotype of the to-be-detected sample, wherein the primer combination consists of 6 primers having nucleotide sequences being sequence 1 to sequence 6 in the sequence table sequentially. Experiments prove that the precision rate for detecting the ABO blood-group genotype by adopting the method is 100 percent; the recombinant plasmid composition provided by the method, serving as a template of an allelic ladder of an ABO blood-group gene locus, can be amplified to obtain the allelic ladder of the ABO blood-group gene locus, and can be applied to various commercial kits. The method and the template have an important application value.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Method for quickly extracting ribonucleic acid (RNA) from ginseng leaf tissues
The invention discloses a method for quickly extracting ribonucleic acid (RNA) from ginseng leaf tissues, and relates to a method for extracting the RNA. The method solves the problems that steps are complex, the consumed time is long, more medicaments are used, and the cost is high existing in the conventional method for extracting the RNA from the ginseng leaf tissues. The method comprises the following steps of: 1, grinding the ginseng leaf tissues into powder in liquid nitrogen, adding extraction buffer solution, beta-mercaptoethanol, Tris balanced phenol and chloroform, oscillating, and centrifuging; 2, taking supernatant, adding the Tris balanced phenol and the chloroform, oscillating, centrifuging, taking supernatant, adding the chloroform, oscillating, and centrifuging; 3, taking supernatant, adding isopropanol, precipitating, centrifuging, and abandoning supernatant; and 4, washing an RNA precipitate by using ethanol, and air-drying the precipitate in the air to obtain the RNA. Compared with the conventional cetyltrimethyl ammonium bromide (CTAB) method and the like, the method has the advantages that the extraction effect is higher, the time is short, and the method is easy to operate; and the extraction cost is low compared with that of a commercial kit.
Owner:NORTHEAST FORESTRY UNIVERSITY
Method for quantitatively detecting pork ingredients in beef products
The invention relates to the field of food detection, in particular to a method for quantitatively detecting pork ingredients in beef products. The method is characterized by including the steps of firstly, preprocessing a sample; secondly, extracting DNA; thirdly, measuring DNA concentration and purity; fourthly, performing a real-time fluorescence PCR reaction; fifthly, calculating the result. The method for quantitatively detecting the pork ingredients in the beef products has the advantages that the method is simple in detection process and accurate in result, and the extracted pork concentration reference substance DNA can be kept for a long time; compared with a method which needs to purchase commercial kits, the method is low in cost, and the needs of pork adulteration identification are satisfied.
Owner:英格尔检测技术服务(上海)有限公司
Preparation method of amino magnetic nanoparticles and application thereof in DNA (desoxyribonucleic acid) extraction
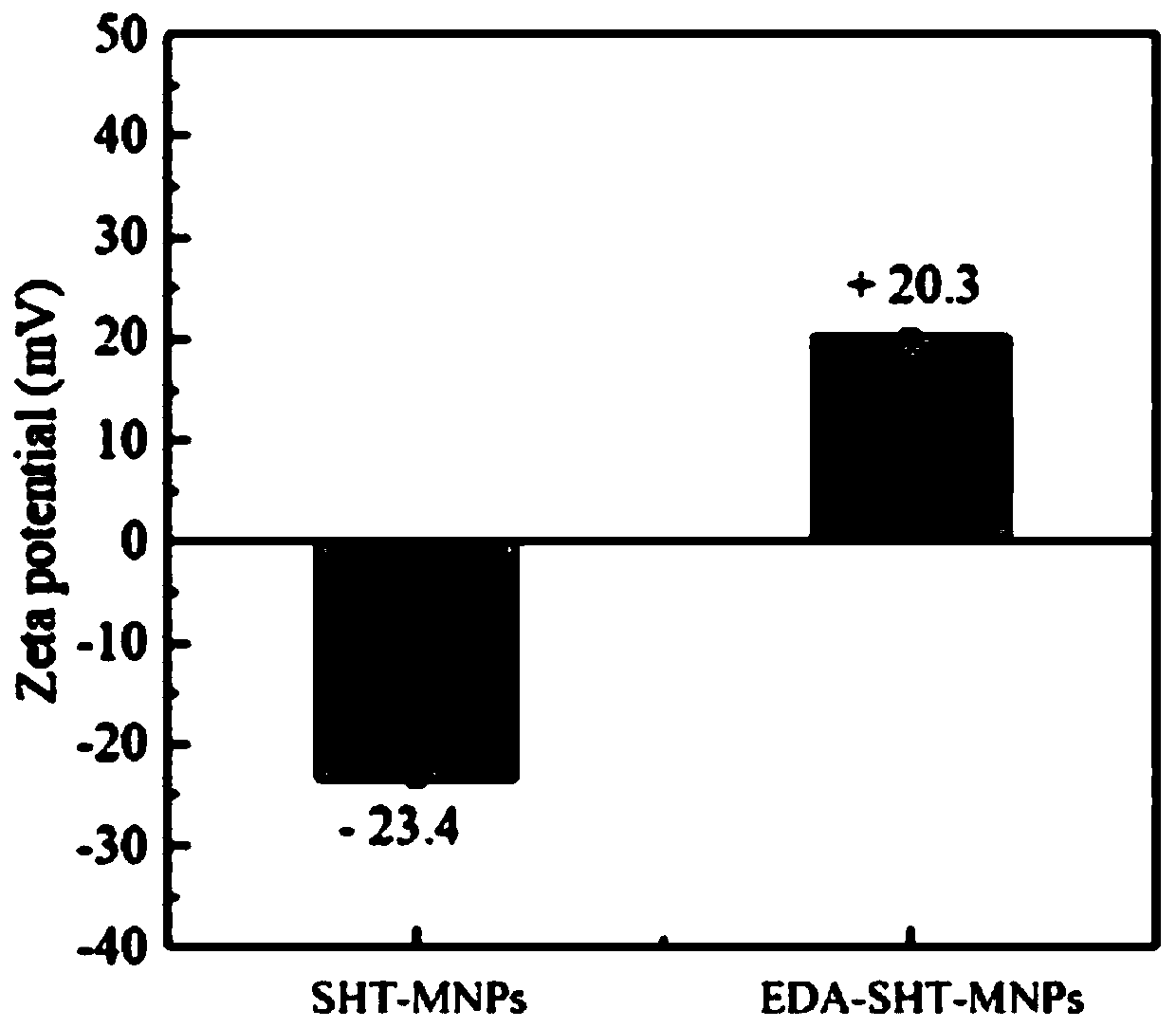
ActiveCN110548489ANo three wastes producedNo GreenOther chemical processesAlkali metal oxides/hydroxidesEthylenediamineMagnetite Nanoparticles
The invention provides a preparation method of amino magnetic nanoparticles and application thereof in DNA (desoxyribonucleic acid) extraction. The preparation method of the amino magnetic nanoparticles comprises the following steps: (1) preparing Fe3O4 magnetic nanoparticles; (2) preparing sodium bitartrate coated Fe3O4 magnetic nanoparticles; and (3) preparing ethidene diamine modified sodium bitartrate coated Fe3O4 magnetic nanoparticles. The amino magnetic nanoparticles prepared by using the preparation method is free of high temperature or high pressure in the synthesis process, are freeof waste, and thus have the characteristics of being green and environment and simple in process; compared with a conventional commercial kit, the nanoparticles are easy in raw material obtaining, lowin price, simple in synthesis step and easy to operate; and the nanoparticles have amino groups on surfaces, are at positive charge states and are beneficial to combination with DNA with negative charges, in the DNA extraction application, the magnetic nanoparticles have good DNA combination capabilities and high extraction efficiency, extraction steps are simple and convenient, and toxic reagents such as chloroform can be avoided.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
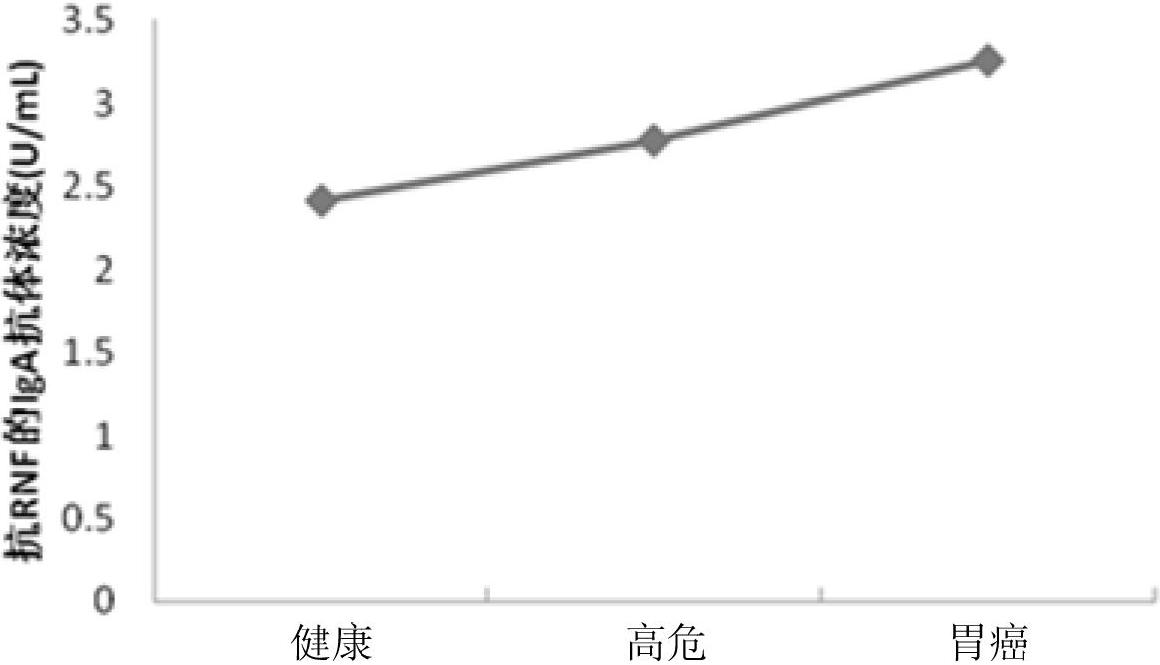
Diagnostic kit and application of RNF19 (ring finger protein 19) in preparation of reagent for early diagnosis of gastric cancers
The invention relates to the field of bioscience, in particular to a diagnostic kit and application of an RNF19 (ring finger protein 19) in preparation of a reagent for early diagnosis of gastric cancers. The diagnostic kit is used for early diagnosis of gastric cancers and comprises an ELISA (enzyme-linked immunosorbent assay) plate, a human protein RNF19, standard serums, an ELISA reagent, an enzyme substrate solution, confining liquid, sample diluent, washing liquid and stop liquid, wherein the human protein RNF19 is enveloped on the ELISA plate. Compared with the prior art, the diagnostic kit has the following advantages: 1. a sensitive, safe, reliable and easy-to-operate commercial kit is provided, is used for qualitatively determining the level of the IgA antibody against RNF19 in the human serums, and is conductive to early diagnosis of gastric cancers in an aided manner; and 2. the provided serum biomarker RNF19 has specificity of 86% and sensitivity of 86% and has the characteristics of high specificity and high sensitivity.
Owner:广州博翀生物科技有限公司
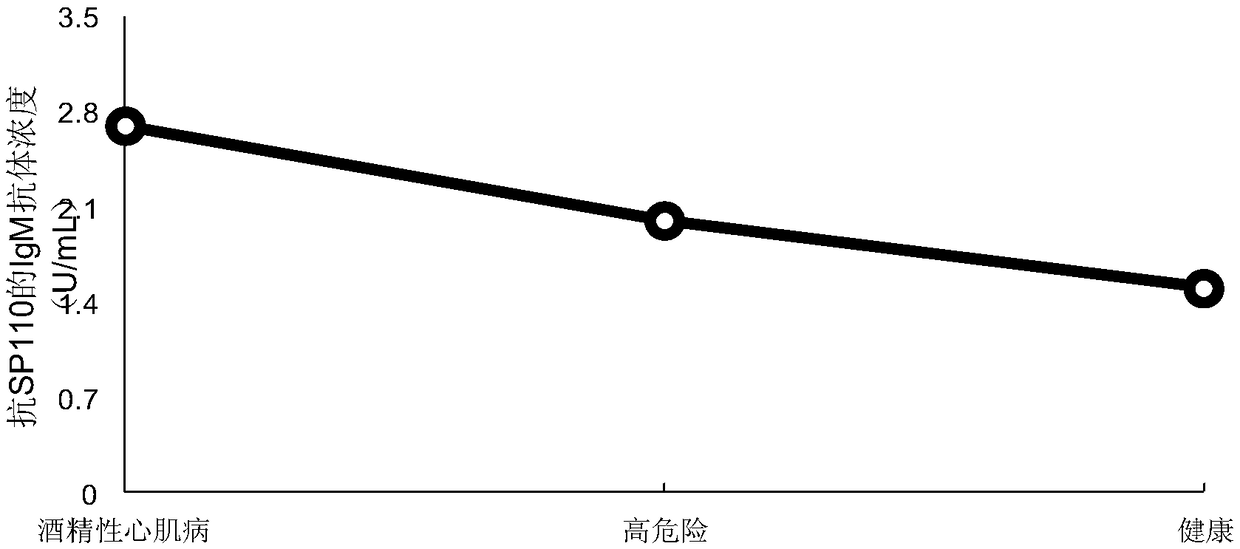
Application of karyosome protein SP110 and kit containing karyosome protein SP110 to preparation of early diagnosis reagent for alcoholic cardiomyopathy
ActiveCN108982868AEasy to operateStrong specificityDisease diagnosisBiological testingIgm antibodyBiomarker (petroleum)
Owner:HARBIN MEDICAL UNIVERSITY
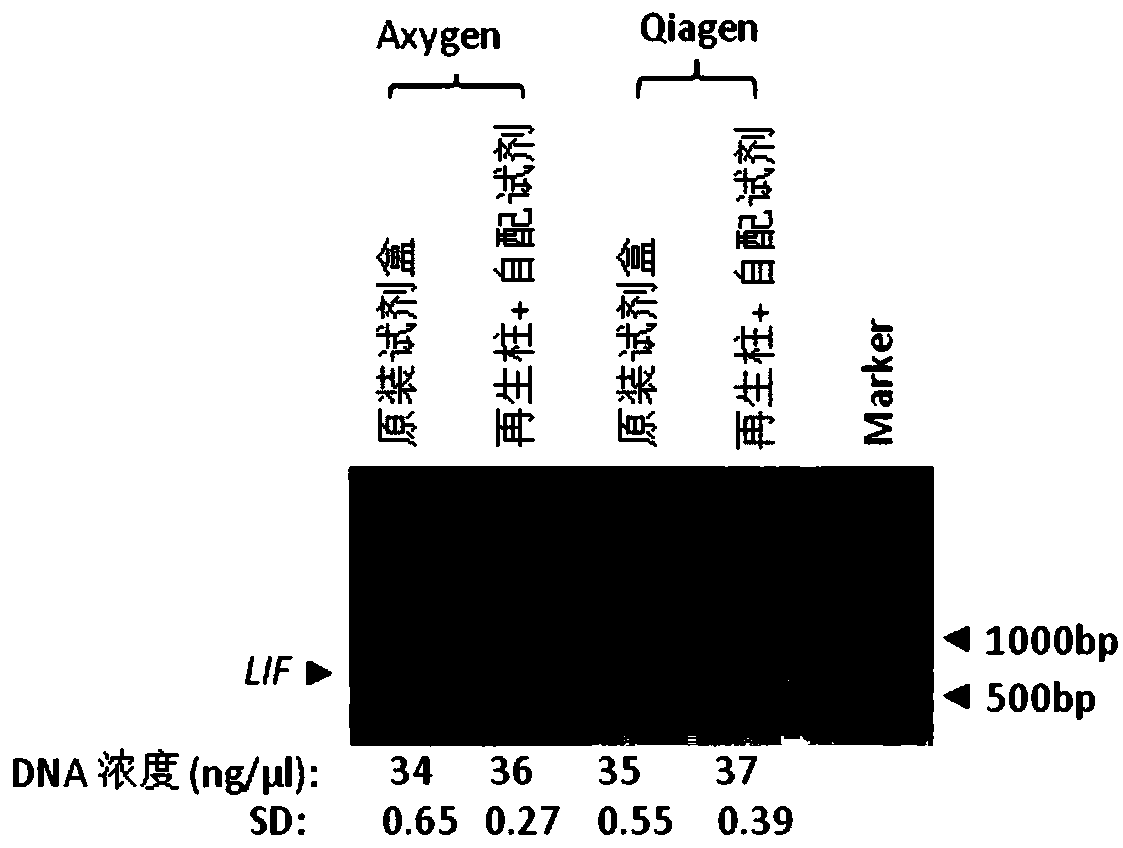
PCR purified reagent and method of purifying PCR product using same
The invention discloses a PCR purified reagent and a method of purifying a PCR product using same. The PCR purified reagent is prepared from a DNA binding buffer and a cleaning solution, wherein the DNA binding buffer is prepared from 3mol / L of sodium chloride and alcohol with volume concentration of 30%, and the cleaning solution is prepared from 10mmol / L of Tris.HCl, 100mmol / L of NaCl, 1mmol / L of EDTA, and alcohol with volume concentration of 80%, and pH of Tris.HCl is 8.0. The PCR purified reagent can be used for purifying DNA successfully without affecting DNA purification output. If the PCR purified reagent is used with a recycled silicon column, fewer disposable commercial kits are used, less laboratory rubbish is generated, the environment is protected, and social resources and usecosts are saved.
Owner:WUHAN UNIV OF SCI & TECH
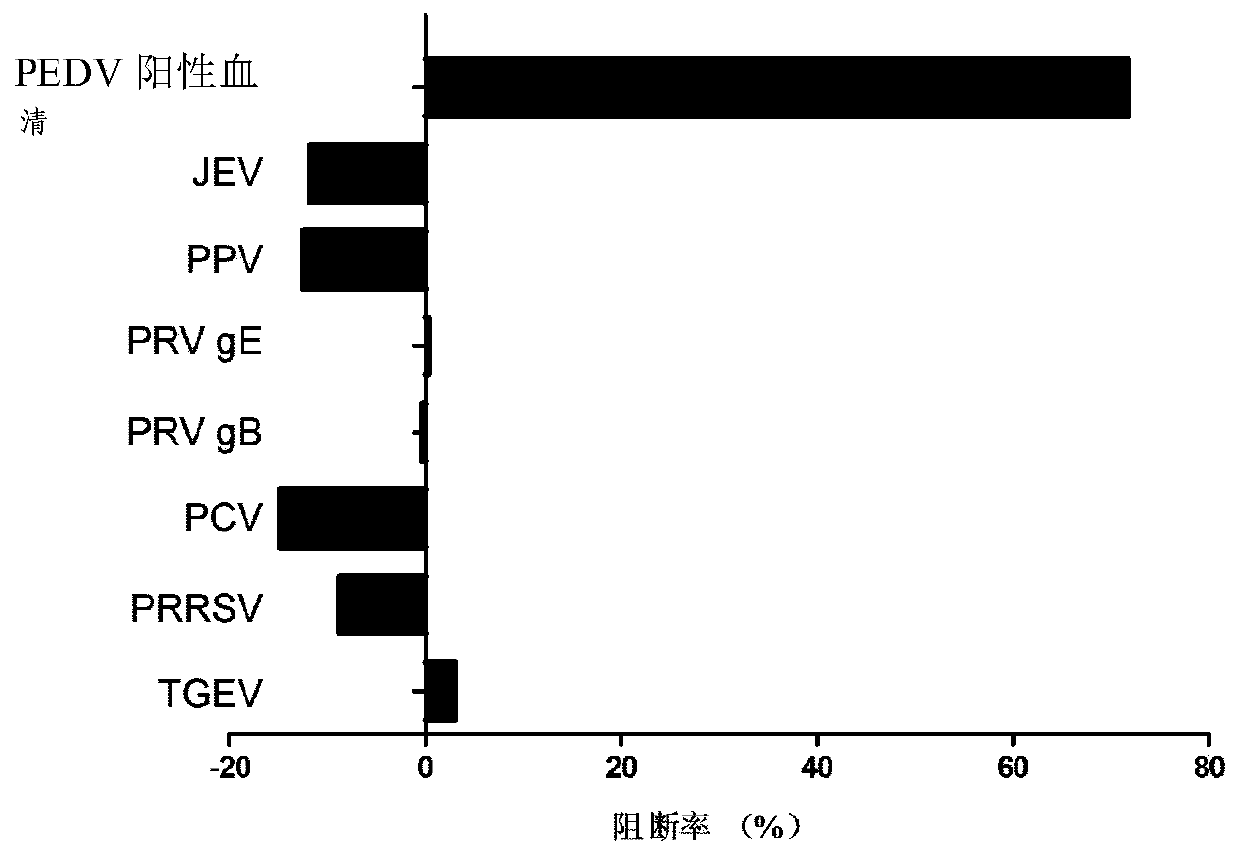
Blocking ELISA detection method based on PEDV N protein specific nano-antibody and application thereof
InactiveCN110016078AStrong specificityGood repeatabilityImmunoglobulins against virusesFermentationElisa methodSpecific antibody
The present invention discloses a blocking ELISA detection method based on PEDV N protein specific nano-antibody and an application thereof. PEDV N proteins are used to coat ELISA plates, the nano-antibody BioNb2 is used as a blocking antibody, and serum samples to be tested are used to inhibit binding of the nano-antibody BioNb2 and PEDV N proteins to detect porcine epidemic diarrhea virus-specific antibody in the serum. The blocking ELISA method is capable of excluding interference of miscellaneous protein components in antigen, has good specificity, is also simple to operate and easy for wide promotion, and has a good application prospect in serodiagnosis of porcine epidemic diarrhea viruses. A large amount of experiments optimize reaction conditions of the blocking ELISA, so that the method used for detection of the porcine epidemic diarrhea virus antibody has good repeatability, specificity and sensitivity, and has advantages of being simple, rapid, low in costs, etc. compared with currently available commercial kits.
Owner:NORTHWEST A & F UNIV
Method and kit for detecting blood-drug concentration of PD-1 antibody
The invention provides a method and a kit for detecting the blood-drug concentration of a PD-1 antibody. With the method provided by the invention, the concentration of the PD-1 antibody is detected by competition of a biotin-labeled PD-1 neutralizing antibody with the PD-1 antibody in a to-be-detected sample through a competitive ELISA method, wherein the biotin-labeled PD-1 neutralizing antibodycompetes with the PD-1 antibody in serum in binding to an antigen immobilized on an elisa plate, and horseradish peroxidase labeled streptavidin is used as a detection antibody to detect the biotin-labeled PD-1 neutralizing antibody, so the PD-1 antibody in the serum is quantified. The method provided by the invention has the advantages of good sensitivity, precision, repeated freeze-thawing stability, room-temperature storage stability, specificity, etc., and facilitates forming a commercial kit. The kit provided by the invention has the advantages of low background, good precision, batch-to-batch consistency, stability, etc.
Owner:ACROBIOSYSTEMS INC
Reagent and kit for detecting 13 CODIS STR gene loci of trace mixed human source DNA sample and application thereof
InactiveCN107557481AReach suitabilityNo knock-on effectMicrobiological testing/measurementCommercial kitAmelogenin
The invention discloses a reagent and a kit for detecting 13 CODIS STR gene loci of a trace mixed human source DNA sample and an application thereof. The reagent comprises amplification primers for 13CODIS STR gene loci and a sex gene locus, wherein the 13 CODIS STR gene loci are separately TPOX, DS31358, FGA, D5S818, CSF1PO, D8S1179, D7S820, TH01, vWA, D13S17, D16S539, D18S51 and D21S11, and thesex gene locus is Amelogenin. The sequences of the amplification primers are separately shown as SEQ ID NO: 1-SEQ ID NO: 28. The 13 CODIS STR gene loci are independent of one another without a chaineffect, and can be matched with the gene loci of an existing commercial kit and a database of the Ministry of Public Security, so that the adaptability of an analytical result is achieved; meanwhile,a single DNA molecule can be amplified and detected; on the premise of ensuring the efficiency of the system, the detection rate of degraded / broken DNA templates can be increased; the reagent and kitcan be widely applied to the fields of individual recognition by legal medical experts, paternity test and other group genetics analysis.
Owner:SUZHOU GENO TRUTH BIOTECHNOLOGY CO LTD +1
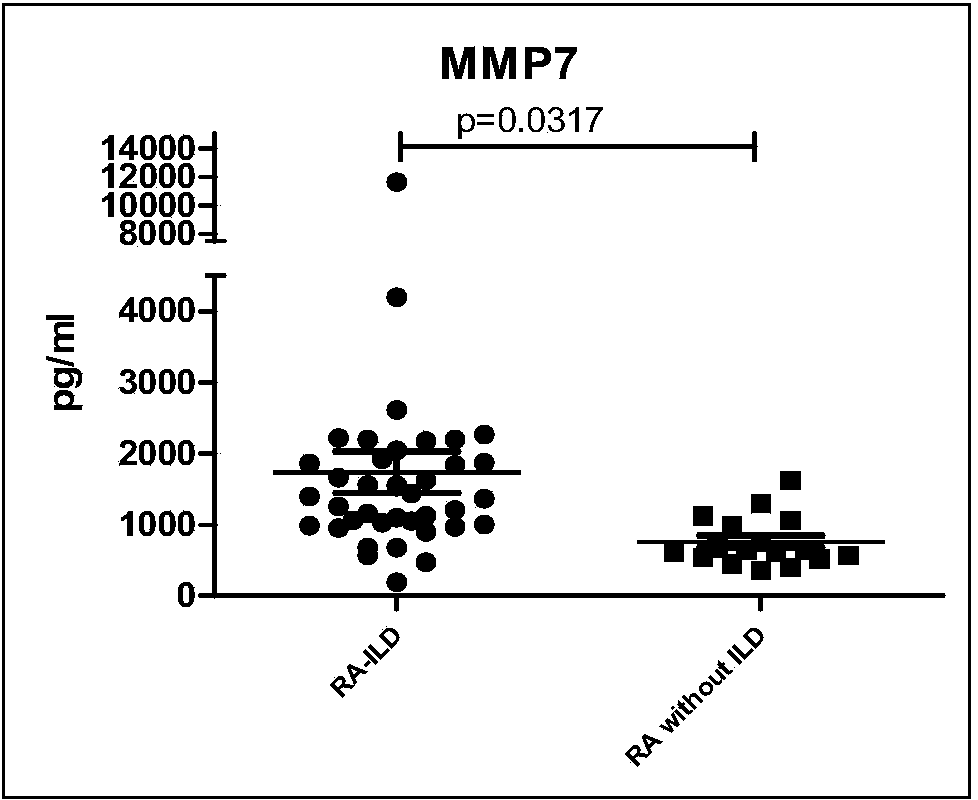
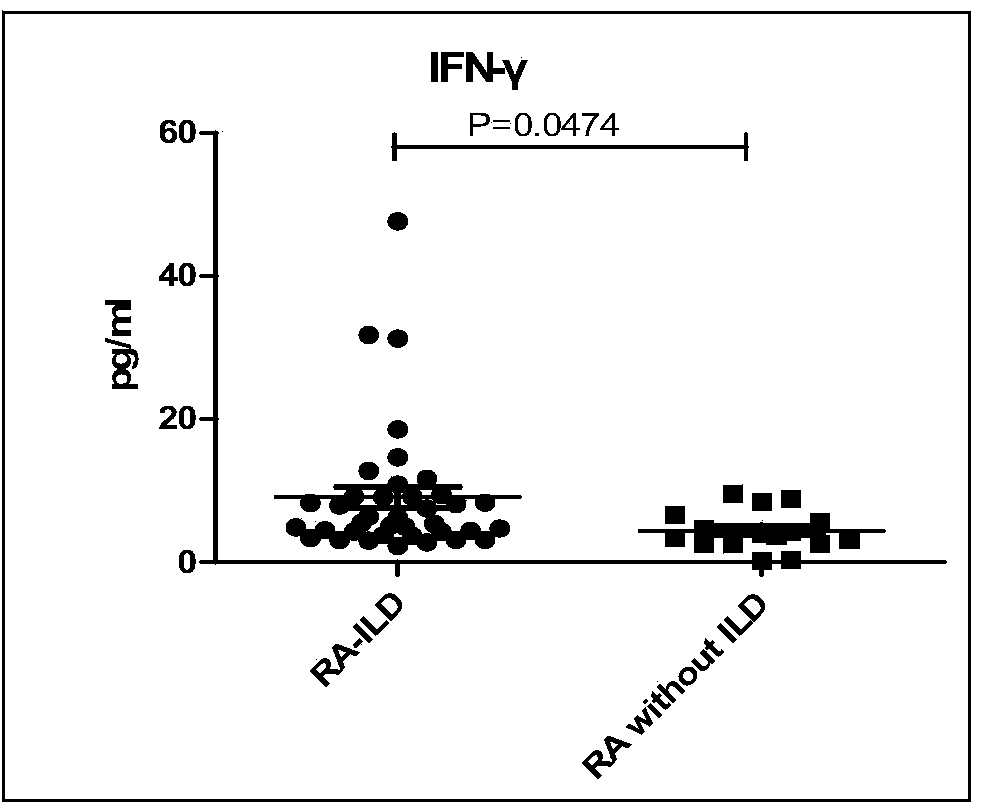
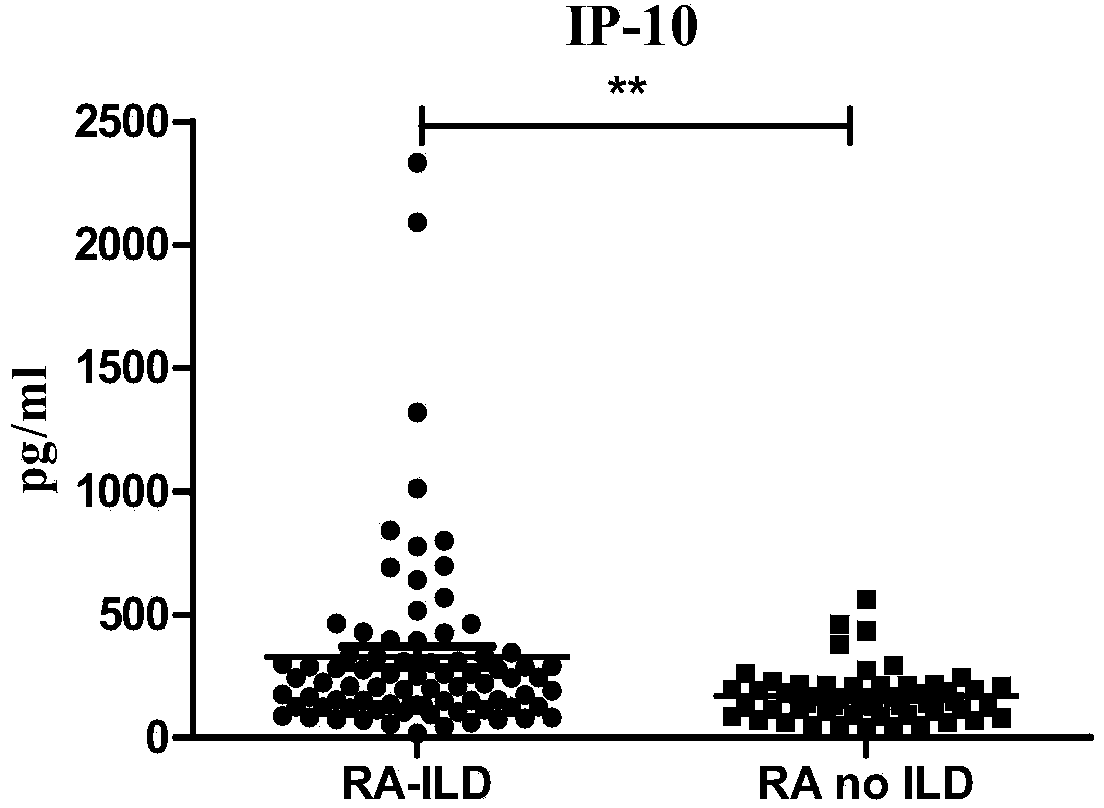
Connective tissue disease-associated early interstitial lung disease diagnostic kit
InactiveCN103698508AEasy to operatePeripheral blood detection method is accurateDisease diagnosisInterstitial lung diseaseSerum ige
The invention discloses a connective tissue disease-associated early interstitial lung disease diagnostic kit, comprising a detection module for detecting human peripheral blood MMP7, IFN-gamma and IP-10. The commercial kit provided by the invention is sensitive, safe, reliable and easy to operate; the kit is capable of quantificationally measuring the level of specific protein cytokines and chemotactic factors in human serum, and is helpful for early diagnosis of the connective tissue disease-associated interstitial lung disease.
Owner:陈娟
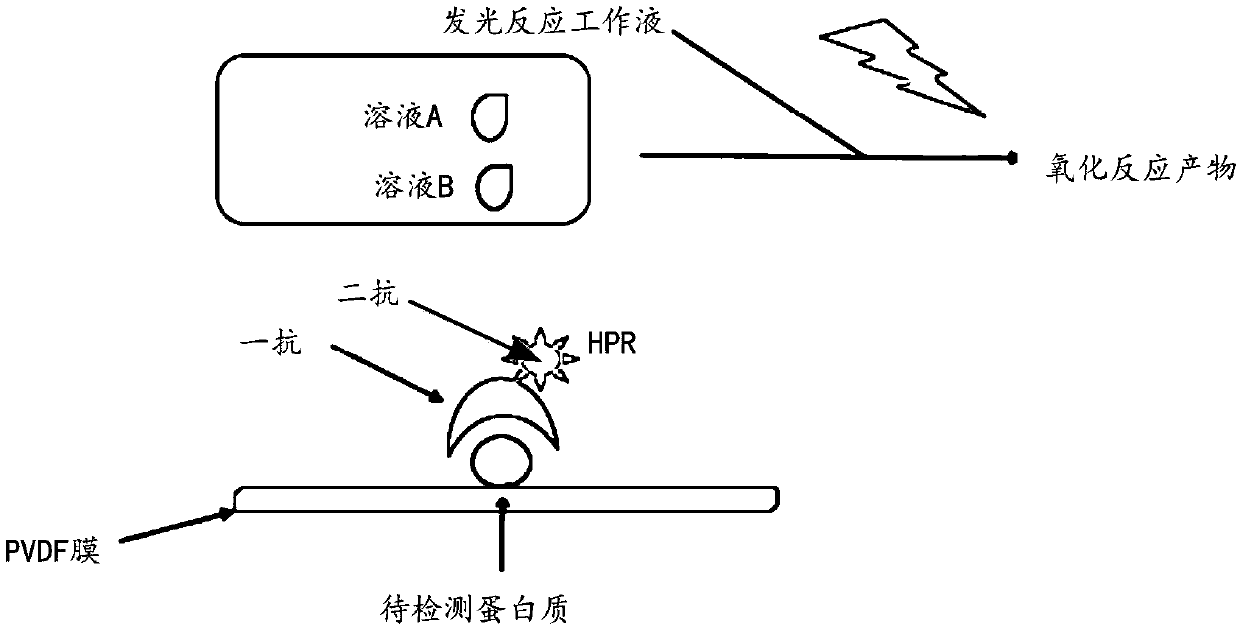
Chemiluminescence detection kit and chemiluminescence detection method thereof
InactiveCN110068568AEasy to detectReduce dosageChemiluminescene/bioluminescenceBiological testingLuminolCommercial kit
The present invention belongs to the field of biochemical experimental reagents, and particularly relates to a chemiluminescence detection kit and a chemiluminescence detection method thereof. The chemiluminescence detection kit is composed of an A solution and a B solution, wherein the A solution is a buffer containing 2.5 mM luminol and 0.396 mM p-coumaric acid, and the B solution is a buffer containing 0.02% hydrogen peroxide. According to the luminescence detection kit provided by the present invention, the protein of each concentration can be better detected, and the detection sensitivityand intensity are nearly 20 times that of the common commercial kit on the market, so that the effects of reducing the dosage of the antibody and saving the experiment cost are achieved.
Owner:深圳市泰锐生物科技有限公司
Colloidal gold immunochromatographic test paper and preparation and application thereof
The invention discloses a colloidal gold immunochromatographic test paper and a preparation and application thereof. The test paper comprises a colloidal gold labelled bovine bluetongue virus vp7 protein, a detection line and a quality control line. The test paper is suitable for detecting a bluetongue virus antibody, a preparation process is optimized through various conditions; the test paper isavailable even when bovine bluetongue standard positive serum is diluted 64 times, and has no cross reaction with bovine tuberculosis, bovine bluetongue, bovine virus diarrhea, bovine pasteurellosisand Enzootic Bovine Leukosis positive serums; and compared with an oversea commercial kit ELISA detection kit (IDEXX), coincidence rate is 98%. The test paper also has relatively better stability, cancomplete a whole detection process within 10 minutes, can quickly detect sample serum and is suitable for field fast check.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
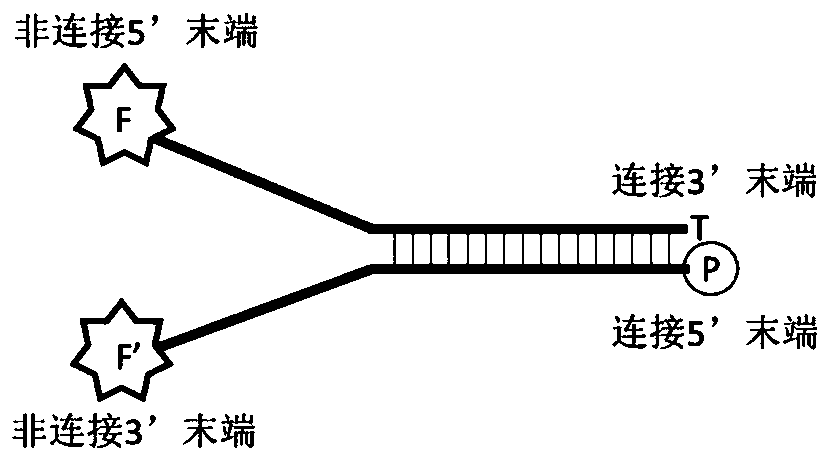
DNA (deoxyribonucleic acid) connector as well as preparation method and application thereof
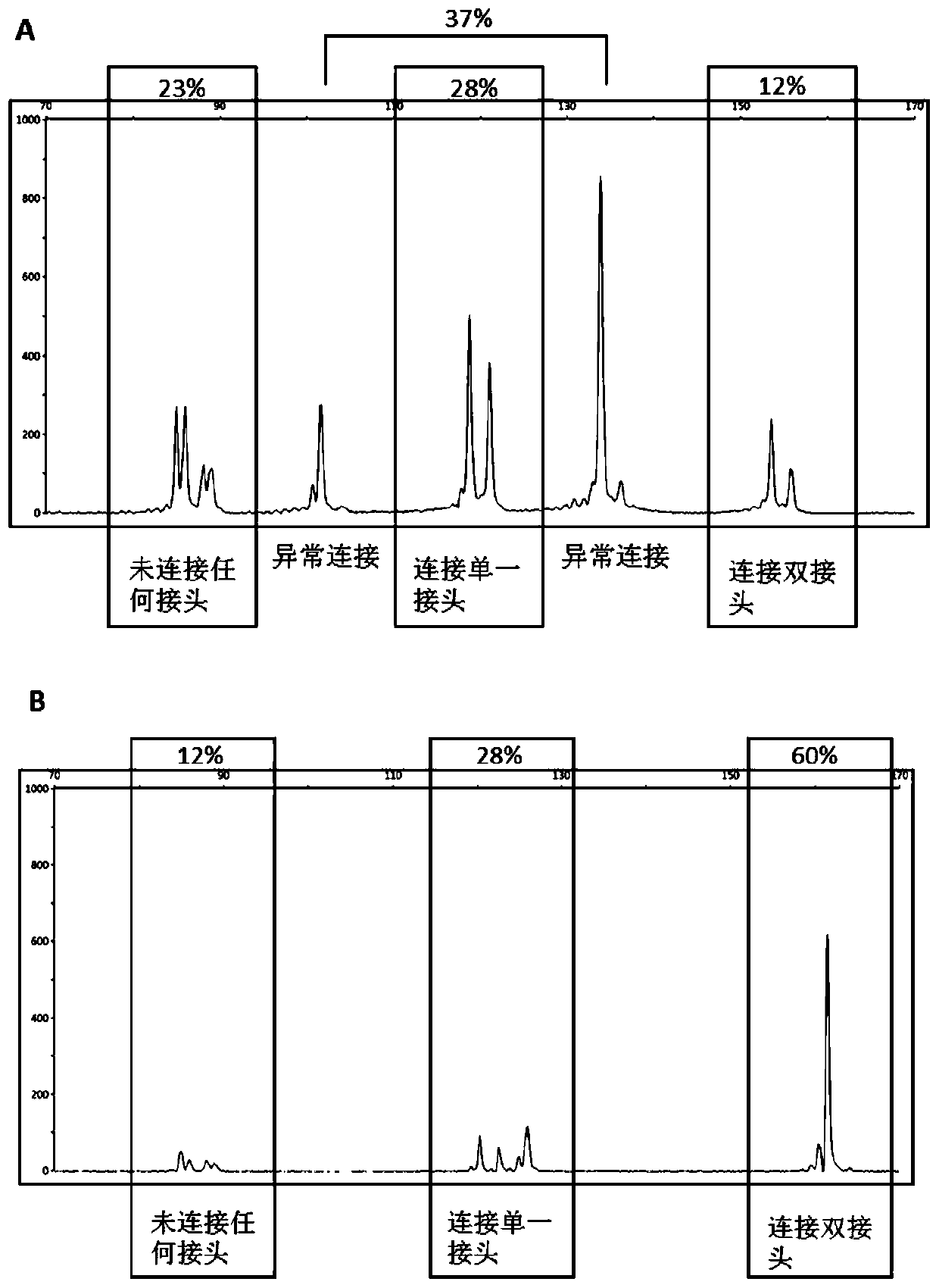
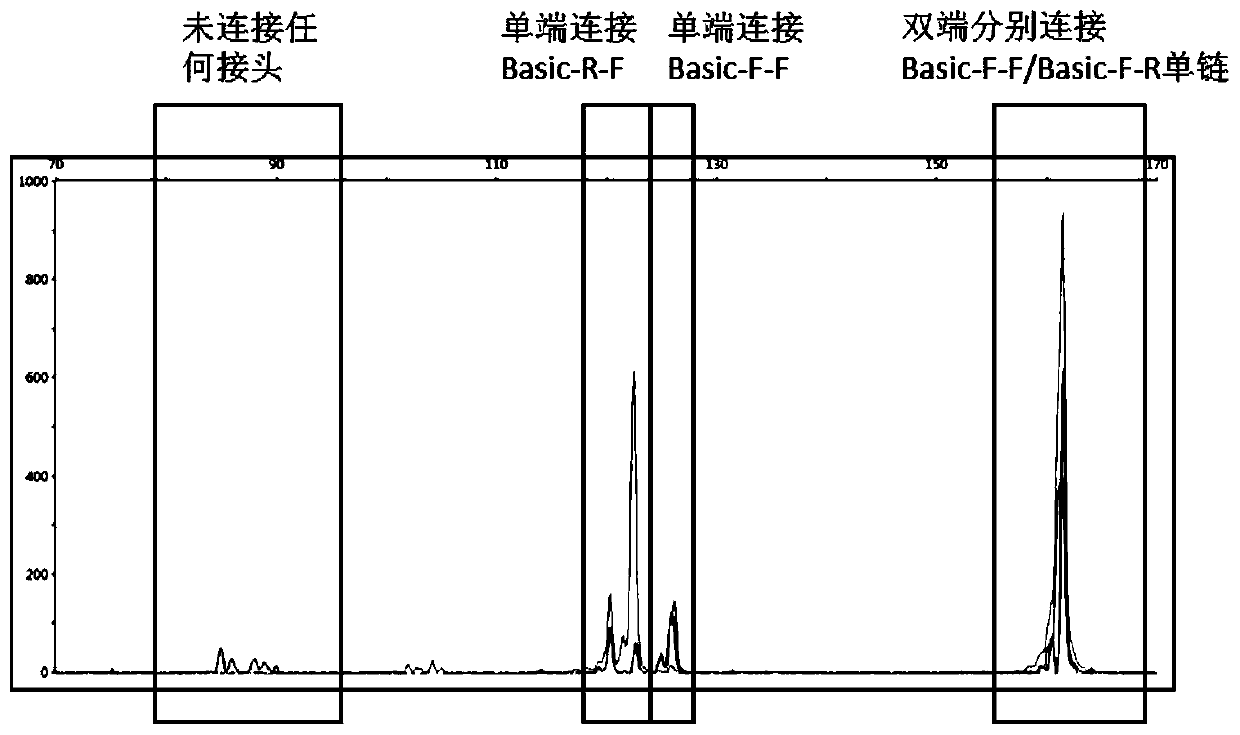
PendingCN111394436AImprove conversion efficiencyLow costMicrobiological testing/measurementLibrary creationTransformation efficiencyEngineering
The invention relates to a DNA connector. 5' and / or 3' at non-connection tail ends of the DNA connector are sealed. By adopting the DNA connector provided by the invention, non-specific connection ofthe non-connection tail end with a DNA fragment can be avoided, the connection efficiency of the connector with the DNA fragment can be improved, and then the library conversion efficiency can be improved. A preparation method of the DNA connector provided by the invention is simple, a library construction method is simple, efficient and low in cost, and the high expense of transformation enzymesor commercial kits can be avoided.
Owner:GENESKY TECH (SUZHOU) INC +1
Method using loop-mediated isothermal amplification technology to detect mycoplasma pneumoniae
InactiveCN107236798AStrong specificityEfficient amplificationMicrobiological testing/measurementConserved sequenceFluorescence
The invention discloses an LAMP (loop-mediated isothermal amplification) detection method of mycoplasma pneumoniae and the special primers and kit of the LAMP detection method. The special primers are used for performing fast isothermal amplification on the mycoplasma pneumoniae. The LAMP primers are designed according to the specific conservative sequence of pneumonia, and each group of primers comprises 4 oligonucleotides. When the special primers are used for mycoplasma pneumoniae detection, the primers are in the form of white precipitate under naked-eye observation; after SYBR GREEN is added during positive reaction, fluorescent green is evidently enhanced under ultraviolet-lamp observation. A real-time turbidity detection result shows that product turbidity increases along with the prolonging of reaction time, and the primers are in the form of trapezoid strips after gel electrophoresis detection. The method has the advantages that a new technical platform is provided for the mycoplasma pneumoniae detection, to-be-detected samples can be DNA extracted by commercial kits or purified DNA and coarsely-extracted DNA extracted by methods represented by a boiling method, and the method is suitable for being popularized and applied in basic units, field monitoring and bedside detection.
Owner:TIANJIN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com