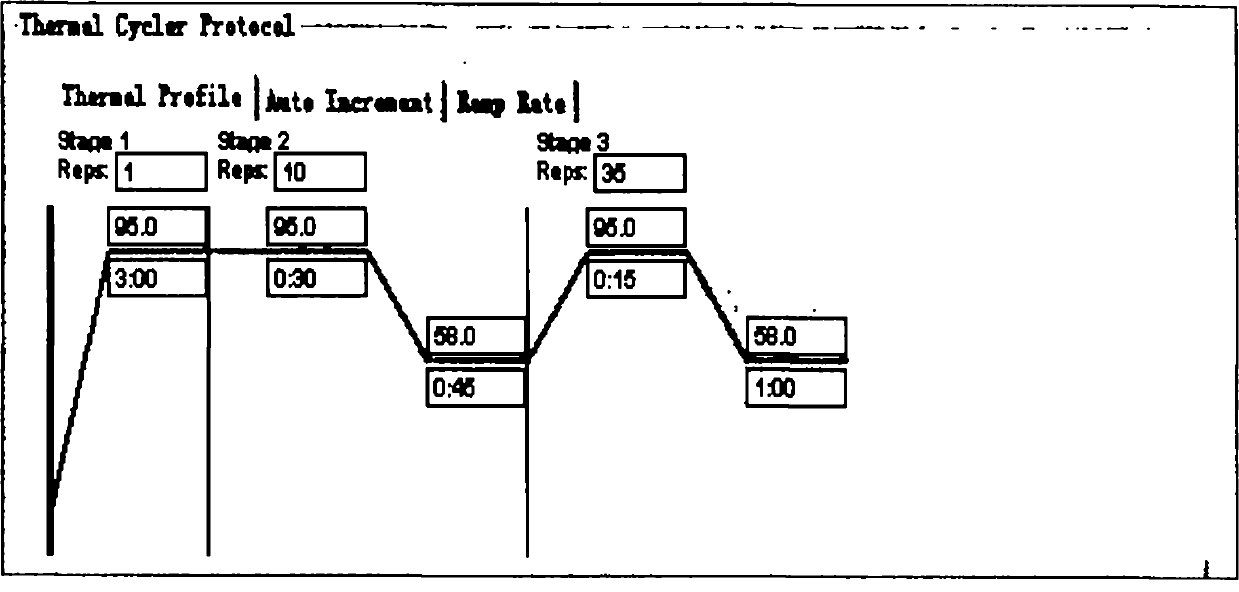
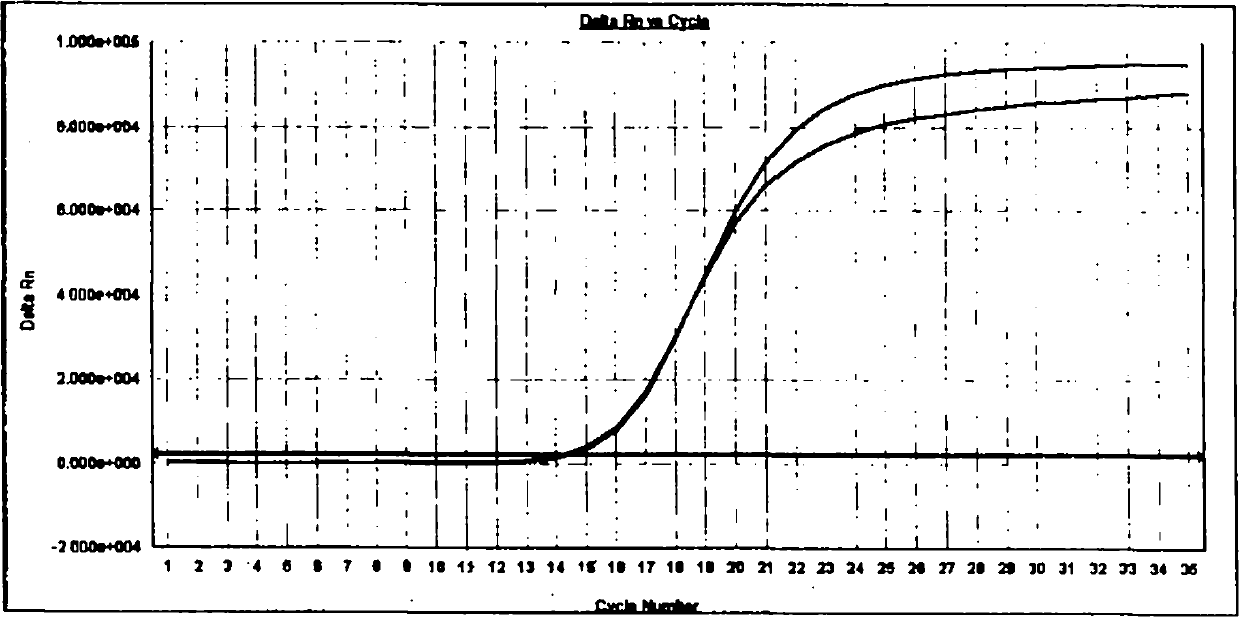
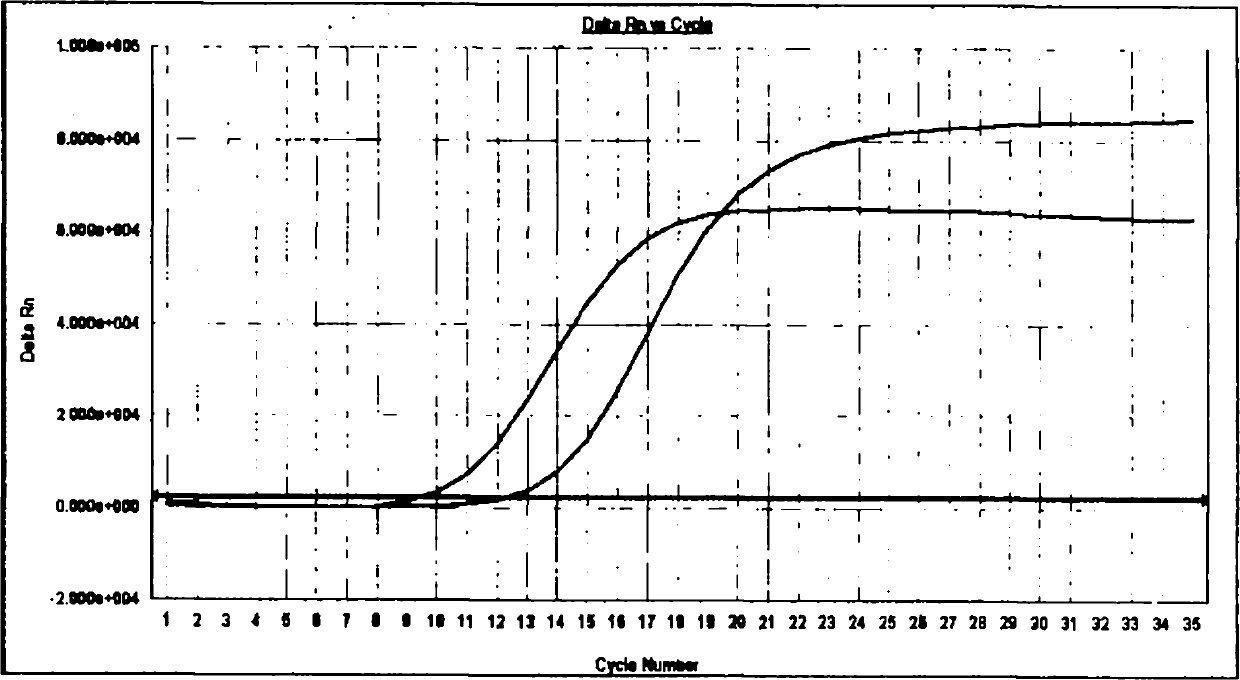
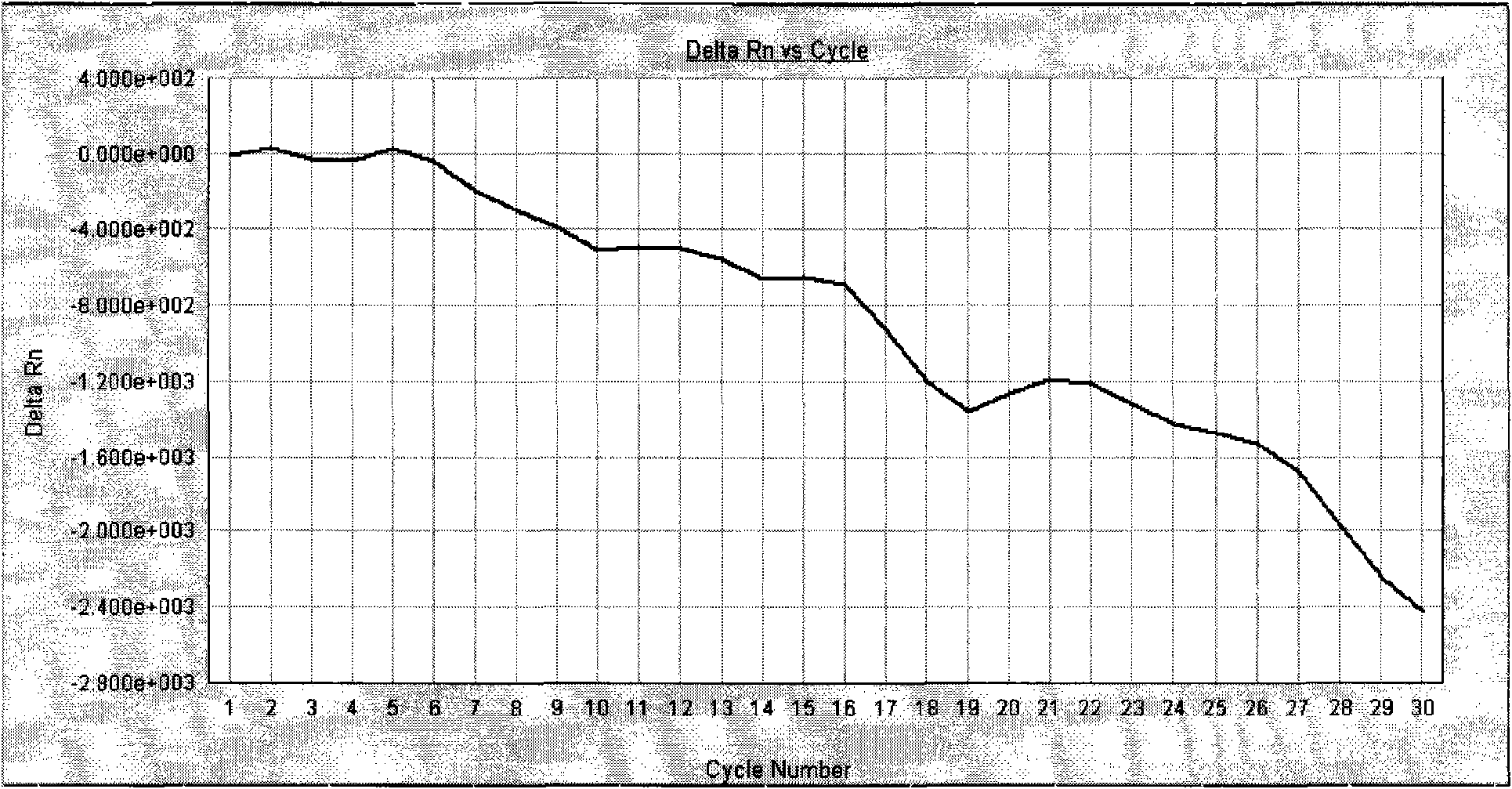
Patents
Literature
35results about How to "Aid in early diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zika virus fluorescent PCR detecting kit
InactiveCN105734171AAccurate detectionEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceEnzyme system
The invention provides a Zika virus fluorescent PCR detecting kit, and relates to a kit for detecting Zika virus, in particular to simultaneous detection of Zika virus RNA by using a real-time fluorescent polymerase chain type reaction technology.The kit mainly comprises RT-PCR reaction liquid, primer probe mixed liquid, an RT-PCR reaction enzyme system, DEPC H2O and a packaging box for separating and packaging reagent bottles or tubes in a centralized manner.The kit adopts a Taqman probe detection mode and a one-step real-time fluorescent PCR reaction mode, can accurately and quickly detect the Zika virus RNA, and can be widely applied to various fields such as Zika virus disease clinic early diagnosis, disease prevention and scientific research.
Owner:DAAN GENE CO LTD
Serum marker MMP-7-based biliary atresia diagnosis kit
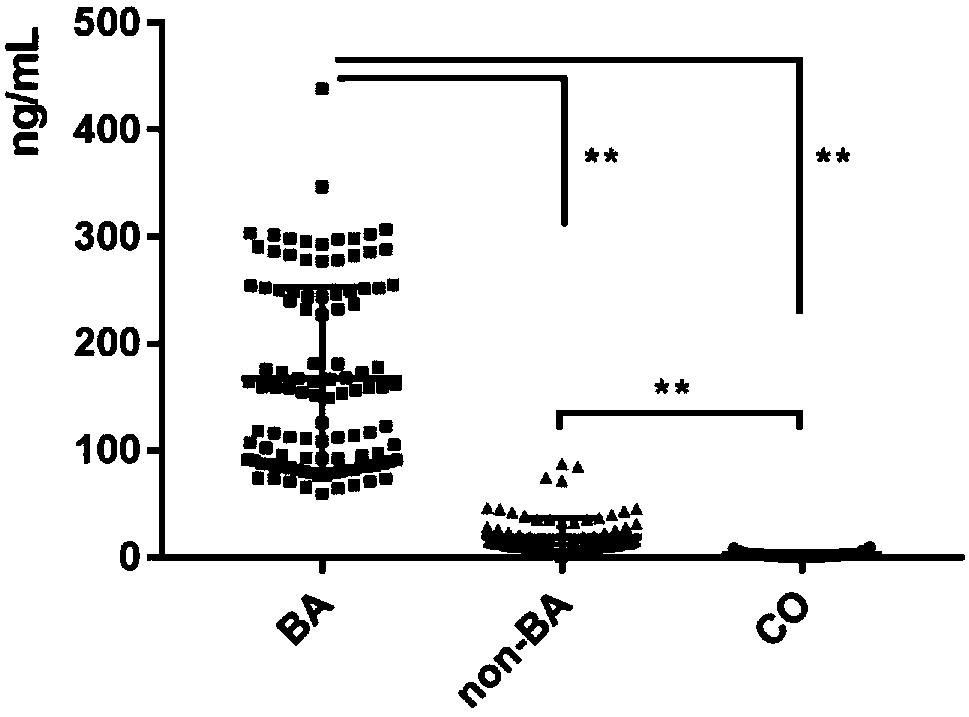
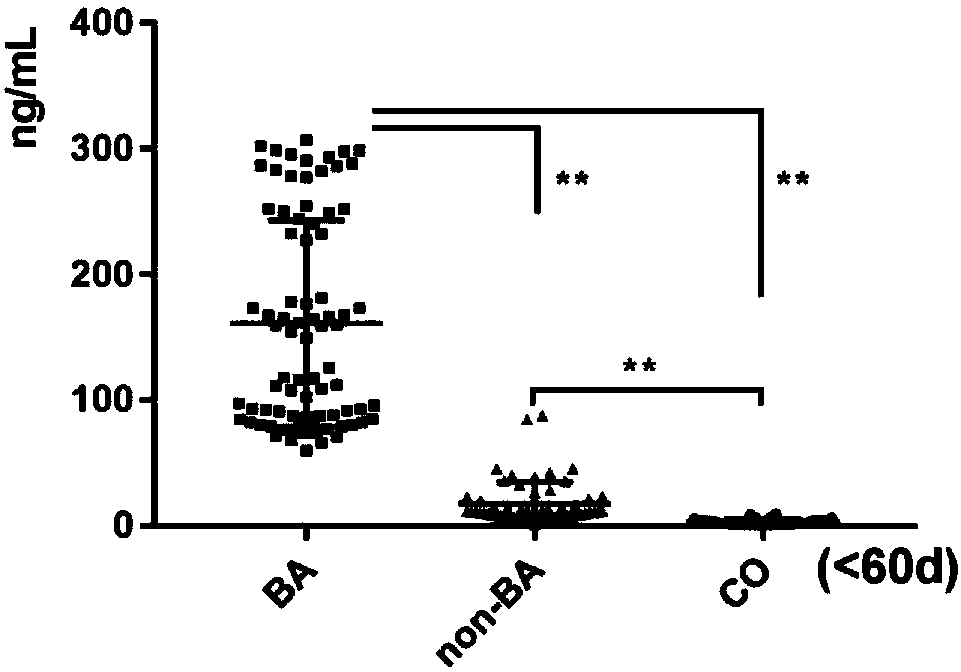
InactiveCN108267585AEasy to operateAid in early diagnosisDisease diagnosisPositive controlPRIMARY BILIARY ATRESIA
The invention relates to a serum marker MMP-7-based biliary atresia diagnosis kit. The diagnosis kit comprises an anti-human MMP-7 monoclonal antibody coated ELISA plate, a negative control solution,a positive control solution, an enzyme labeling reagent, an enzyme substrate solution, a blocking solution, a sample diluent, a washing solution and a stopping solution. The diagnosis kit is a new sensitive, safe, reliable and easily-operated commercial kit. Quantitative detection of the level of MMP-7 in human serum is helpful for early diagnosis of BA; the BA diagnosis sensitivity of the serum biomarker protein MMP-7 is 100%, and the BA diagnosis specificity is 95.6%, so the kit has the characteristics of high specificity and high sensitivity; and the kit improves the early diagnosis rate ofBA, reduces misdiagnosis, and improves the self liver survival rate of the BA.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
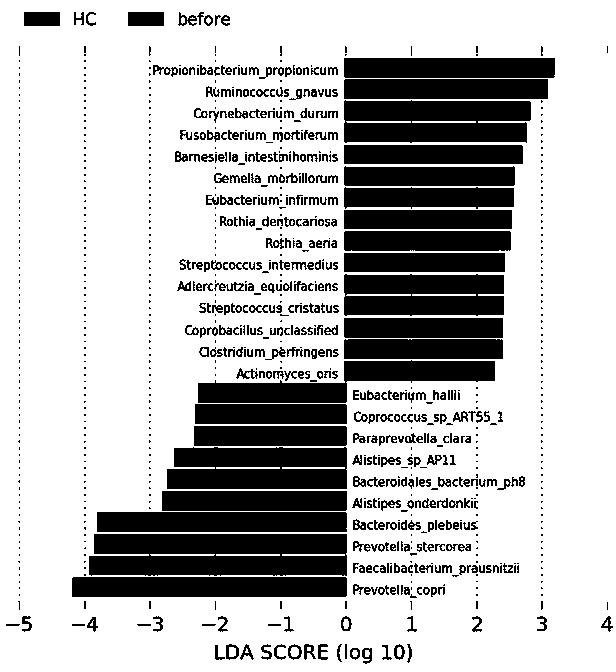
Bipolar affective disorder biomarkers based on enteric microorganism and screening and application thereof
ActiveCN111430027AGuaranteed complianceIncreased sensitivityHealth-index calculationBiostatisticsIntestinal microorganismsPharmaceutical drug
The invention discloses bipolar affective disorder biomarkers based on enteric microorganism and screening and application thereof. The biomarkers includes six types of: 1) Ruminococcus_gnavus; 2) Eubacterium_hallii; 3) Alistipes_onderdonkii; 4) Faecalibacterium_prausnitzii; 5) Prevotella_stercorea; 6) Bacteroidales_bacterium_ph8. In the six biomarkers, the Ruminococcus_gnavus is increased in relative abundance in the bipolar affective disorder patient group, whereas the rest five biomarkers are decreased in relative abundances. The invention also discloses an application of the biomarkers asa detection target point or detection target in preparation of a detection kit, and an application of the biomarkers in the drugs for screening, treating and / or preventing the bipolar affective disorder as target points.
Owner:ZHEJIANG UNIV
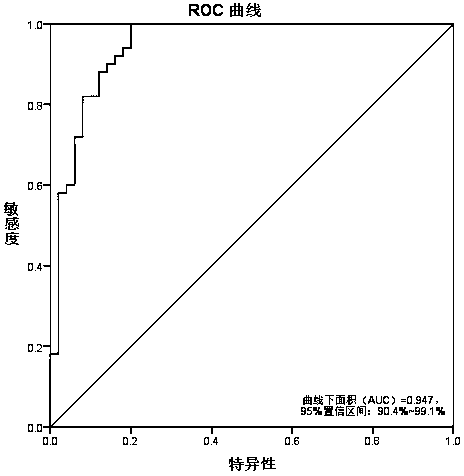
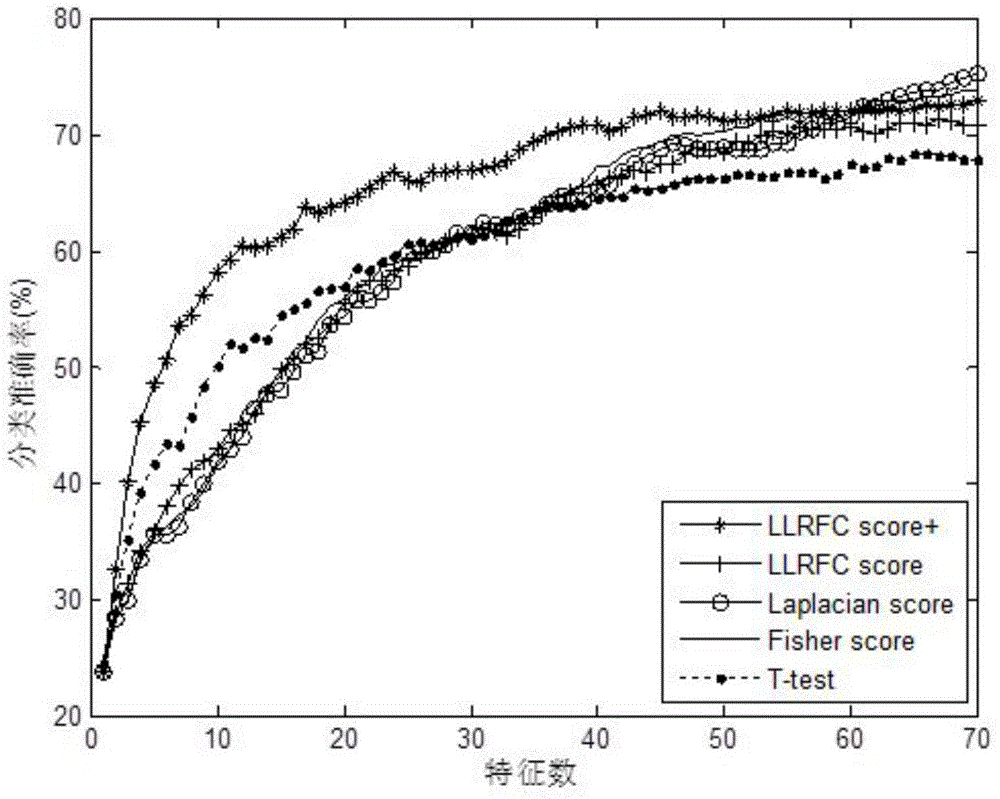
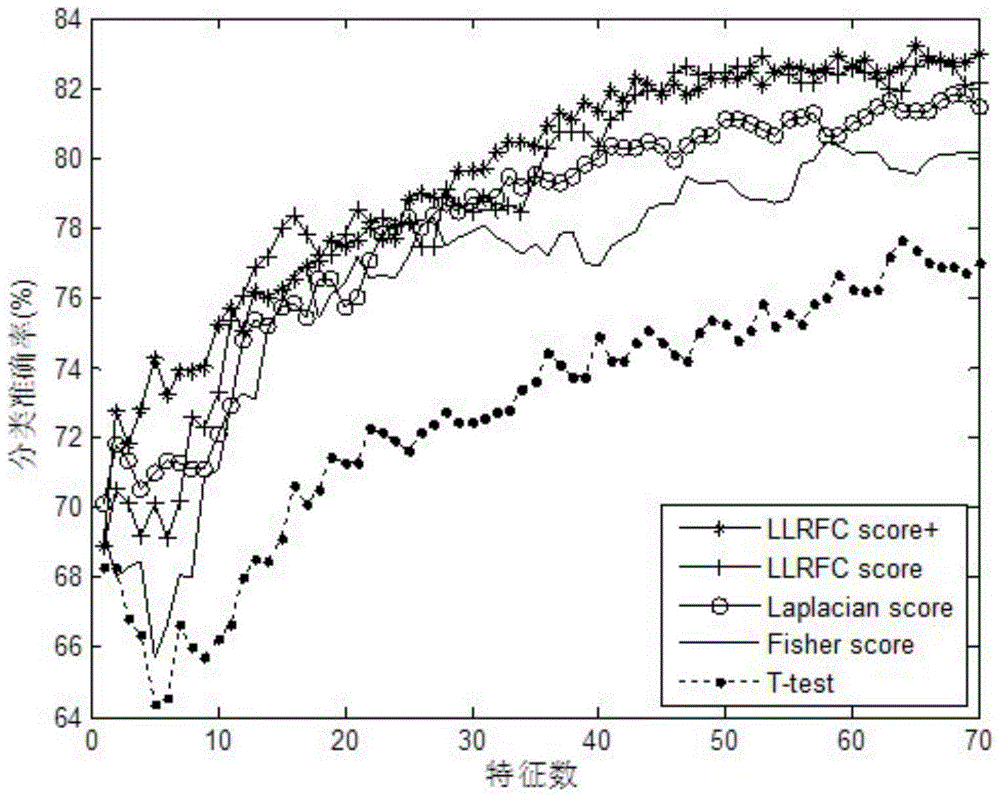
Redundancy removal feature selection method LLRFC score+ based on LLRFC and correlation analysis
InactiveCN105740653AOptimal trait gene subsetImprove classification accuracyBiostatisticsSpecial data processing applicationsDiseaseData set
The invention provides a redundancy removal feature selection method LLRFC (Locally Linear Representation Fisher Criterion) score+ based on LLRFC and correlation analysis. A DNA (Deoxyribonucleic Acid) microarray technology provides a new direction for clinic tumor diagnosis. Performance of gene expression data corresponding to different kinds of tumor is different; through the analysis on the tumor gene expression data, study personnel can realize the accurate recognition on the tumor and the tumor subtype in the molecular level; and important biological significance is realized on the diagnosis and the treatment of the tumor. The feature genes in LLRFC judging criterion descending sort gene expression data is used to be combined with the dynamic correlation analysis strategy for further eliminating redundant features; an LLRFC score+ algorithm is provided; and the optimum feature gene subset is selected. The feature selection method LLRFC score+ has the advantages that the classification precision of a classifier can be effectively improved; a sample data set does not need to meet the normal distribution; and the method is applicable to data in various distribution types. The feature selection method LLRFC score+ can help people to find the virulence gene of cancer, and the early-stage diagnosis, tumor staging and typing, prognosis treatment and the like of clinic tumor diseases are facilitated.
Owner:BEIJING UNIV OF TECH
Kit for detecting HER2 gene amplification
ActiveCN102199659AAid in early diagnosisAids in healingMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceKnowledge Field
The invention relates to a kit for detecting breast cancer HER2 gene amplification, especially to a kit for detecting HER2 gene real-time fluorescence polymerase chain reaction. By the utilization of the real time fluorescence PCR technology, the kit provided by the invention detects the amplification level of the HER2 gene in a breast cancer tissue specimen by calculating Delta Ct value, and canbe applied in a plurality of research fields such as the auxiliary diagnosis, clinic and prognosis of breast cancer.
Owner:DAAN GENE CO LTD
Quantitative detection kit for helicobacter pylori nucleic acid
ActiveCN101608210AEasy to operateShort timeMicrobiological testing/measurementFluorescenceGastric mucosa
The invention relates to a real-time fluorescent polymerase chain reaction detection kit for helicobacter pylori. The kit can quantitatively detect the helicobacter pylori in a gastric mucosa biopsy specimen. A detection method for the kit has the advantages of simple operation, short consumed time, high sensitivity and strong specificity, and can be widely used in various fields such as auxiliary diagnosis of helicobacter pylori infection, guide of clinical medication, epidemiology retrospective study, and the like.
Owner:DAAN GENE CO LTD
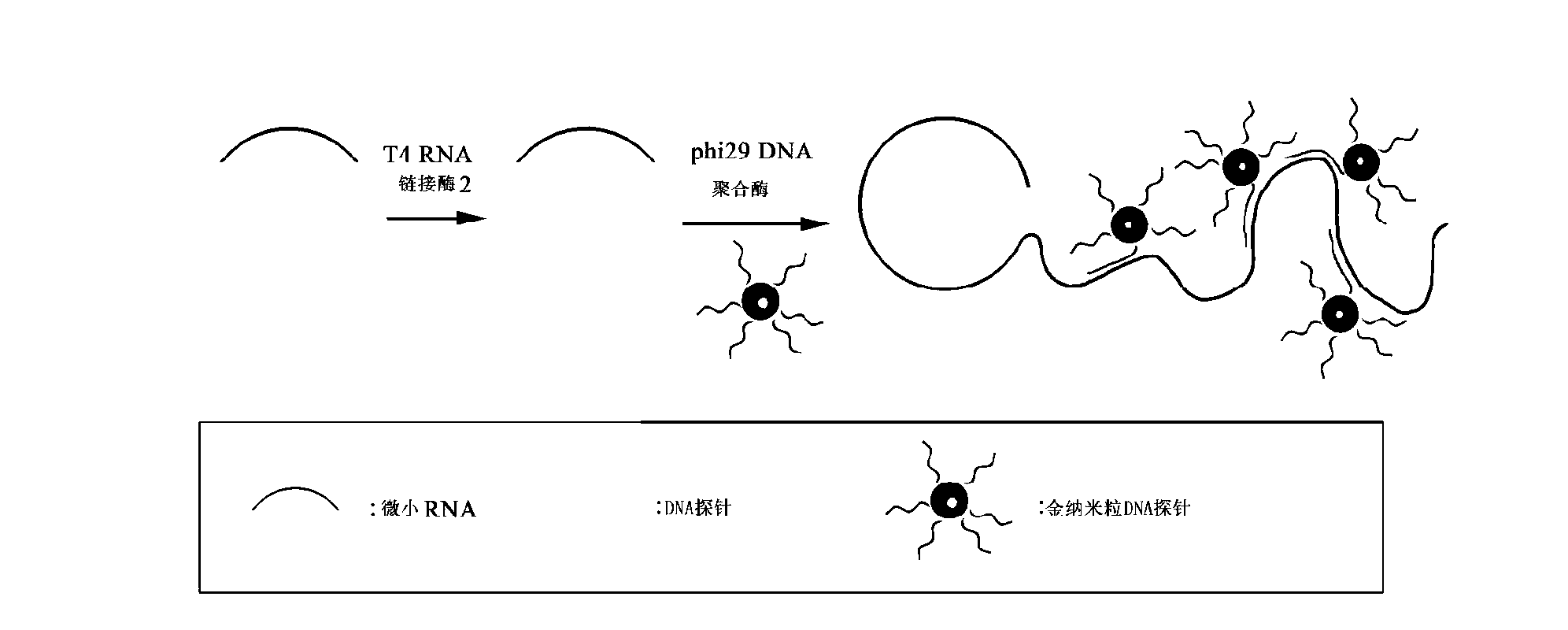
Micro-RNA (Ribonucleic Acid) colorimetric detection method based on rolling circle amplification
ActiveCN103233073AReduce usageAid in early diagnosisMicrobiological testing/measurementDiseaseHybridization reaction
The invention discloses a micro-RNA (Ribonucleic Acid) colorimetric detection method based on rolling circle amplification. The method is characterized by comprising the following steps of: amplifying the target micro-RNA into a long-chain product comprising a plurality of target micro-RNA segments using a rolling circle amplification technology, fixing a gold nanoparticle DNA (Deoxyribonucleic Acid) probe to the long-chain product comprising the plurality of target micro-RNA segments by means of hybridization reaction between nucleic acids, detecting the color change reaction of the gold nanometer particle of the gold nanometer particle DNA probe fixed on the long-chain product comprising the plurality of target micro-RNA segments, and detecting the long-chain product comprising the plurality of target micro-RNA segments so as to achieve the purpose of detecting the target micro-RNA. The detection method disclosed by the invention can be used for preventing the application of expensive instruments such as a PCR (Polymerase Chain Reaction) instrument and reducing the detection cost, and is conductive to the early diagnosis of a relevant disease.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Molecular marker for diagnosing and treating tumors
ActiveCN104975071AAid in early diagnosisAids in healingGenetic material ingredientsMicrobiological testing/measurementNeoplasm diagnosisIndividualized treatment
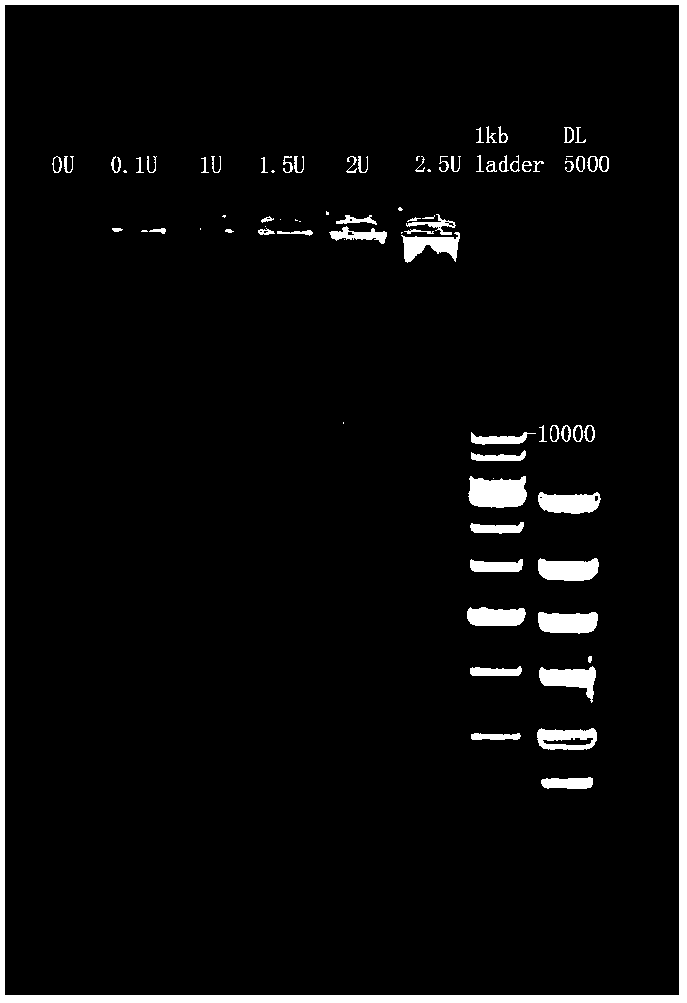
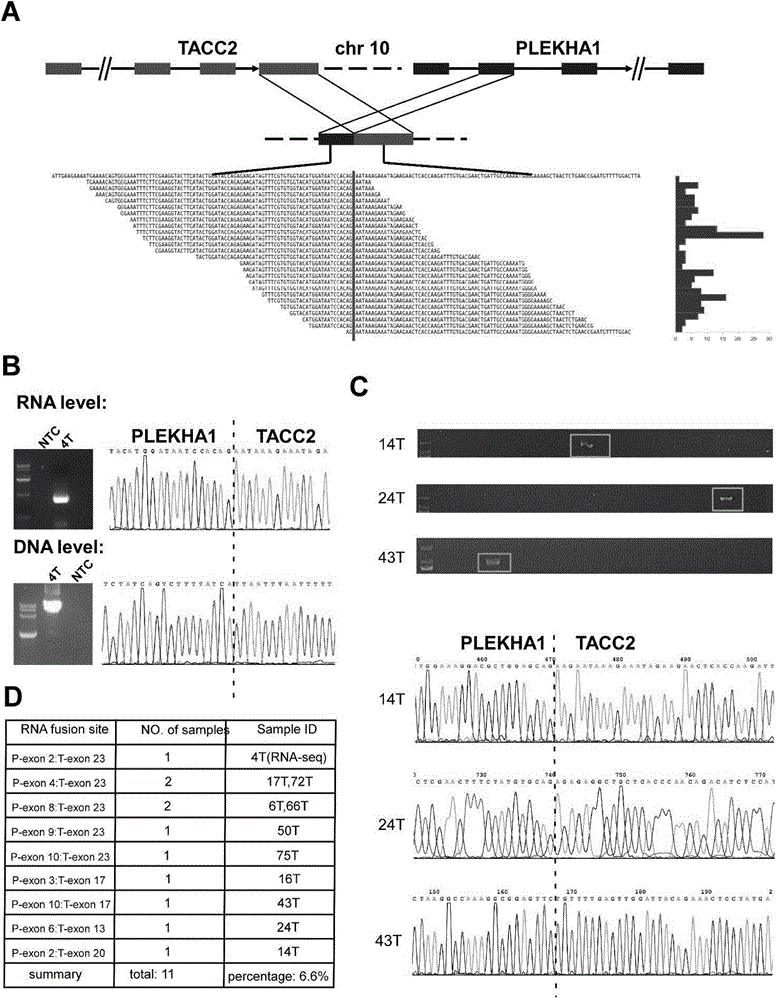
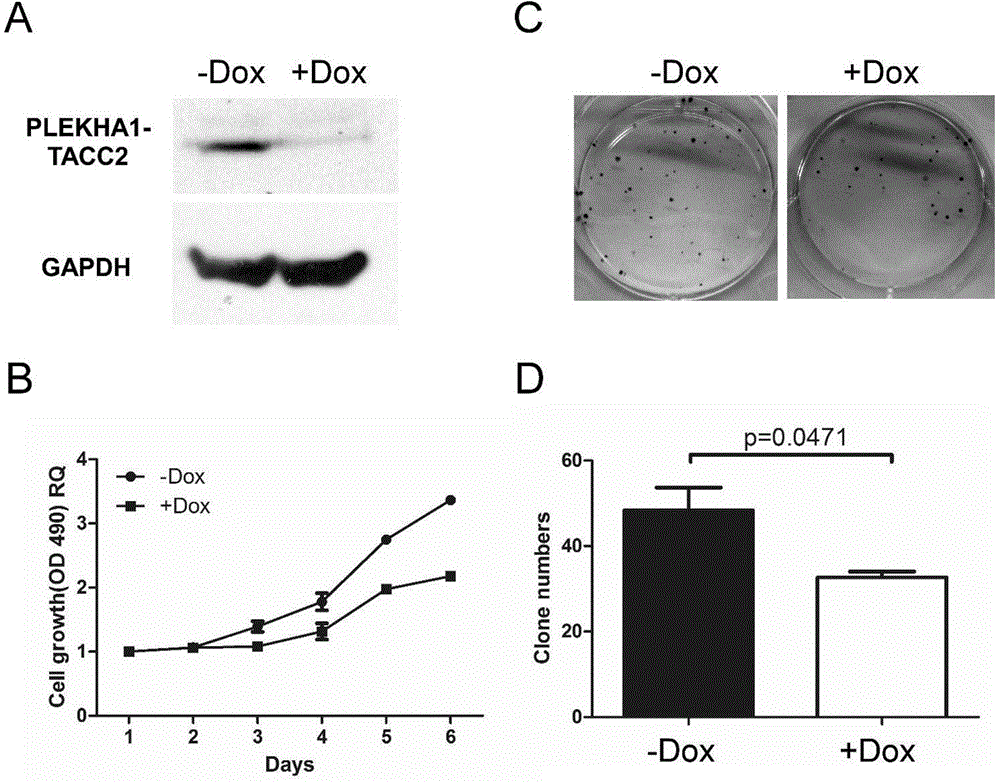
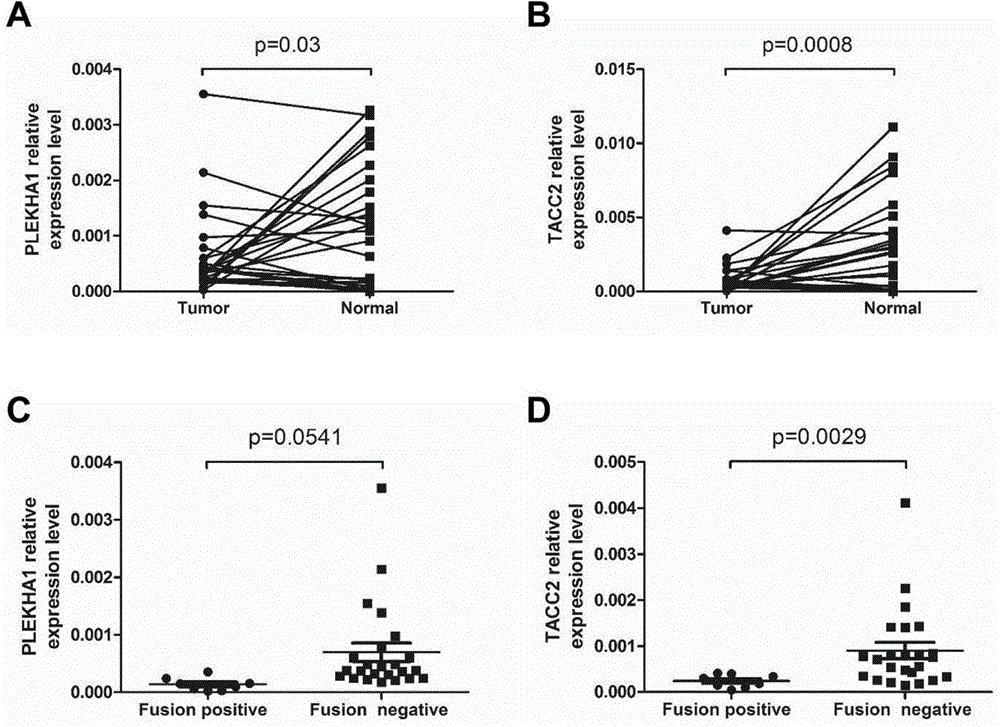
The invention discloses a molecular marker for diagnosing and treating tumors. The molecular marker is a fused gene PLEKHA1-TACC2; and the fused gene simultaneously contains a part of sequences of a gene PLEKHA1 and a gene TACC2. The molecular marker disclosed by the invention is beneficial to early diagnosing and treating tumours, increasing the screening efficiency and reducing the screening cost; a direction is provided for individualized treatment; and the survival rate and the survival quality of patients are improved.
Owner:SUN YAT SEN UNIV +1
Method for measuring number of circulating epithelial tumor cells in blood
InactiveCN105277697AAid in early diagnosisHelp transferMaterial analysisAbnormal tissue growthWhite blood cell
The invention relates to a method for measuring the number of circulating epithelial tumor cells in blood. The method comprises the following steps that A, a blood sample of a test item is obtained from a test object, wherein the sample is anticoagulant whole blood possibly containing the epithelial tumor cells; B, negative enrichment is performed to remove non-target cells in the blood sample, and the non-target cells include red blood cells and most white blood cells; C, the enriched blood sample is mixed with a fluorescently-labeled antibody, and incubation is performed, so that target cells and a fluorescent antibody are sufficiently combined; D, an anti-fall glass sheet is coated with fluorescently-labeled cell suspension, still standing is performed for a moment, and observation is performed under a fluorescence microscope for analyzing samples, so that the existence and the number of the circulating epithelial tumor cells are determined; E, the observed target cells are stored as direct visual images. Compared with the prior art, the method has the beneficial effect that detection is favorable for tumor early-stage diagnosis, transfer and recurrence monitoring, curative effect and prognosis evaluation, drug-resistance monitoring, individual treatment guide and the like.
Owner:SHANG OUTDO BIOTECH CO LTD
Real-time fluorescent quantitative PCR (polymerase chain reaction) kit for detecting candida glabrata
InactiveCN102181567AAid in early diagnosisAids in healingMicrobiological testing/measurementCandida glabrataPcr ctpp
The invention discloses a real-time fluorescent quantitative PCR (polymerase chain reaction) kit for detecting candida glabrata, which comprises the following substances: PCR reaction solution, primer and probe mixed solution, a PCR reaction enzyme, pure H2O and a positive quantitative standard product. The real-time fluorescent quantitative PCR kit is used for detecting the nucleic acid amplification level of the candida glabrata and can reflect the state of the infection of the candida glabrata in a patient body, be used for monitoring, preventing and treating fungal infection, and be conductive to early diagnosis and treatment of diseases; and the real-time fluorescent quantitative PCR technology designs a primer and a probe against the nucleic acid specificity sequence of the candida glabrata, the specificity is strong, the sensitivity is high, and the technology further has the advantages of higher flux, simpleness in operation and relatively low cost, and is applicable to detecting various samples of most of patients. The amplification of the samples to be detected by using the kit is completed by a commercial real-time fluorescent quantitative PCR instrument, the operation is simple, the consumed time is short, and the pollution can be reduced to the maximum extent.
Owner:SOUTHERN MEDICAL UNIVERSITY
High-throughput chromosome and cytoskeleton strain flow analyzer and implementation method
ActiveCN112986063AAddressing Biomedical ResearchSolve problems in clinical applicationBiological particle analysisIndividual particle analysisFluorescenceCytoskeleton
The invention provides a high-throughput chromosome and cytoskeleton strain flow analyzer and an implementation method. The high-throughput chromosome and cytoskeleton strain flow analyzer comprises a sample introduction device, a micro-fluidic chip, a light source module, an imaging device and a control analysis module, wherein the sample introduction device is used for introducing a liquid sample into the micro-fluidic chip; the micro-fluidic chip is used for conveying cells carried by a liquid sample in a single-cell arrangement manner and pushing the cells to pass through a limiting structure; the light source module is used for emitting corresponding wave band laser to the cytoskeleton and chromosome DNA coloring agent of the single cells to obtain single cell fluorescence information; the imaging device is used for carrying out dynamic imaging on the part of the single cells passing through the limiting structure of the micro-fluidic chip; and the control analysis module is positioned right above the imaging device, and is used for identifying and determining a dynamic fluorescence image in which a cytoskeleton and a chromosome in a cell nucleus in the single cell image are extruded by the limiting structure to deform, and analyzing and counting the strain form and structural characteristics of the cytoskeleton and the chromosome in the cell nucleus.
Owner:NORTHWEST UNIV(CN)
Real-time fluorescence quantitative PCR (Polymerase Chain Reaction) kit for detecting candida tropicalis
InactiveCN102181566AAid in early diagnosisAids in healingMicrobiological testing/measurementMicroorganism based processesDiseaseFluorescence
The invention discloses a real-time fluorescence quantitative PCR (Polymerase Chain Reaction) kit for detecting candida tropicalis. The kit comprises the following substances: a PCR solution, a primer and probe mixed solution, a PCR enzyme, pure H2O and a positive quantitative standard product. The kit is used for detecting nucleic acid amplification level of the candida tropicalis, so that state of candida tropicalis infection in a patient can be reflected, and the kit can be used for monitoring, preventing and controlling fungal infection and contribute to early diagnosis and treatment of diseases; and in the real-time fluorescence quantitative PCR technology, a primer and a probe are designed aiming at a specific sequence of the nucleic acid of the candida tropicalis, so that the kit has the advantages of high specificity, high flexibility, higher flux, easiness and convenience in operation and relatively reduced cost, and is suitable for detecting various specimens of most of patients. The amplification of a specimen to be detected by the kit is finished by a commercial real-time fluorescence quantitative PCR instrument; the kit is easy to operate and has low time consumption; and pollution is reduced to the greatest extent.
Owner:SOUTHERN MEDICAL UNIVERSITY
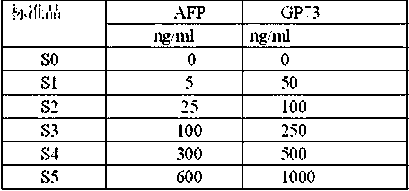
Liver cancer tumour marker AFP, AFP variant and GP73 antigen synchronous immunodetection kit, method and application
The invention relates to a liver cancer tumour marker AFP, AFP variant and GP73 antigen synchronous immunodetection kit, a method and application, and belongs to the technical field of immunodetection. The kit comprises magnetic particles, AFP and GP73 antibody coupled mixed acridinium ester, GP73, an AFP calibration product, first stimulation solution, second stimulation solution, washing solution and all kinds of diluting solution; and the magnetic particles are respectively marked by AFP, LCA-BSA and GP73. By adoption of the kit and the detection method, the sensitivity, the specificity andthe accuracy are high; the detection time is short; identification on benign liver diseases and malignant liver diseases and early diagnosis on liver cancers can be easily carried out; and the kit and the detection method have an important purpose for increasing the early diagnosis rate of patients with liver cancers and prolonging the survival rate, and have wide clinical application value.
Owner:万东山
Nano-fluorescent probe targeting pancreatic cancer circulating tumor cells
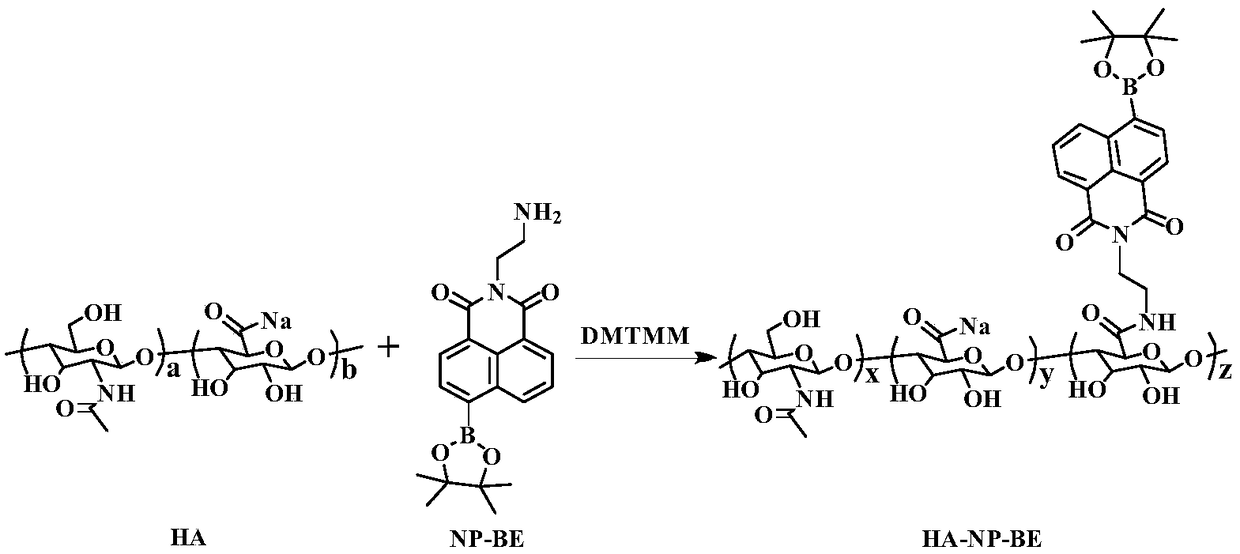
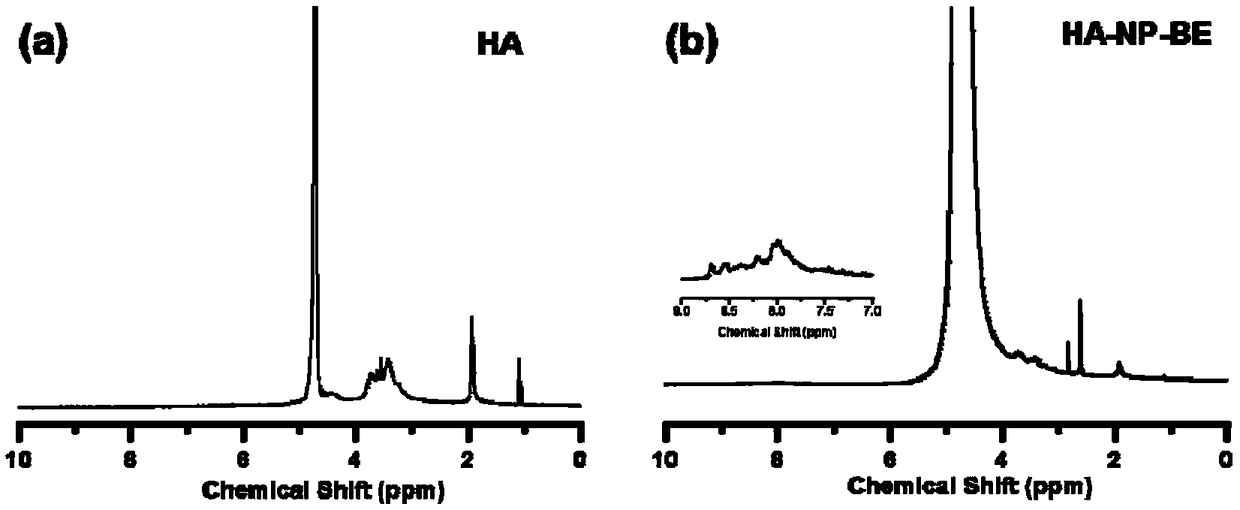
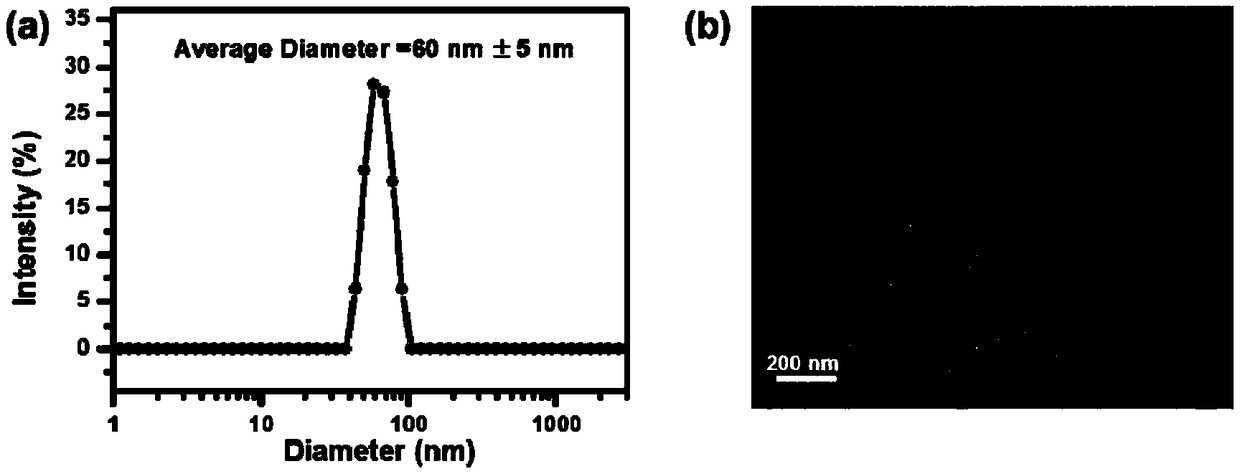
ActiveCN109253990AAid in early diagnosisEasy to distinguishFluorescence/phosphorescenceLuminescent compositionsSurface markerFluorescence
The invention provides a nano-fluorescent probe targeting pancreatic cancer circulating tumor cells. The probe comprises H2O2 responsive fluorescent small molecules and polymers targeting pancreatic cancer surface markers, and the H2O2 responsive fluorescent small molecules and the polymers targeting the pancreatic cancer surface markers have opposite hydrophilicity and hydrophobicity. Through thehigh expression of CD44 antigen specificity binding of hyaluronic acid and the pancreatic cancer tumor surface, the HA-NP-BE nano-fluorescent probe can tragetingly enter pancreatic cancer cells to detect H2O2 in the cells and image H2O2, rapid responsiveness is achieved, the pancreatic cancer tumor cells and normal cells can be effectively distinguished, CTC in pancreatic cancer tumor patients isdetected, and early disgnosis of pancreatic cancer is facilitated.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
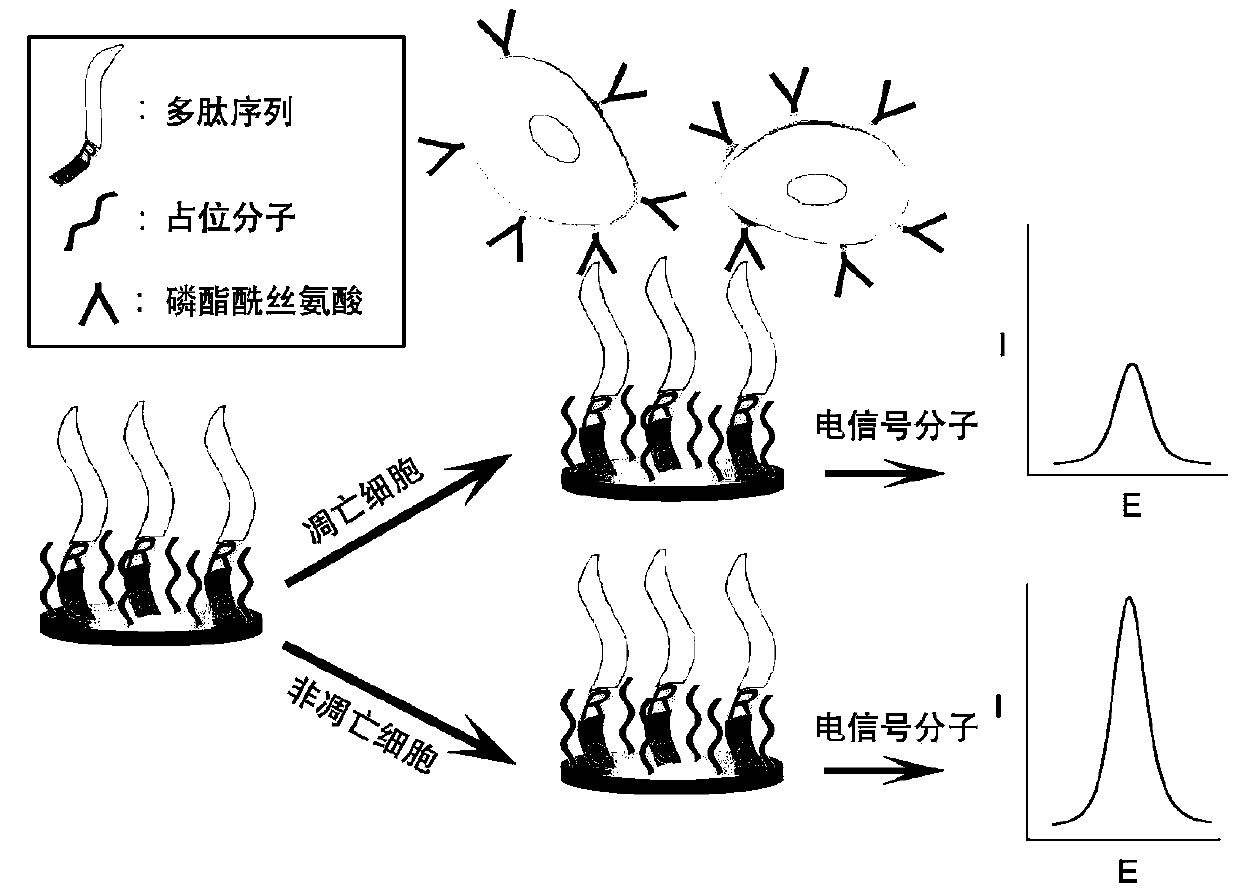
Method for detecting apoptosis degree of cells in solution to be tested
InactiveCN104049010AAvoid dependenceReduce usageMaterial electrochemical variablesAntigenElectrochemical response
The invention discloses a method for detecting an apoptosis degree of cells in a solution to be tested. The method comprises the steps of designing and synthesizing one section of polypeptide sequence, firmly modifying the polypeptide sequence on the surface of a working electrode, orderly arranging the polypeptide sequence through space occupying molecules, identifying the property of phosphatidylserine on surfaces of apoptotic cells in the solution to be tested through the polypeptide specificity, and fixing the apoptotic cells on the surface of the electrode. Because one layer of apoptotic cells is covered on the surface of the electrode, the electrochemical response of the working electrode is correspondingly changed; through comparing degrees of electrochemical signal changes before and after detection, the apoptosis degree of the cells in a sample to be tested can be detected. The method prevents from using the specific binding of an antigen and an antibody, thus the detection cost can be effectively lowered.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
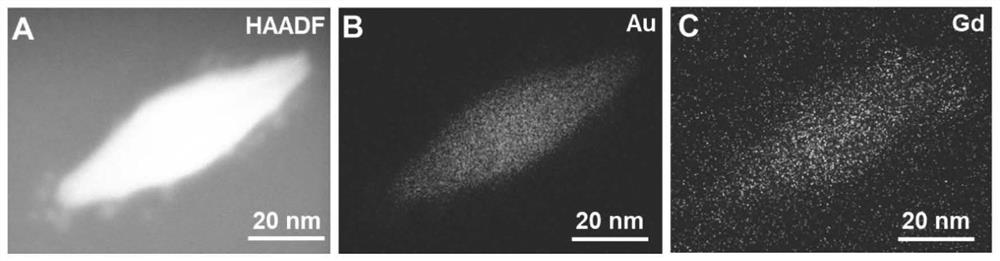
Composite nanoprobe with targeted fluorescence/magnetic resonance bimodal imaging and photothermal therapy functions as well as preparation and application of composite nanoprobe
ActiveCN112156192AGood biocompatibilityGood biological stabilityPowder deliveryEnergy modified materialsNanoprobeBiocompatibility
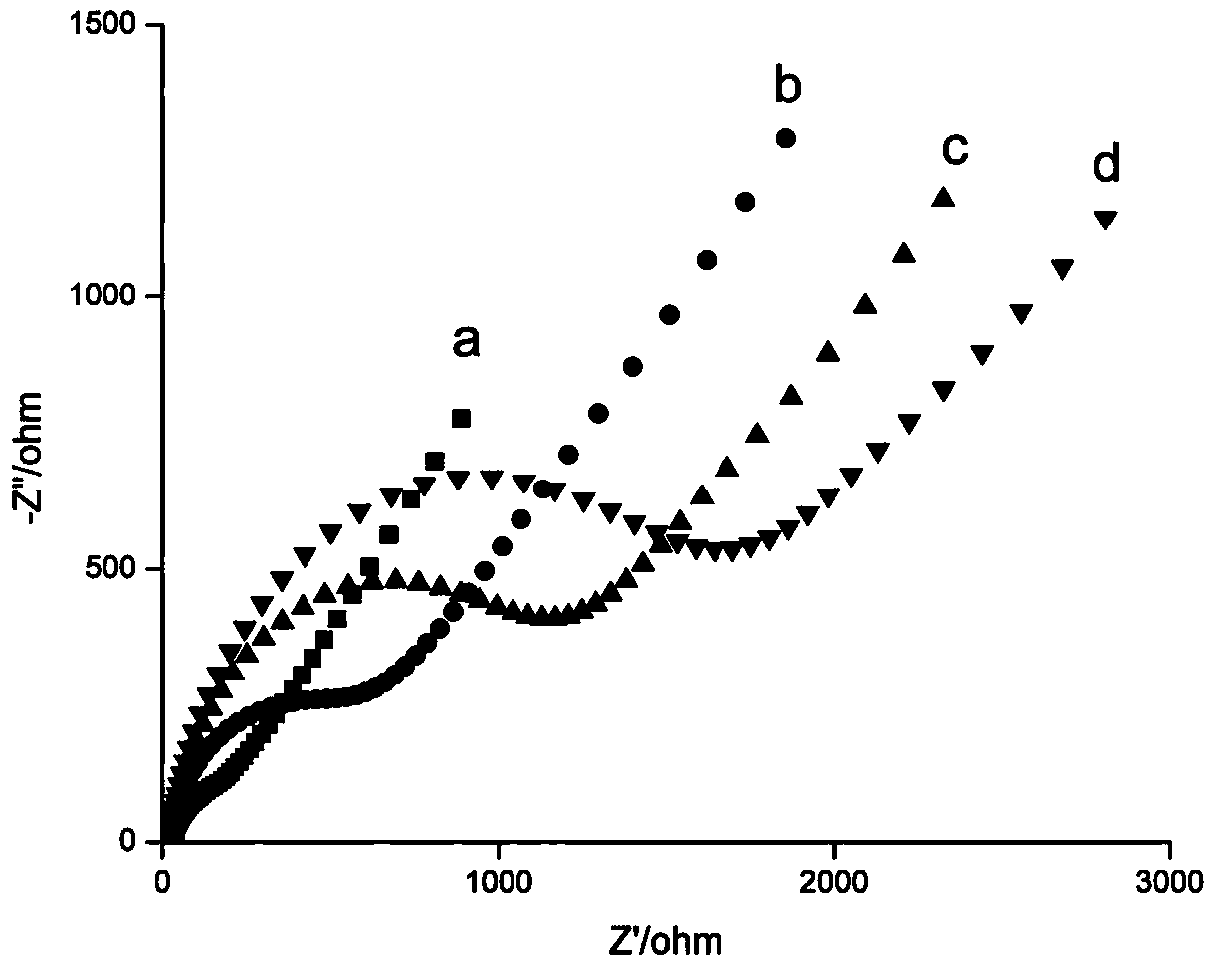
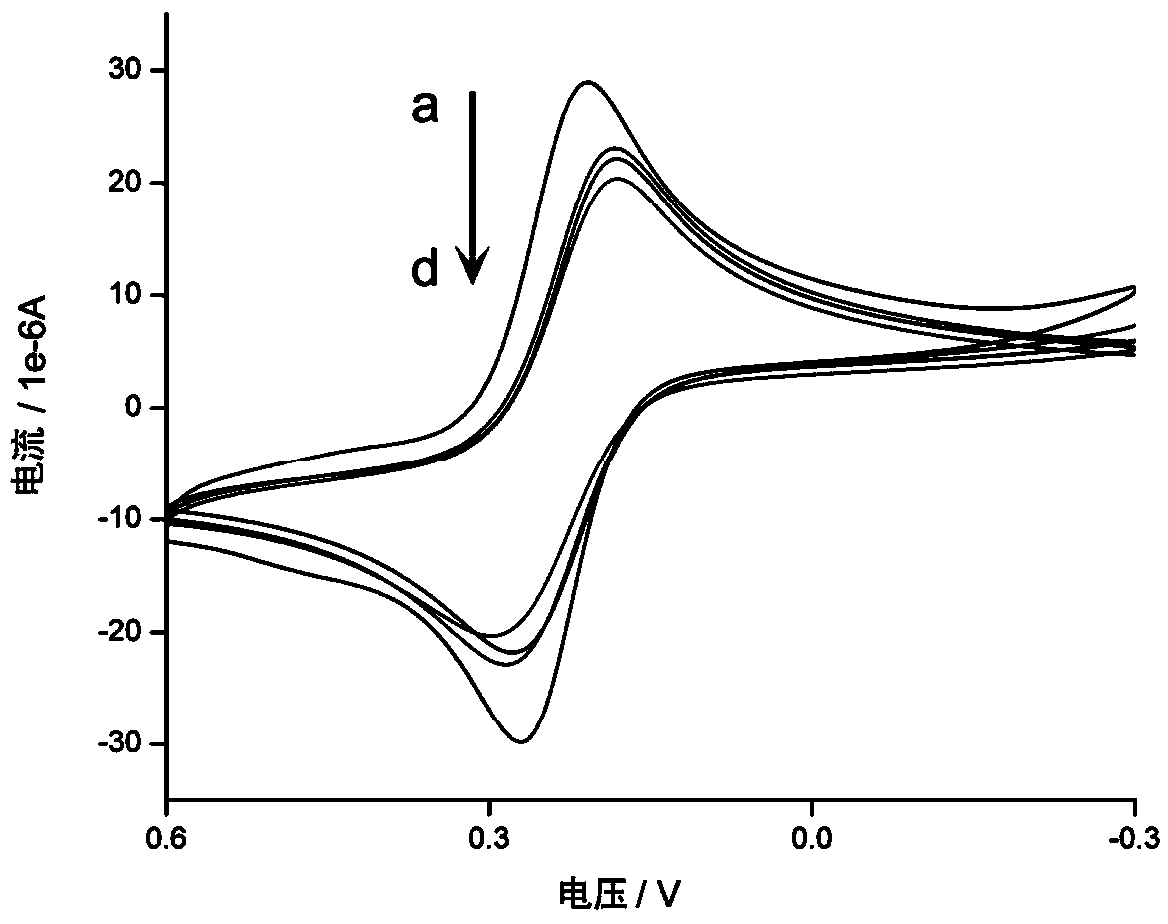
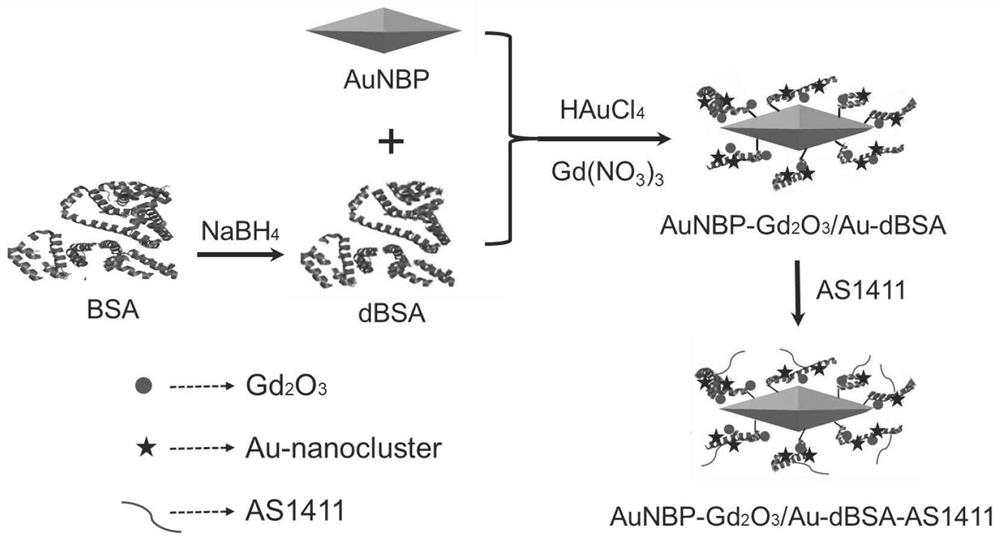
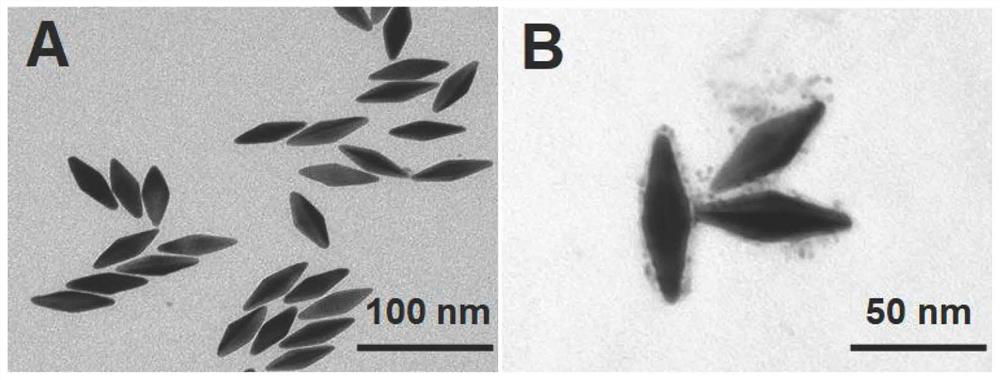
The invention discloses a composite nanoprobe with targeted fluorescence / magnetic resonance bimodal imaging and photothermal therapy functions, as well as a preparation and application of the composite nanoprobe. The preparation method comprises the following steps of firstly, preparing AuNBP by adopting a seed growth method, separating and purifying the AuNBP to obtain pure AuNBP nanoparticles, and then modifying the surfaces of the AuNBP nanoparticles with sulfhydrylated BSA; modifying Gd2O3 nanoparticles and Au nanoclusters on the surfaces of AuNBP nanoparticles with the modified AuNBP nanoparticles as a stabilizer to obtain an AuNBP-Gd2O3 / Au-dBSA composite nanomaterial; and finally enabling an aptamer AS1411 to be connected to the surface of AuNBP-Gd2O3 / Au-dBSA to obtain the AuNBP-Gd2O3 / Au-dBSA-AS1411 targeted composite nanoprobe. The AuNBP-Gd2O3 / Au-dBSA-AS1411 composite nanoprobe provided by the invention has good biocompatibility, fluorescence stability and photothermal stability, also has fluorescence / magnetic resonance bimodal imaging and photothermal therapy functions, can enter tumor cells in a targeting manner, marks and traces the tumor cells, and is beneficial to earlydiagnosis and treatment of tumors.
Owner:XUZHOU MEDICAL UNIV
Kit for detecting multiple influenza viruses by polymerase chain reaction (PCR) microarray
ActiveCN102337352AEasy to operateShort timeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceInfluenza A (H1N1) virus
The invention relates to a kit for detecting multiple influenza viruses by a polymerase chain reaction (PCR) microarray. The kit comprises mainly a PCR microarray reaction plate, a PCR reaction solution, and a packaging box for centralized and partitioned packaging of reagent bottles or reagent tubes. Through the combination of a real-time fluorescence PCR technology and a microarray technology, on the single PCR microarray reaction plate, the kit can detect eight influenza viruses comprising influenza A virus, influenza B virus, seasonal influenza H1 subtype virus, seasonal influenza H3 subtype virus, influenza A H1N1 virus, highly pathogenic avian influenza H5 subtype virus, highly pathogenic avian influenza H7 subtype virus, and highly pathogenic avian influenza H9 subtype virus simultaneously. A result of detection adopting the kit can be utilized for differential diagnosis and monitoring of pathogens capable of causing influenza.
Owner:DAAN GENE CO LTD
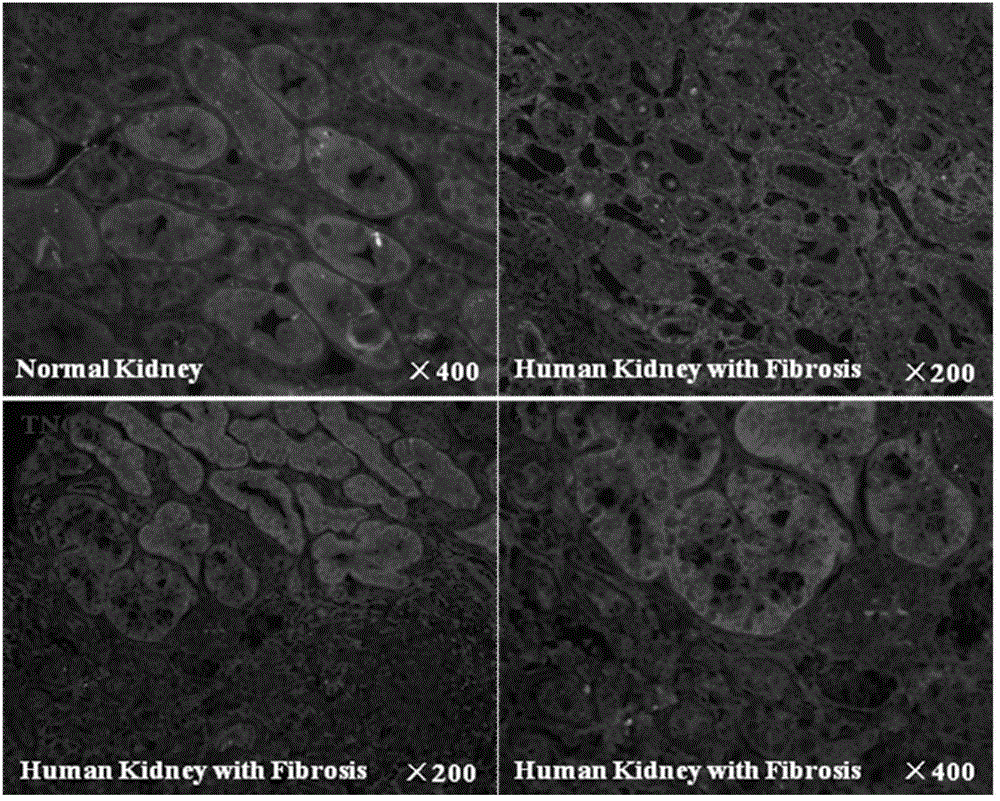
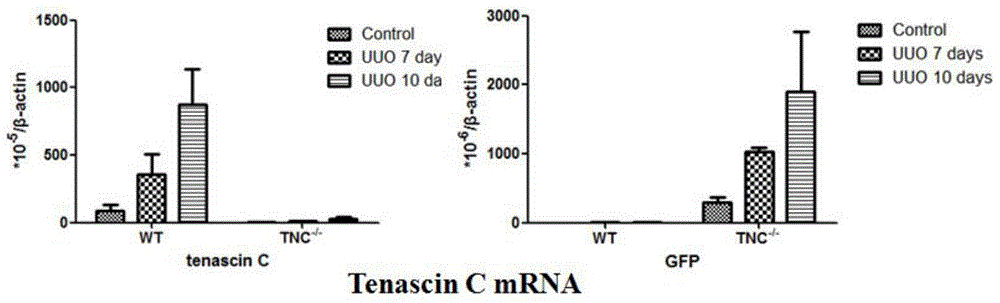
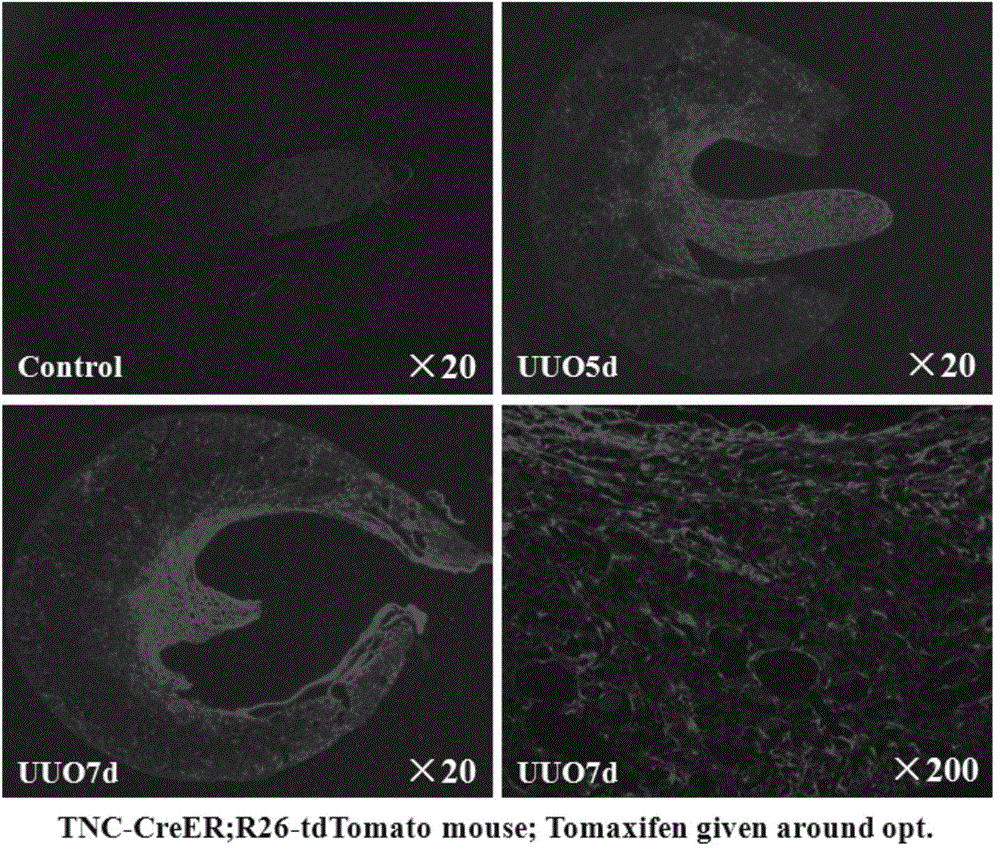
Application of Tenascin-C in preparation of kidney injury diagnosis and treatment preparations
InactiveCN106177907AReduce severityAid in early diagnosisPeptide/protein ingredientsUrinary disorderBacteriuriaIntervention measures
The invention belongs to the technical field of biomedicines, and relates to an application of Tenascin-C in the preparation of kidney injury diagnosis and treatment preparations. The Tenascin-C is used as a kidney injury or kidney fibration biomarker, and especially used as a biomarker in kidney injury or kidney fibration blood and urea. The invention provides an application of the Tenascin-C in the preparation of kits for detecting, diagnosing and treating kidney injury or kidney fibration. The biomarker indexes of the blood and the urea are monitored to reflect the severe degree of the kidney injury and predicate the kidney recovery opportunity, and intervention measures for promoting restoration of acute kidney injury and mitigation of the severe degree of the kidney injury are provided; and the biomarkers of the blood and the urea are monitored to reflect the severe degree of the kidney fibration, and intervention measures for facilitating early stage diagnosis of chronic kidney fibration and monitoring of the kidney fibration progress are provided.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
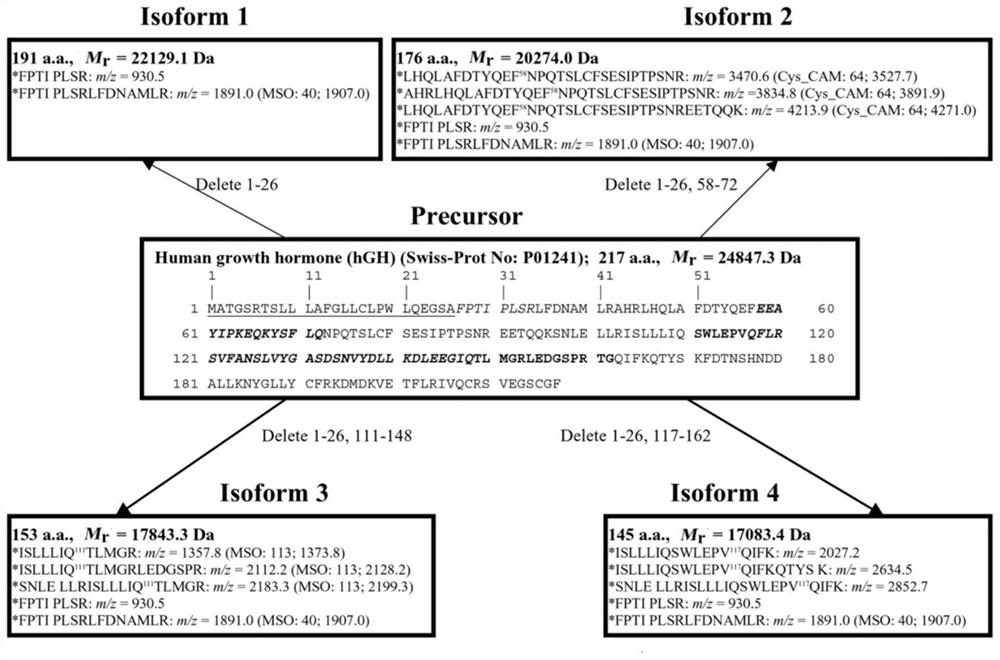
Method for identifying existence form biomarker spectrum of human growth hormone protein
The invention provides a method for identifying a human growth hormone protein existence form biomarker spectrum, which comprises the following steps: collecting human growth hormone secreting pituitary adenoma and normal pituitary tissue samples, respectively extracting tissue proteins, carrying out two-way gel electrophoresis, western blot and coomassie brilliant blue staining, scanning a visual PVDF membrane and a two-way gel into a digital image, digesting corresponding two-way gel protein spots with trypsin, purifying, and identifying a GHP biomarker spectrum through mass spectrum identification and bioinformatics analysis; and identifying post-translational modifications and shear variations on GHP by using quantitative phosphorylated proteomics, ubiquitinated proteomics, and acetylated proteomics analysis in combination with bioinformatics. According to the invention, the GHP change mode between the growth hormone secreting pituitary adenoma and normal pituitary tissues can be identified, 46 kinds of GHPs are identified in the growth hormone secreting pituitary adenoma, 35 kinds of GHPs are identified in the normal pituitary tissues, and 11 kinds of GHPs only appear in the growth hormone secreting pituitary adenoma tissues.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
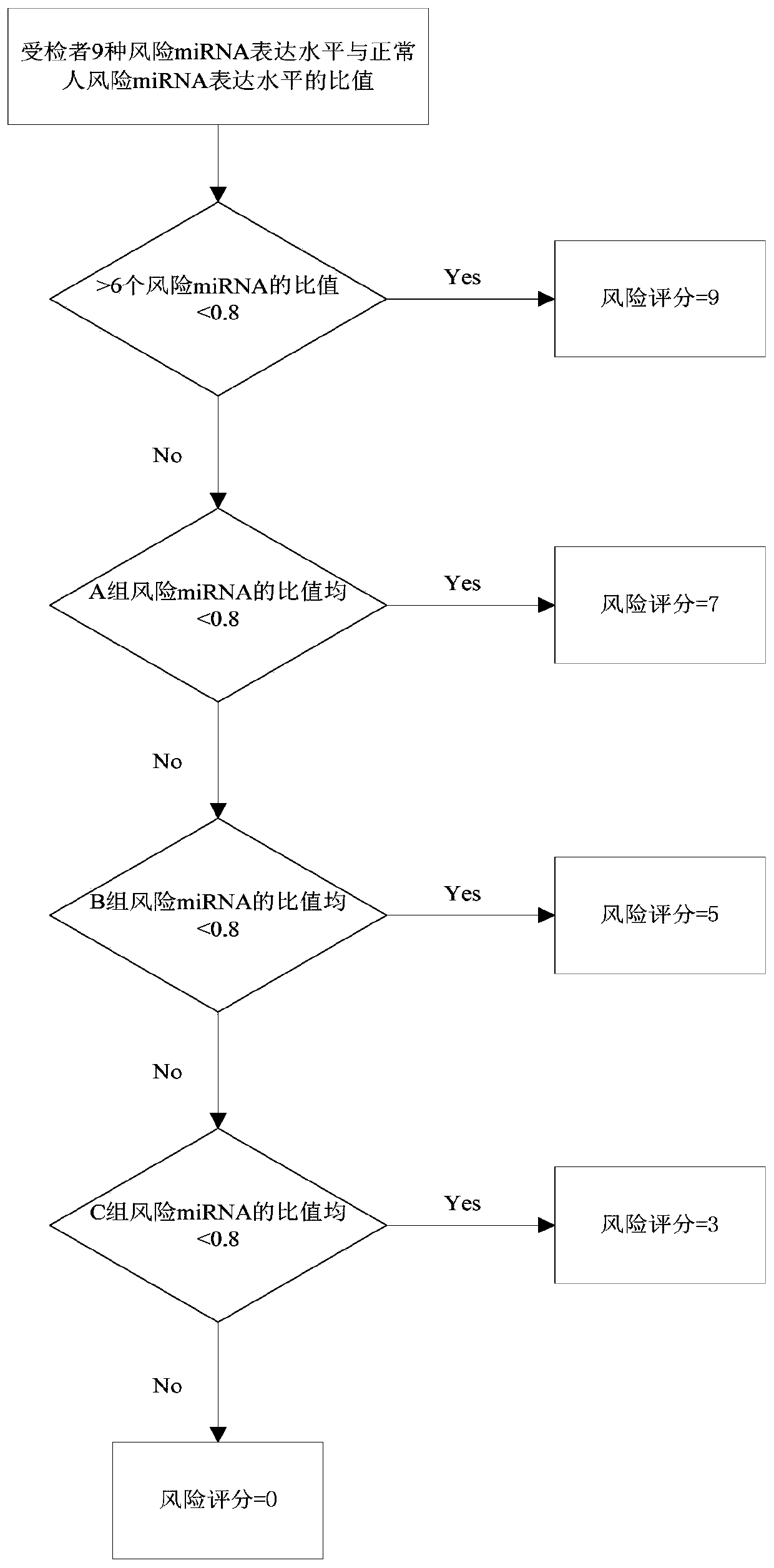
MiRNA group for early diagnosis and/or prognosis monitor of pancreatic caner and application of miRNA
PendingCN111172285AAid in early diagnosisContribute to early warningMicrobiological testing/measurementDNA/RNA fragmentationPancreas CancersPrognosis biomarker
The invention discloses a miRNA group related to the pancreatic cancer. The miRNA group includes the following miRNA: miR-143, miR-218, miR-217, miR-216a, miR-205, miR-145, miR-195, miR-193a and miR-127. The miRNA group can be used as an early detection marker and prognosis biomarker for the pancreatic cancer, and is applied to early diagnosis and / or prognosis monitor of the pancreatic cancer, sothat early diagnosis of the pancreatic cancer is achieved, and the opportunity for early diagnosis and treatment of the pancreatic cancer is seized; and / or whether a pancreatic cancer patient has risks of early metastasis and drug resistance or not can be evaluated, so that a medication plan is adjusted in time, occurrence of early metastasis is prevented, and the survival time of patients is prolonged.
Owner:AFFILIATED HOSPITAL OF JIANGNAN UNIV
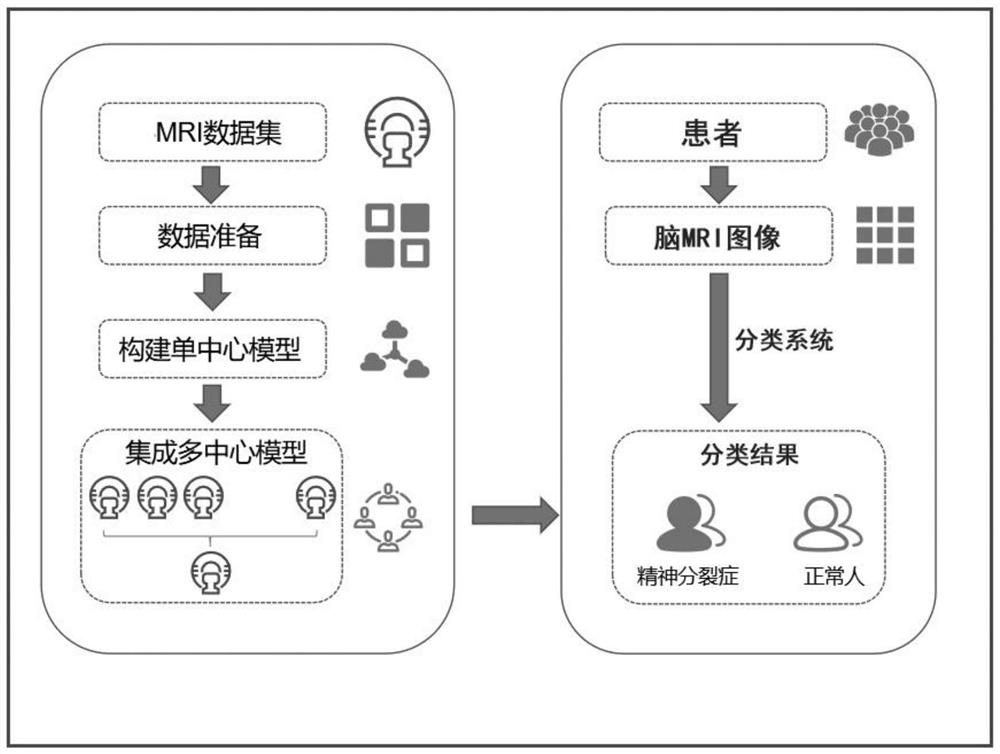
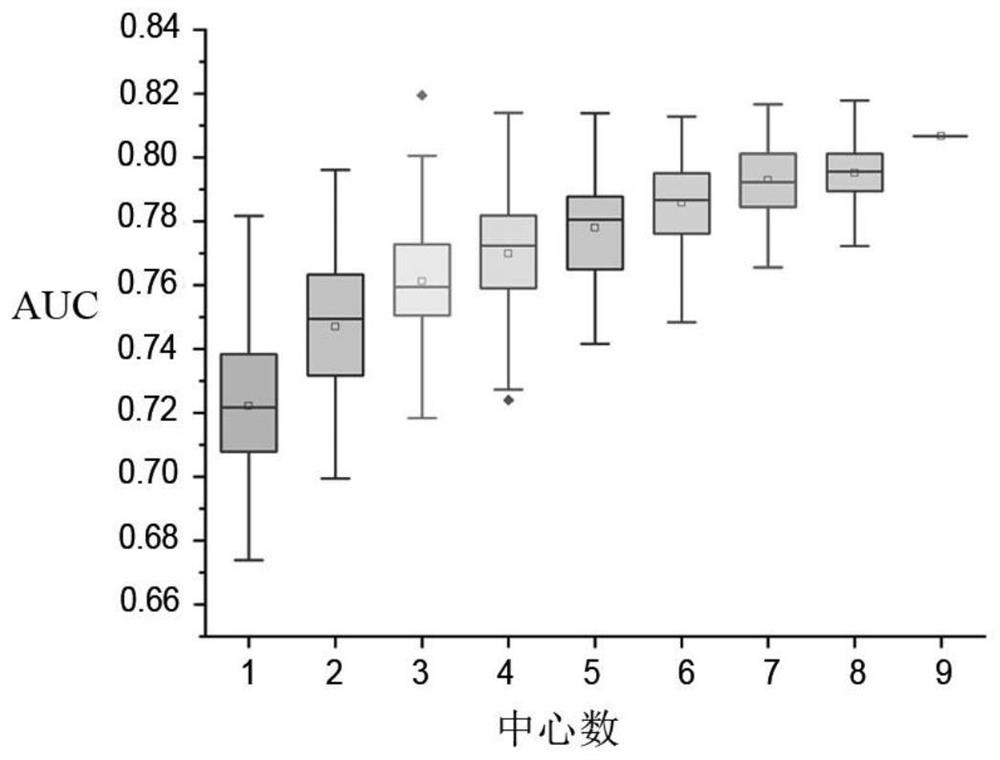
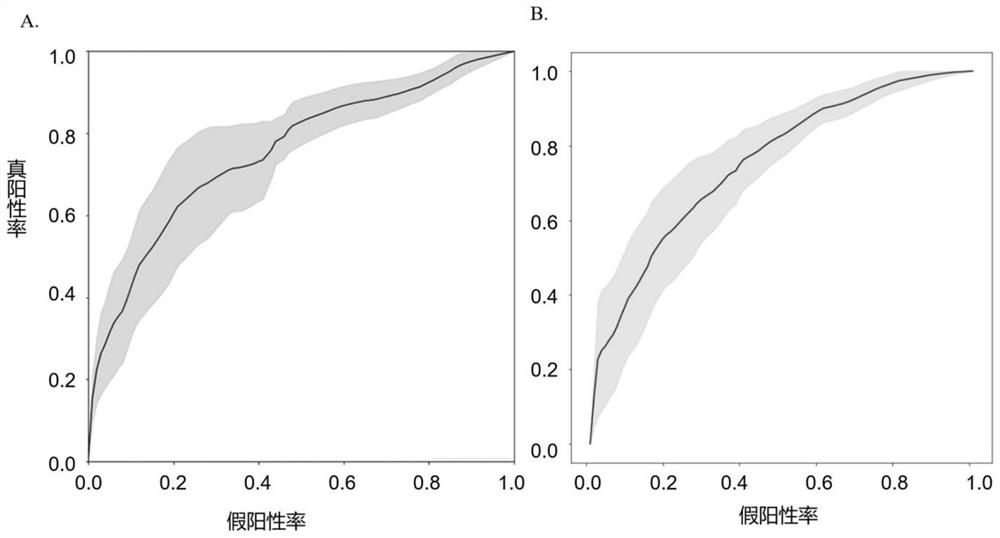
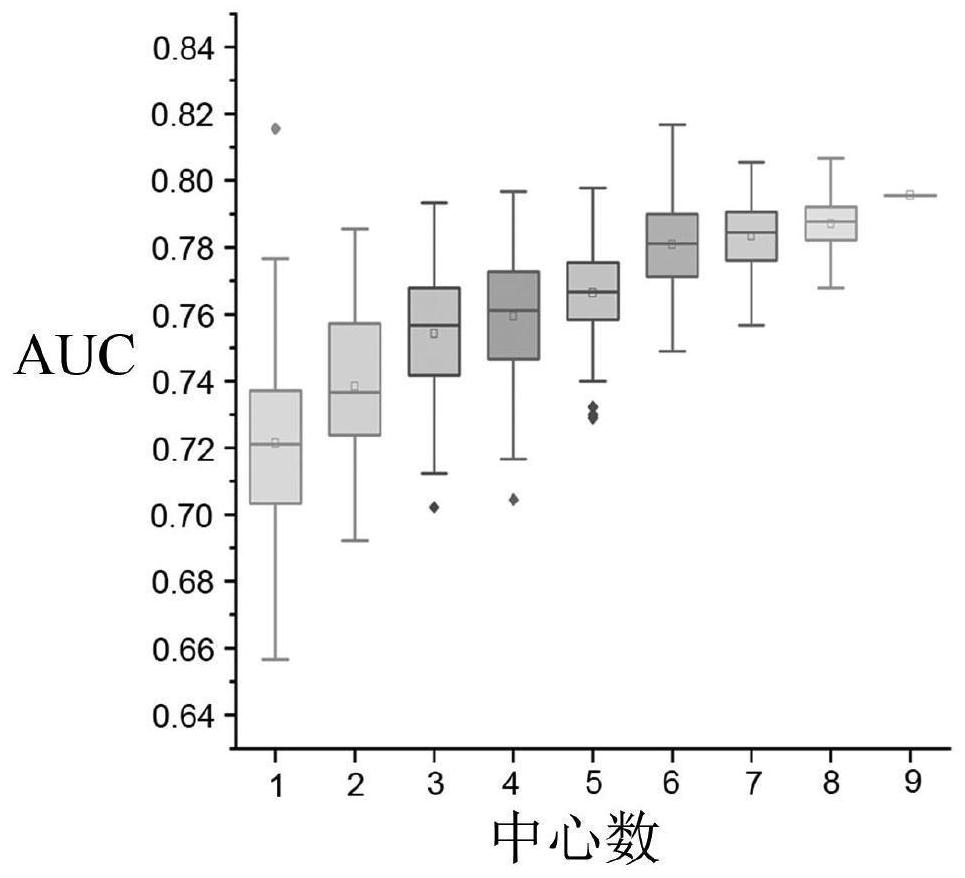
Schizophrenia classification method and system based on multi-center model
ActiveCN113197578AAvoid effectivenessAvoid the defects of poor generalization abilitySensorsPsychotechnic devicesData setAlgorithm
The invention relates to a schizophrenia classification method and system based on a multi-center model, and the method comprises the steps: 1, data preparation: collecting a brain MRI image of a sample, processing the brain MRI image, and extracting a plurality of brain structure features to obtain a feature matrix; carrying out covariant regression processing on the feature matrix, and then carrying out standardization processing; enabling each center to prepare a respective data set according to the step; 2, enabling each center to construct a respective single-center model by using a machine learning classifier, and training the respective single-center model by using the respective data set; 3, classifying to-be-classified test samples by using each single center model to obtain classification probability values of the to-be-classified test samples corresponding to each center; and performing weighted summation on the classification probability value and the weight of each center to obtain a classification probability value based on a multi-center model, and integrating all the single-center models into the multi-center model for classification. Data sharing of each center is realized, and each center does not need to share original data.
Owner:TIANJIN MEDICAL UNIV
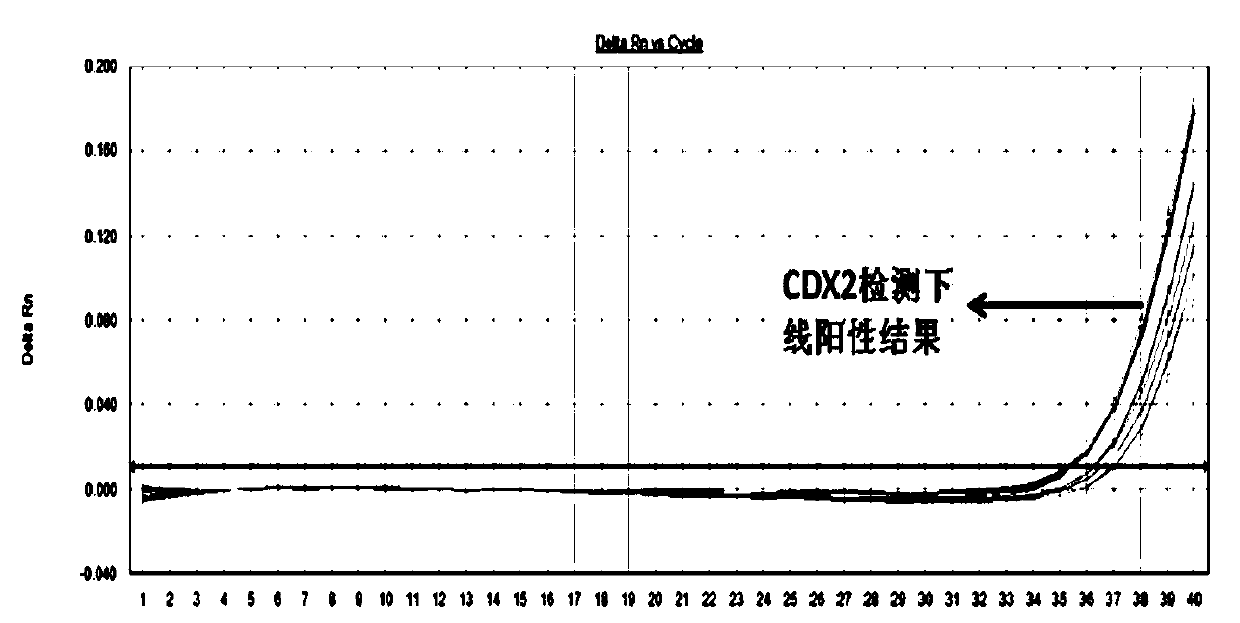
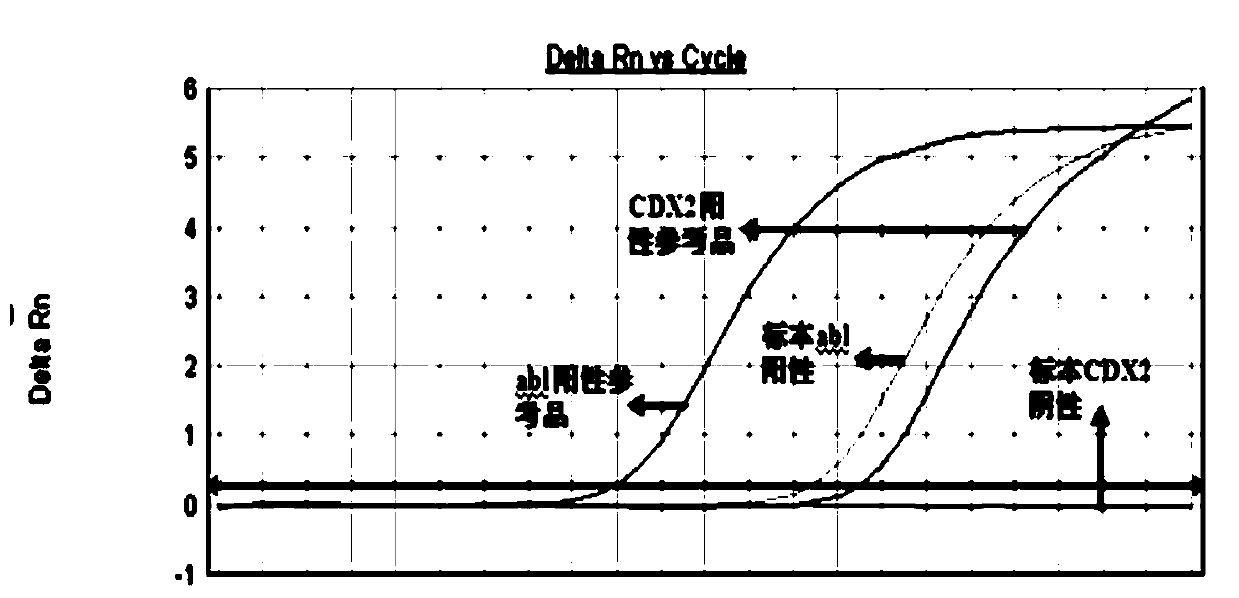
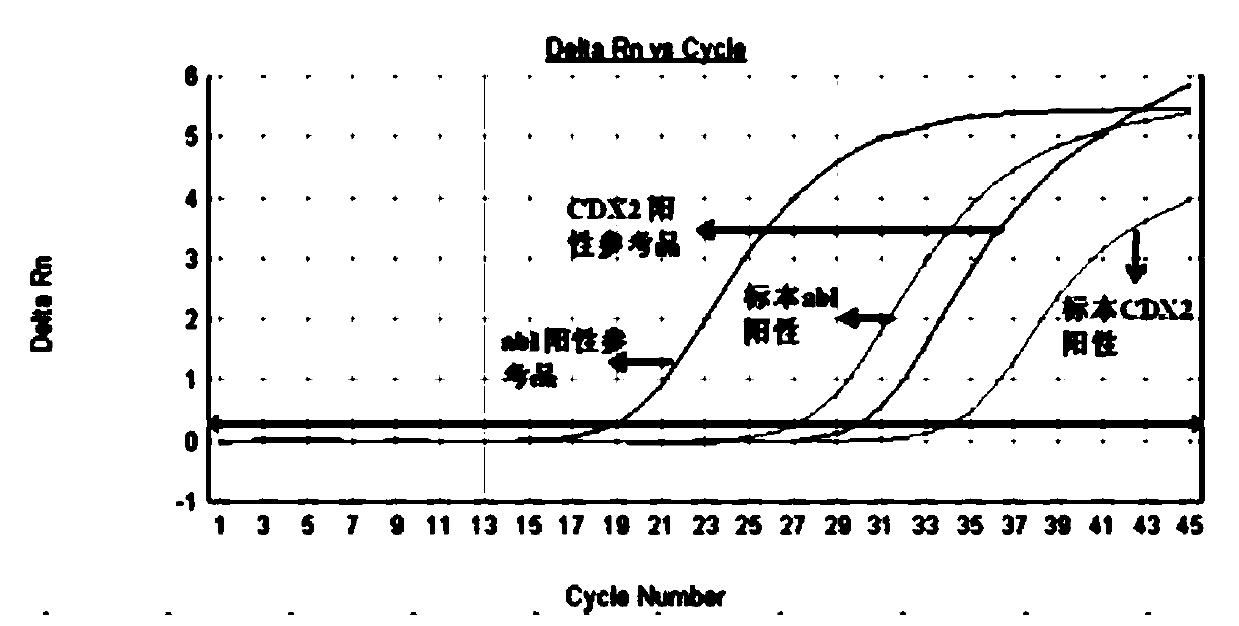
Primers and method for detecting expression level of leukemia cdx2 gene
ActiveCN106906288BHigh precisionEasy to readMicrobiological testing/measurementDNA/RNA fragmentationTherapeutic effectGene expression level
The invention discloses primers and a probe for detecting relative expression quantity of CDX2 gene and a detection method. The forward and reverse primers for amplification coverage of CDX2 gene and the probe are respectively CDX2-F, CDX2-R and CDX2-Probe. The forward and reverse primers and the probe for detecting reference gene Abl are respectively Abl-F, Abl-R and Abl-Probe. The detection reagent and the method have high precision, and results are convenient to read and discriminate. In addition, specificity is good, sensitivity is high, and operation is simple. The invention is helpful for clinical early diagnosis of leukemia and monitoring of minimal residual disease (MRD), and is of great significance for timely intervention of treatment, adjustment of therapeutic schedule, evaluation of treatment effect, prediction of prognosis and prevention of clinical relapse.
Owner:FUZHOU ADICON CLINICAL LAB INC
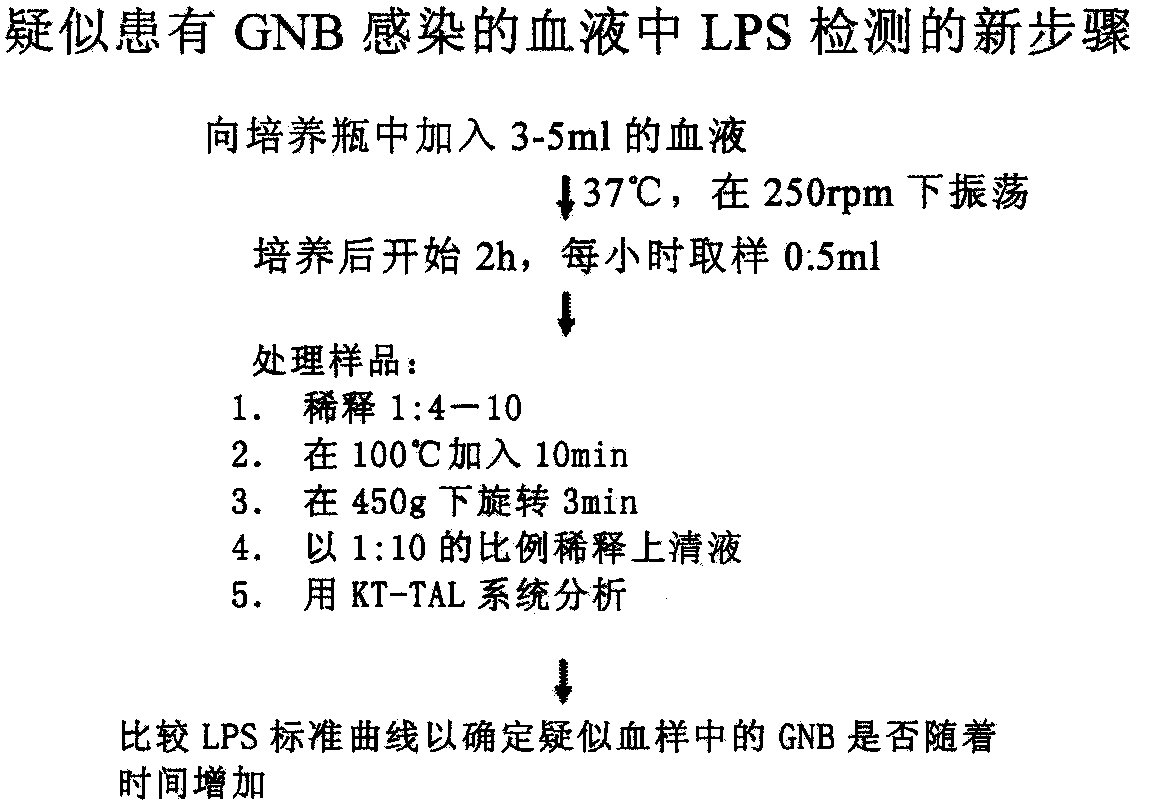
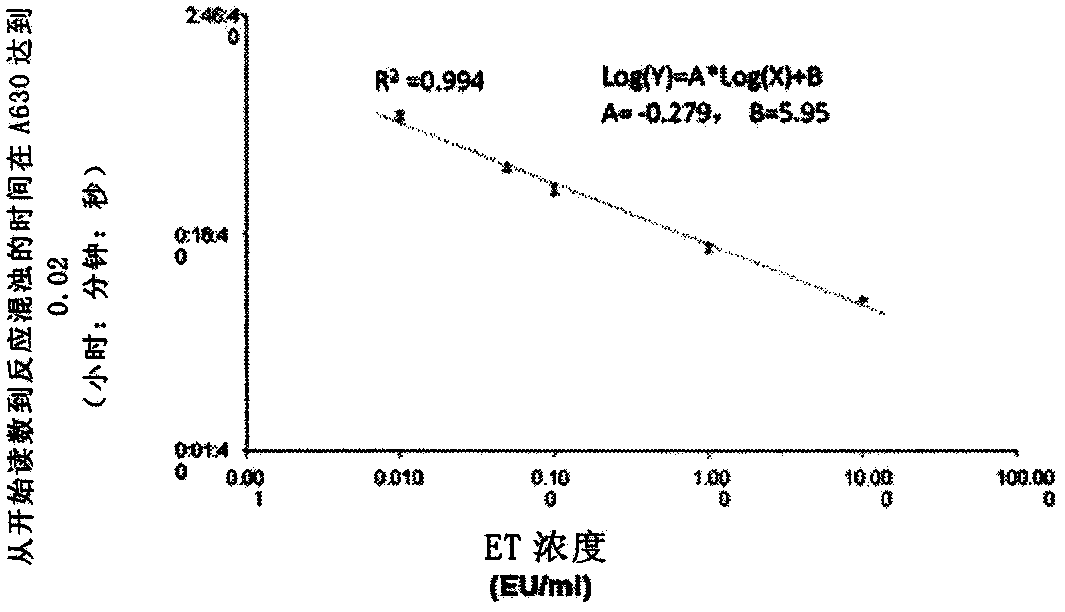
Novel method for rapidly detecting septicopyemia by using gram-negative bacterial infection
ActiveCN111077307AAid in early diagnosisMonitoring Treatment EffectsBiological material analysisGram-negative bacterial infectionsBiologic marker
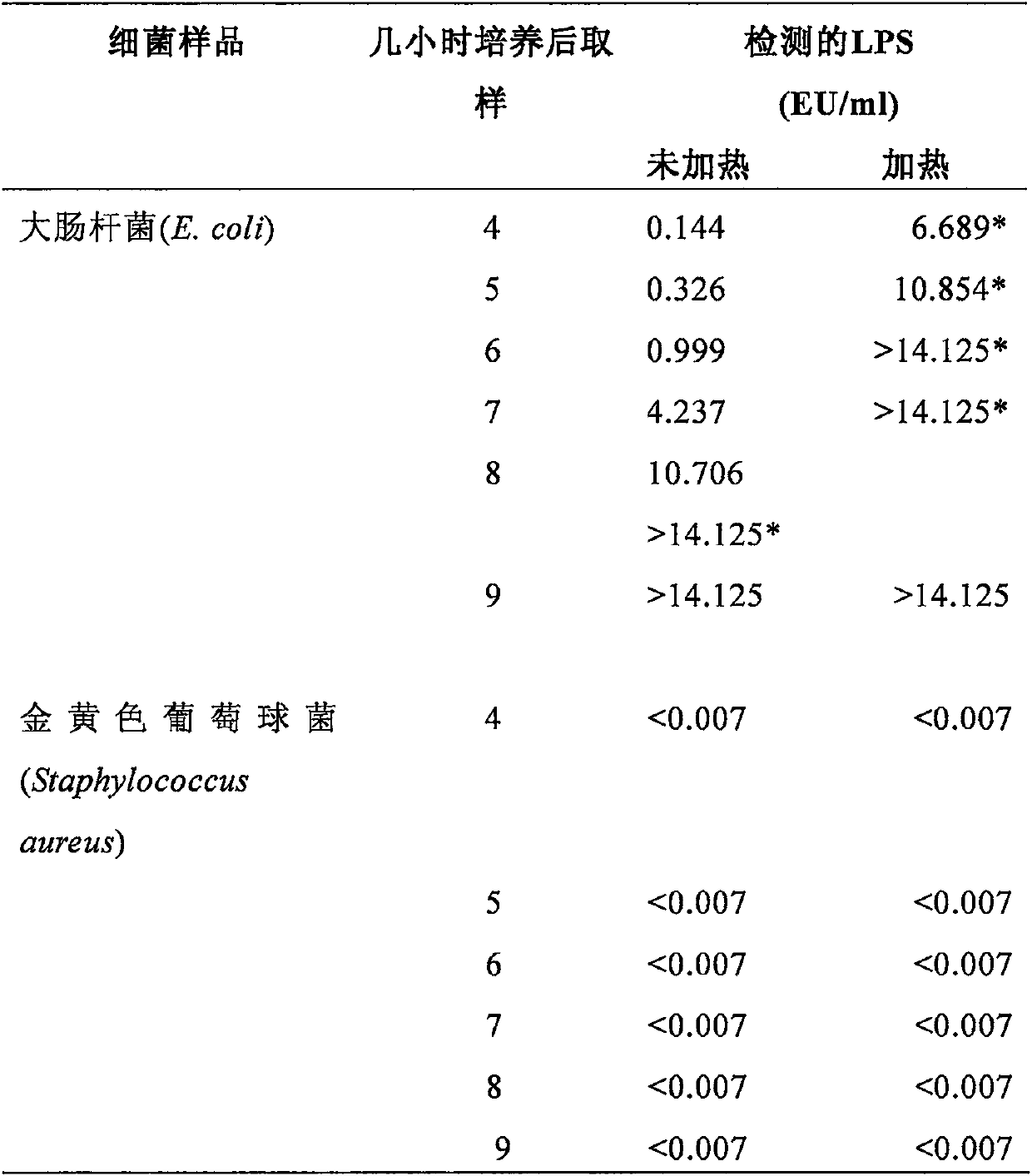
Septicopyemia is a systemic infection endangering life and needs to be properly and quickly treated on the basis of quickly determining pathogens. Gram-negative bacteria (GNB) are the main pathogenicpathogens of endotoxin (LPS) as a characteristic substitute biomarker thereof. In the research, a new method is explored firstly, firstly, LPS is increased through GNB culture, and then the LPS of tachypleus amebocyte lysate (TAL) is detected through a dynamic turbidity measurement method (KT-TALA). Sample preparation procedures are optimized. Results show that under high GNB concentration, LPS can be detected 3 hours after culture, which is 6.5-7.5 hours ahead of time, and 9.5-10.5 hours is for a BD BACTEC positive report; under a low GNB load, a BD BACTEC system needs 22-26 hours to detect GNB, but the KT-TALA system only needs 9 hours to detect LPS of GNB, and the time is advanced by 13-17 hours. Compared with a traditional BD BACTEC system, the novel method for detecting GNB infected LPS is much faster, and especially in the early stage of the septicopyemia with a low bacterial load, and the method is helpful to make appropriate treatment decisions in earlier time.
Owner:XIAMEN BIOENDO TECH CO LTD
A monoclonal antibody for recognizing monohydric phenolic compounds and its preparation method and application
ActiveCN108383910BAid in early diagnosisDetection clearTissue cultureImmunoglobulinsNucleic acid sequenceAmino acid
The present invention relates to a monoclonal antibody that recognizes monohydric phenol compounds. The nucleic acid sequence information of the heavy chain coding region is encoded by the DNA sequence shown in SEQ 1; the amino acid sequence information of the heavy chain coding region is the amino acid shown in SEQ 2 Sequence coding; the nucleic acid sequence information of the light chain coding region is coded by the DNA sequence shown in SEQ 9; the amino acid sequence information of the light chain coding region is coded by the amino acid sequence shown in SEQ 10. The monoclonal antibody for recognizing monohydric phenolic compounds described in the present invention can detect monohydric phenolic compounds in human body fluids, and the content of monohydric phenolic compounds can reflect the degree of cell activity in the body of the subject and the immunity of the body state, thus contributing to the early diagnosis of tumors. The monoclonal antibody for recognizing monohydric phenolic compounds of the present invention can effectively detect gastric cancer, colon cancer, rectal cancer, esophageal cancer, pancreatic cancer and other digestive tract tumors.
Owner:JINAN TAIHE PHARM TECH CO LTD
Construction method and application of morphological fusion classification index of neuroimaging markers
ActiveCN113222001BAvoid security issuesIndicators are simpleMedical automated diagnosisCharacter and pattern recognitionPattern recognitionRadiology
The present invention is a construction method and application of neuroimaging marker morphological fusion classification index. The construction method includes the following contents: obtaining structural MRI data of M centers, extracting brain structural image characteristic data; performing independent training with the data of each center, respectively Establish the classification model of each center and obtain the classification model of M centers; for any sample, in the classification model of all centers, calculate the classification weight value of the sample in each feature of each model, that is, the SHAP matrix; then use each The sample size used in the training of each model is the weight, and a single morphological fusion classification index MICI value is obtained by calculating according to formula (1): where Si represents the sample size of model i, B represents the total number of features, and a i Represents the SHAP value of feature a in model i, i=1~M. The MICI value can well realize the discrimination between patients with mental illness and normal people, and has good interpretability, evolution and scalability.
Owner:TIANJIN MEDICAL UNIV
A Method of Ultrasonic CT Imaging Based on Spatial Coherence
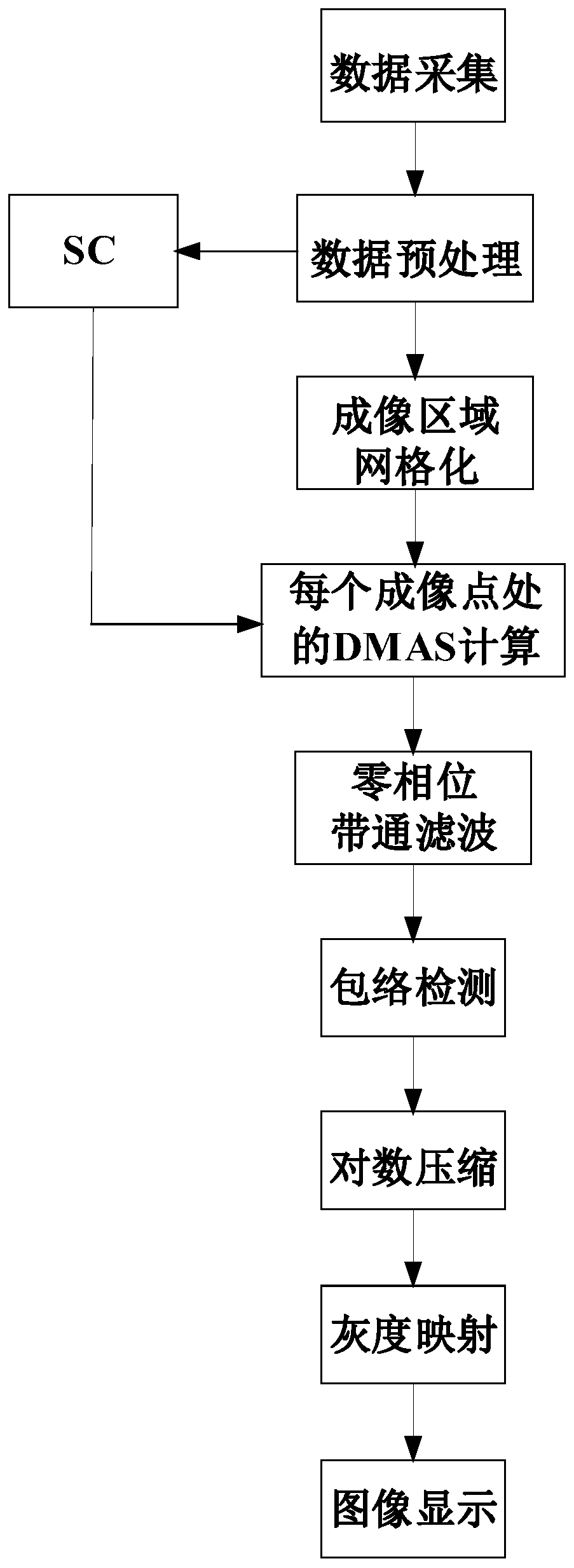
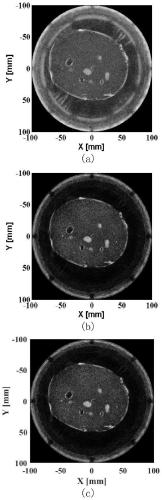
ActiveCN108618799BQuality improvementSuppress interferenceComputerised tomographsInfrasonic diagnosticsUltrasonic imagingRadiology
The invention discloses an ultrasonic CT imaging method based on space coherence. The method includes the following steps of firstly, collecting data to obtain original echo data; secondly, preprocessing the data; thirdly, meshing an imaging area; fourthly, conducting DMAS calculation treatment based on the space coherence for each meshed imaging point to obtain initial imaging signals of the meshed imaging points; fifthly, conducting data postprocessing to finally obtain an ultrasonic CT image. On the basis of the formation characteristics of virtual receiving signals in a filtering delay multiplication and superposition algorithm, by calculating the space coherence of any two signals of the virtual receiving signals in the filtering delay multiplication and superposition algorithm and weighting the virtual received signals, compared with the prior art, the problems of a zero-phase filtering delay multiplication and superposition algorithm applied in the ultrasonic CT reflection imaging field can be effectively solved, and a high-contrast high-noise-ratio and low-sidelobe-level ultrasonic CT image can be reestablished.
Owner:WUHAN WESEE MEDICAL IMAGING CO LTD
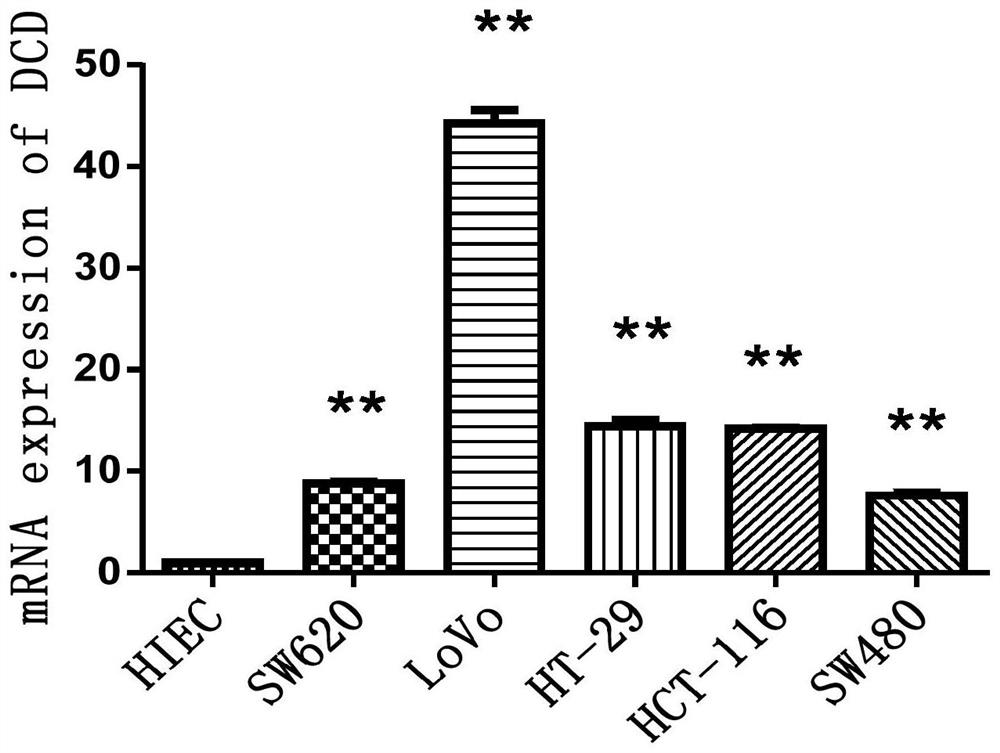
Q-PCR detection method for colorectal cancer marker DCD
PendingCN112831565AAid in early diagnosisSimple procedureMicrobiological testing/measurementDNA/RNA fragmentationRNA extractionColon rectal cancer
The invention relates to the technical field of in-vitro detection, in particular to a Q-PCR detection method of a colorectal cancer marker DCD. The method comprises the steps of RNA extraction of a sample, reverse transcription and Q-PCR amplification. The DCD is used as a colorectal cancer candidate diagnosis index and treatment target, RNA of cells or tissues is firstly extracted, an internal reference primer and a DCD gene primer are set to perform reverse transcription on the RNA to obtain two groups of different amplification products, Q-PCR reaction is performed respectively to obtain melting curves and amplification curves of the two groups of amplification products respectively, and the expression level of the DCD gene is calculated; then, theexpression level of the DCD geneis compared with the mRNA concentration of the DCD in the normal tissue or cell, and if the mRNA concentration of the DCD in the tested tissue or cell is more than one time higher than that of the normal tissue or cell, the tested tissue or cell can be considered to be the colorectal cancer tissue or colorectal cancer cell, so that early diagnosis of the colorectal cancer is facilitated.
Owner:广州医科大学附属中医医院
Monoclonal antibody for identifying monohydroxy phenolic compound as well as preparation method and application thereof
ActiveCN108383910AAid in early diagnosisDetection clearTissue cultureImmunoglobulinsEsophagus CancersCancer research
The invention relates to a monoclonal antibody for identifying a monohydroxy phenolic compound. The nucleotide sequence information of a heavy chain coding region is encoded by DNA sequence shown as SEQ 1; the amino acid sequence information of the heavy chain coding region is encoded by amino acid sequence shown as SEQ 2; the nucleic acid sequence information of a light chain coding region is encoded by the DNA sequence shown as SEQ 9; the amino acid sequence information of the light chain coding region is encoded by amino acid sequence shown as SEQ 10. The monoclonal antibody for identifyingthe monohydroxy phenolic compound has the advantages that the monohydroxy phenolic compound in human body fluid can be detected; the immunity state of the body and the activity degree of cells in thebody of a testee can be reflected through the content of the monohydroxy phenolic compound, so that the early diagnosis of tumor is facilitated. The monoclonal antibody for identifying the monohydroxy phenolic compound can be used for effectively detecting gastrointestinal tumors such as gastric cancer, colon cancer, rectal cancer, esophagus cancer and pancreatic cancer.
Owner:JINAN TAIHE PHARM TECH CO LTD
Colon cancer biomarker and application thereof
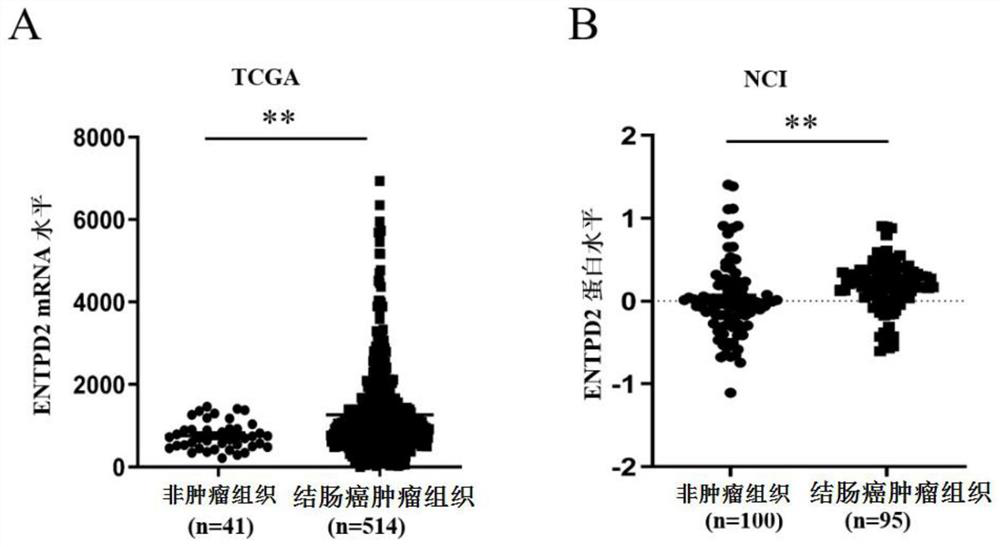
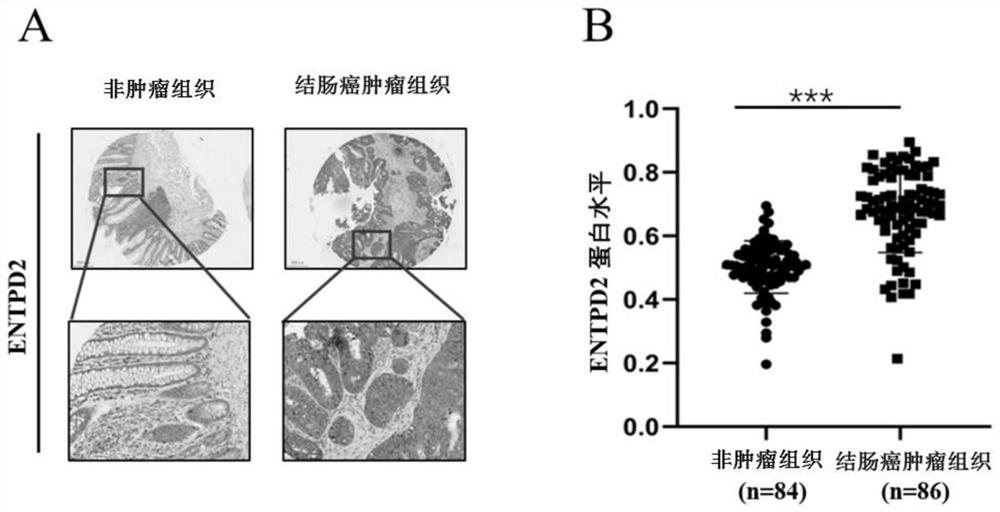
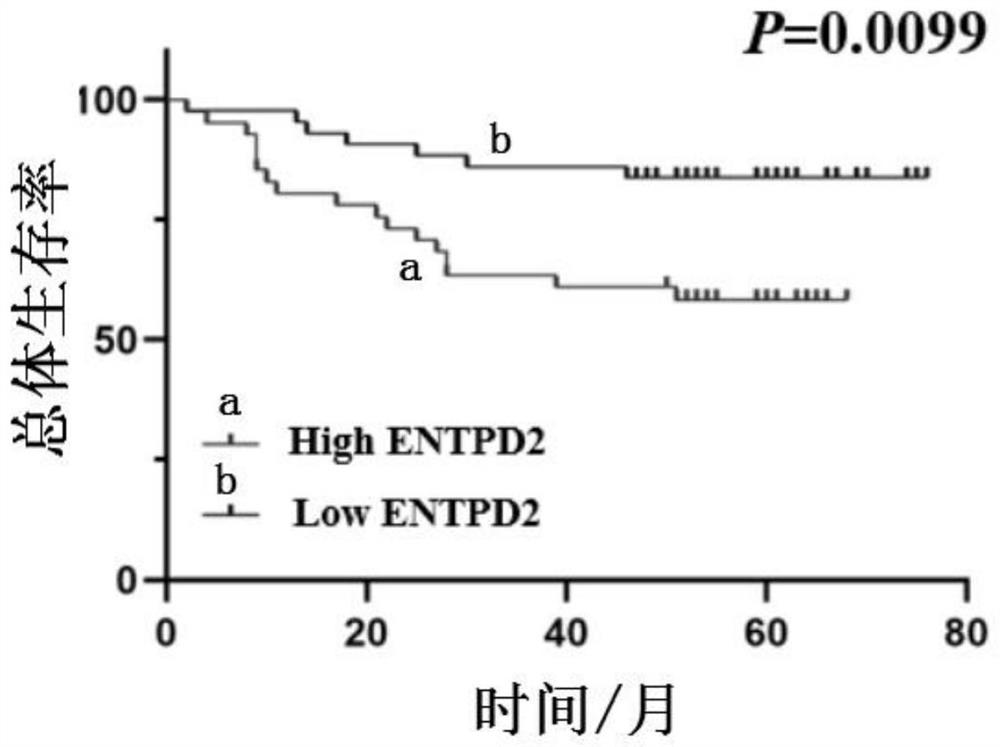
PendingCN114236125AAid in early diagnosisAids in prognosisBiological material analysisCancers diagnosisIndividualized treatment
The invention belongs to the technical field of tumor molecular diagnosis, and particularly relates to a colon cancer biomarker and application thereof.The research finds that an exosome of a colon cancer patient can stably express ENTPD2, the expression level of the exosome is obviously higher than that of a healthy person, and the exosome ENTPD2 with the high level is closely related to TNM staging and tumor infiltration depth of the patient; therefore, the serum exosome ENTPD2 has obvious advantages in colon cancer diagnosis, is expected to become a novel biological marker for colon cancer screening, individualized treatment and prognosis monitoring, and provides a new direction for colon cancer treatment. The invention finds that the ENTPD2 has obvious high expression in colon cancer tissues for the first time, the expression level of the ENTPD2 is closely related to the prognosis of patients, and the ENTPD2 is proved to be beneficial to the early diagnosis and prognosis judgment of the colon cancer patients.
Owner:THE SIXTH AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
The pcr detection primers of spruce dwarf mistletoe and its application and pcr detection method
ActiveCN103923995BAid in early diagnosisQuick checkMicrobiological testing/measurementDNA/RNA fragmentationMistletoe familyMicrobiology
The invention relates to a pair of PCR (polymerase chain reaction) detection primers for Arceuthobium sichuanense based on an ITS2 segment, belonging to the field of molecular biology. The primers comprise the following gene sequences: dwF3:5'-ACAAACTCATTTTCCCA CCACA-3'; dwR3: 5'-ACATTCAAGAAACCTGAC ACCC-3'. The primers provided by the invention have the advantages of short detection period, reliable result, high sensitivity and the like, and is simple to operate, can effectively monitor and early warm infection dynamic of Arceuthobium sichuanense, guide the formulation of scientific disease management policy and have important meaning for preventing and controlling the disease of the Arceuthobium sichuanense by adopting effective measures.
Owner:BEIJING FORESTRY UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com