4- fragrant amido quinazoline derivatives and method of manufacturing the same and application in pharmacy thereof
A technology of aromatic aminoquinazoline and its derivatives, which is applied in antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve problems such as difficult control and increased difficulty of specific inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
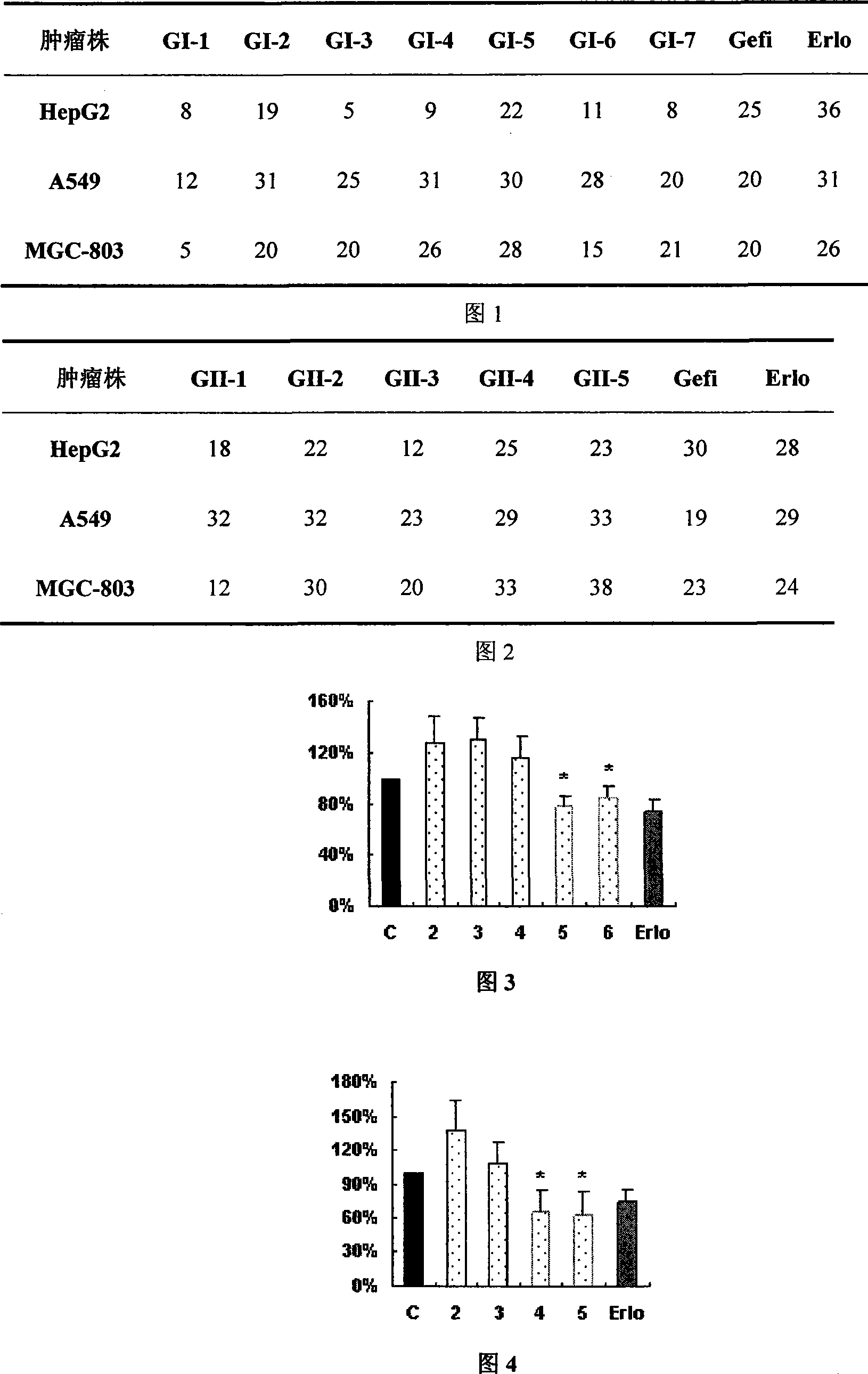
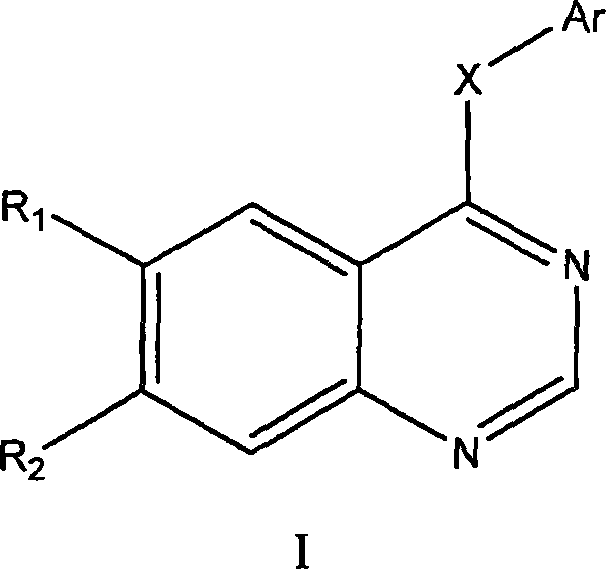
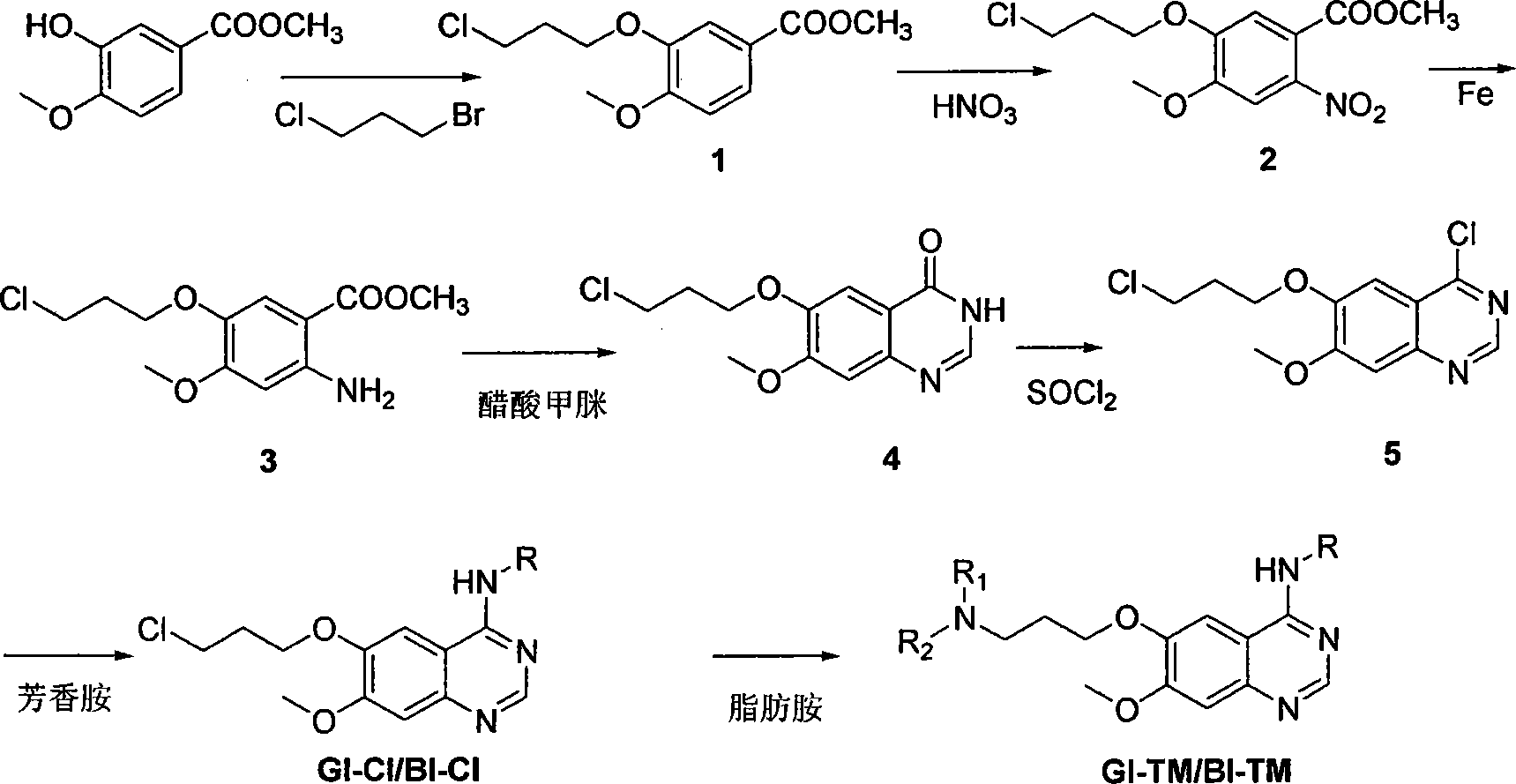
Image
Examples
Embodiment 1
[0137] Example 1: Ethyl 3-(7-methoxy-6-(3-morpholinopropoxy)quinazoline-4-amino)-1H-pyrrole-2-carboxylate (GI-1)
[0138] 3-(6-(3-chloropropoxy)-7-methoxyquinazoline-4-amino)-1H-pyrrole-2-carboxylic acid ethyl ester (GI-Cl, 0.81g, 2mmol), morpholine 5ml, DMF (20ml), reacted at 70°C for 30 minutes, removed the remaining morpholine under reduced pressure, dissolved the residue with 60ml chloroform, washed twice with 20ml water, dried over anhydrous magnesium sulfate, 200-300 mesh silica gel column chromatography, ethyl acetate Ester:triethylamine=20:1 was eluted to obtain 0.78 g of white powder with a yield of 86%. mp: 181-183°C; 1 H-NMR (CDCl 3 )δ: 1.39(t, 3H), 2.04-2.18(m, 2H), 2.51(t, 4H), 2.63(t, 2H), 3.74(t, 4H), 4.00(s, 3H), 4.29(t , 2H), 4.40(q, 2H), 6.90(t, 1H), 7.20(s, 1H), 7.24(s, 1H), 7.45(t, 1H), 8.71(s, 1H), 9.10(b, 1H), 9.95(b, 1H); 13 C-NMR (CDCl 3 )δ: 14.68, 26.19, 53.76, 55.44, 56.10, 60.10, 66.98, 67.76, 101.38, 103.73, 107.92, 108.41, 109.35, 122.36, 147...
Embodiment 2
[0139] Example 2: 3-(7-methoxy-6-(3-(4-methylpiperazin-1-yl)propoxy)quinazoline-4-amino)-1H-pyrrole-2-carboxylic acid Ethyl ester (GI-2)
[0140] Specific implementation method Referring to Example 1, N-methylpiperazine was used instead of morpholine to obtain 0.65 g of white powder with a yield of 71%. mp: 175-178°C; 1 H-NMR (CDCl 3 )δ: 1.43(t, 3H), 1.58(m, 2H), 2.15(t, 2H), 2.34(s, 3H), 2.62-2.69(m, 8H), 4.01(s, 3H), 4.27(t , 2H), 4.41(q, 2H), 6.69(t, 1H), 7.10(s, 1H), 7.24(s, 1H), 7.47(d, 1Hr), 8.58(b, 1H), 8.71(d, 1H), 9.54(b, 1H); 13 C-NMR (CDCl 3 )δ: 14.73, 26.53, 46.01, 53.21, 55.02, 55.20, 56.11, 60.11, 67.89, 101.33, 103.73, 107.95, 108.42, 109.36, 122.35, 147.20, 149.03, 153.87, 156.187 (cm -1 ): 2952, 2936, 2873, 2776, 1659, 1624, 1604, 1585, 1559, 1513, 1471, 1437, 1413, 1398, 1316, 1240, 1212, 1146, 1067, 1044, 987, 926, 844, 777, 617; M / z: 469.2 ([M+H] + , 100%). Elem. Anal. Calcd: C, 61.52; H, 6.88; N, 17.94. Found C, 61.83; H, 6.87; N, 17.32.
Embodiment 3
[0141] Example 3: Ethyl 3-(7-methoxy-6-(3-pyrrolepropoxy)quinazoline-4-amino)-1H-pyrrole-2-carboxylate (GI-3)
[0142] Specific implementation method Referring to Example 1, pyrrole was used instead of morpholine to obtain 0.48 g of yellow powder with a yield of 64%. mp: 192-194°C; 1 H-NMR (CDCl 3 )δ: 1.36(t, 3H), 1.79(b, 4H), 2.16(p, 2H), 2.57(b, 4H), 2.72(t, 2H), 4.00(s, 3H), 4.28(t, 2H ), 4.406(q, 2H), 6.86(t, 1H), 7.17(s, 1H), 7.22(s, 1H), 7, 41(t, 1H), 8.68(s, 1H), 9.10(b, 1H), 9.95(b, 1H); 13 C-NMR (CDCl 3 )δ: 14.69, 23.53, 28.56, 52.97, 54.21, 56.07, 60.05, 67.93, 101.20, 103.68, 107.88, 108.39, 109.34, 122.29, 147.13, 149.02, 153.79, 155.14, 155.27 -1 ): 3410, 3302, 3043, 2959, 2863, 1665, 1622, 1598, 1557, 1508, 1433, 1309, 1208, 1143, 1072, 1044, 896, 835, 774; M / z: 440.2 ([M+H ] + , 100%); Elem. Anal. Calcd: C, 62.85; H, 6.65; N, 15.93. Found C, 62.70; H, 6.62; N, 15.07.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com