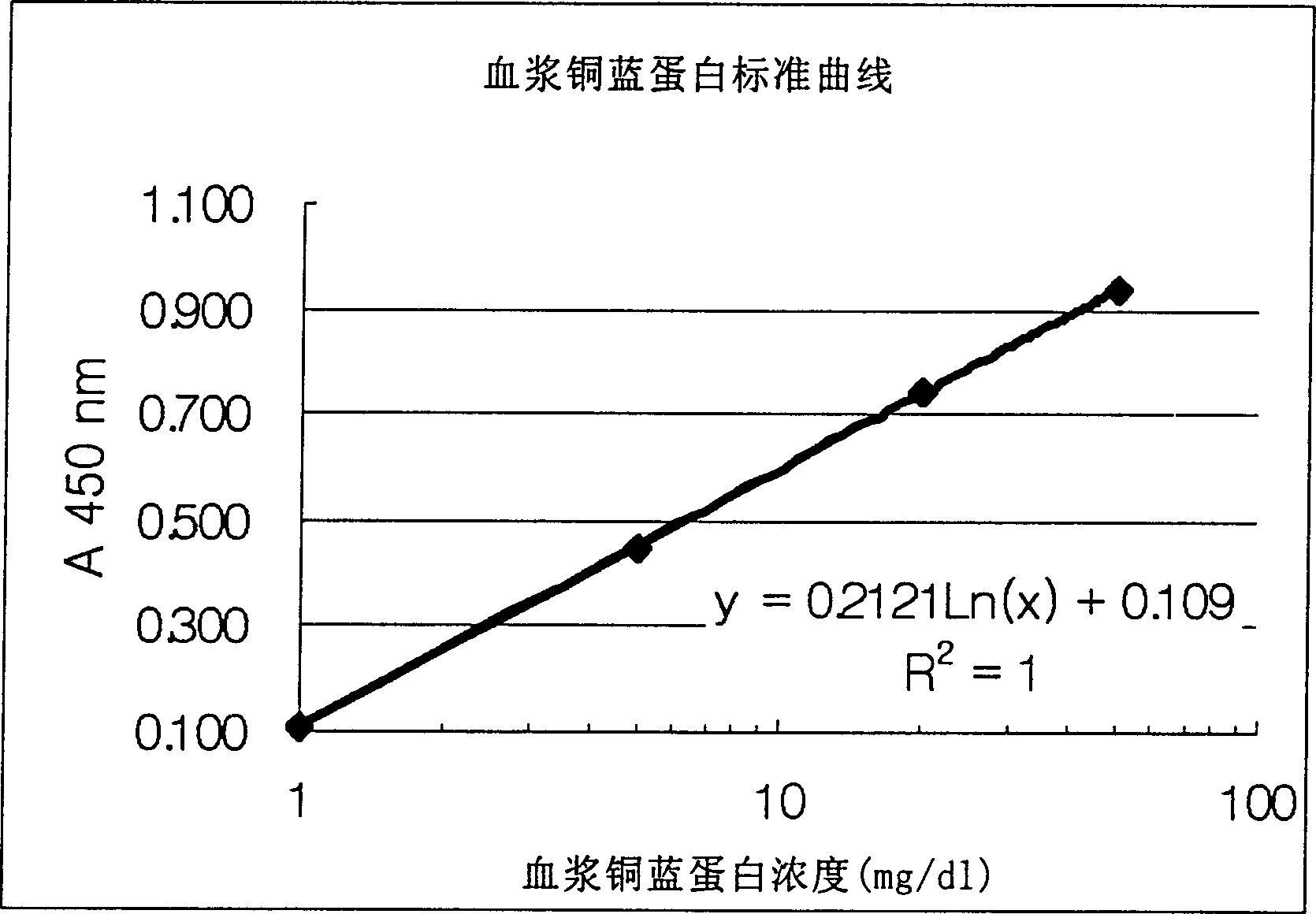
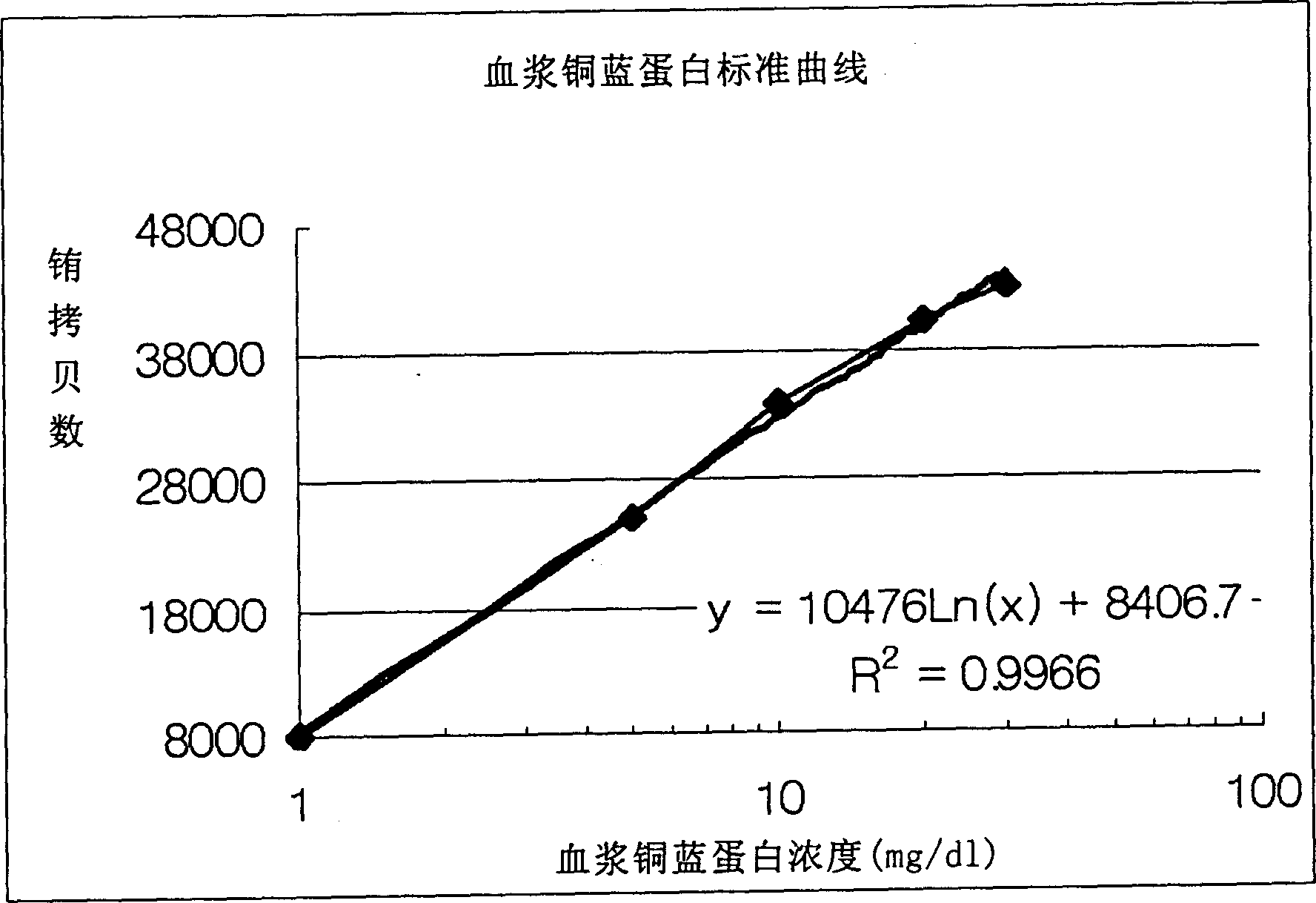
Method of determining ceruloplasm concentration in blood cake and hepatolenticular degeneration disease screening reagent box and diagnostic reagent
A technology of ceruloplasmin concentration and concentration, which is applied in the directions of disease diagnosis, biological testing, biochemical equipment and methods, etc., can solve the problems of lack of ceruloplasmin, difficult to measure ceruloplasmin, and difficult to use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Production of ceruloplasmin-specific polyclonal antibody
[0046] Purified human ceruloplasmin containing whole ceruloplasmin purchased from Sigma was used in this example. In order to generate a polyclonal antibody specific to an antigen, 400 μl of a refined ceruloplasmin solution dissolved in a phosphate buffer was emulsified with Freund's adjuvant (marketed by BRL Company) at a concentration of 1 mg / ml, and given to 10-week-old rabbits received 3 intradermal injections at 14-day intervals. After 14 days, 400 μl of refined ceruloplasmin solution dissolved in phosphate buffer was injected into the ear vein at a concentration of 1 mg / ml, and blood was collected 7 days later by perforating the heart. Place the collected blood at room temperature for 30 minutes, and place it overnight at 4°C. After complete coagulation, centrifuge at 2,500 rpm for 30 minutes, and take supernatant and serum. Ammonium sulfate was added to the collected serum so that the final ...
Embodiment 2
[0047] Example 2: Production of ceruloplasmin-specific monoclonal antibody
[0048] The monoclonal antibody used in the present invention is 100 μl of purified ceruloplasmin at a concentration of 1 mg / ml, vulcanized with the same amount of Freund's adjuvant, and injected 3 times at intervals of 2 weeks after 6-8 weeks. BALB / c mice intraperitoneally. After the final injection, the production of anti-ceruloplasmin antibody was confirmed, and 2 weeks later, the final immunization was performed with 100 μg of ceruloplasmin. After 3 days, spleen cells were extracted from the mice, mixed with Sp2 / 0-Ag14 myeloma cells at a ratio of 10:1, and the mixture was placed in 50% polyethylene glycol 1500 solution for 3 minutes to carry out cell fusion. Then centrifuge this at 1,200rpm for 8 minutes to obtain the cell pellet, suspend it in HAT RPMI-1640 culture medium containing 10% fetal bovine serum, so that each ml contains 3.5×10 6 Cell. Afterwards, dispense into 96-well plates, 0.1ml p...
Embodiment 3
[0050] Example 3: Confirmation of neutralization of ceruloplasmin oxidase activity by antibodies
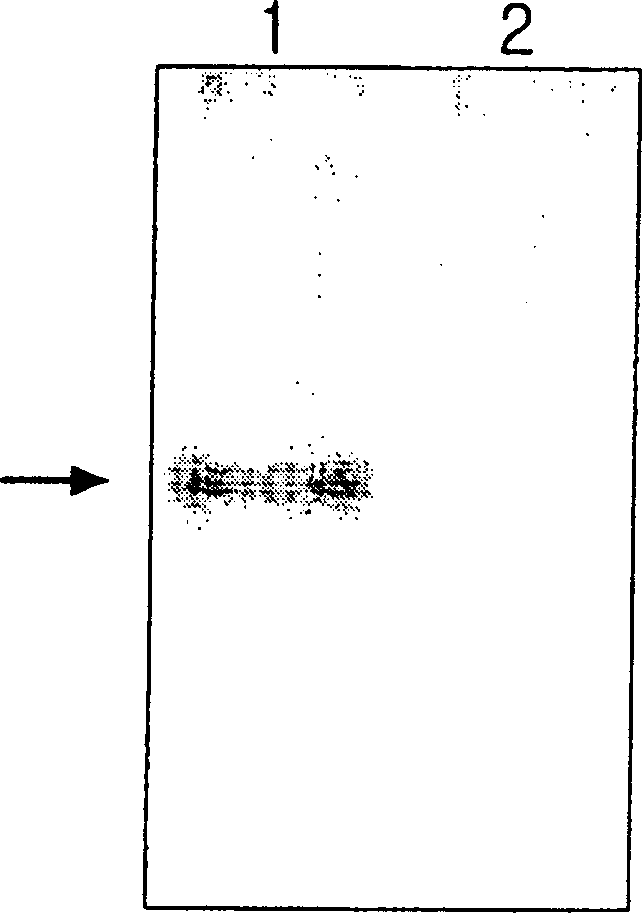
[0051] After mixing 10 μg of purified ceruloplasmin with the hybridoma culture supernatant of the same volume obtained in Example 2, they were reacted at a temperature of 37° C. for 30 minutes, and then, at a temperature of 4° C. ) acrylamide gel (7.5%) electrophoresis. After electrophoresis, in order to confirm the activity of ceruloplasmin oxidase, a sodium acetate (0.1 M, pH 5.7) buffer solution having a concentration of 1 mg / ml p-phenylenediamine was used as a staining solution, and the gel was stained at a temperature of 37°C. Colloid (gel) for 2 hours, decolorized in 50% ethanol solution. ( figure 1 )
[0052] From figure 1 It can be seen from the results that purified ceruloplasmin oxidizes colorless p-phenylenediamine due to its own oxidase activity, forming a purple band. In contrast, in the mixture of ceruloplasmin and ceruloplasmin-specific antibody, no purple ban...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com