Patents
Literature
2003 results about "Positive control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
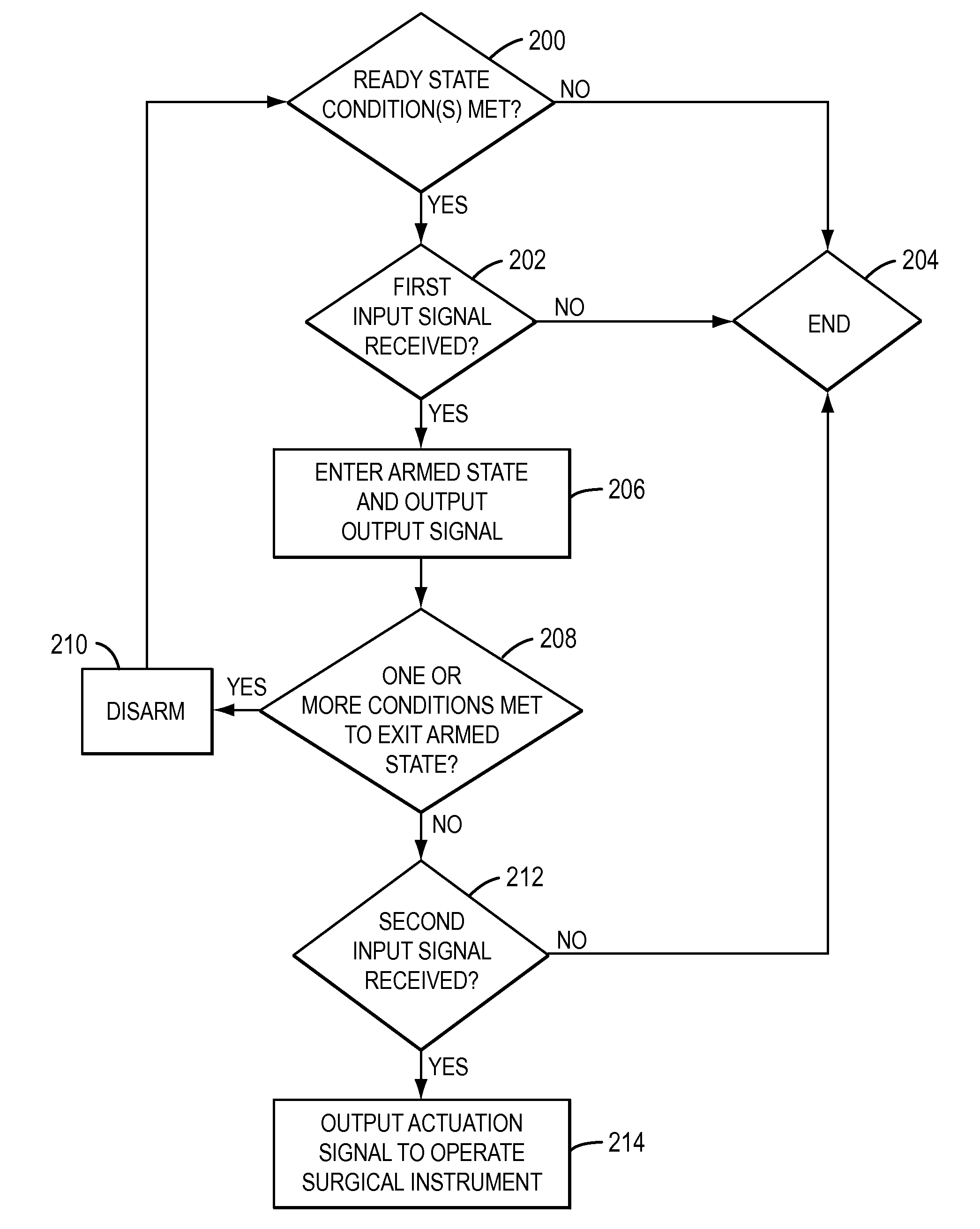
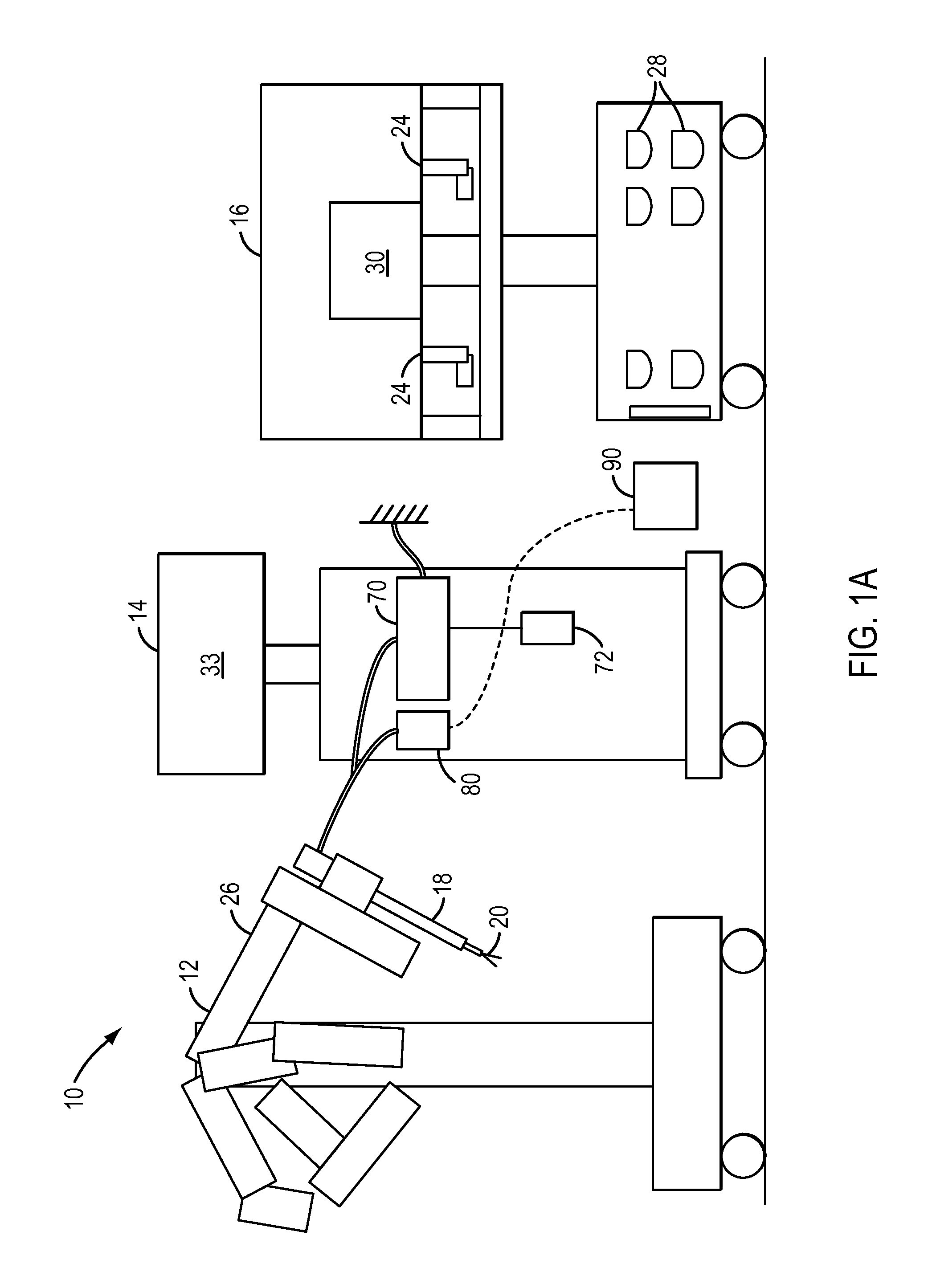
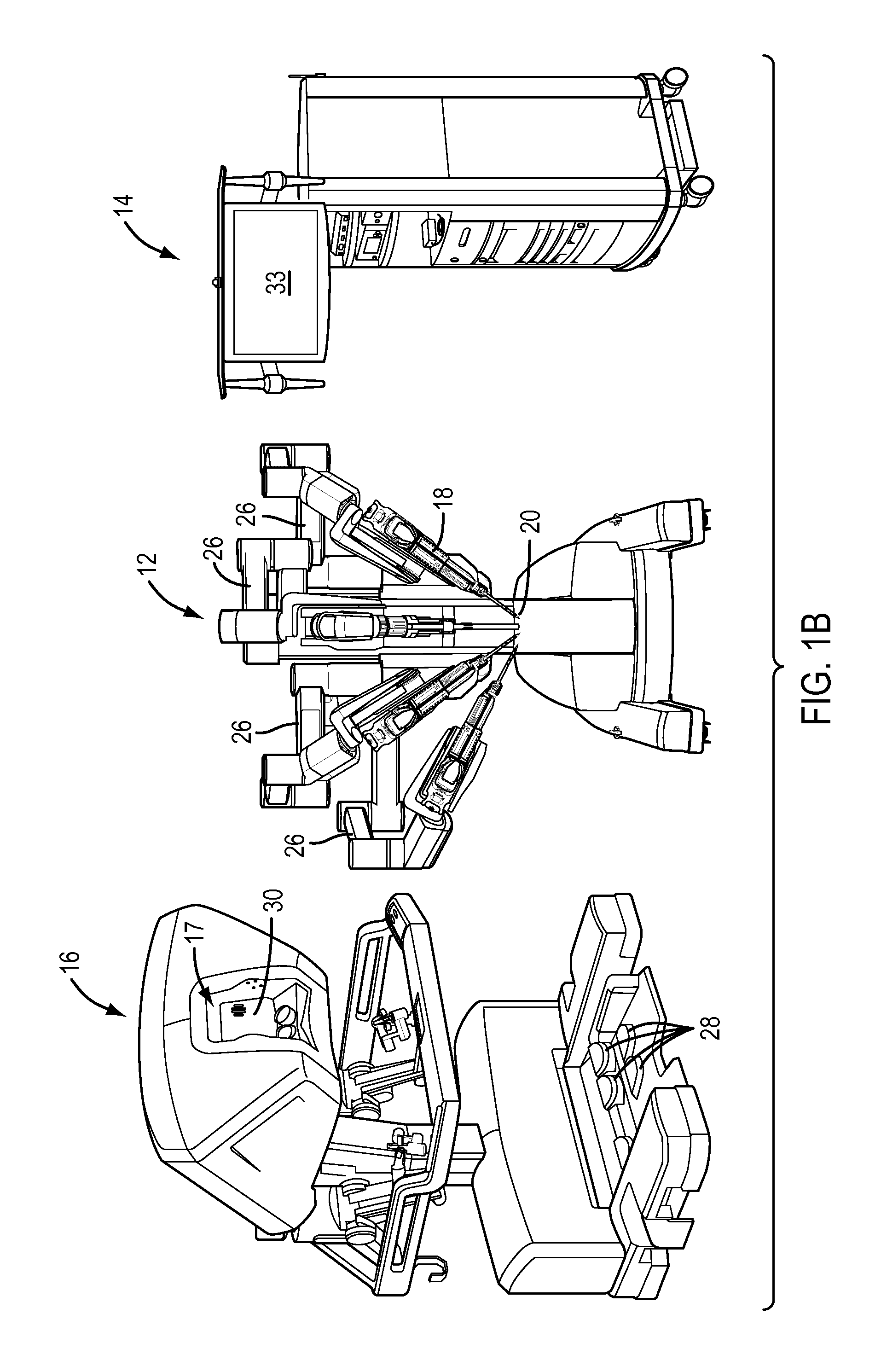
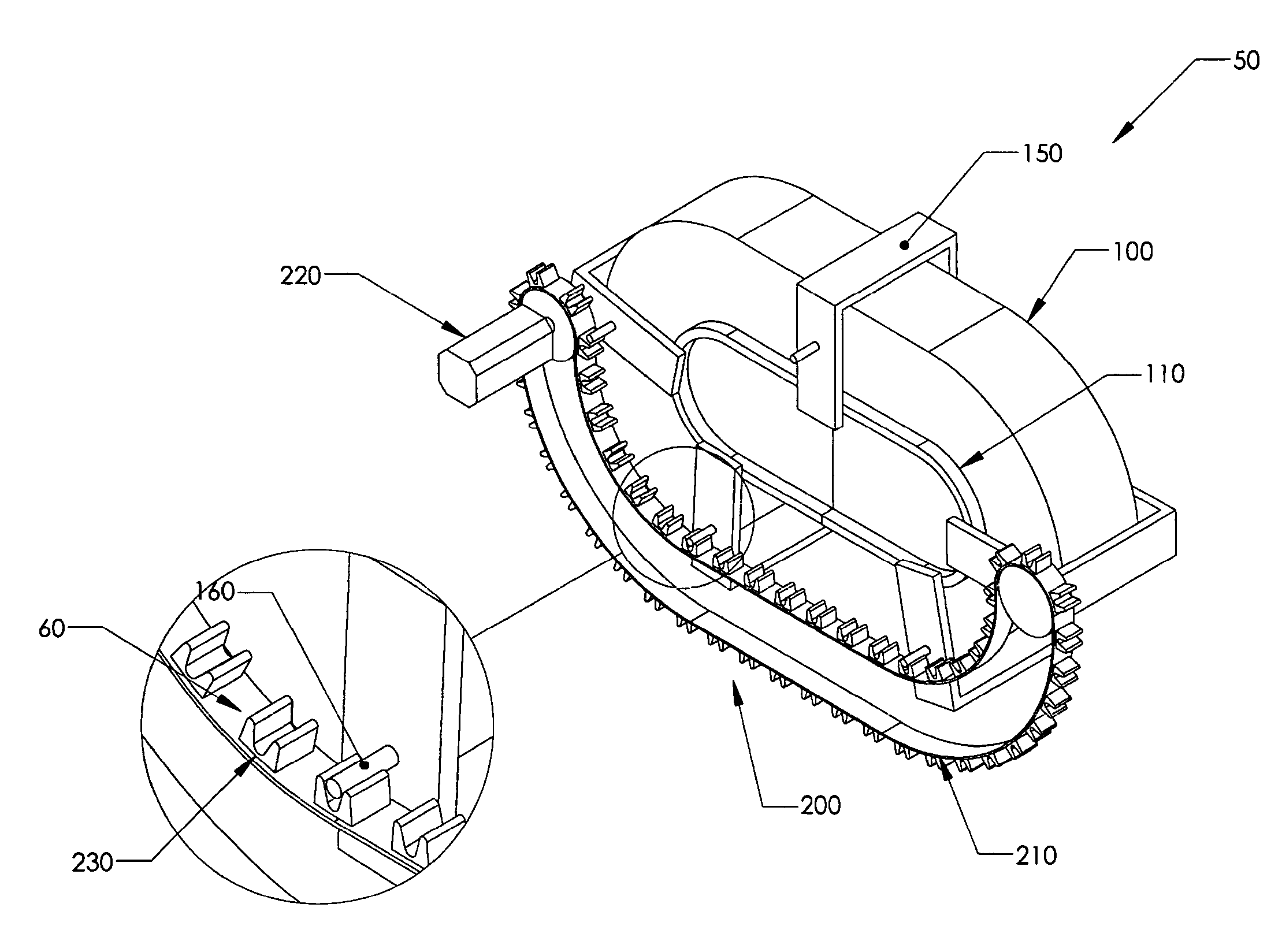
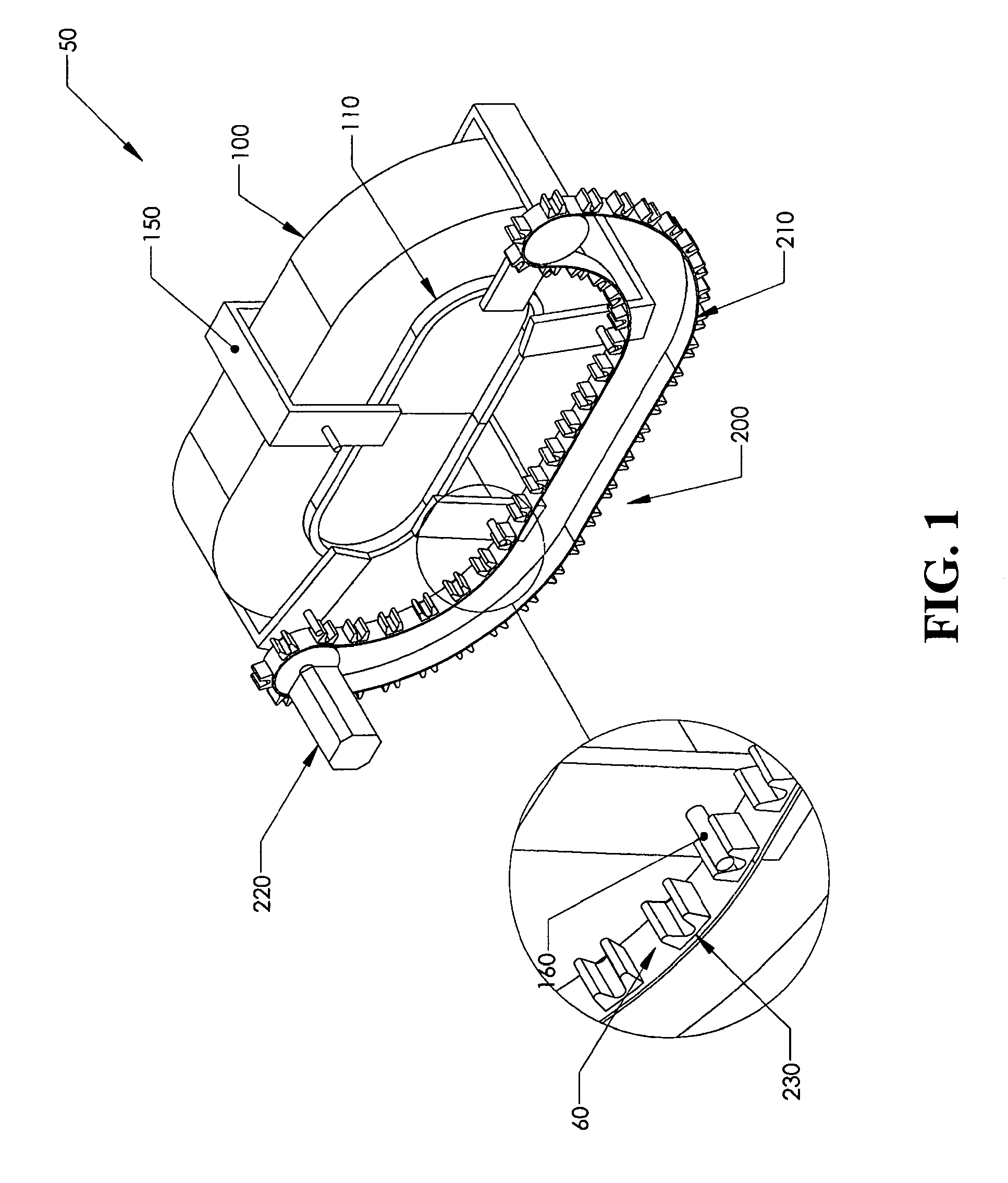
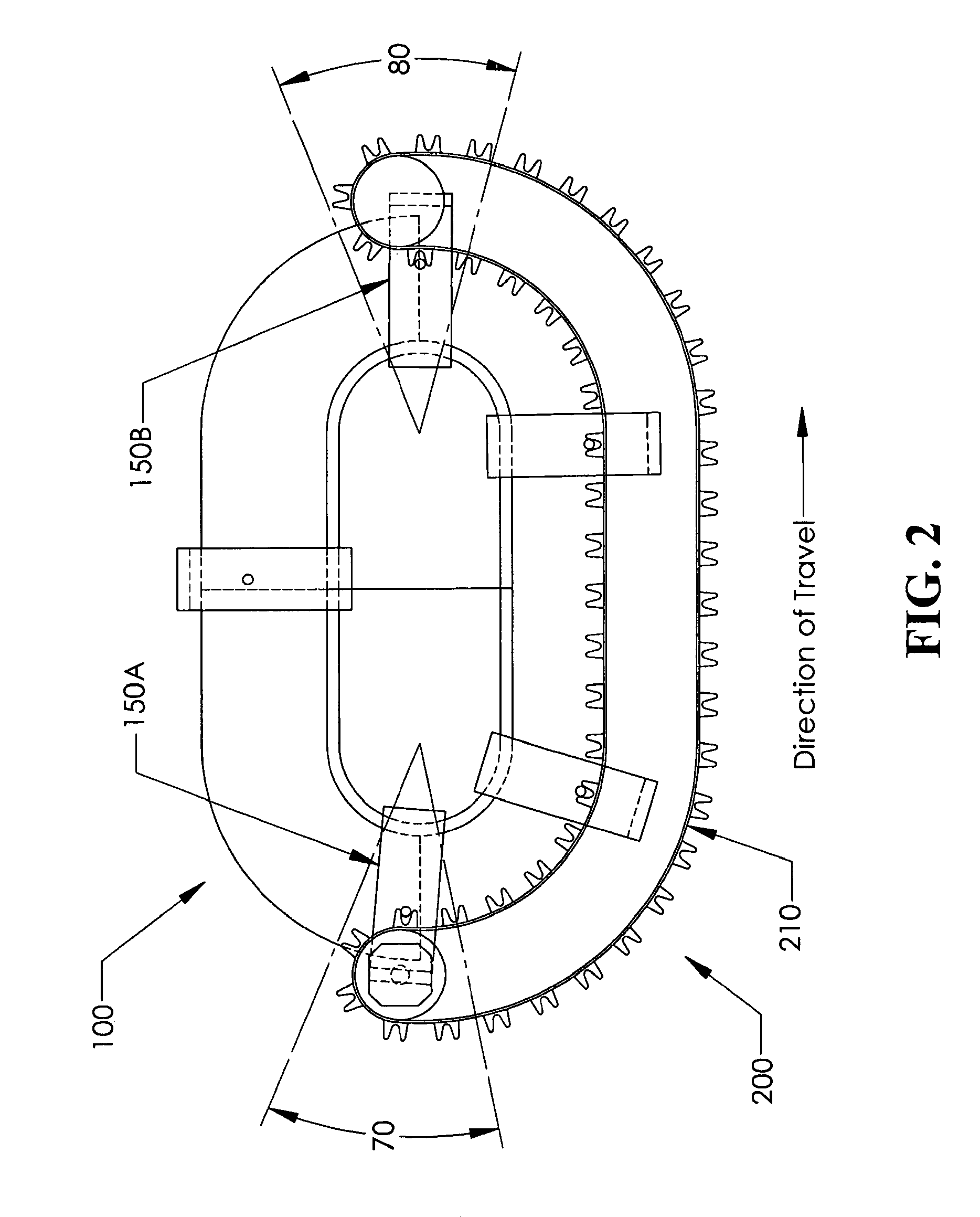
Positive control of robotic surgical instrument end effector
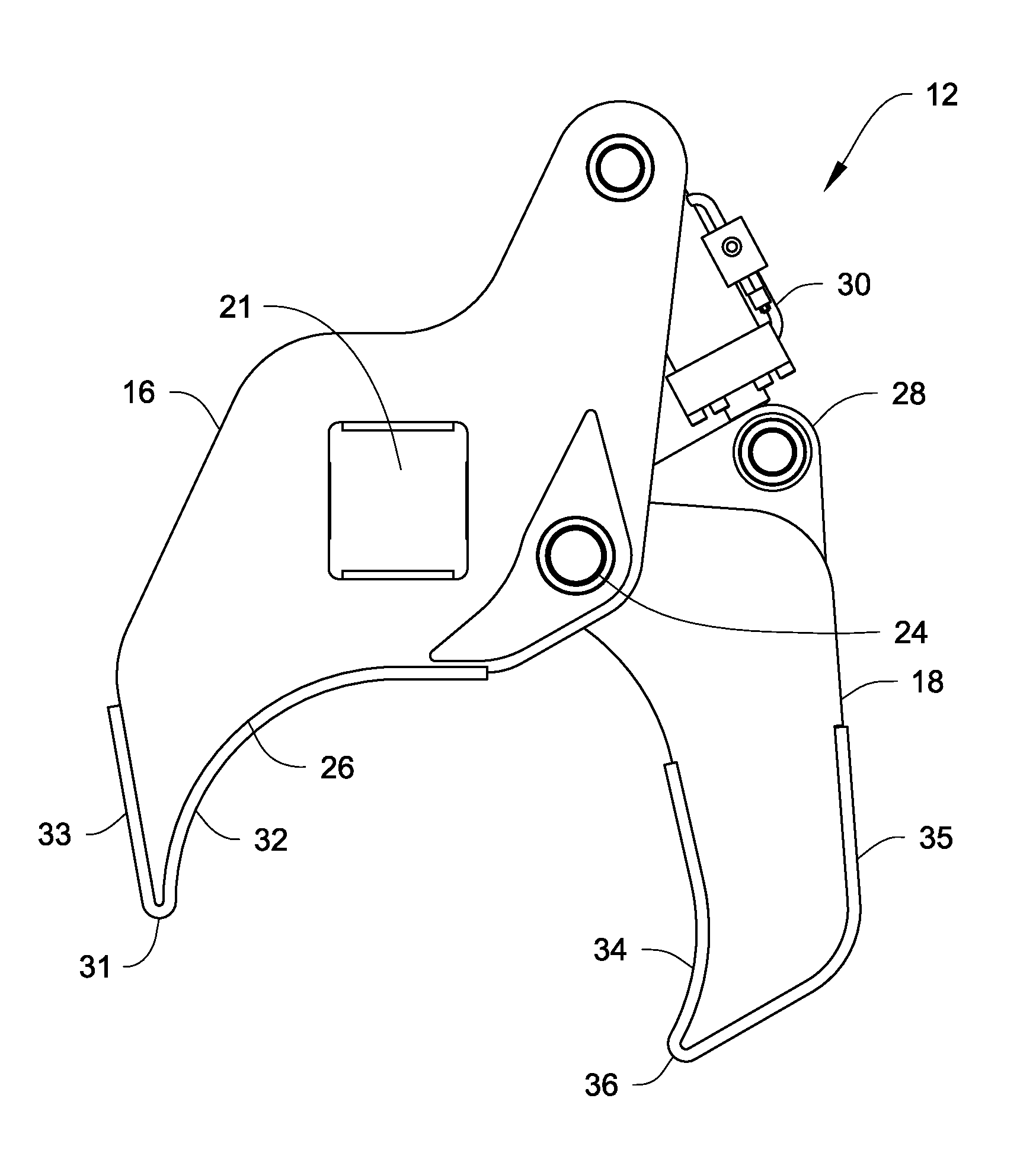
A method of controlling an operation of a robotically-controlled surgical instrument can include receiving a first input signal at a controller indicative of a user's readiness to actuate the surgical instrument to perform a surgical procedure, outputting an output signal from the controller to provide feedback to the user in response to the received first input signal, receiving a second input signal at the controller confirming the user's readiness to actuate the surgical instrument, outputting an actuation signal from the controller in response to receiving the second input signal, and actuating the surgical instrument to perform the surgical procedure based on the actuation signal.
Owner:INTUITIVE SURGICAL OPERATIONS INC
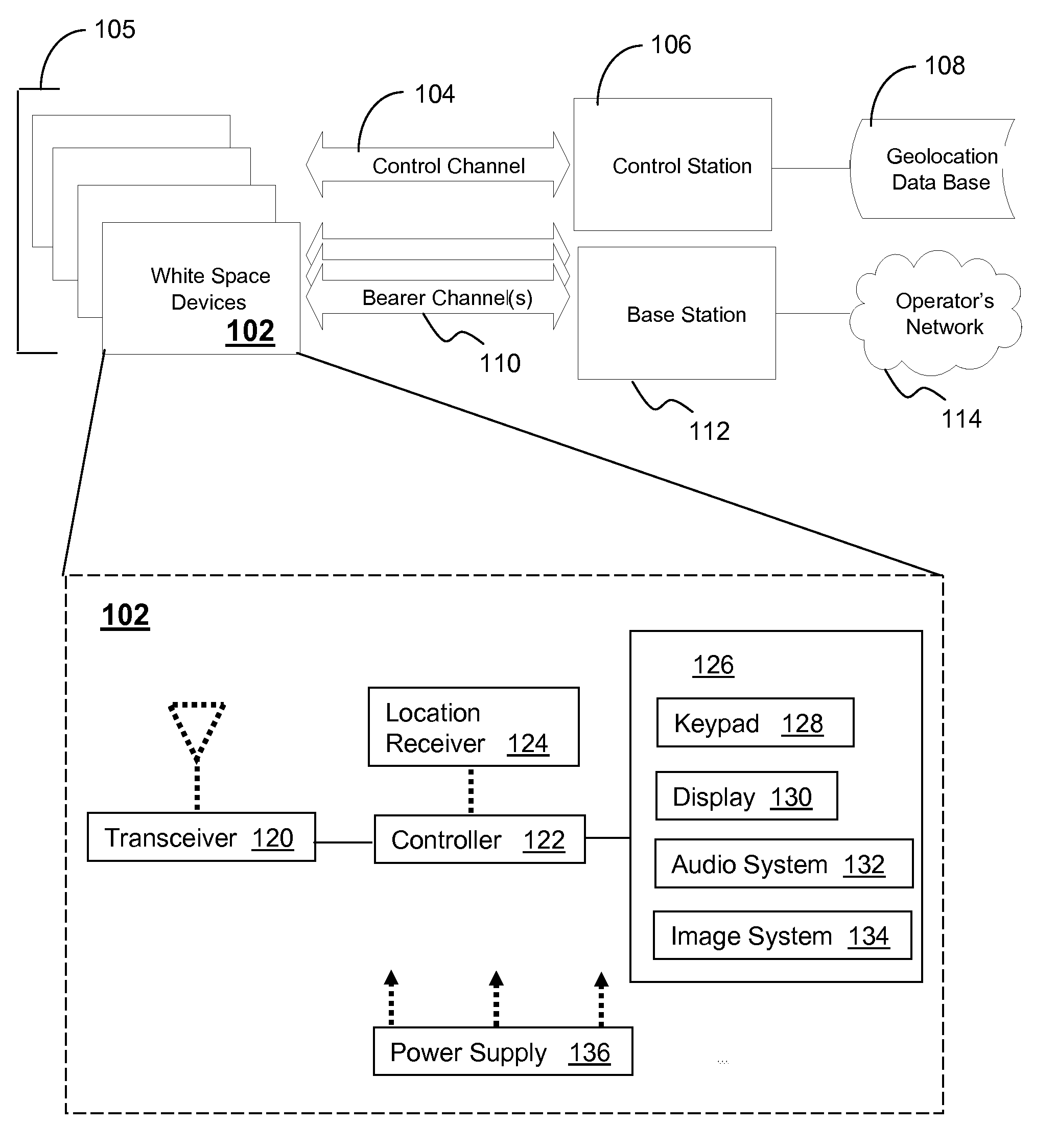
System and method for dynamic frequency assignment
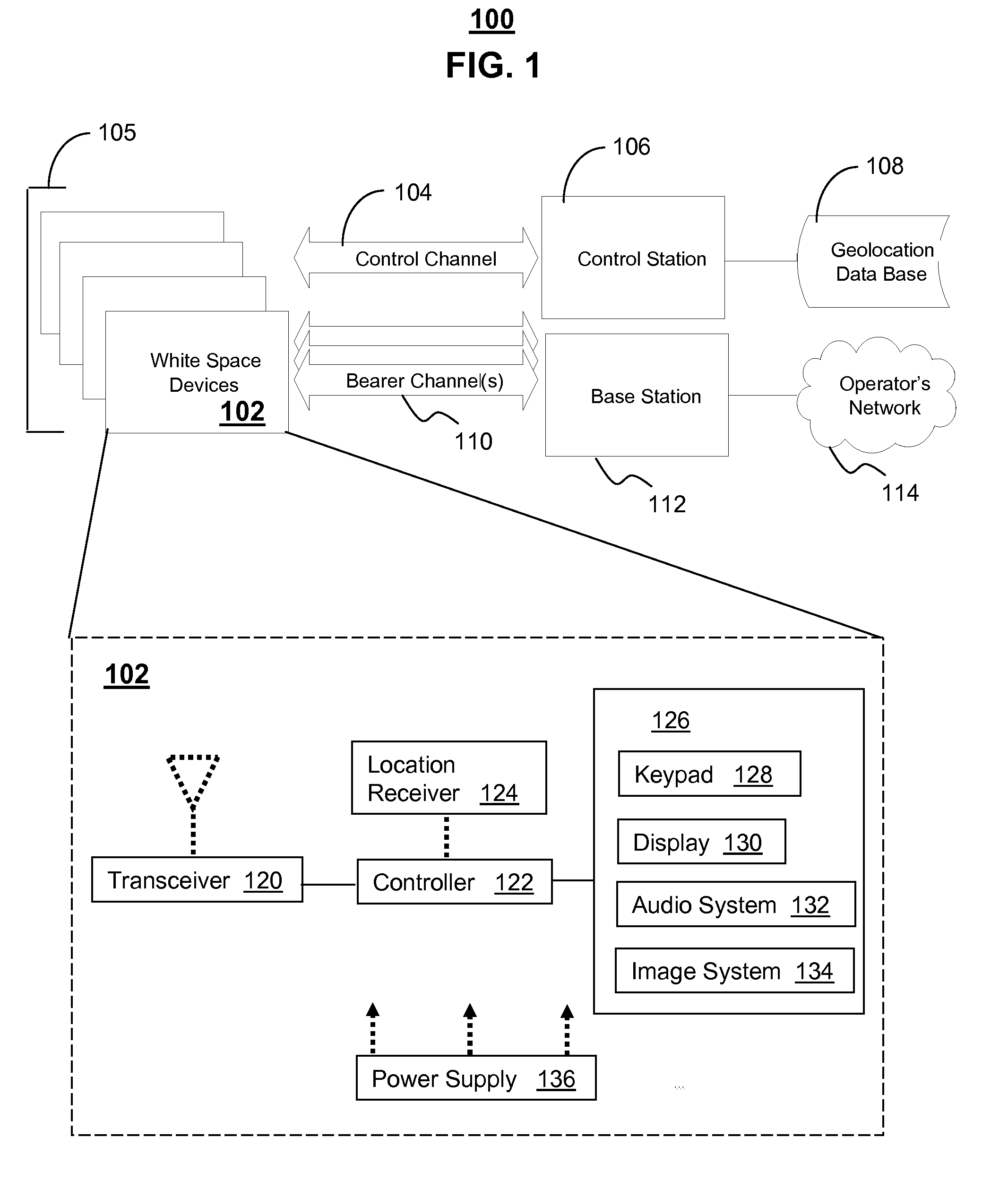
White space devices (102) are unlicensed radiofrequency devices that must have certain capabilities in order to avoid harmful interference to licensed operations. In general, they must be location-aware, must be able to contact a geolocation database (108) and may not operate without receiving a positive control signal. A number of white space devices can use a control channel (104) to communicate with a control station (106). In addition to meeting the geolocation and positive control requirements given above, the control station coordinates the channels used by the white space devices so as to minimize (308) their aggregate interference. In one embodiment, a control channel uses a separate frequency band with high availability and reliability but low throughput. Embodiments optimize channel assignments where the potential interference depends on the mutual distances between the white space devices. Potential interference reductions of 20-30 dB have been found in simulations. Other embodiments are disclosed.
Owner:PROGENY LMS
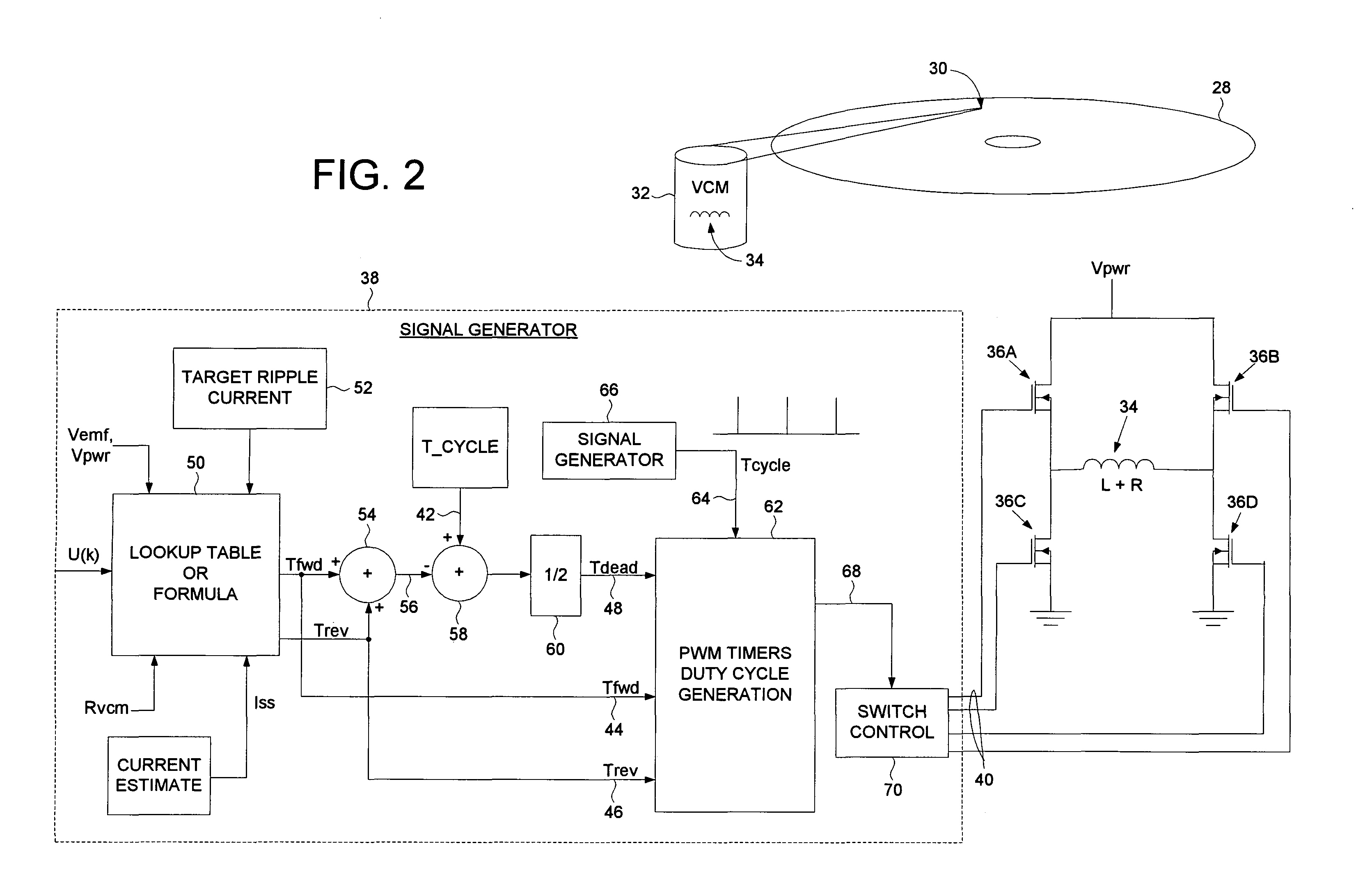
Disk drive controlling ripple current of a voice coil motor when driven by a PWM driver
InactiveUS6965488B1Disposition/mounting of recording headsDriving/moving recording headsNegative controlPositive control
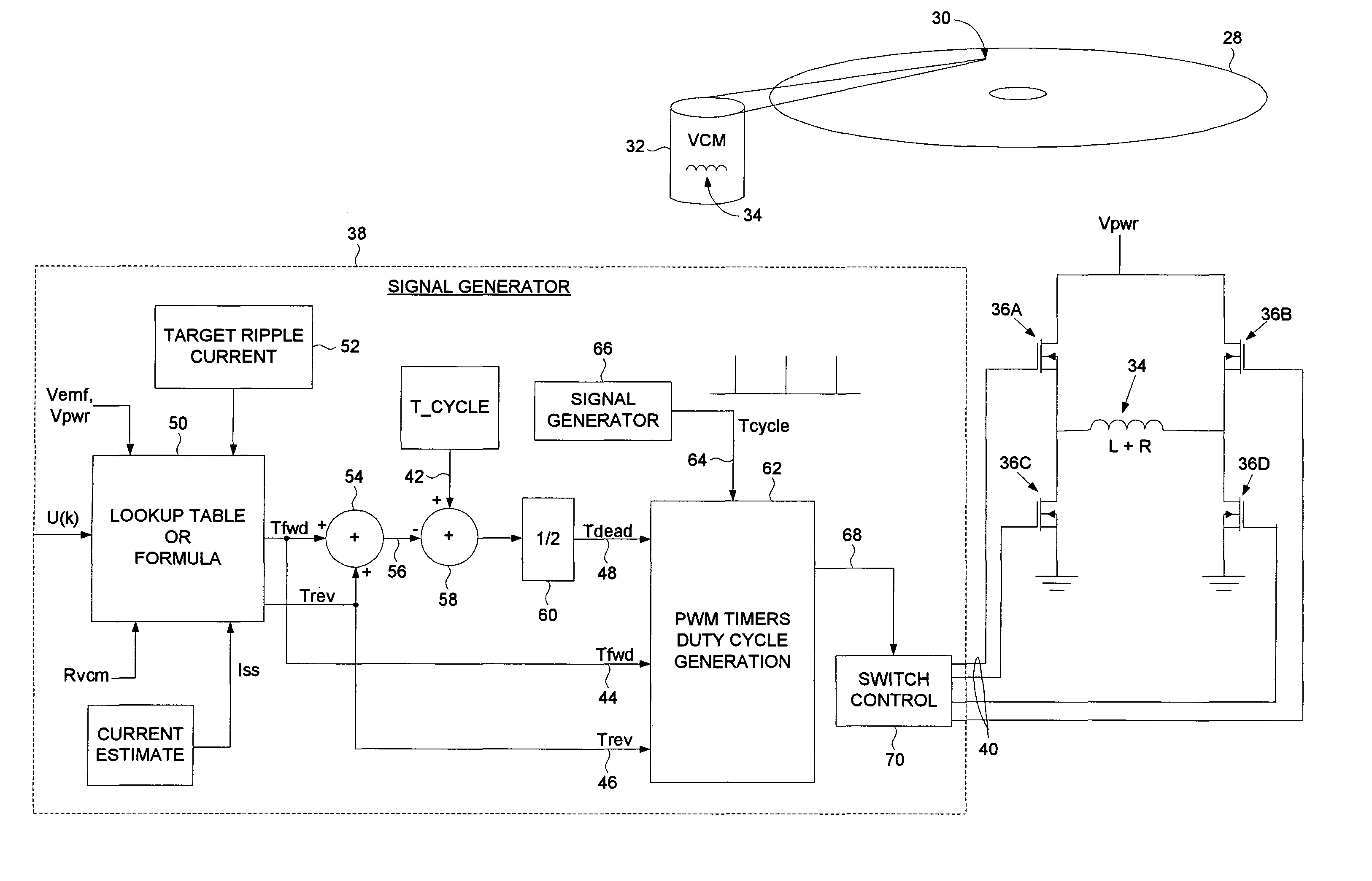
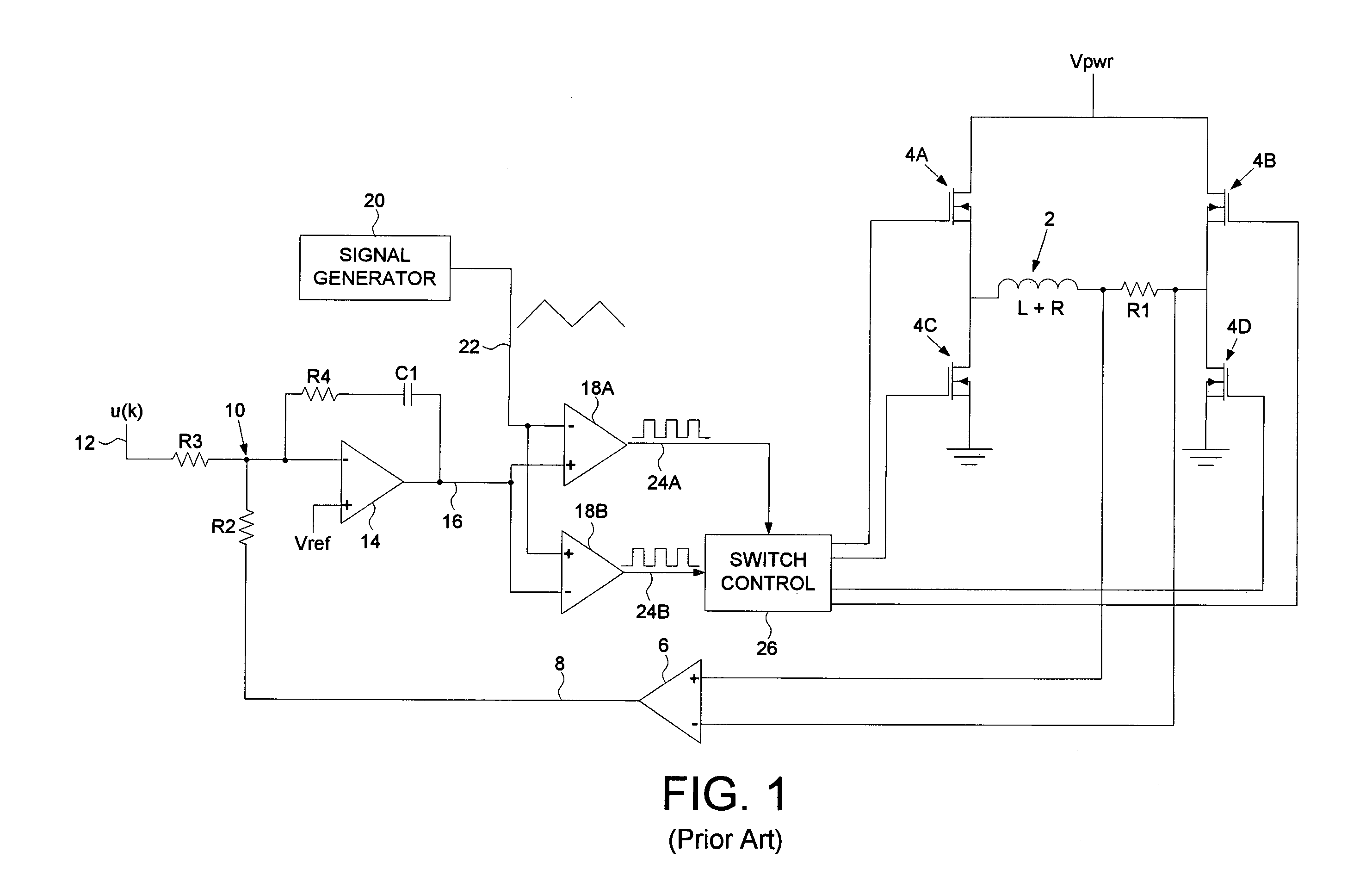
A disk drive is disclosed comprising a pulse width modulated (PWM) signal generator for generating PWM control signals applied to the driver switches of a voice coil motor (VCM). The PWM control signals comprise a PWM cycle time, a Tforward time interval of the PWM cycle time wherein a positive control voltage is applied to the VCM, a Treverse time interval of the PWM cycle time wherein a negative control voltage is applied to the VCM, and a Tdead time interval of the PWM cycle time wherein a substantially zero control voltage is applied to the VCM. The Tdead time interval is adjusted to control a magnitude of an actual ripple current flowing through the VCM.
Owner:WESTERN DIGITAL TECH INC
Medical use of 1beta-keto-5, 11(13)-diene eudesmane-12-acid for inhibiting hepatitis B virus
InactiveCN1927197APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsSugar derivativesDiseasePositive control
The invention involves A 1- beta-keto-5, 11(13)-diene eudesmane-12-acidum who has the structure as formula (1) shows and its medical salt, or solvate and its drug combinations and its medical usage in reducing hepatitis B surface antgien and inhibiting replication activity of aethyl- hepatovirus HBV-DNA medicinal. The invention compounds has strong inhibitory action in hepatitis B surface antgien (HBsAg) externalized by HepG2.2.15 cells and replication of aethyl- hepatovirus deoxyribonucleotide (HBV-DNA) in vitro, its inhibiting ability against HBsAg surpasses that of positive control Lamivudine in the same dose; it has obvious inhibiting ability against replication of aethyl- hepatovirus HBV-DNA under the concentration of 100 mu g / mL,20 mug / mL and 4 mug / mL DNA, it belongs to anti- aethyl- hepatovirus natural products of superactive non-nucleoside, can be expected for producing drugs for treating aethyl- hepatovirus infected disease.
Owner:WENZHOU MEDICAL UNIV
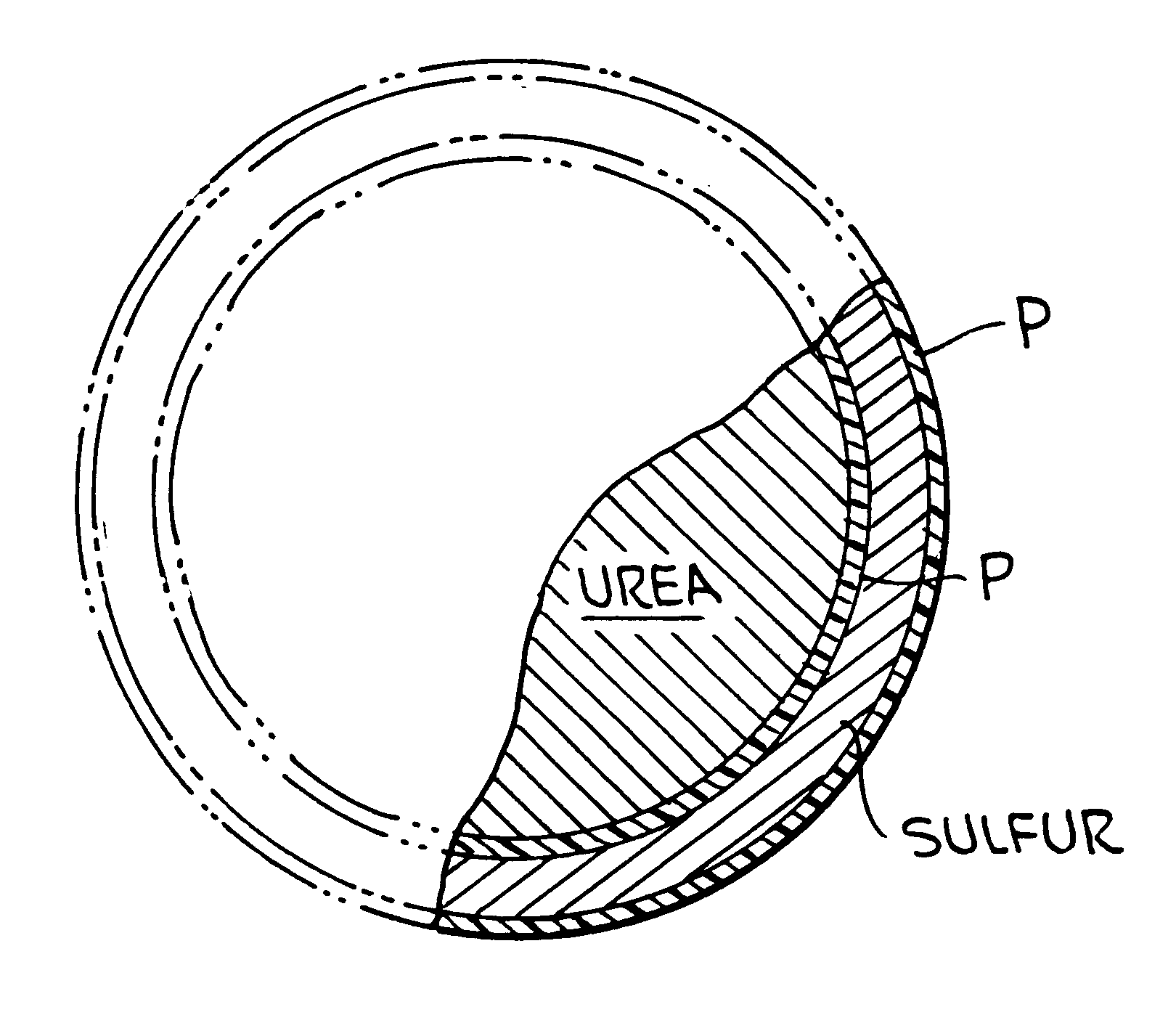
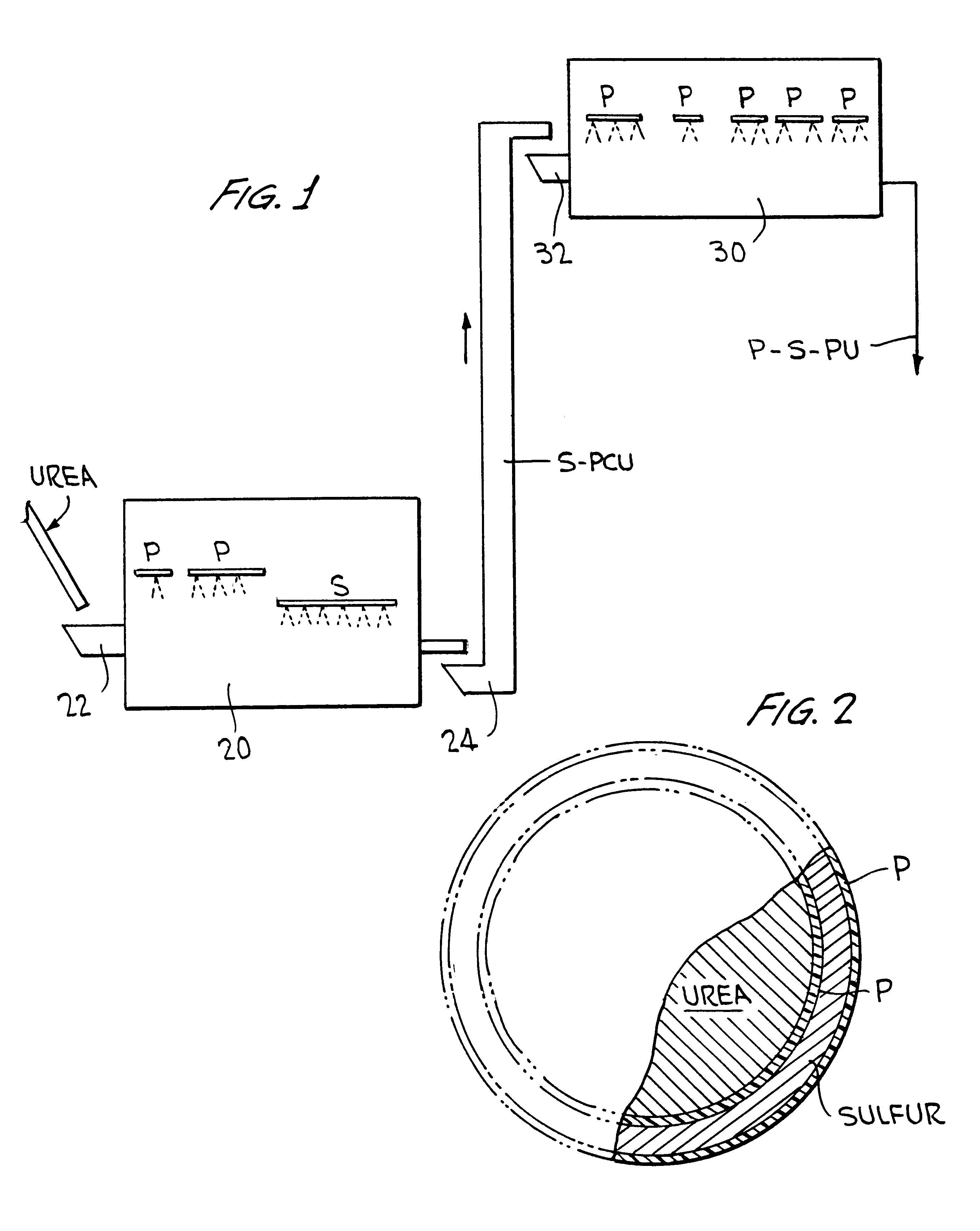
Polymer-sulfur-polymer coated fertilizers
InactiveUS6338746B1Good resistance to abrasionAvoid serious impactBiocideMaterial granulationControlled releasePositive control
The present invention described a polymer coated fertilizer, such as urea, subsequently coated with a layer of sulfur and thereafter a further coating of polymer. Preferably, the polymer coatings are formed by the direct in situ co-polymerization of the components of the polymer on the fertilizer and on the sulfur coating. The compositions provide positive controlled release characteristics, are abrasion and impact resistant and are substantially more economical to produce than polymer coated fertilizers.
Owner:KOCH AGRONOMIC SERVICES LLC
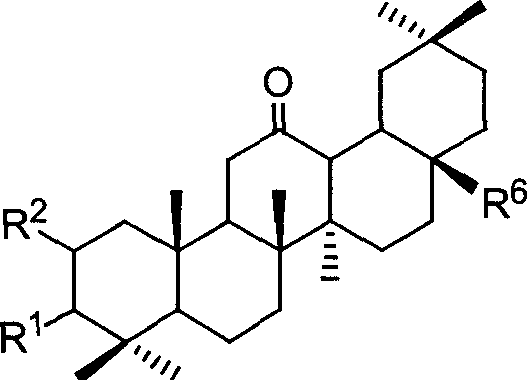
A and C macrocyclic oxidation substituted pentacyclic triterpanoids and preparation method and use thereof
InactiveCN101117348AStrong inhibitory activityOrganic active ingredientsMetabolism disorderDiseaseProstate cancer cell
The present invention relates to a pentacyclic triterpanoid derivative of multiple-oxide substitution of the A ring and the C ring and the medicine salt or solvate of the derivative, and the present invention also relates to the preparation method, the drug combination, and medical use of the derivative. The compound of the present invention has the functions of inhibiting the activity of six human tumor cell strains in vitro, such as human prostate cancer cell (PC-3), nasopharyngeal carcinoma cells (CNE), oral squamous carcinoma cell(KB), human lung cancer cell (A549), human hepatoma cell (BEL-7404), and human cervix cancer cell (Hela), and the function of the invention is at the same magnitude of the positive control of cisplatin, thereby the compound can be used as expected antitumor drug. The compound of the present invention also inhibits the alpha glucosidase strongly, and the inhibiting effect is greater than the positive control of acarbose, thereby the compound can be used as expected medicine for preventing and treating diabetes and the treatment of the virus diseases.
Owner:ZHEJIANG HISUN PHARMA CO LTD
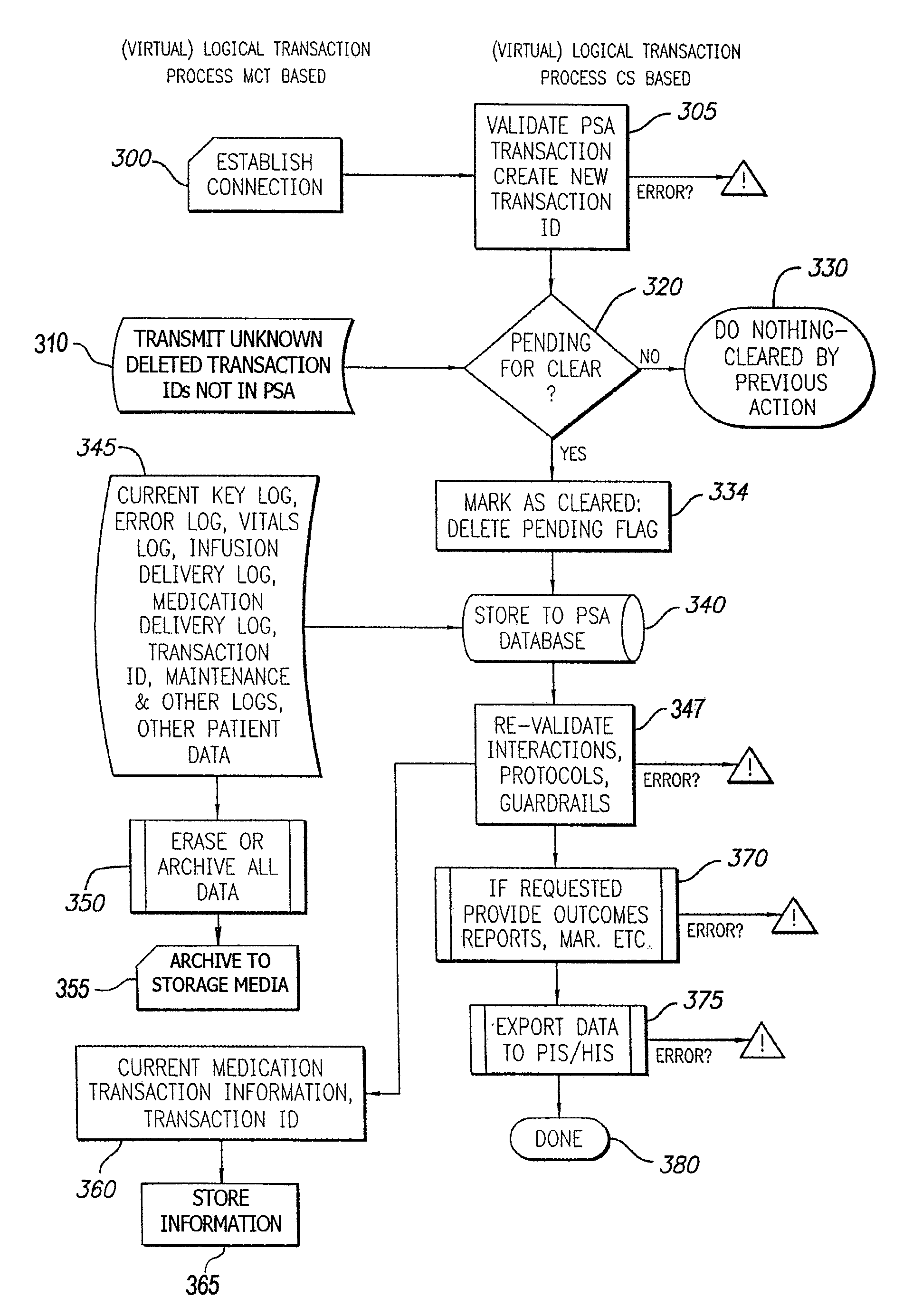
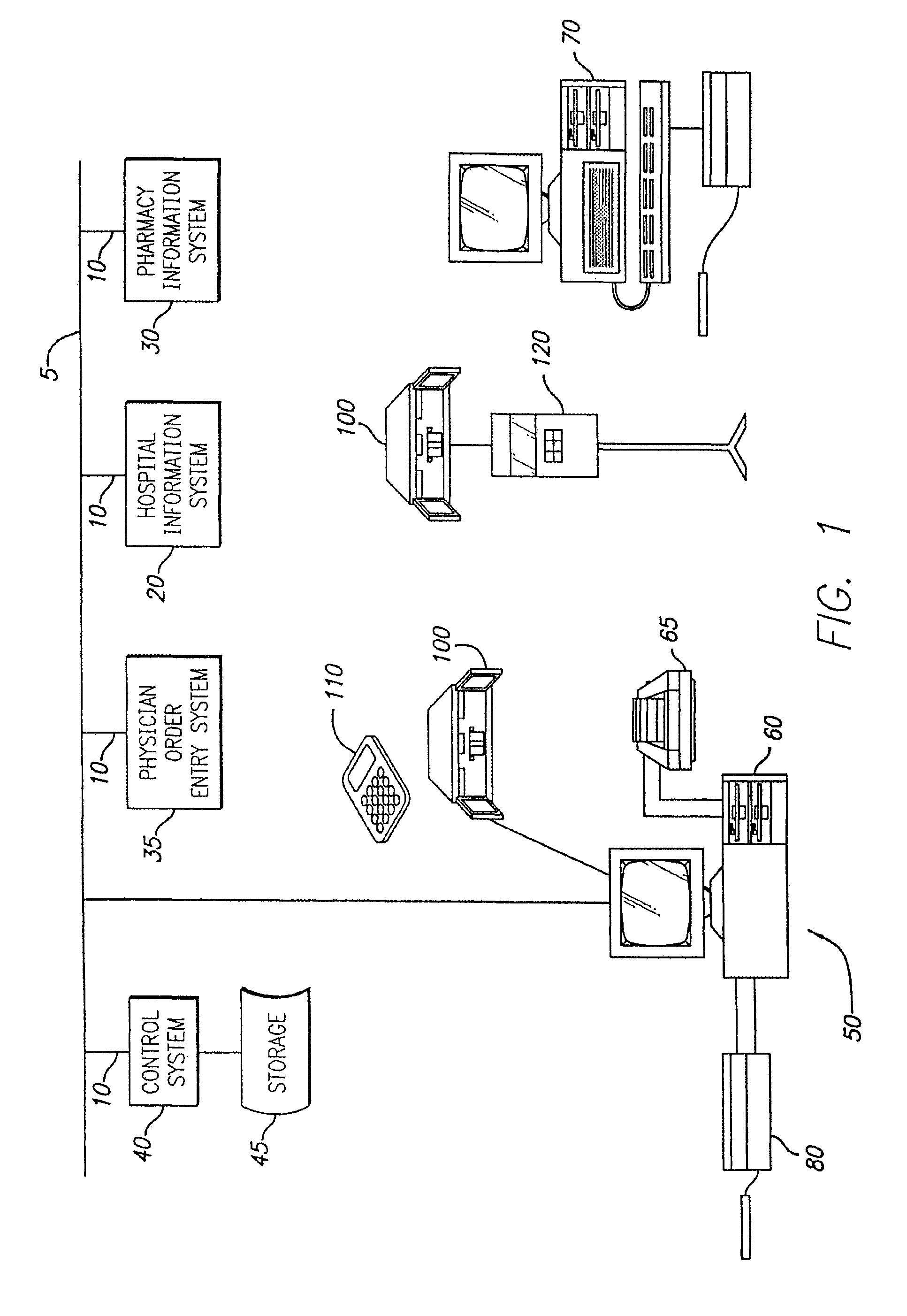
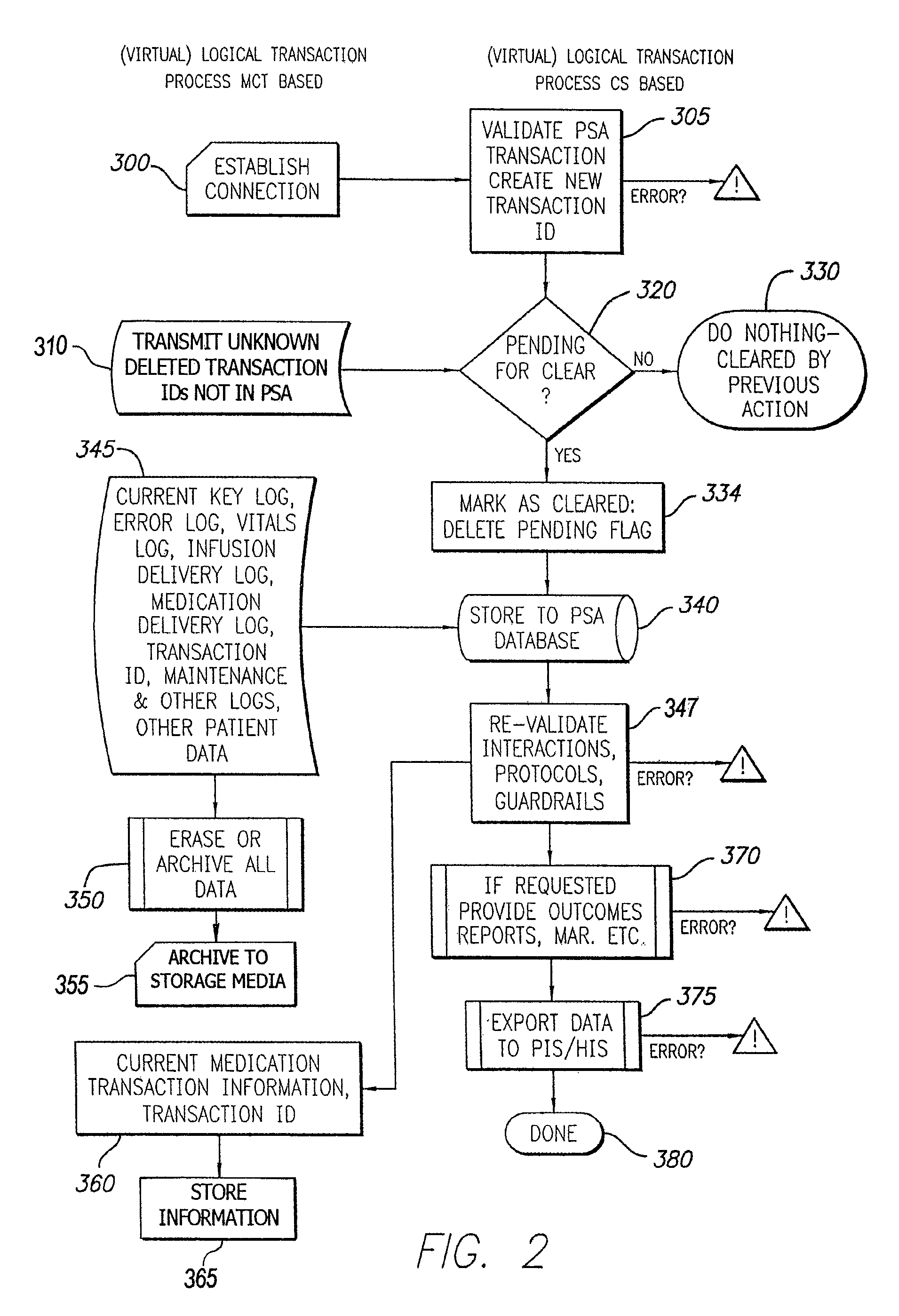
Distributed remote asset and medication management drug delivery system
ActiveUS8005688B2Data processing applicationsDrug and medicationsGlobal information systemPositive control
A system and method for communicating and validating patient information including medication delivery information in a care-giving facility is provided. A medical transaction carrier is used to communicate information regarding medication delivery and other patient information between a control system in communication with the care-giving facility's other information systems and a patient specific asset such as an infusion pump. All information carried by the medical transaction carrier is validated both at the patient specific asset and at the control system. This validation allows for positive control of all transactions even if a medical transaction carrier is lost. The medical transaction carrier may be a smartcard, a PDA such as a Palm™ Pilot, laptop computer, pager, mobile phone, or other device capable of storing and communicating information. The system may use either wired or wireless connections to communicate information between the components of the system.
Owner:CAREFUSION 303 INC
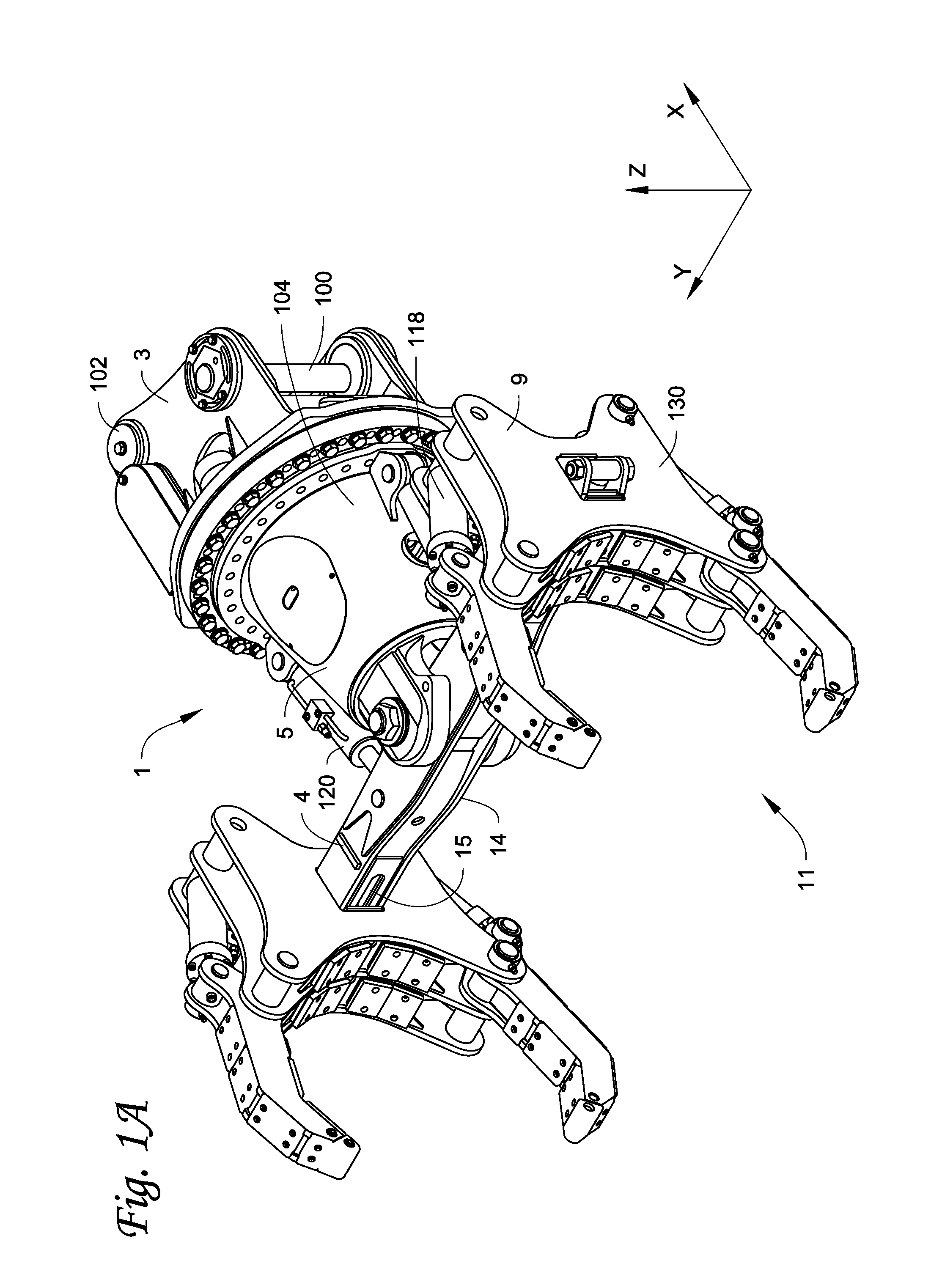
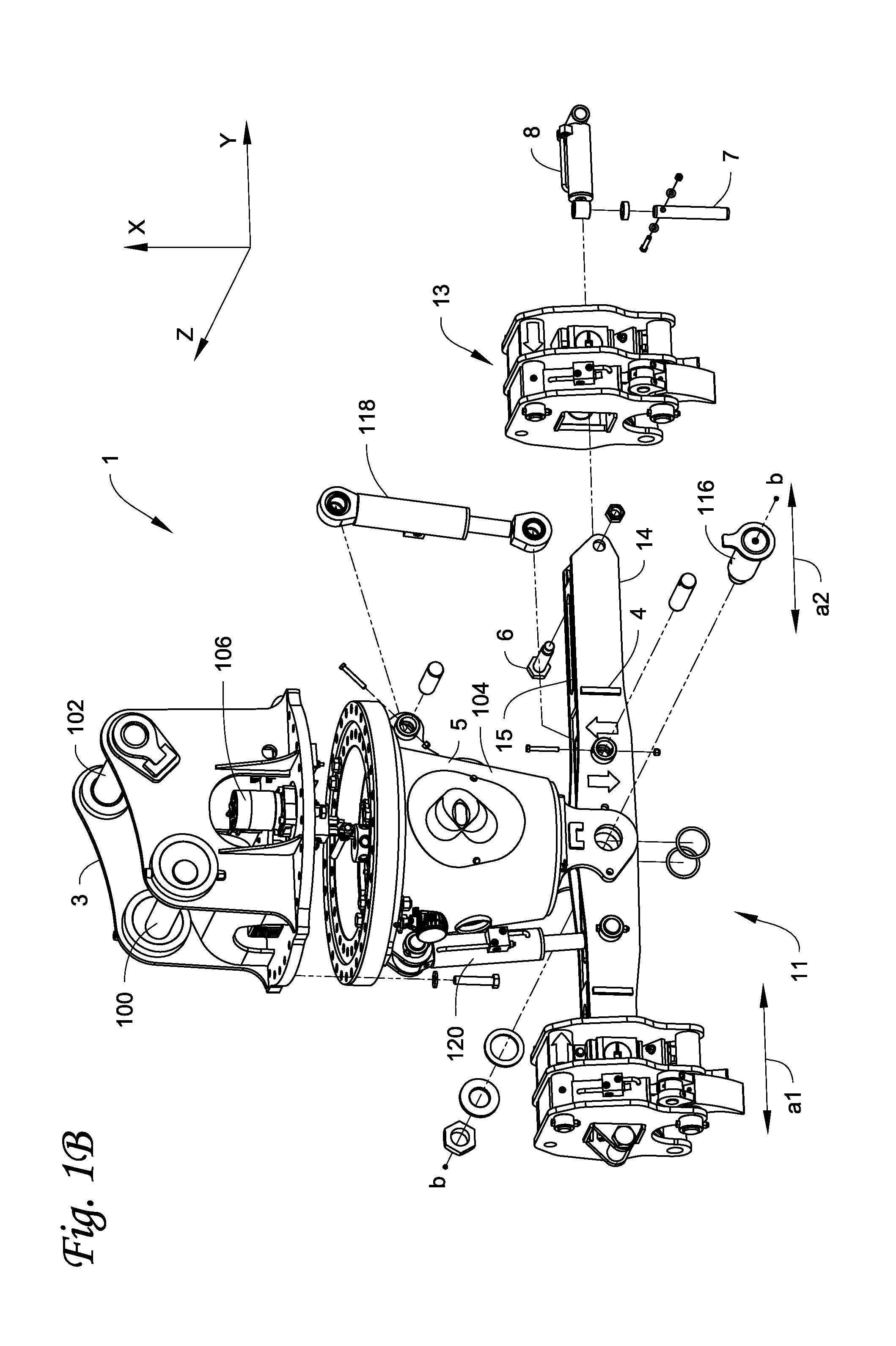
Gripping assembly and gripping members for a grapple attachment
ActiveUS20100308609A1Easy loading and unloadingReduce manpowerDrilling rodsGripping headsPositive controlEngineering
A gripping assembly with gripping members, as part of a grapple attachment, used to grasp and manipulate elongated objects, for example pipe, is described. Due to the gripping action of the gripping members and tilt control capabilities of the grapple attachment, total positive control of the pipe is maintained, even if the gripping assembly picks up pipe off center. The grapple attachment is able to be used on all pipe surface types, including pipe surfaces that are dirty, snow or ice covered. The gripping members are configured to prevent damage to the pipe and to adjacent pipes, and will not crush the pipe.
Owner:LAVALLEY IND
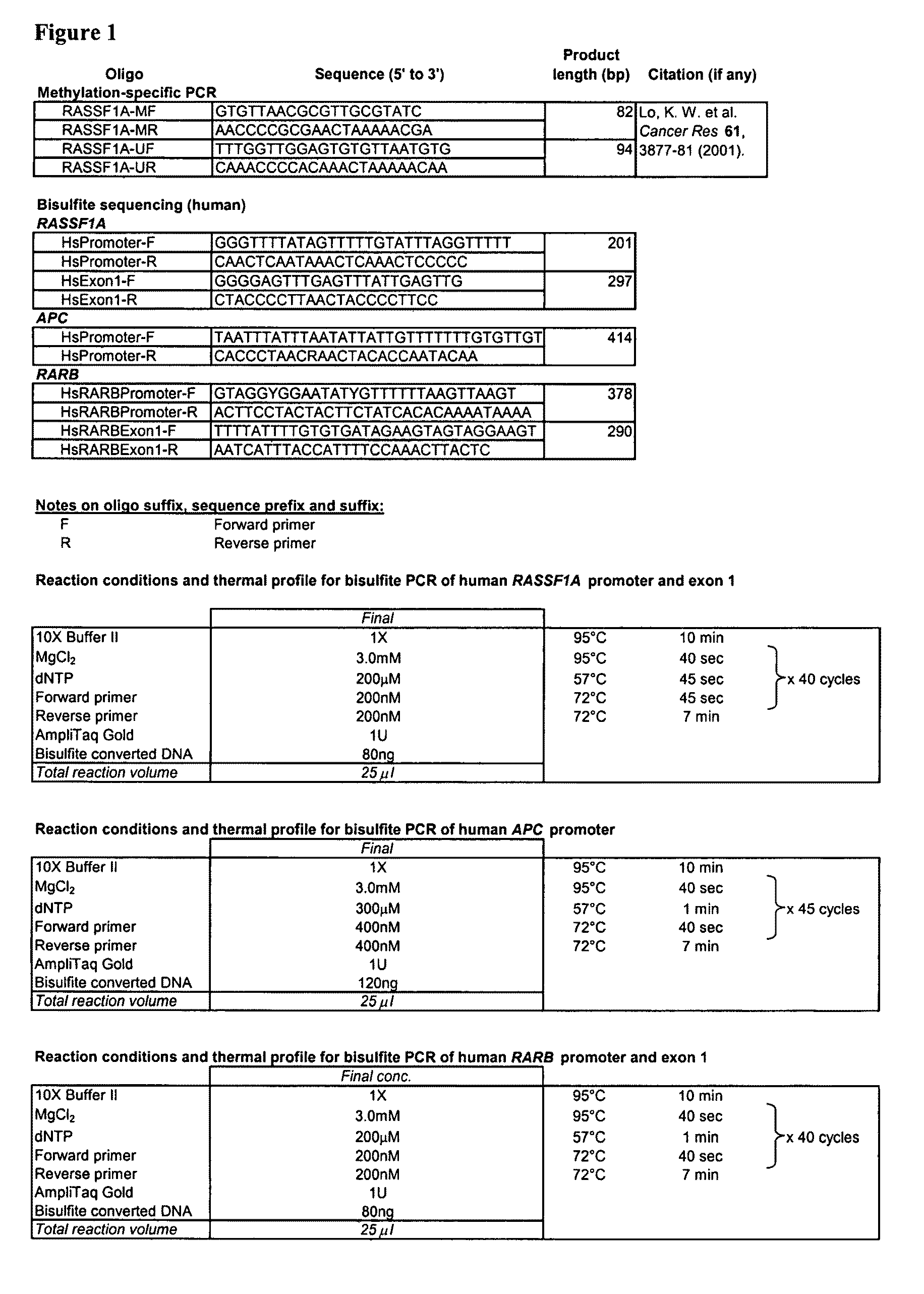
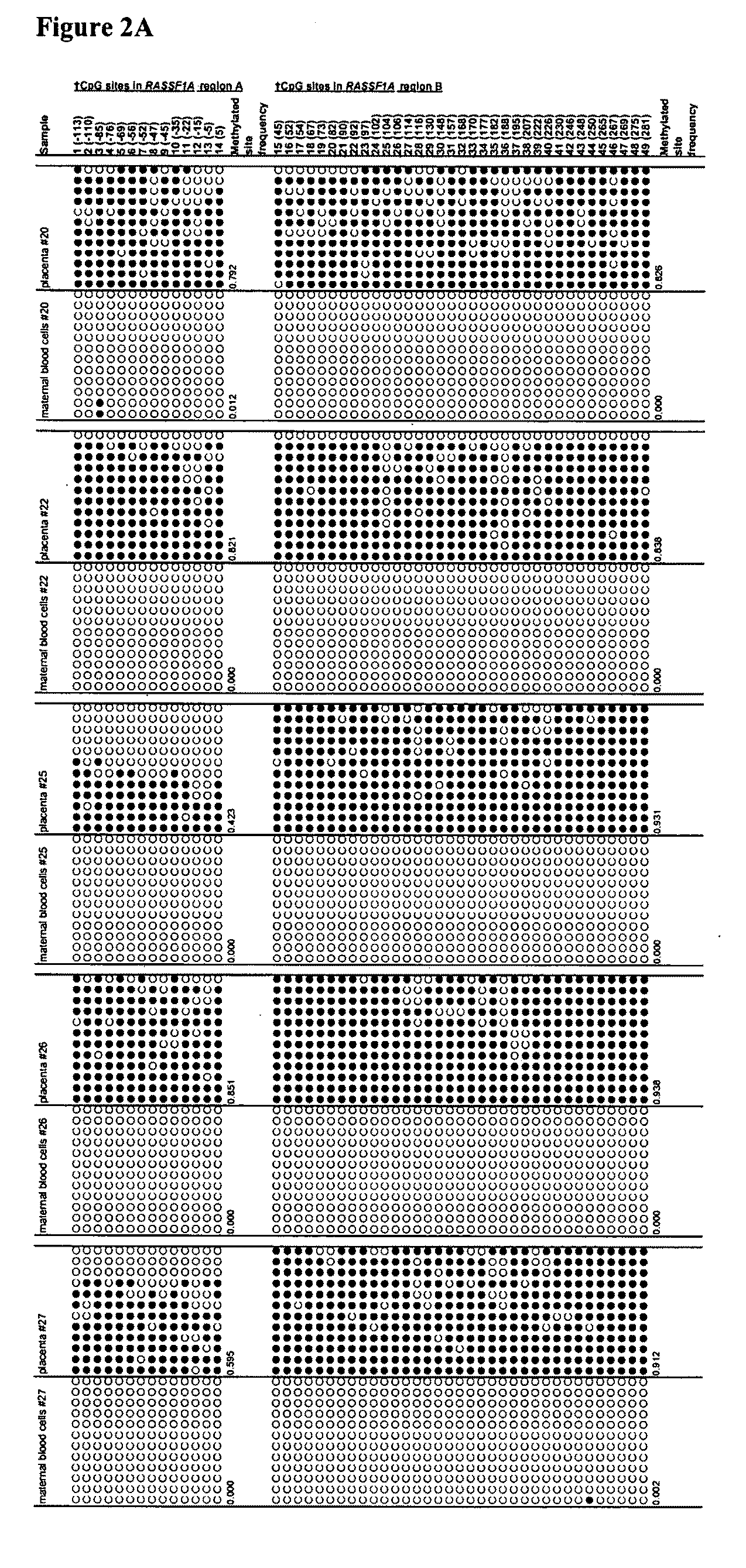
Fetal methylation markers
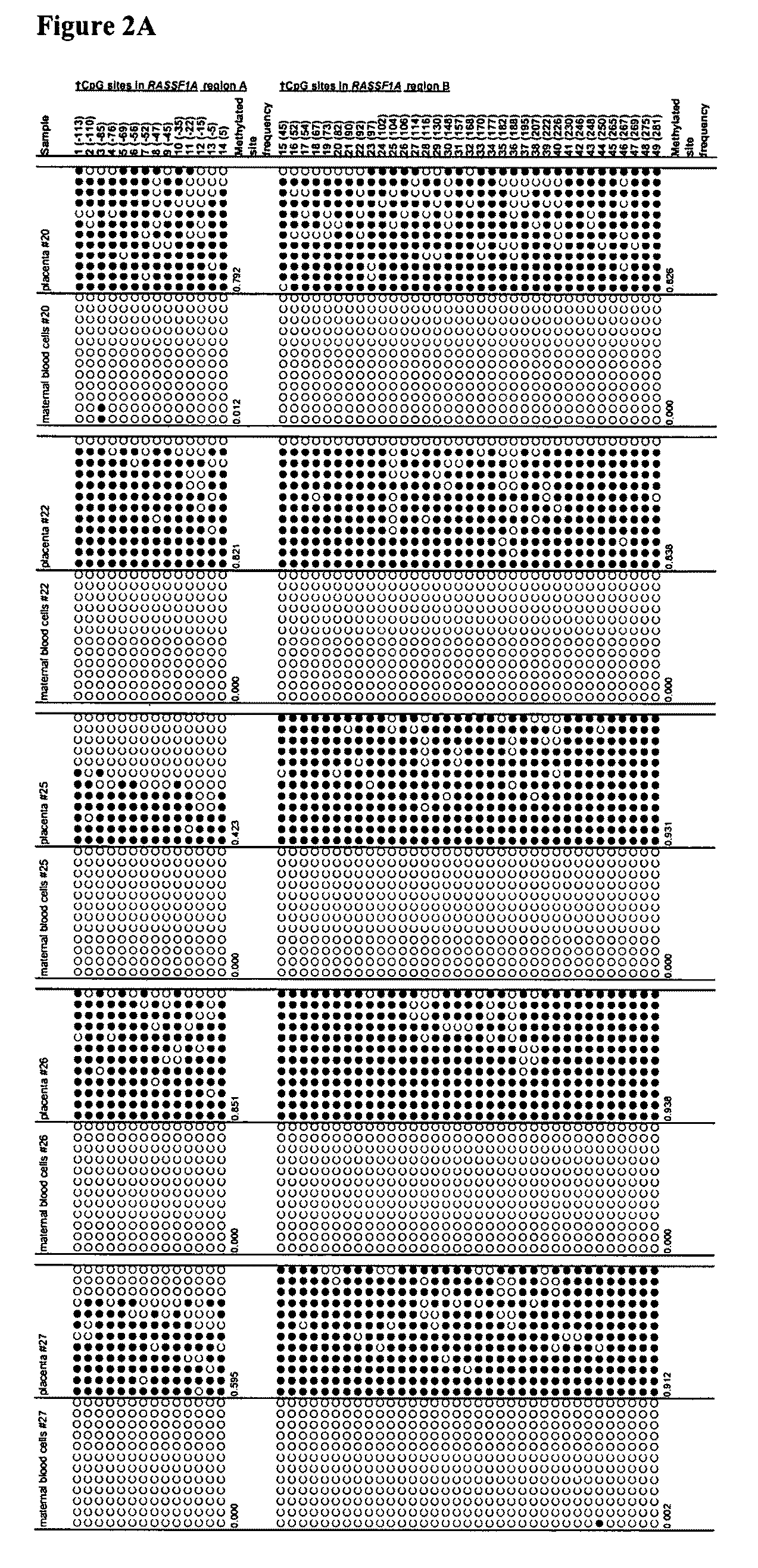
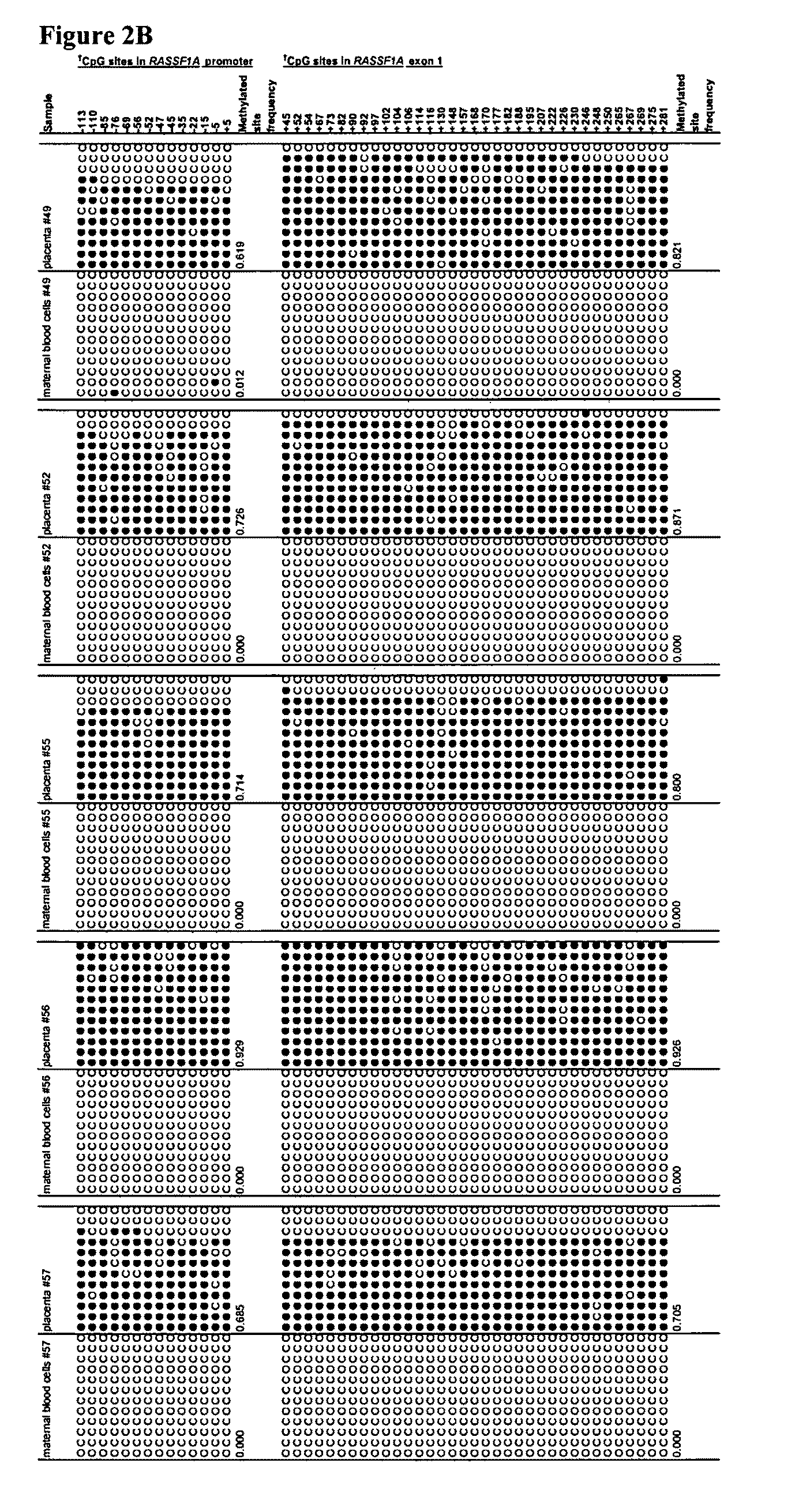
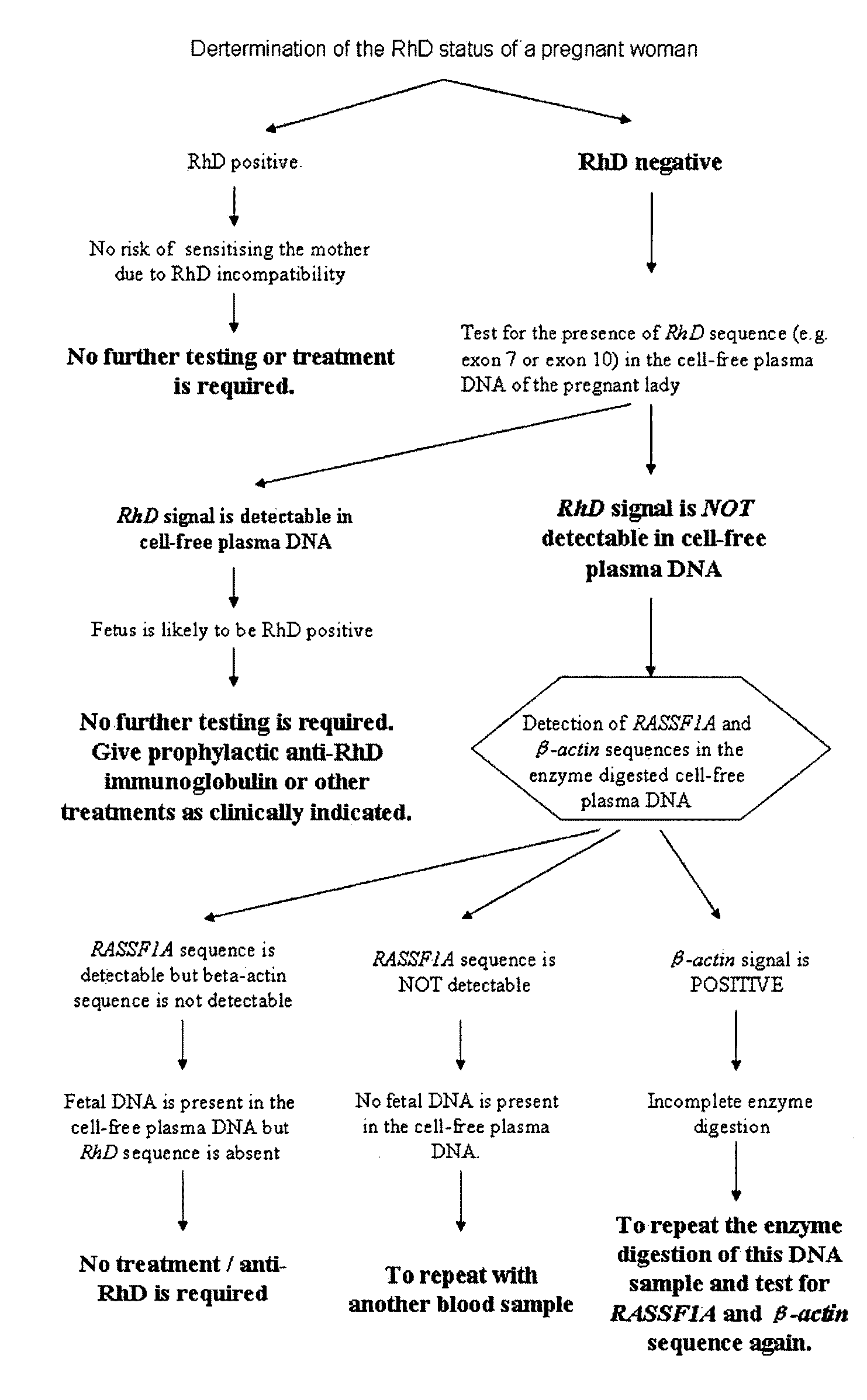
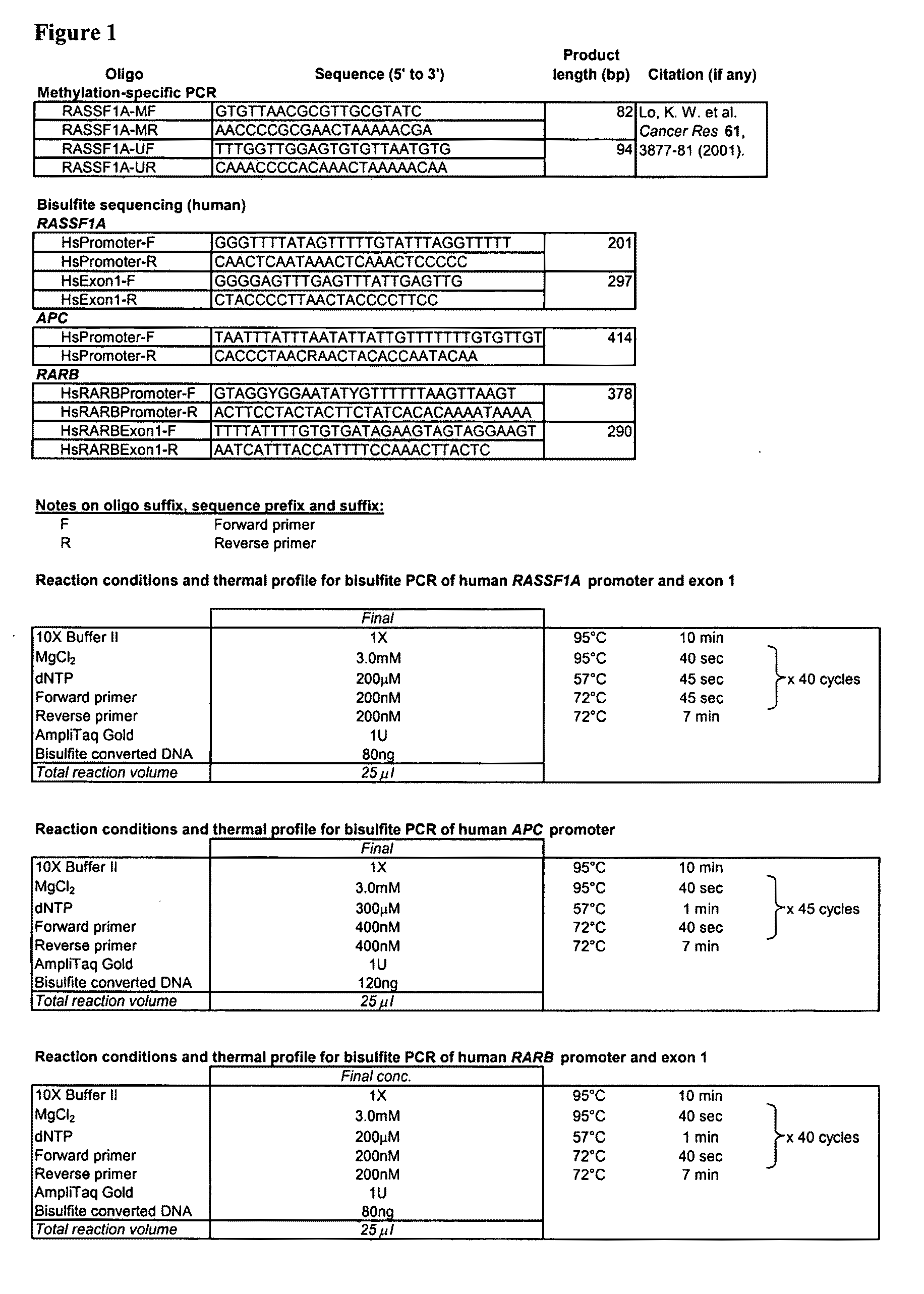
This application describes the discovery that, in a pregnant woman, certain genes (such as RASSF1A, APC, CASP8, RARB, SCGB3A1, DAB2IP, PTPN6, THY1, TMEFF2, and PYCARD) originated from a fetus are highly methylated, whereas the same genes of maternal origin are unmethylated. This discovery allows the easy detection of one or more of these methylated fetal genes in a biological sample from a pregnant woman, serving as a universal indicator of the presence of fetal DNA in the sample. These fetal methylation markers are particularly useful as positive controls for a non-invasive analytical process during which the quality and quantity of fetal DNA are monitored. These newly identified fetal markers can also be measured directly for diagnosis of certain pregnancy-related conditions.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Fetal methylation markers
ActiveUS20090155776A1Increased riskSugar derivativesMicrobiological testing/measurementASC proteinPositive control
This application describes the discovery that, in a pregnant woman, certain genes (such as RASSF1A, APC, CASP8, RARB, SCGB3A1, DAB2IP, PTPN6, THY1, TMEFF2, and PYCARD) originated from a fetus are highly methylated, whereas the same genes of maternal origin are unmethylated. This discovery allows the easy detection of one or more of these methylated fetal genes in a biological sample from a pregnant woman, serving as a universal indicator of the presence of fetal DNA in the sample. These fetal methylation markers are particularly useful as positive controls for a non-invasive analytical process during which the quality and quantity of fetal DNA are monitored. These newly identified fetal markers can also be measured directly for diagnosis of certain pregnancy-related conditions.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
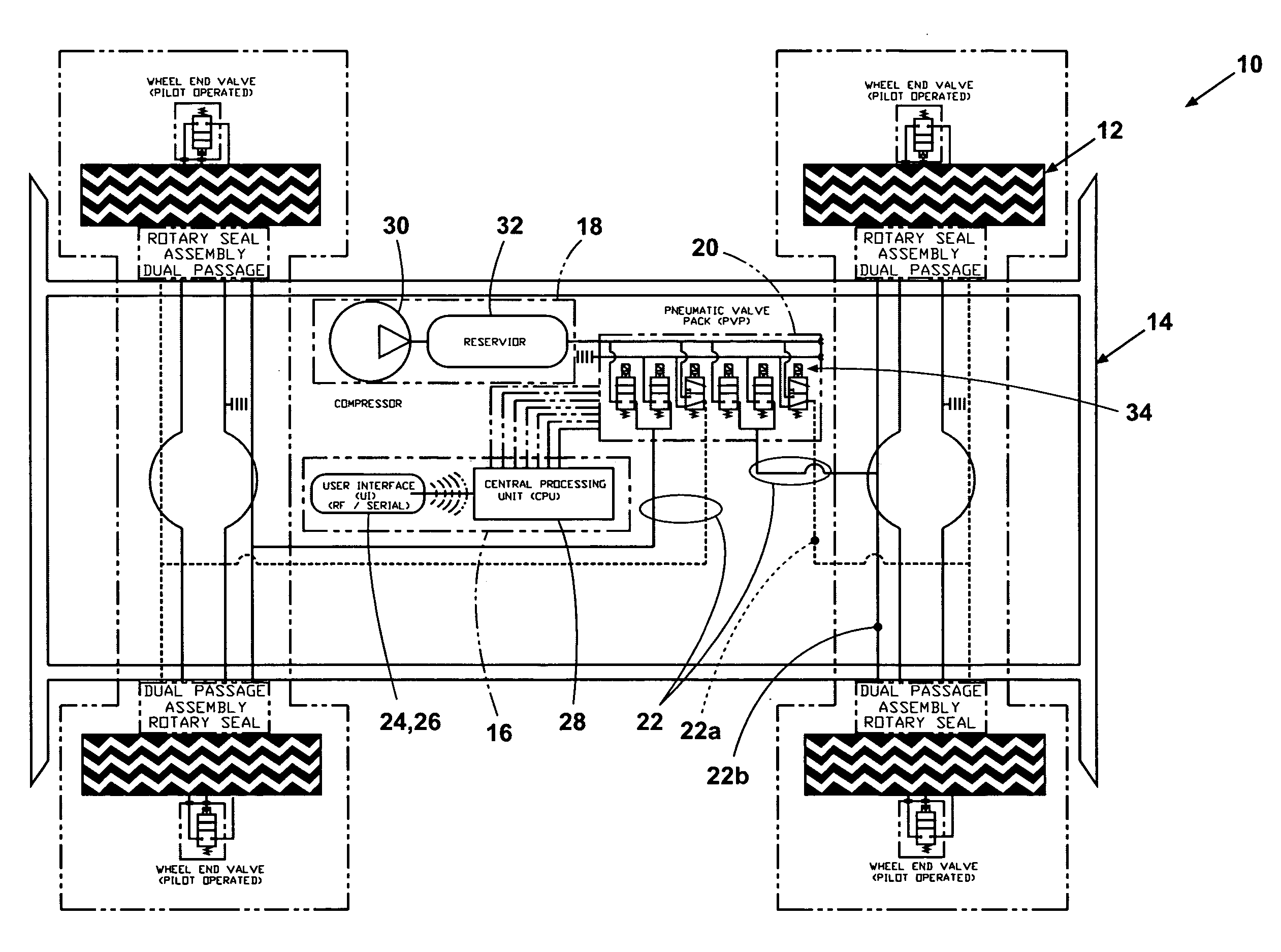
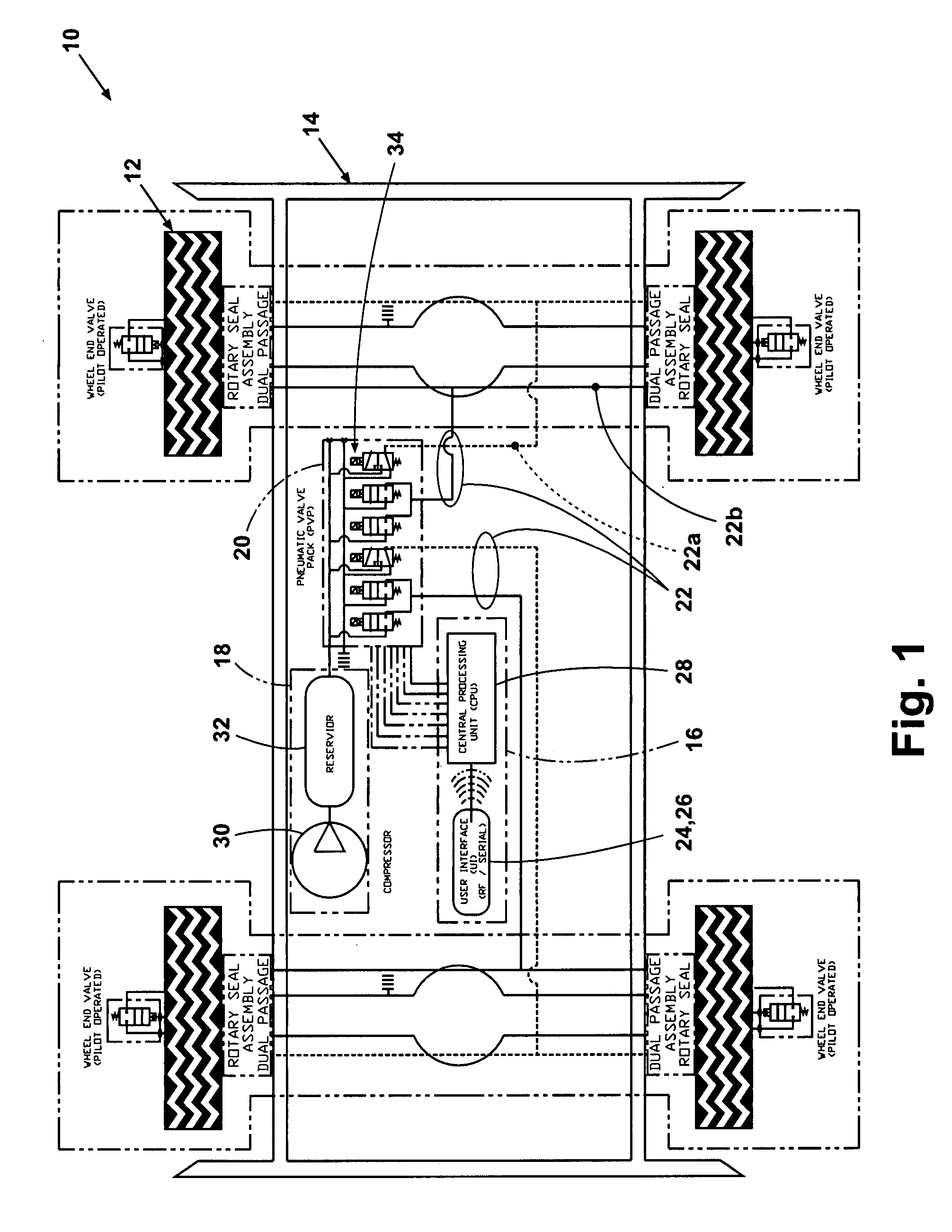
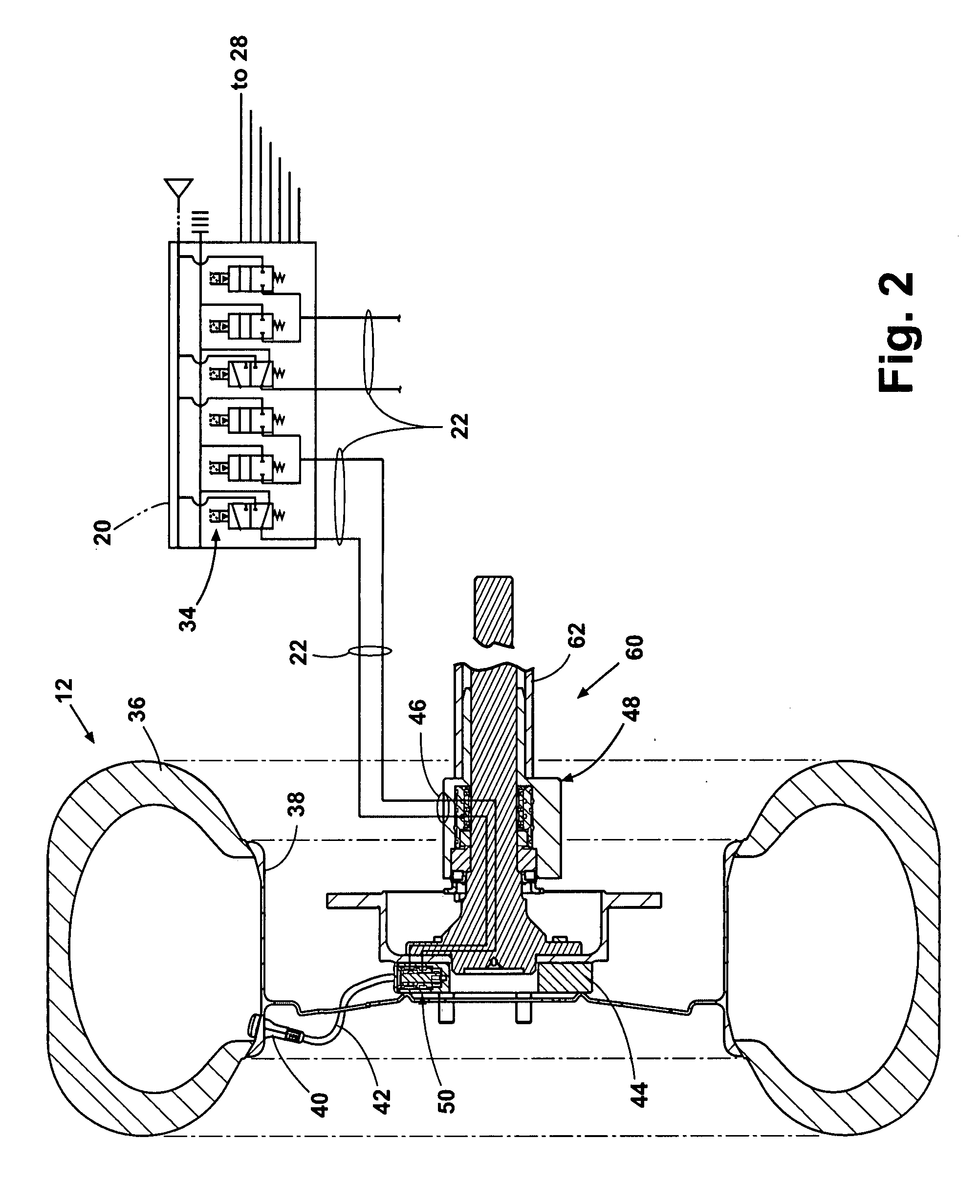
Tire inflation control method and apparatus
An apparatus and method are provided for controlling the air pressure within one or more tires on a vehicle. Means are provided for controlling air pressure dynamically or manually in response to road conditions. Instrumental in the invention is the ability to control the air pressure at each wheel from a remote location using positive control that substantially reduces the chance leaks anywhere else in the system would result in inadvertent flat tires.
Owner:KALAVITZ MICHAEL V
Virtuality reality type visible and controllable intelligent household control system and method
ActiveCN105955042AAvoid transmissionReal working conditionsComputer controlProgramme total factory controlPositive controlControl system
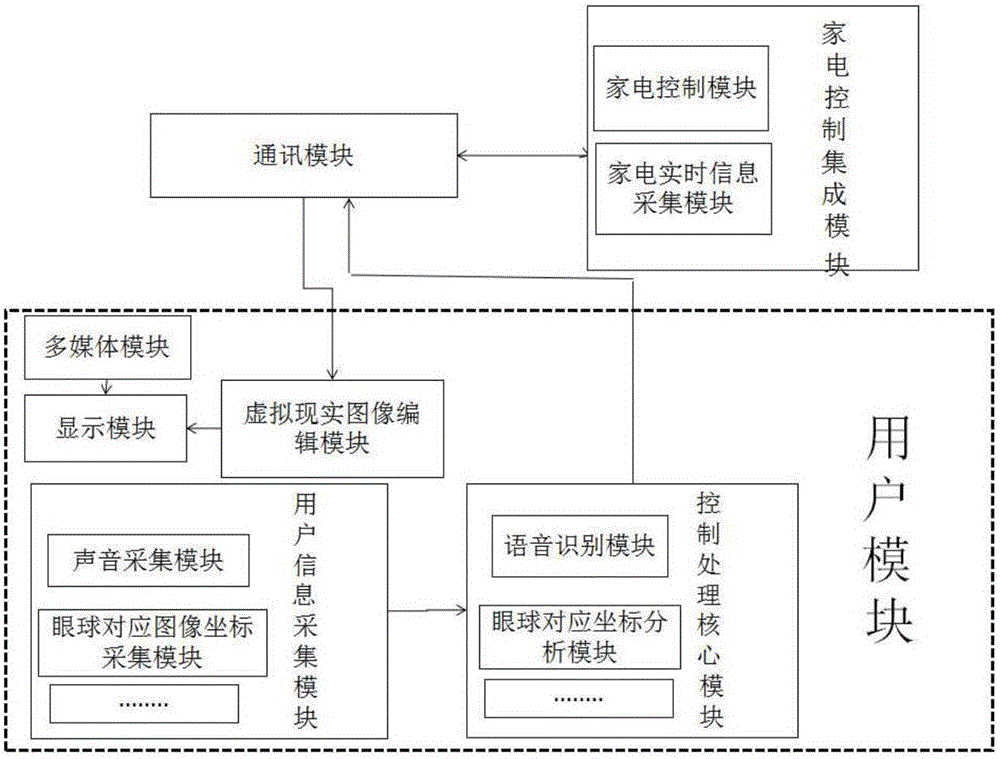
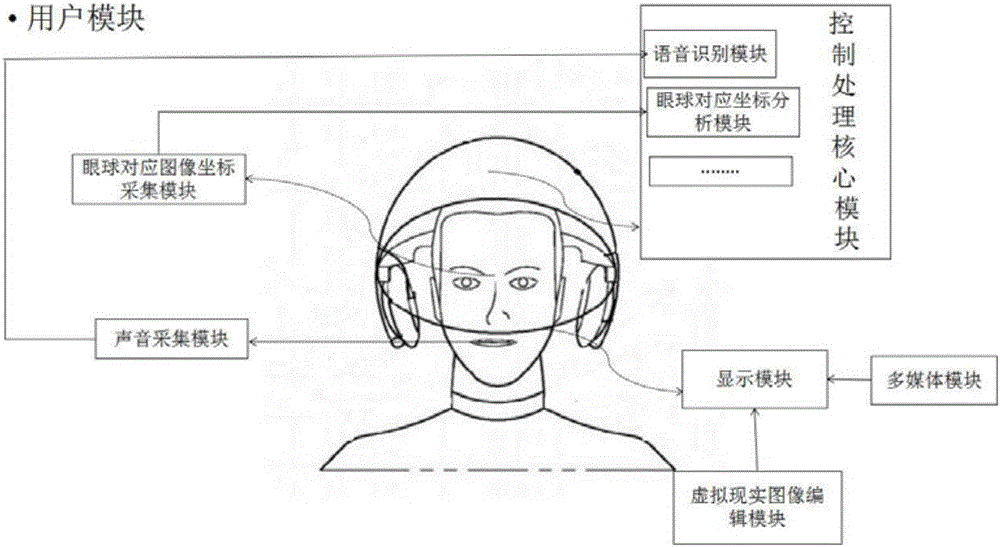
The invention discloses a virtuality reality type visible and controllable intelligent household control system and a method. A virtuality reality household image is constructed by a virtuality reality image edition module and is displayed on a display module, the user information is acquired by a user module, switching to a corresponding room virtuality reality image is carried out according to a room selected by a user, the command information is generated according to controlled household electrical appliance equipment of the room selected by the user and the command content of the user, the command information is transmitted by the user module to an integrated household electrical appliance control module, positive control on the controlled equipment is realized by the integrated household electrical appliance control module according to the command content, the state information of the equipment is read in real time and is fed back to the user module through a communication module, and a closed loop system is formed. Through the system, an integrated visible and controllable man-machine interaction control menu control mode is employed, the intelligent household electrical appliance equipment can be virtually controlled by the user like reality through seeing and thinking, and simplicity and humanization user control experience effects are realized.
Owner:ZHEJIANG UNIV
Curcumin cyclodextrin combination for preventing or treating various diseases
Curcumin has shown anti-inflammatory and anti-angiogenic properties that could be useful in treating various diseases such as those of rheumatology and oncology. However, curcumin is very poorly absorbed and has a very low bioavailability. This patent describes a method of increasing the delivery of curcumin by complexing it with cyclodextrins. Cyclodextrins are well known in the food industry and have been used to carry other drugs to increase bioavailability. The new combination of cyclodextrins and curcumin has been tested in pre-clinical inflammation models where it has demonstrated efficacy superior to both the positive control and curcumin.
Owner:DESAI KETAN
ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and detection method
InactiveCN102367478AIncreased sensitivityQuick checkMicrobiological testing/measurementViral OncogenePositive control
The invention relates to the field of molecular biology and aims to provide an ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and a detection method. The kit comprises a qPCR hybrid reaction solution, a locked nucleic acid retardant probe, a reference primer, an ARMS primer and a positive control sample, wherein the qPCR hybrid reaction solution comprises a PCR buffer solution, dNTPs (Deoxynucleotide Triphosphates), MgCl2, GoldStarbest Taq enzyme, a universal PCR reverse primer and a universal TaqMan probe. The kit provided by the invention can be used for rapidly and accurately detecting specific locus mutation of KRAS genes in various cancer tissues with high sensitivity, has high sensitivity, and can be used for detecting genome DNA with various tissue origins, specially free DNA segments adopting cell-free systems, such as blood serum and blood plasma, orother body fluid origins, wherein the genome DNA is derived from cell systems. Compared with direct sequencing and other mutation detection technologies, the kit and the detection method thereof havethe advantages of strong specificity, high sensitivity, simplicity and rapidness in operation, high throughput, safety, definiteness and objectivity in result identification and the like for detecting the KRAS gene mutation.
Owner:ZHEJIANG UNIV
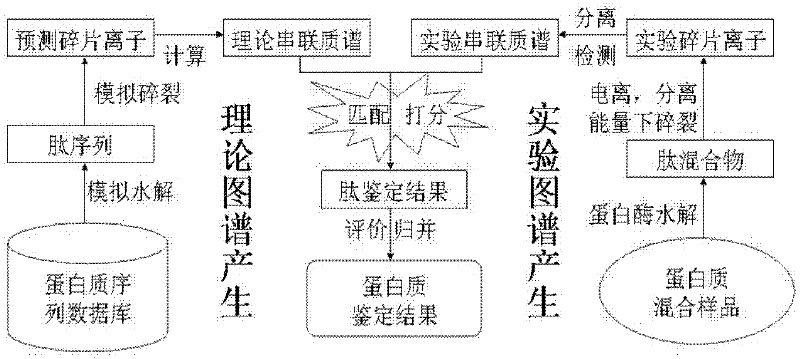
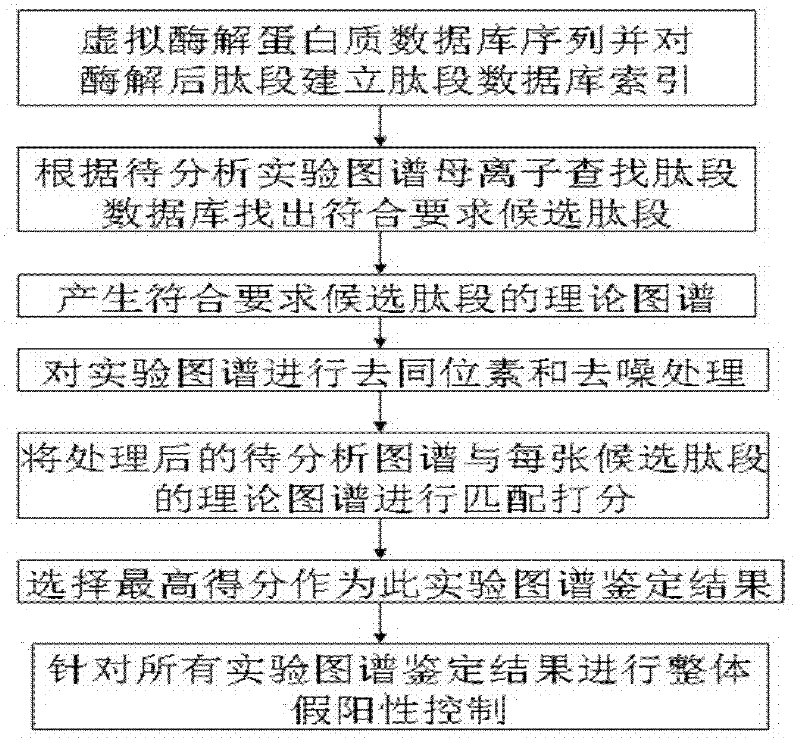
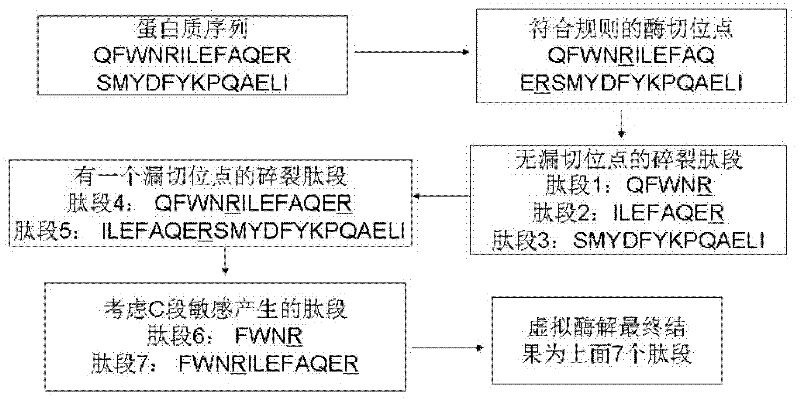
Protein secondary mass spectrometric identification method based on probability statistic model
InactiveCN102495127AMore identificationThe result of the identification method is excellentMaterial analysis by electric/magnetic meansProtein DatabasesMass number
The invention discloses a protein secondary mass spectrometric identification method based on a probability statistic model. The method comprises the following steps of: firstly, virtualizing an enzymolysis protein database array, and establishing a peptide section database and a peptide section database index for peptide sections processed by the enzymolysis according to the mass number of the peptide sections; secondly, finding out standby peptide sections meeting the requirements from the peptide section database according to a nuclear-cytoplasmic ratio of parent ions in an experiment map to be analyzed, and generating a theoretical map meeting the requirements by all the standby peptide sections; thirdly, removing isotopes and noises from the experiment map to be analyzed; matching the processed experiment map to be analyzed and the theoretical map of each standby peptide section and grading, and selecting the standby peptide section with the highest score as an identification result of the experiment map; and finally, carrying out whole false positive control according to all the experiment map identification results. According to the invention, the quantity of effective massspectrums and the quantity of the protein peptide sections are higher than those of an existing algorithm; and the method has the advantages of capability of dynamically selecting peaks and fast operation speed.
Owner:JINAN UNIVERSITY
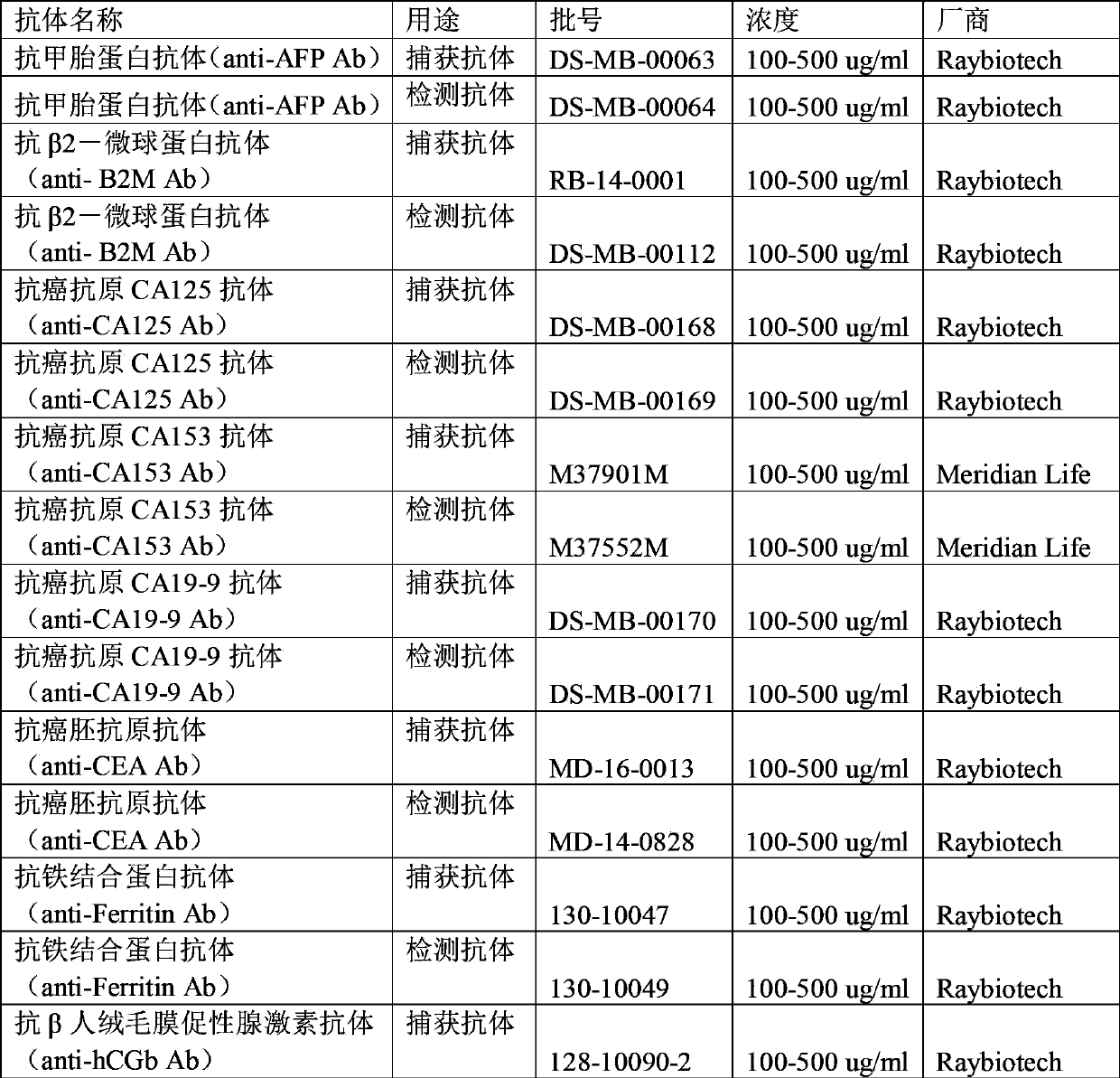
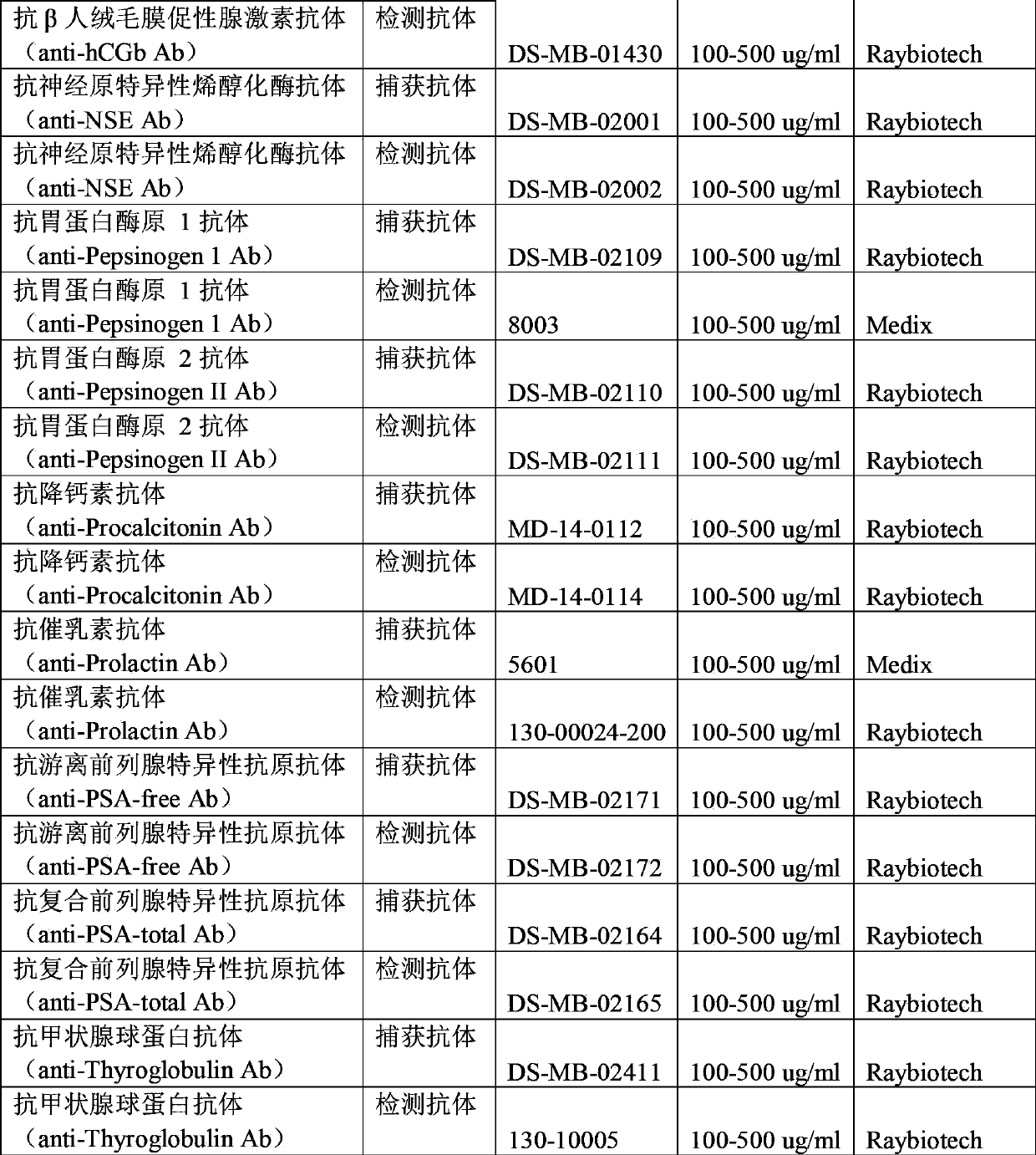
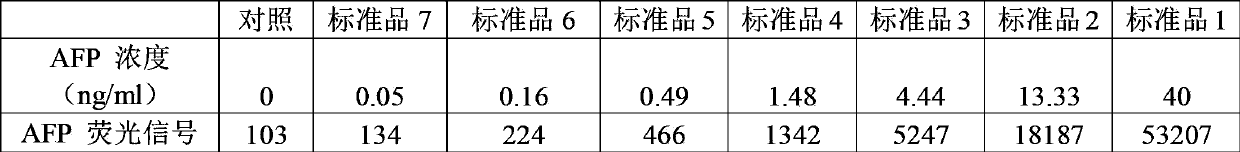
Antibody chip kit for diagnosis of various tumors
ActiveCN103869068AImprove adsorption capacityImprove stabilityMaterial analysisBiotin-streptavidin complexPositive control
The invention relates to an antibody chip kit for diagnosis of various tumors. The kit includes an antibody chip, a tumor marker standard substance mixture, a biotin-labeled tumor marker detection antibody mixture, and a fluorescein Cy3-labeled streptavidin, wherein the antibody chip includes a substrate, 16 kinds of tumor marker specific antibodies fixed on the surface of the substrate, and two kinds of positive controls, and the tumor marker standard substance mixture is a freeze-dried mixture obtained by mixing 16 kinds of standard tumor marker standard substances together according to a certain amount. The kit can detect 16 clinical commonly used tumor markers, overcomes the defects of complicated operation, single detection index, low sensitivity and the like in the prior art, has the advantages of being cheap, convenient, sensitive, accurate, high in throughput, and less in amount of samples, and can be popularized and large-scaled in common laboratories.
Owner:RAYBIOTECH INC GUANGZHOU +1
Controlled motion system
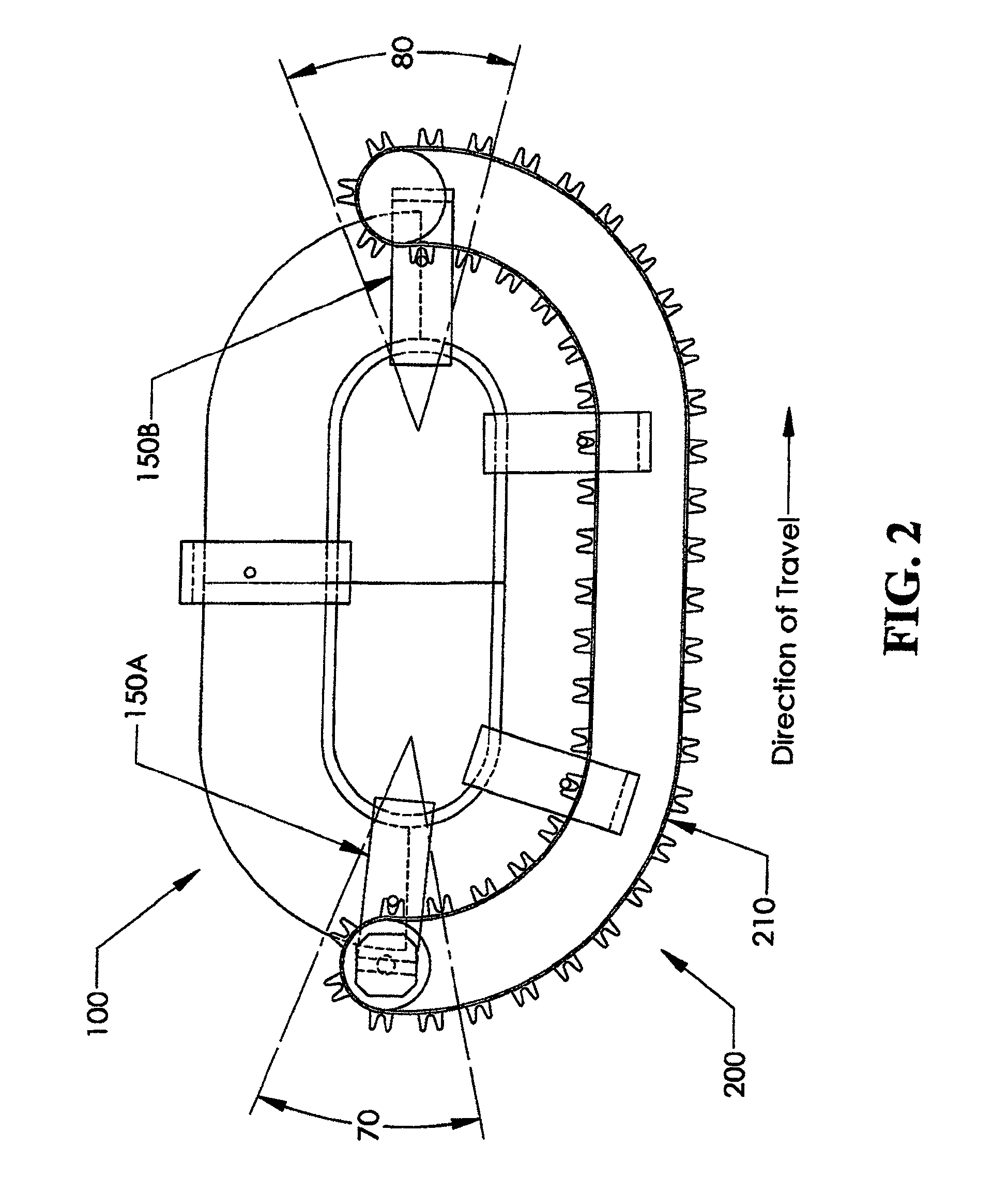
ActiveUS7859139B2Lower assembly costsMotor/generator/converter stoppersDC motor speed/torque controlPositive controlEngineering
A controlled motion system with movers mounted on a hybrid track system comprised of at least one smart section and at least one dumb section. The smart sections control each mover independently, while the dumb sections drive all movers at the same speed. The transition between these sections is characterized by positive control of the movers at all points in the transition. A soft magnetic composite core for the smart sections is disclosed. Also, a single-sided mover for smart sections that is constrained against loads in all direction, except for the direction of motion, is disclosed.
Owner:ROCKWELL AUTOMATION
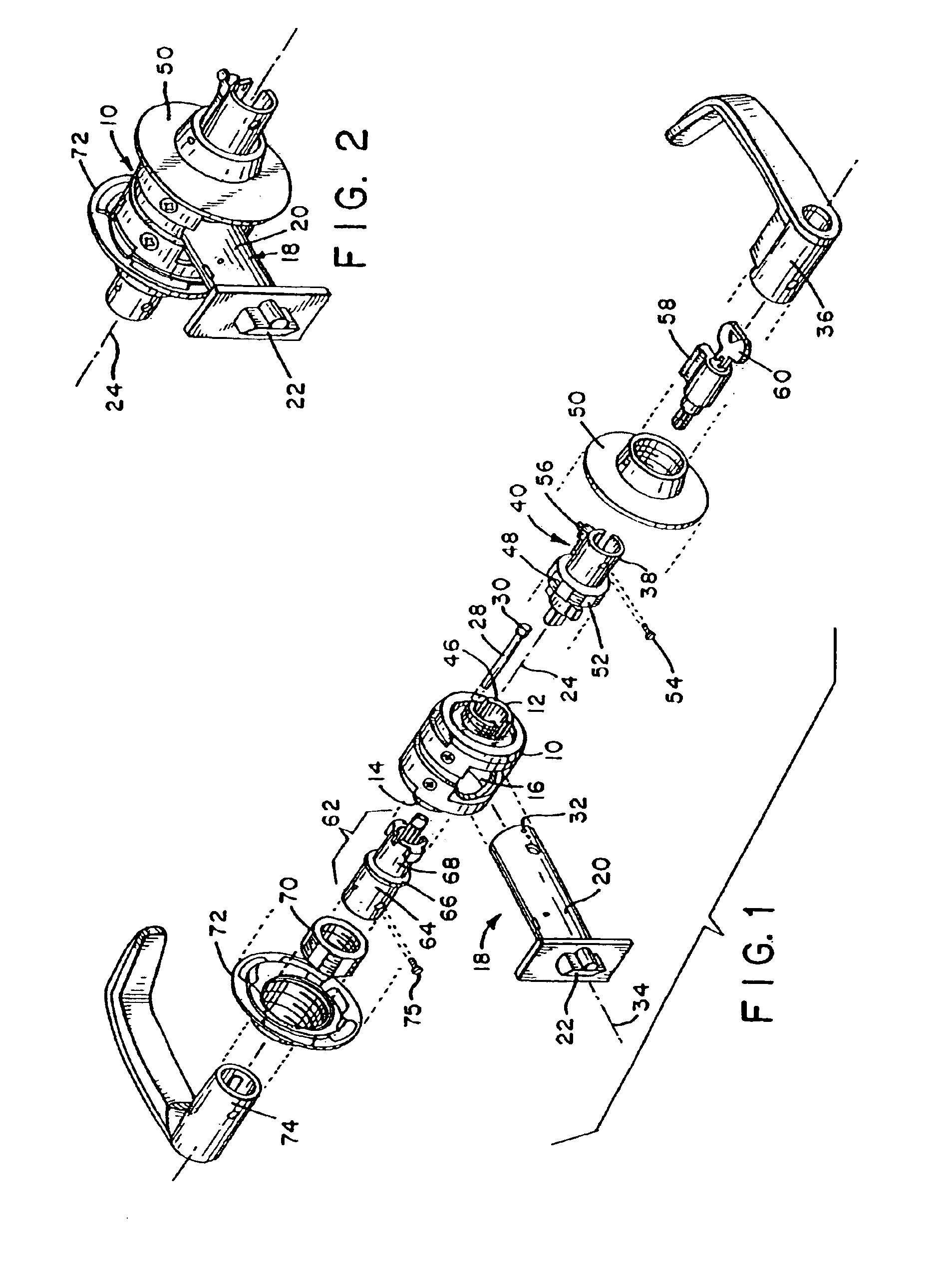
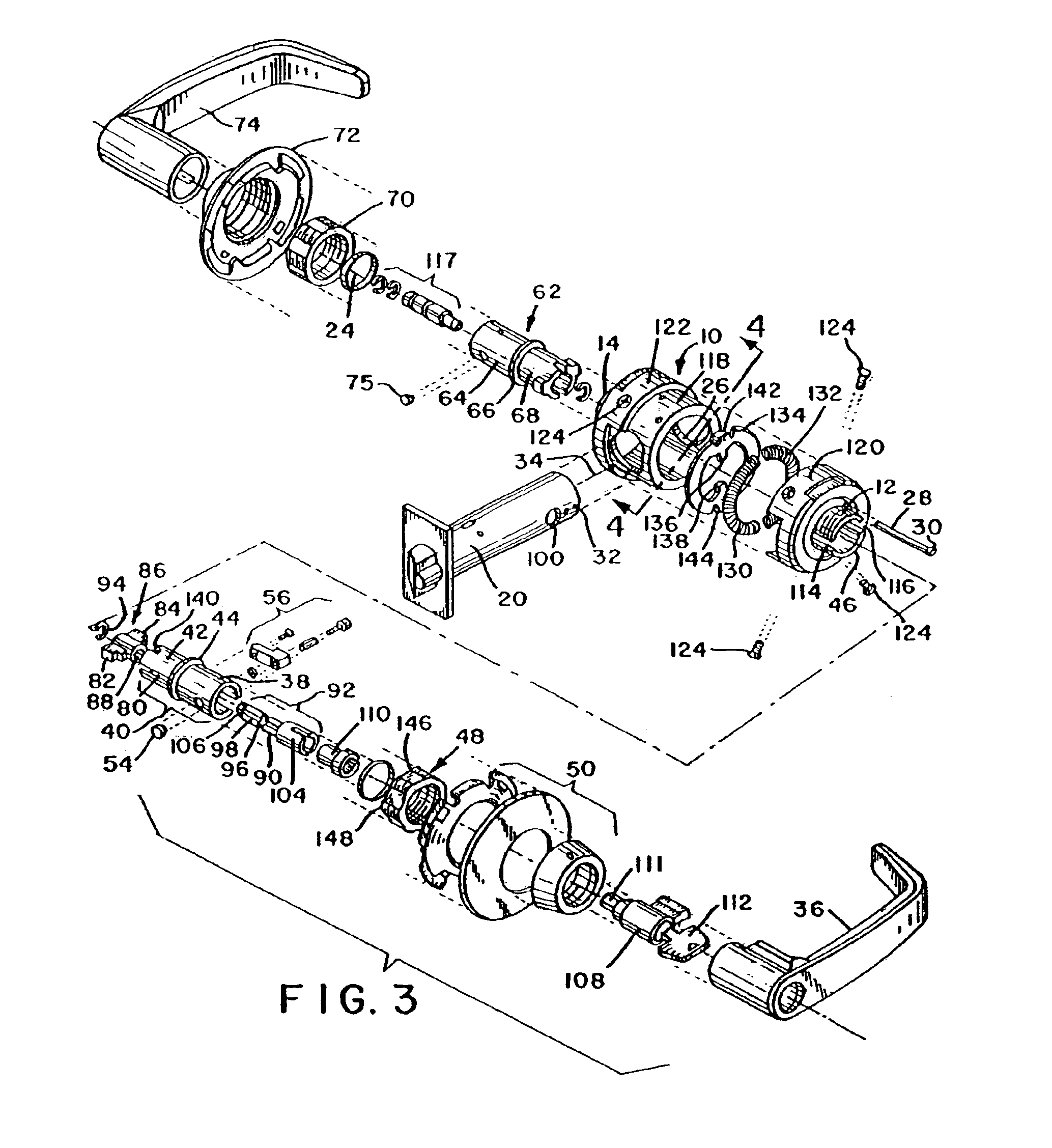
Security classroom function lock mechanism
InactiveUS6860129B2Sufficiently robustPrevent rotationAnti-theft cycle devicesWing handlesPositive controlLocking mechanism
Owner:SARGENT MANUFACTURING CO INC
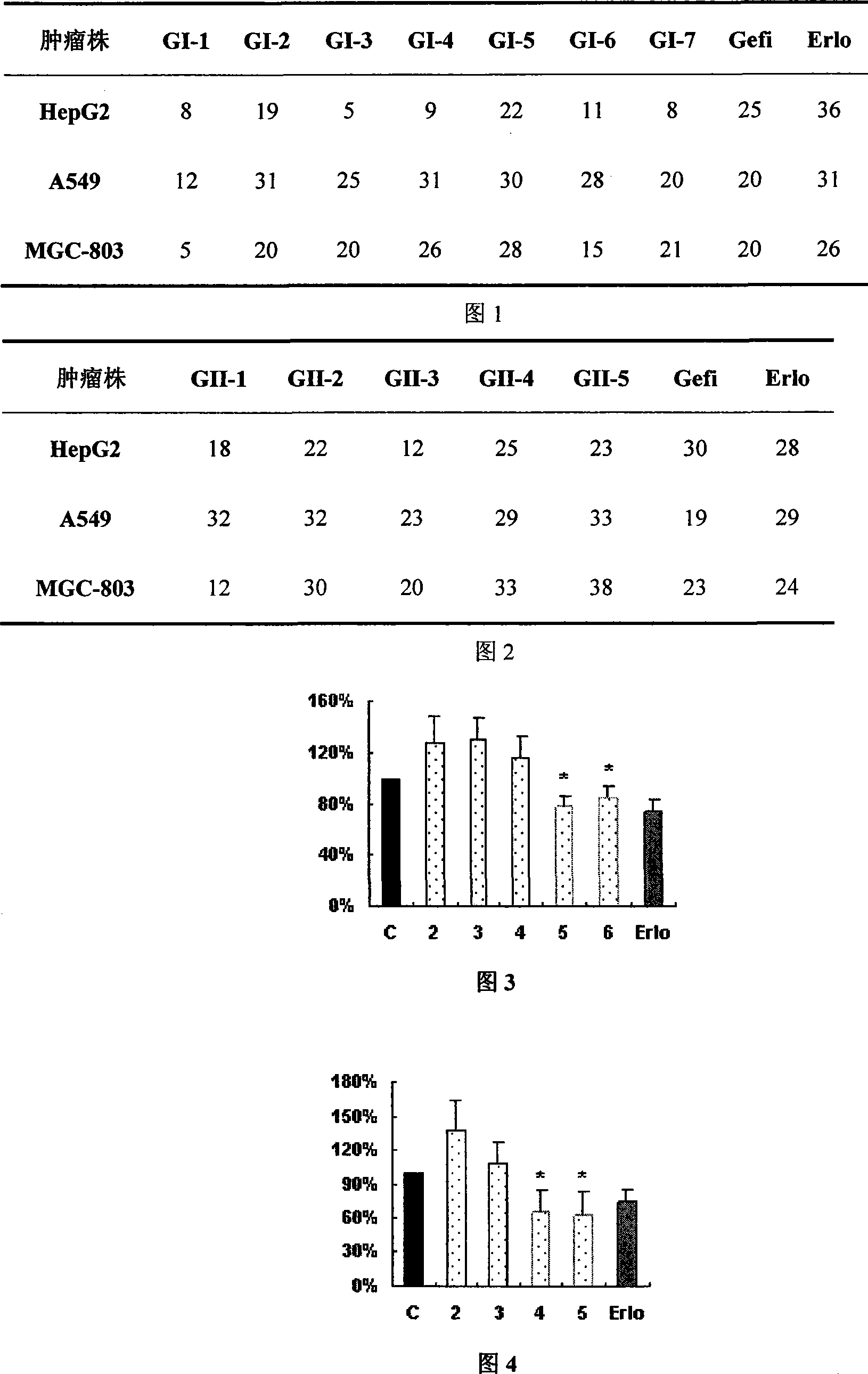
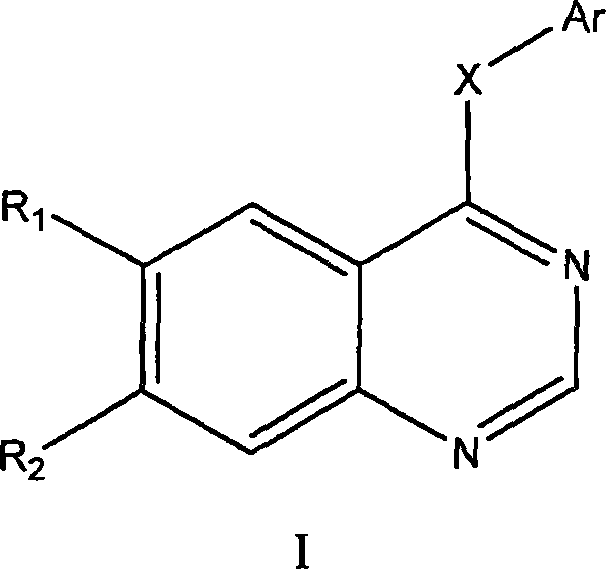
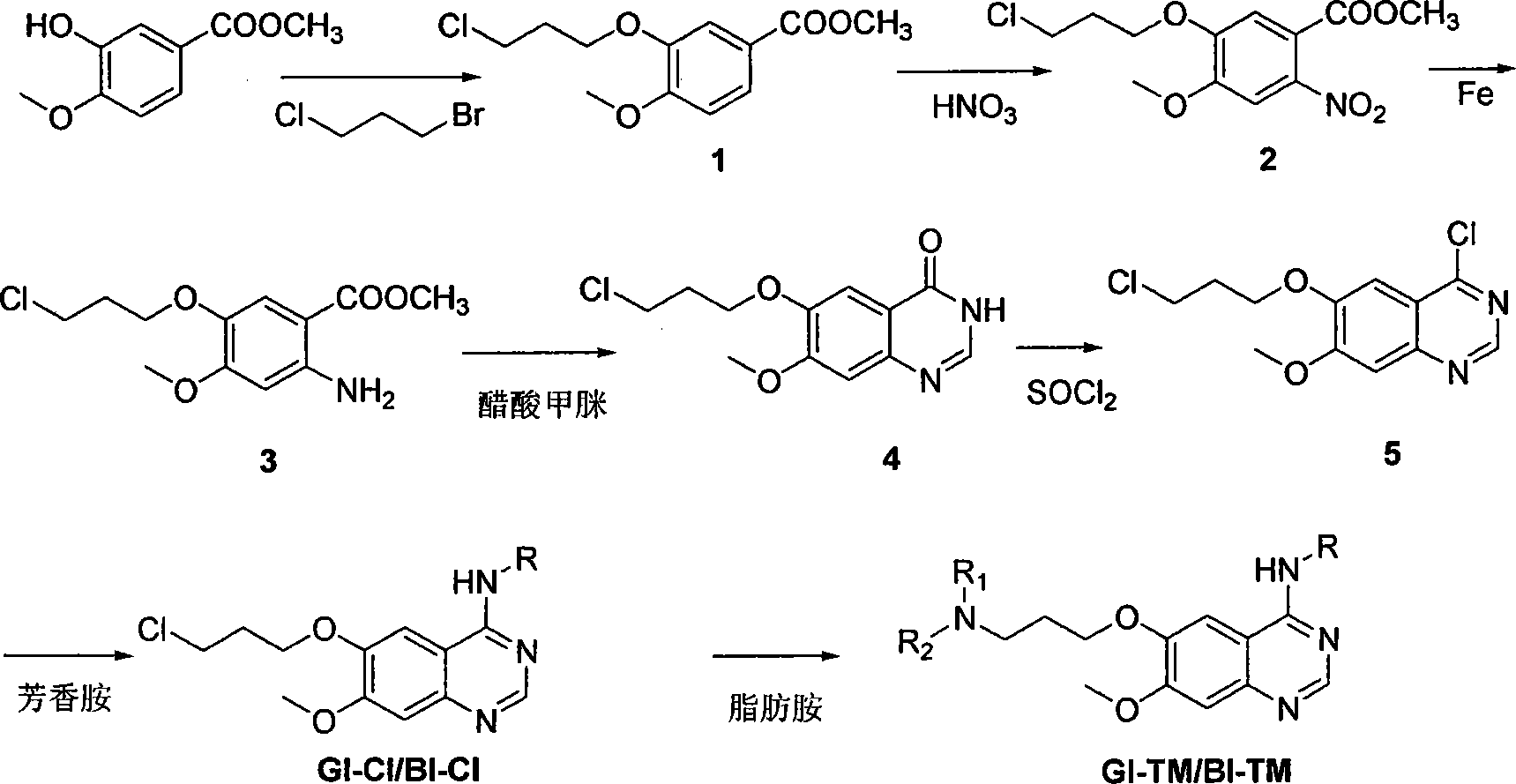
4- fragrant amido quinazoline derivatives and method of manufacturing the same and application in pharmacy thereof
The invention relates to a 4-aromatic aminoquinazoline compounds with general formula of (I) or a receivable salt pharmaceutically and the application in preparing the drugs for treating the tumour. Wherein, R1, R2 are hydrogen, halogen, nitryl, amino, alkyl of C1-6, alkylamino of C1-6 or alkoxy of C1-6, or the group replaced by the alkylamino on the terminal of the alkyl of C1-6, alkylamino and alkoxy; X is N,O or S; Ar is aromatic ring or heteroaromatic groups replaced by the ester of C5 to C8. The invention can inhibit the increase of the tumour cell obviously and has obvious inhibited effect on the tyrosine kinase of the cell, and has activity equal to that of the positive drugs Gefitinib and Erlotinib and even exceeds the positive control.
Owner:SOUTHEAST UNIV
Fluorescence quantitative PCR detection kit of hepatitis B virus and application thereof
ActiveCN101701267AStrong specificityHigh purityMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention discloses a fluorescence quantitative PCR detection kit of hepatitis B virus and an application thereof. The kit is composed of the following independent components: DNA extraction solution I, DNA extraction solution II, DNA extraction solution III, DNA extraction solution IV, positive control interior label, PCR reaction liquid, probe HBV-SP, enzyme mixed liquor containing heat resistant DNA polyase and uracil DNA glycosylase, quantitative hepatitis B virus reference material, hepatitis B virus positive control serum and hepatitis B virus negative control serum, wherein DNA extraction solution I contains 0.2-1.0% of lauryl sodium sulphate (mass / volume), 1.0-4.0% of Triton (volume / volume) and 0.2-1.0mol / L of guanidinium isothiocyanate; DNA extraction solution II contains 100-300mmol / L of 4-HEPES, 100-300mmol / L of sodium chloride with pH of 6.5+ / -0.2 and 100-400 mu g / ml of magnetic beads; DNA extraction solution III contains 0.1-1.0% of Triton (volume / volume) and 100-300mmol / L of sodium chloride; DNA extraction solution IV contains mineral oil. The fluorescence quantitative PCR detection kit of hepatitis B virus of the invention can be used for detecting the HBV-DNA concentration in samples of serum, blood plasma or latex and the like.
Owner:SANSURE BIOTECH
Monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and method for detecting nonstructural protein (NSP) antibody of foot-and-mouth disease virus (FMDV)
The invention discloses a monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and a method for detecting the nonstructural protein (NSP) antibody of a foot-and-mouth disease virus (FMDV) (FMD NSP B-ELISA); the kit comprises ELISA reaction plates, serum diluent, 25 times concentrated detergent, substrate solution, 100* concentrated ELISA detecting antibody, stop buffer, positive control serum and negative control serum; the ELISA reaction plates are two 96-pore high-affinity ELISA reaction plates, firstly 6* groups of amino acid monoclonal antibody or NSP 2C polyclonal antibody, and then FMDV 3ABC or 2C3AB NSP which is expressed by pronucleus and is provided with 6* groups of amino acid labels is captured through the monoclonal antibody or the polyclonal antibody; and compared with other similar kits, the method has higher coincidence rate and higher positive serum detection rate, and is applicable to detecting the serum of cattle, sheep, pigs and other susceptible animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
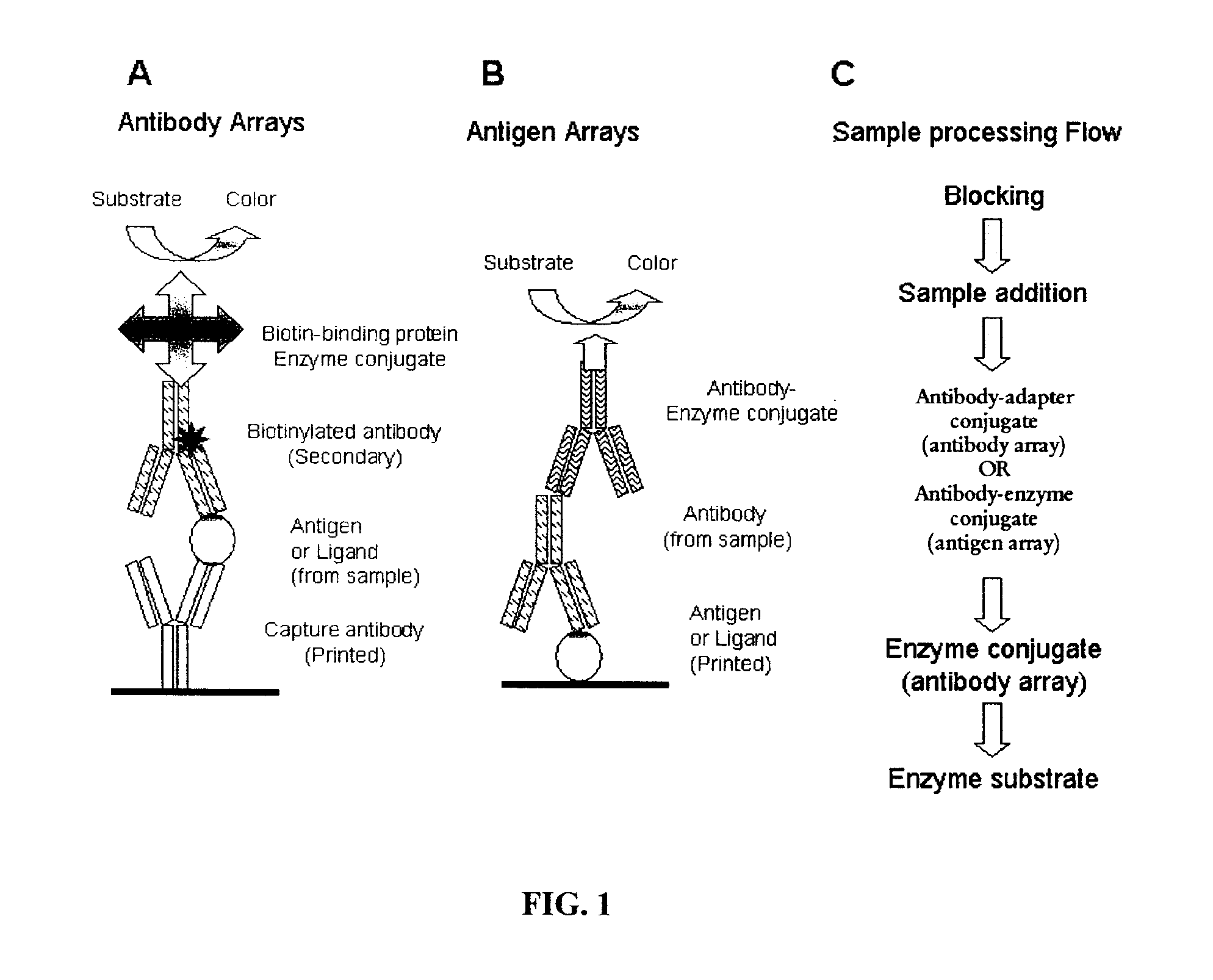
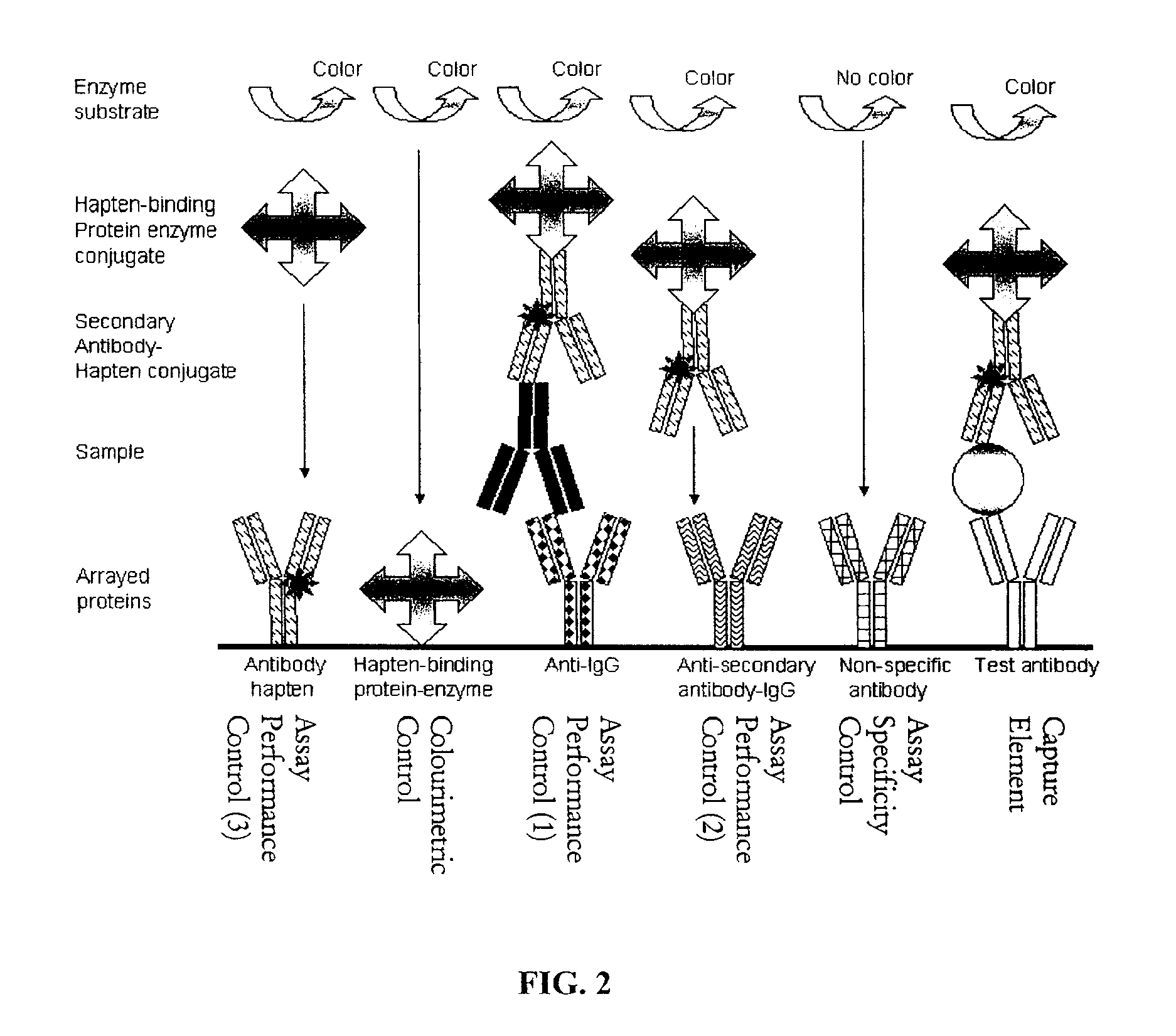
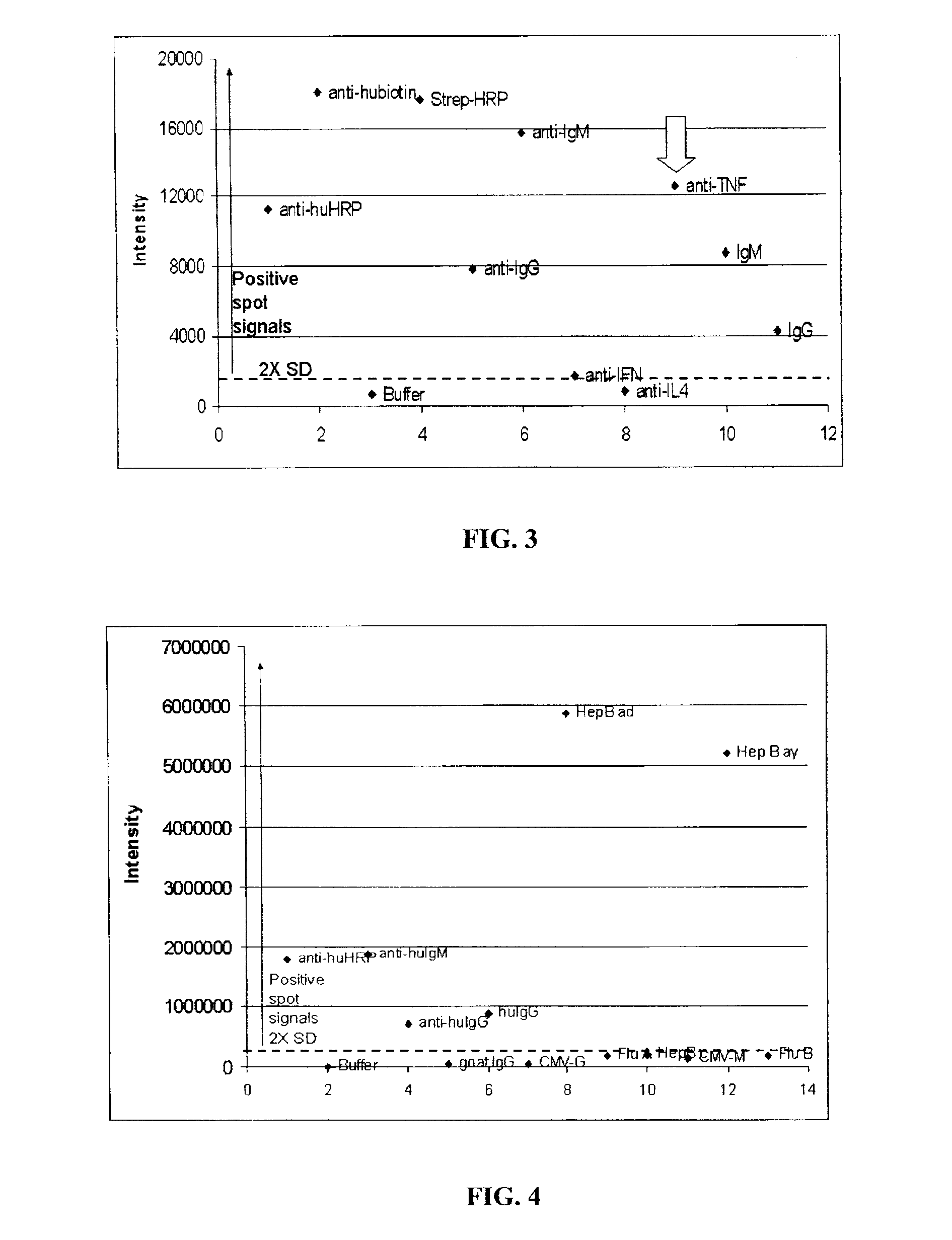
Assay Membrane and Method of Use Thereof
The present invention provides a microporous membrane for detecting at least one target analyte in a sample. The membrane includes an array that comprises at least one capture element and the at least one control element printed on the membrane surface, the at least one capture element corresponding to and being able to bind a target analyte, the plurality of control elements, when present including: i) at least one fiduciary marker, ii) at least one negative control to monitor background signal, iii) at least one negative control to monitor assay specificity, iv) at least one positive colorimetric control, v) at least one positive control to monitor assay performance or any combination thereof.
Owner:PICTOR
Controlled motion system
ActiveUS8896241B2Lower assembly costsIncreasing and decreasing speedMotor/generator/converter stoppersDC motor speed/torque controlPositive controlControl system
A controlled motion system having a plurality of movers controlled as they travel along both smart and simple sections of a track. The controlled motion system comprises a control system for controlling the speed of a mover as it travels along a simple section, and permits the pitch or distance between movers to increase or decrease as they travel along a simple section. In a preferred embodiment the controlled motion system includes at least one coupling feature having a driving feature on a simple section for engaging and operably driving a driven feature on each mover such that positive control of each mover is maintained throughout its transition from a smart section to a simple section.
Owner:ROCKWELL AUTOMATION
Fourteen-food-borne pathogenic bacterium multiplex PCR detection primer set and kit
ActiveCN103484546ATo achieve the detection effectMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesEscherichia coli
The invention discloses a fourteen-food-borne pathogenic bacterium multiplex PCR detection primer set and a kit. The detection primer set is composed of primer pairs for detecting salmonella, Shigella, Vibrio parahemolyticus, campylobacter jejuni, campylobacter coli, staphylococcus aureus, bacillus cereus, Listeria monocytogenes, yersinia enterocolitica, enterobacter sakazakii, Escherichia coli, vibrio cholerae, Escherichia coli O157, aeromonas hydrophila and internal positive control. A multiplex PCR detection method based on an ordinary PCR platform is built, multiplex PCR reactions are carried out on genomic DNA, extracted from a sample to be tested, of bacteria in the same reaction system through the primer pairs acquired through analysis and design, and whether the food-borne pathogenic bacteria are contained in the sample or not is judged through the electrophoretic analysis of reaction products.
Owner:北京卓诚惠生生物科技股份有限公司
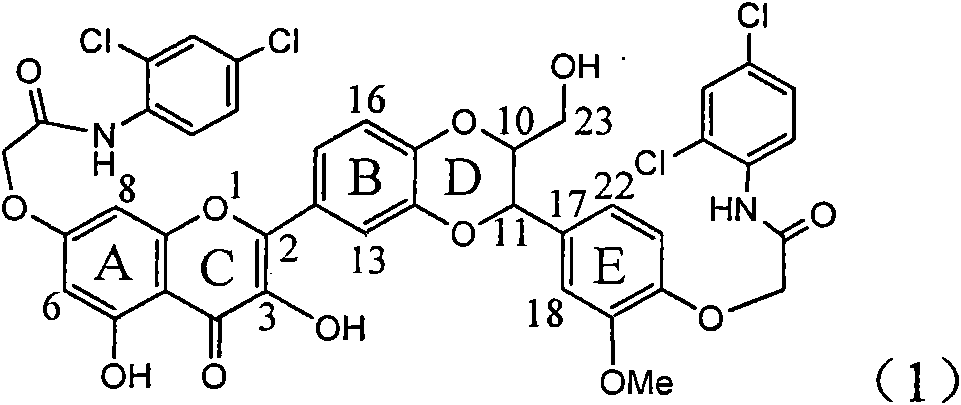
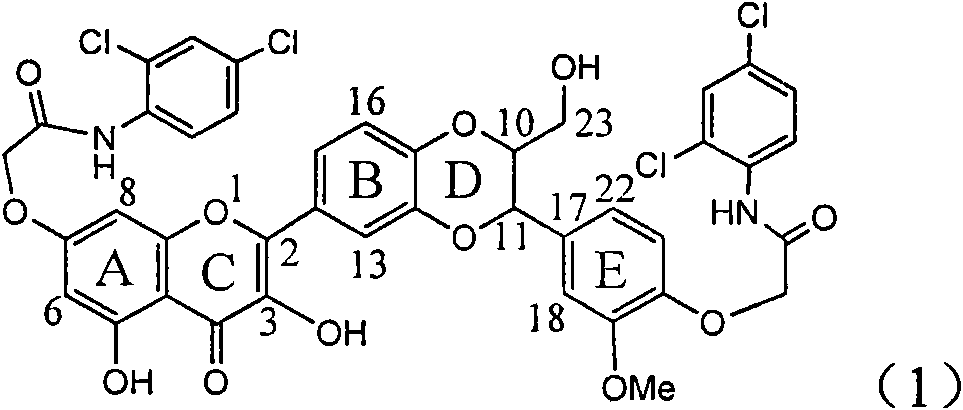
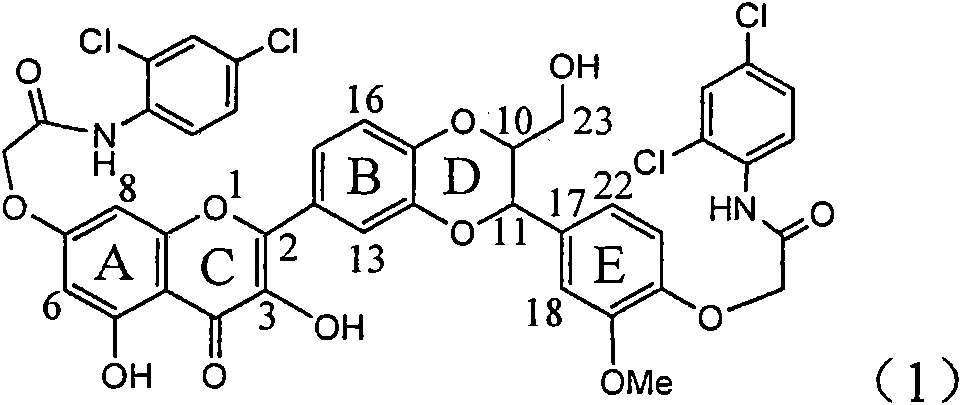
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of flavonoid quercetin dimmer as medicament for treating viral hepatitis B
InactiveCN101829103AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to the application of flavonoid quercetin dimmer as the medicament for treating viral hepatitis B, in particular to the application of flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof to the preparation of the medicament for eliminating HBsAg and HBeAg and inhibiting HBV DNA replication. The flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof has obvious HBsAg and HBeAg inhibiting activity, and at the concentration of 100mcg / ml, the flavonoid quercetin dimmer pharmaceutically acceptable salt thereof has the HBsAg eliminating strength of 65.7% and the HBeAg eliminating strength of 44.8% which are respectively 4.1 times and 2.7 times higher than the positive control medicament of Alpha-interferon and has the HBV DNA inhibiting ratio of 44.8% which is 117% of the HBV DNA inhibiting ratio of the Alpha-interferon at the highest test concentration. Therefore, the flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof can be expectedly used for preparing the non-nucleoside medicament for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating viral hepatitis B.
Owner:DALI UNIV
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
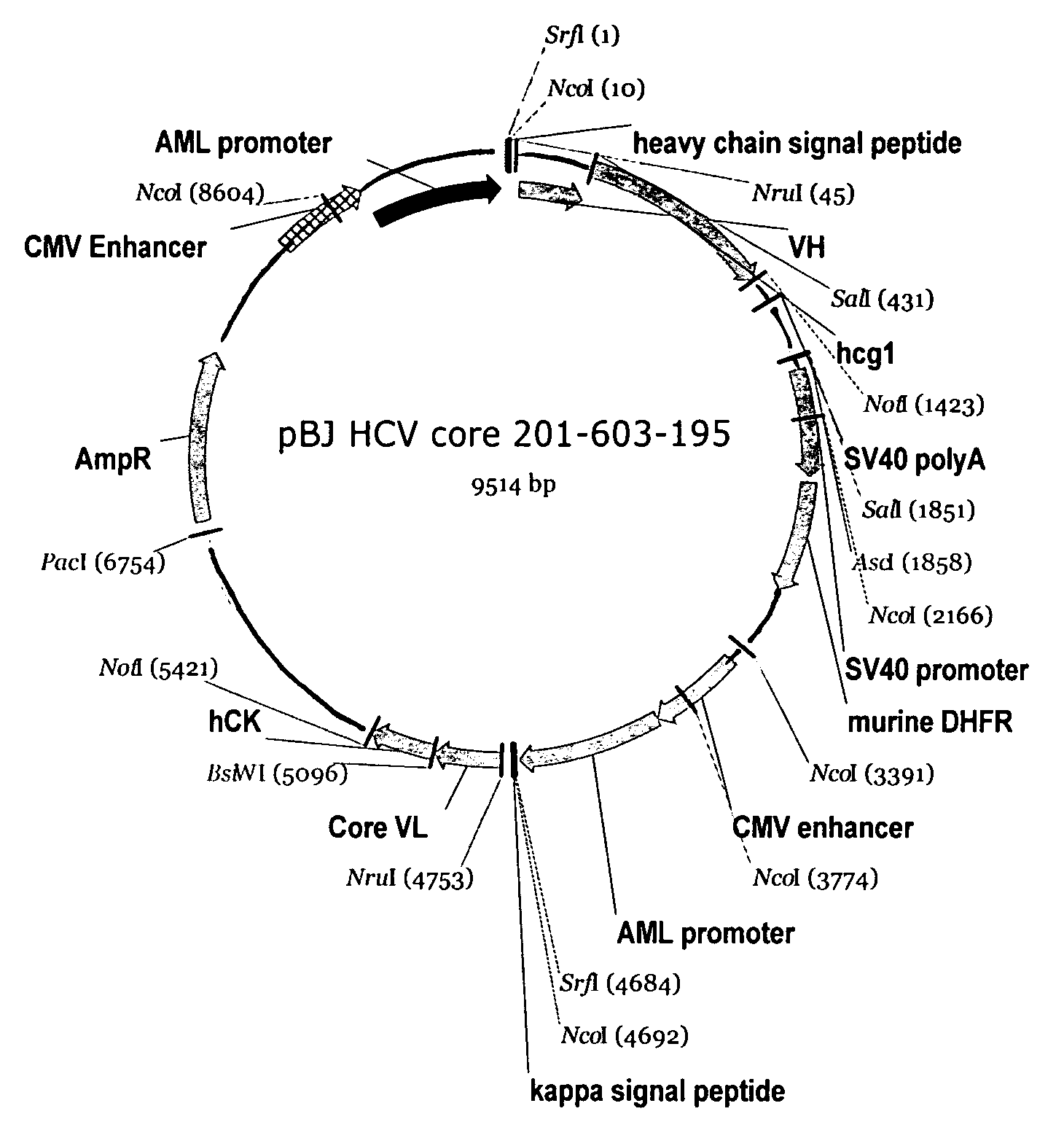
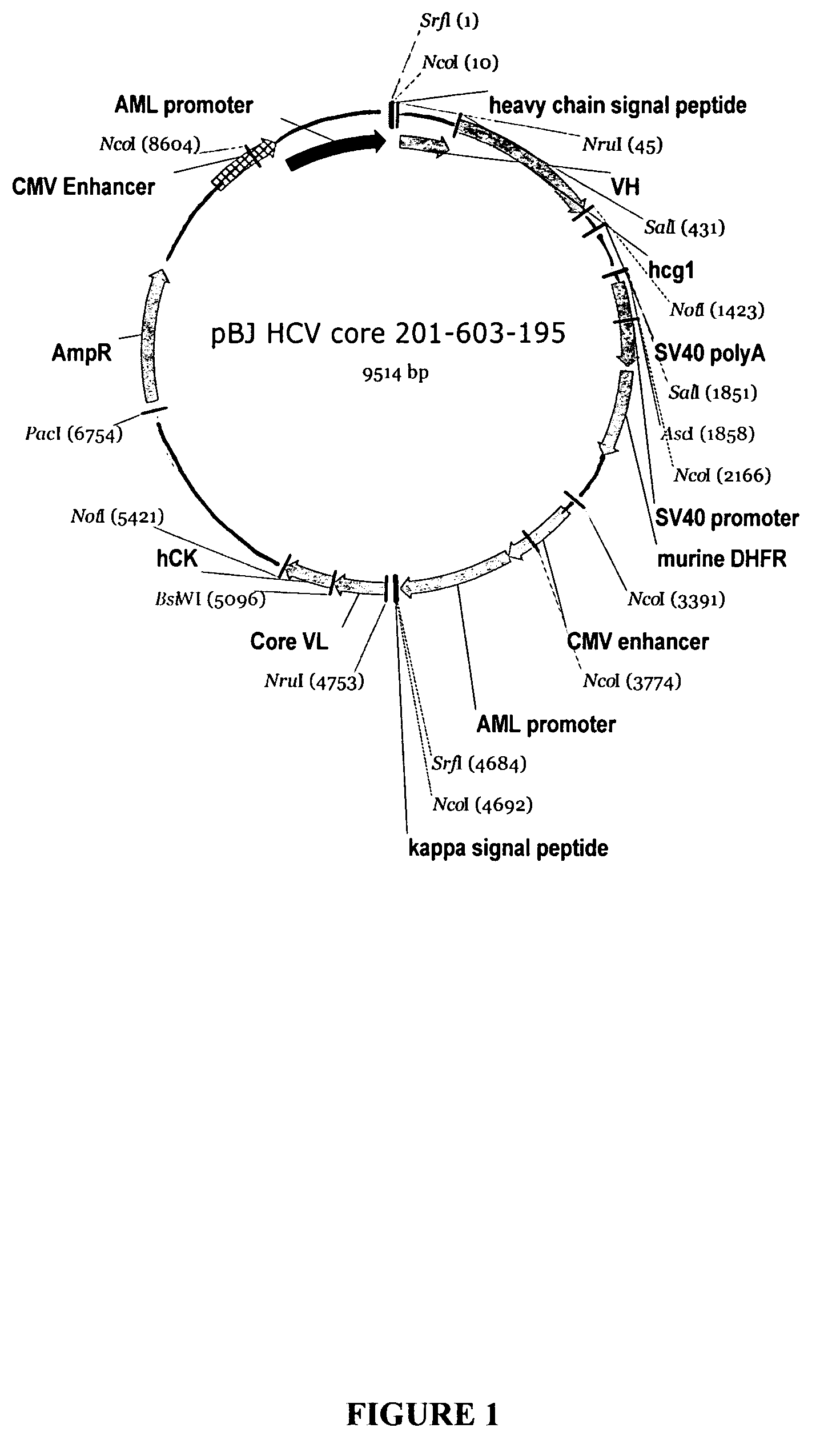
Recombinant antibodies against hepatitis C virus and methods of obtaining and using same
Recombinant antibodies, including chimeric antibodies, specific for hepatitis C (HCV) antigenic proteins are provided. The recombinant antibodies specifically bind to diagnostically relevant regions of HCV proteins and are thus suitable for use, for example, as diagnostic reagents for the detection of HCV, and / or as standardization reagents or positive control reagents in assays for the detection of HCV. The recombinant antibodies can also be used in the treatment or prevention of a HCV infection.
Owner:ABBOTT LAB INC
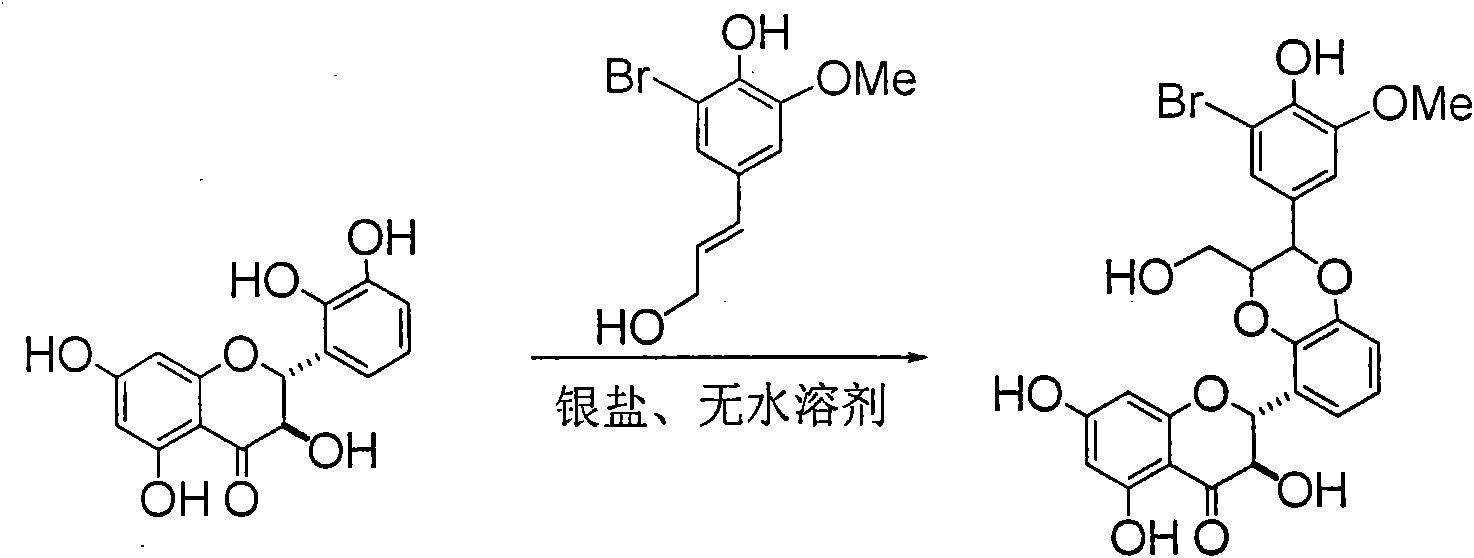
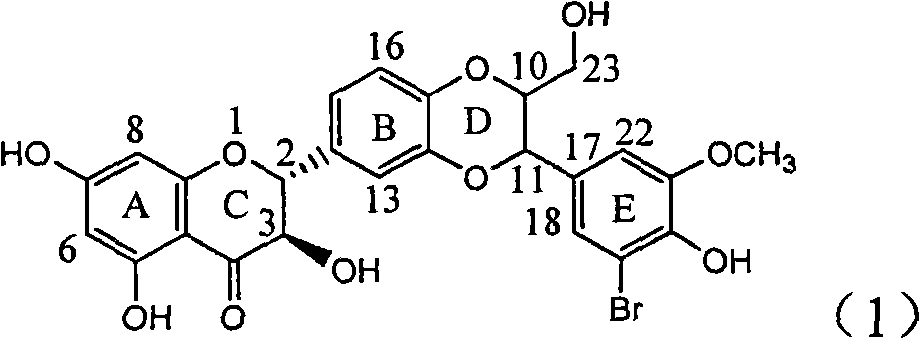
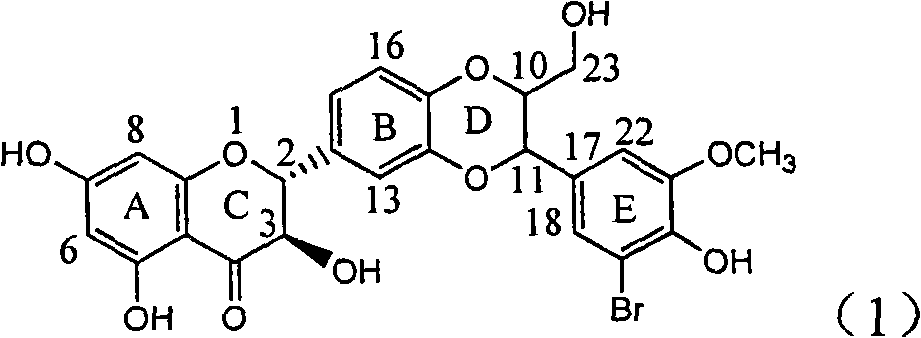
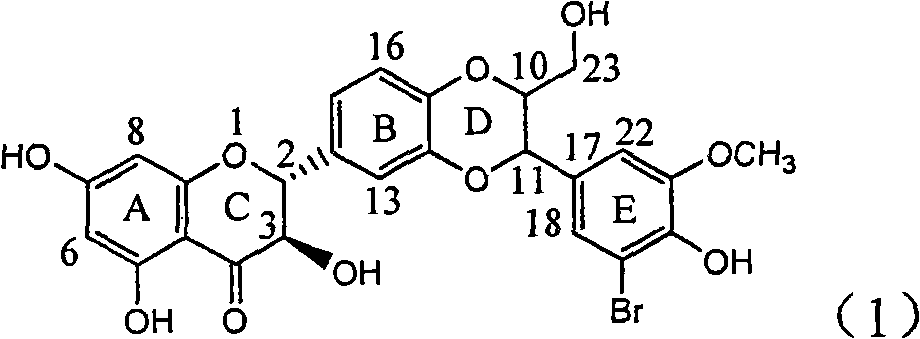
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
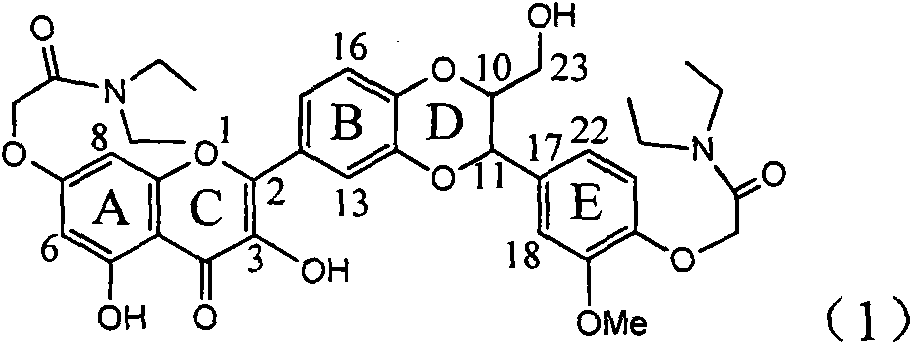
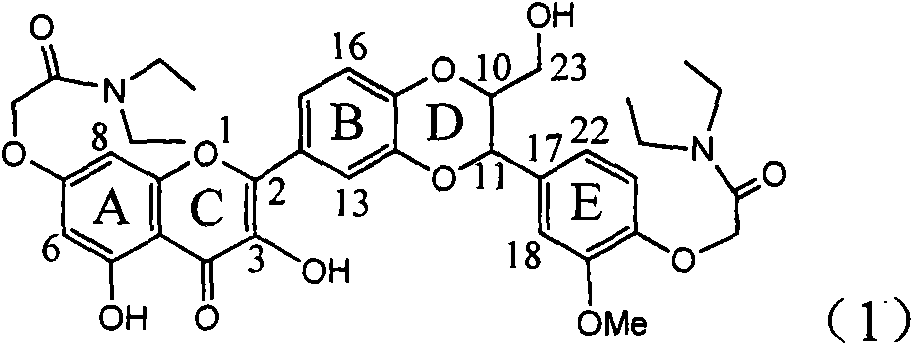
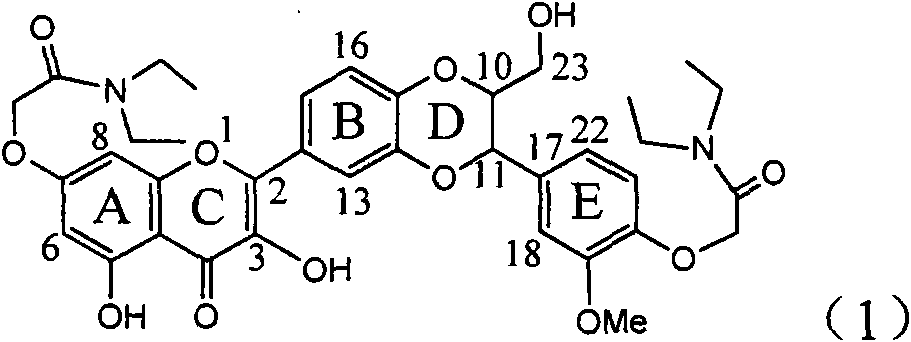
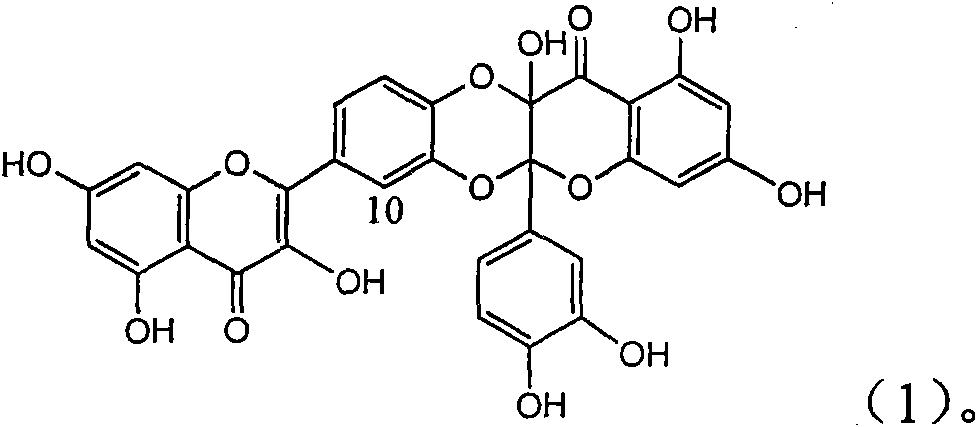
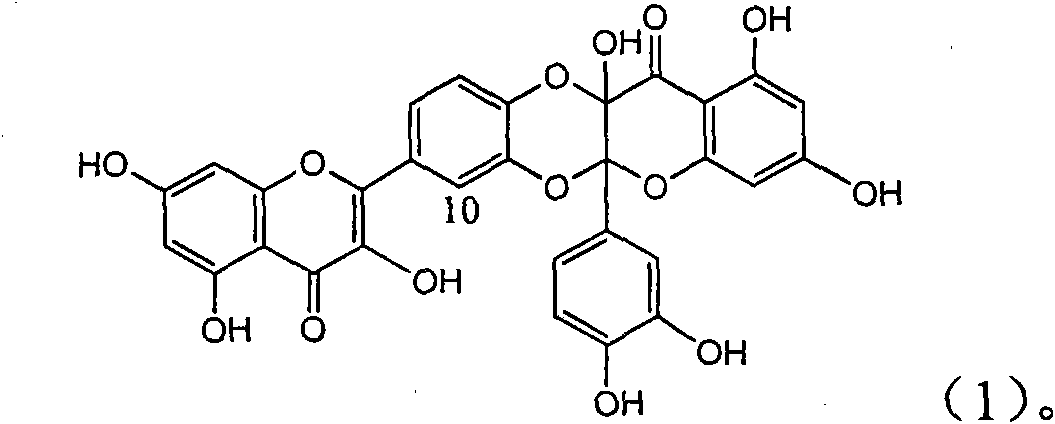
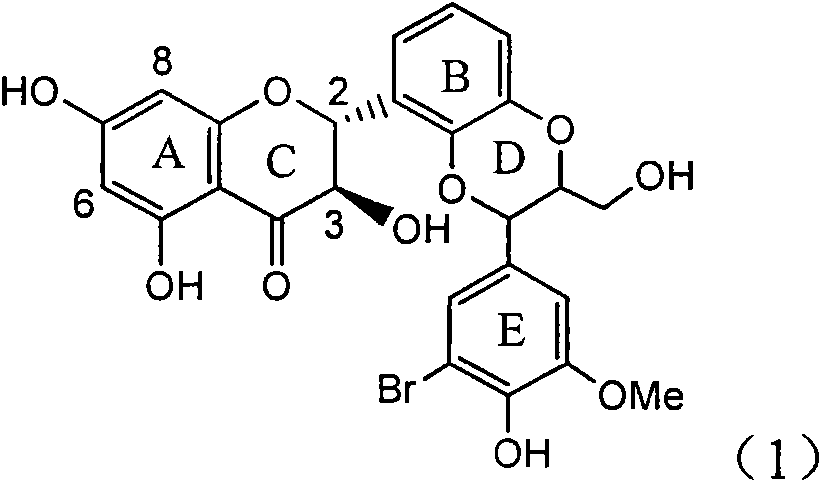
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com