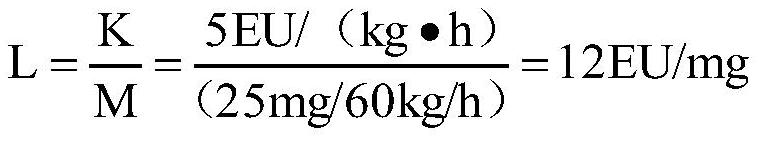Detection method of remimazolam hydrobromide bacterial endotoxin
A technology for remimazolam hydrobromide and bacterial endotoxin, which is applied in the field of bacterial endotoxin detection, can solve the problem of inability to perform remazolam hydrobromide bacterial endotoxin inspection, etc., and achieves the effect of eliminating the interference effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
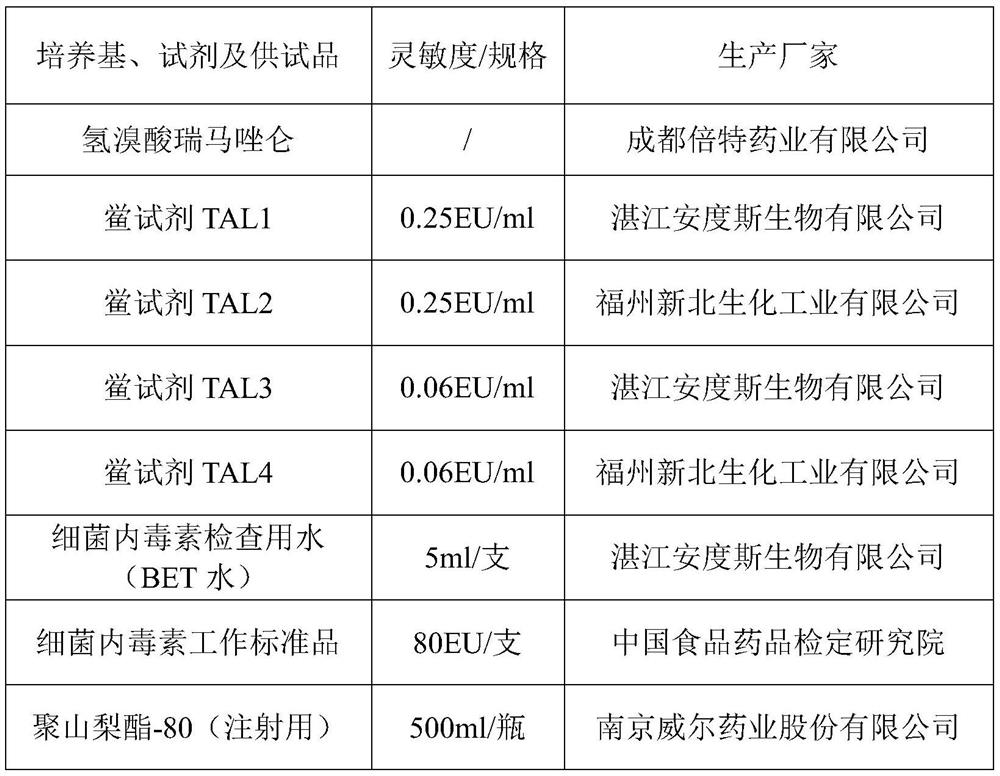
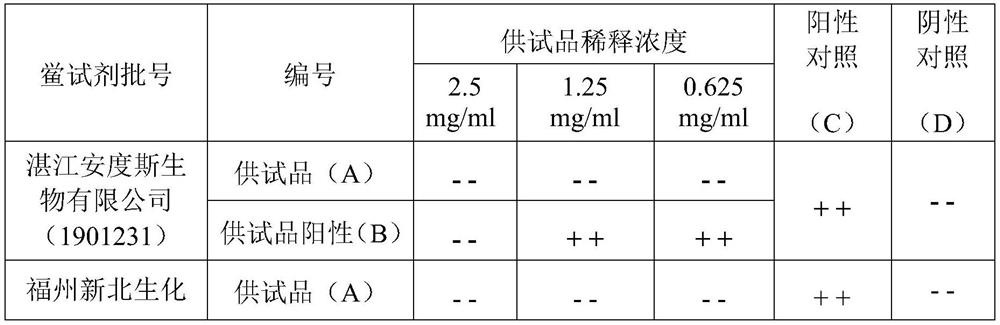
[0031] Interference pre-test: take the test product remazolam hydrobromide, and conduct the interference pre-test with the Limulus reagent (λ=0.25EU / ml) from two manufacturers. The results are shown in Table 2.
[0032] Preparation of the test solution (A): Precisely weigh an appropriate amount of the test product, dissolve the test product with an aqueous solution of 10 mg / mL polysorbate 80 (for injection) to a concentration of 100 mg / mL, and use water for bacterial endotoxin testing. Dilute the test solution to 2.5mg / mL, 1.25mg / mL, 0.625mg / mL.
[0033] Bacterial endotoxin standard solution positive control (C): Take the bacterial endotoxin working standard, add 1.0 mL of test water to dissolve, vortex for 15 minutes, and dilute with bacterial endotoxin test water at 1.0 EU / mL and 0.5 EU / mL.
[0034] Positive control of the test product (B): respectively take an equal volume of the solution containing bacterial endotoxin 1.0EU / ml and the same volume of the test solution 5mg / m...
Embodiment 2
[0043] Interference pre-test: take the test product remazolam hydrobromide, and conduct the interference pre-test with the Limulus reagent (λ=0.25EU / ml) from two manufacturers. The results are shown in Table 3.
[0044] Preparation of test solution (A): Accurately weigh an appropriate amount of the test product, dissolve the test product with an aqueous solution of 20mg / mL polysorbate 80 (for injection) to a concentration of 100mg / mL, and use water for bacterial endotoxin testing. Dilute the test solution to 2.5mg / mL, 1.25mg / mL, 0.625mg / mL.
[0045] Bacterial endotoxin standard solution positive control (C): Take the bacterial endotoxin working standard, add 1.0 mL of test water to dissolve, vortex for 15 minutes, and dilute with bacterial endotoxin test water at 1.0 EU / mL and 0.5 EU / mL.
[0046] Positive control of the test product (B): respectively take an equal volume of the solution containing bacterial endotoxin 1.0EU / ml and the same volume of the test solution 5mg / mL, 2....
Embodiment 3
[0052] Interference pre-test: take the test product remazolam hydrobromide (batch number: 180102), and conduct the interference pre-test with the Limulus reagent (λ=0.25EU / ml) from two manufacturers. The results are shown in Table 4.
[0053] Preparation of test solution (A): Accurately weigh an appropriate amount of the test product, dissolve the test product with an aqueous solution of 30 mg / mL polysorbate 80 (for injection) to a concentration of 100 mg / mL, and use water for bacterial endotoxin testing. Dilute the test solution to 2.5mg / mL, 1.25mg / mL, 0.625mg / mL.
[0054] Bacterial endotoxin standard solution positive control (C): Take the bacterial endotoxin working standard, add 1.0 mL of test water to dissolve, vortex for 15 minutes, and dilute with bacterial endotoxin test water at 1.0 EU / mL and 0.5 EU / mL.
[0055] Positive control of the test product (B): Take an equal volume of the solution containing bacterial endotoxin 1.0EU / ml and the same volume of the test solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com