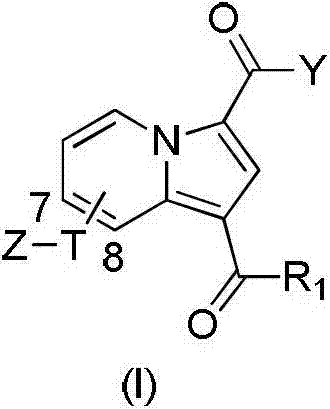
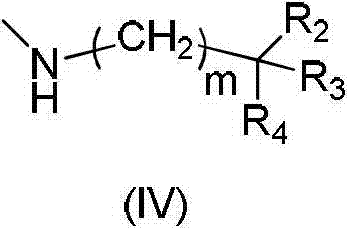
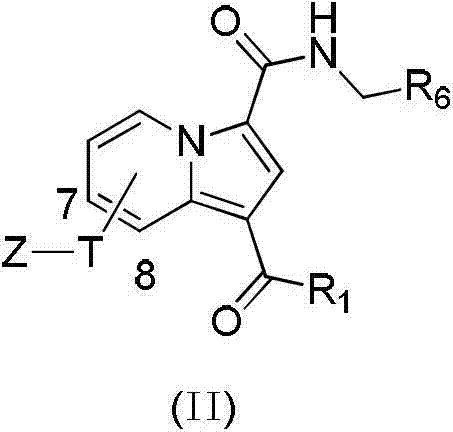
Substituted purrocoline compound and preparation method and application thereof
A compound, C3-C7 technology, applied to substituted indolizine compounds and preparation thereof, in the field of pharmaceutical compositions of the compounds, can solve the problems of not developing antiviral drugs and the like, achieve effective drugs, inhibit virus replication Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1, Synthesis of 7-(2-pyridineformyl)-3-(pyridin-2-yl-methylcarbamoyl)-indolizine-1-carboxylic acid ethyl ester (1)
[0058] The compound 7-(2-pyridinecarbonyl)-3-benzyl carboxylate-indolizine-1-carboxylate ethyl ester (0.8g, 1.8mmol) was dissolved in tetrahydrofuran (THF) (20mL), ethanol (20mL) and In the mixed solution of water (5mL), add lithium hydroxide monohydrate (LiOH·H 2 O) (0.23g, 0.54mmol) react overnight at room temperature, evaporate the solvent under reduced pressure, add 1N hydrochloric acid solution, precipitate a solid, filter, and wash with water to give a yellow solid 7-(2-pyridineformyl)-1-carboethoxy-3 -Carboxylic acid indolizine 0.54g; yield: 88.5%; directly used in the next step without purification.
[0059] Dissolve the product 7-(2-pyridineformyl)-1-carbocarboxylate-3-indolizine (101mg, 0.3mmol) in THF (20mL), add EDC (86mg, 0.45mmol) successively, HOBt (61mg, 0.45mmol), DIPEA (77mg, 0.6mmol) and 2-aminomethylpyridine (39mg, 0.36mmol),...
Embodiment 2
[0060] Example 2, Synthesis of 7-(2-pyridineformyl)-3-(pyridin-3-yl-methylcarbamoyl)-indolizine-1-carboxylic acid ethyl ester (2)
[0061] Using 7-(2-pyridineformyl)-3-carbocarboxylate-indolizine-1-carboxylic acid ethyl ester and 3-aminomethylpyridine as starting materials, according to the similar method of Example 1, the compound was synthesized 2. Yield: 78.1%; Mp: 135-137°C; 1 H NMR (400MHz, CDCl3 )δ9.72(d,J=7.4Hz,1H),9.18(s,1H),8.76(d,J=3.1Hz,1H),8.63(s,1H),8.54(d,J=2.8Hz, 1H), 8.11(d, J=7.8Hz, 1H), 7.95(t, J=7.7Hz, 1H), 7.81(s, 1H), 7.75(d, J=7.8Hz, 1H), 7.61(d, J=7.3Hz,1H),7.54(t,J=5.9Hz,1H),7.30(t,J=5.5Hz,1H),6.90(d,J=4.3Hz,1H),4.69(d,J= 5.1Hz, 2H), 4.33(q, J=6.8Hz, 2H), 1.34(t, J=7.0Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ191.2, 164.0, 161.3, 154.7, 149.5, 149.3, 148.7, 137.5, 136.4, 135.9, 134.1, 131.6, 127.8, 126.8, 125.2, 125.0, 123.9, 120.1, 118.9, 110.4, 10.5, IES -MS m / z:429.0[M+H] + ;Anal.Calcd.for C 24 h 20 N 4 o 4 :C,67.00;H,4.692;N,12.93;Found:C,67.28;...
Embodiment 3
[0062] Example 3, Synthesis of 7-(2-pyridineformyl)-3-(benzylcarbamoyl)-indolizine-1-carboxylic acid ethyl ester (3)
[0063] Using 7-(2-pyridineformyl)-3-benzyl carboxylate-indolizine-1-carboxylate ethyl ester and benzylamine as starting materials, according to the similar method of Example 1, compound 3 was synthesized, and the yield : 78.1%; Mp: 170-173°C; 1 H NMR (400MHz, CDCl 3 )δ9.74(d,J=7.3Hz,1H),9.18(s,1H),8.77(s,1H),8.10(d,J=7.8Hz,1H),7.94(t,J=7.8Hz, 1H),7.74(s,1H),7.60(d,J=7.4Hz,1H),7.56–7.48(m,1H),7.41-7.28(m,5H),6.51(s,1H),4.66(d ,J=4.9Hz,2H),4.33(q,J=6.7Hz,2H),1.34(t,J=7.0Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ191.2, 164.0, 161.1, 154.8, 148.7, 138.2, 137.5, 136.3, 131.4, 129.1, 128.1, 127.9, 127.9, 126.8, 125.3, 125.0, 119.7, 119.2, 113.6, 109, 43.6, IES, 60.5; m / z:428.0[M+H] + ;Anal.Calcd.for C 25 h 21 N 3 o 4 : C, 70.09; H, 4.936; N, 9.755; Found: C, 70.25; H, 4.95; N, 9.83.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com