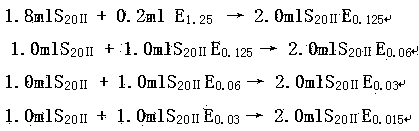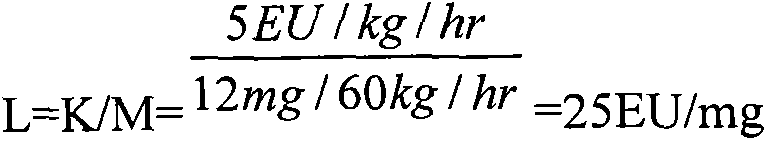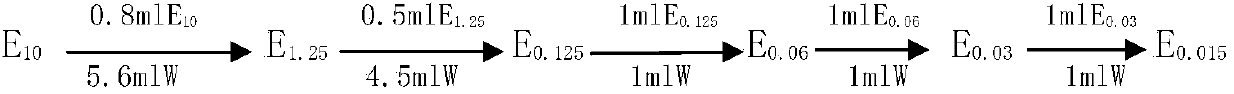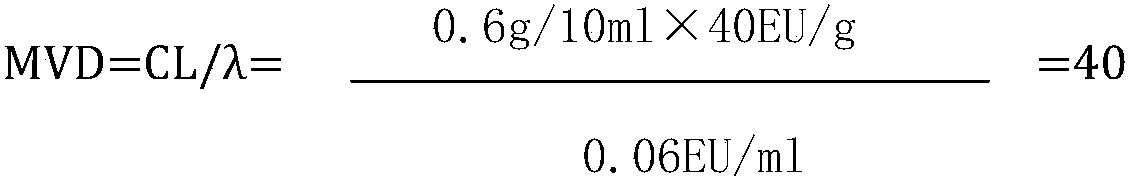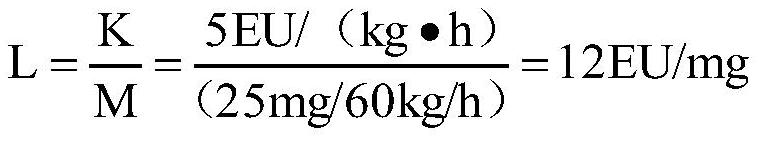Patents
Literature
45 results about "Tachypleus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tachypleus is a genus of south, southeast and east Asian horseshoe crabs in the family Limulidae.
Construction method of cell model for detecting pyrogens, cell model and pyrogen detection kit
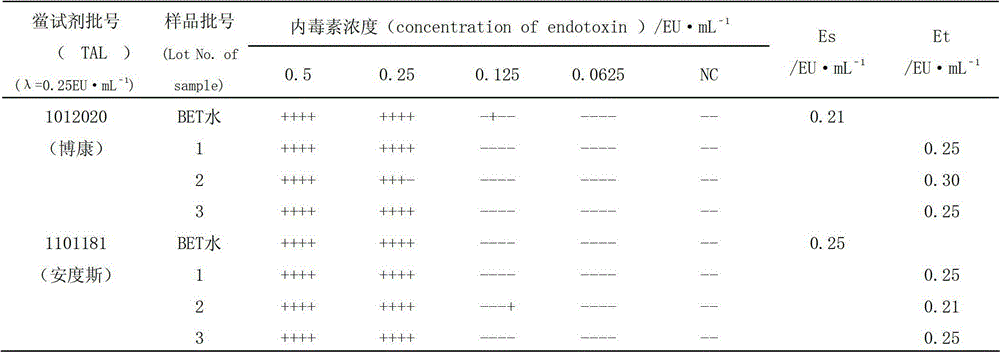
ActiveCN106148286AImprove stabilityIncreased sensitivityCell receptors/surface-antigens/surface-determinantsCulture processWestern blotCytokine
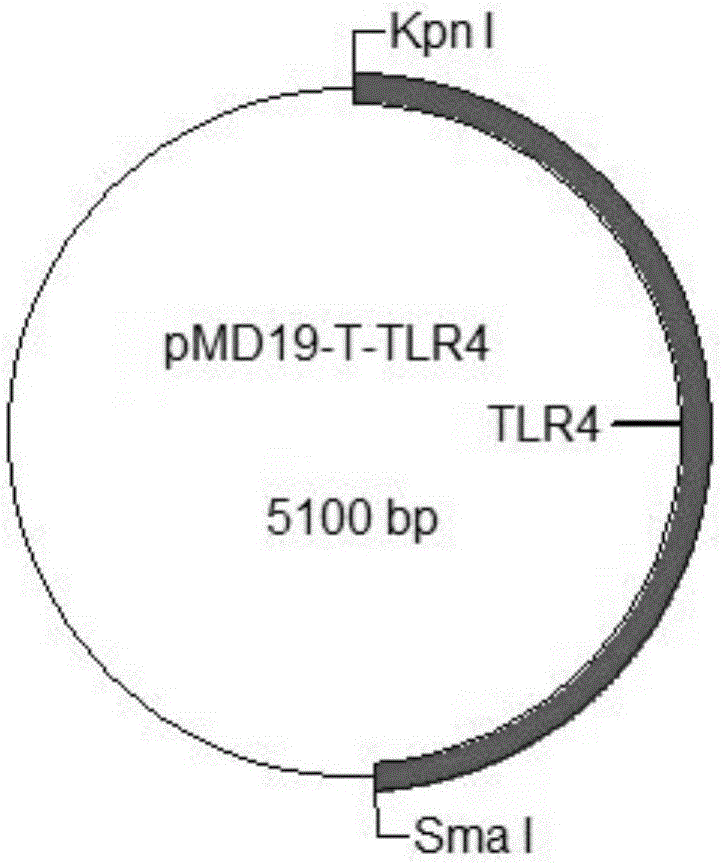
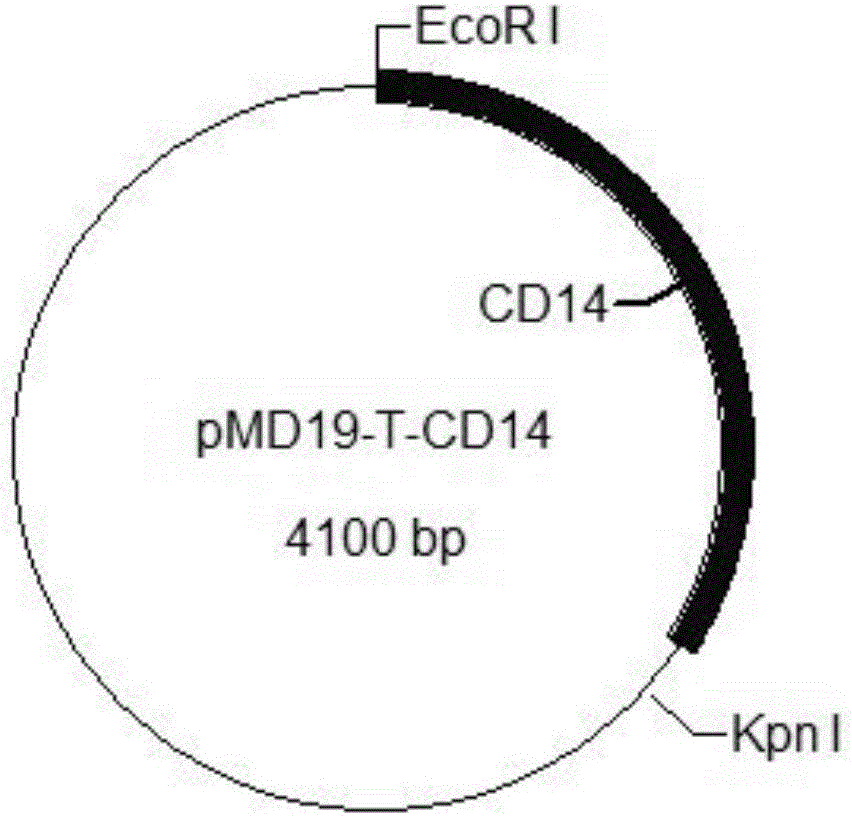
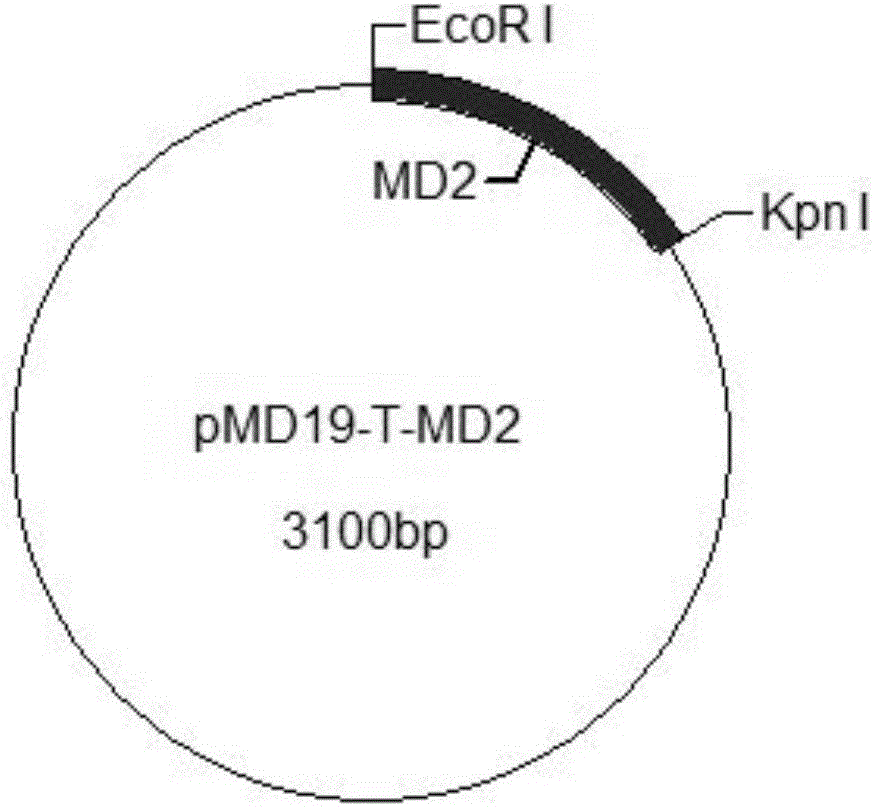
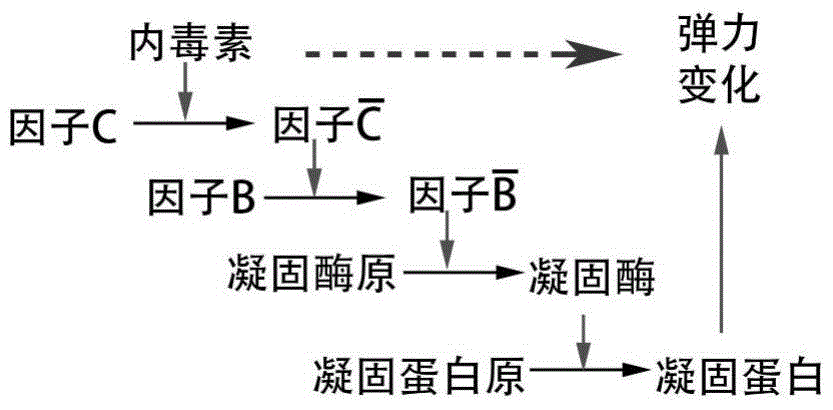
The invention provides a construction method of a cell model for detecting pyrogens, the cell model and a pyrogen detection kit. The cell model utilizes specific locations of CRISPR / CAS9 induced genomes to form double-bond fission, TLR4 and CD14-MD2 are knocked into two chromosomes of a cell line respectively by the aid of the homologous recombination repair principle, green fluorescence GFP and red fluorescence RFP are respectively used for tracing finally successfully constructed TLR4 / CD14 / MD2 fixed-point knocked-in fluorescent tracer cell models, and the LPS stimulating cell model can detect release of IL-6 and TNF-a cytokines by means of ELISA, Western Blot, mass spectrum and immunomagnetic beads. The cell model is good in stability and high in sensitivity, and the lowest detectable limit can reach 0.005EU / mL and is far lower than 0.025EU / mL of the Tachypleus Amebocyte Lysate method.
Owner:牛刚
Method for detecting bacterial endotoxin in citric acid raw material
InactiveCN104232739ASolve inspection problemsEnsure safetyMicrobiological testing/measurementInjection productToxin
The invention discloses a method for detecting bacterial endotoxin in a citric acid raw material. The method comprises the following steps of firstly carrying out interference test to determine the sensitivity of tachypleus amebocyte lysate selected to detect the bacterial endotoxin in the citric acid raw material; and then diluting the to-be-detected citric acid raw material to prepare a to-be-detected solution, adding the tachypleus amebocyte lysate determined in the interference test and detecting the bacterial endotoxin. By virtue of the method for detecting bacterial endotoxin in the citric acid raw material, which is disclosed by the invention, the problem of detection of the bacterial endotoxin in the citric acid raw material for a newly developed injection product is solved; the quality of a sterile product is controlled at the source and the method is of great significance for ensuring the safety of the injection product. Since the method has a theoretical basis of biochemistry, the method is more scientific; molecular and biochemical reactions of an enzyme are applied and the method has the advantages of high specificity, strong reproducibility, high accuracy and sensitivity, no need of special instrument and equipment and strong practicability and is simple and quick to operate.
Owner:CHINA OTSUKA PHARM CO LTD
Method for detecting content of bacterial endotoxin of bromhexine hydrochloride raw material
InactiveCN103837673AAccurate contentReduce inspection costsPreparing sample for investigationBiological testingInterference factorLimit value
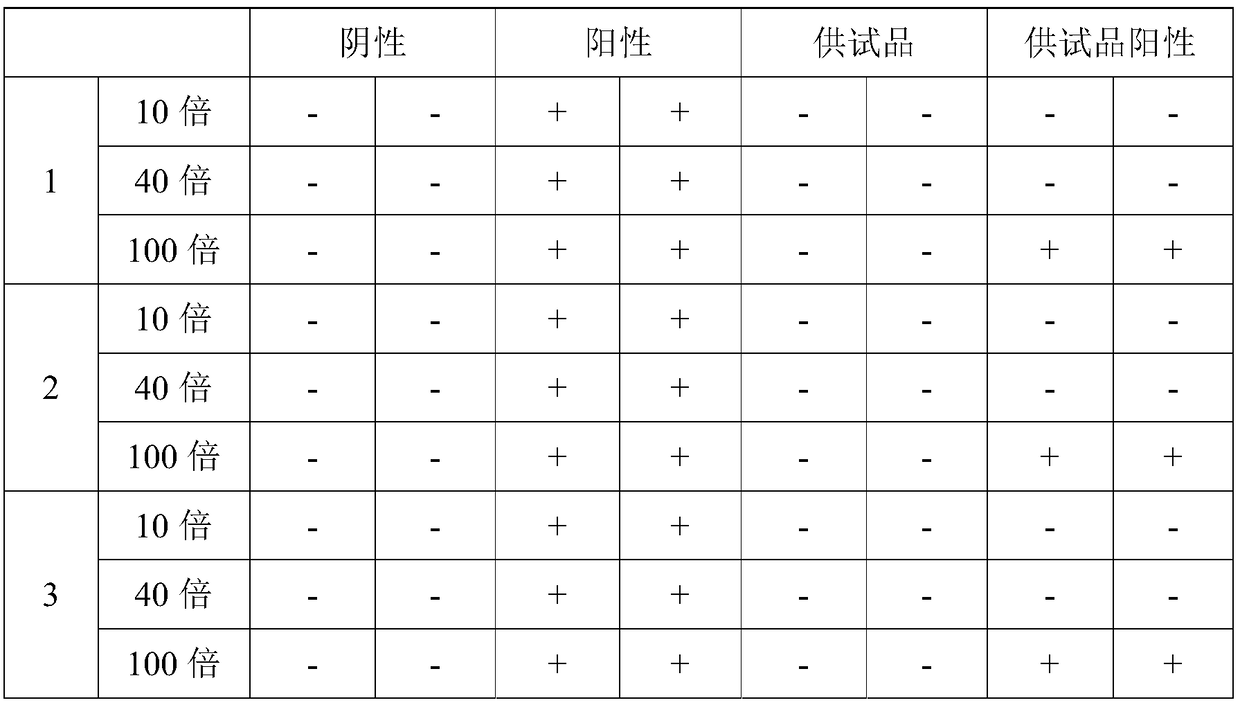
The invention aims at providing a sensitive and reliable method, and particularly relates to a method for quickly detecting the content of bacterial endotoxin of a bromhexine hydrochloride raw material. The method comprises the following steps of measuring bromhexine hydrochloride powder, dissolving the bromhexine hydrochloride powder in DMSO (dimethyl sulfoxide) to have the pre-interference experiment and find out the maximal dilution times; preparing a bromhexine hydrochloride test solution according to 1600 times of the maximal dilution times to have the interference experiment with two TAL (tachypleus amebocyte lysate) with the sensitivity of 0.25EU / ml from two different factories, finally confirming whether the interference factor exists or not, and determining the limit value of the bacterial endotoxin; diluting the bromhexine hydrochloride solution according to the non-interfered maximal valid dilution times, and then examining the bacterial endotoxin.
Owner:张嵩
Method for detecting endotoxin content
ActiveCN104698159AComprehensive detectionConvenient researchBiological testingElectrochemical biosensorDiluent
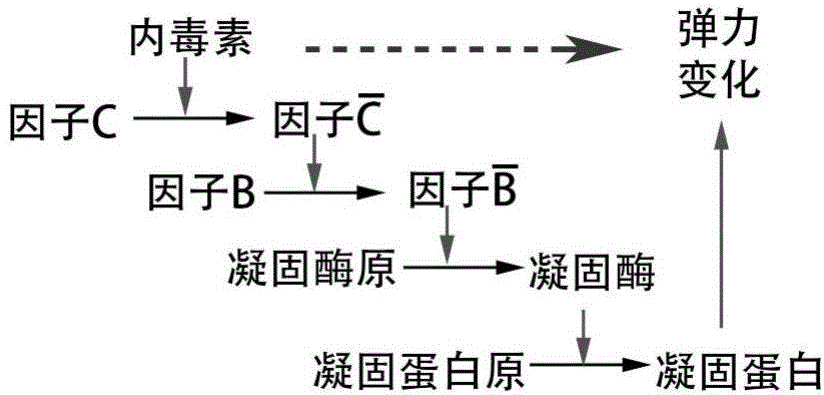
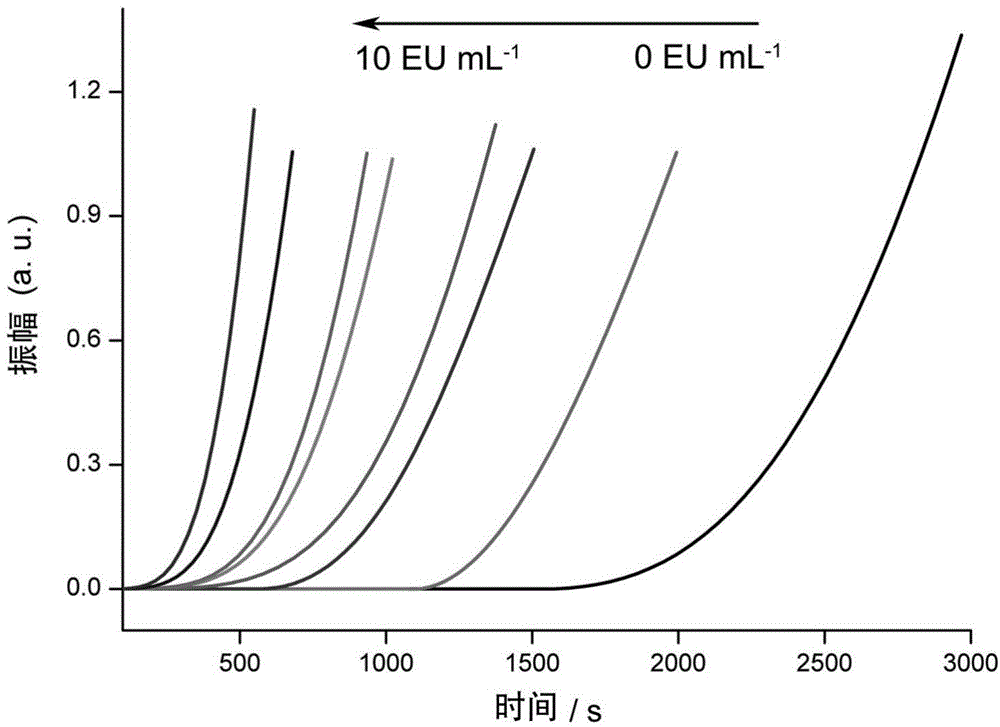
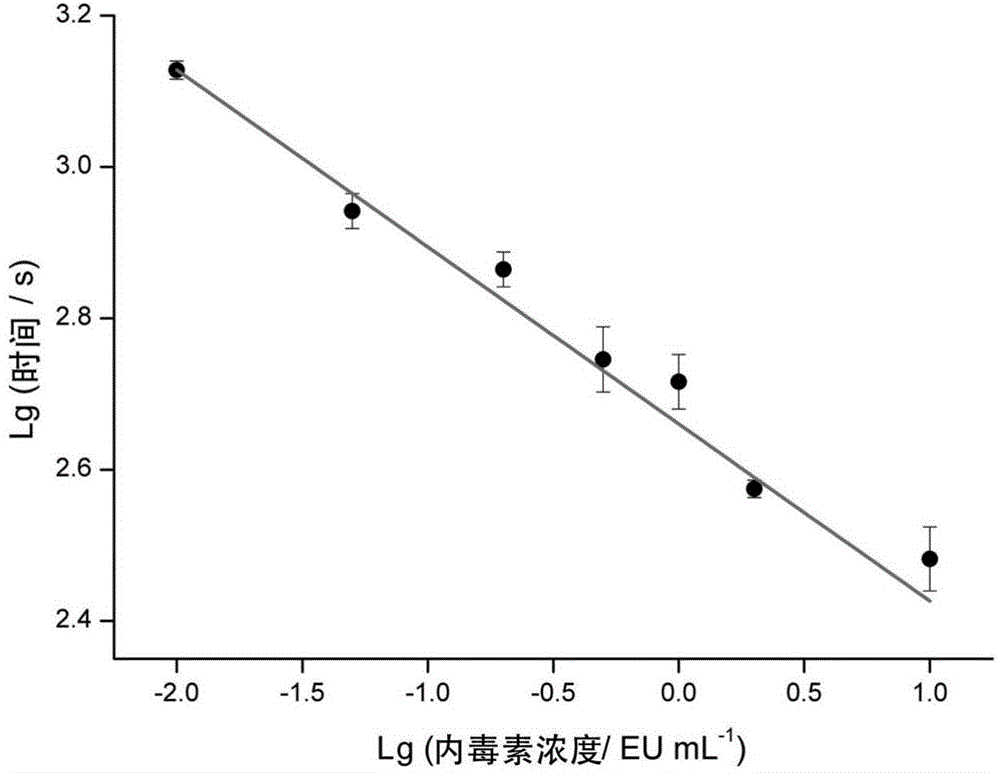
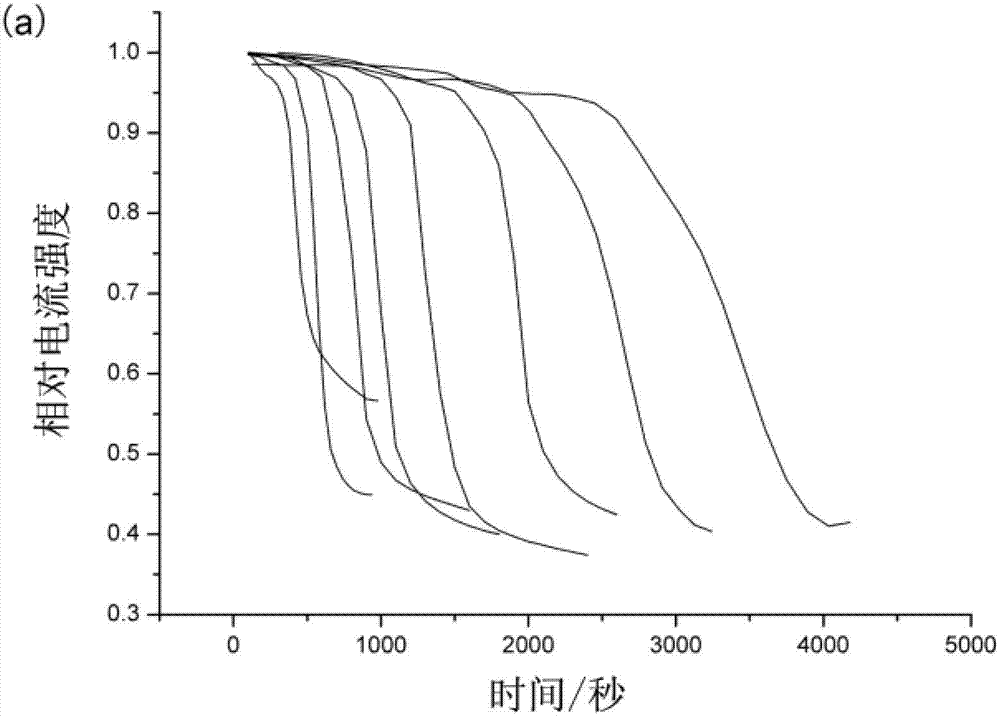
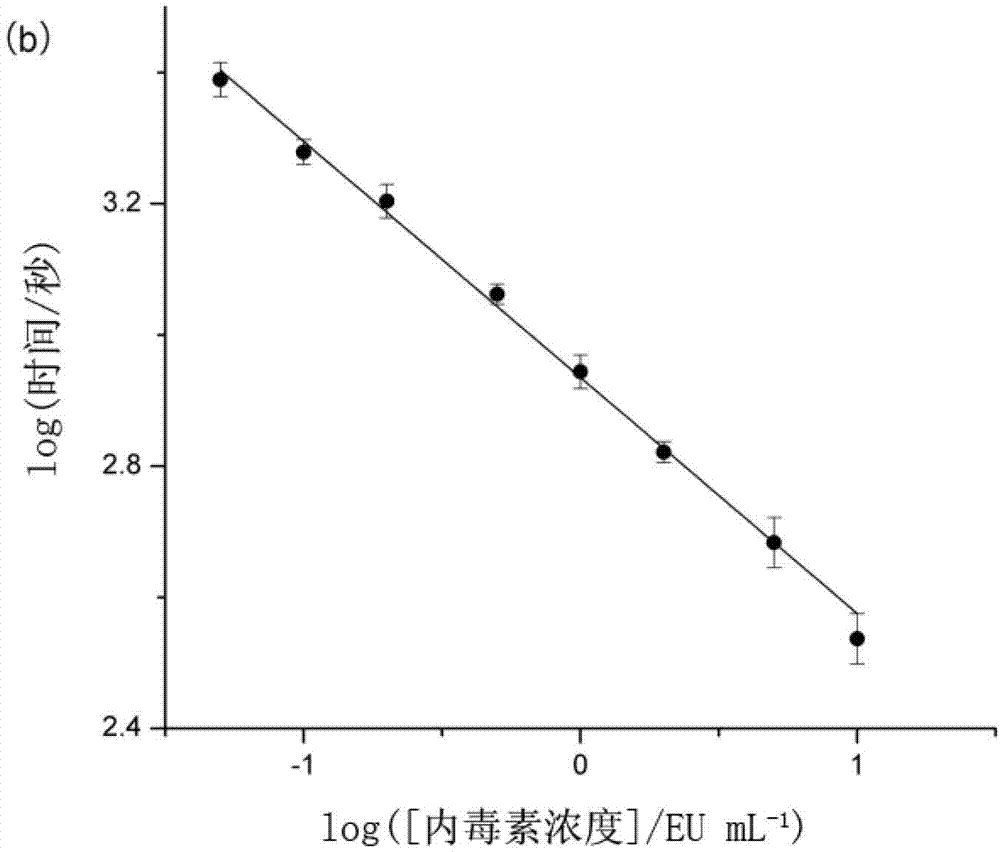
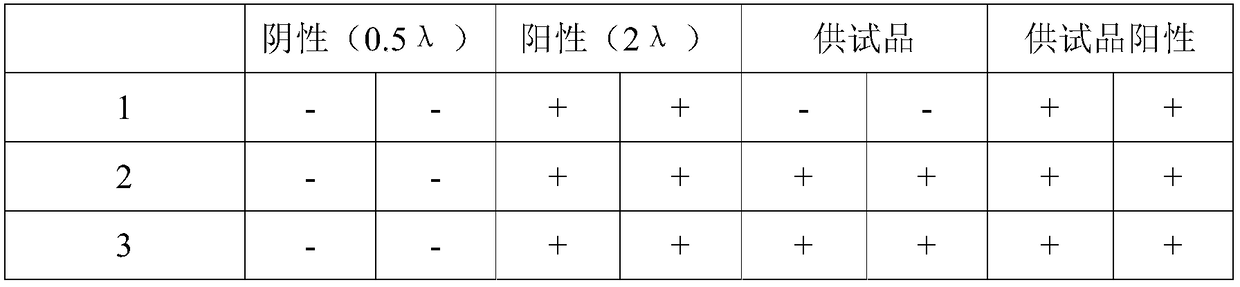
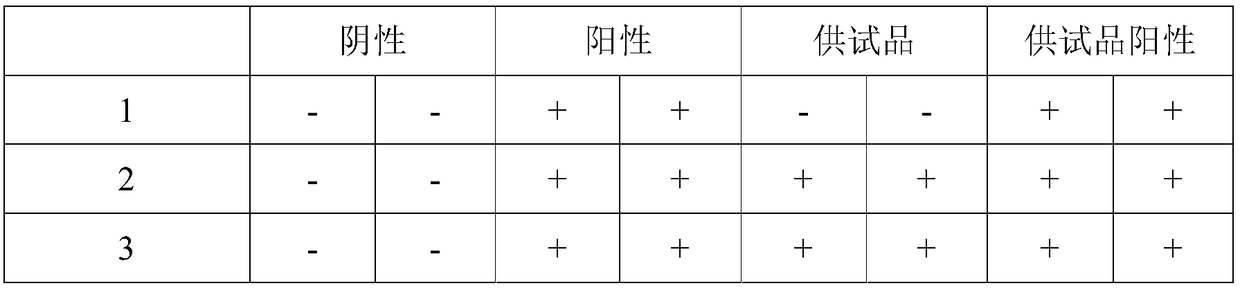
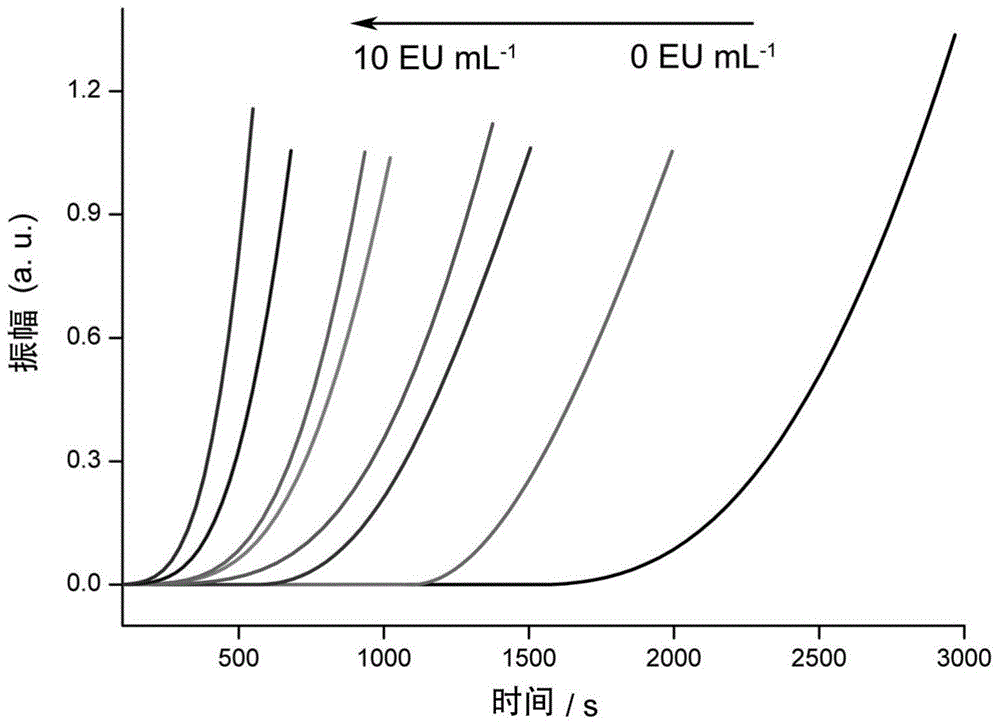
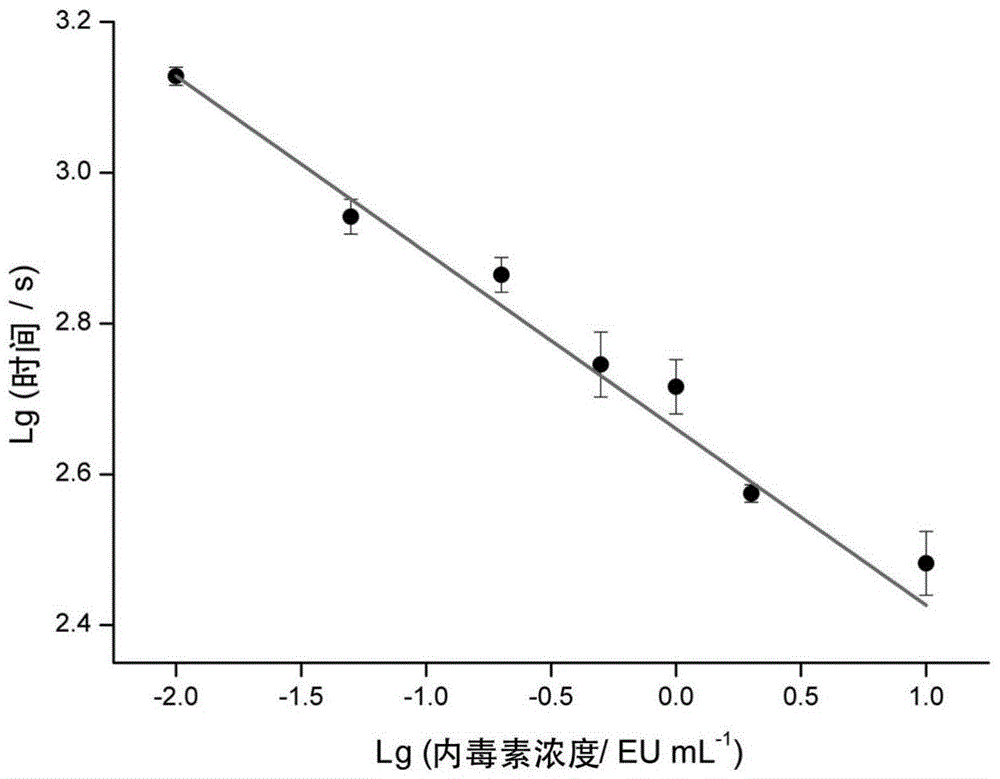
The invention discloses a method for detecting endotoxin content. The method comprises the following steps: S1. preparing at least five endotoxin standard solutions containing different concentrations; S2. respectively mixing a tachypleus amebocyte lysate diluent with each endotoxin standard solution at the ratio; S3. respectively detecting the time required when the elasticity of each mixed liquid reaches 0.1 amplitude by virtue of a thrombelastogram instrument, and drawing a bilogarithmic graph of time-endotoxin concentration to obtain a standard curve graph; and S4. mixing the tachypleus amebocyte lysate diluent with a to-be-detected sample liquid according to a specific ratio, detecting the time required when the elasticity reaches 0.1 amplitude by virtue of the thrombelastogram instrument, and comparing with the standard curve graph, so as to obtain the endotoxin content in the to-be-detected sample liquid. Compared with traditional nephelometry and electrochemical biosensor, relatively comprehensive detection can be carried out on the overall process; research on the special physiological process is facilitated; the detection time is greatly shortened; and the detection sensitivity and accuracy are synchronously improved.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
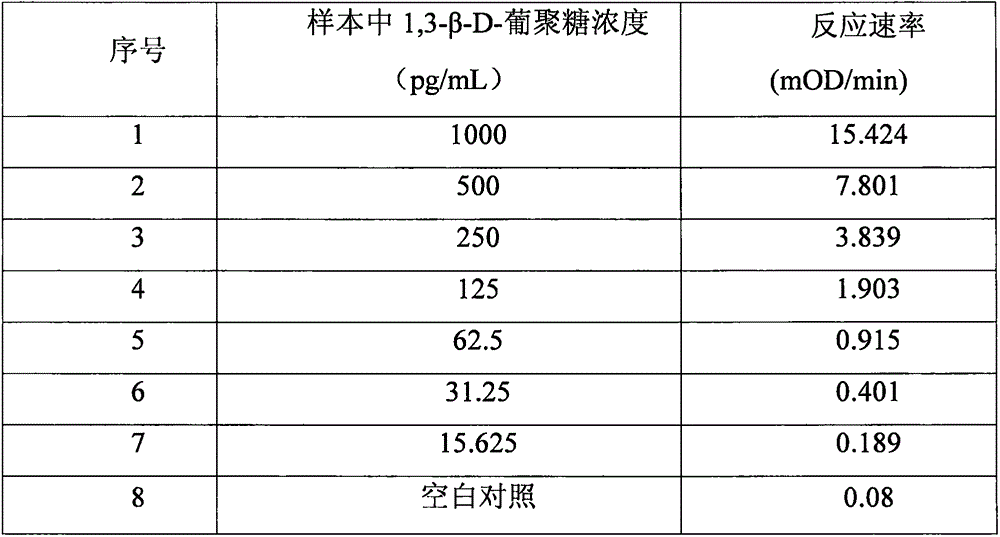
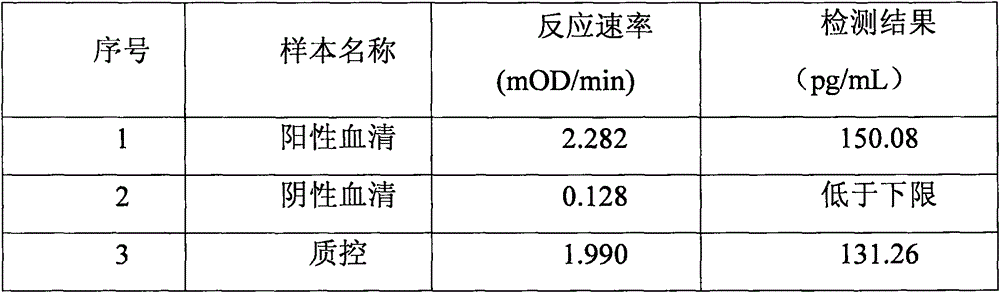
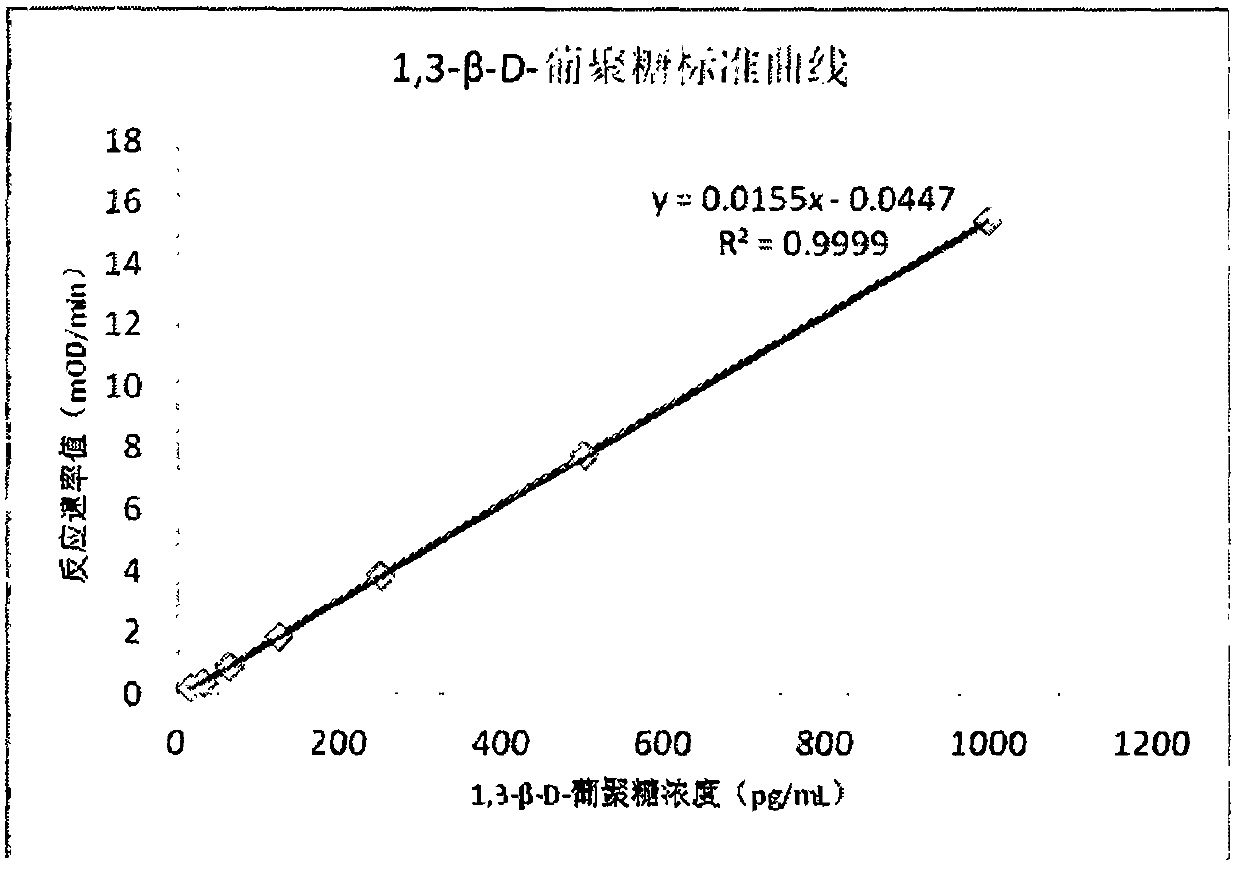
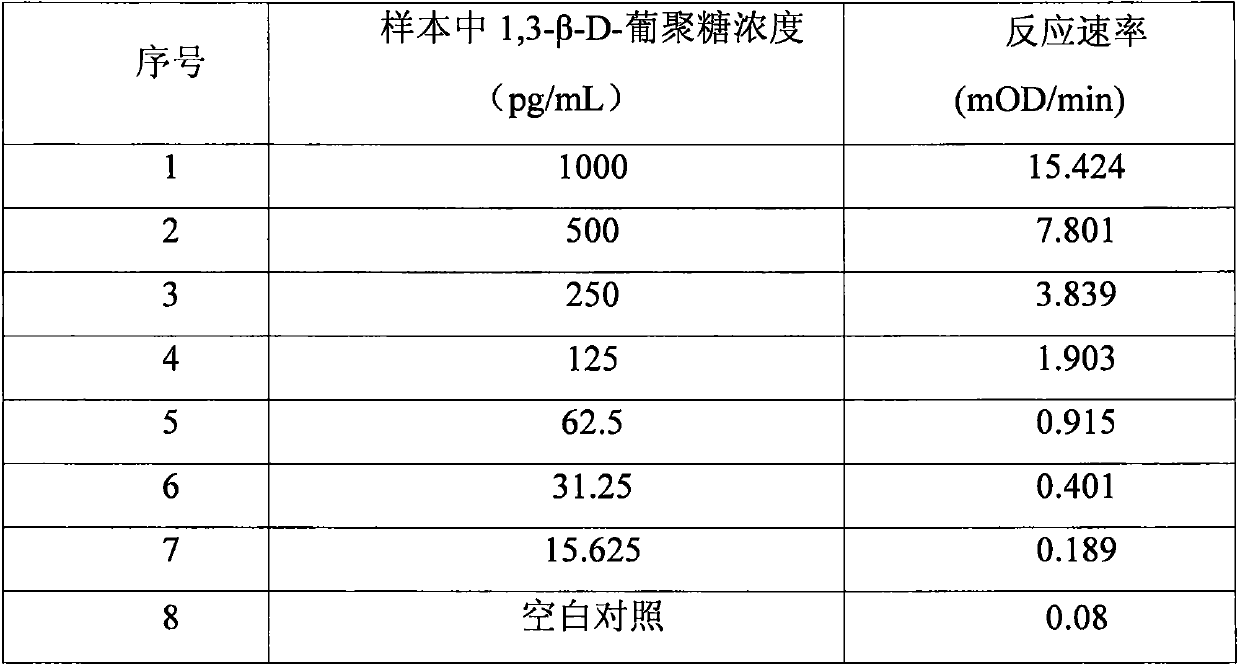
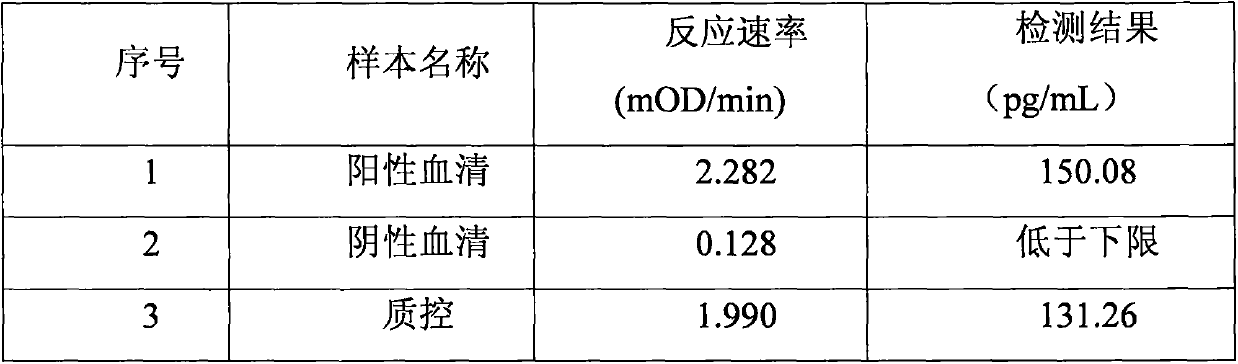
Improved body fluid color-developing fungi 1,3-beta-D-glucan detection kit and application method thereof
ActiveCN106290890ASimple production processImprove stabilityMaterial analysis by observing effect on chemical indicatorC factorBeta d glucan
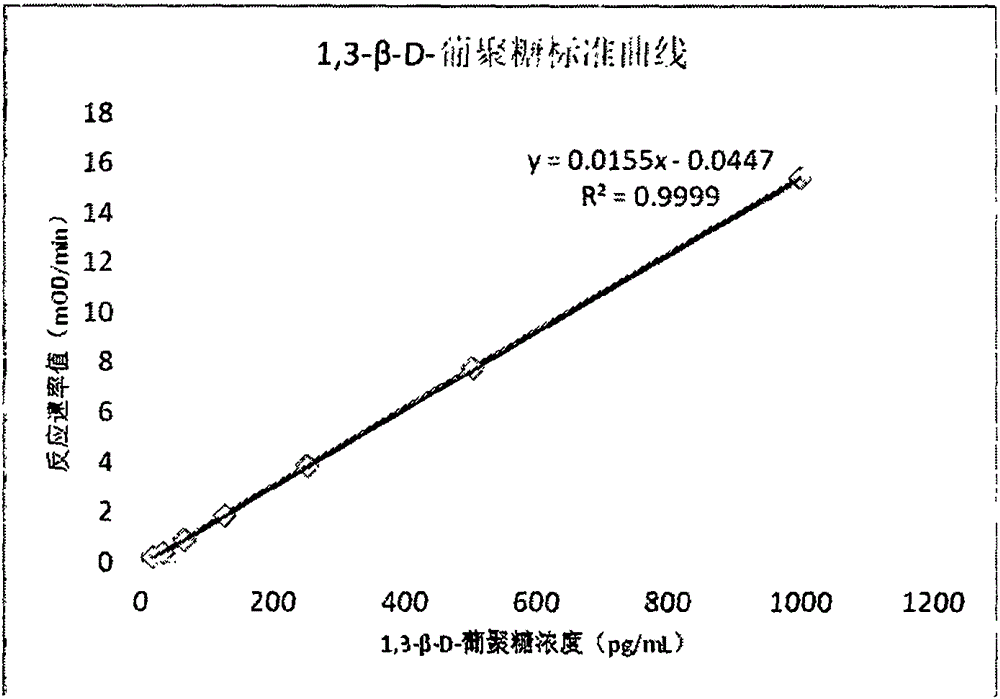
The invention relates to an improved body fluid color-developing fungi 1,3-beta-D-glucan detection kit, which comprises a reaction main-agent, a main agent combination solution, a sample treatment fluid, sterile water, a standard substance and a quality control material. With Tachypleus tridentatus Leach or Limulus polyphemus Linnaeus hemocyte lysate as a main raw material, the reaction main-agent contains G factor, B factor, C factor, clottable protein and a polypeptide chromogenic substrate. By a new preparation process, the reaction main-agent has characteristics of high stability and small intra-assay and inter-assay difference. The main agent combination solution adopts a new formula. After the main agent combination solution is mixed with the reaction main-agent, Tachypleus Amebocyte Lysate endotoxin reaction branch can be effectively shielded. According to the kit, rate-method enzyme kinetics detection is carried out by ELIASA. The detection speed is fast, and anti-interference performance is strong. In addition, the preparation technology is simple, and the product has stronger stability and higher specificity and sensitivity.
Owner:KOCH BIOTECHNOLOGY(BEIJING) CO LTD
Method for amplifying antibody marking signal or nucleic acid probe marking signal
InactiveCN103235116AImprove stabilityNo radioactive contaminationBiological testingNucleic Acid ProbesMicrobiology
The invention discloses a method for amplifying antibody marking signal or nucleic acid probe marking signal, and the main scheme is that: lipopolysaccharide of gram negative bacteria and an antibody are crosslinked, or fungi (1,3)-beta-D-glucan of fungi cell wall and the antibody or a nucleic acid probe are crosslinked, and then tachypleus amebocyte lysate and the gram negative bacteria lipopolysaccharide or fungi cell wall (1,3)-beta-D-glucan which is crosslinked with the antibody are specifically reacted, thereby improving the sensitivity of the antibody or nucleic acid probe detection. The system for amplifying antibody marking signal or nucleic acid probe marking signal can detect object substrates lower than pg level content.
Owner:FUZHOU UNIV
Method for detecting endotoxin
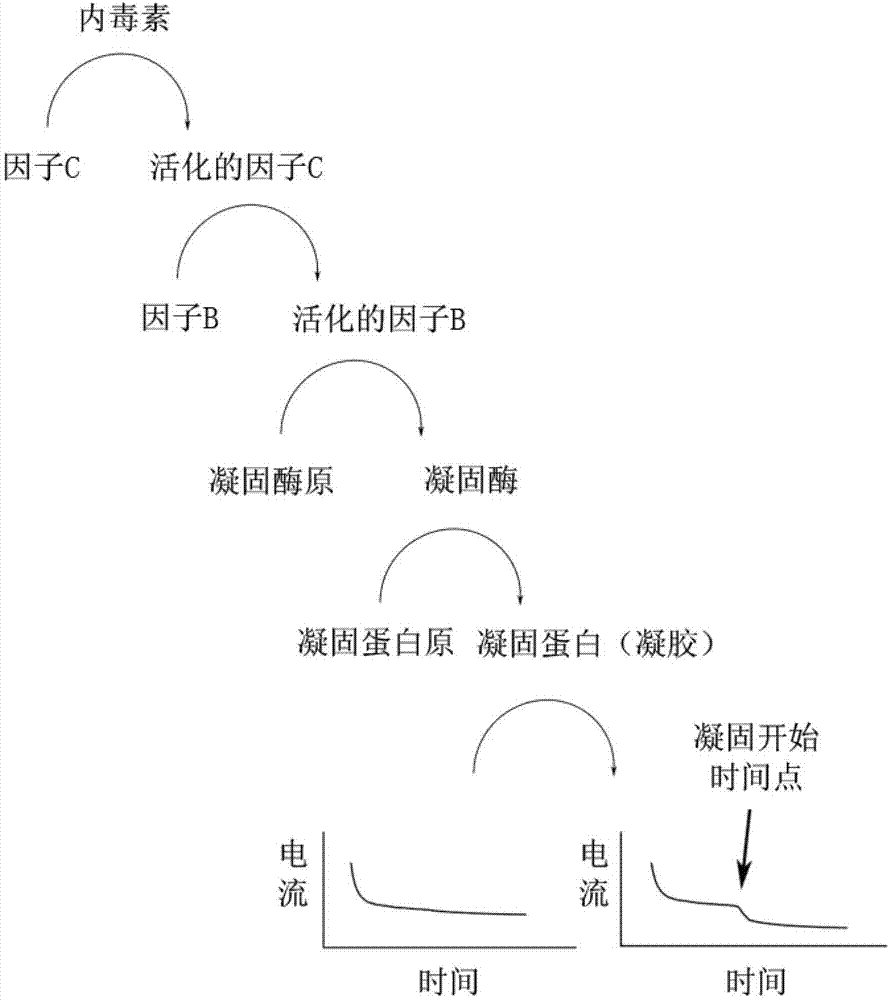
ActiveCN102866187AAccurate detectionRealize detectionMaterial electrochemical variablesOxidation-Reduction ActivityMedical equipment
The invention discloses a method for detecting endotoxin. The method includes a material with oxidation reduction activity is added in tachypleus amebocyte lysate, a sample to be tested is added in the tachypleus amebocyte lysate, the sample to be tested may comprise the endotoxin, an oxidation reduction potential corresponding to the material with the oxidation reduction activity is exerted on the tachypleus amebocyte lysate, size of current passing through the tachypleus amebocyte lysate is monitored in real time simultaneously, and concentration of the endotoxin in the sample to be tested is obtained. Logarithm linear relationship exists between the concentration of the endotoxin added in the tachypleus amebocyte lysate and a time dropping point of the current passing through the tachypleus amebocyte lysate. Viscosity change in a tachypleus amebocyte lysate solidification process is shown through an electrochemical process directly, interference of sample colors is avoided, detection of the endotoxin can be achieved rapidly and accurately, and the method for detecting the endotoxin can be widely applied to the field of drug quality control, medical equipment pyrogen monitoring and the like.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH
Method for detecting fleroxacin bacterial endotoxin used for injection
InactiveCN102841205ASimple and fast operationEliminate distractionsMaterial analysisLuminous intensityTachypleus
The invention relates to a method for detecting fleroxacin bacterial endotoxin used for injection, comprising the steps of detecting fleroxacin used for injection by adopting a gel method or a luminous intensity determination method by utilizing tachypleus amebocyte lysate, diluting the fleroxacin used for injection with a bivalent cation solution and then detecting. The method disclosed by the invention is simple to operate, is sensitive and accurate, can eliminate interference and can avoid defects of a pyrogen inspection method, thus the method disclosed by the invention has positive significance to improvement of quality standard, delivery of finished product and safety of clinical use.
Owner:LIAONING THINKING DECKER MEDICAL TECH
Method using antibacterial composition extracted from tachypleus amebocyte lysate waste to prepare cosmetics
ActiveCN106821935AGrowth inhibitionAvoid drug resistanceCosmetic preparationsToilet preparationsBacteroidesVitamin E Acetate
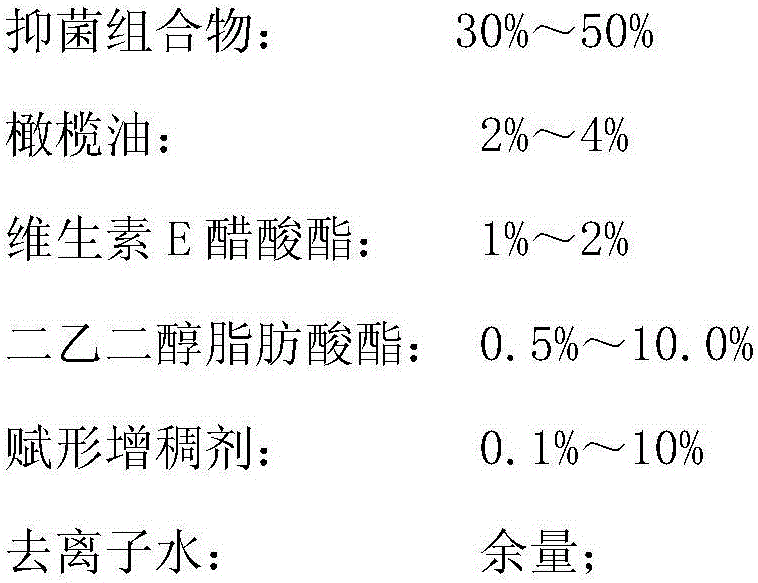
The invention relates to a method using an antibacterial composition extracted from tachypleus amebocyte lysate waste to prepare cosmetics. The method includes the steps of firstly, extracting the antibacterial composition from the tachypleus amebocyte lysate waste, to be more specific, collecting discarded emulsion sediments and discarded plasma in tachypleus amebocyte lysate production, using the discarded emulsion sediments to prepare crude tachyplesin peptide extract, using the discarded plasma to prepare crude hemocyanin extract, using the discarded plasma to prepare crude SOD extract, and mixing the three crude extract to prepare the antibacterial composition; secondly using, by mass percentage, 30-50% of antibacterial composition, 2-4% of olive oil, 1-2% of vitamin E acetate, 0.5-10% of diethylene glycol fatty acid ester, 0.1-10% of shaping thickening agent and the balance deionized water to prepare cosmetic components, and preparing the cosmetics according to a preparation process. The method has the advantages that prepared cosmetics can effectively inhibit the growth the bacteria and fungi, is safe, mild and free of toxic and side effect, has multiple effects of resisting bacteria, diminishing inflammation, moisturizing the skin and preventing ultraviolet and can slow down skin aging.
Owner:GENOBIO PHARM CO LTD
Method for eliminating false positive interference of G test
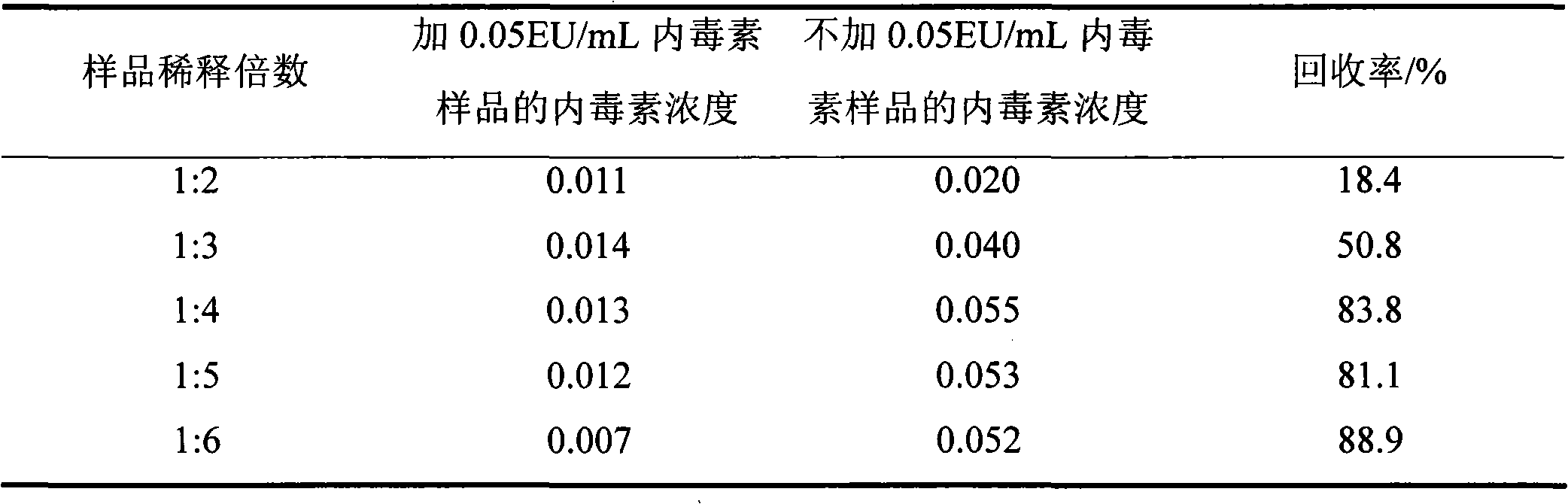
ActiveCN104483495AAccurate detection of β-G concentrationEliminate interferencePreparing sample for investigationBiological testingBlood plasmaTachypleus
The invention relates to the G test detection technical field, and particularly discloses a method for eliminating false positive interference of a G test, that is to say, a buffer solution containing a divalent soluble metal salt and Tris is prepared and is mixed with patient plasma or serum containing an interfering substance to prepare a detection sample, and the detection sample is subjected to the G test. With the use of the method, when the patient plasma / serum containing a nonspecific tachypleus amebocyte lysate is detected, the interference effect can be eliminated, the beta-G concentration in the patient plasma / serum is accurately detected, and patient mental and economic burdens caused by clinical misjudgment are greatly reduced.
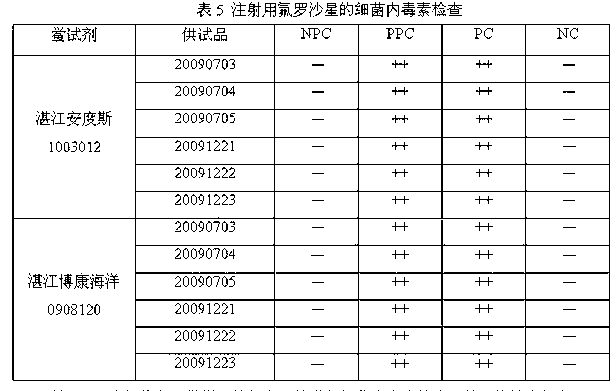
Owner:湛江安度斯生物有限公司
Method for checking bacterial endotoxin in Shenxiong glucose injection
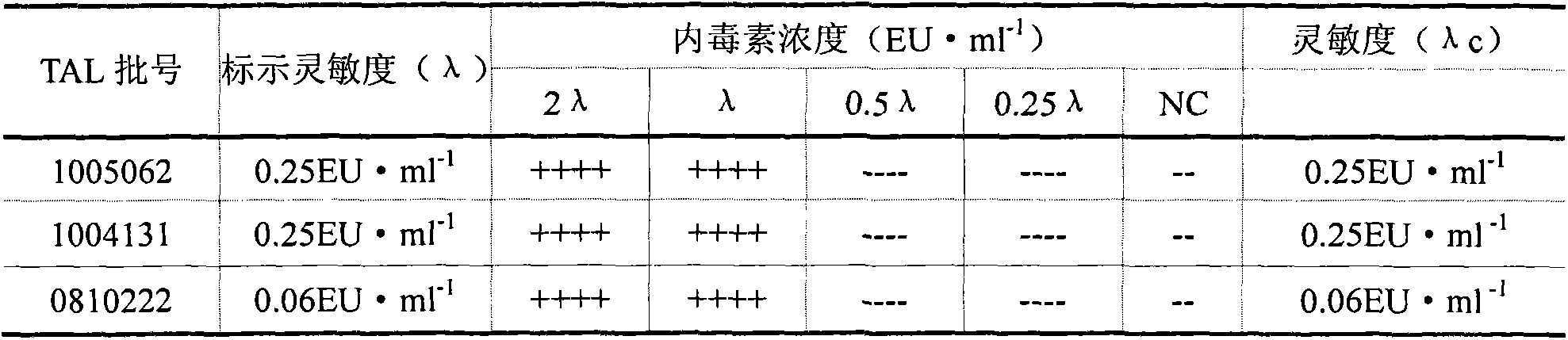
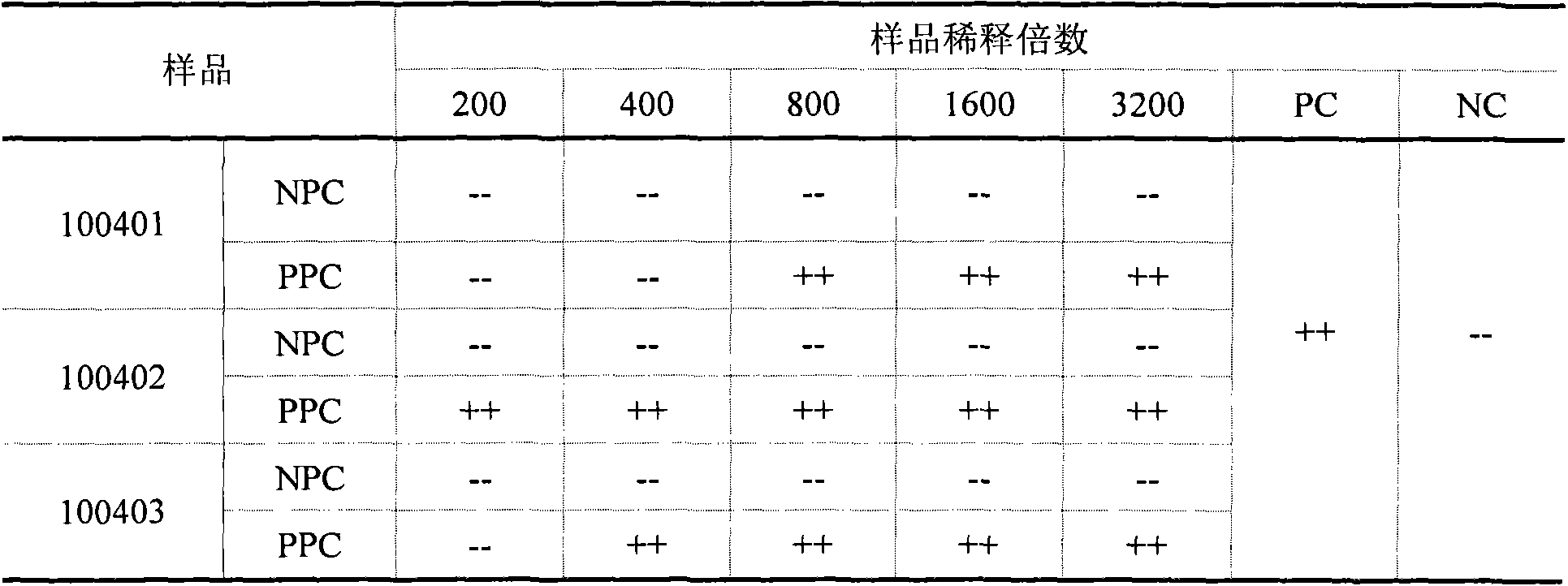
The invention discloses a method for checking bacterial endotoxin in Shenxiong glucose injection, which comprises the following steps: (1) determining the limit value (L) of the bacterial endotoxin in the Shenxiong glucose injection; (2) rechecking the sensitivity of tachypleus amebocyte lysate; (3) carrying out preliminary test of an interference test, i.e. determining the minimum noninterference dilution factor of the Shenxiong glucose injection; (4) carrying out official interference test; and (5) detecting the bacterial endotoxin of a provided sample. When the method disclosed by the invention is utilized to check the bacterial endotoxin in the Shenxiong glucose injection, the method is rapid and sensitive, the detection time is greatly shortened, the detection cost is reduced and the method is particularly suitable to carry out detection work in a laboratory.
Owner:GUIZHOU JINGFENG INJECTION
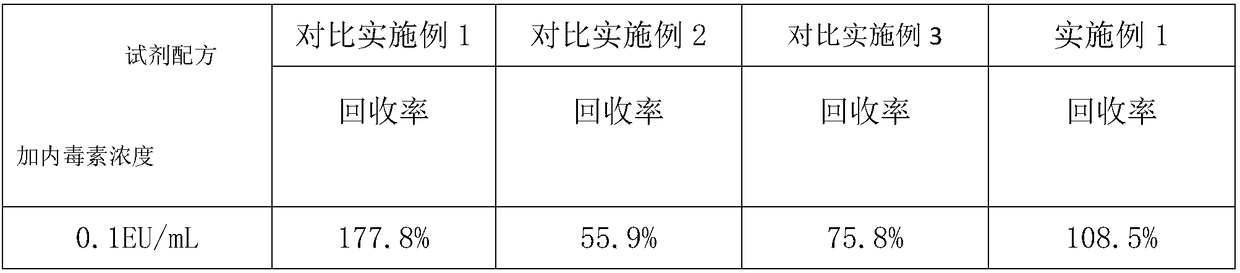
Anti-interference alkali reagent and preparation method and application method thereof
InactiveCN108362542AImprove uniformityImprove stabilityPreparing sample for investigationBiological testingPotassium hydroxideTachypleus
The invention relates to an anti-interference alkali reagent. The anti-interference alkali reagent comprises the following components: potassium hydroxide, sodium chloride, polyprene, bis-glycinate, an ethyleneimine polymer, calcium chloride, magnesium chloride and triton X-100; uniformity and stability of serum can be enhanced by the above components, a lot of protease, blood coagulation factors,and globulin in serum can be simultaneously removed, the protease interference tachypleus amebocyte lysate tests can be greatly reduced, the anti-interference capability is greatly increased, and theideal recovery rate is obtained; an enzyme reaction of a tachypleus amebocyte lysate can be activated at a maximum degree by calcium chloride and magnesium chloride in the formula components, and thereaction capability of endotoxin and the tachypleus amebocyte lysate can be greatly increased; in addition, after usage of the alkali reagent, a Tris-HCl buffer solution is added, the detected serumhas uniformity and stability, is subjected to a cross reaction with endotoxin, an endotoxin structure is protected with a maximum degree, and the endotoxin concentration can be accurately detected.
Owner:FUZHOU XINBEI BIOCHEM IND
Method for measuring bacterial endotoxin content in injection grade granulesten by developing substrate method
InactiveCN102033044AReliable detectionSave upfront workPreparing sample for investigationColor/spectral properties measurementsPhospholipidToxin
The invention relates to a sensitive and accurate method for measuring the bacterial endotoxin content in injection grade granulesten, belonging to the field for detecting the bacterial endotoxin as an auxiliary material for a drug. In the method, the bacterial endotoxin content in the injection grade granulesten is measured by utilizing the developing substrate method of TAL (Tachypleus Amebocyte Lysate); the granulesten is accurately weighed; a 10mg / ml granulesten solution is prepared by utilizing the water for inspecting the bacterial endotoxin and is diluted to each dilution grade not exceeding the maximum effective dilution factor; the result of an interference test indicates that the 10mg / ml granulesten solution which is diluted by more than three times has no interference action on the measurement for the quantitive endotoxin of the developing substrate TAL; and the value of the bacterial endotoxin in the granulesten can be calculated by utilizing the dilution grade. The method provides a method for controlling and measuring the bacterial endotoxin for the production and scientific research of the injection grade granulesten.
Owner:JIANGNAN UNIV
Method for detecting endotoxin content of liquid
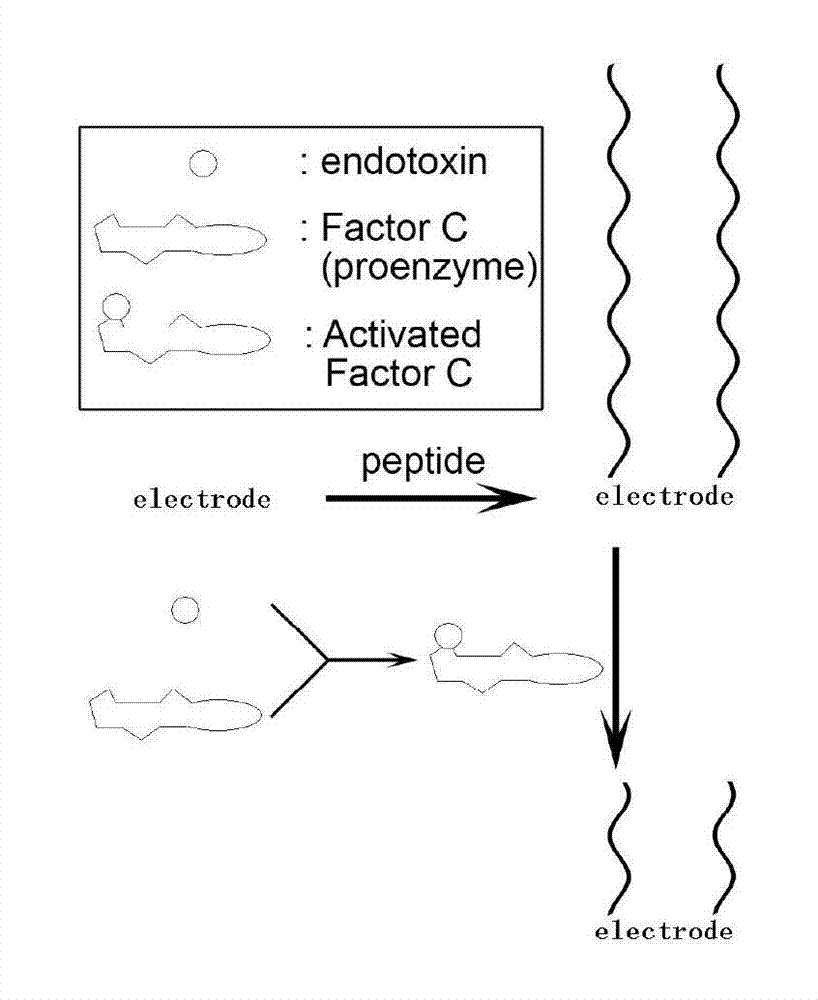
ActiveCN103675051AAvoid interferenceGuaranteed uniformityMaterial electrochemical variablesElectrochemical responseLimulus factor C
The invention discloses a method for detecting the endotoxin content of a liquid. According to the method, the concentration of endotoxin in the liquid to be detected is learned by detecting the electrochemical response of an electrode in electrolyte after the liquid to be detected and a restructuring limulus factor C are mixed and a contact reaction is performed on the mixture and a peptide modified electrode; when the restructuring limulus factor C is prepared, the restructuring limulus factor C is recombined and is expressed by adopting a means of genetic engineering. Through the scheme, a result is obtained directly by an electrochemical technology; the interference of color samples in the conventional methods, such as a colorimetric method, is avoided; the using of tachypleus amebocyte lysate is avoided, so that the detection cost can be reduced, and moreover, the ecological pressure is lightened to a certain degree; the method specifically reacts to the endotoxin, the interference of factor G bypass in the conventional tachypleus amebocyte lysate reaction is avoided; meanwhile, the genetic engineering method is used, so that the uniformity among reagent batches in the detection method is guaranteed, and the industrial production is facilitated.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Method for detecting bacterial endotoxin in Shenmai injection
PendingCN109387638AEasy to carry outFast wayPreparing sample for investigationBiological material analysisTest sampleLimit value
The invention discloses a method for detecting endotoxin in a Shenmai injection, and belongs to the field of medicines, and the method comprises the following steps: (1) determination of endotoxin limit value L; (2) sensitivity check of tachypleus amebocyte lysate; (3) interference test pre-testing for determining that a to-be-tested sample is diluted by a factor of 8; (4) interference tests of the to-be-tested sample for determining that non-interfere dilution factor is 32; and (5) detecting of the bacterial endotoxin in the to-be-tested sample. The method is rapid and sensitive. After determining the endotoxin limit value L and the dilution factor, the to-be-tested sample can be directly tested, no test is required to determine the dilution factor, while the detection accuracy is ensured, the inspection cost is reduced, inspection efficiency is improved, and production process monitoring and inspection work are convenient.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Method for preparing phenoloxidase active protein
The invention discloses a preparation method of a phenoloxidase active protein. The preparation method comprises the following steps: salting out proteins from a raw material waste blood plasma residual on Tachypleus Amebocyte Lysate by a saturated ammonium sulfate solution, performing refrigerating centrifugation to obtain limulus hemocyanin, performing dialysis desalting on a PBS solution of thehemocyanin through an MW 3500 Da dialysis bag, and carrying out Sephacryl S-100 allyl dextran gel column purification and concentrating treatment to obtain high-efficiency phenoloxidase active hemocyanin, wherein the initial reaction rate Vi of the high-efficiency phenoloxidase active hemocyanin is 74.52 nmol.(min.mg). The product obtained in the invention has a high purity and a high activity, and can be used for preparing a phenolic pollutant micro-detector with the advantages of high efficiency, simplicity and low cost; and the preparation method is simple, and simultaneously achieves desalting, separating, purifying and concentrating effects by cooperating the MW 3500 Da dialysis bag with the Sephacryl S-100 allyl dextran gel column.
Owner:湛江博康海洋生物有限公司
Method for detecting bacterial endotoxin of hemoglobin sample
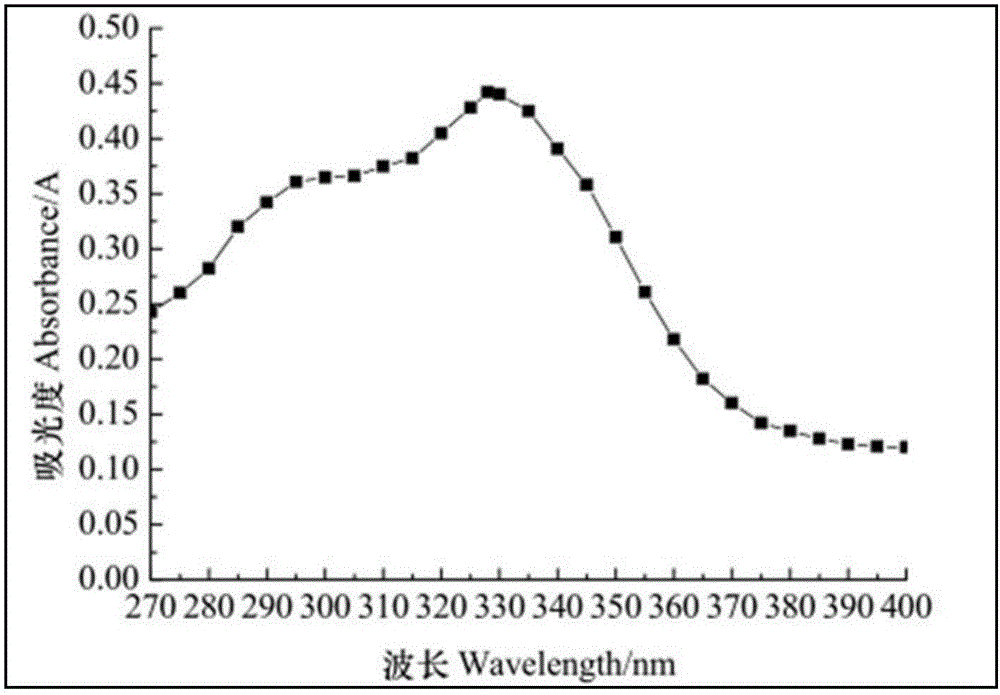
InactiveCN109030486ARealize quantitative detectionReduce distractionsMaterial analysis by observing effect on chemical indicatorAbsorbanceTachypleus
The invention discloses a method for detecting bacterial endotoxin of a hemoglobin sample. The method comprises the following steps of a, dissolving tachypleus amebocyte lysate dry powder by using a Gfactor blocking agent; b, diluting the hemoglobin sample for 20 to 40 times; and c, fully and uniformly mixing a reactant obtained in the step a and b, incubating at 37 DEG C, monitoring absorbance at 660 nm, and carrying out bacterial endotoxin detection. The G factor blocking agent adopted in the step a adds trasylol and pachymaran in purified water, so that each milliliter of blocking agent solution contains 5 to 15 micrograms of trasylol and 10 to 20 micrograms of pachymaran. The method for detecting the bacterial endotoxin of the hemoglobin sample is low in detection limit, high in accuracy and easy to operate, and is capable of realizing accurate detection of the bacterial endotoxin of the hemoglobin sample.
Owner:REDPHARM BEIJING BIOPHARMACEUTICAL INST CO LTD +1
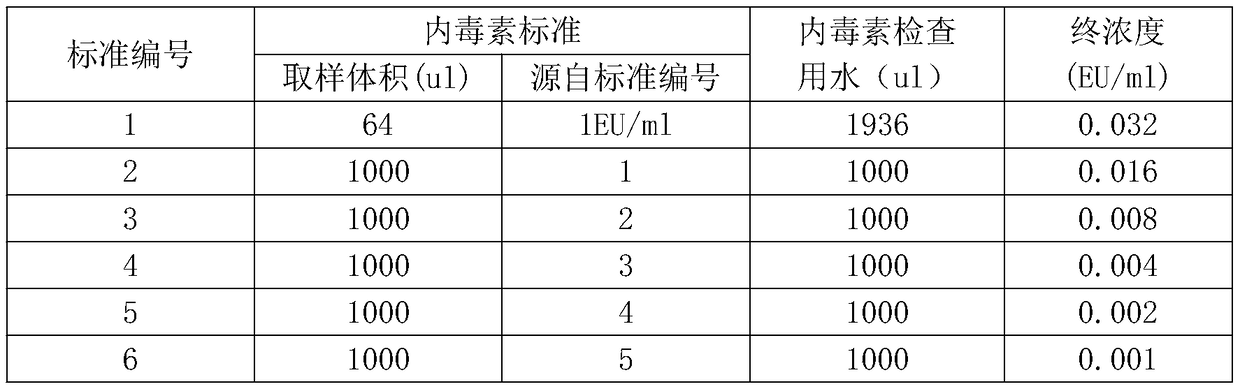
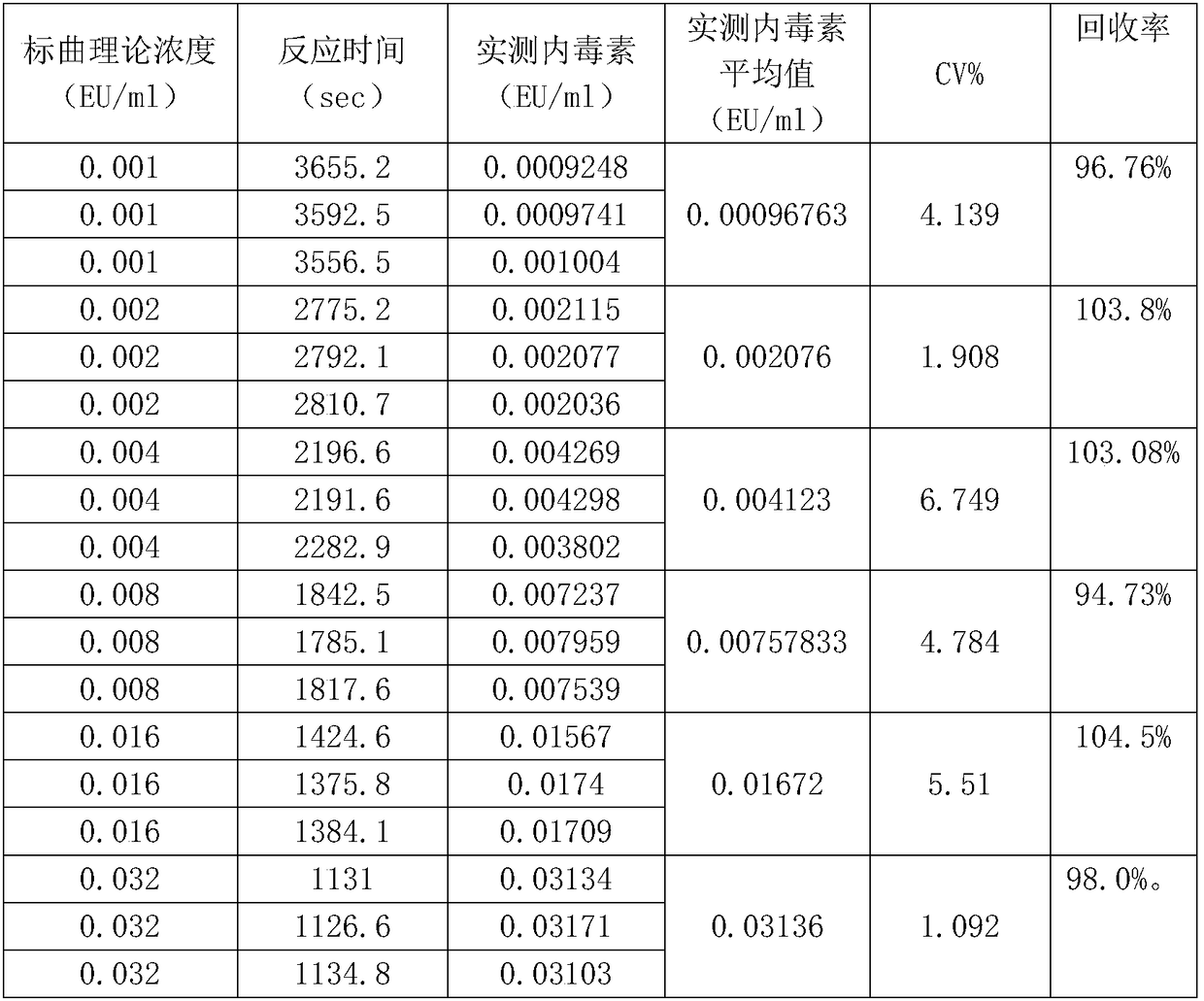
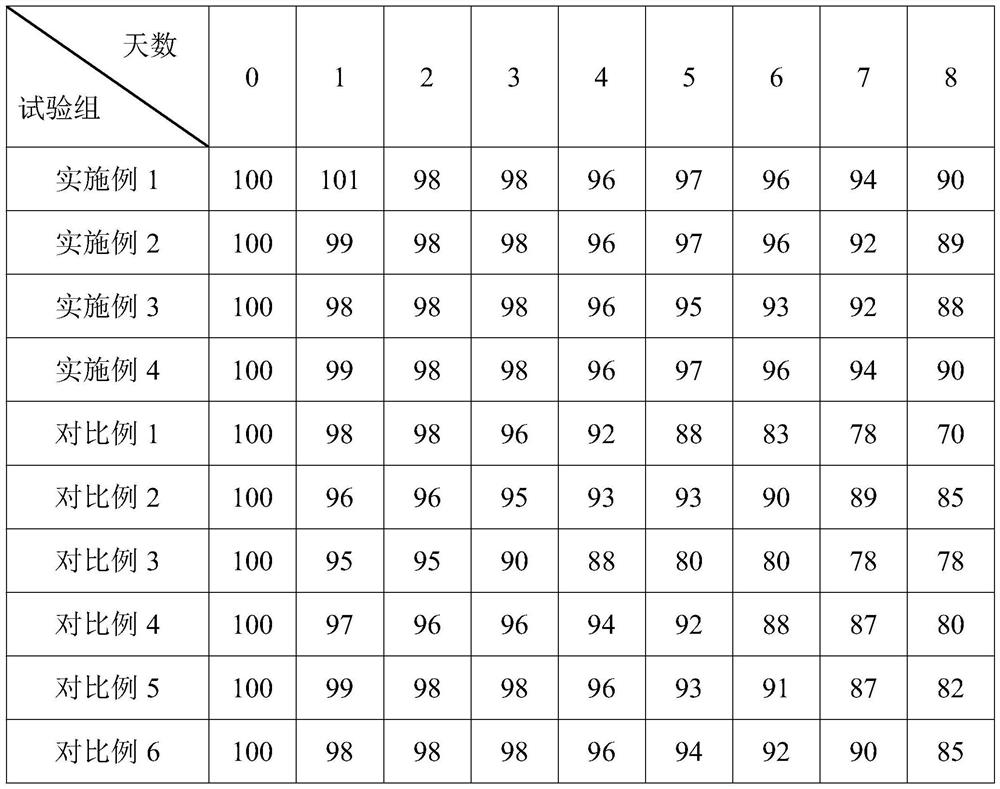
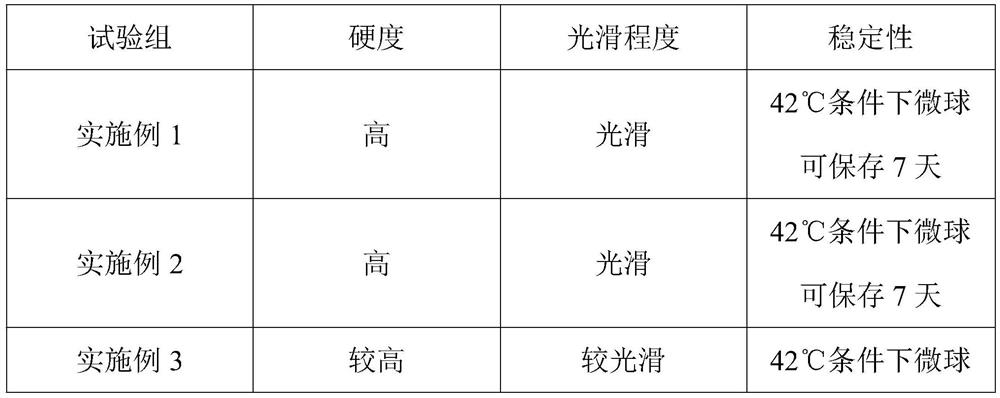
Lyophilized tachypleus amebocyte lyophilized microspheres as well as preparation method and application thereof
PendingCN113092767ADo not interfere with detection functionFast formingMaterial analysisAgainst vector-borne diseasesMicrosphereActive agent
The invention relates to lyophilized tachypleus amebocyte lysate microspheres as well as a preparation method and application thereof. The lyophilized tachypleus amebocyte lyophilized microspheres comprise amebic cell extracts of tachypleus amebocyte lyophilized microspheres and an active agent, wherein the active agent comprises polyvinylpyrrolidone, lactose, mannitol, raffinose, polyethylene glycol and glycine. All the components cooperate with one another to promote the formation of the tachypleus amebocyte lysate, improve hardness of the formed tachypleus amebocyte lysate and protect activity of the tachypleus amebocyte lysate in the precooling period, the freeze-drying period and the storage period, so the tachypleus amebocyte lysate freeze-dried microspheres are high in hardness, smooth in surface, not prone to breaking and chipping, high in stability and convenient for subsequent automatic or manual sub-packaging and storage.
Owner:DYNAMIKER BIOTECH TIANJIN
Method for testing bacterial endotoxin of ferric citrate pyrophosphate raw material
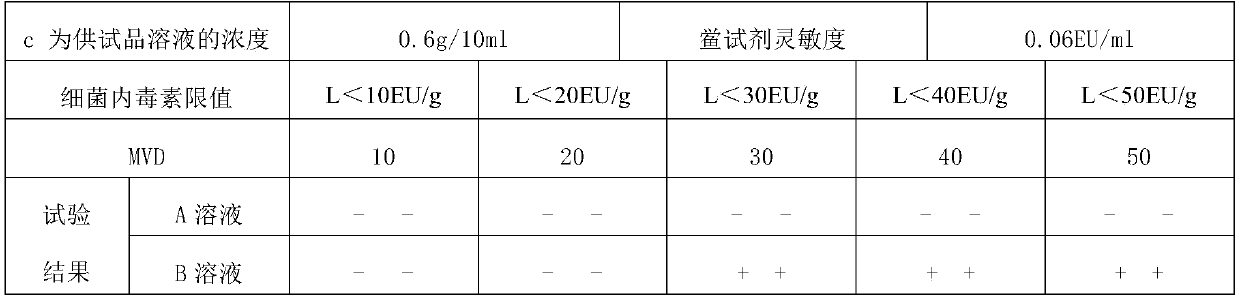
InactiveCN109613250ASolve the problem of difficult limit settingTest method scienceMaterial analysisFerric citratePyrophosphate
The invention discloses a method for testing bacterial endotoxin of a ferric citrate pyrophosphate raw material, comprising the following steps of: preparing the ferric citrate pyrophosphate raw material into a sample solution S having a concentration of 0.6 g / 10 ml by using bacterial endotoxin test water; diluting the sample solution S 40 times with the bacterial endotoxin test water, that is, solution A (S40); performing a bacterial endotoxin test with a TAL (Tachypleus Amebocyte Lysate) having sensitivity of 0.06 EU / ml; keeping warm for 60+ / -2 minutes in a 37+ / -1 DEG C tube thermostat; andgently removing a test tube from the thermostat and slowly reversing by 180 degree. If an in-tube gel does not deform and slip off a tube wall, it is positive. If the in-tube gel does not remain intact and slips off the wall, it is negative. The test method of the invention is scientific, has an accurate test result, fills the blank of the method for testing bacterial endotoxin of the ferric citrate pyrophosphate raw material, and has broad application prospect.
Owner:CHINA OTSUKA PHARM CO LTD
Method for artificially breeding tachypleus tridentatus
InactiveCN111567448AEasy to catchFree movementClimate change adaptationPisciculture and aquariaBlood collectionFishery
The invention provides a method for artificially breeding tachypleus tridentatus. The method for artificially breeding the adult tachypleus tridentatus comprises the following steps: selecting land, at a distance of 50-100 meters to the shore, near a sea area of a subtropical area as a farm; building breeding ponds, dividing each breeding pond into multiple breeding areas, forming channels betweenthe adjacent breeding areas, and dividing each breeding area with corresponding partition nets into multiple breeding units; controlling the water depth in the breeding ponds to be 1.5-1.6 meters; placing 2-4 tachypleus tridentatus into each breeding unit, and stocking ditrema temmincki and / or siganus in the breeding ponds; and feeding shellfish meat or small raw fishes into the breeding ponds every day. According to the method for artificially breeding the tachypleus tridentatus, the tachypleus tridentatus is dispersed into the breeding ponds to avoid excessive gathering, so that death rateincrease due to excessive local density is avoided; the method for artificially breeding the tachypleus tridentatus is suitable for blood collection for production of tachypleus amebocyte lysates, andespecially, repeated catches or catch missing is avoided; a breeding space is fully used, so that maximum of breeding benefits is achieved; and ecological breeding is achieved.
Owner:广西蓝桂水产科技有限公司
Detection method of remimazolam hydrobromide bacterial endotoxin
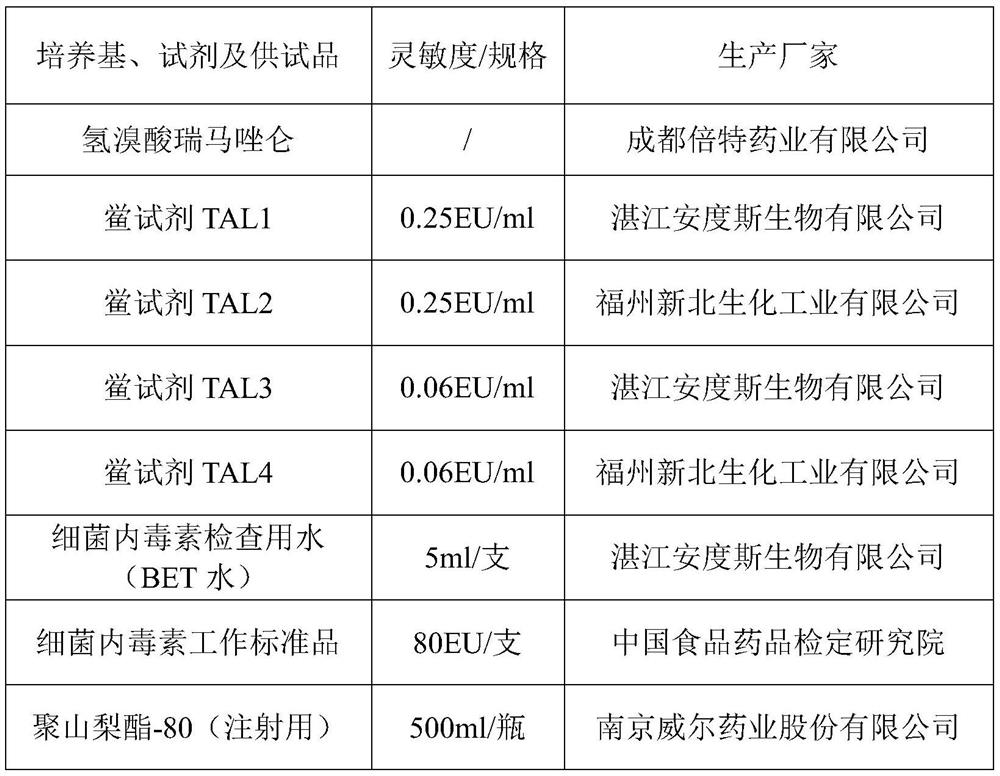
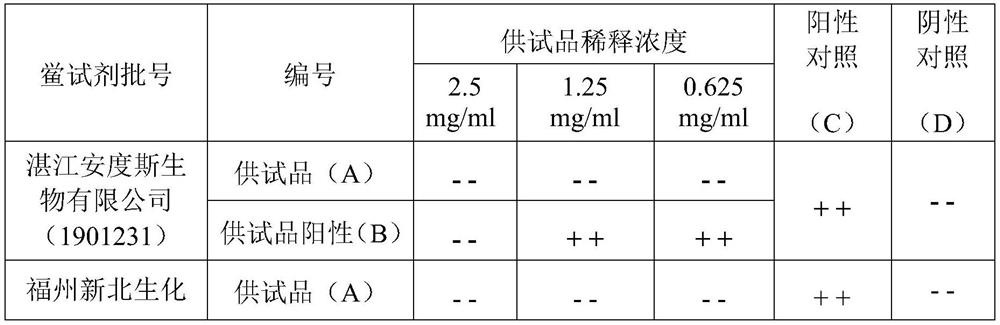
ActiveCN112462015AEliminate interferenceRealize detectionPreparing sample for investigationBiological testingTest sampleTachypleus
The invention discloses a detection method of remimazolam hydrobromide bacterial endotoxin. The detection method comprises the following steps: rechecking the sensitivity of a tachypleus amebocyte lysate; determining the limit value and the minimum dilution concentration of the remimazolam hydrobromide bacterial endotoxin; performing an interference experiment to confirm the concentration of a test sample without interference, wherein in the experiment, the test sample is dissolved by using an aqueous polysorbate-80 solution with a concentration of 10-30 mg / mL, and diluted by using water for bacterial endotoxin detection to obtain a test sample solution; and detecting the remimazolam hydrobromide bacterial endotoxin by dissolving a remimazolam hydrobromide test sample via the aqueous polysorbate-80 solution with the concentration of 10-30 mg / mL, and diluting the test sample to an effective concentration without interference by using water for bacterial endotoxin detection before conduction of detection on the bacterial endotoxin. According to the method, the aqueous polysorbate-80 solution with a certain concentration is used for dissolving the sample, so the interference effect ofthe test sample on bacterial endotoxin can be eliminated; and the method is simple, convenient, easy to operate and high in practicability.
Owner:HAINAN LEVTEC PHARMA
An improved human body fluid chromogenic method fungal 1,3-β-d-glucan detection kit and application method thereof
ActiveCN106290890BSimple production processImprove stabilityMaterial analysis by observing effect on chemical indicatorC factorHuman body
Owner:KOCH BIOTECHNOLOGY(BEIJING) CO LTD
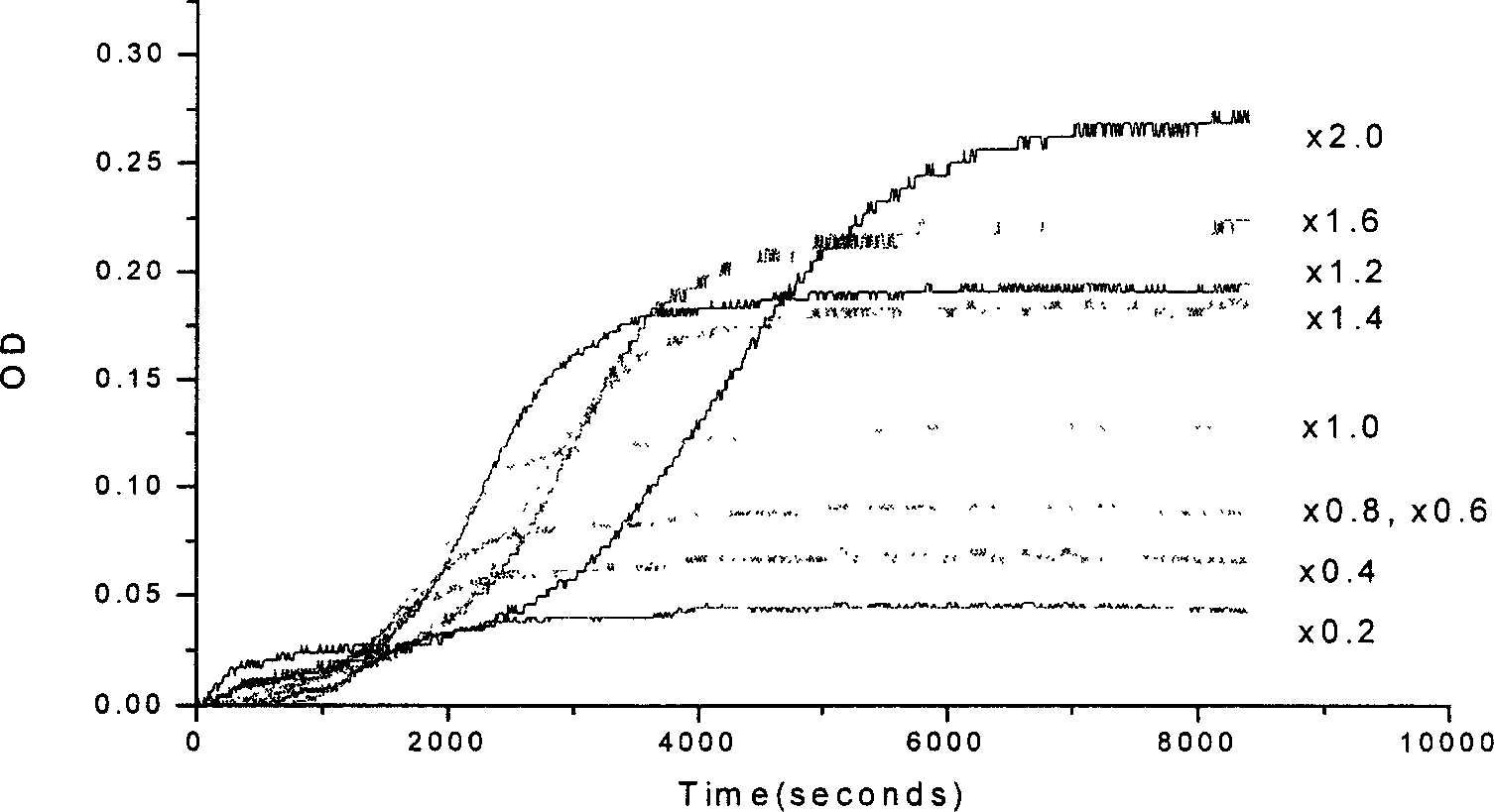
Tachypleus amebocytes lysate quality monitoring method
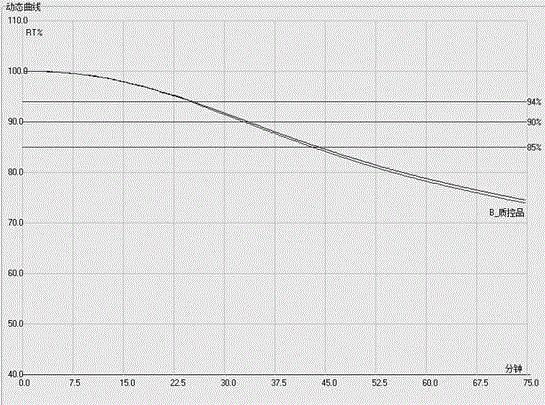
The invention discloses an observation method of limulus reagent quality, which is characterized by the following: putting the same bulk limulus mixture of reagent and standard endotoxin solution in the detection hole of endotoxin determinator to detect turbidity value; defining the turbidity value ranged from 0.08 to 0.27 as on-spec product; recording reaction time of multiple experiment samples and reading turbidity value automatically; judging the experiment result according to the turbidity value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for amplifying antibody marking signal or nucleic acid probe marking signal
InactiveCN103235116BImprove stabilityNo radioactive contaminationBiological testingNucleic Acid ProbesMicrobiology
The invention discloses a method for amplifying antibody marking signal or nucleic acid probe marking signal, and the main scheme is that: lipopolysaccharide of gram negative bacteria and an antibody are crosslinked, or fungi (1,3)-beta-D-glucan of fungi cell wall and the antibody or a nucleic acid probe are crosslinked, and then tachypleus amebocyte lysate and the gram negative bacteria lipopolysaccharide or fungi cell wall (1,3)-beta-D-glucan which is crosslinked with the antibody are specifically reacted, thereby improving the sensitivity of the antibody or nucleic acid probe detection. The system for amplifying antibody marking signal or nucleic acid probe marking signal can detect object substrates lower than pg level content.
Owner:FUZHOU UNIV
A Method to Eliminate the Interference of False Positive in G Test
ActiveCN104483495BAccurate detection of β-G concentrationEliminate interferencePreparing sample for investigationBiological testingBlood plasmaTachypleus
Owner:湛江安度斯生物有限公司
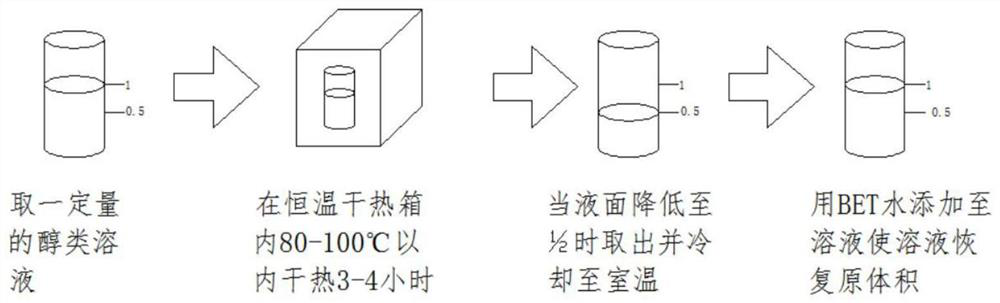
Method for detecting endotoxin in alcohol solution
The invention discloses a method for detecting endotoxin in an alcohol solution. The method comprises the following steps: heating an alcohol solution, and carrying out endotoxin detection on the treated alcohol solution by using a tachypleus amebocyte lysate detection method; and through a heating evaporation method, taking out the alcohol in the solution as much as possible on the premise that endotoxin is not reduced. According to the method, the alcohol solution is heated, then the treated alcohol solution is subjected to endotoxin detection by using a tachypleus amebocyte lysate detectionmethod, and on the basis of the support of the prior art, the pertinence of detection is improved in combination with the expansion of a self mode; and alcohol in the solution is taken out as much aspossible by utilizing a heating evaporation method on the premise of not reducing endotoxin, so that the overall alcohol content in the detection solution is reduced, the overall detection accuracy of the device is improved, and the influence of the alcohol content on a detection result is reduced.
Owner:斯坦利思生物科技(杭州)有限公司
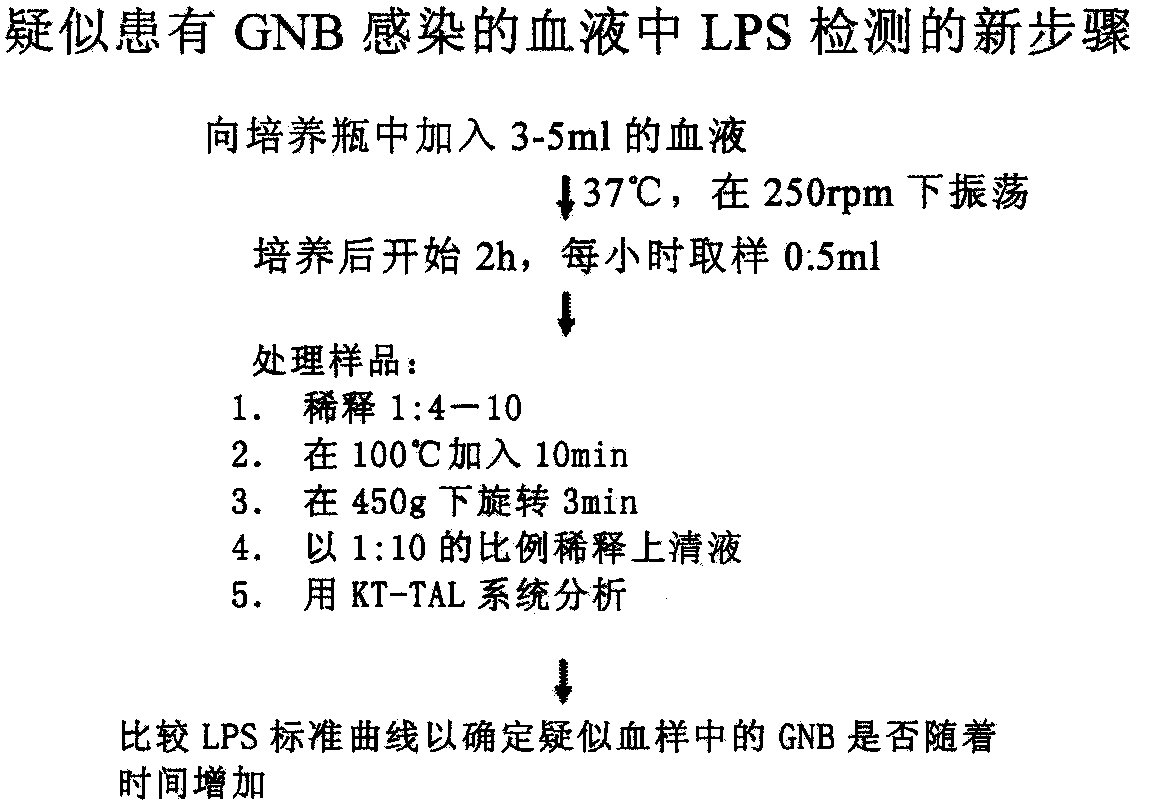
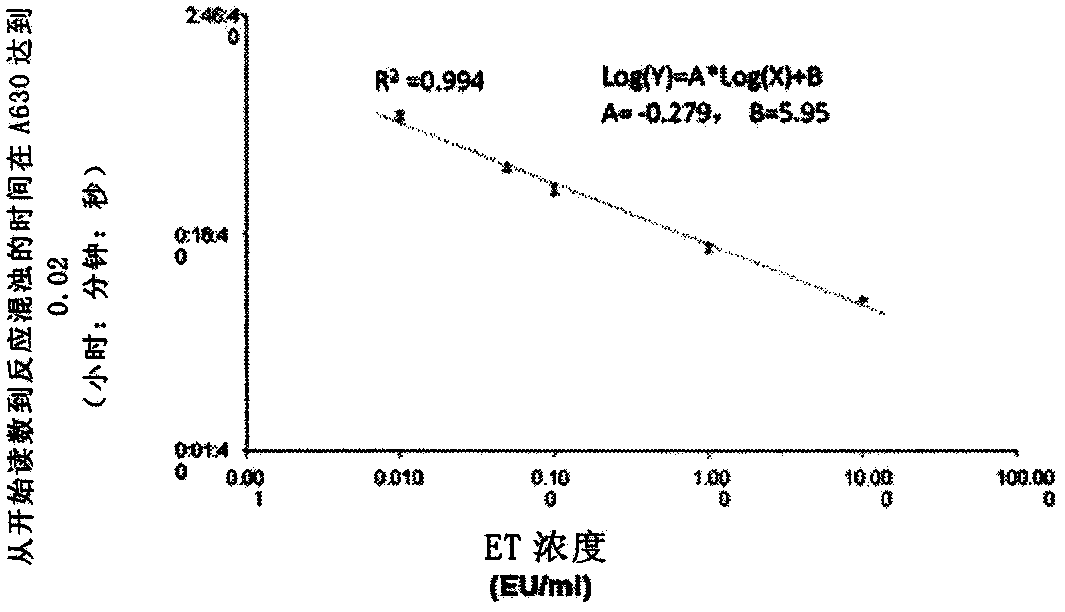
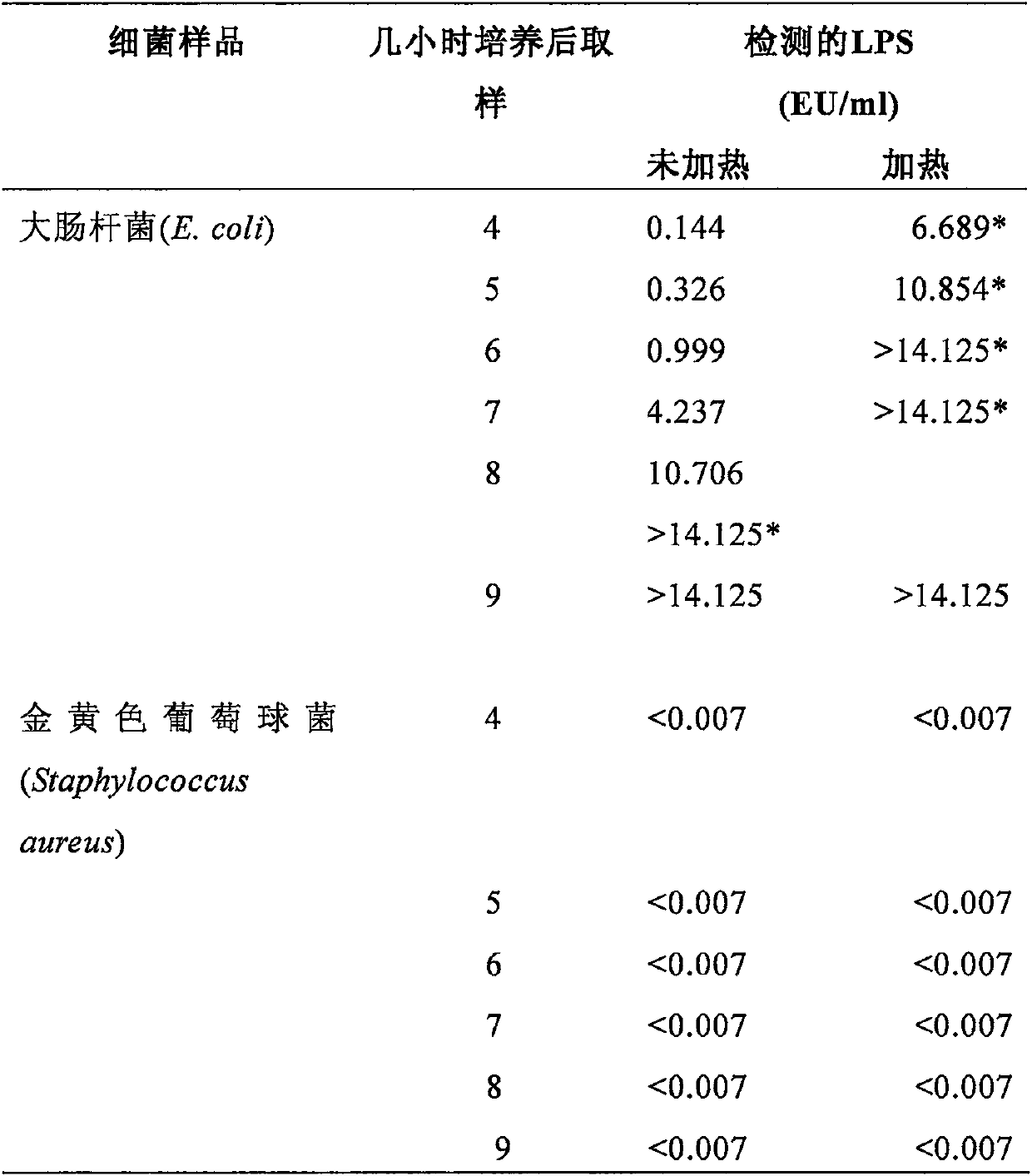
Novel method for rapidly detecting septicopyemia by using gram-negative bacterial infection
ActiveCN111077307AAid in early diagnosisMonitoring Treatment EffectsBiological material analysisGram-negative bacterial infectionsBiologic marker
Septicopyemia is a systemic infection endangering life and needs to be properly and quickly treated on the basis of quickly determining pathogens. Gram-negative bacteria (GNB) are the main pathogenicpathogens of endotoxin (LPS) as a characteristic substitute biomarker thereof. In the research, a new method is explored firstly, firstly, LPS is increased through GNB culture, and then the LPS of tachypleus amebocyte lysate (TAL) is detected through a dynamic turbidity measurement method (KT-TALA). Sample preparation procedures are optimized. Results show that under high GNB concentration, LPS can be detected 3 hours after culture, which is 6.5-7.5 hours ahead of time, and 9.5-10.5 hours is for a BD BACTEC positive report; under a low GNB load, a BD BACTEC system needs 22-26 hours to detect GNB, but the KT-TALA system only needs 9 hours to detect LPS of GNB, and the time is advanced by 13-17 hours. Compared with a traditional BD BACTEC system, the novel method for detecting GNB infected LPS is much faster, and especially in the early stage of the septicopyemia with a low bacterial load, and the method is helpful to make appropriate treatment decisions in earlier time.
Owner:XIAMEN BIOENDO TECH CO LTD
Plasma diluent kit for bacterial endotoxin gel process detection of blood plasma and use method thereof
The invention relates to a plasma diluent kit for bacterial endotoxin gel process detection of blood plasma and a use method thereof. The invention aims to solve the problems of easy influence of heparin sodium, sodium citrate and other anticoagulants in blood plasma and protein in blood plasma on the gel process, complex operation of the turbidimetric process, and expensive equipment. The kit comprises a diluent A, a diluent B and a diluent C. The kit adopts different diluents for dilution of the blood plasma of different anticoagulants, targetedly eliminates the interference of anticoagulants on tachypleus amebocyte lysate gel reaction, also can eliminate protein in blood plasma, and prevent the interference of protein. Also the dilution ratio is smaller, and a dilution ratio of 8 and conventional tachypleus amebocyte lysate sensitivity can achieve detection. The kit provided by the invention can be applied to the plasma endotoxin detection field.
Owner:天晴干细胞股份有限公司
A kind of detection method of endotoxin content
ActiveCN104698159BComprehensive detectionConvenient researchBiological testingElectrochemical biosensorDiluent
The invention discloses a method for detecting endotoxin content. The method comprises the following steps: S1. preparing at least five endotoxin standard solutions containing different concentrations; S2. respectively mixing a tachypleus amebocyte lysate diluent with each endotoxin standard solution at the ratio; S3. respectively detecting the time required when the elasticity of each mixed liquid reaches 0.1 amplitude by virtue of a thrombelastogram instrument, and drawing a bilogarithmic graph of time-endotoxin concentration to obtain a standard curve graph; and S4. mixing the tachypleus amebocyte lysate diluent with a to-be-detected sample liquid according to a specific ratio, detecting the time required when the elasticity reaches 0.1 amplitude by virtue of the thrombelastogram instrument, and comparing with the standard curve graph, so as to obtain the endotoxin content in the to-be-detected sample liquid. Compared with traditional nephelometry and electrochemical biosensor, relatively comprehensive detection can be carried out on the overall process; research on the special physiological process is facilitated; the detection time is greatly shortened; and the detection sensitivity and accuracy are synchronously improved.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Method for detecting bacterial endotoxin in Danshen injection
PendingCN109387637AEasy to carry outFast wayPreparing sample for investigationBiological material analysisTest sampleLimit value
The invention discloses a method for detecting endotoxin in a Danshen injection, and belongs to the field of medicines, and the method comprises the following steps: (1) determination of endotoxin limit value L; (2) sensitivity check of tachypleus amebocyte lysate; (3) interference test pre-testing for determining that a to-be-tested sample is diluted by a factor of 20; (4) interference tests of the to-be-tested sample for determining that non-interfere dilution factor is 80; and (5) detecting of the bacterial endotoxin in the to-be-tested sample. The method is rapid and sensitive. After determining the endotoxin limit value L and the dilution factor, the to-be-tested sample can be directly tested, no test is required to determine the dilution factor, while the detection accuracy is ensured, the inspection cost is reduced, the inspection efficiency is improved, and production process monitoring and inspection work are convenient.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com