Method of reducing impurities in synthesis process of emtricitabine intermediate MGH
A synthesis process and technology of emtricitabine, applied in chemical instruments and methods, preparation of organic compounds, preparation of sulfonates, etc., can solve the problems of long production cycle, increased amount of impurity compound III during holding time, and reduced production capacity, etc. To achieve the effect of reducing the impurity content, shortening the reaction cycle and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
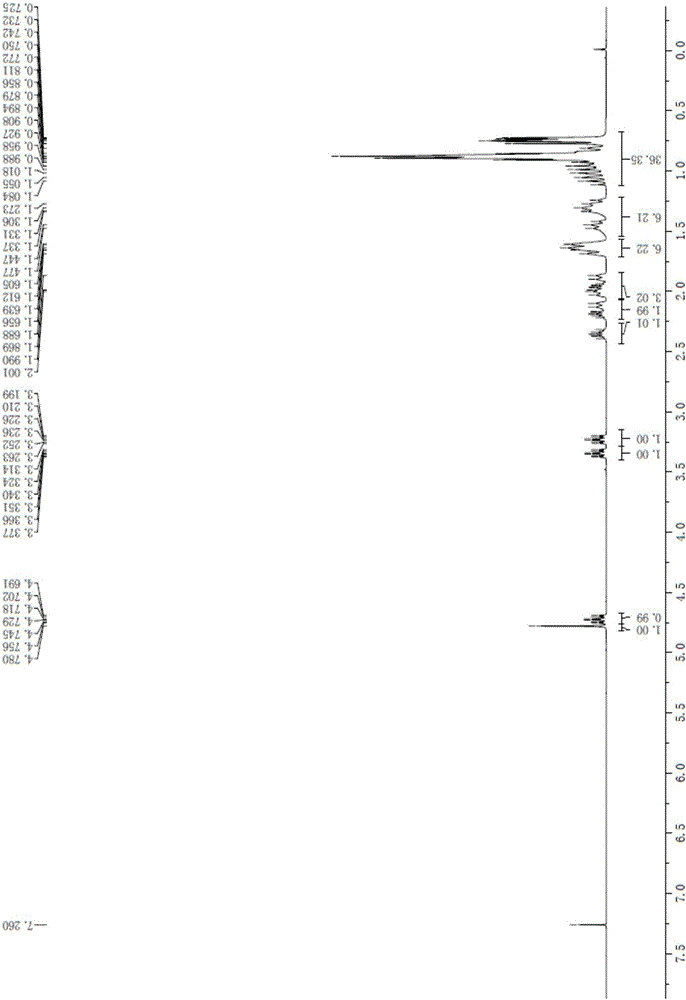
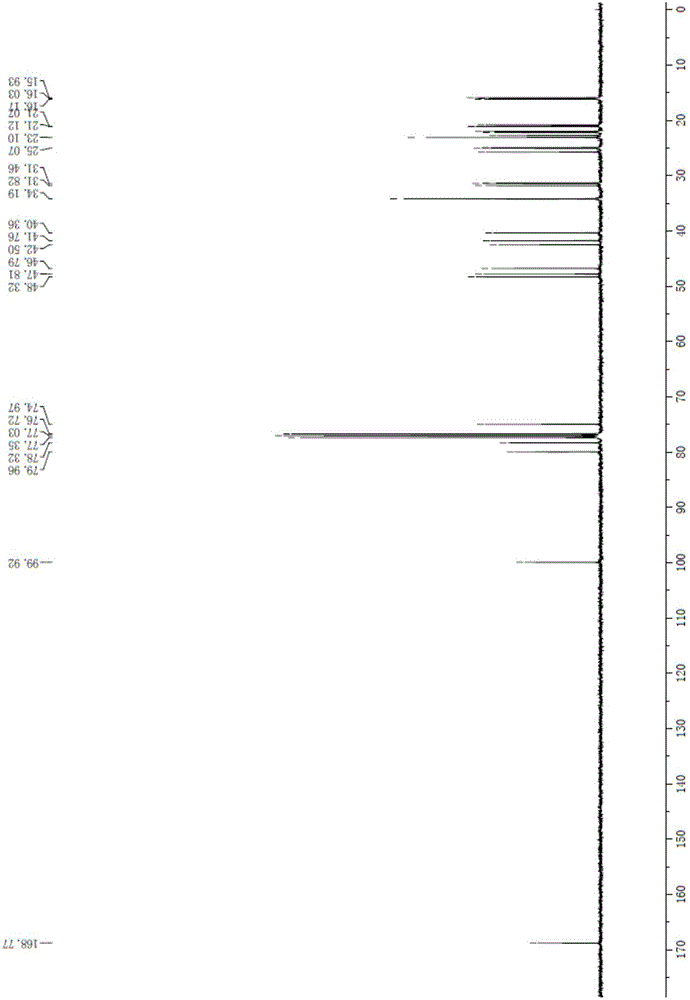
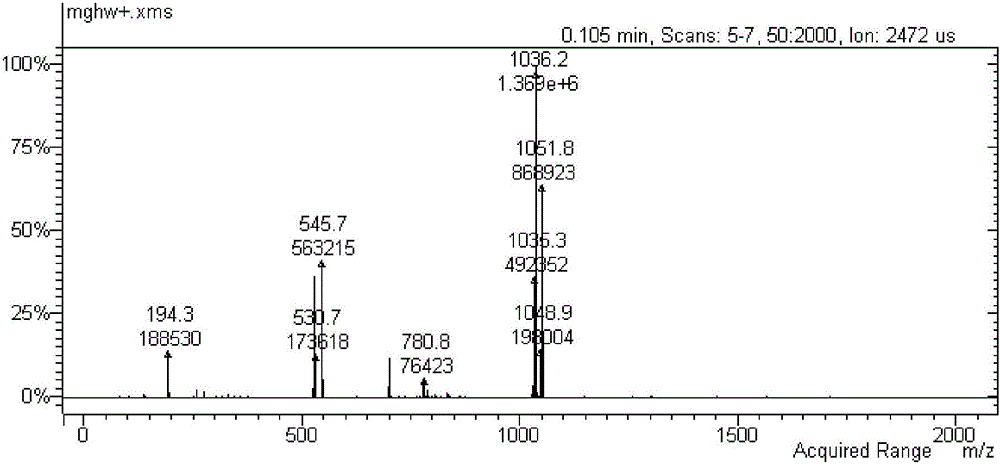
Image
Examples
Embodiment 1
[0039] Add 100g cyclohexane in 1000ml bottle, add 87.2g50% glyoxylic acid (0.59mol) and 5g vitriol oil, heat up and reflux, slowly add the cyclohexane solution of 220gL-menthol (L-menthol 120g (0.77mol) , cyclohexane 100g), reflux and water separation for about 5 hours. The temperature was lowered under the protection of nitrogen, and 30ml of cyclohexane and 60ml of water were added, stirred for 10 minutes, and allowed to stand to separate into layers. The organic phase was washed once with 60 g of water and 50 ml of saturated NaCl aqueous solution, and the organic phase was collected for use.
[0040] Add 150g NaHSO to a 2000ml bottle 3 Aqueous solution (NaHSO 3 0.60mol), stirring and controlling the temperature at 25-30°C, adding the organic phase of the previous step, and using saturated Na 2 CO 3 Adjust pH=4.5-5.5, keep the temperature and pH value at 60°C for 8 hours, and let stand to separate layers. The aqueous layer was washed once with 100g cyclohexane, and then ...
Embodiment 2
[0045] In 1000ml bottle, add 100g cyclohexane, add 87.2g50% glyoxylic acid (0.59mol) and 5g vitriol oil, heat up and reflux, slowly add the cyclohexane solution of 220gL-menthol (L-menthol 120g (0.77mol) , cyclohexane 100g), reflux and water separation for about 5 hours. The temperature was lowered under the protection of nitrogen, and 30ml of cyclohexane and 60ml of water were added, stirred for 10 minutes, and allowed to stand to separate into layers. The organic phase was washed once with 60 g of water and 50 ml of saturated NaCl aqueous solution, and the organic phase was collected for use.
[0046] Add 150g NaHSO to a 2000ml bottle3 Aqueous solution (NaHSO 3 0.60mol), stirring and controlling the temperature at 25-30°C, adding the organic phase of the previous step, and using saturated Na 2 CO 3 Adjust the pH=4.5-5.5, keep the temperature and pH value at 50°C for 6 hours, and let stand to separate layers. The aqueous layer was washed once with 100g cyclohexane, and th...
Embodiment 3
[0050] Add 100g cyclohexane in 1000ml bottle, add 87.2g50% glyoxylic acid (0.59mol) and 5g vitriol oil, heat up and reflux, slowly add the cyclohexane solution of 220gL-menthol (L-menthol 120g (0.77mol) , cyclohexane 100g), reflux and water separation for about 5 hours. The temperature was lowered under the protection of nitrogen, and 30ml of cyclohexane and 60ml of water were added, stirred for 10 minutes, and allowed to stand to separate into layers. The organic phase was washed once with 60 g of water and 50 ml of saturated NaCl aqueous solution, and the organic phase was collected for use.
[0051] Add 150g NaHSO to a 2000ml bottle 3 Aqueous solution (NaHSO 3 0.60mol), stirring and controlling the temperature at 25-30°C, adding the organic phase of the previous step, and using saturated Na 2 CO 3 Adjust the pH=4.5-5.5, keep the temperature and pH value at 55°C for 5 hours, and let stand to separate the layers. The aqueous layer was washed once with 100g cyclohexane, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com