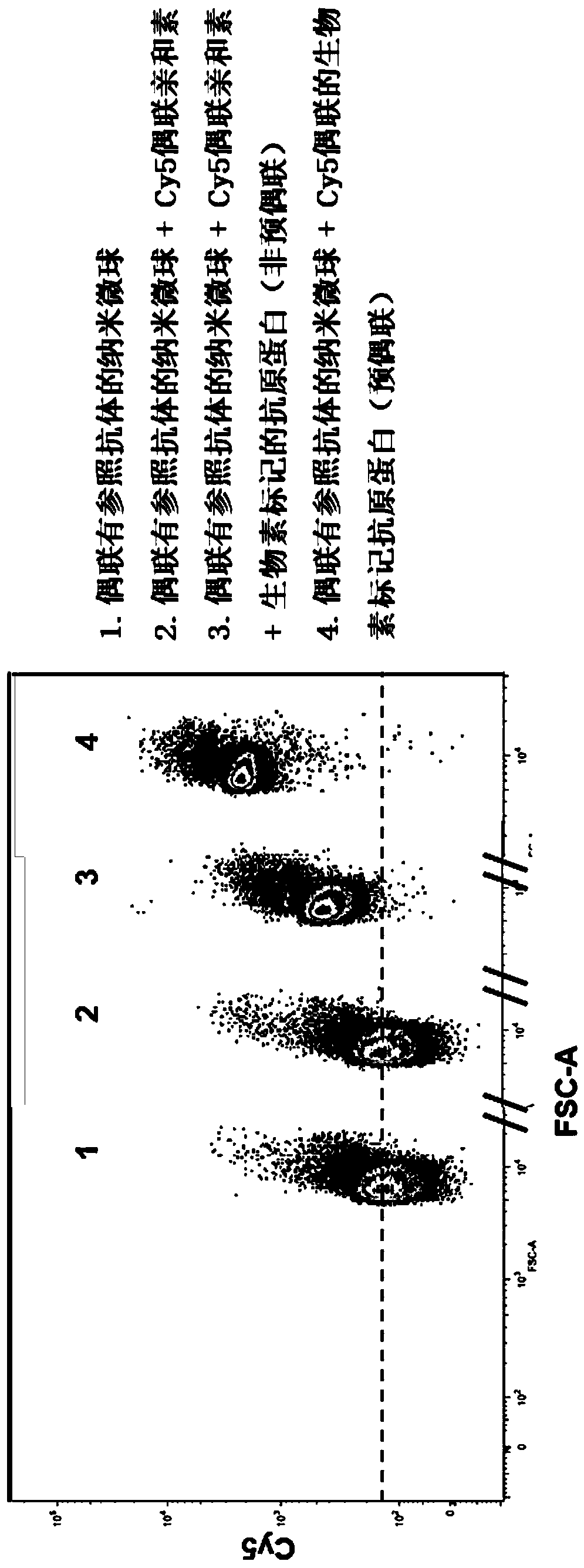
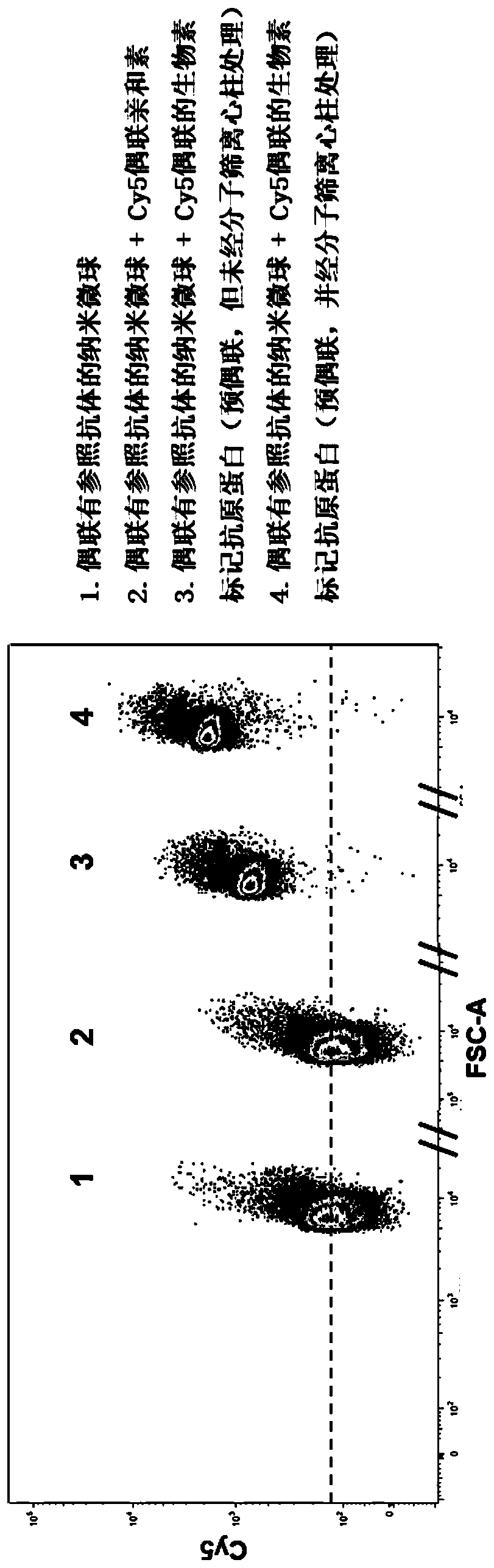
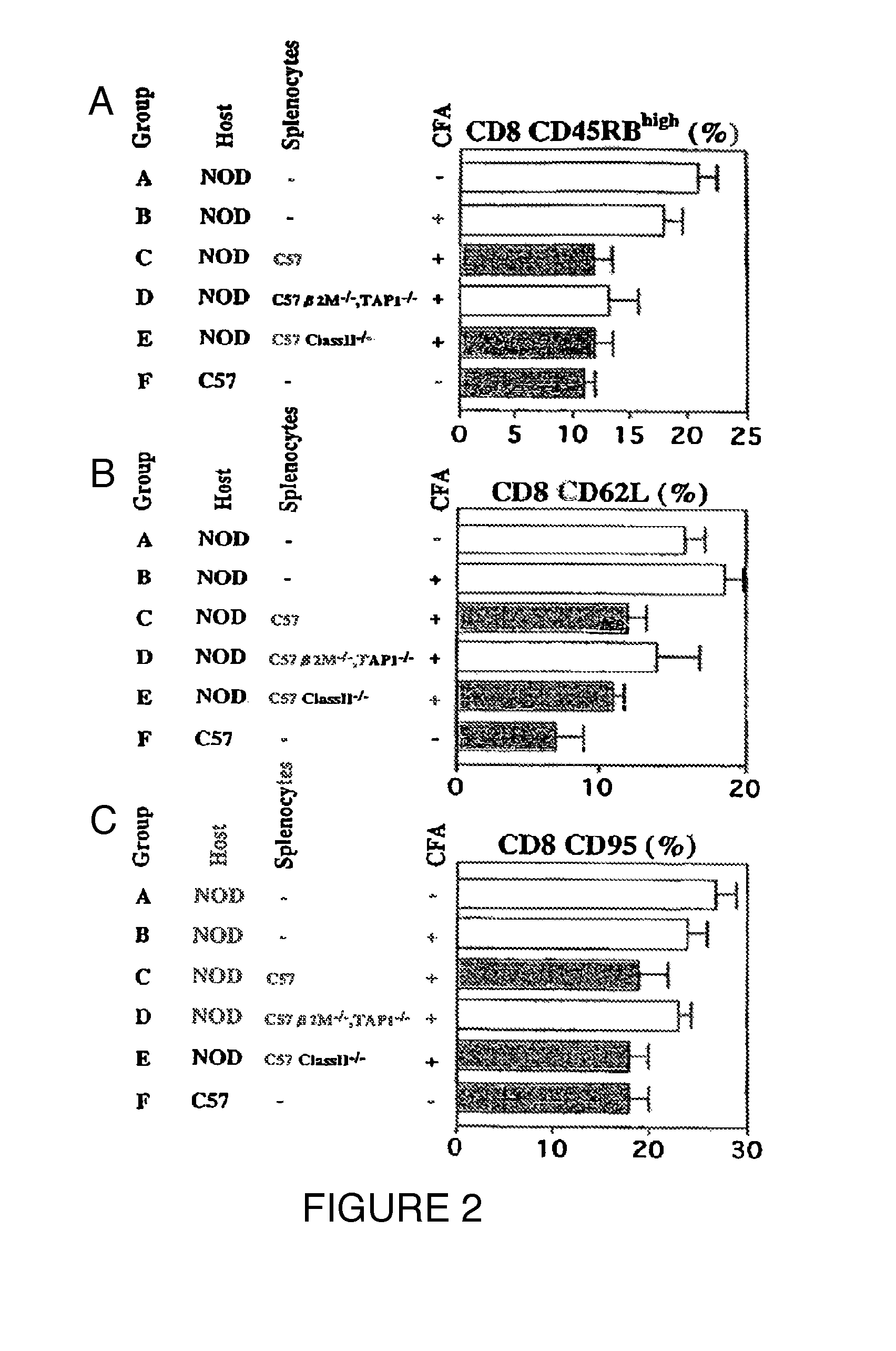
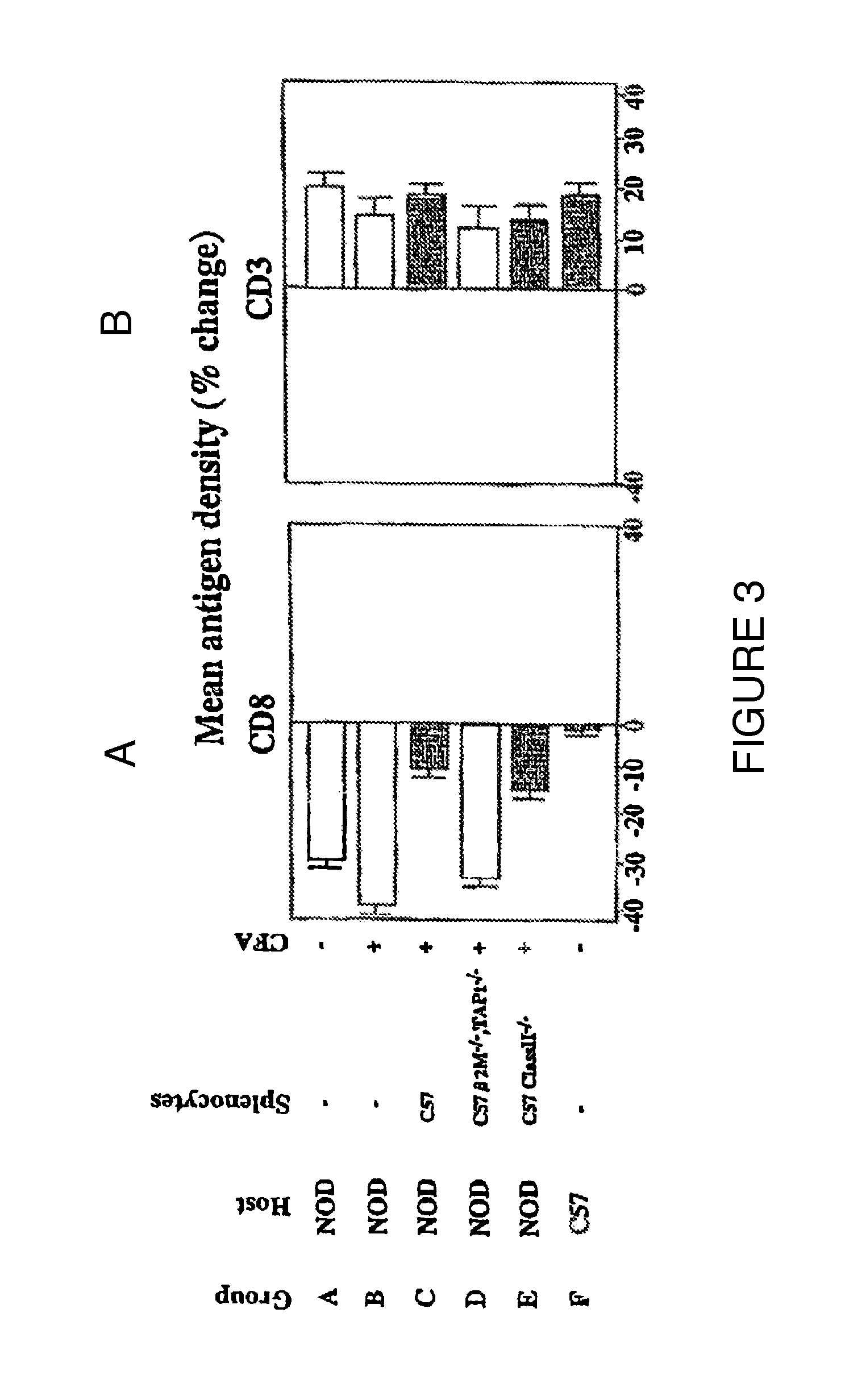
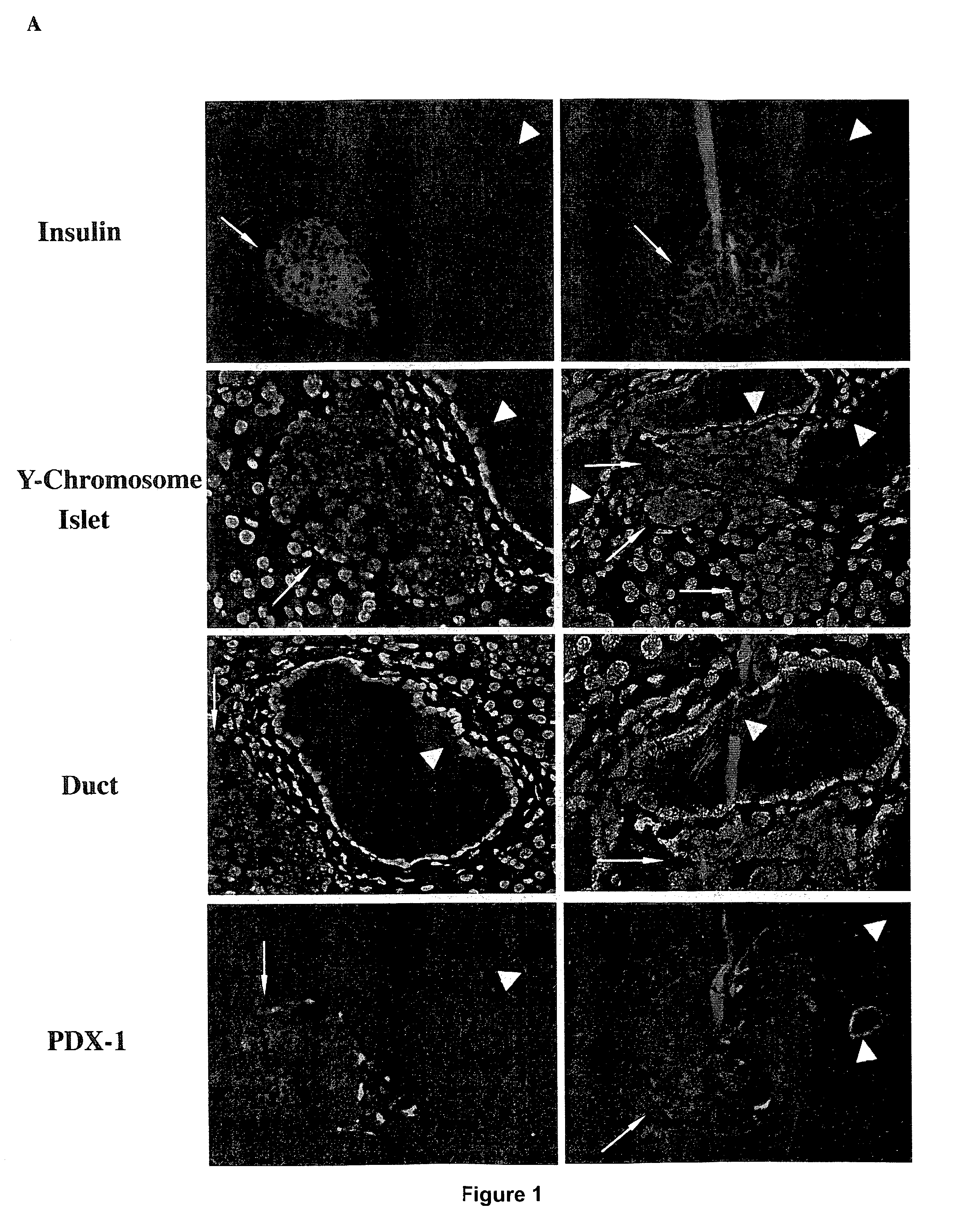
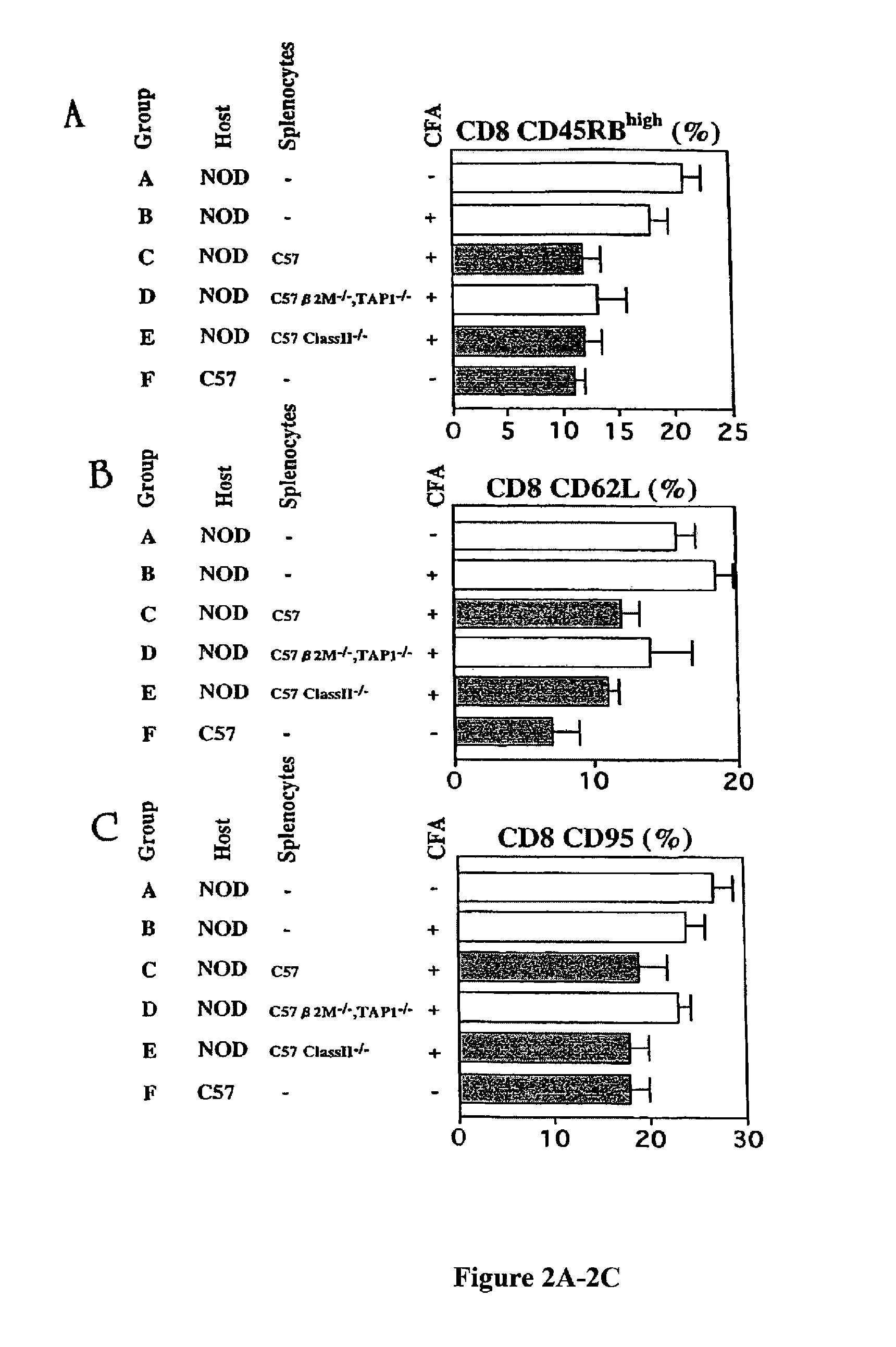
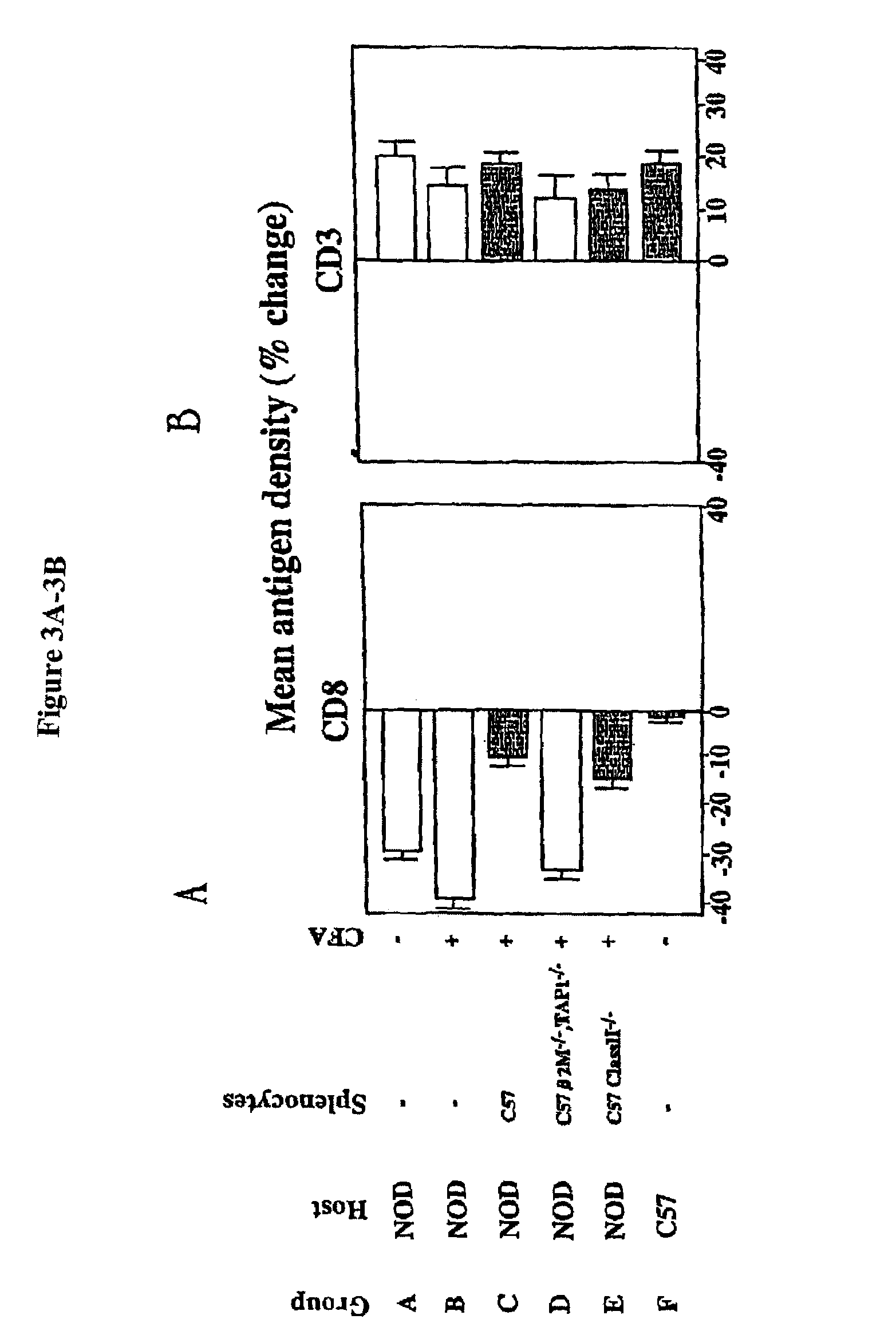
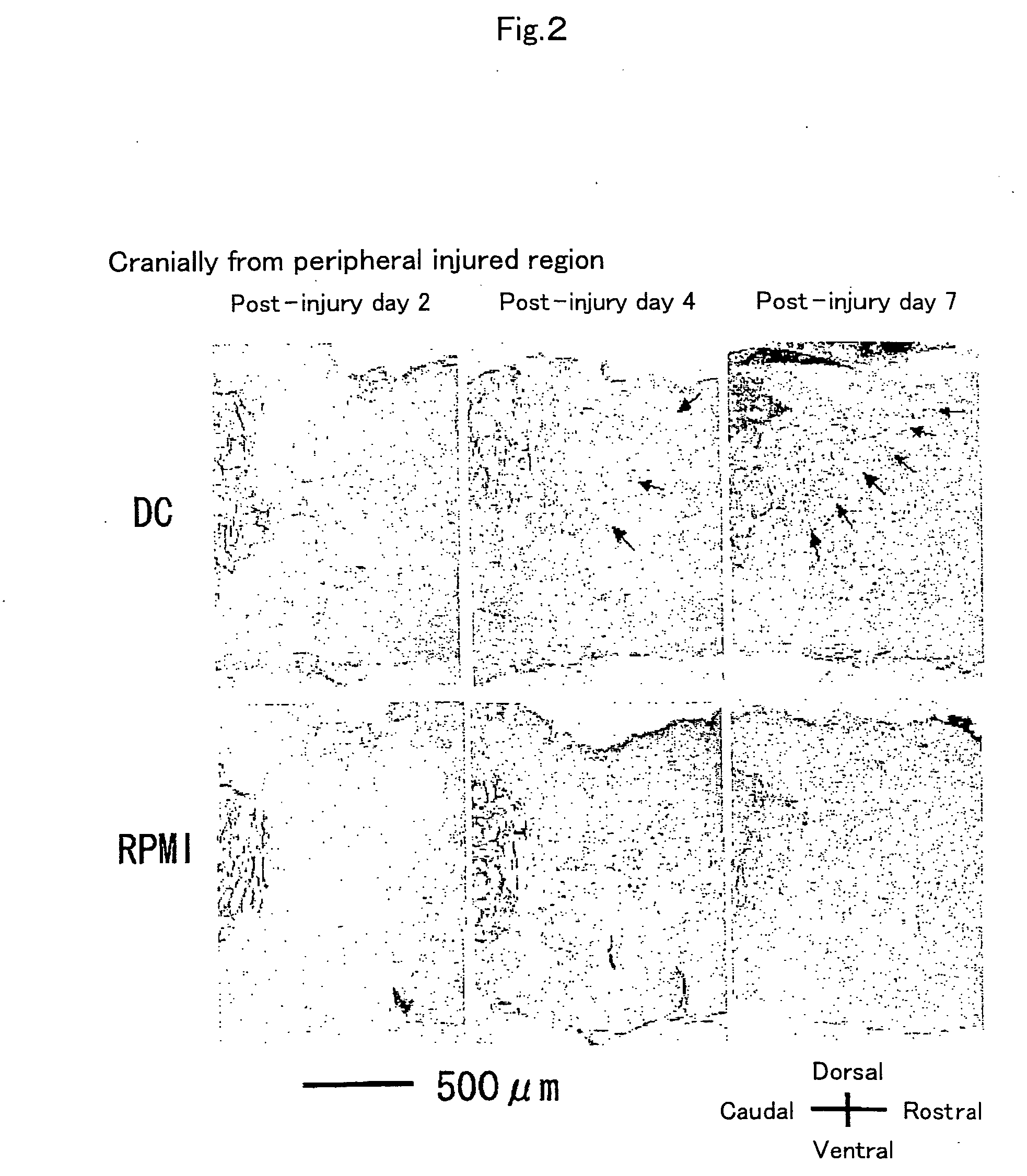
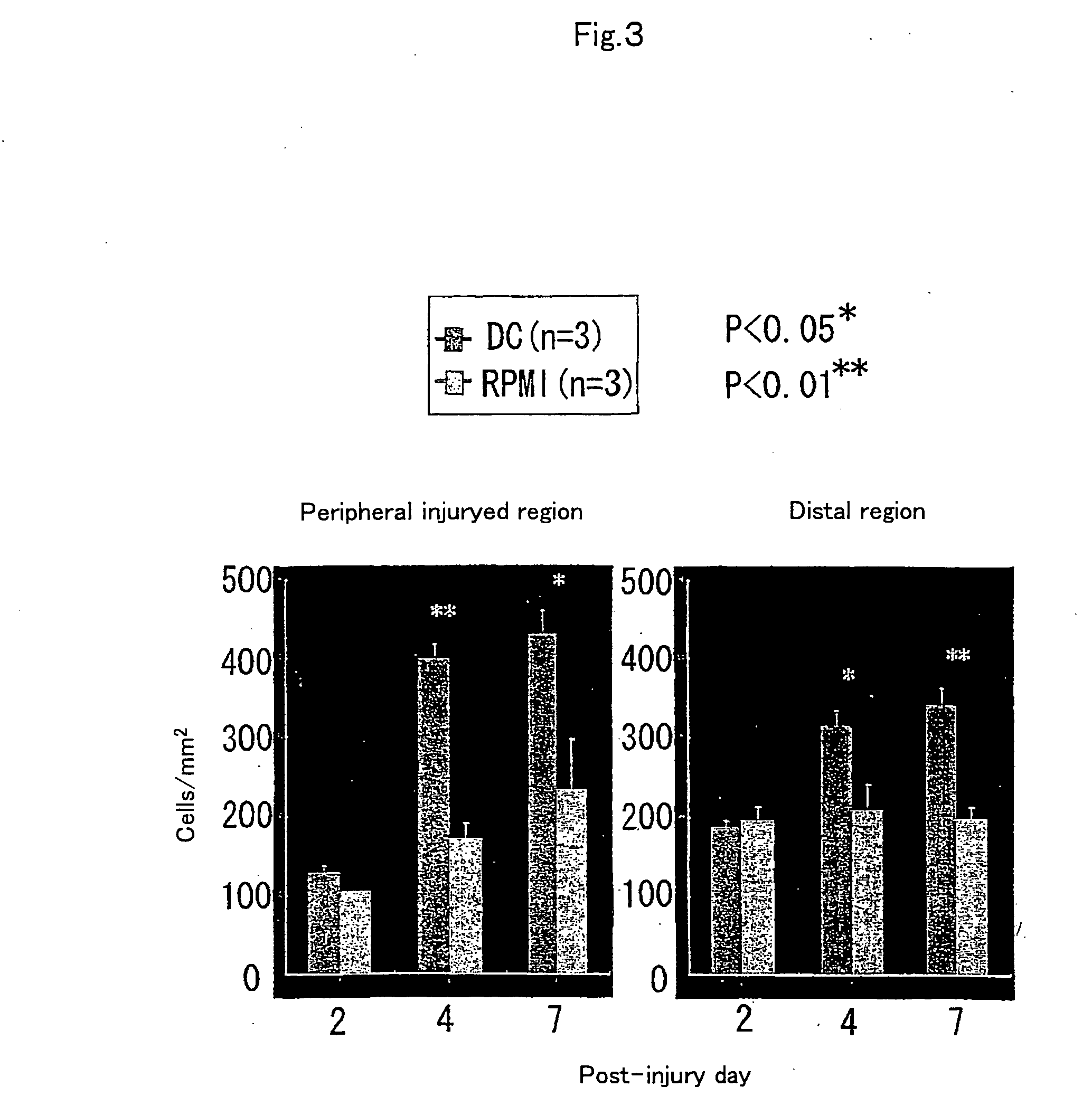
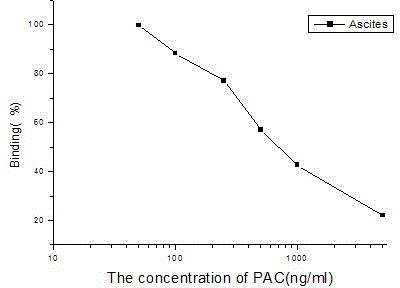
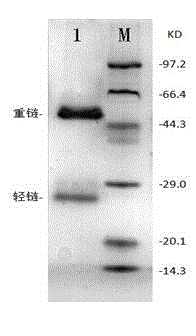
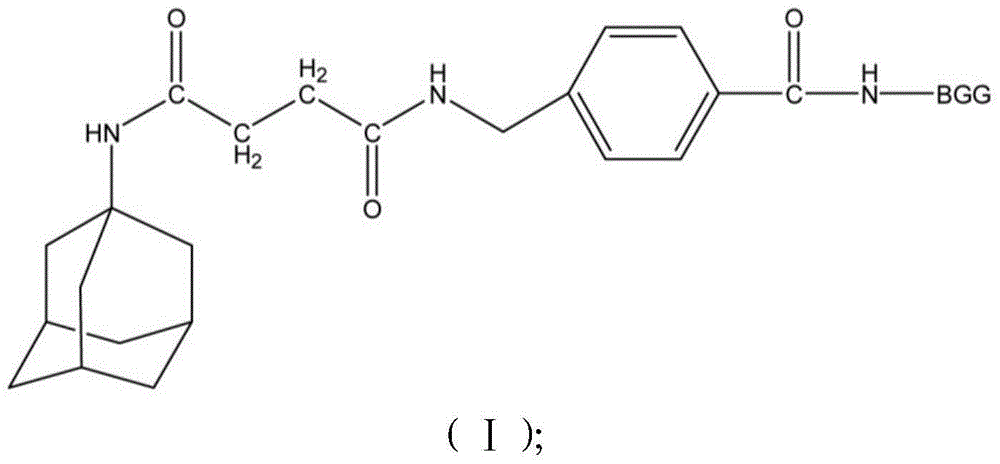
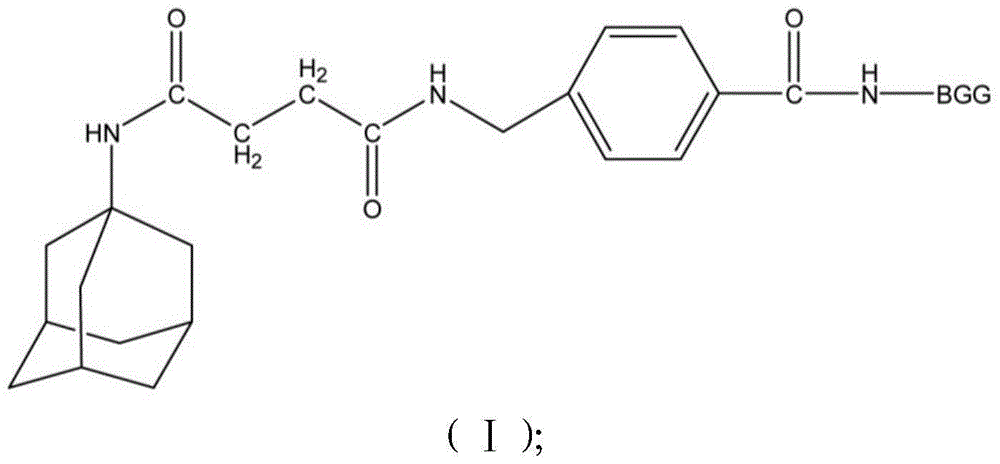
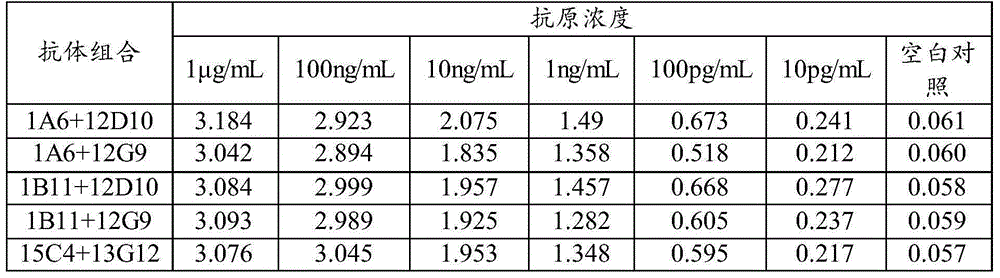
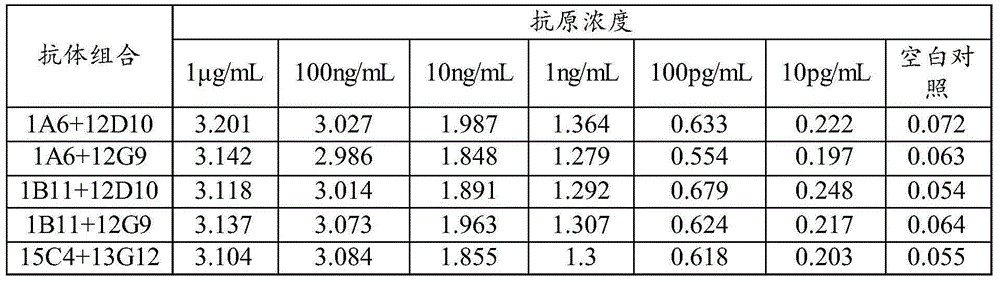
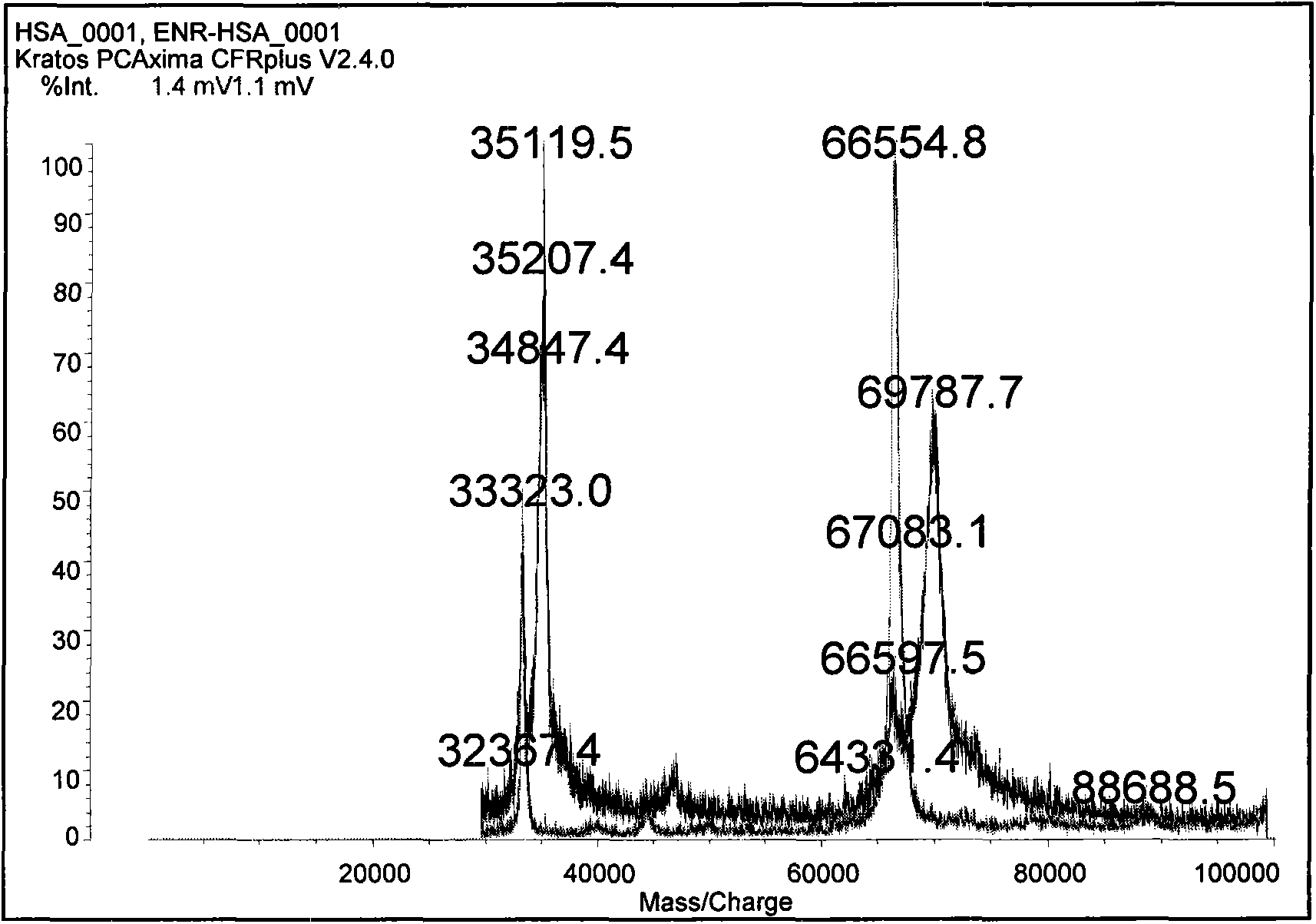
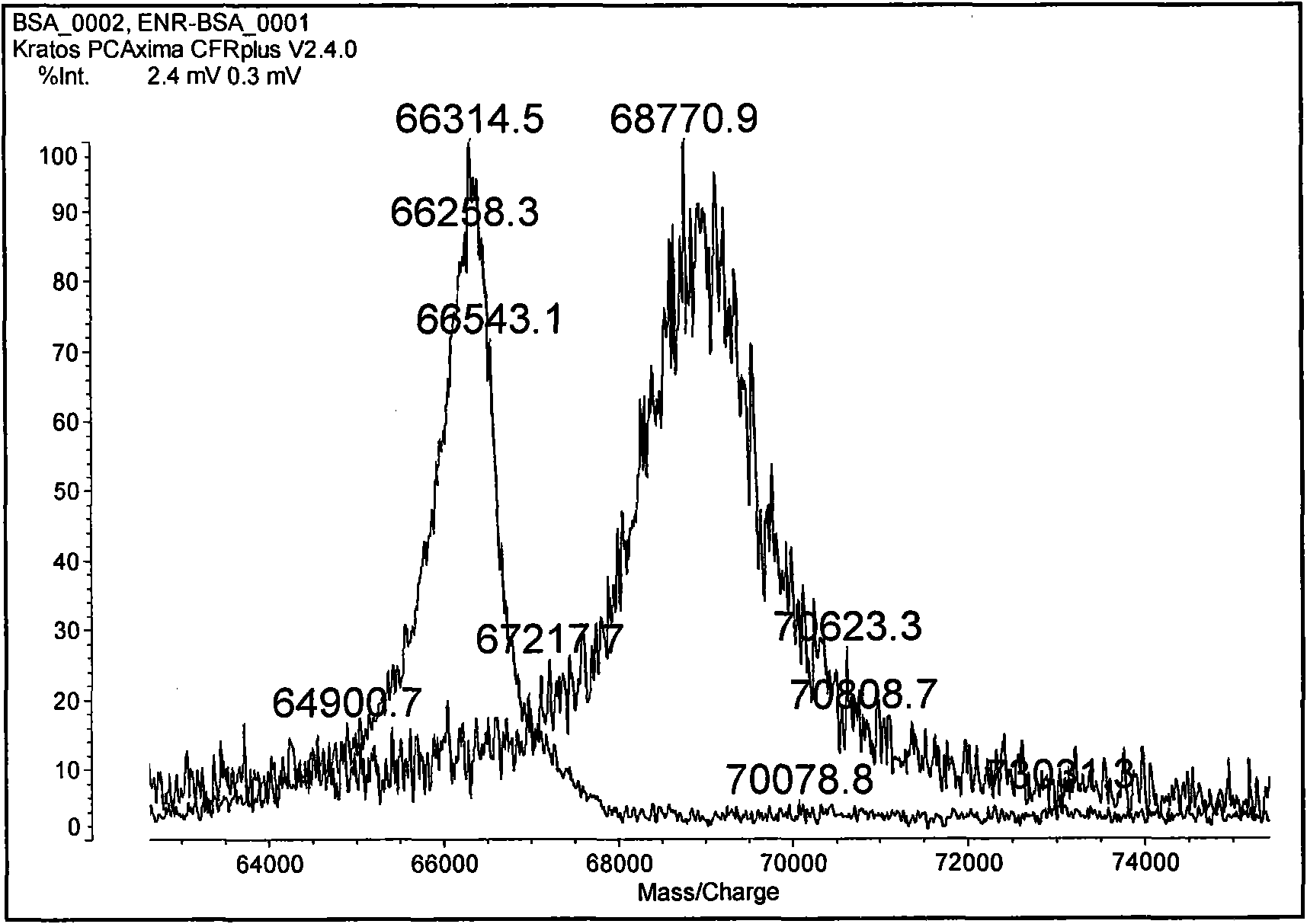
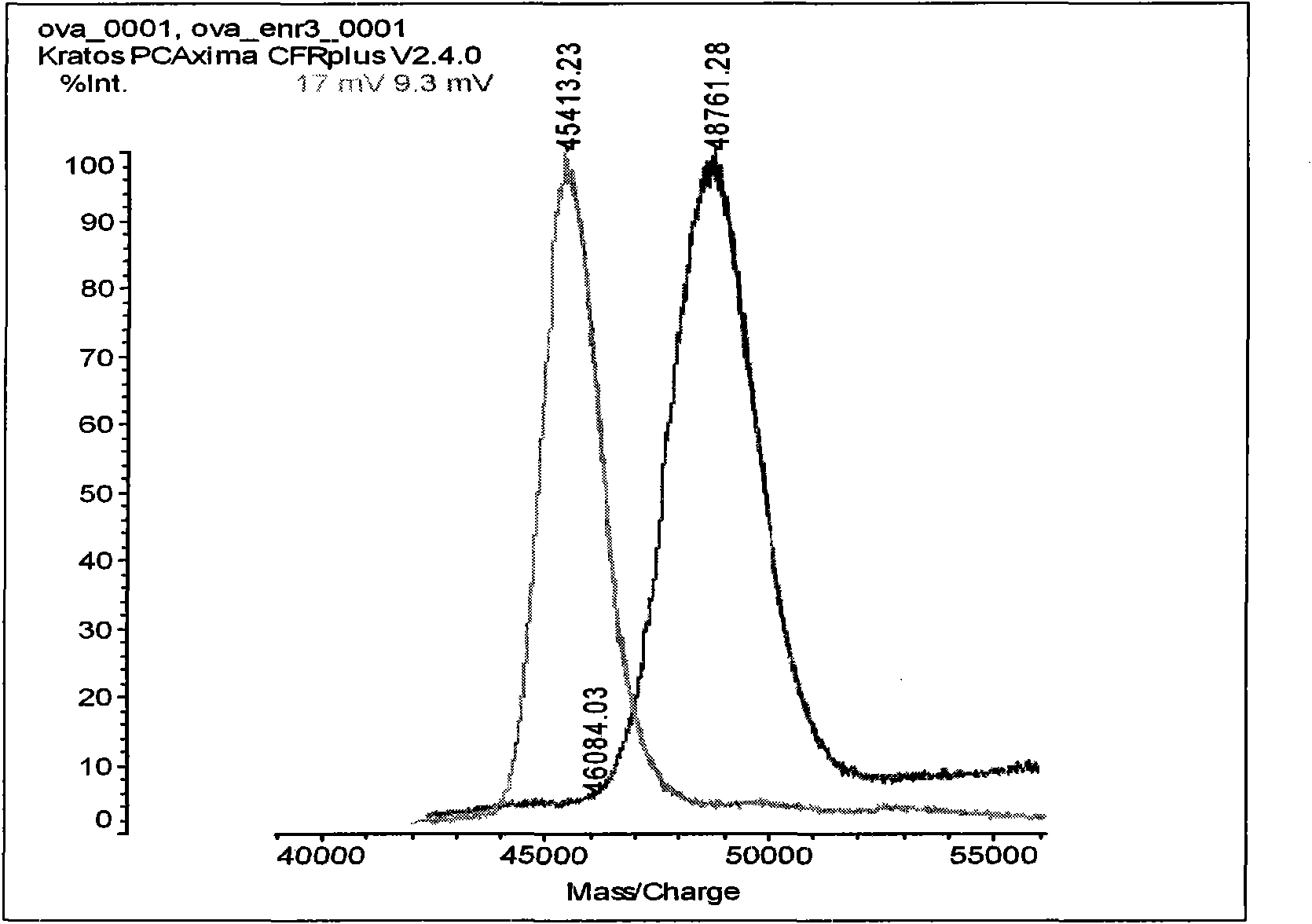
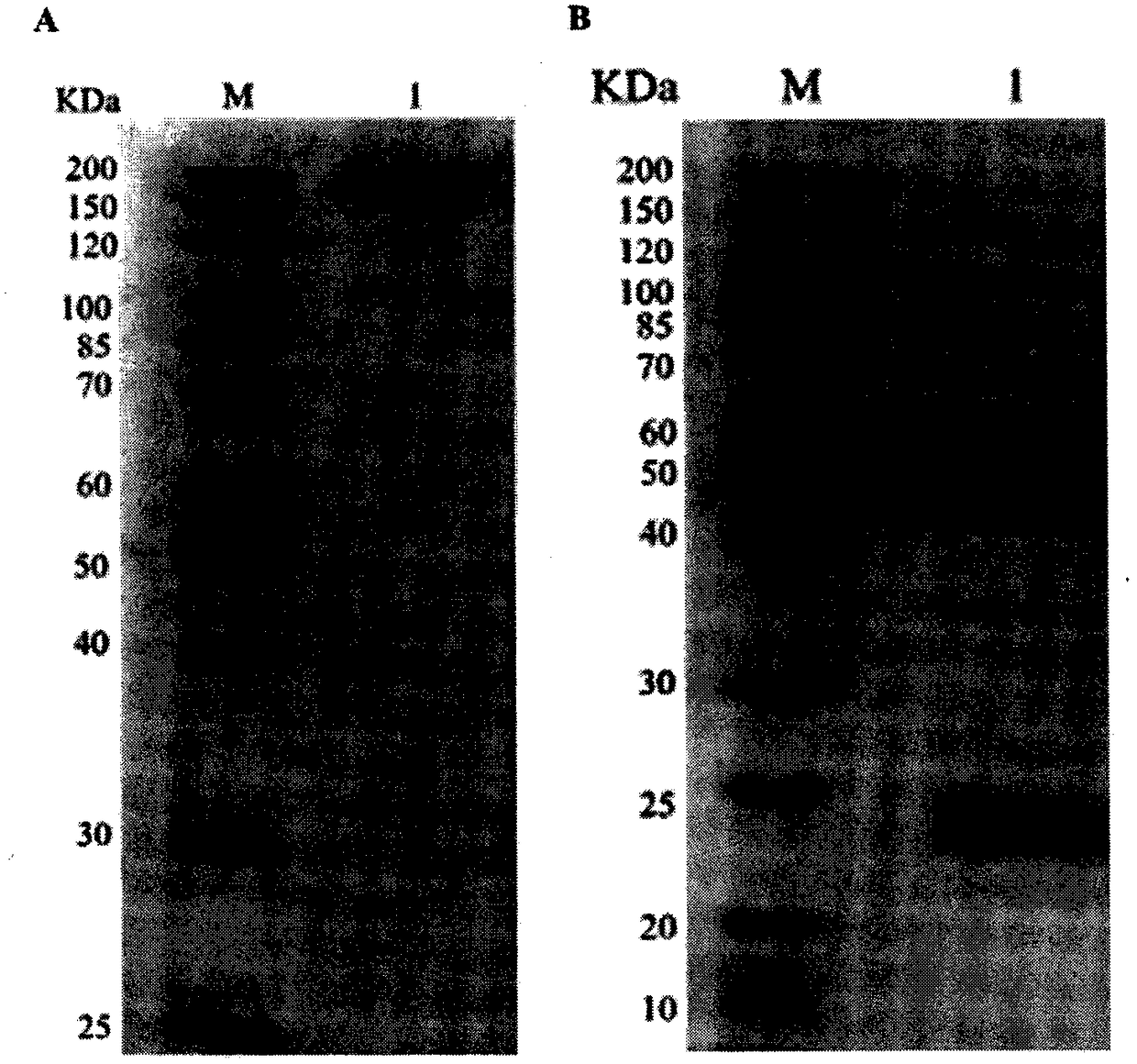
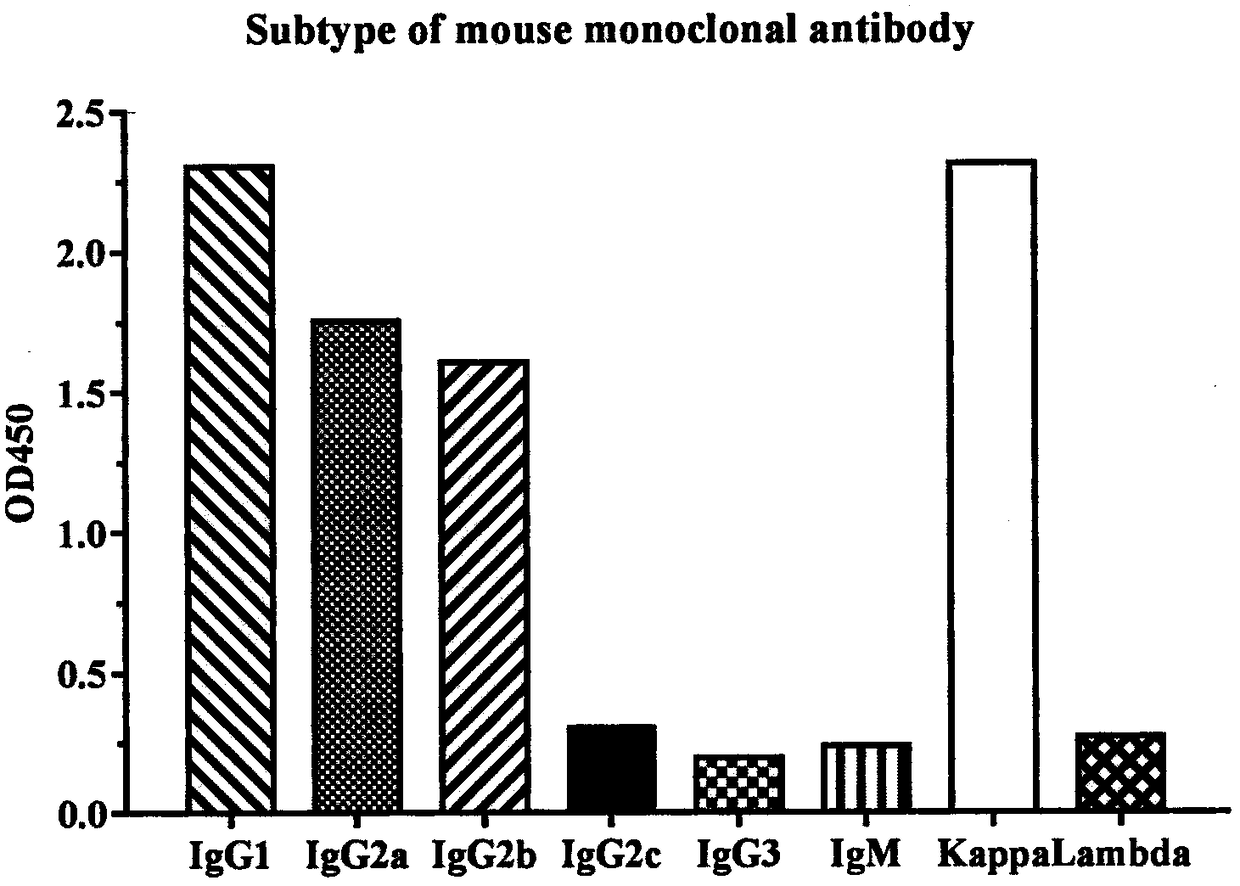
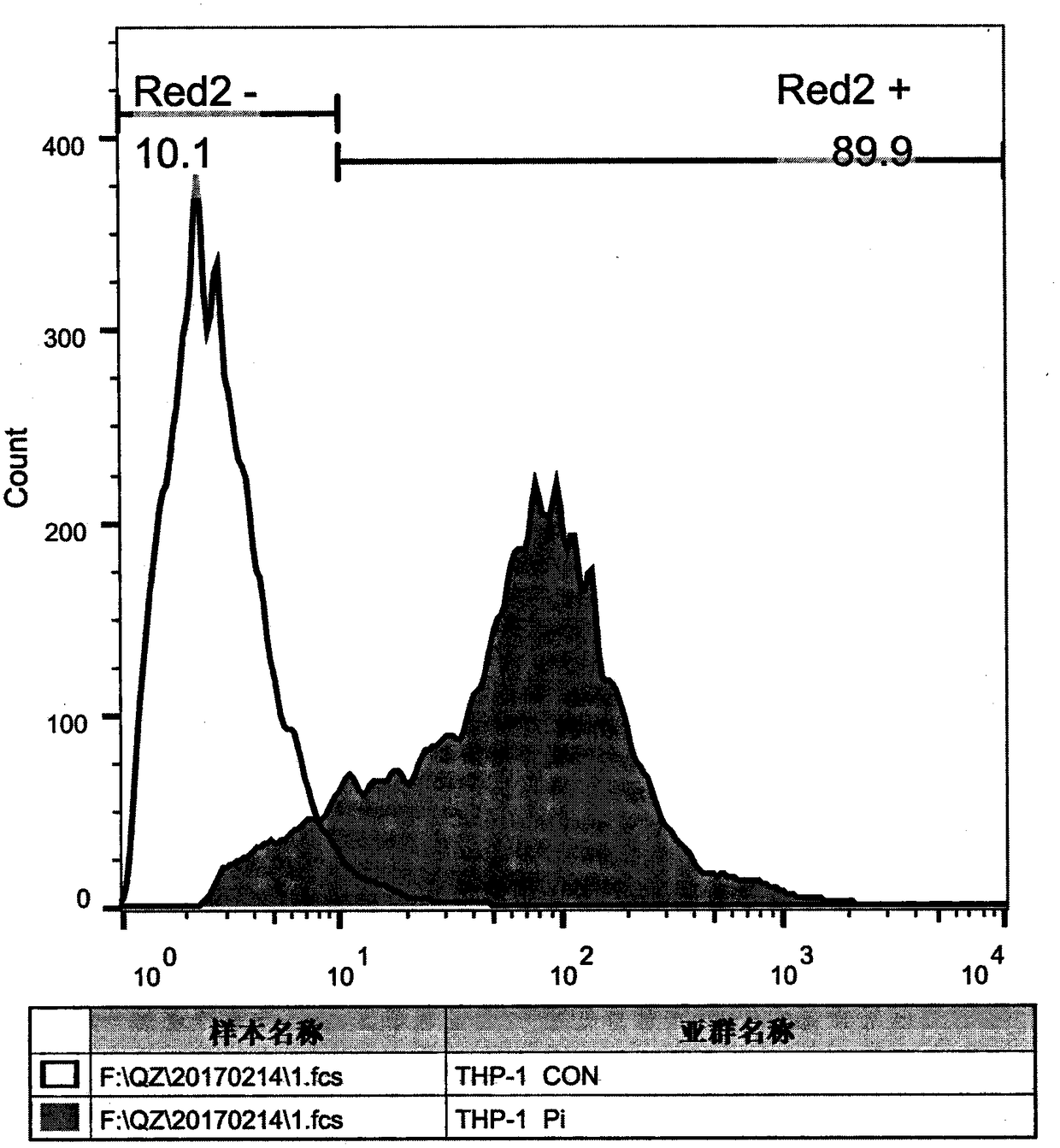
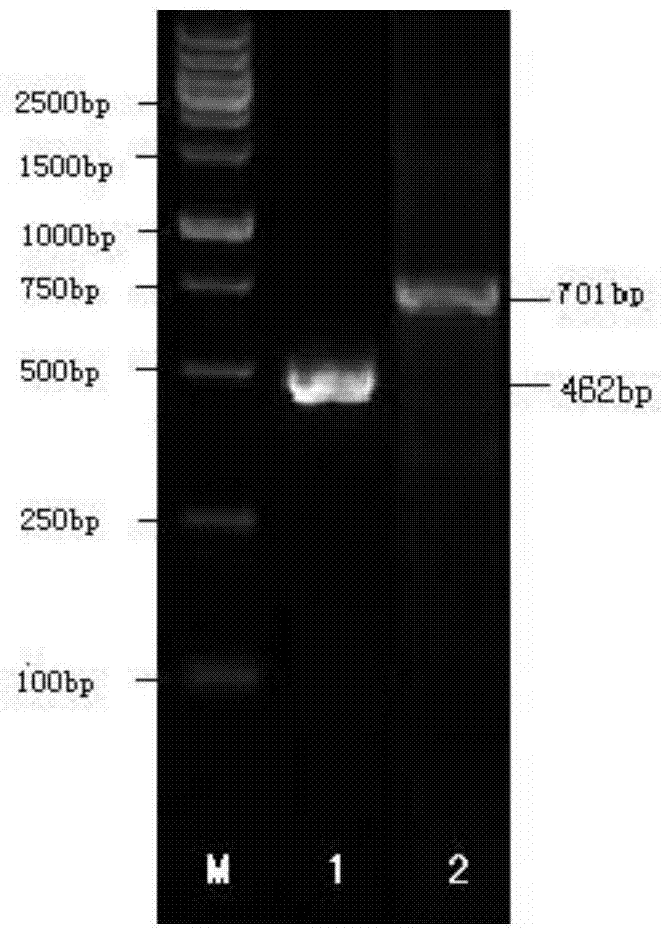
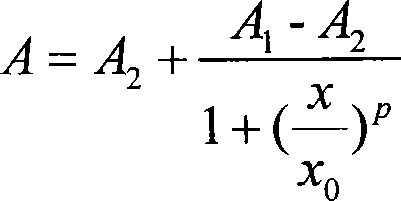Patents
Literature
263 results about "Spleen cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The major role of the spleen is to filter blood. The spleen develops and produces mature immune cells that are capable of identifying and destroying pathogens. Contained within the white pulp of the spleen are immune cells called B and T-lymphocytes. T-lymphocytes are responsible for cell-mediated immunity,...
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
Method for efficiently separating single antigen-specific B lymphocyte from spleen cells
ActiveCN110016462AIncrease positive rateKeep activeCell dissociation methodsBlood/immune system cellsSurface markerSpleen cell
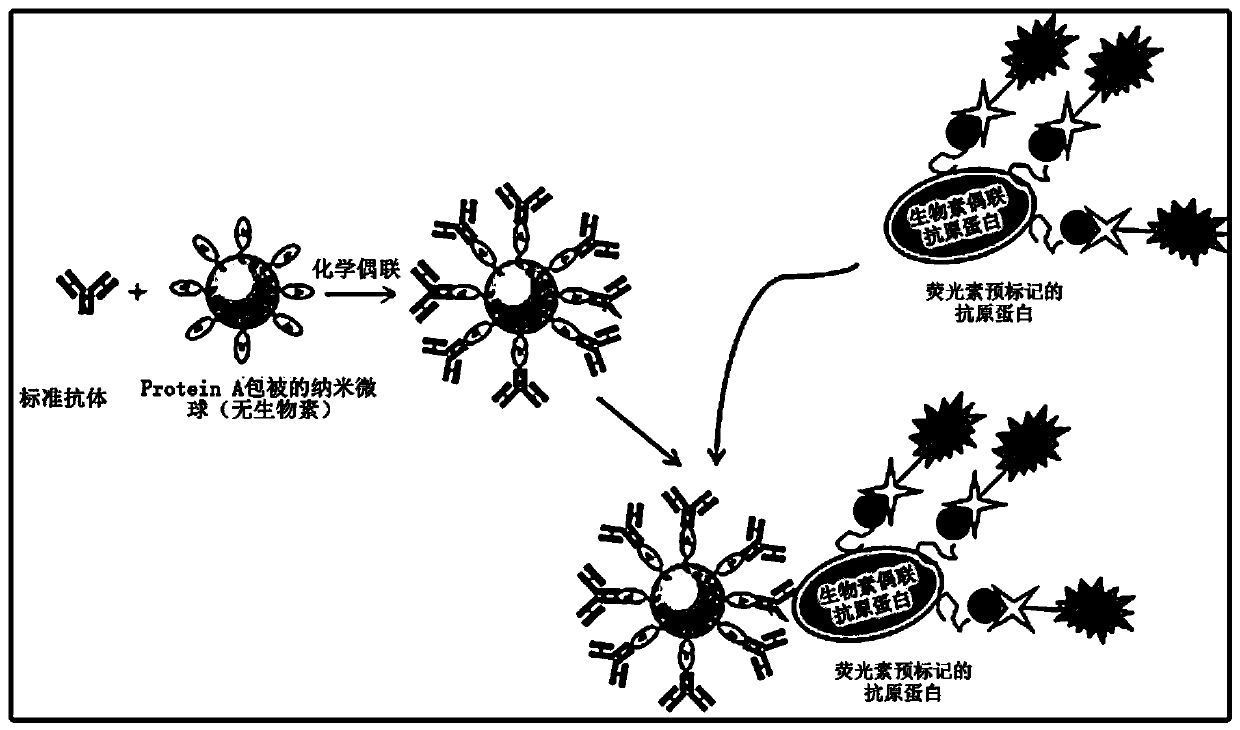
The invention discloses a method for efficiently separating single antigen-specific B lymphocyte from spleen cells. High-throughput rapid screening of single antigen-specific B lymphocyte is realizedby negative screening adopting multiple lymphocyte surface marker antibodies, establishment of a rapid fluorescence marked antigen substance method and high-specificity screening by use of a fluorescence marked antigen substance, and the positive rate of the finally obtained antibody-specific B lymphocyte is increased from less than 5% in a traditional hybridoma method to 30%. Therefore, the scheme can shorten monoclonal antibody development time to a great extent and greatly increase the number and diversity of obtained monoclonal antibodies.
Owner:优睿赛思(武汉)生物科技有限公司
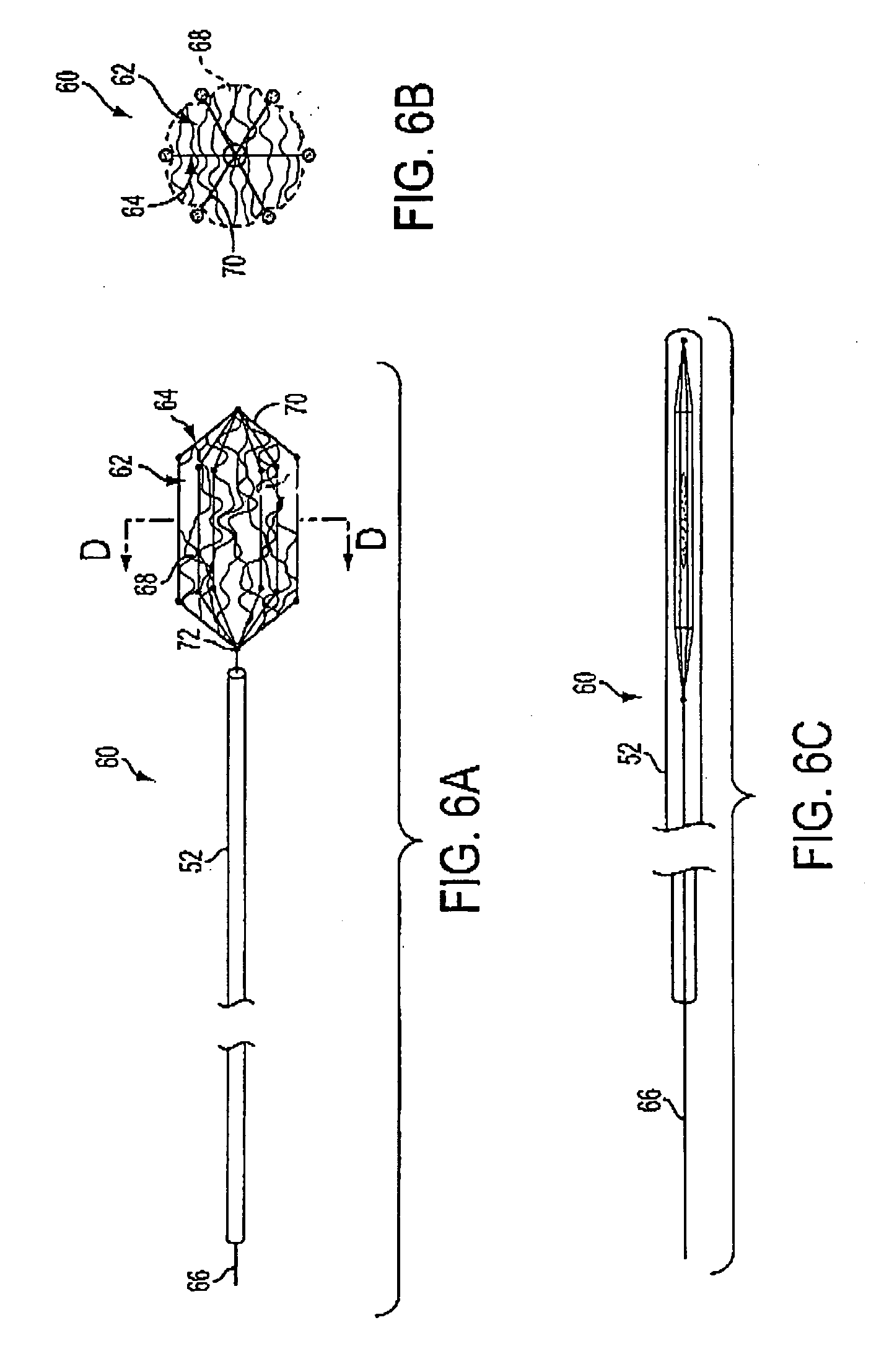
Implantable device for treating disease states and methods of using same
InactiveUS20050064009A1Easy to deployEasy retrievalStentsPharmaceutical delivery mechanismSynthetic HormoneActive agent
Methods and apparatus are provided for delivering drugs, gene vectors, naturally-occurring or synthetic hormones or proteins or other bioactive agents within a patient's vasculature. In a preferred embodiment, the apparatus comprises a material that elutes or secretes a bioactive agent and is held in place within the patient's vessel by an anchor. The material may comprise a biocompatible, and optionally, absorbable matrix, or a culture medium that sustains and nourishes stem cells, spleen cells or pancreatic islets or other beneficial cells. The anchor and material are sized and / or collapsible from a delivery configuration, in which the anchor and material may be delivered into the patient's vasculature within a delivery sheath, to a deployed configuration, wherein the anchor engages an interior wall of the patient's vessel. The apparatus of the invention may be temporarily or permanently implanted, and may in addition self reconfigure after a predetermined period of residency.
Owner:NEXEON MEDSYST
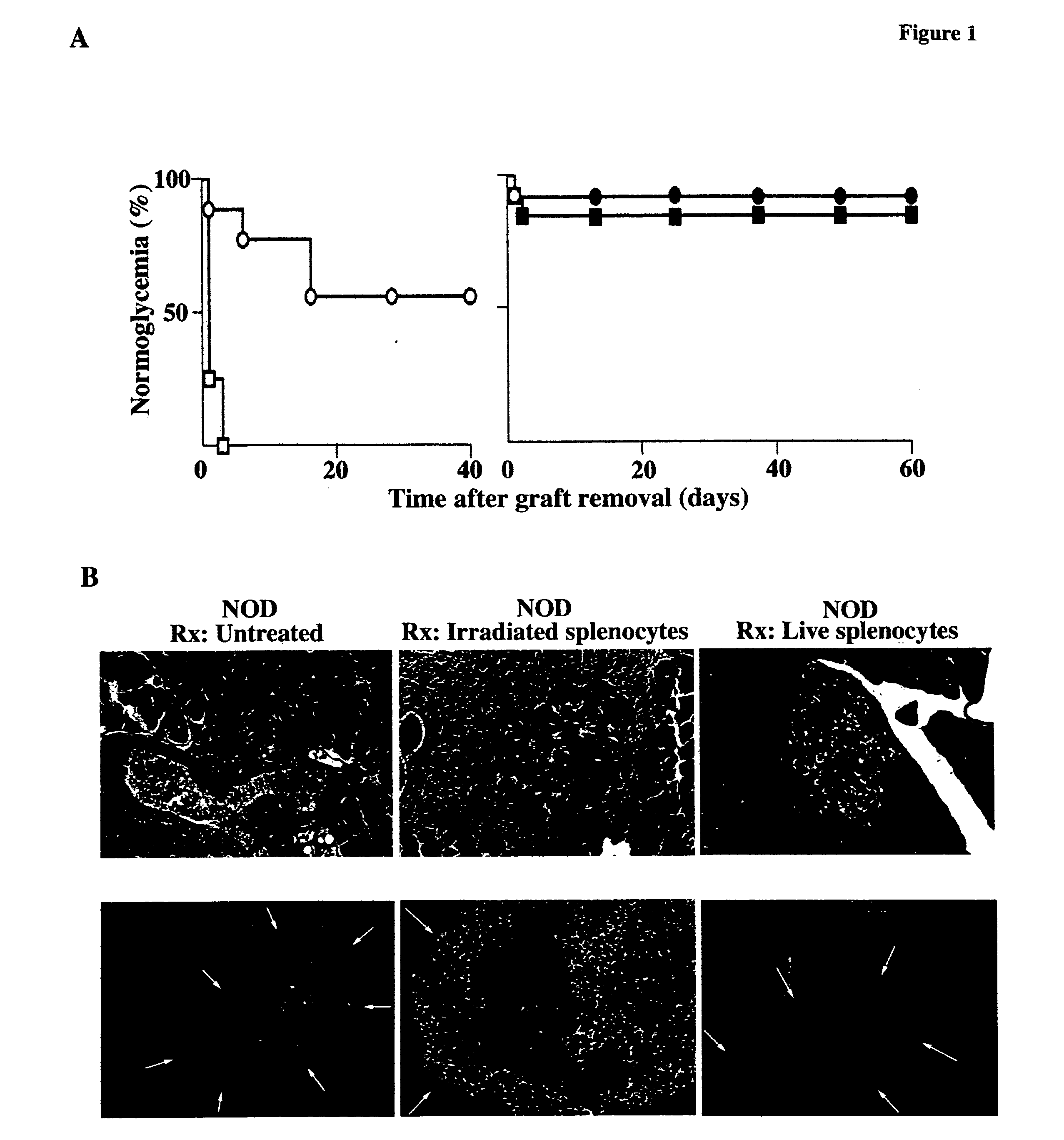
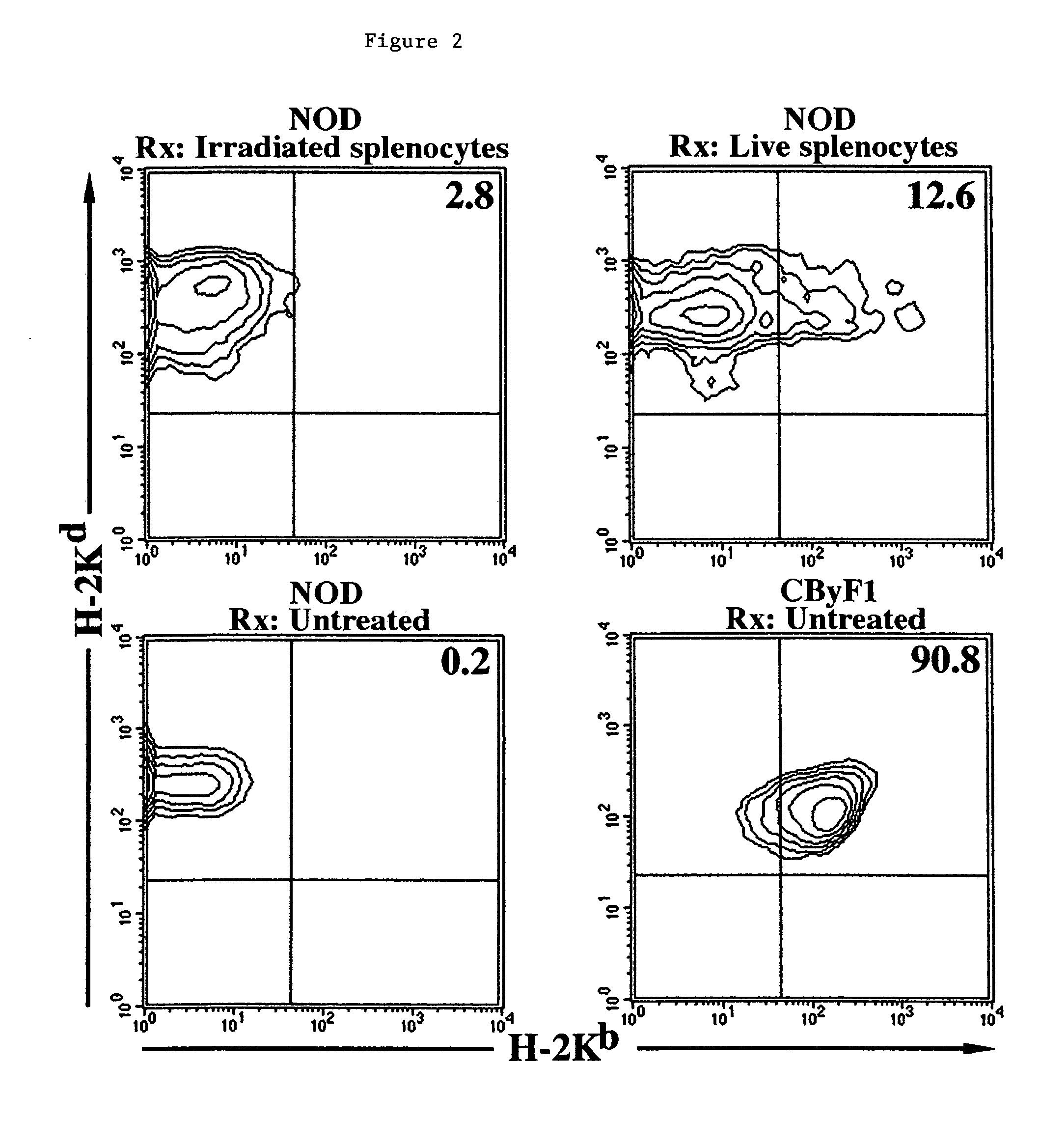
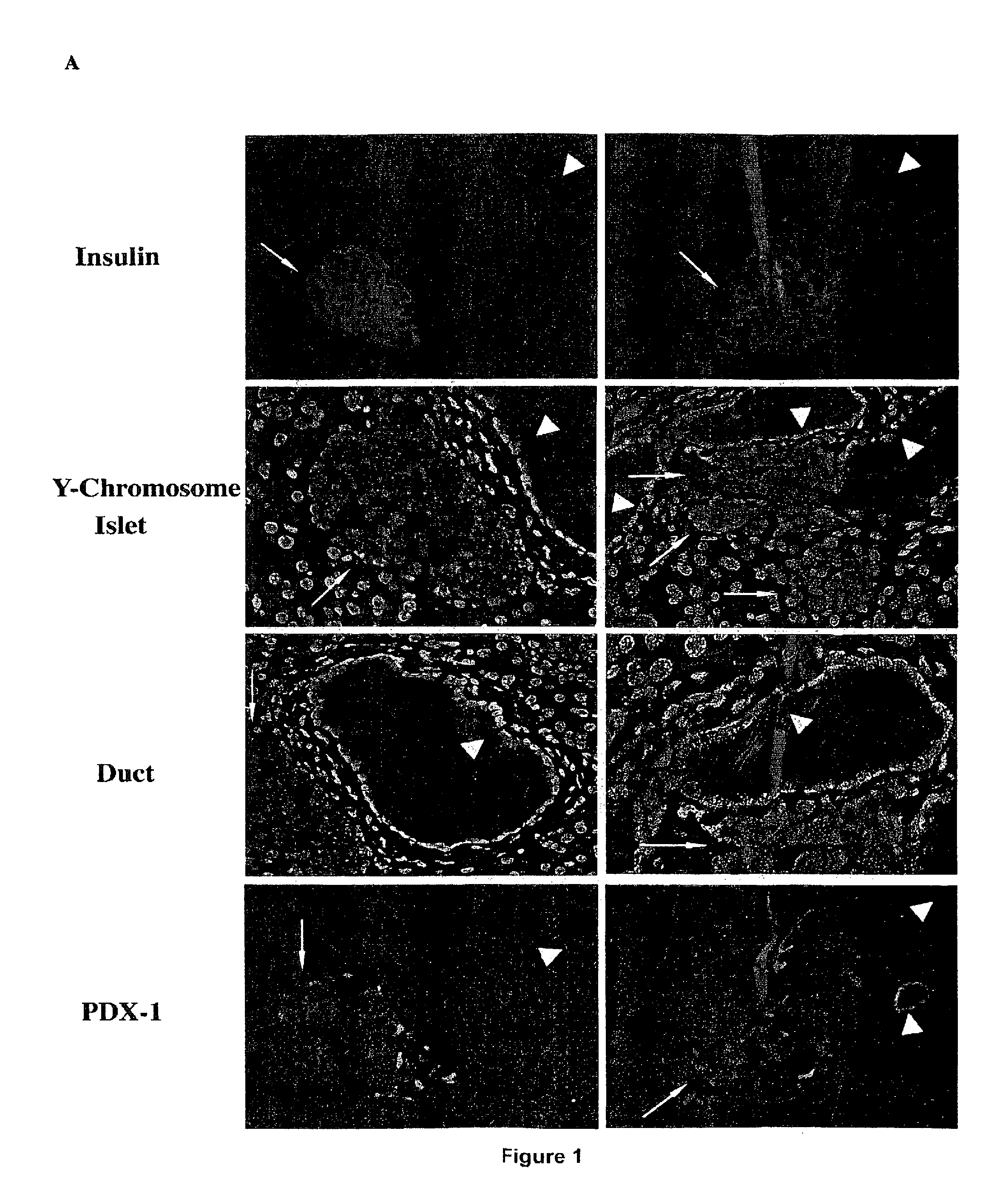
Methods of organ regeneration
InactiveUS20050158288A1Less developmentIncrease secretionOrganic active ingredientsSenses disorderSpleen cellHuman patient
The invention features methods for increasing or maintaining the number of functional cells of a predetermined type in a mammal (e.g., a human patient), for example, the insulin producing cells of the pancreas, liver cells, spleen cells, or bone cells, that has injured or damaged cells of the predetermined type or is deficient in cells of the predetermined type.
Owner:THE GENERAL HOSPITAL CORP
Methods and compositions for treating autoimmune diseases
InactiveUS8173129B2Increasing and maintaining numberTreating and stabilizing and preventing autoimmuneSenses disorderNervous disorderHeart cellsDisease
The invention features methods for increasing or maintaining the number of functional cells of a predetermined type, for example, insulin producing cells of the pancreas, blood cells, spleen cells, brain cells, heart cells, vascular tissue cells, cells of the bile duct, or skin cells, in a mammal (e.g., a human patient) that has injured or damaged cells of the predetermined type.
Owner:THE GENERAL HOSPITAL CORP
Methods and compositions for treating type 1 diabetes
ActiveUS7628988B2Increase secretionImprove bioavailabilitySenses disorderNervous disorderHeart cellsVascular tissue
The invention features methods for increasing or maintaining the number of functional cells of a predetermined type, for example, insulin producing cells of the pancreas, blood cells, spleen cells, brain cells, heart cells, vascular tissue cells, cells of the bile duct, or skin cells, in a mammal (e.g., a human patient) that has injured or damaged cells of the predetermined type.
Owner:THE GENERAL HOSPITAL CORP
Implantable device for treating disease states and methods of using same
InactiveUS7651696B2Reduced Risk of ComplicationsMinimize complicationsStentsPharmaceutical delivery mechanismDiseaseSynthetic Hormone
Methods and apparatus are provided for delivering drugs, gene vectors, naturally-occurring or synthetic hormones or proteins or other bioactive agents within a patient's vasculature. In a preferred embodiment, the apparatus comprises a material that elutes or secretes a bioactive agent and is held in place within the patient's vessel by an anchor. The material may comprise a biocompatible, and optionally, absorbable matrix, or a culture medium that sustains and nourishes stem cells, spleen cells or pancreatic islets or other beneficial cells. The anchor and material are sized and / or collapsible from a delivery configuration, in which the anchor and material may be delivered into the patient's vasculature within a delivery sheath, to a deployed configuration, wherein the anchor engages an interior wall of the patient's vessel. The apparatus of the invention may be temporarily or permanently implanted, and may in addition self reconfigure after a predetermined period of residency.
Owner:NEXEON MEDSYST INC
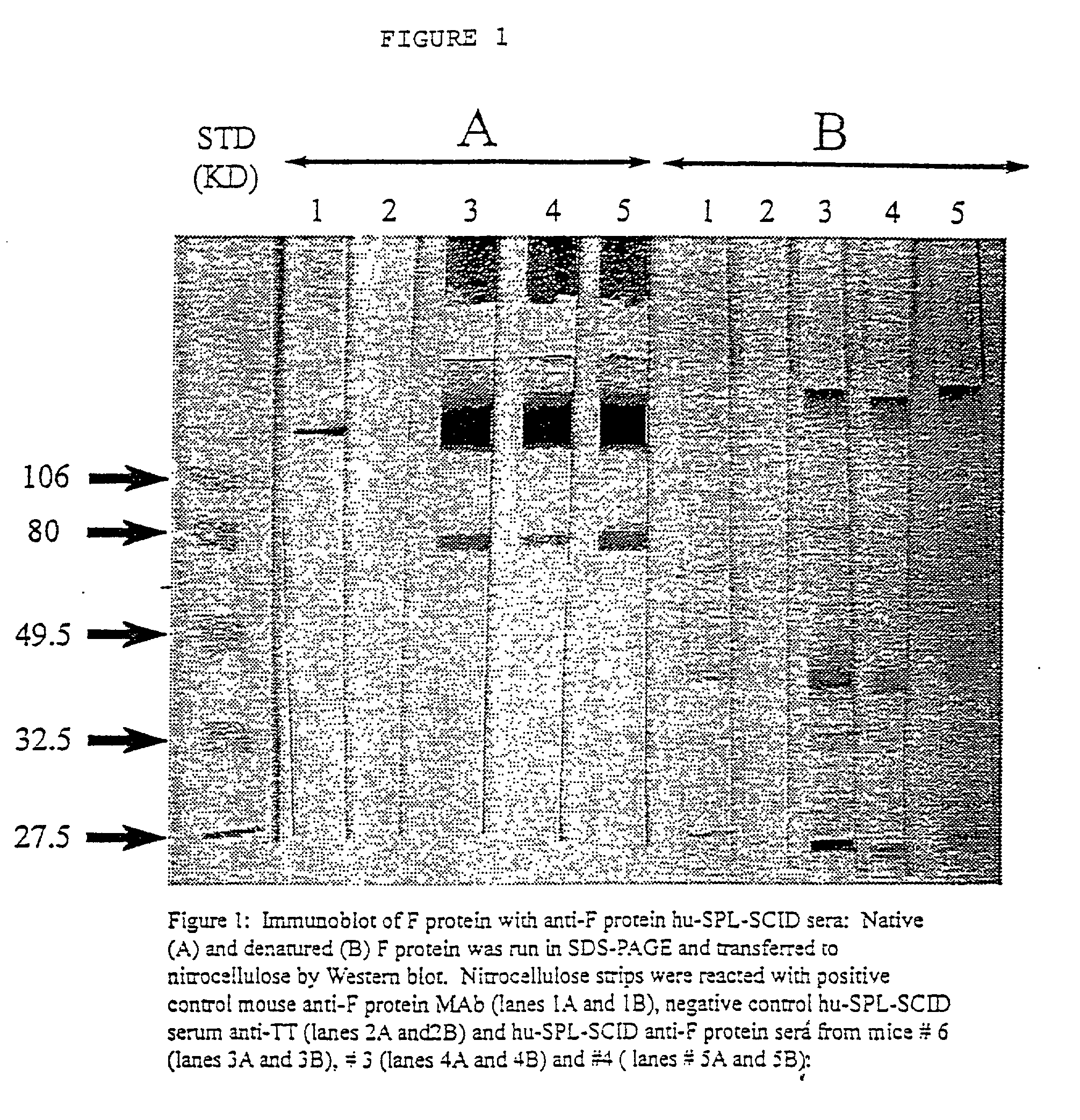
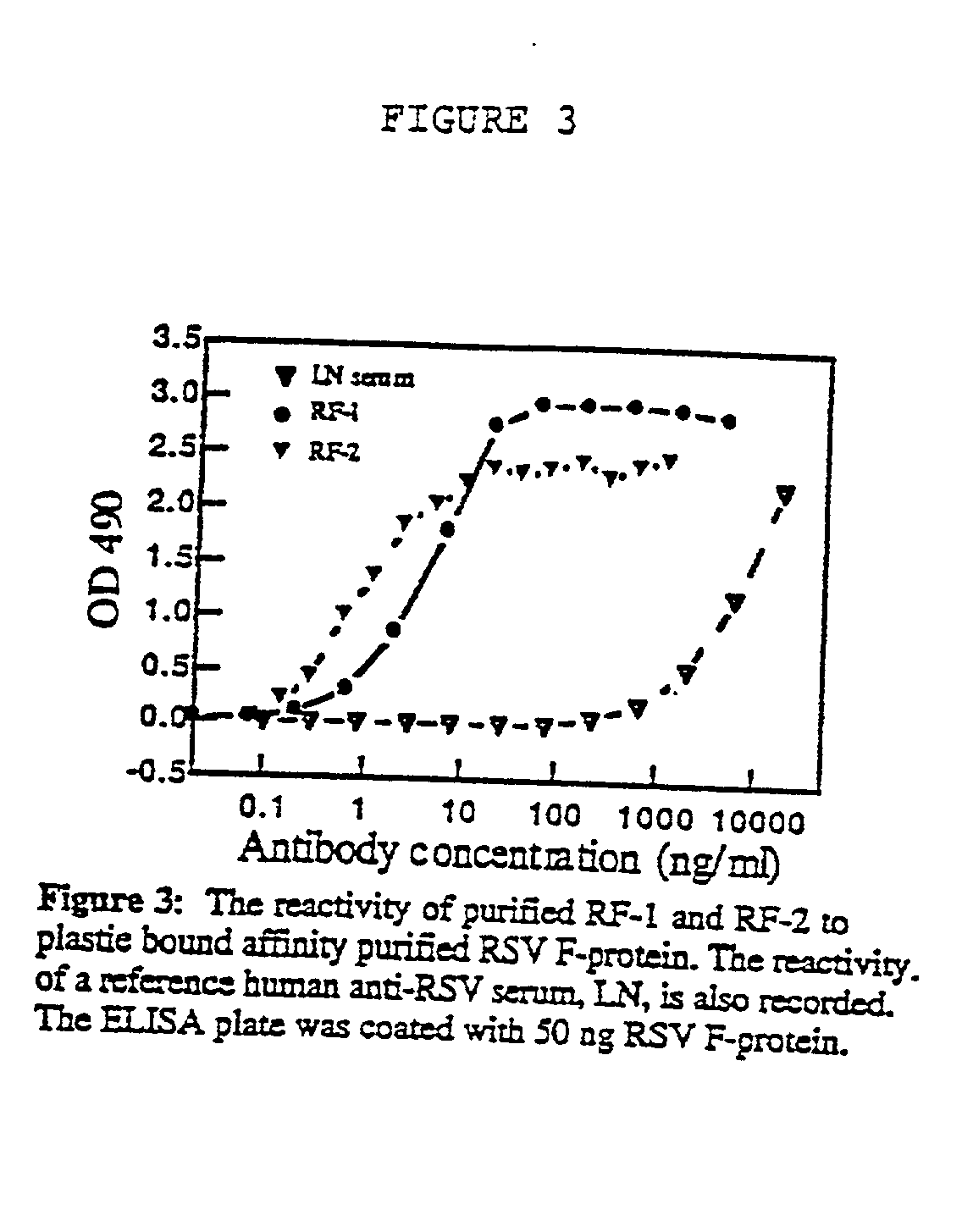
Neutralizing high affinity human monoclonal antibodies specific to RSV F-protein and methods for their manufacture and therapeutic use thereof
InactiveUS20020001798A1Simple methodPromote formationSsRNA viruses negative-sensePeptide/protein ingredientsF proteinRSV Infections
A highly efficient method for generating human antibodies in particular which are specific to be RSV fusion protein which combines in vitro primary of human spleen cells and antigen boosting in SCID mice is taught. This method provides for very high human antibody titers which are predominantly of the IgG isotype which contain antibodies of high specificity and affinity to desired antigens. This method is well suited for generating human monoclonal antibodies for therapeutic and diagnostic applications as well as for rescue of human cells for generation of combinational human antibody gene libraries. Two human monoclonal antibodies, RF-1 and RF-2 which each possess an affinity for RSV F-protein <=2x10--9 Molar are taught as well as their corresponding amino acid and DNA sequences. These antibodies are to be used therapeutically and prophylactically for treating or preventing RSV infection, as well as for diagnosis of RSV in analytes.
Owner:XENEREX BIOSCI +1
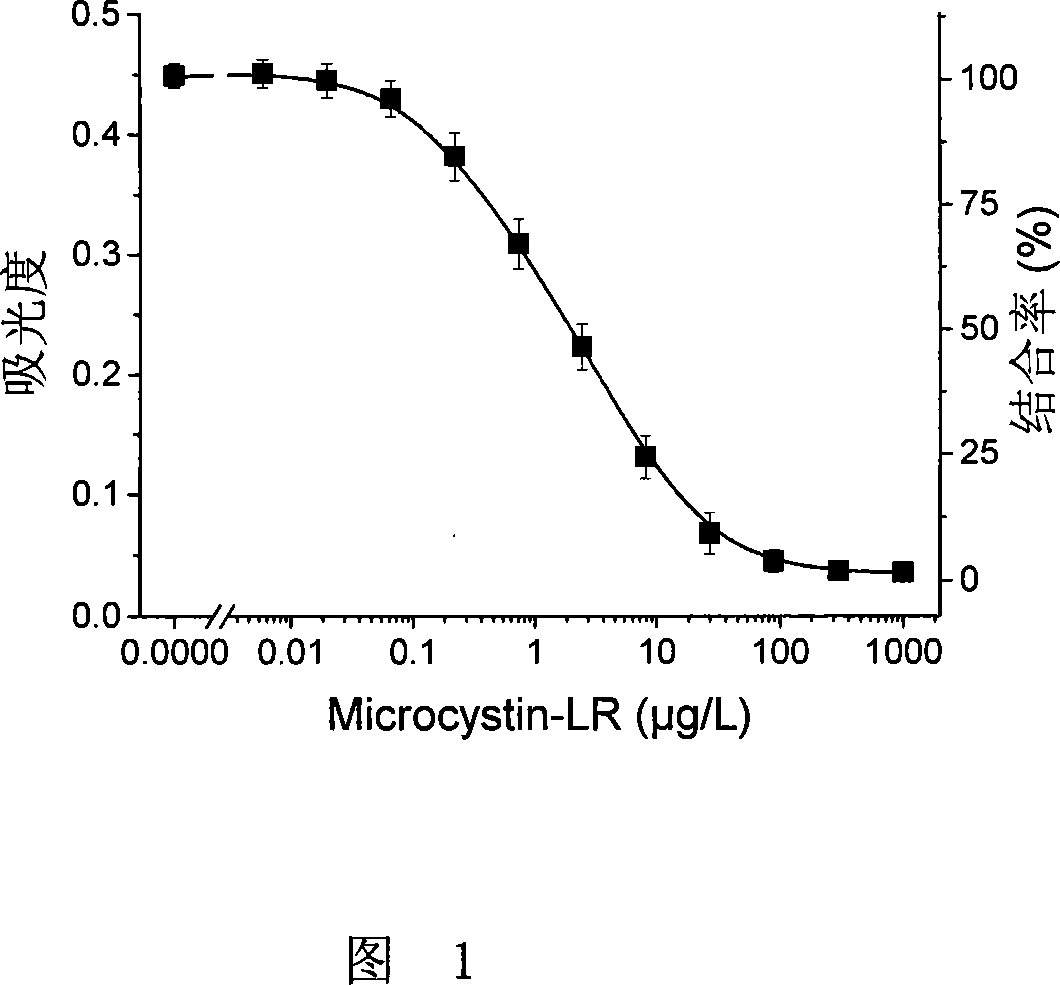
Microcystin monoclonal antibody and its preparation method and application
ActiveCN101125889AUniform textureImprove stabilityImmunoglobulins against animals/humansTissue cultureMicrocystinSpleen cell
The invention discloses a microcystlin monoclonal antibody and the preparation method and the application thereof. The microcystlin monoclonal antibody is a microcystlin-LR monoclonal antibody, and the preparation method thereof is that: 1) an amino group is introduced to a seventh amino acid residue N-methyl dehydroalanine on microcystlin-LR, thus obtaining amino modified microcystlin-LR polypeptide; the amino modified microcystlin-LR polypeptide is coupled with carrier protein to obtain complete A antigen; 2) the immunity mouse of complete A antigen obtained in step 1) to get spleen cell of immunity mouse, which then is fused with myeloma cell of mouse to screen out positive hybrid cancer cell strain; the positive hybrid cancer cell strain is cultured or is injected in sygeneous mouse abdominal cavity to induce ascites to get the microcystlin-LR monoclonal antibody. The monoclonal antibody can be used for examining microcystlin-LR and immunity affinity purification thereof.
Owner:TSINGHUA UNIV
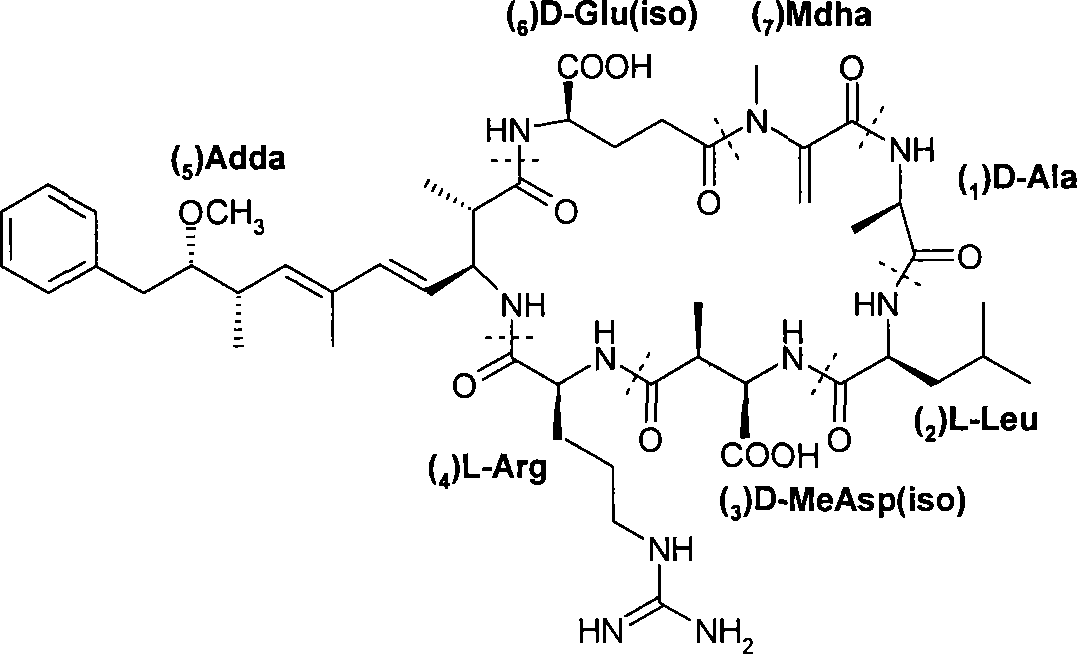
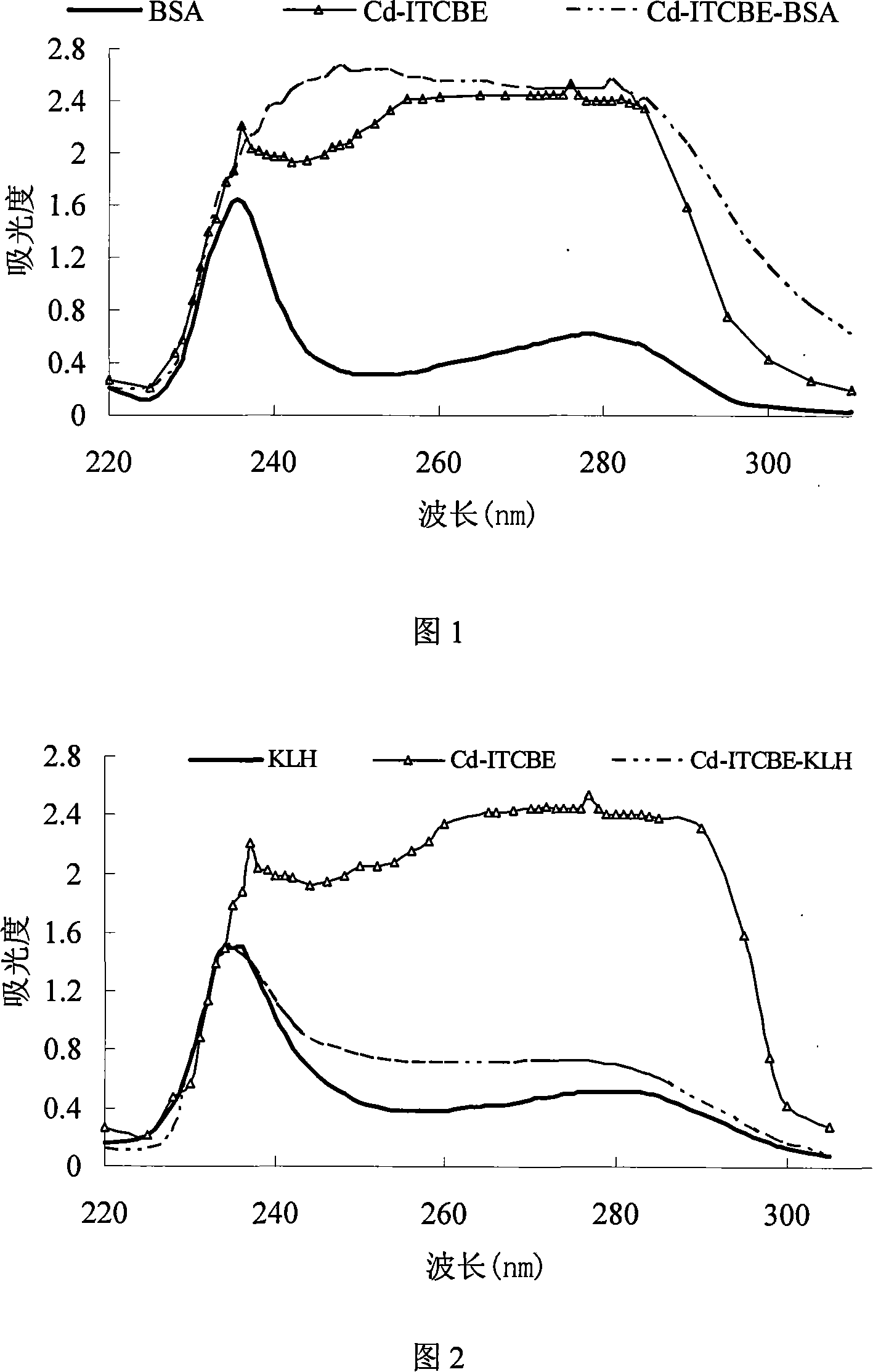
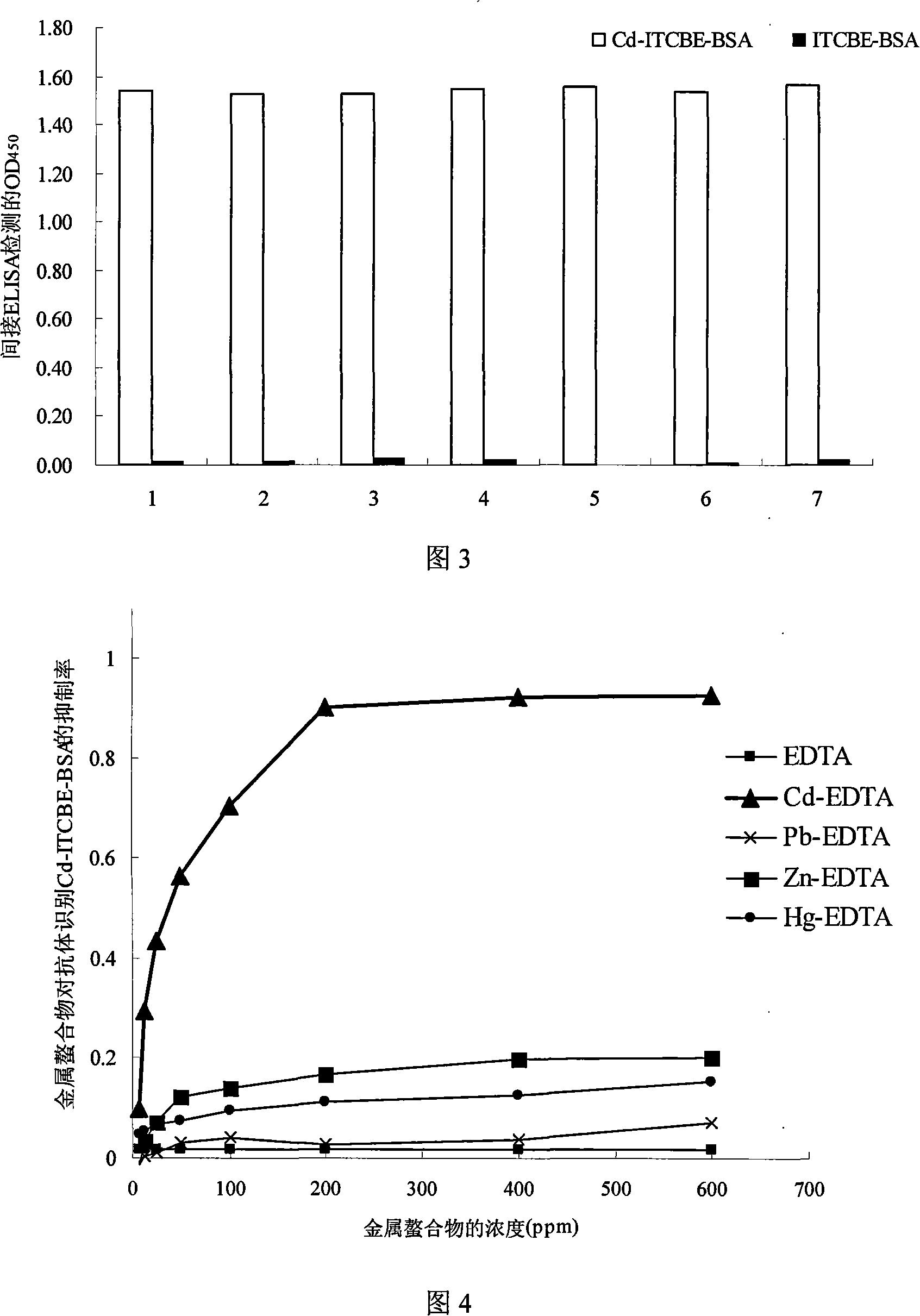
Cadmium chelate complex monoclonal antibody and preparation method and usage thereof
InactiveCN101139396AHigh potencyThe detection process is fastImmunoglobulins against animals/humansMaterial analysisAntiendomysial antibodiesBovine serum albumin
The present invention discloses a cadmium chelate monoclonal antibody and a preparation method as well as application of the monoclonal antibody. The monoclonal antibody is of specificity to cadmium -1 - (4 - isothiocyanate-benzyl) - EDTA - bovine serum albumin and the cadmium metal chelate. The preparation method of the cadmium chelate monoclonal antibody is as following: first of all, hapten is prepared and cadmium chelate complete antigen is formed; spleen cells of mouse which are Cd-ITCBE-KLH-immune are combined with SP2 / 0, and the positive clones are screened through a two-step method; after a plurality of times of subcloning, monoclonal antibody that can stably excrete anti-cadmium chelate is obtained. The cadmium chelate monoclonal antibody is used to measure the concentration of cadmium ion through the linear equation: Y = 0.0081X +0.1115, R 2 = 0.9922. The measurement is fast and the cost is low; the measurement result is accurate and is of high sensitivity and strong selectivity; the equipment is simple and can be easily carried for on-spot testing.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method for PCV-II Cap protein monoclonal antibody, antibody and application
InactiveCN101768218AAvoid distortionThe ability to secrete antibodies is strong and stableImmunoglobulins against virusesFluorescence/phosphorescenceBALB/cIndirect elisa
The invention discloses a preparation method for a PCV-II Cap protein monoclonal antibody, an antibody and application. The invention adopts ultracentrifuged and purified PCV-II as an immunogen to immunize a BALB / c mouse by the conventional method, takes spleen cells of the immunized BALB / c mouse to fuse with SP2 / 0 cells, obtains two strains of hybridoma cells secreting the PCV2-Cap protein monoclonal antibodies by indirect ELISA screening, respectively names the two strains of hybridoma cells as 8-60 and 10-48, identifies biological characteristics of the two strains 8-60 and 10-48, and usesthe two strains 8-60 and 10-48 as the first antibodies to establish an indirect immunofluorescence diagnostic method. The result of the indirect immunofluorescence diagnostic method is basically consistent with that of the PCR diagnostic method, and the positive and negative coincidence rates are respectively 93.75 percent and 100 percent so as to provide reference for preventing and treating theporcine circovirus disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +7
Bursa of Fabricius heptapeptide with immune regulation effect
InactiveCN101830968ASynthetic technology is matureImprove efficiencyPeptide preparation methodsAntibody medical ingredientsSide effectImmunologic Competence
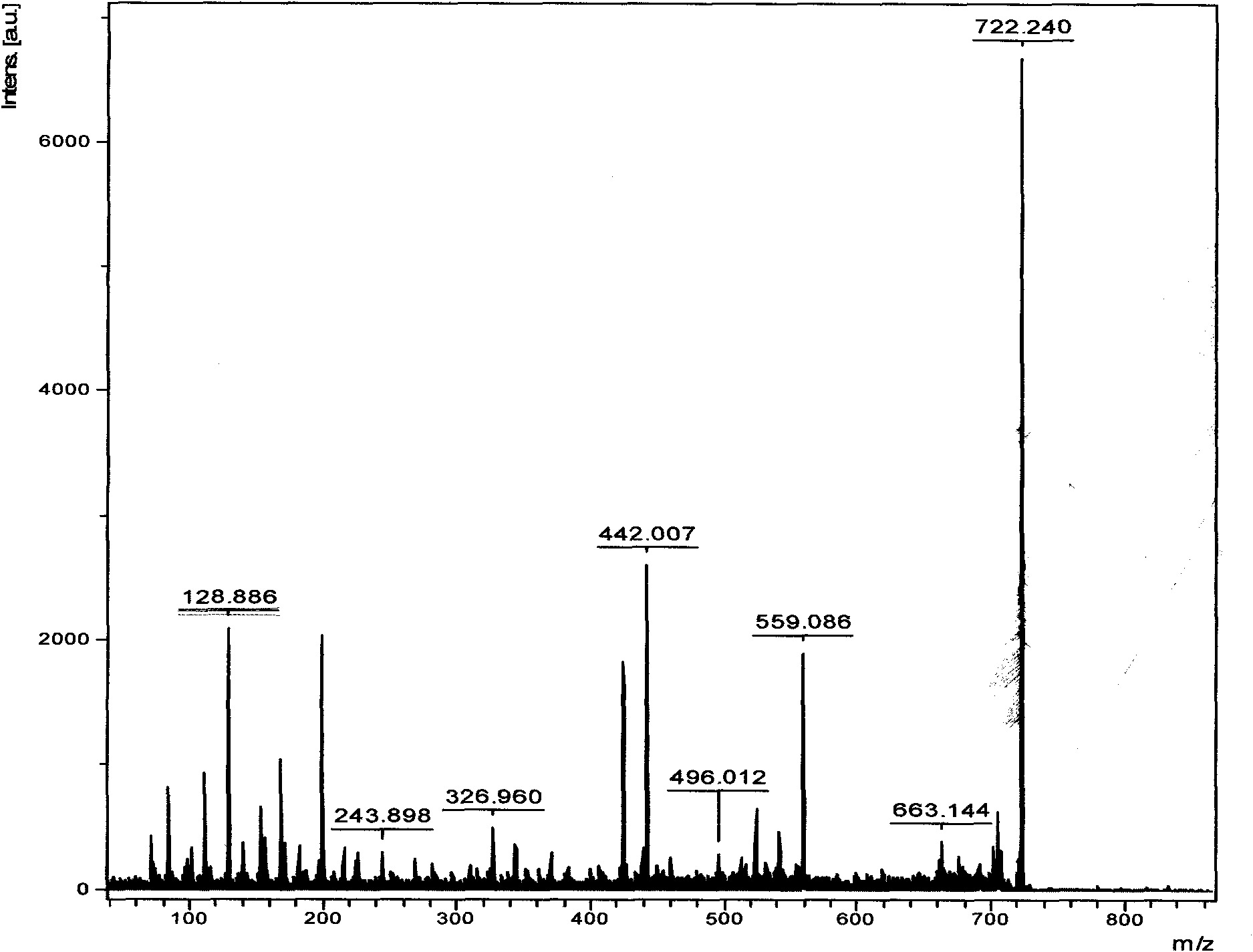
The invention relates to bursa of Fabricius heptapeptide with immune regulation effect and application thereof in immunity (in improving the immune capability of animals, improving the immune effect of vaccines and affecting the activity of tumor cells), and belongs to the field of immunology. The molecular weight of the separated heptapeptide is 722.240, the amino acid sequence is EPASGMM, and the heptapeptide has a simple structure, no toxic or side effect and extremely weak immunogenic property. The heptapeptide can be separated and extracted from bursa of Fabricius, also can be chemically synthesized, has low cost, and can be massively produced. The bursa of Fabricius heptapeptide has induction effect on the production of antibody and subtype thereof, and meanwhile can regulate the production of cell factors, partition of T lymphocytes and proliferation of spleen cells, and promote the immune reaction of the cells. The bursa of Fabricius heptapeptide is an immune regulation factor on functions, has wide application prospect on the aspects of immune regulation, immune therapy and the like, and can be applied in the fields of basic immune research, clinical application research and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Methods of organ regeneration
Owner:THE GENERAL HOSPITAL CORP
Method of inducing growth of nerve stem cells
InactiveUS20050226852A1Improvement of pathologic conditionLow expression of MHCBiocideNervous disorderSurface markerDendritic cell
It is intended to provide a method of efficiently inducing the growth of nerve stem cells, which are most important in transplantation therapy for nerve damage and neurological dysfunction, either in vitro or in vivo, a method of using the nerve stem cells obtained by the above growth induction method, etc. A mammalian nerve tissue containing nerve stem cells is separated and the nerve stem cells are selectively cultured in a medium containing growth factors such as EGF and FGF. Next, the nerve stem cells are co-cultured with dendritic cell such as an immature dendritic cell subset having a CD11c surface marker on the cell surface, spleen cells or blood cell-type cells such as CD8-positive T cells. Alternatively, the nerve stem cells after the culture are further cultured in the presence of GM-CSF or the nerve stem cells after the culture are further cultured in a culture supernatant of dendritic cells or a culture supernatant of blood cell-type cells.
Owner:INST OF GENE & BRAIN SCI
Preparing method for paclobutrazol monoclonal antibodies
The invention discloses a preparing method for paclobutrazol monoclonal antibodies. The preparing method comprises the main steps as follows: (1) synthesizing artificial hapten paclobutrazol hemisuccinates by a microwave solvent free method; (2) performing coupling to obtain artificial antigens of the paclobutrazol by using the paclobutrazol hemisuccinates as raw materials; (3) performing the immune treatment on Balb / c mice by using the synthesized artificial antigens; (4) selecting the mice with optimal serum valence and optimal specificity, and mixing spleen cells and myeloma cells of the mice together in an external manner; (5) culturing and screening the fused cells by using the selective culture medium, and further cloning, propagating and storing by freezing; (7) injecting the expanded cell strains into the abdominal cavities of the mice to generate a lot of abdominal dropsy; (8) purifying the paclobutrazol-resistant monoclonal antibodies in the abdominal dropsy by a caprylic acid-saturated ammonium sulfate method. The prepared paclobutrazol-resistant monoclonal antibodies have high sensitivity and strong specificity, and can be further applied to the construction of technologies of immune sensors, colloidal gold immune chromatographic methods and the like.
Owner:NANCHANG UNIV
GPC3 (glypican-3) monoclonal antibody hybridoma cell strain 7D11 and preparation method and application thereof
ActiveCN102634486AReduce manufacturing costConducive to clinical target validationImmunoglobulins against animals/humansMicroorganism based processesMonoclonalSpleen cell
The invention discloses a GPC3 (glypican-3) monoclonal antibody hybridoma cell strain 7D11 and a preparation method and application thereof, which belong to the technical field of bioengineering. The GPC3 monoclonal antibody hybridoma cell strain 7D11 has a preservation code of CGMCC No.5426. The preparation method of the hybridoma cell strain 7D11 includes: using GPC3 protein as an immunogen to immunize a mouse, subjecting spleen cells of the mouse with serum titer more than 1:104 and myeloma cells SP2 / 0 to fusion, using a HATRPMI-1640 medium to screen fused cells, screening by ELISA (enzyme-linked immuno sorbent assay) and multiple limiting dilution process, and finally obtaining the hybridoma cell strain 7D11. The GPC3 monoclonal antibody hybridoma cell strain 7D11 is high in yield of secreted antibodies and easy to survive, the secreted monoclonal antibodies are high in titer, flexible in reaction, easy to detect, low in production cost and widely applicable to detection of GPC3 protein expression.
Owner:GUANGZHOU DARUI BIOTECH
Agonist antibody against heteroreceptor
InactiveUS20060204496A1Reduced activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSpleen cellHeteroreceptor
Animals were immunized with either the A or B chain of a receptor, and then mRNA was extracted from the spleen cells of the animals, and the variable regions of the L and H chains were isolated by RT-PCR using primers for variable regions comprising CDRs. Single-chain Fv was synthesized by assembly PCR to construct a phage library. Clones for antigen-bound antibodies were concentrated and cloned by panning. An expression vector for scFv-CH1-Fc was prepared by inserting a single-chain variable region between CH1-hinge-CH2-CH3 and the signal sequence for animal cells. Various combinations of such expression vectors were introduced into cells to express antibodies, and antibody clones exhibiting ligand-like activity were selected.
Owner:CHUGAI PHARMA CO LTD
Preparation method of heavy metal mercury monoclonal antibody
InactiveCN101139398AFast pushStrong antigenicityImmunoglobulins against animals/humansBiological testingBALB/cSpleen cell
The present invention relates to a preparation method of heavy metal mercury monoclonal antibody, which belongs to the field of biotechnology. The present invention is particularly used in the preparation of specificity-recognition heavy metal mercury monoclonal antibody and in the fast mercury high-sensitivity measurement remained in the agricultural production and the agricultural production environment. The heavy metal mercury ion and the carrier albumen are coupled into full antigens through bifunctional metal chelate 1-(4-separate-cyano phenyl)-EDTA; the full antigens are used on Balb / C mouse and the spleen cells and the Sp2 / 0 myeloma cells are used to prepare hybridoma cells through the hybridoma technology. And monoclonal antibody which can stably excrete anti-Hg-EDTA is generated. The preparation technology in the present invention is easy and practical: the whole preparation process of the antigen requires no special instrument. The present invention is suitable for factory-scale production.
Owner:NANJING AGRICULTURAL UNIVERSITY
HCMVPP65 antigenemia indirect immunofluorescence method detection reagent kit
InactiveCN101261272AImprove featuresIncreased sensitivityFluorescence/phosphorescenceBALB/cFluorescence
The invention discloses a HCMVPP65 antigenemia indirect immune fluorescence method detection kit, which uses cytomegalovirus-AD169 virus strain pp65 protein as the immunogen to immune a Balb / c mouse. The spleen cells of the immunized mouse and the myeloma cells of the mouse which belongs to the same type with the immune mouse are conventionally integrated, by indirect ELISA screening and finite dilution cloning, the hybridoma cell lines of the mouse cytomegalovirus pp65 protein cloning antibody are obtained, and the characteristics of the hybridoma cell lines are identified by ELISA, immune fluorescence experiment and other methods; two monoclonal antibodies that stably secrete the pp65 protein are established successfully and named respectively as 1A6 and 4A8. A monoclonal antibody which differs from the former report and aims at the pp65 protein of the cytomegalovirus (CMV) is prepared and a method used for preparing erythrocyte fast pyrolysis is established; compared with other detection kits which belongs to the same kind, the detection kit of the invention is faster, simpler and more convenient and has higher specificity and sensitivity.
Owner:天津市秀鹏生物技术开发有限公司
Preparation method of HRPII protein monoclonal antibody of plasmodium falciparum
ActiveCN101659975AGood repeatabilityAchieve serial expressionMicroorganism based processesFermentationChemical synthesisEscherichia coli
The invention relates to a preparation method of HRPII protein monoclonal antibody of plasmodium falciparum. The preparation method comprises the following steps of: adopting HRPII protein of plasmodium falciparum as target antigen and respectively analyzing and selecting two dominant antigen epitopes of A and B; respectively repeating the two dominant antigen epitopes of A and B, then continuously connecting four glycine and forming recombinant protein C; adopting most securest code of escherichia coli and converting the amino acid sequence of the recombinant protein C into corresponding nucleotide sequence; carrying out chemical synthesis to the former step to obtain the nucleotide sequence, and respectively adding enzyme cutting sites BamHI and EcoRI at the upstream and downstream thereof; inserting nucleotide fragment obtained by the former step into expression carrier PET-28a(+), constructing recombinant protein C expression carrier and inducing to express the recombinant proteinC in the escherichia coli BL21 (DE3); carrying out ultrasonic bacteria breaking and low-temperature centrifugation, then taking supernatant of the solution, affining a chromatographic column by nickel-agarose, eluting and obtaining purified recombinant protein C; after immunizing Balb / c mouse with the recombinant protein C for a plurality of times, taking and fusing spleen cells with sp2 / 0 myelomacells, and obtaining six hybridoma cell lines by multiple rounds of screening; and purifying monoclonal antibody, respectively marking horse radish peroxidase and prorating matching and combination of optimum monoclonal antibody by ELISA orthogonal experiment.
Owner:杭州新脉生物科技有限公司
Hybridoma cell strain secreting anti-amantadine monoclonal antibody and application of hybridoma cell strain
ActiveCN104480072AHigh immune titerQuick checkMicroorganism based processesTissue cultureSpleen cellHybridoma cell
The invention discloses a hybridoma cell strain secreting an anti-amantadine monoclonal antibody and application of the hybridoma cell strain. The name of the hybridoma cell strain is hybridoma cell strain 7G4.3, and the collection number is CCTCC No. C2014169. The hybridoma cell strain is obtained by immunizing a mouse with a self-designed amantadine artificial antigen and using spleen cells of the immunized mouse, the anti-amantadine monoclonal antibody secreted by the hybridoma cell strain has high titer and strong specificity, and can be used for fast and accurate immunodetection and immunoassay of amantadine.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES +1
Preparation method of monoclonal antibody and kit thereof
InactiveCN105198996AStrong specificitySame natureImmunoglobulins against hormonesFermentationSpleen cellElisa method
The invention relates to the technical field of preparation of monoclonal antibodies, and in particular relates to a preparation method of a monoclonal antibody and a kit thereof. The preparation method comprises the following steps: performing immunization on an animal by adopting an antigen, and taking immune spleen cells; fusing the immune spleen cells with myeloma cells to obtain fusion cells; detecting the fusion cells by adopting an indirect ELISA (enzyme-linked immunosorbent assay) method to obtain positive hybridoma cells; detecting the positive hybridoma cells by adopting a competitive inhibition ELISA method to obtain hybridoma cells with competitive reactions; sub-cloning the hybridoma cells with competitive reactions to obtain monoclonal antibody hybridoma cells; and excreting the monoclonal antibody hybridoma cells to obtain the monoclonal antibody. According to the preparation method provided by the invention, the monoclonal antibody is screened by adopting a method combining the indirect ELISA method and the competitive inhibition ELISA method, the operation is simple and convenient, the purposiveness is strong, the workload is small, and a high-quality hybridoma cell strain can be locked in a short time.
Owner:SINOCARE
Enrofloxacin monoclonal antibody and application
InactiveCN101565690AStrong specificityRapid and Sensitive DetectionTissue cultureImmunoglobulinsBALB/cIon exchange
The invention relates to an enrofloxacin monoclonal antibody and application, relates to hybridoma strains thereof, and belongs to the technical field of immunochemistry. The enrofloxacin monoclonal antibody is generated by mouse hybridoma strains 6A4 and 8E6. The preparation method comprises the following steps that: enrofloxacin and carrier proteins BSA, HAS and OVA are coupled by a carbodiimide method to synthesize artificial immunogens EnR-BSA, EnR-HSA and coatingen EnR-OVA; a Balb / c mouse is immunized by the synthesized artificial immunogens EnR-BSA and EnR-HSA; a spleen cell of the immunized mouse is extracted to be fused with a SP2 / O myeloma cell and coated by the coatingen EnR-OVA; indirect ELISA method and indirect competition ELISA method are established to screen the hybridoma strains which can stably secrete specific antibody; the obtained cell strain immunized Balb / c mouse is used to prepare ascites; the ascites is purified by a caprylic acid-ammonium method and an ion exchange method; and valences of antibodies of two purified cell strains reach over 1.024*10 and 1.28*10. The monoclonal antibody has strong specificity, can be applied to preparation of enrofloxacin residue inspection kit and aerosol test strip, and can sensibly and quickly inspect the enrofloxacin residue.
Owner:泰州市蛋白质工程研究院
Antibody capable of recognizing HLA class i
InactiveUS20120244142A1Inhibit cell growthProlong lifeImmunoglobulins against animals/humansAntibody ingredientsSpleen cellCell Aggregations
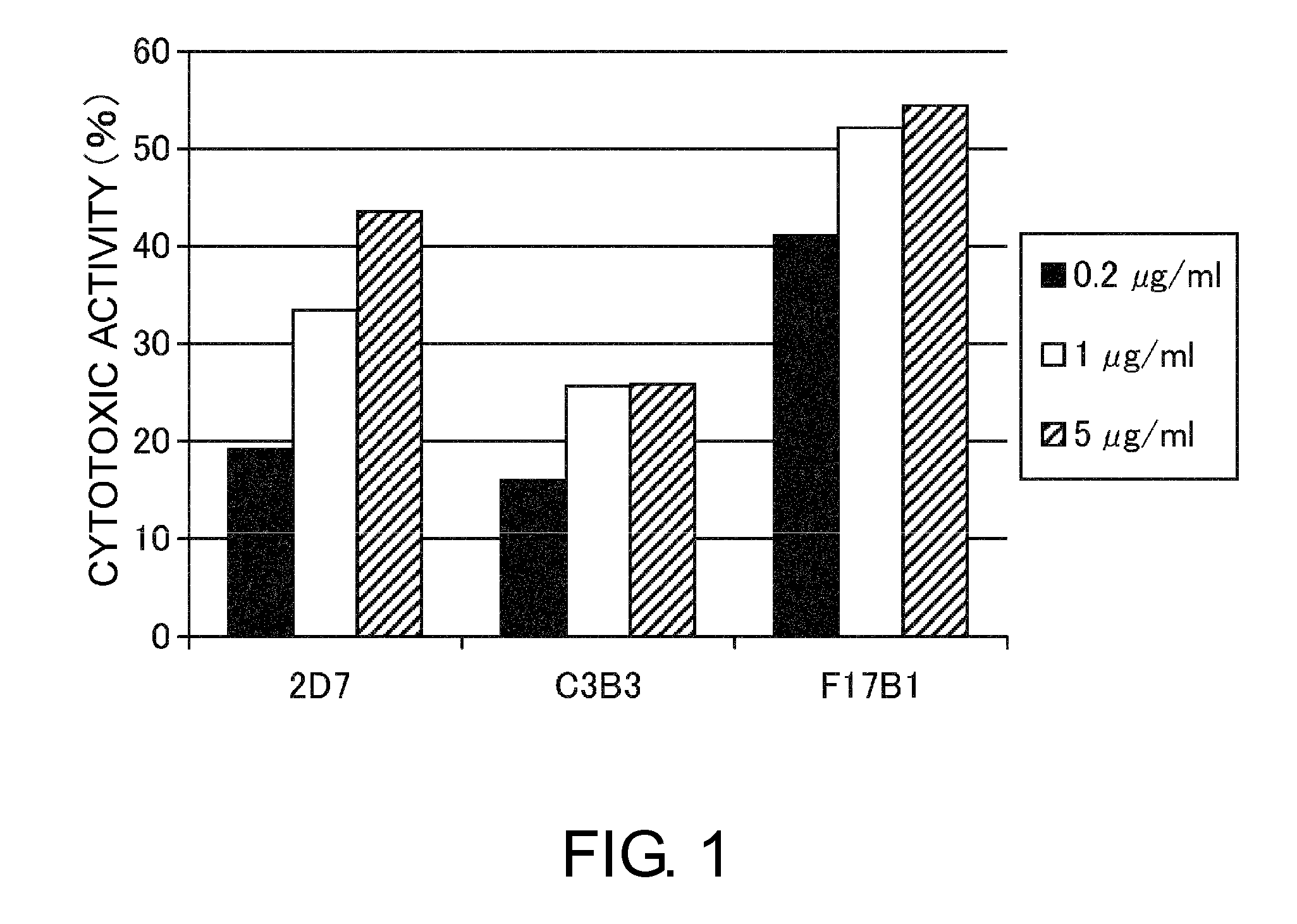
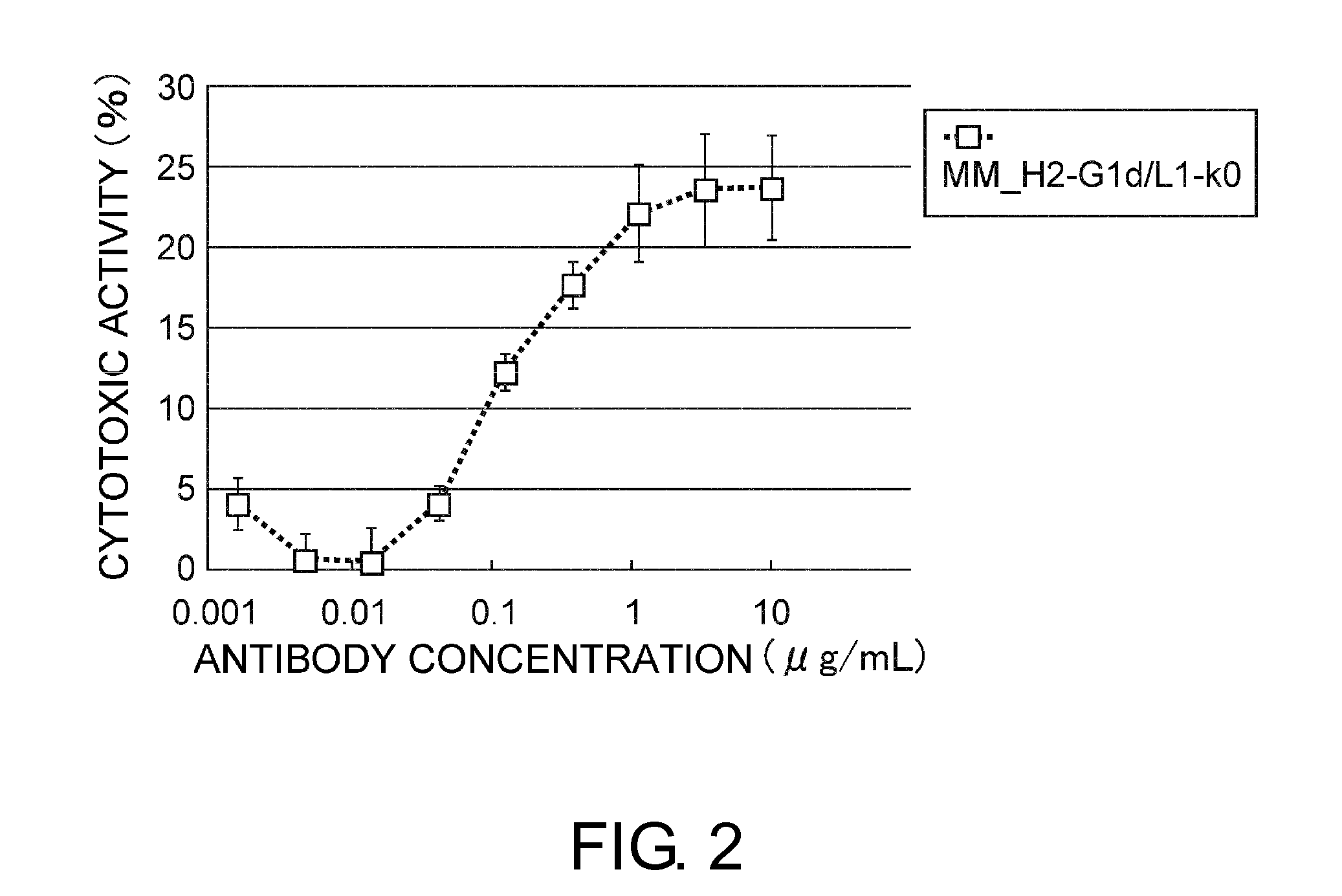
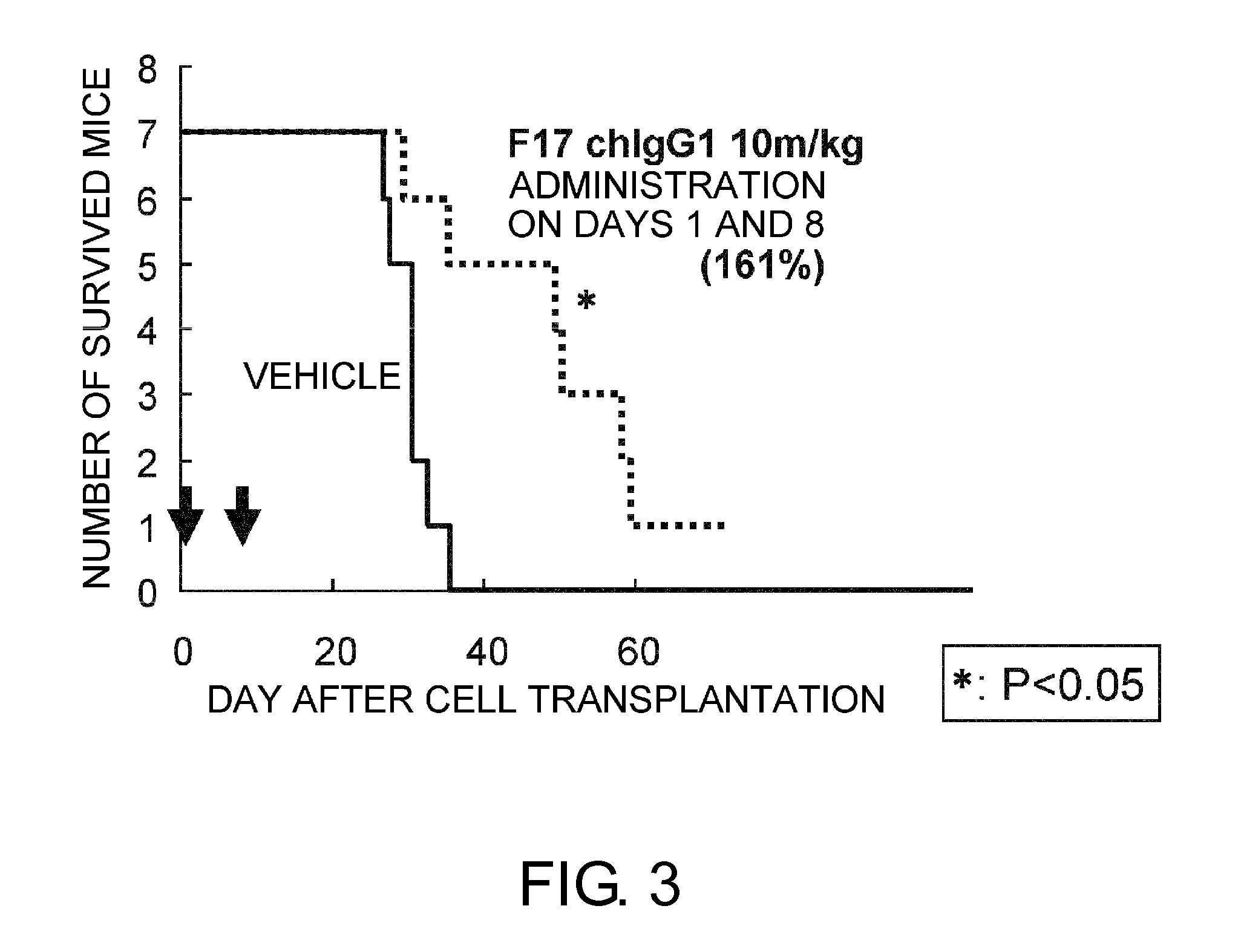
An objective of the present invention is to provide antibodies that recognize HLA class I and have significant cell death-inducing activity. To achieve the above objective, first, the present inventors immunized mice with soluble HLA-A to which a FLAG tag is attached. Four days after the final immunization, spleen cells from the mice were fused with mouse myeloma cells. Then, hybridoma screening was carried out using the cell aggregation-inducing activity and cell death-inducing activity as an index. Thus, the monoclonal antibody F17B1 that has strong cell death-inducing activity was selected. The sequences of the H chain and L chain variable regions of the mouse monoclonal antibody F17B1 were determined to humanize this antibody. Then, the humanized anti-HLA class I antibody H2-G1d_L1-k0 was assessed for cell death induction. The result showed that H2-G1d_L1-k0 suppressed the growth of the IM9 cell line in a concentration dependent manner.
Owner:CHUGAI PHARMA CO LTD
Preparation method of monoclonal antibody and application thereof
ActiveCN1763217AQuick checkEspecially highImmunoglobulins against bacteriaFermentationBALB/cSpleen cell
The present invention is the preparation process and application of one monoclonal antibody. The monoclonal antibody of the present invention is monoclonal antibody of Staphylococcus aureus coat protein. The preparation process includes immunizing Balb / C mouse with sterilized Staphylococcus aureus, fusing the spleen cell and Sp2 / 0 myelomatosis cell, and screening and cloning to obtain myelomatosis strain secreting Staphylococcus aureus resisting monoclonal antibody and the monoclonal antibody. The monoclonal antibody of the present invention is used in preparing ELISA kit or detecting colloidal gold kit.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Preparation of thiacloprid monoclonal antibodies and ELISA detection method
InactiveCN104974255AHigh affinityStrong antibody specificitySerum albuminImmunoglobulinsBALB/cSpleen cell
The invention discloses preparation of thiacloprid monoclonal antibodies. The preparation method comprises the steps of synthesizing thiacloprid haptens, coupling the thiacloprid haptens with bovine serum albumin to prepare immunizing antigens, immunizing a BALB / c mouse, fusing the spleen cells of the immunized mouse with Sp2 / 0 myeloma cells, and performing screening and cloning to obtain hybrid strains of continuous and stable secreting thiacloprid monoclonal antibodies. The thiacloprid monoclonal antibodies have high affinity to thiacloprid and strong specificity, and do not have cross reaction with nicotine insecticide similar to acetamiprid, imidacloprid and the like.
Owner:ZHEJIANG MARINE DEV RES INST
General monoclonal antibody for African swine fever virus strains as well as preparation method and application thereof
InactiveCN104497137AReduce missed detectionQuality improvementMicroorganismsMicroorganism based processesSpleen cellGeographic regions
The invention discloses a general monoclonal antibody for African swine fever virus strains as well as a preparation method and application thereof. The preparation method of the general monoclonal antibody for the African swine fever virus strains comprises the following steps: immunizing a mouse by adopting a purified p54 immune protein, fusing spleen cells of the immunized mouse with myeloma cells to prepare hybridoma cells, screening with three screening antigens so as to obtain the hybridoma cells which can stably secrete a monoclonal antibody, and then preparing the monoclonal antibody by adopting an in vivo or in vitro method, wherein the three screening antigens comprise a prokaryotically expressed p54 recombinant protein, an eukaryotically expressed p54 recombinant protein and an artificially synthesized polypeptide shown in Seq ID No.1. The preparation method of the general monoclonal antibody for the African swine fever virus strains has the advantages that the three screening antigens are adopted for screening the hybridoma cells, and especially an artificially synthesized common polypeptide of all the African swine fever virus strains is taken as the screening antigen, so that the prepared general monoclonal antibody can be used for detecting strains in different geographic regions, the misdetection rate caused by regional difference among the strains is reduced, and the quarantine working quality and efficiency are improved.
Owner:SHENZHEN AUDAQUE DATA TECH
Anti-PD-1 monoclonal antibody and application thereof
ActiveCN108314734AStrong specificityIncrease binding rateImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNucleotidePD-L1
The invention relates to a novel anti-PD-1 monoclonal antibody and a variable region sequence of the antibody and belongs to the technical field of biology. The novel anti-PD-1 monoclonal antibody takes recombinant human PD-1 protein (rhEPD-1) as an immunogen for immunizing a BAL b / c mouse; spleen cells of the mouse are taken and are fused with myeloma cells sp2 / 0-Ag14 to obtain a hybridoma cell line capable of expressing the anti-PD-1 antibody; the cell line can stably secrete the single monoclonal antibody. According to the novel anti-PD-1 monoclonal antibody, variable region nucleotide andamino acid sequences of an H chain and an L chain of the anti-PD-1 monoclonal antibody are cloned at the same time. The anti-PD-1 monoclonal antibody provided by the invention can be specifically combined with PD-1 and the combination of the PD1 and PD-L1 is blocked; functions of T cells are recovered. The novel anti-PD-1 monoclonal antibody can be used as an immune checkpoint inhibitor and is used for treating cancer and infectious diseases.
Owner:CHINA PHARM UNIV
Monoclonal antibody for African swine fever virus gene II type strain as well as preparation method and application thereof
InactiveCN104497136AGuaranteed testingAccurate detectionImmunoglobulins against virusesTissue cultureSpleen cellIn vivo
The invention discloses a monoclonal antibody for an African swine fever virus gene II type strain as well as a preparation method and application thereof. The preparation method of the monoclonal antibody for the African swine fever virus gene II type strain comprises the following steps: immunizing a mouse by adopting a purified p54 immune protein, fusing spleen cells of the immunized mouse with myeloma cells to prepare hybridoma cells, screening with three screening antigens so as to obtain the hybridoma cells which can stably secrete a monoclonal antibody, and then preparing the monoclonal antibody by adopting an in vivo or in vitro method, wherein the three screening antigens comprise a prokaryotically expressed p54 recombinant protein, an eukaryotically expressed p54 recombinant protein and an artificially synthesized polypeptide shown in Seq ID No.1. The preparation method of the monoclonal antibody for the African swine fever virus gene II type strain has the advantages that the three screening antigens are adopted for screening the hybridoma cells, and especially a special artificially synthesized polypeptide for the gene II type strain is adopted as the screening antigen, so that the prepared monoclonal antibody can guarantee the detection rate of the gene II type strain, the misdetection rate is reduced, and the quarantine working quality and efficiency are improved.
Owner:SHENZHEN AUDAQUE DATA TECH
Methods of organ regeneration
InactiveUS20100150893A1Increasing and maintaining numberTreating and stabilizing and preventing autoimmuneBiocideSenses disorderHeart cellsPhysiology
Owner:THE GENERAL HOSPITAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com