Nrti therapies
A product, polymer technology, applied in the direction of drug delivery, powder delivery, antiviral agents, etc., can solve problems such as the route of administration or the frequency of dosing intervals that are rarely discussed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
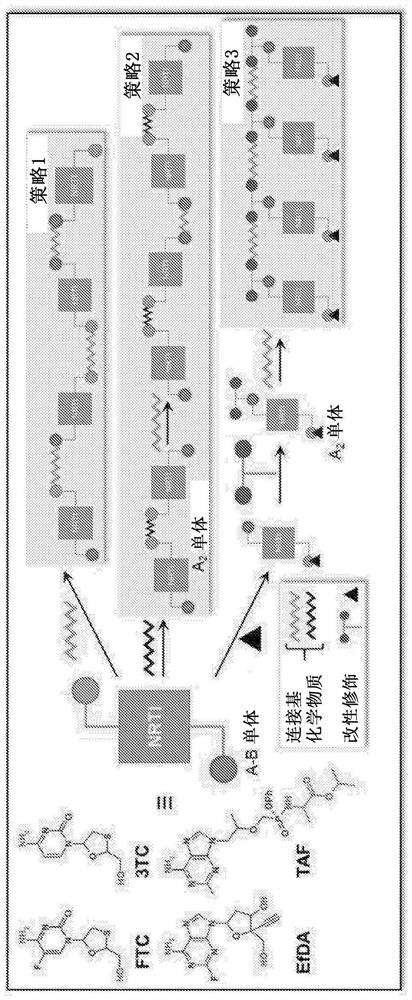
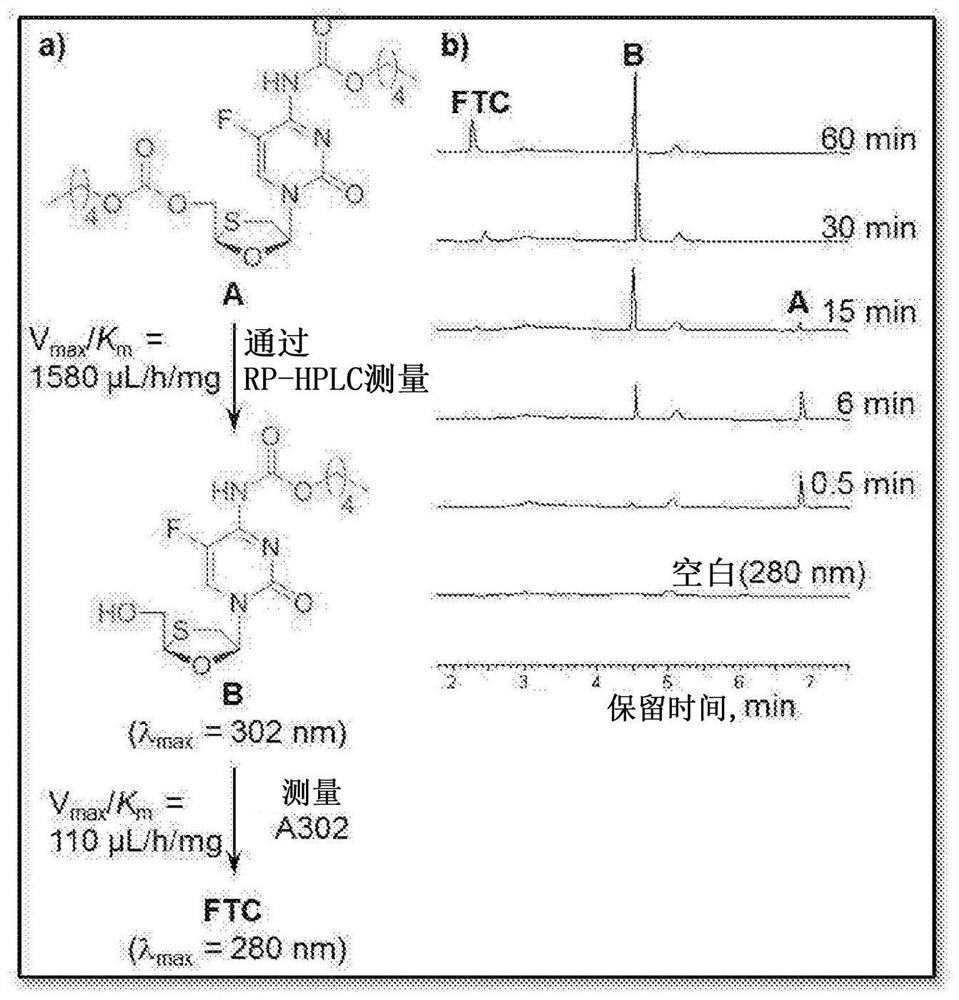
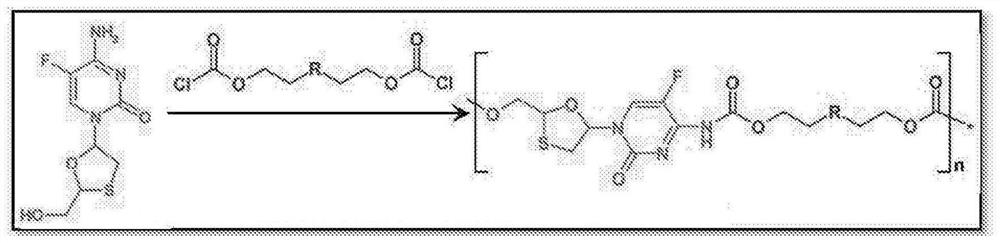
[0038] Figure 1 shows NRTIs (TFV, FTC, 3TC, and EFdA) with multiple functional groups that undergo a step-growth polymerization strategy.
[0039] The present invention offers several novel solutions, including three synthetic strategies (Fig. 1) that exploit the reactivity of NRTIs in polymerization, such as: 1) Reaction of A-B type NRTI monomers with complementary linkers (Strategy 1 ); 2) A 2 Prodrug monomer and B 2 Reaction of the Linker (Strategy 2); and 3) A 2 pendant prodrug monomer with B 2 Linker Reaction (Strategy 3).
[0040] Therefore, a key innovative aspect of the present invention is the use of a "prodrug polymer" (POP) approach, which utilizes NRTI prodrug strategies to address a key need of LA NRTI regimens. These NRTI prodrug strategies enable the synthesis of biodegradable NRTI POP structures. Materials with specific polymer structures are used to create polymer constructs, which are physically assembled implementations of polymer structures (nanopartic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com