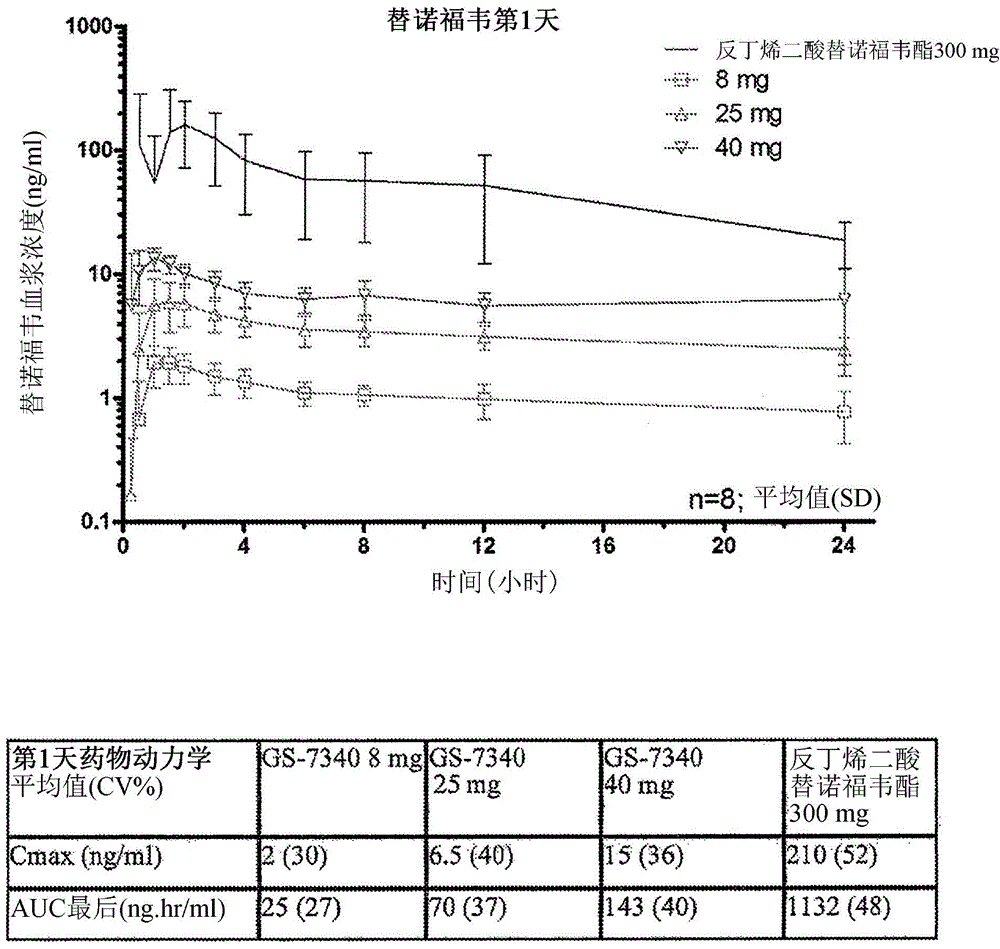
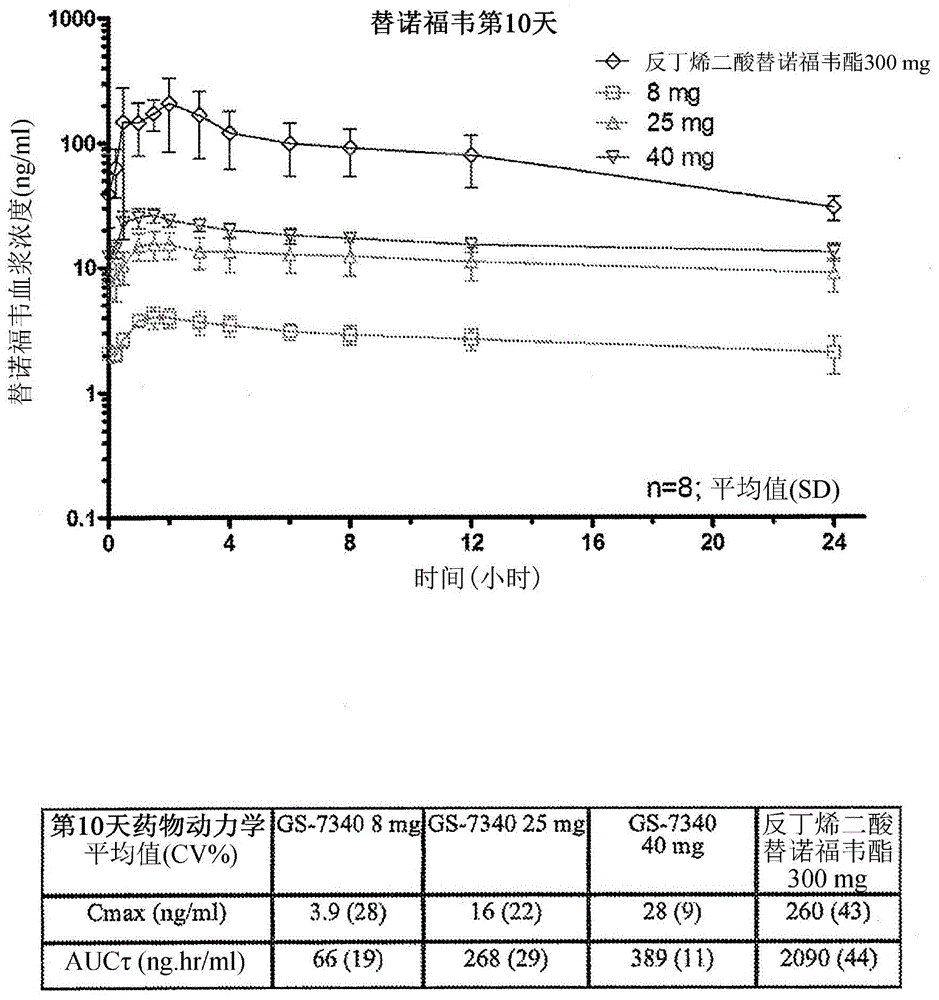
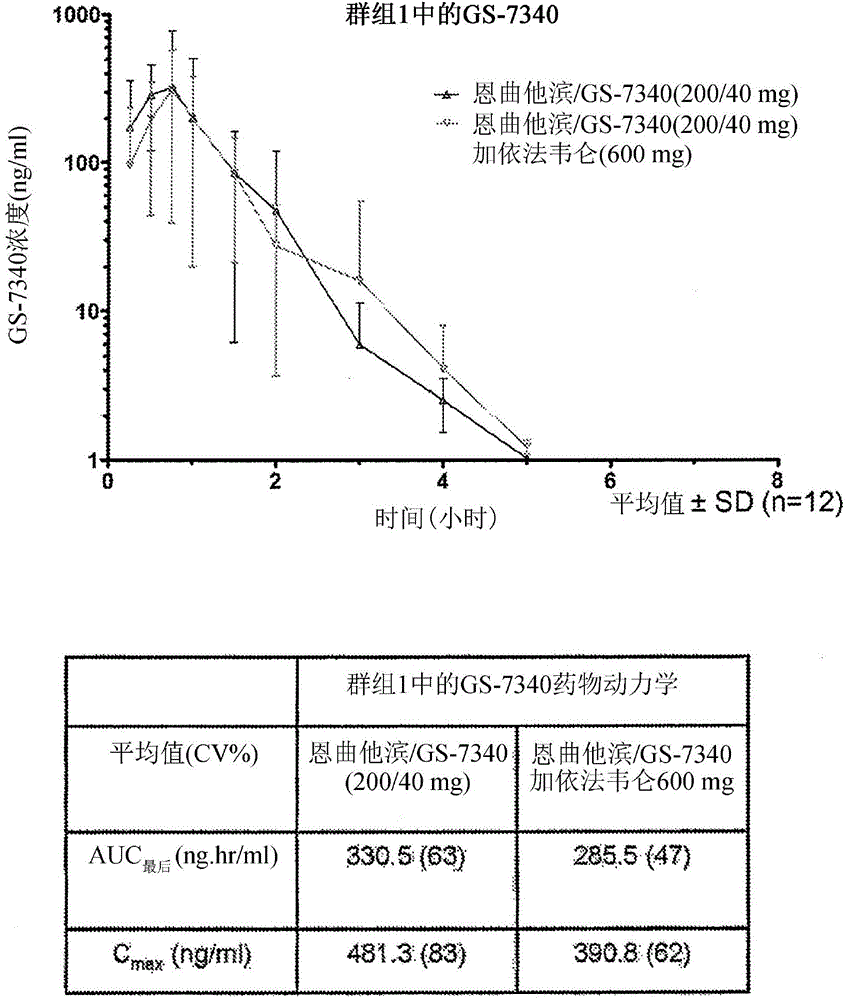
Combination therapy comprising tenofovir alafenamide hemifumarate and cobicistat for use in the treatment of viral infections
A kind of technology of tenofovir alafenamide and fumarate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0307] Synthesis Example 1: Preparation of 9-[(R)-2-[[(R,S)-1-[[(S)-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methane Diastereomeric mixture of oxy]propyl)adenine (15)
[0308]
[0309] a. Preparation of compound 11
[0310] Stir L-alanine isopropyl ester hydrochloride 10 (1kg, 5.97mol, 1.0 equivalent) and potassium bicarbonate (1.45kg, 14.5mol, 2.43 equivalent) under maximum stirring in DCM (4kg) for 10-14 Keep the tank temperature between 19 and 25°C. Then, the mixture was filtered and further rinsed with DCM (2 kg). Filtrate The molecular sieve bed is dried until the water content of the solution is ≤0.05%. The resulting stock solution containing compound 11 was then cooled to a tank temperature of -20°C and kept for further use.
[0311] b. Preparation of compound 13a
[0312] Compound 12 (1 kg, 2.75 mol, 1.00 equivalent) was added to a solution of thionyl chloride (0.72 kg, 6.02 mol, 2.19 equivalents) in acetonitrile (5.5 kg) in 10 equal portions at 60° C. for 2 hour...
Synthetic example 2
[0315] Synthesis Example 2: p-9-[(R)-2-[[(R,S)-1-[[(S)-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methane The diastereomeric mixture of oxy]propyl]adenine (15) undergoes crystallization-induced dynamic resolution to obtain 9-[(R)-2-[[(S)-[[(S)-1- (Isopropoxycarbonyl)ethyl)amino)phenoxyphosphinyl)methoxy)propyl)adenine (16)
[0316]
[0317] (Mixture of diastereoisomers)
[0318] 22% by weight 9-[(R)-2-[[(R,S)-1-[[(S)-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy A solution (2.3kg solution, 0.51kg15, 1.1mol, 1 equivalent) of the diastereomeric mixture of propyl]propyl]adenine (15) in acetonitrile is fed equipped with overhead stirrer, distillation equipment and nitrogen In the inlet of the container. The mixture is concentrated to a final concentration of 30-35% by weight by distillation at 100-300 mbar in the temperature range of 45°C-55°C. The distillation equipment was then removed and the solution was cooled to 20°C. The solution was seeded with 2.0% comp...
Synthetic example 3
[0319] Synthesis Example 3: Preparation of compound 13a with high diastereoisomeric purity
[0320] To a slurry of compound 12 (10.0 g, 27.5 mmol, 1.00 equiv) in toluene (60 mL) was added thionyl chloride (3.0 mL, 41 mmol, 1.5 equiv) at ambient temperature. The slurry was heated to 70°C and agitated for 48-96 hours until the reaction and diastereomeric enrichment were deemed complete according to HPLC (target: the conversion rate of compound 12 to compound 13a>97.0% and the diastereomer of compound 13a Isomer ratio>90:10). The mixture was concentrated to dryness by vacuum distillation, and the dry residue was absorbed in toluene (50 mL). The resulting slurry containing compound 13a is kept at ambient temperature for further use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com