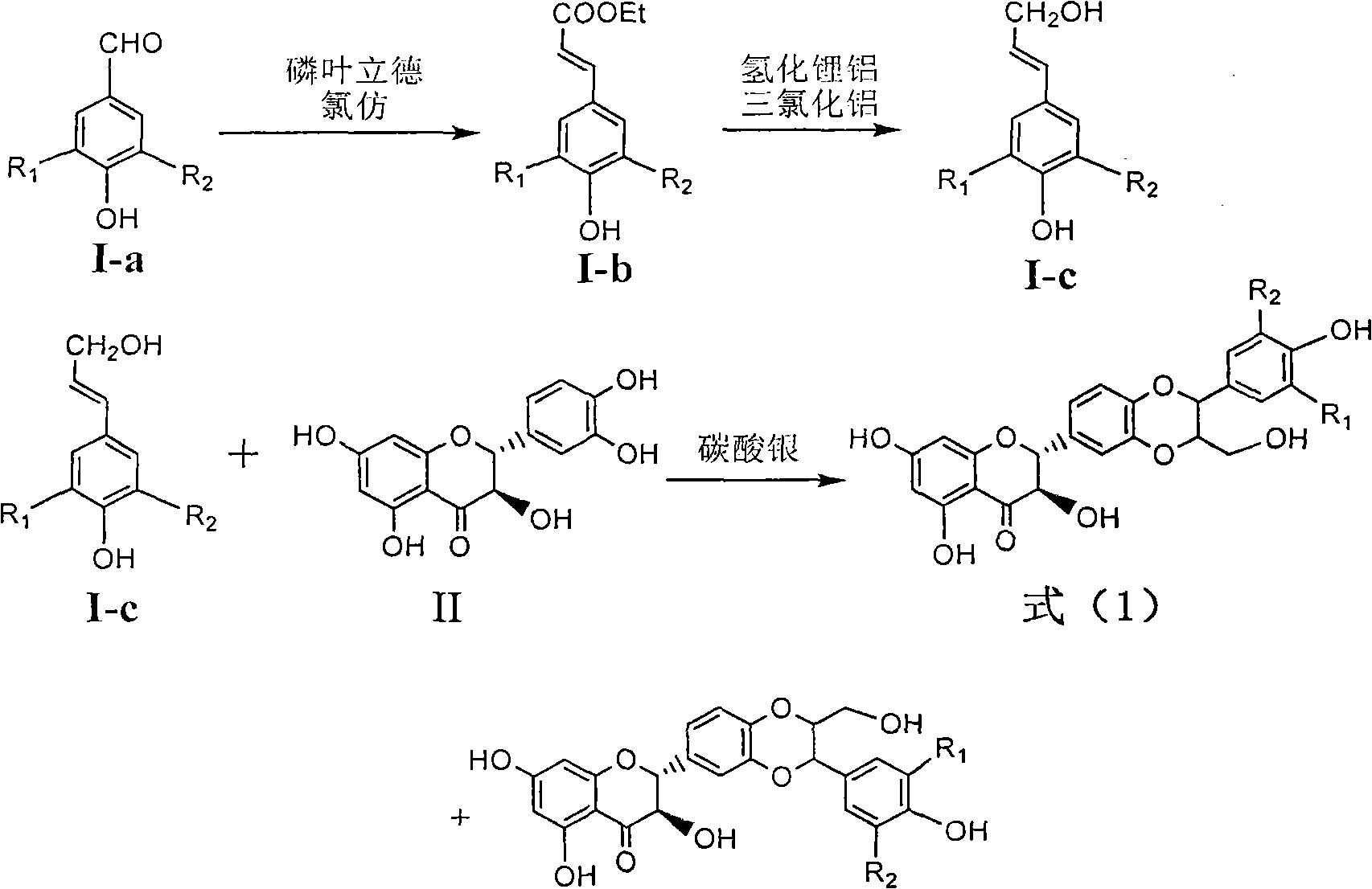
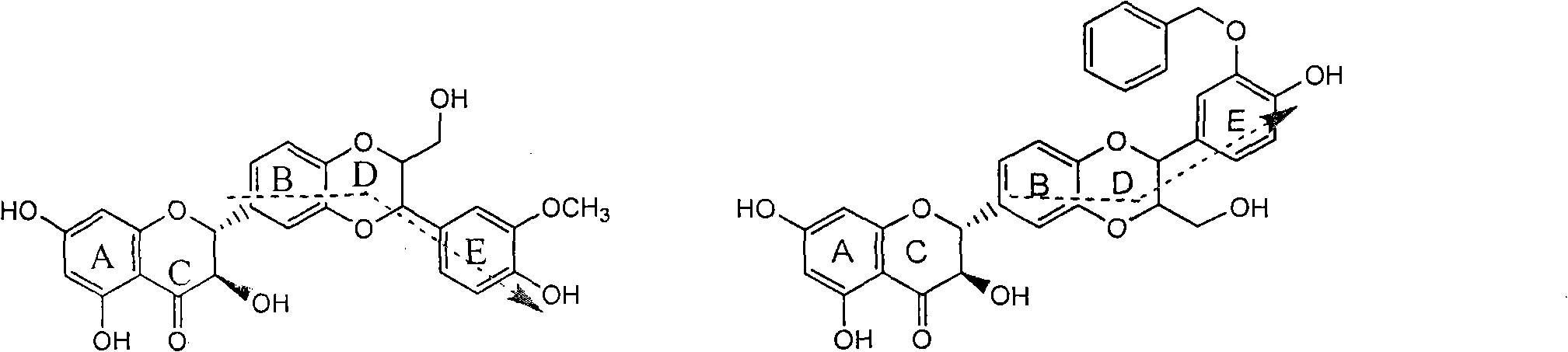
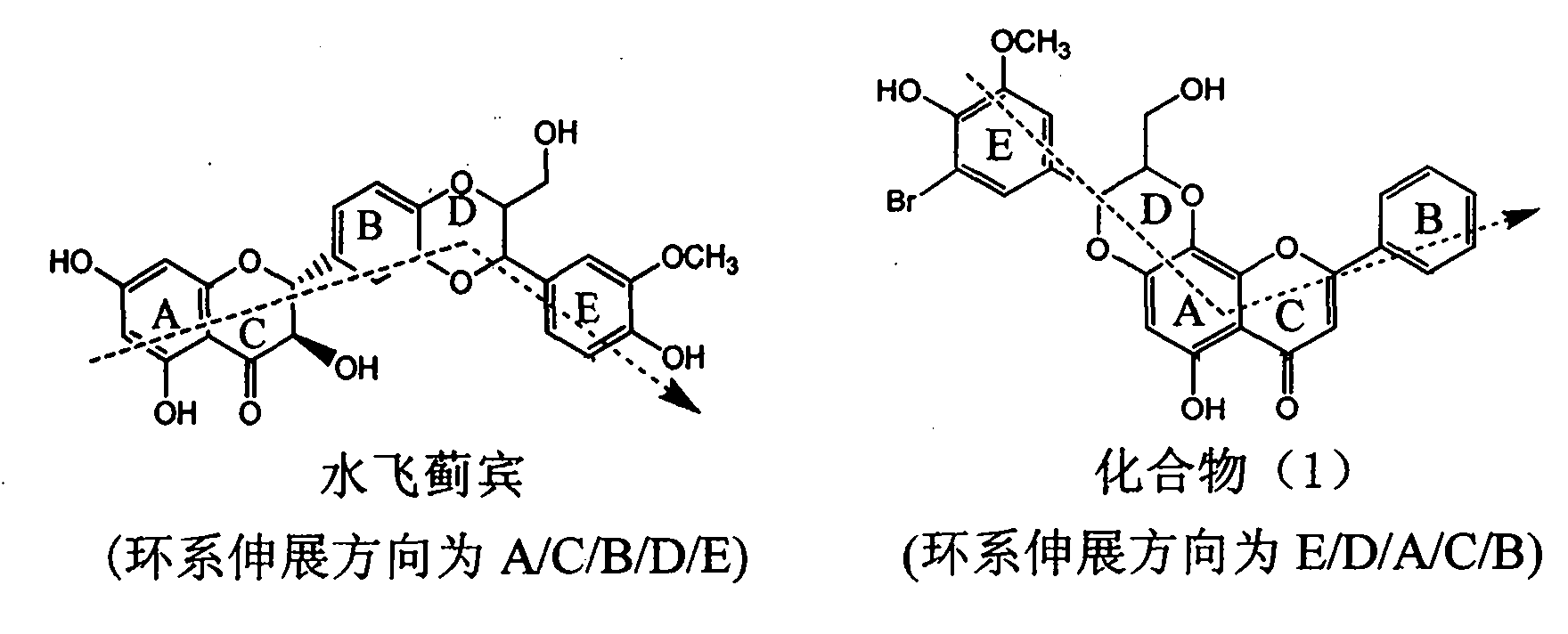
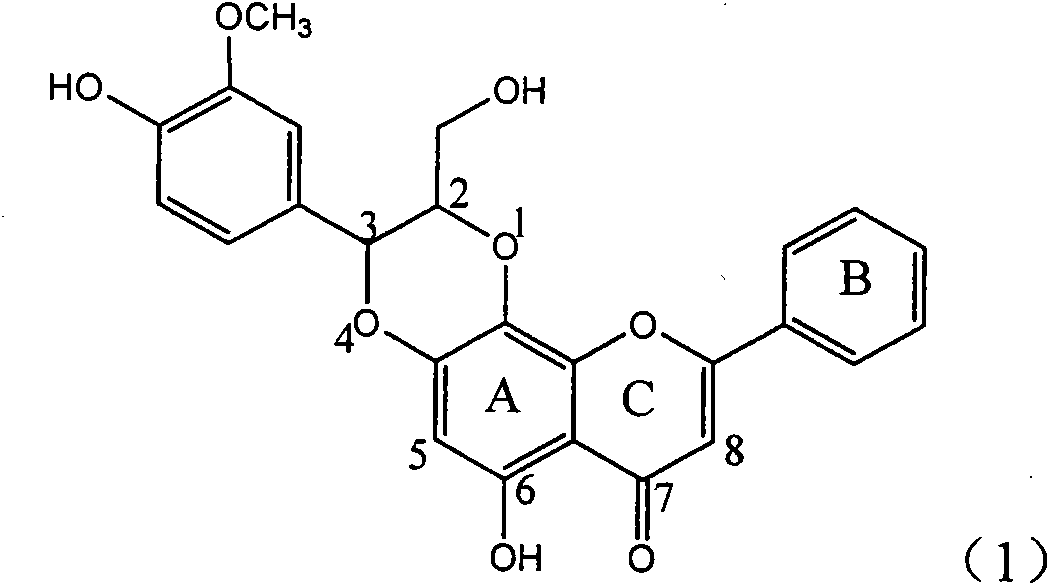
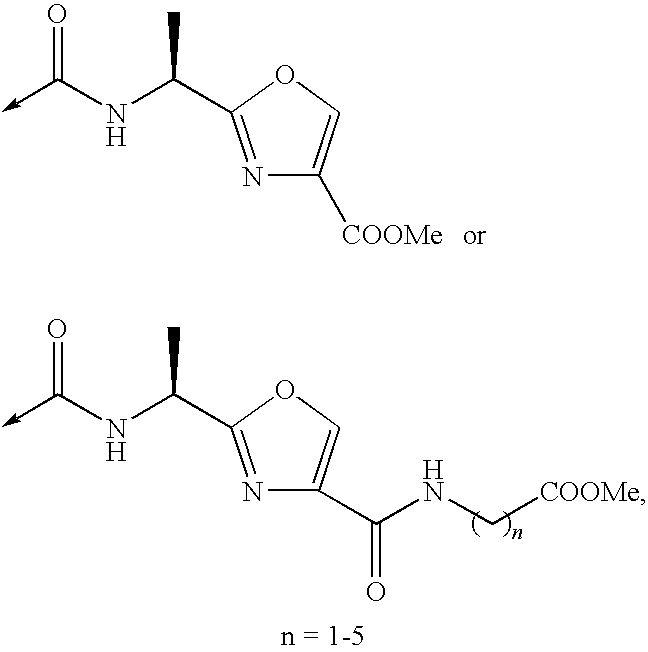
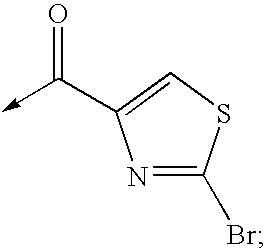
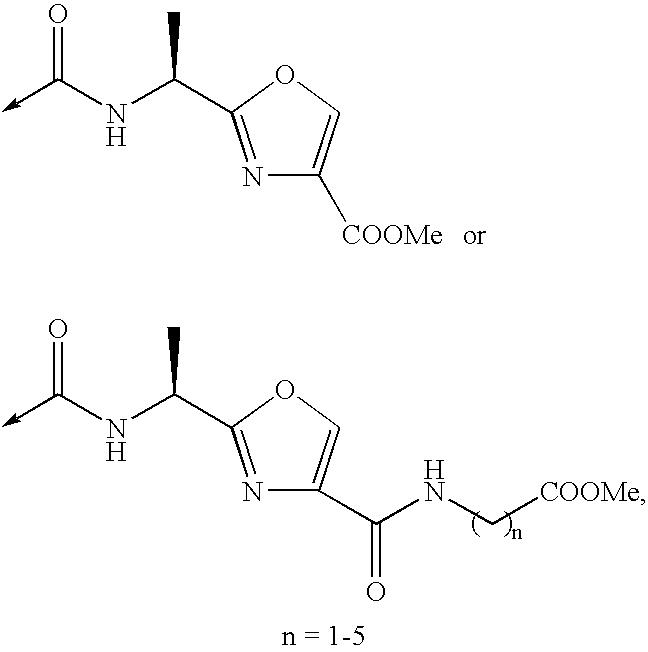
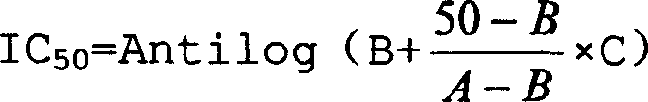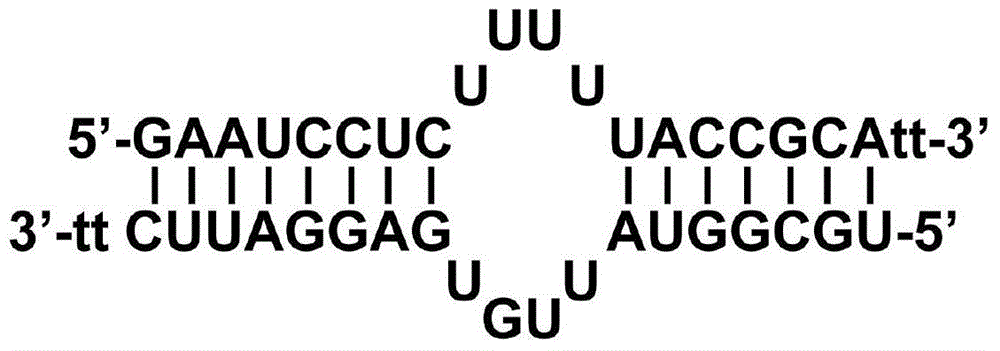Patents
Literature
192 results about "HBeAg" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
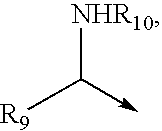
HBeAg is a hepatitis B viral protein. It is an indicator of active viral replication; this means the person infected with Hepatitis B can likely transmit the virus on to another person (i.e. the person is infectious).
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
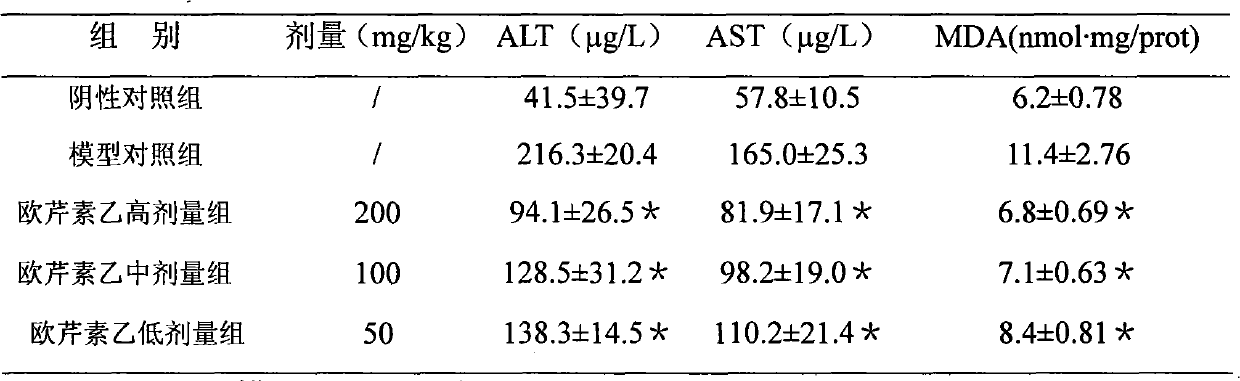
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of flavonoid quercetin dimmer as medicament for treating viral hepatitis B
InactiveCN101829103AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
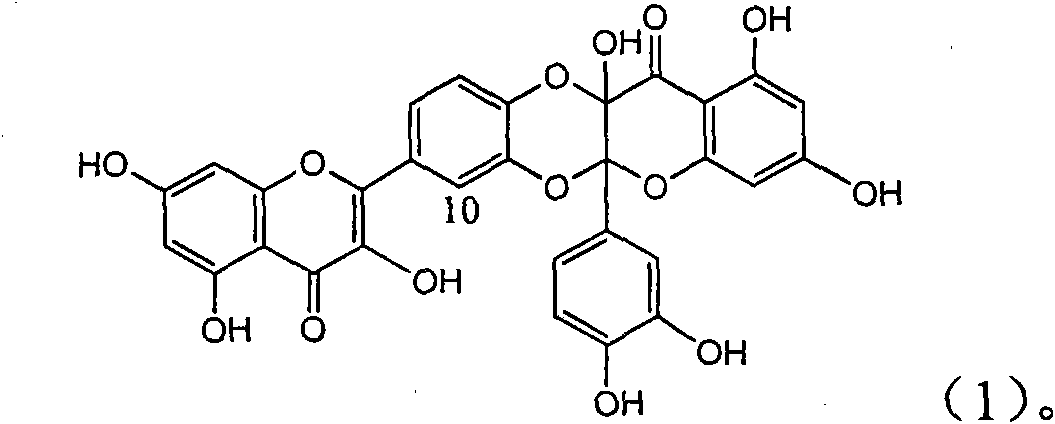
The invention relates to the application of flavonoid quercetin dimmer as the medicament for treating viral hepatitis B, in particular to the application of flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof to the preparation of the medicament for eliminating HBsAg and HBeAg and inhibiting HBV DNA replication. The flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof has obvious HBsAg and HBeAg inhibiting activity, and at the concentration of 100mcg / ml, the flavonoid quercetin dimmer pharmaceutically acceptable salt thereof has the HBsAg eliminating strength of 65.7% and the HBeAg eliminating strength of 44.8% which are respectively 4.1 times and 2.7 times higher than the positive control medicament of Alpha-interferon and has the HBV DNA inhibiting ratio of 44.8% which is 117% of the HBV DNA inhibiting ratio of the Alpha-interferon at the highest test concentration. Therefore, the flavonoid quercetin dimmer or pharmaceutically acceptable salt thereof can be expectedly used for preparing the non-nucleoside medicament for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating viral hepatitis B.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
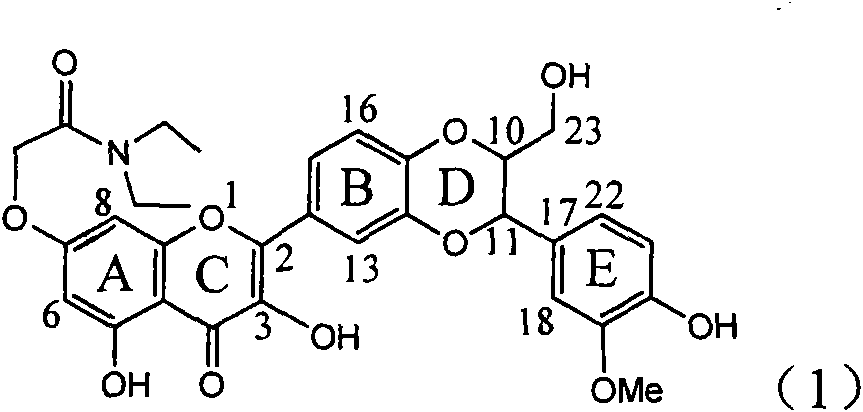
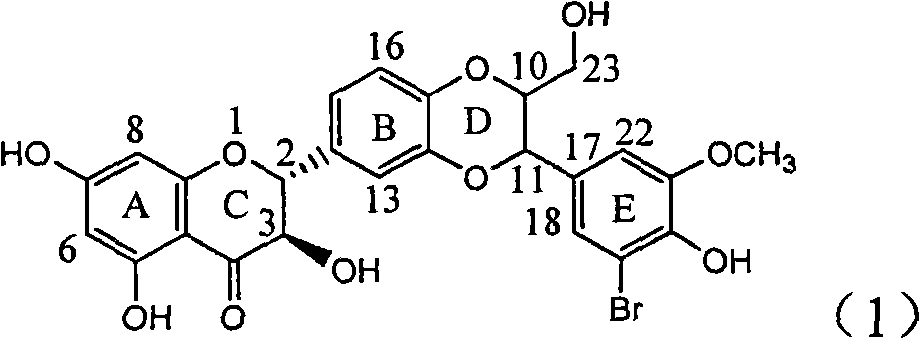
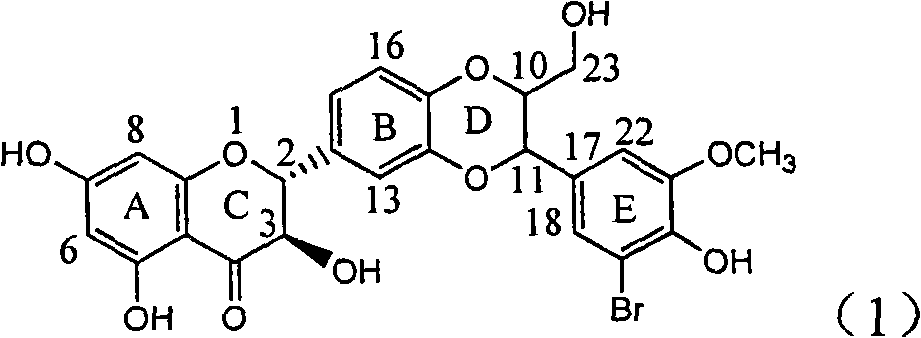
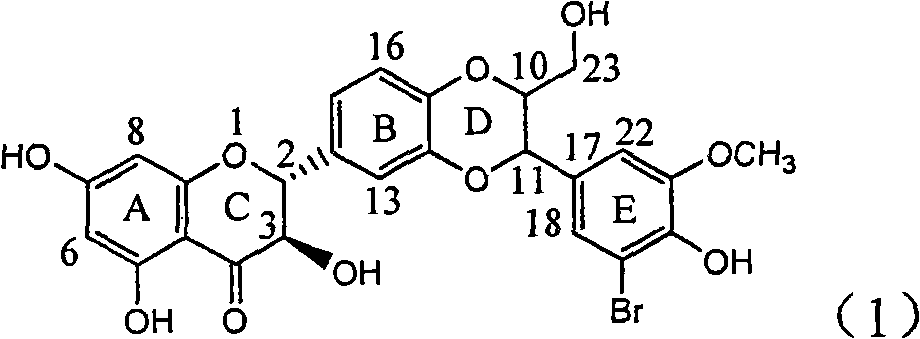
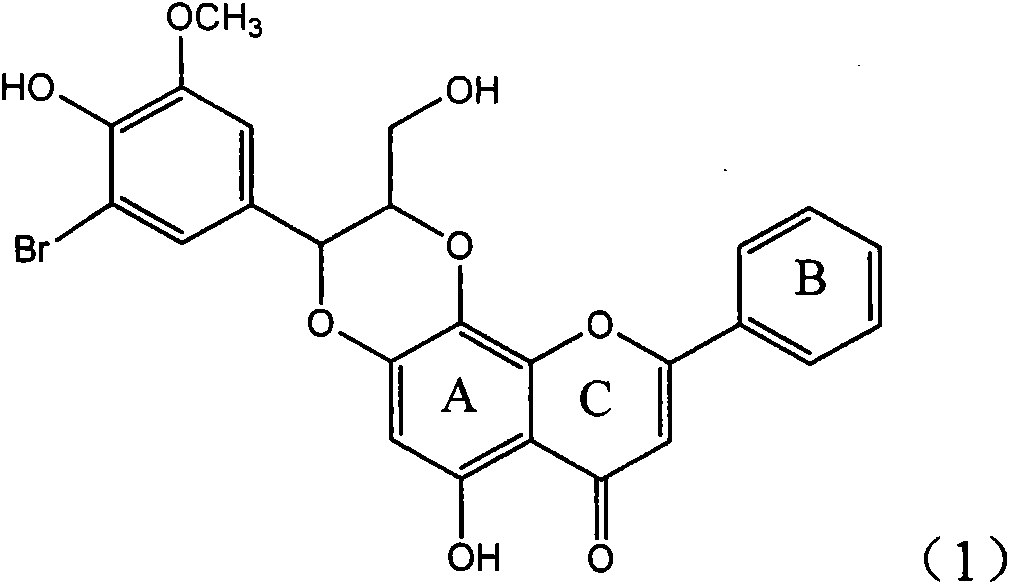
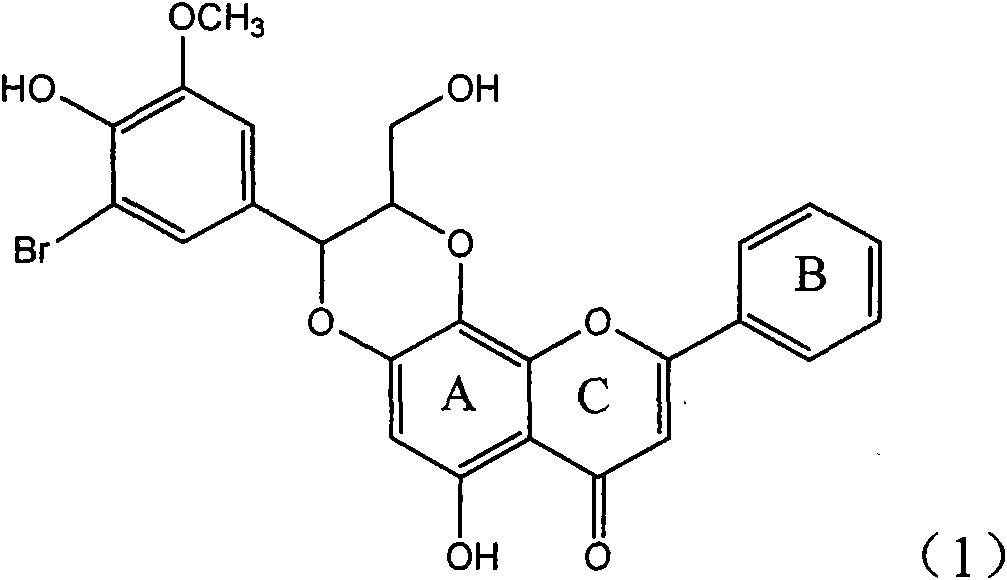
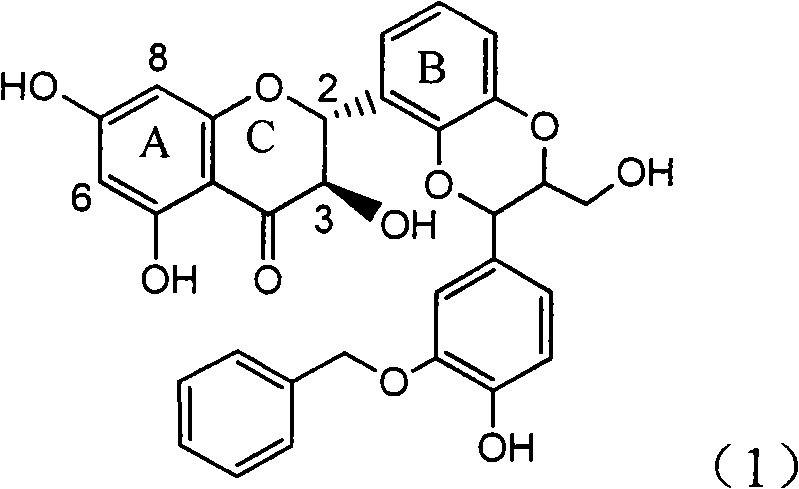
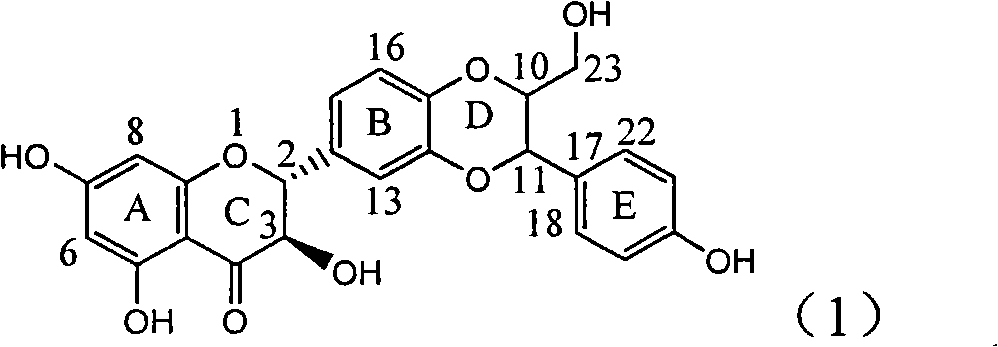
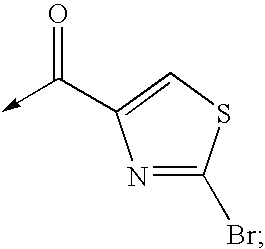
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
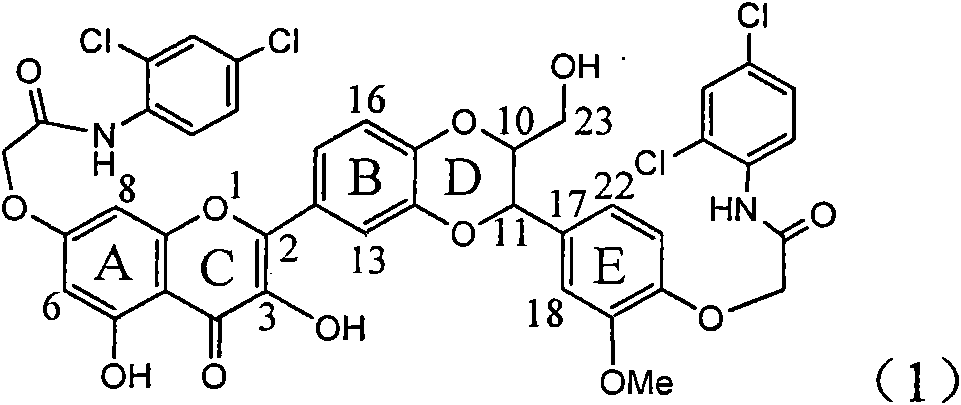
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
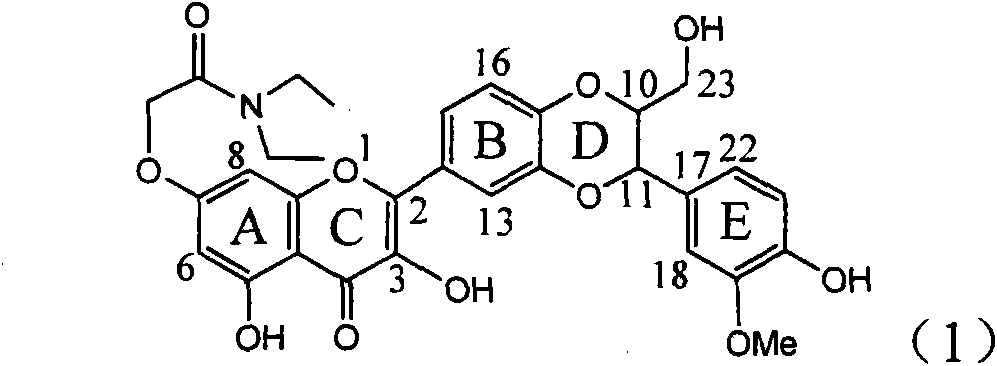
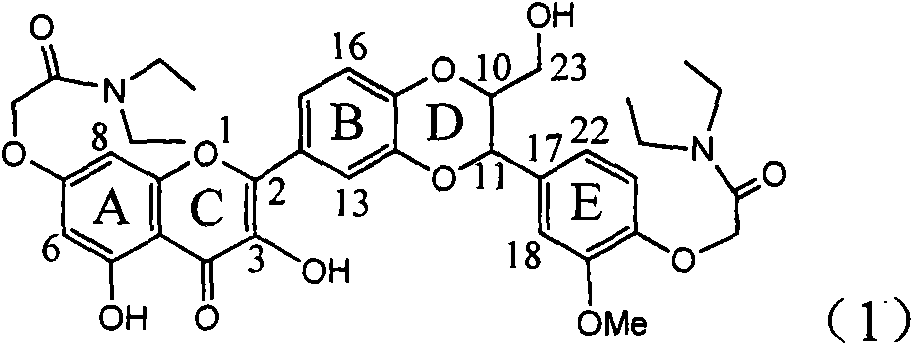
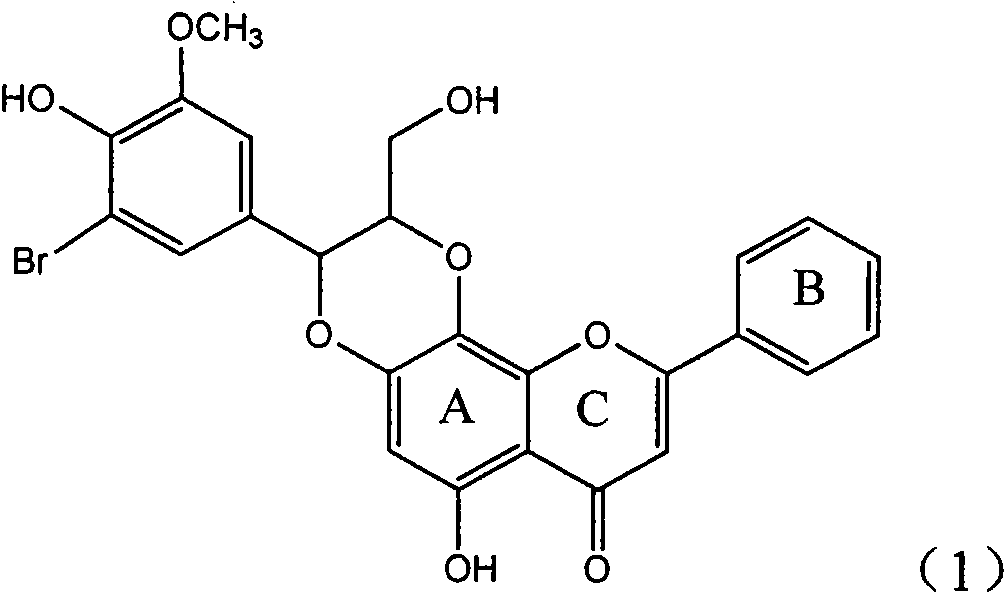
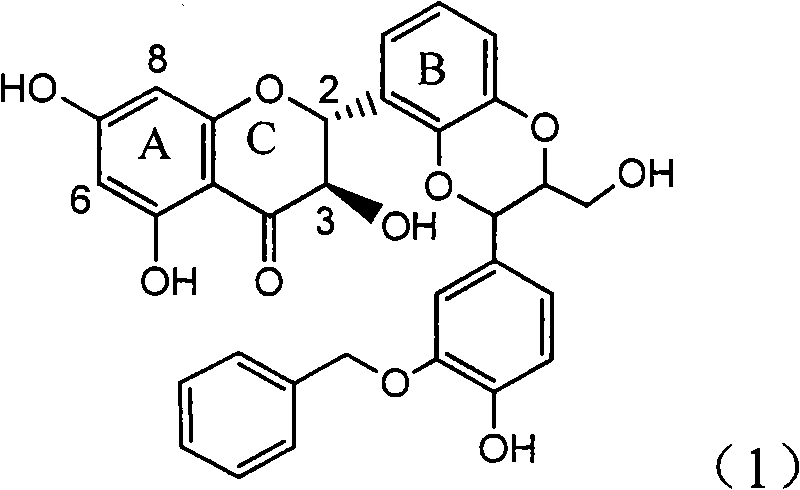
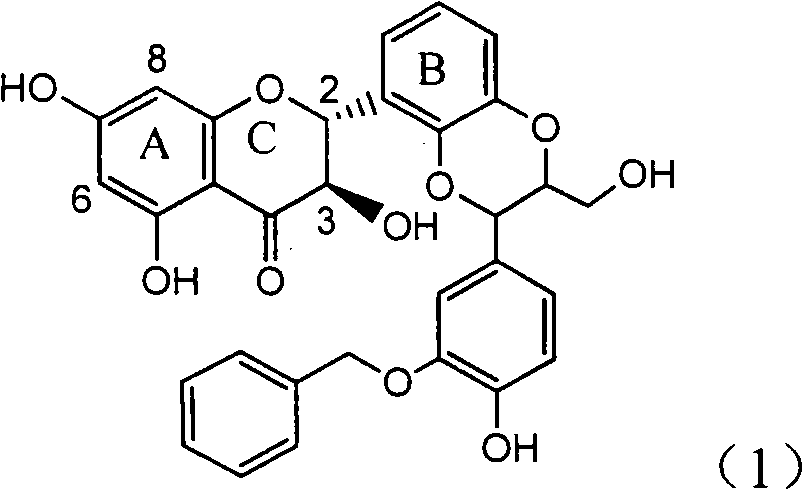
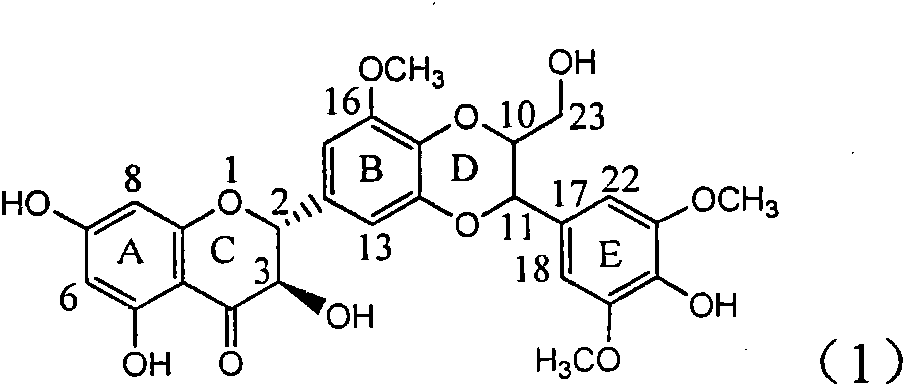
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
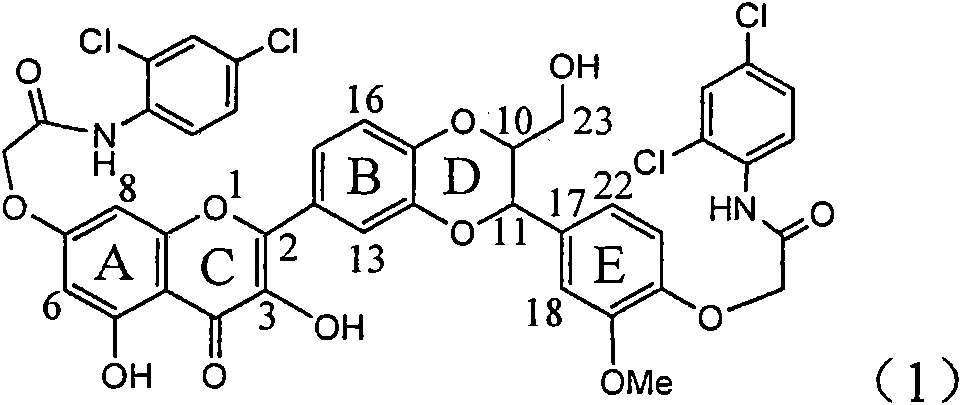
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
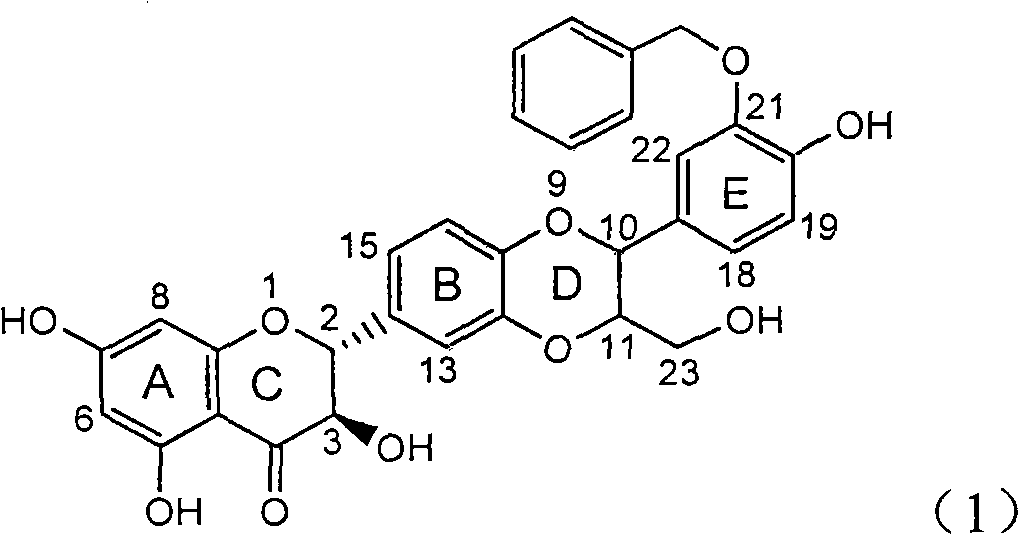
Use of lignanoid containing benzyloxy flavones in preparation of drugs for treating viral hepatitis B
InactiveCN101829095AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to a use of lignanoid containing benzyloxy flavones in the preparation of drugs for treating viral hepatitis B, in particular to the use of a compound as shown in formula (1) or pharmaceutical salts thereof in the preparation of the drugs for eliminating hepatitis B virus surface antigen and hepatitis B e antigen and the drugs for suppressing HBV DNA replication, and the strength of eliminating HBsAg of flavonol lignanoid under the concentration of 20 mu g / ml is 50.8%, which is 3.2 times of the corresponding activity of a positive control drug; the activity of eliminating the HBeAg under the same concentration is equivalent to 10000 units / ml of alpha-interferon; simultaneously, the flavonol lignanoid shows nearly 60% of suppression rate to HBV DNA under the concentration, which is 1.6 times of the corresponding suppression rate of the alpha-interferon. The results show that the lignanoid containing the flavones or the pharmaceutical salts thereof are expected to be used for preparing the non-nucleoside drugs for eliminating the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
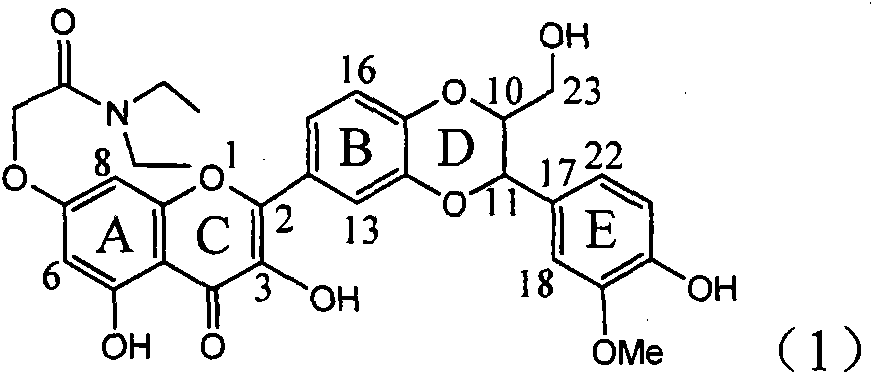
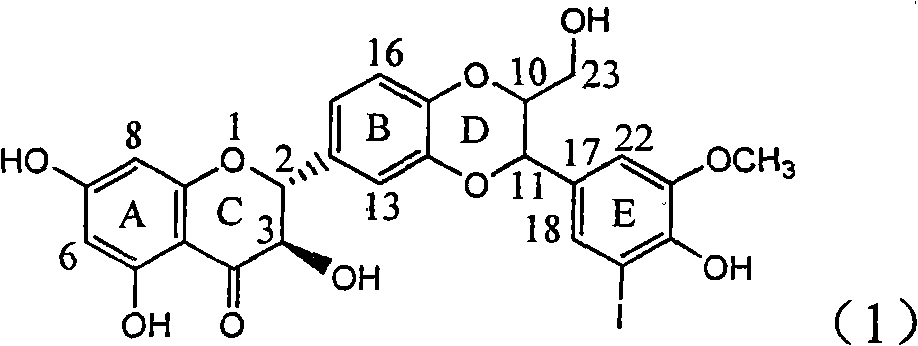
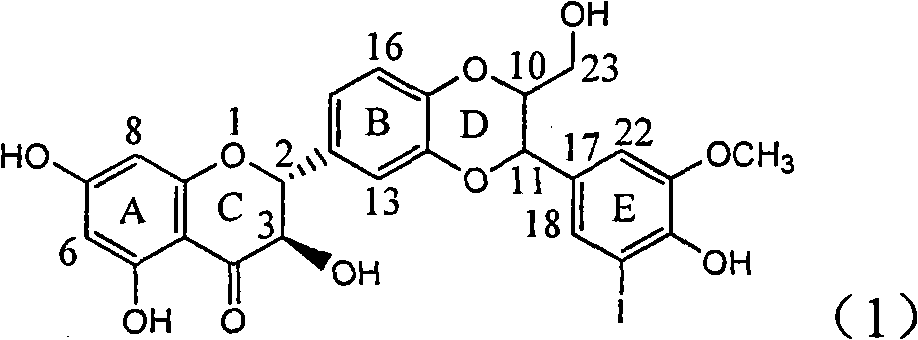
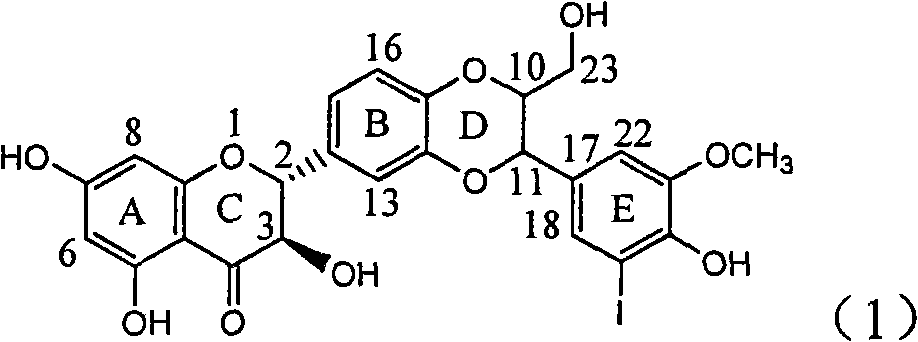
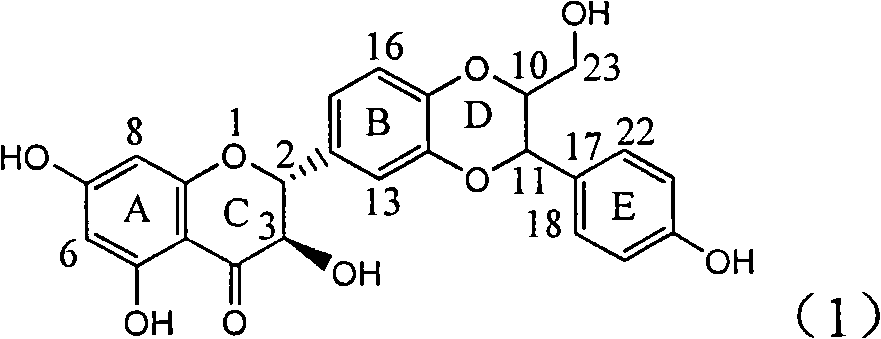
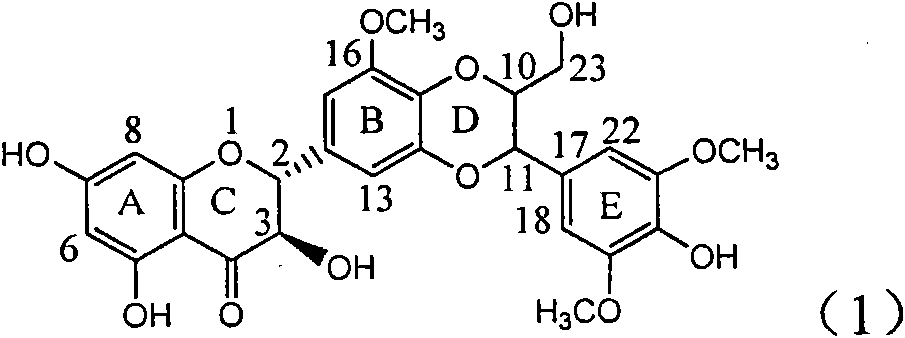
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
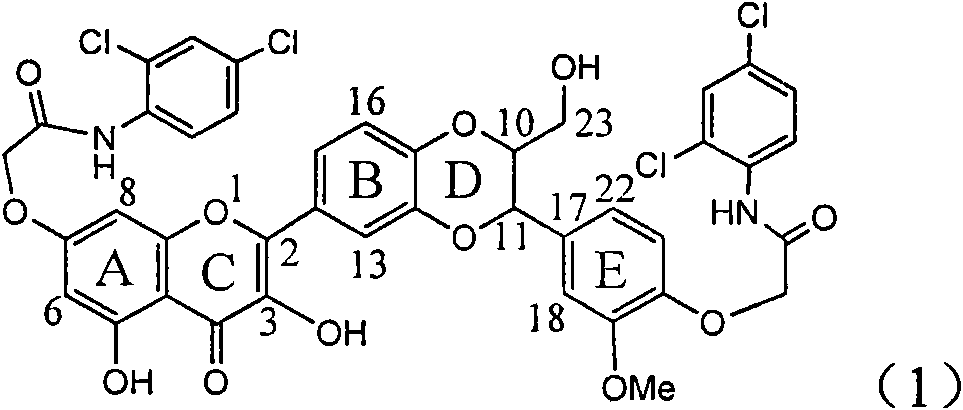
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
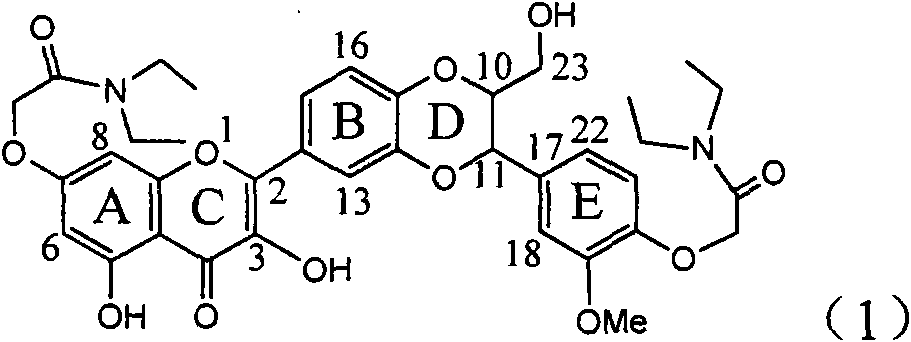
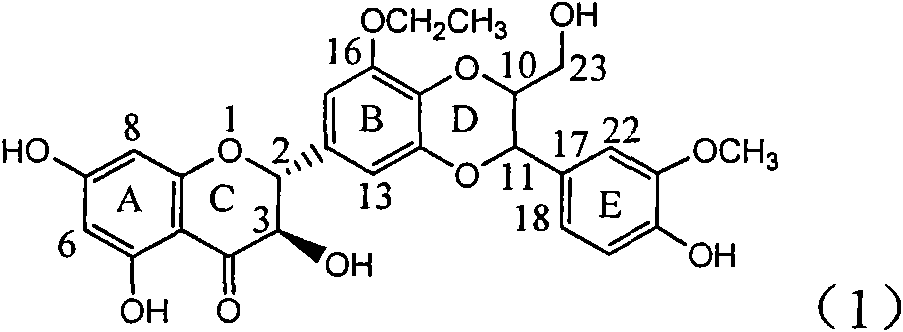
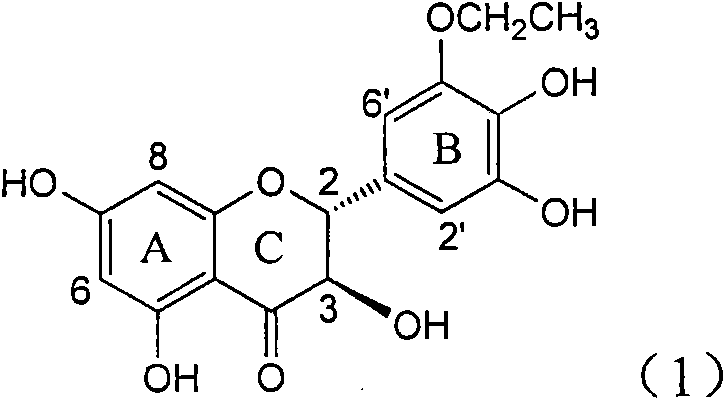
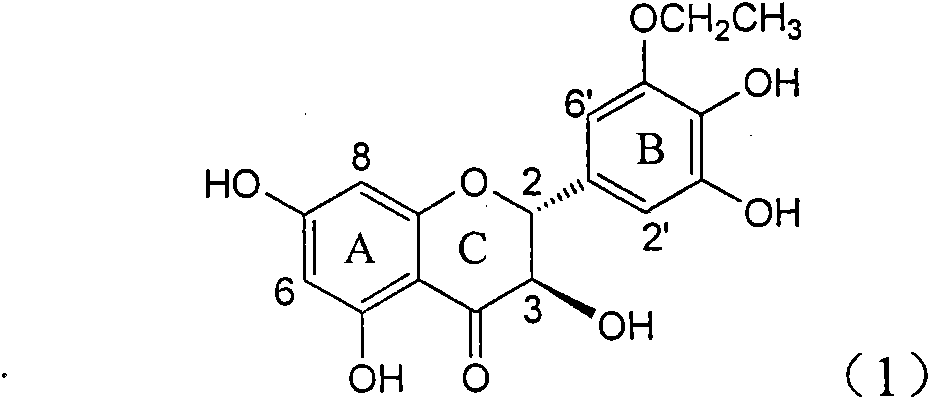
Application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829089AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of ring B ethyoxyl silybin in preparing medicaments for treating viral hepatitis B, in particular to application of ring B ethyoxyl substituted silybin ester or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAG (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has strong activity on suppressing the HBsAG and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing the HBsAg and the HBeAg are respectively 64.6 percent and 44.8 percent which are 4.0 times and 2.7 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression rate of the compound on the HBV DNA is 58.1 percent which is 1.5 times of the corresponding activity of the alpha-interferon. Accordingly, the flavonolignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have strong efficacy on suppressing the HBsAg, the HBeAg and the HBV DNA and can be expected to be used for preparing non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
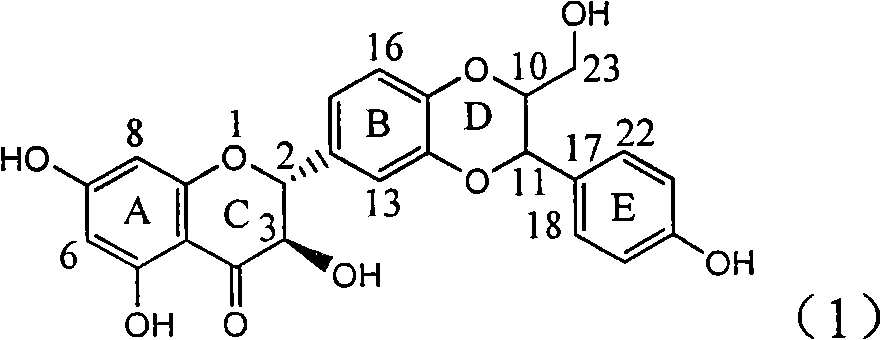
Application of substituted isosilybin in preparing medicament for treating virus hepatitis B
InactiveCN101829098AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of substituted isosilybin in preparing a medicament for treating virus hepatitis B, in particular to application of E-ring substituted isosilybin or medicinal salt thereof in preparing a medicament for clearing hepatitis B e antigen, inhibiting HBV DNA replication and treating hepatitis B virus infected diseases. The E-ring substituted isosilybin has strong effect of inhibiting HBeAg activity, and the strength of the E-ring substituted isosilybin at the concentration of 100 micrograms per milliliter for clearing the HBeAg is 3.5 times that of a positive control front-line medicament (10,000 units per milliliter of alpha-interferon); and moreover, the compound at the concentration of 100 micrograms per milliliter has strong inhibiting rate (97.7 percent) on the HBV DNA. Pharmacodynamical results show that the E-ring substituted isosilybin or the medicinal salt thereof can be expected to be used for preparing the medicament for treating the hepatitis B virus infected diseases.
Owner:DALI UNIV
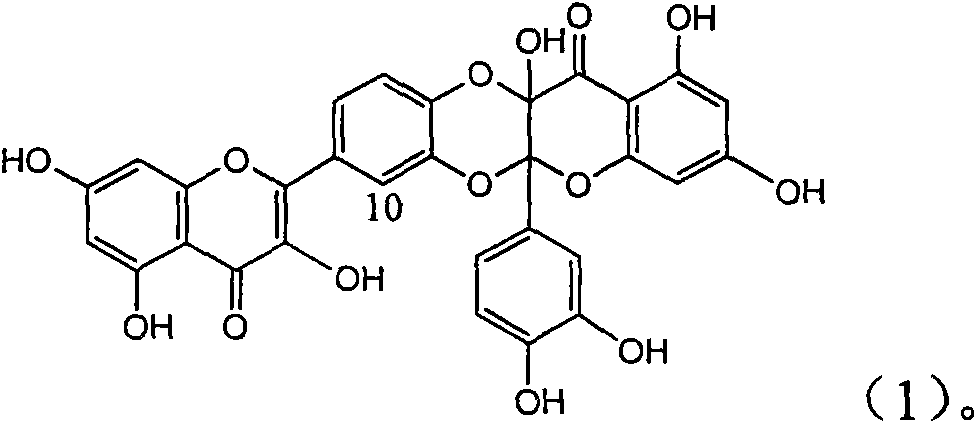
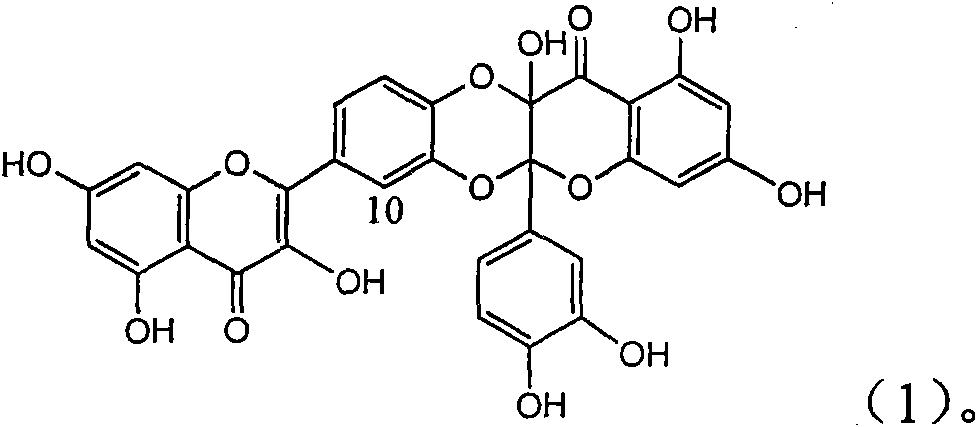
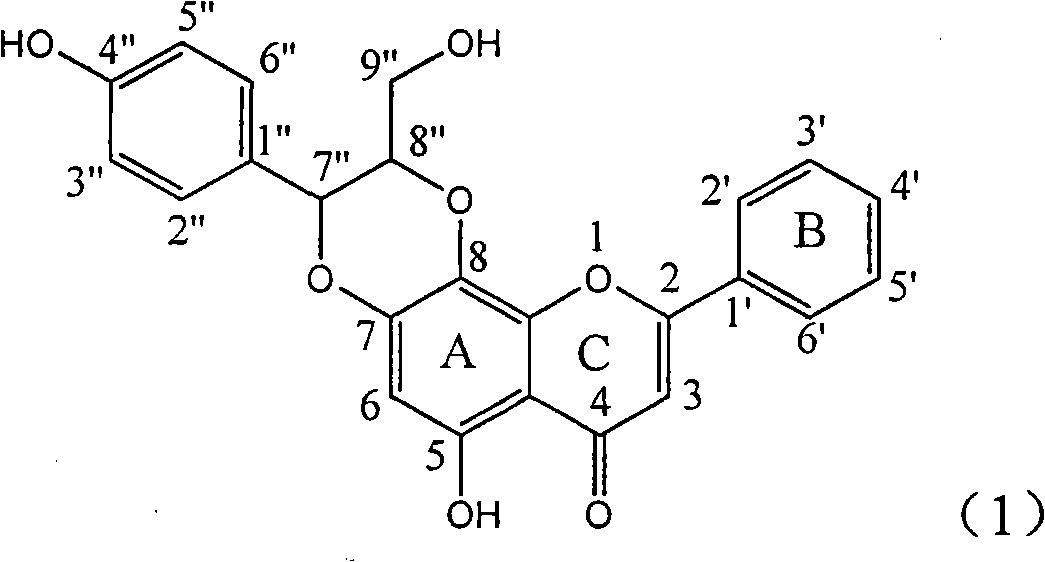
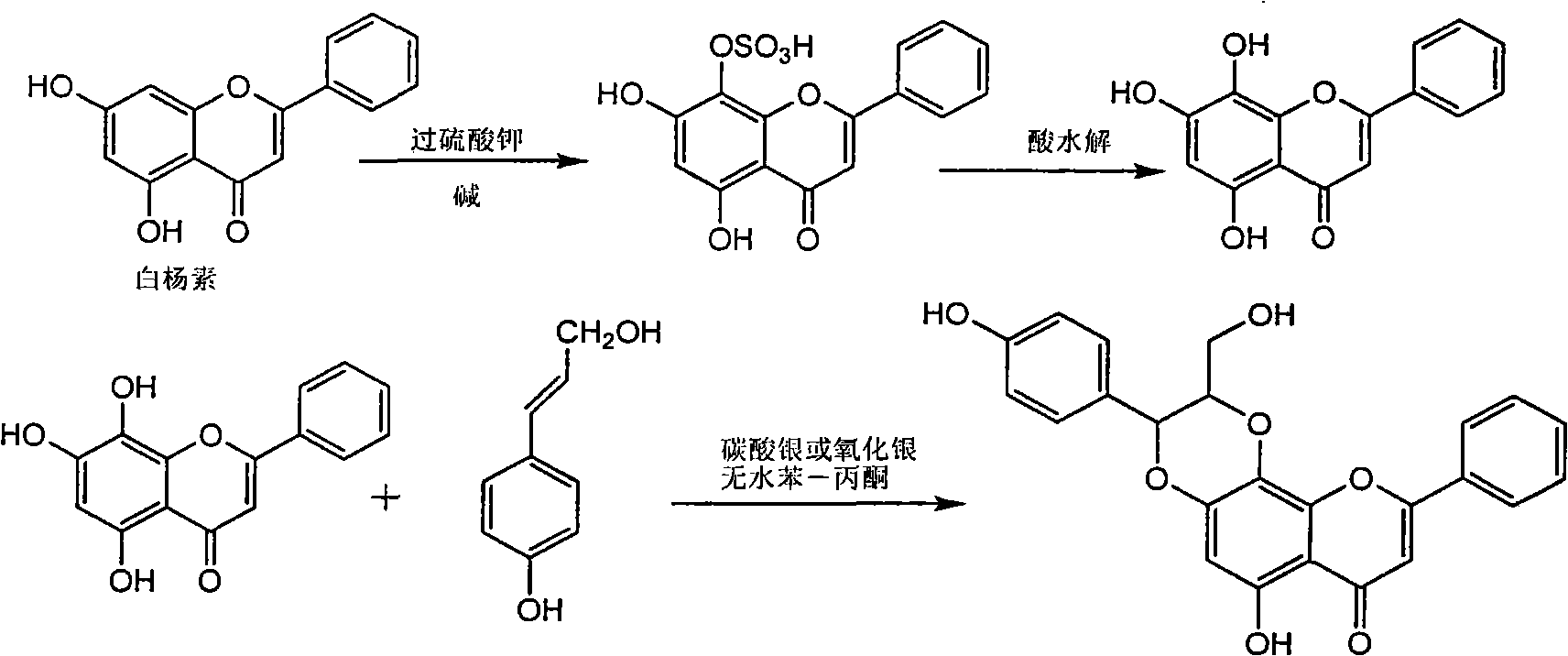
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
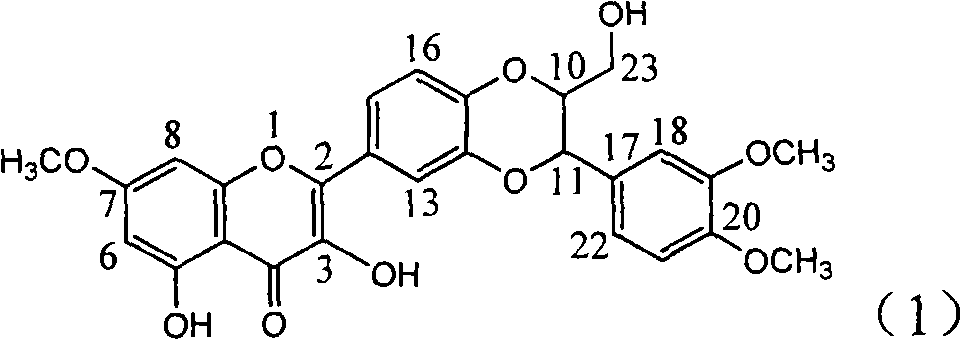
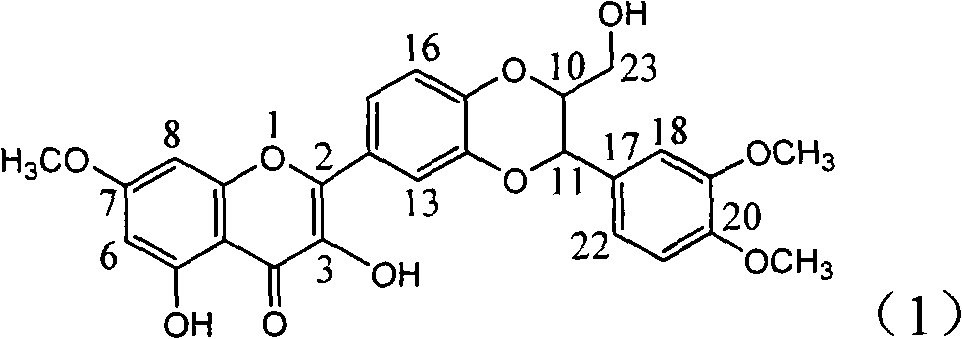
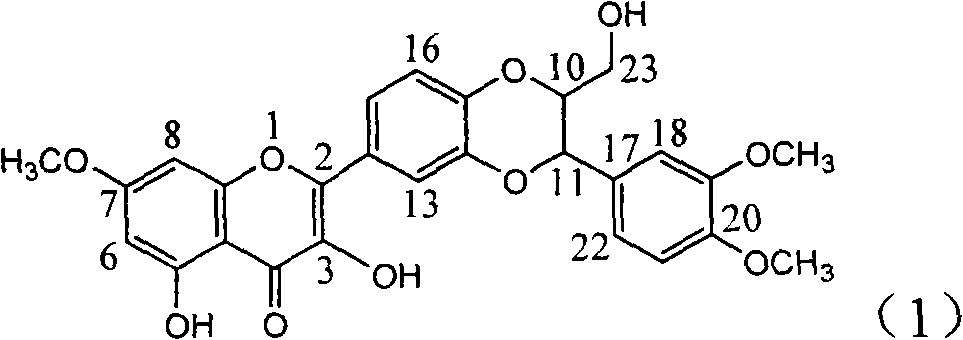
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
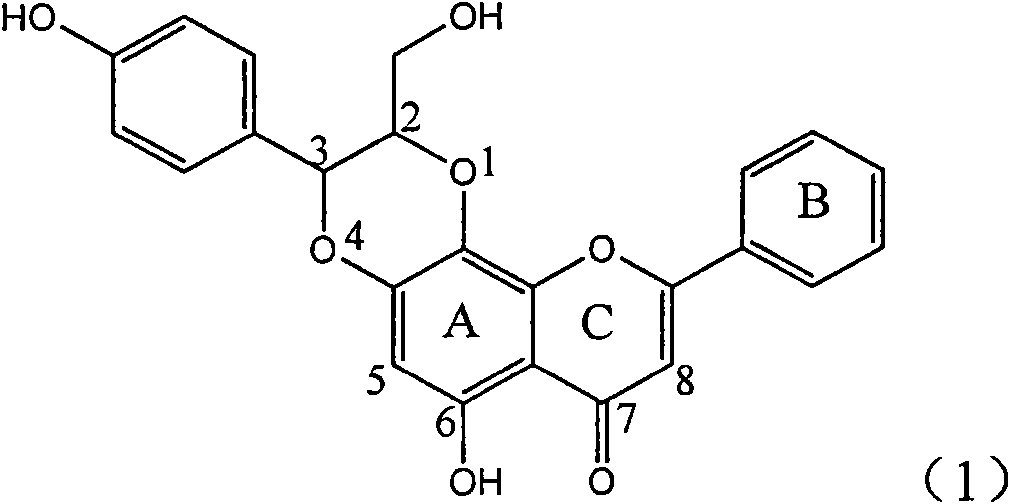
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
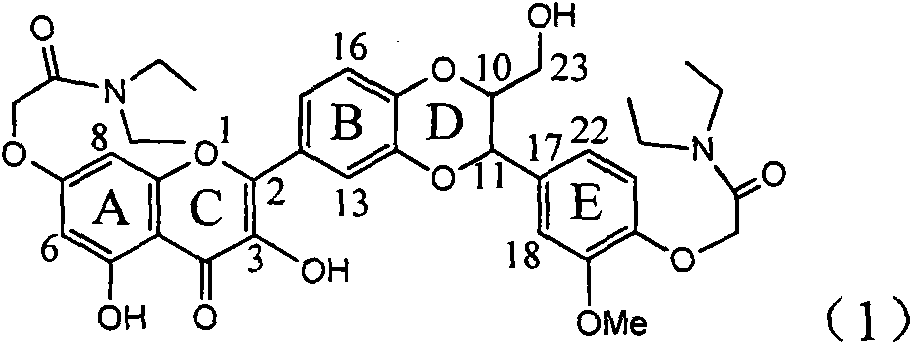
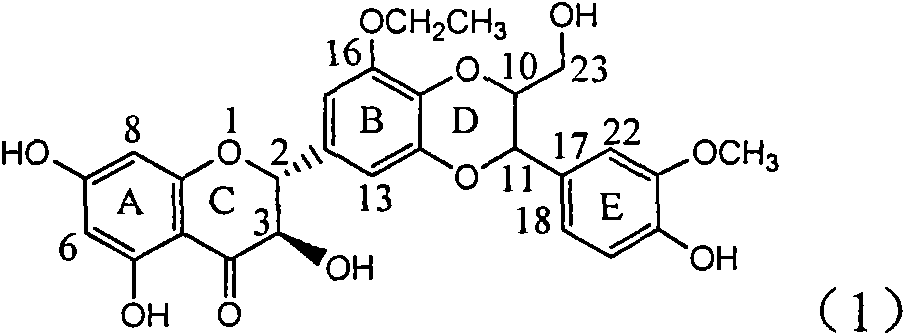
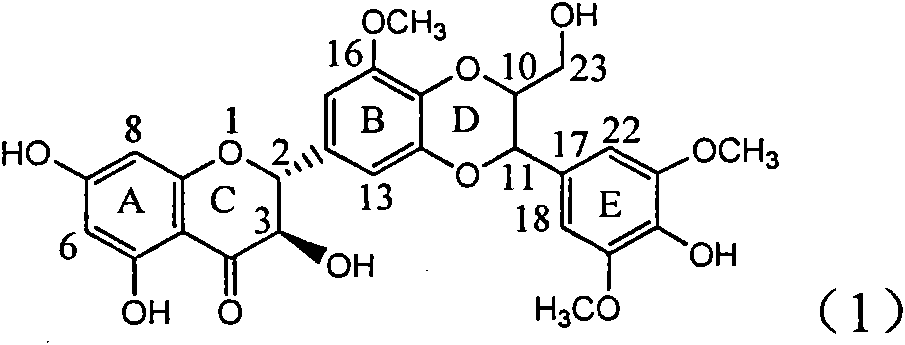
Application of B/E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829088AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of B / E bi-methoxy silybin in preparing medicaments for treating viral hepatitis B, in particular to application of silybin ester substituted by the methoxy on the ring B and the ring E or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The B / E bi-methoxy silybin has strong activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 20 micrograms / milliliter, the intensities for clearing away the HBsAg and the HBeAg are respectively 43.9 percent and 43.7 percent which are 2.7 times and 2.6 times of that of alpha-interferon which is a positive control medicament. In the presence of the concentration, the suppression ratio on the HBV DNA is 68.6 percent, and the suppression activity is 1.8 times of that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to simultaneously have the effects of strongly suppressing the HBsAG, the HBeAg and the HBV DNA and can be expected to be used for preparing the non-nucleoside medicaments for treating HBV infection diseases.
Owner:DALI UNIV
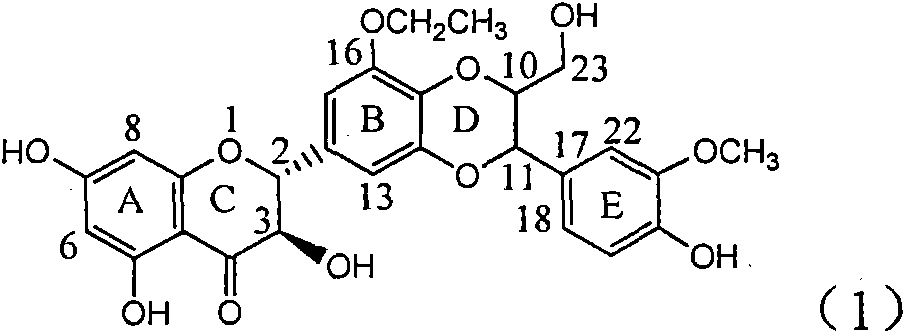
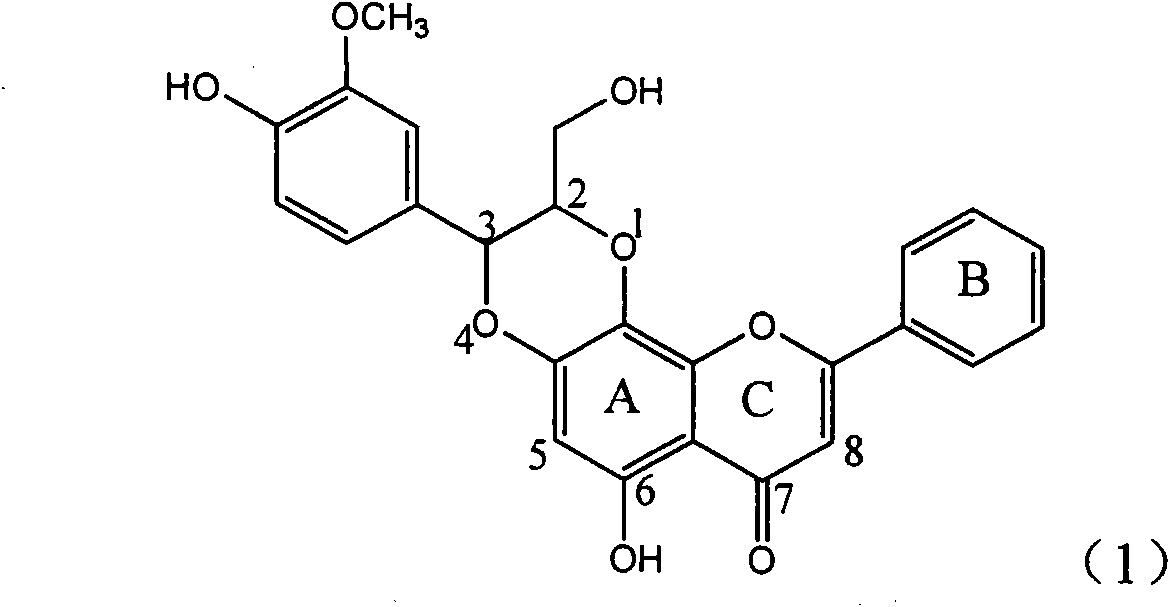
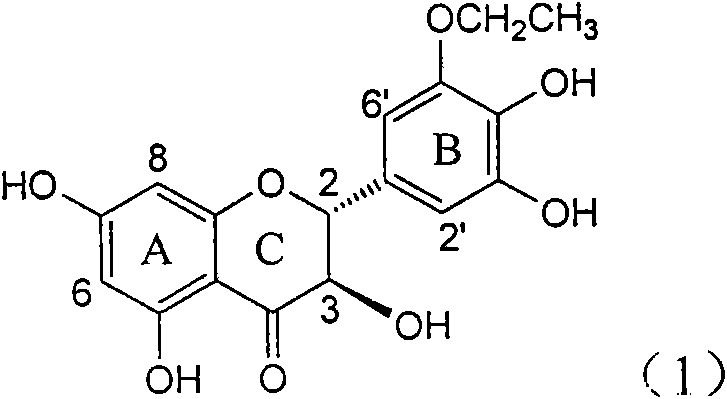
Application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses
InactiveCN101822664AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryDiseasePositive control
The invention relates to application of B-ring ethyoxyl flavanonol in preparing medicaments for treating hepatitis B viruses, in particular to application of a compound as shown in a formula (1) or a medicinal salt thereof in preparing medicaments for clearing away hepatitis B virus surface antigens (HBsAg) and hepatitis B e-antigen (HBeAg) and medicaments for inhibiting the duplication of hepatitis B virus desoxyribonucleic acid (HBV DNA). The compound or the medicinal salt thereof has extremely obvious activity on inhibiting the HBsAg and the HBeAg, and in the presence of a concentration of 20 microgram / milliliter, the intensities for clearing away the HBsAg and the HBeAg of the compound or the medicinal salt thereof are respectively 99.8 percent and 48.5 percent and are 6.2 times and 2.7 times of that of alpha-interferon which is a positive control medicament. More importantly, in the presence of the concentration, the inhibition ratio of the compound or the medicinal salt thereof to the HBV DNA is 64.7 percent, and the activity is 1.7 times of that of the alpha-interferon. Accordingly, the flavone lignan or the medicinal salt thereof can be expected to be used for preparing non-nucleoside medicaments for treating infectious diseases of the hepatitis B viruses.
Owner:DALI UNIV
Application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B
InactiveCN101912385AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention relates to application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B, in particular to the application of 7 and 20-position methyl substituted dehydrated silybin or pharmaceutically acceptable salts thereof in preparing medicaments for removing HBsAG and HBsAg and medicaments for inhibiting HBV DNA replication. The dehydrated silybin has remarkably HBsAg and HBeAg inhibiting activity, wherein the strength for removing the HBsAg and the HBeAg at the concentration of 20 milligram / milliliter is 88.9 percent and 84.1 percent respectively, which are 5.5 times and 5.0 times that of a positive contrast medicament. More importantly, the dehydrated silybin shows the HBV DNA inhibition ratio of about 99.6 percent at the concentration of 20 milligram / milliliter, the activity exceeds lamivudine by 23 percent, which is 2.6 times that of interferon. Therefore, favonolignan or pharmaceutically acceptable salts thereof can be predictably used for preparing the non-nucleoside reverse transcriptase inhibitor medicaments for removing the HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
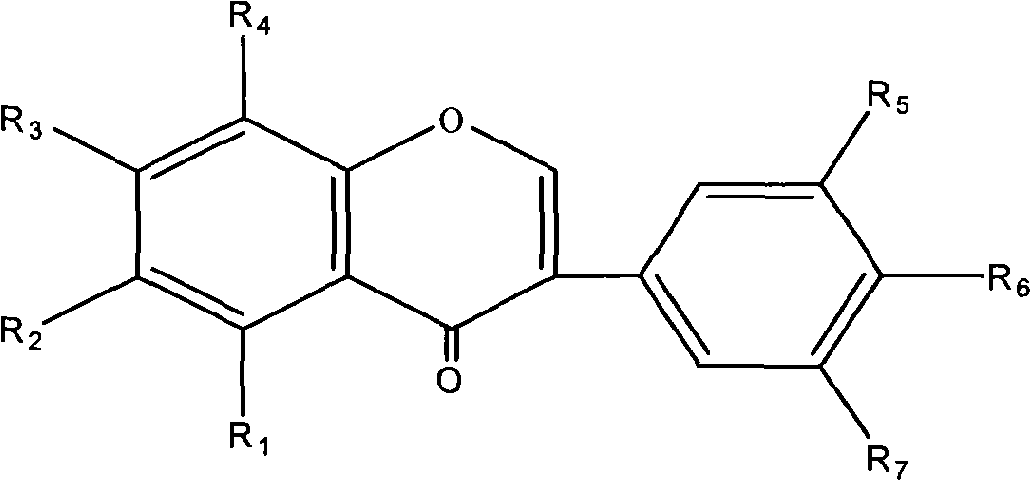
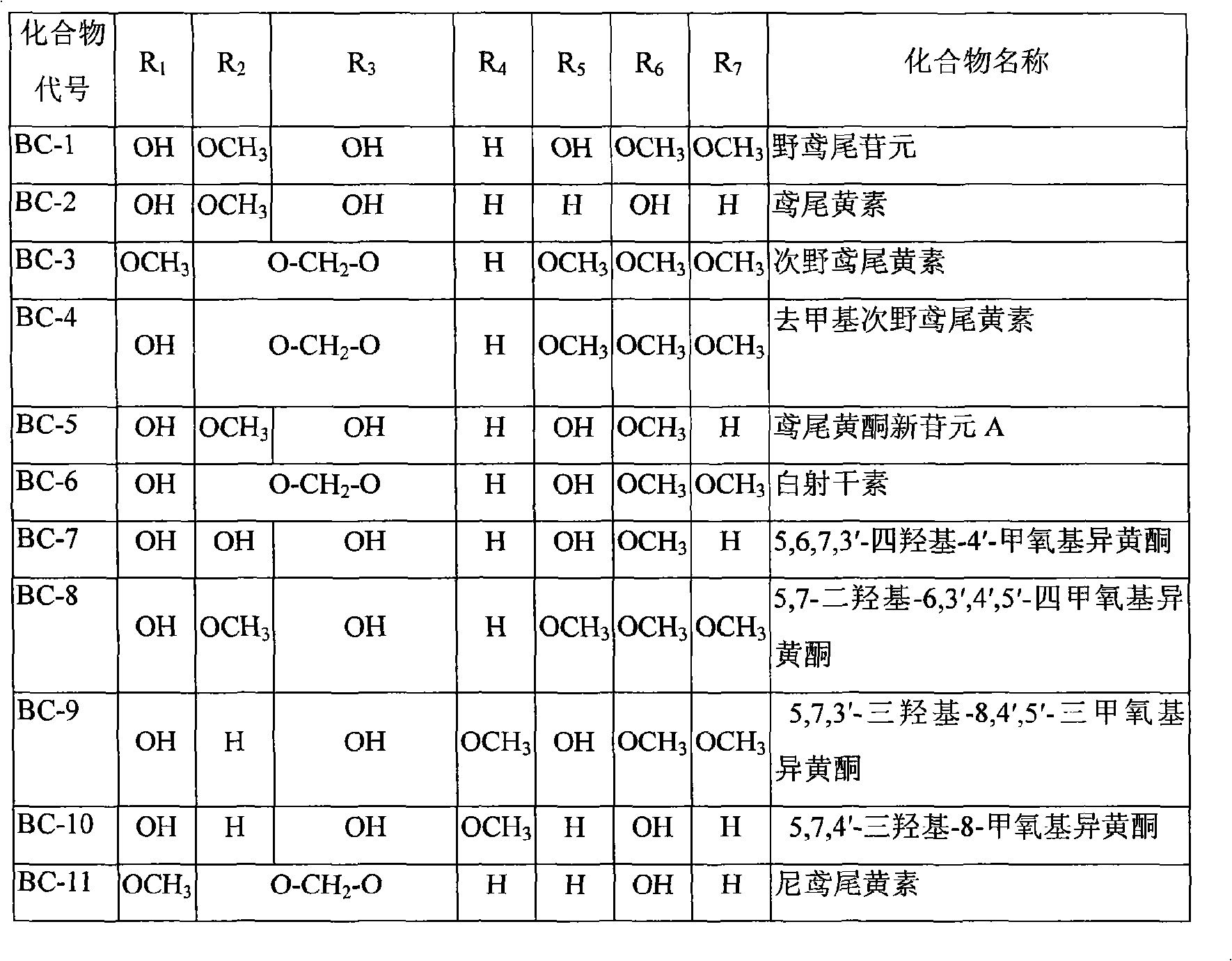
Use of composition of isoflavonoids from Belamcanda chinensis in preparing anti-hepatitis medicament
InactiveCN101301287ASignificant anti-HBV effectOrganic active ingredientsDigestive systemAntigenBelamcanda chinensis
The invention belongs to the medical technical field, in particular relating to an application of isoflavonoids from Belamcanda chinensis in the preparation of anti-hepatitis virus drugs. A Hepatitis B surface antigen (HBsAg) detection reagent test and a hepatitis B core antigen (HBeAg) detection reagent test are performed, and the results indicate that the isoflavonoids from the Belamcanda chinensis can remarkably suppress hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBeAg) and has remarkable effect on preventing and treating hepatitis B viruses. Therefore, the isoflavonoids from the Belamcanda chinensis can be used to prepare medicines or health-care food used to prevent and treat the hepatitis viruses.
Owner:上海双科医药科技有限公司
Recombination interferon with new space conformation and enhanced effect, its preparing method and application
ActiveCN1740197AStrong antiviral activityLittle side effectsPeptide/protein ingredientsAerosol deliveryAntigenSide effect
The invention provides recombination interferon (rSIFN-co) or the functional analog with new space conformation, enhanced effect, and low side effect. It is showed by the external pharmacodynamics, it can not only restrain the DNA replication of the hepatitis b virus, but also restrain the exudation of the surface antigen (HBsAg) and e antigen (HBeAg). The cytology toxicity is 1 / 8 of the clinical interferon and the antiviral activity is 5-20 times to the clinical interferon and has a higher, a more board spectrum and longer time biological response reaction, and prevent the tumor hyperplasia and transmission. The invention also provides the synthetic gene code, gene carrier, expression system of the synthetic gene of the recombination interferon or the functional analog. Finally, the invention also provides the preparing method and application thereof.
Owner:SUPERLAB FAR EAST LTD
Antrodia camphorata mycelium fermented extract and application thereof
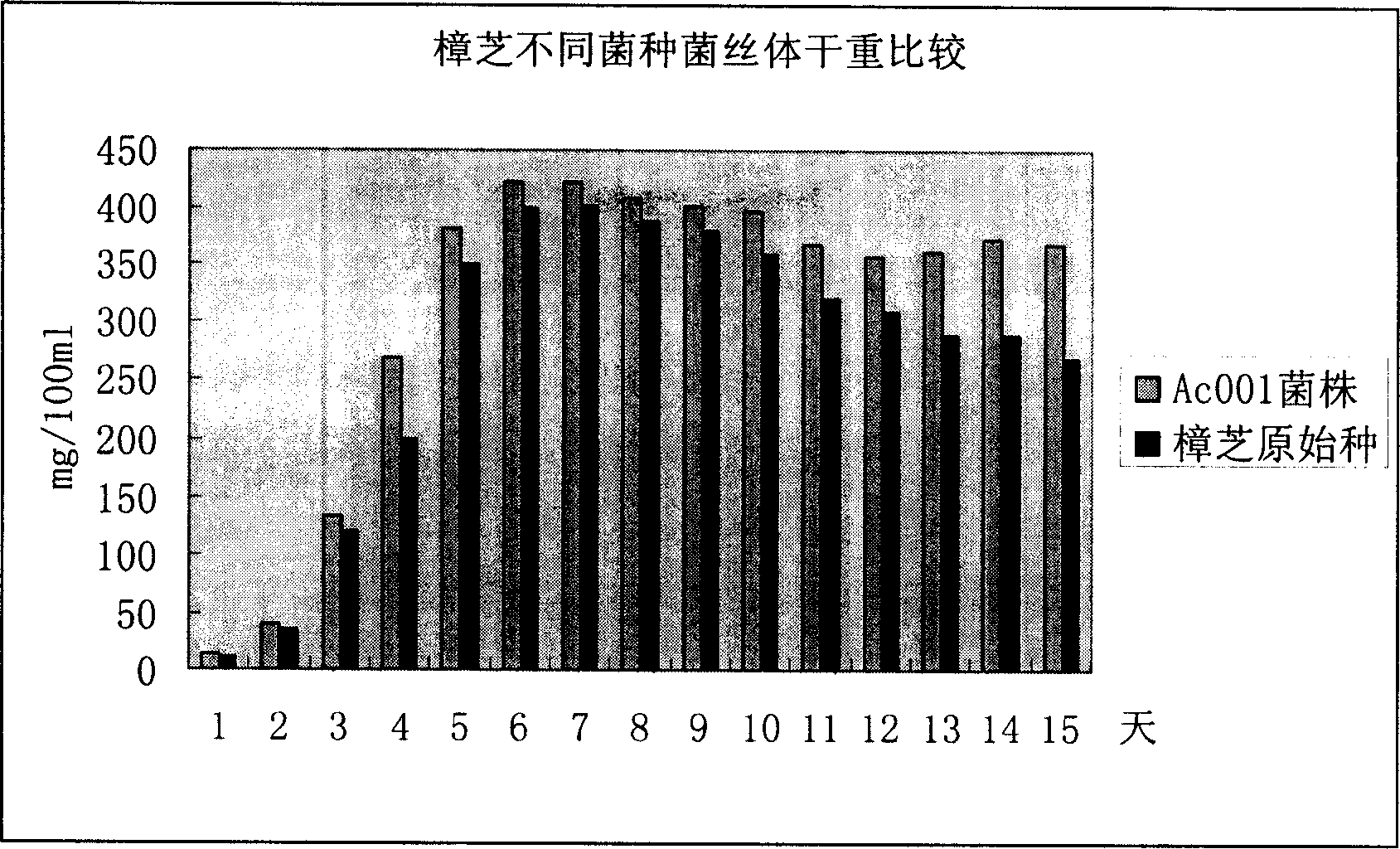
The invention discloses an Antrodia camphorate mycelium fermentation extract abstracted by ethanol, wherein the Antrodia camphorata bacterial strain Ac001 was preserved in the China General Microbiological Culture Collection Center with a docket number of CGMCC No.1460. The Antrodia camphorate mycelium fermentation extract has appreciable actions in inhibiting hepatitis B virus HbsAg, e antigen HbeAg and HBV DNA secretion and resisting cancers especially liver cancer.
Owner:LAIYANG AGRI COLLEGE
Use of 15-methano-substituted-andrographolide derivative in preparing anti-hepatitis B medicine
ActiveCN101416958AExpand the range of optionsTo clarify the anti-HBV activity in vitroOrganic active ingredientsDigestive systemHepatitis B virus core AntigenMedicine
The invention discloses the medical application of a 15-methylene replaced andrographolide derivant as shown in general formula 1, more particularly relates to the application thereof in preparing anti-hepatitis B virus drugs, pertaining to the pharmaceutical chemistry field. HepG2.2.15 cells are used for detecting the secretory volumes of HBsAg and HBeAg and the HBV DNA level related to viral particles in the supernatant liquid of a nutrient solution, and the result shows that the 15-methylene replaced andrographolide derivant has good in-vitro anti-HBV effect. The 15-methylene replaced andrographolide derivant has better development and application prospect by being applied in preparing drugs used for treating and preventing Hepatitis B.
Owner:ZHENGZHOU UNIV
Chemiluminescence method for qualitative and quantitative detection of hepatitis B virus
InactiveCN1963512AAccurate calculationAccurate back calculationChemiluminescene/bioluminescenceHepatitis B Virus AntigenTest agent
This invention relates to hepatitis B virus chemical light qualitative and quantitative test method, which tests hepatitis B surface antigen through its HBsAg and tests hepatitis B surface antibody through antibody HBs chemical test agent case and tests hepatitis B virus antigen through hepatitis antigen HBeAg test agent case and tests hepatitis virus e antibody through virus e antibody chemical property and tests hepatitis virus core antibody through virus core antibody chemical property.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Production method for producing medicine for treating hepatitis B by periplaneta americana extract
ActiveCN102885856AHigh cure rateControlled hydrolysisAnthropod material medical ingredientsDigestive systemAntigenChronic hepatitis
The invention provides a production method for producing a medicine for treating hepatitis B by a periplaneta americana extract, comprising the following steps of: A, mixing, and B, preparing the medicine. A method for extracting the periplaneta americana extract for treating hepatitis B comprises the following steps of: a, inactivating and crushing, b, extracting, c, primarily concentrating, d, degreasing and adsorbing, e, eluting, f, secondarily concentrating and g, drying and crushing. The medicine prepared by adopting the method can effectively control the hydrolysis of active ingredients for generating an inhibition effect on hepatitis B and increase the cure rate of hepatitis B, has an extraction rate of 3-5% for the active ingredients for hepatitis B, and has a total effective rate of 80-95% for the HBeAg (hepatitis Be antigen) negative conversion ratio and the HBV-DNA (hepatitis B virus-deoxyribose nucleic acid) negative conversion ratio of patients with chronic hepatitis B and carriers with hepatitis B virus. Via the method, the physical properties of the medicine are improved and the curative effect of the medicine is enhanced; the own smell of the medicine is effectively controlled, and the patients can conveniently take the medicine; simultaneously, harmful allergens in the medicine can be effectively reduced; and the method is low in cost and high in safety.
Owner:KUNMING SINOWAY NATURAL PHARMA
Bisheterocycle tandem compounds useful as antiviral agents, the uses thereof and the compositions comprising such compounds
InactiveUS7741348B2Inhibition of replicationEasy to prepareBiocideOrganic chemistryCytotoxicityResistant virus
The present invention provides small molecule compounds of bisheterocycle in tandem having the structural formula of P1-P2, and the use thereof as well as a composition containing the compounds, each of P1 and P2 is an unsaturated 5-member heterocyclic ring having one or two heteroatoms. This compound may effectively inhibit the replication of influenza virus, the DNA replication of hepatitis B virus (HBV), and the formation of HBsAg and HBeAg. These compounds can be used for the preparation of a medicament for viral diseases, and may overcome the limitations of the known nucleosides drugs, including cytotoxicity, the requirement of other drugs having different structures for against the drug-resistant virus variants induced by long-term therapy. The structure of the compounds according to the invention is relatively simple and easy to be prepared.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Bisheterocycle Tandem Compounds Useful as Antiviral Agents, the Uses Thereof and the Compositions Comprising Such Compounds
InactiveUS20080306121A1Inhibition of replicationEasy to prepareBiocideOrganic chemistryCytotoxicityResistant virus
The present invention provides small molecule compounds of bisheterocycle in tandem having the structural formula of P1-P2, and the use thereof as well as a composition containing the compounds, each of P1 and P2 is an unsaturated 5-member heterocyclic ring having one or two heteroatoms. This compound may effectively inhibit the replication of influenza virus, the DNA replication of hepatitis B virus (HBV), and the formation of HBsAg and HBeAg. These compounds can be used for the preparation of a medicament for viral diseases, and may overcome the limitations of the known nucleosides drugs, including cytotoxicity, the requirement of other drugs having different structures for against the drug-resistant virus variants induced by long-term therapy. The structure of the compounds according to the invention is relatively simple and easy to be prepared.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Application of imperatorin in preparing medicament for preventing and treating hepatitis or liver injury
ActiveCN101904839AEasy to manufactureWide variety of sourcesDigestive systemAntiviralsHepatitis B Surface AntigensVirus
The invention relates to the technical field of medicament, providing a novel application of coumarin compound imperatorin in preparing a medicament for preventing and treating virus hepatitis and resisting immunological liver injury or chemical liver injury. Pharmacological tests prove that the imperatorin has stronger activity of inhibiting hepatitis B surface antigen (HbsAg) and hepatitis B virus e antigen (HbeAg) and resisting the immunological liver injury or the chemical liver injury, so the imperatorin can be used for preparing the medicament for preventing and treating the virus hepatitis and resisting the immunological liver injury or the chemical liver injury. The imperatorin has the advantages of convenient preparation, wide sources, low price and high safety. The invention provides the novel application of the imperatorin, and provides a new medicinal source for preventing and treating the virus hepatitis and resisting the immunological liver injury or the chemical liver injury.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
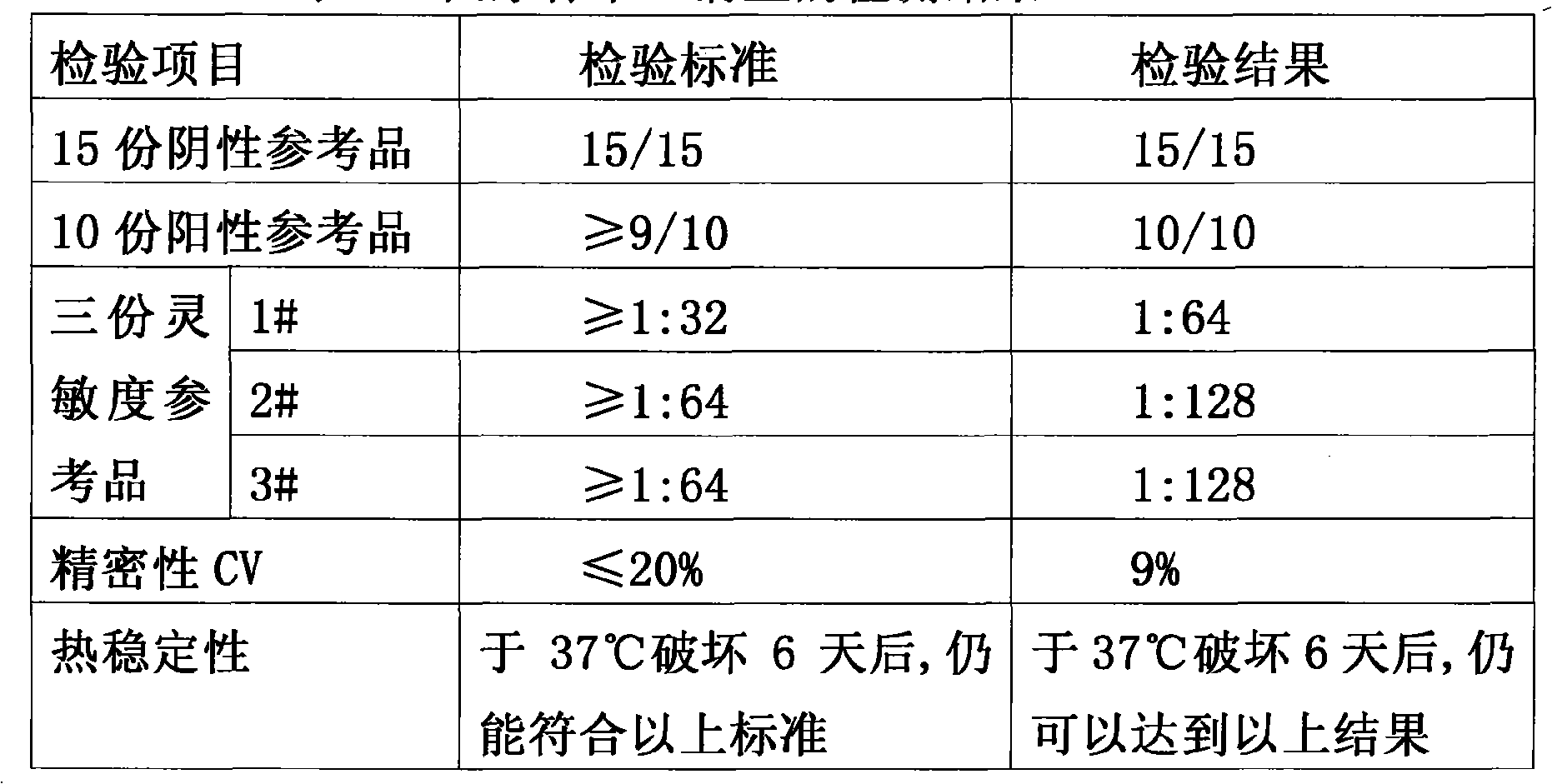
In vitro diagnosis kit of hepatitis B virus E antibody through dual-antigen sandwich method and preparation method thereof
InactiveCN101598730AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceColor/spectral properties measurementsChromogenic SubstratesE Antibody
The invention provides an in vitro diagnosis kit (an ELISA method) of a hepatitis B virus E antibody through a dual-antigen sandwich method, which comprises the following components: 1) a recombinant HBeAg coated in a solid phase carrier; 2) an enzyme-labeled recombinant HBeAg; and 3) a substrate solution, for example, a chromogenic substrate solution applied to the ELISA, or an enzymatic chemiluminescent substrate solution. Besides, the invention provides a method for preparing the kit, which comprises the following steps: 1) coating the recombinant HBeAg into the solid phase carrier; 2) performing enzyme labeling on the recombinant HBeAg; 3) preparing the substrate solution; and 4) assembling the components into a finished kit. The kit has the advantages of high sensitivity, good specificity, accurate result and strong clinical applicability, and can accurately reflect the amount of the E antibody in the body of a hepatitis B patient.
Owner:BEIJING BOSHENGFU BIOTECH CO LTD
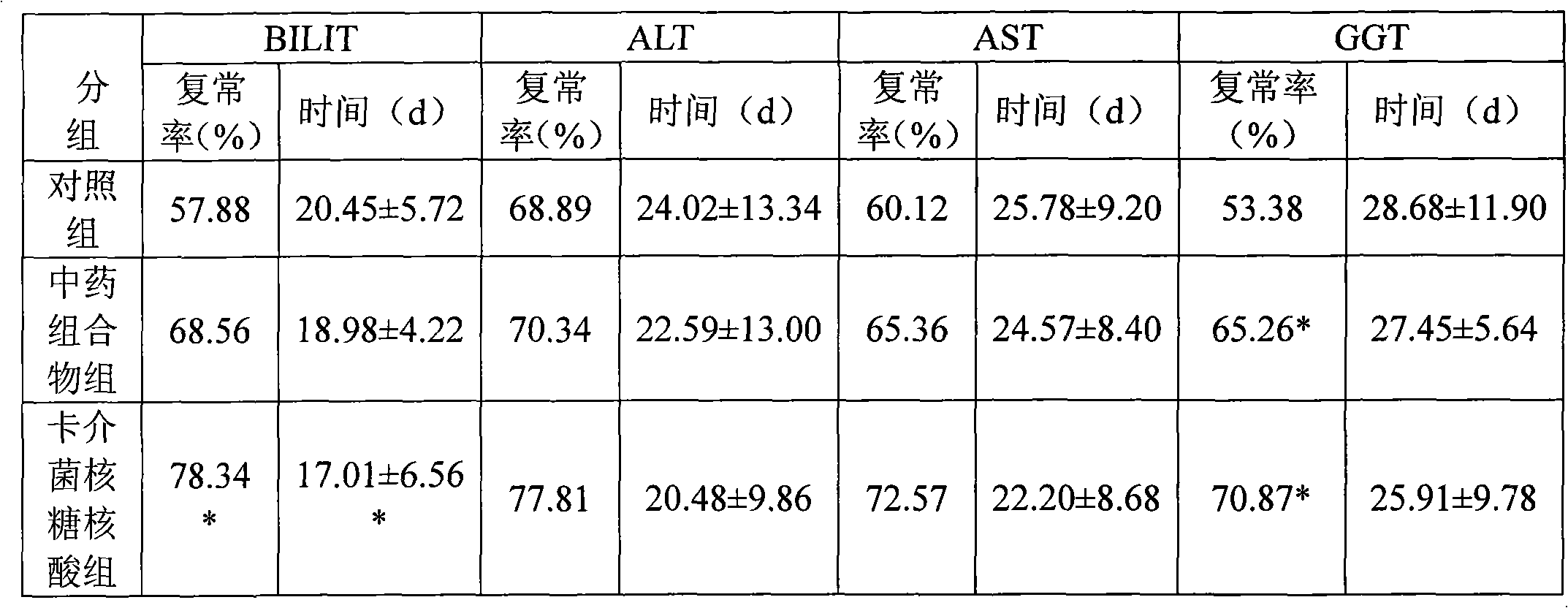
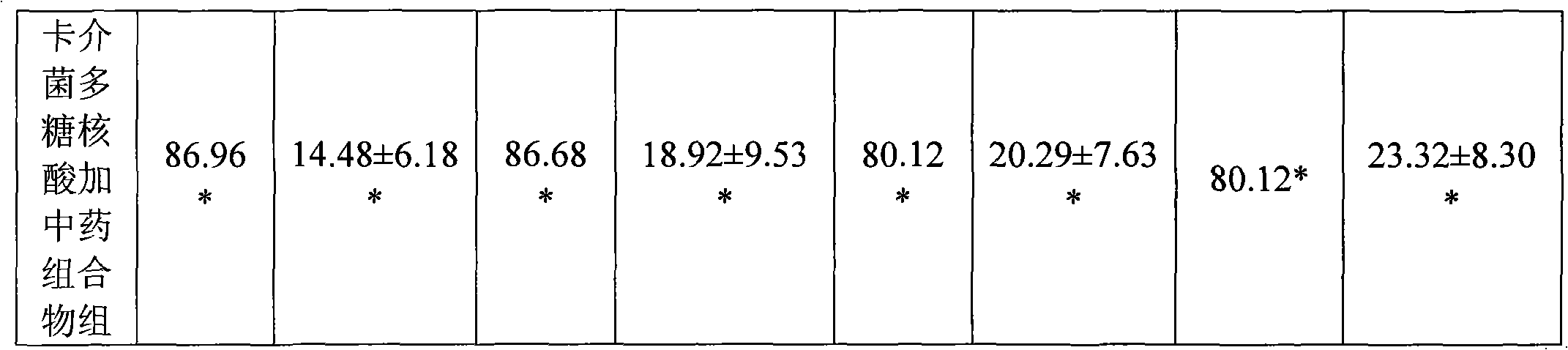
Medicine combination and application thereof in preparing preparations for treating chronic hepatitis B
ActiveCN101628046ARegulate immune functionImprove immunityBacteria material medical ingredientsDigestive systemToxic materialSpleen
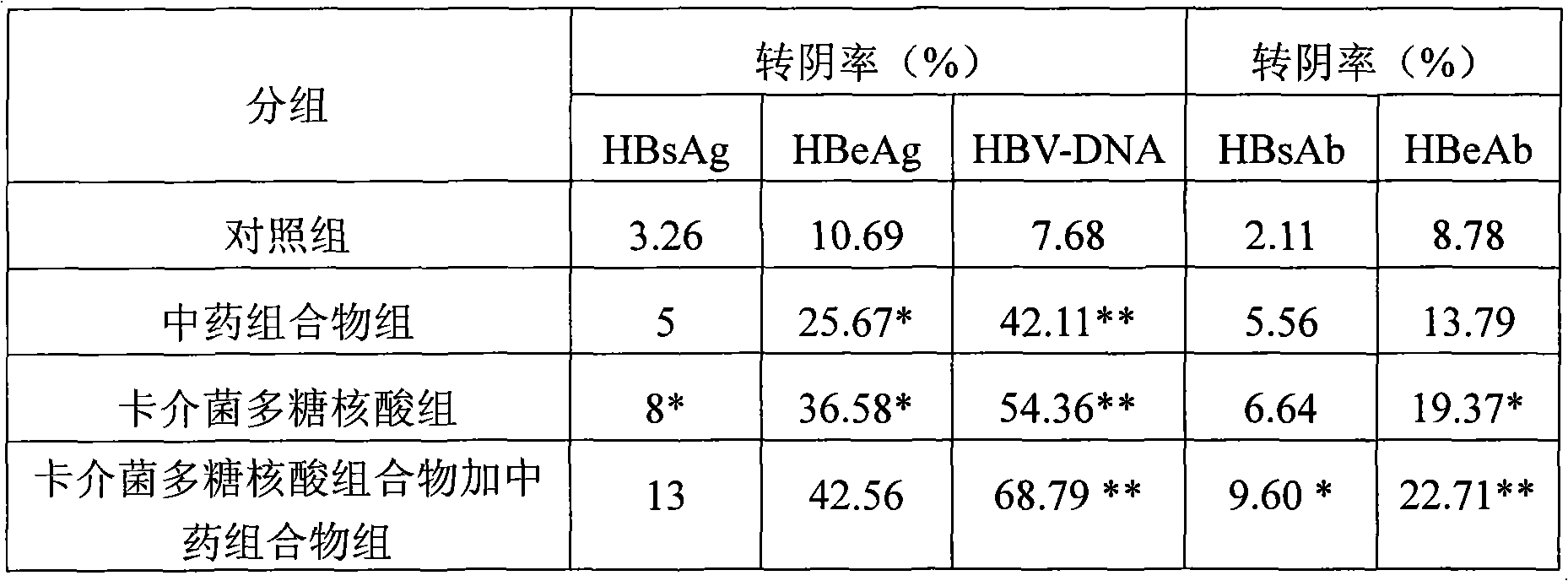
The invention relates to a medicine combination and the application thereof in preparing preparations for treating chronic hepatitis B. The combination comprises a medicine combination with polysaccharide nucleic acid of bacillus calmette guerin as the active component and a Chinese traditional medicine combination, wherein the Chinese traditional medicine combination is prepared from astragalus, herba artemisiae scopariae, oldenlandia diffusa, herba lysimachiae, codonopsis pilosula, processed polygonum capitatum, salvia, radix paeoniae alba, chinaberry fruit, taraxacum, cortex moutan, poria and atractylodes; the polysaccharide nucleic acid of bacillus calmette Guerin is nonspecific immunity active reinforcer, and can effectively restrain the reproduction of hepatitis virus and promote the rapid negative turning of HBV-DNA and HbeAg; and the Chinese traditional medicine combination has the efficacy of invigorating qi and spleen, promoting blood circulation by removing blood stasis and clearing away heat and toxic material. The medicine combination can reduce the incidence rate of variation and drug resistance and effectively promote the improvement under the condition of improving the healing efficacy, and provides a novel, safe and effective drug choice for clinics.
Owner:JIUZHITANG
Construction method and application of HBeAg transgenic mouse model
InactiveCN108424930AStable expressionDoes not affect normal biological functionCompounds screening/testingHydrolasesTreatment effectChronic hepatitis
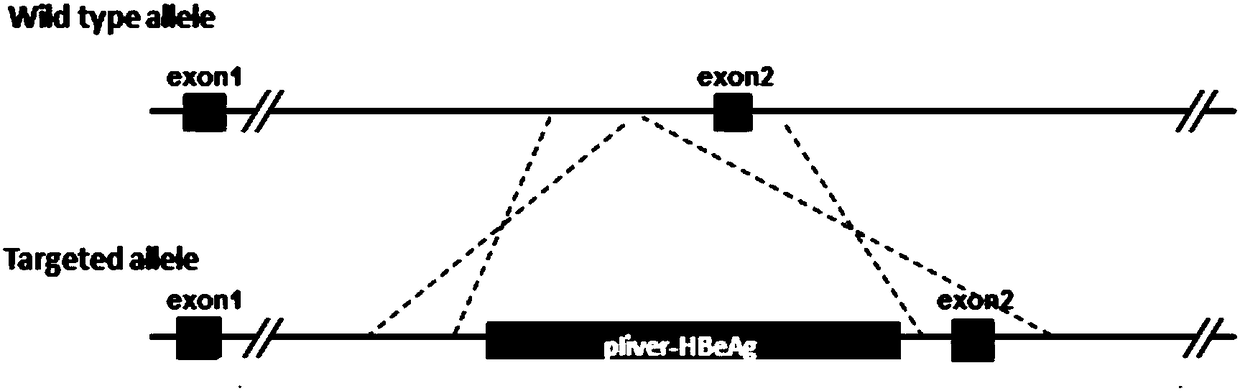
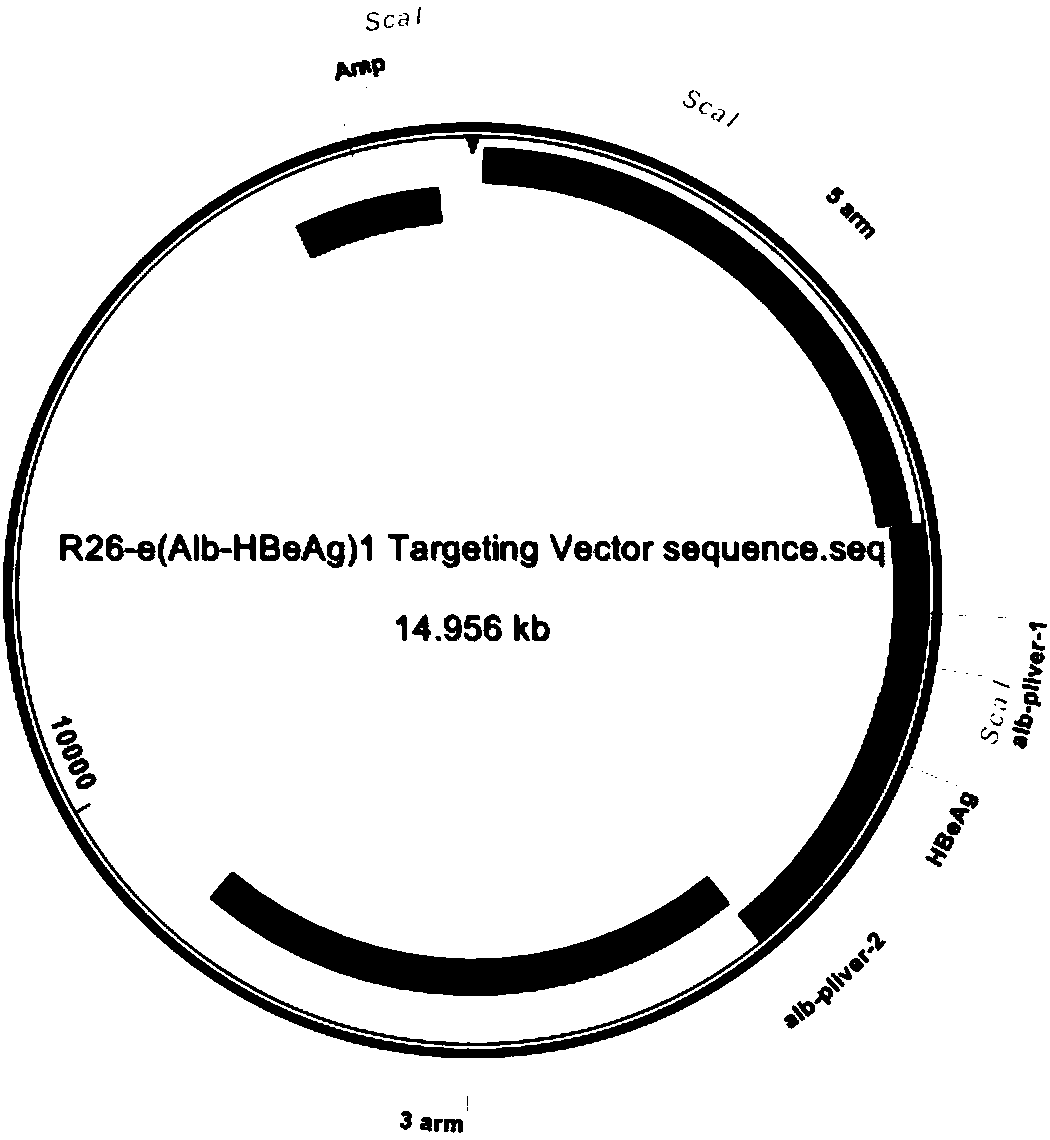
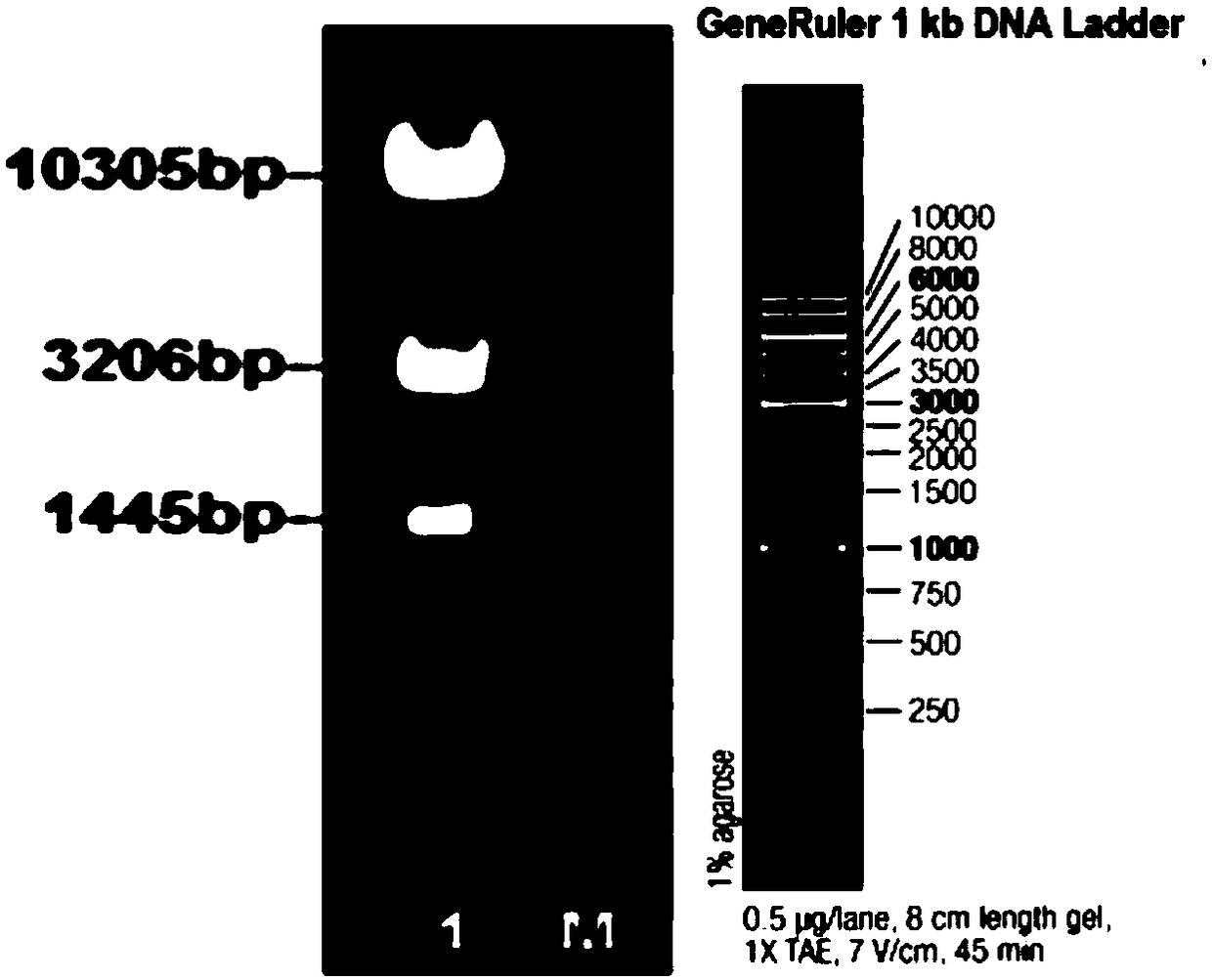
The invention provides a construction method and an application of an HBeAg transgenic mouse model. The method comprises the following steps: CRISPR / Cas9 technology is adopted, an HBeAg gene is inserted into a pliver-HBeAg expression cassette at the Rosa26 gene locus in a fixed-point manner through homologous recombination, and a recombinant R26-e(Alb-HBeAg)1 targeting vector is constructed with an In-Fusion cloning method and contains a 3.3kb 5' homologous arm, pliver-HBeAg and a 3.3kb 3' homologous arm; Cas9mRNA, gRNA and the recombinant R26-e(Alb-HBeAg)1 targeting vector are micro-injectedinto a zygote of a C57BL / 6J mouse, then the zygote is transplanted into the uterus of a C57BL / 6J female mouse, and an HBeAg transgenic mouse is obtained through propagation and purification step by step. The transgenic mouse can specifically express HBeAg in liver, thereby being capable of applying to experimental animal models for researching HBeAg infection mechanism and evaluating treatment effects of therapeutic drugs and vaccines for chronic hepatitis B.
Owner:XI AN JIAOTONG UNIV
Sequence of hepatitis B virus (HBV) specific microRNA like siRNA (msiRNA) and application thereof
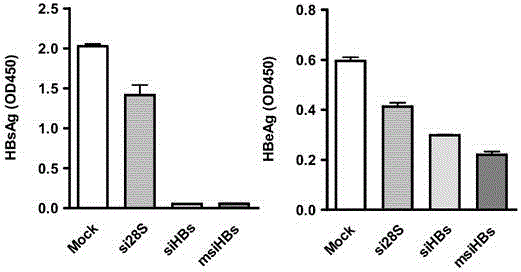
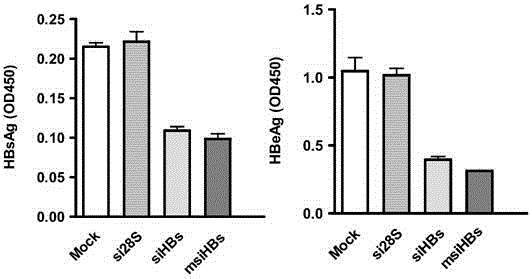
InactiveCN104059916AHas immunostimulatory activityInhibit expressionAntiviralsDNA/RNA fragmentationNucleotideHbv replication
The invention provides a sequence of microRNA like siRNA (msiRNA) aiming at a hepatitis B virus (HBV) S gene and a secondary structure of msiRNA. The sequence is a nucleotide sequence of msiHBs. After co-transfecting HepG2 and Huh7 cells, the msiRNA and a reporter HBV replicating plasmid pHY106+wta can inhibit expression of HBsAg and HBeAg. After transfecting the HepG2.2.15 cell, the msiHBs can also effectively inhibit the expression levels of HBsAg and HBeAg and the load of the HBV in the supernatant. Besides, the msiRNA also has the immunostimulatory activity and can activate innate immunity via a Toll like receptor pathway. Therefore, the msiRNA has double activities of inhibition of HBV gene expression as well as replication and immunostimulation and can be applied to preparation of drugs for treating HBV chronic infection.
Owner:SHIYAN TAIHE HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com