Crystalline form of Adefovir ester and preparation method
A technology of adefovir dipivoxil and new crystallization, applied in the field of AD synthesis, purification and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
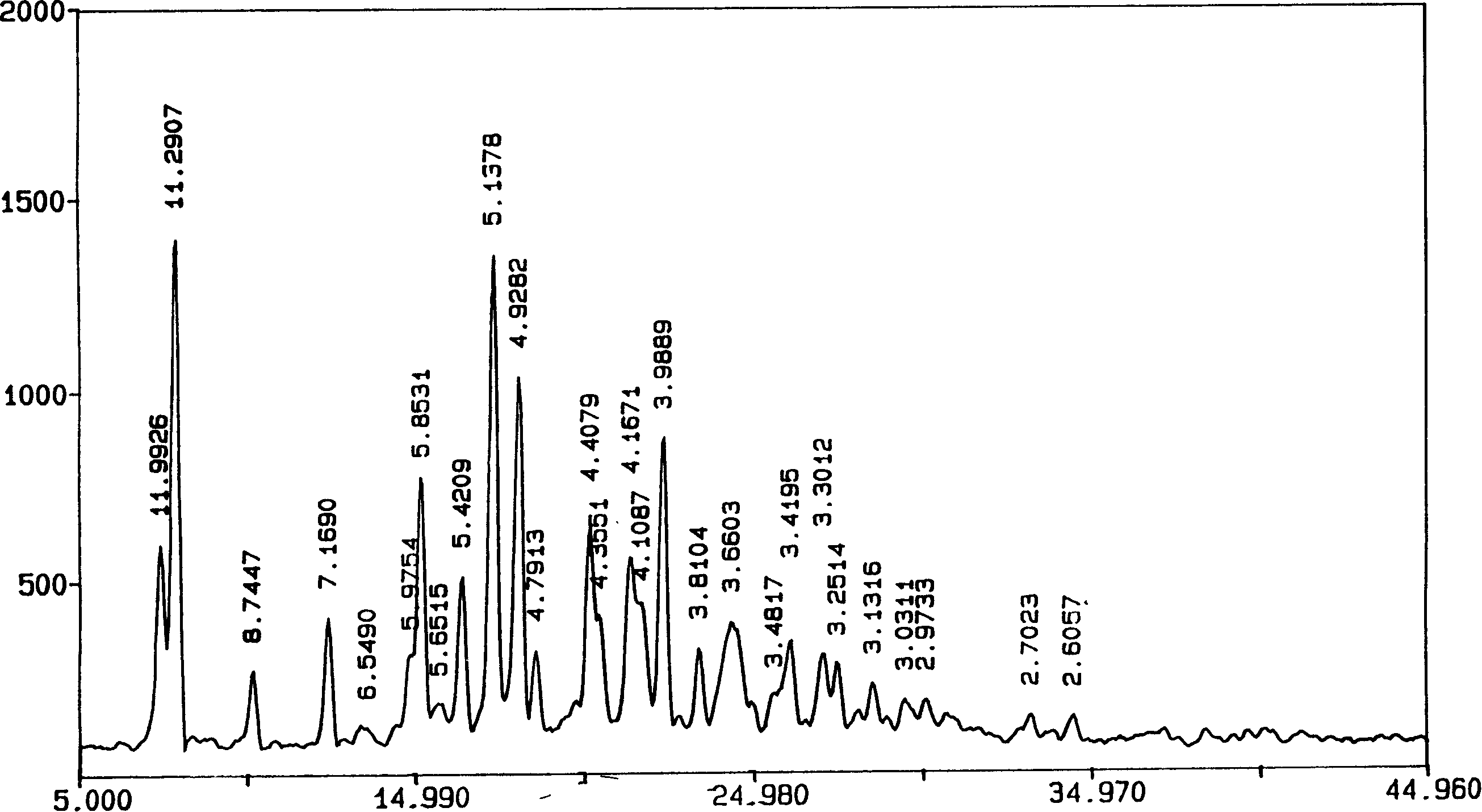
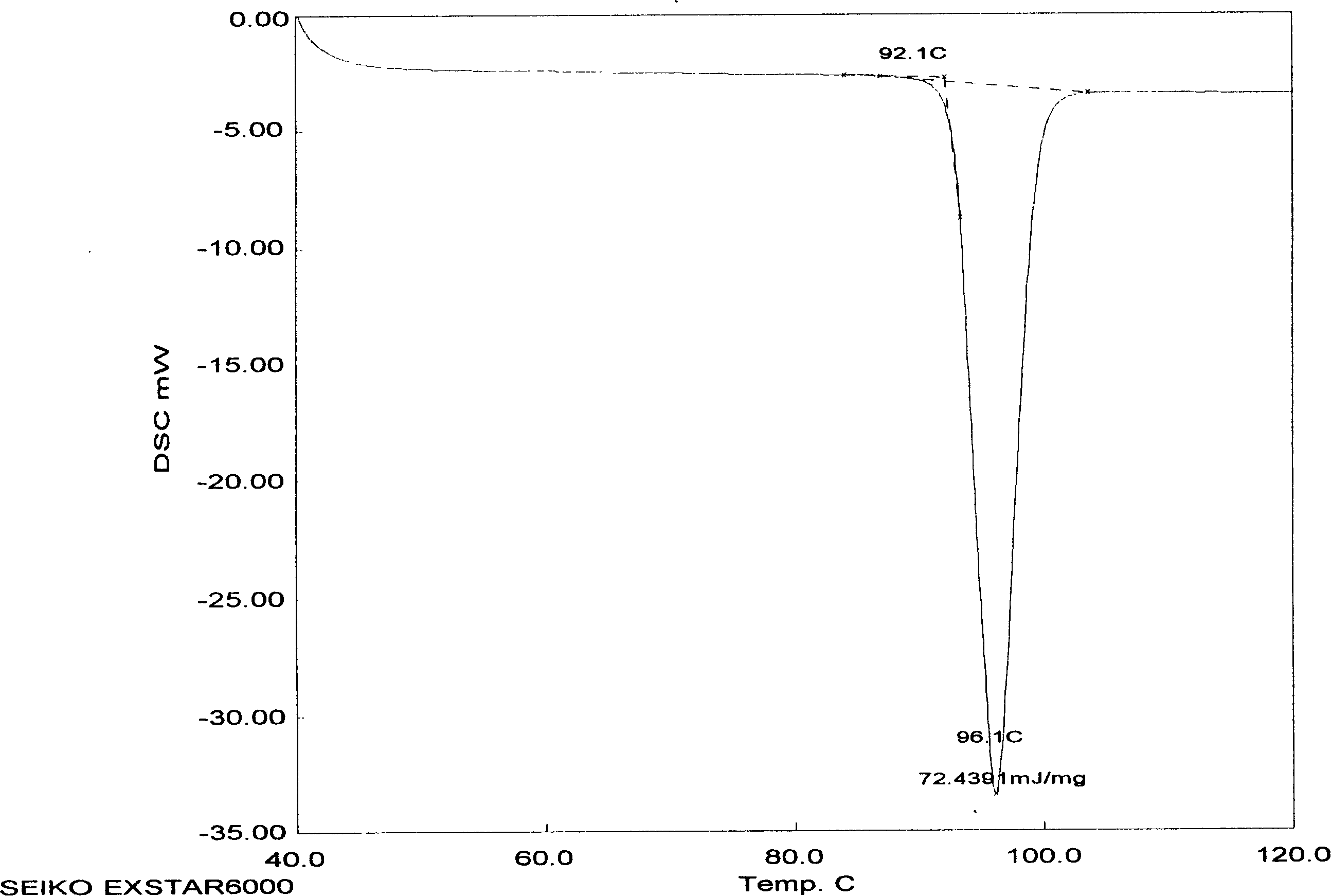
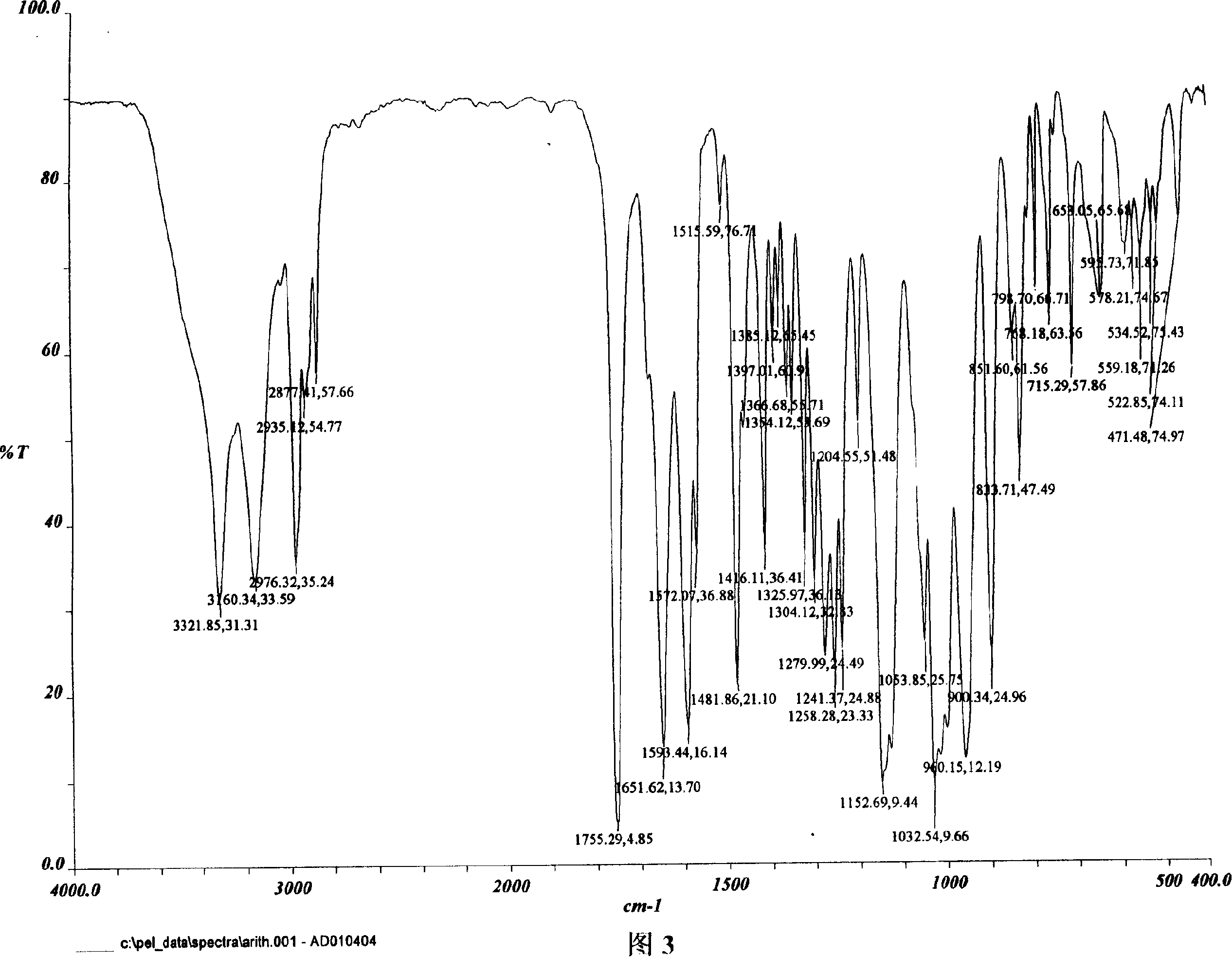
[0029] Characterization of AD Crystalline Morphology
[0030]The inventors used several methods (usually using XRD and DSC differential thermograms, and Fourier transform infrared absorption spectroscopy can also be used to supplement evidence) to characterize the characteristics of the crystal form of AD. Operators generally use XRD to characterize the composition of crystals or to identify them (see "United States Pharmacopoeia" Vol. 23, 1995, Method 941, pp. 1843-1845, published by U.S. Pharmacopeial Convention, Inc., Rockville, MD; Stout et al., X -Ray Structure Determination; A Practical Guide, published by MacMillan Company in New York City, New York, 1968; see also "Pharmacopoeia of the People's Republic of China" 2000 Edition, Part Two, Appendix IX F Method, Appendix 70, Appendix VIII Q Method, Appendix 65 Appendix IV C method, appendix 27 pages, Chemical Industry Press). Diffraction patterns obtained from crystalline compounds are often characteristic of a given crys...
Embodiment 1
[0044] The synthesis of embodiment 1 AD
[0045] Add 2kg (7.3mol) of PMEA, 1.8kg (18mol) of triethylamine and 20kg of DMF into a 50L reactor, raise the temperature to about 45°C, stir for 30 minutes, add 2.1kg (18mol) of chloromethyl pivalate, and continue to stir the reaction 7 hours, lowered to room temperature and continued to stir for 12 hours, added 100kg of ethyl acetate, stirred, filtered, the filtrate was washed with about 80kg×2 saturated sodium bicarbonate solution, about 80Kg×2 saturated salt water, filtered to remove solid impurities, and concentrated to obtain Dissolve the obtained oil in 10L of ethanol, add 20Kg of water to precipitate under stirring, filter, top wash with an aqueous solution containing 25% ethanol, and drain to obtain a wet product AD 3.28Kg.
Embodiment 2
[0047] 200g of the AD wet product obtained in Example 1 was azeotropically crystallized with 2L of ethyl acetate and 50ml of water at an appropriate temperature (40°C-50°C) and under vacuum conditions, and vacuum-dried at 40°C for 4 hours to obtain AD108. 7g. Based on the external standard substance, the purity of the adefovir dipivoxil in the new crystalline form determined by HPLC is 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com