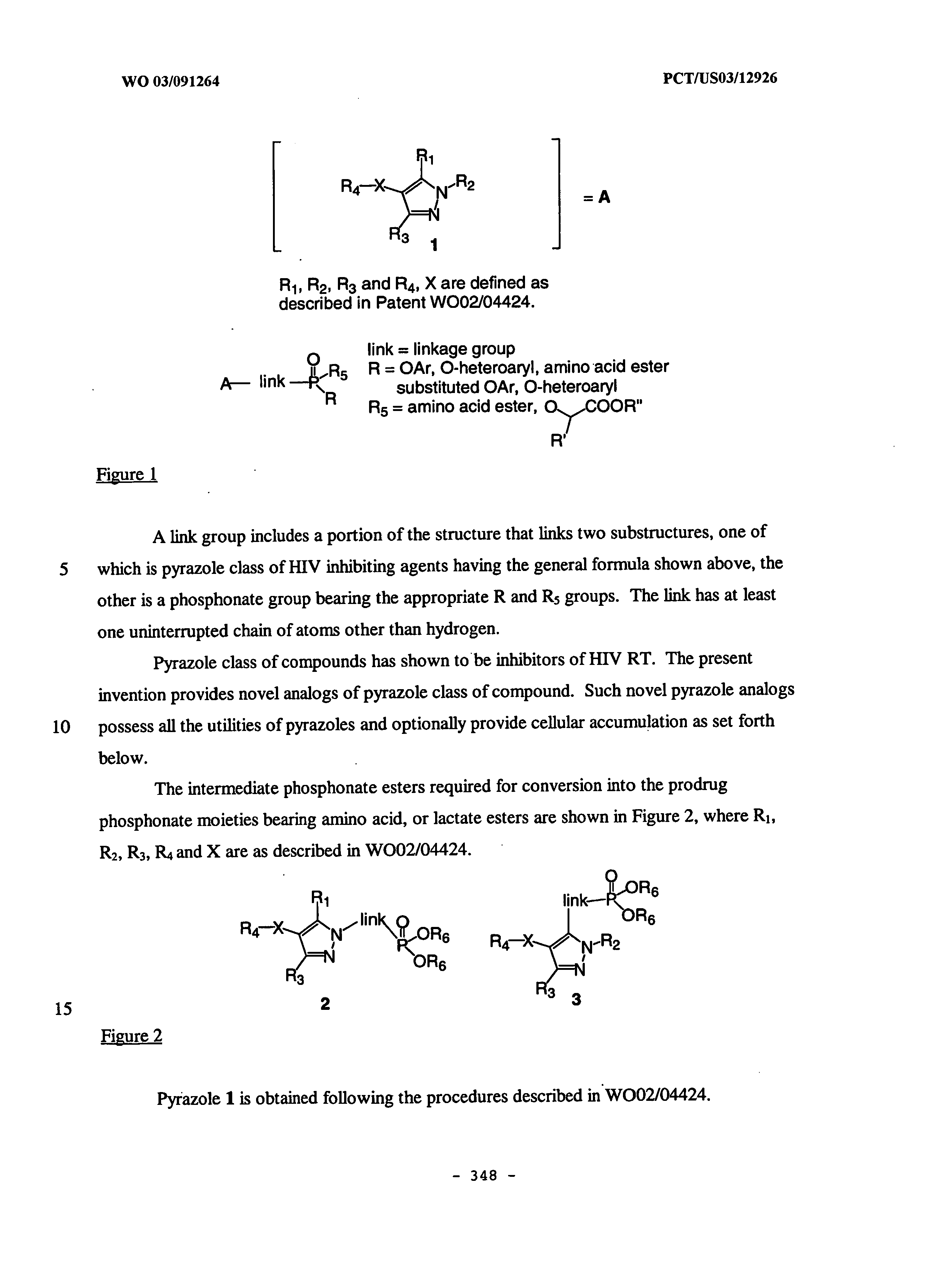
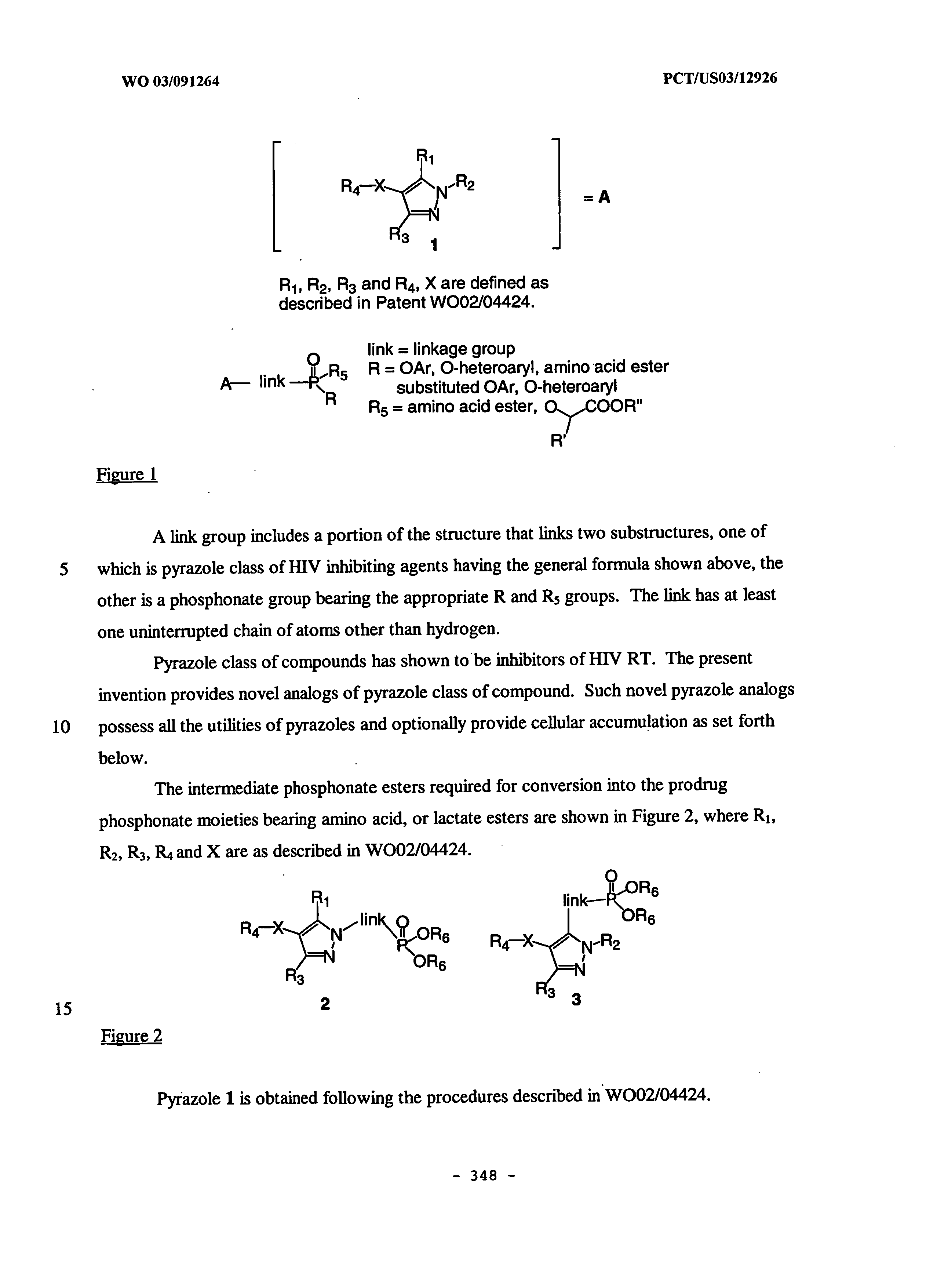
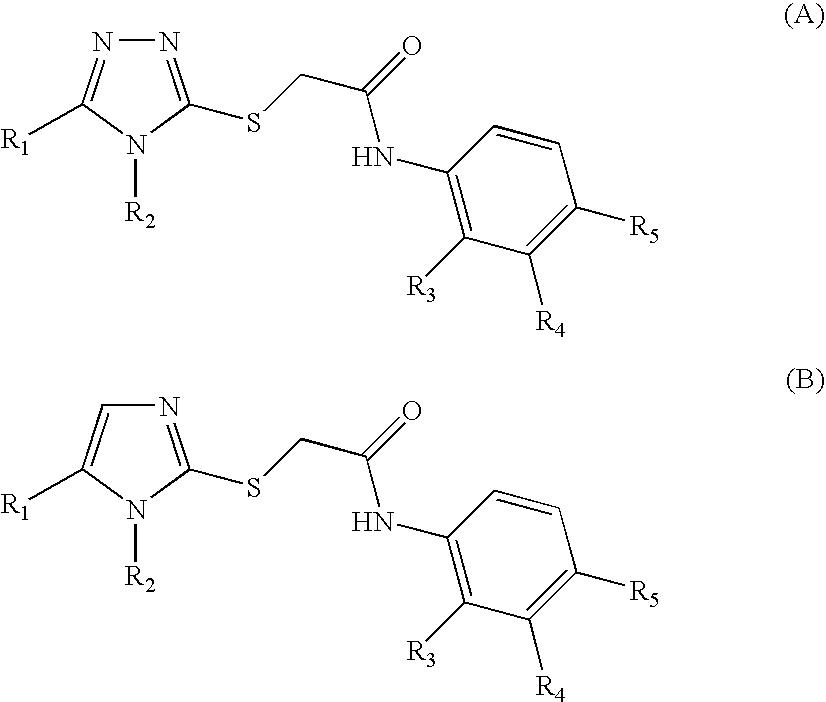
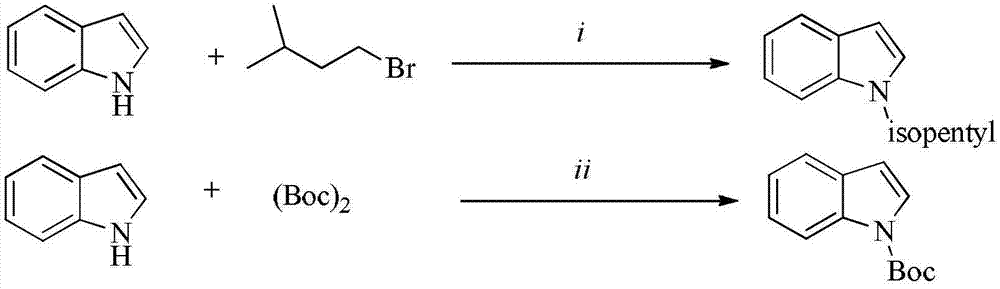
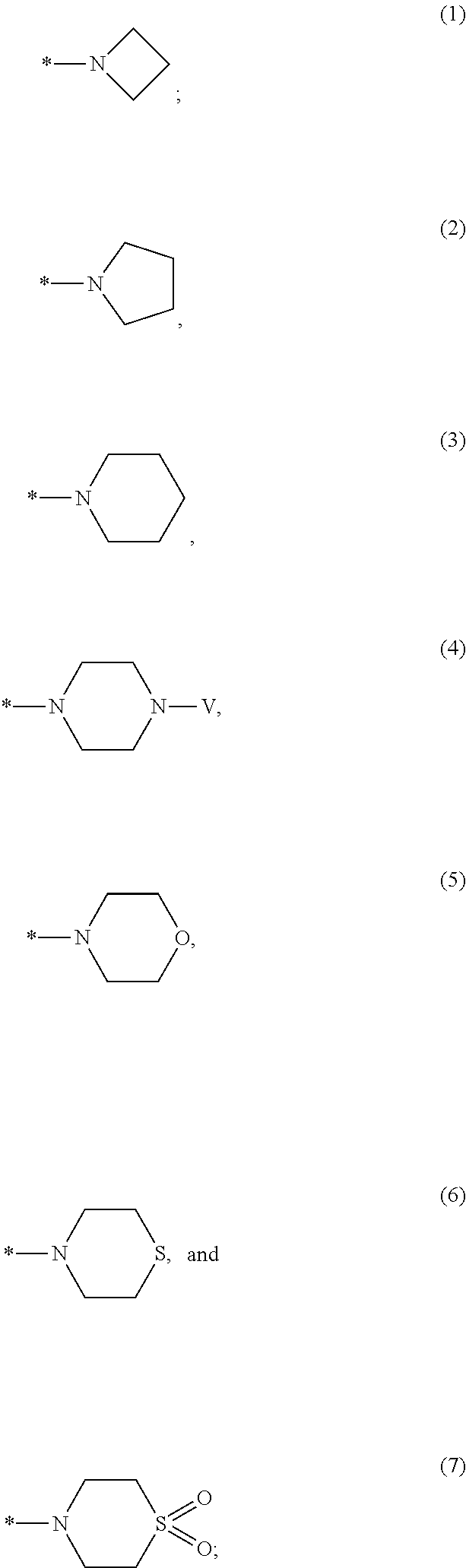
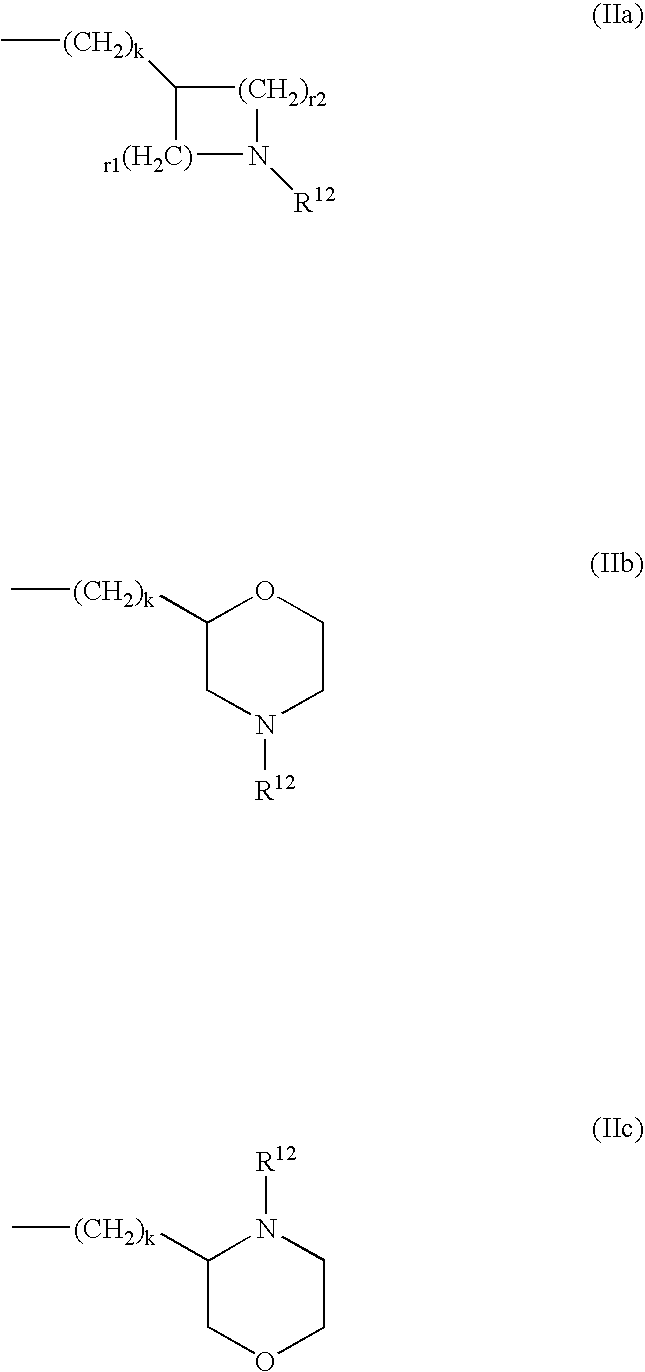
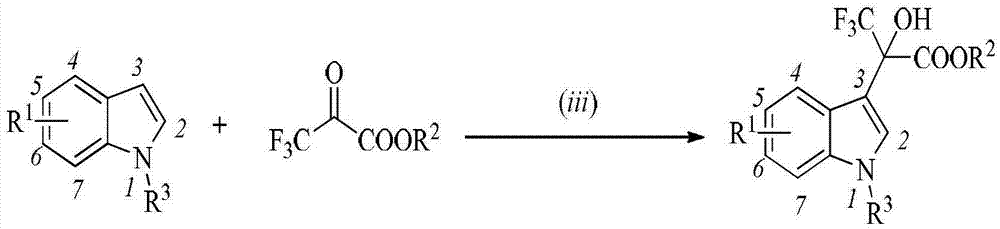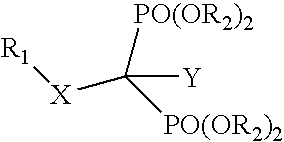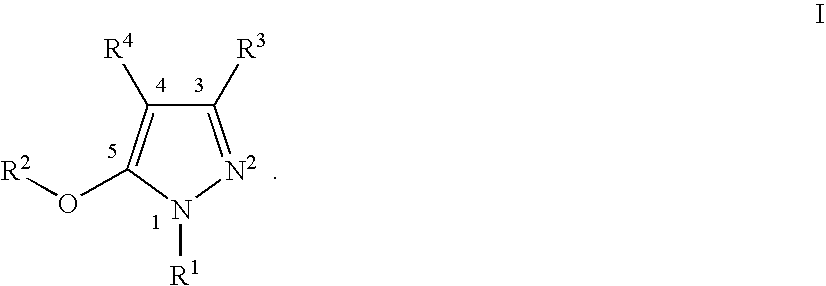Patents
Literature
192 results about "Nucleoside Reverse Transcriptase Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A substance with a structure similar to a nucleoside molecule that is capable of occupying the active catalytic site of a reverse transcriptase resulting in inhibition of enzyme function, chain termination, and inhibition of viral replication. These compounds are converted to their active phosphorylated forms by cellular kinases.
Compressed tablet formulation
InactiveUS7060294B2Organic active ingredientsCapsule deliveryNucleoside Reverse Transcriptase InhibitorClinical study
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
Non-nucleoside reverse transcriptase inhibitors
InactiveUS20070088015A1BiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorPharmaceutical drug
Owner:ROCHE PALO ALTO LLC
Non-nucleoside reverse transcriptase inhibitors
Owner:ROCHE PALO ALTO LLC
Non-nucleoside reverse transcriptase inhibitors
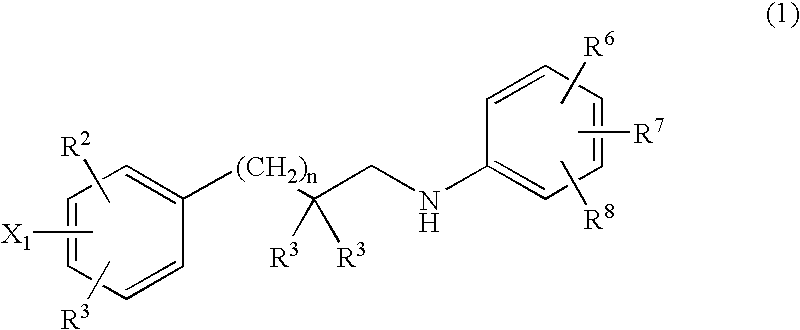
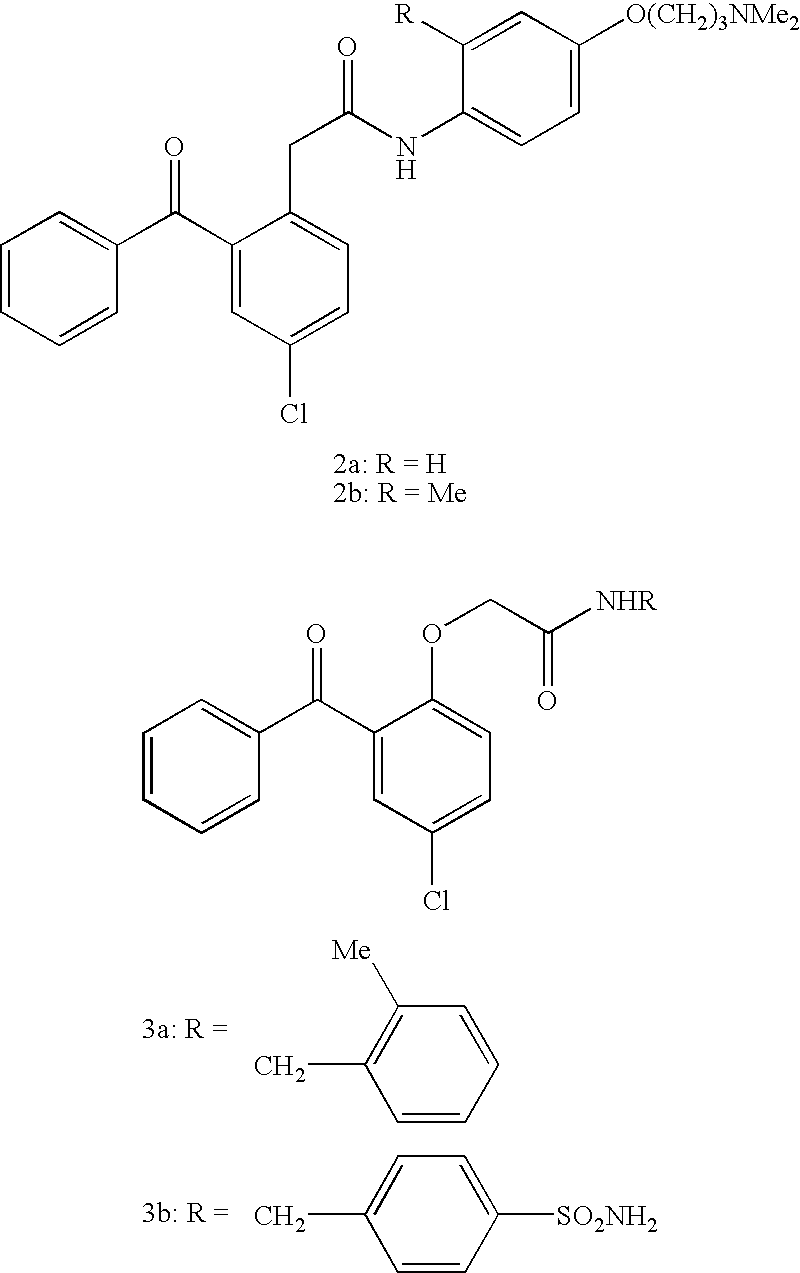
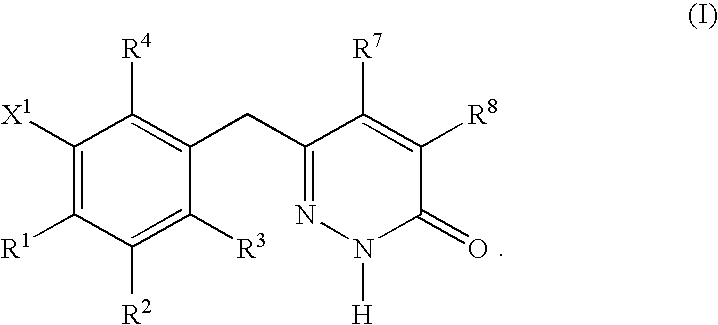
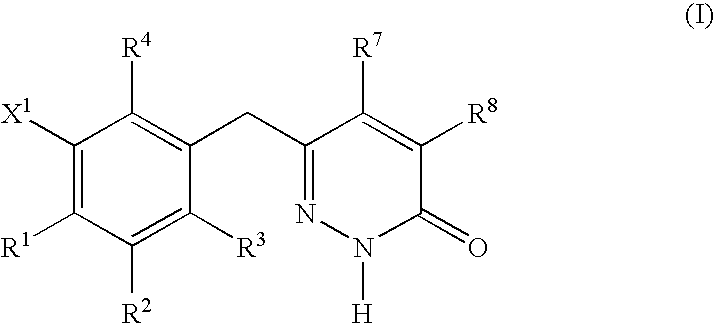
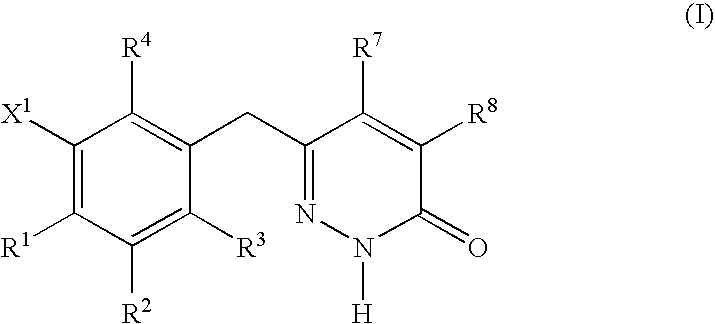
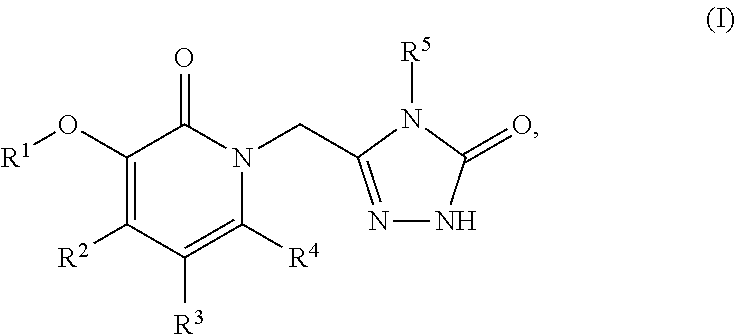
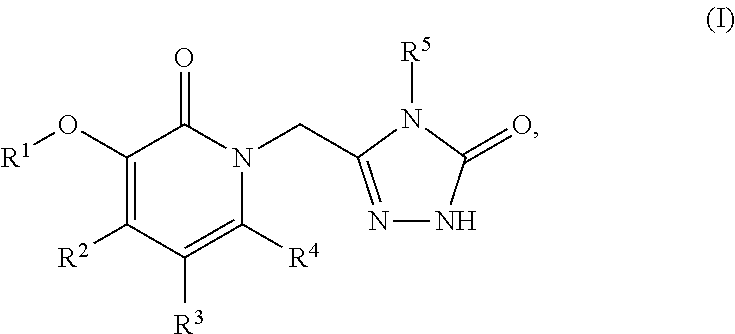
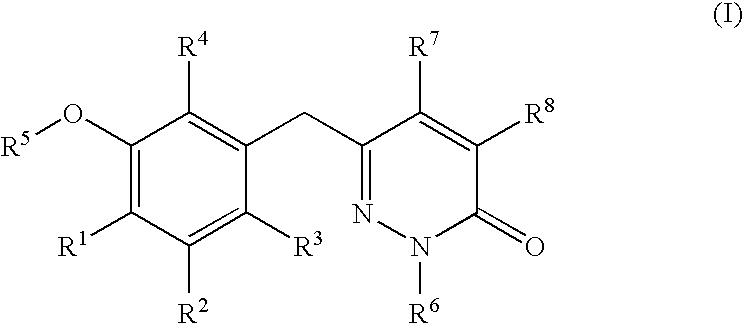
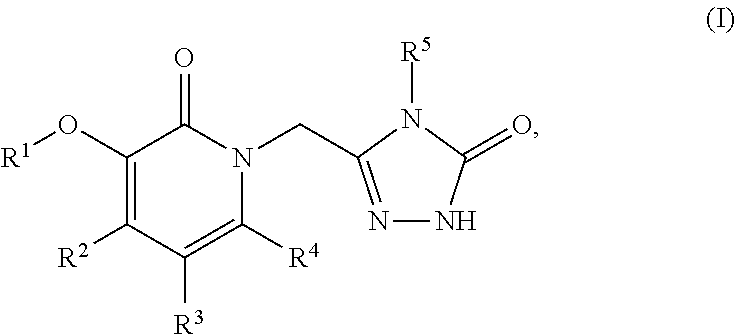
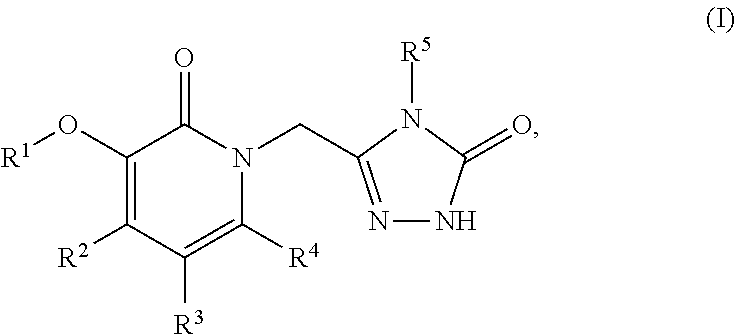
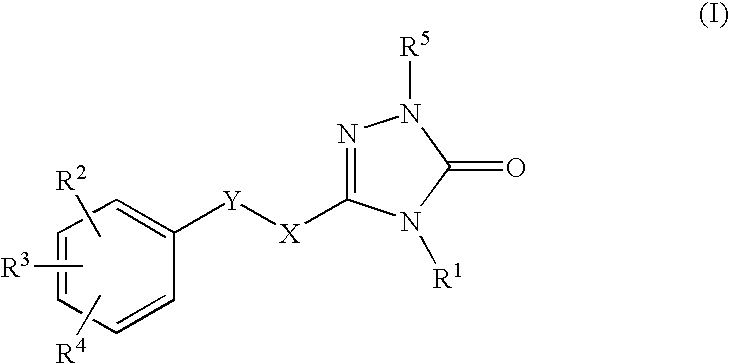
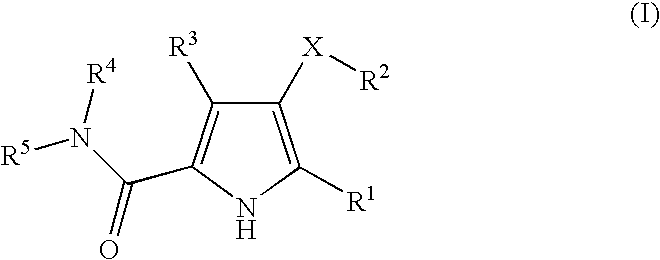
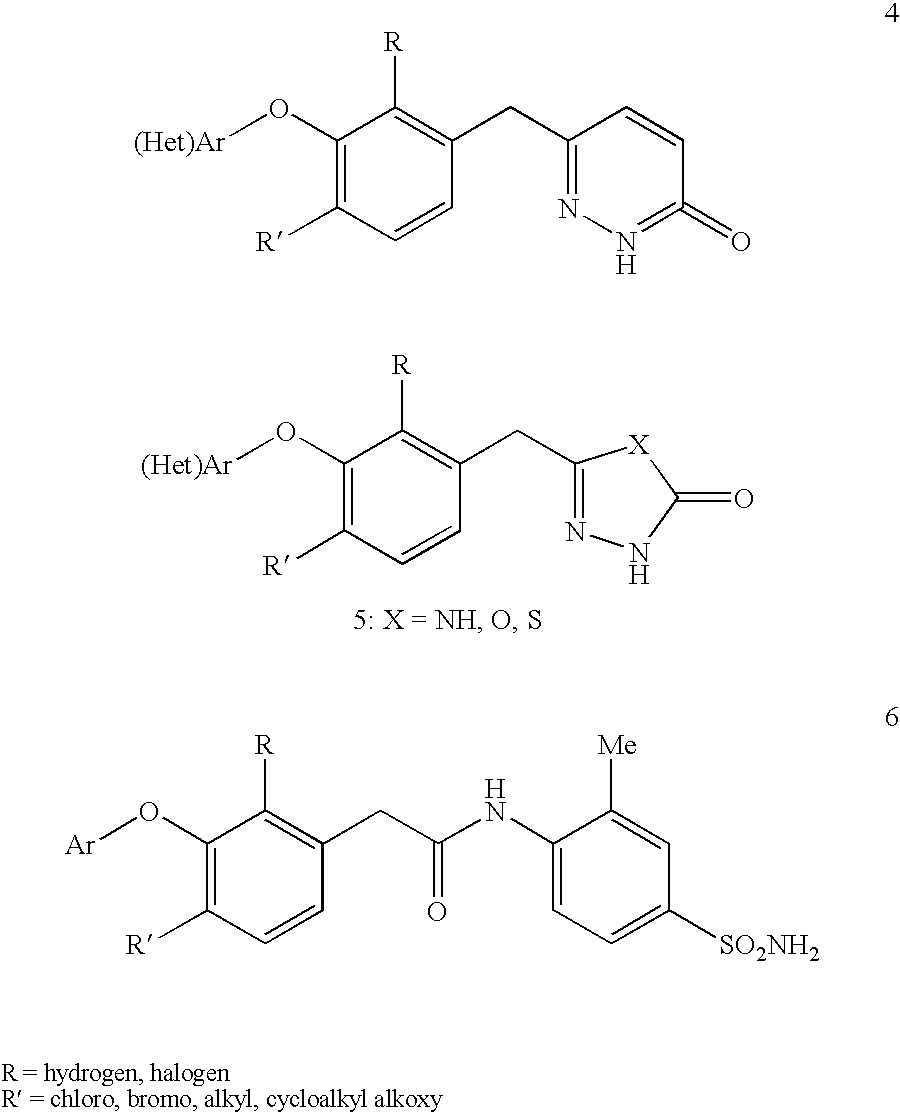
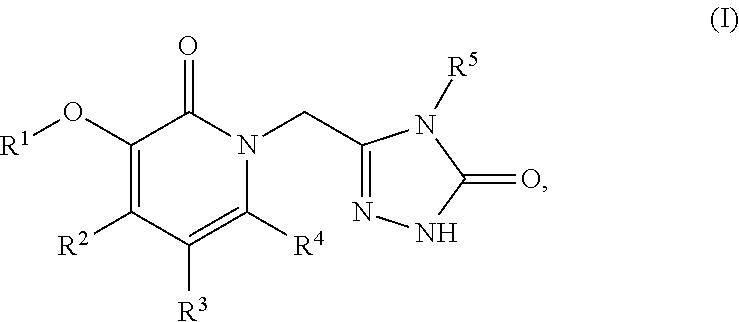
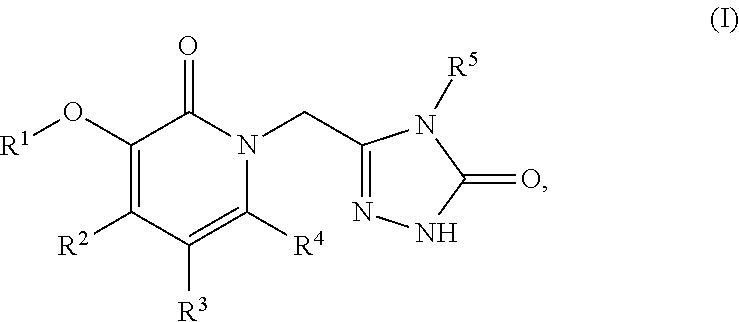
This invention relates to novel pyridazinone derivatives of formula I wherein R1–R4, R7, R8 and X1 are as defined in the summary and pharmaceutically acceptable salts and solvates thereof, methods to inhibit or modulate Human Immunodeficiency Virus (HIV) reverse transcriptase with compounds of formula I, pharmaceutical compositions containing of formula I admixed with at least one solvent, carrier or excipient and processes to prepare compounds of formula I. The compounds are useful for treating disorders in which HIV and genetically related viruses are implicated
Owner:ROCHE PALO ALTO LLC
Non-nucleoside reverse transcriptase inhibitors
ActiveUS20050239881A1Inhibit HIV reverse transcriptaseReduce sensitivityBiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorMedicine
Owner:ROCHE PALO ALTO LLC
Non nucleoside reverse transcriptase inhibitors
InactiveUS20060128692A1Compound screeningBiocideNucleoside Reverse Transcriptase InhibitorReverse transcriptase activity
Phosphorus-substituted imidazole compounds with anti-HIV properties having use as therapeutics and for other industrial purposes are disclosed. The compositions inhibit reverse transcriptase activity and are useful therapeutically for the inhibition of such enzymes, as well as in assays for the detection of such enzymes.
Owner:GILEAD SCI INC
Non-nucleoside reverse transcriptase inhibitors
InactiveUS20080249131A1BiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorNon nucleoside inhibitor
Owner:ARDEA BIOSCIENCES INC
Non-nucleoside reverse transcriptase inhibitors
Heteroaromatic compounds of Formula I:are HIV reverse transcriptase inhibitors, wherein R1, R2, R3, R4 and R5 are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK CANADA
Non-nucleoside reverse transcriptase inhibitors
InactiveUS20060135556A1Reduce virus spreadSuppression problemBiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorNon nucleoside inhibitor
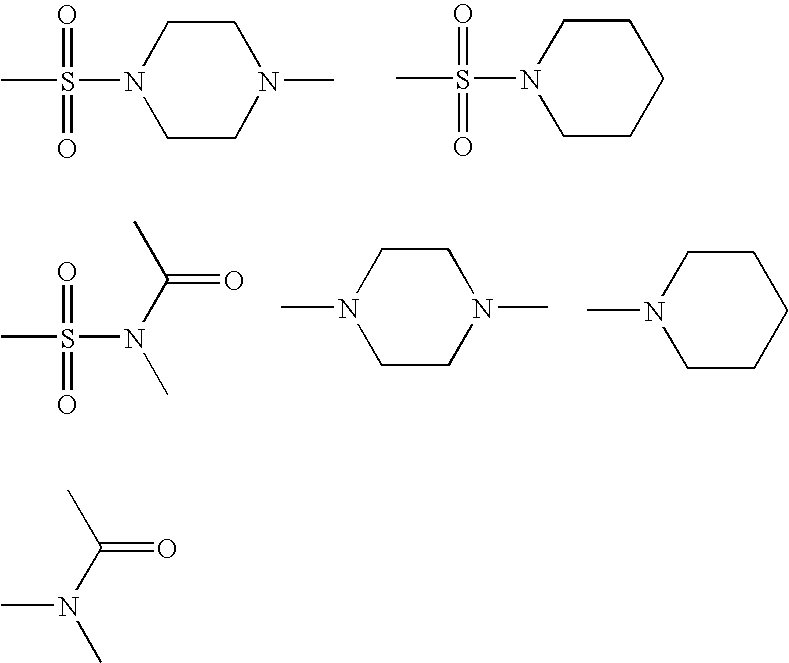
Various carbonyl amides are employed in vitro and in vivo as non-nucleoside inhibitors of a reverse transcriptase, and particularly of HIV reverse transcriptase. Therefore, contemplated compounds may be employed in the treatment of HIV infected patients. Further contemplated aspects include pharmaceutical compositions comprising therapeutically effective amounts of contemplated compounds.
Owner:ARDEA BIOSCI
Non-nucleoside reverse transcriptase inhibitors
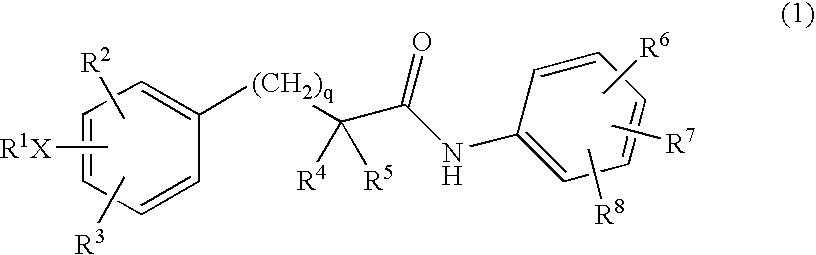
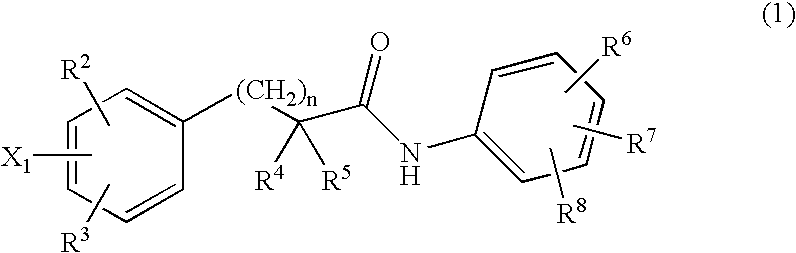
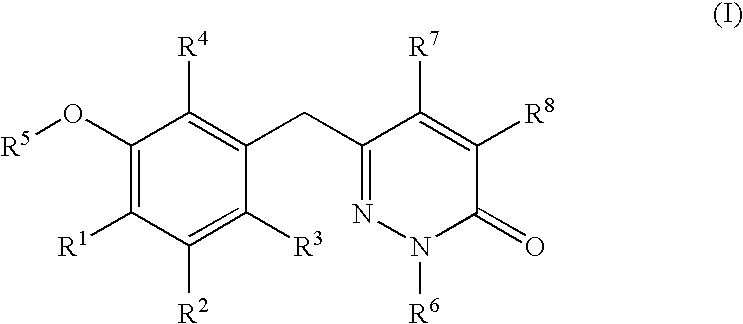
The present invention relates to a compounds according to formula I, methods for treating diseases mediated by human immunodeficieny virus by administration of a compound according to formula I and pharmaceutical compositions for treating diseases mediated by human immunodeficieny virus containing a compound according to formula I where R1, R2, R3, R4, R5, R6, R7 and R8 are as defined herein.
Owner:ROCHE PALO ALTO LLC
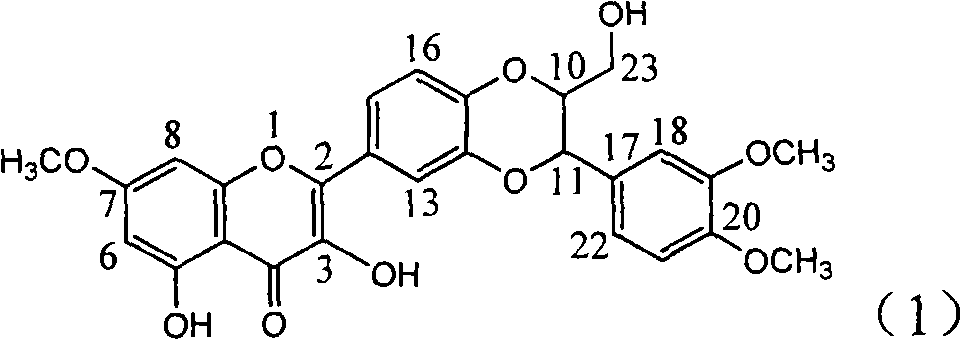
Application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B
InactiveCN101912385AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention relates to application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B, in particular to the application of 7 and 20-position methyl substituted dehydrated silybin or pharmaceutically acceptable salts thereof in preparing medicaments for removing HBsAG and HBsAg and medicaments for inhibiting HBV DNA replication. The dehydrated silybin has remarkably HBsAg and HBeAg inhibiting activity, wherein the strength for removing the HBsAg and the HBeAg at the concentration of 20 milligram / milliliter is 88.9 percent and 84.1 percent respectively, which are 5.5 times and 5.0 times that of a positive contrast medicament. More importantly, the dehydrated silybin shows the HBV DNA inhibition ratio of about 99.6 percent at the concentration of 20 milligram / milliliter, the activity exceeds lamivudine by 23 percent, which is 2.6 times that of interferon. Therefore, favonolignan or pharmaceutically acceptable salts thereof can be predictably used for preparing the non-nucleoside reverse transcriptase inhibitor medicaments for removing the HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
Non-nucleoside reverse transcriptase inhibitors
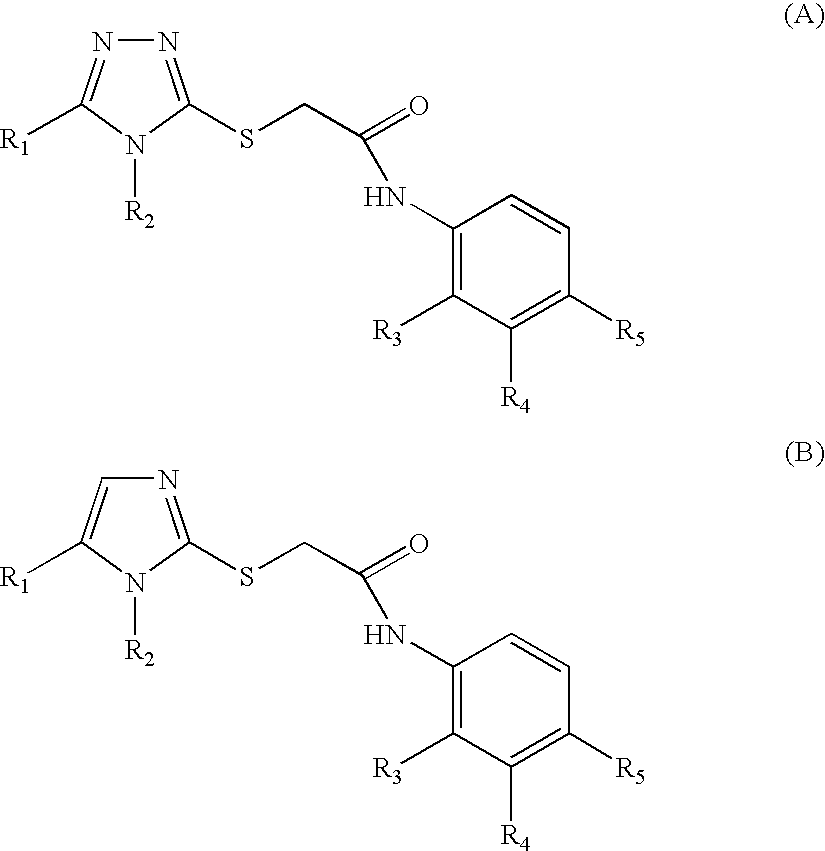
Disclosed herein are compounds of formula Ar1—X—W—Ar2 wherein Ar1 and Ar2 represent aryl groups characterized generally as aromatic heterocycles (e.g. imidazolyl or tetrazolyl) or carbocycles (e.g. phenyl or naphthalenyl); the aryl groups are optionally substituted or fused with other heterocycles or carbocycles; the aryl groups can bear substituents such as alkyl, halo or O-alkyl. X is a heteroatom, a valence bond or an optionally substituted divalent methylene, and W represents a spacer; typical spacers include divalent alkylene or alkylene-amido, -amido or -oxy radicals, which may optionally be substituted (e.g. hydroxyl or oxo). A typical compound is a derivative of 2-(N-napthalenyltetrazolylthio)-N-(2-nitrophenyl)acetamide. The compounds have inhibitory activity against Wild Type and single or double mutant strains of HIV.
Owner:BOEHRINGER INGELHEIM INT GMBH
Combination therapy for treating viral infections
A method of treating viral infections, particularly Hepatitis B (HBV) and Human Immunodeficiency Virus (HIV) infections, by administering Elvucitabine and a second active agent to a patient suffering viral infection is provided herein. The second active agent is, for example, an immunomodulatory compound, an anti-viral agent, or a combination comprising one or more of the foregoing active agents. For example the anti-viral agent may be a tyrosine kinase inhibitor, a CCR5 inhibitor, a non-nucleoside reverse transcriptase inhibitor, a protease inhibitor, an integrase inhibitor. Further provided herein are combination dosage forms comprising Elvucitabine and a second active agent. The combination dosage may be administered once per day. The Elvucitabine may be administered less frequently than the second active agent. Packaged pharmaceutical compositions comprising Elvucitabine, a second active agent, and instructions for using the composition for treating a viral infection by administering Elvucitabine and the second active agent are also provided.
Owner:ACHILLION PHARMA INC
Immunoassays, haptens, immunogens and antibodies for anti-HIV therapeutics
InactiveUS20050244816A1BiocideMicrobiological testing/measurementNucleoside Reverse Transcriptase InhibitorAntiendomysial antibodies
This invention provides compounds, methods, immunoassays, and kits relating to active, metabolically sensitive (“met-sensitive”) moieties of anti-HIV therapeutics, such as HIV protease inhibitors (PI) and HIV non-nucleoside reverse transcriptase inhibitors (NNRTI).
Owner:ARK DIAGNOSTICS
Non-nucleoside reverse transcriptase inhibitors
Heteroaromatic compounds of Formula I:are HIV reverse transcriptase inhibitors, wherein R1, R2, R3, R4 and R5 are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK CANADA
Indole compound and application thereof as HIV-1 reverse transcriptase inhibitor
InactiveCN103113285AOrganic active ingredientsOrganic chemistryNucleoside Reverse Transcriptase InhibitorStereochemistry
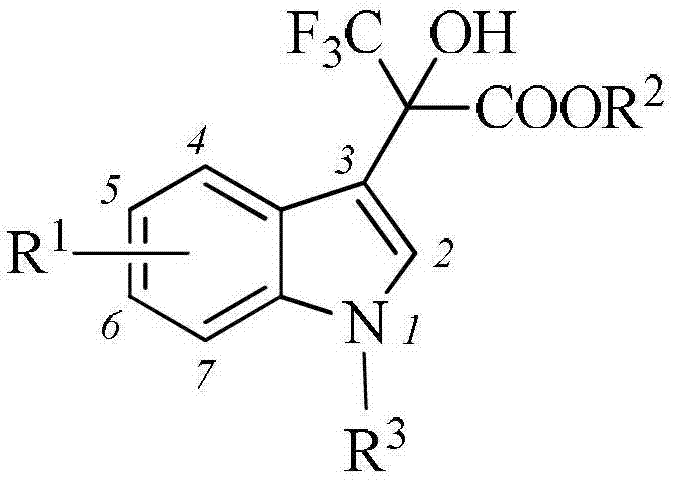
The invention belongs to the field of medical technology, and specifically discloses an indole compound and an application thereof as an HIV-1 reverse transcriptase inhibitor. The indole compound disclosed by the invention has a structural general formula: wherein the formula is described in the specification.
Owner:WUHAN UNIV
Method and composition for the treatment of diseases caused by or associated with HIV
InactiveUS20130171161A1Organic active ingredientsEnergy modified materialsNucleoside Reverse Transcriptase InhibitorCytokine
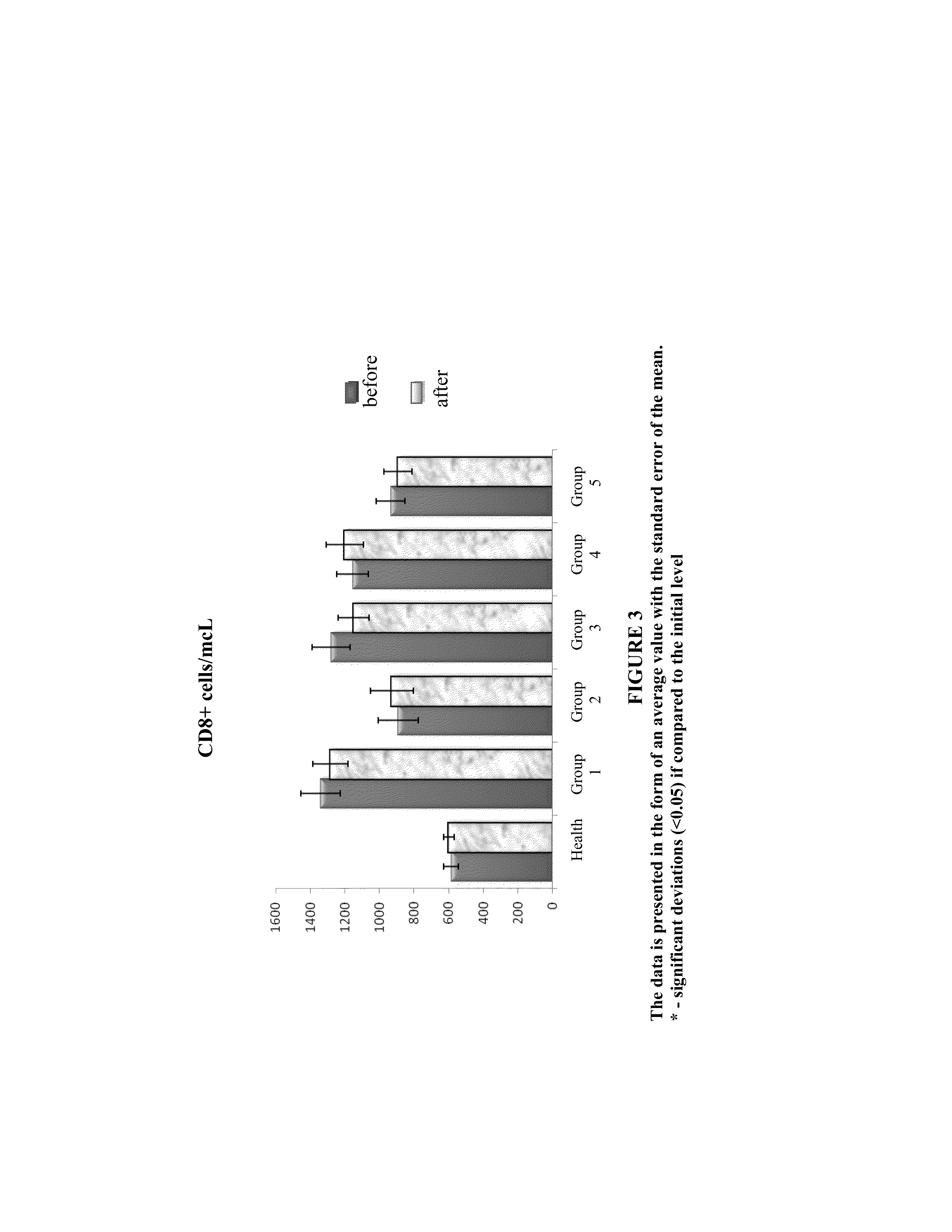
The invention provides a combination pharmaceutical composition comprising a) at least one activated-potentiated form of an antibody to at least one cytokine or at least an activated-potentiated form of an antibody to at least one receptor; and b) an effective amount of a nucleoside reverse transcriptase inhibitor, wherein said at least one cytokine or at least one receptor is participating in the regulation of immune process. Various embodiments and variants are contemplated.The invention also provides a method of treatment or prophylaxis of HIV, including AIDS, which includes administration of the combination pharmaceutical composition described in the specification to the patient in need thereof.
Owner:EPSHTEIN OLEG ILIICH
Compositions and methods for use of antiviral drugs in the treatment of retroviral diseases resistant to nucleoside reverse transcriptase inhibitors
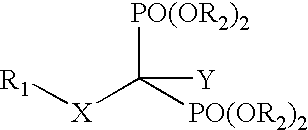
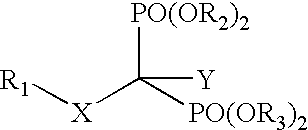
InactiveUS20050113331A1Inhibiting retroviral replicationReduce viral loadBiocidePhosphorous compound active ingredientsNucleoside Reverse Transcriptase InhibitorDisease
The present invention relates to novel compositions comprising an excision-inhibiting bisphosphonate and a nucleoside reverse transcriptase inhibitor. The present invention also relates to methods for preventing or treating retrovirus-related diseases using a composition comprising a bisphosphonate and a nucleoside reverse transcriptase inhibitor. In a specific embodiment, the invention provides methods for preventing or treating AIDS by administering a bisphosphonate-based compound in combination with 3′-azido-3′-deoxythymidine (AZT) to patients infected with AZT-resistant HIV to improve the effectiveness of AZT therapy.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Non-nucleoside reverse transcriptase inhibitors
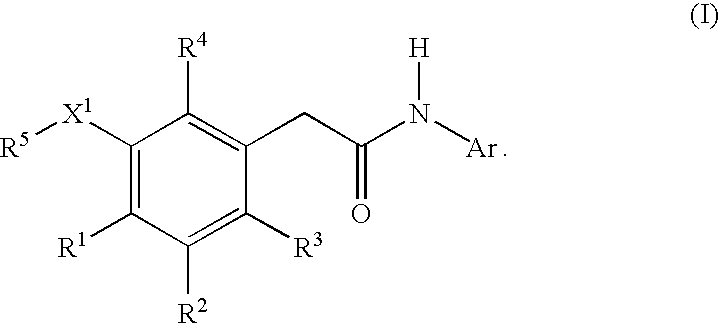
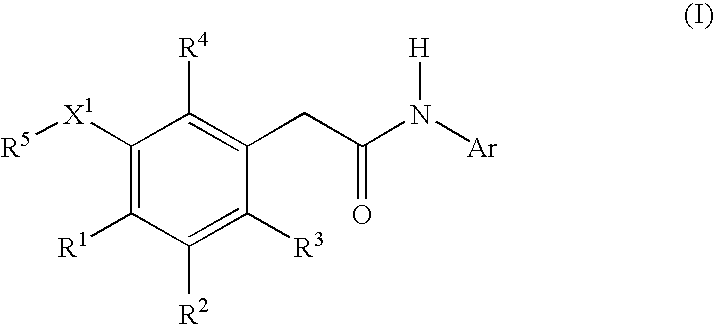
Compounds of formula I, wherein R1, R2, R3, R4, R5, X, Y, and Ar are as defined herein or pharmaceutically acceptable salts thereof, inhibit HIV-1 reverse transcriptase and afford a method for prevention and treatment of HIV-1 infections and the treatment of AIDS and / or ARC. The present invention also relates to compositions containing compounds of formula I useful for the prevention and treatment of HIV-1 infections and the treatment of AIDS and / or ARC.
Owner:ROCHE PALO ALTO LLC
Non-nucleoside reverse transcriptase inhibitors
Compounds of Formula (I) are HIV reverse transcriptase inhibitors, wherein X, R1, R2, R3, R4 and R5 are defined herein. The compounds of Formula (I) and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME CORP
Non-nucleoside reverse transcriptase inhibitors
InactiveUS20080045511A1Reduce sensitivityInhibitor usedBiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorMedicine
Owner:ROCHE PALO ALTO LLC
Method for the treatment of infection with hhv-6 virus and the amelioration of symptoms related to virus using liposomal encapsulation for delivery of reduced glutathione
InactiveUS20090068253A1Increase intracellularIncrease extra cellular antioxidantDispersion deliveryPeptide/protein ingredientsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention is the use of a therapeutically effective amount of glutathione (reduced) in a liposome encapsulation for oral administration to improve symptoms of illnesses that are related to viruses and for the treatment and prevention of virus, particularly HHV-6 and EBV, which liposomal encapsulation of glutathione (reduced) is referred to as liposomal glutathione. The application references specifically reduced glutathione and its importance, and how to stabilize it effectively so it can be taken orally, and need not be refrigerated. New uses for tuberculosis, and asthma are discussed. The combination is proposed of reduced glutathione and Highly Active Anti-Retroviral Therapy having at least one pharmaceutical composition selected from the group of Nucleoside / tide Reverse Transcriptase Inhibitors (NRTIs), Protease Inhibitors (PIs), and Non-nucleoside Reverse Transcriptase Inhibitors (NnRTIs).
Owner:GUILFORD F TIMOTHY
Enhanced method and composition for the treatment of hiv+ tuberculosis patients with Anti-retroviral drugs and liposomal encapsulation for delivery of reduced glutathione
InactiveUS20120244212A1Increase intracellular and extra cellular antioxidantsReduced glutathioneAntibacterial agentsBiocideNucleoside Reverse Transcriptase InhibitorDisease
The invention is the use of a therapeutically effective amount of glutathione (reduced) in a liposome encapsulation for oral administration to improve symptoms of illnesses that are related to tuberculosis and HIV and more generally viruses and for the treatment and prevention of virus, particularly HHV-6 and EBV, which liposomal encapsulation of glutathione (reduced) is referred to as liposomal glutathione. The application references specifically reduced glutathione and its importance, and how to stabilize it effectively so it can be taken orally, and need not be refrigerated. New uses for tuberculosis are discussed. The combination is proposed of reduced glutathione and Highly Active Anti-Retroviral Therapy having at least one pharmaceutical composition selected from the group of Nucleoside / tide Reverse Transcriptase Inhibitors (NRTIs), Protease Inhibitors (PIs), and Non-nucleoside Reverse Transcriptase Inhibitors (NnRTIs), and further anti-tuberculosis drugs.
Owner:GUILFORD FREDERICK TIMOTHY
4-cyanophenylamino-substituted bicyclic heterocyclic compounds as HIV inhibitors
InactiveUS20080176871A1Inhibit HIV replicationBiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorPhenyl group
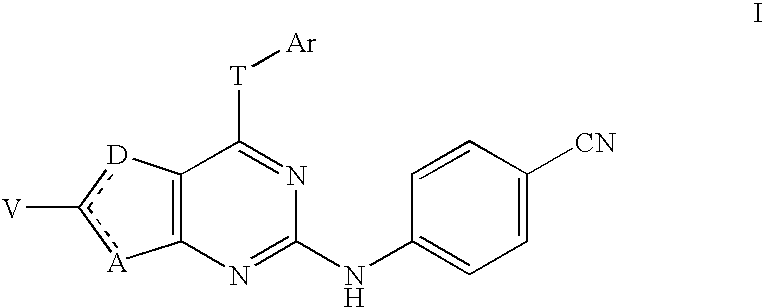
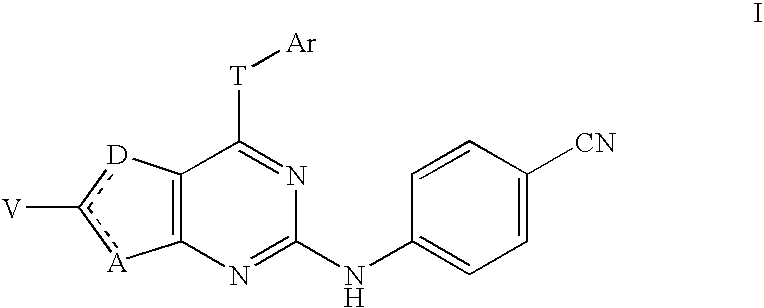
This application concerns certain 4-cyanophenylamino-substituted bicyclic heterocycles of formula Iwhere the dashed line represents a double bond that may be located either between A and C(V) or between C(V) and D, where A is S or C(Z); D is S or C(W); provided that one and only one of A and D is S; where T is NH, O, or S; and where other substituents are defined herein. These compounds are non-nucleoside reverse transcriptase inhibitors and have potential as anti-HIV treatment.
Owner:ARDEA BIOSCI
Non-nucleoside reverse transcriptase inhibitors
ActiveUS20100256181A1BiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorAdditive ingredient
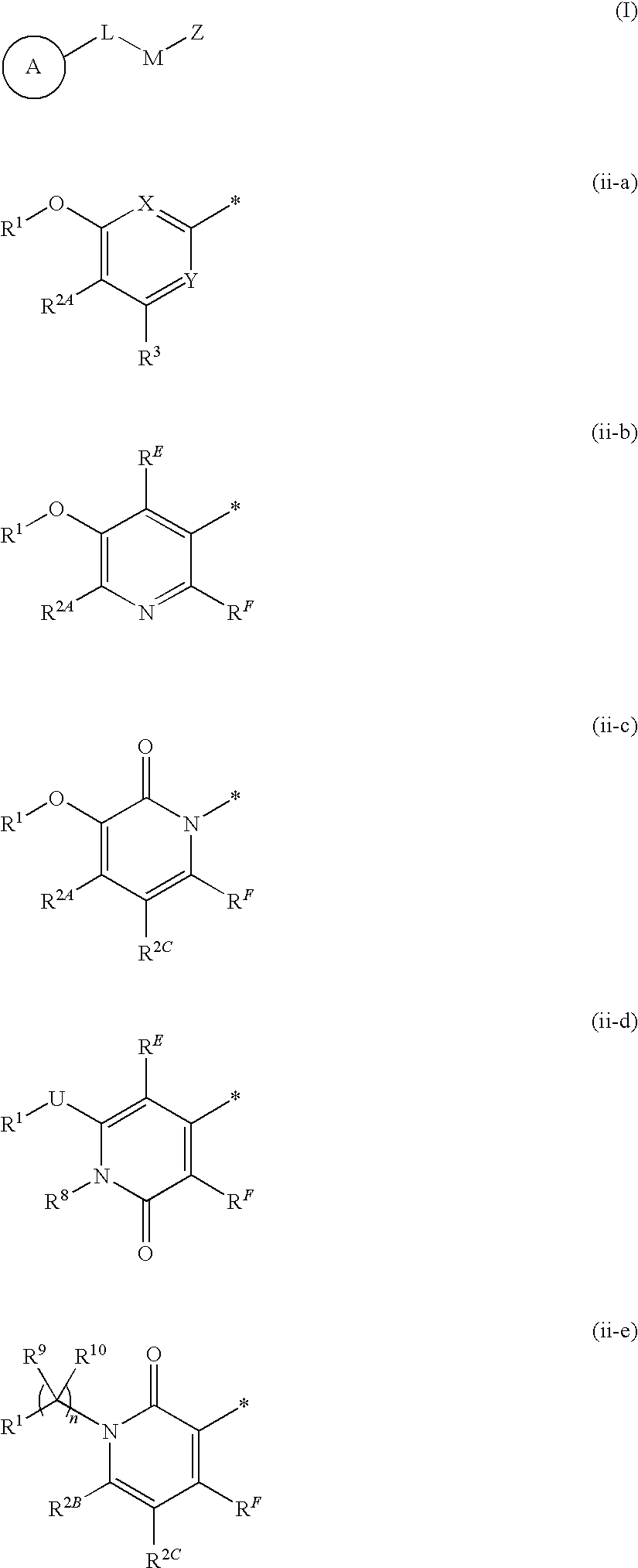
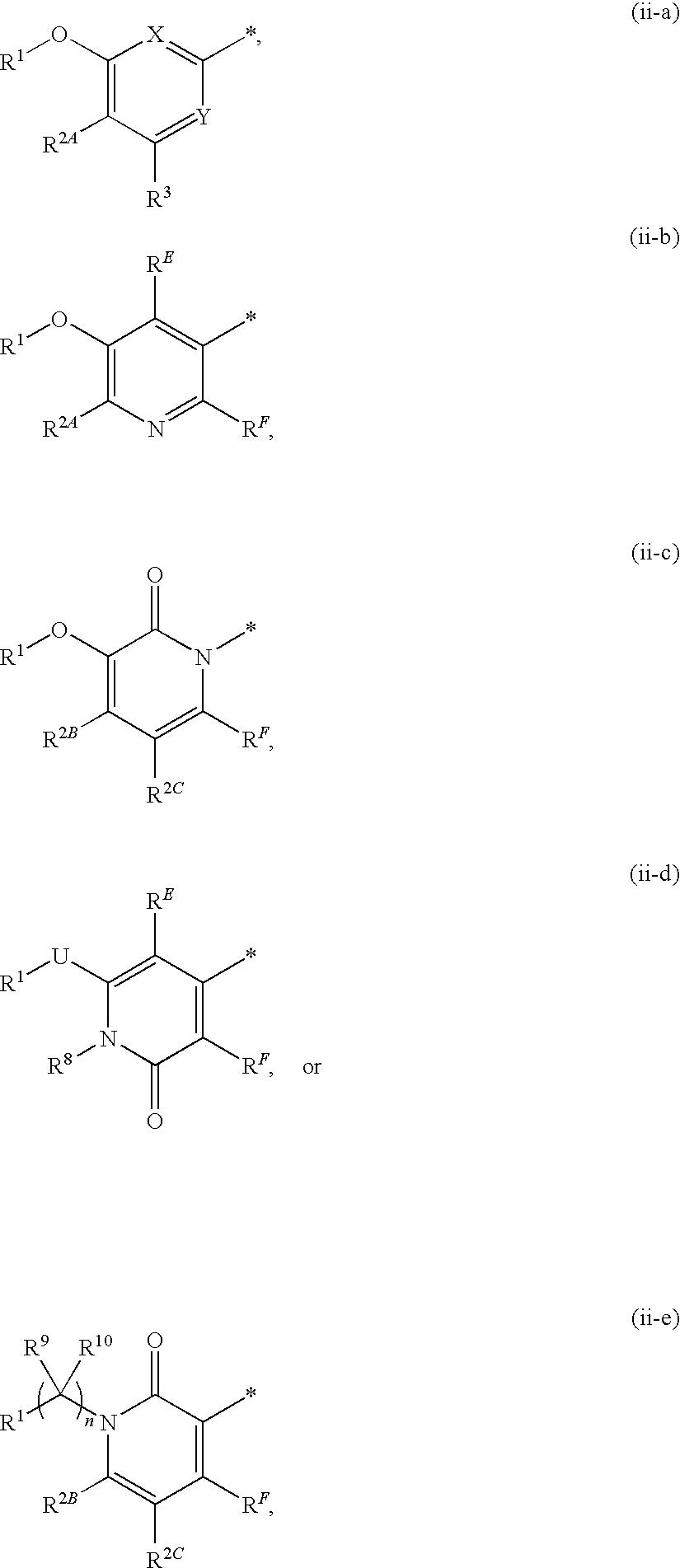
Heteroaromatic compounds of Formula (I) are HIV reverse transcriptase inhibitors, wherein ring A is: (ii-a), (ii-b), (ii-c), (ii-d), or (ii-e); and wherein n, L, M, U, X, Y, Z, RE, RF, R1, R2A, R2B, R2C, R3, R8, R9 and R10 are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts and prodrugs are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts and prodrugs can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME LLC
Non-nucleoside reverse transcriptase inhibitors
Heteroaromatic compounds of Formula I:are HIV reverse transcriptase inhibitors, wherein R1, R2, R3, R4 and R5 are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK CANADA INC
Nonnucleoside reverse transcriptase inhibitors
This invention relates to novel pyrazole derivatives of formula I wherein R1 to R4 are as defined in the summary and pharmaceutically acceptable salts and solvates thereof, methods to inhibit or modulate Human Immunodeficiency Virus (HIV) reverse transcriptase with compounds of formula I and pharmaceutical compositions containing of formula I admixed with at least one solvent, carrier or excipient. The compounds are useful for treating disorders in which HIV and genetically related viruses are implicated
Owner:ROCHE PALO ALTO LLC
Non-nucleoside reverse transcriptase inhibitors
ActiveUS20140100231A1BiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorImmunomodulating Agent
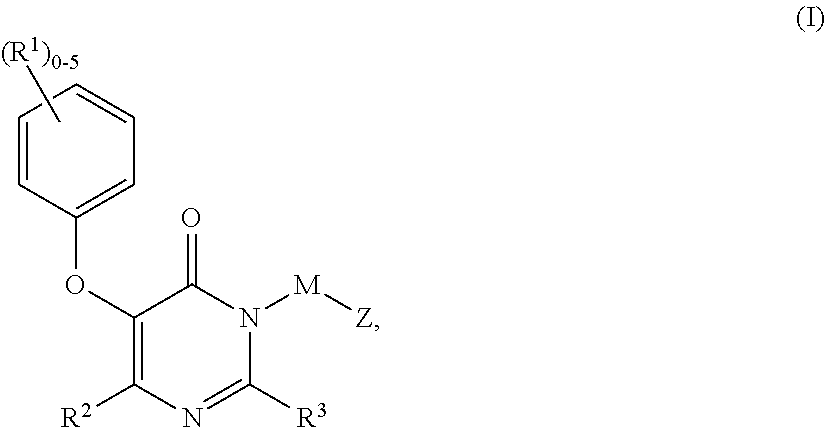
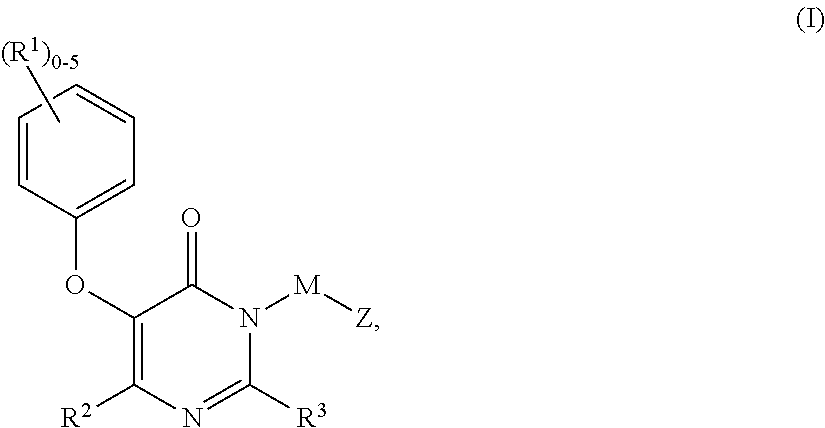
Compounds of Formula I:are HIV reverse transcriptase inhibitors, wherein R1, R2, RE, L, M and Z are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME LLC
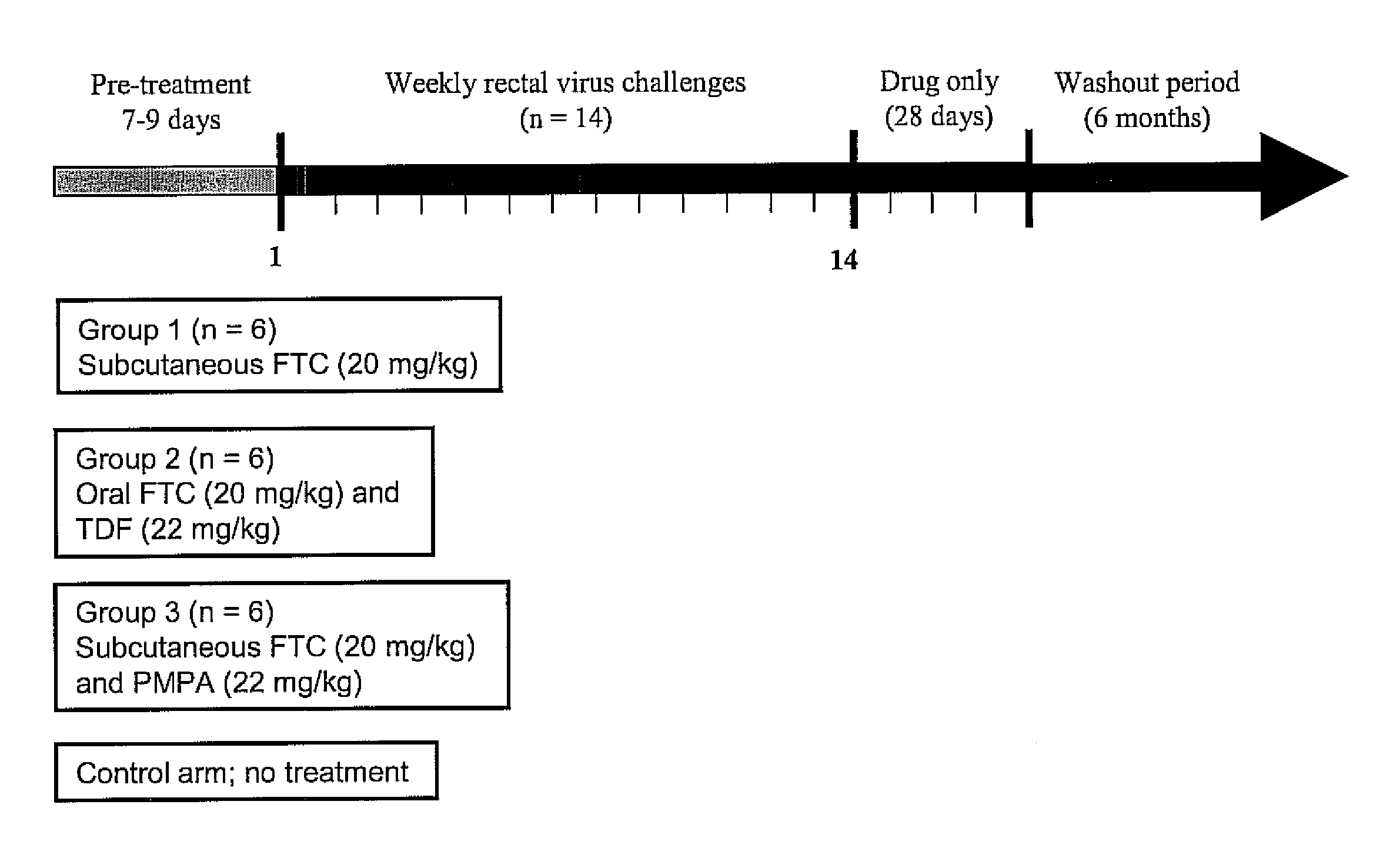
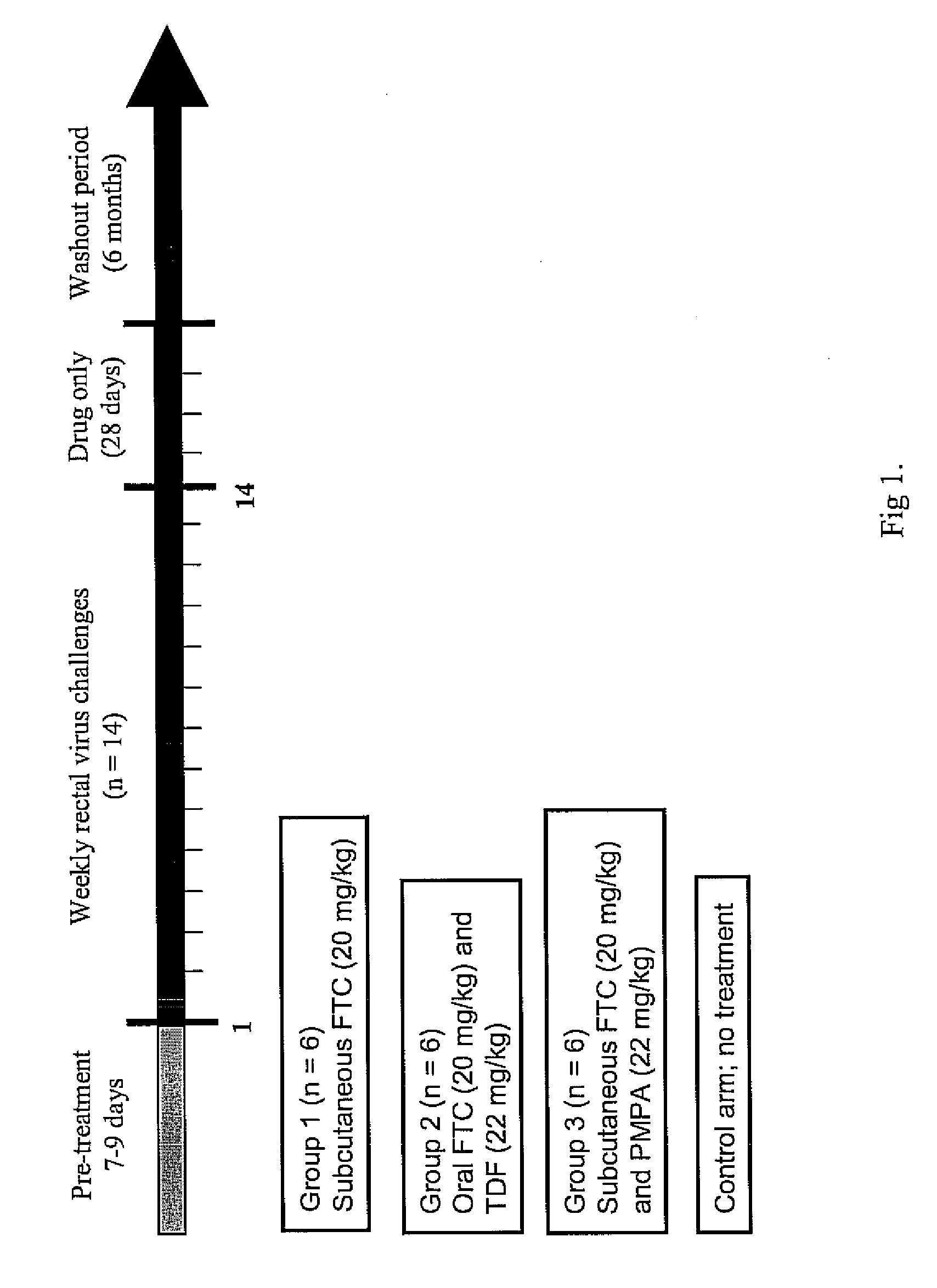
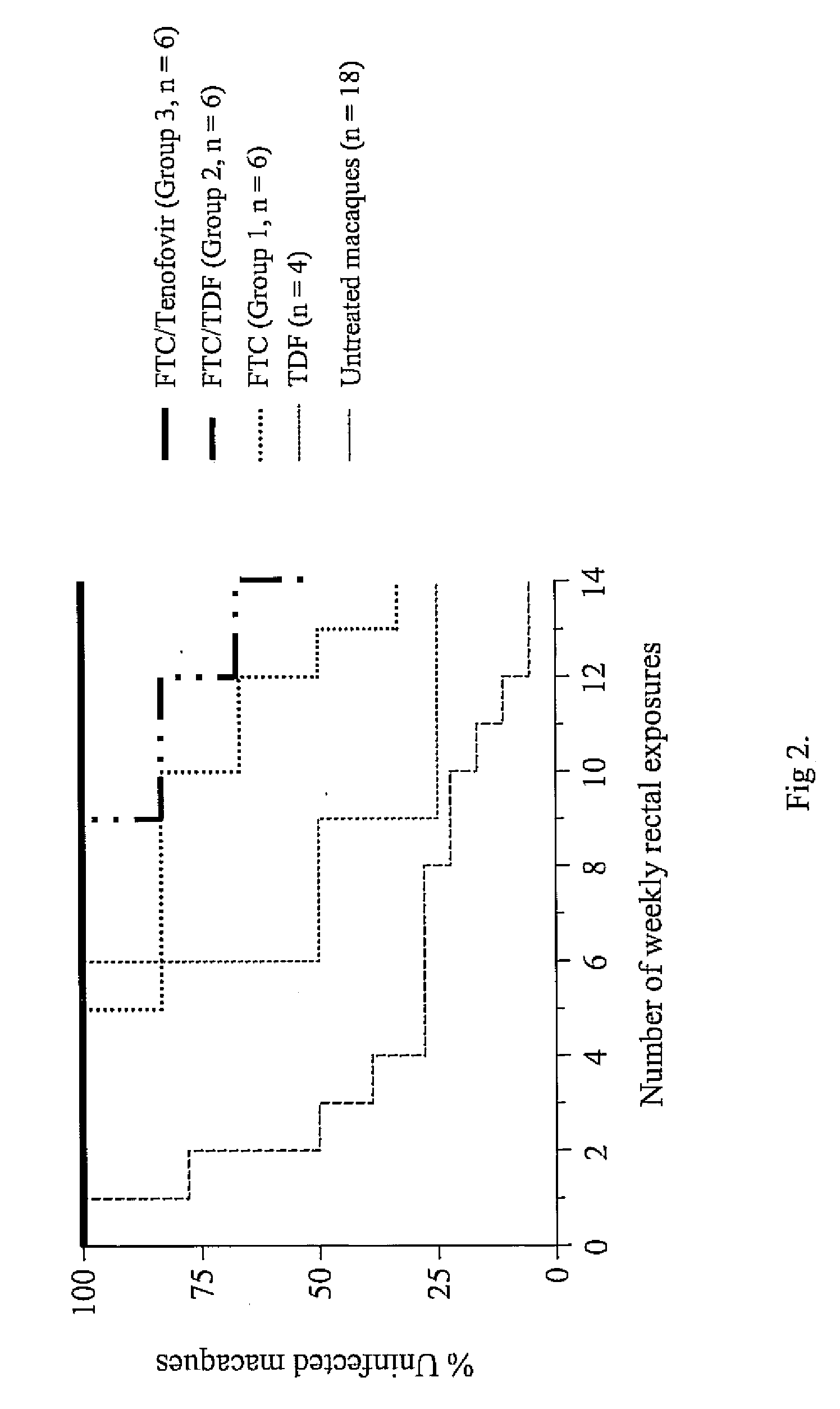
Inhibition of HIV infection fhrough chemoprophyalxis
ActiveUS20070265227A1BiocidePharmaceutical delivery mechanismNucleoside Reverse Transcriptase InhibitorRetroviral infection
A process is provided for protecting a primate host from a self-replicating infection by an immunodeficiency retrovirus. Protection is achieved by administering to the primate host a combination of a pharmaceutically effective amount of a nucleoside reverse transcriptase inhibitor and a pharmaceutically effective amount of a nucleotide reverse transcriptase inhibitor prior to exposure to the immunodeficiency retrovirus. The administration is effective if provided in a single dose within 24 hours of the exposure. A regime of regular daily doses is also effective in providing protection against an immunodeficiency retrovirus becoming self-replicating after infecting a primate host A process for controlling retrovirus transmission within a population includes the administration to a subpopulation at high risk for contracting an immunodeficiency retroviral infection the detailed combination prior to sexual exposure to a source of immunodeficiency retrovirus so as to preclude the immunodeficiency retrovirus from becoming self-replicating in a member of the subpopulation.
Owner:UNITED STATES OF AMERICA
Pharmaceutical antiretroviral composition
InactiveUS20140193491A1Easy to manufactureBiocideOrganic active ingredientsNucleoside Reverse Transcriptase InhibitorEmtricitabine
The present invention relates to a pharmaceutical antiretroviral composition comprising (i) a nucleoside reverse-transcriptase inhibitor selected from lamivudine and emtricitabine, (ii) extended release nevirapine, and (iii) tenofovir; a process for preparing such composition and the use of such composition in medicine, particularly for the prophylaxis and / or treatment of diseases caused by retroviruses.
Owner:CIPLA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com