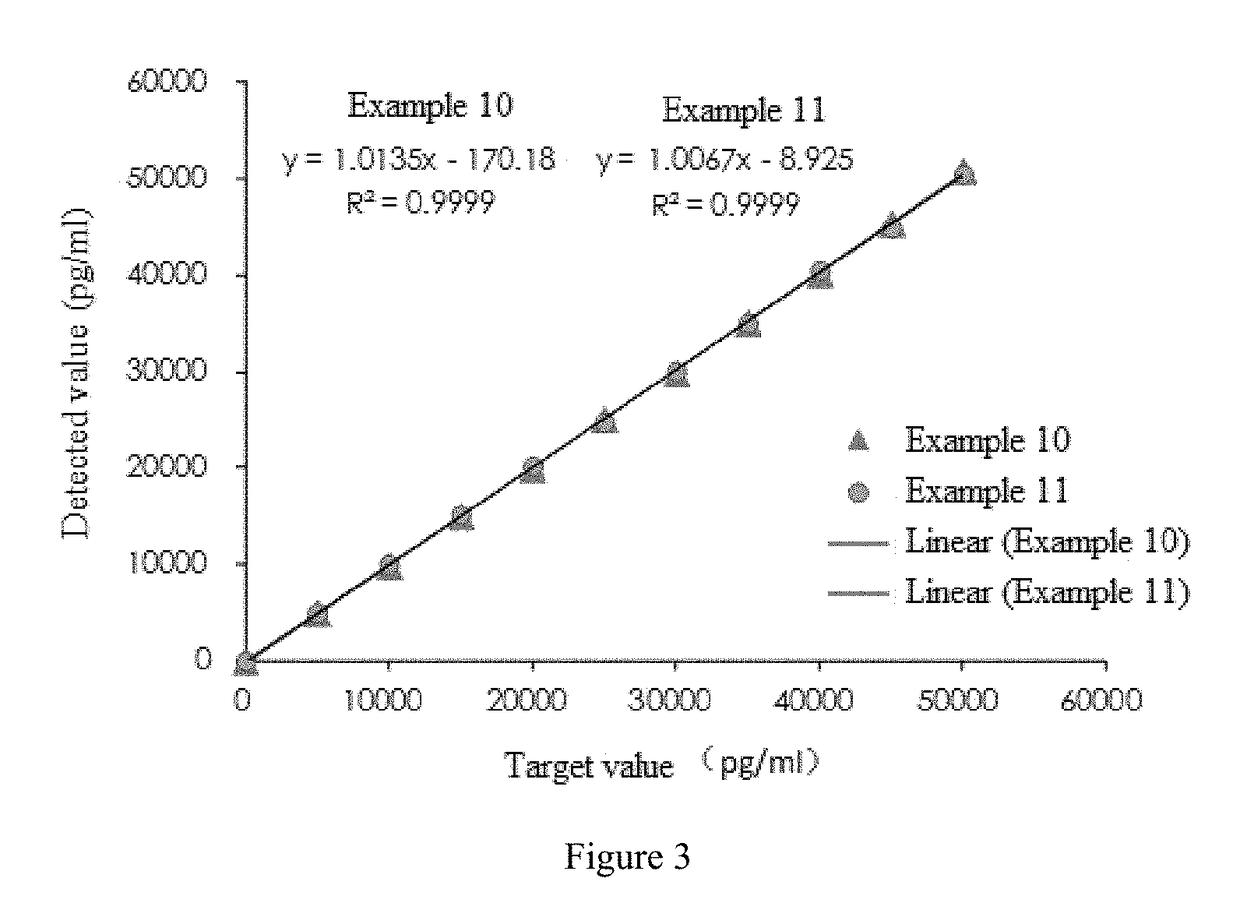
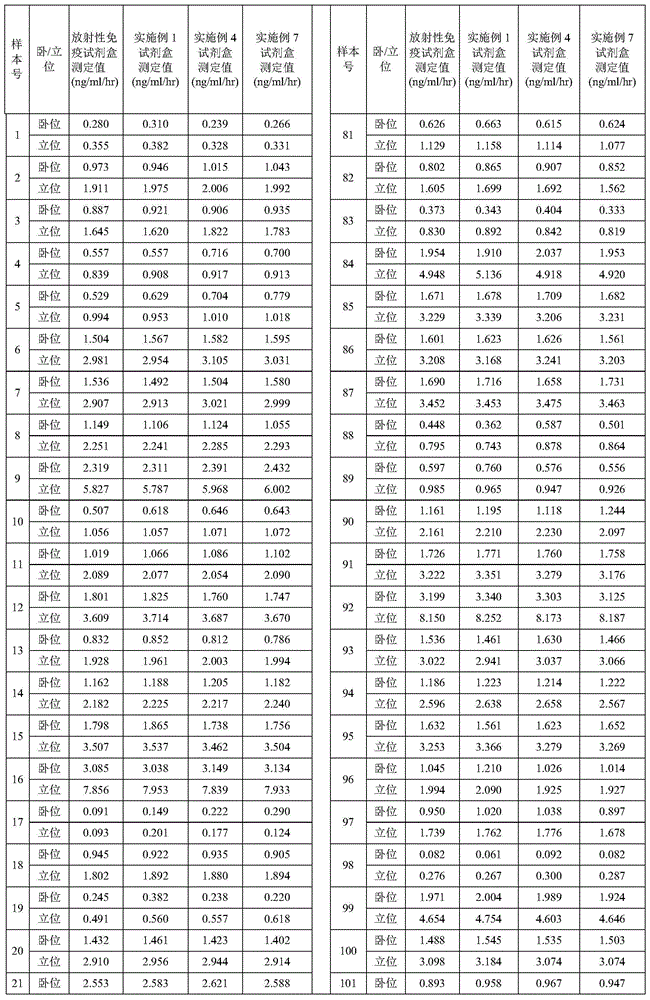
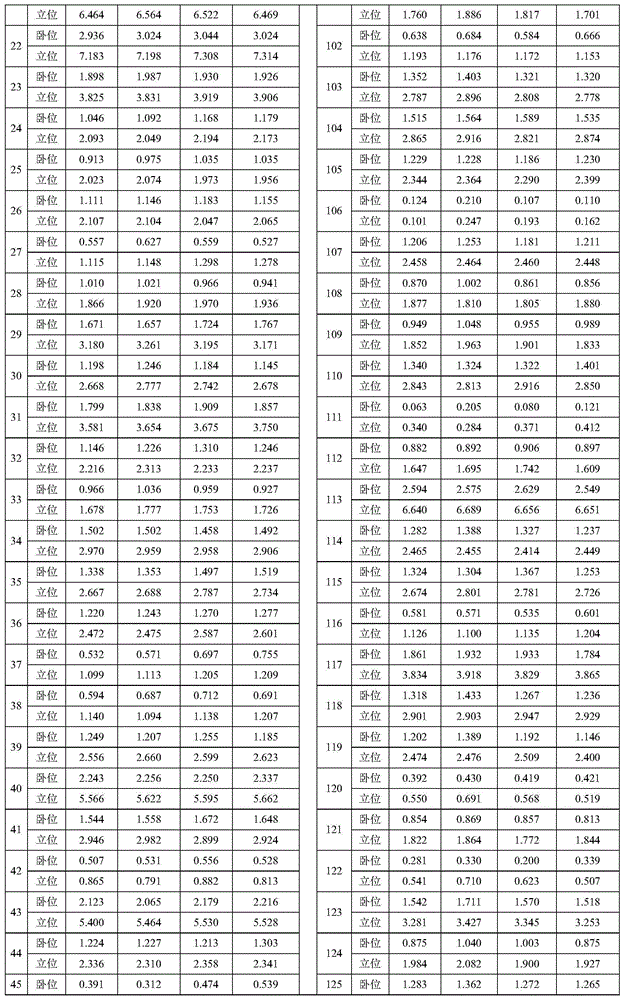
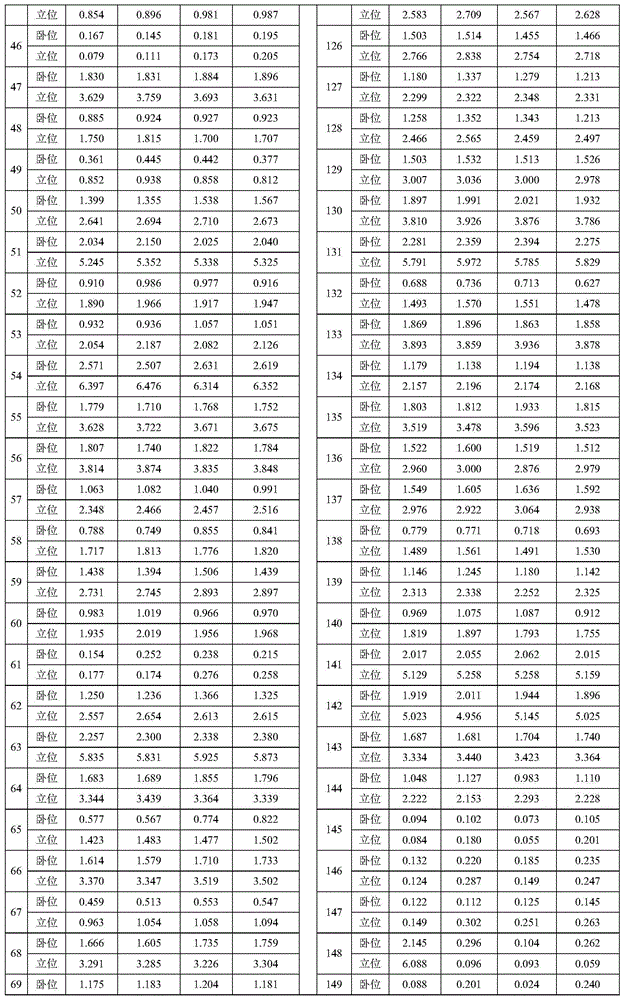
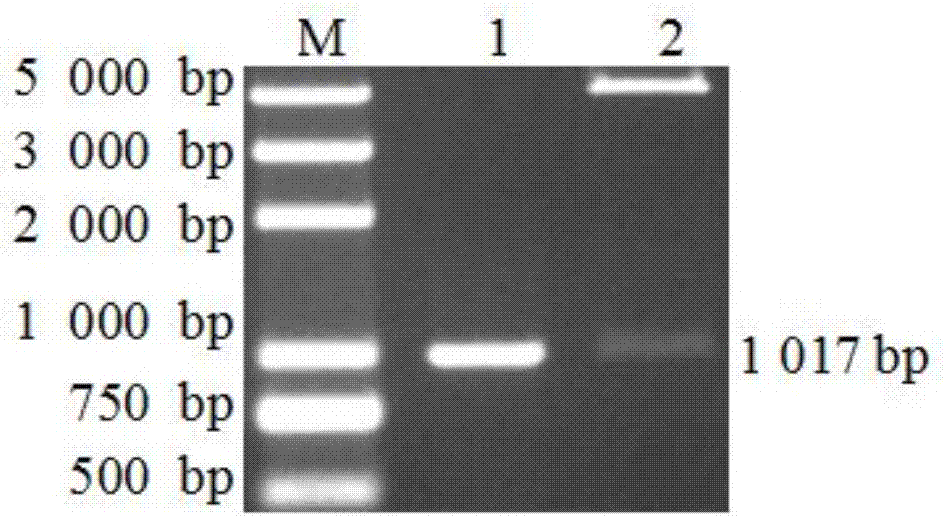
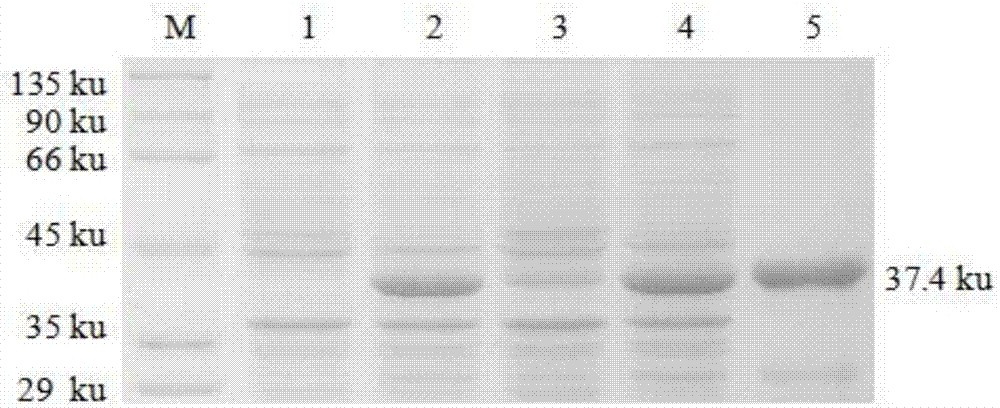
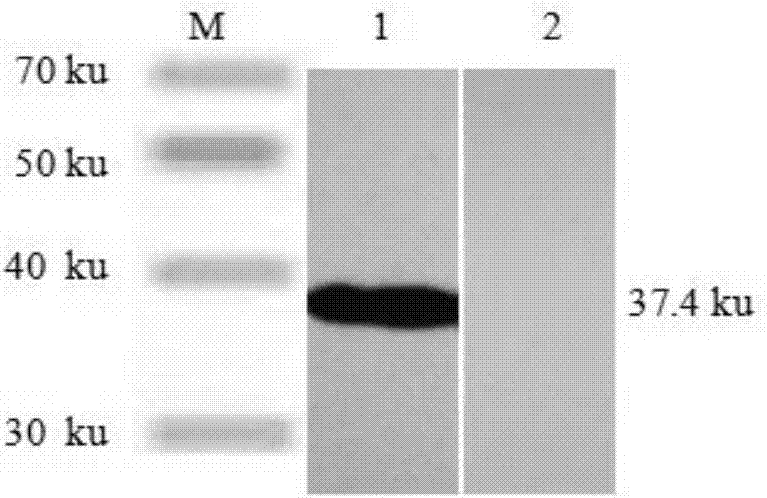
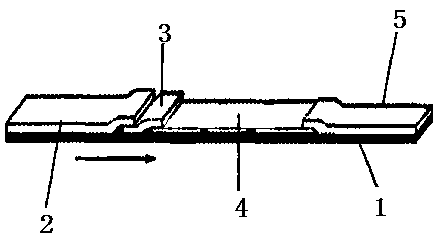
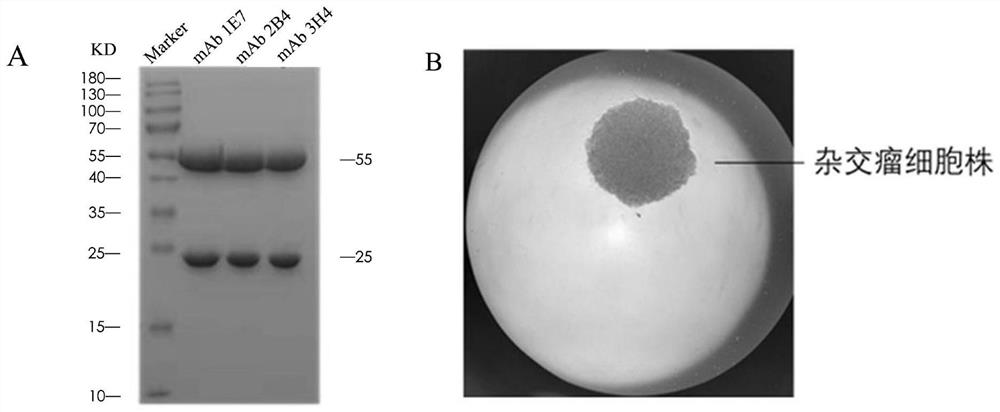
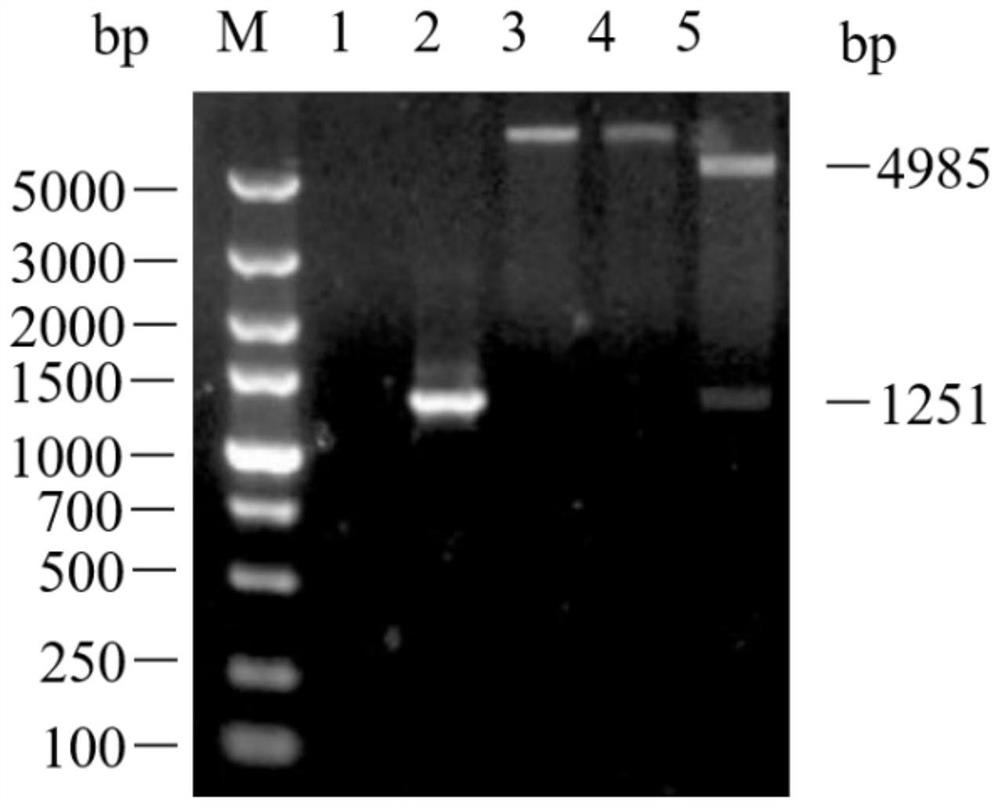
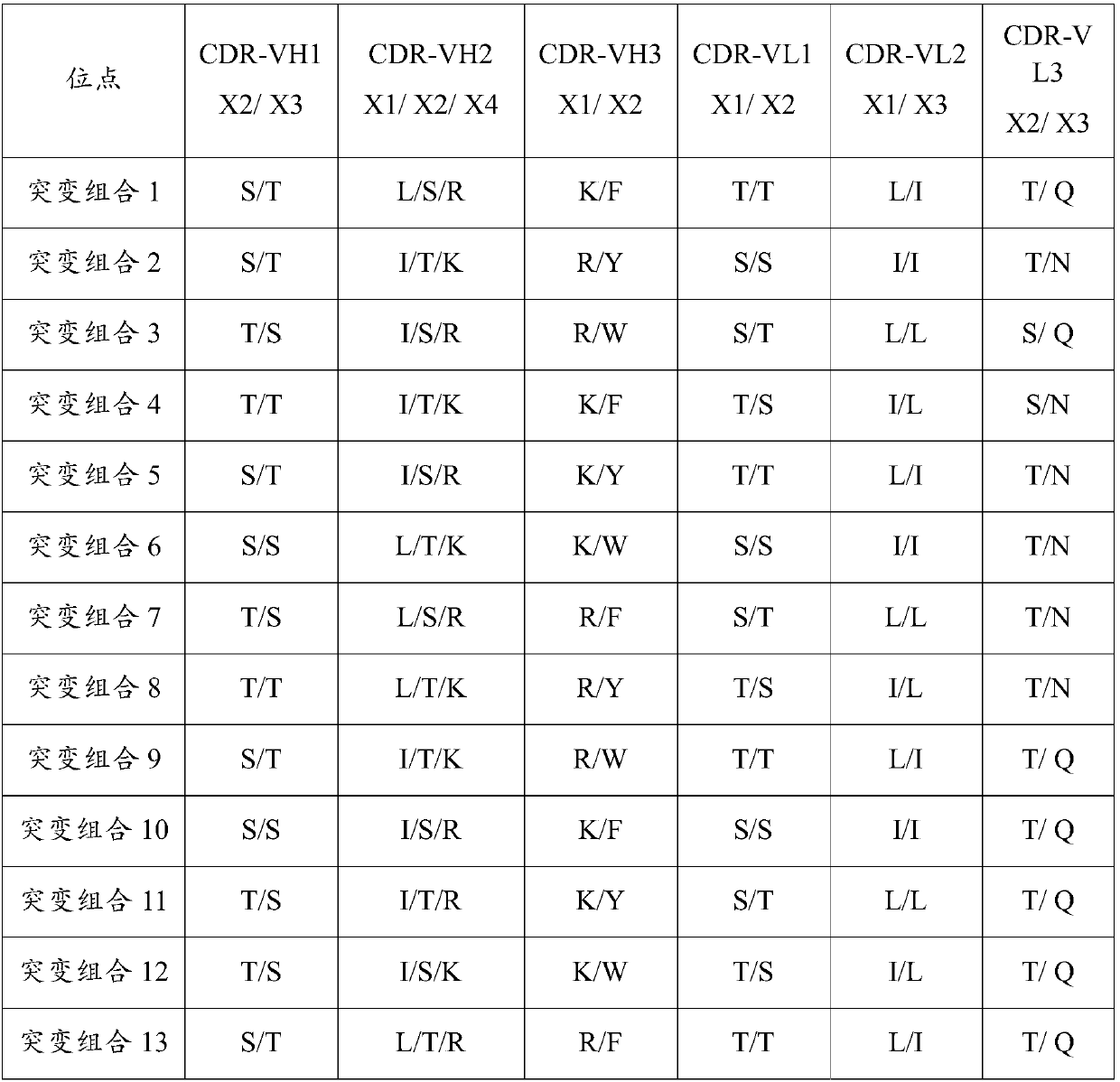
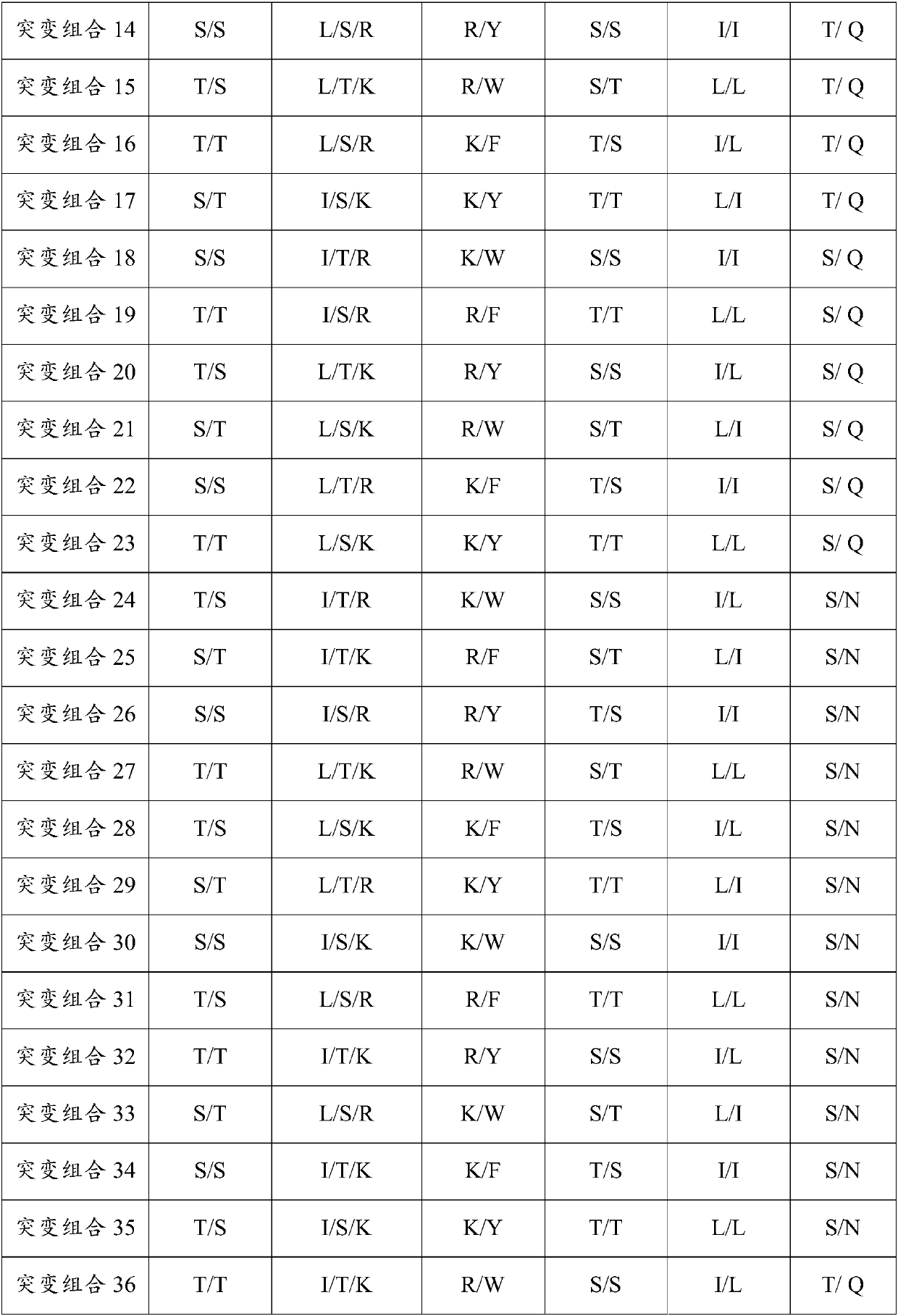
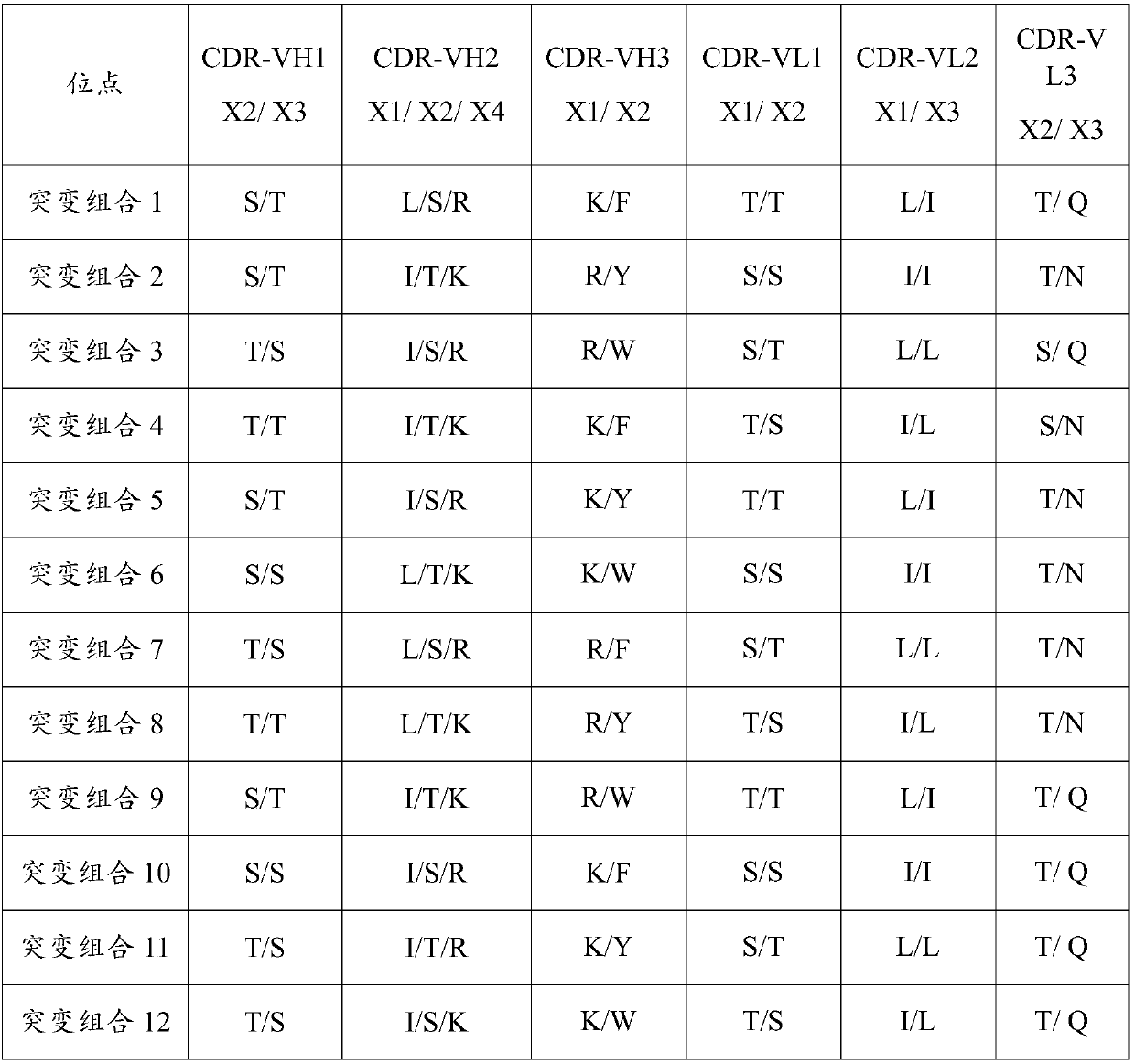
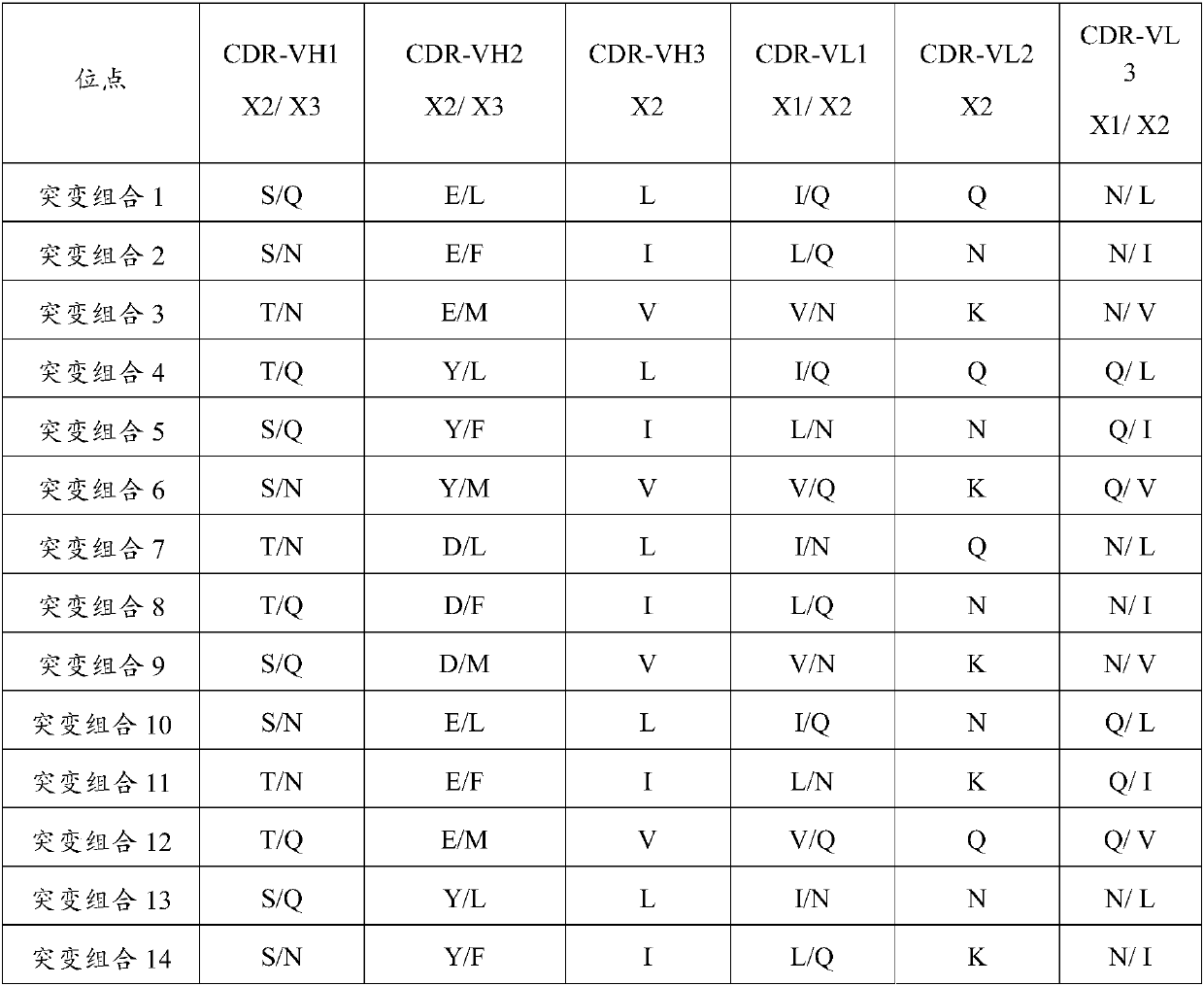
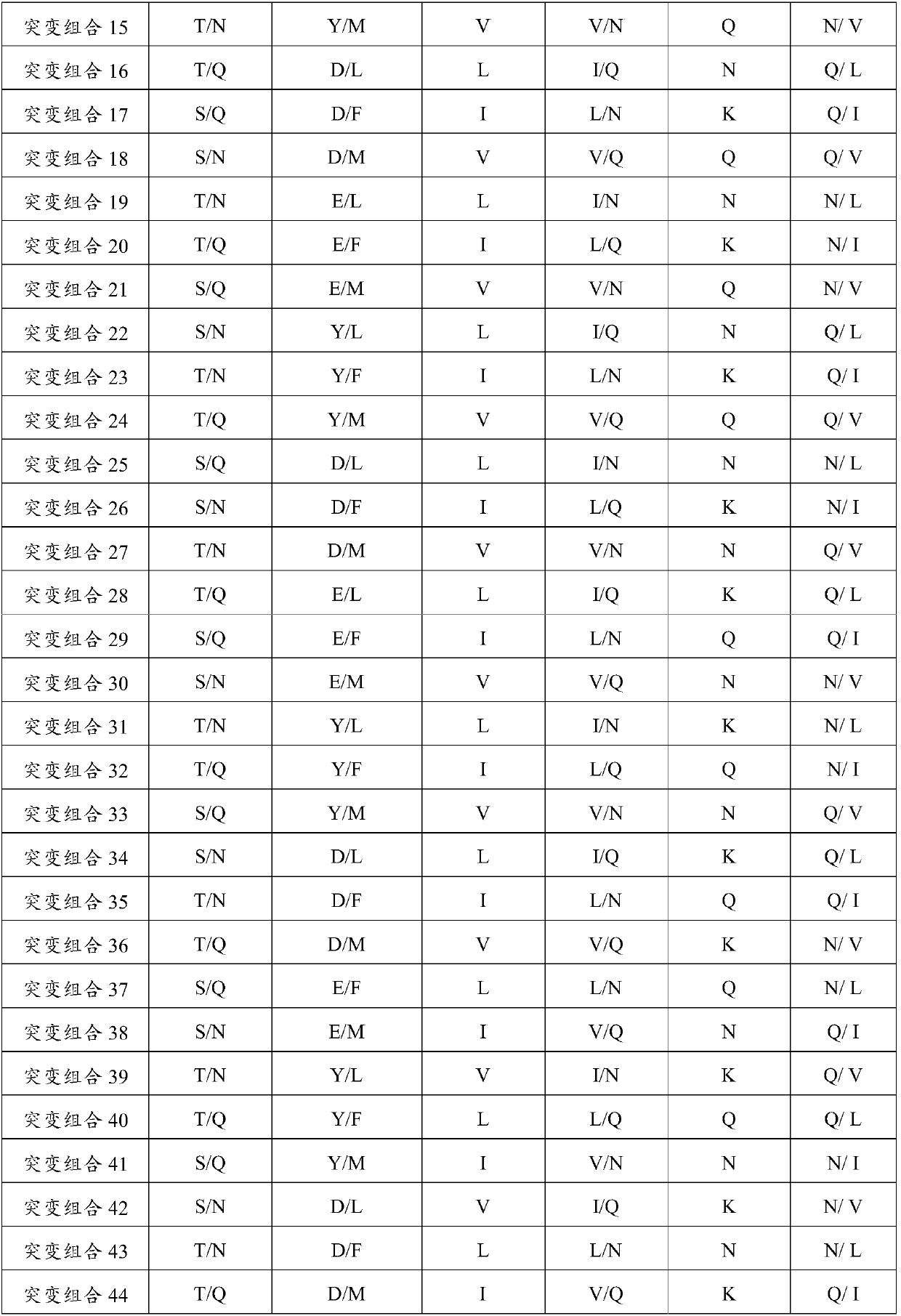
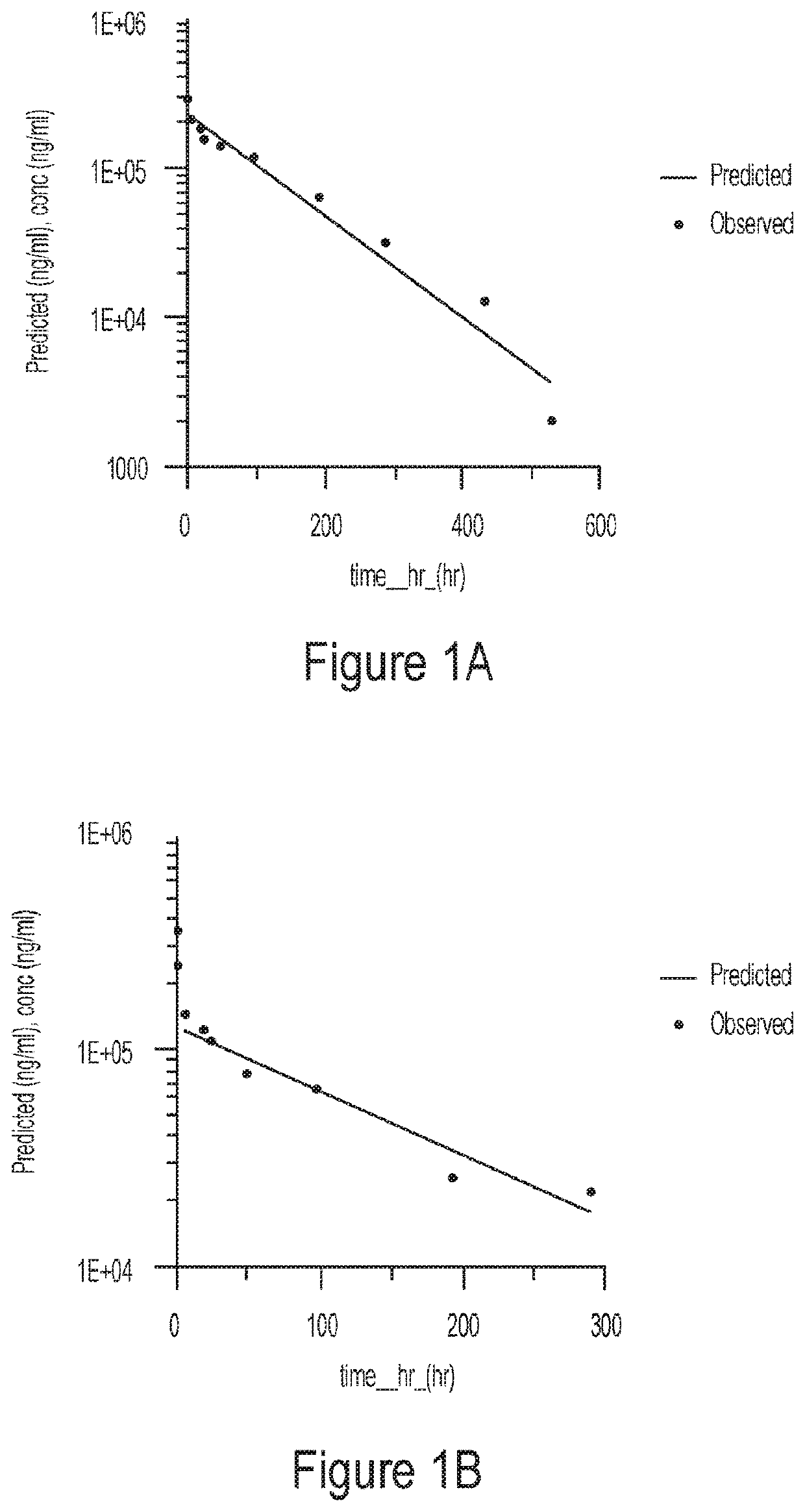
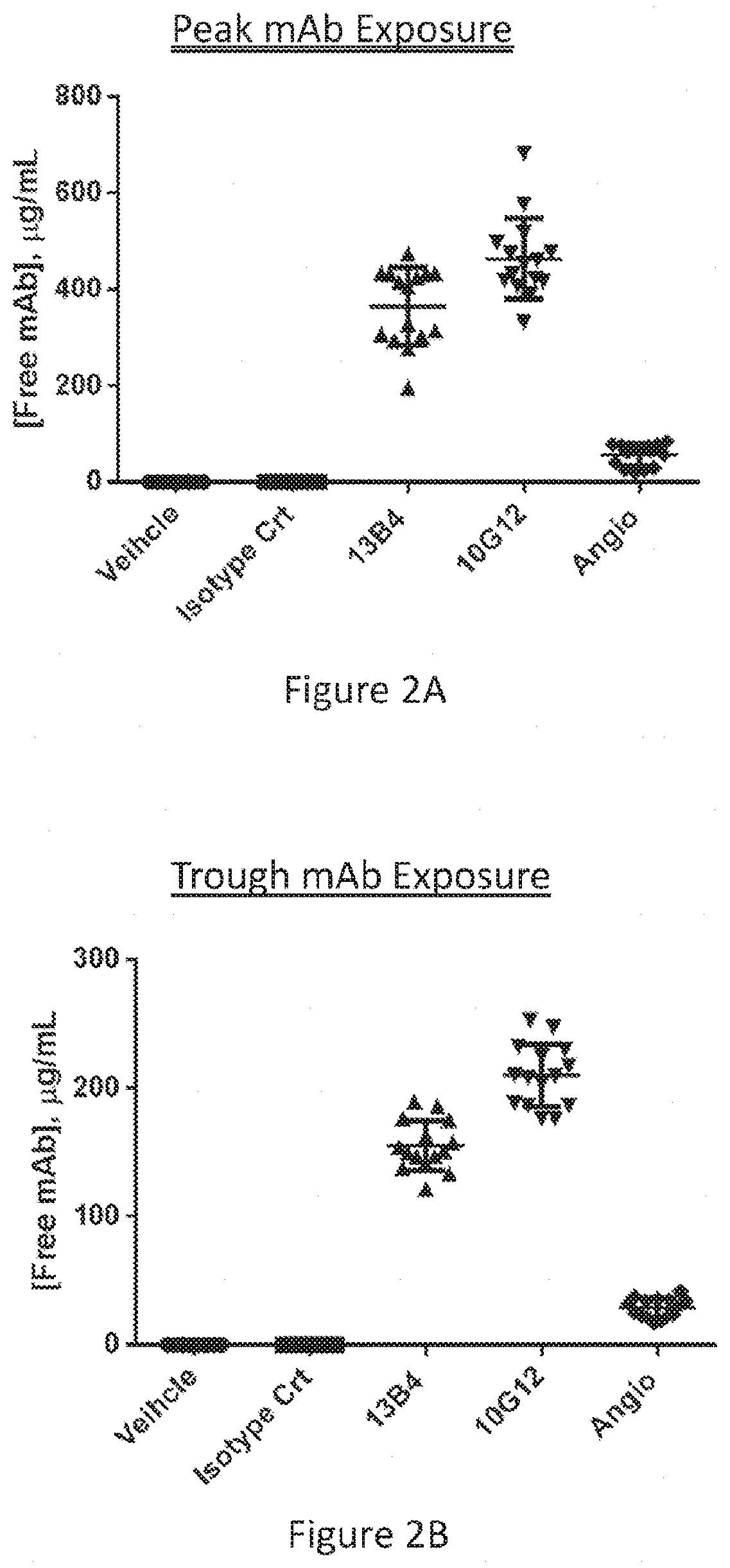
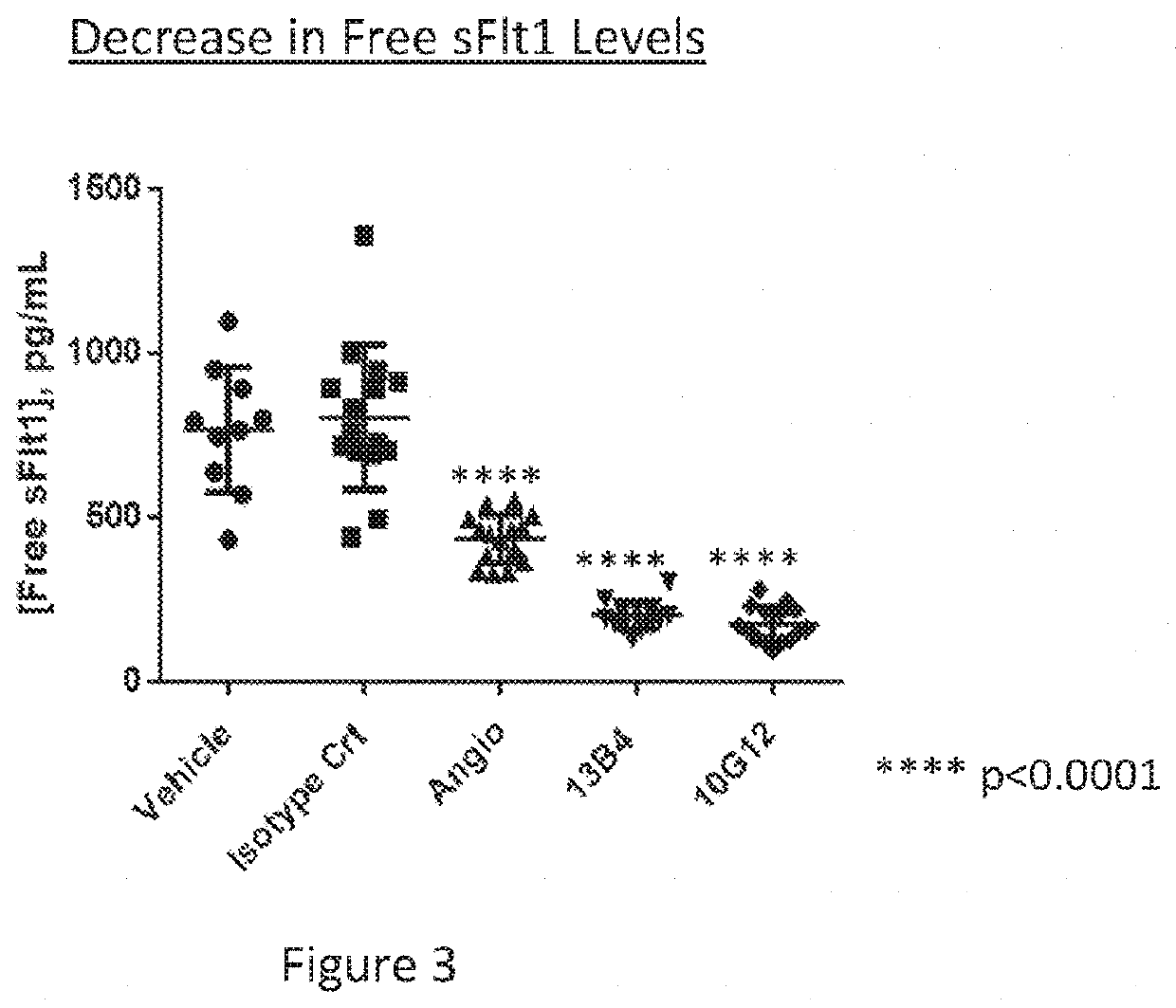
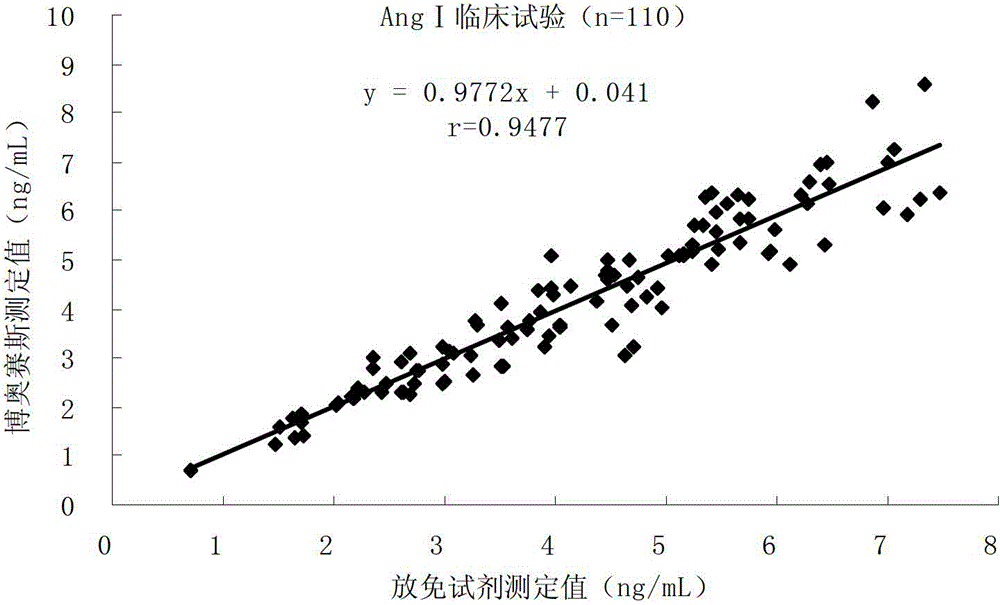
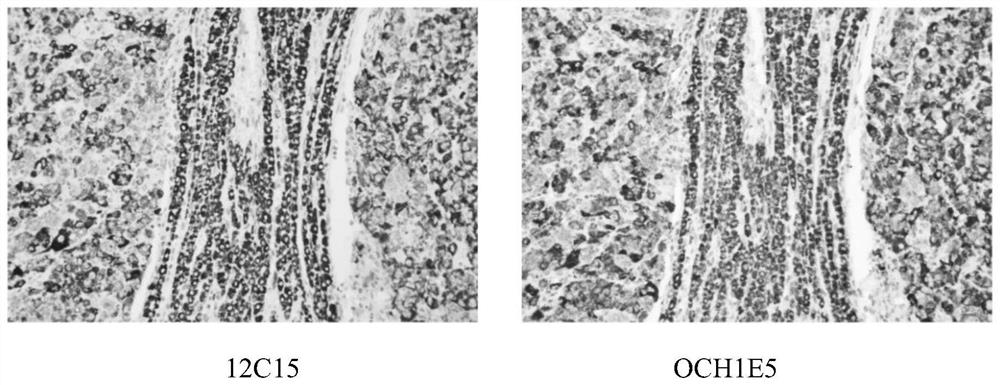
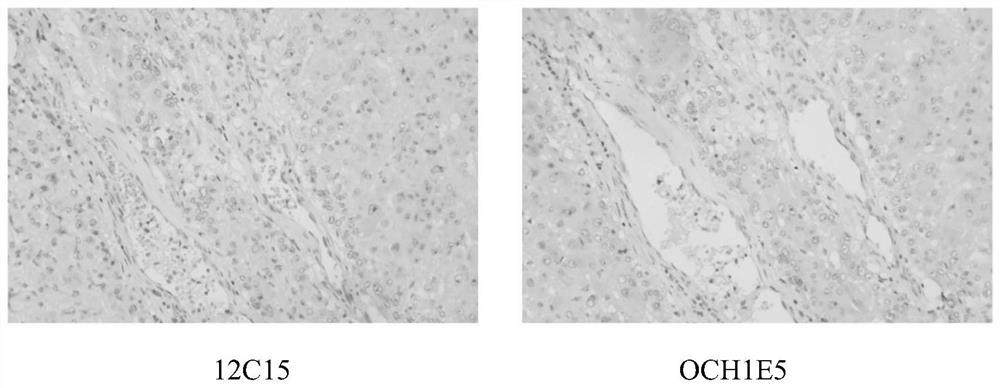
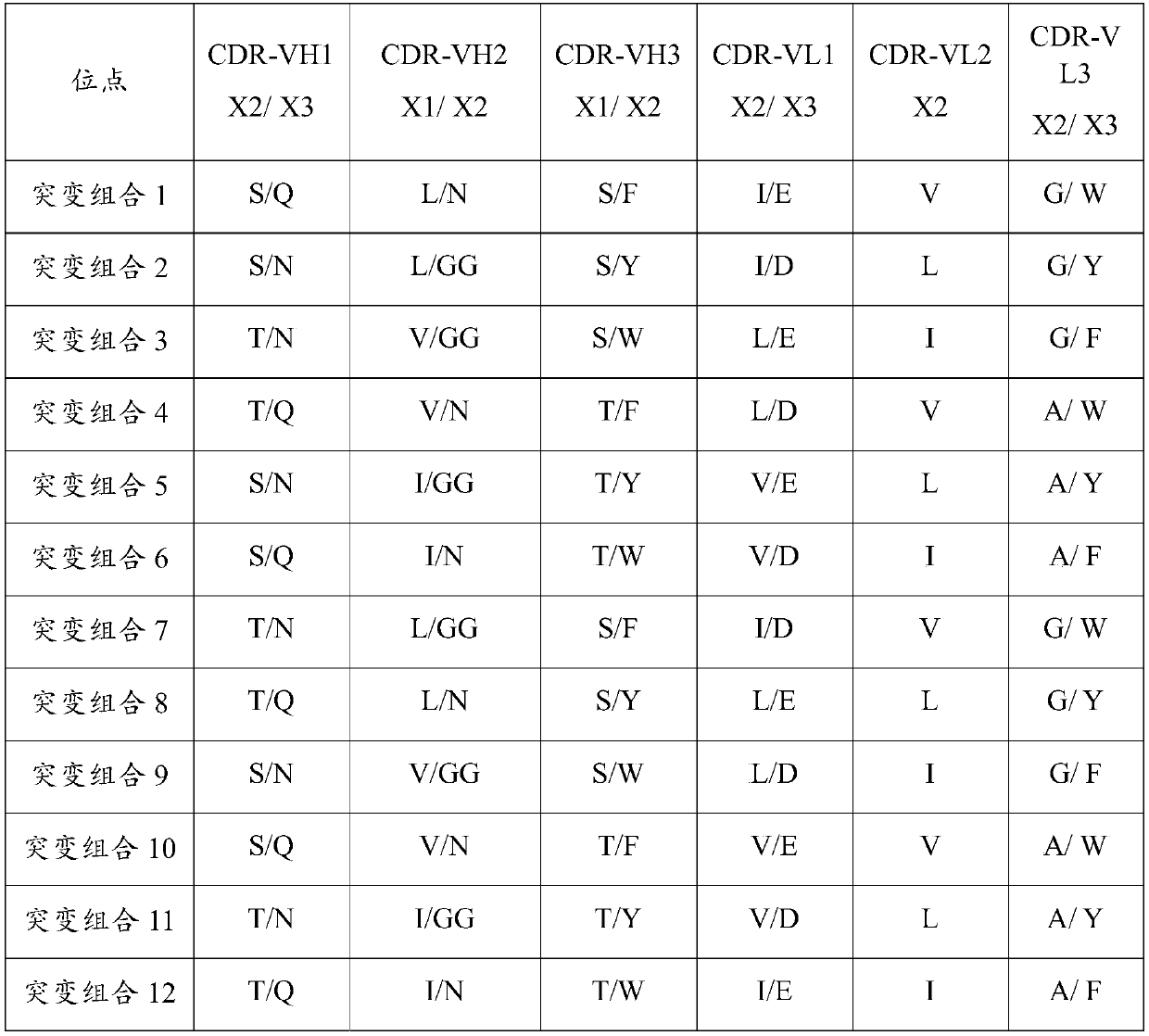
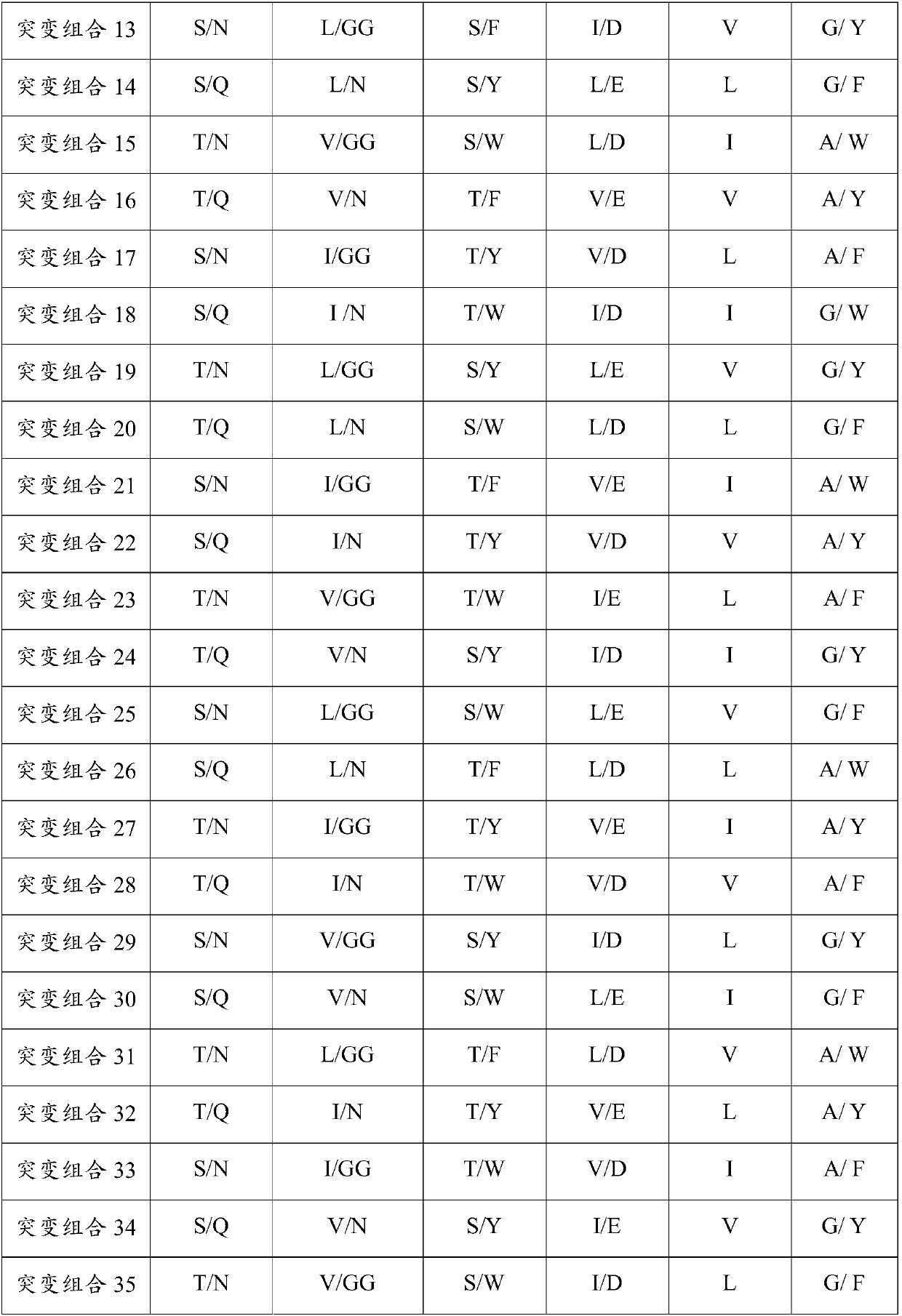
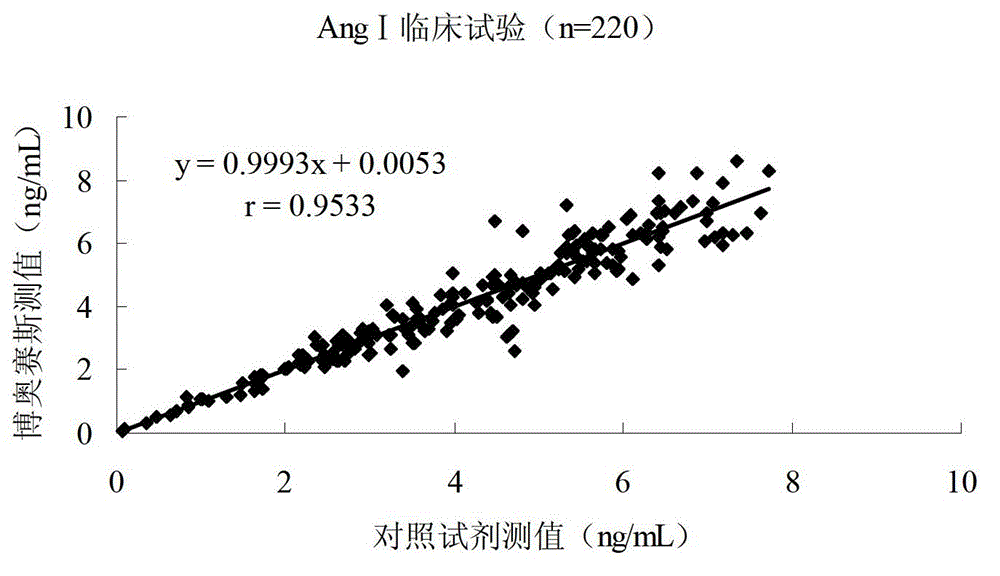
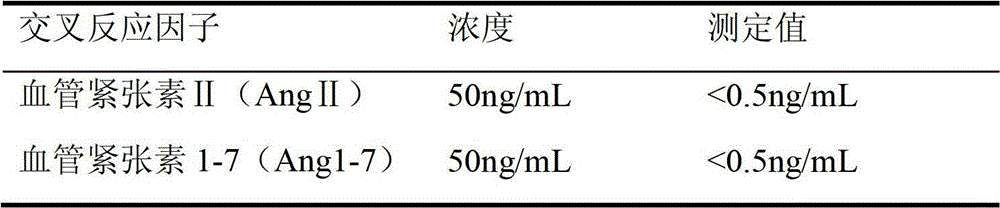
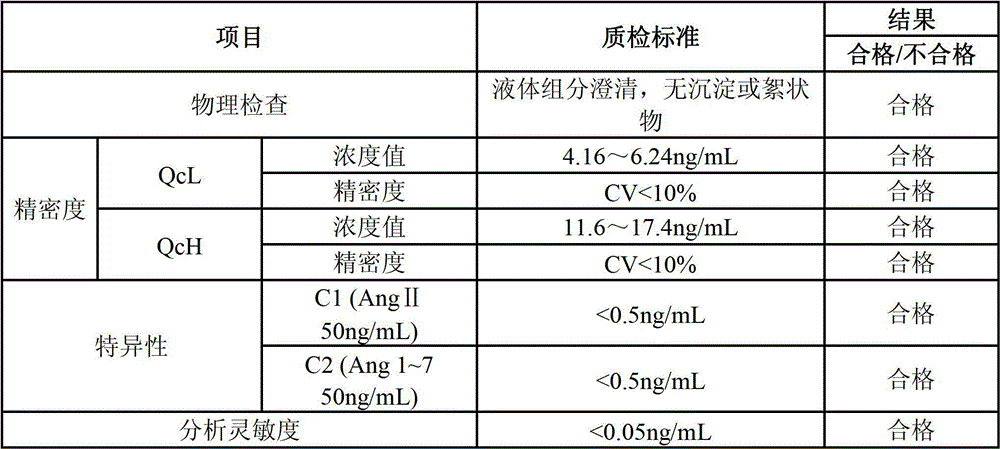
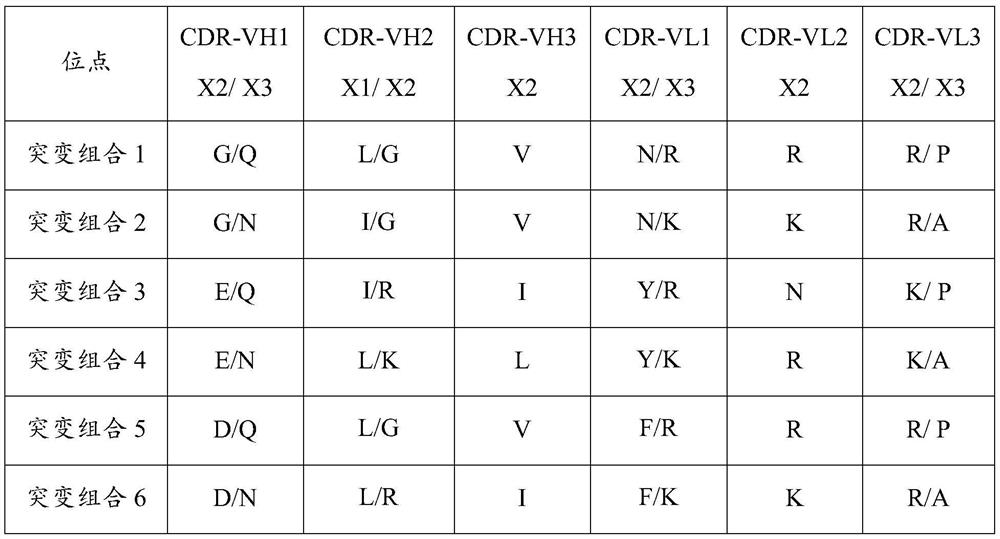
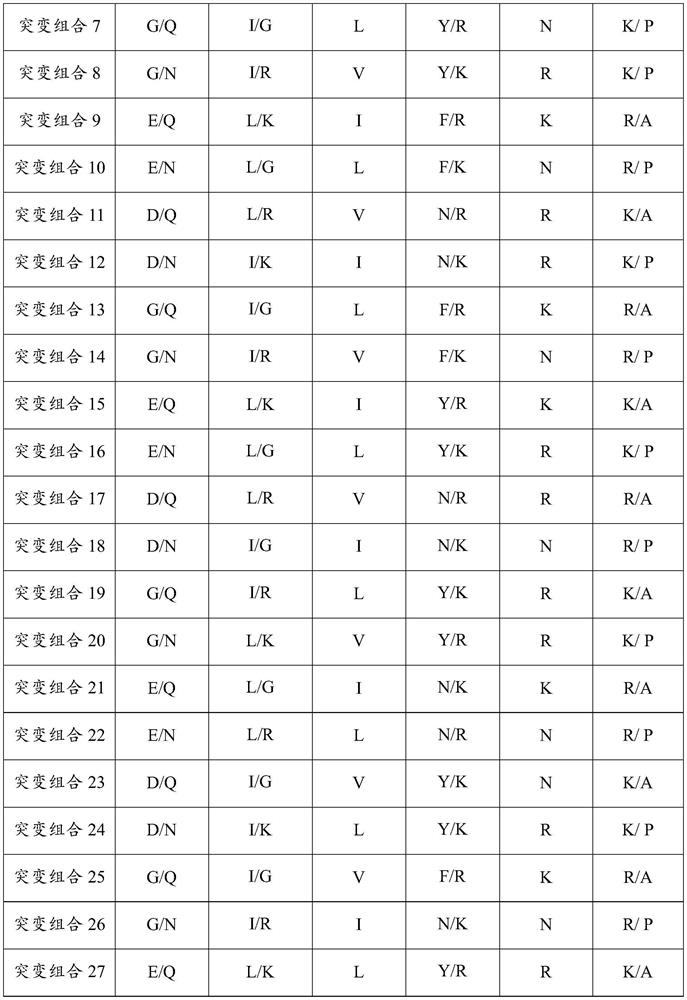
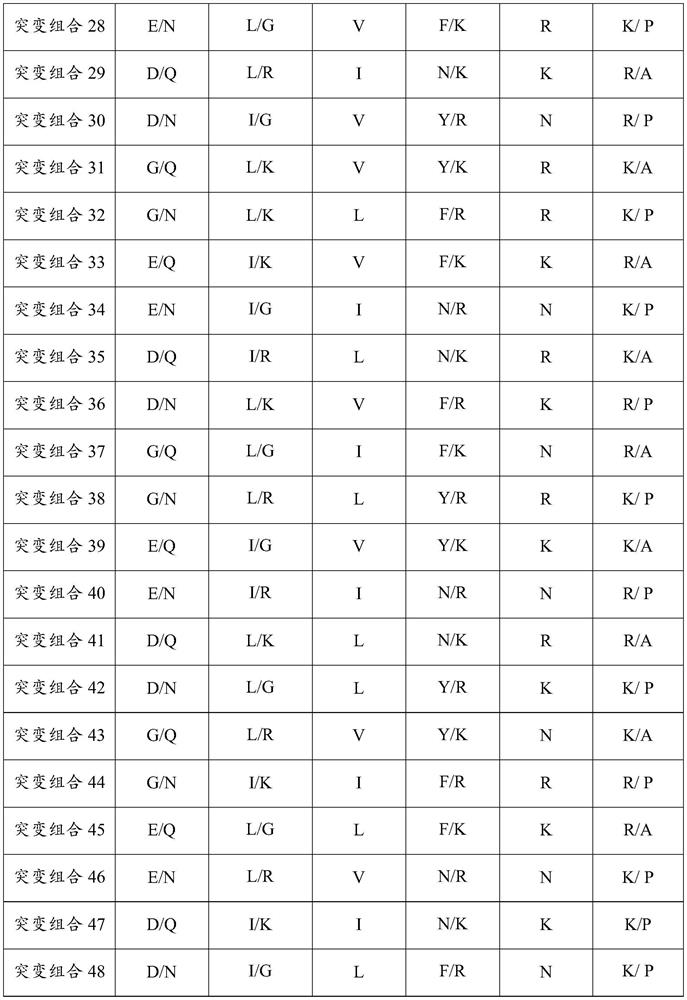
Patents
Literature
41 results about "I antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antibody is a protein produced by the body's immune system when it detects harmful substances, called antigens. Examples of antigens include microorganisms (bacteria, fungi, parasites, and viruses) and chemicals.
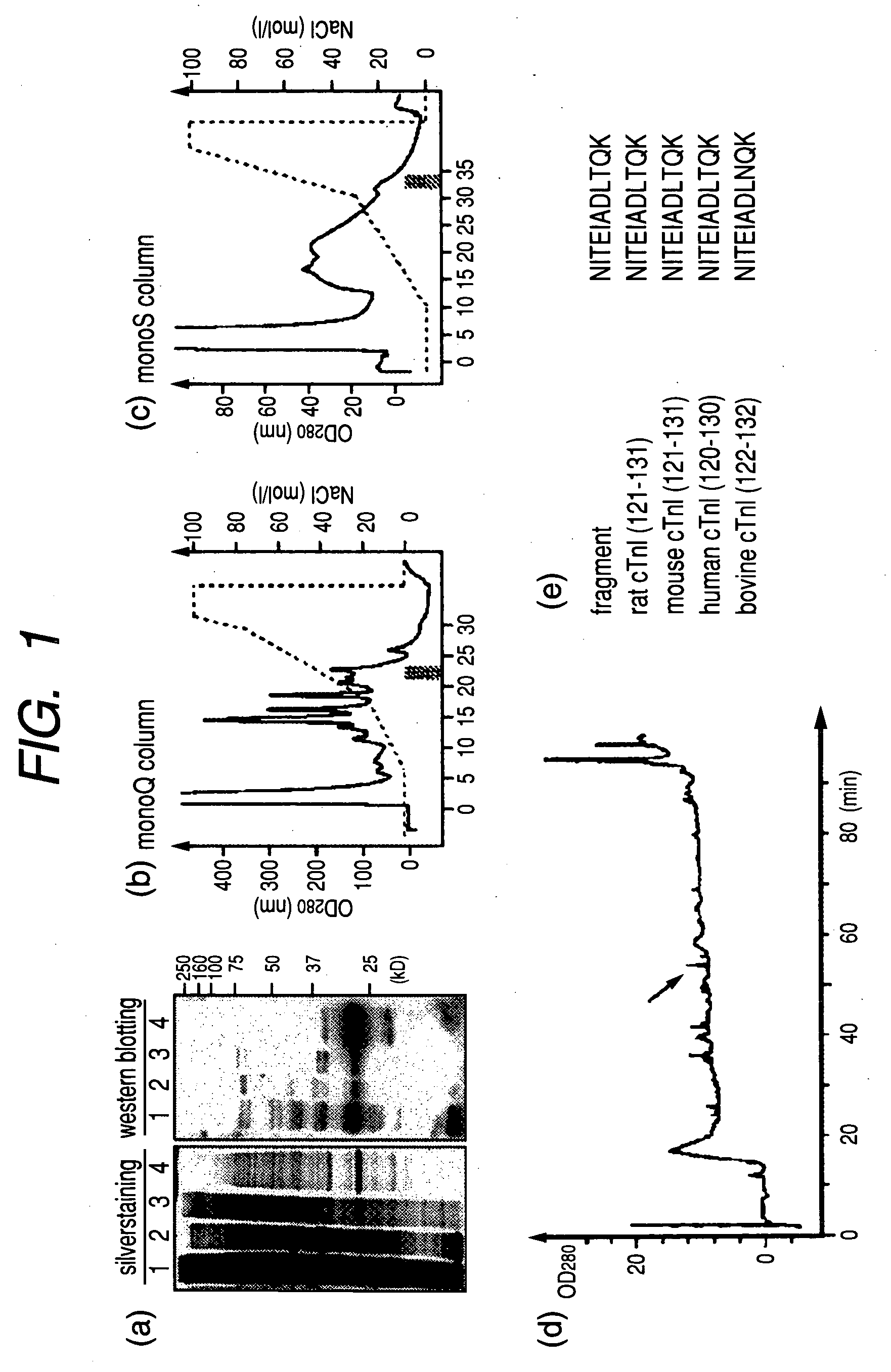
Cardiac troponin I (cTn I) hypersensitive detection kit and hypersensitive detection method
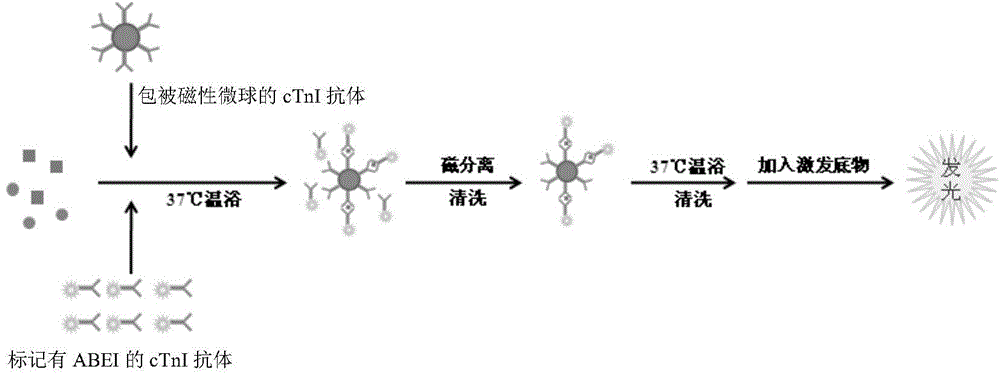
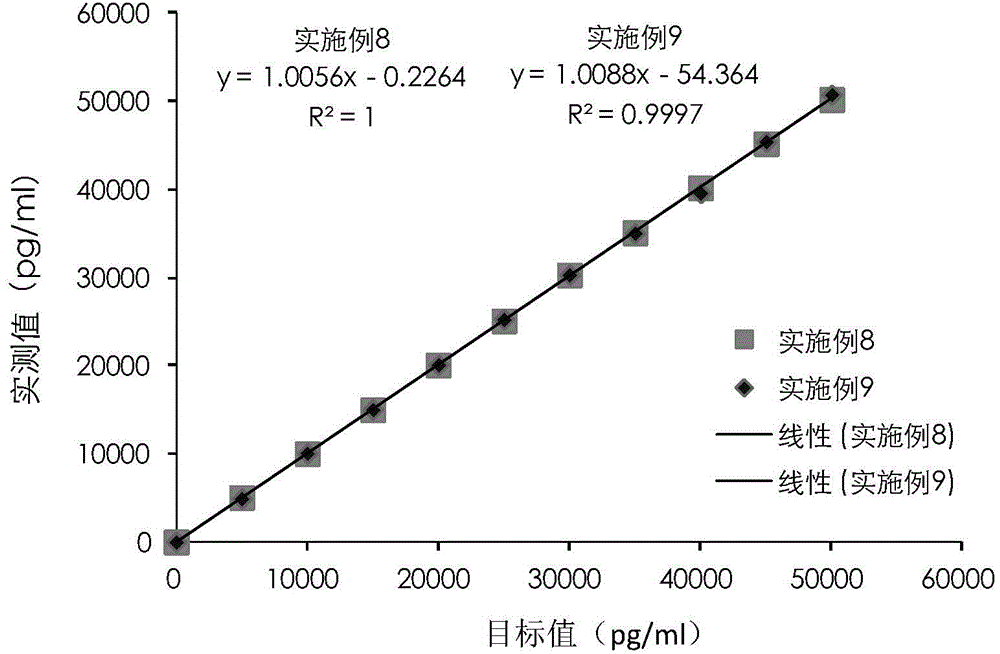
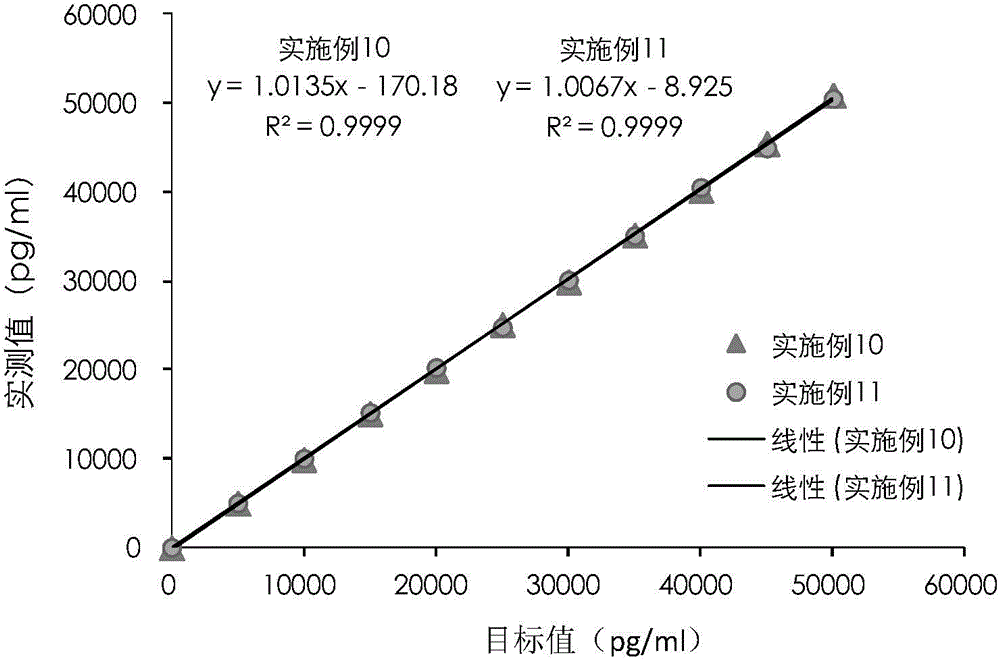
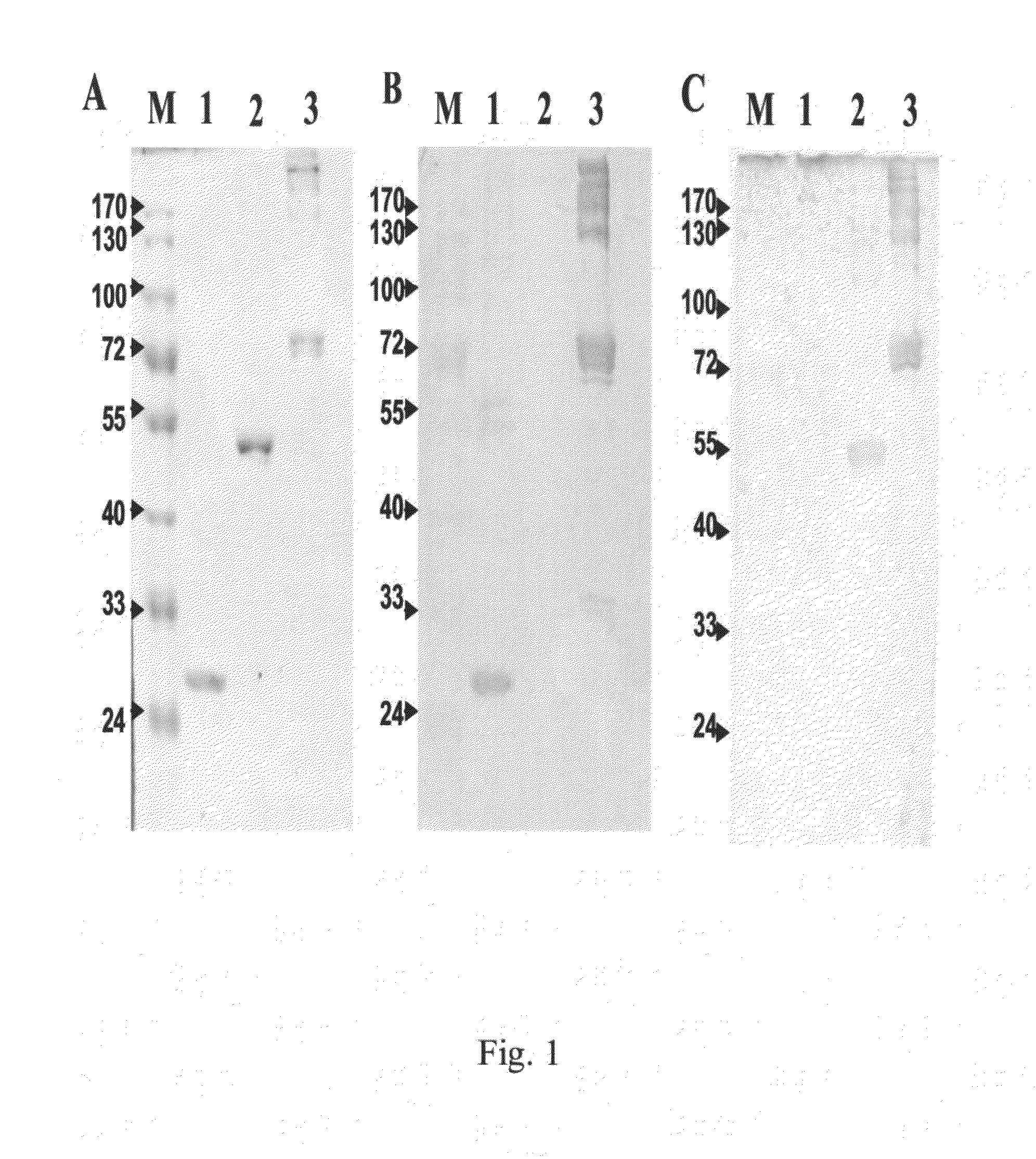
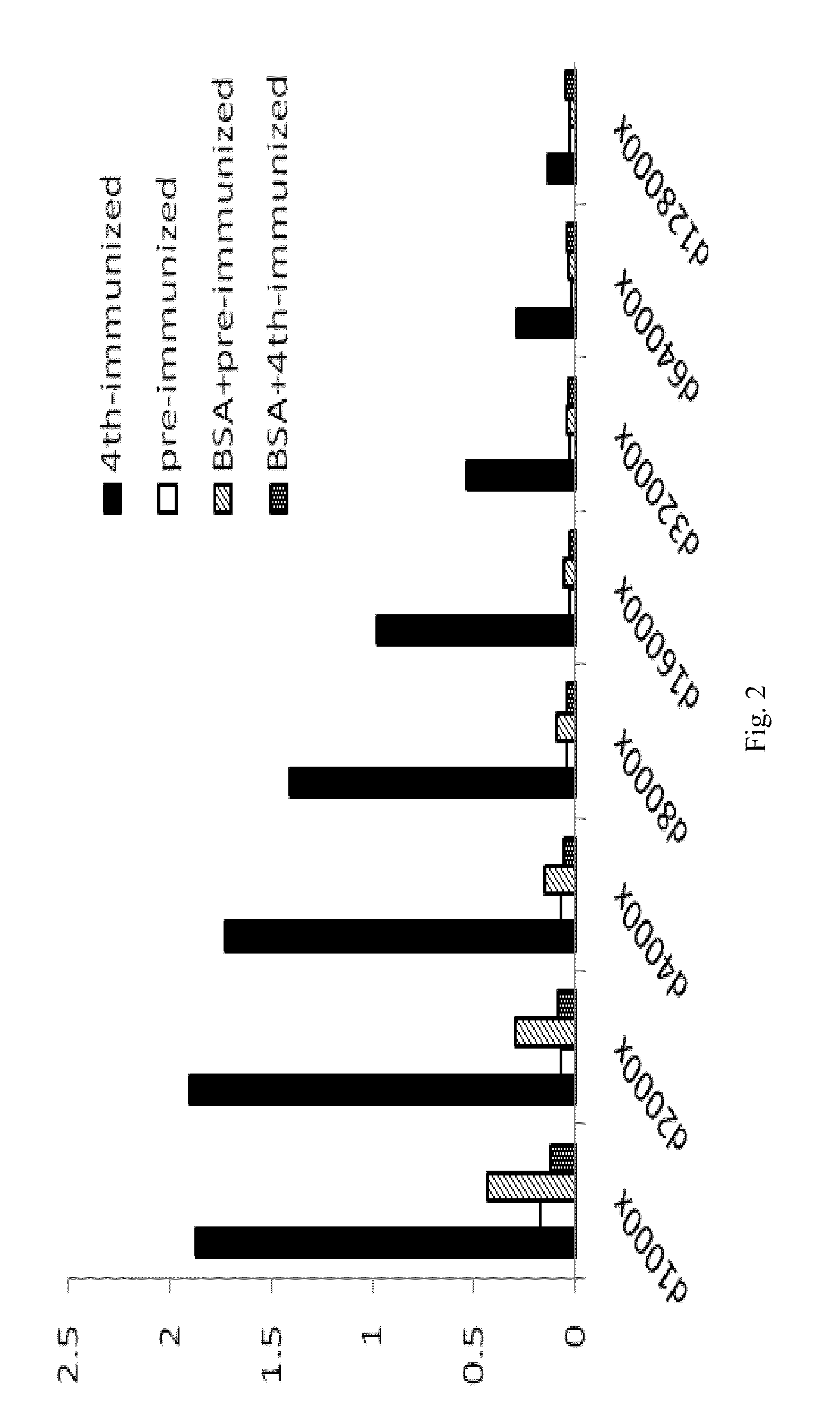
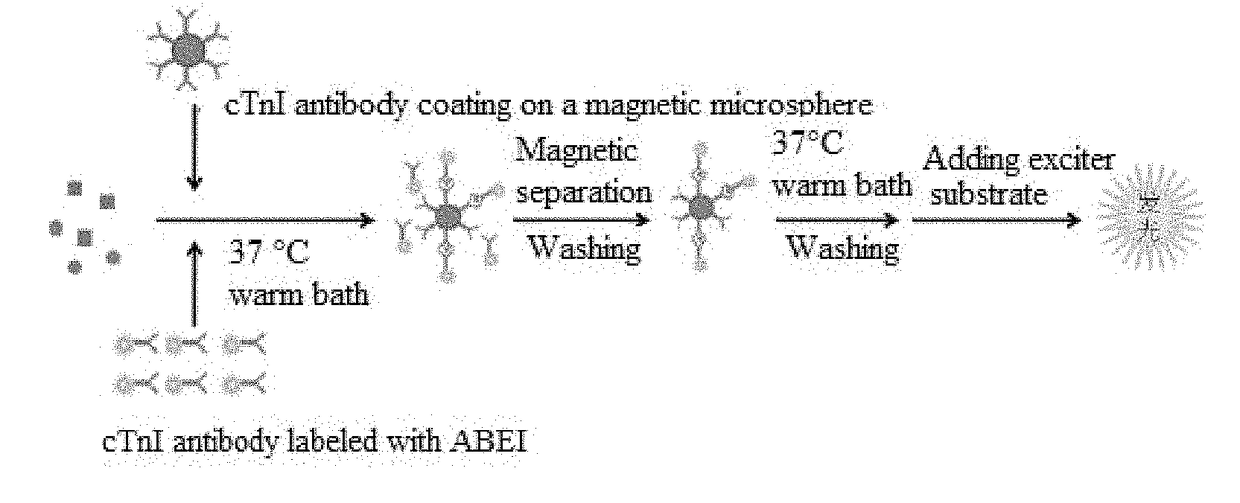
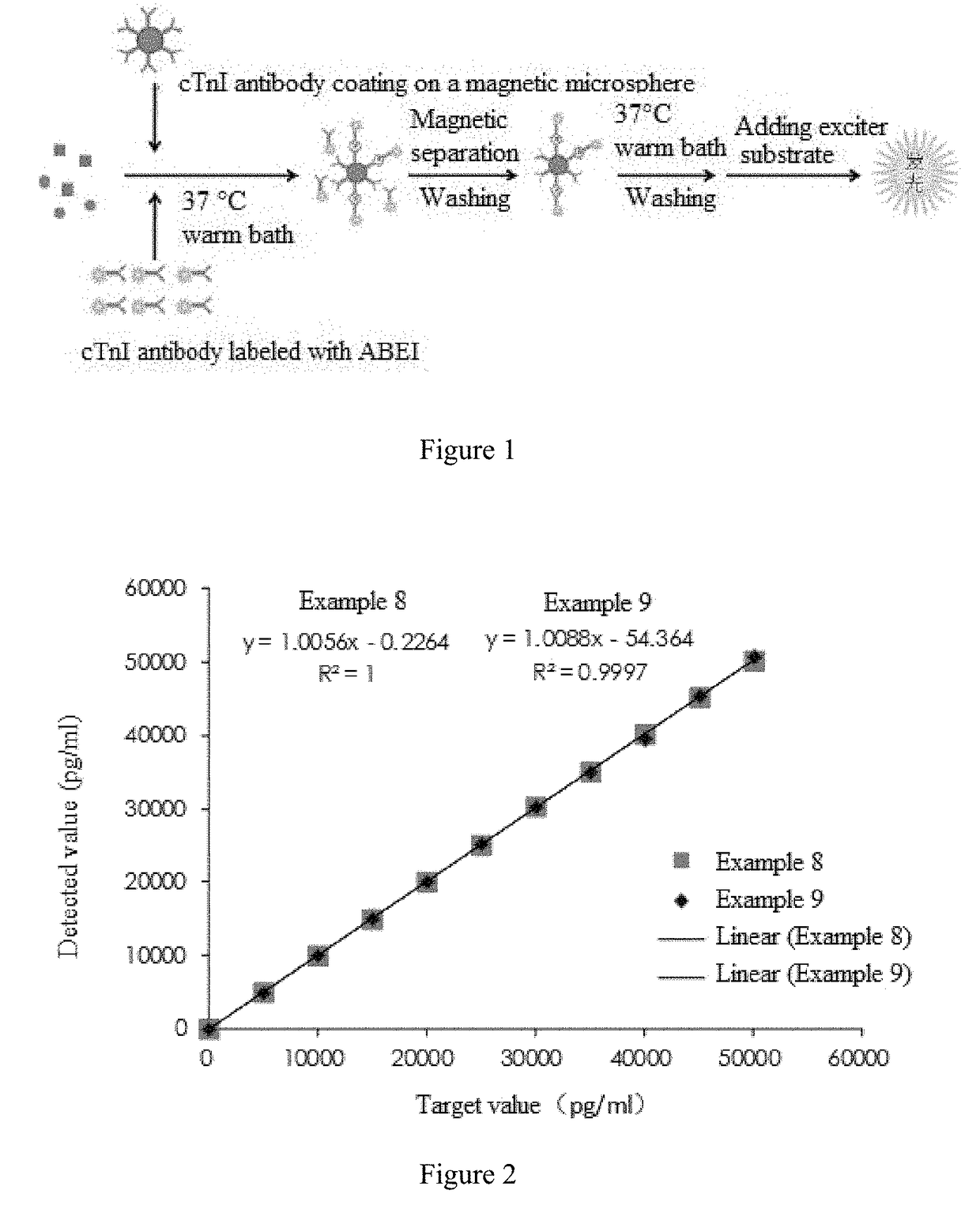
The invention provides a cTn I (cardiac troponin I) hypersensitive detection kit and a preparation method thereof. The kit comprises at least one strain of a first anti-cTn I antibody marked with a tracing marker and at least one strain of a second anti-cTn I antibody coated with magnetic micro-spheres, wherein a binding site between the first anti-cTn I antibody and cTn I is different from a binding site between the second anti-cTn I antibody and cTn I; and the kit can preferably further comprise a diluent, and by adopting the diluent, the non-specific binding in a detection process can be significantly reduced, and the detection accuracy and sensitivity can be further improved. The invention also provides a method for detecting cTn I by using the kit, and the method provided by the invention has very high sensitivity, can be used for sensitively and accurately detecting the cTn I content in a sample, and can provide more timely and reliable information for early diagnosis and treatment of AMI.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
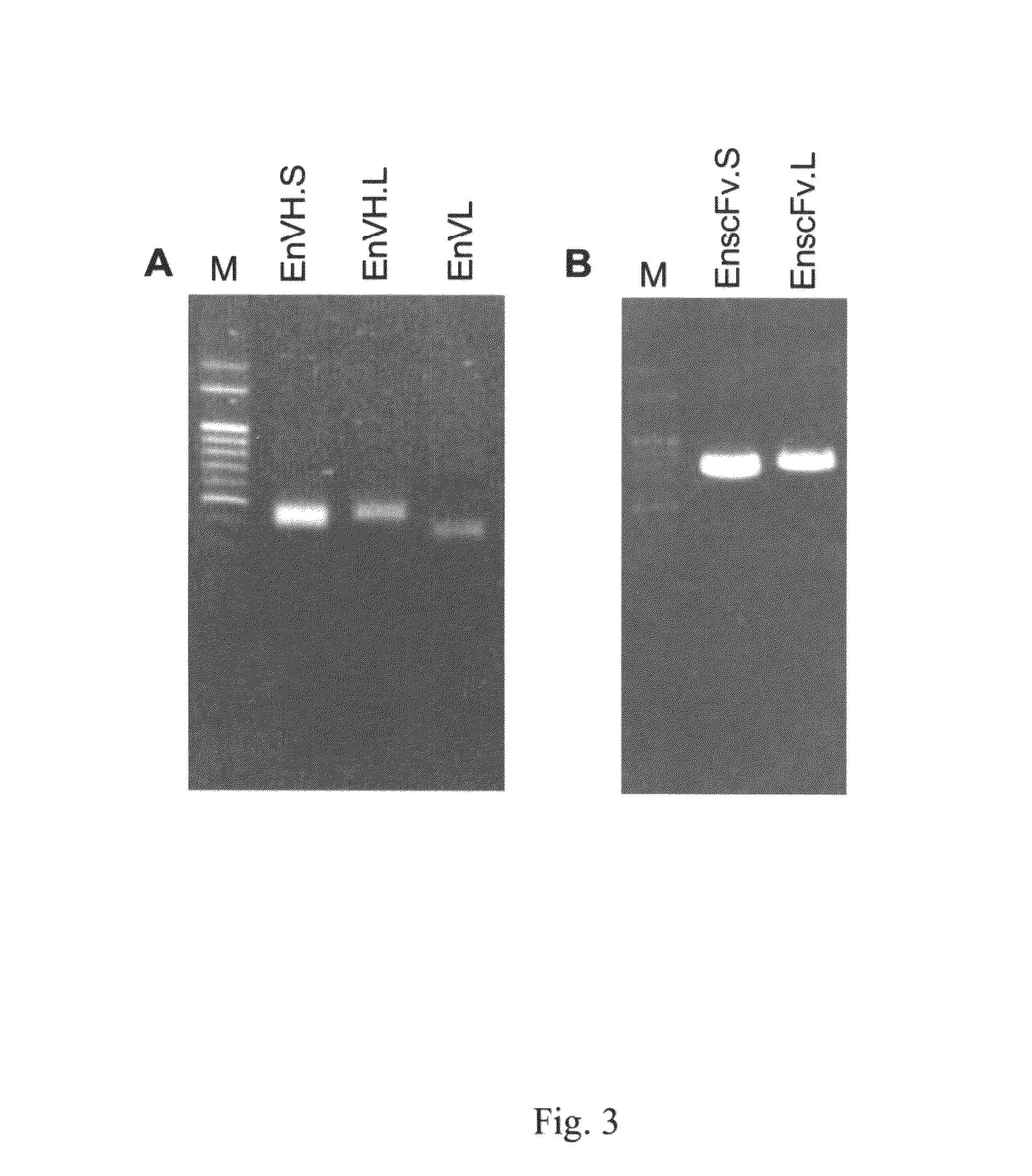
Anti-alpha-Enolase I Antibodies for Diagnosis and Treatment of alpha-Enolase I-Associated Diseases
The invention relates to antibodies against α-enolase I, their pharmaceutical compositions and diagnosis and treatment uses. Particularly, the invention provides polyclonal anti-α-enolase I antibodies and monoclonal single-chain variable fragment (scFv) anti-α-enolase antibodies, pharmaceutical compositions containing the same and their uses in uses in diagnosis and treatment of cancers, autoimmune disorders, ischemia and bacterial infection.
Owner:TAIPEI MEDICAL UNIV
Cardiac troponin i ultra-sensitive detection reagent kit, and ultra-sensitive detection method therefor
ActiveUS20170305983A1The test result is accurateHigh sensitivityChemiluminescene/bioluminescenceAntibody medical ingredientsI antibodyBinding site
A cardiac troponin I ultra-sensitive detection reagent kit, a preparation method, and a detection method. The reagent kit comprises at least one first anti-cardiac troponin I antibody marked with a trace marker and at least one second anti-cardiac troponin I antibody coated on magnetic microspheres, the first anti-cardiac troponin I antibody and cardiac troponin I binding site being different from the second anti-cardiac troponin I antibody and cardiac troponin I binding site. The reagent kit may further comprise a diluent capable of significantly reducing non-specific binding in a detection process, so as to further increase the detection accuracy and sensitivity. The method using the reagent kit to detect cardiac troponin I sensitively and accurately detects the amount of cardiac troponin I in a sample, and provides more timely and reliable information for the early diagnosis and treatment of AMI.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Angiotensin I detection reagent kit as well as preparation method and application thereof
InactiveCN104634965AStrong specificityHigh sensitivityChemiluminescene/bioluminescenceBiological material analysisI antibodyMagnetic sphere
The invention relates to a chemiluminescence immunity detection reagent kit for detecting angiotensin I and a preparation method thereof. The reagent kit comprises a component A and a component B, wherein the component A is an angiotensin I antigen or an adapter of an angiotensin I antigen and a protein carrier, the component B is an anti-angiotensin I antibody, one of the component A and the component B is marked with a trace marker, and the other one is coated with magnetic spheres. The invention further relates to a method for detecting concentration of the angiotensin I by utilizing the reagent kit. According to the angiotensin I detection reagent kit, the concentration of the angiotensin I is determined by utilizing the reagent kit, so that the sensitivity and accuracy in detection are high.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
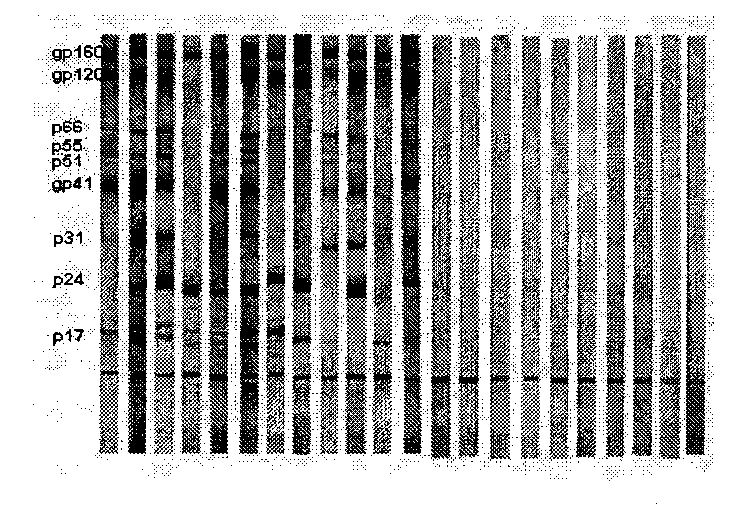
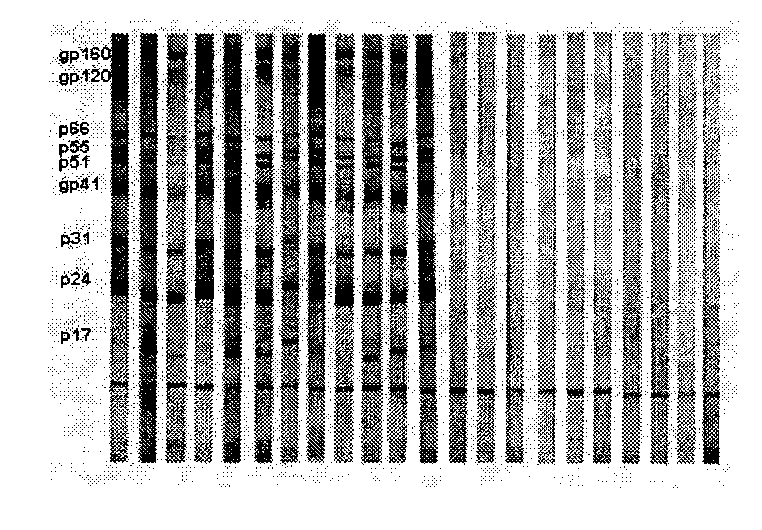
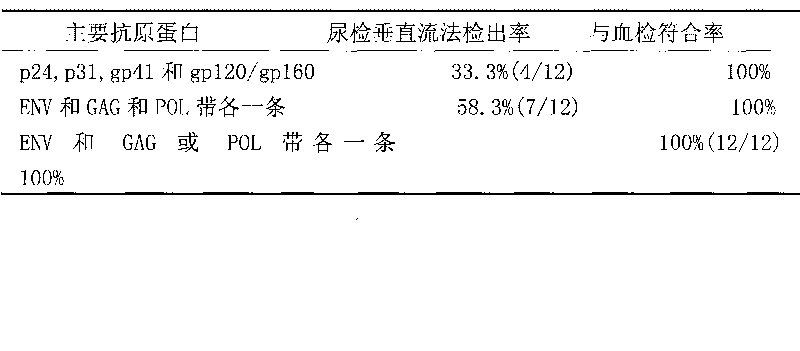
Vertical current western blotting method for quickly detecting human immunodeficiency virus-I antibody in urine
The invention provides an analysis method of immunochemistry and biochemistry, and discloses a vertical current western blotting method for quickly detecting a human immunodeficiency virus-I (HIV-1) antibody in urine, which is particularly suitable for infants and people who are difficult in collecting blood and are unwilling to collect blood. The vertical current western blotting method comprises the following steps: coating HIV-1 antigen to an NC membrane through electrophoresis and electric conversion of lauryl sodium sulfate and polyacrylamide gel, then sticking the film to the bottom of a self-made reaction tank, and combining the reaction tank with a vacuum filtration system. The method enables a urine sample to vertically pass through the NC membrane in a controllable time so as to accelerate the antigen antibody reaction, increase the combination degree of the antigen and the antibody and further improve the detection sensitivity. The method can quickly detect the HIV-1 antibody in the urine, and ensure the HIV-1 infection condition. The method can be used for laboratory diagnosis and confirmation, medicament treatment effect evaluation and the like, is suitable for lower popularization and use, and has large popularization and application values.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Method of screening remedy for heart disease and medicinal composition for treating heart disease
ActiveUS20060246525A1Improve solubilityImprove stabilityPeptide/protein ingredientsMicrobiological testing/measurementI antibodyHeart disease
A method for screening a substance that inhibits the onset of anti-cardiac troponin I autoantibody-related disease, a pharmaceutical composition and a base material for therapy of cardiac disease that contains the substance obtained by aforesaid method thereof, a therapeutic apparatus that removes anti-troponin I autoantibody for aforesaid antibody related disease, a method of making an animal model for evaluating cardiac disease characterized by administrating anti-cardiac troponin I antibody, a method of selection of a therapeutic substance for cardiac disease characterized by using aforesaid animals, and a diagnosis of dilated cardiomyopathy characterized by measuring anti-cardiac troponin I autoantibody. An apparatus of the present invention that removes anti-cardiac troponin I antibody and a pharmaceutical composition for therapy of the antibody related disease may be useful for a therapy and / or prevention of cardiac disease.
Owner:ONO PHARMA CO LTD +1
Quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for angiotensin (Ang) I, and preparation method of kit
ActiveCN102998465ALow cross-specificityImprove performanceChemiluminescene/bioluminescenceBiological testingReaction tubeI antibody
The invention discloses a quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for angiotensin (Ang) I. The kit comprises Ang I calibrators, magnetic particle suspension coupled with streptavidin, an Ang I antibody labeled with biotin, an Ang I enzyme combination, an Ang I quality controller, chemiluminescence liquor A, chemiluminescence liquor B, 20 times concentrated washing liquor, and a reaction tube, wherein enzyme adopted by the Ang I enzyme combination is horse radish peroxidase with the purity RZ being more than or equal to 3.0 and the activity being more than or equal to 250U / ml. The invention also discloses a preparation method of the kit. Compared with the conventional kit, the quantitative detection kit is simple and convenient to operate, is safe, does not cause environment pollution, and also has the advantages of wide concentration range, low cost, good stability and the like of detection samples.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Method for preparing sandwich type photoelectric chemical sensor for cardiac troponin I
ActiveCN108828042AHeavy loadImprove conductivityMaterial analysis by electric/magnetic meansI antibodyBovine serum albumin
The invention relates to a method for preparing a sandwich type photoelectric chemical sensor for cardiac troponin I and belongs to the technical field of nano functional materials, immunoassay and photoelectrochemistry sensation. According to the method, a carboxylation CdS quantum dot is adopted to sensitize a 001 crystal surface of a nano square piece TiO2, then visible light absorption can beimproved, a composite material TiO2 / CdS of which the photoelectric activity is remarkably improved is prepared, by using a layer-by-layer self-assembling method, a cardiac troponin I antibody, bovineserum albumin and cardiac troponin I antigen are assembled on the composite material TiO2 / CdS, Ag@Cu2O shell-core nanoparticles are adopted as a second antibody marker, and by virtue of the excellentphotoelectric activity of the TiO2 / CdS and specific combination of antigen and antibodies of the cardiac troponin I, super-sensitive detection on the cardiac troponin I can be achieved, and the methodhas great significances for analysis and detection on the cardiac troponin I.
Owner:SHANDONG UNIV OF TECH
Method of screening remedy for heart disease and medicinal composition for treating heart disease
ActiveUS7407767B2Improve solubilityImprove stabilityPeptide/protein ingredientsMicrobiological testing/measurementI antibodyAutoantibody production
Owner:ONO PHARMA CO LTD +1
Indirect ELISA kit for detecting aviadenovirus group I antibody, and detection method and application thereof
InactiveCN107102138ALow production costLower control costsMaterial analysis by observing effect on chemical indicatorBiological material analysisSerum igeI antibody
The invention discloses an indirect ELISA kit for detecting an aviadenovirus group I antibody, and a detection method and an application thereof and relates to the technical field of biological detection. The ELISA kit disclosed by the invention comprises a coated elisa plate, a washing liquid, a diluting liquid, a primer developing liquid, a rabbit-anti-chicken elisa second antibody, a terminating liquid, FAVI standard positive serum and FAVI standard negative serum, wherein the coated elisa plate takes a recombinant hexon protein of aviadenovirus group I FAVI as a coating antigen. The ELISA kit disclosed by the invention is used for detecting the aviadenovirus group I antibody, has the advantages of high efficiency, sensitive specificity and repeatability, and is simple and fast to operate, low in cost and suitable for clinical application and popularization.
Owner:YANGLING VOCATIONAL & TECHN COLLEGE +1
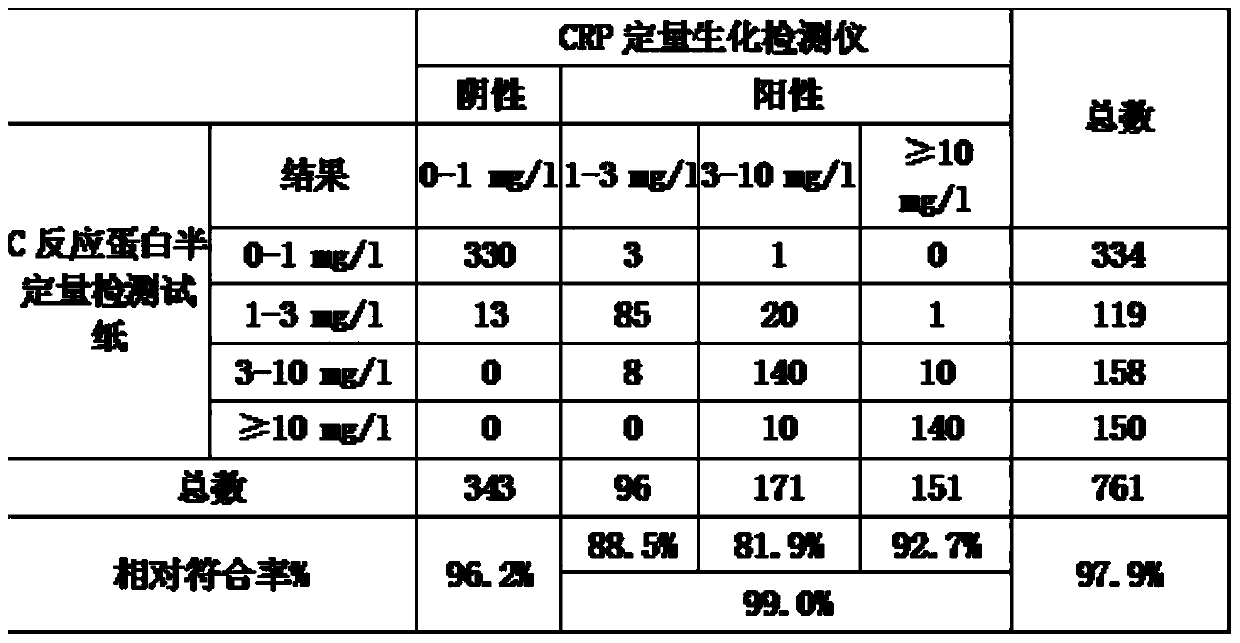
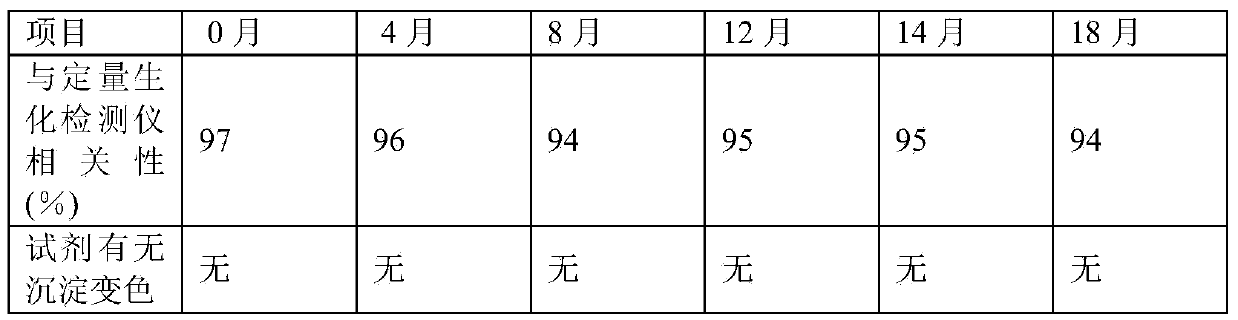
C-reactive protein (CRP) semi-quantitative detection reagent and test paper using reagent
ActiveCN104198731ARapid responseReduce detection application costsBiological testingI antibodyChemistry
The invention discloses a C-reactive protein (CRP) semi-quantitative detection reagent and test paper using the reagent. The CRP semi-quantitative detection reagent comprises the following components: 0.1-3 mg / ml of a coating CRP I antibody mark line, 0.1-1 mg / ml of a coating goat anti rabbit antibody C1 line, 0.1-1 mg / ml of a coating goat anti rabbit antibody C2 line, a marking CRP II antibody, the content of which in per milliliter of colloidal gold is 1-10 micrograms, a marking rabbit IgG antibody, the content of which in per milliliter of colloidal gold is 5-20 micrograms, a gold re-suspension solution which is a Tris-Hcl re-suspension solution with 20mM of Tris and with the pH being 7-10, a sample gasket treatment solution which is a Tris-Hcl solution with 20mM of Tris and with the pH being 7-10, and a sample dilution solution which is a Tris-Hcl solution with 20mM of Tris and the pH being 8. The detection reagent and the test paper are convenient to use, high in detection efficiency, high in response speed, high in sensitivity and high in specificity.
Owner:NINGBO RUI BIO TECH
Anti-human cardiac troponin I antibody and applications thereof
ActiveCN111018983AHigh activityHigh affinityImmunoglobulins against animals/humansDisease diagnosisI antibodyHumanin
The invention relates to a novel isolated binding protein containing a cTnI antigen binding domain, and researches of the preparation, the applications and the like of the binding protein. The bindingprotein is high in activity, has high affinity with human cTnI protein, and can be widely applied to the field of cTnI protein detection.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
Kit for chemilumineseent quantitative immunoassay of angiotensin I and preparation method thereof
ActiveCN102749456ANo pollution in the processWide concentration rangeBiological testingI antibodyTest sample
The invention discloses a kit for chemilumineseent quantitative immunoassay of an angiotensin I. The kit comprises an Ang I antibody-coated plate, a horse radish peroxidase-labeled Ang I, an Ang I calibrator, Ang I quality control materials, a light-emitting solution A, a light-emitting solution B, and a washing liquid concentrated by 20 times. The invention also discloses a preparation method of the kit for chemilumineseent quantitative immunoassay of an angiotensin I. Compared with the existing kit, the kit provided by the invention can be operated more simply, is safe and does not produce pollution on the environment. In addition, the kit provided by the invention allows a wide range of tested sample concentrations and has a long reagent validity period and good stability.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
Protein G coated Sundan red I immunoaffinity column and preparation method thereof
InactiveCN106669638AImprove capture abilityImprove purification efficiencyOther chemical processesSolid sorbent liquid separationChemical LinkageCross-link
The invention provides a Sundan red I immunoaffinity column which comprises a carrier, protein G and a Sundan red I antibody, wherein the protein G is coupled with the carrier through chemical bonds; the Sundan red I antibody is in cross-link with the protein G by using a cross-linking agent after being joined; the protein G is recombinant protein G which is based on GenBank CAA27638.1 and is subjected to gene recombinant expression. Under the condition that detection properties are not affected, the use amount of the Sundan red I antibody of a sepharose gel carrier pretreated with the protein G is about 3 / 4 of that of an antibody for preparing purification column packing by using a direct coupling method. The Sundan red I provided by the invention is very high in purity, can be directly used in high performance liquid chromatography detection without need of extra subsequent purification treatment, therefore, the time and the cost of operators can be saved.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Fluorescence immunochromatographic method for quantitative determination of cardiac troponin I and heart-type fatty acid binding protein
The invention especially relates to a fluorescence immunochromatographic method for quantitative detection of cardiac troponin I and heart-type fatty acid binding protein, belonging to the technical field of immunodetection and analysis. The fluorescence immunochromatographic method employs blood, an anti-cardiac troponin I antibody, an anti-heart-type fatty acid binding protein antibody, a fluorescence reagent and a detector, wherein the anti-cardiac troponin I antibody is one selected from a group consisting of or a combination of an anti-cardiac troponin I monoclonal antibody and an anti-cardiac troponin I polyclonal antibody; the anti-heart-type fatty acid binding protein antibody is one selected from a group consisting of or a combination of an anti-heart-type fatty acid binding protein monoclonal antibody and an anti-heart-type fatty acid binding protein polyclonal antibody; the fluorescence reagent is a liquid-phase and / or solid-phase material containing a fluorescent substance, and the fluorescent substance is one selected from a group consisting of or a combination of an organic fluorescence dye and a rare earth element fluorescence dye; and the detector is a fluorescence detector. According to the invention, human blood is used as a detection sample, and the method can effectively monitor cardiac troponin I and heart-type fatty acid binding protein in bood, has good specificity, good repeatability and high sensitivity, does not need special instrument and equipment and professional training, and is simple to operate, intelligible and distinct in results, easy to promote and applicable to on-site detection.
Owner:CHANGZHOU BIOWIN BIOPHARM
Feline herpes virus type I gB-gD recombinant protein and preparation method and application thereof
PendingCN112457414AHigh sensitivityLow cross-reactivityBacteriaAntibody mimetics/scaffoldsAnimal virusI antibody
The invention discloses a feline herpes virus type I gB-gD recombinant protein, and belongs to the field of animal virus antibody detection. The recombinant protein comprises an amino acid sequence asshown in SEQ ID NO.1 or consists of an amino acid sequence as shown in SEQ ID NO.1. The invention further discloses a gene of the recombinant protein. The gene comprises a vector containing the gene,a host cell. The invention also discloses a preparation method of the recombinant protein and application of the recombinant protein in detection of feline herpes virus type I antibody. The recombinant protein is used for detecting the feline herpes virus type I antibody, is convenient and rapid, has high sensitivity, does not have cross reaction with other pathogens, has high specificity, and has huge clinical significance and wide application prospect.
Owner:杭州爱谨生物科技有限公司
Anti-alpha-enolase I antibodies for diagnosis and treatment of alpha-enolase I-associated diseases
The invention relates to antibodies against α-enolase I, their pharmaceutical compositions and diagnosis and treatment uses. Particularly, the invention provides polyclonal anti-α-enolase I antibodies and monoclonal single-chain variable fragment (scFv) anti-α-enolase antibodies, pharmaceutical compositions containing the same and their uses in uses in diagnosis and treatment of cancers, autoimmune disorders, ischemia and bacterial infection.
Owner:TAIPEI MEDICAL UNIV
Tony red immunodetection test paper and preparation method thereof
The invention discloses a tony red immunodetection test paper. The tony red immunodetection test paper comprises a sample pad, a bonding pad, a cellulose nitrate membrane, a water absorption pad and a backing. The sample pad, the bonding pad, the cellulose nitrate membrane and the water absorption pad are bonded with the backing. Two ends of the bonding pad are in a lap connection relationship with the sample pad and the cellulose nitrate membrane. An end of the cellulose nitrate membrane away from the bonding pad is in a lap connection relationship with the water absorption pad. The cellulose nitrate membrane is provided with a detection line and a quality control line and the detection line and the quality control line are arranged at an interval. The bonding pad is provided with anti-tony red I antibody fluorescent marker. The detection line comprises a tony red I derivative. The quality control line comprises goat anti-mouse IgG. The invention also provides a preparation method of the tony red immunodetection test paper.
Owner:SHENZHEN INST OF ADVANCED TECH
Preparation method for quantum dot-cardiac troponin I antibody immune complex, and preparation method for test strip
InactiveCN110082522AImprove the coupling effectEasy to prepareMaterial analysisI antibodyImmune complex deposition
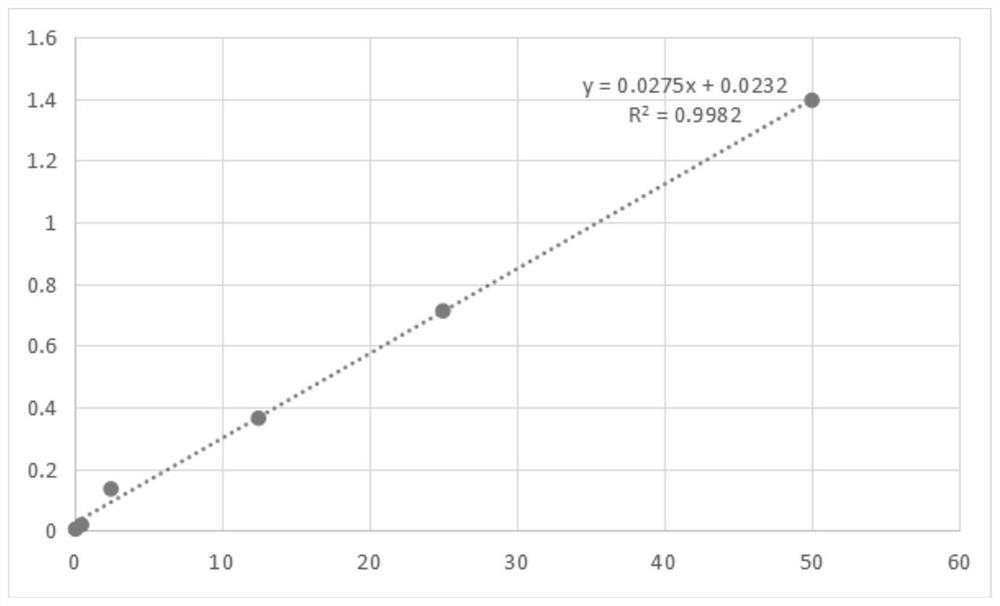
The invention relates to a preparation method for a quantum dot-cardiac troponin I antibody immune complex. A quantum dot and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and N-Hydroxysuccinimide orthe sulfo-product thereof react so as to control the charge molar ratio of the quantum dot, the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, the N-Hydroxysuccinimide or the sulfo-product thereof tobe 1:200-1000:5000-10000; regulating the pH (Potential of Hydrogen) of reaction liquid to be 8-9; and adding the cardiac troponin I antibody into the reaction liquid, and controlling the charge molarratio of the quantum dot to the cardiac troponin I antibody to be 1:42-54. The preparation method has the advantages of good coupling effect, simple preparation method and low cost. The prepared teststrip has a good standard curve, a relevant coefficient is greater than 0.99, detection sensitivity is high, and the accurate quantitative detection of the cardiac troponin I antibody can be realized.
Owner:RES INST OF XIAN JIAOTONG UNIV & SUZHOU
Triple immunofluorescence quantitative detection kit for cardiac troponin I, brain natriuretic peptide and D-dimer chest pain
PendingCN112326975AWide detection rangeLow detection limitDisease diagnosisBiological testingI antibodyReagent strip
The invention provides a triple immunofluorescence quantitative detection kit for cardiac troponin I, brain natriuretic peptide and D-dimer chest pain. The kit for joint quantitative detection of cardiac troponin I, brain natriuretic peptide and D-dimer comprises a reagent strip, and the reagent strip comprises a substrate, and a sample pad, a CP pad, an NC membrane and an absorption pad which aresequentially pasted on the substrate; the CP pad is coated with a cardiac troponin I antibody, a brain natriuretic peptide antibody, a D-dimer antibody and a fluorescein labeled conjugate of chickenIgY; and the NC membrane is respectively provided with a detection line coated with a D-dimer monoclonal antibody, a cTnI monoclonal antibody, a BNP monoclonal antibody and a rabbit anti-chicken IgY monoclonal antibody. According to the detection kit disclosed by the invention, cTnI, BNP and D-dimer can be simultaneously, quickly and accurately detected quantitatively by one-time sampling, so thatclinical diagnosis and treatment on a patient suffering from chest pain are effectively assisted.
Owner:RELIA BIOTECH JIANGSU
Pharmaceutical composition and application thereof
ActiveCN106729705AGrowth inhibitionHas a lethal effectCompounds screening/testingCompound screeningI antibodyComplete remission
The invention relates to the field of researches of anti-tumor drugs, and in particular relates to a pharmaceutical composition and application thereof. The composition comprises a PD-I antibody and a nonspecific amplification activated T cell. The pharmaceutical composition provided by the invention comprises the PD-I antibody and the nonspecific amplification activated T cell which have a synergistic effect to obviously inhibit growth of renal carcinoma cells and kill the renal carcinoma cells. The pharmaceutical composition provided by the invention has an obvious effect on renal carcinoma. The composition evaluated by an RESIST standard reaches PR (partial remission) or is close to CR (complete remission), and the effective rate reaches 100%. Moreover, the composition is quite small in side effects and is free of level-3 or level-4 adverse effects.
Owner:HENAN HUALONG BIOLOGICAL TECH +1
Bovine herpes virus type I antibody blocking ELISA detection method
InactiveCN112180089AStrong specificityEasy to operateVirus peptidesFermentationI antibodyBovine herpesvirus
The invention discloses a bovine herpes virus type I antibody blocking ELISA detection method, belongs to a serological detection method for antibody detection, and is mainly used for detecting bovineherpes virus type I. The detection method is characterized in that the antigen coated by the elisa plate in the ELISA detection method is jointly completed by herpes virus type I gD recombinant protein and horseradish peroxidase labeled monoclonal antibody 2B4. Specifically, the blocking ELISA is established by bovine herpes virus type I recombinant gD protein and horse radish peroxidase labeledmonoclonal antibody 2B4. The detection method is strong in specificity and high in sensitivity, and can directly detect bovine herpes virus type I infected people in an incubation period.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Anti-human cardiac troponin I antibody and applications thereof
ActiveCN111018980AHigh activityHigh affinityImmunoglobulins against animals/humansDisease diagnosisI antibodyHumanin
The invention relates to a novel isolated binding protein containing a cTnI antigen binding domain, and researches of the preparation, the applications and the like of the binding protein. The bindingprotein is high in activity, has high affinity with human cTnI protein, and can be widely applied to the field of cTnI protein detection.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
Anti-human cardiac troponin I antibody and applications thereof
ActiveCN111018979AHigh activityHigh affinityImmunoglobulins against animals/humansDisease diagnosisI antibodyHumanin
The invention relates to a novel isolated binding protein containing a cTnI antigen binding domain, and researches of the preparation, the applications and the like of the binding protein. The bindingprotein is high in activity, has high affinity with human cTnI protein, and can be widely applied to the field of cTnI protein detection.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
Anti-flt-1 antibodies for treating duchenne muscular dystrophy
InactiveUS20210206860A1Improvement in structural and functional characteristicEasy to measureMuscular disorderImmunoglobulins against cell receptors/antigens/surface-determinantsDuchenne muscular dystrophyI antibody
The present invention provides, among other things, anti-Flt-I antibodies and methods for treating muscular dystrophy, in particular, Duchenne muscular dystrophy (DMD). In some embodiments, a method according to the present invention in-cludes administering to an individual who is suffering from or susceptible to DMD an effective amount of an anti-Flt-I antibody or antigen-binding protein thereof such that at least one symptom or feature of DMD is reduced in intensity, severity, or frequency, or has delayed onset.
Owner:TAKEDA PHARMA CO LTD
Kit for chemilumineseent quantitative immunoassay of angiotensin I and preparation method thereof
ActiveCN102749456BNo pollution in the processWide concentration rangeBiological testingI antibodyTest sample
The invention discloses a kit for chemilumineseent quantitative immunoassay of an angiotensin I. The kit comprises an Ang I antibody-coated plate, a horse radish peroxidase-labeled Ang I, an Ang I calibrator, Ang I quality control materials, a light-emitting solution A, a light-emitting solution B, and a washing liquid concentrated by 20 times. The invention also discloses a preparation method of the kit for chemilumineseent quantitative immunoassay of an angiotensin I. Compared with the existing kit, the kit provided by the invention can be operated more simply, is safe and does not produce pollution on the environment. In addition, the kit provided by the invention allows a wide range of tested sample concentrations and has a long reagent validity period and good stability.
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
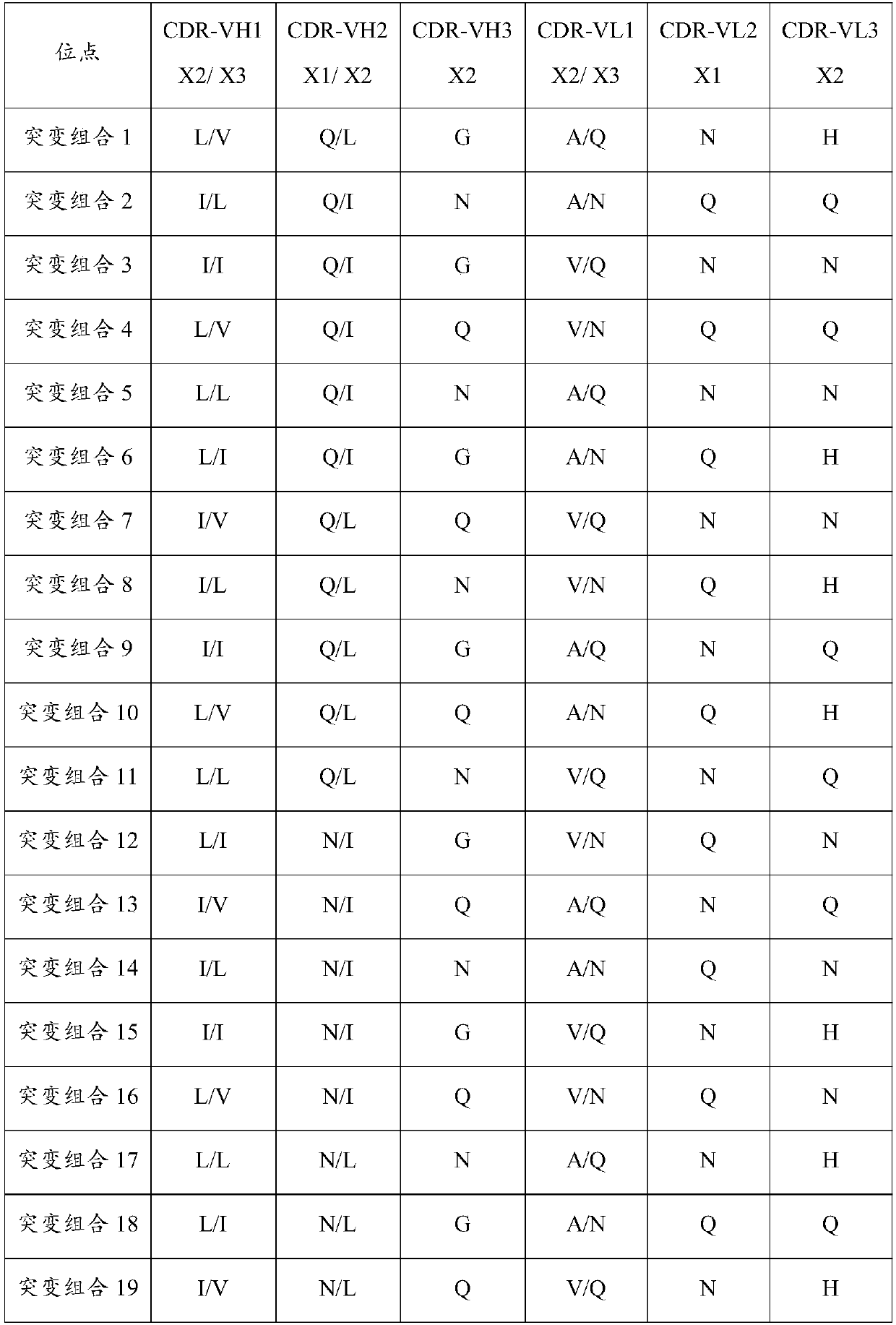
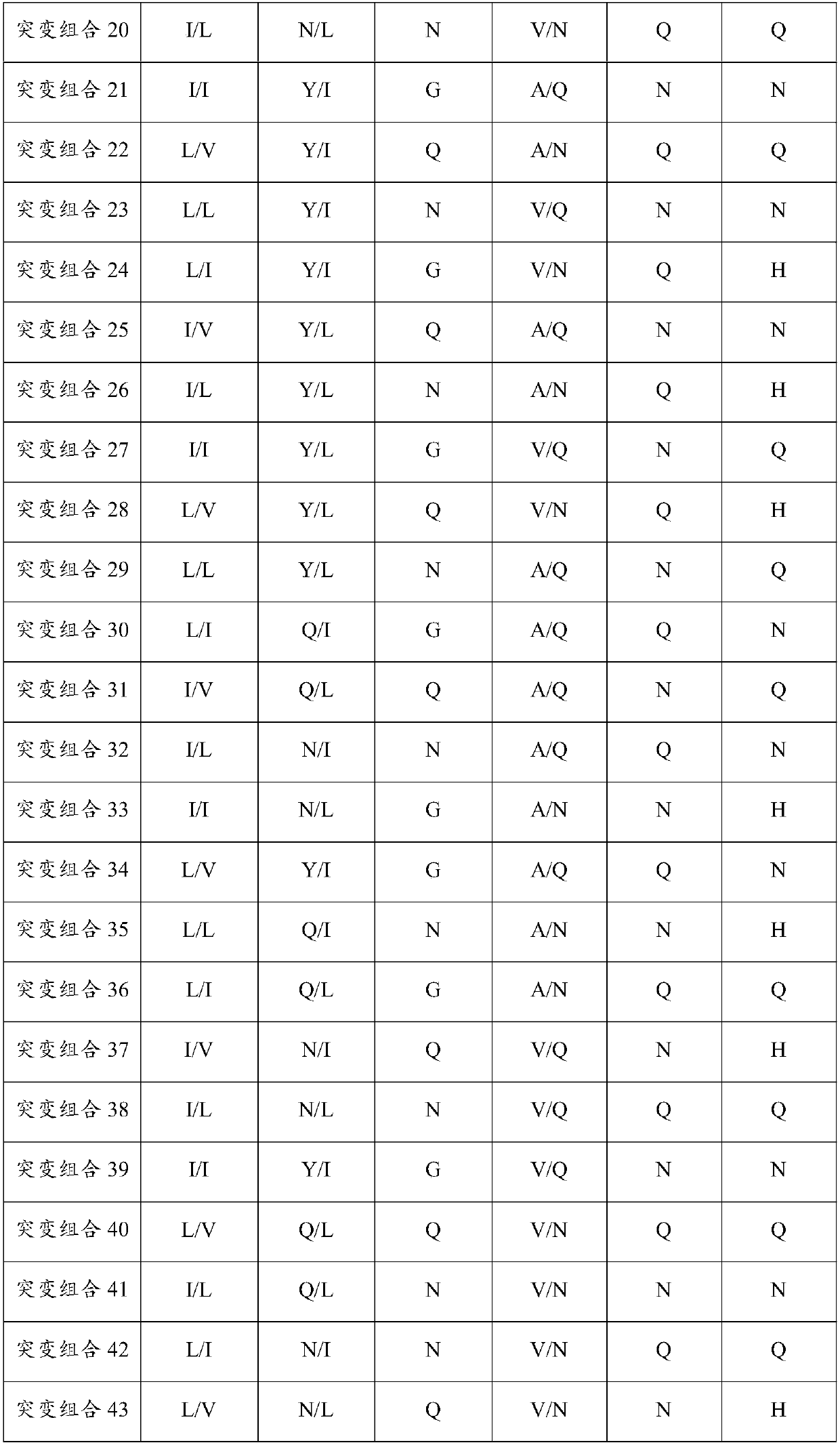
Cps-i antibodies and uses thereof
ActiveCN114213542AImprove featuresIncreased sensitivityHybrid immunoglobulinsBiological material analysisI antibodyAntiendomysial antibodies
The present invention relates to CPS-I antibodies and uses thereof. The present invention relates to an anti-CPS-I antibody or an antigen-binding fragment thereof, said anti-CPS-I antibody comprising at least one CDR selected from the following sequences: SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5, and SEQ ID NO: 6. Experimental results show that the antibody disclosed by the invention can be applied to immune response aiming at CPS-I.
Owner:郑州大学第三附属医院
Anti-human cardiac troponin I antibody and applications thereof
ActiveCN111018978AHigh activityHigh affinityImmunoglobulins against animals/humansDisease diagnosisI antibodyHumanin
The invention relates to a novel isolated binding protein containing a cTnI antigen binding domain, and researches of the preparation, the applications and the like of the binding protein. The bindingprotein is high in activity, has high affinity with human cTnI protein, and can be widely applied to the field of cTnI protein detection.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
Quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for angiotensin (Ang) I, and preparation method of kit
ActiveCN102998465BLow cross-specificityImprove performanceChemiluminescene/bioluminescenceBiological testingI antibodyBiotin-streptavidin complex
Owner:BIOSCIENCE (TIANJIN) DIAGNOSTIC TECH CO LTD
A kind of anti-human cardiac troponin I antibody and its application
The present invention relates to an isolated binding protein comprising cTnI antigen binding domain, and researches on the preparation, application and other aspects of the binding protein. The binding protein has strong activity and high affinity with human cTnI protein, and can be widely used in the detection field of cTnI protein.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com