Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
A technology of dehydrosilibinin and arylcarbamoyl methoxy is applied in the directions of antiviral agents, drug combinations, medical preparations containing active ingredients, etc., and can solve the problems of lack of literature and no effective development, and the like, To achieve the effect of convenient source, convenient source of raw materials and clear industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
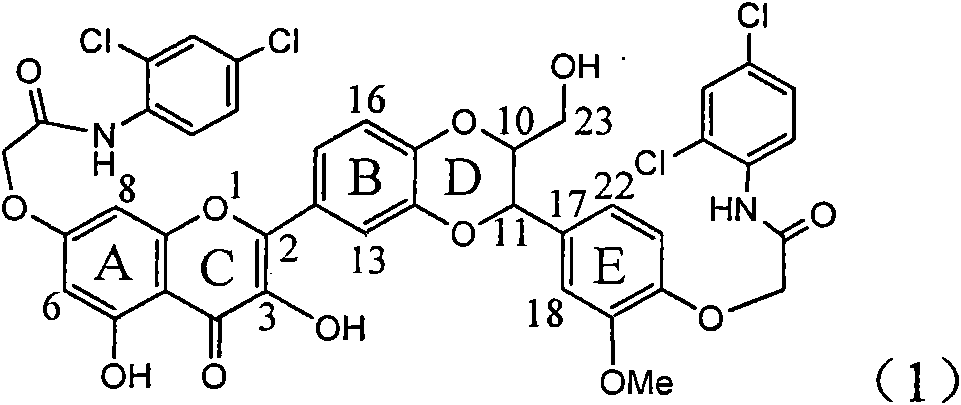
[0027] Example 1: Formula (1) compound N-(2,4-dichloro-phenyl)-2-[2-(3-{4-[(2,4-dichloro-phenylcarbamoyl)-methoxy] -3-methoxy-phenyl}-2-hydroxymethyl-2,3-dihydro-benzo[1,4]dioxane-6-yl)-3,5-dihydroxy-4- Preparation of Oxy-4H-benzopyran-7-yloxy]-acetamide
[0028] 1.1 Instruments and reagents:
[0029] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the hydrogen nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is the internal standard); (100-200, 200-300 and 300-400 mesh) and silica gel GF254 (10-40 mesh) for thin-layer chromatography are produced by Qingdao Ocean Chemical Factory; all reagents used are analytically pure, thin-layer preparative chromatography (PTLC ) uses the aluminum foil silica gel plate of Merck Company; Sephadex LH-20 used for column chromatography adopts the product of Amersham Pharma...
Embodiment 2
[0033] Example 2: Inhibitory Effect of Compound (1) on Hepatitis B Surface Antigen (HBsAg) Secreted by HepG2.2.15 Cells
[0034] 2.1 Cell culture:
[0035] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin, 100 μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity.
[0036] 2.2 The inhibitory effect of the compound of formula (1) on HepG2.2.15 cell growth was measured by MTT method:
[0037] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 cells / ml, seeded in 96-well cell culture plate, 100 μl per well, at 37°C, 5% CO 2 After 24 hours in an incubator with 100% relative humidity, add compound (1) diluted with medium, the concentration is 1000 μg / ml, 200 μg / ml, 40 μg / ml and 8 μg / ml, 200 μg / ml in each well microliter, each concentration was set up in triplicate, placed at 37°C, 5% CO 2 , cultivated in an ...
Embodiment 3
[0046] Example 3: Inhibitory Effect of Compound (1) on Hepatitis B e Antigen (HBeAg) Secreted by HepG2.2.15 Cells
[0047] 3.1 Cell culture: the method is the same as in Example 2.
[0048] 3.2 Determination of the inhibitory effect of the compound of formula (1) on the growth of HepG2.2.15 cells by MTT method: the method is the same as in Example 2.
[0049] 3.3 Determination of the inhibitory effect of the compound on hepatitis B e antigen (HBeAg): take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1 × 10 with the medium 5 / ml, seeded in 96-well cell culture plate, 100ml per well, at 37°C, 5% CO 2 After culturing in an incubator with 100% relative humidity for 24 hours, add samples diluted with culture medium at concentrations of 100 μg / ml, 20 μg / ml and 4 μg / ml, 200 μl per well, and set three concentrations for each Multiple wells were placed at 37°C, 5% CO 2 , cultivated in an incubator with 100% relative humidity, change the culture med...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com