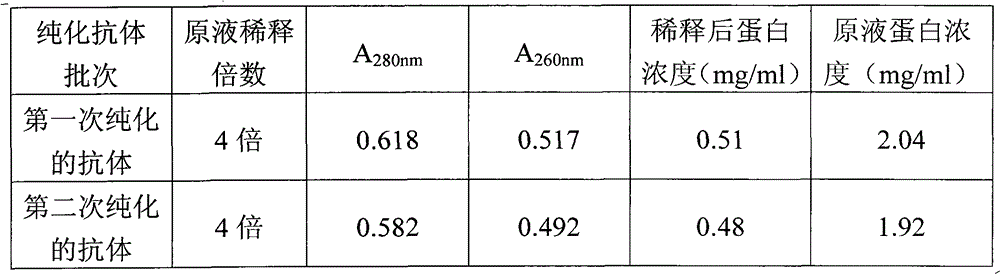
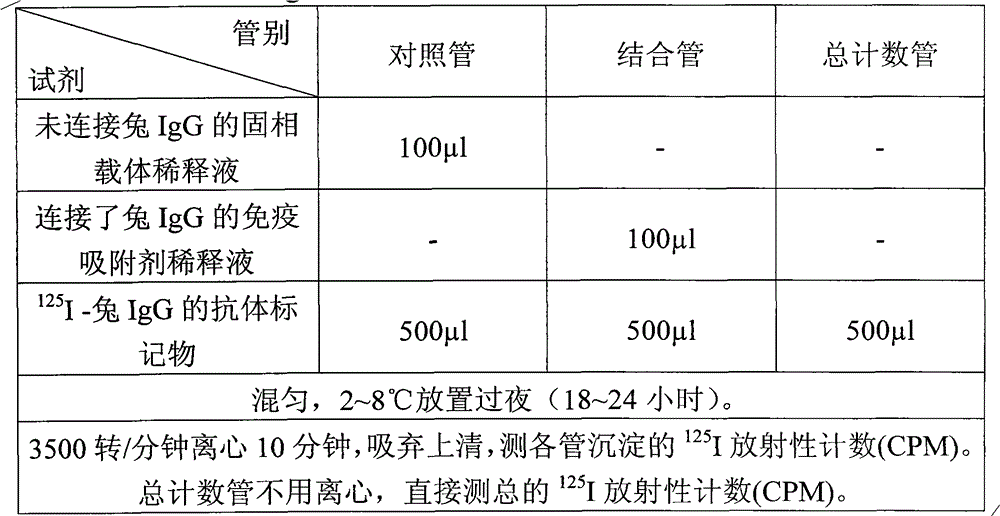
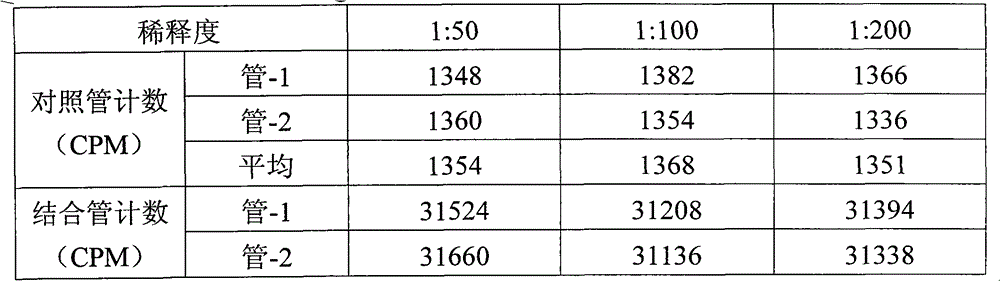
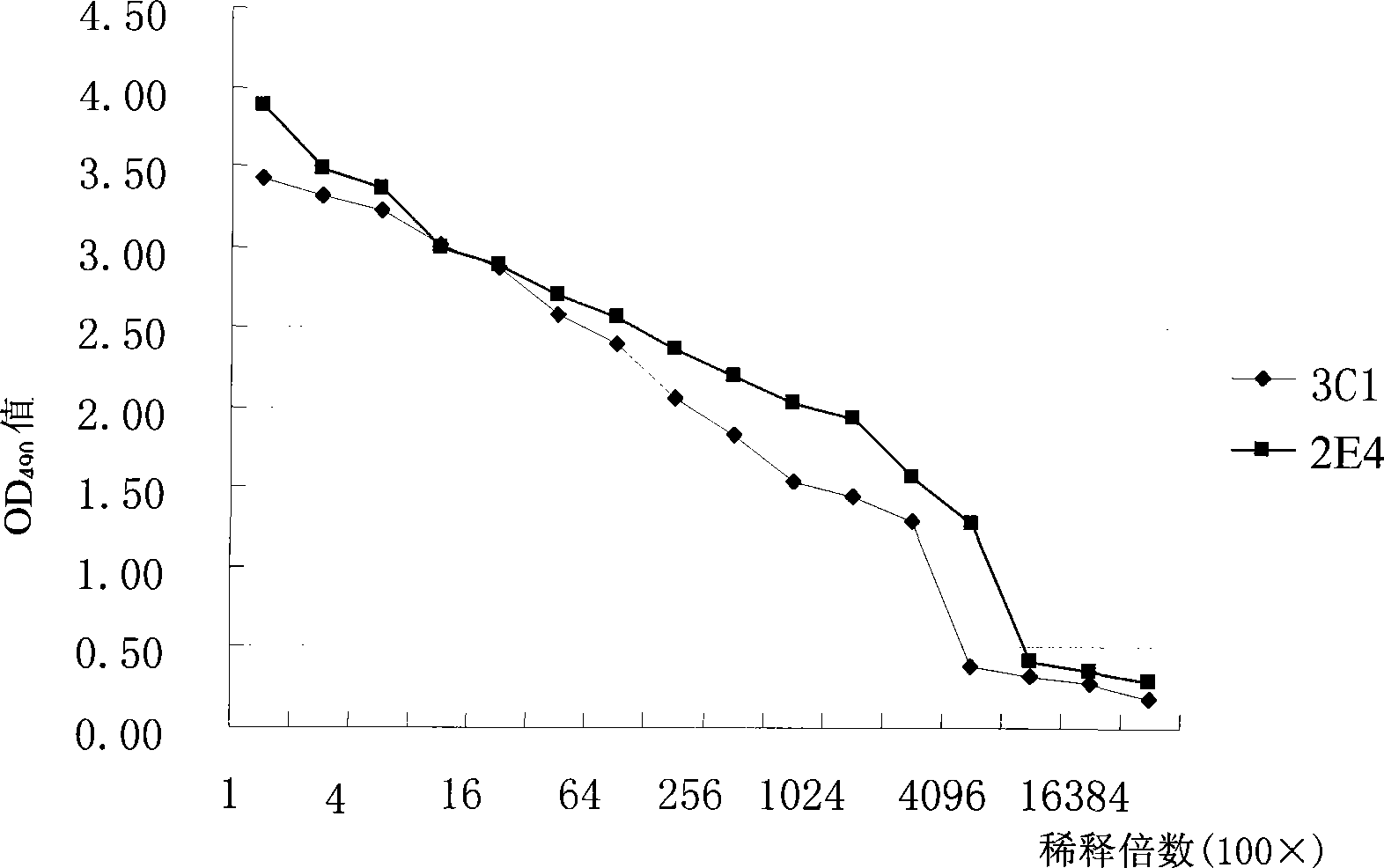
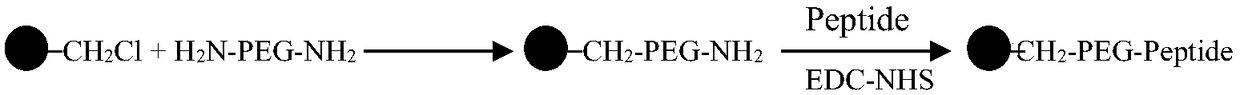Patents
Literature
111 results about "Immunosorbents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An insoluble support for an ANTIGEN or ANTIBODIES that is used in AFFINITY CHROMATOGRAPHY to adsorb the homologous antibody or antigen from a mixture. Many different substances are used, among them SEPHAROSE; GLUTARALDEHYDE; copolymers of ANHYDRIDES; polyacrylamides, etc.
Integrated method for enriching and detecting rare cell in biological fluid sample
ActiveCN101587043APromote universal applicationLow costMicrobiological testing/measurementPreparing sample for investigationRed blood cellFluorescence
Owner:CYTTEL BIOSCI BEIJING
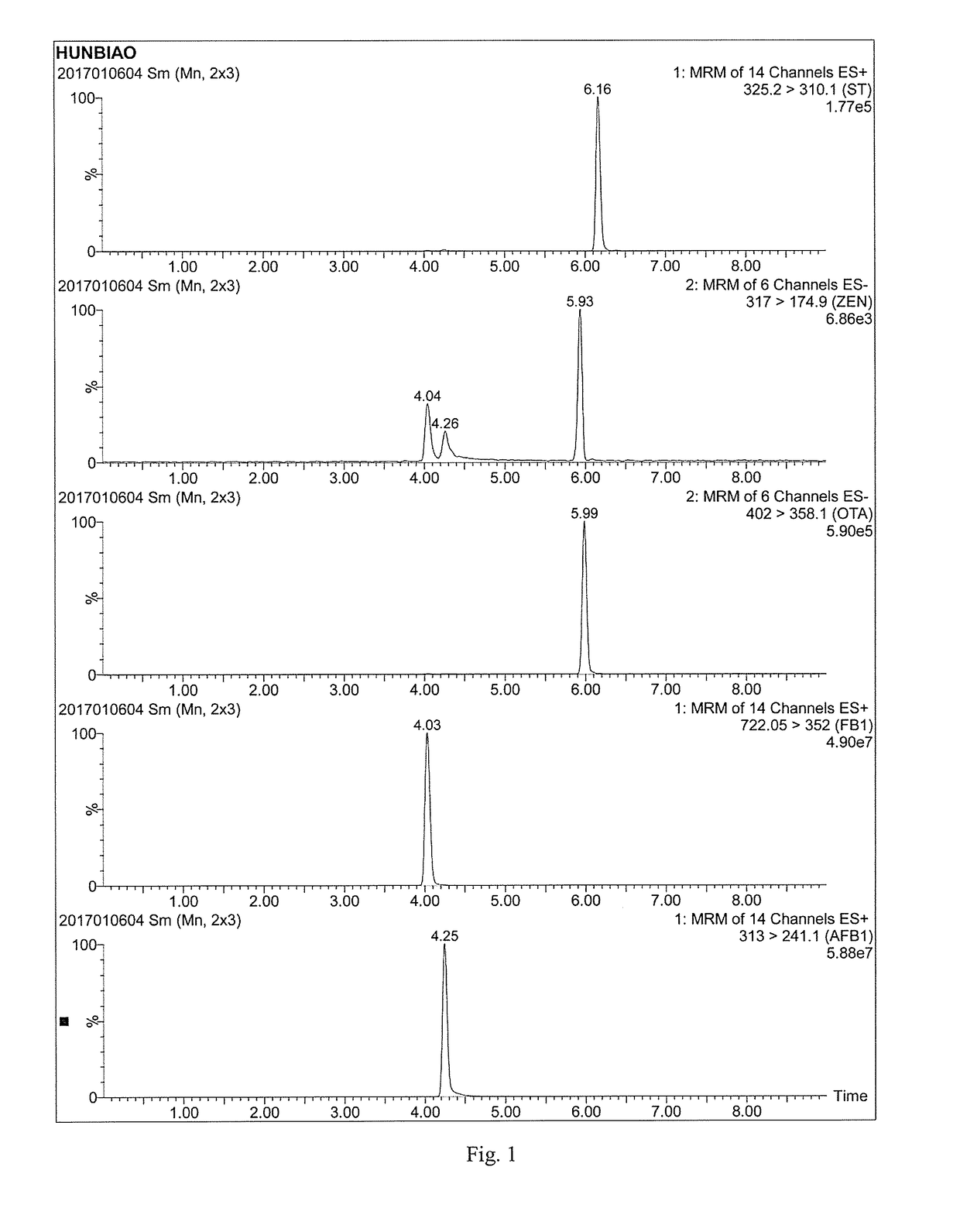
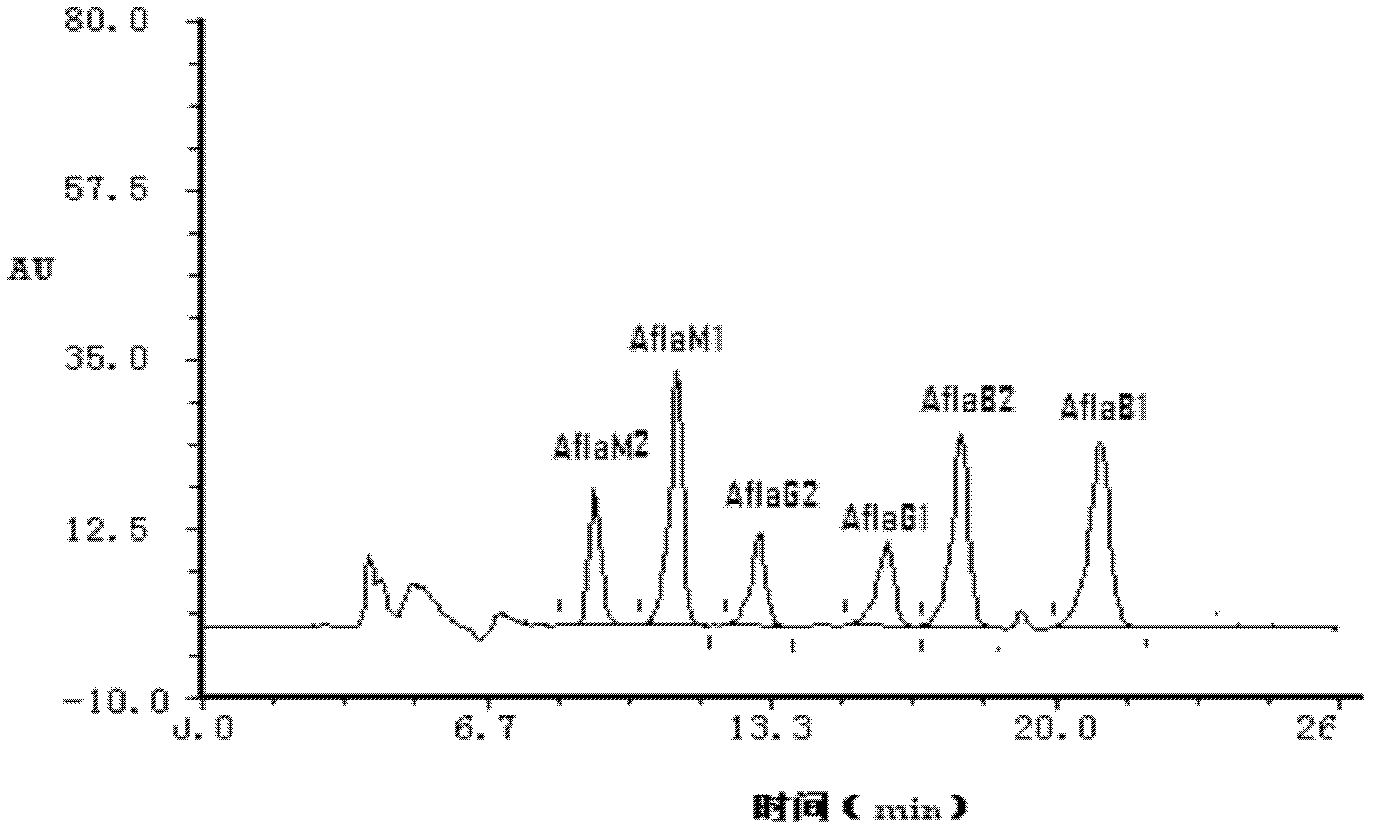
Aflatoxin nano-antibody immunosorbent, immunoaffinity column and preparation methods and application of aflatoxin nano-antibody immunosorbent and immunoaffinity column
ActiveCN103861569AImprove stabilityHigh temperature resistantOther chemical processesComponent separationImmunosorbentsGene
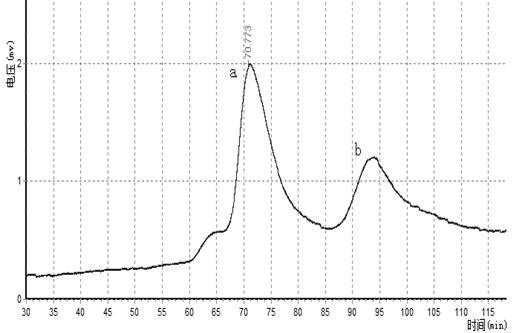
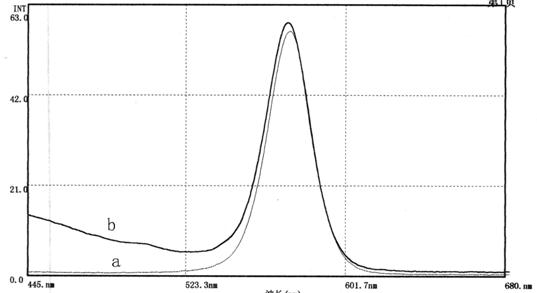
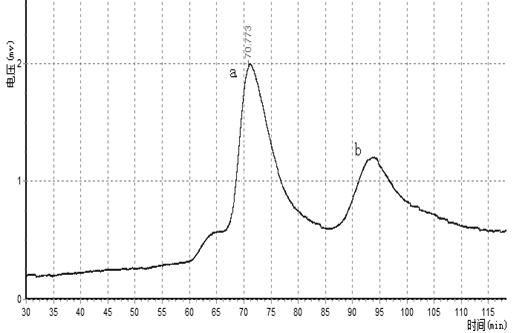
The invention relates to an aflatoxin nano-antibody immunosorbent, an aflatoxin nano-antibody immunoaffinity column and preparation methods and application of the two. The immunosorbent comprises a solid phase carrier and an aflatoxin B1 nano-antibody 2014AFB-G15 coupled with the solid phase carrier, the half maximal inhibitory concentration (IC50) of the aflatoxin B1 nano-antibody 2014AFB-G15 to aflatoxin B1 is 0.66ng / mL, and the cross reaction rates of the aflatoxin B1 nano-antibody 2014AFB-G15 to aflatoxins B2, G1, G2 and M1 are 22.6%, 10.95%, 32.1% and 26%, respectively; an amino acid sequence of the aflatoxin B1 nano-antibody 2014AFB-G15 is represented by SEQIDNO:7, and a coding gene sequence of the aflatoxin B1 nano-antibody 2014AFB-G15 is represented by SEQIDNO:8. The aflatoxin nano-antibody immunoaffinity column disclosed by the invention can be used for purifying and concentrating a sample extracting solution before machinery detection and can be repeatedly used.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
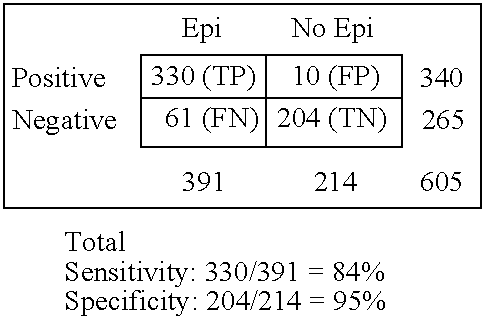
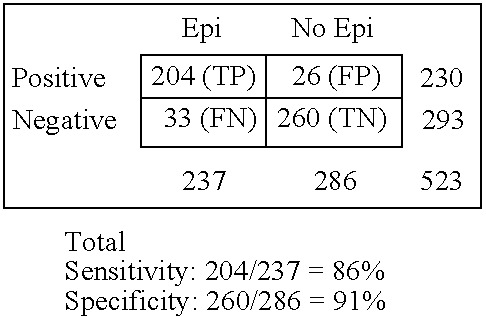
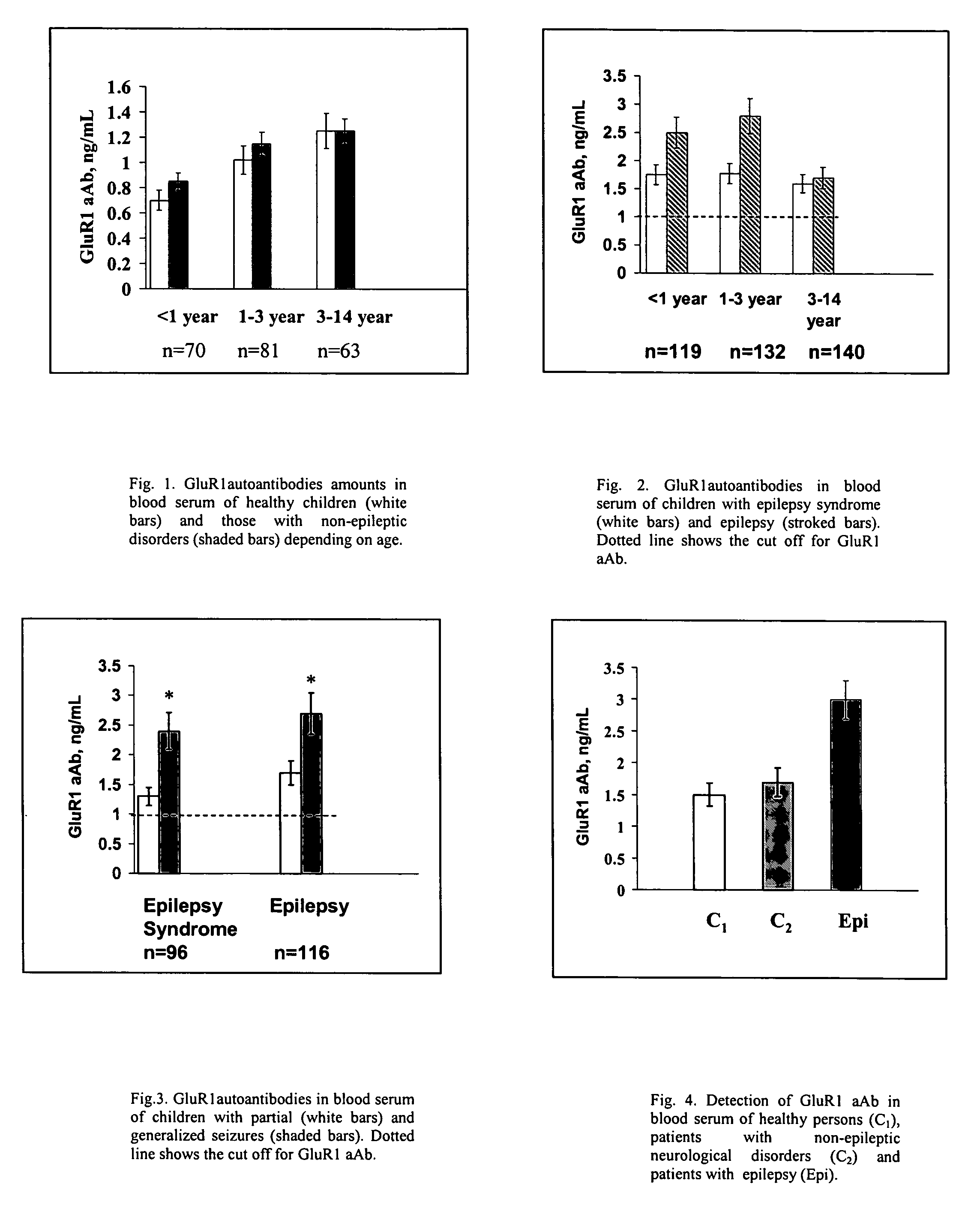
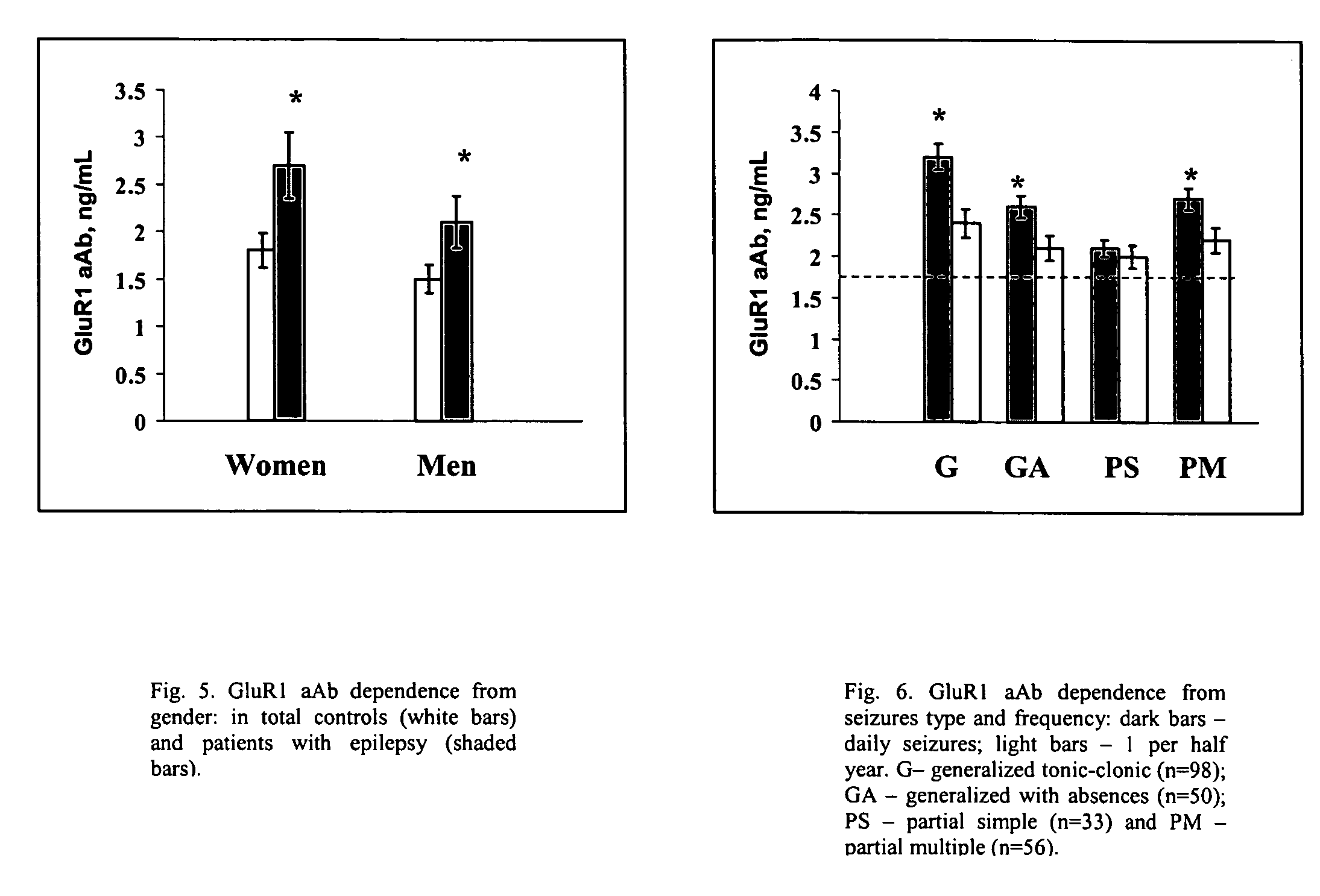
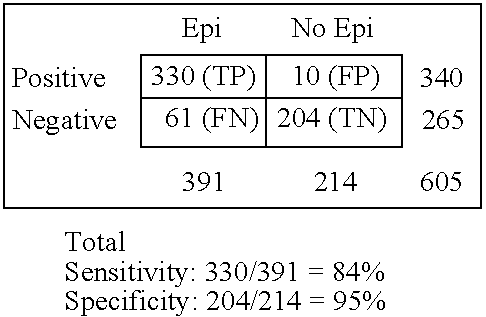
Immunosorbent blood tests for assessing paroxysmal cerebral discharges
InactiveUS20050181466A1Effective therapeutic interventionRapid inexpensiveNervous disorderDisease diagnosisDiseaseParoxysmal AF
Immunosorbents, kits and compositions for diagnosing a central nervous system disorder, particularly paroxysmal cerebral discharges and epilepsy, comprising measuring the concentration of GluR1 or fragment thereof and / or GluR1 antibodies in a biological sample from a human subject. The method is particularly useful for identifying individuals that are at risk for brain related seizures and epilepsy, for distinguishing epilepsy from pseudo-epilepsy and epilepsy-like disorders, for following up after anticonvulsive treatment, and for the adjustment of adequate therapy and doses.
Owner:GRACE LAB
Application of microdrop control in virus detection and detection method and chip
InactiveCN1912625ARealize online on-wafer inspectionNo damageMaterial analysisImmunosorbentsEngineering
A liquid micro drop control chip used in virus detection consists of top and bottom substrates as well as support structure. It is featured as preparing said substrates and said structure by micromechanical means; forming bottom substrate by bottom base plate, electrode array and insulated hydrophobic layer; forming top substrate by top base plate and hydrophobic layer; setting liquid channel, liquid storage region and detection region between top and bottom substrates.
Owner:TSINGHUA UNIV
Integrated Method for Enriching and Detecting Rare Cells from Biological Body Fluid Sample
InactiveUS20110195413A1Good cell shapeHigh recovery rateBioreactor/fermenter combinationsBiological substance pretreatmentsStainingSorbent
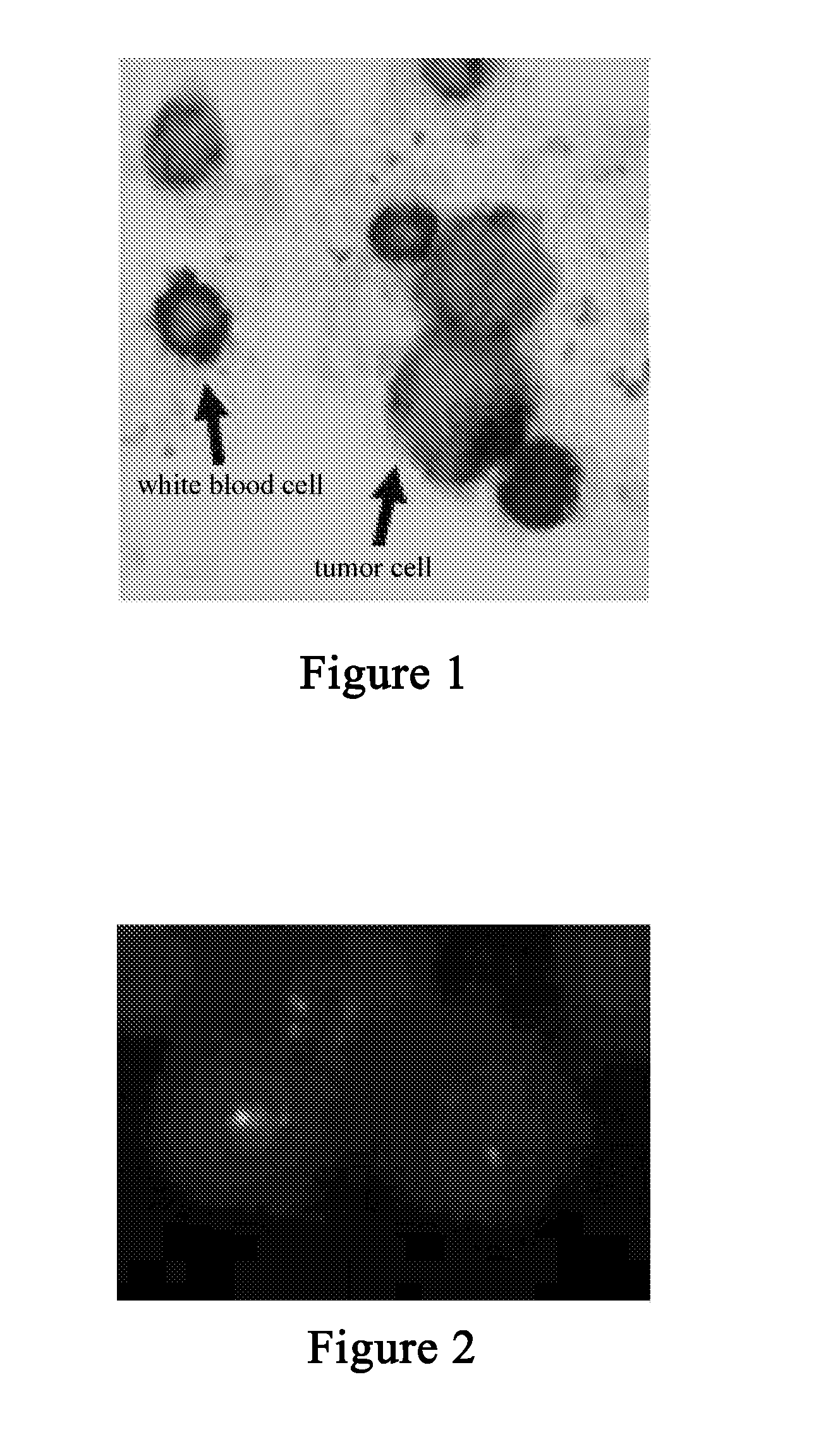
The present invention relates to an integrated method for enriching and detecting rare cells in biological body fluid sample. The enriching method comprises: (a) removing plasma protein by centrifugation; (b) optionally adding a red cell lysis solution to carry out red cell lysis so as to remove the red blood cells; (c) adding immunomicrospheres or immunoadsorbent to incubate; and (d) carrying out density centrifugation based on a special cell separation medium for separating the circulating rare cells, residual red blood cells after removing red blood cells and the white blood cells combined on the immunomicrospheres. The method for detecting the enriched rare cells according to the present invention comprises combining immunohistochemistry based staining with immunofluorescence, or bicolor, tricolor or multicolor staining based on immunohistochemistry, and observing and identifying by fluorescence or ordinary optical microscope or a scanner based on microscope principle. The novel and unique method for enriching and staining has been proved to have low cost and can rapidly, effectively and high specifically enrich and quantitatively detect the rare cells in blood.
Owner:CYTTEL BIOSCI BEIJING
Preparation of heavy metal cadmium polyclonal antibody and method for measuring enzyme linked immunity absorption
InactiveCN101240023ALow cross-reactivityTimely remedyImmunoglobulins against animals/humansBiological testingImmunosorbentsCadmium Cation
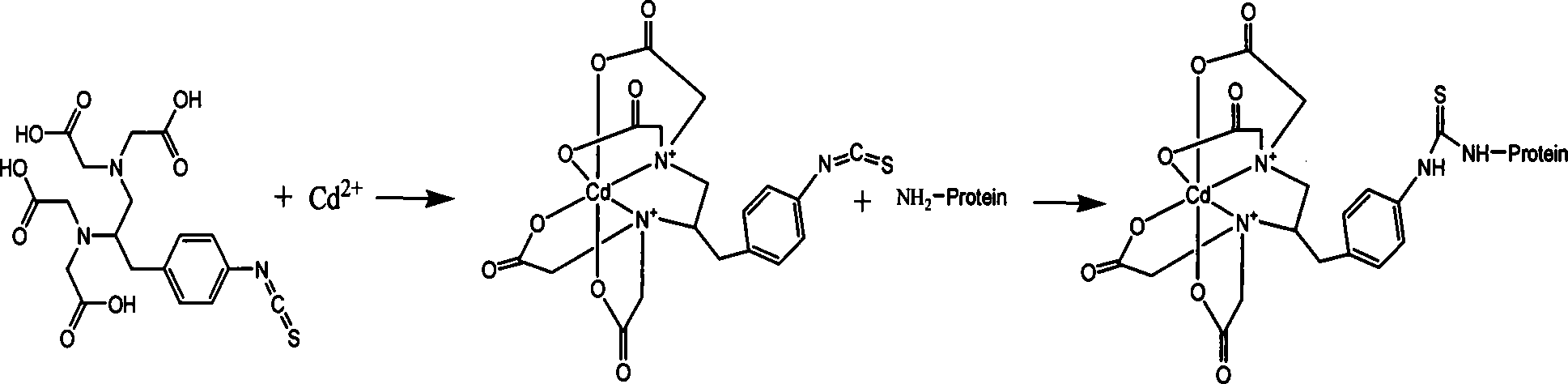
The invention discloses a preparation of polyclonal antibody of heavy metal cadmium, and a measurement method for enzyme linked immunosorbent. The preparation of polyclonal antibody of heavy metal cadmium includes steps of : (A) synthesis of complete antigen, which is critical for preparation of the polyclonal antibody; (B) preparation of the antibody. The measurement method for enzyme linked immunosorbent includes: (1) determining optimal reaction concentration of coating source Cd-ITCBE-OVA and antibody by matrix titration, and coating 96-well enzyme label plate by coating source Cd-ITCBE-OVA with optimal concentration; (2) direct competitive enzyme-linked immunosorbent assay; (3) examining impact of various reaction condition on CI-ELISA, and optimizing reaction condition; (4) establishing CI-ELISA, drawing standard inhibition curve under optimized reaction condition. Detection limit of the invention is 0.04 mu g / L, linear range is 10-1-103mu g / L. The invention is specific to cadmium, suitable for measurement of trace cadmium in ambient water. The invention also provides technical support in fast in-situ detection for sudden accident of heavy metal pollution, in order to carry out remedy and recovery for pollution accident in time.
Owner:NANJING UNIV
Method for quantitatively detecting allergen alpha-lactalbumin based on quantum dot fluorescence
InactiveCN102680705AIncreased sensitivitySimple and fast operationBiological testingFluorescence/phosphorescenceBALB/cFluorescence
The invention discloses a method for quantitatively detecting allergen alpha-lactalbumin based on quantum dot fluorescence and application of the allergen alpha-lactalbumin. The method comprises the following steps of: preparing a monoclonal antibody from alpha-lactalbumin as active immunization BALB / c mice; carrying out conjugation labeling on the alpha-lactalbumin monoclonal antibody by using a fluorescent quantum dot; forming an immunofluorescence complex by adopting a competitive immunosorbent assay; then detecting a fluorescent signal under a full-wavelength multifunctional ELIASA (Enzyme-Linked Immunosorbent Assay Apparatus); and quantitatively detecting the allergen alpha-lactalbumin in the food by establishing a standard curve. The method constructed by the invention can be widely applied to detection of relevant allergens in various powders and liquid milk products, has the characteristics of quickness, accuracy, high sensitivity, favorable repeatability, excellent specificity and the like, provides an effective means for high-throughput detection of relevant allergens in various foods as well as has a favorable popularization and application prospect.
Owner:NANCHANG UNIV
Mycotoxin composite immunosorbent, immunoaffinity column and kit, and applications thereof
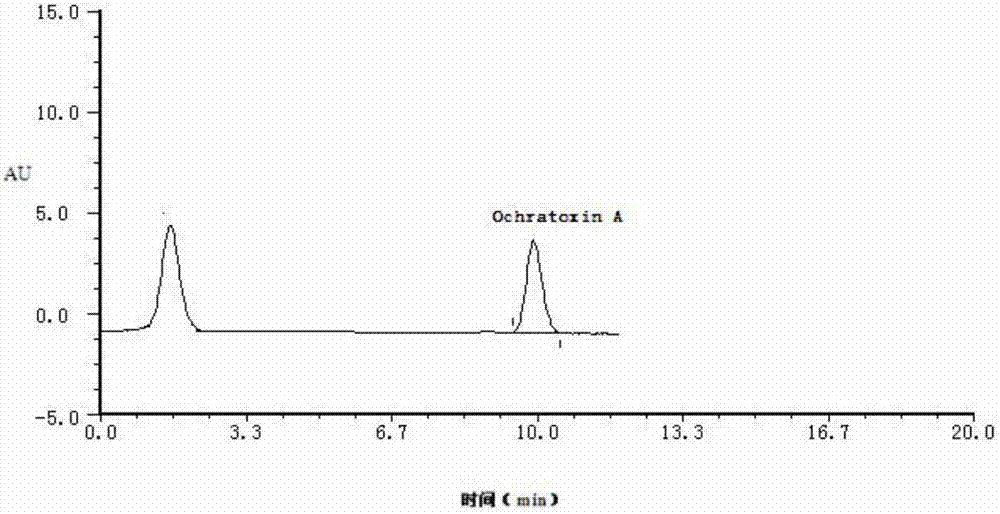
ActiveCN103157439AAchieving Simultaneous PurificationEasy to detectOrganic compounds purification/separation/stabilisationOther chemical processesImmunosorbentsOchratoxin A
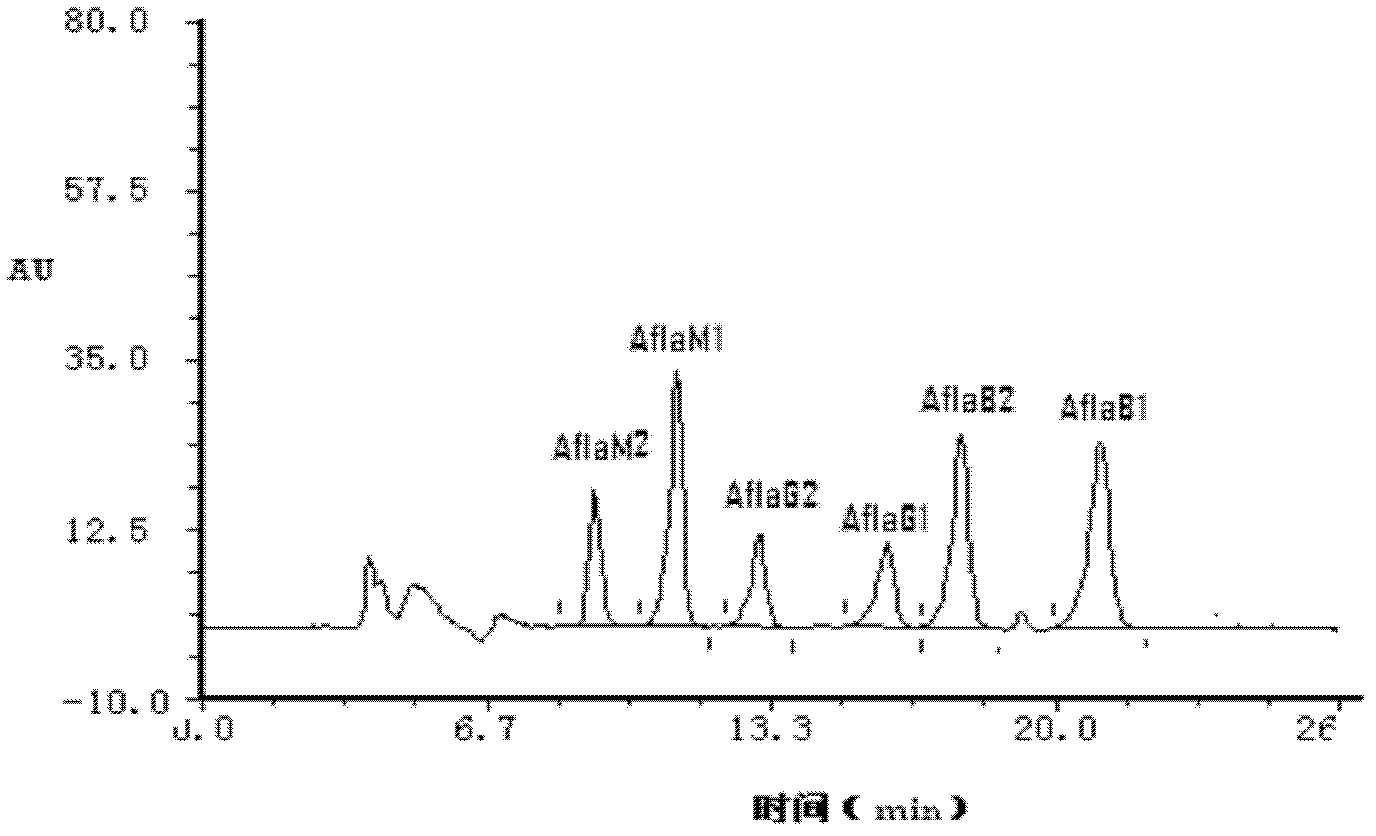
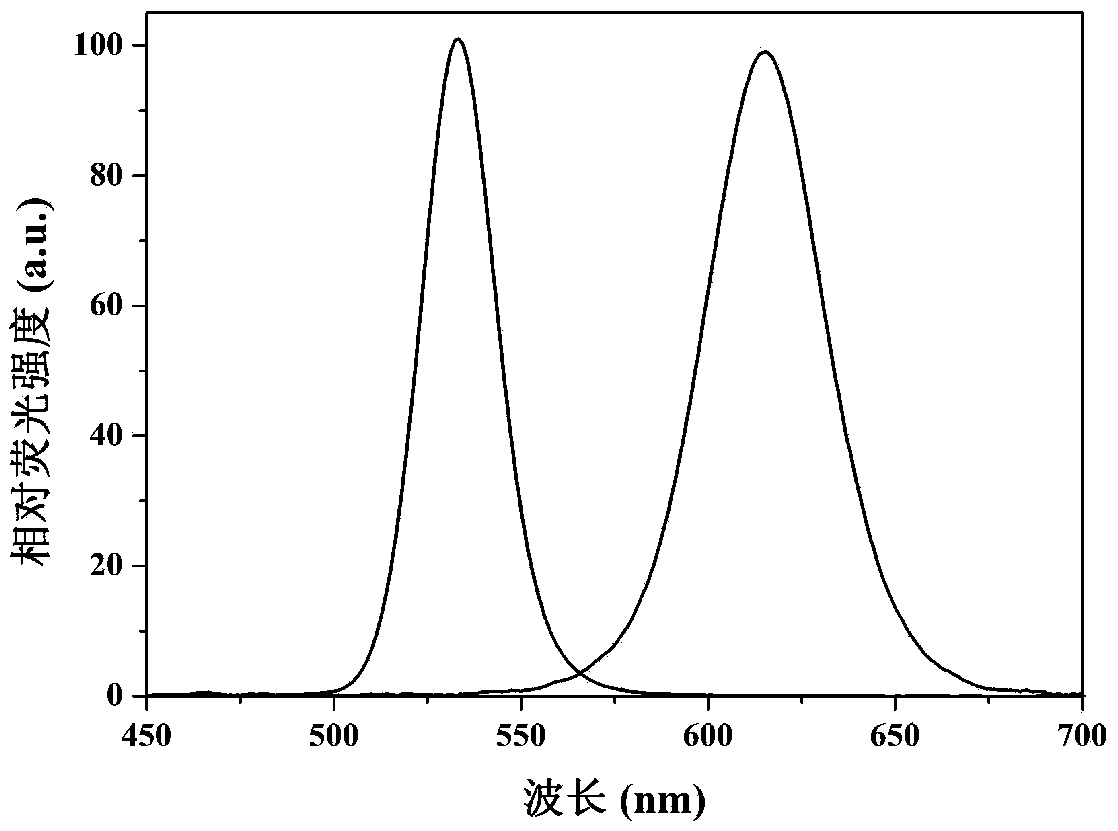
The invention discloses an immunosorbent comprising a solid-phase carrier and an antibody coupled with the solid-phase carrier. The antibody comprises a monoclonal antibody secreted by a hybridoma cell line CGMCC NO.5504 and a monoclonal antibody secreted by a hybridoma cell line CGMCC NO.5505. The invention also provides a kit comprising the immunosorbent or an immunoaffinity column. Also, the invention provides applications of the immunosorbent, immunoaffinity column, and kit in detecting aflatoxins, sterigmatocystin, zearalenone congeners and ochratoxin A. The invention specifically provides separation and detection methods. According to the invention, the specific monoclonal antibodies with stable performances are developed, such that simultaneous purification and detection of 6 aflatoxins, sterigmatocystin, 6 zearalenone congeners and ochratoxin A are realized.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +2
Treatment of B cell diseases using anti-germline antibody binding agents
InactiveUS20070081989A1Reduce amountReduce in quantityAntibody ingredientsDisease diagnosisAutoimmune conditionImmunosorbents
Methods for reducing the number of pathologic antibody producing B cells in a patient suffering from an autoimmune disease by administration of an anti-germline antibody are described. Methods for removing pathologic antibodies and B cells and plasma cells producing pathologic antibodies from the body of a patient suffering from autoimmune disease are provided, comprising contacting the blood or plasma of the patient with an immunoadsorbent having specific binding for an epitope present on germline antibodies, particularly VH4-34 antibodies, wherein said contacting results in the reduction in the amount of germline antibodies present in the blood or bone marrow or lymphoid tissue of the patient or the amount of germline antibody producing B cells present in the blood, lymphoid tissues or bone marrow of the patient. Methods for treating a patient suffering from a B cell cancer expressing cell surface germline antibodies by similar methods are also provided. Methods for ex vivo purging bone marrow of pathologic antibody producing B-cells and cancerous B-cells expressing germline antibodies are provided. Methods for monitoring the efficacy of a therapeutic treatment in a patient suffering from an autoimmune disease or B cell cancer are also provided. Kits and uses in preparation of a medicament are also described.
Owner:SANDERS MARTIN E
Composite affinity column for purifying 3-acetyl deoxynivalenol, aflatoxin, ochratoxin A and zearalenone
InactiveCN104707362AImprove performanceEconomic detection methodComponent separationOther chemical processesImmunosorbentsOchratoxin A
The invention discloses a composite affinity column for purifying 3-acetyl deoxynivalenol, aflatoxin, ochratoxin A and zearalenone, a preparation method and an application thereof. According to the invention, by employing 4% of cylindrical agarose gel as a solid phase carrier, agarose gel and an antibody are coupled to form an immunoadsorbent, and filling in a column to prepare the immunization affinity column. When a sample containing 3-acetyl deoxynivalenol, aflatoxin, ochratoxin A and zearalenone passes through the immunization affinity column, 3-acetyl deoxynivalenol, aflatoxin, ochratoxin A and zearalenone can be specifically adsorbed by the immunoadsorbent, other impurities flows out of the immunization affinity column, then methanol is used for eluting 3-acetyl deoxynivalenol, aflatoxin, ochratoxin A and zearalenone from the column, so that the sample can be better purified.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +3
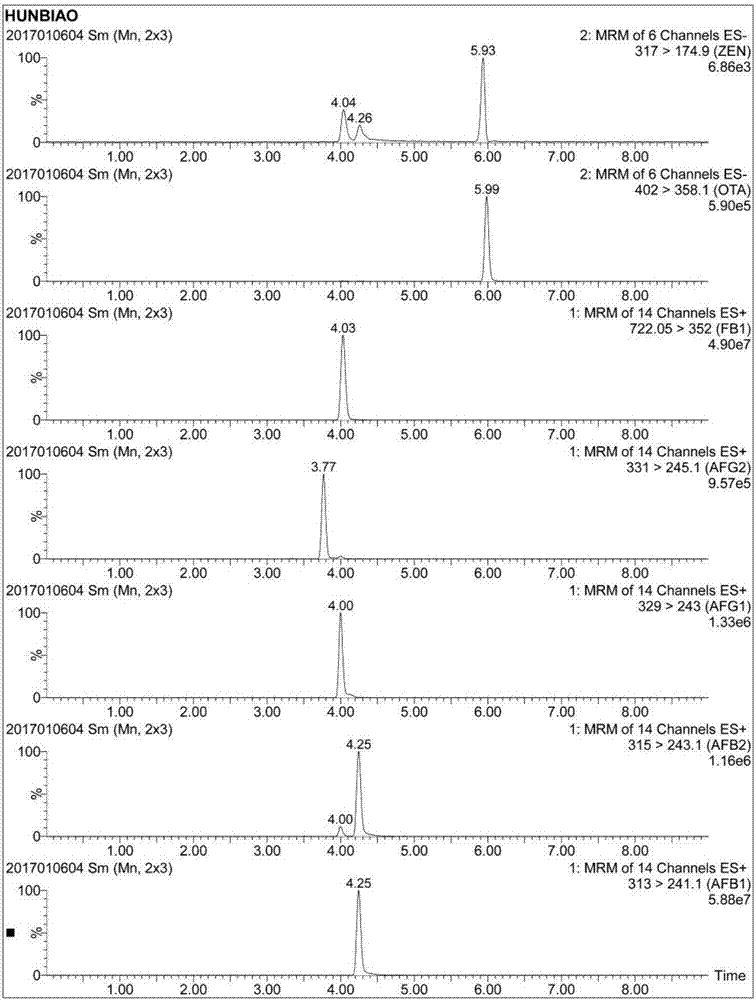
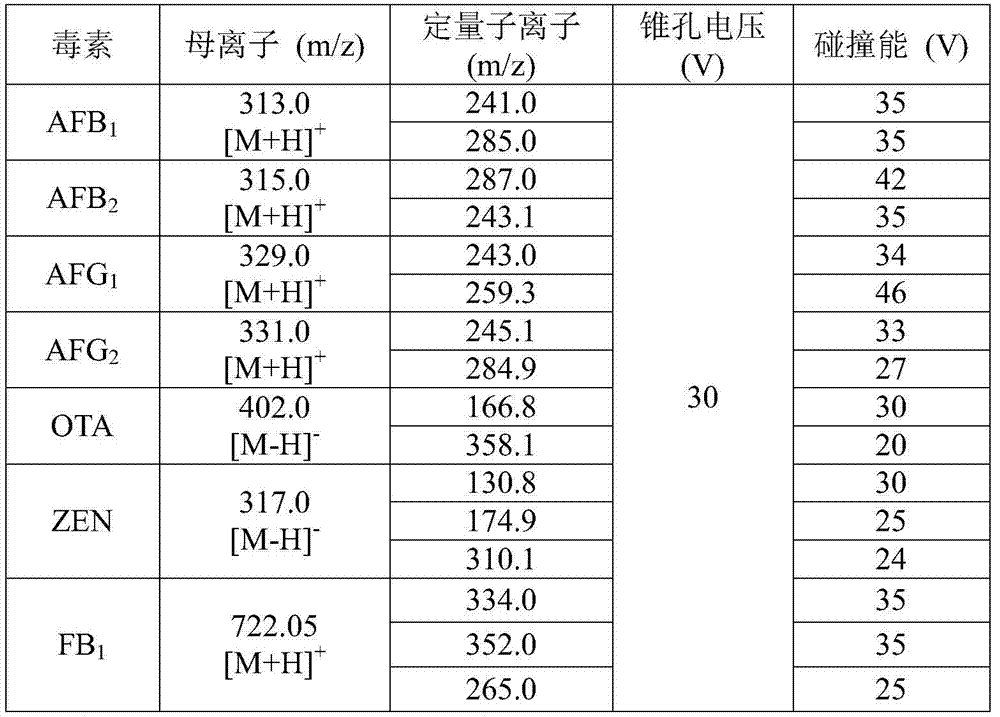
Immunoadsorbent and composite affinity column for purification of fumonisin B1, aflatoxin, ochratoxin A and zearalenone
ActiveCN106977600AImprove performanceFast detection methodComponent separationOther chemical processesFumonisin B1Immunosorbents
The present invention relates to an immunoadsorbent and a composite affinity column for purification of fumonisin B1, aflatoxin, ochratoxin A and zearalenone. The immunoadsorbent comprises a solid carrier and fumonisin B1, aflatoxin, ochratoxin A and zearalenone monoclonal antibodies coupled with the solid carrier, the fumonisin B1 monoclonal antibody is secreted from hybridoma cell line Fm7A11 with the preservation number of CCTCCNO.C201636, the hybridoma cell line Fm7A11 is preserved in CCTCC in March 29, 2016 in Wuhan University Wuhan China. The composite affinity column is loaded with the immunoadsorbent with the fumonisin B1, aflatoxin, ochratoxin A and zearalenone monoclonal antibodies, and is used for purification of the fumonisin B1, aflatoxin, ochratoxin A and zearalenone. The composite affinity column can be used for purification and detection of fumonisin B1, aflatoxin, ochratoxin A and zearalenone toxin samples.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Affinity absorption column for specially removing virions from blood and preparing method thereof
InactiveCN101628135AStrong specificityReduce harmIon-exchange process apparatusOther blood circulation devicesTreatment effectSide effect
The invention discloses an affinity absorption column for specially removing virions from blood and preparing method thereof, in particular relates to an affinity absorption column for blood purification. The affinity absorption column comprises an absorption carrier, an activator and antivirus surface protein antibody; the activator is added into the absorption carrier to obtain an activated carrier; the antivirus surface protein antibody is added into the activated carrier to perform antibody coupling to obtain an immune absorber, and the immune absorber is loaded into the absorption column to obtain the affinity absorption column. The affinity absorption column has the advantages of strong specificity, little toxic side effect, good therapeutic effect, good repeatability and low cost, and is suitable for blood purification; besides, being a separation technology, the affinity absorption column can be widely applied to other fields for the purification and separation of virus antibodies.
Owner:WUHAN UNIV
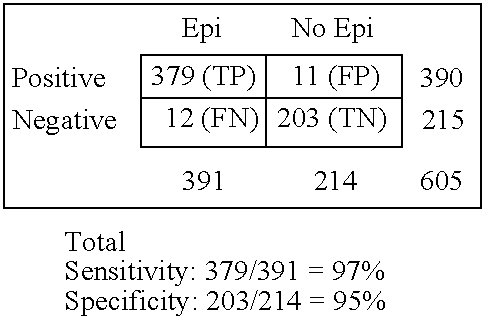
Immunosorbent blood tests for assessing paroxysmal cerebral discharges
InactiveUS7820398B2Improve accuracyImprove diagnostic certainty of brain related seizuresNervous disorderDisease diagnosisParoxysmal AFAnticonvulsant therapy
Owner:GRACE LAB
Kit for in vitro detection of anti-cyclic citrullinated peptide (CCP) antibody and preparation method thereof
InactiveCN102323402ANo biological toxicitySignificant technological progressMaterial analysisAnti ccp antibodiesImmunosorbents
The invention belongs to the field of biological engineering, and provides a kit for in vitro detection of an anti-cyclic citrullinated peptide (CCP) antibody, which comprises a detection plate, a CCP antigen, a colloidal gold-labeled recombinant gold staphylococcus aureus A protein conjugate, a confining liquid, a washing liquid, a positive reference product and a negative reference product. The invention also provides a preparation method of the kit for the in vitro detection of the anti-CCP antibody. The kit adopts an indirect method immunosorbent assay principle to detect the anti-CCP antibody in a human serum, the CCP antigen is coated on a nitrocellulose membrane to be made into a solid-phase antigen for capturing the anti-CCP antibody in the human serum, and then colloidal gold is labeled on staphylococcus aureus A protein (SPA) to form the colloidal gold conjugate as a tracer; and if the detected human serum contains the anti-CCP antibody, then a solid phase antigen-anti-CCP antibody-colloid gold conjugate is formed and has red spots. The invention can be applied to the auxiliary diagnosis of rheumatoid arthritis.
Owner:上海精臻生物科技有限公司
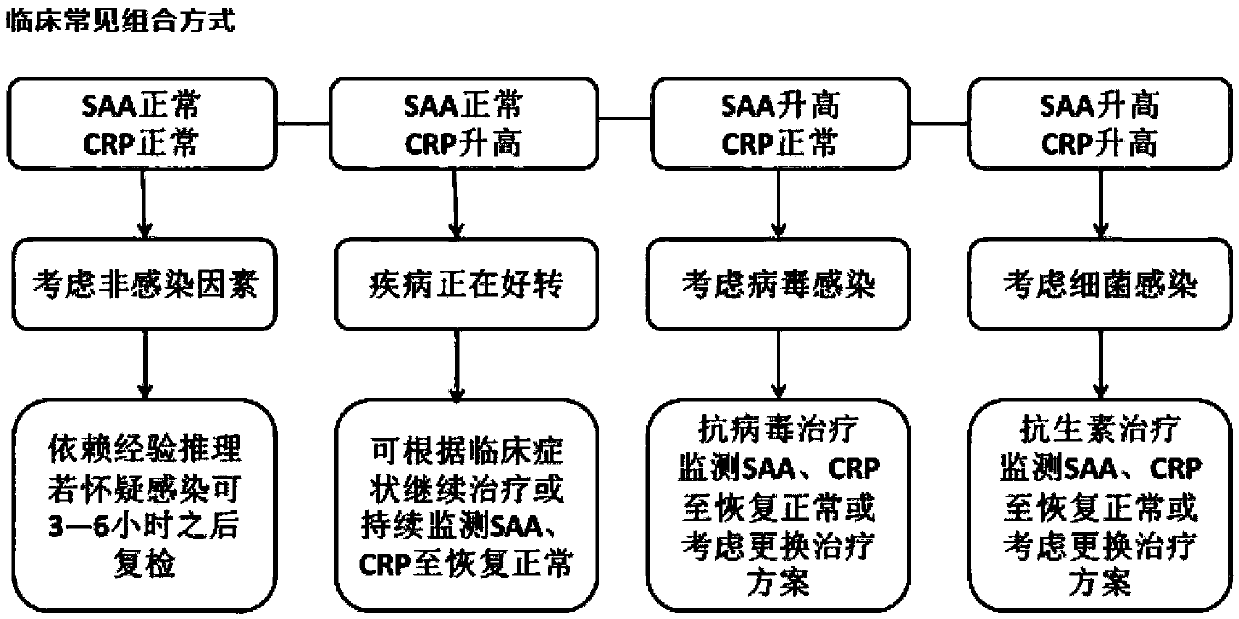
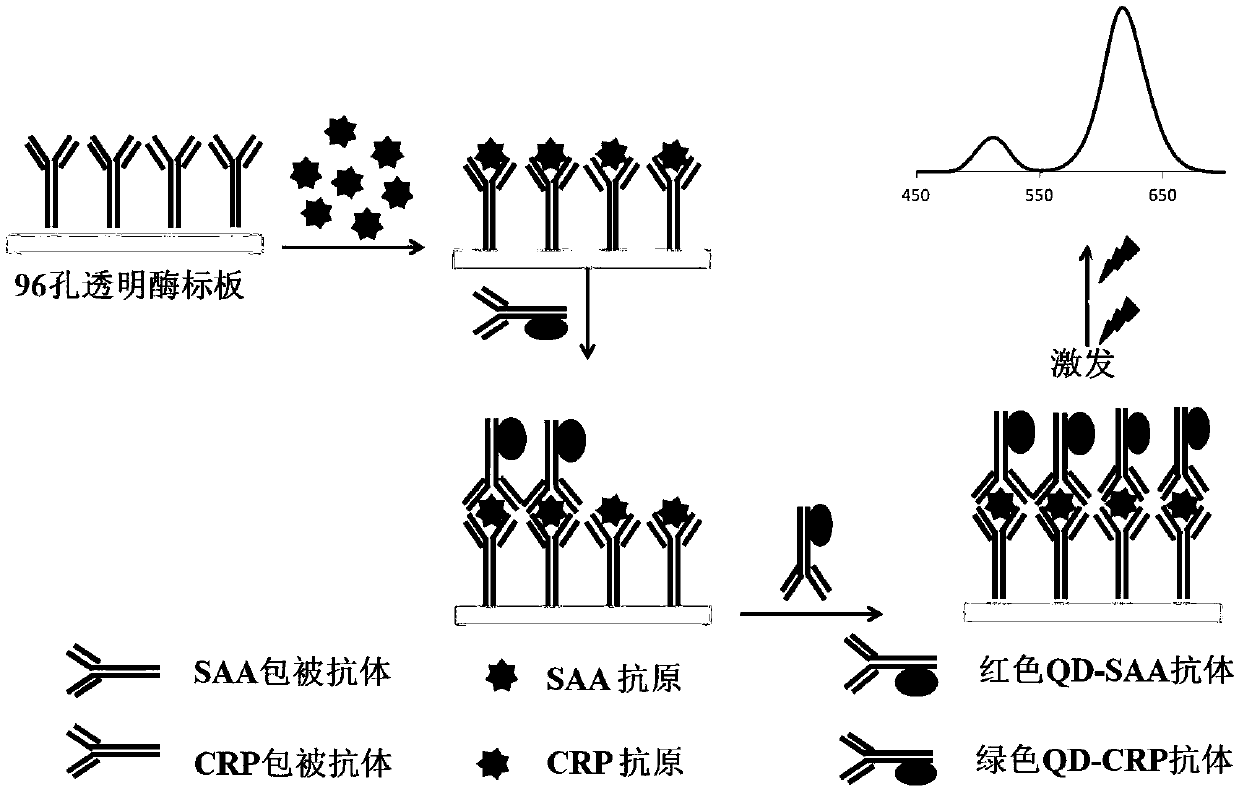
Fluorescence immunosorbent assay kit for joint detection of SAA (Serum Amyloid A) and CRP (C-reactive protein) based on two-color quantum dots and preparation method of fluorescence immunosorbent assay kit
InactiveCN109669044AHigh quantum yieldHalf maximum widthBiological testingImmunosorbentsFluorescence
The invention provides a fluorescence immunosorbent assay kit for joint detection of SAA (Serum Amyloid A) and CRP (C-reactive protein) based on two-color quantum dots and a preparation method of thefluorescence immunosorbent assay kit, and belongs to the technical field of in vitro diagnostic detection. According to the fluorescence immunosorbent assay kit, the advantages of quantum dot multicolor marking and the characteristics of antibody antigen specific reaction are fully utilized; and two antibodies are coated onto the same enzyme coated plate hole to form two kinds of double antibody sandwich methods of coated antibody-to-be-tested antigen-quantum dot labeled antibodies, and a novel simple, sensitive and stable detection method of various markers is established. Compared with a traditional ELISA (Enzyme-linked immunosorbent assay) method, the method has the advantages that the use amount of detection samples is reduced and the detection time is shortened; in addition, the detection efficiency can be improved; the detection cost is favorably saved; and the medical cost is reduced.
Owner:HENAN UNIVERSITY
Immunoadsorbent for purifying five kinds of mycotoxins including fumonisin b1 and aflatoxin b1, and complex affinity column
ActiveUS20180259526A1Improve performanceFast and accurate and safeComponent separationOther chemical processesImmunosorbentsFumonisin B1
The invention relates to an immunoadsorbent for purifying five kinds of mycotoxins including fumonisin B1 and aflatoxin B1, and a complex affinity column. The immunoadsorbent comprises a solid-phase support, and an anti-fumonisin B1 monoclonal antibody, an anti-aflatoxin B1 monoclonal antibody, an anti-ochratoxin A monoclonal antibody, an anti-zearalenone monoclonal antibody and an anti-sterigmatocystin monoclonal antibody which are coupled to the solid-phase support, wherein the anti-fumonisin B1 monoclonal antibody is secreted by a hybridoma cell strain Fm7A11, and the hybridoma cell strain Fm7A11 has been preserved at China Center for Type Culture Collection, Wuhan University, Wuhan, China on Mar. 29, 2016 with the preservation number of CCTCC No. C201636. The complex affinity column can be used for purification and detection of a fumonisin B1 sample, an aflatoxin B1 sample, an ochratoxin A sample, a zearalenone sample and a sterigmatocystin sample at the same time.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Method for preparing a carbonized resin DNA immunoadsorbent
InactiveUS6262172B1Quality improvementAvoid damageOrganic active ingredientsGenetic material ingredientsBenzoyl peroxideSorbent
A method for preparing a carbonized resin DNA immunoadsorbent, which uses styrene, acrylonitrile, divinylbenzene, toluene, liquid paraffin, benzoyl peroxide and water solution of polyvinyl alcohol as raw materials, produces a DNA immunoadsorbent with 0.3-0.5 mg DNA per milliliter of resin using two-staged procedures for preparation of first carbonized resin, and secondly carbonized resin DNA immunoadsorbent. The resultant immunoadsorbent is high in adsorbent capacity, low in production cost, and can be synthesized without pyrogens. It therefore satisfies the demands of medical application for the treatment of systemic lupus erythematosus by immunoadsorption.
Owner:JAFRON BIOMEDICAL
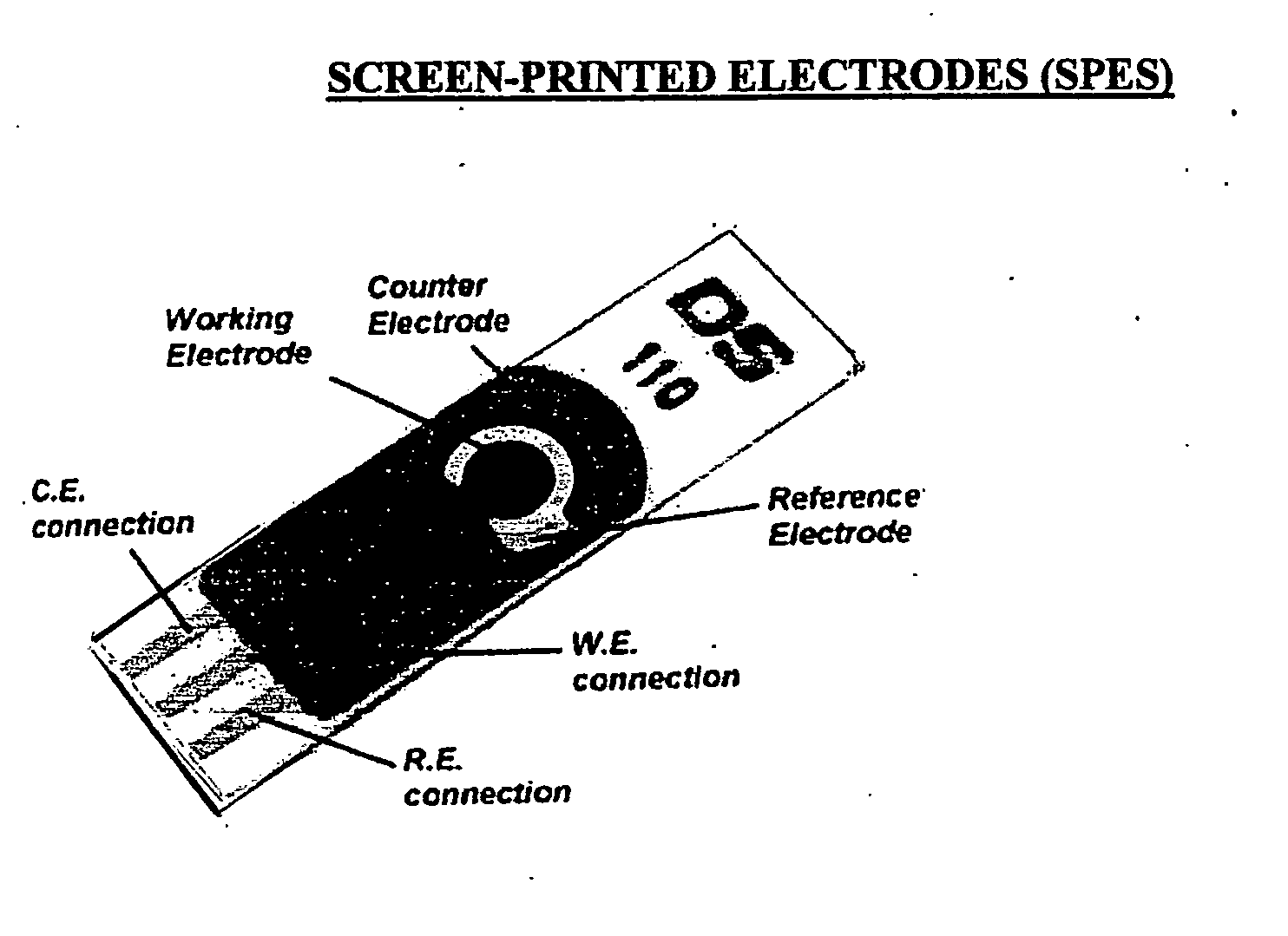
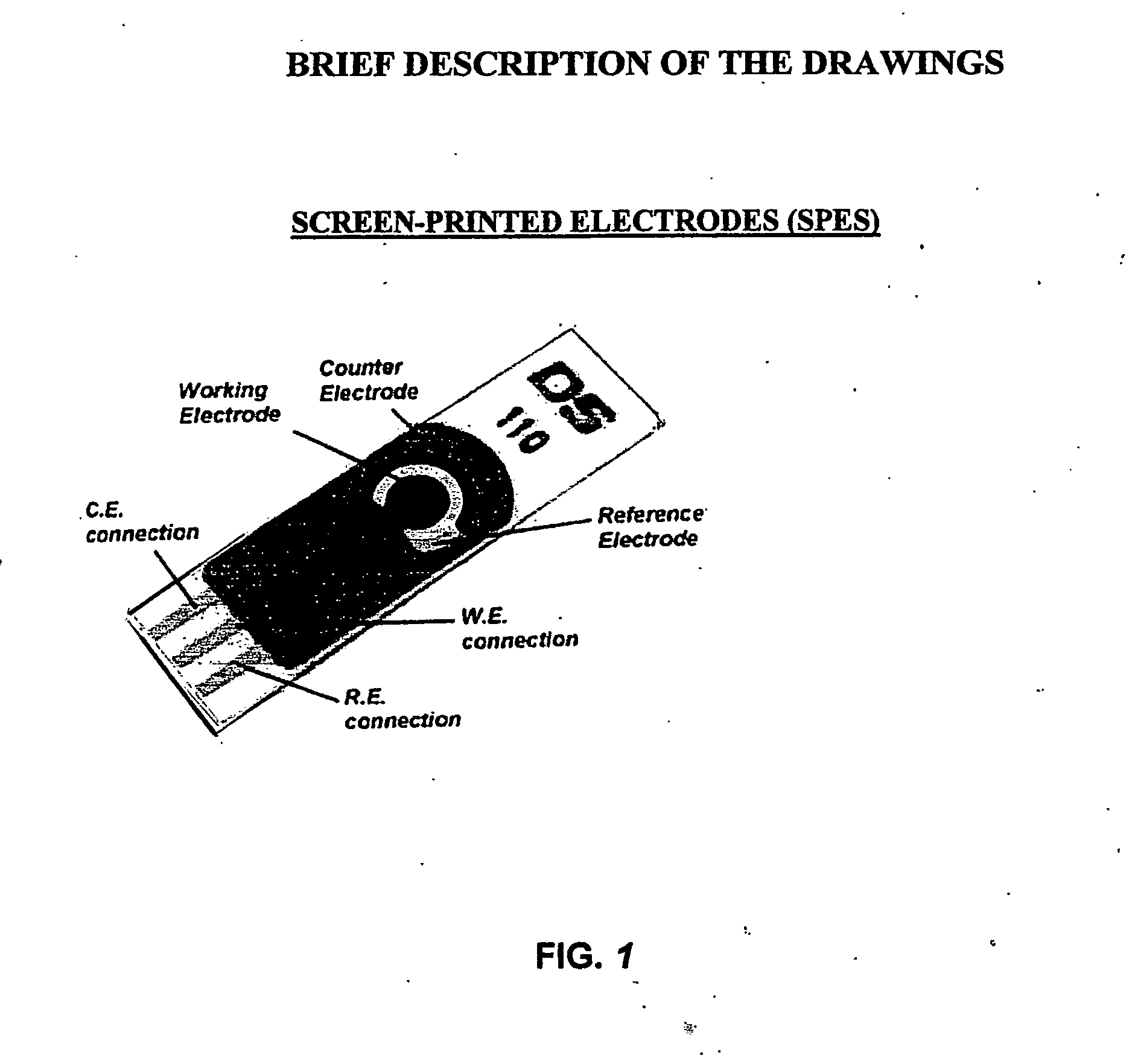
Chip for a pathogens, parasites, toxins and desired chemical compounds detection
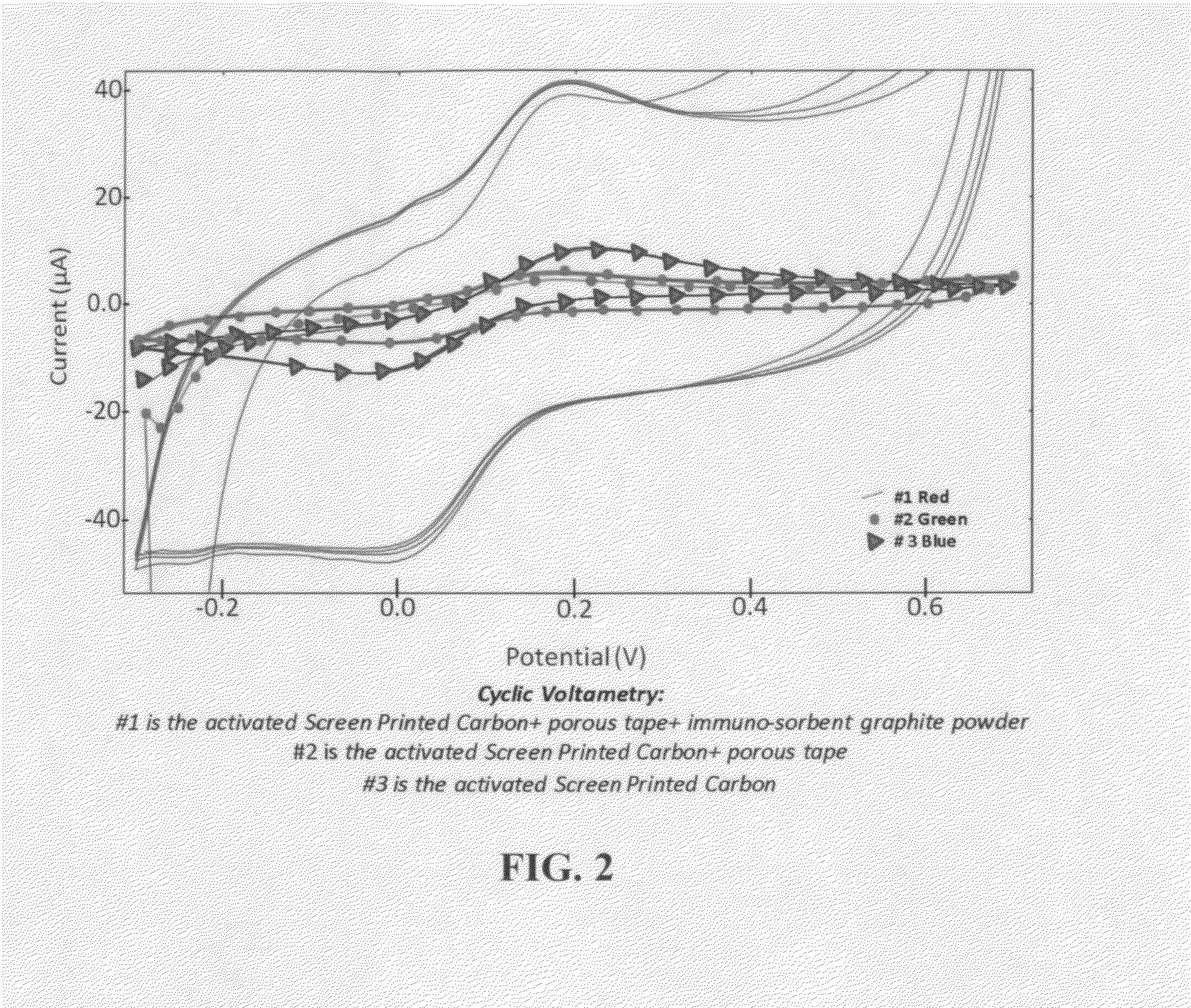
ActiveUS20140332409A1Fast and sensitive and inexpensive and portable immunoassayFaster and cheap methodImmobilised enzymesBioreactor/fermenter combinationsImmunosorbentsSorbent
The present invention is directed to a method and apparatus for an immunoassay technique that uses amperometric measurements to rapidly analyze different pathogenic microorganisms, including bacteria, viruses, toxins, and parasites and chemical compounds using a disposable element. In accordance with one aspect of the present invention, at least one conductive immunosorbent is used to provide support for antibody immobilization and placed on the top of the working electrode; it could also be used by itself as a working electrode. This immunosorbent or powder can be fabricated of conductive material or nonconductive material over which a conductive material is coated. A membrane cover of the working electrode forms a fluidic chamber having a pore size that is suited to the particular application to insure no contact between the working electrode and counter or silver electrode. The immunoassay can be automated using microprocessor control to reduce the amount of human intervention.
Owner:WILKINS EBTISAM
Kit for in vitro detection of GPI (Glucose-6-Phosphate Isomerase) antigen and preparation method thereof
ActiveCN102323401ASignificant technological progressThe test results are accurate and convenientMaterial analysisReference productImmunosorbents
The invention belongs to the field of biological engineering, and provides a kit for in vitro detection of a GPI (Glucose-6-Phosphate Isomerase) antigen, which comprises a detection plate, a goat anti-GPI antibody, a colloidal gold conjugate, a confining liquid, a washing liquid, a positive reference product and a negative reference product. The invention also provides a preparation method of the kit for the in vitro detection of the GPI antigen. The kit adopts a double antibody sandwich colloidal gold immunosorbent assay method principle to detect the GPI antigen in a human serum, the goat anti-GPI antibody is coated on a nitrocellulose membrane to be made into a solid phase antibody for capturing the GPI antigen which possibly exists in a human peripheral serum, and then colloidal gold is labeled on the goat anti-GPI antibody to form the colloidal gold conjugate as a tracer; and if the detected human serum contains the GPI antigen, then a solid phase goat anti-GPI antibody-GPI antigen-colloid gold conjugate is formed. The invention can be applied to the auxiliary diagnosis of rheumatoid arthritis.
Owner:上海北加生化试剂有限公司
Immune affinity precipitation method for purification of antibodies
ActiveCN102942627AReduce manufacturing costEasy to make costPeptide preparation methodsImmunoglobulinsImmunosorbentsSorbent
The invention discloses an immune affinity precipitation method for purification of antibodies. The immune affinity precipitation method comprises that copolymer solid-phase microballoons containing acrylamide and acrylic acid as monomers are used as solid-phase carriers; antigens are connected to the solid-phase carriers by a coupling agent of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) so that an immunosorbent is formed; an antibody stock solution needing to be purified is mixed with the immunosorbent and the mixture undergoes a full reaction to produce an antibody-immunosorbent conjugate; the antibody-immunosorbent conjugate is subjected to centrifugal washing so that other unbound mixtures are removed and a pure antibody-immunosorbent conjugate is obtained; a system pH value is reduced so that antibodies are dissociated in the solution; the solution with the dissociated antibodies is subjected to centrifugal separation; and a supernatant is collected and then is dialyzed so that the purified antibodies are obtained. The immune affinity precipitation method has simple processes, is suitable for volume production and realizes preparation of antibodies of which purity reaches to an immune affinity chromatography level.
Owner:BEIJING NORTH INST OF BIOLOGICAL TECH
Preparation and use of antibacterial peptide PMAP-23 monoclonal antibody
InactiveCN101481724ASolve the real problemImmunoglobulins against animals/humansFermentationBALB/cChemical synthesis
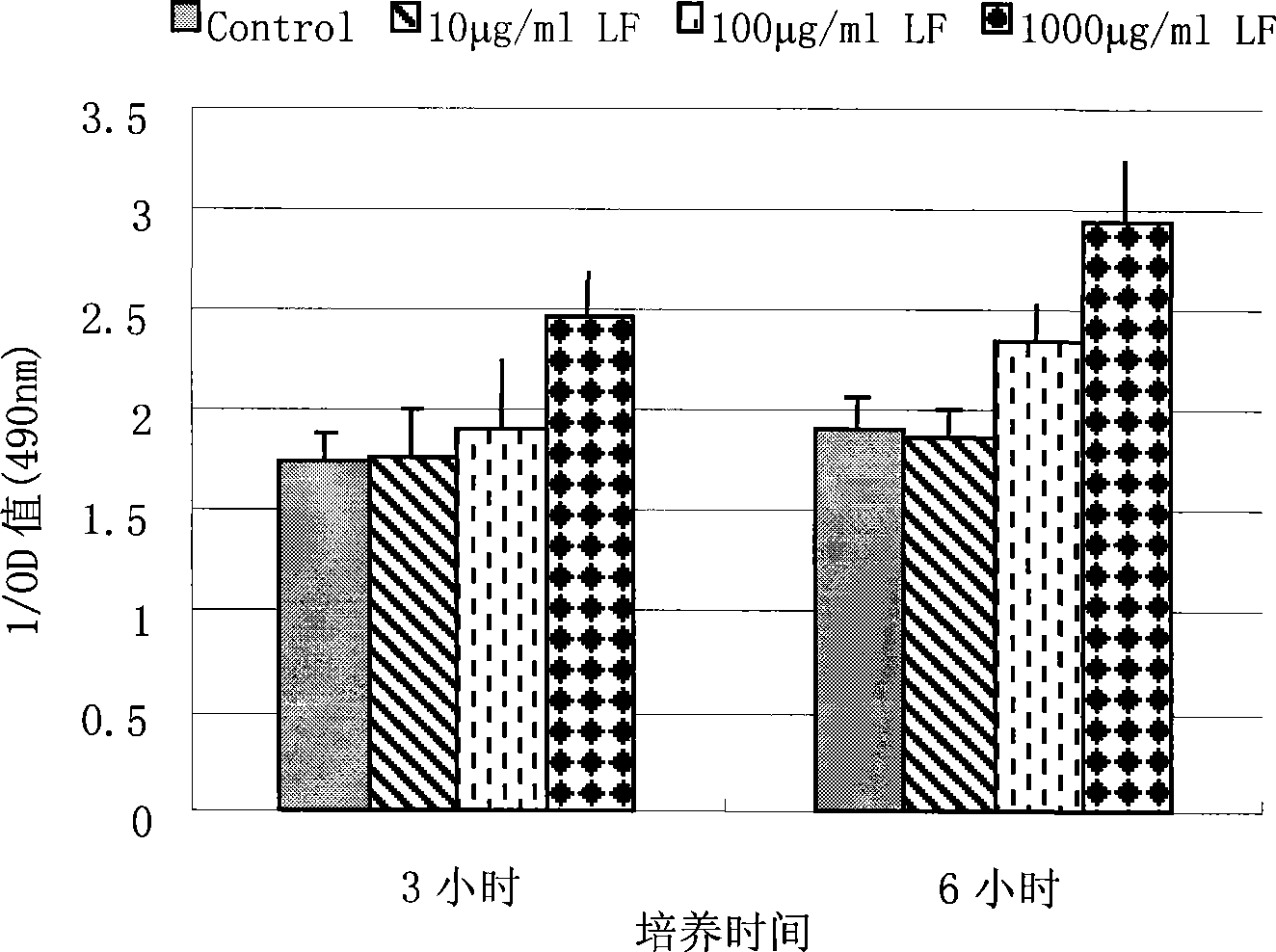
The invention discloses a preparing method of an antibacterial peptide PMAP-23 monoclonal antibody. Amino fragment of chemically synthesized antibacterial peptide PMAP-23 is used for immunizing Balb / c mouse. Then, spleen cells of the immunized mouse are used to be integrated with Sp2 / 0 myeloma cells. After screening and cloning, hybridoma cell strains for secreting anti-pig antibacterial peptide PMAP-23 monoclonal antibody are obtained. And finally, the required antibody is obtained. The monoclonal antibody of the invention can be used for constructing an enzyme linked immunosorbent assay method for detecting influences of lactoferrin on the PMAP-23 secreting level of the myeloma cells.
Owner:ZHEJIANG UNIV
Novel coronavirus SARS-CoV-2 detection proteome and application thereof
PendingCN112666348AStrong specificityEfficient and accurate determinationMaterial analysisAssayImmunosorbents
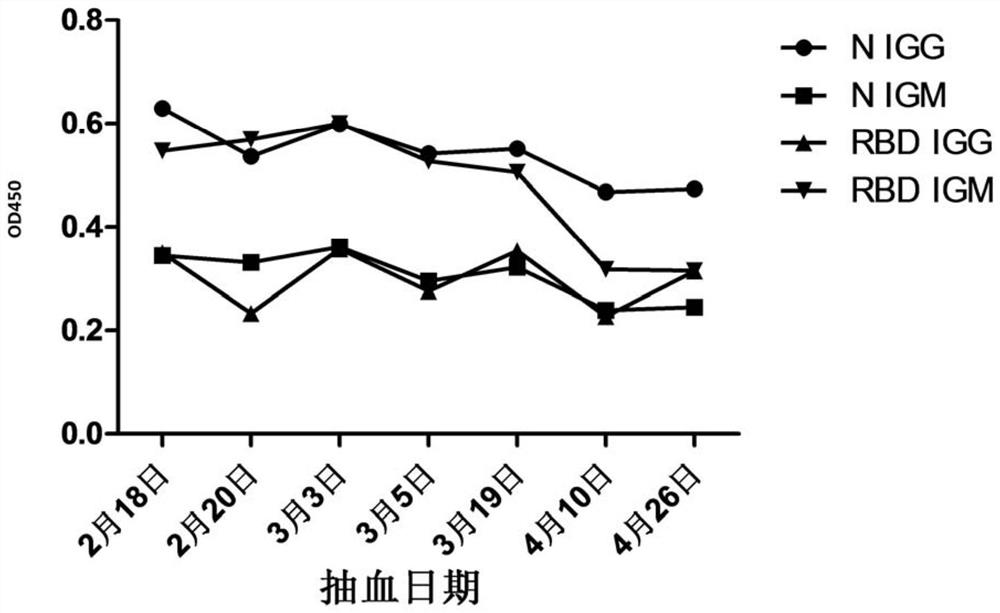
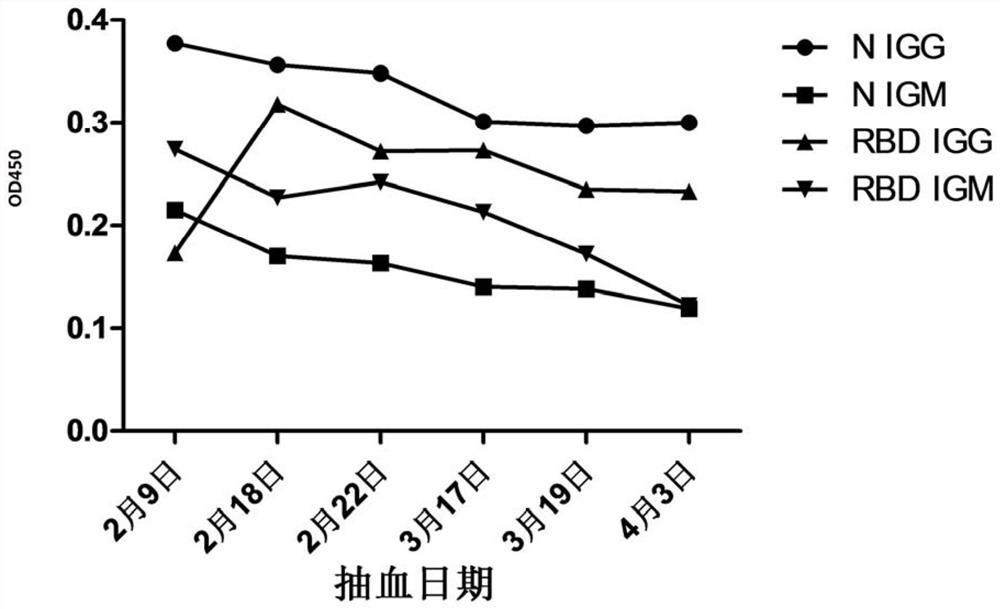
The invention discloses a novel coronavirus SARS-CoV-2 detection proteome and an application thereof. A detection method for detecting novel coronavirus SARS-CoV-2 is an enzyme-linked immunosorbent assay method. The N protein is optimized, and the receptor binding region (RBD 331aa-532aa) of the S protein is selected for expression through structural analysis aiming at the S gene of the novel coronavirus, so that the detection specificity can be further improved; an enzyme-linked immunosorbent assay method is used, and whether serum to be detected is positive or not can be efficiently and accurately determined by optimizing conditions such as coating concentration and time, primary antibody and secondary antibody incubation time and developing time.
Owner:SHANXI ACAD OF ADVANCED RES & INNOVATION
Immunosorbent and immunoaffinity column for aflatoxin m1 nanobody and preparation method thereof
ActiveUS20150276729A1Improve stabilityExtended shelf lifeComponent separationOther chemical processesImmunosorbentsAflatoxin M
An aflatoxin M1 nanobody, an immunosorbent and an immunoaffinity column. The aflatoxin M1 nanobody 2014AFM-G2 has the amino acid sequence of SEQ ID NO:7, is encoded by the nucleic acid sequence of SEQ IDNO:8, has a 50% inhibiting concentration IC50 to aflatoxin M1 of 0.208 ng / mL, and has cross reaction rates with aflatoxins B1, B2, G1, and G2 of 9.43%, 5.93%, 4.87% and 6.17%, respectively. The immunosorbent includes a solid phase carrier and aflatoxin M1 nanobody 2014AFM-G2 coupled with the solid phase carrier. The immunoaffinity column is loaded with the aflatoxin M1 nanobody immunosorbent. It can be used for purifying and concentrating an extracting solution of a sample before loading to a machine for detection and the immunoaffinity column can be used repeatedly for many times.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Immunosorbent for rheumatoid factors for blood perfusion and preparation method thereof
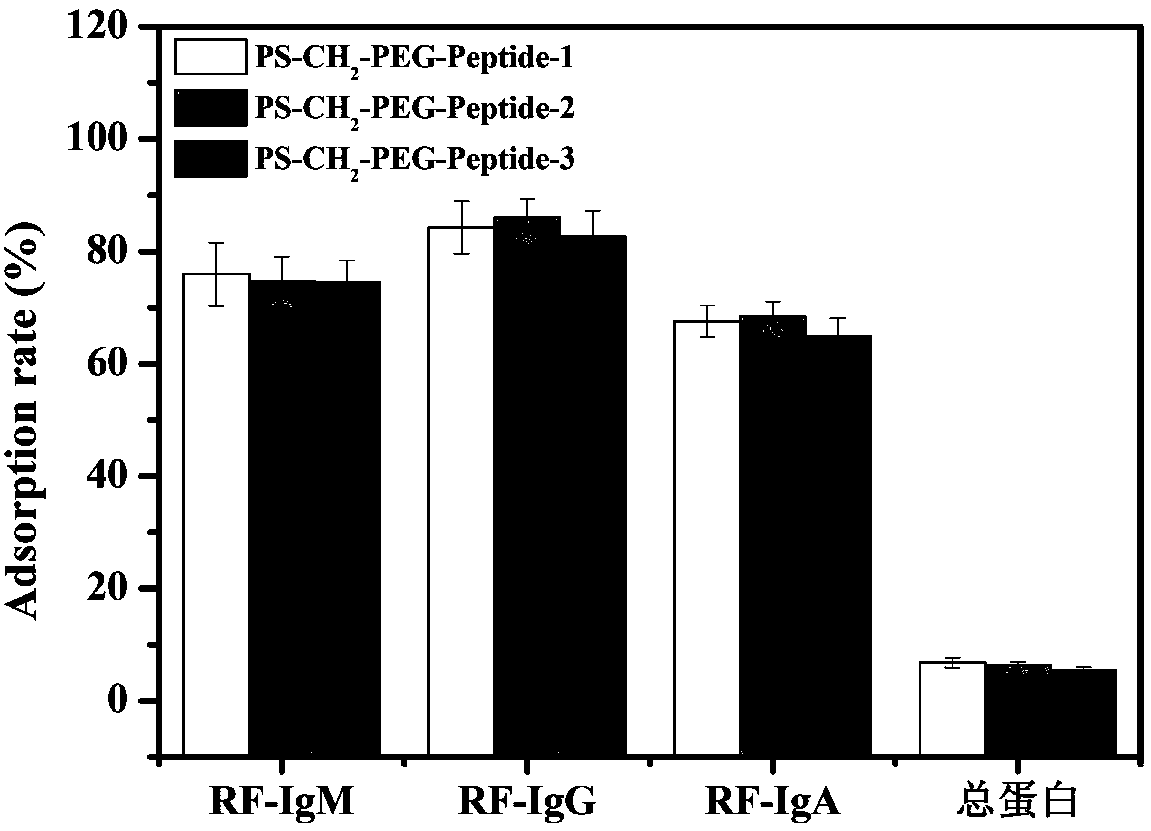
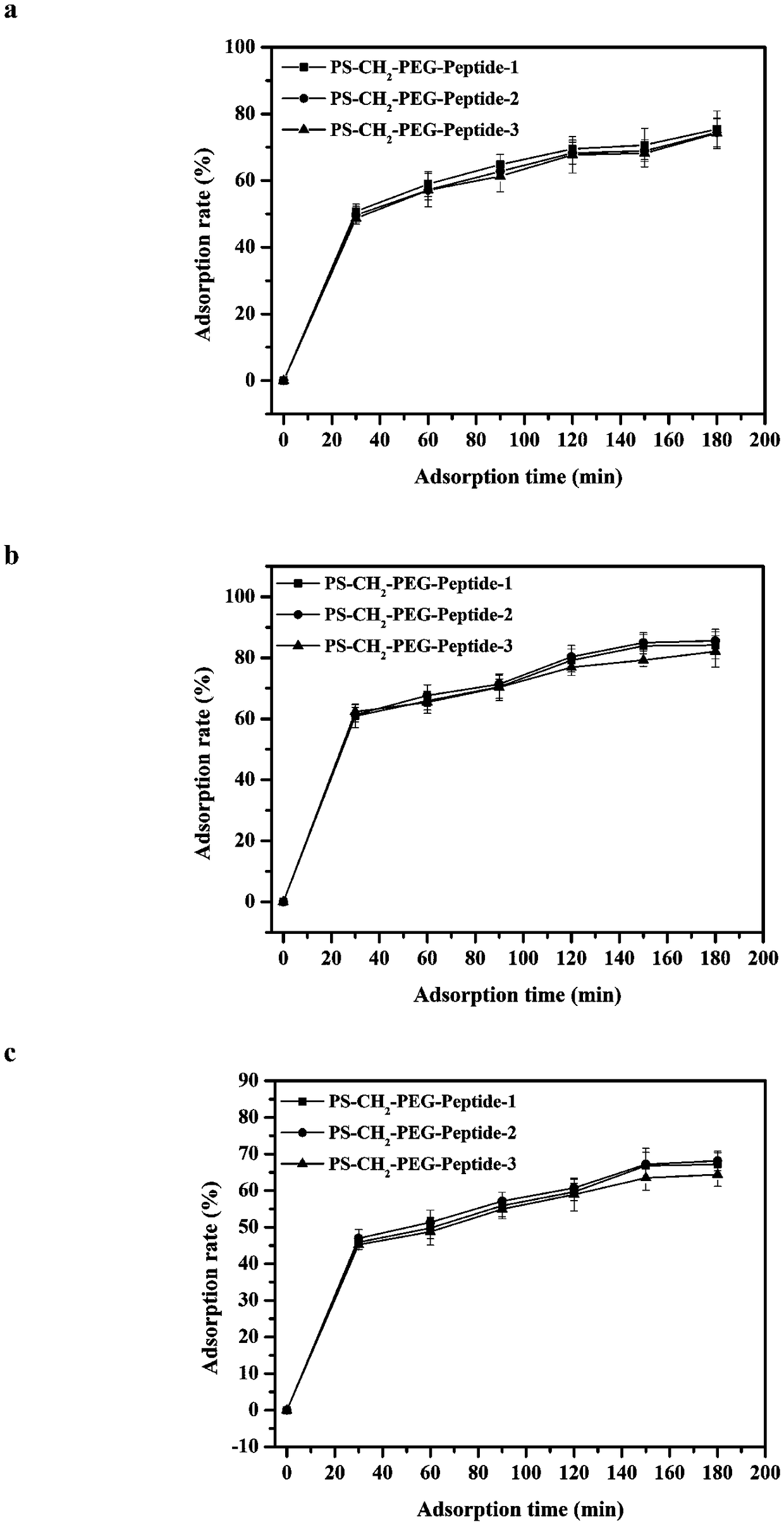
ActiveCN108586794AImprove adsorption capacityHigh adsorption selectivityOther chemical processesMicroballoon preparationSpecific adsorptionMicrosphere
The invention relates to an immunosorbent for rheumatoid factors for blood perfusion and a preparation method thereof. The immunosorbent uses chloromethylation macroporous structure styrene-divinylbenzene resin as carriers, uses micro-molecule polypeptides (consisting of 6 to 14 amino acids) as ligands, uses amino-functionalization polyethylene glycol (H2N-PEG-NH2) with different molecular weightas arms, so that carrier microspheres are coupled with the ligands through the arms. The preparation of the immunosorbent is simple; on the basis of maintaining the specific adsorption on the rheumatoid factors, the relevant side effects of potential immunogenicity and the like of the macromolecule (antigen or antibody) ligand immunosorbent is avoided; the cost is low; the immunosorbent is suitable for being used for the excessive rheumatoid factors in a body of the patient through blood or plasma perfusion.
Owner:NANKAI UNIV
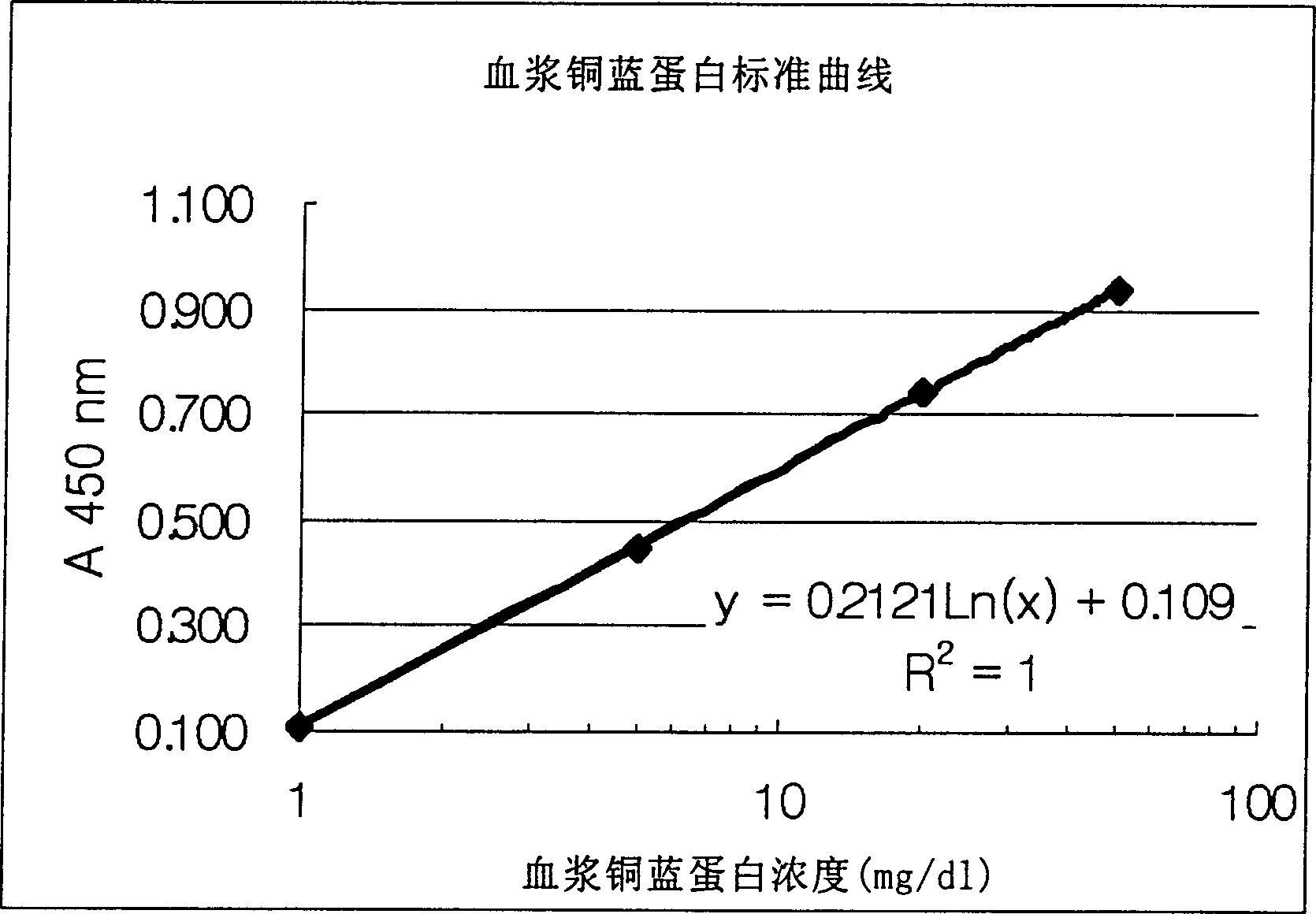
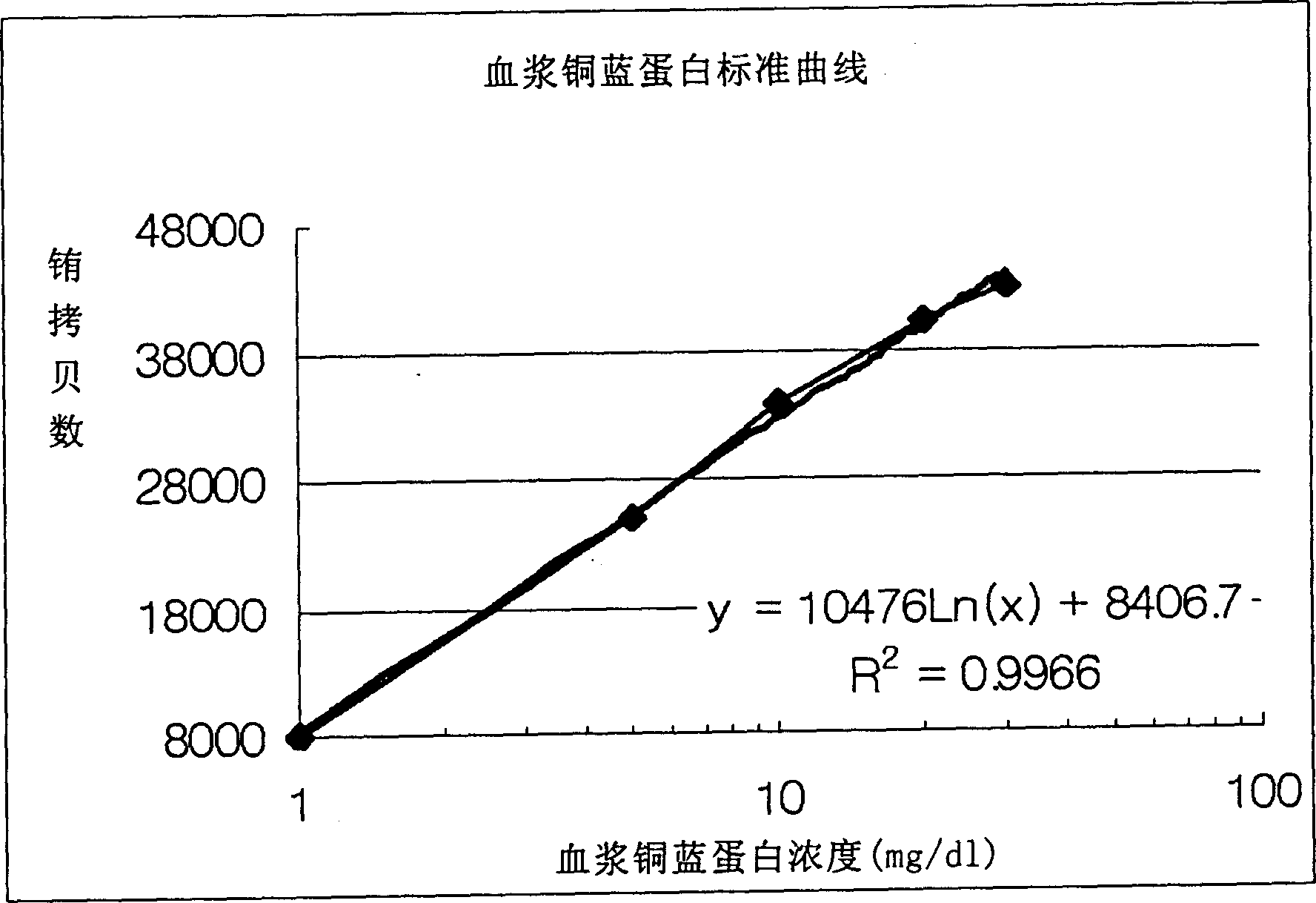
Method of determining ceruloplasm concentration in blood cake and hepatolenticular degeneration disease screening reagent box and diagnostic reagent
The present invention relates to a method for measuring the concentration of whole plasma ceruloplasmin, in particular to the use of whole plasma ceruloplasmin-specific polyclonal antibody and whole plasma ceruloplasmin-specific monoclonal antibody, by using enzyme-linked immunosorbent assay (enzyme-linked immunosorbent assay method) Immunosorbent assay; ELISA) or dissociation-enhanced time-resolved fluorescent immunoassay (dissociation-enhanced time-resolved fluoroimmunoassy) to obtain the standard concentration curve, the method of measuring the concentration of whole plasma ceruloplasmin.
Owner:CHI NAK BEK
Aflatoxin nanobody immunoabsorbent and immunoaffinity column and preparation method and use thereof
ActiveUS20160318998A1Extended shelf lifeRepeat usageBiological material analysisPeptide preparation methodsImmunosorbentsAflatoxin B
An aflatoxin nanobody immunoabsorbent and immunoaffinity column and preparation method and use thereof. The immunoabsorbent comprises a solid phase carrier and aflatoxin B1 nanobody 2014AFB-G15 coupled with the solid phase carrier. The 50% inhibiting concentration IC50 of aflatoxin B1 nanobody 2014AFB-G15 to aflatoxin B1 is 0.66 ng / mL, and the cross-reactivity of aflatoxin B1 nanobody 2014AFB-G15 to aflatoxins B2, G1, G2, and M1 are respectively 22.6%, 10.95%, 32.1% and 26%. The amino acid sequence of aflatoxin B1 nanobody 2014AFB-G15 is as depicted by SEQ ID NO: 7, and the coding gene sequence thereof is as depicted by SEQ ID NO: 8. The aflatoxin nanobody immunoaffinity column can be used for purification and concentration of sample extract prior to computer testing, and the immunoaffinity column can be reused repeatedly.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Aflatoxin sterigmatocystin hybridoma cell strain, antibody, immunoadsorbent, immunoaffinity column, kit and applications of immunoadsorbent, immunoaffinity column and kit
InactiveCN103160472AImprove performanceAchieving Simultaneous PurificationComponent separationOther chemical processesImmunosorbentsSecretion
The invention discloses a hybridoma cell strain CGMCC NO. 5506 and a monoclonal antibody obtained by secretion of the cell strain. The invention also provides an immunoadsorbent comprising a solid phase vector and an antibody coupled with the solid phase vector, wherein the antibody is the above monoclonal antibody; and an immunoaffinity column loaded with the immunoadsorbent. The invention also provides a kit containing the above immunoadsorbent or the above immunoaffinity column, and applications of the above immunoadsorbent, the above immunoaffinity column and the kit in detecting aflatoxins and sterigmatocystins. Besides, the invention provides a separation method and a detection method specifically. The monoclonal antibody with stable and specific performance is developed by the invention, so that purification and detection for six aflatoxins and sterigmatocystins at the same time are realized.
Owner:北京中检维康技术有限公司 +2
Automatic fluid device for detecting protein in biological sample and its method
An automated microfluidic system that includes a cartridge reservoir part, a cartridge, a compressed-air storage tank, a buffer storage tank, and a reader is provided. The automated microfluidic system that detects a particular protein in a biological sample by enzyme-linked immunosorbent assay (ELISA) can automate a series of assaying processes, beginning with sample injection and ending with detection, with a simple structure, thereby allowing for convenient and quick detection of a particular protein in a biological sample without requiring skillful, elaborate manipulations by an operator.
Owner:SAMSUNG ELECTRONICS CO LTD
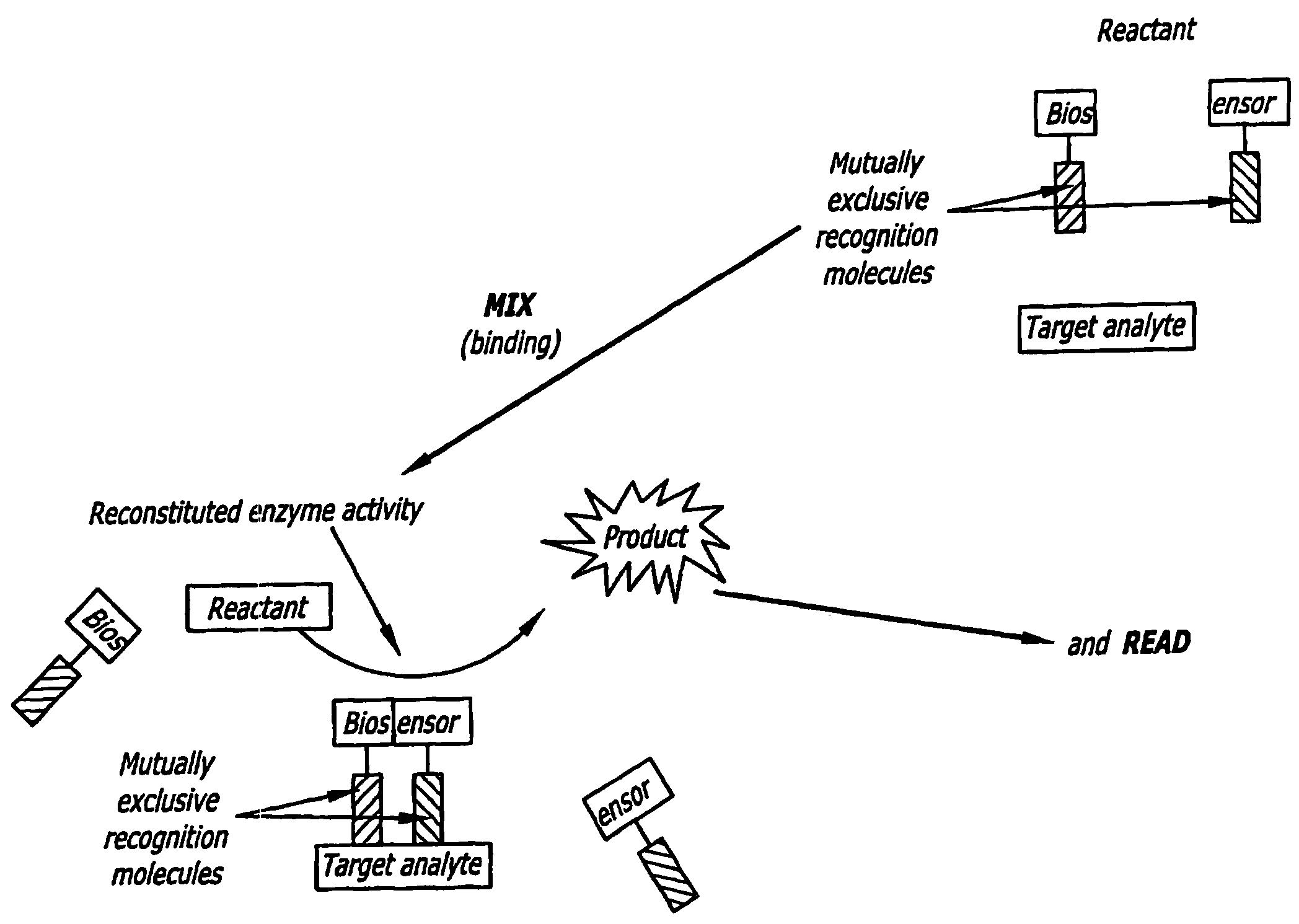
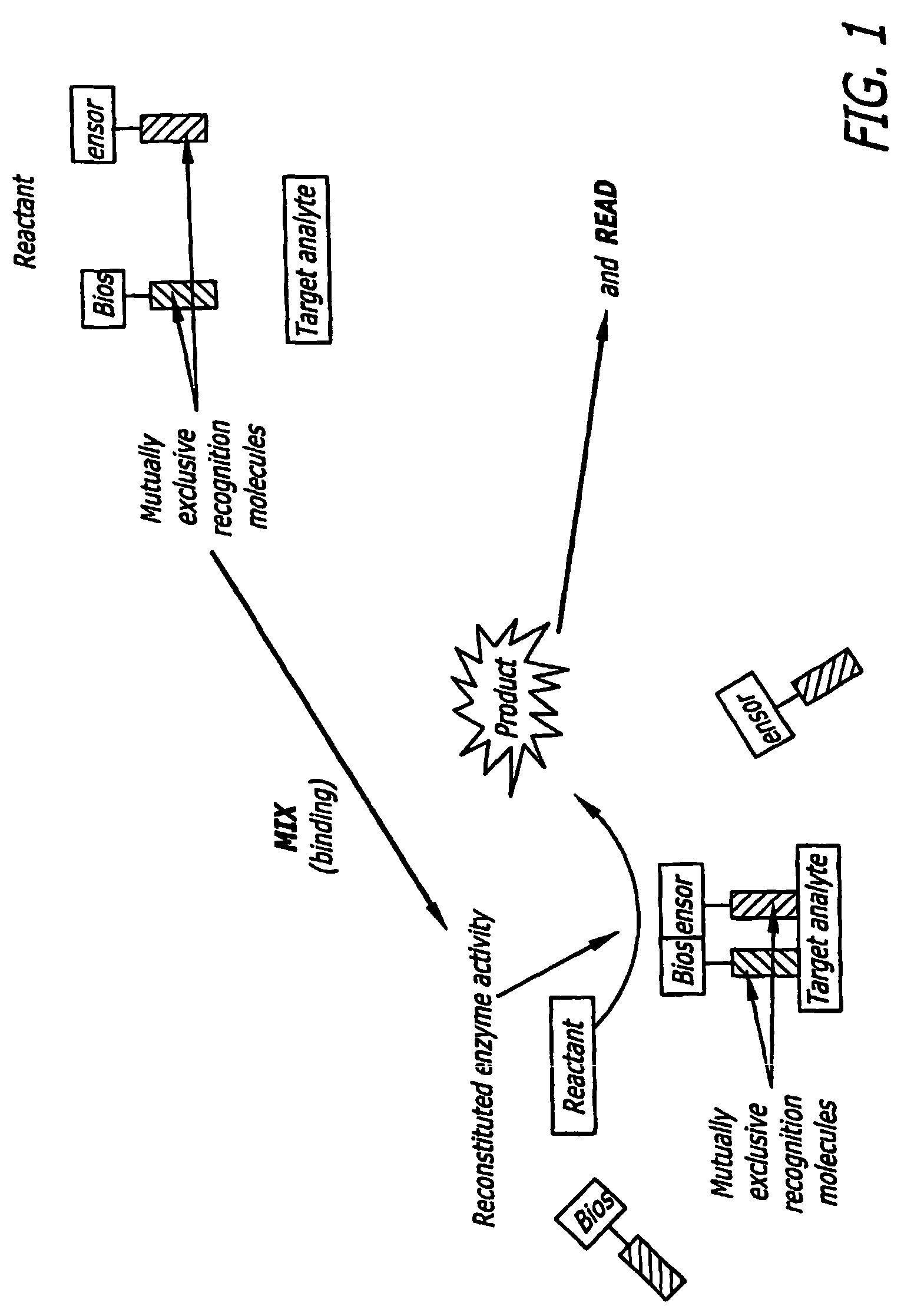
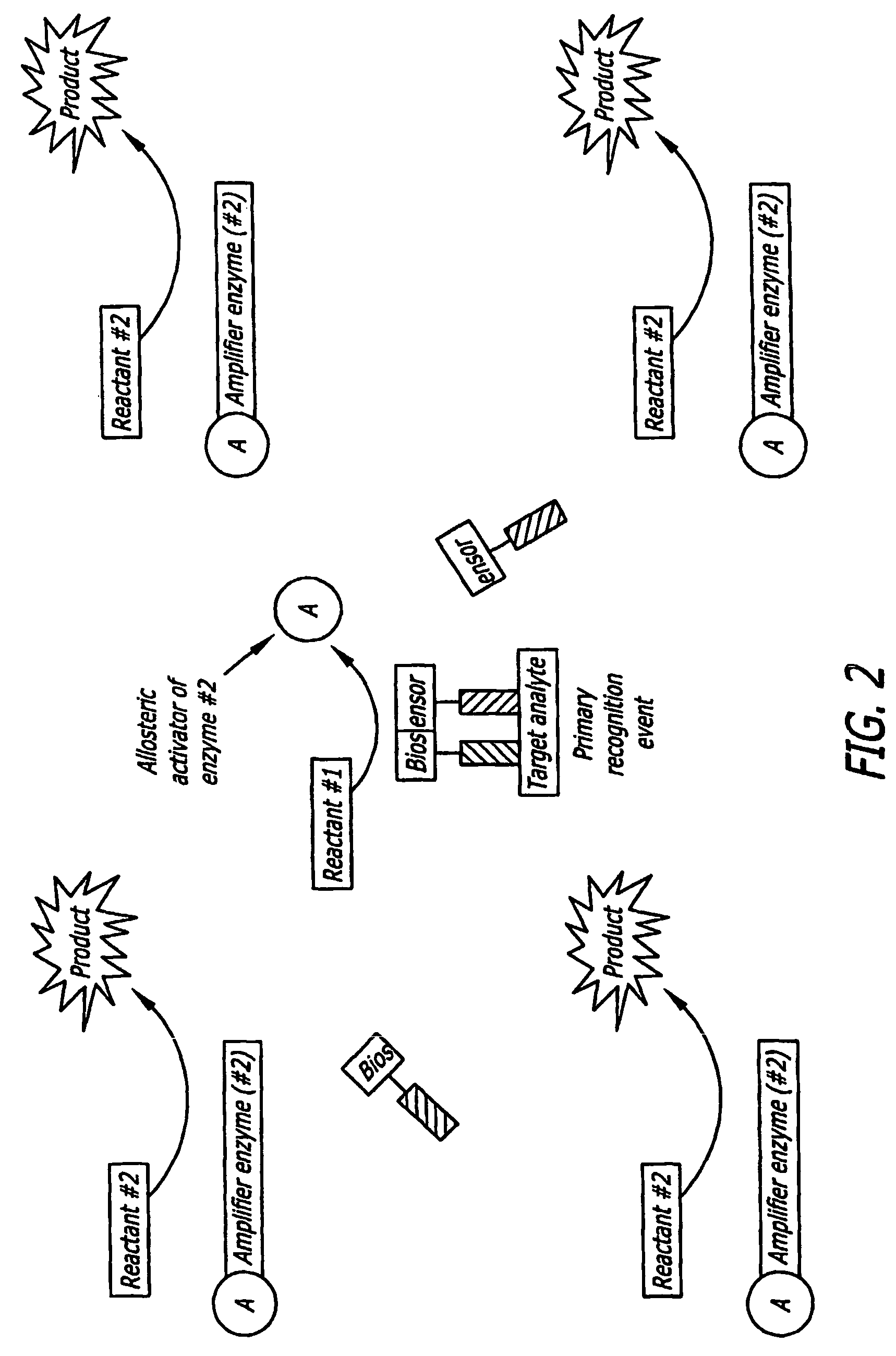
Split Enzyme Linked Immunosorbent and Nucleic Acid Assays
InactiveUS20080248463A1Simple methodHigh gainMicrobiological testing/measurementLibrary screeningAnalyteActive enzyme
A method for detecting the presence of an analyte in a solution. The analyte includes at least two mutually exclusive recognition sites that are capable of binding to corresponding recognition molecules. Biosensors are provided that include the recognition molecules, which are attached to the inactive portions of a split enzyme. The recognition sites are located such that the inactive enzyme portions combine to form a detectable biologically active enzyme when the recognition molecules bind to recognition sites.
Owner:RGT UNIV OF CALIFORNIA
Immunosorbent for removing inflammatory factors in blood and preparation method thereof
ActiveCN108855003AGood blood compatibilityGood biocompatibilityOther chemical processesOther blood circulation devicesAlkaneImmunosorbents
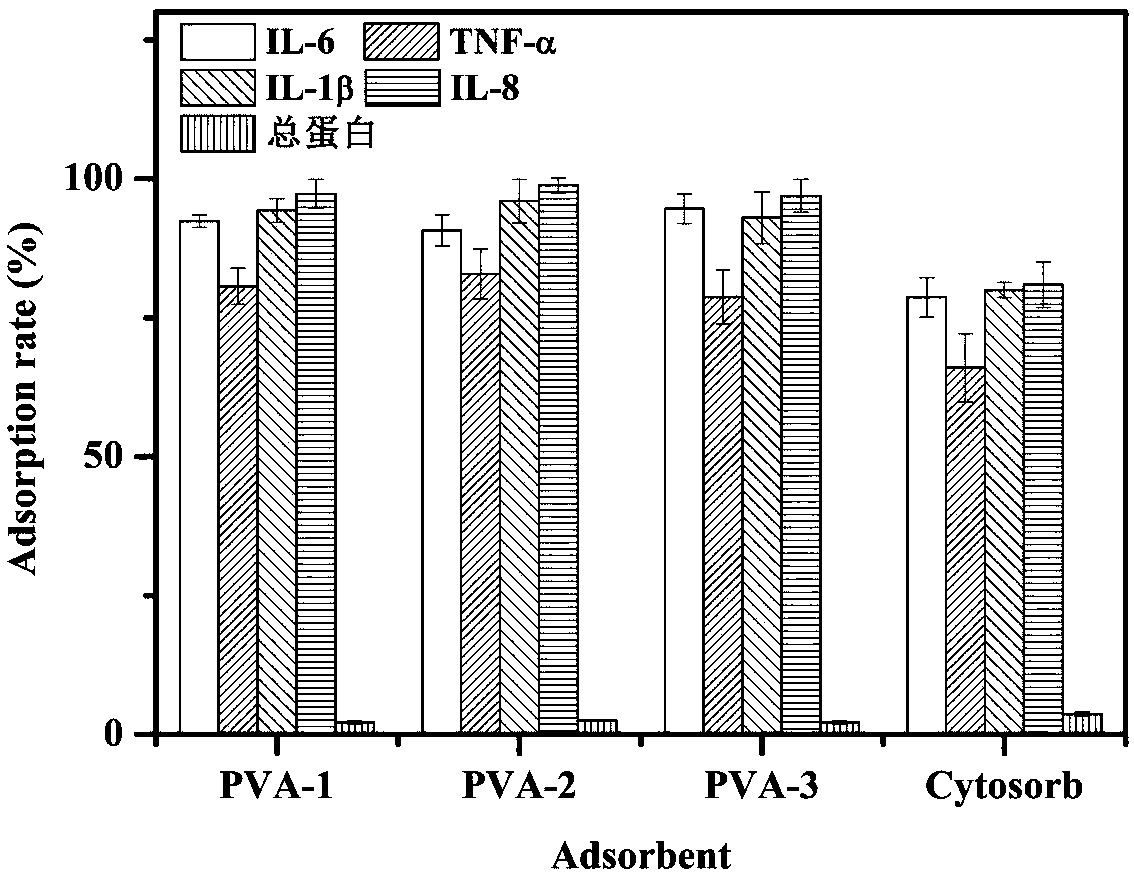
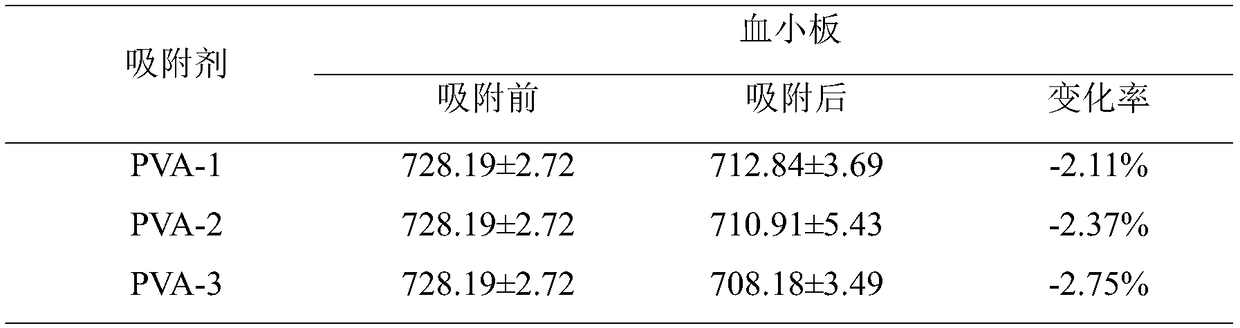
The invention discloses an immunosorbent for removing inflammatory factors in blood and a preparation method thereof. Based on a variety of intermolecular interactions between the antigenic epitope ofinflammatory factors such as IL-6, TNF-alpha, IL-1 beta and IL-8 and corresponding receptors thereof, affinity ligand of small molecule polypeptide with 5-18 selective amino acids is designed througha computer-assisted drug design method. Specifically, with vinyl acetate-triallyl isocyanurate as a basic skeleton, a mixture of ethyl acetate and alkane as a pore-forming agent, and a synthetic resin with benzoyl peroxide as an initiator as a carrier, alcoholysis activation is carried out, and then the small molecule polypeptide is grafted to obtain the small-molecule ligand immunosorbent whichdoes not adsorb macromolecular proteins and the like. The immunosorbent is simple to prepare, has high adsorbing capacity, high selectivity, good biological and blood compatibility, and relatively lowcost, and provides a new therapeutic method for removing excessive pathogenic inflammatory factors in patients.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com