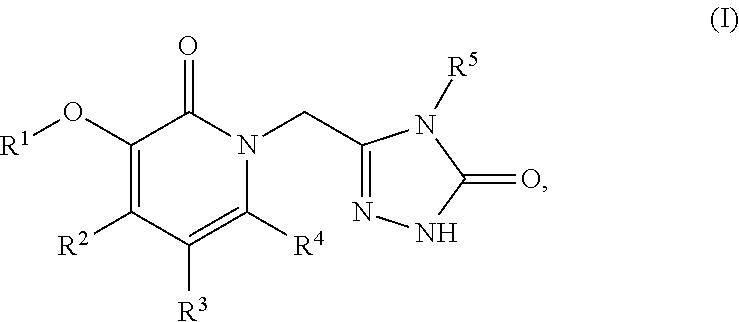
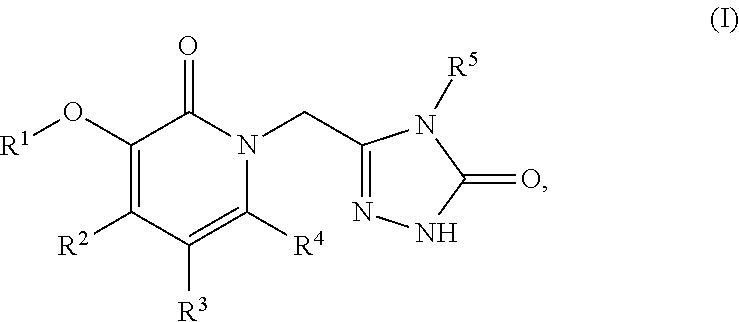
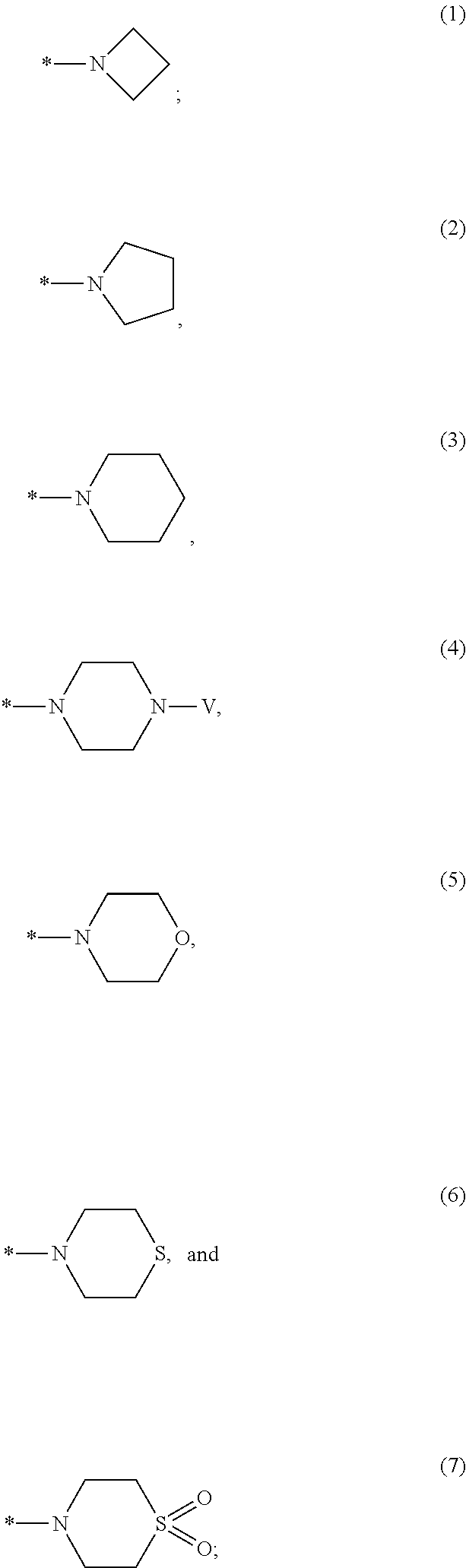
Non-nucleoside reverse transcriptase inhibitors
a reverse transcriptase inhibitor and non-nucleoside technology, applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of mutant hiv strains that are resistant to known inhibitors, and are highly susceptible to debilitating and ultimately fatal opportunistic infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-Chloro-5-({1-[(4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methyl]-2-oxo-4-(trifluoromethyl)-1,2-dihydropyridin-3-yl}oxy)benzonitrile (1-1)
[0493]
Step 1(a): 3-(3-bromo-5-chlorophenoxy)-2-chloro-4-(trifluoromethyl)pyridine (1-2)
[0494]
[0495]A mixture of the 3-bromo-5-chlorophenol (3.74 g; 18.0 mmol), 2-chloro-3-fluoro-4-(trifluoromethyl)pyridine (3.00 g; 15.0 mmol) and K2CO3 (2.49 g; 18.0 mmol) in NMP (15 mL) was heated to 120° C. for one hour, then cooled to room temperature. The mixture was then diluted with 250 mL EtOAc and washed with 3×250 mL 1:1 H2O:brine. The organic extracts were dried (Na2SO4) and concentrated in vacuo. Purification by ISCO CombiFlash (120 g column; load with toluene; 100:0 to 0:100 hexanes:CH2Cl2 over 40 minutes) provided title compound (1-2) as a white solid. Repurification of the mixed fractions provided additional title compound. 1H NMR (400 MHz, CDCl3): δ 8.55 (d, J=5.0 Hz, 1H); 7.64 (d, J=5.0 Hz, 1H); 7.30 (s, 1H); 6.88 (s, 1H); 6.77 (s, 1H).
Step ...
example 1a
3-Chloro-5-({1-[(4-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methyl]-2-oxo-4-(trifluoromethyl)-1,2-dihydropyridin-3-yl}oxy)benzonitrile (1-1)
[0505]
Step 1A(a): 2-chloro-3-(3-chloro-5-iodophenoxy)-4-(trifluoromethyl)pyridine (1A-2)
[0506]
[0507]A mixture of the 3-chloro-1-iodophenol (208 g; 816.0 mmol), 2-chloro-3-fluoro-4-(trifluoromethyl)pyridine (155 g; 777.0 mmol) and K2CO3 (161 g; 1165.0 mmol) in NMP (1.5 L) was held at 60° C. for 2.5 hours, and then left at room temperature for 2 days. The mixture was then re-heated to 60° C. for 3 hours, then cooled to room temperature. The mixture was then diluted with 4 L EtOAc and washed with 2 L water+1 L brine. The combined organics were then washed 2× with 500 mL half brine then 500 mL brine, dried over MgSO4 and concentrated to afford crude 1A-2. 1H NMR (500 MHz, DMSO) δ 8.67 (d, J=5.0 Hz, 1H), 7.98 (d, J=5.0 Hz, 1H), 7.63-7.62 (m, 1H), 7.42-7.40 (m, 1H), 7.22 (t, J=2.1 Hz, 1H).
Step 1A(b): 2-chloro-3-(3-chloro-5-iodophenoxy)-4-(triflu...
example 2
3-Chloro-5-({1-[(4-ethyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methyl]-2-oxo-4-(trifluoromethyl)-1,2-dihydropyridin-3-yl}oxy)benzonitrile (2-1)
[0513]
[0514]The title compound was prepared using the procedure described in Example, wherein the iodomethane employed in Step 1(f) was replaced with iodoethane. 1H NMR (400 MHz, DMSO): δ 11.68 (s, 1H); 7.92 (d, J=7.3 Hz, 1H); 7.76 (s, 1H); 7.60 (s, 1H); 7.52 (s, 1H); 6.69 (d, J=7.3 Hz, 1H); 5.20 (s, 2H); 3.65-3.56 (m, 2H); 1.11 (t, J=7.1 Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| room temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com