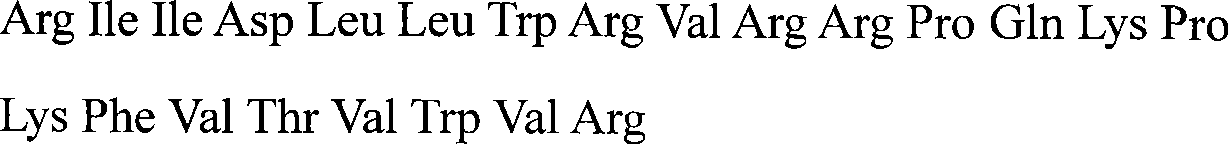Preparation and use of antibacterial peptide PMAP-23 monoclonal antibody
A technology of PMAP-23 and monoclonal antibody, which is applied in the direction of anti-animal/human immunoglobulin, instruments, analytical materials, etc., and can solve the problems of low content, small molecular weight of natural antimicrobial peptides, and difficulty in obtaining antimicrobial peptide immunogens, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1, the preparation of artificial immunogen:
[0012] Artificially synthesized fragments containing amino acid residues of porcine antimicrobial peptide PMAP-23: Arg-Ile-Ile-Asp-Leu-Leu-Trp-Arg-Val-Arg-Arg-Pro-Gln-Lys-Pro-Lys-Phe-Val -Thr-Val-Trp-Val-Arg. The immunogen PMAP-23-OVA was prepared by the carbodiimide (EDC) method, and the protein concentration of the coupled PMAP-23-OVA was measured by a full-wavelength ultraviolet / visible light scanning spectrophotometer, and then stored in -20°C. The artificially coated original PMAP-23-BSA was prepared by the same method, and stored at -20°C in aliquots.
[0013] Healthy female Balb / c pure-line mice aged 8-12 weeks were immunized by direct intraperitoneal injection with two basic immunizations and one booster immunization. On the first day, 100 μl PMAP-23-OVA+100 μl Freund’s complete adjuvant was used for basic immunization; on the 16th day, 100 μl PMPA-23-OVA+100 μl Freund’s incomplete adjuvant was used for ...
Embodiment 2
[0020] Embodiment 2, the clone of positive hybridoma cell:
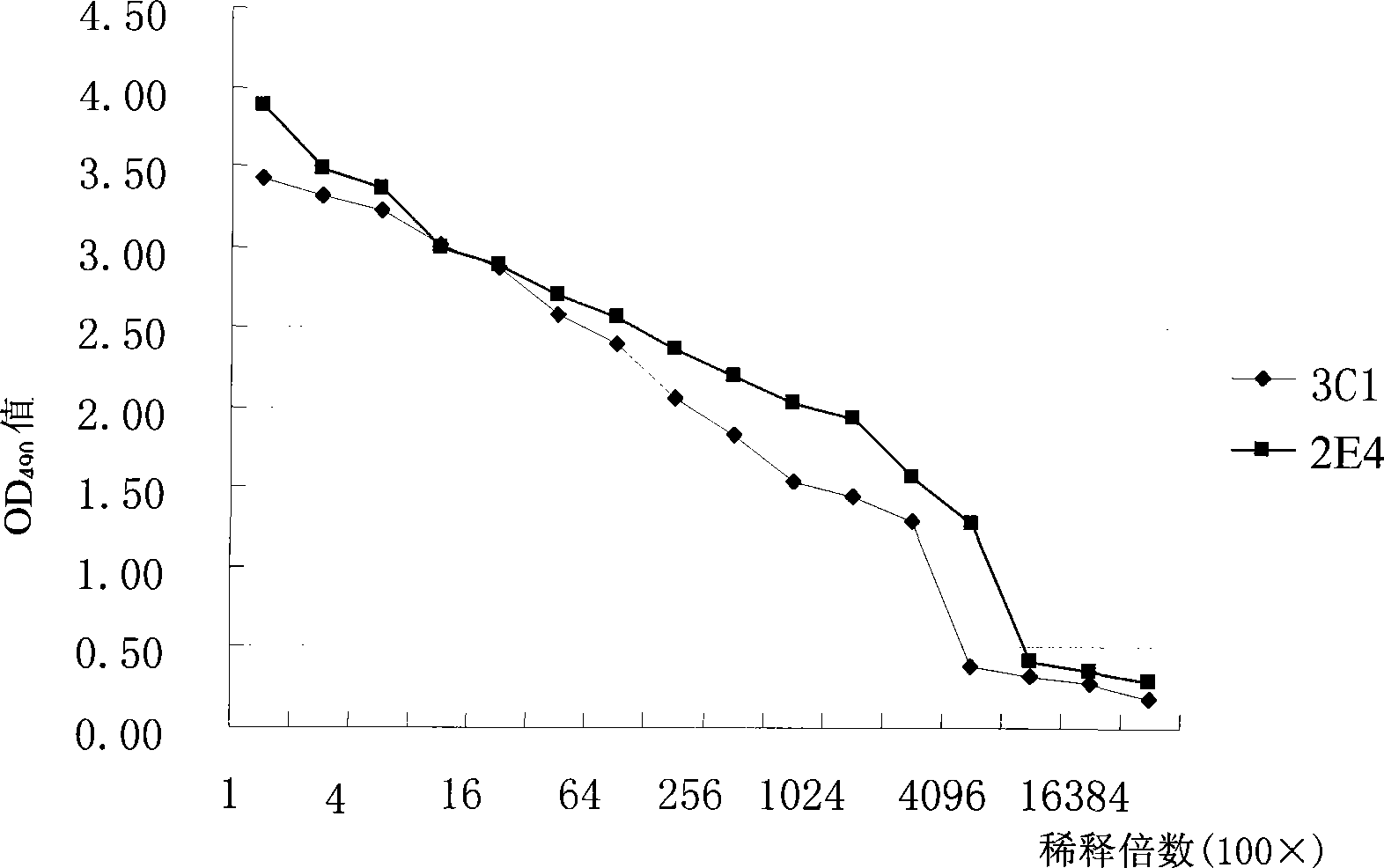
[0021] Use a pasteurized tube to gently blow and blow the cells in the target well to be cloned to prepare a cell suspension. Prepare a series of double-ratio cell suspensions in a 96-well cell culture plate, 0.2ml per well, cell pellet for 2-3 hours, and select under an inverted microscope Wells with the appropriate number of cells. Pick 80-100 cells / well for the first clone, and 60-80 cells / well for the second clone. Transfer the cells in the selected wells to a cell culture flask, then add 10ml of HT medium, and inoculate 0.1ml / well into a 96-well cell culture plate, that is, each well contains about 1 cell on average. After 9-10 days of cloning, single-cell cloning lines were selected for positive detection, and the obtained positive cloning wells were further cloned for the second and third times until the positive rate of the supernatant was 100% after cloning and the degree of reaction was basically the same ...
Embodiment 3
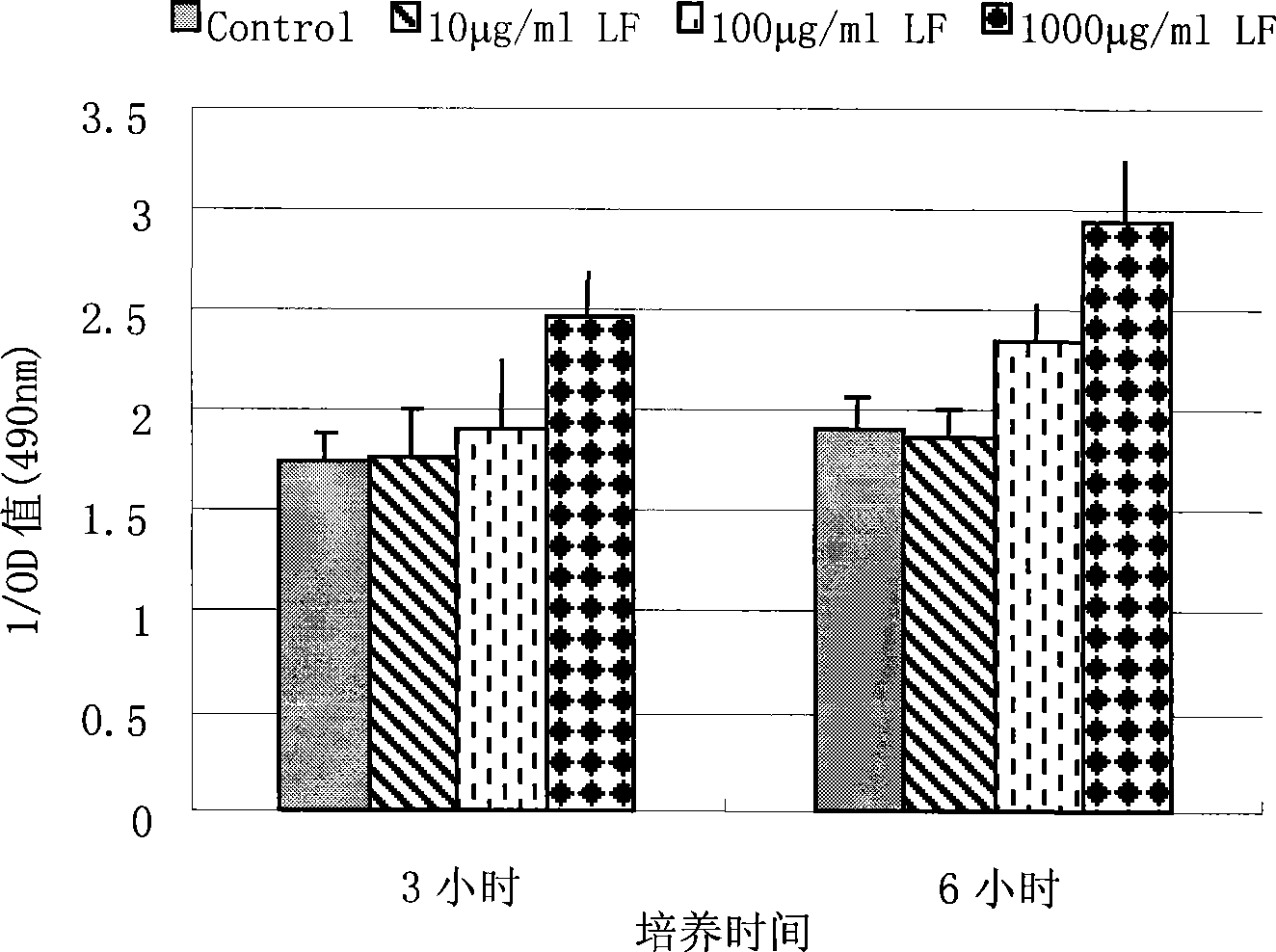
[0042] Example 3, application of PMAP-23 monoclonal antibody: establishment of an inhibitory ELISA experimental method to detect the effect of lactoferrin on the level of PMAP-23 secreted by bone marrow cells:
[0043] The culture supernatant of porcine bone marrow cells contains the natural antimicrobial peptide PMAP-23. Taking the culture supernatant of bone marrow cells as the inhibitory object, the prepared positive supernatant was used to establish an inhibitory ELISA to detect the effect of lactoferrin on PMAP-23 in the culture supernatant of bone marrow cells. level of impact. Extract 30ml of bone marrow from the femur of a 30-day-old piglet by puncture, place it in RPMI 1640 culture medium (containing 10% fetal bovine serum), use a pipette tip to separate the bone marrow cells into single cells, and then spread them on the same volume with a specific gravity of 1.077g Centrifuge at 2000rpm for 30min, take the granulocyte layer into a new centrifuge tube, wash twice wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com