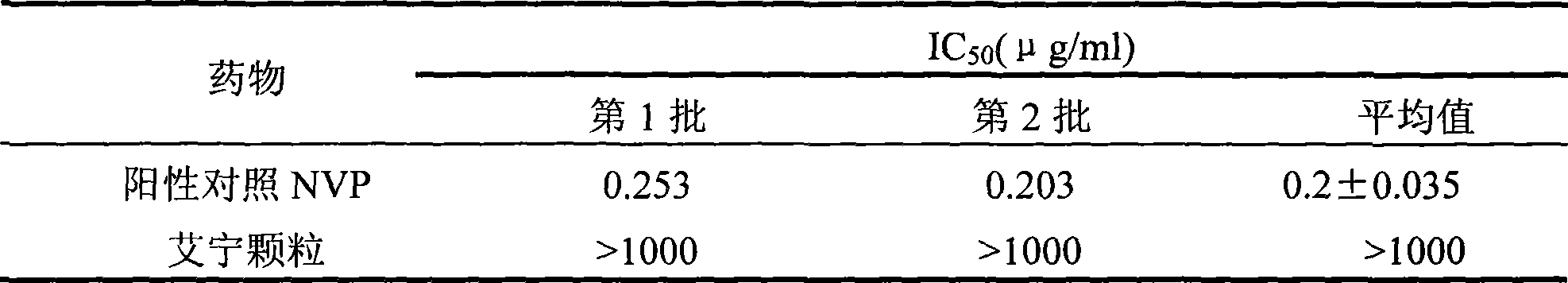
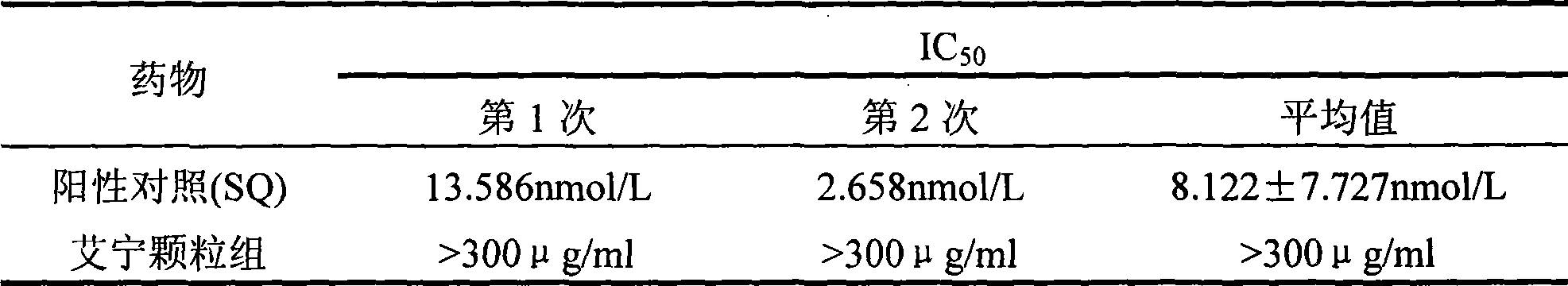
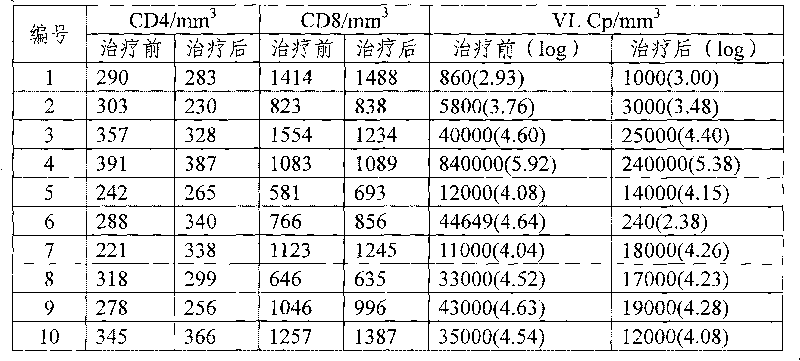
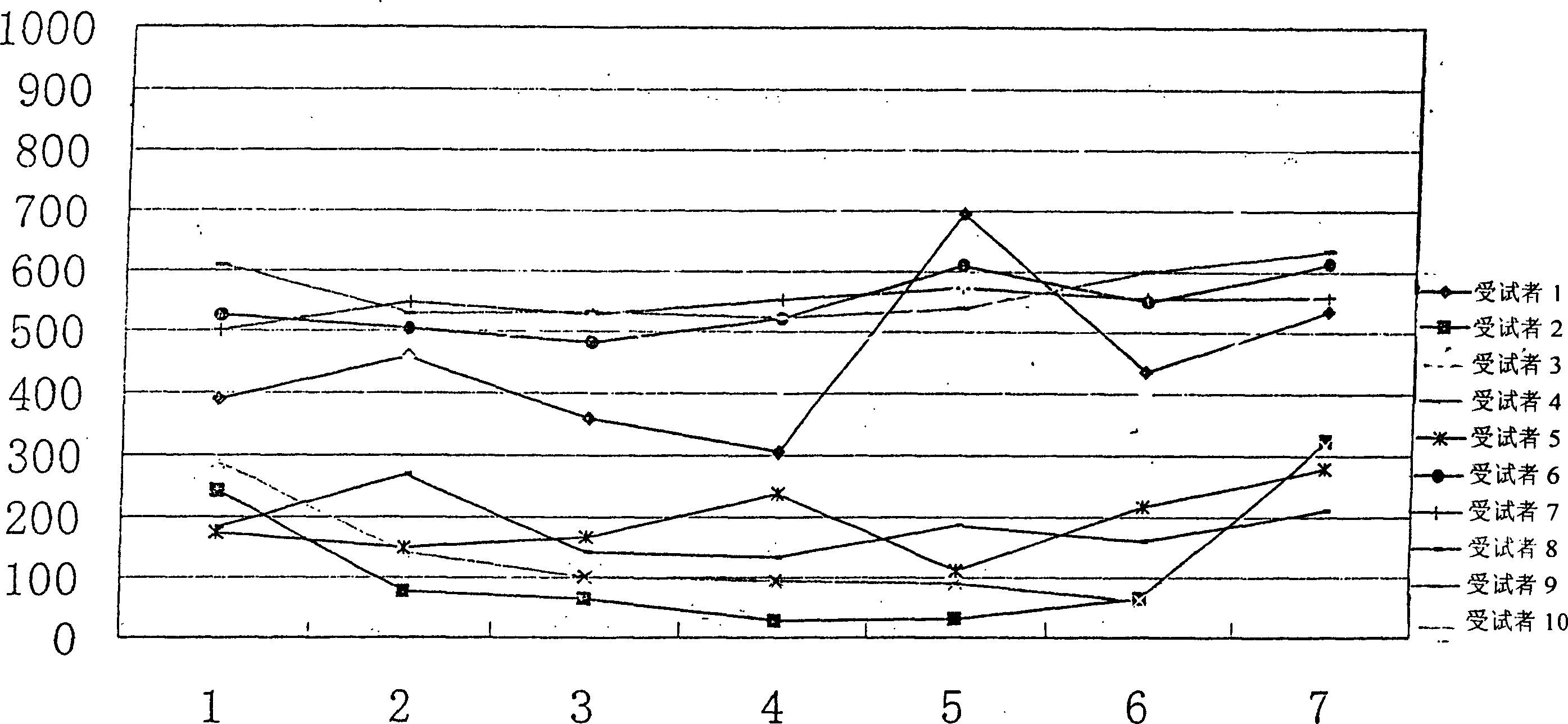
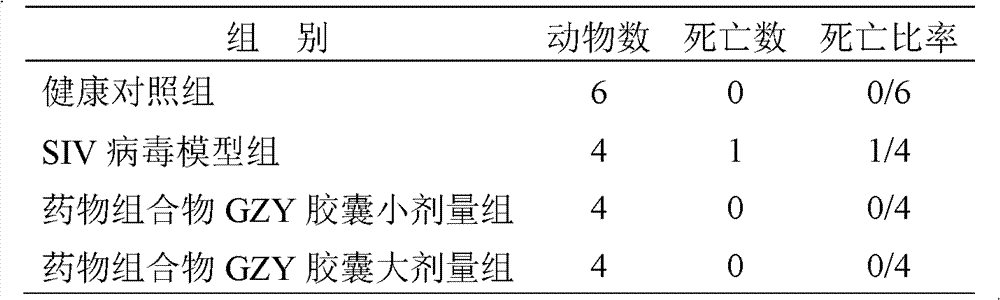
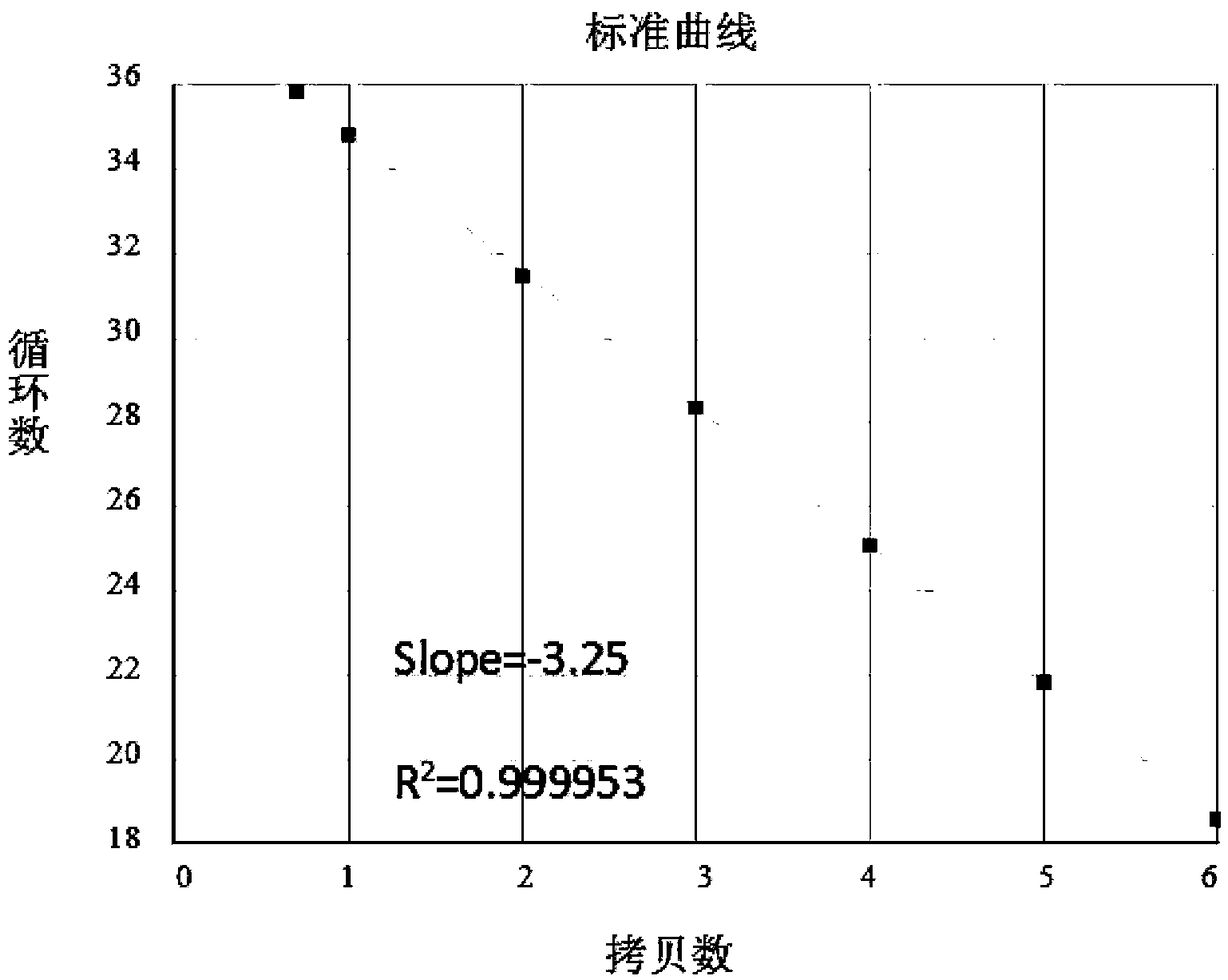
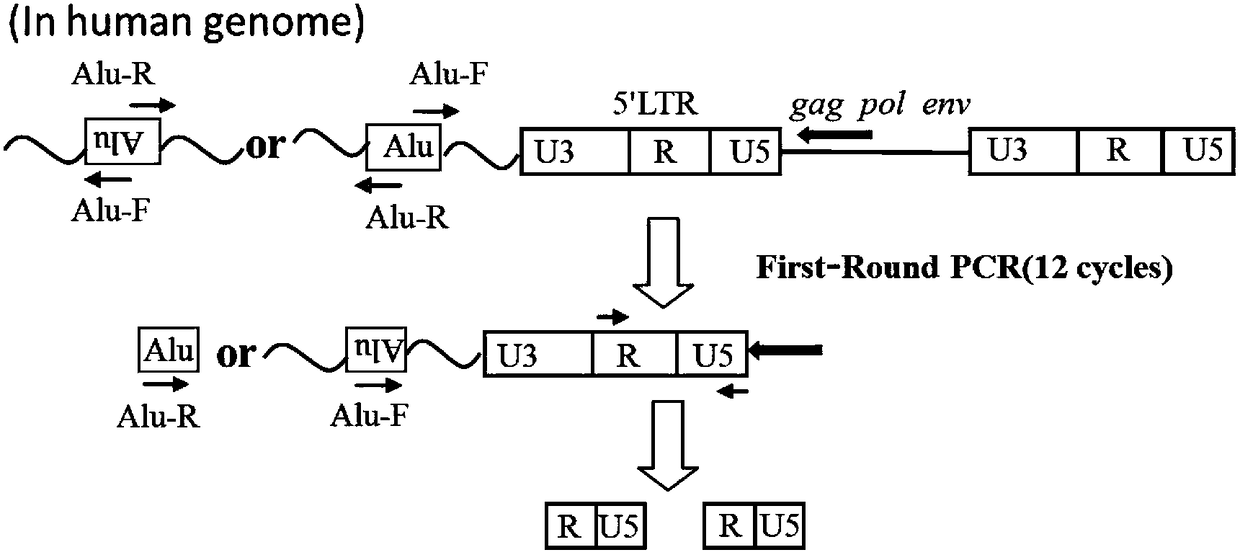
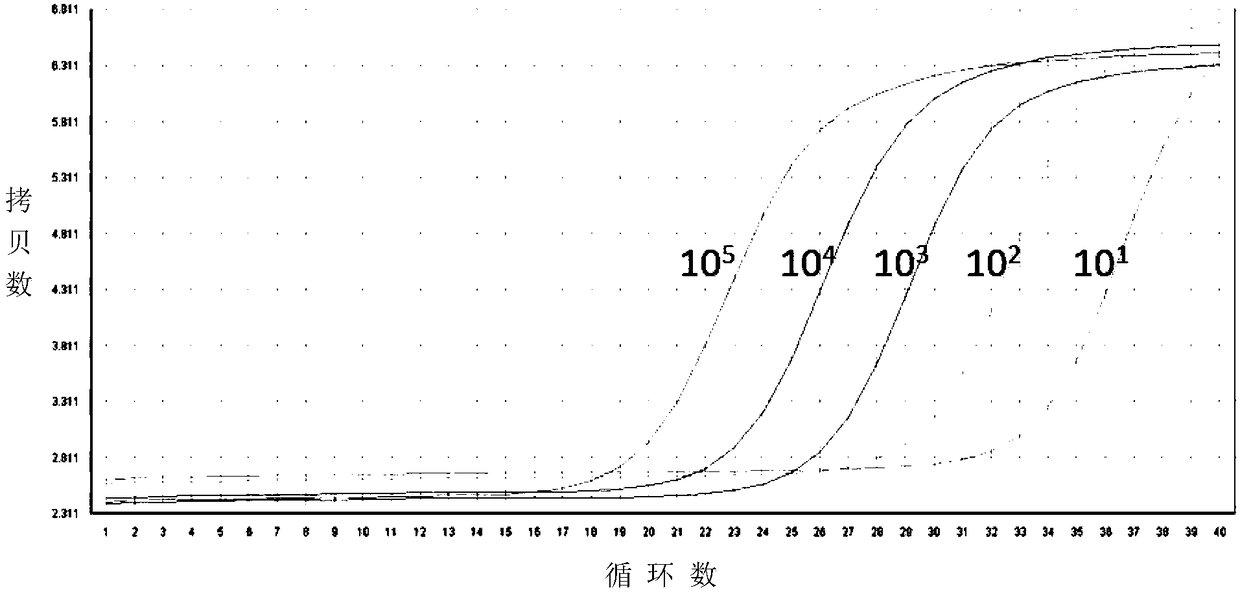
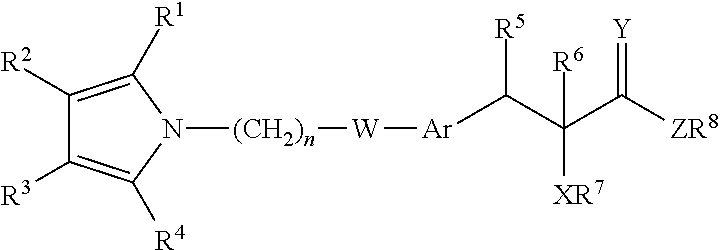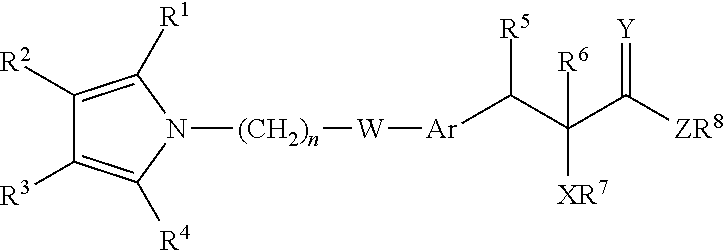Patents
Literature
105 results about "Hiv patients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment for lipodystrophy
ActiveUS20130338209A1Prevention and treatment and alleviation of symptomPrevention alleviationBiocideOrganic chemistryLipoatrophyReverse transcriptase
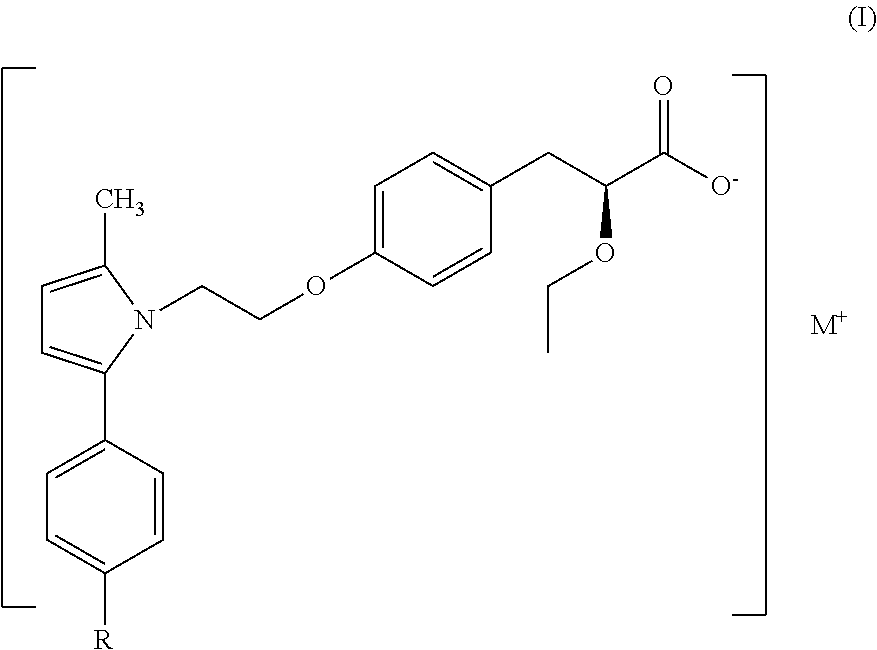
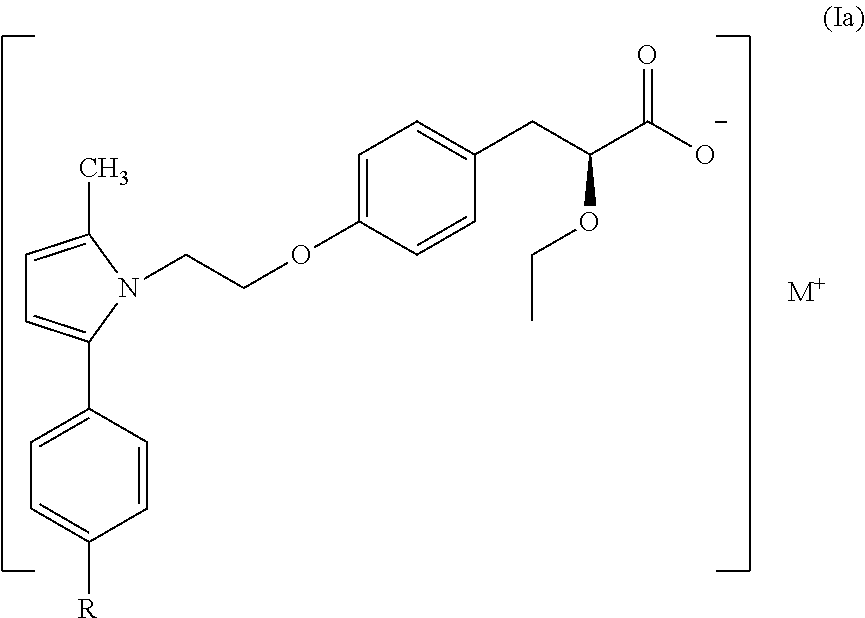
The present invention provides a therapeutic compound of formula (I) and their pharmaceutically acceptable salts for the prevention and treatment of lipodystrophy caused because of HIV infection or combination therapy of HIV-1 protease inhibitors (Pis) and / or reverse transcriptase inhibitors (nRTIs) by neutralizing lipohypertrophy, lipoatrophy and metabolic abnormalities in HIV patient.
Owner:CADILA HEALTHCARE LTD
Nutritional supplement for a category of HIV patients
InactiveUS20090082249A1Relieve symptomsReduce infectionBiocidePeptide/protein ingredientsPhysiologyAntiretroviral therapy
The present invention relates to a nutritional product for HIV patients that are not on Highly Active Antiretroviral Therapy. More specifically the invention relates to a nutritional composition comprising oligosaccharides. This invention also relates to the manufacture of a nutritional supplement for use in HIV patients.
Owner:NUTRICIA
Human immunodeficiency virus affinity adsorption column, and preparation method and uses thereof
InactiveCN102631891ARich sourcesQuality improvementIon-exchange process apparatusOther blood circulation devicesMicrosphereHuman immunodeficiency
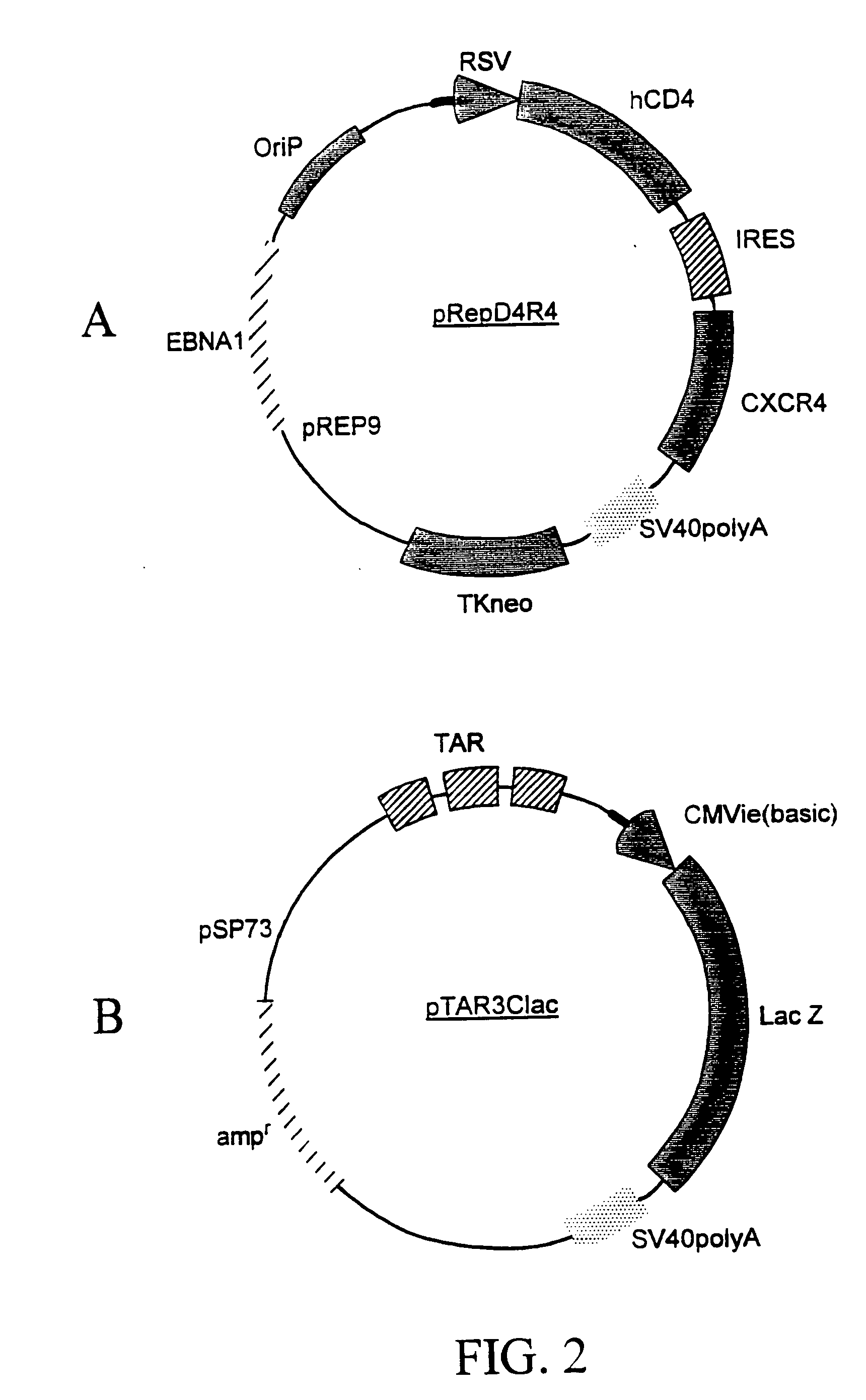
The invention discloses a human immunodeficiency virus (HIV) affinity adsorption column, comprising a column body and at least one activated affinity microsphere located in the column body, wherein the activated affinity microsphere is connected with a human immunodeficiency virus affinity protein, and the affinity protein can be bound with human immunodeficiency virus. The affinity protein comprises a main receptor CD4 molecule, a gp120 antibody, an auxiliary receptor CXC chemokine receptor 4 (CXCR-4) and a CC chemokine receptor 5 (CCR-5). The affinity microsphere may be a glass microsphere with the size of 1mm, a chitosan crosslinking microsphere with the diameter more than 500 microns, or a gluosan microsphere. The human immunodeficiency virus affinity adsorption column disclosed by the invention is applicable for eliminating the human immunodeficiency virus in the blood of HIV patients, and relieving and treating the immunodeficiency syndrome of HIV patients; and compared with the traditional treatment methods, the immunoadsorption column has high safety, good specificity, small toxic or side effects and good operation simplicity.
Owner:WUHAN UNIV
Method of treating cancer using dithiocarbamate derivatives
InactiveUS20050096304A1Readily available easily used treatmentInhibitionHeavy metal active ingredientsBiocideAdjuvantAnticarcinogen
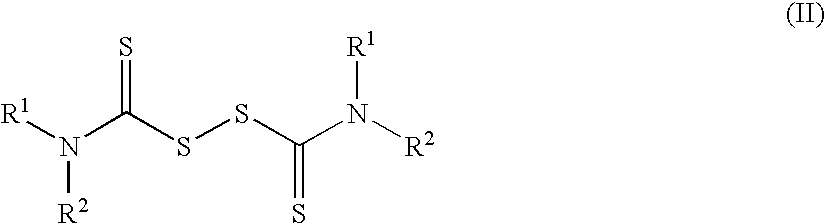
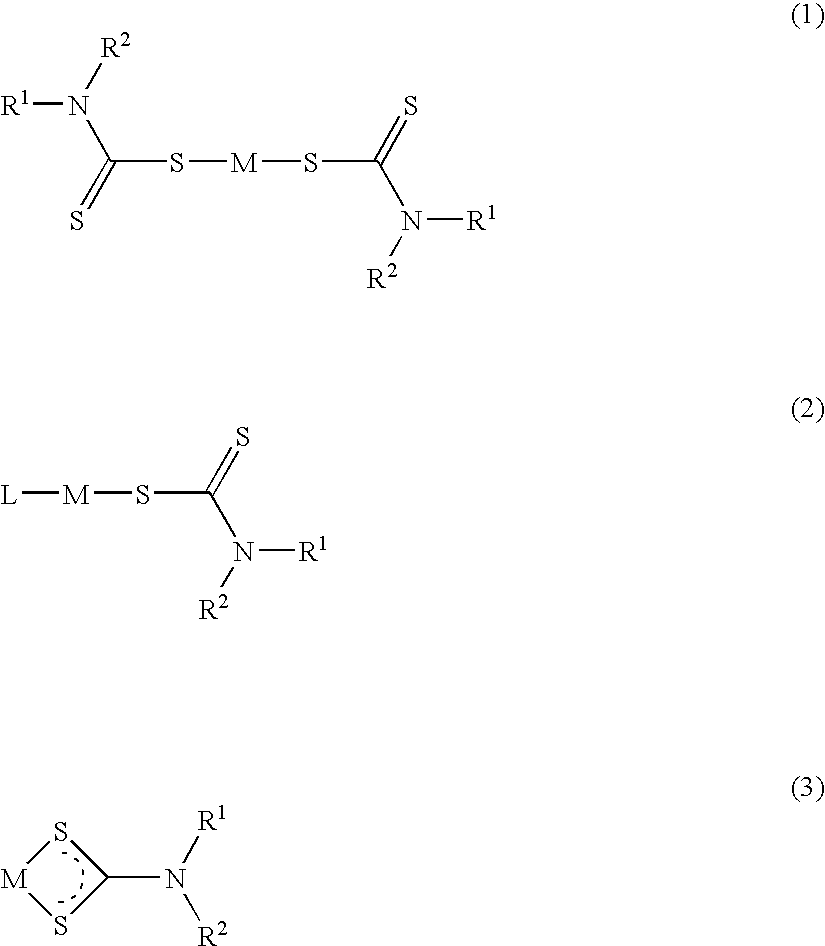
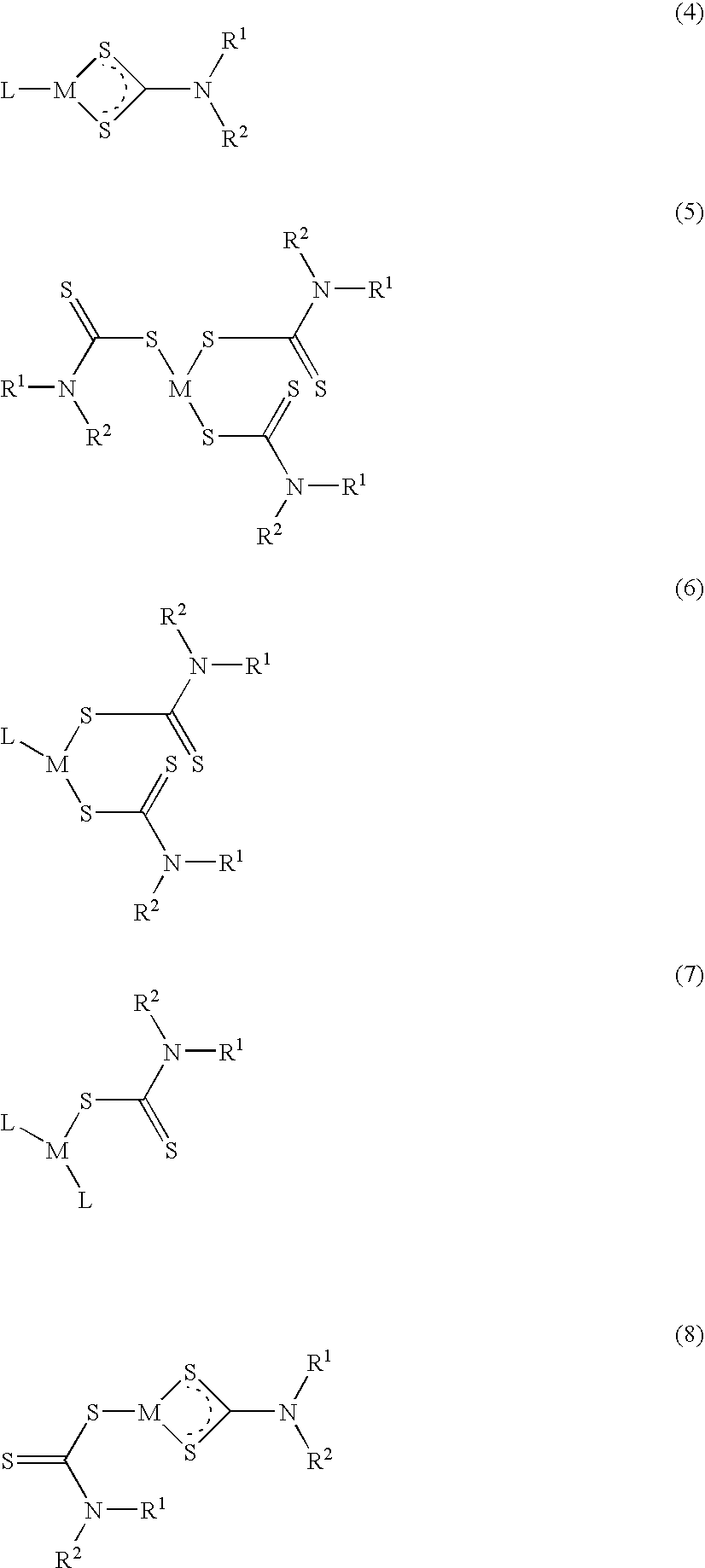
The invention encompasses neutral dithiocarbamate metal compounds and methods of treating cancer using such compounds, along with methods for sensitizing AIDS / HIV patients to anti-retroviral therapy by blocking the P-glycoprotein membrane toxin extrusion pump using such compounds. Compounds inhibit the growth of cancer cells of a variety of cell types. A method is presented for using the neutral compounds disclosed herein, amongst other uses disclosed herein, to reduce tumor growth, and to potentiate the effect of other anticancer agents. The invention also encompasses pharmaceutical compositions comprising the neutral compounds and a pharmaceutically acceptable excipient, diluent, solubilizer, solvent, adjuvant or carrier, or a mixture thereof.
Owner:AAIPHARMA SERVCIES CORP
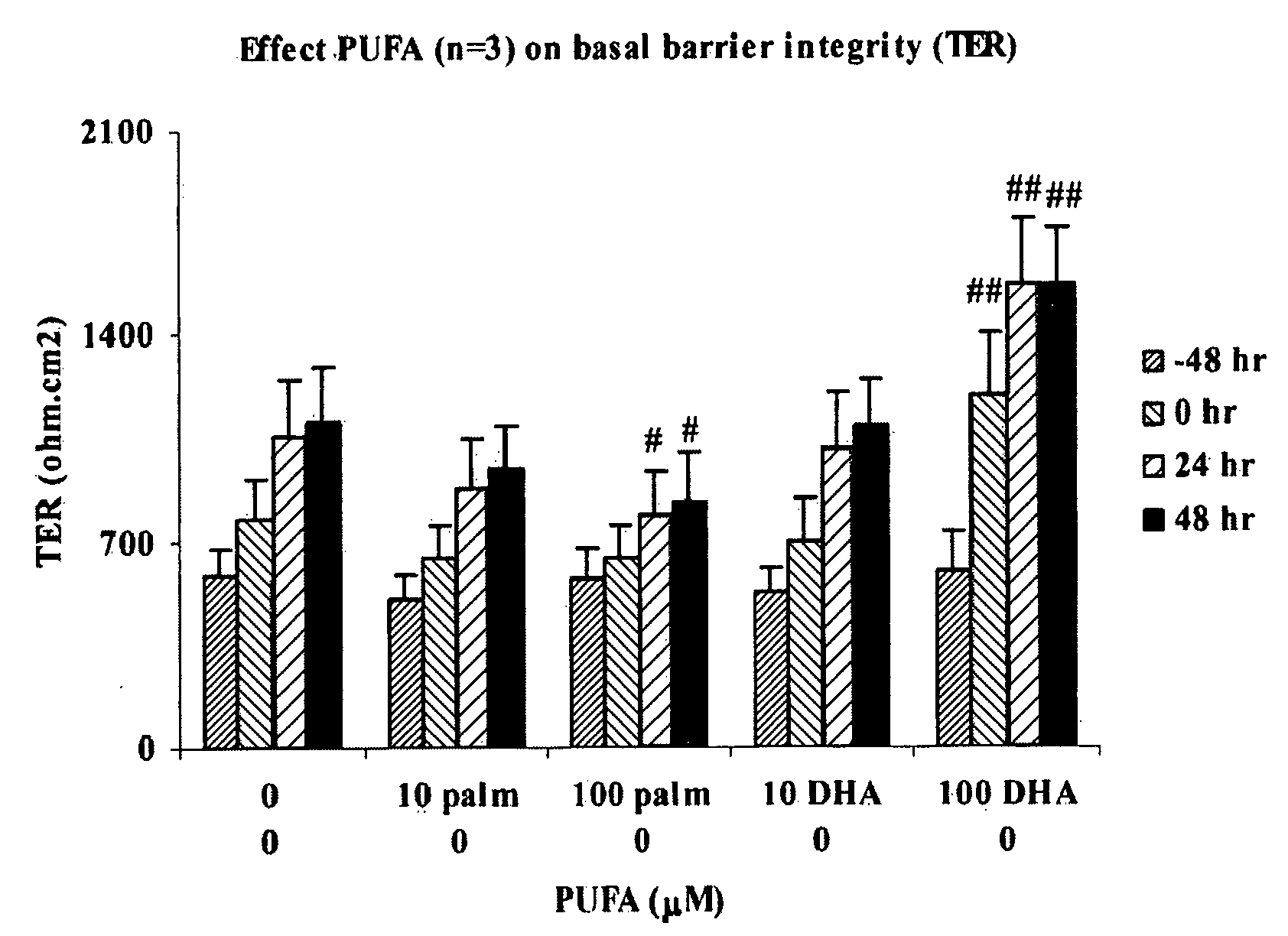
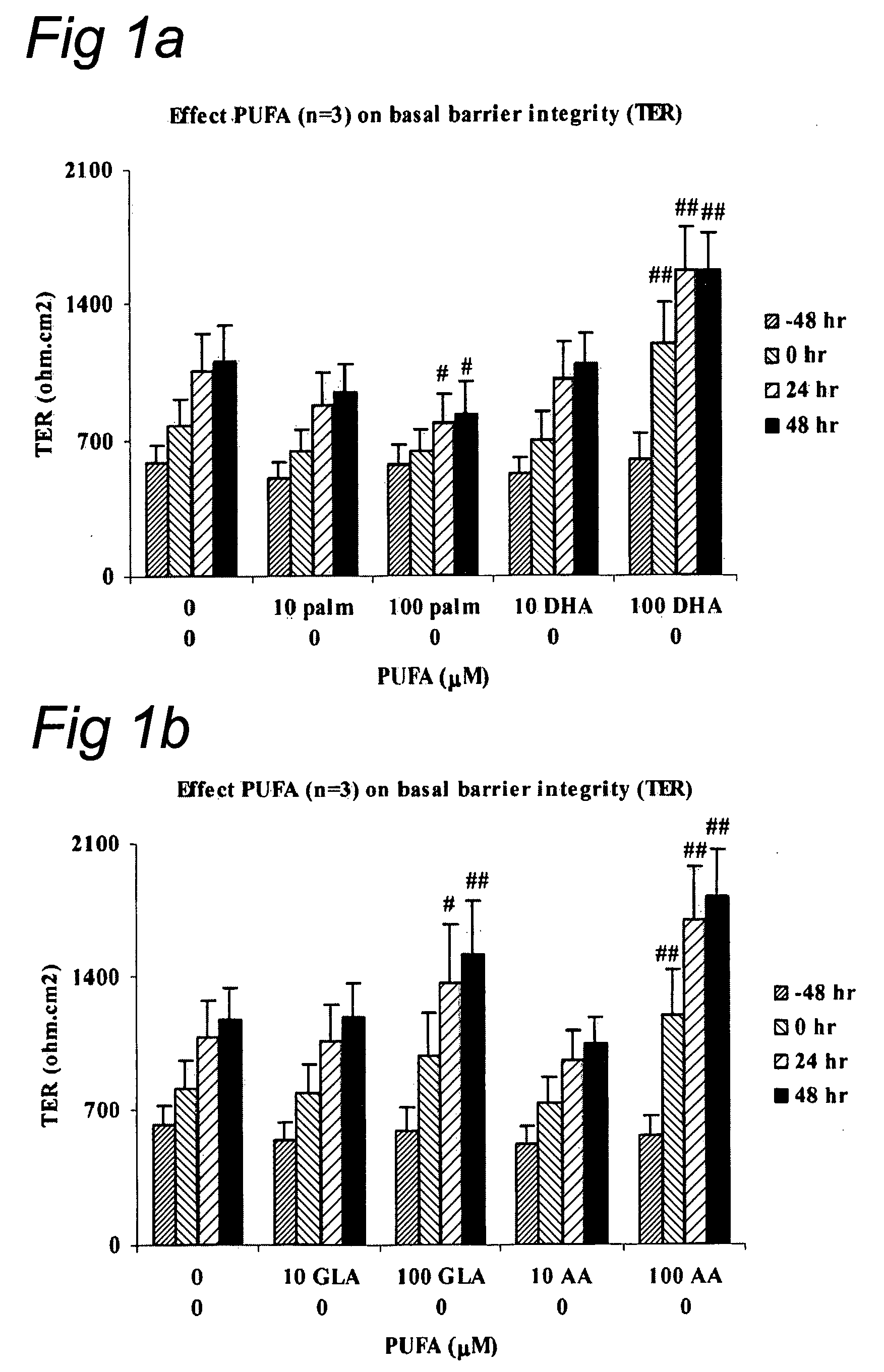
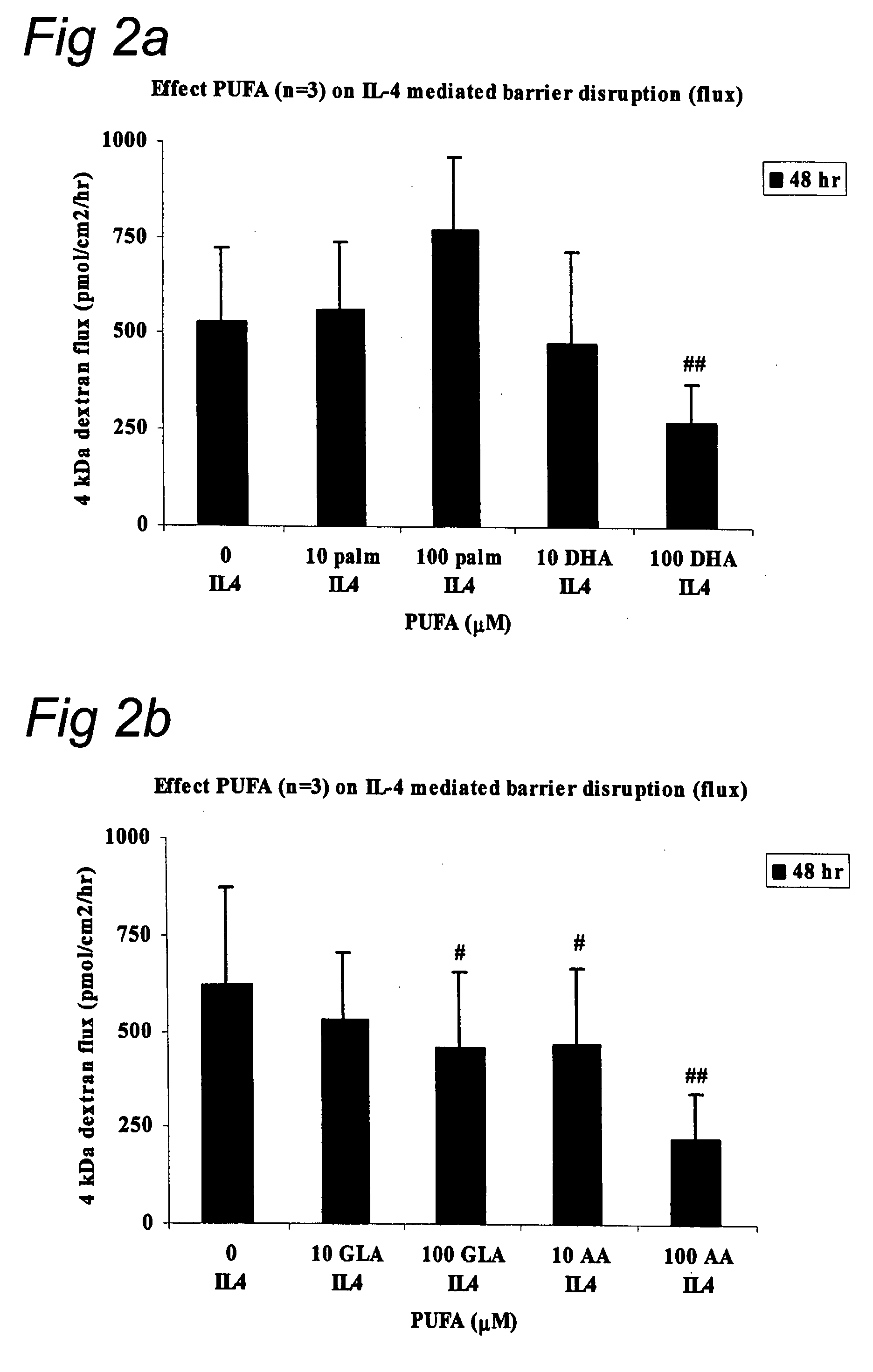
Barrier Integrity in Hiv Patients
InactiveUS20080015166A1Reduce molecular fluxImprove epithelial resistanceBiocideAntipyreticDHA - Docosahexaenoic acidEPA - Eicosapentaenoic acid
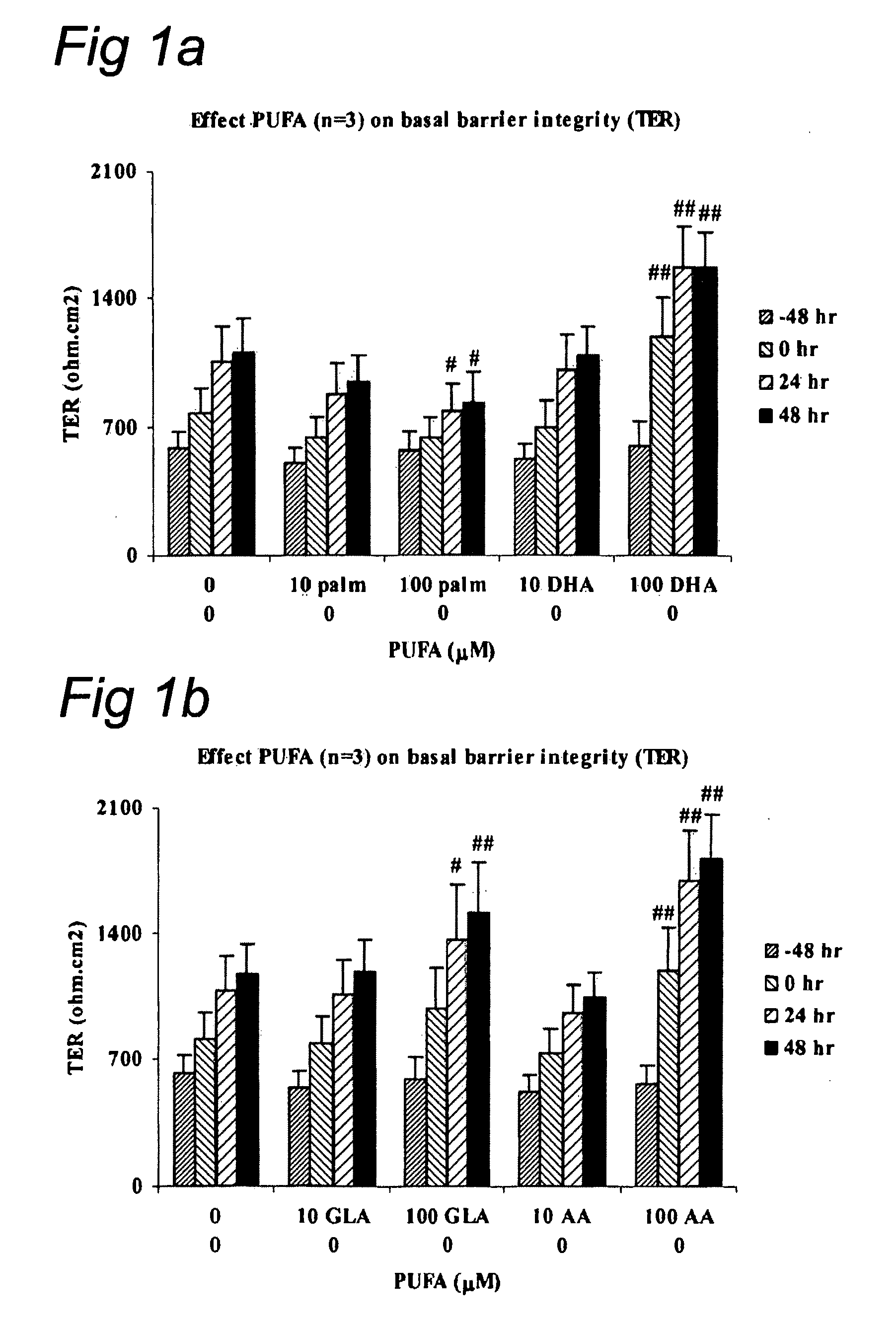
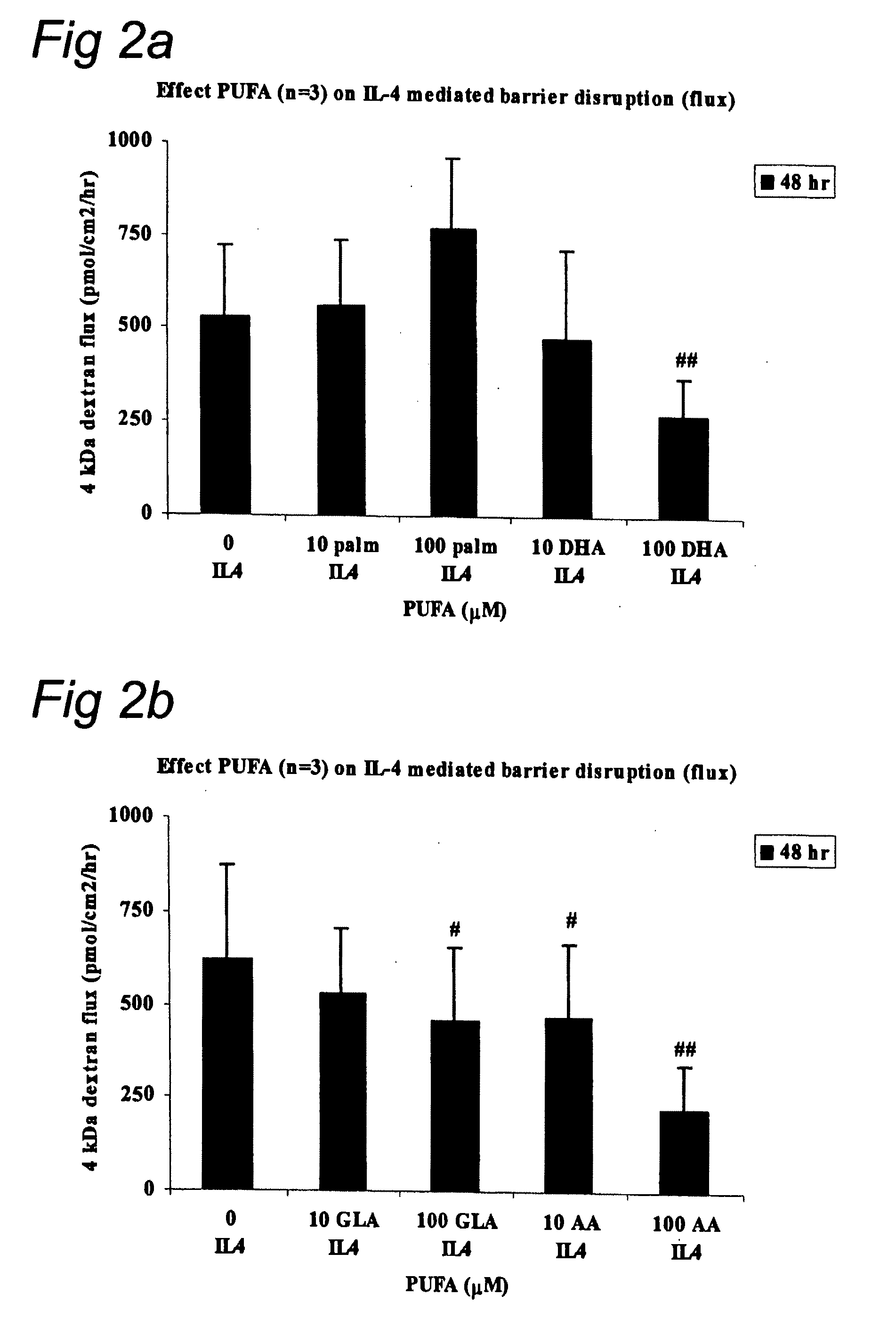
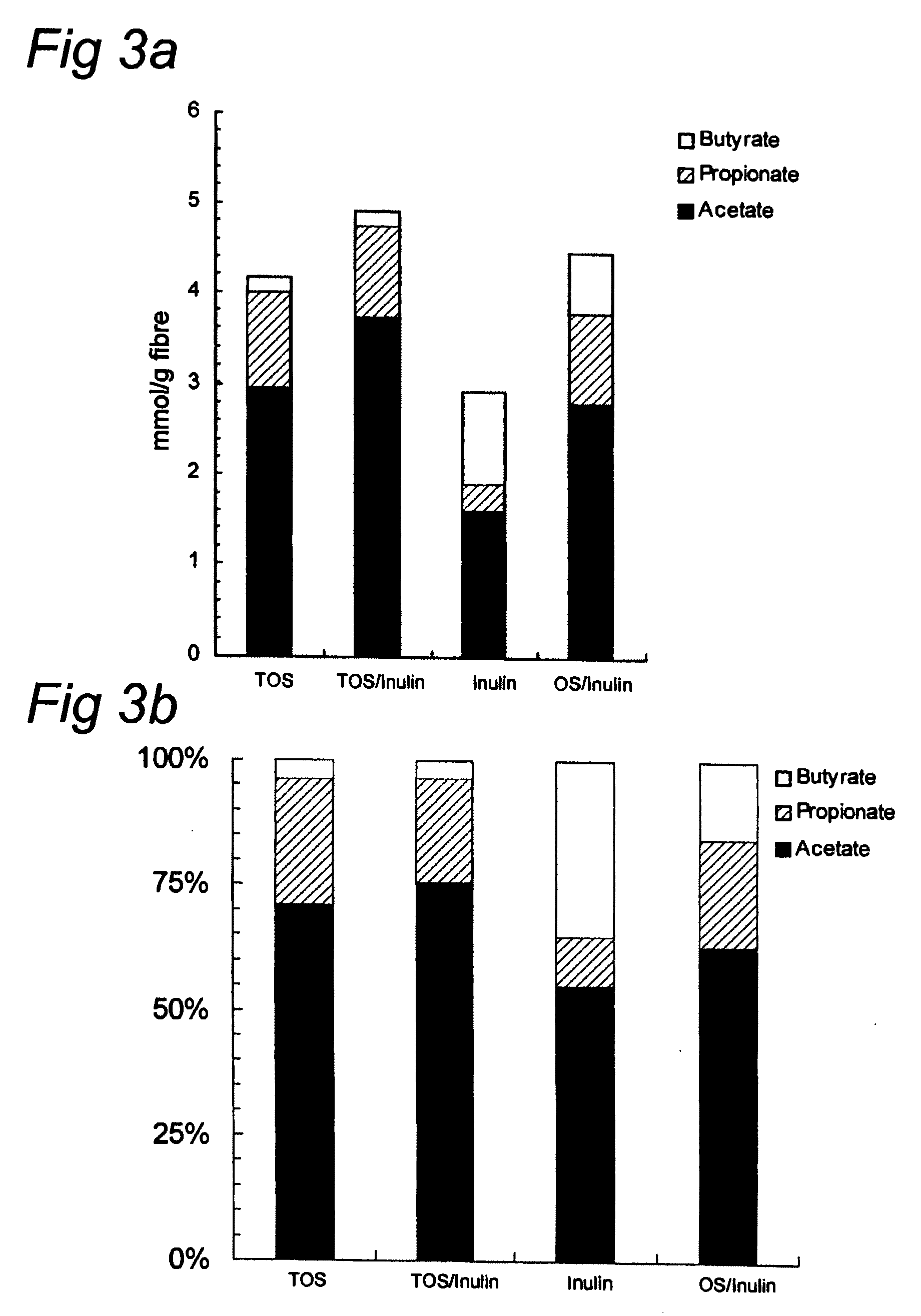
The invention concerns a method for stimulating intestinal barrier integrity in a patient infected with HIV by administering to said patient composition comprising: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and arachidonic acid (ARA), and at least two distinct oligosaccharides.
Owner:NUTRICIA
Combination of medication of containing kurarinone and glycyrrhetic acid, and application
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Modified erythrocytes and uses thereof
InactiveUS20070082392A1Genetically modified cellsArtificial cell constructsRed cell acanthocytosisHIV receptor
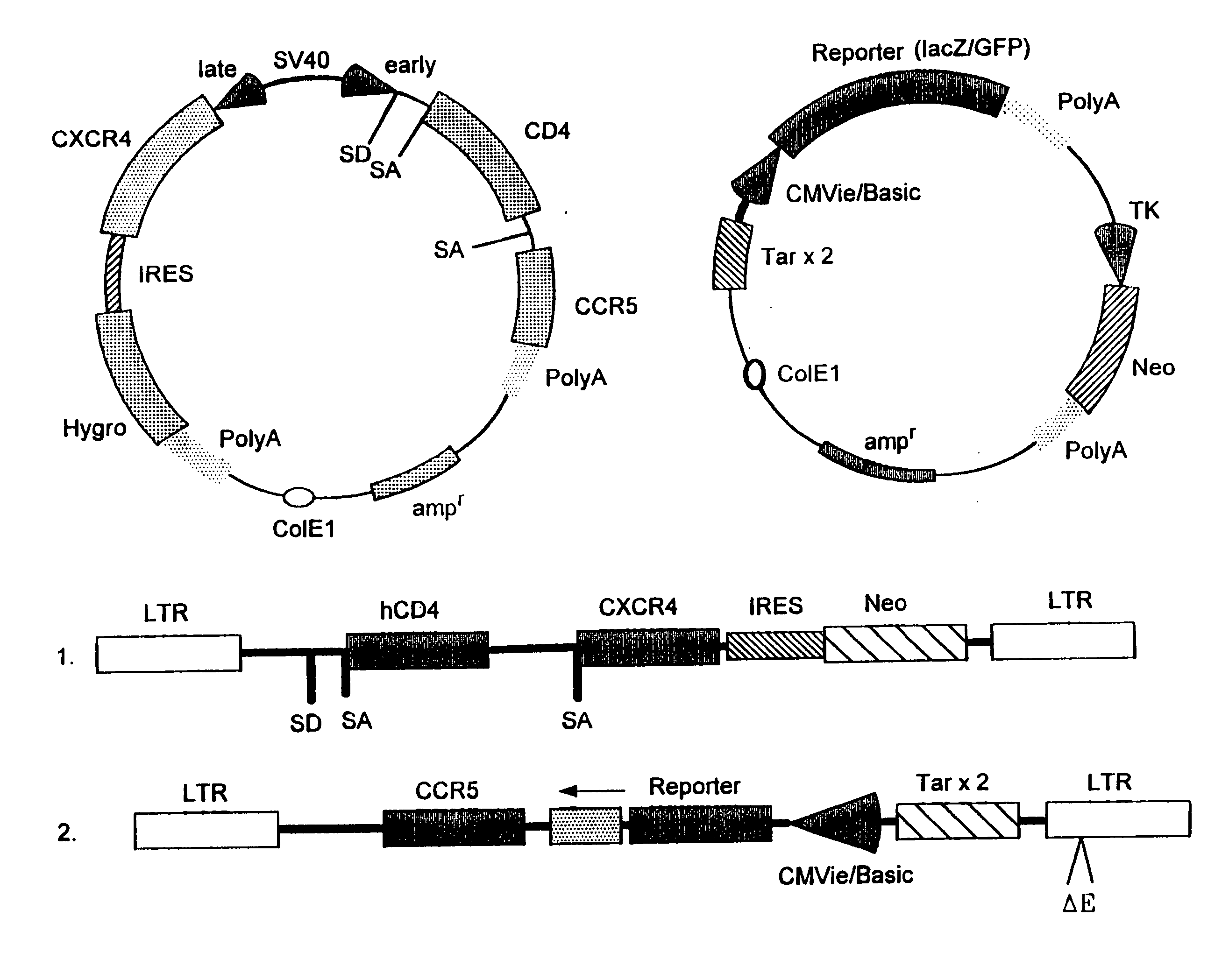
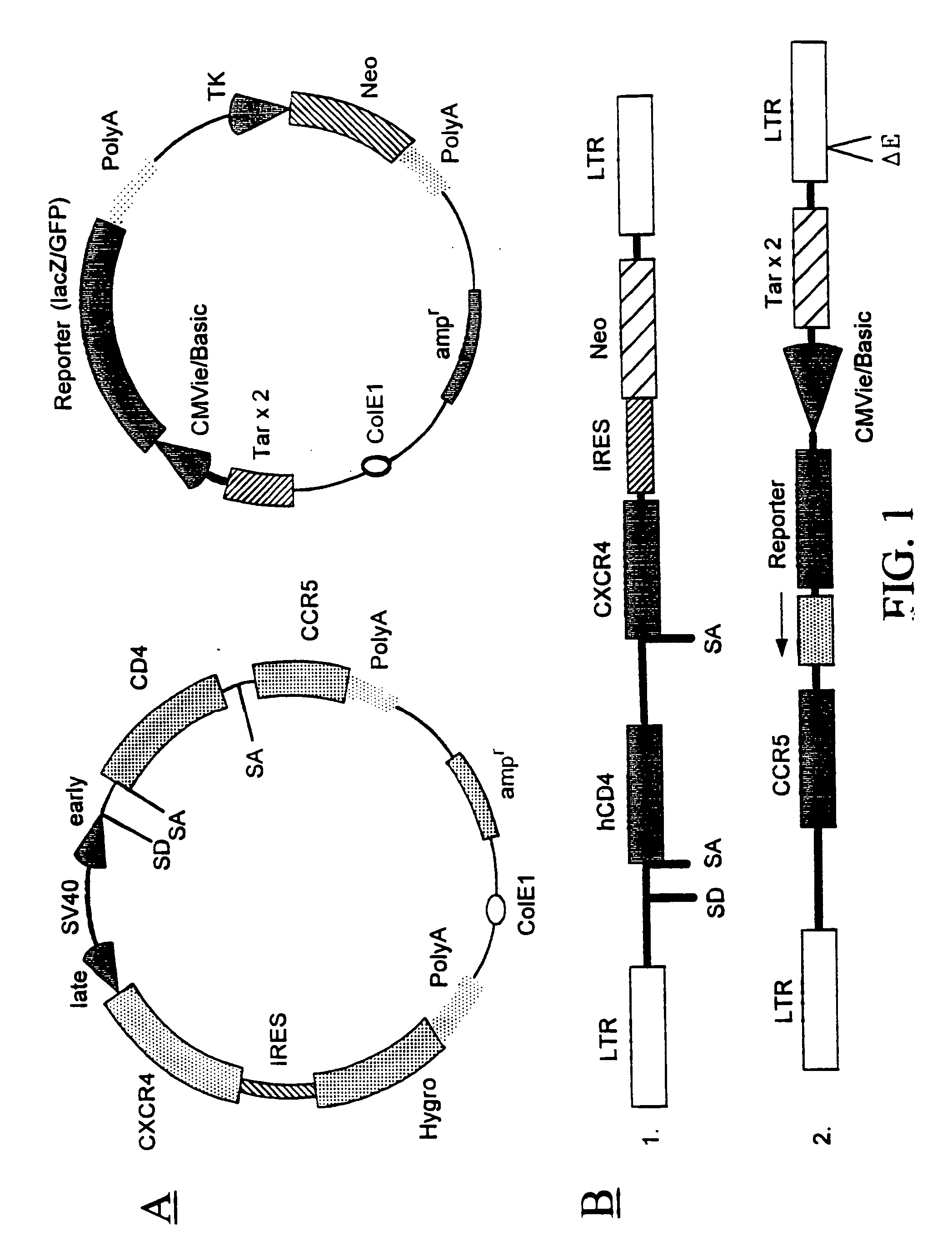
The present invention provides modified erythrocytes which comprise viral receptor proteins capable of mediating entry of respective viruses into the modified erythrocytes. The present invention also provides methods of using the modified erythrocytes for the treatment or prevention of viral infections. In one embodiment, the modified erythrocytes of the present invention comprise CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is either degraded or deactivated within the erythrocytes, or destroyed by erythrophagocytosis.
Owner:GLASER LAWRENCE F
Method for testing drug susceptibility of HIV
InactiveUS20040106136A1Convenient and cost-effective and ultra sensitiveImprove throughputMicrobiological testing/measurementDisease diagnosisHigh-Throughput Screening MethodsHIV receptor
Methods, compositions and kits are provided for testing susceptibility of HIV to drug treatment, such as drug resistance of HIV and inhibition of HIV replication by a drug candidate. In one aspect of the invention, a method is provided for detecting drug resistance of HIV contained in a sample from an individual infected with HIV. In one embodiment, the method employs an indicator cell line which over-expresses CD4 and one or more co-receptors for HIV such as CXCR4 and CCR5 at high levels to render the cells susceptible to productive infection of various strains, subtypes or clades of HIV from both laboratory and clinical isolates. The methods, compositions and kits can be used for high throughput screening of HIV patient samples, anti-HIV agents, and for designing customized HIV therapy.
Owner:MUSC FOUND FOR RES DEV
Barrier integrity in HIV patients
InactiveUS20100167982A1Reducing mucosal productionImproving intestinal integrityBiocidePeptide/protein ingredientsDocosahexaenoic acidEicosapentaenoic acid
The invention concerns a method for stimulating intestinal barrier integrity in a patient infected with HIV by administering to said patient composition comprising: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and arachidonic acid (ARA), and at least two distinct oligosaccharides.
Owner:NV NUTRICIA
Modified erythrocytes and uses thereof
InactiveUS7462485B2Genetically modified cellsArtificial cell constructsViral ReceptorRed cell acanthocytosis
The present invention provides modified erythrocytes which comprise viral receptor proteins capable of mediating entry of respective viruses into the modified erythrocytes. The present invention also provides methods of using the modified erythrocytes for the treatment or prevention of viral infections. In one embodiment, the modified erythrocytes of the present invention comprise CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is either degraded or deactivated within the erythrocytes, or destroyed by erythrophagocytosis.
Owner:GLASER LAWRENCE F
Methods and compositions for the treatment of lipodystrophy
InactiveUS20070161551A1Reduce the possibilityBiocidePeptide/protein ingredientsGrowth hormoneCombination therapy
The present invention is directed to methods and compositions for the treatment of lipodystrophy. The methods contemplate treatment of lipodystrophy in both HIV and non-HIV patients. More specifically, the methods are directed to a combination therapy that employs growth hormone and statins to effect treatment of lipodystrophy.
Owner:LAB SERONO SA
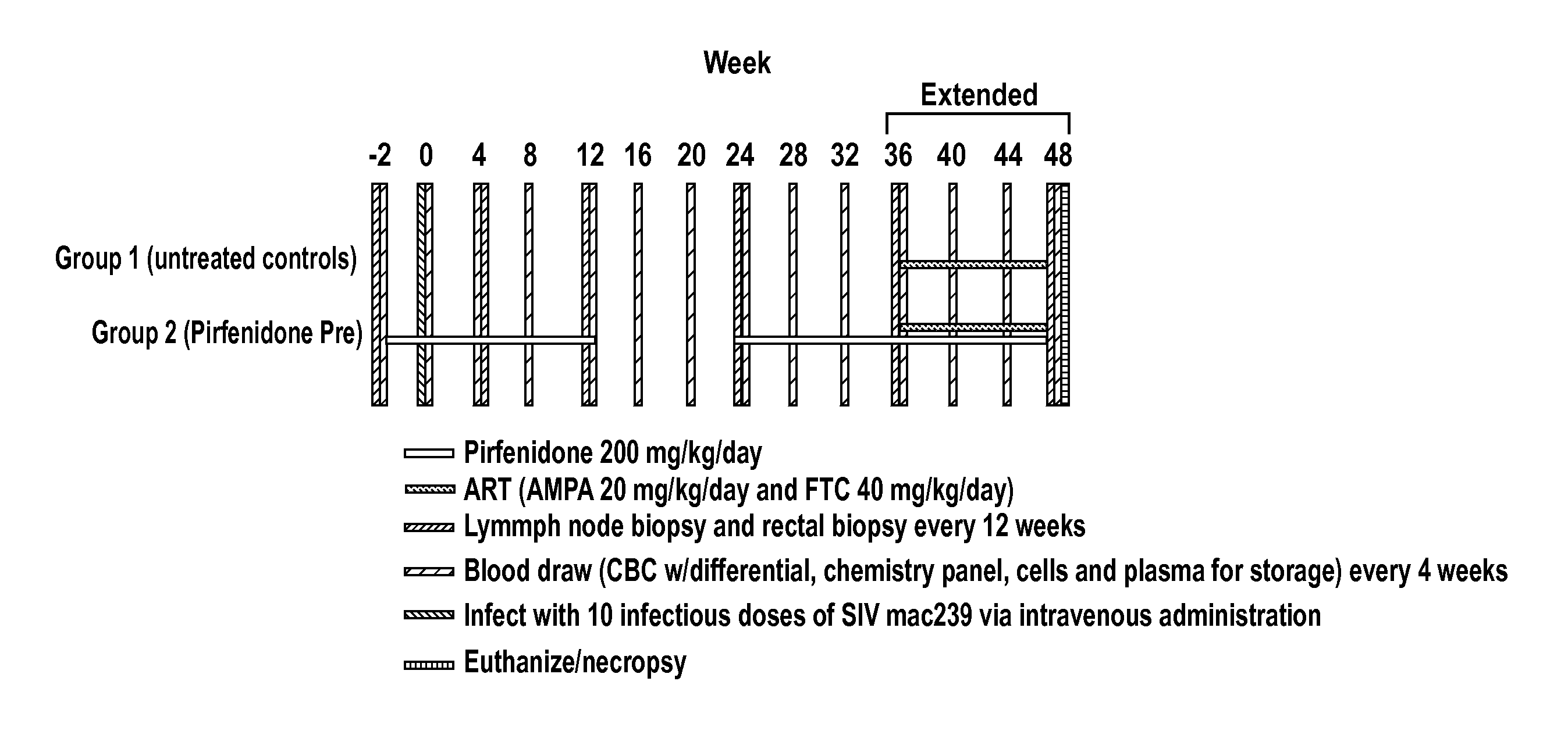
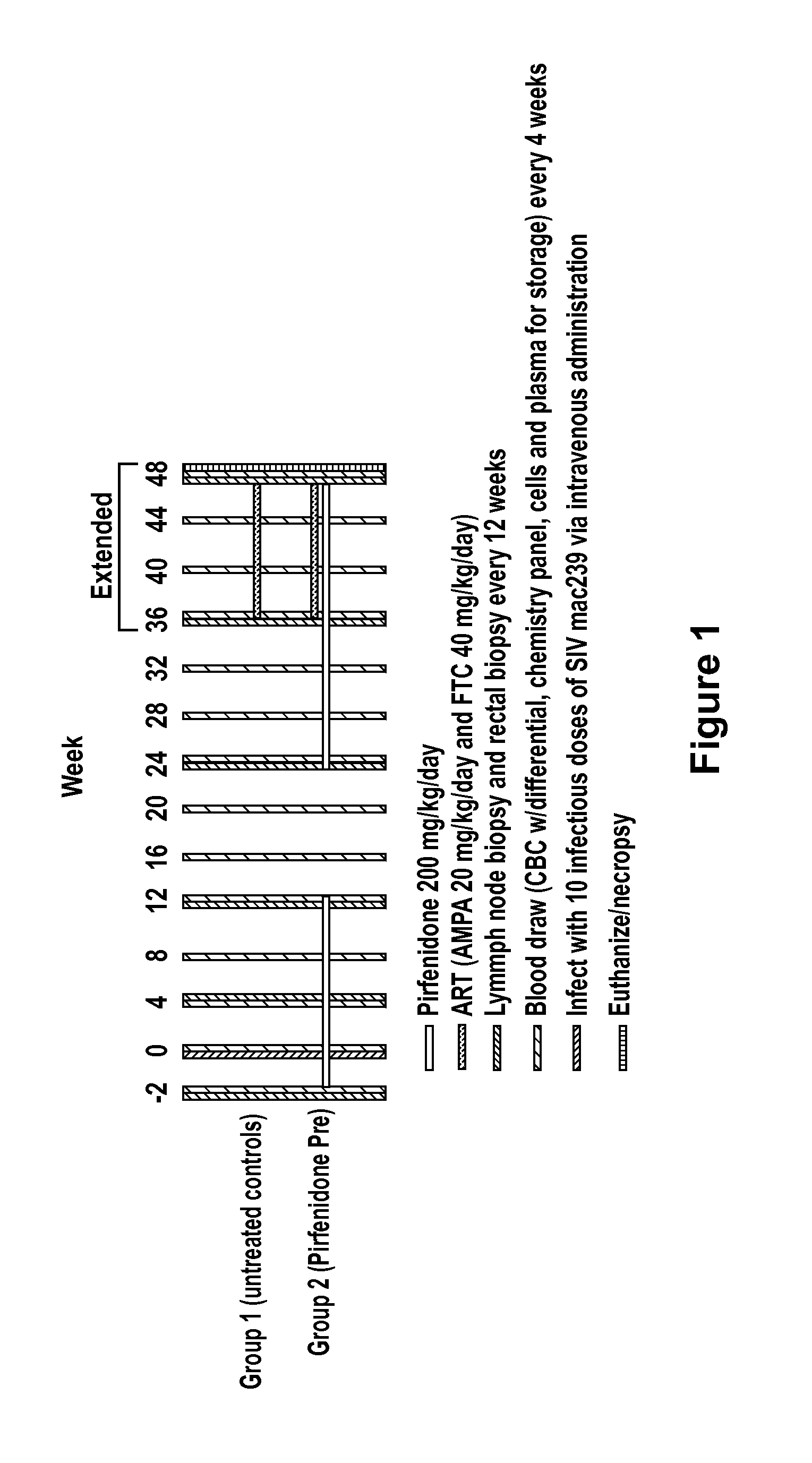
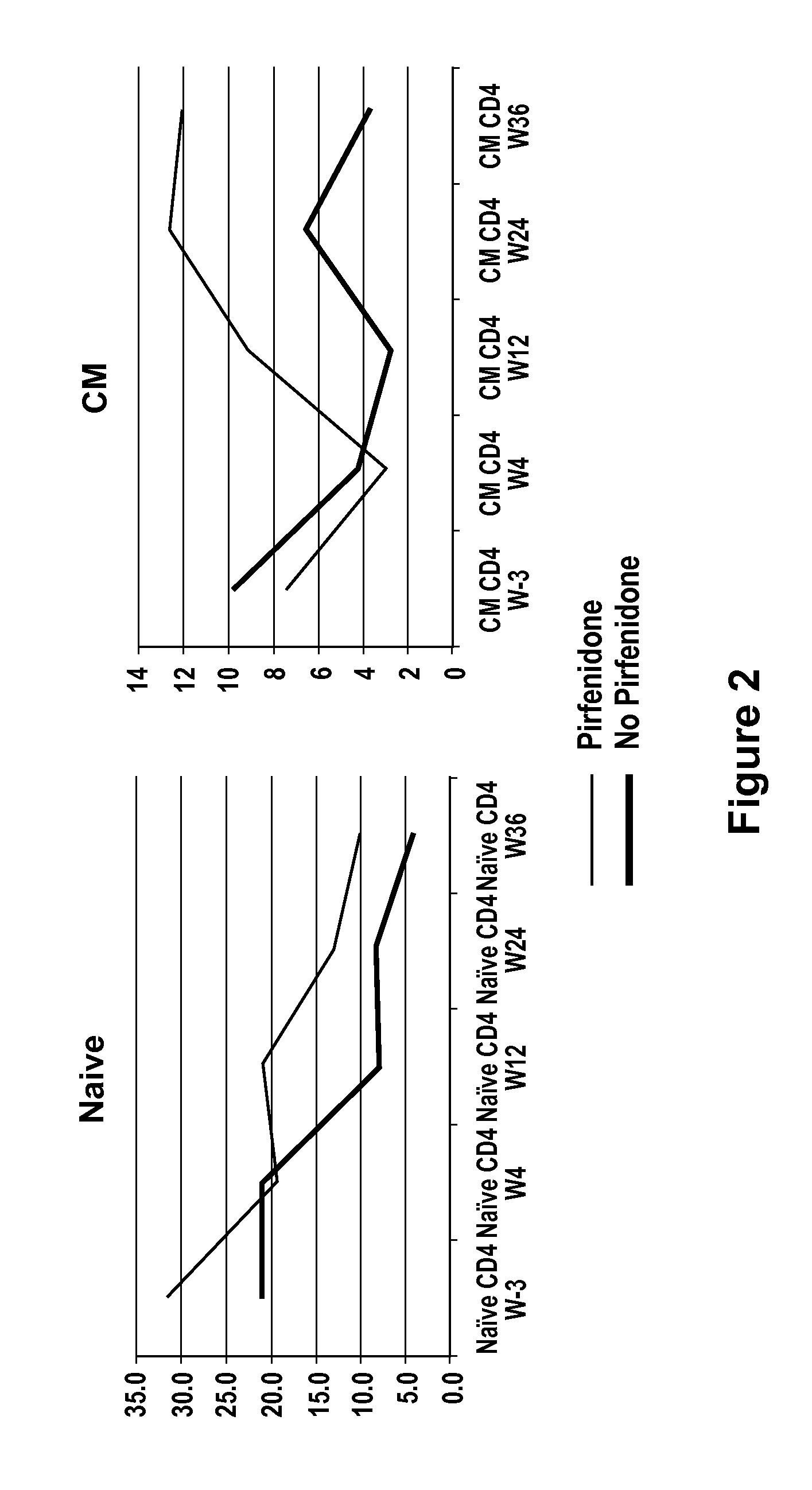
Methods of treating HIV patients with Anti-fibrotics
InactiveUS20120014917A1Good effectReduce areaBiocideOrganic chemistryImmunodeficiency virusAnti fibrotic
The invention relates to methods of treating patients infected with human immunodeficiency virus (HIV) with a therapeutic that has anti-fibrotic effects, for example, pirfenidone and analogs thereof.
Owner:INTERMUNE INC
Modified and fusion enhanced erythrocytes, cells and uses thereof
InactiveUS20110091973A1Short half-lifeGenetically modified cellsBlood/immune system cellsADAMTS ProteinsChemokine receptor CCR5
Modified fusion enhanced erythrocytes (or other cell types and synthetic cells) including human viral receptor proteins, human viral coreceptor proteins and viral derived proteins capable of mediating entry of respective viruses into the modified erythrocytes, cells or pseudo-cells and the method of using the fusion enhanced modified erythrocytes, cells or pseudo-cells for the treatment or prevention of viral infections. The fusion enhanced modified erythrocytes comprises CD4 and at least one HIV coreceptor, such as CXCR4 or CCR5 and as well, at least one of cholesterol rafts, fusin, actin, a viral derived protein such as fusion peptide derived from HIV GP120 or HIV GP41 or a shorter protein derived from a long viral protein, such as a portion of HIV derived GP120, or HIV GP41 such as the 23 N-terminal peptide of the HIV-1 gp 41 protein (AVGIGALFLGFLGAAGSTMGARS) called FP23 (Fusion Peptide). These viral-fusion enhanced cells may also be electrostatic charge enhanced through further additions named in this invention. The modified erythrocytes, when administered to an HIV patient, bind to the plasma virus and induce the injection of the HIV ribonucleoprotein complex into the cells. The entrapped viral content is sequestered within said cell for at least the period of time that the cell maintains its outer membrane integrity. The virus is thereafter either degraded or deactivated within the erythrocytes, cells or pseudo-cells, or destroyed by erythrophagocytosis.
Owner:GLASER LARRY F
Traditional Chinese medicine composition and preparation method and use thereof
InactiveCN101450173AEnhance immune functionHas anti-HIV effectAntinoxious agentsAntiviralsLycium barbarum fruitOphiopogon japonicus
The invention discloses a traditional Chinese medicine composition for preventing and treating AIDS and preparation method thereof. The medicament composition is mainly made from the following raw material medicaments of weight proportions of: astragalus 15-20 parts, Ginseng 7-12 parts, Chinese angelica 8-13 parts, wolfberry 9-14 parts, Chinese magnoliavine 7-12 parts, Tuber of Dwarf Lilyturf 7-12 parts, trichosanthes root 8-13 parts, Poria cocos 7-12 parts, licorice 7-12 parts, radix bupleuri 3-8 parts and cimicifuga foetida 3-8 parts. The traditional Chinese medicine composition of the invention has the functions of supplementing center, boosting qi, nourishing blood and nourishing yin; it can not only resist AIDS virus, but also increase the immunization function of patient substantially, and can eliminate the common symptoms of AIDS patients.
Owner:HUNAN YANDI BIOLOGICAL ENG
Chinese herbal composition for curing HIV and preparation method thereof
InactiveCN101700321ANormal respiratory functionAbundant resourcesAnthropod material medical ingredientsHydroxy compound active ingredientsCD4 LymphocyteTherapeutic effect
The invention relates to a Chinese herbal composition for curing HIV and a preparation method thereof. The Chinese herba composition is prepared by the following raw medicinal materials in parts by weight: 300-400 parts of ginseng, 200-300 parts of pseudo-ginseng, 120-220 parts of radices trichosanthis, 120-220 parts of indigo naturalis, 10-25 parts of cornu bubali, 120-220 parts of Chinese violet, 120-220 parts of earthworm, 100-200 parts of cutch, 10-25 parts of borneol and 120-220 parts of liquorice. The Chinese herba composition in the invention can increase CD4 lymphocyte counting, has certain inhibiting function on HIV virus, has better effect on symptoms such as acratia, low heat, emaciation, cough, slow wit and the like of HIV patients, especially has obvious effect on acratia and cough.
Owner:NANTONG AIQIKANG PHARMA TECH
Chinese medicine for treating AIDS
InactiveCN1806834AImprove the body's immunityReduce loadAntiviralsUnknown materialsTherapy HIVLycium barbarum fruit
The invention provides a Chinese medicament for treating AIDS, wherein the constituents comprise (by weight ratio): Chinese angelica root 0.6-1.5, astragalus root 1.6-2.5, wolfberry fruit 1.6-2.5, root of large-flowered skullcap 1.6-2.5, root of Chinese trichosannthes 1.6-2.5, kuh-seng 1.6-2.5, cow's gall 0.6-1.5, licorice root 1.6-2.5, rhodiola root 1.6-2.5, ligustrum japonicum 0.6-1.5, capsule of weeping forsythia 1.6-2.5, and Radix Ranunculi Ternati 1.6-2.5.
Owner:李恩
Chinese medicine for treating AIDS
InactiveCN101199749AImprove immunityImprove the immunityAntiviralsSolution deliverySide effectRadix Rehmanniae Preparata
The invention relates to a Chinese traditional preparation for treating AIDS. The medicine is prepared by the following raw materials according to certain quality proportions: Radix Trichosanthis, Radix Astragali, Rabdosia Rubescens, Herba Houttuyniae, Radix Paeoniae Rubra, Herba Solani Nigri, Indigo Naturalis, Radix Ginseng, Radix Codonopsis, Fructus Ligustri Lucidi, Semen Cuscutae, Rhizoma Polygonati, Fructus Psoraleae, Rhizoma Coptidis, Cortex Moutan, Ganoderma Lucidumseu Sinensis, Poria, Radix Sanguisorbae, Radix Rehmanniae Preparata, Fructus Aurantii, Radix Achyranthis Bidentatae, Radix Salviae Miltiorrhizae, Radix Angelicae Sinensis, Fructus Gleditsiae Abnormalis and Radix Glycyrrhizae. The raw materials are balanced according to the quality proportions and then are prepared into granules, powder, pills, oral liquid or tablets according to the conventional Chinese traditional medicine preparation method, better into granules or oral liquid. The invention can evidently improve the immunity function and resistance of the AIDS patients and can effectively improve the clinical physical symptoms, with accurate curative effect, few side effects and low cost for treatment.
Owner:宋世昌
Specificity detection primers of Aids related intestinal tract Megamonas funiformis translocation and application
InactiveCN105950780AShift quicklyEasy to moveMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyTreatment strategy
The invention discloses specificity detection primers of Aids related intestinal tract Megamonas funiformis translocation and application. The sequence of an upstream primer is Meg-F5'-AGTTCCTTCTCTTCGGAGAAC-3', and the sequence of a downstream primer is Meg-R5'-TAAGGAGGTGATCCAGCC-3'; the specificity detection primers have the advantages that the detection is accurate, simple, convenient and rapid, the sensitivity is high, and the specificity is high and have excellent detectability and a wide application prospect; the technology can be widely applied to early stage monitoring before symptom appearance of Aids patient intestinal tract Megamonas funiformis flora translocation, and a scientific basis is provided for formulating the treatment strategy.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
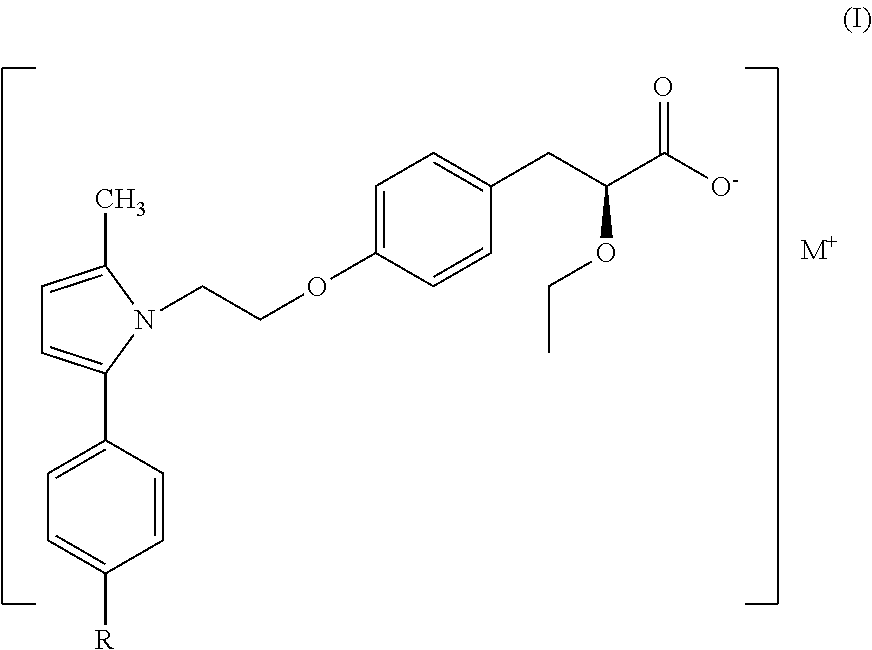
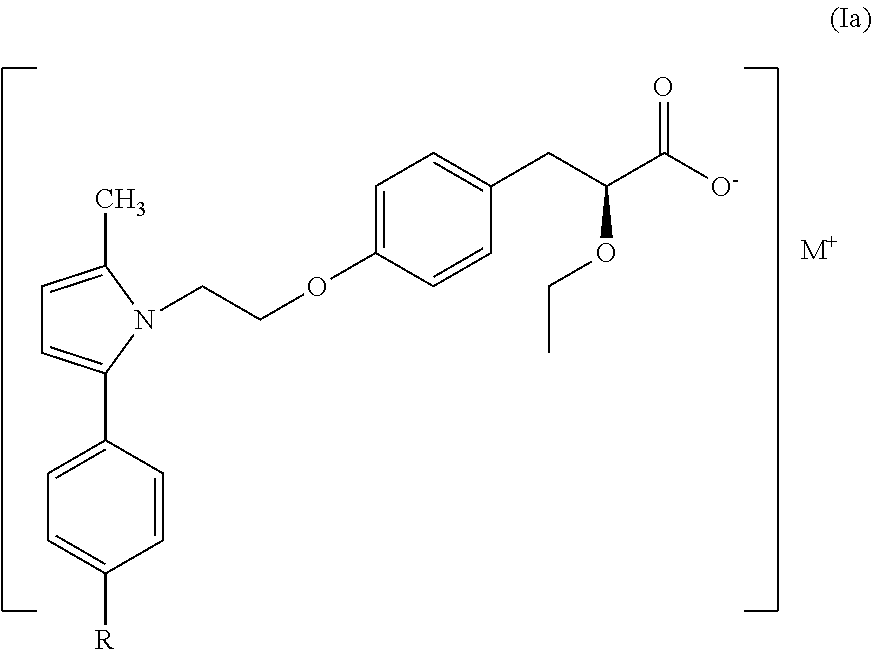
Treatment for lipodystrophy
ActiveUS20170088514A1Reduce productionHigh affinityOrganic chemistryMetabolism disorderLipoatrophyReverse transcriptase
The present invention provides a therapeutic compound of formula (I) and their pharmaceutically acceptable salts for the prevention and treatment of lipodystrophy caused because of HIV infection or combination therapy of HIV-1 protease inhibitors (PIs) and / or reverse transcriptase inhibitors (nRTIs) by neutralizing lipohypertrophy, lipoatrophy and metabolic abnormalities in HIV patient.
Owner:CADILA HEALTHCARE LTD
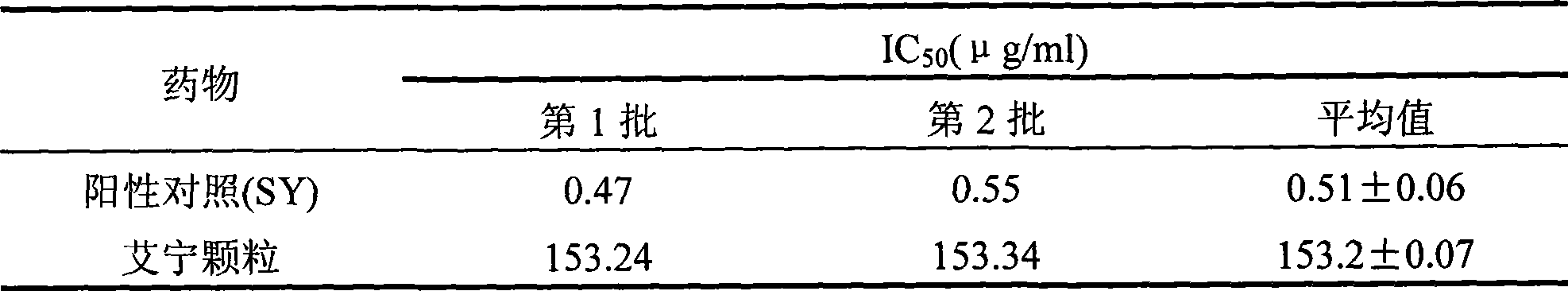
Disinfectant bactericidal Chinese medicine preparation and producing process thereof
InactiveCN1439404ARelieve painImprove treatmentUnknown materialsAntiinfectivesDisinfectantChrysanthemum Flower
Owner:朱俊
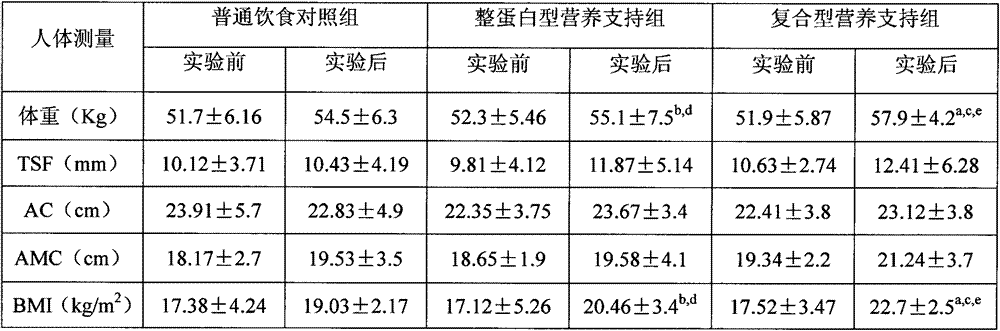
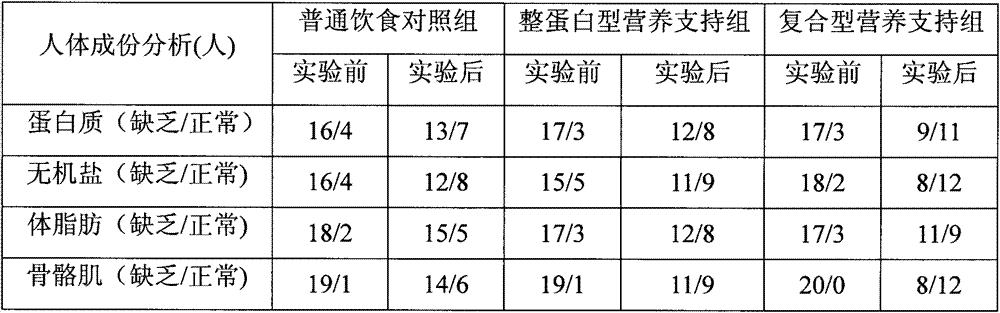
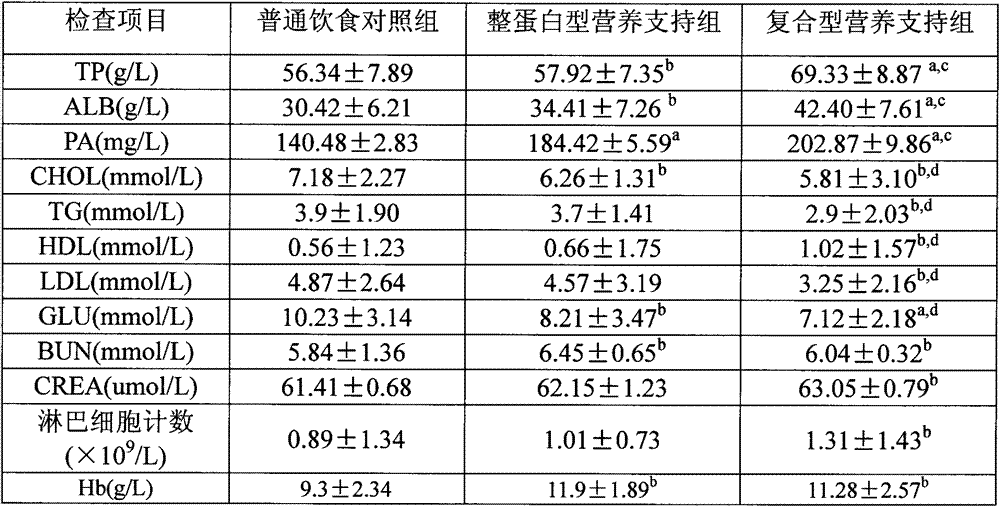
Composite enteral nutrition applicable to AIDS patients
ActiveCN103082167AProtect Nutritional StatusImprove nutritional statusFood preparationNutritional statusGut flora
The invention discloses a composite enteral nutrition applicable to AIDS patients. The composite enteral nutrition comprises nutrients such as proteins, fat, carbohydrates, vitamins, minerals and dietary fibers required by human bodies. The nutrition has a good clinical nutritional supporting effect on the AIDS patients, is beneficial to absorption of the AIDS patients and improvement of poor nutrient conditions of the patients compared with a common whole protein type nutrition, and can obviously improve the living quality of the patients; compared with a common nutrient group, the nutrition has the advantages of remarkably improving the nutrient conditions of the patients and lightening the decline of various physical measurement indexes such as weight caused by AIDS; various biochemical indexes of blood of the patients can be remarkably improved, the blood sugar and the blood fat of the patients are adjusted, and the insulin resistance and the lipid metabolism disorder of the patients are lightened; and the intestinal floras of the patients are adjusted, the intestinal barrier function of the patients is protected, diarrhea caused by the flora disorder of the patients is reduced, the immunity of the patients is enhanced, and the opportunistic infections caused by intestinal flora translocation are reduced.
Owner:昆明理工大学附属医院
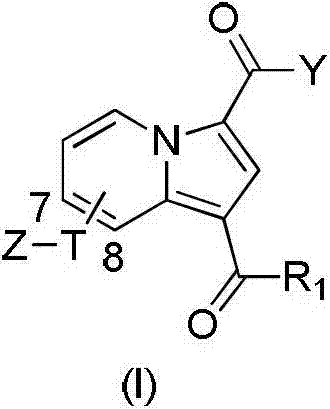
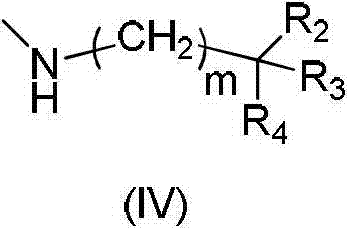
Substituted purrocoline compound and preparation method and application thereof
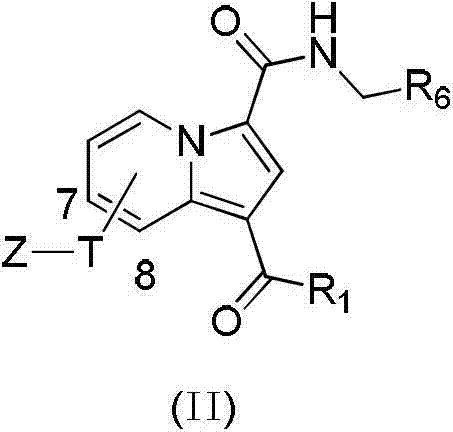
InactiveCN103087061AInhibit bindingStrong antiviral activityOrganic active ingredientsOrganic chemistryMechanism of actionNitrogen
The invention discloses a substituted purrocoline compound shown as a formula (I) and a preparation method and application thereof. The definition of each substituent group in the formula (I) is detailed in the specification. The experimental data proves that the compound has the function of inhibiting the VIF (virus infection factor) activity due to the interaction of inhibiting VIF / ElonginC and has the function of inhibiting virus replication. The compound with the acting mechanism is expected to reduce the tolerance problem in the HIV (human Immunodeficiency virus) treatment process, and a novel medicine is provided for treatment of a HIV patient.
Owner:PEKING UNIV +1
Kit and method for detecting genetic mutation of PR regions and RT regions of HIV-1 genes
ActiveCN109439800AEfficient amplificationStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesPol genesMutation detection
The invention provides a kit and method for detecting genetic mutation of PR regions and RT regions of HIV-1 genes, and particularly discloses method for detecting genetic mutation of PR regions and RT regions of HIV-1 virus pol genes in plasmas of HIV patient, a primer and a kit comprising mixed liquor of the primer. Based on a generation of sequencing platform, the method has the advantages of being multiple in mutation detection sites, high in accuracy and easy in sample acquisition.
Owner:DAAN GENE CO LTD
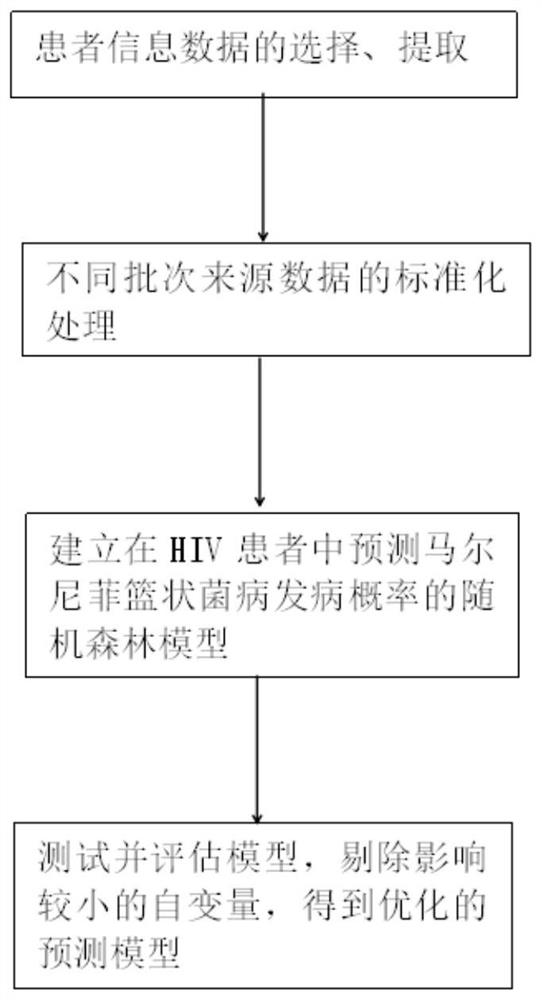
Establishment method of HIV patient talaromyces marneffei disease incidence probability prediction model
PendingCN113140325AEasy to operateAdaptableHealth-index calculationEpidemiological alert systemsDisease ratesData mining
The invention provides an establishment method of a HIV patient talaromyces marneffei disease incidence probability prediction model, and belongs to the technical field of disease prediction models. The prediction model is a model based on a random forest algorithm. The method comprises the following steps: selecting and extracting patient information data; performing standardization processing on different batches of source data; establishing a random forest model for predicting the incidence probability of the talaromyces marneffei disease in the HIV patient; the model is tested and evaluated, independent variables with small influences are removed, and an optimized prediction model is obtained. According to the method, the collected patient information data is subjected to unified standardization processing and then input into software, the relation between the independent variable and the dependent variable is established, the obtained model prediction result is more reliable and accurate, and an effective method is provided for predicting the incidence rate of the talaromyces marneffei disease of the HIV patient.
Owner:LIUZHOU PEOPLES HOSPITAL
Medicinal composition for treating acquired immune deficiency syndrome (Aids) as well as preparation method, quality control method and application thereof
ActiveCN102641489BHas therapeutic effectInhibition efficacy indexAntiviralsCapsule deliveryRadix AconitiTherapy HIV
A pharmaceutical composition for treating AIDS and preparation method, quality controlling method and use thereof. The raw material of the composition consists of Radix Aconiti Lateralis Preparata, Herba Epimedii, Rhizoma Zingiberis, Radix Glycyrrhizae, Radix Ginseng, Radix Salviae Miltiorrhizae, Rhizoma Polygoni Cuspidati, Poria, Cortex Phellodendri and Radix Scutellariae.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
CCR5 antagonist for enhancing immune reconstitution and treating opportunistic infection in HIV patients
InactiveCN101466376AImprove immune reconstitutionPrevent opportunistic symptomsAntibacterial agentsOrganic active ingredientsCXCR4HIV receptor
The present invention relates to the use of a CCR5 antagonist in an HIV infected patient to enhance their immune reconstitution and so treat to HIV related opportunistic conditions resulting from the immunocompromised state of the HIV patient. The invention also allows treatment with a CCR5 antagonist of patients having a CXCR4 using viral population since such patients will also benefit from an increase in their CD4 and / or CD8 cell count.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Nucleic acid amplification fluorescent quantitation method for detecting and integrating HIV-1 virus genomes and application thereof
InactiveCN108130382AIncrease coverageHighly conservativeMicrobiological testing/measurementTreatment effectFluorescence
The invention provides a nucleic acid amplification fluorescent quantitation method for detecting and integrating an HIV-1 virus genomes and application thereof. The method comprises the following steps of (1) building a real-time absolute quantitative HIV total DNA method; (2) preparing a standard product used for integrating an HIV genome; using the method in the step (1) for quantification; (3)performing gradient dilution for integrating an HIV standard product; performing nested fluorescence-quantitative PCR for building an HIV integration standard curve; (4) simultaneously preparing an HIV integration standard product and a sample to be tested; installing an Alu-primer-free reference pipe for each sample to be tested; after the PCR operation in the step (3), comparing the amplification result of the same to be tested with the standard curve to obtain the absolute copy number of the sample to be tested, wherein the virus copy number of the sample to be tested before the HIV integration equals to the copy number difference of the Alu primer sample and the Alu-primer-free reference pipes. The method can be used for distinguishing the integrated and non-integrated HIV virus genome; the virus before the specificity detection has very high sensitivity (5 to 10 copy / mul); the technical guarantee is provided for accurately evaluating HAART treatment effect and precisely quantifying the virus storage base in bodies of HIV patients.
Owner:WUHAN UNIV OF SCI & TECH
Chinese herbal prescription for treating AIDS
InactiveCN1654054AChange the history of no cureImprove faithAntiviralsUnknown materialsCurative effectTherapy HIV
The present invention belongs to the field of Chinese medicine technology, and is especially one kind of powdered Chinese medicine for treating AIDS. The powdered Chinese medicine is prepared with seven kinds of Chinese medicinal materials, including musk, realgar, frankincense, gypsum, etc. and through grinding, mixing and other steps. The Chinese medicine has certain curative in treating AIDS.
Owner:高平
Oral liquor for treating acquired immunodeficiency syndrome lung infection
The invention discloses oral liquor for treating acquired immunodeficiency syndrome lung infection. The oral liquor is prepared from the following raw materials in parts by weight: 5-15 parts of indigowoad leaves, 10-20 parts of Indian buead, 5-15 parts of dwarf lilyturf tuber, 15-25 parts of prepared pinellia tuber with ginger, 10-20 parts of szechuan-fritillary bulbs, 10-20 parts of Chinese magnoliavine fruits, 5-15 parts of Chinese thorowax root, 5-15 parts of milkvetch root, 10-20 parts of fructus forsythiae and 5-15 parts of heartleaf houttuynia herb. The oral liquor disclosed by the invention has the effects of clearing away heat and toxic materials, reducing phlegm, relieving cough, driving away pathological factors, benefiting qi, nourishing yin, strengthening body resistance, eliminating the pathogenic factors and the like, thereby having anti-inflammation and anti-infection effects. Thus, the normal physiological functions of the lung of a patient with acquired immunodeficiency syndrome can be restored as soon as possible. By clinically applying the oral liquor to the patient with acquired immunodeficiency syndrome lung infection, cough, coughing of foam sputum, chest distress, shortness of breath, fever, stuffy nose, throat itching and other uncomfortable symptoms can be obviously alleviated.
Owner:邓鑫
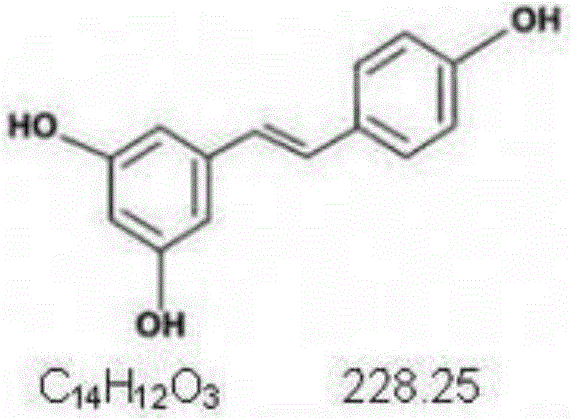
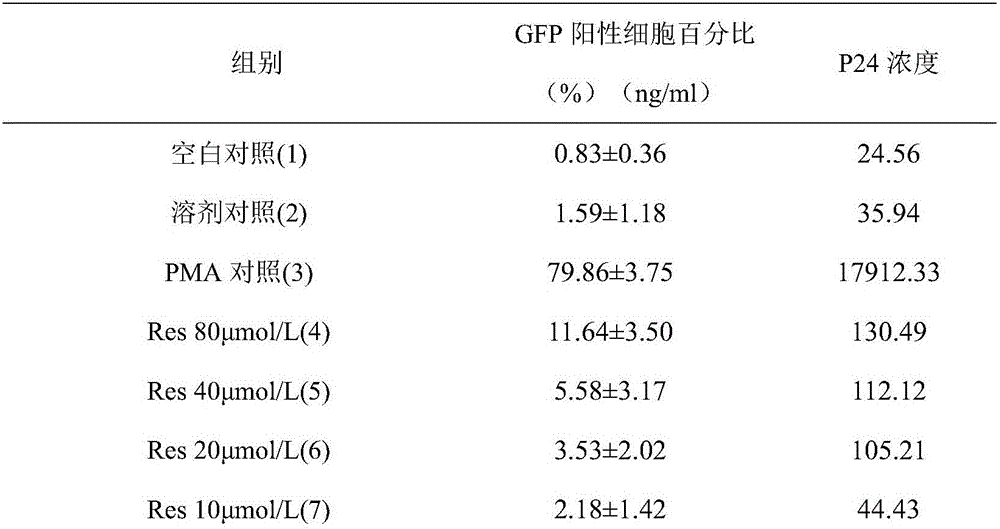
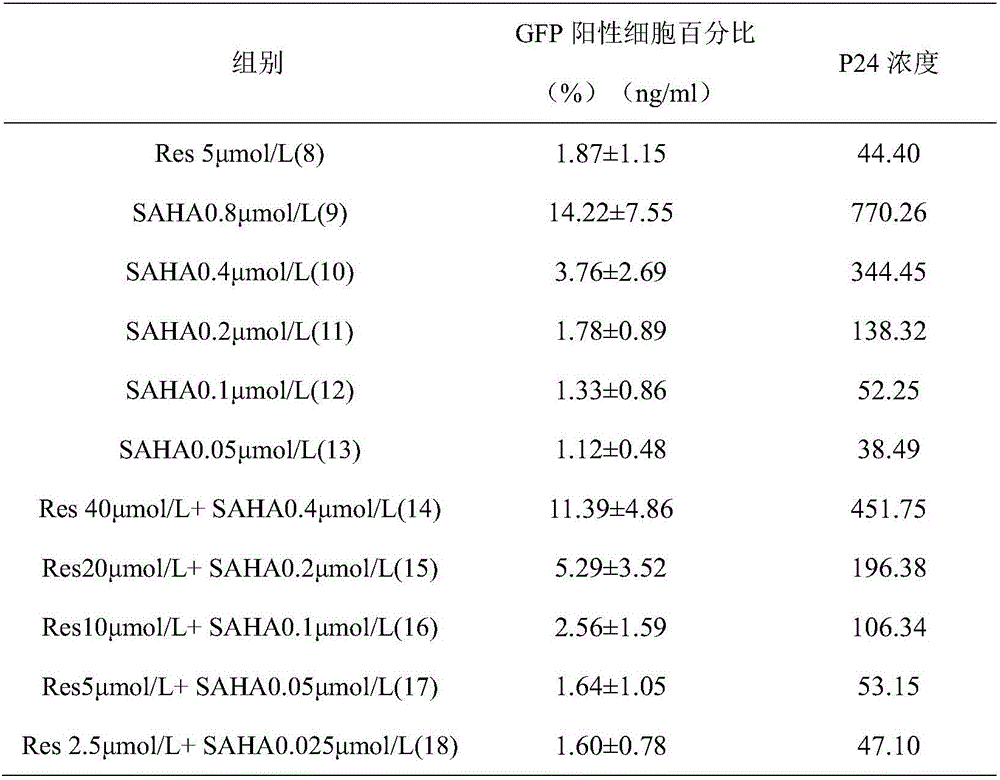
Application of resveratrol and analogs of resveratrol in serving as HIV latent virus reservoir activating agents
The invention discloses application of resveratrol and analogs of resveratrol in serving as HIV latent virus reservoir activating agents.The inventor proves that resveratrol can effectively stimulate rising of expression of GFP protein and a P24 antigen in a cell model through the HIV latent cell model J-lat full length clone 10.6.Thus, resveratrol and derivatives thereof can be independently used or jointly used with other activating medicine for activating the HIV latent virus reservoir in the body of an HIV patient and can serve as an alternative medicine molecule for curing aids.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com