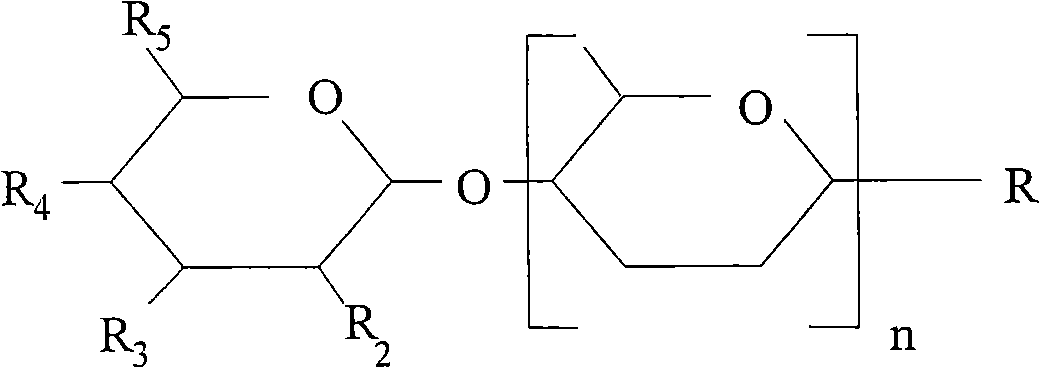
Nutritional supplement for a category of HIV patients
A kind of use, oligosaccharide technology, applied in antiviral agents, applications, metabolic diseases, etc., can solve problems such as improving nutritional status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0139] The present invention also provides a method for preparing the composition for treating and / or preventing HIV, the method comprising:
[0140] - providing a suitable amount of one or more oligosaccharides;
[0141] - formulation of the aforementioned components into a suitable food or food supplement or pharmaceutical composition.
[0142] The invention is illustrated in the following examples. The practice of the present invention will employ, unless otherwise indicated, standard conventional methods of molecular biology, pharmacology, immunology, virology, microbiology or biochemistry. These techniques are described in Sambrook and Russell (2001) Molecular Cloning: A Laboratory Manual, Third Edition, Cold Spring Harbor Laboratory Press, NY, in Volumes 1 and 2 of Ausubel et al. (1994) Current Protocols in Molecular Biology, Current Protocols, USA and Remington' s Pharmaceutical Sciences, Mack Publishing Company, Philadelphia, Pa., 17th ed. (1985), Microbiology: A Lab...
Embodiment 1
[0144] Example 1. Blocking the binding of DC-SIGN-Fc with acid oligosaccharides and GOS
[0145] Blocking DC-SIGN has been found to prevent viral translocation from dendritic cells to CD4 T cells. The inventors have surprisingly found that oligosaccharides can block DC-SIGN with varying potency. As shown in Table 1, the acid oligosaccharides (AOS) pectin hydrolyzate had the greatest potency. These results indicated that AOS could prevent the binding of Fc fragment to DC-SIGN at the lowest concentration.
[0146] Table 1. Potency of oligosaccharides for DC-SIGN binding
[0147] Oligosaccharides
I.C.50(μg / ml)
Acid Oligosaccharides (Pectin Hydrolyzate)
200
Galacto-oligosaccharides (trans-galacto-oligosaccharides)
600
FOS (Inulin HP)
>1000
[0148] Materials and methods:
[0149] The serially diluted oligosaccharide preparations were coated on ELISA plates. Binding of DC-SIGN-Fc was determined by ELISA using anti-DC...
Embodiment 2
[0150] Example 2. Nutritional Bar Composition
[0151] Raw Material Code g / day Protein g / 100g
[0152] Colostrum SR 20.00 15.00 27.38
[0153] Borage Oil (Ropufa 25n-6) 2000342 4.00 0.00 5.48
[0154] EPA-DHA Oil (Maruha) 2001292 6.00 0.00 8.21
[0155] Galacto-oligosaccharide elixir or syrup 2001189 15.38 0.00 21.06
[0156] Inulin (Raftiline HP) 2001190 0.79 0.00 1.08
[0157] Acid oligosaccharides (pectin hydrolyzate) SR 8.54 0.11 11.69
[0158] N-acetyl-cysteine SR 1.83 1.34 2.50
[0159] Fructosestroop JJ 13.20 0.00 18.07
[0160] Glycerin JJ 3.30 0.00 4.52
[0161] kcal En% per day
[0162] Energy Protein 66 26.9
[0163] Energy carbohydrates 82 33.4
[0164] Energy Fat 97 39.7
[0165] 245
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com