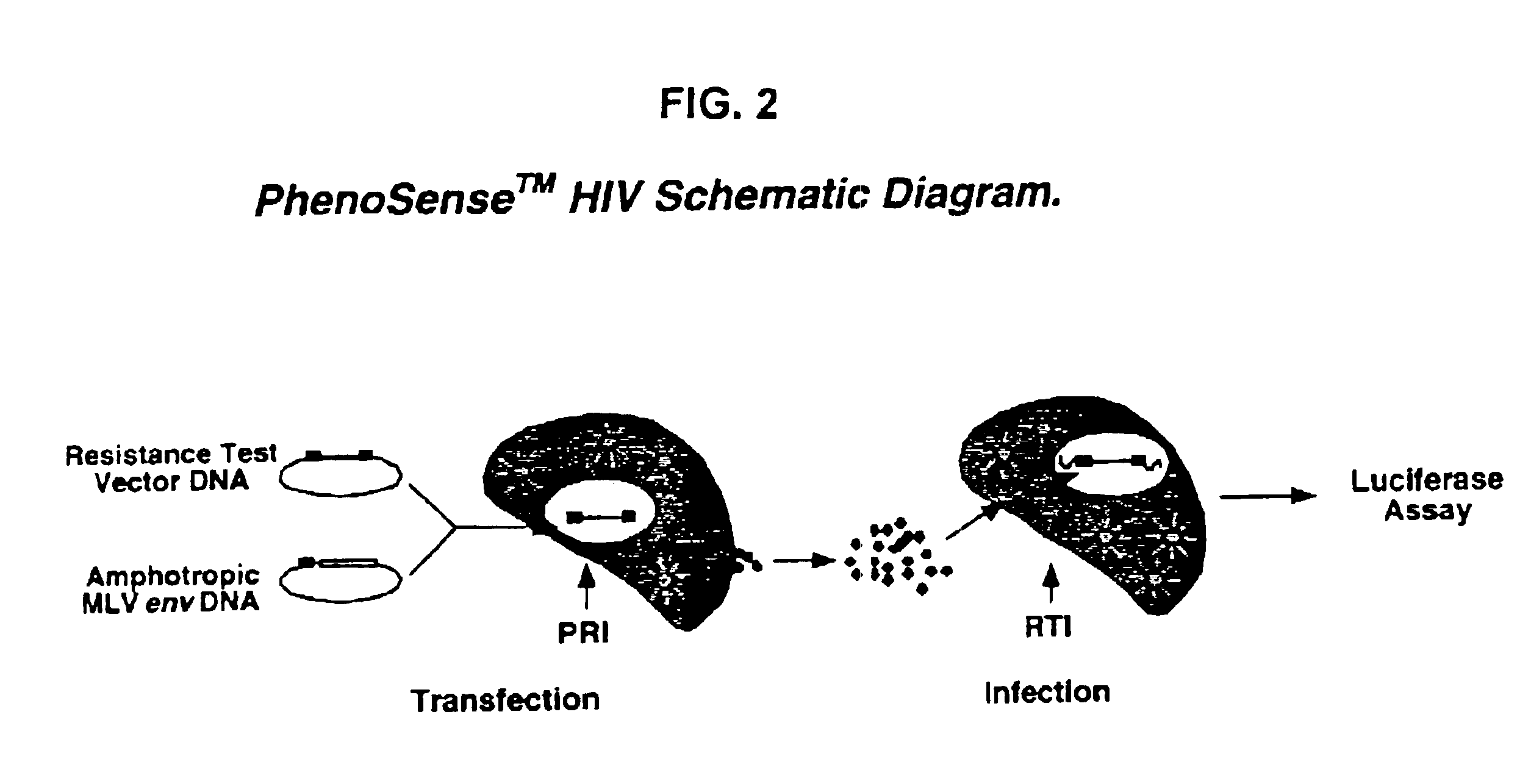
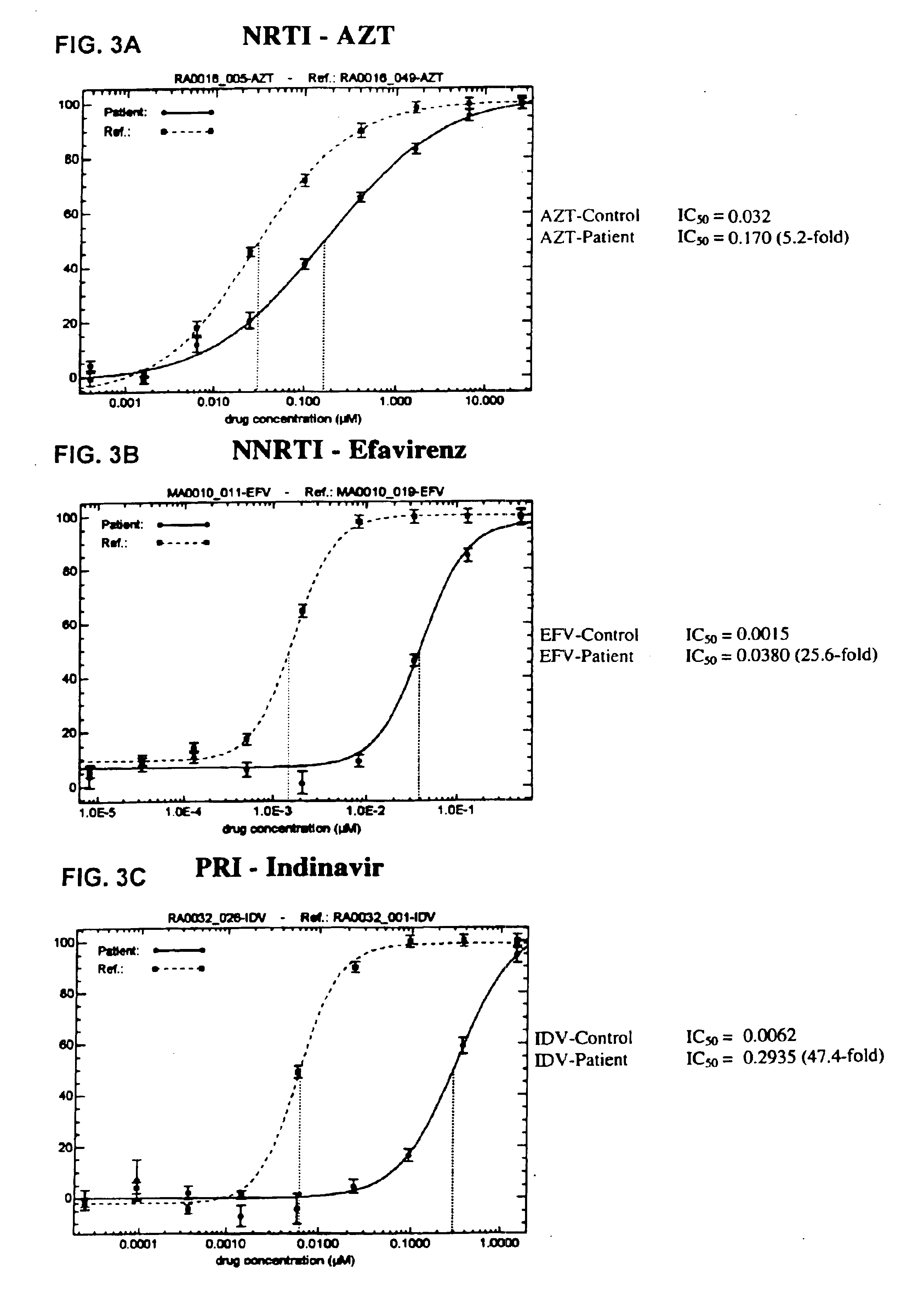
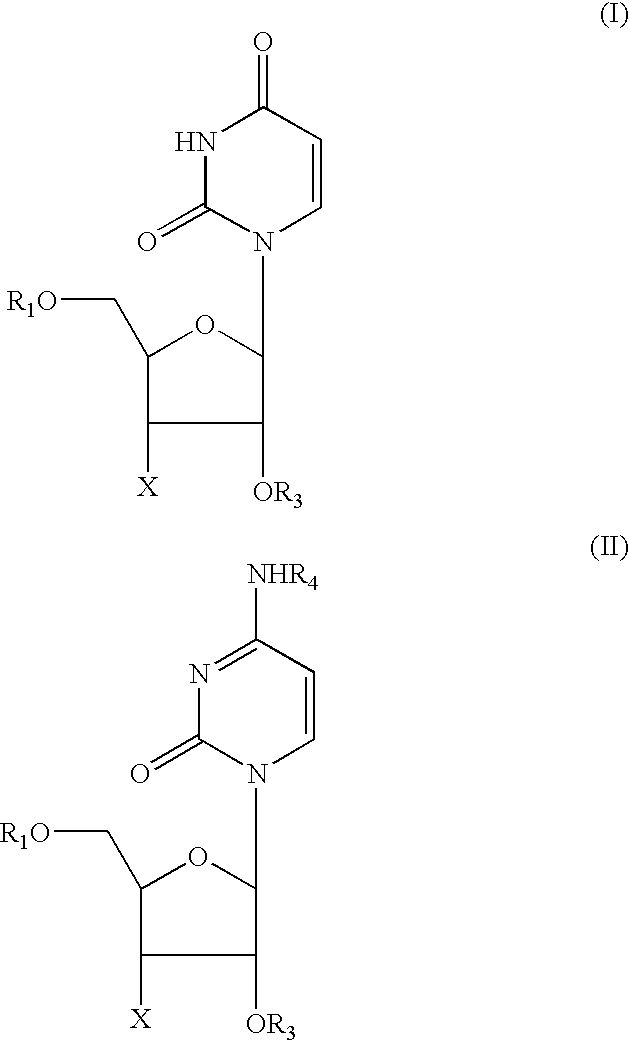
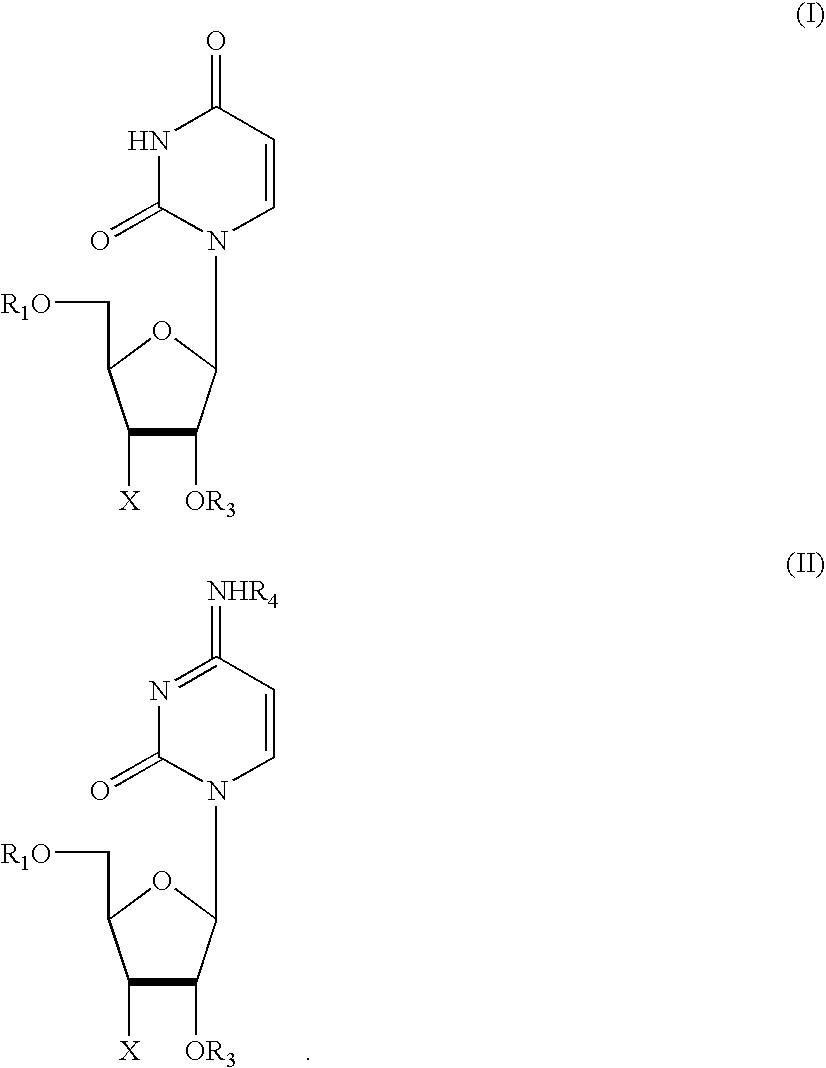
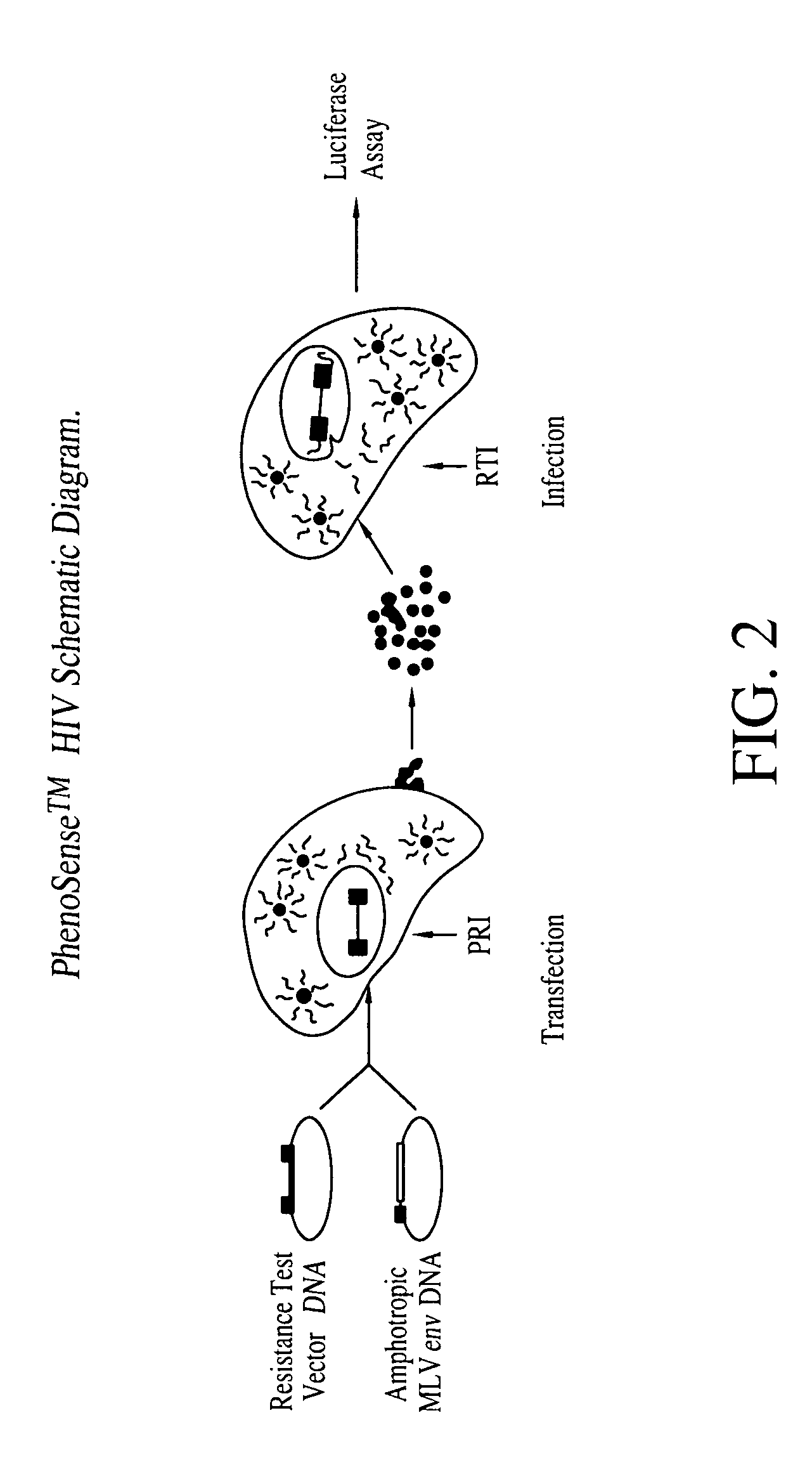
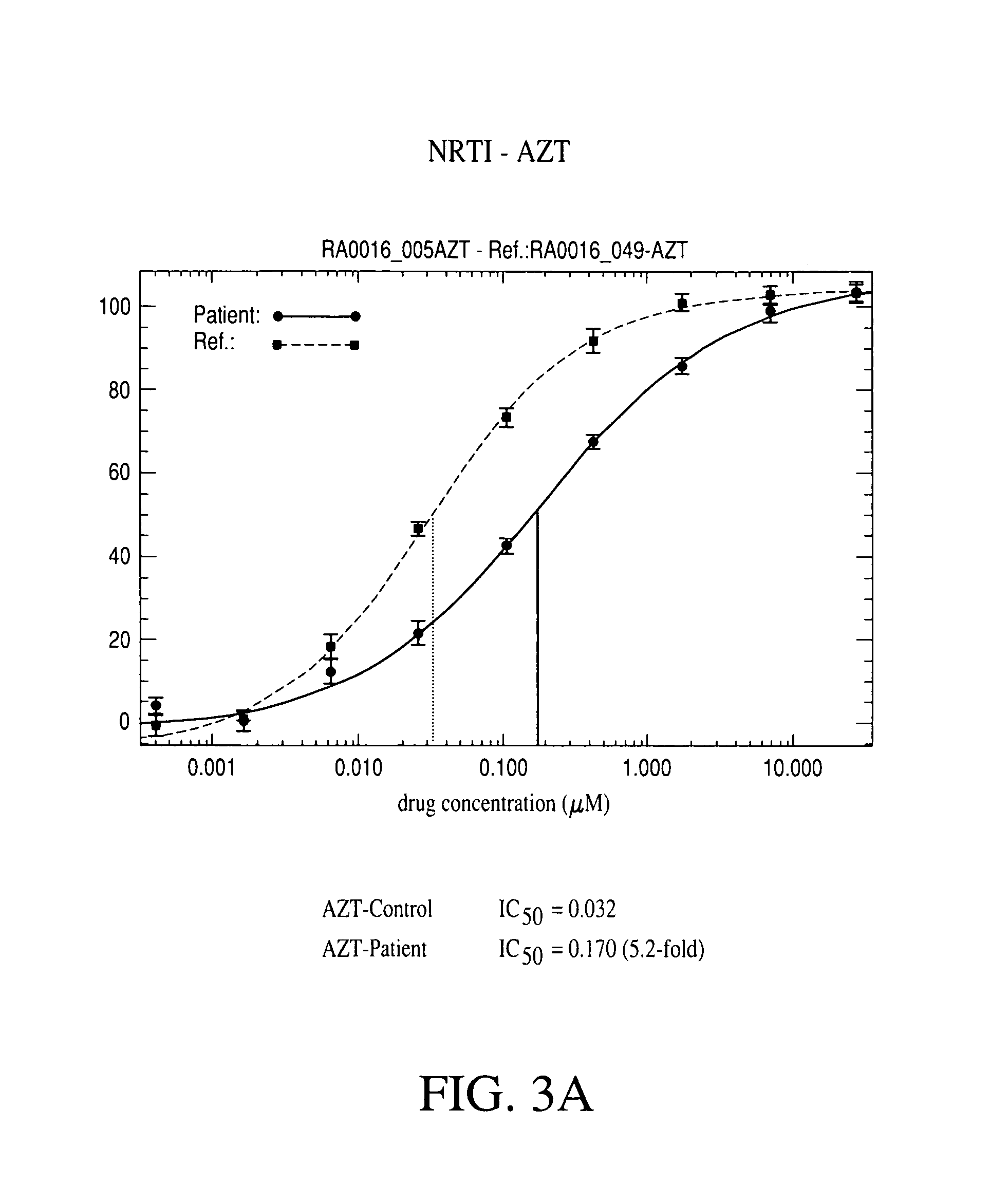
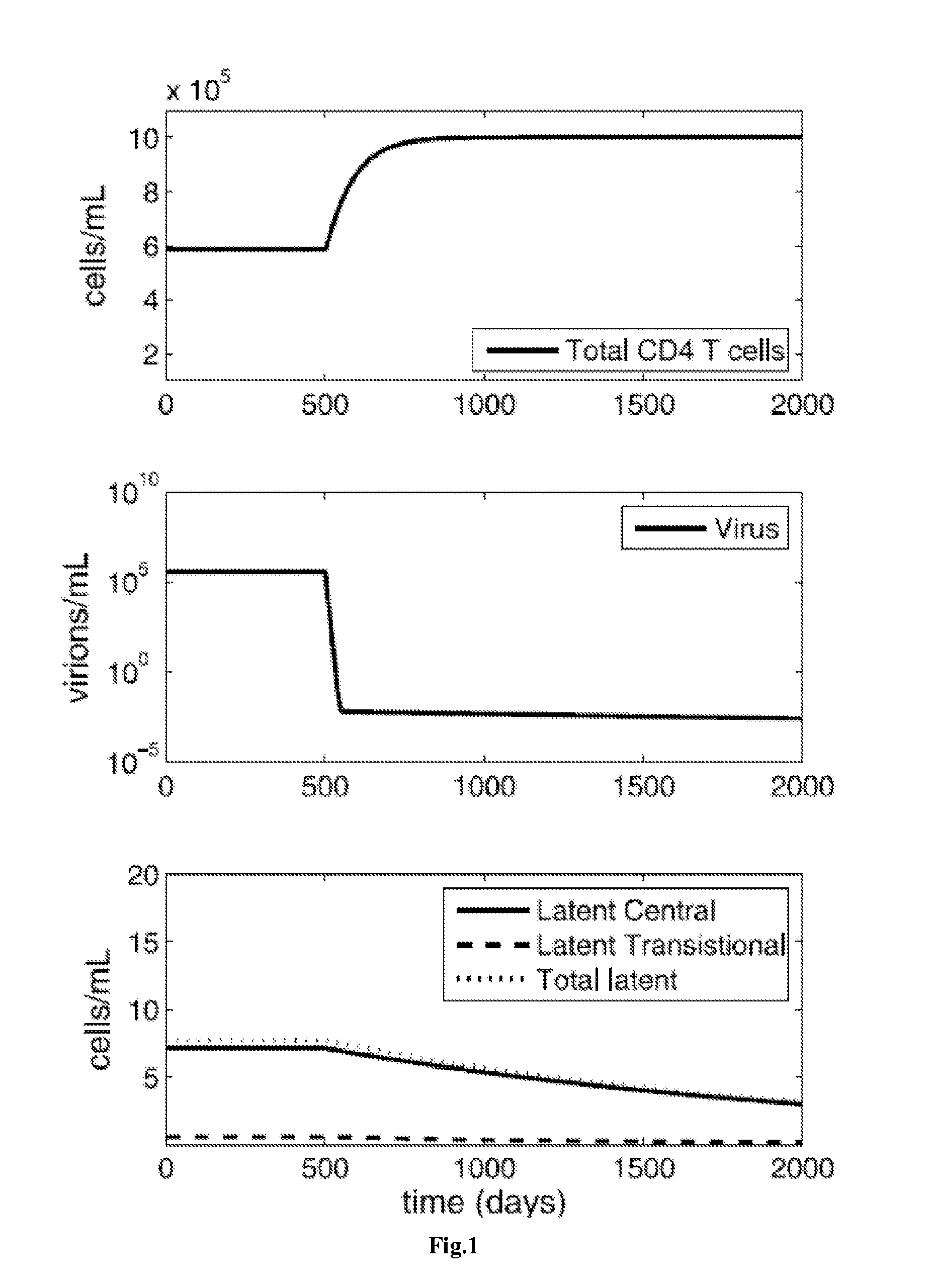
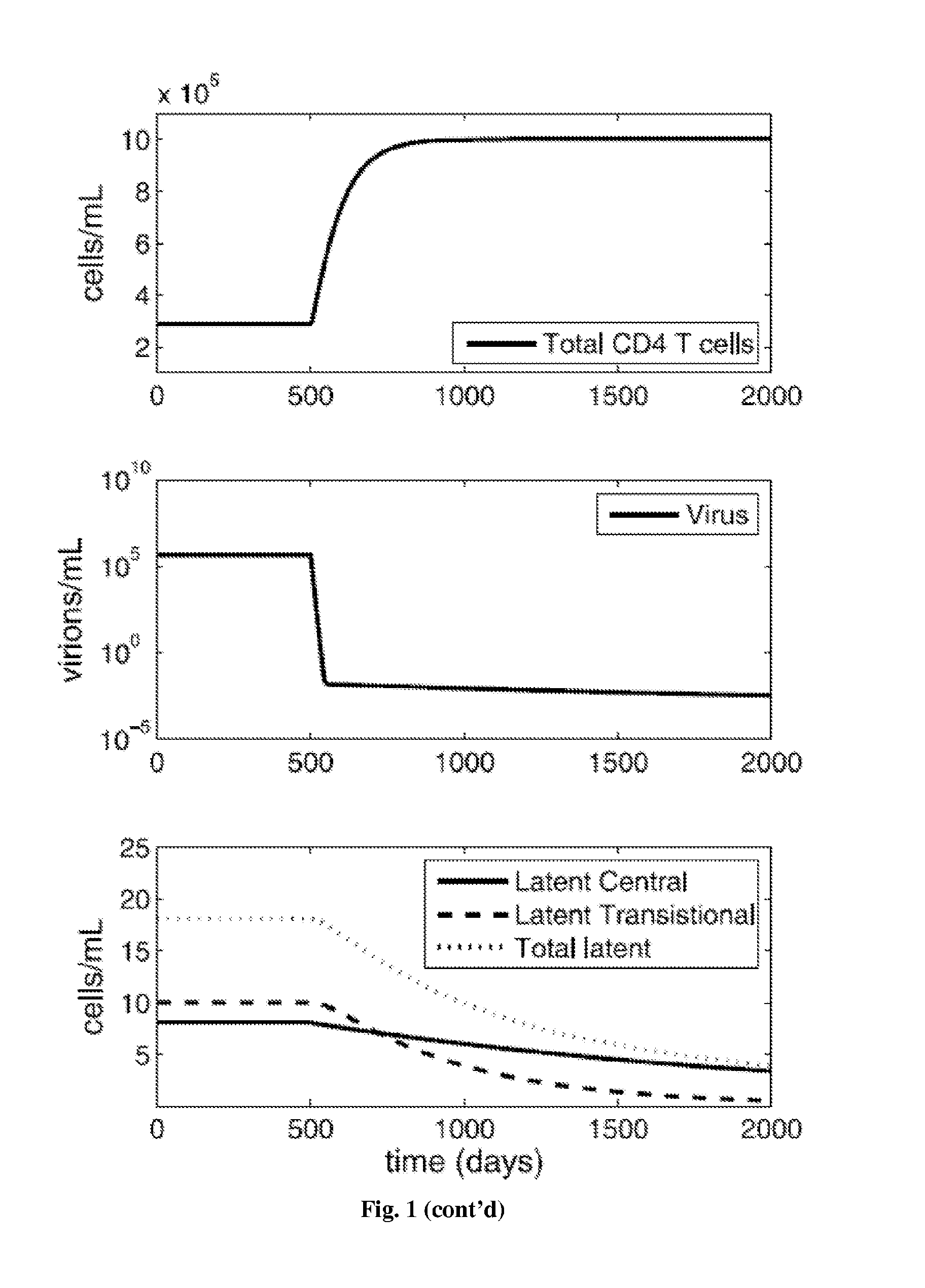
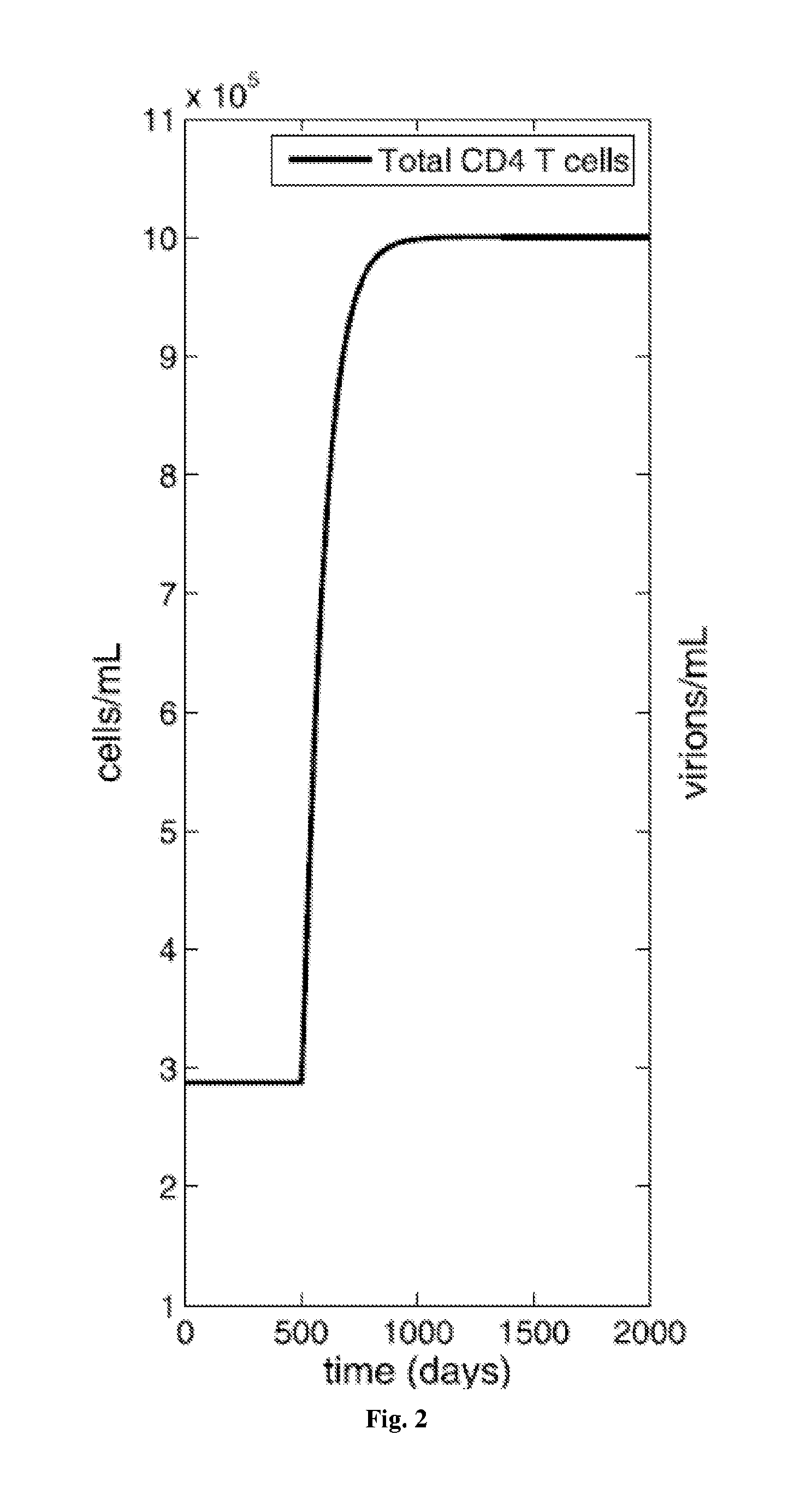
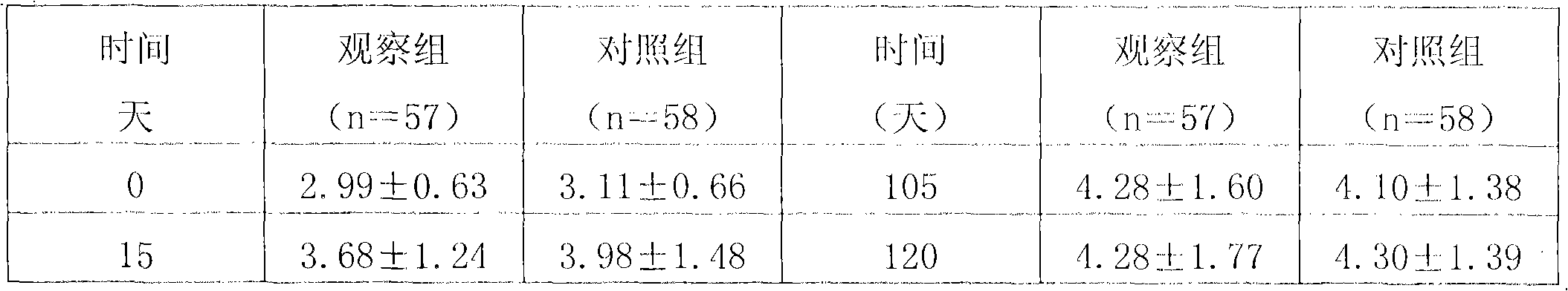
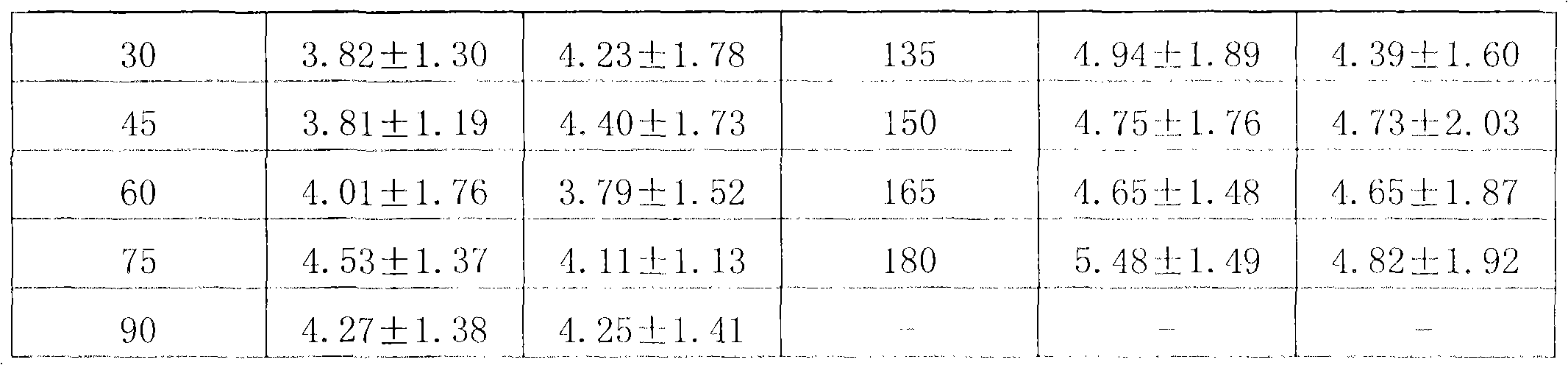
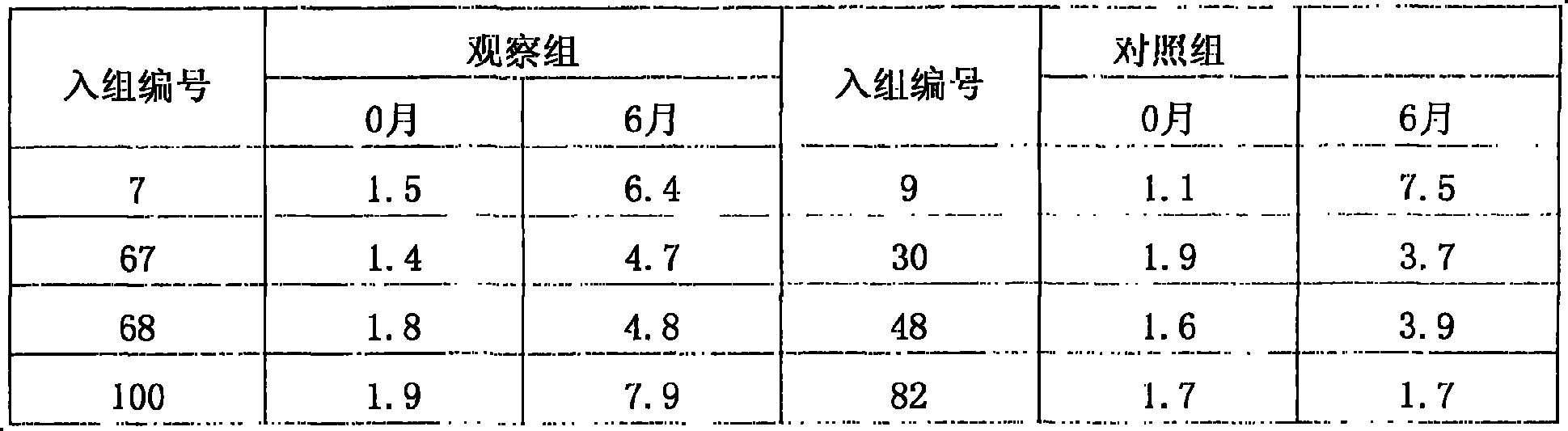
Patents
Literature
40 results about "Antiretroviral therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antiretroviral Therapy (ART) Synonym(s): Combination Therapy, Combined Antiretroviral Therapy (cART), Highly Active Antiretroviral Therapy (HAART) The daily use of a combination of HIV medicines (called an HIV regimen) to treat HIV infection. A person's initial HIV regimen generally includes three antiretroviral (ARV) drugs from at least two different HIV drug classes.
Nutritional supplement for a category of HIV patients
InactiveUS20090082249A1Relieve symptomsReduce infectionBiocidePeptide/protein ingredientsPhysiologyAntiretroviral therapy
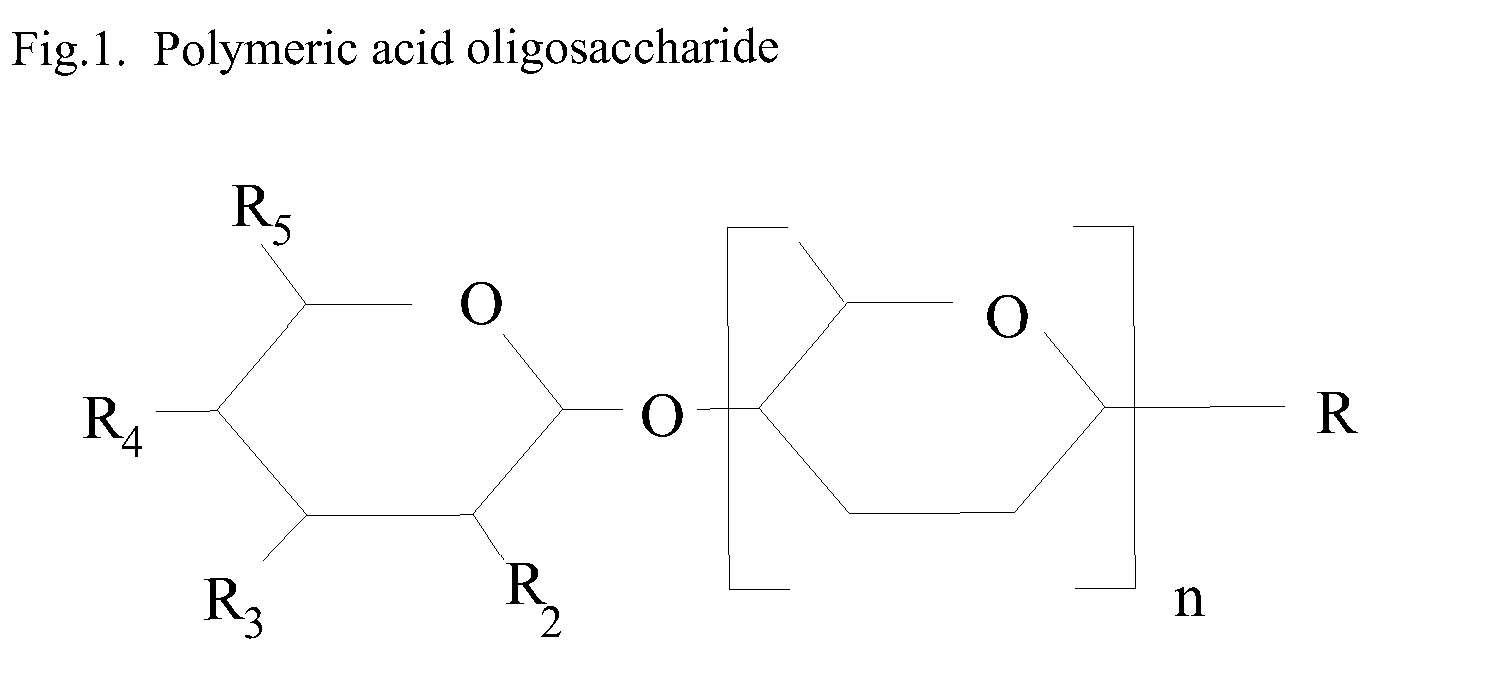
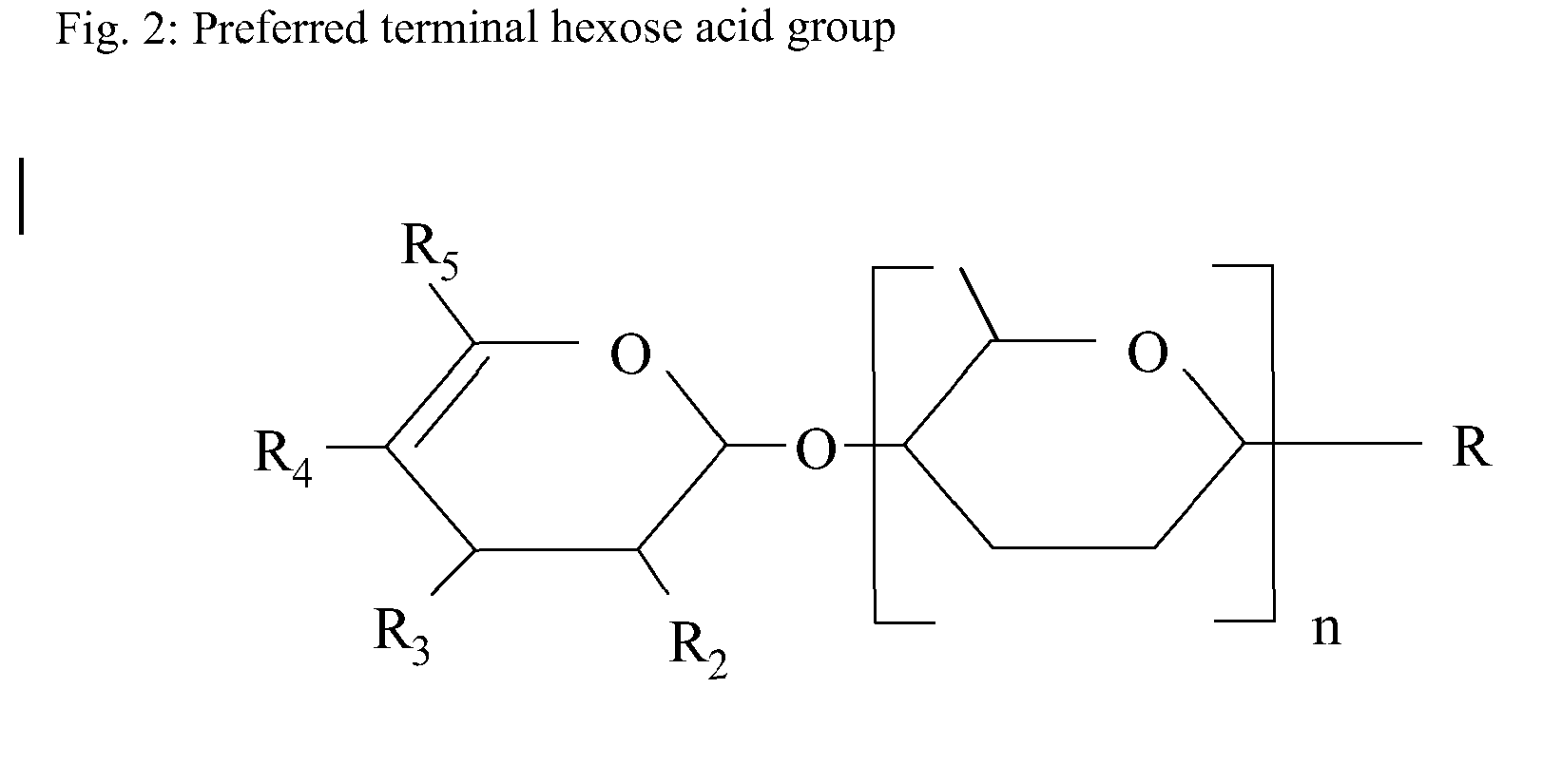
The present invention relates to a nutritional product for HIV patients that are not on Highly Active Antiretroviral Therapy. More specifically the invention relates to a nutritional composition comprising oligosaccharides. This invention also relates to the manufacture of a nutritional supplement for use in HIV patients.
Owner:NUTRICIA
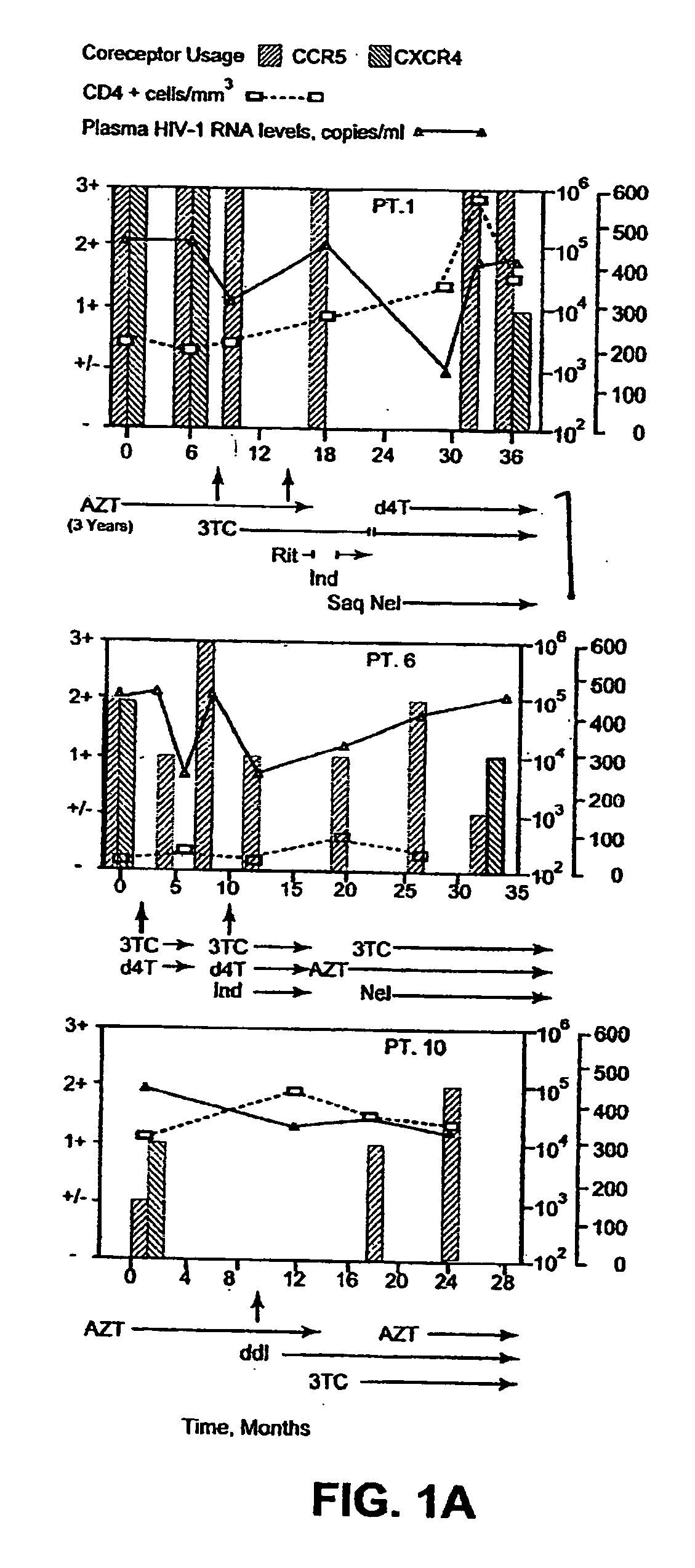
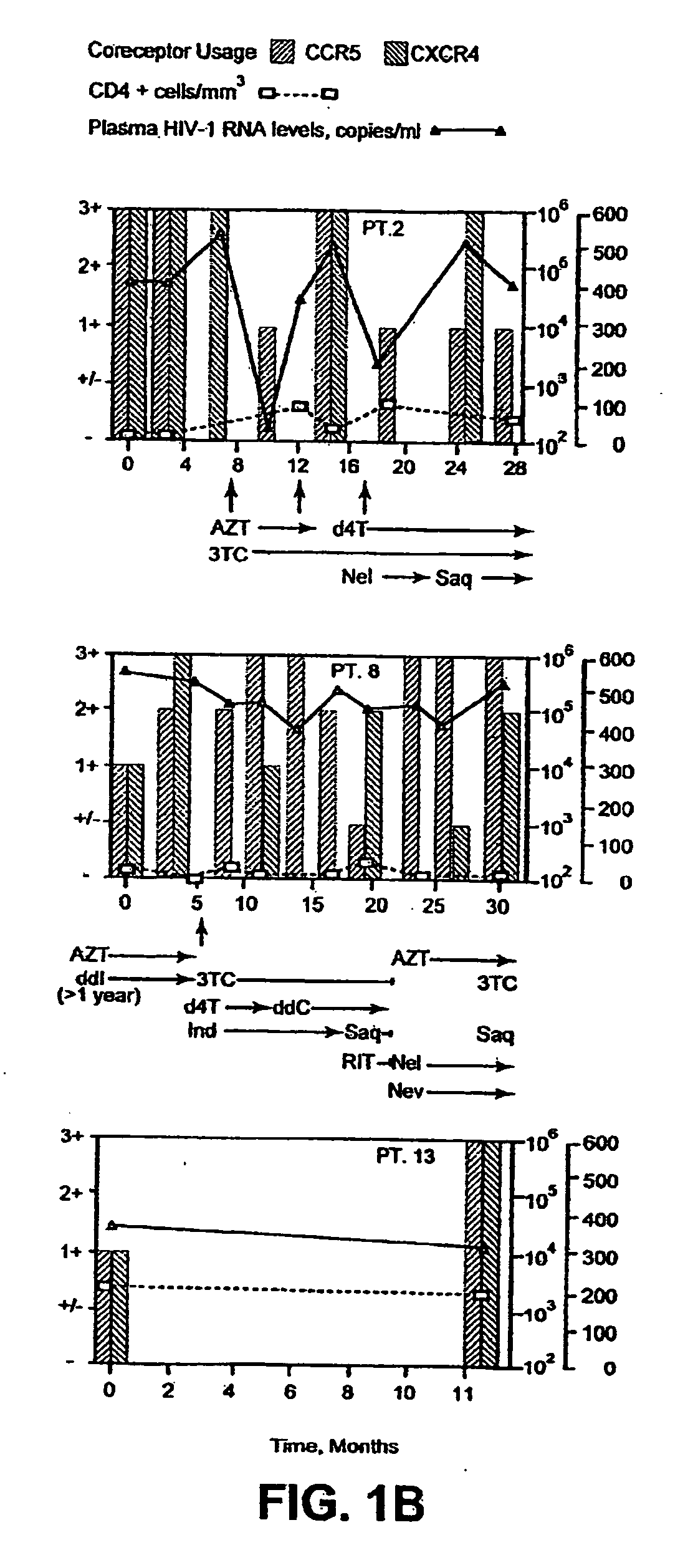
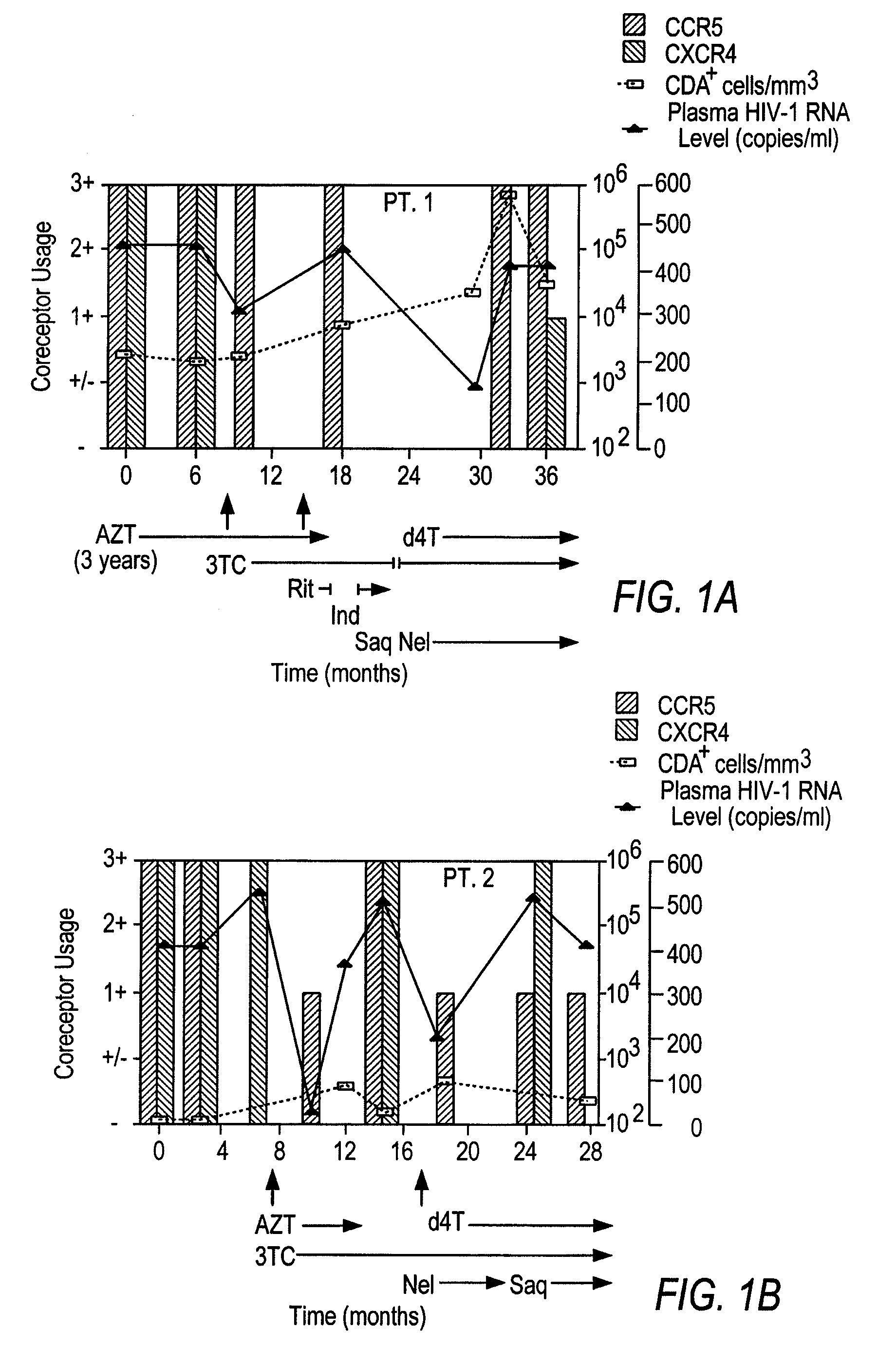
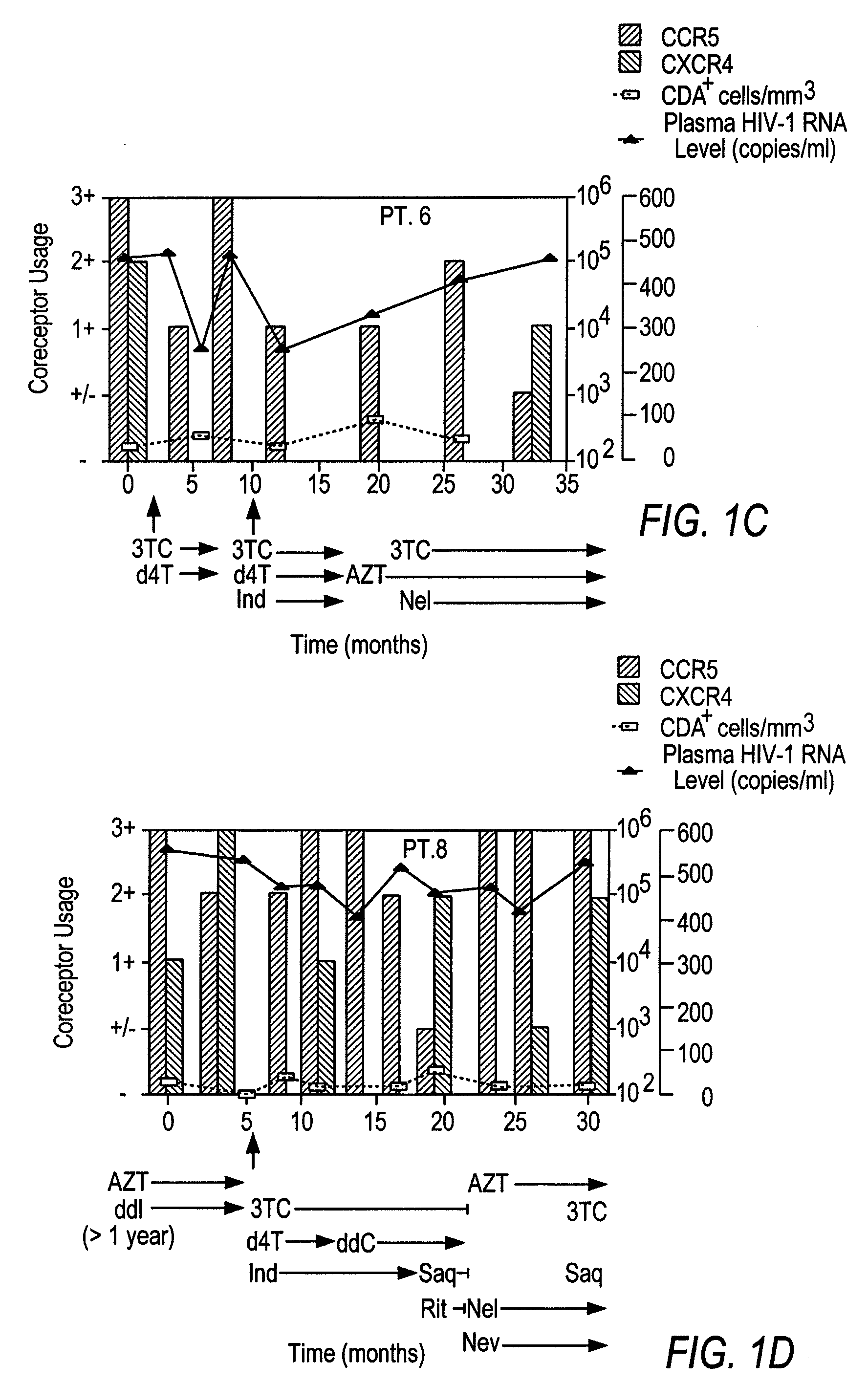
Analysis of HIV-1 coreceptor use in the clinical care of HIV-1-infected patients
InactiveUS6727060B2Accurate predictionMore effectivenessMicrobiological testing/measurementArtificial cell constructsImmunodeficiency virusHIV positives
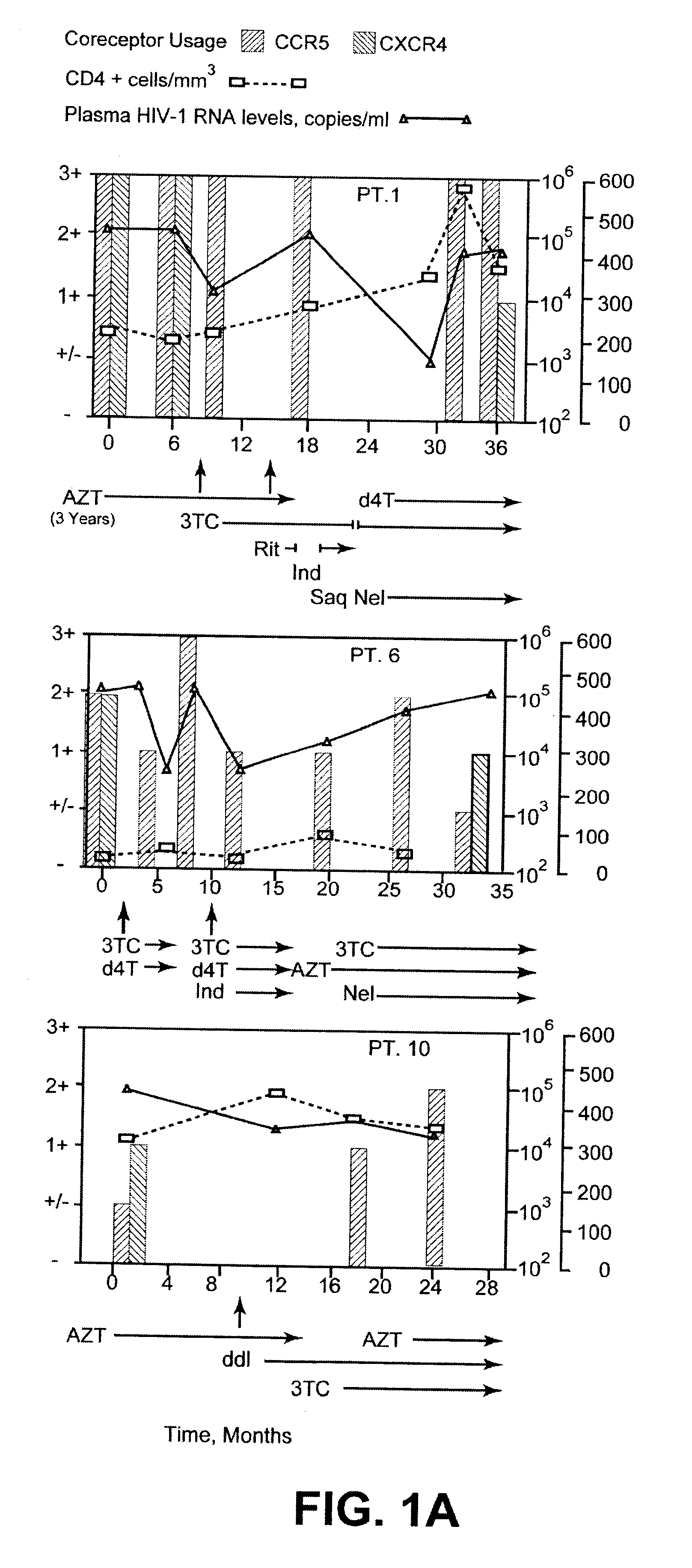
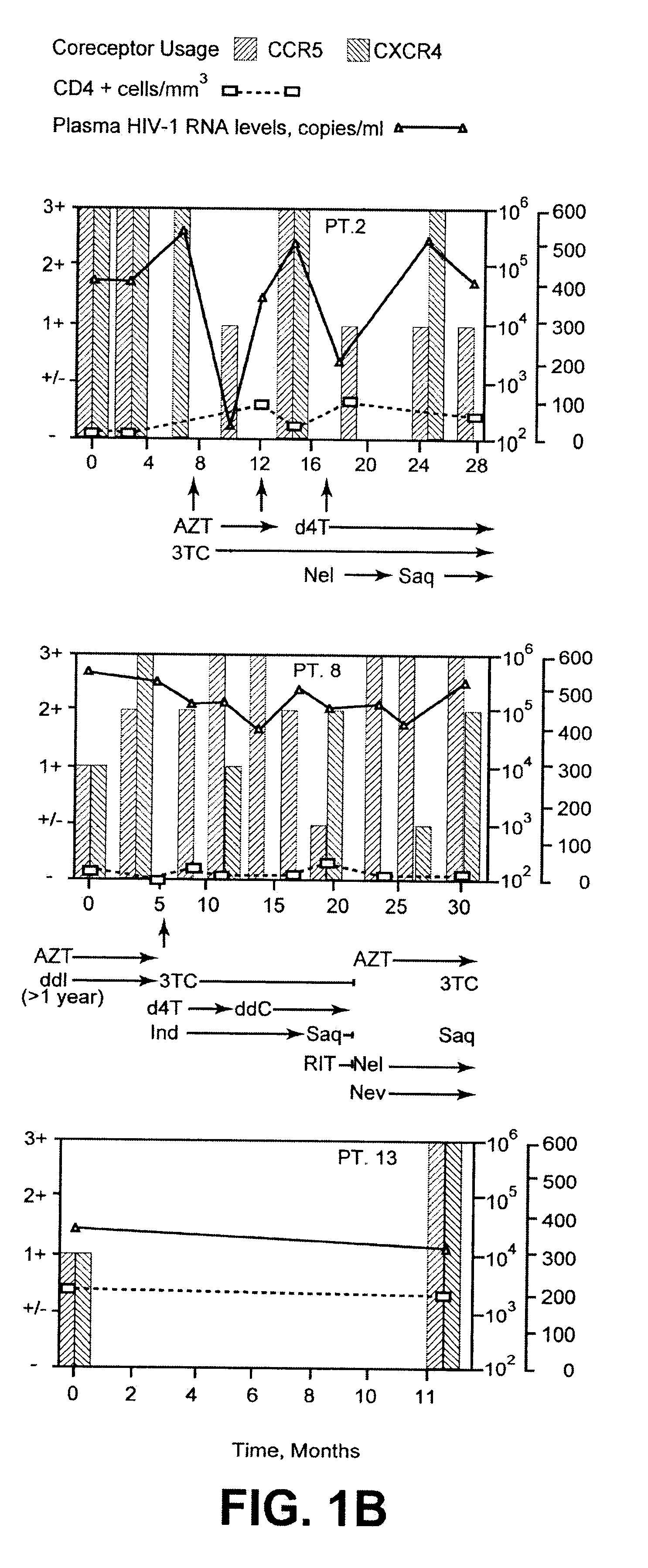
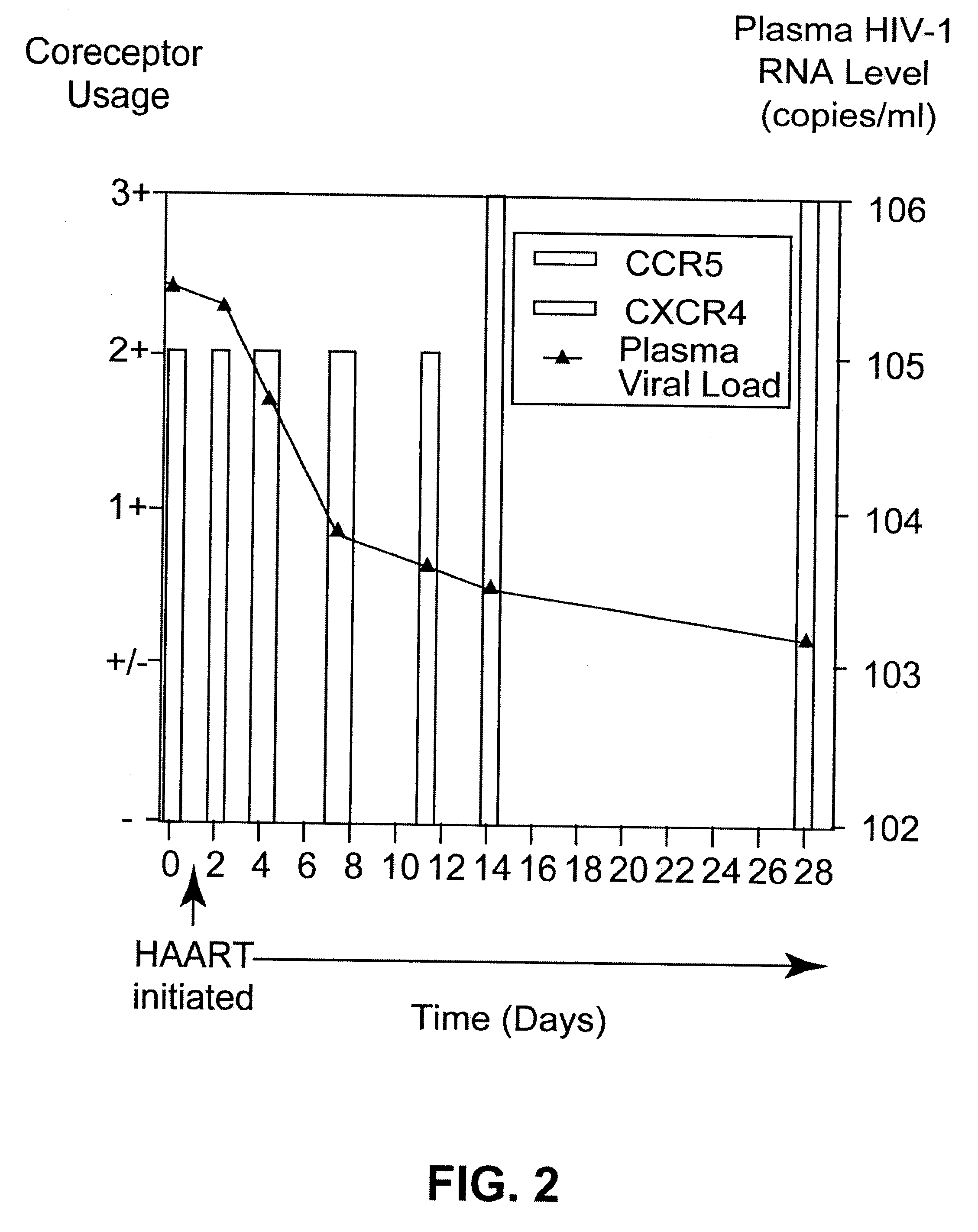
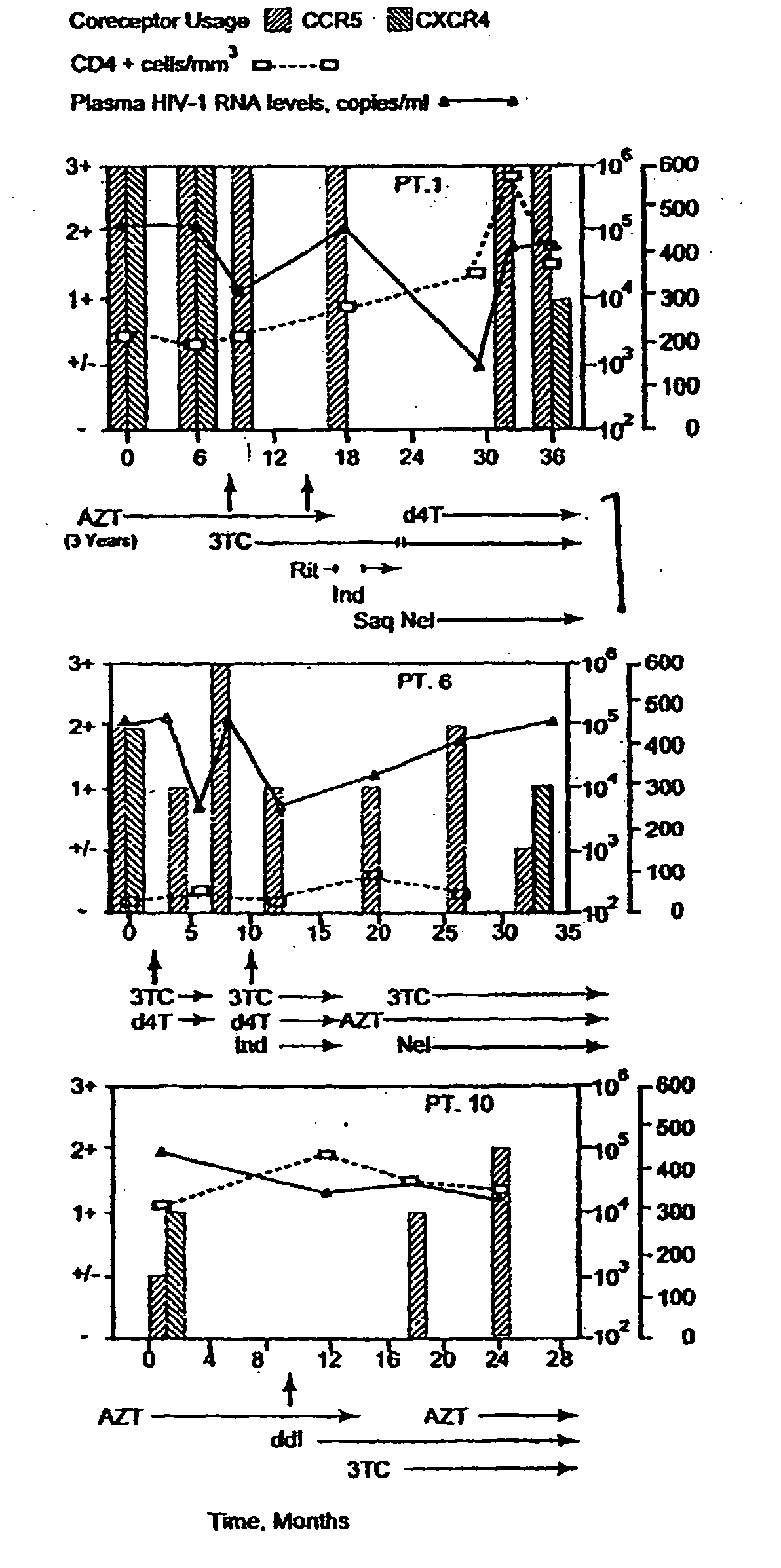
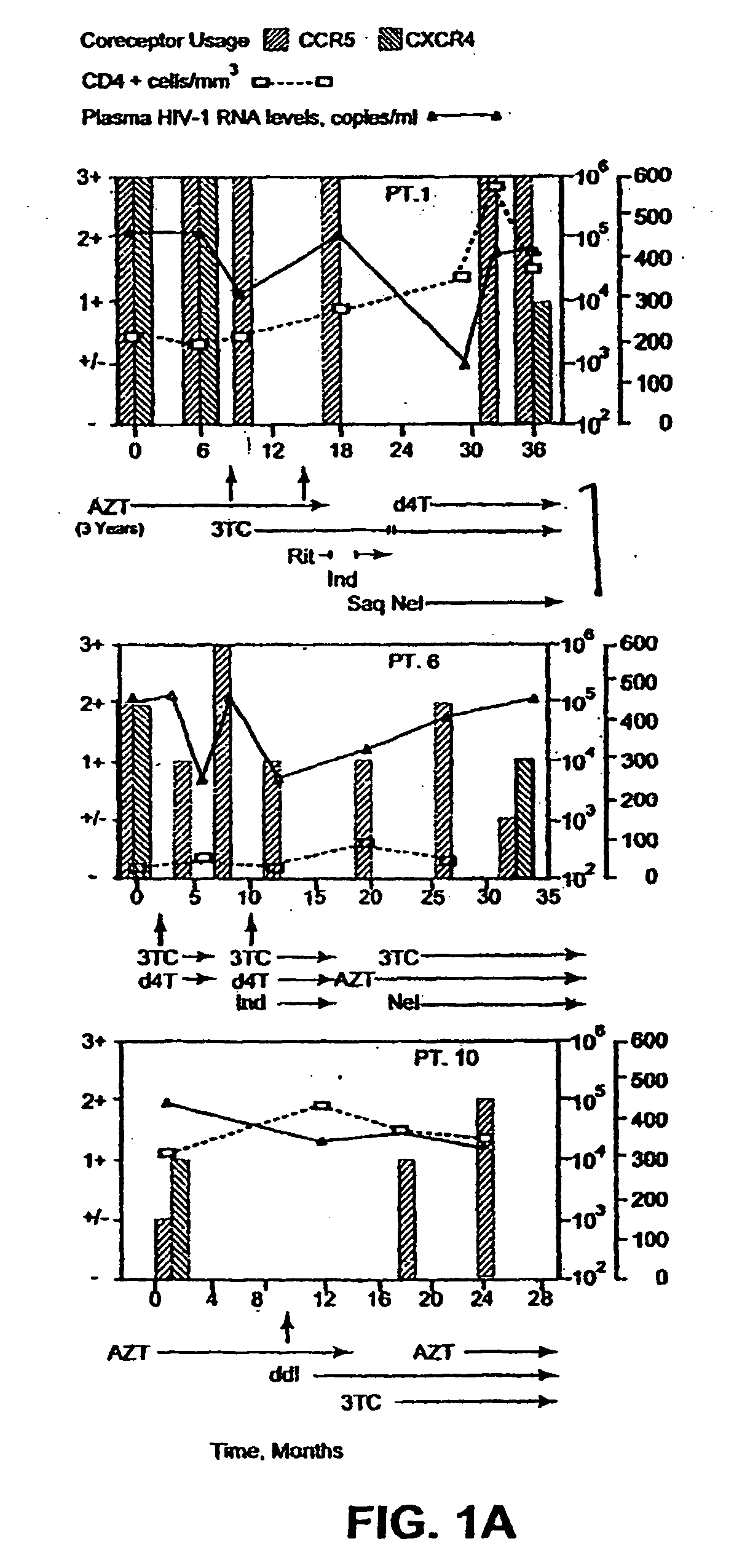
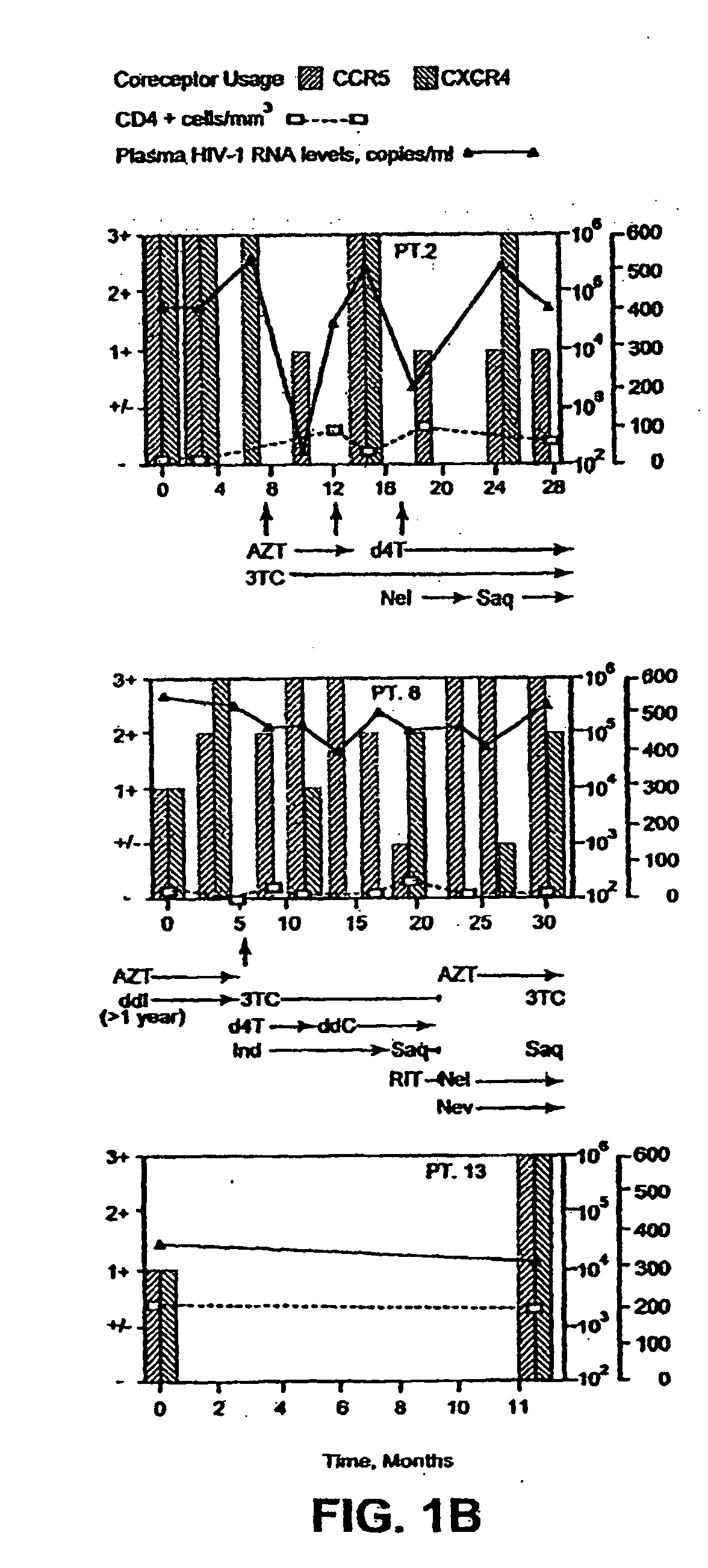
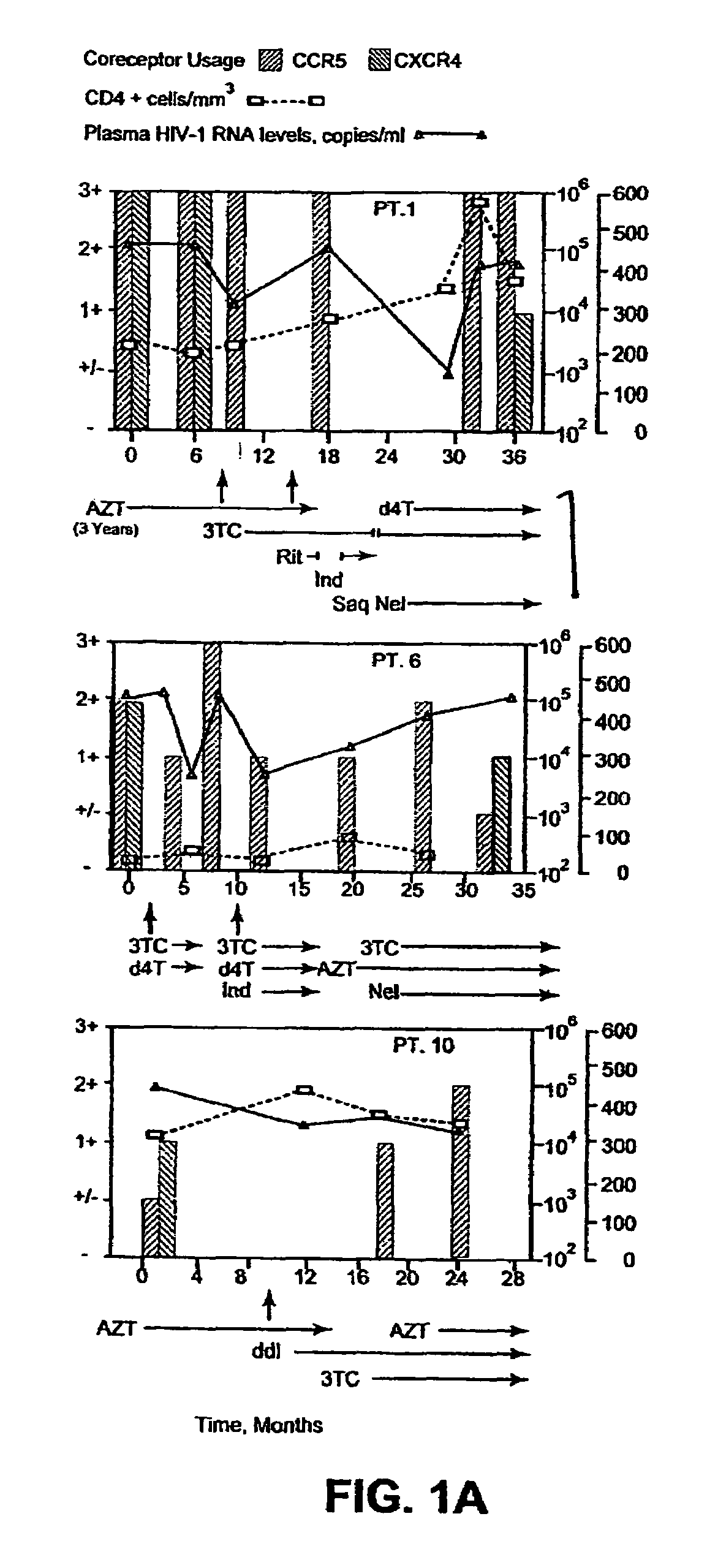
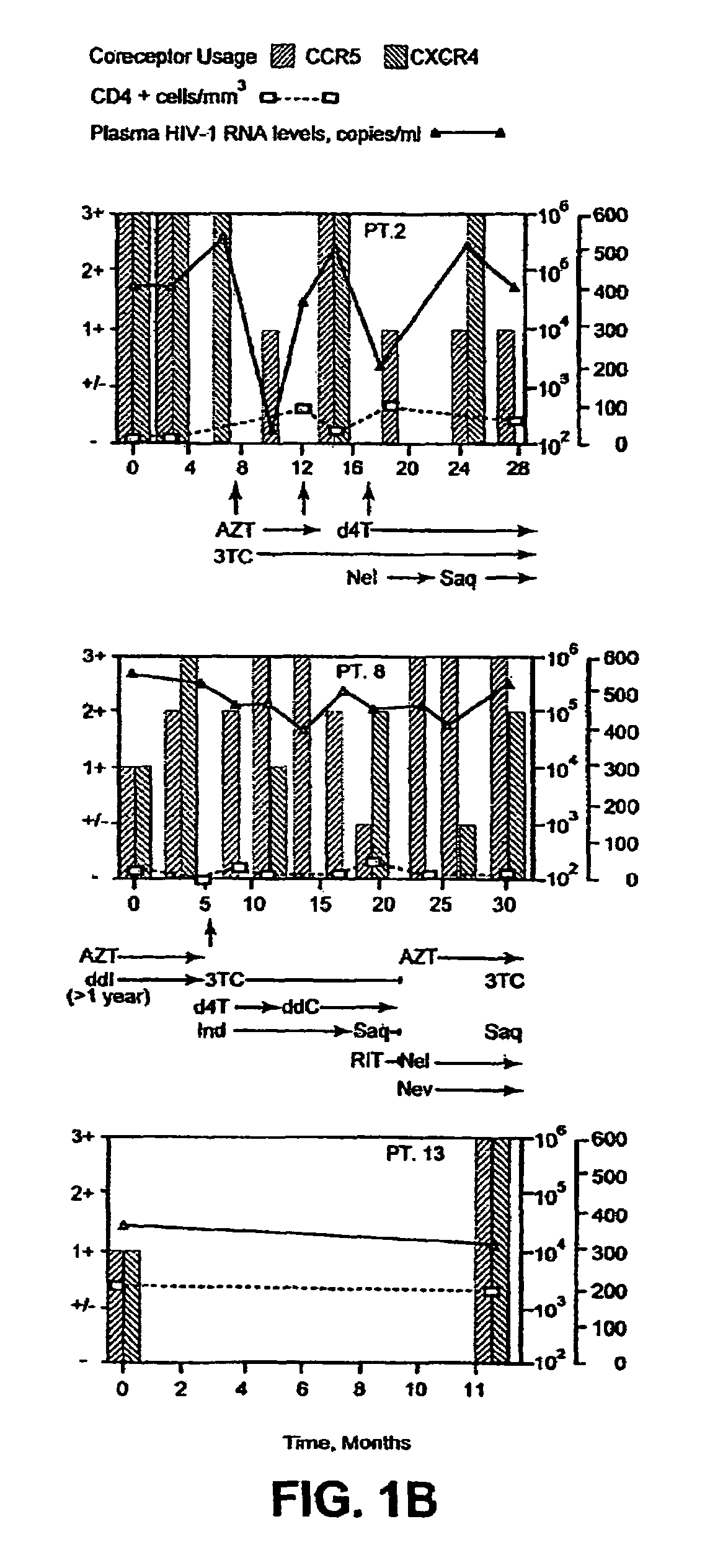
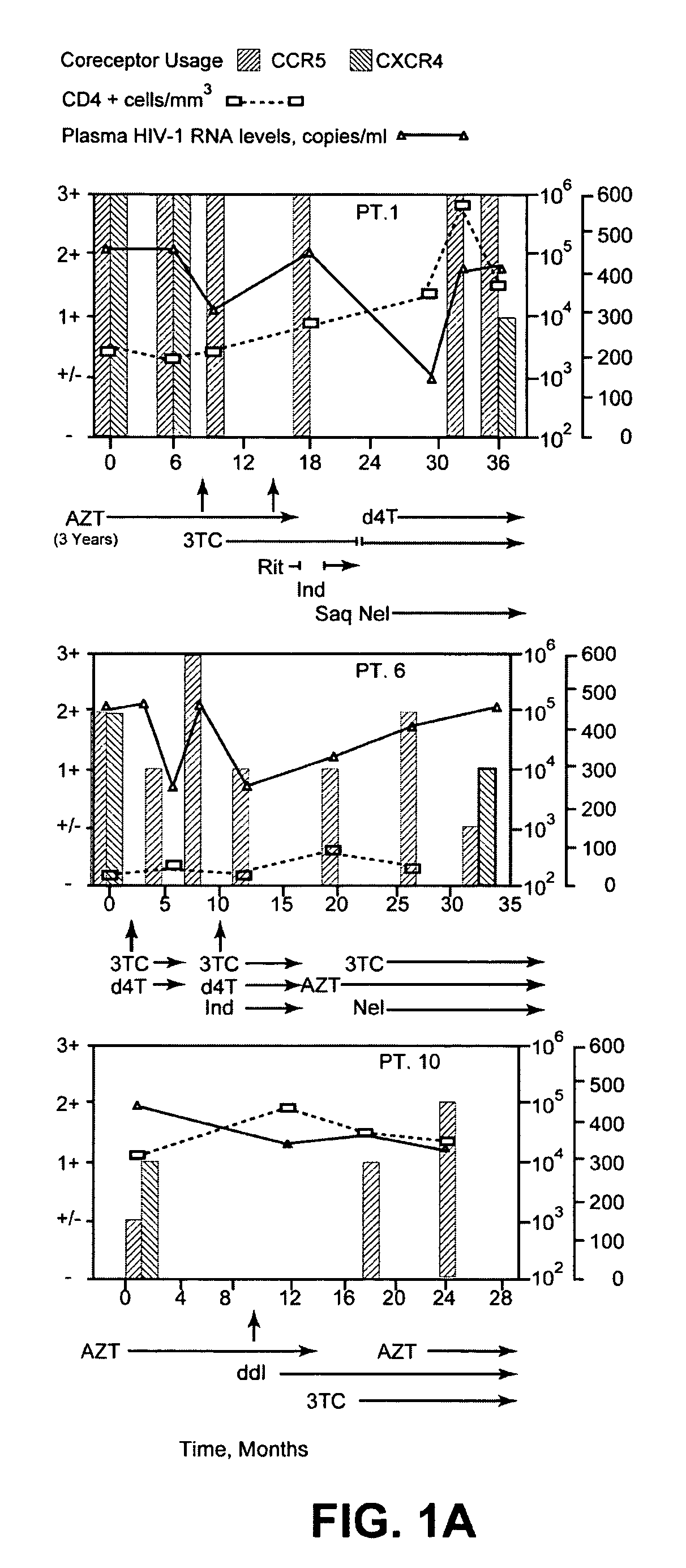
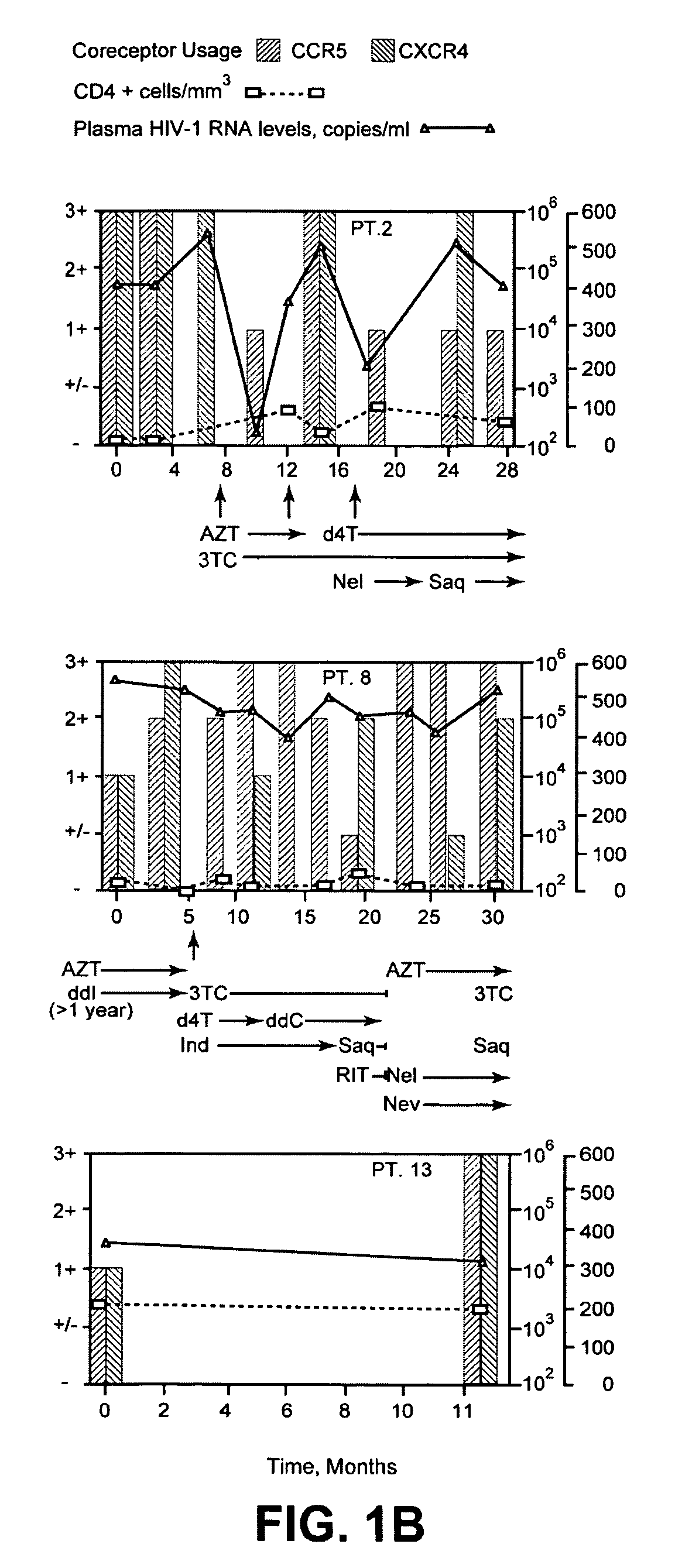
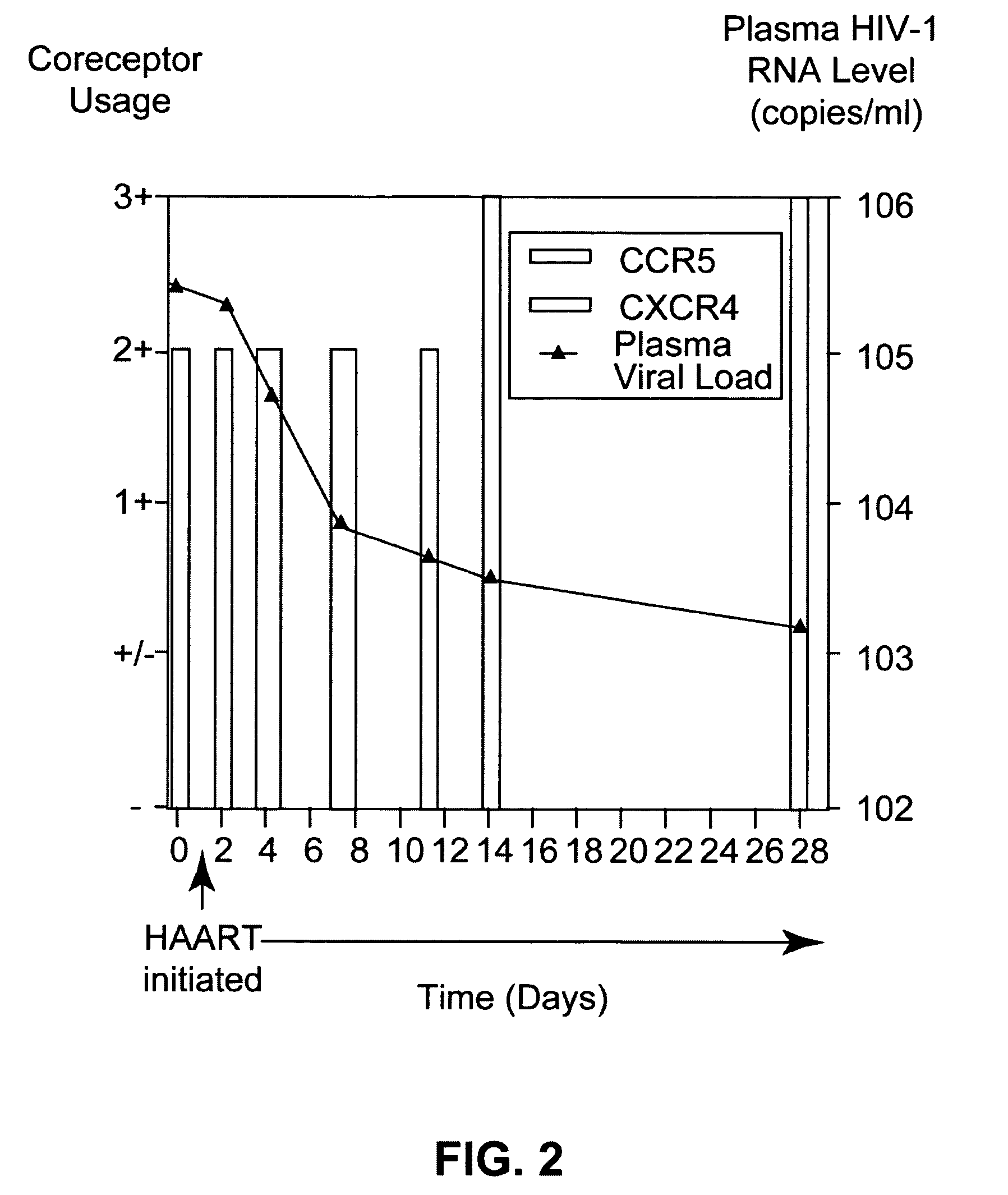
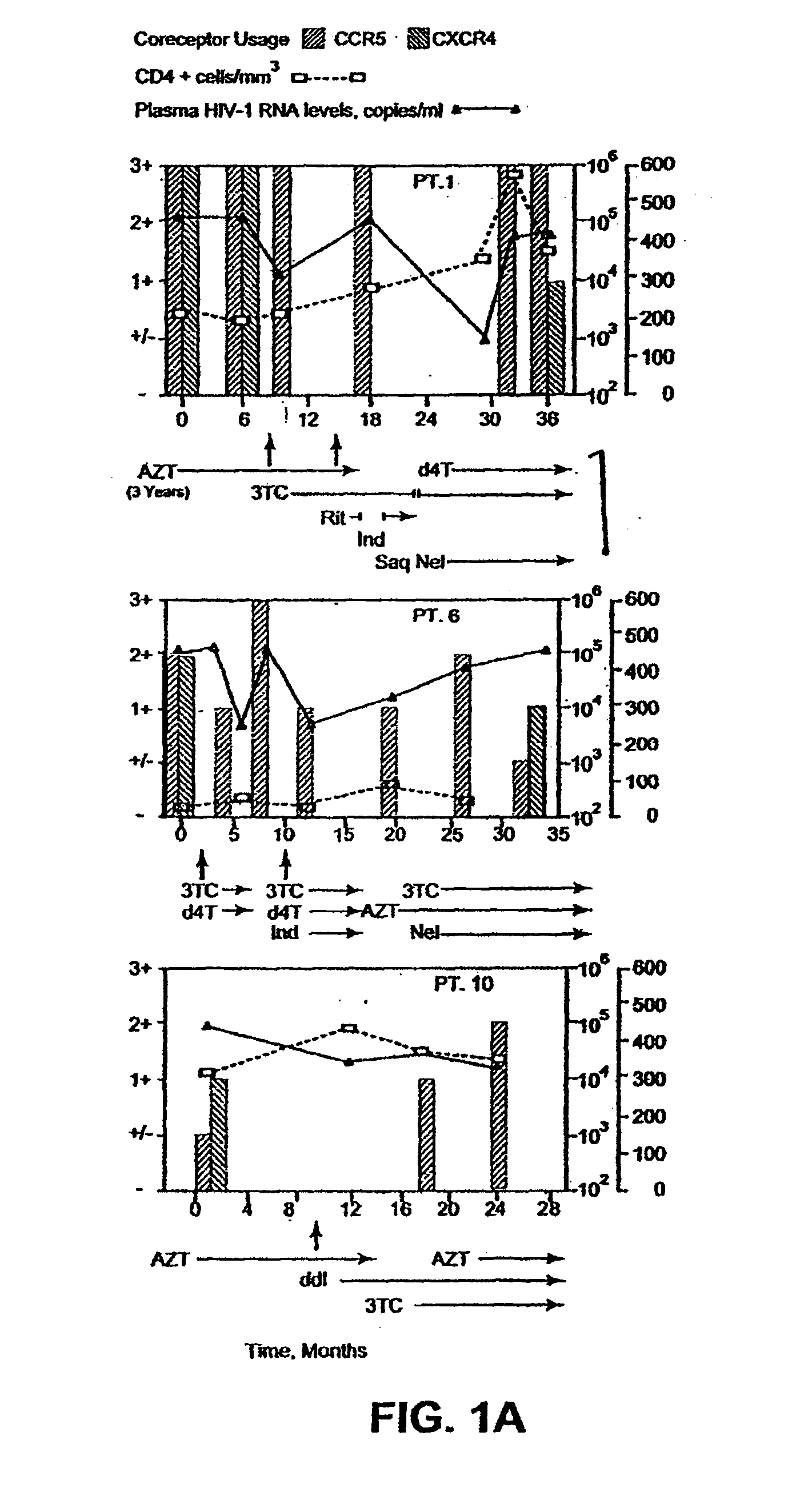
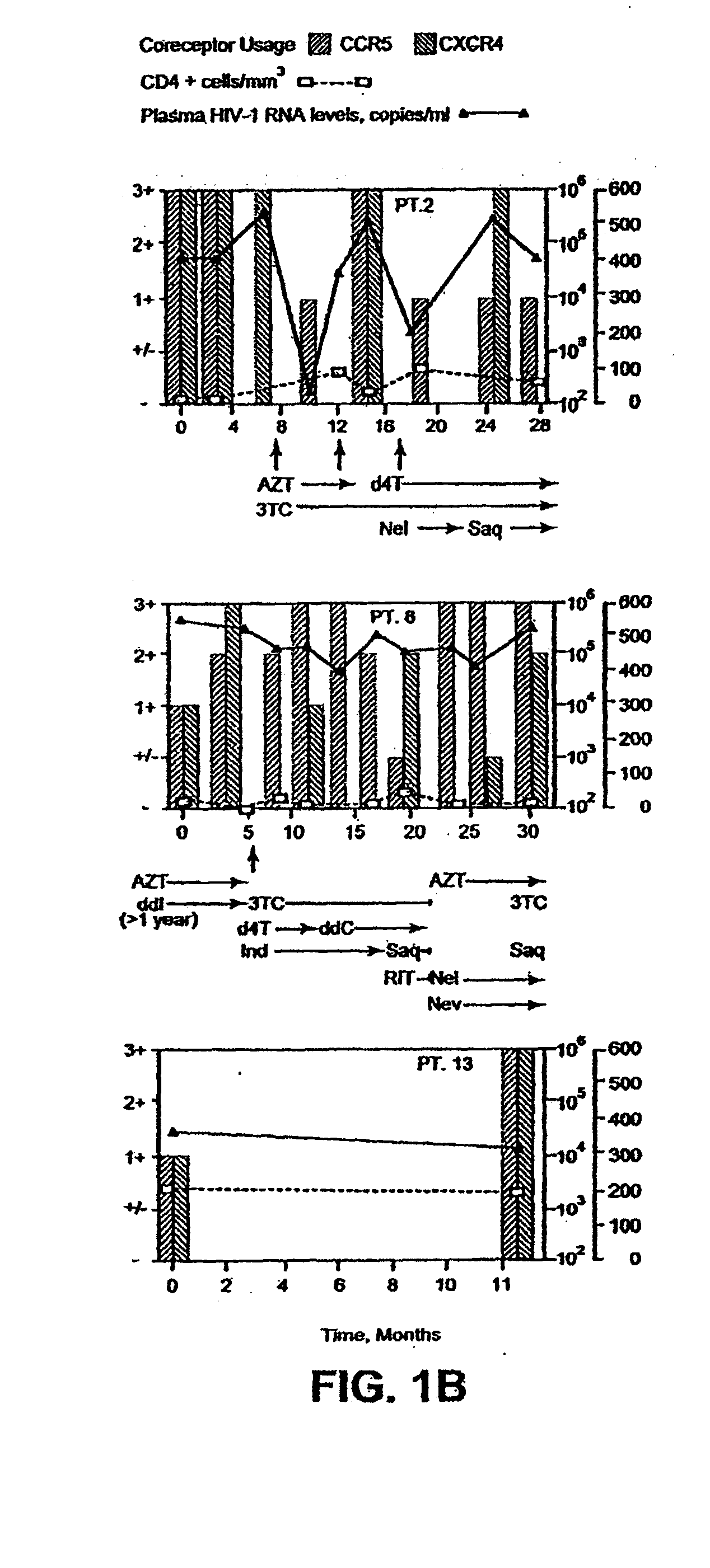
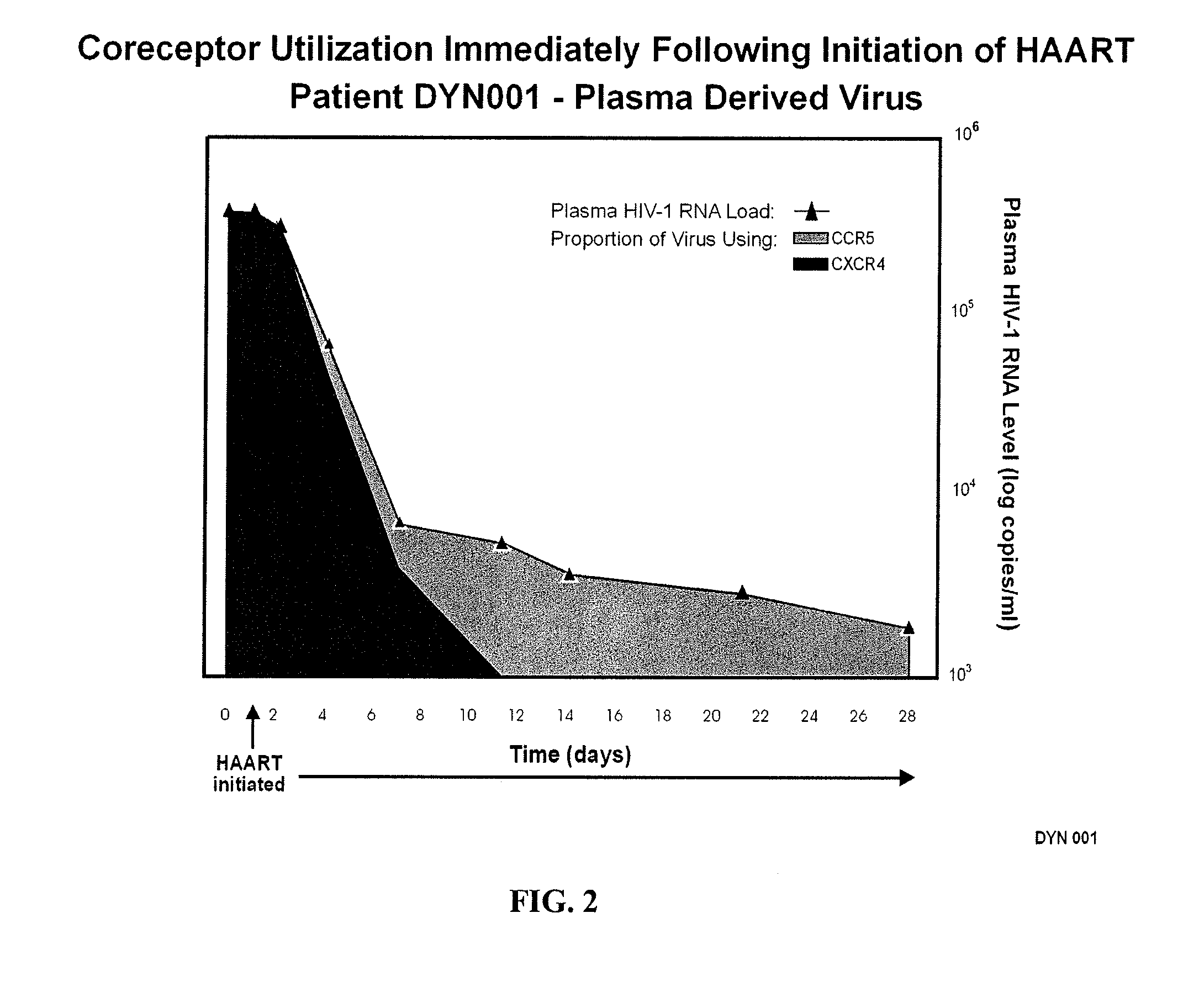
A change in viral tropism occurs in many HIV positive individuals over time and can be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus can be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CXCR4 specific strains. The diagnostic methods can be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time.
Owner:HEALTH RES INC
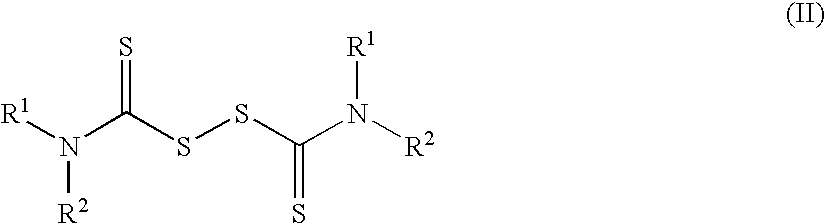
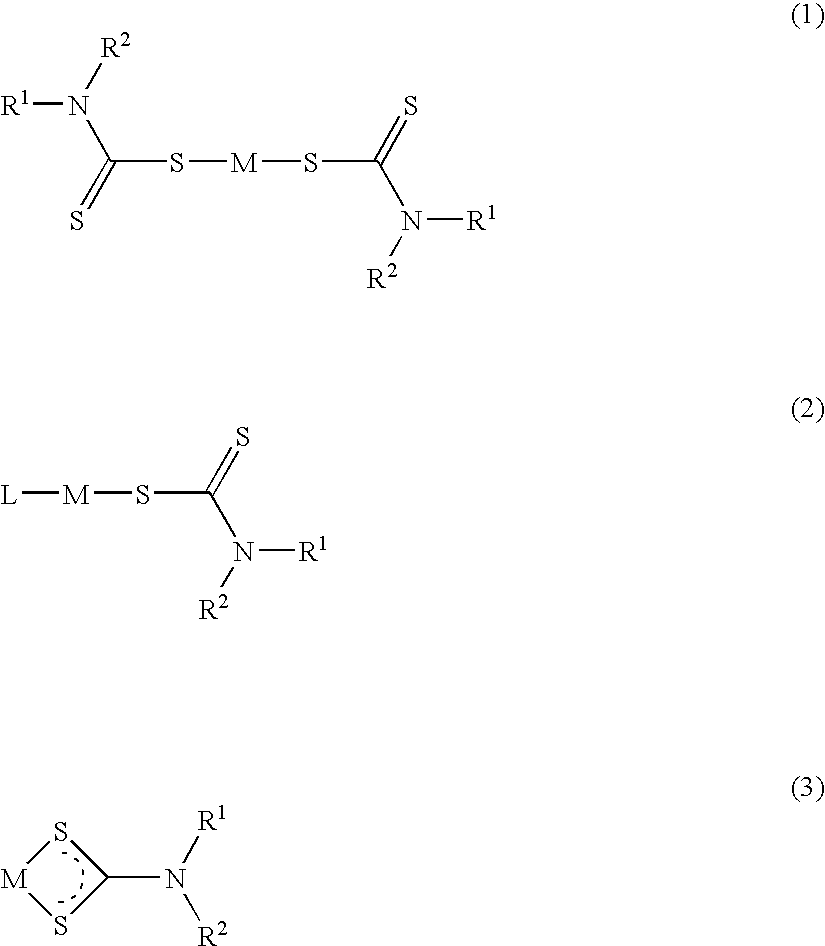
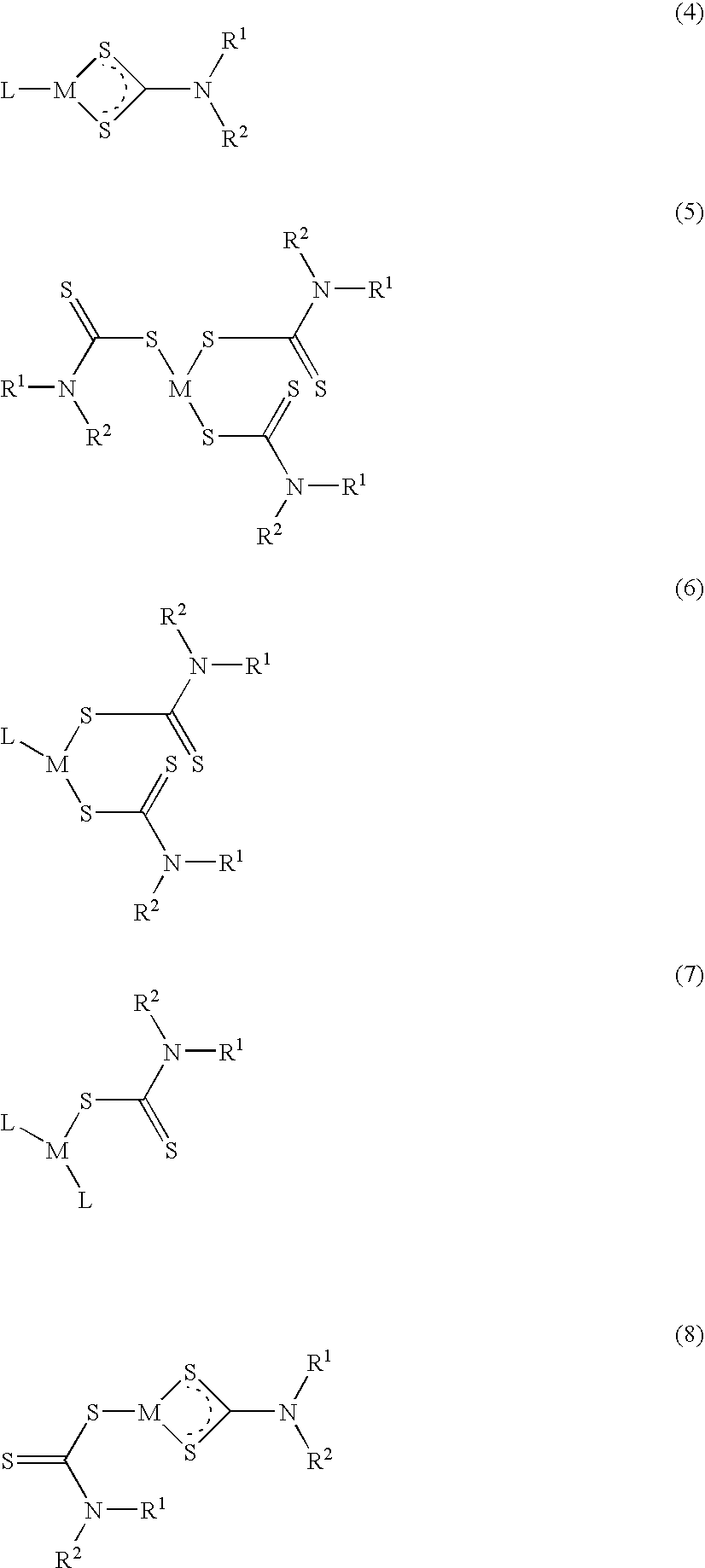
Method of treating cancer using dithiocarbamate derivatives
InactiveUS20050096304A1Readily available easily used treatmentInhibitionHeavy metal active ingredientsBiocideAdjuvantAnticarcinogen
The invention encompasses neutral dithiocarbamate metal compounds and methods of treating cancer using such compounds, along with methods for sensitizing AIDS / HIV patients to anti-retroviral therapy by blocking the P-glycoprotein membrane toxin extrusion pump using such compounds. Compounds inhibit the growth of cancer cells of a variety of cell types. A method is presented for using the neutral compounds disclosed herein, amongst other uses disclosed herein, to reduce tumor growth, and to potentiate the effect of other anticancer agents. The invention also encompasses pharmaceutical compositions comprising the neutral compounds and a pharmaceutically acceptable excipient, diluent, solubilizer, solvent, adjuvant or carrier, or a mixture thereof.
Owner:AAIPHARMA SERVCIES CORP
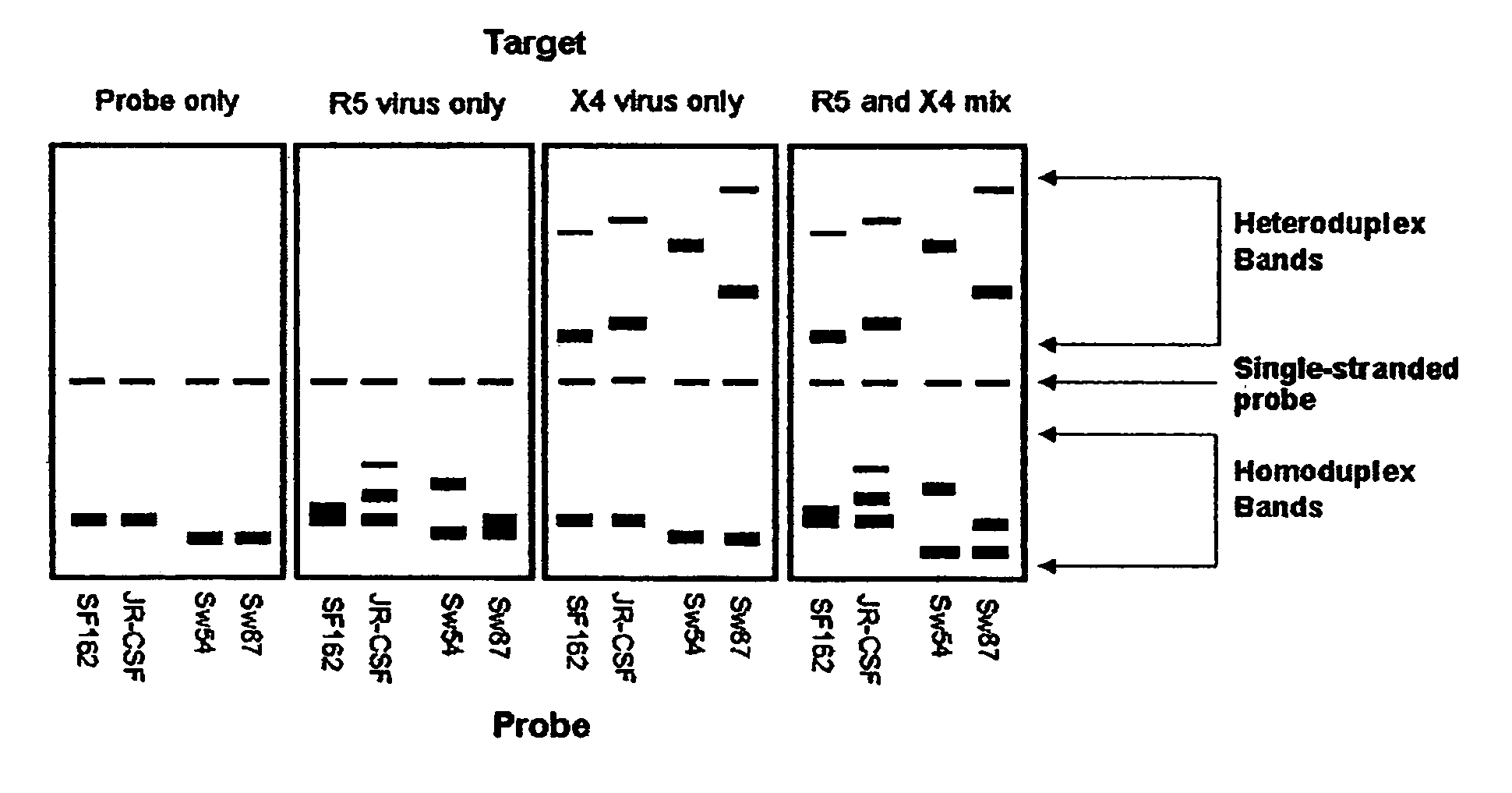
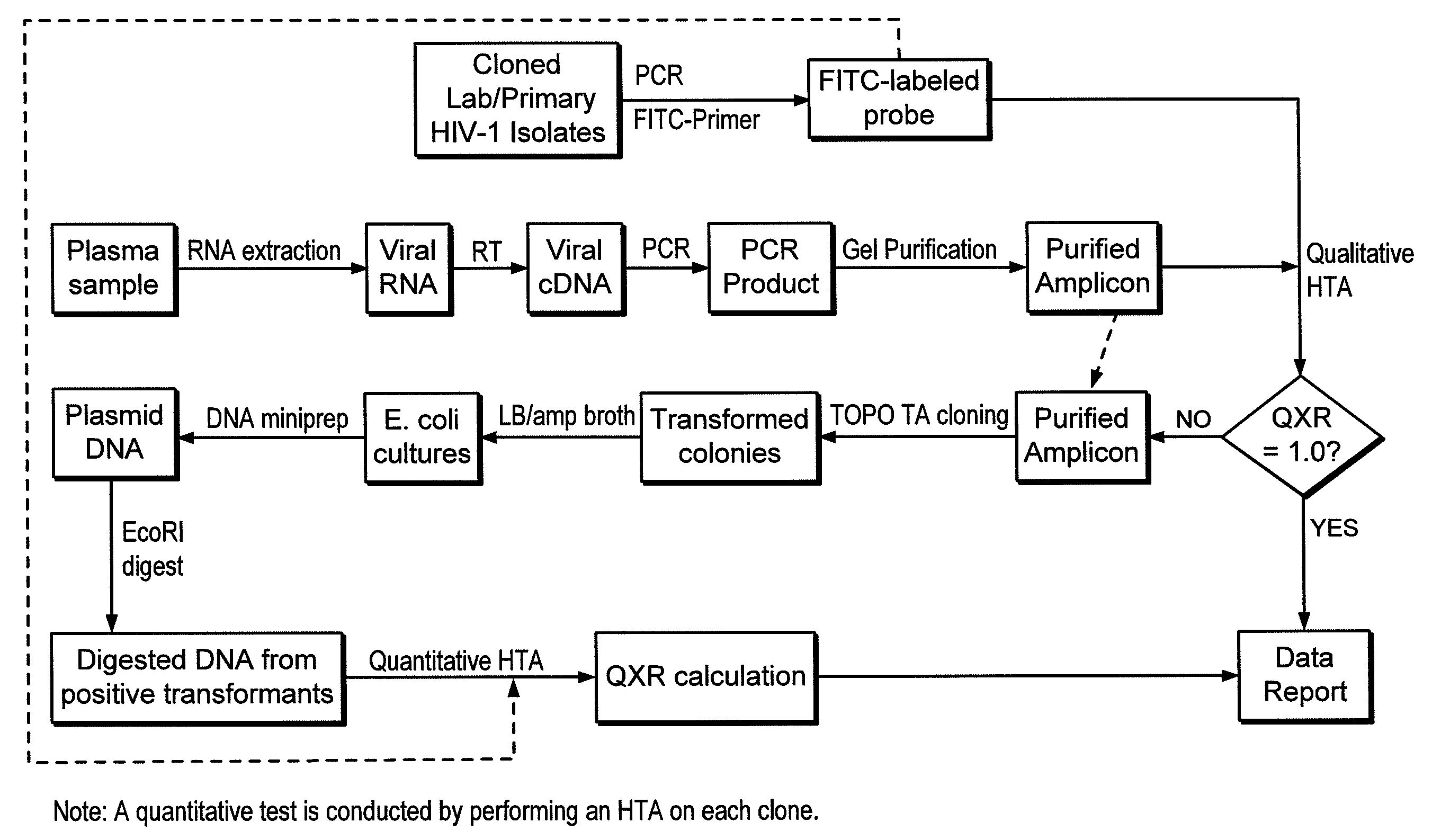
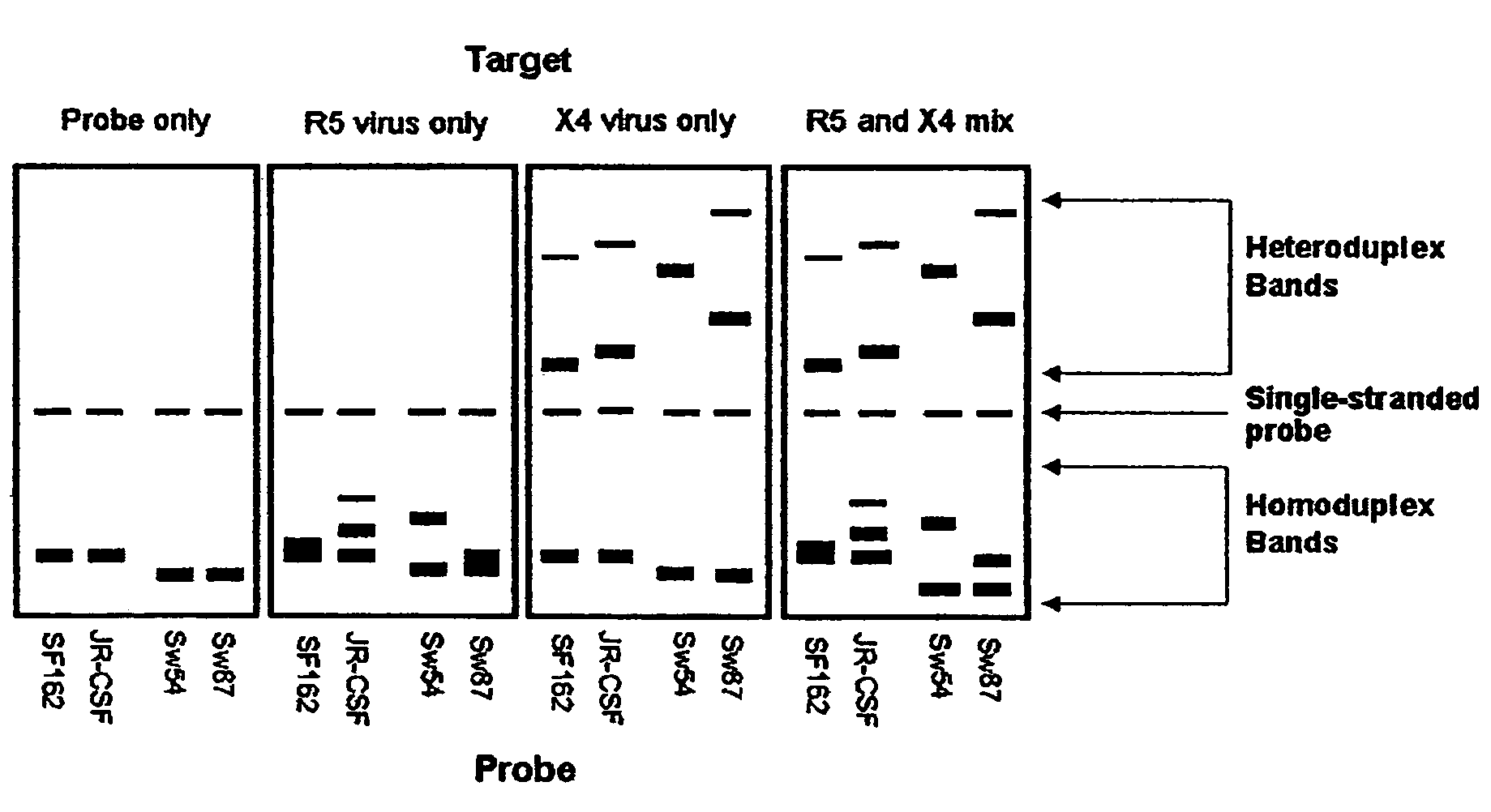
Heteroduplex tracking assay
InactiveUS20060194227A1Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
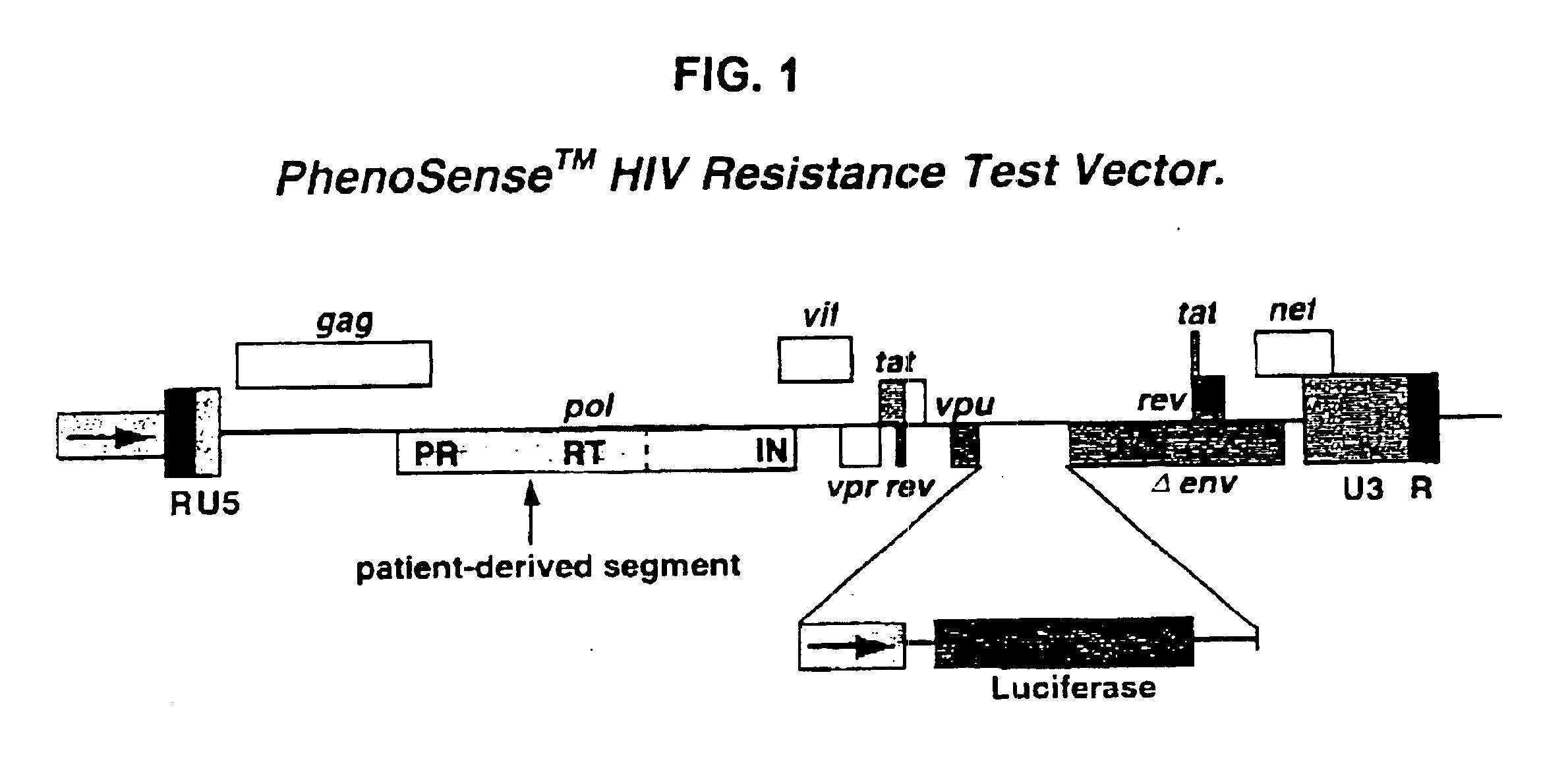
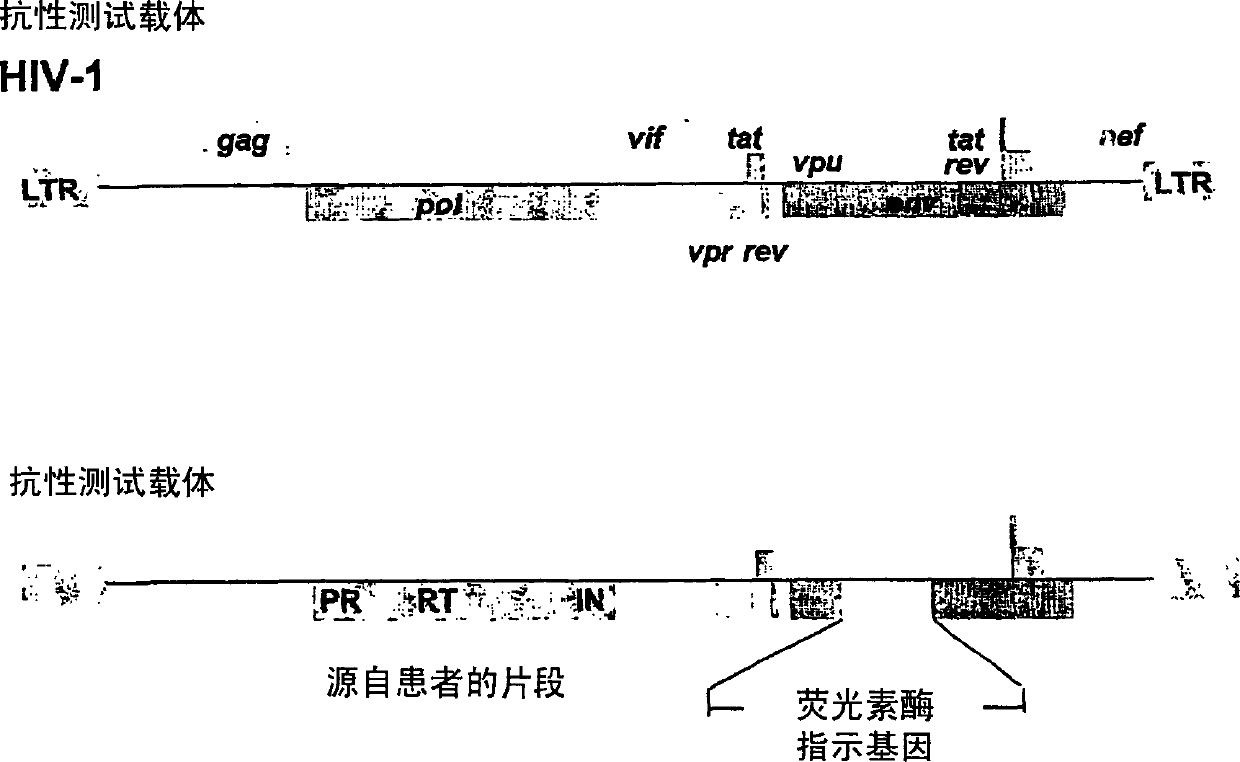
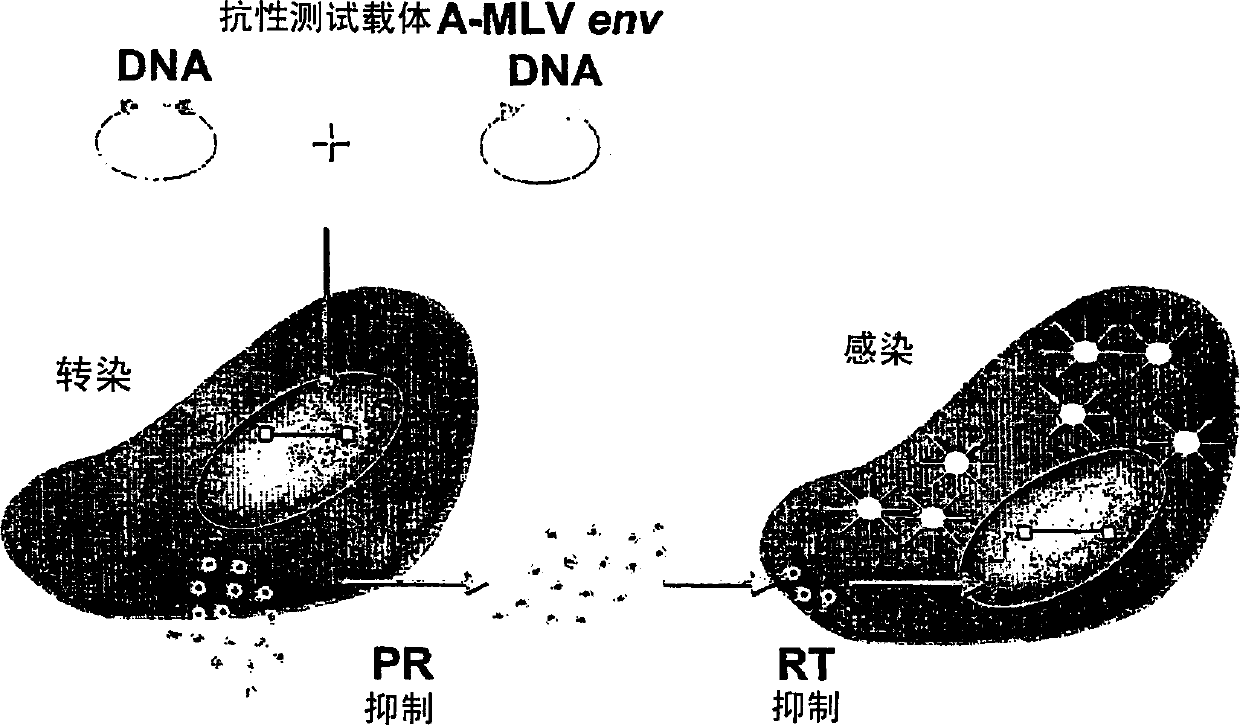
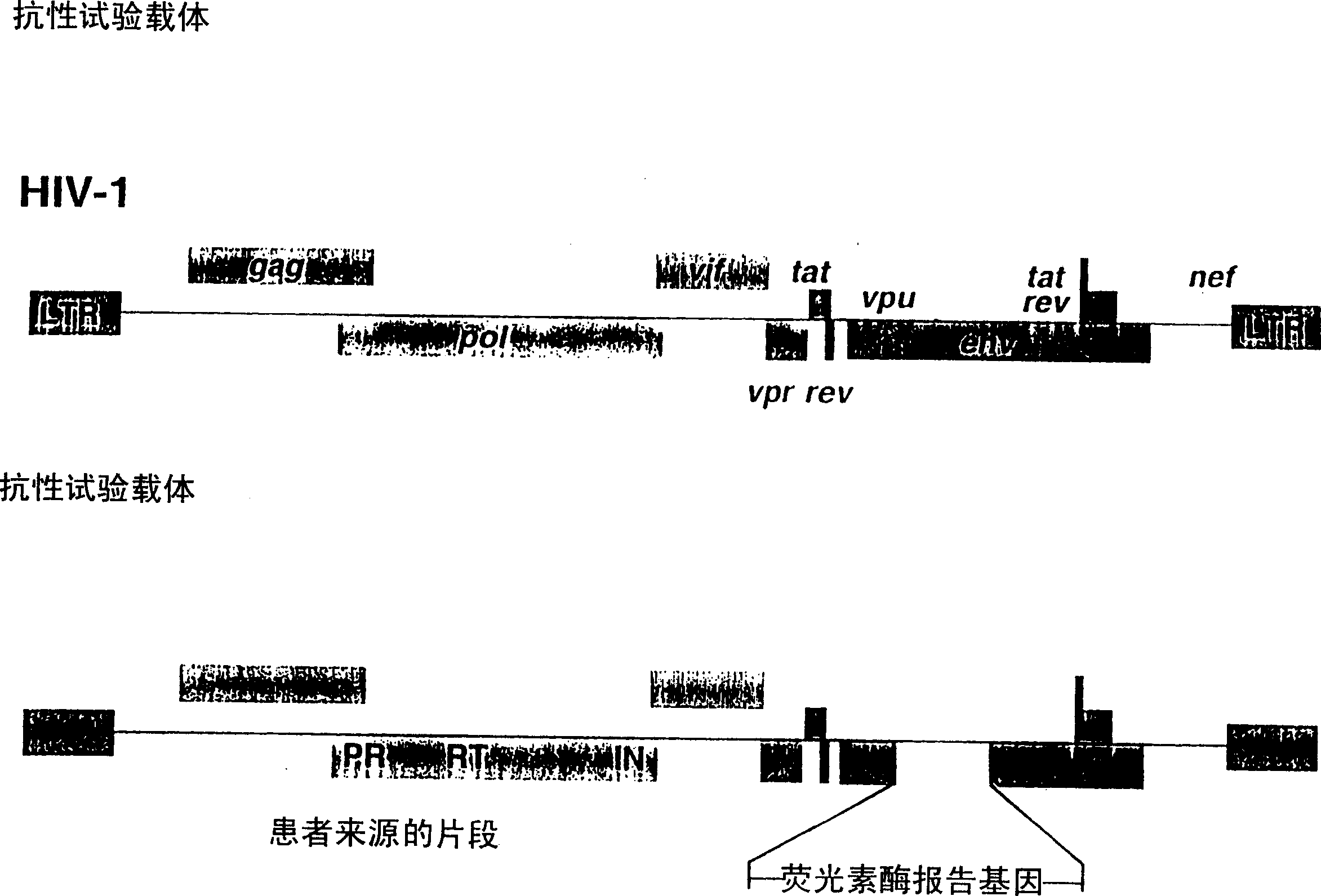
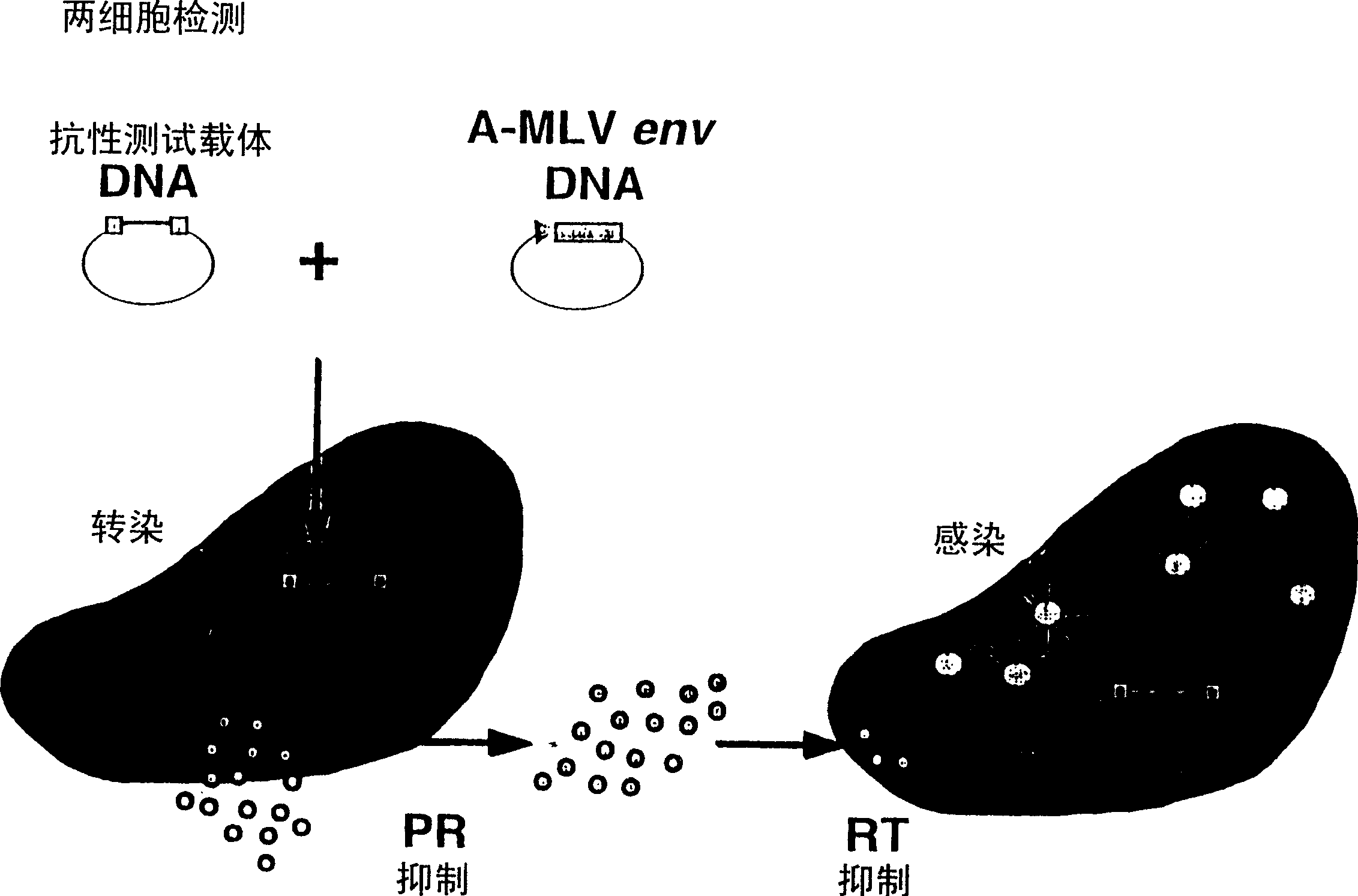
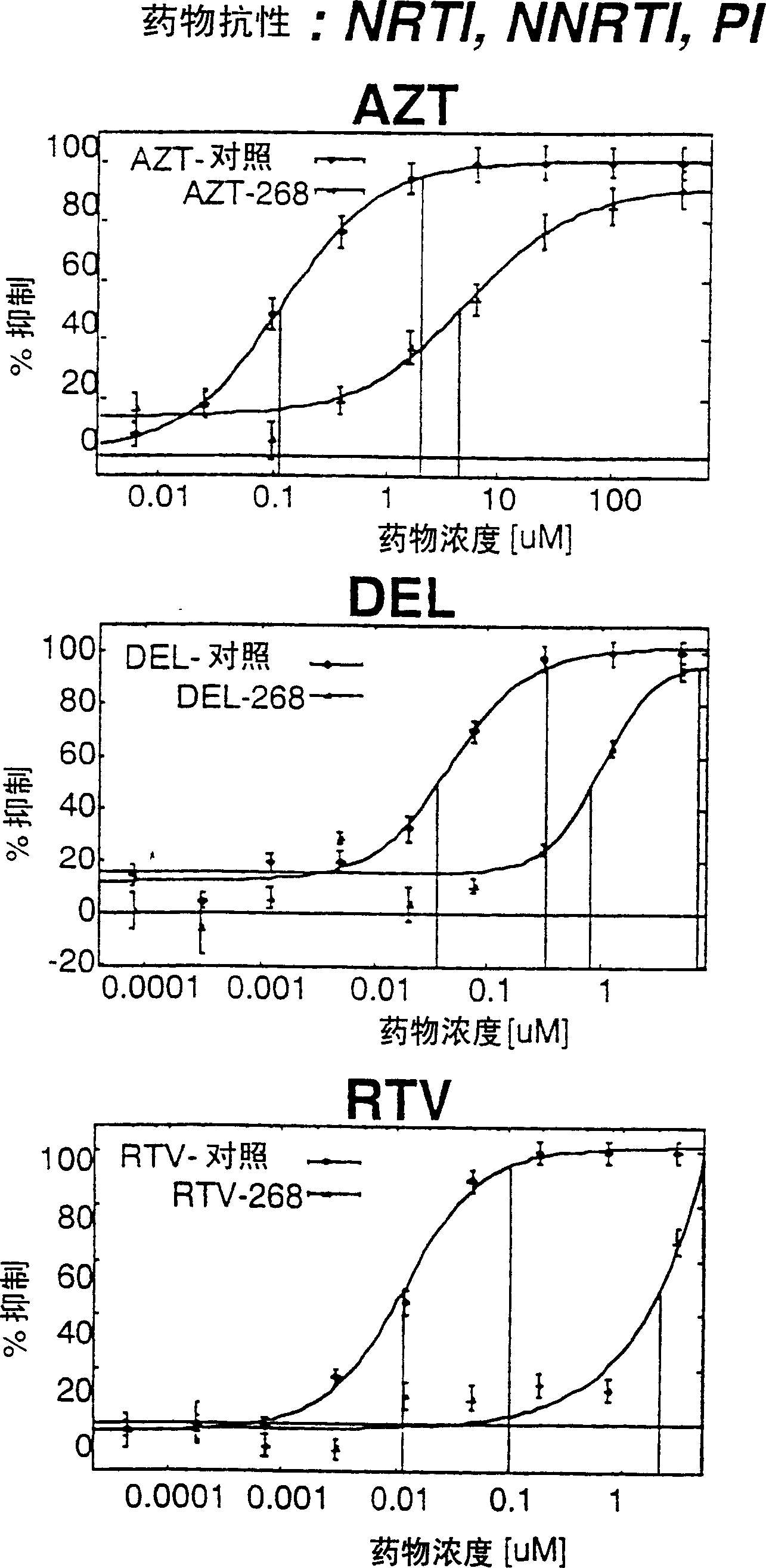
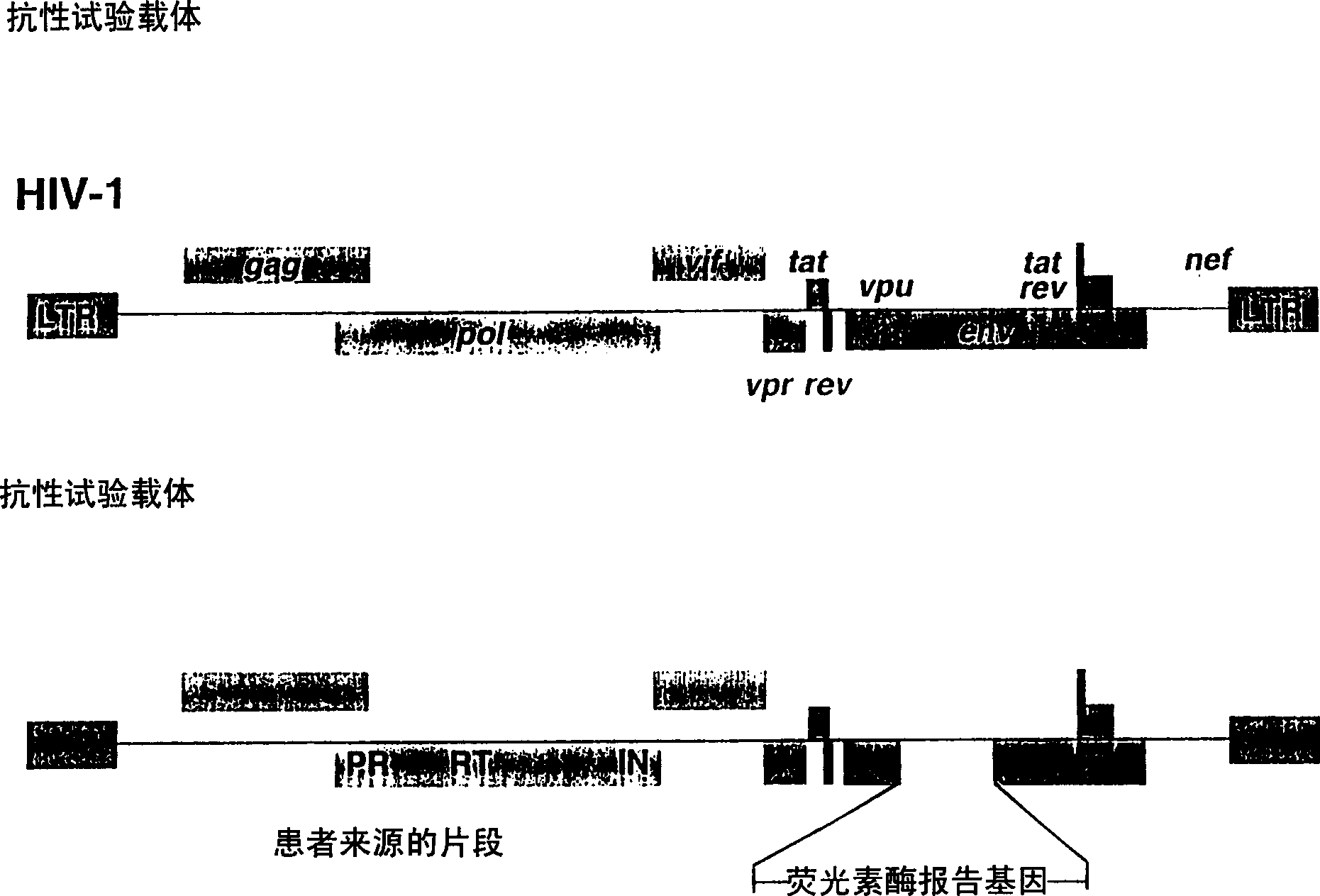
Means and methods for monitoring protease inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
InactiveUS6869759B1Identification and of fitnessSugar derivativesMicrobiological testing/measurementAcquired immunodeficiencyImmunodeficiency virus
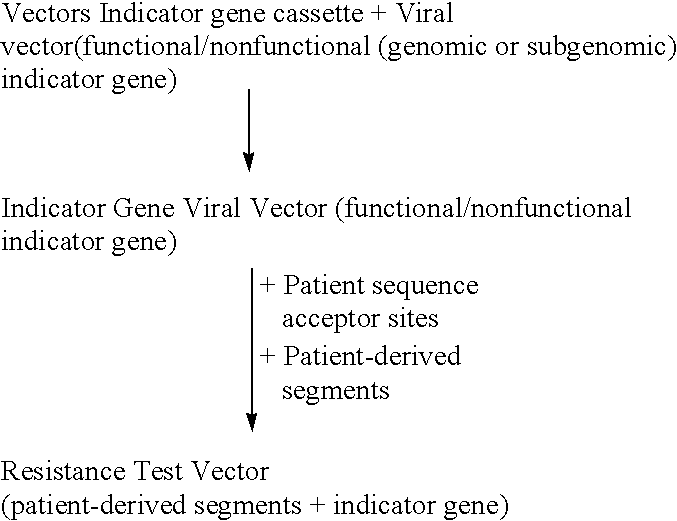
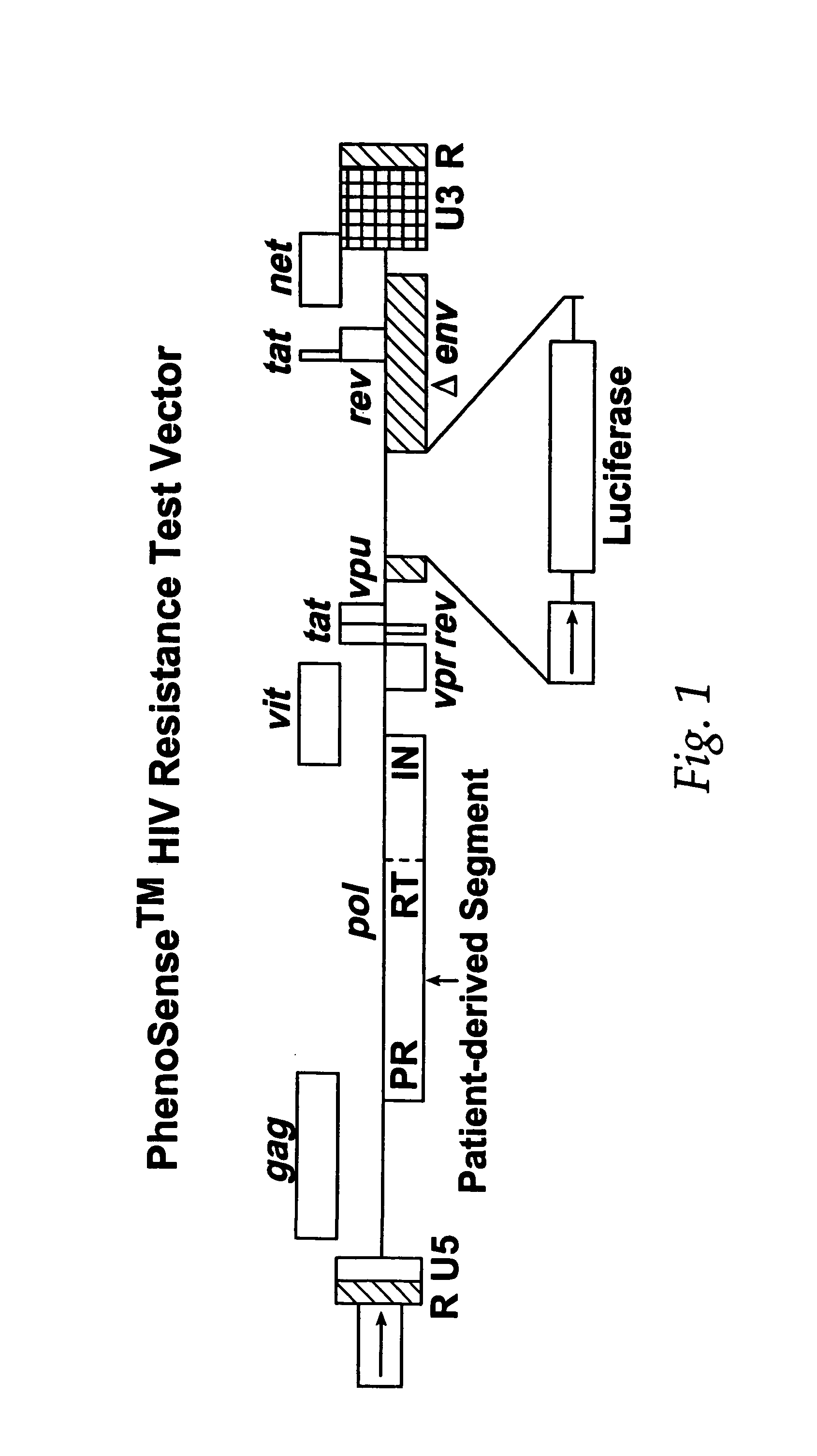
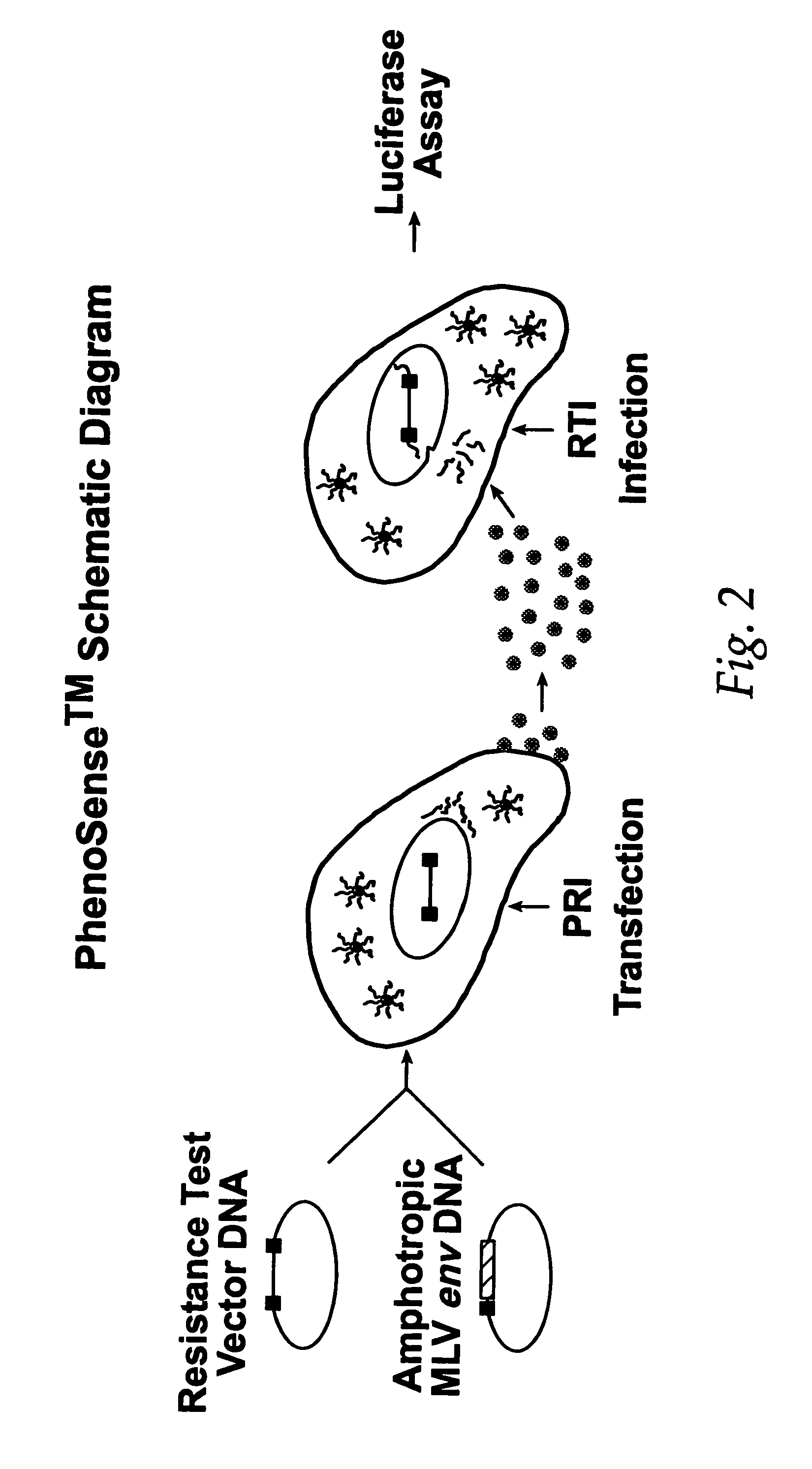
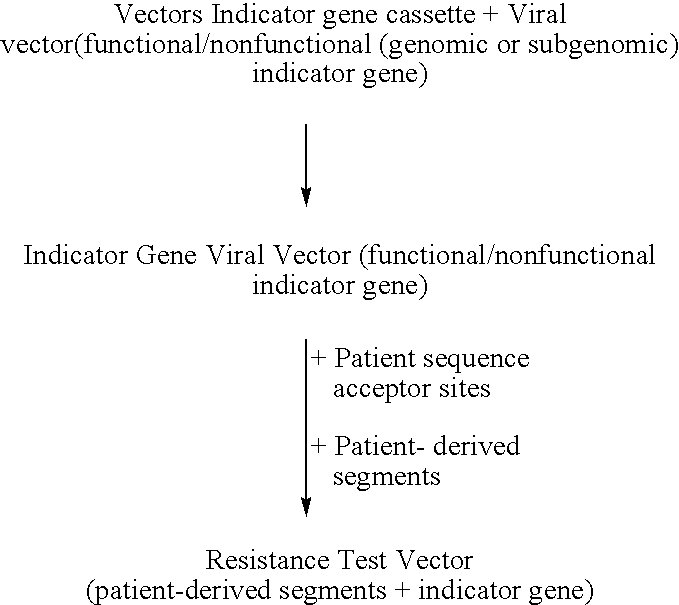
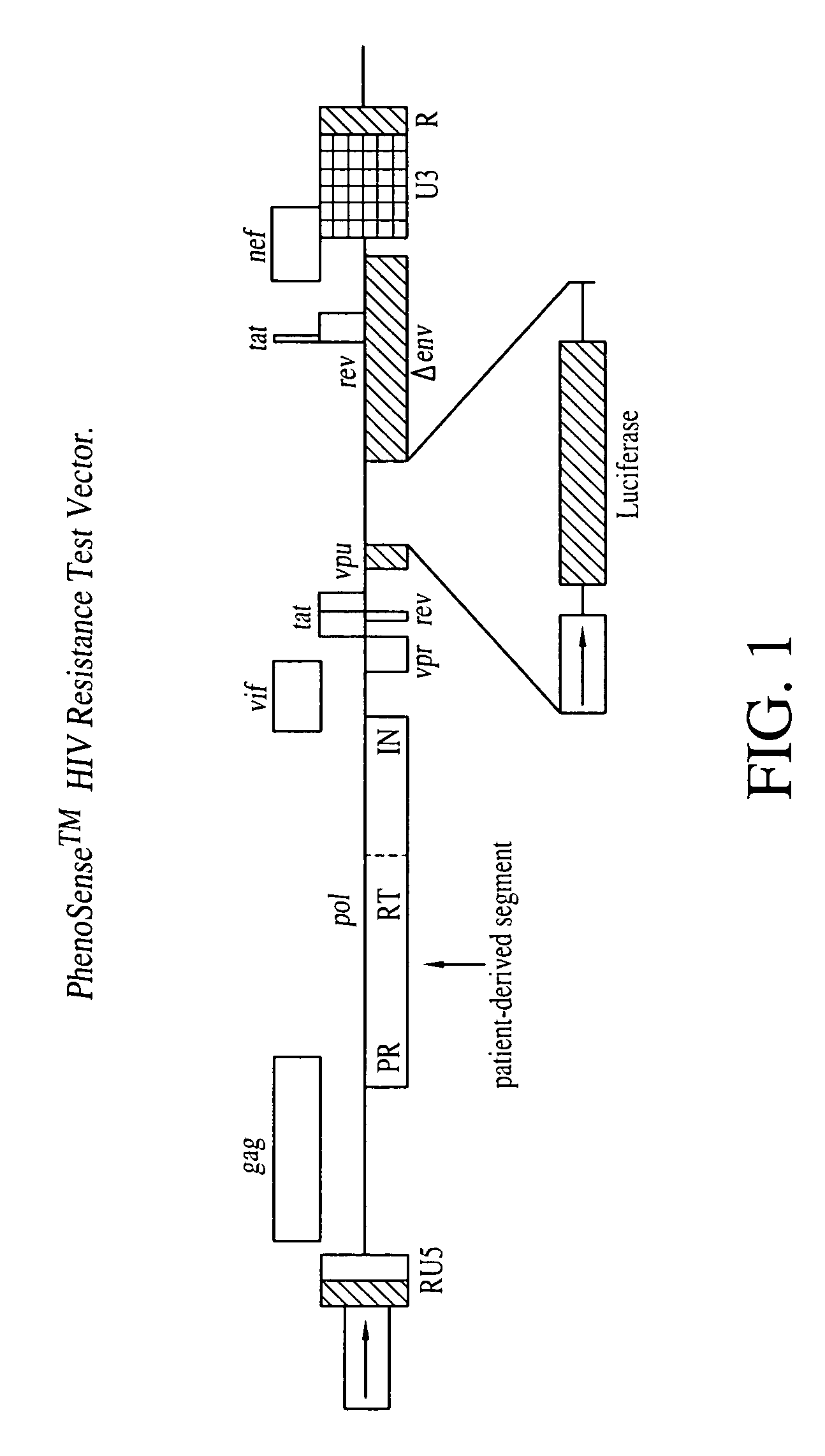
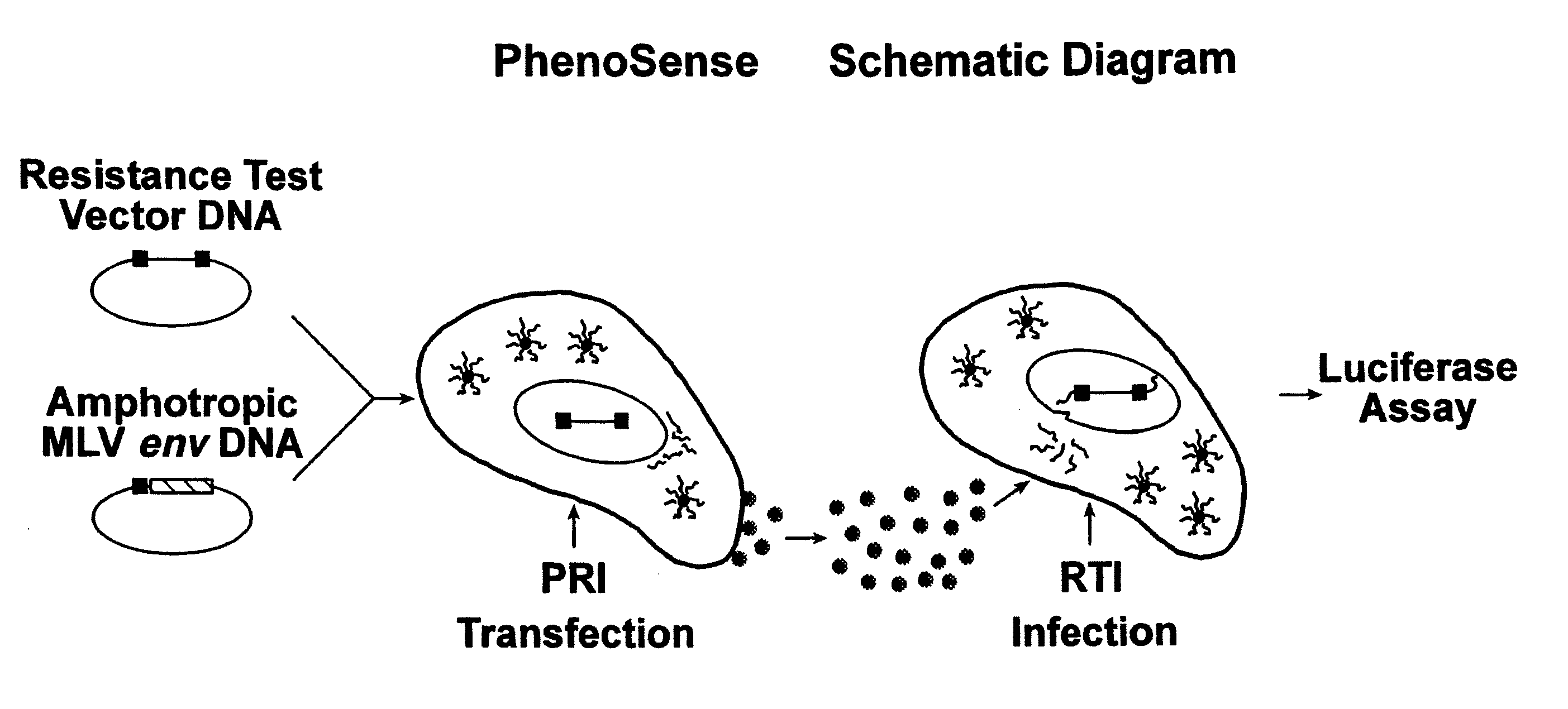
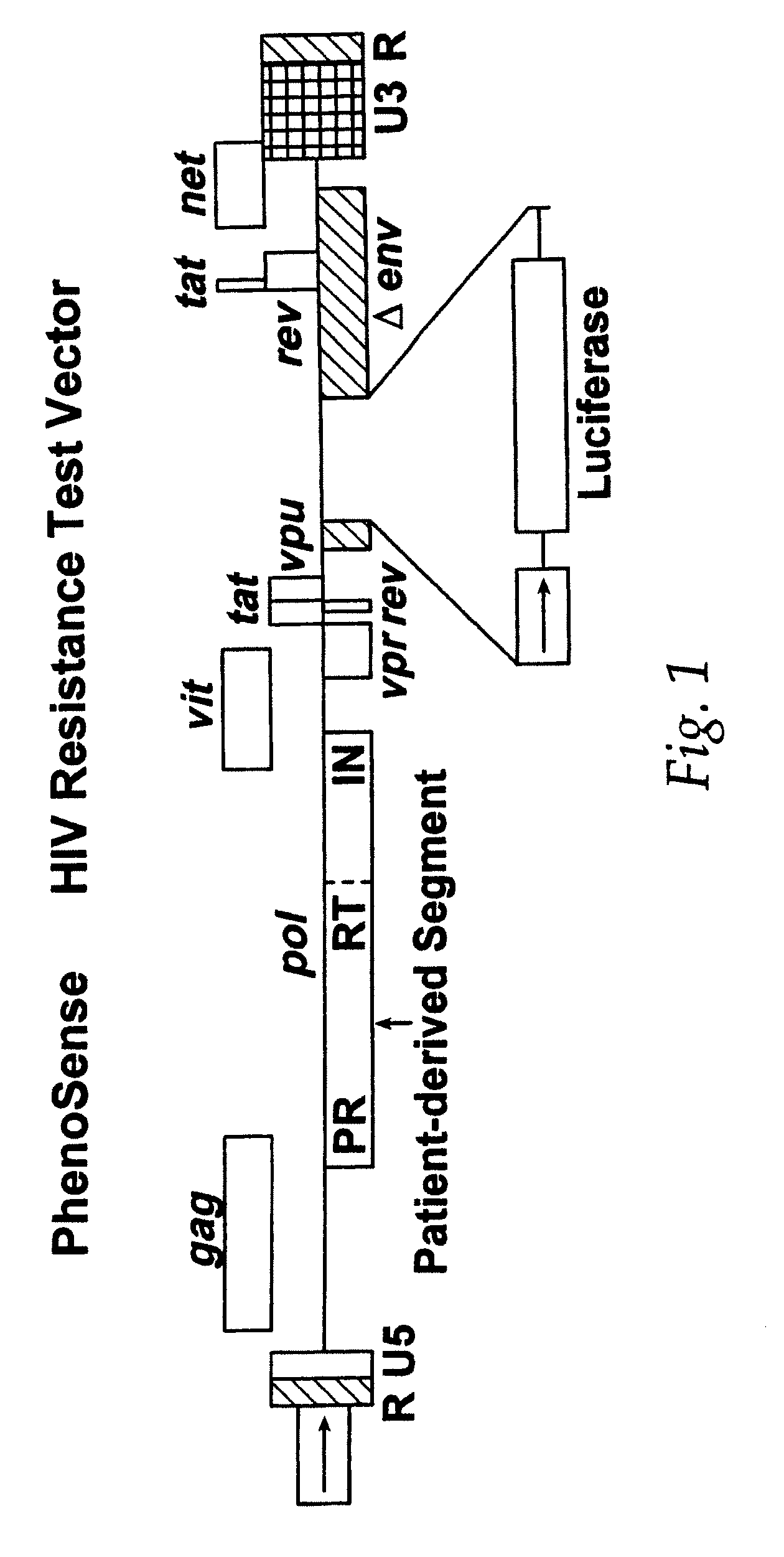
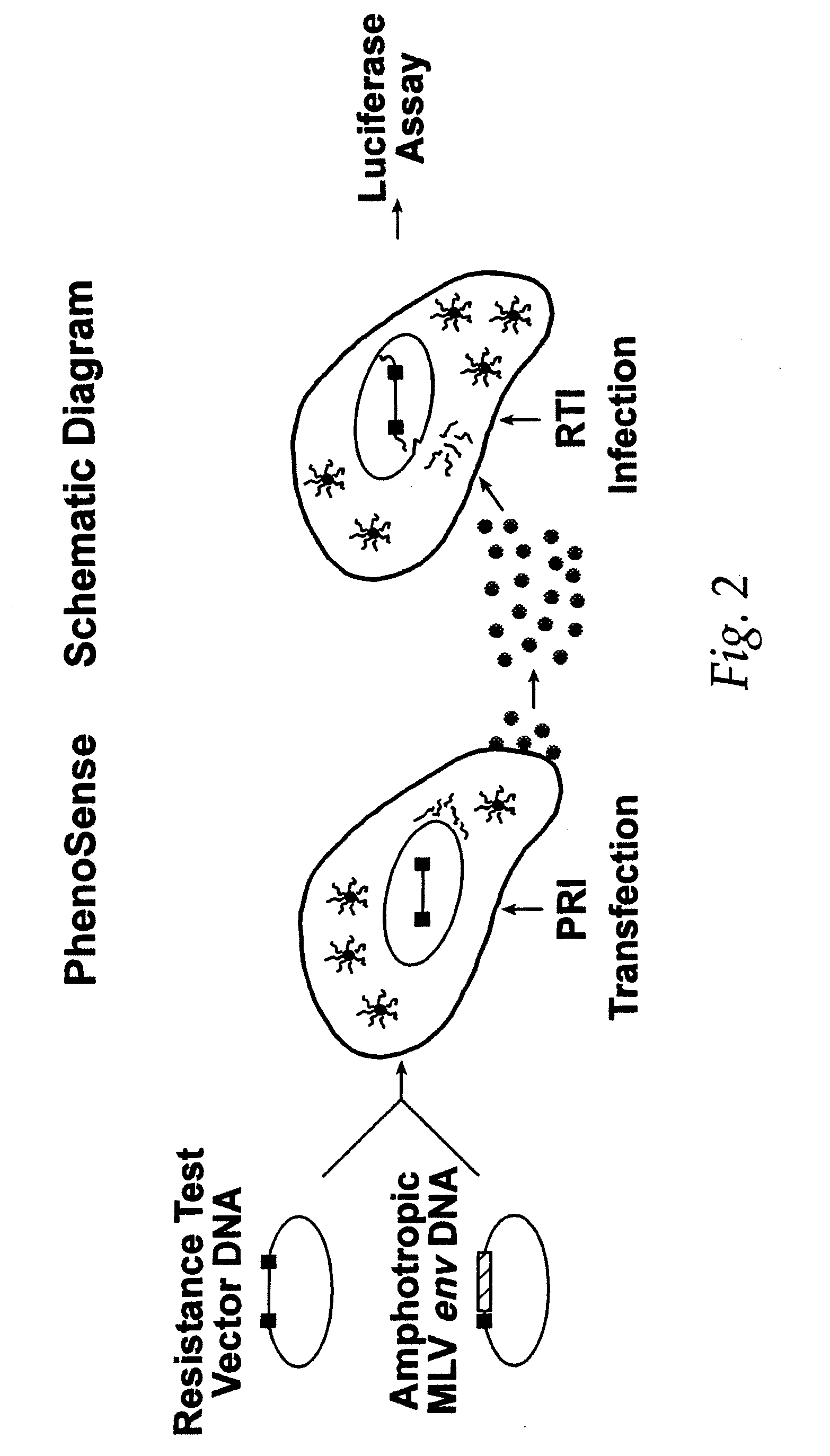
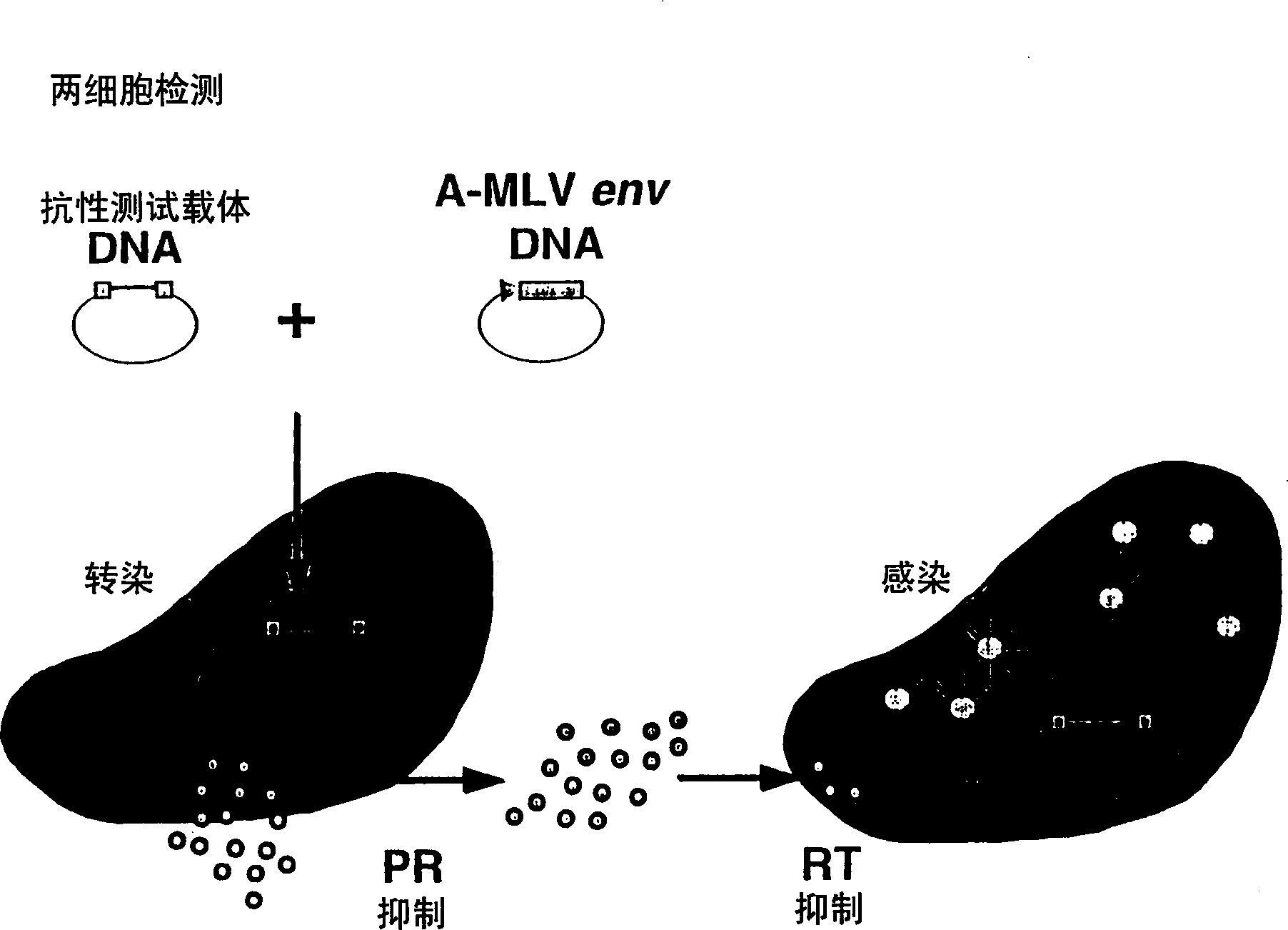
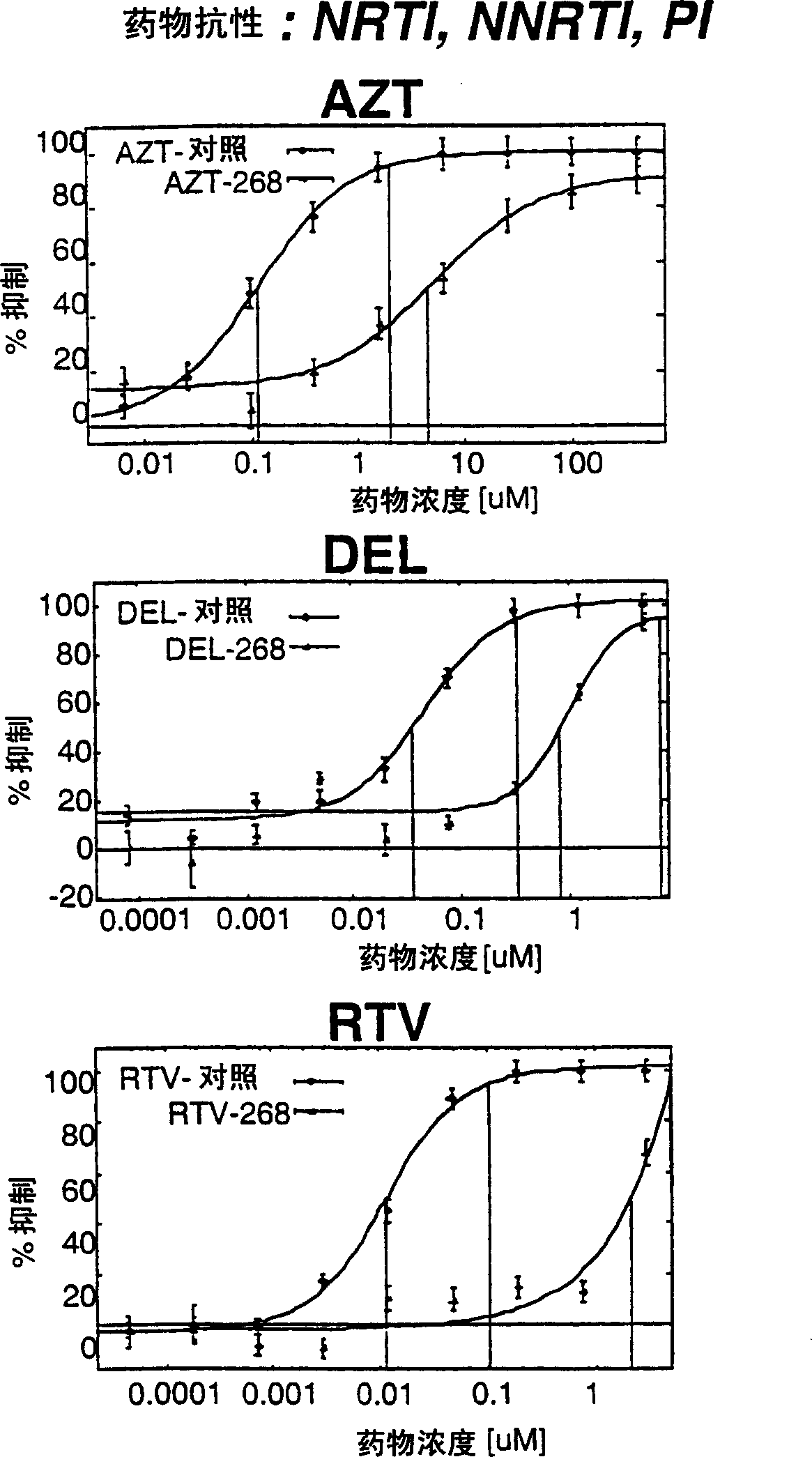
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), particularly treatment regimens including a protease inhibitor. The invention further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy using phenotypic or genotypic susceptibility assays.
Owner:MONOGRAM BIOSCIENCES
Treatment of AIDS
InactiveUS20060194212A1Peptide/protein ingredientsMicrobiological testing/measurementTherapy HIVAntiretroviral therapy
The invention includes methods of treating HIV infection in a patient where the method includes administration of an antibody to TNF-alpha and an antibody to interferon-gamma to the patient and administering antiretroviral therapy to a patient. The invention further includes methods of treating HIV infection in a patient where the method comprises administration of an antibody to TNF-alpha and an antibody to alpha interferon to the patient and administering antiretroviral therapy to a patient. The invention further includes a method of treating HIV infection in a patient where the method includes administering an antibody to alpha interferon and antiretroviral therapy to a patient. The invention further includes a method of treating an HIV infection in a patient where the method comprises administering a chimeric TNF-alpha receptor and anti-retroviral therapy to a patient.
Owner:ADVANCED BIOTHERAPY
Combating side-effects
The invention relates to the use of active ingredients, which increase the concentration of pyrimidine-based elements for nucleic acid biosynthesis in the body, in particular to the use of pyrimidine nucleosides and / or prodrugs produced therefrom, for reducing the side-effects of inhibitors of nucleic acid biosynthesis or their precursors, in particular by activating the biosynthesis of mitochondrial DNA (mtDNA). The invention also relates to the use of said active ingredients, in particular pyrimidine nucleosides and / or prodrugs for producing pharmaceutical preparations for reducing the aforementioned side-effects and to combinations or products for administering active ingredients of this type, in particular pyrimidine nucleosides and / or prodrugs produced therefrom, comprising inhibitors of nucleic acid biosynthesis or their precursors. The invention further relates to methods for treating the side-effects of inhibitors of nucleic acid biosynthesis or their precursors using the aforementioned active ingredients, in particular pyrimidine nucleosides and / or prodrugs produced therefrom, or the aforementioned combinations or products and to corresponding pharmaceutical preparations. Side-effects of HAART (Highly Active Anti-Retroviral Therapy) and side-effects of other anti-viral nucleoside analogous agents, which inhibit the mitochondrial γ-polymerases, can in particular be prophylactically and / or therapeutically treated in this manner.
Owner:PHARMA NORD
Heteroduplex tracking assay
Owner:HEALTH RES INC
Medicine composition for preventing and treating AIDS and its prepn process and use
The present invention discloses one kind of medicine composition for preventing and treating AIDS and its preparation process and use. The medicine composition consists of active ingredient and auxiliary ingredient, and may be prepared into granule, tablet, capsule, oral liquid or injection. It has the functions of inhibiting the copying of HIV, promoting immunologic reconstruction of the AIDS patient, delaying disease and clinical complication, and synergizing and attenuating in high efficiency retrovirus resisting therapy. The present invention has wide application foreground.
Owner:广州中医药大学热带医学研究所
Methods and compositions for treating hiv-associated diarrhea
InactiveUS20140163096A1Not cause deterioration of immune statusBiocideDigestive systemDrug-drug interactionAntiretroviral therapy
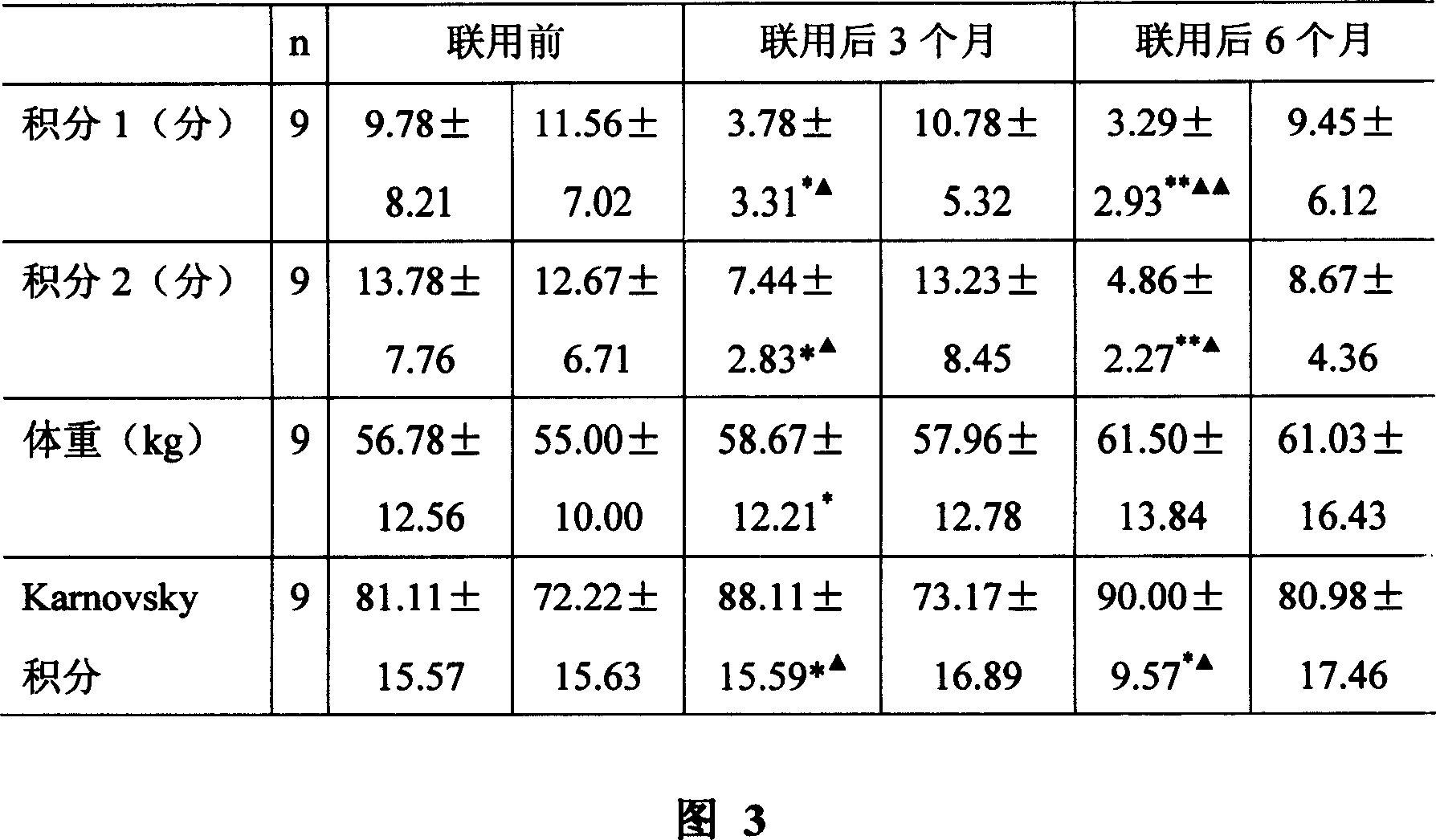
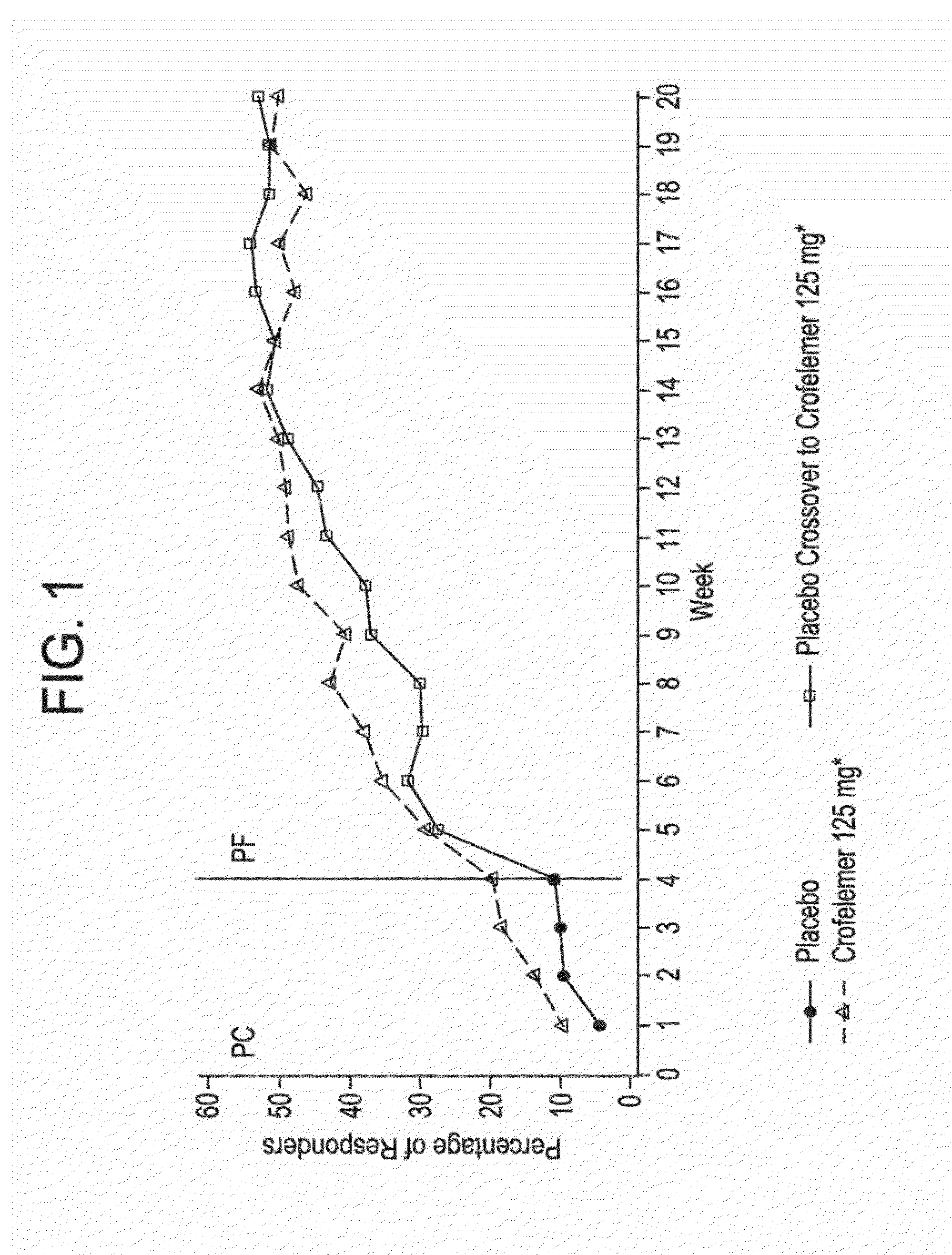
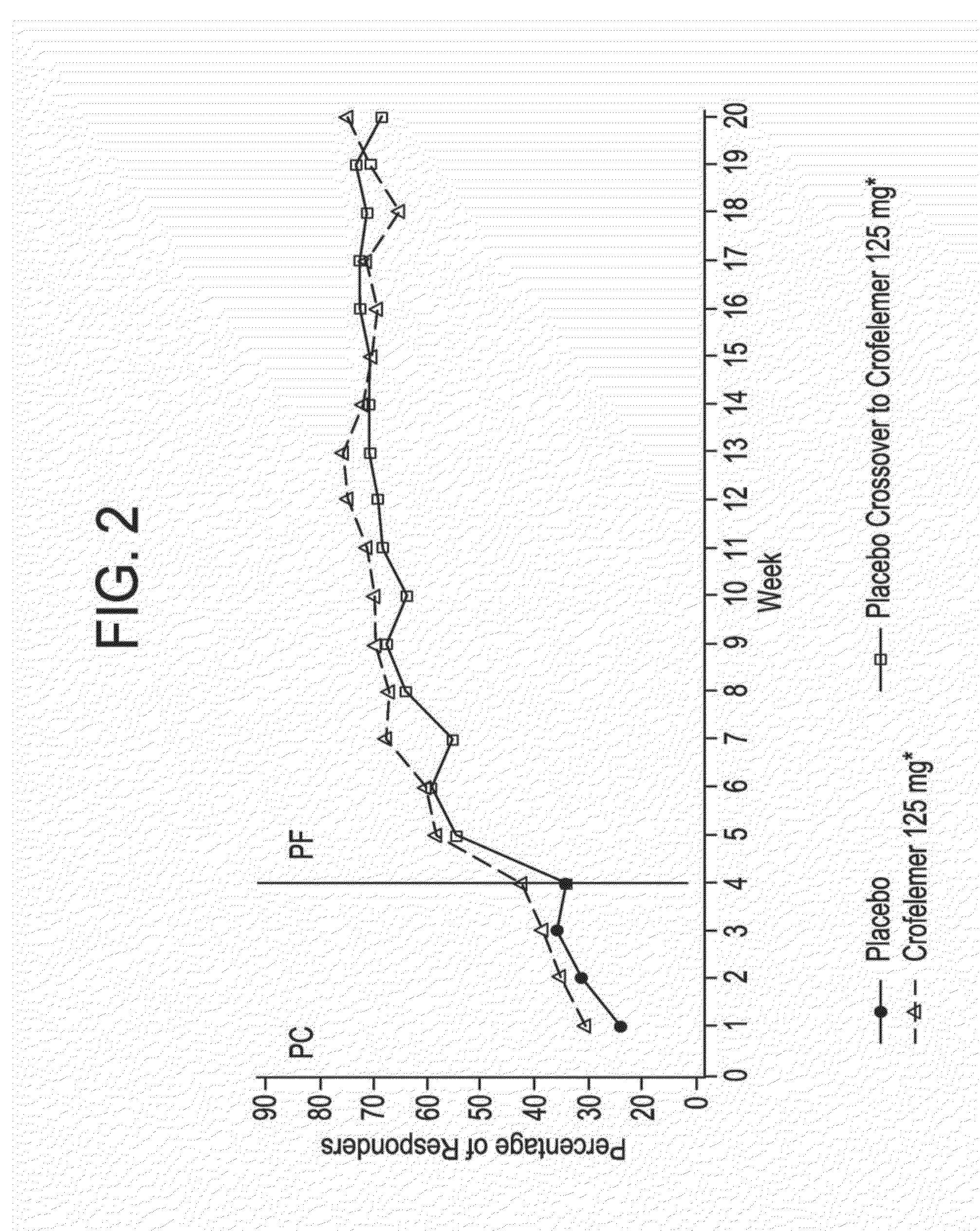
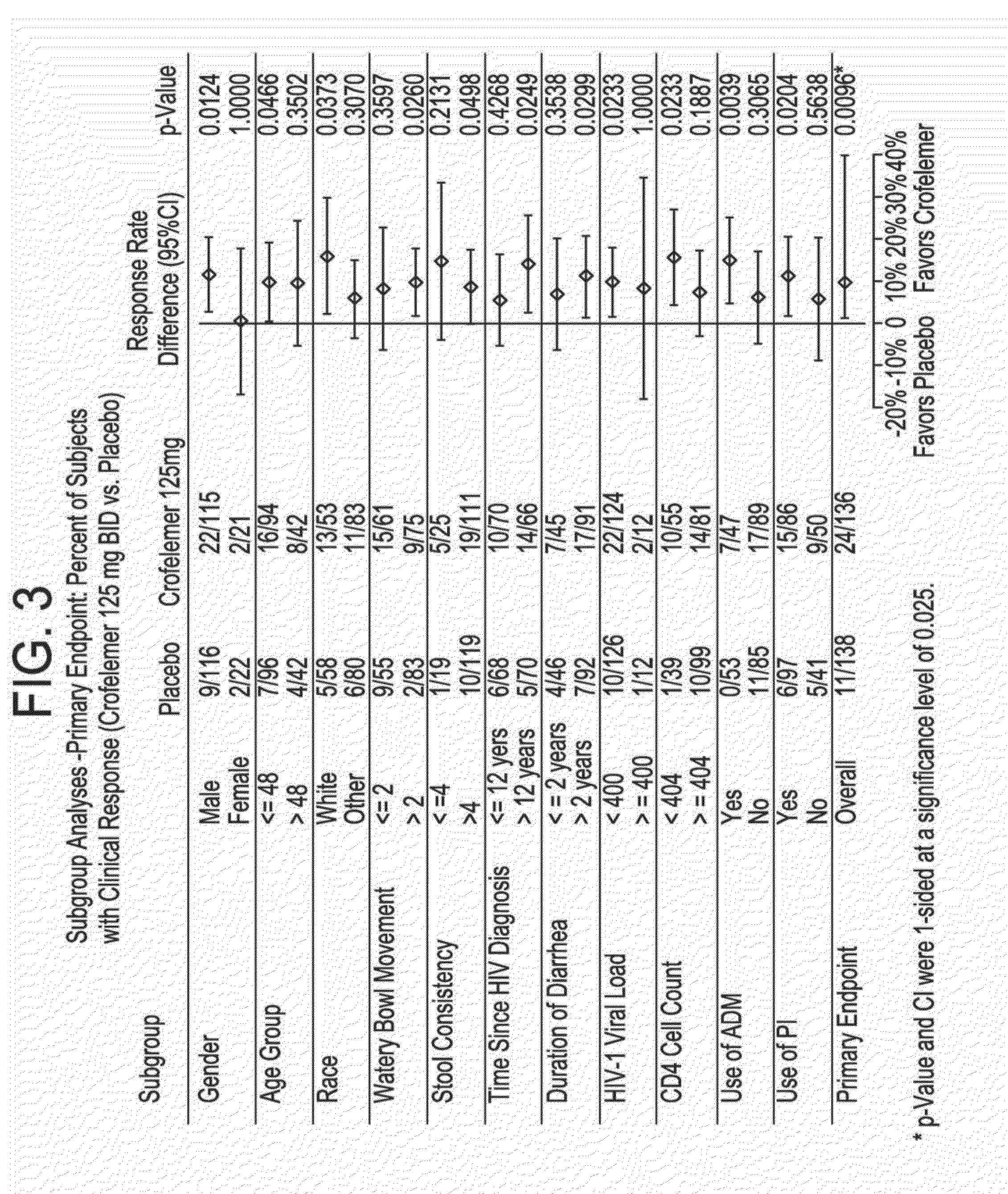
Provided herein are methods for treating HIV-associated or highly active antiretroviral therapy (HAART)-associated diarrhea in an HIV positive subject by administering a composition comprising crofelemer to the subject wherein the composition has minimal drug-drug interactions with at least one other compound concurrently administered to the subject to treat an HIV infection. Also provided are methods for treating HIV-associated or highly active antiretroviral therapy (HAART)-associated diarrhea in an HIV positive subject by administering a composition comprising crofelemer to the subject, wherein the composition does not significantly inhibit the activity of at least one other compound concurrently administered to the subject to treat an HIV infection.
Owner:NAPO PHARMA INC
Detection of DNA sequences as risk factors for HIV infection
A method for identifying a risk factor for diseases, disorders or conditions, such as those caused by human immunodeficiency virus, using the polymerase chain reaction and specific primers. Methods for treating patients having these diseases, disorders or conditions by antimicrobial treatment of the risk factor by combined antiviral and antibacterial treatment or by sustaining or stimulating the subject's immune system. Methods for screening biological products including red blood cell preparations. Primers and methods for detecting nucleic acids or microbial agents associated with red blood cells, such as those associated with red blood cells in subjects infected with HIV and undergoing antiretroviral therapy.
Owner:MONTAGNIER LUC
Heteroduplex tracking assay
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry soon after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
Owner:HEALTH RES INC
Means and method for monitoring antiretroviral therapy and guiding therapeutic decisions in treatment of HIV/AIDS
InactiveCN1446265AReduce sensitivityMicrobiological testing/measurementVirus peptidesAcquired resistanceNucleoside Reverse Transcriptase Inhibitor
The present invention relates to antiviral drug susceptibility and resistance assays for identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), and to monitoring the clinical progression of HIV infection and Modes and methods of HIV infection response to antiretroviral therapy, in particular nucleoside reverse transcriptase inhibitor therapy using phenotypic sensitivity analysis and genotypic analysis.
Owner:病毒科学公司
Detection of DNA sequences as risk factors for HIV infection
Owner:MONTAGNIER LUC
Heteroduplex tracking assay
InactiveUS7344830B2Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
Owner:HEALTH RES INC
Analysis of HIV-1 coreceptor use in the clinical care of HIV-1-infected patients
InactiveUS20040121318A1Improve efficacyReduce the possibilityMicrobiological testing/measurementVertebrate cellsImmunodeficiency virusHIV positives
A change in viral tropism occurs in many HIV positive individuals over time and can be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus can be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CXCR4 specific strains. The diagnostic methods can be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time.
Owner:HEALTH RES INC
Means and methods for monitoring protease inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
InactiveUS7138231B2Identification and of fitnessSugar derivativesHydrolasesAcquired immunodeficiencyImmunodeficiency virus
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), particularly treatment regimens including a protease inhibitor. The invention further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy using phenotypic or genotypic susceptibility assays.
Owner:MONOGRAM BIOSCIENCES
Means for the treatment of HIV
The invention relates to a non-coding sequence of deoxyribonucleic acids comprising at least one sequence motif N1N2CGN3N4, wherein N is a nucleotide comprising A, C, T, or G, and C is deoxycytidine, G is deoxyguanosine, A is deoxyadenosine and T is deoxy-thymidine for the treatment of viral infections. In particular, the non-coding sequence of deoxyribonucleic acids is used in combination with antiretroviral therapy and / or histone de-acetylase inhibitors.
Owner:GILEAD SCI INC
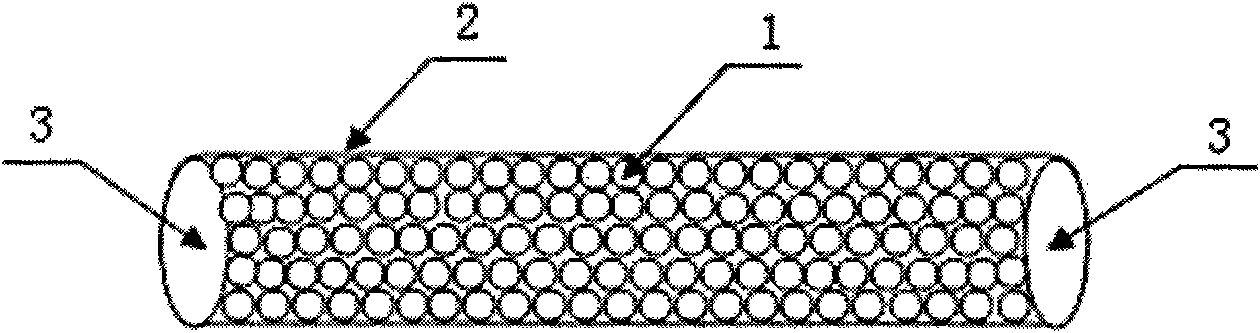
Hepatitis B virus affinity adsorption column and preparation method thereof
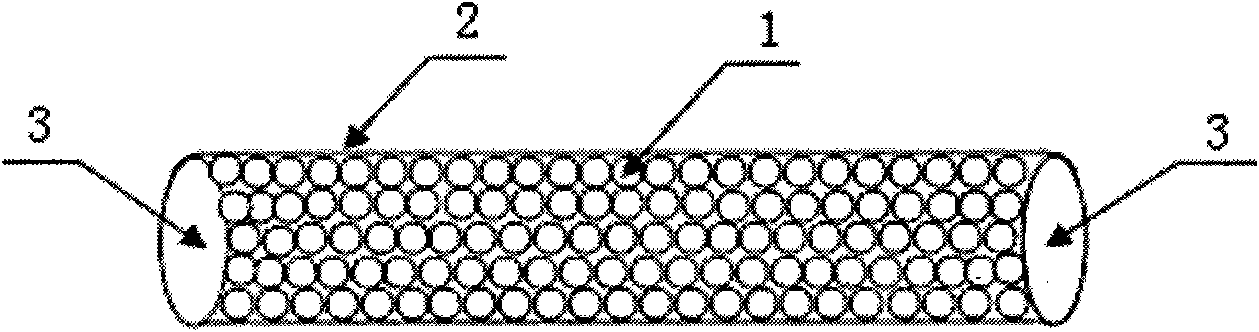
InactiveCN101862483AWide variety of sourcesLow priceIon-exchange process apparatusOther blood circulation devicesPolyesterMicrosphere
The invention discloses a hepatitis B virus affinity adsorption column and a preparation method thereof, and relates to blood perfusion technology. The adsorption column comprises affinity microspheres (1), a polyester column (2) and separation membranes (3), wherein the polyester column (2) is filled with the affinity microspheres (1); and the two ends of the polyester column (2) are sealed by using the separation membranes (3). The method comprises the following steps of: (1) preparing adsorption microspheres; (2) activating the adsorption microspheres to obtain activated microspheres; (3) fixing an anti-body to obtain the affinity microspheres; (4) sealing unreacted aldehyde groups and epoxy groups; (5) filling the column; and (6) sealing membranes. The hepatitis B virus affinity adsorption column can assist or replace antiretroviral therapy of hepatitis B, has a remarkable effect, is suitable for clearing hepatitis B virus in the blood of a hepatitis B virus carrier, a patient with chronic hepatitis B, a patient with severe hepatitis B and a patient with liver cancer, and fulfills the aims of remission, treatment and saving the lives of patients.
Means and methods for monitoring protease inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
InactiveUS20060035249A1Identification and of fitnessCompound screeningApoptosis detectionAcquired immunodeficiencyGenotype
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), particularly treatment regimens including a protease inhibitor. The invention further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy using phenotypic or genotypic susceptibility assays.
Owner:VIROLOGIC INCORPORATED
Means and methods for monitoring protease inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
InactiveUS7186506B1Microbiological testing/measurementVertebrate cellsAcquired immunodeficiencyImmunodeficiency virus
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), particularly treatment regimens including a protease inhibitor. The invention further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy using phenotypic or genotypic susceptibility assays.
Owner:MONOGRAM BIOSCIENCES
Treatment of latent HIV infection
InactiveUS20150320893A1EliminateEncourage viral productionBiocideAntiviralsReverse transcriptaseProstaglandin analog
Methods for treating HIV positive patients, and purging and eradicating latent HIV virus from a patient's system, are disclosed. The bulk of viral load is eradicated using conventional antiretroviral (ARV) therapy. Compounds that encourage viral production in the latent cells are then administered, preferably without activating those cells, while maintaining the ARV therapy. The administration of compounds that encourage viral production in latent cells is cycled, and after around 7-10 cycles, the methods can virtually eliminate latent HIV in the patient. Ideally, the ARV regimen includes at least one integrase inhibitor, at least one entry inhibitor, such as a CCR5 antagonist, and at least one, and preferably two, reverse transcriptase inhibitors. The compounds that encourage viral production in latent cells ideally include a combination of prostratin or a prostratin analog and an HDAC inhibitor, such as butyrate, valproate, or SAHA.
Owner:VOLPE JOSEPH M
Traditional Chinese medicine capsule for treating bone marrow suppression caused by highly active antiretroviral therapy (HAART) for AIDS (acquired immure deficiency syndrome)
InactiveCN102145067AFormulation ScienceAbundant raw materialsCapsule deliveryBlood disorderFine powderEssence oil
The invention relates to traditional Chinese medicine capsule for treating bone marrow suppression caused by an highly active antiretroviral therapy (HAART) for AIDS (acquired immure deficiency syndrome), which effectively solves the treatment medication problem of the bone marrow suppression caused by the HAART for the AIDS, wherein the capsule is prepared by ginseng, angelica, fructus ligustri lucidi, caulis spatholobi, radix astragali, herba epimedii, pericarpium citri reticulatae and oldenlandia diffusa, wherein the angelica and the pericarpium citri reticulatae are crushed, added with the water and distilled to respectively collect essential oil and distilled water solution; the dregs of the angelica and the pericarpium citri reticulatae are mixed with the fructus ligustri lucidi, the caulis spatholobi, the radix astragali, the herba epimedii and the oldenlandia diffusa, and the mixture is added with water to decoct twice; during the decocting process, the decocting liquid is combined with the distilled water solution of the angelica and the pericarpium citri reticulatae; the mixture is filtered, condensed and added with the ginseng powder for evenly mixing; then the mixture is dried, crushed into fine powder and sieved; the essential oil of the angelica and the pericarpium citri reticulatae is injected and mixed uniformly, and the capsule is filled. The traditional Chinese medicine capsule in the invention have scientific prescription and good edible effect, can effectively solve the treatment medication problem of the bone marrow suppression caused by the HAART for the AIDS, and are innovation of the traditional Chinese medicine.
Owner:HENAN UNIV OF CHINESE MEDICINE
Means and methods for monitoring protease inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), particularly treatment regimens including a protease inhibitor. The invention further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy using phenotypic or genotypic susceptibility assays.
Owner:MONOGRAM BIOSCIENCES
Nutritional supplement for a category of HIV patients
The present invention relates to a nutritional product for HIV patients that are not on highly active antiretroviral therapy. More specifically the invention relates to a nutritional composition comprising oligosaccharides. This invention also relates to the manufacture of a nutritional supplement for use in HIV patients.
Owner:NV NUTRICIA
Methods for reversing HIV latency using baf complex modulating compounds
PendingUS20210315876A1Prevent nucleosomal positioningRelieving transcriptional repressionOrganic active ingredientsOrganic chemistryMechanism of actionAntiretroviral therapy
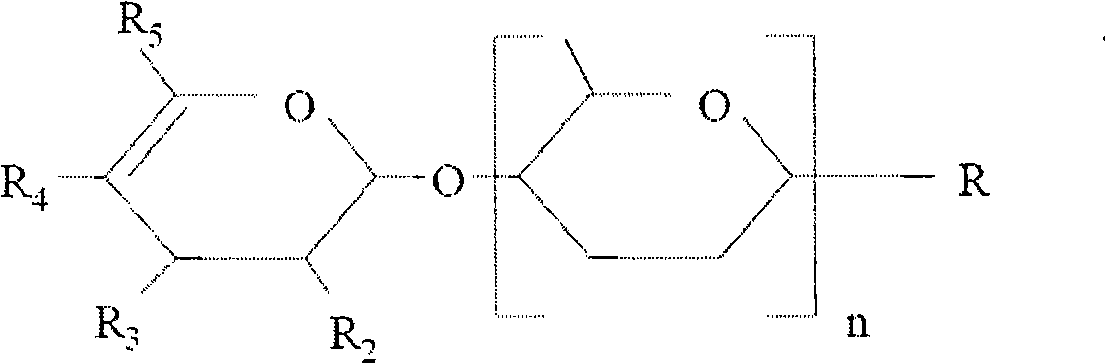
This disclosure provides methods of using BAF complex modulating compounds as inhibitors of BAF-mediated transcription in target cells. The BAF complex modulating compounds include 12-membered macrolactam compounds that can target a BAF-specific subunit (e.g., ARID1A) to prevent nucleosomal positioning, relieving transcriptional repression of HIV-1. The subject methods can provide for reversal of latency of HIV-1 in cells in vitro or in vivo. Use of the macrolactam BAF complex modulating compounds represent a method of HIV latency reversal with a unique mechanism of action, which can be optionally combined with other Latency Reversal Agents to improve reservoir targeting. The subject methods can be utilized in conjunction with any convenient methods of treating HIV or HIV latency, including methods related to immune system activation, antiretroviral therapies and / or anti-HIV agents.
Owner:PURDUE RES FOUND INC +2
Path and method for monitoring non nucleotide reverse transcriptive enzyme inhibitor anti retrovirus therapy
InactiveCN1532290ASignificant reductionReduce sensitivityMicrobiological testing/measurementRecombinant DNA-technologyNucleoside Reverse Transcriptase InhibitorGenotype Assay
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodificiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) and further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy, particularly non-nucleoside reverse transcriptase inhibitor therapy using phenotypic susceptibility assays or genotypic assays.
Owner:病毒科学公司
Means and method for monitoring non-nucleoside reverse transcriptase inhibitor antiretroviral therapy
InactiveCN1311823ASignificant reductionReduce sensitivitySugar derivativesMicrobiological testing/measurementNucleoside Reverse Transcriptase InhibitorGenotype Assay
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodificiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) and further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy, particularly non-nucleoside reverse transcriptase inhibitor therapy using phenotypic susceptibility assays or genotypic assays.
Owner:病毒科学公司
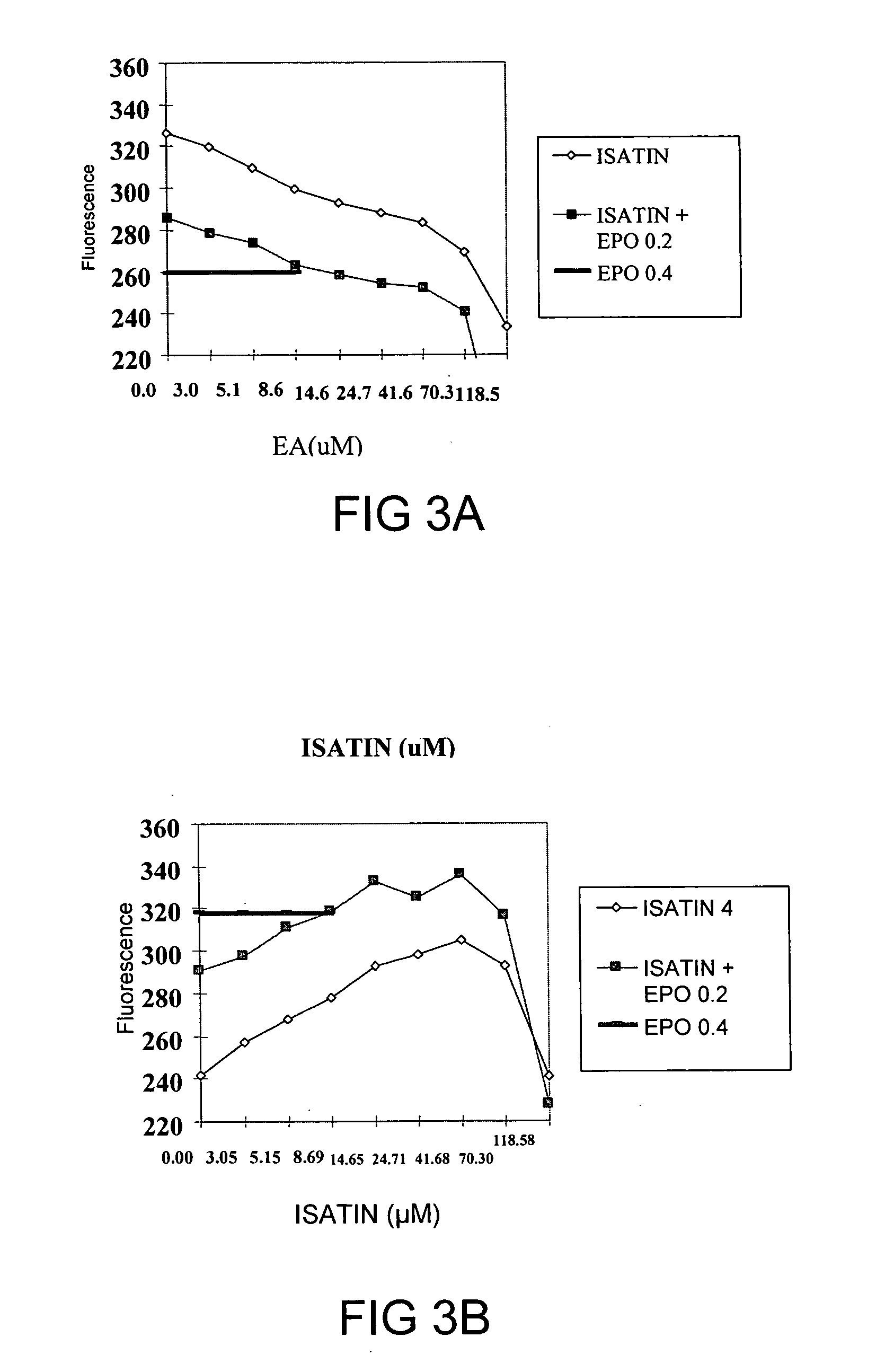
Use of Isatin in stimulating red blood cell production and treatment of anemia
InactiveUS20060229354A1Promote productionBiocideAnimal repellantsMyelosuppressive therapyDisease cause
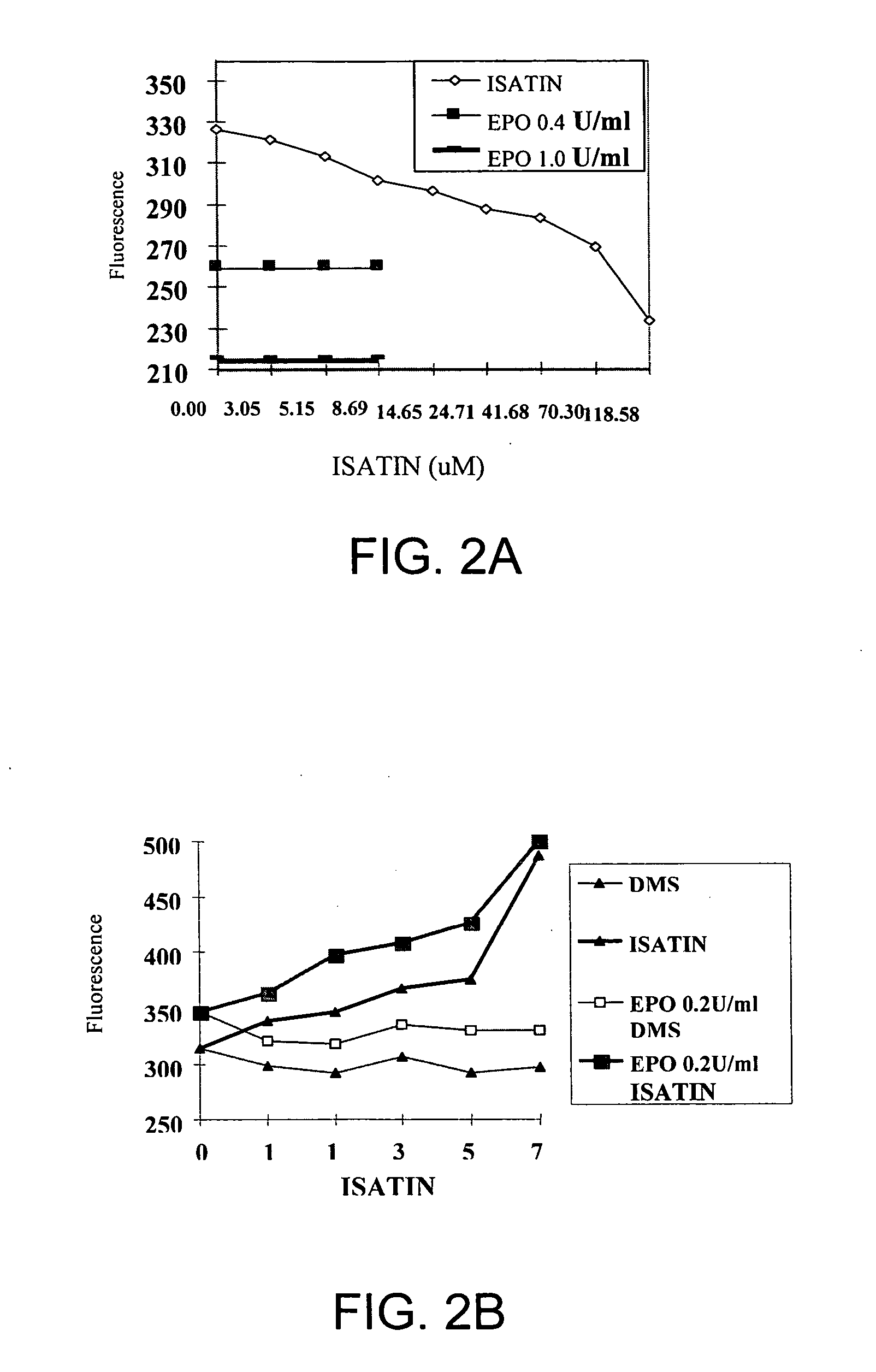
The present invention concerns small chemical compounds—Isatin and its derivatives, and a novel, safer and more convenient methods for the treatment of anemia resulting from various underlying conditions or diseases. The invention provides new methods to stimulate red cell production, and the new methods to prevent the development of anemia. The invention provides a new therapeutic approach for the treatment of chronic anemia patients, anemia patients who are receiving bone marrow suppressive therapies, HIV / AIDS patients who develop anemia upon the treatment of anti-retroviral therapies or from underlying disease itself, and anemia with other causes. The invention also provides methods for preventing anemia in many relatively healthy people such as young women who loss blood from menstrual blood.
Owner:ZEBROTECH
Heteroduplex tracking assay
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry soon after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
Owner:HEALTH RES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com