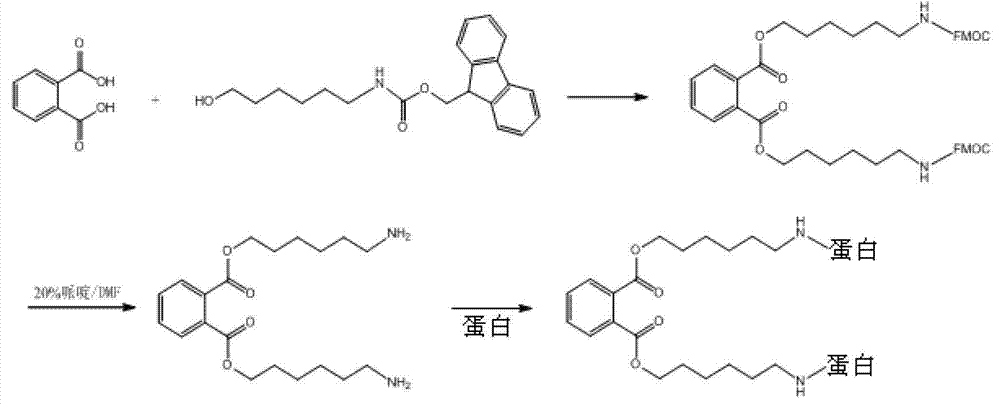
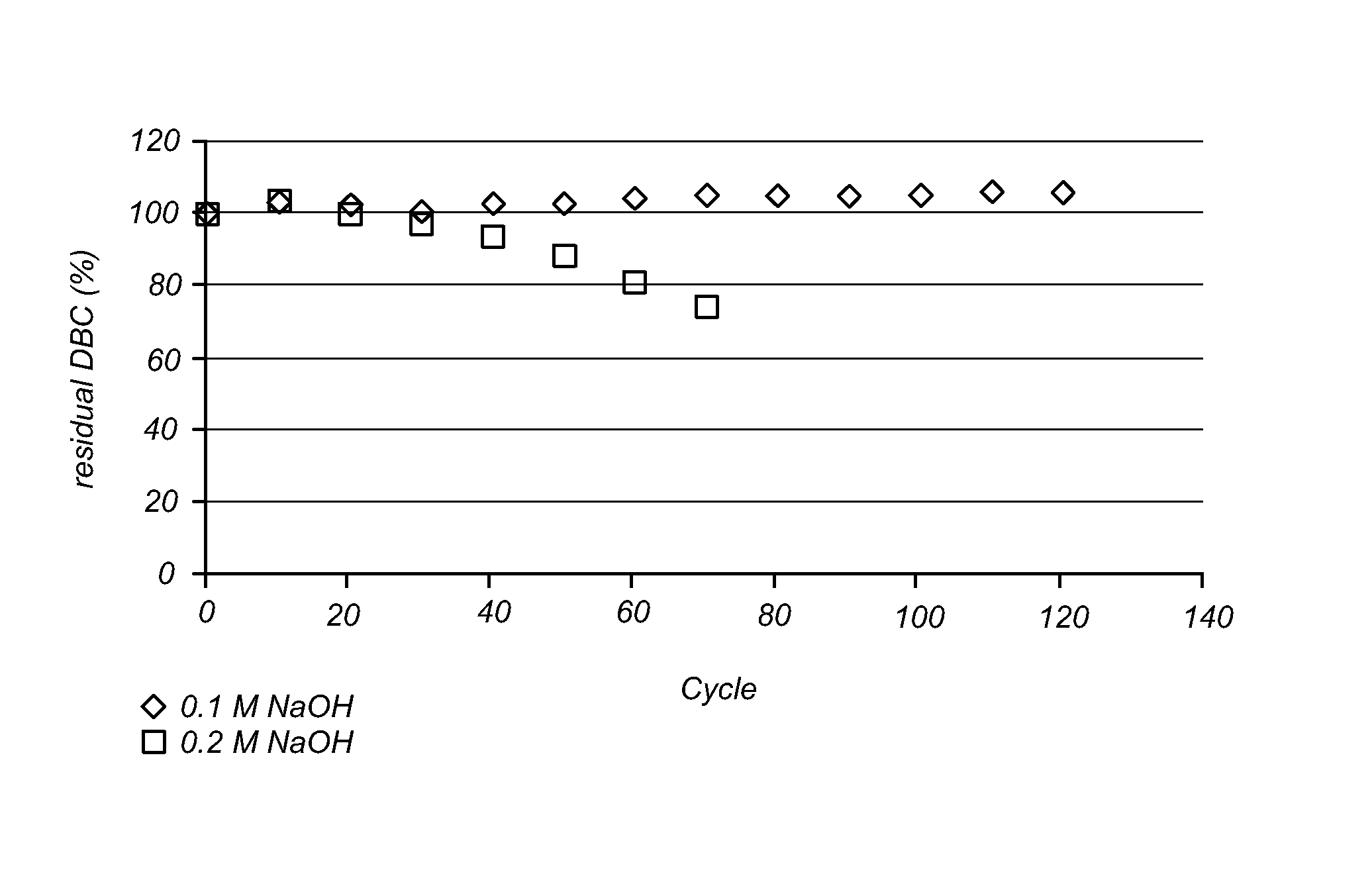
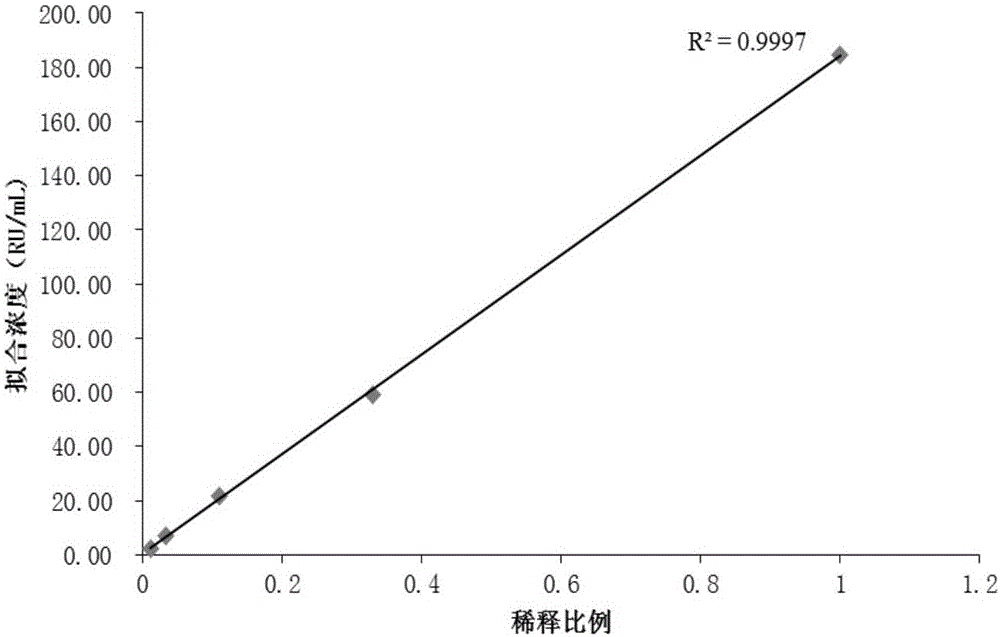
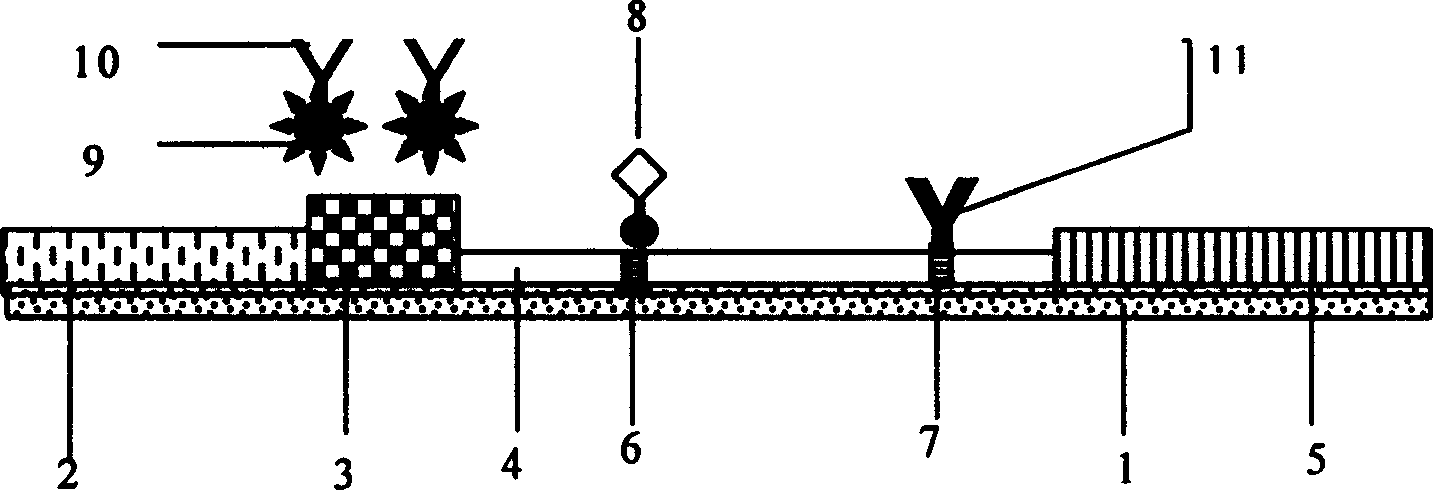
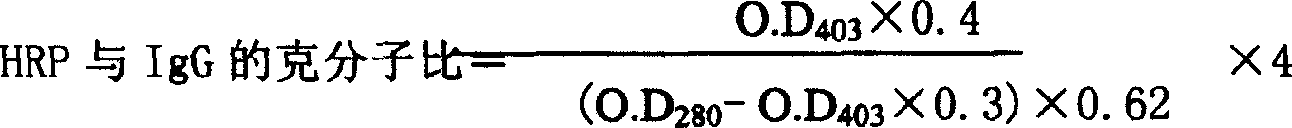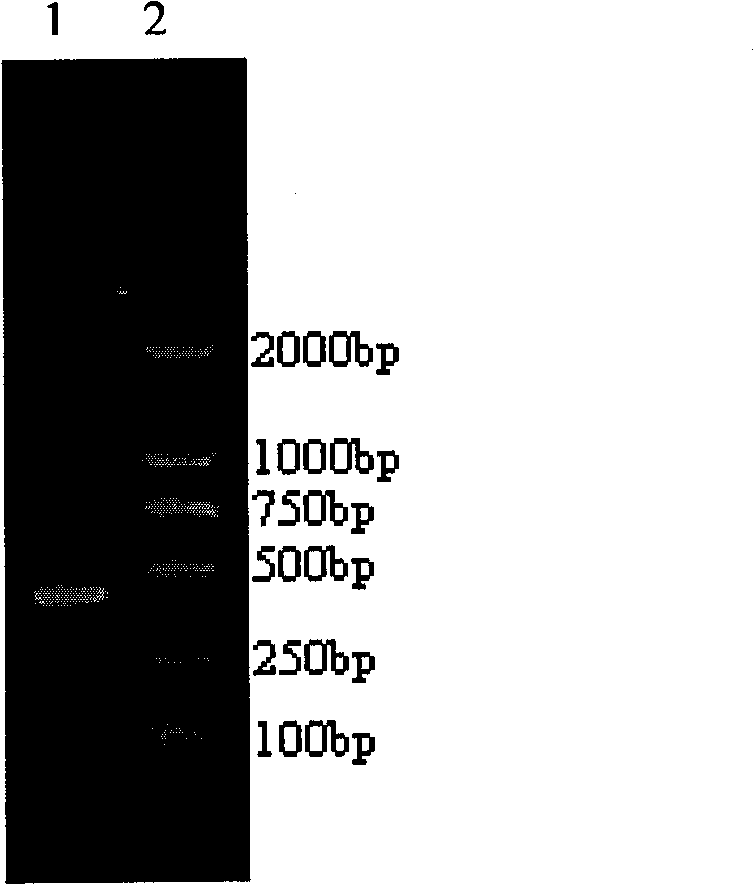Patents
Literature
745 results about "Immunoadsorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoadsorption is an alternative blood purification technique used to eliminate pathogenic antibodies. It may be used as an alternative to plasma exchange in certain conditions. Evidence of benefit is lacking in those with kidney problems. Concerns include that it is expensive.
Hybridoma cell strain ST03, anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof
ActiveCN103849604ANo cross reactionHigh sensitivityTissue cultureImmunoglobulins against fungi/algae/lichensAbzymeAflatoxin biosynthesis
The invention relates to a hybridoma cell strain ST03, an anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof. The hybridoma cell strain ST03 with the preservation number of CCTCCNO.C2013187 can be used for preparing high-valence anti-aflatoxin biosynthesis precursor ST monoclonal antibody, wherein valence measured by an enzyme linked immunosorbent assay (ELISA) method of the anti-aflatoxin biosynthesis precursor ST mouse ascites antibody can reach 6.4*10<5>. The anti-aflatoxin biosynthesis precursor ST monoclonal antibody is high in sensitivity, has 50% inhibition concentration IC50 of 0.36 ng / mL on the aflatoxin biosynthesis precursor ST, has no cross reaction with the aflatoxin B1, aflatoxin B2, aflatoxin G1 and G2, and can be applied to measuring content of the aflatoxin biosynthesis precursor ST.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Hybridoma cell line 1C11 and anti-aflatoxin general monoclonal antibody generated by same as well as applications thereof
ActiveCN101993855AHigh sensitivityHigh practical application valueMicroorganism based processesTissue cultureCell strainHybridoma cell
The invention provides a hybridoma cell line 1C11 and an anti-aflatoxin general monoclonal antibody secreted by the same as well as the applications thereof. The hybridoma cell line 1C11 can be used for preparing a high-titer aflatoxin antibody, and a mouse hydroperitoneum antibody is measured to reach 5.12*106 by using an ELISA (Enzyme-Linked Immunosorbent Assay). The anti-aflatoxin general monoclonal antibody has high sensitivity, respectively reaches the IC50 (50% inhibiting concentration) of aflatoxin B1, B2, G1 and G2 to be 1.2, 1.3, 2.2 and 18.0 pg / mL, is the antibody with highest sensitivity among currently reported four aflatoxin antibodies, is used for measuring the total aflatoxin amounts, i.e. the total amounts of the aflatoxin B1, B2, G1 and G2 and has great practical application values.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Centrifugal micro-fluidic device and method for detecting target in fluid sample
InactiveUS20110124132A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCentrifugal microfluidicsMicro fluidic
Disclosed are a centrifugal micro-fluidic device and an immunosorbent assay method using the same. In particular, a centrifugal micro-fluidic device having a plurality of micro-fluidic structures placed in a disc type platform to simultaneously conduct several immunosorbent assays, as well as an immunosorbent assay method using the same are provided.
Owner:SAMSUNG ELECTRONICS CO LTD
Biofunctional magnetic nanoparticles for pathogen detection
InactiveUS20060292555A1High sensitivityEasy to reportNanotechMicrobiological testing/measurementMagnetite NanoparticlesBiology
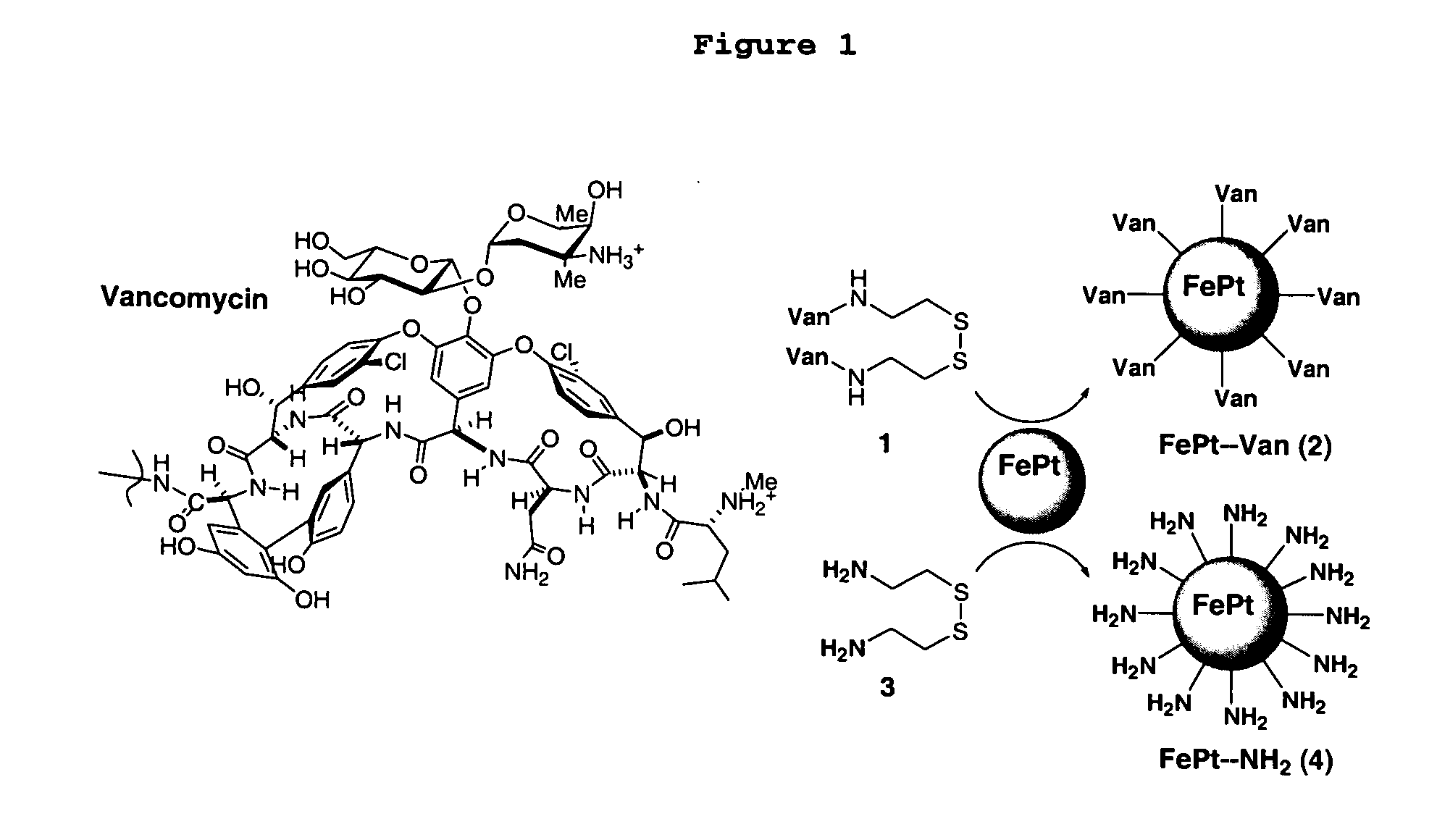
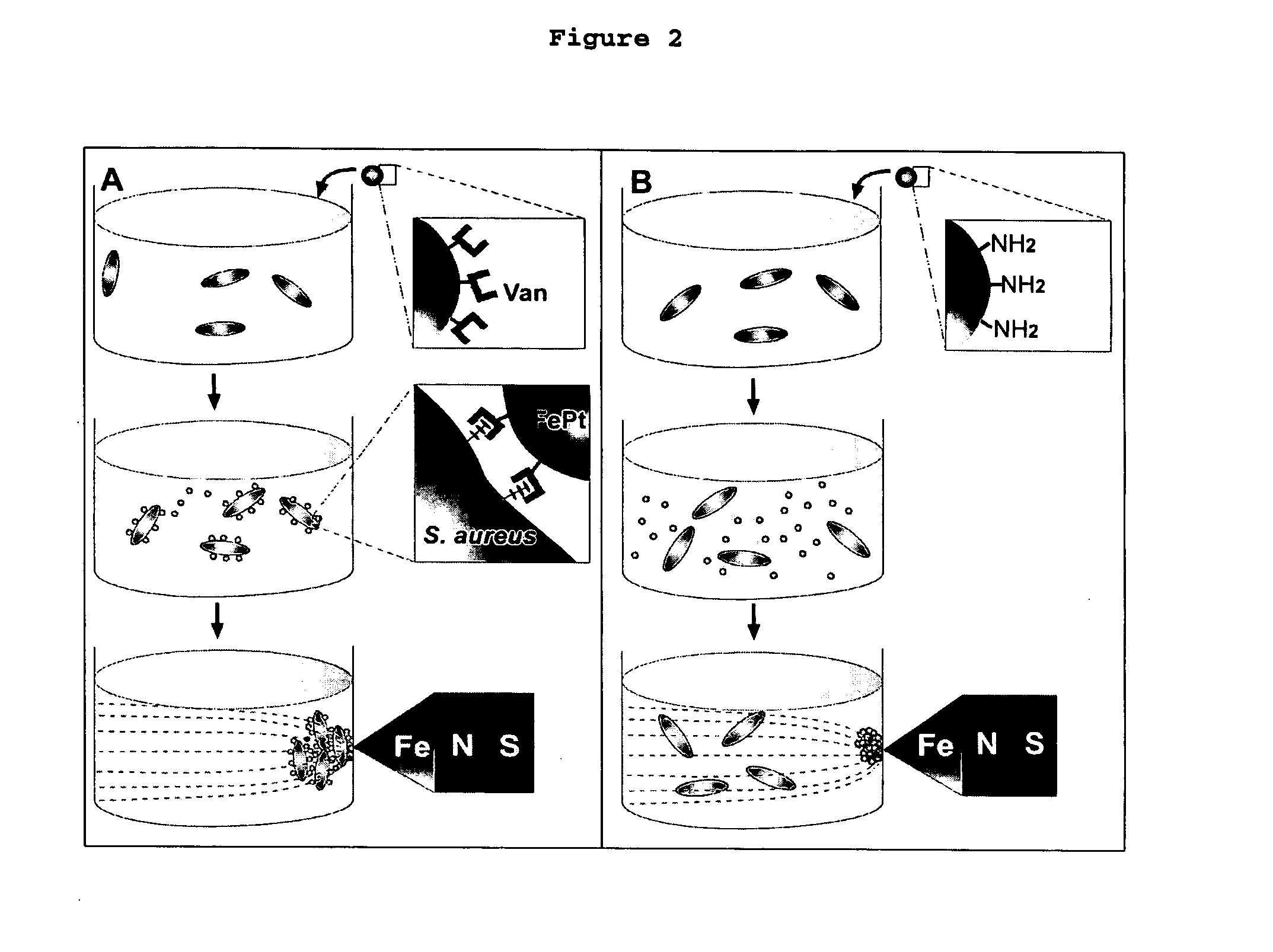
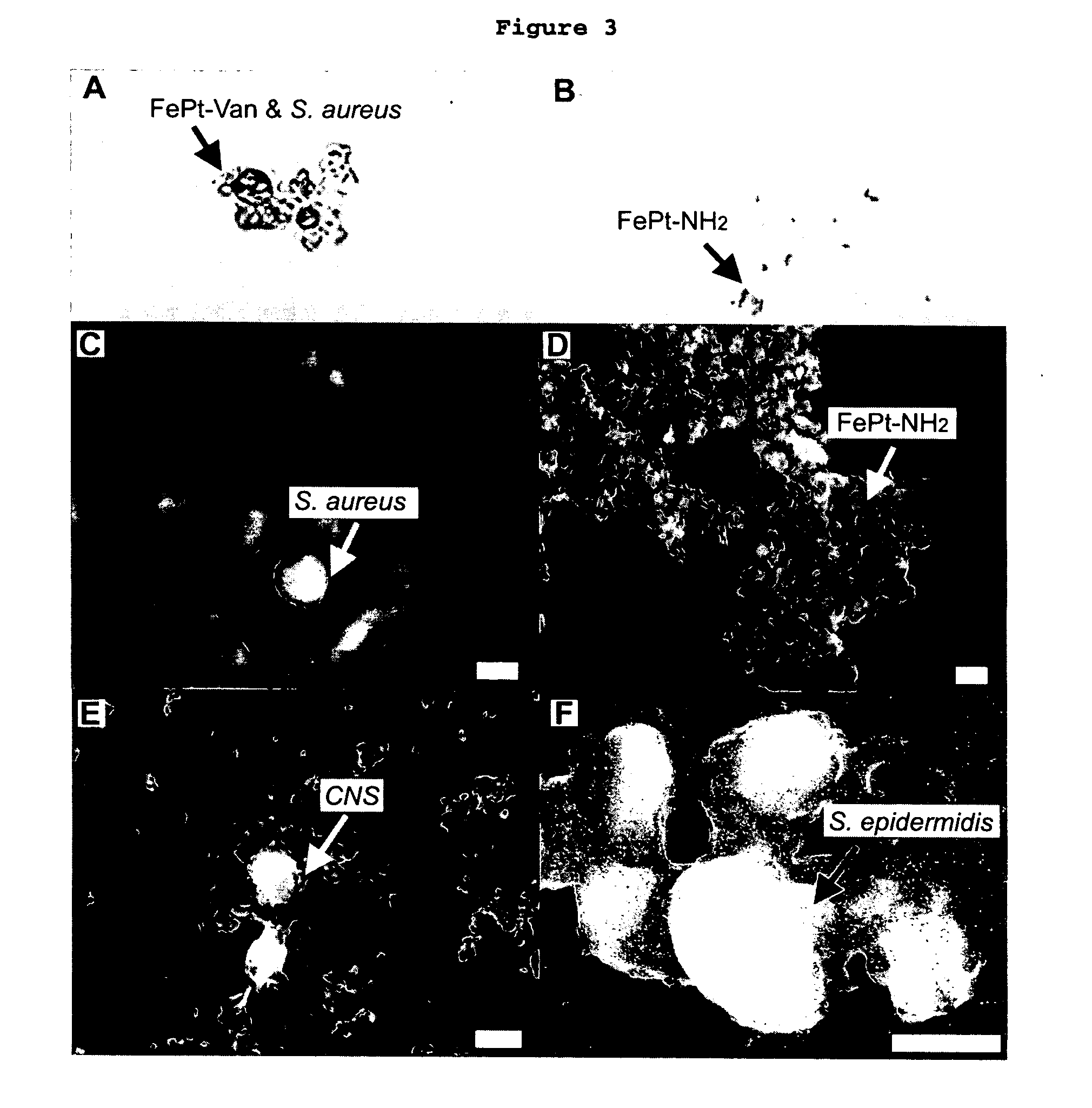
This invention provides a method of detecting pathogens comprising the steps of: (a) contacting a sufficient amount of biofunctional magnetic nanoparticles with an appropriate sample for an appropriate period of time to permit the formation of complexes between the pathogens in the sample and the nanoparticles; (b) using a magnetic field to aggregate said complexes; and (c) detecting said complexes. The method may further comprise the additional step of removing said complexes. The biofunctional magnetic nanoparticles are preferably a conjugate of vancomycin and FePt. The pathogens may be bacteria or viruses, and the sample may be a solid, liquid, or gas. Detection may involve conventional fluorescence assay, enzyme-linked immunosorbent assay (ELISA), optical microscope, electron microscope, or a combination thereof. The sensitivity of detection for the method is at least as low as 10 colony forming units (cfu) of the pathogens in one milliliter of solution within one hour.
Owner:THE HONG KONG UNIV OF SCI & TECH +1
Human immunodeficiency virus affinity adsorption column, and preparation method and uses thereof
InactiveCN102631891ARich sourcesQuality improvementIon-exchange process apparatusOther blood circulation devicesMicrosphereHuman immunodeficiency
The invention discloses a human immunodeficiency virus (HIV) affinity adsorption column, comprising a column body and at least one activated affinity microsphere located in the column body, wherein the activated affinity microsphere is connected with a human immunodeficiency virus affinity protein, and the affinity protein can be bound with human immunodeficiency virus. The affinity protein comprises a main receptor CD4 molecule, a gp120 antibody, an auxiliary receptor CXC chemokine receptor 4 (CXCR-4) and a CC chemokine receptor 5 (CCR-5). The affinity microsphere may be a glass microsphere with the size of 1mm, a chitosan crosslinking microsphere with the diameter more than 500 microns, or a gluosan microsphere. The human immunodeficiency virus affinity adsorption column disclosed by the invention is applicable for eliminating the human immunodeficiency virus in the blood of HIV patients, and relieving and treating the immunodeficiency syndrome of HIV patients; and compared with the traditional treatment methods, the immunoadsorption column has high safety, good specificity, small toxic or side effects and good operation simplicity.
Owner:WUHAN UNIV
Anti-viral compositions comprising heterocyclic substituted phenyl furans and related compounds
InactiveUS20060287319A1Potent anti-HIV activityInhibit HIV replicationBiocideAntiviralsFuranFluorescence
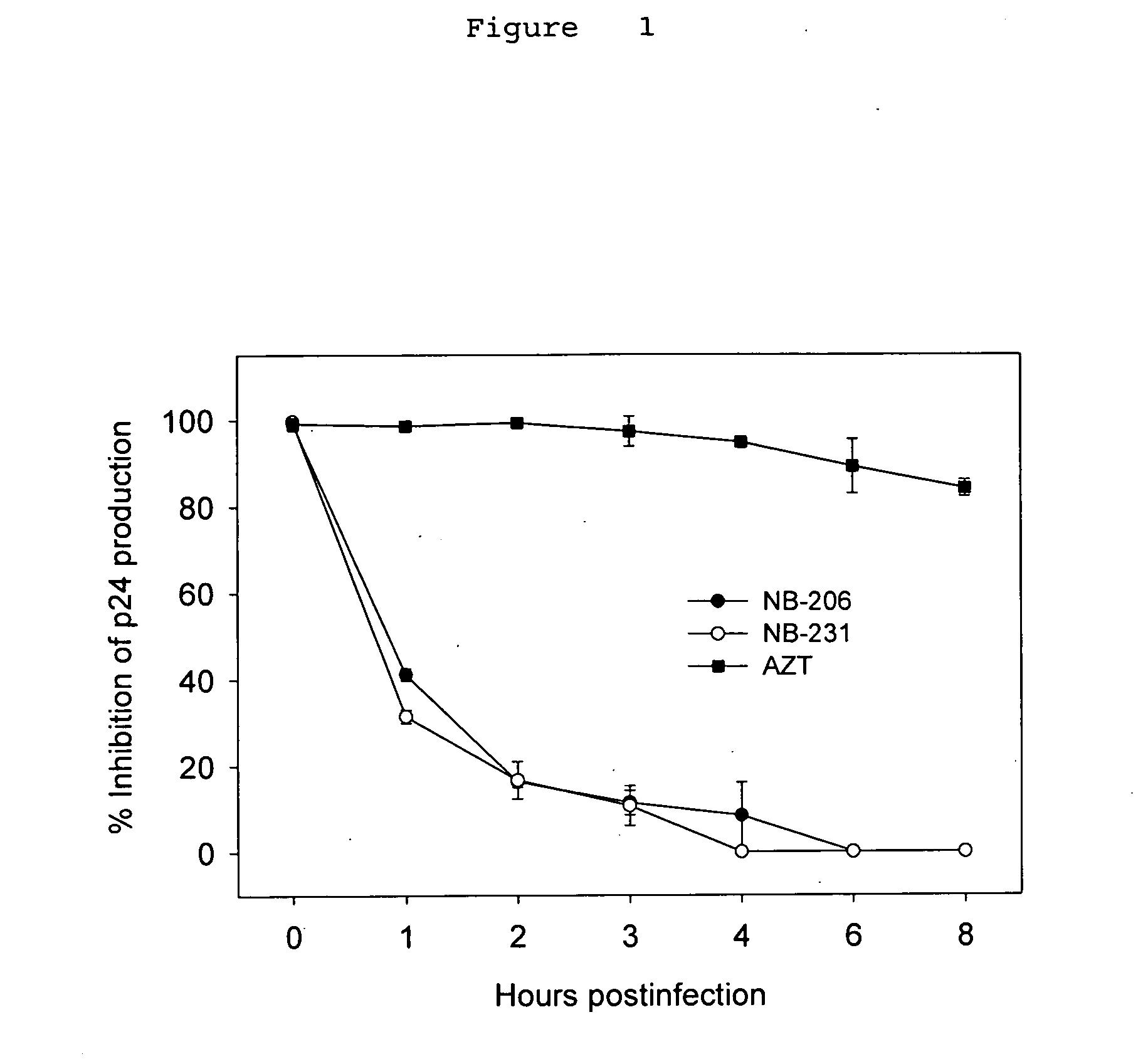
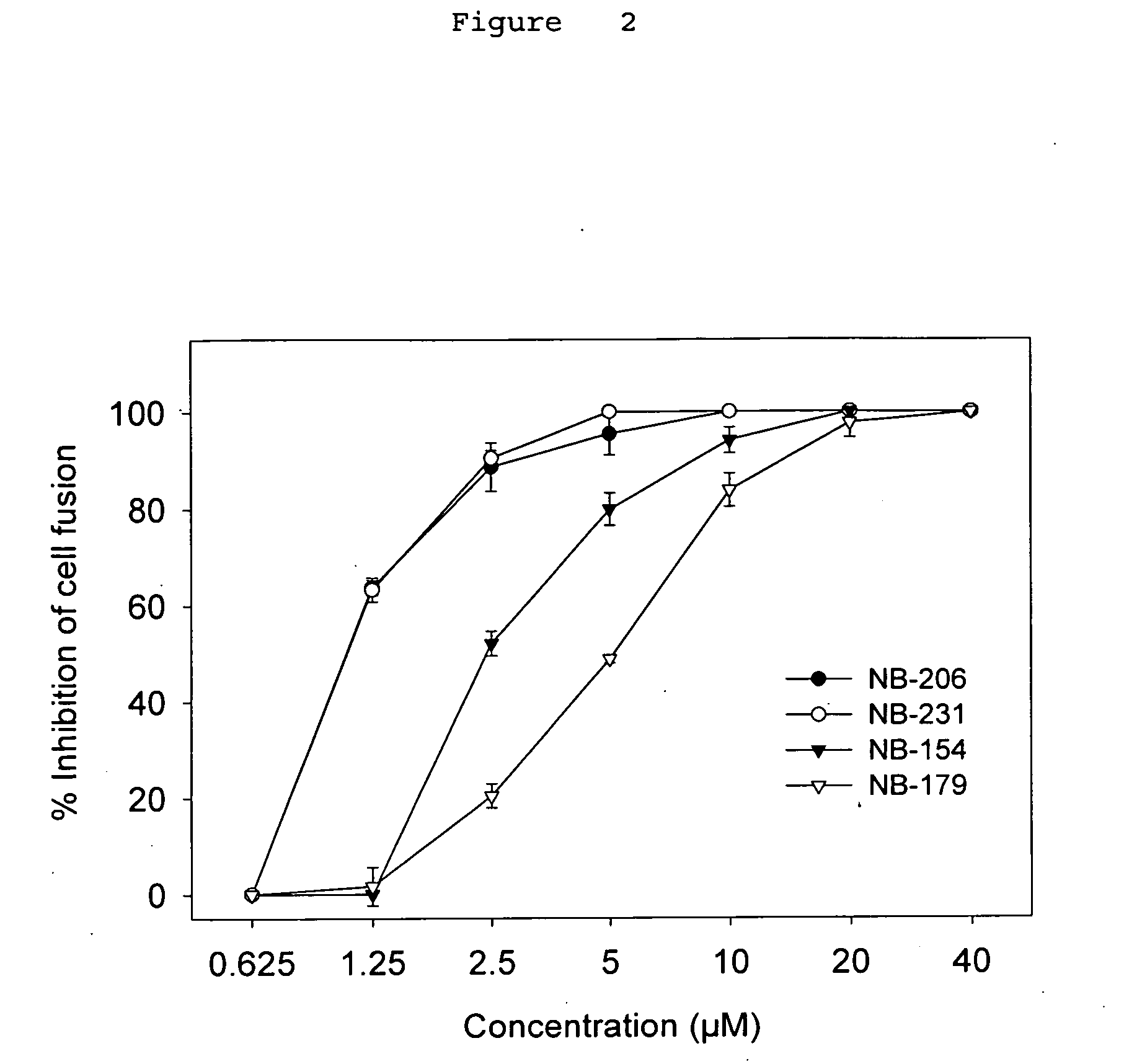
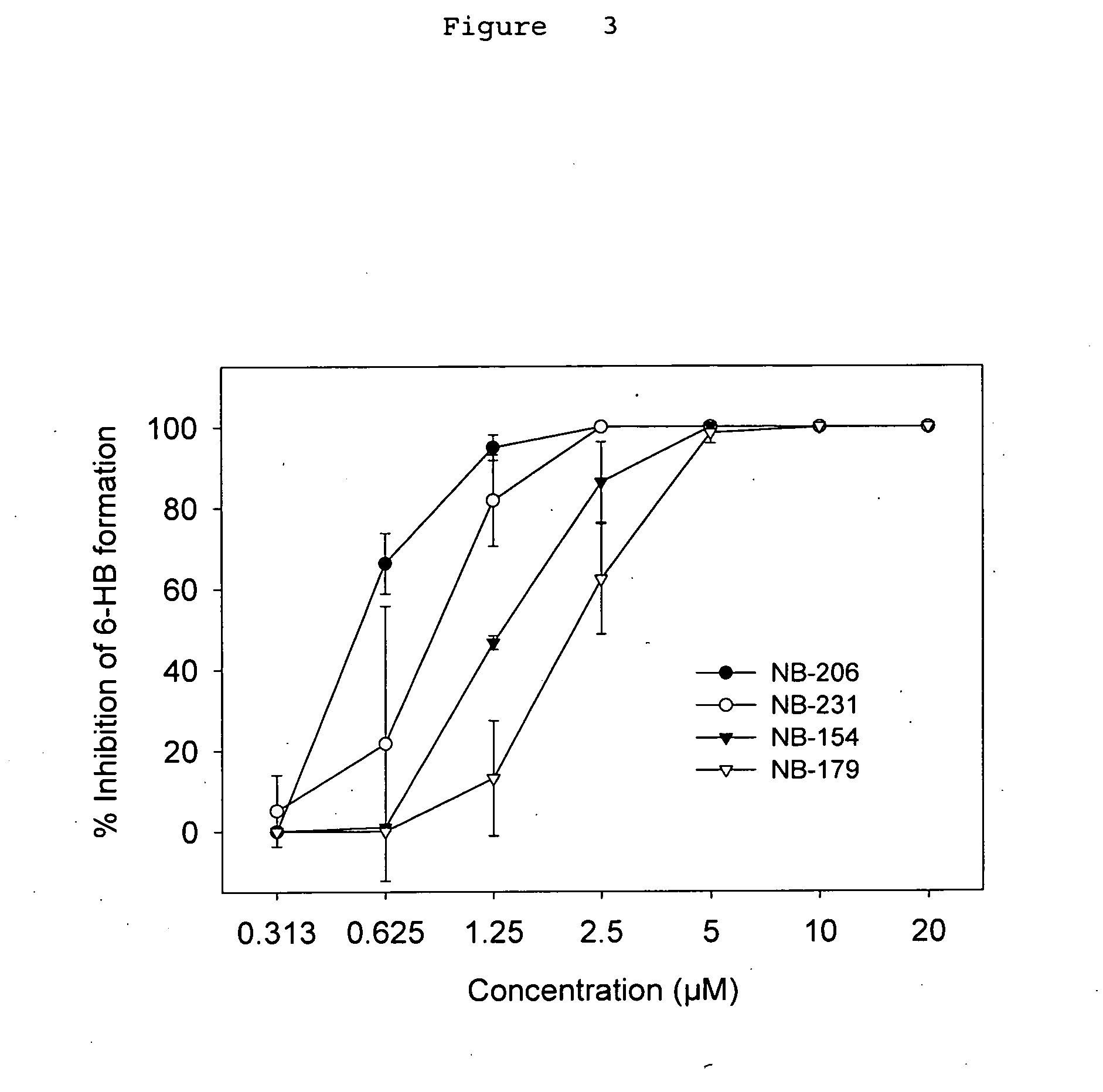
A group of compounds that inhibit HIV replication by blocking HIV entry was identified. One representative compound, designated NB-206, and its analogs inhibited HIV replication (p24 production) with IC50 values at nanomolar levels. It was proved that NB-206 and its analogs are HIV entry inhibitors by targeting the HIV gp41 since: 1) they inhibited HIV-mediated cell fusion; 2) they inhibited HIV replication only when they were added to the cells less than one hour after virus addition; 3) they blocked the formation of the gp41 core that is detected by sandwich enzyme linked immunosorbent assay (ELISA) using a conformation-specific MAb NC-1; and 4) they inhibited the formation of the gp41 six-helix bundle revealed by fluorescence native-polyacrylamide gel electrophoresis (FN-PAGE). These results suggested that NB-206 and its analogs may interact with the hydrophobic cavity and block the formation of the fusion-active gp41 coiled coil domain, resulting in inhibition of HIV-1 mediated membrane fusion and virus entry.
Owner:NEW YORK BLOOD CENT
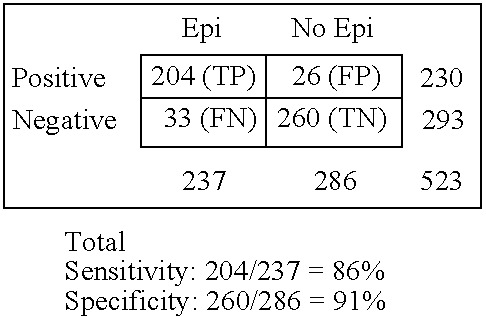
Immunosorbent blood tests for assessing paroxysmal cerebral discharges
InactiveUS20050181466A1Effective therapeutic interventionRapid inexpensiveNervous disorderDisease diagnosisDiseaseParoxysmal AF
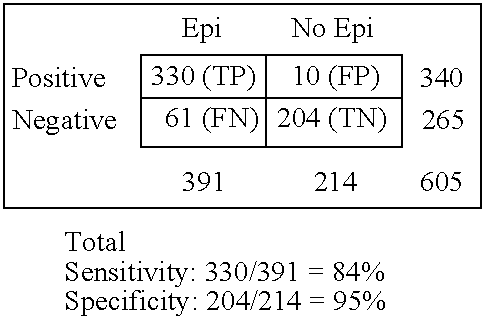
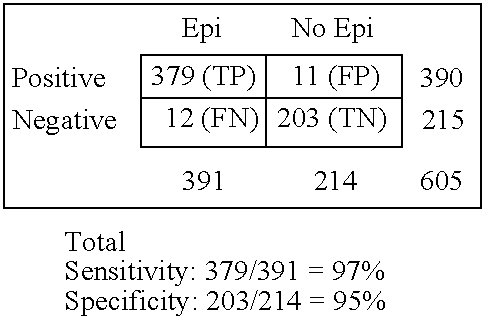
Immunosorbents, kits and compositions for diagnosing a central nervous system disorder, particularly paroxysmal cerebral discharges and epilepsy, comprising measuring the concentration of GluR1 or fragment thereof and / or GluR1 antibodies in a biological sample from a human subject. The method is particularly useful for identifying individuals that are at risk for brain related seizures and epilepsy, for distinguishing epilepsy from pseudo-epilepsy and epilepsy-like disorders, for following up after anticonvulsive treatment, and for the adjustment of adequate therapy and doses.
Owner:GRACE LAB
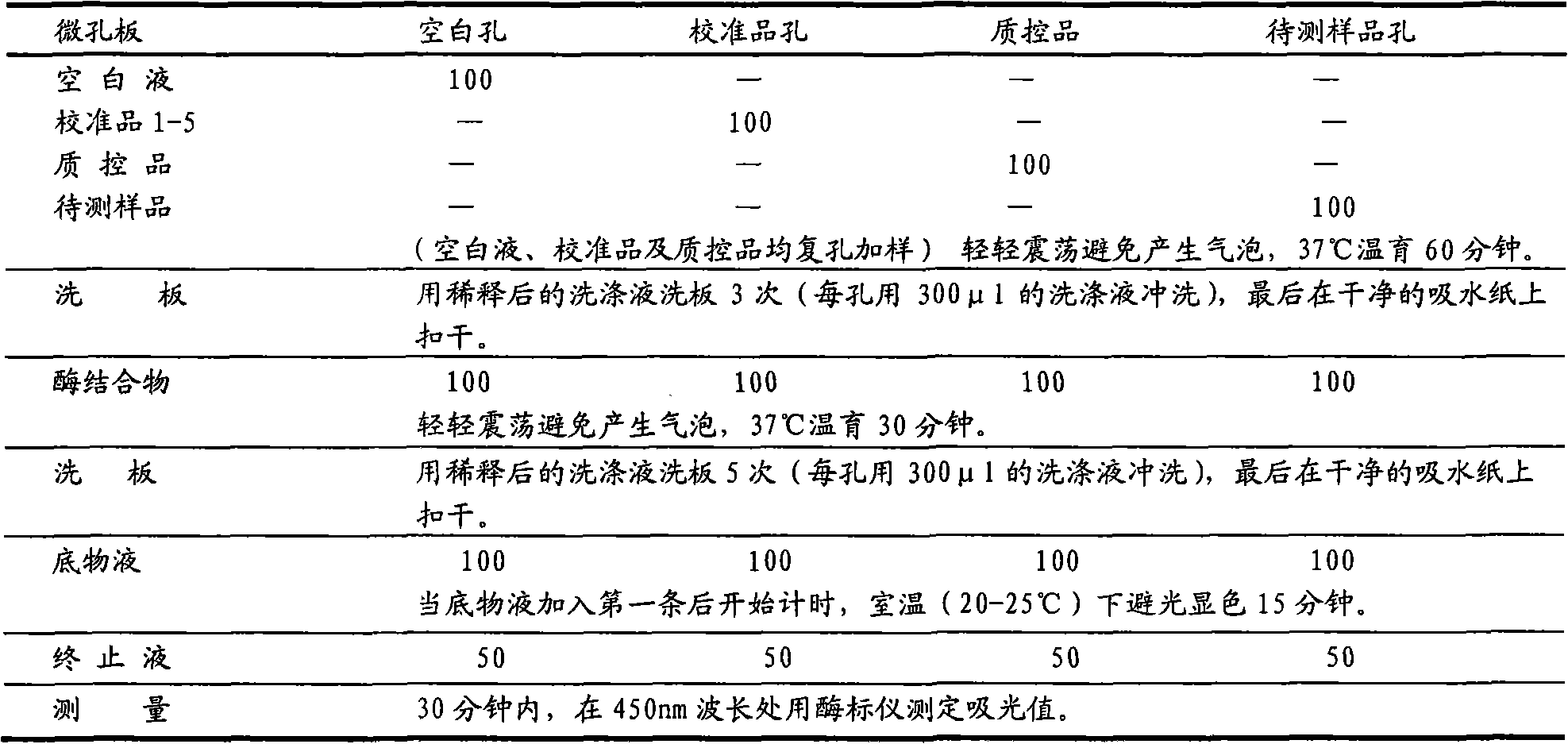
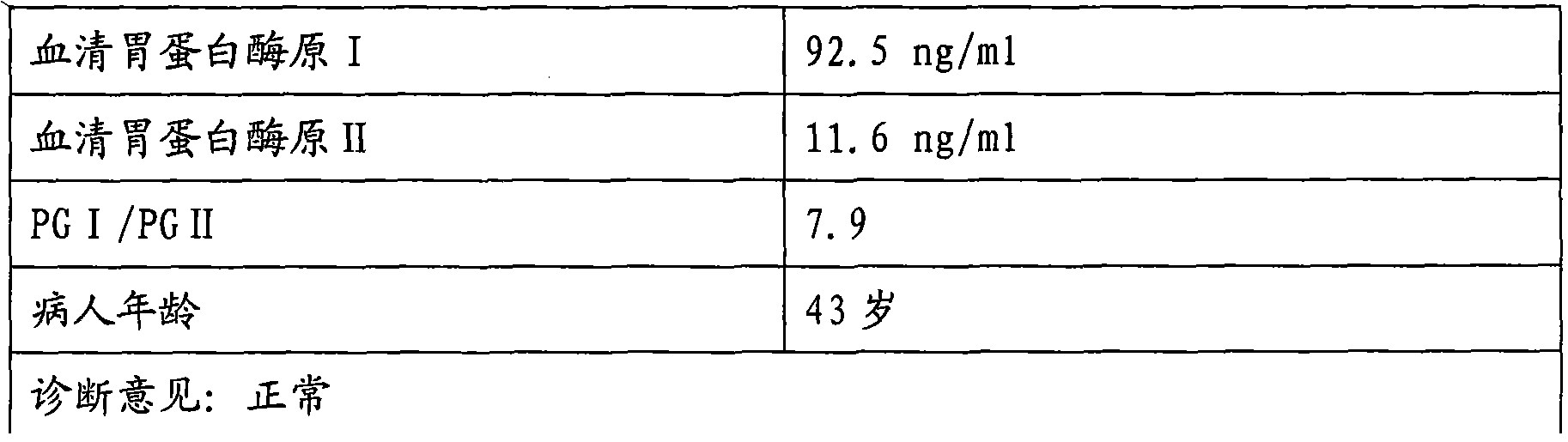
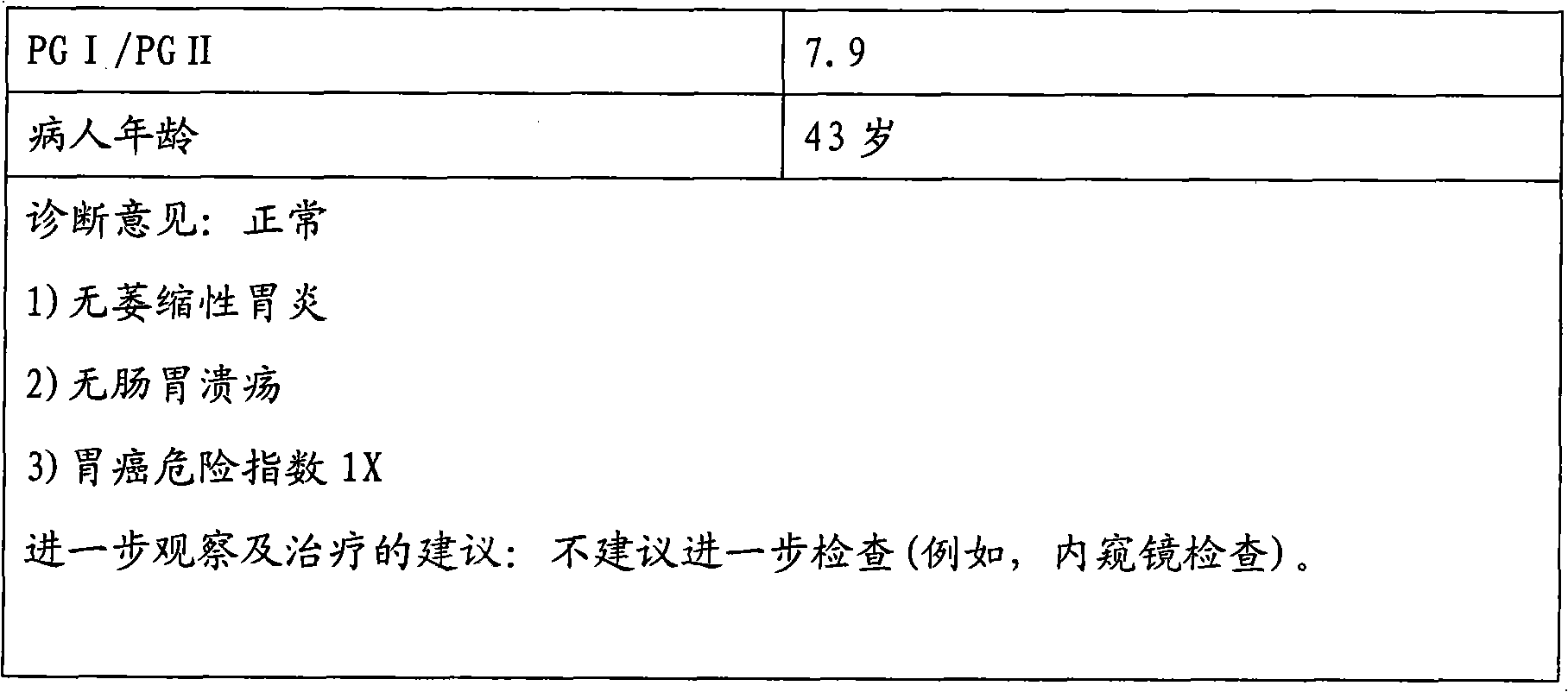
Enzyme linked immunosorbent assay kit for combined diagnosis of gastrosis or evaluation of gastric cancer risks
InactiveCN102087279AIncreased sensitivityImprove featuresComponent separationTissue cultureAntigenPepsinogen I
The invention discloses an enzyme linked immunosorbent assay kit for combined diagnosis of the gastrosis or evaluation of gastric cancer risks and a preparation method thereof. The kit comprises a micropore plate coated with an antibody against a pepsin antigen I or an antibody against a pepsin antigen II, an enzyme labeled antibody, a color-developing agent, a stop solution and a concentrated cleaning solution, wherein the pepsin antigen I or the pepsin antigen II is a natural protein obtained from extraction of human gastric mucosa tissue. The kit disclosed by the invention adopts a mouse immunized with pepsinogen I and pepsinogen II which are separated from human gastric mucosa to prepare immunogen of a monoclonal antibody, the used standard sample also adopts the pepsin antigen I or the pepsin antigen II separated from the human gastric mucosa, thereby the defects caused by adopting different structures of animal pepsinogen and human pepsinogen are filled. The kit can be used for accurately diagnosing the gastrosis or early gastric cancer and has the advantages of high sensitivity, strong specificity, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
IgG kit for detecting streetvirus of dogs using indirect enzyme immunosorbent assay and preparation method thereof
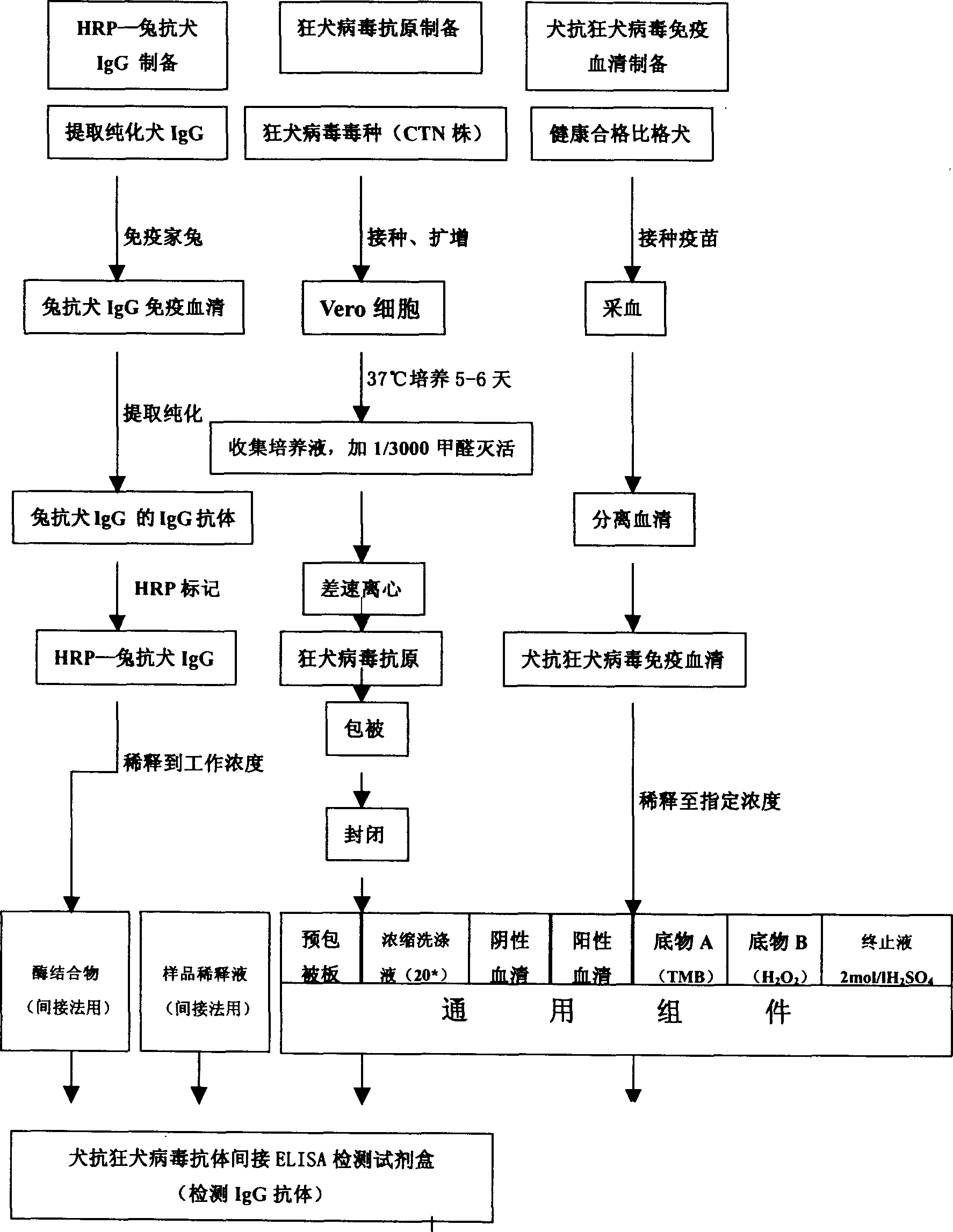
The invention refers to a kind of detecting reagent box and the manufacturing method, concretely refers to the reagent which is indirect enzyme immune sorption experiment for detecting rabies virus IgG and the manufacturing method. The reagent box compositions are: beforehand enclosed rabies virus antigen enzyme label board, sample diluting solution, HRP-rabies resisting IgG enzyme compound, condensed washer solvent, substrate and stopping liquid. The specificity of the reagent can reach 100%; the sensitivity is 1:640; the accuracy (the variation coefficient) is 6.98%. The reagent uses indirect ELISA to detect the rabies virus IgG antibody.
Owner:湖北省预防医学科学院
Reagent kit and enzyme-linked immunochromatography for detecting various organophosphorus pesticide residues
InactiveCN102539753AHigh detection sensitivityHigh resolutionMaterial analysisColloidOrganophosphorus pesticides
The invention discloses a reagent kit and an enzyme-linked immunochromatography for detecting various organophosphorus pesticide residues and particularly relates to the reagent kit and the enzyme-linked immunochromatography for detecting organophosphorus pesticide residues in the production of vegetative agricultural products. The enzyme-linked immunochromatography for detecting the organophosphorus pesticide residues uses the high-sensitivity characteristic of enzyme to carry out detection by adopting the immunochromatography; compared with a traditional enzyme-linked immunoserbent assay (ELISA) for detecting organophosphorus pesticides, the enzyme-linked immunochromatography has the advantages of simplicity and convenience in operation, and visual result, and no special instrument equipment is needed; and compared with a colloidal gold immunochromatography, the enzyme-linked immunochromatography has the advantages of high sensitivity, strong specificity, accuracy in quantification by combining with a readout instrument and the like.
Owner:SHENZHEN KANGMEI BIOTECH
Hybridoma cell line 3G1 and anti-alfatoxin B1 monoclonal antibody produced by the same
ActiveCN102747043AHigh sensitivityStrong specificityBiological material analysisTissue cultureAflatoxin BELISA unit
The present invention relates to a hybridoma cell line 3G1 and an anti-alfatoxin B1 monoclonal antibody produced by the hybridoma cell line 3G1. The hybridoma cell line 3G1 (CCTCCNO.C201014) can be used for preparation of a high titer anti-aflatoxin B1 monoclonal antibody, wherein an enzyme-linked immunosorbent assay (ELISA) method is adopted to determine a titer, and the titer is 6.40*10<6>. The anti-aflatoxin B1 monoclonal antibody of the present invention has characteristics of high sensitivity and good specificity, wherein 50% inhibiting concentration on aflatoxin B1 by the monoclonal antibody is 1.6 ng / mL, cross reaction rate with aflatoxin B2 is 6.4%, and cross reaction rates with aflatoxin G1 and G2 are less than 1%. In addition, the anti-aflatoxin B1 monoclonal antibody of the present invention can be used for determination of aflatoxin B1.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Rapid method for enzyme-linked immunosorbent assay
InactiveUS6498016B1Fast wayRapid diagnosis of diseaseDielectric heatingOptical radiation measurementAntigenFood technology
This invention relates to a rapid and efficient method for carrying out enzyme-linked immunosorbent assay for detection of minute quantities of biomolecules such as antigen, antibody etc. This invention particularly relates to microwave mediated immobilization of antigen or antibody on to the activated surface followed by performing subsequent steps of ELISA by controlled microwave irradiation. The invented procedure has dramatically reduced the total time required for ELISA to less than 10 minutes from hours to days. The invented ELISA procedure is rapid, economical, reproducible and simple and can be automated. The invented procedure is useful for carrying out ELISA in clinical diagnostics, molecular biology, agriculture, sericulture, food technology, environmental science, biomedical research and other related fields.
Owner:COUNCIL OF SCI & IND RES
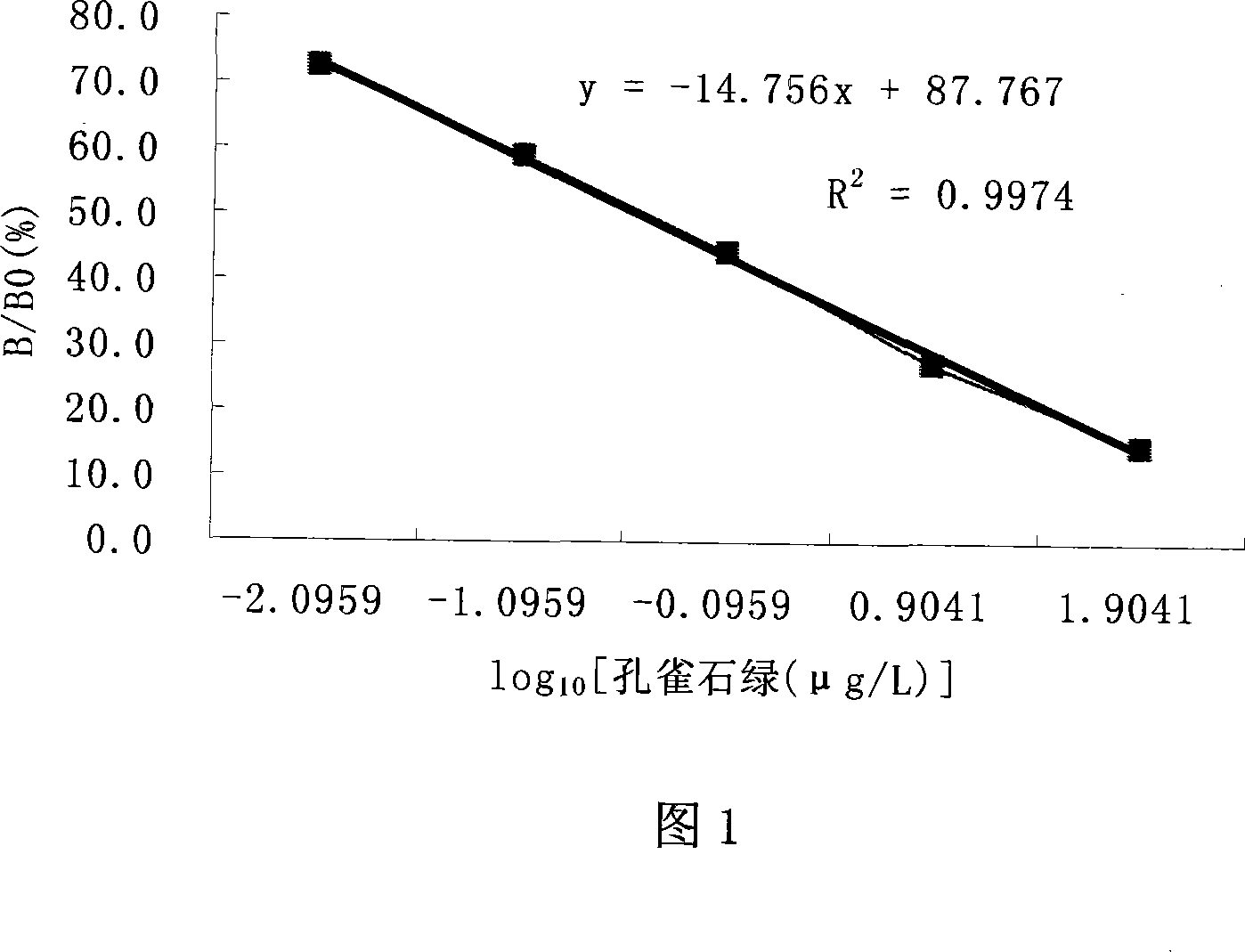
Malachite green vestigial ELISA detection kit and usage method thereof
The invention discloses an enzyme immunoassay of testing the bice green residues in animal derived food, which comprises an enzyme label plate covering bice green antigen, enzyme label bice green antibody working solution, bice green standard solution, substrate solution, substrate buffer solution, reaction termination solution, concentration washing liquid and sample dilute solution. The invention further discloses a method for using the immunoassay to test bice green residues, which comprises sample pretreatment, testing via the immunoassay, processing and analyzing result. The inventive immunoassay of bice green test uses direct competition enzyme-linked immunoassay adsorption analysis technique, with high sensitivity, high stability, simplified operation, reduced reaction time, reduced error caused by complex operation, reduced cost, wide application for testing samples and high practicality.
Owner:SOUTH CHINA AGRI UNIV
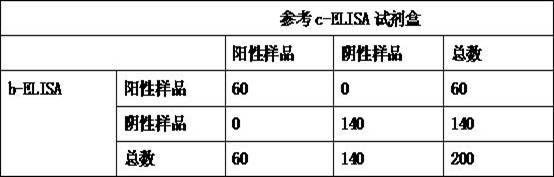
Kit for detecting antibody against Peste des petits ruminants virus b-ELISA and preparation method thereof
InactiveCN102419369AReduce economic costsLow costMaterial analysisViral antibodyEpidemiologic survey
The invention relates to the technical field of biology, particularly the field of viral antibody detection. A kit for detecting the antibody against Peste des petits ruminants virus b-ELISA comprises the following ingredients which are arranged respectively: Peste des petits ruminants nucleoprotein antigen, Peste des petits ruminants monoclonal antibody, diluent, strong positive serum, weak positive serum, negative serum, HRP sheep anti-mouse secondary antibody, 20 times the concentration of washing liquid, substrate liquid, stopping solution and enzyme-linked immunosorbent plate. The optimum proportion of each ingredient in the kit is determined by experiments. The kit can be used for rapid diagnosis and detection of animal Peste des petits ruminants virus antibody, especially for the antibody detection of a lot of samples in the epidemiological survey of Peste des petits ruminants. The detection method of Peste des petits ruminants virus b-ELISA has different detection principle and experiment operating procedures and the like from those of a c-ELISA detection method in a BIRAD laboratory. The Peste des petits ruminants nucleoprotein antigen and Peste des petits ruminants monoclonal antibody in the kit are self-developed. The detection sensitivity, singularity and other indexes of the kit are the same with those of the c-ELISA detection method in the internationally recognized BIRAD laboratory.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR +1
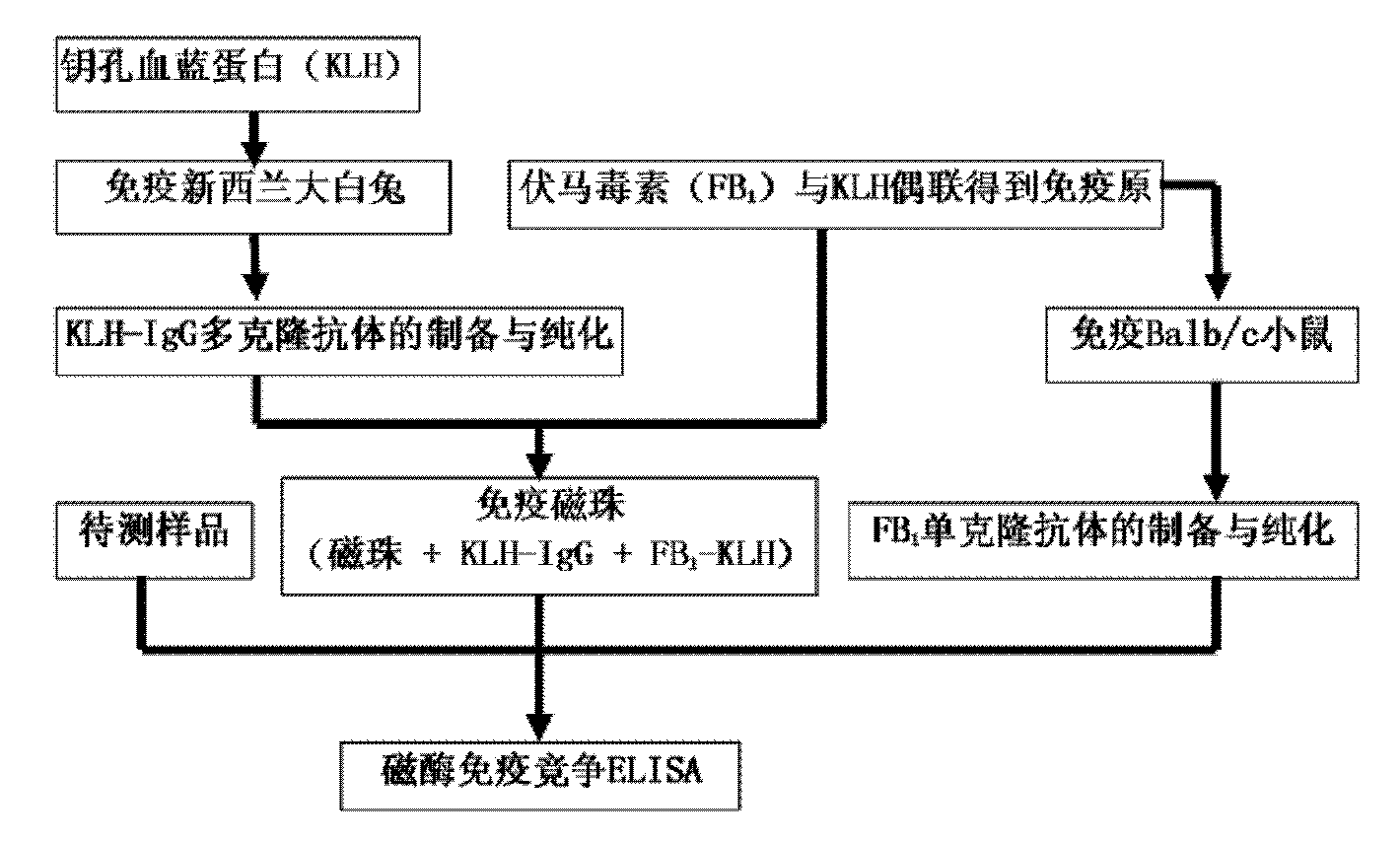
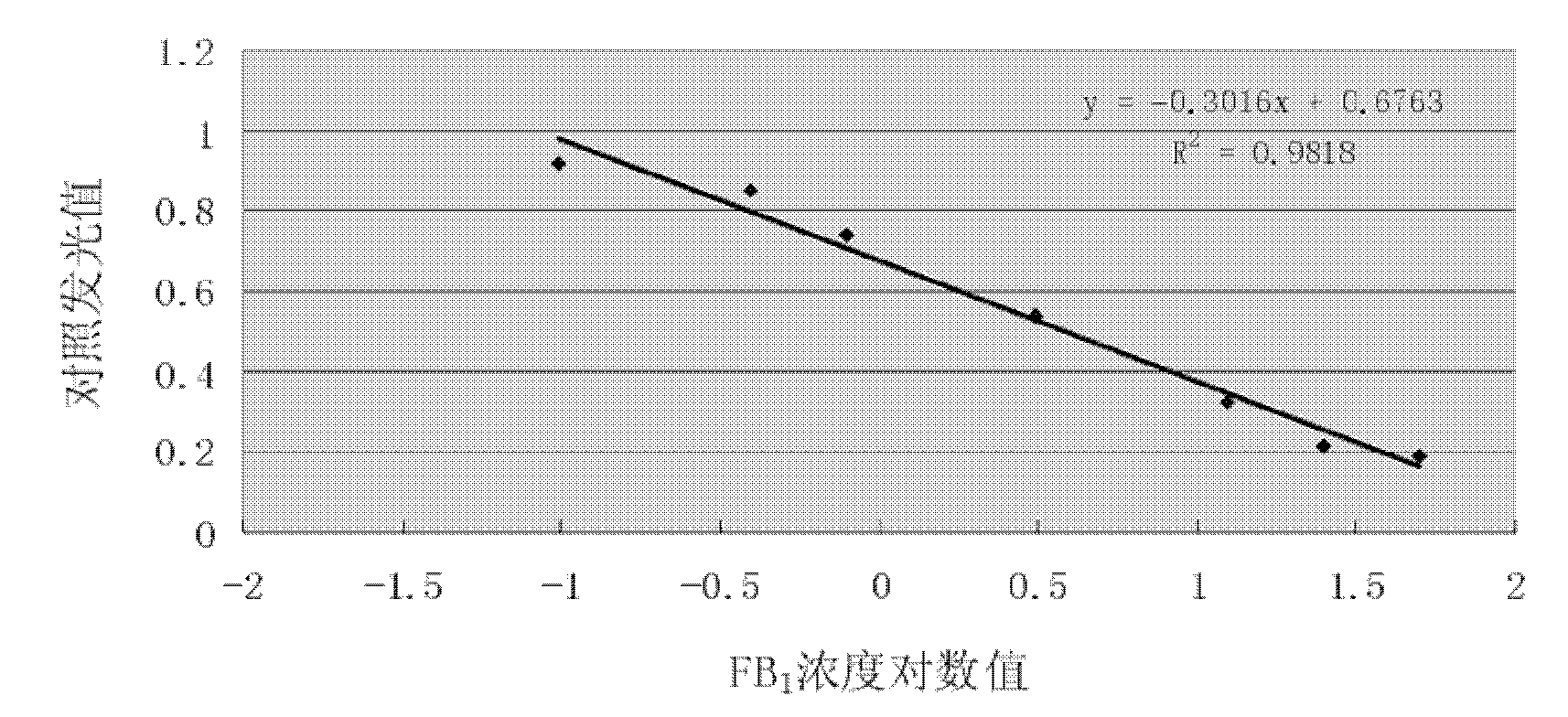
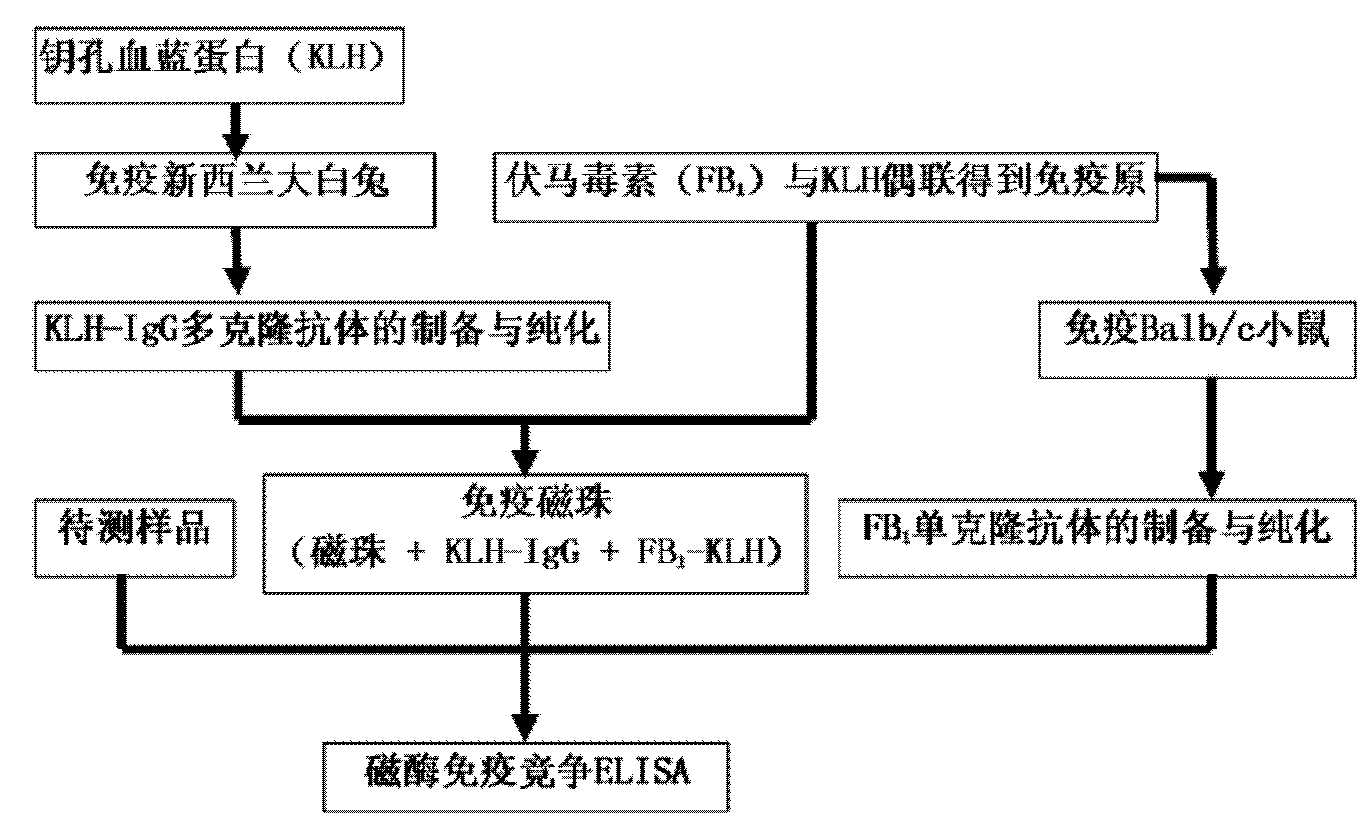
Method for detecting fumonisins based on immuno-magnetic bead combined enzyme-linked immunosorbent assay
InactiveCN101865924AStrong specificityEasy to separateMaterial analysisMagnetic beadMonoclonal antibody
The invention relates to a method for detecting fumonisins based on immuno-magnetic bead combined enzyme-linked immunosorbent assay, belonging to the technical field of chemical detection. The method comprises the steps of: preparing fumonisins-KLH conjugate and fumonisins-OVA conjugate, combining the fumonisins-KLH conjugate with an immuno-magnetic bead, and preparing a magnetic bead for detecting the fumonisins; then, using the fumonisins-KLH conjugate to prepare fumonisins monoclonal antibody, applying a competition ELISA method for detecting the fumonisins together with the magnetic bead for detecting the fumonisins, and obtaining the magnetic bead for detecting the fumonisins by a magnetic separating method; and developing and obtaining the detection result by an enzyme linked immunosorbent assay. The method is used for the fumonisins sample which is lower than detection limit, and enlarges the combination superficial area by enrichment of the immuno-magnetic bead and the full diffusion of the magnetic bead in the liquid, thus indirectly changing the detection limit, improving the detection sensitivity and avoiding undetected error.
Owner:SHANGHAI JIAO TONG UNIV
Enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody, test method and application thereof
InactiveCN101839917AStrong specificityIncreased sensitivityDepsipeptidesFermentationAntigenDuck hepatitis A virus
The invention relates to an enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody and relates to a test method and application of the kit. The kit comprises an enzyme label plate coated by the recombinant VP1 (virus protein) protein, a rabbit anti-duck IgY antibody marked by horseradish peroxidase, a TMB substrate colour reagent, a positive serum, a negative serum and a kit specification. In the invention, by adopting the polymerase chain reaction, the VP1 genes are amplified from the DHV-1genome and the VP1 gene-containing recombinant expression plasmid pET32a-VP1 is constructed; the plasmid is transferred to host cells BL21 (DE3), and the in-vitro expression VP1 protein is purified by a nickel column and then used as the antigen; the enzyme-linked immunosorbent assay kit is established; the positive serum is the standard positive serum of duck hepatitis virus type-I and the negative control is the standard negative serum of duck. The test kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale popularization and application, very important application value in diagnosis of duck hepatitis virus type-I, survey of epidemiology and immunization survey and the like.
Owner:HENAN UNIV OF SCI & TECH
Hybridoma cell strain 2D3, monoclonal antibody to zearalenone secreted by same and application of monoclonal antibody
The invention provides a hybridoma cell strain 2D3, a monoclonal antibody to zearalenone secreted by the hybridoma cell strain 2D3 and application of the monoclonal antibody. The hybridoma cell strain 2D3 is preserved in China Center for Type Culture Collection with an accession number of CCTCC No. C201328 and can be used for preparation of a high-titer monoclonal antibody to zearalenone. According to detection results of enzyme linked immunosorbent assay (ELISA), the titer of the monoclonal antibody to zearalenone prepared through purification of mouse ascites can reach 1.5 * 10<5>. The monoclonal antibody to zearalenone has high sensitivity, half maximal inhibitory concentration IC50 of 20 pg / mL to zearalenone and cross reactivity of 4.9%, 3.3% and 3.2% with beta-zearalanel, alpha-zearalanel and beta-zeranol, respectively. The monoclonal antibody to zearalenone can be used for determination of the content of zearalenone.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
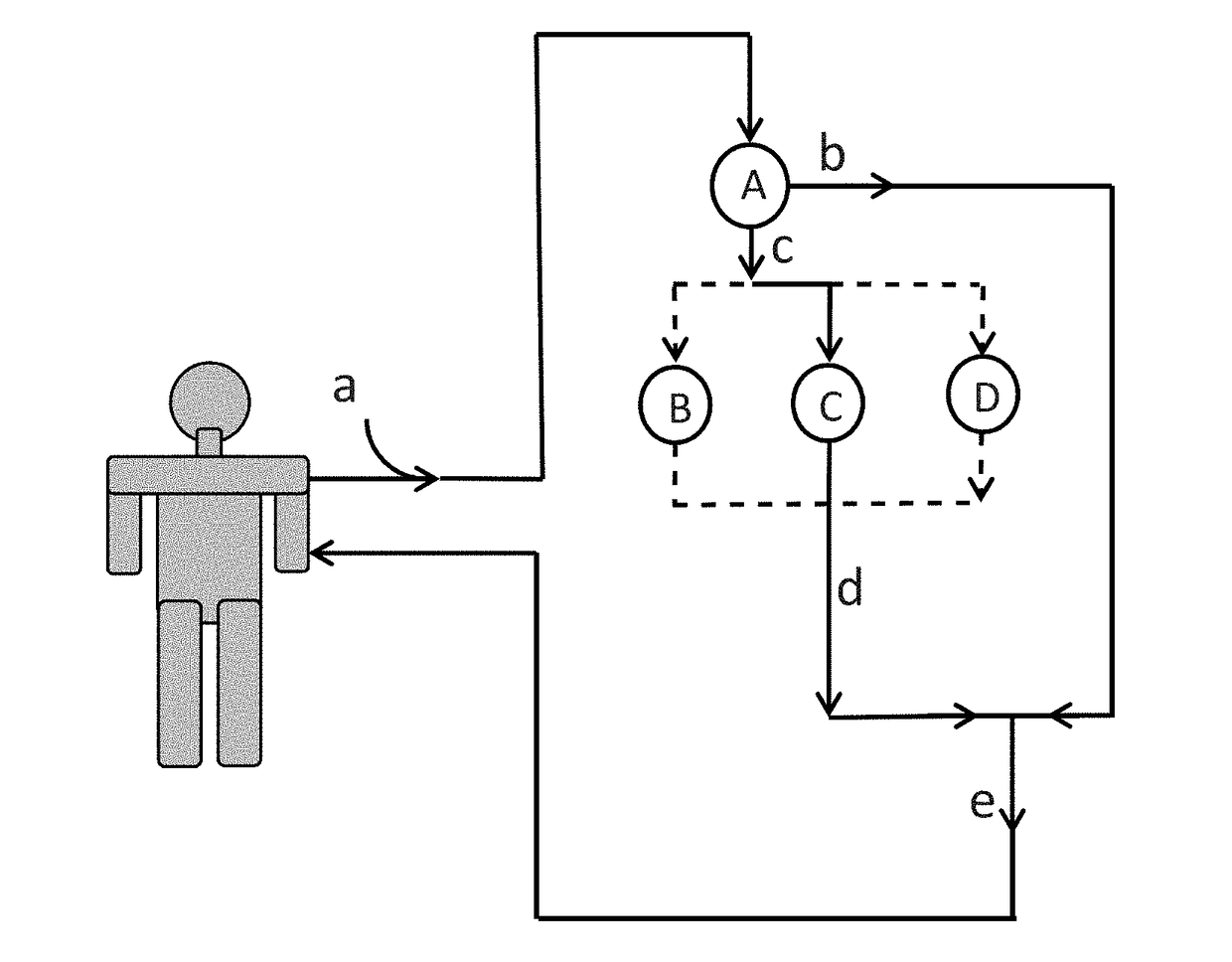
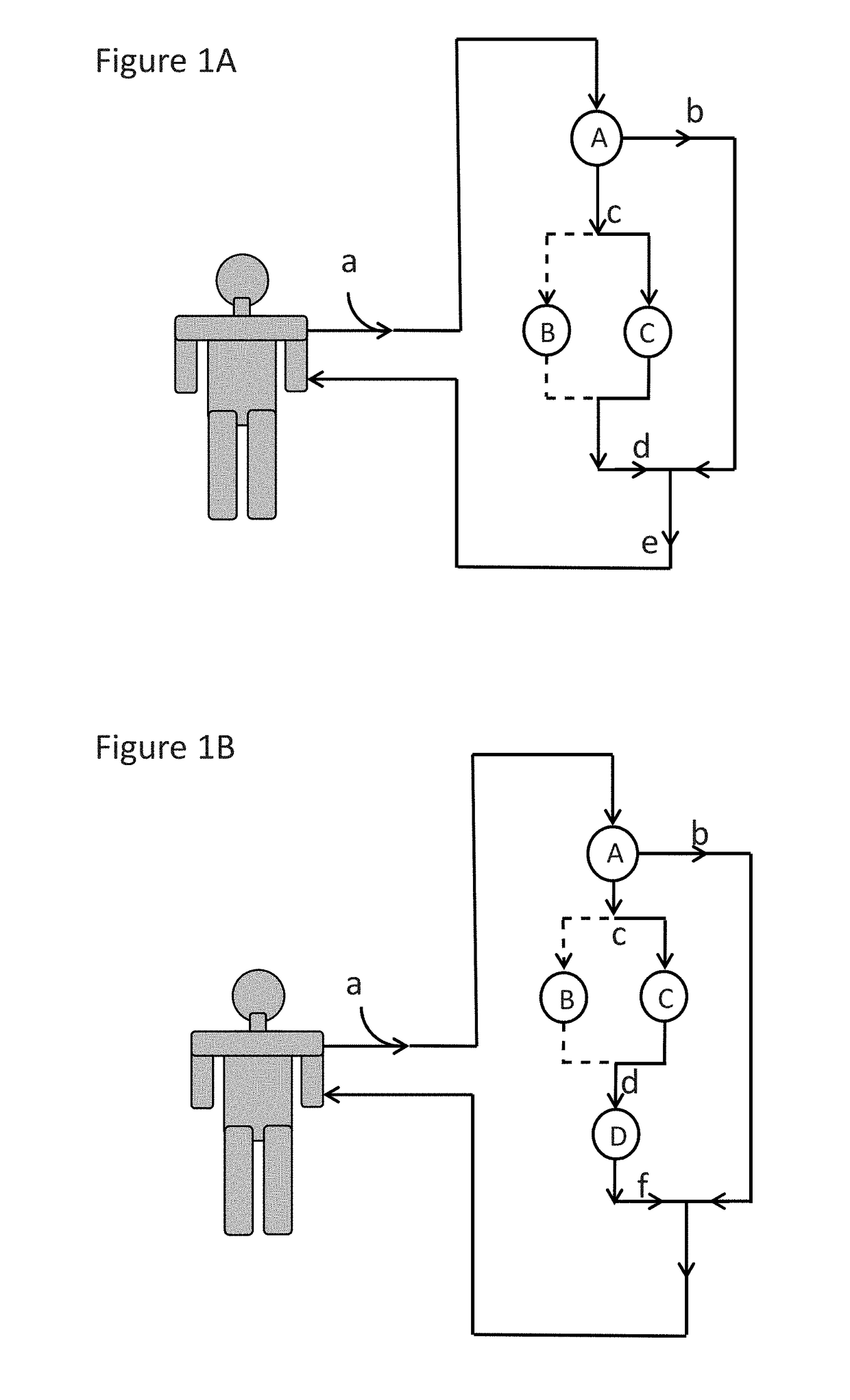
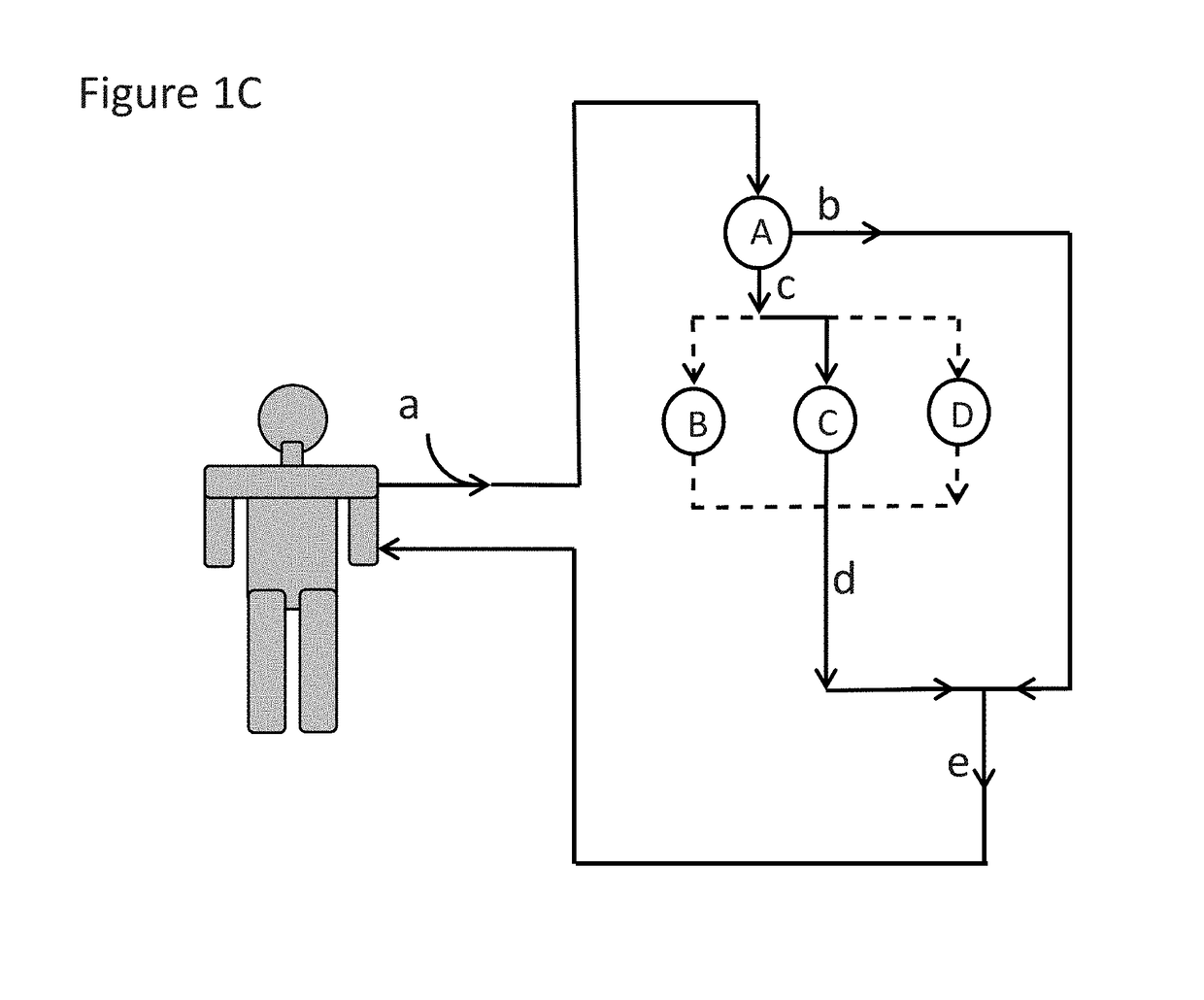
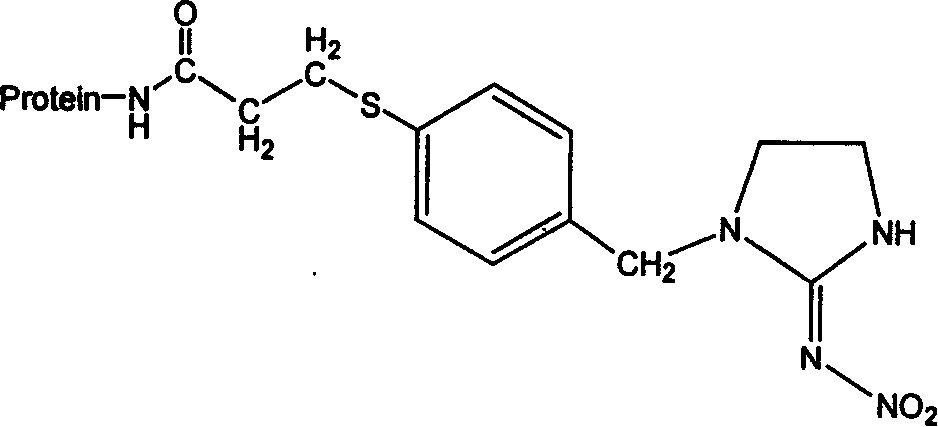
Immunoadsorption
ActiveUS20180169273A1Reduces nAb levelSevere riskInfusion devicesPharmaceutical delivery mechanismBlood plasmaNeutralizing antibody
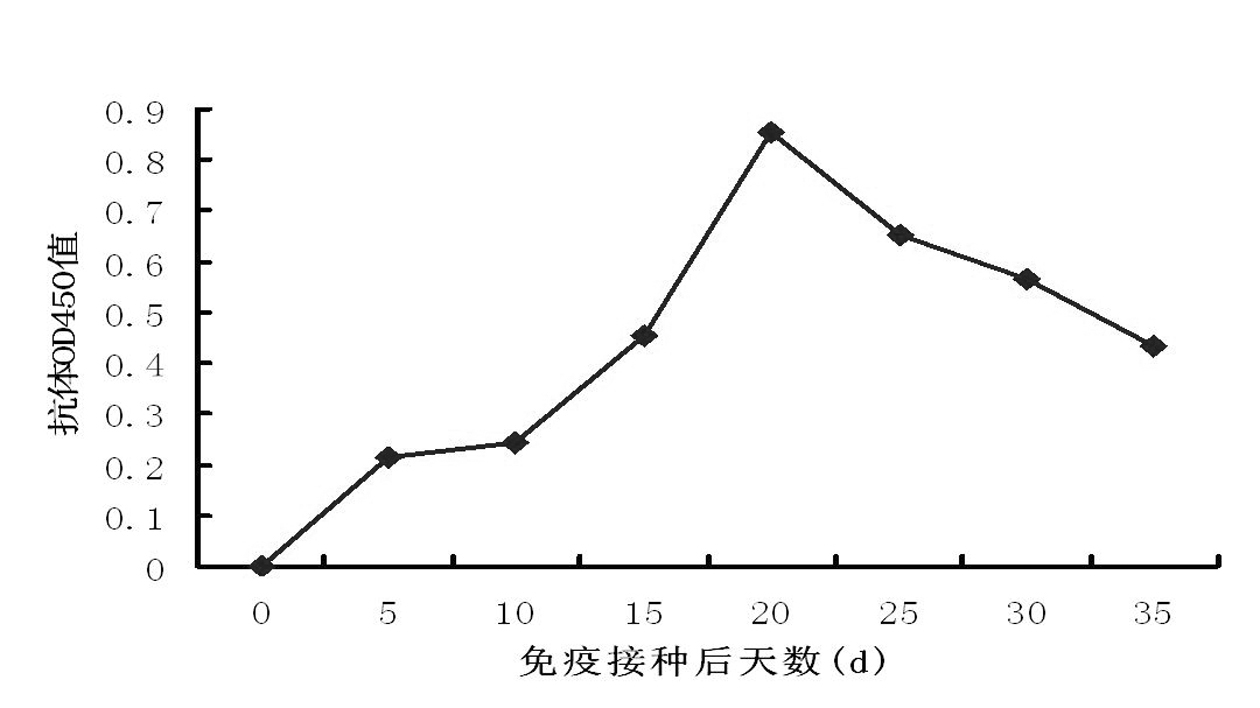
Upon administration of rAAV vectors the humoral immune response (neutralizing antibodies) is the first barrier that needs to be overcome. Surprisingly it was found that by using immunoadsorption for depletion of immunoglobulins from the blood (plasma), subjects can be highly efficiently treated with rAAV vectors, i.e. obtain highly efficient transduction after rAAV vector administration, in spite of the presence of high levels of nAb.
Owner:UNIQURE IP BV
Rapid and automated electrochemical method for detection of viable microbial pathogens
InactiveUS20040175780A1Quick checkImprove automationMicrobiological testing/measurementVolume/mass flow measurementBiotechnologyMicrobial disease
A method for in situ detection of viable pathogenic bacteria in a selective medium by measuring cathodic peak current of oxygen on cyclic voltammograms during bacterial proliferation with an electrochemical voltammetric analyzer. The rapid oxygen consumption at a time during the growth of bacteria resulted in a sharp decline of the cathodic peak current curves. The detection times (threshold values) obtained from the cathodic peak current curve were inversely related to the concentrations of the pathogenic bacteria in the medium. This method for detection of pathogenic bacteria is more sensitive than nucleic acid-based polymerase chain reaction (PCR) methods and any of antibody-based methods such as enzyme-linked immunosorbent assay (ELISA) technology, electrochemical immunoassays, immunosensors, and it has a sensitivity similar to conventional culture methods and impedimetric methods but is more rapid than both of them. A calibration curve was obtained by plotting initial cell concentrations (CFU / ml) determined by conventional plate counting, as a function of the detection time.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Vitamin D synthetic antigen, and preparation method and preparation thereof
ActiveCN103588872AQuick checkAchieve batchOvalbuminSerum albuminVitamin D synthesisBovine serum albumin
The invention provides a vitamin D synthetic antigen and a preparation method thereof. The vitamin D synthetic antigen is a conjugate of vitamin D and a protein carrier. The vitamin D is 25-hydroxy vitamin D3 or 1,25-dihydroxy vitamin D3; and the protein carrier is one or more selected from bovine serum albumin, ovalbumin, hemocyanin and human serum albumin. The invention also provides application of the vitamin D synthetic antigen to vitamin D immunological detection. The invention further provides a vitamin D detection kit, which integrates the advantages of existing clinical vitamin D detection methods; and the kit can be applied to all enzyme mark instruments, chemiluminescence instruments and time-resolved analyzers, and has greatly shortened detection time, and sensitivity, accuracy and precision met the detection requirements. The immunosorbent assay kit with strong versatility provided by the invention can realize batch and rapid detection on vitamin D in serum (or plasma).
Owner:BEIJING BOHUI INNOVATION TECH
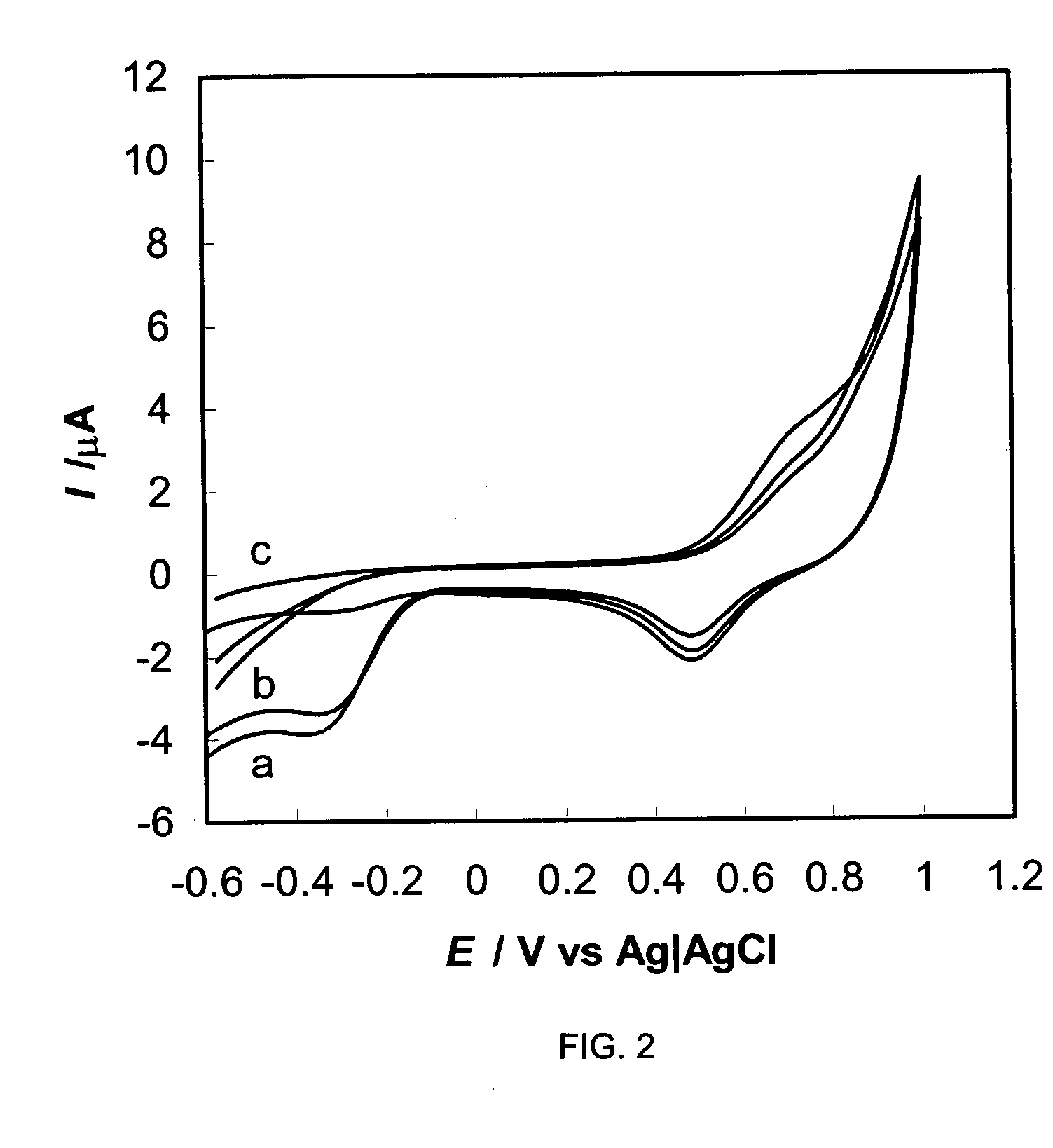
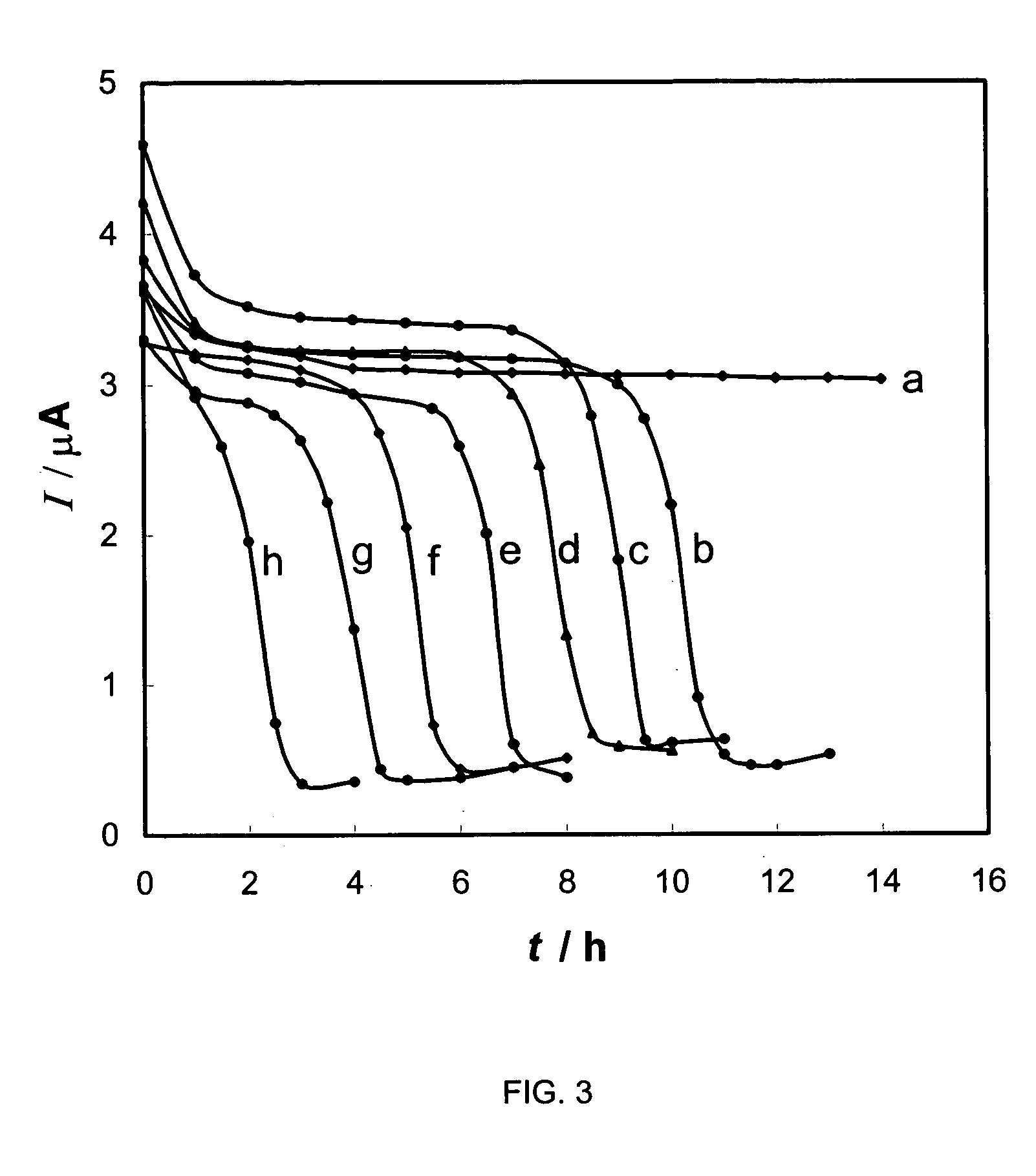
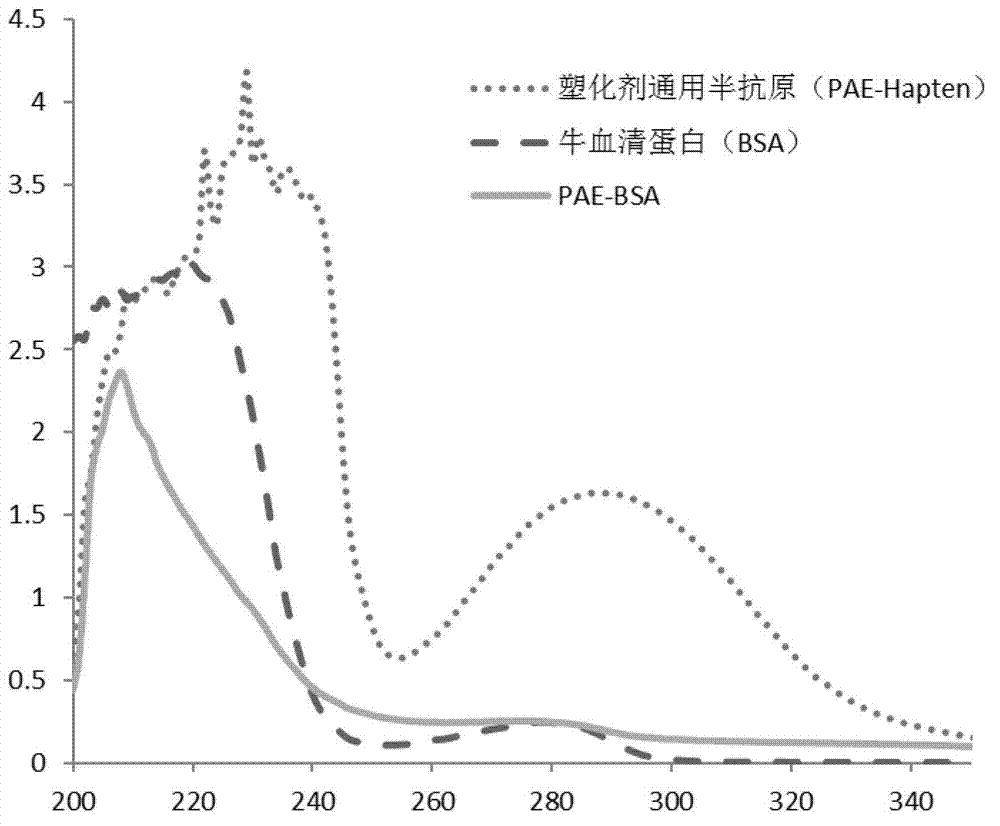
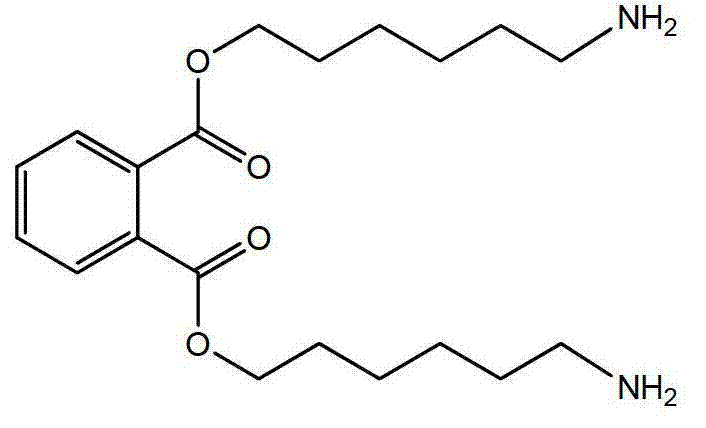
Synthetic method of general artificial antigen of phthalate plasticizers for immunodetection
ActiveCN103204925AImprove versatilityHigh sensitivityOrganic compound preparationOvalbuminCarrier proteinAntiserum
A synthetic method of a general artificial antigen of phthalate plasticizers for immunodetection belongs to the field of bio-chemical engineering technology. The synthetic method of the present invention comprises the following steps of carrying out an esterification reaction between phthalic acid and 6-(fluorenyl methoxy carbonyl acyl-amino)-1-hexanol, removing fluorene methoxy carbonyl acyl protecting groups to form a hapten, and coupling with amino from carrier protein to obtain the general artificial antigen of phthalate plasticizers. Experimental results disclose that titer of antiserum obtained by immunizing animals by the antigen of the invention is up to 160000;the 50% inhibiting concentration IC50 for DBP, DEHP and DINP is less than 500 ng / ml; and the generated antibodies have good generality and high sensitivity. The antigen or antibodies of the invention can be used for the establishment of enzyme-linked immunosorbent assay and colloidal gold test paper rapid detection methods, thus can be used for rapid detection of residues of phthalic acid ester plasticizers in food, and have broad application prospects.
Owner:JIANGNAN UNIV
Single-domain antigen-binding proteins that bind mammalian IgG
The present application relates to antigen-binding proteins that are capable of binding to mammalian IgG. The frame-work regions of the antigen-binding proteins of the application preferably correspond to those of antibodies naturally that are devoid of light chains as may e.g. be found in camelids. The application further relates to nucleic acids that encode such antigen-binding proteins, to immunoadsorbent materials that comprise such proteins, to the uses of such immunoadsorbent materials for the purification of mammalian IgG antibodies and for therapeutic apheresis.
Owner:BAC IP
Magnetic particle-based quantitative chemiluminescent assay kit for anti-ribosome P protein antibody IgG, and preparation and detection methods thereof
InactiveCN105954266AEasy to useGuaranteed detection effectChemiluminescene/bioluminescencePolyclonal antibodiesBovine serum albumin
The invention discloses a magnetic particle chemiluminescence quantitative assay kit for anti-ribosomal P protein antibody IgG. The kit includes: anti-ribosomal P protein antibody IgG calibrator; Ribosomal P protein antigen and bovine serum albumin-labeled Tris buffer; alkaline phosphatase-labeled goat anti-human polyclonal antibody and bovine serum albumin in Tris buffer; streptavidin-labeled magnetic particles and bovine serum albumin Serum albumin in Tris buffer; wash solution. Based on the traditional membrane strip immunoassay and enzyme-linked immunosorbent assay, the detection method of the kit increases the sensitivity and linear range by 3-5 orders of magnitude, and realizes quantitative detection in a real sense, with rapid response, reliable results, and It can be used in conjunction with a fully automatic chemiluminescence immunoassay analyzer to realize fully automatic use, and has irreplaceable important value for clinical diagnosis.
Owner:北京贝尔医疗设备有限公司
Fabrication method and application for citrinin immune chromatography detection test paper
InactiveCN1603823ASuitable for on-site rapid detectionSimple and fast operationBiological testingAntigenCitrinin
This invention discloses a citrinin immune chromatography test paper and its process method and utility. The test paper comprises pad, sample pad, tracing particle compound pad, pyroxylin film, adsorption pad, test thread, and control thread. The method is the following: to orderly put sample pad, tracing particle compound pad, pyroxylin film and adsorption pad on the pad; the tracing particle compound pad has citrinin antibody with tracing particle mark; the pyroxylin film has test thread and control thread; the test thread has citrinin antigen and antibody of citrinin antigen.
Owner:AIBIT BIOTECH INSTR
Human adiponectin enzyme-linked immunosorbent detection kit, preparation method and application thereof
The invention provides a human adiponectin enzyme-linked immunosorbent detection kit, a preparation method and application thereof. The kit comprises antihuman APN monoclonal antibody-coated ELISA plates, recombinant gAPN protein standard, antihuman APN monoclonal detection antibodies, HRP-streptomycin, 10 mM of phosphate buffer with a pH of 7.4, TMB enzyme-substrate chromogenic solution, 0.1 M of phosphate buffer with a pH of 7.4 and 2 M of H2SO4. The invention also comprises the preparation method of the kit and the application of the kit in the detection of human-blood APN content. The kit has the advantages that the kit is convenient and fast to use and operate, capable of getting detection results within 2 hours, high in specificity, low in cost and convenient for clinical large-scale use.
Owner:袁洪 +2
Synthetic method used for preparing adsorbent for clearing pathogenic antibody by oxidizing periodate
ActiveCN102000550ASimple methodEase of mass productionOther chemical processesAntibody adsorptionOrganic chemistry
The invention relates to a synthetic method used for preparing an adsorbent for clearing a pathogenic antibody by oxidizing periodate, and discloses a method for preparing a protein immunoadsorption material by oxidizing the periodate by taking agarose gel as a carrier. The method comprises the following steps of: preparing aldehyde group-containing agarose gel by oxidizing the agarose gel by using the periodate; coupling the aldehyde group-containing agarose gel and immunoglobulin binding protein; and blocking a material and reducing or reducing directly without blocking to obtain the immunoadsorption blood purification material. The method has the characteristics of simple process, environmental friendliness, low cost and the like; and simultaneously the product has high specificity, adsorptive property, regenerability and the like, and can be used for clinical immunoadsorption treatment practically.
Owner:GUANGZHOU KONCEN BIOSCI
ELISA adsorption analysis method for measuring gross amount of malachite green and colorless malachite green in water sample and aquatic products
InactiveCN101482559AHigh sensitivityEasy to handleComponent separationBiological testingMalachite greenMalachite green stain
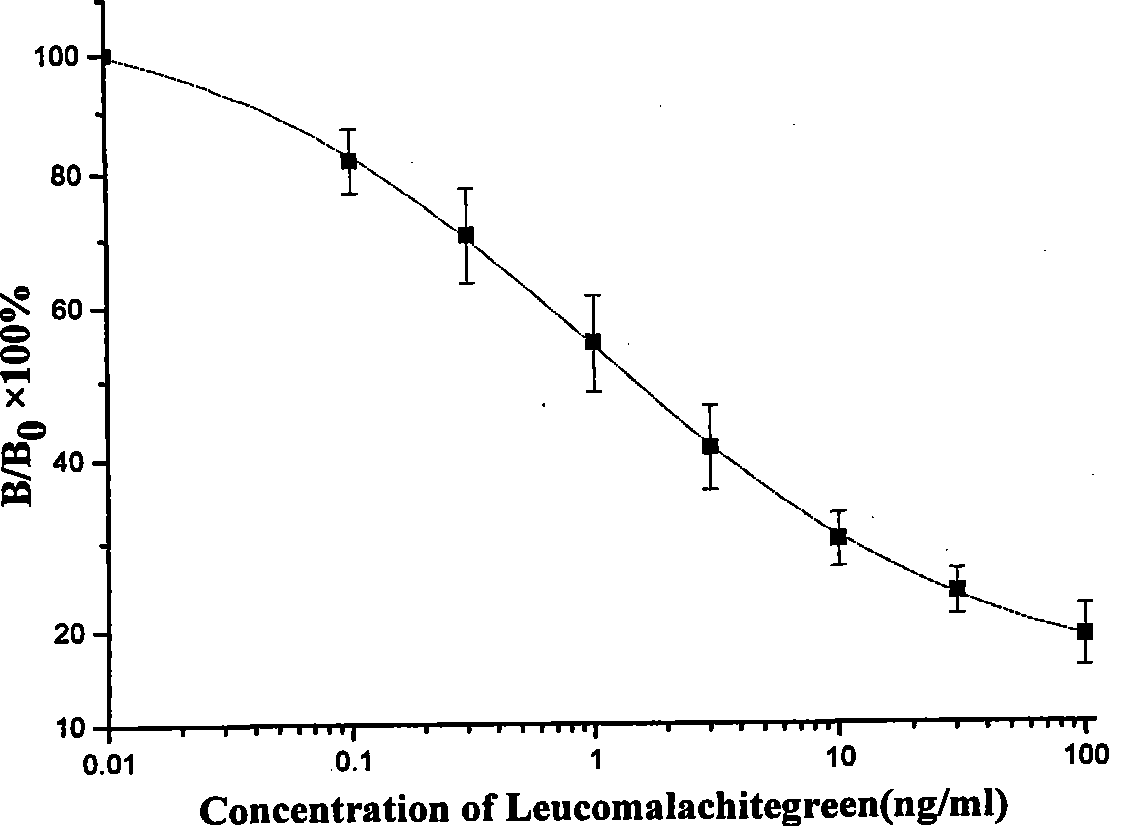
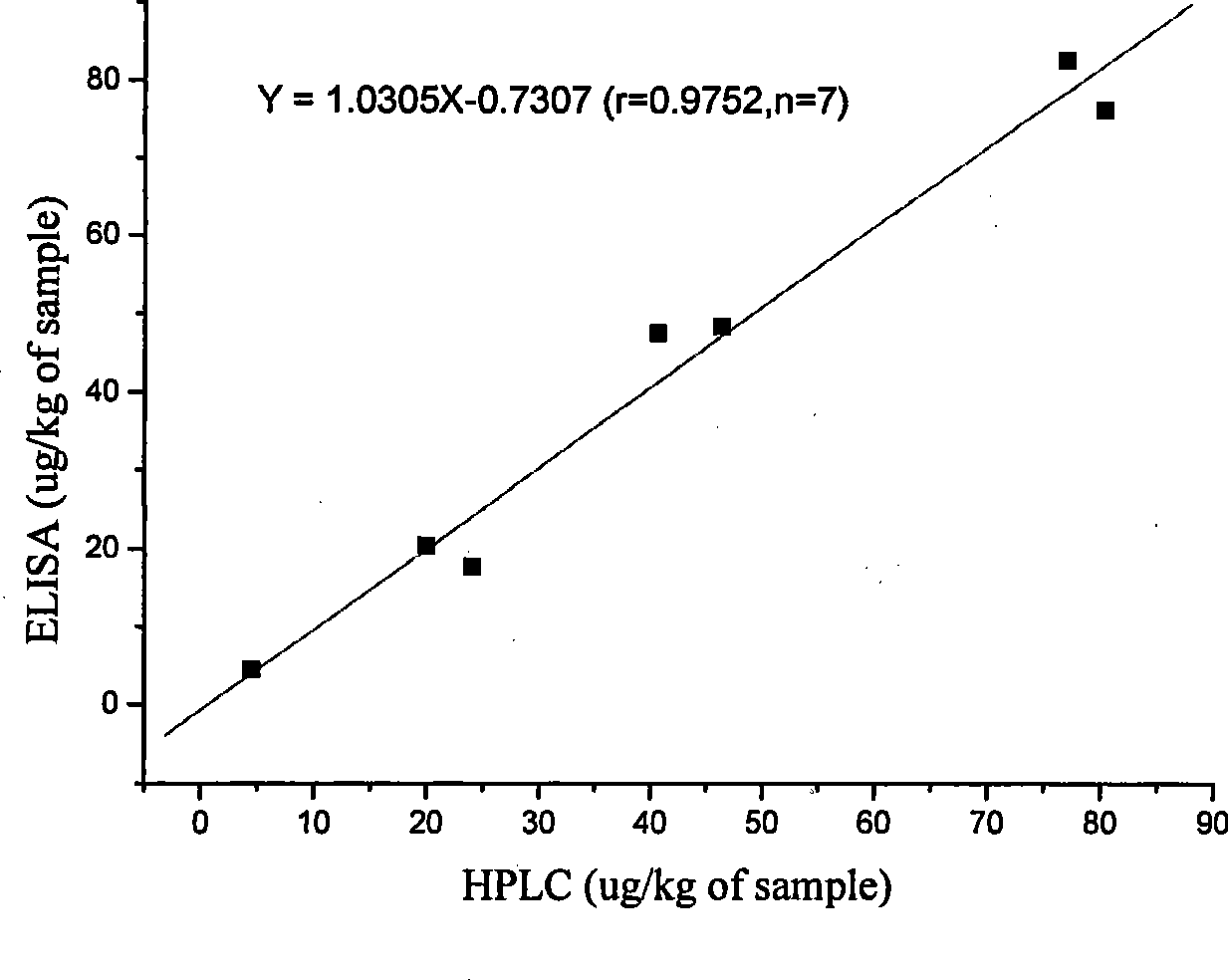
The invention discloses an enzyme linked immunosorbent assay method (ELISA) for detecting the total amount of diamond green and blank diamond green in measurement water sample and aquatic products, characterized in that: the modified compound of amido blank diamond green is synthesized and linked with the protein to produce immunogen and envelope antigen, and the polyclonal antibody of rabbit-anti colorless diamond green is obtained by immuning animal and the concentration range of ELISA standard curve is 0.1-100ng / mL, IC50 is 0.9-2.6ng / mL and the recovery of standard addition is 76.2-95%, the correlation coefficient of ELISA and HPLC is 0.975, n=7; because the cross-reaction rates of the prepared antibody and diamond green are respectively 95.25%, the ELISA can be used for measuring the total amount of the diamond green and blank diamond green without any oxidation step.
Owner:SICHUAN UNIV
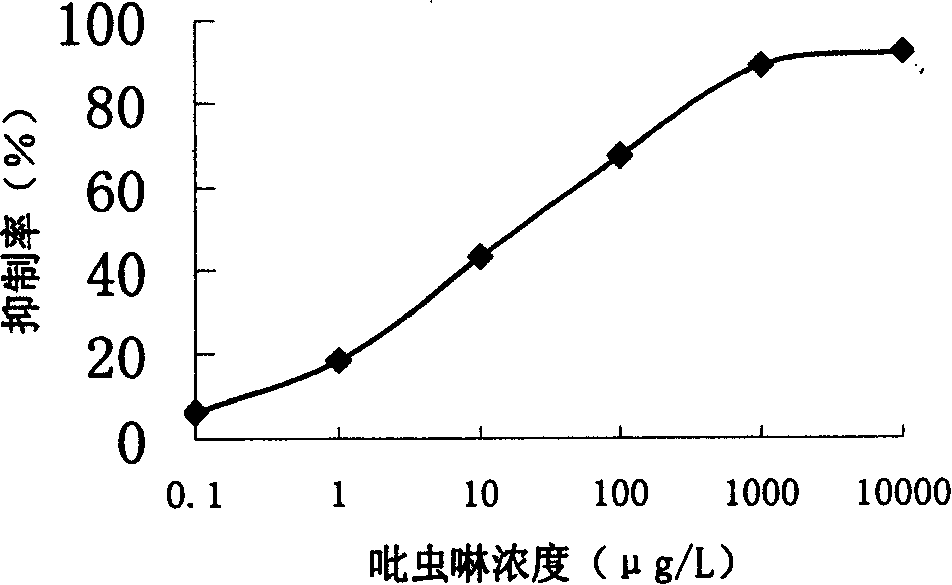
Production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody
InactiveCN1569840AEasy to handleFast and accurate analysis and detectionImmunoglobulinsTesting food2-ImidazolineImidacloprid
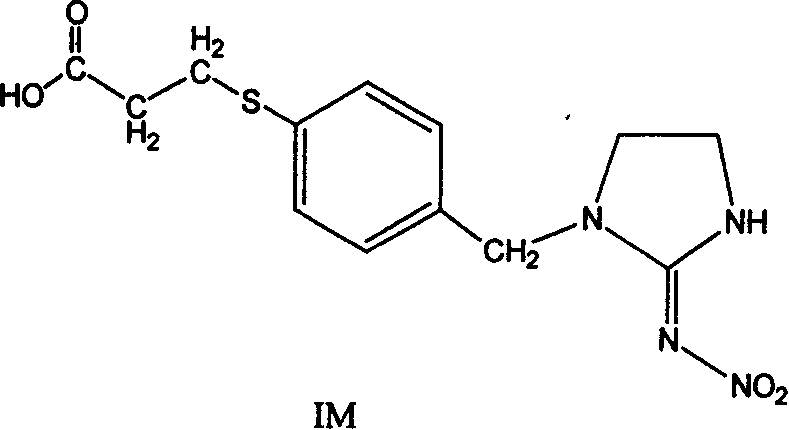
The invention discloses the production method and use for imidacloprid artificial hapten, artificial antigen and specific antibody, wherein the production method comprises, using imidacloprid (1-(6-chlorine-3-picolyl)-N-nitro-2-imidazoline imine) as raw material for reaction with 3-mercaptopropionic acid under alkaline condition, thus synthesizing hapten 1-(6-(2-carboxyethyl) sulfo-3-picolyl)-N-nitro-2-imidazoline imines (IM), then coupling with proteins through carbodiimide method and mixed anhydride method to prepare artificial antigens (immunogens and peridium antigens).
Owner:ZHEJIANG UNIV
Kit for detecting cyclic citrullinated peptide (CCP) and immunoglobulin G (IgG) resistant bispecific antibody
InactiveCN101957365ANo pollution threatSimple and fast operationColor/spectral properties measurementsSerum igeBispecific antibody
The invention discloses a kit for detecting a cyclic citrullinated peptide (CCP) and immunoglobulin G (IgG) resistant bispecific antibody, which belongs to the field of clinical laboratory science. The kit of the invention is developed based on an enzyme-linked immunosorbent assay technology, utilizes a double-antigen sandwich method, comprises two antigens of CCP and IgG and can detect a naturalbispecific antibody existing in the serum of a rheumatoid arthritis patient, is convenient for use and can simply and easily detect the bispecific antibody.
Owner:BEIJING HOSPITAL
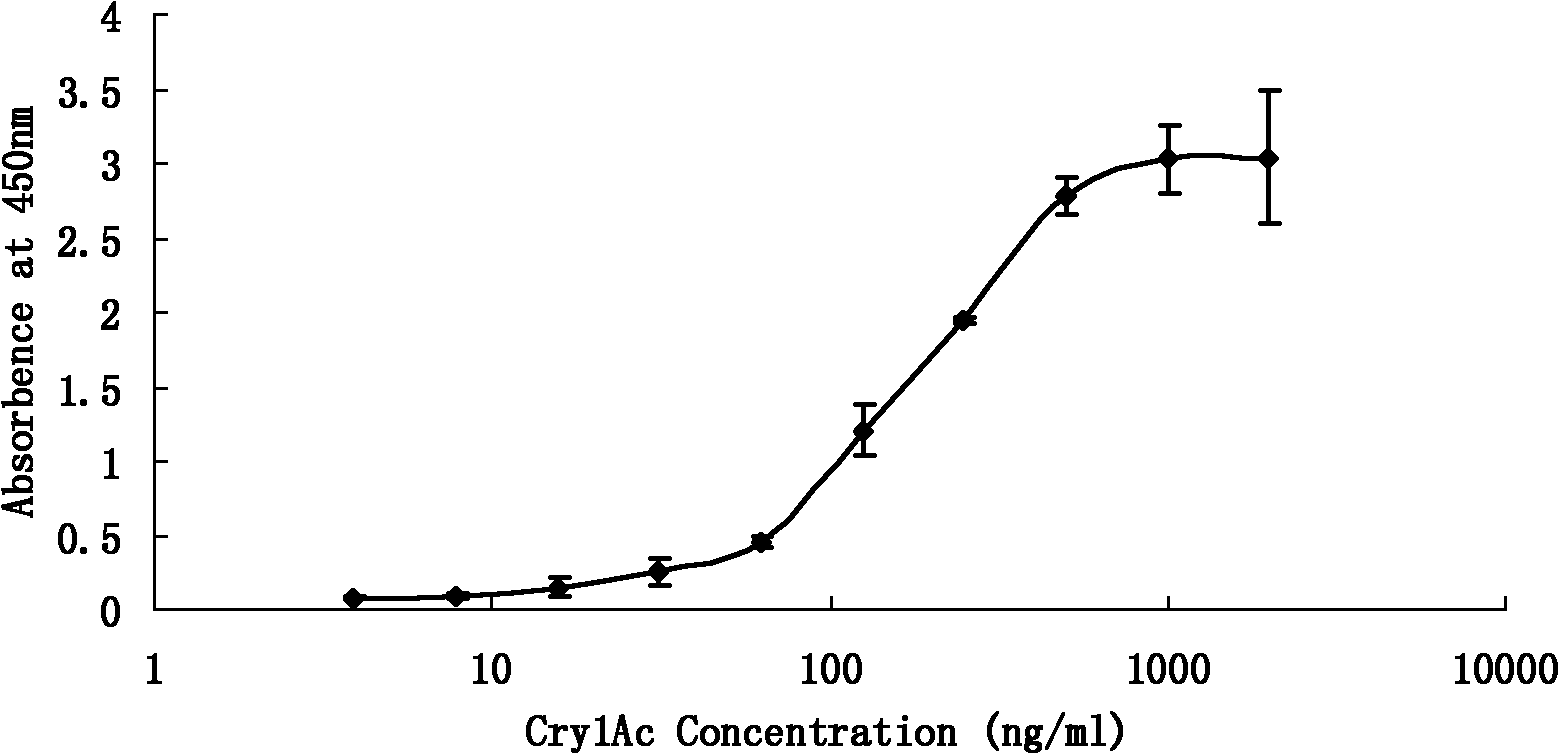
Insecticidal crystal protein CrylAc enzyme linked immunosorbent detection kit
InactiveCN102095855AReduce testing costsMaterial analysis by observing effect on chemical indicatorBiotechnologyProtein detection
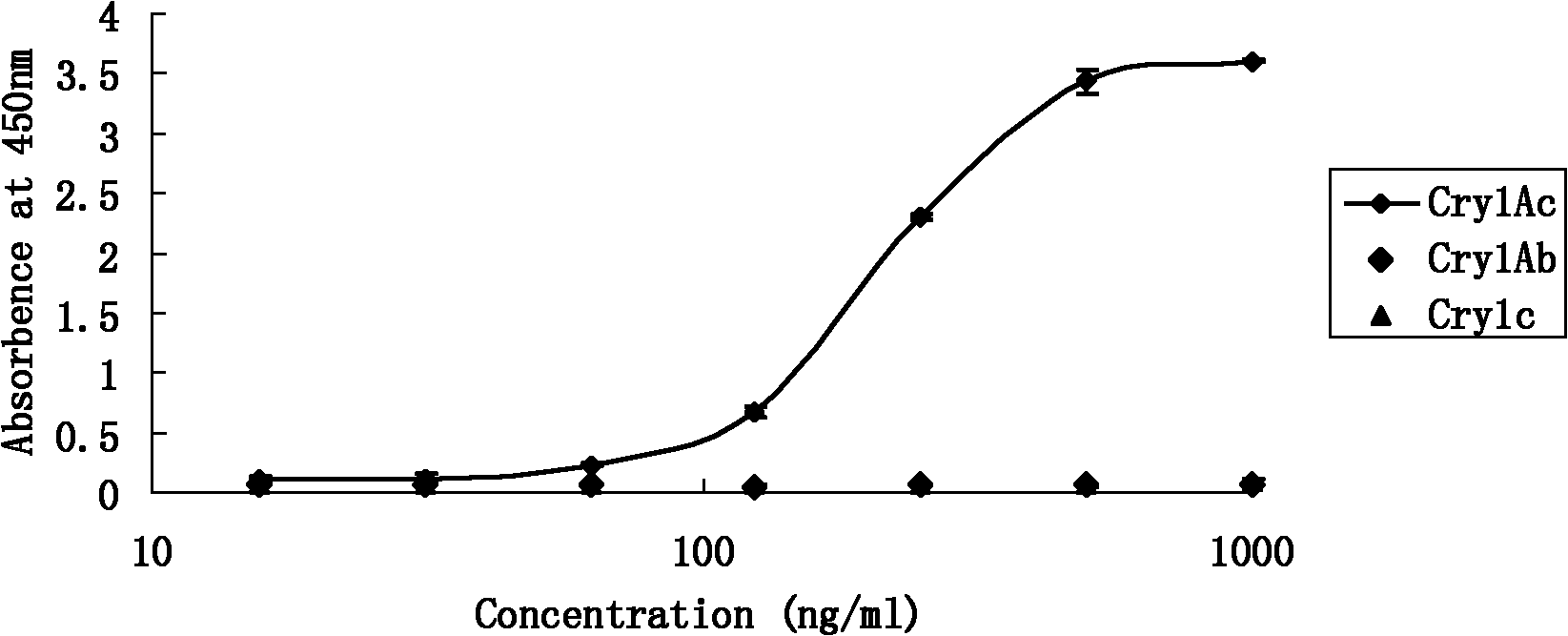
The invention discloses an insecticidal crystal protein CrylAc enzyme linked immunosorbent detection kit. In the CrylAc protein detection of the kit, the lowest detection limit is 7.81ng / mL, the linear detection range is 62.5-500ng / mL, the linear interval fitted equation y is equal to 1.1136Ln(x)-4.164, and R2 is equal to 0.9991. No cross reaction occurs among the kit and CrylAb and Crylc proteins. The kit is suitable for qualitative detection of the CrylAc protein in transgenic plant leaves, fruits and derivatives. The kit can detect large batch of samples simultaneously, is convenient and fast, has very important realistic significance in analyzing related transgenic articles, simultaneously ensures the detection cost to be greatly reduced and has potential economic value.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com