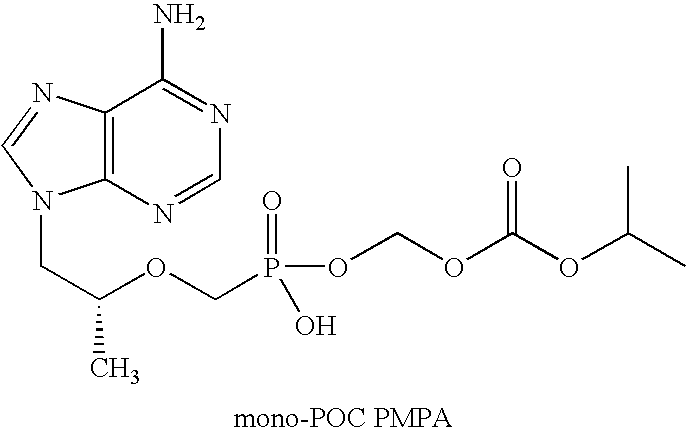
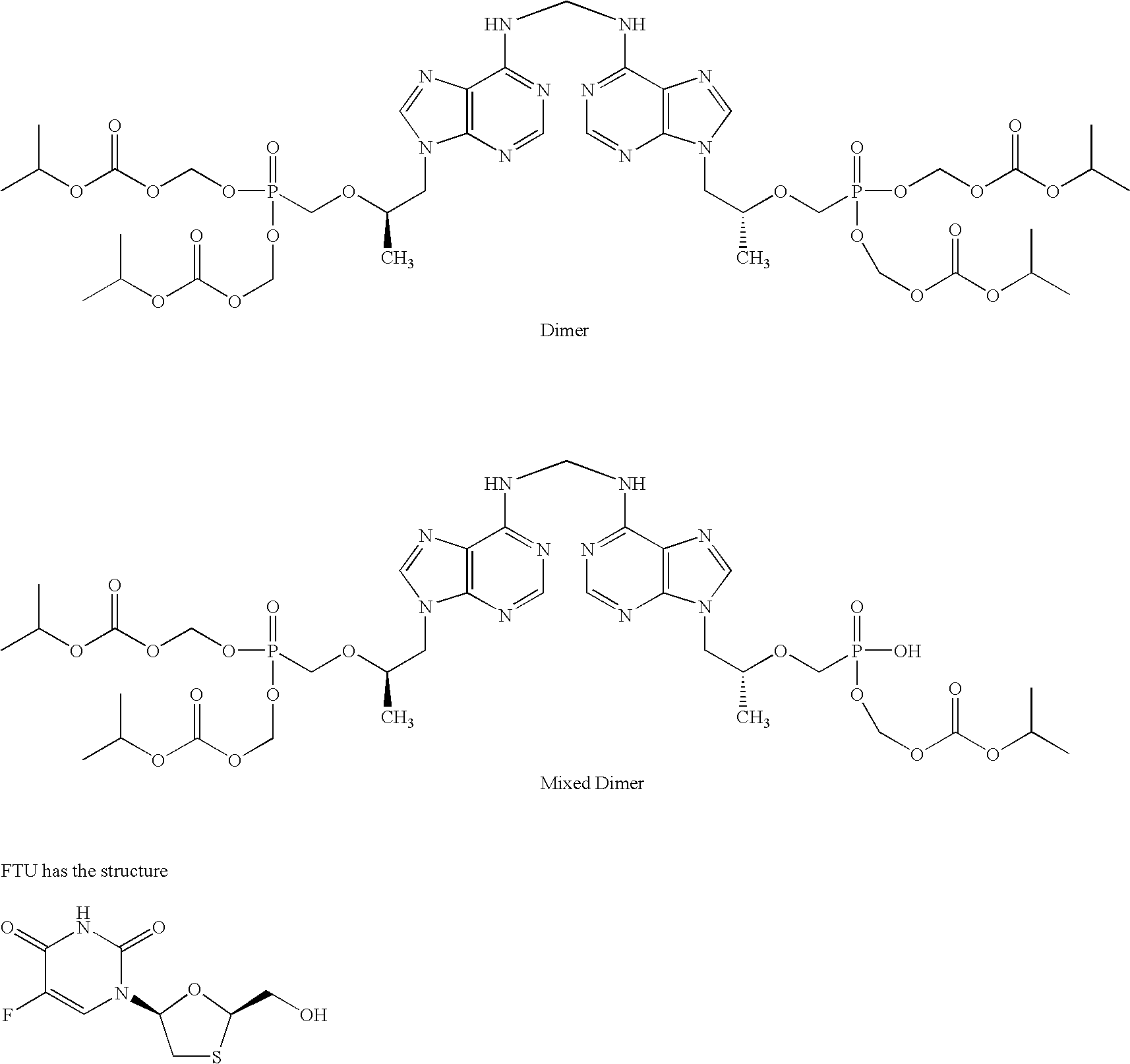
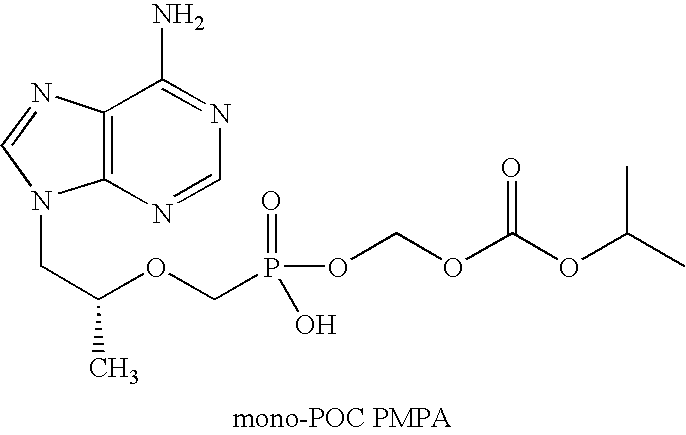
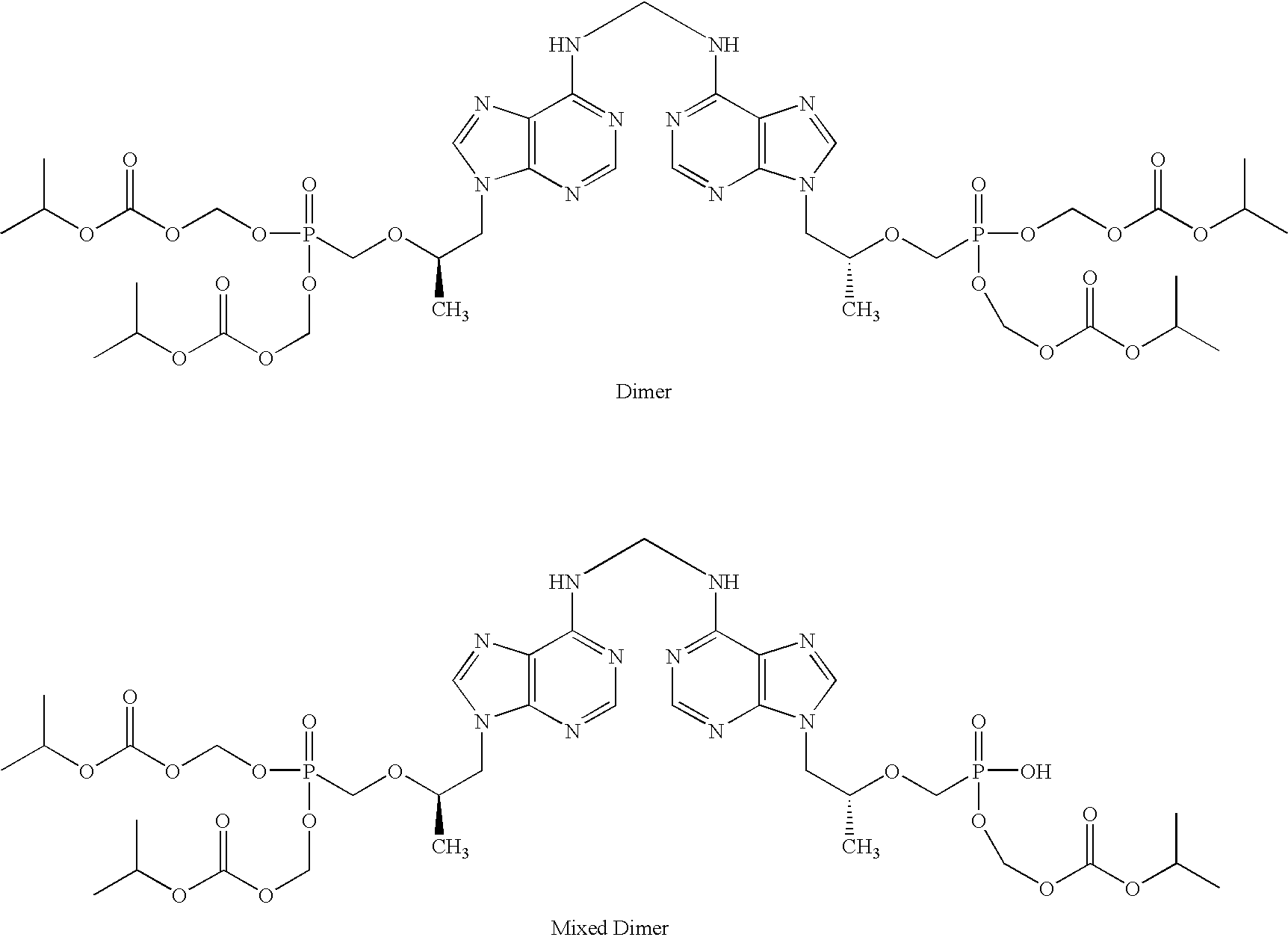
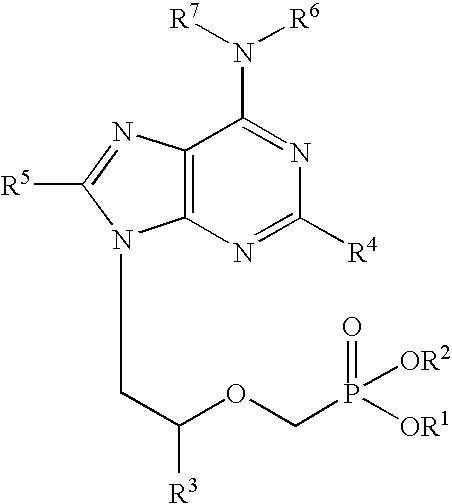
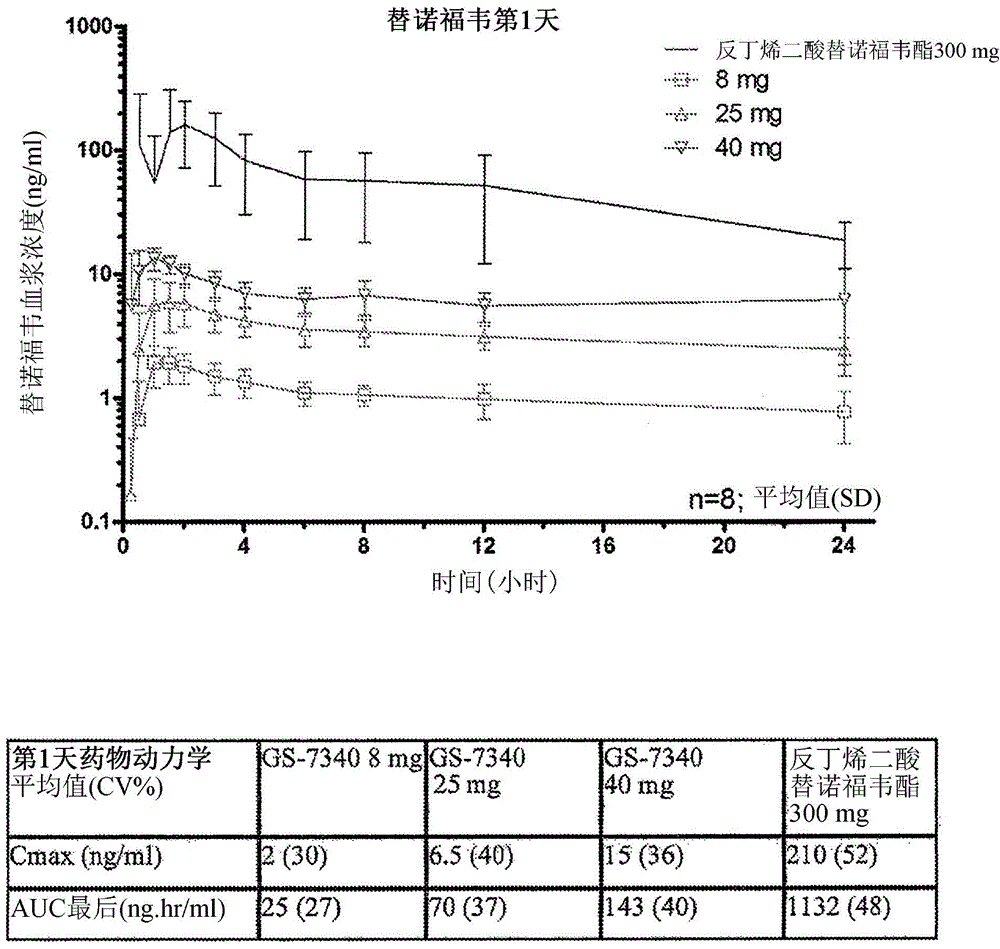
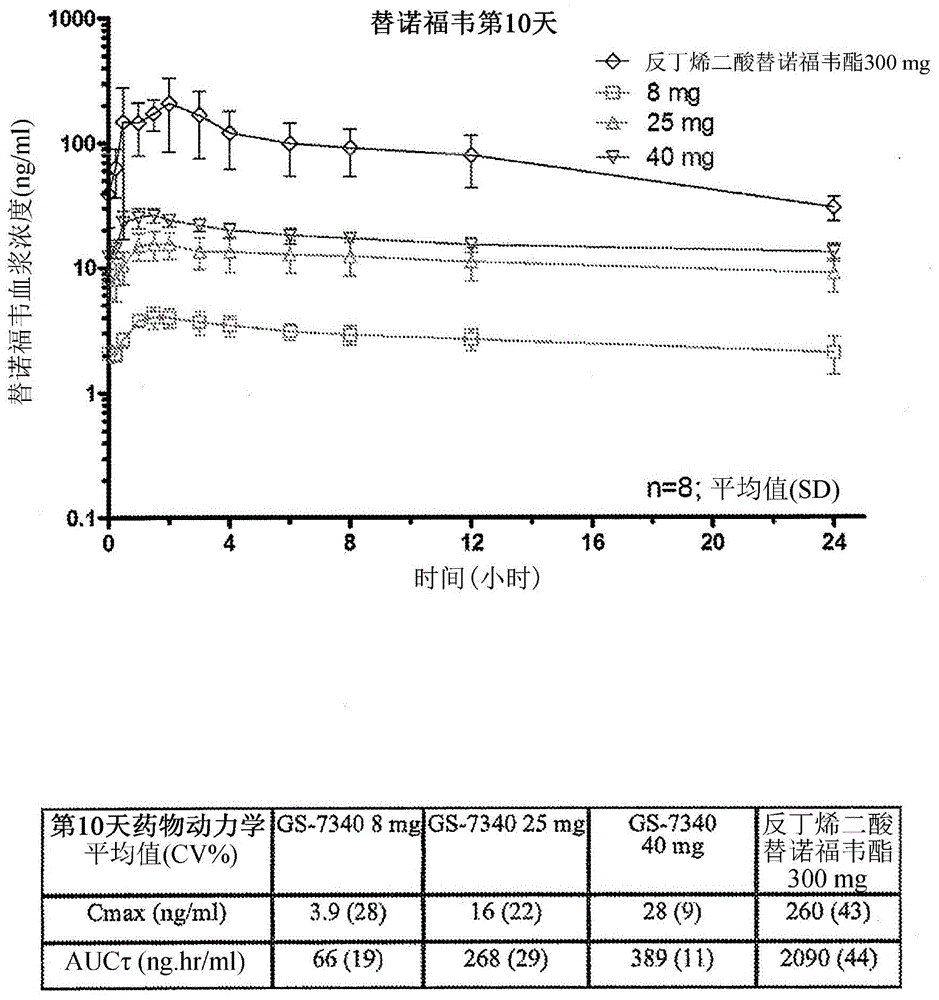
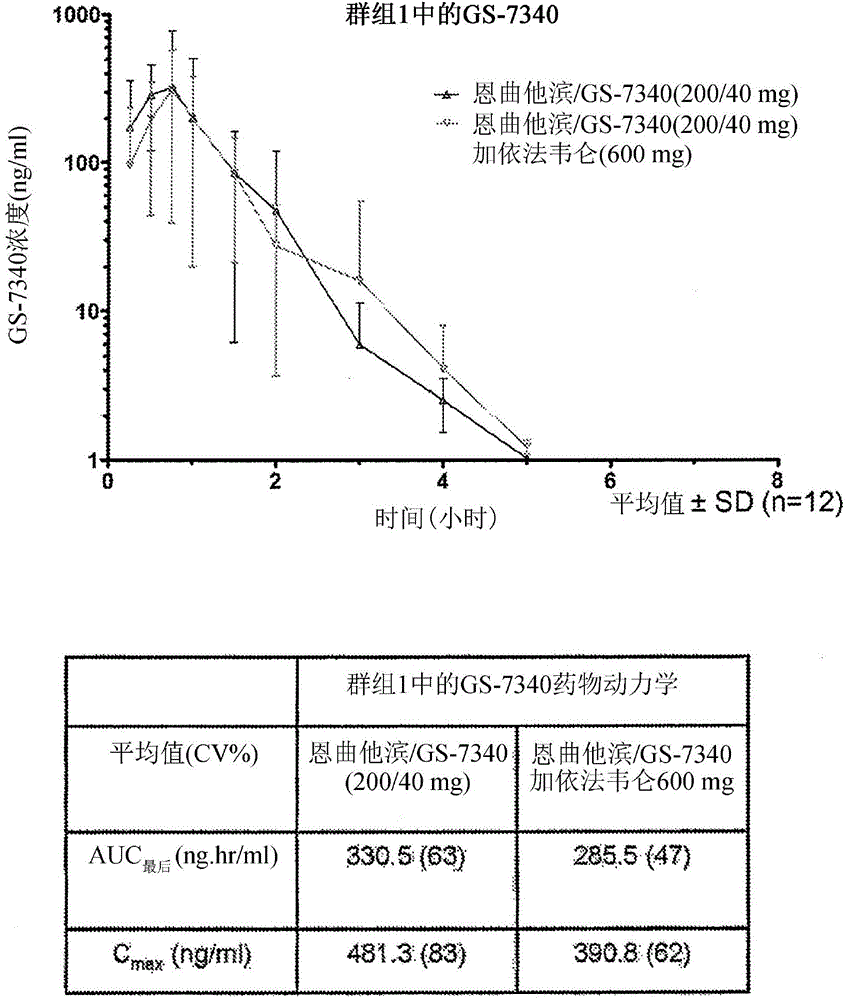
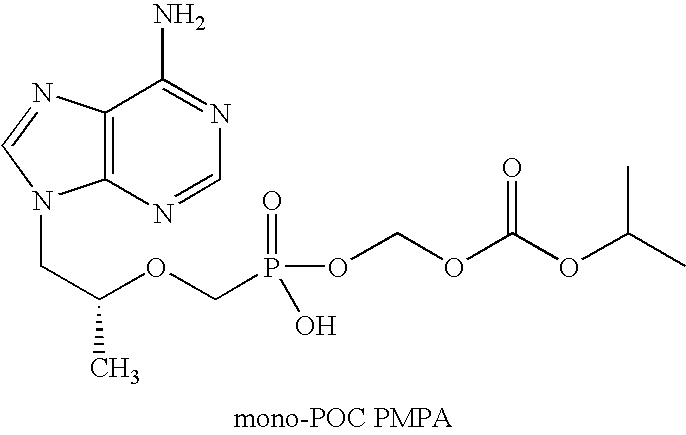
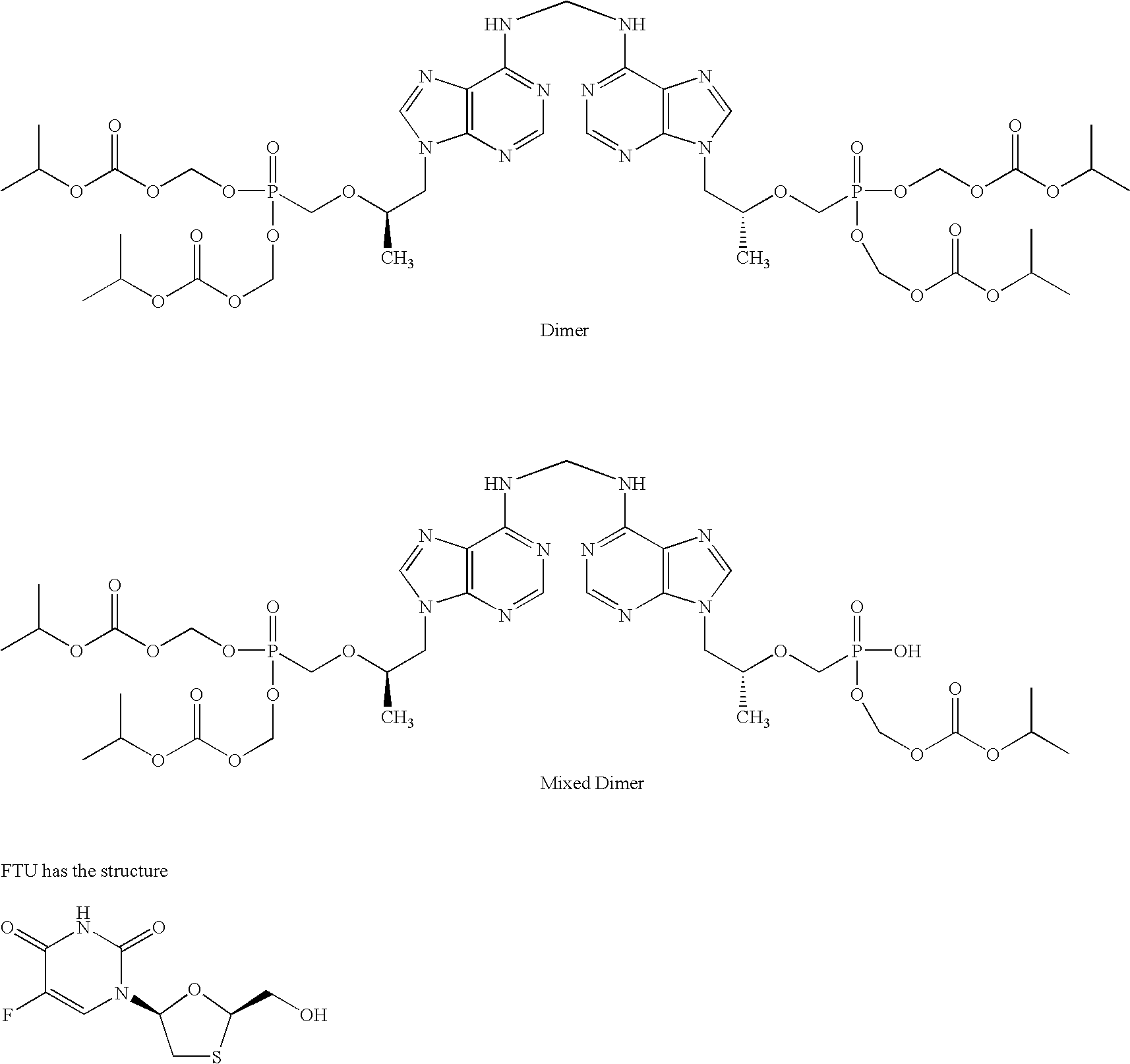
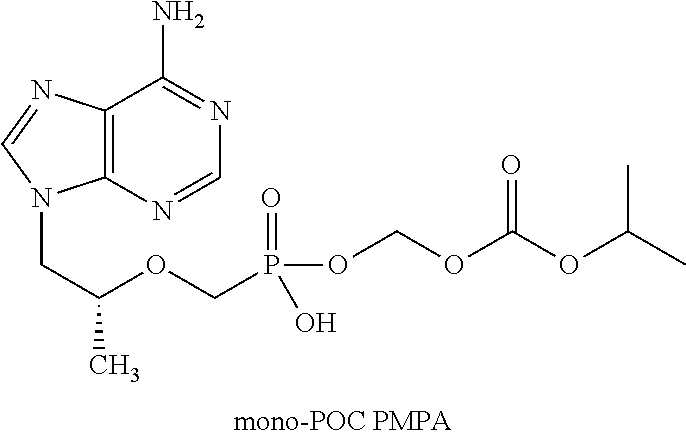
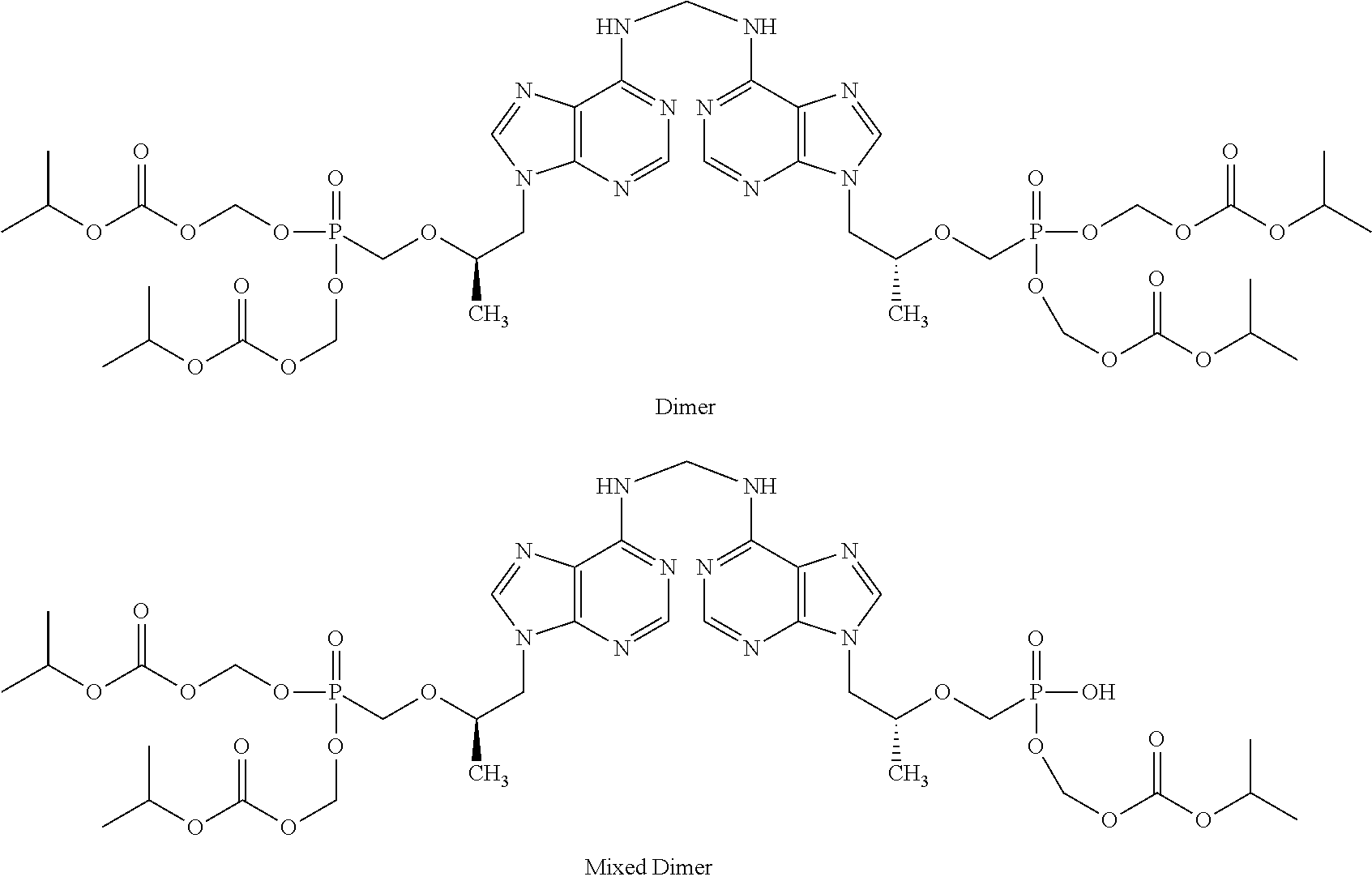
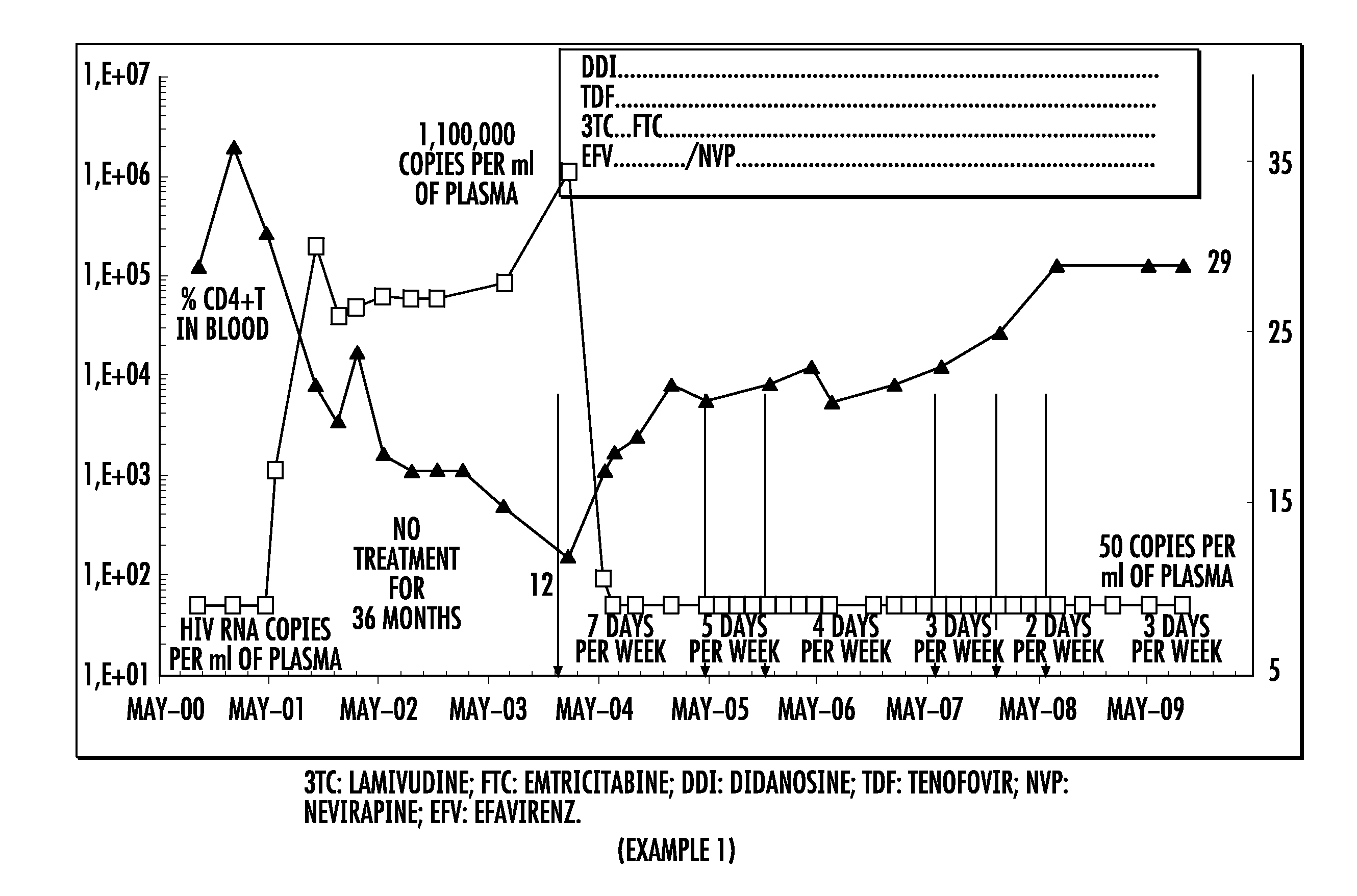
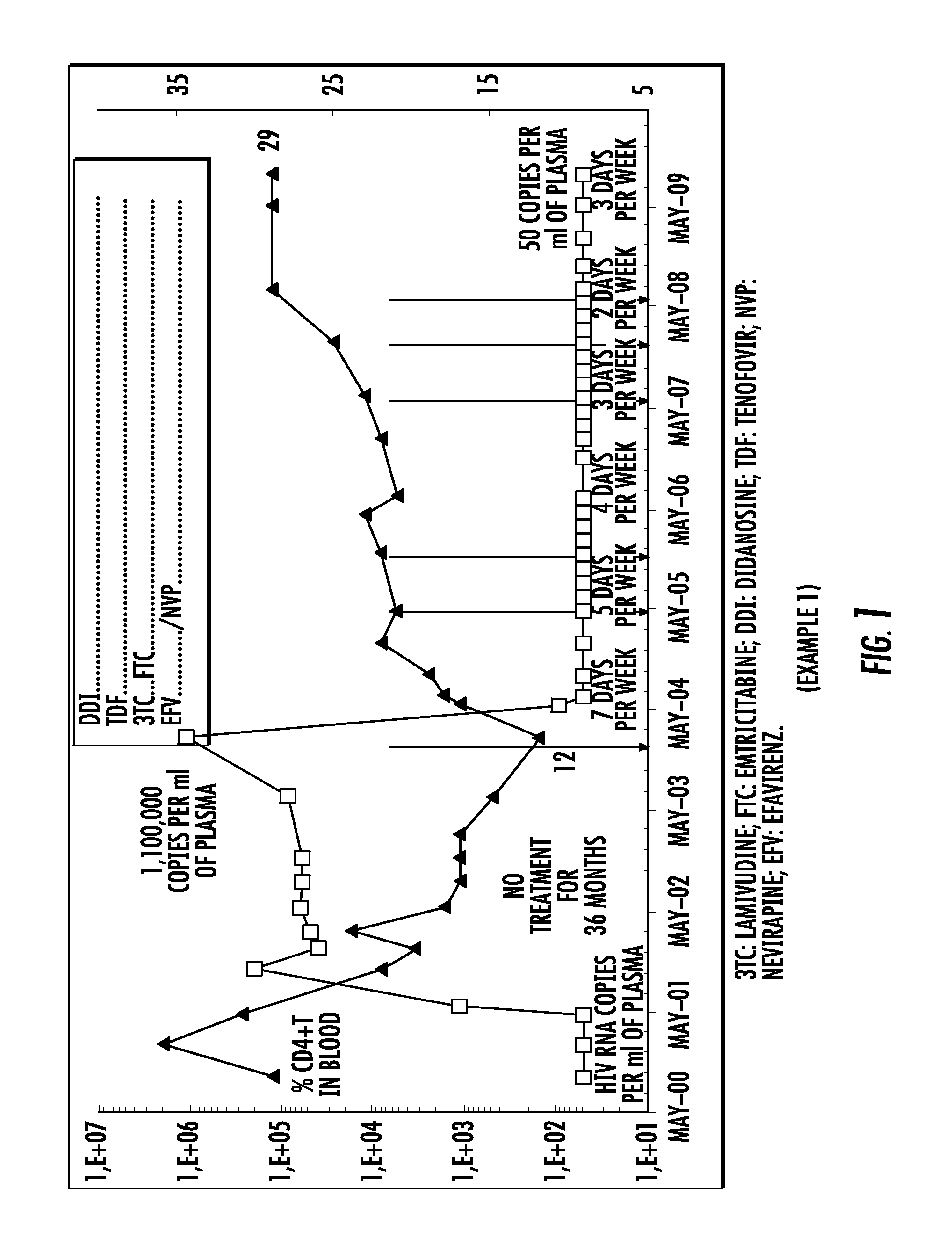
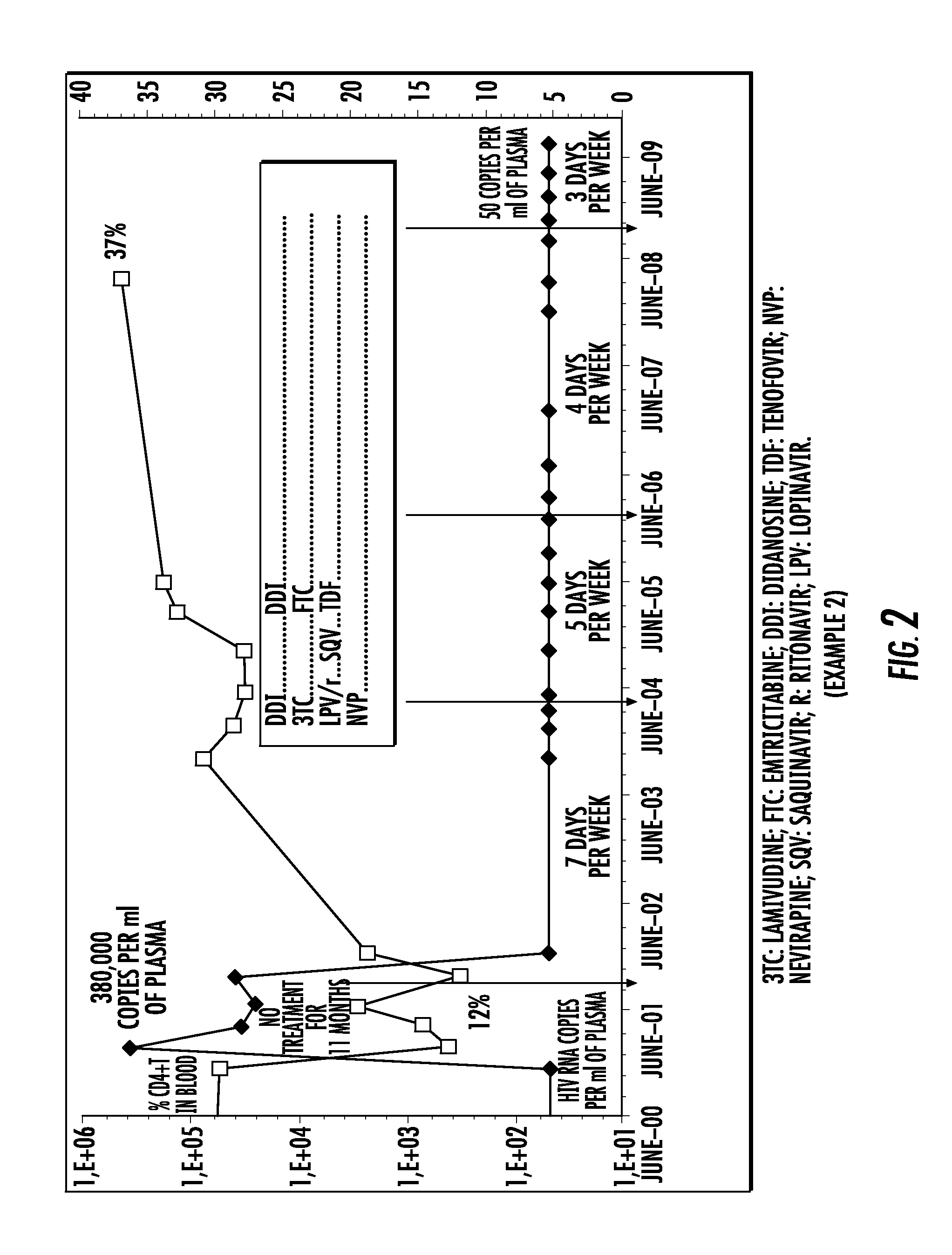
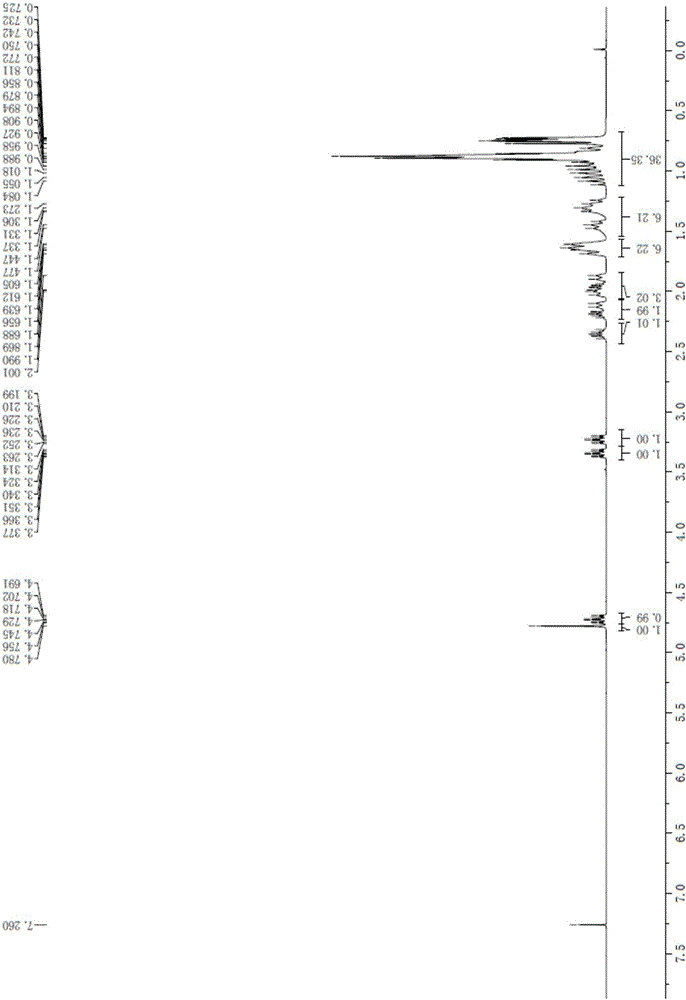
Patents
Literature
76 results about "Emtricitabine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
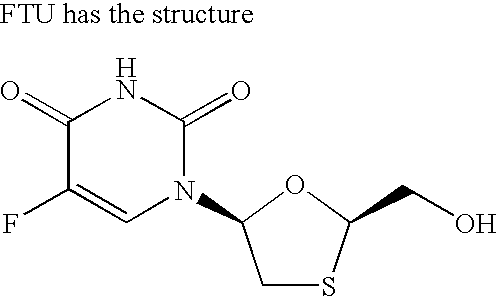
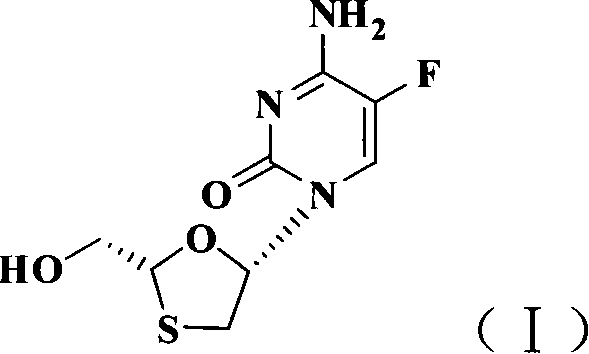
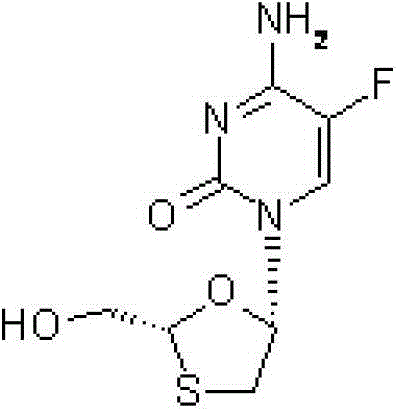
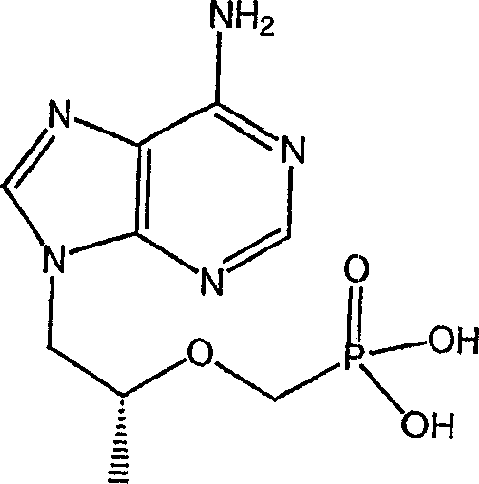
Emtricitabine is used with other HIV medications to help control HIV infection.
Unitary pharmaceutical dosage form
In accordance with this invention a novel pharmaceutical product containing efavirenz, emtricitabine and tenofovir DF are provided as a multicomponent unitary oral dosage form, component 1 comprising tenofovir DF (and, optionally, emtricitabine) and component 2 comprising efavirenz, wherein components 1 and 2 are in a stabilizing configuration. In preferred embodiments component 1 is made by dry granulation.
Owner:GILEAD SCI LLC
Method and composition for pharmaceutical product
InactiveUS20070077295A1Improve stabilityFormulation stabilityOrganic active ingredientsBiocideMedicineEmtricitabine
This invention is directed to a composition comprising dry granulated tenofovir DF and emtricitabine, and a method for making same. Dry granulation was unexpectedly found to be important in preparing a tenofovir DF containing composition suitable for inclusion in a combination dosage form containing emtricitabine, efavirenz and tenofovir DF.
Owner:GILEAD SCI INC
Suitqable to industrialized method for preparing emtricitabine
ActiveCN1563002ARaw materials are easy to getHigh yieldOrganic chemistryDigestive systemMentholEmtricitabine
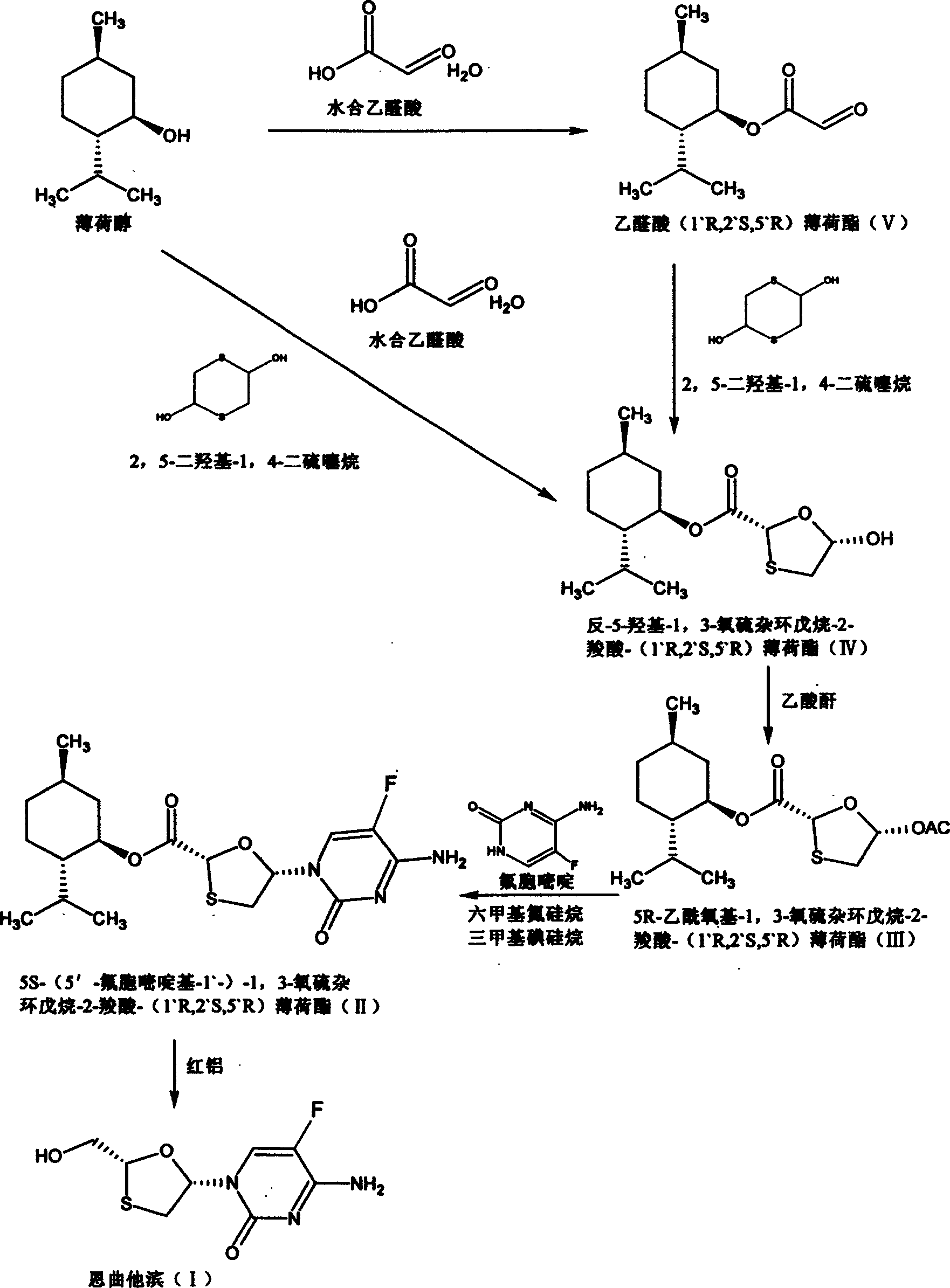
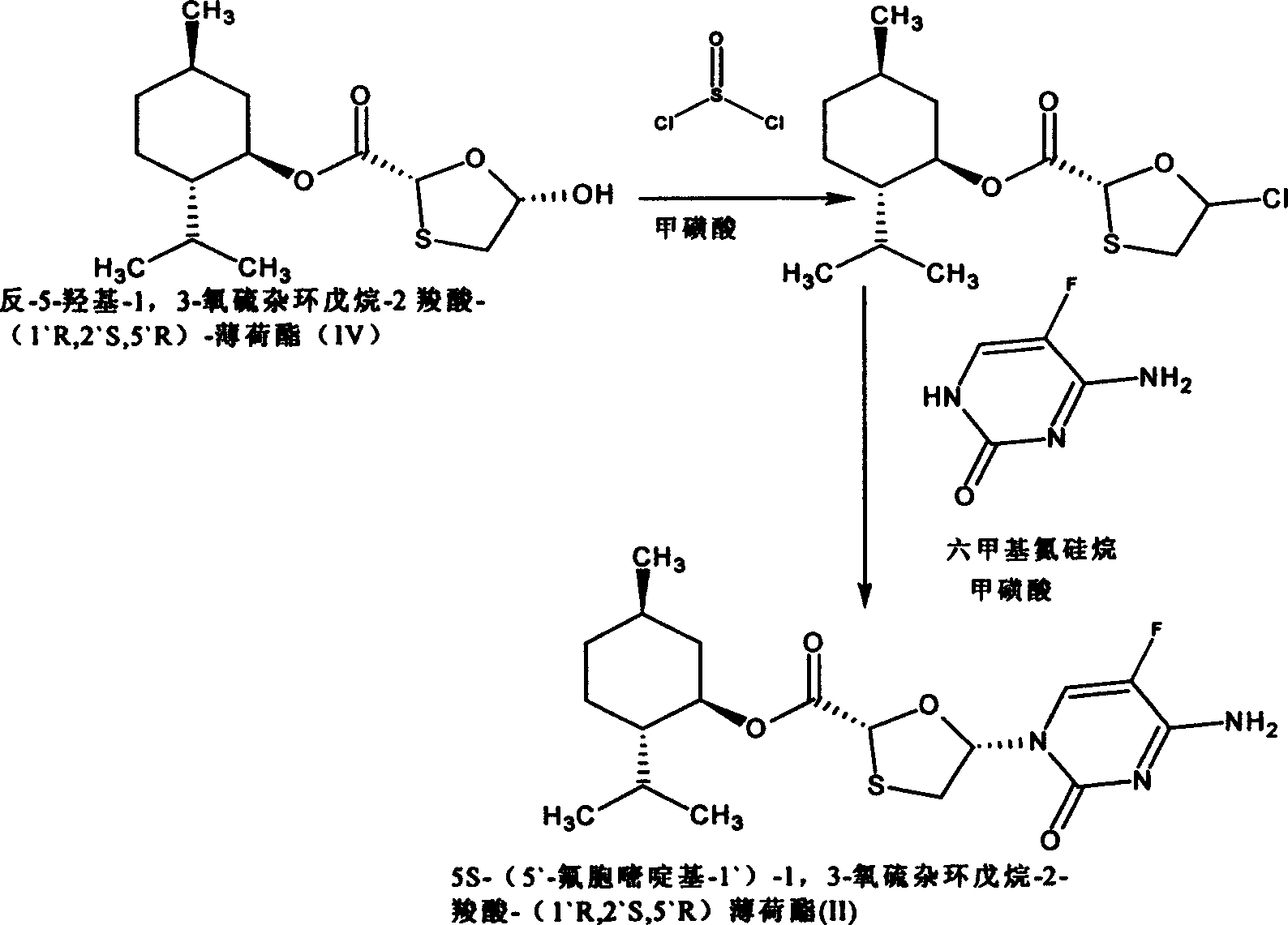
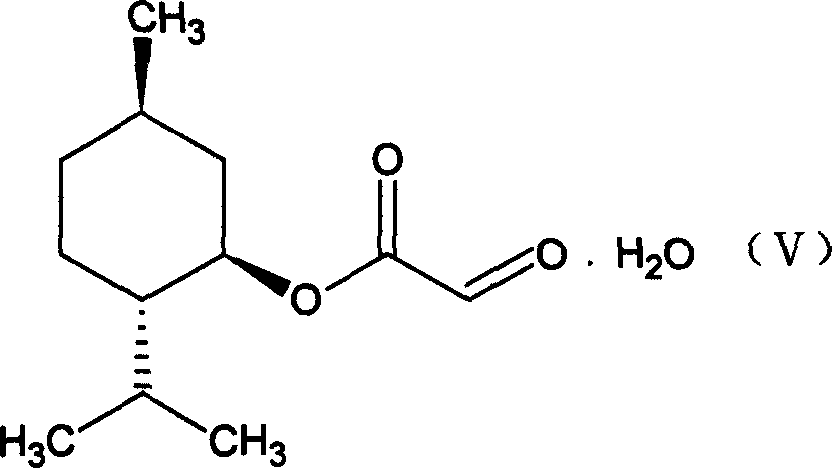
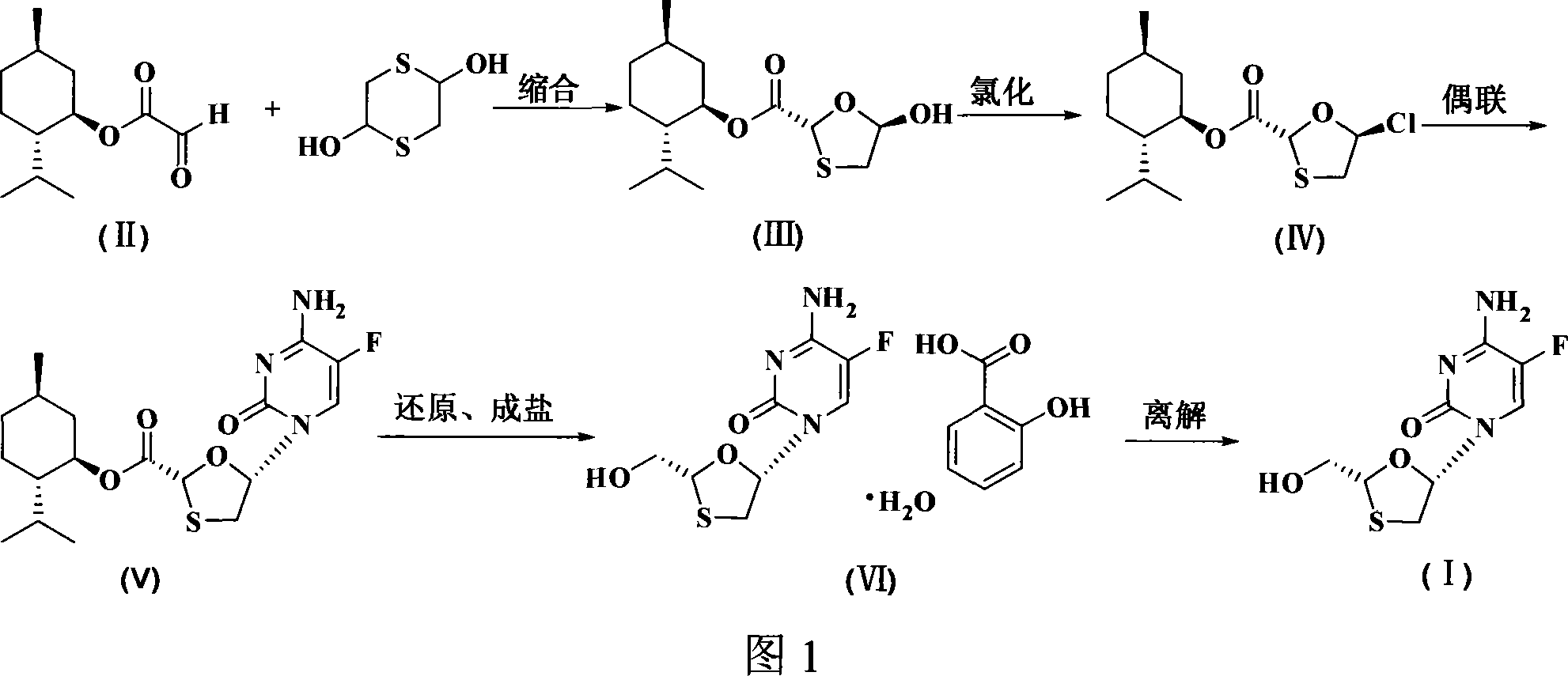
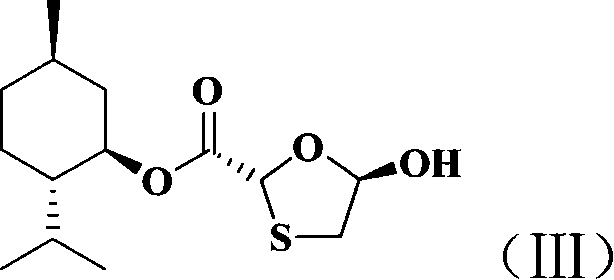
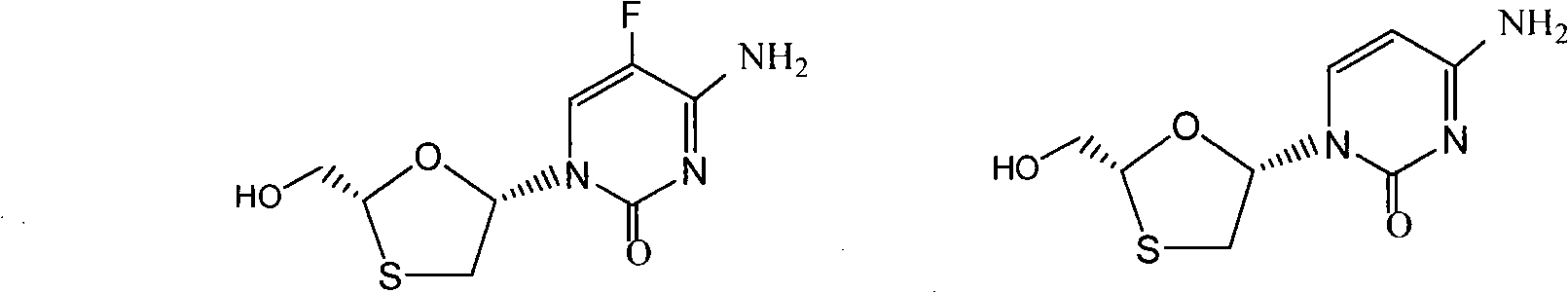
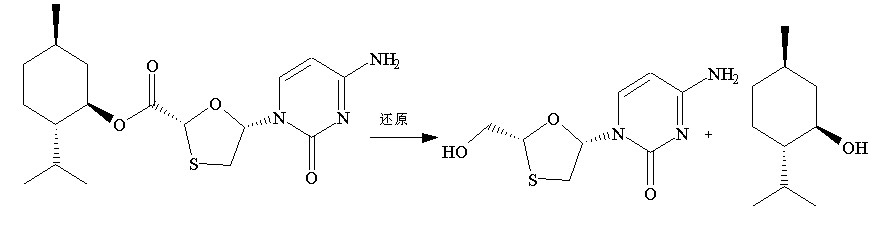
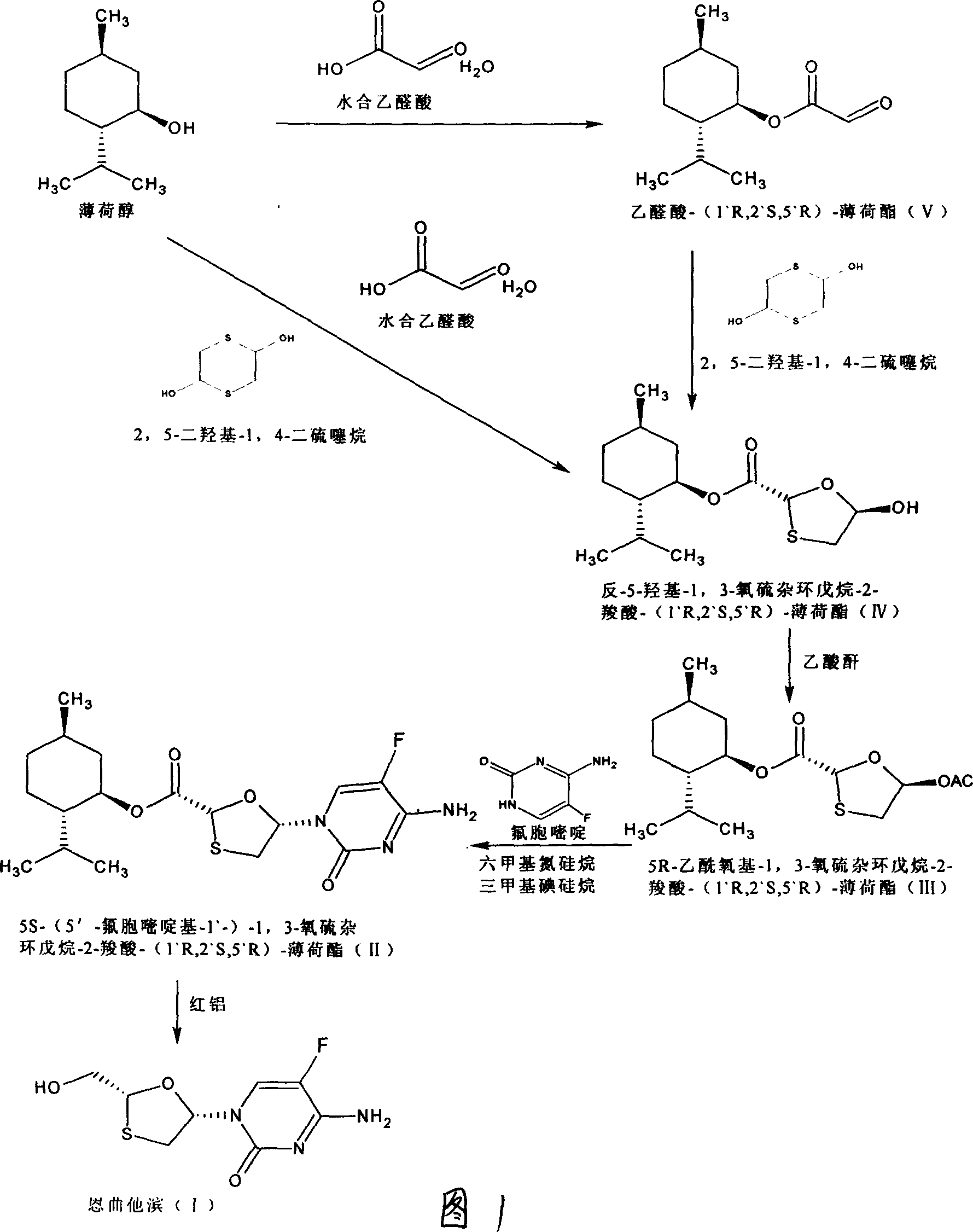
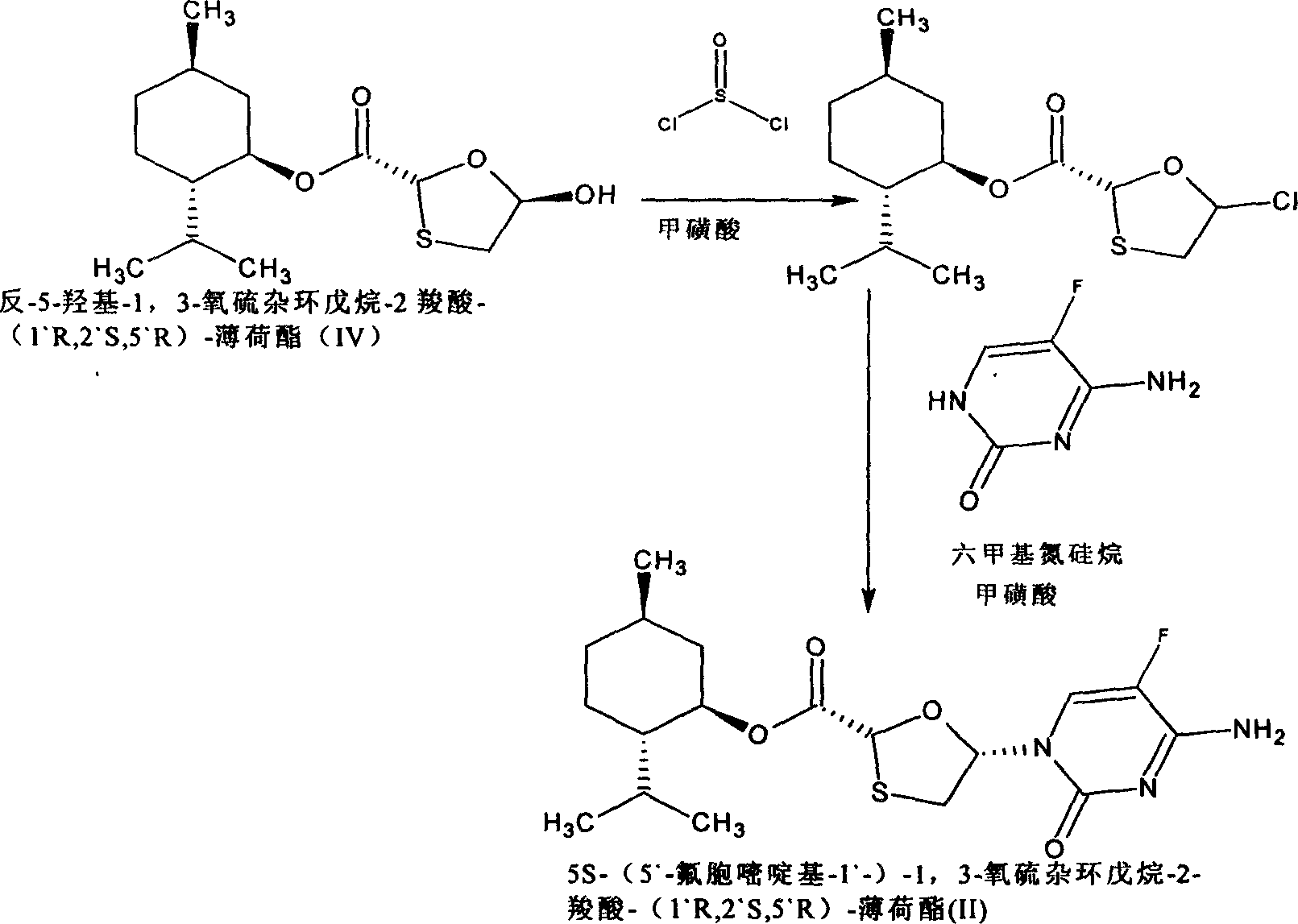
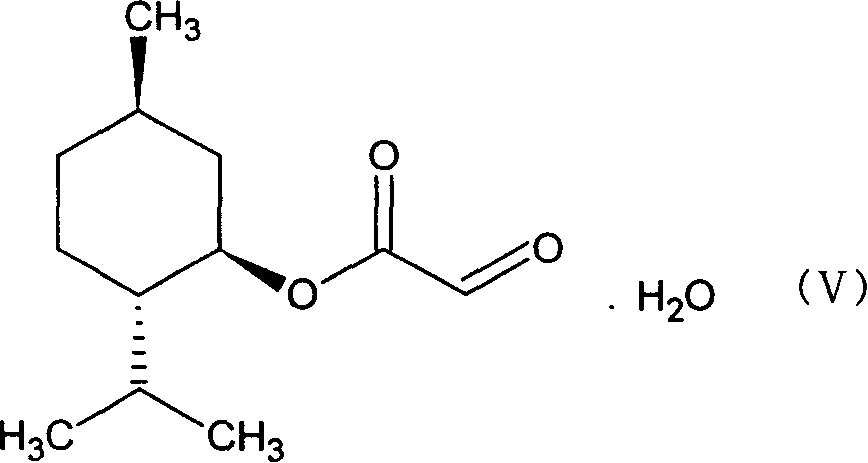
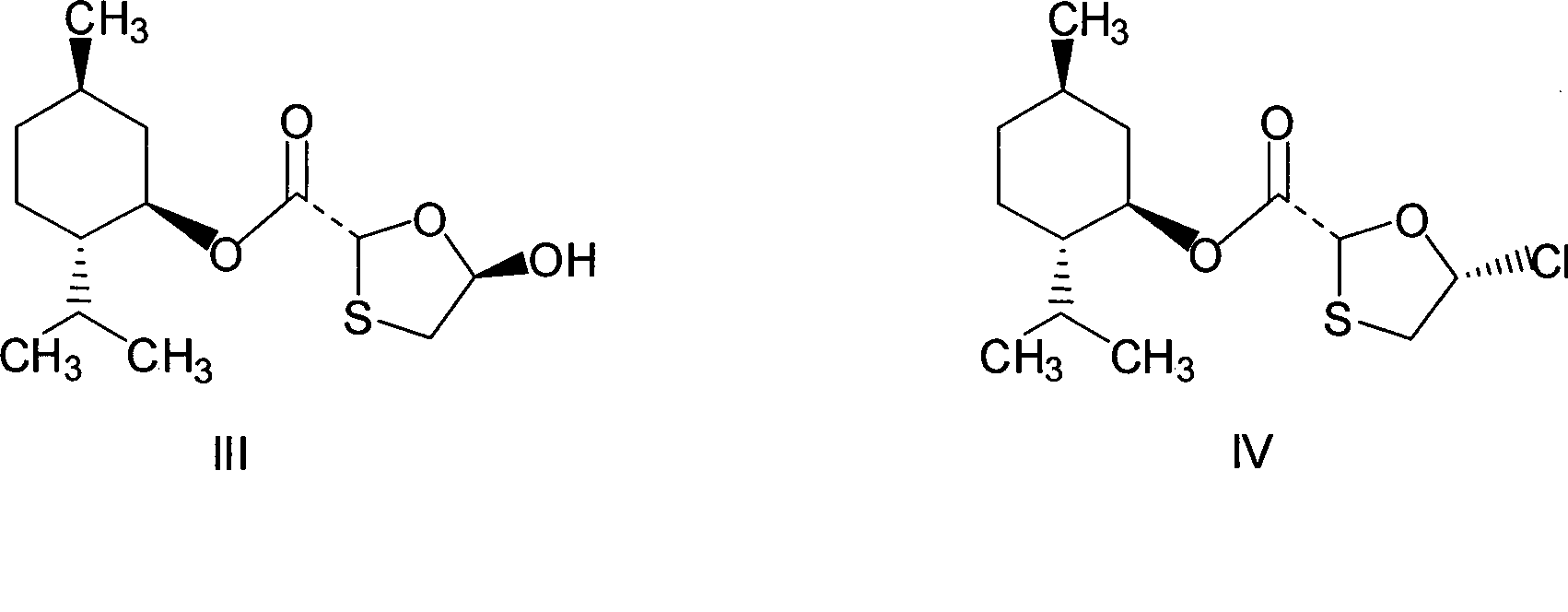
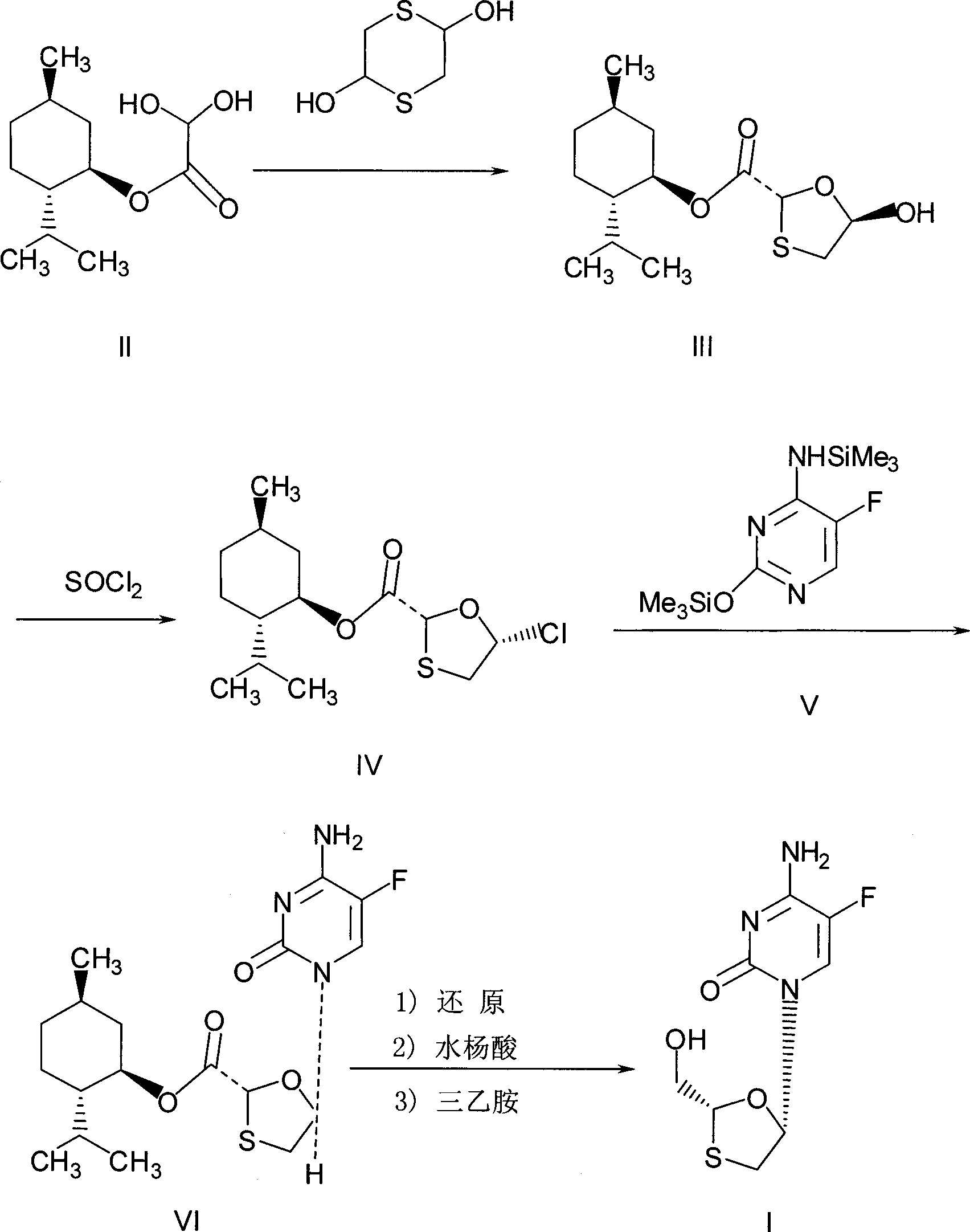
This invention prepn. method consists procedures (as shown in route chart) of: (1). hydrated glyoxalic acid is reacted with menthol in solvent and catalyst to produce stable intermediate-glyoxalic (1,R,2,5,5,R) menthol ester (V); then, reacting with 2,4-dihydroxy-1,4-dithiothiane to produce menthol ester (IV); (2). said product being proceeded acidylation by hydroxy to produce menthol ester (III); (3) being condensed with 5-fluorocytosine under the protection of silanized reagent, after purification to obtain menthol ester (II); (4). above-said product is reduce by reducing agent to obtain final invention product enqutabin (I) This invention has advantages of: available raw material, high yield, safety and commercialization prodn.
Owner:SHANDONG WEIFANG PHARMA FACTORY
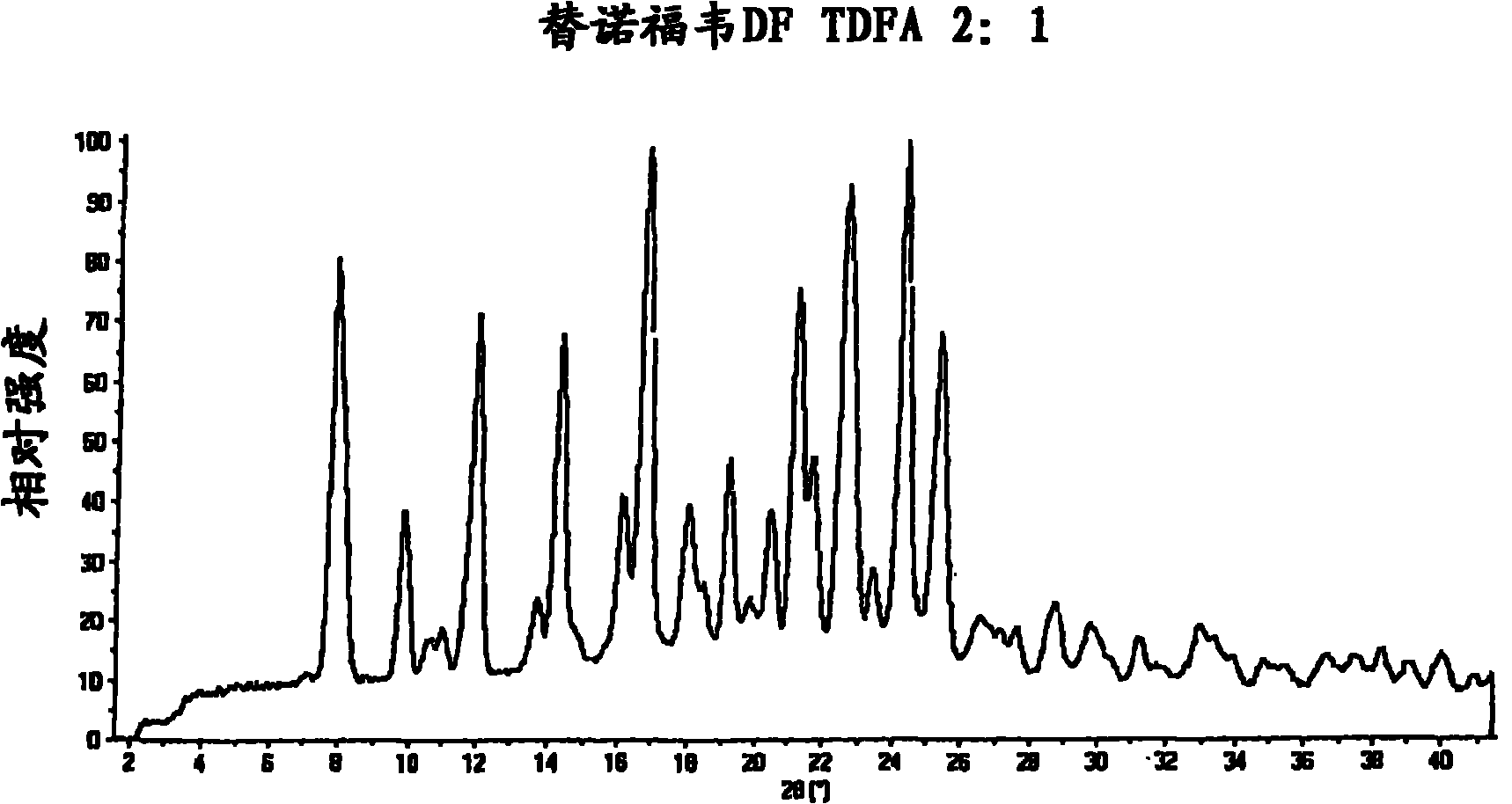
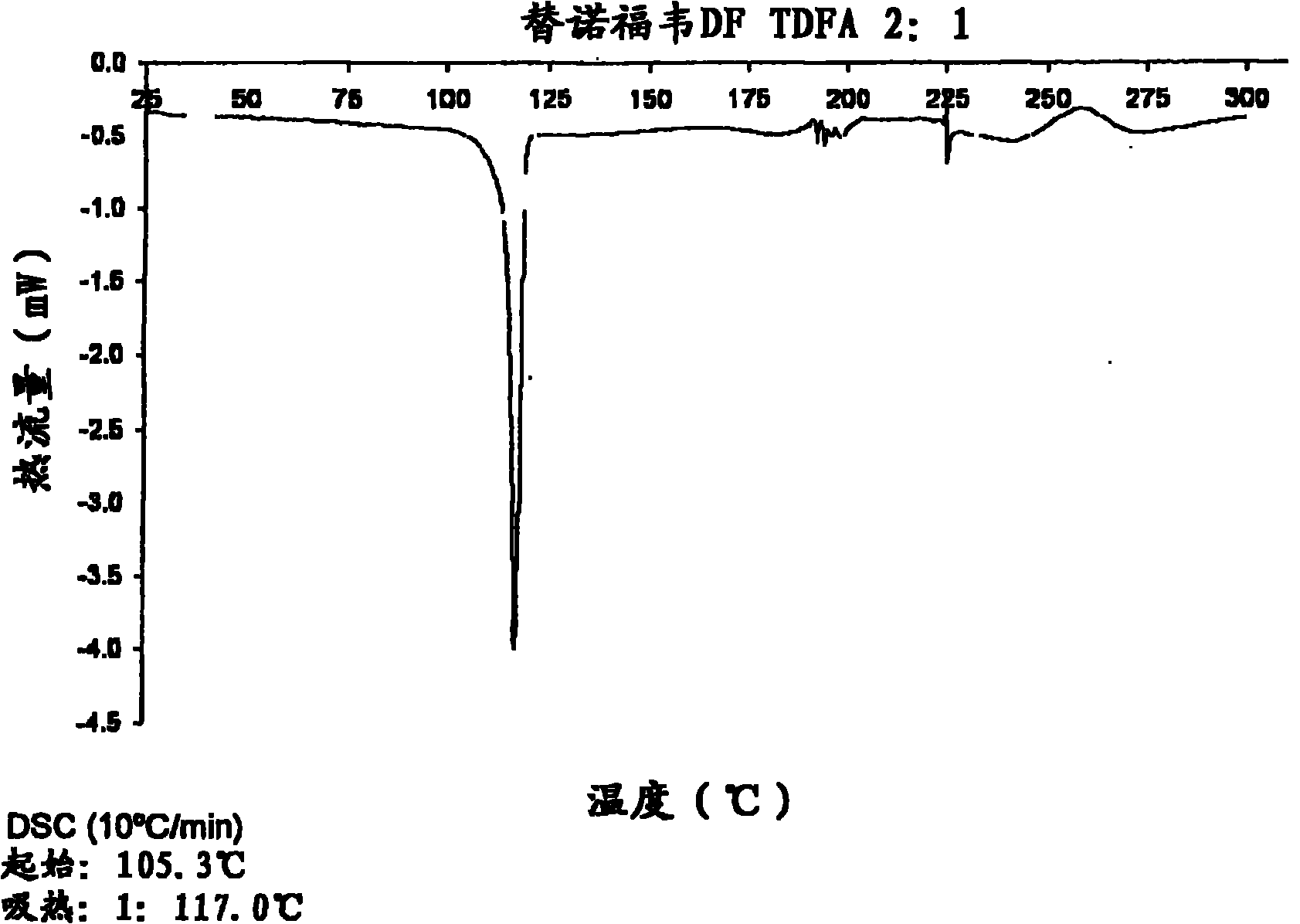
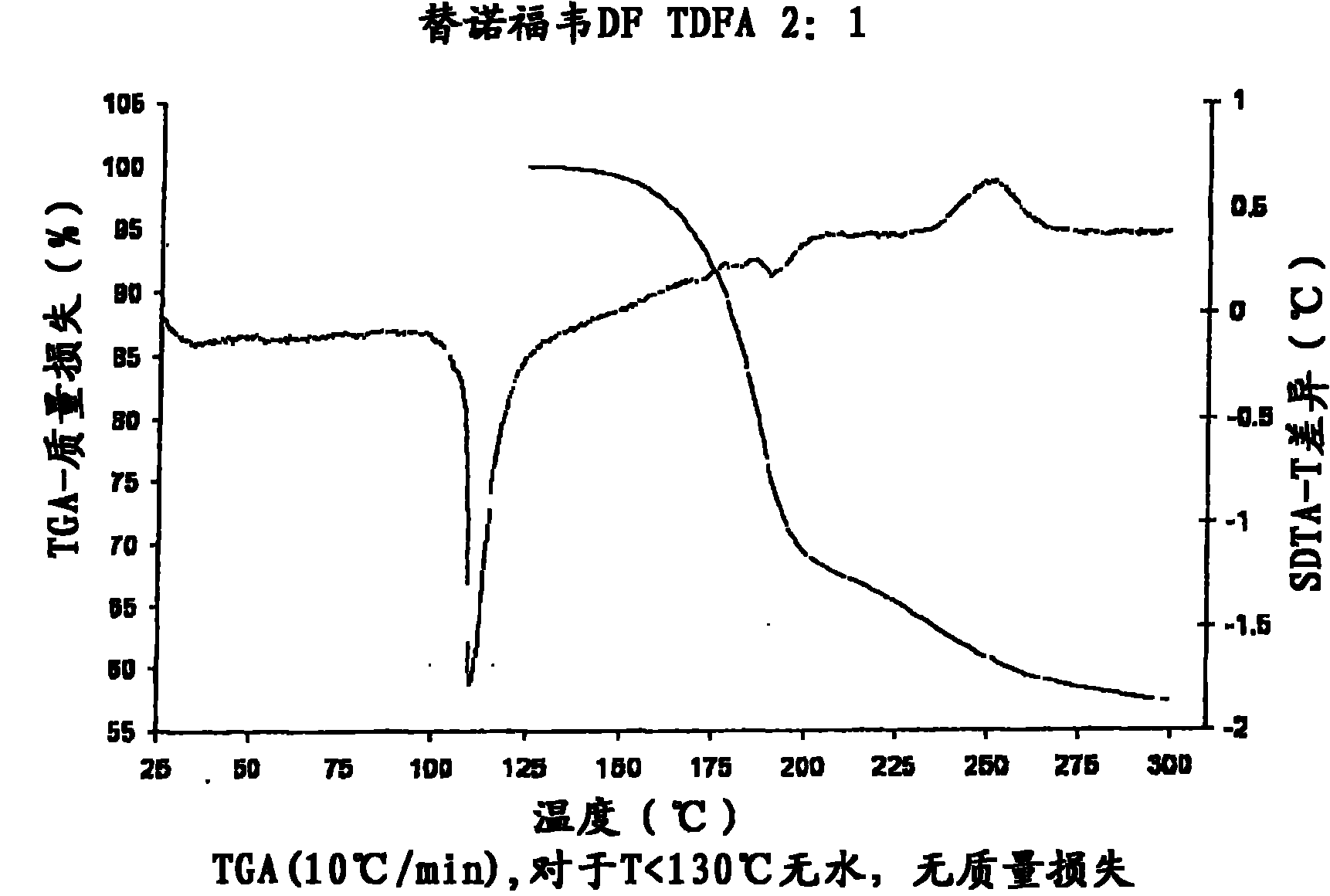
Tenofovir disoproxil hemi-fumaric acid co-crystal
InactiveCN101778855AOrganic active ingredientsGroup 5/15 element organic compoundsEmtricitabineTenofovir DF
The present invention provides a novel crystalline form of Tenofovir disoproxil fumarate (Tenofovir DF), designated Co-crystal TDFA 2:1, methods for the preparation thereof and its use in pharmaceutical applications, in particular in anti-HIV medicaments. The crystalline form TDFA 2:1 can be used in combination with other anti-HIV medicaments such as Efavirenz, Emtricitabine, Ritonavir and / or TMC114.
Owner:ULTIMORPHIX TECH
Non-enantioselective prepn process of emtricitabine
InactiveCN101066971AMild reaction conditionsSimple and fast operationOrganic chemistryDithianeAlcohol
The present invention discloses non-enantioselective preparation process of emtricitabine. The preparation process includes condensation of glyoxalic acid as the initial material and 2, 5-dihydroxy-1, 4-dithiane under the asymmetric induction of optically active alcohol ester to obtain trans-5-hydroxy-1, 3-oxythiacyclopentane-2-carboxylate; halogenating and coupling with silylated 5-flucytosine, and reducing to obtain initial product; reaction with salicylic acid to form salt, purification and separation to obtain optically pure emtricitabine. The present invention has mild reaction condition, simple operation, low cost, less environmental pollution and high product purity reaching medicinal standard, and is suitable for industrial production. The product is used in treating hepatitis B and AIDS.
Owner:葛建利
Compositions and methods for combination antiviral therapy
InactiveUS20060246130A1Eliminate side effectsPatient compliance is goodBiocideOrganic active ingredientsMedicineEmtricitabine
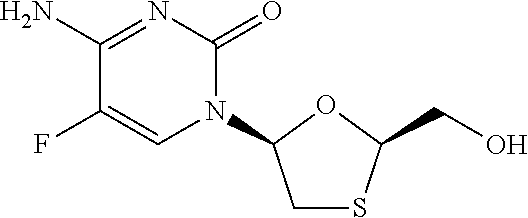
The present invention relates to therapeutic combinations of [2-(6-amino-purin-9 yl)-1-methyl-ethoxymethyl]-phosphonic acid diisopropoxycarbonyloxymethyl ester (tenofovir disoproxil fumarate, Viread®) and (2R,5S,cis)-4-amino-5-fluoro-1-(2 hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one (emtricitabine, Emtriva™, (−)-cis FTC) and their physiologically functional derivatives. The combinations may be useful in the treatment of HIV infections, including infections with HIV mutants bearing resistance to nucleoside and / or non-nucleoside inhibitors. The present invention is also concerned with pharmaceutical compositions and formulations of said combinations of tenofovir disoproxil fumarate and emtricitabine, and their physiologically functional derivatives, as well as therapeutic methods of use of those compositions and formulations.
Owner:GILEAD SCI INC
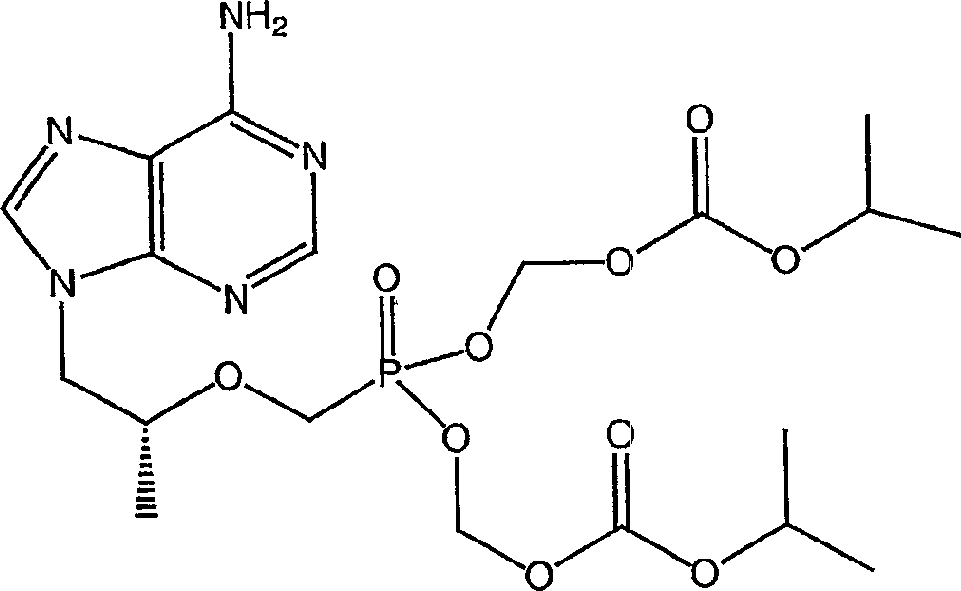
Combination therapy comprising tenofovir alafenamide hemifumarate and cobicistat for use in the treatment of viral infections
InactiveCN104105484AAntiviralsHeterocyclic compound active ingredientsEmtricitabineTenofovir alafenamide
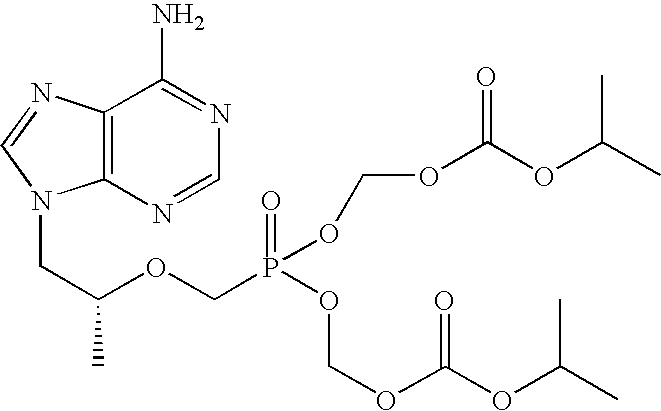
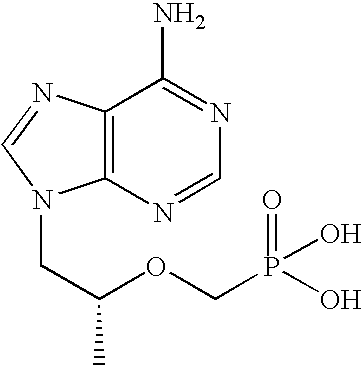
The use of the hemifumarate form of {9-[(R)-2-[[(S)-[[(S)-l- (isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine} (tenofovir alafenamide hemifumarate) in combination with cobicistat is disclosed. In addition, the combination of tenofovir alafenamide hemifumarate, cobicistat, emtricitabine, and elvitegravir, and the combination of tenofovir alafenamide hemifumarate, cobicistat, emtricitabine, and darunavir, are disclosed.
Owner:GILEAD SCI INC
Pharmaceutical antiretroviral composition
InactiveUS20140193491A1Easy to manufactureBiocideOrganic active ingredientsNucleoside Reverse Transcriptase InhibitorEmtricitabine
The present invention relates to a pharmaceutical antiretroviral composition comprising (i) a nucleoside reverse-transcriptase inhibitor selected from lamivudine and emtricitabine, (ii) extended release nevirapine, and (iii) tenofovir; a process for preparing such composition and the use of such composition in medicine, particularly for the prophylaxis and / or treatment of diseases caused by retroviruses.
Owner:CIPLA LTD
Unitary pharmaceutical dosage form
In accordance with this invention a novel pharmaceutical product containing efavirenz, emtricitabine and tenofovir DF are provided as a multicomponent unitary oral dosage form, component 1 comprising tenofovir DF (and, optionally, emtricitabine) and component 2 comprising efavirenz, wherein components 1 and 2 are in a stabilizing configuration. In preferred embodiments component 1 is made by dry granulation.
Owner:GILEAD SCI LLC
Slow released anticancer injection with both antimetabolite and its synergist
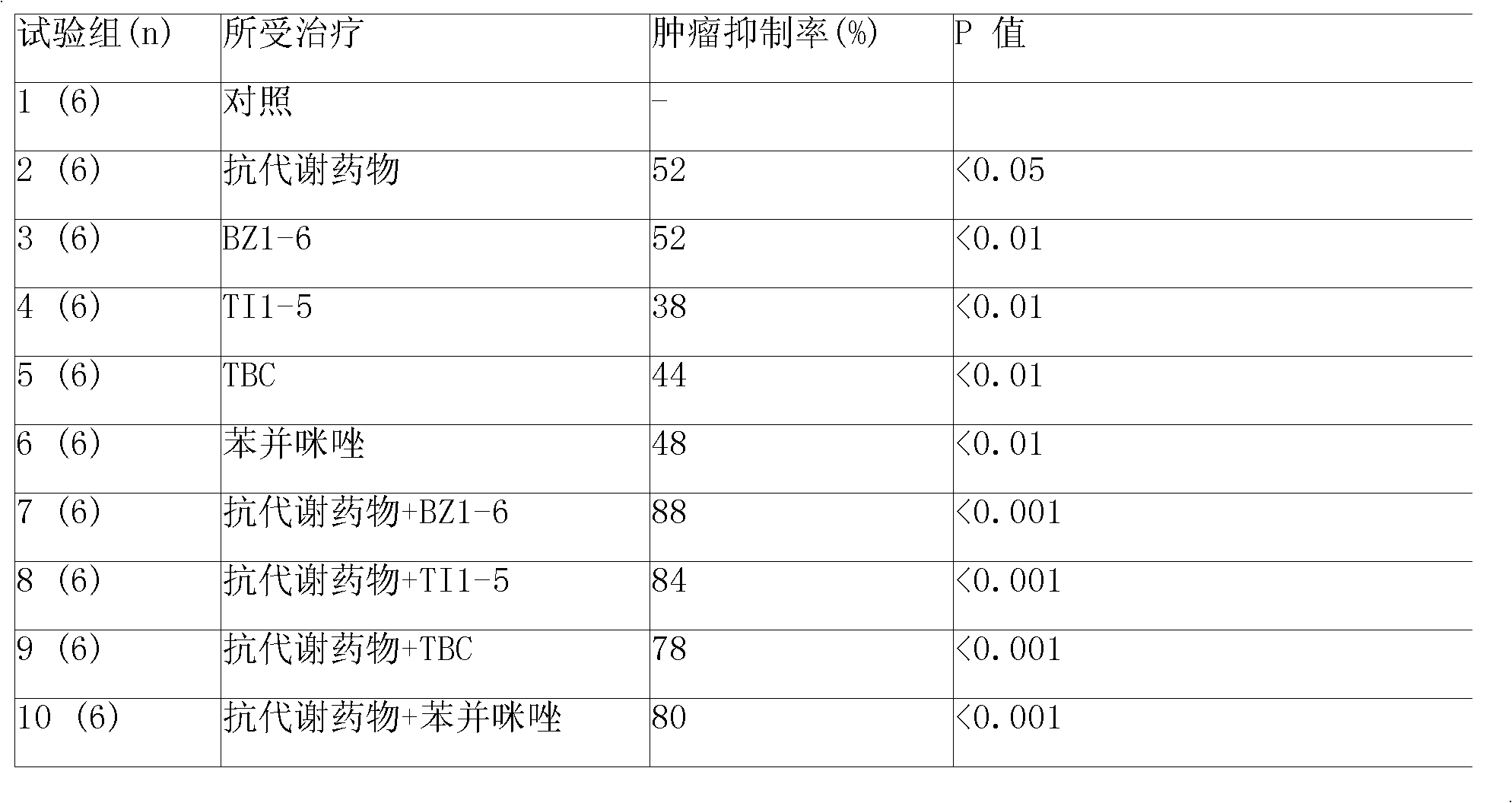
The slow released anticancer injection consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is antimetabolite Zalcitabine, Emtricitabine, etc and / or antimetabolite synergist selected from hormone anticarcinogen and / or platinum compound. The slow releasing supplementary material is selected from difatty acid-sebacic acid copolymer, poly (erucic acid dipolymer-sebacic acid), poly(fumaric acid-sebacic acid), etc or their composition. The suspending agent is carboxymethyl cellulose, etc. and has viscosity of 80-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent for use alone or together with chemotherapeutic medicine, radiotherapeutic medicine, etc.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Method and composition for pharmaceutical product
ActiveUS20140037732A1Improve stabilityFormulation stabilityPowder deliveryOrganic active ingredientsEmtricitabineMedicine
This invention is directed to a composition comprising dry granulated tenofovir DF and emtricitabine, and a method for making same. Dry granulation was unexpectedly found to be important in preparing a tenofovir DF containing composition suitable for inclusion in a combination dosage form containing emtricitabine, efavirenz and tenofovir DF.
Owner:GILEAD SCI INC
Method for separating emtricitabine
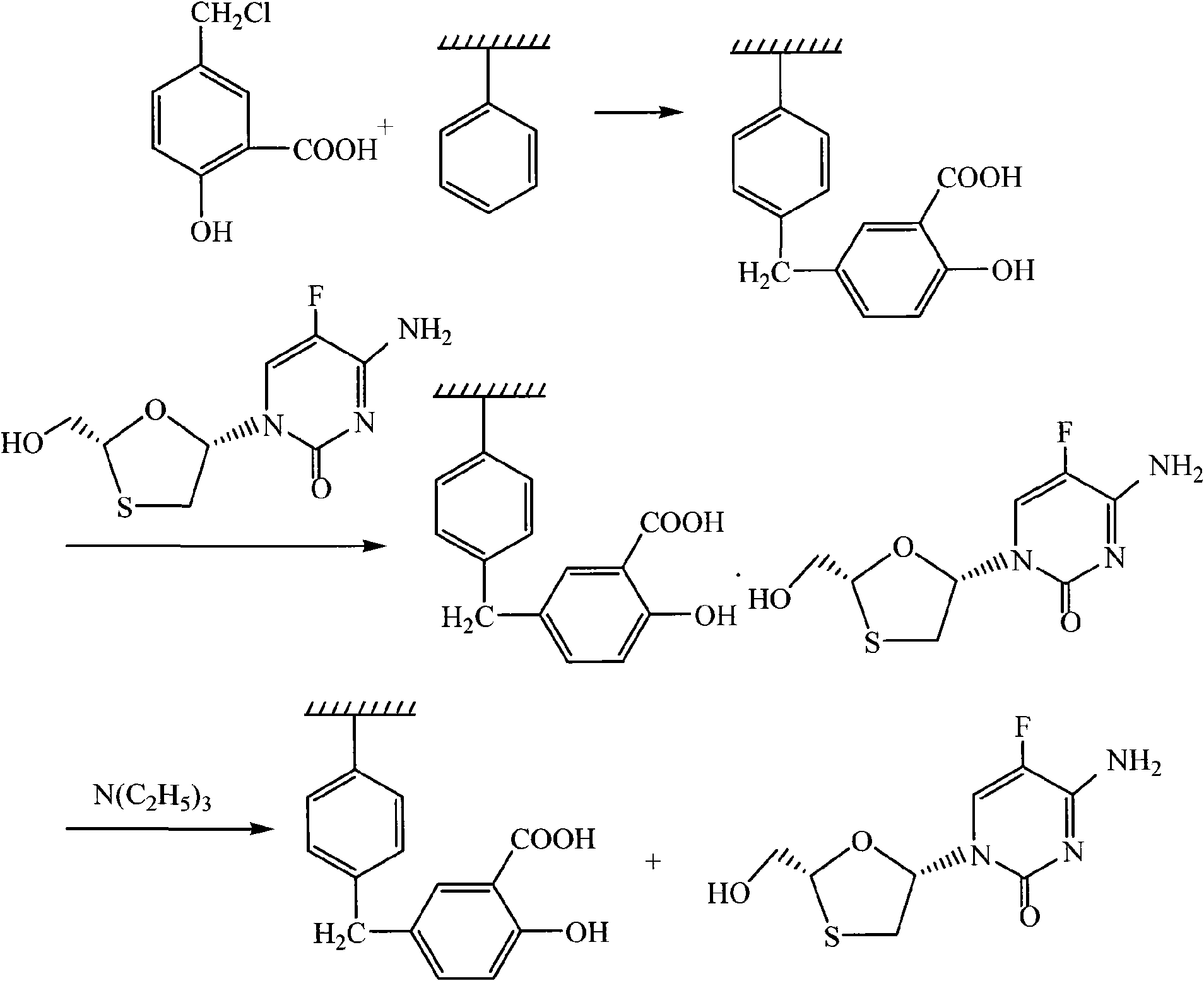
The invention discloses a method for separating emtricitabine. Salicylic acid chloromethylate and polystyreneresin are adopted to have Friedel-Crafts alkylation reaction under the catalysis of aluminium trichloride to obtain salicylic acid functionalized polystyreneresin; salicylic acid functionalized polystyreneresin and the reaction mixture of synthetic emtricitabin are heated together, the emtricitabin in the mixture is complexed with the salicylic acid on the resin, the resin combined with the emtricitabine is separated by filtering, and then emtricitabine is analyzed with triethylamine; and the used resin is processed into regenerated resin with hydrochloric acid 1mol / L and reused again. The invention has the advantages of safe and reliable operation and simple and easy operation, andis suitable for mass production; and the resin can be reused after regeneration so as to reduce the production cost and also reduce the pollution to the environment.
Owner:CANGZHOU SENARY CHEM SCI TEC
Method for preparing emtricitabine
ActiveCN109438432AStarting materials are cheap and readily availableMild reaction conditionsOrganic chemistry methodsEmtricitabineL menthol
The invention discloses a method for preparing emtricitabine. The method comprises the following steps: refining so as to obtain 5S-(5'-flucytosine-1')-1,3-oxythiacyclopentane-2-carbethoxy-(1'R,2'S,3'R)-menthyl lactate; under a weak alkali and solvent condition, removing a chiral aid L-menthol, thereby obtaining a product of emtricitabine. The initial raw material used in the method is low in price, easy to obtain, mild in reaction condition, high in atom utilization rate and simple and convenient in operation process, the reagent is environmentally friendly, the obtained product is high in chemical purity, meets medicine standards, and is applicable to industrial production of emtricitabine.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Method for purifying and refining lamivudine and emtricitabine
The invention discloses a method for purifying and refining lamivudine and emtricitabine. In the method, the lamivudine or the emtricitabine in a material system after reduction reaction is purified and refined by a physical method. The method comprises the following basic steps: flocculation, filtration, adsorption, desorption, decoloration, crystallization and the like, and finally the high-purity lamivudine or emtricitabine product is obtained. The method disclosed by the invention is safe and reliable to operate, is simple and feasible and is applicable to large-scale production. By using the method, the product quality is improved and environmental pollution is reduced.
Owner:刘建红
Unit dosage form comprising emtricitabine, tenofovir, darunavir and ritonavir and a monolithic tablet comprising darunavir and ritonavir
The present invention relates to an oral unit dosage form comprising Emtricitabine, Tenofovir, Darunavir and Ritonavir and a monolithic tablet comprising Darunavir and Ritonavir and their use to treat HIV infection.
Owner:TEVA PHARMA IND LTD
Antiretroviral pharmaceutical composition
ActiveCN107334772AImprove stabilityReduce degradationOrganic active ingredientsDigestive systemEmtricitabineDissolution
The invention provides an antiretroviral pharmaceutical composition in the form of tablets. The antiretroviral pharmaceutical composition comprises main drugs efavirenz, emtricitabine and tenofovir disoproxil fumarate, as well as pharmaceutically acceptable excipients microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, magnesium stearate and the like. A preparation method of the antiretroviral pharmaceutical composition comprises the steps of (1) preparation of a tenofovir disoproxil fumarate-beta-cyclodextrin inclusion complex; (2) preparation of efavirenz pellets; (3) preparation of emtricitabine microparticles; and (4) preparation of tables from the three preparations through one-step granulation. The prepared pharmaceutical composition can effectively avoid the degradation of active ingredients in the preparation process and improve the dissolution and stability of drugs.
Owner:ANHUI BIOCHEM BIO PHARMA
Pharmaceutical compositions of anti-viral compounds and process for preparation thereof
InactiveUS20150141376A1Improve efficacyImprove securityBiocideOrganic active ingredientsInfected patientEmtricitabine
Pharmaceutical compositions of anti-viral compounds, process for preparation and method of using the same are provided. Particularly, the present invention relates to chemically stable pharmaceutical compositions of efavirenz, emtricitabine and tenofovir disoproxil fumarate with optionally one or more pharmaceutically acceptable excipients, process for preparation and method for the treatment or prevention of the symptoms or effects of an HIV infection in an infected patient.
Owner:AUROBINDO PHARMA LTD
Anti-cancer drug slow release injection containing gemcitabine
The invention relates to an anti-cancer drug slow release injection containing gemcitabine, which comprises slow release microspheres and a menstruum, wherein the microsphere comprises anti-cancer active ingredients and slow release auxiliary materials, and the menstruum is a special menstruum containing a suspending agent; the anti-cancer active ingredients are gemcitabine, or antimetabolites selected from zalcitabine, emtricitabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazole gemcitabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite potentiating agents selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogue and / or DNA repair enzyme inhibitor; the slow release auxiliary materials are polifeprosan, dienoic fatty acid, decanedioic acid copolymer, polylactic acid copolymer, EVAc and the like; the suspending agent has a viscosity of 100cp-3000cp (20-30 DEG C), and is selected from sodium carboxymethylcellulose and the like. The slow release microspheres can be prepared into slow release implants. When injected or placed into tumors or at peripheries of tumors, the slow release microspheres can enhance the treatment effect of non-operative treatment methods, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Wet granulation of tenofovir, emtricitabine and efavirenz
The present invention describes a method and composition for a pharmaceutical product based on Tenofovir disoproxil hemifumarate, Emtricitabine and Efavirenz. The composition can be prepared by a process comprising a wet granulation step to produce a stable dosage form suitable for the treatment of HIV in essential absence of known degradation products.
Owner:ULTIMORPHIX TECH
Therapeutic compositions for treatment of human immunodeficiency virus
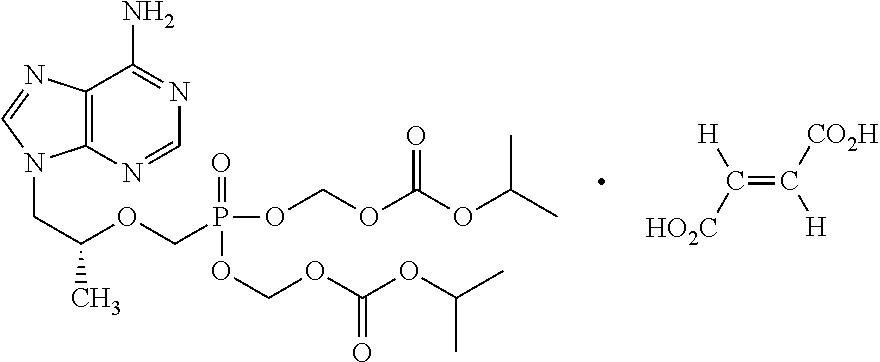
A solid oral dosage form is provided, comprising a compound of Formula I or a pharmaceutically acceptable salt thereof, tenofovir alafenamide or a pharmaceutically acceptable salt thereof, and emtricitabine or a pharmaceutically acceptable salt thereof.
Owner:GILEAD SCI INC
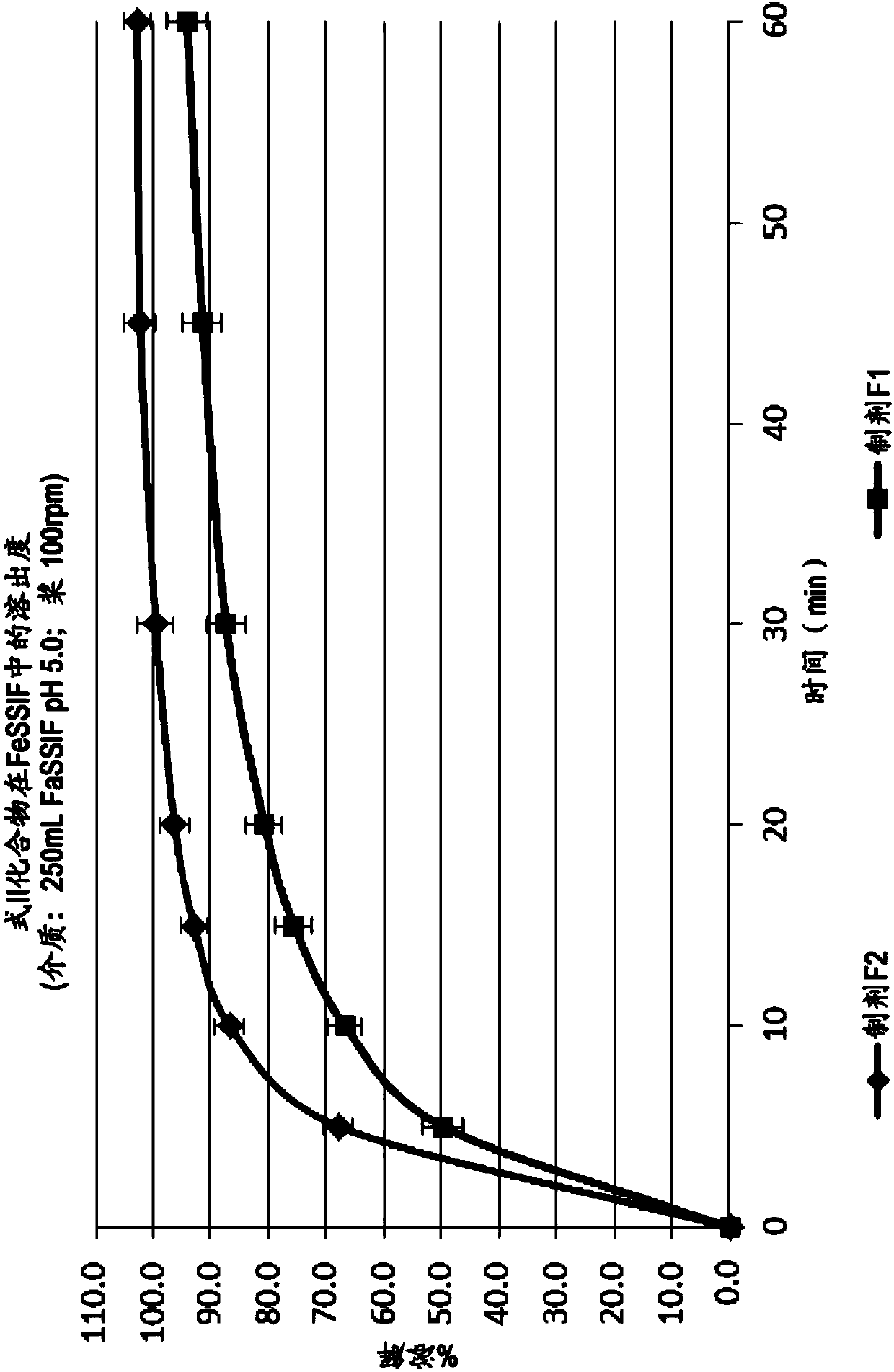
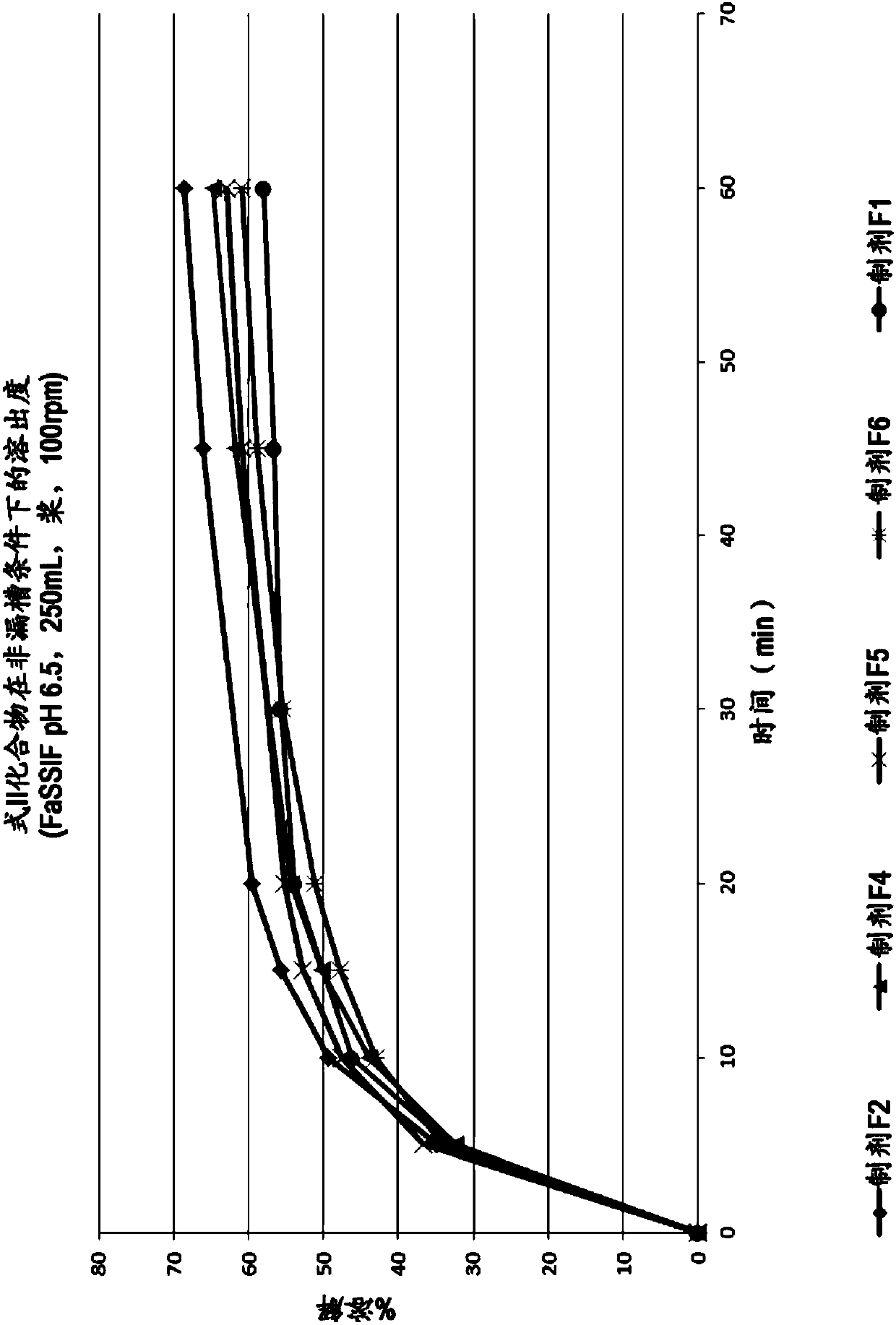
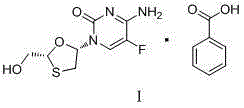
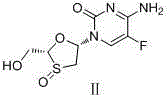
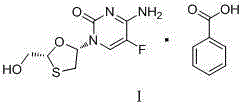
Emtricitabine benzoate, preparation method thereof, and method of preparing emtricitabine from emtricitabine benzoate
The invention discloses emtricitabine benzoate, a preparation method thereof, and a method of preparing emtricitabine from emtricitabine benzoate, and belongs to the biochemistry field. Emtricitabine benzoate is a novel compound, has not been reported before, and is not recorded in any product catalogue. The emtricitabine benzoate is prepared by combining benzoic acid and emtricitabine in a water phase or an organic phase. When benzoic acid and emtricitabine are combined in a water phase or an organic phase, emtricitabine benzoate can be easily separated from the reaction system. By adding organic base or inorganic base into the water phase / organic phase reaction system, emtricitabine benzoate can be decomposed to obtain emtricitabine. By preparing emtricitabine from emtricitabine benzoate, after the reactions, emtricitabine can be easily separated from the reaction system, the purity of the obtained emtricitabine is more than 99%, and the yield is usually more than 80%.
Owner:SHANDONG WEIFANG PHARMA FACTORY
Suitqable to industrialized method for preparing emtricitabine
ActiveCN1274687CRaw materials are easy to getHigh yieldOrganic chemistryDigestive systemHydration reactionPtru catalyst
This invention prepn. method consists procedures (as shown in route chart) of: (1). hydrated glyoxalic acid is reacted with menthol in solvent and catalyst to produce stable intermediate-glyoxalic (1,R,2,5,5,R) menthol ester (V); then, reacting with 2,4-dihydroxy-1,4-dithiothiane to produce menthol ester (IV); (2). said product being proceeded acidylation by hydroxy to produce menthol ester (III); (3) being condensed with 5-fluorocytosine under the protection of silanized reagent, after purification to obtain menthol ester (II); (4). above-said product is reduce by reducing agent to obtain final invention product enqutabin (I) This invention has advantages of: available raw material, high yield, safety and commercialization prodn.
Owner:SHANDONG WEIFANG PHARMA FACTORY
Emtricitabine-containing tablet and preparation method thereof
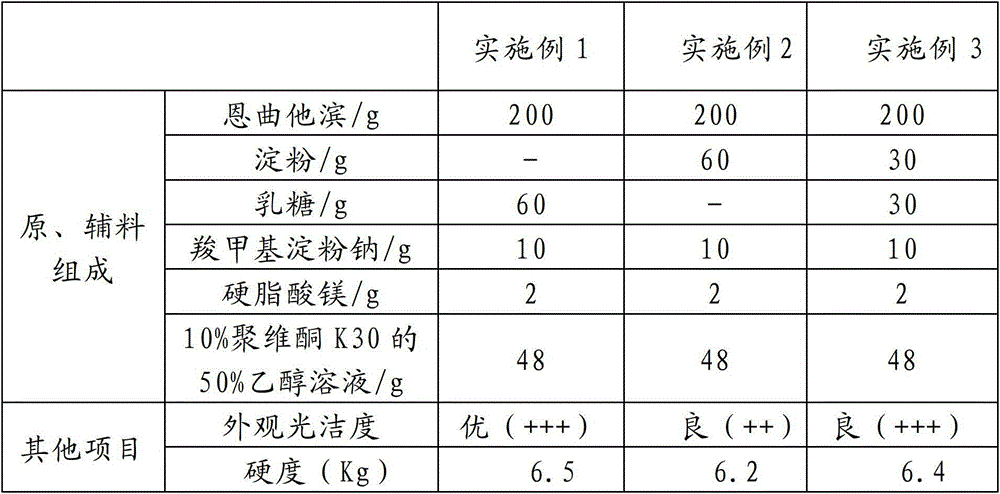
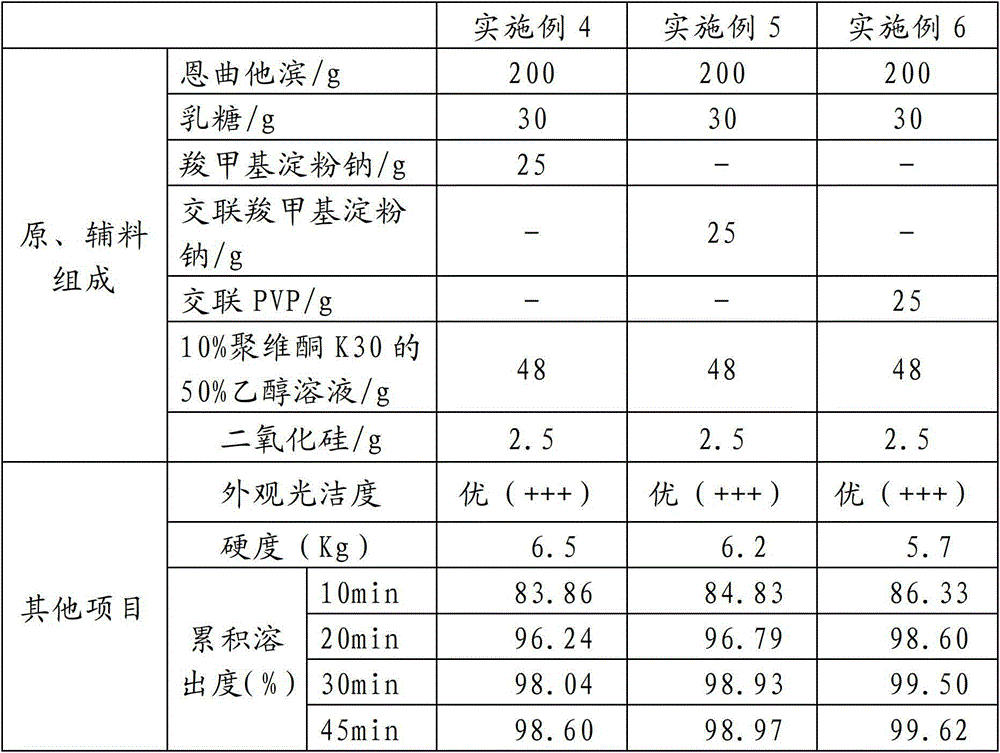
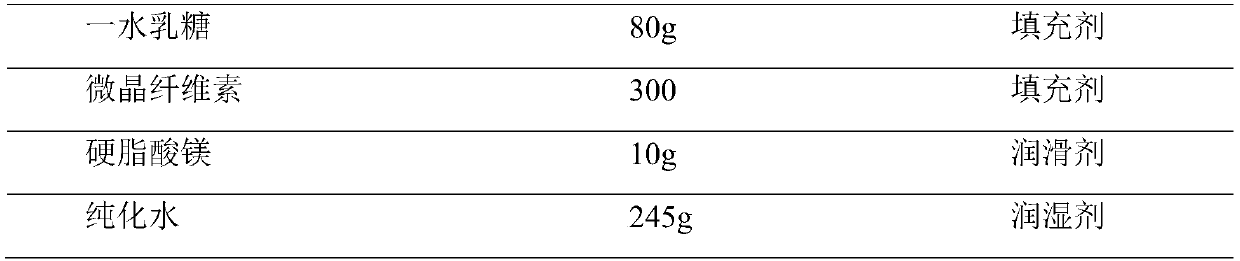
InactiveCN104000788AImprove stabilityHigh dissolution rateOrganic active ingredientsAntiviralsAntiviral drugEmtricitabine
The invention relates to an emtricitabine-containing tablet, which is composed of emtricitabine accounting for 30-80% of the total weight of the tablet and pharmaceutical adjuvants accounting for 20-70% of the total weight of the tablet. The pharmaceutical adjuvants contain a diluents accounting for 10-30% of the total weight of the tablet and a disintegrating agent accounting for 3-10% of the total weight of the tablet. The tablet provided by the invention has the characteristics of good stability, high l dissolution rate, appropriate tablet weight, and convenient drinking. Due to adoption of the single active component emtricitabine, the dosage is accurate. During use, the dosage can be adjusted according to the actual situation, thus facilitating combined use with other antiviral drugs. The preparation method provided by the invention has the advantages of simple process, cheap and easily available adjuvants, and easy industrial production.
Owner:BEIJING UNION PHARMA FACTORY +1
Compositions and methods for combination antiviral therapy
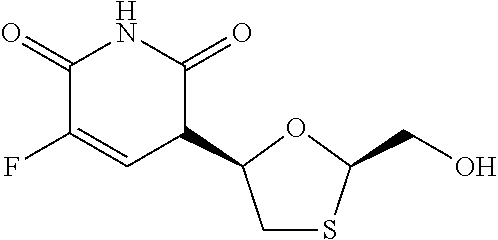
The present invention relates to therapeutic combinations of [2-(6-amino-purin-9-yl)-1-methyl-ethoxymethyl]-phosphonic acid diisopropoxycarbonyloxymethyl ester (tenofovir disoproxil fumarate, Viread <*>) and (2R, 5S, cis)-4-amino-5-fluoro-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one (emtricitabine, Emtriva <TM>, (-)-cis FTC) and their physiologically functional derivatives. The combinations may be useful in the treatment of HIV infections, including infections with HIV mutants bearing resistance to nucleoside and / or non-nucleoside inhibitors. The present invention is also concerned with pharmaceutical compositions and formulations of said combinations of tenofovir disoproxil fumarate and emtricitabine, and their physiologically functional derivatives, as well as therapeutic methods of use of those compositions and formulations.
Owner:GILEAD SCI INC
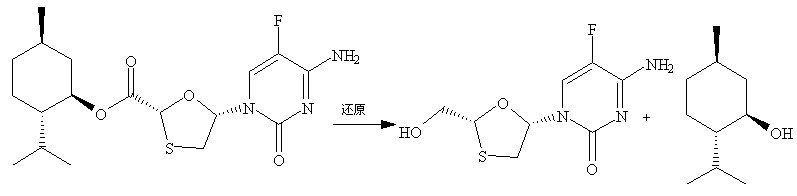
Method for synthesizing emtricitabine intermediate
ActiveCN101391997AOperational securityThe reaction steps are simpleOrganic chemistryState of artEmtricitabine
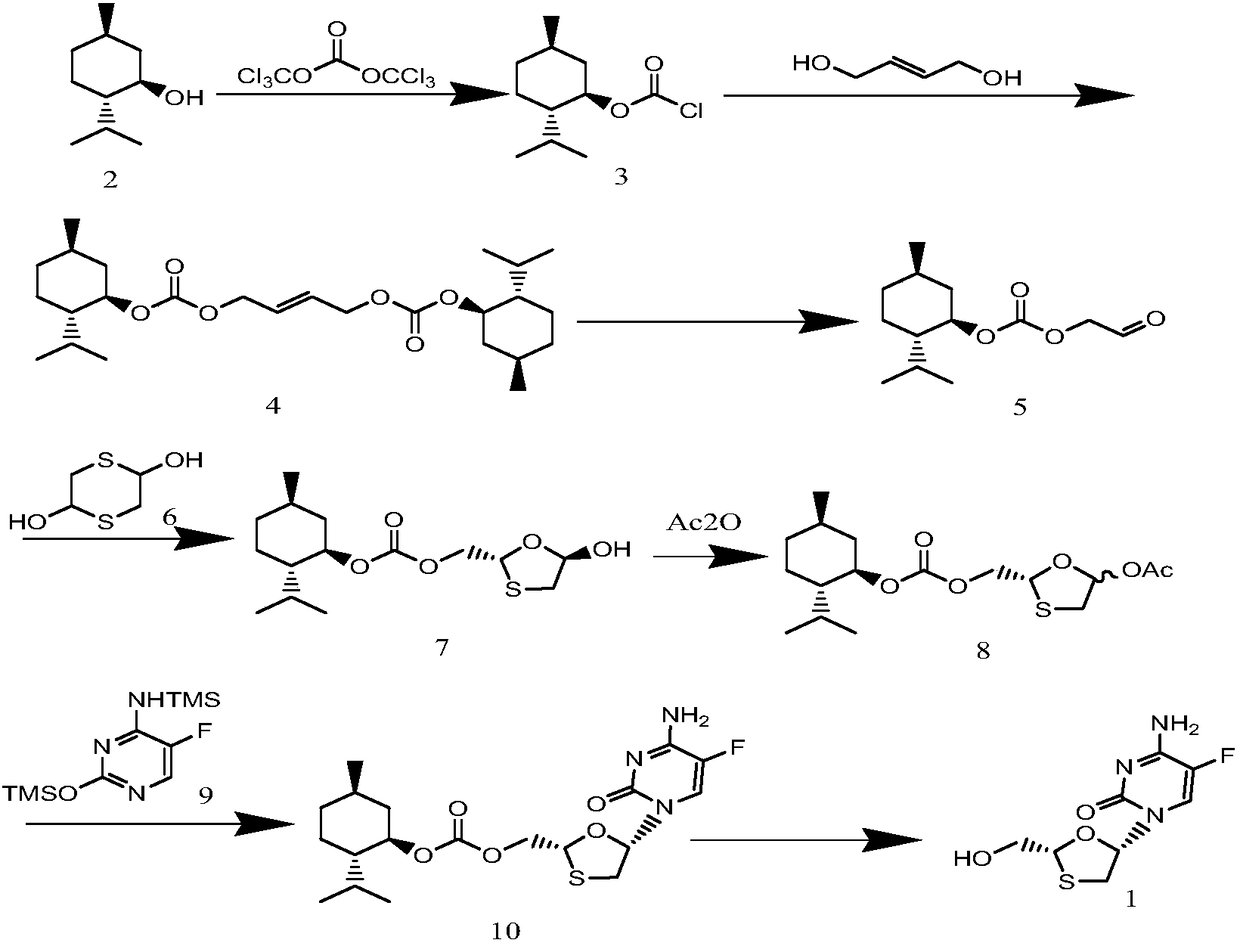
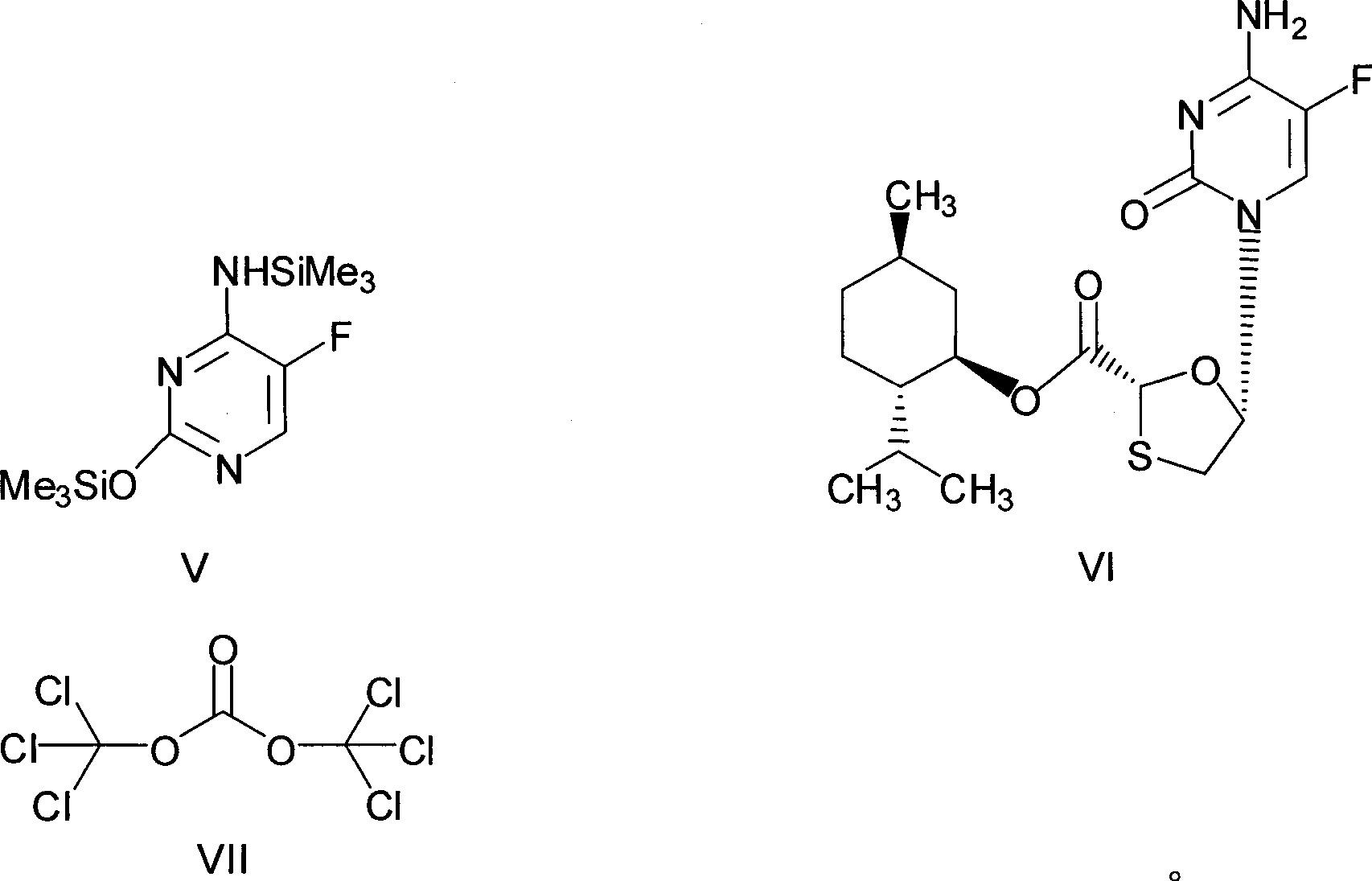
The invention discloses a synthetic method of emtricitabine intermediates, namely, (2R, 5S)-5-(5'-fluoro-cytimidine-1-group)-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester having the structural formula of (VI), (2R, 5S)-5-hydroxy-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester having the structural formula of (III) is taken as raw material to obtain chloro compounds having the structural formula of (IV) by chlorination reaction, then the chloro compounds (IV) are treated with condensation and hydrolyzation reactions with N, O-bis(trimethoxy)5-flurocytosin having the structural formula of (V), thus obtaining the (2R, 5S)-5-(5'-fluoro-cytimidine-1-group)-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester (VI). The invention uses bis(trichloromethyl) carbonic ester to replace the thionyl chloride in the prior art so as to be taken as a chlorination reagent, thus having the advantages of safe and reliable operation and environmental protection.
Owner:JIANGSU COBEN PHARMA CO LTD +1
Preparation method for emtricitabine isomer
ActiveCN109553610AGuarantee processQuality assuranceOrganic chemistry methodsBulk chemical productionEmtricitabineAcetylation
The invention discloses a preparation method for an embritabine isomer. The preparation method comprises the following steps: with Solketal as a starting material, allowing the Solketal to undergo a six-step reaction of esterification, hydrolysis, oxidation, condensation cyclization, acetylation and glycosylation condensation so as to synthesize four mixture intermediates of emtricitabine; and splitting the four isomer intermediates into a cis-isomer mixture and a trans-isomer mixture through a chiral reagent. According to the invention, by adoption of a simple starting material, a mixture forsplitting key intermediates of four optical isomers of the emtricitabine is synthesized through the six-step reaction, and chiral acid is utilized to split the four isomers into a mixture of cis andtrans isomers, so the preparation method provided by the invention has the advantages of simple and convenient operation, high yield and high isomer chiral purity.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Quadruple therapy useful for treating persons afflicted with the human immunodeficiency virus (HIV)
InactiveUS20150305983A1Reduce in quantityMaintain curative effectComputer controlDrug and medicationsEmtricitabineNucleotide
The present invention relates to a pharmaceutical composition for treating the human immunodeficiency virus (HIV) in a human being, comprising four active principles selected as being: one nucleoside inhibitor of reverse transcriptase (NRTI) selected from lamivudine and emtricitabine; two nucleoside or nucleotide inhibitor of reverse transcriptase (NRTI) selected from didanosine, abacavir and tenofovir; and the fourth active principle is selected from (i) a non-nucleoside inhibitor of reverse transcriptase (NNRTI) selected from nevirapine, efavirenz and etravirine; or (ii) a protease inhibitor selected from atazanavir, lopinavir, saquinavir, ritonavir, indinavir, amprenavir, nelfinavir, fosamprenavir, tipranavir and darunavir.The present invention also relates to an electronic portable pillbox comprising a multidrug therapy for treating the immunodeficiency virus (HIV) in human beings allowing improving the observance of medication intake.
Owner:UNIV VERSAILLES SAINT QUENTIN EN YVELINES
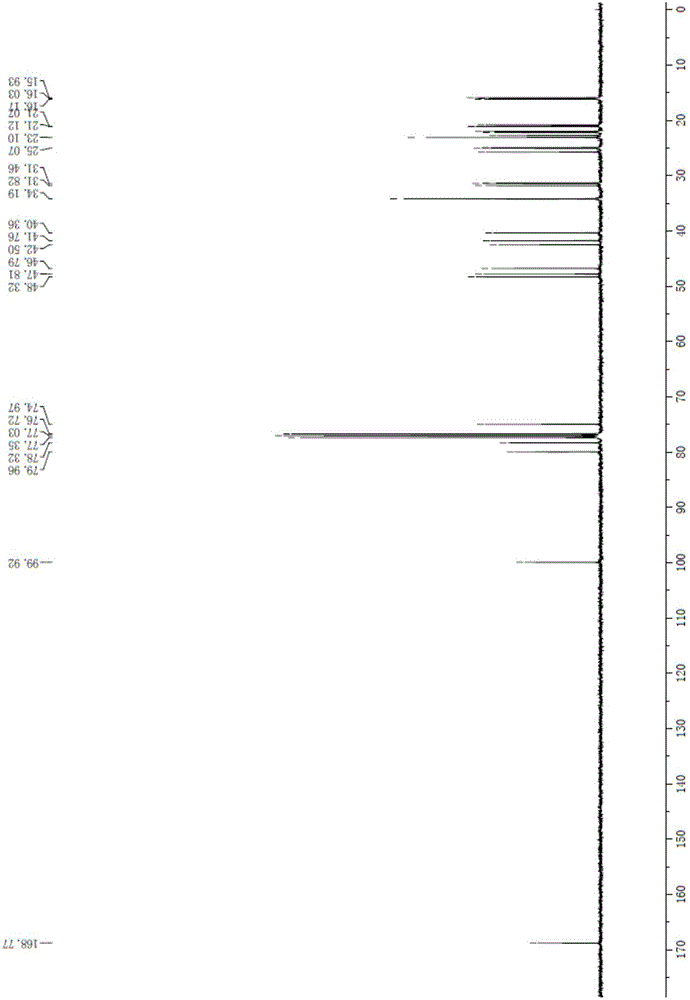
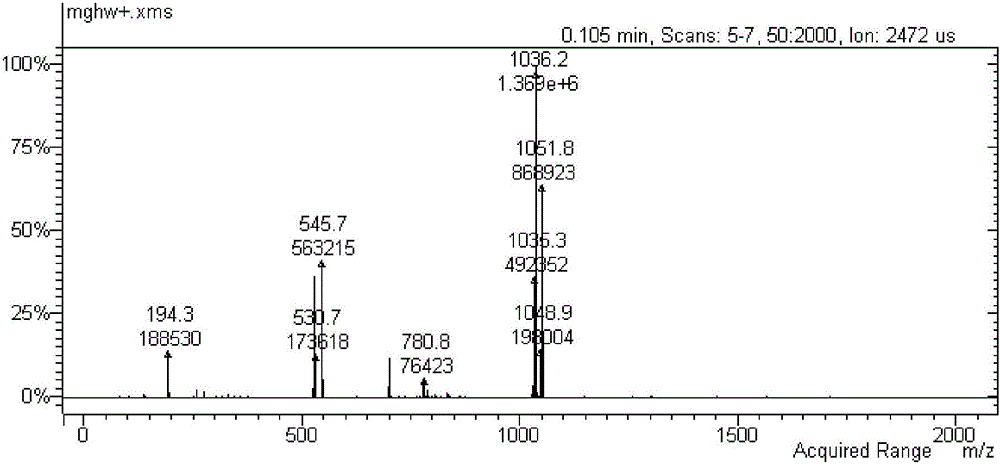
Method of reducing impurities in synthesis process of emtricitabine intermediate MGH
ActiveCN105130806ARaise quality standardsShort reaction timeOrganic compound preparationCarboxylic acid esters preparationEmtricitabineReaction temperature
The invention discloses a method of reducing impurities in a synthesis process of an emtricitabine intermediate MGH. The method comprises the following steps: taking L-menthol and glyoxylic acid as raw materials; carrying out an esterification reaction under acid catalysis; carrying out an addition reaction under the action of sodium hydrogen sulfite; carrying out a hydroxylation reaction under the action of formaldehyde; carrying out post-treatment to obtain L-menthyl dyhydroxy acetate, wherein in the process of the addition reaction, the pH value is 4-6, and the reaction temperature is 40-80 DEG C and the reaction time is 4-10 hours. With the adoption of the method, the reaction time is greatly shortened and a reaction period is shortened; meanwhile, in a reaction process, cyclohexane is only used as an organic solvent, so that the operation is simple and the post-treatment is convenient; by controlling process conditions and post-treatment conditions, the content of impurities is greatly reduced and the yield is improved. Meanwhile, certain helps are provided for registration and application, and production and center control of emtricitabine, and improvement of quality standards of the emtricitabine intermediate.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Emtricitabine and tenofovir medicine composition
ActiveCN110251476AImprove physical stabilityGood chemical stabilityOrganic active ingredientsAntiviralsMedicineEmtricitabine
The invention provides a stable emtricitabine and tenofovir medicine composition. The prepared medicine composition can effectively control degradation of effective components in the preparation and storage process, and the physical and chemical stability of medicines is improved. The invention further relates to a preparation method and an application of the medicine composition.
Owner:海思科制药(眉山)有限公司
Nrti therapies
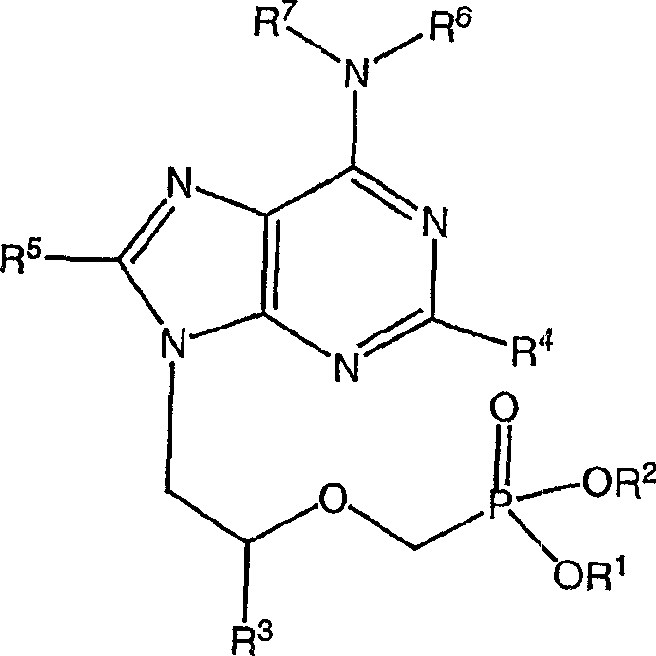
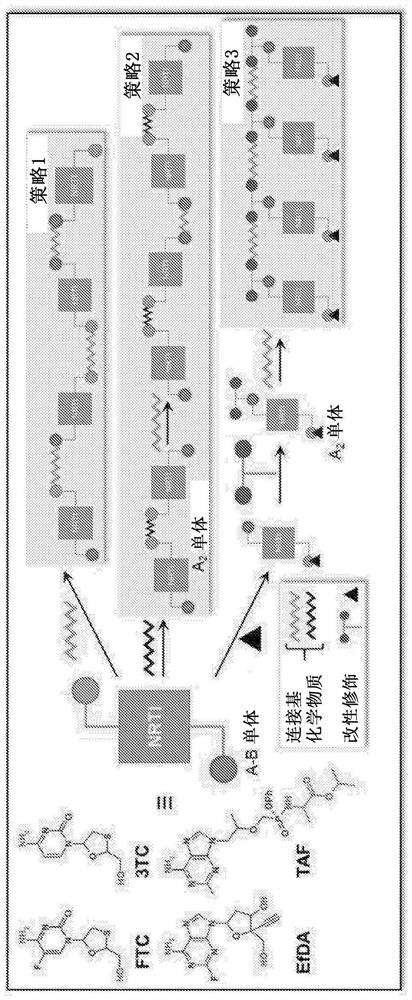
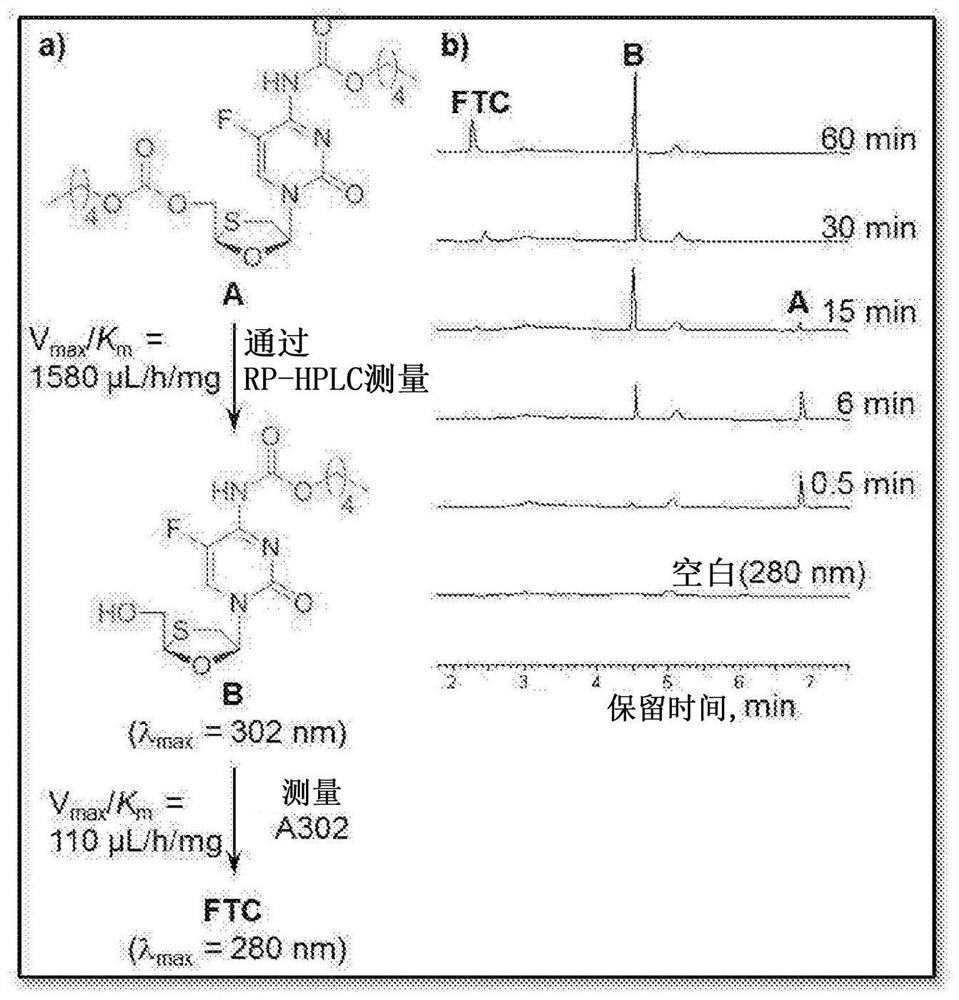
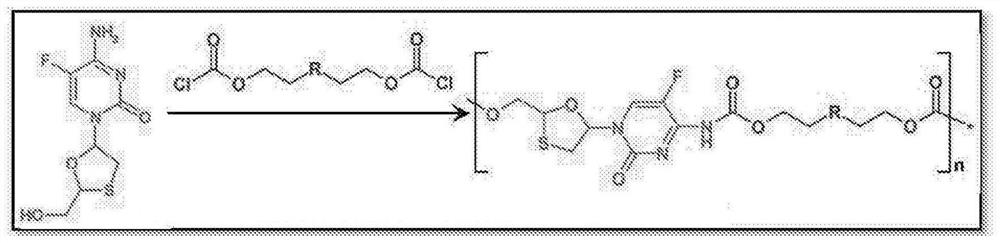
Polymer-of-prodrug (POP) materials enable new nucleoside reverse transcriptase inhibitor (NRTI) therapy strategies. The materials are prodrugs of NRTIs in the form of polymers. Suitable materials include products which are polymeric NRTI delivery systems comprising polymeric materials which are capable of degradation after administration to release NRTIs or NRTI prodrugs which themselves are capable of metabolism to the parent NRTIs. The NRTIs may optionally be selected from tenofovir (TFV), emtricitabine (FTC), lamivudine (3TC) and MK-8591 (EFdA). The invention facilitates long-acting (LA) regimens. Constructs of the materials may be in the form of injectable compositions or implants.
Owner:UNIV OF LIVERPOOL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com